Abstract
Cholangiocarcinoma (CCA) is most strongly associated with risk factors characterized by chronic inflammatory states. However, most CCAs are sporadic, with no identifiable risk factors. In this chapter, we provide a detailed review of the current evaluation and management of CCA, with a particular emphasis on the imaging features. Imaging features of CCA are dependent on the location, size, morphologic growth pattern, and degree of intratumoral fibrosis, necrosis, or mucin content. Ultrasound (US), endoscopic US, computed tomography (CT), magnetic resonance imaging (MRI)/ magnetic resonance cholangiopancreatography (MRCP), and positron emission tomography (PET) are the imaging modalities typically used to diagnose and monitor CCA. Histologically, CCAs are primarily adenocarcinomas. Generally, the more centrally located perihilar or distal CCAs are more likely to have well-formed glands lined by columnar epithelial cells with mucin production, whereas CCAs located at the liver periphery are more likely to grow as irregular, anastomosing tubular structures lined by low cuboidal cells that do not produce mucin. Treatment options for CCA include surgical resection, local image-guided percutaneous thermal and nonthermal ablative therapies, locoregional therapies such as transarterial chemoembolization, transarterial radioembolization and stereotactic body radiation therapy, and systemic therapies, which are increasingly targeted to characteristic oncogenic mutations or fusion proteins.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cholangiocarcinoma
- CCA
- Imaging
- CT
- MRI
- Pathology
- Treatment
- Therapy
- Resection
- Surgery
- Transplant
- Ablation
- Chemoembolization
Epidemiology of BTC
Cholangiocarcinoma (CCA) is a malignancy that arises from the epithelium lining the bile ducts. CCAs are subdivided based on anatomic criteria into intrahepatic (iCCA), perihilar (pCCA), and distal cholangiocarcinoma (dCCA). The most recent estimates from the Global Burden of Disease Study are of 184,000 incident cases of biliary tract cancer (BTC) worldwide in 2016, with 108,000 (59%) occurring in women and 76,000 (41%) in men [1]. Particularly concerning is the evidence for increasing incidence of CCA in younger birth cohorts. The risk factors most strongly associated with CCA are those characterized by chronic inflammatory states. However, most CCAs are sporadic, with no identifiable risk factors. In Western countries, primary sclerosing cholangitis (PSC) is the most recognized risk factor for CCA. Even though PSC is a well-established risk factor for CCA, the incidence of CCA in patients with PSC is relatively low at 0.5–1.5% per year, reflecting the fact that most of the CCA cases diagnosed in Western countries arise de novo [2,3,4,5,6,7].
At present, there are no effective screening protocols for early detection of sporadic CCA, nor explicit recommendations for screening of PSC patients for CCA. The most effective strategy for detecting early CCA in PSC involves annual magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP) or ultrasound and CA 19-9, followed by endoscopic retrograde cholangiopancreatography (ERCP) and brush cytology or forceps biopsy for evaluation of suspicious strictures [8]. This strategy, however, is invasive and costly [9]. The only curative modality for CCA is surgical resection or liver transplantation for early-stage disease [10]. The vast majority of patients with CCA have late-stage disease not amenable to surgical resection with curative intent [11]. The standard of care for intermediate to advanced stage CCA, which is chemotherapy, is typically accompanied by significant toxicity and a high rate of recurrence. In this chapter, we provide a detailed review of the current practices employed to evaluate and manage patients diagnosed with CCA, with a particular emphasis on the imaging features.
Imaging of Cholangiocarcinoma (CCA)
Imaging Classification Overview: Anatomic Location and Morphology
Cholangiocarcinoma (CCA) can be classified at imaging by anatomic location and morphologic growth pattern [12]. CCA classified by location includes (i) intrahepatic CCA (iCCA) occurring proximal to the second-order bile ducts, (ii) perihilar CCA (pCCA) occurring from the second-order bile ducts to the level of the cystic duct origin, and (iii) distal CCA (dCCA) occurring from the cystic duct origin to the ampulla of Vater [13, 14]. CCA classified by morphologic growth pattern includes (i) mass-forming exophytic subtype which appears as a focal hepatic mass, (ii) periductal infiltrating subtype which appears as longitudinal tumor with growth along the bile ducts, (iii) intraductal polypoid type which appears as a focal intraluminal mass, and (iv) mixed pattern [15]. The initial imaging modality for detection of CCA may vary from incidental detection such as in a patient with new onset jaundice versus screening detection in high-risk populations such as those with known cirrhosis or primary sclerosing cholangitis [16] (Figs. 2.1, 2.2, 2.3, 2.4, 2.5, and 2.6).
Imaging Features
Intrahepatic CCA (iCCA)
iCCA or peripheral cholangiocarcinoma occurs proximal to the second-order bile ducts. The mass-forming exophytic type is the most common subtype of iCCA (80%) followed by periductal infiltrating, intraductal growth with or without papillary features, and mixed subtypes [15, 17]. Imaging features of iCCA are dependent on size, morphologic growth pattern, and degree of intratumoral fibrosis, necrosis, or mucin content (Figs. 2.1, 2.2, 2.3, and 2.4).
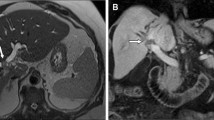
Small mass-forming intrahepatic cholangiocarcinoma (iCCA) evaluated with extravascular contrast on MRI. The mass shows (a) peripheral DWI hyperintensity (white arrow), (b) T2-weighted hyperintensity, (c) T1-weighted hypointensity, (d) peripheral arterial phase hyperenhancement (white arrow), and (e, f) progressive centripetal enhancement on (e) portal venous and (f) delayed phase MRI (white arrow). DWI diffusion-weighted imaging, MRI magnetic resonance imaging
Small mass-forming intrahepatic cholangiocarcinoma (iCCA) evaluated with hepatobiliary contrast on MRI. The mass shows (a) T1-weighted hypointensity (white arrow), (b) peripheral arterial phase hyperenhancement (white arrow), (c, d) progressive centripetal enhancement on (c) portal venous and (d) delayed phases (white arrow), and (e, f) a rim of peripheral hypointensity with a central hypointense focus on the hepatobiliary phase imaging. DWI diffusion-weighted imaging, MRI magnetic resonance imaging
Large mass-forming intrahepatic cholangiocarcinoma (iCCA) CT, 18F-FDG PET, and MRI. The mass is (a) isodense with areas of central hypodensity on noncontrast CT (white arrow). (b) Axial 18F-FDG-PET/CT show heterogeneous hypermetabolism in the periphery of the hepatic mass (white arrow). The mass shows (c) DWI hyperintensity (white arrow), (d) heterogenous T2-weighted hyperintensity, (e) T1-weighted hypointensity, (f) peripheral arterial phase hyperenhancement (white arrow), and (g–i) progressive centripetal enhancement on portal venous and delayed phases (white arrow). 18F-FDG 8F-fluorodeoxyglucose, PET positron emission tomography, CT computed tomography, MRI magnetic resonance imaging, DWI diffusion-weighted imaging
Intraductal intrahepatic cholangiocarcinoma (iCCA) in a patient with ulcerative colitis and primary sclerosing cholangitis evaluated with CT and MRI/MRCP. The ill-defined mass in the left hepatic lobe is (a) hypodense with minimal enhancement on portal venous phase CT (white arrows) and shows (b) T2-weighted hyperintensity (white arrow) and (c) DWI-weighted hyperintensity (white arrow) on MRI. (d) Coronal MIP MRCP shows marked intraductal/periductal irregularity of the intrahepatic bile ducts in the left hepatic lobe (white arrows). CT computed tomography, MRI magnetic resonance imaging, MRCP magnetic resonance cholangiopancreatography, DWI diffusion-weighted imaging, MIP maximum intensity projection
Ultrasound (US)
On US, mass-forming iCCA may appear as a nonspecific focal mass with variable echogenicity ranging from a hypoechoic (<3 cm) to hyperechoic (>3 cm) mass with heterogeneous echotexture depending on degree of fibrosis, necrosis, mucin content, or calcification and occasional peripheral echogenic rim [18, 19]. The periductal infiltrating subtype may appear as a small mass or with diffuse bile duct thickening with or without a bile duct stricture and peripheral dilated ducts [18, 20]. The intraductal subtype may demonstrate focal or diffuse biliary ductal dilatation with or without an echogenic intraluminal mass with papillary features [18]. A few studies have examined contrast-enhanced US (CEUS) in iCCA and hepatocellular carcinoma (HCC), with conflicting results. Although studies have identified similar arterial phase enhancement between iCCA and HCC, both overlapping and nonoverlapping portal venous and delayed phase washout kinetics have been reported with dynamic CEUS [21, 22].
