Abstract
Purpose
The purpose of the research is to investigate the applicability of the low-cost natural biosorbents for the removal of Pb(II) ions from aqueous solution and effluent from battery industry.
Methods
Six different biosorbents namely rice straw, rice bran, rice husk, coconut shell, neem leaves, and hyacinth roots have been used for the removal of Pb(II) ions from aqueous solution in batch process. All the biosorbents were collected from local area near Kolkata, West Bengal, India. The removal efficiency was determined in batch experiments for each biosorbent.
Results
The biosorbents were characterized by SEM, FTIR, surface area, and point of zero charge. The sorption kinetic data was best described by pseudo-second-order model for all the biosorbents except rice husk which followed intraparticle diffusion model. Pb(II) ions adsorption process for rice straw, rice bran, and hyacinth roots were governed predominately by film diffusion, but in the case of rice husk, it was intraparticle diffusion. Film diffusion and intraparticle diffusion were equally responsible for the biosorption process onto coconut shell and neem leaves. The values of mass transfer coefficient indicated that the velocity of the adsorbate transport from the bulk to the solid phase was quite fast for all cases. Maximum monolayer sorption capacities onto the six natural sorbents studied were estimated from the Langmuir sorption model and compared with other natural sorbents used by other researchers. The Elovich model, the calculated values of effective diffusivity, and the sorption energy calculated by using the Dubinin–Radushkevich isotherm were indicated that the sorption process was chemical in nature. The thermodynamic studies indicated that the adsorption processes were endothermic. FTIR studies were carried out to understand the type of functional groups responsible for Pb(II) ions binding process. Regeneration of biosorbents were carried out by desorption studies using HNO3. Battery industry effluents were used for the application study to investigate applicability of the biosorbents.
Conclusion
The biosorbents can be utilized as low-cost sorbents for the removal of Pb(II) ions from wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metals in aqueous system such as copper, lead, cadmium, chromium, zinc, mercury, etc. are not biodegradable and tend to accumulate in living organisms, causing various diseases and disorders (Liao et al. 2010; Mahmoud et al. 2010). Increasing concentration of these metals in the water constitutes a severe health hazard for their toxicity, persistent in nature particularly when it exceeds the permissible limits. These metal ions exist in wastewater from many industries such as smelting, metal plating, mining, galvanizing, glass operations, paints, pigments, lead batteries, insecticides, electroplating, cosmetics, stabilizer, alloy manufacturing, and agricultural activities (Naiya et al. 2009a; Wang et al. 2010).
The tolerance limits of Pb(II) ion concentration in drinking water and discharge in inland surface water are 0.05 and 0.10 mg/L, respectively, according to the Indian Standard (IS 10500 1992). The Environmental Protection Agency and World Health Organization have suggested 0.015 and 0.01 mg/L, respectively, for safe drinking water containing Pb(II) ions (http://www.epa.gov/safewater/contaminants/index.html, 09 Aug 2007; http://www.who.int/water_sanitation_health/dwq/gdwq3rev/en/index.html, 09 Aug 2007). Therefore, the removal of Pb(II) in wastewater has received much attention in recent years.
Many methods have been developed to remove heavy metals from wastewater, namely adsorption, precipitation, ion exchange, flotation, membrane-related process, electrochemical technique and biological process, etc. Among these methods, adsorption is quite promising due to its high efficiency, easy handling, availability of different sorbents, and cost-effectiveness (Naiya et al. 2009b). Lead-ion removal from aqueous solution has been reported in many recent publications where different inexpensive and locally abundantly available biosorbents such chaff, hazelnut shells, rice husk, sawdust, crab shell, arca shell, olive stone, coir, coca shells, apple residues, hazelnut husk, barley straws, and de-oiled allspice husk, etc. were used (Lee et al. 1998; Cimino et al. 2000; Khalid et al. 2001; Meunier et al. 2003; Han et al. 2005; Conrad and Hansen 2007; Li et al. 2007; Dahiya et al. 2008; Imamoglu and Tekir 2008; Pehlivan et al. 2009; Blazquez et al. 2010; Cruz-Olivares et al. 2010). Studies have been reported in literature for the removal of heavy metals using different industrial waste and algae as adsorbents (Ali and Gupta 2007; Ali 2010; Gupta et al. 1997, 1998, 1999, 2001, 2004, 2007, 2009, 2010a, b, 2011; Gupta and Andimranali 2008; Gupta and Ali 2004; Gupta and Rastogi 2008a, b, 2009; Gupta and Sharma 2003; Khan et al. 2009; Srivastava et al. 1997).
