Abstract
Food and nutritional security, agrarian sustainability, biotic and abiotic stress tolerance are the key requirements for sustainable agriculture. To attain these goals and to utilize the existing genetic diversity/allelic variation in a particular crop, huge germplasm collections need to be characterized using genomic approaches along with phenotypic and biochemical evaluation. Finger millet is one such crop loaded with massive nutritional and nutraceutical properties and climate resilience. Earlier characterization of diversity was confined to morphological, cytological, biochemical, and molecular markers including random amplified polymorphic DNA (RAPD), inter-simple sequence repeats (ISSRs); simple sequence repeats (SSRs), etc., but now there is a paradigm shift in the way we characterize and utilize the available genetic diversity in finger millet. Genotyping-by-sequencing (GBS) and genome-wide association study (GWAS) have been used to identify candidate genes and marker-trait associations for grain micronutrient, protein and tryptophan content, blast resistance and agro-morphological traits, etc. With the availability of finger millet whole-genome sequence, resequencing of the selected lines is being done to capture the complete and accurate genetic variation, single-nucleotide polymorphisms (SNPs), insertion/deletions (InDels), etc. Further, associative transcriptomics, genomic selection, pan-genome sequencing, and haplotype-based breeding approaches could be applied to dissect complex traits associated with agronomic performance, stress tolerance, and nutritional aspects in finger millet.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cytological markers
- Genome-wide association studies
- Molecular markers
- Morphological markers
- Next-generation sequencing
- Pan-genome sequencing
4.1 Introduction
Finger millet [(Eleusine coracana (L.) Gaertn.); family: Poaceae; subfamily: Chloridoideae; allotetraploid (2n = 4x = 36, AABB); A genome donor: E. indica; B genome donor: unknown; assembled genome size: 1.2 Gb; genome size based on flow cytometry: 1.5 Gb; self-pollinated] was domesticated from its wild progenitor, E. coracaca subsp. africana, in Ethiopian highlands and western Uganda more than 5,000 years ago (Hilu 1988; Liu et al. 2011, 2014; Hittalmani et al. 2017; Hatakeyama et al. 2018). Finger millet also known as ragi is cultivated in semiarid, arid, tribal, and hilly regions of Africa and India. India is the secondary center of finger millet diversity, where the crop was introduced in 3000 BC in Western Ghats. Finger millet is a crop of immense importance in view of its climate resilience, massive nutritional and nutraceutical properties (high protein; essential amino acids particularly enriched in lysine and methionine; minerals especially high calcium content, vitamins; dietary fiber; phytochemicals; glycoproteins; gluten free; low glycaemic index) (Saleh et al. 2013; Chandra et al. 2016; Kumar et al. 2016a). The crop is also a storehouse of vital genomic resources because of its excellent adaptability to harsh conditions. Large germplasm collections of finger millet are available in genebanks [ICRISAT genebank: ~6000 accessions (http://exploreit.icrisat.org/profile/Small%20millets/187); National Genebank, ICAR-NBPGR, New Delhi, India: ~11,352 accessions (http://www.nbpgr.ernet.in:8080/PGRPortal/(Shtfofsuczs3o44ns55eh2h45))/SimpleSearch.aspx)], which needs to be comprehensively characterized (high throughput) to know the level of phenotypic, genotypic, and nutritional diversity as well as adaptability under difficult conditions for future food and nutritional security, agrarian sustainability, biotic and abiotic stress tolerance. These large germplasm holdings of genebanks are the repositories of genetic variation and novel alleles/traits, which were earlier characterized through morphological markers to know the level of genetic diversity and develop core/minicore sets (Upadhyaya et al. 2006, 2010). These huge collections are now being characterized through molecular markers and next-generation sequencing (NGS) technologies to develop molecular cores, identify candidate genes and marker-trait associations. So, there is a paradigm shift in the way we characterize and utilize the available genetic diversity/allelic variation in a particular crop.
A number of reports from the genetics era are available wherein characterization of diversity was carried out and variation levels and genetic structure were assessed using different genetic markers, including classical markers such as morphological, cytological, and biochemical markers, and DNA/molecular markers including hybridization-based markers such as restriction fragment length polymorphisms (RFLPs) and polymerase chain reaction (PCR)-based markers such as random amplified polymorphic DNA (RAPDs); amplified fragment length polymorphisms (AFLPs); inter-simple sequence repeats (ISSRs); simple sequence repeats (SSRs); sequence-related amplified polymorphism (SRAP); start codon targeted (SCoT), etc.
But in today’s scenario, high throughput, in terms of amount of genetic and genomic data generated and bioinformatic analysis are the backbone of characterization for enhanced utilization of genetic resources. In the era of genomics, information has been generated in finger millet in terms of whole-genome sequence (Hittalmani et al. 2017; Hatakeyama et al. 2018), genome-wide molecular markers/genes discovery, genotyping-by-sequencing (GBS), and genome-wide association study (GWAS), which represents a paradigm shift from genetics to genomics. These genomics approaches need to be augmented by using associative transcriptomics, genomic selection, pan-genome sequencing, etc. to dissect complex traits associated with agronomic performance, stress tolerance, and nutritional aspects and to promote haplotype-based breeding in finger millet.
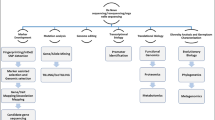
4.2 Genetics to Genomics: Characterization of Diversity in Finger Millet
Characterization of diversity is the foundation of all crop improvement programs. The factors responsible for creating such variation/diversity are genetic drift and/or recombination, selection, migration, and mutation. Evaluation of genetic diversity of plant genetic resources is very crucial due to bourgeoning population, food insecurity, associated risks with narrowing genetic base of the existing cultivars and climate change. Finger millet is one such crop that can fulfill the criteria of nutritional and future food security as well climate resilience and needs to be thoroughly characterized for its enhanced utilization. A number of reports are available wherein classical markers, viz. morphological, cytological and biochemical markers; DNA/molecular markers and next-generation sequencing approaches have been used to characterize diversity in finger millet showing a paradigm shift from genetics to genomics.
Morphological markers though monitored easily, are limited in number, and affected by the environment, hence limiting their usefulness. Similar restrictions are put on to the usage of isozyme markers also (Andersen and Lubberstedt 2003). Among morphological, isozyme (biochemical), and molecular markers, the latter give superior power of detection due to recognition of genotypic differences, genomic abundance, level of polymorphism, and non-influence of environmental factors.
Molecular characterization based on DNA molecular markers can be carried out using Random DNA Markers (RDMs-RAPD, RFLP, AFLP, ISSR, SSR, etc.), gene-targeted markers (GTMs), and functional markers (FMs).
In the genomic era, NGS approaches including whole-genome sequencing (WGS) and resequencing, RNA sequencing, metagenomics, high throughput genotyping, pan-genome sequencing, etc., and bioinformatic analysis tools are available to molecularly characterize the germplasm at the sequence level and to best utilize them for crop improvement. The most common complexity reduction high-throughput genotyping technologies are: restriction-site-associated DNA (RAD), GBS, diversity array technology (DArT) and are being frequently used in diversity analysis.
Characterization of all the germplasm holdings of a particular crop in a genebank using genotyping at the sequence level will answer many questions. Duplicates can be identified to mark the redundant germplasm and to decrease the load on genebanks in terms of usage of different resources (financial, manpower, etc.). Diversity level and population structure of the whole germplasm could be assessed and the molecular cores/subsets representing maximum diversity of the whole set could be designed. These cores can then be easily handled for their multilocation morpho-agronomic performance, biotic and abiotic stress resistance evaluation in the field as well as under artificial conditions. These can also be evaluated for quality traits for adding nutritional value to them. Once the whole germplasm set or molecular cores or subsets are characterized for different traits of economic importance, the genotyping along with phenotypic/biochemical data could be used for GWAS to identify SNPs, gene(s), or quantitative trait loci (QTLs) linked to these traits.
One such report (Bharati 2011) is available wherein the global composite collection of finger millet was characterized molecularly as well as for morpho-agronomic traits and a reference set of 300 most genetically diverse accessions capturing 89.2% of the alleles of the whole collection (959 accessions) was constituted using 20 SSR markers. A total of 11.6 alleles per locus along with 121 common alleles and 110 rare alleles at 1% and a mean gene diversity of 0.560 were reported. Wild spontanea race accessions showed maximum gene diversity of 0.611. A large number of alleles ranging from 10 to 21 were observed using UGEP81, UGEP10, UGEP102, UGEP26, and UGEP77 SSR loci. UGEP3, UGEP5, UGEP31, and UGEP104 SSR loci detected large numbers of multiple alleles. High PIC values of more than 0.636 were reported using UGEP15, UGEP5, UGEP18, UGEP102, UGEP12, and UGEP77 SSRs. Race and region-specific unique alleles were also reported. QTL UGEP56 in LG6 and UGEP8 in LG3 for days to 50% flowering indicated strong marker-trait associations (MTAs). Accessions were identified for high grain yield, early flowering, more fingers, fodder yield, ear head length, basal tiller number, high iron, and zinc content for finger millet improvement program. More such studies involving entire genebank germplasm and NGS-based genotyping along with morpho-agronomic and biochemical characterization are the need of the hour to characterize and utilize finger millet germplasm.
4.3 Morphological Markers
The establishment of genetic and genomic resources is a crucial step for crop improvement for desired traits (Ceasar et al. 2018). With the onset of studies on diversity analysis in crops, morphological markers have emerged as potential markers that are dependent on agromorphological characters. Morphological characteristics could facilitate the determination of the agronomic parameters as well as the taxonomic classification of plant species (Ortiz et al. 2008). These markers also play a key role in the maintenance and management of plant genetic resources (PGR), as well as in the Plant Breeders’ Rights (PBR) system (Babic et al. 2016).
A number of reports are available wherein morphological markers have been used to characterize genetic diversity in finger millet using either a smaller or larger set of germplasm. Upadhyaya et al. (2006) developed a core subset of 622 accessions representing the diversity of the entire collection of finger millet germplasm based on their geographical origin and data on 14 quantitative traits from the global collection of 5,940 accessions (Africa, Asia, America, Europe, and unknown origin) available at the genebank at ICRISAT, Patancheru, India. All the five races and breeding materials, improved cultivars, landraces, and wild types were represented in the core subset. Upadhyaya et al. (2007) characterized 909 finger millet accessions introduced from the genebank ICRISAT, Bulawayo, Zimbabwe, and grown at ICRISAT, Patancheru. Variability was recorded for plant pigmentation and growth characters (time to 50% flowering, plant height) and inflorescence characters (inflorescence width, length, and exsertion). Dominance of green-type plant pigmentation, erect-type growth habit, light brown grain color was observed. Dwarf plant (up to 75 cm) accessions were mostly from Zimbabwe. Early flowering accessions were reported from Kenya and late flowering from Tanzania and Zaire.
