Abstract
Intramedullary spinal cord surgery is a particularly challenging field due to the delicate balance between the aim to remove a harmful lesion and the need to preserve the healthy, functional tissue in which the lesion is growing. Over the decades, a spectrum of techniques with the scope to monitor sensory and motor functions during a surgical operation, hence maximizing resection while minimizing damage to the surrounding nervous tissue, have been developed. These techniques are known as intraoperative neurophysiological monitoring (IONM) and the most common include somatosensory-evoked potentials (SSEPs), transcranial motor-evoked potentials (TcMEPs), free running electromyography (EMG), and D wave monitoring. Along with these, mapping techniques to localize specific functions in the spinal cord can be employed, such as dorsal columns and cortico-spinal tract mapping. The ever-growing experience in using these techniques allowed the development of warning criteria that are currently used in a context of teamwork and efficient communication within the OR team in order to optimize surgical strategy and patient outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Intraoperative neurophysiological neuromonitoring
- Spinal cord tumor
- Spinal cord monitoring
- Spinal cord mapping
- D-wave
- Motor-evoked potentials
-
The pre-operative clinical assessment is instrumental in ensuring adequate neurophysiological monitoring during an intramedullary spinal cord operation.
-
TcMEPs, ideally combined with D wave monitoring, constitute the gold standard to assess and preserve motor function during a surgical operation to the spinal cord.
-
Baseline monitoring features, anesthesia settings, physiological variables, and phase of surgery are important factors to consider in IONM signal interpretation.
-
Efficient communication and common language within the OR team are needed to ensure prompt surgical strategy adaptation and optimization of clinical outcomes.
Intramedullary spinal cord surgery (IMSCS) mainly consists of the removal of tumoral lesions and, to a lesser extent, of vascular malformations. Surgery on the spinal cord is particularly delicate due to the very high density of functional tissue and the very limited possibility for nervous structure manipulation [1,2,3].
Intraoperative neurophysiological monitoring (IONM), both in the form of mapping and monitoring techniques, proved to be a reliable tool to guide surgeons during IMSCS with the aim to preserve function (sensory and motor) while enabling the most complete resection possible. Over the years multiple IONM techniques have evolved and the correlations between IONM data and clinical outcomes have been addressed in various studies [4,5,6,7,8,9,10]. The growing experience in using these techniques allowed for the definition of warning IONM criteria that are currently used to make IMSCS safer.
The most common monitoring modalities in IMSCS are SSEP and TcMEP, which have all been covered in detail in other sections of this book. The use of free running EMG has also been reported [11]. D-wave monitoring is an additional technique which offers the strongest prediction of long-term motor outcome and, in our opinion, should become a standard monitoring technique for IMSCS [12]. In terms of spinal cord mapping techniques, the mapping of the dorsal columns (Chap. 1) is the most used, followed by the cortico-spinal tract (CST) mapping [13,14,15,16].
Given the very small margin for mistakes, good communication and efficient teamwork between surgeon, neurophysiologist, and anesthesiologist are essential to ensure the best results for the patient [17, 18]. In this chapter, we will guide the reader through the different phases of IMSCS, providing specific insights about each of them, reviewing monitoring and mapping techniques and providing useful tips to optimize efficiency and safety of each surgical case.
Preoperative Considerations
Clinical Picture
The first information to know about a patient with a spinal cord lesion with surgical indication is the clinical picture. A carefully conducted clinical examination will inform the team on the presence of both sensory and motor deficits which may affect the recording of IONM baselines and monitorability throughout surgery. For example, the presence of a significant motor deficit of the abductor pollicis brevis or the tibialis anterior will likely correspond to a low amplitude or even absent TcMEP, pushing the neurophysiologist to use higher stimulation intensities or increase the number of stimuli to obtain a monitorable response that can be used during surgery. Knowing the clinical picture of the patient and its correlation to IONM data allows one to plan the monitoring strategy accordingly, ruling out other possible causes (technical or physiological) for weak or absent signals.
Tumor Features
The MRI scan of the spinal cord is by far the most informative radiological exam when it comes to intramedullary lesions [19]. It allows the definition of the lesion’s location, extension, and morphologic characteristics which will in turn, influence the surgical strategy and most importantly, the possibility to obtain a complete resection versus a partial one. Ependymomas, which comprise 45% of all spinal cord tumors, are amenable to radical resection thanks to their non-infiltrative behavior and the possible presence of a capsule surrounding the lesion. They are usually centrally located and can present satellite cysts and syrinx. Similar to ependymomas, hemangioblastomas are amenable to radical resection, but they are much rarer lesions (5%) arising from blood vessels and with an increased risk of neurophysiological changes compared to ependymomas [20]. Astrocytomas, which comprise 40% of all spinal cord tumors, present with an infiltrative nature which often does not allow a complete resection and, especially in high grade tumors, results in a poorer prognosis. Their location tends to be eccentric, more commonly in the thoracic section of the spinal cord and they are more common in children [2, 19].
