Abstract
Water and solutes are essential ingredients of root pressure. For root pressure to develop, the entry into root cells including xylem tissues of these ingredients is necessary. Therefore, in the light of latest research findings, the integrated synthesis of the combined “osmotic” and “energetically driven uphill water transport” in plants against water potential gradient and the gravity has been presented with the hope to halt or at least dilute for some time to come the prevalent legacy of “riddle” or “enigma” of root pressure. Further, various techniques, both invasive and noninvasive including new ones, have been described focusing on the factors affecting and consequences and implications of root pressure for agriculture, horticulture, and forestry.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The evolution and progression of unicellular to multicellular aquatic plants and to the present-day giant terrestrial tallest known living tree measuring 115.61 m (379.3 ft) (Preston 2007) on the Earth essentially call for an interdependency of their various organs deploying the principle of division of labor to drive their complex life activities through the integration of different structurally specialized functions for survival and growth under continually click-by-click changing environment since the birth of this planet about 4.5 billion years ago (Darwin 1859; Chamberlin 1916; Singh et al. 2009a). Obviously and quite understandably, in this long route of evolutionary journey of progression, the higher plants strategically through adaptation opted for the formation of two distinct organs, i.e., root and shoot, to perform interdependent functions translating them into growth and development through their mutual negotiations. These negotiations include, in the main, the supply of water and nutrients by root to the shoot and, in return, the supply of manufactured food and energy by shoot to the root making the existence of plants themselves as well as animals and humans possible. However, not surprisingly, we hear and read more of shoot functionings than those of root—the supplier of basic ingredients such as water and nutrients, for photosynthesis—the basis of life on this planet. This discrepancy for root victimization seems to have resulted from the general principle of human behavior under the rule of a well-known dictum—out of sight, out of mind—roots being invisible to human eyes due to their hidden habitat beneath the soil. However, of late, the increasing demand for food to feed the ever-rising population, both the diminishing availability of water and the restrictions imposed on the increasing use of fertilizers to provide nutrients to agricultural crops in the main, has attracted the attention of most plant biologists including physiologists, agronomists, geneticists, breeders, biotechnologists, and biometricians toward root research vis-a-vis shoot research worldwide (Barber and Bouldin 1984; Kirk 1994; Baluska 1995; Singh and Singh 1999, 2000; Singh et al. 1999; Henry 2013; Eshel and Beekchman 2013; Ahmed et al. 2014). Among root studies, the investigations on the physiology of uptake and transport of water and nutrients by roots have been in the past and the present time too the topic of great interest among plant physiologists on account of their economic and ecological significance for achieving global ecosystem sustainability (Jackson et al. 2000; Javot et al. 2003; Draye et al. 2010; Maurel et al. 2010; Aroca et al. 2012; Lobet et al. 2013). In this context, however, it has long been recognized that water and solute absorption by roots may either result from forces arising up in the leaves due to transpiration pull and transmitted to the roots and nearing soil solution (Steudle 2000, 2001; Tyree 2003a) or from forces developed down in the roots themselves called root pressure visibly evidenced by stem stump exudation or guttation or bleeding (Renner 1915, 1925; Kramer 1945; Singh and Singh 2013; Singh 2013, 2014a, b).
Pertinent to the present topic, there appear no variant opinions that it was Stephen Hales who first discovered the phenomenon of liquid exudation from the heavily pruned grapevines (Vitis vinifera) as early as 1727 and ascribed this event to some kind of force arising in the root which propelled the liquid up to exude from the cut surfaces terming this force as root pressure. Since then, it has engaged the mind of experimental botanists and plant physiologists who began to investigate this curious, complex, and intriguing physiological phenomenon, albeit with long intervals between investigations (Pfeffer 1881; Sachs 1887). It was only in the first quarter of the twentieth century that some definitive experimental data were provided to explain this phenomenon (Renner 1915; Priestley 1920; Overton 1921; Bose 1923; Kramer 1932) which followed, with the passage of time drawing the twentieth century to close, its detailed and accredited accounts written by a number of workers (White 1938; White et al. 1958; Lundegardh 1944; Kramer and Currier 1950; Stocking 1956; Davis 1961; Barrs 1966; O’Leary 1966; O’Leary and Kramer 1964; Singh and Singh 1989; Zholkevich 1991) leaving much scope for further work at the experimental front to explain the unresolved phenomenon of root pressure for this and perhaps, as it appears, the next century as well. Accordingly, the dawn of twenty-first century witnessed some illuminating studies advancing the concepts of “two-compartments,” “three-interphases” (Pickard 2003a, b), “plant heart theory” (Kundt and Gruber 2006), “pushing water upward-like mechanism” (Singh et al. 2009a), and very recently proposed energetically driven uphill “water co-transport theory” (Wegner 2014) to explain the mechanism of root pressure. The problem of how plants manage to lift their water from the soil to their leaves has been with biologists at least since 1726, when Stephen Hales discussed it in the first edition of the Proceedings of the Royal Society of London. So much has been thought about it that present-day scientists often believe that the problem has been solved long ago or that there was no problem at all with mentions of osmotic suction, capillary forces, transpiration, and coherence. For plants, this supply problem is not answered in the biological textbooks, at least not satisfactorily so leaving the mechanism unresolved (Pickard 2003a, b; Kundt and Gruber 2006; Zholkevich et al. 2007; Wegner 2014).
I, therefore, in efforts to getting as close to the bottom of this physiological phenomenon as possible, have attempted to organize this chapter in various heads and subheads in the light of new and novel research findings which have led to the formulation of modern thermodynamically sound physical, physiological, metabolic, and molecular concepts and explanations based on the art-of-the-state technology of the techniques and instrumentation, to account for “pushing” the sap up in plants under “positive pressure” of roots in the absence or reduced transpiration which otherwise “pulls” the water up under “negative pressure” generated by cohesion–tension on the water present in the leaves at the cost of solar energy.
2 Definition of Root Pressure
The hydrostatic pressure developed in the roots of plants, causing exudation of sap from cut stems and/or guttation of water from uninjured leaves, is known as root pressure. This phenomenon is also known as “root exudation pressure” or “stump exudation pressure” or simply “sap pressure.” It is inclusive of “radial cell pressure” as well as “axial xylem pressure.” The pressure is assumed to be generated in combination by osmotically and energetically driven uphill transport of water across the plasma membrane of xylem parenchyma cells possibly taking advantage of the free energy gradients of ions and sugars (Wegner 2014).
3 Taxonomic Distribution of Root Pressure
Root pressures in temperate climate most frequently develop during warm nights though most of water transport occurs during daytime. Significant and consistent root pressures are developed in a wide range of plant species. Moreover, root pressure develops not only in the herbaceous species but in deciduous trees as well. Both dicotyledonous and monocotyledonous species have been reported to exhibit root pressure (Zachary 2009). Among dicots, prominent examples include several important agricultural plants such as tomato (Solanum lycopersicum) (White 1938), grapes (Vitis vinifera) (Sperry et al. 1987), sugar maple (Acer saccharum) (Sperry et al. 1988), birch tree (Betula cordifolia) (Sperry 1993), oak tree (Quercus robur and Q. petraea) (Steudle and Meshcheryakov 1996), walnut tree (Juglans regia) (Ameglio et al. 2001), sunflower (Helianthus annuus) (Dustmamatov et al. 2004), kiwifruit tree (Actinidia spp.) (Clearwater et al. 2007), and Betula lenta L. and B. populifolia Marsh. (Miller-Rushing and Primack 2008). However, in many of the above dicots, the observed root pressure was seasonal, being synced with the onset of spring. Such pressures are demonstrable in the spring before the buds open, but once the leaves have expanded and rapid water movement through the plant begins, root pressure can no longer be detected.
Further, a group of recent studies found root pressure in 61 of 109 tropical vine-like species despite the lack of freezing temperatures, much higher percentage of species with positive xylem pressures than those reported earlier by Ewers et al. (1997), suggesting this to be a regular, if not daily, occurrence (Fisher et al. 1997), both monocotyledonous and dicotyledonous vines evinced the phenomenon. These studies imply that root pressures are less common in lianas than in more herbaceous climbers and that certain families may have a strong tendency for root pressures, including the monocotyledonous family Araceae (ten of ten species) and the dicotyledonous families Vitaceae (ten of ten species) and Dilleniaceae (three of five species) (Fisher et al. 1997). In contrast to dicotyledonous lianas, the climbing fern Lygodium venustum and the viny bamboo Rhipidocladum racemiflorum also exhibit root pressures that might be adequate to refill embolized vessels throughout the entire plant. In addition, significant daily root pressure has been observed in herbaceous dicots (Milburn and McLaughlin 1974; Kramer and Kozlowski 1979), palms (Davis 1961), and banana (Davis 1961; Lu et al. 2002). Grasses in particular have widely demonstrated a daily pattern of root pressure, including Rhodes grass (Chloris gayana Kunth) (Ogata et al. 1985), corn (Zea mays) (Miller 1985; Tyree et al. 1986), sugarcane (Saccharum spp.) (Tyree et al. 1986; Neufeld et al. 1992), the vine-like bamboo (R. racemiflorum) (Cochard et al. 1994), rice (Oryza sativa) (Stiller et al. 2003), and several others (Phleum pratense and Festuca pratensis) (Macduff and Bakken 2003).
Davis (1961) reported positive root pressures in ten species of palm trees and in banana (Musa sapientum) but a lack of root pressure in five dicotyledons as trees and in a cycad. Root pressure, therefore, appears to be intimately linked to the hydraulics of plants, and it was suggested that monocotyledons, and other taxa with reduced or absent secondary growth for the production of new vascular tissue in their stems, may often depend on root pressures for water transport. Thus, far from a rare phenomenon, root pressure appears to be a widespread, if not common, factor in sap flow. It is, however, generally agreed that root pressures are not directly important for the high-volume daytime transpiration in vascular plants, as was implied by Davis (1961) for monocotyledons though Kundt and Gruber (2006) argue for a bigger role of root pressure in upward transport of water both in tall and short plants.
4 Quantification of Root Pressure
Root pressure is one of several important plant activities which serves a number of purposes required for growth and development. It is, however, affected by chemical, physical, environmental, edaphic, and genetic factors. Its quantification and determination of sap composition are essential for ecological, physiological, genetic, and metabolic studies on the one hand and for agricultural, horticultural, and forestry research on the other. It can be measured both invasively and noninvasively directly and computed and quantified indirectly as well.
4.1 Direct Measurements of Root Pressure
4.1.1 Invasive Techniques
4.1.1.1 Manometric Method
4.1.1.1.1 Stem Stump Method
Root pressure may be demonstrated and measured by attaching a mercury manometer by vinyl tubing to the cut end of the stem called stump. Within a short time, the level of mercury rises up indicating upward movement of sap due to root pressure. Bubble manometers can also be used to measure xylem pressure by attaching them to branch stumps (Ewers et al. 1997). The manometers made from glass tubes (1-mm internal diameter) are sealed at the distal end by flame. The distal half is filled with air, while water fills the basal half. The base is connected to the stump by a tight fitting of vinyl tube and hose clamps. Prior to attachment, the freshly cut stump is shaved with a new razor blade to permit unobstructed fluid flow between the stump and manometer. Each evening the cut stumps are reshaved and the manometers reattached. After allowing for equilibration overnight, the bubble length (L pd) in the manometer is measured at predawn. The vinyl tubing is then cut and the bubble length (L atm) immediately remeasured at atmospheric pressure. The xylem water pressure (P x in kPa) is calculated from a relation derived from the ideal gas law:
when L pd is >L atm (including situations where all the water from the manometer is absorbed by the shoot), the P x is recorded as negative, though manometers generally are not able to accurately measure the negative pressures that can occur in plants. The bubble manometers give P x values near those often recorded by electronic pressure transducers (Cochard et al. 1994) and are used as such because they cost much less for especially surveys involving many species. Observations may be repeated three consecutive mornings at predawn.
4.1.1.1.2 Excised Root Method
Root exudation of an individual root may be quantified by excising the root at the base under water and inserting it 10 mm into a glass capillary (diameter = 0.55 mm) and sealing the capillary with a drop of super glue. The root is bathed in nutrient solution from the pot in which the plant had grown. The rise of xylem sap in the capillary may be recorded at time intervals (arbitrary) of 5 min for 1 h, and osmotic flow rate (Q ros) may be calculated from the linear part of the flow vs time plot. At the end, the exudate in the capillary may be collected with a hypodermic needle attached to a syringe. The exudate may be analyzed for osmolality using picoliter osmometry separately.
4.1.1.2 Root Pressure Probe Techniques
4.1.1.2.1 Stem Stump Method
For measuring the root pressure by this technique which can be deployed on stump after cutting off the stem just below the first leaves, the remaining stump is fixed to the probe, a miniature pressure sensor “root pressure probe” with a rubber seal (Steudle and Jeschke 1983; Steudle et al. 1993). The pot with the root system is supplied continuously with nutrient solution or water as the case may be. With some root systems, root pressure could be recorded for several days. Curves may be digitized using a digitizing tablet.
4.1.1.2.2 Excised Root Method
The individual seminal roots are excised at their base under water. The root is fixed to the root pressure probe using a cylindrical silicon seal made from liquid silicon material (Knipfer and Fricke 2010). Seals are tightened using a screw, which compresses the seal and the root cortex. The root is then bathed in the same medium in which the plant had been grown. The medium is circulated with the aid of a peristaltic pump to ensure the uniformity of bathing medium. When a stable root/xylem pressure is ensured to have been reached (after 0.5–2 h), pressure relaxations are induced. Hydrostatic pressure relaxations are produced by turning the metal rod of the probe rapidly to induce a hydrostatic pressure pulse (0.05 MPa) in the root xylem. Osmotic relaxations are induced by adding 20 or 25 mM NaCl to the root medium. Root hydraulic conductivity is calculated from the halftimes of overall hydrostatic (T 1/2hy) and osmotic pressure (T 1/2os) relaxations. Root radial hydraulic conductivity (LpHYP and LpHOP, in m s−1 MPa−1) is related to hydrostatic and osmotic T 1/2 as follows:
The elastic modulus of the measuring system (β, 5.1 × 10−9 MPa m−3) is determined by inducing step changes of volume and measuring the resulting changes in root pressure. The total root surface area (A r) is calculated from root length and diameter including lateral roots. The reflection coefficient (r) for NaCl during osmotic pressure relaxations is calculated by relating the measured maximum decrease in root pressure caused by addition of NaCl to the expected decrease in pressure (20 or 25 mM NaCl; 0.1 or 0.125 MPa).
