Abstract
Climate change is strong in the Amazon basin. Climate models consistently predict widespread warmer and drier conditions by the end of the 21st century. As a consequence, water stress will increase throughout the region. We here review current understanding of the impact of climate change on forests’ distribution patterns, species diversity and ecosystem functioning of lowland rainforests in the Amazon basin. We reviewed 192 studies that provide empirical evidence, historical information and theoretical models. Over millions of years rainforests expansions and contractions have been accompanied by changes in the diversity and productivity of forests. In the future, drought will produce forest contractions along the forest edges and the savanna ecotone, causing an extensive savannization, particularly in the east. In terms of diversity, warming will reduce plant species survival by decreasing their productivity, but extinctions may also occur as a result of vegetation disequilibrium, as many plants, dispersal and pollinator species will fail to track changing climate; mild drought kills understory trees and severe drought may eliminate canopy trees as well. Severe droughts will thus produce directional changes in species composition, although these shifts may vary among forests on different soil types. In terms of ecosystem functioning, droughts will reduce root growth and standing biomass and may shift the Amazonian forest from being CO2 sinks to become CO2 sources. Physiological and ecological responses to warming and the feedback between vegetation and climate are still not completely understood. In particular, experimental assays that allow direct conclusions on the response of Amazonian plants to the predicted climatic conditions are needed. Such studies could make possible more reliable estimates of future climatic and vegetation responses.
Resumen
El cambio climático es intenso en la cuenca Amazónica. Los modelos climáticos predicen condiciones más secas y cálidas para finales del siglo 21. Como consecuencia, el estrés hídrico aumentará a través de la región. Aquí revisamos el conocimiento actual del impacto del cambio climático en los patrones de distribución, diversidad y funcionamiento de los bosques en la cuenca Amazónica. Examinamos 192 estudios basados en evidencia empírica, información histórica y modelos teóricos. Durante millones de años, las expansiones y contracciones de los bosques húmedos han estado acompañadas por cambios en su diversidad y productividad. En el futuro, la sequía provocará contracciones de los bosques húmedos a lo largo del límite con las sabanas, causando una extensa sabanización, particularmente en el oriente. En términos de diversidad, el calentamiento puede afectar la sobrevivencia de las especies vegetales al disminuir su productividad; sin embargo, podrían ocurrir extinciones como resultado de un desequilibrio en la vegetación, pues muchas especies vegetales, dispersores y polinizadores sucumbirán ante el cambio climático; sequías leves podrían eliminar los árboles del sotobosque y sequías severas podrían a su vez eliminar las especies del dosel. Intensas sequías producirán entonces cambios direccionales en la composición de las especies vegetales, pero estos cambios podrían variar de acuerdo al tipo de suelo. En términos del funcionamiento de los ecosistemas, las sequías reducirían el crecimiento de las raíces y la biomasa existente y transformarían los bosques Amazónicos en fuentes en lugar de sumideros de CO2. Aún no entendemos completamente las respuestas fisiológicas y ecológicas al calentamiento, así como la retroalimentación entre vegetación y clima. En particular, se requieren ensayos experimentales que permitan conclusiones directas sobre la respuesta de las plantas Amazónicas a las futuras condiciones climáticas. Tales estudios podrían dar lugar a estimativos más confiables de la futura distribución climática y de la vegetación en la cuenca Amazónica.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Shifts in plant community composition (Enquist & Enquist, 2011) and accelerated carbon cycles (Allen et al., 2010; Laurance et al., 2004; Lewis et al., 2004; Phillips et al., 2002) show that climate change is already impacting tropical rainforests. Paleoecological records show that similar shifts occurred in the past (Behling & Hooghiemstra, 2000; Mayle & Power, 2008; Moreira et al., 2013), but often over time frames of hundreds or thousand of years instead of decades as they currently occur (Malhi & Wright, 2004; Vincent et al., 2005).
Among rainforest areas, the Amazon basin is of particular interest in this context because it influences precipitation and carbon cycles at global and regional scales (Cox et al., 2008). Through evapotranspiration, Amazonian rainforests subsidize 15–30% of the total precipitation in the region (Trenberth, 1999; Betts et al., 2004). In addition, Amazonian plant communities release, capture (Saleska et al., 2003) and store (Malhi et al., 2006; Saatchi et al., 2007) large amounts of carbon. Amazonian forests are highly threatened by two main factors; high deforestation rates (Soares-Filho et al., 2006; Hubbell et al., 2008), and the environmental changes caused by the increasing accumulation of heat-trapping gases in the atmosphere (Johns et al., 2003; Scholze et al., 2006; Malhi et al., 2008; Cook et al., 2012).
Amazonian warming has been evident since the late 20th century, particularly in the eastern parts of the region (Victoria et al., 1998; Jones et al., 1999). Currently, warming occurs at an average rate of 0.25°C decade−1 for the whole region (Malhi & Wright, 2004). Climate models predict increasing warming rates that will lead to a regional temperature increase of 2.5°C by 2050 (Feeley & Rehm, 2012). Increasing frequency and intensity of drought periods in the region are also projected for the coming decades (Phillips et al., 2002; Phillips et al., 2009a). Yet, drought trends are difficult to predict because data are only available for the last 60 years and from a few areas within the basin (Malhi & Wright, 2004).
Changes in temperature, precipitation and seasonality are widely recognized as major threats to Amazonian diversity and stability (Cox et al., 2004; Malhi & Wright, 2004). However, many studies are confronted with major methodological and funding limitations, and have focused on short-term impacts, small forest areas (Williamson et al., 2000; Laurance et al., 2001; Metcalfe et al., 2010) or a few species (Lloyd & Farquhar, 2008). Yet, to fully understand climate change effects on species distributions and ecosystem functioning, research at multiple scales ranging from physiological mechanisms that determine individuals and species fitness to the large-scale distribution patterns is needed. Here, we aim to provide a synthesis of studies that evaluate effects of warming, drought and prolonged dry seasons across Amazonian rainforests.
This review is related to those of Bush & Flenley (2007) and Bush et al. (2011), particularly to the chapters therein by Phillips et al. (2007) and Marengo et al. (2011). However, our review has a different focus, analyzing the relative effects of warming, drought and dry season length on forest extension, species diversity and ecosystem functioning. We cite 192 references of which 115 were not included in the mentioned reviews and 44 were published after Bush and collaborators’ work was published.
Concerning the paleoecological evidence, we restricted our review to studies that evaluate climatic change impacts from the Last Glacial Maximum (LGM) ca. 21,000 years ago and onwards through the Holocene, to ensure that species and communities were similar to those found in the region today. We reviewed literature found on Web of Science and Google Scholar using combinations of terms regarding the Amazonian region (Amazon basin, Amazon rainforest); climate changes (drought, climate change, warming); and the types of impact we focused on (biomass, plant distribution changes, plant richness, plant diversity).
Diversity and Distribution of Rainforest Communities in the Amazon basin
After the Andean uplift (65–7 Ma BP), the Amazon basin became a dynamic system mobilizing large amounts of sediments and nutrients. This dynamic system, coupled with a heterogeneous landscape, fostered the diversification of entire animal and plant linages (Hoorn et al., 2010; Fernandes et al., 2014; Honorio et al., 2014). Pleistocene glaciations such as the LGM also caused large-scale changes in forest distribution and species composition (van der Hammen & Hooghiemstra, 2000). As such, interactions between montane biota and the existing lowland elements resulted in various forest types similar to the modern ones (Graham, 2011). Subsequently, after overcoming a period of intensive drought during the early-mid Holocene (11.7–6 ka), the Amazon forests reached their current extent (Fig. 1).
The Amazon basin. The Amazon basin sensu stricto refers to the area occupied by the Amazon and part of the Tocantins river watersheds, which includes parts of Brazil, Bolivia, Peru, Ecuador, Colombia and Venezuela and demarcated by a solid black line on this map. In total, the Amazon basin covers 5,569,174 km2, with Brazil harbouring the largest proportion (Eva & Huber, 2005). The Guiana and Brazilian Shields are Precambrian geological formations consisting of well-weathered sedimentary rocks; the rivers Negro, Xingú and Tapajós draining these areas tend to be nutrient-poor, in contrast to the Amazon and Madeira rivers that drain the fertile Andean slopes. White lines represent the isopleths of mean daily precipitation during the three driest months of the year (in mm) (Davidson et al., 2012). Isopleths are only available for Brazil; however, they illustrate the increase of drought conditions towards the east and south of the Amazon basin.
For our analysis, we subdivided the Amazon basin in western and eastern regions at the 60°W longitude, and we also differentiate the Guiana Shield, and the northwest, southwest, south, east, and central Amazon basin (Fig. 1). We focused on the five major forest types in Amazonian lowlands: terra firme, várzea, white-sand forest, swamps and igapó, and we exclude areas above 700 m on the Andean slopes in which other factors determine the overall climatic variation and hence plant species distribution patterns, species diversity and ecosystem functioning.
Although only 227 tree species dominate in the whole Amazon basin (ter Steege et al., 2013), diversity varies between regions, and types of forest follow patterns in soil fertility and dry season length (Gentry, 1988; Terborgh & Andresen, 1998; van der Hammen & Hooghiemstra, 2000; Higgins et al., 2011). High diversity is reached in the central and western Amazon basin, whereas the eastern part and the Guiana Shield (Fig. 1) have lower diversity (Malhi et al., 2004; ter Steege et al., 2006). Three principal vegetation types are recognized across the basin: terra firme, floodplains and swamp forests. These formations are primarily differentiated by local topography and hydrology. Terra firme forests dominate the Amazon basin occupying more than 70% of the total area (4,587,000 km2, Eva & Huber, 2005). They are found at low elevations, generally below 200 m, in areas that are not flooded during the wet season (Pires & Prance, 1985). Within the terra firme, two formations are distinguished, terra firme proper and white-sand. Floodplain forests cover a smaller area of about 190,000 km2 (Eva & Huber, 2005) and are located in low-lying areas that are periodically inundated by rivers. Floodplains are subdivided in formations named várzea and igapó, reflecting differences in geology, soil texture and water chemistry. Swamps are also a common vegetation type. Located either close to the rivers or embedded within terra firme forests, swamps are depressed areas that never fully drain during the dry season.
Across the Amazon basin, terra firme forests are the most diverse in all regions followed by white sand formations (Malhi et al., 2004; ter Steege et al., 2006). Low diversity is found in inundated vegetation types, in particular in the eastern parts of the basin and the Guiana and Brazilian Shields (Campbell et al., 1986; Richards, 1996; Parolin, 2010). Like diversity, the physiognomy of the vegetation is controlled by the intensity of the dry season in the Amazon basin (Gentry, 1988; ter Steege et al., 2000). Basal area and stem density are higher in the northwest Amazon basin where the dry season is weak (Butt et al., 2008). In contrast, highly seasonal forests in the southern and eastern parts of the basin have lower stem densities (Pires & Prance, 1985; ter Steege et al., 2000). Basal area can exceed 40 m2 hectare−1 in dense terra firme forests, whereas it may be less than 25 m2 hectare−1 in more open forests (Pires & Prance, 1985).
Terra Firme Forest
This vegetation type is mainly found on ultisols in the west and oxisols in the eastern Amazon basin (Malhi et al., 2004). Both types of soils are acid, deep and well drained (Sombroek, 2000). Although terra firme is a general term for the non-inundated forests, high variation exists within them. Tall and dense forests develop in aseasonal and humid areas of western terra firme forests (e.g. Korning & Balslev, 1994). Large tree species are abundant and form a continuous and dense canopy. Arecaceae, Fabaceae, Moraceae, Myristicaceae and Rubiaceae are the most abundant families in the dense forest of the western Amazon basin (Balslev et al., 1987; ter Steege et al., 2000). There are also more open terra firme forests, particularly in the east, where the vegetation is lower and the canopy less dense, and, as a consequence, biomass (Pires & Prance, 1985) and productivity (Aragão et al., 2009a) are lower in these formations. Lower humidity and seasonality as well as substrate features, such as low permeability or poor drainage affect the composition of these open terra firme forests (Pires & Prance, 1985).
