Abstract
Long-term changes in soil phosphorus influence ecosystem development and lead to a decline in the productivity of forests in undisturbed landscapes. Much of the soil phosphorus occurs in a series of organic compounds that differ in their availability to organisms, but changes in the relative abundance of these compounds during pedogenesis remain unknown. We used alkaline extraction and solution phosphorus-31 nuclear magnetic resonance spectroscopy to assess the chemical nature of soil organic phosphorus along a 120,000-year post-glacial chronosequence at Franz Josef, New Zealand. Inositol phosphates, DNA, phospholipids, and phosphonates accumulated rapidly during the first 500 years of soil development characterized by nitrogen limitation of biological productivity, but then declined slowly to low concentrations in older soils characterized by intense phosphorus limitation. However, the relative contribution of the various compounds to the total organic phosphorus varied along the sequence in dramatic and surprising ways. The proportion of inositol hexakisphosphate, conventionally considered to be relatively recalcitrant in the environment, declined markedly in older soils, apparently due to a corresponding decline in amorphous metal oxides, which weather to crystalline forms during pedogenesis. In contrast, the proportion of DNA, considered relatively bioavailable in soil, increased continually throughout the sequence, due apparently to incorporation within organic structures that provide protection from biological attack. The changes in soil organic phosphorus coincided with marked shifts in plant and microbial communities, suggesting that differences in the forms and bioavailability of soil organic phosphorus have ecological significance. Overall, the results strengthen our understanding of phosphorus transformations during pedogenesis and provide important insight into factors regulating the composition of soil organic phosphorus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil development is characterized by a long-term loss of phosphorus by leaching at a greater rate than it is added in rainfall or released by the weathering of bedrock (Walker and Syers 1976). Simultaneously, primary mineral phosphate (for example, apatite) present at the onset of soil formation is converted by biological and chemical processes into organic and ‘occluded’ forms, some of which are of limited availability to plants. In the absence of catastrophic disturbance, these processes alter the nutrient status of the ecosystem: young soils contain relatively large amounts of phosphorus and biological productivity is limited by nitrogen availability (Chapin and others 1994), whereas older soils contain little phosphorus and are characterized by intense phosphorus limitation (Wardle and others 2004). This pattern has been documented in several chronosequences, defined as a series of soils developed on the same bedrock and under similar climate and vegetation, but that vary in the time since the onset of pedogenesis (Stevens and Walker 1970). These include chronosequences formed on wind-blown sand (Syers and Walker 1969), glacial forelands (Stevens 1968; Richardson and others 2004), uplift terraces (Coomes and others 2005), volcanic tephra (Parfitt and others 2005), and volcanic islands (Crews and others 1995; Chadwick and others 1999).
The changes in soil phosphorus during pedogenesis have important ecological consequences, because phosphorus limitation can become sufficiently intense in the late stages of ecosystem development to cause a decline in forest biomass and productivity (Wardle and others 2004). This ‘retrogressive’ phase may also coincide with changes in plant community composition and a decline in species diversity. For example, the Franz Josef post-glacial chronosequence in New Zealand is characterized by a decrease in the total number of species and a gradual shift from evergreen angiosperms to conifers of the Podocarpaceae, which are well-adapted to survival under phosphorus-limited conditions (Richardson and others 2005). The exception seems to be lowland rain forests in the mainland tropics, which exhibit hyper-diverse tree communities on old, infertile soils (Losos and Leigh 2004; Kitayama 2005).
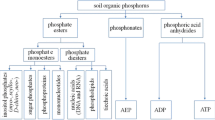
A key aspect of the pedogenic changes in soil phosphorus is its accumulation in organic forms. For example, Syers and Walker (1969) reported that organic phosphorus increased from 3 to 63% of the total phosphorus during 10,000 years of soil development on a wind-blown sand chronosequence at Manawatu, New Zealand. Similarly, Parfitt and others (2005) reported that organic phosphorus was greater than 90% of the total soil phosphorus in a series of contrasting chronosequences formed on volcanic tephra, glacial forelands, and uplift terraces. This accumulation of soil organic phosphorus is rarely assigned ecological significance, because models that describe changes in phosphorus during pedogenesis consider organic phosphorus as a single functional pool of limited availability to plants (for example, Walker and Syers 1976). This limits our understanding of ecosystem development for two reasons. First, soil organic phosphorus represents a dynamic pool of phosphorus in forest soils that is potentially available to plants through a variety of mechanisms, including the synthesis of phosphatase enzymes, secretion of organic acids, and association with mycorrhizal fungi (Attiwill and Adams 1993). Second, soil organic phosphorus occurs in a broad spectrum of compounds that differ markedly in their behavior in soil and, therefore, the degree to which they are available to organisms (Condron and others 2005). For example, phosphate diesters, such as nucleic acids and phospholipids, turn over relatively rapidly in soils and may provide an important source of nutrition for organisms (Bowman and Cole 1978; Harrison 1982). In contrast, inositol phosphates bind strongly to metal oxides and other soil components, so they accumulate in soils and are considered to be of limited bioavailability (Turner 2007).
Changes in the chemical composition of soil organic phosphorus during ecosystem development, although currently unknown, seem likely given that pedogenesis involves changes in mineralogy and other soil properties linked to phosphorus stabilization (Vitousek and others 1997). As the expression of traits involved in the utilization of organic phosphorus varies widely among organisms (see chapters in Turner and others 2005a), it also seems possible that changes in soil phosphorus composition during pedogenesis influence the composition of plant and microbial communities.
The Franz Josef post-glacial chronosequence includes soils up to 120,000-year-old and offers an opportunity to assess changes in soil nutrients during pedogenesis. We used solution 31P nuclear magnetic resonance (NMR) spectroscopy to determine the chemical nature of soil organic phosphorus along this sequence, including the retrogressive phase characterized by extreme phosphorus limitation and marked changes in the forest community.
Methods
Site Description
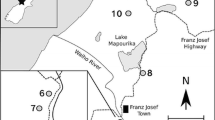
The Franz Josef glacial chronosequence (approximately 43°S and 170°E) consists of a series of post-glacial schist outwash surfaces on the South Island of New Zealand. Some site characteristics are reported in Table 1, with further details available elsewhere (Stevens 1968; Almond and others 2001). The climate is cool-temperate and soil development is rapid due to the high rainfall (>6,000 mm at the younger sites) and associated intense leaching.
