Abstract
Fifty-two genotypes of Eleusine coracana collected from Uttarakhand hills were subjected to simple sequence repeat (SSR), random amplified polymorphic DNA (RAPD)-PCR and protein profiling analysis to investigate the variation in protein content. The main objective of the present study was to detect variability among E. coracana and also assess the discriminating ability of these three molecular methods. A total of 21 RAPD and 24 SSR primers were assayed for their specificity in detecting genetic variability in E. coracana, of which 20 RAPD and 21 SSR primers were highly reproducible and were found suitable for use in PCR analysis. Assessing genetic diversity among E. coracana genotypes by RAPD-PCR using 20 polymorphic primers yielded 56 different RAPD markers which clustered the genotypes into different groups on the basis of protein content. Similarly, SSR-PCR with 21 polymorphic primers clustered the genotypes into different groups. On the other hand, biochemical typing of E. coracana using whole seed proteins generated profiles that showed no major difference indicating the technique to be not useful in typing genotypes of this crop. However, a few of the genotypes showed the presence of a unique band of 32 kDa that needs to be further investigated to understand the role of the protein from nutritional point of view, if any. In the present study, significant negative correlation (r = −0.69*) was found between the protein and calcium content of finger millet genotypes. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis based seed storage proteins generated profiles showed no major differences in banding pattern among 52 finger millet genotypes while quantitative estimation of seed storage protein fractions using Lowry method revealed that glutelin was highest followed by prolamin, globulin and albumin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Finger millet, Eleusine coracana L. Gaertn., is a tetraploid crop (2n = 4x = 36; genome constitution AABB) belonging to the grass family Poaceae, subfamily Chloridoideae. The crop is adapted to a wide range of environments, can withstand significant levels of salinity, is relatively resistant to water logging, and has few serious diseases. Finger millet is grown mainly by subsistence farmers and serves as a food security crop because of its high-nutritional value and excellent storage qualities. Genetic research in finger millet has been limited to studying the mode of inheritance of a few qualitative traits reviewed by Rachie and Peters [1] and biodiversity analyses. Isozyme and DNA marker analyses have indicated that cultivated finger millet has a narrow genetic base and most likely went through a bottleneck during domestication [2–5]. As expected, variation in the wild subsp. africana was considerably higher [1, 6]. Although the finger millet germplasm pool remains largely uncharacterized, small-scale analyses of the nutritional value of seeds of wild and cultivated E. coracana lines have shown a wide variation in protein, calcium and iron content [7, 8]. Phenotypic variation for blast resistance, early vigor and other yield-related characters has also been observed.
Molecular marker technology provides information that can help to define the distinctiveness of germplasm and their ranking according to the number of close relatives and their phylogenetic position. DNA marker is a new approach based on DNA polymorphism among tested genotypes, and thus applicable to biological research. Several molecular markers viz. RFLP, RAPD [9], SSRs [10], amplified fragment length polymorphism (AFLP) and SNPs are presently available to assess the variability and diversity at molecular level [11]. Simple sequence repeat (SSR) markers or microsatellites are tandem repeats interspersed throughout the genome and can be amplified using primers that flank these regions [12]. More recently molecular markers, such as SNPs and SSRs, which are genetically linked to fragrance and to identify the nature of the locus (homozygous or heterozygous condition), and have the advantage of being inexpensive, simple, rapid and only requiring small amount of tissue, may also be useful for the rapid incorporation of the scent character into breeding lines [13]. On the other hand random amplified polymorphic DNA (RAPD) is the widely used molecular marker where DNA fragments are amplified by the polymerase chain reaction (PCR) using short (usually 10 bases in length) synthetic primers of random sequence. RAPD markers tend to estimate intra- or intergenetic distances among more distantly related individuals. Inspite of many weaknesses, it is relatively easy, speedy, high degree of polymorphisms as well as virtually inexhaustible pool of possible genetic markers make the technique advantageous over other molecular techniques [14]. Randomly amplified polymorphic DNA (RAPD) have been extensively used for the assessment of genetic diversity in a variety of plants like Saxifraga cernua [15], Zea mays [16], Ziziphus spp. [17], Saccharum and Erianthus [18], Panax quinquefolius [19], etc. Inter-simple sequence repeat (ISSR) markers are much more informative than RAPDs and have been used for the analysis of genetic diversity in Cicer sp. [20], Morus alba [21], Pisum sativum [22], Asparagus acutifolius [23], Corchorus species [24] and others. AFLP has helped unravel genetic diversity in Azadirachta indica [25], Brassica nigra [26], Ranunculus acris [27], Nicotiana attenuate [28], Brassica rapa [29], Cicer sp. [30], Z. mays [31], Cynodon [32], Glycine soja [33], Myricaria laxiflora [34], Gardenia jasminoides [35], Chimonanthus spp. [36] and others.