4.1.1.2.3 Root Vacuum Perfusion Method
Individual roots are excised under water. The excised root is inserted at its base into a 10-mm-long glass capillary (diameter = 0.55 mm), which is fixed to the bottom of a 100-mm-long water-filled glass capillary (diameter = 0.4 mm). The root is pushed through the small capillary piece until the root base protruded into the water-filled capillary. The root is sealed with a drop of super glue on the outer surface between the root and the capillary. The water-filled capillary is connected to a vacuum pump via rigid silicon tubing and is held by a clamp attached to a stand in such a way that the weight of the root is supported by the stand and does not contribute to the weight of nutrient solution in which the root is bathed. The beaker containing nutrient solution is placed on a balance, and root water uptake is measured gravimetrically as the decrease in weight with time. Before the application of hydrostatic pressure gradients, the osmotic flow rate Q ros is measured from the linear part of the water uptake vs time plot. The driving force for this osmotic water flow is the difference in osmotic pressure (D p, MPa) between root xylem and medium multiplied by the corresponding reflection coefficient for solutes (a value of 1.0 is used). Water loss from the beaker as a result of evaporation (Q eva) is generally negligible and is corrected accordingly. To measure the hydrostatic flow rate Q rhy, a partial vacuum of 20, 30, 40, 60, and 80 kPa is applied at the open end of the capillary in succession for 10 min each. Q rhy is determined from the linear part of the water uptake vs time plot after Q eva and Q ros had been subtracted (Knipfer and Fricke 2010).
4.1.1.2.4 Externally Applied Pneumatic Pressure Method
This method also involves cutting off stem with a sharp razor blade above the ground level and allowing it to exude freely for some time. Then the cut end of the stump is attached to a vinyl or tygon thick-walled tubing sealing with silicon glue or parafilm properly as described earlier on the one end and to a pressure applying system equipped with a pressure gauge on the other. Subsequently, the pressure is gently applied to stop the exudation confirming it by viewing through a hand lens. At this point of time, the magnitude of pressure applied is noted which is interpreted to be equal to the root pressure.
4.1.2 Noninvasive Techniques
The minimally invasive measurements in the xylem of trunks remain the greatest challenge. Knowledge of the forces and flows in this compartment are crucial to unearthing the truth about water lifting because branches, twigs, and petioles may be segmented and separated from the main stream in the trunk. However, independent of the outcome of such experiments, in the light of the evidences reviewed here, it is obvious that nature has developed a broad spectrum of complementary strategies for water transport against gravity to cope with various water deficiency situations without the necessity of developing incredibly negative pressures (Zimmermann et al. 1995). Various noninvasive techniques are briefly described hereunder.
4.1.2.1 Isopiestic Method
This is a noninvasive technique for measuring root pressure. By adapting this technique, the root pressure can be measured isopiestically in intact plants by applying solutions of different concentrations separately of non-penetrable osmoticum such as polyethylene glycol (MW ≥ 4,000) to the root medium with the observations on the guttation process (Klepper and Kaufmann 1966; Steudle and Jeschke 1983; Zhu et al. 1995). The magnitude of the osmotic potential (negative) of the solution that stops the guttation is considered equal to the root pressure (positive).
4.1.2.2 Xylem Pressure Probe Method
The xylem pressure probe method allows the direct measurement of diurnal and seasonal changes in xylem pressure, xylem flow, and solute composition in intact plants (Balling and Zimmermann 1990). Briefly, the xylem pressure probe is filled with denucleated water and incorporates a water-wettable pressure transducer. The microcapillary of the probe is advanced through the tissue (Fig. 1).
A schematic diagram of the xylem pressure probe [Source: Balling and Zimmermann (1990)]
Penetration is stopped immediately when the transducer registers a pressure below atmospheric. In order to verify that the probe is placed in a vessel, the microcapillary is loaded with a dye/water mixture prior to insertion. This results in staining of the penetrated vessel. Furthermore, injection of volume pulses into a probed vessel by appropriate displacement of the metal rod results in a rapid dissipation of the pressure provided that the tip is unobstructed and placed in the lumen of a large vessel. The probe can accurately read both the negative and positive pressures. Clearwater et al. (2007) measured root pressure using pressure transducers that were installed inside the xylem of kiwi roots. To my knowledge, this is the only existing nondestructive continuous method to measure root pressure in a herbaceous plant. However, the application of this method on herbaceous plants with limited root and stem diameters such as tomato may be difficult, if not impossible, because this method requires installation of the system 10–15 mm inside the xylem.
4.1.2.3 Cell Pressure Probe Method
For this purpose, a cell pressure probe is used to measure hydrostatic pressure of the intact root cortical cells (Azaizeh et al. 1992). An oil-filled capillary (outer tip diameter, 4 ± 0.7 mm) is attached to a pressure chamber which contains an electronic transducer (silicon chip). The probe is fixed on a micromanipulator which allows insertion of the tip into individual cells. Cells may be probed at distances between 30 and 100 mm from the apex of roots grown in any growth medium. Thus, by using the pressure probe, the hydrostatic pressure of individual cells may be measured directly. However, the primary limitation of this method is that some cells are too small to measure. Furthermore, some cells tend to leak after being stabbed with the capillary, and others plug up the tip of the capillary, thereby preventing valid measurements. By measuring the depth of insertion of the tip in the cortex, the location of punctured cells could be measured. Prior to insertion into root tissue, the pressure probe is completely filled with silicon oil. When a cell is punctured, a meniscus is formed between oil and cell sap, and this meniscus is kept at a certain position. The pressure transducer converts the pressure signal into a proportional voltage. Pressure/time curves are recorded on a chart recorder. Happily, other hydraulic traits such as turgor, hydraulic conductivity, and elastic modulus can also be quantified by this method. For processing the data on such traits, recorder strips are digitized using a digitizing tablet. Cell elastic moduli are evaluated from changes in cell volume which caused changes in cell turgor pressure.
4.1.2.4 Axial and Radial Root Growth Confinement Method
A method was developed by Misra et al. (1986) for estimating radial root growth pressure of intact seedlings. Under this method, each root of desired seedling is confined both in the axial and radial directions in a cylindrical chalk sample at a constant water potential. In doing so, the root exerts radial stress which causes tensile failure in a proportion of the chalks. The measurement of tensile strength of duplicate chalks enables estimation of the maximum radial pressure exerted by the roots. The axial and radial root growth pressures measured in this manner were, for example, of comparable magnitudes registering 497, 289, and 238 kPa for pea, cotton, and sunflower seedlings of similar size, respectively (Misra et al. 1986).
4.2 Indirect Measurements of Root Pressure in Intact Plants
4.2.1 Root Pressure Computation by Difference in Leaf Thickness Under Different Relative Humidities
Recently, a technique was developed to measure the root pressure in which measurements of leaf thickness are compared with predictions of it using a mechanistic model (De Swaef and Bleyaert 2012). This model predicts diurnal variations in leaf thickness based on variations in transpiration and a concept of growth in relation to turgor. The principle is that when measurement of leaf thickness exceeds the predictions during the night, this difference could be attributed to root pressure. If this difference is not present, the diurnal differences in leaf thickness are entirely explained by the transpiration and growth concepts present in the model, and root pressure is expected not to be present. Thus, the difference between measured and predicted leaf thickness could mathematically be translated into an absolute value for root pressure. The leaf patch clamp pressure probes can also be used for this purpose (Zimmermann et al. 2008; Lee et al. 2012). The practical implication of these methods is that these can be used to relate the occurrence of tip burn in lettuce head to the occurrence of root pressure by manipulating nighttime relative humidity around the plant (De Swaef and Bleyaert 2012).
4.2.2 Root Pressure Computation by Using Sap Flow and Stem Diameter
Very recently, a nondestructive estimation of root pressure using sap flow, stem diameter measurements, and mechanistic modeling was developed by De Swaef et al. (2013). The magnitude of the root pressure destructively measured using a manometer installed on excised tomato stems has been found to agree well with the model-based estimations made by these authors. Under this technique, however, destructively measured root pressure showed a decrease during the night, presumably as a result of decreasing substrate temperature, whereas estimated root pressure in intact leafed plants showed an increasing pattern toward the end of the night. It is therefore hard to relate diurnal dynamics of destructively measured root pressure to diurnal dynamics of transpiring plants, because the excised stems did not transpire during the day; root pressure enhanced during the day because of the higher temperature in the greenhouse, whereas root pressure is not allowed to develop in transpiring plants. With critical considerations concurrent with the root pressure-induced nighttime increase in diameter, it might be expected that water flow must increase. This was, however, not always visible in the experiments conducted by the above referred authors but could be clarified by the model calculations. The measured nighttime diameter increase corresponds approximately to a calculated maximum water mass inflow of approximately 250 mg h−1 for an 8-m-long tomato stem, which is 400 times smaller compared with daytime water flow rates. Because of the low vapor pressure deficit at the end of the nights, plant water loss via transpiration could be neglected in such cases. It is, therefore, hypothesized that nearly all of the upward pushed water flow in the xylem is stored in the plant itself and thus results in the increase in diameter. However, the sensitivity of the heat balance sensors may not always allow detection of these low amounts of water flow (van Bavel and van Bavel 1990). The approach described above was validated in an extra experiment on tomato plants in which relative humidity of the air was manipulated to be high during the night. The same model was used for the forward simulation using sap flow as input variable and shows comparison between measured and simulated diameter for the forward simulation. The resulting estimates of root pressure were then compared with actual measurements of root pressure on excised shoots.
From the foregoing discussion, it is clear that no single method is available which is applicable to all situations. Therefore, depending upon the plant species and their age, different strategies are required for the measurement of root pressure which need to be noninvasive, simple, easy to use, cheap, and dependable.
5 Magnitude of Root Pressure
The exudation of liquid water from passive hydathodes, such as in Colocasia, and the secretion of water and solutes from wounds show that the roots of many plants under certain conditions can develop considerable pressure displaying a daily rhythm and a yearly rhythm: the mechanisms seem to be working only when required in an unspectacular way (Kramer and Kozlowski 1979; Kundt and Gruber 2006). The magnitude of pressures also depends upon the techniques used. For example, pressures measured by root pressure probe were higher (in the range of 0–0.5 MPa) than the values produced by the pressure chamber, although new experiments have recently been conducted with the pressure probe (Wei et al. 1999) and were found to agree with the pressure chamber. Over seven decades ago, White (1938) recorded pressures of 600–700 kPa in excised tomato roots. A pressure of 700 kPa, though capable of causing a flow of water in the xylem of tall trees, in view of existence of resistance to flow, cavitation, etc., may not be sufficient to push the water in most of the tallest trees. However, such magnitudes of root pressure would certainly be no problem for either agricultural and horticultural crops (Singh and Singh 1989; Tanner and Beevers 1999, 2001; Singh et al. 2009a) or woody as well as vine-like lianas (Fisher et al. 1997) and bamboos (Zachary 2009) or deciduous forest trees (Feild et al. 2005; Feild and Arens 2007) acting as supplementary device to the cohesion–tension mechanism (Steudle 2001) for upward movement of sap. A root pressure in the root xylem/stele has been typically found within the range 0.1–0.4 MPa (Steudle et al. 1987; Knipfer et al. 2007) and attributable to active solute uptake and subsequent passive water inflow. Actually, plants depending upon their habitat require an excess pressure inside a plant, not to be established by suction, which can amount to 0.6 MPa in the tomato, 1 MPa in grass stalks, or even 6 MPa in certain desert plants studied since many decades (White et al. 1958; Kundt and Gruber 2006). Positive xylem pressures in the stems of plants usually are attributed to “root pressure,” i.e., the osmotic water uptake caused by solute uptake into roots (Tyree et al. 1994; Ewers et al. 1997; Fisher et al. 1997). The water flow caused by root pressure is normally much less than that caused by transpiration pull. Apparently, when transpiration is high, the osmotic force causing root pressure tends to disappear but not nonexistent because solutes are diluted by an influx of soil water in the xylem. Thus, root pressure is highest when transpiration is minimal, such as predawn and during rainstorms (Cochard et al. 1994).
Data for Tetracera recorded by Cochard et al. (1994) are similar to results of Scholander et al. (1957) for this genus at the same site, where they found xylem pressures of 10–80 kPa. However, as noted by Scholander et al. (1957), the root pressure values need to be considered in the context of the height of the plants. The measured values of root pressure near the base of the stems of Dilleniaceae (a maximum of 64 kPa in Doliocarpus major) was modest considering that those plants reached the canopy height of 18 m. Based on the above argument and these data, Ewers et al. (1997) concluded that the root pressure of 64 kPa would be adequate, given enough time, to refill embolized vessels of the roots and lower stems, at maximum height of just 7.1 m. L. venustum with 7.5-m height exhibited root pressure values up to 66 kPa, suggesting that xylem pressures might be quite adequate for refilling of the tracheids even in the upper parts of the leaves. On the other hand, R. racemiflorum climbed to only 4.5 m but it had xylem pressure values up to 120 kPa. Furthermore, Cochard et al. (1994) found positive root pressure values even at the most distal part of the stems in this species, suggesting that root pressures could serve to refill embolized vessels throughout the shoot. Further, tropical palm trees whose saps are used as beverages have been reported to have root pressures sufficient to pump water up to heights as large as 12.5 m (Davis 1961), and there is evidence that at least some tropical lianas have positive water pressure in their stem xylem at certain times (Scholander et al. 1957; Putz 1983). Since the wide vessels of tropical liana stems remain conductive for many years, it has been suggested that they might be refilled (reversal of air embolism) as a result of root pressure sufficient to dissolve emboli in vessels (Putz 1983; Ewers et al. 1991). It is, therefore, clear from the foregoing discussion that the root pressure phenomenon is of common occurrence and that its magnitude may vary in space and time and go up to generally 0.6–0.8 MPa depending upon the plant species and their habitat. Occasionally, root pressures amounting up to 6 MPa (White et al. 1958), sufficient to satisfy the need for refilling the freeze- and drought-induced embolism in plants (Tyree 2003a, b; Kundt and Gruber 2006; Holbrook and Zwieniecki 1999; Zwieniecki and Holbrook 2009), seem to establish and signify their existence as necessary mechanism for upward water transport in plants.
6 Morphology and Structural Anatomy of Root Per Se and Root Pressure
Undoubtedly, in recent years, our understanding of water uptake and transport within plant roots has been substantially improved by new tools, which operate at the molecular, cell, tissue, organ, plant, and ecosystem levels. Techniques such as cell and root pressure probes, stopped flow, and the use of transgenic plants and of stable isotopes provided a fast progress in water transport research (Steudle 1993, 2001; Kramer and Boyer 1995; Maurel 1997; Steudle and Peterson 1998; Tyerman et al. 1999, 2002; Ehleringer et al. 2000). A better understanding of the mechanisms of water uptake by plant roots should be vital for improving water-use efficiency in agriculture, horticulture, and forestry. The morphological and anatomical features of the roots, such as their diameter or length, the cell layer from exodermis to endodermis, and the degree of their suberization and the radial and axial water transport pathway have a great influence both on the hydraulic conductivity and root pressure. The recent works highlight the progress for those who want to update their understanding of basic mechanisms of root hydraulics and plant water relations (Zhao et al. 2004; Aroca et al. 2012; Lobet et al. 2013).
