Abstract
Pollution resulting from industrial wastewater imposes a significant threat to human life and the environment. Adsorption is recognized as a suitable tool to overcome the water contamination problem of industrial origin. Nevertheless, high costs of commercial adsorbents like activated carbon led to eco-friendly low-cost natural biosorbents such as plants, microbes (e.g. bacteria, fungi, microalgae), biomaterials (e.g. chitosan, chitin), agricultural wastes, etc. The present chapter covers biosorption as a useful technique for industrial wastewater treatment. Different types of biosorbents and mechanisms of the biosorption process are initially explained. Afterwards, regeneration of biosorbent achieved by desorption is explained, followed by a cost estimation of biosorbents for wastewater treatment. Finally, probable challenges for industrial implementation of biosorption for wastewater treatment and prospects are explained.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Developments in technology and industrialization have revolutionized the world toward an easier lifestyle but have caused various environmental problems instead. Modernization cannot occur without direct/indirect influence on the environment (Esmaeili et al. 2015; Gonçalves et al. 2017; Kunjirama et al. 2017). The foremost issue drawing the attention of researchers in the 21 century is the water contamination caused by the disposal of industrial effluents into water bodies. Textile industries, Tannery, dye industries, fertilizer industries, pharmaceutical industries, mining industries, etc., are among the important industries reported to produce a huge amount of wastewater. Industrial effluents are mainly composed of a huge amount of hazardous compounds like toxic metals (Pb, Zn, Cd, Co, Cu, As, Hg, etc.), organic contaminants, and microbial contaminants. Also, the presence of dyes and their intermediates, various salts, nitrogen, and phosphorous is confirmed in some types of industrial effluents (Sörme and Lagerkvist 2002; Sun et al. 2020).
The United States Environmental Protection Agency (US EPA) has listed the chemicals from different industries which might threaten both humans and the environment. For example, effluents of the oil industry contain considerable amounts of phenol and cobalt (Khraisheh et al. 2020). Hexavalent chromium is applied in industries such as electroplating, stainless steel, dyes, and leather tanneries is also introduced by the US EPA as one of the seventeen chemicals threatening humans (Jobby et al. 2018). Several standards have been applied to solve the problems raised by the excessive amount of water pollutants that endanger human health. For instance, in the case of heavy metals, the World Health Organization (WHO) has specified guideline values as well as the maximum permitted level of these substances in the European Union (EU) and the USA (US EPA) (Table 1).
Disposal of industrial wastewater into water bodies without undergoing proper treatment is dangerous for human health and the whole ecosystem. It may cause several deficiencies in the human body, such as carcinogenic properties, skin irritation, poor eyesight, neurotoxicity, genotoxicity, breathing difficulty, etc., for human beings and lead to degradation of the ecosystem (Saha et al. 2019). Therefore, industrial wastewater treatment containing compounds with complicated and complex structures is substantially urgent (Li et al. 2020; Karri et al. 2021; Dehghani et al. 2021).
Currently, complete treatment of wastewater is done using physical (ion exchange, membrane separation, adsorption, etc.), chemical (chemical oxidation, coagulation/flocculation, etc.), and biological (aerobic/anaerobic reactors, phytoremediation, mycoremediation) processes (Crini and Lichtfouse 2019; Sarode et al. 2019). Although the mentioned methods are useful in treating wastewater, they have their limitations. The common problems with these methods are the persistence of the applied chemicals in the treated water and the feasibility of reaction between the added chemicals leading to the generation of secondary products with unknown influence on human beings (Bhattacharjee et al. 2020). For example, oxidants such as O3, Cl2, ClO2, H2O2, and KMnO4 are used in chemical oxidation. Although this method is advantageous in terms of integration of physicochemical processes, being facile, efficient, and on-site formation of ozone, it has some drawbacks like requirement of chemicals, problems associated with oxidants (e.g. production, transport, and management), necessity for pretreatment, and dependence of process efficiency on the type of oxidant applied. Moreover, involvement of non-reusable chemicals such as coagulants, flocculants, etc. in the coagulation/flocculation method is considered as the main disadvantage of this method (Crini and Lichtfouse 2019). Adsorption is the increasingly being used technique for the treatment of wastewater owing to low cost, high removal efficiency, manageability, ability to remove different types of contaminants, and ease of regeneration (Yusof et al. 2020; Koduru et al. 2019; Putro et al. 2017; Mehmood et al. 2021; Khan et al. 2021; Ahmed et al. 2021). Activated carbon is a famous adsorbent used to remove toxic compounds from wastewater in the adsorption process (Saha et al. 2019). Due to its porous structure, high surface area, easy operation, sensitivity to toxic compounds, and high efficiency, activated carbon was selected as the first-choice adsorbent in adsorption researches for wastewater treatment (Saraswat et al. 2020; Dehghani et al. 2021; Karri and Sahu 2018). Nevertheless, the drawbacks such as high costs and limited reusability have persuaded the researchers to work on other adsorbents, which are more environmentally friendly and cost efficient (Saraswat et al. 2020; Saha et al. 2019).
Limitations with activated carbon and environmental considerations led to the development of alternative treatment approaches based on green chemistry principles, emphasising minimizing contamination by maximizing the use of biodegradable and naturally available resources. The use of such approaches in wastewater treatment is in good compliance with sustainability. Biosorption has come into existence for sustainable wastewater treatment by using different types of biomass (Bhattacharjee, Dutta, and Saxena 2020). Dead or alive microorganisms and biological substances have been used since 1980 for the treatment of wastewater contaminated by heavy metals and dyes (Atar et al. 2008; Çolak et al. 2009). Biosorption can be applied for heavy metals removal (Papirio et al. 2017), dyes (Mishra et al. 2020), pesticides (ul Haq et al. 2021), phenolic compounds (Mallek et al. 2018), etc., from wastewater. Up to the present, various agro-industrial and food wastes (chitosan, sugarcane bagasse, cocoa shell, sawdust, mungbean husk, rice husk, straw, coconut waste, coffee waste, etc.), plant materials, and microbes (algae, fungi, bacteria) have been utilized successfully as biosorbents for the treatment of wastewater (Mella et al. 2017; Nazir et al. 2019; Sahu et al. 2019).
The chapter covers biosorbents in industrial wastewater treatment. It aims at providing a good understanding of the mechanism of wastewater treatment using different types of naturally occurring biosorbents. Also, the regeneration of biosorbents, which is the foremost challenge of a good treatment procedure, is discussed in detail. To continue, costs of the treatment process needed to determine the economic feasibility of the operation were evaluated. Finally, challenges to perform the projects related to the use of biosorbents in wastewater treatment on an industrial scale were explained and discussed, while the outlook for the future of this approach was clarified.
2 Biosorption
Biosorption is defined as the capability of biological substances to accumulate toxic materials from wastewater (Fard et al. 2011). Biosorption is a physicochemical process that occurs in natural biomasses, leading to the passive binding of cellular constituents with the contaminants through biological, physical, and chemical mechanisms resulting in industrial wastewater treatment. The process has long been recognized but has appeared as an inexpensive, environmentally safe method for wastewater treatment during recent decades (Ubando et al. 2021; Eletta and Ighalo 2019; Jobby et al. 2018).
2.1 Biosorption History
The ability to live microorganisms for metal uptake from aqueous solutions was known during the eighteenth and nineteenth centuries. But it was only during the twentieth century that the ability of some living/non-living microorganisms for metal removal leading to the elimination of pollutants and their subsequent regeneration was studied by different researchers (Modak and Natarajan 1995). However, the use of dead or live microorganisms is mechanistically different. The term bioaccumulation is usually used for the live biomass, while adsorption by dead microorganisms is typically called biosorption (de Freitas et al. 2019).
Wutrich was the first one who carried out a well-organized study in 1892 reporting the interaction between fungal spores and metals. Later in 1902, the first research study on the biosorption of metals was published (Muraleedharan et al. 1991). Sewage and wastewater treatment were among the earliest technological applications of the biosorption process (Ullrich and Smith 1951). The first patent (specification number GB 1,324,358) was granted in 1973 to Mills and Sanderson for the biosorption apparatus they have designed and used for the treatment of sewage (de Freitas et al. 2019). Since then, scientists and researchers worldwide have shown different attitudes toward the biosorption process. For example, regarding heavy metals, scientists of the life sciences have studied the toxicological effects of different concentrations of heavy metals on microorganisms; while, environmental engineers and scientists used this capability of microorganisms to monitor the pollution of water by heavy metals, removal of heavy metals from the wastewaters and their subsequent recovery/regeneration (Muraleedharan et al. 1991).
2.2 Biosorption in Industrial Wastewater Treatment
Industrial wastewater should go through a chain of treatment steps before being discharged into water bodies so that the concentration of its pollutants reaches acceptable standards (Shafiq et al. 2018). Since industrial wastewater contains pollutants with various concentrations, its treatment is a rather complex task. Thus, selective removal of specific pollutants from industrial wastewater with high efficiency is challenging. Due to different affinities of the contaminants toward the adsorbent’s active sites, which rises from different electronegativity, different ionic strength, etc., various adsorptive behaviors of pollutants may be observed.
Although biosorption could be used for different purposes, its usage is strongly recommended for industrial wastewater treatment based on multitudes of reports regarding the decisive role of this technique in removing pollutants like heavy metals and some organic materials from industrial wastewater (Crini 2006). For example, in the research conducted by Adenuga et al. (2019), simultaneous removal of metal ions including Pb(II), Cd(II), and Zn(II) from industrial wastewater using the Spent Seedcake of Calophyllum inophyllum as a sustainable and low-cost biosorbent was investigated. The adsorption capability of the biosorbent was evaluated using the batch adsorption process. Also, carbonization and microwaving the biosorbent resulted in optimum operation at solution pH of 9, dosage of 10 g/L, temperature of 30 °C, and equilibrium time of 60 min. Maximum adsorption capacity for Pb(II), Cd(II) and Zn(II) was calculated as 52.63, 51.28, and 17.99 mg/g, respectively. Real industrial wastewater was tested for the effectiveness of the biosorbent, and it was found that the metal removal percentage was between 55 and 71%. In another study, removal of BOD, COD, Oil & Grease was considered in the derivatives of oil and soap in the effluents of industrial wastewater using a cost-effective environmentally benign biosorbent, namely Phragmites australis. Their results demonstrated that biosorption capacity for BOD, COD, and Oil & Grease was maximized at neutral pH. SEM image clarified that the surface texture of the plant converted from being smooth and entire into coarse and irregular after the biosorption process (Fig. 1). Furthermore, P. australis exhibited good performance in removing organic pollutants such as BOD, COD, Oil & Grease from the industrial wastewater samples (El Shahawy and Heikal 2018).
a and b SEM images of raw dried P. australis (magnification power 2500 ×), c and d SEM images of dried P. australis biomass after adsorption (magnification power 2500 × and 1000 × ) (El Shahawy and Heikal 2018)
3 Types of Biosorbents for Industrial Wastewater Treatment
Adsorbents with biological origins are called biosorbents. Biosorbents usually belong to the family of bacteria, fungi, algae, plant wastes, fruits, active sludge, and biopolymers such as chitosan. Some of the main features of commercial biosorbents include high biosorption capacity, good adsorption kinetics, proper physical properties (size, shape, etc.), cost-effectiveness and availability of biosorbents, facile separation of the biosorbents from the solution, good thermal stability and chemical resistance, strong mechanical properties, and regeneration and reusability of biosorbents (Beni and Esmaeili 2020). Following, different types of biosorbents are classified and explained by giving useful examples.