Another molecular technique that has proved to be useful in typing crop genotypes is sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) of whole seed proteins, wherein differences seen in protein bands have been successfully used to group the genotypes. SDS-PAGE is used due to its validity and simplicity for describing genetic structure of crop germplasm, but its implication has been limited mainly to cereals due to less polymorphism in most of the legumes [37]. Seed storage proteins have been used as genetic markers obtained by electrophoresis to resolve the taxonomic and evolutionary problems of several crop plants [38, 39]. Researchers can use genetic similarity information to make decisions regarding the choice for selecting superior genotypes for improvement or to be used as parents for the development of future cultivars through hybridization.
The present study was initiated to study genetic diversity on the basis of seed protein profile and its relationship with protein content in E. coracana. RAPD and SSR markers were also used to study the genetic diversity and relatedness of 52 finger millet genotypes in relation to variation in protein content.
Materials and methods
Germplasm collection
A total of 52 genotypes were used in the present study. Seed of 52 genotypes of E. coracana (collected from different districts of Uttarakhand were obtained from Ranichauri Hill Campus G. B. Pant University of Agriculture and Technology). Protein of each sample was estimated by Kjeldhal method. The pass port data of 52 genotypes is presented in (Table 1).
DNA extraction and PCR amplification
The genomic DNA of different accessions of finger millets were isolated by standard method [40] quantified and analyzed on agarose gel electrophoresis [41].
RAPD and SSR markers assay
A total of 21 random primers and 24 SSR primers were used for the polymorphism survey (Table 2). PCR amplification was performed as per the standard protocol using 50–100 ng of template DNA, 30 ng of primer (Life Tech), 0.1 mM dNTPS, 1.5 U Taq DNA polymerase (Bangalore Genei pvt. Bangalore, India), 1× PCR buffer (10 mM Tris pH 8.0, 50 mM KCl and 1.8 mM MgCl2) in a volume of 25 μl. Amplification was performed with thermal cycler (Eppendorf Germany). The standardized amplification was: Initial denaturation 95°C for 5 min followed by 40 cycles of denaturation 94°C for 1 min; Primer annealing based on T m value for 1 min; primer extension at 72°C for 2 min; and final primer extension at 72°C for 7 min. The annealing temperatures of the cycling parameter were readjusted for each microsatellite primers according to their calculated melting temperature (T m) based on the sequence composition [T m = 4°C (G + C) + 2°C (A + T) − 3°C].
PCR amplified products of all the primers were subjected to gel electrophoresis using 1.8% agarose gel in 1× TAE buffer at 100 V. The fragment sizes, ranged from 0.3 to 4.0 kb were detected by comparing the amplicons with a 100 bp DNA ladder and EcoRI/HindIIIdouble digest marker (Genei Pvt., Bangalore, India) and the ethidium bromide stained gels were documented using Alpha Imager 1200™ (Alpha Innotech Corporation, USA). Duplicated independent DNA preparations for each sample were done and only major bands consistently amplified were scored.
Statistical analysis
DNA fingerprints were scored for the presence (1) or absence (0) of bands of various molecular weight sizes in the form of binary matrix. Data were analyzed to obtain Jaccard’s coefficients [42] among the genotypes by using NTSYS-pc (version 2.11 W; Exeter Biological Software, Setauket, NY, [43]. The SIMQUAL program was used to calculate the Jaccard’s coefficient, a common estimator of genetic identity and was calculated as follows: Jaccard’s coefficient = N AB/(N AB + N A + N B).
Similarity matrices were utilized to construct the UPGMA (unweighted pair- group method with arithmetic average) dendrograms. Polymorphic information content (PIC) or average heterozygosity was calculated as per the formula: PIC = 2fi (1 – fi), where ‘fi’ is the frequency of the amplified allele and ‘1 – fi’ is the frequency of null allele. Principal coordinate analysis was performed in order to highlight the resolving power of the ordination. To determine robustness of the dendrogram, the data were bootstrapped with 2000 replications along with Jaccard’s coefficient by the computer programme WINBOOT [44].