Forests in the region are dominated by evergreen angiosperms (for example, Griselinia littoralis, Metrosideros umbellata, Weinmannia racemosa) and conifers in the Podocarpaceae (for example, Dacrydium cupressinum, Podocarpus hallii), while tree ferns such as Dicksonia squarrosa are also present (Wardle 1980). However, there are marked changes in forest community composition along the Franz Josef chronosequence (Richardson and others 2004). Angiosperms account for 100% of the total canopy cover in the early stages of ecosystem development, but eventually decline to only 46% of the total by the oldest stage of the sequence (Figure 1). In contrast, conifers appear after 500 years and gradually increase as the ecosystem ages, representing 54% of total canopy cover after 120,000 years (Figure 1). Overall diversity and forest height also decline strongly in the later stages of the sequence, characteristic of ecosystem retrogression (Wardle and others 2004).
Changes in the proportion of angiosperms and conifers (Podocarpaceae) in the forest canopy along the 120,000-year Franz Josef post-glacial chronosequence, New Zealand (data from Richardson and others 2004 ).
Soil was sampled from nine locations along the chronosequence. Soil properties (Table 2) were described previously (Richardson and others 2004), except for amorphous aluminum and iron, which were determined by oxalate extraction (Loeppert and Inskeep 1996). The oxalate procedure extracts ‘active’ (amorphous or non-crystalline) aluminium and iron oxides, plus some organically-bound forms, all of which are important in phosphorus retention due to their large surface area (Vitousek and others 1997).
Briefly, the soils are characterized by a relatively rapid loss of approximately half the total phosphorus during the first 500 years of pedogenesis, followed by a gradual decline to the end of the sequence. Soil at the oldest stage contains approximately one-eighth of the phosphorus present initially. The initial loss of phosphorus is associated with a decline in pH and the loss of acid-extractable inorganic phosphate (that is, primary calcium-phosphate minerals such as apatite). Amorphous aluminum and iron increase to maximum values after 130 years and then decline slowly. The decline in total phosphorus occurs in parallel with an increase in soil organic matter; the resulting increases in the ratios of carbon-to-phosphorus and nitrogen-to-phosphorus indicate that the ecosystem becomes progressively more phosphorus limited, especially in the late stages of the sequence. This is supported by leaf tissue nutrient concentrations and resorption proficiency, which indicate that plants are extremely phosphorus-limited during the retrogressive phase (Richardson and others 2004, 2005).
It is important to acknowledge that observed changes in soil properties across the Franz Josef chronosequence may be partly attributed to factors other than progressive soil development with time, including temporal and spatial differences in climate and substrate. In particular, the two oldest sites of the chronosequence have experienced at least one glacial–interglacial cycle, and soil formation and loess accumulation are likely to have occurred simultaneously during the last glaciation (Almond and Tonkin 1999; Richardson and others 2004). Despite this, the Franz Josef chronosequence exhibits surprisingly clear trends in soil nutrient pools, vegetation, and soil organisms (for example, Walker and Syers 1976; Richardson and others 2004; Wardle and others 2004; Allison and others 2007). Biologically, therefore, the Franz Josef sequence represents a clear sequence of age-related nutrient stresses that are reflected in both above- and belowground communities.
Solution 31P NMR Spectroscopy
Mineral soil (0–10 cm) was collected from five plots at each location (5 cores per plot), sieved (<4 mm), air-dried (30°C), re-sieved (<2 mm) and ground prior to analysis. Soil from each of the replicate plots at each location was then pooled for NMR analysis. Phosphorus was extracted by shaking 2 g of air-dried soil with 40 ml of a solution containing 0.25 M NaOH and 0.05 M Na2EDTA (ethylenediaminetetraacetate) for 16 h at 22°C (Cade-Menun and Preston 1996). This was an adaptation of the original method (Bowman and Moir 1993), which involved a 2-h extraction at 85°C. It is assumed that the procedure extracts organic phosphorus quantitatively from soil (Bowman and Moir 1993; Turner and others 2005b), although this is impossible to confirm because no direct method exists for the determination of soil organic phosphorus.
Extracts were centrifuged at 10,000g for 30 min and an aliquot taken for determination of total phosphorus by ignition and acid digestion (Anderson 1976). The remaining solution was frozen at −80°C, lyophilized, and ground. Each freeze-dried extract (∼100 mg) was re-dissolved in 0.1 ml of deuterium oxide and 0.9 ml of a solution containing 1.0 M NaOH and 0.1 M Na2EDTA, and then transferred to a 5-mm NMR tube.
Solution 31P NMR spectra were obtained using a Bruker Avance DRX 500 MHz spectrometer operating at 202.456 MHz for 31P. Samples were analyzed using a 6-μs pulse (45°), a delay time of 2.0 s, an acquisition time of 0.4 s, and broadband proton decoupling. The delay time used here allows sufficient spin-lattice relaxation between scans for phosphorus compounds in NaOH–EDTA (Cade-Menun and others 2002). Approximately 30,000 scans were acquired for each sample. Chemical shifts of signals were determined in parts per million (ppm) relative to an external standard of 85% H3PO4. Signals were assigned to phosphorus compounds or functional groups based on literature reports (Turner and others 2003b) and signal areas calculated by integration. Spectra were plotted with a line broadening of 5 Hz. Concentrations of myo-inositol hexakisphosphate (Turner and others 2003c) and scyllo-inositol hexakisphosphate (Turner and Richardson 2004) were determined by spectral deconvolution and quantification of the appropriate signals (see below). All spectral processing was done using NMR Utility Transform Software (NUTS) for Windows (Acorn NMR Inc., Livermore, California, USA). Analysis of replicate extracts was not performed due to the time and expense involved, although error for solution 31P NMR spectroscopy of soil extracts and similar samples is approximately 5% for the larger signals and 10% for smaller signals (Cade-Menun and others 2005; Turner and others 2007). A blank sample containing only 0.25 M NaOH and 0.05 M Na2EDTA taken through the entire procedure contained only 1 mg P/kg as phosphate.
Statistical Analysis
Concentrations of phosphorus compounds are expressed on the basis of oven-dried soil (105°C). A correlation matrix (R values) was calculated to investigate relationships between soil properties and phosphorus compounds. Regression models were calculated using least squares linear regression. All analyses were performed using standard procedures in Sigma Plot 6.0.
