Abstract
The frequency and magnitude of drought is expected to increase in the US Great Plains under future climate regimes. Although semiarid systems are considered highly resistant to water limitation, novel drought events could alter linkages among biogeochemical processes, and result in new feedbacks that influence the timescale of ecosystem recovery. We examined changes in carbon and nitrogen cycling in the last 2 years of an 11-year drought manipulation in the shortgrass steppe, and under the first 2 years of recovery from drought. We measured plant production, plant tissue chemistry, soil trace gas flux, and soil inorganic nitrogen dynamics to test the extent that this magnitude of drought altered carbon and nitrogen fluxes and how these changes affected post-drought dynamics. We found that soil inorganic nitrogen was up to five times higher under severe drought than under control conditions, but that this nitrogen may not have been accessible to plants and microbial communities during drought due to diffusion limitations. Drought plots had higher N2O flux when they received equal rainfall pulses, showing that this accumulated N may be vulnerable to loss. In addition, plants in drought plots had higher tissue nitrogen for 2 years following drought. These results show that decadal-length droughts that may occur under future precipitation regimes are likely to alter ecosystem properties through interactions among precipitation, vegetation, and N cycling. Shifts in plant N, vulnerability of nitrogen to loss, and rainfall use efficiency that we observed are likely to affect the recovery time of semiarid systems subject to droughts of this magnitude.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Precipitation is the major control over ecosystem processes in semiarid ecosystems (Noy-Meir 1973), where it is also highly variable within and among years (Lauenroth and Bradford 2009). Because organisms in these ecosystems have adapted to variable rainfall and frequent water limitation, ecosystem processes, including processes that couple carbon (C) and nitrogen (N) cycles such as the accumulation of biomass, remain relatively stable under historical ranges of precipitation variability. However, future climate regimes in semiarid systems are expected to be characterized by more frequent summer droughts, increases in temperature (IPCC 2007; CCSP 2008) and possibly, decadal-scale “megadroughts” with no known recent analogues (Cook and Seager 2010; Woodhouse and others 2010). So although semiarid systems are highly resistant to drought, processes such as biomass accumulation and decomposition will likely be altered, and perhaps in different ways, by these extreme events. Major changes due to disturbance, and different responses among ecosystem processes, can cause C and N to “decouple” (Asner and others 1997). This decoupling causes asynchrony in N supply and demand that can increase nutrient loss and create new biogeochemical feedbacks. Such changes in fundamental ecosystem properties can intensify the impact of disturbances such as drought, and result in legacies that impact ecosystem processes beyond the timescale of the disturbance.
Because N that is linked to C in biomass is less vulnerable to loss (Vitousek and Reiners 1975), C and N decoupling as a result of disruptions of biomass accumulation can alter N retention. In semiarid systems, the majority of N flux occurs through internal cycling; rates of “open” fluxes, such as leaching and gaseous emissions, are low (Burke and others 2008), or limited to brief precipitation pulses (Austin and others 2004). Although these low loss rates suggest that seasonal supply and demand of N are highly synchronized in drier ecosystems (Risser and Parton 1982), N loss rates may increase relative to internal fluxes as precipitation decreases (Austin and Sala 2002; McCulley and others 2009). In addition, N has been seen to accumulate in soil as inorganic N during dry periods (Jackson and others 1988; Whitford and others 1995; Reynolds and others 1999; Augustine and McNaughton 2004) and under short-term drought manipulations (Yahdjian and others 2006). These studies suggest that water limitation in semiarid systems may cause N availability to be asynchronous with plant and microbial N demand, just as short-term fluctuations in precipitation can lead to periods of greater N loss and limitation (Austin and others 2004).
Long-term changes in N retention as a result of precipitation changes may cause organisms to be more frequently N limited, and alter plant-N–soil interactions. After water, N is most likely to limit productivity in semiarid systems (Burke and others 1997; Hooper and Johnson 1999; Ronnenberg and Wesche 2011), and N and water availability are highly interdependent (Harpole and others 2007; Bai and others 2008). Because of this N limitation, N that has accumulated in dry years may result in higher-than-expected plant productivity in years following drought (Briggs and Knapp 1995). In addition, new plant–soil-N feedbacks following drought could cause increased variability in production (Haddad and others 2002). Alternatively, plant, root, and tiller mortality, and reduced meristem density that occur as a result of drought can also generate “structural vegetative constraints” (Lauenroth and Sala 1992) that reduce the capacity of plants to respond to both ambient moisture conditions and any increases in N availability that occurred under drought (Benson and others 2004). In addition, C and N decoupling could occur in the short-term, but changes in plant species composition and plant tissue C:N that occur as long-term drought persists could cause a “re-coupling” of C or N in a way that more closely resembles a drier system. In sum, although previous studies have documented possible ecosystem impacts of C and N decoupling under moisture limitation, the integrated effect of a long-term drought on C and N dynamics and drought recovery is unclear.
Although there has been much previous work on interactions among rainfall, N, and C cycling in grasslands (Austin and others 2004; Burke and others 2008; Yahdjian and Sala 2008), most findings are based either on the monitoring of natural variability across space and time (Augustine and McNaughton 2004; McCulley and others 2009), and therefore not ideally suited to investigate responses to novel events, or on short-term rainfall manipulations (1–4 years) (Yahdjian and others 2006), which may not sufficiently test the limits of drought-resistance in semiarid systems. The shortgrass steppe, a semiarid ecosystem on the drier Western edge of the US Great Plains, is resistant to interannual drought (Milchunas and others 1988; Burke and others 2008; Peters and others 2008), but there is little evidence of its resistance to multi-year drought events that are likely to occur in this area in the future (IPCC 2007).
In this study, we use an 11-year rainfall manipulation in the shortgrass steppe to ask how a drought of unprecedented duration and severity affects C and N coupling, and how this decoupling interacts with ecosystem recovery. To address this, we measure C and N dynamics in the 10th and 11th year of a long-term drought experiment where plots received 25 and 50% of growing season rainfall, then in the first and second year of recovery, when plots received ambient rainfall.
