Abstract
Several studies have been conducted on the response of crops to greater concentrations of atmospheric CO2 (CO2 fertilization) as a result of climate change, but only few studies have evaluated this effect on multipurpose agroforestry tree species in tropical environments. The objectives of this study were to quantify differences in growth parameters and in leaf carbon (C) and nitrogen (N) concentrations of Cedrela odorata L. and Gliricidia sepium (Jacq.) Walp. seedlings under current ambient temperature (32°C daytime, 22°C night time) and CO2 (360 ppm) (AMB); CO2 fertilization (800 ppm, 32°C daytime, 22°C night time) (fCO2); elevated ambient temperature (360 ppm, 34°C daytime, 25°C night time) (TEMP); and a combination of elevated temperature (32°C daytime, 22°C night time) and CO2 fertilization (800 ppm) (TEMPxfCO2). Results showed significant differences (P < 0.05) in seedling growth parameters (seedling height, number of stem leaves, leaf area ratio, shoot and root biomass, and shoot/root ratio) between treatments for both tree species. The greatest increases in growth parameters occurred in the TEMP and TEMPxfCO2 treatments compared to the AMB treatment for both tree species. However, growth parameters were significantly lower (P < 0.05) in the fCO2 treatment compared to that of the AMB treatment. Leaf N concentration was 1.1 to 2.1 times lower (P < 0.05) in all treatments when compared to current ambient conditions (AMB) in both tree species, but no significant changes in leaf C concentrations were observed. Results from our study suggested that fCO2 had the greatest negative impact on tree growth parameters, and leaf N concentrations were affected negatively in all treatments compared to current ambient conditions. It is expected that such changes in growth parameters and plant N content may impact the long-term cycling of nutrients in agroforestry systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The effects of climate change as a result of rising ambient temperatures and atmospheric CO2 concentrations are currently observed in a range of ecosystems and species on a global level (Warren et al. 2011), including tropical central America (Walther et al. 2002) where temperatures are predicted to increase between 1.5 and 5.6°C compared to current conditions (IPCC 2007). The majority (95%) of plants including agricultural crops, fruit and nut bearing trees, timber trees and service trees such as those used in agroforestry practices are characterized by the C3 photosynthesis pathway (Ainsworth and Long 2005). However, the ecological competitive advantage of C3 plant species may be negatively affected under greater than current ambient temperatures and atmospheric CO2 concentrations (Lee 2011). C3 species may be negatively affected under elevated temperatures but may respond positively under elevated CO2. For example, Lee (2011) found that higher temperatures may inhibited specific plant growth stages, including flowering and fertilization, and lead to greater tree mortality (Lee 2011).
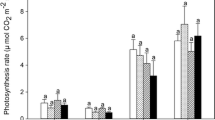
In tropical environments, remarkably few studies have been conducted on the response of C3 agricultural crops and tropical forest trees to elevated levels of atmospheric CO2 concentrations, commonly referred to as CO2 fertilization or enrichment (Lovelock et al. 1999; Keller and Lerdau 1999; Chambers and Silver 2004; Lloyd and Farquhar 2008). In Panama, Luehea seemanii Planch. and Triana branchlets had a greater rate of photosynthesis as a result of CO2 fertilization (Lovelock et al. 1999). Lovelock et al. (1999) suggested that enhanced rates of photosynthesis may lead to a greater tree growth rate. However, Chambers and Silver (2004) suggested that a greater rate of photosynthesis among other physiological responses due to CO2 fertilization in C3 trees may be dependent on changes in ambient temperature, and could also be species specific. For example, Clark (2004) and Cramer et al. (2004) found that CO2 fertilization reduced tree growth rates and their potential to sequester carbon (C) in two tropical forest tree species [Beilschmiedia pendula (Sw.) Hemsl., Tachigalia versicolor Standl. and L.O. Wms.]. Cox et al. (2000) noted that it is important to understand the combined effects of higher temperatures and CO2 fertilization on plant productivity because of their collective occurrence as a result of global warming. As such, changes in ambient temperatures and atmospheric CO2 concentrations may result in a lower tree productivity and C sequestration potential, and altered tree growth patterns in some species (Bradley and Pregitzer 2007). In agroforestry systems, this may also negatively affect timber production and wood quality (Zieche and Overdieck 2004), impact fruit and nut yield and quality, and influence productivity and quality of livestock fodder and pruning biomass commonly used as green manure or mulch to maintain soil fertility.