Protein profiling
All 52 genotypes of finger millet were tested for their protein profiles. Total proteins were extracted by grinding seed (50 mg) with 2% (w/v) SDS, 5% (v/v) 2- mercaptoethanol, 10% (v/v) glycerol, 0.0625 M Tris–HCl, pH 6.8 (1 ml) followed by boiling for 5 min and centrifuged at 10,000 rpm for 15 min. Total protein in the form of supernatant was collected and resuspended in 50 μl of 2× sample buffer (0.5% sodium dodecyl sulphate, 1.25% 2-β-mercaptoethanol, 0.03% bromophenol blue, 2.5% glycerol in 15 mM Tris–C1 at pH 6.8) and incubated in a dry bath at 98°C for 15 min. Approximately 25 μg of the protein sample was taken and subjected to SDS–polyacrylamide gel electrophoresis (SDS-PAGE) containing 5% stacking and 15% of resolving gels and separated based on Laemeli discontinuous buffer system (Harlow and Lane 1988). After electrophoresis on a vertical slab unit under a constant voltage of 150 V for 7 h, the gels were stained with coomassie brilliant blue R-250 (HiMedia, Mumbai, India). A medium protein marker calibration kit (Bangalore Genei, Bangalore, India) was used to estimate the molecular weight of protein bands.
Seed storage proteins (Albumin, Globulin, Prolamin and Glutelin) were also extracted and subjected for their protein profiles. Prolamins were extracted from ball-milled seed (10 g), which was defatted with chloroform (2 × 100 ml) and air-dried. Albumins and globulins were extracted by stirring with 1 M NaCl (2 × 100 ml) for 1 h and centrifuged (10,000×g for 15 min), the supernatant solutions were dialysed and freeze-dried. The pellet was washed with water and prolamins extracted with 70% (v/v) aqueous ethanol (2 × 100 ml for 1 h each), followed by 50% (v/v) aqueous propan-1-ol, 2% (v/v) acetic acid and 2% (v/v) 2-mercaptoethanol (100 ml for 1 h). The respective supernatants were dialysed in a low Mr cutoff membrane (Spectra/Por 3, Pierce and Warriner) and freeze-dried. Glutelin-alkali soluble fraction, the insoluble residue obtained after the above extraction was extracted with 20 ml of 0.2% NaOH [45]. Proteins were analysed on 15% (w/v) acrylamide SDS–PAGE gels, based on the system of Laemmli [46].
Results
RAPD analysis
In this study, RAPD-PCR fingerprints were generated for 52 genotypes of finger millet. Eighteen randomly designed 10-mer oligonucleotide primers were initially used for screening DNA samples to obtain reproducible RAPD fingerprints. RAPD-PCR was run thrice to evaluate and check for the repeatability of the fingerprints generated. Out of the 21 primers tested, only 20 primers provided consistent well resolved and reproducible band patterns and were therefore selected for further analysis.
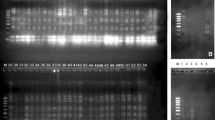
The total number of fragments observed among the finger millet genotypes based on RAPD analysis with 20 polymorphic primers was 146. The number of scorable fragments produced per primer ranged from 3 to 15 and size of the products ranged from 100 to 3034 bp. A representative RAPD profile obtained by primer RAPD-10 is shown in (Fig. 1a). Of a total of 11 bands (0.15–2.5 kb), 7 were polymorphic (64%). Marked ‘A’ a 0.8 kb band and marked ‘B’, a 0.18 kb band, is unique to genotypes containing high protein. The similarity coefficients based on RAPD markers ranged from 0.64 to 0.999 with an average value of 0.819. The PIC values, a reflection of allele diversity and frequency among the varieties, were not uniformly higher for all the RAPD loci tested. The PIC value ranged from 0.141(RAPD-09) to 0.5 (RAPD-030) with a mean of 0.351.
Cluster analysis of RAPD primers generated RAPD profiles separated the genotypes at an average similarity values of 73% respectively (data not shown). A dendrogram based on the similarity matrix generated with the RAPD primers is presented in Fig. 1a. The dendrograms at an average similarity value of 73% grouped all genotypes in different clusters showing high diversity in profiles. Besides, the RAPD profiles also enabled a few of the genotypes to be discriminated based on their protein content. The remaining clusters consisted of mixed genotypes. The dendrogram generated were also support bootstrap value (Fig. 1b) which indicates the accuracy of the tree.
