Abstract
Aims
Explore how soil CO2 efflux and its components change after moving sand dunes are stabilized with shrubs, and how abiotic factors affect those components at different scales.
Methods
Soil CO2 efflux from a sand-stabilized area was compared to that from moving sand dunes in the Tengger Desert. To partition rhizosphere respiration (RR) from soil basal respiration (RB), a root-isolation plot was established.
Results
Compared to moving sand dunes, total soil respiration (RT) in the sand-stabilized area increased 3.2 fold to 0.28 ± 0.08 μmol CO2 m-2 s-1, two thirds of which was from RB. Shrub patchiness produced spatial variation in soil respiration, whereas temporal dynamics of soil respiration were affected mainly by soil water content. Shallow soil water content (0–20 cm) influenced RT and RB, whereas deep soil water content (30–210 cm) influenced RR and the ratio RR/RT. During most of the year when soil water content was below field capacity, diurnal changes in soil respiration were partially decoupled from soil temperature but could be modeled using soil temperature and photosynthetic active radiation.
Conclusions
Sand-dune stabilization increased soil respiration, and increased RB from biological soil crust and altered soil properties such as increased soil organic matter contributed more than increased RR from increased shrubs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vegetation restoration is a common and effective method to combat desertification and to prevent sand encroachment into adjacent areas in many arid regions of the world (Fullen and Mitchell 1994; Fearnehough et al. 1998). To date, ecological engineering projects have successfully reduced hazards of sandstorms and desertification in northern China. For example, a non-irrigated revegetation system was initiated in the 1950s to protect 40 km of the Baotou-Lanzhou railway line from sand burial by mobile dunes at the southeastern fringe of the Tengger Desert. This protective system first used straw-checkerboards to stabilize the dune surface and then was followed by planting xerophytic shrubs to fix sand dunes in place (Li et al. 2004, 2007). Revegetation and subsequent plant-soil succession along the rail line changed the landscape from moving sand dunes to one covered by sand-stabilizing shrubs, regenerated shrubs and annual plants, and biological soil crusts (BSCs) (Li et al. 2004, 2007; Zhang et al. 2008a).
Soil respiration or soil CO2 efflux is often thought to be a sensitive indicator of ecosystem functions (Raich and Tufekcioglu 2000; Tang and Baldocchi 2005; Rey et al. 2011; Talmon et al. 2011). For example, vegetation patches in water-limited ecosystems generally have greater soil respiration rates than interspaces between vegetation because vegetation patches have greater substrate availability, greater organic matter and nutrient contents, better micro-climatic conditions, and greater root and microbial biomass and activity (Maestre and Cortina 2003; Tang and Baldocchi 2005; McCulley et al. 2007; Berg and Steinberger 2008; Cable et al. 2008, 2011). Because stabilizing sand dunes in the Tengger Desert increased plant cover and biomass, improved edaphic properties, and ameliorated microclimate (Fullen and Mitchell 1994; Fearnehough et al. 1998; Li et al. 2004, 2007, 2010; Zhang et al. 2008b, 2009a), soil respiration is expected to increase. However, no information is available on soil respiration in sand-stabilized areas.
Soil respiration in arid and semiarid ecosystems is characterized by high spatial and temporal heterogeneity (Cable et al. 2011). Spatial heterogeneity of soil respiration in deserts mainly results from discontinuous or patchy distribution of vegetation as discussed above. On the other hand, temporal variation of soil respiration is driven by both climatic factors such as soil moisture and temperature (Raich and Schlesinger 1992; Raich and Tufekcioglu 2000) and biological factors such as photosynthetic activity (Maestre and Cortina 2003; Cable et al. 2008; Belnap et al. 2005; Vargas and Allen 2008; Almagro et al. 2009; Castillo-Monroy et al. 2011). Furthermore, mosses, algae, and cyanobacteria that comprise BSCs respond more rapidly to episodic precipitation than vascular plants (Belnap et al. 2005; Sponseller 2007). Thus, CO2 exchange associated with BSCs begins with little rainfall or even with dew and fog (Zaady et al. 2000; Sponseller 2007; Wilske et al. 2008; Thomas and Hoon 2010), whereas soil CO2 efflux from roots requires a large precipitation pulse or a series of closely-spaced pulses. Thus, BSCs may contribute to the temporal dynamics of soil respiration in arid ecosystems.
Ultimately, temporal and spatial variability of soil respiration in arid ecosystems is due to how the functionally different components of soil respiration respond to abiotic and biotic factors. Total soil respiration (RT) has two major components: rhizosphere respiration (RR, including root respiration and associated heterotrophic rhizomicrobial respiration) and basal respiration (RB, including heterotrophic microbial respiration and respiration by autotrophic BSCs) (Tang and Baldocchi 2005; Cable et al. 2011). RB is influenced primarily by soil temperature and moisture, whereas RR is influenced more by plant eco-physiological processes such as photosynthesis and carbon availability to roots (Hanson et al. 2000; Cheng et al. 2005). Thus, responses of RB and RR to abiotic factors likely differ, suggesting that partitioning soil respiration into its two main components, RR and RB, will help us understand factors that drive temporal and spatial variation in soil respiration (Tang and Baldocchi 2005).
The two main objectives of our study were to determine: (1) how the two major components of soil respiration (RR or RB) contribute to RT after moving sand dunes are stabilized with straw-checkerboards and revegetation; and (2) how RR and RB change at different temporal (daily and seasonal) scales and how abiotic factors such as soil temperature and moisture affect them. We hypothesized that RB has a greater contribution to RT across the landscape in sand-stabilized areas because of high BSCs cover and their rapid response to rainfall. Also, we hypothesized that soil temperature and moisture have different influences on RR and RB. To investigate these objectives, a sand-stabilized area at the southeastern fringe of the Tengger Desert that was revegetated with Artemisia ordosica Krasch. was selected for study. A root-isolation plot was established in the sand-stabilized area to measure RB, and a nearby moving sand dune was used as a reference site.
Materials and methods
Study site
The study area, Shapotou, is located at the southeastern fringe of the Tengger Desert (37°32′N, 105°02′E; altitude 1300 m) and has a typical temperate desert environment. Annual mean temperature is 10.0 °C, January mean temperature is −6.9 °C, and July mean temperature is 24.3 °C. Annual mean precipitation is 186 mm, and about 80 % of this precipitation falls between May and September. Annual potential evaporation is about 2900 mm. Groundwater lies more than 80 m below the surface and therefore cannot be used by plants. Predominant soils are Orthic Sierozem and aeolian sandy soil (Li et al. 2010).