Results
Total Phosphorus Extraction in NaOH–EDTA
Phosphorus concentrations in NaOH–EDTA extracts increased from 18 mg P/kg in the youngest soil to a maximum of 397 mg P/kg in the 500-year soil, and then declined to 95 mg P/kg by the end of the sequence (Table 3). Recovery of total soil phosphorus was 2% in the 5-year-old soil, increased to 87% in the 500-year soil, and then remained at similar values until the end of the sequence (Table 3). The low recovery in the initial sample was due to its mainly mineral content, with most phosphorus present in primary minerals (that is, extractable in acid rather than alkali). The concentrations of total phosphorus extracted in NaOH–EDTA were almost identical to the organic phosphorus concentrations determined by the ignition method (Figure 2A). The remaining phosphorus not extracted in NaOH–EDTA was quantitatively similar to the acid-extractable inorganic phosphate (Figure 2B).
The relationship between A soil organic phosphorus determined by the ignition procedure and the total phosphorus extracted in NaOH–EDTA, and B acid-extractable inorganic phosphate and the phosphorus not extracted in NaOH–EDTA. In both figures, the 1:1 relationship is shown by the solid line. Data on the ignition procedure are from Richardson and others (2004 ).
Phosphorus Determination by Solution 31P NMR Spectroscopy
Identification of compounds
Several groups of compounds were identified in NaOH–EDTA extracts by solution 31P NMR spectroscopy, including phosphate esters, inorganic phosphates, and phosphonates (Figure 3).
Solution 31P NMR spectra of NaOH–EDTA extracts of soils from the 120,000-year Franz Josef post-glacial chronosequence, New Zealand. The signals were assigned as follows: A, phosphate; B, phosphate monoesters; C, pyrophosphate; D, phosphonates; E, DNA; F, phospholipids. The spectra are plotted with 5 Hz line broadening.
Of the inorganic phosphates, phosphate was detected as the strong signal at approximately 6.4 ppm, whereas pyrophosphate, an inorganic polyphosphate with a chain length of two, was detected as the signal at approximately −4.0 ppm (Figure 3). Long-chain polyphosphate was not detected in any sample.
A variety of organic phosphorus compounds were detected (Figure 3). Phosphate esters were classified into monoesters and diesters. Phosphate monoesters were identified as a complex group of signals between 3 and 7 ppm (excluding the inorganic phosphate signal at 6.4 ppm). Within this group, several compounds were identified, including two inositol phosphates (Figure 4). The presence of myo-inositol hexakisphosphate was indicated by a signal at 5.9 ppm from the phosphate at the C-2 position on the inositol ring (Turner and others 2003c), whereas scyllo-inositol hexakisphosphate was identified as a strong signal at 4.2 ppm (Turner and Richardson 2004). Signals at around 6.8 ppm slightly downfield of the phosphate signal probably also represent inositol phosphates (Turner and Richardson 2004), but were not quantified separately.
Solution 31P NMR spectrum of an NaOH–EDTA extract of a 130-year-old soil from the Franz Josef post-glacial chronosequence, New Zealand. The spectrum shows only the phosphate monoester region and is truncated vertically. The signals were assigned as follows: A, unidentified inositol phosphate; B, phosphate; C, the C−2 phosphate of myo-inositol hexakisphosphate; D, the degradation products of phospholipids in alkali (phosphatidic acid at 5.2 ppm, β-glycerophosphate at 4.9 ppm); E, the three remaining signals of myo-inositol hexakisphosphate; F, scyllo-inositol hexakisphosphate. The spectrum is plotted with 5 Hz line broadening.
Other strong signals in the phosphate monoester region at 4.9 and 5.2 ppm were assigned to β-glycerophosphate and phosphatidic acid, respectively (Figure 4). These are the degradation products of phospholipids, such as phosphatidyl choline, in alkaline solution (Turner and others 2003b). Ribonucleic acid (RNA), a phosphate diester, also degrades rapidly in strong alkali and is detected as a series of signals in the phosphate monoester region between 4.3 and 4.8 ppm (Makarov and others 2002a; Turner and others 2003b). Consequently, many of the ‘other’ phosphate monoesters (that is, those not identified as inositol phosphates) were the degradation products of RNA and phospholipids and would have occurred as phosphate diesters in the original sample.
Some phospholipids, such as phosphatidyl ethanolamine, are relatively stable in strong alkali (Turner and others 2003b) and were detected intact as broad and weak signals between 0.5 and 2 ppm in some extracts (Figure 3). The other form of phosphate diester present in extracts was deoxyribonucleic acid (DNA), which occurred as a broad signal at 0 ppm in all soils except the youngest (Figure 3).
Phosphonates, which contain a direct carbon–phosphorus bond, were identified as signals at approximately 20 ppm in all but the youngest and oldest soils (Figure 3). Two phosphonates were always present, probably representing 2-aminoethylphosphonic acid at 20.8 ppm and a phosphonolipid, such as phosphonoethanolamine, at 19.3 ppm (Turner and others 2003b).
Concentrations of compounds
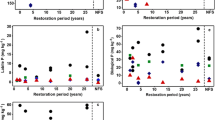
The concentrations of all compounds followed a similar trend through 120,000 years of pedogenesis, being low or undetectable at the onset of soil development, increasing rapidly to a maximum after no more than 500 years, and then declining slowly to the end of the sequence (Figure 5; Table 4). With the exception of the initial sample, which contained mainly acid-extractable phosphate in primary minerals, approximately one-third of the phosphorus extracted in NaOH–EDTA was inorganic, and two thirds was organic (Table 3). The concentration of total inorganic phosphorus (the sum of phosphate and pyrophosphate) in the extracts increased to a maximum of 137 mg P/kg after 500 years, and then declined to 36 mg P/kg by the end of the sequence (Table 3). Phosphate and pyrophosphate both increased to a maximum after 500 years. Phosphate reached a maximum of 105 mg P/kg and then declined to 22 mg P/kg by the end of the sequence. Pyrophosphate concentrations were smaller, increasing to a maximum of 32 mg P/kg after 500 years, but then remaining relatively stable.
