Abstract
Drylands are arid and semi-arid areas whose main feature is their low level of precipitation. They cover nearly half of Earth’s land surface and are distributed worldwide, constituting the planet’s largest biome. Dryland soils have low fertility, are greatly affected by climate variability, and are vulnerable to wind and water erosion. The phosphorus-rich soil dust traveling by aeolian processes from drylands is the main source of P for the global primary productivity in P-limited areas, a fact that highlights the importance of arid and semiarid areas in the global nutrient budget. This review discusses the development of dryland soils, the sources of P in drylands, the C, N, P, relation in dryland soils, the fractionation and bioavailability of P, and biotic and abiotic factors affecting the P in drylands. Finally, the dynamic of P in biological soil crusts and resource islands is discussed. Combined, they contribute to the surface organic matter pools, alter the soil fertility, and are determinant factors in P’s availability in dryland soils. We conclude the review by highlighting the gaps in knowledge that should be addressed in future research regarding biological and abiotic processes that determine the dynamics of P in drylands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
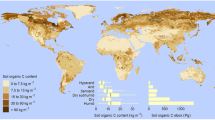
The term drylands refers to areas with dry climate conditions characterized by low amounts of precipitation in the form of rainfall or snow (Monger et al. 2005; United Nations Environment Management Group 2011). Drylands cover about 41% of Earth’s ice-free land surface and are widely distributed in Africa, Asia, America, and Oceania, considerably less common in Europe (Fig. 1; Schimel 2010; Osman 2018) and constitute the largest biome on the planet. They harbor nearly 38% of the global human population, 74% of the global pastures, and 50% of the croplands. Despite having a high biodiversity of specialized life forms, the drylands are among the most vulnerable to degradation and most disregarded ecosystems in the world (Monger et al. 2005; Safriel and Zafar 2005; Schimel 2010; Plaza et al. 2018).
Distribution of drylands and their subtypes. Based on UNEP-WCMC (2007). A spatial analysis approach to the global delineation of drylands areas of relevance to the CBD Programme of Work on Dry and Subhumid Lands. Dataset based on the spatial analysis between WWF terrestrial ecoregions and aridity zones. United Nations Environment Programme-World Conservation Monitoring Centre (UNEP-WCMC)
Drylands are characterized by long dry periods interrupted by high-intensity and short-duration rainfalls (Noy-Meir 1973; Kalma and Franks 2003; Osman 2018). Erosion as a possible consequence of climate change could produce heterogeneous patterns of vegetation dominated by sparse vegetation growing in patches, which play a critical role in the distribution of below-ground resources such as organic matter and nutrients (Hartley et al. 2007; Hutchinson and Herrmann 2008; Osman 2018).
Phosphorus (P) is one of the main limiting nutrients that affects plant growth and establishment in terrestrial ecosystems (Tunesi et al. 1999; Turner et al. 2002; Buckingham et al. 2010; Ahmad et al. 2017). The main sources of P in drylands are the deposition and redistribution of atmospheric dust coming from the same drylands, the weathering of parent materials, and the deposition of plant litter (Belnap 2011; Azadi and Baghernejad 2019; Lopez and Bacilio 2020). P-rich dust from drylands stimulates global primary productivity in P-depleted marine and terrestrial ecosystems (Barkley et al. 2019; Bigio et al. 2020). These findings highlight the impact of P on productivity and raise new questions about how global ecosystems are connected to the P cycle. Consequently, understanding the fate of P in drylands could contribute to understanding the future fertility of terrestrial and oceanic ecosystems across the globe.
Belnap (2011) reviewed the biological P cycling in drylands, suggesting that the redistribution of P due to gaps between bare soil and soil covered by vegetation is higher in drylands than in areas with a moderate or high supply of moisture and that the redistribution of P is a major determining factor in soil P levels. Moreover, the availability of P is lower in dry soils, as compared to more humid soils, due to alkaline pH values, abundant content of CaCO3, and the presence of compounds that bind P. While the amount of inorganic P is higher, organic matter, microbial mobilization, and phosphatase activities are lower in drylands than in more humid areas.
Considering the studies carried out on both the biotic and abiotic P processes in arid soils since 2011, we believe that a review of the different elements defining the sources, importance, and dynamics of P in drylands is timely and that the knowledge gained from such a review may be important for the sustainable management of these soils. Thus, in this review, we discuss (i) the development and surfaces of dryland soils; (ii) dust deposition and weathering as the main sources of P in drylands, and the carbon (C)–nitrogen (N)–P relation in dryland soils; (iii) the fractionation and bioavailability of P in dryland soils; (iv) the abiotic and biotic processes that affect P availability and dynamics in dryland soils, such as the effect of dry-wetting cycles, solubilization of inorganic P, mineralization of organic P, phosphatase activities, and ATP and adenylate energy charge; and v) the P dynamics in biological soil crust (BSC) and resource islands (RI), because they are two natural microbial-floral entities dominating P functionality in dryland ecosystems (Bashan and de-Bashan 2010; Garcia et al. 2018; Wang et al. 2019). Finally, we suggest the direction of future research regarding the biological and abiotic processes that determine the dynamics of P in drylands.
Development and surfaces of dryland soils
Soil formation in drylands is a complex process with a polygenic nature strongly influenced by environmental change. With few exceptions (i.e., the Atacama and Namib Deserts), dry soils likely originated from the major climatic events that occurred during the Quaternary, when fluctuations of arid and humid conditions were accompanied by active aeolian dust deposition and carbonate accumulation, leading to intervals of relatively rapid soil formation (Chadwick and Davis 1990; Dixon 2009; Fookes et al. 2013; Fig. 2a). In addition to geogenic processes, the weathering of rocks and minerals determines the chemical profile of soils, while variable climate influences the rate and magnitude of soil formation, resulting in a great diversity of soil types (Warke 2013, Fig. 2b–c). Rock weathering contributes to soil formation in drylands through processes such as fragmentation by mechanical forces, thermal fluctuations, chemical degradation by salts, and the pioneering activity of microorganisms and plants (Dixon 2009; Dietze et al. 2012; Fookes et al. 2013; Lopez and Bacilio 2020; Fig. 2c).
Pedogenesis of drylands with an emphasis on P distribution (a–c) and soil P transformations in humid (d) and arid ecosystems (e). During Quaternary, global climate changes and massive dust deposition of Ca–P produced ecosystems’ succession. With time, the regional climatic variability gave rise to different levels of aridity (a). Low rainfall (Ppt) and high evapotranspiration (EvT) induce salt accumulation and eventually calcrete formation in subsurface horizons. The main P inputs in contemporary drylands come from rock/mineral weathering and active dust deposition while P losses occur mainly by wind and water erosion. Dust exportation is the main source of P for wetter ecosystems (b, c). The traditional model of Walker and Syers (1976) (d) illustrates changes in P-fractions through time during the pedogenesis of an ecosystem, and panel e describes the P-distribution in the same model adapted to drylands according to Selmants and Hart (2010), where residual-P plays an important role in the long-term process of adsorption/complexation and therefore in P-bioavailability. Panel d, adapted from Walker and Syers (1976); panel e, adapted from Selmants and Hart (2010); dust-dep, dust deposition; dust-exp, dust exportation of P-containing minerals; PCa input, primary minerals bearing calcium phosphates; Pp, rainfall; EvT, evapotranspiration
The most widely known surfaces in drylands are pavements, abiogenic and biological crusts, and vesicular horizons (Dixon 2009). Pavements are surfaces composed of gravel or boulders overlying mantles of sand, silt, and clay-sized materials of multifactorial origin, including climate-driven weathering, sediment transport caused mainly by wind deflation, episodic flash floods, and dust deposition with airborne salts (Dixon 2009; Dietze et al. 2012; Fookes et al. 2013; Knight and Zerboni 2018). These stony surfaces represent niches for lithobionts (Warke 2013), which can form cryptogamic ground covers such as those, almost endemic, documented by Jung et al. (2019) in the Atacama Desert.
The abiogenic salt crusts are formed by the alternating dissolution and recrystallization of salts (i.e., gypsum), resulting in a thin and hard layer on the soil surface (Gutiérrez 2005; Dixon 2009). Surface cracks are associated with strong changes in the temperature and volume of the crust, resulting in polygons with elevated edges (Dixon 2009). The biological soil crusts are defined as complex communities of cyanobacteria, bacteria, algae, lichens, and mosses (Pointing and Belnap 2012; Williams et al. 2012) that significantly impact water infiltration, runoff, and nutrient cycling (Pointing and Belnap 2012). The ecological importance of BSC for P dynamics will be further discussed in detail in this review.
Last, the upper horizons under the pavements or the crusts consist of vesicles isolated or interconnected within a matrix of fine-grained materials whose structure confers surface stability to soils and resistance to mechanical disturbance (Anderson et al. 2002; Dietze and Kleber 2012).
Sources of P and C–N–P relation in dryland soils
As presented earlier, the abundance and availability of P in drylands result from dust deposition and losses through wind or water, weathering, and biotic processes (Belnap 2011). Atmospheric sources of P include mineral aerosols, P-rich materials derived from fires, biogenic particles, sea salt aerosols, and volcanic aerosols (Okin et al. 2001), with mineral aerosols and biogenic particles contributing 80% and 12%, respectively, to the total atmospheric P (Okin et al. 2001; Mahowald et al. 2008).
