Abstract
The response of tropical trees to rising temperatures represents a key uncertainty that limits our ability to predict biosphere-atmosphere feedbacks in a warming world. We review the current understanding of temperature effects on the ecophysiology of tropical trees from organelle to biome level, where we distinguish between short-term responses, acclimation, and adaptation . We present new data on short-term temperature responses of photosynthesis and dark respiration , and temperature acclimation of photosynthesis. We also compare new field and laboratory-obtained photosynthesis-temperature response data. We identify several priority study areas. (1) Acclimation: We need to better understand photosynthetic acclimation, for example to determine whether the adjustment of the thermal optimum of photosynthesis (TOpt) is consistently negated by a decrease in photosynthesis at TOpt, as we observed. (2) Growth: Whereas tropical seedlings may grow better with warming, canopy trees reportedly grow worse; we do not currently know what explains these contrasting temperature effects. (3) Reproduction: Tropical trees may be close to reproductive temperature thresholds, as heat sterility in crops occurs in the upper 30 °C range. Nonetheless, the temperature sensitivity of tropical tree reproduction is virtually unstudied. (4) Mortality: How does heat-induced atmospheric drought (high leaf-to-air vapor pressure deficit ) affect tropical tree mortality? (5) Stomatal behavior: What is the specific role of temperature in the induction of midday-stomtal closure on sunny days? Better knowledge in these areas will improve our ability to predict carbon fluxes in tropical forests experiencing ongoing warming.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- respirationRespiration
- photosynthesisPhotosynthesis
- Short-term Temperature Response
- Isoprene Emission
- Tropical Canopy Tree
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Tropical forests cover only 15 % of the planet’s terrestrial surface (Pan et al. 2013), yet they account for more than one-third of its net primary productivity (NPP) (Saugier et al. 2001) and two-thirds of its plant biomass (Pan et al. 2013). Given this disproportionally large contribution to the global carbon cycle, it is important that we increase our understanding of the effects of global warming on tropical forest trees. Recent analyses have suggested that global variation in temperature and precipitation have no direct effect on global patterns of NPP after accounting for stand age and biomass (Michaletz et al. 2014), but this does not mean that changes in climatic variables will not affect NPP within a given biome. The coming decades will see ongoing warming in tropical regions, leading to unprecedented temperature regimes (Diffenbaugh and Scherer 2011) that currently do not support closed-canopy forests (Wright et al. 2009). Tropical regions have previously experienced warming—most notably leading up to the Paleocene-Eocene Thermal Maximum (~56 million years ago) when temperatures rose by 3–5 °C—but even the most rapid of such historical warming events took place over thousands to tens of thousands of years, timescales during which gradual changes in species composition, adaptive responses, and speciation are possible (Jaramillo et al. 2010). Current warming, on the other hand, occurs over the lifetime of individual trees, necessitating a high degree of thermal plasticity to maintain long-term growth and survival.
Warming effects on the physiology of woody plants have been studied primarily in mid- and high-latitiude ecosystems. Many of these effects are expected to be broadly generalizable, given the universal effects of temperature on enzyme kinetics, and the common principles of the biochemical pathways of both photosynthesis and mitochondrial respiration, the key drivers of terrestrial biosphere-atmosphere carbon exchange. However, there are several important differences between tropical and higher latitude ecosystems that might differentially affect plant responses to climate warming.
First, seasonal temperature fluctuations are minimal in the tropics, and such thermally stable conditions may not favor evolution of the capacity to acclimate to temperature changes (Janzen 1967; Cunningham and Read 2003). If tropical species have limited thermal plasticity, current warming may be highly detrimental to their performance. Second, for most of the past 2.6 million years tropical regions have experienced lower temperatures than today. Natural selection under such conditions would not have favored heat-protective traits (Corlett 2011). Temperate and boreal climates have also been cooler over this period, but current warming is more likely to expose tropical vegetation to temperatures near the thermal limit of photosynthesis than higher latitude vegetation. Indeed, tropical forests might be close to their thermal optimum already (Doughty and Goulden 2008; but see Lloyd and Farquhar 2008). Third, warming does not lengthen the growing season in the tropics as it does at higher latitutes (Menzel and Fabian 1999; Menzel et al. 2006). This means that a longer growing period will not compensate for any negative effect of temperature on carbon uptake in the tropics. Given the above three points, temperature relationships of tropical trees deserve special attention.
Here we review the current understanding of the effects of elevated temperature on the ecophysiology of tropical trees and forests. We present new data on instantaneous effects and growth-temperature effects on seedling gas exchange ; we compare laboratory-based measurements with in situ measurements of temperature effects on foliar physiology; we review experimental, observational, and modeling studies; and we identify areas of study that should be prioritized to improve our ability to predict carbon fluxes in gradually warming tropical forests. We will focus on temperature responses at the ecophysiological level, emphasizing the effects of warming in the non-damaging temperature range. For information on molecular responses to heat stress we refer readers to several reviews on this subject that have been published in the past decade (e.g., Wahid et al. 2007; Allakhverdiev et al. 2008; Ashraf and Harris 2013). Our primary concern here is the effect that warming has on tropical lowlands, which are currently the warmest ecosystems that support closed-canopy forests (Wright et al. 2009). The ~15 % of tropical forests that are montane are beyond the scope of this chapter.
We distinguish between different temporal scales at which temperature may affect tropical trees, and between the organizational scales at which these temperature response processes operate (Table 1). We will start our review at the organelle and leaf level—with a strong focus on photosynthesis and respiration—then work our way up through the whole-tree level out to stand-, ecosystem-, and biome-level temperature effects. Along the way we go from a highly mechanistic understanding of organelle- and leaf-level processes—based on foundational work on temperate species and backed up by a large body of experimental work—to more speculation as we attempt to predict temperature effects at higher organizational scales and over longer time periods for which no experimental work exists to date.
Leaf-Level Temperature Effects
Photosynthesis
Net photosynthesis (ANet) increases with short-term warming before reaching a maximum CO2 assimilation rate (AOpt) at optimum temperature (TOpt), beyond which net CO2 uptake rates decline and eventually drop to zero at the upper CO2 compensation point (Box 1). We will first discuss the short-term temperature response over non-harmful, reversible temperature ranges. After briefly addressing heat damage, we discuss temperature responses at timescales over which acclimation may occur.
Direct Effects: CO2 Fixation
At a given light level, photosynthesis is limited by one of three factors: (1) the capacity of ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) to carboxylate ribulose bisphosphate (RuBP) (Rubisco-limited, or RuBP-carboxylation-limited photosynthesis); (2) the capacity to regenerate RuBP through the Calvin cycle and the thylakoid reactions (RuBP-regeneration-limited photosynthesis ); or (3) the capacity for triose phosphate use (TPU) through starch and sucrose synthesis, where low TPU limits the regeneration of inorganic phosphate necessary for photophosphorylation (TPU-limited photosynthesis) (Harley and Sharkey 1991; von Caemmerer 2000). At light levels that saturate photosynthesis, RuBP-regeneration capacity generally reflects the maximum electron transport capacity (Jmax), whereas the rate of RuBP-carboxylation-limited photosynthesis represents the maxium capacity for Rubisco carboxylase activity (VCmax). TPU limitation at current atmospheric CO2 concentrations only occurs at low temperatures (Sage and Kubien 2007), and is expected to have minimal impact on photosynthesis in lowland tropical forest trees (but see Ellsworth et al. 2015 for potential TPU limitation driven by foliar phosphorus limitation). At current ambient CO2 concentrations, RuBP-caboxylation limitation is common at intermediate temperatures, whereas RuPB-regeneration can become limiting at high temperatures (Sage and Kubien 2007). High temperature can also impede Rubisco functioning through its effect on Rubsico activase, the enzyme that promotes the dissociation of inhibitory sugar phosphates from the active site of Rubisco, thereby activating it (Portis 2003). Rubisco activase has lower heat tolerance than Rubisco itself, so high-temperature impairment of Rubisco activity may represent reduced activation of Rubsico, rather than reduced functioning of activated Rubisco (Salvucci and Crafts-Brandner 2004).
Rubsico can not only carboxylate RuBP , it can also oxygenate it, leading to photorespiration , a process that results in loss rather than gain of CO2. Photorespiration, although associated with reduced carbon gain, appears to play a beneficial role under high temperatures by maintaining electron flow and preventing photo-oxidation (Osmond and Björkman 1972; Kozaki and Takeba 1996), and by providing a substrate for the synthesis of isoprene (Jardine et al. 2014), a volatile organic compound believed to be associated with thermoprotection of photosynthesis (see “Volatile organic compounds and thermoprotection”). Increasing temperature promotes photorespiration in two ways. First, the solubility of CO2 decreases more strongly with temperature than that of O2, so proportionally more O2 can reach the active sites of Rubisco and stimulate RuBP oxygenation. Second, the relative specificity of Rubisco for CO2 compared to O2 decreases with increasing temperature, so at higher temperatures, higher CO2 concentrations are needed to achieve a given RuBP-carboxylation rate (von Caemmerer and Quick 2000).