3.1 Use of Plants as Biosorbents
Biomass obtained from plants is frequently used for decontaminating synthetic and industrial effluents. The cellulosic content of plants has made them strong biosorbents for removing wastewater contaminants. So far, different plant organs like seed, leaf, root, bark, and peel are used as biosorbents in removing pollutants from wastewater (Jain et al. 2016).
3.1.1 Plant Leaves
Plant leaves are excellent biosorbents as they are cheap, biodegradable, non-toxic, and highly efficient in wastewater treatment (Anastopoulos et al. 2019; Kyzas and Kostoglou 2014). Thus, lots of research was devoted to exploring the role of this organ of plants in removing contaminants from wastewater bodies through the last two decades. Before being utilized as biosorbents, most of the leaves go through some preparation steps, including cleaning, drying, grinding, and sieving. Chemical modification may also be needed in some cases. Modification is usually done by soaking the plant leaves in acid (nitric acid, sulphuric acid, hydrochloric acid, and formic acid) or alkali (sodium hydroxide) for a specific time (often several hours) under stirring. For some plant species, chemical activation and carbonization are done instead of chemical modification to produce activated carbon out of plant leaves. Activation is achieved by chemicals such as zinc chloride, sulphuric acid, and phosphoric acid in a period ranging between 1 and 24 h.
Lots of plant species have been used as biosorbents to decontaminant wastewater from heavy metals, dyes, etc. The removal efficiency of plant leaves to remove heavy metals such as chromium, lead, cadmium, zinc, nickel, and copper was greater than 90%. For example, alkali-modified spent tea leaves were used as cost-effective biosorbents to remove Cu(II) from its containing solution. The pseudo-second-order model well represented adsorption kinetics, and equilibrium data followed Langmuir adsorption isotherm. The maximum removal efficiency was calculated as 96.12% (Ghosh et al. 2015). Removal of other pollutants like dyes, especially methylene blue, was done with approximately 80% efficiency by most leaves obtained from different plant species.
Furthermore, a high adsorption capacity was achieved using plant leaves as biosorbents. Das et al. (2020) used Butea monosperma leaf powder to remove methylene blue dye from an aqueous solution. Maximum adsorption (98.70%) was achieved at the adsorbent dosage of o.5 g L−1, pH value of 8, and contact time of 120 min. In another study, Ficcus palmate leaves were used as effective plant adsorbents to remove methylene blue. Operating conditions, including initial dye concentration of 15 mg L−1, the adsorbent dosage of 0.45 g, contact time of 80 min, the temperature of 318 K, and neutral pH, led to maximum removal efficiency of 98% (Fiaz et al. 2019).
3.1.2 Plant Seed
Plant seeds have been reported for their ability in serving as potential biosorbents for contaminants elimination from wastewater. The textile industry is a typical example for which the seeds of plants have been used as biosorbents for their treatment. For example, sunflower seed hull previously treated with alkali was used in the research performed by Oguntimein (2015) for dye removal from the effluent of textile factory (decolorization of the effluent). The Pseudo-second order model conformed well to the kinetic data at all tested dye concentrations. Activation parameters between the sunflower seed hull and the textile dye such as activation energy (8.79 kJ/mol), enthalpy (8.79 kJ/mol), the entropy change (−39.57 kJ/mol/K), and Gibbs free energy (6.27–8.11 kJ/mol) were calculated. In another research, biosorption of cadmium was studied using the seeds of Alhaji Maurorum. The seeds went through some preparation steps before being used as adsorbents. They were thoroughly washed with distilled water followed by drying at ambient temperature for 48 h. A grinder then powdered the seeds and sieved to reach the desired particle size. Maximum removal of cadmium (85.5%) occurred at pH 6, adsorption dosage of 20 g/L, and duration of 45 min. Also, the mean adsorption energy determined from the Dubinin-Radushkevitch isotherm algorithm confirmed the physical nature of the biosorption process (Ebrahimi et al. 2015).
Powdered seeds of Annona crassiflora (araticum fruit) were used as efficient biosorbents for the adsorption of crystal violet dye as well as simulated textile wastewater. Characterization of biosorbent showed that seeds of araticum had an amorphous nature with irregular and heterogeneous shapes. Maximum removal of crystal violet occurred at pH 7.5 and araticum seed powder dosage of 0.7 g L−1. A maximum biosorption capacity of 300.96 mg g−1 was achieved at 328 K. Also, the efficiency of the biosorbent was confirmed by the 87.8% removal of the dye from simulated textile wastewater (Franco et al. 2020).
Brazilian berry seeds (Eugenia uniflora) were also used as cost-effective and eco-friendly biosorbents to effectively treat textile effluents containing methylene blue. For lower methylene blue concentrations, equilibrium reached after 20 min, while 120 min were required to reach the higher concentrations. Meanwhile, kinetic data were well presented by the general and pseudo-second-order models. The biosorbents were also successful in treating two different simulated textile effluent (92.2 and 88.7% removal). The Brazilian berry seeds also served as packing in a fixed-bed column reaching to biosorption capacity of 88.7 mg g−1 within 840 min (Georgin et al. 2020). Allium Cepa seed biomass (ASCB) was prepared and used as an effective biosorbent for biosorption of Cr(VI), Cd(II), Cu(II), Zn(II), Pb(II) from aqueous media. Before being applied as biosorbent, the biomass went through some preparation steps such as cleaning (in a granular bed with the flow of deionized water), drying (under natural convection mode for 10 days followed by oven drying for 1 h at 100 °C), and grinding process. FTIR spectrum revealed the hydroxyl group as the source of metal uptake. Maximum adsorption of metal ions was obtained at 4 gL−1 of ASCB, 50 mgL−1 adsorbates, and neutral pH. Maximum removal efficiency under these conditions was determined as high as 99% for Pb(II), Cu(II), while rather lower removal efficiencies were obtained for Zn(II) and Cr(VI) (Fig. 2) (Sheikh et al. 2021).
Schematic of whole biosorption process including biomass preparation and the diagram of removal efficiency for the removal of Cr(VI), Cd(II), Cu(II), Zn(II), Pb(II) using Allium Cepa seed biomass (ASCB) (Sheikh et al. 2021)
3.1.3 Plant Root
Plant roots have been used as biosorbents to remove heavy metals, dyes, salinity, etc., from the wastewater. For example, water hyacinth (Eichhornia crassipes) roots were used as biosorbents to remove BF-4B red reactive dye from the aqueous solution. Dye removal was conducted with 95% efficiency until the equilibrium time of 110 min was reached. The biosorption kinetic data conformed well to the Pseudo-second order and Elovich models. A maximum biosorption capacity of 43.28 mg g−1 was obtained with the biosorption process tending to the Langmuir model (Rigueto et al. 2020). In another study, biosorption of fluoride from water using coconut tree (Cocus Nucifera Linn.) was investigated. Equilibrium studies disclosed that the biosorption capacity of coconut roots for fluoride was significantly high and linearly increased with the enhancement of the initial concentration of adsorbate. Kinetic data fitted well to the Pseudo-second order model signifying the chemical nature of the adsorption process. Also, the thermodynamic study confirmed the endothermic and spontaneous nature of the biosorption process (ΔH = 12.728 kJ · mol) (George and Tembhurkar 2019).
The roots of plants also adsorb heavy metals as biosorbents. Heavy metals including Pb, Cu, Zn, and Cd were removed by the root powder of Long-root Eichhornia crassipes from the aqueous media. Analysis of the energy spectrum revealed that the mentioned heavy metals were adsorbed on the biosorbent after their adsorption. The adsorption of metals was investigated in the single-metal and multi-metal systems (competitive adsorption). It was found that in the competitive adsorption, an increase in the concentration of metals led to a significant decline in the adsorption of Zn, Pb, and Cd. Also, the adsorption of every two metals on the plant powder showed that the involvement of either Cu or Pb in the binary metal group masters the adsorption proficiency of Cd and Zn (Li et al. 2016).
3.1.4 Plant Bark
Plant bark has an excellent absorption capacity for pollutants such as heavy metals, salts, pharmaceuticals, pesticides, etc. However, about two-thirds of the literature regarding biosorption using this plant organ is devoted to heavy metals. Based on the preparation technique, plant barks are subdivided into unmodified biosorbents, pre-modified biosorbents, chemically modified biosorbents, physically modified biosorbents, and bio-based activated carbon. The latter is obtained when the biomaterials (plant barks) are sent into a furnace to go through the carbonization process.
Heavy metals like Cd, Cr, Ni, Pb, and Cu are mostly studied in plant barks’ biosorption. The adsorption capacity of plant barks for heavy metals depends upon several factors, including the type of the biomaterial (plant), the method used to modify the biosorbent, type of the heavy metal, temperature, and pH. Dyes studied as pollutants include methylene blue, methyl orange, methyl red, congo red, solar blue, and remazol. Also, pharmaceutical compounds such as ibuprofen, amoxicillin, naproxen, ketoprofen, and diclofenac were among pharmaceutical pollutants being removed so far with certain efficiencies using plant barks. Sorption of plant bark usually fits Langmuir isotherm. Also, the kinetic data generally obeys the Pseudo-second order model. Search into the thermodynamics of the biosorption process utilizing plant barks showed that the process is spontaneous, having a physical nature. Regeneration studies show good potential for these biosorbents, making them excellent biosorbents for industrial applications (Ighalo and Adeniyi 2020).