White-sand Forest
These forests are scattered throughout the central and eastern Amazon basin, where podsol or spodosol soils are found. Given the low nutrient content of these soils, white sand forests have low species diversity and high endemism. Large areas of forests are dominated by single species like Micrandra sprucei and Eperua leucantha (Myster, 2009). Slender trees up to 20 m high characterize these forests; herbs and shrubs are abundant in the understory because the canopy is rather open (Anderson, 1981). Members of Malvaceae, Clusiaceae, Fabaceae and Euphorbiaceae are abundant in the western white-sand forests (Honorio et al., 2008; Myster, 2009). In the east, also different epiphytes such as orchids and bromeliads are common (Anderson, 1981).
Várzea
Rivers such as the Amazonas and the Madeira inundate floodplains known as várzeas. These rivers transport large amounts of sediments that originate in the nutrient rich soils of the Andean slopes. Várzeas located in the west are flooded during the rainy season and inundation may last up to five months. In contrast, tidal movements rather than precipitation regimes determine the flooding level in várzeas in the east close to the Atlantic Ocean (Pires & Prance, 1985; ter Steege et al., 2000). These muddy waters charged with clay sediments are sometimes called “white water” because of their characteristic light brown colour. Sediments accumulate along the river forming fertile levees. The fertile alluvial soils support plant communities with high abundance of Arecaceae, Heliconiaceae, Marantaceae and Urticaceae. Overall species diversity is lower than in terra firme forests, but within várzeas diversity, trees density and basal area are determined by gradients in soil fertility and inundation levels (Damasco et al., 2013). Hence, species diversity is higher in várzeas of the western part of the basin and in places with short periods of inundation. Yet, productivity in várzeas is higher than in terra firme forests, reaching up to 100 tons ha−1 year−1 in contrast to 27 tons ha−1 year−1 in terra firme (Myster, 2009).
Igapó
This term defines the floodplains inundated by rivers of black or crystalline nutrient poor waters. Blackwater rivers (as the Rio Negro) originate within the Amazon basin in areas of white podsolic soils; waters are black because the vegetation and litterfall in this region have high concentrations of tannins that are leached into the water. Crystalline rivers (e.g., Tapajós river) originate and flow over the hard rocks of the ancient Precambrian areas of the Guiana and Brazilian shields. As a consequence, their igapós are located on sandy soils, and, since almost no sediments are transported in the river water, no levees are formed. Hence, during the flood season stems, and sometimes crowns, of many trees are completely underwater. Members of Myrtaceae are abundant in these floodplains and Leguminosae species are particularly abundant (Pires & Prance, 1985; ter Steege et al., 2000). Asteranthus brasiliensis, Glandonia williamsii, Henriquezia nitida, Leopoldinia piassaba, Mauritia carana, Ocotea esmeraldana and Vitex calothyrsa are just a few examples of the many species that exclusively inhabit igapó forests (Junk & Piedade, 2011).
Swamps
They may be inundated by either white waters, which is most common or by black waters. In general, swamps have low species diversity and low density of tall trees. Palms are important in swamp forests (Prance, 1979). Although many Amazonian swamps are dominated by Mauritia flexuosa, a palm that reaches up to 30 m height, other palm genera such as Euterpe and Bactris are also common (Richards, 1996) as are other tree genera such as Triplaris (Polygonaceae) and Virola (Myristicaceae). Herbaceous genera are characteristic of non-forested swamps; among them are Hydrocotyle (Apiaceae), Pistia (Araceae), Eichornia (Pontederiaceae) and Azolla (Salviniaceae) (Kalliola et al., 1991; Richards, 1996).
Past, Current and Future Climates in the Amazon basin
The uplift of the Andes started 65 Ma ago with plate subduction along the Pacific coastline, and it represents a geologic milestone explaining most of the current climatic and physiognomic characteristics of the Amazonian forests; although other aspects of Amazonian vegetation physiognomy also relate to even deeper-time factors. Mountain uplift resulted in the formation of fluvial systems that covered a large area in northwestern and northeastern South America, and changes in climatic regimes included increased precipitation in the east (Hoorn et al., 2010). Later, Pleistocene glaciations (2 Ma–11.7 ka BP) reshaped moisture and temperature gradients across the Amazon basin (van der Hammen & Hooghiemstra, 2000). Dry conditions persisted until the mid-Holocene (9–6 ka) (Maslin & Burns, 2000). During that time, the eastern Amazon basin was particularly dry with 15–30% less rainfall than today, while temperatures were similar (van Breukelen et al., 2008). Causes of the early-mid Holocene drought relate to solar radiative forcing fluctuations (Silva Dias et al., 2009) that characterize the multi-millennial orbital cycle (Wanner et al., 2008). The decrease in solar forcing would account for a decline in moisture transportation from the Atlantic Ocean to the Amazon basin and an increase of sea surface temperature (SST). These processes ultimately resulted in a weak monsoon responsible for an extensive dry period (Silva Dias et al., 2009). The displacement of the Intertropical Convergence Zone (ITCZ) southwards at 2.2–0.7 ka BP reversed the situation, increasing convective rainfall in the Amazon basin (Mayle et al., 2000; Moreira et al., 2013). Wet climatic conditions, which prevail until today (Behling et al., 2001), allowed Amazonian rainforests to reach their current geographical extent (Mayle et al., 2000; Bush et al., 2007).
Today, the climate of the Amazon basin is characterized by mean annual temperatures around 26°C and mean annual precipitation around 2,400 mm with 0–3 dry months with precipitation <100 mm (Malhi & Wright, 2004). Still, some parts of the eastern Amazon basin are highly seasonal, with up to six months of low rainfall (<100 mm, Fisher et al., 2008). High variation exists among the regions. Precipitation is highest and most constant in northwestern Amazon, whereas the east experiences shallow dry seasons in the northeast and more intense dry seasons in the southeast. Although high variation in precipitation exists across the Amazon basin, the main patterns are determined by the correlation between precipitation and dry season length along both latitudinal and longitudinal gradients (Gentry, 1988; ter Steege et al., 2003). The northwestern Amazon basin (at 2–4°S) is the wettest with approx. 3,000 mm of rain per year and no dry season. To the north and south, precipitation declines and variation increases. Highly seasonal climates occur around 15°S where precipitation is <1,500 mm yr−1, and this trend becomes stronger eastwards (Silman, 2007). Interannual variation in Amazonian climate is to a large extent determined by a 3–5 year oscillation of the SST and the atmospheric pressure patterns in the equatorial Pacific Ocean; a phenomenon currently known as El Niño Southern Oscillation (ENSO). During ENSO events the Amazon basin in general experiences less precipitation and higher temperatures (Fig. 2), particularly in the northern and eastern parts (Ropelewski & Halpert, 1987), whereas the western Amazon basin experiences almost no effect (Malhi & Wright, 2004).
Drivers of climate change in the Amazon basin. The cumulative effects of the disequilibrium between local and regional circulation patterns determine climate change. Warming and drought are largely controlled by atmospheric circulation patterns (a), but local factors provide positive feedbacks that enhance and prolong their effects (b). Deforestation and reduced evaporation increase warming and decrease precipitation, providing positive feedback that enhances and prolongs droughts, which in turn increases fires frequency and intensity. Planned development including strategies to increase reforestation as well as unsettled and protected areas could offset local stress factors. Slow economic growth and environmental law enforcement are needed to counteract large-scale threats. SSTs: Sea Surface Temperatures; ITCZ: Intertropical Convergence Zone; ENSO: El Niño Southern Oscillation.
Among the processes that govern the Amazon basin climate, the ITCZ deserves particular attention. Throughout a year, the air at the ITCZ moves along gradients in altitude and latitude, and that migration determines the annual distribution of rainfall across the Amazon basin (Fearnside, 2009). In the northwestern Amazon basin (10°S–5°N; 70–60°W) the ITCZ crosses the equator twice a year, producing two precipitation maxima. In contrast, the south and central Amazon basin have a single rainy season from December–March (Sturm et al., 2007). If the water in the North Atlantic Ocean is warmer than usual, a strong gradient in SST emerges and forces the ITCZ to migrate further north. In consequence, dry airflows disperse through the Amazon basin, causing devastating droughts in the southern part of the basin, as was the case in 2005 (Marengo et al., 2008).
For future climatic conditions, predictions are based on the IPCC CO2 emission models. Four broad groups of scenarios apply, named A1, A2, B1 and B2 (IPCC, 2001, 2007). Currently, CO2 concentration in the atmosphere has reached 400 ppm (NOAA, 2013) with an emissions rate of approximately 34 billion tons CO2 year−1 (Le Quéré et al., 2013). IPCC scenarios A2 and B1 are commonly used for future climate projections. Scenario A2 assumes that emissions maintain their current rate and CO2 concentration reaches approximately 860 ppm by 2100, whereas in B1 a decrease in CO2 emissions, through strong environmental policies, leads to a concentration of 550 ppm (IPCC, 2001). For most of the Amazon basin, models predict higher temperatures, a significant reduction in precipitation and longer dry seasons for the period 2071–2100 (Malhi & Wright, 2004; Coppola & Giorgi, 2005; Rojas et al., 2006). Under an optimistic scenario B2, temperature would increase by 2–3°C and precipitation would decrease by 5% by 2100. Under A2 scenarios, however, temperature is expected to increase by 4–6°C and precipitation to decrease by 20–30% by the end of this century (Marengo, 2007; Harris et al., 2008). In this case, the southeastern Amazon basin would experience an extreme decline of >10mm day−1, whereas an increase by >2 mm day−1 would occur in the west (Cook & Vizy, 2008). Many studies predict that changes in precipitation are likely to enhance seasonal regimes, again as a result of increasing SSTs in the Atlantic and Pacific Oceans (Malhi et al., 2009; Cook et al., 2012) (Fig. 2), although projected precipitation changes differ among models (Harris et al., 2008; Cook et al., 2012). There are also models that predict an increase in rainfall across the basin as a consequence of increasing accumulation of CO2. According to these simulations, rainfall will increase and be evenly distributed throughout the year in the west. In the east, however, increases will only occur during wet seasons, again causing stronger seasonality (Cook et al., 2012). The increasing precipitation effect would, however, be cancelled if deforestation rates continue at their current level (Costa & Foley, 2000) (Fig. 2). The lack of forest cover due to deforestation causes poor moisture recycling (Eltahir, 1996; Costa & Foley, 1997; Betts et al., 2004; Stickler et al., 2013). If current deforestation rates continue, the Amazon basin will lose 40% of forest cover by 2050 (Soares-Filho et al., 2006). In this case, an overall decrease of 12 and 25% in precipitation would occur during wet season and dry seasons respectively, across the whole region (Spracklen et al., 2012; Stickler et al., 2013).
Another human-induced feedback that must be considered is fire. If precipitation decreases and dry periods strengthen, anthropogenic fires will become more intense and frequent (Cochrane & Barber, 2009). Under these conditions, both forest areas and regional precipitation would disappear at a high pace (Cochrane & Laurance, 2002) and would supress Amazonian forests in the mid-term (Cochrane & Schulze, 1999; Cochrane, 2003) (Fig. 2).
It is important to note that current data on precipitation are inaccurate, because large areas of Amazonian forests are still uncovered by climatic stations and usually records exist for even less than six decades (Malhi & Wright, 2004). Thus, many of the studies we reviewed pointed to difficulties in obtaining reliable inferences for this factor (e.g., Costa & Foley, 1997). For further discussion of the causal mechanisms of changes in temperature and precipitation regimes see Marengo (2004, 2007), Cook & Vizy (2008), and Dai (2011). Overall, however, there is an increasing consensus pointing to widespread drier and warmer conditions across the Amazon basin by the end of the 21st century.
In order to preserve some of the natural ecosystems and mitigate the impacts of climate change, detailed knowledge on the past and current ecosystem responses to climate is needed. In the next sections, we discuss crucial findings on the effects of warming, drought and prolonged dry seasons on three levels of impact in Amazonian forests.
Effects of Warming
Plant diversity in the Neotropics appear to have been considerably higher than today during past periods of maximum warmth (e.g., Bermingham & Dick, 2001; Svenning & Condit, 2008), namely during most of the Eocene (55–40 Ma; Jaramillo et al., 2006). Today, tropical lowlands are subject to the highest temperature estimated for the last two million years (Bush, 2002). However, this warming is on a time scale much too short for effects on species origination rates to be realized (Svenning & Sandel, 2013). Instead, changes in vegetation distribution (Feeley, 2012), functional composition (Nepstad et al., 2007) and demographic rates (Lewis et al., 2004) have been documented and are expected to increase as climate change intensifies.