Along with the tumor features, modifying features that affect the “healthy” spinal cord beyond the limits of the lesions, such as the presence of perilesional edema or the presence of a syrinx, should be taken into account. Perilesional edema, for example, is known to slow down neural conductance, therefore a longer latency can be expected in the TcMEP baseline along with a reduced amplitude.
Monitoring and Mapping Techniques
Monitoring Techniques
IONM monitoring techniques are used during IMSCS to assess the integrity of the sensory-motor function of the spinal cord.
Somatosensory-evoked potentials (SSEPs) are a measure of electrophysiological activity of the sensory pathways which propagates rostrally from the periphery to the primary sensory cortex, passing through the posterior columns of the spinal cord and the higher gateway stations (thalamic nuclei). The stimulation typically consists of bipolar, non-noxious, peripheral electric stimulation at the level of ulnar and tibial nerves [4, 5, 21]. In case of a highly located cervical lesion, it can be useful to add the median nerve to the setting in order to obtain a better triangulation of the signal in case a change occurs. While recording of the response can be made at multiple sites (periphery, spinal cord, and scalp) in the case of an IMSCS it is important to record from either the cortex or, at least, at the level of C1 in order to detect all changes that would happen at the level of the spinal cord where the surgery is taking place. The initial phase of the myelotomy (see below) is the time in which the posterior columns are at the highest risk of being injured, while later in the surgery the risk progressively moves toward damaging the motor tracts, located more anteriorly. The most important limitation of SSEPs is that they do not provide information on motor function. In addition, as SSEPs require averaging in order to limit the influence of electric noise on the actual signal, to rely solely on this technique may result in a delay in informing the surgeon and the team of a significant change in the signal.
Transcranial motor-evoked potentials (TcMEPs) are the gold standard for monitoring motor function in IMSCS. They consist of electric activity that propagates through the motor pathway starting from the primary motor cortex, where the stimulation is provided, through the scalp electrodes, travels through the CST into the spinal cord, and is recorded at the periphery, at the level of muscles [4,5,6,7,8, 21]. The level of the spinal cord where the lesion is located as well as the preoperative neurological condition of the patient, are important variables to consider in order to decide which muscles should be monitored during surgery. TcMEPs are generated by a train of stimuli which are needed to trigger the action potential at the level of lower motor neurons, which are characterized by a higher capacitance compared to the upper motor neuron. In terms of montage setting at the scalp level, a more medial setting has the advantages to activate the lower extremities more selectively and to reduce the occurrence of unwanted tongue or mouth bite injuries [22]; on the other hand, there is the risk to sacrifice too much the ability to monitor the upper extremities; therefore, all these elements should be carefully considered when choosing the IONM strategy. TcMEPs are normally large amplitude, bi-multiphasic, high-frequency responses. Latency, useful to differentiate between muscles thanks to the difference in the distance that separates the primary motor cortex to each muscle (the farther the muscle the longer the latency), can be abnormally prolonged due to the presence of a spinal cord lesion due to both direct damage to the motor fibers and the presence of perilesional edema. In general, recording from a wide variety of muscles is recommended, with two to four muscles employed per limb. The most common muscles include biceps, extensor digitorum, abductor pollicis brevis in the upper limb, and quadriceps, tibialis anterior, and abductor hallucis in the lower limb.
Electromyography (EMG) consists of the recording of free muscle activity through the use of intramuscular electrodes. In physiological conditions, the trace is quiescent with low level background activity. The importance of including free running EMG in the IONM setting for an IMSCS case, is that irritation to the CST fibers due to direct manipulation triggers sustained bursts of the EMG that can be detected and communicated by the neurophysiologist. While the clinical relevance of such signal modifications is yet to be fully determined, the observation of changes can suggest the need to check for integrity of TcMEPs and D wave before proceeding with the surgery [2, 11].
