Abstract
In spite of advancements in neuro-imaging and microsurgical techniques, surgery for intramedullary spinal cord tumors (ISCT) remains a challenging task. The rationale for using intraoperative neurophysiological monitoring (IOM) is in keeping with the goal of maximizing tumor resection and minimizing neurological morbidity. For many years, before the advent of motor evoked potentials (MEPs), only somatosensory evoked potentials (SEPs) were monitored. However, SEPs are not aimed to reflect the functional integrity of motor pathways and, nowadays, the combined used of SEPs and MEPs in ISCT surgery is almost mandatory because of the possibility to selectively injury either the somatosensory or the motor pathways. This paper is aimed to review our perspective in the field of IOM during ISCT surgery and to discuss it in the light of other intraoperative neurophysiologic strategies that have recently appeared in the literature with regards to ISCT surgery. Besides standard cortical SEP monitoring after peripheral stimulation, both muscle (mMEPs) and epidural MEPs (D-wave) are monitored after transcranial electrical stimulation (TES). Given the dorsal approach to the spinal cord, SEPs must be monitored continuously during the incision of the dorsal midline. When the surgeon starts to work on the cleavage plane between tumor and spinal cord, attention must be paid to MEPs. During tumor removal, we alternatively monitor D-wave and mMEPs, sustaining the stimulation during the most critical steps of the procedure. D-waves, obtained through a single pulse TES technique, allow a semi-quantitative assessment of the functional integrity of the cortico-spinal tracts and represent the strongest predictor of motor outcome. Whenever evoked potentials deteriorate, temporarily stop surgery, warm saline irrigation and improved blood perfusion have proved useful for promoting recovery, Most of intraoperative neurophysiological derangements are reversible and therefore IOM is able to prevent more than merely predict neurological injury. In our opinion combining mMEPs and D-wave monitoring, when available, is the gold standard for ISCT surgery because it supports a more aggressive surgery in the attempt to achieve a complete tumor removal. If quantitative (threshold or waveform dependent) mMEPs criteria only are used to stop surgery, this likely impacts unfavorably on the rate of tumor removal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intramedullary spinal cord tumors (ISCTs) are rare neoplasms, accounting for only 2–4% of central nervous system tumors. The surgical removal of ISCT is still believed to carry a significant risk for surgical damage and, therefore, neurologic dysfunction.
In 1907 Anton von Eiselsberg, in Vienna, performed the first successful resection of an ISCT but the first report about such a resection appeared in 1911 by Charles Elsberg in New York who described a two-stage strategy for the removal of these tumors [11]. Thereafter, since post-operative neurological deficits were significant, many neurosurgeons recommended a conservative strategy with biopsy, dural grafting and radiation therapy regardless of histological diagnosis [51]. With the introduction of the operating microscope, the development of ultrasonic aspiration and laser technologies, and the advent of magnetic resonance (MR) imaging, the strategy for the treatment of ISCTs has dramatically changed. Nowadays the microsurgical resection of ISCTs is the primary treatment modality for these neoplasms, while radiotherapy should be indicated only for recurrent or malignant tumors [14]. The observation that the majority of ISCTs are benign [6] and, consequently, a gross total removal may result in long-term survival [12, 16] further supports the need for a “safe” surgery. Nevertheless, surgery for these neoplasms remains a challenging task and the rationale for using intraoperative neurophysiological monitoring (IOM) is in keeping with the goal of maximizing tumor resection and minimizing neurological morbidity. IOM should therefore not only predict a post-operative neurological deficit but, most important, identify an impending injury to the cord in time for corrective measures to be taken.
For many years, before the advent of motor evoked potentials (MEPs), only somatosensory evoked potentials (SEPs) were monitored during spinal cord procedures. However, SEPs are not aimed to reflect the functional integrity of motor pathways and the assumption that they could do so has resulted in a number of so called “false negative” results, meaning post-operative motor deficit in spite of unchanged intraoperative SEPs [15, 19, 28, 38].
As a semantic annotation, we are not in favor of the misleading terminology of “false negative SEPs” to indicate the occurrence of a post-operative motor deficit in spite of intraoperative unchanged SEPs. A SEP result should be labeled as “false negative” only when post-operative sensory deficits occur and were not predicted by intraoperative SEP changes. Similarly, a MEP result should be labeled as “false negative” if the patient wakes up with a new or worsened motor deficit in spite of intraoperatively unchanged MEPs.
Theoretically, the possibility that SEPs may indirectly provide information on the motor tracts integrity could exist for those procedures (like scoliosis surgery) where motor and sensory pathways are expected to be injured simultaneously due to distracting maneuvers of the spinal cord. Even so, Nuwer reported a number of false negative SEP results in a large series of scoliosis surgeries [34]. For other procedures like spinal cord tumor surgery or endovascular procedures for the embolization of spinal cord arteriovenous malformations, the combined used of SEPs and MEPs is almost mandatory because the possibility to selectively injury either the somatosensory or the motor pathways exists, and there is no scientific justification for a selective use of either SEPs or MEPs [44]. From this perspective, we should keep in mind that dorsal columns, monitored by SEPs, are in the vascular territory of the posterior spinal arteries, while anterior, and lateral corticospinal tract (CT) are in the territory of the anterior spinal artery.