Total organic phosphorus (the sum of phosphate monoesters, phosphate diesters, and phosphonate) in the NaOH–EDTA extracts increased to a maximum of 260 mg P/kg after 500 years, and then declined to only 59 mg P/kg at the end of the sequence (Table 3). The carbon to organic phosphorus and nitrogen to organic phosphorus ratios calculated from these values increased markedly in the late stages of the sequence (Table 3). The concentration of myo-inositol hexakisphosphate increased rapidly to 46 mg/P kg after 130 years, declined to 28 mg P/kg after 280 years, and then declined gradually to only 6 mg P/kg by the end of the sequence (Figure 5; Table 4). The concentration of scyllo-inositol hexakisphosphate increased to 30 mg/kg after 130 years and then remained at about this level until the late stages of the sequence, where concentrations decreased markedly to only 3 mg P/kg after 120,000 years (Figure 5).
Young soils contained less scyllo-inositol hexakisphosphate than myo-inositol hexakisphosphate (ratio < 1), whereas between 280 and 60,000 years the opposite was true (ratio > 1). The ratio steadily increased for the first 12,000 years of soil development, indicating the preferential accumulation of scyllo-inositol hexakisphosphate compared to the myo isomer. However, it declined steadily in the later stages, and after 120,000 years the concentration of myo-inositol hexakisphosphate was twice that of the scyllo isomer, although both concentrations at this stage were small. Phosphate monoesters other than the inositol hexakisphosphates constituted up to 126 mg P/kg, declining to 35 mg P/kg by the end of the sequence (Figure 5; Table 4).
Concentrations of DNA increased steadily to a maximum of 45 mg P/kg after 500 years, and then declined to 15 mg P/kg by the end of the sequence (Figure 5; Table 4). DNA therefore occurred in similar concentrations to the inositol phosphates. Intact phospholipids were detected only in samples between 130 and 5,000 years at concentrations up to 13 mg P/kg (Figure 5; Table 4). As discussed above, the phospholipids were almost certainly under-estimated due to degradation of some compounds during extraction and analysis in strong alkali. The maximum concentration of phosphonates was 25 mg P/kg after 500 years, with none detected in the youngest and oldest soils (Figure 5; Table 4).
Proportions of the total inorganic/organic phosphorus
There were marked differences among the various inorganic and organic phosphorus compounds when expressed as a proportion of the total inorganic (Figure 6) or total organic (Figure 7) phosphorus, respectively. Similar patterns were evident for compounds expressed as a proportion of the total extracted phosphorus (Table 4). However, we consider that expression as a proportion of the inorganic/organic phosphorus is more meaningful given that small changes in the solubility of inorganic phosphorus, which is not extracted quantitatively by the NaOH–EDTA solution, can have a large impact on values for organic phosphorus when expressed as a proportion of the total extracted phosphorus.
Phosphate and pyrophosphate extracted in NaOH–EDTA displayed opposite trends (Figure 6). Phosphate declined rapidly from 96% of the inorganic phosphorus in the initial sample to 77% after 280 years, and then declined slowly to 62% by the end of the sequence. In contrast, pyrophosphate increased rapidly from only 4% of the inorganic phosphorus initially to 23% after 500 years, and then continued to increase steadily to 38% by the end of the sequence.
Of the organic phosphorus compounds, inositol phosphates initially increased rapidly in the first 130 years of soil development, with myo- and scyllo-inositol hexakisphosphate constituting 21 and 13% of the organic phosphorus, respectively (Figure 7). The proportion of myo-inositol hexakisphosphate then remained relatively constant at 10–13% until the end of the sequence, whereas scyllo-inositol hexakisphosphate increased to 17% after 5000 years, then declined gradually to only 4% by the end of the sequence. As a proportion of the phosphate monoesters, myo-inositol hexakisphosphate constituted up to 25% and scyllo-inositol hexakisphosphate up to 23%. Other phosphate monoesters constituted between 40 and 60% of the organic phosphorus for most of the sequence. The exception was the youngest soil, in which inositol phosphates could not be identified due to the low concentration of phosphate monoesters (see Figure 3).
Of the phosphate diesters, intact phospholipids increased to 5% of the total organic phosphorus after 500 years, but then declined to an undetectable level after 12,000 years. In contrast, DNA increased rapidly to 14% of the organic phosphorus after only 60 years, but continued to increase steadily until the end of the sequence, where it represented 26%. Phosphonates followed a similar trend to phospholipids and scyllo-inositol hexakisphosphate, increasing rapidly to 10% of the organic phosphorus after 500 years, but then declining to zero by the end of the sequence (Figure 7).
Relationships between Soil Properties and Phosphorus Compounds
There was a strong and positive correlation between total carbon and total nitrogen, and a negative correlation between these values and soil pH. Both inorganic phosphates were correlated significantly and positively with total carbon and nitrogen, although only pyrophosphate was correlated significantly (negatively) with soil pH and neither compound was correlated significantly with amorphous metals (Table 5). Inorganic phosphate compounds were also correlated with NaHCO3-extractable phosphate, but neither was correlated significantly with total soil phosphorus or the ratios of elements in organic matter.
Of the organic phosphorus compounds, DNA was correlated significantly and positively with total carbon and nitrogen, and negatively with soil pH (Table 5). However, it was not correlated significantly with amorphous metals. DNA was therefore associated with similar soil properties as pyrophosphate. In contrast, myo-inositol hexakisphosphate was not correlated significantly with total carbon or nitrogen, but was correlated significantly and positively with amorphous metals. Concentrations of scyllo-inositol hexakisphosphate were correlated with amorphous aluminum (but not iron) and also with total carbon and nitrogen. However, the inositol phosphates were not correlated significantly with soil pH, total phosphorus, or element ratios. Phospholipids were only correlated significantly with total nitrogen, whereas phosphonates were correlated positively with total carbon, total nitrogen, and NaHCO3-extractable phosphate.
Discussion
Organic phosphorus is quantitatively important during long-term soil development, but the possibility that its composition and, therefore, ecological significance varies through time is rarely considered. Our detailed assessment of soil phosphorus speciation along a developmental chronosequence, including the retrogressive phase, demonstrates clearly that the chemical nature of soil organic phosphorus varies dramatically during pedogenesis. This allows a more detailed description of the Walker and Syers (1976) model of changes in soil phosphorus during pedogenesis (Figure 8). The revised model includes accurate information not only on the changes in total soil organic phosphorus (Figure 8a), overestimated using conventional ignition procedures (see below), but also on changes in individual organic phosphorus compounds and functional groups (Figure 8b).