Umar and Kwon-Ndung (2014) characterized 10 finger millet accessions collected from diverse locations in northern Nigeria using morphological characters (plant height, leaf length and diameter, finger length and width, number of fingers, and 1000 seed weights). Significant genetic diversity was observed for the traits studied.
Variability for 19 agromorphological characters between 60 exotic and 89 Indian accessions was reported and flag leaf sheath length, peduncle length, panicle exsertion, ear head width, fingers per head, and 1000-grain weight were found more in the Indian accessions (Babu et al. 2017). Significant and positive correlations were observed between days to 50% flowering and days to maturity as well as for peduncle length and panicle exsertion. The genotypes (IE 7320, IE 4491, GE 1437, VHC 3911, and VHC 3898) and (GE 1437, GE 5192, and IE 5367) were identified as better parents for high photosynthetic efficiency and tryptophan content, respectively.
The germplasm from Plant Genetic Resource Center, Gannoruwa, Sri Lanka, was characterized using morphological markers (Dasanayaka 2016; Kaluthanthri and Dasanayaka 2016; Kumari et al. 2018). Kaluthanthri and Dasanayaka (2016) characterized 20 finger millet accessions and on principal component analysis (PCA), characteristics such as days to flowering, finger number, and yield per plant were found as important traits for variability among the studied genotypes. A set of 24 accessions were characterized using 14 quantitative characters (Dasanayaka 2016). Kumari et al. (2018) morphologically characterized 139 accessions (100 local collections from 15 districts of Nepal, 26 accessions from India, Zimbabwe, and unknown exotic origin, 9 from farmer’s fields, 2 standards, and 4 others) using 14 quantitative characters and the highest variability was observed in grain yield, panicle exertion, weight of 20 mature ears, number of productive tillers and length of the longest finger. A significant and positive correlation was observed between grain yield and the number of productive tillers, threshing ratio, weight of 20 mature ears, and panicle exertion. The reported studies could be used for finger millet improvement in Sri Lanka.
Anuradha et al. (2017) evaluated genetic diversity among 25 finger millet genotypes through PCA and cluster analysis. The characteristics, viz., plant height, number of productive tillers, days to 50% flowering, fodder, and grain yield showed significant variability. Genotypes VR 1101, VR 1098, VR 1112, VR 1113, VR 1111, VR 1115, VR 1116, and VR 1117 were found better for different traits could be further utilized for breeding programs. A set of 27 accessions were morphologically evaluated and a high degree of similarity was observed between IC49979A and IC49974B genotypes, whereas IC204141 and IC49985 showed a low level of similarity (Prabhu et al. 2018).
Mohan et al. (2018) evaluated 38 finger millet genotypes (18 released varieties and 20 landraces from India) using morphological markers (13 qualitative and 14 quantitative traits). Among the qualitative traits studied, diversity was observed for ear shape and size, however, most of the quantitative traits showed significant differences among the genotypes.
Kumar et al. (2019) evaluated 92 accessions for 16 qualitative morphological descriptors at G.B. Pant University of Agriculture and Technology, Pantnagar, India. Erect growth, dark green glume, droopy ears, non-pigmented leaf juncture, nonculm stem branching, lodging susceptibility, non-pubescent leaf sheath, non-branched fingers with multiple whorls, in thumb position branched fingers, and seeds with enclosed glume cover, brown color, round shape, rough surface, unpersistent pericarp with shattering nature were the predominant characters.
Morphology, plant growth, and yield contributing characteristics have been evaluated to characterize 20 accessions of finger millet in the Palghar district of Maharashtra (Patil et al. 2019). Characters such as erect growth habit, light brown seed color, partially enclosed seeds by glumes, and semi-compact ear were found dominant among the studied accessions. Productive tiller number followed by ear head length and finger number were the most varied traits and the lowest variation was shown by a finger width.
4.4 Cytological Markers
Cytological markers are based on variation in chromosomal morphology and have been used to discover progenitors or parents to the cultivated finger millet and also to study relatedness between different species. They can also be used in physical mapping and identification of linkage groups, however, their direct use has been very limited.
Chennaveeraiah and Hiremath (1974) concluded the subspecies africana as the direct progenitor of finger millet based on chromosome pairing data and no contribution of E. indica toward finger millet genome on the basis of lack of chromosome pairing. The report from this study was contrary to the recent reports wherein E. indica is considered one of the genome donors of domesticated finger millet. The reason for this disparity may be due to the fact that they did not mention the number of crosses made and extensive cytogenetical studies are required to conclude phylogenetic affinities (Dewey 1982). Further lack of chromosome pairing does not always show a lack of genomic resemblances (De Wet and Harlan 1972).
Hiremath and Salimath (1992) reported E. floccifolia not to be the genome donor to E. coracana and E. multiflora as a distinct species, with genomic symbol “C” based on mean chromosome pairing. Identification of the “B” genome donor to cultivated E. coracana is yet to be identified.
Bisht and Mukai (2000) mapped ribosomal DNA (rDNA) sites of four diploid and two tetraploid species of Eleusine by fluorescence in situ hybridization (FISH) and the similarity of the rDNA sites and their location on chromosomes in the studied species showed that diploid species might be the possible genome donors to tetraploid species. E. multiflora was differentiated from the rest of the species due to the presence of 18S-5.8S-26S rDNA on the largest pair of the chromosomes, 5S rDNA at four sites on two pairs of chromosomes, and 18S-5.8S-26S and 5S rDNA at the same location on one pair of chromosomes. Tetraploid species, namely, E. coracana and E. africana were found to possess the same number of 18S-5.8S-26S and 5S rDNA sites located at a similar position on the chromosomes. Diploid species, E. floccifolia, E. indica, and E. tristachya were found to possess the same 18S-5.8S-26S sites and locations that showed resemblance with the two pairs of 18S-5.8S-26S rDNA locations in tetraploid species, E. africana and E. coracana. The 5S rDNA sites on chromosomes of E. floccifolia and E. indica were found comparable to those of E. africana and E. coracana.
Bisht and Mukai (2001) revealed E. indica and E. floccifolia as genome donor/contributor to E. coracana (an allotetraploid species) on the basis of in situ hybridization of E. coracana genome with the genomic DNA of different diploid species of the same genus. A close genomic relationship was observed between four diploid species, namely, E. floccifolia, E. indica, E. intermedia and E. tristachya, and the tetraploid species E. coracana. Based on the common genomic in situ hybridization (GISH) signals, it was found that E. indica and E. tristachya shared close similarities and E. intermedia as the intermediate species of E. indica and E. floccifolia.
Liu et al. (2014) reported E. indica as the primary A-genome parent and E. tristachya (or its extinct sister or ancestor) as the secondary A-genome donor to finger millet based on multicolor genomic in situ hybridization (McGISH).
4.5 Biochemical Markers
Biochemical markers generally involve the analysis of seed storage proteins and isozymes (enzymes differing in the sequence of amino acids but catalyzing the same biochemical reaction). These markers are based on enzymatic functions and allow the measuring of allele frequencies for specific genes. Studies involving isozymes and seed proteins for characterization of genetic diversity specifically for E. coracana are scanty.
Werth et al. (1994) employed 16 isozyme loci coding nine enzymes to analyze genetic variability among seven Eleusine species. Genetic variability differed considerably among members of diploid species (E. indica and E. jaegeri). Both the subspecies of the tetraploid E. coracana (subsp. coracana and subsp. africana) displayed fixed heterozygosity at several loci. Moreover, both the tetraploids also possessed E. indica marker alleles at all loci, confirming that they were derived from E. indica by hybridization with an unknown diploid.
Chong et al. (2011) studied genetic diversity within and among six glyphosate-resistant (R) and eight glyphosate-susceptible (S) E. indica populations from Peninsular Malaysia using isozyme markers encoding acid phosphatase (Acp), glutamate dehydrogenase (Gdh), glucose-6-phosphate isomerase (Pgi or Gpi), glycerate dehydrogenase (Gly), isocitrate dehydrogenase (Idh), malate dehydrogenase (Mdh) phosphoglucomutase (Pgm), and uridine diphosphogluconate pyrophosphate (Ugp). Genetic variations at 13 enzyme loci from studied enzyme systems were evaluated in a set of 840 accessions. Low levels of isozyme diversity in R and S populations of E. indica were observed with a small percentage of polymorphism and the number of alleles per locus. The results inferred that the populations might possess a background of severe or long-lasting population bottlenecks that have shrunken the genetic diversity.
Kumar et al. (2012) studied seed storage proteins profiles of 52 finger millet genotypes from Uttarakhand. Clear and distinct polypeptide bands (15–25) having molecular weights in the range of 10–100 kDa were observed. No major differences in banding pattern among 52 finger millet genotypes were reported based on sodium dodecyl sulfate polyacrylamide gel electrophoresis. However, an additional band of 32 kDa was detected in a few genotypes that need to be studied further from a nutritional point of view.
4.6 Molecular Markers
On the basis of the method of detection, molecular markers are classified into hybridization-based and PCR-based markers. The molecular markers, gene discovery, and advancement of genomic resources were also reviewed by Sood et al. (2016).
4.6.1 Hybridization-Based Markers
RFLP is the first marker system based on hybridization, which relies on polymorphism as a result of insertion/deletions (known as InDels), point mutations, translocations, duplications, and inversions (Nadeem et al. 2018).
Salimath et al. (1995) analyzed genome origins and genetic diversity in 22 accessions belonging to five species of Eleusine from Africa and Asia using an eight probes-three enzyme RFLP combination, revealing 14% polymorphism (low level of sequence variability) in 17 accessions of E. coracana from Asia and Africa. Along with RFLP, RAPD and ISSR patterns were also studied based on which three species including E. coracana, E. indica, and E. tristachya showed a close genetic assemblage within the genus, whereas E. floccifolia and E. compressa were found most divergent.
Muza et al. (1995) classified 26 finger millet lines belonging to Africa and India into cytotype groups on the basis of the Southern blot hybridization patterns obtained with maize and sorghum mitochondrial cloned gene probes. Five restriction endonuclease enzymes were used, giving a total of 20 enzyme/probe combinations. A low level of polymorphism was detected with RFLP banding patterns. However, the data based on mitochondrial DNA clone atp9 hybridization allowed the classification of the lines into three cytotype groups.
Parani et al. (2001) studied 119 accessions belonging to 7 small millet species using the chloroplast trnS-psbC gene regions to generate PCR–RFLP with 8 restriction enzymes individually as well as in combinations of two enzymes. A combination of two enzymes distinguished all the species. Species-specific differential banding patterns were observed.