Computed Tomography (CT)
On CT, mass-forming iCCA may appear as a well-defined focal mass or a poorly defined infiltrative mass that is typically homogeneously hypo- to iso-attenuating to the normal hepatic parenchyma on unenhanced CT [19, 21, 23,24,25]. Typical enhancement characteristics include peripheral or rim arterial phase enhancement followed by gradual centripetal enhancement in the portal venous and delayed phases relative to the normal background liver [18, 26,27,28,29,30]. The viable tumor at the periphery of iCCA may show arterial phase enhancement with subsequent iso- to hypoenhancement during the portal venous phase [28]. This appearance, which is sometimes referred to as the “target pattern,” is not specific to iCCA and may also be seen in colon carcinoma metastases. Conversely, the central portion of the iCCA may show central hypoenhancement with centrally necrotic tumors and/or those with higher mucin content [28, 30]. Associated findings include satellite lesions, hepatic capsular retraction which may be seen in up to 20% of patients, and vascular invasion [20]. Some small mass-forming iCCA may demonstrate arterial phase hyperenhancement (APHE), similar to HCC; as such, portal venous and delayed phase enhancement characteristics become important for differentiating these two. The periductal infiltrating subtype may show diffuse biliary ductal mural thickening and enhancement as well as dilated or narrowed ducts [18]. The CT appearance of the intraductal subtype is the most variable and depends on the presence of an intraluminal mass and the degree of biliary ductal obstruction. Different patterns include (i) an intraluminal mass that is hypo- to isoattenuating to liver on unenhanced CT and enhances with contrast associated with concomitant diffuse severe upstream biliary ductal dilatation, (ii) severe intrahepatic biliary ductal dilatation without an intraductal mass, (iii) an intraductal mass with only localized or mild biliary ductal dilatation, or (iv) a focal biliary stricture with mild proximal biliary ductal dilatation [18]. Primary hepatic tumors with biphenotypic characteristics of both CCA and HCC may demonstrate overlapping imaging features and may ultimately require biopsy for diagnosis and treatment planning [31].
Magnetic Resonance Imaging/Magnetic Resonance Cholangiopancreatography (MRI/MRCP)
The morphologic, signal, and enhancement features of iCCA at MRI depend on the morphologic growth pattern and degree of intratumoral fibrosis, necrosis, mucin content, and/or hemorrhage [18, 26]. Mass-forming iCCA is typically iso- to hypointense on T1-weighted (T1W) imaging relative to background liver with possible foci of T1 hyperintensity when intratumoral hemorrhage is present. Additionally, mass-forming iCCA typically shows variable hypo- to hyperintensity on T2-weighted (T2W) imaging depending on the degree of fibrosis (more T2 hypointense) versus necrosis or mucin content (more T2 hyperintense) [18, 25, 27]. On diffusion-weighted imaging (DWI), 50–75% of iCCA may demonstrate target-like central hypointensity with peripheral high signal intensity at high b-values [25, 32, 33]. Additionally, MRCP may show bile duct invasion [20]. Similar to CT, gadolinium-enhanced MRI with extracellular-based agents shows peripheral or rim arterial phase enhancement with patchy central enhancement on portal venous phase and progressive central enhancement on delayed phase imaging [18, 20, 25, 34] with peripheral washout in the portal venous and/or delayed phases. Areas of early enhancement correlate with viable tumor, whereas areas of delayed enhancement correlate with the relatively hypovascular fibrosis. Conversely, gadolinium-enhanced MRI with hepatobiliary-specific contrast agents shows relative hypoenhancement of iCCA relative to the background liver [18, 35]. A target sign has been described in intrahepatic cholangiocarcinoma in the hepatobiliary phase with hepatocyte-specific agents. This is due to circulating contrast agents that tend to remain in the extracellular space associated with fibrosis. This appearance is not specific to iCCA but can also be seen in fibrous tumors such as fibrolamellar hepatocellular carcinoma or treated colorectal metastases. CT and MRI have similar diagnostic performance in the detection of primary and satellite iCCA lesions, but the spatial resolution of CT is superior for the detection of vascular involvement [36]. The morphologic appearance and enhancement characteristics of the periductal infiltrating and intraductal subtypes of iCCA are similar between CT and MRI [18].
Positron Emission Tomography (PET)
18F-florodeoxyglucose (18F-FDG) PET/CT can provide metabolic information related to tumoral glucose uptake and is the most common radiotracer investigated in iCCA. Viable tumor shows FDG uptake, while centrally necrotic or fibrotic portions of the tumor will appear as a photopenic defect. 18F-FDG PET/CT has been shown to be accurate for the evaluation and detection of primary tumors as well as both lymph node and distant metastases in patients with iCCA [37]. Moreover, quantitative tumor standardized uptake value max (SUV-max) has been shown to be an independent prognostic factor for oncological outcomes in patients with resectable iCCA, with tumor SUV-max >8 associated with worse disease-free and overall survival after surgical resection [38, 39]. Moreover, iCCA has been shown to have greater SUV-max compared to extrahepatic CCA [40].
Perihilar CCA (pCCA)
pCCA develops from the second-order bile ducts to the common bile duct at and above the cystic duct origin and may be nodular, sclerosing (periductal infiltrating), or papillary morphologic subtypes [12, 27] (Fig. 2.5). Nodular pCCA tends to grow intraluminally with bile duct invasion resulting in significant fibrotic reaction [41]. Conversely, papillary pCCA grows intraluminally without invasion of the bile duct wall [42]. Sclerosing pCCA produces concentric thickening of the bile duct and eventual duct obliteration without a discrete mass [43]. Early pCCA is very difficult to detect due to their small tissue volume that causes only focal thickening of the bile duct wall and relatively less stricturing or complete biliary obstruction.
Perihilar cholangiocarcinoma—“Klatskin” tumor—evaluated with 18F-FDG PET/CT and MRI/MRCP. (a) Coronal 18F-FDG-PET/CT shows a hypermetabolic mass in the central liver (white arrow). (b) Coronal MIP MRCP shows dilatation of intrahepatic bile ducts beginning at the confluence of the left and right hepatic ducts secondary to an hilar obstructing mass and no filling of the extrahepatic bile duct (white arrow). (c–e) MRI shows an ill-defined mass in the porta hepatis that shows (c) peripheral arterial phase hyperenhancement (white arrow) and (d, e) slight progressive centripetal enhancement on (d) portal venous and (e) delayed phases (white arrow). 18F-FDG 18F-fluorodeoxyglucose, PET positron emission tomography, CT computed tomography, MRI magnetic resonance imaging, MRCP magnetic resonance cholangiopancreatography, MIP maximum intensity projection
Ultrasound (US)
Because patients with pCCA often present with obstructive jaundice, US is often the initial imaging modality for evaluation of biliary duct obstruction and is helpful for identifying the level of obstruction [27]. US has a reported sensitivity and specificity of 89% and 80–95% for detection of pCCA [27, 44, 45]. An intraluminal mass with variable echogenicity ranging from hypo- to hyperechoic with upstream ductal dilatation may been seen. Nonetheless, while a mass or stricture may not be directly visualized, US is useful for detecting invasion of the liver parenchyma or portal veins and can help guide next steps for invasive or noninvasive imaging evaluation.
Computed Tomography (CT)
Unenhanced CT may show a variably defined hypodense mass centered near the porta hepatis, which demonstrates variable progressive enhancement of contrast-enhanced imaging. The overall diagnostic accuracy of CT has been reported at 79–92%, and it may be particularly useful for demonstrating the level of biliary obstruction, extent of local invasion into adjacent tissue, and metastatic disease in the abdomen and pelvis [27, 28]. Moreover, CT with angiogram (CTA) and venogram (CTV) protocols are accurate for detection of hepatic arterial and portal vein involvement by pCCA in up to 87–93% of cases [26, 27, 46, 47]. Nevertheless, CT may underestimate the longitudinal extension of tumor along the bile duct for periductal infiltrating subtypes as well as regional lymphadenopathy and peritoneal metastases in up to 50% of cases [36, 46]. Streak artifact from metallic biliary stents may further limit evaluation of locoregional disease extent [46]. As such, CT cholangiography may provide further detail on the biliary anatomy and is an option when MRCP is not available [47]. Nonetheless, CT cholangiography is dependent on a functioning secretory system of the biliary tree, which may be limited in patients with severe biliary obstruction or hyperbilirubinemia.
Magnetic Resonance Imaging/Magnetic Resonance Cholangiopancreatography (MRI/MRCP)
Periductal infiltrating pCCA may be difficult to directly visualize at MRI in the absence of a mass-like lesion. Consequently, tumor extent may be inferred by secondary signs, including proximal biliary ductal dilatation, periductal thickening, and enhancement. Intraductal mass is rare but when present often appears as hyperintense on T2-weighted imaging, hypointense on T1-weighted imaging, and with mild hypoenhancement relative to the liver with the use of extracellular gadolinium-based contrast agents [12, 16, 18, 27]. In addition, hepatobiliary-specific contrast agents may provide the dual benefit of dynamic contrast-enhanced imaging followed by delayed hepatobiliary phase imaging for better delineation of the biliary tree [48]. The utility of hepatobiliary contrast alone for evaluation of pCCA still needs further evaluation as dynamic contrast-enhanced phases are often limited or of inferior quality compared to standard extracellular contrast agents.
MRCP is a particularly accurate method for imaging the biliary tree and is the imaging modality of choice in patients with suspected CCA in conjunction with MRI, particularly for periductal infiltrating tumors [12, 16, 25, 27, 49]. MRCP is ideally performed prior to decompression of the biliary tree by percutaneous or endoscopic techniques. MRCP and ERCP serve complementary roles with MRCP being better able to evaluate the peripheral hepatic ducts. Overall, MRI/MRCP has an overall diagnostic accuracy of 66% for detection of locoregional lymph node metastases, 78% sensitivity and 91% specificity for portal vein invasion, and 58–73% sensitivity and 93% specificity for hepatic arterial invasion, slight less than CT [50,51,52]. As such, CT and MRI/MRCP serve complementary roles in the diagnosis and staging of pCCA with CT better at demonstrating vascular involvement while MRI/MRCP better demonstrates extent of biliary neoplastic invasion [51, 53].