D Wave
Among the IONM techniques, D wave recording reflects the electrical activity of the CST fibers at the level of the spinal cord following a single stimulus delivered to the primary motor cortex through the scalp electrodes. D waves are recorded as near field potentials and the ideal setting would entail the use of two epidural or subdural electrodes, one cranially and one caudally located to the lesion [5,6,7,8, 12]. In higher cervical tumors, the cranial electrode might not be placed due to the lack of room. For caudally located thoracic lesions, D wave can be monitored roughly down to T11, as the progressive reduction in the number of CST fibers makes it progressively more difficult to obtain a satisfactory, monitorable signal. Differently from TcMEPs, which need a train of stimuli able to generate muscle contraction, D waves can be monitored continuously because single stimuli will not be able to cause unwanted movement during the surgery. Another difference from TcMEPs is that the stimuli to generate D waves are delivered to activate both left and right CST allowing the whole motor axis to be captured. In order to permit this, stimulation intensity should be increased to obtain maximal response. Similar to SSEPs, D waves may need to be averaged in order to cancel noise or artifacts. While the latency of the D wave can vary based on electrode location (the lower the electrode placement, the longer the latency), its shape has specific morphological features. It is characterized by a large negativity (upward spike) bounded by smaller positivity (downward spikes); in case a higher intensity of stimulation is used, D waves may bifurcate or even trifurcate. With regard to amplitude, it progressively reduces the more distal the D wave is recorded, due to the progressively lower number of fibers that compose the CST. There are some physiological conditions in which D waves might be difficult to obtain due to dispersion of the signal, as in the case of infants of 18 months of age or younger, in which myelination is still not complete. In cases where D wave recording is challenging due to artifacts or noise, possible strategies to be employed include reduction of stimulus intensity, the use of low dose of muscle relaxants (although this may affect TcMEPs recordings), or increasing anesthesia. Being an asynaptic recording, D waves are quite resistant to anesthetic type and concentration, while SSEPs and TcMEPs are not, and any change in anesthesia setting during the surgery might heavily impact the recordings of both these monitoring modalities [23, 24].
Mapping Techniques
Mapping the spinal cord is of crucial importance for the localization and preservation of both sensory and motor functions. Dorsal column mapping is helpful during the first phase of the myelotomy because it allows the localization of the posterior median sulcus which will be the entry point to the cord itself; CST mapping is helpful during the advanced phases of surgery in which tumor debulking and dissection progressively bring the surgeon closer to the motor pathways.
Dorsal column mapping, as extensively explained in Chap. 1, is a method of distinguishing between the right and left gracile fasciculi and it can employ different techniques. We will briefly describe here the two most commonly used [16, 25].
The first method consists in measuring a regular SSEP at the level of the spinal cord, following stimulation of the tibialis anterior or median nerve [25]. A micro-grid electrode is positioned perpendicular to the long axis of the cord. The posterior median sulcus is localized between the two electrodes that record the highest amplitude signals after the respective stimulation of the left and right nerves. The assessment of such signals needs a trained eye to accurately determine the difference.
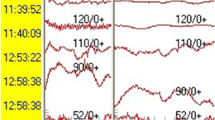
The second method is called phase reversal method [16]. It entails the direct stimulation of the spinal cord using a hand-held bipolar stimulator and recording the response at the level of the scalp electrodes where the activity of the primary sensory cortex is recorded. Starting on one side of the posterior aspect of the spinal cord and identified the gracile fasciculus on that side, the stimulation is then progressively moved toward the other side of the cord until the phase of the trace is reversed, which determines the position of the gracile fasciculus on the other side (Fig. 34.1). The posterior median sulcus is localized in the point where the scalp potentials flatten.
Dorsal column mapping. Top row. On the left, intraoperative photograph of the monopolar probe stimulating the right dorsal column; on the right the correspondent evoked potential. Middle row. On the left, intraoperative photograph of the monopolar probe stimulating the left dorsal column; on the right the correspondent evoked potential which demonstrates phase reversal. Bottom row. On the left, intraoperative photograph of the monopolar probe locating the dorsal midline guided by the mapping which finds an area with correspondent isoelectric activity (on the right)
CST mapping can be useful to determine the distance between the surgical plane on which the surgeon is working and the motor fibers; such information is precious as it contributes to the decision on how aggressive to be in removing a tumor, which is of particular importance in the case of an infiltrative lesion where boundaries of the tumor are less clear or completely nonexistent [13, 26]. One method of CST mapping consists in the use of a hand-held bipolar micro-stimulator that delivers electric pulses in a range intensity of 0.1–1 mA. The response is recorded in the periphery at the level of the monitored muscles. In order to have a reliable result, it is crucial to have a wide set of recording muscles, otherwise there would be the risk of obtaining a false negative, where a functional bundle is stimulated but no response is recorded, therefore leading to the possibility of damaging the bundle during the surgery. Some authors have recently proposed the use of an electrified ultrasonic aspirator for a continuous dynamic mapping of the CST [13]. However, unless a double stimuli mapping technique is used, as recently proposed by Deletis et al., it is not possible to differentiate whether a muscle response following direct cord stimulation is truly elicited by orthodromic stimulation of the CST rather than antidromic stimulation of the dorsal columns [14].