We hypothesized that C–N decoupling induced by high moisture limitation under drought results in increased rainfall use efficiency (RUE) and decreased nitrogen use efficiency in the shortgrass steppe. We predicted that (1) after 11 years of drought, plant production decreases and inorganic N accumulates in the soil, leaving it more vulnerable to loss through gaseous flux; and (2) under recovery, structural vegetative constraints result in lags in plant production such that plants assimilate less biomass C per unit N.
Methods
Study Site and Rainfall Manipulations
We conducted this study in the semiarid shortgrass steppe at the Central Plains Experimental Range (CPER) Long Term Ecological Research Site (Lauenroth and others 2008a), 60 km northeast of Fort Collins, Colorado (40°49′ N latitude, 104°46′ W longitude). Mean annual temperature is 8.2°C, and mean annual precipitation is 341 mm (65-year average), with 83% of precipitation occurring between April and September (Sala and others 1992). Soils are frequently dry but experience brief wet periods, and as a result, soil water content is highly variable (Lauenroth and Bradford 2006), and leaching to deeper soil layers is minimal (Sala and others 1992). Precipitation patterns are dominated by small events (<5 mm), but differences in the size of large events (>30 mm) accounts for most of the interannual variability in rainfall (Lauenroth and Sala 1992). In this system, the most frequently wet layer is between 4 and 15 cm, and root depth distribution closely matches that of available water (Sala and others 1992).
Dominant vegetation in the shortgrass steppe includes short-stature C4 grasses Bouteloua gracilis (blue grama) and Bouteloua dactyloides (buffalograss). Other common plant species are Opuntia polyacantha (plains pricklypear cactus), Artemisia frigida (prairie sagewort), Eriogonum effusum (spreading buckwheat), Chrysothamnus nauseosus (rubber rabbitbrush), and Gutierrezia sarothrae (broom snakeweed) (Lauenroth 2008). Soil types at this site are Renohill and Ascalon fine sandy loams (Aridic Argiustoll and Ustic Haplargid) (Natural Resource Conservation Service 2008).
In spring of 1998, two blocks with similar vegetation were identified near the headquarters of the SGS LTER field site, and divided into four 3.5 m by 1.7 m plots (Figure 1). Blocks were selected to represent slightly different topographies (slight slope and toeslope) and soil textures, although differences in soil texture between blocks were not significant. During the growing season (average dates 26 April–7 October), two rainout shelters automatically covered plots with a sliding roof when rainfall was detected by an electronic rainfall sensor (AeroChem Metrics, Bushnell, FL). Each week, a proportion (100, 25, or 50%) of ambient rainfall measured by a rain gauge was added to the rainfall exclusion plots (using well water from a hose) to simulate drought. Thus, these treatments received 25 and 50% of growing season rainfall for 11 years (Figure 2). The 100% treatment was used to test the shelter effect and specifically, the effect of changes in rainfall timing caused by the re-additions. We also excluded a 25-cm buffer strip in our sampling to exclude portions of the plot where rainfall may have blown in from the side. The control plot was never covered by shelters and received ambient precipitation.
Experimental design and sampling scheme. Rainout shelters cover two blocks (not shown in Block 2 for clarity) during rainfall events and a proportion of rainfall is re-added weekly. Various parameters describing C and N coupling were measured in the last 2 years of an 11-year drought, and the first 2 years of recovery, when shelters were removed.
Growing season precipitation in 25, 50, and total (100% and control) treatments during the 11-year drought experiment and 2-year recovery, when shelters were removed. Rainout shelters were engaged May–October, when treatments received a proportion of the growing season precipitation (also shown). Lines represent long-term (1959–2009) mean annual precipitation (341 mm, solid line) and growing season precipitation (241 mm, dashed line).
We monitored soil moisture in these plots throughout the 4-year study period to evaluate the effectiveness of drought treatments. In 2008, we monitored soil moisture every 2 weeks using a handheld 10 cm TDR Probe (Campbell Scientific, Logan, UT). In 2009, we inserted one 10 cm TE probe (Decagon Devices, Pullman, WA) in each plot vertically (10 cm depth) and collected hourly soil temperature and moisture data in each of the eight plots (2 blocks × 4 treatments) from 2009 to 2011 (Figure 3).
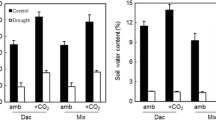
Soil moisture dynamics under rainfall manipulations during 2008 and 2009 growing season, and after manipulations were removed after the growing season of 2009 and remained disengaged in 2010 and 2011. Soil moisture was measured every 2 weeks in the summer of 2008, and hourly from spring of 2009 through 2011. Error bars are standard errors of means (N = 2). The asterisk indicates that there was a significant difference among drought treatments and control (P < 0.05). Treatments (25 and 50%) were significantly different in May and August 2008; Control and 100% were significantly different in May 2008 and September 2009.
These 11-year drought manipulations gave us the unique opportunity to experimentally investigate the cumulative effects of a long-term drought. However, the large investment required to set up and maintain rainfall manipulations often results in smaller plot sizes that make careful sampling essential to preserve the long-term integrity of the plots. In an effort to use the most accurate but least destructive method for measuring plant and biogeochemical dynamics, our methods sometimes differed between years, but properties and processes were always measured consistently across plots within years.
Overall, to test how drought affected C–N decoupling in these plots, we monitored plant production, soil N dynamics, and soil trace gas flux, which are good indicators of biogeochemical decoupling and important for ecosystem function.
Plant Production and N Content
We measured aboveground and belowground vegetation dynamics each year between the 1st and 15th of August, which is within range of expected peak biomass in the shortgrass steppe (Lauenroth and others 2008b). In 2008, we estimated aboveground net primary productivity (ANPP) using a First Growth digital canopy camera (Decagon Devices 2004), establishing a calibration between measured ANPP and percent cover as determined by the camera using a greenness index. Because the relationship between greenness and ANPP was significant (P < 0.05) but weak (R 2 = 0.45), and this method has since been found to be less accurate than other non-destructive measures of production in the shortgrass steppe (Byrne and others 2011), we estimated 2009–2011 ANPP using a point-intercept method (Jonasson 1988; Frank and McNaughton 1990) modified by Byrne and others (2011) (Figure 1). The frequency of graminoid, shrub, and forb contact with intercept points on a 62 × 80 cm grid was a good predictor of harvested ANPP values in calibrated plots (2009: R 2 = 0.84, 2010: R 2 = 0.79, 2011: R 2 = 0.87, all P values < 0.05). Plant cover was also monitored throughout this drought experiment (1999–2009) and is reported in Evans and others (2011).