The area near Shapotou is a typical ecotone between natural sandy desert and sand-stabilized steppe. The natural sandy desert is characterized by high, dense, and continuous reticulate barchan dunes with about 1 % cover of natural vegetation. Dominant plants in the moving sand dunes are Hedysarum scoparium Fisch. and Agriophyllum squarrosum Moq (Li et al. 2007). For a sand-stabilized study area, we used a portion of the Water Balance Experimental Field (WBEF) that was established in April 1989. The WBEF was constructed by first leveling sand dunes, next erecting sand barriers of 1 × 1 m wheat-straw checkerboards, and finally planting Artemisia ordosica Krasch. and Caragana korshinskii Kom. (Zhang et al. 2008a). As typically occurred during the 15 years after sand-stabilization and revegetation, deep-rooted shrubs such as C. korshinskii gradually were lost from the sand-stabilized area due to decreasing deep soil water content and replaced by the shallow-rooted shrub A. ordosica and annual plants (Li et al. 2004). Dominant annual species in the sand-stabilized areas are Eragrostis poaeoides Beauv., Bassia dasyphylla O. Kuntze, Corispermum patelliforme Iljin, and Salsola ruthenica Iljin (Zhang et al. 2008b). In addition to vegetation changes after sand stabilization, BSCs on the soil surface greatly increase. At the WBEF, BSCs are primarily algal crusts, composed mainly of filamentous cyanobacteria (accounting for 46 %), with smaller amounts of diatoms (27 %), green algae (18 %), and Euglena sp. (9 %) (Li et al. 2010).
Experimental design and soil respiration measurements
For our study, an A. ordosica monoculture stand within WBEF was selected as the sand-stabilized experimental site (hereafter referred to as “A. ordosica stand”), and the windward slope of moving sand dunes near WBEF was selected as representative of a moving sand dune site (hereafter referred to as “MSD”). In September 2007, we characterized vegetation of both sites. MSD had few plants (< 1 % plant cover), but the A. ordosica stand had ~31 % shrub cover and ~12 % cover for annuals. Cover of BSCs was similar throughout the A. ordosica stand at 35 % cover, and BSCs were 1.2 ± 0.3 cm thick.
At each site, three 10 × 10 m quadrats were established to characterize soils in December 2006. Within each quadrat, five soil samples were collected along one diagonal using an auger (5 cm diameter). Soil samples were divided into 0–5, 5–10 and 10–20 cm depth increments, and then the five samples from each depth increment were mixed to form one composite sample. Soil samples were air-dried, then sieved through a 2 mm screen to remove coarse material before further analyses. Particle size was analyzed by the pipette method; soil pH was measured in 1:5 soil:water suspensions; soil organic matter was measured using the K2Cr2O7 method (Agriculture Chemistry Specialty Council, Soil Science Society of China 1983). Total N was measured using the Kjeltec system 1026 Distilling Unit (Tecator AB, Höganäs, Sweden). Five neutron probe access tubes previously installed at the two sites in April 2003 (Zhang et al. 2008b) were used to monitor deep soil water content.
To measure total soil respiration rates (RT), five A. ordosica shrubs were selected randomly within the A. ordosica stand at WBEF. Sampled individuals were similar in size and averaged 0.66 m in height and 1.15 × 1.07 m in crown size. For each of the five A. ordosica shrubs, three PVC collars were inserted 3 cm into the soil, with one collar each at 10, 50 and 100 cm from the sample shrub trunk (corresponding to beneath shrub’s crown, at the edge of shrub’s crown, and within the interspace between shrubs). BSCs were included in each PVC collar, and BSC cover at the A. ordosica stand in July 2010 was not significantly different among the microsites at 10, 50, and 100 cm from shrub trunks. This sampling design resulted in a maximum of five measurements of RT at three different spatial locations within the vegetated sand-stabilized area. In addition, five PVC collars were installed in unvegetated locations at MSD prior to measurement. Note that total CO2 flux from soil at the A. ordosica stand (RT) has both rhizosphere (RR) and soil basal (RB) components (as described in the Introduction), but total soil respiration measurements at MSD (denoted as RS in this paper) is assumed to have only a heterotrophic microbial component because plant roots are sparse and care was taken to select sampling locations without plants or BSCs.
To estimate soil basal respiration (RB, including heterotrophic soil respiration and respiration by autotrophic BSCs), a plot was established in September 2005 at the WBEF that consisted of a 1 × 4 m root-isolation plot (hereafter referred to as “RIP”; Figure S1). One m deep trenches were excavated along the four sides of the plot, and nylon nets with 300 meshes per cm2 were placed along the trench walls and buried to prevent roots of plants outside the RIP from penetrating into the soil volume of the RIP. Aboveground biomass of shrubs and annuals were completely removed from the RIP, and any subsequent plant regrowth was removed weekly to maintain a completely barren plot. Five PVC collars were placed in the RIP, and a neutron probe access tube also was installed to monitor deep soil water content.
Soil respiration rates were measured using the LI-6400-09 soil chamber (Li-Cor, Inc., Lincoln, Nebraska, USA), beginning 1 year after establishment of the root-isolation plot. From September 2006 to April 2008, daily courses of soil respiration rates were measured nearly once a month, and a total of 19 daily courses were conducted. During warmer months (April to September), daily courses of soil respiration rate were measured during the entire day (daytime and nighttime) at 2 h intervals beginning at predawn. During colder months (October to March), daily courses of soil respiration rate were measured at 2 h intervals beginning at predawn, but measurements only were made during daylight hours because the Li-Cor soil chamber did not function reliably at low nighttime temperatures (< 0 °C). Three to five shrubs were randomly selected on each sampling date, and soil respiration rates were measured at each canopy location for those shrubs.
During the study, photosynthetic active radiation (PAR), air temperature (Ta), and soil temperature at 2.5 cm depth (T2.5, with 6000-09TC soil temperature probe) also were measured. Shallow soil water content (θS, the 0–20 cm portion of the soil profile) was measured using a Theta probe (HH2, Delta-T Devices, Cambridge, UK). Soil water contents at 30, 50, 70, 90, 120, 150, 180 and 210 cm depth were measured twice monthly using a neutron probe (CNC503DR, Beijing Nucleon Instrument Company, Beijing, China). Deep soil water content (θD, the 30–210 cm portion of the soil profile) was calculated as the average water content over all eight depths. Rainfall was recorded with a siphon rain gauge.
