Abstract
Guttation is one of the most conspicuous visible phenomena in plants occurring in a wide range of plants. The guttation fluids, though look clear and translucent, carry a number of organic and inorganic constituents. The organic component may include sugars, amino acids, general proteins, antimicrobial phylloplane proteins, transport proteins for transporting sucrose, purine and cytokinins, toxic elements etc. and enzymes such as peroxidases, dehydrogenases, ATPases, in addition to mRNA, ATP, reductants and other important ingredients of plant life. Guttation fluids also contain a number of natural plant hormones such as auxins, gibberellins, cytokinins, abscisic acid etc., apart from several vitamins. Recent discoveries have revealed the presence of a number of salts, ions, nutrients and macromolecules in guttation fluid playing significant role in enhancing disease resistance, tolerance to toxic elements, photosynthetic efficiency, biomass production and economic yield of agricultural crops. In the light of aforementioned discoveries in guttation transgenic plants have been created to serve as bio-factories for producing various kinds of phytochemicals of immense agricultural, pharmaceutical, nutriceutical, therapeutic, cosmeceutic and commercial significance impacting food productivity and human health adding happiness to life.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
All forms of life revolve round the chemicals. Some of these chemicals, among a vast array of their listing, are specifically tuned to internal flow of energy on account of their conversion from one form to another empowering various visible and invisible activities in plants and animals (Stryer 1989; Moore et al. 1998). The process of exudation and trickling of water drops from leaves, often noticed during early morning or late hours of the day, is one of the most conspicuous visible phenomena in plants. These drops of water oozing out of uninjured leaves through special structures known as hydathodes, is called guttation (Burgerstein 1887). This physiological phenomenon is now known to occur in a wide range of plant species belonging to angiosperms, gymnosperms, pteridophytes, algae and fungi (Stocking 1956a; Dieffenbach et al. 1980a, b; Sperry 1983; Tarakanova et al. 1985; Pedersen 1998; Chen and Chen 2005; Feild et al. 2005; Singh et al. 2008, 2009a). Among crop plants, rice, wheat, barley, oats, maize, sorghum, tobacco, tomato, Colocasia, strawberry etc.(Arisz et al. 1951; Baba 1957; Maeda and Maeda 1987, 1988; Bugbee and Koerner 2002), submerged aquatic plants (Pedersen 1993), perennial herbs and rainforest trees (Feild et al. 2005; Feild and Arens 2007) guttate heavily, some of them exuding a few milliliter to one liter or more from a single leaf in one day depending upon soil, weather and climatic conditions (Kramer 1949; Wolfe 1993; Zaitseva et al. 1998; Singh et al. 2009a). These guttation waters, though look clear and translucent, contain a maggot of organic and inorganic chemicals of immense agricultural, pharmaceutical, nutriceutical, therapeutic, cosmeceutic and commercial significance (Rybicki 2009). The modern cutting-edge science and art-of-the-state technology and now biotechnology have enabled scientists isolate, characterize and produce some of these plant-derived chemicals and macromolecules as directed and desired by man (Fischer and Emans 2000; Fischer and Schillberg 2004; Rybicki 2009). In this review, we have attempted to describe as how the recent discoveries and new information on the process of guttation and its fluid travelling and carrying all along from roots via shoot to leaves and reproductive organs, different dissolved inorganic salts, ions, nutrients and organic compounds and macromolecules such as sugars, amino acids, lipoids, various kinds of proteins, enzymes, hormones, vitamins, antibodies, toxins etc. have led to the creation of transgenic plants now much in existence and in future, are likely to open new vistas in yet another era of miracle plants impacting crop yield, human health and social life.
Path of guttation: hydathodes as channels of guttation
Transpiration is the loss of water as vapour from stomata of leaves but guttation is exudation of liquid water via special structures called ‘hydathodes’ and sometimes also known as ‘water stomata’ or ‘water pores’ (Fig. 1A–C). They are located at the tips, along the margins and adaxial and abaxial surfaces of leaves as well and found in a wide range of plant species (Dieffenbach et al. 1980a, b; Lersten and Curtis 1982, 1985, 1991; Sperry 1983; Maeda and Maeda 1987, 1988; Chen and Chen 2005; Singh et al. 2009a). Morphologically, they form natural openings and unlike stomata, they are permanently open representing a pathway of least resistance to the flow of fluid out of leaf. Quite often they are found in groups of one to several at each site of their occurrence in the leaves.
External and internal features of hydathodes in F. formosana Maxim. f. Shimadai Hayata. A Hydathodes on the adaxial surface of leaf and scattered in a linear arrangement between mid-rib and leaf margin. White points indicate hydathodes. B Magnification of hydathodes found in group (encircled). C Drawing of a longitudinal section of a hydathode (Chen and Chen 2005)
Recent genetic studies have demonstrated that the basic helix-loop-helix (bHLH) protein, MUTE, is required for the formation of hydathode pore in Arabidopsis thaliana. MUTE consistently displays expression at the tip of cotyledons and leaves, thus co-localizing with the auxin maxima. Thus, the bHLH protein, MUTE, evolutionarily controls differentiation of stomata as well as hydathode pore at least in this plant species (Pillitteri et al. 2008; Peterson et al. 2010). Interestingly, further studies on the genetic control of hydathode development with mutants having defective leaf polarity or with loss of function in the multiple auxin-biosynthetic YUCCA (YUC) genes exhibited a similar abnormal leaf margin and less expanded leaves and had fewer hydathodes and an increased number of cell patches in which the patterns of epidermal cells resembled those of hydathodes (Wang et al. 2011). Anatomically, each hydathode is formed by colorless cells, while always piping exteriorly they are stomata-like pores in the epidermis or epithem connected by tracheary endings at the interior having large chamber with masses of thin-walled parenchymatous and loose tissue surrounded by sheath layer. It is the parenchymatous loose tissue lying beneath the hydathode which is known as ‘epithem’ or ‘transfer tissue’ is involved in absorption and secretion. Functionally, there are two types of hydathodes viz., epidermal hydathodes and epithemal hydathodes exuding actively or passively, respectively. Ultrastructurally, the epithem cell has a dense cytoplasm, numerous mitochondria, an extended endoplasmic reticulum system, many small vesicles derived from Golgi bodies and proliferate peroxisomes with abundant plasmodesmata interconnecting the cells (Chen and Chen 2005). Plasmalemmasomes are also found on the plasmamemebrane of the epithem cells whose structures are variable as a result of endocytosis caused by the plasmolysis-deplasmolysis cycle induced by repeating transpiration and guttation day and night. The epithem cells being lobed in shape providing opportunity to sinuous cell wall to increase the contact surface area between the cell wall and xylem sap, can improve the absorption rate of epithem cell (Sattelmacher 2001). According to the studies of Komis et al. (2002), hyperosmotic stress can induce the reorganisation of active filaments in leaf cells of Chlorophyton comosum. Question remains unresolved however, whether osmotic pressure induces a special epithemal ontogenesis through the rearrangement of microtubules or actin filaments (Telewski 2006).
It is interesting that plasmamembrane of epithem cell is very unstable after transpiration. The transpiration triggers increase of the solute concentration in the xylem sap within hydathodes that cause high salt and high osmotic conditions in the epithem cell. These stresses can affect the morphology of the plasmalemma. On account of variability of plasmalemmosomes in epithem cells, it appears that these structures might be a result of the membrane invagination of epithem cells under high salt and osmotic stress (Gordon-Kamm and Steponkus 1984; Oparka et al. 1990; Chen and Chen 2005). At the same time, the sinuous cell walls of the epithem cells have the potential to propose the enlargement of membrane surfaces to regulate a unique membrane area/cell volume ratio. Especially, repetition of guttation and transpiration cycles seems to induce solute concentration change and let epithem cells undergo plasmolysis and deplasmolysis cycles, making fluid-phase endocytosis more likely for guttation to occur. However, this needs further studies to clarify the role of these physiological processes in guttation.
Physiology of guttation
Natural guttation is often observed during early morning or late hours of the day but it seems to be always at work. However, due to high rate of evaporation from leaf surfaces during day hours guttation fluid is not visible but it can be induced and seen as desired at any time in the intact as well as excised leaves or branches under pneumatic pressure applied either to the root system (Wei et al. 1999) or the excised leaves/branches (Singh and Singh 1989). For quantitative measurement of guttation, the drippings of fluid from leaves can be collected either in the test tubes or glass micro-capillaries or more accurately and rapidly by blotting paper technique (Singh et al. 2008). This makes it possible to use for practical applications in large scale screening of guttation-efficient genotypes at field level with a view to enhancing crop productivity as a positive correlation has been found between the rate of guttation and the sink potential of rice panicles (Singh et al. 2008, 2009a). Though the phenomenon of guttation in plants is genetically controlled but regulated by a number of internal, external and edaphic factors present in the habitat of their existence (Singh et al. 2009a). The internal factors include genetic makeup of the plant, growth phenology, leaf age and size, hormonal status etc. whereas external factors include mechanical stimuli, wind, turgor, light, temperature, humidity etc. Apart from this, the edaphic factors such as soil temperature, moisture, nutrients, aeration, mycorrhizae etc. also play significant role in the regulation of guttation. However, it is generally agreed that the root pressure provides impactus for guttation whose magnitude often ranges between 3 and 4 bars but at times it may go up to 7 bars or more (Singh and Singh 1989; Pedersen 1993; Canny 1995, 2001; Tanner and Beevers 2001; Kundt and Gruber 2006; Singh et al. 2009b). The role of ions and solutes accumulation and transport in and out of cells is vital for the development of root pressure (Stocking 1956b). Root pressure consists of osmotic as well as metabolic components by which continued uptake of nutrients, ions and water take place (Zholkevich 1992; Pickard 2003a, b; Katsuhara et al. 2008; Knipfer et al. 2011). Most proteins responsible for nutrients, ions and metabolites uptake and transport in plant cells are energized by electrochemical gradients, a prerequisite for life of both plant and animal, by protons across the plasma membrane. The function of these gradients is due to the action of plasmamembrane H + pumps fuelled by ATP. The plasmamemebrane H + -ATPases share a membrane topography and general mechanism of action with other P-type ATPases, but differ in regulatory properties (Palmgren 2001). Spectacular advances made particularly by workers in Denmark, Belgium and Germany in this field include the identification of the complete H+-ATPase gene family in Arabidopsis (Duby and Boutry 2009; Bobik et al. 2010), analysis of H+-ATPase function by the methods of reversed genetics, an improved understanding of the posttranslational regulation of pump activity by 14-3-3 proteins (Duby et al. 2009; Yang et al. 2010), novel insight into H+ transport mechanism (Buch-Pedersen and Palmgren 2003; Niittylä et al. 2007), and progress in structural biology and enzymology of H+-ATPases with respect to ions and solutes uptake, a prerequisite for the development of root pressure causing guttation (Pedersen et al. 2007; Duby et al. 2009; Duby and Boutry 2009). Summarily, plasmamembrane H+-ATPases which constitute a family of proton pumps driven by hydrolysis of ATP and found in the plasmamembrane of plants and fungi play a major role in the transport of nutrients into the cell. The plasmamembrane H+-ATPases are electrogenic enzymes since they extrude positive charges (H+) and thus form a membrane potential gradient (negative on the inside). The combined electrochemical gradient and matter constitute driving force for solutes to enter the cell. Cations, anions, and neutral solutes are able to enter the cell through various carrier proteins as described earlier through which transport is energized by the concomitant uptake of protons. Thus, most of the hundred of membrane-bound transport proteins that have been identified in plants are energized indirectly through the plasma membrane H+-ATPases and therefore, have been traditionally assumed to be general endpoints of all signalling pathways affecting membrane polarization and transport (Merlot et al. 2007). Taking root (invisible) and shoot including leaves (visible) as a whole unit, the mode and mechanism of guttation may be summarized as: in the presence of conditions favoring absolute reduction in and inhibition of transpiration (high humidity, still air, favorable air and soil temperature, abundant soil moisture) → signal transduction (mechanosensors/temperature/light/hormones) → metabolic activities → energy coupling → contractile proteins/ion channels gating influenced → influx of ions → osmotic potential gradient established → aquaporins gating influenced → absorption of water follows → hydrostatic pressure, i.e. root pressure develops (root pressure provides the impectus for the flow) → xylem sap + phloem sap (xylem-to-phloem and phloem-to-xylem transmission/inter-trafficking) intermingled and upward movement → finally pushed out of hydathodes → fluid flows out in the form of droplets which is guttation. However, it is not clear at the moment whether stem pressure are some other local pressures are also involved in guttation which need to be investigated at depth to unravel the mechanism of guttation or forces aiding it.