Invasive Cholangiography: Endoscopic Retrograde Cholangiopancreatography (ERCP) or Percutaneous Transhepatic Cholangiography (PTC)
Both ERCP and PTC are invasive techniques that can be both diagnostic and therapeutic [54, 55]. Both techniques are useful diagnostically for delineating the biliary tree, location of biliary pathology and/or strictures, and obtaining tissue for histologic, cytologic, or molecular testing. Moreover, ERCP and PTC can be therapeutic with the ability to place internal biliary stents or internal–external biliary drains to decompress an obstructed biliary system [54]. Overall sensitivity and specificity of invasive cholangiography is ~75% with an accuracy of 95% for diagnosis of pCCA [56]. Percutaneous transhepatic biliary drainage (PTBD) has been shown to have a lower complication rate compared to endoscopic biliary drainage (EBD) in the preoperative setting prior to CCA resection [57].
Endoscopic Ultrasound (EUS)
EUS has emerged as an important modality for assessment of pCCA with advantages for evaluating extent of local periductal tumor invasion and regional lymph nodes [58].
Positron Emission Tomography (PET)
Experience with 18F-FDG PET/CT or PET/MRI in pCCA is much more limited than for iCCA, but it may be helpful in detection of distant metastatic disease (Fig. 2.5).
Distal CCA (dCCA)
dCCA develops in the common bile duct between the cystic duct origin and the ampulla of Vater. In general, imaging findings of dCCA and pCCA are similar, with the two subclasses often referred to together as extrahepatic cholangiocarcinoma [12, 18, 27] (Fig. 2.6).
Periductal infiltrating cholangiocarcinoma of the distal common bile duct evaluated with ERC/EUS and MRI. (a) Endoscopic retrograde cholangiography (ERC) shows irregular narrowing of the distal common bile duct (CBD) (white arrow). (b) Endoscopic ultrasound shows marked mural thickening of the distal common bile duct (white arrow) and a large porta hepatis lymph node (white asterisk). (c–e) MRI shows (c) the periductal infiltrating soft tissue with T2-weighted iso- to hypointensity and (d) diffuse enhancement as well as (e) multiple enlarged porta hepatis lymph nodes that demonstrate DWI hyperintensity. MRI magnetic resonance imaging, DWI diffusion-weighted imaging
Ultrasound (US)
Similar to pCCA, US may be the first imaging modality in the setting of new obstructive jaundice and is helpful for identifying the level of obstruction, upstream biliary ductal dilatation, and guiding subsequent invasive and noninvasive imaging.
Computed Tomography (CT) and Magnetic Resonance Imaging/Magnetic Resonance Cholangiopancreatography (MRI/MRCP)
CT and MRI/MRCP may demonstrate thickening, enhancement, or stricturing of the common bile duct, with or without an enhancing intraluminal mass. Contrast-enhanced CT or MRI provides important information about tumor involvement of the duodenum and pancreas as well as vascular involvement of the portal vein (PV) or hepatic artery (HA) and locoregional lymph node metastases.
Endoscopic Retrograde Cholangiopancreatography (ERCP) and Endoscopic Ultrasound (EUS)
ERCP has a high diagnostic accuracy for detection of dCCA and evaluating the extent of tumor involvement of the biliary tree. Moreover, EUS is helpful for evaluating invasion of the biliary wall, hepatic vasculature, and pancreas, as well as detecting porta hepatis lymph node metastases. EUS with fine-needle aspiration (FNA) can be used for sampling the primary tumor as well as locoregional lymph nodes and is often diagnostic. Importantly, individuals who are potential candidates for liver transplantation for their pCCA or dCCA should not have FNA sampling of the primary tumor through the bile duct wall, as that increases the risk of tumor dissemination and is a contraindication to liver transplantation.
Pathology
Grossly, intrahepatic cholangiocarcinoma is typically firm and not encapsulated (Fig. 2.7). It is usually mass forming but can sometimes grow in a diffusely infiltrating periductal distribution or display an intraductal growth pattern [13, 18, 59, 60]. Histologically, cholangiocarcinomas are primarily adenocarcinomas, but other rare histologic variants also exist [61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84]. Extrahepatic cholangiocarcinoma, particularly perihilar cholangiocarcinoma, is typically rich in fibrous stroma, and often has a dense desmoplastic response; this results from extracellular matrix production by activated myofibroblasts present in the stroma [85] (Fig. 2.8). In most cholangiocarcinomas, the tumor forms glands or tubules which are lined by epithelial cells (Fig. 2.9). Generally, the more centrally located hepatic cholangiocarcinomas are more likely to have well-formed glands lined by columnar epithelial cells with mucin production, whereas cholangiocarcinomas located at the liver periphery are more likely to grow as irregular, anastomosing tubular structures lined by low cuboidal cells that do not produce mucin [65]. Cholangiocarcinoma can also have a variety of growth patterns, often present in the same tumor, including irregular tubules, infiltrating glands, solid nests, trabeculae, and micropapillary structures (Fig. 2.10). Cholangiocarcinoma can grow along sinusoids and spread extensively throughout the liver through the portal venous or lymphatic system (Fig. 2.11). Perineural invasion is usually seen only where the larger nerves of the liver are located, in the large portal areas close to the liver hilum, and is more frequently seen in perihilar cholangiocarcinoma.
Although there is not a universally adopted grading system for cholangiocarcinoma, many pathologists in the United States utilize a four-tier grading system adapted by the College of American Pathologists (CAP) and American Joint Committee on Cancer (AJCC), based on the percentage of the glandular component in the tumor [86]. According to this schema, adenocarcinomas are graded as follows: well-differentiated (grade 1), moderately differentiated (grade 2), poorly differentiated (grade 3), and undifferentiated (grade 4). Tumor grade is an independent predictor of patient survival and cancer recurrence [87, 88]. Of note, rare variants of cholangiocarcinoma cannot be graded according to this scheme and are usually not assigned a specific grade.
Immunohistochemical stains are frequently performed on cholangiocarcinoma to exclude metastatic disease to the liver or hepatocellular carcinoma. Cholangiocarcinoma shows positive cytoplasmic staining with polyclonal carcinoembryonic antigen (CEA). CK19 is also positive in 70–80% of cases, and MOC31, a monoclonal antibody that recognizes an epithelial-associated glycoprotein also known as Epithelial Specific Antigen/Ep-CAM, is positive in 90% of cases [89]. Intrahepatic cholangiocarcinoma is virtually always positive for CK7, but varies in CK20 expression. Interestingly, the immunoprofile of cholangiocarcinoma can depend on its location. For example, 50% of peripheral cholangiocarcinomas are CK20 negative, while central and extrahepatic cholangiocarcinomas tend to be CK20 positive [90]. Likewise, peripheral cholangiocarcinomas, especially those with a “bile ductular” pattern, tend to express CD56 [91]. Finally, focal positive staining for HepPar-1, though rare, is more commonly seen in peripherally located tumors.
The histologic differential diagnosis for cholangiocarcinoma includes metastatic adenocarcinoma to the liver, hepatocellular carcinoma, epithelioid hemangioendothelioma, bile duct adenoma, bile duct hamartoma, and biliary adenofibroma. Immunohistochemical stains, when necessary, are used in conjunction with tumor morphology to distinguish cholangiocarcinoma from these tumors. Cholangiocarcinoma has an immunoprofile that is similar to that of other pancreaticobiliary and upper gastrointestinal adenocarcinomas. In recent times, however, in situ hybridization for albumin has shown high sensitivity and specificity in distinguishing hepatic cholangiocarcinoma from metastatic adenocarcinoma to the liver or carcinoma of unknown origin [92]. Hence, the pathologic diagnosis is based on exclusion of other tumors using morphology, immunostains, in situ hybridization, imaging studies, and clinical findings; once other carcinomas have been excluded, a diagnosis of cholangiocarcinoma can be made.
Diagnosis Using Fluorescence In Situ Hybridization (FISH)
Infiltrating extrahepatic cholangiocarcinoma and pancreatic ductal cancers are often sampled by endoscopic brushing cytology. Although routine cytology has been the primary tool for detecting pancreatobiliary tract malignancy and has near perfect clinical specificity, the diagnostic sensitivity of routine cytology is limited and varies considerably based on stage at diagnosis, cytology collection type, and patient cohort (PSC vs. non-PSC, mass presenting lesions, etc.). More specifically, a review demonstrated a wide range of performance characteristics based on cytology preparation, including pancreatobiliary brushings (sensitivity range, 26–89%; specificity range, 80–100%; accuracy range, 48–96%), bile duct brushings (sensitivity range, 33–54%; specificity range, 100%; accuracy range, 43–67%), pancreatic duct brushings (sensitivity range, 47–66%; specificity range, 100%; accuracy range, 67–79%), and bile cytology (sensitivity range, 6–50%; specificity range, 100%; accuracy range, 31–57%) [93].