To date, only a few studies have evaluated the response of multipurpose agroforestry trees to CO2 fertilization in temperate (Gebauer et al. 1996; Tupker et al. 2003; Scarascia-Mungozza et al. 2005) and tropical (Tissue et al. 1997; Carswell et al. 2000) biomes. Uncertainty still remains on how an interactive effect of increased temperature and CO2 fertilization will impact tree ecophysiology in tropical environments (Chambers and Silver 2004; Clark 2004); and to date only a few studies have investigated this concept in tropical forest ecosystems (Lloyd and Farquhar 2008; Wertin et al. 2009; Lukac et al. 2010; Lee 2011). However, no information is currently available on the response of tropical multipurpose agroforestry trees to a combined effect of enhanced ambient temperature and CO2 fertilization.
This study quantified the effect of CO2 fertilization, enhanced ambient temperature, and their combined effect on two commonly used multipurpose agroforestry tree species in Costa Rica. It was hypothesized that CO2 fertilization, and greater ambient temperature or the combined effect would negatively affect tree seedling growth parameters compared current ambient conditions. The objectives of this study were to quantify differences in growth parameters and changes in leaf C and nitrogen (N) concentrations of Cedrela odorata L. and Gliricidia sepium (Jacq.) Walp. tree seedlings under current ambient temperature and CO2 conditions, under CO2 fertilization, elevated ambient temperatures, and a combination of elevated temperature and CO2 fertilization.
Materials and methods
Experimental design
Seeds of C. odorata and G. sepium were obtained from the tree seed bank at the Centro Agronómico Tropical de Investigacíón y Enseñanza (CATIE), located in Turrialba, Costa Rica in August 2008. The experiment was conducted at the University of Waterloo, Canada and the experimental design was a complete randomized design (CRD) with four treatments per tree species and each treatment was replicated eight times. The treatments used were (1) experimental control using current ambient conditions (AMB) at a CO2 concentration of 360 ppm, a mean day time temperature of 32°C and a mean night time temperature of 22°C; (2) CO2 fertilization (fCO2) using a CO2 concentration of 800 ppm with mean day and night time temperatures of 32 and 22°C; (3) elevated temperature (TEMP) at mean day and night time temperatures of 34 and 25°C, and at a CO2 concentration of 360 ppm; and (4) a combination of elevated temperature and CO2 (TEMPxfCO2) with mean day and night time temperatures of 34 and 25°C and a CO2 concentration of 800 ppm. Relative humidity was 80% during the day and 90% at night for all treatments throughout the experiment. The different mean day and night time temperatures and CO2 concentrations were based on the predictions of the UK Hadley Centre Global Climate Model (A2 scenario) predicted for the year 2100 in central America, including Costa Rica (IPCC 2007). Climate data from Puntarenas, Costa Rica was used to establish ambient day and night time temperatures, and provided a baseline for establishing predicted increases in temperature under the A2 scenario for the TEMP and TEMBxfCO2 treatments (IPPC 2007).
Tree seedling germination and growth
Prior to initiating the experiment, and after transportation to Canada, the tree seedlings were germinated under controlled environmental conditions using the same germination procedure for all treatments. Seeds were submerged in water at room temperature (21°C) and kept in darkness for 12 (C. odorata) and 24 h (G. sepium), and subsequently covered in moist sand and kept in darkness.