Additional methods of CST mapping entail the possibility to measure the “electrical collision” between signals coming from direct spinal cord stimulation and D waves [27]. While some of these CST mapping methods have been scientifically validated in terms of reliability in truly reflecting CST activity, it should be pointed out that there are only anecdotal reports with regard to the correlation with clinical outcome and the true value of these techniques in improving the safety of IMSCS remained undetermined. All in all, for IMSCS monitoring of SEPs, Tc-MEPs, and D-wave still play, by far, the most important role when compared to mapping techniques.
Patient Positioning and Baseline Determination
The most common approach for IMSCS is posteriorly with the patient positioned prone. After induction and intubation by the anesthesiologist and careful positioning of scalp and muscle/peripheral electrodes by the neurophysiologist, the patient is ready to be positioned.
According to the location of the tumor, the prone position can have specific positional nuances. In the case of a cervical or upper thoracic lesion, the head would be preferably held by a Mayfield head clamp to minimize unwanted movement during surgery and during electrical stimulation. The arms should be tucked along the sides of the body wrapped and secured with an overriding sheet and with the thumbs in anatomical position pointing downward. It is worth mentioning a couple of considerations with regard to the placement of the Mayfield head clamp which involve both the neurophysiologist and the anesthesiologist. On one hand, when placing the scalp electrodes, the neurophysiologist must foresee the position of the Mayfield’s pins, which will coincide with the superior temporal line on both side of the head; on the other hand, the anesthesiologist must always be warned when the surgeon intends to place the Mayfield in order to allow for adjustment of the anesthesia setting, to avoid causing pain, tachycardia, and blood pressure spikes.
In case of a lesion located in the mid-lower thoracic spinal cord, the head clamp can be avoided, and the head can simply rest on a padded doughnut. In this case, the arms should be placed in the “superman” position, parallel to the ground, with a 90° angle between the sides of the body and the arms, and again a 90° angle between arm and forearm, with forearm and hands resting on two arm holders attached to the OR bed.
In both types of positioning, a careful check of the electrodes on arms and legs should be done before wrapping and securing is complete because later access to them will be very limited.
With regard to the IONM baseline determination, both pre-positioning and post-positioning baseline should be part of the IONM strategy in order to allow the neurophysiologist and the whole team to address potential causes of signal reduction or disappearance. Once again, the awareness of the patient’s neurological condition becomes crucial in order to know what to expect and explain the findings of the baseline. In general, the more “damaged” the spinal cord and the worse the clinical condition, the more difficult it will be to obtain a monitorable baseline response.
Surgical Technique
Once a satisfactory (or the best possible) baseline is obtained and with the patient correctly positioned, the surgery can begin. In the initial phase of muscle dissection to reveal the posterior bony elements of the spine, the use of muscle relaxants may be needed to reduce the tension on the tissue and facilitate the opening. While the use of muscle relaxants does not affect the SSEP, it will affect TcMEPs. In general, it would be best to avoid their use altogether in these surgeries, but in case the surgeon deems them necessary, the whole team should be aware of the request, and specifically the anesthesiologist should communicate the dosage used, so the neurophysiologist is aware of what to expect in terms of changes in the signals and when a return to the original baseline can be expected. The train-of-four technique can be used to assess the degree of recovery following muscle relaxation.
Muscle dissection is followed by laminotomy or laminectomy depending on patient anatomy and surgeon’s preference. The exposure of the spinal canal is extended cranially and caudally to the limits of the lesions to allow sufficient room for a comfortable working space. Once exposure and hemostasis are satisfactory, SSEPs and TcMEPs are checked before durotomy. This is important as the opening of the dura itself can cause a change in the signals as the pressure on the spinal cord starts to be relieved. Durotomy usually starts by performing a small hole with a scalpel, paying attention to not pierce the arachnoid, which now serves as a protective layer for the spinal cord. The rest of the durotomy is usually completed with the same blade and then traction is applied to the two dural flaps using multiple anchor points on both edges. Sutures can be tied to the homolateral muscles or clamped with hemostatic forceps. Once the dura is opened, the arachnoid is incised, and its flaps are secured to the dural edges through small hemostatic clips.
In the case D wave monitoring is involved, in this phase of the surgery the electrode is placed epidurally or subdurally, caudal to the lesion. If possible, an additional electrode is placed cranially to the lesion. It is of paramount importance to have enough space above and below the working area in order to avoid inadvertent mobilization of these electrodes as the D wave is notoriously affected by electrode displacement. In order to minimize this risk, the cables that connect the electrodes to the IONM machine should be stabilized using sutures of surgical clamps but always paying attention not to clamp the cable directly, as it can cause damage to the wires which would result in suboptimal or biased electrical recordings. For obvious reasons, D wave monitoring is the only technique for which the baseline is obtained in the middle of the surgery and therefore attention must be paid to the current anesthesia settings which can be different from the ones present during pre-positioning baseline determination [12].