Forest Distribution
An analysis of herbarium records from 1970–2009 revealed temperature-related distribution changes in South American plants across the last decades (Feeley, 2012). Such warming-induced changes in distributions may be particularly strong when climate change occurs at rates that are too fast for species to acclimatize or adapt (Svenning & Sandel, 2013). In such cases, species have to migrate to track their suitable climatic conditions, or they may become extinct if they fail to do so (Feeley & Silman, 2010b; Feeley et al., 2012a). The lack of high elevations across the Amazon basin would constrain species to cooler and wetter small-scale refuges within the lowlands under warmer conditions (Bush et al., 2004). Even if upslope migration were possible and migration rates could keep pace with temperature increases, analogous climates may not exist in the uplands in the coming decades (Williams et al., 2007) or deforestation could restrict the access to them (Higgins, 2007; Feeley & Rehm, 2012; Feeley et al., 2012b). This, in consequence, could result in regional extinction of many plant species and the habitat loss for others, which might depend on the formers as providers of suitable microenvironmental conditions (Feeley et al., 2012a).
Model projections of future habitat areas have been made with respect to the distribution of plant species across the Amazon basin (Feeley et al., 2012b). Different scenarios of climate change, and different levels of plant species adaptability and migration rates were assessed. The first scenario assumed a low rate of deforestation, positive effect of increased CO2 on plant water use efficiency (WUE), and ability of plants to migrate and adapt to higher temperatures. This scenario showed that an increase of 2–4°C would cause species to lose a small percentage (8–12%) of their current habitat. In contrast, the second scenario assumed deforestation rates similar as those of today, and that neither temperature adaptation nor migration occur. In this case species would lose 82–99% of their current habitat under 2–4°C global warming. The largest percentage of habitat loss occurred under the assumption that species are not able to adapt to increasing temperatures (Feeley et al., 2012b).
Another model that does not assume adaptation indicated that a progressive warming of 2–6°C may cause complete depletion of Amazonian tree coverage in less than 500 years (Hirota et al., 2010). The earliest changes in forest cover are expected to occur at the end of this century and in areas with high variation in temperature or precipitation (Cook et al., 2012; Feeley et al., 2012b). Hence, the southern and eastern Amazon basin will most likely experience changes to open savanna vegetation by the end of the 21st century (Hutyra et al., 2005; Salazar et al., 2007). In the west changes will be moderate, but the combined effect of deforestation (Feeley et al., 2012b) and increased temperatures may accelerate the loss of forest coverage (Salazar et al., 2007).
Species diversity
The Amazon basin experienced strong environmental changes since the Paleogene with likely effects on plant species diversity in the region. Despite intense periods of cooling and warming, plant species diversity has increased throughout the Amazon basin over the past 56 Ma, except in periods when water was a limiting factor (Svenning & Condit, 2008; Jaramillo et al., 2010). Changes in tectonics, sea level and climate produced heterogeneous habitats that contributed to plant diversification in the region (Hoorn et al., 2010). While low temperatures allowed the entrance of montane taxa, warm and humid periods allowed the diversification of many other species by providing habitat heterogeneity (van der Hammen & Hooghiemstra, 2000). Thus, if a high percentage of current Amazonian plants already lived during the warm period in the Pliocene (2.6–5 Ma), they may be capable of tolerating or adapting to such warm conditions again (Colwell et al., 2008). In fact, various abundant and widespread Amazonian tree species descend from ancestor populations that lived through warm periods similar to what is estimated for 2100 without upslope migration (Dick et al., 2013), suggesting that other factors than warming itself may be the main drivers of diversity losses in the Amazon basin. Diversity losses might instead occur when warming causes disequilibrium in demographical and ecological factors such as population sizes and fragmentation or a decline in pollinators and dispersal availability (Svenning & Sandel, 2013).
Ecosystem Functioning
At the cellular level, temperature modulates enzymatic activity and the electron transport rate. Temperature also affects photosynthesis by modulating stomata responses to the evaporative demand. At high temperatures stomata closure reduces transpiration losses. Prolonged stomata closure causes a decrease of intercellular CO2 and therefore it reduces photosynthetic rates (Koch et al., 1994; Tribuzy, 2005). Leaves with high exposure to sunlight and higher leaf temperatures, reach the threshold of stomatal closure faster than shaded leaves (Doughty & Goulden, 2008). The relative importance of temperature-direct and indirect effects on photosynthesis differs between models. In some models, it is the indirect effect of temperature on stomatal conductance that causes the highest decline in photosynthesis (Doughty & Goulden, 2008; Lloyd & Farquhar, 2008), but others showed that direct effects on photorespiration and photosynthetic machinery are more important (Galbraith et al., 2010; Doughty, 2011).
Even if tropical trees have high physiological heat tolerance (Krause et al., 2010), they are often close to their temperature optima for carbon acquisition at temperatures higher than 28 °C (e.g., Doughty & Goulden, 2008; Lloyd & Farquhar, 2008). In gas exchange chambers, tropical leaves showed sharp decline in photosynthesis at temperatures of 26−34°C (Koch et al., 1994; Keller & Lerdau, 1999; Doughty, 2011). Over a period of 13 weeks at 30°C, Amazonian tree leaves showed decrease in maximum photosynthetic rate compared to the control, and no signs of acclimatization appeared. When heated to 37°C over more than three weeks leaves suffered necrosis and abscission (Doughty, 2011). CO2 uptake in entire Amazonian plant communities is considerably lower during warmer than average periods (Grace et al., 1995; Goulden et al., 2004). For instance, net ecosystem exchange declined by 12 μmol m2s−1 during days that were 3°C warmer than average in the central Amazon basin and further increases caused sharp decline in CO2 uptake (Goulden et al., 2004).
Under high temperatures, plant productivity decreases because stomatal closure causes CO2 shortage (Lloyd & Farquhar, 2008), an effect that higher atmospheric concentrations of CO2 might counteract (Clark, 2004; Wright, 2005; Lloyd & Farquhar, 2008; Huntingford, et al., 2013). However, empirical evidence supporting this in tropical plants is scarce (Würth et al., 1998). Increased CO2 causes only a slight and transient increase in the rate of carbon fixation, which is not sufficient to increase plant growth (Lovelock et al., 1999; Wright, 2005). Furthermore, there is no evidence that tropical trees are CO2 limited (Cernusak et al., 2013). Under an optimistic scenario, an increase in CO2 concentration from 270 ppm to700 ppm would increase productivity only by 25% (Chambers & Silver, 2004). Although increased CO2 concentrations may — in part — offset the impact of warming, the ability of Amazonian plants to shift their photosynthesis and respiratory rates would determine the stability of individuals’ growth (Ghannoum & Way, 2011) and the resilience of entire plant communities (Feeley & Silman, 2010a; Feeley et al., 2012a; Huntingford et al., 2013).
Effects of Drought
Drought conditions are not new to Amazonian forests. First, drier periods during the LGM and the Holocene modified the spatial distribution (Mayle et al., 2000; Mayle & Power, 2008; Silva Dias et al., 2009) and diversity (van der Hammen & Hooghiemstra, 2000) of Amazonian forests. Second, a current climatic gradient exists from the aseasonal west to the highly seasonal eastern Amazon. Thus, the predicted increase of water stress throughout the region might — in part — cause similar changes as during the past and will vary along the gradient in seasonality. Nevertheless, the interaction between drought, warming and deforestation could lead to a non-analogous scenario. The effects of drought are discussed from a historical perspective in the two following subsections.
(i) Past and Current Effects of Drought
Despite past changes in the spatial distribution (Mayle et al., 2000; Mayle & Power, 2008; Silva Dias et al., 2009) and diversity (van der Hammen & Hooghiemstra, 2000), Amazonian forests have endured such changes and persisted for at least 55 million years (Bush et al., 2007; Hoorn et al., 2010).
Forest Distribution
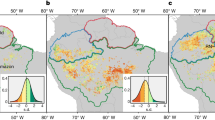
During the LGM, climate was cold and dry. Temperatures were 4.5 ±1°C lower than today across the basin (Stute et al., 1995; van der Hammen & Hooghiemstra, 2000). As a consequence of the decrease in condensation, precipitation was 20% and 40% lower than today in the central and eastern Amazon basin respectively (Anhuf et al., 2006) (Fig. 3a and c). Hence, the area covered by rainforests during the LGM is thought to be smaller than today (François et al., 1999) (Fig. 3e), although other estimates suggest relatively little fragmentation during the LGM (e.g., Mayle et al., 2000). During the Holocene, drought was strong in the east and along the ecotones, whereas the northwest (Cheng et al., 2013) and central Amazon were less affected (Behling & Hooghiemstra, 2000; De Freitas et al., 2001; Moreira et al., 2013) (Fig. 3b). Indeed, minor floristic changes occurred in the northwest, where rainforest communities prevailed (Bush et al., 2007), but savannas were extensive in the south (Mayle et al., 2000), inducing a contraction of forests in the southeastern Amazon basin (Ledru et al., 1998) (Fig. 3f). Finally, rainforest expanded in the southwest during late-Holocene (2.2–0.7 ka BP, Mayle et al., 2000), reaching their current extent (Fig. 3g).
Climate and rainforests distribution in the Amazon basin through time. Maps (a-d) show drought distribution in the Amazon basin since the Last Glacial Maximum (LGM, 21 ka BP) to the end of the 21st century based on the distribution of precipitation anomalies (mm/month) with respect to the present for the LGM (a) and for the mid-Holocene (9–6 ka) (b) (drawn after François et al., 1999); and based on the mean annual self calibrated Palmer Drought Severity Index (scPDSI) for years 2000–2009 (c) and for years 2090–2099 (d) (drawn after Dai, 2011). Maps (e-h) show rainforest distribution during the same periods and relative to present-day; (e) drawn after Anhuf et al. (2006); (f) drawn after Jonathan Adams at http://www.esd.ornl.gov/projects/qen/nercSOUTHAMERICA.html; (g) Amazon basin, current distribution area (h) drawn after Cook & Vizy (2008)
Today as well as in the past, changes in precipitation regimes are likely to affect the extension of rainforests mainly along the ecotones and where there are steep moisture gradients. The south-eastern ecotone is particularly vulnerable because of its high seasonality and because it holds one of the highest rates of forest loss worldwide (Hansen et al., 2013).
Species Diversity
As a consequence of dry conditions during the Holocene, fire events were frequent (Bush et al., 2008; Mayle & Power, 2008). Depending on fire extents, smaller or larger shifts in forests distribution and composition occurred (Silman, 2007). Pollen and charcoal records attest to regular fire events during early-mid Holocene across the Amazon basin that had little impact on forest composition and coverage (Urrego et al., 2013). This was, however, not the case during the late-Holocene, when fires of human origin became more frequent and covered larger areas (da Silva Meneses et al., 2013). Late-Holocene fires caused changes in rainforests composition and structure. Abundance of herbaceous (e.g., Asteraceae, Lamiaceae) and shoreline taxa (e.g., Cyperaceae, Sagittaria) increased, whereas mature forest elements (Anacardiaceae, Lecythidaceae, Myristicaceae) declined (Urrego et al., 2013).
While the total amount of rainfall previously was seen as the main predictor of current diversity patterns in the Amazon basin (Clinebell et al., 1995), more recent interpretations suggest that current diversity patterns are driven by seasonal variability in the amount of precipitation throughout the region (Silman, 2007; Gentry, 1988; ter Steege et al., 2003). Thus, today the highest diversity of vascular plants occurs where there is high rainfall and no dry seasons (Gentry, 1988). This explains the gradient of diversity from the rich very wet and aseasonal forests in the western Amazon basin, to the poor drier and highly seasonal forests in the eastern Amazon. Similarly, it explains the high diversity found in the central forests, where the amount of precipitation is low but more evenly distributed over the year (ter Steege et al., 2000). In the absence of competition for water, forest communities reach high stem density and complete canopy coverage (Pires & Prance, 1985). Such conditions provide moist-shade habitats for numerous species in the lower strata (Gentry, 1988; Wright, 1992; Butt et al., 2008).