Chemical constituents of guttation
Secretion is a fundamental process providing plants with the means for disposal of solutes, improvement of hormone and nutrient acquisition, and attraction for pollination etc. or repulsion of animals for defense purposes. Specific secretory organs, such as nectaries, hydathodes, and trichomes, use a combination of secretory and retrieval mechanisms, which are poorly understood at present. The guttation fluids, though look clear and translucent, are not simply water. The guttation liquids and guttation deposits on hydathodes have been shown to contain both organic and inorganic constituents in varying concentrations (Table 1). In this context, xylem and phloem saps can be equated to guttation fluid constituents. Of particular importance in determining the composition of this liquid are the age, physiological activity, and species of plant involved as well as the solute composition and concentration in the medium from which the plant is absorbing. Most samples of guttate or of solutes deposited on hydathodes have been obtained under relatively uncontrolled conditions so that an examination of their composition can give at best only an indication of general trends. The composition of guttated liquid appears to vary in quantity and quality of inorganic and organic solutes, which roughly constitute 0.05–0.5 % of the liquid (Curtis 1943, 1944a, b; Ivanoff 1963; Pedersen 1998).
Organic constituents
Organic constituents of guttation fluid may contain sugars, amino acids, amides, reductants, different types of proteins and enzymes such as simple proteins, transport proteins, signal transduction proteins, isoprenyltransferase, peroxidases, ATPases, dehydrogenases, recombinant proteins, antibodies, ATP, mRNA, lipophilic materials, volatile oils, herbicides, insecticides, fungicides, purines, pyrimidines, alkaloids, toxins etc. (Fig. 2) (Fischer et al. 2004; Ma et al. 2005; Twyman et al. 2005). In an excellent study, Goatley and Lewis (1966) determined the kinds and amounts of substances that would account for differential growth of Claviceps purpurea in guttation fluids from Rosen rye, Genesee wheat, and Traill barley seedlings. Chromatographic methods were used by these authors for determining amino acids and sugars, spot tests and spectrometric methods for inorganic materials, and microbiological methods for vitamins. Total sugar content was about equal in rye and barley fluids, but lower in wheat. Glucose was the principal sugar component of the rye and barley fluids and galactose was highest in wheat. The amino acid mostly present in all three fluids was either aspartic acid or asparagine. Barley fluid was far higher than the other two species in total amino acids, with wheat being the lowest. Most inorganic elements were found to be highest in barley and lowest in wheat, with the exception of iron where rye was highest and barley lowest. Barley fluid was highest in choline, p-aminobenzoic acid, thiamine, and uracil, while rye was highest in inositol and pyridoxine. Wheat was much lower than the other two in choline and inositol. Recently, signal transduction proteins, putative transcription factors and stress response factors as well as metabolic enzymes have been identified in xylem and phloem saps which constitute guttation fluid. These results imply that proteomics on a nano-scale is a potent tool for investigation of biological processes in plants and that ample inter- trafficking of chemical substances of immense metabolic significance from phloem to xylem and vice versa takes place in various plant organs. Gareis and Gareis (2007) found eight of eleven ochratoxigenic isolates of Penicillium nordicum and Penicillium verrucosum produced in guttation droplets when grown on Czapek yeast extract agar (CYA) for 10–14 days at 25 °C. This shows that high amounts of mycotoxins could be excreted from toxigenic Penicillium isolates into guttation droplets which could be accounted for the variation in the intensity of disease.
Guttation fluid containing a number of organic and inorganic compounds including metabolites, enzymes, hormones, vitamins, salts, ions, nutrients etc. impacting plant behavior (modified from Salisbury and Ross 1992)
Enzymes
It has long been recognized that some proteins are naturally secreted into plant guttation fluid. In the first quarter of the last century, for the first time, Wilson (1923) reported that proteins (catalase and peroxidase) were present in the guttation fluid of maize (Zea mays) and oats (Avena sativa), whereas reductase was released into the guttation fluid of timothy (Phleum pratense). Recent studies have revealed the presence of a number of proteins and enzymes of immense significance in guttation water. However, it would be particularly interesting to study the chemical composition of guttation fluid collected at different times with particular reference to various proteins at genotypic and varietal levels in order to establish their physiological efficiencies.
Phylloplane proteins
Shepherd and Wagner (2007) have described the physical structures and biochemicals of the phylloplane and discussed protein-based surface defenses of animals. They have also reviewed the emerging evidence pertaining to antimicrobial phylloplane proteins and mechanisms by which proteins can be released to the phylloplane, including biosynthesis (e.g. phylloplanins) by specific trichomes and delivery in guttation fluid from hydathodes. It is advisable to extend these studies to investigate the production and secretion of such and other proteins at cultivar levels of various guttating agricultural crops.
Transport proteins
A number of transport proteins have been detected in guttation fluid. Transport studies in cultured Arabidopsis cells indicate that adenine and cytokinin are transported by a common H+-coupled high-affinity purine transport system involving AtPUP1 and AtPUP2, transport properties being similar to that of Arabidopsis purine transporters (Bürkle et al. 2003). Promoter-reporter gene studies point towards AtPUP1 expression in the epithem of hydathodes and the stigma surface of siliques, suggesting a role in retrieval of cytokinins from xylem sap to prevent their loss during guttation. As for sugars particularly glucose, fructose, galactose and sucrose have been found in guttation fluid of cereals (Goatley and Lewis 1966). The data obtained by Slewinski et al. (2009) demonstrate that SUT1 is crucial for efficient phloem loading of sucrose and it may get its way into xylem saps hence, guttation as well due to inter-trafficking of contents between xylem and phloem tissues in maize leaves. Transporter proteins may hopefully be found in guttation fluid for other sugars as well. The mRNA localization was diversified in leaf, stem, flower and drupe, but recurred in all organ sieves, suggesting a role in sap nutrient transport. Kinetic studies showed a pattern similar to that of sucrose content variation (Testone et al. 2009).
Recently, a number of transporter proteins for toxic elements like Zn, Cd, Ni, B, As, Se etc. have been detected in plant exudates which may serve as a measure of injurious and toxic elements on the one hand and enhancement of tolerance via restriction on absorption and exclusion, on the other (Ghosh and Singh 2005; Meagher and Heaton 2005; Schmidt et al. 2009). In an important study, Sutton et al. (2007) recently identified Bot1, a BOR1 ortholog, as the gene responsible for the superior boron-toxicity tolerance of the Algerian barley landrace Sahara 3771 (Sahara) in plant exudates. Bot1 was located at the tolerance locus by high-resolution mapping which provided a higher capacity to tolerance in yeast. Bot1 transcript levels identified in barley tissues are consistent with a role in limiting the net entry of boron into the root and in the disposal of boron from leaves via hydathode guttation. Therefore, enhancement in tolerance to elemental toxic effects via restriction on absorption by special transport protein production is of immense significance in crop production on marginal and waste lands. Similarly, the nickel (Ni) hyper accumulator Alyssum murale has been developed as a commercial crop for phytoremediation and phytomining Ni from metal-enriched soils. Tappero et al. (2007) studied guttation fluid for metal co-tolerance, accumulation and localization in A. murale exposed to metal co-contaminants. Mihucz et al. (2005) studied arsenic speciation of xylem sap of cucumber plants (Cucumis sativus L.) and found arsenite, arsenate and dimethylarsinic acid (DMA) in the sap of the plants. These arsenic compounds are very likely to find their ways in guttation fluid and may serve as detoxification mechanism on the one hand and may recycle through soil-root system on the other. Thus, guttation is a means of excreting excess of harmful elements such as Ni thereby promoting plant growth. It is presumed that apart from above-mentioned proteins and enzymes, a number of other important transport proteins, in addition to those mentioned earlier, are probably also present in guttation fluid which must be explored to elucidate the role of guttation in plant productivity. The future research should hopefully lead to exciting advances in our understanding of the phylloplane proteins, transport proteins etc. to useful biotechnological interventions for improving crop productivity, utilization of waste lands and freeing environment from the clutches of pollution. Therefore, one way in which plants are able to dispose of unwanted compounds is via the mechanism of guttation.
Pathogenesis-related proteins
An analysis of the guttation fluid using one- and two-dimensional electrophoresis has shown a clustering of approximately 200 proteins, primarily with isoelectric points in the acidic pH range (Grunwald et al. 2003). The protein profile of the guttation fluid was remarkably modified by treating plants with methyl jasmonic acid suggesting that the protein composition of the guttation fluid is controlled by internal and/or external stimuli. They are also involved, however, in whole plant events including stress responses and long-distance signaling. Phloem and xylem saps therefore include a variety of proteins. Taking advantage of the complete and available genomic information for rice plant, Aki et al. (2008) performed a shotgun analysis of the proteome of phloem and xylem saps obtained from this crop. Using a mass spectrometer, the authors identified 118 different proteins and eight different peptides in xylem sap and 107 different proteins and five different peptides in phloem sap, very likely to be present in guttation fluid travelling up through shoot. Such analyses of guttation fluid indeed provide opportunity for understanding the mechanism of plant strategies to fight against diseases.
Peroxidases and isozymes
Peroxidases are enzymes that catalyze the oxidative cross-linking and polymerization of certain organic compounds by hydrogen peroxide and other organic peroxides. A number of acidic peroxidases and other proteins have been detected in xylem saps obtained from several temperate fruit plants (Biles and Abeles 1991). Two other sources of xylem sap used in this study were stem exudates and guttation fluid. Similar peroxidases were also found in stem exudates and guttation fluids of strawberry (Fragaria ananassa Duch.), tomato (Lycopersicum esculentum L.), and cucumber (Cucumis sativus L.). Interestingly, the physiological mechanisms associated with resistance of cabbage to black rot disease seem to be associated with the hydathodes. Hydathodal fluids of resistant varieties of cabbage (Brassica oleracea L.) had greater peroxidase activity during pathogenesis with Xanthomonas campestris pv. campestris when compared to susceptible ones, with infected plants having higher peroxidase levels than non-infected plants (Gay and Tuzun 2000). Lignin deposition in and around the hydathodes was found to be associated with the accumulation of isoelectric isozymes in hydathodal fluids. Undoubtedly, these studies throw light on the involvement of hydathodal secretion of defense proteins against bacterial diseases.
Amino acids and amides
The amino acids commonly present in guttation fluid include aspartic acid, glutamic acid and their amides particularly asparagine. An Arabidopsis thaliana activation tagged mutant, glutamine dumper1 (gdu1), was identified that accumulates as salt crystals at the hydathodes (Pilot et al. 2004). Chemical analysis demonstrated that, in contrast with the amino acid mixture normally present in guttation droplets, the crystals mainly contained glutamine (Gln). Gdu1 was cloned and found to encode a novel 17-kD protein containing a single putative transmembrane span. Gdu1 is expressed in the vascular tissues and in hydathodes. Gln content is specifically increased in xylem sap and leaf apoplasm, whereas the content of several amino acids is increased in leaves and phloem sap. Indeed, studies such as this may help to shed light on the secretory mechanisms for amino acids in plants.
Lipoids, alkaloids and glucosides
The lipid materials consisting of fatty acids, sesquiterpene antidepressant, monoterpenes, sclareol, volatile oils etc. have been found in leaf exudates and the significance of some of these compounds is not clear and understood (Sparrow et al. 2007). Novel cyanogenic glucosides such as dhurrin and diglucoside, dhurrin-6-glucoside have been isolated from guttation droplets of young seedlings of Sorghum bicolor (Selmar et al. 1996). Many grasses live in association with asymptomatic fungi (Neotyphodium spp. endophytes) which grow in the intercellular spaces of the grass. These endophytes produce a range of alkaloids that protect the grass against grazing by mammals and insects. One of these alkaloids found in guttation fluid is an unusual pyrrolopyrazine, peramine (Koulman et al. 2007). Peramine appears to be continuously produced by the endophyte, but does not progressively accumulate which needs further studies.
Hormones
A number of natural plant hormones such as auxins, gibberellins, cytokinins, abscisic acid, ethylene etc. have been recently detected in xylem and phloem saps and exudates of roots and guttation (Fletcher and Mader 2007). The physiological significance of hormones and other compounds in terrestrial as well as submerged aquatic plants lies in an efficient transport system for acropetal translocation of water to satisfy their demand for root-derived inorganic nutrients and hormones for active growth and that this system is influenced by the developmental stage of the leaf hydathode (Pedersen et al. 1997). The plant growth regulators indole-butyric acid, 6-benzylaminopurine, and kinetin were also demonstrated to increase rhizosecretion of Guy’s 13 (Drake et al. 2009). The effect of the growth regulators differed, as alpha-naphthalene acetic acid and indole-butyric acid increased the root dry weight of hydroponic plants, whereas the cytokinins benzylaminopurine and kinetin increased rhizosecretion without affecting root mass. It would be interesting to investigate the effect of gibberellins and auxins in view of their importance in differentiation and development of stomata and hydathodes (Pillitteri et al. 2008; Peterson et al. 2010; Wang et al. 2011).
Auxins
Exogenous application of auxin indole-3-acetic acid (IAA) to the cut stumps of sunflower accelerated the bleeding throughout the 24 h cycle during the first few days of bleeding. Later the auxin effect was more pronounced during the maximum period of bleeding (Skoog et al. 1938). The mechanism responsible for the periodicity of bleeding and root pressure is not well understood. If a purely osmotic explanation for root pressure is accepted, then a diurnal cycle must result from a cyclic rate of secretion of solutes into the xylem followed by the osmotic influx of water. It must then follow that endogenous auxin acts in conjunction with the periodic secretion and removal of solutes from the xylem stream, in addition to its other physiological roles in hydathode initiation and its further development (Pillitteri et al. 2008; Peterson et al. 2010; Wang et al. 2011).
