Abstract
The term epigenetics is generally referred to phenotype modifications occurring in the DNA or the chromatin’s structure, which may influence the transcription of many genes independently of their primary nucleotide sequences. Although epigenetics is still in its infancy in the field of physical exercise, some studies convincingly suggest that epigenetic regulations may play an important role in modulating the favorable effects of exercise on development and progression of cancer. Several lines of evidence demonstrated that regular physical activity decreased the risk of several types of malignancies, and some of these beneficial effects are seemingly mediated by epigenetic modifications. More specifically, it has been clearly demonstrated that physical exercise is effective to induce histone modifications, methylation and acetylation of DNA, modulatory expression of microRNAs (miRNAs), as well as additional influences on proteins and biological pathways implicated in cancer biology such as tumor suppressor p53, lipoprotein(a), and hypoxia-inducible factor-1 (HIF-1). Although the available evidence does not support the notion that exercise-induced epigenetic changes always follow a unidirectional path in terms of cancer risk, the favorable effects of reduced cancer development and progression probably overwhelm cancer-promoting activities. If preliminary findings are confirmed in larger studies, physical exercise may hence be regarded as an appealing perspective for reducing the risk of cancer in different populations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Cancer and Exercise: Review of Epidemiological Evidence
The association between physical activity (PA) and health is an old theory, having been first speculated more than 2000 years ago by the Greek physician Hippocrates, who advised: “eating alone will not keep a man well; he must also take exercise” [1]. With the decline of the Hellenic civilization, the interest in the relationship between PA, fitness, and health faded for centuries and then rose again with the advent of the Industrial Revolution . During that period scientists began to measure the benefits of exercise more objectively, thus inspiring a new era in which the association between exercise and human health could be analyzed more scientifically, by using numerical quantification. Since then, evidence accumulated from a number of epidemiological studies unequivocally supports the existence of an inverse, independent, and graded association between PA health and overall mortality , especially in the field of cardiovascular medicine [2, 3].
The earliest pioneer studies postulating the inverse relationship between PA and cancer risk were concomitantly published in 1922 by two independent groups [4, 5]. The authors assessed cancer mortality rates in men with different occupations and concluded that those employed in physically demanding jobs experienced lower cancer mortality rates than those engaged in less strenuous activities.
Thereafter, modest progress was made until the mid-1980s , when the interest on this topic re-emerged, thus leading to a rapid increase of scientific literature on this topic. Since then, more than 600 epidemiological studies were carried out in both genders, in different ethnic groups, in broad age classes, in a variety of social and occupational groups, and in most continents around the world. Although individual studies have demonstrated that PA might be virtually protective against all types of cancer, the evidence emerged was judged as mostly inconsistent for some cancer types because of the impossibility to perform high-quality meta-analyses from existing data. In particular, the use of different definition of PA, the lack of consistency in the methods used to quantify it, the difference in the study design, the lack of uniformity in adjusting for confounding factors, as well as the intrinsic bias associated with self-reported questionnaires, represent some of the main drawbacks which hampered cumulative data analysis due to the large differences in risk estimates across the different studies [6]. Following a rigorous evaluation of the available literature, the report published in 2007 by the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR ) judged that the evidence supporting a protective role of PA against cancer was “convincing” for colon cancer, “probable” for postmenopausal breast and endometrial cancer and “limited-suggestive” for lung, pancreas, and premenopausal breast cancer [6, 7].
The best evidence for a protective influence of PA against cancer currently emerges from epidemiologic studies on colon cancer . Wolin and colleagues conducted the first formal estimation of the magnitude of association between PA and reduction of colon cancer risk in 2009. This meta-analysis of 24 cohort studies and 28 case−control studies reported a significant 24 % reduced risk when comparing the most vs. the least active individuals across all studies (relative risk [RR], 0.76; 95 % CI, 0.72–0.81). The effect was similar in men and women. The findings from case−control studies were stronger (RR, 0.69; 95 % CI, 0.65–0.74) than from cohort studies (RR, 0.83; 95 % CI, 0.78–0.88) [8]. More recent results of an ensuing meta-analysis provide convincing evidence that the association between PA and the risk of colon cancer does not differ by anatomical subsite. By calculating the summary risk estimates from 21 studies, the authors observed that the risks of both proximal colon cancer and distal colon cancer were, respectively, 27 and 26 % lower among the most physically active people compared with the least active people [9]. Results from the two meta-analyses were substantially similar, although evidence emerged from the recent one appears more consistent due to the low grade of statistical heterogeneity in the estimates across studies (I 2, 31.3 %; p = 0.057 and I2, 0.0 %; P = 0.473 for proximal and distal colon cancer, respectively). The lower risk of colon cancer associated with PA appears not consistently modified by other well-known risk factors , including body mass index (BMI), cigarette smoking, energy intake, and high-risk diet [10, 11]. In contrast to colon cancer, most studies on rectal cancer reported no significant relation between increased PA and cancer risk.
There is substantial evidence that breast cancer risk is statistically significant decreased among physically active individuals. The best confirmation for this association comes from studies of postmenopausal breast cancer for which the evidence has been judged as ‘probable’ by both the WCRF/AICR [12], and the IARC [13].
Studies on premenopausal women reached fewer certain results, thus making the association “limited-suggestive” according to experts’ conclusions. However, a recent meta-analysis pooling results of 31 studies reported that premenopausal active women had a stronger reduction of breast cancer risk [RR, 0.72; 95 % CI, 0.65–0.81] than those in the postmenopausal state (RR; 0.87; 95 % CI, 0.87–0.92 [14]. At variance with these findings, another meta-analysis published in 2014 reconfirmed that PA seemingly prevents breast cancer, especially in postmenopausal women [15]. Notably, the homogeneity with which the menopausal status has been defined and applied to stratify study populations in these investigations is probably the main cause of the different conclusions reached in the two meta-analyses. Therefore, further investigations using a clear and unique definition of menopausal status to cluster study population will be needed to clarify this important aspect. Regardless the influence of menopausal status, an agreement in literature exists for assuming that women who were most active in their occupational and/or recreational activities may have a lower incidence of breast cancer than their sedentary counterparts. In addition, the housework seems to reduce the risk of breast cancer by itself as well as in combination with spare time activity such as walking, cycling, or playing sport [16, 17]. The reported reduction in the risk ranges from 10 to 80 %, is on average 25 %, and appears to be stronger for subjects with BMI < 30 kg/m2, parous women, women without a family history of breast cancer, and estrogen and progesterone receptor-negative women [18, 19]. Finally, most prospective studies found evidence for a dose−response relationship showing a lower risk of breast cancer with higher levels of PA [20, 21].