The germinated seeds were planted in large pots (20 cm diameter, 14 cm height) using a general purpose soil (Premier Horticulture, Pro-mix Bx Mycorise Pro, Canada) with mycorrhizal inoculum (Glomus intraradices). The soil was derived from Canadian sphagnum peat moss (75–85%/volume), horticultural grade perlite and vermiculite and dolomitic and calcitic limestone. The pots with the germinated seeds were placed into controlled environmental growth chambers (Conviron PGR-15, Controlled Environments Inc., Winnipeg, Manitoba) to determine their response to the different treatments (AMB, fCO2, TEMP, and TEMPxfCO2) over a 35-day period. Light intensity, temperature, humidity and CO2 were monitored hourly, and environmental conditions were assumed to be identical between treatments and chambers except for differences in atmospheric CO2 concentrations and temperature for the actual treatments (fCO2, TEMP and TEMPxfCO2).
The seedlings were watered every second day, with each tree receiving 120 ml of ultra-pure water. On a weekly basis, the tree order within and between chambers was rotated randomly to avoid systematic bias due to slight differences between the chambers (Thomas and Strain 2000). As a nutrient supplement to the potting soil, a general NPK (10-15-10) fertilizer (Schultz Liquid Plant Food, Spectrum Brands, Madison, WI) was added to the 120 ml of pure water on day 21 using a dilution of four drops for every 1,000 ml of ultra-pure water.
Quantification of tree seedling growth, and Carbon and Nitrogen concentrations
Observations on tree height (cm) and leaf number were quantified every second day over the entire 35 days of growth. Leaf surface area for each tree was measured on day 34, one day prior to destructive sampling (Beerling and Fry 1990). Leaf surface area was quantified by tracing each leaf onto grid paper, and subsequently cutting out the shapes of the individual leaves and analyzing these with the LI-COR (LI-3000, Lincoln, Nebraska).
Trees were removed from their potting medium after 35 days of growth and separated into above- and below-ground components. Soil was carefully removed from seedling roots (Oelbermann et al. 2005), and both shoots and roots were weighed (wet weight). Subsequently, seedling shoot and root components were placed in an oven for 48 h at 72°C and weighed to determine their dry weight and to quantify the shoot/root ratio (S/R). The leaf area ratio [(LAR (cm2/g)] was determined with a LI-COR LI-3000. The root mass faction (RMF) was quantified by dividing the root biomass into the total seedling biomass (Raizada et al. 2009). The dried shoots from each tree species within each treatment were ground using a Kinematica Polymix plant grinder (Px-MFC 90D, Lucerne, Switzerland) using a 2 mm sieve and subsequently ground in a Retsch Ball Mill (MM-200, Haan, Germany) and prepared for C and N analysis on a Costec Elemental Analyzer (ECS-1410, Cernusco, Italy).
Statistical analysis
All data were examined for homogeneity of variance using Levene’s Test and normal distribution was determined with the Kolmogorov–Smirnov (K–S) Test and the Shapiro-Wilks Test. If the data did not meet Levene’s Test it was natural log transformed (Steel et al. 1997). For all statistical analyses, the threshold probability level for determining significant differences was P < 0.05. Data for the growth parameters and leaf nutrient content was evaluated using the general linear model (ANOVA) in SPSS (SPSS Science Inc. 2009). Significant differences were evaluated using Tukey’s least significant difference multiple comparison test (Steel et al. 1997). Prior to statistical analyses, data for tree height was corrected by subtracting the first day of measurement from all of the subsequent days of measurement in order to quantify the change in growth over the 5 week period.
Results
Tree seedling growth
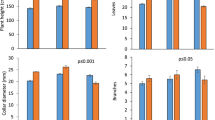
Both tree species showed a steady increase in seedling growth over the 35-day growing period in all treatments. The final tree seedling heights were significantly different between treatments for C. odorata (Fig. 1), and followed a sequence of TEMPxfCO2 > TEMP > AMB > fCO2. The TEMPxfCO2 and TEMP treatments had a 58 and 26% greater height compared to the AMB treatment, whereas in the fCO2 treatment, C. odorata tree height was 43% lower compared to that of the AMB treatment. Differences in tree height for G. sepium seedlings were also significant and followed a sequence of TEMP ≈ TEMPxfCO2 > AMB > fCO2 (Fig. 2). Differences in seedling height at the end of the 35 days were less pronounced compared to that of C. odorata. For example, TEMPxfCO2 and TEMP treatments showed a 21 and 22% greater seedling height compared to the AMB treatment whereas the fCO2 treatment had a reduction in height by 21% compared to the AMB treatment.