SSR analysis
A total of 168 scorable markers were yielded by the 21 polymorphic primers with an average of 08 bands per primer. 112 (66.6%) with an average of 5.3 per primer were polymorphic. A representative fingerprint pattern generated by primer SSR, UTR-36 is shown in (Fig. 2a). Out of 11 alleles generated by this primer (size range 0.1–2.0 kb), four were monomorphic. A 0.2 kb allele ‘A’ and 0.1 kb allele ‘B’ are present in genotypes containing high protein but absent in genotypes containing low protein content. The PIC value ranged from 0.274 (SSR-10) to 0.758 (SSR-02) with a mean of 0.557. The similarity coefficients based on SSR markers ranged from 0.55 to 0.999 with an average value of 0.774. Jaccard’s pair-wise similarity coefficient values ranged from 0.255 to 0.950 with an average value of 0.602. Cluster analysis of SSR primers generated SSR profiles separated the genotypes at an average similarity values of 75% respectively (data not shown). A dendrogram based on the similarity matrix generated with the SSR primers is presented in Fig. 2a. The dendrograms at an average similarity value of 75% grouped all genotypes in different clusters showing high diversity in profiles. The dendrogram generated were also support bootstrap value (Fig. 2b) which indicates the accuracy of the tree.
Protein profiling
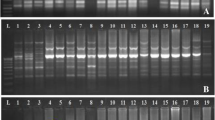
On 15% SDS-PAGE electrophoresis, the analysis of total seed crude protein in 52 genotypes of finger millet yielded approximately 15–25 clear and distinct polypeptide bands with molecular weights ranging from 10 to 100 kDa. The total seed crude protein banding patterns were observed to be identical for all the genotypes tested. However, in few genotypes, an additional band of 32 kDa was detected (Fig. 3a). Although, significant difference was found on the basis of comparative quantitative analysis of total seed protein content of 52 finger millet genotypes (Fig. 3b).
a Representative protein profiles of Eleusine coracana genotypes. M, protein marker; lanes 1–20, finger millet genotypes, Arrows indicate the 32 kDa unique band observed in the fingerprints, b Graphical representation of total seed protein variation in the 52 genotypes of Eleusine coracana collected from Uttarakhand
In the previous studies calcium content of 52 finger millet genotypes was estimated using atomic absorption Spectrophotometry (AAS) [47].
The genotypes of finger millet collected from different districts of Uttarakhand grouped according to high, medium and low calcium contents, estimated by atomic absorption Spectrophotometry (AAS). These results were also supported by RAPD, SSR and cytochrome P450 gene based DNA marker profiles. In E. coracana analysis of all the three markers (RAPD, SSR and cytochrome P450 gene based markers) grouped the finger millet genotypes into three distinct clusters. The first cluster had genotypes containing low calcium (100–200 mg/100 g). Second cluster included genotypes containing high calcium (300–450 mg/100 g). Third cluster included genotypes containing medium calcium (200–300 mg/100 g).
In the present study protein content of all 52 genotypes was estimated by Kjeldhal method. When the total calcium content of 52 genotypes was compared with total crude protein content, it was found that negative correlation was present between the total calcium and protein content of 52 finger millet genotypes (Table 3).
In terms of the total crude protein, 52 genotypes were divided into three groups (High, Medium and Low). In these three groups, the first group had 36 genotypes having protein content ranging from 10 to 14%. The protein content of first group of genotype GPHCPB-1 was found highest and three genotypes (GPHCPB-16, GPHCPB-32 and GPHCPB-43) were found with lowest protein content. In second group, there were 12 genotypes having protein content ranging between 8 and 10% and it was observed that in this group genotype GPHCPB-52 had higher protein content while two genotypes (GPHCPB-18 and GPHCPB-49) with minimum protein content. In third group, there were four genotypes with protein content below 8% and it was observed that in this group the protein content was maximum in genotype GPHCPB-12 and minimum in genotype GPHCPB-45 (Fig. 3b).
Comparative seed storage protein profiling: Seed storage protein fractions i.e. Albumin, globulin, prolamin and glutelin were separated on the basis of their solubility in their respective solvent. SDS-PAGE based seed storage proteins generated profiles showed no major difference in banding pattern of 52 finger millet genotypes (Fig. 4a) while quantitative estimation of seed storage protein fractions using Lowry method revealed that glutelin was highest followed by prolamin, globulin and albumin (Fig. 4b).