To date, five publications have summarized the available epidemiologic evidence regarding the association between PA and risk of endometrial cancer [22–26]. Concertedly, findings from these studies suggest that PA is associated with an 18–30 % reduction in endometrial cancer risk with high versus low PA levels. The association was observed for broad range of activity domains, including both recreational and occupational PA, and for different intensities, including light, moderate, and vigorous activities. Particular protection from endometrial cancer through PA participation was found for women who were overweight or obese [26].
Recent epidemiologic investigations support the hypothesis that PA might reduce the risk of many other cancers other than those previously discussed, thus including malignancies of lung, pancreas, prostate, and stomach. Nevertheless, the current evidence remains limited and additional data are needed [27].
Observational studies evaluating the association between PA and risk of death among survivors of cancer suggest that PA prolongs overall and cancer-specific survival [28–30]. Even in such cases, most convincing data come from studies on colon and breast cancer. In particular, a meta-analysis of prospective studies published through June 2013 showed that engaging in the approximate equivalent of 150 min of at least moderate intensity PA per week after cancer diagnosis was associated with a 24 % reduced risk of total mortality among breast cancer survivors and 28 % decreased total mortality risk among colorectal cancer survivors [31]. The apparent protection afforded by PA was observed even after adjustments for tumor stage, cancer treatment, smoking, and adiposity and was confirmed in different geographical settings, in both large and small studies, as well as in surveys using self-reported and interview-based PA assessments. The authors also confirmed previous findings suggestive of a beneficial effect of PA performed before cancer diagnosis on both total and cancer mortality. They found that high versus low prediagnosis PA was associated with decreased risk of total mortality. More specifically, higher prediagnosis PA was associated with a 13 % decreased risk of total mortality among breast cancer survivors and a 14 % decreased total mortality risk among colorectal cancer survivors. Finally, they showed that an increase in PA from pre- to postdiagnosis further reduces total mortality risk.
Recommended Dose of Physical Activity for Health Benefits
According to a real biological perspective, physical exercise should indeed be regarded as a form of probably “favorable” stress . The large experience accumulated during the past suggests in fact that PA induces a large number of metabolic adaptations that are magnified in the process of transition from a sedentary to a physically active state [32]. What has become rather clear after decades of research in this field is that a linear dose−response relationship seemingly exists between physical exercise and metabolic changes . Interestingly, it was recently shown that former participants in the Tour de France (i.e., the most famous worldwide professional 3-week cycling race) have a considerable increase in average longevity (+17 %) compared to the general population [33], thus underpinning that the burden of the most frequent causes of death (especially cancer and cardiovascular disease) may be consistently reduced by PA [34].
The recent guidelines of the American College of Sports Medicine and the American Heart Association recommend that health promotion and maintenance in healthy adults aged 18–65 years can be achieved by engagement in moderate-intensity aerobic (endurance) PA for not less than 30 min on 5 days each week or vigorous-intensity aerobic PA for not less than 20 min on 3 days each week [35]. In older subjects, these indications should also consider the aerobic fitness and risk of fall and should include exercise to promote or enhance flexibility [36]. Additional recommendations have been published by the World Health Organization (WHO) [37]. Specifically, children and youth aged 5–17 years should be engaged in at least 60 min of moderate- to vigorous-intensity PA (preferably aerobic) every day; amounts greater than 60 min provide additional health benefits. Adults aged 18–64 years should be engaged in not less than 150 min of moderate-intensity aerobic PA per week or not less than 75 min of vigorous-intensity aerobic PA per week or an equivalent combination of moderate- and vigorous-intensity activity. Moderate-intensity aerobic PA and vigorous-intensity aerobic PA may be increased to 300 min and 150 min per week, respectively, for gaining additional health benefits. These recommendations also apply to subjects aged 65 years or older, although it is clearly stated that older adults who are unable to perform the minimum amount of PA due to health conditions should be as physically active as their abilities and conditions allow. As specifically regards cancer prevention, the American Cancer Society (ACS) endorsed that adults should be engaged in not less than 150 min of moderate intensity or 75 min of vigorous intensity activity per week (or a combination of these), preferably spread throughout the week, whereas children and teens should be engaged in not less than 1 h of moderate or vigorous intensity activity per day, with vigorous activity on at least 3 days each week [38]. Overall, the time spent on sedentary behavior such as sitting, lying down, watching television, and other forms of screen-based entertainment should be very limited. Interestingly, the ACS has also released PA guidelines for cancer survivors [39], indicating that these individuals should avoid inactivity (i.e., aiming to exercise not less than 150 min per week, including strength training exercises not less than 2 days per week), and return to normal daily activities as soon as possible after diagnosis.
Therefore, despite universal recommendations do exist, a generalization seems inappropriate wherein the number and type of congenital and acquired risk factors for both cancer and cardiovascular disease varies widely across the population. As especially regards cancer, a more personalized approach seems advisable, which should take into account the family history (i.e., the genetic predisposition to develop some forms of cancer), demographic determinants (i.e., age, gender, and racial origin), and the exposure to environmental risk factors (e.g., diet and ambient pollutants). In particular, although the relationship between exercise, health and fitness seems now virtually unquestionable, a major dilemma remains, that is to definitively establish which is the adequate amount of physical exercise needed for the single patient (regardless of the age) for improving health without reaching the so-called wrecking point, after which the potential benefits may be outweighed by the adverse consequence of excess stress (Fig. 6.1).
Physical Activity and Epigenetic Modulation of Cancer
PA is thought to act in a variety of ways to affect cancer risk [40]. Indeed, one of the most important mechanisms mediating the association between PA and cancer prevention is the direct and indirect effects that exercise has on weight control. Regular PA, in fact, helps maintain a healthy body weight by balancing caloric intake with energy expenditure and by regulating the circulating levels of sex hormones, adipokines, insulin, and pro-inflammatory cytokines. PA has also been hypothesized to reduce carcinogenic prostaglandin production. In addition, PA increases colon motility, leading to decreased transit time and, perhaps, reduced mucosal exposure time to carcinogens. Another potential mechanism is through the beneficial effects of chronic PA on DNA oxidative damage or repair and on immune system [27, 41].