This paper is aimed to review our perspective in the field of IOM during ISCT surgery and to discuss it in the light of other intraoperative neurophysiological strategies that have recently appeared in the literature with regards to ISCT surgery.
Methods
Anesthesia
The anesthesia management that allows intraoperative monitoring particularly of MEPs consists of a constant infusion of propofol (usually in a dose of about 100–150 μg/kg/min) and fentanyl (usually around 1 μg/kg/h). The use of propofol for anesthesia with MEP monitoring has been reported with various stimulation techniques [13, 18, 21, 47]. Nitrous oxide not exceeding 50 vol.% can be used. Bolus injections of both iv. agents should be avoided because this temporarily disrupts muscle MEP (mMEP) recordings, which is particularly problematic during the critical part of the operation. Short acting muscle relaxants are given for intubation but not thereafter to allow continuous mMEP monitoring.
Halogenated anesthetics should not be used [48, 49]. They elevate mMEP stimulus thresholds and block mMEPs in a dose-dependent fashion at cortical and spinal levels.
Neurophysiological monitoring
To follow is a brief, critical, summary of our SEPs, and MEPs methods that have been described in details elsewhere [9, 43].
Somatosensory evoked potentials
We elicit cortical and subcortical SEPs by stimulation of the median nerve at the wrist and the posterior tibial nerve at the ankle (intensity 40 mA, duration 0.2 ms, and repetition rate of 4.3 Hz). Recordings are performed via corkscrew-like electrodes inserted in the scalp (CS electrode, Nicolet Biomedical, Madison, WI, USA) at CZ′-FZ (legs) and C3′/C4′-FZ (arms), according to the 10–20 International EEG system.
Motor evoked potentials (Fig. 1)
Multipulse technique: transcranial electrical stimulation and recordings from limb muscle (mMEPs).
Short trains of 5–7 square-wave stimuli of 0.5 ms duration and interstimulus interval (ISI) of 4 ms are delivered at a repetition rate up to 2 Hz through CS electrodes placed at C1 and C2 scalp sites, according to the 10/20 EEG system. A C1/C2 montage is preferentially used to elicit right extremity mMEPs, while C2/C1 is preferable for left extremity mMEPs. For monitoring leg muscles, sometimes a Cz-Fz montage is adopted, which produces less intense muscle twitching. The stimulation intensity never exceeds 240 mA and rarely goes higher than 200 mA. We record mMEPs via needle electrodes 3 cm apart inserted into upper and lower extremity muscles. We usually monitored mMEPs from the abductor pollicis brevis and the extensor digitorum communis for the arm and the tibialis anterior and the abductor hallucis for the leg.
Motor evoked potentials for spinal cord surgery. Left: schematic illustration of electrode positions for transcranial electrical stimulation of the motor cortex according to the International 10–20 EEG system. The site labeled “6 cm” is 6 cm anterior to CZ. Top right: schematic diagram of the position of the epidural catheter electrode placed caudal to the lesion to monitor the incoming signal (D-wave) passing through the site of surgery. A single stimulus of 0.5 ms duration is used. Bottom right: recording of mMEPs from the abductor pollicis brevis and tibialis anterior muscles after eliciting them with a short train of electrical stimuli (multipulse technique), 4 ms apart. Modified from [25]
Single pulse technique: transcranial electrical stimulation and epidural (D-wave) recordings.
A single TES stimulus is applied, using the same montage as for mMEPs, to elicit a D-wave that is recorded by an electrode placed in the epi- or subdural space of the spinal cord caudal to the tumor. Signals are amplified 10,000 times and the bandwidth 1.5–1,700 Hz baseline D-waves are recorded after exposing the spinal cord.
For cervical tumors we routinely monitor mMEPs from all four extremities (abductor pollicis brevis, tibialis anterior, and abductor hallucis) and, when recordable, the D-wave from an epidural electrode placed caudal to the tumor. Below the level of T1, only muscles from lower extremities should be monitored but, methodologically, we favor the adjunctive monitoring of an upper extremity muscle as a control parameter. This allows to discriminate whether or not an intraoperative neurophysiological event is related to surgical maneuvers or general infuences such as anesthesia or cardiovascular factors. For the same reason, whenever possible, we place an epidural electrode also rostral to the tumor as a control recording.
We use the Axon Sentinel-4 evoked potential system with modified software and hardware (AXON Systems Inc., Hauppage, NY, USA) for stimulation and recording.
Discussion
Evolution of IOM in ISCT surgery
Intramedullary spinal cord tumors are rare and reports on the use of IOM techniques during their surgical removal remained anecdotal for many years. Nevertheless, ISCTs recently accounted for the most eventful monitoring in a series of 423 neurosurgical monitored cases [50].
Before the advent of MEP in ISCT surgery [32] SEPs only were used with the assumption that changes in SEPs specifically represent spinal cord dysfunction. Kearse Jr et al. [23] reported good sensitivity but poor specificity of SEPs; this high rate of false positive results (changes in SEPs without changes in neurological outcome) suggests that, monitoring only SEPs, may unjustifiably stop surgery precluding a complete resection of the tumor. Recently, Skinner et al. [46] reported on a patient with a C7–T1 intramedullary cavernous malformation with unchanged posterior tibial nerve SEPs in spite of lost transcranial MEPs from both legs at the end of surgery. The patient woke up with a new paraparesis that would not have been recognized by monitoring SEPs only.