6.1 Distribution of Root Systems in Soil and Collection of Water and Solutes
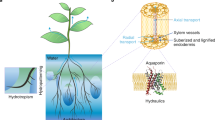
Root morphology and root pressure are intimately linked to each other. The spatial distribution of roots in soil determines the ability of plants to take up soil water and nutrients in order to sustain plant growth and development (Fig. 2). Water uptake by plant roots is controlled or is even regulated by different physical and physiological processes. Water supplied to plants by their roots has a major influence on root pressure, hence the shoot water status, influencing plant growth and development (Meister et al. 2014). A large number of studies confirm that deeper root systems enable plants to access and collect water not available to shallow rooted plants and to pump into the main stem or trunk. Coarse as well as fine regulation may coexist for water uptake. Coarse regulation is physical in nature and strongly depends on root structure. These regulations mainly involve minimizing water loss and maximizing water uptake. Water uptake, on the other hand, is maximized by adjusting the allocation pattern, namely, increasing investment in the roots, enhancing root depth, or extending root system distribution (Fig. 2). Since root hairs are unicellular functional units of root system architecture, modulating root hair number and length is an alternative to improving root function depending on the soil type. The development of Arabidopsis root hairs (type 3 striped pattern) differs from rice root hair development due to the asymmetric cell division (type 2). The known root hair loci in rice share homology to Arabidopsis genetic counterparts. These include the auxin-regulated OsWOX3A, which negatively regulates root hair number and length but is a positive regulator of lateral root number, and a putative mannosyl-oligosaccharide glucosidase (OsMOGS), controlling both initiation and elongation of root hairs (Zhao et al. 2004; Baluska et al. 2000; Baluska and Mancuso 2013). Root hairs are an important component of root system architecture since they increase the surface area for uptake of water and nutrients and are one of the sites for plant–microbe interactions as well. Since the ontogeny of root hairs varies between species, know-how regarding how cell fate is specified will shed light on the ways to achieve epidermal cell type independent of root hair differentiation and whether it benefits productivity (Baluska et al. 2000; Baluska and Mancuso 2013). Fine regulation of water flow is achieved by aquaporins by phosphorylation and dephosphorylation or indirectly by regulating protein kinases and phosphatases or gating (Maurel et al. 2008).
Morphology and anatomy of root systems and water and solute collection and transport. (a) Whole plant with root and shoot [Source: O’Toole and Chang (1978)]. (b) Root system (Source: http://www.greenmanspage.com/guides/logistics.html). (c) Prolific root hairs (Source: https://www.pinterest.com/pin/506232814336189676/). (d) Magnified view of root hairs (Source: http://www.daviddarling.info/encyclopedia/T/trichome.html). (e) Radial flow of water and solutes in roots (Source: http://www.pleasanton.k12.ca.us/avhsweb/thiel/apbio/labs/plant_transport.html)
6.2 Conduction of Water and Nutrients
Xylem is specialized for the conduction of water and mineral substances in the plant body. It is a heterogenous tissue made up of four different types of cellular elements such as xylem tracheids, xylem tracheae, xylem fibers, and xylem parenchyma. Of these, the tracheids and the tracheae are described as essential elements since they are directly involved in the translocation of water and mineral substances. Xylem fibers and xylem parenchyma are described as associated elements, since they are only supporting structures. The tracheids, the trachea, and the xylem fibers are nonliving components, while xylem parenchyma represents the only living component of the tissue. Xylem is commonly described as a dead, complex permanent tissue. Secondary xylem (wood) performs many functions, but chief among them is long-distance transport and mechanical support of the canopy. The studies on the comparison of the inter-conduit pits between angiosperm vessels and gymnosperm tracheids showed that the different torus–margo structure of conifer pit membrane was 60 times more efficient at water conduction than the homogenous pit membranes between angiosperm vessels and equally as safe against air seeding. The more efficient pits of conifers compensate for the shorter length of the single-celled tracheid. Consequently, a single-celled tracheid has approximately the same conducting capacity as a multicellular vessel of the same diameter (Tyree and Zimmermann 2002). In addition, perhaps the circulation of water and sugars via the phloem also seems to contribute to building up root pressure (Wegner 2014).
6.2.1 Physiological Anatomy of Root Pressure
Normally, it is not only water in the soil that is of significance to plant life, but nutrients are of vital importance, too. Soil consists of particles that contain masses of elements in solid form; these are not available to plants unless they are dissolved in the soil solutions (Fig. 2e). Chemical and physical erosion and fertilizers release elements from the soil particles, so making them available to plant. Also, soil particles, which normally have a negative electric charge, bind positively charged soil ions to their surface. Plant roots have developed an ingenious strategy to release these absorbed ions. Roots extrude protons through specific proton-ATPase complexes on the plasma membrane. These released protons are exchanged to the nutrient ions bound on the soil particles, since protons have a higher affinity to the soil particles than other ions. It is often thought that plants take up nutrients from the soil with the flow of water from the soil to the roots. This is not so. Charged nutrient ions cannot pass the plant plasma membrane; they have to be taken in through protein pores or channels in the membrane along an electrochemical gradient (Palmgren 2001). If they are taken in against a concentration gradient, the transport consumes energy, which is available in the form of the proton gradient formed by the proton-ATPase pump or by direct involvement of ATP in the transport process (Pedersen et al. 2007).
The water and solutes intake in the roots can follow two ways, i.e., apoplastic and symplastic with plasmodesmata (transcellular) linking the protoplasts from cell to cell. Movements in relation to the route of the epidermis to the endodermis of the root are called radial water transport (Fig. 3). The relative importance of these pathways is still a cause of much discussion, but there is some evidence for the suggestion that plants displaying low transpiration activity predominantly witness symplastic transport, while those displaying high transpiration activity witness a greater proportion of apoplastic transport (Boyer 1985; Steudle 2001). Another important detail in relation to these different pathways is relevant only in the outer layers of the root tissue, because in the endodermis, the water apoplastic flow is limited due to the Casparian strip. In this hydrophobic barrier, the radial and transverse endodermal cell walls are impregnated with lignin, suberin, structural wall proteins, and wax. In many plants such a barrier also occurs in the epidermal cells, forming a double-layered hydrophobic barrier in the roots (Enstone et al. 2003). It is important to note that the Casparian strip does not always establish a barrier that is totally impermeable to water and solutes coming from the soil. This can be observed, for example, by the development of young roots where pericycle growth can break parts of the endodermis and allow free access to water until the reconstitution of the tissue.
Apoplastic, symplastic, and transcellular movement of water and solutes finally reaching the xylem of roots (Source: http://bankofbiology.blogspot.com/2014/07/comparison-between-active-absorption.html)
6.2.1.1 Apoplast Pathway
Here water passes from root hairs to xylem through the walls of intervening cells and middle lamella without crossing any membrane or cytoplasm. The pathway provides the least resistance to movement of water. However, it is interrupted by the presence of impermeable lignosuberin Casparian strips in the walls of endodermal and exodermal cells.
6.2.1.2 Symplast Pathway
In the plasma membrane, there are at least two pathways along which water is believed to move. One is diffusion through the lipid bilayer, a process which depends on the thermal motion of the membrane lipids, and the other is conduction through water channels which are established by polypeptides of the aquaporin type. Flow through these two pathways may be independently regulated (Henzler and Steudle 1995; Steudle and Henzler 1995). When considering water flow across a structure as complex as a root, the principal resistance in the pathway is often envisaged as being due to plasma membranes of cells in the pathway. A common view is that the plasma membrane of the endodermis having Casparian band may contribute the major part of this resistance, but there are different opinions about this matter (Kramer and Boyer 1995; Steudle and Peterson 1998). Water passes from cell to cell through their protoplasm with water and solutes also entering vacuoles en route. As mentioned earlier, the cytoplasms of the adjacent cells are connected through bridges called plasmodesmata. For entering into symplast, water has to pass through plasmalemma (cell membrane) at least at one place. It is also called transmembrane pathway. Symplastic movement is aided by cytoplasmic streaming of individual cells (Baluska et al. 2000; Baluska and Mancuso 2013). It is, however, slower than apoplastic movements. Both the pathways are involved in the movement across the root. Water flows via apoplast in the cortex, finally getting into the pericycle from where it enters the xylem. Mineral nutrients also have the same pathways as that of water. However, their absorption and passage into symplast mostly occurs through active absorption (Palmgren 2001; Pedersen et al. 2007). There exist numerous reports in the literature that show that radial uptake of water and that of solutes are uncoupled from each other (Munns 1985) and which suggests that ions (Na+ and Cl−) are transported across roots along the symplast (Lauchli et al. 2008). Once inside the xylem, the movement is purely along the pressure gradient, but there is different opinion about it too (Enns et al. 2000).
Pickard (2003a, b) presented a model of water flow through roots which incorporates hydrostatic pressure-driven flow through plasmodesmata. If turgor pressure in endodermal cells is in the range of pressures in other plant cells (0.4–1 MPa), a gradient in hydrostatic pressure should exist between the xylem/stele and the endodermis and enable water flow into the xylem even during endosmotic hydrostatic pressure relaxations. We cannot rule out the possibility that this water flow, being part of the cell-to-cell path and not mediated through aquaporins, contributes to slightly shorter halftimes in hydrostatic compared with osmotic experiments. However, the observations of Knipfer and Fricke (2010) emphasize membranes (and aquaporins) as control points for radial water transport in roots. The results also question the generally accepted idea that a special apoplastic, low-resistance pathway of water movement driven by hydrostatic gradients is required in roots to meet the transpirational water demand of the shoot. Since the present review is concerned with the “push-driven water ascent” in plants, the issue of “pull-driven water ascent” including the composite model of water transport (Steudle 2001) which has fallen under criticism requiring its revision (Zimmermann et al. 2004; Kundt and Gruber 2006; Knipfer and Fricke 2010) will not be discussed further.
6.2.2 Ultrastructural Anatomy of a Root Cell Per Se, Root-to-Shoot and Vice Versa Signaling, and Root Pressure
This section relates to the regulation of transport of water, nutrients, food, organic substances including hormones, and signaling between roots and shoots. After the upward transport, water is again required for taking the photosynthesis products from the leaves through the phloem to all sites of growth, in the buds, branches, stems, roots, blooms, and fruits. Water must therefore permanently circulate through every plant parts, as sap with dissolved materials of varying concentrations. Emphasizing the intricate relation between root and shoot, Kundt and Gruber (2006) opined that the “overlapping double saw-cut” experiment proves that a tree does not die when all its xylem vessels are severed (Preston 1952; Zimmermann et al. 2004), giving the theory of xylem functioning for water transport in isolation a deadly blow. The same is true for phloem’s functioning when a young maple tree, whose stem was cut all the way around its periphery some 10 years ago, has continued growing ever since (Kundt and Gruber 2006). This proofs the strong interconnectivity between xylem and phloem. Although root pressures are generally the mechanism for embolism reversal in most of the studied plants (Tyree and Sperry 1989; Ewers et al. 1997; Fisher et al. 1997; Tyree and Zimmermann 2002; Tyree 2003a, b; Voicu et al. 2008), however, these may not be the only mechanism by which embolisms can be reversed in the xylem. Because xylem cavitations are largely a product of winter freezing (Cobb et al. 2007) and drought stress (Stiller et al. 2003; Singh et al. 2009a), the seasonal occurrence and species variation in root pressure are believed to function as a mechanism to repair cavitations in most of forest trees and crop plants. A recent study indicates that in the evergreen shrub Laurus nobilis xylem embolism can be reversed in the absence of positive root pressures, even at xylem water potentials of 30 kPa (Salleo et al. 1996). For that species, phloem transport through inter-trafficking between xylem and phloem appears to be essential to the refilling process, although the exact mechanism of refilling is unknown.
At the cellular level, the results of fluorescence resonance energy transfer (FRET) imaging in living maize protoplasts co-expressing plasma membrane intrinsic proteins 1 (PIP1s) and 2 (PIP2s) further support a model in which aquaporins of the two classes directly interact, very likely by heterotetramerization, to facilitate PIP1 trafficking. Whereas interaction-dependent trafficking of PIP1s and PIP2s offers a broad range of combinatorial regulations, a future challenge is to determine to what extent this process can dominate the expression of PIP1 or PIP2 homotetramers. Similar to other membrane proteins, PIP2 aquaporins are subjected to constitutive cycling. Their endocytosis is clathrin-dependent (Dhonukshe et al. 2007) and reduced by auxin (Siefritz et al. 2002). Export of PIP2 aquaporins from the endoplasmic reticulum is also critically controlled, and the role of a diacidic motif contained in the N-terminal tail of PIP2s was recently uncovered in maize and Arabidopsis (Fig. 4) (Maurel et al. 2008). The cellular mechanisms that determine aquaporin trafficking and their subcellular relocalization in response to stimuli will surely fuel intense investigations in the coming years. Additionally, the interplay of phytohormones produced in the roots and shoots in long-distance signaling evidenced by variations in xylem sap cytokinin concentrations, shoot auxin level, auxin transport, and auxin response seems to be operative for induction of root pressure. For instance, chemical signals, altered under and originating from roots, play an important role in the root-to-shoot communication in the movement of water from soil layers through roots and shoots to sustain plant growth. Therefore, the manner, not yet fully understood, for the initiation and control of root pressure is important in root-to-shoot signaling and vice versa. For example, expression of AtNCEDs, AtABA2, and AAO3 genes in phloem companion cells and xylem parenchyma cells of turgid plants is probably the main site of ABA biosynthesis in unstressed plants and ABA, and its precursors might be synthesized in vascular tissues and transported to target cells such as stomata, hydathodes, and sites of root pressure (Koiwai et al. 2004). ABA by way of its presence in vascular bundles, roots, and leaves might influence gating of aquaporins resulting in increased permeability to water hence its increased transport pressing the water to exude from stump and guttation.