Interestingly, it has been recently postulated that PA may regulate molecular pathways related to inflammatory processes, metabolism, and energy consumption through the induction of epigenetic modifications. Changes in the concentrations of metabolites, such as oxygen, tricarboxylic acid (TCA) intermediates, 2-oxoglutarate, 2-hydroxyglutarate, and β-hydroxybutyrate, are potentially dependent upon epigenetic modifications and many epigenetic enzymes [42]. PA induces fluctuations in these enzymes in a tissue-dependent manner. Many of these changes are regulated by epigenetic modifiers such as DNA methyl transferases, histone acetyltransferases, histone deacetylases, and histone demethylases, between others [42]. Therefore, these substrates and signaling molecules, regulated by PA, affect important epigenetic mechanisms which ultimately control the gene expression involved in metabolism [42].
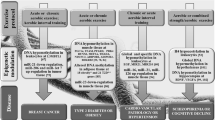
The term epigenetics is generally referred to phenotype modifications occurring in the DNA or the chromatin’s structure that can influence the transcription of several genes independently of their primary nucleotide sequences [43]. The most common epigenetic changes induced by exercise include histone modifications, such as methylation and acetylation, DNA methylation, and synthesis of noncoding mRNA [44]. The role of such exercise-induced modifications in cancer is discussed below.
Physical Activity and DNA Methylation
DNA methylation is a process in which methyl groups are added to the DNA, thus modifying its function, most frequently by suppressing gene transcription [45]. The process is usually catalyzed by DNA methyltransferases and entails the covalent linkage of methyl groups in cytosine within CpG dinucleotides concentrated in large clusters conventionally known as “CpG islands.” Interestingly, although hypermethylation within the promoter region may lead to inactivation of tumor-suppressor genes, generalized hypomethylation is also associated with genomic instability and may hence contribute to make the cell more vulnerable to transformation. Therefore, both aspects are deeply involved in the pathogenesis of human cancer, wherein hypomethylation may be associated with hyperactivation of oncogenes whereas hypermethylation may be linked to transcriptional silencing of critical growth regulators such as tumor suppressor genes, [46].
Brown recently performed a meta-analysis of 16 studies, totaling 387 genes and 1580 subjects, to comprehensively summarize exercise-associated DNA methylation changes [47]. Overall, DNA methylation was found to be significantly reduced with exercise (as many as 478 genetic elements showed exercise-associated DNA methylation patterns), especially with ageing (i.e., after the age of 40). Five exercise-associated imprinted loci could also be identified, including KCNQ1 (potassium channel, voltage-gated KQT-like subfamily Q, member), MEG3 (maternally expressed 3), GRB10 (growth factor receptor-bound protein 10), L3MBTL1 (l(3)mbt-like 1), and PLAGL1 (pleomorphic adenoma gene-like 1). DNA methylation decreased with exercise (60 % of loci). Importantly, the genes displaying reduction of DNA methylation were part of a microRNA-regulated gene network functioning to suppress cancer. More specifically, hypermethylation was found for the genes CXCL10 (C-X-C motif chemokine 10 also known as chemokine interferon-γ inducible protein 10), DCC (deleted in colorectal carcinoma), PPP2R3A (protein phosphatase 2, regulatory subunit B, alpha), RASA1 (RAS p21 protein activator (GTPase activating protein) 1), SULF1 (Sulfatase 1), TMEM100 (transmembrane protein 100), and WNT7A (Protein Wnt-7a), whereas hypomethylation was found for the genes GAB1 (the GRB2-associated binding protein 1), L3MBTL1 (Lethal(3)malignant brain tumor-like protein 1), PLAGL1 (pleomorphic adenoma gene-like 1), WNK3 (With-No-Lysine Kinase 3), BCL2L11 (Bcl-2-like protein 11), and CACNA2D3 (calcium channel, voltage-dependent, alpha 2/delta subunit 3). Although the real significance of most of these changes remains to be elucidated, some of these genes are particularly interesting for cancer biology.
CXCL10 belongs to the large CXC chemokine family, composed by elements that bind to the chemokine (C-X-C motif) receptor 3 (CXCR3) and exert a wide spectrum of either carcinogenic or anticancer activities. In particular, it has been shown that CXCL10 is capable to bind to G-protein-coupled receptors, thus inducing a wide spectrum of biological and physiological activities which include an enhancement of cell growth and proliferation, as well as overresponsiveness to cytokines synthesized by malignant and inflammatory cells [48]. WNT7A is a glycoprotein which not only plays an important role in modulating cellular proliferation and differentiation, but also influences carcinogenesis and tumor progression. Overexpression of this protein has recently been observed in patients with endometrial cancer, and its expression strongly correlates with disease progression [49]. Lethal(3)malignant brain tumor-like protein 1, the transcription product of the L3MBTL1 gene, has been shown to bind histones, thus increasing the order of chromatin structure and generating transcriptional repression. Recent evidence shows that depletion of this gene is associated with replicative stress, enhancement of DNA injury, development of DNA damage response, and overall genomic instability [50]. L3MBTL1 can hence be considered a powerful tumor suppressor gene, and it is conceivable that hypomethylation of this gene during exercise should be regarded as protective mechanism against cancer machinery. The PLAGL1 is another (putative) tumor suppressor gene. More specifically, this gene is frequently silenced in both breast and ovarian malignancies, whereas its overexpression (as may result from hypomethylation during exercise) may be associated with antiproliferative effects [51].
It is also noteworthy, however, that the relationship between sport , DNA methylation and cancer may be complex and not unidirectional. In fact, although the hypermethylated genes are indeed involved in cancer biology, most of them exert tumor suppressor functions rather than tumor promoting activities. DCC is a putative tumor suppressor gene and has been found to be frequently mutated or downregulated in colorectal cancer and esophageal carcinoma [52]. PPP2R3A encodes one of the regulatory subunits of the protein phosphatase 2 (PP2), which is involved in negative modulation of cell growth and division [53]. Interestingly, silencing of RASA1 is also associated with unregulated cell proliferation and carcinogenesis [54], whereas enhanced expression of SULF1 seemingly reduces cell proliferation, migration, and invasion [55]. TMEM100, an activin receptor-like kinase-1 (ALK1) signaling-dependent gene essential for arterial endothelium differentiation and vascular morphogenesis, inhibits metastasis and cancer cell proliferation [56]. The GAB1 gene encodes the GRB2-associated binding protein 1, which is a pivotal mediator of cellular growth, transformation and apoptosis. Recent evidence suggests that overexpression of GAB1 enhances cell growth and strongly promotes tumorigenesis [57], so that hypomethylation of this gene during exercise may influence vulnerability to developing cancer. WNK3 belongs to the “with no lysine” family of serine−threonine protein kinases. Its expression has been found consistently increased in several human cancers [58]. Importantly, overtranscription of this gene (e.g., as a result of hypomethylation) may increase cell survival by delaying apoptosis.