Gibberellins (GAs)
Gibberellins affect a number of plant growth and development processes whose presence is found in plant saps, fruits, leaves, meristematic tissues, developing panicles etc. (Paleg 1965; Coombe and Iland 2004). High concentrations of GA7 in apical and lateral buds and low concentrations of GA1, 3, & 9 in xylem sap in combination with xylem sap cytokinins may regulate water relations hence guttation however, it needs further work before this can be said with certainty. Fletcher and Mader (2007) using high-performance liquid chromatography quadruple time-of-flight tandem mass spectrometry (LC-QToF-MS/MS) analyzed multiple plant hormone groups in small samples for the study of the physiology of abnormal vertical growth (AVG) in Macadamia integrifolia (cv. HAES344). Cytokinins (CKs), gibberellins (GAs), abscisic acid (ABA), and auxins were all detected in xylem sap and apical and lateral buds. However, the distribution and function of gibberellins as mentioned earlier particularly hydathodal tissues have not been investigated.
Cytokinins
The importance of CKs, bioactive forms of cytokinin, for shoot development and the identification of CKs, mainly of zeatin, zeatin riboside, and isopentenyladenine were discovered several decades ago (Letham 1994). The fact of the apparent absence of free CK in the buds of wind-protected plants and the typical upward decreasing gradients of free and conjugated CKs suggest that the bulk of the CK is synthesized in the root cap, exported through the xylem and accumulates at sites of highest transpiration where cuticles do not yet exist or do not protect against water loss (Dodd et al. 2004; Aloni et al. 2005). The authors have demonstrated transpiration-dependent transport of root-produced cytokinins in xylem sap of transgenic Arabidopsis thaliana. Factors like xylem sap zeatin riboside concentration and shoot auxin levels as evidenced in rms5 mutant pea plants may control water relations hence guttation in plants. This opens a new field for fruitful investigation into the role of cytokinins in the development of hydathodes and guttation. Further, increase in root-derived hormonal status in leaves and exudates derived from them correlated with delayed leaf senescence may result in increased photosynthetic potential (PSII efficiency) leading to increased yield and biomass production. The decreased X-CK and transpiration rate of rms2 following N deprivation suggests that changes in xylem-supplied CKs may modify water use. Under such conditions the process of guttation is also very likely to be affected. Further, an important question that arises at the moment is whether photoperiod cytokinin (CK) is to do something with the initiation of guttation. All these exudation- and guttation-aided studies including those of Fletcher and Mader (2007) should provide basic platform for unraveling the complex nature of the mechanism of hormones action in the regulation of plant growth and development impacting plant productivity and agricultural production.
Abscisic acid
Abscisic acid (ABA) by way of its presence in root exudates and vascular bundles, roots, and leaves might influence gating of aquaporins resulting in increased permeability of water hence, its increased transport pressing the water to exude as guttation. Although ABA may reduce assimilation by limiting growth, it may also improve growth by increasing water status and cell turgor, or may influence growth and development through direct effects on signaling pathways and cross-talk with other hormones (Thompson et al. 2007). Studies utilizing ABA-deficient mutants and inhibitors of ABA synthesis to decrease endogenous ABA levels, and experimental strategies to circumvent variation in plant water status with concomitant effect on plant exudation with ABA deficiency, are changing the view of the role of ABA from the traditional idea that this hormone is generally involved in growth inhibition. Interestingly, ABA seems to increase guttation whereas cytokinins decrease it.
Ethylene
Like other naturally-occurring plant hormones found in plant exudates, ethylene is also considered a hormone which regulates quite a few physiological phenomena and interact with other hormones (Fletcher and Mader 2007; Thompson et al. 2007). In particular, there appears to be no work on the effect and involvement of ethylene in fluid exudation in plant. However, its involvement in exudation and guttation may be expected to occur via ABA and vice versa.
Vitamins
Plant-derived vitamins are of great interest because of their impact on human health. They are essential for metabolism because of their redox chemistry and role as enzymatic cofactors, not only in animals but also in plants. Several vitamins have strong antioxidant potential, including both water-soluble (vitamins B and C) and lipid-soluble (vitamins A, E and K) compounds (Asensi-Fabado and Munné-Bosch 2010). However, there is no work except those of Goatley and Lewis (1966) on the composition of guttation fluid with respect to vitamins. These authors determined chemical composition of guttation fluid from barley, rye and wheat. Barley fluid was highest in choline, p-aminobenzoic acid, thiamine, and uracil, while rye was highest in inositol and pyridoxine. Wheat was much lower than the other two species in choline and inositol. Most successfully cultured roots of dicotyledons require thiamine. Many plant roots require both vitamin B1 and niacin. The tomato requires thiamine and pyridoxine (vitamin B6). Thiamine is synthesized in leaves and is transported downward to the roots where it is essential for their growth. Even though the B vitamins are so clearly root growth hormones for those species that require them for continued growth in culture, it is interesting that thiamine, for example, has not been generally classified as a plant hormone. Yet, there appears to be great paucity of work on the involvement and role of vitamins in guttation. There is, therefore, urgent need for further work on vitamins with regard to their natural occurrence and effects on guttation by various genotypes of field crops.
Inorganic constituents
Guttation is indeed associated with salt absorption and salt movement into the xylem. Therefore, the liquid of guttation is not pure water but a dilute solution of organic and inorganic compounds. Elemental and mineral constituents such as Na, K, Ca, Mg, Mn, B, Co, Zn, Se, Ni, Fl, Si, As, Al, Cl, NH4, NO3, PO4, SO4, CO3, HCO3 etc. have been found in guttation fluid, leaf leachates and leaf surface secretions of several plant species by a number of workers (Wilson 1923; Curtis 1943, 1944a, b; Ivanoff 1963). In most instances, guttation liquid consists of nutrient salts usually found in the plant sap, sugars and other organic substances, enzymes, hormones etc.
Mode of effect of guttation fluids on crop husbandry
Chemicals of guttation impacting soil fertility
The analysis of composition of guttation fluid provides an opportunity for non-invasive test for nutritional status of both soils and plants (Curtis 1943, 1944a, b; Ivanoff 1963). Interestingly, guttation water has been found to exercise control over solubility of organic and inorganic nutrients and polymerization of organic compounds aiding soil fertility (Magwa et al. 1993). The plants provide the organic matter in the form of leaves and flowers dropped on the surface of the soil and they also provide water to the microorganisms by wetting the soil surface through water of guttation. All plant species have a network of roots just a few centimetres below the soil surface and the deep roots as well. It is deep root systems which draw water from lower soil profile. This water is dropped through the leaves by guttation on the ground and the roots near the surface of the soil reabsorb this water, which is enriched with the mineral ions and nutrients. Some of the plants even add organic compounds to the water of guttation in order to provide the soil microorganisms with more energy. While studying the biochemistry of soils, Magwa et al. (1993) characterized peroxidases, enzymes that catalyze the oxidative cross-linking and polymerization of certain organic compounds by hydrogen peroxide and other organic peroxides. The total peroxidase activity of root bleed was very similar to that of guttation fluid of Helianthus annuus taken from higher up the hypocotyl. These observations suggest that the root is the main, if not the sole, source of the peroxidases in guttation fluid. These findings motivated other scientific studies and Kerstetter et al. (1998) demonstrated that peroxidases are present in guttation collected from Bermuda grass hybrids 419 and Tifway 2 [Cynodon dactylon (L.) × Cynodon transvaalensis Davy], which are warm-season C4 grasses, and Kentucky bluegrass (Poa pratensis L.), which is a cool-season C3 grass. Peroxidase activity in guttational fluids collected from grasses during early morning was in the range of 80–120 μg/L and found most abundant in the top 5 cm layer of field soils impacting thereby their fertility and productivity. It would be interesting to investigate the effects of other enzymes found in guttation water on other soil activities and characteristics aiding its improvement.
Chemicals of guttation impacting plant nutrition
Water transport carrying and distributing required inorganic nutrients and phytohormones, both derived from the roots and exuded in guttation fluid, ensures optimal plant growth in the absence of a transpiration stream. Of particular interest are the findings of recent studies that have confirmed that guttation fluid may be used as a measure of plant nutritional status (Palzkill and Tibbitts 1977; Dieffenbach et al. 1980a, b). The evidence also came from the studies of Pedersen (1994, 1998) who used guttation as a non-destructive way to track the flow of water and mineral ions from the roots. In fact, guttation may be useful to plants in that it ensures a continued supply of nutrients even when normal transpiration cannot take place to carry the nutrients in its stream. It thus appears that convective water transport in the xylem, brought about by root pressure and the resultant guttation is in itself sufficient for long-distance mineral supply and that transpiration is not required for this function (Tanner and Beevers 2001). Further, for assessing the nutritional status of plants and monthly variation in nutrient contents recently guttation droplets were used for assessing K, Ca, Mg, Na, Fe, Zn, Cu, NH3, NH4 and total N status and the author concluded that the management practice has a major impact on the potential plant-atmosphere relation (Necmi 2005). Other recent studies have also confirmed the involvement of hydathodes, i.e. the guttation pores, in retrieval of plant nutrients from xylem sap. Testone et al. (2009) studied the localization of mRNA in apple plants and found that it was diversified in leaf, stem, flower and drupe, but recurred in all organ sieves, suggesting a role in sap nutrient transport and its delivery to guttation fluid. Cytochemical data indicated that the epithem cell might also use the coated-vesicles endocytosis to retrieval of nutrient from the guttated solution in addition to using proton pump (Chen and Chen 2005). These studies undoubtedly prove the utility of guttation in plant nutrition.
Chemicals of guttation protecting plants from herbivores and insects
Recently, considerable researches have focused on glandular secreting trichomes to understand and exploit their ability to secrete phytochemicals that might improve resistance to insects, microbes, herbivores and modify gland metabolism towards improving properties of exudates, e.g. flavor and aroma in herbs (Shepherd and Wagner 2007). Plants produce a wide variety of substances which at first sight seem to be excretory product. However, many of them have been found to have a function, for example, in defense against herbivores and parasites. The accumulation of toxic compounds at the surface allows their direct contact with insects, pathogens and herbivores. Many grasses live in association with asymptomatic fungi (Neotyphodium spp. endophytes), which grow in the intercellular spaces of the grass. These endophytes produce a range of alkaloids that protect the grass against grazing by mammals and insects. A good report showing the mobilization of fungal alkaloids into plant fluids by the host plant in grass-endophyte associations has been prepared (Koulman et al. 2007). One of these alkaloids is an unusual pyrrolopyrazine, peramine. Peramine appears to be continuously produced by the endophyte, but does not progressively accumulate. No mechanism for the removal of peramine by its further metabolism or any other process has been reported. Peramine was detected in the cut leaf fluid as well as in the guttation fluid of all grass-endophyte associations. In some associations the lolines and ergot peptide alkaloids have also been detected. Thus it seems that the guttation and trichome exudates are ideally positioned to provide a first line of defense against attacking organisms, perhaps providing time for activation of induced defenses (Wagner et al. 2004). Shepherd and Wagner (2007) have described the physical structures and biochemicals of the phylloplane and discussed protein-based surface defenses of animals. They have also reviewed the emerging evidence pertaining to antimicrobial phylloplane proteins and mechanisms by which proteins can be released to the phylloplane, including biosynthesis (e.g. phylloplanins) by specific trichomes and delivery in guttation fluid from hydathodes. In fact, the future research on phylloplane proteins should hopefully lead to exciting advances in our understanding of these proteins and to useful biotechnological interventions for overall improvement of crop productivity. Recent advancements in plant biotechnology have led to spectacular results of practical utility. The recent demonstration that cyanogenic glycoside biosynthesis can be engineered into a non-cyanogenic plant forces us to remain open-minded about the flexibility of plant metabolism. Tattersall et al. (2001) transformed Arabidopsis with three genes that encode biosynthetic enzymes for the cyanogenic glycoside dhurrin. Transgenic plants produced low-level, but sufficient dhurrin to provide resistance to the flea beetle, Phyllotreta nemorum, demonstrating that an endogenous primary metabolite precursor (in this case tyrosine) can be tapped by an introduced metabolic pathway. Therefore, plants during the course of evolution develop adaptive traits to ensure their survival in the habitat of their existence.