The multiple cellular functions of plant aquaporins. The figure illustrates the variety of transport functions achieved by aquaporins in various subcellular compartments. The different aquaporins subclasses or isoforms are identified below the illustration in distinct colors. Isoforms of the plasma membrane intrinsic protein 1 (PIP1) and PIP2 subfamilies are thought to follow the secretory pathway, which carries cargo from the endoplasmic reticulum (ER) toward the plasma membrane through the Golgi apparatus. PIPs also undergo repeated cycles of endocytosis and recycling through endosomal compartments before being eventually targeted to the lytic vacuole through the multivesicular body [Source: Maurel et al. (2008), for further explanation, see Maurel et al. (2008)]
7 Mechanism of Root Pressure
Till the last date of writing this review paper, there was no unanonymous agreement about the mechanism of root pressure in plants. Here, I am presenting a synthesis of osmotic and metabolic including biophysical and molecular aspects in an integrated manner stressing the need for both the water and solutes entering the plant roots to account for the mechanism of root pressure. The presence of solutes constitutes the osmotic aspect, but their uptake along with energy-driven uphill uptake of water and transport constitutes the metabolic including biophysical and molecular aspects (Dustmamatov et al. 2004; Zholkevich et al. 2007; Wegner 2014).
7.1 Osmotic Aspect of Root Pressure
The most generally accepted explanation of root pressure is based on active accumulation of solutes by root cells, their secretion into the xylem, and the subsequent osmotic movement of water along the water potential gradient thus established (Bai et al. 2007; Zhu et al. 2010). Thus, the “osmometer model” considers the tissue separating the xylem sap from the external medium as a semipermeable transport barrier or “membrane”; alternatively, the root symplast is accepted as a third, transitory compartment. At night, when there is almost no transpiration, root cells continue pumping mineral ions into the xylem of the stele. Meanwhile, the endodermis and exodermis help prevent the ions from leaking out. The resulting accumulation of minerals lowers the water potential within the stele. Water flows in from the root cortex, generating root pressure, a push of xylem sap. In brief, the development of root pressure seems to follow or accompany an active transport of salt into the xylem conducting system, where the osmotic value (more negative osmotic potential) rises above that of the external solution. Thus, water so withdrawn from the external solution will depend on the difference between the osmotic pressure of the latter and that of the xylem vessels after intervening cells have reached full turgor. Since the parenchyma within the endodermis, being confined, is limited in extensibility on account of the structure of the endodermal cells, a strong hydrostatic pressure will therefore develop in this core of cells, sufficient to cause an excretion of water and solutes into the xylem vessels so long as the osmotic gradient persists. When the water and solutes enter the xylem, they are free to move upward in the vessels. Water may leak backward as far as the endodermis but no further on account of the suberized walls of the endodermis, which structure has been shown to prevent such a backward leakage. Priestley (1920) showed that the apical region of the root did not permit a backward leakage.
7.2 Molecular Mechanism of Root Pressure
There is a very strong group of Russian workers headed by Zholkevich who proposed the metabolic concept of root pressure apart from the osmotic concept discussed above which could not justify fully the uptake and upward movement of water and solutes implicated in root pressure (Dustmamatov et al. 2004; Zholkevich et al. 2007; Dustmamatov and Zholkevich 2008). Actually, it is the isotonic water flow or even radial water flow against an osmotic pressure gradient between the external medium and the guttation fluid or the exudate secreted by the root stump which paved the path for the involvement of metabolic process in root pressure (Oertli 1966; Zholkevich 1991; Schwenke and Wagner 1992; Enns et al. 2000; Pickard 2003a, b). It was Oertli (1966) who first proposed active water transport in plants taking place at the expense of metabolic energy defining the process characterized by the increased water potential, and this gain, he explained, must depend on the decrease in free energy in some metabolic process. This followed the work of Ginsburg (1971) on active water transport in a corn preparation who stated that water flow had two components, one osmotic and one non-osmotic. The non-osmotic flow was inhibited by cyanide. No correlation was found between water flow and solute flow suggesting that active water transport occurred in the root pressure. The Russian workers headed by Zholkevich at the K. A. Timiryazev Institute of Plant Physiology, Moscow, went steps further ahead accumulating a huge amount of data which suggested the involvement of metabolic process triggered by G-proteins during stimulatory action of neurotransmitters such as adrenalin and noradrenalin in root exudation, water transport, and creation of the root pressure (Mozhaeva and Pil’shchikova 1972; Zholkevich 1991; Dustmamatov et al. 2004; Zholkevich et al. 2007; Dustmamatov and Zholkevich 2008).
Now, very recently has come the work of Wegner (2014) on the horizon of root pressure research who has proposed an energetically driven “uphill water co-transport hypothesis” integrating osmotic and metabolic mechanisms with aquaporin-facilitated water transport within xylem parenchyma taking advantage of the free energy gradients of ions and sugars. In fact, the energy source for such pumping (ultimately ATP) was not exhausted for several days after supplies of photosynthate and reducing power from the shoot. The source of ATP must have been from substrate reserves and from tissue degradation. If the latter was significant, the author explained, it was also remarkable that the integrity of membranes, critical in maintaining the diffusion barrier between the xylem and the outside solution, was preserved for so long. This process could drive volume flow “energetically uphill” against the free energy gradient of water. According to the model of co-transport of water, solutes released by xylem parenchyma cells are subsequently retrieved from the sap at the expense of metabolic energy to maintain the concentration gradient that drives the water secretion (Fig. 5a, b). The salt release by co-transporters is an electroneutral process (Zeuthen and McAulay 2012) and would not interfere with K+ reuptake by ion channels that require a membrane potential more negative than the Nernst potential of K+, which is maintained by proton pump activity (Fig. 5a). Evidence for “simultaneous” uptake and release of K+ has indeed been obtained for root tissue, using refined radioactive tracer techniques (Britto and Kronzucker 2006). Rapid, seemingly “futile cycling” of ions is apparently a common phenomenon at root membranes that was found for K+, Na+, and Cl− and becomes more prominent at elevated concentrations of these ions. Futile cycling consumes metabolic energy, but its benefit for the plant seemed to be elusive and so far has remained an open question. Water secretion may be part of the answer. In his quantitative biophysical analysis of coupled ion and water transport by cation–chloride co-transporters type (CCC type), the framework of the thermodynamics of irreversible processes was conveniently applied to arrive at a quantitative expression for the hypothesis on root pressure developed by Wegner (2014). Overall volume flow across the plasma membrane of xylem parenchyma cells can be described as the sum of two separate components, namely, volume flow driven by the chemical potential of water and volume flow driven by the CCC transporter (Teakle and Tyerman 2010). Transporters of the CCC type known to mediate water secretion in mammalian cells have also been found in Arabidopsis and in rice. The mechanism proposed here for root pressure could also explain refilling of embolized vessels and contribute to long-distance water transport in trees when the cohesion–tension mechanism of water ascent fails. Possibly, several pathways for co-transport of water and ions (solutes) may coexist in the membrane to achieve the required rate of water secretion under various conditions, as previously also described for epithelia (Zeuthen 2010), though the reality is even more complex. However, the present hypothesis needs rigorous testing and verification in plants of different habitats and statures. Embolism repair requires vessels to be filled with xylem sap secreted by adjacent cells. It is generally believed that some overpressure has to be built up in a vessel during the refilling process in order to dissolve residual inclusions of gas completely; removal of cavitation nuclei appeared to be a prerequisite for a vessel to regain functionality (Holbrook and Zwieniecki 1999; Zwieniecki and Holbrook 2009; Nardini et al. 2011; Secchi and Zwieniecki 2011; Brodersen and McElrone 2013). Usually refilling occurs overnight when transpiration is low and little or no tension prevails in adjacent, functional vessels. This issue has been taken into account by this mechanism of root pressure.
(a) Hypothetical interplay of membrane transporters in the plasma membrane of xylem parenchyma cells for water secretion. Coupling between water and ion transport occurs in a potassium–chloride co-transporter type (KCC type) that translocates K+ and Cl− together with a fixed number of water molecules. Note that this transport is electrically silent. The ions are at least partly recycled via a K+ inward-rectifying channel and a Cl−−2H+ symporter, respectively. These processes are energized by the activity of a H+ ATPase that maintains the H+ gradient and hyperpolarizes the membrane to values more negative than E K+. Aquaporins may to some extent short-circuit co-transport-driven water flow if their activity is not downregulated. Note that all transporters have been demonstrated to coexist in the plasma membrane of root stelar cells. ΔV M = membrane potential of xylem parenchyma cell. E K+ = Nernst potential for K+ [Source: Wegner (2014), for more details, see Wegner (2014)]. (b) Alternative model for water secretion that makes use of different water–ion coupling ratios in outward- and inward-rectifying K+ channels. Arbitrarily, the K+ outward rectifier is thought to carry three water molecules together with one K+ ion, whereas the inward rectifier transports water and K+ on a 1:1 basis. Both rectifiers operate alternately, coordinated by membrane potential oscillations. In this way, futile K+ cycling is organized that drives a net water flow from the cytosol into the apoplast. Note that this K+ cycling consumes metabolic energy when the membrane potential is hyperpolarized by proton pump activity. ΔV M = membrane potential of xylem parenchyma cell. E K+ = Nernst potential for K [Source: Wegner (2014), for more details, see Wegner (2014)]
8 Factors Affecting Root Pressure
Several factors such as drought, salinity, soil compaction, flooding, low temperatures, and light intensity; a number of mechanosignals such as pressure, wind, gravity, mechanical loading, etc.; and the nutritional status affect the hydraulic conductivity of the tissues, hence root pressure. The root pressure keeps on fluctuating generally within a range of 0.05–0.3 MPa and often goes up to 0.8 MPa or even 6 MPa (White et al. 1958; Kundt and Gruber 2006) varying with species, growing conditions, environmental and edaphic factors, etc. accompanied by seasonal and diurnal periodicity. Therefore, more than one cause of root pressure can perhaps be effective in sap exudation, bleeding, or guttation due possibly to increased root pressure (Raleigh 1946). As described earlier, with regard to water absorption control in the roots, plants also present a family of water channel proteins, called aquaporins. These proteins have a critical role in water absorption, reducing the resistance to the water flow along the transcellular path. The number of these proteins available for the root surface is variable throughout the day. The aquaporins are controlled by many endogenous and exogenous factors of the roots including environmental factors that interfere in hydraulic conductance along the water flow by the plant (Siefritz et al. 2002; Maurel et al. 2008; Heinen et al. 2009).
8.1 Chemical Factors
At the molecular level, factors affecting directly the gating of aquaporins include phosphorylation, heterotetramerization, pH, cations, solute gradients, etc. apart from the kinetic energy of water molecules (Maurel et al. 2008; Benga 2009). In addition, the permeability of water channel proteins is influenced by nutrient stress, plant hormones, and attack by pathogens. The phosphorylation sites are located in the N-terminal and C-terminal segments and also in loop B. Calcium-dependent protein kinases are involved in phosphorylation that results in the pore opening. Hydroxyl radicals also induce a marked (≥90 %) and reversible inhibition of water transport in Chara cells, which was interpreted in terms of direct oxidative gating of aquaporins (Henzler et al. 2004). On the other hand, cytosolic proteins decrease the water permeability of PIPs and tonoplast intrinsic proteins (TIPs). A coordinated inhibition of PIPs and, as a consequence, a general block of root water transport during anoxic stress (resulting from soil flooding) was attributed to closure of the channel after cell acidosis.
8.2 Nutrient Stress Factors
An unexplained interaction between cell and root hydraulic conductivity and the supply of certain mineral nutrients has been described in many species. At the cellular level, NO3 − deprivation decreased hydraulic conductivity of cortical cells in cotton roots to a period of 4 days (Radin and Matthews 1989). At the whole-plant level, it decreased by a similar extent grown in P-deficient conditions (Radin and Eidenbock 1984) and by 20 % of control values over a 4-day period in SO4 2− deprived barley roots (Karmoker et al. 1991). Short periods of oxygen starvation (Birner and Steudle 1993; Else et al. 1995) and variation in the supply of nutrients (N, P, S) and of NaCl (high salinity) are all characterized by marked changes in the hydraulic conductivities of roots or root cells. In the future, it will be an obvious challenge to find out how the diurnal variation in root hydraulic conductivity and aquaporin expression fit into the broader picture of variable root pressure which has been known for a long time.
8.3 Physical Factors
Physical pressure has been shown by “patch clamp” technique very clearly to cause prevention of ion transport across the membrane affecting turgor pressure of cells. Wind, gravity, touch, sound, snow loading, etc. have been found to affect aquaporins gating, hence water transport, affecting root pressure (Telewski 2006).
8.4 Genetic Factor
As described earlier, the phenomenon of root exudation occurs in a wide range of plant species which include herbaceous mesophytes, shrubs, and woody trees in angiosperms (Singh 2014b). Though there is lack of information on genotypic differences in root exudation among field and horticultural crops yet, the rate of root pressure differs among rice varieties (Lafitte and Courtois 2002). Similar varietal and species responses to root pressure were observed in tomato, orange, and watermelon (Mitchell et al. 1991; Dorais et al. 2001). Fujii and Tanaka (1957) examined the difference in the guttation and bleeding of seedlings of various varieties of rice. The increased guttation of the late-maturing varieties as compared to early-maturing ones was possible due to increased root pressure on account of efficient root metabolism. Recently, Singh et al. (2008, 2009b) also found large genotypic variability in guttation rate among modern rice cultivars which was correlated with their panicle sink potentials. This could also be due to perhaps increased root pressure though this trait was not quantified. Obviously, the genetic basis of variation in root pressure is not known and so is the case with ion channel types. Therefore, our knowledge is hampered by the lack of such information.
8.5 Aquaporin Factor
Globally, there appear to be eight major research centers, three in Germany, one each headed by Steudle (sadly, he died a few years ago), Schaffner, and Kaldenhoff, respectively, Maurel in France, Tyree in the USA (though he assumes multinational locations), Chaumont in Belgium, Tyerman in Australia, and Maeshima in Japan engaged in groundbreaking research in discovering, characterization, localization, structural chemistry, mechanism of action, and physiological role of aquaporins in fine-tuning of water uptake and transport in plants influenced by a number of chemico–mechanosensors for their survival and productivity in changing environment (Maurel et al. 2008; Maeshima and Ishikawa 2008; Kaldenhoff et al. 2008, 2014; Vandeleur et al. 2009; Heinen et al. 2009). Evidently, Steudle’s laboratory has been the hub of attraction for aquaporin research in plants where scientists from all corners of the world tended to converge at one time or another, for example, Lafitte from IRRI, Tyerman from Australia, Chaumont from Belgium, Bohnert from the USA, Maurel from France, and Smith and Clarkson from England can be seen in a number of publications under joint authorships. By this narration, however, under no circumstances I mean to undermine the significance of excellent work currently being done in other laboratories as well (Benga 2009) which have been duly credited and described as and when necessary in this chapter.