Under current ambient CO2 concentrations, photosynthesis is controlled by TPU capacity at low temperature (<~20 °C for the tropical species Ipomoea batatas (L.) Lam. (sweet potato; Sage and Kubien 2007). At intermediate temperatures RuBP carboxylation becomes the rate-limiting step. With further warming (>~32 °C for I. batatas) increased photorespiration reduces photosynthetic efficiency, and the electron transport processes of RuBP regeneration become limiting. Ultimately, at temperatures exceeding the thermal tolerance of Rubisco activase, Rubsico activation may become limiting. Increasing CO2 concentrations will reduce photorespiration , thus increasing the rates of both RuBP-regeneration-limited photosynthesis and RuBP-caboxylation-limited photosynthesis, without affecting TPU-limited photosynthesis. RuBP-caboxylation-limited photosynthesis is more strongly stimulated by CO2 than RuBP-regeneration limited photosynthesis. Consequently, at higher CO2, there is a shift in what controls net photosynthesis at a given temperature, with control exerted by TPU extending to higher temperatures than at low CO2, and RuBP regeneration starting to limit photosynthesis at lower temperatures, resulting in a reduced or disappearing role of RuBP-caboxylation-limitation in constraining net photosynthesis (Sage and Kubien 2007). In other words, at elevated CO2 , Jmax exerts stronger control over the temperature response of photosynthesis than VCmax. The remainder of our discussion will focus primarily on temperature effects at current ambient CO2 concentration (~400 ppm).
Figure 1a illustrates how temperature affects net CO2 assimilation at 21 % versus 2 % O2—i.e., with and without photorespiration—in two early-successional tropical tree species, Ochroma pyramidale (Cav. ex Lam.) Urb. and Ficus insipida Willd., and in the late-successional species Calophyllum longifolium Willd. Because photorespiration is proportionally higher at higher temperatures (Slot et al. 2016), TOpt of net photosynthesis is higher at 2 % O2, similar to what has been observed under elevated CO2 (e.g., Berry and Björkman 1980). C. longifolium has the lowest rates of photosynthesis regardless of temperature, consistent with its conservative growth strategy. O. pyramidale and F. insipida, in contrast, are fast growing species with higher TOpt and AOpt values. They germinate in forest gaps where irradiance levels as well as temperatures are higher than in the understory where C. longifolium typically regenerates.
Temperature response curves of net photosynthesis under photorespiratory (21 % O2, dashed lines, closed symbols), and non-photorespiratory conditions (2 % O2, solid lines, open symbols) a, and dark respiration b of recently fully expanded leaves of Calophyllum longifolium (circles), Ficus insipida (triangles), and Ochroma pyramidale (squares) seedlings grown outdoors under full natural radiation in Panama (mean annual temperature 27 °C). Arrows indicate increases in TOpt and AOpt with removal of photorespiration . Non-photorespiratory conditions were created by mixing air entering the cuvette with nitrogen gas at a 1:9.5 ratio. After passing the mixture through soda lime, CO2 was added to generate a CO2 concentration of 400 ppm. Measurements were made on attached leaves (n = 3–6) in a temperature-controlled Walz cuvette (Walz GmbH, Eiffeltrich, Germany) attached to an LI-6252 infrared gas analyzer (Licor). Photosynthesis curves were fit as: net photosynthesis \( = b \times \left( {{\text{T}}_{\text{Leaf}} - {\text{T}}_{\text{Min}} } \right) \times \left( {1 - e^{{c \times \left( {{\text{T}}_{\text{Leaf}} - {\text{T}}_{\text{Max}} } \right)}} } \right) \). TMin and TMax are the hypothetical low- and high-temperature CO2 compensation points, and b and c are constants. All four variables were estimated using a non-linear solver function. Respiration fits are 3rd order polynomials. For clarity only means are shown in a; in b means ± 1 SEM are shown. Modified after Slot et al. (2016)
Direct Effects: Thermodamage
One of the most temperature-sensitive components of plants is photosystem II (PSII), a protein complex located in the thylakoid membrane of the chloroplast. PSII efficiency can be readily determined using chlorophyll a-fluorescence techniques (e.g., Krause and Weis 1991). Thermodamage assessment from chlorophyll fluorescence measurements correlates well with classical analysis of post-heating necrosis (Krause et al. 2010, 2013). The critical temperature for irreparable leaf damage is about 52 or 53 °C, depending on whether thermal damage is determined in the dark or in the light (Krause et al. 2015). Such temperatures are only a few degrees above leaf temperatures that already occur occassionally in situ in sun-exposed leaves of tropical forest trees (Krause et al. 2010; Fig. 2). There is some variation across species in reported thermotolerance, but this may in part reflect differences in methodology used to assess thermotolerance. In a study comparing tropical and temperate rainforest tree species in Australia , the leaf temperature at which irreversible damage occurred was independent of growth temperature and biome of origin (Cunningham and Read 2006), suggesting minimal acclimation and phylogeographic predisposition to thermal damage.
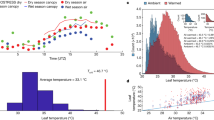
Comparison of field and laboratory based measurements of net photosynthesis versus leaf temperature (TLeaf) of Ficus insipida a, and the frequency distribution of daytime TLeaf for this species in the field b based on 1 month (Jan–Feb, dry season) of continuous monitoring of 6 leaves with thermocouple wires touching the abaxial surfaces. The inset in a shows the temperature response of stomatal conductance (GS). Lab measurements were made as described in Fig. 1. Field measurements were made with an LI-6400 (Licor) between 8 a.m. and noon on a typical dry season day. The leaves of the ~30 m tall study tree were accessed using a canopy crane. The response curve of field-based measurements is overlaid on the frequency distribution of TLeaf in b to illustrate the fact that TOpt occurs at the most frequently occurring TLeaf. Error bars represent 1 SEM (n = 4–6). Modified after Slot et al. (2016)
Indirect Effects: CO2 Fixation
Photosynthetic carbon uptake may also be affected by temperature via leaf-to-air vapor pressure deficit (VPD). Warm air can accomodate more water vapor than cool air, so at a given absolute moisture content of the air, VPD increases with leaf temperature, potentially causing stomatal closure. It is very difficult with standard gas-exchange equipment to keep VPD constant over a large range of leaf temperatures. One study that appears to have successfully controlled for VPD reported TOpt values of 28–29 °C for two rainforest tree species in Costa Rica measured in situ (Vargas and Cordero 2013), values that are comparable to those reported in Fig. 1, in which VPD was not controlled. These observations also roughly correspond to decreasing ANet values at temperatures >30 °C that Doughty and Goulden (2008) observed on three species measured in situ in the Brazilian Amazon, and with observations of Cheesman and Winter (2013a) of decreasing ANet when TLeaf was >27–30 °C in three tropical tree species grown in controlled-environment chambers in Panama . Whereas Vargas and Cordero (2013) maintained VPD < 2.0 kPa at leaf temperatures above TOpt, VPD in the experiment depicted in Fig. 1 increased from about 2.0 kPa at TOpt to 8.0 kPa at the upper CO2 compensation point. Nonetheless, these two studies show similar decreases in net photosynthesis at temperatures above ~30 °C. Insufficient data on VPD-controlled temperature responses of ANet of tropical trees complicate the interpretation of the role of VPD-induced stomatal closure in determining TOpt and photosynthetic decline above TOpt.