4.6.2 PCR-Based Markers
PCR allows amplification of the region of DNA, targeted by the regions of high homology with the primers. PCR-based markers can be categorized as: (i) arbitrary primer-based markers, and (ii) sequence-based markers. As per the published reports, RAPD and SSRs have been the markers of choice for genetic diversity and population structure analyses in finger millet, and to some extent, ISSR and SNP makers have also been used for diversity studies. Very few reports are available on genomic SSR marker development (Dida et al. 2007; Gimode et al. 2016; Hittalmani et al. 2017; Lee et al. 2017) in finger millet. Large-scale genomic SSR marker (18,514) development based on finger millet genome sequence was reported by Hittalmani et al. (2017) and 35 SSRs were validated in 26 E. coracana and 14 wild species accessions, the markers identified can be used for diversity and marker-trait association studies to hasten marker-assisted breeding programs in finger millet. Functional/gene-based markers such as expressed sequence tag-simple sequence repeats (EST-SSRs)/transcriptome sequencing-based markers, nucleotide-binding site-leucine rich repeat (NBS-LRR) based markers, cytochrome P450 gene-based markers, Aspartate kinase2 gene based SSRs, calcium transporters and calmodulin based anchored-SSRs, drought stress related genic SSRs, SRAP, SCoT have also been developed and used for molecular characterization of finger millet (Arya et al. 2009; Panwar et al. 2010a, b; Panwar et al. 2011; Naga et al. 2012; Nirgude et al. 2014; Kumar et al. 2015a; Saha et al. 2017; Panda et al. 2020). The different markers employed to study genetic diversity and population structure in finger millet are detailed in Table 4.1.
4.7 Prospects of Molecular Markers in the Post-genomics Era
4.7.1 Genetic Diversity and Population Structure Analysis
Genetic diversity and population structure studies based on RFLP, RAPD, ISSR, and SSR markers, etc. have already been discussed above. Here we will focus on the use of GBS in genotyping followed by genetic diversity and population structure analysis. The technique is highly cost-effective, robust, and was developed by Elshire et al. (2011). GBS is an NGS-based approach employed for discovering and genotyping SNPs in crop genomes and populations (He et al. 2014). SNP markers generated through GBS possess outstanding genetic attributes such as high reproducibility, wide genome coverage, codominant mode of inheritance, and chromosome-specific location, and thus extensive genotyping followed by the selection of diverse parents/alleles for breeding programs is achievable. GBS has been employed in finger millet for diversity and population structure analysis for extensive characterization at the sequence level.
Kumar et al. (2016b) studied 113 accessions from Africa, India, Nepal, Maldives, and Germany through GBS and generated 33 GB of data (160 million raw reads). Genome-wide set of 23,000 SNPs segregating across the entire collection and several thousand SNPs segregating within each accession were observed. Based on phylogenetic analysis and model-based STRUCTURE program three groups/subpopulations: Subpopulation 1 (southern Asia (India, Nepal, and the Maldives), followed by eastern Africa, Europe, unknown origin); Subpopulation 2 (southern Asia, followed by eastern Africa, western Africa); Subpopulation 3 (southern Asia, followed by eastern Africa, unknown origin) consistent with geographical distribution with some exceptions were inferred. The results also confirmed the hypothesis of African domestication of finger millet followed by its introduction to India.
Nyongesa et al. (2018) genotyped 95 genotypes from Kenya, India, Uganda, Malawi, Zambia, Zimbabwe, Nigeria, Nepal, and Germany using GBS. The genotypes were divided into three subpopulations (A, B, and C) and all three showed an admixture of alleles based on 117,542 SNPs. Cluster B comprised of genotypes showing high resistance to Striga, clusters A and C contained the most susceptible genotypes. Existing genetic variation can be used for marker-assisted breeding for Striga resistance. The highly diverse nature of the composite collection was revealed based on racial and regional diversity. Structure analysis closely corresponded with the phylogenetic analysis.
In another study by Puranik et al. (2020), 190 genotypes of Asian (India, Nepal, Pakistan, Sri Lanka, and the Maldives); east African (Burundi, Ethiopia, Kenya, Tanzania, and Uganda); south African (Malawi, Zambia, and Zimbabwe) and European or American origin were characterized by GBS (169,365 SNPs including 16,000 putative SNPs in the stringent and 73,419 putative SNPs in the relaxed parameter). The less diverse genetic background between the East and South African accessions indicated their common evolutionary lineage and evolution from the same natural population. Three subpopulations: Subpopulation 1 (East African origin); Subpopulation 3 (the South African origin) and Subpopulation 2 belonging to Asian origin (India, Nepal, Pakistan, Sri Lanka, and the Maldives) were identified. European or American origin genotypes were not clustered with any particular group. This kind of clear geographic distinctions was also reported based on random markers systems.
4.7.2 Phylogenetic Relationships
Chloroplast DNA sequence analysis is a reliable tool for concluding phylogenetic relationships in polyploid species as compared to cytogenetic studies. Hilu (1988) revealed E. indica as the maternal genome donor of finger millet based on chloroplast DNA sequence analysis as chloroplast and its genome are predominantly maternally inherited.
Hilu (1995) used RAPD markers and reported the close genetic affinity of E. tristachya to the E. coracana-E. indica group and the distinctness of E. multiflora. A loose correlation between geographic distribution and pattern of genetic variability was observed. The allotetraploid nature of the finger millet was also confirmed.
In another study by Neves et al. (2005), E. indica was suggested as the A-genome (maternal) donor of E. coracana based on nuclear ITS and plastid trnT-trnF sequences. And also reported that E. floccifolia is not the second genome donor and the B genome donor is unidentified or extinct.
Liu et al. (2011) reported E. indica-E. tristachya clade as possible A-genome progenitors to E. coracana based on a biparentally inherited nuclear Pepc4 gene tree and a maternally inherited plastid 6-gene tree.
Liu et al. (2014) suggested a single allotetraploid origin for the E. africana-E. coracana subclade based on the low-copy nuclear gene (waxy). E. indica and E. tristachya were found as the A-genome donors, with a differential degree of relatedness to E. coracana.
Zhang et al. (2019) also concluded E. indica as the maternal parent of E. coracana and E. africana group, based on transcriptome analysis of E. multiflora, E. floccifolia, E. tristachya, E. intermedia, E. africana, E. coracana, and E. indica. The study also supported a close relationship between E. indica and E. tristachya. The close relationship between E. multiflora and E. floccifolia was unexpected since they have distinct nuclear genomes, CC and BB, respectively.
4.7.3 Generation of Linkage Maps
The genetic maps provide important information for genetic analysis and crop improvement. Large numbers of highly variable markers are required to generate maps useful for trait analysis and eventually plant breeding. The first linkage map of finger millet based on 332 loci and F2 population developed by crossing between Okhale-1 (a cultivated accession) and MD-20 (a wild accession), was generated by Dida et al. (2007). This group used RFLP, AFLP, EST, and SSR markers to generate the map of the tetraploid finger millet. The map spans A genome: 721 cM and B genome: 787 cM and covers almost all 18 finger millet chromosomes. The map was also used for a comparative study between rice and finger millet genomes.
The first high-density genetic map (4,453 SNP markers/18 linkage groups) of finger millet was developed by Qi et al. (2018) using the same population as discussed above. Paired-end GBS reads (278,880,767) and a new pipeline (UGbS-Flex) was used to generate the map. This pipeline can be used in species having different breeding systems, ploidy, and polymorphism levels, and even in the absence of a reference genome sequence.
4.7.4 Genetic Purity Testing of Hybrids
RAPD, ISSR, and SSR markers were used to find polymorphism between two parental lines, viz. PR 202 and IE 2606 of finger millet (Ajeesh Krishna et al. 2020). Twelve RAPD, 4 ISSR, and 21 SSR markers showed polymorphism and were used for genetic purity testing of the F1 hybrids. Molecular markers were found useful in the identification of true hybrids and comparing the efficacy of hybridization methods. Hot-water-based emasculation was found much better compared to the hand-emasculation method of hybridization in finger millet.
4.7.5 Whole-Genome Sequencing and High-Throughput Genotyping by Resequencing
A paradigm shift from marker-based to genomics-based high-throughput sequencing approaches is generating sequencing data by several orders of magnitude with the advantage of dense marker coverage, less time requirement, and cost-effectiveness. Availability of whole-genome sequence in a particular crop is a boon since it provides a reference for resequencing of genotypes constituting core/minicore/trait-specific reference sets/mapping populations, etc. in a particular crop. This will deliver extensive and quality information at the sequence level for estimation of diversity, marker-trait associations, identification of candidate genes, taxonomic and evolutionary studies, high mapping accuracy and resolution, comparative mapping, etc. The data generated will further aid in functional genomics, forward and reverse genetics, and proteomic studies.
Developments of NGS tools have progressed the WGS and transcriptome sequencing in several crop species (Ceasar et al. 2018). The trend and timeline of genome sequencing in millets have been summarized in Fig. 4.1. Presently there are 2 reports available on WGS in finger millet, 1 using ML 365 yielded 1,196 Mb (~82% of the total estimated genome size) and predicted 85,243 genes and 1,14,083 SSRs using Illumina and SOLiD platforms (Hittalmani et al. 2017). Another group, Hatakeyama et al. (2018) reported 1.2 Gb as against 1.5 Gb genome size estimated based on flow cytometry and 62,348 genes in PR 202 based on NGS combined with single-molecule sequencing and by whole-genome optical mapping with Bionano Irys® system.
This whole-genome sequence data in finger millet has opened up the door for resequencing and utilization of huge variability available in genebanks. Resequencing data of 88 finger millet genotypes accomplished by GoK-funded Finger Millet Genomics Project is available at NCBI. This is being used for the development of superior finger millet varieties for Karnataka using Integrated genomics-assisted breeding approaches. But the whole germplasm of finger millet available in national genebanks needs to be characterized by NGS-based genotyping followed by the development of molecular cores/minicores and then these cores can be resequenced along with phenotypic and biochemical characterization for genome-wide marker-trait associations studies and identification of candidate genes.