The first water channel protein (WCP), called today aquaporin 1 (AQP1), was discovered in the red blood cell (RBC) membrane by Benga’s group in 1985 in Romania (Benga et al. 1986a, b) followed by the first evidence regarding the existence of a WCP in plant membranes (Wayne and Tazawa 1990). In 1993, a protein from the vacuolar membrane (tonoplast) of Arabidopsis thaliana was identified as a WCP by Maurel and coworkers in France. Aquaporin activity is regulated at both the transcriptional and the posttranslational levels. Aquaporins are encoded by genes which display a remarkable degree of conservation across taxa and kingdoms, the most obvious homology stems from two loops that both harbor the signature amino acid motif Asn–Pro–Ala (NPA) (Maurel et al. 2008; Heinen et al. 2009). Based on structural studies with mammalian aquaporins, the NPA loops are presumed to form the pore which confers permeability to water driven by osmotic or hydraulic gradients across the membrane (Jung et al. 1994).
It is noted that responses to a change in environmental conditions can also be realized by other mechanisms, including aquaporin gating influenced by kinetic energy of water molecules, translocation of aquaporins into the membrane, and interactions of membrane proteins (Hedfalk et al. 2006; Zelazny et al. 2007; Maurel et al. 2008; Aroca et al. 2012). Finally, aquaporin functions need to be further integrated in the whole-plant physiology. This will first require a better understanding of how the various transport activities of aquaporins are coupled with those of other transport proteins. The chains of events that lead to control of aquaporin functions by hormones, local or long-distance signals, in response to mechanosensory stimuli, will also have to be elucidated. Finally, although the field of aquaporin research has already enlarged considerably, we may not be at the end of our surprises because of novel primary functions as diverse as cell proliferation. Although much has been learned about the possible physiological roles of aquaporins in plants, many questions remain unanswered (Baiges et al. 2002; Aroca et al. 2012).
8.6 Environmental Factors
The environmental conditions in general affect root pressure, but because of genetic differences related to internal cellular sensing networks, responses vary between plants in terms of the effect on root pressure. Which is why, conditions that discourage root pressure such as cold, dry aerated soil, etc. also reduce guttation which itself is the expression of root pressure. The change in humidity brings about variation in aquaporin activities. Thus, the change in humidity resulted in an accumulation of water channel proteins, and those proteins were still present 24 h later. This conclusion is supported by immunolabeling experiments, which revealed that PIP1 protein remained highly abundant in root cross-sections 28 h after the transfer to lower humidity. How exactly changes in the aboveground environment are transmitted to and sensed by roots remains unknown. The most parsimonious hypothesis is that root cells sense xylem pressure pulses (McElrone et al. 2007) or changes in water potential (Levin et al. 2009), and/or cell turgor (Hill et al. 2004), which all would correspond to changes in hydraulic conductivity affecting root pressure. Future work is required at unraveling the nature of this signaling process as how the signal is perceived by root aquaporins.
8.7 Soil Factors
8.7.1 Soil and Root Temperature
Root pressure being an energy-dependent process as discussed earlier, root temperature plays a dominant role in its regulation. Pedersen (1993, 1994) showed by measuring the rate of guttation, which is the manifestation of root pressure, that in submerged aquatic plants Sparganium emersum and Lobelia dortmanna, the acropetal water transport is clearly an active process confined to the roots. The flow is stopped by cooling the root compartment to 4 °C, and lowering the temperature from 15 to 10 °C reduced the guttation rate fivefold. This indicates that the water transport is dependent on root metabolism and that the driving force is restricted to the roots. Although periodicity in bleeding appears to be automatic in origin, a sharp increase in temperature will determine the time of occurrence of the maxima and minima (Fujii and Tanaka 1957). Under spring conditions, soil temperatures may remain several degrees higher than air temperatures at night. Here, intense radiation may warm the soil during the day, and a rapid cooling of the air at night produces optimal conditions for root pressure to occur (Frey-Wyssling 1941). Tropical areas with humid night air and warm soil also are particularly favorable for rapid root exudation. Thus, the phenomenon of root pressure does appear to be affected significantly by prevalent soil and root temperature suggesting the involvement of metabolic control of minerals uptake via membrane ATPases on the one hand and functioning of aquaporins for water influx into the roots, on the other.
8.7.2 Soil Moisture
Root exudation is very common during warm humid nights in plants growing in high soil moisture. These conditions favor low transpiration and high root pressure. Even at night after periods of water stress, absorption may not completely replace the water deficit in the plant, and then actual pressures would not be developed in the xylem (Stocking 1956). Thus, no root exudation was observed under conditions of soil moisture stress (Kramer and Boyer 1995). The root pressure mechanism, as measured by exudation, ceased in Coleus, sunflower, and tomato plants growing in sandy soil when about 45 % of the moisture available to intact plants still remained in the soil. It appears that root pressure probably is not developed in plants growing in soil containing less than about 45 % of the moisture in the range from moisture equivalent to permanent wilting percentage (Zaitseva et al. 1998). If in these instances water is added to the soil, root exudation soon follows. More recently, Singh et al. (2009b) have provided quantitative data on the relationship between soil moisture stress and guttation caused by root pressure in rice. The volumes of guttation fluid, an indirect measure of root pressure, were 19 μL, 56 μL, and 93 μL at leaf water potentials of −1.0, −0.5, and −0.2 MPa (watered), respectively. Lowered water potentials of roots seem to affect the gating as well as distribution of various isoforms of aquaporins (Katsuhara et al. 2008; Heinen et al. 2009) inhibiting the entry of water becoming not enough to cause hydrostatic pressure in the roots on the one hand and cause cavitation and embolism in plants on the other (Holbrook et al. 2001; Brodribb and Holbrook 2006).
9 Consequences and Implications of Root Pressure
The ascent of water in terrestrial transpiring plants, both tall and short, has been extensively studied and explained to occur in response to increasing water potential gradient between top and bottom of the plants, a concept popularly known as “cohesion–tension” mechanism (Dixon and Joly 1894; Scholander et al. 1965; Tyree 2003a). However, the upward movement of water in the absence or reduced transpiration, for example, in seedlings emerging through soil crust (Misra et al. 1986), upward movement of water and nutrients in submerged aquatic plants wherein no transpiration takes place (Pedersen 1993, 1994), nocturnal ascent of sap in tall bamboos (32–40-m high) (Zachary 2009; Cao et al. 2012), climbing forest vines (Ewers et al. 1997; Fisher et al. 1997), rice plants (Singh and Singh 1989; Singh et al. 2009a), and cracking of pavements and walls by grasses and tree roots (Grabosky et al. 2011) are known to take place due to positive hydrostatic pressure in roots. Water rises in plants not only during sunny daytime but also at night, during dry epochs, and in winter, before the leaves have unfolded. It also rises at the bottom of rain forests—manifested by guttation where transpiration is prevented by 100 % air moisture and almost complete darkness, as well as in plants kept locked up in closed glass containers for a whole season. In all such thirsty intervals, plants depend uniquely on their root pumps (Kundt and Gruber 2006). The significance and implications of root pressure in various fields of studies are briefly described hereunder.
9.1 Agronomic Significance
The phenomenon of root pressure has a number of practical and agronomic significance which will be briefly described here. It is a crucial factor to control tipburn in head lettuce by optimizing its magnitude in polyhouses (De Swaef and Bleyaert 2012). The radial and axial pressure components of root pressure of emerging seedlings help in reducing and overcoming resistance of compacted and sodic soils (Misra et al. 1986; Bengough and Mullins 1990) leading to proper seedling growth and optimum plant density. The roots exert radial as well as axial stress which causes tensile failure in significant magnitude helping the emergence of seedlings through the hard and compacted soils. The axial and radial root growth pressures measured in this manner were, for example, of comparable magnitudes registering 497, 289 and 238 kPa for pea, cotton, and sunflower seedlings of similar size, respectively (Misra et al. 1986). Obviously, this excess pressure allows plants to penetrate into the ground, split rock, lift concrete plates, and push away obstacles. Shoots from coconuts pierce their tough enclosing shells on account of high magnitude of hydrostatic pressure developed in the shoot and root of emerging seedlings. Root pressure helps in root growth causing its turgor-induced elongation through soil matrix. It also helps in pushing soils apart facilitating proper soil aeration which is essential for nutrient uptake and root respiration leading to proper root growth and its metabolism. Further, by pushing the soils apart, root pressure also enlarges pore spaces in soils increasing their water storage capacity, hence increasing the soil moisture content. Root pressure has also been implicated in some plant diseases (Johnson 1936). Further, due to increased root pressure, bursting of cells allows pathogens such as Botrytis and Mycosphaerella to infect the damaged tissue and spread around in the plant. Failure of refilling embolized tissues can lead to crown dieback disease.
Root pressure also aids in inter-trafficking of xylem and phloem saps. Recently, xylem and phloem saps have been reported to carry a number of proteins including transport proteins, enzymes, amino acids, and hormones apart from inorganic compounds (Singh and Singh 2013). The distribution of these chemicals is facilitated through inter-trafficking by root pressure and by circulating in the entire plant system to be utilized in organs they are required most (Burkle et al. 2003). Out of these two processes, the former is undoubtedly regulated by root pressure, at least under the conditions of the absence or reduced transpiration facilitating inter-trafficking of organic and inorganic substances through these conducting tissues. Therefore, root pressure is an essential component of inter-trafficking of chemicals required for proper plant growth and yield.
9.2 Horticultural Significance
In the wake of global warming and climate change, the reduction of energy use has become an important issue in glasshouse cultivation. It is, therefore, imperative to develop a modified crop management and new energy-efficient climate strategies that may create conditions favoring the buildup of optimum root pressure. Because the occurrence of excessive root pressure may cause considerable damage to the harvestable product (Heuvelink et al. 2008), the technique developed by De Swaef et al. (2013) could be of direct significance to growers, as an indicator when root pressure-associated problems might occur. Because these new energy-efficient technologies allow better glasshouse climate control, potentially unfavorable conditions could be avoided using an intelligently managed compromise between energy saving and optimal growing conditions. Because some important physiological problems in horticulture such as watery fruits in tomato, watermelon, muskmelon, etc. (Mitchell et al. 1991; Johnson et al. 1992; De Swaef et al. 2012) and glassiness in lettuce (Maaswinkel and Welles 1986) are currently attributed to excess root pressure, a critical level at which root pressure becomes undesired should be determined.
To date, the main problem for investigating this critical level, as well as the environmental factors driving root pressure, is the lack of appropriate systems for determining root pressure. Therefore, a nondestructive technique could allow root pressure to be estimated continuously while imposing a wide range of growing conditions. Recently, Clearwater et al. (2007) unraveled a correlation between the rootstock affinity to build up root pressure and the scion vigor after grafting in kiwi. For tomato, it is known in practice that rootstocks have an important effect on scion vigor and on the occurrence of root pressure-related problems. The noninvasive technique could be used to select rootstocks quantitatively with a different affinity for root pressure. As a result, better combinations of rootstocks and productive grafts could be made. Root pressure is also expected to play a beneficial role in the distribution of calcium to the leaves of cabbage and lettuce (Palzkill and Tibbitts 1977).
9.3 Use of Root Pressure in Physiological Investigations
9.3.1 Use of Root Pressure in Measuring Hydraulic Properties
The phenomenon of root pressure can be used for a number of physiological research investigations. Accordingly, the root pressure can be used as a tool to measure the hydraulic properties of the whole-plant system (Liu et al. 2009) in deciding the nature of root water flow, i.e., apoplastic or symplastic, in a number of crop plants of economic importance (Knipfer and Fricke 2010).
9.3.2 Use of Root Pressure in the Determination of Reflection Coefficients
The reflection coefficients may be determined by using root pressure as a tool. Deviation of the reflection coefficient from 1.0 may be attributable to a nonperfect semipermeability of the water uptake pathway between the medium and the xylem or to bypass flow at the endodermis (Steudle et al. 1993; Steudle and Peterson 1998). However, one should be cautious as the possibility of induction of some artifact may change the interpretation.
9.3.3 Use of Root Pressure in the Determination of Nutrients and Hormones Movement in Various Plant Organs
The root pressure can be used for tracing the path, velocity, and quantum of different nutrients, injurious heavy metals, pesticides, and hormones in plant organs at various growth stages for deciding their fate (Singh and Singh 2013). A convective viscous mass transport system can move nutrients in a bulk flow of water from root to shoot. Such a mass transport of water could be driven by root pressure which has been demonstrated in a wide range of terrestrial (Sperry 1983; Singh and Singh 1989; Zholkevich 1991; Dustmamatov et al. 2004; Feild et al. 2005; Singh et al. 2009a) and submerged aquatic plants (Pedersen 1993, 1997; Pedersen and Sand-Jensen 1997). Under moist or saturated soil conditions with high relative humidity of the atmosphere, the root systems of plants like rice, tomato, potato, etc. absorb excess of water by active uptake giving rise to the buildup of root pressure. Palzkill and Tibbitts (1977) and Dieffenbach et al. (1980) used successfully the root exudation carrying plant nutrients as a way to track the flow of water and mineral ions from the roots. It thus appears that the water transport in the xylem, brought about by root pressure, is in itself sufficient for long-distance mineral supply and that transpiration is not required for this function (Tanner and Beevers 2001). Root pressure-directed water transport, distributing required inorganic nutrients and phytohormones, both derived from the roots, ensures optimal plant growth in the absence of a transpiration stream.
Further, the important natural plant hormones such as auxins, GAs, cytokinins, and ABA have been characterized in xylem saps, hence transported to the sites of their requirement for regulating growth, development, and fruiting by root pressure (Fletcher and Mader 2007). The abovementioned physiological effects attributed to root pressure indicate the need to investigate this intriguing phenomenon further in depth. However, currently a “bottleneck” in root pressure research is the lack of a system that allows continuous and nondestructive measurements, especially for herbaceous plants.
9.3.4 Use of Root Pressure in the Maintenance of Shoot Water Storage
Undoubtedly, root pressure is a mechanism to “push” xylem sap up the plant. The contribution of root pressure giving rise to guttation in the maintenance of leaf water potential has been realized (Barrs 1966; O’Leary 1966), because within the vascular bundles, pressure pushes water into the main space available in the vessels, repairing embolisms caused by water stress (Singh and Singh 1989; Holbrook et al. 2001; Brodribb and Holbrook 2006; Singh et al. 2009a). Observations of strawberry plants suggest that the presence or absence of root pressure-generated guttation is related to plant water status (Takeda and Glenn 1989). Leaves with guttation had a predawn leaf water potential (PLWP) higher than −0.1 MPa, and those without guttation had PLWP lower than −0.1 MPa. Also, root pressure supplies water to the shoots of rice (Singh et al. 2009a), bamboos (Zachary 2009), and other trees as well (Sperry et al. 1987) during spring when plants are devoid of leaves, left with no provision for pulling water up by transpiration. Hence, it saves trees and crops from collapse due to water deficits (Sperry et al. 1987; Clearwater et al. 2007).