Ecosystem Functioning
Seasonality in precipitation appears to be the most important factor driving regional variation in vegetation patterns. Unfortunately, studies on the strategies of Amazonian plants to endure seasonality have been addressed mostly at local scales, which prevent us from drawing conclusions that refer to the entire Amazon basin. However, we draw attention to a coarse geographical contrast between different growth phenologies and growth strategies, assuming that studies performed at each local area represent the pattern at a wider scale. Hence, plants in the eastern forests are often deciduous which reduces the transpiring surface area, or they increase water uptake by producing fine-large roots, which have larger area per unit mass (Metcalfe et al., 2008). In central, less seasonal forests, overstory species access water from deep soil layers (to depths of 8 m or more) to counteract drought and maintain their crowns throughout the year (Nepstad et al., 1994; Jipp et al., 1998; Oliveira et al., 2005). Deep soil moisture is in turn redistributed in the upper layers, a process known as water uplift or hydraulic redistribution that delays drought in entire plant communities (Oliveira et al., 2005; Nepstad et al., 2008). In understory species, survival is mainly limited by the lack of long roots, which limits their access to deep-water sources (Butt et al., 2008). Thus, during dry periods understory species strongly depend on overstory species, because they provide a source of water and also because canopy coverage prevents desiccation by sunlight. This drought-avoiding mechanism is only possible under medium-term water deficits. Its maintenance during long dry seasons represents a major hydraulic limitation.
Biomass stocks in the Amazon basin forests vary along precipitation gradients, because restrictions to development in tropical plants are usually related to the lack or excess of water (Pires & Prance, 1985). Hence, tree basal area (Malhi et al., 2006) and Leaf Area Index (LAI) (Spracklen et al., 2012) are higher in areas of high rainfall. At very low moisture levels, resistance to water transport through the vascular system increases and plants usually undergo water column disruptions or embolisms (Hacke et al., 2001). Embolisms increase with tree height (Hartmann, 2011; Choat et al., 2012), and inhibit efficient transport of water from roots to leaves (Taiz & Zeiger, 1998). Therefore they constitute a common cause for loss of twigs and branches. Yet, embolism by itself is not a major cause for mortality of entire plants. Instead, mortality of trees during drought events may be the result of limited water transport from the xylem to the phloem, which may cause a shortage of assimilates (Hartmann, 2011).
During droughts trees’ growth-rates decline and mortality increases. This results in the loss of large amounts of biomass (da Costa et al., 2010; Lewis et al., 2011) that significantly contribute as CO2 sources (Phillips et al., 2010), particularly when drought kills the largest trees (da Costa et al., 2010; Phillips et al., 2010). Amazonian plant communities are resilient to biomass losses when precipitation shortage lasts less than three years (Nepstad et al., 2002; da Costa et al., 2010). After three years, losses are massive and may modify forest structure (Nepstad et al., 2007; da Costa et al., 2010). The LAI is also susceptible to dry conditions. An 8% decrease in soil moisture produced a decline of about 1% in LAI of forests in the central and southern Amazon basin (Cook et al., 2012).
The transport of water and assimilates through the vascular system involves physiological constraints that increase at larger distances (Hartmann, 2011). Large trees may be susceptible to drought because of physiological mechanisms related to roots functioning (McElrone et al., 2007), a possible relationship between wood density and resistance to cavitation (Hacke et al., 2001), and the apparent carbon starvation effect, caused by prolonged stomatal closure (McDowell et al., 2008; Sevanto et al., 2013). Yet, there is no consistent evidence for any of these arguments in Amazonian trees, preventing direct conclusions on the direction of changes in forest communities under drier conditions. Although the impacts of water shortage alone on ecosystem functioning do not seem large, rainforests may undergo major and differential changes caused by differences in forest composition (da Costa et al., 2010; Fisher et al., 2010; van der Molen et al., 2011), as well as the interaction between drought and seasonality (Wright, 1992), soil texture (Fisher et al., 2008) and land use (Higgins, 2007). Importantly, the effects of drought on soil organic matter decomposition, nitrogen mineralisation, carbohydrate reserves and carbon allocation to defense compounds are well understood in temperate forests (van der Molen et al., 2011 and references therein) but remain elusive in Amazonian forests. As such, further studies are needed, with more ecophysiological data that will make it possible to determine and quantify the underlying functional relationships.
(ii) Effects of Future Prolonged Droughts
Drought and longer dry seasons will intensify the effects of warming (Bush et al., 2008; Colwell et al., 2008) and exacerbate the contraction of rainforest communities (Salazar et al., 2007). In the central and southern Amazon basin, hypothetical increases in rainfall are not expected to alleviate water stress caused by warming, because during wet seasons excess rainfall will drain as runoff, and during dry periods soil water depletion would cause severe drought (Zeng et al., 2008; Phillips et al., 2009a; Cook et al., 2012).
Forest Distribution
Models predict a range of potential forest distributions by the end of the 21st century depending on the grid size and the assumptions considered. A whole range from more seasonal forests (Cook et al., 2012) to a complete dieback across the Amazon basin (Cox et al., 2004; Zeng et al., 2013) exists. Models using coarse grid scales tend to underestimate the amount of rainfall and misrepresent local climate features. They generally suggest the replacement of large areas of rainforests with open savanna vegetation. Instead, less radical effects are suggested by fine-grained projections. In this case, local feedbacks increase the overall moisture conditions and result in the loss of smaller forest areas (Li et al., 2006; Malhi et al., 2009; Poulter et al., 2010).
With these finer grain projections, prolonged dry seasons in large areas of Peru, Brazil, Guyana and Suriname are predicted to cause expansion of savannas by the end of this century (2081–2100), while arid landscapes will dominate in areas of low precipitation in Bolivia, Argentina and Paraguay (Cook & Vizy, 2008). There is, however, little consensus concerning changes in the central Amazon basin forests, where droughts are expected to occur less frequently (Salazar et al., 2007) but where changes in temperature and rainfall regimes are strongly dependent on the synergistic effects of deforestation and fire (Hutyra et al., 2005). Although model simulations are only indicators of the potential distribution shifts caused by climate change, the models’ results coincide with distribution patterns that occurred in the Amazon basin rainforests during the LGM (Fig. 3d and h).
The numerous models for future Amazon landcover show that the dry eastern part of the basin will most likely suffer from a replacement of evergreen forest by savanna-like vegetation (e.g., Nepstad et al., 2008; Cook et al., 2012) (Fig. 3h). Assuming that (i) future climatic conditions are similar to the period between 1996–2005 (i.e., drier and warmer) (ii) precipitation decreases by 10% and (iii) tree drought tolerance is exceeded when available soil water is less than 30%; then a 4% decrease in forest cover will occur in the eastern and southern parts of the basin by 2030 (Nepstad et al., 2008). Other models showed that the effect of 2°C warming combined with 20% less precipitation will cause a reduction in forest cover by 11%, and further increases of up to 4°C will result in the permanent loss of 20% forest cover (Hirota et al., 2010). Nevertheless, it is also possible that a similar decline in precipitation will not cause major changes in the distribution of rainforests, but a decrease in total biomass of up to 4.6% (Galbraith et al., 2010). Still, current estimates may be rather conservative. Abundant georeferencing errors inflating species ranges and increasing temperature and precipitation tolerances of tropical plants exist and might be included in some of the models (Feeley & Silman, 2010b). Identification errors are also an important issue that causes differences in plants’ distribution ranges; however, currently no assessment of this factor and its effects on modelling outcomes exist. Additionally, not all models include the significant effect of vegetation on rainfall (Spracklen et al., 2012) and the feedbacks between deforestation, forest fires (Aragão et al., 2008; Cochrane & Laurance, 2002) and drought (Hutyra et al., 2005) that largely determine the moisture circulation patterns in Amazonian forests. In fact, South America lost ~16% of rainforests from 2000–2012 mainly due to deforestation (Hansen et al., 2013). As such, during the coming decades deforestation feedback on drought could be the main driver of future biomass and forest cover decline in the Amazon basin (Spracklen et al., 2012; Stickler et al., 2013).
Species diversity
If the dry season is prolonged, increasing water stress will cause directional shifts in species composition throughout the Amazonian region. Forests would support less moisture-adapted species and in general less and lower vegetation (Butt et al., 2008; Hartmann, 2011), an effect previously documented in Central American forests (Condit et al., 1996; Enquist & Enquist, 2011). Nevertheless, there is evidence that this would be a consequence of species’ drought sensitivity and not of the trade-off between shade and drought tolerance (Engelbrecht et al., 2007). A pervasive increase in dominance of lianas across terra firme forest in the Amazon basin occurred over the period 1979–1999 (Phillips et al., 2002). Lianas were hypothesized to thrive in rainforest communities at the expense of trees, because they efficiently extract water from deep soil layers (Restom & Nepstad, 2001), and also because they seem to benefit from increasing CO2 concentrations (Phillips et al., 2002). However, in sharp contrast to this hypothesis, lianas were shown to be highly sensitive to experimental drought conditions, with mortality rates of 70% or higher than the control (Nepstad et al., 2007). High mortality in lianas may be the result of physical constraints, such as wide vessels and narrow stems, which make them more vulnerable than trees to xylem embolism (Putz et al., 1989). Hence, whether lianas are prone to thrive or suffer with longer dry seasons remains elusive. Fast-growing canopy tree species have also increased in dominance over the last decades in the Amazon basin despite the high tree mortality caused after numerous ENSO events (Laurance et al., 2004). However, increases in lianas and fast-growing trees may only be a temporary effect caused by drought events (Table 1).
Species of Eschweilera and Inga are hyperdominant canopy trees in Amazonian forests (ter Steege et al., 2013) and both are highly sensitive to drought (Nepstad et al., 2007; da Costa et al., 2010). During 1997, an intense drought in the central Amazon basin caused high mortality of mostly canopy and overstory tree species, which suggests low tolerance of large trees to high water stress (Phillips et al., 2010; Laurance et al., 2001). In contrast, other studies showed that, despite numerous drought events, basal area and density of fast-growing, canopy and emergent trees increased over the last two decades, whereas some slow-growing, subcanopy or understory trees declined in numerous terra firme forests (Laurance et al., 2004). The discrepancy between these findings could be due to different drought intensities. Initially, moderate drought events cause mortality of several understory trees. Then reduced competition promotes the growth of the surviving trees (usually tall trees) (Butt et al., 2012; Williamson et al., 2000; Laurance et al., 2004; Phillips et al., 2009b), but mortality of tall trees magnifies when 60% of the available soil water is depleted (Nepstad et al., 2007). Thus, under low intensity and frequency of drought events, we might expect large trees to persist and dominate. Indeed, current distribution patterns of diversity (Wright, 1992) and long-term monitoring studies (Condit, 1998; Enquist & Enquist, 2011) attest to the decline of understory species with the increase in dry season length. The loss of understory drought-intolerant species would result in generalist communities with low species diversity (Butt et al., 2012). Highly intense and frequent droughts would result in larger changes in community composition. The loss of canopy and emergent trees results in open canopies, where subcanopy and understory species would disappear by warming and drought in the forest interior (Nepstad et al., 2008). In addition, increased fire frequency and proportional changes in species diversity are currently expected more as a consequence of land use than as a consequence of climatic shifts (e.g., Aragão et al., 2008; Cochrane & Barber, 2009). Although high variation in fire-related mortality exists across the Amazon basin, small-statured species might be particularly vulnerable (e.g., Balch et al., 2011).
Drought responses in Amazonian flooded forests have been less studied. Drought tolerance is expected in some flood-tolerant species, such as Nectandra amazonum, Pseudobombax munguba and Astrocaryum jauari. These species either maintain or decrease CO2 assimilation and decrease root respiration (Parolin et al., 2010). However, high mortality of seedlings observed in floodplains during extended dry periods show that tolerance level might be moderate (Parolin, 2001). The physiological mechanisms that determine drought resistance in flood-tolerant trees are similar to those on the terra firme forests. These mainly include the access to water layers through deep roots and the maintenance of carbon assimilation under mild-drought conditions (Parolin et al., 2010).
It is important to note that the length of the dry season may not be a cause for water deficit itself. One study compared the effect of dry season length by comparing two seasonal forests in the eastern Amazon basin (Fisher et al., 2008). The first site located in Manaus, received less rainfall (2,088 mm year−1) than the second site in Caxiuanã (2,350 mm year−1), and the dry season lasted about 7 and 5 months respectively. Unlike Caxiuanã, the Manaus site experienced a strong decline in evapotranspiration during the dry period but was unrelated to soil water deficit, which was highly similar (−214 mm at Manaus vs. –210 mm at Caxiuanã). The availability of soil water was however at least two times lower in Manaus (with low porosity clay soils) than in Caxiuanã (with highly porous sandy soils). As a consequence plants exhibit different responses to drought depending on soil porosity. Overall, in soils with low water holding capacity, trees are more vulnerable and likely to suffer water stress (Fisher et al., 2008, and references therein). Finally, it is interesting to note that in sandy soils the access of trees to deep-water layers is more frequent than in their clayey counterparts (Hacke et al., 2000), which must also be taken into account when assessing drought responses among different plant communities. We still have to document and thoroughly study plant responses to seasonal drought to draw robust conclusions on which species are particularly vulnerable (Table 1).