There have been previous attempts to assess the organic phosphorus composition of soils from the Franz Josef chronosequence. Baker (1976) reported small concentrations of phospholipids (≤14 mg P/kg) and larger concentrations of inositol penta- and hexakisphosphates (the stereoisomeric composition was not determined), which in young surface soils accounted for up to 38% of the total organic phosphorus. However, the author regarded the latter values with caution, due to analytical difficulties in the extraction and analysis of soil inositol phosphates using conventional column chromatography. It should be noted that subsequent revision of the chronology of the Franz Josef chronosequence (Almond and others 2001) means that the oldest site in Baker (1976) corresponds to the 120,000-year site reported here.
Soil organic phosphorus followed a unimodal pattern along the Franz Josef chronosequence that was clearly related to ecosystem nutrient status. Leaf tissue analysis indicates that productivity in woody plant communities is limited by nitrogen in the early stages of ecosystem development (approximately the first 500 years) and by phosphorus in the remainder of the sequence (Richardson and others 2004, 2005). Soil organic phosphorus therefore accumulated under nitrogen limitation and declined under phosphorus limitation. Similar patterns were found on the basaltic soils of Hawaii during four million years of ecosystem development (Crews and others 1995; Kitayama and Mueller-Dombois 1995). The nutrient status of the ecosystem is therefore of key importance for interpreting information on soil organic phosphorus. However, expression of data as a proportion of the total organic or inorganic phosphorus revealed dramatic and surprising differences in the various compounds along the chronosequence that challenge our conventional understanding of soil organic phosphorus. In particular, the proportion of inositol phosphates, considered conventionally to be recalcitrant in soils (Turner 2007), declined markedly during the late stages of the sequence. In contrast, the proportions of DNA and pyrophosphate, considered conventionally to be relatively labile in soils (Condron and others 2005), increased continually throughout the sequence.
Phosphorus speciation along the sequence is probably regulated by three main factors: changes in the potential for phosphorus stabilization, changes in phosphorus inputs, and differences in the biological utilization of the various compounds. Pedogenic changes in mineralogy are likely to be of key importance, because they affect the potential for phosphorus stabilization on the surfaces of metal oxides. For the basaltic soils of the Hawaiian Islands (Vitousek and others 1997), primary minerals present at the onset of soil formation (for example, plagioclase and feldspars) weather relatively rapidly during the first 20,000 years to amorphous (non-crystalline) minerals (for example, ferrihydrite and allophane) with large surface areas that retain phosphorus strongly. These amorphous minerals then undergo further weathering to crystalline minerals (for example, kaolinite and hematite) that are less effective in retaining phosphorus. There is little information on such mineralogical changes along the Franz Josef chronosequence, although the decline in plagioclase and feldspar (Stevens 1968), as well as amorphous aluminum and iron oxides (Table 2), with soil age indicate that similar processes have occurred.
Of the soil organic phosphorus compounds detected along the Franz Josef chronosequence, the inositol phosphates are most likely to be influenced by mineralogical changes during pedogenesis, because correlations between inositol phosphates and oxalate-extractable aluminum and iron reported here and elsewhere (McKercher and Anderson 1968; Anderson and others 1974; Turner and others 2003c) suggest that inositol phosphates are stabilized mainly by association with amorphous metal oxides rather than through incorporation in organic matter structures. In addition, amorphous iron oxides such as ferrihydrite have a greater capacity to retain myo-inositol hexakisphosphate than more crystalline oxides such as goethite (reviewed in Celi and Barberis 2007). The decline in amorphous metals in the late stages of the sequence would therefore reduce the potential for stabilization of inositol phosphates, rendering them susceptible to biological attack (Lung and Lim 2006). The association of inositol phosphates with metal oxides may also mean that they are lost from surface horizons by leaching during podzol development (Skjemstad and others 1992), which occurs towards the end of the Franz Josef chronosequence (Stevens 1968). Walker and Syers (1976) previously noted a small accumulation of organic phosphorus in the deeper horizons of old soil profiles, although the majority of the total organic phosphorus remained in the surface horizons.
The changes in inositol phosphate concentrations reported here also offer an explanation for the fact that soils in temperate regions, being formed mainly since the last glaciation approximately 10,000 years ago, often contain a large proportion of their organic phosphorus as inositol phosphates (Turner 2007). The accumulation of scyllo-inositol hexakisphosphate compared to the myo isomer (that is, an increasing scyllo to myo ratio) in the early stages of soil development is consistent with the greater apparent recalcitrance of the scyllo structure (Cosgrove 1966), despite potentially larger inputs of myo-inositol hexakisphosphate from plant material (Raboy 2007).
In contrast to the inositol phosphates, the proportion of the organic phosphorus as DNA increased continually throughout the sequence and was strongly correlated with soil organic carbon and nitrogen, suggesting that DNA was stabilized by association with stable soil organic matter. This is supported by the presence of DNA in precipitated humic acids (Makarov and others 1997; Mahieu and others 2000), including those isolated from soils from the first 5,000 years of the Franz Josef chronosequence (Baker 1977), and in high organic matter soils under strong phosphorus limitation (Turner and others 2003a; Turner and Newman 2005). Pyrophosphate also increased continually as a proportion of the inorganic phosphate and was strongly correlated with soil organic carbon and nitrogen, indicating that it behaves in a functionally similar manner to DNA in soil, despite being an inorganic phosphate. Strong correlations between soil organic matter and the concentrations of DNA and pyrophosphate were reported previously for a series of temperate pasture soils (Turner and others 2003d) and there is evidence that pyrophosphate can become incorporated into humic structures when it is used to extract organic matter from soil (Francioso and others 1998). These findings challenge the common assumption that DNA and pyrophosphate are relatively labile and degrade rapidly in soil, because it appears that they can be preferentially stabilized to the extent that they persist under conditions of extreme phosphorus limitation.