In upper-canopy tree leaves of tropical forest canopies, the combination of high temperature, high irradiance and high VPD can lead to pronounced midday depressions of stomatal conductance and CO2 uptake (e.g., Zotz et al. 1995; Cernusak et al. 2013; Goldstein et al. this volume, Santiago et al. this volume). Figure 2 shows changes in net CO2 uptake of F. insipida leaves measured in situ in the upper-canopy of a tropical forest in Panama as photosynthetic photon flux density (PPFD), temperature and VPD all increase from morning to noon on a sunny day. Compared to laboratory-measured seedling leaves (see Fig. 1 for methods), AOpt of canopy leaves was much higher because these leaves were acclimated to higher PPFD and had higher leaf mass per area. TOpt was also higher in the field, probably owing to generally higher leaf temperatures in situ. Whereas stomatal conducantance and photosynthesis in the field decreased to zero when leaf temperatures reached 45 °C, in the laboratory net photosynthesis remained positive up to 50 °C and conductance—after a marked decrease between 30 and 45 °C—started to increase above 45 °C (Fig. 2). This re-opening of stomata at high temperatures in the laboratory was also observed for O. pyramidale and C. longifolium (Slot et al. 2016). Nonetheless, in exposed canopy leaves, high heat load caused by high solar radiation can become so stressful that photosynthetic carbon uptake may take place mainly during morning hours. During the course of the day depicted in Fig. 2a, 50 % of the time leaf temperatures exceeded TOpt of F. insipida (Fig. 2b), stomata were partially closed, and ANet was reduced. Depending on leaf orientation, the extent to which midday depression occurs may vary considerably, even between neighboring leaves, or within leaves if they are undulated (e.g., Cecropia; K. Winter, unpublished data). The variability in leaf-level irradiance and leaf temperature makes stomatal conductance particularly challenging to model and predict with high spatial and temporal resolution. Given the frequent occurrence of midday stomatal reductions in tropical trees on sunny days, further understanding of this phenomenon is warranted to better predict global carbon fluxes in a warming world.
Acclimation
Thermal acclimation is a biochemical, physiological, or morphological adjustment by individual plants in response to a change in the temperature regime, which results in an alteration in the short-term response to temperature (Smith and Dukes 2013). Thermal acclimation of photosynthesis commonly leads to a shift of the thermal optimum of photosynthesis towards the new growth temperature (Box 1). This shift results from a change in relative contribution of RuBP carboxylation and RuBP regeneration in controlling net photosynthesis. The optimum temperature of RuBP regeneration is higher than that of RuBP carboxylation (Kirschbaum and Farquhar 1984; Hikosaka et al. 2006). Warm-acclimated plants tend to have a lower Jmax/VCmax ratio than cool-grown control plants (e.g., Bernacchi et al. 2003). When Jmax/VCmax is low, RuBP regeneration—with its relatively high TOpt— exerts greater control over net photosynthesis than when Jmax/VCmax is high in cool-grown plants, and as a result TOpt of net photosynthesis is higher in warm-acclimated plants (Hikosaka et al. 2006). If the Jmax/VCmax ratio is not reduced by high temperature, TOpt may still increase if the activation energy of VCmax increases more with growth temperature than that of Jmax (Hikosaka et al. 2006).
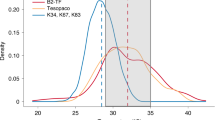
Consistent with thermal acclimation, TOpt of C. longifolium seedlings grown in controlled-environment chambers increased with growth temperature (Fig. 3), but there is a limit to this adjustment. Plants grown at 25/19 °C (day/night) and 30/24 °C had TOpt values close to their daytime growth temperature, whereas plants grown at 35/29 °C had a TOpt of 30 °C, suggesting that, at least for this late-successional species, 30 °C represents the maximum TOpt at current CO2 concentrations. While TOpt shifted towards the growth temperature, AOpt decreased with increasing growth temperature. Way and Yamori (2014) call this detractive adjustment to warming, as opposed to constructive adjustment (see Box 1). The exception to this pattern was the 35/29 °C treatment associated with elevated relative humidity (~90 % as opposed to ~45 %), for which both TOpt and AOpt were highest among all treatments. This may indicate that part of the decrease in AOpt relative to growth temperature resulted from temperature effects on VPD, with higher stomatal conductance being maintained when plants were grown at elevated relative humidity.
The optimal temperature of photosynthesis (TOpt) (a), and net photosynthesis at TOpt (AOpt) (b) plotted against growth temperature of Calopyllum longifolium seedlings (mean ± 1 SEM). Seedlings were grown in individual pots (2.8 l) in commercial potting soil in growth cabinets (Environmental Growth Chambers, Chagrin Falls, OH, USA) at day/night temperatures of 25/19, 30/24, 35/29, 35/29 + elevated RH, and 39/33 °C. Plants from the 39/33 °C did not have enough live leaves for photosynthesis measurements (see Fig. 4). Net photosynthesis was measured with an LI-6400 (Licor) at 20, 25, 30, 35, and 40 °C on one leaf per seedling (n = 6/treatment) after equilibration for at least 45 min in growth cabinets set to the target temperature. TOpt was calculated as described in the caption of Fig. 1. In one 35/29 °C cabinet relative humidity was increased to ~90 % (as opposed to ~45 %) by maintaining a pot of water near boiling point in the cabinet during the light period
Kositsup et al. (2009) grew Hevea brasiliensis Müll. Arg. seedlings at 18 and 28 °C and also found that TOpt adjusted to growth temperature. They further observed that ANet, VCmax and Jmax all increased with increased growth temperature, suggesting constructive acclimation. However, 18 °C is probably sub-optimal for lowland tropical species such as H. brasiliensis, and the observed adjustments may indicate that 28 °C is closer to the optimum temperature for this species, rather than indicate strong constructive thermal acclimation . Doughty (2011) warmed leaves of trees and lianas (woody vines) in the Brazilian Amazon and found no acclimation of ANet. The decrease in photosynthesis with warming was assigned to leaf damage caused by occasionally very high leaf temperatures of experimentally warmed leaves during sunny spells. Similarly, C. longifolium plants grown in controlled-environment chambers at 39/33 °C (day/night) showed severe leaf damage and photosynthesis could not be measured (Fig. 4).
Dusenge et al. (2015) reported that VCmax was similar between leaves of trees from two tropical rainforest sites in Rwanda differing in temperature regime, whereas Jmax was significantly lower at the warmer site, resulting in a reduced Jmax/VCmax, in line with observations on temperate species. The physiological plasticity notwithstanding, ANet was reduced at the warmer site compared to the cool site, suggesting detractive adjustment, similar to observations on C. longifolium (Fig. 3). We still know little about the thermal acclimation potential of VCmax and Jmax of lowland tropical species, and to date no study has compared the acclimation potential of VCmax and Jmax of tropical and temperate tree species. Such experimental studies are needed to better predict the effect of long-term warming on the photosynthetic properties of tropical forest vegetation.
Adaptation
There is at least one comparative study that points to fundamental differences in the physiological properties of species adapted to temperate versus tropical conditions. Cunningham and Read (2002) reported higher TOpt values for tropical than temperate species at a given growth temperature. Furthermore, the temperature response curve for temperate species was wider, i.e., temperate species had photosynthesis rates of >80 % of AOpt over a larger temperature range. These results are consistent with the absence of strong selection for a broad temperature range of photosynthesis for tropical species. We found that TOpt of tropical species is lower than growth temperature when growth temperatures exceed ~30 °C (Fig. 3). The narrower curves of tropical species and the apparent limit to increasing AOpt, despite a small increase in TOpt, suggest that significant warming in the tropics may cause a decline in photosynthetic carbon uptake.
Dark Respiration
Temperature Response
About 30 % of the carbon fixed by tropical forests through photosynthesis is released back into the atmosphere by foliar respiration (Chambers et al. 2004; Malhi 2012). Respiration is vital for plant growth and survival, supporting biosynthesis, cellular maintenance and repair, but from a carbon-balance perspective respiration represents a loss.
In the short term, dark respiration increases with temperature, peaks with a TOpt > 50 °C, and then steeply declines. The initial increase is often assumed to be exponential, and can be expressed as a Q10 value—the proportional increase in respiration with 10 °C warming (Box 1). The general assumption is that respiration rates double for every 10 °C warming, resulting in a Q10 of 2.0, although in reality a wide range of Q10 values—from ~1.4 to 4.2— has been reported (Atkin et al. 2005, and references therein). Leaf respiration of tropical forest species is at least as sensitive to short-term temperature increase as that of temperate species, with Q10 values ranging from 1.5 to 4.1 (Meir et al. 2001; Cavaleri et al. 2008; Slot et al. 2013, 2014b), and averaging above 2.0 (Slot et al. 2013, 2014b). Figure 1b shows respiration-temperature response curves for leaves of C. longifolium, F. insipida, and O. pyramidale. Notably, the response-curves (n = 3–6) are not exponential. Over a relatively narrow temperature range it is difficult to distinguish different shapes of temperature response curves, but the examples in Fig. 1b cover a range of ~25 °C, and an exponential curve is clearly not an appropriate approximation. We do not know whether the data shown in Fig. 1b reflect a uniquely tropical phenomenon, but a recent study with Oryza sativa L. (rice) reported similarly linear temperature responses of respiration over a ~14 °C range (Peraudeau et al. 2015). In contrast, measurements over a broad temperature range on the temperate Eucalyptus pauciflora suggest a good exponential fit up to critical temperatures around 50 °C (O’Sullivan et al. 2013), whereas Hüve et al. (2012) showed considerable variation in the shape of the response curve over a ~25 °C range across three temperate herbs. Modeling temperature responses using an exponential Q10 or Arrhenius-type fit when a linear fit better describes the actual response could lead to overestimation of respiration rates at high temperatures. Future research will need to assess how widely distributed the non-exponential temperature responses of dark respiration are among tropical species.