Estimations of weathering rates and P stocks in dry soils are scarce and challenging because most mass balance approaches depend on inputs and losses due to erosion and dust deposition whose determination is difficult (Newman 1995; Hartley et al. 2007; Plaza et al. 2018). The least weathered soils, such as entisols and aridisols which dominate arid lands, show higher inputs (0.05 g P m−2 year−1) than the less common and intermediate weathered soils, mollisols, alfisols, and vertisols, whose inputs (0.01 g P m−2 year−1) are similar to those of humid soils such as spodsols (Wang et al. 2010; Soil Survey Staff 2014).
Although bioweathering is often underestimated in arid lands, low amounts of moisture support the activity of N fixers, chemolithotroph microorganisms, and plants, which play an important role in establishing the initial pools of nutrients for primary production and nutrient cycling (Jung et al. 2019; Lopez and Bacilio 2020), especially of the strong interlinked C–N–P dynamics (Delgado-Baquerizo et al. 2013). Thus, the initial stages of ecosystem succession are characterized by the prevalence of geochemical processes, where P originates from the weathering of primary minerals, and C and N largely depend on the mineralization of soil organic matter through microbial activity (Walker and Syers 1976; Delgado-Baquerizo et al. 2013). As the ecosystem develops, P is subjected to several processes, such as incorporation into the biomass, interaction with secondary minerals, leaching, erosion, and solubilization/precipitation, eventually coupling the P dynamics to the N and C cycles (Fig. 2b). However, intense weathering over long time scales in drylands may result in an increase in P, and as a result, the C, N, and P cycles eventually become decoupled (Ippolito et al. 2010; Delgado-Baquerizo et al. 2018; Fig. 2c).
Using geo-databases from around the world, Plaza et al. (2018) showed that, unlike C and N, total P content was higher in dryland soils than in soils of humid regions, with larger labile inorganic and apatite P content but lower organic P due to lower biological activity. Furthermore, the imbalance in the C, N, and P stoichiometry fluctuates along with aridity (Delgado-Baquerizo et al. 2013; Gong et al. 2017). Delgado-Baquerizo et al. (2013) analyzed 224 dryland sites across all continents and found that, as aridity increased, biological processes became more limited, and the bioavailability of C and N decreased, while P was continuously released through weathering processes. This C–N–P stoichiometric imbalance results in the accumulation of P and is sometimes related to the calcium carbonate content of arid soils. Another example was provided by Gong et al. (2017), who found that drought, especially in the upper soil horizon (0–20 cm), inhibited the uptake of nutrients by the plants in a desert community, impacting the N–P stoichiometric balance. A comprehensive study by Eldridge et al. (2020) showed that, in addition to the C, N, and P content in soils, soil functions and the C–N–P stoichiometric balance were affected by (i) climatic features: annual temperature, aridity index, and seasonal precipitation; (ii) soil properties: roughness of the soil surface, crust resistance, crust brokenness, surface integrity, deposited materials, soil aggregates, and pH; (iii) biocrust cover; and (iv) plant-related features such as vegetation cover, foliage, and litter incorporation.
Fractionation and bioavailability of P in dryland soils
The development of ecosystems largely determines the distribution and bioavailability of P in soils (Walker and Syers 1976, Fig. 2d). Thus, P resulting from weathering is subjected to incorporation into the biomass and interaction with other chemical constituents. Essentially, these transformations comprise four forms: inorganic P (mostly calcium-bound P, P–Ca), non-occluded P, occluded P, and organic P (Po). According to the Walker and Syers model, the distribution of these P forms changes during long-term pedogenesis because, as soil develops, P–Ca declines, whereas the occluded P and non-occluded P peak and then decrease, while the Po increases with time and is controlled by the biological activity (Fig. 2d).
Following the distribution of P forms proposed in the model of Walker and Syers, it is now generally recognized that the total P can be divided into the following fractions (or P pools): (i) labile or bioavailable, where P is free in the soil or weakly associated with surfaces; (ii) moderately labile, where P is reversibly adsorbed or bound to the mineral surfaces; (iii) stable, where P is associated with Ca or inorganic P (Pi) occluded within sesquioxides; and (iv) non-extractable P and stable organic P (Walker and Syers 1976; Buckingham et al. 2010; Hou et al. 2017; Baumann et al. 2018). This fractionation is based on sequential extraction with specific extractants solubilizing specific P fractions. It is important to point out that the distribution and the estimated reactivity of the P pools depend on the fractionation procedure (Condron and Newman 2011).
Selmants and Hart (2010) tested the model of Walker and Syers in semiarid ecosystems and found that the overall distribution of P formed along the chronosequences of volcanic substrates agreed with the distribution of pools suggested by the model (Fig. 2d). Specifically, the total soil P and mineral P declined with soil development, the organic P increased at a low rate, and the non-occluded P peaked during early to mid-term soil development. Nonetheless, in their study, the occluded P was highest in the youngest soils and then declined to an almost steady state instead of increasing and then declining, as described in the traditional model. Selmants and Hart (2010) called this fraction residual P, with P bound to Al or Fe hydrous oxides. They also suggested that in some drylands, the organic P, the P–Ca, and the non-occluded inorganic P are still in the early stages of the Walker and Syers model and have not yet reached the stage where P–Ca is completely depleted (Fig. 2e).
Moreover, based on the conceptual framework proposed by the Walker and Syers model, Vitousek et al. (2010) identified six mechanisms of P limitation in terrestrial ecosystems: (i) depletion driven: P losses due to the leaching and exhausting of P-rich minerals; (ii) soil barriers: physical layers preventing the roots from accessing P; (iii) transactional: the release of P is slower than that of inputs; (iv) low P concentration in the parent materials; (v) sink driven: accumulation of P into inaccessible forms; and (vi) anthropogenic, through the alteration of other nutrients, such as N. In more humid climates, P depletion is perhaps the most widely known mechanism of P limitation in soils. In contrast, the main mechanism in drylands is sink driven, where P is precipitated with Al, Fe, or minerals, or sorbed onto the soil’s particles, such as organic matter and clays (Buckingham et al. 2010; Vitousek et al. 2010; Moradi et al. 2020). P bioavailability is regulated by the soil constituents through processes such as biological immobilization/mineralization, adsorption/desorption, and precipitation/dissolution (Hou et al. 2017).
As discussed earlier, generally the total P pool in the initial stages of soil development is dominated by primary minerals, such as apatite (Ca–P). Then, during soil development, the apatite is weathered by physicochemical and biological factors, releasing inorganic P forms as secondary P fractions (secondary minerals P, Al–P, and Fe–P), which interact with the soil particles (Bruker and Spohn 2019) and finally become occluded P over a large time scale (Fig. 2d). However, in dry soils originating from sedimentary rocks, the most unavailable forms of P are secondary apatite minerals, and P is weathered mostly from Fe/Al oxides. Therefore, P content is related to the bedrock and Fe and Al content, suggesting that Fe and Al also play an important role in P availability in dryland soils (Neff et al. 2006).
Aridity can also affect the P fractionation in drylands. Feng et al. (2016) conducted a study to understand the P fractionation in a large-scale climosequence in grasslands in northern China. The calcium phosphate content in the primary minerals was higher (being about 80% of the total P) in the more arid sites compared to the wetter sites, and the organic P, non-occluded inorganic P, and occluded P represented more than 50% of the total P in the wetter sites. These findings confirmed that pedogenesis depends on the volume of water leached through soils and that the model of Walker and Syers applies not only to chronosequences but also to climosequences (Feng et al. 2016).
Since P fractions are affected by multiple factors and because a major source of P is dust deposition, it is difficult to conclude that the bioavailable P will behave in the same manner across different dry soils. For example, Gu et al. (2019) found that Ca–P and P fixed with Al or Fe oxides contributed 20–45% and 55–82% respectively, of the total soil inorganic P in cool semi-arid regions in Arizona. However, in dust, the main fraction was Ca–P, whereas Al–P was negligible, and Fe–P accounted for only 10–20% of the dust inputs. Thus, although these results support the Walker and Syers model, where the Ca–P pool declines during pedogenesis due to weathering and acidification, they highlight the important contribution of aeolian dust, which impacts the bioavailable P fraction, increasing Ca–P and decreasing Fe–P and Al-P.
In summary, the distribution and bioavailability of P in drylands depend on geological history, pedogenesis, climatological and physicochemical factors in soil, and important contributions by dust deposition. However, dryland soils are dynamic and complex systems harboring a large group of biological components, such as microbes and plants, which also play a relevant role in P bioavailability.
Abiotic and biotic factors affecting the dynamics of P in dryland soils
The biological activity in arid soils is limited by several factors, including soil moisture, pH, micro- and micronutrients availability, the presence of calcium and sodium, and osmotic effects (Brady and Weil 2002). Soil moisture is generally scarce, but this can change depending on the rainfall (Belnap 2011). Micronutrient deficiencies occur as soil pH increases; the availability of Fe, Zn, Mn, and Cu decreases over pH 7.0 because they form insoluble hydroxides, while that of B is particularly low at pH 9.0 because the strength of the inner sphere complexes on the surfaces of Fe and Al oxides and silicate clays is strong (Brady and Weil 2020). The presence of free CaCO3 at pH 8.4 can inhibit plant growth, decreasing rhizodeposition and the plant debris input to the soil. The increase in soil pH enhances the inorganic P adsorption to Ca, Fe and Al oxides, and silicates, decreasing the amount of P available to soil microorganisms, which favors the synthesis of phosphatases by microorganisms (Nannipieri et al. 2011). Also, the presence of Na negatively affects the soil structure in alkaline soils, and lastly, the osmotic effects limit both the biological activity and the plant activity (Brady and Weil 2002).