Ecosystem Functioning
Initial studies evaluating the effect of ENSO drought events reported mortality rates of 1.1% yr−1 (Williamson et al., 2000; Laurance et al., 2001) and 2.4% yr−1 (Laurance et al., 2001) for old growth and fragmented central Amazon forests respectively. These studies reported a weak effect of drought on forest dynamics, because tree mortality rates recover to pre-drought values after one year, and neither composition nor structure were significantly affected. Yet, projections agree on the negative effect of drought on above ground biomass (Cowling & Shin, 2006; Poulter et al., 2010). Extensive drought in 2010 caused the loss of 2.2 Pg C through the interruption of growth and high tree mortality (Lewis et al., 2011). Additional surveys found that long dry periods cause reduced LAI values (Nepstad et al., 2002; Fisher et al., 2007; Meir et al., 2008), lower Above Ground Net Primary Productivity (Brando et al., 2008) and lower root growth (Metcalfe et al., 2008).
In sum, longer dry seasons can alter rainforests structure and carbon dynamics due to high biomass losses and decreased canopy coverage. A caveat is that most studies relate to short monitoring periods, which may not adequately capture forests behaviour trends (Enquist & Enquist, 2011). Long-term surveys over several decades would be needed to evidence any directional or selective effect of drought on tropical forest functioning.
Synthesis
We here reviewed current understanding of the processes and mechanisms involved in observed changes in distribution, diversity and functioning of the Amazon basin’s forests. Overall, longer and intense dry periods will contract forests along the eastern and south-eastern borders of the region, and perhaps to a similar extent as under the LGM. These droughts might cause directional changes in species composition, and in turn switch forests from CO2 sinks to sources due to high biomass losses and decreased canopy coverage. Warming causes a significant decline in plants productivity. Models predict that the ability of plants to adapt to rising temperatures and increasing drought will be a strong determinant of future forests distribution, diversity and ecosystem functioning in the Amazon basin.
During the early Holocene, when the Amazon became warmer, temperatures increased by approximately 0.01°C decade−1 (Bush et al., 2004). Currently, temperature increases by 0.25°C decade−1 (Malhi & Wright, 2004) and models predict a rate of 0.6°C decade−1 by the end of the 21st century (IPCC, 2007). Compared to the Holocene, current-warming rates in the Amazon basin are considerably higher and additional changes in precipitation regimes and dry season length are expected over the coming decades. By the end of the 21st century, longer and more intense dry periods as well as more frequent fires would be the mechanisms driving distribution-range shifts (Hutyra et al., 2005; Cook & Vizy, 2008) and diversity changes (Wright, 1992) in rainforest communities. Although it is uncertain which taxa will benefit from the novel climate, we may expect analogous changes to the ones experienced during Holocene dry periods. Climate scenarios suggest that reductions in precipitation are likely to promote savannization. As such, it is plausible that there will be small areas of rainforests in the wetter localities surrounded by a rather dry area dominated by savanna vegetation.
Savannization is more likely to affect species with small range sizes (Wang et al., 2013). which usually concentrate in areas that have been climatically stable in the past, but are susceptible to future changes (Sandel et al., 2011). Such areas include western and central Amazon basin, where deforestation could change the regional water balance. In addition, those species whose range includes the southeastern extensions of the Amazon forests are under severe risk of extinction due to high rates of habitat loss (Feeley & Silman, 2009). Consequently, local extinctions and constrained rainforest areas make a more plausible scenario than adaptation for most Amazonian plant species. In this scenario the stability of plant assemblages and their relation to animal communities could be highly threatened (Parmesan, 2006; Walther et al., 2002). The decline in tree abundance and the loss of large areas of rainforest will cause a decrease in evaporation rates and carbon storage, which at the ecosystem functioning level will generate shifts in carbon and water dynamics. If drought, fires and deforestation continue the suppression of large tree species and in general of large forest areas, Amazonian rainforests will release significant amounts of CO2 (Nepstad et al., 2007, 2008) and have poor water recycling (Spracklen et al., 2012; Stickler et al., 2013).
Preservation of Amazonian forests depends on prompt actions to (1) reduce greenhouse gas emissions which would prevent or, at least, postpone warming by two degrees that would lead to prolonged and more frequent dry periods, (2) reduce deforestation rates through effective land use planning, (3) reforest and preserve forest areas and (4) maintain corridors connecting the lowlands to upslope environments (Fig. 2). Further studies are needed on the physiological and ecological responses of plant communities to warming and edaphic changes, and also on the feedbacks between vegetation and climate (Table 1). In particular, experimental assays that allow direct conclusions on the response of plants to the predicted climatic conditions are needed. The results of these studies will enable us to produce more reliable estimates of future climatic and vegetation responses, and in doing so to formulate and strengthen effective conservation strategies.
Literature Cited
Allen, C. D., A. K. Macalady, H. Chenchouni, D. Bachelet, N. McDowell, M. Vennetier, T. Kitzberger, A. Rigling, D. D. Breshears, E. Hogg, P. Gonzalez, R. Fensham, et al. 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management 259: 660–684.
Anderson, A. B. 1981. White-sand vegetation of Brazilian Amazonia. Biotropica 13: 199–210.
Anhuf, D., M.-P. Ledru & H. Behling. 2006. Paleo-environmental change in Amazonian and African rainforest during the LGM. Palaeogeography, Palaeoclimatology, Palaeoecology 239: 510–527.
Aragão, L. E. O., Y. Malhi, N. Barbier, A. Lima, Y. Shimabukuro, L. Anderson & S. Saatchi. 2008. Interactions between rainfall, deforestation and fires during recent years in the Brazilian Amazonia. Philosophical Transactions of the Royal Society B: Biological Sciences 363: 1779–1785.
———, ———, D. Metcalfe, J. Silva-Espejo, E. Jiménez, D. Navarrete, S. Almeida, A. Costa, N. Salinas & O. Phillips. 2009a. Above-and below-ground net primary productivity across ten Amazonian forests on contrasting soils. Biogeosciences 6: 2759–2778.
Balch, J. K., D. C. Nepstad, L. M. Curran, P. M. Brando, O. Portela, P. Guilherme, J. D. Reuning-Scherer & O. de Carvalho. 2011. Size, species, and fire behavior predict tree and liana mortality from experimental burns in the Brazilian Amazon. Forest Ecology and Management 261: 68–77.
Balslev, H., J. Luteyn, B. Ilgaard & L. Im-Nielsen. 1987. Composition and structure of adjacent unflooded and floodplain forest in Amazonian Ecuador. Opera Botanica 92: 37–57.
Behling, H. & H. Hooghiemstra. 2000. Holocene Amazon rainforest-savanna dynamics and climatic implications: high-resolution pollen record from Laguna Loma Linda in eastern Colombia. Journal of Quaternary Science 15: 687–695.
———, G. Keim, G. Irion, W. Junk & J. De Mello. 2001. Holocene environmental changes in the central Amazon Basin inferred from Lago Calado (Brazil). Palaeogeography, Palaeoclimatology, Palaeoecology 173: 87–101.
Bermingham, E. & C. Dick. 2001. The Inga–Newcomer or Museum Antiquity? Science 293: 2214–2216.
Betts, R., P. Cox, M. Collins, P. Harris, C. Huntingford & C. Jones. 2004. The role of ecosystem-atmosphere interactions in simulated Amazonian precipitation decrease and forest dieback under global climate warming. Theoretical and Applied Climatology 78: 157–175.
Brando, P. M., D. C. Nepstad, E. A. Davidson, S. E. Trumbore, D. Ray & P. Camargo. 2008. Drought effects on litterfall, wood production and belowground carbon cycling in an Amazon forest: results of a throughfall reduction experiment. Philosophical Transactions of the Royal Society B: Biological Sciences 363: 1839–1848.
Bush, M. B. 2002. Distributional change and conservation on the Andean flank: a palaeoecological perspective. Global Ecology and Biogeography 11: 463–473.
——— & J. Flenley. 2007. Tropical rainforest responses to climatic change. Springer-Verlag, Berlin, Heidelberg.
———, M. R. Silman & D. H. Urrego. 2004. 48,000 years of climate and forest change in a biodiversity hot spot. Science 303: 827–829.
———, M. Silman & C. Listopad. 2007. A regional study of Holocene climate change and human occupation in Peruvian Amazonia. Journal of Biogeography 34: 1342–1356.
———, Flenley, J. & W. D. Gosling. 2011. Tropical rainforest responses to climatic change, ed. 2nd. Springer-Verlag, Berlin, Heidelberg.
———, M. Silman, C. McMichael & S. Saatchi. 2008. Fire, climate change and biodiversity in Amazonia: a Late-Holocene perspective. Philosophical Transactions of the Royal Society B: Biological Sciences 363: 1795–1802.
Butt, N., Y. Malhi, O. Phillips & M. New. 2008. Floristic and functional affiliations of woody plants with climate in western Amazonia. Journal of Biogeography 35: 939–950.
———, ———, M. New, M. J. Macía, S. L. Lewis, G. Lopez-Gonzalez, W. F. Laurance, S. Laurance, R. Luizão & A. Andrade. 2012. Shifting dynamics of climate–functional groups in old-growth Amazonian forests. Plant Ecology & Diversity: 1–13.
Campbell, D. G., D. C. Daly, G. T. Prance & U. N. Maciel. 1986. Quantitative ecological inventory of terra firme and várzea tropical forest on the Rio Xingu, Brazilian Amazon. Brittonia 38: 369–393.
Cernusak, L. A., K. Winter, J. W. Dalling, J. A. Holtum, C. Jaramillo, C. Körner, A. D. Leakey, R. J. Norby, B. Poulter & B. L. Turner. 2013. Tropical forest responses to increasing atmospheric CO2: current knowledge and opportunities for future research. Functional Plant Biology 40: 531–551.
Clark, D. A. 2004. Sources or sinks? The responses of tropical forests to current and future climate and atmospheric composition. Philosophical Transactions of the Royal Society B: Biological Sciences 359: 477–491.
Clinebell, R. R., O. L. Phillips, A. H. Gentry, N. Stark & H. Zuuring. 1995. Prediction of neotropical tree and liana species richness from soil and climatic data. Biodiversity and Conservation 4: 56–90.
Cochrane, M. A. 2003. Fire science for rainforests. Nature 421: 913–919.
——— & M. D. Schulze. 1999. Fire as a recurrent event in tropical forests of the eastern Amazon: effects on forest structure, biomass, and species composition. Biotropica 31: 2–16.
——— & W. F. Laurance. 2002. Fire as a large-scale edge effect in Amazonian forests. Journal of Tropical Ecology 18: 311–325.
——— & C. P. Barber. 2009. Climate change, human land use and future fires in the Amazon. Global Change Biology 15: 601–612.
Colwell, R. K., G. Brehm, C. L. Cardelús, A. C. Gilman & J. T. Longino. 2008. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 322: 258–261.
Condit, R. 1998. Ecological implications of changes in drought patterns: shifts in forest composition in Panama. Climatic Change 39: 413–427.
———, S. P. Hubbell & R. B. Foster. 1996. Changes in tree species abundance in a neotropical forest: impact of climate change. Journal of Tropical Ecology 12: 231–256.
Cook, K. H. & E. K. Vizy. 2008. Effects of twenty-first-century climate change on the Amazon rain forest. Journal of Climate 21: 542–560.
Cook, B., N. Zeng & J. H. Yoon. 2012. Will Amazonia dry out? Magnitude and causes of change from IPCC climate model projections. Earth Interactions 16: 1–27.
Coppola, E. & F. Giorgi. 2005. Climate change in tropical regions from high-resolution time-slice AGCM experiments. Quarterly Journal of the Royal Meteorological Society 131: 3123–3145.
Costa, M. H. & J. A. Foley. 1997. Water balance of the Amazon Basin: Dependence on vegetation cover and canopy conductance. Journal of Geophysical Research: Atmospheres 102: 23973–23989.
——— & ———. 2000. Combined effects of deforestation and doubled atmospheric CO2 concentrations on the climate of Amazonia. Journal of Climate 13: 18–34.
