Abstract
Castor (Ricinus communis; family: Euphorbiaceae) oil extracted from castor seed is a nonedible, nontoxic, yellowish color liquid that has become an essential bioresource material for industrial uses. The castor oil is rich in ricinoleic acid; this is a key precursor of the production of lactones. The presence of a double bond and hydroxyl and carboxylic groups with a long hydrocarbon chain in ricinoleic acid proposes several possibilities for converting it into valuable compounds. γ-Decalactone is an aroma compound having peach-like essence, generally utilized in food industries. Lipase-mediated biotransformation is used to produce γ-decalactone from ricinoleic acid under controlled conditions. Several studies and industrial approaches have explained the genetic and metabolic engineering and bioprocess engineering strategies in the enrichment of aroma compounds, but few studies have been available on the utilization of castor oil as a natural raw material for the synthesis of aroma compounds. As a result, this review draws attention to the importance of castor oil in the production of value-added aroma compounds with their estimated global market prospective. The review gives information about the properties of castor oil and its geographical accessibility and its exploitation as a bio-based resource for the production of various value-added materials. In addition, this review emphasizes the utilization of ricinoleic acid or castor oil as a renewable source for the production of aroma compounds. Though chemical transformation for the production of lactone derivatives is known, the products are chiral mixtures. On the other hand, the lipase-based conversion is enantiospecific, and this product is categorized as nature-identical and considered safe for using in food products.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction: Why Castor Oil?
Castor plant (Ricinus communis) belongs to the family Euphorbiaceae and is commonly known as Arandi in Hindi. Castor plants are cultivated in South America, India, and Africa and are widely grown in tropical regions [1]. Brazil, China, and India are the major castor-producing countries in the world [2, 3]. Over 90% of castor oil is exported by India. On the other hand, about 84% of castor oil is imported by the United States, European Union, and China [4]. The oil obtained from castor beans is nonvolatile fatty oil. It is a pale yellow semi-transparent liquid having a high boiling point and density. Castor oil and its derivatives are generally used in making soaps, paints, surface coating, inks, waxes, cold-resistant plastic, biodiesel, and lubricants. The importance of castor oil in the pharmaceutical industry is growing in terms of its use as an anti-inflammatory and antimicrobial agent. It also has good moisturizing properties, due to which it is regarded as safe for hair and skin [5].
Furthermore, castor oil is also considered as a major raw material for the industrial sector in the development of various products like perfumes and flavors, receiving increased attention nowadays. Castor oil is recognized as a low-priced, biodegradable, and low-toxic oil compared to other vegetable oils. Due to this reason, it has attained an impetus in the food industry in terms of food additives, mold inhibitors, and flavoring agents [5]. Thus, all these characteristics of castor oil drive it toward high market growth and valued products.
Castor oil, rich in ricinoleic acid (RA) , is generally used as a substrate for the production of γ-decalactone through biotransformation using efficient microbial catalysts. RA having a long-chain fatty acid composes around 85% of the fatty acids in castor oil [6, 7]. Consequently, it is promising to use castor oil as an economical way and in copious amounts for industrial production of γ-decalactone [8]. Recently, the use of the biotransformation method to produce aroma compounds has achieved significant global attention. This biotechnological conversion method is of low cost to produce flavor compounds. Before the bioconversion process, castor oil is subjected to the hydrolysis process to produce 85% of pure RA-enriched castor oil [9]. Several microorganisms such as Candida, Yarrowia lypolytica, Pseudomonas, and Rhodotorula have been reported to potentially produce aroma compounds [10]. RA enters the mitochondria within the cells of microorganism and degrade to 4-hydroxy-decanoyl-CoA after four β-oxidation cycles, which subsequently cyclizes to γ-decalactone in the biotransformation process [11].
The current review mainly focuses on the properties, market value of castor oil, and biotechnology methods to make value-added products from castor oil.
9.2 Geographical Distribution of Castor Plants
Castor is a perennial plant adapted to grow in tropical climates with a height of 10–12 m, whereas in temperate regions it is an annual plant with a height of 1–3 m [12]. Castor plants grow well in low humidity seasons and fertile, slightly acidic, and sandy loam soil. The optimum temperature required to for their growth is about 20–25 °C, but a temperature of above 35 °C can badly affect the seed setting. Cold temperature can trigger chilling injury reducing plant growth [13]. In ancient times, nearly about 6000 years ago, castor plants were cultivated for their oil content in Egypt [14]. Castor plants are native to the Ethiopian region of east African tropical regions and considered to be a weed in the south western United States. In India, it grows abundantly on wastelands [15]. India produced 54% while China 23.4% of castor seeds, followed by Brazil, i.e., 11.9%, in 2000–2009. Other countries that produce castor seeds are Mozambique, Thailand, Paraguay, Cambodia, Ethiopia, Indonesia, Madagascar, Peru, Russia, Philippines, Haiti, South Africa, Tanzania, Vietnam, Uganda, Syria, Pakistan, Ecuador, and Colombia [16]. The highest castor-producing states in India are Gujarat (7.02 lakh ha), Rajasthan (1.54 lakh ha), Andhra Pradesh (0.33 lakh ha), Telangana (0.22 lakh ha), and Odisha (0.04 lakh ha). Narayanpet (6973 ha), Wanaparthy (5567 ha), Mahabubnagar (5104 ha), and Gadwal (2163 ha) are the major growing districts in Telangana.
9.3 Castor Oil Scenario
In 2020, the production of global castor market has reached to 740.5 kilotons (https://www.imarcgroup.com/castor-oil-manufacturing-plant). It is anticipated to show stable growth during the next 5 years. Asia Pacific leads the global market in the growth of castor oil due to the constant expansion in the field of pharmaceutical, surface coatings, and cosmetics industries. The largest market for castor oil has been acquired by China itself because of having large geographical area [17]. In the current pandemic (COVID-19) situation, many castor oil-producing entities have been closed down due to the lockdown imposed by the government of different countries, which has badly affected the market growth.
Due to the rising consumption of castor oil, its demand has increased globally at a rate of 7320 tons per year. Based on the increased demand, it is not sufficient to meet the expected rate of castor oil production for supply in the current pandemic situation. Therefore, as the previous trends have shown that castor oil costs and demand will increase gradually and are predicted to reach 1.81 billion by 2020 in the global market [18]. The increasing use of castor oil can be found due to its application in biopolymer and biofuels industries, cosmetics, and pharma industries. Some companies working in the production of castor oil and its derivatives in the global market are Adani Wilmer Ltd., Gokul Agri international Ltd. (GAIL), Jayant Agro Organics Ltd., Kandla Agro and Chemicals Pvt. Ltd. (KACPL), Hokoku Corporation, Thai Castor Oil Industries Co., Liaoyang Huaxing Chemical Co. Ltd., Bom Brazil, ITOH Oil Chemicals Co. Ltd., and Kanak Castor Products Pvt. Ltd. [19].
The international castor oil market was recorded as $1180 million in 2018 and is projected to reach $1470 million by the end of 2025, rising at a CAGR (compound annual growth rate) of 2.8% between 2019 and 2025, according to global reports. India exports castor oil to Europe, Japan, China, and the USA [20]. Due to the trade war between USA and China, the demand from China has been slowed down. Moreover, due to the shortage in the supply of castor oil in 2019, the export rate is less as compared to 2018, when the country had exported 5.5–6 lakh tons of castor oil. In the local market, its price has grown by 27% to Rs 1150 per 10 kg from Rs 900 a year ago, although the local market is very small compared to overseas demand [21].
The castor seed production increased during 2018–2019 because its prices enhanced up to 30% to Rs 54,750 a quintal. However, due to the COVID-19 pandemic across the globe, the prices have dropped to below Rs. 40,000 a quintal. Since then, the prices have improved after the increase of exportation up to 71,900 tons in May. From the commercial point of view, having hydroxylated fatty acid, castor oil is a vital component for the chemical industry worldwide [22].
9.4 Composition, Properties, and Applications of Castor Oil
Different methods have been applied for the extraction of castor oil from castor beans, such as mechanical pressing, solvent extraction, or a combination of both. Depending on the geographical distribution, extraction methods, and varieties, castor seeds contain around 44–55% of oil by weight. The alterations in the castor oil properties can be a result of the extraction methods, different varieties, seasonal variations, and type of soil. For example, low iodine value, low acid value, and high saponification value have been reported in cold-pressed castor oil compared to solvent extracted oil. In addition, the physicochemical property and composition have also been observed to be changed due to seasonal variations [23]. Resembling other vegetable oils, castor oil also contains unsaturated and saturated fatty acids attached to a glycerol [24, 25]. The castor oil mixture has a higher content of fatty acids, ricinoleic acid of around 90%, and other constituents in small amounts, as given in Table 9.1. The commercial value of castor oil has increased due to the presence of the high content of ricinoleic acid (RA), which can be utilized in various applications in the chemical industry.
Castor oil is colorless to pale yellow thick liquid with a unique taste, having a mild odor, and have a boiling point at 586 K [26]. The distinctive property of castor oil is due to the occurrence of the hydroxyl group in ricinoleic acid, which makes it complementary with plasticizers of a broad range of synthetic and natural resins, polymers, waxes, and elastomers [27]. For example, the oil has high specific gravity and viscosity; it has little solubility in aliphatic petroleum solvents while highly soluble in alcohols [28]. The physicochemical properties of castor oil are given in Table 9.2. In comparison to other oils, castor oil has the highest viscosity and is reported to be different in other parameters like cloud point, flash point, etc. As compared to standard engine oil (SAE 40), the values of thermal conductivity, pour point, viscosity, and density of castor oil were found to be higher [32].
