Abstract
Drylands play a significant role in the global biogeochemical cycling of nutrients (carbon, nitrogen, and phosphorus) through abiotic (geological, atmospheric, and hydrological) and biotic (animals, insects, plants, and microorganisms) pathways. They act as important carbon reservoirs and are estimated to store over 30% of the global soil organic carbon reserve. However, nitrogen and phosphorus availability are major limiting factors for biological activity in these oligotrophic environments, affecting community structure, species diversity, and other ecosystem functions (e.g., nutrient cycling and productivity). Nutrient cycling in desert soils is primarily achieved by plant and microbial communities, in particular soil microbial communities, biological soil crusts, hypoliths, and endoliths. Drylands are highly sensitive and prone to disturbance and land degradation resulting from desertification. Changes induced by climate (e.g., precipitation and temperature), structural and temporal variability (nutrient accumulation and distribution of minerals, seasonal variation, and differences in turnover rates), and human activity often alter nutrient cycles that negatively affect the structure and function of these ecosystems (e.g., decreasing carbon storage capacity, increasing NOx emissions, and reducing phosphorus cycling). Comprehending the extent, nature, magnitude, and reversibility of such changes is urgent, given the global importance of drylands in terms of carbon sequestration, greenhouse gas emissions, ecology and biodiversity, and human habitation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
The focus of this chapter is motivated by the high sensitivity of drylands to perturbations affecting the past and current nutrient cycling of most of these ecosystems around the world. It shows how changes in the abiotic and mostly biotic components and their interactions of each particular dryland’s microclime ecosystems can affect the dynamics of their nutrient cycling both in time and space. Thus, in this chapter, we present a general discussion of the dynamics of the soil C, N, and P cycles in drylands with special attention to the role of Biological Soil Crusts, the influence of hydration-desiccation pulses, and the impact of Climate Change on nutrient cycling.
7.1 Carbon in Drylands Soils
Studies have indicated that drylands currently act as a large carbon (C) sink and play a more significant role in the terrestrial C balance than previously expected (Lal 2001; Wohlfahrt et al. 2008; Rotenberg and Yakir 2010). Dryland ecosystems are an important component of the global C cycle as they store ~32% (~470 Pg; Plaza et al. 2018a) of the global soil organic carbon (SOC), account for about one-third of the global vegetation C storage (Allen-Diaz et al. 1996), and represent 30–35% of terrestrial net primary production (NPP; Field et al. 1998). Drylands, therefore, dominate the positive global land CO2 sink, although large fractions of the interannual variability (IAV) of net CO2 flux have been mostly associated with variations in gross primary production (GPP; Beer et al. 2010; Yao et al. 2020). Model projects showed that the majority of dryland GPP variability is attributed to precipitation and air temperature (Ahlström et al. 2015; Zhang et al. 2016a; Yao et al. 2020), although other environmental variables such as changes in ecosystem types also play relatively small roles in regulating GPP variability (Yao et al. 2020). With the increasing frequency of extreme climatic events, the IAV of GPP is also projected to increase and will likely cause significant impacts on the global C cycle (Zscheischler et al. 2014). Although the physical-chemical properties of dryland soils limit their potential to store C (Ewing et al. 2006; Serrano-Ortiz et al. 2012; Weil and Brady 2017; Plaza et al. 2018a), their large coverage of the global land area, and the fact that many of these soils have been degraded, means that drylands have the greatest potential to sequester C (Scurlock and Hall 1998; Rosenberg et al. 1999). In fact, the amount of SOC present in drylands is ~42 times more than what is added into the atmosphere through anthropogenic activities, estimated at 11.3 Pg C year–1 in 2017 (Lal 2004a; Le Quéré et al. 2018). Studies have indicated that drylands currently act as a large C sink and play a more significant role in the terrestrial C balance than previously expected (Lal 2001; Wohlfahrt et al. 2008; Rotenberg and Yakir 2010).
In this chapter, we present a general discussion of the dynamics of the soil C cycle in drylands with special attention to C sequestration and climate change in arid environments. Although greenhouse gases methane (CH4) and nitrous oxide (N2O) are important components in the C cycle, carbon dioxide (CO2) is the most prevalent greenhouse gas in the atmosphere and will be the primary gas considered when discussing C sequestration.
Soil C pools in arid environments exist as three main components: (1) soil organic C (SOC); (2) soil inorganic C (SIC); and (3) biomass organic C (Serrano-Ortiz et al. 2012). Important features and current stocks will be briefly discussed for each pool in the following sections.
7.1.1 Soil Organic Carbon in Drylands Soils
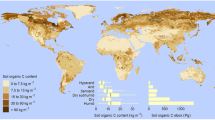
SOC is a strong determinant of soil quality, particularly in arid environments, as it influences the physical, chemical, and biological properties of soil (Gaitán et al. 2019) and is critical for improving soil fertility (Zhang and Shao 2014). As the SOC pool is strongly affected by precipitation and temperature (Lal 2002), SOC storage tends to decrease exponentially with an increase in temperature, but increases with increments in soil water content (Lal 2002; Follett et al. 2012; Yan et al. 2017; Chatterjee et al. 2019). Consequently, dryland soils contain small amounts of SOC, often less than 0.5% of the soil mass resulting in typical densities of 0–15 kg m–2 (Lal 2002, 2004a). However, due to the vast extension of dryland ecosystems soils are estimated to contain ~ 646 ± 9 Pg of SOC to a 2-m depth, representing about 32% of the world’s total SOC pool (Plaza et al. 2018a; Lal 2019).
Arid and hyper-arid soils contain ~113 Pg organic C, whereas semiarid and sub-humid areas store ~ 318 Pg SOC (Plaza et al. 2018b). Studies have demonstrated that the SOC density is lower for bare soils (1–3 kg C m–2; Rasmussen 2006; Woomer et al. 2004) compared to shrublands (2–6 kg C m–2; Chen et al. 2007; Wiesmeier et al. 2011) and grasslands (5 ± 2 kg C m–2; Chen et al. 2007; He et al. 2008).
7.1.2 Soil Inorganic C in Drylands Soils
SIC is also a critical component of arid regions and plays an important role in carbon sequestration and climate alleviation (Lal 2004b). Dryland SIC pools are estimated to be higher (1237 ± 15 Pg; Plaza et al. 2018a) than SOC pools and may even exceed SOC by a factor of 10 in some arid areas (Schlesinger 1985, 2006; Scharpenseel et al. 2000). The SIC stocks are generally highest for arid (487 ± 7 Pg) and semiarid (456 ± 7 Pg) environments (Plaza et al. 2018a) where the SIC pool is mainly present as lithogenic inorganic carbonates (LIC) that originates as detritus from parent materials (i.e., limestone), and pedogenic inorganic carbonates (PIC) formed by dissolution and re-precipitation of LIC or by the dissolution of CO2 into bicarbonate (HCO3–) followed by precipitation with Ca2+ and/or Mg2+ originating from non-LIC minerals (e.g., silicate weathering, dust, and fertilizers) (Marion 1989; Schlesinger 1985, 2002; Tan et al. 2014; Wang et al. 2015; Sahrawat 2003; An et al. 2019).
These carbonates tend to precipitate at relatively shallow depths as a result of low precipitation and poor leaching of soils (Gocke et al. 2011). Consequently, about 80% of the global SIC pool is found in drylands (Eswaran et al. 2000; Plaza et al. 2018a). Reported SIC stocks can be modified significantly taking into account anthropogenic practices (Serrano-Ortiz et al. 2012). For example, the SIC pool can be affected by land management practices such as afforestation, irrigation, fertilization, and liming (Sanderman 2012; Gao et al. 2017). Such activities can cause elevated levels of CO2 in soils, resulting in significant formation and precipitation of secondary carbonates, thereby contributing to soil C sequestration (Denef et al. 2008; Sanderman 2012; Gao et al. 2017).
7.1.3 Biomass Organic C in Dryland Soils
7.1.3.1 Vegetation
Dryland vegetation is highly variable and can range from barren or sparsely vegetated desert to grasslands, shrublands and savannahs, croplands, and dry woodlands (FAO 2004; Lal 2004a; Mander et al. 2017). Vegetation cover in drylands is influenced by various factors including drought stress, variations in annual temperatures, and precipitation intensity and frequency (FAO 2004; Lal 2004a; Mander et al. 2017). These factors strongly impact above-ground biomass productivity and therefore the level of SOC in soil.
Carbon storage for dryland vegetation is overall low (Eswaran et al. 2000), with the global average and maximum stock estimated at ~65 and 81 Pg C, respectively (Safriel et al. 2005; Serrano-Ortiz et al. 2012). However, these values can be modified significantly when considering anthropogenic activities, like grazing and desertification, which may reduce the biomass C pool by 10–20% of the given value (Serrano-Ortiz et al. 2012; An et al. 2019). Biomass C density can differ between arid regions, depending on vegetation coverage and land use. For example, hyper-arid and arid deserts have the capacity to store 0.04–0.40 kg C m–2 in biomass (Woomer et al. 2004; Fan et al. 2008; Perez-Quezada et al. 2011), while grasslands and sub-humid forested drylands have a biomass C density of ~1 (He et al. 2008) and 4–5 kg C m–2 (Glenday 2008), respectively.
7.1.3.2 Microbial Autotrophs
As most drylands represent sparse vegetation cover, autotrophic microbes (soil and BSC) act as important primary producers (Liu et al. 2018). Autotrophic CO2 fixation processes are significant for C accumulation in dryland soils that may influence ecosystem succession processes (Agarwal et al. 2017; Liu et al. 2018). In fact, microbial autotrophy accounts for ~ 4% of total C sequestered by terrestrial ecosystems per year, of which drylands comprise a substantial fraction (Yuan et al. 2012). Microbial autotrophs are distributed amongst both bacteria and archaea with highly diverse phylogeny, metabolic activities, and ecological variants (Hügler and Sievert 2011). For example, studies have shown that autotrophic microbes in drylands belong to the taxa Actinobacteria, Proteobacteria, Chloroflexi, Acidobacteria, Gemmatimonadetes, Firmicutes, Thaumarchaeota, Nitrospirae, Planctomycetes, and Bacteroidetes (Yang et al. 2017; Liu et al. 2018).
Even though the Calvin cycle is the predominant pathway utilized by microbes in nutrient-rich conditions, many autotrophic microbes in drylands fix C through the energy-conserving reductive citrate cycle, reductive acetyl-CoA cycle and 3-hydroxypropionate bi-cycle pathway (Agarwal et al. 2017; Liu et al. 2018). Liu et al. (2018) were able to demonstrate the capacity of desert microbial autotrophs to directly incorporate 13CO2 into SOC using the three above-mentioned pathways, which accounted for ~4% of the atmospheric CO2 absorbed by desert soil. In addition, the study showed that the efficiency of autotrophic CO2 fixation was impacted by soil properties, the autotrophic composition, and the abundance of genes associated with the CO2 fixation pathways. Their results highlighted the underestimated importance of microbial autotrophy in the C cycle and storage in drylands.
7.1.4 Carbon Sequestration and Loss: The Impact of Abiotic and Biotic Factors
Carbon sequestration in drylands involves the transfer of atmospheric CO2 into both SOC and SIC pools via management of vegetation, soil, and water resources that lead to a positive C budget (Lal 2009). Mechanisms involved in SOC and SIC sequestration, and the rate of each process are discussed by Lal (2009). In general, primary producers/plants convert atmospheric CO2 to complex organic molecules through photosynthesis; these molecules enter the soil C cycle as decaying organic matter (OM; Batlle-Aguilar et al. 2011; Serrano-Ortiz et al. 2012). A significant portion of the OM is directly used to sustain energy for pedo-and microfauna metabolism and is released back to the atmosphere as a by-product of autotrophic (plant) and heterotrophic (e.g., microbial) respiration (Batlle-Aguilar et al. 2011; Thomey et al. 2014). Some of the soil C is assimilated by vegetation and finally transferred to the soil as plant litter, becoming part of SOM (Porporato et al. 2003). In addition to biological processes, there is growing evidence to suggest that abiotic processes contribute to CO2 fluxes in soils and may even dominate the flux during dry seasons (Kowalski et al. 2008; Serrano-Ortiz et al. 2012; Cueva et al. 2019). The exact physical and biogeochemical mechanisms that promote CO2 capture in soils are still uncertain and warrant future research to accurately understand and quantify the C balance, from local to global scales (Cueva et al. 2019).
Soil C stock in drylands is strongly impacted by biotic and abiotic processes, factors (e.g., climate, vegetation, soil properties), causes (e.g., urbanization, wildfires), and interactions among them (Lal 2019), that primarily control C fluxes between the atmosphere and both SOC and SIC pools via management of vegetation, soil and water resources that lead to a positive C budget (Lal 2019). The C stock is also affected by erosional processes, both aeolian and hydrologic, which influence transport, redistribution, and deposition of C over the landscape (Lal 2019).
Significant progress has been made to understand the controls on regional and global patterns of soil SOC in drylands. Correlative analyses and/or structural equation modeling (SEM) across natural environmental gradients indicate that SOC is largely controlled by climatic factors such as precipitation and temperature, as well as soil properties and plant productivity (White et al. 2009; He et al. 2014; Wang et al. 2014a–d; Mureva et al. 2018; Gaitán et al. 2019; Smith and Waring 2019; Zhu et al. 2019). For example, precipitation influences SOC storage by constraining primary productivity and decomposition (Wynn et al. 2006; Yang et al. 2007), whereas higher temperatures accelerate the microbial decomposition of SOM, thereby causing C loss (Giardina and Ryan 2000).
In addition to climate, soil properties, such as soil mineralogical characteristics (Smith and Waring 2019), pH (Min et al. 2014; Ou et al. 2017), bulk density (Feng et al. 2002), total phosphorus (Tian et al. 2017), and soil moisture content (Wang et al. 2014a–d), are strongly related to SOC. It should be noted that the main factors controlling SOC concentration may differ between regions and even within the same ecosystem (Cable et al. 2011), or can be scale dependent (Dai and Huang 2006; Liu et al. 2006; Evans et al. 2011; Wang et al. 2013; Qin et al. 2016). An important reason for such discrepancies is that soil CO2 efflux is a combined result of biotic processes and abiotic factors (Ma et al. 2010, 2017; Gaitán et al. 2019), each of which exhibits its own flux behavior at various time scales and responds differently to the environment (Ryan and Law 2005; Li et al. 2010) to control SOC concentration and dynamics.
7.2 Nitrogen in Dryland Soils
Second to water, nitrogen (N) availability is the main limiting factor of biological activity in oligotrophic arid environments (Whitford 2002). Microorganisms in open soils, root systems, or cryptic niches (e.g., biological soil crusts, hypoliths, and endoliths) are the main providers of atmospheric fixed N but also act as the key players in other N-cycling processes such as nitrification and denitrification.
7.2.1 Biological Nitrogen Fixation (BNF) as N Input in Drylands
The main nitrogen input in many hyperarid environments comes from biological nitrogen fixation (BNF) (Johnson et al. 2005; Pointing and Belnap 2012). The BNF is an anaerobic process that is performed only by prokaryotes. The oxygen-sensitive nitrogenase complex catalyzes the reduction of atmospheric N2 to bioavailable ammonia (NH3). This is a highly energy-demanding process for cells (16 ATP molecules and 8 electrons per reduced N2 molecule; Zehr et al. 2003), particularly in desert environments that are nutrient deprived. Acetylene reduction assays (ARA) are commonly used to measure the nitrogenase activity in oligotrophic environments via the reduction of acetylene to ethylene (Aranibar et al. 2003) and calculating the rates by which 15N is incorporated into cells (Mayland and McIntosh 1966; Caputa et al. 2013; Alcamán-Arias et al. 2018).
The most widely used phylogenetic marker to study N-fixation is the nifH gene as its phylogeny is fairly concordant with that of the 16S rRNA gene (Zehr et al. 2003). Nitrogen-fixing bacteria (diazotrophs) belong to several phyla including the autotrophic Cyanobacteria and Chlorobi, as well as to heterotrophic Actinobacteria, Firmicutes, and Proteobacteria (α-, γ-, ε-classes; see Hartley et al. 2007); all of which have been detected in hot and cold deserts (Cowan et al. 2011; Ronca et al. 2015; Crits-Christoph et al. 2016; Lacap-Bugler et al. 2017; Meslier et al. 2018). Also, several archaeal taxa such as Methanobacteria, Methanococci, and Methanomicrobia have the potential to fix N (Chan et al. 2013; Wei et al. 2016) as recently demonstrated in wetland soils (Bae et al. 2018).
Biological soil crusts (BSCs) are abundant in cold and hot deserts (Belnap 2001a; Pointing and Belnap 2012; Makhalanyane et al. 2015; Chap. 3) and can contribute to ~30% of biologically fixed nitrogen in terrestrial ecosystems (Elbert et al. 2012). In cyanobacterial dominated crusts N is primarily fixed by filamentous heterocystous (e.g., Anabaena, Nostoc, Scytonema) and non-heterocystous (e.g., Microcoleus, Chroococcidiopsis, Phormidium) cyanobacteria (Belnap 2002; Wei et al. 2016; Lacap-Bugler et al. 2017; Mogul et al. 2017), although other organisms such as chlorophyte algae, heterotrophic bacteria, fungi, mosses and/or lichens are also present (Belnap 2003; Pointing and Belnap 2012). Studies have shown that N-fixation in cyanobacterial–algal crusts from the Tengger and the Gurbantunggut Deserts presented the highest nitrogenase activity (up to 16.6 mmol m−2 h−1), followed by lichens (up to 6.9 mmol m−2 h−1) and mosses (up to 2.6 mmol m−2 h−1; Wu et al. 2009; Su et al. 2011). In contrast, lichen-dominated crusts in the Colorado Plateau were found to be the main N-fixers and the amount of N fixed was ten-fold higher than that of cyanobacteria-dominated crusts (Belnap 2002). Looking at ammonium oxidation (AO) in two types of BSCs from the Colorado Plateau, Johnson et al. (2005) found that N2 fixation potential was appreciably higher (6.5–48 μmol C2H2 m–2 h–1) in dark crusts, dominated by heterocystous cyanobacteria, than those in light crusts.
N-fixation and N-fixing activity in drylands are influenced by several environmental factors, of which water availability and temperature appear to be the most important parameters (Belnap 2001b; Hartley et al. 2007). Cyanobacteria and other heterotrophic bacteria are physiologically active only when water is available, thereby controlling photosynthesis and ultimately N-fixation (Belnap 2001b; Hartley et al. 2007). Moisture levels needed to initiate and optimize N-fixation vary widely with species, habitats, and pre-existing conditions (Belnap 2001b). N-fixation rates are also limited by temperature extremes. Most nitrogenase activity occurs at –5 to 30 °C, with the optimum at 20–28 °C for the majority of drylands (Belnap 2001b; Wu et al. 2009; Schwabedissen et al. 2017). Low temperatures can reduce photosynthetic rates and thus available ATP and reductant pools, creating a lag time before N-fixation is initiated (Belnap 2001b). In contrast, at higher temperatures (i.e., above minimum temperature for species) N-fixation rates show a strong, positive response to increasing air temperature until an upper limit is reached, after which rates quickly decline (Belnap 2001b).