The idea to have neurophysiological parameters as a major outcome predictor in spinal cord surgery emerged in 1997 when Morota introduced the use of D-wave (epidural MEP) monitoring after transcranial electrical stimulation and concluded that this appeared as a better predictor of functional outcome than the patient’s pre-operative motor status [32]. Since the 1950s it is known that a small but essential fiber population in the corticospinal tract gives rise to a recordable traveling wave, the D-wave [36, 39]. This population accounts for the pool of high conduction velocity fibers supporting locomotion. After Merton’s description of transcranial electrical motor cortex stimulation in man [31] this knowledge was applied in the operating room [1, 3, 22, 29]. Muscle recording techniques were introduced with magnetic [10] and electric [55] motor cortex stimulation, but the intraoperative use of mMEPs is more recent because anesthesia-induced blocking of the alpha-motoneurons posed a major problem. The multipulse technique introduced by Taniguchi et al. in the mid-90s [48, 49] resolved the problem and, since then, mMEPs have been routinely and successfully used intraoperatively in different neurosurgical procedures, including ISCTs [5, 26, 37].
Current IOM strategy for SEPs and MEPs
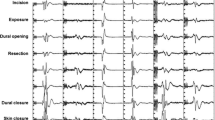
We have developed our IOM strategy according to the progress of neurophysiological techniques and adapting the strategy to the steps of surgery (Fig. 2).
Neurophysiological monitoring during surgery for ISCTs. Incision of the dorsal median raphe (left panels): myelotomy is carried out by using a fine blade or laser. In spite of any attempt to stay within the median raphe (Panel I) to avoid damage to the dorsal column, SEPs are frequently compromized or loss during this surgical step (Panel II). Although the drop in amplitude is usually reversible, SEPs may remain unmonitorable for several hours. Removal of the tumor (right panels): after dorsal columns are separated, there is direct acccess to the tumor. If there is not adequate lateral visualization to safely remove the tumor without excessive retraction of normal neural tissues, ultrasonic aspiration can be used to debulk the central part of the tumor (Panel III). At this point it is possible to gently dissect the tumor from the neural tissue. In doing so, traction on the corticospinal and other descending motor tracts can occur (Panel IV). Accordingly, muscle MEPs as well as epidural MEPs (D-wave) should be strictly monitored during this surgical step. The upper right panel shows the disappearance of the left tibialis anterior MEP during tumor removal. The lower right panel illustrates a stable D-wave, which warrants good long-term motor outcome (see text for more details) Finally, the ventral part of the tumor is detached from the anterior spinal cord where perforating vessels from the anterior spinal artery are located (Panel V). Here again it is critical to monitor motor pathways since a vascular injury to the cord may result in an irreversible severe motor deficit (combined from refs. [8, 12, 43, 45])
Incision of the dorsal median raphè
Visual identification of the midline may be difficult when the cord anatomy is distorted by the tumor. If needed, a so-called “dorsal column mapping” technique [27] can be used to identify the “physiologic midline.” With very thin wire-electrodes mounted on an array electrode which is placed transversely over the cord it is possible to record the traveling SEP waves in the dorsal columns very selectively. SEP stimulation on the right posterior tibial nerve allows recording of a traveling wave which will have its highest amplitude close to the midline because of the somatotopic distribution of afferent fibers in the dorsal column. The same happens for the contralateral side so that a “physiologic midline” can be identified between these two amplitude peaks.
Given the dorsal approach to the spinal cord, SEPs must be monitored continuously during the incision of the dorsal midline. Bipolar coagulation can be used to coagulate the veins on the surface of the cord and usually this maneuver does not disrupt somatosensory pathways. Then the surgeon separates the dorsal columns to access the tumor. During the incision of the dorsal median raphe, there is the possibility of changes in or even loss of SEPs. SEP amplitude may drop very quickly during this very early stage of surgery. Even though loss of SEPs is usually transient and does not necessarily result in post-operative ataxia, SEPs may remain unmonitorable during the most critical steps of tumor removal. Furthermore, due to the need for signal averaging, a time delay occurs before SEP traces are updated and the identification of injury can lag behind the progress of the surgery, so that a potentially irreversible injury may occurr before it is even detected. Nevertheless, this should be recognized by the monitoring team and this information should be passed to the surgeon. In many istances, temporarily ceasing the retraction of the dorsal columns or irrigating the surgical field with warm saline may be enough to facilitate SEP recovery. Sometimes SEPs recover only later, at the end of the case, or not at all. Late recovery is probably frequent as one may detect only subtle sensory deficits or no deficits at all post-operatively. Overall, the preservation of SEPs is highly recommendable but their loss during splitting of the dorsal columns is never used as criteria to abandon surgery [2, 24].
Dissection of the interface between tumor and spinal cord
When the surgeon starts to work on the cleavage plane between tumor and spinal cord, attention must be paid to MEPs.