Other growth parameters, including number of leaves, shoot and root biomass, S/R and RMF were significantly different between treatments for C. odorata (Table 1). Shoot biomass was 58 and 83% greater for the TEMP and TEMPxfCO2 treatments respectively, whereas shoot biomass in the fCO2 treatment was 40% lower than that of the AMB treatment. Root biomass was 55% greater in the TEMPxfCO2 treatment compared to the AMB treatment whereas the TEMP and fCO2 treatments were 9 and 80% lower than the AMB treatment. The number of C. odorata leaves was lower by 54 to 24% in the treatments compared to the AMB control, however S/R was the greatest in the TEMP and fCO2 treatments compared to the control (AMB). The RMF was significantly different between treatments with the greatest fraction of the root mass occurring in the TEMPxfCO2 and fCO2 treatments. However, the LAR was 6% (TEMP), 21% (fCO2), and 27% (TEMPxfCO2) lower compared to the AMB treatment.
For G. sepium, tree seedling growth parameters were significantly different between treatments and showed a similar pattern between the number of leaves and leaf area ratio (Table 2). For example, the greatest number of leaves and the highest LAR occurred in the AMB treatment followed by the TEMP, fCO2 and TEMPxfCO2 treatments (Table 2). Shoot biomass accumulation was 55 and 36% greater in the TEMP and TEMPxfCO2 treatments compared to the AMB treatment, whereas the fCO2 had a 43% lower biomass accumulation compared to that of the AMB treatment. Comparatively, root biomass allocation for G. sepium tree seedlings was 30% greater in the TEMP treatment compared to that of AMB, but was 29 and 74% lower in the TEMPxfCO2 and fCO2 treatments than that of AMB. The S/R and RMF were significantly greater in the TEMPxfCO2 and fCO2 treatments followed by the TEMP and AMB treatments.
Leaf nutrient concentrations
Leaf C concentrations for both tree species were not significantly different between treatments (Table 3). However, leaf N concentrations were significantly different between treatments showing a 9% greater leaf N concentration in the TEMP treatment compared to that of the AMB treatment for C. odorata. However, the TEMPxfCO2 and fCO2 treatments had a 1 and 62% lower leaf N concentration compared to that of the AMB treatment. For G. sepium tree seedlings, leaf N concentrations were significantly lower compared to that of the AMB treatment. For example, leaf N concentration was 12, 48 and 52% lower in the TEMP, TEMPxfCO2 and fCO2 treatments respectively (Table 3).
Discussion
Tree seedling growth
Tree height is necessary for the survival and successful establishment of agroforestry trees, and rapid seedling growth is valued by farmers because it is an indicator of successful agroforest establishment and survival (Nair 1993). In our study, tree seedlings from both species had the greatest response to the TEMP and TEMPxfCO2 treatments compared to AMB conditions with respect to height. Similarly, Overdieck et al. (2007) also found a greater tree height of European beech (Fagus sylvatica L.) seedlings when grown under warmer temperatures (+4°C from current ambient conditions). Allen and Vu (2008) showed an increase in citrus tree (Citrus reticulate B., Citrus limon (L.) Burm.) height in Florida when grown under a combination of enhanced temperature and CO2 fertilization.