Cowling, S. A. & Y. Shin. 2006. Simulated ecosystem threshold responses to co‐varying temperature, precipitation and atmospheric CO2 within a region of Amazonia. Global Ecology and Biogeography 15: 553–566.
Cox, P. M., R. Betts, M. Collins, P. Harris, C. Huntingford & C. Jones. 2004. Amazonian forest dieback under climate-carbon cycle projections for the 21st century. Theoretical and Applied Climatology 78: 137–156.
———, P. P. Harris, C. Huntingford, R. A. Betts, M. Collins, C. D. Jones, T. E. Jupp, J. A. Marengo & C. A. Nobre. 2008. Increasing risk of Amazonian drought due to decreasing aerosol pollution. Nature 453: 212–215.
Chambers, J. Q. & W. L. Silver. 2004. Some aspects of ecophysiological and biogeochemical responses of tropical forests to atmospheric change. Philosophical Transactions of the Royal Society B: Biological Sciences 359: 463–476.
Cheng, H., A. Sinha, F. W. Cruz, X. Wang, R. L. Edwards, F. M. d’Horta, C. C. Ribas, M. Vuille, L. D. Stott & A. S. Auler. 2013. Climate change patterns in Amazonia and biodiversity. Nature 4: 1411.
Choat, B., S. Jansen, T. J. Brodribb, H. Cochard, S. Delzon, R. Bhaskar, S. J. Bucci, T. S. Feild, S. M. Gleason & U. G. Hacke. 2012. Global convergence in the vulnerability of forests to drought. Nature 491:752–755.
da Costa, A. C. L., D. Galbraith, S. Almeida, B. T. T. Portela, M. da Costa, J. de Athaydes Silva Junior, A. P. Braga, P. H. de Gonçalves, A. A. de Oliveira & R. Fisher. 2010. Effect of 7 yr of experimental drought on vegetation dynamics and biomass storage of an eastern Amazonian rainforest. New Phytologist 187: 579–591.
da Silva Meneses, M. E. N., M. L. da Costa & H. Behling. 2013. Late Holocene vegetation and fire dynamics from a savanna-forest ecotone in Roraima state, northern Brazilian Amazon. Journal of South American Earth Sciences 42: 17–26.
Dai, A. 2011. Drought under global warming: a review. Wiley Interdisciplinary Reviews: Climate Change 2: 45–65.
Damasco, G., A. Vicentini, C. V. Castilho, T. P. Pimentel & H. E. Nascimento. 2013. Disentangling the role of edaphic variability, flooding regime and topography of Amazonian white‐sand vegetation. Journal of Vegetation Science 24: 384–394.
Davidson, E. A., A. C. de Araújo, P. Artaxo, J. K. Balch, I. F. Brown, M. M. Bustamante, M. T. Coe, R. S. DeFries, M. Keller, M. Longo, J. W. Munger & W. Schroeder. 2012. The Amazon basin in transition. Nature 481: 321–328.
De Freitas, H. A., L. C. R. Pessenda, R. Aravena, S. E. M. Gouveia, A. S. Ribeiro & R. Boulet. 2001. Late Quaternary vegetation dynamics in the southern Amazon Basin inferred from carbon isotopes in soil organic matter. Quaternary Research 55: 39–46.
Dick, C. W., S. L. Lewis, M. Maslin & E. Bermingham. 2013. Neogene origins and implied warmth tolerance of Amazon tree species. Ecology and evolution 3: 162–169.
Doughty, C. E. 2011. An in situ leaf and branch warming experiment in the Amazon. Biotropica 43: 658–665.
——— & M. L. Goulden. 2008. Are tropical forests near a high temperature threshold? Journal of Geophysical Research 113: 1–12.
Eltahir, E. A. 1996. Role of vegetation in sustaining large-scale atmospheric circulations in the tropics. Journal of Geophysical Research 101: 4255–4268.
Engelbrecht, B. M., L. S. Comita, R. Condit, T. A. Kursar, M. T. Tyree, B. L. Turner & S. P. Hubbell. 2007. Drought sensitivity shapes species distribution patterns in tropical forests. Nature 447: 80–82.
Enquist, B. J. & C. A. Enquist. 2011. Long-term change within a Neotropical forest: assessing differential functional and floristic responses to disturbance and drought. Global Change Biology 17: 1408–1424.
Eva, H. D. & O. Huber (eds). 2005. A proposal for defining the geographical boundaries of Amazonia, Rep. EUR 21808-E. Office for Official Publications of the European Communities, Luxembourg.
Fearnside, P. M. 2009. Global warming in Amazonia: impacts and mitigation. Acta Amazonica 39: 1003–1011.
Feeley, K. J. 2012. Distributional migrations, expansions, and contractions of tropical plant species as revealed in dated herbarium records. Global Change Biology 18: 1335–1341.
——— & M. R. Silman. 2009. Extinction risks of Amazonian plant species. Proceedings of the National Academy of Sciences 106: 12382–12387.
——— & ———. 2010a. Biotic attrition from tropical forests correcting for truncated temperature niches. Global Change Biology 16: 1830–1836.
——— & ———. 2010b. Modelling the responses of Andean and Amazonian plant species to climate change: the effects of georeferencing errors and the importance of data filtering. Journal of Biogeography 37: 733–740.
——— & E. M. Rehm. 2012. Amazon’s vulnerability to climate change heightened by deforestation and man-made dispersal barriers. Global Change Biology 18: 3606–3614.
———, ——— & B. Machovina. 2012a. The responses of tropical forest species to global climate change: acclimate, adapt, migrate, or go extinct? Frontiers of Biogeography 4: 69–84.
———, Y. Malhi, P. Zelazowski & M. R. Silman. 2012b. The relative importance of deforestation, precipitation change, and temperature sensitivity in determining the future distributions and diversity of Amazonian plant species. Global Change Biology 18: 2636–2647.
Fernandes, A. M., M. Wink, C. H. Sardelli & A. Aleixo. 2014. Multiple speciation across the Andes and throughout Amazonia: the case of the spot-backed antbird species complex (Hylophylax naevius/Hylophylax naevioides). Journal of Biogeography 41: 1094–1104.
Fisher, R. A., M. Williams, M. de Lourdes Ruivo, A. L. de Costa & P. Meir. 2008. Evaluating climatic and soil water controls on evapotranspiration at two Amazonian rainforest sites. Agricultural and Forest Meteorology 148: 850–861.
———, ———, A. L. da Costa, Y. Malhi, R. F. da Costa, S. Almeida & P. Meir. 2007. The response of an Eastern Amazonian rain forest to drought stress: results and modelling analyses from a throughfall exclusion experiment. Global Change Biology 13: 2361–2378.
———, N. McDowell, D. Purves, P. Moorcroft, S. Sitch, P. Cox, C. Huntingford, P. Meir & F. Ian Woodward. 2010. Assessing uncertainties in a second-generation dynamic vegetation model caused by ecological scale limitations. New Phytologist 187: 666–681.
François, L. M., Y. Goddéris, P. Warnant, G. Ramstein, N. de Noblet & S. Lorenz. 1999. Carbon stocks and isotopic budgets of the terrestrial biosphere at mid-Holocene and last glacial maximum times. Chemical geology 159: 163–189.
Galbraith, D., P. E. Levy, S. Sitch, C. Huntingford, P. Cox, M. Williams & P. Meir. 2010. Multiple mechanisms of Amazonian forest biomass losses in three dynamic global vegetation models under climate change. New Phytologist 187: 647–665.
Gentry, A. H. 1988. Changes in plant community diversity and floristic composition on environmental and geographical gradients. Annals of the Missouri Botanical Garden 75: 1–34.
Ghannoum, O. & D. A. Way. 2011. On the role of ecological adaptation and geographic distribution in the response of trees to climate change. Tree Physiology 31: 1273–1276.
Goulden, M. L., S. D. Miller, H. R. Da Rocha, M. C. Menton, H. C. de Freitas, A. M. E. Silva Figueira & C. A. D. de Sousa. 2004. Diel and seasonal patterns of tropical forest CO2 exchange. Ecological Applications 14: 42–54.
Grace, J., J. Lloyd, J. Mcintyre, A. Miranda, P. Meir, H. Miranda, J. Moncrieff, J. Massheder, I. Wright & J. Gash. 1995. Fluxes of carbon dioxide and water vapour over an undisturbed tropical forest in south-west Amazonia. Global Change Biology 1: 1–12.
Graham, A. 2011. The age and diversification of terrestrial New World ecosystems through Cretaceous and Cenozoic time. American Journal of Botany 98: 336–351.
Hacke, U., J. Sperry, B. Ewers, D. Ellsworth, K. Schäfer & R. Oren. 2000. Influence of soil porosity on water use in Pinus taeda. Oecologia 124: 495–505.
———, ———, W. T. Pockman, S. D. Davis & K. A. McCulloh. 2001. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126: 457–461.
Hansen, M., P. Potapov, R. Moore, M. Hancher, S. Turubanova, A. Tyukavina, D. Thau, S. Stehman, S. Goetz & T. Loveland. 2013. High-Resolution Global Maps of 21st Century Forest Cover Change. Science 342: 850–853.
Harris, P. P., C. Huntingford & P. M. Cox. 2008. Amazon basin climate under global warming: the role of the sea surface temperature. Philosophical Transactions of the Royal Society B: Biological Sciences 363: 1753–1759.
Hartmann, H. 2011. Will a 385 million year-struggle for light become a struggle for water and for carbon?–How trees may cope with more frequent climate change-type drought events. Global Change Biology 17: 642–655.
Higgins, P. A. 2007. Biodiversity loss under existing land use and climate change: an illustration using northern South America. Global Ecology and Biogeography 16: 197–204.
Higgins, M. A., K. Ruokolainen, H. Tuomisto, N. Llerena, G. Cardenas, O. L. Phillips, R. Vásquez & M. Räsänen. 2011. Geological control of floristic composition in Amazonian forests. Journal of Biogeography 38: 2136–2149.
Hirota, M., C. Nobre, M. D. Oyama & M. Bustamante. 2010. The climatic sensitivity of the forest, savanna and forest-savanna transition in tropical South America. New Phytologist 187: 707–719.
Honorio, C. E. N., T. R. Pennington & L. A. Freitas. 2008. Análisis de la composición florística de los bosques de Jenaro Herrera, Loreto, Perú. Revista Peruana de Biología 15: 53–60.
———, K. G. Dexter, M. F. Poelchau, P. M. Hollingsworth, O. L. Phillips & T. R. Pennington. 2014. Ficus insipida subsp. insipida (Moraceae) reveals the role of ecology in the phylogeography of widespread Neotropical rain forest tree species. Journal of Biogeography. doi:10.1111/jbi.12326.
Hoorn, C., F. P. Wesselingh, H. ter Steege, M. A. Bermudez, A. Mora, J. Sevink, I. Sanmartín, A. Sanchez–Meseguer, C. L. Anderson, J. P. Figueiredo, C. Jaramillo, D. Riff, et al. 2010. Amazonia through time: Andean uplift, cimate change, landscape evolution, and biodiversity. Science 330: 927–931.
Hubbell, S. P., F. He, R. Condit, L. Borda-de-Agua, J. Kellner & H. ter Steege. 2008. How many tree species are there in the Amazon and how many of them will go extinct? Proceedings of the National Academy of Sciences 105: 11498–11504.
Huntingford, C., P. Zelazowski, D. Galbraith, L. M. Mercado, S. Sitch, R. Fisher, M. Lomas, A. P. Walker, C. D. Jones & B. B. Booth. 2013. Simulated resilience of tropical rainforests to CO2-induced climate change. Nature Geoscience 6: 268–273.
Hutyra, L., J. Munger, C. Nobre, S. Saleska, S. Vieira & S. Wofsy. 2005. Climatic variability and vegetation vulnerability in Amazonia. Geophysical Research Letters 32, L24712.
IPCC. 2001. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change (IPCC). University Press, Cambridge, UK, Cambridge. Climatic Change 2001: The Scientific Basis.
——— 2007. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC). University Press, Cambridge, UK, Cambridge. Climate Change 2007: The Physical Science Basis.
Jaramillo, C., M. J. Rueda & G. Mora. 2006. Cenozoic plant diversity in the Neotropics. Science 311: 1893–1896.