Some of the compounds detected here must have originated from live microbial cells at the time of extraction, although microbial phosphorus typically represents only a few per cent of the total soil organic phosphorus (Brookes and others 1984). Phospholipids and DNA displayed different patterns along the chronosequence, of which only phospholipids corresponded to changes in microbial biomass (Allison and others 2007). This suggests that the phospholipids most closely reflected live microbial cells, whereas DNA was present mainly as stable extracellular compounds, supporting results from a previous detailed study of the origins of phosphate diesters in alkaline soil extracts (Makarov and others 2002b).
Changes in microbial community composition, notably a shift dominance by bacteria to fungi, occur along the Franz Josef chronosequence (Allison and others 2007), as well as along a sequence of uplift terraces in Fjordland National Park, New Zealand (Williamson and others 2005). Such changes could influence the concentrations of compounds such as myo- and scyllo-inositol phosphates, which are both synthesized by soil microbes (Turner 2007). It is also possible that inputs of myo-inositol hexakisphosphate, the dominant phosphorus compound in seeds (Raboy 2007), are altered by shifts in tree community composition from angiosperms to gymnosperms, especially if changes in nutrient status influence allocation of phosphorus to reproductive parts. Phosphonates only tend to accumulate under acidic or waterlogged conditions (Condron and others 2005), but they declined to undetectable levels in the late stages of the Franz Josef chronosequence (soil pH < 4.0), suggesting that phosphorus limitation is an additional factor influencing their abundance. It is also of interest to note that previous work on soil organisms along the Franz Josef chronosequence indicated a rich protozoan fauna, especially in the younger soils (Stout and Dutch 1967). As soil protozoa are an important source of phosphonates (Hilderbrand 1983) and possibly also the phosphorylated inositol stereoisomers such as scyllo-inositol hexakisphosphate (Turner 2007), the changes in the concentrations of these compounds could be due in part to corresponding changes in protozoan abundance.
The changes in soil phosphorus composition during pedogenesis have ecological significance for both above- and belowground communities. In particular, the decline in the availability of inorganic phosphate may favor organisms that can access soil organic phosphorus. This could, for example, involve a shift towards soil microbes that express phytase activity that enables them to utilize inositol phosphates (Hill and Richardson 2007), and may also influence the shift in dominance from angiosperms to Podocarps that occurs along the Franz Josef sequence. Many Podocarp species are characteristic of New Zealand soils that contain little available phosphate, so their increasing dominance along the Franz Josef chronosequence is thought to reflect adaptation to low soil nutrient concentrations, conferred by traits such as efficient nutrient resorption from senescing tissues, long leaf lifespan, and slow growth (Richardson and others 2004, 2005; Wardle and others 2004). As soil organic phosphorus was depleted in the late stages of the chronosequence where the abundance of Podocarps was greatest, an additional factor may be that these trees have a greater ability to access soil organic phosphorus. If so, it might be expected that differences in mycorrhizal infection would be important, although the angiosperm and Podocarp species that occur along the Franz Josef chronosequence form associations with the same species of arbuscular mycorrhizae (Russell and others 2002). Such fungi are not widely considered to use organic forms of phosphorus (Joner and others 2000b), although some species express phosphatase activity and can grow on a range of organic phosphates, including myo-inositol hexakisphosphate (Joner and others 2000a; Koide and Kabir 2000). Ectomycorrhizae, in contrast, are well-known for their ability to use organic forms of nutrients (Read and Perez-Moreno 2003), so it is of interest that Leptospermum scoparium, the only species found along the chronosequence that can associate with such fungi, occurs only at the oldest site. It is not known whether L. scoparium is associated with arbuscular or ectomycorrhizae at that location, although examples growing in Podocarp forests in the west coast region of New Zealand are mainly associated with arbuscular mycorrhizae (Moyersoen and Fitter 1999).
Soil organic phosphorus in often measured by ignition procedures, in which organic phosphorus is calculated as the difference in acid-extractable inorganic phosphate before and after a high temperature treatment to destroy organic matter (for example, Saunders and Williams 1955). Such methods are considered to provide a reasonable approximation of the organic phosphorus in most soils (Condron and others 2005), but are known to be in error for strongly-weathered soils (Williams and others 1970; Condron and others 1990) and are therefore inappropriate for comparing soils with marked differences in degree of weathering, such as those represented by the Franz Josef chronosequence. Furthermore, a large component of the soil inorganic phosphorus (both phosphate and pyrophosphate) is alkali-extractable, but as this fraction is not recovered in the initial acid extraction of the ignition procedure, it contributes to the increase in acid-extractable phosphate following ignition. Given these two sources of error in the ignition procedure it seems likely that some previous studies of soil chronosequences have overestimated soil organic phosphorus and underestimated carbon to organic phosphorus ratios.
In summary, we used solution 31P NMR spectroscopy to quantify changes in the chemical nature of soil organic phosphorus during 120,000 years of ecosystem development along the Franz Josef post-glacial chronosequence. Organic phosphorus accumulated rapidly during the first 500 years of pedogenesis when biological productivity was limited by nitrogen availability, but then declined gradually as the degree of phosphorus limitation increased. There were surprising changes in the soil organic phosphorus composition along the chronosequence, with DNA being preferentially stabilized in older soils compared to the inositol phosphates. The decline in inositol phosphates may be linked to the weathering of amorphous metal oxides to more crystalline forms, whereas DNA and pyrophosphate appeared to be stabilized by incorporation into organic matter structures. The changes in soil organic phosphorus occurred in parallel with changes in plant and microbial communities, suggesting that the ability to access soil organic phosphorus may influence the distribution of species surviving on older surfaces. Future research should aim to assess the importance of organic phosphorus in sustaining biological activity on old soils.