3.1.5 Plant Peel
In recent years, fruit peel has been investigated for its adsorption ability to eliminate pollutants like heavy metals, dyes, and organic pollutants from industrial wastewater. The most studied fruit peels are orange and banana peels, while Pb and methylene blue biosorption is done with greater removal efficiencies than the rest of the pollutants. Adsorption isotherms usually obey the Langmuir and Freundlich, isotherm models. Like most biosorbents, kinetic data are well represented by the Pseudo-second order model. Thermodynamic studies usually reveal the exothermic nature of the biosorption process using fruit peels which is consistent with the nature of the adsorption process.
Citrullus colocynthis peels were used as adsorbents to remove methylene blue dye from an aqueous solution. Langmuir isotherm followed by Temkin isotherm best fitted to equilibrium data. Maximum adsorption capacity and removal efficiency were found to be 4.48 mg g−1 and 91.43%, respectively (Alghamdi and El Mannoubi 2021). In another study, the peels of Artocarpus Nobilis exhibited good adsorption ability toward Ni(II) ions. Air-dried particles led to 50% removal when the reaction mixture was kept at both static and dynamic conditions. After optimizing shaking time, settling time, and process temperature, the removal efficiency increased 71%. Also, it was found that linearized Langmuir isotherm better described equilibrium data compared to the Freundlich model. Further enhancement of removal efficiency up to 93% was achieved at dynamic mode by optimising the packing bed's height and flow rate (Priyantha and Kotabewatta 2019).
Pretreatment of fruit peels enhances their adsorption capacity but influences the total cost of the process, which is definitely of great importance for the scale-up of the process. The use of fruit peels as biosorbents has not been commercialized yet and remains in batch mode. Surface characteristics of fruit peels as natural biomaterials depend on several factors, including location and geological conditions of the source fruit, season, ripening state of the fruit, and its quality. These properties directly affect the biosorption capability of fruit peels, which makes comparisons between similar fruit peels of different origins difficult (Pathak et al. 2015).
3.2 Use of Microbes as Biosorbents
Microbial biosorbents fall into the following category: Bacterial biosorbents, fungal biosorbents, and microalgal biosorbents. Biosorption of pollutants with microbial biosorbents offers some advantages compared to the rest of biosorbents. In the case of metal removal, due to their selectivity in binding to specific metal ions, they possess high metal removal efficiency. Besides, the small size of microorganisms provides a large surface area per volume ratio, which favors the adsorption process. Other advantages include low cost and environmentally friendliness of the microbial biosorbent and the feasibility of using dead or live biomass in the biosorption of pollutants from the wastewater (Yu et al. 2020; Vijayaraghavan and Yun 2008).
3.2.1 Bacterial Biosorbents
Bacteria, which are single-cell microbes, are the most abundant microorganisms. Bacterial cells lack membrane-bound organelles and nuclei. Therefore, the cell structure is simpler compared to other organisms. Bacteria can grow under severe conditions. Thus, bacterial biomass is abundantly available. Due to bacterial biomass's distinctive properties like high efficiency and low cost, they can serve as suitable adsorbents. For example, biosorption of heavy metals from wastewater capabilities of bacterial biomass in binding to metals through its functional groups and high biosorption capacity have made them excellent adsorbents. In addition, the reusability of bacterial biomass aids in the cost-effectiveness of the process and minimises the remaining wastes (Priyadarshanee and Das 2020). Bacterial biosorbents are mostly used in treating metal/dye-containing wastewaters. Metal removal is extensively done using bacterial biosorbents such as Bacillus sp., Pseudomonas sp., Sphaerotilus sp., Thiobacillus sp., Staphylococcus sp., Streptomyces sp., Desulfovibrio sp., and Corynebacterium sp.; while bacterial species such as Corynebacterium glutamicum, Streptomyces rimosus, Bacillus catenulatus, and Acidithiobacillus thiooxidans were mostly involved in the biosorption of dyes (Vijayaraghavan and Yun 2008; Kim et al. 2015; Nguyen et al. 2016).
In bacteria, the sorption ability of biomass could be enhanced by modification methods through enhancement or activation of the binding sites on the surface of bacterial biomass (Suazo-Madrid et al. 2011). The typical pretreatment methods recommended for bacterial biomass are physical, chemical, and biological pretreatment methods.
3.2.2 Fungal Biosorbents
Fungal biosorption is defined as the ability of fungal biomass, especially its cell wall, to accumulate dangerous wastes (pharmaceuticals, heavy metals, and dyes). Fungi offer several advantages in the treatment of wastewater, including being facile, inexpensive, high efficient, and flexible in operation (Legorreta-Castañeda et al. 2020). These groups of microbes are formed as unicellular and multicellular organisms. The latter shows quite different development mechanisms compared to plants and animals due to the formation of long filaments called hyphae (Riquelme et al. 2018). The filaments are like eukaryotic cells and are surrounded by a cell wall composed mainly of glycoproteins, chitin, and glycans. Also, the functional groups responsible for the biosorption process, including carboxyl, amine, and hydroxyl, are present in great amounts in the cell wall (Lo et al. 2014). It is worthy to note that most fungi used in the biosorption process to remove pollutants are non-pathogenic, so they can be used without safety recommendations. Fungal biomass is accessible in great amounts, yielding an abundance of functional groups that can bind to metals, thereby preparing a strong biosorptive agent (Ayele et al. 2021).
Filamentous fungi biomass being recognized as a valuable source for the adsorption of heavy metals greatly threatens human health and the ecosystem. Fungal biomass should satisfy some requirements before being applied as heavy metal biosorbents. For instance, factors such as bioavailability, cost-effectiveness, reusability, and high adsorption capacity are necessary for an efficient biosorption process using these biosorbents with microbial origin. In this sense, effluents from large-scale bioprocess industries such as antibiotic industries, which contain a great amount of filamentous biomass, maybe a great help. Various heavy metals have been removed by fungal biosorbents mainly from the fungal strains of Aspergillus carbonarius, Aspergillus lentulus, Aspergillus flavus, Aspergillus niger, Aspergillus fumigatus, Funalia trogii, Aspergillus japonicas, Lentinus edodes, Penicillium chrysogenum, Phanerochaete chrysosporium, Pleurotus ostreatus, Rhizopus arrhizus, Rhizopus nigricans, Rhizopus oryzae, and Trametes versicolor.
Fungal biosorbents have been applied extensively for the removal of dyes. High resistance and survival under extreme conditions of highly toxic dye contaminants and reusability of the fungal biosorbent after several treatment cycles have made these biosorbents an attractive choice for dye removal. Fungal species such as Aspergillus sp., Penicillium sp., Trametes versicolor, Phanerochaete chrysosporium, and Funalia trogii have been used for the removal of a variety of dyes from dye-contaminated effluents.
Phenolic compounds such as phenol, 2-chlorophenol, pentachlorophenol, bisphenol A, benzophenone, 1-naphthalenamine, naphthol, etc., were successfully removed by the adsorption capability of some fungi such as Anthracophyllum discolor, Funalia trogii, Penicillium oxalicum, Phanerochaete chrysosporium, Phanerochaete chrysosporium, and Trametes versicolor. However, the biosorption capacity of fungi for phenolic compounds is lower than that of dyes mainly due to the greater molecular weight of dyes than phenolic compounds, as adsorption is directly calculated based on the molecular weight of the adsorbate. Another reason for better adsorption of dye may be high values for solubility and pKa of phenolic compounds; since enhancement in these factors leads to a significant decline in the adsorption ability of the adsorbate. Other pollutants yet to be removed by fungal biosorbents are known as organic compounds, such as pesticides, humic substances, and pharmaceuticals (Legorreta-Castañeda et al. 2020).
3.2.3 Microalgal Biosorbents
Microalgae are known as photosynthetic microorganisms with the ability to change solar energy into biomass. Microalgae use sunlight as a source of energy to fix CO2 and produce different biomaterials in turn. The selection of suitable microalgae strain is vital to use these biofactories’ capability for wastewater treatment. Those microalgae species able to undergo mixotrophic or chemoheterotrophic metabolism satisfy the requirements for wastewater treatment (Ubando et al. 2021). The metal-binding sites of a microalgae cell are depicted in Fig. 3. When metal accumulates inside the cell, the metal ions are instantly placed in specific organelles and attach to metal-binding ligands like phytochelatins and metallothioneins. Although microalgae are less used to remove contaminants from wastewater than other microbes such as bacteria and fungi, interest in this field has been increasing in the recent decade.
Binding site of metals in microalgae cell (Chai et al. 2020)
The cell wall in microalgae is usually composed of polysaccharides, glycoproteins, and an external layer composed mainly of chitin in some species. The functional groups present in the microalgae cell wall are responsible for binding to contaminants, especially metals existing in the wastewater. Different laboratory conditions lead to the production of microalgae with various functional groups, which can be utilized for the biosorption of various contaminating agents in wastewater (Escudero et al. 2019). Wastewater treatment using microalgae is preferred over seaweeds. The reason lies in the potential of cultivating microalgae on a large scale and thus reaching a tremendous amount of biomass. Furthermore, wastewater treatment using microalgae can be done simultaneously with other value-added operations like the production of biofuels (Chu and Phang 2019).
Microalgae have been successfully utilized in the biosorption of heavy metals and dyes from wastewater. In the case of heavy metals, Chlorella, chlamydomonas and Desmodesmus (green algae), Phaeodactylum, Cyclotella and Aulosira (diatoms), Spirulina, Oscillatoria, and Phormidium (cyanobacteria) have been evaluated for their ability in the adsorption of heavy metals. Chlorella vulgaris is the most researched species used as non-living, living, free, or immobilized cells (Kumar et al. 2015). It is worthy to note that various pretreatments (physical or chemical) on microalgae before being used as biosorbents favor the sorption capacity of microalgae by improving cell wall features. Different dyes, especially malachite green and methylene blue, are reported to be removed with the biosorptive performance of various types of microalgae. Studies mostly involve non-living algal biomass with a synthetic dye solution. Nevertheless, some of the reports focus on using living microalgae and cyanobacteria (Chu and Phang 2019).