The main limitation of cytology is false-negative results in patients with pancreatobiliary tract cancer. As a result, ancillary molecular markers can be utilized to increase diagnostic sensitivity. Cancer genomes contain a wide assortment of genetic alterations that activate oncogenes or that inactivate tumor suppressor genes, including single-nucleotide substitutions, structural rearrangements, small insertions, small deletions, and copy number variation. Fluorescence in situ hybridization (FISH) is a technique that uses fluorescently labeled DNA probes to detect chromosomal copy number variation. For pancreatobiliary testing, FISH probes are specifically designed to assess for neoplastic cells with chromosomal abnormalities (i.e., aneuploidy) in neoplastic cells among a background of diploid nonneoplastic cells. Nonneoplastic cells generally show disomy, with two copies for each of the FISH probes, because each probe targets the two alleles in an individual cell (Fig. 2.12). Specimens are interpreted as abnormal when the number of cells demonstrating losses or gains of probes exceeds the thresholds established in normal value studies for the FISH probes used.
The majority of publications have focused on one of two FISH probe sets. The UroVysion probe set (Abbott Molecular, Inc., Des Plaines, IL) contains a probe directed to the CDKN2A gene located at 9p21 and chromosome enumeration probes directed to chromosomes 3, 7, and 17. In 2009, Fritcher et al. published the most comprehensive report of FISH testing with UroVysion and indicated that the sensitivity of FISH was significantly higher than cytology for detecting malignancy (43% vs 20%; P < 0.001) [94]. Many other institutions have also reported the improved performance characteristics of FISH using the UroVysion probe set for detecting pancreatobiliary tract malignancy [3]. More recently, a newly tailored pancreatobiliary FISH probe set targeting chromosomal regions 1q21 (MCL1), 7p12 (EGFR), 8q24 (MYC), and 9p21 (CDKN2A) has gained acceptance clinically. In a comparison study, the newer tailored pancreatobiliary FISH probe set had a significantly higher sensitivity (64.7%) than the UroVysion FISH probe set (45.9%) for detecting malignancy and is now the preferred probe set for these specimens [95]. Representative examples of the pancreatobiliary probe set are shown in Fig. 2.12. Future molecular markers and newer technologies for assessing cytology specimens for malignancy will likely continue to improve detection and direct therapeutic decisions.
Treatments with Curative Intent
Surgical resection is the standard treatment for CCA. The goal is complete removal of the tumor with a negative margin and an adequate functional liver remnant (FLR). The use of strategies such as portal vein embolization, preoperative biliary drainage, and complex vascular reconstructive techniques has improved outcomes following surgical resection [96,97,98,99,100,101]. Staging laparoscopy prior to laparotomy is recommended, especially in patients with high CA 19-9 to assess for evidence of peritoneal metastasis, given that resection is not beneficial in this setting [102].
For iCCA, surgical therapy usually consists of hemi-hepatectomy with excision of regional nodes to ensure adequate staging [103]. Most guidelines recommend resection only for single iCCA tumors, though recent reports have also noted benefit in patients with two or three lesions [104, 105]. Outcomes following resection in patients with iCCA are related to the extent of disease and the ability to obtain a complete resection. In a recent large multicenter series of 1013 patients, those with a single completely resected tumor had a 43% 5-year survival, compared to 28% 5-year survival for those with two tumors [105]. The use of liver transplantation (LT) has been recently described in a multicenter retrospective series of 15 patients with small unresectable (<2 cm) iCCA occurring in the setting of decompensated cirrhosis, achieving a 65% 5-year survival [106, 107]. On the opposite end of the spectrum, favorable outcomes following LT for patients with large, indolent unresectable iCCA occurring in the setting of normal background liver with no evidence of metastasis and a prolonged period of disease stability following chemotherapy have also recently been reported [108]. Prospective data collection from larger series will be needed to confirm these preliminary findings.
Resection is also the standard therapy for patients with pCCA though unfortunately many patients present with unresectable disease either due to metastatic disease, extensive bi-lobar involvement precluding resection, or advanced underlying liver disease such a primary sclerosing cholangitis (PSC). For those who are eligible, resection typically involves an (extended) hemi-hepatectomy including the caudate lobe with en bloc resection of the extrahepatic bile duct as well as regional lymph nodes [109]. Even in those thought to be resectable, a complete resection is only achieved in approximately 70% of cases [109,110,111]. Outcomes following resection of pCCA depend on the ability to obtain a complete resection as well as the presence of nodal disease, and typically range from 25% to 45% 5-year survival [109,110,111].
Liver transplantation was initially considered an ideal strategy to improve the likelihood of complete resection for patients with pCCA, but outcomes for LT alone were poor due to a high rate of disease recurrence [112, 113]. Because of this unacceptable rate of disease recurrence for LT alone, a protocol combining neoadjuvant chemoradiotherapy followed by LT for patients with early-stage unresectable pCCA was developed [114, 115]. The use of this combined protocol has achieved 5-year survival rates of 65–70%, leading to the adoption of neoadjuvant chemoradiotherapy followed by liver transplantation as a part of standard organ transplant allocation policy for patients with early-stage pCCA [116,117,118,119].
The benefit of combined neoadjuvant therapy and LT for patients with unresectable pCCA has led to the question of whether the same therapy should be offered to patients with resectable pCCA. The severe shortage of available liver allografts and the need for lifelong immunosuppression are key obstacles to this strategy. Recently, a multicenter retrospective analysis found that those with unresectable pCCA undergoing combined neoadjuvant therapy + LT protocol had superior 5-year survival (64% vs 18%; P < 0.001), compared to patients undergoing resection who otherwise met LT criteria, and this remained even after accounting for tumor size, nodal status, and PSC (P = 0.049) [120].
Surgical resection is often feasible for patients with early-stage distal cholangiocarcinoma with no evidence of local invasion, lymph node, peritoneal, or distant metastases. The most common operation is pancreatoduodenectomy with hepaticojejunostomy (the Whipple procedure). In selected patients in whom the tumor is located above the upper pancreatic border, an extrahepatic bile duct resection may be performed as an alternative [121].
Locoregional Interventional Radiologic Therapies for Treatment of iCCA
Local and locoregional interventional radiologic therapies include image-guided percutaneous thermal and nonthermal ablative therapies using energy-based devices and transarterial chemoembolization (TACE) or radioembolization (TARE).
Percutaneous Ablation
Image-guided percutaneous radiofrequency (RFA) and microwave (MWA) ablation have been shown to be safe and effective for treatment of iCCA in the palliative setting in patients with unresectable tumors or in those patients whose tumors have recurred after surgical resection (Fig. 2.13). Local tumor recurrence has been reported in up to 22% of patients following RFA and MWA with a greater risk of local tumor progression with primary tumors and superficially located tumors [122].
Small mass-forming intrahepatic cholangiocarcinoma (iCCA) evaluated with ultrasound and CT and treated with percutaneous microwave ablation (MWA). (a) Grayscale and color Doppler ultrasound shows a small, well-circumscribed hypoechoic mass with mild vascularity (white arrow). (b) The mass shows peripheral enhancement on contrast-enhanced CT (white arrow). (c) The mass was treated with percutaneous microwave ablation using two microwave antennae (white arrow). (d, e) Immediate postablation contrast-enhanced CT shows the hypoechoic ablation zone encompassing the tumor without any residual enhancing tumor on (d) arterial or (e) portal venous phase (white arrow). CT computed tomography
Irreversible Electroporation (IRE)
Percutaneous irreversible electroporation (IRE) is a nonthermal-based ablation treatment option that induces pores in cell membranes, leading to cell death by complex mechanisms. Few reports have demonstrated the safety, feasibility, and early local tumor control of image-guided percutaneous IRE in patients with iCCA and pCCA [123, 124]. Currently, there is an ongoing phase I/II multicenter trial of ablation with IRE in patients with advanced pCCA [125].
Transarterial Chemoembolization (TACE) and Radioembolization (TARE)
Transarterial chemoembolization (TACE) may be performed with drug-eluting beads (DEB-TACE) or with conventional embolic agents mixed with chemotherapeutics (cTACE). Transarterial radioembolization (TARE) is performed with yttrium-90 (Y90) beta-emitting radioactive glass or resin microspheres (Fig. 2.14). DEB-TACE, cTACE, and Y90-TARE have all been shown to be safe and effective for treatment of unresectable CCA in the palliative setting. Median overall survival for TACE ranges from 12 to 15 months with an improved toxicity profile of DEB-TACE compared to cTACE [126,127,128,129,130,131]. Similarly, median overall survival for TARE ranges from 11 to 22 months [130, 132,133,134,135].
Large mass-forming intrahepatic CCA with hepatic vein invasion evaluated with CT and catheter angiography and treated with transarterial radioembolization (TARE). The mass in the central superior right hepatic lobe shows (a) minimal central enhancement on contrast-enhanced CT (white arrow) and (b) mild enhancement on selective right hepatic arteriogram (white arrow). The mass was treated with yttrium-90 (Y90) transarterial radioembolization (TARE). (c) Post-Y90 SPECT/CT bremsstrahlung scan shows intense uptake within the tumor corresponding with the region of treated tumor. SPECT single-photon emission computed tomography, CT computed tomography
Radiation Therapy
External Beam Radiotherapy
External beam radiotherapy (EBRT) plays a role in the treatment of localized intrahepatic (iCCA) and extrahepatic (eCCA) cholangiocarcinoma. Advances in diagnostic imaging and EBRT planning and delivery allow for potential radiotherapy dose escalation and/or improved protection of normal tissues, which may improve the therapeutic ratio for treatment of CCA.