Other factors that affect N-fixation in drylands include pH, salinity, and nutrient content (Hartley et al. 2007). Nitrogenase activity in soil cyanobacteria and BSCs are greatest above pH 7 (Hartley et al. 2007; Schwabedissen et al. 2017); however, nitrogenase activity is reduced at high (8–10) and low pH (see Belnap 2001a for further explanations). Effects of salinity on nitrogenase activity in drylands are not well studied and show mixed results. For example, N-fixing cyanobacteria in Utah deserts preferred soils with high electrical conductivity (70 dS m–1; Anderson et al. 1982). In contrast, experimental addition of NaCl to cyanobacterial lichen crusts in the Chihuahuan desert inhibited nitrogenase activity (Delwiche and Wijler 1956). Nutrient effects on nitrogenase activity vary in drylands and largely depend on the element. Phosphorus (P) and potassium (K) additions can stimulate cyanobacterial nitrogenase activity, likely through the stimulation of ATP synthesis (Dodds et al. 1995). Low amounts of zinc (Zn), cobalt (Co), molybdenum (Mo), and iron (Fe) can also stimulate cyanobacterial nitrogenase activity, whereas higher levels of the same elements have adverse effects (Granhall 1981; Dodds et al. 1995). Glucose (i.e., carbon) additions to soils and BSCs have been shown to increase heterotrophic nitrogenase activity, suggesting that C sources are essential in N dynamics (Hartley and Schlesinger 2002; Billings et al. 2003; Hartley et al. 2007).
Hypolithic microbial communities (biofilms on quartz and translucent rocks) represent major contributions to organic biomass in extreme deserts where communities are dominated by cyanobacterial photoautotrophs (Chan et al. 2012), although red Chloroflexi-dominated hypoliths have been encountered in the Atacama Desert (Lacap et al. 2011). In the Mojave Desert, hypolithic dinitrogen fixation potential was demonstrated using ARA measurements (Schlesinger et al. 2003), while a recent shotgun metagenome study revealed that Namib Desert hypolithic communities could mediate the full N-cycle (apart from ANAMMOX; Vikram et al. 2016). These results were later supported by stable N isotope (δ15N) measurements of Namib Desert hypolithic biomass, and surface and subsurface soils over a 3 year period across dune and gravel plain biotopes (Ramond et al. 2018). The authors demonstrated that photoautotrophic hypolithic communities are the main contributors to N2 fixation for plant productivity events in the Namib Desert. In accordance with these findings, metatranscriptome profiling of Namib Desert soils revealed an active microbial community dominated by non-photosynthetic bacteria where nitrate appeared to be the source of bioavailable N (Leon-Sobrino et al. 2019).
7.2.2 Atmospheric N Deposition and N Discharges as N Inputs in Drylands
Although BNF is the most studied and described process to generate N in drylands, other inputs such as atmospheric depositions and accumulations, environmental runoff, discharge of N-rich untreated wastewater, and/or agriculture processes are also important N sources.
Drylands act as large reservoirs for nitrate-rich salts. N is delivered to desert landscapes by either wet or dry atmospheric deposition of particles produced by gas to particle conversion, and by biological N2 fixation (Böhlke et al. 1997; Michalski et al. 2004; Graham et al. 2008). Wet deposition of N (e.g., precipitation, sea spray, and fog) has been implicated as sources of NH4+, NO3– and other salts in the Turpan-Hami area, northwestern China (Qin et al. 2012), and the Atacama (Michalski et al. 2004; Wang et al. 2014c, d), Mojave (Michalski et al. 2004), and Chihuahuan Deserts (Báez et al. 2007). Additionally, drylands receive external N inputs via the discharge of untreated, ammonia-rich wastewater (e.g., Negev and Tengger Deserts), or through agricultural processes where poultry manure is used for soil amendments (Abed et al. 2010; Posmanik et al. 2017; Zuo et al. 2018). Large N inputs have been deposited in areas surrounding the Gurbantunggut Desert following farming activities (Li et al. 2012a). All these practices induce nitrification and denitrification with the subsequent release of greenhouse gasses (N2O).
7.2.3 Nitrogen Losses in Drylands
Biological fixed N does not accumulate over time and is lost to the atmosphere from drylands through several mechanisms including ammonia volatilization, wind erosion, leaching, nitrification, and denitrification (Peterjohn and Schlesinger 1990; Walvoord et al. 2003; McCalley and Sparks 2009; Wang et al. 2014a, b; Jin et al. 2015). Volatilization is considered to be the primary cause of N loss in deserts, with wind erosion contributing to 6.6% of total N loss (Peterjohn and Schlesinger 1990; McCalley and Sparks 2009). Volatile losses of N include nitric oxide (NO), nitrous oxide (N2O), ammonia (NH3), and dinitrogen (N2) gas (Peterjohn and Schlesinger 1990). Of these compounds, NO, N2O, and NH3 are of particular consequence to the atmosphere affecting ozone levels and contributing to global warming (Logan et al. 1981; Peterjohn and Schlesinger 1990; van Amstel and Swart 1994). N loss from dryland soils through leaching was previously considered to be negligible, however, several studies challenged this assumption and demonstrated significant NH3 leaching and accumulation in subsoil zones (Walvoord et al. 2003; Jin et al. 2015).
Nitrification and denitrification processes, especially in BSCs, are considered major contributors to N loss in dryland soils (Austin et al. 2004). BSCs have been shown to emit NO, N2O, and nitrous acid (HONO) with amounts dependent on N2 fixation rates, crust type, and season (Barger et al. 2005; Abed et al. 2013; Weber et al. 2015; Maier et al. 2018). Weber et al. (2015) showed that dark cyanobacteria-dominated crusts exhibit the highest NO and HONO emission fluxes and can even exceed those of neighboring uncrusted soils by 20 times. Using laboratory, field, and satellite measurement data, the authors estimated a global emission of reactive N from BSCs at ~1.7 Tg year–1 (1.1 Tg year–1 of NO-N and 0.6 Tg year–1 of HONO-N), corresponding to ∼20% of global NOx emissions from soils under natural vegetation. In the desert of northern Oman (Arabian Desert), N2O gas was emitted from incomplete denitrification at very high potential rates of 387 ± 143 and 31 ± 6 μmol N m–2 h–1 from cyanobacterial and lichen crusts, respectively (Abed et al. 2013). This contributed up to 54–66% of the total produced gases during denitrification in both types of crusts. Although lower N2O emissions have been detected from other drylands such as the Sonoran Desert (Guilbault and Matthias 1998), Great Basin (Mummey et al. 1994), and southeastern Utah (Belnap 2001b), their environmental implications should not be underestimated.
The potential for N loss from drylands is primarily affected by precipitation, substrate availability, and high soil-surface temperatures (McCalley and Sparks 2008, 2009; Yahdjian and Sala 2010). In the Mojave Desert, NH3 dominated reactive N gas emissions were shown to be higher during the summer season, while a fall precipitation event yielded even larger N fluxes (McCalley and Sparks 2008). Further laboratory manipulations of environmental conditions on N gas emissions showed a large transient NH3 pulse (~70–100 ng N m−2 s−1) following water addition, presumably driven by an increase in soil NH4 concentrations. As such, NO production was boosted with maximum NO flux rates of 34 ng N m−2 s−1. Similar results have been observed for rainfall manipulated experiments in the Chihuahuan Desert (Hartley and Schlesinger 2000) and Patagonian steppe (Yahdjian and Sala 2010). N2O flux in drylands is relatively constant over the dry seasons; however, N2O emissions have been shown to considerably increase following precipitation and/or irrigation events (Hall et al. 2008; Chen et al. 2013; Liu et al. 2014; Yue et al. 2020). These brief “wetting-pulse” patterns of N2O fluxes can account for the majority of annual N2O emissions, making dryland N2O patterns unique compared with other terrestrial ecosystems (Hu et al. 2017). Interestingly, studies have found that N gas fluxes are responsive to additions of labile C and elevated CO2 (Schaeffer and Evans 2005; McCalley et al. 2011). Increased availability of C alters soil N dynamics by increasing biological (plant and microbial) activity and immobilization of N, thereby reducing N availability for nitrification and denitrification, and NH3 volatilization (Schaeffer and Evans 2005; McCalley et al. 2011).
7.2.4 Nitrification and Denitrification in Desert Soils
Nitrification is a biological two-step process in which NH3 is oxidized to nitrite (NO2−) followed by the oxidation of NO2− to NO3–. The transformation of ammonia to NO2− is usually the rate-limiting step of the process (Kowalchuk and Stephen 2001) performed by ammonia-oxidizing bacteria (AOB) and/or archaea (AOA) (reviewed by Offre et al. 2013; Stein 2019). Subsequent oxidation of NO2− to NO3– is performed by nitrite-oxidizing bacteria (NOB), mostly from the genera Nitrobacter and Nitrospira. Both steps produce energy coupled to ATP synthesis. Ammonia- and nitrite oxidation may also occur simultaneously in Comamox bacteria (Daims et al. 2015; van Kessel et al. 2015).
Nitrification potential for drylands has been demonstrated in both soils and BSCs across several deserts and drylands (e.g., Colorado Plateau, Great Basin, and Negev and Sonoran Deserts; Nejidat 2005; Strauss et al. 2012; Marusenko et al. 2013, 2015; Delgado-Baquerizo et al. 2016). Interestingly, results suggest that temperature and aridity are important modulators of AO communities and that AOA abundance dominates over that of AOB in bare soils at more extreme conditions (i.e., higher temperature and aridity; Marusenko et al. 2013; Delgado-Baquerizo et al. 2016). Conversely, vegetated dryland soils show a higher abundance and richness of AOB than AOA due to higher OM and available N contents (Delgado-Baquerizo et al. 2016). Collectively, results suggest that niche differentiation plays a key role between AOA and AOB communities (Marusenko et al. 2013, 2015; Zhou et al. 2016; Delgado-Baquerizo et al. 2016). Furthermore, AO estimates (mean of 110 μmol N m–2 h–1) in BSCs from the Colorado Plateau and the Mojave, Sonoran, and Chihuahuan Deserts indicate that the magnitude of N cycling processes (e.g., nitrification) is subject to seasonal changes where soil temperature and moisture levels affect microbial activity (Strauss et al. 2012). Crust type and oxygen availability have also been implicated as important factors in ammonia oxidation fluxes (Johnson et al. 2005). For example, Johnson et al. (2005) found that AO rates (measured at depth of 2–3 mm) from light cyanobacterial crusts (53.4 ± 28.1 mmol N m–2 h–1 in light and 28.6 ± 13.0 mmol N m–2 h–1 in dark) were higher than for dark cyanobacterial crusts (42.0 ± 21.1 mmol N m–2 h–1 in light and 14.8 ± 10.2 mmol N m–2 h–1 in dark), irrespective of the incubation strategy (Johnson et al. 2005). It should be noted that potential rates were based on wet crusts and are likely to be overestimated.
Nitrate is removed from the immediate environment by processes such as denitrification, assimilation, or anaerobic ammonia oxidation (anammox). Denitrification, the biological process in which NO2–, NO, and N2O are successively reduced to N2 gas, is a facultative anaerobic process performed by heterotrophic bacteria (Williams et al. 1992). This process is highly dependent on NO3– and C source availability (Williams et al. 1992). In addition to bacteria, several fungi are able to perform denitrification during anaerobic respiration (Shoun et al. 2012). Drylands are not considered as denitrification hotspots, although denitrification potential and denitrifying enzyme activity have been detected in grasslands (Santa Barbara, CA; Homyak et al. 2016), semiarid (“El Romeral,” Cajón del Maipo, Chile; Orlando et al. 2012) and hyperarid soils (Atacama Desert; Orlando et al. 2012), and BSCs (Abed et al. 2013). Studies suggest that semiarid soils have a higher denitrification potential (based on the presence of nirK and nirS genes) than hyperarid soils attributable to its higher soil moisture and nutrient content (Orlando et al. 2012). Denitrification rates in BSCs are likely to surpass those in uncrusted soils due to the formation of SOC in crusts (Barger et al. 2016; Brankatschk et al. 2013), however, contradictory results exist to support this hypothesis (see Barger et al. 2016 for full review on denitrification in BSCs).
7.3 Phosphorus in Dryland Soils
Phosphorus (P) is one of the less-abundant macronutrients in the lithosphere (0.1% of total) and then considered as a limiting nutrient (Bate et al. 2008), thus turnover of organic phosphorus (Po) is critical for community structure, species diversity, and ecosystem processes (Runge-Metzger 1995; Sardans et al. 2012). P concentration is fundamental in the cycling of nutrients such as soil organic matter (SOM), nitrogen (N), and C (Stevenson and Cole 1999; Mackenzie et al. 2002; Vitousek et al. 2010; Griffiths et al. 2012; George et al. 2018), and vital to cellular organization (phospholipids) and genetic material (nucleic acids), intracellular signaling molecules, and for metabolism and energy transfer (ATP; Ruttenberg 2003; Butusov and Jernelöv 2013).
In mesic terrestrial environments, soil P is derived from three sources: (1) chemical and biological weathering of bedrock; (2) aeolian dust deposition; and (3) decomposition of biomass (Walker and Syers 1976; Lajtha and Schlesinger 1988; Okin et al. 2004; Turner et al. 2007; Yang and Post 2011; Porder and Ramachandran 2013). In most soils, P is the result of geochemical weathering of parent material (Lajtha and Schlesinger 1988), with relatively small inputs from aeolian deposition (Okin et al. 2004; Selmants and Hart 2010). Plants and microbes incorporate P into biomass and return it to the soil in organic forms, which can then be recycled by phosphatase enzymes to release inorganic phosphate for biological uptake (Walker and Syers 1976; Cross and Schlesinger 1995; Turner et al. 2007). Contrariwise, the input of P into dryland soils is primarily the result of atmospheric dust deposition (see Belnap 2011 for a comprehensive description), followed by biological weathering of parent materials (Reynolds et al. 2006; Belnap 2011), and then topsoil (0–15 cm) typically contain high concentrations of total P (Jobbagy and Jackson 2001; He et al. 2014).
7.3.1 P Stocks and Redistribution by Biological Processes in Drylands
Phosphorus stocks in dryland soils range from ~200 to 1200 mg P kg–1 (Tiessen et al. 1984; Turner et al. 2003a; Plaza et al. 2018a). More than 50% of the total P consists of labile inorganic and apatite P, that are mostly unavailable for plant or microbial uptake (Cross and Schlesinger 2001; Jones and Oburger 2011). Since Po is mostly absent or at low concentrations, variation in P fractions can be closely related to the Pi content of parent materials (Neff et al. 2006; Buckingham et al. 2010). In addition, Pi is more likely to be found in mineral-associated forms such as oxides (e.g., aluminum and iron), clays, or carbonates (Sinsabaugh et al. 2008). In fact, calcium (Ca) minerals (e.g., calcium carbonate-and phosphate) are the predominant reservoir of P (Lajtha and Schlesinger 1988; Cross and Schlesinger 1995; Sims and Pierzynski 2005; Belnap 2011) in drylands due to reduced water inputs and high evapotranspiration.
The ability of P to change from one fraction to another is mainly controlled by mineralogy (Cross and Schlesinger 2001; Lajtha and Schlesinger 1988; Neff et al. 2006). Several different minerals play important roles in the stabilization and release of P (Buckingham et al. 2010) in the soil matrix. For example, studies have shown that carbonates sorbs P moderately due to its positive electrostatic charge, although the association can be reversed by changes in pH (Tiessen and Moir 1993), where goethite quickly sorbs P via ligand exchange until the sorption requirements of the mineral are met (Goldberg and Sposito 1985). Some minerals, such as aluminum (Al) and iron (Fe) oxides, may stabilize P through several mechanisms (Buckingham et al. 2010). These oxides can sorb P onto mineral surfaces that are reversible or may exchange ligands that sequester P into the physical structure of the oxides (Sollins et al. 1988). Amorphous minerals tend to sorb P more rapidly than crystalline materials (Ryan et al. 1985) and form tighter bonds with P because of their larger surface area (Carreira et al. 2006). Ultimately, the type and intensity of P-mineral bonds are important characteristics that determine its mobility and plant availability in soils (Buckingham et al. 2010).
In un-weathered or moderately weathered soils with neutral to alkaline pH, calcium-phosphates are the main supply of Pi. In contrast, Al and Fe phosphates and Pi bound and/or occluded by Al and Fe oxy(hydr)oxides predominate in acidic and more progressively weathered soils (Sims and Pierzynski 2005). In neutral and acidic soils, Al and Fe oxides and hydroxides exert a great impact on P availability given that various identified Fe and Al phosphates (e.g., wavellite, variscite, and strengite) are generally rare in occurrence (Harris 2002). In addition, the increased positive surface charge of the oxides forms strong covalent bonds (chemisorption) with the negatively charged P under acidic conditions, rendering it rather recalcitrant to exchange reactions (Jones and Oburger 2011). Nevertheless, changes in pH might directly or indirectly affect the oxides’ surface potential and consequently Pi solubility. Also, LMW organic anions (e.g., gluconate, oxalate, etc.) released by P solubilizing organisms are capable of competing with Pi for sorption sites (Jones and Oburger 2011).
Po influences P availability by contributing to the labile Pi pool, which is important to NPP as mentioned previously (Cross and Schlesinger 2001). Certain fractions of Po are relatively labile and accessible for biological uptake (Haas et al. 1961; Wild and Oke 1966; Dalal 1977), while unavailable Po is mineralized to labile Pi by simple autolysis or enzymatic phosphorylation (Cosgrove 1977). On average, Po can comprise 30–65% of total P in mineral soils, while Po approaches up to 90% in organic soils (Dalal 1977; Jones and Oburger 2011). The main Po compounds in soil include inositol phosphates (>80%), phospholipids (0.5–7.0%), and nucleic acids (<3%; Dalal 1977; Quiquampoix and Mousain 2005; Turner et al. 2002; Jones and Oburger 2011). Other, less abundant forms of Po include sugar-P (Anderson 1961), monophosphorylated carboxylic acids (Anderson and Malcolm 1974), and teichoic acids (Zhang et al. 1998).
The redistribution of P into various mineral and chemical fractions is impacted by mechanical and biological weathering, biological uptake and cycling, adsorption onto secondary minerals or soil constituents, or leaching (Walker and Syers 1976; Tiessen et al. 1984; Ruttenberg 2003). These P pools are typically represented as soluble phosphate, Po, and primary and secondary mineral fractions that vary in their potential mobility, relative quantities, and availability to biota (Hedley et al. 1982; Tiessen et al. 1984; Kuo 1996). The horizontal (e.g., wind, water, wildlife, livestock, human activities) and vertical (e.g., animals, insects, and plants) redistribution of soil P in dryland systems is imperative for plant productivity. Redistribution of P can determine the community, biomass, and distribution of a given plant community, which in turn affects animal distributions (Belnap 2011). For a comprehensive review on redistribution of soil P in drylands see Belnap (2011).