All the fractions (albumin, globulin, prolamin and glutelin) were divided into three groups on the basis of percentage of crude protein. In the first group, it was observed that the albumin content was maximum in genotype GPHCPB-4 and minimum in genotype GPHCPB-19, globulin content was maximum in genotype GPHCPB-23 and minimum in genotype GPHCPB-20, prolamin content was maximum in genotype GPHCPB-21 and minimum in genotype GPHCPB-1 and glutelin content was maximum in genotype GPHCPB-4 and minimum in genotype GPHCPB-20.
Similarly in the second group, the albumin content was highest in genotype GPHCPB-6 and lowest in GPHCPB-17, globulin content was highest in genotype GPHCPB-6 and lowest in GPHCPB-22, prolamin content was highest in genotype GPHCPB-22 and lowest in GPHCPB-47 and glutelin content was highest in genotype GPHCPB-28 and lowest in GPHCPB-30.
On the other hand, in the third group the albumin content was highest in genotype GPHCPB-46 and lowest in GPHCPB-45, globulin content was highest in genotype GPHCPB-46 and lowest in GPHCPB-45, prolamin content was highest in genotype GPHCPB-45 and lowest in GPHCPB-12 and glutelin content was highest in genotype GPHCPB-45 and lowest in GPHCPB-12.
Discussion
Finger millet is an excellent source of calcium (seven times more than rice) and also has good amounts of phosphorous. Among cereals, it possesses a reasonably high level of methionine, the major limiting amino acid of tropical regions, and the component least correctable by the addition of pulses to the diet [1]. Hence research efforts needs to be directed to utilize the full potential of this crop in terms of seed storage proteins containing high amount of essential amino acids. In the present study molecular characterization of 52 genotypes was carried out by using various markers to understand the genetic basis of this important character.
Molecular characterization techniques are now widely used for the categorization of genotypes on the basis of specific traits and location. In this study, we have evaluated three molecular methods, RAPD-PCR, SSR-PCR and whole seed protein profiling to differentiate genotypes on the basis of protein content. Finger millet genotypes were arbitrarily grouped into three classes viz. very low, moderate, and high in terms of protein content using standard statistical programs. Significant differences between the genotypes were observed for protein content and calcium content. Protein content as high as 14.2% [48] and as low as 5.85% [49] have been reported in finger millet. Wide variations in protein content have also been reported [6, 48]. In the present study total crude Protein content of the 52 finger millet genotypes were found to be ranged from 6.4 to 14%. Thirty-two genotypes possessed significantly higher protein content than the general mean of 10.3 g per 100 g of grain. Calcium content of the 52 finger millet genotypes ranged from 117 to 452 mg/100 g. Twenty genotypes possessed significantly higher calcium content than the overall mean of 260 mg/100 g of grain. The protein content had a negative and highly significant (P < 0.01) genotypic correlation with calcium content [3].
Molecular characterization led to the amplification of various specific bands, like 0.8 and 0.18 kb band amplified by primer RAPD-10, a 1.0 kb SSR band amplified by primer SSR-01, and 0.2 kb band amplified by primer SSR UTR-36 which are present only in genotypes containing high protein but absent in genotypes containing low protein content similarly a 0.1 kb band was amplified by SSR UTR-36 present only in genotypes containing low protein. Dendrograms generated from RAPD and SSR primers data showed similarity in relative placement of genotypes. Cluster analysis was carried out on three sets of marker profiling data based on RAPD and SSR. The results based on these DNA marker profiles analysis broadly grouped the 52 genotypes into distinct clusters showing relation on the basis of protein content. The genotypes containing high protein, medium protein and low protein grouped in different clusters.
These markers demonstrated striking genetic differentiation between pairs of finger millet varieties examined. This study reveals the average genetic variation among the finger millet varieties and emphasizes the need for stock/variety wise cultivation, conservation and propagation assisted rehabilitation and selection of the natural populations of finger millet. These varieties have expressed nearly similar characteristic features to some extent, while molecular markers revealed maximum similarities between high protein content biochemical characteristics. There have been initiatives for finger millet improvement using classical plant breeding approach for high yielding, early maturing, resistance to biotic stress, tolerance to abiotic stresses particularly cold and drought to enhance nutritional quality [55]. The prerequisite for attaining this goal involves screening of different germplasms to obtain desired traits to be utilized in making crosses [56]. The acquisition of primary information about plant genetic diversity is an important fundamental work to sustain genetic conservation i.e., in situ and ex situ for gene bank management. Consequently, exploiting the genetic diversity existing in the available germplasms could be quite beneficial to breeders in crop improvement through genome-based utilization of unexploited gene pools [8, 57] because, so far, a very small fraction of the total available collections of finger millets have been used in the national breeding programs of India [58].