9.3.5 Use of Root Pressure in Developing Drought-Resistant Cultivars
This trait has been found to be associated with the amount of sap exuded from single cut tillers of rice, and initial analyses indicated lower root pressure to be linked with drought sensitivity (Lafitte and Courtois 2002; Lian et al. 2004). Xylem vessel cavitation was observed, and nighttime root pressure was hypothesized to be important for refilling of cavitated xylem vessels (Stiller et al. 2003). Cavitation-resistant juniper continues gas exchange during drought, extracting more water from the soil. But in doing so, the juniper eventually nevertheless suffers considerable cavitation, making it less competitive for water after the next rain. In this context, understanding the extensive mortality of piñon pine across the Southeastern USA in recent years is imperative (McDowell et al. 2008).
9.3.6 Use of Root Pressure in Enhancing Production of Recombinant Proteins, Allelochemicals, and Chelatins
Guttation is the best-known phenomenon attributed to root pressure which has a number of agricultural and pharmaceutical implications (Singh and Singh 2013; Singh 2014a, b). Recently, plants have emerged as one of the most promising general production platforms for pharmaceuticals and are now gaining widespread acceptance for the large-scale production of recombinant proteins (Komarnytsky et al. 2000). These problems have been addressed by engineering two related tobacco plant production systems in Raskin’s laboratory in the USA to continuously secrete recombinant proteins from their roots called “rhizosecretion” into a simple hydroponic medium (Borisjuk et al. 1999). There is also leaf secretion called “phyllosecretion” which is caused by increased root pressure (Komarnytsky et al. 2000). Undoubtedly, these processes of nondestructive secretions are governed by root pressure. Apart from these, secretions of organic acids and transport of heavy metal chelatins (Sears 2013) and allelochemicals (Xiao et al. 2006) from roots are also regulated by root pressure. Therefore, the optimization of root pressure can play a significant role in the noninvasive production of pharmaceuticals, allelochemicals, and heavy metal chelatins which are of immense economic importance.
9.4 Ecological Significance
Ecological adaptation has enabled desert plants to even achieve root pressures of 6 MPa. They require an excess pressure inside the plant body, not to be established by suction (White et al. 1958; Kundt and Gruber 2006). Further, lianas (woody vines) are much more common in tropical than temperate ecosystems, but the reasons for this are unknown (Gentry 1991). Lianas have long been known to have thin stems and a high ratio of leaf area to transverse stem area (Putz 1983; Ewers and Fisher 1991). Wide xylem vessels, which in temperate areas are quite prone to freezing-induced embolism (Cochard and Tyree 1990; Sperry and Sullivan 1992; Sperry et al. 1994; Tyree et al. 1994), are also one of the characteristic features of tropical lianas (Berger 1931; Ewers and Fisher 1991). It is not known whether the wide vessels of tropical vines usually avoid embolism throughout the life of the stems or if they become embolized and are periodically refilled. If a perennial plant such as a liana could not refill its embolized vessels, its distribution might be limited to environments where embolism induction would be minimal. For instance, some species might be limited to environments with a very consistent water supply and without freezing temperatures. However, more is required to be known about the vulnerability of lianas to embolism and about the possibility of embolism reversal by means other than root pressure as well (Lens et al. 2013). In terms of global distribution, there is a strong inverse correlation between latitude and liana abundance. Thus, root pressure appears to play an important role in the distribution of plant species suiting to prevalent environment. Also, nocturnal refilling in bamboos is another example of ecological significance. Positive root pressure is the primary mechanism for hydraulic movement in Guadua angustifolia (Zachary 2009). Nocturnally pumped sap is stored within internodal cavities for daily use. Differences among tissue sap flow profiles suggest that root pressure-induced sap flow is mediated by internodal tissue, while subsequent distribution to leaves and branches is carried out by nodal tissue. The evolution of sophisticated micro-valves in gymnosperms is crucial to the success of this lineage and helps conifers compete effectively for water with angiosperms (Pittermann et al. 2005). Hydraulic lift, also a consequence of root pressure which adds moisture to otherwise dry soils, dramatically alters the microenvironment and influences community composition (Jackson et al. 2000; Meinzer et al. 2001).
10 Concluding Remarks and Future Perspectives
It has been very exciting to see the progress made over the past about 15 years in elucidating the molecular mechanisms of water uptake, transport, and the root pressure. Modification of root function should continue to be a productive area for future applications to agriculture, horticulture, and forestry which may eventually enhance yield and quality of these crops under sustainable ecosystem. To ensure success in this area, we will need to more fully understand how to best regulate the expression of water co-transporters and their substrates to create more efficient plants for water use that do not have any soil and yield penalties. The combination of continuous measurements of water flow and stem diameter and the mechanistic water flow and storage model allowed elucidation of the effects on mechanisms other than the C–T theory, such as root pressure, which is as yet difficult to measure noninvasively in situ. Therefore, the techniques described in this chapter could have important implications both in practice and in future root pressure research.
The study of roots has advanced to the point where modifications can be made to both architecture and function using molecular tools. However, a challenge will be to find the winning combination of shoot and root traits that can be successfully combined to benefit the whole-plant growth and productivity by optimizing root pressure. The development and use of noninvasive quantification of root pressure and high-throughput techniques such as pressure probe, radioactive tracer isotopes, fluorescence resonance energy transport, magnetic resonance imaging, computed tomography, X-ray crystallography, knockout gene transfer technology, etc. have helped elucidate the mechanisms involved in root morphology and physiology. However, for stress-induced embolism in plants of economic importance, simple, cheap, and dependable techniques are required for use in laboratory and field. Although a link has been established between root hydraulics and expression and function of aquaporins, the knowledge of cell-specific expression, specified location, and function of root aquaporins is largely lacking, mainly due to the high diversity of aquaporin isoforms in plants. In the future, analysis of single knockout aquaporin mutants will hopefully provide evidence for the multiple functions of aquaporins in water transport, their distribution, and root pressure development affecting growth and development of plants and in their adaptive response to chemical, physical, environmental, edaphic, and genetic factors. Further investigations are necessary to determine the molecular structure of the water pores and the mechanisms of their selectivity and gating. There are, to date, no novel approaches to estimate the relative contribution of the two water transport pathways (e.g., apoplastic and symplastic) to the overall water and solute uptake and hydraulic conductivity of roots. With respect to having a clear picture, models of water uptake by root systems are still only semiquantitative and require completion. In order to set up good quantitative physical models, we must combine mathematical principles and computer technology with biological principles, including some biophysics and biochemistry. Studies of root physiology and of key genes that regulate water transport and root pressure should complement traditional studies of shoot physiology and stomatal control of water loss, fundamental for the understanding of plant water balance and overall productivity.
Many researchers have contributed a large amount of valid work in the mechanisms of water uptake by roots. Yet, there are still a lot of aspects which need perfection and improvement. Intense research is under way in different laboratories to clarify mechanisms of plant hydraulics. Therefore, the future promises to see a much clearer picture of the basic mechanisms for water uptake by plant roots and root pressure. Since roots play an essential role in the acquisition of water and minerals from soils, modifying root traits such as root pressure in crops for enhanced agricultural, horticultural, and forest produce is imperative. The future for the modification of crop plant roots for water and nutrients collection for optimizing root pressure looks promising, but there are important challenges too, though examples have emerged showing that modifications to roots result in higher yield, enhanced quality of roots, and increased water productivity. Using combined “patch clamp” and “biosensor” techniques to determine the uptake and movement of ions in roots from the soil solution as well as within the roots could provide novel tools. How dynamic aboveground changes are perceived by roots and how root aquaporins are subsequently regulated is not well understood. Although much has been learned about the possible physiological roles of aquaporins in plants, many questions remain unanswered. It is hoped that the combination of aquaporin genetics integrated with plant physiology and energetics of root pressure will provide critical insights into the hydraulic conductance architecture in response to chemico–mechanosensory systems for the induction of root pressure.
While closing the chapter, I believe that the materials presented in this review would contribute significantly to a future solution of root pressure and its implications in the wake of advancing biotechnological and nanotechnological innovations and arrest, at least temporarily for some time to come, the legacy of the use of such terms as “riddle” and “enigma” of root pressure.
References
Ahmed AM, Kroener E, Holz M, Zarebanadkouki M, Carminati A (2014) Mucilage exudation facilitates root water uptake in dry soils. Funct Plant Biol 41:1129–1137
Ameglio T, Ewers FW, Cochard H, Martignac M, Vandame M, Bodet C, Cruiziat P (2001) Winter stem xylem pressure in walnut trees: effects of carbohydrates, cooling and freezing. Tree Physiol 21:387–394
Aroca R, Porcel R, Ruiz-Lozano JM (2012) Regulation of root water uptake under abiotic stress conditions. J Exp Bot 63:43–57
Azaizeh H, Gunse B, Steudle E (1992) Effects of NaCl and CaCl2 on water transport across root cells of maize (Zea mays L.) seedlings. Plant Physiol 99:886–894
Bai X-F, Zhu J-J, Zhang P, Wang Y-H, Yang L-Q, Zhang L (2007) Na+ and water uptake in relation to radial reflection coefficient of root in arrowleaf saltbush under salt stress. J Integr Plant Biol 49:1334–1340
Baiges I, Schäffner AR, Affenzeller MJ, Mas A (2002) Plant aquaporins. Physiol Plant 115:175–182
Balling A, Zimmermann U (1990) Comparative measurements of the xylem pressure of Nicotiana plants by means of the pressure bomb and pressure probe. Planta 182:325–338
Baluska F (1995) Structure and function of roots. Springer, Berlin
Baluska F, Mancuso S (2013) Root apex transition zone as oscillatory zone. Front Plant Sci 4:354. doi:10.3389/fpls.2013.00354
Baluska F, Salaj J, Mathur J, Braun M, Jasper F, Samaj J, Chua N-H, Barlow PW, Volkmann D (2000) Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev Biol 227:618–632
Barber SA, Bouldin DR (1984) Roots, nutrients and water influx, and plant growth. ASA Publication, American Society of Agronomy, Madison, WI
Barrs HD (1966) Root pressure and leaf water potential. Science 152:1266–1268
Benga G (2009) Water channel proteins (later called aquaporins) and relatives: past, present, and future. IUBMB Life 61:112–133
Benga G, Popescu O, Pop VI, Holmes RP (1986a) p-Chloromercuribenzene-sulfonate binding by membrane proteins and the inhibition of water transport in human erythrocytes. Biochemistry 25:1535–1538
Benga G, Popescu O, Borza V, Pop VI, Muresan A, Mocsy I, Brain A, Wrigglesworth J (1986b) Water permeability of human erythrocytes. Identification of membrane proteins involved in water transport. Eur J Cell Biol 41:252–262
Bengough AG, Mullins CE (1990) Mechanical impedance to root growth—a review of experimental techniques and root growth responses. J Soil Sci 41:341–358
Berger W (1931) Das Wasserleitungssystem von krautigen Pflanzen, Zwergstaäuchern und Lianen in quantitativer Betrachtung. Beih Bot Centralbl 48:363–390
Birner TP, Steudle E (1993) Effects of anaerobic conditions on water and solute relations, and on active transport in roots of maize (Zea mays L.). Planta 190:474–483
Borisjuk NV, Borisjuk LG, Logendra S, Petersen F, Gleba YY, Raskin I (1999) Production of recombinant proteins in plant root exudates. Nat Biotechnol 17:466–469
Bose JC (1923) The physiology of the ascent of sap. Longmans, Green and Co., London
Boyer JS (1985) Water transport. Annu Rev Plant Physiol 36:473–516
Britto DT, Kronzucker HJ (2006) Futile cycling at the plasma membrane: a hallmark of low-affinity nutrient transport. Trends Plant Sci 11:529–534
Brodersen CR, McElrone AJ (2013) Maintenance of xylem network transport capacity: a review of embolism repair in vascular plants. Front Plant Sci 4:198
Brodribb TJ, Holbrook NM (2006) Declining hydraulic efficiency as transpiring leaves desiccate: two types of response. Plant Cell Environ 29:2205–2215
Burkle L, Cedzich A, Dopke C, Stransky H, Okumoto S, Gillissen B, Kuhn K, Frommer WB (2003) Transport of cytokinins mediated by purine transporters of the PUP family expressed in phloem, hydathodes, and pollen of Arabidopsis. Plant J 34:13–26
Cao KF, Yang SJ, Zhang YJ, Brodribb TJ (2012) The maximum height of grasses is determined by roots. Ecol Lett 15:666–672