Photosynthesis and respiration are highly sensitive to temperature, but the effects depend on the timescale of exposure. Long term warming (e.g., weeks to months) may modify the short-term response through acclimation, i.e., the biochemical, or structural adjustment by individual plants in response to a new temperature regime
Acclimation
Nighttime temperatures increase more rapidly than daytime temperatures (e.g., Easterling et al. 1997), potentially leading to increased respiration costs in tropical forest systems. However, acclimation of respiration might mitigate this. Respiration at a given temperature is generally decreased in warm-acclimated plants compared to control plants, either through down-regulation of respiratory capacity at all temperatures, or because the short-term temperature sensitivity in warm-acclimated plants is reduced, leading to lower rates at intermediate to high temperatures (Atkin and Tjoelker 2003; see Box 1). Recently, we showed that upper-canopy leaves of tropical canopy trees and lianas acclimate to nighttime warming (Slot et al. 2014a). Acclimation has also been shown in seedlings of tropical tree species grown under different temperature regimes in controlled-environment chambers and open-top chambers (Cheesman and Winter 2013b). In fact, when accounting for the degree and duration of warming there is no indication that tropical species differ from species from other biomes in their capacity for thermal acclimation of respiration (Slot and Kitajima 2015).
At the whole-plant level, respiratory acclimation can be affected by interactions with mycorrhizal symbionts (Atkin et al. 2009). The only study to date that has addressed these interactions for tropical tree species indicated a strong species-specific effect of mycorrhizal colonization of roots on acclimation of whole-plant respiration to nighttime warming (Fahey et al. 2016). The host species-specific nature of the effects of mycorrhizae may complicate generalizations of acclimation trends in vegetation models, and calls for more research into the mechanisms behind these interactions.
Adaptation
Respiration rates at ambient temperature vary considerably across biomes, with rates increasing twofold from the Arctic to the tropics (Atkin et al. 2015). This doubling over a ~20 °C range in growing season temperature represents a considerable deviation from the much steeper instantaneous temperature response. Respiration standardized to 25 °C is threefold lower in the tropics than in the Arctic (Atkin et al. 2015), suggesting significant downregulation of respiratory capacity in warm-adapted species. These global patterns may reflect adaptation to different climates, but model simulations by Vanderwel et al. (2015) show that accounting for acclimation to local temperature regimes produces very similar patterns in simulated respiration across global temperature gradients. Given that the capacity for acclimation is common across the globe (Slot and Kitajima 2015) and sufficient to explain global patterns, it appears that there may not have been further selective pressure leading to adaptive temperature responses of respiration.
Volatile Organic Compounds and Thermoprotection
Volatile organic compounds (VOC) can contribute significantly to the carbon emissions from forests. The most common VOCs are isoprenes and monoterpenes, and the emission of both increases with short-term warming in the tropics (Keller and Lerdau 1999; Bracho-Nuñez et al. 2013). Isoprene emission is hypothesized to protect photosynthesis from short, high-temperature episodes (Sharkey and Yeh 2001; Sasaki et al. 2007) by helping stabilize thylakoid membranes (Velikova et al. 2011), quenching reactive oxygen species (Velikova et al. 2012; Jardine et al. 2013, 2014), and/or increasing heat dissipation (Sasaki et al. 2007; Pollastri et al. 2014). In the short term (minutes to hours), isoprene emission increases exponentially with temperature, both in temperate (Sharkey and Monson 2014) and tropical trees (Keller and Lerdau 1999), but above 38–40 °C emissions may decrease (Tingey et al. 1979; Alves et al. 2014; Jardine et al. 2013, 2014). TOpt of isoprene emission is ~10 °C higher than TOpt of net photosynthesis. Consequently, at high temperatures isoprene synthesis requires carbon sources other than recent photosynthates. Photorespiration may provide such an alternative source at temperatures exceeding TOpt of photosynthesis (Jardine et al. 2014). Controlled-environment studies with temperate species have shown that plants acclimated to higher growth temperature have a higher capacity for isoprene release than plants grown at moderate temperatures (Tingey et al. 1979; Monson et al. 1992), but there is currently no evidence to suggest that warm-adapted tropical species have higher emission rates than temperate species.
Despite its potential importance in thermoprotection, isoprene emission does not occur in all plants. It only occurs in perennial plants with C3 photosynthesis (Loreto and Fineschi 2015), but without a clear phylogenetic distribution, due in part to frequent loss and secondary acquisition of the trait (Monson et al. 2013). In the tropics isoprene emission is equally common in deciduous and evergreen plants, whereas emission is comparatively rare in temperate evergreens (Loreto and Fineschi 2015). Tambunan et al. (2006) studied isoprene emission in 42 tropical tree species and reported high rates in 4 species, very low rates (<10 µg g−1 h−1) in 28 species, and non-detectable rates in 10 species. Of the 51 tropical tree and lianas studied by Keller and Lerdau (1999), only 15 emitted isoprene at detectable rates. Even for the latter species, the total contribution of emission of isoprene to the carbon balance was relatively small, with an overall mean rate of 24 nmol m−2 s−1 (~65 µg g−1 h−1 at mean leaf mass per area of 90 g m−2) at 30 °C and PPFD of 1000 µmol m−2 s−1, which is ~500 times lower than the mean photosynthesis rate. It is likely that long-term warming will increase rates of isoprene emission from leaves of isoprene-emitting species (Sharkey and Monson 2014), but given the thermoprotective role these emissions appear to play, the loss of isoprene carbon is probably more than compensated for by the maintenance or recovery of photosynthetic functioning during episodic heat.
Whole-Plant Temperature Effects
Biomass Accumulation
Two meta-analyses suggest that plant growth decreases with warming when starting temperatures are already high (Way and Oren 2010; Lin et al. 2010); in both studies tropical species were underrepresented. Figure 4 shows the effect of combined day and nighttime warming on growth of C. longifolium. Growth was optimal at 35/29 °C (day/night), i.e., at temperatures well above those in its natural habitat in the forest understory. Several studies show that nighttime warming stimulates growth in seedlings of tropical tree species (Esmail and Oelbermann 2011; Cheesman and Winter 2013a, b). These seedling responses contrast with results obtained for tropical canopy trees, which suggest a negative correlation between nighttime temperature and tree growth (Clark et al. 2003, 2010, 2013; Vlam et al. 2014; Anderegg et al. 2015a). A recent study on Eucalyptus species across a temperature gradient in Australia reported that, compared to small trees, big trees show a disproportionally strong decline in growth with increasing mean annual temperature (Prior and Bowman 2014). Perhaps ontogenetic differences in temperature sensitivity govern the contrasting temperature effects on tropical canopy trees and seedlings. Leaf area ratio (leaf area divided by total plant mass) decreases with ontogeny, so canopy trees have proportionally less photosynthetic material with which to support the maintenance of non-photosynthetic tissue than seedlings. If the capacity for thermal acclimation of root and stem respiration is smaller than that of leaf respiration, then warming would increase the respiration load in canopy trees more strongly than in seedlings. There is no indication that acclimation of root respiration is lower than that of leaf respiration in temperate species (Loveys et al. 2003), but little is known about thermal acclimation of root and stem respiration in the tropics beyond the observation by Fahey et al (2016) that acclimation of whole-plant- and root respiration varied greatly among species.
In in situ studies of growth-temperature relationships—using latitudinal or elevational temperature gradients, or inter-annual trends in temperature—temperature is one of multiple factors that change, and patterns of biological response cannot be assigned to temperature differences alone. Controlled-environment chambers, on the other hand, enable the isolated study of temperature effects. However, in controlled-environment studies with potted seedlings, soil temperature may track changes in air temperature more closely than in the field, and soil warming itself may increase growth (Königer and Winter 1993; Holtum and Winter 2014). Forest soils are thermally very stable, so soil processes may explain at least part of the observed contrasting responses of seedlings and canopy trees to nighttime warming. Soil warming may increase nutrient mineralization rates (Jarvis and Linder 2000), thereby stimulating growth. However, we found that nighttime warming stimulated growth of O. pyramidale seedlings equally in fertilized and non-fertilized soils (Slot and Winter unpublished data). Altered source-sink relationships (Pilkington et al. 2015) may provide an alternative, though not yet tested explanation for the observed responses.