The total seed protein banding patterns were observed to be identical for all the genotypes tested. However, in few genotypes, an additional band of 32 kDa was detected. It is important to note that a low level of intra-specific variation has been reported in various legumes, i.e., chickpea [50], lentil [51, 52], groundnut [53], pigeon pea [54] and black gram [50] but in the case of E. coracana, a considerable low amount of variation was also observed based on SDS-PAGE. Although, significant difference was found on the basis of comparative quantitative analysis of total seed protein content of 52 finger millet genotypes.
SDS-PAGE based seed storage proteins generated profiles showed no major difference in banding pattern of 52 finger millet genotypes while quantitative estimation of seed storage protein fractions using Lowry method revealed that glutelin was highest followed by prolamin, globulin and albumin.
In this study, although SSR-PCR, RAPD-PCR and SDS-PAGE profiles were reproducible and generated several bands, the banding patterns observed with protein profiling were almost similar and not discriminatory as observed with DNA fingerprinting. Thus, it can be concluded that RAPD-PCR and SSR-PCR which is a rapid and simple tool could be used in typing and differentiating a large number of E. coracana genotypes which could be useful in their characterization.
References
Rachie KO, Peters LV (1977) The Eleusines: a review of the world literature. International Crops Research Institute for the Semi Arid Tropics (ICRISAT), Hyderabad, pp 118–136
Hilu KW, Johnson JL (1992) Ribosomal DNA hybridization in finger millet and wild species of Eleusine (Poaceae). Theor Appl Genet 83:895–902
Worth C, Sniegowski BP, Stephan W (1994) The evolutionary dynamics of repetitive DNA in eukaryotes. Nature 371:215–220
Muza SR, Fulco CS, Lyons T, Rock PB, Beidleman BA, Kenney J, Cymerman A (1995) Augmented chemosensitivity at altitude and after return to sea level: impact on subsequent return to altitude. Acta Andina 4:109–112
Salimath SS, Olivera ACD, Godwin ID, Bennetzen JL (1995) Assessment of genome origins and diversity in the genus Eleusine with DNA markers. Genome 38:757–763
Vadivoo AS, Joseph R, Ganesan NM (1998) Genetic variability and diversity for protein and calcium contents in finger millet Eleusine coracana (L. Gaertn.) in relation to grain color. Plant Foods Hum Nutr 52:353–364
Tingey SV, del Tufo JP (1993) Genetic analysis with random amplified polymorphic DNA markers. Plant Physiol 101:349–352
Mccouch SR, Tanksley SD (1997) Seed banks and molecular maps: unlocking genetic potential from the wild. Science 277:1063–1066
Joshi SP, Gupta VS, Aggarwal RK, Ranjekar PK, Brar DS (2000) Genetic diversity and phylogenetic relationship as revealed by Inter simple sequence repeat polymorphism in the genus Oryza. Theor Appl Genet 100:1311–1320
Giovannoni JJ, Wing RA, Ganal MW, Tanksley S (1991) Isolation of molecular markers from specific chromosomal intervals using DNA pools from existing mapping populations. Nucl Acids Res 19:6553–6558
Cordeiro GM, Christopher MJ, Henry RJ, Reinke RF (2002) Identification of microsatellite markers for fragrance in rice by analysis of the rice genome sequence. Mol Breed 9(4):245–250
Fristsch P, Rieseberg LH (1995) The use of random amplified polymorphic DNA (RAPD) in conservation genetics. In: Smither TB, Wayne RK (eds) Molecular genetic approaches in conservation. Oxford University Press, Oxford, pp 54–73
Kjølner S, Såstad SM, Taberlet P, Brochmann C (2004) Amplified fragment length polymorphism versus random amplified polymorphic DNA markers: clonal diversity in Saxifraga cernua. Mol Ecol 13:81–86
Garcia AAF, Benchimol LL, Barbosa AMM, Geraldi IO, Souza CLJ, Souza AP (2004) Comparison of RAPD, RFLP, AFLP and SSR markers for diversity studies in tropical maize inbred lines. Genet Mol Biol 27:579–588
Singh AK, Sharma RK, Singh NK, Bansal KC, Koundal KR, Mohapatra T (2006) Genetic diversity in ber (Ziziphus spp.) as revealed by AFLP markers. J Hortic Sci Biotechnol 81:205–210