For patients with resected iCCA or eCCA and features suggestive of a high risk for local/regional recurrence, such as positive surgical margins and/or regional lymph node involvement, postoperative EBRT with concurrent chemotherapy has been utilized, with suggestion of benefit in reducing risk of recurrence and possible improvement in survival [136]. A recent multi-institutional phase II trial evaluated the safety and efficacy of an adjuvant therapy regimen for resected eCCA consisting of initial gemcitabine and capecitabine for 3 months, followed by EBRT (52.5–59.4 Gray in 25–33 fractions) with concurrent capecitabine [137]. The regimen was reasonably well tolerated and associated with promising efficacy, with 2-year overall and disease-free survival of 68% and 54%, respectively. Local/regional recurrence was uncommon, and the most common pattern of recurrence was distant metastasis.
For patients with early stage but unresectable perihilar CCA, a novel treatment approach has been utilized in select patients, consisting of preoperative EBRT (45 Gy in 30 fractions delivered twice per day over 3 weeks) with concurrent 5-flurouracil chemotherapy, followed by intracavitary bile duct brachytherapy, maintenance chemotherapy, and orthotopic liver transplantation. Favorable outcomes have been reported from Mayo Clinic and other institutions [116].
For patients with localized, unresectable iCCA, focal high-dose EBRT, using conformal, hypofractionated photon, or proton techniques, has emerged as a safe and efficacious treatment approach (Fig. 2.15). In a multi-institution phase II trial conducted in the United States, 37 patients with localized, unresectable iCCA were treated with high-dose focal proton beam radiotherapy (median dose 58.05 Gy in 15 fractions). The median overall survival was 22.5 months, and the 2-year local control rate was 94% [138].
For patients with localized eCCA not amenable to resection or liver transplantation, EBRT with concurrent chemotherapy may provide modest benefit in overall survival [139].
Chemotherapy and Other Targeted Therapies
Most patients with biliary tract cancers (BTCs) present with advanced stage disease and are only candidates for systemic therapy. Gemcitabine combined with cisplatin has emerged as a standard-of-care regimen for patients with advanced BTCs [140]. Here, we summarize recent advances in systemic and targeted therapies for the treatment of BTCs.
Taxanes have emerged as a class of cytotoxic therapies with promising efficacy in BTCs. In a single-arm Phase 2 clinical study, gemcitabine in combination with nab-paclitaxel yielded a response rate of 30%, progression-free survival (PFS) of 7.7 months, and overall survival (OS) of 12.4 months [141]. A parallel, single-arm Phase 2 trial using a triplet combination of gemcitabine, cisplatin, and nab-paclitaxel (GAP) demonstrated a response rate of 45%, PFS of 11.8 months, and OS of 19.2 months [142]. These promising data have formed the basis for a prospective, multicenter Phase 3 study comparing the GAP triplet to standard-of-care gemcitabine/cisplatin (S1815, NCT03768414) [143]. Similarly, gemcitabine has been tested in combination with fluoropyrimidines using agents such as S-1 (response rate: 15.8%, PFS: 5.8 months, OS: 15.9 months) [144] or capecitabine (PFS: 8 months, OS: 13 months) [145]. Definitive Phase 3 studies comparing these regimens have not been conducted. Nevertheless, these preliminary data are encouraging and provide alternatives for patients who are not suitable for or are found to be intolerant of platinum-based regimens. For patients who progress or have intolerance while on first-line therapies, a Phase 3 trial of modified FOLFOX (5-FU, leucovorin, oxaliplatin) versus best supportive care (ABC-06, NCT01926236) showed a benefit for the combination over best supportive care, and is currently considered the standard of care in the second-line [146].
While therapies that are currently in use in advanced BTCs largely comprise empirical use of cytotoxic therapies, precision medicine has been an area of increasing investigation. Genomic profiling of cancers has become feasible on a large scale, and initial application has been in the context of therapy selection for patients with advanced disease. Tractable targets include receptor tyrosine kinases such as fibroblast growth factor 2 (FGFR2) fusions, HER2/neu amplifications/mutations, epidermal growth factor receptor amplifications/mutations, and MET amplifications. Mutations in the metabolic enzymes isocitrate dehydrogenase 1 and 2 (IDH1/IDH2), RAS/RAF pathway (KRAS/NRAS mutations, BRAF mutations), PI3K-mTOR signaling pathway, and chromatin modifiers have also been observed.
Oncogenic fusions of FGFR2 with other proteins have been found predominantly in patients with iCCA at a frequency of ~10–15%. In this group of patients, promising clinical efficacy has been observed with a number of FGFR small molecule kinase inhibitors. These include infigratinib (BGJ398), derazantinib, and pemigatinib, which have exhibited response rates of 14–48% in single-arm Phase 2 studies [147,148,149]. Class effects have included hyperphosphatemia, rash, and eye toxicities. Resistance mechanisms are a subject of intense investigation. Emergence of gatekeeper, polyclonal mutations has been observed [150]. In April 2020 pemigatinib was approved by the US FDA for previously treated unresectable locally advanced or metastatic CCA with an FGFR2 fusion or other rearrangement.
Drugs targeting IDH1 (ivosidenib) and IDH2 (enasidenib) are approved for clinical use in patients with acute myeloid leukemia bearing these alterations. IDH1 mutations occur at a frequency of approximately 10–15% in patients with iCCA, predominantly in codon 132 [151]. IDH2 mutations are less common (~5%) and are typically seen in codon 172. In an early-phase clinical trial with ivosidenib (AG-120), a response rate of 6% and 6-month PFS of 40% were observed [152]. This led to a pivotal Phase 3 trial (ClarIDHy, NCT02989857) which demonstrated a significant improvement in progression free survival in patients with advanced IDH1 mutant CCA who had progressed on previous treatment (median 2.7 months [95% CI 1.6–4.2] vs 1.4 months [1.4–1.6]; hazard ratio 0.37; 95% CI 0.25–0.54; one-sided p < 0.0001) [153].
While not separately approved for use in advanced BTCs, tumor-agnostic drug approvals have provided a mechanism for rapid availability of promising therapies with genetic alterations amenable to therapeutic intervention. Currently, this includes pembrolizumab in patients with microsatellite instability (MSI-high) or mismatch repair deficiency (MMR). Patients with MSI-high or MMR exhibited deep and durable responses to pembrolizumab, irrespective of the organ of origin of the tumor [154]. Similarly, patients with fusions involving NTRK1, NTRK2, or NTRK3 who received larotrectinib experienced durable tumor-agnostic responses [155]. Both of these trials included patients with advanced BTCs. The prevalence of both sets of markers is only 2–3% in advanced BTC patients, but due the durability of the responses seen, the data are felt to be meaningful in nature.
As highlighted, advances in novel cytotoxic combinations, precision medicine, and immunotherapies are transforming the care of patients with advanced BTCs.
References
Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016. JAMA Oncol. 2018;4(11):1553–68. https://doi.org/10.1001/jamaoncol.2018.2706.
Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995;332(14):924–33. https://doi.org/10.1056/NEJM199504063321406.
Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Loof L, Danielsson A, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36(3):321–7. https://doi.org/10.1016/s0168-8278(01)00288-4.
Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004;99(3):523–6. https://doi.org/10.1111/j.1572-0241.2004.04067.x.
Boberg KM, Bergquist A, Mitchell S, Pares A, Rosina F, Broome U, et al. Cholangiocarcinoma in primary sclerosing cholangitis: risk factors and clinical presentation. Scand J Gastroenterol. 2002;37(10):1205–11.
Bergquist A, Glaumann H, Persson B, Broome U. Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: a case-control study. Hepatology. 1998;27(2):311–6.
Chapman MH, Webster GJM, Bannoo S, Johnson GJ, Wittmann J, Pereira SP. Cholangiocarcinoma and dominant strictures in patients with primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 2012;24(9):1051–8.
Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660–78.
Razumilava N, Gores GJ. Surveillance for cholangiocarcinoma in patients with primary sclerosing cholangitis: effective and justified? Clin Liver Dis. 2016;8(2):43–7.
Vogel A, Wege H, Caca K, Nashan B, Neumann U. The diagnosis and treatment of cholangiocarcinoma. Dtsch Arztebl Int. 2014;111(44):748–54.
Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234(4):507–17; discussion 517–9.
Hennedige TP, Neo WT, Venkatesh SK. Imaging of malignancies of the biliary tract- an update. Cancer Imaging. 2014;14:14.
Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013;11(1):13–21.e1; quiz e3–4.
Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma — evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 018;15(2):95–111.
Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003;10(4):288–91.
Sandrasegaran K, Menias CO. Imaging and screening of cancer of the gallbladder and bile ducts. Radiol Clin North Am. 2017;55(6):1211–22.
Nathan H, Aloia TA, Vauthey J-N, Abdalla EK, Zhu AX, Schulick RD, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16(1):14–22.
Chung YE, Kim MJ, Park YN, Choi JY, Pyo JY, Kim YC, et al. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics. 2009;29(3):683–700.
Ros PR, Buck JL, Goodman ZD, Ros AM, Olmsted WW. Intrahepatic cholangiocarcinoma: radiologic-pathologic correlation. Radiology. 1988;167(3):689–93.
Lim JH. Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings. Am J Roentgenol. 2003;181(3):819–27.
Vilana R, Forner A, Bianchi L, García-Criado Á, Rimola J, Rodríguez de Lope C, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology. 2010;51(6):2020–9.