The objective of this study was to investigate the feasibility of Pb(II) ion removal from aqueous solution onto six natural biosorbents namely rice straw, rice bran, rice husk, hyacinth roots, neem leaves, and coconut shell as low-cost adsorbent in the batch process. The desorption study and application study using battery industry effluents were also reported.
2 Materials and methods
2.1 Sorbent
Rice straw, rice bran, rice husk, coconut shell, neem leaves, and hyacinth roots were used as low-cost natural or agricultural wastes for Pb(II) ion removal from aqueous solution. All the sorbents were collected from local area near Kolkata, West Bengal, India.
Rice straw, rice bran, rice husk, and hyacinth roots were boiled for 6 h to remove color materials. Coconut shell was crashed in roll crusher and then grinded. Neem leaves and coconut shell were treated with 0.1 N NaOH to remove lignin-based color materials followed by 0.1 N H2SO4. Finally all the sorbents were washed with distilled water several times and dried at 105°C for 6 h. After drying, all the sorbents were sieved to obtain particle size of 250–350 μm prior to use for sorption studies.
2.2 Sorbate
The stock solution containing 1,000 mg/L of Pb(II) ions was prepared by dissolving 1.61 g of lead nitrate [Pb(NO3)2] in 1,000 mL of double distilled water. The range of Pb(II) ions concentration was varied from 5 to 300 mg/L by diluting stock solution.
2.3 Reagent and equipment
All the necessary chemicals used in the study were of analytical grade and obtained from E. Merck Limited, Mumbai, India. The pH of the solution was measured with EUTECH make digital microprocessor-based pH meter previously calibrated with standard buffer solutions. Analysis of Pb(II) ions and other metal ions were carried out using atomic absorption spectrophotometer (AA 240 VARIAN, Australia). Scanning electron microscope (S-3400N; Hitachi, Japan) study was conducted to observe the surface texture of the sorbents. Fourier transform infrared spectroscopy (FTIR; Jasco FT/IR-670 Plus) studies were carried out to determine the type of functional group responsible for Pb(II) ions sorption. The surface area was measured by Micromeritics surface area analyzer (ASAP 2020). The moisture content determination was carried out with a digital microprocessor-based moisture analyzer (Metteler LP16). The point of zero charge was determined by solid addition method (Srivastava et al. 2006) and reported in Table 1.
2.4 Characterization of sorbents
Scanning electron micrographs of all the biosorbents are shown in Fig. 1a–f. These figures show that the sorbents were irregular and porous in nature. FTIR study was also carried out to identify the functional groups presents in the biosorbents at 4,000–400 cm−1 range. Surface area, bulk density, dry matter, moisture content, point of zero charge, and ash content of all the biosorbents were measured and shown in Table 1.
2.5 Batch sorption studies
Batch sorption experiments were carried out to determine the optimum Pb(II) ion sorption conditions onto biosorbents in a 250-mL stopper conical flask. The pH of the solutions was adjusted by adding HCl or NaOH solution as per required at the beginning of the experiment. Necessary amount of sorbent was then added, and content in these flasks was shaken for the desired contact time in an electrically thermostated reciprocating shaker at 110–125 strokes/minute at 30°C. The time required to reach the equilibrium was estimated by withdrawing conical flask containing treated solution at regular intervals of time. After completion of the sorption process, the sorbent was separated from the solution using filter paper (Whatman no. 42, pore size—2.5 μm) and the supernatant was analyzed for residual Pb(II) concentration by atomic absorption spectrophotometer at the wavelength of 283.3 nm (APHA 1998). The percentage of removed Pb(II) ions in solution was calculated using following equation:
The amount of Pb(II) ions adsorbed (q) per unit of empty biosorbents at any time was obtained by the following equation:
All the investigations were carried out in triplicate, and average result was reported to avoid any discrepancy. The reproducibility and the relative deviation were of the order of ± 0.5% and ± 2.5%, respectively.