———, D. Ochoa, L. Contreras, M. Pagani, H. Carvajal-Ortiz, L. M. Pratt, S. Krishnan, A. Cardona, M. Romero, L. Quiroz, G. Rodriguez, M. J. Rueda, et al. 2010. Effects of rapid global warming at the Paleocene-Eocene boundary on Neotropical vegetation. Science 330: 957–961.
Jipp, P. H., D. C. Nepstad, D. Cassel & C. R. De Carvalho. 1998. Deep soil moisture storage and transpiration in forests and pastures of seasonally-dry Amazonia. Climatic Change 39: 395–412.
Johns, T., J. Gregory, W. Ingram, C. Johnson, A. Jones, J. Lowe, J. Mitchell, D. Roberts, D. Sexton & D. Stevenson. 2003. Anthropogenic climate change for 1860 to 2100 simulated with the HadCM3 model under updated emissions scenarios. Climate Dynamics 20: 583–612.
Jones, P., M. New, D. Parker, S. Martin & I. Rigor. 1999. Surface air temperature and its changes over the past 150 years. Reviews of Geophysics 37: 173–199.
Junk, W. J. & M. T. F. Piedade. 2011. An introduction to South American wetland forests: distribution, definitions and general characterization. Pp 3–25. In: W. J. Junk, M. T. F. Piedade, F. Wittmann, J. Schöngart, & P. Parolin (eds). Amazonian floodplain forests. Ecophysiology, biodiversity and sustainable management. Springer, New York.
Kalliola, R., M. Puhakka, J. Salo, H. Tuomisto & K. Ruokolainen. 1991. The dynamics, distribution and classification of swamp vegetation in Peruvian Amazonia. Annales Botanici Fennici 28: 225–239.
Keller, M. & M. Lerdau. 1999. Isoprene emission from tropical forest canopy leaves. Global Biogeochemical Cycles 13: 19–29.
Koch, G. W., J. S. Amthor & M. L. Goulden. 1994. Diurnal patterns of leaf photosynthesis, conductance and water potential at the top of a lowland rain forest canopy in Cameroon: measurements from the Radeau des Cimes. Tree Physiology 14: 347–360.
Korning, J. & H. Balslev. 1994. Growth rates and mortality patterns of tropical lowland tree species and the relation to forest structure in Amazonian Ecuador. Journal of Tropical Ecology 10: 151–166.
Krause, G. H., K. Winter, B. Krause, P. Jahns, M. García, J. Aranda & A. Virgo. 2010. High-temperature tolerance of a tropical tree, Ficus insipida: methodological reassessment and climate change considerations. Functional Plant Biology 37: 890–900.
Laurance, W. F., G. B. Williamson, P. Delamónica, A. Oliveira, T. E. Lovejoy, C. Gascon & L. Pohl. 2001. Effects of a strong drought on Amazonian forest fragments and edges. Journal of Tropical Ecology 17: 771–785.
———, A. A. Oliveira, S. G. Laurance, R. Condit, H. E. Nascimento, A. C. Sanchez-Thorin, T. E. Lovejoy, A. Andrade, S. D’Angelo & J. E. Ribeiro. 2004. Pervasive alteration of tree communities in undisturbed Amazonian forests. Nature 428: 171–175.
Le Quéré, C., R. J. Andres, T. Boden, T. Conway, R. A. Houghton, J. I. House, G. Marland, G. P. Peters, G. van der Werf, A. Ahlström, R. M. Andrew, L. Bopp, et al. 2013. The global carbon budget 1959–2011. Earth System Science Data Discussions 5: 1107–1157.
Ledru, M. P., M. L. Salgado-Labouriau & M. L. Lorscheitter. 1998. Vegetation dynamics in southern and central Brazil during the last 10,000 yr BP. Review of Palaeobotany and Palynology 99: 131–142.
Lewis, S. L., O. L. Phillips, T. R. Baker, J. Lloyd, Y. Malhi, S. Almeida, N. Higuchi, W. F. Laurance, D. A. Neill, J. N. M. Silva, J. Terborgh, A. Torres Lezama, et al. 2004. Concerted changes in tropical forest structure and dynamics: evidence from 50 South American long-term plots. Philosophical Transactions of the Royal Society B: Biological Sciences 359: 421–436.
———, P. M. Brando, O. L. Phillips, G. M. van der Heijden, D. Nepstad, et al. 2011. The 2010 Amazon Drought. Science 331: 554–554.
Li, W., R. Fu & R. E. Dickinson. 2006. Rainfall and its seasonality over the Amazon in the 21st century as assessed by the coupled models for the IPCC AR4. Journal of Geophysical Research 111: 1–14.
Lloyd, J. & G. D. Farquhar. 2008. Effects of rising temperatures and [CO2] on the physiology of tropical forest trees. Philosophical Transactions of the Royal Society B: Biological Sciences 363: 1811–1817.
Lovelock, C. E., A. Virgo, M. Popp & K. Winter. 1999. Effects of elevated CO2 concentrations on photosynthesis, growth and reproduction of branches of the tropical canopy tree species, Luehea seemannii Tr. & Planch. Plant, Cell & Environment 22: 49–59.
Malhi, Y. & J. Wright. 2004. Spatial patterns and recent trends in the climate of tropical rainforest regions. Philosophical Transactions of the Royal Society B: Biological Sciences 359: 311–329.
———, J. T. Roberts, R. A. Betts, T. J. Killeen, W. Li & C. A. Nobre. 2008. Climate change, deforestation, and the fate of the Amazon. Science 319: 169–172.
———, L. E. Aragão, D. Galbraith, C. Huntingford, R. Fisher, P. Zelazowski, S. Sitch, C. McSweeney & P. Meir. 2009. Exploring the likelihood and mechanism of a climate-change-induced dieback of the Amazon rainforest. Proceedings of the National Academy of Sciences 106: 20610–20615.
———, T. R. Baker, O. L. Phillips, S. Almeida, E. Alvarez, L. Arroyo, J. Chave, C. I. Czimczik, A. Di Fiore & N. Higuchi. 2004. The above-ground coarse wood productivity of 104 Neotropical forest plots. Global Change Biology 10: 563–591.
———, D. Wood, T. R. Baker, J. Wright, O. L. Phillips, T. Cochrane, P. Meir, J. Chave, S. Almeida & L. Arroyo. 2006. The regional variation of aboveground live biomass in old-growth Amazonian forests. Global Change Biology 12: 1107–1138.
Marengo, J. A. 2004. Interdecadal variability and trends of rainfall across the Amazon basin. Theoretical and Applied Climatology 78: 79–96.
——— 2007. Climate change and hydrological modes of the wet tropics. Pp 236–268. In: M. Bush & J. Flenley (eds). Tropical rainforest responses to climatic change. Springer-Verlag, Berlin, Heidelberg.
———, Nobre, C. A., G. Sampaio, L. Salazar & L. Borma. 2011. Climate change in the Amazon Basin: tipping points, changes in extremes, and impacts on natural and human systems. Pp 259–283. In: M. Bush, J. Flenley, & W. D. Gosling (eds). Tropical rainforest responses to climatic change, ed. 2nd. Springer-Verlag, Berlin, Heidelberg.
———, ———, J. Tomasella, M. D. Oyama, G. de Sampaio Oliveira, R. de Oliveira, H. Camargo, L. M. Alves & I. F. Brown. 2008. The drought of Amazonia in 2005. Journal of Climate 21: 495–516.
Maslin, M. A. & S. J. Burns. 2000. Reconstruction of the Amazon Basin effective moisture availability over the past 14,000 years. Science 290: 2285–2287.
Mayle, F. E. & M. J. Power. 2008. Impact of a drier Early-Mid-Holocene climate upon Amazonian forests. Philosophical Transactions of the Royal Society B: Biological Sciences 363: 1829–1838.
———, R. Burbridge & T. J. Killeen. 2000. Millennial-scale dynamics of southern Amazonian rain forests. Science 290: 2291–2294.
McDowell, N., W. T. Pockman, C. D. Allen, D. D. Breshears, N. Cobb, T. Kolb, J. Plaut, J. Sperry, A. West & D. G. Williams. 2008. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist 178: 719–739.
McElrone, A. J., J. Bichler, W. T. Pockman, R. N. Addington, C. R. Linder & R. B. Jackson. 2007. Aquaporin-mediated changes in hydraulic conductivity of deep tree roots accessed via caves. Plant, Cell & Environment 30: 1411–1421.
Meir, P., D. B. Metcalfe, A. C. L. Costa & R. A. Fisher. 2008. The fate of assimilated carbon during drought: impacts on respiration in Amazon rainforests. Philosophical Transactions of the Royal Society B: Biological Sciences 363: 1849–1855.
Metcalfe, D. B., P. Meir, L. E. O. Aragão, A. C. da Costa, A. P. Braga, P. H. Gonçalves, J. D. A. S. Junior, S. S. de Almeida, L. A. Dawson & Y. Malhi. 2008. The effects of water availability on root growth and morphology in an Amazon rainforest. Plant and Soil 311: 189–199.
———, ———, ———, R. Lobo-do-Vale, D. Galbraith, R. Fisher, M. M. Chaves, J. Maroco, A. da Costa & S. de Almeida. 2010. Shifts in plant respiration and carbon use efficiency at a large-scale drought experiment in the eastern Amazon. New Phytologist 187: 608–621.
Moreira, L., P. Moreira-Turcq, R. Cordeiro, B. Turcq, S. Caquineau, J. Viana & N. Brandini. 2013. Holocene paleoenvironmental reconstruction in the eastern Amazonian basin: Comprido Lake. Journal of South American Earth Sciences 44: 55–62.
Myster, R. W. 2009. Plant communities of western Amazonia. The Botanical Review 75(3): 271–291.
Nepstad, D. C., C. M. Stickler, B. Soares-Filho & F. Merry. 2008. Interactions among Amazon land use, forests and climate: prospects for a near-term forest tipping point. Philosophical Transactions of the Royal Society B: Biological Sciences 363: 1737–1746.
———, I. M. Tohver, D. Ray, P. Moutinho & G. Cardinot. 2007. Mortality of large trees and lianas following experimental drought in an Amazon forest. Ecology 88: 2259–2269.
———, C. R. de Carvalho, E. A. Davidson, P. H. Jipp, P. A. Lefebvre, G. H. Negreiros, E. D. da Silva, T. A. Stone, S. E. Trumbore & S. Vieira. 1994. The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 372: 666–669.
———, P. Moutinho, M. B. Dias, E. Davidson, G. Cardinot, D. Markewitz, R. Figueiredo, N. Vianna, J. Chambers & D. Ray. 2002. The effects of partial throughfall exclusion on canopy processes, aboveground production, and biogeochemistry of an Amazon forest. Journal of Geophysical Research 107: 1–18.
NOAA. 2013. U.S. Department of Commerce, National Oceanic and Atmospheric Administration. Earth System Research Laboratory – Global Monitoring Division. Research news. http://www.esrl.noaa.gov/gmd/news/7074.html
Oliveira, R. S., T. E. Dawson, S. S. Burgess & D. C. Nepstad. 2005. Hydraulic redistribution in three Amazonian trees. Oecologia 145: 354–363.
Parmesan, C. 2006. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics 37: 637–669.
Parolin, P. 2001. Morphological and physiological adjustments to waterlogging and drought in seedlings of Amazonian floodplain trees. Oecologia 128: 326–335.
——— 2010. Flood-tolerant trees of Amazonian floodplains also tolerate drought. Pesquisas Botânica 61: 7–38.
———, C. Lucas, M. T. F. Piedade & F. Wittmann. 2010. Drought responses of flood-tolerant trees in Amazonian floodplains. Annals of botany 105: 129–139.
Phillips, O. L., S. Lewis, T. Baker & Y. Malhi. 2007. The response of South American tropical forests to contemporary atmospheric change. Pp 317–332. In: M. Bush & J. Flenley (eds). Tropical rainforest responses to climatic change. Springer Springer-Verlag, Berlin, Heidelberg.
———, N. Higuchi, S. Vieira, T. R. Baker, K. J. Chao & S. L. Lewis. 2009b. Changes in Amazonian forest biomass, dynamics, and composition, 1980–2002. Pp 373–387. In: M. Keller, M. Bustamante, J. Gash, & P. S. Dias (eds). Amazonia and global change. American Geophysical Union, Washington, D. C., USA.