Chamberlin TC (1916) The origin of the Earth. The University of Chicago Press, Chicago, IL
Clearwater MJ, Blattmann P, Luo Z, Lowe RG (2007) Control of scion vigor by kiwifruit rootstocks is correlated with spring root pressure phenology. J Exp Bot 58:1741–1751
Cobb AR, Choat B, Holbrook NM (2007) Dynamics of freeze-thaw embolism in Smilax rotundifolia (Smilacaceae). Am J Bot 94:640–649
Cochard H, Tyree MT (1990) Xylem dysfunction in Quercus: vessel sizes, tyloses, cavitation and seasonal changes in embolism. Tree Physiol 6:393–407
Cochard H, Ewers FW, Tyree MT (1994) Water relations of a tropical vine-like bamboo (Rhipidocladum racemiflorum): root pressures, vulnerability to cavitation and seasonal changes in embolism. J Exp Bot 45:1085–1089
Darwin C (1859) On the origin of species by means of natural selection or the preservation of favoured races in the struggle for life. John Murray, London
Davis TA (1961) High root pressure in palms. Nature 192:227–228
De Swaef T, Bleyaert P (2012). Is root pressure the crucial factor to control tipburn in head lettuce? http://www.inagro.be/ophalen_popup.aspx?lijst=Research&ID=11. Accessed 28 Jan 2015
De Swaef T, Verbist K, Cornelis W, Steppe K (2012) Tomato sap flow, stem and fruit growth in relation to water availability in rockwool growing medium. Plant Soil 350:237–252
De Swaef T, Hanssens J, Cornelis A, Steppe K (2013) Non-destructive estimation of root pressure using sap flow, stem diameter measurements and mechanistic modeling. Ann Bot 111:271–282
Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J et al (2007) Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol 17:520–527
Dieffenbach H, Kramer D, Luttege U (1980) Release of guttation fluid from passive hydathodes of intact barley plants. I. Structural and cytological aspects. Ann Bot 45:397–401
Dixon HH, Joly J (1894) On the ascent of sap. Philosophical transactions of the royal society. Biol Sci 186:563–576
Dorais M, Gosselin A, Papadopoulos AP (2001) Greenhouse tomato fruit quality. Hortic Rev 26:239–306
Draye X, Kim Y, Lobet G, Javaux M (2010) Model-assisted integration of physiological and environmental constraints affecting the dynamic and spatial patterns of root water uptake from soils. J Exp Bot 61:2145–2155
Dustmamatov AG, Zholkevich VN (2008) Effects of HgCl2 on principal parameters of exudation from maize detached root systems. Russ J Plant Physiol 55:814–820
Dustmamatov AG, Zholkevish VN, Kuznetsov VV (2004) Water pumping activity of the root system in the process of cross-adaptation of sunflower plants to hyperthermia and water deficiency. Russ J Plant Physiol 51:822–826
Ehleringer JR, Roden J, Dawson TE (2000) Assessing ecosystem-level water relations through stable isotopes ratio analysis. In: Sala OE, Jackson RB, Mooney HA, Howarth RW (eds) Methods in ecosystem science. Springer, New York, NY, pp 181–198
Else MA, Davies WJ, Malone M, Jackson MB (1995) A negative hydraulic message from oxygen deficient roots of tomato plants? Influence of soil flooding on leaf water potential, leaf expansion, and synchrony between stomatal conductance and root hydraulic conductivity. Plant Physiol 109:1017–1024
Enns LC, Canny MJ, McCully ME (2000) An investigation of the role of solutes in the xylem sap and in the xylem parenchyma as a source of root pressure. Protoplasma 211:183–197
Enstone DE, Peterson CA, Ma F (2003) Root endodermis and exodermis: structure, function, and responses to the environment. J Plant Growth Regul 21:335–351
Eshel M, Beeckman T (2013) Plant roots: the hidden half, 4th edn. CRC Press, Boca Raton, FL
Ewers FW, Fisher JB (1991) Why vines have narrow stems: histological trends in Bauhinia (Fabaceae). Oecologia 8:233–237
Ewers FW, Fisher JB, Fichtner K (1991) Water flux and xylem structure in vines. In: Putz FE, Mooney HA (eds) The biology of vines. Cambridge University Press, Cambridge, pp 127–160
Ewers FW, Cochard H, Tyree MT (1997) A survey of root pressures in vines of a tropical lowland forest. Oecologia 110:191–196
Feild TS, Arens NC (2007) The ecophysiology of early angiosperms. Plant Cell Environ 30:291–309
Feild TS, Sage TL, Czerniak C, Iles WJD (2005) Hydathodal leaf teeth of Chloranthus japonicus (Chloranthaceae) prevent guttation-induced flooding of the mesophyll. Plant Cell Environ 28:1179–1190
Fisher JB, Angeles GA, Ewers FW, López-Portillo J (1997) A survey of root pressure in tropical vines and woody species. Int J Plant Sci 158:44–50
Fletcher AT, Mader JC (2007) Hormone profiling by LC-QToF-MS/MS in dormant Macadamia integrifolia: correlations with abnormal vertical growth. Plant Growth Regul 26:351–361
Frey-Wyssling A (1941) Die guttation als aligemeine erscheinung. Berichte Der Schweizerischen Botanischen Gesellschaft 51:321–325
Fujii Y, Tanaka N (1957) Intensity of guttation in rice seedlings in relation to earliness or lateness of the variety. Jpn J Crop Sci 25:131–132
Gentry AH (1991) The distribution and evolution of climbing plants. In: Putz FE, Mooney HA (eds) The biology of vines. Cambridge University Press, Cambridge, pp 3–50
Ginsburg H (1971) Model for iso-osmotic water flow in plant roots. J Theor Biol 32:147–158
Grabosky JC, Smiley ET, Dahle GA (2011) Observed symmetry and force of Plantanus × acerifolia (Ait.) Willd. Roots occurring between foam layers under pavement. Arboricult Urban For 37:35–40
Hedfalk K, Hosefield T, Nyblom S, Johanson U, Kjellbom D, Neutze R (2006) Aquaporin gating. Curr Opin Struct Biol 16:447–456
Heinen RB, Ye Q, Chaumont F (2009) Role of aquaporins in leaf physiology. J Exp Bot 60:2971–2985
Henry A (2013) IRRI’s drought stress research in rice with emphasis on roots: accomplishments over the last 50 years. Plant Root 7:5–19
Henzler T, Steudle E (1995) Reversible closing of water channels in Chara internodes provides evidence for a composite transport model of the plasma membrane. J Exp Bot 46:199–209
Henzler T, Ye Q, Steudle E (2004) Oxidative gating of water channels (aquaporins) in Chara by hydroxyl radicals. Plant Cell Environ 27:1184–1195
Heuvelink E, Bakker MJ, Marcelis LFM, Raaphorst M (2008) Climate and yield in a closed greenhouse. Acta Hortic 801:1083–1092
Hill AE, Shachar-Hill B, Shachar-Hill Y (2004) What are aquaporins for? J Membr Biol 197:1–32
Holbrook NM, Zwieniecki MA (1999) Embolism repair and xylem tension: do we need a miracle? Plant Physiol 120:7–10
Holbrook NM, Ahrens ET, Burns MJ, Zwieniecki MA (2001) In vivo observation of cavitation and embolism repair using magnetic resonance imaging (MRI). Plant Physiol 126:27–31
Jackson RB, Sperry JS, Dawson TE (2000) Root water uptake and transport: using physiological processes in global predictions. Trends Plant Sci 5:482–488
Javot H, Lauvergeat V, Santoni V, Laurent M, Guclu J, Vinh J, Heyes J, Franck KI, Schaffner AR, Bouchez D, Maurel C (2003) Role of a single aquaporin isoform in root water uptake. Plant Cell 15:509–522
Johnson J (1936) Relation of root pressure to plant disease. Science 84:135–136
Johnson RW, Dixon MA, Lee DR (1992) Water relations of the tomato during fruit growth. Plant Cell Environ 15:947–953
Jung JS, Preston GM, Smith BL, Guggino WB, Agre P (1994) Molecular structure of the water channel through aquaporins CHIP. The hourglass model. J Biol Chem 269:14648–14654
Kaldenhoff R, Ribas-Carbo M, Sans JF, Lovisolo C, Heckwolf M, Uehlein N (2008) Aquaporins and plant water balance. Plant Cell Environ 31:658–666
Kaldenhoff R, Kai L, Uehlein N (2014) Aquaporins and membrane diffusion of CO2 in living organisms. Biochim Biophys Acta 1840:1592–1595
Karmoker JL, Clarkson DT, Saker LR, Rooney JM, Purves JV (1991) Sulphate deprivation depresses the transport of nitrogen to the xylem and hydraulic conductivity of barley (Hordeum vulgare L.) roots. Planta 185:2269–2278
Katsuhara M, Hanba YT, Shiratake K, Maeshima M (2008) Expanding roles of plant aquaporins in plasma membranes and cell organelles. Funct Plant Biol 35:1–14
Kirk GJD (1994) Rice roots: nutrient and water use. International Rice Research Institute, Los Banos, Philippines
Klepper B, Kaufmann MR (1966) Removal of salt from xylem sap by leaves and stems of guttating plants. Plant Physiol 41:1743–1747
Knipfer T, Fricke W (2010) Root pressure and a solute reflection coefficient close to unity exclude a purely apoplastic pathway of radial water transport in barley (Hordeum vulgare). New Phytol 187:159–170
Knipfer T, Das D, Steudle E (2007) During measurements of root hydraulics with pressure probe, the contribution of unstirred layers is minimized in the pressure relaxation mode: comparison with pressure clamp and high pressure flow meter. Plant Cell Environ 30:845–860
Koiwai H, Nakaminami K, Seo M, Toyomasu T, Koshiba T (2004) Tissue-specific localization of an abscisic acid biosynthetic enzyme, AAO3, in Arabidopsis. Plant Physiol 134:1697–1707
Komarnytsky S, Borisjuk N, Borisjuk L, Alam M, Raskin I (2000) Production of recombinant proteins in tobacco guttation fluid. Plant Physiol 124:927–933
Kramer PJ (1932) The absorption of water by root systems of plants. Am J Bot 19:148–164
Kramer PJ (1945) Absorption of water by plants. Bot Rev 11:310–355
Kramer PJ, Boyer JS (1995) Water relations of plants and soils. Academic, San Diego, CA
Kramer PJ, Currier HB (1950) Water relations of plant cells and tissues. Annu Rev Plant Physiol 1:265–284
Kramer PJ, Kozlowski TT (1979) The physiology of woody plants. Academia, Orlando, FL
Kundt W, Gruber E (2006) The water circuit of the plants. Do plants have hearts? Quant Biol 0603019:1–19
Lafitte HR, Courtois B (2002) Interpreting cultivar environment interactions for yield in upland rice: assigning value to drought-adaptive traits. Crop Sci 42:1409–1420
Lauchli A, James RA, Munns R, Huang C, McCully M (2008) Cellspecific localization of Na+ in roots of durum wheat, and possible control points for salt exclusion. Plant Cell Environ 31:1565–1574
Lee KM, Driever SM, Heuvelink E et al (2012) Evaluation of diel patterns of relative changes in cell turgor of tomato plants using leaf patch clamp pressure probes. Physiol Plant 146:439–447
Lens F, Tixier A, Cochard H, Sperry JS, Jansen S, Herbette S (2013) Embolism resistance as a key mechanism to understand adaptive plant strategies. Curr Opin Biotechnol 16:287–292
Levin M, Resnick N, Rosianskey Y, Kolotilin I, Wininger S, Lemcoff JH, Cohen S, Galili G, Koltai H, Kapulnik Y (2009) Transcriptional profiling of Arabidopsis thaliana plants’ response to low relative humidity suggests a shoot–root communication. Plant Sci 177:450–459
Lian HL, Yu X, Ye Q, Ding XS, Kitagawa Y et al (2004) The role of aquaporin RWC3 in drought avoidance in rice. Plant Cell Physiol 45:481–489
Liu BB, Steudle E, Deng X-P, Zhang S-Q (2009) Root pressure probe can be used to measure the hydraulic properties of whole root systems of corn (Zea mays L.). Bot Stud 50:303–310
Lobet G, Hachez C, Chaumont F, Javaux M, Draye X (2013) Root water uptake and water flow in the soil-root domain. In: Eshel M, Beeckman T (eds) Plant roots: the hidden half, 4th edn. CRC Press, New York, NY, pp 18–24
Lu P, Woo KC, Liu ZT (2002) Estimation of whole-plant transpiration of bananas using sap flow measurements. J Exp Bot 53:1771–1779
Lundegardh H (1944) Bleeding and sap movement. Arkiv for Botanik 31:1–56
Maaswinkel RHM, Welles GWH (1986) Factors influencing glassiness in lettuce. Neth J Agr Sci 34:57–65
Macduff JH, Bakken AK (2003) Diurnal variation in uptake and xylem contents of inorganic and assimilated N under continuous and interrupted N supply to Phleum pretense and Festuca pratensis. J Exp Bot 54:431–444
Maeshima M, Ishikawa F (2008) ER membrane aquaporins in plants. Eur J Physiol 456:709–716
Maurel C (1997) Aquaporins and water permeability of plant membranes. Annu Rev Plant Physiol Plant Mol Biol 48:399–429
Maurel C, Verdoucq L, Luu DT, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59:595–624
Maurel C, Simonneau T, Sutka M (2010) The significance of roots as hydraulic rheostats. J Exp Bot 61:3191–3198
McDowell N, Pockman W, Allen C, Breshears D, Cobb N, Kolb T, Sperry JS, West A, Williams D, Yepez E (2008) Mechanisms of plant survival and mortality during drought. Why do some plants survive while others succumb to drought? New Phytol 178:719–739
McElrone AJ, Bichler J, Pockman WT, Addington RN, Linder CR, Jackson RB (2007) Aquaporin-mediated changes in hydraulic conductivity of deep tree roots accessed via caves. Plant Cell Environ 30:1411–1421
Meinzer FC, Clearwater MJ, Goldstein G (2001) Water transport in trees: current perspectives, new insights and some controversies. Environ Exp Bot 45:239–262
Meister R, Rajani MS, Ruzicka D, Schachtman DP (2014) Challenges of modifying root traits in crops for agriculture. Trends Plant Sci 19:779–788
Milburn JA, McLaughlin ME (1974) Studies of cavitation in isolated vascular bundles and whole leaves of Plantago major L. New Phytol 73:861–871
Miller DM (1985) Studies of root function in Zea mays: III. Xylem sap composition at maximum root pressure provides evidence of active transport into the xylem and a measurement of the reflection coefficient of the root. Plant Physiol 77:162–167
Miller-Rushing AJ, Primack RB (2008) Effects of winter temperatures on two birch (Betula) species. Tree Physiol 28:659–664
Misra RK, Dexter AR, Alston AM (1986) Maximum axial and radial growth pressures of plant roots. Plant Soil 95:315–326
Mitchell JP, Shennan C, Grattan SR, May DM (1991) Tomato fruit yields and quality under water deficit and salinity. J Am Soc Hort Sci 116:215–221
Mozhaeva LV, Pil’shchikova NV (1972) Nature of pumping water process by plant roots. Izvestiya Timiryazevskol Sel’skokhozyaistven-noi Akademii 3:3–15
Munns R (1985) Na+, K+ and Cl in xylem sap flowing to shoots of NaCl-treated barley. J Exp Bot 36:1032–1042
Nardini AL, Gullo MA, Salleo S (2011) Refilling embolized xylem conduits. Is it a matter of xylem unloading? Plant Sci 180:604–611
Neufeld HS, Grantz DA, Meinzer FC, Goldstein G, Crisosto GM, Cristosto C (1992) Genotypic variability in vulnerability of leaf xylem to cavitation in water-stressed and well-irrigated sugarcane. Plant Physiol 100:1020–1028
O’Leary JW (1966) Root pressure exudation from apical root segments. Nature 212:96–97
O’Leary JW, Kramer PJ (1964) Root pressure in conifers. Science 145:284–285
O’Toole JC, Chang TT (1978) Drought and rice improvement in perspective. IRRI Res Pap Ser Los Baños, Philippines 14:27
Oertli JJ (1966) Active water transport in plants. Physiol Plant 19:809–817