According to this evidence, it seems hence more reasonable to conclude that PA-induced DNA methylation does not follow a unidirectional path in terms of cancer risk, and additional studies may be needed to clearly define this issue.
Physical Activity and Histone Modification
Histone modifications are posttranslational alterations on the lysine-rich tail region of histones. They mainly include not only acetylation and methylation, but also some less-studied modifications such as phosphorylation, ubiquitylation, sumoylation, ADP ribosylation, deamination, and proline isomerization. Each of these histone modifications directly or indirectly affects chromatin structure, thereby leading to alterations in DNA repair, replication, and gene transcription.
Histone acetylation is regulated by a balance between histone acetyl-transferase (HAT) and histone deacetylase (HDAC) activities, with the former involved in a process of addiction of an acetyl group to the α-amino groups on the N-terminal tails of histones, whereas the activity of the latter enzyme entails removing acetyl groups from DNA. In chromatin, DNA is tightly wrapped around histones. Lysine acetylation by HATs is believed to neutralize the positive charge of histone tails, weakening histone-DNA or nucleosome-nucleosome bindings, and inducing an open (euchromatin-like) conformational change. As a consequence, the access of transcription factors to DNA is facilitated and gene expression concomitantly enhanced. On the other hand, deacetylation of histones by HDACs reduces the space between nucleosome and DNA, thus leading to a closed (heterochromatin-like) chromatin conformation that lower the accessibility for transcription factors , ultimately decreasing gene expression [59, 60].
Histone methylation is a reversible process that occurs through histone methyltransferases (HMTs), which are enzymes that add methyl groups to lysine and arginine tail regions of histones. The most heavily methylated histone is H3, followed by H4. Both arginine and lysine methylation can occur in mono-, di-, and tri-methylated forms. Although, the current knowledge on the biological role of this modification is still rather limited, it has been suggested that it may reflect transcriptionally active euchromatin or transcriptionally repressed heterochromatin [61].
Current data on the effect of PA on histone modifications are limited and mainly come from studies exploring molecular pathways implicated in metabolic processes. For example, it has been concluded that histone modifications do regulate glucose transporter type 4 (GLUT4) expression in response to exercise [62–64]. In human skeletal muscle , the highly expressed class IIa HDACs is known to interact with the myocyte-specific enhancer factor 2 (MEF2) by creating a complex which removes acetyl groups from MEF2 and represses the expression of GLUT4, a MEF2-dependent transcription.
Following acute exercise, the HDACs activity is reduced as a consequence of a ubiquitin-mediated proteasomal degradation, and of its phosphorylation by Ca2+/Calmodulin-dependent protein kinase (CaMK), AMP-dependent protein kinase (AMPK), or protein kinase D (PKD), which both cause a dissociation between HDACs and MET2 and the exit of HDACs from the nucleus [65]. Upon removal of the transcriptional repressive function of HDACs, the expression of GLUT4 increases. Such enhancement of skeletal muscle GLUT4 seems to occur as rapidly in response to an exercise stimulus as it declines with cessation of training [66]. The epigenetic modifications of GLUT4 expression by exercise may have remarkable clinical implications. The GLUT4 glucose transporter is the major mediator of glucose removal from the circulation, and a key regulator of whole-body glucose homeostasis. Therefore, the ability of PA to interfere with its trafficking pathways may have therapeutic potential in obesity, type II diabetes, metabolic syndrome as well as in associated comorbidities including obesity-related cancers.
Another intriguing effect of exercise-induced histone modification is that involved in the activation of the hypothalamus−adipocyte axis . In 2010 Cao and colleagues demonstrated that physical as well as social cognitive stimulations may trigger brain-derived neurotrophic factor (BDNF) expression in the hypothalamus leading to preferential sympathoneural activation of white adipose tissue. The elevated sympathetic drive is then effective to activate adipocyte β-adrenergic receptors, inhibiting leptin expression and release, and ultimately suppressing cancer growth and preventing metastasis [67]. More specifically, exercise is able to increase the expression of BDNF through at least two pathways: by inducing the acetylation of histone H3 in the BDNF promoter IV, which result in the transcription of BDNF gene, as well as by increasing the phosphorylation levels of cAMP response element binding protein (CREB) and CaMKII, that once activated, acquire a strong histone acetylation transferase-promoting activity and, in turn, activate BDNF transcription [68].
Physical Activity and microRNA
A growing body of evidence suggests that PA may affect the production of microRNAs (miRNA), small noncoding single-stranded RNA of approximately 20 nucleotide in length that play critical roles in many biological processes including cell development, differentiation, proliferation, and apoptosis. At variance with protein-coding RNA, miRNAs represent a large portion of eukaryotic transcript and do not result in protein production. Instead, they are known to regulate about 30 % of human gene expression. The miRNAs act at a posttranscriptional level by targeting the 3′ untranslated region of mRNAs, thus regulating translation of mRNA to amino acids. MiRNA regulation is dynamic. Their effects can be temporary, when the miRNA temporarily binds an mRNA to suppress translation, or permanent, causing degradation of the mRNA strand.
What is currently known about the association between exercise and microRNAs has been summarized in a systematic review published in the early 2015 by Flowers et al., and including as many as 14 studies [69]. With the exception of few miRNAs which have been found repeatedly modulated across studies, most articles reported different findings. The reasons for this discrepancy mainly reside in the different type of exercise that has been evaluated (i.e., cardiorespiratory fitness vs. resistance training) and in the timing of miRNAs evaluation (i.e., acute-phase vs. long-term responses). For example, miR-146a was increased immediately following acute exercise, but consistently declined after resistance training. Similarity, miR-20a and miR-20b both decreased following cycle ergometry, but their concentration was enhanced after completion of a 90-day exercise training program. Among the over 100 miRNAs which have been found to be up- or downregulated in response to exercise, some may play an important role in tumorigenesis. For example, it has been reported that miR-15a and miR-16-1 that target BCL2, an antiapoptotic gene, were downregulated in B-cell chronic lymphocytic leukemia [70], whereas miR-145 was downregulated in colorectal cancer [71, 72]. Amplification or overexpression of the miR-17-92 cluster has been reported in patients with lymphomas [73].
According with a recent hypothesis, miRNAs may also represent a plausible mechanistic link between PA, telomeres, and improved health [74]. Telomeres are specialized nucleoprotein structures that protect the ends of linear chromosomes and progressively shorten with each round of cellular division. Telomere length, shorter than the average length for a specific age group, has been associated with increased incidence of age-related diseases and decreased lifespan in humans. Moreover, excessive or accelerated telomere shortening can induce genomic instability by mediating interchromosomal fusion and may contribute to telomere stabilization and development of cancer [75]. Several studies indicate that individuals with shorter telomeres have a greater risk for development of lung, bladder, renal cell, gastrointestinal, head, and neck cancers [76–78].