Data processing and modeling soil respiration
Daily soil respiration rates were calculated with a trapezoidal integration method. To estimate nighttime soil respiration during the winter (October to March) when cold temperatures precluded Li-Cor measurements, first and last measurements of daily courses (i.e. the values measured at predawn and evening) were averaged and assumed to represent soil respiration rate for the entire nighttime. Total respiration rate (RT) in the A. ordosica stand was calculated at the landscape level by a weighting method, where soil respiration rate at each canopy location was weighted by its proportional cover across the landscape. Weighting coefficients for locations beneath crown, at edge of crown, and interspace among shrubs were 0.1, 0.4 and 0.5, respectively. Rhizosphere respiration rate (RR) in the A. ordosica stand was calculated by subtracting RB from RT. Daily averaged shallow soil water contents (θS), PAR, Ta, and T2.5 for the A. ordosica stand were calculated with the weighted average method.
Based on previous studies of how environmental factors influence soil respiration (Vargas and Allen 2008; Almagro et al. 2009; Castillo-Monroy et al. 2011), we modeled the dependency of soil respiration rate on environmental factors using the following relationships:
where R is the measured soil respiration rate (μmol m-2 s-1), T2.5 is soil temperature at 2.5 cm depth (°C), θS and θD are shallow and deep soil water contents (m3 m-3), PAR is photosynthetic active radiation (μmol m-2 s-1), and β0, β1, β2 and β3 are model parameters. Equations (1)–(5) were fit to each of the daily integrated values of soil respiration (e.g. RS, RB, RT, RR and RR/RT,). In addition, Eqs. (1) and (6) were used to model the dependency of R on T2.5 and PAR during daily courses of soil respiration that were measured in April, June, August and October 2007 at moving sand dunes (MSD), a root-isolation plot (RIP), and three canopy locations [interspaces among shrubs (Space), edge of shrub (Edge) and beneath shrub (Under)]. Equation (6) was a linear model based on PAR to explain the daily temperature-independent variation in soil respiration as described by Vargas and Allen (2008). The best model to predict R was selected based on the root mean squared error (RMSE) and the Akaike information criterion (AIC), which is a penalized likelihood criterion (Burnham and Anderson 2002):
where L is the likelihood of the fitted model, and p is the total number of parameters in the model. The best statistical model minimized the value of AIC.
Statistical analyses
Because our experimental design only had one plot for each type of site (MSD, RIP, and A. ordosica stand), the use of individual sample locations within a plot to compare sites would typically be considered as pseudo-replication (Hurlbert 1984). However, auto-correlation analyses indicated that each collar was an independent unit, and thus statistical comparisons among sites using individual sample locations were valid. Nonetheless, we have limited statistical justification to extrapolate from our three sites to other similar sites, although individual plots used in our studies were representative of each type of site near Shapotou.
A general linear model (GLM) was used to compare soil respiration rates among sample locations in the A. ordosica stand. In the models, integrated daily and maximum soil respiration rates were dependent factors, and location was a fixed factor. To account for any inherent differences in environment among plots that may influence soil respiration, corresponding θS, PAR, Ta, and T2.5 were included in the GLM as covariate factors. Similarly, GLM also was used to compare differences among sites (RS at MSD, RB at RIP and weighted RT at A. ordosica stand). Multivariate GLM was used to compare θS, Ta and T2.5 among locations and among sites, with location and sites as the respective fixed factors. Finally, differences in soil physiochemical properties between MSD and the A. ordosica stand were analyzed with one-way ANOVA.
To compare seasonal variation of soil respiration rates and environmental factors, split-plot ANOVAs were used, with month as the main plot factor, with location or site as the split-plot factor, and with daily integrated and maximum soil respiration rates and corresponding environmental factors as dependent variables. Differences in deep soil water contents (θD) among three sites were analyzed with repeated measures ANOVA. For all ANOVAs, multiple comparisons of means were conducted at α = 0.05 level using Tukey’s post hoc test when equal variance occurred and Tamhane's T2 test when the assumption of equal variance was not satisfied.
Pearson’s correlations between RT, RB, RR, and ratio of RR to RT (RR/RT) with PAR, Ta, T2.5, θS, and θD also were performed. Data were transformed as needed to ensure that they met normal distribution requirements. Statistical analyses and graphic plotting were conducted using SPSS 13.0 for Windows (SPSS, Chicago, IL, USA) and Origin 7.0 (OriginLab Corporation, Northampton, MA, USA), respectively.
Results
Effects of sand-stabilization on soil respiration and environmental factors
Stabilization of moving sand dunes with straw checkerboards and followed by shrub establishment altered soil physicochemical properties, particularly for the top 10 cm of soil (Table 1). Silt content, clay content, soil organic matter and total N were significantly greater in the A. ordosica stand than MSD for the 0–5 and 5–10 cm soil depth increments, and the trend for these soil properties in the 10–20 cm depth increment was usually similar. For topsoil (0–5 cm), soil organic matter and total N at the A. ordosica stand was about 10 and 20 times greater, respectively, than MSD. Sand content of the A. ordosica stand consistently was lower than MSD at all depth increments, although differences were significant only for the 0–5 and 5–10 cm depth increments. Soil pH was not significantly changed by sand stabilization.
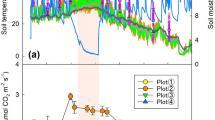
Both sand-stabilization and shrub restoration significantly increased daily integrated and maximum soil respiration rates (Table 2, Fig. 1). Daily integrated soil respiration rate at MSD averaged 0.088 ± 0.012 μmol CO2 m-2 s-1. After 18 years of stabilizing moving sand dunes with straw checkerboards and shrubs, daily soil respiration rate increased 2.3 fold to 0.199 ± 0.045 μmol CO2 m-2 s-1 at RIP and 3.2 fold to 0.284 ± 0.079 μmol CO2 m-2 s-1 at the A. ordosica stand (Fig. 1). Daily integrated soil respiration also significantly varied among the three microsite locations within the A. ordosica stand, with greater soil respiration rates for locations nearer to the base of a shrub (Table 2, Fig. 1). Maximum soil respiration rates showed similar trends among sites and locations, but maximum rates were not significantly different between RIP and A. ordosica stand (Fig. 1).