Chemicals of guttation impacting flower pollination
Guttation fluids may be used as a non-invasive quantitative assessment test for insecticide/fungicide/weedicide residues present in the plant by their chemical analysis (Harris 1999). Such tests may be imperative for the understanding of mortality, if any, of bees and other insect pollinators. Guttation droplets collected from the tips of winter leaves, previously treated with a xylem-mobile fungicide (ExpF) that was known to elute readily in guttation fluid, were analyzed for the presence of radiolabel. Not all xylem-mobile fungicides elute significantly, however. Guttation through passive hydathodes was used as a non-destructive xylem sampling technique to study the effect of the herbicide diuron [3-(3,4-dichloro-phenyl)1,1-dimethyl urea] on potassium translocation in intact barley seedlings (Riedell and Schmid 1987). Guttation drops may also be used as a means of test for the translocation of fungicides acting as systemic fungicide and not secondarily by altering the host metabolism as evidenced by the uptake and translocation of griseofulvin by wheat seedlings (Stokes 1954). The concentration of griseofulvin in the guttation drops was directly related to the concentration in the nutrient solution; there was evidence of griseofulvin accumulation in the leaves, the concentration in the guttation drops being frequently higher than that in the nutrient solution. The guttation fluid from squash plants was shown to bring Cu into solution from Cu(OH)2; furthermore, the guttation fluid from all plants tested increased the toxicity of yellow Cu2O and Bordeaux mixture when sprayed on glass slides and seeded with known dilutions of Macrosporium sarcinaeforme. This behaviour can explain also the process by which plants previously sprayed with Bordeaux the Cu content of potato leaves can be built up to concentrations toxic to the potato leaf hopper. As pointed out earlier, the guttation liquid exuded from leaf margins has been considered to be xylem sap mixed with phloem constituents. Identification of the radioactive component in the guttation fluid should indicate the form of amiben transported from roots to shoots. Amiben (3-amino-2,5-dichlorobenzoic acid) is widely used as a herbicide for weed control in soybeans. Stoller (1970) experimented with amiben in wheat collecting about 20 mg of fluid (an equivalent amount of nutrient solution containing about 90 dpm) and found that amiben-treated wheat did not guttate; untreated wheat guttated sparingly. Thus, amiben seems to inhibit guttation in wheat.
Here the points of interest are that plants lacking guttation are slow-growing and very often are mycotrophic. In members of Rosaceae heavy guttation has been implicated in high pollen production which could be of far-fetching significance. A current subject of discussion is whether the active secretions of water by plants (guttation) might be of significance for bees on account of residues of neonicotinoid pesticides possibly contained in them. In the light of its significance in crop husbandry, the possibility of the acute and chronic toxicity to honeybees (Apis mellifera) of guttation water and dew collected from winter rape plants treated with the insecticide Nurelle D® (a.i. chlorpyriphos + cypermethrin) was investigated (Shawki et al. 2006). The mortality of bees treated with guttation water and dew collected from the treated plants did not exceed 10 %. The chlorpyriphos residue found in contaminated guttation water and dew were below the limit of detection (0.8 μg kg−1) and the cypermethrin residue was below the detection levels as well. Additionally, the facts collected by Bayer Crop Science (Anonymous 2009) and the expert assessments currently available to them, guttation from treated plants with neonicotinoid pesticides possibly contained in them, revealed no effect on the health of bee colonies under conditions of normal agricultural practice. The German bee monitoring programme from 2004 to 2008 found no causal connection between neonicotinoid pesticides and bee health irrespective of the source of exposure. A new scientific study performed by the French Food Safety Authority (AFSSA) has made it very clear that a multitude of factors is responsible for the continuing bee mortality in parts of France. On the contrary, Valente and Bologna (2011) went on hunger strike in Italy urging total ban on manufacturing, sales and use of neonicotenoid insecticides which killed, according to them, 80 % of their bee colonies on account of bees feeding on plant saps, i.e. guttation fluid. Obviously, guttation fluids can serve as easy and dependable assessment test for pesticide residues in plants.
Chemicals of guttation elevating disease resistance of crop plants
Interestingly, guttation fluid also serves as vehicle for the inhibition of disease-causing bacterial, fungal and viral growth due to production of toxins, mycotoxins and alkaloids etc. produced by these microorganisms. Lewis (1962) studied the susceptibility of three cereals to Claviceps purpurea using their guttation fluids as media for the growth of germinating spores of this fungus. Rye guttation fluid produced the most growth and barley fluid the least. The degree of susceptibility was correlated to the variation in the production of organic and inorganic compounds including vitamins by these crop species (Goatley and Lewis 1966). In fact, high amounts of mycotoxins could be excreted from toxigenic Penicillium isolates into guttation droplets which could be accounted for the variation in the intensity of disease incidence (Gareis and Gareis 2007). Secretion of antimicrobial phylloplane proteins by specific trichomes and delivering in guttation fluid from hydathodes protecting plants from diseases have been reported (Shepherd and Wagner 2007). Also, guttation has been demonstrated to reduce wood moisture thereby preventing wood initial decay by fungus (Schmidt and Czeschlik 2006). Guttation fluid has also been used to differentiate varietal resistance to bacterial diseases (Fukui et al. 1999). Growth and survival of Xanthomonas campestris pv. dieffenbachiae in guttation fluids, i.e. xylem sap exuded from leaf margins of Anthuriums have been found to be suppressed by several bacterial strains indigenous to leaves of various Anthurium cultivars. Inhibition of growth was not observed in filter-sterilized guttation fluids and was restored to original levels only by reintroducing specific mixtures of bacteria into filter-sterilized guttation fluids. These findings are of great significance as this bacterial community has potential for biological control of Anthurium blight. The epithem can also add substances to the guttation fluid, such as excess calcium and proteins that protect vulnerable hydathodes from microbial and fungal attack.
When guttation occurs, the plant surface is wetted. These are the conditions that will allow epiphytic living motile bacteria to move and to eventually enter the plant’s interior via the hydathodes. Grunwald et al. (2003) investigated brilliantly as to whether the plant has capacity to develop a protection mechanism against motile bacteria in the vicinity of the hydathodes. Such a protection mechanism could use the well known pathogenesis-related (PR) proteins. Indeed, an analysis of the guttation fluid showed a clustering of approximately 200 proteins which belonged mostly to the family of PR-proteins suggesting a role in plant protection against invaders. The protein profile of the guttation fluid was remarkably modified by treating plants with methyl jasmonic acid suggesting that the protein composition of the guttation fluid is controlled by both internal and external stimuli. The anti-POC1, an antibody, reacted only with a protein of the same mobility as POC1 in extracellular and guttation fluids from plants undergoing incompatible responses. In effort to understand the physiological mechanisms associated with resistance of rice to Xanthomonas oryzae pv oryzae (Young et al. 1995) and resistance of cabbage to black rot disease (Gay and Tuzun 2000), the role of hydathodes in disease resistance with respect to total peroxidase activities, anionic peroxidase isozyme expression, and lignin deposition was investigated. Hydathodal fluids of resistant varieties had greater peroxidase activity when compared to susceptible ones, with infected plants having higher peroxidase levels than noninfected plants. Lignin deposition in and around the hydathodes was found to be associated with the accumulation of this particular isozyme in hydathodal fluids. Thus, it is of immense significance that guttation fluid can be used to differentiate varietal resistance to fungal as well as bacterial diseases.From the foregoing discussion it seems increasingly clear that guttation fluid plays important role in defense against various bacterial and fungal diseases and that it also elevates disease resistance in plants.
Chemicals of guttation impacting fungal classification as an aid to crop protection
It is important to point out that guttation fluid may provide an excellent opportunity for studying host-parasite relationship in plants. The color of guttation droplets is often crystal clear and translucent. However, it can vary depending upon the strains, races and forms of fungi invading leaf tissues. The color and intensity of guttation fluid can be utilized to identify and distinguish various strains, races and forms of Penicillium chysogenum. By using this technique, Raper and Thom (1949) examined hundreds of isolates of the P. chysogenum “series” and considered P. griseoroseum Dierckx to be a synonym of P. notatum and Samson and Gams (1984) reinforced the broad concept of P. chysogenum by further reducing P. cyaneofulvum, P. meleagrinum and P. notatum to synonym with P. chysogenum. Very recently, Scott et al. (2004) observed yellow guttation droplets and they utilized color variations as the basis of classification and categorization of Penicillium sps which may aid greatly the control measures for the disease caused by this fungus. Further studies may look for other disease-causing fungi or otherwise producing variants of colors to be used for crop protection purposes.
Chemicals of guttation impacting human health
Production of plant-derived pharmaceutical proteins is gaining momentum these days (Schillberg et al. 2003; Fischer et al. 2004; Rybicki 2009). Several crops like tobacco, potato, rice, wheat, barley and maize have been investigated as potential hosts for recombinant protein production but tobacco has been a suitable crop for the production of antibodies and other recombinant proteins impacting human health through guttation and root secretions (Borisjuk et al. 1999; Komarnytsky et al. 2000, 2004, 2006; Gaume et al. 2003; Drake et al. 2009). Examination of guttation fluid may also indicate the pathogenic status of fruits and developing grains. In addition, the authors of this review suggest the collection and utilisation of guttation water, particularly from medicinal plants, to be used as mix for drinking as it may remove malnutrition of growing babies particularly those belonging to low economic strata, because of its organic and inorganic constituents including antibodies and other secreting materials of vital metabolic significance. The water so discerned might be the equivalent of half-a-litre or so, but enough to fuel hopes of finding more such fluids in the unexplored aspect of guttation. However, these areas of research remain unexplored and therefore, prospective field for research which warrant immediate attention of investigators.
Chemicals of guttation exerting deleterious effects on crop plants
Guttation water seated on the tips, margins and surfaces of leaves (laminar hydathodes) is likely to cause injury due to deposition of salts present therein, ingress of pesticides and disinfecting gases (SO2) used in the crops or entrance of pathogenic microorganisms. As early as 1804, DeSaussure reported the injury and death of leaves of certain garden plants associated with salt deposits of guttation fluid on leaves. Much later Ivanoff (1941, 1963) and Curtis (1943, 1944a, b) working on different species of plants under varied climatic conditions arrived at similar conclusions as reported by DeSaussure about 137 years ago. An examination of guttation fluid of maize seedlings showed the presence of nitrates, nitrites, potassium, calcium and other elements therein and it was, therefore, deduced that the climatic conditions resulting in too rapid a loss of water from lettuce leaves were probably responsible for the injury “tip burn”, an abnormality found most troublesome during hottest part of summer (Ivanoff 1941).
Chemicals of guttation causing non-pathogenic abnormalities
Injury caused by chlorotic and necrotic lesions
As stated earlier, the water that is lost in this process is not pure but contains a number of minerals, organic acids, sugars, proteins, enzymes etc. The estimation of solutes lost in this process, revealed that certain plants lose about 200–500 mg of solutes per liter of water. On evaporation, the solutes such as Cd, Zn, Hg, Al, Ni, Na, Ca, B, Se, NO3, NO2, Cl, SO4 etc. that remain at the margins or tips of leaves cause salt burning, which is often called ‘guttation burn’. The liquid drops that are exuded from the leaves always pass through hydathodes and as such it may be assumed to have physiological implications of significance in plants (Heringa 1971).
Injury caused by pesticide residues
Plant pathologists and entomologists have been concerned for a long time with the mode of action of certain pesticides and their sometime injurious effects, particularly with injuries along the periphery of leaves (see "Chemicals of guttation impacting flower pollination"). Curtis (1944a, b) found that guttation fluid from squash plants brought copper into solution from copper hydroxide. He advanced the hypothesis that salts or compounds contained in the guttation fluid react or combine with materials in sprays and dusts applied to plants as pesticides to form new compounds that either alter the effectiveness of the pesticide or bring the copper into solution from the insoluble form from copper oxide and Bordeaux mixture. The dissolved pesticide is then sucked back into the leaf in the guttation drops and the injury occurs from the inside of the leaf. In actual laboratory tests the guttation fluid increased the toxicity of two fungicides against known dilution of Macrosporium sarcinaeforme seeded on glass slides. However, such experiments are required to be conducted with new high yielding cultivars of agricultural crops.
Injury caused by loss of nutrients from leaves
It is important to understand the significance of guttation in order to appreciate large losses of nutrients by plants through guttation and leaching and the deleterious effects they may induce (Ingham 1950; Tamm 1951; Tibbitts 1986). As early as 1909, LeClerc and Breazeale attempted to understand the manner in which plants lose nutrients from living leaves and other above-ground parts. Great losses of nutrients, brought about by recurrent guttation periods and associated with drastic changes in plant behavior, have been observed by Ivanoff (1941) in the Winter Garden region of Texas, where conditions for frequent and profuse guttations are particularly favorable. Analyses of wheat plant indicated that the rains, which amounted to one inch, “dissolved” from the plants the following nutrient constituents: nitrogen, 27–32; phosphoric acid, 20–22; potash, 63–66; soda, 46–65; lime, 51–59; magnesia, 54–62; and chlorine, 90 % of these constituents acquired by the leaf tissues (LeClerc and Breazeale 1909). The loss of soluble substances reported from uninjured leaf surfaces by the percolating action of rains and dews may in most cases be interpreted as a part of guttation phenomena. Certain specific detrimental effects have been reported to follow excessive loss of nutrients, in some instances, guttation is specifically mentioned. In other cases the authors suggested or implied a simple diffusion action or gave no consideration as to how these substances were liberated.