7.3.2 Abiotic and Biotic Control of P Cycling
Biogeochemical cycling of P in drylands is ultimately controlled by precipitation and temperature (Belnap 2011; Hou et al. 2018). The timing, intensity, and amount of water input directly affect the availability of P by influencing rates of geochemical reactions, ion diffusion, and biological activity (Belnap 2011). Because precipitation in arid environments is highly variable and inherently low, pulses of P-releasing activities vary on both spatial and temporal scales (Belnap 2011). While precipitation affects soil P leaching, temperature can directly enhance soil P sorption and desorption (Barrow 1983). Climate may also affect soil P availability through additional factors including: (1) different soil P forms; (2) soil properties such as soil particle size, pH, and SOM; and (3) plant and soil microbial activities (e.g., P uptake and return; Hou et al. 2018). For instance, high temperature facilitates the transformation of labile P and secondary mineral P to occluded P in soil (Barrow 1983; Siebers et al. 2017), whereas high precipitation may drive the loss of secondary mineral P or even occluded P in very humid environments (annual precipitation >2000 mm; Austin and Vitousek 1998). The interaction of precipitation and temperature can also affect the release of bound P (see Belnap 2011). Essentially, cool and wet conditions result in greater production of carbonic acid (H2CO3) that causes the soil pH to decrease, thus dissolving carbonates and increasing the transition of solid-phase P to solution-phase P (Lajtha and Schlesinger 1988; Magid and Nielsen 1992; Jungk and Claassen 1997; Miller et al. 2006a, b).
While the availability of Pi in dryland soils is mainly regulated by the dissolution properties of P-bearing minerals, the availability of P derived from Po is largely governed by microbial activity (Jones and Oburger 2011). Soil microorganisms can solubilize P via three mechanisms including: (1) the active or passive release of protons, CO2, and secondary organic metabolites (e.g., sugars, organic acid anions, amino acids, siderophores, enzymes, phenols); (2) the release of extracellular phosphatase enzymes (biochemical Po mineralization); and (3) the release of Po during substrate degradation (biological Po mineralization) (Mcgill and Cole 1981; Jones and Oburger 2011). P incorporated into microbial biomass, to allow the use of organic C and root exudates for energy (Wu et al. 2007), may be temporarily immobilized but remains in a bioavailable form that can be released via microbial turnover (re-mineralization; Jones and Oburger 2011).
Approximately 1–50% of soil bacteria and 0.5–0.1% of soil fungi can be classified as P-solubilizing microorganisms (PSM; Kucey et al. 1989; Gyaneshwar et al. 2002); however, their population and activity vary with environment and depend upon various environmental factors (Chatli et al. 2008). P-solubilizing organisms are capable of hydrolyzing Po and Pi compounds from insoluble P (Kalayu 2019). Among the PSM group, species from bacterial genera (Bacillus, Pseudomonas, Rhizobium, Burkholderia, Achromobacter, Agrobacterium, Microccocus, Aereobacter, Flavobacterium, and Erwinia), fungal general (Penicillium, Aspergillus, and Trichoderma), and actinomycetes (Streptomyces and Streptoverticillium) are significant in solubilizing phosphate (Rodriguez and Fraga 1999; Whitelaw 2000; Kumar et al. 2018; Kalayu 2019). Although the number of bacteria in soil classified as PSM generally outnumber those of fungi, the fungal isolates generally exhibit a greater P-solubilizing capacity (Banik and Dey 1982; Gyaneshwar et al. 2002). The mechanisms and processes involved in P mobilization by PSM in soil are discussed elsewhere (Jones and Oburger 2011; Richardson and Simpson 2011).
Microbial immobilization of P constitutes a significant component of the total soil P and is generally equivalent to, or exceeds that held in plant biomass (Richardson and Simpson 2011). Concentrations of microbial P in dryland soils typically account for 2–4% of total soil P (Lajtha and Schlesinger 1988; Xu et al. 2013; Perroni et al. 2014). Importantly, microbial P is a highly dynamic pool of soil P and release of P (i.e., orthophosphate and in organic forms) during the microbial biomass turnover is subject to significant change in response to environmental factors such as seasonal conditions, soil temperature and moisture, and C availability (Patra et al. 1990; He et al. 1997; Butterly et al. 2009; Richardson and Simpson 2011).
Carbon dynamics in soil have been closely linked to microbial biomass P (Achat et al. 2009) where immobilization of P is highly regulated by C availability (C:P ratio; Spohn and Kuzyakov 2013; Heuck et al. 2015). Microbial phosphatases and other enzymes are involved in organic C mineralization to promote the availability of P and C from organophosphorylated compounds (Spohn and Kuzyakov 2013; Heuck et al. 2015). Thus, P immobilization and transformation is inherently coupled, to a certain extent, with C mineralization (Luo et al. 2020). As soil microbial activity increases with the availability of C (i.e., SOM), immobilized P is released to increase the available P during microbial biomass turnover (Yang et al. 2010; Haripal and Sahoo 2014). When C sources become limited microbial biomass P will subsequently decrease (low C:P ratio; Yang et al. 2010; Haripal and Sahoo 2014). Given that the turnover of microbial P is largely driven by the C availability it is of particular importance in the rhizosphere (Richardson and Simpson 2011). As orthophosphate diffuses through the rhizosphere/mycorrhizosphere (Jakobsen et al. 2005) it is in direct competition for uptake and immobilization by microorganisms. Subsequently, the rate of release of P from microorganisms or microbial biomass turnover time within the rhizosphere will have major implications for P availability to plants (Richardson and Simpson 2011).
7.4 Role of Biological Soil Crust in Nutrient (C, N, and P) Cycles
Biological soil crusts (BSCs) or biocrust consist of photoautotrophic (green microalgae and cyanobacteria) and heterotrophic (bacteria and fungi) organisms, stable layers predominantly on bare soil surfaces in which inorganic particles are bound together by sticky extracellular polymeric substances (EPS; Belnap et al. 2001). The importance of biocrusts in the functioning of ecosystems in arid environments is well documented (Veste et al. 2001; Belnap et al. 2003; Maestre et al. 2011; Chap. 3).
It is estimated that BSCs are responsible for global C fixation of ~7% of the terrestrial vegetation, and for N fixation of ~50% of terrestrial biological N fixation (Elbert et al. 2012). Much of the C that is photosynthetically assimilated by biocrusts is released to the underlying soil shortly after fixation, thereby increasing the total amount of C and organic matter in soil (Pointing and Belnap 2012). Biocrusts also regulate the temporal dynamics of soil CO2 efflux and net CO2 uptake (Castillo-Monroy et al. 2011; Wilske et al. 2008, 2009). Studies have shown that temporal increase in soil moisture (i.e., higher and more intense precipitation frequencies) significantly increases C inputs in BSCs (Wilske et al. 2008; Huang et al. 2014), although the moisture levels at which organisms become photosynthetically active are highly variable (Belnap et al. 2004). Concurrently, higher moisture levels and temperature induce CO2 emissions (Maestre et al. 2013) by several factors:
-
(i)
Infiltration of water may physically displace the CO2 accumulated in soil pore spaces during the dry season.
-
(ii)
Rewetting triggers microbial activity in both biocrusts and underlying soil matrix that results in increased respiration rates.
-
(iii)
Water addition may induce CO2 release from microbial biomass pool (Li et al. 2018).
Biocrusts may also influence the activity of C-related soil enzymes, depending on the environmental conditions (e.g., β-glucosidase; Bowker et al. 2011; Yuan and Yue 2012; Miralles et al. 2013). When moisture and organic C content increases enough, sufficient resources are available to support higher microbial biomass and thus higher enzyme activity. High organic C content also allows existing enzymes to be stabilized by their absorption to SOM (Yuan and Yue 2012). Conversely, low moisture content and organic C result in relatively low microbial growth, enzyme stabilization, and activity (Yuan and Yue 2012). BSCs type plays an important role in C fixation at sites (Sancho et al. 2016; Maier et al. 2018). For example, Miralles et al. (2018) have demonstrated that late-successional biocrusts (i.e., lichens and mosses) had higher gross photosynthesis than early-successional biocrusts (cyanobacteria) in two semiarid ecosystems. Similar results were obtained by Housman et al. (2006) for the Colorado Plateau and Chihuahuan Desert where late-successional biocrusts (i.e., cyanobacteria [Nostoc and Scytonema] and lichens [Placidium and Collema]) had higher C fixation rates (1.2–1.3-fold) than early-successional biocrusts (cyanobacteria [Microcoleus]). Estimates for annual C inputs range from 0.4 to 2.3 g C m–2 year–1 for early successional crusts to 12–37 g C m–2 year–1 for late-successional crusts (Evans and Lange 2003; Li et al. 2012b; Yan-Gui et al. 2013), representing 1% of the NPP of terrestrial vegetation (~56 Pg year–1; Zhao et al. 2005). In terms of dryland systems, biocrusts account for ~9% of the total NPP, corresponding to ~0.07 Pg year–1 compared to the total NPP of ~0.8 Pg year–1 (Zhao et al. 2005; Elbert et al. 2012; Sancho et al. 2016). In terms of C release, studies have found that 40–60% of soil respiration in dryland ecosystems were attributable to (algal) biocrust-dominated sites (Castillo-Monroy et al. 2011; Zhang et al. 2013). However, soil respiration is highly dependent on the type of crust, temperature, and precipitation (Tucker and Reed 2016; Zhang et al. 2016b; Guan et al. 2018; Li et al. 2018).
Given that most BSC components can fix C, the availability of P increases as organic matter accumulates in soil (Belnap 2011). The availability of P is further intensified through the secretion of extracellular phosphatases, organic acids (e.g., oxalic acid, citric acid, and malic acid), and metal chelators (e.g., siderochromes; Lange 1974; Belnap 2001a, 2011; Crain et al. 2018). Most BSC organisms (soil and hypolithic cyanobacteria, green algae, lichens, and mosses) contain phosphatases in their cell walls and mucilaginous sheaths, but they also release extracellular phosphatases into the surrounding soil (Belnap 2011). Phosphatases hydrolyze organic phosphates to subsequently release P (Turner et al. 2003b; Nannipieri et al. 2011; Baumann et al. 2017; Crain et al. 2018) which can then be immobilized by microbes, transferred to plant host roots, or stabilized by humic substances (Sinsabaugh 1994; Lindahl et al. 2005). As phosphatase activity is highly correlated with SOM, which occurs at low concentrations in dryland soils, reduced phosphatase activity is expected compared to more mesic environments (Sinsabaugh et al. 2008).
In addition to nutrient cycling, BSCs control the water balance (Warren 2003; Chamizo et al. 2016) by regulating the following factors (Chap. 3):
-
(i)
Water infiltration and runoff (Belnap 2006; Chamizo et al. 2012, 2016; Zaady et al. 2013)
-
(ii)
Increase soil moisture retention
-
(iii)
Protect soil surface aggregates
-
(iv)
Prevent soil erosion by water or wind
-
(v)
Facilitate colonization by vascular plants (Belnap 2003)
-
(vi)
Contribute to the stabilization of sand dunes (Eldridge and Greene 1994; Belnap 2002)
The presence of BSCs can also alter soil surface conditions in ways that affect the suitability of the habitat for other organisms (Bowker et al. 2005, 2006). However, their metabolic activity is strongly linked to moisture availability (Sancho et al. 2016). Due to low and highly variable precipitation pulses in drylands, biocrusts are sporadically active (Johnson et al. 2012; Yu et al. 2014; Sancho et al. 2016; Fernandes et al. 2018; Miralles et al. 2018). Nevertheless, their distinguishing characteristics highlight their overwhelming ecological importance in dryland environments, particularly in element cycling where the C, N, and P cycles are interlinked (Delgado-Baquerizo et al. 2013).
7.5 Influence of Hydration-Desiccation Pulses on Nutrient (C, N, and P) Cycles
Infrequent precipitation events occur in drylands; thus, soil surfaces are most often dry. During desiccation periods, nutrients accumulate on the soil surface due to dust deposition and the degradation and/or death of organisms from UV exposure (Belnap 2011). When precipitation occurs at higher temperatures, microbial diversity and function tend to rapidly increase thus affecting the rate of nutrient cycling in soils (Belnap 2011; Bell et al. 2014; Montiel-González et al. 2017; Chap. 11). Should precipitation occur at the proper time to stimulate annual plant activity for a few weeks to months, subsequent C inputs will stimulate soil microbial activity, including processes that release bound P (Whitford 1999). However, when a small amount of precipitation occurs (<5 mm rain) there is a shallow penetration of water into the soil, and substantial buildup of nutrients at the surface, that elicit more responses from soil surface organisms and their associated processes than vascular plants. The temporal dynamics of water use between microbial and plant communities can result in decoupling between their responses to precipitation and nutrients (Stursova et al. 2006; Belnap et al. 2004), but also in the accumulation of nutrients (hydrolyzable Po and C) at the soil surface between large precipitation events (Whitford 2002; White et al. 2004).
In that sense, much attention has been given to hydration-desiccation cycles in drylands and the impact of rewetting on microbial communities and nutrient cycling (emphasis on C) in both laboratory conditions and field studies (Austin et al. 2004, Bell et al. 2014; Frossard et al. 2015; Montiel-González et al. 2017; Schimel 2018; Chap. 11). As soils dry, diffusion rates decline and semi-soluble materials precipitate (Schimel 2018) causing water stress. Microbes will increase their intracellular solute concentration to compensate for the extracellular concentration and counterbalance the increased osmotic pressure (Stark and Firestone 1995; Bell et al. 2008). This high concentration of solutes results in an inhibition of the enzymatic activity and therefore decreases cellular activity (Batlle-Aguilar et al. 2011). As a consequence, microbial activity and respiration is reduced (Stark and Firestone 1995; Austin et al. 2004; Schimel 2018), and a portion of microbial biomass is killed under such conditions (Austin et al. 2004). However, the magnitude of the respiration decrease may vary; in some cases, substantial reductions in soil moisture have limited effects on respiration (Lu et al. 2017).
Rewetting of soils after a drought period can result in large pulses of CO2, also known as the “Birch effect” (Birch 1958). The CO2 pulse can be many times greater than the basal respiration level (Schimel 2018), although the size of the pulse may largely depend on the labile C soil pools and the soil’s history of physical disturbance (Austin et al. 2004; Schimel 2018). The exact mechanism that drives the Birch effect remains unclear and contrasting patterns emerge from rewetting experiments (see Schimel 2018 for a comprehensive overview). A study by Fierer et al. (2003) suggests that during each rewetting cycle, carbon is immediately lost from the (dead) microbial biomass and labile SOM is simultaneously mobilized from the soil matrix. The mobilized material may then be utilized by microbes to replace lost biomass C and to promote additional growth (Schimel 2018). Therefore, through multiple cycles, the C mobilized may ultimately be from stable soil C, but within each cycle, the C originates immediately from microbial biomass (Schimel 2018).
In addition to C, wet–dry cycles have also important consequences on N cycling processes. In drylands covered with BSCs, water pulses have a temperature-dependent impact: cold desert soils remain moist for longer periods of time than hot desert soils, thereby increasing the N inputs into soils (Austin et al. 2004). Since rates and duration of nitrogenase activity are mainly controlled by water availability (Hartley and Schlesinger 2000; Belnap 2002; Hartley and Schlesinger 2002; Wu et al. 2009; Zhou et al. 2016), N fixation in drylands may start within a few hours following a wetting event (Abed et al. 2010) as organic matter increases due to primary production (Wierenga et al. 1987). For example, nitrogenase activity was detected within 2 h after wetting dry cyanobacteria-dominated crusts (i.e., Nostoc, Scytonema, and Microcoleus) from Sahel, Niger (Malam Issa et al. 2001). In the Great Basin, nitrogenase activity was observed within 3 days after wetting cyanolichen crusts (Jeffries et al. 1992).
Wetting of dry soils also induces NO emissions, which can reach 400-fold compared to dry soils (Austin et al. 2004). Likewise, water pulses have a critical effect on denitrification where rates can increase significantly after precipitation events (Austin et al. 2004 and references thereof). Even smaller water pulses, either by rainfall, fog, or dew, following a large precipitation event can readily activate soil and BSC microorganisms to respire, photosynthesize, and perform other metabolic processes (Belnap and Lange 2001; Belnap 2003; Veste et al. 2008). N formed by metabolically active BSCs, mainly as NH4+, may leak to the environment increasing nutrient availability for nearby vegetation and microorganisms (reviewed in Belnap 2001b). Increased N will also lead to higher N loss through nitrification and denitrification processes as discussed above (Zaady 2005; Barger et al. 2005; Johnson et al. 2007; Strauss et al. 2012; Brankatschk et al. 2013; Liu et al. 2016).
An important aspect to consider in soil respiration is that of microbial community composition. Bacteria and fungi are ecologically and physiologically distinct groups, support different soil food-chains (De Vries et al. 2006) and differ in their C use efficiency (Sakamoto and Oba 1994). Studies have demonstrated that fungi have a higher resistance to drought and that fungal growth is largely unresponsive to drying-rewetting cycles (Bapiri et al. 2010; Evans and Wallenstein 2012; Manzoni et al. 2012; Barnard et al. 2013; Canarini et al. 2017). However, new evidence suggests that fungal communities are more strongly influenced by drying-wetting cycles than previously considered and that communities shift toward a more Ascomycota-dominated population (Hicks et al. 2019; Liu et al. 2019). In contrast, bacterial communities are more responsive to wetting pulses and may show an immediate and linear growth response following rewetting, or bacterial growth starts exponentially after a prolonged lag-period of no growth (Meisner et al. 2013, 2015). This causes changes in the fungal and bacterial biomass, as well as the fungal:bacterial (F:B) ratio, which ultimately influences the C use efficiency and C loss from soils (Fig. 7.1; Canarini et al. 2017).
Conceptual model of C dynamics during a drying-rewetting cycle summarizing the observed patterns obtained in a meta-analysis by Canarini et al. (2017). Fungi maintain higher respiration rates during the drying period, while bacteria show higher resilience and faster growth rates to return to pre-drought conditions when diffusion is restored (rewetting), causing a burst in respiration. Carbon-rich soils may boost this mechanism due to a higher fungal population, and by a greater release of available substrates (accumulated during the drying phase) to stimulate the bacterial community. A drought intensity threshold must be reached to initiate this response, possibly by regulating diffusion and therefore modulating the connection between available substrate and microorganisms. Adapted from Canarini et al. (2017)
7.6 Impact of Climate Change on Nutrient Cycling
Climate models estimate that global warming will likely aggravate dryland expansion due to increases in evaporative demand and a global hydrological cycle with longer and more severe droughts (Schlaepfer et al. 2017; Koutroulis 2019). Of particular concern is the decrease in soil moisture that can result in: (1) changes in vegetation due to shifts in plant functional types (Harrison et al. 2015), woody plant mortality (Allen et al. 2010), and encroachment (D’Odorico et al. 2012), and resistance of some vegetation types (Craine et al. 2013); and (2) rise in temperatures within 3.0–4.0 °C (IPCC 2014; Huang et al. 2017; Koutroulis 2019).
Also, increase in aridity along chrono- and climosequences has been shown to cause a decline in the concentration of both the total and the most biologically available forms of C and N, but an increase in biologically available forms of P, hence distorting soil C, N, and P cycles (Finzi et al. 2011; Delgado-Baquerizo et al. 2013; Feng et al. 2016). This results in an overall decrease in C:P and N:P ratios and decoupling of C and N cycles from P (Fig. 7.2; Evans and Burke 2013; Delgado-Baquerizo et al. 2013; Wardle 2013). Such changes to the environment and uncoupling of nutrient cycles may have detrimental effects on ecosystem function (Delgado-Baquerizo et al. 2013). Reduced availability of C and N may skew C:N:P stoichiometry in soil, constraining plant and microbial activity and diversity (Finzi et al. 2011; Peñuelas et al. 2012). This may negatively impact biogeochemical reactions that control key ecosystem functions (e.g., primary production, respiration, and decomposition) and services (e.g., food production and C sequestration; (Dodds et al. 2004; Schimel and Bennett 2004; Schimel 2010; Finzi et al. 2011; Delgado-Baquerizo et al. 2013; Maestre et al. 2016; Lal 2019), that can jeopardize human well-being and nature conservation (Schlaepfer et al. 2017).