Castor oil and its derivatives play a vital role in the production of soaps, lubricating agents, coating, pharma products, paints, plasticizers, food, pesticide, perfumery, purgative, disinfectant, and germicidal agents [33]. Its high ricinoleic acid content allows its ready derivatization through the presence of the hydroxyl group. Biodiesel is also produced through the transesterification process. Due to these multiple applications, it is considered an industrial crop. Industrial uses of castor oil are given in Table 9.3.
9.5 Castor Oil Processing Techniques for Aromatic Compounds
As compared to other vegetable oils, castor oil is considered the most suitable raw material for industrial purposes to convert various high value-added products. Due to the presence of the high amount of RA in castor oil along with the occurrence of hydroxyl and carboxyl groups and double bonds, it has more potential and versatile applications in various industries [14]. It is also used in the formation of aroma compounds where the oil needs to undergo a biotransformation process to obtain value-added aroma compounds by using microbial catalysts. Before the biotransformation method, the castor oil undergoes chemical hydrolysis to purify the RA for maximum conversion of aroma compounds. Cosmetics and pharmaceuticals accounted for over 25% of total market volume in 2013 and was the largest application segment. Growing demand for bioingredient-based cosmetics would continue to remain a key driver for this segment. Castor oil and its derivatives are used in a number of industries for the production of a wide variety of products. It is utilized as a raw material in the production of a number of chemicals, which are further used in the fabrication of surfactants, soaps, cosmetics, surface coatings, pharmaceuticals, perfumes, plasticizers, greases, lubricants, and rubber (Table 9.3). Its basic derivatives, undecylenic acid and heptaldehyde, are used to produce various perfumery compounds [41]. In pharmaceuticals and cosmetics, it is used as an ingredient in various formulations. The demand of around 4% of flavors and fragrance from castor oil per annum is estimated globally. In Asia/Pacific region, the demand for flavors and fragrances is estimated to be rising at a rate of about 7% per annum through 2008. The current research studies mainly focus on the development of flavor and fragrance from natural sources and have a preference to utilize raw materials that are safe and biodegradable. In light of this fact, there is an excellent possibility for castor oil derivatives in the international market.
9.5.1 Hydrolysis and Purification of Castor Oil to Form Ricinoleic Acid
Some chemical and biochemical methods have been performed for the isolation of RA from castor oil. RA and glycerol have been yielded upon hydroxylation of the ester linkages in the triglyceride molecules. Under chemical methods, castor oil hydrolysis reaction is usually performed by the gradual addition of 80% sodium hydroxide in castor oil for 1 h, and fatty acids are formed. The fatty acids are released by adding distilled water and acidified with concentrated hydrochloric acid. Further fatty acids are extracted in ethyl acetate and dried over magnesium sulfate. Clearing up of fatty acids is performed by addition of n-hexane (1:5 w/v) and kept at −4 °C for 3 days in darkness. This reaction produces ricinoleic acid and glycerol. The purity of RA is 88%, and the rest are the palmitic, stearic, oleic, vaccenic, linoleic, and linolenic acids.
Another method to isolate RA from castor oil is the use of biocatalysts such as lipase enzymes. Some lipase-producing microbes such as Geotrichum candidum, Candida rugosa, and Pseudomonas cepacia have been reported to be used for hydrolysis of castor oil [42]. In this study, the hydrolysis of oil was performed in a reaction including oil (100 mg), 0.5 M phosphate buffer (pH 7) (600 μL), and about 2–5 mg of lipase enzyme at 30 °C at 500 rpm for 1–4 h in an incubator shaker. The level of hydrolysis was assessed by titration method against 0.1 N NaOH solutions (pH 12), taking the hydrolysis mixture in diethyl ether:ethanol:water solution (3:3:2). In this reaction, P. cepacia lipase showed successful conversion of castor oil to RA up to 27% in comparison to other microbial enzymes like C. rugosa and G. candidum that are recorded to be 13%. Another study also exhibited that when immobilized C. rugosa was applied for the lipolysis of castor oil, the yield of RA was about 20–40% under controlled conditions such as pH, temperature, and the quantity of enzymes and substrate [43]. One more remarkable study was done by Piazza and Farrell [44] on hydrolysis of castor oil using ground oats (Avena sativa L.) lipase, which has shown a conversion of 90% of RA.
Another green method using microwave-assisted extraction of RA from castor oil has been described [45] wherein hydrolysis of castor oil was done by ethanolic KOH solution kept in a microwave oven with a few pieces of ice. A water condenser and a magnetic stirrer were connected in the microwave oven and heating for 19 min was given continuously for the reaction, which has yielded 89% of RA. The above-mentioned methods for the separation of RA from castor oil were good for RA yield while using different catalysts and heat sources.
9.5.2 Chemical Transformation of Castor Oil
Due to the presence of long-chain hydrocarbon, OH, and COOH groups and double bonds in RA, there are lots of possibilities of modifications and transformations of the castor oil into a number of value-added products. Some chemical reactions have been performed, for instance, esterification [46] and amidation [24], leading to transformation due to the presence of carboxylic group, while double bond allows the transformation of the oil through a number of reactions like carbonylation [47], hydrogenation [24], and epoxidation [48]. In addition, the presence of OH group also drives the chemical reactions of dehydration [49] to remove the hydroxyl group and acetylation [48] and alkoxylation [50] to enhance the unsaturation of the oil. A new double bond formation occurs in the chain of ricinoleic acid upon catalytic dehydration reaction, which results in good color maintenance, fast drying, and water resistance for protective coatings [49]. Further heating at 249.85 °C in the presence of NaOH, capryl alcohol (2-octanol) and sebacic acid (a 10-carbon dicarboxylic acid) are formed. Both capryl alcohol and sebacic acid have several applications [51]. A study has reported that when a co-solvent system involving ethanol and isopropyl ether (35:65; v/v) applied to castor oil produced a purified RA (about 98.6% purity). The recovery of RA was recorded at about 70%, and the total yield of 55.5 ± 2.5% was obtained [51].
9.5.3 Biotransformation of Castor Oil to Form Enriched Flavored Products
The synthesis of aroma compounds can be done by the biotechnological methods following two important techniques: one is to use biotransformation method, and another is via de novo synthesis. Complex molecules are synthesized from simple molecules (sugars, amino acids, nitrogen salts, minerals, etc.) using intricate metabolic pathways of organisms, and this is defined as the de novo synthesis method. Biotransformation refers to the formation of value-added products from structurally similar substrates in a single reaction catalyzed enzymatically (microbial cells or pure enzymes) [52]. Biotransformation or bioconversion is considered a greener, cheaper, and commercial approach in comparison to de novo synthesis methods for the production of bioflavors [53]. In 1950, microbial production of blue cheese-note compounds was discovered the first time, and since then, numerous bio-based aroma compounds have been developed during the time [54].
γ-Decalactone is a peach-like aroma compound extensively used in food and beverages, which is the cause of the big interest in its biotechnological production [55]. To enhance the accessibility of ricinoleic acid to cells, castor oil can be hydrolyzed by lipases [56], generating esters such as methyl ricinoleate [55]. The process comprises the breakage of ricinoleic acid into 4-hydroxy-decanoic acid, a precursor of γ-decalactone, which is achieved by the process of peroxisomal 𝛽-oxidation enzymes [57].
9.5.3.1 Lipase-Mediated Biotransformation
A variety of microbial strains are able to convert substrates into valuable aroma compounds, and the examples of some microbial strains involved in the bioconversion process are given in Table 9.4, which demonstrate the potential of microbes for the synthesis of aroma compounds. Some microorganisms such as Pseudomonas, Candida, Rhodotorula, and Yarrowia lipolytica have been reported for higher productivity of aroma compounds through biotransformation [10]. Lactones
are well recognized for their wonderful flavor and fragrance (like pineapple, peach, raspberry, apricot, mango, coconut, papaya, grapes), which are produced industrially hundreds of tons yearly [64]. γ- and δ-lactones are the highly valuable five- and six-membered rings, respectively, with 12 carbons equal or less. It comprises compounds like γ-decalactone/4-decanolide (peach-like) [65], 4-dodecanolide (fruity-coconut like) [66], 4-octanolide (sweet herbaceous coconut-like) [67], 5-decanolide/ -decalactone (creamy-coconut peach-like) [68], 5-dodecanolide (fruit-oily peach-like) [69], and 6-pentyl-α-pyrone (6PP, strong coconut-like) [70]. Although lactone can be synthesized chemically from keto acids, production of δ-decalactone (DDL) and γ-decalactone (GDL) through the biotransformation method is growing currently due to their generally recognized as safe (GRAS) values reaching between US$ 1400 kg−1 and US$ 6000 kg−1 [71]. However, the prices reduced up to US$ 300 per kilogram due to the growing production of both lactone compounds over the years [65].