Removal of nutrients from crowns of living forest trees by rains has also been studied. Tamm (1951) collected from beneath pine, oak, birch, and spruce trees, considerable amounts of calcium, potassium, and sodium, together with smaller amounts of nitrogen and phosphorus. It was estimated that in about 40 days, after a total rainfall of 98 mm beneath pine, the amounts of nutrients supplied to the ground, calculated in kg ha−1, was calcium 2.5, potassium 2.7, and sodium 3.2. Ingham (1950) compared the composition of open field rain with rain drops from trees on the seacoast of Natal and found that the rain from trees contained 6 times the amount of P2O5 and 3–4 times the amount of CaO and ammonium nitrogen that the field rain did. In 12 consecutive monthly collections, after a total rainfall of 1244.6 mm; he obtained 12.06 kg of ammonium nitrogen, 0.49 kg of nitrate nitrogen, 9.97 kg of P and 153.31 kg of CaO, calculated on per acre basis. Loss of nutrients by apple foliage through the “leaching action” of rains has also been investigated by Dalbro (1955). He found the following amounts of constituents leached (exclusive of the constituents in rain water) in kg per hectare of planted area: potassium, 25–30; sodium, 9; calcium, 105; and organic matter, 7.4—equivalents KMnO4. The amount leached seemed to be a function of the amount of precipitation. Some organic-reducing substances were also collected from both rain and leaching water, though they seemed to differ from one another but remarkable amounts were leached during the dormant period of the trees. Importantly, the magnitude of the nutrient losses, the nature of the compounds lost, and the physiological factors that may affect the rate of this loss have been studied by radioisotopes (Long et al. 1956). Several amino acids and large quantities of reducing substances were also identified in the leachate. The authors suggested that such losses may be of sufficient magnitude to alter markedly the behavior of the plant but it needs integrated studies on soil–plant-nutrient recycling system.
Chemicals of guttation causing pathogenic abnormalities
As stated earlier, guttation fluid is considered to be an ideal medium for bacterial, fungal and viral growth. It constitutes the habitats that appear to be favorable for pathogens development (Bald 1952). Besides providing a liquid vehicle for bacterial and other pathogens to enter the host plant, the water of guttation causes an increase of relative humidity under and around the plant parts in a manner similar to the action of dews and other kinds of precipitation. Guttation, therefore, should be considered and taken into account in explaining disease epidemiology in dry climates and in areas where irrigation is practiced. Although guttation and dew precipitation have been frequently confused by casual observers, the two phenomena are causatively and otherwise distinct and do not necessarily occur at the same time. It is therefore, likely that sometimes in certain cases guttation may cause disease infections by releasing toxins in the host plants. However, the early views and belief about injurious effects of guttation water have been set aside in the light of recent findings and new discoveries relating to its overall beneficial effects which have been elaborately described in previous sections of this review.
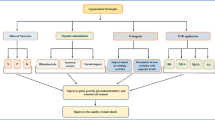
Gene mining and molecular farming for guttation
Molecular farming for pharmaceutical products
Commercial protein production has traditionally relied on microbial fermentation and mammalian cell lines, but these systems have disadvantages in terms of cost, scalability and safety that have prompted research into alternatives. Therefore, plants have emerged as one of the most promising general production platforms for pharmaceuticals and are now gaining widespread acceptance for the large-scale production of recombinant proteins. Plant-based biopharmaceuticals, nutriceuticals and cosmeceuticals have been described in detail (Conrad and Fiedler 1998; Ma et al. 2003; Fischer et al. 2004) and the technological basis of molecular farming in plants, with a focus on proteins that can be used for diagnostic, therapeutic and prophylactic applications has been recently discussed (Rybicki 2009). In fact, easy cultivation and the ability of plants to carry out numerous post-translational protein modifications make them suitable bioreactors for the production of many valuable recombinant proteins used as pharmaceuticals, industrial enzymes, or fine chemicals. Numerous heterologous proteins have been expressed in different plant organs and plant cell compartments (Twyman et al. 2004). This principle has been demonstrated by the commercial success of several first-generation products, and many others are currently under development. Over the past 10 years, several efficient plant-based expression systems have emerged, and more than 100 recombinant proteins have now been produced in a range of different plant species (Rybicki 2009). However, several constraints that hinder the widespread use of plants as bioreactors remain to be addressed. Important factors include quality and homogeneity of the final product, the challenge of processing plant-derived pharmaceutical macromolecules under good manufacturing practice conditions and concerns about biosafety etc. Molecular farming in plants will only realize its huge potential if these constraints are removed through rigorous and detailed science-based studies (Raghuram et al. 2006; Pathak et al. 2008). In attempt to solving these problems there has been significant success in tackling some of the limitations of plant bioreactors, such as low yields and inconsistent product quality that have limited the approval of plant-derived pharmaceuticals. Another advantage of the use of plants in recombinant protein production is that vaccine candidates can be expressed in edible plant organs, allowing them to be administered as unprocessed or partially processed material. However, tobacco being a ‘non-food’, ‘non-feed’ crop carries a reduced risk of transgenic material or recombinant proteins contaminating feed and human food chains. Yet, the hunt for other suitable crops is in progress and lettuce has been used for clinical trials with a hepatitis B virus subunit vaccine. Rice may be another preferred pharmaceutical-producing crop as it guttates profusely (Singh et al. 2008, 2009a).
In general, plant-based systems compare favorably well with alternative expression platforms, both in terms of quality and cost of complex therapeutic proteins. Most first-generation recombinant proteins were well-characterized peptides, such as insulin and other hormones, which functioned as therapeutic agents just as they normally would. Many second- and third-generation recombinant products, however, are complex monoclonal antibodies (mAbs) that require multiple processing steps to preserve their original bioactivity. Therefore, high costs and limited production capacities remain the major obstacles to many long-term therapies based on mAb treatments. The expression of foreign genes is now routine for many plant species. Now a variety of antibody fragments and/or full-length mAbs have been produced in plants (Komarnytsky et al. 2006). Biologically active mAbs require a number of assembly steps and post-translational modifications that are carried out in the endoplasmic reticulum (ER). Once the recombinant protein is directed to the ER, it is generally secreted to the apoplast following the default secretion pathway, targeted to the vacuole, or retained in the ER by the addition of the KDEL C-terminal sequence. However, proteases released during plant tissue harvesting, extraction, and downstream protein purification often result in antibody degradation. Therefore, the large-scale production of recombinant proteins in plants is limited by relatively low yields and difficulties in extraction and purification. These problems were addressed by engineering two related tobacco plant production systems in Raskin’s laboratory in USA to continuously secrete recombinant proteins from their roots called ‘rhizosecretion’ into a simple hydroponic medium (Borisjuk et al. 1999) and from leaf secretion called ‘phyllosecretion’ or guttation (Komarnytsky et al. 2000). These two nondestructive secretion processes that provide high yields of recombinant proteins over the lifetime of a plant and facilitate downstream purification have enabled to circumvent the manufacturing challenges. The rhizosecretion of a functional murine mAb from the roots of previously transformed tobacco plants, resulting in a mean antibody yield of 12 μg/g root dry weight per day, was demonstrated subsequently (Drake et al. 2003). Rhizosecretion is an attractive technology for the production of recombinant proteins from transgenic plants. Such a secretion of a target protein in the hydroponic medium provides an alternative manufacturing platform that simplifies the downstream purification procedure and increases protein yield. However, to date, yields of plant-derived recombinant pharmaceuticals by this method have been too low for commercial viability. In order to increase the production rates of rhizosecreted proteins, Raskin laboratory has exploited the ability of Agrobacterium rhizogenes to induce the formation of large amounts of root tissue on transgenic tobacco plants engineered to secrete a model recombinant protein, human secreted alkaline phosphatase (SEAP). The secretion of SEAP from hairy roots induced on the stems of transgenic tobacco plants was 5-7 times higher than that from adventitious transgenic roots (Gaume et al. 2003). Komarnytsky et al. (2004) have further developed an optimized antibiotic-free transformation and rhizosecretion system for stable high-yield production of complex proteins based on the pRYG transformation vector. Taking this a step further these authors engineered the system to provide enhanced levels of tissue-specific expression of the human single-chain IgG1 and full-length IgG4 immunoglobulin complexes and improve production rates based on the use of plant-derived signal peptides (Komarnytsky et al. 2006). Additionally, they demonstrated that cosecretion of the Bowman-Birk Ser protease inhibitor (BBI) into the plant growth medium significantly enhanced antibody stability and yield such that it increased antibody production to 36.4 μg/g root dry weight per day for single-chain IgG1 and 21.8 μg/g root dry weight per day for full-size IgG4 antibodies. These results suggest that constitutive cosecretion of a protease inhibitor combined with the use of the plant signal peptide and the antibiotic marker-free transformation system offers a novel strategy to achieve high yields of complex therapeutic proteins secreted from plant roots. Further studies conducted focused on three transgenic plant lines grown in hydroponic culture medium, two expressing monoclonal antibodies Guy’s 13 and 4E10 and one expressing a small microbicide polypeptide cyanovirin-N. Rhizosecretion rates increased significantly by the addition of the plant growth regulator alpha-naphthalene acetic acid (Drake et al. 2009). The maximum rhizosecretion rates achieved were 58 μg/g for Guy’s 13, 10.43 μg/g for 4E10, and 766 μg/g root dry weight/24 h for cyanovirin-N respectively, the highest figures so far reported for a full-length antibody and a recombinant protein. The plant growth regulators indole-butyric acid, 6-benzylaminopurine, and kinetin were also demonstrated to increase rhizosecretion of Guy’s 13. The effect of the growth regulators differed, as alpha-naphthalene acetic acid and indole-butyric acid increased the root dry weight of hydroponic plants, whereas the cytokinins benzylaminopurine and kinetin increased rhizosecretion without affecting root mass. A comparative glycosylation analysis between MAb Guy’s 13 purified from either hydroponic culture medium or from leaf extracts demonstrated a similar pattern of glycosylation comprising high mannose to complex glycoforms. Analysis of the hydroponic culture medium at harvest revealed significantly lower and less complex levels of proteolytic enzymes, in comparison with leaf extracts, which translated to a higher proportion of intact Guy’s 13 IgG in relation to other IgG products. These authors further suggested that hydroponic medium could be added directly to a chromatography column for affinity purification, allowing simple and rapid production of high purity Guy’s 13 antibody. In addition to the attractiveness of controlled cultivation within a contained environment for pharmaceutical-producing plants, their study demonstrates advantages with respect to the quality and downstream purification of recombinant proteins. The phyllosecretion, i.e. guttation fluid is another easily collectable solution exuded daily by plants. In a natural environment, guttation fluid is most often observed at dawn after cool, still nights when conditions for absorption of water by roots are very favorable while transpiration is suppressed. Komarnytsky et al. (2000) worked on tobacco (Nicotiana tabacum L. cv Wisconsin) plants engineered to secrete human placental secreted alkaline phosphatase (SEAP), green fluorescent protein (GFP) from jellyfish (Aequorea victoria), and xylanase from Clostridium thermocellum through the plant cell default secretion pathway. The volume of guttation fluid they collected was at the highest rate of 1–2 mL/g of leaf dry weight per day (up to 5 mL/cm2 of leaf area) from the 2-month-old tobacco plants. The fluid contained up to 20 mg/mL (40 mg/g of leaf dry weight per day) of total soluble protein. Interestingly, a clearing zone indicating an enzymatic cleavage of RBB-xylan by active bacterial xylanase released into the guttation fluid of transgenic tobacco plants developed in 3 h. No clearing area was detected around the control sample. The leaves of transgenic tobacco plants released 60, 30, 30, and 15 ng GFP/g of leaf dry weight. These results demonstrated that recombinant GFP might be secreted into the apoplast space and released into guttation fluid of transgenic tobacco following the expression of GFP under the control of mas29 promoter. Further, the guttation fluid of SEAP-transgenic tobacco contained the recombinant protein at concentrations of 0.15–1.1 mg/g of leaf dry weight per day which was 0.3–2.8 % of total soluble protein. These numbers are somewhat below the levels of SEAP production observed in the rhizosecretion system (Borisjuk et al. 1999). Their results indicated that recombinant proteins directed to the leaf intercellular space (apoplast) were effectively released into the plant guttation fluid. Because guttation fluid contains less total protein as compared to apoplast fluid and it can be collected continuously throughout the plant’s lifetime, guttation can be successfully used as a vehicle for recombinant protein production in plants. In addition, expression of image friendly proteins, such as GFP, in the plant guttation fluid could provide a novel tool to study various molecular and physiological aspects of guttation phenomenon in plants. However, the tobacco guttation reported by these authors was not optimized for the maximum protein recovery or yield. The guttation-based system of recombinant protein production can be operated continuously throughout the life of the plant, capturing its total capacity to synthesize recombinant proteins. It is nondestructive and abolishes the need for tissue extraction and simplifies complex protein purification procedures. The production of these pharmaceuticals can be further optimized by taking additional parameters such as optimum temperature, light, nutrients, aeration, hormone supply, humidity etc. that affect the plant guttation process into consideration. In contrast to tobacco, tomato leaves exude large guttation drops at their tips and margins, which can be easily shaken off the leaves. Monocots, notably grasses and rice, guttate profusely under field conditions (Singh et al. 2008, 2009a) and are capable of producing larger leaf surface area for guttation drops. Uniform drops at the tip and along the margins of the grass blades might represent an excellent collectable target for production of recombinant proteins by means of phyllosecretion technology (Singh et al. 2009a). In addition, optimization of transgene expression and tissue specificity will play an important role in optimizing phyllosecretion for all plant species. Another important step in future system optimization might include assessing the ability of the simultaneous use of both the phyllosecretion and rhizosecretion systems. Going a step further, hairy roots on the stems of transgenic tobacco may be 5-7 times more efficient in producing therapeutic antibodies and other recombinant proteins than adventitious transgenic roots (Gaume et al. 2003). If successful, the combination of all these techniques could significantly increase the total yield of heterologous proteins produced by plants in the easily accessible form of a water solution (Hehle et al. 2011).