Increase in aridity causes element cycles (C, N, and P) to decouple. Aridity is predicted to increase in many dryland ecosystems worldwide due to climate change. Delgado-Baquerizo et al. (2013) reported that, as aridity increases, available soil carbon (C) and nitrogen (N) decline, whereas available soil phosphorus (P) increases. This is a result of the impairment of biological processes that contribute to the C and N levels, and of an increase in the relative importance of abiotic processes that contribute to P availability. Adapted from Wardle (2013)
Changes in temperature, soil moisture, and vegetation structure and composition will likely modify the C sequestration capacity in high latitude drylands via direct warming effects on photosynthesis and decomposition of primary producers (Sjögersten and Wookey 2009), affecting the amount of biomass SOC stored in soils. In addition, evidence suggest that increase in temperature will affect biocrust communities that will consequently reduce their capacity to act as a carbon sink (Maestre et al. 2013; Darrouzet-Nardi et al. 2015; Escolar et al. 2015). For example, warming will reduce biocrust cover, richness, and diversity (Maestre et al. 2013; Ladrón de Guevara et al. 2018; Lafuente et al. 2018; Eldridge and Delgado-Baquerizo 2019), and communities will shift from late (i.e., dominated by mosses and lichens) toward early successional states dominated by cyanobacteria (Ferrenberg et al. 2015). This has critical implications for C sequestration and ecosystem functioning as a reduction in biocrust cover and the formation of early successional biocrusts will lessen the C storage capacity (Housman et al. 2006; Ferrenberg et al. 2015; Darrouzet-Nardi et al. 2018) of soils. This decrease may act synergistically with other warming-induced effects, such as the increase in CO2 efflux (Maestre et al. 2013; Darrouzet-Nardi et al. 2015, 2018; Escolar et al. 2015) and changes in microbial communities, to alter C cycling in drylands and ultimately reduce soil C stocks in the mid to long term (Maestre et al. 2013).
Importantly, drylands are sensitive to land degradation and desertification which dramatically reduce the amount of SOC stored in soils (Lal 2004a; Serrano-Ortiz et al. 2012). Previous studies have estimated that the historic SOC loss from drylands due to desertification ranges between 13 and 29 Pg C (Ojima et al. 1995; Lal et al. 1999; Lal 2001). Additionally, changes in vegetation structure induced by overgrazing promote C losses that may significantly change both plant and SOC pools (Gaitán et al. 2017; Abdalla et al. 2018). As SOC has a major impact on soil physical structure and ecosystem function (e.g., nutrient retention, water storage, pollutant attenuation), its reduction can lead to reduced soil fertility and further land degradation (Abdalla et al. 2018). The magnitude of this loss underscores the importance of appropriate management systems and best strategies to restore, preserve, or increase dryland SOC to enhance ecosystem functions and services, and mitigate climate changes (Plaza et al. 2018b).
Climate change will also significantly alter N cycling processes in drylands as NOx emissions are related to temperature (McCalley and Sparks 2009; Suddick et al. 2013). Optimal temperatures for N processes (e.g., N fixation) generally range between 20 and 30 °C (Trolldenier 1982; Montañez et al. 1995). With global temperatures rising, BNF and thus N input will consequently induce larger NOx emissions in hot deserts where summer temperatures already exceed 40 °C (Makhalanyane et al. 2015). In addition, higher N losses will further contribute to soil infertility, therefore unable to support most plant life. Although some climate models predict higher summer precipitation events for desert areas (e.g., arid regions of central Asia), the combination of moisture and heat would greatly enhance N losses. One should also consider that most climate models only consider biological factors to predict N gases losses from soils and rarely account for abiotic impacts on the N budget (McCalley and Sparks 2009).
P cycling will be also strongly influenced by climate change, as P availability is directly and indirectly impacted by both temperature and precipitation in drylands (Belnap 2011). Plants whose distribution is limited by available P will likely undergo spatial shits in habitat (Belnap 2011). Consequently, biological cycling of P will be reduced due to manifold factors: (1) a decline in soil moisture will not only negatively affect abiotic processes by slowing the release of bio-unavailable P, but also biotic processes (Belnap 2011). Reduced soil moisture will decrease microbial abundance and activity (Sardans et al. 2006), resulting in reduced acidification and production/excretion of enzymes, chelators, and other compounds to release bound P (Belnap 2011). Lower plant biomass, including root biomass, will further reduce soil acidification and root exudates to release bound P (Delucia et al. 1997; Li and Sarah 2003); (2) low soil moisture will also affect phosphatase activity as their effectiveness is more dependent on water availability than substrate availability (Sardans et al. 2008); (3) infrequent hydration-desiccation events will cause less P to be released from physical processes or from dead microbes (Belnap 2011); (4) severe droughts and higher temperatures will increase plant resorption of P, resulting in less P in detritus material (Killingbeck and Whitford 2001; Sardans et al. 2006; Luo et al. 2018); and (5) reduced plant cover can intensify dust emissions (Field et al. 2010; Duniway et al. 2019), resulting in substantial loss of many plant-essential nutrients including P, Na, K, and Mg from soil (Li et al. 2007; Belnap 2011; Katra et al. 2016).
7.7 Conclusion
Nutrient cycling in dryland soils and BSCs is a complex, yet active, process largely controlled by water availability (i.e., the size and frequency of precipitation events). Other abiotic factors such as temperature, C content and light, but also microbial species composition determine the rate by which nutrients are cycled. As aridity in drylands increases with climate change, the coupling between nutrient cycles will weaken and many arid environments can observe abrupt declines in organic C and total N content, while inorganic P will accumulate. Such changes are likely to have adverse effects on key ecosystem functions (e.g., primary production, respiration, and decomposition) and food services (e.g., food production and C storage). As such, comprehending the role and responses of microbial communities in drylands (soil and BSCs) can offer vital insights/predictions into future C and N fluxes, with important implications for future sustainable management and conservation policies.
References
Abdalla M, Hastings A, Chadwick DR, Jones DL, Evans CD, Jones MB et al (2018) Critical review of the impacts of grazing intensity on soil organic carbon storage and other soil quality indicators in extensively managed grasslands. Agr Ecosyst Environ 253:62–81. https://doi.org/10.1016/j.agee.2017.10.023
Abed RM, Al Kharusi S, Schramm A, Robinson MD (2010) Bacterial diversity, pigments and nitrogen fixation of biological desert crusts from the Sultanate of Oman. FEMS Microbiol Ecol 72(3):418–428. https://doi.org/10.1111/j.1574-6941.2010.00854.x
Abed RM, Lam P, De Beer D, Stief P (2013) High rates of denitrification and nitrous oxide emission in arid biological soil crusts from the Sultanate of Oman. ISME J 7(9):1862–1875. https://doi.org/10.1038/ismej.2013.55
Achat DL, Bakker MR, Morel C (2009) Process-based assessment of phosphorus availability in a low phosphorus sorbing forest soil using isotopic dilution methods. Soil Sci Soc Am J 73(6):2131–2142. https://doi.org/10.2136/sssaj2009.0009
Agarwal L, Dafale NA, Purohit HJ (2017) Microbial CO2 fixation bioprocesses and desert as future carbon sink. In: Purohit HJ (ed) Optimization and applicability of bioprocesses. Springer, Singapore
Ahlström A, Raupach MR, Schurgers G, Smith B, Arneth A, Jung M et al (2015) The dominant role of semi-arid ecosystems in the trend and variability of the land CO2 sink. Science 348(6237):895–899. https://doi.org/10.1126/science.aaa1668
Alcamán-Arias ME, Pedrós-Alió C, Tamames J, Fernández C, Pérez-Pantoja D, Vásquez M, Díez B (2018) Diurnal changes in active carbon and nitrogen pathways along the temperature gradient in porcelana hot spring microbial mat. Front Microbiol 9(2353):1–17. https://doi.org/10.3389/fmicb.2018.02353
Allen CD, Macalady AK, Chenchouni H, Bachelet D, Mcdowell N, Vennetier M et al (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecol Manag 259(4):660–684. https://doi.org/10.1016/j.foreco.2009.09.001
Allen-Diaz B, Chapin FS, Díaz S, Howden SM, Puigdefábregas J, Stafford Smith M (1996) Rangelands in a changing climate: impacts, adaptations, and mitigation. In: Climate change 1995: impacts, adaptations and mitigation of climate change: scientific-technical analyses. Cambridge University Press, Cambridge, pp 131–158
An H, Li Q-L, Yan X, Wu X-Z, Liu R-T, Fang Y (2019) Desertification control on soil inorganic and organic carbon accumulation in the topsoil of desert grassland in Ningxia, northwest China. Ecol Eng 127:348–355. https://doi.org/10.1016/j.ecoleng.2018.12.014
Anderson G (1961) Estimation of purines and pyrimidines in soil humic acid. Soil Sci 91(3):156–161
Anderson G, Malcolm RE (1974) The nature alkali-soluble soil organic phosphates. J Soil Sci 25(3):282–297. https://doi.org/10.1111/j.1365-2389.1974.tb01124.x
Anderson DC, Harper KT, Holmgren RC (1982) Factors influencing development of cryptogamic soil crusts in Utah deserts. J Range Manag 35(2):180–185
Aranibar JN, Anderson IC, Ringrose S, Macko SA (2003) Importance of nitrogen fixation in soil crusts of southern African arid ecosystems: acetylene reduction and stable isotope studies. J Arid Environ 54(2):345–358. https://doi.org/10.1006/jare.2002.1094
Austin AT, Vitousek PM (1998) Nutrient dynamics on a precipitation gradient in Hawai’i. Oecologia 113(4):519–529. https://doi.org/10.1007/s004420050405
Austin AT, Yahdjian L, Stark JM, Belnap J, Porporato A, Norton U et al (2004) Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141(2):221–235. https://doi.org/10.1007/s00442-004-1519-1
Bae HS, Morrison E, Chanton JP, Ogram A (2018) Methanogens are major contributors to nitrogen fixation in soils of the Florida everglades. Appl Environ Microbiol 84(7). https://doi.org/10.1128/AEM.02222-17
Báez S, Fargione J, Moore DI, Collins SL, Gosz JR (2007) Atmospheric nitrogen deposition in the northern Chihuahuan desert: temporal trends and potential consequences. J Arid Environ 68(4):640–651. https://doi.org/10.1016/j.jaridenv.2006.06.011
Banik S, Dey BK (1982) Available phosphate content of an alluvial soil as influenced by inoculation of some isolated phosphate-solubilizing microorganisms. Plant Soil 69(3):353–364. https://doi.org/10.1007/Bf02372456
Bapiri A, Baath E, Rousk J (2010) Drying-rewetting cycles affect fungal and bacterial growth differently in an arable soil. Microb Ecol 60(2):419–428. https://doi.org/10.1007/s00248-010-9723-5
Barger NN, Belnap J, Ojima DS (2005) NO gas loss from biologically crusted soils in Canyonlands National Park, Utah. Biogeochemistry 75:373–391. https://doi.org/10.1007/s10533-005-1378-9
Barger NN, Weber B, Garcia-Pichel F, Zaady E, Belnap J (2016) Patterns and controls on nitrogen cycling of biological soil crusts. In: Weber B, Büdel B, Belnap J (eds) Biological Soil crusts: an organizing principle in drylands. Ecological studies (analysis and synthesis). Springer, Cham, pp 257–285. https://doi.org/10.1007/978-3-319-30214-0_14
Barnard RL, Osborne CA, Firestone MK (2013) Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J 7(11):2229–2241. https://doi.org/10.1038/ismej.2013.104
Barrow NJ (1983) A mechanistic model for describing the sorption and desorption of phosphate by soil. J Soil Sci 34(4):733–750. https://doi.org/10.1111/j.1365-2389.1983.tb01068.x
Bate DB, Barrett JE, Poage MA, Virginia RA (2008) Soil phosphorus cycling in an Antarctic polar desert. Geoderma 144(1–2):21–31. https://doi.org/10.1016/j.geoderma.2007.10.007
Batlle-Aguilar J, Brovelli A, Porporato A, Barry DA (2011) Modelling soil carbon and nitrogen cycles during land use change. A review. Agron Sustain Dev 31(2):251–274. https://doi.org/10.1051/agro/2010007
Baumann K, Glaser K, Mutz JE, Karsten U, Maclennan A, Hu YF et al (2017) Biological soil crusts of temperate forests: their role in P cycling. Soil Biol Biochem 109:156–166. https://doi.org/10.1016/j.soilbio.2017.02.011
Beer C, Reichstein M, Tomelleri E, Ciais P, Jung M, Carvalhais N et al (2010) Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. Science 329(5993):834–838. https://doi.org/10.1126/science.1184984
Bell C, Mcintyre N, Cox S, Tissue D, Zak J (2008) Soil microbial responses to temporal variations of moisture and temperature in a Chihuahuan Desert Grassland. Microb Ecol 56(1):153–167. https://doi.org/10.1007/s00248-007-9333-z
Bell CW, Tissue DT, Loik ME, Wallenstein MD, Acosta-Martinez V, Erickson RA, Zak JC (2014) Soil microbial and nutrient responses to 7 years of seasonally altered precipitation in a Chihuahuan Desert grassland. Glob Chang Biol 20(5):1657–1673. https://doi.org/10.1111/gcb.12418
Belnap J (2001a) Microbes and microfauna associated with biological soil crusts. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function, and management. Ecological studies (analysis and synthesis), vol 150. Springer, Berlin, pp 167–174. https://doi.org/10.1007/978-3-642-56475-8_14
Belnap J (2001b) Factors influencing nitrogen fixation and nitrogen release in biological soil crusts. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function, and management. ecological studies (analysis and synthesis). Springer, Heidelberg, pp 241–261. https://doi.org/10.1007/978-3-642-56475-8_19
Belnap J (2002) Nitrogen fixation in biological soil crusts from southeast Utah, USA. Biol Fert Soils 35(2):128–135. https://doi.org/10.1007/s00374-002-0452-x
Belnap J (2003) The world at your feet: desert biological soil crusts. Front Ecol Environ 1(4):181–189. https://doi.org/10.1890/1540-9295(2003)001[0181:Twayfd]2.0.Co;2
Belnap J (2006) The potential roles of biological soil crusts in dryland hydrologic cycles. Hydrol Process 20(15):3159–3178. https://doi.org/10.1002/hyp.6325
Belnap J (2011) Biological phosphorus cycling in dryland regions. In: Bünemann EK, Oberson A, Frossard E (eds) Phosphorus in action. biological processes in soil phosphorus cycling, vol 26. Springer, Heidelberg, pp 371–406. https://doi.org/10.1007/978-3-642-15271-9
Belnap J, Lange OL (2001) Structure and functioning of biological soil crusts: a synthesis. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function, and management. Springer, Heidelberg, pp 471–479
Belnap J, Büdel B, Lange OL (2001) Biological soil crusts: characteristics and distribution. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function, and management. Ecological studies (analysis and synthesis), vol 150. Springer, Berlin, pp 3–30. https://doi.org/10.1007/978-3-642-56475-8_1
Belnap J, Phillips SL, Duniway M, Reynolds RE (2003) Soil fertility in deserts: a review on the influence of biological soil crusts and the effect of soil surface disturbance on nutrient inputs and losses. In: Alsharhan AS, Wood WW, Goudie A, Fowler AR, Abdellatif EM (eds) Desertification in the third millennium. Swets & Zeitlinger (Balkema), Lisse, The Netherlands, pp 245–252
Belnap J, Phillips SL, Miller ME (2004) Response of desert biological soil crusts to alterations in precipitation frequency. Oecologia 141(2):306–316. https://doi.org/10.1007/s00442-003-1438-6
Billings SA, Schaeffer SM, Evans RD (2003) Nitrogen fixation by biological soil crusts and heterotrophic bacteria in an intact Mojave Desert ecosystem with elevated CO2 and added soil carbon. Soil Biol Biochem 35:643–649. https://doi.org/10.1016/S0038-0717(03)00011-7
Birch HF (1958) The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 10(1):9–31. https://doi.org/10.1007/BF01343734
Böhlke JK, Ericksen GE, Revesz K (1997) Stable isotope evidence for an atmospheric origin of desert nitrate deposits in northern Chile and southern California, U.S.A. Chem Geol 136(1–2):135–152. https://doi.org/10.1016/S0009-2541(96)00124-6
Bowker MA, Belnap J, Davidson DW, Phillips SL (2005) Evidence for micronutrient limitation of biological soil crusts: importance to arid-lands restoration. Ecol Appl 15(6):1941–1951. https://www.jstor.org/stable/4543496
Bowker MA, Belnap J, Miller ME (2006) Spatial modeling of biological soil crusts to support rangeland assessment and monitoring. Rangeland Ecol Manag 59(5):519–529. https://doi.org/10.2111/05-179r1.1
Bowker MA, Mau RL, Maestre FT, Escolar C, Castillo-Monroy AP (2011) Functional profiles reveal unique ecological roles of various biological soil crust organisms. Funct Ecol 25(4):787–795. https://doi.org/10.1111/j.1365-2435.2011.01835.x
Brankatschk R, Fischer T, Veste M, Zeyer J (2013) Succession of N cycling processes in biological soil crusts on a Central European inland dune. FEMS Microbiol Ecol 83(1):149–160. https://doi.org/10.1111/j.1574-6941.2012.01459.x