To assess root dynamics, we measured belowground net primary production (BNPP) in 2008, and standing root biomass in 2010 and 2011. Because data from these methods may not be comparable (Lauenroth 2000), we did not attempt to compare 2008 data to other years, but focused on relative differences among treatments within years. We estimated BNPP (in 2008) using a root in-growth core technique (Vogt and Persson 1991; Lauenroth 2000), quantifying new root production that grew into a 5 × 15 cm core lined with 1 mm mesh (McCulley and others 2009). Under drought recovery (2010 and 2011), we destructively sampled eight (2010) and five (2011) 5 × 15 cm randomly sampled cores from each plot to measure standing root biomass. We did not attempt to measure new (net) annual root production by sampling multiple cores in a year because we wanted to capture spatial variability so treatment comparisons were robust, but still minimize plot destruction. In both years, cores were dried for 1 week at 50°C, and roots were separated from cores by sieving and hand-picking roots for a standard amount of time, then weighed.
We measured the C and N content of above and belowground plant biomass sampled in August 2010 and August 2011. Because we did not harvest ANPP, we collected aboveground biomass for tissue analysis by collecting leaves from grasses, shrubs and forbs nearest to the soil core sampled. We dried leaves and roots from biomass cores at 50°C for 1 week, ground them on a Wiley mill, and analyzed tissues for total C and N content on a LECO CHN-1000 analyzer (St. Joseph, MI). We also estimated the N yield of different plant growth forms by multiplying plant tissue N by production, using plant species cover data (described in Evans and others 2011) to partition total ANPP among growth forms. We calculated RUE as the plant production per cm rainfall plots received in the previous year (for example, September 2010 to August 2011 for 2011 RUE).
Soil N Dynamics
To evaluate the extent of C and N decoupling under drought and how these dynamics affect recovery, we measured both net N mineralization and the total soil inorganic N that is vulnerable to loss at any given time. We used ion-exchange membrane probes or “Plant Root Simulators” (Western Ag Innovations, Inc., Saskatoon, Canada) as an index of net N mineralization and to quantify plant- and microbial-available N (Dodd and others 2000; Hook and Burke 2000). In 2008 (during drought) and 2010 (first year of drought recovery), we buried three pairs (an anion and cation probe) of 10 cm membranes for two roughly 2-month periods (2008: May 24–July 29 and July 31–October 14 and 2010: June 11–July 23 and July 23–September 1) (Figure 1). Probes were analyzed for NH4-N and NO3-N on an Alpkem Analyzer (Pulse Instruments Ltd., Saskatoon, SK) at Western Ag. We report inorganic N accumulation per day to account for small variation (±2 days) in the time the probes remained in the soil, and per cm rainfall to control for differences in rainfall in the different sampling periods, and to investigate the effect of water on nitrogen availability in these plots.
In addition to measuring available N, we quantified N that is potentially available at any one time by sampling soil and extracting it with 2 M potassium chloride (KCl) at multiple time points. Although the number of cores sampled per plot differed each year (2008, 2 cores, 2009, 3 cores, 2010, 8 cores, 2011, 5 cores), core sampling and lab analyses were identical. We extracted ammonium (NH4 +) and nitrate (NO3 −) from 15 × 5 cm cores, filtered extracts with Whatman (#40) filter paper, and analyzed them on a Perstorp Analytical Alpkem autoanalyzer (Wilsonville, OR).
Trace Gas Flux
We installed three flux chambers in each plot (24 chambers total) in the 2008 and 2009 growing season. Trace gas measurements were carried out following Livingston and Hutchinson (1995) with modifications similar to von Fischer and others (2009). In brief, we measured the concentration of gas in the headspace of PVC base rings (3140 cm3) every 5 min for 20 min by extracting 30 mL of gas and analyzing them on a Shimadzu GC14B gas chromatograph with N2 carrier gas.
In 2008, we measured CO2 and N2O every 2 weeks throughout the growing season, but N2O flux was too small to be detected (detection limit < 24.4 μg N/m2/day). In 2009, we also tested for the vulnerability of C and N to gaseous loss under equal-moisture conditions (simulating recovery) by subjecting manipulated plots to equal pulses of moisture (100% of ambient rainfall from previous week) and measuring CO2 and N2O flux. We did not subject control plots to these pulses so that the rainfall received by this treatment would not deviate from ambient. In addition, to ensure manipulated plots still received the correct overall reductions of growing season rainfall (that is, 25 and 50%) after these three pulses (June 12, June 30, July 14), we adjusted the following week’s water additions to account for additional rainfall received during the experimental moisture pulse.
Statistical Analysis
We used a randomized block model (SAS, proc mixed) to analyze C and N responses under drought and recovery, estimating responses using the restricted maximum likelihood (REML) method. Overall, our data consisted of measurements from four treatment types in two blocks (N = 2), some of which were repeated within plots to capture additional variation, and some of which were repeated in the same plots over time (Figure 1). We adjusted each randomized block model according to the data structure. For variables that were measured at a single time point (for example, BNPP in 2008), responses were averaged by plot and the randomized block mixed model included treatment as a fixed factor, and block as a random factor. For variables measured at several time points throughout the experiment but in different locations within a plot (inorganic N, PRS probes, ANPP, root biomass), plot averages were computed (at each time point) and used for statistical analyses. To account for correlations among measurements on the same plot over time, we used a repeated measures model that included treatment, time and their interactions as fixed factors, and block as a random factor.
For most sampling times, we collected more than one sample in each plot (for example, 5 cores or 3 quadrats per plot, Figure 1). In the above models, we averaged across these samples to obtain one parameter measurement per plot. In this way, these measurements served to capture within-plot variation and strengthen our assessment of treatment effects, but did not add statistical power as independent replication. When samples were taken in the same location within plots over time (such as trace gas measurements within a year), locations within plots were not averaged and a random effect of location (for example, trace gas base 1, 2, 3) nested within the block by treatment interaction was added to the repeated measures model to account for additional variation produced by the sampling location.