Effect of dry-wetting cycles
According to Belnap (2011), the biological P cycle in dry soil is controlled by the intensity, timing, and amount of precipitation. Water associated with decreased temperature, as occurs in winter, can decrease soil pH due to the increase in H2CO3, thus increasing the solubility of inorganic P. The timing of rainfall is important because if rainfall occurs during a warm period, it will stimulate microbial and plant activity (Belnap 2011). Although the amount of rainfall is also a parameter that controls the biological P cycle, a precipitation event rarely exceeds 5 mm of rain; as such, only the surface soil organisms respond to the increase in soil moisture, not the vascular plants, which require a greater amount of precipitation depending on their rooting depth. Likewise, the length of time between two rainfall events and the rapid soil wetting and drying processes are also important. P inputs to the soil surface occur during the dry period due to the high microbial mortality from desiccation and radiation damage, dust accumulation, and degradation of organic matter by UV (Jobbagy and Jackson 2001; Belnap 2011). The available P can move from the surface soil to lower layers but usually not more than 5 mm. In addition, soil surface materials are brought to the deeper soil layers by fauna, especially ants and termites, which are present in dry soils worldwide (Belnap 2011). Pulses of available P can occur in surface soil as the result of the drying–wetting cycles. According to Kieft et al. (1987), 58% of the microbial biomass is killed upon rapid drying due to the high temperature that follows the wetting of soils by rainfall. According to Cosentino et al. (2006), fungi are less affected than bacteria by drying and rewetting, although Butterly et al. (2009) and Gordon et al. (2008) observed the opposite. To the best of our knowledge, the underlying mechanisms behind the release of P from the drying and rewetting of arid soils are poorly known. We speculate that the amount of released P from killed microbial cells is low in arid soils due to the low microbial biomass, as shown by the low ATP content of air-dried soils (see below). After rewetting soil, P can be released from P-rich microbial cells (usually bacteria having higher P concentrations than fungi) by active phosphatases (Bünemann et al. 2013). In addition to the mineralization of dead (killed by drying) microbial cells, the stimulation of microbial activity, mineralization of photodegraded organic matter, and mineralization of solutes released upon wetting are also biotic mechanisms responsible for the release of P (Barnard et al. 2020). Typically, CO2 pulses occur during the first hours after rewetting because water connectivity is restored among the huge numbers of microsites (they are separated during the dry periods), facilitating the access of available nutrients to microbes (Barnard et al. 2020). Abiotic mechanisms can also release physically protected P after the drying and rewetting of soils because slaking can disrupt macroaggregates (Bünemann et al. 2013). However, future research should address the mechanisms responsible for the release of P after drying and rewetting events of arid soils.
Microbial solubilization of inorganic P
Microorganisms solubilize P from less available inorganic forms, making it bioavailable for plants and other microorganisms (Magallon-Servin et al. 2020). The role of P-solubilizing bacteria in drylands is complex and poorly understood, especially in desert soils (Ameen et al. 2019). The bacterial ability to solubilize P from sparingly soluble P sources in drylands is limited by C availability (Bruker et al. 2020); therefore, the capacity of specific microorganisms to solubilize P will differ between the rhizosphere and the bulk soil. Currently, it is recognized that chelation (due to secretion of siderophores) and/or acidification (through the ionization of organic acids or the release of H+ accompanying respiration or NH4+ assimilation) is the main mechanisms of microbial P solubilization (Reyes et al. 2001; Hamdali et al., 2008; Song et al. 2008). Some organic acids (e.g., oxalic, citric, tartaric, and lactic acid) can efficiently solubilize P by dissolving the mineral phosphate directly as a result of the exchange of the anion PO43− by an acid anion or by the chelation of Ca, Fe, and Al ions associated with phosphate, lowering the pH (Khan et al. 2007; Belnap 2011). Bacterial genera, such as Bacillus, Pantoea, Pseudomonas, Burkholderia, Asaia, Rahnella, Streptomyces, Serratia, and Micromonospora (Perez et al. 2007; Hamdali et al. 2008; Song et al. 2008; Sulbaran et al. 2009; Magallon-Servin et al. 2020), and fungal genera, such as Trichoderma, Aspergillus, Penicillium, and Mortierella (Osorio and Habte 2014; Zuñiga-Silgado et al. 2020), can solubilize phosphates through the production of organic acids. However, not all organic acids have the same solubilization effect on the different insoluble forms of P. The effectiveness of P solubilization by organic acids depends on their chelating and complexing abilities, which are affected by the type and position of the functional ligand (Kpomblekou-A and Tabatabai 1994). Since organic acids are weak acids, the P-solubilization process by microorganisms cannot be explained only by protonation (Sagoe et al. 1998); other factors, such as the reactivity of the P minerals, play a major role. This reactivity, or potential to be solubilized, depends on the amount of available Ca, Fe, Al, and accessory minerals (Kpomblekou-A and Tabatabai 1994; 2003). Therefore, P solubilization by microorganisms is a complex process that involves many factors in the soil system.
Most studies have evaluated P solubilization in cropped arable soils using different types of P fertilizers, but this approach does not provide a mechanistic understanding of the relative microbial processes. Few studies have focused on the microbial solubilization of inorganic P pools and the effect of factors such as the availability of C, N, and water. Brucker et al. (2020) examined the microbial P solubilization of apatite and weathered rock (saprolites) in different soils (ranging from the Mediterranean to arid) from the Coastal Cordillera of Chile, finding that the concentrations of C and N, regardless of the soluble P concentration, play an indirect role in P solubilization through effects on soil microbial activity. These results suggest that P availability is not particularly important for microbial P solubilization. In soils from arid regions in Jordan, Ameen et al. (2019) observed that, although certain arid soils contained microbiota that could solubilize P, the process was controlled by water availability. Considering that drying–wetting cycles affect the concentration of P in drylands, their effect on P solubilizers should be further investigated.
Organic P and phosphatase activities
According to Nedeau et al. (2007) and Turner et al. (2003a), 87% of organic P is hydrolysable in arid soils. However, in these soils, more than 50% of the soil is inorganic P, whereas, in mesic soil, organic P can prevail over inorganic P (Belnap 2011). Water-soluble P represents 5% of the organic P and is mainly present as nucleic acid and phospholipids released by lysed cells and can be easily used by the active microbial cells in soil (Turner et al. 2003b). Other pulses of dissolved organic P can occur independently from the cells because drying–wetting cycles can cause the detachment of organic P from the organic matter coating the soil particles (Blackwell et al. 2009). The presence of available P can stimulate microbial P immobilization, but this process is minimal in dry soil; for example, it accounted for 3% of the total P in the Chihuahuan Desert (Lajtha and Schlesinger 1988), whereas it accounted for 36–55% of the total P in peatlands (Walbridge 1991). This difference is likely due to the fact that dry conditions affect microbial activity, microbial biomass, and the diffusion of the substrates and extracellular enzymes needed to catalyze the extracellular hydrolysis of polymers, including those containing organic P. Moreover, the mineralization of organic P is very low in dry soils, as shown by the low phosphatase activity (Margalef et al. 2017). Generally, both acid and alkaline phosphatase activities decrease after the drying of soil (Nannipieri et al. 2011), while they increase from bulk soil to rhizosphere soil (Taradfar and Jungk 1987).
The most studied phosphatase activities in soil are acid and alkaline phosphatases, which are phosphomonoesterases hydrolyzing phosphomonoesters such as β-glycerophosphate, phenylphosphate, β-naphthyl phosphate, and p-nitrophenyl phosphate (Acosta-Martinez and Tabatabai 2011; Nannipieri et al. 2011). The correct biochemical names are acid and alkaline phosphomonoesetrase, but in soil enzymology they are termed acid and alkaline phosphatase. Here, we will use the latter terms to reflect what was reported in the cited references.
In acid soils, acid phosphatase activities surpass alkaline phosphatase activities, while the opposite is true for alkaline soils (Acosta-Martinez and Tabatabai 2011; Nannipieri et al. 2011). The phosphodiesterase activity can give insights into the degradation of nucleic acids and phospholipids because it hydrolyzes one of the two phosphoesters of the molecule and can precede the activity of the phosphomonoesterases, which release inorganic P (Nannipieri et al. 2011).