The surgical strategy varies, to some extent, according to different tumor histology. We will here discuss only the two commonest histological types, namely ependymomas and astrocytomas.
Ependymomas are more common in adults [30, 33], centrally located in the spinal cord and often associated with caudal and rostral cysts. At surgery these tumors usually offer a favorable cleavage plane between tumor and surrounding neural tissue. However, since ependymomas receive their vascular supply mainly from branches of the anterior spinal artery axis, any attempt to detach the anterior part of the tumor from the spinal cord may result in mechanical or vascular derangement, and consequently neurological injury. This typically occur during the later stages of tumor removal and this is also the time when MEPs may be significantly affected. An abrupt injury to the anterior spinal artery axis due to inappropriate traction or coagulation when attempting the removal of “the last piece of tumor” can result in a sudden deterioration of the D-wave; this event is rather rare but can obviously have disastrous consequences for the patient.
Astrocytomas are the most common ISCT in children [6], and are mainly low-grade tumors. They have a more heterogeneous enhancement pattern on MRI, and are usually eccentrically located. Unlike ependymomas, a true cleavage plane between the tumor and the spinal cord is uncommon and therefore to achieve a radical tumor removal is more challenging, even when surgery is assisted by IOM [45]. The location of the corticospinal tract in the anterolateral aspects of the cord make them mechanically vulnerable while detaching the tumor from the lateral aspects of the cord and this is especially true for astrocytomas, due to the poor cleavage plane.
During tumor removal, we alternatively monitor D-wave and mMEPs, sustaining the stimulation during the most critical steps of the procedure.
D-wave recordings are very robust even under general anesthesia and are not impaired by the use of muscle relaxants. These recordings provide specific and semi-quantitative information on the functional integrity of the fast conducting fibers of the CT and they are essential in predicting the prognosis and establishing reliable warning criteria. However D-wave recordings still do not provide specific information on each of the lateral CT and cannot differentiate between different muscle groups. Furthermore, D-wave cannot be recorded below the level of T12 because there is not enough CT fibers to record from. Previous scarring can sometimes impair electrode placement. Finally, in about one-third of the cases the D-wave is not recordable because of a desynchronization phenomenon [7].
Muscle MEPs are generated by CT as well as by polysynaptic pathway, and are therefore sensitive to anesthesia and can be entirely blocked by muscle relaxants. Moreover, they can induce some muscle twitching that can be disturbing for the surgeon. However, they can provide specific information on different muscle groups from the left and right side and from upper and lower extremities. Finally, they are recordable also in patients with spinal cord lesion encompassing the most caudal spinal cord levels.
Criteria to interpret MEPs changes during ISCT surgery have been established on the basis of hundreds of monitored cases [24, 26, 32, 45] and, up to now, no exceptions have been reported. To achieve a good long-term post-operative outcome, it is imperative to maintain the D-wave amplitude above 50% of its initial value. When this criterion is satisfied, the patient will either have an unchanged motor status at the end of the procedure if mMEPs have been preserved or will have a so-called transient paraparesis/paraplegia if mMEPs have been lost uni- or bi-laterally. This transient post-operative motor deficit, occasionally as severe as a true plegia, invariably recovers over a period of days, weeks, or months after surgery. One possible explanation is that the D-wave is generated exclusively by fast neurons of the CTs, while mMEPs are generated by CT and other descending tracts within the spinal cord. An injury to these non-CT tracts can be functionally compensated post-operatively by the CTs, but not vice versa.
Based on previous experience of several hundreds of monitored ISCT surgeries, we currently adopt intraoperative corrective measures based on the criteria presented in Table 1.
Stable mMEP and D-wave recordings are a significant and useful information because the surgeon is reassured on the functional integrity of motor pathways and feels comfortable in proceeding with the tumor debulking.
If there is a loss of mMEPs the surgeon will stop resection and manipulation. However, before a complete loss of mMEPs occurs, we have often observed an increase in the stimulating threshold necessary to elicit a response or fluctuations in the amplitude with recordings in a “on and off” fashion. At a given stimulation intensity, a decrease in amplitude is usually more common than latency shifts. Disappearance of mMEPs usually precedes changes in the D-wave although the D-wave may remain stable or drop insignificantly in spite of complete mMEP loss. Very rarely, the D-wave amplitude decreases without significant changes in the mMEPs. In any case, D-wave deterioration occurs gradually so that, if this event is recognized, there is usually time to take corrective measures.
Whenever the D-wave amplitude decreases and reach a level of about 50% of its baseline value, then the surgery will be terminated because a further, permanent, deterioration of the D-wave amplitude will invariably correlate with a permanent motor deficit. However, even a decline in D-wave amplitude is potentially reversible with warm irrigation and induced mild hypertension. Consequently, as long as the D-wave amplitude recovers above the 50% threshold, surgery can be resumed.