In all repeated measures models, we also considered whether to include an autoregressive correlation between plots over time, but did not include this additional parameter when it resulted in higher or nearly equal corrected Akaike Information Criterion (AICc) values compared to the model without it. When significant treatment by time interactions occurred in any model (P < 0.05), we compared treatments within each year or time point.
Results
Effectiveness of Treatments
When we monitored soil moisture in drought plots in the 10 years of drought, we found plots receiving 25 and 50% of ambient rainfall resulted in significantly lower soil moisture (N = 2, Figure 3), but soil moisture was not significantly different among plots when shelters were removed. Plots that received 100% of rainfall (the same amount as control plots, but re-added weekly) displayed higher variation in soil moisture, and sometimes lower soil moisture than control plots (significant differences in monthly soil moisture in May 2008 and September 2009, Figure 3). The three water additions in 2009 (data not shown) increased soil moisture in all treatments, and there was no significant difference in soil moisture among treatments (P > 0.05) within each water addition.
Plant Production Under Drought and Recovery
Long-term drought significantly reduced ANPP in 2008 and 2009, the 10th and 11th year of the rainfall manipulation (Figure 4A). ANPP decreased with drought severity in both years, but 25 and 50% treatments were only significantly different from each other in 2009 (P = 0.03). Decreases in ANPP were not proportional to rainfall reductions; treatments receiving 25 and 50% of rainfall resulted in an average drought-year ANPP of 50 and 65% of control ANPP, respectively. Reductions in ANPP persisted 2 years after drought ended, but only in 25% drought plots. In rainout shelter treatments that received 100% of rainfall, ANPP and root biomass were often lower and sometimes significantly lower than the control treatments, following patterns of lower soil moisture in 100% treatments compared to control (Figure 3).
Aboveground primary productivity (A) and root dynamics (B) in drought manipulations receiving 25, 50, and 100% of ambient precipitation on the shortgrass steppe for a period of 10 and 11 years (2008 and 2009), then released from drought treatments (indicated by dashed line, 2010 and 2011). Root dynamics (B) were described by estimating belowground net primary production (BNPP) in 2008 using an in-growth core, and by harvesting root biomass in 2010 and 2011 (B, second axis). Bars show mean (N = 2) and standard error, and different letters indicate significant differences between treatments (P < 0.05).
Root in-growth cores revealed that BNPP (up to a 15 cm depth) in the 25% drought plots was 16% lower than the BNPP in control (P = 0.007), but there was no significant difference between BNPP in 50% and control plots (Figure 4B). In 2010 and 2011, after drought had ended, root biomass in 25 and 50% plots was significantly lower than root biomass in control plots (P < 0.01).
Under drought, RUE (plant production per cm rainfall) was higher in drought plots than the control in 2008 (P < 0.05), but not in 2009 (Figure 3). RUE was lower in drought plots in 2010 and 2011 (P < 0.05), when all plots received equal rainfall (Figure 5).
Mean rainfall use efficiency (RUE, bars, N = 2) under drought manipulations in 2008 and 2009, and recovery from drought in 2010 and 2011 (after dashed line) in the shortgrass steppe. RUE was as calculated by dividing ANPP for each treatment by annual rainfall each treatment received in the previous year, shown by open dots.
Nitrogen Dynamics Under Drought and Recovery
Nitrogen content (%) of plant tissue varied by vegetation type, and in general, drought plots had higher N content in plants and roots than control and 100% treatments in both recovery years (Figure 6A, B, inset). Due to differences in production among plots, when we calculated ANPP-N, or the overall aboveground N yield (gN/m2), there were fewer trends among treatments (Figure 6). However, 25% drought plots did have significantly lower total ANPP-N and root biomass N than control plots, largely mediated by overall greater production in control plots, and shifts in plant species composition (Evans and others 2011) (Figure 6).
Aboveground and belowground N yield of vegetation in the first (2010, A) and second year (2011, B) of recovery after an 11-year drought in the shortgrass steppe. N yield was determined using the percent N in vegetation (inset) and the belowground biomass and aboveground production for each growth form. (P < 0.05, N = 2).
There were no significant differences in plant available N (as measured by resin probes) among treatments under drought in 2008 and 2009 (Figure 7). However, when differences in rainfall among treatments were accounted for by expressing plant available N per cm rainfall received, plant available N was higher in drought plots (Figure 7, inset). In 2010, the first year of recovery, drought plots had significantly higher available N than control plots in the June–July measurement period, and this trend continued in the August–September period, although was not significant. Changes in the ratio of available NO3 − to NH4 + did not vary among treatments.
Total inorganic N (NO3 −-N + NH4 +-N) captured on ion-exchange membrane probes in the 10th year of a long-term drought manipulation in the shortgrass steppe (2008), when rainfall the plots received varied (shown by open dots) and the first year of recovery (2010), when all plots received the same amount of rainfall. Bars show μg-N accumulated per 10 cm probe per day in each 2-month period of burial, whereas inset shows μg-N per cm rainfall during 2008, when treatments received unequal rainfall. Where significant differences among treatments occurred, significant pairwise differences (P < 0.05) are indicated by letters. Ratios of nitrate to ammonium were consistent across treatments within any given time point, so are not shown separately.
KCl-extracted (as opposed to available) NO3 − was consistently higher under drought, and up to 5 times that of NO3 − in control plots (Figure 8), but KCl-extracted NH4 + did not accumulate under drought. Nitrate continued to be higher under drought than control treatments for both sampling dates in 2010 and in 2011.
NO3 −-N (A) and NH4 +-N (B) extracted with 2 M KCl in the 10th and 11th year of a drought manipulation (2008 and 2009), and after release from the manipulation (indicated by dashed line, 2010 and 2011). Bars show means (N = 2) and standard error and different letters indicate significant differences between treatments (P < 0.05).
Trace Gas Flux Under Drought and Moisture Pulses
CO2 flux in 25 and 50% drought plots was an average of 39 and 28% lower than control treatments across all measurement dates in 2008, respectively (Figure 9). Soil moisture differed among treatments at the time of sampling, and variation in soil moisture explained 42% of the variance in CO2 flux. The water pulses executed at 3 time points in 2009 resulted in average volumetric soil moistures of 22.0, 19.6, and 14.5% (moisture data not shown), respectively, and within each time point, soil moisture content was not significantly different among treatments. After water additions, N2O flux in the 25% drought treatment was nearly double N2O flux in 100% plots on June 12 (P = 0.006), and remained significantly higher than N2O in 100% plots on June 30 (P = 0.01), but there was no significant difference among treatments on July 14 (P = 0.21) (Figure 10B). In contrast to N2O, CO2 flux in drought plots was consistently lower than 100% plots after 2009 water additions (Figure 10A), though we cannot conclude whether this was due to changes in heterotrophic or autotrophic respiration.