4.7.6 Trait Mapping
Finger millet germplasm collections available with national and international genebanks are the repertoire of useful variations important for crop improvement. Previous studies on finger millet germplasm/core sets have identified genotypes for desirable traits such as high grain mineral content (Fe, Zn, and Ca content), protein content, resistance to biotic and abiotic stresses, etc. (Upadhyaya et al. 2011; Babu et al. 2013; Krishnamurthy et al. 2014). However, the true potential of finger millet germplasm collections has not been realized due to the lack of information about genes/alleles associated with these traits. In plants, the linkage mapping approach has been widely used to identify QTL(s)/gene(s), however, this method was not much successful in millets due to the difficulty involved in the generation of mapping populations. Recent advancements in genomics particularly the availability of high throughput genotyping technologies (GBS and resequencing), have made it possible to map diverse traits using a complementary gene mapping technique, association mapping (AM). AM approach exploits linkage disequilibrium at adjacent loci in diverse sets of genotypes, mostly natural populations to establish the correlation between genotype and phenotype (Gupta et al. 2014). This approach is highly efficient for genetic dissection of complex traits and has been used widely for mapping traits in a wide range of crops and would also be useful in the case of finger millet. There are different variants of association mapping, which include candidate gene association mapping, genome-wide association mapping, and associative transcriptomics. Potential applications of these approaches in finger millet are described below.
4.7.6.1 Candidate Gene-Based Association Mapping
Candidate gene association approach involves sequencing of candidate genes from diverse genotypes in order to discover variants which are then tested for their association with the target trait using statistical models. The application of this approach has allowed the identification of allelic variants for many useful traits in many crops including rice (Mishra et al. 2016; Abbai et al. 2019), wheat (Sukumaran et al. 2015; Ma et al. 2016), maize (Cook et al. 2012), soybean (Ikram et al. 2020). The approach can be very well applied in finger millet to identify novel allelic variants of candidate genes associated with various desirable traits. Since, some studies in finger millet have already identified some putative candidate genes for economically important traits including grain protein, micronutrient, tryptophan content, and blast resistance (Babu et al. 2014b, c; Puranik et al. 2020; Tiwari et al. 2020), candidate association mapping studies can be conducted for these genes using genebank collections/core sets. For nutritional traits, the important genes that can be potentially targeted for association analysis work are: gene encoding for no apical meristem-associated (NAM) protein, a member of the NAC family associated with iron content (Puranik et al. 2020), gene encoding for aspartyl protease exhibiting strong association with grain protein content (GPC) (Tiwari et al. 2020) and genes involved in lysine and tryptophane biosynthesis (Babu et al. 2014b). Similarly, candidate gene association analysis can also be attempted for orthologues of NBS-LRR family blast resistance genes of rice such as PiKh and Pita as the pathogen Magnaporthae grisea causes blast disease of rice and finger millet (Babu et al. 2014d). Besides, the above-described traits, drought tolerance is another desirable trait in finger millet as it is mainly grown as a rainfed crop. Candidate gene association mapping can play important role in the identification of novel alleles for abiotic stress tolerance genes. To date, a host of abiotic tolerance genes and transcription factors (DREB, MYB, NAC) have been identified in other cereals such as rice, wheat, foxtail millet, etc. (Lata et al. 2011). Orthologues of these genes can be annotated from the finger millet genome and targeted for candidate gene-based association mapping. Importantly, there is a need to identify more number of functionally validated candidate genes in order to unravel superior alleles/haplotypes for various traits from germplasm collections using candidate gene based association mapping approach.
4.7.6.2 Genome-Wide Association Study
Genome-wide association study (GWAS) is a very powerful technique and does not require mapping populations’ generation step which is a laborious, time-consuming, and technically demanding exercise. Moreover, since GWAS uses a diverse set of lines that may capture many historical recombinant events, linkage disequilibrium (LD) mapping can enable mapping resolution up to 100–200 Kb as compared to 10–20 cM in the case of biparental mapping, and sometimes it may even identify causative genes associated with the target trait. GWAS has been widely used in many plant species including, arabidopsis (Togninalli et al. 2018), rice (Lekklar et al. 2019), wheat (Chaurasia et al. 2020; Kumar et al. 2020), foxtail millet (Jaiswal et al. 2019), soybean (Fang et al. 2017), maize (Mazaheri et al. 2019), pearl millet (Srivastava et al. 2019), etc. for identification of genomic regions/genes associated with economically important traits. Recently, with the availability of finger millet genome sequence and high throughput genotyping methods, it is possible to conduct large-scale genome-wide association studies in this crop for diverse traits. As of now, GWAS studies in finger millet have been reported for various traits such as agro-morphological characteristics, blast resistance, nutritional traits, and low phosphorus tolerance (Babu et al. 2014a, b, c; Kumar et al. 2015b; Ramakrishnan et al. 2016b, 2017; Tiwari et al. 2020). Babu et al. (2014a) conducted AM on a panel of 190 diverse lines and identified markers associated with agromorphological traits including flag leaf width, plant height, basal tiller number, and 50% flowering. Grain protein and tryptophan content associated genomic regions have also been identified using GWAS (Babu et al. 2014b; Tiwari et al. 2020). Moreover, integration of AM with comparative mapping has proved very effective in the identification of genomic region for tryptophan and grain protein content. Using this approach, Babu et al. (2014b) have identified 2 QTLs for tryptophan and 1 QTL for grain protein content (GPC) in a global collection of finger millet. Of the 2 QTLs controlling tryptophan, 1 was major, explained 11% of phenotypic variance and was found associated with SSR marker OM5 designed from 27-kD γ-zein gene of OPM (opaque-2 modifiers). It was a very significant finding as OPM influences tryptophan content to a large extent (Babu et al. 2014b). GWAS has also been conducted for mapping of blast disease which is caused by Magnaporthe grisea and is considered to be one of the major limiting factors in finger millet production. A total of four QTLs for finger blast and one QTL for neck blast have been mapped in global finger millet collection using SSR markers (Babu et al. 2014c). The marker FMBLEST32 and RM262 explained 8% and 10% of the phenotypic variance, respectively, for blast resistance. UGEP81 and UGEP18 were associated with finger and neck blast and explained 7.5% and 11% of phenotypic variance, respectively. The above-described studies indicate the great potential of GWAS in the genetic dissection of important traits in finger millet. GWAS was also done to find the association of SNPs with Striga reaction based on field Striga resistance and GBS using 95 finger millet genotypes and markers TP 85,424 and TP 88,244 were identified for Striga resistance (Nyongesa et al. 2018). In another study, 14 agromorphological traits were mapped in a panel of 113 finger millet accessions using GBS-derived SNP markers and three different GWAS models, viz., SLST, MLMM, and MTMM. A total of 109 novel associations were identified for 14 different agomorphological traits. Further, among these 109 novel MTAs, 9 were common across 3 different models (Sharma et al. 2018). Recently, Tiwari et al. (2020) applied association mapping on 113 diverse finger millet lines to dissect complex genetic regulation of GPC and uncovered 5 reliable genomic regions for GPC. Out of these five regions, one contained gene encoding for aspartyl protease, which was considered a major promising candidate gene contributing to variation in GPC content. In a recent study (Puranik et al. 2020), the application of a combination of GBS and GWAS on a panel of 190 finger millet genotypes revealed genomic regions underlying putative candidate genes associated with grain micronutrient content (iron, zinc, calcium, magnesium, potassium, and sodium). A total of 34 highly reliable MTAs were identified, out of which 18 markers showed homology with the candidate genes and suggested to have putative functions in remobilization, binding, and transport of metals.
4.7.6.3 Associative Transcriptomics
Associative transcriptomics is a variant of GWAS which involves the analysis of transcripts across association panels to discover genomic regions controlling complex traits (Harper et al. 2012). This approach has allowed the identification of both transcript-level sequence variation (SNPs/InDels) and changes in the expression (Gene expression markers; GEM) that could be associated with diverse traits. Initially established in Brassica, this approach has also been used in other species such as wheat (Miller et al. 2016; Wang et al. 2017) maize (Azodi et al. 2020), and chestnut (Kang et al. 2019) for mapping complex traits. Indeed, associative transcriptomics has opened a way to identify expression-level variations in genomic regions/genes critical for the development of important traits. In the case of finger millet, this approach can be used to identify differentially expressed genomic regions associated with variations in grain mineral nutrients such as Ca, Fe, Zn, etc. as for these traits it is the changes in the expression of individual genes that could have more role than sequence-level variation (Kumar et al. 2016a).
4.7.6.4 Genomic Selection
The main factors limiting the utilization of large finger millet germplasm resources conserved in genebanks are the requirement of huge financial resources and technical expertise to identify potential lines for various desirable traits. In recent years, with the availability of cost-effective high throughput genotyping methods, genomic selection (GS) holds great promise for the selection of superior germplasm lines from the gene bank collections. (Muleta et al. 2017). The GS approach involves estimation of genomic estimated breeding values (GEBV) of traits in a reference population using genome-wide markers and phenotypic data, and subsequently, these GEBVs are used to predict the performance of respective traits in a related genotype sets exclusively based on the genomic data. Since the GS approach does away with multilocation/multiyear evaluation of germplasm required for trait identification, it can facilitate early selection of useful germplasm from genebank collections and accelerate genetic gain in the breeding program. In the beginning, the GS approach was mainly considered for the selection of lines in the breeding population, however, recently a few studies have reported the potential of GS in the identification of desirable traits in a germplasm collection of crops such as soybean, wheat, rice, etc. (Muleta et al. 2017; Kehel et al. 2020). In soybean, a GS model for white mold disease developed using a reference population of a diverse set of lines, could reliably identify white mold resistance genotypes from the United States Department of Agriculture (USDA) soybean germplasm collection using their genotyping data. In the case of finger millet, GS has not been reported so far as there was very limited genomic resources information available in this crop. However, with the availability of finger millet genome sequence and advanced genotyping methods, this approach can be potentially considered for selecting potential lines for yield contributing traits, blast resistance, and rich in micronutrient contents from genebank collection. The development of GS prediction models in finger millet would require constituting diverse finger millet germplasm lines with variations for various traits. The panel can be densely genotyped and phenotyped for various traits under various environments and the GEBV is calculated. The GEBV values for traits are used to select useful lines from the genebanks exclusively based on genomic information.