A particularly challenging situation occurs where baseline mMEPs are present while the D-wave is absent from the beginning. Others interpreted and previously described this phenomenon—which occurs in about 30% of the cases—as a “desynchronization” of the D-wave [7, 24, 32]. We observed desynchronization of the D-wave more commonly either after irradiation of the spinal cord, or in cases where ISCTs are associated to extended syringomyelic cysts. It seems that, under these circumstances, fast fibers of the corticospinal tracts conduct D-waves at different speeds and therefore the recording epidural electrode will pick-up only desynchronized descending volleys. When the D-wave is desynchronized or the epidural electrode cannot be placed due to anatomical reasons (e.g., dural scars due to previous surgery), monitoring can rely only on mMEP. Unfortunately, mMEP loss during tumor removal would not allow distinguishing between a permanent and a transient post-operative motor deficit. In this case, to continue surgery despite mMEP disappearance exposes the patient to a significant risk of complete and permanent motor deficit.
Although mMEP loss usually indicates a post-operative impairment of voluntary movement, about 10% of cases exhibit false positive results (i.e., patients who intraoperatively lost mMEPs but did not show any significant motor deficit after surgery) [26]. One possible explanation for this discrepancy, which essentially represents an exception to the “transient paraplegia” phenomenon, is the possibility that preserved fast conducting CT fibers can immediately compensate for an injury that is most likely limited to the supportive motor system. Thus no clinical motor deficits are present post-operatively. This has almost exclusively happened for ISCTs at the thoracic level, and very rarely occurred during cervical ISCT surgeries.
What to do when MEP deteriorate?
A frequently asked question in IOM is: “What to do when potentials get worse?” There are at least three factors we have found useful for promoting the recovery of lost MEPs or deteriorated SEPs during ISCT surgery, and they can be easily recalled using the acronym T.I.P.: time, irrigation, papaverine/pressure (blood pressure) [43].
Time: we have consistently observed that if surgery is transiently stopped immediately after mMEP have disappeared or D-wave has significantly deteriorated, these potentials often spontaneously recover. At this point the spinal cord is again able to sustain the further manipulation necessary to remove the remaining tumor. Conversely, to ignore these events and continue or, even worse, speed up the use of cavitron ultrasound aspirator (CUSA) or any other cord manipulation would likely transform a reversible injury into an irreversible one. We have therefore adopted a sort of “stop and go” strategy that we did not use before the neurophysiological feedback became available. As a result, surgery can sometimes be transiently stopped for half an hour or more, to allow mMEPs and/or D-wave to recover; at that point further manipulation of the cord is possible. Doing so, we attempt to adapt the surgical strategy to the changes in the level of tolerance of the spinal cord along the procedure.
Irrigation: irrigation of the surgical field with warm saline solution dilutes potassium which accumulates in the extracellular space possibly inducing a block of conduction, and generally clears out irritating blood products and metabolites [53, 54].
Local application of papaverine and increasing the mean arterial pressure are both methods to improve local perfusion to counteract an incipient ischemia. Sometimes, MEPs are dramatically correlated with blood pressure values and a sustained hypotension may affect MEPs and unfavorably affect the outcome [35, 40].
Does monitoring make a difference?
The real impact of neurophysiological monitoring on the neurological outcome after ISCT surgery remains debated and very difficult to prove based on control studies. In fact, those neurosurgeons who operate with the assistance of IOM, and believe in its efficacy to prevent neurological deficit, would not accept a prospective randomized study given the ethical and medico-legal concerns of designating a “control group”.
We recently tried to address the question concerning the real impact of IOM by comparing the neurological outcome of 50 patients operated on with the assistance of IOM (SEPs, mMEPs, and D-wave) with that of 50 patients selected from 301 ISCTs previously operated on by the same team without IOM [45]. We matched the two groups by the pre-operative neurological status, tumor histology, location and extent of removal. Matching was blind to outcome. Conclusion of this study was that a combined mMEP and D-wave monitoring protocol significantly improves motor outcome at a follow-up of at least 3 months. Interestingly, however, we also observed that short-term evaluation, early after surgery, did not show an advantage for the monitored versus non-monitored group, and this was likely due to the transient paraplegia phenomenon that would mask the beneficial role of monitoring, when patients are evaluated in the early post-operative stage. From our study emerged also the observation that patients who arrive to surgery in severe neurological conditions are poor candidates to IOM because the monitorability rate of both mMEPs and D-wave is almost 50% less than that observed in neurologically intact patients. Still, we believe that it is worthwhile to attempt neurophysiological monitoring also in these patients because, on an individual basis, monitoring can be successful and help to prevent further neurological injury.
Criteria for MEP interpretation
For mMEP monitoring during ISCT surgery, unlike the 50% amplitude criteria used for the D-wave, we use yes/no criteria. In other words, only presence/absence responses are considered but not changes in the morphology, amplitude, or latency due to the extreme variability of these parameters even in the neurologically intact patient. By applying these criteria, no false negative results (patient being paraplegic with present MEP at the end of surgery) and a <10% rate of false positive results (patient being neurologically intact post-operatively in spite of intraoperative MEP loss) have been observed by us and other groups [26]. The rate of total tumor removal in our study is about 76%, and it is similar to the rates reported by other recent series of ISCTs.
Over the past decade, however, different criteria for mMEP monitoring have been proposed.