3.3 Use of Agricultural Wastes as Biosorbents
Agricultural wastes, especially cellulosic materials, show outstanding capacity for biosorption of pollutants from wastewater. Agricultural wastes are mainly composed of hemicellulose, lignin, lipids, proteins, a simple sugar, starch, hydrocarbons, and water. Most agricultural wastes contain various functional groups such as aldehyde, amine, keto groups, etc. Due to their distinct chemical composition, cost-effectiveness, high removal efficiency, availability, simple operation, and regeneration, agricultural wastes are suitable options for wastewater treatment. The use of agricultural wastes as biosorbents may be helpful in two ways: first, it can help solve environmental issues concerning the use of chemical adsorbents; second, it can help reduce the operation costs (Mo et al. 2018).
3.3.1 Rice Waste
Rice is the seed of the grass species Oryza sativa grown extensively worldwide. Owing to millions of tones of rice world production industry, numerous by-products of rice like rice husk, rice hull, and rice bran are produced. Bažant and Estenssoro (1979) have used alkali-modified rice husk to eliminate copper from its containing solution. Experimental results showed that approximately 90–98% copper was removed using the modified rice husk. In another study, the ability of rice straw, rice husk, and rice bran was evaluated in the biosorption of Pb(II) ions both from its containing solution and wastewater produced from the battery industry. The Pseudo-second order model well represented kinetic data. However, rice husk was an exception that was best fitted to the intraparticle diffusion model. Biosorption capacity for rice straw, rice bran, and rice husk were calculated as 24.17, 20.54, and 21.38 mg g−1, respectively (Singha and Das 2012).
Dyes are other pollutants to be adsorbed onto rice wastes. For instance, several studies have studied the biosorption of methylene blue using rice husk. Ahmad et al. (2019) studied the influence of sorption parameters, including initial dye concentration (10–50 mg L−1), contact time (10–120 min), pH (2–10), and adsorbate dosage (0.05–0.25 g) on the removal efficiency of methylene blue. Acid Yellow 17 dye left from the textile and paper industries is toxic to human health and the environment. This dye was removed by the biosorption ability of rice husk with the removal efficiency of 97.97% at the optimum operating conditions when 0.7 g of biosorbent was present (Patil et al. 2015). Other compounds removed by rice wastes include herbicides, phenolic (mono aromatic), and poly aromatic compounds. As expected, pretreatments (chemical, physical, and biological) positively impact the biosorption ability of rice waste (Shamsollahi and Partovinia 2019).
3.3.2 Tea and Coffee Waste
Coffee and tea are the most popular beverages in the world. Tea and coffee wastes are the product of their manufacturing companies and cafeterias. Cellulose, hemicellulose, lignin, condensed tannins, and functional groups including carboxylate, aromatic carboxylate, phenolic, hydroxyl, and oxyl groups make the chemical composition of tea leaves. Generally, removal of contaminants present in the wastewater is done with the mentioned functional groups (Amarasinghe and Williams 2007). Çelebi et al. (2020) examined the adsorption ability of tea wastes in the removal of lead (II), cadmium (II), nickel (II), and zinc (II). Study of the parameters such as pH (2.0–6.0), adsorbent amount (0.1–5.0 g), and contact time (1–150 min) revealed that the removal efficiency of heavy metals was inversely affected by pH; while increase in other parameters resulted in linear enhancement of removal efficiency. Maximum adsorption capacity for Pb, Zn, Ni, and Cd at the optimum pH value (between 4.0 and 5.0) was found to be 1.20, 1.46, 1.16, and 2.47 mg g−1. In another study, tea waste was utilized for methylene blue dye removal from its containing solution. The adsorption process is composed of two stages. The first stage included adsorption with a fast trend, while a rather slow adsorption process was in progress throughout the second stage. Based on Langmuir isotherm, the maximum adsorption capacity of the tea waste was found to be 113.15 mg g−1 (Liu et al. 2018).
The coffee tree belongs to the family Rubiaceae. Two plant species, namely Coffea Arabica (Arabica) and Coffea canephora (Robusta), supply 75 and 25% of the world’s total coffee production. The main by-products from the coffee industry include spent coffee grounds, the coffee silverskin, and the coffee husks. Microscopic tests confirm the presence of fibrous tissues in the surface layer of the coffee wastes. These fibrous tissues are mainly composed of cellulose and hemicellulose. Glucose has the greatest amount among the monosaccharides present in the coffee wastes. Proteins and extractives also constitute a major part of coffee wastes (Anastopoulos et al. 2017). The Biosorption ability of different types of coffee wastes is proved in literature. For example, coffee husk biomass was used for heavy metal removal from wastewater. Coffee waste was used to produce biochar, which was modified with sodium hydroxide after that to create functional groups on the surface of the biosorbent and enhance the specific surface area. The maximum adsorption capacity for Cd and Pd was 116.3 and 139.5 mg g−1, respectively. The produced biochar removed 89.6% of Pb (II) and 81.5% of Cd (II) from wastewater (thi Quyen et al. 2021). Ahsan et al. (2018) primarily used a facile method for the synthesis of sulfonated coffee waste and then utilized the produced biosorbent for the adsorption of bisphenol A (endocrine-disrupting chemical) and sulfamethoxazole (antibiotic) from the solution. Biosorption capacity for bisphenol A and sulfamethoxazole biosorption was calculated as 271 and 256 mg g−1. Efficient biosorption of both contaminants is provided by the formation of π–π interaction and electrostatic interaction between sulfonated coffee waste and the contaminants.
3.3.3 Sawdust
Sawdust is a waste mainly produced in the paper, carpentry, and furniture industries (Dolatabadi et al. 2018). Wood is generally composed of hemicellulose (20–30%), lignin (20–30%), and extractives (1–5.5%). However, the exact composition strongly depends on the specific tree species from which the wood is obtained. Different bonding exists among hemicellulose, cellulose, and lignin. The three compounds are bound with hydrogen bonding primarily, while a chemical bonding exists between hemicellulose and lignin. This bonding results in the involvement of a small number of carbohydrates in the lignin structure. Various functional groups, including hydroxyl, carboxyl, amid, and phenolic groups, are present in the chemical structure of sawdust. These functional groups pose the adsorptive ability to sawdust and provide binding to pollutants, especially heavy metals (Ouafi et al. 2017). Typically, sawdust treatment with acid or alkali is carried out to enhance its adsorption capacity.
During the last decades, sawdust has been utilized as an efficient precursor to produce activated carbon with improved characteristics compared to the typical activated carbon, such as higher porosity, larger surface area, and more functional groups. The produced activated carbon is used for various applications like heavy metal removal, dye removal, etc. Various types of water-soluble dyes, such as methylene blue, methyl violet, congo red, malachite green, etc., are reported to be removed by the sawdust-derived activated carbon. Akhouairi et al. (2019) used the sawdust-derived activated carbon as a natural, widespread, and low-cost biosorbent for Eriochrome Black T (EBT) adsorption from aqueous media its adsorption onto sawdust. A maximum adsorption capacity of 40.96 mg g−1 corresponding to 80% removal was obtained at pH 4. In another study, sulfonic acid incorporated pine sawdust (APSD) was used as a cost-efficient adsorbent for the batch removal of Maxilon Red GRL (MR GRL) from a synthetic dye solution. Under the optimal conditions (pH value in the range between 5.7 and 6.0, the temperature of 298 k, dye concentration of 250 mg L−1, and adsorbent dosage of 8 g L−1 maximum MR GRL removal of 99.35% was obtained within 180 min (Şentürk and Yıldız 2020).
Sawdust was also applied for the uptake and removal of heavy metal ions. Literature on the adsorption ability of sawdust is available in great number. For example, Alhumaimess et al. (2019) firstly enhanced the uptake capacity of metal ions by sawdust through grafting phosphorus oxychloride over the surface of raw sawdust. The resulting biosorbent was used to remove Cd(II), Cr(III), ad Pb(II) metal ions from an aqueous medium with a maximum adsorption capacity of 244.3, 325, and 217 mg g−1. Sawdust-derived biochar was used for the treatment of gold tailings wastewater. Using the biosorbent, concentration of CN−, Cr3+, Fe3+, Zn2+, Ni3+, Pb2+, Mn2+, and Cu2+ was reduced to 0.76, 0.74, 0.75, 0.83, 0.85 and 0.87, respectively. While the reduced concentrations for Cr3+, Fe2+, Zn2+, Mn2+, and Cu2+ met the WHO guidelines, More retention time and biochar dosage were needed to satisfy WHO guidelines of CN−, Ni3+, and Pb2+ (Manyuchi et al. 2021).
3.3.4 Sugarcane Bagasse
Ease of access and availability are important factors that determine whether an agro-based waste product is suitable to be used as a biosorbent or not. Sugarcane bagasse, the main agro-waste from the sugar industry being utilized worldwide, seems reasonably eligible for this purpose. Sugarcane bagasse was found to be capable of adsorption of heavy metals in wastewater treatment. Improvement of biosorption ability of this agro-waste for heavy metal removal could be achieved considerably by its binding into functional groups such as carboxylic, amin, etc., or by removing soluble organic compounds (Pereira et al. 2010; Martín-Lara et al. 2010; Karri et al. 2020; Lingamdinne et al. 2020; Kaur et al. 2020). Removal of dyes was also conducted using sugarcane bagasse. In research performed by Gusmão et al. (2012), removal of methylene blue as the most common dye in the textile and paper industries was achieved utilizing succinylated sugarcane bagasse. The negative charge of carboxylate function in the chemical structure of sugarcane bagasse interacts with cationic dyes. In this sense, succinic anhydride and sodium bicarbonate solutions could be used to improve the functionality. FTIR spectroscopy revealed that carboxylate group and symmetric stretching of ester group were responsible for dye biosorption.
The Biosorption ability of sugarcane bagasse is also observed in removing petroleum, phenolic compounds, and organic nutrients. Like other agro-waste products, modification of sugarcane bagasse conducted by different methods (mechanical, chemical, magnetic, immobilization), enhances its biosorption capacity noticeably (Sarker et al. 2017). Kamel et al. (2012) modified sugarcane bagasse by different methods (chemical bleaching, acrylonitrile grafting, and thermal charring) to remove phenol. They found that the maximum potential of each of these methods for modifying bagasse (186.50, 160.64, and 195.00 mg L−1) is achieved when the initial concentration of adsorbate is high (500 ppm).
3.4 Use of Other Biomaterials as Biosorbents
3.4.1 Chitin and Chitosan
Chitin, chitosan, and their derivatives are natural, low-cost biopolymers available in abundance. During recent years, these biopolymers have been used extensively to eliminate toxic contaminants from wastewater due to their nontoxicity, biodegradability, biocompatibility, and bioactivity (Xiong Chang et al. 2021; Dehghani et al. 2020a, b). In addition, the excellent physical and chemical performance of chitin and chitosan has made them excellent candidates to serve as metal chelating agents for the biosorption of toxic metals present in the wastewaters (Sarode et al. 2019; Saha et al. 2019).