-decalactone (creamy-coconut peach-like) [68], 5-dodecanolide (fruit-oily peach-like) [69], and 6-pentyl-α-pyrone (6PP, strong coconut-like) [70]. Although lactone can be synthesized chemically from keto acids, production of δ-decalactone (DDL) and γ-decalactone (GDL) through the biotransformation method is growing currently due to their generally recognized as safe (GRAS) values reaching between US$ 1400 kg−1 and US$ 6000 kg−1 [71]. However, the prices reduced up to US$ 300 per kilogram due to the growing production of both lactone compounds over the years [65].
Castor oil is generally utilized as a substrate to produce γ-decalactone by yeast cells through β-oxidation cycles of fatty acids recurring in the cells [11, 72]. However, prior to entering the biotransformation process, the castor oil is hydrolyzed to get refined castor oil having 86% of ricinoleic acid as the major component [9]. In the microbial cells, RA inserts inside the microbial cells, and after recurring four β-oxidation cycles, RA breaks into 4-hydroxy-decanoyl-CoA, which subsequently cyclizes to γ-decalactone [11]. In a study, it has been observed under bioconversion reaction that γ-decalactone concentration was 52.9 and 62.4 mg/L from 2.5% castor oil and 1.5% RA, respectively [73].
Currently, nonconventional oleaginous yeast, Yarrowia lipolytica , is getting more importance in the bioconversion of castor oil or RA into γ-decalactone. Six-member family of acyl-CoA oxidases (Aox) encoded by POX1 to 6 gene, which plays a vital role in β-oxidation of fatty acids, is present in Y. lipolytica [74]. This four-step procedure involves mainly two oxidation steps, one hydration, and another cleavage reaction, which are catalyzed by three enzymes. At each cycle, the compound confers two-carbon shorter metabolite and an acetyl group [75]. One more study has reported about Aox family and revealed that Aox2 is long-chain specific (C18 to C10), whereas Aox3 is short-chain specific (C10 to C4) [74, 76, 77], both showing strong activity [60]. Furthermore, some other studies utilized modified strains of Y. lipolytica W29 (POX2 overexpressed and POX3–5 disrupted) and were competent to make more lactone without any degradation [60, 76].
It has been illustrated that extracellular lipases, specifically the endogenous lipase of Y. lipolytica (W29) and extracellular lipases (Lipozyme TL IM), were able to release ricinoleic acid from castor oil, and as a result, γ-decalactone produces rapidly [56, 78]. However, this method is not much appropriate as per industrial approaches since it is time- and cost-consuming. Therefore, to fill the gap of this problem, overexpression of the Lip2 enzyme would improve the production rate of γ-decalactone with no additional expenses. This was further investigated by Braga et al. [59] that Y. lipolytica (JMY3010) has the ability to synthesize extracellular lipase due to the presence of an additional copy of LIP2 gene necessary for the production of γ-decalactone. From a biotechnological point of view, gene expression studies during bioconversion of RA into γ-decalactone are still very less; here, a few studies involving upregulation and downregulation or disruption of some genes that play an important role in γ-decalactone production are given in Table 9.4.
9.5.3.2 Bioconversion of Ricinoleic Acid (RA) to γ-Decalactone (GDL)
The most essential determining factor to produce γ-decalactone and 3-hydroxy-γ-decalactone is β-oxidation pathway in microbial cells, which was first discovered by Okui et al. [79]. It is four-step reaction sequences, yielding an acyl CoA, which has two carbons less and an acetyl-CoA. This reaction recurred many times until the total breakdown of the compounds. Lactonization can take place at the C10 stage resulting in the formation of strong fruity notes [80]. Under anoxic conditions, the activity of 3-hydroxy-acyl-CoA dehydrogenase is minimized, because regeneration of its cofactor (NAD+) is not enough. When the regeneration of NAD is not enough, lactone accumulation takes place because a reduction in the β-oxidation flux decreases the requirement for NAD and thus the cofactor is not necessary any longer, a limiting compound for the pathway. This phenomenon also happens when the aeration of cells is changed, and this accumulation can be a signal of upscaling complexities in industry. 3-Hydroxy lactone is released when aeration is reduced with the declination in the β-oxidation pathway flux, and as a result, NAD is again regenerated in plenty amount and 3-hydroxy lactone does not accumulate [81, 82]. A variety of microbial cells have been isolated from different sources by various researchers for the bioconversion of lactones from different substrates (Table 9.5).
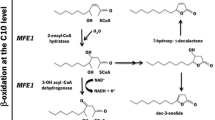
A study on bioconversion of methyl ricinoleate into γ-decalactone was carried out using Pichiu guilliermondii through the peroxisomal beta-oxidation pathway [102]. This study showed that enzymes of peroxisomes and peroxisomal β-oxidation, i.e., catalase and acyl-CoA oxidase activities, respectively, were stimulated by methyl ricinoleate. The activity of acyl-CoA oxidase increased approximately 40 times when this yeast was transferred in glucose medium in which fatty acid methyl ester act as the only carbon source. The acetyl-CoA molecules are afterward moved from the peroxisomes to the mitochondria by the acetyl-carnitine-transferase system. It has been reported that peroxisomal β-oxidation has a detoxification role because the peroxisomal reaction occurs in the absence of a mitochondrial respiratory chain involved in fatty acid consumption [103]. On the other hand, due to the accumulation of hydroxy fatty acids in the culture medium, β-oxidation process appears to be obstructed. Current research studies have focused on gene function that encodes acyl-CoA oxidase isozymes, oxygen mass transfer rates, lipid metabolism, interactions of cell–substrate to increase the production of γ- decalactone, and selection of overproducing mutants. β-Oxidation is a four-reaction cycle that shortens the carbon chain by two after catalyzed in peroxisomes by the following enzymatic activities: acyl-CoA oxidase, 2-enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and 3-ketoacyl-CoA thiolase (Fig. 9.1). A 10-carbon compound (4-hydroxydecanoic acid) is obtained from the 18-carbon chain in the last reaction, which is then cyclized to γ-decalactone. The products obtained from biotransformation reaction also fluctuate according to the precursor fatty acid: ricinoleic acid is degraded to an 8-carbon acid, whereas homoricinoleic acid degradation is guided to synthesize an intermediate of 11-carbon. The uncertainty of hydroxyl fatty acid oxidation relies both on the carbon chain length and hydroxy group position [104].
Even though some attempts have been made so far, the products yields are too low; therefore, additional studies are required to overcome the constraints observed at present to make the biotechnological process more useful. The necessary step toward higher production of aroma compounds is mainly done by focusing on the optimization attempts on fermentation technologies and downstream processes.
9.5.3.3 Optimal Conditions for Biotransformation
The biotransformation process is dependent on various parameters such as temperature, pH, time, enzyme amount (microbial cells), substrate concentration, and types of additives for γ-decalactone production. The first parameter is carbon source (substrate such as castor oil or ricinoleic acid) that acts as an activator of enzymes in the pathway of γ-decalactone production. The second parameter is nitrogen sources comprising peptone and yeast extract that are imperative to enhance cell growth and biotransformation process. The last factor is pH, which should be optimized to maintain the highest cell growth and γ-decalactone production.
9.5.3.3.1 Effect of Carbon Source
Synthesis of lipase, which is responsible for the bioconversion of castor oil into lactones, can be induced by the carbon sources in the culture medium. Some examples of carbon sources are carbohydrates, glycerol, organic acids, oils or fats, and other alcohols [105]. Some oils such as soybean oil, olive oil, oleic acid, and tributyrin have been observed to be effective in inducing lipase activity [106, 107]. For instance, the use of oleic acid exhibited increased production of lipase and biomass content in comparison to other fatty acids of different carbonic chains [108]. Another study showed that vegetable oil possessing linoleic and oleic acids was found superlative in lipase production from Candida species [109]. Olive oil used as a carbon source was also found to induce lipase activity by Y. lipolytica [110].
9.5.3.3.2 Effect of Nitrogen Source
Like carbon source, nitrogen source is also a chief factor influencing the synthesis of lipase enzymes. Both the inorganic and organic nitrogen have an essential role in the synthesis of the enzyme [109, 111]. Yeast extract, malt extract, peptone, amino acids, urea, nitrate and ammonium salts, agroindustrial waste, such as water soy flour and corn, are nitrogenous compounds generally used for lipase production [111, 112]. The selection of nitrogen compounds mostly depends on the microorganism used and the addition of other ingredients in the culture medium [112]. Nitrogen sources are the necessary component for cellular physiology because they provide vitamins and amino acids as enzyme cofactors [113]. For instance, cell growth and lipase synthesis were found to be increased by the addition of yeast extract and tryptone. In a study, various nitrogen sources have been examined on the growth of Y. lipolytica for lipase synthesis, and it was observed that mineral nitrogen did not show any significant effect on the growth of yeast possessing lipase activity [114]. Tryptone N1 (a casein hydrolysate) showed maximum production of lipase compared with other nitrogen-containing organic or mineral substrates [105].
9.5.3.3.3 Effects of Temperature
The temperature influences the growth parameters of microbes, such as the specific growth rate and the adaptation time (lag phase), and affects the biosynthesis of primary and secondary metabolites [115]. It has been observed in a study that lipase production by Y. lipolytica (681) was most influenced by the temperature [116]. The optimum temperature for significant lipase activity was 29.5 °C.