By investigating the acute effects of 30 min of intense cardiorespiratory exercise on the expression of genes involved in telomere regulation in white blood cells (WBCs), Chilton and colleagues were able to identify four miRNAs (miR-186, miR-181, miR-15a, and miR-96) that potentially targeted telomeric gene mRNA [79]. In particular, telomeric repeat binding factor 2 interacting protein (TERF2IP), a protein that is part of a complex involved in telomere length shortening, was identified as a potential binding target for miR-186 and miR-96 [80, 81]. The expression of both miR-186 and miR-96 was found to be increased from immediately after to 60 min postexercise and was accompanied by a parallel and simultaneous downregulation of TERF2IP mRNA expression. Such findings suggest that intense cardiorespiratory exercise may be sufficient to block the oncogenic insult of TERF2IP, and that the effect may be mediated by microRNAs.
Using a similar exercise protocol , Tonevitsky and coauthors [82] identified four miRNA−mRNA networks dynamically regulated by 30 min of exercise. The target mRNAs were involved in immune function, transcription regulation, and membrane traffic of proteins. Most importantly, some of these miRNA−mRNA networks including hsa-miR-24-2-5p-MYC and hsa-miR-21-5p-TGFBR3 have a role in cancer development and progression, since they were found to be involved in cell proliferation, apoptosis, transformation, migration invasion, angiogenesis, and metastasis.
Physical Activity and Modulation of the Tumor Suppressor p53 Pathway
The p53 tumor suppressor protein is a transcription factor that regulates the expression of stress response genes and exerts multiple, antiproliferative functions [83]. Tumor protein p53 is one of the most important proteins that protect against cancer and has also been identified as the most important guardian of the human genome. Therefore, it is not a surprise that the p53 gene is mutated or dysfunctional in the majority of human tumors. The disruption of normal p53 function represents one of the main prerequisite for the initiation and/or progression of tumors.
Recently, p53 has been described as an important regulator of miRNAs [84]. In 2007, several independent groups identified different miRNAs as direct transcriptional targets of p53 [85]. Among all miRNAs, the members of the miR-34 family displayed the highest induction by p53 [86]. Some years later, evidence suggests that miRNAs not only mediate the downstream effect of p53, but are also involved in the upstream regulation of p53, thus further highlighting the importance of miRNAs in human tumors. An overview of the role of miRNAs in the p53 network is shown in Fig. 6.2. Interestingly, some of these regulators of p53 may in turn be modulated by stress and exercise [87]. In particular, depending on the intensity of training, these miRNAs may cause upregulation or downregulation of the tumor suppressor p53.
Exercise-induced regulation of p53 protein by miRNAs. miR-29 upregulates p53 by inhibiting the expression of negative regulators of p53 (CDC42, PI3K, PPM1D). miR-125b and miR-504 downregulate p53 by binding the 3′ untraslated region of p53 mRNA. CDC42 cell division control protein 42 homolog , PI3K phosphatidylinositol-4,5-bisphosphate 3-kinase, PPM1D protein phosphatase Mg2+/Mn2+-dependent 1D; UTR 3′ untraslated region
The miR29a, whose changes in expression levels are associated with immediate response to cardiorespiratory fitness [88], is effective to increase p53 activity by targeting two negative regulators of p53, namely p85α, the regulatory subunit of phosphatidylinositol-3 kinase (P13K), and CDC42 (cell division control protein 42 homolog), a Rho GTPase [89]. Furthermore, miR‑29 was shown to target the protein phosphatase Mg2+/Mn2+-dependent 1D (PPM1D) during ageing, which is another negative regulator of p53. Thus, miR‑29 and p53 form a positive feedback loop that reinforces p53 functions, such as apoptosis and senescence.
The first report suggesting a negative regulation of p53 by miRNAs was published in 2009. Le and colleagues, by performing an in silico search for putative miRNA binding site in the p53 3′ untranslated region, demonstrated that miR-125b can bind to human and zebrafish p53 mRNA, thus reducing p53 expression. Overexpression of miR-125b is capable to repress the endogenous level of p53 protein, thus suppressing apoptosis in human neuroblastoma cells and human lung fibroblast cells. In contrast, knockdown of miR-125b is effective to enhance the concentration of p53 protein, thus inducing apoptosis in human lung fibroblasts and in the zebrafish brain [90]. Elevated expression levels of miR-125b were associated with increased tumor size and invasion in 89 colorectal cancer samples, and also correlated with poor prognosis and decreased survival [91]. It is also noteworthy that miR125b belongs to a class of inflammatory microRNAs whose expression levels are modulated by acute exercise [92–94]. One year later, Hu et al. demonstrated that miR-504, a stress-induced miRNA [95], can regulate p53 expression through its binding to two binding sites in human p53 3′-untranslated region [96]. Experiments presented by the authors demonstrated that overexpression of miR-504 reduces p53 protein levels and impairs p53 functions, including apoptosis and cell cycle arrest. Furthermore, miR-504 promotes tumorigenicity of cells in vivo.
Taken together, these findings suggested that PA and stress may positively or negatively regulate the activity and function of the p53 signaling pathway by modulating the effect of tumor suppressor or oncogenic miRNAs.
Physical Activity and Hypoxia-Inducible Factor-1 Pathway
The hypoxia-inducible factor-1 (HIF-1) pathway plays a pivotal role in cancer biology. More specifically, hyperactivation of this pathway has been associated with increased angiogenesis, enhanced cell survival and local or distant cancer spread, so that inhibitors of HIF-1 are increasingly developed and used as anticancer therapeutics [97]. In brief, the HIF pathway is composed by two different proteins (HIF-1α and HIF-1β). After synthesis, HIF-1α can only exert its transcription activities at the DNA level by stabilization by HIF-1β, so that the HIF-1α/HIF-1β complex can cross the nuclear membrane, bind to intranuclear proteins and trigger gene transcription. The activity of HIF-1 pathway is hence modulated at multiple levels, which entail protein stabilization, transactivation, and target gene availability. More specifically, prolyl-hydroxylase (PHD) and asparaginyl hydroxylase both promote HIF-1α degradation, whereas histone deacetylase sirtuin-6 (SIRT6) and factor-inhibiting HIF (FIH) substantially inhibit its transcriptional activity.