3 Results and discussion
3.1 Effect of initial pH
The acidity of the solution is one of the most important factors affecting the sorption process (Lalhruaitluanga et al. 2010). In general, the sorption of cation is favored at pH > pHpzc. Figure 2 shows the effect pH on the removal of Pb(II) ions from aqueous solution. The results showed that the sorption capacities of Pb(II) ions increased significantly as pH increased from 2.0 to 5.0 for all the sorbents. At low pH (pH 2–3) less metal ions sorption occurred, this may be explained on the basis of the binding sites being protonated, resulting in a competition between H+ and Pb(II) ions for occupancy of binding sites (Naiya et al. 2009a; Hasana et al. 2010; Reddy et al. 2010). As the pH increases and biosorbents surface functional groups are activated, it results in increased Pb(II) ions sorption. The Pb(II) ions may undergo hydrolysis and salvation in aqueous solution as follows (Naiya et al. 2009b):
In the present study, maximum removal was occurred at pH 5. With further increase of pH, Pb(II) ions started to precipitate out from the solution as lead hydroxide. So at pH > 5, removal efficiency increased may be a combination of both sorption onto biosorbents surface and precipitation of lead hydroxide from the solution. Therefore experiments were not conducted at pH > 5. A similar trend has also been reported in the removal of Pb(II) ions onto other biosorbents (Low et al. 2000; Saeeda et al. 2005). After sorption the final pH was changed from 5 to 6.45, 6.23, 5.97, 6.20, 6.15, and 6.69 for rice straw, rice bran, rice husk, coconut shell, neem leaves, and hyacinth roots, respectively.
3.2 Effect of contact time, initial Pb(II) ions concentration, and biosorbent dosage
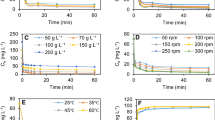
The effect of contact time on the sorption of Pb(II) ions onto different biosorbents is shown in Fig. 3. The initial rate of adsorption was rapid, it indicated to give away a very slow approach to equilibrium and accounted for the major part in the total Pb(II) ions sorption. Then sorption reached a plateau value, i.e., equilibrium which showed that the saturation of the active sites of the biosorbent. The time required to reach equilibrium for the sorption of Pb(II) ions were 2.5 h for rice straw, rice bran, and rice husk, and 2.0 h for hyacinth roots, neem leaves, and coconut shell.
Percent removal decreases with the increase in initial Pb(II) ions concentration from 5 to 300 mg/L. At the lower concentration, all the Pb(II) ions in the solution would react with the binding sites and thus facilitated almost complete sorption (Naiya et al. 2009a, b). At higher concentration more Pb(II) ions left unsorbed in the solution due to the saturation of the binding sites. This indicates that the energetically less favorable sites become involve with increasing Pb(II) ions concentration in aqueous solution. Sorption capacity increases from the range of 0.4 to 21.0 mg/g when initial concentration of Pb(II) ions increases from 5 to 300 mg/L.
The effect of sorbent types and dosage (1–15 g/L) for the removal of Pb(II) ions from aqueous solution using initial metal ion concentration at 25 mg/L were carried out. The removal efficiency was found to increase rapidly and decreases sorption capacity at optimum pH for all the cases which may be attributed to increase surface area of the biosorbent and availability of more sorption sites due to increase amount of biosorbent. Table 2 represents the range of variables and optimum condition obtained from batch experiment for the process.
3.3 Sorption kinetics
In order to determine kinetic parameters and to explain the mechanism of biosorption process, pseudo-first-order model, pseudo-second-order model, and intraparticle diffusion model are used (Lagergren 1898; Weber and Morris 1963; Ho et al. 2000). The mechanism of sorption depends on the physical and chemical characteristics of the sorbent as well as on the mass transfer process (Naiya et al. 2009b). The results obtained from the effect of contact time variation were used to study the kinetics on the Pb(II) ions sorption. The conformity between experimental data and the model predicted values was expressed by statistical parameter. The results of different rate constants along with statistical parameter are listed in Table 3.
The linearity of the Lagergren, pseudo-second-order, and intraparticle diffusion model indicated the applicability of these models. The high correlation coefficients (r 2) and low chi-square (\( \chi_t^2 \)) values indicate that the sorption of Pb(II) ions onto biosorbents follow the pseudo-second-order model except rice husk. Sorption of Pb(II) ions onto rice husk better follows the intraparticle diffusion model.