Calancie et al. suggested threshold-level parameters during multipulse TES to assess intraoperative mMEPs changes [4, 5]. One problem, with threshold-level criteria is that threshold to elicit mMEPs after TES is highly variable because mMEPs are generated through a polysynaptic pathway and are very sensitive to the effects of anesthesia. Wide variation in amplitude and latency therefore do not necessarily indicate an impending injury to the motor tracts [20]. The authors provide no information with regards to the rate of tumor removal and we therefore do not know whether the applied MEP criteria prevented or favored a radical tumor removal in some patients.
More recently Quinones-Hinojosa et al. [41] proposed mMEP criteria based on alterations in mMEP morphology (from polyphasic to biphasic and from biphasic to loss). D-wave was not monitored. Changes in MEP morphology correlated with motor grade loss in the immediate post-operative period, at discharge and at follow-up. Further refinement of mMEPs criteria to predict post-operative outcome and prevent neurological deficits is clearly desirable. However, the degree of motor impairment in patients whose MEP changed from polyphasic to biphasic was mild and mostly transient. At the follow-up all these patients had a McCormick grade 4 or up and therefore were all ambulatory without assistance. By applying these MEP criteria we can therefore protect the patient from a mild, mostly transient, impairment in motor strength. From a neurooncological perspective, however, the rate of total tumor removal in this study—where the majority of tumors were ependymomas—was only 57% and some patients with incomplete tumor removal were sent to radiotherapy. This rate compares unfavorably with other series [17, 42, 52], especially if we consider that ependymomas are amenable of gross total removal, must be eradicated to achieve cure, and radiotherapy should be limited to malignant tumors.
In our opinion, the benefit of more sensitive MEP criteria should be always balanced with the risk of stopping resection too early. From a neurosurgical stand-point, the possibility of a mild, often transient, motor impairment may be an acceptable price for the patient to pay, if this is rewarded by a complete tumor removal.
Free running EMG has been also recently applied as a method to detect early motor tract injury during ISCT surgery [46]. In this study, changes in free-running EMG anticipated mMEPs changes in three cases and were the only intraoperative finding in two patients whose mMEPs remained unchanged. These last two patients presented a mild post-operative worsening, which completely recovered at follow-up. This report represents the first hint to the possibility of using free-running EMG criteria to improve the reliability of IOM during ISCT surgery, but needs to be confirmed on a larger group of patients.
Conclusion
Only a decade ago, intraoperative neurophysiological monitoring was considered of limited value during ISCT surgery because motor and sensory deficits can occur independently and SEPs were not reliable to provide information on motor pathways. The introduction of D-wave and mMEP monitoring has dramatically changed this picture and we can today rely on a very reliable combined SEP-MEP IOM strategy. In our opinion combining mMEPs and D-wave monitoring, when available, remains nowadays the gold standard for ISCT surgery because it supports a more aggressive surgery in the attempt to achieve a complete tumor removal. Without D-wave monitoring, in the case of mMEP loss it is impossible to predict whether the neurological deficit will be transient or permanent. Moreover, if quantitative (threshold or waveform dependent) mMEPs criteria only are used to stop surgery, this likely impacts unfavorably on the rate of tumor removal. Different MEP methods and warning criteria still exist (mMEP, D-wave, absence/presence criteria, amplitude criteria, and morphology criteria…) and future studies should clarify which method provides the strongest reliability to support both an aggressive tumor removal while preserving the long-term neurological outcome of the patient. What also needs to be discerned is the value of IOM in patients who arrive to surgery with severe neurological deficits and the role played by IOM in different tumor types such as ependymomas, astrocytomas, and hemangioblastomas.
References
Boyd SG, Rothwell JC, Cowan JMA, Webb PJ, Morley T, Asselman P, Marsden CD (1986) A method of monitoring function in corticospinal pathways during scoliosis surgery with a note on motor conduction velocities. J Neurol Neurosurg Psychiatr 49:251–257
Brotchi J (2002) Intrinsic spinal cord tumor resection. Neurosurgery 50:1059–1063
Burke D, Hicks RG, Stephen JPH (1990) Corticospinal volleys evoked by anodal and cathodal stimulation of the human motor cortex. J Physiol (Lond) 425:283–299
Calancie B, Harris W, Brindle GF, Green BA, Landy HJ (2001) Threshold-level repetitive transcranial electrical stimulation for intraoperative monitoring of central motor conduction. J Neurosurg (Spine 1) 95:161–168