Root and shoot biomass accumulation responded positively under greater than ambient temperature (TEMP) or a combination effect (TEMPxfCO2), and was similar to the findings of Lee (2011). This may be due to a higher than normal soil temperature (Lewis et al. 2009), suggesting that the necessary resources required for seedling growth were available over the short-term (Khurana and Singh 2001) due to greater rates of soil nutrient mineralization (Lewis et al. 2009), and changes in soil microbial activity and community structure (Lukac et al. 2010). However, the various tree seedling growth parameters in our study responded negatively to fCO2 compared to the AMB treatment. Previous studies from tropical and temperate environments reported a positive effect on tree seedling growth parameters under CO2 fertilization relative to current ambient conditions (Wurth et al. 1998; Raizada et al. 2009; Xiao et al. 2005; Lindroth 2010). For example, Sigurdsson et al. (2001) found a 45% increase in Populus trichocarpa Torr. and Gray ex Hook. shoot and a 58% increase in root biomass under CO2 fertilization. Similarly, Carswell et al. (2000) found that when C. odorata was exposed to a CO2 concentration of 700 ppm compared to the current ambient concentration, shoot and root biomass accumulation increased by 12% when a high level of fertilizer was added, but observed a much lower growth response under low soil fertility conditions relative to current ambient conditions. Raizada et al. (2009) suggested that seedling growth may vary among species and found a decrease or increase in biomass accumulation under CO2 fertilization relative to current ambient conditions. Sigurdsson et al. (2001) proposed that such differences may be due to nutrient availability. They noted that biomass accumulation of P. trichocarpa under CO2 fertilization only occurred when N availability was very high, but showed no effect or a decrease in biomass, compared to current ambient conditions, when N availability was low (Sigurdsson et al. 2001). Results from these studies and our study illustrated a discrepancy in plant growth with respect to CO2 fertilization and suggested there is likely no 1:1 effect (CO2 fertilization:plant growth) (Körner 2009).
A significantly lower number of leaves in all treatments compared to AMB suggested that smaller amounts of organic matter from litterfall or tree prunings may be returned to the soil in an agroforestry system. This could negatively affect the long-term cycling of organic matter, C and nutrients. For example, Oelbermann et al. (2004) found that 28% of the C returned to the soil via pruning biomass was derived from G. sepium leaves. Similarly, Gielen et al. (2001) and Na et al. (2011) also found a lower number of stem leaves on tree seedlings exposed to greater than ambient temperatures and CO2 concentrations.
An increase in the S/R ratio for both tree species in the TEMP, fCO2 and TEMPxfCO2 treatments compared to AMB conditions was also reported in other studies (Wilson 1988; Shenglei and Ferris 2006; Xiao et al. 2005; Overdieck et al. 2007; Allen and Vu 2008). Markesteijn and Poorter (2009) suggested that an increase in S/R may occur to maximize growth under stress conditions. A significant increase in RMF in the TEMPxfCO2 and TEMP treatments compared to ambient conditions was also reported by Xiao et al. (2005) and Raizada et al. (2009). For example, Raizada et al. (2009) found a greater RMF when Caragana intermedia Kuang and H.C. Fu was exposed to 700 ppm, and suggested that this may indicate insufficient nutrient availability.
A decrease in LAR in both G. sepium and C. odorata, was also reported by others (Hui et al. 2002; Xiao et al. 2005; Raizada et al. 2009; Körner 2009), and may be due to an increased productivity on a smaller photosynthetic area (Xiao et al. 2005). As such, fCO2 and TEMPxfCO2 may be more efficient photosynthetically compared to seedlings grown under AMB conditions. A decrease in LAR may also be due to increased levels of non-structural carbohydrates, or starch content in leaves, or changes in leaf anatomy (Raizada et al. 2009). Körner (2004) suggested that such changes in levels of non-structural carbohydrates or leaf starch content may also lead to shorter-lived trees, and a higher tree mortality rate.
Leaf nutrient concentrations
Our study showed no change in leaf C concentration for either tree species under any of the different treatments, and was consistent with results observed by Liu et al. (2009). A decrease in leaf N concentration however, was also reported in other studies (Hui et al. 2002; Hoorens et al. 2003; Liu et al. 2009; Norby et al. 2010). Carswell et al. (2000) found a decrease in leaf N and phosphorus (P) concentrations when C. odorata was exposed to 700 ppm compared to AMB conditions. Cotrufo et al. (2005) suggested that CO2 fertilization may alter litter quality leading to a reduction in the long-term availability of nutrients. Lukac et al. (2010) showed that changes in tree litter quality may be far-reaching and may depend on the differential responses of nutrient demand under increased concentrations of atmospheric CO2. Any change in nutrient availability, utilization or cycling in plant biomass, soil and soil water may affect the nutrient status of other ecosystem components causing a potentially cascading effect that may be most prevalent for non-leguminous species (Bradley and Pregitzer 2007).