The available P in arid surface soil is controlled by microorganisms in the biological soil biocrusts, which have the capacity to solubilize inorganic P and can also synthesize and release extracellular phosphatases (Belnap 2011). These enzyme activities are increased by Ca and decreased by Mg in cyanobacteria (Belnap 2011). The presence of extracellular polysaccharides from cyanobacteria preserves the extracellular phosphatase activities against proteolysis (de Caire et al. 2000). This is particularly important because it suggests that extracellular phosphatases can be stabilized and protected against proteolysis even if the organic content of the soil is low. This extracellular and stabilized enzyme activity is independent of the extant microbial activity and can thus be active even under conditions hostile to microbial activity (Nannipieri et al. 2018). Unfortunately, current enzyme assays do not distinguish the activity associated with these extracellular and stabilized enzymes from that due to microbial activity, which includes the activity of free extracellular enzymes, enzymes attached to the outer surfaces of viable cells with the active site extended into the extracellular environment, and intracellular enzymes (Nannipieri et al. 2018). Future research should determine the origin of the measured phosphatase activities—likely the alkaline phosphatase activity prevalent in dry soil—by using molecular techniques measuring alkaline–phosphatase-encoding genes (Wei et al. 2019). The alkaline pH of arid soils, due to the presence of carbonate, is also responsible for the higher phenol oxide activities compared to those in mesic soils, whereas the opposite is true for glycosidase activities (Garcia et al. 2017). Indeed, the optimal pH values of phenol oxidases are alkaline, while those of glycosidases range from 4 to 6.
ATP and adenylate energy charge
Adenosine 5’-triphosphate (ATP) is an important organic P compound in the cell, and its content has been used to determine the microbial biomass of soil because it is quite constant, ranging from 10 to 11 μmol ATP g−1 biomass (Qiu et al. 2019). Air-drying markedly reduces the ATP content of the soil, but it increases upon the rewetting of the dry soil; for example, the ATP contents of oven-dried fresh organic soil, air-dried organic soil, and air-dried and rewetted organic soil (4.3% C) were 7.82, 2.11, and 4.40 nmol g−1, respectively, and 1.43, 0.10, and 0.99 nmol g−1 in oven-dried fresh sandy soil, air-dried sandy soil, and air-dried and rewetted sandy soil (1.8% C) (Brookes et al. 1983). The concentrations of the three adenylate nucleotides are used to determine the adenylate energy charge (AEC), which provides a measure of the metabolic energy stored in the cells, according to the following equation: AEC = [ATP] + [1/2 ADP]/[ATP] + [ADP] + [AMP (adenosine monophosphate)]. It can vary from 1.0 (all ATP) to 0 (all AMP), but the important values are (i) the highest ones (0.8–0.85), which are indicative of actively growing cells; (ii) the intermediate ones (0.5–0.75), which suggest cells under the stationary phase; and (iii) the low ones (less than 0.5), which are indicative of dead or dying cells (Nannipieri et al. 1990). The high AEC values of temperate soils are indicative of microbial cells that are metabolically active and can thus respond to nutrient additions and seem to contradict the assumption that most soil microorganisms are inactive (Brookes et al. 1987; Nannipieri et al. 1990). However, the AEC of a clay loam subjected to the Mediterranean climate with hot, dry summers, and with a low organic-matter content (1.3%) was 0.65, whereas that of the organic clay soil with a high organic matter content (24.1%) was 0.8 when nucleotides were extracted with an acidic extracting solution inhibiting enzyme reactions involving ATP, ADP, and AMP, with both soils used at 50% of their water-holding capacity (Ciardi et al. 1990). The rate of desiccation had a greater effect than the absolute value of the water potential on the decrease in the AEC values of the surface soils sampled from the Mediterranean climate area (Rosacker and Kieft 1990). Although the AEC value can indicate the energy state of soil microorganisms, it has been scarcely studied, and it would be interesting to examine the AEC data of desert soils subjected to wetting.
Dynamic of P in biological soil crusts
BSCs significantly affect major environmental processes, such as soil stability and erosion, atmospheric nitrogen fixation, nutrient uptake by plants, soil–plant–water relations, water infiltration, seedling germination, and plant growth, due to their strategic location at the soil–air interface of the desert (Bashan and de-Bashan 2010). Therefore, they contribute to surface organic matter pools, alter soil fertility through C and N fixation, and are a determining factor in the availability of P in dryland soils (Belnap 2011). According to Williams et al. (2012), the mechanisms of BSC formation are (i) stabilization and precipitation of authigenic minerals, (ii) drying–wetting along with expansion–contraction, (iii) capture of deposited dust, (iv) microscale mass waste produced by weakened aggregates, and (v) vesicular horizon formation.
Little is known about P pools in BSC in drylands, as compared to BSC in temperate regions. In a gradient of soils (arid, semi-arid, Mediterranean, humid) in Chile, Baumann et al. (2018) found that labile P was higher in BSC from semi-arid and Mediterranean soils than in BSC from humid soils. For all sites, the main P pools were stable and non-extractable, with Ca–P species dominating in arid, semi-arid, and Mediterranean areas, and Al–P more common in humid areas.
The microbial components of crusts, particularly the cyanobacterial filaments, secrete polysaccharides that bind soil particles and form microaggregates, which are harder to move by the wind, compared to single grains (Crain et al. 2018, Fig. 3). Thus, well-developed crusts can efficiently capture P from dust and prevent its loss, allowing plants to have a higher P uptake compared to the adjacent interspaces (Belnap 2011). They may also counteract RI formation by preventing the redistribution of the soil and seeds. When present, plants are worse competitors for P uptake than microorganisms of the biocrust soil (Housman et al. 2007).
Schematic representation of the complex P dynamic in arid soils as affected by resource islands (RI) and biological soil crusts (BSC). In RI, rhizosphere acidification, CEC of the root system, and mycorrhizal associations allow increase P concentration under the canopy of nurse trees. In RI and BSC, beneficial microorganisms such as N2 fixers, P solubilizers, siderochrome releasers, and the release of phosphatases by cyanobacteria and lichens contribute to stimulating P cycling. RI, resource island; P, phosphorus; OM, organic matter; CEC, cation exchange capacity. Red ˂: lower concentration in comparison with RI or BSC. Blue ˃: higher concentration or activity in comparison with interspaces areas
As has been shown earlier, in drylands, most of the bound P is in an inorganic form that must be dissolved to be available for plant growth. Soil crust microorganisms have the capacity to solubilize P in the following ways: (i) they can excrete H+ during respiration, which decreases the soil pH and releases recalcitrant carbonate-bond P; (ii) they can secrete metal chelators, such as siderochromes, which increase the P availability by maintaining the metals in the solution and preventing the formation of P-bound complexes (Belnap 2011); and (iii) some have bioweathering capacity by secreting weak organic acids (e.g., citric, malic, pyruvic, lactic, formic, and fumaric acids) that solubilize P from surface rock or dust deposits (Belnap 2011; Crain et al., 2018).
The effect of BSCs on soil physicochemical properties may depend on the species composition. Crusts dominated by the lichen Diploschistes diacapsis and by Lecidella spp. had higher concentrations of P, N, and Cu and a lower pH than bare and semi-arid soil in Jalisco, Mexico (Concostrina-Zubiri et al. 2013); however, the microsites in crusts dominated by the lichen Acarospora socialis had lower P and higher Fe concentrations than bare soil. According to Bowker et al. (2006), the negative correlation between P content and lichen-moss crust communities could be explained by uptake and conversion of P to unavailable organic forms. The acidification of the soil in D. diacapsis crusts may be due to the secretion of organic acids by lichen. BSC dominated by lichens also has the capacity to extend vertically into the uppermost soil layer (Maier et al. 2014).
As presented earlier, the availability of P in drylands is mostly due to bioweathering and the solubilization of inorganic P and to a lesser extent to the organic-matter decomposition by enzyme activity. BSC organisms have phosphatases in their cell walls and mucilaginous sheaths that are released to the surroundings (Crain et al. 2018). It is reasonable to hypothesize that these sheaths may protect the phosphatases against proteolysis, thus allowing the phosphatases to hydrolyze organic P in the extracellular soil environment. A particular feature of cyanobacteria and sometimes of green algae is the presence of multicellular hairs, which are phosphatase coated and can extend beyond the colony to look for organic P to be hydrolyzed (Whitton et al. 2005).
The alkaline phosphatase activity seems to be related to the type of biocrust, being higher in cyanobacterial-dominated crusts than in lichen-dominated crusts (Miralles et al. 2012). Moreover, in lichen-dominated crusts, the species seems to further influence the type of enzyme activity. In a multi-soil study in semi-arid regions in Spain, crusts growing in gypsiferous soils showed phosphatase activity negatively related to the abundance of D. diacapsis and positively related to that of the lichen Squamarina lentigera, while the ß-glucosidase and urease activities were less responsive to the crust community (Bowker et al. 2011). In the soils of Jalisco, Mexico, D. diacapsis reduced phosphatase activity (Concostrina-Zubiri et al. 2013). Conversely, in calcareous soils, phosphatase activity, urease activity, and soil respiration were unaffected by the abundance of mosses and lichens (Bowker et al. 2011).
Conditions such as mechanical disturbances and increased aridity can also affect the P distribution inside the BSC. In the biocrust-dominated soils in the Chihuahuan Desert in North America, the concentrations of available P were higher in areas with disturbances and lower in intact soil crusts (Crain et al. 2018). A possible explanation for this is that in intact crusts, cyanobacteria and other organisms may be incorporating P into biomass or helping in the creation of biotic P pools, thereby avoiding P accumulation in comparison to the disturbed areas. Thus, the higher P concentration in disturbed areas compared to biocrusts may be explained by the lack of microbial activity in the former. In the dryland agricultural landscape of northwest Victoria, Australia, a statistical model identified relationships between crust abundance and available P, soil C, and perennial grass, showing that disturbances from grazing and camping are the main cause of a loss of BSC coverage, whereas proximity to the windward edge, litter cover, and tree cover were secondary to crust abundance (Read et al. 2008). A multifunctional analysis of biocrust done in North America, Europe, and Australia indicated that phosphatase activity as well as the available P, soil organic carbon, total N, and ß-glucosidase activity increased with aridity (Delgado-Baquerizo et al. 2016).