Mortality
A quantitative trait locus (QTL) for thermotolerance has been identified for the comparatively heat-tolerant Oryza glaberrima Steud. (African rice), the overexpression of which in O. sativa L., Arabidopsis thaliana L., and Festuca arundinacea Schreb., significantly reduced their mortality following a 12 day 38/35 °C treatment (Li et al. 2015). The QTL is associated with degradation of cytotoxic denatured proteins that accumulate during heat exposure, suggesting an important role for the proteins encoded by the QTL in mitigating warming-induced cell death and plant mortality. Several other factors may play a role in warming-induced mortality. For example, increased rates of respiration and decreased rates of photosynthesis could lead to carbon starvation . Atmospheric drought caused by temperature-induced increase in VPD may also kill plants. It is hard to disentangle drought and temperature effects, as soil moisture availability may prevent warming-induced carbon starvation by enabling transpirational leaf cooling and maintenance of a positive carbon balance during heat events (Bauweraerts et al. 2014), and carbon reserves in the form of soluble carbohydrates may prevent drought-induced hydraulic failure (O’Brien et al. 2014). Drought is a common agent of mortality in the tropics (e.g., Condit et al. 1995; Allen et al. 2010), subject of observational (e.g., Condit et al. 1995; Phillips et al. 2010) and experimental studies (e.g., Slot and Poorter 2007; Nepstad et al. 2007), but atmospheric drought, such as occurs during warming, is much less studied (Breshears et al. 2013).
In the C. longifolium study depicted in Fig. 4, daytime temperature of 39 °C and relative humidity of ~29 % was lethal for most seedlings. We do not know whether the well-watered seedlings died from cytotoxicity, carbon starvation, or hydraulic failure, but the fact that TOpt and AOpt at 35/29 °C were higher under elevated relative humidity (Fig. 3), shows that atmospheric drought can negatively impact the maintenance of a positive carbon balance, and potentially survival. Tropical sapling mesocosms grown outdoors in Panama, with daytime warming of ~5 °C did not negatively impact biomass accumulation and photosynthetic traits as long as plants had access to soil water (Slot and Winter unpublished data), further highlighting the interconnected nature of heat and drought as agents of plant mortality.
Elevated CO2 improves both the carbon balance and water use efficiency of plants, but a recent greenhouse study on Eucalyptus radiata A.Cunn. ex DC suggests that negative effects of elevated temperature on drought-induced mortality are not alleviated by elevated CO2 , despite higher leaf-level water use efficiency in the high CO2 treatment (Duan et al. 2014). Thus, drought-induced mortality may occur in parts of the tropics where warming is accompanied by decreased precipitation (Olivares et al. 2015). Furthermore, abiotic climatic changes are likely to interact with biotic sources of mortality such as insect outbreaks (Anderegg et al. 2015b). For example, warming can affect phytochemistry and the feeding efficiency of invertebrate herbivores (Jamieson et al. 2015), potentially leading to increased leaf loss and plant mortality.
Reproduction
There have not been any detailed experimental studies on how rising temperatures affect reproduction of tropical vegetation, although the reproductive phase is one of the most temperature-sensitive parts of the lifecycle of plants. Pollen production, pollen viability and pollen tube growth all decrease with warming in a range of crop species (Sage et al. 2015, and references therein). There is considerable variation in temperature sensitivity across genotypes and species, but no clear relationship between ambient growth environment and thermal sensitivity (Prasad et al. 2006). Pollen viability of the tropical tree Mangifera indica L. (mango) decreases above 33 °C (Issarakraisila and Considine 1994), which is not an unusually high temperature in the tropics. Many tropical species flower for only short periods and risk reproductive failure if flowering coincides with anomalously high temperatures. Surprisingly, flower production correlated positively with temperature both seasonally and interannually in tropical forests in Panama and Puerto Rico (Pau et al. 2013). Increased flower production could compensate for decreased pollen viability but detailed study is required to better understand temperature effects on tropical tree reproduction and to disentangle direct warming effects on plant fertility, and warming effects on reproductive allocation.
Temperature may also affect reproduction through its effect on pollinators. For example, fig-pollinating wasps—which develop inside developing figs—die at temperatures only a few degrees above current ambient temperatures (Patiño et al. 1994). Transpirational cooling helps maintain fruit temperatures in non-lethal ranges, but further warming could have major negative consequences for fig reproduction.
Exposure to high temperature is necessary to break physical dormancy of seeds of many species. This is especially common for species that form seed banks, as the high temperature is associated with high light conditions favorable for seedling establishment of these early-successional species. More intense heatwaves (Meehl and Tebaldi 2004) could potentially trigger germination under conditions not favorable for seedling establishment, leading to mortality. The potential effects of climate change on the seed ecology of tropical forest trees is not well understood (Walck et al. 2011).
Stand-, Ecosystem-, and Biome-Level Temperature Effects
An important aspect of tropical forests is their very high species diversity. All trees ultimately use—and often directly compete for—the same resources, and species coexistence is at least in part maintained by niche specialization along resource gradients (e.g., Kitajima and Poorter 2008; Condit et al. 2013). This means that climate warming may shift the competitive balance within a forest community if rising temperature differentially affects mortality and reproduction across species. For example, warming may increase soil mineralization rates in the tropics (Salinas et al. 2011). Tropical tree species vary widely in their phosphorus affinity (Condit et al. 2013), and temperature-induced changes in nutrient availability potentially contribute to shifts in species composition. This may result in reduced diversity, or a change in the dominant species and functional types associated with a particular nutrient regime. Climate-induced changes in species composition will affect forest growth (Coomes et al. 2014). Systematic changes in growth rates of trees may also cause turnover rates in tropical forests to increase (Phillips and Gentry 1994). Nevertheless, changes in tree growth rates and turnover do not necessarily change ecosystem carbon storage if the size structure of trees within the ecosystem is maintained (Körner 2009).
Tropical ecosystems have lower ratios of NPP over gross primary productivity (GPP) than most higher latitude ecosystems (Zhang et al. 2009, 2014). Furthermore, rising temperatures decrease the NPP/GPP ecosystem carbon use efficiency (Zhang et al. 2014) suggesting that, despite observed thermal acclimation capacity at the leaf level, warming causes ecosystem-level respiration cost to increase. Soil respiration is a major component of ecosystem respiration, and soil respiration rates in tropical forests are higher than in any other ecosystem in the world (Raich and Schlesinger 1992). The capacity of tropical soil respiration to acclimate to higher temperatures is unknown.
Concluding Remarks
Because most research on temperature effects on the ecophysiology of plants has been done on non-tropical species, much is still to be discovered about how tropical trees will respond to climate change . We have a fairly sound understanding of general principles of temperature responses at the organelle and leaf level (Table 1), but we do not know whether tropical trees differ systematically from non-tropical species in how warming affects leaf and whole-plant performance. For example, more research is needed to establish the generality of detractive acclimation of photosynthesis in tropical species, as observed in C. longifolium (Fig. 3). We have shown that the commonly assumed exponential increase of respiration with temperature may not apply to tropical leaves over wide temperature ranges, a finding that requires further confirmation. At this point, we can only speculate on the whole tree-level effects of warming, apart from noting that warming is associated with atmospheric drought and may affect reproduction. At the stand level, warming may exacerbate midday reductions in stomatal conductance . A better mechanistic understanding of the causes and consequences of midday stomatal closure will improve our ability to translate understanding based on individual leaf responses to stand-level photosynthesis simulations.
In the decades to come the tropics will experience further increases in atmospheric CO2 concentrations and warming, and significant alterations in the timing and amount of rainfall (Diffenbaugh and Scherer 2011; Mora et al. 2013). The vulnerability of tropical forests to climate change is under much debate (e.g., Clark 2004; Lloyd and Farquhar 2008, Lewis et al. 2009; Booth et al. 2012; Randerson 2013). Because of the large contribution of tropical forests to the global carbon cycle, a better understanding of how these forests respond to climate change drivers will improve our ability to predict future climate and biogeochemical cycling at the global scale (Booth et al. 2012; Huntingford et al. 2013; Piao et al. 2013). Information on thermal acclimation of photosynthetic parameters VCmax and JMax will be crucial to improve predictive models De Kauwe et al. (2016). We can learn a lot about plant-temperature interactions from elevation gradients in the tropics (e.g., Malhi et al. 2010), but these gradients do not reveal how plants at the high-temperature end of the gradient will respond to further warming, and that is precisely the key in determining the fate of tropical lowland forests in a warming world. As global warming generates novel climate regimes in tropical latitutes, maintenance of current forest structure requires processes of thermal acclimation to occur in conjuction with adjustments to changing precipitation regimes (Mora et al. 2013)—and possibly changes in cloud cover—under gradually rising ambient CO2 concentrations and increasing atmospheric deposition of nutrients (Hietz et al. 2011). Clearly, in situ warming experiments of entire forest segments are needed to better assess the effects of rising temperatures on tropical vegetation (Cavaleri et al. 2015).