By boiling chitin in potassium hydroxide, acid-soluble chitosan would be synthesized. Chitin, the second most plentiful polysaccharide worldwide, is usually extracted from the exoskeleton of aquatic organisms like lobster, crayfish, prawns, shrimp, and crab. The chemical composition of chitin and chitosan is demonstrated in Fig. 4. Chitosan is not soluble in water, alkaline solutions, and organic solvents due to hydrogen bonding among its molecules. This biopolymer is soluble in acidic solutions due to the protonation of its amine functional groups. Due to having various functional groups (e.g. dhydroxyl and amino groups), chitosan has a high affinity toward the biosorption of pollutants like heavy metals and dyes. Cationization of amino groups in chitosan enriches its affinity to adsorb anionic dyes through electrostatic attraction in acidic solutions. Furthermore, the sensitivity of chitosan to pH has made it feasible to form either gel or solution based on the pH of the solution.
Chemical structures of chitin and chitosan (free base and cationic form) (Fini and Orienti 2003)
However, the use of these biomaterials has some drawbacks as well. For instance, low mechanical resistance, solubility in acidic medium, and low surface area have limited the performance of chitosan as an efficient biosorbent. Therefore, modification of this biomaterial is needed to reach the standard requirements for proper biosorption performance (Sheth et al. 2021; Knidri et al. 2018).
4 Mechanism of Biosorption
Biosorption is generally defined based on the interaction between a sorbate (i.e. atom, ion, or molecule) and a biosorbent (with biological origin), resulting in biosorption and accumulation of the sorbate in the sorbate-biosorbent interface. This phenomenon usually leads to a considerable decline in the sorbate concentration inside the primary solution (Gadd 2009). Due to the lack of precise scientific evidence, the exact mechanism of the biosorption process remains unknown. The mechanisms proposed by the scientists so far are mostly based on presumptions and experimental results (Saha et al. 2019). Four primary mechanisms, namely chemisorption, physisorption, precipitation, and oxidation/reduction, comprise the biosorption mechanism. However, the complex process of biosorption sometimes necessitates more than one mechanism.
4.1 Biosorption of Metals
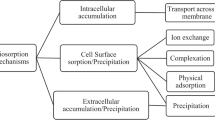
Due to the diversity of biosorbent material and contaminants present in wastewater. Complexation, chelation, reduction, precipitation, and ion exchange are the most common mechanisms, especially for the adsorption of heavy metals (Tsezos et al. 2006). Feasible mechanisms for decontaminating wastewater via the biosorption process are schematically represented in Fig. 5.
Schematic diagram of prevalent adsorption mechanisms (Huang et al. 2020)
Functional groups involved in the adsorption of metal ions include carboxyl, hydroxyl, sulfate, phosphoryl, and amino groups. Therefore, pH has a key role in metals biosorption by influencing the charge of the mentioned functional groups, which directly affects the amount of metals biosorbed by the biomass. Since most of the metals present in the wastewater are in their cationic form, the intense negative charge of the biosorbent results in better biosorption of the metal ion. Thus, pH in the range between 7.0 and 8.0 is recognized as the most suitable pH for the adsorption of heavy metals. At lower pH values, there is competition between hydrogen ions and metal ions for binding sites of the adsorbent, while at higher pH values, metal ions are precipitated in the form of hydroxides, resulting in the reduction of biosorbed metal ions. However, for metals, which are predominantly in their anionic form, acidic pH values in the range between 2.0 and 4.0 represent the best pH range for the biosorption process. Anions are attracted onto the biomass with more positively charged functional groups at acidic pHs more easily.
Temperature is another important factor, which greatly affects the rate of reaction. Generally, the rate of biosorption increases at higher temperatures by enhancing surface activity and kinetic energy of adsorbate. An increase in temperature usually increases the maximum amount of metals biosorbed by the adsorbent. This happens when the process is endothermic. In the case of the exothermic process, an increase in temperature has a negative effect on biosorption capacity, which is most probably a result of the damage imposed to the surface of the biosorbent. This effect of temperature is more considerable in living biomass compared to dead biomass. Finally, enhancement of ionic strength negatively affects the biosorption capacity as a result of competition between other cations for the binding sites of the functional groups. This is a crucial problem in real wastewater solutions where great amounts of various cations are involved in the solution (Torres 2020).
4.2 Biosorption of Organic Compounds
Many organic compounds, such as antibiotics, dyes, phenolic compounds, etc., have adverse effects on human health and the ecosystem. Some of the persistent organic pollutants (POPs), such as pesticides, organochlorines, insecticides, herbicides, etc., have been developed and known for a long time; while a group of these compounds are created due to the advances in analytical methods and are called emerging organic contaminants (EOCs). Even at low temperatures, such compounds should be eliminated from wastewaters because of their toxicity. The biosorptiontion process for eliminating these contaminants is similar to the metal biosorption process, and the factors influencing the biosorption capacity are identical. However, the response to the changes in these factors may be different, which necessitates a thorough study of each case.
The complex composition of organic compounds, unlike metals, shows that they have different functional groups with different charges and degrees of ionization based on the solution pH. Thus, optimization of pH is very important in these compounds. Hydrophobicity is another important factor that should be considered in the case of organic pollutants. Hydrophobic materials can interact with the biosorbent via hydrophobic interactions. They can even go through the cell membrane when the biosorbent is a living organism. Therefore, this property of organic pollutants contributes to their efficient removal through biosorption.
The effect of temperature on biosorption of organic pollutants is the same as metals, meaning that for endothermic biosorption, an increase in temperature enhances the adsorption effectiveness, while the opposite result is obtained when the process is exothermic. Although the effect of ionic strength on the biosorption capacity is less studied compared to metals, it is known that a high concentration of salts is required in the solution so that the biosorption of organic compounds is decreased considerably (Torres 2020).
5 Desorption and Regeneration of Biosorbents
Reusability of the biosorbent means the removal of adsorbate from the biosorbent surface after usage and its return to its primary state, especially in terms of morphology and efficiency. Recovery of biosorbent after usage is one of the key issues in selecting suitable biosorbents. In other words, the reusability of biosorbent is as important as its good biosorption performance. Thus, desorption and regeneration of biosorbents are two fundamental processes that should be checked, so that effectiveness of a biosorbent is confirmed.
Several methods have been proposed for the regeneration of biosorbents, among which the use of eluents is the most promising method. The selection of appropriate eluents, which is based on the composition of biosorbent, adsorbate, and the mechanism of biosorption, is of great importance. A good eluent does not vary or harm the structure of biosorbent, is environmentally friendly, inexpensive, has a high affinity toward the particular adsorbate, and easily separates from the adsorbate. Mineral acids (HCl, H2SO4, and HNO3), organic acids (citric, acetic, and lactic acids), and complexing agents (EDTA, thiosulphate, etc.) are some examples of eluents used in recent years for the regeneration of biosorbents.
It is feasible to perform desorption in batch or column. However, the packed column system provides an easier mode for this process, especially when the biosorption process is performed in the column as well. Desorption efficiency is defined by the S/L ratio, where S represents solid (biosorbent), and L represents liquid (eluent volume). It is worthy of mentioning that a high S/L ratio favors the desorption process (Adewuyi 2020; Kanamarlapudi et al. 2018). Table 2 summarizes information on the reusability potential of some plant leaves as an example of biosorbents used in many kinds of research for wastewater treatment. To examine this, biosorbents obtained from plant leaves go through some adsorption/desorption cycles until lost most of their adsorption capacity. It can be inferred from the information given in Table 1 that except for some rare cases, most of the plants maintained their removal efficiency up to the fifth cycle. After 5 cycles, the removal efficiency reduced by 5–15% in most species. These findings indicate that plant leaves are interestingly efficient in removing pollutants from wastewater (Adeniyi and Ighalo 2019).
6 Cost Estimation of Biosorbents for Wastewater Treatment
Evaluating the cost of the biosorption process is not a simple task since it relies on several factors such as required pretreatments on biosorbent, transportation, maintenance, energy consumption, regeneration, desorption, etc. The nature of wastewater to be treated by biosorption and the operational volume are other key factors to be considered. However, the type and size of the treatment plant specify capital costs. To minimize process costs, it is recommended to use waste materials such as agro-industrial wastes as biosorbents (see Sect. 3).
The dependence of cost estimation on numerous factors discussed earlier makes it hard to generalise and reach a specified relation. The composition of biosorbent has a major effect on the cost of the biosorption process. Pretreatment of biosorbent before use increases operational costs. To minimize cost, it is better to place a pretreatment facility close to where the waste is kept. Energy consumption is another important factor influencing process cost related to the biosorbent used and its biosorption capacity. For example, less energy is consumed with a fast biosorbent capable of completing the biosorption process within a short time. In other words, more energy consumption equals to high biosorption cost. Disposal of biosorbent, which is inevitable after several cycles and its replacement by new biosorbent, also increases process costs (Adewuyi 2020).
Overall, compared to other methods for wastewater treatment like ion exchange, adsorption, membrane separation, chemical oxidation, coagulation/flocculation, process cost is much less in the case of the biosorption process. The reason mainly lies in eliminating chemicals and the use of microorganisms or agricultural/food wastes instead.
7 Challenges for Industrial Implementation
Typically, all the biosorbents show promising results in terms of their adsorption capacity for particular contaminants at laboratory and pilot scales. However, few reports are available on the feasibility of their application at an industrial scale. Industrial application of biosorption process is faced by several challenges as follows:
-
Since wastewater from some industries like electroplating contains heavy metals with different pHs, it is impossible to use biofilters that require stable conditions for their growth and maintenance at an industrial scale.
-
Unlike the lab scale in which an increase in agitation rate enhances the biosorption capacity due to providing additional turbulence in the solution, industrial-scale biosorption is not affected by this parameter due to the process’s rapid rate and surface saturation.
-
Lab-scale studies reveal that increase in contact time results in enhancement of pollutant removal through active sites till saturation is reached with an increase in time. On the industrial scale, the contact time is much less, with millions of tons of pollutants normally present in industrial wastewaters, resulting in less pollutant biosorption.
Therefore, it is reasonably rejected to directly transfer information and practices on a laboratory scale into an industrial scale. Further efforts are needed to commercialize and scale the technology on a lab scale (Singh et al. 2020).