Daily and maximum soil respiration rate and averaged air temperature (Ta), soil temperature at 2.5 cm depth (T2.5) and shallow soil water contents (θS) at moving sand dunes (MSD), a root-isolation plot (RIP) and A. ordosica stand, and three canopy locations [interspaces among shrubs (Space), edge of shrub (Edge) and beneath shrub (Under)] in the A. ordosica stand. Different lower case letters and upper case letters indicate significant differences among locations and sites at α = 0.05 level, respectively; bars are standard errors
Although sand-stabilization and shrub restoration also significantly affected the environmental factors θS, Ta and T2.5 (Table 2), the patterns of site and location differences for these factors did not mirror those for soil respiration (Fig. 1). Average daily θS was not significantly different among the three sites or among the three microsites within the A. ordosica stand. Average daily Ta at RIP was significantly warmer than that at MSD, but the A. ordosica stand was not significantly different from the other two sites nor was average Ta significantly different among microsites within the A. ordosica stand. However for average daily T2.5, both the MSD and RIP sites were significantly warmer than the A. ordosica stand; furthermore, interspaces among shrubs (Fig. 1 “Space”) were significantly warmer than under shrub canopies (Fig. 1 “Under”).
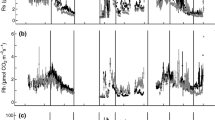
Diurnal variation of soil respiration and temperature
Soil respiration rates (R) and temperatures (T2.5) typically varied during the day (Fig. 2), although the extent of diurnal variation changed during the year as well as among locations or sites. Diurnal variations in both R and T2.5 typically were greater during the plant growing season (April to September) than during the non-growing season (October to March). However, diurnal variation in soil respiration rates at MSD was typically much smaller than at other sample locations, whereas diurnal variation in T2.5 at MSD was typically greater than at other sample locations. Thus, MSD experienced the greatest diurnal variation in temperature but the smallest variation in soil respiration.
Diurnal soil respiration rates and T2.5 for representative measurements dates during April, June, August and October 2007. Diurnal data for all measurements dates are shown in Figure S2
The correspondence of diurnal variation in R with that in T2.5 appeared to be influenced by θS. When soils were wettest (for example, June 2007 when θS exceeded 0.10 m3 m-3 at all sites and locations; field capacity is 0.11 m3 m-3 for the top 10 cm layer at the sand-stabilized area), daily courses of soil respiration rates generally corresponded with those of T2.5, and both R and T2.5 peaked in mid-afternoon (14:00) (Fig. 2). However, when soils were dryer (average θS in April, August and October 2007 were 0.089, 0.040 and 0.062 m3 m-3, respectively), soil respiration was partially decoupled from soil temperature, and a hysteresis occurred between R and T2.5 during the afternoon. During the morning, both R and T2.5 increased, but soil respiration rates peaked between 10:00 and 13:00 for all locations and sites whereas soil temperature continued to increase into the afternoon. Thus, soil respiration rate at a particular soil temperature during the morning, which occurred while soil temperatures were increasing, was greater than corresponding points during the afternoon, which occurred while soil temperatures were decreasing. For example in August 2007 at RIP, T2.5 at 10:00 was similar to that at 14:00 (both were ~23 °C), but RB at 10:00 during the morning increase in soil temperature was 6 times greater than that at 14:00 during the afternoon decrease in soil temperature (0.542 ± 0.032 vs. 0.090 ± 0.019 μmol CO2 m-2 s-1).
Seasonal variation of soil respiration and environmental factors
Soil respiration rates and environmental factors significantly varied among months (Table S1). Daily integrated soil respiration rates (RS, RB, RT, and RR) had similar seasonal trends (Fig. 3, bottom panel), with consistently low rates during the non-growing season (October – March) and higher rates during the growing season (April – September) that were punctuated by a sharp peak for all respiration rates except RS in June 2007. Both Ta and T2.5 had gradual changes during the year that corresponded with seasons, where minimum values occurred in mid-winter (January) and maximum in mid-summer (July). PAR had seasonal trends similar to that of temperature, but partial cloudiness reduced daily integrated PAR during August 2007 and April 2008. Unlike temperature and PAR, both shallow and deep soil water (θS and θD, respectively) had less predictable seasonable patterns and were affected by precipitation. During spring and early summer, small rain events were sufficient to increase θS, but during late summer and early autumn when temperatures and PAR (i.e. two important components of potential evapotranspiration) were high, even large rain events did not increase θS for long time periods. For θD, large rain events are needed to increase soil water content (e.g. June and September 2007).
Seasonal variations of PAR, Ta, T2.5, rainfall, θS, θD and daily integrated soil respiration rates. PAR, Ta, T2.5, and θS observed at mid-morning were used to create the graph. For convenient reference in the soil respiration panel, a dotted line is shown where soil respiration rate equals to zero. RS: soil respiration rate at MSD, RB: basal soil respiration rate at RIP, RT: total soil respiration rate at A. ordosica stand, RR: rhizosphere respiration rate at A. ordosica stand
During the plant growing season, the ratio of RB to RT (RB/RT) in the A. ordosica stand averaged 66.8 ± 2.1 %, and monthly averages consistently exceeded 50 %. In contrast, the ratio of RR to RT (RR/RT) averaged 33.2 ± 2.1 %. Thus, the majority of CO2 efflux from the soil in a sand-stabilized area with age of 18 years was from soil basal respiration. Interestingly, RR/RT peaked twice during the year, in June and in September (Fig. 4).
Daily integrated and maximum soil respiration rates also were significantly different among locations and sites. During the plant growing season (April – September), soil respiration rates were greater near shrubs (Figure S3). During the non-growing season (October – March), however, soil respiration rates beneath shrubs were not always greater than those in the interspaces, and rates in the A. ordosica stand were not always greater than those in RIP, especially during November and December 2007.
Location and site were not always significant in the ANOVAs for environmental factors (Table S1). T2.5 was consistently significant among locations or sites and both PAR and Ta were often significantly different among locations or sites, but θS was not significantly different among locations and among sites. Thus, results shown in Fig. 1 that average over the entire study period were representative of observations in each month. However, significant differences among sites for deep soil water contents (θD) varied during the study. MSD had greater θD than the A. ordosica stand over the entire study experimental period. However, θD at RIP was significantly greater than that at MSD before April 2007 and greater than that at A. ordosica stand before July 2007. After those dates, θD at RIP was no longer significantly different from MSD and the A. ordosica stand.