The greater decrease in leaf N concentration in the leguminous tree seedling G. sepium compared to the non-leguminous C. odorata was also observed in other studies. For example, Rogers et al. (2006) found leaf N content in soybean (Glycine max L.) was significantly lower at elevated concentrations of CO2 in the first 50 days after emergence, which they equated to the initial growth phase of the soybean crop. However, as soybean growth and maturation proceeded, leaf N concentration was similar to that of other non-leguminous plants exposed to ambient levels of CO2 (Rogers et al. 2006). In our study, tree seedling leaf N concentration was evaluated after 35 days of growth, which likely corresponded to the initial growth phase described by Rogers et al. (2006). This suggested that leguminous species commonly used as multipurpose agroforestry trees may not exhibit reduced leaf N content under CO2 fertilization, or a combination effect, after their initial growth phase. Thomas and Strain (2000) suggested that legumes may respond to increased N demands induced by elevated CO2 through increased N2-fixation, even if soil N levels are low or if conditions of drought occur. They found that under CO2 fertilization (700 ppm), the net uptake of N by G. sepium seedlings stimulated N2-fixation (Thomas and Strain 2000).
Conclusions
This study demonstrated the effect of elevated ambient temperature,CO2 fertilization or the combined effect on two different tree species commonly used in Costa Rican agroforestry practices. Both tree species responded similarly to the TEMP and TEMPxfCO2 treatments with respect to growth parameters (height, number of leaves, LAI, shoot and root biomass, and S/R ratio) and leaf C and N concentrations. Results from our study suggested that CO2 fertilization may negatively impact tree growth parameters, but a combined effect likely results in a positive growth effect. However, leaf N concentration was affected negatively in all treatments in both tree species compared to ambient conditions. This may impact the long-term cycling of nutrients in agroforestry systems, especially those with non-leguminous tree species, which may alter litter quality and ultimately lead to changes in nutrient availability in agroforestry systems. As such, further studies using common leguminous and non-leguminous multipurpose agroforestry tree species are required. Additionally, changes in soil dynamics as a result of a combined effect of CO2 fertilization and enhanced temperature are required over the long-term to advance our understanding of the response of these plants to changing atmospheric and environmental conditions. It is especially important that pot trials in growth chambers are paralleled with field trials in order to evaluate plant response to climate change under conditions that resemble as close as possible the plant’s natural environment. Additionally, knowledge of these ecophysiological responses to a combined effect of CO2 fertilization and temperature under different conditions of moisture may also play a key role in understanding the functioning of the whole agroforest.
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analysis review of the responses of photosynthesis, canopy. New Phytol 165:351–371
Allen LH, Vu JCV (2008) Carbon dioxide and high temperature effects on growth of young orange trees in a humid, subtropical environment. Agr Forest Meteorol 149:820–830
Beerling DJ, Fry JC (1990) A comparison of the accuracy, variability and speed of five different methods for estimating leaf area. Ann Bot London 65:483–488
Bradley KL, Pregitzer KS (2007) Ecosystem assembly and terrestrial carbon balance under elevated CO2. Trends Ecol Evol 10:538–547
Carswell FB, Grace J, Lucas ME, Jarvis PG (2000) Interaction of nutrient limitation and elevated CO2 concentration on carbon assimilation of a tropical tree seedling (Cedrela odorata). Tree Physiol 20:977–986