In conclusion, the effect of biocrusts is important in environments with increased aridity because of the low microbial activity and nutrient availability of arid soils. Increases in enzyme activities by BSC are important to improve the function of arid soils, but the origin of the increased activities should be investigated by comparing enzyme activities and the relative enzyme-encoding genes.
Effects of plants on P dynamics in dryland soils: The resource island phenomenon
Like microorganisms, plants play a major role in the release of P into the soil. Plants in drylands access the mineral P from the deep soil, translocate it from the roots to the shoots, and then deposit P as organic P in the surface soil. Thus, P transformation and distribution are controlled by the vegetation during soil genesis and ecosystem succession, but this is dependent on how deeply the roots can penetrate the soil to uplift inorganic P and transform it into organic P (Gao et al. 2019).
The most common natural vegetation pattern in drylands is a RI, a complex habitat buildup where many plants modify adverse environmental growth conditions by changing their surroundings, causing changes in the microclimate and soil properties (Bashan and de-Bashan 2010). The RIs associated with perennial trees or shrubs influence the concentrations of soil nutrients through physical and biotic mechanisms (Mudrak et al. 2014), and this increases the spatial heterogeneity of the soil nutrients. Indeed, plants maintain a tight internal cycle, absorbing nutrients underneath their canopy (Salazar et al. 2019) and accumulating resources from the adjacent open spaces via their roots and through the physical redistribution of litter on the soil surface to prevent the loss of deposits due to wind (Kondo et al. 2012). Shade and organic matter contribute to the process of the stabilization of fine-textured soils, which will, in turn, become microhabitats for entire communities of organisms (Bashan and de-Bashan 2010), facilitating the establishment of other plants under their canopy and accelerating microbial activity (Fig. 3). Thus, vegetation patches concentrate the biogeochemical cycles of P, C, N, and K in the RI, whereas the adjacent inter-shrub spaces (henceforth called interspaces) show very low biotic activities (Kondo et al. 2012) (Fig. 3).
Dryland plants use several mechanisms to increase P levels in the associated RI under their canopies. First, the most common way for plants to access bound P is the acidification of the rhizosphere through proton extrusion (Belnap 2011), mainly carried out by the electrogenic proton pumps localized in the plasma membrane. Such proton effluxes may act as a driving force for the energy-dependent transmembrane uptake of essential substances, such as amino acids, sugars, and various ions (Bashan and Levanony 1989). It is well established that a P deficiency in soils affects the proton efflux (Carrillo et al. 2002), leading to enhanced acidification, which may have two effects: mobilization of solid phosphate, which supplies part of the plant’s needs, and enhancement of root growth because cells grow faster in acid pH (Carrillo et al. 2002). Second, plants in drylands with a higher cation exchange capacity (CEC) can access more recalcitrant P sources (Belnap 2011); legume trees seem to be efficient in obtaining P from insoluble sources such as Ca–P compounds due to their root system with a high CEC, which lowers the calcium activity in the soil, facilitating the release of P (Geesing et al. 2000). Third, plants with a mycorrhizal association can increase phosphatase activity under their canopies, but such activity depends on the type of mycorrhiza because ectomycorrhiza can produce phosphatases (Lambers et al. 2008), while the role of arbuscular mycorrhiza fungi (AMF) in the mineralization of organic P is unclear (Joner et al. 2000). A visualization of the mechanisms is presented in Fig. 3.
One of the most well-known nurse plant–seedling associations occurs between leguminous trees and shrubs and desert succulents (Bashan and de-Bashan 2010). It has been reported that leguminous trees such as the mesquite tree (Prosopis spp.) can modify the spatial arrangement of shrubs and herbaceous plant species and the chemical conditions of the soil, thereby generating local vegetative heterogeneity (Rossi and Villagra 2003). Higher concentrations of P, organic matter, and N in the soils under the canopy of mesquite than those in open adjacent areas have been reported in several dryland areas in North, Central, and South America. Such is the case of Prosopis flexuosa in the Monte Desert, Argentina, and Prosopis articulata in the Mexican Sonoran Desert (Bashan et al. 2000; Rossi and Villagra 2003). In a study on Prosopis pallida in a dryland forest in Northern Peru, concentrations of P, N, and C were positively affected by tree size, and the relationships between the nutrient concentrations in the leaf and soil were significant for P and Mn, suggesting that the RI effect was due to the quantitative increase in the amount of leaf litter (Salazar et al. 2019). Under the canopy of Prosopis glandulosa in South Texas, the P and N concentrations in both soil and leaves were significantly correlated with the trunk diameter. The pumping of nutrients from deeper levels of the soil probably explains the increase in soil P under the large canopy related to the larger trunk diameter (Geesing et al. 2000). In a 13-year silvo-pastoral system in Brazil with a planted Prosopis juliflora and two trees preserved from the native thorn forest (Ziziphus joazeiro and Spondias tuberosa), analysis of the organic and inorganic P fractions indicated that P. juliflora and Z. joazeiro enriched the soil under their canopy with P, whereas S. tuberosa had no positive effect on the P content (Tiessen et al. 2003). A similar pattern has been found with other legume trees. Studies of Caragana microphylla in Mongolian grassland showed higher available P inside the mound formed under the canopy of the RI, than below and outside the mound (Kondo et al. 2012). The differences in the available P pools, soil electrical conductivity, inorganic N pool, and total C and N pools between the mound and the outside were significantly and positively related to canopy size. The higher P, N, and C contents beneath the canopy with increased tree size were probably due to the increased primary production or litter supply per unit of soil area (Kondo et al. 2012). Similarly, the contents of available P, total N, organic matter, and moisture were generally higher under the canopies of C. microphylla and the non-legume Salix gardejerii than in open spaces. However, the enrichment ratios of the total and available P, N, and organic matter were significantly higher under C. microphylla than under S. gardejerii (Zhao et al. 2007).
Plant species seems to play a key role in the fractionation of P under the canopy. For example, the RI under piñon pine (Pinus edulis) and one-seed juniper (Juniperus monosperma) in Northern Arizona showed no effect on the pools of recalcitrant bound Ca–P, but a strong effect on the distribution of the labile inorganic P (Pi) and intermediate P pools (organic P [Po] and NaOH Pi) (Selmants and Hart 2010). The concentrations of Pi under piñon pine were higher than those under one-seed juniper and in the interspaces, probably due to enhanced phosphatase activity, as piñon pine was associated with ectomycorrhiza, while juniper was associated with AMF. The spatial pattern of P fractions in the RI of two native shrubs, Guiera senegalensis and the legume Piliostigma reticulatum, in Senegal showed that the P. reticulatum soil was dominated by the intermediate available NaOH–Po fraction, whereas G. senegalensis had a higher concentration of labile P, particularly organic P (bicarb–Po) (Dossa et al. 2010). In general, regardless of the shrub, the amount of bicarb–Po fraction under the canopy was significantly higher than that in the soils outside the canopy. Bicarb–Po is recognized as an organic P fraction easily accessible to microbes and positively correlated with phosphatase activity, which controls the Po mineralization in soils (Dossa et al. 2010). Therefore, a higher level of this fraction under the shrub canopy reflects the ability of these plants to cycle and maintain biologically active P.
As might be expected, a gradient of fertility seems to form from the core of the patch to the exterior due to microclimates created by the canopy (Segoli et al. 2012a, Fig. 3). In the RI under the shrub Sarcopoterium spinosum in the northern Negev Desert of Israel, the contents of soil nutrients (P, organic matter, K, nitrate, and ammonium) and the temperature regimes were higher at the core of the shrub patch than outside the patch (Segoli et al. 2012b). The patch type had a significant effect on the P content. The interspace had significantly lower levels of P than the periphery, which, in turn, had lower levels than the core.
Conclusions and future perspectives
This review discussed not only the state of the art regarding P cycling in drylands but also the knowledge gaps that need to be filled by future research. The effects of plants or microbial residues with different C:P ratios on the P pools in dry soils should be investigated both in BSCs and RIs because they can markedly affect P cycling in soils (Erinle et al. 2020). Molecular studies are also needed, not only to increase the knowledge about microbial diversity, but also to investigate the distribution of microorganisms bearing phosphatase-encoding genes, to understand the origin of the phosphatase activities of soil (Wei et al. 2019). Adenylate energy charge can represent the energetic status of the soil microbiome, and it may be interesting to compare the AEC values of soils under RIs or BSCs with the values of the respective bare soils when rainfall events occur followed by rapid drying. This could verify the hypothesis that AEC values are high only under optimal conditions, such as temperate and usually wet soils, as well as provide insights into the microbial metabolism of soils under RIs or BSCs and bare soils. In this context, metagenome studies should be combined with metatranscriptomic studies, which, to the best of our knowledge, have never been conducted in arid soil in the context of P dynamics. This review underlined the fact that there are also knowledge gaps concerning the abiotic processes; for example, weathering rates with P release in dry soils need to be monitored under field conditions, although this is challenging due to the inputs from erosion and dust deposition (Newman, 1995; Hartley et al. 2007). Soil-surface-reactive particles can affect the amount of available P in temperate soils, but their effects in dry soils are unknown. Improved knowledge of P dynamics may be important for the sustainable management of these soils, which cover about 41% of Earth’s ice-free land surface and are among the most threatened and disregarded ecosystems in the world (Monger et al. 2005; Safriel and Zafar 2005; Schimel 2010; Plaza et al. 2018).