Wildner D, Pfeifer L, Goertz R, Bernatik T, Sturm J, Neurath M, et al. Dynamic contrast-enhanced ultrasound (DCE-US) for the characterization of hepatocellular carcinoma and cholangiocellular carcinoma. Ultraschall der Medizin - Eur J Ultrasound. 2014;35(06):522–7.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2.
Loyer EM, Chin H, DuBrow RA, David CL, Eftekhari F, Charnsangavej C. Hepatocellular carcinoma and intrahepatic peripheral cholangiocarcinoma: enhancement patterns with quadruple phase helical CT—A comparative study. Radiology. 1999;212(3):866–75.
Fábrega-Foster K, Ghasabeh MA, Pawlik TM, Kamel IR. Multimodality imaging of intrahepatic cholangiocarcinoma. HepatoBiliary Surg Nutr. 2017;6(2):67–78.
Sainani NI, Catalano OA, Holalkere N-S, Zhu AX, Hahn PF, Sahani D V. Cholangiocarcinoma: current and novel imaging techniques. RadioGraphics. 2008;28(5):1263–87.
Joo I, Lee JM, Yoon JH. Imaging diagnosis of intrahepatic and perihilar cholangiocarcinoma: recent advances and challenges. Radiology. 2018;288(1):7–13.
Kim TK, Choi BI, Han JK, Jang HJ, Cho SG, Han MC. Peripheral cholangiocarcinoma of the liver: two-phase spiral CT findings. Radiology. 1997;204(2):539–43.
Iavarone M, Piscaglia F, Vavassori S, Galassi M, Sangiovanni A, Venerandi L, et al. Contrast enhanced CT-scan to diagnose intrahepatic cholangiocarcinoma in patients with cirrhosis. J Hepatol. 2013;58(6):1188–93.
Lacomis JM, Baron RL, Oliver JH, Nalesnik MA, Federle MP. Cholangiocarcinoma: delayed CT contrast enhancement patterns. Radiology. 1997;203(1):98–104.
Wells ML, Venkatesh SK, Chandan VS, Fidler JL, Fletcher JG, Johnson GB, et al. Biphenotypic hepatic tumors: imaging findings and review of literature. Abdom Imaging. 2015;40(7):2293–305.
Kim R, Lee JM, Shin C-I, Lee ES, Yoon JH, Joo I, et al. Differentiation of intrahepatic mass-forming cholangiocarcinoma from hepatocellular carcinoma on gadoxetic acid-enhanced liver MR imaging. Eur Radiol. 2016;26(6):1808–17.
Park HJ, Kim YK, Park MJ, Lee WJ. Small intrahepatic mass-forming cholangiocarcinoma: target sign on diffusion-weighted imaging for differentiation from hepatocellular carcinoma. Abdom Imaging. 2013;38(4):793–801.
Kim SH, Lee CH, Kim BH, Kim WB, Yeom SK, Kim KA, et al. Typical and atypical imaging findings of intrahepatic cholangiocarcinoma using gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid–enhanced magnetic resonance imaging. J Comput Assist Tomogr. 2012;36(6):704–9.
Ringe KI, Husarik DB, Sirlin CB, Merkle EM. Gadoxetate disodium–enhanced MRI of the liver: part 1, protocol optimization and lesion appearance in the noncirrhotic liver. Am J Roentgenol. 2010;195(1):13–28.
Vilgrain V. Staging cholangiocarcinoma by imaging studies. HPB. 2008;10(2):106–9.
Hu J-H, Tang J, Lin C-H, Chu Y-Y, Liu N-J. Preoperative staging of cholangiocarcinoma and biliary carcinoma using 18F-fluorodeoxyglucose positron emission tomography: a meta-analysis. J Investig Med. 2018;66(1):52–61.
Ma KW, Cheung TT, She WH, Chok KSH, Chan ACY, Dai WC, et al. Diagnostic and prognostic role of 18-FDG PET/CT in the management of resectable biliary tract cancer. World J Surg. 2018;42(3):823–34.
Yoh T, Seo S, Morino K, Fuji H, Ikeno Y, Ishii T, et al. Reappraisal of prognostic impact of tumor SUVmax by 18F-FDG-PET/CT in intrahepatic cholangiocarcinoma. World J Surg. 2019;43(5):1323–31.
Sabaté-Llobera A, Gràcia-Sánchez L, Reynés-Llompart G, Ramos E, Lladó L, Robles J, et al. Differences on metabolic behavior between intra and extrahepatic cholangiocarcinomas at 18F-FDG–PET/CT: prognostic implication of metabolic parameters and tumor markers. Clin Transl Oncol. 2019;21(3):324–33.
Cleary SP, Dawson LA, Knox JJ, Gallinger S. Cancer of the gallbladder and extrahepatic bile ducts. Curr Probl Surg. 2007;44(7):396–482.
Lim JH, Yoon K-H, Kim SH, Kim HY, Lim HK, Song SY, et al. Intraductal papillary mucinous tumor of the bile ducts. RadioGraphics. 2004;24(1):53–66.
Choi BI, Lee JM, Han JK. Imaging of intrahepatic and hilar cholangiocarcinoma. Abdom Imaging. 2004;29(5):548–57.
Slattery JM. What is the current state-of-the-art imaging for detection and staging of cholangiocarcinoma? Oncologist. 2006;11(8):913–22.
Sharma MP, Ahuja V. Aetiological spectrum of obstructive jaundice and diagnostic ability of ultrasonography: a clinician’s perspective. Trop Gastroenterol. 1999;20(4):167–9.
Choi J-Y, Kim M-J, Lee JM, Kim KW, Lee JY, Han JK, et al. Hilar cholangiocarcinoma: role of preoperative imaging with sonography, MDCT, MRI, and direct cholangiography. Am J Roentgenol. 2008;191(5):1448–57.
Lee HY, Kim SH, Lee JM, Kim S-W, Jang J-Y, Han JK, et al. Preoperative assessment of resectability of hepatic hilar cholangiocarcinoma: combined CT and cholangiography with revised criteria. Radiology. 2006;239(1):113–21.
Seale MK, Catalano OA, Saini S, Hahn PF, Sahani D V. Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. RadioGraphics. 2009;29(6):1725–48.
Manfredi R, Masselli G, Maresca G, Brizi MG, Vecchioli A, Marano P. MR imaging and MRCP of hilar cholangiocarcinoma. Abdom Imaging. 2003;28(3):319–25.
Hänninen EL, Pech M, Jonas S, Ricke J, Thelen A, Langrehr J, et al. Magnetic resonance imaging including magnetic resonance cholangiopancreatography for tumor localization and therapy planning in malignant hilar obstructions. Acta Radiol. 2005;46(5):462–70.
Masselli G, Manfredi R, Vecchioli A, Gualdi G. MR imaging and MR cholangiopancreatography in the preoperative evaluation of hilar cholangiocarcinoma: correlation with surgical and pathologic findings. Eur Radiol. 2008;18(10):2213–21.
Lee M-G, Park KB, Shin YM, Yoon HK, Sung KB, Kim MH, et al. Preoperative evaluation of hilar cholangiocarcinoma with contrast-enhanced three-dimensional fast imaging with steady-state precession magnetic resonance angiography: comparison with intraarterial digital subtraction angiography. World J Surg. 2003;27(3):278–83.
Ruys AT, van Beem BE, Engelbrecht MRW, Bipat S, Stoker J, Van Gulik TM. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta-analysis. Br J Radiol. 2012;85(1017):1255–62.
Anderson MA, Appalaneni V, Ben-Menachem T, Decker GA, Early DS, Evans JA, et al. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest Endosc. 2013;77(2):167–74.
Saad WEA, Wallace MJ, Wojak JC, Kundu S, Cardella JF. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy. J Vasc Interv Radiol. 2010;21(6):789–95.
Park M-S, Kim TK, Kim KW, Park SW, Lee JK, Kim J-S, et al. Differentiation of extrahepatic bile duct cholangiocarcinoma from benign stricture: findings at MRCP versus ERCP. Radiology. 2004;233(1):234–40.
Al Mahjoub A, Menahem B, Fohlen A, Dupont B, Alves A, Launoy G, et al. Preoperative biliary drainage in patients with resectable perihilar cholangiocarcinoma: is percutaneous transhepatic biliary drainage safer and more effective than endoscopic biliary drainage? A meta-analysis. J Vasc Interv Radiol. 2017;28(4):576–82.
Nguyen K, James T Sing Jr. Review of endoscopic techniques in the diagnosis and management of cholangiocarcinoma. World J Gastroenterol. 2008;14(19):2995-9.
Sasaki A, Aramaki M, Kawano K, Morii Y, Nakashima K, Yoshida T, et al. Intrahepatic peripheral cholangiocarcinoma: mode of spread and choice of surgical treatment. Br J Surg. 1998;85(9):1206–9.
Shimada K, Sano T, Sakamoto Y, Esaki M, Kosuge T, Ojima H. Surgical outcomes of the mass-forming plus periductal infiltrating types of intrahepatic cholangiocarcinoma: a comparative study with the typical mass-forming type of intrahepatic cholangiocarcinoma. World J Surg. 2007;31(10):2016–22.