Clark DA (2004) Sources or sinks? the responses of tropical forests to current and future climate and atmospheric composition. Philos Trans Royal Soc B 359:477–491
Cotrufo MF, De Angelis P, Polle A (2005) Leaf litter production and decomposition in a poplar short-rotation coppice exposed to free air CO2 enrichment (POPFACE). Glob Change Biol 11:971–982
Cox PM, Betts RA, Jones CD, Spall SA, Totterdell LJ (2000) Acceleration of global warming due to carbon-cycle feedbacks in coupled climate models. Nature 408:184–187
Cramer W, Bondeau A, Schaphoff S, Lucht W, Smith B, Sitch S (2004) Tropical forests and the global carbon cycle: impacts of atmospheric carbon dioxide, climate change and rate of deforestation. Philos Trans Royal Soc B 359:331–343
Gebauer R, Reynolds J, Strain BR (1996) Allometric relations and growth in Pinus taeda: the effect of elevated CO2 and changing N availability. New Phytol 134:85–93
Gielen B, Calfapietra C, Sabatti M, Ceulemans R (2001) Leaf area dynamics in a closed poplar plantation under free-air carbon dioxide enrichment. Tree Phys 21:1245–1255
Hoorens B, Aerts R, Stroetenga M (2003) Is there a trade-off between the plant’s growth response to elevated CO2 and subsequent litter decomposability? Oikos 105:17–30
Hui D, Sims DA, Johnson DW, Cheng W, Luo Y (2002) Effects of gradual versus step increases in carbon dioxide on Plantago photosynthesis and growth in a microcosm study. Environ Exp Bot 47:51–66
Intergovernmental Panel for Climate Change (IPCC) 2007. Climate change 2007-the physical science basis, contribution of working group I to the fourth assessment report of the IPCC
Keller M, Lerdau M (1999) Isoprene emission from tropical forest canopy leaves. Glob Biogeochem Cycles 13:19–29
Khurana EKTA, Singh JS (2001) Ecology of seed and seedling growth for conservation and restoration of tropical dry forests: a review. Environ Conserv 28:39–52
Körner C (2004) Through enhanced tree dynamics carbon dioxide enrichment may cause tropical forests to lose carbon. Philos Trans Royal Soc B 443:493–498
Körner C (2009) Responses of humid tropical trees to rising CO2. Annu Rev Ecol Syst 40:61–79
Lee JS (2011) Combined effect of elevated CO2 and temperature on the growth and phenology of two annual C3 and C4 weedy species. Agr Ecosyst Environ 140:484–491
Lewis SL, Lloyd J, Sitch S, Mitchard ETA, Laurance WF (2009) Changing ecology of tropical forests: evidence and divers. Annu Rev Ecol Syst 40:529–549
Lindroth RL (2010) Impacts of elevated atmospheric CO2 and O3 on forests: phytochemistry, trophic interactions and ecosystem dynamics. J Chem Ecol 36:2–21
Liu L, King JS, Brooker FL, Giardina CP, Allen HL, Hu S (2009) Enhanced litter input rather than changes in litter chemistry drive soil carbon and nitrogen cycles under elevated CO2: a microcosm study. Glob change Biol 15:441–453
Lloyd J, Farquhar GD (2008) Effects of rising temperatures and [CO2] on the physiology of tropical forest trees. Philos Trans Royal Soc B 363:1811–1817
Lovelock CE, Virgo A, Popp M, Winter K (1999) Effects of elevated CO2 concentrations on photosynthesis, growth and reproduction of branches of the tropical canopy tree species, Luehea seemannii Tr. and Planch. Plant Cell Environ 22:49–59
Lukac M, Calfapietra C, Lagomarsino A, Loreto F (2010) Global climate change and tree nutrition: effects of elevated CO2 and temperature. Tree Physiol 1093:1–12
Markesteijn L, Poorter L (2009) Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought- and shade-tolerance. J Ecol 97:311–325
Na L, Genxu W, Yan Y, Yongshen G, Guangsheng L (2011) Plant production, and carbon and nitrogen source pools, are strongly intensified by experimental warming in alpine ecosystems in the Qunghai-Tibet plateau. Soil Biol Biochem 43:942–953
Nair PKR (1993) An introduction to agroforestry. Kluwer, Dordrecht
Norby RJ, Warren JM, Iversen CM, Medlyn BE, McMurtrie RE (2010) CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc Natl Acad Sci USA 107:19368–19373