Selvi A, Nair NV, Noyer JL, Singh NK, Balasundaram N, Bansal KC, Koundal KR, Mohapatra T (2006) AFLP analysis of phonetic organization and genetic diversity in the sugarcane complex, Saccharum and Erianthus. Genet Resour Crop Evol 53:831–842
Lim W, Mudge KW, Weston LA (2007) Utilization of RAPD markers to assess genetic diversity of wild populations of North American Ginseng (Panax quinquefolius). Planta Med 73:71–76
Souframanien J, Gopalakrishna T (2004) A comparative analysis of genetic diversity in blackgram genotypes using RAPD and ISSR markers. Theor Appl Genet 109:1687–1693
Awasthi AK, Nagaraja GM, Naik GV, Kanginakudru S, Thangavelu K, Nagaraju J (2004) Genetic diversity and relationships in mulberry (genus Morus) as revealed by RAPD and ISSR marker assays. BMC Genet 5:1
Baranger A, Aubert G, Arnau G, Lainé AL, Deniot G, Potier J, Weinachter C, Lejeune-Hé naut I, Lallemand J, Burstin J (2004) Genetic diversity within Pisum sativum using proteinand PCR-based markers. Theor Appl Genet 108:1309–1321
Sica M, Gamba G, Montieri S, Gaudio L, Aceto S (2005) ISSR markers show differentiation among Italian populations of Asparagus acutifolius L. BMC Genet 6:17
Roy A, Bandyopadhyay A, Mahapatra AK, Ghosh SK, Singh NK, Bansal KC, Koundal KR, Mohapatra T (2006) Evaluation of genetic diversity in jute (Corchorus species) using STMS, ISSR and RAPD markers. Plant Breed 125:292–297
Singh A, Chaudhury A, Srivastava PS, Lakshmikumaran M (2002) Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci 162:17–25
Negi MS, Sabharwal V, Bhat SR, Lakshmikumaran M (2004) Utility of AFLP markers for the assessment of genetic diversity within Brassica nigra germplasm. Plant Breed 123(1):13–16
Odat N, Jetschke G, Hellwig FH (2004) Genetic diversity of Ranunculus acris L. (Ranunculaceae) populations in relation to species diversity and habitat type in grassland communities. Mol Ecol 13(5):1251–1257
Bahulikar RA, Stanculescu D, Preston CA, Baldwin IT (2004) ISSR and AFLP analysis of the temporal and spatial population structure of the post-fire annual, Nicotiana attenuata, in SW Utah. BMC Ecol 4:12
Zhao J, Wang X, Deng B, Lou P, Wu J, Sun R, Xu Z, Vromans J, Koornneef M, Bonnema G (2005) Genetic relationships within Brassica rapa as inferred from AFLP fingerprints. Theor Appl Genet 110:1301–1314
Shan F, Clarke HC, Plummer JA, Yan G, Siddique KHM (2005) Geographical patterns of genetic variation in the world collections of wild annual Cicer characterized by amplified fragment length polymorphisms. Theor Appl Genet 110:381–391
Menkir A, Kling JG, Badu-Apraku B, Ingelbrecht I (2005) Molecular marker-based genetic diversity assessment of Striga-resistant maize inbred lines. Theor Appl Genet 110:1145–1153
Wu YQ, Taliaferro CM, Bai GH, Martin DL, Anderson JA, Anderson MP, Edwards RM (2006) Genetic analyses of Chinese Cynodon accessions by flow cytometry and AFLP markers. Crop Sci 46:917–926
Ru Z, Zhou C, Weifeng L, Baorong L (2006) Estimating genetic diversity and sampling strategy for a wild soybean (Glycine soja) population based on different molecular markers. Chin Sci Bull 51:1219–1227
Liu Y, Wang Y, Huang H (2006) High interpopulation genetic differentiation and unidirectional linear migration patterns in Myricaria laxiflora (Tamaricaceae), an endemic riparian plant in the Three Gorges Valley of the Yangtze River. Am J Bot 93:206–215
Han J, Zhang W, Cao H, Chen S, Wang Y (2007) Genetic diversity and biogeography of the traditional Chinese medicine, Gardenia jasminoides, based on AFLP markers. Biochem Syst Ecol 35:138–145
Zhou M-Q, Zhao K-G, Chen L-Q (2007) Genetic diversity of Calycanthaceae accessions estimated using AFLP markers. Sci Hortic (in press)
Ghafoor A, Afzal M, Anwar R (2002) Diversity in food legumes for sustainable utilization of plant genetic resources. In: Anwar R, Takahashi J, Bhatti MS, Masood S (eds) Sustainable utilization of plant genetic resources. PARC/IPGRI/JICA, Islamabad, pp 238–250