Once a satisfactory D wave is obtained, the intradural part of the surgery can begin. First, the midline is identified to properly access the tumor between the two dorsal columns. Usually, the dorsal median raphe is clearly identified by small vessels diving into the pia at 90°. If the anatomy is not clear, then dorsal column mapping can be done, as described above.
Myelotomy is usually started through bipolar coagulation of the superficial small veins located above the posterior median sulcus. Progressive opening of the spinal cord is then performed to and beyond the limits of the lesion using a scalpel. From this moment on, coagulation should be limited as much as possible while irrigation and gentle pressure to ensure adequate hemostasis should be preferred in order to minimize damage to the “healthy” spinal cord tissue.
Tumor removal is usually performed through a combination of debulking and, when possible, direct dissection from the surrounding healthy tissue. The surgeon can use a combination of surgical forceps, coagulation, aspiration, or Cavitron Ultrasonic Surgical Aspirator (CUSA) to remove the tumor piece by piece or en-block. As during the opening phase, coagulation on the healthy tissue surrounding the tumor should be limited as much as possible, favoring the use of foam-based hemostatics, irrigation with warm saline, and gentle pressure. Working at the edges of the tumor is the most critical phase of the surgery where D-wave and TcMEPs monitoring are crucial. When the tumor is completely removed or criteria for stopping the surgery are met (see below), careful hemostasis is performed, followed by watertight dural closure (Fig. 34.2). D wave monitoring should continue until the dura is closed, then the electrodes can be removed. At this stage, the surgeon may ask the anesthesiologist to perform a Valsalva maneuver on the patient in order to confirm adequate, watertight closure. TcMEPs and SSEPs monitoring should continue until skin closure is completed [28].
Four key moments of the intradural phase of the tumor removal operation. (a) Opening of the spinal cord at the midline using a scalpel. The midline, while anatomically clear, has been confirmed through the mapping of the dorsal columns (see Fig. 34.1). (b) The opening of the spinal cord progressively reveals the posterior aspect of the tumor. The opening should extend for the whole length of the tumor. (c) The tumor should be progressively and carefully dissected from the surrounding healthy tissue. The dissection will allow the tumor to slowly emerge from the surgical opening on the spinal cord. (d) Once the tumor has been removed, careful hemostasis should be performed, and the cavity explored for possible residues. On the bottom of the surgical field, the anterior vascular structures of the spinal cord can be appreciated, proof that a complete split of the spinal cord was needed to ensure radical resection
IONM Changes: Contributing Factors, Warning Criteria, and Reaction Strategies
Contributing Factors
As the only functional guidance during IMSCS, the neurophysiologist is the team member who sets the pace of the surgery when unexpected changes in the IONM happen. It is of paramount importance to be able to correctly evaluate these changes in order to minimize the occurrence of false negatives, which would lead to the development of postoperative deficits, as well as false positives, which would in turn reduce the opportunity to completely eradicate a lesion. Several factors need to be considered when evaluating a change in monitoring signals:
-
Quality of the signal
-
Characteristics of the baseline for that patient
-
Anesthesia protocol and current setting (medications and ventilation settings)
-
Physiological variables (blood flow, temperature)
-
Phase of the surgery
The thorough assessment of these factors involves all the team members. Once an alarm is raised the surgeon should stop and irrigate the surgical field with warm saline (see details below). The one element that mostly helps differentiate between a surgically induced versus a change due to systemic causes is the presence of a localized change versus the presence of a widespread alteration on the IONM signal panel. Particularly in the second scenario, the anesthesiologist should readily check the anesthesia settings. As explained in Chap. 17, different anesthetic medications have different influences on IONM signals [23, 24]. Halogenated inhalational agents increase latency and reduce amplitude of SSEPs while easily abolishing TcMEPs; conversely, D wave is highly resistant to such medications being an non-synaptic pathway (the effect of anesthetics increases with the number of synapses present between stimulating and recording electrodes). With regard to intravenous agents, propofol and opioids present minor influence on both SSEPs and TcMEPs and a combination of these medications is considered adequate. As reported above, muscle relaxants, with their direct action on the neuromuscular junction, have a significant effect on TcMEPs while leaving SSEPs and D-waves substantially unaltered. The anesthesiologist is also in charge of evaluating additional physiological variables such as patient’s blood flow and temperature. The presence of systemic or local events that reduce blood flow of the spinal cord can produce reduction of both SSEPs and TcMEPs, with SSEPs remaining normal to approximately 20 mL/min/100 g. Ventilation itself can contribute to variation on blood flow to the spinal cord and subsequent signal alteration, and specifically in case of very low values of carbon dioxide, which have been suggested to induce ischemia due to vasoconstriction. Change in body and local temperature can, as well, induce changes in the IONM signals, and specifically both general (due to exposure) and local (due to cold water irrigation) hypothermia will tend to slow down both SSEPs and TcMEPs conduction. Finally, the neurophysiologist should always consider the possibility of a technical issue and check the different components of the monitoring setting.