References
Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, Mohanty P (2008) Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res 98:541–550
Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A et al (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Man 259:660–684
Alves EG, Harley P, Gonçalves JFC, da Silva CE, Moura KJ (2014) Effects of light and temperature on isoprene emission at different leaf developmental stages of Eschweilera coriacea in central Amazon. Acta Amazonica 44:9–18
Anderegg WR, Ballantyne AP, Smith WK, Majkut J, Rabin S, Beaulieu C, Birdsey R, Dunne JP et al (2015a) Tropical nighttime warming as a dominant driver of variability in the terrestrial carbon sink. Proc Nal Acad Sci USA 112:15591–15596
Anderegg WRL, Hicke JA, Fisher RA, Allen CD, Aukema J, Bentz B, Hood S, Lichstein JW et al (2015b) Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol 208:674–683
Ashraf M, Harris PJC (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51:163–190
Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8:343–351
Atkin OK, Bruhn D, Tjoelker MG (2005) Response of plant respiration to changes in temperature: mechanisms and consequences of variations in Q10 values and acclimation. In: Lambers H, Ribas-Carbo M (eds) Plant respiration: from cell to ecosystem. Springer, Dordrecht, pp 95–135
Atkin OK, Sherlock D, Fitter A, Jarvis S, Hughes J, Campbell C, Hurry V, Hodge A (2009) Temperature dependence of respiration in roots colonized by arbuscular mycorrhizal fungi. New Phytol 182:188–199
Atkin OK, Bloomfield KJ, Reich PB, Tjoelker MG, Asner GP, Bonal D, Bönisch G, Bradford M et al (2015) Global variability in leaf respiration among plant functional types in relation to climate and leaf traits. New Phytol 206:614–636
Bauweraerts I, Ameye M, Wertin TM, McGuire MA, Teskey RO, Steppe K (2014) Water availability is the decisive factor for the growth of two tree species in the occurrence of consecutive heat waves. Agr Forest Meteorol 189:19–29
Bernacchi CJ, Pimentel C, Long SP (2003) In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant Cell Environ 26:1419–1430
Berry J, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31:491–543
Booth BBB, Jones CD, Collins M, Totterdell IJ, Cox PM, Sitch S, Huntingford C, Betts RA, Harris GR, Lloyd J (2012) High sensitivity of future global warming to land carbon cycle processes. Environ Res Lett 7:024002
Bracho-Nuñez A, Knothe NM, Welter S, Staudt M, Costa WR, Liberato MAR, Piedade MTF, Kesselmeier J (2013) Leaf level emissions of volatile organic compounds (VOC) from some Amazonian and mediterranean plants. Biogeosci 10:5855–5873
Breshears DD, Adams HD, Eamus D, McDowell NG, Law DJ, Will RE, Williams AP, Zou CB (2013) The critical amplifying role of increasing atmospheric moisture demand on tree mortality and associated regional die-off. Front Plant Sci 4:266
von Caemmerer S (2000) Biochemical models of leaf photosynthesis (2). CSIRO Publishing, Collingwood, Australia
von Caemmerer S, Quick WP (2000) Rubisco: physiology in vivo. In: Leegood RC, Sharkey TD, von Caemmerer S (eds) Photosynthesis. Springer, Dordrecht, pp 85–113
Cavaleri MA, Oberbauer SF, Ryan MG (2008) Foliar and ecosystem respiration in an old-growth tropical rain forest. Plant Cell Environ 31:473–483
Cavaleri MA, Reed SC, Smith WK, Wood TE (2015) Urgent need for warming experiments in tropical forests. Glob Change Biol 21:2111–2121
Cernusak LA, Winter K, Dalling JW, Holtum JA, Jaramillo C, Körner C, Leakey ADB, Norby RJ et al (2013) Tropical forest responses to increasing atmospheric CO2: current knowledge and opportunities for future research. Funct Plant Biol 40:531–551
Chambers JQ, Tribuzy ES, Toledo LC, Crispim BF, Higuchi N, Dos Santos J, Araújo AC, Kruijt B et al (2004) Respiration from a tropical forest ecosystem: partitioning of sources and low carbon use efficiency. Ecol Appl 14:S72–S88
Cheesman AW, Winter K (2013a) Growth response and acclimation of CO2 exchange characteristics to elevated temperatures in tropical tree seedlings. J Exp Bot 64:3817–3828
Cheesman AW, Winter K (2013b) Elevated night-time temperatures increase growth in seedlings of two tropical pioneer tree species. New Phytol 197:1185–1192
Clark DA (2004) Sources or sinks? The responses of tropical forests to current and future climate and atmospheric composition. Philos Trans Roy Soc B 359:477–491
Clark DA, Piper SC, Keeling CD, Clark DB (2003) Tropical rain forest tree growth and atmospheric carbon dynamics linked to interannual temperature variation during 1984–2000. Proc Natl Acad Sci USA 100:5852–5857
Clark DA, Clark DB, Oberbauer SF (2013) Field-quantified responses of tropical rainforest aboveground productivity to increasing CO2 and climatic stress, 1997–2009. J Geophys Res-Biogeo 118:783–794
Clark DB, Clark DA, Oberbauer SF (2010) Annual wood production in a tropical rain forest in NE Costa Rica linked to climatic variation but not to increasing CO2. Glob Change Biol 16:747–759
Condit R, Hubbell SP, Foster RB (1995) Mortality rates of 205 neotropical tree and shrub species and the impact of a severe drought. Ecol Monogr 65:419–439
Condit R, Engelbrecht BM, Pino D, Pérez R, Turner BL (2013) Species distributions in response to individual soil nutrients and seasonal drought across a community of tropical trees. Proc Natl Acad Sci USA 110:5064–5068
Coomes DA, Flores O, Holdaway R, Jucker T, Lines ER, Vanderwel MC (2014) Wood production response to climate change will depend critically on forest composition and structure. Glob Change Biol 20:3632–3645
Corlett RT (2011) Impacts of warming on tropical lowland rainforests. Trends Ecol Evol 27:145–150
Cunningham SC, Read J (2002) Comparison of temperate and tropical rainforest tree species: photosynthetic responses to growth temperature. Oecologia 133:112–119
Cunningham SC, Read J (2003) Do temperate rainforest trees have a greater ability to acclimate to changing temperatures than tropical rainforest trees? New Phytol 157:55–64
Cunningham SC, Read J (2006) Foliar temperature tolerance of temperate and tropical evergreen rain forest trees of Australia. Tree Physiol 26:1435–1443
De Kauwe MG, Lin YS, Wright IJ, Medlyn BE, Crous KY, Ellsworth DS, Maire V, Prentice IC et al (2016) A test of the ‘one‐point method’ for estimating maximum carboxylation capacity from field‐measured, light‐saturated photosynthesis. New Phytol In Press. doi:10.1111/nph.13815
Diffenbaugh NS, Scherer M (2011) Observational and model evidence of global emergence of permanent, unprecedented heat in the 20th and 21st centuries. Clim Change 107:615–624
Doughty CE (2011) An in situ leaf and branch warming experiment in the Amazon. Biotropica 43:658–665
Doughty CE, Goulden ML (2008) Are tropical forests near a high temperature threshold? J Geophys Res Biogeosci 113:G00B07
Duan H, Duursma RA, Huang G, Smith RA, Choat B, O’Grady AP, Tissue DT (2014) Elevated [CO2] does not ameliorate the negative effects of elevated temperature on drought-induced mortality in Eucalyptus radiata seedlings. Plant Cell Environ 37:1598–1613
Dusenge ME, Wallin G, Gårdesten J, Niyonzima F, Adolfsson L, Nsabimana D, Uddling J (2015) Photosynthetic capacity of tropical tree species in relation to leaf nutrients, successional group identity and growth temperature. Oecologia 177:1183–1194
Easterling DR, Horton B, Jones PD, Peterson TC, Karl TR, Parker DE, Salinger MJ, Razuvayev V et al (1997) Maximum and minimum temperature trends for the globe. Science 277:346–366
Ellsworth DS, Crous KY, Lambers H, Cooke J (2015) Phosphorus recycling in photorespiration maintains high photosynthetic capacity in woody species. Plant Cell Environ 38:1142–1156
Esmail S, Oelbermann M (2011) The impact of climate change on the growth of tropical agroforestry tree seedlings. Agrofor Syst 83:235–244
Fahey C, Winter K, Slot M, Kitajima K (2016) Influence of arbuscular mycorrhizal fungi on whole-plant respiration and thermal acclimation of tropical tree seedlings. Ecol & Evol. In Press. doi:10.1002/ece3.1952