Mean soil CO2 flux (bars) in the growing season of 2008, the 10th year of a long-term drought manipulation in the shortgrass steppe. Soil moisture is shown for control plots (symbols and line) to display the variation in soil moisture at the time of sampling; drought treatments had reduced soil moistures. The asterisk indicates a significant difference (P < 0.05) among treatments.
Mean and standard error of soil CO2 (A) and N2O (B) flux after three experimental water pulses in the summer of 2009. The same amount of water was added to each plot during a pulse and soil moisture was not significantly different among treatments within a moisture pulse date. Different letters indicate significant differences among treatments.
Discussion
We found that long-term drought causes significant reductions in plant biomass and CO2 flux, and an accumulation of inorganic N in the shortgrass steppe. This decoupling of C and N under long-term drought altered N dynamics during recovery, decreasing both nitrogen use efficiency (NUE) and rainfall use efficiency (RUE) when rainfall returned to normal levels. The accumulation of soil N and reductions in plant biomass persisted 2 years after manipulations were removed, and was associated with increased N in plant tissue. This suggests that biogeochemical decoupling that occurs under a decade-long drought could alter patterns of drought recovery in the shortgrass steppe.
C and N Decoupling Under Long-Term Drought
Under drought, we observed an increase in the amount of N in inorganic form. Carbon flux—ANPP, BNPP, and soil CO2 flux—were lower under drought. Although inorganic N pools at any single time point are not useful in interpreting rates of processes (Schlesinger 1997), we consistently found higher inorganic N in soil subject to drought (Figure 8), as other studies have (Garcia-Mendez and others 1991; Davidson and others 1993; Whitford and others 1995; Reynolds and others 1999; Yahdjian and others 2006), providing evidence that long-term drought induces C–N decoupling in the shortgrass steppe.
The decoupling we observed may be caused by different ecosystem compartments or processes having different sensitivities to drought, or by abiotic mechanisms. Aboveground and belowground production (up to 15 cm depth) were significantly reduced by long-term drought (Figure 4). Although production was reduced, microbial biomass was not significantly different among treatments in the last year of drought (data not shown). Microorganisms may be capable of decomposing plant litter at moisture levels that are not high enough to stimulate plant production (Sala and Lauenroth 1982; Austin and others 2004; Ogle and Reynolds 2004), and continue mineralizing plant material in dry periods (Giese 2009), although recent studies have not found evidence of this in the shortgrass steppe (Dijkstra and others 2012). Further, different microbial processes could also have different sensitivities to drought, resulting in accumulation of certain pools. We found that under drought net N mineralization (as indexed by N accumulated on exchange membranes) was not significantly different, that inorganic N accumulated, and that CO2 flux was reduced, which indirectly supports findings that N mineralization and decomposition may have different sensitivities to moisture (Hook and Burke 2000; Barrett and Burke 2002; Yahdjian and others 2006; Yahdjian and Sala 2008). In addition, different microbial groups can have different sensitivities to moisture stress. Specifically, ammonium-oxidizing bacteria may recover more quickly from stressful conditions than nitrite-oxidizing bacteria (Tappe and others 1999). We did not separate nitrite from nitrate in our analysis, but it is possible that increased abundance of ammonium-oxidizers relative to nitrite-oxidizers could have caused nitrite accumulation under drought (Gelfand and Yakir 2008). However, community composition analysis from these plots does not support this mechanism; we did not find evidence that Nitrosomonas abundance increased relative to Nitrobacter under drought (Evans and Wallenstein 2012, submitted).
Alternatively, physical properties of the products of these processes, rather than differences in process sensitivity to moisture, could explain N accumulation and C–N decoupling under drought (Yahdjian and others 2006). Specifically, nitrate, which dominates inorganic N pools (relative to ammonium) in the shortgrass steppe and that accumulated under drought (Figure 8A) is highly mobile under high water content. Reduced soil water content could have altered nitrate mobility, slowing the movement of nitrate to deeper soil layers and limiting diffusion of nitrate to plants and microbes.
Although we did observe decoupling of C and N in the 10th and 11th year of a long-term drought, we did not monitor C and N dynamics throughout the drought. Therefore, we cannot conclude that this decoupling is due to the length of the drought per se. Indeed, other studies have seen such decoupling in shorter-term droughts (Yahdjian and others 2006; Landesman and Dighton 2010), across seasons (Augustine and McNaughton 2004), or even between rainfall pulses (Dijkstra and others 2012). However, it is also likely that re-synchronization of C and N occurs more quickly after these shorter-term dry periods, because reduced plant activity (not biomass) may be the cause of decoupling. It is possible the novelty of the length of the drought we induced is (and will continue to be) revealed by the persistence of this decoupling into recovery.
Effectiveness of Treatments and Implications for Altered Rainfall Timing
Overall, our drought treatments effectively reduced rainfall and resulted in consistently lower soil moisture in drought treatments than control treatments. Occasionally, we found small effects associated with the shelter manipulation, in that variables responded differently to control (ambient rainfall) and 100% treatments that received weekly re-additions. Although rarely statistically significant, these differences could have been caused by differences in the water used to irrigate plots (well water vs. rainfall) or the altered timing of water inputs to manipulated plots. The timing of precipitation plays a large role in ecosystem dynamics in the shortgrass steppe (Sala and Lauenroth 1982), and changes in the timing of rainfall timing can have as significant of an effect on grassland C cycling as changes in rainfall amount (Knapp and others 2002; Heisler-White and others 2009). In the shortgrass steppe, Heisler-White and others (2008) showed that higher rainfall variability can lead to greater ANPP, possibly because water from larger rainfall events infiltrates to deeper soil layers where it is not vulnerable to evaporation (Heisler-White and others 2008). Interestingly, in our experiment, ANPP in 100% treatment was often lower than ANPP in control, as was soil moisture, suggesting that if anything, our rainout design exacerbated drought effects, rather than negating them.