Ladizinsky G, Hymowitz T (1979) Seed protein electrophoresis in taxonomic and evolutionary studies. Theor Appl Genet 54:145–151
Das S, Mukarjee KK (1995) Comparative study on seed proteins of Ipomoea. Seed Sci Technol 23:501–509
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucl Acids Res 8:4321–4326
Maniatis T, Sambrook J, Fritsch EF (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Jaccard P (1908) Nouvelles recherches sur la distribution florale. Bull Soc Vaudoise Sci Nat 44:223–270
Rohlf FJ (1997) NTSYS-pc numerical taxonomy and multivariate analysis system, version 2.0. Exeter Publications, New York
Yap IV, Nelson RJ (1996) Winboot: a program for performing bootstrap analysis of binary data to determine the confidence of UPGMA-based dendrograms. IRRI, Manilla
Tatham AS et al (1996) Characterisation of the Major Prolamins of Tef (Eragrostis tef) and Finger Millet (Eleusine coracana). J Cereal Sci 24:65–71
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Panwar P, Saini RK, Sharma N, Yadav D, Kumar A (2010) Efficiency of RAPD, SSR, and Cytochrome P450 gene based marker in accessing genetic variability amongst finger millet (Eleusine coracana) accessions. Mol Biol Rep. doi:10.1007/s11033-010-0067-5
Ayyangar GNR (1934) Recent work on the genetics of millets in India. Madras Agric J 22:16–26
Adrian J, Jaquot P (1964) Le sorgho et les mils en alimentation humaine et animale. Centre de Recherches sur la Nutrition, Bellevue (Seine et Olse). Vigot Frères, Editeurs
Ramiah PV, Satyanarayana P (1936) Biological values of ragi proteins. Proc Soc Biol Chem 1:7–8
Sastri BN (1939) Ragi (Eleusine coracana L. Gaertn), a new ragi material for the malting industry. Curr Sci 8:34–35
Ghafoor A (2003) [Vigna mungo (L.) Hepper] Identification of early maturing and high yielding lines of black gram. Proc Pak Acad Sci 40:127–134
Piergiovanni AR, Taranto G (2003) Geographic distribution of genetic variation in a lentil collection as revealed by SDS-PAGE fractionation of seed storage proteins. J Genet Breed 57:39–46
Sultana T, Ghafoor A, Ashraf M (2006) Geographic patterns of diversity of cultivated lentil germplasm collected from Pakistan, as assessed by seed protein assays. Acta Biol Crac Ser Bot Pol 48:77–78
Javaid A, Ghafoor A, Anwar R (2004) Seed storage protein electrophoresis in groundnut for evaluating genetic diversity. Pak J Bot 36(1):25–29
Jha SS, Ohri D (1996) Phylogenetic relationships of Cajanus cajan (L.) Millsp. (pigeonpea) and its wild relatives based on seed protein profiles. Genet Res Crop Evol 43:275–281
First International Small Millet Workshop (1986). http://www.fsifee.u-akugei.ac.jp/millets/1stintsym.htm
Project All India Coordinated Small Millets Improvement (1996) Annual Report University of Agriculture Sciences. Bangalore, India
Watanabe KN, Iwanaga M (1999) Plant genetic resources and its global contribution. Plant Biotechnol 16:7–13
Evaluation of finger millet germplasm (1996) Project Report All India Coordinated Research Project, Gandhi Krishi Vigyan Kendra, University of Agricultural Sciences. Bangalore, India
Acknowledgments
The authors wish to acknowledge the Department of Biotechnology, Govt. of India for providing financial support in the form of Programme Support for research and development in Agricultural Biotechnology at G.B. Pant University of Agriculture and Technology, Pantnagar (Grant No. BT/PR7849/AGR/02/374/2006). The authors thank the, Ranichauri Hill Campus G.B. Pant University of Agriculture and Technology for providing the seed samples of the germplasm analyzed in present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, A., Sharma, N., Panwar, P. et al. Use of SSR, RAPD markers and protein profiles based analysis to differentiate Eleusine coracana genotypes differing in their protein content. Mol Biol Rep 39, 4949–4960 (2012). https://doi.org/10.1007/s11033-011-1291-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1291-3