4.8 Concluding Remark and Future Prospects
4.8.1 Pan-Genome Sequencing: Constitution of Pan and Super Pan Genomes of Finger Millet and Its Wild Relatives
The actual assessment of the extent and pattern of genetic diversity in finger millet germplasm collection is critical for its conservation and efficient utilization in the breeding program. However, so far, the genetic diversity studies in finger millet have used traditional PCR-based markers and a limited number of genotypes (Vetriventhan et al. 2020), which have failed to provide a comprehensive picture of the actual genomic diversity in genepool of finger millet at the level of total genes. Recently, with a decrease in sequencing costs, NGS-based genotyping approaches such as GBS/resequencing are becoming popular for characterizing genetic diversity in crop genebank collections (Milner et al. 2019). However, both these approaches have some limitations. The GBS, approach which involves only partial sequencing of individual genomes, may not be able to capture genomic diversity/sequence variation at a large portion of the genome across the studied set of genotypes/genebank collection. Similarly, in the resequencing approach, SNP/InDel variants are identified by aligning sequencing reads of each accession against the single reference genome so the genomic regions/genes that are present in one or some individuals but absent in the reference genome cannot be analyzed/compared using this approach. Therefore, currently, the emphasis is on pan-genome sequencing in which individuals of the targeted species are sequenced, de novo assembled, and compared to unravel the total genes present in the genepool of that species (Bayer et al. 2020). The pan-genome represents total genomic diversity available in a species collection and is comprised of a core set of genes that are present in all the individuals as well as variable genes which are absent in some individuals. The first pan genome in plants was constituted using seven wild individuals, which revealed a variable number of genes for seed composition, organ size and biomass, flowering and maturity time genes, etc. in soybean (Li et al. 2014). Thereafter pan genomes have been constituted in many plant species including rice (Stein et al. 2018), sunflower (Hübner et al. 2019), and sesame (Yu et al. 2019). Likewise, pan-genome sequence of finger millet collections could be constituted that would unravel novel genes/alleles for different traits and accelerate their utilization in breeding programs. Pan-genome sequencing can enable the comparison of genomes of ancestral and cultivated species and tracking of gene frequency during the domestication process and breeding.
4.8.2 Haplotype-Based Breeding
Recently, haplotype-based breeding is emerging as a potential strategy for designing tailor-made crops. The approach involves mining of superior haplotypes of genes controlling agronomically useful traits from germplasm collection and their deployment in the best combinations for developing high-yielding superior varieties (Sinha et al. 2020). Some studies in this direction have already been initiated in rice and pigeon pea. In the coming years, resequencing of finger millet germplasm resources/core set is expected to unravel allelic diversity and thus would aid in harnessing genetic diversity. This would facilitate the identification of genotypes with novel superior alleles for agronomical and yield traits that could in turn be used in finger millet improvement. For finger millet, low productivity is one of the major factors limiting its wide-scale cultivation, haplotype-based breeding may enable mobilization of superior alleles and would pave the way for the development of tailor-made finger millet varieties.
The developments like GWAS, genome sequencing, transcriptome analysis, trait mapping, etc. have ensued in finger millet but not to the extent as required (Fig. 4.2). Enhanced usage of genomic tools and approaches is the need of the hour to efficiently utilize the huge amount of diversity available in the genebanks for food and nutritional security, biotic and abiotic stress resilience, and overall sustainability. Further genomic selection, associative transcriptomics, pan-genome sequencing, and haplotype-based breeding approaches should be employed to accelerate finger millet crop improvement.
References
Abbai R, Singh VK, Nachimuthu VV, Sinha P, Selvaraj R, Vipparla AK, Singh AK, Singh UM, Varshney RK, Kumar A (2019) Haplotype analysis of key genes governing grain yield and quality traits across 3K RG panel reveals scope for the development of tailor-made rice with enhanced genetic gains. Plant Biotechnol J 17(8):1612–1622. https://doi.org/10.1111/pbi.13087
Ajeesh Krishna TP, Maharajan T, Victor Roch G, Ramakrishnan M, Antony Ceasar S, Ignacimuthu S (2020) Hybridization and hybrid detection through molecular markers in finger millet [Eleusine coracana (L.) Gaertn.]. J Crop Impr 34(3):335–355. https://doi.org/10.1080/15427528.2019.1709596
Andersen JR, Lubberstedt T (2003) Functional markers in plants. Trends Plant Sci 8:554–560. https://doi.org/10.1016/j.tplants.2003.09.010
Anuradha N, Patro TSSK, Divya M, Sandhya Rani Y, Triveni U (2017) Morphological diversity analysis in finger millet [Eleusine coracana (L.) Gaertn.]. Front Crop Impr 5(1):67–70
Arya L, Solanki IS, Verma M, Seetharam A (2016) Population structure and genetic variation in Indian and African Eleusine coracana (L.) Gaertn. Indian J Plant Genet Res 29(2):114–120. https://doi.org/10.5958/0976-1926.2016.00016.4
Arya L, Verma M, Gupta VK, Karihaloo JL (2009) Development of EST-SSRs in finger millet (Eleusine coracana ssp coracana) and their transferability to pearl millet (Pennisetum glaucum). J Plant Biochem Biot 18:97–100. https://doi.org/10.1007/BF03263303
Arya L, Verma M, Gupta VK, Seetharam A (2013) Use of genomic and genic SSR markers for assessing genetic diversity and population structure in Indian and African finger millet (Eleusine coracana (L.) Gaertn.) germplasm. Plant Syst Evol 299:1395–1401. https://doi.org/10.1007/s00606-013-0822-x
Azodi CB, Pardo J, VanBuren R, de los Campos G, Shiu SH (2020) Transcriptome-based prediction of complex traits in maize. Plant Cell 32(1):139–151. https://doi.org/10.1105/tpc.19.00332
Babic V, Ana Nikolic A, Andjelkovic V, Kovacevic D, Filipovic M, Vasic V, Mladenovic-Drinic S (2016) UPOV morphological versus molecular markers for maize inbred lines variability determination. Chil J Agric Res 76(4):417–426. https://doi.org/10.4067/S0718-58392016000400004
Babu BK, Agrawal PK, Pandey D, Jaiswal JP, Kumar A (2014a) Association mapping of agromorphological characters among the global collection of finger millet genotypes using genomic SSR markers. Mol Biol Rep 41(8):5287–5297. https://doi.org/10.1007/s11033-014-3400-6
Babu BK, Agrawal PK, Pandey D, Kumar A (2014b) Comparative genomics and association mapping approaches for opaque2 modifier genes in finger millet accessions using genic, genomic and candidate gene-based simple sequence repeat markers. Mol Breed 34:1261–1279. https://doi.org/10.1007/s11032-014-0115-2
Babu BK, Dinesh P, Agrawal PK, Sood S, Chandrashekara C, Bhatt JC, Kumar A (2014c) Comparative genomics and association mapping approaches for blast resistant genes in finger millet using SSRs. PLoS ONE 9(6):e99182. https://doi.org/10.1371/journal.pone.0099182
Babu BK, Pandey D, Agrawal PK, Sood S, Kumar A (2014d) In-silico mining, type and frequency analysis of genic microsatellites of finger millet (Eleusine coracana (L.) Gaertn.): a comparative genomic analysis of NBS-LRR regions of finger millet with rice. Mol Biol Rep 41:3081–3090. https://doi.org/10.1007/s11033-014-3168-8
Babu BK, Senthil N, Gomez SM, Biji KR, Rajendraprasad NS, Satheesh Kumar S, Babu RC (2007) Assessment of genetic diversity among finger millet (Eleusine coracana (L.) Gaertn.) accessions using molecular markers. Genet Resour Crop Evol 54:399–404. https://doi.org/10.1007/s10722-006-0002-8
Babu BK, Sood S, Agrawal PK, Chandrashekara C, Kumar A, Kumar A (2017) Molecular and phenotypic characterization of 149 finger millet accessions using microsatellite and agro-morphological markers. Proc Natl Acad Sci, India Sect B Biol Sci 87:1217–1228. https://doi.org/10.1007/s40011-015-0695-6
Babu TK, Thakur RP, Upadhyaya HD, Reddy PN, Sharma R, Girish AG, Sarma NDRK (2013) Resistance to blast (Magnaporthe grisea) in a mini-core collection of finger millet germplasm. Eur J Plant Pathol 135(2):299–311. https://doi.org/10.1007/s10658-012-0086-2
Bayer PE, Golicz AA, Scheben A, Batley J, Edwards D (2020) Plant pangenomes are the new reference. Nat Plant 6:914–920. https://doi.org/10.1038/s41477-020-0733-0
Bezaweletaw K (2011) Genetic diversity of finger millet [Eleusine coracana (L.) Gaertn.] landraces characterized by random amplified polymorphic DNA analysis. Innov Syst Des Eng 2(4):207–218
Bharathi A (2011) Phenotypic and genotypic diversity of global finger millet (Eleusine coracana (L.) Gaertn.) composite collection. PhD thesis, Tamil Nadu Agricultural University, Coimbatore, India
Bisht MS, Mukai Y (2000) Mapping of rDNA on the chromosomes of Eleusine species by fluorescence in situ hybridization. Genes Genet Syst 75(6):343–348. https://doi.org/10.1266/ggs.75.343
Bisht MS, Mukai Y (2001) Genomic in situ hybridization identifies genome donor of finger millet (Eleusine coracana). Theor Appl Genet 102:825–832. https://doi.org/10.1007/s001220000497