Therefore, although we are confident drought treatments successfully induced moisture limitation for 11 years, the shelter effect prevents us from making direct predictions about specific amounts of rainfall reductions, and low replication (spatial and temporal) limits our scope and ability to make conclusions about specific mechanisms and account for all N pools. These results can, therefore, be used to direct future research toward investigating full ecosystem N budgets under drought and further implications of these changes for recovery.
Ecosystem Legacies Under Drought Recovery
We continued to see differences among drought and control treatments—both in plant production and nitrogen cycling—in the two recovery years we monitored. Both aboveground and belowground production remained lower after drought ended, when plants were less efficient with nitrogen and more efficient with water. These lags in production following dry years have been observed in response to natural precipitation variability in the shortgrass steppe (Lauenroth and Sala 1992), and could be due to structural vegetative constraints that occur after dry years. This is supported by lower root biomass in drought plots in the 2 years following drought treatments, although the measurement of total biomass could also be biased by previous years’ differences. Soil inorganic N also remained higher in drought plots after shelters were removed. Greater soil water content under recovery may have made this inorganic N more accessible to plants and microbes after shelters were removed, as indicated by greater N captured by ion-exchange probes in plots that had previously experienced drought (Figure 7).
A greater N availability after drought may explain why plant tissue N was higher in drought plots under recovery. Other studies in semiarid grasslands have shown increases in plant available N results in greater plant N uptake when water is available (Giese and others 2011). A lower plant tissue C:N could alter plant–soil interactions as well as forage quality and land use in the shortgrass steppe. High-N litter produced from inefficient plant N use can lead to increased net N mineralization and increased available N that is then advantageous for N inefficient plants (Vitousek 1982; Vinton and Burke 1995). This positive feedback could be mediated by changes in plant community composition. In this drought study, “weedy” species have increased relative to grasses (Evans and others 2011). As shown in N addition experiments, these changes can persist long after N additions have ceased (Lauenroth and others 1978; Milchunas and Lauenroth 1995).
Interestingly, although foliar plant N increased the year after drought, the reduced ANPP and altered species composition in drought plots did not result in a significant difference in N yield (g biomass N/m2, Figure 4), suggesting that a reduction in forage quantity under drought is not necessarily compensated for by increased forage quality.
Implications for N Conservation Under Drought and Recovery
The decoupling of C and N under drought, and altered cycling of N under recovery, has implications for shortgrass steppe responses to future droughts. Our findings of reduced plant NUE after drought suggest that drought may cause nitrogen to be less efficiently cycled, when accumulated nitrogen is available to plants under recovery. In addition to this, we also observed greater N2O flux in plots that had been experiencing drought for 11 years (Figure 8B) when we added equal-moisture pulses to each plot to simulate recovery. Like changes in plant tissue N, greater N2O flux in drought plots was likely mediated by greater inorganic N in these plots (Figure 6), similar to flux patterns seen after soil is wet following a drying period (Dijkstra and others 2012), or simply under N additions (Mosier and others 1996). Overall, this suggests drought can lead to greater vulnerability of inorganic N to loss via N2O flux. Although the data are clear, we have limited inference for this conclusion, in that sampling (3 time points) may not be representative of dynamics under drought recovery, or capture intraannual variation, like a peak N2O flux that could occur during spring thaw (Wolf and others 2010).
These changes we observed in nitrogen dynamics under drought and recovery highlight the need to determine the long-term fate of the soil inorganic N that accumulates under drought. For example, N accumulated under drought can stimulate plant production in mesic systems (Briggs and Knapp 1995; Haddad and others 2002), but we did not find evidence for this. The higher concentration of plant tissue N we observed (and accompanying plant–soil-N feedbacks) could increase interannual variability in production (Haddad and others 2002), or cause inertia in the shrub- and forb-dominated plant communities that have emerged under drought (Evans and others 2011). Our data also suggest that a drought-induced soil inorganic pool also makes N more vulnerable to loss, potentially for many years if vegetation is slow to recover. Thus, drought also has the potential to result in greater N loss that could leave vegetation N limited once plants can fully respond to rainfall (Yahdjian and others 2006).
Conclusions
We found that an 11-year rainfall manipulation in the drought-resistant shortgrass steppe significantly altered C and N dynamics, and affected ecosystem function at least 2 years into recovery. Soil inorganic N accumulated under drought, whereas plant production decreased, thus decoupling C and N cycles. Following drought, lower plant production persisted under conditions of high N availability, resulting in high plant N concentrations. We also observed higher gaseous N loss from drought plots when water was available. These experimental results have implications for N conservation under drought and recovery. Decoupling of C and N dynamics can alter the response and recovery of the shortgrass steppe to decade-long droughts that are likely to occur in the future.
References
Asner GP, Seastedt TR, Townsend AR. 1997. The decoupling of terrestrial carbon and nitrogen cycles. BioScience 47:226–34.
Augustine DJ, McNaughton SJ. 2004. Temporal asynchrony in soil nutrient dynamics and plant production in a semiarid ecosystem. Ecosystems 7:829–40.
Austin AT, Sala OE. 2002. Carbon and nitrogen dynamics across a natural precipitation gradient in Patagonia, Argentina. J Veg Sci 13:351–60.
Austin AT, Yahdjian L, Stark JM, Belnap J, Porporato A, Norton U, Ravetta DA, Schaeffer SM. 2004. Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141:221–35.
Bai YF, Wu JG, Xing Q, Pan QM, Huang JH, Yang DL, Han XG. 2008. Primary production and rain use efficiency across a precipitation gradient on the Mongolia plateau. Ecology 89:2140–53.
Barrett JE, Burke IC. 2002. Nitrogen retention in semiarid ecosystems across a soil organic-matter gradient. Ecol Appl 12:878–90.
Benson EJ, Hartnett DC, Mann KH. 2004. Belowground bud banks and meristem limitation in tallgrass prairie plant populations. Am J Bot 91:416–21.
Briggs JM, Knapp AK. 1995. Interannual variability in primary production in tallgrass prairie: climate, soil-moisture, topographic position, and fire as determinants of aboveground biomass. Am J Bot 82:1024–30.