Ogata S, Saneoka H, Matsumoto K (1985) Nutritional-physiological evaluation of drought resistance of warm season forage species: comparative studies on root development water and nutrient absorption of forage species at various soil moisture levels. J Jpn Grassl Sci 31:263–271
Overton JB (1921) The mechanism of root pressure and its relation to sap flow. Am J Bot 8:369–374
Palmgren MG (2001) H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Mol Biol 52:817–845
Palzkill DA, Tibbitts TW (1977) Evidence that root pressure flow is required for calcium transport to head leaves of cabbage. Plant Physiol 60:854–856
Pedersen O (1993) Long-distance water transport in aquatic plants. Plant Physiol 103:1369–1375
Pedersen O (1994) Acropetal water transport in submerged plants. Bot Acta 107:61–65
Pedersen O (1997) The nature of water transport in aquatic plants. In: Pedersen O (ed) Freshwater biology. Priorities and development in Danish research. Gad, København, pp 196–207
Pedersen O, Sand-Jensen K (1997) Transpiration does not control growth and nutrient supply in the amphibious plant, Mentha aquatica. Plant Cell Environ 20:117–123
Pedersen BP, Buch-Pedersen MJ, Morth JP, Palmgren MG, Nissen P (2007) Crystal structure of the plasma membrane proton pump. Nature 450:1111–1114
Pfeffer W (1881) Pflanzenphysiologie. Ein Handbuch des Stoffwechsels und Kraftwechsels in der Pflanze. Erster Band: Stoffwechsel. Verlag Wilhelm Engelmann, Leipzig
Pickard WF (2003a) The riddle of root pressure. I. Putting Maxwell’s demon to rest. Funct Plant Biol 30:121–134
Pickard WF (2003b) The riddle of root pressure. II. Root exudation at extreme osmolalities. Funct Plant Biol 30:135–141
Pittermann J, Sperry JS, Hacke UG, Wheeler JK, Sikkema E (2005) Torus-margo pits help conifers compete with angiosperms. Science 310:1924
Preston RD (1952) Movement of water in higher plants. In: Frey-Wyssling A (ed) Deformation and flow in biological systems. North Holland Publishing, Amsterdam, pp 257–321
Preston R (2007) The wild trees: a story of passion and daring. Anchor Books, California
Priestley JH (1920) The mechanism of root pressure. New Phytol 19:153–212
Putz FE (1983) Liana biomass and leaf area of a “tierra firme” forest in the Rio Negro Basin, Venezuela. Biotropica 15:185–189
Radin JW, Eidenbock MP (1984) Hydraulic conductance as a factor limiting leaf expansion of phosphorus-deficient cotton. Plant Physiol 75:372–377
Radin JW, Matthews MA (1989) Water transport properties of cortical cells in roots of nitrogen- and phosphorus-deficient cotton seedlings. Plant Physiol 89:264–268
Raleigh GJ (1946) The effect of various ions on guttation of the tomato. Plant Physiol 21:194–200
Renner O (1915) Theoretisches und Experimentelles zur Kohasionstherie der [Theoretical and experimental contributions to the cohesion theory of water transport]. Jahrbuch Wissenschaftliche Botanik 56:617–667
Renner O (1925) Zum Nachweis negativer Drucke im Gefäßwasser bewurzelter Holzgewächse. Flora 119:402–408
Sachs J (1887) Vorlesunguber Pflanzen-Physiologie, 2nd edn. Verlag Wilhelm Engelmann, Leipzig
Salleo S, Logullo MA, Depaoli D, Zippo M (1996) Xylem recovery from cavitation-induced embolism in young plants of Laurus nobilis: a possible mechanism. New Phytol 132:47–56
Scholander PS, Ruud B, Leivestad H (1957) The rise of sap in a tropical liana. Plant Physiol 32:1–6
Scholander PS, Bradstreet E, Hemmingsen E, Hammel H (1965) Sap pressure in vascular plants: negative hydrostatic pressure can be measured in plants. Science 148:339–346
Schwenke H, Wagner E (1992) A new concept of root exudation. Plant Cell Environ 15:289–299
Sears ME (2013) Chelation: harnessing and enhancing heavy metal detoxification—a review. Sci World J 2013:219840
Secchi F, Zwieniecki MA (2011) Sensing embolism in xylem vessels: the role of sucrose as a trigger for refilling. Plant Cell Environ 34:514–524
Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R (2002) PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants. Plant Cell 14:869–876
Singh S (2013) Guttation: path, principles and functions. Aust J Bot 61:497–515
Singh S (2014a) Guttation: quantification, microbiology and implications for phytopathology. In: Luttge U et al (eds) Progress in Botany, vol 75. Springer, Berlin, pp 187–214
Singh S (2014b) Guttation: new insights into agricultural implications. Adv Agron 128:7–135
Singh G, Singh TN (1989) Root-mediated water transport to the shoot of rice. Curr Sci 58:1134–1138
Singh S, Singh TN (1999) Root growth of wheat in simulated vertical and lateral splits of layered salt profiles in soil. Indian J Plant Physiol 4:73–78
Singh S, Singh TN (2000) Rooting ability and water relations of rice plant. Indian J Plant Physiol 5:1–6
Singh S, Singh TN (2013) Guttation: chemistry, crop husbandry and molecular farming. Phytochem Rev 12:147–172
Singh G, Singh TN, Singh S (1999) Trinodal rooting in rice: a new parameter on drought resistance. Indian J Plant Physiol 4:232–235
Singh S, Chauhan JS, Singh TN (2008) Guttation: a potential yield enhancing trait in rice. Curr Sci 95:455–456
Singh S, Singh TN, Chauhan JS (2009a) Water transport in crop plants with special reference to rice: key to crop production under global water crisis. J Crop Improv 23:194–212
Singh S, Singh TN, Chauhan JS (2009b) Guttation in rice: occurrence, regulation and significance in varietal improvement. J Crop Improv 23:351–365
Sperry JS (1983) Observations on the structure and function of hydathodes in Blechnum lehmannii. Am Fern J 73:65–72
Sperry JS (1993) Winter xylem embolism and spring recovery in Betula cordifolia, Fagus grandifolia, Abies balsamea and Picea rubens. In: Borghetti M, Grace J, Raschi A (eds) Water transport in plants under climatic stress. Cambridge University Press, Cambridge, pp 87–98
Sperry JS, Sullivan JEM (1992) Xylem embolism in response to freeze-thaw cycles and water stress in ring-porous, diffuse porous, and conifer species. Plant Physiol 100:603–613
Sperry JS, Holbrook NM, Zimmermann MH, Tyree MT (1987) Spring filling of xylem vessels in wild grapevine. Plant Physiol 83:414–417
Sperry JS, Donnelly JR, Tyree MT (1988) Seasonal occurrence of xylem embolism in sugar maple (Acer saccharum). Am J Bot 75:1212–1218
Sperry JS, Nichols KL, Sullivan JEM, Eastlack SE (1994) Comparative studies of xylem embolism in ring-porous, diffuse-porous and coniferous trees of northern Utah and interior Alaska. Ecology 75:1736–1752
Steudle E (1993) Pressure probe techniques: basic principles and application to studies of water and to studies of water and solute relations at cell, tissue and organ. In: Smith JAC, Griffihs H (eds) Water deficits: plant responses from cell to community. Bios Scientific Publishers, Oxford, pp 5–36
Steudle E, Jeschke WD (1983) Water transport in barley roots. Planta 158:237–248
Steudle E, Henzler T (1995) Water channels in plants: do basic concepts of water transport change? J Exp Bot 46:1067–1076
Steudle E (2000) Water uptake by roots: effects of water deficit. J Exp Bot 51:1531–1542
Steudle E (2001) The cohesion-tension mechanism and the acquisition of water by plant roots. Annu Rev Plant Physiol Plant Mol Biol 52:847–875
Steudle E, Meshcheryakov AB (1996) Hydraulic and osmotic properties of oak roots. J Exp Bot 47:387–401
Steudle E, Peterson C (1998) How does water get through roots? J Exp Bot 49:775–788
Steudle E, Oren R, Schulze ED (1987) Water transport in maize root. Plant Physiol 84:1220–1232
Steudle E, Murrmann M, Peterson CA (1993) Transport of water and solutes across corn roots modified by puncturing the endodermis. Plant Physiol 103:335–349
Stiller V, Lafitte HR, Sperry JS (2003) Hydraulic properties of rice and the response of gas exchange to water stress. Plant Physiol 132:1698–1706
Stocking CR (1956) Root pressure. In: Ruhland W (ed) Handbuch der pflazenphysiologie. Springer, Berlin, pp 581–595
Takeda F, Glenn DM (1989) Hydathode anatomy and the relationship between guttation and plant water status in strawberry (Fragaria x ananassa duch.). Acta Hortic (ISHS) 265:387–392
Tanner W, Beevers H (1999) Does transpiration have an essential function in long-distance ion transport in plants? Plant Cell Environ 13:745–750
Tanner W, Beevers H (2001) Transpiration, a prerequisite for long-distance transport of minerals in plants? Proc Natl Acad Sci USA 98:9443–9447
Teakle NL, Tyerman SD (2010) Mechanisms of chloride transport contributing to salt tolerance. Plant Cell Environ 33:566–589
Telewski FW (2006) A unified hypothesis of mechanoperception in plants. Am J Bot 93:1466–1476
Tyerman SD, Bohnert H, Maurel C, Steudle E, Smith JAC (1999) Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. J Exp Bot 50:1055–1071
Tyerman SD, Niemietz CM, Bramley H (2002) Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant Cell Environ 25:173–194
Tyree MT (2003a) Plant hydraulics: the ascent of water. Nature 423(6943)
Tyree MT (2003b) Hydraulic properties of roots. In: Kroon DH, Visser EJW (eds) Root ecology. Springer, Berlin, pp 125–149
Tyree MT, Sperry JS (1989) Vulnerability of xylem to cavitation and embolism. Annu Rev Plant Biol 40:19–36
Tyree MT, Zimmermann MH (2002) Xylem structure and the ascent of sap. Springer, New York, NY
Tyree MT, Fiscus EL, Wullschleger SD, Dixon MA (1986) Detection of xylem cavitation in corn under field conditions. Plant Physiol 82:597–599
Tyree MT, Yang S, Cruiziat P, Sinclair B (1994) Novel methods of measuring hydraulic conductivity of tree root systems and interpretation using AMAIZED: a maize-root dynamic model for water and solute transport. Plant Physiol 104:189–199
van Bavel MG, van Bavel CHM (1990) Dynagage installation and operational manual. Dynamax, Houston, TX
Vandeleur RK, Mayo G, Shelden MC, Gilliham M, Kaiser BN, Tyerman SD (2009) The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol 149:445–460
Voicu MC, Zwiazek JJ, Tyree MT (2008) Light response of hydraulic conductance in bur oak (Quercus macrocarpa) leaves. Tree Physiol 28:1007–1015
Wayne R, Tazawa M (1990) Nature of the water channels in the internodal cells of Nitellopsis. J Membr Biol 116:31–39
Wegner LH (2014) Root pressure and beyond: energetically uphill water transport into xylem vessels? J Exp Bot 65:381–393
Wei C, Tyree MT, Steudle E (1999) Direct measurement of xylem pressure in leaves of intact maize plants. A test of the cohesion-tension theory taking hydraulic architecture into consideration. Plant Physiol 121:1191–1205
White PR (1938) “Root pressure”—an unappreciated force in sap movement. Am J Bot 25:223–227
White PR, Schuler E, Kern JR, Fuller FH (1958) “Root pressure” in gymnosperms. Science 128:308–309
Xiao H, Peng S, Zheng Y, Mo J, Luo W, Zeng X, He X (2006) Interactive effects between plant allelochemicals, plant allelopathic potential and soil nutrients. J Appl Ecol 17:1747–1750
Zachary M (2009) Sap flow dynamics of a tropical, woody bamboo: deductions of physiology and hydraulics within Guadua angustifolia. PhD thesis, Washington University in St. Louis, USA
Zaitseva RI, Minashina NG, Sudnitsyn II (1998) Influence of capillary-sorptive and osmotic moisture pressure in chernozem on the growth and guttation of barley. Eurasian Soil Sci 31:1075–1082
Zelazny E, Borst JW, Muylaert M, Batoko H, Hemminga MA, Chaumont F (2007) FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proc Natl Acad Sci USA 104:12359–12364
Zeuthen T (2010) Water-transporting proteins. J Membr Biol 234:57–73
Zeuthen T, McAulay N (2012) Cotransport of water by Na+–K+–2Cl– cotransporters expressed in Xenopus oocytes: NKCC1 versus NKCC2. J Physiol 590:1139–1154
Zhao C-X, Xi-Ping D, Sui-QI Z, Qing Y, Steudle E, Lun S (2004) Advances in the studies on water uptake by plant roots. Acta Bot Sin 46:505–514
Zholkevich VN (1991) Root Pressure. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots, the hidden half. Marcel-Dekker, New York, NY, pp 589–603
Zholkevich VN, Popova MS, Zhukovskaya NV (2007) Stimulatory effects of adrenalin and noradrenalin on root water-pumping activity and the involvement of G-proteins. Russ J Plant Physiol 54:790–796
Zhu JJ, Zimmermann U, Thurmer F, Haase A (1995) Xylem pressure in maize roots subjected to osmotic stress: determination of radial reflection coefficients by using the xylem pressure probe. Plant Cell Environ 18:906–912
Zhu JJ, Bai XF, Bu QM, Jiang XM (2010) An analysis to the driving forces for water and salt absorption in roots of maize seedlings under salt stress. Agr Sci China 9:806–812
Zimmermann U, Zhu JJ, Benkert R, Schneider H, Thurmer F, Zimmermann G (1995) Xylem pressure measurements in intact laboratory plants and excised organs: a critical evaluation of methods in the literature and the xylem pressure probe. In: Terazawa M, McLeoad CA, Tamia Y (eds) Tree sap. Hokkaido University Press, Sapporo, pp 59–70
Zimmermann U, Schneider H, Wegner LH, Haase A (2004) Water ascent in tall trees: does evolution of land plants rely on a highly metastable state? New Phytol 162:575–615
Zimmermann D, Reuss R, Westhoff M et al (2008) A novel, non-invasive, online-monitoring, versatile and easy plant-based probe for measuring leaf water status. J Exp Bot 59:3157–3167
Zwieniecki MA, Holbrook NM (2009) Confronting Maxwell’s demon: biophysics of xylem embolism repair. Trends Plant Sci 14:530–534
Acknowledgments
The author is highly delighted and most grateful to Prof. U. Luettge, Editor of Progress in Botany for having being invited by him to write this chapter. He also extends his most sincere thanks to him for the pains taking and critical reading of the manuscript and forwarding useful comments and constructive suggestions for the improvement of the text. At the local level, the author wishes to thank Prof. Fassil Kebede, Dean, College of Agriculture & Rural Transformation, and Mr. Tesfaye Wossen, Head, Department of Plant Sciences, for continued moral support during the write-up of this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Singh, S. (2016). Root Pressure: Getting to the Root of Pressure. In: Lüttge, U., Cánovas, F., Matyssek, R. (eds) Progress in Botany 77. Progress in Botany, vol 77. Springer, Cham. https://doi.org/10.1007/978-3-319-25688-7_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-25688-7_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-25686-3
Online ISBN: 978-3-319-25688-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)