9.5.3.3.4 Effect of pH
Microbial growth can be affected by the hydrogen ion concentration in a culture medium indirectly, affecting nutrient accessibility or directly acting on cellular surfaces [115]. It is reported that under low pH, the synthesis of lipase by liquid substrate fermentation, with diverse microorganisms, is highest at the end of cultivation [117, 118]. Mooradi et al. [73] have optimized that under acidic pH, nitrogen source, and increased amount of yeast extract and castor oil, the production of γ-decalactone will be enhanced. Other researchers have also observed that acidic pH is more suitable for bioconversion of RA into 4-hydroxydecanoic acid and then γ-decalactone [57, 85, 91, 119].
9.5.3.4 Significance of Bioreactors
Bioreactors are devices for biotransformation or any fermentation process in which biological or biochemical reactions are performed under closely monitored and controlled conditions. A number of different types of bioreactors have been used for fermentation methods. Among these, two are commonly categorized as solid-state or stirred-tank bioreactors with two different fermentation methods, such as SSF and SmF, respectively. For research and development, both SmF and SSF have been employed for the production of bioflavor compounds. Approaches for the development of new types of reactors are going on continuously for special purposes [120]. In SmF type of bioreactor, mostly the reactions of microorganisms and substrate are performed in a liquid medium and the products produced by microbial enzymes reaction are recovered from the liquid medium and purified. Gas/air mixture required for fermentation reaction is delivered to a culture medium in sterilized environments. During the bioconversion process in bioreactors, optimized conditions for pH, temperature, aeration, and foam sensors are needed. Mechanical agitation is required for mixing and bubble dispersion. In addition, nutritional factors such as carbon and nitrogen sources are also important for the growth and synthesis of flavor compounds in lab as well as industrial scale [81, 121]. Several researchers have studied the microbial conversion of bioflavor compounds by SmF-type bioreactors [120,121,122,123,126].
De novo synthesis or transformation methods are generally performed in solid-state fermentation (SSF) system for the aroma compound production using various yeasts and fungi such as Kluyveromyces marxianus, Ceratocystis fimbriata, Moniliella suaveolens, Trichoderma harzianum, Pityrosporum ovale, Ceratocytis oniliformis, Aspergillus niger, and Rhizopus oryzae [125,126,127,128,131]. Furthermore, compatible combinations of yeasts and fungi, which cannot be cultured in submerged fermentation (SmF), can yield many aroma-active compounds in SSF [132]. In SSF, the fermentation process takes place in the absence of water on solid support generating important metabolites in the presence of microbes. SmF is a technique in which microorganisms can grow in liquid broth consuming the nutrients to release the desired metabolites into solution during fermentation. Although SSF has been used since ancient times and has many biotechnological advantages, there have been some limitations involved during scaling up in this technology, usually due to the heterogeneous nature of the mass, substrate, and troubles in heat transfer [127, 132, 133]. Besides the development of digital imaging technologies for assessing the growth kinetics in filamentous fungi in SSF, further improvement in mathematical modeling tools has been achieved to describe the scale-up studies [134].
Braga et al. [58, 59] have used stirred tanks and airlift bioreactors for the production of γ-decalactone using castor oil in batch cultures of Y. lipolytica (W29). Airlift bioreactors are generally considered for the developing processes on the basis of aerobic cultures because of the requirement for high oxygen transfer rates. Air was introduced at the bottom of the bioreactor using a five-hole sparger. γ-Decalactone concentration (around 3 g/L) was twofold increased in the airlift compared to the STR (stirred tank reactor) . In a study, it has been reported that when castor oil (substrate) was reacted with microbial enzymes, the production of GDL (11 g/L) was recorded in 55 h [65].
For immobilized enzymatic reactions for the production of bioflavor in industry, packed-bed bioreactors, especially operated in continuous mode, and fluidized bed reactors are the most often utilized [127, 135]. In addition, studies have led to designing new reactors such as immersion bioreactors, rotating drum bioreactors, and mixed solid-state bioreactors, which would succeed over the troubles for large-scale productions [10, 131].
Fed-batch strategy is an interesting strategy to avoid degradation of γ-decalactone and cut down the inhibitory impacts of ricinoleic acid on the cells [136]. Using intermittent feed in fed-batch, aroma concentration of 6.7 g L−1 was obtained as compared to 1.9 g L−1 in batch fermentation and the formation of the side product 3-hydroxy-γ-decalactone augmented concurrently to 10 g L−1 in the bioreactor. However, in this system, the maintenance of an emulsion causes several restraints to ensure the supply of fresh medium and remove an emulsion with the same characteristics. Thus, the substrate addition by step-wise fed-batch (pulses) is a method of avoiding this problem. In a step-wise fed-batch method, the productivity of γ-decalactone of 0.043 g L−1 h−1 has been reported when methyl ricinoleate (30 g L−1) was fed twofold to the bioreactor using Y. lipolytica W29 [136]. Braga et al. [59] also attempted a step-wise fed-batch method with MTLY40-2P strain, in which castor oil (60 g L−1) was supplemented in two pulses, leading to a twofold increase in γ-decalactone synthesis (about 7 g L−1) as compared to batch mode.
9.6 Castor Oil: Advantages and Disadvantages
9.6.1 Advantages
Castor is regarded as the most important industrial and medicinal plant since thousands of compounds can be extracted from it. These compounds have been reported to have antimicrobial activity against diverse pathogenic bacteria [137, 138]. Castor oil is extensively utilized in the industry with diverse applications [23]. The usage of castor oil is safe from broad historical usage to industrial application. Some ground-breaking technologies have been developed for the production of value-added castor oil chemicals and their derivatives. Food-grade castor oil is utilized in the formation of food additives, flavorings, and mold inhibitors and in packaging [139]. The products obtained by castor oil are not dangerous to the environment. It is biodegradable, nontoxic, and agriculture oriented. The export of castor oil can boost the economy of the country and also encourage the development of the agricultural sector.
Castor cake can be used as organic manure, which prevents soil from exhausting. Castor cake has a high content of N (6.4%), potash (1%), and phosphoric acid (2.55%) and has moisture retention capacity. It also contains crude protein (20%), ash (15%), and sugar (50%) [140]. Castor cake is used to control nematodes in soils [141]. Ricin can remain in the soil for about 2 years after castor harvesting [142]. However, the effect of the residual toxin on flora and fauna in the soil is yet to be determined.
9.6.2 Disadvantages
Castor contains toxic ricin in seeds. However, these toxic compounds can be utilized to formulate drugs to treat many diseases globally [143]. Soil micro flora is one of the most imperative factors that augment soil fertility in various ways. Antimicrobial activity of castor was also observed against soil microbial community, which in turn disturbs soil health and fertility during its cultivation in soil.
It has been observed that microbial diversity declined in soils when castor was cultivated as compared to other plants [144]. There may be possibilities of the occurrence of some residual inhibitors in the soil sensitive to certain fungal and bacterial species affecting the growth of plants and soil health [145]. However, certain microorganisms have the ability to degrade and survive at high concentrations of inhibitors. It has been observed in a study that Pseudomonas and Erwinia sp. can efficiently degrade the protein in an in vitro assay. Consequently, these bacteria can be utilized as biofertilizers for castor cultivation without damaging microbial diversity in soil enhancing plant growth and soil health [144].
9.6.3 Safety Assessment or Toxicity Study of Castor
The seeds of castor contain two types of proteins, R. communis agglutinin (RCA120) and ricin (RCA 60) , which are toxic to eukaryotic cells [146]. Ricin is a strong cytotoxin with weak haemagglutinin properties, while RCA120 is less toxic and has powerful haemagglutinin, which functions as an allergen causing a health hazard during harvesting and processing [147]. Ricin remains in the meal after its oil extraction. There are various methods for the detoxification of castor toxins [148, 149]. Ricin can be detoxified with the help of proteolytic enzymes, sodium ricinoleate, H2O2, KMnO4, autolyzed yeast or Azotobacter, halogens, ethanol, high temperature, and UV irradiation. In addition, other methods like steam treatment, NaCl, Ca (OH)2, formaldehyde, NH4OH, (NH4)2SO4, KMnO4, or urea can also be employed for detoxification of ricin [150]. US Department of Health and Human Services (NIH) has established safety measures for the usage of castor oil. In 1990, no harmful side effects of castor oil have been noticed in the feed studies on rats or mice. In 90 days, studies on dietary concentrations showed no effect on survival or body weight gains.
9.7 Concluding Remarks
Various types of aroma compounds are isolated from natural and synthetic sources to meet the market demand. The aroma industry has a great interest in natural products, especially fragrance and flavor compounds; therefore, an alternative method has been generated in the metabolic engineering field to obtain natural aroma compounds. Microbial biosynthesis and biotransformations are considered safe and most suitable technologies for the production of bioflavors and fragrances. This method has a number of advantages over traditional methods because microbes can be genetically and metabolically altered to improve the production of desired important compounds.
Castor oil is a significant and potential nonedible crop, producing numerous industrially important chemicals and products. Due to its lots of functional value in agriculture, industry, pharmaceutical, and cosmetics sectors, castor oil is proved to be a potential bio-based preliminary material increasing the economy of a country. Castor oil has unique properties of the presence of a double bond, carboxylate group, and hydroxyl group in the ricinoleic acid, which is useful in its easy derivatization into vital industrial raw materials. Due to the manufacture of large-scale end products from castor oil and being a green bioresource for chemical transformations, castor seed has global demand for enhanced production rate.