Recent evidence suggests that training may promote a negative regulation of the HIF-1 pathway . Lindholm et al. studied skeletal muscle tissue in matched populations of moderately active individuals and elite athletes [98]. When compared with moderately active individuals, elite athletes displayed a significantly higher expression of all negative HIF-1 modulators, including PHD (73.5 ± 9.5 vs. 98.0 ± 6.6), FIH (4.3 ± 0.2 vs. 31.0 ± 8.0), and SIRT6 (0.2 ± 0.1 vs. 11.4 ± 2.2). Similar evidence was previously published in an animal model, wherein Koltai et al. showed that exercise training was effective to reduce the expression of carbonylated proteins, including HIF-1-alpha, in rats [99].
The Intriguing Relationship Between Lipoprotein[a] , Physical Exercise, and Cancer
An interesting aspect in the intriguing relationship between sports, epigenetics, and cancer recently emerged from studies on lipoprotein[a] (Lp[a]) metabolism. Lp[a] is a highly atherogenic lipoprotein which strictly resembles a low-density lipoprotein (LDL) particle since it is composed by apolipoprotein B100 (i.e., the main protein moiety of LDL) covalently linked to a single copy of the unique and enigmatic apolipoprotein[a] (Apo[a]). The latter protein is unique to humans, Old World monkeys and apes, although an Apo[a]-like protein also exists in the blood of the hedgehog, in which it probably appeared independently, as result of a process of convergent evolution. The appearance of Apo[a] in the hedgehog genome, its preservation throughout the evolution of this small animal and primates, combined with the evidence that high levels of Lp[a] are compatible with longevity, do suggest that Lp[a] may confer some kinds of evolutionary advantage to those species who are capable to produce it. Although the enigma remains still inexplicable, it seems reasonable to conceive that the negative impact of Lp[a] on the cardiovascular system may be somehow offset by some favorable biological effects. Indeed, the large cholesterol content of this lipoprotein has been identified as beneficial for cell regeneration and organism recovery after trauma. Nevertheless, a more intriguing biological pathway has recently been elucidated, according to which Lp[a] may enhance survivor by decreasing mortality for cancer. The biochemical structure of Apo[a] is homologous to that of plasminogen, since this protein contains a protease domain, a single copy of plasminogen kringle V and multiple repeats of domains similar to plasminogen kringle IV (Fig. 6.3). Angiostatin is a natural modulator of angiogenesis, which is prevalently produced by catabolism of kringle-containing precursor proteins, which also include Apo[a]. Angiostatin exerts a kaleidoscope of anticancer effects such as upregulation of p53 protein, stimulation of FasL-mediated signaling pathways, and inhibition of Akt. All these activities ultimately converge to promote apoptosis of endothelial cell and inhibition of angiogenic signaling pathway activated by a number of angiogenic factors including fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF). Several lines of evidence now attest that Lp[a] is an active phase protein, wherein the transcription of the Apo[a] gene can be substantially amplified by various types of stress, including physical exercise [100]. The main effector regulating Apo[a] synthesis is probably interleukin-6 (IL-6), which activates the promoter region of the gene and ultimately increases the blood concentration of Apo[a], thus magnifying its putative anticancer potential [101] (Fig. 6.3). This hypothesis has recently been supported by epidemiological evidence attesting that low Lp[a] levels are associated with both all-cause and cancer death [102], and that elderly patients display equivalent or even higher values of Lp[a] than those of the general population aged 75 years or younger.
Future Perspectives
Although epigenetics is still in its infancy in the field of exercise, studies have already suggested that epigenetic regulation may play an important role in modulating the effect of exercise on cancer development and progression . Research findings demonstrate that the benefits from PA occur when activity is at least of moderate intensity and performed regularly and is sustained over lifetime or at least for a long term. However, the effects of various modalities of exercise in modulating epigenetic modifications in different cancer sites remain largely unknown. Future studies will focus on the effect of cardiorespiratory versus resistant training and of acute versus prolonged exercise on epigenetics changes measured in acute phase and at long term. Findings derived from such studies might have two clinically relevant implications. First they may prompt the development of cancer-specific recommendations and guidelines establishing the exact type, intensity, and duration of exercise required for improved health outcomes in different group populations. Second, they will provide a number of epigenetic markers which could be used to monitor patients’ response to exercise interventions and predict health benefits.
References
Hippocrates (1953) Hippocrates: with an English translation by W. H. S. Jones. William Heinemann, London
Morris JN, Heady JA, Raffle PA et al (1953) Coronary heart-disease and physical activity of work. Lancet 265:1111–1120
Kokkinos P, Myers J (2010) Exercise and physical activity: clinical outcomes and applications. Circulation 122:1637–1648
Cherry T (1922) A theory of cancer. Med J Aust 1:435–438
Sivertsen I, Dahlstrom AW (1922) The relation of muscular activity to carcinoma: a preliminary report. J Cancer Res 6:365–378
Wiseman M (2008) The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc 67:253–256
WCRF/AICR (2007) Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. AICR, Washington DC
Wolin KY, Yan Y, Colditz GA et al (2009) Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer 100:611–616
Boyle T, Keegel T, Bull F et al (2012) Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. J Natl Cancer Inst 104:1548–1561
Slattery ML, Potter JD (2002) Physical activity and colon cancer: confounding or interaction? Med Sci Sports Exerc 34:913–919
Giovannucci E, Ascherio A, Rimm EB et al (1995) Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 122:327–334
WCRF/AICR (2014) Continuous Update Project Report: Food. Nutrition, Physical Activity, and the Prevention of Breast Cancer, http://www.dietandcancerreport.org/cup/cup_resources.php. Accessed 16 Sept 2015
IARC/WHO (2002) IARC Handbooks of Cancer Prevention: Weight Control and Physical Activity. IARC Press, Lyon