Correlation between different components of soil respiration and environmental variables
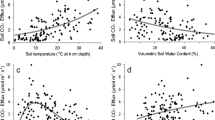
RS, RB, RR and RT and ratios of RR to RT (RR/RT) were significantly correlated with many of the environmental variables (Table S2). Major exceptions were the lack of significant correlations between θD and both RS and RB and between PAR and RR. Pearson’s correlation coefficients for RT and RR were greatest with θS compared to other environmental factors, and θS was second greatest for RS and RB, suggesting strong correlations between surface soil water and all components of soil respiration. Interestingly, Pearson’s correlation coefficient for RR/RT was greatest with θD.
The two best models to explain seasonal variation of RS, RB and RT were Eqs. (2) and (3), i.e. functions that included θS only or both θS and T2.5. Including T2.5 in the model (i.e. Eq. (3)) increased R2 values over the model with only θS (Eq. (2)) for most soil respiration components, although AIC scores of Eq. (3) were not always smaller than those of Eq. (2). The two best models to explain seasonal variations of RR and RR/RT were the equations with only θD as a variable (Eq. 4) or with both θD and T2.5 (Eq. 5) (Table S3).
When θS was high and diurnal changes in R were concordant with diurnal changes in soil temperature during June 2007, Eq. (1) had the lowest AIC and thus was the best model to explain diurnal changes in soil respiration (R2 ranged 0.51–0.84 for all sites and location). However, when θS was low or diurnal changes in R were decoupled from diurnal changes in soil temperature during August and October 2007, a model including PAR (Eq. 6) better represented the observed diurnal changes in soil respiration with R2 values between 0.41 and 0.99 (P < 0.001), increasing predictability than if only temperature (Eq. 1) was used. For diurnal changes observed during April 2007 when θS was moderate, Eq. (1) had the lower AIC in some cases, but Eq. (6) typically had higher R2 values. Considering the hysteresis often observed between soil respiration and soil temperature, Eq. (6) is generally the better model to predict diurnal changes in soil respiration.
Discussion
Effects of revegetation and succession on soil respiration
Total soil CO2 flux (RT) increased greatly after sand dune stabilization with straw checkerboards and xerophytic shrubs. Although our measurements of RT were generally smaller than those reported in other deserts (Raich and Schlesinger 1992; Fernandez et al. 2006; Almagro et al. 2009; Zhang et al. 2010; Cable et al. 2011; Talmon et al. 2011), RT at our sand-stabilized area with age of 18 years was 3.2 fold greater than that of moving sand dunes. Furthermore, both components of RT, rhizosphere respiration (RR) and soil basal respiration (RB), also increased with sand stabilization. As plant cover increased from <1 % at the moving sand dunes to ~31 % at the sand-stabilized area dominated by A. ordosica, RR increased from almost zero to an average 0.09 μmol CO2 m-2 s-1. In addition, RB, which included soil respiration both from heterotrophic microbes and from autotrophic BSCs, increased 2.3 fold with sand stabilization. Although RR often was an important component of RT in water-limited ecosystem (Tang and Baldocchi 2005; Wang et al. 2005; Jia et al. 2006; Chen et al. 2009; Bao et al. 2010), the contribution of RR to RT averaged 33 % in the sand-stabilized area, whereas the contribution of RB to RT was typically twice that of RR. Thus as we predicted, a greater proportion of the increased soil CO2 flux with sand stabilization was due to respiration from heterotrophic soil microbes and autotrophic BSCs than from root systems of sand-fixed shrubs.
The large increase in RB in our study was likely due to both increased heterotrophic microbial respiration and increased BSCs respiration. Although other soil respiration studies removed or avoided BSCs in their experimental designs (McCulley et al. 2007; Sponseller 2007; Berg and Steinberger 2008; Thomas and Hoon 2010; Cable et al. 2011; Castillo-Monroy et al. 2011), BSCs were not removed or avoided in our study because BSCs were an important component of the sand-stabilization process (Li et al. 2004, 2007) and because BSCs removal would have greatly disturbed the soil and confounded our measurements. Thus, our study was not designed to partition heterotrophic microbial respiration from BSCs respiration; only to partition RR and RB. Nonetheless, results from our studies coupled with evidence in the literature indicated high likelihood for increased heterotrophic and BSCs respiration, as discussed below.
Factors such as increased C input from restored vegetation and improvements of edaphic properties on sand-fixed dunes could have increased heterotrophic soil respiration. Soil microbial activity in desert ecosystems such as the Mojave Desert of western North America are C limited, and additional C inputs into these desert ecosystems increase soil respiration (Schaeffer et al. 2003, 2007; Billings et al. 2004). In addition, stabilization and revegetation of moving sand dunes promote the capacity of dust entrenchment (Fearnehough et al. 1998), increase substrate availability (Li et al. 2004; Zhang et al. 2008b, 2009a), and improve topsoil physiochemical properties (Table 1, Li et al. 2007). Together, these changes result in greater soil respiration in shrub dominated areas (Maestre and Cortina 2003; Tang and Baldocchi 2005; McCulley et al. 2007; Sponseller 2007; Berg and Steinberger 2008; Talmon et al. 2011). Because similar increased C input and improved edaphic properties also occurred in our sand-stabilized areas (Table 1), increased heterotrophic microbial respiration likely occurred.
The extent that BSCs contribute to increase RB in the sand-stabilized area is more difficult to assess because BSCs are autotrophic and hence reduce soil CO2 efflux during periods when BSCs are photosynthetically active. Although maximum photosynthetic rates of BSCs can rival that of vascular plants occupying the same ecosystem, BSCs net photosynthesis is restricted to infrequent and short periods when they are hydrated following sufficient rainfall (Lange 2001). Thus, RB was likely underestimated in our study. Nonetheless, a study from a Mediterranean semiarid ecosystem where BSCs cover was similar to our sand-stabilized area reported that 42 % of annual soil CO2 efflux was from BSCs dominated microsites (Castillo-Monroy et al. 2011). Furthermore, BSCs can be a substantial input of C into desert ecosystems (Evans and Lange 2001), and this C from BSCs often becomes available to heterotrophic soil microbes. Clearly, BSCs cover increased greatly after sand stabilization (Li et al. 2004, 2007). Thus, BSCs were likely direct and indirect contributors to increased soil respiration in sand-stabilized dunes.