Castor oil conversion in aroma compound γ-decalactone by the biotransformation method using efficient microorganisms is a striking method, but the production time is high and quantity is very low. Various approaches have been adopted to overcome these problems by applying suitable microbial enzymes for the synthesis of aroma compounds. Fed-batch fermentation method can be applied, choosing suitable microorganisms for higher yields of lactone. This method is also useful to overcome the inhibitory effect of the substrate in lactone production. Solid-state fermentation is the most appropriate method in producing microbial metabolites in large quantities, which also provides high-quality yields with improved product characteristics accompanied by low economic costs. A much higher research thrust on productivity improvement is needed for mitigating the demand–supply gap of castor seeds. Besides breeding, efforts should be focused on three other prime objectives: (1) improvement of castor oil quantity and quality, (2) development of ricin-free castor, and (3) production of value-added aroma products from castor crop.
Next to these strategies, the application of bioprocess engineering has countless benefits to attain higher yields and product concentrations by a comprehensive understanding of the regulation of different pathways involved in aroma production. However, so far, the products yields are very poor to make the biotechnological method feasible, and more research studies are needed to overcome these limitations. Furthermore, selection of mutant or new potential strains, fermentation methods, downstream processes, and up-scaling from lab to industrial levels need severe studies to maximize the yield as well as lessen the limitations. The continuous development of genetic engineering and systems biology tools, in association with advanced strategies, will allow more bioflavors to be produced in this manner in the future.
References
Thomas A (2000) Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & KGaA; Fats and fatty oils.
Gad-Elkareem, M. A., Abdelgadir, E. H., Badawy, O. M., & Kadri, A. (2019). Potential antidiabetic effect of ethanolic and aqueous-ethanolic extracts of Ricinus communis leaves on streptozotocin-induced diabetes in rats. PeerJ, 7, e6441.
Perdomo, F. A., Acosta-Osorio, A. A., Herrera, G., Vasco-Leal, J. F., Mosquera-Artamonov, J. D., Millan-Malo, B., & Rodriguez-Garcia, M. E. (2013). Physicochemical characterization of seven Mexican Ricinus communis L. seeds & oil contents. Biomass and Bioenergy, 48, 17–24.
McKeon, T., Hayes, D., Hildebrand, D., & Weselake, R. (2016). Chapter 1: Imtroduction to industrial oil crops. In Ind Oil Crops (pp. 1–13). Elsevier.
Ibeagha, O. A. (2015). Strategies for improving the value chain of castor as an industrial raw material in Nigeria. Agricultural Engineering International: CIGR Journal, 17(3).
Gomes, M. C. S., Pereira, N. C., & Barros, S. T. D. (2010). Separation of biodiesel and glycerol using ceramic membranes. Journal of Membrane Science, 352, 271–276.
Maume, K. A., & Cheetham, P. S. J. (1991). The production of gamma decalactone by fermentation of castor oil. Biocatalysis, 5, 79–97.
Moradi, H., Asadollahi, M. A., & Nahvi, I. (2013). Improved γ-decalactone production from castor oil by fed-batch cultivation of Yarrowia lipolytica. Biocatalysis and Agricultural Biotechnology, 2(1), 64–68.
Gomes, N., Braga, A., Teixeira, J. A., & Belo, I. (2013). Impact of lipasemediated hydrolysis of castor oil on γ-decalactone production by Yarrowia lipolytica. Journal of the American Oil Chemists' Society, 90, 1131–1137.
Longo, M. A., & Sanroman, M. A. (2006). Production of food aroma compounds: Microbial and enzymatic methodologies. Food Technology and Biotechnology, 44, 335–353.
Braga, A., & Belo, I. (2015). Production of Ƴ-decalactone by Yarrowia lipolytica: Insights into experimental conditions and operating mode optimization. Journal of Chemical Technology and Biotechnology, 90, 559–565.
Salihu, B. Z., Gana, A. K., & Apuyor, B. (2014). Castor oil plant (Ricinus communis L.): Botany, ecology and uses. IJSR, 3, 1333–1341.
Patel, V. R., Dumancas, G. G., Viswanath, L. C. K., Maples, R., & Subong, B. J. J. (2016). Castor oil: Properties, uses, and optimization of processing parameters in commercial production. Lipid Insights, 9(1), 1–12.
Mutlu, H., & Meier, M. A. R. (2010). Castor oil as a renewable resource for the chemical industry. European Journal of Lipid Science and Technology, 112(1), 10–30.
Ladda, P. L., & Kamthane, R. B. (2014). Ricinus communis (castor): An overview. Int j res Pharmacol pharmacother, 3(2), 136–144.
Food and Agricultural Organization of the United Nations. (2011). FAOSTAT. Retrieved November 15, 2013, from http://faostat.fao.org/.
Guan, S., Rong, S., Wang, M., Cai, B., Li, Q., & Zhang, S. (2019). Enhanced biotransformation productivity of gamma decalactone from ricinoleic acid based on the expanded vermiculite delivery system. Microbial Biotechnology, 29(7), 1071–1077.
James, S. (2016). Castor oil and derivatives market trend, growth, research and analysis to 2020. Retrieved from https://www.linkedin.com/pulse/castor-oil-derivatives-market-trend-growth-research-analysis-james.
Anonymous. (2020). Grand view research. Castor oil and derivatives market analysis by product (sebacic acid, undecylenic acid, castor wax, dehydrated castor oil), by application (lubricants, surface coatings, biodiesel, cosmetics & pharmaceuticals, plastics & resins) and segment forecasts to 2020. Retrieved from http://www.grandviewresearch.com/industry-analysis/castor-oil-derivatives-industry.
Castor Outlook. (2019). Retrieved from https://www.pjtsau.edu.in/files/AgriMkt/2019/oct/Castor-October-2019.pdf.
The Economic Times. (n.d.). Retrieved from https://economictimes.indiatimes.com/markets/commodities/news/castor-oil-prices-spike-23-in-globalmarket/articleshow/69089709.cms?utm_source=contentofinterest&utm_medium=text&utm_campaign=cppst.
Vora, R. (2020). Agri business: Global castor oil consumption to dip amid shrinking economies.
Ogunniyi, D. S. (2006). Castor oil: A vital industrial raw material. Bioresource Technology, 97, 1086–1089.
Alwaseem, H., Donahue, C. J., & Marincean, S. (2014). Catalytic transfer hydrogenation of castor oil. Journal of Chemical Education, 91, 575–578.
Nangbes, J. G., Nvau, J. B., Buba, W. M., & Zukdimma, A. N. (2013). Extraction and characterization of castor (Ricinus Communis) seed oil. IJES, 2, 105–109.
Yeganeh, H., & Hojati-Talemi, P. (2007). Preparation and properties of novel biodegradable polyurethane networks based on castor oil and poly(ethylene glycol). Polymer Degradation and Stability, 92(3), 480–489.
Imankulov, N. (2012). Preparation and research on properties of castor oil as a diesel fuel additive. ATI, 6, 30–37.
Gana, A. K., Yusuf, A. F., & Apuyor, B. (2013). Castor oil plant and its potentials in transformation and industrialization of under developing nations in the world. Advanced Journal of Agricultural Research, 1, 72–79.
Ahmad, M. H., Ibrahim, W. A., Sazali, J., Izhab, I., & Hassan, Z. (2020). Review: Thermal process of castor and plant-based oil. Indonesian Journal of Chemistry, 20(1), 237–224.
Olasheu, T. I., Adebiyi, K. A., Durowoju, M. O., & Odesanya, K. O. (2014). Characterization of Castor (Ricinus communis) and Jatropha (Jatropha curcas) oils as alternative base oil for automotive lubricants. Journal of Scientific and Engineering Research, 5(8), 1318–1336.
Bobade, S. N., & Khyade, V. B. (2012). Detail study on the properties of Pongamia pinnata (Karanja) for the production of biofuel. Research Journal of Chemical Sciences, 7, 16–20.
Kazeem, O., Taiwo, O., Kazeem, A., & Mondiu, D. (2014). Determination of some physical properties of castor (Ricirus communis) oil. International Journal of Engineering, Science and Technology, 3(12), 1503–1508.
Prasad, R. B. N., & Rao, B. V. S. K. (2017). Chapter 8: Chemical derivatization of castor oil and their industrial utilization. In Fatty acids, chemistry, synthesis and applications (pp. 279–303).
Bello, E. I., & Makanju, A. (2011). Production, characterization and evaluation of castor oil biodiesel as an alternative fuel for diesel engines. Journal of Engineering and Applied Science, 2(3), 525–530.
Yenwo, G. M., Manson, J. A., Pulido, J., Sperling, L. H., Conde, A., & Devia, N. (1977). Castor-oil-based interpenetrating polymer networks: Synthesis and characterization. Journal of Applied Polymer Science, 21(6), 1531–1541.
Burt, B. G., & Mealy, W. C. (1942). U.S. Patent No. 2,271,619. Washington, DC: U.S. Patent and Trademark Office.
Lehrer, S. B., Karr, R. M., Müller, D. J., & Salvaggio, J. E. (1980). Detection of castor allergens in castor wax. Clinical Allergy, 10(1), 33–41.
Dwivedi, M. C., & Sapre, S. (2002). Total vegetable-oil based greases prepared from castor oil. Journal of Synthetic Lubrication, 19(3), 229–241.