Calancie B, Harris W, Broton JG, Alexeeva N, Green BA (1998) Threshold-level multipulse transcranial electrical stimulation of motor cortex for intraoperative monitoring of spinal motor tracts: description of method and comparison to somatosensory evoked potential monitoring. J Neurosurg 88:457–470
Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, Epstein FJ (2000) Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg (Spine) 93:183–193
Deletis V (2002) Intraoperative neurophysiology and methodologies used to monitor the functional integrity of the motor system. In: Deletis V, Shils J (eds) Neurophysiology in neurosurgery: a modern intraoperative approach. Academic, San Diego, pp 25–51
Deletis V (2000) Neuromonitoring. In: McLone DG (ed) Pediatric neurosurgery: surgery of the developing nervous system. Saunders, Philadelphia, pp 1204–1213
Deletis V, Kothbauer K (1998) Intraoperative neurophysiology of the corticospinal tract. In: Stålberg E, Sharma HS, Olsson Y (eds) Spinal cord monitoring. Springer, Vienna, pp 421–444
Edmonds HL, Paloheimo MPJ, Backman MH, Johnson JR, Holt RT, Shields CB (1989) Transcranial magnetic motor evoked potentials (tcMMEP) for functional monitoring of motor pathways during scoliosis surgery. Spine 14:683–686
Elsberg CA, Beer E (1911) The operability of intramedullary tumors of the spinal cord. A report of two operations with remarks upon the extrusion of intraspinal tumors. Am J Med Sci 142:636–647
Epstein FJ, Farmer J-P, Freed D (1993) Adult intramedullary spinal cord ependymoma: the result of surgery in 38 patients. J Neurosurg 79:204–209
Fennelly ME, Taylor BA, Hetreed M (1993) Anaesthesia and the motor evoked potential. In: Jones SJ, Boyd S, Hetreed M, Smith NJ (eds) Handbook of spinal cord monitoring. Proceedings of the fifth international symposium on spinal cord monitoring, London, UK, June 2–5, 1992. Kluwer, Dordrecht, pp 272–276
Fischer G, Brotchi J, Mahla K (2005) Surgical management of intramedullary spinal cord tumors in adults. In: Schmidek H, Roberts D (eds) Schmidek and sweet operative neurosurgical techniques: indications, methods, and results. Saunders Elsevier, Philadelphia, pp 1945–1954
Ginsburg HH, Shetter AG, Raudzens PA (1985) Postoperative paraplegia with preserved intraoperative somatosensory evoked potentials. J Neurosurg 63:296–300
Guidetti B, Mercuri S, Vagnozzi R (1981) Long-term results of the surgical treatment of 129 intramedullary spinal gliomas. J Neurosurg 54:323–330
Hanbali F, Fourney DR, Marmor E, Suki D, Rhines LD, Weinberg JS, McCutcheon IE, Suk I, Gokaslan ZL (2002) Spinal cord ependymoma: radical surgical resection and outcome. Neurosurgery 51:1162–1174
Jellinek D, Jewkes D, Symon L (1991) Noninvasive intraoperative monitoring of motor evoked potentials under propofol anesthesia: effect of spinal surgery on the amplitude and latency of motor evoked potentials. Neurosurgery 29:551–557
Jones SJ, Buonamassa S, Crockard HA (2003) Two cases of quadriparesis following anterior cervical discectomy, with normal perioperative somatosensory evoked potentials. J Neurol Neurosurg Psychiatry 74:273–276
Jones SJ, Harrison R, Koh KF, Mendoza N, Crockard HA (1996) Motor evoked potential monitoring during spinal surgery: responses of distal limb muscles to transcranial cortical stimulation with pulse trains. Electroencephalogr Clin Neurophysiol 100:375–383
Kalkman CJ, Drummond JC, Ribberink AA, Patel PM, Sano T, Bickford RG (1992) Effects of propofol, etomidate, midazolam and fentanyl on motor evoked responses to transcranial electrical or magnetic stimulation in humans. Anesthsiology 76:502–509
Katayama Y, Tsubokawa T, Maemjima S, Hirayama T, Yamamoto T (1988) Corticospinal direct response in humans: identification of the motor cortex during intracranial surgery under general anesthesia. J Neurol Neurosurg Psychiatr 51:50–59
Kearse LA Jr, Lopez-Bresnahan M, McPeck K, Tambe V (1993) Loss of somatosensory evoked potentials during intramedullary spinal cord surgery predicts postoperative neurologic deficits in motor function [corrected] [published erratum appears in J Clin Anesth 1993 Nov-Dec; 5(6):529]. J Clin Anesth 5:392–398
Kothbauer K, Deletis V, Epstein FJ (1997) Intraoperative spinal cord monitoring for intramedullary surgery: an essential adjunct. Pediatr Neurosurg 26:247–254
Kothbauer K, Deletis V, Epstein FJ (2000) Intraoperative neurophysiological monitoring. In: Crockard A, Hayward R, Hoff JT (eds) Neurosurgery: the scientific basis of clinical practice, 3rd edn. Blackwell, Oxford, p 1042
Kothbauer KF, Deletis V, Epstein FJ (1998) Motor-evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg Focus 4:Article 1
Krzan M, Deletis V, Isgum V (1996) Intraoperative neurophysiological mapping of dorsal columns. A new tool in the prevention of surgically induced sensory deficit? Electroencephalogr Clin Neurophysiol 102:37P [Abstract]
Lesser RP, Raudzens P, Lüders H, Nuwer MR, Goldie WD, Morris HH, Dinner DS, Klem G, Hahn JF, Shetter AG, Ginsburg HH, Gurd AR (1986) Postoperative neurological deficits may occur despite unchanged intraoperative somatosensory evoked potentials. Ann Neurol 19:22–25