Brhane H, Haileselassie T, Tesfaye K (2017) Genetic diversity and population structure of Ethiopian finger millet (Eleusine coracana (L.) Gaertn.) genotypes using inter simple sequence repeat (ISSR) markers. Afr J Biotechnol 16(21):1203–1209. https://doi.org/10.5897/AJB2016.15262
Ceasar SA, Maharajan T, Ajeesh Krishna TP, Ramakrishnan M, Victor Roch G, Satish L, Ignacimuthu S (2018). Finger millet [Eleusine coracana (L.) Gaertn.] improvement: current status and future interventions of whole genome sequence. Front Plant Sci 9:1054. https://doi.org/10.3389/fpls.2018.01054
Chandra D, Chandra S, Arora P, Sharma AK (2016) Review of finger millet (Eleusine coracana (L.) Gaertn.): a power house of health benefiting nutrients. Food Sci Hum Wellness 5:149–155. https://doi.org/10.1016/j.fshw.2016.05.004
Chaurasia S, Singh AK, Songachan LS, Sharma AD, Bhardwaj R, Singh K (2020) Multi-locus genome-wide association studies reveal novel genomic regions associated with vegetative stage salt tolerance in bread wheat (Triticum aestivum L.). Genomics 112(6):4608–4621. https://doi.org/10.1016/j.ygeno.2020.08.006
Chennaveeraiah MS, Hiremath SC (1974) Genome analysis of Eleusine coracana (L.) Gaertn. Euphytica 23:489–495. https://doi.org/10.1007/BF00022469
Chong JL, Wickneswari R, Ismail BS, Salmijah S (2011) Genetic diversity of glyphosate-resistant and glyphosate-susceptible Eleusine indica (L.) Gaertn. (Poaceae) populations from Peninsular Malaysia. Malays Appl Biol 40(2):27–36
Cook JP, McMullen MD, Holland JB, Tian F, Bradbury P, Ross-Ibarra J, Buckler ES, Flint-Garcia SA (2012) Genetic architecture of maize kernel composition in the nested association mapping and inbred association panels. Plant Physiol 158(2):824–834. https://doi.org/10.1104/pp.111.185033
Das S, Mishra RC, Rout GR, Aparajita S (2007) Genetic variability and relationships among thirty genotypes of finger millet (Eleusine coracana L. Gaertn.) using RAPD markers. Z Naturforsch 62:116–122. https://doi.org/10.1515/znc-2007-1-220
Das S, Misra RC, Routi GR, Pattanaik MC, Aparajita S (2009) Relationship of status of polymorphic RAPD bands with genotypic adaptation in early finger millet genotypes. Afr Crop Sci J 17:61–69. https://doi.org/10.4314/acsj.v17i2.54199
Dasanayaka PN (2016) Characterization of some ex situ conserved finger millet (Eleusine coracana (L.)) germplasm accessions in Sri Lanka. Intl J Multidiscip Stud 3(2):141–150. https://doi.org/10.4038/ijms.v3i2.16
De Wet JMJ, Harlan JR (1972) Chromosome pairing and phylogenetic affinities. Taxon 21:67–70. https://doi.org/10.2307/1219224
Dewey DR (1982) Genomic and phylogenetic relationships among North American perennial Triticeae. In: Est JR, Tyrl RJ, Brunken JN (eds) Grasses and grasslands. University of Oklahoma Press, Norman, pp 55–58
Dida MM, Srinivasachary RS, Bennetzen JL, Gale MD, Devos KM (2007) The genetic map of finger millet, Eleusine coracana. Theor Appl Genet 114(2):321–332. https://doi.org/10.1007/s00122-006-0435-7
Dida MM, Wanyera N, Harrison Dunn ML, Bennetzen JL, Devos KM (2008) Population structure and diversity in finger millet (Eleusine coracana) germplasm. Trop Plant Biol 1:131–141. https://doi.org/10.1007/s12042-008-9012-3
Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, Mitchell SE (2011) A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 6:e19379. https://doi.org/10.1371/journal.pone.0019379
Fakrudin B, Kulkarni RS, Shashidhar HE, Hittalmani S (2004) Genetic diversity assessment of finger millet, Eleusine coracana (Gaertn.), germplasm through RAPD analysis. PGR Newsl 138:50–54
Fang C, Ma Y, Wu S, Liu Z, Wang Z, Yang R, Hu G, Zhou Z, Yu H, Zhang M, Pan Y et al (2017) Genome-wide association studies dissect the genetic networks underlying agronomical traits in soybean. Genom Biol 18(1):1–14. https://doi.org/10.1186/s13059-017-1289-9
Gimode D, Odeny DA, de Villiers EP, Wanyonyi S, Dida MM, Mneney EE, Muchugi A, Machuka J, de Villiers SM (2016) Identification of SNP and SSR markers in finger millet using next generation sequencing technologies. PLoS ONE 11(7):e0159437. https://doi.org/10.1371/journal.pone.0159437
Gupta PK, Kulwal PL, Jaiswal V (2014) Association mapping in crop plants: opportunities and challenges. Adv Genet 85:109–147. https://doi.org/10.1016/B978-0-12-800271-1.00002-0
Harper AL, Trick M, Higgins J, Fraser F, Clissold L, Wells R, Hattori C, Werner P, Bancroft I (2012) Associative transcriptomics of traits in the polyploid crop species Brassica napus. Nat Biotechnol 30(8):798–802. https://doi.org/10.1038/nbt.2302
Hatakeyama M, Aluri S, Balachadran MT, Sivarajan SR, Patrignani A, Grüter S, Poveda L, Shimizu-Inatsugi R, Baeten J, Francoijs KJ, Nataraja KN, Reddy Y, Phadnis S, Ravikumar RL, Schlapbach R, Sreeman SM, Shimizu KK (2018) Multiple hybrid de novo genome assembly of finger millet, an orphan allotetraploid crop. DNA Res 25(1):39–47. https://doi.org/10.1093/dnares/dsx036
He J, Zhao X, Laroche A, Lu Z, Liu H, Li Z (2014) Genotyping-by-sequencing (GBS), an ultimate marker-assisted selection (MAS) tool to accelerate plant breeding. Front Plant Sci 5:484. https://doi.org/10.3389/fpls.2014.00484
Hilu KW (1988) Identification of the “A” genome of finger millet using chloroplast DNA. Genetics 118:163–167
Hilu KW (1995) Evolution of finger millet: evidence from random amplified polymorphic DNA. Genome 38:232–238. https://doi.org/10.1139/g95-028
Hiremath SC, Salimath SS (1992) The ‘A’ genome donor of Eleusine coracana (L.) Gaertn. (Gramineae). Theor Appl Genet 84:747–754. https://doi.org/10.1007/BF00224180
Hittalmani S, Mahesh HB, Shirke MD, Biradar H, Uday G, Aruna YR, Lohithaswa HC, Mohanrao A (2017) Genome and transcriptome sequence of finger millet (Eleusine coracana (L.) Gaertn.) provides insights into drought tolerance and nutraceutical properties. BMC Genomics 18:465. https://doi.org/10.1186/s12864-017-3850-z
Hübner S, Bercovich N, Todesco M, Mandel JR, Odenheimer J, Ziegler E, Lee JS, Baute GJ, Owens GL, Grassa CJ, Ebert DP et al (2019) Sunflower pangenome analysis shows that hybridization altered gene content and disease resistance. Nat Plant 5(1):54–62. https://doi.org/10.1038/s41477-018-0329-0
Ikram M, Han X, Zuo JF, Song J, Han CY, Zhang YW, Zhang YM (2020) Identification of QTNs and their candidate genes for 100-seed weight in soybean (Glycine max L.) using multi-locus genome-wide association studies. Genes 11(7):714. https://doi.org/10.3390/genes11070714
Jaiswal V, Bandyopadhyay T, Gahlaut V, Gupta S, Dhaka A, Ramchiary N, Prasad M (2019) Genome-wide association study (GWAS) delineates genomic loci for ten nutritional elements in foxtail millet (Setaria italica L.). J Cereal Sci 85:48–55. https://doi.org/10.1016/j.jcs.2018.11.006
Joshi BK, Joshi D, Ghimire SK (2020) Genetic diversity in finger millet landraces revealed by RAPD and SSR markers. Nepal J Biotechnol 8(1):1–11. https://doi.org/10.3126/njb.v8i1.30204
Kaluthanthri DVS, Dasanayaka PN (2016) Assessment of genetic diversity of some finger millet (Eleusine coracana (L.) Gaertn. accessions using morphological markers. J Trop Forest Environ 6(2):25–35. https://doi.org/10.31357/jtfe.v6i2.2940
Kang MJ, Shin AY, Shin Y, Lee SA, Lee HR, Kim TD, Choi M, Koo N, Kim YM, Kyeong D, Subramaniyam S, Park EJ (2019) Identification of transcriptome-wide, nut weight-associated SNPs in Castanea crenata. Sci Rep 9:13161. https://doi.org/10.1038/s41598-019-49618-8
Kehel Z, Sanchez-Garcia M, El Baouchi A, Aberkane H, Tsivelikas A, Charles C, Amri A (2020) Predictive characterization for seed morphometric traits for genebank accessions using genomic selection. Front Ecol Evol 8:32. https://doi.org/10.3389/fevo.2020.00032
Krishnamurthy L, Upadhyaya HD, Purushothaman R, Gowda CLL, Kashiwagi J, Dwivedi SL, Singh S, Vadez V (2014) The extent of variation in salinity tolerance of the minicore collection of finger millet (Eleusine coracana L. Gaertn.) germplasm. Plant Sci 227:51–59. https://doi.org/10.1016/j.plantsci.2014.07.001
Kumar A, Chawla HS, Jeena AS, Rohit (2019) Morphological characterization of finger millet germplasm collected from Uttarakhand hills for qualitative traits. Intl J Curr Microbiol App Sci 8(9):2105–2109. https://doi.org/10.20546/ijcmas.2019.809.243
Kumar A, Gaur VS, Goel A, Gupta AK (2015a) De novo assembly and characterization of developing spikes transcriptome of finger millet (Eleusine coracana): a minor crop having nutraceutical properties. Plant Mol Biol Rep 33:905–922. https://doi.org/10.1007/s11105-014-0802-5
Kumar A, Metwal M, Kaur S, Gupta AK, Puranik S, Singh S, Singh M, Gupta S, Babu BK, Sood S, Yadav R (2016a) Nutraceutical value of finger millet [Eleusine coracana (L.) Gaertn.], and their improvement using omics approaches. Front Plant Sci 7:934. https://doi.org/10.3389/fpls.2016.00934
Kumar A, Sharma D, Tiwari A, Jaiswal JP, Singh NK, Sood S (2016b) Genotyping-by-sequencing analysis for determining population structure of finger millet germplasm of diverse origins. Plant Genom 9(2). https://doi.org/10.3835/plantgenome2015.07.0058
Kumar A, Sharma N, Panwar P, Gupta AK (2012) Use of SSR, RAPD markers and protein profile based analysis to differentiate Eleusine coracana genotypes differing in their protein content. Mol Biol Rep 39:4949–4960. https://doi.org/10.1007/s11033-011-1291-3
Kumar A, Yadav S, Panwar P, Gaur VS, Sood S (2015b) Identification of anchored simple sequence repeat markers associated with calcium content in finger millet (Eleusine coracana). Proc Natl Acad Sci India Sect B Biol Sci 85(1):311–317. https://doi.org/10.1007/s40011-013-0296-1