———, R. V. Martínez, L. Arroyo, T. R. Baker, T. Killeen, S. L. Lewis, Y. Malhi, A. M. Mendoza, D. Neill & P. N. Vargas. 2002. Increasing dominance of large lianas in Amazonian forests. Nature 418: 770–774.
———, Aragão, L. E., S. L. Lewis, J. B. Fisher, J. Lloyd, G. López-González, Y. Malhi, A. Monteagudo, J. Peacock & C. A. Quesada. 2009a. Drought sensitivity of the Amazon rainforest. Science 323: 1344–1347.
———, G. van der Heijden, S. L. Lewis, G. López-González, L. E. Aragão, J. Lloyd, Y. Malhi, A. Monteagudo, S. Almeida & E. A. Dávila. 2010. Drought–mortality relationships for tropical forests. New Phytologist 187: 631–646.
Pires, J. M. & G. T. Prance. 1985. The vegetation types of the Brazilian Amazon. Pp 109–145. In: G. T. Prance & T. E. Lovejoy (eds). Key environments – Amazonia. Pergamon press, Oxford, England.
Poulter, B., F. Hattermann, E. Hawkins, S. Zaehle, S. Sitch, N. Restrepo-Coupe, U. Heyder & W. Cramer. 2010. Robust dynamics of Amazon dieback to climate change with perturbed ecosystem model parameters. Global Change Biology 16: 2476–2495.
Prance, G. T. 1979. Notes on the vegetation of Amazonia III. The terminology of Amazonian forest types subject to inundation. Brittonia 31: 26–38.
Putz, F. E., H. A. Mooney & S. H. Bullock. 1989. Biology of vines. Trends in Ecology & Evolution 4: 224–224.
Restom, T. G. & D. C. Nepstad. 2001. Contribution of vines to the evapotranspiration of a secondary forest in eastern Amazonia. Plant and Soil 236: 155–163.
Richards, P. W. 1996. Pp 352–359. The Tropical Rain Forest: An Ecological Study, ed. 2nd. Cambridge University Press, Cambridge, UK.
Rojas, M., A. Seth & S. A. Rauscher. 2006. Relationship between precipitation and moisture flux changes in the SRES A2 scenario for the South American monsoon region. CLARIS News 3: 14–20.
Ropelewski, C. F. & M. S. Halpert. 1987. Global and regional scale precipitation patterns associated with the El Niño/Southern Oscillation. Monthly Weather Review 115: 1606–1626.
Saatchi, S., R. Houghton, R. Dos Santos Alvala, J. Soares & Y. Yu. 2007. Distribution of aboveground live biomass in the Amazon basin. Global Change Biology 13: 816–837.
Salazar, L. F., C. A. Nobre & M. D. Oyama. 2007. Climate change consequences on the biome distribution in tropical South America. Geophysical Research Letters 34: 1–6.
Saleska, S. R., S. D. Miller, D. M. Matross, M. L. Goulden, S. C. Wofsy, H. R. da Rocha, P. B. de Camargo, P. Crill, B. C. Daube & H. C. de Freitas. 2003. Carbon in Amazon forests: unexpected seasonal fluxes and disturbance-induced losses. Science 302: 1554–1557.
Sandel, B., L. Arge, B. Dalsgaard, R. Davies, K. Gaston, W. Sutherland & J.-C. Svenning. 2011. The influence of Late Quaternary climate-change velocity on species endemism. Science 334: 660–664.
Scholze, M., W. Knorr, N. W. Arnell & I. C. Prentice. 2006. A climate-change risk analysis for world ecosystems. Proceedings of the National Academy of Sciences 103: 13116–13120.
Sevanto, S., N. G. McDowell, L. T. Dickman, R. Pangle & W. T. Pockman. 2013. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant, Cell & Environment.. doi:10.1111/pce.12141.
Silman, M. 2007. Plant species diversity in Amazonian forests. Pp 269–294. In: M. Bush & J. Flenley (eds). Tropical rainforest responses to climatic change. Springer Springer-Verlag, Berlin, Heidelberg.
Silva Dias, P. L., B. Turcq, M. A. F. Silva Dias, P. Braconnot & T. Jorgetti. 2009. Mid–Holocene climate of tropical South America: a model-data approach. Pp 259–281. In: F. Vimeux, F. Sylvestre, & M. Khodri (eds). Past climate variability in South America and surrounding regions. Springer-Verlag, Berlin, Heidelberg.
Soares-Filho, B. S., D. C. Nepstad, L. M. Curran, G. C. Cerqueira, R. A. Garcia, C. A. Ramos, E. Voll, A. McDonald, P. Lefebvre & P. Schlesinger. 2006. Modelling conservation in the Amazon basin. Nature 440: 520–523.
Sombroek, W. 2000. Amazon landforms and soils in relation to biological diversity. Acta Amazonica 30: 81–100.
Spracklen, D. V., S. R. Arnold & C. M. Taylor. 2012. Observations of increased tropical rainfall preceded by air passage over forests. Nature 482: 282–285.
Stickler, C. M., M. T. Coe, M. H. Costa, D. C. Nepstad, D. G. McGrath, L. C. Dias, H. O. Rodrigues & B. S. Soares-Filho. 2013. Dependence of hydropower energy generation on forests in the Amazon Basin at local and regional scales. Proceedings of the National Academy of Sciences 110: 9601–9606.
Sturm, C., G. Hoffmann & B. Langmann. 2007. Simulation of the stable water isotopes in precipitation over South America: Comparing regional to global circulation models. Journal of climate 20: 3730–3750.
Stute, M., M. Forster, H. Frischkorn, A. Serejo, J. Clark, P. Schlosser, W. Broecker & G. Bonani. 1995. Cooling of tropical Brazil (5°C) during the Last Glacial Maximum. Science 269: 379–383.
Svenning, J.-C. & R. S. Condit. 2008. Biodiversity in a warmer world. Science 322: 206–207.
——— & Sandel, B. 2013. Disequilibrium vegetation dynamics under future climate change. American Journal of Botany 100: 1–21.
Taiz, L. & E. Zeiger. 1998. Plant Physiology. Sinauer Associates Inc, Sunderland, Massachusetts.
ter Steege, H., D. Sabatier, H. Castellanos, T. van Andel, J. Duivenvoorden, A. A. de Oliveira, R. Ek, R. Lilwah, P. Maas & S. Mori. 2000. An analysis of the floristic composition and diversity of Amazonian forests including those of the Guiana Shield. Journal of Tropical Ecology 16: 801–828.
———, N. C. A. Pitman, O. L. Phillips, J. Chave, D. Sabatier, A. Duque, J. F. Molino, M. F. Prévost, R. Spichiger, H. Castellanos, P. von Hildebrand & R. Vásquez. 2006. Continental-scale patterns of canopy tree composition and function across Amazonia. Nature 443: 444–447. doi:10.1038/nature05134.
———, ———, D. Sabatier, H. Castellanos, P. van Der Hout, D. C. Daly, M. Silveira, O. L. Phillips, R. Vasquez & T. van Andel. 2003. A spatial model of tree α-diversity and tree density for the Amazon. Biodiversity and Conservation 12: 2255–2277.
———, ———, ———, C. Baraloto, R. P. Salomão, J. E. Guevara, O. L. Phillips. C. V. Castilho, W. E. Magnusson, J.-F. Molino, A. Monteagudo, P. Núñez Vargas, et al, C. Baraloto, R. P. Salomão, J. E. Guevara, O. L. Phillips. C. V. Castilho, W. E. Magnusson, J.-F. Molino, A. Monteagudo, P. Núñez Vargas, et al. 2013. Hyperdominance in the Amazonian tree flora. Science 342.
Terborgh, J. & E. Andresen. 1998. The composition of Amazonian forests: patterns at local and regional scales. Journal of Tropical Ecology 14: 645–664.
Trenberth, K. E. 1999. Atmospheric moisture recycling: role of advection and local evaporation. Journal of Climate 12: 1368–1381.
Tribuzy, E. 2005. Variacões da temperatura foliar do dossel eo seu efeito na taxa assimilatória de CO2 na Amazônia Central. PhD dissertation, Universidade de Sao Paulo.
Urrego, D. H., M. B. Bush, M. R. Silman, B. A. Niccum, P. La Rosa, C. H. McMichael, S. Hagen & M. Palace. 2013. Holocene fires, forest stability and human occupation in south-western Amazonia. Journal of Biogeography 40: 521–533.
van Breukelen, M., H. Vonhof, J. Hellstrom, W. Wester & D. Kroon. 2008. Fossil dripwater in stalagmites reveals Holocene temperature and rainfall variation in Amazonia. Earth and Planetary Science Letters 275: 54–60.
van der Hammen, T. & H. Hooghiemstra. 2000. Neogene and Quaternary history of vegetation, climate, and plant diversity in Amazonia. Quaternary Science Reviews 19: 725–742.
van der Molen, M., A. Dolman, P. Ciais, T. Eglin, N. Gobron, B. Law, P. Meir, W. Peters, O. Phillips & M. Reichstein. 2011. Drought and ecosystem carbon cycling. Agricultural and Forest Meteorology 151: 765–773.
Victoria, R., L. Matinelli, J. Moraes, M. Ballester, A. Krusche, G. Pellegrino, R. Almeida & J. Richey. 1998. Surface air temperature variations in the Amazon region and its border during this century. Journal of Climate 11: 1105–1110.
Vincent, L. A., T. Peterson, V. Barros, M. Marino, M. Rusticucci, G. Carrasco, E. Ramirez, L. Alves, T. Ambrizzi & M. Berlato. 2005. Observed trends in indices of daily temperature extremes in South America 1960–2000. Journal of Climate 18: 5011–5023.
Walther, G. R., E. Post, P. Convey, A. Menzel, C. Parmesan, T. J. Beebee, J. M. Fromentin, O. Hoegh-Guldberg & F. Bairlein. 2002. Ecological responses to recent climate change. Nature 416: 389–395.
Wang, S., A. Chen, J. Fang & S. W. Pacala. 2013. Why abundant tropical tree species are phylogenetically old. Proceedings of the National Academy of Sciences 110: 16039–16043.
Wanner, H., J. Beer, J. Bütikofer, T. J. Crowley, U. Cubasch, J. Flückiger, H. Goosse, M. Grosjean, F. Joos & J. O. Kaplan. 2008. Mid-to Late Holocene climate change: an overview. Quaternary Science Reviews 27: 1791–1828.
Williams, J. W., S. T. Jackson & J. E. Kutzbach. 2007. Projected distributions of novel and disappearing climates by 2100 AD. Proceedings of the National Academy of Sciences 104: 5738–5742.
Williamson, G. B., W. F. Laurance, A. A. Oliveira, P. P. Delamônica, C. Gascon, T. E. Lovejoy & L. Pohl. 2000. Amazonian tree mortality during the 1997 El Niño drought. Conservation Biology 14: 1538–1542.
Wright, S. J. 1992. Seasonal drought, soil fertility and the species density of tropical forest plant communities. Trends in ecology & evolution 7: 260–263.
——— 2005. Tropical forests in a changing environment. Trends in Ecology & Evolution 20: 553–560.
Würth, M., K. Winter & C. Körner. 1998. In situ responses to elevated CO2 in tropical forest understorey plants. Functional Ecology 12: 886–895.
Zeng, N., J. H. Yoon, J. A. Marengo, A. Subramaniam, C. A. Nobre, A. Mariotti & J. D. Neelin. 2008. Causes and impacts of the 2005 Amazon drought. Environmental Research Letters 3: 1–9.
Zeng, Z., S. Piao, A. Chen, X. Lin, H. Nan, J. Li & P. Ciais. 2013. Committed changes in tropical tree cover under the projected 21st century climate change. Scientific Reports 3: 1–6.
Acknowledgments
We thank Flemming Nørgaard for drawing the figures and Naia Morueta-Holme, Rodrigo Cámara-Leret and Benjamin Blonder for their useful comments. We are also grateful to the reviewers for providing insight and posing critical questions. This work was funded by a training fellowship from the Department of Bioscience at Aarhus University and a student fellowship from Alberta Mennega Stichting. JCS was supported by the European Research Council (ERC-2012-StG-310886-HISTFUNC) and HB by a grant from the Danish Natural Science Research Council (10–083348).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olivares, I., Svenning, JC., van Bodegom, P.M. et al. Effects of Warming and Drought on the Vegetation and Plant Diversity in the Amazon Basin. Bot. Rev. 81, 42–69 (2015). https://doi.org/10.1007/s12229-014-9149-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12229-014-9149-8