Wu Y, Zhang D, Kang S (2013) Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat 137:869–882
Goncalves AK, Dantas Florencio GL, Maisonnette de Atayde Silva MJ et al (2014) Effects of physical activity on breast cancer prevention: a systematic review. J Phys Act Health 11:445–454
Fournier A, Dos Santos G, Guillas G et al (2014) Recent recreational physical activity and breast cancer risk in postmenopausal women in the E3N cohort. Cancer Epidemiol Biomarkers Prev 23:1893–1902
Steindorf K, Ritte R, Eomois PP et al (2013) Physical activity and risk of breast cancer overall and by hormone receptor status: the European prospective investigation into cancer and nutrition. Int J Cancer 132:1667–1678
Monninkhof EM, Velthuis MJ, Peeters PH et al (2009) Effect of exercise on postmenopausal sex hormone levels and role of body fat: a randomized controlled trial. J Clin Oncol 27:4492–4499
Hirose K, Hamajima N, Takezaki T et al (2003) Physical exercise reduces risk of breast cancer in Japanese women. Cancer Sci 94:193–199
Thune I, Furberg AS (2001) Physical activity and cancer risk: dose-response and cancer, all sites and site-specific. Med Sci Sports Exerc 33:S530–550; discussion S609-510
Monninkhof EM, Elias SG, Vlems FA et al (2007) Physical activity and breast cancer: a systematic review. Epidemiology 18:137–157
Cust AE, Armstrong BK, Friedenreich CM et al (2007) Physical activity and endometrial cancer risk: a review of the current evidence, biologic mechanisms and the quality of physical activity assessment methods. Cancer Causes Control 18:243–258
Keum N, Ju W, Lee DH et al (2014) Leisure-time physical activity and endometrial cancer risk: dose-response meta-analysis of epidemiological studies. Int J Cancer 135:682–694
Moore SC, Gierach GL, Schatzkin A et al (2010) Physical activity, sedentary behaviours, and the prevention of endometrial cancer. Br J Cancer 103:933–938
Voskuil DW, Monninkhof EM, Elias SG et al (2007) Physical activity and endometrial cancer risk, a systematic review of current evidence. Cancer Epidemiol Biomarkers Prev 16:639–648
Schmid D, Behrens G, Keimling M et al (2015) A systematic review and meta-analysis of physical activity and endometrial cancer risk. Eur J Epidemiol 30:397–412
Leitzmann M, Powers H, Anderson AS et al (2015) European Code against Cancer 4th edition: Physical activity and cancer., Cancer Epidemiol
Ballard-Barbash R, Friedenreich CM, Courneya KS et al (2012) Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst 104:815–840
Ibrahim EM, Al-Homaidh A (2011) Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol 28:753–765
Je Y, Jeon JY, Giovannucci EL et al (2013) Association between physical activity and mortality in colorectal cancer: a meta-analysis of prospective cohort studies. Int J Cancer 133:1905–1913
Schmid D, Leitzmann MF (2014) Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol 25:1293–1311
Sanchis-Gomar F, Lippi G (2014) Physical activity - an important preanalytical variable. Biochem Med (Zagreb) 24:68–79
Sanchis-Gomar F, Olaso-Gonzalez G, Corella D et al (2011) Increased Average Longevity among the "Tour de France" Cyclists. Int J Sports Med 32:644–647
Lippi G, Plebani M (2013) Biomarker research and leading causes of death worldwide: a rather feeble relationship. Clin Chem Lab Med 51:1691–1693
Haskell WL, Lee IM, Pate RR et al (2007) Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 39:1423–1434
Nelson ME, Rejeski WJ, Blair SN et al (2007) Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 116:1094–1105
Organization WH (2010) Global Recommendations on Physical Activity for Health. WHO Press, Geneva, Switzerland
Kushi LH, Doyle C, McCullough M et al (2012) American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 62:30–67
Rock CL, Doyle C, Demark-Wahnefried W et al (2012) Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 62:243–274
McTiernan A, Ulrich C, Slate S et al (1998) Physical activity and cancer etiology: associations and mechanisms. Cancer Causes Control 9:487–509
Friedenreich CM, Orenstein MR (2002) Physical activity and cancer prevention: etiologic evidence and biological mechanisms. J Nutr 132:3456S–3464S
Pareja-Galeano H, Sanchis-Gomar F, Garcia-Gimenez JL (2014) Physical exercise and epigenetic modulation: elucidating intricate mechanisms. Sports Med 44:429–436
Garcia-Gimenez JL, Sanchis-Gomar F, Lippi G et al (2012) Epigenetic biomarkers: A new perspective in laboratory diagnostics. Clin Chim Acta 413:1576–1582
Horsburgh S, Robson-Ansley P, Adams R et al (2015) Exercise and inflammation-related epigenetic modifications: focus on DNA methylation. Exerc Immunol Rev 21:26–41
Kulis M, Esteller M (2010) DNA methylation and cancer. Adv Genet 70:27–56
Baylin SB (2005) DNA methylation and gene silencing in cancer. Nat Clin Pract Oncol 2(Suppl 1):S4–11
Brown WM (2015) Exercise-associated DNA methylation change in skeletal muscle and the importance of imprinted genes: a bioinformatics meta-analysis., Br J Sports Med
Liu M, Guo S, Stiles JK (2011) The emerging role of CXCL10 in cancer (Review). Oncol Lett 2:583–589
Liu Y, Meng F, Xu Y et al (2013) Overexpression of Wnt7a is associated with tumor progression and unfavorable prognosis in endometrial cancer. Int J Gynecol Cancer 23:304–311
Gurvich N, Perna F, Farina A et al (2010) L3MBTL1 polycomb protein, a candidate tumor suppressor in del(20q12) myeloid disorders, is essential for genome stability. Proc Natl Acad Sci U S A 107:22552–22557
Abdollahi A, Pisarcik D, Roberts D et al (2003) LOT1 (PLAGL1/ZAC1), the candidate tumor suppressor gene at chromosome 6q24-25, is epigenetically regulated in cancer. J Biol Chem 278:6041–6049
Fearon ER, Pierceall WE (1995) The deleted in colorectal cancer (DCC) gene: a candidate tumour suppressor gene encoding a cell surface protein with similarity to neural cell adhesion molecules. Cancer Surv 24:3–17
Sablina AA, Hector M, Colpaert N et al (2010) Identification of PP2A complexes and pathways involved in cell transformation. Cancer Res 70:10474–10484
Liu Y, Liu T, Sun Q et al (2015) Downregulation of Ras GTPaseactivating protein 1 is associated with poor survival of breast invasive ductal carcinoma patients. Oncol Rep 33:119–124
Lai JP, Sandhu DS, Shire AM et al (2008) The tumor suppressor function of human sulfatase 1 (SULF1) in carcinogenesis. J Gastrointest Cancer 39:149–158
Ou D, Yang H, Hua D et al (2015) Novel roles of TMEM100: inhibition metastasis and proliferation of hepatocellular carcinoma. Oncotarget 6:17379–17390
Holgado-Madruga M, Emlet DR, Moscatello DK et al (1996) A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature 379:560–564