Lima, R. L. S., Severino, L. S., Sampaio, L. R., Sofiatti, V., Gomes, J. A., & Beltrão, N. E. M. (2011). Blends of castor meal and castor husks for optimized use as organic fertilizer. Industrial Crops and Products, 33(2), 364–368.
Grummitt, O., & Marsh, D. (1953). Alternative methods for dehydrating castor oil. Journal of the American Oil Chemists' Society, 30(1), 21–25.
Nautiyal, O. P. H. (2018). Castor oil and its derivatives with market growth and commercial perspective: Review. Organic & Medicinal, 6(4), 555692.
Foglia, T. A., Jones, K. C., & Sonnet, P. E. (2000). Selectivity of lipases: Isolation of fatty acids from castor, coriander, and meadowfoam oils. European Journal of Lipid Science and Technology, 102, 612–617.
Ozcan, A., & Sagiroglu, H. M. (2009). Production of ricinoleic acid from castor oil by immobilised lipases. Preparative Biochemistry & Biotechnology, 39, 170–182.
Piazza, G. J., & Farrell, H. M. (1991). Generation of ricinoleic acid from castor oil using the lipase from ground oat (Avena sativa L.) seeds as a catalyst. Biotechnology Letters, 13, 179–184.
Karpakavalli, M., Arthi, I., & Seena, K. X. (2012). Microwave assisted isolation of hesperidin, ricinoleic acid and piperic acid. Journal of Scientific Research in Pharmacy, 1, 76–79.
Deshpande, D. P., Haral, S. S., Gandhi, S. S., & Ganvir, V. N. (2012). Transesterification of castor oil. ISCA Journal of Engineering Sciences, 1, 2–7.
Marc, R. L., Furst, M. R. L., Le Goff, R., Quinzler, D., Mecking, S., Botting, C. H., & Cole-Hamilton, D. J. C. (2012). Polymer precursors from catalytic reactions of natural oils. Green Chemistry, 14, 472–477.
Sinadinovic-Fiser, S., Jankovic, M., & Borota, O. (2012). Epoxidation of castor oil with peracetic acid formed in situ in the presence of an ion exchange resin. Chemical Engineering and Processing, 62, 106–113.
Nezihe, A., Elif, D., Yılmaz, O., & Tuncer, E. A. (2011). Microwave heating application to produce dehydrated castor oil. Industrial and Engineering Chemistry Research, 50, 398–403.
Zhang, Q., Sun, Y., Zhi, L., Zhang, Y., & Di Serio, M. (2015). Properties of ethoxylated castor oil acid methyl esters prepared by ethoxylation over an alkaline catalyst. Journal of Surfactants and Detergents, 18, 365–370.
Vaisman, B., Shikanov, A., & Domb, A. J. (2008). The isolation of ricinoleic acid from castor oil by salt-solubilitybased fractionation for the biopharmaceutical applications. Journal of the American Oil Chemists' Society, 85, 169–184.
Dionísio, A. P., Molina, G., de Carvalho, D. S., Dos Santos, R., Bicas, J. L., & Pastore, G. M. (2012). Natural flavourings from biotechnology for foods and beverages. In Natural food additives, ingredients and flavourings (pp. 231–259). Woodhead Publishing.
Bicas, J. L., Molina, G., Barros, F. F. C., & Pastore, G. M. (2015). Chapter 12: Production of aroma compounds by white biotechnology. In M. A. Coelho & B. D. Ribeiro (Eds.), White biotechnology for sustainable chemistry. Green Chemistry, RSC.
Braga, A., Guerreiro, C., & Belo, I. (2018). Generation of flavors and fragrances through biotransformation and de novo synthesis. Food and Bioprocess Technology, 11(12), 2217–2228.
Coelho, M. A. Z., Amaral, P. F. F., & Belo, I. (2010). Lipolytica: An industrial workhorse. In Current research, technology and education topics in applied microbiology and microbial biotechnology (pp. 930–944).
Braga, A., Gomes, N., & Belo, I. (2012). Lipase induction in Yarrowia lipolytica for castor oil hydrolysis and its effect on Ƴ-decalactone production. Journal of the American Oil Chemists' Society, 89(6), 1041–1047.
Aguedo, M., Ly, M. H., Belo, I., Teixeira, J. A., Belin, J. M., & Wache, Y. (2004). The use of enzymes and microorganisms for the production of aroma compounds from lipids. Food Technology and Biotechnology, 42(4), 327–336.
Braga, A., Mesquita, D. P., Amaral, A. L., Ferreira, E. C., & Belo, I. (2015a). Aroma production by Yarrowia lipolytica in airlift and stirred tank bioreactors: Differences in yeast metabolism and morphology. Biochemical Engineering Journal, 93, 55–56.
Braga, A., Coq, C.-L., Dulermo, R., Nicaud, J. M., & Belo, I. (2015b). Effect of POX genotype and Lip2p over expression on lactone production and reconsumption by Yarrowia lipolytica using castor oil as substarte. Process Biochemistry, 50, 1357–1362.
Guo, T., Kong, J., Zhang, L., Zhang, C., & Hu, S. (2012). Fine tuning of the lactate and diacetyl production through promoter engineering in Lactococcus lactis. PLoS One, 7, e36296. https://doi.org/10.1371/journal.pone.0036296
Guo, Y., Feng, C., Song, H., Wang, Z., Ren, Q., & Wang, R. (2011). Effect of POX3 gene disruption using self-cloning CRF1 cassette in Yarrowia lipolytica on the γ-decalactone production. World Journal of Microbiology and Biotechnology, 27(12), 807–2812.
Peng, B., Xu, J., Cai, Z., Zhang, B., Yu, M., & Ma, R. (2020). Different roles of the five-alcohol acyltransferase in peach fruit aroma development. Journal of the American Society for Horticultural Science, 145(6), 374–381.
Waché, Y., Laroche, C., Bergmark, K., Møller-Andersen, C., Aguedo, M., Le Dall, M. T., Wang, H., Nicaud, J. M., & Belin, J. M. (2000). Involvement of acyl coenzyme A oxidase isozymes in biotransformation of methyl ricinoleate into γ-decalactone by Yarrowia lipolytica. Applied and Environmental Microbiology, 66(3), 1233–1236.
Huang FC, Schwab W (2011) Cloning and characterization of a 9-lipoxygenase gene induced by pathogen attack from Nicotiana benthamiana for biotechnological application. BMC Biotechnology 11(1):1–5.
Schrader, J., Etschmann, M. M. W., Sell, D., Hilmer, J. M., & Rabenhorst, J. (2004). Applied biocatalysis for the synthesis of natural flavour compounds–current industrial processes and future prospects. Biotechnology Letters, 26, 463–472.
Cheetham, P. (2007). Combining the technical push and the business pull for natural flavours. In Biotechnology of aroma compounds. Advances in biochemical engineering/biotechnology (Vol. 55). Springer. https://doi.org/10.1007/BFb0102061
Cardillo, R., Fuganti, C., Sacerdote, G., Barbeni, M., Cabella, P., & Squarcia, F. (1990). Process for the microbiological production of gamma (R) decanolide and gamma (R) octanolide. US Patent 4950607.
Surburg, H., Panten, J., & Bauer, K. (2006). Common fragrance and flavor materials: Preparation, properties and uses. Wiley-VC.
Gocho, S., Rumi, K., & Tsuyoshi, K. (1998). Process for the production of 𝛿-decalactone. US Patent 5763233 A.
Collins, R. P., & Halim, A. F. (1972). Characterization of the major aroma constituent of the fungus Trichoderma viride. Journal of Agricultural and Food Chemistry, 20, 437–438. https://doi.org/10.1021/jf60180a010
Hui, Y. H. (2006). Handbook of food science, technology, and engineering, CRCNET books. Taylor & Francis.
Barth, G. (2013). Yarrowia lipolytica: Biotechnological applications. Springer Science & Business Media.
Mooradi, H., Asadollahi, M. A., & Nahvi, I. (2016). Optimization of gamma-decalactone production by yeast Yarrowia lipolytica using the Taguchi method. Journal of Microbiology, Biotechnology and Food Sciences, 6(1), 685–688.
Waché, Y., Aguedo, M., Choquet, A., Gatfield, I. L., Nicaud, J. M., & Belin, J. M. (2001). Role of β-oxidation enzymes in γ-decalactone production by the yeast Yarrowia lipolytica. Applied and Environmental Microbiology, 67(12), 5700–5704.
Nelson, D. L., & Cox, M. M. (2008). Lehninger principles of biochemistry (5th ed.). Book. https://doi.org/10.2307/1309148
Groguenin, A., Waché, Y., Garcia, E. E., Aguedo, M., Husson, F., LeDall, M. T., Nicaud, J. M., & Belin, J. M. (2004). Genetic engineering of the β-oxidation pathway in the yeast Yarrowia lipolytica to increase the production of aroma compounds. Journal of Molecular Catalysis B: Enzymatic, 28(2–3), 75–79.
Waché, Y., Aguedo, M., LeDall, M. T., Nicaud, J. M., & Belin, J. M. (2002). Optimization of Yarrowia lipolytica’s β-oxidation pathway for γ-decalactone production. Journal of Molecular Catalysis B: Enzymatic, 347–351.