References
Acosta-Martinez V, Tabatabai MA (2011) Phosphorus cycle enzymes. In: Dick RP (Ed) Methods of soil enzymology. Soil Science Society of America, Madison, WI, pp 161-183
Ahmad M, Ahmad M, El-Naggar AH, Usman ARA, Abduljabbar A, Vithanage M, Elfaki J, Al-Faraj A, Al-Wabel M (2017) Aging effect of organic and inorganic fertilizer on phosphorus fractionation in calcareous sandy loam soil. Pedosphere 28:873–883
Ameen F, AlYahya SA, AlNadhari S, Alasmari H, Alhoshani F, Wainwright M (2019) Phosphate solubilizing bacteria and fungi in desert soils: species, limitations and mechanisms. Arch Agron Soil Sci 65:1446–1459
Anderson K, Wells S, Graham R (2002) Pedogenesis of vesicular horizons, cima volcanic field, Mojave Desert, California. Soil Sci Soc Am J 66:878–887
Azadi A, Baghernejad M (2019) Application of kinetic models in describing soil phosphorus release in relation with soil phosphorus fractions across three soil toposequences of calcareous soils. Soil Chem 52:778–792
Barkley AE, Prospero JM, Mahowald N, Hamilton DS, Popendor KJ, Oehlert AM, Pourmand A, Gatineau A, Panechou-Pulcherie K, Blackwelder P, Gaston CJ (2019) African biomass burning is a substantial source of phosphorus deposition to the Amazon, Tropical Atlantic Ocean, and Southern Ocean. PNAS 116:16216–16221
Barnard RL, Blazewicz SJ, Firestone MK (2020) Rewetting of soil: revisiting the origin of soil CO2 emissions. Soil Biol Biochem 147:107819
Bashan Y, de-Bashan LE (2010) Microbial populations of arid lands and their potential for restoration of deserts. In: Dion P (Ed) Soil Biology and Agriculture in the tropics. Chapter 6. Soil Biology Series 21. Springer, Berlin, pp. 109–137.
Bashan Y, Levanony H (1989) Effect of root environment on proton efflux in wheat roots. Plant Soil 119:191–197
Bashan Y, Davis EA, Carrillo-Garcia A, Linderman RG (2000) Assessment of VA mycorrhizal inoculum potential in relation to the establishment of cactus seedlings under mesquite nurse-trees in the Sonoran Desert. Appl Soil Ecol 14:165–176
Baumann K, Jung P, Samolov E, Lehnert LW, Büdel B, Karsten U, Bendix J, Achilles S, Schermer M, Matus F, Oses R (2018) Biological soil crusts along a climatic gradient in Chile: richness and imprints of phototrophic microorganisms in phosphorus biogeochemical cycling. Soil Biol Biochem 127:286–300
Belnap J (2011) Biological phosphorus cycling in dryland regions. In: Bunemann EK, Oberson A, Frossard E (Eds) Phosphorus in action, biological processes in soil phosphorus cycling. Springer-Verlag, Berlin, pp 371-406
Bigio L, Mayol-Bracero OL, Santos G, Fishman A, Angert A (2020) Are the phosphate oxygen isotopes of Saharan dust a robust tracer of atmospheric P source? Atmos Environ 235:117561
Blackwell MSA, Brookes PC, de la Fuente-Martinez N, Murray PJ, Snars KE, Williams JK, Haygarth PM (2009) Effects of soil drying and rate of re-wetting on concentrations and forms of phosphorus in leaching. Biol Fertil Soils 45:635–643
Bowker MA, Belnap J, Davidson DW, Goldstein H (2006) Correlates of biological soil crust abundance across a continuum of spatial scales: support for a hierarchical conceptual model. J Appl Ecol 43:152–163
Bowker MA, Mau RL, Maestre FT, Escolar C, Castillo-Monroy AP (2011) Functional profiles reveal unique ecological roles of various biological soil crust organisms. Funct Ecol 25:787–795
Brady NC, Weil RR (2002) The nature and properties of soils, 3rd edn. Prentice Hall, Upper Saddle River, NJ
Brookes PC, Tate KR, Jenkinson DJ (1983) The adenylate energy charge of the soil microbial biomass. Soil Biol Biochem 15:9–16
Brookes PC, Newcombe AD, Jenkinosn DS (1987) Adenylate energy charge measurements in soil. Soil Biol Biochem 19:211–217
Brucker E, Spohn M (2019) Formation of soil phosphorus fractions along a climate and vegetation gradient in the Coastal Cordillera of Chile. CATENA 180:203–211
Brucker E, Kerchen S, Spohn M (2020) Release of phosphorus and silicon from minerals by soil microorganisms depends on the availability of organic carbon. Soil Biol Biochem 143:107737
Buckingham SE, Neff J, Titiz-Maybach B, Reynolds RL (2010) Chemical and textural controls on phosphorus mobility in drylands of southeastern Utah. Biogeochemistry 100:105–120
Bunëmann EK, Keller B, Hoop D, Jud K, Bolvin P, Frossard E (2013) Increased availability of phosphorus after drying and rewetting of a grassland soil: process and plant use. Plant Soil 370:511–526
Butterly CR, Bunëmann EK, McNeill AM, Baldock JA, Marschner P (2009) Carbon pulses but not phosphorus pulses are related to decreases in microbial biomass during repeated drying and rewetting of soils. Soil Biol Biochem 41:1406–1416
Carrillo AE, Li CY, Bashan Y (2002) Increased acidification in the rhizosphere of cactus seedlings induced by Azospirillum brasilense. Naturwissenschaften 89:428–432
Chadwick OA, Davis JO (1990) Soil-forming intervals caused by eolian sediment pulses in the Lahontan basin, northwestern Nevada. Geology 18:243–246
Ciardi C, Ceccanti B, Nannipieri P (1990) Method to determine the adenylate energy charge in soil. Soil Biol Biochem 23:1099–1101
Concostrina-Zubiri L, Huber-Sannwald E, Martínez I, Flores JF, Escudero A (2013) Biological soil crusts greatly contribute to small-scale soil heterogeneity along a grazing gradient. Soil Biol Biochem 64:28–36
Condron LM, Newman S (2011) Revisiting the fundamentals of phosphorus fractionation of sediments and soils. J Soils Sediments 11(830):840
Cosentino D, Chenu C, Le Bissonnais Y (2006) Aggregate stability and microbial community dynamics under drying-wetting cycles in a silt loam soil. Soil Biol Bochem 38:2053–2062
Crain GM, McLaren JR, Brunner B, Darrouzet-Nardi A (2018) Biologically available phosphorus in biocrust-dominated soils of the Chihuahuan Desert. Soil Systems 2:56
De Caire GZ, de Cano MS, Palma RM, de Mulè CZ (2000) Changes in soil enzyme activities following additions of cyanobacterial biomass and exopolysaccharides. Soil Biol Biochem 32:1985–1987
Delgado-Baquerizo M, Maestre F, Gallardo A, Bowker MA, Wallestein MD, Quero JL, Ochoa V, Gonzalo B, García-Gómez M, Soliveres S, García-Palacios P, Berdugo M, Valencia E, Esoclar C, Arredondo T, Barraza-Zepeda C, Bran D, Carreira JA, Chaieb M, Conceiçao AA, Derak M, Eldridge DJ, Escudero A, Espinosa CI, Gaitán J, Gatica MG, Gómez-González S, Guzman E, Gutiérrez JR, Florentino A, Hepper E, Hernández RM, Huber-Sannwaldk E, Jankju M, Liu J, Pucheta E, Ramírez E, Ramírez-Collantes DA, Romao R, Tighe M, Torres D, Torres-Diaz C, Ungar ED, Val J, Wamiti W, Wang D, Zaady E (2013) Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 502:672–676
Delgado-Baquerizo M, Maestre FT, Eldridge DJ, Bowker MA, Ochoa V, Gozalo B, Berdugo M, Val J, Singh BK (2016) Biocrust-forming mosses mitigate the negative impacts of increasing aridity on ecosystem multifunctionality in drylands. New Phytol 209:1540–1552
Delgado-Baquerizo M, Eldridge DJ, Maestre FT, Ochoa V, Gonzalo B, Reich PB, Singh BK (2018) Aridity decouples C:N: P stoichiometry across multiple trophic levels in terrestrial ecosystems. Ecosystems 21:459–468
Dietze M, Bartel S, Lindner M, Kleber A (2012) Formation mechanisms and control factors of vesicular soil structure. CATENA 99:83–96
Dietze M, Kleber A (2012) Contribution of lateral processes to stone pavement formation in deserts inferred from clast orientation patterns. Geomorphology 139–140:172–187
Dixon JC (2009) Aridic soils, patterned ground, and desert pavements. In: Parsons AJ, Abrahams AD (Eds) Geomorphology of desert environments, Springer, Dordrecht, Netherlands. pp. 101–122
Dossa EL, Diedhiou S, Compton JE, Assigbetse KB, Dick RP (2010) Spatial patterns of P fractions and chemical properties in soils of two native shrub communities in Senegal. Plant Soil 327:185–198
Eldridge DJ, Delgado-Baquerizo M, Quero JL, Ochoa V, Gonzalo B, García-Palacios P, Escolar C, García-Gómez M, Prina A, Bowker MA, Bran DE, Castro I, Cea A, Derak M, Espinosa CI, Florentino A, Gaitán JJ, Gatica G, Goómez-González S, Ghiloufi W, Gutierrez JR, Gusmán-Montalván E, Hernández RM, Hughes FM, Muiño W, Monerris J, Ospina A, Ramírez DA, Ribas-Fernández YA, Romão RL, Torres-Díaz C, Koen TB, Mestre FT (2020) Surface indicators are correlated with soil multifunctionality global drylands. J Appl Ecol 57:424–435
Erinle KO, Doolette A, Marschner P (2020) Changes in phosphorus pools in the detritusphere induced by removal of P or switch of residues with low and high C/P ratio. Biol Fertil Soils 56:1–10
Feng J, Turner BL, Lü X, Chen Z, Wei K, Tian J, Wang Ch, Luo W, Chen L (2016) Phosphorus transformation along a large-scale climosequence in arid and semiarid grasslands of northern China. Global Biogeochem Cy 30:1264–1275
Fookes PG, Hart AB, Lee EM (2013) Some near-surface desert features of significance in engineering geology evaluations. Q J Eng Geol Hydroge 46:259–266
Gao X, Li X, Zhao L, Kuzykov Y (2019) Regulation of soil phosphorus cycling in grassland by shrubs. Soil Biol Biochem 133:1–11
Garcia DE, Lopez BR, de-Bashan LE, Hirsch M, Maymon M, Bashan Y (2018) Functional metabolic diversity of the bacterial community in undisturbed resource island soils in the southern Sonoran Desert. Land Degrad Dev 29:1467–1477
Garcia C, Moreno JL, Hernandez T, Bastida F (2017) Soils in arid and semiarid environments: the importance of organic carbon and microbial population. Facing the future. In: Blire S (Ed) The Biology of Arid Soils. Walter de Gruyter GmbH.