Moniz S, Jordan P (2010) Emerging roles for WNK kinases in cancer. Cell Mol Life Sci 67:1265–1276
Walkinshaw DR, Tahmasebi S, Bertos NR et al (2008) Histone deacetylases as transducers and targets of nuclear signaling. J Cell Biochem 104:1541–1552
Lee KK, Workman JL (2007) Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol 8:284–295
Feng Q, Wang H, Ng HH et al (2002) Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain. Curr Biol 12:1052–1058
Frosig C, Rose AJ, Treebak JT et al (2007) Effects of endurance exercise training on insulin signaling in human skeletal muscle: interactions at the level of phosphatidylinositol 3-kinase, Akt, and AS160. Diabetes 56:2093–2102
Host HH, Hansen PA, Nolte LA et al (1985) (1998) Rapid reversal of adaptive increases in muscle GLUT-4 and glucose transport capacity after training cessation. J Appl Physiol 84:798–802
Kraniou GN, Cameron-Smith D, Hargreaves M (2004) Effect of short-term training on GLUT-4 mRNA and protein expression in human skeletal muscle. Exp Physiol 89:559–563
Richter EA, Hargreaves M (2013) Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 93:993–1017
McGee SL, Hargreaves M (2011) Histone modifications and exercise adaptations. J Appl Physiol 110:258–263
Cao L, Liu X, Lin EJ et al (2010) Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell 142:52–64
Liu X, McMurphy T, Xiao R et al (2014) Hypothalamic gene transfer of BDNF inhibits breast cancer progression and metastasis in middle age obese mice. Mol Ther 22:1275–1284
Flowers E, Won GY, Fukuoka Y (2015) MicroRNAs associated with exercise and diet: a systematic review. Physiol Genomics 47:1–11
Cimmino A, Calin GA, Fabbri M et al (2005) miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A 102:13944–13949
Cummins JM, He Y, Leary RJ et al (2006) The colorectal microRNAome. Proc Natl Acad Sci U S A 103:3687–3692
Michael MZ, O’ Connor SM, van Holst Pellekaan NG et al (2003) Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res 1:882–891
He L, Thomson JM, Hemann MT et al (2005) A microRNA polycistron as a potential human oncogene. Nature 435:828–833
Epel ES, Lin J, Dhabhar FS et al (2010) Dynamics of telomerase activity in response to acute psychological stress. Brain Behav Immun 24:531–539
De Lange T (2005) Telomere-related genome instability in cancer. Cold Spring Harb Symp Quant Biol 70:197–204
Wu X, Amos CI, Zhu Y et al (2003) Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst 95:1211–1218
McGrath M, Wong JY, Michaud D et al (2007) Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev 16:815–819
Meeker AK (2006) Telomeres and telomerase in prostatic intraepithelial neoplasia and prostate cancer biology. Urol Oncol 24:122–130
Chilton WL, Marques FZ, West J et al (2014) Acute exercise leads to regulation of telomere-associated genes and microRNA expression in immune cells. PLoS One 9, e92088
Oh BK, Kim YJ, Park C et al (2005) Up-regulation of telomere-binding proteins, TRF1, TRF2, and TIN2 is related to telomere shortening during human multistep hepatocarcinogenesis. Am J Pathol 166:73–80
Matsutani N, Yokozaki H, Tahara E et al (2001) Expression of telomeric repeat binding factor 1 and 2 and TRF1-interacting nuclear protein 2 in human gastric carcinomas. Int J Oncol 19:507–512
Tonevitsky AG, Maltseva DV, Abbasi A et al (2013) Dynamically regulated miRNA-mRNA networks revealed by exercise. BMC Physiol 13:9
Vogelstein B, Lane D, Levine AJ (2000) Surfing the p53 network. Nature 408:307–310
Hermeking H (2012) MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat Rev Cancer 12:613–626
Rokavec M, Li H, Jiang L et al (2014) The p53/miR-34 axis in development and disease. J Mol Cell Biol 6:214–230
Hermeking H (2007) p53 enters the microRNA world. Cancer Cell 12:414–418
Sanchis-Gomar F, Garcia-Gimenez JL, Perez-Quilis C et al (2012) Physical exercise as an epigenetic modulator: Eustress, the “positive stress” as an effector of gene expression. J Strength Cond Res 26:3469–3472
Bye A, Rosjo H, Aspenes ST et al (2013) Circulating microRNAs and aerobic fitness--the HUNT-Study. PLoS One 8, e57496
Park SY, Lee JH, Ha M et al (2009) miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol 16:23–29
Le MT, Teh C, Shyh-Chang N et al (2009) MicroRNA-125b is a novel negative regulator of p53. Genes Dev 23:862–876
Nishida N, Yokobori T, Mimori K et al (2011) MicroRNA miR-125b is a prognostic marker in human colorectal cancer. Int J Oncol 38:1437–1443
Radom-Aizik S, Zaldivar F Jr, Leu SY et al (2012) Effects of exercise on microRNA expression in young males peripheral blood mononuclear cells. Clin Transl Sci 5:32–38
de Gonzalo-Calvo D, Davalos A, Montero A et al (1985) (2015) Circulating inflammatory miRNA signature in response to different doses of aerobic exercise. J Appl Physiol 119:124–134
Radom-Aizik S, Zaldivar F, Haddad F et al (1985) (2013) Impact of brief exercise on peripheral blood NK cell gene and microRNA expression in young adults. J Appl Physiol 114:628–636
Zhang Y, Zhu X, Bai M et al (2013) Maternal deprivation enhances behavioral vulnerability to stress associated with miR-504 expression in nucleus accumbens of rats. PLoS One 8, e69934
Hu W, Chan CS, Wu R et al (2010) Negative regulation of tumor suppressor p53 by microRNA miR-504. Mol Cell 38:689–699
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3:721–732
Lindholm ME, Fischer H, Poellinger L et al (2014) Negative regulation of HIF in skeletal muscle of elite endurance athletes: a tentative mechanism promoting oxidative metabolism. Am J Physiol Regul Integr Comp Physiol 307:R248–255
Koltai E, Szabo Z, Atalay M et al (2010) Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev 131:21–28
Mackinnon LT, Hubinger LM (1999) Effects of exercise on lipoprotein(a). Sports Med 28:11–24
Muller N, Schulte DM, Turk K et al (2015) IL-6 blockade by monoclonal antibodies inhibits apolipoprotein (a) expression and lipoprotein (a) synthesis in humans. J Lipid Res 56:1034–1042
Lippi G (2005) Lipoprotein(a)-lowering therapies: A double edged sword? Atherosclerosis 242:504–505
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lippi, G., Danese, E., Sanchis-Gomar, F. (2016). Stress, Exercise, and Epigenetic Modulation of Cancer. In: Berger, N. (eds) Epigenetics, Energy Balance, and Cancer. Energy Balance and Cancer, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-319-41610-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-41610-6_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-41608-3
Online ISBN: 978-3-319-41610-6
eBook Packages: MedicineMedicine (R0)