Braga, A., Gomes, N., Teixeira, J. A., & Belo, I. (2013). Impact of lipasemediated hydrolysis of castor oil on Ƴ-decalactone production by Yarrowia lipolytica. Journal of the American Oil Chemists' Society, 90(8), 1131–1137.
Okui, S., Uchiyama, M., & Mizugaki, M. (1963). Metabolism of hydroxy fatty acids: Intermediates of the oxidative breakdown of ricinoleic acid by genus Candida. Journal of Biochemistry, 54, 536–540.
Gatfield, I. L., Güntert, M., Sommer, H., & Werkhoff, P. (1993). Some aspects of the microbiological production of flavour-active lactones with particular reference to c-decalactone. Chem Microbiol Technol Lebensm, 15, 165–170.
Romero-Guido, C., Belo, I., Ta, T. M., Cao-Hoang, L., Alchihab, M., Gomes, N., Thonart, P., Teixeira, J. A., Destain, J., & Wache, Y. (2011). Biochemistry of lactone formation in yeast and fungi and its utilisation for the production of flavour and fragrance compounds. Applied Microbiology and Biotechnology, 89, 535–547.
Swizdor, A., Panek, A., Milecka-Tronina, N., & Kołek, T. (2012). Biotransformations utilizing b-oxidation cycle reactions in the synthesis of natural compounds and medicines. International Journal of Molecular Sciences, 13, 16514–16543.
An, J. U., Joo, Y. C., & Oh, D. K. (2013). New biotransformation process for the production of the fragrance compound γ-dodecalactone from 10- hydroxystearate by permeabilized Waltomyces lipofer cells. Applied and Environmental Microbiology, 79, 2636–2641.
An, J. U., & Oh, D. K. (2013). Increased production of Ƴ-lactones from hydroxy fatty acids by whole Waltomyces lipofer cells induced with oleic acid. Applied Microbiology and Biotechnology, 97(18), 8265–8272.
Alchihab, M., Destain, J., Aguedo, M., Majad, L., Ghalfi, H., Wathelet, J. P., & Thonart, P. (2009). Production of gamma-decalactone by a psychrophilic and a mesophilic strain of the yeast Rhodotorula aurantiaca. Applied Biochemistry and Biotechnology, 158(1), 41–50.
Rabenhorst, J., & Gatfield, I. (2002). Method of producing γ-decalactone using Yarrowia lipolytica strain HR 145 (DSM 12397). United States Patent 6451565.
Carrau, F. M., Medina, K., Farina, L., Boido, E., Henschke, P. A., & Dellacassa, E. (2008). Production of fermentation aroma compounds by Saccharomyces cerevisiae wine yeasts: Effects of yeast assimilable nitrogen on two model strains. FEMS Yeast Research, 8, 1196–1207.
Pagot, Y., Endrezzi, A., Nicaud, J. M., & Belin, J. M. (1997). Utilization of an auxotrophic strain of the yeast Yarrowia lipolytica to improve g-decalactone production yields. Letters in Applied Microbiology, 25, 113–116.
Neto, R. S., Pastore, G. M., & Macedo, G. A. (2004). Biocatalysis and biotransformation producing γ-decalactone. Journal of Food Science, 69(9), C677–C680.
Lanza, E., Ko, K. H., & Palmer, J. K. (1976). Aroma production by cultures of Ceratocystis monliformis. Journal of Agricultural and Food Chemistry, 24, 1247–1250.
Lee, S. L., Lin, S. J., & Chou, C. C. (1995). Growth of and production of γdecalactone by Sporobolomyces odorus in jar fermentors as affected by pH, aeration and fed-batch technique. Journal of Fermentation and Bioengineering, 80(2), 195–199.
Dufossé, L., Feron, G., Mauvais, G., Bonnarme, P., Durand, A., & Spinnler, H. E. (1998). Production of γ-decalactone and 4-hydroxy-decanoic acid in the Genus Sporidiobolus. Journal of Fermentation and Bioengineering, 86(2), 169–173.
Farbood, M. I., Morris, J. A., & Mclean, L. B. (1998). Fermentation process for preparing 10-hydroxy-C18-carboxylic acid and gamma-dodecalactone derivatives. European patent 0578388.
Han, O., & Han, S. R.. (1995). Process for production of C10 and/or C12 gamma-lactones from the corresponding C10 and/or C12 carboxylic acids by means of microbial biotransformation in the presence of mineral oil. US patent 5457036.
Gocho, S., Tabogami, N., Inagaki, M., Kawabata, C., & Komai, T. (1995). Biotransformation of oleic acid to optically active γ-dodecalactone. Bioscience, Biotechnology, and Biochemistry, 59, 1571–1572.
Haffner, T., & Tressl, R. (1996). Biosynthesis of (R)-γ-decanolactone in the yeast Sporobolomyces odorus. Journal of Agricultural and Food Chemistry, 44, 1218–1223.
Pereira de Andrade, D., Carvalho, B. F., Schwan, R. F., & Dias, D. R. (2017). Production of γ-decalactone by yeast strains under different conditions. Food Technology and Biotechnology, 55(2), 225–230.
Rong, S., Yang, S., Li, Q., Cai, B., Guan, S., Wang, J., Zhou, Y., & Chen, Y. (2017). Improvement of γ-decalactone production by stimulating the import of ricinoleic acid and suppressing the degradation of γ-decalactone in Saccharomyces cerevisiae. Biocatalysis and Biotransformation, 35(2), 96–102.
Jo, Y. S., An, J. U., & Oh, D. K. (2014). γ-Dodecelactone production from safflower oil via 10-hydroxy-12 (Z)-octadecenoic acid intermediate by whole cells of Candida boidinii and Stenotrophomonas nitritireducens. Journal of Agricultural and Food Chemistry, 62(28), 6736–6745.
Farbood, M. I., & Willis, B. J. (1983). Production of g-decalactone. WO Patent, 1983001072.
Chalier, P., & Crouzet, J. (1992). Production of lactones by Penicillium roqueforti. Biotechnology Letters, 14, 275–280.
Endrizzi, A., Awad, C. A. C., & Belin, J. M. (1993). Presumptive involvement of methyl ricinoleate betii-oxidation in the production of gammadecalactone by the yeast Pichia guilliermondii. FEMS Microbiology Letters, 114, 153–160.
Skoneczny, M., Cheltowska, A., & Rytka, J. (1988). Study of the coinduction by fatty acids of catalase A and acyl-CoA oxidase in standard and mutant Saccharomyces cerevisiae strains. European Journal of Biochemistry, 174, 297–302.
Mizugaki, M., Uchiyama, M., & Okui, S. (1965). Metabolism of hydroxy fatty acids. V: Metabolic conversion of homoricinoleic and homoricinelaidic acids by Escherichia coli K12. Journal of Biochemistry (Tokyo), 58, 273–278.
Fickers, P., Nicaud, J. M., Gaillardin, C., Destain, J., & Thonart, P. (2004). Carbon and nitrogen sources modulate lipase production in the yeast Yarrowia lipolytica. Journal of Applied Microbiology, 96(4), 742–749.
Deive, F. J., Costas, M., & Longo, M. A. (2003). Production of a thermostable extracellular lipase by Kluyveromyces marxianus. Biotechnology Letters, 25(17), 1403–1406.
Freitas, L., Bueno, T., Perez, V. H., Santos, J. C., & de Castro, H. F. (2007). Enzymatic hydrolysis of soybean oil using lipase from different sources to yield concentrated of polyunsaturated fatty acids. World Journal of Microbiology and Biotechnology, 23(12), 1725–1731.
Obradors, N., Montesinos, J. L., Valero, F., Lafuente, F. J., & Sola, C. (1993). Effects of different fatty acids in lipase production by Candida rugosa. Biotechnology Letters, 15(4), 357–360.
Tan, T., Zhang, M., Wang, B., Ying, C., & Deng, L. (2003). Screening of high lipase producing Candida sp. and production of lipase by fermentation. Process Biochemistry, 39(4), 459–465.
Najjar, A., Robert, S., Guérin, C., Violet-Asther, M., & Carrière, F. (2011). Quantitative study of lipase secretion, extracellular lipolysis, and lipid storage in the yeast Yarrowia lipolytica grown in the presence of olive oil: Analogies with lipolysis in humans. Applied Microbiology and Biotechnology, 89(6), 1947–1962.
Treichel, H., de Oliveira, D., Mazutti, M. A., Di Luccio, M., & Oliveira, J. V. (2010). A review on microbial lipases production. Food and Bioprocess Technology, 3(2), 182–196.
Montesinos, J. L., Obradors, N., Gordillo, M. A., Valero, F., Lafuente, J., & Sola, C. (1996). Effect of nitrogen sources in batch and continuous cultures to lipase production by Candida rugosa. Applied Biochemistry and Biotechnology, 59(1), 25–37.
Almeida, A. F., Taulk-Tomisielo, S. M., & Carmona, E. C. (2012). Influence of carbon and nitrogen sources on lipase production by a newly isolated Candida viswanathii strain. Annales de Microbiologie, 63(4), 1225–1234.
Shirazi, H., Rahman, S. R., & Rahman, M. M. (1998). Short communication: Production of extracellular lipases by Saccharomyces cerevisiae. Journal of Microbiology and Biotechnology, 14(4), 595–597.
Carlile, M., & Watkinson, S. C. (1997). The Fungi. Academic Press.