Warning Criteria
In general, abrupt changes in the signals are more worrisome than gradual ones, as these usually reflect vascular more than mechanical injury. With regard to SSEPs, the gold standard refers to reduction of 50% in amplitude and increase of 10% in latency as a warning criterion. While it is rare to abort a surgery only based on SSEPs change, as it is the status of motor-related responses that mostly inform the decision, it must be kept in mind that the ability to walk and properly use and coordinate the different body segments heavily rely on the very sensory information that is monitored through SSEPs, therefore every effort to try and preserve them should be made [29].
As said, motor-related signals, specifically tcMEPs and D waves, are the signals that contribute most to the decision to continue, temporarily stop or permanently stop the surgery. The reason for this is the body of evidence that correlates the change of intra-operative motor signals with the presence of temporary or permanent postoperative motor deficits. For this reason, both TcMEPs and D waves should be sampled frequently, in order to detect changes with the least possible delay. TcMEPs warning criteria can be determined by different modalities. They can be based on a presence/absence criterion [7, 8], a reduction in amplitude criterion, or a change in morphology criterion [10]. Of the three, the change in amplitude is the most widely used, with the raising of an alarm in case of a reduction of 50–80% in amplitude [30, 31]. The neurophysiologist may concomitantly apply the morphology criterion where a reduction in the complexity of the trace (from polyphasic to biphasic) reflects a deterioration of the signal. However, in our opinion, morphology criteria are very sensitive and may result in suboptimal tumor resection. The presence/absence criteria are potentially the less sensitive and the one that requires the greatest and most ready reaction of the team. Nevertheless, as long as the D-wave is recordable and not decreased below 50% of its baseline amplitude, it is acceptable to consider absence/presence criteria only for the Tc-MEPs, as this strategy would offer the best chance of a total resection of the tumor [7, 8, 32] (Table 34.1). It should be emphasized that the absence/presence criteria for Tc-MEPs should apply only when the D-wave is preserved and exclusively for IMSCS, not for other spine procedures.
So, in the context of predicting motor outcome and avoiding irreversible severe paresis, TcMEPs and D waves are best evaluated together. In case of loss of Tc-MEP with full preservation of D wave surgery could continue as only a temporary deficit is expected. Vice versa, in case of a loss of Tc-MEPs and reduction of D wave amplitude more than 50% of baseline values, there is a substantial risk of permanent deficit. In this case, surgery should be promptly stopped and if corrective measures are not efficient, abandoned. Typically, progressive D-wave decrement anticipates or parallels progressive loss of Tc-MEPs uni- or bi-laterally.
Although D-wave preservation is the strongest predictor of long-term motor outcome, D-wave does not predict short-term outcome and does not differentiate between right and left side changes. Therefore, TcMEPs retain a great value in IMSCS because they predict the short-term motor outcome and allow to differentiate between left and right loss, therefore offering an important information to the neurosurgeon for the fine tuning of the surgical strategy.
Free-running EMG usually does not present specific warning parameters; in general, the presence of bursts in relation to surgical maneuvers and prolonged discharge should be communicated, and assessment of TcMEPs and D waves should be readily performed.
Reaction Strategies
When a significant IONM change occurs, communication should be fast and specific, and adequate reaction strategies should follow. In this scenario, a practical checklist to “assess, communicate and execute” is useful to readily address any type of change in IONM [1, 5, 8, 25, 28]. Years ago we established the T (time), I (irrigation), P (pressure/papaverine) protocol to summarize the corrective measures in case of signal deterioration [33].