Increased shrub cover in the sand-stabilized likely had both direct and indirect effects on soil respiration and its components, and vegetation patchiness resulted in small-scale spatial heterogeneity of soil respiration. Previous studies at our sites showed that coarse root biomass increased near shrubs (Zhang et al. 2008b, 2009a), which was consistent with our observations of greater RR in the sand-stabilized area as well as greater RR near shrubs. Furthermore, studies from water-limited ecosystem (Titus et al. 2002) showed that shrubs create fertile islands beneath shrub crowns, which improved edaphic properties conducive to increased heterotrophic microbial respiration, as discussed above. Finally, shrubs ameliorate microclimate (Fullen and Mitchell 1994), which in turn can affect soil respiration by modifying soil temperature and soil water contents (Raich and Tufekcioglu 2000; Maestre and Cortina 2003; Berg and Steinberger 2008; Rey et al. 2011; Talmon et al. 2011). In our study, shrubs typically influenced soil and air temperatures but had little effect on shallow soil water contents. With sparse crown and low leaf area index, A. ordosica has little effect on soil evaporation under its canopy (Zhang et al. 2008a) as well as intercepts only ~6 % of rainfall (Zhang et al. 2009b) and therefore has little influence on soil water balance beneath its crown. However, A. ordosica shrubs provide sufficient shade to decrease soil temperature beneath their crown compared to interspaces or the root-isolation plot, especially in winter. This combination of lower temperature but similar soil water under shrubs led to smaller soil respiration under shrubs than in interspaces during winter (Fig. 1, Table S3). Nonetheless, the variation among our and previously published studies (Maestre and Cortina 2003; Berg and Steinberger 2008; Rey et al. 2011) in how soil respiration differed between shrub and interspaces microsites indicated that plant crowns in arid ecosystems do not have consistent effects on soil CO2 efflux because of different influences on soil temperature and shallow soil water contents, especially in winter. Given the importance of soil respiration in C cycles, the effects of shrubs on the carbon cycle in arid ecosystems need more thorough investigations.
Although RB contributed approximately 2-fold more to total soil respiration than RR at 18 years after sand stabilization, we suspected that these relative contributions changed during succession of plant-soil systems after establishment of sand-stabilized shrubs. For example, shrub biomass and cover in sand-fixed areas peaked about 15 years after restoration and then gradually degraded due to reductions in deep soil moisture (Li et al. 2004). Thus, we expected that RR also initially increased after revegetation, then decreased after peak cover at 15 years. Over the same period, the depth of BSCs and subsoil also gradually increased as sand-fixed dunes aged (Li et al. 2004, 2007), which led to increased respiration from heterotrophic soil microbes and BSCs through time. Given decreased plant abundance and its likely decreased root abundance coupled with the gradual change in soil surface structure, continued succession of plant-soil system in sand-fixed dunes will gradually increase the contribution of RB to RT and decrease that of RR to RT. Finally, despite the large increase in soil CO2 efflux with sand stabilization and revegetation, the capacity of carbon sequestration likely increased as these sand-fixed dunes aged because the 10-fold increase in soil organic matter was much greater than the 3-fold increase in soil respiration.
Temporal variation of soil respiration
Seasonal variations of soil respiration rates in our study sites (MSD, RIP and A. ordosica stand) appeared to be more affected by soil water content than by temperature. Both soil respiration and θS had very similar seasonal trends (Fig. 3), and the correlation coefficient between them was greater than those between soil respiration rates and either Ta or T2.5 (Table S4). More importantly, seasonal patterns of RS, RB and RT were best modeled by functions that combine θS and T2.5. However, θS alone explained much of the variation in RS, RB and RT, and models with θS alone had AIC scores nearly as small, or in some cases such as RT, smaller than equations with both θS and T2.5 (Table S3). Results from other arid and semiarid ecosystems were similar (Maestre and Cortina 2003; Fernandez et al. 2006; McCulley et al. 2007; Jia et al. 2006; Wilske et al. 2008; Almagro et al. 2009; Chen et al. 2009; Thomas and Hoon 2010; Rey et al. 2011; Shi et al. 2011; Talmon et al. 2011), suggesting that soil respiration was triggered by moisture and then further influenced by temperature (Maestre and Cortina 2003; Wilske et al. 2008).
Seasonal courses of RR and RR/RT were significantly correlated with θD, and θD alone explained most of their seasonal variations (Table S3). Thus, the contribution of RR to RT was strongly influenced by θD. Other studies also found relationships between RR and soil water (Chen et al. 2009; Shi et al. 2011). In addition, RR/RT had very similar seasonal trends as fine roots during the growing season at the site (Zhang et al. 2008b, 2009a); both peaked in June and September with lowest values in July. Other studies in arid or semiarid lands also found that RR corresponds well with root biomass (Wang et al. 2005; Jia et al. 2006; Vargas and Allen 2008; Bao et al. 2010; Shi et al. 2011). Thus, θD likely affected the seasonal dynamics of RR and RR/RT through effects of θD on fine root growth.
For daily courses of soil respiration, soil respiration and T2.5 had similar diurnal changes only when θS was close to field capacity. However during most of the year (i.e. at moderate and low soil water conditions), soil respiration decoupled from T2.5, and soil respiration rates were always higher during increasing temperatures in the morning than during decreasing temperatures in the afternoon. A function with T2.5 alone was enough to explain most diurnal changes in soil respiration at high soil moisture condition. However, when soil respiration decoupled from temperature at low and moderate soil water conditions, diurnal changes in soil respiration were predicted well by diurnal changes in both temperature and PAR, as observed by Vargas and Allen (2008). These results further emphasized that soil respiration links to biotic processes, such as linkage between microbial decomposition or root respiration with photosynthesis (McCulley et al. 2007; Vargas and Allen 2008).
Conclusion
After establishment of straw checkerboards and shrubs to stabilize moving sand dunes in the Tengger Desert of northern China, soil CO2 efflux increased. At 18 years after sand stabilization, soil basal respiration contributed ~2/3 to total soil respiration. Shrubs patchiness in sand-stabilized areas produced spatial variations in soil respiration, whereas temporal courses in soil respiration were affected mainly by soil water content. Total and basal respiration rates were more affected by soil water content in upper soil layers (0–20 cm layer), whereas rhizosphere respiration was influenced by deep soil water content (30–210 cm layer). Diurnal changes in soil respiration rates during most of the year when soil water contents were below field capacity were partially decoupled from soil temperature, but diurnal changes in soil respiration could be modeled by both temperature and PAR. Despite increased soil respiration rates, the sand-stabilized area had sequestered additional soil C. Circumstantial evidence indicated that BSCs are major contributors to increased soil respiration and that the relative contribution of RB to RT has increased in the past and is likely to continue increasing into the future, but these results need further study.