8 Prospects
Removing contaminants like toxic industrial compounds, heavy metals, dyes, hydrocarbons, pesticides, drugs, etc., from wastewater requires methods with high efficiency, low operational costs, high selectivity, and simplicity. Biosorption satisfies these requirements in its best condition. Although a lot of research has been done regarding using several biosorbents for wastewater treatment, commercialization has not been done yet. Commercialization of the biosorption process will not occur unless important factors such as industrial requirements, problems raised in the scale-up of the process, and the operational costs are managed technically and scientifically. Biosorbents such as agro-industrial wastes, plant/fruit wastes, and microorganisms (bacteria, fungi, and algae) are highly efficient in removing different pollutants from the wastewater by their functional groups and characteristics such as high pore volume and large surface area. Several pretreatments may be done on the biosorbents to enhance their biosorption capacity. In addition to this, researchers are seeking new materials to increase the efficiency of the typical biosorbents and design hybrid biosorption systems such as nano biocomposites through which biosorption of pollutants from wastewaters would be feasible with high efficiency. Future will witness biosorbents at domestic and industrial scales as an efficient and eco-friendly method for wastewater treatment.
References
Abedi S, Mousavi HZ, Asghari A (2016) Investigation of heavy metal ions adsorption by magnetically modified aloe vera leaves ash based on equilibrium, kinetic and thermodynamic studies. Desalin Water Treat 57:13747–13759
Adeniyi, George A, Ighalo JO (2019) Biosorption of pollutants by plant leaves: an empirical review. J Environ Chem Eng 7:103100
Adenuga, Abiodun A, Amos OD, Oyekunle JAO, Umukoro EH (2019) Adsorption performance and mechanism of a low-cost biosorbent from spent seedcake of Calophyllum inophyllum in simultaneous cleanup of potentially toxic metals from industrial wastewater. J Environ Chem Eng 7:103317
Adewuyi A (2020) Chemically modified biosorbents and their role in the removal of emerging pharmaceutical waste in the water system. Water 12:1551
Ahmad LN, Zakariyya UZ, Garba Zaharaddeen N (2019) Rice husk as Biosorbent for the adsorption of methylene blue. Sci World J 14:66–70
Ahmed S, Khan FSA, Mubarak NM, Khalid M, Tan YH, Mazari SA, Karri RR, Abdullah EC (2021) Emerging pollutants and their removal using visible-light responsive photocatalysis—a comprehensive review. J Environ Chem Eng 9
Ahsan MA, Islam MT, Imam MA, Hyder AG, Jabbari V, Dominguez N, Noveron JC (2018) Biosorption of bisphenol A and sulfamethoxazole from water using sulfonated coffee waste: Isotherm, kinetic and thermodynamic studies. J Environ Chem Eng 6:6602–6611
Ajmal M, Rao RAK, Ahmad R (2011) Adsorption studies of heavy metals on Tectona grandis: removal and recovery of Zn (II) from electroplating wastes. J Dispersion Sci Technol 32:851–856
Akhouairi S, Ouachtak H, Addi AA, Jada A, Douch J (2019) Natural sawdust as adsorbent for the eriochrome black T dye removal from aqueous solution. Water Air Soil Pollut 230:1–15
Alghamdi WM, El Mannoubi I (2021) Investigation of seeds and peels of Citrullus Colocynthis as efficient natural adsorbent for Methylene Blue Dye. Processes 9:1279
Alhumaimess MS, Alsohaimi IH, Alqadami AA, Kamel MM, Naushad Mu, Ahamad T, Alshammari H (2019) Synthesis of phosphorylated raw sawdust for the removal of toxic metal ions from aqueous medium: adsorption mechanism for clean approach. J Sol-Gel Sci Technol 89:602–615
Amarasinghe BMWPK, Williams RA (2007) Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem Eng J 132:299–309
Anastopoulos I, Karamesouti M, Mitropoulos AC, Kyzas GZ (2017) A review for coffee adsorbents. J Mol Liq 229:555–565
Anastopoulos I, Robalds A, Tran HN, Mitrogiannis D, Giannakoudakis DA, Hosseini-Bandegharaei A, Dotto GL (2019) Removal of heavy metals by leaves-derived biosorbents. Environ Chem Lett 17:755–766
Atar N, Olgun ASİM, Çolak F (2008) Thermodynamic, equilibrium and kinetic study of the biosorption of basic blue 41 using Bacillus maceran. Eng Life Sci 8:499–506
Ayele A, Haile S, Alemu D, Tesfaye T, Kamaraj M (2021) Mycoremediation: fungal-based technology for biosorption of heavy metals—a review. Strat Tools Pollut Mitigat: Avenues Clean Environ 355
Bažant ZP, Estenssoro LF (1979) Surface singularity and crack propagation. Int J Solids Struct 15:405–426
Beni AA, Esmaeili A (2020) Biosorption, an efficient method for removing heavy metals from industrial effluents: a review. Environ Technol Innov 17:100503
Bhattacharjee C, Dutta S, Saxena VK (2020) A review on biosorptive removal of dyes and heavy metals from wastewater using watermelon rind as biosorbent. Environ Adv 2:100007
Calderón OA, Ramírez OM, Abdeldayem AP, Rene ER (2020) Current updates and perspectives of biosorption technology: an alternative for the removal of heavy metals from wastewater. Curr Pollut Rep 6:8–27
Çelebi H, Gök G, Gök O (2020) Adsorption capability of brewed tea waste in waters containing toxic lead (II), cadmium (II), nickel (II), and zinc (II) heavy metal ions. Sci Rep 10:1–12
Chai WS, Tan WG, Munawaroh HSH, Gupta VK, Ho S-H, Show PL (2020) Multifaceted roles of microalgae in the application of wastewater biotreatment: a review. Environ Pollut 116236
Chakravarty S, Ashok Mohanty T, Nag Sudha AK, Upadhyay JK, Sircar JK, Madhukar A, Gupta KK (2010) Removal of Pb (II) ions from aqueous solution by adsorption using bael leaves (Aegle marmelos). J Hazard Mater 173:502–509
Cheraghi E, Ameri E, Moheb A (2015) Adsorption of cadmium ions from aqueous solutions using sesame as a low-cost biosorbent: kinetics and equilibrium studies. Int J Environ Sci Technol 12:2579–2592
Choudhary BC, Paul D, Borse AU, Garole DJ (2017) Recovery of palladium from secondary waste using soluble tannins cross-linked Lagerstroemia speciosa leaves powder. J Chem Technol Biotechnol 92:1667–1677
Chu W-L, Phang S-M (2019) Biosorption of heavy metals and dyes from industrial effluents by microalgae. In: Microalgae biotechnology for development of biofuel and wastewater treatment. Springer
Çolak F, Atar N, Olgun A (2009) Biosorption of acidic dyes from aqueous solution by Paenibacillus macerans: kinetic, thermodynamic and equilibrium studies. Chem Eng J 150:122–130
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Biores Technol 97:1061–1085
Crini G, Lichtfouse E (2019) Advantages and disadvantages of techniques used for wastewater treatment. Environ Chem Lett 17:145–155
Das M, Samal AK, Mehar N (2020) Butea monosperma leaf as an adsorbent of methylene blue: recovery of the dye and reuse of the adsorbent. Int J Environ Sci Technol 17:2105–2112
de Freitas G, Rocha MG, da Silva C, Vieira MGA (2019) Biosorption technology for removal of toxic metals: a review of commercial biosorbents and patents. Environ Sci Pollut Res 26:19097–19118
Dehghani MH, Karri RR, Alimohammadi M, Nazmara S, Zarei A, Saeedi Z (2020a) Insights into endocrine-disrupting Bisphenol-A adsorption from pharmaceutical effluent by chitosan immobilized nanoscale zero-valent iron nanoparticles. J Mol Liq 311
Dehghani MH, Karri RR, Yeganeh ZT, Mahvi AH, Nourmoradi H, Salari M, Zarei A, Sillanpää M (2020b) Statistical modelling of endocrine disrupting compounds adsorption onto activated carbon prepared from wood using CCD-RSM and DE hybrid evolutionary optimization framework: comparison of linear vs non-linear isotherm and kinetic parameters. J Mol Liq 302:112526
Dehghani MH, Omrani GA, Karri RR (2021) Solid waste—sources, toxicity, and their consequences to human health. In: Soft computing techniques in solid waste and wastewater management. Elsevier
Dolatabadi M, Mehrabpour M, Esfandyari M, Alidadi H, Davoudi M (2018) Modeling of simultaneous adsorption of dye and metal ion by sawdust from aqueous solution using of ANN and ANFIS. Chemom Intell Lab Syst 181:72–78
Ebrahimi A, Ehteshami M, Dahrazma B (2015) Isotherm and kinetic studies for the biosorption of cadmium from aqueous solution by Alhaji maurorum seed. Process Saf Environ Prot 98:374–382
Eletta OAA, Ighalo JO (2019) A review of fish scales as a source of biosorbent for the removal of pollutants from industrial effluents. J Res Inf Civ Eng 16:2479–2510
El Shahawy A, Heikal G (2018) Organic pollutants removal from oily wastewater using clean technology economically, friendly biosorbent (Phragmites australis). Ecol Eng 122:207–218
El-Sayed M, Nada AA (2017) Polyethylenimine−functionalized amorphous carbon fabricated from oil palm leaves as a novel adsorbent for Cr (VI) and Pb (II) from aqueous solution. J Water Process Eng 16:296–308
Escudero LB, Quintas PY, Wuilloud RG, Dotto GL (2019) Recent advances on elemental biosorption. Environ Chem Lett 17:409–427
Esmaeili A, Saremnia B, Kalantari M (2015) Removal of mercury (II) from aqueous solutions by biosorption on the biomass of Sargassum glaucescens and Gracilaria corticata. Arab J Chem 8:506–511
Fard RF, Azimi AA, Nabi Bidhendi GR (2011) Batch kinetics and isotherms for biosorption of cadmium onto biosolids. Desalinat Water Treat 28:69–74