Burke IC, Lauenroth WK, Parton WJ. 1997. Regional and temporal variation in net primary production and nitrogen mineralization in grasslands. Ecology 78:1330–40.
Burke IC, Mosier AR, Hook PB, Milchunas DG, Barrett JE, Vinton MA, McCulley RL, Kaye JP, Gill RA, Epstein HA, Kelly RH, Parton WJ, Yonker CM, Lowe P, Lauenroth WK. 2008. Soil organic matter and nutrient dynamics of shortgrass steppe ecosystems. In: Lauenroth WK, Burke IC, Eds. Ecology of the shortgrass steppe. New York: Oxford University Press. p 84–118.
Byrne KM, Lauenroth WK, Adler PB, Byrne CM. 2011. Estimating aboveground net primary production in grasslands: a comparison of nondestructive methods. Rangel Ecol Manag 64:498–505.
CCSP, 2008. Effects of climate change on energy production and use in the United States. In: Research, U.C.C.S.C.a.t.S.o.G.C. (Ed.).
Cook ER, Seager R. 2010. Megadroughts in North America: placing IPCC projections of hydroclimatic change in a long-term paleoclimate context. J Quat Sci 25:48–61.
Davidson EA, Matson PA, Vitousek PM, Riley R, Dunkin K, Garciamendez G, Maass JM. 1993. Processes regulating soil emissions of NO and N2O in a seasonally dry tropical forest. Ecology 74:130–9.
Dijkstra F, Augustine D, Brewer P, Fischer J. 2012. Nitrogen cycling and water pulses in semiarid grasslands: are microbial and plant processes temporally asynchronous? Oecologia 1–10. doi:10.1007/s00442-012-2336-6.
Dodd MB, Lauenroth WK, Burke IC. 2000. Nitrogen availability through a coarse-textured soil profile in the shortgrass steppe. Soil Sci Soc Am J 64:391–8.
Evans SE, Byrne KM, Burke IC, Lauenroth WK. 2011. Defining the limit to resistance in a drought-tolerant grassland: long-term severe drought significantly reduces the dominant species and increases ruderals. J Ecol 99:1500–07.
Evans SE, Wallenstein MA. 2012. Does moisture niche partitioning drive shifts in microbial community composition under long-term drought in the shortgrass steppe? International Society for Microbial Ecology (ISME) Journal (submitted).
Frank DA, McNaughton SJ. 1990. Above-ground biomass estimation with the canopy intercept method: a plant-growth form caveat. Oikos 57:57–60.
Garcia-Mendez G, Maass JM, Matson PA, Vitousek PM. 1991. Nitrogen transformations and nitrous-oxide flux in a tropical deciduous forest in Mexico. Oecologia 88:362–6.
Gelfand I, Yakir D. 2008. Influence of nitrite accumulation in association with seasonal patterns and mineralization of soil nitrogen in a semi-arid pine forest. Soil Biol Biochem 40:415–24.
Giese M, Gao Y, Lin S, Brueck H. 2011. Nitrogen availability in a grazed semi-arid grassland is dominated by seasonal rainfall. Plant Soil 340:157–67.
Haddad NM, Tilman D, Knops JMH. 2002. Long-term oscillations in grassland productivity induced by drought. Ecol Lett 5:110–20.
Harpole WS, Potts DL, Suding KN. 2007. Ecosystem responses to water and nitrogen amendment in a California grassland. Glob Change Biol 13:2341–8.
Heisler-White JL, Blair JM, Kelly EF, Harmoney K, Knapp AK. 2009. Contingent productivity responses to more extreme rainfall regimes across a grassland biome. Glob Change Biol 15:2894–904.
Heisler-White JL, Knapp AK, Kelly EF. 2008. Increasing precipitation event size increases aboveground net primary productivity in a semi-arid grassland. Oecologia 158:129–40.
Hook PB, Burke IC. 2000. Biogeochemistry in a shortgrass steppe landscape: control by topography, soil texture, and microclimate. Ecology 81:2686–703.
Hooper DU, Johnson L. 1999. Nitrogen limitation in dryland ecosystems: responses to geographical and temporal variation in precipitation. Biogeochemistry 46:247–93.
IPCC. 2007. Climate change 2007: synthesis report. Geneva: Intergovernmental Panel on Climate Change.
Jackson LE, Strauss RB, Firestone MK, Bartolome JW. 1988. Plant and soil-nitrogen dynamics in California annual grassland. Plant Soil 110:9–17.
Jonasson S. 1988. Evaluation of the point intercept method for the estimation of plant biomass. Oikos 52:101–6.
Knapp AK, Fay PA, Blair JM, Collins SL, Smith MD, Carlisle JD, Harper CW, Danner BT, Lett MS, McCarron JK. 2002. Rainfall variability, carbon cycling, and plant species diversity in a mesic grassland. Science 298:2202–5.
Landesman WJ, Dighton J. 2010. Response of soil microbial communities and the production of plant-available nitrogen to a two-year rainfall manipulation in the New Jersey Pinelands. Soil Biol Biochem 42:1751–8.
Lauenroth WK. 2000. Methods for estimating belowground net primary production. In: Sala OE, Jackson RB, Mooney HA, Howard RW, Eds. Methods in ecosystem science. New York: Springer. p 58–71.
Lauenroth WK. 2008. Vegetation of the shortgrass steppe. In: Lauenroth WK, Burke IC, Eds. Ecology of the shortgrass steppe. New York: Oxford University Press. p 70–83.
Lauenroth WK, Bradford JB. 2006. Ecohydrology and the partitioning AET between transpiration and evaporation in a semiarid steppe. Ecosystems 9:756–67.
Lauenroth WK, Bradford JB. 2009. Ecohydrology of dry regions of the United States: precipitation pulses and intraseasonal drought. Ecohydrology 2:173–81.
Lauenroth WK, Burke IC, Morgan JA. 2008a. The shortgrass steppe. New York: Oxford University Press.
Lauenroth WK, Dodd JL, Sims PL. 1978. Effects of water-induced and nitrogen-induced stresses on plant community structure in a semi-arid grassland. Oecologia 36:211–22.
Lauenroth WK, Milchunas DG, Sala OE, Burke IC, Morgan JA. 2008b. Net primary production in the shortgrass steppe. In: Lauenroth WK, Burke IC, Eds. Ecology of the shortgrass steppe. New York: Oxford University Press. p 270–305.