Abbreviations
- MSD:
-
Moving sand dunes
- A. ordosica stand:
-
The sand-stabilized area planted with Artemisia ordosica Krasch. in 1989
- BSCs:
-
Biological soil crusts
- RIP:
-
Root-isolation plot
- Space:
-
Interspaces among shrubs
- Edge:
-
Edge of shrub
- Under:
-
Beneath shrub
- R:
-
Soil respiration rate
- RR :
-
Rhizosphere respiration at sand-stabilized area
- RB :
-
Soil basal respiration at sand-stabilized area
- RT :
-
Total soil respiration rate at sand-stabilized area
- RS :
-
Total soil respiration rate at moving sand dunes
- RB/RT :
-
Ratio of RB to RT
- RR/RT :
-
ratio of RR to RT
- θS :
-
Shallow soil water content (0–20 cm)
- θD :
-
deep soil water content (30–210 cm)
- T2.5 :
-
Soil temperature at 2.5 cm depth
- Ta :
-
Air temperature
- PAR:
-
Photosynthetic active radiation
References
Agriculture Chemistry Specialty Council, Soil Science Society of China (1983) General analysis methods of soil agriculture chemistry. Science Press, Beijing
Almagro M, López J, Querejeta JI, Martínez-Mena M (2009) Temperature dependence of soil CO2 efflux is strongly modulated by seasonal patterns of moisture availability in a Mediterranean ecosystem. Soil Biol Biochem 41:594–605
Bao F, Zhou GS, Wang FY, Sui XH (2010) Partitioning soil respiration in a temperate desert steppe in Inner Mongolia using exponential regression method. Soil Biol Biochem 42:2339–2341
Belnap J, Welter JR, Grimm NB, Barger N (2005) Linkages between microbial and hydrologic processes in arid and semi-arid watersheds. Ecology 86:298–307
Berg N, Steinberger Y (2008) Role of perennial plants in determining the activity of the microbial community in the Negev Desert ecosystem. Soil Biol Biochem 40:2686–2695
Billings S, Schaeffer SM, Evans RD (2004) Soil microbial activity and N availability with elevated CO2 in Mojave Desert soils. Global Biogeochem Cycles 18:GB1011. doi:10.1029/2003GB002137
Burnham KP, Anderson DR (2002) Model selection and inference. A practical information theoretic approach. Springer, Berlin Heidelberg New York
Cable JM, Ogle K, Williams DG, Weltzin JF, Huxman TE (2008) Soil texture drives responses of soil respiration to precipitation pulses in the Sonoran Desert: implications for climate change. Ecosystems 11:961–979
Cable JM, Ogle K, Lucas RW, Huxman TE, Loik ME, Smith SD (2011) The temperature responses of soil respiration in deserts: a seven desert synthesis. Biogeochemistry 103:71–90
Castillo-Monroy AP, Maestre FT, Rey A, Soliveres S, García-Palacios P (2011) Biological soil crust microsites are the main contributor to soil respiration in a semiarid ecosystem. Ecosystems 14:835–847
Chen S, Lin G, Huang JH, Jenerette GD (2009) Dependence of carbon sequestration on the differential responses of ecosystem photosynthesis and respiration to rain pulses in a semiarid steppe. Global Change Biol 15:2450–2461
Cheng W, Fu S, Susfalk RB, Mitchell RJ (2005) Measuring tree root respiration using 13C natural abundance: rooting medium matters. New Phytol 167:297–307
Evans RD, Lange OL (2001) Biological soil crusts and ecosystem nitrogen and carbon dynamics. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function and management. Ecological studies series. Springer Verlag, New York, pp 263–279
Fearnehough W, Fullen MA, Mitchen DJ, Trueman IC, Zhang J (1998) Aeolian deposition and its effect on soil and vegetation changes on stabilized desert dunes in northern China. Geomorphology 23:171–182
Fernandez DP, Neff JC, Belnap J, Reynolds RL (2006) Soil respiration in the cold desert environment of the Colorado Plateau (USA): abiotic regulators and thresholds. Biogeochemistry 78:247–265
Fullen MA, Mitchell DJ (1994) Desertification and reclamation in north-central China. Ambio 23:131–135
Hanson PJ, Edwards NT, Garten CT, Andrews JA (2000) Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 48:115–146
Hurlbert SH (1984) Pseudoreplication and the design of ecological field experiments. Ecol Monogr 54:187–211
Jia B, Zhou G, Wang F, Wang Y, Yuan W, Zhou L (2006) Partitioning root and microbial contributions to soil respiration in Leymus chinensis populations. Soil Biol Biochem 38:653–660
Lange OL (2001) Photosynthesis of soil-crust biota as dependent on environmental factors. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function and management. Ecological studies series. Springer Verlag, New York, pp 217–240
Li XR, Ma FY, Xiao HL (2004) Long-term ecosystem effects of vegetation on soil water content of sand dunes in arid region of Northern China. J Arid Environ 57:1–16
Li XR, He MZ, Duan ZH, Xiao HL, Jia XH (2007) Recovery of topsoil physicochemical properties in revegetated sites in the sand-burial ecosystems of the Tengger Desert, northern China. Geomorphology 88:254–265
Li XR, He MZ, Stefan Z, Li XJ, Liu LC (2010) Micro-geomorphology determines community structure of biological soil crusts at small scales. Earth Surf Proc Land 35:932–940
Maestre FT, Cortina J (2003) Small-scale spatial variation in soil CO2 efflux in a Mediterranean semiarid steppe. Appl Soil Ecol 23:199–209
McCulley RL, Boutton TW, Archer SR (2007) Soil respiration in a subtropical savanna parkland: Response to water additions. Soil Sci Soc Amer J 71:820–828
Raich JW, Schlesinger WH (1992) The global carbon dioxide flux in soil respiration and its relation to vegetation and climates. Tellus 44:81–99
Raich JW, Tufekcioglu A (2000) Vegetation and soil respiration: correlations and controls. Biogeochemistry 48:71–90
Rey A, Pegoraro E, Oyonarte C, Werea A, Escribanob P, Raimundo J (2011) Impact of land degradation on soil respiration in a steppe (Stipa tenacissima L.) semi-arid ecosystem in the SE of Spain. Soil Biol Biochem 43:393–403
Schaeffer SM, Billings SA, Evans RD (2003) Responses of soil nitrogen dynamics in a Mojave Desert ecosystem to manipulations in soil carbon and nitrogen availability. Oecologia 134:547–553