Fiaz R, Hafeez M, Mahmood R (2019) Ficcus palmata leaves as a low-cost biosorbent for methylene blue: Thermodynamic and kinetic studies. Water Environ Res 91:689–699
Fini A, Orienti I (2003) The role of chitosan in drug delivery. Am J Drug Deliv 1:43–59
Franco DSP, Georgin J, Drumm FC, Netto MS, Allasia D, Oliveira MLS, Dotto GL (2020) Araticum (Annona crassiflora) seed powder (ASP) for the treatment of colored effluents by biosorption. Environ Sci Pollut Res 27:11184–11194
Gadd G (2009) Heavy metal pollutants: environmental and biotechnological aspects. In: Encyclopedia of microbiology. Elsevier
Geng J, Chang J (2020) Synthesis of magnetic Forsythia suspensa leaf powders for removal of metal ions and dyes from wastewater. J Environ Chem Eng 8:104224
George AM, Tembhurkar AR (2019) Analysis of equilibrium, kinetic, and thermodynamic parameters for biosorption of fluoride from water onto coconut (Cocos nucifera Linn.) root developed adsorbent. Chin J Chem Eng 27:92–99
Georgin J, Franco DSP, Netto MS, Allasia D, Oliveira MLS, Dotto GL (2020) Treatment of water containing methylene by biosorption using Brazilian berry seeds (Eugenia uniflora). Environ Sci Pollut Res 27:20831–20843
Ghosh A, Das P, Sinha K (2015) Modeling of biosorption of Cu (II) by alkali-modified spent tea leaves using response surface methodology (RSM) and artificial neural network (ANN). Appl Water Sci 5:191–199
Gonçalves AL, Pires JCM, Simões M (2017) A review on the use of microalgal consortia for wastewater treatment. Algal Res 24:403–415
Gusmão KA, Guimarães LV, Gurgel A, Melo TMS, Gil LF (2012) Application of succinylated sugarcane bagasse as adsorbent to remove methylene blue and gentian violet from aqueous solutions–kinetic and equilibrium studies. Dyes Pigm 92:967–974
Hossain MA, Ngo HH, Guo W, Zhang J, Liang S (2014) A laboratory study using maple leaves as a biosorbent for lead removal from aqueous solutions. Water Qual Res J Can 49:195–209
Huang D, Li B, Ou J, Xue W, Li J, Li Z, Li T, Chen S, Deng R, Guo X (2020) Megamerger of biosorbents and catalytic technologies for the removal of heavy metals from wastewater: preparation, final disposal, mechanism and influencing factors. J Environ Manag 261:109879
Ighalo JO, Adeniyi AG (2020) Adsorption of pollutants by plant bark derived adsorbents: an empirical review. J Water Process Eng 35:101228
Jain CK, Malik DS, Yadav AK (2016) Applicability of plant based biosorbents in the removal of heavy metals: a review. Environmental Processes 3:495–523
Jobby R, Jha P, Yadav AK, Desai N (2018) Biosorption and biotransformation of hexavalent chromium [Cr (VI)]: a comprehensive review. Chemosphere 207:255–266
Kamel S, Abou-Yousef H, Yousef M, El-Sakhawy M (2012) Potential use of bagasse and modified bagasse for removing of iron and phenol from water. Carbohyd Polym 88:250–256
Kanamarlapudi SLRK, Chintalpudi VK, Muddada S (2018) Application of biosorption for removal of heavy metals from wastewater. Biosorption 18:69
Karri RR, Sahu JN, Meikap BC (2020) Improving efficacy of Cr (VI) adsorption process on sustainable adsorbent derived from waste biomass (sugarcane bagasse) with help of ant colony optimization. Indus Crops Prod 143:111927
Karri RR, Ravindran G, Dehghani MH (2021) Wastewater—sources, toxicity, and their consequences to human health. In: Soft computing techniques in solid waste and wastewater management. Elsevier
Karri RR, Sahu JN (2018) Process optimization and adsorption modeling using activated carbon derived from palm oil kernel shell for Zn (II) disposal from the aqueous environment using differential evolution embedded neural network. J Mol Liq 265:592–602
Kaur M, Mubarak NM, Chin BLF, Khalid M, Karri RR, Walvekar R, Abdullah EC, Tanjung FA (2020) Extraction of reinforced epoxy nanocomposite using agricultural waste biomass. In: IOP conference series: materials science and engineering
Khan FSA, Mubarak NM, Tan YH, Khalid M, Karri RR, Walvekar R, Abdullah EC, Nizamuddin S, Mazari SA (2021) A comprehensive review on magnetic carbon nanotubes and carbon nanotube-based buckypaper for removal of heavy metals and dyes. J Hazard Mater 413
Khraisheh M, Al-Ghouti MA, AlMomani F (2020) P. putida as biosorbent for the remediation of cobalt and phenol from industrial waste wastewaters. Environ Technol Innov 20:101148
Kim SY, Jin MR, Chung CH, Yun Y-S, Jahng KY, Yu K-Y (2015) Biosorption of cationic basic dye and cadmium by the novel biosorbent Bacillus catenulatus JB-022 strain. J Biosci Bioeng 119:433–439
Knidri El, Hakima RB, Addaou A, Laajeb A, Lahsini A (2018) Extraction, chemical modification and characterization of chitin and chitosan. Int J Biol Macromol 120:1181–1189
Koduru JR, Karri RR, Mubarak NM (2019) Smart materials, magnetic graphene oxide-based nanocomposites for sustainable water purification. Sustain Polym Comp Nanocomp 759–781
Kumar KS, Dahms H-U, Won E-J, Lee J-S, Shin K-H (2015) Microalgae–a promising tool for heavy metal remediation. Ecotoxicol Environ Saf 113:329–352
Kunjirama M, Saman N, Johari K, Song S-T, Kong H, Cheu S-C, Lye JWP, Mat H (2017) Adsorption affinity and selectivity of 3-ureidopropyltriethoxysilane grafted oil palm empty fruit bunches towards mercury ions. Environ Sci Pollut Res 24:15167–15181
Kyzas GZ, Kostoglou M (2014) Green adsorbents for wastewaters: a critical review. Materials 7:333–364
Legorreta-Castañeda AJ, Lucho-Constantino CA, Beltrán-Hernández RI, Coronel-Olivares C, Vázquez-Rodríguez GA (2020) Biosorption of water pollutants by fungal pellets. Water 12:1155
Li Q, Chen Bo, Lin P, Zhou J, Zhan J, Shen Q, Pan X (2016) Adsorption of heavy metal from aqueous solution by dehydrated root powder of long-root Eichhornia crassipes. Int J Phytorem 18:103–109
Li J, Ninh Pham A, Dai R, Wang Z, David Waite T (2020) Recent advances in Cu-Fenton systems for the treatment of industrial wastewaters: role of Cu complexes and Cu composites. J Hazard Mater 392:122261
Lingamdinne LP, Vemula KR, Chang Y-Y, Yang J-K, Karri RR, Koduru JR (2020) Process optimization and modeling of lead removal using iron oxide nanocomposites generated from bio-waste mass. Chemosphere 243:125257
Liu Li, Fan S, Li Y (2018) Removal behavior of methylene blue from aqueous solution by tea waste: kinetics, isotherms and mechanism. Int J Environ Res Pub Health 15:1321
Lo Y-C, Cheng C-L, Han Y-L, Chen B-Y, Chang J-S (2014) Recovery of high-value metals from geothermal sites by biosorption and bioaccumulation. Biores Technol 160:182–190
Mahmoud AE, Din MF, Radwan A (2016) Optimization of Cadmium (CD2+) removal from aqueous solutions by novel biosorbent. Int J Phytorem 18:619–625
Makeswari M, Santhi T (2013) Optimization of preparation of activated carbon from Ricinus communis leaves by microwave-assisted zinc chloride chemical activation: competitive adsorption of Ni2+ ions from aqueous solution. J Chem 2013:314790
Makeswari M, Santhi T (2014) Adsorption of Cr (VI) from aqueous solutions by using activated carbons prepared from ricinus communis leaves: Binary and ternary systems. Arab J Chem 57:57–69
Mallek M, Chtourou M, Portillo M, Monclús H, Walha K, Salah A, Salvadó V (2018) Granulated cork as biosorbent for the removal of phenol derivatives and emerging contaminants. J Environ Manag 223:576–585
Manyuchi MM, Sukdeo N, Stinner W, Mutusva TN (2021) Influence of sawdust based biochar on gold tailings wastewater heavy metal contaminants removal. S Afr J Chem Eng 37:81–91
Martín-Lara M Á, Rico ILR, de la Caridad Alomá Vicente I, García GB, de Hoces MC (2010) Modification of the sorptive characteristics of sugarcane bagasse for removing lead from aqueous solutions. Desalination 256:58-63
Mehmood A, Khan FSA, Mubarak NM, Tan YH, Karri RR, Khalid M, Walvekar R, Abdullah EC, Nizamuddin S, Mazari SA (2021) Magnetic nanocomposites for sustainable water purification—a comprehensive review. Environ Sci Pollut Res 28:19563–19588
Mella B, Puchana-Rosero MJ, Costa DES, Gutterres M (2017) Utilization of tannery solid waste as an alternative biosorbent for acid dyes in wastewater treatment. J Mol Liq 242:137–145
Mishra S, Cheng L, Maiti A (2020) The utilization of agro-biomass/byproducts for effective bio-removal of dyes from dyeing wastewater: a comprehensive review. J Environ Chem Eng 104901
Mo J, Yang Q, Zhang N, Zhang W, Zheng Y, Zhang Z (2018) A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment. J Environ Manag 227:395–405
Modak JM, Natarajan KA (1995) Biosorption of metals using nonliving biomass—a review. Min Metall Explor 12:189–196
Mondal DK, Nandi BK, Purkait MK (2013) Removal of mercury (II) from aqueous solution using bamboo leaf powder: equilibrium, thermodynamic and kinetic studies. J Environ Chem Eng 1:891–898
Muraleedharan TR, Iyengar L, Venkobachar C (1991) Biosorption: an attractive alternative for metal removal and recovery. Curr Sci 61:379–385
Nag S, Mondal A, Mishra U, Bar N, Das SK (2016) Removal of chromium (VI) from aqueous solutions using rubber leaf powder: batch and column studies. Desalin Water Treat 57:16927–16942
Nazir H, Salman M, Athar M, Farooq U, Wahab A, Akram M (2019) Citric acid functionalized Bougainvillea spectabilis: a novel, sustainable, and cost-effective biosorbent for removal of heavy metal (Pb2+) from waste water. Water Air Soil Pollut 230:1–16