In conclusion, we sum up the story by expressing that to succeed, the technology of rhizosecretion and phyllosecretion will have to compete with bacterial, cell culture, and transgenic animals technologies as well as with other methods of recombinant protein production in plants. However, the continuous and nondestructive recovery of recombinant proteins from a living plant potentially allows much higher yield than the single time harvesting, which captures only a fraction of proteins synthesized over the lifetime of a plant. An additional advantage of the guttation-based technology is the containment of the recombinant plants in the greenhouse. Most other plant-based production technologies rely on field-grown plants, which pose a potential risk to the environment. It is clear that more work will be required to develop this technology and to increase the yield of recombinant proteins. These revelations only demonstrate the feasibility of the approach, leaving the optimization for the maximum production of antibodies and recombinant proteins for the future. In addition, engineering solutions to the large-scale collection of guttation fluid must be developed, which might include shaking off the guttation droplets into a collection vessel or removing them from the leaf surface with a vacuum or blotting paper, the easiest and most successful technique recently developed by Singh et al. (2008) which is applicable for large scale collection of guttation fluid and elution of products from the blotting paper for further chemical characterization thereof.
Molecular farming for industrial and commercial products
Significant advances over the last few years have seen plant-made pharmaceuticals move from the exploratory research phase towards clinical trials, with the first commercial products for human use has either already arrived in the market or likely to come soon (Rybicki 2009). During the last few years registrations have been obtained for the industrial production of certain plan-derived antibodies and vaccines in USA and Cuba. In a significant achievement, a trichome-specific promoter that might be useful for the secretion of recombinant proteins into the leaf guttation fluid has also been described in tobacco. Commercially important products produced by secreting organs such as simple trichomes and glandular-secreting types (GSTs) include, among others, cotton fibers (Sparrow et al. 2007). Since the commercial value of cotton fibers is dependent on fiber length (Kim and Triplett 2001), mechanisms underlying cotton fiber elongation (turgor-driven expansion and cellulose synthesis, in particular) and trichome differentiation are currently key targets for improving production of this crop. Cotton fiber gene libraries have been created to identify transcription factors regulating these mechanisms (Jaradat and Allen 1999) and the mechanisms of turgor generation underlying fiber expansion have been studied by monitoring expression of turgor-related genes during fiber elongation (Smart et al. 1998). These studies are undoubtedly source of inspiration and encouragement for future investigations into guttation for hormones and other phytochemicals found therein.
Molecular farming for enhanced food production
As stated earlier, molecular farming in plants has already begun and proven to be a successful way of producing a range of technical proteins. Exploiting the ooze through engineering secretion systems of plants is an exciting and fascinating field of plant molecular biology (Borisjuk et al. 1999; Komarnytsky et al. 2000, 2004, 2006). Molecular farming through gene mining involves the use of genetically enhanced plants to produce particular biochemicals for increasing pest-resistance, biological and economic yield. Not surprisingly, plants can be engineered to produce everything from vaccines to plastics (Giddings et al. 2000). Secretion systems of plants may be molecular farming for pest and disease resistance and increased yield sink potential factor factories of the future, hence for enhanced crop production (Singh et al. 2008, 2009a).
Molecular cloning
The expression of image-friendly proteins, hormones and other macromolecules in the plant secretions could provide a novel tool to study various molecular and physiological aspects of guttation contributing to enhanced biological and economic yield of plants (Slewinski et al. 2009; Testone et al. 2009). Considerable recent research, as described earlier, has focused on glandular secreting trichomes (GSTs) to understand and exploit their ability to secrete phytochemicals that might improve resistance to insects, microbes and herbivores, modify gland metabolism towards improving properties of exudates (e.g. flavor and aroma in herbs) and allow commercial production of useful compounds by guttation (Kim et al. 2003).
Modification of trichome and hydathode metabolism
Efforts are now being made to modify trichome metabolism for exploiting trichomes and glands for commercial and agronomic purposes (Wagner and Wang 2001; Wagner et al. 2004). Genome mining and directed gene knockdown are two approaches being used to investigate and modify glandular-secreting types (GSTs). It might be thought that similar studies of hydathodes, for example, would be more difficult or impossible because these structures are embedded in the epidermal plane. However, EST libraries have been prepared from guard cell protoplasts (Kwak et al. 1997) and crystal idioblasts have been isolated from Pistia stratiotes (Kostman et al. 2001). Thus, while it may not be feasible to isolate secreting structures embedded in the epidermal plane or elsewhere using mechanical means, these may be accessible via protoplast technology. Finally, laser-capture micro-dissection has been used to isolate resin blisters (resin ducts) from grand fir (Abies grandis). Theoretically, this method could be applied to isolation of any surface or subsurface structure or cell type. Methods for preparing high quality RNA from plants have improved greatly in recent years, so once such structures are isolated, they may, like trichomes, yield EST libraries for applying the genomics approach (Harada et al. 2010). Despite some possible disadvantages of using trichomes and possibly hydathodes for molecular farming, there are many advantages that should allow their exploitation for production of pharmaceuticals, nutriceuticals and cosmeceuticals and so is the need for molecular farming of guttation-efficient cultivars of field crops for enhanced production of food which does not involve any additional processing. Future studies of course, are required to understand the hormonal status of various genotypes of crop plants differing in guttation efficiency. Significantly, Singh et al. (2008) have developed a new technique of measuring guttation that is easy, simple, accurate, non-invasive and quick to perform and does not need costly and cumbersome equipment and requires much less time than other known laboratory or field methods or techniques. They have found a positive relationship between the rate of guttation and the yield sink potential of rice panicle of six cultivars (Fig. 3). These findings on guttation in rice are novel (http//www.google.com Google → images → guttation in rice). Therefore, it holds out good promise for selecting; for example, by setting a lower limit at 90 μL in the present case, genotypes exhibiting guttation rates higher than the cutoff point, from a large pool of germplasm for breeding crop varieties for yield improvement. Further, these findings provide opportunity for mapping the genes controlling this trait and using them for creating efficient transgenic rice plants with increased guttation as success has been achieved in increasing and regulating the expression of recombinant proteins in plants that further increase efficiency of guttation in tobacco (Komarnytsky et al. 2000, 2004, 2006). There is great potential for progress in understanding the genetic and molecular basis of guttation. Identification of the significance or function of guttation is important to prioritization and identifying gaps in present knowledge. Such a selection tool may be useful in identifying key interactions in a systematic and transparent way and hopefully for rice, staple food for half of the world population of 7 billion (as of 21st July 2012) and for its ever rising trend in future infinite.
The relationship for six rice cultivars between the rate of guttation during pre-heading stage and their panicle weights (the yield sink potential) (Singh et al. 2008)
Enhancement of ABA biosynthesis: a factor for increased guttation
Transgenic tobacco and tomato plants both were produced containing the wild-type allele of notabilis, LeNCED1 coding region under the control of one of two strong constitutive promoters, either the doubly enhanced CaMV 35S promoter or the chimaeric ‘Super-Promoter’ (Thompson et al. 2000, 2007). Many of these plants were wilty, suggesting co-suppression of endogenous gene activity; however three transformants displayed a common, heritable phenotype that could be due to enhanced ABA biosynthesis, showing increased guttation and seed dormancy. Progeny from two of these transformants also exhibited reduced stomatal conductance, increased NCED mRNA enhancing thereby 9-cis-epoxycarotenoid dioxygenase and elevated seed ABA content. Apart from P450 monooxygenase BcABA1 which is essential for ABA biosynthesis (Siewers et al. 2004) NCED is indeed a key regulatory enzyme in the leaves for this synthetic pathway (Thompson et al. 2000). These authors found strong evidence that demonstrates for the first time that plant ABA content can be increased through manipulating NCED. Thus, guttation fluid could provide a novel tool to study various molecular and physiological aspects of guttation in plants unravelling several unresolved questions in plant biology which might lead to new biotechnological interventions to cater the human needs for food, health and happiness.
Conclusions
In the light of new discoveries and recent information the earlier belief and opinion on guttation have been set aside. Among several biological phenomena, guttation remains one of the most powerful engines for chemical mobility in plants. This review has highlighted recent researches in guttation having chemical impact on crop husbandry and molecular farming for enhanced production of food, pharmaceuticals, nutriceuticals and cosmeceuticals. The main physiological roles of guttation consisting of essentially xylem and phloem saps involve the acquisition, retention and transport of essential ingredients of life such as water, nutrients, metabolites, hormones, vitamins, enzymes, transport proteins, pathogen-related proteins etc. within the plants interacting with the environment and impacting their influence on the growth, development, biological and economic yield of plants. Thus, it serves the purpose of a barometer for gauging internal chemical status and plant productivity. It can also prove an effective tool for screening varieties having increased guttation with a view to creating transgenic plants for molecular farming of recombinant proteins and enhanced crop productivity. The screening and development of new varieties would greatly add to enhanced food production as there is absolute lack and paucity of information on varietal differences in guttation among field crops though the rate of guttation differs widely not only among species but among varieties as well. There is, therefore, need to tap potential of such varieties for enhanced economic yield under ecofriendly and sustainable agriculture system. We hope and firmly believe that future work on guttation would be exciting and challenging and would bring laurel to the science of plant secretions. In addition, guttation fluid would serve as a non-invasive tool for the discovery of new macromolecules and assessment of their function unravelling several unresolved questions in plant biology which might lead to new biotechnological interventions to cater the human needs for food, health and happiness.