The first step consists in pausing the surgery, as this simple act may be enough to allow the nervous system to re-establish its balance, for as long as it is needed for the signals to recover. Along with halting the surgery, irrigation of the surgical field with warm saline helps re-establish the ionic equilibrium; during the surgery in fact, the extracellular concentration of potassium ions tends to increase and this change in ionic equilibrium has been observed to impair the local electrical conduction, resulting in suboptimal monitoring outputs. The use of warm saline can also be used to stabilize the temperature as local hypothermia during the surgery may occur. It is important to observe how this strategy of trying to improve neural function through warm saline irrigation is in perfect contrast with the strategy of reducing excessive neuronal firing using cold ringer in case of an emerging seizure following cortical stimulation during the removal of brain tumors. In both these scenarios, the readiness of the OR nurse staff becomes critical in order to have both solutions readily available at the right temperature. An additional action that can be taken is to release tension on the spinal cord if traction was maintained for a long period of time.
From the systemic perspective, the most common strategy to address a change in IONM signals is to increase perfusion. Blood flow can be reduced both systemically and locally, and accordingly both systemic and local strategies to restore it can be applied. The anesthesiologist can increase perfusion through pharmacological intervention and/or optimizing ventilation parameters, while the surgeon can irrigate the surgical field with papaverine, a smooth muscle relaxant that increases blood flow locally through vasodilation.
The use of intravenous steroids can be used in case of persistent reduction in IONM signals, but its value is undetermined.
Finally, after performing all possible and adequate measures to resolve a change in IONM data, if recurrent severe deterioration occurs or no significant result is obtained, the possibility to permanently stop the surgery must be considered. Once the postoperative neurological condition is assessed and in case the surgical indication persists, there could be the option to return to the operating theater.
References
Takami T, Naito K, Yamagata T, Ohata K. Surgical management of spinal intramedullary tumors: radical and safe strategy for benign tumors. Neurol Med Chir (Tokyo). 2015;55(4):317–27.
Tobin MK, Geraghty JR, Engelhard HH, Linninger AA, Mehta AI. Intramedullary spinal cord tumors: a review of current and future treatment strategies. Neurosurg Focus. 2015;39(2):E14.
Brotchi J. Intrinsic spinal cord tumor resection. Neurosurgery. 2002;50(5):1059–63.
Choi I, Hyun S-J, Kang J-K, Rhim S-C. Combined muscle motor and somatosensory evoked potentials for intramedullary spinal cord tumour surgery. Yonsei Med J. 2014;55(4):1063.
Sala F, Bricolo A, Faccioli F, Lanteri P, Gerosa M. Surgery for intramedullary spinal cord tumors: the role of intraoperative (neurophysiological) monitoring. Eur Spine J. 2007;16(S2):130–9.
Sutter M, Deletis V, Dvorak J, Eggspuehler A, Grob D, MacDonald D, et al. Current opinions and recommendations on multimodal intraoperative monitoring during spine surgeries. Eur Spine J. 2007;16(S2):232–7.
Kothbauer KF, Deletis V, Epstein FJ. Motor-evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg Focus. 1998;4(5):E3.
Sala F, Palandri G, Basso E, Lanteri P, Deletis V, Faccioli F, et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery. 2006;58(6):1129–43.
Daniel JW, Botelho RV, Milano JB, Dantas FR, Onishi FJ, Neto ER, et al. Intraoperative neurophysiological monitoring in spine surgery: a systematic review and meta-analysis. Spine. 2018;43(16):1154–60.
Quiñones-Hinojosa A, Lyon R, Zada G, Lamborn KR, Gupta N, Parsa AT, et al. Changes in transcranial motor evoked potentials during intramedullary spinal cord tumor resection correlate with postoperative motor function. Neurosurgery. 2005;56(5):982–93; discussion 982–993.
Skinner SA, Nagib M, Bergman TA, Maxwell RE, Msangi G. The initial use of free-running electromyography to detect early motor tract injury during resection of intramedullary spinal cord lesions. Oper Neurosurg. 2005;56(suppl_4):ONS-299–314.
Costa P, Peretta P, Faccani G. Relevance of intraoperative D wave in spine and spinal cord surgeries. Eur Spine J. 2013;22(4):840–8.
Barzilai O, Lidar Z, Constantini S, Salame K, Bitan-Talmor Y, Korn A. Continuous mapping of the corticospinal tracts in intramedullary spinal cord tumor surgery using an electrified ultrasonic aspirator. J Neurosurg Spine. 2017;27(2):161–8.
Deletis V, Seidel K, Sala F, Raabe A, Chudy D, Beck J, et al. Intraoperative identification of the corticospinal tract and dorsal column of the spinal cord by electrical stimulation. J Neurol Neurosurg Psychiatry. 2018;89(7):754–61.
Mehta AI, Mohrhaus CA, Husain AM, Karikari IO, Hughes B, Hodges T, et al. Dorsal column mapping for intramedullary spinal cord tumor resection decreases dorsal column dysfunction. J Spinal Disord Tech. 2012;25(4):205–9.