Kajiyama K, Maeda T, Takenaka K, Sugimachi K, Tsuneyoshi M. The significance of stromal desmoplasia in intrahepatic cholangiocarcinoma: a special reference of “scirrhous-type” and “nonscirrhous-type” growth. Am J Surg Pathol. 1999;23(8):892–902.
Shiota K, Taguchi J, Nakashima O, Nakashima M, Kojiro M. Clinicopathologic study on cholangiolocellular carcinoma. Oncol Rep. 2001;8(2):263-8.
Nakajima T, Kondo Y, Miyazaki M, Okui K. A histopathologic study of 102 cases of intrahepatic cholangiocarcinoma: histologic classification and modes of spreading. Hum Pathol. 1988;19(10):1228–34.
Chow LT, Ahuja AT, Kwong KH, Fung KS, Lai CK, Lau JW. Mucinous cholangiocarcinoma: an unusual complication of hepatolithiasis and recurrent pyogenic cholangitis. Histopathology. 1997;30(5):491–4.
Shimonishi T, Miyazaki K, Nakanuma Y. Cytokeratin profile relates to histological subtypes and intrahepatic location of intrahepatic cholangiocarcinoma and primary sites of metastatic adenocarcinoma of liver. Histopathology. 2000;37(1):55–63.
Tsou Y-K, Wu R-C, Hung C-F, Lee C-S. Intrahepatic sarcomatoid cholangiocarcinoma: clinical analysis of seven cases during a 15-year period. Chang Gung Med J. 2008;31(6):599–605.
Craig JR, Peters RL, Edmondson HA AFI of P, (U.S.) O. Tumors of the liver and intrahepatic bile ducts. Armed Forces Inst Pathol Supt Docs, US GPO.
Haas S, Gütgemann I, Wolff M, Fischer H-P. Intrahepatic clear cell cholangiocarcinoma: immunohistochemical aspects in a very rare type of cholangiocarcinoma. Am J Surg Pathol. 2007;31(6):902–6.
Isa T, Kusano T, Muto Y, Furukawa M, Kiyuna M, Toda T. Clinicopathologic features of resected primary adenosquamous carcinomas of the liver. J Clin Gastroenterol. 1997;25(4):623–7.
Maeda T, Takenaka K, Taguchi K, Kajiyama K, Shirabe K, Shimada M, et al. Adenosquamous carcinoma of the liver: clinicopathologic characteristics and cytokeratin profile. Cancer. 1997;80(3):364–71.
Takahashi H, Hayakawa H, Tanaka M, Okamura K, Kosaka A, Mizumoto R, et al. Primary adenosquamous carcinoma of liver resected by right trisegmentectomy: report of a case and review of the literature. J Gastroenterol. 1997;32(6):843–7.
Sasaki M, Nakanuma Y, Nagai Y, Nonomura A. Intrahepatic cholangiocarcinoma with sarcomatous transformation: an autopsy case. J Clin Gastroenterol. 1991;13(2):220–5.
Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47(5):1544–56.
74. Bloustein PA, Silverberg SG. Squamous cell carcinoma originating in an hepatic cyst. Case report with a review of the hepatic cyst-carcinoma association. Cancer. 1976;38(5):2002–5.
Gresham GA, Rue LW. Squamous cell carcinoma of the liver. Hum Pathol. 1985;16(4):413–6.
Lynch MJ, McLeod MK, Weatherbee L, Gilsdorf JR, Guice KS, Eckhauser FE. Squamous cell cancer of the liver arising from a solitary benign nonparasitic hepatic cyst. Am J Gastroenterol. 1988;83(4):426–31.
Pliskin A, Cualing H, Stenger RJ. Primary squamous cell carcinoma originating in congenital cysts of the liver. Report of a case and review of the literature. Arch Pathol Lab Med. 1992;116(1):105–7.
Kanamoto M, Yoshizumi T, Ikegami T, Imura S, Morine Y, Ikemoto T, et al. Cholangiolocellular carcinoma containing hepatocellular carcinoma and cholangiocellular carcinoma, extremely rare tumor of the liver: a case report. J Med Invest. 2008;55(1–2):161–5.
Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, et al. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30(6):1425–33.
Nakanuma Y, Sasaki M, Ikeda H, Sato Y, Zen Y, Kosaka K, et al. Pathology of peripheral intrahepatic cholangiocarcinoma with reference to tumorigenesis. Hepatol Res. 2008;38(4):325–34.
Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol. 2010;2(12):419–27.
Nakanuma Y, Sato Y, Ikeda H, Harada K, Kobayashi M, Sano K, et al. Intrahepatic cholangiocarcinoma with predominant “ductal plate malformation” pattern: a new subtype. Am J Surg Pathol. 2012;36(11):1629–35.
Jeng YM, Chen CL, Hsu HC. Lymphoepithelioma-like cholangiocarcinoma: an Epstein-Barr virus-associated tumor. Am J Surg Pathol. 2001;25(4):516–20.
Chen TC, Ng KF, Kuo TT. Intrahepatic cholangiocarcinoma with lymphoepithelioma-like component. Mod Pathol. 2001;14(5):527–32.
Terada T, Makimoto K, Terayama N, Suzuki Y, Nakanuma Y. Alpha-smooth muscle actin-positive stromal cells in cholangiocarcinomas, hepatocellular carcinomas and metastatic liver carcinomas. J Hepatol. 1996;24(6):706–12.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657–69.
Nuzzo G, Giuliante F, Ardito F, De Rose AM, Vellone M, Clemente G, et al. Intrahepatic cholangiocarcinoma: prognostic factors after liver resection. Updates Surg. 2010;62(1):11–9.
Chan ES, Yeh MM. The use of immunohistochemistry in liver tumors. Clin Liver Dis. 2010;14(4):687–703.
Rullier A, Le Bail B, Fawaz R, Blanc JF, Saric J, Bioulac-Sage P. Cytokeratin 7 and 20 expression in cholangiocarcinomas varies along the biliary tract but still differs from that in colorectal carcinoma metastasis. Am J Surg Pathol. 2000;24(6):870–6.
Kozaka K, Sasaki M, Fujii T, Harada K, Zen Y, Sato Y, et al. A subgroup of intrahepatic cholangiocarcinoma with an infiltrating replacement growth pattern and a resemblance to reactive proliferating bile ductules: “bile ductular carcinoma”. Histopathology. 2007;51(3):390–400.
Ferrone CR, Ting DT, Shahid M, Konstantinidis IT, Sabbatino F, Goyal L, et al. The ability to diagnose intrahepatic cholangiocarcinoma definitively using novel branched DNA-enhanced albumin RNA in situ hybridization technology. Ann Surg Oncol. 2016;23(1):290–6.
Volmar KE, Vollmer RT, Routbort MJ, Creager AJ. Pancreatic and bile duct brushing cytology in 1000 cases: review of findings and comparison of preparation methods. Cancer. 2006;108(4):231–8.
Fritcher EG, Kipp BR, Halling KC, Oberg TN, Bryant SC, Tarrell RF, et al. A multivariable model using advanced cytologic methods for the evaluation of indeterminate pancreatobiliary strictures. Gastroenterology. 2009;136(7):2180-6.
Barr Fritcher EG, Voss JS, Brankley SM, Campion MB, Jenkins SM, Keeney ME, et al. An optimized set of fluorescence in situ hybridization probes for detection of pancreatobiliary tract cancer in cytology brush samples. Gastroenterology. 2015;149(7):1813–1824.
Abbas S, Sandroussi C. Systematic review and meta-analysis of the role of vascular resection in the treatment of hilar cholangiocarcinoma. HPB (Oxford). 2013;15(7):492–503.
van Vugt JLA, Gaspersz MP, Coelen RJS, Vugts J, Labeur TA, de Jonge J, et al. The prognostic value of portal vein and hepatic artery involvement in patients with perihilar cholangiocarcinoma. HPB (Oxford). 2018;20(1):83–92.
Farges O, Regimbeau JM, Fuks D, Le Treut YP, Cherqui D, Bachellier P, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg. 2013;100(2):274–83.
Coelen RJS, Roos E, Wiggers JK, Besselink MG, Buis CI, Busch ORC, et al. Endoscopic versus percutaneous biliary drainage in patients with resectable perihilar cholangiocarcinoma: a multicentre, randomised controlled trial. Lancet Gastroenterol Hepatol. 2018;3(10):681–90.
Esposito F, Lim C, Lahat E, Shwaartz C, Eshkenazy R, Salloum C, et al. Combined hepatic and portal vein embolization as preparation for major hepatectomy: a systematic review. HPB (Oxford) 2019;21(9):1099-1106.
Nuzzo G, Giuliante F, Ardito F, Giovannini I, Aldrighetti L, Belli G, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012;147(1):26–34.
Bird N, Elmasry M, Jones R, Elniel M, Kelly M, Palmer D, et al. Role of staging laparoscopy in the stratification of patients with perihilar cholangiocarcinoma. Br J Surg. 2017;104(4):418–25.
Weber SM, Ribero D, O’Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17(8):669–80.
Buettner S, Ten Cate DWG, Bagante F, Alexandrescu S, Marques HP, Lamelas J, et al. Survival after resection of multiple tumor foci of intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2019;23(11):2239-2246.
Conci S, Ruzzenente A, Viganò L, Ercolani G, Fontana A, Bagante F, et al. Patterns of distribution of hepatic nodules (single, satellites or multifocal) in intrahepatic cholangiocarcinoma: prognostic impact after surgery. Ann Surg Oncol. 2018;25(12):3719–27.