Buckingham SE, Neff J, Titiz-Maybach B, Reynolds RL (2010) Chemical and textural controls on phosphorus mobility in drylands of southeastern Utah. Biogeochemistry 100(1–3):105–120. https://doi.org/10.1007/s10533-010-9408-7
Butterly CR, Bünemann EK, Mcneill AM, Baldock JA, Marschner P (2009) Carbon pulses but not phosphorus pulses are related to decreases in microbial biomass during repeated drying and rewetting of soils. Soil Biol Biochem 41(7):1406–1416. https://doi.org/10.1016/j.soilbio.2009.03.018
Butusov M, Jernelöv A (2013) Phosphorus in the organic life: cells, tissues, organisms. Phosphorus: an element that could have been called Lucifer, vol 9. Springer, New York. https://doi.org/10.1007/978-1-4614-6803-5
Cable JM, Ogle K, Lucas RW, Huxman TE, Loik ME, Smith SD et al (2011) The temperature responses of soil respiration in deserts: a seven desert synthesis. Biogeochemistry 103(1–3):71–90. https://doi.org/10.1007/s10533-010-9448-z
Canarini A, Kiaer LP, Dijkstra FA (2017) Soil carbon loss regulated by drought intensity and available substrate: a meta-analysis. Soil Biol Biochem 112:90–99. https://doi.org/10.1016/j.soilbio.2017.04.020
Caputa K, Coxson D, Sanborn P (2013) Seasonal patterns of nitrogen fixation in biological soil crusts from British Columbia’s Chilcotin grasslands. Botany 91(9):631–641. https://doi.org/10.1139/cjb-2013-0014
Carreira JA, Vinegla B, Lajtha K (2006) Secondary CaCO3 and precipitation of P-Ca compounds control the retention of soil P in arid ecosystems. J Arid Environ 64(3):460–473. https://doi.org/10.1016/j.jaridenv.2005.06.003
Castillo-Monroy AP, Maestre FT, Rey A, Soliveres S, García-Palacios P (2011) Biological soil crust microsites are the main contributor to soil respiration in a semiarid ecosystem. Ecosystems 14(5). https://doi.org/10.1007/s10021-011-9449-3
Chamizo S, Canton Y, Lazaro R, Sole-Benet A, Domingo F (2012) Crust composition and disturbance drive infiltration through biological soil crusts in semiarid ecosystems. Ecosystems 15(1):148–161. https://doi.org/10.1007/s10021-011-9499-6
Chamizo S, Canton Y, Rodriguez-Caballero E, Domingo F (2016) Biocrusts positively affect the soil water balance in semiarid ecosystems. Ecohydrology 9(7):1208–1221. https://doi.org/10.1002/eco.1719
Chan Y, Lacap DC, Lau MC, Ha KY, Warren-Rhodes KA, Cockell CS et al (2012) Hypolithic microbial communities: between a rock and a hard place. Environ Microbiol 14(9):2272–2282. https://doi.org/10.1111/j.1462-2920.2012.02821.x
Chan Y, Van Nostrand JD, Zhou J, Pointing SB, Farrell RL (2013) Functional ecology of an Antarctic Dry Valley. Proc Natl Acad Sci U S A 110(22):8990–8995. https://doi.org/10.1073/pnas.1300643110
Chatli AS, Beri V, Sidhu BS (2008) Isolation and characterisation of phosphate solubilising microorganisms from the cold desert habitat of Salix alba Linn. in trans Himalayan region of Himachal Pradesh. Indian. J Microbiol 48(2):267–273. https://doi.org/10.1007/s12088-008-0037-y
Chatterjee D, Kuotsu R, Ao M, Saha S, Kumar Ray S, Ngachan SV (2019) Does rise in temperature adversely affect soil fertility, carbon fractions, microbial biomass and enzyme activities under different land uses? Curr Sci 116(12):2044–2054. https://doi.org/10.18520/cs/v116/i12/2044-2054
Chen L, Gong J, Fu B, Huang Z, Huang Y, Gui L (2007) Effect of land use conversion on soil organic carbon sequestration in the loess hilly area, loess plateau of China. Ecol Res 22(4). https://doi.org/10.1007/s11284-006-0065-1
Chen W, Zheng X, Chen Q, Wolf B, Butterbach-Bahl K, Brüggemann N, Lin S (2013) Effects of increasing precipitation and nitrogen deposition on CH4 and N2O fluxes and ecosystem respiration in a degraded steppe in Inner Mongolia, China. Geoderma 192:335–340. https://doi.org/10.1016/j.geoderma.2012.08.018
Cosgrove DJ (1977) Microbial transformations in the phosphorus cycle. In: Alexander M (ed) Advances in microbial ecology. Advances in microbial ecology, vol 1. Springer, Boston, MA, pp 95–134. https://doi.org/10.1007/978-1-4615-8219-9_3
Cowan DA, Sohm JA, Makhalanyane TP, Capone DG, Green TG, Cary SC, Tuffin IM (2011) Hypolithic communities: important nitrogen sources in Antarctic desert soils. Environ Microbiol Rep 3(5):581–586. https://doi.org/10.1111/j.1758-2229.2011.00266.x
Crain GM, Mclaren JR, Brunner B, Darrouzet-Nardi A (2018) Biologically available phosphorus in biocrust-dominated soils of the Chihuahuan Desert. Soil Syst 2(4):56. https://doi.org/10.3390/soilsystems2040056
Craine JM, Ocheltree TW, Nippert JB, Towne EG, Skibbe AM, Kembel SW, Fargione JE (2013) Global diversity of drought tolerance and grassland climate-change resilience. Nat Clim Change 3(1):63–67. https://doi.org/10.1038/Nclimate1634
Crits-Christoph A, Gelsinger DR, Ma B, Wierzchos J, Ravel J, Davila A et al (2016) Functional interactions of archaea, bacteria and viruses in a hypersaline endolithic community. Environ Microbiol 18(6):2064–2077. https://doi.org/10.1111/1462-2920.13259
Cross AF, Schlesinger WH (1995) A literature-review and evaluation of the Hedley fractionation—applications to the biogeochemical cycle of soil-phosphorus in natural ecosystems. Geoderma 64(3–4):197–214. https://doi.org/10.1016/0016-7061(94)00023-4
Cross AF, Schlesinger WH (2001) Biological and geochemical controls on phosphorus fractions in semiarid soils. Biogeochemistry 52(2):155–172. https://doi.org/10.1023/A:1006437504494
Cueva A, Volkmann THM, Van Haren J, Troch PA, Meredith LK (2019) Reconciling negative soil CO2 fluxes: insights from a large-scale experimental hillslope. Soil Syst 3(1):10. https://doi.org/10.3390/soilsystems3010010
Dai WH, Huang Y (2006) Relation of soil organic matter concentration to climate and altitude in zonal soils of China. Catena 65(1):87–94. https://doi.org/10.1016/j.catena.2005.10.006
Daims H, Lebedeva EV, Pjevac P, Han P, Herbold C, Albertsen M et al (2015) Complete nitrification by Nitrospira bacteria. Nature 528(7583):504–509. https://doi.org/10.1038/nature16461
Dalal RC (1977) Soil organic phosphorus. Adv Agron 29:83–117. https://doi.org/10.1016/S0065-2113(08)60216-3
Darrouzet-Nardi A, Reed SC, Grote EE, Belnap J (2015) Observations of net soil exchange of CO2 in a dryland show experimental warming increases carbon losses in biocrust soils. Biogeochemistry 126(3):363–378. https://doi.org/10.1007/s10533-015-0163-7
Darrouzet-Nardi A, Reed SC, Grote EE, Belnap J (2018) Patterns of longer-term climate change effects on CO2 efflux from biocrusted soils differ from those observed in the short term. Biogeosciences 15(14):4561–4573. https://doi.org/10.5194/bg-15-4561-2018
Delgado-Baquerizo M, Maestre FT, Gallardol A, Bowker MA, Wallenstein MD, Quero JL et al (2013) Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 502(7473):672–676. https://doi.org/10.1038/nature12670
Delgado-Baquerizo M, Maestre FT, Eldridge DJ, Singh BK (2016) Microsite differentiation drives the abundance of soil ammonia oxidizing bacteria along aridity gradients. Front Microbiol 7:505. https://doi.org/10.3389/fmicb.2016.00505
Delucia EH, Callaway RM, Thomas EM, Schlesinger WH (1997) Mechanisms of phosphorus acquisition for ponderosa pine seedlings under high CO2 and temperature. Ann Bot 79(2):111–120. https://doi.org/10.1006/anbo.1996.0320
Delwiche CC, Wijler J (1956) Non-symbiotic nitrogen fixation in soil. Plant Soil 7:113–129
Denef K, Stewart CE, Brenner J, Paustian K (2008) Does long-term center-pivot irrigation increase soil carbon stocks in semi-arid agro-ecosystems? Geoderma 145(1–2):121–129. https://doi.org/10.1016/j.geoderma.2008.03.002
De Vries FT, Hoffland E, Van Eekeren N, Brussaard L, Bloem J (2006) Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biol Biochem 38(8):2092–2103. https://doi.org/10.1016/j.soilbio.2006.01.008
Dodds WK, Gudder DA, Mollenhauer D (1995) The ecology of Nostoc. J Phycol 31:2–18
Dodds WK, Marti E, Tank JL, Pontius J, Hamilton SK, Grimm NB et al (2004) Carbon and nitrogen stoichiometry and nitrogen cycling rates in streams. Oecologia 140(3):458–467. https://doi.org/10.1007/s00442-004-1599-y
D’Odorico P, Okin GS, Bestelmeyer BT (2012) A synthetic review of feedbacks and drivers of shrub encroachment in arid grasslands. Ecohydrology 5(5):520–530. https://doi.org/10.1002/eco.259
Duniway MC, Pfennigwerth AA, Fick SE, Nauman TW, Belnap J, Barger NN (2019) Wind erosion and dust from US drylands: a review of causes, consequences, and solutions in a changing world. Ecosphere 10(3). https://doi.org/10.1002/ecs2.2650
Elbert W, Weber B, Burrows S, Steinkamp J, Budel B, Andreae MO, Poschl U (2012) Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat Geosci 5(7):459–462. https://doi.org/10.1038/NGEO1486
Eldridge DJ, Delgado-Baquerizo M (2019) The influence of climatic legacies on the distribution of dryland biocrust communities. Glob Chang Biol 25(1):327–336. https://doi.org/10.1111/gcb.14506
Eldridge DJ, Greene RSB (1994) Assessment of sediment yield by splash erosion on a semi-arid soil with varying cryptogam cover. J Arid Environ 26(3):221–232. https://doi.org/10.1006/jare.1994.1025
Escolar C, Maestre FT, Rey A (2015) Biocrusts modulate warming and rainfall exclusion effects on soil respiration in a semi-arid grassland. Soil Biol Biochem 80:9–17. https://doi.org/10.1016/j.soilbio.2014.09.019
Eswaran H, Reich PF, Kimble JM, Beinroth E, Padmanabhan E, Moncharoen P (2000) Global carbon stocks. In: Lal R (ed) Global climate change and pedogenic carbonate. CRC, Boca Raton, pp 15–26
Evans SE, Burke IC (2013) Carbon and nitrogen decoupling under an 11-year drought in the shortgrass steppe. Ecosystems 16(1):20–33. https://doi.org/10.1007/s10021-012-9593-4
Evans RD, Lange OL (2003) Biological soil crusts and ecosystem nitrogen and carbon dynamics. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function, and management. 150. Springer, Berlin, pp 263–279. https://doi.org/10.1007/978-3-642-56475-8_20
Evans SE, Wallenstein MD (2012) Soil microbial community response to drying and rewetting stress: does historical precipitation regime matter? Biogeochemistry 109(1–3):101–116. https://doi.org/10.1007/s10533-011-9638-3
Evans SE, Burke IC, Lauenroth WK (2011) Controls on soil organic carbon and nitrogen in Inner Mongolia, China: a cross-continental comparison of temperate grasslands. Glob Biogeochem Cycle 25:GB3006. https://doi.org/10.1029/2010gb003945
Ewing SA, Sutter B, Owen J, Nishiizumi K, Sharp W, Cliff SS et al (2006) A threshold in soil formation at Earth’s arid–hyperarid transition. Geochimica et Cosmochimica Acta 70(21):5293–5322. https://doi.org/10.1016/j.gca.2006.08.020
Fan J, Zhong H, Harris W, Yu G, Wang S, Hu Z, Yue Y (2008) Carbon storage in the grasslands of China based on field measurements of above- and below-ground biomass. Clim Change 86(3–4):375–396. https://doi.org/10.1007/s10584-007-9316-6
Fao (2004) Carbon sequestration in dryland soils, World soil resources reports no. 102. Rome, Italy
Feng Q, Endo KN, Cheng GD (2002) Soil carbon in desertified land in relation to site characteristics. Geoderma 106(1–2):21–43. https://doi.org/10.1016/S0016-7061(01)00099-4
Feng J, Turner BL, Lu XT, Chen ZH, Wei K, Tian JH et al (2016) Phosphorus transformations along a large-scale climosequence in arid and semiarid grasslands of northern China. Glob Biogeochem Cycle 30(9):1264–1275. https://doi.org/10.1002/2015gb005331
Fernandes VMC, Machado De Lima NM, Roush D, Rudgers J, Collins SL, Garcia-Pichel F (2018) Exposure to predicted precipitation patterns decreases population size and alters community structure of cyanobacteria in biological soil crusts from the Chihuahuan Desert. Environ Microbiol 20(1):259–269. https://doi.org/10.1111/1462-2920.13983
Ferrenberg S, Reed SC, Belnap J (2015) Climate change and physical disturbance cause similar community shifts in biological soil crusts. Proc Natl Acad Sci USA 112(39):12116–12121. https://doi.org/10.1073/pnas.1509150112
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281(5374):237–240. https://doi.org/10.1126/science.281.5374.237
Field JP, Belnap J, Breshears DD, Neff JC, Okin GS, Whicker JJ et al (2010) The ecology of dust. Front Ecol Environ 8(8):423–430. https://doi.org/10.1890/090050
Fierer N, Allen AS, Schimel JP, and Holden PA. (2003) Controls on microbial CO2 production: a comparison of surface and subsurface soil horizons. Glob Change Biol 9(9):1322–1332. https://doi.org/10.1046/j.1365-2486.2003.00663.x
Finzi AC, Austin AT, Cleland EE, Frey SD, Houlton BZ (2011) Responses and feedbacks of coupled biogeochemical cycles to climate change: examples from terrestrial ecosystems. Front Ecol Environ 9(1):61–67. https://doi.org/10.1890/100001
Follett RF, Stewart CE, Pruessner EG, Kimble JM (2012) Effects of climate change on soil carbon and nitrogen storage in the US Great Plains. J Soil Water Conserv 67(5):331–342. https://doi.org/10.2489/jswc.67.5.331
Frossard A, Ramond JB, Seely M, Cowan DA (2015) Water regime history drives responses of soil Namib Desert microbial communities to wetting events. Sci Rep 5:12263. https://doi.org/10.1038/srep12263
Gaitán JJ, Bran DE, Oliva GE, Aguiar MR, Buono GG, Ferrante D et al (2017) Aridity and overgrazing have convergent effects on ecosystem structure and functioning in Patagonian rangelands. Land Degrad Dev 29(2):210–218. https://doi.org/10.1002/ldr.2694
Gaitán JJ, Maestre FT, Bran DE, Buono GG, Dougill AJ (2019) Biotic and abiotic drivers of topsoil organic carbon concentration in drylands have similar effects at regional and global scales. Ecosystems 22(7):1445–1456. https://doi.org/10.1007/s10021-019-00348-y
Gao Y, Tian J, Pang Y, Liu J (2017) Soil inorganic carbon sequestration following afforestation is probably induced by pedogenic carbonate formation in Northwest China. Front Plant Sci 8:1282. https://doi.org/10.3389/fpls.2017.01282
George TS, Giles CD, Menezes-Blackburn D, Condron LM, Gama-Rodrigues AC, Jaisi D et al (2018) Organic phosphorus in the terrestrial environment: a perspective on the state of the art and future priorities. Plant Soil 427(1–2):209–211. https://doi.org/10.1007/s11104-017-3488-2
Giardina CP, Ryan MG (2000) Soil warming and organic carbon content—Reply. Nature 408(6814):790–790. https://doi.org/10.1038/35048675
Glenday J (2008) Carbon storage and emissions offset potential in an African dry forest, the Arabuko-Sokoke Forest, Kenya. Environ Monit Assess 142(1–3):85–95. https://doi.org/10.1007/s10661-007-9910-0
Gocke M, Pustovoytov K, Kuzyakov Y (2011) Carbonate recrystallization in root-free soil and rhizosphere of Triticum aestivum and Lolium perenne estimated by C-14 labeling. Biogeochemistry 103(1–3):209–222. https://doi.org/10.1007/s10533-010-9456-z
Goldberg S, Sposito G (1985) On the mechanism of specific phosphate adsorption by hydroxylated mineral surfaces—a review. Commun Soil Sci Plan 16(8):801–821. https://doi.org/10.1080/00103628509367646
Graham RC, Hirmas DR, Wood YA (2008) Large near-surface nitrate pools in soils capped by desert pavement in the Mojave Desert, California. Geology 36(3):259–262. https://doi.org/10.1130/G24343A.1
Granhall U (1981) Biological nitrogen fixation in relation to environmental factors and functioning of natural ecosystems. In: Clark FE, Rosswall T (eds) Terrestrial nitrogen cycles. Ecological bulletin. Swedish Natural Science Research Council, Stockholm, pp 131–144
Griffiths BS, Spilles A, Bonkowski M (2012) C:N:P stoichiometry and nutrient limitation of the soil microbial biomass in a grazed grassland site under experimental P limitation or excess. Ecol Process 1:6. https://doi.org/10.1186/2192-1709-1-6
Guan C, Li X, Zhang P, Chen Y (2018) Diel hysteresis between soil respiration and soil temperature in a biological soil crust covered desert ecosystem. PLoS One 13(4):e0195606. https://doi.org/10.1371/journal.pone.0195606
Guilbault MR, Matthias AD (1998) Emissions of N2O from Sonoran Desert and effluent-irrigated grass ecosystems. J Arid Environ 38(1):87–98. https://doi.org/10.1006/jare.1997.0300
Gyaneshwar P, Kumar GN, Parekh LJ, Poole PS (2002) Role of soil microorganisms in improving P nutrition of plants. Plant Soil 245(1):83–93. https://doi.org/10.1023/A:1020663916259
Haas HJ, Grunes DL, Reichman GA (1961) Phosphorus changes in Great Plains soils as influenced by cropping and manure applications. Soil Sci Soc Am Proc 25(3):214–218. https://doi.org/10.2136/sssaj1961.03615995002500030022x
Hall SJ, Huber D, Grimm NB (2008) Soil N2O and NO emissions from an arid, urban ecosystem. J Geophys Res 113(G01016). https://doi.org/10.1029/2007JG000523
Haripal K, Sahoo S (2014) Microbial biomass carbon, nitrogen, and phosphorus dynamics along a chronosequence of abandoned tropical agroecosystems. Int J Curr Microbiol App Sci 3(9):956–970
Harris WG (2002) Phosphate minerals. In: Dixon JB, Schulze DG (eds) Soil mineralogy with environmental applications. SSSA book series 7. Soil Science Society of America, Madison, WI, pp 637–665
Harrison SP, Gornish ES, Copeland S (2015) Climate-driven diversity loss in a grassland community. Proc Natl Acad Sci USA 112(28):8672–8677. https://doi.org/10.1073/pnas.1502074112
Hartley AE, Schlesinger WH (2000) Environmental controls on nitric oxide emission from northern Chihuahuan desert soils. Biogeochemistry 50(3):279–300. https://doi.org/10.1023/A:1006377832207