Kumar S, Kumari J, Bhusal N, Pradhan AK, Budhlakoti N, Mishra DC, Chauhan D, Kumar S, Singh AK, Reynolds M, Singh GP, Singh K, Sareen S (2020) Genome-wide association study reveals genomic regions associated with ten agronomical traits in wheat under late-sown conditions. Front Plant Sci 11:549743. https://doi.org/10.3389/fpls.2020.549743
Kumari K, Pande A (2010) Study of genetic diversity in finger millet (Eleusine coracana L. Gaertn) using RAPD markers. Afr J Biotechnol 9(29):4542–4549
Kumari WMR, Pushpakumara, DKNG, Weerakoon WMW, Senanayake DMJB, Upadhyaya HD (2018) Morphological characterization of local and introduced finger millet (Eleusine coracana (L.) Gaertn.) germplasm in Sri Lanka. Trop Agric Res 29(2):167–183. https://doi.org/10.4038/tar.v29i2.8287
Lata C, Bhutty S, Bahadur RP, Majee M, Prasad M (2011) Association of a SNP in a novel DREB2-like gene SiDREB2 with stress tolerance in foxtail millet [Setaria italica (L.)]. J Exp Bot 62(10):3387–3401. https://doi.org/10.1093/jxb/err016
Lee KJ, Yoon MS, Shin MJ, Lee JR, Cho YH, Lee HS, Ma KH, Lee GA (2017) Development of SSR markers and their use in studying genetic diversity and population of finger millet (Eleusine coracana L. Gaertn.). Plant Breed Biotechnol 5(3):183–191. https://doi.org/10.9787/PBB.2017.5.3.183
Lekklar C, Pongpanich M, Suriya-Arunroj D, Chinpongpanich A, Tsai H, Comai L, Chadchawan S, Buaboocha T (2019) Genome-wide association study for salinity tolerance at the flowering stage in a panel of rice accessions from Thailand. BMC Genomics 20(1):76. https://doi.org/10.1186/s12864-018-5317-2
Li YH, Zhou G, Ma J, Jiang W, Jin LG, Zhang Z, Guo Y, Zhang J, Sui Y, Zheng L, Zhang SS et al (2014) De novo assembly of soybean wild relatives for pangenome analysis of diversity and agronomic traits. Nat Biotechnol 32(10):1045–1052. https://doi.org/10.1038/nbt.2979
Liu Q, Jiang B, Wen J, Peterson PM (2014) Low-copy nuclear gene and McGISH resolves polyploid history of Eleusine coracana and morphological character evolution in Eleusine. Turk J Bot 38:1–12. https://doi.org/10.3906/bot-1305-12
Liu Q, Triplett JK, Wen J, Peterson PM (2011) Allotetraploid origin and divergence in Eleusine (Chloridoideae, Poaceae): evidence from low-copy nuclear gene phylogenies and a plastid gene chronogram. Ann Bot London 108:1287–1298. https://doi.org/10.1093/aob/mcr231
Lule D, de Villiers S, Fetene M, Odeny DA, Rathore A, Das RR, Tesfaye K (2018) Genetic diversity and association mapping of Ethiopian and exotic finger millet accessions. Crop Pasture Sci 69(9):879–891. https://doi.org/10.1071/CP18175
Ma L, Li T, Hao C, Wang Y, Chen X, Zhang X (2016) TaGS5-3A, a grain size gene selected during wheat improvement for larger kernel and yield. Plant Biotechnol J 14(5):1269–1280. https://doi.org/10.1111/pbi.12492
Manjappa, Deshpande S, Rangaiah S, Gowda MVC (2018) Assessment of molecular diversity in an elite set of finger millet (Eleusine coracana (L.) Gaertn.) genotypes using SSR markers. Elect J Plant Breed 9(2):564–576. https://doi.org/10.5958/0975-928X.2018.00069.8
Manyasa EO, Tongoona P, Shanahan P, Mgonja MA, de Villiers S (2015) Genetic diversity in East African finger millet (Eleusine coracana (L.) Gaertn.) landraces based on SSR markers and some qualitative traits. Plant Genet Resou Characteriz Utiliz 13(1):45–55. https://doi.org/10.1017/S1479262114000628
Mazaheri M, Heckwolf M, Vaillancourt B, Gage JL, Burdo B, Heckwolf S, Barry K, Lipzen A, Ribeiro CB, Kono TJ, Kaeppler HF (2019) Genome-wide association analysis of stalk biomass and anatomical traits in maize. BMC Plant Biol 19(1):1–17. https://doi.org/10.1186/s12870-019-1653-x
Miller CN, Harper AL, Trick M, Werner P, Waldron K, Bancroft I (2016) Elucidation of the genetic basis of variation for stem strength characteristics in bread wheat by associative transcriptomics. BMC Genomics 17:500. https://doi.org/10.1186/s12864-016-2775-2
Milner SG, Jost M, Taketa S, Mazón ER, Himmelbach A, Oppermann M, Weise S, Knüpffer H, Basterrechea M, König P, Schüler D et al (2019) Genebank genomics highlights the diversity of a global barley collection. Nat Genet 51(2):319–326. https://doi.org/10.1038/s41588-018-0266-x
Mishra S, Singh B, Panda K, Singh BP, Singh N, Misra P, Rai V, Singh NK (2016) Association of SNP haplotypes of HKT family genes with salt tolerance in Indian wild rice germplasm. Rice 9(1):15. https://doi.org/10.1186/s12284-016-0083-8
Mohan H, Arya L, Verma M, Bisht IS, Saha D, Dhillon BS (2018) Morpho-molecular variation in Indian finger millet (Eleusine coracana (L.) Gaertn.) varieties and landraces. Indian J Plant Genet Resour 31(3):276–285. https://doi.org/10.5958/0976-1926.2018.00032.3
Muleta KT, Rouse MN, Rynearson S, Chen X, Buta BG, Pumphrey MO (2017) Characterization of molecular diversity and genome-wide mapping of loci associated with resistance to stripe rust and stem rust in Ethiopian bread wheat accessions. BMC Plant Biol 17:134. https://doi.org/10.1186/s12870-017-1082-7
Mundada PS, Umdale SD, Nikam TD, Ahire ML (2019) Genetic diversity using RAPD markers, mineral composition and their correlation in selected local landraces of finger millet [Eleusine coracana (L.) Gaertn.]. Vegetos 32:1–10. https://doi.org/10.1007/s42535-019-00001-y
Muza FR, Lee DJ, Andrews DJ, Gupta SC (1995) Mitochondrial DNA variation in finger millet (Eleusine coracana L. Gaertn). Euphytica 81:199–205. https://doi.org/10.1007/BF00025434
Nadeem MA, Nawaz MA, Shahid MQ, Doğan Y, Comertpay G, Yıldız M, Hatipoğlu R, Ahmad F, Alsaleh A, Labhane N, Özkan H, Chung G, Baloch FS (2018) DNA molecular markers in plant breeding: current status and recent advancements in genomic selection and genome editing. Biotechnol Biotechnol Equip 32:261–285. https://doi.org/10.1080/13102818.2017.1400401
Naga BLRI, Mangamoori LN, Subramanyam S (2012) Identification and characterization of EST-SSRs in finger millet (Eleusine coracana (L.) Gaertn.). J Crop Sci Biotechnol 15(1):9–16. https://doi.org/10.1007/s12892-011-0064-9
Neves SS, Swire-Clark G, Hilu KW, Baird WV (2005) Phylogeny of Eleusine (Poaceae: Chloridoideae) based on nuclear ITS and plastid trnT–trnF sequences. Mol Phylogenet Evol 35:395–419. https://doi.org/10.1016/j.ympev.2004.12.005
Nirgude M, Babu BK, Shambhavi Y, Singh UM, Upadhyaya HD, Kumar A (2014) Development and molecular characterization of genic molecular markers for grain protein and calcium content in finger millet (Eleusine coracana (L.) Gaertn.). Mol Biol Rep 41(3):1189–1200. https://doi.org/10.1007/s11033-013-2825-7
Nyongesa SP, Simiyu WD, Chrispus O, Achieng OD, George DO (2018) Molecular characterization of global finger millet (Eleusine coracana, L. Gaertn.) germplasm reaction to Striga in Kenya. Asian J Biochem Genet Mol Biol 1(2):1–14. https://doi.org/10.9734/ajbgmb/2018/v1i2493
Ortiz R, Crossa J, Franco J, Sevilla R, Burgueño J (2008) Classification of Peruvian highland maize races with plant traits. Genet Resour Crop Evol 55(1):151–162. https://doi.org/10.1007/s10722-007-9224-7
Panda D, Sailaja NH, Behera PK, Lenka K, Sharma SS, Lenka SK (2020) Genetic diversity of under-utilized indigenous finger millet genotypes from Koraput, India for crop improvement. J Plant Biochem Biot 30:99–116. https://doi.org/10.1007/s13562-020-00557-w
Pandian S, Satish L, Rameshkumar R, Muthuramalingam P, Rency AS, Rathinapriya P, Ramesh M (2018a) Analysis of population structure and genetic diversity in an exotic germplasm collection of Eleusine coracana (L.) Gaertn. using genicSSR markers. Gene 653:80–90. https://doi.org/10.1016/j.gene.2018.02.018
Pandian S, Marichelvam K, Satish L, Ceasar SA, Pandian SK, Ramesh M (2018b) SPAR markers-assisted assessment of genetic diversity and population structure in finger millet (Eleusine Coracana (L.) Gaertn.) mini-core collection. J Crop Sci Biotechnol 21:469–481. https://doi.org/10.1007/s12892-018-0034-0
Panwar P, Jha AK, Pandey PK, Gupta AK, Kumar A (2011) Functional markers based molecular characterization and cloning of resistance gene analogs encoding NBS-LRR disease resistance proteins in finger millet (Eleusine coracana). Mol Biol Rep 38:3427–3436. https://doi.org/10.1007/s11033-010-0452-0
Panwar P, Nath M, Yadav VK, Kumar A (2010a) Comparative evaluation of genetic diversity using RAPD, SSR and cytochrome P450 gene based markers with respect to calcium content in finger millet (Eleusine coracana L. Gaertn.). J Genet 89:121–133. https://doi.org/10.1007/s12041-010-0052-8
Panwar P, Saini RK, Sharma N, Yadav D, kumar A, (2010b) Efficiency of RAPD, SSR and Cytochrome P450 gene based markers in accessing genetic variability amongst finger millet (Eleusine coracana) accessions. Mol Biol Rep 37:4075–4082. https://doi.org/10.1007/s11033-010-0067-5
Parani M, Rajesh K, Lakshmi M, Parducci L, Szmidt AE, Parida A (2001) Species identification in seven small millet species using polymerase chain reaction–restriction fragment length polymorphism of trnS-psbC gene region. Genome 44:495–499
Paterson A, Bowers J, Bruggmann R, Dubchak I, Grimwood I, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A et al (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457:551–556. https://doi.org/10.1038/nature07723
Patil S, Kauthale V, Aagale S, Pawar M, Nalawade A (2019) Evaluation of finger millet [Eleusine coracana (L.) Gaertn.] accessions using agro-morphological characters. Indian J Agric Res 53(5):624–627. https://doi.org/10.18805/IJARe.A-5239