Geesing D, Felker P, Bingham RL (2000) Influence of mesquite (Prosopis glandulosa) on soil nitrogen and carbon development: implications for global carbon sequestration. J Arid Environ 46:157–180
Gong Y, Lv G, Guo Zh, Chen Y, Cao J (2017) Influence of aridity and salinity on plant nutrients scales up from species to community level in a desert ecosystem. Sci Rep 7:6811
Gordon H, Haygarth PM, Bardgett RD (2008) Drying and rewetting effects on soil microbial community composition and nutrient leaching. Soil Biol Biochem 40:302–311
Gu Ch, Hart S, Turner BL, Hu Y, Meng Y, Zhu M (2019) Aeolian dust deposition and the perturbation of phosphorus transformations during long-term ecosystem development in a cool, semi-arid environment. Geochim Cosmochim Ac 246:498–514
Gutiérrez M (2005) Desert surfaces: pavements, patterned ground, varnishes and crusts. In: Gutiérrez M (Ed) Developments in Earth Surface Processes, Vol. 8, Elsevier, Amsterdam, pp 259–284
Hamdali H, Bouizgarne B, Hafidi M, Lebrihi A, Virolle MJ, Ouhdouch Y (2008) Screening for rock phosphate solubilizing actinomycetes from Moroccan phosphate mines. Appl Soil Ecol 38:12–19
Hartley A, Barger N, Belnap J, Okin GS (2007) Dryland ecosystems. In: Marschner P, Rengel Z (Eds) Nutrient cycling in terrestrial ecosystems, Springer Berlin Heidelberg, pp. 271–307
Hou E, Chen Ch, Luo Y, Zhou G, Kuang Y, Zhang Y, Heenan M, Lu X, Wen D (2017) Effects on climate on soil phosphorus cycle and availability in natural terrestrial ecosystems. Glob Change Biol 24:3344–3356
Housman DC, Yeager CM, Darby BJ, Sanford RL Jr, Kuske CR, Neher DA, Belnap J (2007) Heterogeneity of soil nutrients and subsurface biota in a dryland ecosystem. Soil Biol Biochem 39:2138–2149
Hutchinson CF, Herrmann SM (2008) The future of arid lands — revisited: a review of 50 years of drylands research. Advances in Global Change Research Series, 32. Springer Netherlands.
Ippolito JA, Blecker SW, Freeman CL, McCulley RL, Blair JM, Kelly EF (2010) Phosphorus biogeochemistry across a precipitation gradient in grasslands of central North America. J Arid Environ 74:954–961
Jobbagy EG, Jackson RB (2001) The distribution of soil nutrients with depth: global patterns and the in print of plants. Biogeochemistry 53:51–77
Joner EJ, van Aarle IM, Vosatka M (2000) Phosphatase activity of extra-radical arbuscular mycorrhizal hyphae: a review. Plant Soil 226:199–210
Jung P, Schermer M, Briegel-Williams L, Baumann K, Leinweber P, Karsten U, Lehnert L, Achilles S, Bendix J, Büdel B (2019) Water availability shapes edaphic and lithic cyanobacterial communities in the Atacama Desert. J Phycol 55:1306–1318
Kalma JD, Franks S (2003) Rainfall in arid and semi-arid regions. In: Simmers I (Ed) Understanding water in a dry environment: hydrological processes in arid and semi-arid zones, Vol. 23. Balkema, Lisse, Netherlands, pp. 15-56
Khan MS, Zaidi A, Wani PA (2007) Role of phosphate-solubilizing microorganism in sustainable agriculture- a review. Agron Sustain Dev 27:29–43
Kieft TL, Soroker E, Firestone MJ (1987) Microbial biomass response to a rapid increase in water potential when dry soil is wetted. Soil Biol Biochem 19:119–126
Knight J, Zerboni A (2018) Formation of desert pavements and the interpretation of lithic-strewn landscapes of the central Sahara. J Arid Environ 153:39–51
Kondo J, Hirobe M, Yamada Y, Undarmaa J, Sakamoto K, Yoshikawa K (2012) Effects of Caragana microphylla patch and its canopy size on “islands of fertility” in a Mongolian grassland ecosystem. Lands Ecol Eng 8:1–8
Kpomblekou-A K, Tabatabai MA (1994) Effect of organic acids on release of phosphorus from phosphate rocks. Soil Sci 158:442–453
Kpomblekou-A K, Tabatabai MA (2003) Effect of low-molecular weight organic acids on phosphorus release and phytoavailabilty of phosphorus in phosphate rocks added to soils. Agr Ecosyst Environ 100:275–284
Lajtha K, Schlesinger WH (1988) The biogeochemistry of phosphorus cycling and phosphorus availability along a desert soil chronosequence. Ecology 6:24–39
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103
Lopez BR, Bacilio M (2020) Weathering and soil formation in hot, dry environments mediated by plant–microbe interactions. Biol Fertil Soils 56:447–459
Magallon-Servin P, Antoun H, Taktek S, Bashan Y, de-Bashan LE (2020) The maize mycorrhizosphere as a source for isolation of arbuscular mycorrhizae-compatible phosphate rock-solubilizing bacteria. Plant Soil 451:169–186
Mahowald N, Jickells TD, Baker AR, Artaxo P, Benitez-Nelson CR, Bergametti G, Bond TC, Chen Y, Cohen DD, Herut B, Kubilay N, Losno R, Luo C, Maenhaut W, Mcgee KA, Okin GS, Siefert RL (2008) Global distribution of atmospheric phosphorus sources concentrations and deposition rates and anthropogenic impacts. Global Biogeochem Cy 22:GB4026
Maier S, Schmidt TS, Zheng L, Peer T, Wagner V, Grube M (2014) Analyses of dryland biological soil crusts highlight lichens as an important regulator of microbial communities. Biodivers Conserv 23:1735–1755
Margalef O, Sardans J, Fernandez-Martinez M, Molowny-Horas R, Janssens IA, Ciais P, Goll D, Ritcher A, Obersteiner M, Asensio D, Peñuelas J (2017) Global pattern of phosphatase activity in natural soils. Sci Rep 7:1337–1357
Miralles I, Domingo F, Cantón Y, Trasar-Cepeda C, Leirós MC, Gil-Sotres F (2012) Hydrolase enzyme activities in a successional gradient of biological soil crusts in arid and semi-arid zones. Soil Biol Biochem 53:124–132
Monger HC, Martinez-Rios JJ, Khresat SA (2005) Tropical Soils - Arid and Semiarid. In: Hillel D (Ed) Encyclopedia of soils in the environment, Elsevier, London, pp. 182–187
Moradi G, Bol R, Trbojevic L, Missong A, Mörchen R, Fuentes B, May SM, Lehndorff E, Klumpp E (2020) Contrasting depth distribution of colloid-associated phosphorus in the active and abandoned sections of an alluvial fan in a hyper-arid region of the Atacama Desert. Global Planet Change 185.