Hartley AE, Schlesinger WH (2002) Potential environmental controls on nitrogenase activity in biological crusts of the northern Chihuahuan Desert. J Arid Environ 52(3):293–304. https://doi.org/10.1006/jare.2002.1007
Hartley AE, Barger N, Belnap J, Okin GS (2007) Dryland ecosystems. In: Marschner P, Rengel Z (eds) Nutrient cycling in terrestrial ecosystems. Soil biology, vol 10. Springer, Heidelberg, pp 271–307. https://doi.org/10.1007/978-3-540-68027-7_10
He ZL, Wu J, Odonnell AG, Syers JK (1997) Seasonal responses in microbial biomass carbon, phosphorus and sulphur in soils under pasture. Biol Fert Soils 24(4):421–428. https://doi.org/10.1007/s003740050267
He N, Yu Q, Wu L, Wang Y, Han X (2008) Carbon and nitrogen store and storage potential as affected by land-use in a Leymus chinensis grassland of northern China. Soil Biol Biochem 40(12):2952–2959. https://doi.org/10.1016/j.soilbio.2008.08.018
He N-P, Wang RM, Zhang YH, Chen QS (2014) Carbon and nitrogen storage in inner Mongolian grasslands: relationships with climate and soil texture. Pedosphere 24(3):391–398. https://doi.org/10.1016/S1002-0160(14)60025-4
Hedley MJ, Stewart JWB, Chauhan BS (1982) Changes in inorganic and organic soil-phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci Soc Am J 46(5):970–976. https://doi.org/10.2136/sssaj1982.03615995004600050017x
Heuck C, Weig A, Spohn M (2015) Soil microbial biomass C:N:P stoichiometry and microbial use of organic phosphorus. Soil Biol Biochem 85:119–129. https://doi.org/10.1016/j.soilbio.2015.02.029
Hicks LC, Ang R, Leizeaga A, Rousk J (2019) Bacteria constrain the fungal growth response to drying-rewetting. Soil Biol Biochem 134:108–112. https://doi.org/10.1016/j.soilbio.2019.03.006
Homyak PM, Blankinship JC, Marchus K, Lucero DM, Sickman JO, Schimel JP (2016) Aridity and plant uptake interact to make dryland soils hotspots for nitric oxide (NO) emissions. Proc Natl Acad Sci U S A 113(19):E2608–E2616. https://doi.org/10.1073/pnas.1520496113
Hou EQ, Chen CR, Luo YQ, Zhou GY, Kuang YW, Zhang YG et al (2018) Effects of climate on soil phosphorus cycle and availability in natural terrestrial ecosystems. Glob Change Biol 24(8):3344–3356. https://doi.org/10.1111/gcb.14093
Housman DC, Powers HH, Collins AD, Belnap J (2006) Carbon and nitrogen fixation differ between successional stages of biological soil crusts in the Colorado Plateau and Chihuahuan Desert. J Arid Environ 66(4):620–634. https://doi.org/10.1016/j.jaridenv.2005.11.014
Hu HW, Trivedi P, He JZ, Singh BK (2017) Microbial nitrous oxide emissions in dryland ecosystems: mechanisms, microbiome and mitigation. Environ Microbiol 19(12):4808–4828. https://doi.org/10.1111/1462-2920.13795
Huang L, Zhang Z, Li X (2014) Carbon fixation and influencing factors of biological soil crusts in a revegetated area of the Tengger Desert, northern China. J Arid Environ 6(6):725–734. https://doi.org/10.1007/s40333-014-0027-3
Huang JP, Yu HP, Dai AG, Wei Y, Kang LT (2017) Drylands face potential threat under 2 degrees C global warming target. Nat Clim Change 7(6):417–425. https://doi.org/10.1038/Nclimate3275
Hügler M. and Sievert SM. (2011) Beyond the Calvin cycle: autotrophic carbon fixation in the ocean. Ann Rev Mar Sci 3:261–289. https://doi.org/10.1146/annurev-marine-120709-142712
IPCC (2014) Synthesis report. Contribution of Working Groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland, p 151
Jakobsen I, Leggett ME, Richardson AE (2005) Rhizosphere microorganisms and plant phosphorus uptake. In: Sims JT, Sharpley AN (eds) Phosphorus: agriculture and the environment. Agronomy monograph, vol 46. ASA, CSSA, and SSSA, Madison, WI, pp 437–494
Jeffries DL, Klopatek JM, Link SO, Bolton H (1992) Acetylene reduction by cryptogamic crusts from a blackbrush community as related to resaturation and dehydration. Soil Biol Biochem 24(11):1101–1105. https://doi.org/10.1016/0038-0717(92)90059-7
Jin Z, Zhu Y, Li X, Dong Y, An Z (2015) Soil N retention and nitrate leaching in three types of dunes in the Mu Us desert of China. Sci Rep 5:14222. https://doi.org/10.1038/srep14222
Jobbagy EG, Jackson RB (2001) The distribution of soil nutrients with depth: global patterns and the imprint of plants. Biogeochemistry 53(1):51–77. https://doi.org/10.1023/A:1010760720215
Johnson SL, Budinoff CR, Belnap J, Garcia-Pichel F (2005) Relevance of ammonium oxidation within biological soil crust communities. Environ Microbiol 7(1):1–12. https://doi.org/10.1111/j.1462-2920.2004.00649.x
Johnson SL, Neuer S, Garcia-Pichel F (2007) Export of nitrogenous compounds due to incomplete cycling within biological soil crusts of arid lands. Environ Microbiol 9(3):680–689. https://doi.org/10.1111/j.1462-2920.2006.01187.x
Johnson SL, Kuske CR, Carney TD, Housman DC, Gallegos-Graves L, Belnap J (2012) Increased temperature and altered summer precipitation have differential effects on biological soil crusts in a dryland ecosystem. Glob Chang Biol 18(8):2583–2593. https://doi.org/10.1111/j.1365-2486.2012.02709.x
Jones DL, Oburger E (2011) Solubilization of phosphorus by soil microorganisms. In: Büunemann EK, Oberson A, Frossard E (eds) Phosphorus in action. biological processes in soil phosphorus cycling. Springer, Heidelberg, pp 169–198. https://doi.org/10.1007/978-3-642-15271-9
Jungk A, Claassen N (1997) Ion diffusion in the soil-root system. Adv Agron 61:53–110. https://doi.org/10.1016/S0065-2113(08)60662-8
Kalayu G (2019) Phosphate solubilizing microorganisms: promising approach as biofertilizers. Int J Agron. https://doi.org/10.1155/2019/4917256
Katra I, Gross A, Swet N, Tanner S, Krasnov H, Angert A (2016) Substantial dust loss of bioavailable phosphorus from agricultural soils. Sci Rep 6. https://doi.org/10.1038/srep24736
Killingbeck KT, Whitford WG (2001) Nutrient resorption in shrubs growing by design, and by default in Chihuahuan Desert arroyos. Oecologia 128(3):351–359. https://doi.org/10.1007/s004420100668
Koutroulis AG (2019) Dryland changes under different levels of global warming. Sci Total Environ 655:482–511. https://doi.org/10.1016/j.scitotenv.2018.11.215
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu Rev Microbiol 55:485–529. https://doi.org/10.1146/annurev.micro.55.1.485
Kowalski AS, Serrano-Ortiz P, Janssens IA, Sánchez-Moral S, Cuezva S, Domingo F et al (2008) Can flux tower research neglect geochemical CO2 exchange? Agric For Meteorol 148(6–7):1045–1054. https://doi.org/10.1016/j.agrformet.2008.02.004
Kucey RMN, Janzen HH, Leggett ME (1989) Microbially mediated increases in plant-available phosphorus. Adv Agron 42:199–228
Kumar A, Kumar A, Patel H (2018) Role of microbes in phosphorus availability and acquisition by plants. Int J Curr Microbiol App Sci 7(5):1344–1347. https://doi.org/10.20546/ijcmas.2018.705.161
Kuo S (1996) Phosphorus. In: Sparks DL (ed) Methods of soil analysis part 3: chemical methods, SSSA book series 5. Soil Science Society of America, Madison, WI, pp 869–920
Lacap DC, Warren-Rhodes KA, Mckay CP, Pointing SB (2011) Cyanobacteria and chloroflexi-dominated hypolithic colonization of quartz at the hyper-arid core of the Atacama Desert, Chile. Extremophiles 15(1):31–38. https://doi.org/10.1007/s00792-010-0334-3
Lacap-Bugler DC, Lee KK, Archer S, Gillman LN, Lau MCY, Leuzinger S et al (2017) Global diversity of desert hypolithic cyanobacteria. Front Microbiol 8:867. https://doi.org/10.3389/fmicb.2017.00867
Ladrón De Guevara M, Gozalo B, Raggio J, Lafuente A, Prieto M, Maestre FT (2018) Warming reduces the cover, richness and evenness of lichen-dominated biocrusts but promotes moss growth: insights from an 8 yr experiment. New Phytol 220(3):811–823. https://doi.org/10.1111/nph.15000
Lafuente A, Berdugo M, De Guevara ML, Gozalo B, Maestre FT (2018) Simulated climate change affects how biocrusts modulate water gains and desiccation dynamics after rainfall events. Ecohydrology 11(6):e1935. https://doi.org/10.1002/eco.1935
Lajtha K, Schlesinger WH (1988) The biogeochemistry of phosphorus cycling and phosphorus availability along a desert soil chronosequence. Ecology 69(1):24–39. https://doi.org/10.2307/1943157
Lal R (2001) Potential of desertification control to sequester carbon and mitigate the greenhouse effect. Clim Change 51(1):35–72. https://doi.org/10.1023/A:1017529816140
Lal R (2002) Soil carbon dynamics in cropland and rangeland. Environ Pollut 116(3):353–362. https://doi.org/10.1016/s0269-7491(01)00211-1
Lal R (2004a) Carbon sequestration in dryland ecosystems. Environ Manag 33(4):528–544. https://doi.org/10.1007/s00267-003-9110-9
Lal R (2004b) Soil carbon sequestration impacts on global climate change and food security. Science 304(5677):1623–1627. https://doi.org/10.1126/science.1097396
Lal R (2009) Sequestering carbon in soils of arid ecosystems. Land Degrad Dev 20(4):441–454. https://doi.org/10.1002/ldr.934
Lal R (2019) Carbon cycling in global drylands. Curr Clim Change Rep 5(3):221–232. https://doi.org/10.1007/s40641-019-00132-z
Lal R, Hassan HM, Dumanski J (1999) Desertification control to sequester C and mitigate the greenhouse effect. In: Rosenberg NJ, Izaurralde RC, Malone EL (eds) Carbon sequestration in soils: science, monitoring and beyond. Battelle, Columbus, OH, pp 83–151
Lange W (1974) Chelating agents and blue-green algae. Can J Microbiol 20(10):1311–1321. https://doi.org/10.1139/m74-204
Leon-Sobrino C, Ramond JB, Maggs-Kolling G, Cowan DA (2019) Nutrient acquisition, rather than stress response over diel cycles, drives microbial transcription in a hyper-arid namib desert soil. Front Microbiol 10:1054. https://doi.org/10.3389/fmicb.2019.01054
Le Quéré C, Andrew RM, Friedlingstein P, Sitch S, Hauck J, Pongratz J et al (2018) Global carbon budget. Earth Syst Sci Data 10:2141–2194. https://doi.org/10.5194/essd-10-2141-2018
Li XZ, Sarah P (2003) Enzyme activities along a climatic transect in the Judean Desert. Catena 53(4):349–363. https://doi.org/10.1016/S0341-8162(03)00087-0
Li J, Okin GS, Hartman LJ, Epstein HE (2007) Quantitative effects of vegetation cover on wind erosion and soil nutrient loss in a desert grassland of southern New Mexico, USA. Biogeochemistry 85(3):317–332. https://doi.org/10.1007/s10533-007-9142-y
Li XD, Fu H, Guo D, Li XD, Wan CG (2010) Partitioning soil respiration and assessing the carbon balance in a Setaria italica (L.) Beauv. Cropland on the Loess Plateau, Northern China. Soil Biol Biochem 42(2):337–346. https://doi.org/10.1016/j.soilbio.2009.11.013
Li KH, Song W, Liu XJ, Shen JL (2012a) Atmospheric reactive nitrogen concentrations at ten sites with contrasting land use in an arid region of central Asia. Biogeosciences 9:4013–4021. https://doi.org/10.5194/bg-9-4013-20
Li XR, Zhang P, Su YG, Jia RL (2012b) Carbon fixation by biological soil crusts following revegetation of sand dunes in arid desert regions of China: a four-year field study. CATENA 97:119–126. https://doi.org/10.1016/j.catena.2012.05.009
Li XJ, Zhao Y, Yang HT, Zhang P, Gao YP (2018) Soil respiration of biologically-crusted soils in response to simulated precipitation pulses in the Tengger Desert, Northern China. Pedosphere 28(1):103–113. https://doi.org/10.1016/S1002-0160(17)60307-2
Lindahl BD, Finlay RD, Cairney JWG (2005) Enzymatic activities of mycelia in mycorrhizal fungal communities. In: Dighton J, White JF, Oudemans P (eds) The fungal community: its organization and role in the ecosystem, vol 23. Taylor & Francis, Boca Raton, FL, pp 331–348
Liu Q, Edwards NT, Post WM, Gu L, Ledford J, Lenhart S (2006) Temperature-independent diel variation in soil respiration observed from a temperate deciduous forest. Glob Chang Biol 12(11):2136–2145. https://doi.org/10.1111/j.1365-2486.2006.01245.x
Liu X, Qi Y, Dong Y, Peng Q, He Y, Sun L et al (2014) Response of soil N2O emissions to precipitation pulses under different nitrogen availabilities in a semiarid temperate steppe of Inner Mongolia, China. J Arid Land 6(4):410–422. https://doi.org/10.1007/s40333-013-0211-x
Liu YR, Delgado-Baquerizo M, Trivedi P, He JZ, Singhad BK (2016) Species identity of biocrust-forming lichens drives the response of soil nitrogen cycle to altered precipitation frequency and nitrogen amendment. Soil Biol Biochem 96:128–136. https://doi.org/10.1016/j.soilbio.2016.01.021
Liu Z, Sun Y, Zhang Y, Feng W, Lai Z, Fa K, Qin S (2018) Metagenomic and 13C tracing evidence for autotrophic atmospheric carbon absorption in a semiarid desert. Soil Biol Biochem 125:156–166. https://doi.org/10.1016/j.soilbio.2018.07.012
Liu D, Keiblinger KM, Leitner S, Wegner U, Zimmermann M, Fuchs S et al (2019) Response of microbial communities and their metabolic functions to drying-rewetting stress in a temperate forest soil. Microorganisms 7(5):129. https://doi.org/10.3390/microorganisms7050129
Logan JA, Prather MJ, Wofsy SC, Mcelroy MB (1981) Tropospheric chemistry: a global perspective. J Geophys Res 86(C8):7210–7254. https://doi.org/10.1029/JC086iC08p07210
Lu HB, Liu SR, Wang H, Luan JW, Schindlbacher A, Liu YC, Wang Y (2017) Experimental throughfall reduction barely affects soil carbon dynamics in a warm-temperate oak forest, central China. Sci Rep 7. https://doi.org/10.1038/s41598-017-15157-3
Luo W, Xu C, Ma W, Yue X, Liang X, Zuo X et al (2018) Effects of extreme drought on plant nutrient uptake and resorption in rhizomatous vs bunchgrass-dominated grasslands. Oecologia 188(2):633–643. https://doi.org/10.1007/s00442-018-4232-1
Luo G, Xue C, Jiang Q, Xiao Y, Zhang F, Guo S et al (2020) Soil carbon, nitrogen, and phosphorus cycling microbial populations and their resistance to global change depend on soil C:N:P stoichiometry. MSystems 5(3):e00162-00120. https://doi.org/10.1128/mSystems.00162-20
Ma WH, He JS, Yang YH, Wang XP, Liang CZ, Anwar M et al (2010) Environmental factors covary with plant diversity-productivity relationships among Chinese grassland sites. Glob Ecol Biogeogr 19(2):233–243. https://doi.org/10.1111/j.1466-8238.2009.00508.x
Ma J, Liu R, Li Y (2017) Abiotic contribution to total soil CO2 flux across a broad range of land-cover types in a desert region. J Arid Land 9(1):13–26. https://doi.org/10.1007/s40333-016-0061-4
Mackenzie FT, Vera LM, Lerman A (2002) Century-scale nitrogen and phosphorus controls of the carbon cycle. Chem Geol 190(1–4):13–32. https://doi.org/10.1016/S0009-2541(02)00108-0
Maestre FT, Bowker MA, Canton Y, Castillo-Monroy AP, Cortina J, Escolar C et al (2011) Ecology and functional roles of biological soil crusts in semi-arid ecosystems of Spain. J Arid Environ 75(12):1282–1291. https://doi.org/10.1016/j.jaridenv.2010.12.008
Maestre FT, Escolar C, De Guevara ML, Quero JL, Lazaro R, Delgado-Baquerizo M et al (2013) Changes in biocrust cover drive carbon cycle responses to climate change in drylands. Glob Chang Biol 19(12):3835–3847. https://doi.org/10.1111/gcb.12306
Maestre FT, Eldridge DJ, Soliveres S, Kefi S, Delgado-Baquerizo M, Bowker MA et al (2016) Structure and functioning of dryland ecosystems in a changing world. Annu Rev Ecol Evol Syst 47:215–237. https://doi.org/10.1146/annurev-ecolsys-121415-032311
Magid J, Nielsen NE (1992) Seasonal-variation in organic and inorganic phosphorus fractions of temperate-climate sandy soils. Plant Soil 144(2):155–165. https://doi.org/10.1007/Bf00012872
Maier S, Tamm A, Wu D, Caesar J, Grube M, Weber B (2018) Photoautotrophic organisms control microbial abundance, diversity, and physiology in different types of biological soil crusts. ISME J 12(4):1032–1046. https://doi.org/10.1038/s41396-018-0062-8
Makhalanyane TP, Valverde A, Gunnigle E, Frossard A, Ramond JB, Cowan DA (2015) Microbial ecology of hot desert edaphic systems. FEMS Microbiol Rev 39(2):203–221. https://doi.org/10.1093/femsre/fuu011
Malam Issa O, Stal LJ, Défarge C, Couté A, Trichet J (2001) Nitrogen fixation by microbial crusts from desiccated Sahelian soils (Niger). Soil Biol Biochem 33(10):1425–1428. https://doi.org/10.1016/S0038-0717(01)00046-3
Mander L, Dekker SC, Li M, Mio W, Punyasena SW, Lenton TM (2017) A morphometric analysis of vegetation patterns in dryland ecosystems. R Soc Open Sci 4(2):160443. https://doi.org/10.1098/rsos.160443
Manzoni S, Schimel JP, Porporato A (2012) Responses of soil microbial communities to water stress: results from a meta-analysis. Ecology 93(4):930–938. https://doi.org/10.1890/11-0026.1
Marion GM (1989) Correlation between long-term pedogenic CaCO3 formation rate and Modern precipitation in deserts of the America southwest. Quat Res 32(3):291–229. https://doi.org/10.1016/0033-5894(89)90095-1
Marusenko Y, Bates ST, Anderson I, Johnson SL (2013) Ammonia-oxidizing archaea and bacteria are structured by geography in biological soil crusts across North American arid lands. Ecol Process 2(9). https://doi.org/10.1186/2192-1709-2-9
Marusenko Y, Garcia-Pichel F, and Hall SJ. (2015) Ammonia-oxidizing archaea respond positively to inorganic nitrogen addition in desert soils. FEMS Microbiol Ecol 91(2):1–11. https://doi.org/10.1093/femsec/fiu023
Mayland HF, Mcintosh TH (1966) Availability of biologically fixed atmospheric nitrogen-15 to higher plants. Nature 209:421–422
Mccalley CK, Sparks JP (2008) Controls over nitric oxide and ammonia emissions from Mojave Desert soils. Oecologia 156(4):871–881. https://doi.org/10.1007/s00442-008-1031-0