Sapisochin G, de Lope CR, Gastaca M, de Urbina JO, López-Andujar R, Palacios F, et al. Intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma in patients undergoing liver transplantation: a Spanish matched cohort multicenter study. Ann Surg. 2014;259(5):944–52.
Sapisochin G, Facciuto M, Rubbia-Brandt L, Marti J, Mehta N, Yao FY, et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology 2016;64(4):1178–88. Sapisochin G, Facciuto M, Rubbia-Brandt L, Marti J, Mehta N, Yao FY, et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology. 2016;64(4):1178–88.
Lunsford KE, Javle M, Heyne K, Shroff RT, Abdel-Wahab R, Gupta N, et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol. 2018;3(5):337–48.
Hartog H, Ijzermans JNM, van Gulik TM, Groot Koerkamp B. Resection of perihilar cholangiocarcinoma. Surg Clin North Am. 2016;96(2):247–67.
Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258(1):129–40.
Ebata T, Mizuno T, Yokoyama Y, Igami T, Sugawara G, Nagino M. Surgical resection for Bismuth type IV perihilar cholangiocarcinoma. Br J Surg. 2018;105(7):829–38.
Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation. 2000;69(8):1633–7.
Robles R, Figueras J, Turrión VS, Margarit C, Moya A, Varo E, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265–71.
Rea DJ, Heimbach JK, Rosen CB, Haddock MG, Alberts SR, Kremers WK, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242(3):451–8; discussion 458–61.
Sudan D, DeRoover A, Chinnakotla S, Fox I, Shaw B, McCashland T, et al. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2(8):774–9.
Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143(1):88–98.e3.
Darwish Murad S, Kim WR, Therneau T, Gores GJ, Rosen CB, Martenson JA, et al. Predictors of pretransplant dropout and posttransplant recurrence in patients with perihilar cholangiocarcinoma. Hepatology. 2012;56(3):972–81.
Duignan S, Maguire D, Ravichand CS, Geoghegan J, Hoti E, Fennelly D, et al. Neoadjuvant chemoradiotherapy followed by liver transplantation for unresectable cholangiocarcinoma: a single-centre national experience. HPB. 2014;16(1):91–8.
Policies – OPTN [Internet]. [cited 2019 Aug 30]. Available from: https://optn.transplant.hrsa.gov/governance/policies
Ethun CG, Lopez-Aguiar AG, Anderson DJ, Adams AB, Fields RC, Doyle MB, et al. Transplantation versus resection for hilar cholangiocarcinoma: an argument for shifting treatment paradigms for resectable disease. Ann Surg. 2018;267(5):797–805.
Schreuder AM, Engelsman AF, van Roessel S, Verheij J, Besselink MG, van Gulik TM, et al. Treatment of mid-bile duct carcinoma: local resection or pancreatoduodenectomy? Eur J Surg Oncol. 2019;45(11):2180–7.
Takahashi EA, Kinsman KA, Schmit GD, Atwell TD, Schmitz JJ, Welch BT, et al. Thermal ablation of intrahepatic cholangiocarcinoma: safety, efficacy, and factors affecting local tumor progression. Abdom Radiol. 2018;43(12):3487–92.
Melenhorst MCAM, Scheffer HJ, Vroomen LGPH, Kazemier G, van den Tol MP, Meijerink MR. Percutaneous irreversible electroporation of unresectable hilar cholangiocarcinoma (Klatskin tumor): a case report. Cardiovasc Intervent Radiol. 2016;39(1):117–21.
Mafeld S, Wong JJ, Kibriya N, Stenberg B, Manas D, Bassett P, et al. Percutaneous irreversible electroporation (IRE) of hepatic malignancy: a bi-institutional analysis of safety and outcomes. Cardiovasc Intervent Radiol. 2019;42(4):577–83.
Coelen RJS, Vogel JA, Vroomen LGPH, Roos E, Busch ORC, van Delden OM, et al. Ablation with irreversible electroporation in patients with advanced perihilar cholangiocarcinoma (ALPACA): a multicentre phase I/II feasibility study protocol. BMJ Open. 2017;7(9):e015810.
Kiefer M V., Albert M, McNally M, Robertson M, Sun W, Fraker D, et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol. Cancer. 2011;117(7):1498–505.
Park S-Y, Kim JH, Yoon H-J, Lee I-S, Yoon H-K, Kim K-P. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol. 2011;66(4):322–8.
Vogl TJ, Naguib NNN, Nour-Eldin N-EA, Bechstein WO, Zeuzem S, Trojan J, et al. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: results and prognostic factors governing treatment success. Int J Cancer 2012;131(3):733–40.
Kuhlmann JB, Euringer W, Spangenberg HC, Breidert M, Blum HE, Harder J, et al. Treatment of unresectable cholangiocarcinoma. Eur J Gastroenterol Hepatol. 2012;24(4):437-43.
Boehm LM, Jayakrishnan TT, Miura JT, Zacharias AJ, Johnston FM, Turaga KK, et al. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol. 2015;111(2):213–20.
Aliberti C, Carandina R, Sarti D, Pizzirani E, Ramondo G, Mulazzani L, et al. Chemoembolization with drug-eluting microspheres loaded with doxorubicin for the treatment of cholangiocarcinoma. Anticancer Res. 2017;37(4):1859–63.
Hoffmann R-T, Paprottka PM, Schön A, Bamberg F, Haug A, Dürr E-M, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol. 2012;35(1):105–16.
Rafi S, Piduru SM, El-Rayes B, Kauh JS, Kooby DA, Sarmiento JM, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol. 2013;36(2):440–8.
Mosconi C, Gramenzi A, Ascanio S, Cappelli A, Renzulli M, Pettinato C, et al. Yttrium-90 radioembolization for unresectable/recurrent intrahepatic cholangiocarcinoma: a survival, efficacy and safety study. Br J Cancer. 2016;115(3):297–302.
Reimer P, Virarkar MK, Binnenhei M, Justinger M, Schön MR, Tatsch K. Prognostic factors in overall survival of patients with unresectable intrahepatic cholangiocarcinoma treated by means of yttrium-90 radioembolization: results in therapy-naïve patients. Cardiovasc Intervent Radiol. 2018;41(5):744–52.
Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30(16):1934–40.
El-Khoueiry AB, Rankin CJ, Ben-Josef E, Lenz HJ, Gold PJ, Hamilton RD, et al. SWOG 0514: a phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Investig New Drugs. 2012;30(4):1646–51.
Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blaszkowsky LS, et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2016;34(5):460–8.
Torgeson A, Lloyd S, Boothe D, Cannon G, Garrido-Laguna I, Whisenant J, et al. Chemoradiation therapy for unresected extrahepatic cholangiocarcinoma: a propensity score-matched analysis. Ann Surg Oncol. 2017;24(13):4001–8.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract Cancer. N Engl J Med. 2010;362(14):1273–81.
Sahai V, Catalano PJ, Zalupski MM, Lubner SJ, Menge MR, Nimeiri HS, et al. Nab-paclitaxel and gemcitabine as first-line treatment of advanced or metastatic cholangiocarcinoma. JAMA Oncol. 2018;4(12):1707.
Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: a phase 2 clinical trial. JAMA Oncol. 2019;5(6):824-830.
Gemcitabine hydrochloride and cisplatin with or without nab-paclitaxel in treating patients with newly diagnosed advanced biliary tract cancers - ClinicalTrials.gov. [cited 2019 May 13]. Available from: https://clinicaltrials.gov/ct2/show/NCT03768414
Arima S, Shimizu K, Okamoto T, Toki M, Suzuki Y, Okano N, et al. A multicenter phase II study of gemcitabine plus S-1 chemotherapy for advanced biliary tract Cancer. Anticancer Res. 2017;37(2):909–14.
Gabriel E, Gandhi S, Attwood K, Kuvshinoff B, Hochwald S, Iyer R. Gemcitabine and capecitabine for advanced biliary cancer. J Gastrointest Oncol. 2017;8(4):728–36.
Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. ABC-06 | A randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previouslytreated with cisplatin/gemcitabine (CisGem) chemotherapy. Journal of Clinical Oncology. 2019;37(15_suppl):4003–4003.
Mazzaferro V, El-Rayes BF, Droz Dit Busset M, Cotsoglou C, Harris WP, Damjanov N, et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br J Cancer. 2019;120(2):165–71.
Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol. 2018;36(3):276–82.
Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–84.
Goyal L, Saha SK, Liu LY, Siravegna G, Leshchiner I, Ahronian LG, et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov. 2017;7(3):252–63.
Kipp BR, Voss JS, Kerr SE, Barr Fritcher EG, Graham RP, Zhang L, et al. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum Pathol. 2012;43(10):1552–8.
Lowery MA, Abou-Alfa GK, Burris HA, Janku F, Shroff RT, Cleary JM, et al. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol. 2017;35(15_suppl):4015–4015.
Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796-807.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13.
Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Thompson, S.M. et al. (2020). Cholangiocarcinoma. In: Roberts, L., Yang, J., Venkatesh, S. (eds) Evaluation and Management of Liver Masses. Springer, Cham. https://doi.org/10.1007/978-3-030-46699-2_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-46699-2_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-46698-5
Online ISBN: 978-3-030-46699-2
eBook Packages: MedicineMedicine (R0)