References
Allison VJ, Condron LM, Peltzer DA, Richardson SJ, Turner BL. 2007. Changes in enzyme activities and soil microbial community composition along carbon and nutrient gradients at the Franz Josef chronosequence, New Zealand. Soil Biol Biochem 39:1770–1781
Almond PC, Tonkin PJ. 1999. Pedogenesis by upbuilding in an extreme leaching and weathering environment, and slow loess accretion, south Westland, New Zealand. Geoderma 92:1–36
Almond PC, Moar NT, Lian OB. 2001. Reinterpretation of the glacial chronology of South Westland, New Zealand. New Zealand J Geol Geophys 44:1–15
Anderson JM. 1976. An ignition method for determination of total phosphorus in lake sediments. Water Res 10:329–331
Anderson G, Williams EG, Moir JO. 1974. A comparison of the sorption of inorganic orthophosphate and inositol hexaphosphate by six acid soils. J Soil Sci 25:51–62
Attiwill PM, Adams MA. 1993. Nutrient cycling in forests. New Phytol 124:561–582
Baker RT. 1976. Changes in the chemical nature of soil organic phosphate during pedogenesis. J Soil Sci 27:504–512
Baker RT. 1977. Humic acid-associated organic phosphate. New Zealand J Sci 20:439–441
Bowman RA, Cole CV. 1978. Transformations of organic phosphorus substrates in soils as evaluated by NaHCO3 extraction. Soil Sci 125:49–54
Bowman RA, Moir JO. 1993. Basic EDTA as an extractant for soil organic phosphorus. Soil Sci Soc Am J 57:1516–1518
Brookes PC, Powlson DS, Jenkinson DS. 1984. Phosphorus in the soil microbial biomass. Soil Biol Biochem 16:169–175
Cade-Menun BJ, Preston CM. 1996. A comparison of soil extraction procedures for 31P NMR spectroscopy. Soil Sci 161:770–785
Cade-Menun BJ, Liu CW, Nunlist R, McColl JG. 2002. Soil and litter phosphorus-31 nuclear magnetic resonance spectroscopy: extractants, metals, and phosphorus relaxation times. J Environ Qual 31:457–465
Cade-Menun BJ, Benitez-Nelson CR, Pellechia P, Paytan A. 2005. Refining 31P nuclear magnetic resonance spectroscopy for marine particulate samples: Storage conditions and extraction recovery. Mar Chem 97:293–306
Celi L, Barberis E. 2007. Abiotic reactions of inositol phosphates in soil. In: Turner BL, Richardson AE, Mullaney EJ, Eds. Inositol phosphates: linking agriculture and the environment. Wallingford (UK): CAB International. pp. 207–220
Chadwick OA, Derry LA, Vitousek PM, Huebert BJ, Hedin LO. 1999. Changing sources of nutrients during four million years of ecosystem development. Nature 397:491–497
Chapin FS III, Walker LR, Fastie CL, Sharman LC. 1994. Mechanisms of primary succession following deglaciation at Glacier Bay, Alaska. Ecol Monogr 64:149–175
Condron LM, Moir JO, Tiessen H, Stewart JWB. 1990. Critical evaluation of methods for determining total organic phosphorus in tropical soils. Soil Sci Soc Am J 54:1261–1266
Condron LM, Turner BL, Cade-Menun BJ. 2005. The chemistry and dynamics of soil organic phosphorus. In: Sims JT, Sharpley AN, Eds. Phosphorus: agriculture and the environment. Madison (WI): ASA-CSSA-SSSA. pp. 87–121
Coomes DA, Allen RB, Bentley WA, Burrows LE, Canham CD, Fagan L, Forsyth DM, Gaxiola-Alcantar A, Parfitt RL, Ruscoe WA, Wardle DA, Wilson DJ, Wright EF. 2005. The hare, the tortoise and the crocodile: the ecology of angiosperm dominance, conifer persistence and fern filtering. J Ecol 93:918–935
Cosgrove DJ. 1966. Synthesis of the hexaphosphates of myo-, scyllo-, neo-, and D-inositol. J Sci Food Agric 17:550–554
Crews TE, Kitayama K, Fownes JH, Riley RH, Herbert DA, Mueller-Dombois D, Vitousek PM. 1995. Changes in soil phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology 76:1407–1424
Francioso O, Ciavatta C, Tugnoli V, Sanchez-Cortes S, Gessa C. 1998. Spectroscopic characterization of pyrophosphate incorporation during extraction of peat humic acids. Soil Sci Soc Am J 62:181–187
Harrison AF. 1982. Labile organic phosphorus mineralization in relationship to soil properties. Soil Biol Biochem 14:343–351
Hilderbrand RL. 1983. The role of phosphonates in living systems. Boca Raton (FL): CRC Press
Hill JE, Richardson AE. 2007. Isolation and assessment of microorganisms that utilize phytate. In: Turner BL, Richardson AE, Mullaney EJ, Eds. Inositol phosphates: linking agriculture and the environment. Wallingford (UK): CAB International. pp. 61–77
Joner EJ, Ravnskov S, Jakobsen I. 2000a. Arbuscular mycorrhizal phosphate transport under monoxenic conditions using radio-labelled inorganic and organic phosphate. Biotechnol Lett 22:1705–1708
Joner EJ, van Aarle IM, Vosatka M. 2000b. Phosphatase activity of extra-radical arbuscular mycorrhizal hyphae: a review. Plant Soil 226:199–210
Kitayama K. 2005. Comment on “Ecosystem properties and forest decline in contrasting long-term chronosequences”. Science 308:633b
Kitayama K, Mueller-Dombois D. 1995. Vegetation changes along gradients of long-term soil development in the Hawaiian montane rainforest zone. Vegetatio 120:1–20
Koide RT, Kabir Z. 2000. Extraradical hyphae of the mycorrhizal fungus Glomus intraradices can hydrolyse organic phosphate. New Phytol 148:511–517
Loeppert RH, Inskeep WP. 1996. Iron. In: Sparks DL et al. Eds. Methods of soil analysis, part 3: chemical methods. Madison (WI): Soil Science Society of America, Inc., American Society of Agronomy, Inc. pp. 639–664
Losos EC, Leigh Jr EG, Eds. 2004. Tropical forest diversity and dynamism: findings from a large-scale plot network. Chicago (IL): University of Chicago Press
Lung S-C, Lim BL. 2006. Assimilation of phytate-phosphorus by the extracellular phytase activity of tobacco (Nicotiana tabacum) is affected by the availability of soluble phytate. Plant Soil 279:187–199
Mahieu N, Olk DC, Randall EW. 2000. Analysis of phosphorus in two humic acid fractions of intensively cropped lowland rice soils by 31P-NMR. Eur J Soil Sci 51:391–402