Corzo, G., & Revah, S. (1999). Production and characteristics of the lipase from Yarrowia lipolytica 681. Bioresource Technology, 70(2), 173–180.
Bussamara, R., Fuentefria, A. M., de Oliveira, E. S., Broetto, L., Simcikova, M., Valente, P., Schrank, A., & Vainstein, M. H. (2010). Isolation of a lipase-secreting yeast for enzyme production in a pilot-plant scale batch fermentation. Bioresource Technology, 101(1), 268–275.
Yadav, K. S., Adsul, M. G., Bastawde, K. B., Jadhav, D. D., Thulasiram, H. V., & Gokhale, D. V. (2011). Differential induction, purification and characterization of cold active lipase from Yarrowia lipolytica NCIM 3639. Bioresource Technology, 102(22), 10663–10670.
Waché, Y., Aguedo, M., Nicaud, J. M., & Belin, J. M. (2003). Catabolism of hydroxyacids and biotechnological production of lactones by Yarrowia lipolytica. Applied Microbiology and Biotechnology, 61(5), 393–404.
Pescheck, M., Mirata, M. A., Brauer, B., Krings, U., Berger, R. G., & Schrader, J. (2009). Improved monoterpene biotransformation with Penicillium sp. by use of a closed gas loop bioreactor. Journal of Industrial Microbiology & Biotechnology, 36, 827–836.
Yilmaztekin, M., Cabaroglu, T., & Erten, H. (2013). Effects of fermentation temperature and aeration on production of natural isoamyl acetate by Williopsis saturnus var. saturnus. BioMed Research International, 1–6.
Barghini, P., Di Gioia, D., Fava, F., & Ruzzi, M. (2007). Vanillin production using metabolically engineered Escherichia coli under non-growing conditions. Microbial Cell Factories, 6, 13–113.
Löser, C., Urit, T., Förster, S., Stukert, A., & Bley, T. (2012). Formation of ethyl acetate by Kluyveromyces marxianus on whey during aerobic batch and chemostat cultivation at iron limitation. Applied Microbiology and Biotechnology, 96, 685–696.
Medeiros, A. B. P., Pandey, A., Christen, P., Fontoura, P. S. G., De Freitas, R. J. S., & Soccol, C. R. (2001). Aroma compounds produced by Kluyveromyces marxianus in solid state fermentation on a packed bed column bioreactor. World Journal of Microbiology and Biotechnology, 17, 767–771.
Tai, Y. N., Xu, M., Ren, J. N., Dong, M., Yang, Z. Y., Pan, S. Y., & Fan, G. (2016). Optimisation of α-terpineol production by limonene biotransformation using Penicillium digitatum DSM 62840. Journal of the Science of Food and Agriculture, 96, 954–961.
Urit, T., Löser, C., Wunderlich, M., & Bley, T. (2011). Formation of ethyl acetate by Kluyveromyces marxianus on whey: Studies of the ester stripping. Bioprocess and Biosystems Engineering, 34, 547–559.
Akacha, N. B., & Gargouri, M. (2015). Microbial and enzymatic technologies used for the production of natural aroma compounds: Synthesis, recovery modeling, and bioprocesses. Food and Bioproducts Processing, 94, 675–706.
Mantzouridou, F. T., Paraskevopoulou, A., & Lalou, S. (2015). Yeast flavour production by solid state fermentation of orange peel waste. Biochemical Engineering Journal, 101, 1–8.
Medeiros, A. B. P., Christen, P., Roussos, S., Gern, J. C., & Soccol, C. R. (2003). Coffee residues as substrates for aroma production by Ceratocystis fimbriata in solid state fermentation. Brazilian Journal of Microbiology, 34, 245–248.
Medeiros, A. B. P., Pandey, A., Freitas, R. J. S., Christen, P., & Soccol, C. R. (2000). Optimization of the production of aroma compounds by Kluyveromyces marxianus in solid-state fermentation using factorial design and response surface methodology. Biochemical Engineering Journal, 6, 33–39.
Medeiros, A. B. P., Pandey, A., Vandenberghe, L. P. S., Pastore, G. M., & Soccol, C. R. (2006). Production and recovery of aroma compounds produced by solid-state fermentation using different adsorbents. Food Technology and Biotechnology, 44, 47–51.
Hölker, U., Höfer, M., & Lenz, J. (2004). Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Applied Microbiology and Biotechnology, 64, 175–186.
Singhania, R. R., Patel, A. K., Soccol, C. R., & Pandey, A. (2009). Recent advances in solid-state fermentation. Biochemical Engineering Journal, 44, 13–18.
Couri, S., Mercês, E. P., Neves, B. C. V., & Senna, L. F. (2006). Digital image processing as a tool to monitor biomass growth in Aspergillus niger 3T5B8 solid-state fermentation: Preliminary results. Journal of Microscopy, 224, 290–297.
Ju, H. Y., Yang, C. K., Yen, Y. H., & Shieh, C. J. (2009). Continuous lipase-catalyzed synthesis of hexyl laurate in a packed-bed reactor: Optimization of the reaction conditions in a solvent-free system. Journal of Chemical Technology and Biotechnology, 84, 29–33.
Gomes, N., Teixeira, J. A., & Belo, I. (2012). Fed-batch versus batch cultures of Yarrowia lipolytica for γ-decalactone production from methyl ricinoleate. Biotechnology Letters, 34, 649–654.
Naz, R., & Bano, A. (2012). Antimicrobial potential of Ricinus communis leaf extracts in different solvents against pathogenic bacterial and fungal strains. Asian Pacific Journal of Tropical Biomedicine, 2(12), 944–947.
Rashmi, Pathak, D. V., & Kumar, R. (2019). Effect of Ricinus communis L on microorganisms: Advantages and disadvantages. International Journal of Current Microbiology and Applied Sciences, 8(4), 878–884.
Hemant, Y., Shrirame, N. L., Panwar, B. R., & Bamniya. (2011). Bio diesel from castor oil—A green energy option. Low Carbon Economy, 2, 1–6.
Rao, M. S., Parvatha Reddy, P., Sukhada, M., & Nagesh, M. P. (1998). Management of root-knot nematode on egg plant by integrating endomycorrhiza (Glomus fasciculatum) and castor (Ricinus communis) cake. Nematologia Mediterranea, 26, 217–219.
Tiyagi, S. A., & Ajaz, S. (2004). Biological control of plant parasitic nematodes associated with chickpea using oil cakes and Paecilomyces lilacinus. Journal of Nematology, 34(1), 44–48.
Boroda, E. (2002). Quantifying ricin in agricultural soils. Texas Tech University.
Mathur, A., Verma, S. K., Yousuf, S., Singh, S. K., Prasad, G., & Dua, V. K. (2011). Antimicrobial potential of roots of Ricinus communis against pathogenic microorganisms. International Journal of Pharma and Bio Sciences, 2, 545–548.
Zartman, R., Green, C., Francisco, M. S., Zak, J., Jaynes, W., & Boroda, E. (2003). Mitigation of ricin contamination in soils: Sorption and degradation. DTIC documents. Retrieved from http://www.dtic.mil/cgibin/GetTRDoc?AD=ADA482765.
Venkateswarlu, B., Hari, K., & Katyal, J. C. (1997). Influence of soil and crop factors on the native rhizobial populations in soils under dryland farming. Applied Soil Ecology, 7(1), 1.
Lord, M. J., Roberts, L. M., & Robertus, J. D. (1994). Ricin: Structure, mode of action, and some current applications. The FASEB Journal, 8, 201–208.
Chen, G. Q., He, X., & McKeon, T. A. (2005). A simple and sensitive assay for distinguishing the expression of ricin and Ricinus communis agglutinin genes in developing castor seed (R. communis L). Journal of Agricultural and Food Chemistry, 53, 2358–2361.
Anandan, S., Kumar, G., Ghosh, J., & Ramachandran, K. (2004). Effect of different physical and chemical treatments on detoxification of ricin in castor cake. Animal Feed Science and Technology, 120, 159–168.
Horton, J., & Williams, M. A. (1989). A cooker-extruder for deallergenation of castor bean meal. Journal of the American Chemical Society, 66, 227–231.
Rao, H. K. (1970). Toxic factors and their detoxification in castor. Journal of Food Science and Technology, 7, 77–82.
Acknowledgments
The authors are grateful to the Directors of CSIR-CIMAP, Lucknow, and IIT Delhi. The authors are thankful to CSIR India for funding the project PME/FTT-FTC/FC2020-23/MLP-09/2020. The author (SS) would like to acknowledge the contribution of FTT project MLP-009 for the funding. The author (NS) is thankful for the DST-INSPIRE fellowship (IF:180344), and the author (SC) is grateful for the UGC WM PDF fellowship (F.15-1/2017/PDFWM-2017-18-DEL-3915 (SA-II) from UGC, India).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Singh, S., Syed, N., Chaturvedi, S., Nannaware, A.D., Rout, P.K., Hung, YT. (2022). Castor Oil: A Promising Source for the Production of Flavor and Fragrance Through Lipase-Mediated Biotransformation. In: Wang, L.K., Wang, MH.S., Hung, YT. (eds) Waste Treatment in the Biotechnology, Agricultural and Food Industries. Handbook of Environmental Engineering, vol 26. Springer, Cham. https://doi.org/10.1007/978-3-031-03591-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-03591-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-03589-0
Online ISBN: 978-3-031-03591-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)