Prabhu KS, Das AB, Dikshit N (2018) Assessment of genetic diversity in ragi [Eleusine coracana (L.) Gaertn.] using morphological, RAPD and SSR markers. Z Naturforsch C J Biosci 73(5–6):165–176. https://doi.org/10.1515/znc-2017-0182
Puranik S, Sahu PP, Beynon S, Srivastava RK, Sehgal D, Ojulong H, Yadav R (2020) Genome-wide association mapping and comparative genomics identifies genomic regions governing grain nutritional traits in finger millet (Eleusine coracana L. Gaertn.). Plant Peop Plan 2:649–662. https://doi.org/10.1002/ppp3.10120
Qi P, Gimode D, Saha D, Schröder S, Chakraborty D, Wang X, Dida MM, Malmberg RL, Devos KM (2018) UGbS-Flex, a novel bioinformatics pipeline for imputation-free SNP discovery in polyploids without a reference genome: finger millet as a case study. BMC Plant Biol 18:117. https://doi.org/10.1186/s12870-018-1316-3
Rajendran HAD, Muthusamy R, Stanislaus AC, Krishnaraj T, Kuppusamy S, Ignacimuthu S, Al-Dhabi NA (2016) Analysis of molecular variance and population structure in southern Indian finger millet genotypes using three different molecular markers. J Crop Sci Biotechnol 19:275–283. https://doi.org/10.1007/s12892-016-0015-6
Ramakrishnan M, Antony Ceasar S, Duraipandiyan V, Al-Dhabi NA, Ignacimuthu S (2016a) Assessment of genetic diversity, population structure and relationships in Indian and non-Indian genotypes of finger millet (Eleusine coracana (L.) Gaertn.) using genomic SSR markers. Springerplus 5:120. https://doi.org/10.1186/s40064-015-1626-y
Ramakrishnan M, Antony Ceasar S, Duraipandiyan V, Al-Dhabi NA, Ignacimuthu S (2015) Using molecular markers to assess the genetic diversity and population structure of finger millet (Eleusine coracana (L.) Gaertn.) from various geographical regions. Genet Resour Crop Evol 63:361–376. https://doi.org/10.1007/s10722-015-0255-1
Ramakrishnan M, Antony Ceasar S, Duraipandiyan V, Vinod KK, Kalpana K, Al-Dhabi NA, Ignacimuthu S (2016b) Tracing QTLs for leaf blast resistance and agronomic performance of finger millet (Eleusine coracana (L.) Gaertn.) genotypes through association mapping and in silico comparative genomics analyses. PLoS One 11(7):e0159264. https://doi.org/10.1371/journal.pone.0159264
Ramakrishnan M, Antony Ceasar S, Vinod KK, Duraipandiyan V, Ajeesh Krishna TP, Upadhyaya HD, Al-Dhabi NA, Ignacimuthu S (2017) Identification of putative QTLs for seedling stage phosphorus starvation response in finger millet (Eleusine coracana L. Gaertn.) by association mapping and cross species synteny analysis. PloS One 12(8):e0183261. https://doi.org/10.1371/journal.pone.0183261
Saha D, Rana RS, Arya L, Verma M, Gowda MVC, Upadhyaya HD (2017) Genetic polymorphisms among and between blast disease resistant and susceptible finger millet, Eleusine coracana (L.) Gaertn. Plant Genet Resour 15(4):355–365. https://doi.org/10.1017/S1479262116000010
Saleh AS, Zhang Q, Chen J, Shen Q (2013) Millet grains: nutritional quality, processing, and potential health benefits. Comp Rev Food Sci Food Safety 12:281–295. https://doi.org/10.1111/1541-4337.12012
Salimath SS, de Oliveira AC, Godwin ID, Bennetzen JL (1995) Assessment of genome origins and genetic diversity in the genus Eleusine with DNA markers. Genome 38(4):757–763. https://doi.org/10.1139/g95-096
Sharma D, Tiwari A, Sood S, Jamra G, Singh NK, Meher PK, Kumar A (2018) Genome wide association mapping of agro-morphological traits among a diverse collection of finger millet (Eleusine coracana L.) genotypes using SNP markers. PLoS One 13(8):e0199444. https://doi.org/10.1371/journal.pone.0199444
Sinha P, Singh VK, Saxena RK, Khan AW, Abbai R, Chitikineni A, Desai A, Molla J, Upadhyaya HD, Kumar A, Varshney RK (2020) Superior haplotypes for haplotype-based breeding for drought tolerance in pigeonpea (Cajanus cajan L.). Plant Biotechnol J 18:2482–2490. https://doi.org/10.1111/pbi.13422
Srivastava RK, Singh RB, Srikanth B, Satyavathi CT, Yadav R, Gupta R (2019) Genome-wide association studies (GWAS) and genomic selection (GS) in pearl millet: advances and prospects. Front Genet 10:1389. https://doi.org/10.3389/fgene.2019.01389
Stein JC, Yu Y, Copetti D, Zwickl DJ, Zhang L, Zhang C, Chougule K, Gao D, Iwata A, Goicoechea JL, Wei S (2018) Genomes of 13 domesticated and wild rice relatives highlight genetic conservation, turnover and innovation across the genus Oryza. Nat Genet 50(2):285–296. https://doi.org/10.1038/s41588-018-0040-0
Sood S, Kumar A, Kalyana Babu B, Gaur VS, Pandey D, Kant L, Pattnayak A (2016) Gene discovery and advances in finger millet [Eleusine coracana (L.) Gaertn.] genomics—an important nutri-cereal of future. Front Plant Sci 7:1634
Sukumaran S, Dreisigacker S, Lopes M, Chavez P, Reynolds MP (2015) Genome-wide association study for grain yield and related traits in an elite spring wheat population grown in temperate irrigated environments. Theor Appl Genet 128(2):353–363. https://doi.org/10.1007/s00122-014-2435-3
Tiwari A, Sharma D, Sood S, Jaiswal JP, Pachauri SP, Ramteke PW, Kumar A (2020) Genome-wide association mapping for seed protein content in finger millet (Eleusine coracana) global collection through genotyping by sequencing. J Cereal Sci 91:102888. https://doi.org/10.1016/j.jcs.2019.102888
Togninalli M, Seren Ü, Meng D, Fitz J, Nordborg M, Weigel D, Borgwardt K, Korte A, Grimm DG (2018) The AraGWAS catalog: a curated and standardized Arabidopsis thaliana GWAS catalog. Nucl Acids Res 46(D1):D1150–D1156. https://doi.org/10.1093/nar/gkx954
Umar ID, Kwon-Ndung EH (2014) Assessment of variability of finger millet (Eleusine coracana (L) Gaertn.) landraces germplasm in Northern Nigeria. Niger J Genet 28(2):48–51. https://doi.org/10.1016/j.nigjg.2015.09.005
Upadhyaya HD, Gowda CLL, Gopal Reddy V (2007) Morphological diversity in finger millet germplasm introduced from Southern and Eastern Africa. SAT eJournal 3(1), online
Upadhyaya HD, Gowda CLL, Pundir RPS, Reddy VG, Singh S (2006) Development of core subset of finger millet germplasm using geographical origin and data on 14 quantitative traits. Genet Resour Crop Evol 53:679–685. https://doi.org/10.1007/s10722-004-3228-3
Upadhyaya HD, Ramesh S, Sharma S, Singh SK, Varshney SK, Sarma NDRK, Ravishankar CR, Narasimhudu V, Reddy VG, Sahrawat KL, Dhanalakshmi TN, Mgonja MA, Parzies HK, Gowda CLL, Singh S (2011) Genetic diversity for grain nutrients contents in a core collection of finger millet (Eleusine coracana (L.) Gaertn.) germplasm. Field Crop Res 121(1):42–52. https://doi.org/10.1016/j.fcr.2010.11.017
Upadhyaya HD, Sarma NDRK, Ravishankar CR, Albrecht T, Narasimhudu Y, Singh SK, Varshney SK, Reddy VG, Singh S, Dwivedi SL, Wanyera N, Oduori COA, Mgonja MA, Kisandu DB, Parzies HK, Gowda CLL (2010) Developing a minicore collection in finger millet using multilocation data. Crop Sci 50:1924–1931
Varshney R, Shi C, Thudi M, Mariac C, Wallace J, Qi P, Zhang H, Zhao Y, Wang X, Rathore A et al (2017) Pearl millet genome sequence provides a resource to improve agronomic traits in arid environments. Nat Biotechnol 35:969–976. https://doi.org/10.1038/nbt.3943
Vetriventhan M, Azevedo VCR, Upadhyaya HD, Nirmalakumari A, Kane-Potaka J, Anitha S, Antony Ceaser S, Muthamilarasan M, Bhat BV, Hariprasanna K, Bellundagi A, Cheruku D, Backiyalakshmi C, Santra D, Vanniarajan C, Tonapi VA (2020) Genetic and genomic resources, and breeding for accelerating improvement of small millets: current status and future interventions. Nucleus 63:217–239. https://doi.org/10.1007/s13237-020-00322-3
Wang Y, Yu H, Tian C, Sajjad M, Gao C, Tong Y, Wang X, Jiao Y (2017) Transcriptome association identifies regulators of wheat spike architecture. Plant Physiol 175:746–757. https://doi.org/10.1104/pp.17.00694
Werth CR, Hilu KW, Langner CA (1994) Isozyme of Eleusine (Gramineae) and the origin of finger millet. Amer J Bot 81:1186–1197. https://doi.org/10.2307/2445481
Yu J, Golicz AA, Lu K, Dossa K, Zhang Y, Chen J, Wang L, You J, Fan D, Edwards D, Zhang X (2019) Insight into the evolution and functional characteristics of the pangenome assembly from sesame landraces and modern cultivars. Plant Biotechnol J 17(5):881–892. https://doi.org/10.1111/pbi.13022
Zhang G, Liu X, Quan Z, Cheng S, Xu X, Pan S, Xie M, Zeng P, Yue Z, Wang W et al (2012) Genome sequence of foxtail millet (Setaria italica) provides insights into grass evolution and biofuel potential. Nat Biotechnol 30:549–554. https://doi.org/10.1038/nbt.2195
Zhang H, Hall N, Goertzen LR, Chen CY, Peatman E, Patel J, McElroy JS (2019) Transcriptome analysis reveals unique relationships among Eleusine species and heritage of Eleusine coracana. G3 Gene Genome Genet 9:2029–2036. https://doi.org/10.1534/g3.119.400214
Zou C, Li L, Miki D, Li D, Tang Q, Xiao L, Rajput S, Deng P, Peng L, Jia W et al (2019) The genome of broomcorn millet. Nat Commun 10:436. https://doi.org/10.1038/s41467-019-08409-5
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Arya, L., Singh, M., Singh, A.K., Verma, M. (2022). Paradigm Shift from Genetics to Genomics: Characterization of Diversity and Prospects of Molecular Markers. In: Kumar, A., Sood, S., Babu, B.K., Gupta, S.M., Rao, B.D. (eds) The Finger Millet Genome. Compendium of Plant Genomes. Springer, Cham. https://doi.org/10.1007/978-3-031-00868-9_4
Download citation
DOI: https://doi.org/10.1007/978-3-031-00868-9_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-00867-2
Online ISBN: 978-3-031-00868-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)