Mudrak EL, Schafer JL, Fuentes-Ramirez A, Holzapfel C, Moloney KA (2014) Predictive modeling of spatial patterns of soil nutrients related to fertility islands. Landscape Ecol 29:491–505
Nannipieri P, Grego S, Ceccamti B (1990) Ecological significance of the biological activity in soil. In: Bollag J-M, Stotzky G (Eds) Soil biochemistry vol 6. Marcel Dekker, New York, pp 293-356
Nannipieri P, Giagnoni L, Landi L, Renella G (2011) Role of phosphatase enzymes in soil. In: Bunemann EK, Oberson A, Frossard E (Eds) Phosphorus in action, biological processes in soil phosphorus cycling. Springer-Verlag Berlin, pp. 215-241
Nannipieri P, Trazar-Cepeda C, Dick RP (2018) Soil enzyme activity: a brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol Fertil Soils 54:11–19
Nedeau J, Qualls R, Nowak R, Blank R (2007) The potential bioavailability of organic C, N, and P through enzyme hydrolysis in soils of the Mojave Desert. Biogeochemistry 82:305–320
Neff JC, ReynoldsSanford RRL Jr, Fernandez D (2006) Controls of bedrock geochemistry on soil and plant nutrients in Southeastern Utah. Ecosystems 9:879–893
Newman EI (1995) Phosphorus inputs to terrestrial ecosystems. Ecology 83:713–726
Noy-Meir I (1973) Desert ecosystems: environment and producers. Annu Rev Ecol Syst 4:25–51
Okin GS, Murray B, Schlesinger WH (2001) Degradation of sandy arid shrubland environments: observations, process modelling, and management implications. J Arid Environ 47:123–144
Osman KT (2018) Management of soil problems. In: Osman KT (Ed) Management of Soil Problems. Springer, Cham, Switzerland, pp. 15–36
Osorio NW, Habte M (2014) Soil phosphate desorption induced by a phosphate-solubilizing fungus. Commun Soil Sci Plant Anal 45:451–460
Perez E, Sulbarán M, Ball MM, Yarzábal LA (2007) Isolation and characterization of mineral phosphate-solubilizing bacteria naturally colonizing a limonitic crust in the south-eastern Venezuelan region. Soil Biol Biochem 39:2905–2914
Plaza C, Zaccone C, Sawicka K, Méndez AM, Tarquis A, Gascó G, Heuvelink GBM, Schuur EAG, Maestre FT (2018) Soil resources and element stocks in drylands to face global issues. Sci Rep 8:1–8
Pointing SB, Belnap J (2012) Microbial colonization and controls in dryland systems. Nature Rev Microbiol 10:551–562
Qiu G, Zhu M, Meng J, Luo Y, Di H, Xu J, Brookes P (2019) Changes in soil microbial biomass C, ATP and microbial P concentration due to increasing soil Cd levels in Chinese paddy soils growing rice (Oryza sativa). Plant Soil 436:1–12
Read CF, Duncan DH, Vesk PA, Elith J (2008) Biological soil crust distribution is related to patterns of fragmentation and land use in a dryland agricultural landscape of southern Australia. Landscape Ecol 23:1093–1105
Reyes I, Baziramakenga R, Bernier L, Antoun H (2001) Solubilization of phosphate rocks and minerals by a wild-type strain and two UV-induced mutants of Penicillium rugulosum. Soil Biol Biochem 33:1741–1747
Rosacker LL, Kieft TL (1990) Biomass and adenylkate energy charge of a grassland soil during drying. Soil Biol Biochem 22:1121–1127
Rossi BE, Villagra PE (2003) Effects of Prosopis flexuosa on soil properties and the spatial pattern of understory species in arid Argentina. J Veg Sci 14:543–550
Safriel U, Zafar A (2005) Dryland Systems. In: Hassan R, Scholes R, Ash N (Eds) Ecosystems and human well-being: current state and trends. Island Press, Washington, DC, pp. 625–662
Sagoe CI, Ando T, Kouno K, Nagaoka T (1998) Relative importance of protons and solution calcium concentration in phosphate rock dissolution by organic acids. Soil Sci Plant Nutr 44:617–625
Salazar PC, Navarro-Cerrillo RM, Grados N, Cruz G, Barrón V, Villar R (2019) Tree size and leaf traits determine the fertility island effect in Prosopis pallida dryland forest in Northern Peru. Plant Soil 437:117–135
Schimel DS (2010) Drylands in the earth system. Science 327(418):419
Segoli M, Ungar ED, Giladi I, Arnon A, Shachak M (2012) Untangling the positive and negative effects of shrubs on herbaceous vegetation in drylands. Landscape Ecol 27:899–910
Segoli M, Ungar ED, Shachak M (2012) Fine-scale spatial heterogeneity of resource modulation in semi-arid “Islands of Fertility.” Arid Land Res Manag 26:344–354
Selmants PC, Hart SC (2010) Phosphorus and soil development: does the Walker and Syers model apply to semiarid ecosystems? Ecology 91:474–484
Soil Survey Staff (2014) Keys to soil taxonomy. Soil Conservation Service, 12, 410. http://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_051546.pdf. Accessed 11 Sept 2020
Song O, Lee S, Lee Y, Lee S, Kim K, Choi Y (2008) Solubilization of insoluble inorganic phosphate by Burkholderia cepacia DA23 isolated from cultivated soil. Braz J Microbiol 39:151–156
Sulbaran M, Perez E, Ball MM, Bahsas A, Yarzabal LA (2009) Characterization of the mineral phosphate-solubilizing activity of Pantoea aglomerans MMB051 isolated from an iron-rich soil in southeastern Venezuela (Bolivar state). Curr Microbiol 58:378–383
Taradfar JC, Jungk A (1987) Phsophatase activity in the rhizosphere and its relation to the depletion of soil organic phosphorus. Biol Fertil Soils 3:199–204
Tiessen H, Menezes RSC, Salcedo IH, Wick B (2003) Organic matter transformations and soil fertility in a treed pasture in semiarid NE Brazil. Plant Soil 252:195–205
Tunesi S, Poggi V, Gessa C (1999) Phosphate adsorption and precipitation in calcareous soils: the role of calcium ions in solution and carbonate minerals. Nutr Cycl Agroecosys 53:219–227
Turner BA, Papházy MJ, Haygarth PM, McKelvie IA (2002) Inositol phosphates in the environment. Philos T Roy Soc B 357:449–469
Turner BL, Cade-Menun BJ, Wastermann DT (2003) Organic phosphorus composition and potential bioavailability in semi-arid arable soils of the western United States. Soil Sci Soc Am J 67:1168–1179
Turner BL, Driessen JP, Haygarth PM, McKelvie ID (2003) Potential contribution of lysed bacterial cells to phosphorus solubilisation in two rewetted Australian pasture soils. Soil Biol Biochem 35:187–189
UNEMG (2011) Global drylands: a UN system-wide response. United Nations Environment Management Group. Online [accessed 8 august 2020]: https://www.unep-wcmc.org/resources-and-data/global-drylands--a-un-system-wide-response
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl 20:5–15
Walbridge MR (1991) Phosphorus availability in acid organic soils of the lower North Carolina coastal plain. Ecology 72:2083–2100
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19
Wang YP, Law RM, Pak B (2010) A global model of carbon, nitrogen and phosphorus cycles for the terrestrial biosphere. Biogeosciences 2261–2282.
Wang H, Cai Y, Yang Q, Gong Y, Lv G (2019) Factors that alter the relative importance of abiotic and biotic drivers on the fertile island in a desert-oasis ecotone. Sci Total Environ 697:134096
Warke PA (2013) Weathering in arid regions. In: Shroder J (Ed) Treatise on geomorphology, Vol 4. Academic Press, San Diego, CA, pp. 197-227
Wei X, Hu Y, Razavi BS, Zhou J, Shen J, Nannipieri P, Wu J, Ge T (2019) Rare taxa of alkaline phosphomonoesterase-harboring microorganisms mediate soil phosphorus mineralization. Soil Biol Biochem 131:62–70
Whitton BA, Al-Shehri AM, Ellwood NTW, Turner BL (2005) Ecological aspects of phosphatase activity in cyanobacteria, eukaryotic algae and bryophytes. In: Turner BL, Frossard E, Baldwin DS (Eds) Organic phosphorus in the environment. CABI, Cambridge, MA, pp. 205-241
Williams AJ, Buck BJ, Beyene MA (2012) Biological soil crusts in the Mojave Desert, USA: Micromorphology and pedogenesis. Soil Sci Soc Am J 76:1685–1695
Zhao HL, Zhou RL, Su YZ, Zhang H, Zhao LY, Drake S (2007) Shrub facilitation of desert land restoration in the Horqin Sand Land of Inner Mongolia. Ecol Eng 31:1–8
Zúñiga-Silgado D, Rivera-Leyva J, Coleman J, Sanchez-Reyez A, Valencia-Díaz S, Serrano M, de-Bashan L, Folch-Mallol JL (2020) Soil mineralogy affects organic acid production and phosphorus solubilization efficiency mediated by several native fungal strains from Mexico. Microorganisms 8:1337
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dedication: This study is dedicated to the memory of Prof. Yoav Bashan (1951–2018) a leading figure in the field of microbial ecology of desert areas and founder of the Environmental Microbiology Group At CIBNOR and Bashan Institute of Science
Rights and permissions
About this article
Cite this article
de-Bashan, L.E., Magallon-Servin, P., Lopez, B.R. et al. Biological activities affect the dynamic of P in dryland soils. Biol Fertil Soils 58, 105–119 (2022). https://doi.org/10.1007/s00374-021-01609-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-021-01609-6