Ngah WSW, Hanafiah MAKM (2009) Surface modification of rubber (Hevea brasiliensis) leaves for the adsorption of copper ions: kinetic, thermodynamic and binding mechanisms. J Chem Technol Biotechnol: Int Res Process Environ Clean Technol 84:192–201
Nguyen TA, Chun-Chieh Fu, Juang R-S (2016) Biosorption and biodegradation of a sulfur dye in high-strength dyeing wastewater by Acidithiobacillus thiooxidans. J Environ Manage 182:265–271
Oguntimein GB (2015) Biosorption of dye from textile wastewater effluent onto alkali treated dried sunflower seed hull and design of a batch adsorber. J Environ Chem Eng 3:2647–2661
Ouafi R, Rais Z, Taleb M, Benabbou M, Asri M (2017) Sawdust in the treatment of heavy metals-contaminated wastewate. Environ Res J 11
Papirio S, Frunzo L, Mattei MR, Ferraro A, Race M, D’Acunto B, Pirozzi F, Esposito G (2017) Heavy metal removal from wastewaters by biosorption: mechanisms and modeling. In: Sustainable heavy metal remediation. Springer
Pathak PD, Mandavgane SA, Kulkarni BD (2015) Fruit peel waste as a novel low-cost bio adsorbent. Rev Chem Eng 31:361–381
Patil C, Ratnamala GM, Channamallayya ST (2015) Adsorption studies for removal of acid yellow 17 using activated rice husk. Int Res J Eng Technol 2:769
Pereira FV, Gurgel LVA, Gil LF (2010) Removal of Zn2+ from aqueous single metal solutions and electroplating wastewater with wood sawdust and sugarcane bagasse modified with EDTA dianhydride (EDTAD). J Hazard Mater 176:856–863
Peydayesh M, Rahbar-Kelishami A (2015) Adsorption of methylene blue onto Platanus orientalis leaf powder: kinetic, equilibrium and thermodynamic studies. J Ind Eng Chem 21:1014–1019
Priyadarshanee M, Das S (2020) Biosorption and removal of toxic heavy metals by metal tolerating bacteria for bioremediation of metal contamination: a comprehensive review. J Environ Chem Eng 104686
Priyantha N, Kotabewatta PA (2019) Biosorption of heavy metal ions on peel of Artocarpus nobilis fruit: 1—Ni (II) sorption under static and dynamic conditions. Appl Water Sci 9:1–10
Priyanthaa N, Romzib AA, Chanb CM, Limb LBL (2021) Enhancing adsorption of crystal violet dye through simple base modification of leaf adsorbent: isotherm, kinetics, and regeneration. Desalinat Water Treat 215:194–208
Putri KN, Atika SK, Keereerak A, Chinpa W (2021) Facile green preparation of Lignocellulosic Biosorbent from Lemongrass Leaf for Cationic Dye Adsorption. J Polym Environ 29:1681–1693
Putro JN, Kurniawan A, Ismadji S, Yi-Hsu J (2017) Nanocellulose based biosorbents for wastewater treatment: study of isotherm, kinetic, thermodynamic and reusability. Environ Nanotechnol Monitori Manag 8:134–149
Ramírez-Rodríguez AE, Reyes-Ledezma JL, Chávez-Camarillo GM, Cristiani-Urbina E, Morales-Barrera L (2018) Cyclic biosorption and desorption of acid red 27 onto Eichhornia crassipes leaves. Revista Mexicana De Ingeniería Química 17:1121–1134
Reddy DH, Kumar YH, Seshaiah K, Reddy AVR (2010) Biosorption of Pb (II) from aqueous solutions using chemically modified Moringa oleifera tree leaves. Chem Eng J 162:626–634
Rigueto CVT, Piccin JS, Dettmer A, Rosseto M, Dotto GL, de Oliveira Schmitz AP, Perondi D, de Freitas TSM, Loss RA, Geraldi CAQ (2020) Water hyacinth (Eichhornia crassipes) roots, an amazon natural waste, as an alternative biosorbent to uptake a reactive textile dye from aqueous solutions. Ecol Eng 150:105817
Riquelme M, Aguirre J, Bartnicki-García S, Braus GH, Feldbrügge M, Fleig U, Hansberg W, Herrera-Estrella A, Kämper J, Kück U (2018) Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. Microbiol Mol Biol Rev 82:e00068-e117
Saha S, Muhammad Zubair MA, Khosa SS, Ullah A (2019) Keratin and chitosan biosorbents for wastewater treatment: a review. J Polym Environ 27:1389–1403
Sahu JN, Karri RR, Zabed HM, Shams S, Qi X (2019) Current perspectives and future prospects of nano-biotechnology in wastewater treatment. Separat Purificat Rev 1–20
Sangi MR, Shahmoradi A, Zolgharnein J, Azimi GH, Ghorbandoost M (2008) Removal and recovery of heavy metals from aqueous solution using Ulmus carpinifolia and Fraxinus excelsior tree leaves. J Hazard Mater 155:513–522
Saraswat SK, Demir M, Gosu V (2020) Adsorptive removal of heavy metals from industrial effluents using cow dung as the biosorbent: kinetic and isotherm modeling. Environ Qual Manage 30:51–60
Sarker TC, Md Golam Gousul Azam S, El-Gawad AMA, Gaglione SA, Bonanomi G (2017) Sugarcane bagasse: a potential low-cost biosorbent for the removal of hazardous materials. Clean Technol Environ Policy 19:2343–2362
Sarode S, Punita Upadhyay MA, Khosa TM, Shakir A, Song S, Ullah A (2019) Overview of wastewater treatment methods with special focus on biopolymer chitin-chitosan. Int J Biol Macromol 121:1086–1100
Şentürk İ, Yıldız MR (2020) Highly efficient removal from aqueous solution by adsorption of Maxilon Red GRL dye using activated pine sawdust. Korean J Chem Eng 37:985–999
Shafiq M, Alazba AA, Amin MT (2018) Removal of heavy metals from wastewater using date palm as a biosorbent: a comparative review. Sains Malaysiana 47:35–49
Shamsollahi Z, Partovinia A (2019) Recent advances on pollutants removal by rice husk as a bio-based adsorbent: a critical review. J Environ Manage 246:314–323
Sheikh Z, Amin M, Khan N, Khan MN, Sami SK, Khan SB, Hafeez I, Khan SA, Bakhsh EM, Cheng CK (2021) Potential application of Allium Cepa seeds as a novel biosorbent for efficient biosorption of heavy metals ions from aqueous solution. Chemosphere 279:130545
Sheth Y, Dharaskar S, Khalid M, Sonawane S (2021) An environment friendly approach for heavy metal removal from industrial wastewater using chitosan based biosorbent: a review. Sustain Energy Technol Assess 43:100951
Singh S, Kumar V, Datta S, Dhanjal DS, Sharma K, Samuel J, Singh J (2020) Current advancement and future prospect of biosorbents for bioremediation. Sci Total Environ 709:135895
Singha B, Das SK (2012) Removal of Pb (II) ions from aqueous solution and industrial effluent using natural biosorbents. Environ Sci Pollut Res 19:2212–2226
Sörme L, Lagerkvist R (2002) Sources of heavy metals in urban wastewater in Stockholm. Sci Total Environ 298:131–145
Suazo-Madrid A, Morales-Barrera L, Aranda-García E, Cristiani-Urbina E (2011) Nickel (II) biosorption by Rhodotorula glutinis. J Ind Microbiol Biotechnol 38:51–64
Sun Y, Zhou S, Sun W, Zhu S, Zheng H (2020) Flocculation activity and evaluation of chitosan-based flocculant CMCTS-gP (AM-CA) for heavy metal removal. Separat Purificat Technol 241:116737
thi Quyen V, Pham T-H, Kim J, Thanh DM, Thang PQ, Le QV, Jung JSH, Kim T (2021) Biosorbent derived from coffee husk for efficient removal of toxic heavy metals from wastewater. Chemosphere 131312
Torres E (2020) Biosorption: a review of the latest advances. Processes 8:1584
Tsezos M, Remoundaki E, Hatzikioseyian A (2006) Biosorption-principles and applications for metal immobilization from waste-water streams. In: Proceedings of EU-Asia workshop on clean production and nanotechnologies, Seoul, pp 23–33
Ubando AT, Africa ADM, Maniquiz-Redillas MC, Culaba AB, Chen W-H, Chang J-S (2021) Microalgal biosorption of heavy metals: a comprehensive bibliometric review. J Hazard Mater 402:123431
ul Haq A, Saeed M, Usman M, Zahoor AF, Anjum MN, Maqbool T, Naheed S, Kashif M (2021) Mechanisms of halosulfuron methyl pesticide biosorption onto neem seeds powder. Sci Re 11:1–13
Ullrich AH, Smith MW (1951) The biosorption process of sewage and waste treatment. Sewage Indus Wastes 1248–1253
Van Suc N, Son LN (2016) Mistletoe leaves as a biosorbent for removal of Pb (II) and Cd (II) from aqueous solution. Desalinat Water Treat 57:3606–3618
Vijayaraghavan K, Yun Y-S (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291
Xiong Chang X, Mujawar Mubarak N, Ali Mazari S, Sattar Jatoi A, Ahmad A, Khalid M, Walvekar R, Abdullah EC, Karri RR, Siddiqui MTH, Nizamuddin S (2021) A review on the properties and applications of chitosan, cellulose and deep eutectic solvent in green chemistry. J Ind Eng Chem 104:362–380
Yu Z, Han H, Feng P, Zhao S, Zhou T, Kakade A, Kulshrestha S, Majeed S, Li X (2020) Recent advances in the recovery of metals from waste through biological processes. Bioresourc Technol 297:122416
Yusof NH, Foo KY, Hameed BH, Hazwan Hussin M, Lee HK, Sabar S (2020) One-step synthesis of chitosan-polyethyleneimine with calcium chloride as effective adsorbent for Acid Red 88 removal. Int J Biol Macromol 157:648–658
Zaidi M, Hazirah NA, Lim LBL, Priyantha N, Usman A (2018) Artocarpus odoratissimus leaves as an eco-friendly adsorbent for the removal of toxic Rhodamine B Dye in aqueous solution: equilibrium isotherm, kinetics, thermodynamics and regeneration studies. Arab J Sci Eng (Springer Science & Business Media BV) 43
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nematollahzadeh, A., Vaseghi, Z. (2022). Biosorbents in Industrial Wastewater Treatment. In: Karchiyappan, T., Karri, R.R., Dehghani, M.H. (eds) Industrial Wastewater Treatment . Water Science and Technology Library, vol 106. Springer, Cham. https://doi.org/10.1007/978-3-030-98202-7_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-98202-7_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-98201-0
Online ISBN: 978-3-030-98202-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)