Harley PC, Sharkey TD (1991) An improved model of C3 photosynthesis at high CO2: reversed O2 sensitivity explained by lack of glycerate reentry into the chloroplast. Photosyn Res 27:169–178
Hietz P, Turner BL, Wanek W, Richter A, Nock CA, Wright SJ (2011) Long-term change in the nitrogen cycle of tropical forests. Science 334:664–666
Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onoda Y (2006) Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. J Exp Bot 57:291–302
Holtum JA, Winter K (2014) Limited photosynthetic plasticity in the leaf-succulent CAM plant Agave angustifolia grown at different temperatures. Funct Plant Biol 41:843–849
Huntingford C, Zelazowski P, Galbraith D, Mercado LM, Sitch S, Fisher R, Lomas M, Walker AP et al (2013) Simulated resilience of tropical rainforests to CO2-induced climate change. Nat Geosci 6:268–273
Hüve K, Bichele I, Ivanova H, Keerberg O, Pärnik T, Rasulov B, Tobias M, Niinemets Ü (2012) Temperature responses of dark respiration in relation to leaf sugar concentration. Physiol Plant 144:320–334
Issarakraisila M, Considine JA (1994) Effects of temperature on pollen viability in mango cv. ‘Kensington’. Ann Bot-London 73:231–240
Jamieson MA, Schwartzberg EG, Raffa KF, Reich PB, Lindroth RL (2015) Experimental climate warming alters aspen and birch phytochemistry and performance traits for an outbreak insect herbivore. Glob Change Biol 21:268–2710
Janzen DH (1967) Why mountain passes are higher in the tropics. Am Nat 101:233–249
Jaramillo C, Ochoa D, Contreras L, Pagani M, Carvajal-Ortiz H, Pratt LM, Krishnan S, Cardona A et al (2010) Effects of rapid global warming at the Paleocene-Eocene boundary on neotropical vegetation. Science 330:957–961
Jardine K, Meyers K, Abrell L, Alves EG, Serrano AMY, Kesselmeier J, Karl T, Guenther A et al (2013) Emissions of putative isoprene oxidation products from mango branches under abiotic stress. J Exp Bot 64:3669–3679
Jardine K, Chambers JQ, Alves EG, Teixeira A, Garcia S, Holm J, Niguchi N, Abrell L et al (2014) Dynamic balancing of isoprene carbon sources reflects photosynthetic and photorespiratory responses to temperature stress. Plant Physiol 166:2051–2064
Jarvis P, Linder S (2000) Constraints to growth of boreal forests. Nature 405:904–905
Keller M, Lerdau M (1999) Isoprene emission from tropical forest canopy leaves. Glob Biogeochem Cycles 13:19–29
Kirschbaum MUF, Farquhar GD (1984) Temperature dependence of whole-leaf photosynthesis in Eucalyptus pauciflora Sieb. ex Spreng. Funct Plant Biol 11:519–538
Kitajima K, Poorter L (2008) Functional basis for resource niche partitioning by tropical trees. In: Carson WP, Schnitzer SA (eds) Tropical forest community ecology. Blackwell, Oxford, pp 172–188
Königer M, Winter K (1993) Growth and photosynthesis of Gossypium hirsutum L. at high photon flux densities: effects of soil temperatures and nocturnal air temperatures. Agronomie 13:423–431
Körner C (2009) Responses of humid tropical trees to rising CO2. Annu Rev Ecol Evol Syst 40:61–79
Kositsup B, Montpied P, Kasemsap P, Thaler P, Améglio T, Dreyer E (2009) Photosynthetic capacity and temperature responses of photosynthesis of rubber trees (Hevea brasiliensis Müll. Arg.) acclimate to changes in ambient temperatures. Trees 23:357–365
Kozaki A, Takeba G (1996) Photorespiration protects C3 plants from photooxidation. Nature 384:557–560
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Biol 42:313–349
Krause GH, Winter K, Krause B, Jahns P, García M, Aranda J, Virgo A (2010) High-temperature tolerance of a tropical tree, Ficus insipida: methodological reassessment and climate change considerations. Funct Plant Biol 37:890–900
Krause GH, Cheesman AW, Winter K, Krause B, Virgo A (2013) Thermal tolerance, net CO2 exchange and growth of a tropical tree species, Ficus insipida, cultivated at elevated daytime and nighttime temperatures. J Plant Physiol 170:822–827
Krause GH, Winter K, Krause B, Virgo A (2015) Light-stimulated heat tolerance in leaves of two neotropical tree species, Ficus insipida and Calophyllum longifolium. Funct Plant Biol 42:42–51
Lewis SL, Lloyd J, Sitch S, Mitchard ET, Laurance WF (2009) Changing ecology of tropical forests: evidence and drivers. Annu Rev Ecol Evol Syst 40:529–549
Lin D, Xia J, Wan S (2010) Climate warming and biomass accumulation of terrestrial plants: a meta-analysis. New Phytol 188:187–198
Li XM, Chao DY, Wu Y, Huang X, Chen K, Cui LG, Su L, Ye W-W, et al. (2015). Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat Genet 47:827–833
Lloyd J, Farquhar GD (2008) Effects of rising temperatures and [CO2] on the physiology of tropical forest trees. Phil Trans R Soc B 363:1811–1817
Loreto F, Fineschi S (2015) Reconciling functions and evolution of isoprene emission in higher plants. New Phytol 206:578–582
Loveys BR, Atkinson LJ, Sherlock DJ, Roberts RL, Fitter AH, Atkin OK (2003) Thermal acclimation of leaf and root respiration: an investigation comparing inherently fast-and slow-growing plant species. Glob Change Biol 9:895–910
Makino A, Nakano H, Mae T (1994) Effects of growth temperature on the responses of ribulose-1,5-bisphosphate carboxylase, electron-transport components, and sucrose synthesis enzymes to leaf nitrogen in rice, and their relationships to photosynthesis. Plant Physiol 105:1231–1238
Malhi Y (2012) The productivity, metabolism and carbon cycle of tropical forest vegetation. J Ecol 100:65–75
Malhi Y, Silman M, Salinas N, Bush M, Meir P, Saatchi S (2010) Introduction: elevation gradients in the tropics: laboratories for ecosystem ecology and global change research. Glob Change Biol 16:3171–3175
Meehl GA, Tebaldi C (2004) More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305:994–997
Meir P, Grace J, Miranda AC (2001) Leaf respiration in two tropical rainforests: constraints on physiology by phosphorus, nitrogen and temperature. Funct Ecol 15:378–387
Menzel A, Fabian P (1999) Growing season extended in Europe. Nature 397:659
Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, Ahas R, Alm-Kübler K, Bissolli P et al (2006) European phenological response to climate change matches the warming pattern. Glob Change Biol 12:1969–1976
Michaletz ST, Cheng D, Kerkhoff AJ, Enquist BJ (2014) Convergence of terrestrial plant production across global climate gradients. Nature 512:39–43
Monson RK, Jaeger CH, Adams WW, Driggers EM, Silver GM, Fall R (1992) Relationships among isoprene emission rate, photosynthesis, and isoprene synthase activity as influenced by temperature. Plant Physiol 98:1175–1180
Monson RK, Jones RT, Rosenstiel TN, Schnitzler J-P (2013) Why only some plants emit isoprene. Plant Cell Environ 36:503–516
Mora C, Frazier AG, Longman RJ, Dacks RS, Walton MM, Tong EJ, Sanchez TJ, Kaiser LR et al (2013) The projected timing of climate departure from recent variability. Nature 502:183–187
Murakami Y, Tsuyama M, Kobayashi Y, Kodama H, Iba K (2000) Trienoic fatty acids and plant tolerance of high temperature. Science 287:476–479
Nepstad DC, Tohver IM, Ray D, Moutinho P, Cardinot G (2007) Mortality of large trees and lianas following experimental drought in an Amazon forest. Ecology 88:2259–2269
O’Brien MJ, Leuzinger S, Philipson CD, Tay J, Hector A (2014) Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat Clim Change 4:710–714
Olivares I, Svenning J-C, van Bodegom PM, Balslev H (2015) Effects of warming and drought on the vegetation and plant diversity in the Amazon basin. Bot Rev 81:42–69
Osmond CB, Björkman O (1972) Simultaneous measurements of oxygen effects on net photosynthesis and glycolate metabolism in C3 and C4 species of Atriplex. Carnegie Inst Wash Yearb 71:141–148
O’Sullivan OS, Weerasinghe KK, Evans JR, Egerton JJ, Tjoelker MG, Atkin OK (2013) High-resolution temperature responses of leaf respiration in snow gum (Eucalyptus pauciflora) reveal high-temperature limits to respiratory function. Plant Cell Environ 36:1268–1284
Pan Y, Birdsey RA, Phillips OL, Jackson RB (2013) The structure, distribution, and biomass of the world’s forests. Annu Rev Ecol Evol Syst 44:593–622