References
Aki T, Shigyo M, Nakano R, Yoneyama T, Yanagisawa S (2008) Nano-scale proteomics revealed the presence of regulatory proteins including three FT-like proteins in phloem and xylem saps from rice. Plant Cell Physiol 49:767–790
Aloni R, Langhans M, Aloni E, Dreieicher E, Ullrich CI (2005) Root-synthesized cytokinin in Arabidopsis is distributed in the shoot by the transpiration stream. J Exp Bot 56:1535–1544
Ameziane R, Bernhard K, Lightfoot D (2000) Expression of the bacterial gdhA gene encoding a NADPH glutamate dehydrogenase in tobacco affects plant growth and development. Plant Soil 221:47–57
Anonymous (2009) Guttation water of no relevance for agricultural practice as a route of exposure to pesticide residues. Bayer Crop Science, Press release, 16 Feb, Monheim, available at www.bayercropscience.com
Arisz WH, Helder RJ, Nie RV (1951) Analysis of the exudation process in tomato plants. J Exp Bot 2:257–297
Asensi-Fabado MA, Munné-Bosch S (2010) Vitamins in plants: occurrence, biosynthesis and antioxidant function. Trends Plant Sci 15:582–592
Baba I (1957) Studies on the nutrition of the rice plant with special reference to nitrogen and silica. IV. On silica in the exudation and guttation sap. Japan J Crop Sci 25:139–140
Bald JG (1952) Stomatal droplets and the penetration of leaves by plant pathogens. Am J Bot 39:97–99
Biles CL, Abeles FB (1991) Xylem sap proteins. Plant Physiol 96:597–601
Bobik K, Duby G, Nizet Y, Vandermeeren C, Stiernet P, Kanczewska J, Boutry M (2010) Two widely expressed plasma membrane H(+)-ATPase isoforms of Nicotiana tabacum are differentially regulated by phosphorylation of their penultimate threonine. Plant J 62:291–301
Borisjuk NV, Borisjuk LG, Logendra S, Petersen F, Gleba YY, Raskin I (1999) Production of recombinant proteins in plant root exudates. Nature Biotechnol 17:466–469
Buch-Pedersen MJ, Palmgren MG (2003) Mechanism of proton transport by plant plasma membrane proton ATPases. J Plant Res 116:507–515
Bugbee B, Koerner G (2002) Yield comparisons and unique characteristics of the dwarf wheat cultivar ‘USU-Apogee’ research: super dwarf cultivar studies: ‘APOGEE’ WHEAT. http://www.usu.edu/cpl/research_dwarf_wheat.htm
Burgerstein A (1887) Materialien zu einer monographie betreffend die erscheinungen der transpiration der pflanzen. Zool-bot Ges Wien 37:691–782
Bürkle L, Cedzich A, Dopke C, Stransky H, Okumoto S, Gillissen B, Kuhn C, Frommer WB (2003) Transport of cytokinins mediated by purine transporters of the PUP family expressed in phloem, hydathodes, and pollen of Arabidopsis. Plant J 34:13–26
Canny MJ (1995) A new theory for the ascent of sap—cohesion supported by tissue pressure. Ann Bot 75:343–357
Canny MJ (2001) Contributions to the debate on water transport. Am J Bot 88:43–46
Chen C-C, Chen Y-R (2005) Study on laminar hydathodes of Ficus formosana (Moraceae) I. Morphology and ultrastructure. Bot Bull Acad Sin 46:205–215
Conrad U, Fiedler U (1998) Compartment-specific accumulation of recombinant immunoglobulins in plant cells: an essential tool for antibody production of physiological functions and pathogen activity. Plant Mol Biol 38:101–109
Coombe BG, Iland PG (2004) Grape berry development and winegrape quality. In: Coombe BG, Dry P (eds) Viticulture, vol I-resources. Winetitles Pty Ltd, Australia, pp 210–248
Curtis LC (1943) Deleterious effects of guttated fluids on foliage. Am J Bot 30:778–781
Curtis LC (1944a) The exudation of glutamine from lawn grass. Plant Physiol 19:1–5
Curtis LC (1944b) The influence of guttation fluid on pesticides. Phytopathology 34:196–205
Dalbro S (1955) Leaching of apple foliage by rain. In: Proceedings of 14th international horticultural congress. The Hague, Scheveningen, pp 770–778
De Saussure T (1804) Recherches chimique sur la vegetation. Gautheir-Villars, Paris, pp 264–265
Dieffenbach H, Kramer D, Lüttge U (1980a) Release of guttation fluid from passive hydathodes of intact barley plants. I. Structural and cytological aspects. Ann Bot 45:397–401
Dieffenbach H, Lüttge U, Pitman MG (1980b) Release of guttation fluid from passive hydathodes of intact barley plants. II. The effects of abscisic acid and cytokinins. Ann Bot 45:703–712
Dodd I, Ngo CN, Turnbull CG, Beveridge CA (2004) Effects of nitrogen supply on xylem cytokinin delivery, transpiration and leaf expansion of pea genotypes differing in xylem-cytokinin concentration. Funct Plant Biol 31:903–911
Drake PMW, Chargelegue DM, Vine ND, van Dolleweerd CJ, Obregon P, Ma JK (2003) Rhizosecretion of a monoclonal antibody protein complex from transgenic tobacco roots. Plant Mol Biol 52:233–241
Drake PMW, Barbi T, Sexton A, McGowan E, Stadlmann J, Navarre C, Paul MJ, Ma JKC (2009) Development of rhizosecretion as a production system for recombinant proteins from hydroponic cultivated tobacco. FASEB J 23:3581–3589
Duby G, Boutry M (2009) The plant plasma membrane proton pump ATPase: a highly regulated P-type ATPase with multiple physiological roles. Pflugers Arch 457:645–655
Duby G, Poreba W, Piotrowiak D, Bobik K, Derua R, Waelkens E, Boutry M (2009) Activation of plant plasma membrane H + -ATPase by 14–3-3 proteins is negatively controlled by two phosphorylation sites within the H + -ATPase C-terminal region. J Biol Chem 284:4213–4221
Feild TS, Arens NC (2007) The ecophysiology of early angiosperms. Plant, Cell Environ 30:291–309
Feild TS, Sage TL, Czerniak C, Iles WJD (2005) Hydathodal leaf teeth of Chloranthus japonicus (Chloranthaceae) prevent guttation-induced flooding of the mesophyll. Plant, Cell Environ 28:1179–1190
Fischer R, Emans N (2000) Molecular farming of pharmaceutical proteins. Transgenic Res 9:279–299
Fischer R, Schillberg S (2004) Molecular farming: plant-made pharmaceuticals and technical proteins. Wiley, New York
Fischer R, Stoger E, Schillberg S, Christou P, Twyman RM (2004) Plant-based production of biopharmaceuticals. Curr Opin Plant Biol 7:152–158
Fletcher AT, Mader JC (2007) Hormone profiling by LC-QToF-MS/MS in dormant Macadamia integrifolia: correlations with abnormal vertical growth. Plant Growth Regul 26:351–361
Fukui R, Fukui H, Alvarez AM (1999) Suppression of bacterial blight by a bacterial community isolated from the guttation fluids of Anthuriums. Appl Environ Microbiol 65:1020–1028
Gareis M, Gareis E (2007) Guttation droplets of Penicillium nordicum and Penicillium verrucosum contain high concentrations of the mycotoxins ochratoxin A and B. J Mycopathologia 163:207–214
Gaume A, Komarnytsky S, Borisjuk N, Raskin I (2003) Rhizosecretion of recombinant proteins from plant hairy roots. Plant Cell Rep 21:1188–1193
Gay PA, Tuzun S (2000) Involvement of a novel peroxidase isozyme and lignification in hydathodes in resistance to black rot disease in cabbage. Can J Bot 78:1144–1149
Ghosh M, Singh SP (2005) A review on phytoremediation of heavy metals and utilization of its byproducts. Appl Ecol Environ Res 3:1–18
Giddings G, Allison G, Brooks D, Carter A (2000) Transgenic plants as factories for biopharmaceuticals. Nature Biotechnol 18:1151–1155
Goatley JL, Lewis RW (1966) Composition of guttation fluid from rye, wheat, and barley seedlings. Plant Physiol 41:373–375
Gordon-Kamm WJ, Steponkus PL (1984) The behaviour of the plasma membrane following osmotic contraction of isolated protoplasts: implications in freezing injury. Protoplasma 123:83–94
Grunwald I, Rupprecht I, Schuster G, Kloppstech K (2003) Identification of guttation fluid proteins: the presence of pathogenesis-related proteins in non-infected barley plants. Physiol Plant 119:192–202
Harada E, Kim J-A, Meyer AJ, Hell R, Clemens S, Choi Y-E (2010) Expression profiling of tobacco leaf trichomes identifies genes for biotic and abiotic stresses. Plant Cell Physiol 51:1627–1637
Harris RI (1999) Guttation—the basis of an assay for evaluating formulation behaviour in vivo. Pesticide Sci 55:582–583
Hehle VK, Paul MJ, Drake PM, Ma JKC, Dolleweerd CJV (2011) Antibody degradation in tobacco plants: a predominantly apoplastic process. BMC Biotechnol 11:128–140
Heringa JW (1971) Guttation and dewdrops as contribution to some physiological disorders. Landbouwkd Tijdschr 83:7
Ingham G (1950) Effect of materials absorbed from the atmosphere in maintaining soil fertility. Soil Sci 70:205–212
Ivanoff SS (1941) Chemical deposits on foliage of citrus and other plants and their possible relation to chlorosis and yield. Texas agricultural experiment station, 54th annual report, pp 181–182
Ivanoff SS (1963) Guttation injuries of plants. Bot Rev 29:202–229
Jaradat TT, Allen RD (1999) Isolation of a novel cDNA encoding an auxin-induced basic helix–loop–helix transcription factor (Accession no. AF 165924) from cotton (Gossypium hirsutum L.). Plant Physiol 121:685–686
Katsuhara M, Hanba YT, Shiratake K, Maeshima M (2008) Expanding roles of plant aquaporins in plasma membranes and cell organelles. Functional Plant Biol 35:1–14
Kerstetter RE, Zepp RG, Carreira LH (1998) Peroxidases in grass dew derived from guttation: possible role in polymerization of soil organic matter. Biogeochemistry 42:311–323
Kim HJ, Triplett BA (2001) Cotton fiber growth in plants and in vitro: models for plant cell elongation and cell wall biogenesis. Plant Physiol 127:1361–1366
Kim KY, Kwon SY, Lee HS, Hur Y, Bang JW, Kwak SS (2003) A novel oxidative stress-inducible peroxidase promoter from sweet potato: molecular cloning and characterization in transgenic tobacco plants and cultured cells. Plant Mol Biol 51:831–838
Knipfer T, Besse M, Verdeil J-L, Fricke W (2011) Aquaporin-facilitated water uptake in barley (Hordeum vulgare L.) roots. J Exp Bot 62:4115–4126
Komarnytsky S, Borisjuk N, Borisjuk L, Alam M, Raskin I (2000) Production of recombinant proteins in tobacco guttation fluid. Plant Physiol 124:927–933
Komarnytsky S, Gaume A, Garvey A, Borisjuk N, Raskin I (2004) A quick and efficient system for antibiotic-free expression of heterologous genes in tobacco roots. Plant Cell Rep 22:765–773
Komarnytsky S, Borisjuk N, Yakoby N, Garvey A, Raskin I (2006) Co-secretion of protease inhibitor stabilizes antibodies produced by plant roots. Plant Physiol 141:1185–1193
Komis G, Apostolakos P, Galatis B (2002) Hyperosmotic stress-induced actin filament reorganisaton in leaf cells of Chlorophyton comosum. J Exp Bot 53:1699–1710
Kostman TA, Tarlyn NM, Loewus FA, Franceschi VR (2001) Biosynthesis of L-Ascorbic acid and conversion of carbons 1 and 2 of L-ascorbic acid to oxalic acid occurs within individual calcium oxalate crystal idioblasts. Plant Physiol 125:634–640
Koulman A, Lane GA, Christensen MJ, Fraser K, Tapper BA (2007) Peramine and other fungal alkaloids are exuded in the guttation fluid of endophyte-infected grasses. Phytochemistry 68:355–360
Kramer PJ (1949) Plant and soil water relationships. McGraw Hill Book Co Inc, New York
Kundt W, Gruber E (2006) The water circuit of the plants. Do plants have hearts? Quant Biol 0603019:1–19
Kwak JM, Kim SA, Hong SW, Nam HG (1997) Evaluation of 515 expressed sequence tags obtained from guard cells of Brassica campestris. Planta 202:9–17
LeClerc JA, Breazeale JF (1909) Plant food removed from growing plants by rain or dew. US Department of Agriculture, Yearbook, pp 389–402
Lersten LR, Curtis JD (1982) Hydathodes in Physocarpus (Rosaceae: Spiraeoideae). Can J Bot 60:850–855
Lersten LR, Curtis JD (1985) Distribution and anatomy of hydathodes in Asteraceae. Bot Gaz 146:106–114
Lersten LR, Curtis JD (1991) Laminar hydathodes in Urticaceae: survey of tribes and anatomical observation on Pilea pumila and Urtica dioica. Plant Syst Evol 176:179–203
Letham DS (1994) Cytokinins as phytohormones: sites of biosynthesis, translocation, and function of translocated cytokinin. In: Mok DWS, Mok MC (eds) Cytokinins: chemistry, activity and function. CRC Press, Boca Raton, FL, pp 57–80
Lewis RW (1962) Guttation fluid: effects on growth of Claviceps purpurea in vitro. Science 138:690–691
Long WC, Sweet DV, Turkey HB (1956) Loss of nutrients from plant foliage by leaching as indicated by radioisotopes. Science 123:1039–1040
Ma JK-C, Drake PMW, Christou P (2003) The production of recombinant pharmaceutical proteins in plants. Nature Rev Genetics 4:794–805
Ma JK-C, Barros E, Bock R, Christou P, Dale PJ, Dix PJ, Fischer R, Irwin J, Mahoney R, Pezzotti M, Schillberg S, Sparrow P, Stoger E, Twyman RM (2005) Molecular farming for new drugs and vaccines—current perspectives on the production of pharmaceuticals in transgenic plants. EMBO Rep 6:593–599
Maeda E, Maeda K (1987) Ultrastructural studies of leaf hydathodes. I. Wheat (Triticum aestivum) leaf tips. Japan J Crop Sci 56:641–651