Nair D, Kumaraswamy VM, Braver D, Kilbride RD, Borges LF, Simon MV. Dorsal column mapping via phase reversal method. Neurosurgery. 2014;74(4):437–46.
Skinner S, Sala F. Communication and collaboration in spine neuromonitoring: time to expect more, a lot more, from the neurophysiologists. J Neurosurg Spine. 2017;27(1):1–6.
Skinner S, Holdefer R, McAuliffe JJ, Sala F. Medical error avoidance in intraoperative neurophysiological monitoring: the communication imperative. J Clin Neurophysiol. 2017;34(6):477–83.
Chung JY, Lee JJ, Kim HJ, Seo HY. Characterization of magnetic resonance images for spinal cord tumors. Asian Spine J. 2008;2(1):15.
Feletti A, Boaro A, Giampiccolo D, Casoli G, Moscolo F, Ferrara M, et al. Spinal hemangioblastomas: analysis of surgical outcome and prognostic factors. Neurosurg Rev. 2021;45(2):1645–61. https://doi.org/10.1007/s10143-021-01696-x.
Park T, Park J, Park YG, Lee J. Intraoperative neurophysiological monitoring for spinal cord tumor surgery: comparison of motor and somatosensory evoked potentials according to tumor types. Ann Rehabil Med. 2017;41(4):610.
Tamkus A, Rice K. The incidence of bite injuries associated with transcranial motor-evoked potential monitoring. Anesth Analg. 2012;115(3):663–7.
Sloan TB, Heyer EJ. Anesthesia for intraoperative neurophysiologic monitoring of the spinal cord. J Clin Neurophysiol. 2002;19(5):430–43.
Sloan TB, Jäntti V. Anesthetic effects on evoked potentials. In: Handbook of clinical neurophysiology. Elsevier; 2008. p. 94–126. https://linkinghub.elsevier.com/retrieve/pii/S1567423107080057.
Yanni DS, Ulkatan S, Deletis V, Barrenechea IJ, Sen C, Perin NI. Utility of neurophysiological monitoring using dorsal column mapping in intramedullary spinal cord surgery: clinical article. J Neurosurg Spine. 2010;12(6):623–8.
Gandhi R, Curtis CM, Cohen-Gadol AA. High-resolution direct microstimulation mapping of spinal cord motor pathways during resection of an intramedullary tumor. J Neurosurg Spine. 2015;22(2):205–10.
Deletis V, Bueno De Camargo A. Interventional neurophysiological mapping during spinal cord procedures. Stereotact Funct Neurosurg. 2001;77(1–4):25–8.
Forster M-T, Marquardt G, Seifert V, Szelényi A. Spinal cord tumor surgery—importance of continuous intraoperative neurophysiological monitoring after tumor resection. Spine. 2012;37(16):E1001–8.
MacDonald DB, Dong C, Quatrale R, Sala F, Skinner S, Soto F, et al. Recommendations of the International Society of Intraoperative Neurophysiology for intraoperative somatosensory evoked potentials. Clin Neurophysiol. 2019;130(1):161–79.
Calancie B. Intraoperative neuromonitoring and alarm criteria for judging MEP responses to transcranial electric stimulation: the threshold-level method. J Clin Neurophysiol. 2017;34(1):12–21.
Kothbauer KF. The interpretation of muscle motor evoked potentials for spinal cord monitoring. J Clin Neurophysiol. 2017;34(1):32–7.
Skrap B, Tramontano V, Faccioli F, Meglio M, Pinna G, Sala F. Surgery for intramedullary spinal cord ependymomas in the neuromonitoring era: results from a consecutive series of 100 patients. J Neurosurg Spine. 2021:1–11.
Sala F, Lanteri P, Bricolo A. Motor evoked potential monitoring for spinal cord and brain stem surgery. In: Pickard JD, Di Rocco C, Dolenc VV, Fahlbusch R, Lobo Antunes J, Sindou M, et al., editors. Advances and technical standards in neurosurgery, vol. 29. Vienna: Springer Vienna; 2004. p. 133–69. https://doi.org/10.1007/978-3-7091-0558-0_4.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Boaro, A., Sala, F. (2023). Intraoperative Neurophysiology During Intramedullary Spinal Cord Tumor Surgery. In: Seubert, C.N., Balzer, J.R. (eds) Koht, Sloan, Toleikis's Monitoring the Nervous System for Anesthesiologists and Other Health Care Professionals. Springer, Cham. https://doi.org/10.1007/978-3-031-09719-5_34
Download citation
DOI: https://doi.org/10.1007/978-3-031-09719-5_34
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-09718-8
Online ISBN: 978-3-031-09719-5
eBook Packages: MedicineMedicine (R0)