Mccalley CK, Sparks JP (2009) Abiotic gas formation drives nitrogen loss from a desert ecosystem. Science 326(5954):837–840. https://doi.org/10.1126/science.1178984
Mccalley CK, Strahm BD, Sparks KL, Eller ASD, Sparks JP (2011) The effect of long-term exposure to elevated CO2 on nitrogen gas emissions from Mojave Desert soils. J Geophys Res 116(G3). https://doi.org/10.1029/2011JG001667
Mcgill WB, Cole CV (1981) Comparative aspects of cycling of organic C, N, S and P through soil organic-matter. Geoderma 26(4):267–286. https://doi.org/10.1016/0016-7061(81)90024-0
Meisner A, Baath E, Rousk J (2013) Microbial growth responses upon rewetting soil dried for four days or one year. Soil Biol Biochem 66:188–192. https://doi.org/10.1016/j.soilbio.2013.07.014
Meisner A, Rousk J, Baath E (2015) Prolonged drought changes the bacterial growth response to rewetting. Soil Biol Biochem 88:314–322. https://doi.org/10.1016/j.soilbio.2015.06.002
Meslier V, Casero MC, Dailey M, Wierzchos J, Ascaso C, Artieda O et al (2018) Fundamental drivers for endolithic microbial community assemblies in the hyperarid Atacama Desert. Environ Microbiol 20(5):1765–1781. https://doi.org/10.1111/1462-2920.14106
Michalski G, Böhlke JK, Thiemens M (2004) Long term atmospheric deposition as the source of nitrate and other salts in the Atacama Desert, Chile: new evidence from mass-independent oxygen isotopic compositions. Geochim Cosmochim Acta 68(20):4023–4038. https://doi.org/10.1016/j.gca.2004.04.009
Miller ME, Belnap J, Beatty SW, Webb BL (2006a) Effects of water additions, chemical amendments, and plants on in situ measures of nutrient bioavailability in calcareous soils of southeastern Utah, USA. Plant Soil 288(1–2):19–29. https://doi.org/10.1007/s11104-006-9014-6
Miller ME, Belnap J, Beatty SW, Reynolds RL (2006b) Performance of Bromus tectorum L. in relation to soil properties, water additions, and chemical amendments in calcareous soils of southeastern Utah, USA. Plant Soil 288(1–2):1–18. https://doi.org/10.1007/s11104-006-0058-4
Min K, Lehmeier CA, Ballantyne F, Tatarko A, Billings SA (2014) Differential effects of pH on temperature sensitivity of organic carbon and nitrogen decay. Soil Biol Biochem 76:193–200. https://doi.org/10.1016/j.soilbio.2014.05.021
Miralles I, Trasar-Cepeda C, Leiros MC, Gil-Sotres F (2013) Labile carbon in biological soil crusts in the Tabernas desert, SE Spain. Soil Biol Biochem 58:1–8. https://doi.org/10.1016/j.soilbio.2012.11.010
Miralles I, De Guevara ML, Chamizo S, Rodriguez-Caballero E, Ortega R, Van Wesemael B, Canton Y (2018) Soil CO2 exchange controlled by the interaction of biocrust successional stage and environmental variables in two semiarid ecosystems. Soil Biol Biochem 124:11–23. https://doi.org/10.1016/j.soilbio.2018.05.020
Mogul R, Vaishampayan P, Bashir M, Mckay CP, Schubert K, Bornaccorsi R et al (2017) Microbial community and biochemical dynamics of biological soil crusts across a gradient of surface coverage in the Central Mojave Desert. Front Microbiol 8:1974. https://doi.org/10.3389/fmicb.2017.01974
Montañez A, Danso SKA, Hardarson G (1995) The effect of temperature on nodulation and nitrogen fixation by five Bradyrhizobium japonicum strains. Appl Soil Ecol 2(3):165–174. https://doi.org/10.1016/0929-1393(95)00052-M
Montiel-González C, Tapia-Torres Y, Souza V, Garcia-Oliva F (2017) The response of soil microbial communities to variation in annual precipitation depends on soil nutritional status in an oligotrophic desert. PeerJ 5:e4007. https://doi.org/10.7717/peerj.4007
Mummey DL, Smith JL, Bolton H (1994) Nitrous oxide flux from a shrub-steppe ecosystem: sources and regulation. Soil Biol Biochem 26(2):279–286. https://doi.org/10.1016/0038-0717(94)90168-6
Mureva A, Ward D, Pillay T, Chivenge P, Cramer M (2018) Soil organic carbon increases in semi-arid regions while it decreases in humid regions due to woody-plant encroachment of grasslands in South Africa. Sci Rep 8. https://doi.org/10.1038/s41598-018-33701-7
Nannipieri P, Giagnoni L, Landi L, Renella G (2011) Role of phosphatase enzymes in soil. In: Bünemann E, Oberson A, Frossard E (eds) Phosphorus in action: biological processes in soil phosphorus cycling. Soil biology, vol 26. Springer, Heidelberg, pp 215–243. https://doi.org/10.1007/978-3-642-15271-9_9
Neff JC, Reynolds R, Sanford RL, Fernandez D, Lamothe P (2006) Controls of bedrock geochemistry on soil and plant nutrients in southeastern Utah. Ecosystems 9(6):879–893. https://doi.org/10.1007/s10021-005-0092-8
Nejidat A (2005) Nitrification and occurrence of salt-tolerant nitrifying bacteria in the Negev desert soils. FEMS Microbiol Ecol 52(1):21–29. https://doi.org/10.1016/j.femsec.2004.10.011
Offre P, Spang A, Schleper C (2013) Archaea in biogeochemical cycles. Annu Rev Microbiol 67:437–457. https://doi.org/10.1146/annurev-micro-092412-155614
Ojima DS, Smith MS, Beardsley M (1995) Factors affecting carbon storage in semi-arid and arid ecosystems. In: Squires VR, Glenn EP, Ayoub AT (eds) Combating global climate change by combating land degradation. Nairobi, Kenya, UNEP, pp 93–115
Okin GS, Mahowald N, Chadwick OA, Artaxo P (2004) Impact of desert dust on the biogeochemistry of phosphorus in terrestrial ecosystems. Glob Biogeochem Cycle 18(2). https://doi.org/10.1029/2003gb002145
Orlando J, Caru M, Pommerenke B, Braker G (2012) Diversity and activity of denitrifiers of Chilean arid soil ecosystems. Front Microbiol 3:101. https://doi.org/10.3389/fmicb.2012.00101
Ou Y, Rousseau AN, Wang LX, Yan BX (2017) Spatio-temporal patterns of soil organic carbon and pH in relation to environmental factors-A case study of the Black Soil Region of Northeastern China. Agr Ecosyst Environ 245:22–31. https://doi.org/10.1016/j.agee.2017.05.003
Patra DD, Brookes PC, Coleman K, Jenkinson DS (1990) Seasonal-changes of soil microbial biomass in an arable and a grassland soil which have been under uniform management for many years. Soil Biol Biochem 22(6):739–742. https://doi.org/10.1016/0038-0717(90)90151-O
Peñuelas J, Sardans J, Rivas-Ubach A, Janssens IA (2012) The human-induced imbalance between C, N and P in Earth’s life system. Glob Change Biol 18(1):3–6. https://doi.org/10.1111/j.1365-2486.2011.02568.x
Perez-Quezada JF, Delpiano CA, Snyder KA, Johnson DA, Franck N (2011) Carbon pools in an arid shrubland in Chile under natural and afforested conditions. J Arid Environ 75(1):29–37. https://doi.org/10.1016/j.jaridenv.2010.08.003
Perroni Y, Garcia-Oliva F, Tapia-Torres Y, Souza V (2014) Relationship between soil P fractions and microbial biomass in an oligotrophic grassland-desert scrub system. Ecol Res 29(3):463–472. https://doi.org/10.1007/s11284-014-1138-1
Peterjohn WT, Schlesinger WH (1990) Nitrogen loss from deserts in the southwestern United States. Biogeochemistry 10(1):67–79. https://doi.org/10.1007/BF00000893
Plaza C, Zaccone C, Sawicka K, Mendez AM, Tarquis A, Gasco G et al (2018a) Soil resources and element stocks in drylands to face global issues. Sci Rep 8(1):13788. https://doi.org/10.1038/s41598-018-32229-0
Plaza C, Gascó G, Méndez AM, Zaccone C, Maestre FT (2018b) Soil organic matter in dryland ecosystems. In: Garcia C, Nannipieri P, Hernandez T (eds) The future of soil carbon. Its conservation and formation. Academic, London, pp 39–70. https://doi.org/10.1016/C2016-0-01797-7
Pointing SB, Belnap J (2012) Microbial colonization and controls in dryland systems. Nat Rev Microbiol 10(8):551–562. https://doi.org/10.1038/nrmicro2831
Porder S, Ramachandran S (2013) The phosphorus concentration of common rocks-a potential driver of ecosystem P status. Plant Soil 367(1–2):41–55. https://doi.org/10.1007/s11104-012-1490-2
Porporato A, D’Odorico P, Laio F, Rodriguez-Iturbe I (2003) Hydrologic controls on soil carbon and nitrogen cycles. I. Modeling scheme. Adv Water Resour 26(1):45–58. https://doi.org/10.1016/S0309-1708(02)00094-5
Posmanik R, Nejidat A, Dahan O, Gross A (2017) Seasonal and soil-type dependent emissions of nitrous oxide from irrigated desert soils amended with digested poultry manures. Sci Total Environ 1(593–594):91–98. https://doi.org/10.1016/j.scitotenv.2017.03.115
Qin Y, Li Y, Bao H, Liu F (2012) Massive atmospheric nitrate accumulation in a continental interior desert, northwestern China. Geology 40(7):623–626. https://doi.org/10.1130/G32953.1
Qin FY, Shi XZ, Xu SX, Yu DS, Wang DD (2016) Zonal differences in correlation patterns between soil organic carbon and climate factors at multi-extent. Chin Geogr Sci 26(5):670–678. https://doi.org/10.1007/s11769-015-0736-3
Quiquampoix H, Mousain D (2005) Enzymatic hydrolysis of organic phosphorus. In: Turner BL, Frossard E, Baldwin DS (eds) Organic phosphorus in the environment. CABI, Wallingford, pp 89–112
Ramond JB, Woodborne S, Hall G, Seely M, Cowan DA (2018) Namib Desert primary productivity is driven by cryptic microbial community N-fixation. Sci Rep 8(1):6921. https://doi.org/10.1038/s41598-018-25078-4
Rasmussen C (2006) Distribution of soil organic and inorganic carbon pools by biome and soil taxa in Arizona. Soil Sci Soc Am J 70(1):256–265. https://doi.org/10.2136/sssaj2005.0118
Reynolds R, Neff J, Reheis M, Lamothe P (2006) Atmospheric dust in modern soil on aeolian sandstone, Colorado Plateau (USA): variation with landscape position and contribution to potential plant nutrients. Geoderma 130(1–2):108–123. https://doi.org/10.1016/j.geoderma.2005.01.012
Richardson AE, Simpson RJ (2011) Soil microorganisms mediating phosphorus availability. Plant Physiol 156(3):989–996. https://doi.org/10.1104/pp.111.175448
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17(4–5):319–339. https://doi.org/10.1016/S0734-9750(99)00014-2
Ronca S, Ramond JB, Jones BE, Seely M, Cowan DA (2015) Namib Desert dune/interdune transects exhibit habitat-specific edaphic bacterial communities. Front Microbiol 6:845. https://doi.org/10.3389/fmicb.2015.00845
Rosenberg NJ, Izaurralde RC, Malone EL (1999) Carbon sequestration in soils: science, monitoring and beyond. Battelle, Columbus, OH
Rotenberg E, Yakir D (2010) Contribution of semi-arid forests to the climate system. Science 327(5964):451–454. https://doi.org/10.1126/science.1179998
Runge-Metzger A (1995) Closing the cycle: obstacles to efficient P management for improved global food security. In: Scope-Scientific Committee on Problems of the Environment International Council of Scientific Unions, Indianapolis, pp 27–42
Ruttenberg KC (2003) The global phosphorus cycle. In: Schlesinger WH, Holland HD, Turekian KK (eds) Treatise on geochemistry, vol 8. Elsevier, New York, pp 585–643. https://doi.org/10.1016/B0-08-043751-6/08153-6
Ryan MG, Law BE (2005) Interpreting, measuring, and modeling soil respiration. Biogeochemistry 73(1):3–27. https://doi.org/10.1007/s10533-004-5167-7
Ryan J, Hasan HM, Baasiri M, Tabbara HS (1985) Availability and transformation of applied phosphorus in calcareous lebanese soils. Soil Sci Soc Am J 49(5):1215–1220. https://doi.org/10.2136/sssaj1985.03615995004900050029x
Safriel U, Adeel Z, Niemeijer D, Puigdefabregas J, White R, Lal R et al (2005) Dryland systems. In: Hassan R, Scholes R, Ash N (eds) Ecosystems and human well-being: current state and trends: findings of the Condition and Trends Working Group, vol 1. Island Press, Washington, DC, pp 623–662
Sahrawat KL (2003) Importance of inorganic carbon in sequestering carbon in soils of the dry regions. Curr Sci 84(7):864–865
Sakamoto K, Oba Y (1994) Effect of fungal to bacterial biomass ratio on the relationship between CO2 evolution and total soil microbial biomass. Biol Fert Soils 17(1):39–44. https://doi.org/10.1007/Bf00418670
Sancho LG, Belnap J, Colesie C, Raggio J, Weber B (2016) Carbon budgets of biological soil crusts at micro-, meso-, and global scales. In: Weber B, Büdel B, Belnap J (eds) Biological soil crusts: an organizing principle in drylands. Ecological studies (analysis and synthesis), vol 226. Springer, Cham, pp 287–304. https://doi.org/10.1007/978-3-319-30214-0_15
Sanderman J (2012) Can management induced changes in the carbonate system drive soil carbon sequestration? A review with particular focus on Australia. Agr Ecosyst Environ 155:70–77. https://doi.org/10.1016/j.agee.2012.04.015
Sardans J, Penuelas J, Estiarte M (2006) Warming and drought alter soil phosphatase activity and soil P availability in a Mediterranean shrubland. Plant Soil 289(1–2):227–238. https://doi.org/10.1007/s11104-006-9131-2
Sardans J, Penuelas J, Ogaya R (2008) Experimental drought reduced acid and alkaline phosphatase activity and increased organic extractable P in soil in a Quercus ilex Mediterranean forest. Eur J Soil Biol 44(5–6):509–520. https://doi.org/10.1016/j.ejsobi.2008.09.011
Sardans J, Rivas-Ubach A, Penuelas J (2012) The elemental stoichiometry of aquatic and terrestrial ecosystems and its relationships with organismic lifestyle and ecosystem structure and function: a review and perspectives. Biogeochemistry 111(1–3):1–39. https://doi.org/10.1007/s10533-011-9640-9
Schaeffer SM, Evans RD (2005) Pulse additions of soil carbon and nitrogen affect soil nitrogen dynamics in an arid Colorado Plateau shrubland. Oecologia 145(3):425–433. https://doi.org/10.1007/s00442-005-0140-2
Scharpenseel HW, Mtimet A, Freytag J (2000) Soil inorganic carbon and global change. In: Lal R, Kimble JM, Eswaran H, Stewart BA (eds) Global climate change and pedogenic carbonates. CRC, Boca Raton, FL, pp 27–42
Schimel DS (2010) Drylands in the earth system. Science 327(5964):418–419. https://doi.org/10.1126/science.1184946
Schimel JP (2018) Life in dry soils: effects of drought on soil microbial communities and processes. Annu Rev Ecol Evol Syst 49:409–432. https://doi.org/10.1146/annurev-ecolsys-110617-062614
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85(3):591–602. https://doi.org/10.1890/03-8002
Schlaepfer DR, Bradford JB, Lauenroth WK, Munson SM, Tietjen B, Hall SA et al (2017) Climate change reduces extent of temperate drylands and intensifies drought in deep soils. Nat Commun 8. https://doi.org/10.1038/ncomms14196
Schlesinger WH (1985) The formation of caliche in soils of the Mojave Desert, California. Geochimica et Cosmochimica Acta 49(1):57–66. https://doi.org/10.1016/0016-7037(85)90191-7
Schlesinger WH (2002) Inorganic carbon and the global C cycle. In: Lal R (ed) Encyclopedia of soil science. Marcel Dekker, New York, pp 706–708
Schlesinger WH (2006) Inorganic carbon and the global C cycle. In: Lal R (ed) Encyclopedia of soil science, 3rd edn. CRC, Boca Raton, FL. https://doi.org/10.4324/9781315161860
Schlesinger WH, Pippen JS, Wallenstein MD, Hofmockel KS, Klepeis DM, Mahall BE (2003) Community composition and photosynthesis by photoautotrophs under quartz pebbles, Southern Mojave Desert. Ecology 84(12):3222–3231. https://doi.org/10.1890/02-0549
Schwabedissen SG, Lohse KA, Reed SC, Aho KA, Magnuson TS (2017) Nitrogenase activity by biological soil crusts in cold sagebrush steppe ecosystems. Biogeochemistry 134:57–76. https://doi.org/10.1007/s10533-017-0342-9
Scurlock JMO, Hall DO (1998) The global carbon sink: a grassland perspective. Glob Chang Biol 4:229–233. https://doi.org/10.1046/j.1365-2486.1998.00151.x
Selmants PC, Hart SC (2010) Phosphorus and soil development: does the Walker and Syers model apply to semiarid ecosystems? Ecology 91(2):474–484. https://doi.org/10.1890/09-0243.1
Serrano-Ortiz P, Sánchez-Cañete EP, Oyonarte C (2012) The carbon cycle in drylands. In: Lal R, Lorenz K, Hüttl R, Schneider B, Von Braun J (eds) Recarbonization of the biosphere. Springer, Dordrecht, pp 347–368. https://doi.org/10.1007/978-94-007-4159-1_15
Shoun H, Fushinobu S, Jiang L, Kim SW, Wakagi T (2012) Fungal denitrification and nitric oxide reductase cytochrome P450nor. Philos Trans R Soc Lond B Biol Sci 367(1593):1186–1194. https://doi.org/10.1098/rstb.2011.0335
Siebers N, Sumann M, Kaiser K, Amelung W (2017) Climatic effects on phosphorus fractions of native and cultivated North American grassland soils. Soil Sci Soc Am J 81(2):299–309. https://doi.org/10.2136/sssaj2016.06.0181
Sims JT, Pierzynski GM (2005) Chemistry of phosphorus in soils. Chemical processes in soils. SSSA book series, vol 8. SSSA, Madison, WI, pp 151–192
Sinsabaugh RS (1994) Enzymic analysis of microbial pattern and process. Biol Fert Soils 17(1):69–74. https://doi.org/10.1007/BF00418675
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C et al (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11(11):1252–1264. https://doi.org/10.1111/j.1461-0248.2008.01245.x
Sjögersten S, Wookey PA (2009) The impact of climate change on ecosystem carbon dynamics at the Scandinavian mountain birch forest-tundra heath ecotone. Ambio 38(1):2–10. https://www.jstor.org/stable/25515792
Smith KR, Waring BG (2019) Broad-scale patterns of soil carbon (C) pools and fluxes across semiarid ecosystems are linked to climate and soil texture. Ecosystems 22(4):742–753. https://doi.org/10.1007/s10021-018-0299-0
Sollins P, Robertson GP, Uehara G (1988) Nutrient mobility in variable-charge and permanent-charge soils. Biogeochemistry 6(3):181–199. https://doi.org/10.1007/Bf02182995