Levy WJ, York DH, McCaffrey M, Tanzer F (1984) Motor evoked potentials from transcranial stimulation of the motor cortex in humans. Neurosurgery 15:287–302
McCormick PC, Torres R, Post KD, Stein BM (1990) Intramedullary ependymoma of the spinal cord. J Neurosurg 72:523–532
Merton PA, Morton HB (1980) Stimulation of the cerebral cortex in the intact human subject. Nature 285:227
Morota N, Deletis V, Constantini S, Kofler M, Cohen H, Epstein FJ (1997) The role of motor evoked potentials during surgery for intramedullary spinal cord tumors. Neurosurgery 41:1327–1336
Nadkarni TD, Rekate HL (1999) Pediatric intramedullary spinal cord tumors: critical review of the literature. Childs Nerv Syst 15:17–28
Nuwer MR, Dawson EG, Carlson LG, Kanim LE, Sherman JE (1995) Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter study. Electroencephalogr Clin Neurophysiol 96:6–11
Owen JH (1999) The application of intraoperative monitoring during surgery for spinal deformity. Spine 24:2649–2662
Patton HD, Amassian VE (1954) Single-and multiple unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol 17:345–363
Pechstein U, Cedzich C, Nadstawek J, Schramm J (1996) Transcranial high-frequency repetitive electrical stimulation for recording myogenic motor evoked potentials with the patient under general anesthesia. Neurosurgery 39:335–344
Pelosi L, Lamb J, Grevitt M, Mehdian S, Webb J, Blumhardt L (2002) Combined monitoring of motor and somatosensory evoked potentials in orthopaedic spinal surgery. Clin Neurophysiol 113:1082–1091
Philips CG, Porter R (1964) The pyramidal projection to motoneurones of some muscle groups of the baboon’s forelimb. In: Eccles JC, Schadé JP (eds) Progress in brain research. Elsevier, Amsterdam, pp 222–243
Polo A, Tercedor A, Paniagua-Soto J, Acosta F, Canadas A (2000) Neurophysiological monitoring during scoliosis surgery using control hypotension. Rev Esp Anestesiol Reanim 47:367–370
Quinones-Hinojosa A, Lyon R, Zada G, Lamborn KR, Gupta N, Parsa AT, McDermott MW, Weinstein PR (2005) Changes in transcranial motor evoked potentials during intramedullary spinal cord tumor resection correlate with postoperative motor function. Neurosurgery 56:982–993
Raco A, Esposito V, Lenzi J, Piccirilli M, Delfini R, Cantore G (2005) Long-term follow-up of intramedullary spinal cord tumors: a series of 202 cases. Neurosurgery 56:972–981
Sala F, Lanteri P, Bricolo A (2004) Motor evoked potential monitoring for spinal cord and brain stem surgery. Adv Tech Stand Neurosurg 29:133–169
Sala F, Niimi Y, Berenstein A, Deletis V (2001) Neuroprotective role of neurophysiological monitoring during endovascular procedures in the spinal cord. Ann NY Ac Sci 939:126–136
Sala F, Palandri G, Basso E, Lanteri P, Deletis V, Faccioli F, Bricolo A (2006) Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery 58:1129–1143
Skinner S, Nagib M, Bergman T, Maxwell R, Msangi G (2005) The initial use of free-running electromyography to detect early motor tract injury during resection of intramedullary spinal cord lesions. Neurosurgery 56:299–314
Sloan TB (2002) Intraoperative neurophysiology and anesthesia management. In: Deletis V, Shils J (eds) Neurophysiology in neurosurgery: a modern intraoperative approach. Academic, San Diego, pp 451–474
Taniguchi M, Nadstawek J, Langenbach U, Bremer F, Schramm J (1993) Effects of four intravenous anesthetic agents on motor evoked potentials elicited by magnetic transcranial stimulation. Neurosurgery 33:407–415
Taniguchi M, Schramm J, Cedzich C (1991) Recording of myogenic motor evoked potentials under general anesthesia. In: Schramm J, Møller ÅR (eds) Intraoperative neurophysiologic monitoring in neurosurgery. Springer, Berlin, pp 72–87
Wiedemayer H, Fauser B, Sandalcioglu IE, Schafer H, Stolke D (2002) The impact of neurophysiological intraoperative monitoring on surgical decisions: a critical analysis of 423 cases. J Neurosurg 96:255–262
Wood E, Berne A, Taveras J (1954) The value of radiation therapy in the management of intrinsic tumors of the spinal cord. Radiology 63:11–24
Xu Q, Bao W, Mao R, Yang G (1996) Aggressive surgery for intramedullary tumor of cervical spinal cord. Surg Neurol 46:322–328
Young W, Koreh I (1986) Potassium and calcium changes in injured spinal cords. Brain Res 365:42–53
Young W, Rosenbluth J, Wojak JC, Sakatani K, Kim H (1989) Extracellular potassium activity and axonal conduction in spinal cord of the myelin-deficient mutant rat. Exp Neurol 106:41–51
Zentner J, Kiss I, Ebner A (1989) Influence of anesthetics-nitrous oxide in particular-on electromyographic response evoked by transcranial electrical stimulation of the cortex. Neurosurgery 24:253–256
Conflict of interest statement None of the authors has any potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sala, F., Bricolo, A., Faccioli, F. et al. Surgery for intramedullary spinal cord tumors: the role of intraoperative (neurophysiological) monitoring. Eur Spine J 16 (Suppl 2), 130–139 (2007). https://doi.org/10.1007/s00586-007-0423-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-007-0423-x