Makarov MI, Malysheva TI, Haumaier L, Alt HG, Zech W. 1997. The forms of phosphorus in humic and fulvic acids of a toposequence of alpine soils in the northern Caucasus. Geoderma 80:61–73
Makarov MI, Haumaier L, Zech W. 2002a. Nature of soil organic phosphorus: an assessment of peak assignments in the diester region of 31P NMR spectra. Soil Biol Biochem 34:1467–1477
Makarov MI, Haumaier L. Zech W. 2002b. The nature and origins of diester phosphates in soils: a 31P-NMR study. Biol Fertil Soils 35:136–146
McKercher RB, Anderson G. 1968. Content of inositol penta- and hexaphosphates in some Canadian soils. J Soil Sci 19:47–55
Moyersoen B, Fitter AH. 1999. Presence of arbuscular mycorrhizas in typically ectomycorrhizal host species from Cameroon and New Zealand. Mycorrhiza 8:247–253
Parfitt RL, Ross DJ, Coomes DA, Richardson SJ, Smale MC, Dahlgren RA. 2005. N and P in New Zealand soil chronosequences and relationships with foliar N and P. Biogeochemistry 75:305–328
Raboy V. 2007. Seed phosphorus and the development of low-phytate crops. In: Turner BL, Richardson AE, Mullaney EJ, Eds. Inositol phosphates: linking agriculture and the environment. Wallingford (UK): CAB International. pp. 111–132
Read DJ, Perez-Moreno J. 2003. Mycorrhizas and nutrient cycling in ecosystems—a journey towards relevance? New Phytol 157:475–492
Richardson SJ, Peltzer DA, Allen RB, McGlone MS, Parfitt RL. 2004. Rapid development of phosphorus limitation in temperate rainforest along the Franz Josef soil chronosequence. Oecologia 139:267–276
Richardson SJ, Peltzer DA, Allen RB, McGlone MS. 2005. Resorption proficiency along a chronosequence: responses among communities and within species. Ecology 86:20–25
Russell AJ, Bidartondo MI, Butterfield BG. 2002. The root nodules of the Podocarpaceae harbour arbuscular mycorrhizal fungi. New Phytol 156:283–295
Saunders WMH, Williams EG. 1955. Observations on the determination of total organic phosphorus in soils. J Soil Sci 6:254–267
Skjemstad JO, Fitzpatrick RW, Zarcinas BA, Thompson CH. 1992. Genesis of podzols on coastal dunes in Southern Queensland: II. Geochemistry and forms of elements as deduced from various soil extraction procedures. Aust J Soil Res 30:615–644
Stevens PR. 1968. A chronosequence of soils near the Franz Josef Glacier. Unpublished PhD Thesis, Lincoln College, University of Canterbury, New Zealand
Stevens PR, Walker TW. 1970. The chronosequence concept and soil formation. Q Rev Biol 45:333–350
Stout JD, Dutch ME. 1967. Changes in biological activity in the Franz Josef and Manawatu sand chronosequences. Soil News 1:35–40
Syers JK, Walker TW. 1969. Phosphorus transformations in a chronosequence of soils developed on wind-blown sand in New Zealand I. Total and organic phosphorus. J Soil Sci 20:57–64
Turner BL. 2007. Inositol phosphates in soil: amounts, forms and significance of the phosphorlyated inositol stereoisomers. In: Turner BL, Richardson AE, Mullaney EJ, Eds. Inositol phosphates: linking agriculture and the environment. Wallingford (UK): CAB International. pp. 186–206
Turner BL, Richardson AE. 2004. Identification of scyllo-inositol phosphates in soils by solution phosphorus-31 nuclear magnetic resonance spectroscopy. Soil Sci Soc Am J 68:802–808
Turner BL, Newman S. 2005. Phosphorus cycling in wetlands: the importance of phosphate diesters. J Environ Qual 34:1921–1929
Turner BL, Chudek JA, Whitton BA, Baxter R. 2003a. Phosphorus composition of upland soils polluted by long-term atmospheric nitrogen deposition. Biogeochemistry 65:259–274
Turner BL, Mahieu N, Condron LM. 2003b. Phosphorus-31 nuclear magnetic resonance spectral assignments of phosphorus compounds in soil NaOH–EDTA extracts. Soil Sci Soc Am J 67:497–510
Turner BL, Mahieu N, Condron LM. 2003c. Quantification of myo-inositol hexakisphosphate in alkaline soil extracts by solution 31P NMR spectroscopy and spectral deconvolution. Soil Sci 168:469–478
Turner BL, Mahieu N, Condron LM. 2003d. The phosphorus composition of temperate pasture soils determined by NaOH–EDTA extraction and solution 31P NMR spectroscopy. Org Geochem 34:1199–1210
Turner BL, Frossard E, Baldwin DS, Eds. 2005a. Organic phosphorus in the environment. Wallingford (UK): CAB International
Turner BL, Cade-Menun BJ, Condron LM, Newman S. 2005b. Extraction of soil organic phosphorus. Talanta 66:294–306
Turner BL, Newman S, Cheesman AW, Reddy KR. 2007. Sample pretreatment and phosphorus speciation in wetland soils. Soil Sci Soc Am J 71:1538–1546
Vitousek PM, Chadwick OA, Crews TE, Fownes JH, Hendricks DM, Herbert D. 1997. Soil and ecosystem development across the Hawaiian Islands. GSA Today 7:1–8
Walker TW, Syers JK. 1976. The fate of phosphorus during pedogenesis. Geoderma 15:1–19
Wardle P. 1980. Primary succession in Westland National Park and its vicinity, New Zealand. New Zealand J Bot 18:221–232
Wardle DA, Walker LR, Bardgett RD. 2004. Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 305:509–513
Williams JDH, Syers JK, Walker TW, Rex RW. 1970. A comparison of methods for the determination of soil organic phosphorus. Soil Sci 110:13–18
Williamson WM, Wardle DA, Yeates GW. 2005. Changes in soil microbial and nematode communities during ecosystem decline across a long-term chronosequence. Soil Biol Biochem 37:1289–1301
Acknowledgements
We thank Alex Blumenfeld (University of Idaho, USA) for analytical support and Peter Almond for invaluable discussion of the Franz Josef chronosequence. The project was funded by J.S. Wright Estate through Lincoln University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turner, B.L., Condron, L.M., Richardson, S.J. et al. Soil Organic Phosphorus Transformations During Pedogenesis. Ecosystems 10, 1166–1181 (2007). https://doi.org/10.1007/s10021-007-9086-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-007-9086-z