Maeda E, Maeda K (1988) Ultrastructural studies of leaf hydathodes II. Rice (Oryza sativa) leaf tips. Japan J Crop Sci 57:733–742
Magwa ML, Lindner WA, Brand JM (1993) Guttation fluid peroxidases from Helianthus annuus. Phytochemistry 32:251–253
Meagher RB, Heaton AC (2005) Strategies for the engineered phytoremediation of toxic element pollution: mercury and arsenic. J Ind Microbiol Biotechnol 32:502–513
Merlot S, Leonhardt N, Fenzi F, Valon C, Costa M, Piette L, Vavasseur A, Genty B, Boivin K, Müller A, Giraudat J, Leung J (2007) Constitutive activation of a plasma membrane H(+) ATPase prevents abscisic acid-mediated stomatal closure. EMBO J 26:3216–3226
Mihucz VG, Tatár E, Virág I, Cseh E, Fodor F, Záray G (2005) Arsenic speciation in xylem sap of cucumber (Cucumis sativus L.). Anal Bioanal Chem 383:461–466
Mizuno N, Takahashi A, Wagatsuma T, Mizuno T, Obata H (2002) Chemical composition of guttation fluid and leaves of Petasites japonicus v. giganteus and Polygonum cuspidatum growing on ultramafic soil. Soil Sci Plant Nutr 48:451–453
Moore R, Clark WD, Vodopich DS (1998) Botany, 2nd edn. WCB/McGraw-Hill Co Inc, New York
Necmi P (2005) Investigation of monthly variation in some plant-nutrient contents of guttation fluid samples taken from Dieffenbachia plants. J Plant Nutr 28:1375–1382
Niittylä T, Fuglsang AT, Palmgren MG, Frommer WB, Schulze WX (2007) Temporal analysis of sucrose-induced phosphorylation changes in plasma membrane proteins of Arabidopsis. Mol Cell Proteomics 6:1711–1726
Nolte SA, Young BG, Mungur R, Lightfoot DA (2004) The glutamate dehydrogenase gene gdhA increased the resistance of tobacco to glufosinate. Weed Res 44:335–339
Oparka KJ, Prior DAM, Harris N (1990) Osmotic induction of fluid-phase endocytosis in onion epidermis cells. Planta 180:555–561
Paleg LG (1965) Physiological effects of gibberellins. Ann Rev Plant Physiol 16:291–322
Palmgren MG (2001) H+-ATPases: powerhouses for nutrient uptake. Ann Rev Plant Physiol Plant Mol Biol 52:817–845
Palzkill DA, Tibbitts TW (1977) Evidence that root pressure flow is required for calcium transport to head leaves of cabbage. Plant Physiol 60:854–856
Pathak RR, Ahmad A, Lochab S, Raghuram N (2008) Molecular physiology of plant nitrogen-use-efficiency and biotechnological options for its enhancement. Curr Sci 94:1394–1403
Pedersen O (1993) Long-distance water transport in aquatic plants. Plant Physiol 103:1369–1375
Pedersen O (1994) Acropetal water transport in submerged plants. Bot Acta 107:61–65
Pedersen O (1998) The nature of water transport in aquatic plants. In: Pedersen O (ed) Freshwater biology. Blackwell Publishing, New Jersey, pp 196–207
Pedersen O, Jùrgensen LB, Sand-Jensen K (1997) Through-flow of water in leaves of a submerged plant is influenced by the apical opening. Planta 202:43–50
Pedersen BP, Buch-Pedersen MJ, Morth JP, Palmgren MG, Nissen P (2007) Crystal structure of the plasma membrane proton pump. Nature 450:1111–1114
Peterson KM, Rychel AL, Torii KU (2010) Out of the mouths of plants: the molecular basis of the evolution and diversity of stomatal development. Plant Cell 22:296–306
Pickard WF (2003a) The riddle of root pressure. I. Putting Maxwell’s demon to rest. Funct Plant Biol 30:121–134
Pickard WF (2003b) The riddle of root pressure. II. Root exudation at extreme osmolalities. Funct Plant Biol 30:135–141
Pillitteri LJ, Bogenschutz NL, Torii KU (2008) The bHLH protein, MUTE, controls differentiation of stomata and the hydathode pore in Arabidopsis. Plant Cell Physiol 49:934–943
Pilot G, Stransky H, Bushey DF, Pratelli R, Ludewig U, Wingate VP, Frommer WB (2004) Overexpression of GLUTAMINE DUMPER1 leads to hypersecretion of glutamine from hydathodes of Arabidopsis leaves. Plant Cell 16:1827–1840
Raghuram N, Pathak RR, Sharma P (2006) Signalling and the molecular aspects of N-use-efficiency in higher plants. In: Singh RP, Jaiswal PK (eds) Biotechnological approaches to improve nitrogen-use-efficiency in plants. Studium Press LLC, Houston, TX, pp 19–40
Raper KB, Thom C (1949) A manual of the Penicillia. Williams and Wilkins, Baltimore, MD
Riedell WE, Schmid WE (1987) Diuron decreases light-stimulated potassium translocation to shoots of barley seedlings. J Plant Nutr 10:25–32. http://www.informaworld.com/smpp/title~db=all~content=t713597277~tab=issueslist~branches=10
Rybicki EP (2009) Third international conference on plant-based vaccines and antibodies. Expert Rev Vaccines 8:1151–1155
Salisbury FB, Ross CW (1992) Plant physiology, 4th edn. Wadsworth Publishing Co, Belmont, CA
Samson RA, Gams W (1984) The taxonomic situation in the hyphomycete genera Penicillium, Aspergillus and Fusarium. Antonie Van Leeuwenhoek 50:815–824
Sattelmacher B (2001) The apoplast and its significance for plant mineral nutrition. Tansley Review no. 22. New Phytol 149:167–192
Schillberg S, Fischer R, Emans N (2003) Molecular farming of recombinant antibodies in plants. Cell Mol Life Sci 60:433–445
Schmidt O, Czeschlik D (2006) Wood and tree fungi. Springer, Berlin
Schmidt AC, Steier S, Otto M (2009) Evaluation of the arsenic binding capacity of plant proteins under conditions of protein extraction for gel electrophoretic analysis. Talanta 77:1830–1836
Scott J, Untereiner WA, Wong B, Straus NA, Malloch D (2004) Genotypic variation in Penicillium chysogenum from indoor environments. Mycologia 96:1095–1105
Selmar D, Irandoost Z, Wray V (1996) Dhurrin-6′-glucoside, a cyanogenic diglucoside from Sorghum bicolor. Phytochemistry 43:569–572
Shawki MA-A, Kazda TD, Kohoutkova JJ, Taborsky V (2006) Toxicity to honeybees of water guttation and dew collected from winter rape treated with Nurelle D. Plant Protect Sci Prague 42:9–14
Shepherd RW, Wagner GJ (2007) Phylloplane proteins: emerging defenses at the aerial frontline? Trends Plant Sci 12:51–56
Siewers V, Smedsgaard J, Tudzynski P (2004) The P450 monooxygenase BcABA1 is essential for abscisic acid biosynthesis in Botrytis cinerea. Appl Environ Microbiol 70:3868–3876
Singh G, Singh TN (1989) Root-mediated water transport to the shoot of rice. Curr Sci 58:1134–1138
Singh S, Chauhan JS, Singh TN (2008) Guttation: a potential yield enhancing trait in rice. Curr Sci 95:455–456
Singh S, Singh TN, Chauhan JS (2009a) Guttation in rice: occurrence, regulation, and significance in varietal improvement. J Crop Improv 23:351–365
Singh S, Singh TN, Chauhan JS (2009b) Water transport in crop plants with special reference to rice: key to crop production under global water crisis. J Crop Improv 23:194–212
Skoog F, Broyer TC, Grossenbacher KA (1938) Effects of auxin on rates, periodicity and osmotic relations in exudation. Amer J Bot 25:749–759
Slewinski TL, Meeley R, Braun DM (2009) Sucrose transporter1 functions in phloem loading in maize leaves. J Exp Bot 60:881–892
Smart LB, Vojdani F, Maeshima M, Wilkins TA (1998) Genes involved in osmoregulation during turgor-driven cell expansion of developing cotton fibers are differentially regulated. Plant Physiol 116:1539–1549
Sparrow PAC, Irwin JA, Dale PJ, Twyman RM, Ma JKC (2007) Pharma-planta: road testing the developing regulatory guidelines for plant-made pharmaceuticals. Transgenic Res 16:147–161
Sperry JS (1983) Observations on the structure and function of hydathodes in Blechnum lehmannii. Amer Fern J 73:65–72
Stocking CR (1956a) Guttation and bleeding. In: Ruhland W (ed) Encycl plant physiol III. Springer, Berlin, pp 489–502
Stocking CR (1956b) Root pressure. In: Ruhland W (ed) Handbuch der Pflazenphysiologie III. Springer, Berlin, pp 581–595
Stokes A (1954) Uptake and translocation of griseofulvin by wheat seedlings. Plant Soil 5:132–142
Stoller EW (1970) Mechanism for the differential translocation of amiben in plants. Plant Physiol 46:732–737
Stryer L (1989) Biochemsitry, 3rd edn. WH Freeman, Sanfrancisco, CA
Sutton T, Baumann U, Hayes J, Collins NC, Shi B-J, Schnurbusch T, Hay A, Mayo G, Pallotta M, Tester M, Langridge P (2007) Boron-toxicity tolerance in barley arising from efflux transporter amplification. Science 318:1446–1449
Tamm CO (1951) Removal of plant nutrients from tree crowns by rain. Physiol Plant 4:184–188
Tanner W, Beevers H (2001) Transpiration, a prerequisite for long-distance transport of minerals in plants? Proc Natl Acad Sci USA 98:9443–9447
Tappero R, Peltier E, Gräfe M, Heidel K, Ginder-Vogel M, Livi KJT, Rivers ML, Marcus MA, Chaney RL, Sparks DL (2007) Hyperaccumulator Alyssum murale relies on a different metal storage mechanism for cobalt than for nickel. New Phytol 175:641–654
Tarakanova GA, Shvedova OY, Zholkevich VN (1985) Electrochemical parameters and guttation of Pilobolus umbonatus Buller cells. Dokl Akad Nauk SSSR 280:1277–1280
Tattersall DB, Bak S, Jones PR, Olsen CE, Nielsen JK, Hansen ML, Høj PB, Møller BL (2001) Resistance to herbivore through engineered cyanogenic glucoside synthesis. Science 293:1826–1828
Telewski FW (2006) A unified hypothesis of mechanoperception in plants. Amer J Bot 93:1466–1476
Testone G, Condello E, Verde I, Caboni E, Iannelli MA, Bruno L, Mariotti D, Bitonti MB, Giannino D (2009) The peach (Prunus persica [L.] Batsch) homeobox gene KNOPE3, which encodes a class 2 knotted-like transcription factor, is regulated during leaf development and triggered by sugars. J Mol Genetics Genomics 282:47–64
Thompson AJ, Jackson AC, Symonds RC, Mulholland BJ, Dadswell AR, Blake PS, Burbidge A, Taylor IB (2000) Ectopic expression of a tomato 9-cis-epoxycarotenoid dioxygenase gene causes overproduction of abscisic acid. Plant J 23:363–374
Thompson AJ, Andrews J, Mulholland BJ, McKee JMT, Hilton HW, Horridge JS, Farquhar GD, Smeeton RC, Smillie IRA, Black CR, Taylor IB (2007) Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiol 143:1905–1917
Tibbitts TW (1986) Controlled environment life support system: calcium-related leaf injuries on plants. NASA Contractor Report, p, 37
Twyman RM, Stoger E, Schillberg S (2004) http://74.125.153.132/search?q=cache:Fwj4MjTk1d8J:www.pharma-planta.org/resources_documents/COPB_2004.pdf+plant+based+production+of+biopharmaceuticals&cd=1&hl=en&ct=clnk-1
Twyman RM, Schillberg S, Fischer R (2005) Transgenic plants in the biopharmaceutical market. Expert Opin Emerg Drugs 10:185–218
Valente M, Bologna R (2011) www.rfb.it/bastaveleni/chisiamo.htm. Published daily “live” updates
Wagner GJ, Wang E (2001) Exploiting the ooze-engineering surface secretion systems of plants: secretion systems of plants may be molecular farming and pest/disease resistance factor factories of the future. Agric Biotechnol Net 3:1–3
Wagner GJ, Wang E, Shepherd RW (2004) New approaches for studying and exploiting an old protuberance, the plant trichome. Ann Bot 93:3–11
Wang W, Ben X, Wang H, Li J, Huang H et al (2011) YUCCA genes are expressed in response to leaf adaxial-abaxial juxtaposition and are required for leaf margin development. Plant Physiol 157:1805–1819
Wei C, Tyree MT, Steudle E (1999) Direct measurement of xylem pressure in leaves of intact maize plants: a test of the cohesion-tension theory taking hydraulic architecture into consideration. Plant Physiol 121:1191–1206
Wilson JK (1923) The nature and reaction of water from hydathodes. Cornell Agric Exp Sta Memoirs 65, USA
Wolfe JA (1993) A method of obtaining climatic parameters from leaf assemblages. US Geol Surv Bull 2040:1–71
Yang Y, Qin Y, Xie C, Zhao F, Zhao J, Liu D, Chen S, Fuglsang AT, Palmgren MG, Schumaker KS, Deng XW, Guo Y (2010) The Arabidopsis chaperone J3 regulates the plasma membrane H + -ATPase through interaction with the PKS5 kinase. Plant Cell 22:1313–1332
Young SA, Guo A, Guikema JA, White FF, Leach JE (1995) Rice cationic peroxidase accumulates in xylem vessels during incompatible interactions with Xanthomonas oryzae pv oryzae. Plant Physiol 107:1333–1341
Zaitseva RI, Minashina NG, Sudnitsyn II (1998) Influence of capillary-sorptive and osmotic moisture pressure in chernozem on the growth and guttation of barley. Eurasian Soil Sci 31:1075–1082
Zholkevich VN (1992) Root pressure. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half. Marcel Dekker, New York, pp 589–603
Acknowledgments
The authors extend their deep sense of gratitude to Dr. Amare Ayalew, Head of School of Plant Sciences, Dr. Kindie Tesfaye, Dean, College of Agriculture and Environmental Sciences and Dr. Nigussie Dechassa, Vice-President, Research Affairs & Extension Services of Haramaya University, Ethiopia for improving the text of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, S., Singh, T.N. Guttation 1: chemistry, crop husbandry and molecular farming. Phytochem Rev 12, 147–172 (2013). https://doi.org/10.1007/s11101-012-9269-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-012-9269-x