Oelbermann M, Voroney RP, Kass DCL (2004) Gliricidia sepium carbon inputs and soil carbon pools in a Costa Rican alley cropping system. Int J Agri Sustain 2:33–42
Oelbermann M, Voroney RP, Kass DCL, Schlönvoigt AM (2005) Above- and below-ground carbon inputs in 19-, 10-, and 4-year old Costa Rican alley cropping systems. Agr Ecosyst Environ 105:163–172
Overdieck D, Ziche D, Bottcher-Jungclaus K (2007) Temperature response of growth of wood anatomy in European beech saplings grown in different carbon dioxide concentrations. Tree Physiol 27:261–268
Raizada P, Singh A, Raghubanshi AS (2009) Comparative response of seedlings of selected native dry tropical and alien invasive species to CO2 enrichment. J Plant Ecol 2:69–75
Rogers A, Gibon Y, Stitt M, Morgan PB, Bernacchi CJ, Ort DR, Long SP (2006) Increased C availability at elevated carbon dioxide concentration improves N assimilation in a legume. Plant Cell Environ 29:1651–1658
Scarascia-Mungozza G, De Angelis P, Sabatti M, Calfapietra C, Miglietra F, Raines C, Godbold D, Hoosbeek M, Taylor G, Polle A, Ceulemans R (2005) Global change and agro-forest ecosystems: adaptation and mitigation in a FACE experiment on a poplar plantation. Plant Biosyst 139:253–264
Shenglei F, Ferris H (2006) Plant species, atmospheric CO2 and soil N interactively or additively control C allocation within plant-soil systems. Sci China Ser C 49:603–612
Sigurdsson BD, Thorgeirsson H, Linder S (2001) Growth and dry matter partitioning of young Populus trichocarpa in response to carbon dioxide concentration and mineral nutrient availability. Tree Physiol 21:941–950
Steel GD, Torrie JH, Dickey DA (1997) Principles and procedures of statistics: a biometrical approach. McGraw-Hill, NY
Thomas RB, Strain BR (2000) Root restriction as a factor in photosynthetic acclimation of cotton seedlings grown in elevated carbon dioxide. Plant Physiol 96:627–634
Tissue DT, Megonigal JF, Thomas RB (1997) Nitrogenase activity and N2 fixation are stimulated by elevated CO2 in a tropical N2-fixing tree. Oecologia 109:1–28
Tupker KA, Thomas BR, Macdonald SE (2003) Propagation of trembling aspen and hybrid poplar for agroforestry: potential benefits of elevated CO2 in the greenhouse. Agrofor Sys 59:61–71
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Formentin JM, Hoegh-Gugdberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
Warren R, Price J, Fischlin A, de la Nava Santos S, Midgley G (2011) Increasing impacts of climate change upon ecosystems with increasing global mean temperature rise. Climat Change 106:141–177
Wertin TM, McGuire MA, Teskey RO (2009) The influence of elevated temperature, elevated atmospheric CO2 concentration and water stress on net photosynthesis of loblolly pine (Pinus taeda L.) at northern, central and southern sites in its native range. Glob Change Biol 16:2089–2103
Wurth MKR, Winter K, Körner CH (1998) In situ responses to elevated CO2 in tropical forest understory plants. Funct Ecol 12:886–895
Xiao CW, Sun OJ, Zhou GS, Zhao JA, Wu G (2005) Interactive effects of elevated CO2 and drought stress on leaf water potential and growth in Caragana intermedia. Trees 19:711–720
Zieche D, Overdieck D (2004) CO2 and temperature effects on growth, biomass production, and stem wood anatomy of juvenile Scots pine (Pinus sylvestris L.). J Appl Bot Angew Bot 78:120–132
Acknowledgments
We thank the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Inter-American Institute for Cooperation on Agriculture Canada (IICA-Canada) for providing financial assistance for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esmail, S., Oelbermann, M. The impact of climate change on the growth of tropical agroforestry tree seedlings. Agroforest Syst 83, 235–244 (2011). https://doi.org/10.1007/s10457-011-9424-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-011-9424-1