Spohn M, Kuzyakov Y (2013) Phosphorus mineralization can be driven by microbial need for carbon. Soil Biol Biochem 61:69–75. https://doi.org/10.1016/j.soilbio.2013.02.013
Stark JM, Firestone MK (1995) Mechanisms for soil-moisture effects on activity of nitrifying bacteria. Appl Environ Microbiol 61(1):218–221
Stein LY (2019) Insights into the physiology of ammonia-oxidizing microorganisms. Curr Opin Chem Biol 49:9–15. https://doi.org/10.1016/j.cbpa.2018.09.003
Stevenson FJS, Cole MA (1999) Cycles of soils: carbon, nitrogen, phosphorus, sulfur, micronutrients, 2nd edn. Wiley, New York
Strauss SL, Day TA, Garcia-Pichel F (2012) Nitrogen cycling in desert biological soil crusts across biogeographic regions in the Southwestern United States. Biogeochemistry 108(1/3):171–182. https://doi.org/10.1007/s10533-011-9587-x
Stursova M, Crenshaw CL, Sinsabaugh RL (2006) Microbial responses to long-term N deposition in a semiarid grassland. Microb Ecol 51(1):90–98. https://doi.org/10.1007/s00248-005-5156-y
Su Y-G, Zhao X, Li A-X, Li X-R, Huang G (2011) Nitrogen fixation in biological soil crusts from the Tengger desert, northern China. Eur J Soil Biol 47(3):182–187. https://doi.org/10.1016/j.ejsobi.2011.04.001
Suddick EC, Whitney P, Townsend AR, Davidson EA (2013) The role of nitrogen in climate change and the impacts of nitrogen–climate interactions in the United States: foreword to thematic issue. Biogeochemistry 114:1–10. https://doi.org/10.1007/s10533-012-9795-z
Tan WF, Zhang R, Cao H, Huang CQ, Yang QK, Wang M-K, Koopal LK (2014) Soil inorganic carbon stock under different soil types and land uses on the Loess Plateau region of China. Catena 121:22–30. https://doi.org/10.1016/j.catena.2014.04.014
Thomey ML, Ford PL, Reeves MC, Finch DM, Litvak ME, Collins SL (2014) Climate change impacts on future carbon stores and management of warm deserts of the United States. Rangelands 36(1):16–24. https://doi.org/10.2111/RANGELANDS-D-13-00045.1
Tian JH, Boitt G, Black A, Wakelin S, Condron LM, Chen LJ (2017) Accumulation and distribution of phosphorus in the soil profile under fertilized grazed pasture. Agr Ecosyst Environ 239:228–235. https://doi.org/10.1016/j.agee.2017.01.022
Tiessen H, Moir JO (1993) Characterization of available p by sequential extraction. In: Carter MR (ed) Soil sampling and methods of analysis. Lewis, Boca Raton, FL, pp 75–86
Tiessen H, Stewart JWB, Cole CV (1984) Pathways of phosphorus transformations in soils of differing pedogenesis. Soil Sci Soc Am J 48(4):853–858. https://doi.org/10.2136/sssaj1984.03615995004800040031x
Trolldenier G (1982) Effect of soil temperature on nitrogen fixation on roots of rice and reed. Plant Soil 68(2):217–222. https://doi.org/10.1007/BF02373707
Tucker CL, Reed SC (2016) Low soil moisture during hot periods drives apparent negative temperature sensitivity of soil respiration in a dryland ecosystem: a multi-model comparison. Biogeochemistry 128(1–2):155–169. https://doi.org/10.1007/s10533-016-0200-1
Turner BL, Mckelvie ID, Haygarth PM (2002) Characterisation of water-extractable soil organic phosphorus by phosphatase hydrolysis. Soil Biol Biochem 34(1):27–35. https://doi.org/10.1016/S0038-0717(01)00144-4
Turner BL, Cade-Menun BJ, Westermann DT (2003a) Organic phosphorus composition and potential bioavailability in semi-arid arable soils of the western United States. Soil Sci Soc Am J 67(4):1168–1179. https://doi.org/10.2136/sssaj2003.1168
Turner BL, Baxter R, Ellwood NTW, Whitton BA (2003b) Seasonal phosphatase activities of mosses from Upper Teesdale, northern England. J Bryol 25:189–200. https://doi.org/10.1179/037366803235001670
Turner BL, Condron LM, Richardson SJ, Peltzer DA, Allison VJ (2007) Soil organic phosphorus transformations during pedogenesis. Ecosystems 10(7):1166–1181. https://doi.org/10.1007/s10021-007-9086-z
Van Amstel AR, Swart RJ (1994) Methane and nitrous oxide emissions: an introduction. Fertil Res 37:213–225. https://doi.org/10.1007/BF00748940
Van Kessel MA, Speth DR, Albertsen M, Nielsen PH, Op Den Camp HJ, Kartal B et al (2015) Complete nitrification by a single microorganism. Nature 528(7583):555–559. https://doi.org/10.1038/nature16459
Veste M, Littmann T, Breckle SW, Yair A (2001) The role of biological soil crusts on desert sand dunes in the Northwestern Negev, Israel. In: Breckle SW, Veste M, Wucherer W (eds) Sustainable land use in deserts. Springer, Berlin, pp 357–367. https://doi.org/10.1007/978-3-642-59560-8_38
Veste M, Heusinkveld BG, Berkowicz SM, Breckle SW, Littmann T, Jacobs AFG (2008) Dew formation and activity of biological soil crusts. In: Breckle SW, Yair A, Veste M (eds) Arid dune ecosystems. Ecological studies (analysis and synthesis). Springer, Heidelberg
Vikram S, Guerrero LD, Makhalanyane TP, Le PT, Seely M, Cowan DA (2016) Metagenomic analysis provides insights into functional capacity in a hyperarid desert soil niche community. Environ Microbiol 18(6):1875–1888. https://doi.org/10.1111/1462-2920.13088
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl 20(1):5–15. https://doi.org/10.1890/08-0127.1
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15(1):1–19. https://doi.org/10.1016/0016-7061(76)90066-5
Walvoord MA, Phillips FM, Stonestrom DA, Evans RD, Hartsough PC, Newman BD, Striegl RG (2003) A reservoir of nitrate beneath desert soils. Science 302(5647):1021–1024. https://doi.org/10.1126/science.1086435
Wang ZY, Sun G, Luo P, Mou CX, Wang J (2013) A study of soil-dynamics based on a simulated drought in an alpine meadow on the Tibetan Plateau. J Mt Sci 10(5):833–844. https://doi.org/10.1007/s11629-013-2697-2
Wang B, Zha TS, Jia X, Wu B, Zhang YQ, Qin SG (2014a) Soil moisture modifies the response of soil respiration to temperature in a desert shrub ecosystem. Biogeosciences 11(2):259–268. https://doi.org/10.5194/bg-11-259-2014
Wang C, Wang X, Liu D, Wu H, Lu X, Fang Y et al (2014b) Aridity threshold in controlling ecosystem nitrogen cycling in arid and semi-arid grasslands. Nat Commun 5:4799. https://doi.org/10.1038/ncomms5799
Wang F, Michalski G, Seo J-H, Ge W (2014c) Geochemical, isotopic, and mineralogical constraints on atmospheric deposition in the hyper-arid Atacama Desert, Chile. Geochim Cosmochim Ac 135:29–48. https://doi.org/10.1016/j.gca.2014.03.017
Wang M, Su YZ, Yang X (2014d) Spatial distribution of soil organic carbon and its influencing factors in desert grasslands of the Hexi Corridor, Northwest China. PLoS One 9(4). https://doi.org/10.1371/journal.pone.0094652
Wang X, Wang J, Xu M, Zhang W, Fan T, Zhang J (2015) Carbon accumulation in arid croplands of northwest China: pedogenic carbonate exceeding organic carbon. Sci Rep 5:11439. https://doi.org/10.1038/srep11439
Wardle DA (2013) Drivers of decoupling in drylands. Nature 502(7473):628–629. https://doi.org/10.1038/502628a
Warren SD (2003) Synopsis: influence of biological soil crusts on arid land hydrology and soil stability. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function and management. Springer, Heidelberg, pp 349–360
Weber B, Wu D, Tamm A, Ruckteschler N (2015) Biological soil crusts accelerate the nitrogen cycle through large NO and HONO emissions in drylands. PNAS 112(50):15384–15389. https://doi.org/10.1073/pnas.1515818112
Wei ST, Lacap-Bugler DC, Lau MC, Caruso T, Rao S, De Los Rios A et al (2016) Taxonomic and functional diversity of soil and hypolithic microbial communities in Miers Valley, McMurdo Dry Valleys, Antarctica. Front Microbiol 7:1642. https://doi.org/10.3389/fmicb.2016.01642
Weil RR, Brady NC (2017) The nature and properties of soils, 15th edn. Pearson, Boston
White CS, Moore DI, Craig JA (2004) Regional-scale drought increases potential soil fertility in semiarid grasslands. Biol Fert Soils 40(1):73–78. https://doi.org/10.1007/s00374-004-0744-4
White DA, Welty-Bernard A, Rasmussen C, Schwartz E (2009) Vegetation controls on soil organic carbon dynamics in an arid, hyperthermic ecosystem. Geoderma 150(1–2):214–223. https://doi.org/10.1016/j.geoderma.2009.02.011
Whitelaw MA (2000) Growth promotion of plants inoculated with phosphate-solubilizing fungi. Adv Agron 69:99–151. https://doi.org/10.1016/S0065-2113(08)60948-7
Whitford WG (1999) Comparison of ecosystem processes in the Nama-karoo and other deserts. In: Deand WRJ, Milton SJ (eds) The Karoo: ecological patterns and processes. Cambridge University Press, Cambridge, pp 291–313
Whitford WG (2002) Ecology of desert systems. Academic, San Diego, CA
Wierenga PJ, Hendrickx JMH, Nash MH, Ludwig JA, Daugherty LA (1987) Variation of soil and vegetation with distance along a transect in the Chihuahuan Desert. J Arid Environ 13(1):53–63
Wiesmeier M, Barthold F, Blank B, Kögel-Knabner I (2011) Digital mapping of soil organic matter stocks using Random Forest modeling in a semi-arid steppe ecosystem. Plant Soil 340(1–2):7–24. https://doi.org/10.1007/s11104-010-0425-z
Wild A, Oke OL (1966) Organic phosphate compounds in calcium chloride extracts of soils: identification and availability to plants. J Soil Sci 17(2):356–371. https://doi.org/10.1111/j.1365-2389.1966.tb01479.x
Williams EJ, Guenther A, Fehsenfeldi FC (1992) An inventory of nitric oxide emissions from soils in the United States. J Geophys Res 97(D7):7511–7519. https://doi.org/10.1029/92JD00412
Wilske B, Burgheimer J, Karnieli A, Zaady E, Andreae MO, Yakir D, Kesselmeier J (2008) The CO2 exchange of biological soil crusts in a semiarid grass-shrubland at the northern transition zone of the Negev desert, Israel. Biogeosciences 5(5):1411–1423. https://doi.org/10.5194/bg-5-1411-2008
Wilske B, Burgheimer J, Karnieli A, Zaady E (2009) Modeling the variability in annual carbon fluxes related to biological soil crusts in a Mediterranean shrubland. Biogeosci Discus 6:7295–7324. https://doi.org/10.5194/bgd-6-7295-2009
Wohlfahrt G, Fenstermaker LF, Arnone JA (2008) Large annual net ecosystem CO2 uptake of a Mojave Desert ecosystem. Glob Chang Biol 14:1475–1487. https://doi.org/10.1111/j.1365-2486.2008.01593.x
Woomer PL, Touré M, Sall M (2004) Carbon stocks in Senegal’s Sahel transition zone. J Arid Environ 59(9):499–510. https://doi.org/10.1016/j.jaridenv.2004.03.027
Wu JS, Huang M, Xiao HA, Su YR, Tong CL, Huang DY, Syers JK (2007) Dynamics in microbial immobilization and transformations of phosphorus in highly weathered subtropical soil following organic amendments. Plant Soil 290(1–2):333–342. https://doi.org/10.1007/s11104-006-9165-5
Wu N, Zhang YM, Downing A (2009) Comparative study of nitrogenase activity in different types of biological soil crusts in the Gurbantunggut Desert, Northwestern China. J Arid Environ 73(9):828–833. https://doi.org/10.1016/j.jaridenv.2009.04.002
Wynn JG, Bird MI, Vellen L, Grand-Clement E, Carter J, Berry SL (2006) Continental-scale measurement of the soil organic carbon pool with climatic, edaphic, and biotic controls. Glob Biogeochem Cycle 20(1):GB1007. https://doi.org/10.1029/2005gb002576
Xu XF, Thornton PE, Post WM (2013) A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob Ecol Biogeogr 22(6):737–749. https://doi.org/10.1111/geb.12029
Yahdjian L, Sala O (2010) Size of precipitation pulses controls nitrogen transformation and losses in an arid Patagonian ecosystem. Ecosystems 13(4):575–585. https://doi.org/10.1007/s10021-010-9341-6
Yan D, Li J, Pei J, Cui J, Nie M, Fang C (2017) The temperature sensitivity of soil organic carbon decomposition is greater in subsoil than in topsoil during laboratory incubation. Sci Rep 7(1):5181. https://doi.org/10.1038/s41598-017-05293-1
Yang X, Post WM (2011) Phosphorus transformations as a function of pedogenesis: a synthesis of soil phosphorus data using Hedley fractionation method. Biogeosciences 8(10):2907–2916. https://doi.org/10.5194/bg-8-2907-2011
Yang YH, Mohammat A, Feng JM, Zhou R, Fang JY (2007) Storage, patterns and environmental controls of soil organic carbon in China. Biogeochemistry 84(2):131–141. https://doi.org/10.1007/s10533-007-9109-z
Yang K, Zhu JJ, Zhang M, Yan QL, Sun OJ (2010) Soil microbial biomass carbon and nitrogen in forest ecosystems of Northeast China: a comparison between natural secondary forest and larch plantation. J Plant Ecol 3(3):175–182. https://doi.org/10.1093/jpe/rtq022
Yang J, Kang Y, Sakurai K, Ohnishi K (2017) Fixation of carbon dioxide by chemoautotrophic bacteria in grassland soil under dark conditions. Acta Agric Scand B Soil Plant Sci 67(4):362–371. https://doi.org/10.1080/09064710.2017.1281433
Yan-Gui S, Xin-Rong L, Ying-Wu C, Zhi-Shan Z, Yan L (2013) Carbon fixation of cyanobacterial–algal crusts after desert fixation and its implication to soil organic carbon accumulation in desert. Land Degrad Dev 24(4):342–349. https://doi.org/10.1002/ldr.1131
Yao J, Liu H, Huang J, Gao Z, Wang G, Li D et al (2020) Accelerated dryland expansion regulates future variability in dryland gross primary production. Nat Commun 11(1):1665. https://doi.org/10.1038/s41467-020-15515-2
Yu J, Glazer N, Steinberger Y (2014) Carbon utilization, microbial biomass, and respiration in biological soil crusts in the Negev Desert. Biol Fertil Soils 50(2):285–293. https://doi.org/10.1007/s00374-013-0856-9
Yuan BC, Yue DX (2012) Soil microbial and enzymatic activities across a chronosequence of chinese pine plantation development on the Loess Plateau of China. Pedosphere 22(1):1–12. https://doi.org/10.1016/S1002-0160(11)60186-0
Yuan H, Ge T, Chen C, O’Donnell AG, Wu J (2012) Significant role for microbial autotrophy in the sequestration of soil carbon. Appl Environ Microbiol 78(7):2328–2336. https://doi.org/10.1128/AEM.06881-11
Yue P, Cui X, Wu W, Gong Y, Li K, Misselbrook T, Liu X (2020) Are annual nitrous oxide fluxes sensitive to warming and increasing precipitation in the Gurbantunggut Desert? Land Degrad Dev. https://doi.org/10.1002/ldr.3636
Zaady E (2005) Seasonal change and nitrogen cycling in a patchy Negev Desert: a review. Arid Land Res Manag 19(2):111–124. https://doi.org/10.1080/15324980590916512
Zaady E, Arbel S, Barkai D, Sarig S (2013) Long-term impact of agricultural practices on biological soil crusts and their hydrological processes in a semiarid landscape. J Arid Environ 90:5–11. https://doi.org/10.1016/j.jaridenv.2012.10.021
Zehr JP, Jenkins BD, Short SM, Steward GF (2003) Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ Microbiol 5(7):539–554
Zhang P, Shao M (2014) Spatial variability and stocks of soil organic carbon in the Gobi desert of Northwestern China. PLoS One 9(4):e93584. https://doi.org/10.1371/journal.pone.0093584
Zhang XD, Amelung W, Ying YA, Zech W (1998) Amino sugar signature of particle-size fractions in soils of the native prairie as affected by climate. Soil Sci 163(3):220–229. https://doi.org/10.1097/00010694-199803000-00007
Zhang ZS, Li XR, Nowak RS, Wu P, Gao YH, Zhao Y et al (2013) Effect of sand-stabilizing shrubs on soil respiration in a temperate desert. Plant Soil 367(1–2):449–463. https://doi.org/10.1007/s11104-012-1465-3
Zhang Y, Xiao X, Guanter L, Zhou S, Ciais P, Joiner J et al (2016a) Precipitation and carbon-water coupling jointly control the interannual variability of global land gross primary production. Sci Rep 6:39748. https://doi.org/10.1038/srep39748
Zhang ZS, Zhao Y, Dong XJ, Shi YF, Chen YL, Hu YG (2016b) Evolution of soil respiration depends on biological soil crusts across a 50-year chronosequence of desert revegetation. Soil Sci Plant Nutr 62(2):140–149. https://doi.org/10.1080/00380768.2016.1165597
Zhao MS, Heinsch FA, Nemani RR, Running SW (2005) Improvements of the MODIS terrestrial gross and net primary production global data set. Remote Sens Environ 95(2):164–176. https://doi.org/10.1016/j.rse.2004.12.011
Zhou X, Smith H, Giraldo Silva A, Belnap J, Garcia-Pichel F (2016) Differential responses of dinitrogen fixation, diazotrophic cyanobacteria and ammonia oxidation reveal a potential warming-induced imbalance of the N-cycle in biological soil crusts. PLoS One 11(10):e0164932. https://doi.org/10.1371/journal.pone.0164932
Zhu SH, Li CF, Shao H, Ju WM, Lv NN (2019) The response of carbon stocks of drylands in Central Asia to changes of CO2 and climate during past 35 years. Sci Total Environ 687:330–340. https://doi.org/10.1016/j.scitotenv.2019.06.089
Zscheischler J, Reichstein M, Von Buttlar J, Mu M, Randerson JT, Mahecha MD (2014) Carbon cycle extremes during the 21st century in CMIP5 models: future evolution and attribution to climatic drivers. Geophys Res Lett 41:8853–8861. https://doi.org/10.1002/2014GL062409
Zuo R, Jin S, Chen M, Guan X, Wang J, Zhai Y et al (2018) In-situ study of migration and transformation of nitrogen in groundwater based on continuous observations at a contaminated desert site. J Contam Hydrol 211:39–48. https://doi.org/10.1016/j.jconhyd.2018.03.003
Acknowledgments
KJ is supported by a CONICYT-Fondecyt Postdoctorado 3190464 grant.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jordaan, K., Stucken, K., Díez, B. (2022). C, N, and P Nutrient Cycling in Drylands. In: Ramond, JB., Cowan, D.A. (eds) Microbiology of Hot Deserts. Ecological Studies, vol 244. Springer, Cham. https://doi.org/10.1007/978-3-030-98415-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-98415-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-98414-4
Online ISBN: 978-3-030-98415-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)