Pau S, Wolkovich EM, Cook BI, Nytch CJ, Regetz J, Zimmerman JK, Wright SJ (2013) Clouds and temperature drive dynamic changes in tropical flower production. Nat Clim Change 3:838–842
Patiño S, Herre EA, Tyree MT (1994) Physiological determinants of Ficus fruit temperature and implications for survival of pollinator wasp species: comparative physiology through an energy budget approach. Oecologia 100:13–20
Peraudeau S, Lafarge T, Roques S, Quiñones CO, Clement-Vidal A, Ouwerkerk PB, van Rie J, Fabre D et al (2015) Effect of carbohydrates and night temperature on night respiration in rice. J Exp Bot 66:3931–3944
Phillips OL, Gentry AH (1994) Increasing turnover through time in tropical forests. Science 263:954–958
Phillips OL, Van der Heijden G, Lewis SL, López‐González G, Aragão LE, Lloyd J. Malhi Y, Monteagudo A et al (2010) Drought–mortality relationships for tropical forests. New Phytol 187:631–646
Piao S, Sitch S, Ciais P, Friedlingstein P, Peylin P, Wang X, Ahlström A, Anav A et al (2013) Evaluation of terrestrial carbon cycle models for their response to climate variability and to CO2 trends. Glob Change Biol 19:2117–2132
Pilkington SM, Encke B, Krohn N, Hoehne M, Stitt M, Pyl ET (2015) Relationship between starch degradation and carbon demand for maintenance and growth in Arabidopsis thaliana in different irradiance and temperature regimes. Plant Cell Environ 38:157–171
Pollastri S, Tsonev T, Loreto F (2014) Isoprene improves photochemical efficiency and enhances heat dissipation in plants at physiological temperatures. J Exp Bot 65:1565–1570
Portis AR Jr (2003) Rubisco activase–Rubisco’s catalytic chaperone. Photosyn Res 75:11–27
Prasad PVV, Boote KJ, Allen LH Jr, Sheehy JE, Thomas JMG (2006) Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Res 95:398–411
Prior LD, Bowman DM (2014) Big eucalypts grow more slowly in a warm climate: evidence of an interaction between tree size and temperature. Glob Change Biol 20:2793–2799
Raich JW, Schlesinger WH (1992) The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 44:81–99
Randerson JT (2013) Climate science: global warming and tropical carbon. Nature 494:319–320
Sage RF, Kubien DS (2007) The temperature response of C3 and C4 photosynthesis. Plant Cell Environ 30:1086–1106
Sage TL, Bagha S, Lundsgaard-Nielsen V, Branch HA, Sultmanis S, Sage R (2015) The effect of high temperature stress on male and female reproduction in plants. Field Crop Res 182:30–42
Salinas N, Malhi Y, Meir P, Silman M, Roman Cuesta R, Huaman J, Salinas D, Huaman V et al (2011) The sensitivity of tropical leaf litter decomposition to temperature: results from a large-scale leaf translocation experiment along an elevation gradient in Peruvian forests. New Phytol 189:967–977
Salvucci ME, Crafts-Brandner SJ (2004) Relationship between the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiol 134:1460–1470
Sasaki K, Saito T, Lämsä M, Oksman-Caldentey KM, Suzuki M, Ohyama K, Muranaka T, Ohara K, Yazaki K (2007) Plants utilize isoprene emission as a thermotolerance mechanism. Plant Cell Physiol 48:1254–1262
Saugier B, Roy J, Mooney HA (2001) Estimations of global terrestrial productivity: converging toward a single number? In: Roy J, Saugier B, Mooney HA (eds) Terrestrial global productivity. Academic Press, New York, pp 543–557
Sharkey TD, Monson RK (2014) The future of isoprene emission from leaves, canopies and landscapes. Plant Cell Environ 37:1727–1740
Sharkey TD, Yeh S (2001) Isoprene emission from plants. Annu Rev Plant Biol 52:407–436
Slot M, Poorter L (2007) Diversity of tropical tree seedling responses to drought. Biotropica 39:683–690
Slot M, Wright SJ, Kitajima K (2013) Foliar respiration and its temperature sensitivity in trees and lianas: in situ measurements in the upper canopy of a tropical forest. Tree Physiol 33:505–515
Slot M, Rey-Sánchez C, Gerber S, Lichstein JW, Winter K, Kitajima K (2014a) Thermal acclimation of leaf respiration of tropical trees and lianas: response to experimental canopy warming, and consequences for tropical forest carbon balance. Glob Change Biol 20:2915–2926
Slot M, Rey-Sánchez C, Winter K, Kitajima K (2014b) Trait-based scaling of temperature-dependent foliar respiration in a species-rich tropical forest canopy. Funct Ecol 28:1074–1086
Slot M, Kitajima K (2015) General patterns of thermal acclimation of leaf respiration across biomes and plant types. Oecologia 177:885–900
Slot M, Garcia MN, Winter K (2016) Temperature response of CO2 exchange in three tropical tree species. Funct Plant Biol. doi:10.1071/FP15320
Smith NG, Dukes JS (2013) Plant respiration and photosynthesis in global-scale models: incorporating acclimation to temperature and CO2. Glob Change Biol 19:45–63
Tambunan P, Baba S, Kuniyoshi A, Iwasaki H, Nakamura T, Yamasaki H, Oku H (2006) Isoprene emission from tropical trees in Okinawa Island, Japan. Chemosphere 65:2138–2144
Tingey DT, Manning M, Grothaus LC, Burns WF (1979) The influence of light and temperature on isoprene emission rates from live oak. Physiol Plant 47:112–118
Vanderwel MC, Slot M, Lichstein JW, Reich PB, Kattge J, Atkin OK, Bloomfield K, Tjoelker M, Kitajima K (2015) Global convergence in projected leaf respiration from estimates of thermal acclimation across time and space. New Phytol 207:1026–1037
Vargas GG, Cordero SR (2013) Photosynthetic responses to temperature of two tropical rainforest tree species from Costa Rica. Trees 27:1261–1270
Velikova V, Várkonyi Z, Szabó M, Maslenkova L, Nogues I, Kovács L, Peeva V, Busheva M et al (2011) Increased thermostability of thylakoid membranes in isoprene-emitting leaves probed with three biophysical techniques. Plant Physiol 157:905–916
Velikova V, Sharkey T, Loreto F (2012) Stabilization of thylakoid membranes in isoprene-emitting plants reduces formation of reactive oxygen species. Plant Signal Behav 7:139–141
Vlam M, Baker PJ, Bunyavejchewin S, Zuidema PA (2014) Temperature and rainfall strongly drive temporal growth variation in Asian tropical forest trees. Oecologia 174:1449–1461
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P (2011) Climate change and plant regeneration from seed. Glob Change Biol 17:2145–2161
Wang D, Li XF, Zhou ZJ, Feng XP, Yang WJ, Jiang DA (2010) Two Rubisco activase isoforms may play different roles in photosynthetic heat acclimation in the rice plant. Physiol Plant 139:55–67
Way DA, Oren R (2010) Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiol 30:669–688
Way DA, Yamori W (2014) Thermal acclimation of photosynthesis: on the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosynth Res 119:89–100
Wright SJ, Muller-Landau HC, Schipper J (2009) The future of tropical species on a warmer planet. Conserv Biol 23:1418–1426
Zhang Y, Xu M, Chen H, Adams J (2009) Global pattern of NPP to GPP ratio derived from MODIS data: effects of ecosystem type, geographical location and climate. Glob Ecol Biogeogr 18:280–290
Zhang Y, Yu G, Yang J, Wimberly MC, Zhang X, Tao J, Jiang Y, Zhu J (2014) Climate-driven global changes in carbon use efficiency. Glob Ecol Biogeogr 23:144–155
Zotz G, Harris G, Königer M, Winter K (1995) High rates of photosynthesis in a tropical pioneer tree, Ficus insipida. Flora 190:265–272
Acknowledgements
This work was supported by the Smithsonian Tropical Research Institute. M.S. was recipient of a CTFS-Forest-GEO postdoctoral fellowship. Milton Garcia assisted with in situ canopy measurements.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Slot, M., Winter, K. (2016). The Effects of Rising Temperature on the Ecophysiology of Tropical Forest Trees. In: Goldstein, G., Santiago, L. (eds) Tropical Tree Physiology. Tree Physiology, vol 6. Springer, Cham. https://doi.org/10.1007/978-3-319-27422-5_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-27422-5_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27420-1
Online ISBN: 978-3-319-27422-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)