Lauenroth WK, Sala OE. 1992. Long-term forage production of North-American shortgrass steppe. Ecol Appl 2:397–403.
Livingston GP, Hutchinson GL. 1995. Enclosure-based measurement of trace gas exchange: applications and sources of error. In: Matson PA, Harris RC, Eds. Biogenic trace gases: measuring emissions from soil and water. Cambridge: Blackwell Science. p 394.
McCulley RL, Burke IC, Lauenroth WK. 2009. Conservation of nitrogen increases with precipitation across a major grassland gradient in the Central Great Plains of North America. Oecologia 159:571–81.
Milchunas DG, Lauenroth WK. 1995. Inertia in plant community structure: state changes after cessation of nutrient-enrichment stress. Ecol Appl 5:452–8.
Milchunas DG, Sala OE, Lauenroth WK. 1988. A generalized-model of the effects of grazing by large herbivores on grassland community structure. Am Nat 132:87–106.
Mosier AR, Parton WJ, Valentine DW, Ojima DS, Schimel DS, Delgado JA. 1996. CH4 and N2O fluxes in the Colorado shortgrass steppe. 1. Impact of landscape and nitrogen addition. Global Biogeochem Cycles 10:387–99.
Noy-Meir I. 1973. Desert ecosystems: environment and producers. Annu Rev Ecol Syst 4:25–52.
Ogle K, Reynolds JF. 2004. Plant responses to precipitation in desert ecosystems: integrating functional types, pulses, thresholds, and delays. Oecologia 141:282–94.
Peters DPC, Lauenroth WK, Burke IC. 2008. The role of disturbance in shortgrass steppe community and ecosystem dynamics. In: Lauenroth WK, Burke IC, Eds. Ecology of the shortgrass steppe. New York: Oxford University Press. p 84–118.
Reynolds JF, Virginia RA, Kemp PR, de Soyza AG, Tremmel DC. 1999. Impact of drought on desert shrubs: effects of seasonality and degree of resource island development [review]. Ecol Monogr 69(1):69–106.
Risser PG, Parton WJ. 1982. Ecosystem analysis of the tallgrass prairie: nitrogen-cycle. Ecology 63:1342–51.
Ronnenberg K, Wesche K. 2011. Effects of fertilization and irrigation on productivity, plant nutrient contents and soil nutrients in southern Mongolia. Plant Soil 340:239–51.
Sala OE, Lauenroth WK. 1982. Small rainfall events: an ecological role in semi-arid regions. Oecologia 53:301–4.
Sala OE, Lauenroth WK, Parton WJ. 1992. Long-term soil-water dynamics in the shortgrass steppe. Ecology 73:1175–81.
Schlesinger WH. 1997. Biogeochemistry: an analysis of global change. Oxford: Elsevier.
Tappe W, Laverman A, Bohland M, Braster M, Ritterhaus S, GRoeneweg J, van Verseveld HW. 1999. Maintenance energy demand and starvation recovery dynamics of Nitrosomonas europaea and Nitrobacter winogradskyi cultivated in a retentostat with complete biomass retention. Appl Environ Microbiol 65:2471–7.
Vinton MA, Burke IC. 1995. Interactions between individual plant species and soil nutrient status in shortgrass steppe. Ecology 76:1116–33.
Vitousek P. 1982. Nutrient cycling and nutrient use efficiency. Am Nat 119:553–72.
Vitousek PM, Reiners WA. 1975. Ecosystem succession and nutrient retention: a hypothesis. BioScience 25:376–81.
Vogt KA, Persson H. 1991. Root methods. In: Lassoie H, Hinkley TM, Eds. Techniques and approaches in forest tree ecophysiology. Boca Raton: CRC Press. p 477–501.
von Fischer, JC, Butters, G, Duchateau, PC, Thelwell, RJ, Siller, R, 2009. In situ measures of methanotroph activity in upland soils: A reaction-diffusion model and field observation of water stress. Journal of Geophysical Research-Biogeosciences 114.
Whitford WG, MartinezTuranzas G, MartinezMeza E. 1995. Persistence of desertified ecosystems: explanations and implications. Environ Monit Assess 37:319–32.
Wolf B, Zheng X, Brüggemann N, Chen W, Dannenmann M, Han X, Sutton MA, Wu H, Yao Z, Butterbach-Bahl K. 2010. Grazing-induced reduction of natural nitrous oxide release from continental steppe. Nature 464:881–4. doi:10.1038/nature08931.
Woodhouse CA, Meko DM, MacDonald GM, Stahle DW, Cooke ER. 2010. A 1,200-year perspective of 21st century drought in southwestern North America. Proc Natl Acad Sci USA 107:21283–8.
Yahdjian L, Sala O, Austin AT. 2006. Differential controls of water input on litter decomposition and nitrogen dynamics in the Patagonian steppe. Ecosystems 9:128–41.
Yahdjian L, Sala OE. 2008. Do litter decomposition and nitrogen mineralization show the same trend in the response to dry and wet years in the Patagonian steppe? J Arid Environ 72:687–95.
Acknowledgments
This work was conducted at the Central Plains Experimental Range (CPER), which is administered by the USDA Agricultural Research Service (ARS) and is a Long Term Ecological Research site (SGS-LTER) funded by the National Science Foundation (NSF DEB 0823405 and NSF DEB 0217631). This work would not have been possible without the 1998–2011 SGS field crews and staff, the SGS-LTER Information Manager Nicole Kaplan, and Kenneth Murphy, who conceived and implemented the rainfall manipulation plots. We also would like to thank Dr. Joe von Fischer for assistance in trace gas analysis, Dr. Phillip Chapman for statistical advising, and two anonymous reviewers and the associate editor for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
IC conceived and designed the original long-term rainfall manipulations at SGS-LTER, SE designed the biogeochemical investigation with guidance from IC, and SE performed field and lab measurements, analyzed the data and wrote the paper.
Rights and permissions
About this article
Cite this article
Evans, S.E., Burke, I.C. Carbon and Nitrogen Decoupling Under an 11-Year Drought in the Shortgrass Steppe. Ecosystems 16, 20–33 (2013). https://doi.org/10.1007/s10021-012-9593-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-012-9593-4