Schaeffer SM, Billings SA, Evans RD (2007) Laboratory incubations reveal potential responses of soil nitrogen cycling to changes in soil C and N availability in Mojave Desert soils exposed to elevated atmospheric CO2. Global Change Biol 13:854–865
Shi WY, Tateno R, Zhang JG, Wang YL, Norikazu Y, Du S (2011) Response of soil respiration to precipitation during the dry season in two typical forest stands in the forest–grassland transition zone of the Loess Plateau. Agr Forest Meteorol 151:854–863
Sponseller RA (2007) Precipitation pulses and soil CO2 flux in a Sonoran Desert ecosystem. Global Change Biol 13:426–436
Talmon Y, Sternberg M, Grünzweig J (2011) Impact of rainfall manipulations and biotic controls on soil respiration in Mediterranean and desert ecosystems along an aridity gradient. Global Change Biol 17:1108–1118
Tang J, Baldocchi DD (2005) Spatial–temporal variation in soil respiration in an oak–grass savanna ecosystem in California and its partitioning into autotrophic and heterotrophic components. Biogeochemistry 73:183–207
Thomas AD, Hoon SR (2010) Carbon dioxide fluxes from biologically-crusted Kalahari Sands after simulated wetting. J Arid Environ 74:131–139
Titus JH, Nowak RS, Smith SD (2002) Soil resource heterogeneity in the Mojave Desert. J Arid Environ 52:269–292
Vargas R, Allen MF (2008) Environmental controls and the influence of vegetation type, fine roots and rhizomorphs on diel and seasonal variation in soil respiration. New Phytol 179:460–471
Wang W, Ohse K, Liu J, Mo WH, Takehisa O (2005) Contribution of root respiration to soil respiration in a C3/C4 mixed grassland. J Biosciences 30:507–514
Wilske B, Burgheimer J, Karnieli A, Zaady E, Andreae MO, Yakir D, Kesselmeier J (2008) The CO2 exchange of biological soil crusts in a semiarid grass-shrubland at the northern transition zone of the Negev desert. Israel Biogeosciences 5:1411–1423
Zaady E, Kuhn U, Wilske B, Sandoval-Sotob L, Kesselmeier J (2000) Patterns of CO2 exchange in biological soil crusts of successional age. Soil Biol Biochem 32:959–966
Zhang ZS, Liu LC, Li XR, Zhang JG, He MZ, Tan HJ (2008a) Evaporation properties of a revegetated area of the Tengger Desert, North China. J Arid Environ 72:964–973
Zhang ZS, Li XR, Wang T, Wang XP, Xue QW, Liu LC (2008b) Distribution and seasonal dynamics of roots in a revegetated stand of Artemisia ordosica Kracsh. in the Tengger Desert (North China). Arid Land Res Manag 22:195–211
Zhang ZS, Li XR, Liu LC, Jia RL, Zhang JG, Wang T (2009a) Distribution, biomass, and dynamics of roots in a revegetated stand of Caragana korshinskii in the Tengger Desert, northwestern China. J Plant Res 122:109–119
Zhang ZS, Li XR, Dong XJ, Jia XH, He MZ, Tan HJ (2009b) Rainfall interception by sand-stabilizing shrubs related to crown structure. Sciences in Cold and Arid Regions 1:0107–0119
Zhang LH, Chen YN, Zhao RF, Li WH (2010) Significance of temperature and soil water content on soil respiration in three desert ecosystems in Northwest China. J Arid Environ 74:1200–1211
Acknowledgements
This research was financially supported by the Chinese National Natural Sciences Foundation (Grant No. 40971033 and 31170385) and the Innovation Project from the Chinese Academy of Sciences (KZCX2-EW-301-3). RSN gratefully acknowledges support from the Nevada Agricultural Experiment Station. We appreciate Prof. Shiping Wang for reviewing the manuscript. We thank two anonymous reviewers and Dr. Elizabeth M Baggs for valuable comments that greatly improved the presentation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Photos showing the root-isolation plot (upper) and the A. ordosica stand (lower) with grayish biological soil crusts. (DOC 151 kb)
Figure S2
Soil temperature at 2.5 cm depth (T2.5) and soil respiration rates (R) from September 2006 to April 2008 at moving sand dunes (MSD), a root-isolation plot (RIP), and three canopy locations [interspaces among shrubs (Space), edge of shrub (Edge) and beneath shrub (Under)] in an area where sand was stabilized in 1989 (A. ordosica stand). (DOC 185 kb)
Figure S3
Seasonal variations of daily and maximum soil respiration rates at three locations (Space, Edge and Under) and three sites (MSD, RIP and A. ordosica stand). (DOC 238 kb)
Table S1
Results from two-way ANOVA of seasonal variations in environmental factors and in daily integrated and maximum soil respiration rates. (DOC 52.0 kb)
Table S2
Pearson correlation coefficients between soil resperation rates and envirnomental factors. (DOC 16.0 kb)
Table S3
Results of regression analyses relating seasonal changes in daily integrated soil respiration rates (R) and RR/RT to T2.5, θS and θD. The equations used to fit data are (1)\( R = {\beta_0}{e^{({\beta_1}{T_{2.5}})}} \), (2)\( R = {e^{({\beta_0} + {\beta_1}{\theta_S} + {\beta_2}{\theta_S}^2)}} \), (3)\( R = {\beta_0}{e^{({\beta_1}{T_{2.5}})}}{e^{({\beta_2}{\theta_S})}} \), (4)\( R = {e^{({\beta_0} + {\beta_1}{\theta_D} + {\beta_2}{\theta_D}^2)}} \) and (5)\( R = {\beta_0}{e^{({\beta_1}{T_{2.5}})}}{e^{({\beta_2}{\theta_D} + {\beta_3}{\theta_D}^2)}} \). (DOC 63.0 kb)
Table S4
Results of regression analyses relating diurnal changes in R to T2.5 and PAR for daily courses measured in April, June, August and October 2007 at MSD, RIP, Space, Edge and Under. The equations used to fit data are (1)\( R = {\beta_0}{e^{({\beta_1}{T_{2.5}})}} \) and (6)\( R = {\beta_0}{e^{({\beta_1}{T_{2.5}})}} + {\beta_2}(PAR + {\beta_3}) \). (DOC 88 kb)
Rights and permissions
About this article
Cite this article
Zhang, ZS., Li, XR., Nowak, R.S. et al. Effect of sand-stabilizing shrubs on soil respiration in a temperate desert. Plant Soil 367, 449–463 (2013). https://doi.org/10.1007/s11104-012-1465-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1465-3