3.4 Reichenberg model
The rate of sorption also determined by applying well-known equation for the diffusion and mass transfer phenomena. For the fast reaction, the sorption may be due to film diffusion (Reichenberg 1953) and occur within the micropore of the sorbents. The Reichenberg equation is as follows:
The above equation may be written as
Where, F(t) is the ratio of amount of Pb(II) ions adsorbed per gram of biosorbent at any time to that of at equilibrium time. B is the time-dependent factor. The plot of Bt versus t was linear with a correlation coefficient of 0.8320, 0.8045, 0.7655, 0.9276, 0.9476, and 0.9869 for the sorption of Pb(II) ions on rice straw, rice bran, rice husk, coconut shell, neem leaves, and hyacinth roots, respectively, as shown in Fig. 4. The higher correlation coefficient for hyacinth roots indicates that the sorption process was controlled by film diffusion.
3.5 Elovich model
The Elovich model (Low 1960) is initially proposed for the description of the kinetics of the chemisorption of gases on solids. In this model, a variation in the energy of chemisorption is attributed to a change in the surface coverage or to a continuous and specific range of site reactivities (Teng and Hsieh 1999). The equation is formulated as
where a 1, b 1, and \( {t_0} = \frac{1}{{{a_1} \cdot {b_1}}} \)are constants. Parameter a 1 gives an idea of the reaction rate constant, b 1 represents the rate of chemisorption at zero coverage, and t 0 is an adjustable parameter that makes the plot liner over the entire range (Fig. 5). In these cases of sorption process, the linear correlation coefficients were 0.9842, 0.9803, 0.9909, 0.9737, 0.9811, and 0.9442 for the sorption onto rice straw, rice bran, rice husk, coconut shell, neem leaves, and hyacinth roots, respectively, which indicated the process of sorption was chemical in nature (Ho and McKay 2002).
3.6 Prediction of rate limiting step
Fick’s equation is used (Eq. 9) to describe the diffusion process of Pb(II) ions onto different biosorbents surface (Seeber et al. 1998; Zaki et al. 2000; Qin et al. 2007). Generally, the sorption rate is controlled by film diffusion (outer diffusion) or intraparticle diffusion (inner diffusion) or both (Kalavathy et al. 2005).
The plot of \( \frac{{{q_t}}}{{{q_{\infty }}}} \) (\( {q_{\infty }} \)was replaced by \( {q_e} \)) versus \( \sqrt {t} \) is shown in Fig. 6 for hyacinth roots. The first linear portion indicated to the film diffusion, the second linear portion related the intraparticle diffusion, and the last linear portion suggested sorption–desorption equilibrium. Figure 6 showed that the diffusion took about 75 min while the intraparticle diffusion took 45 min. The ratio of the time taken by film diffusion to intraparticle diffusion was 5:3. A similar type of result is also found for other biosorbents. The ratio of time taken by film diffusion to intraparticle were 4:1, 4:1, 1:2, 1:1, and 1:1 for rice straw, rice bran, rice husk, coconut shell, and neem leaves, respectively. So in the case of rice straw, rice bran and hyacinth roots film diffusion was predominated over the intraparticle diffusion but in case of rice husk it was reverse. Film diffusion and intraparticle diffusion were equally responsible for coconut shell and neem leaves.
3.7 Mass transfer analysis
Mass transfer analysis for the sorption of Pb(II) ions from aqueous solutions by the six different natural sorbents are carried out by using the following equation (McKay et al. 1981):
Figure 7 represents the plot of \( \ln \left( {\frac{{{C_t}}}{{{C_0}}} - \frac{1}{{1 + M{K_{{bq}}}}}} \right) \) versus t resulted a straight line of slope of \( \left( {\frac{{1 + M{K_{{bq}}}}}{{M{K_{{bq}}}}}} \right)\beta {S_S} \). Table 4 shows the values of mass transfer coefficients, and it indicated that the velocity of the adsorbate transport from the bulk to the solid phase was quite fast.
3.8 Determination diffusivity
Experimental kinetic data could be treated by the Boyd et al. (1947) model. With the diffusion rate-controlling sorption process in the sorption of Pb(II) ions onto biosorbents, the solution of the simultaneous set of differential and algebraic equation leads to
Assuming spherical shape of the sorbents, the Vermeulen’s approximation (Vermeulen 1953) of Eq. 11 fits the whole range 0 < F(t) < 1 as
The plot of \( \ln \left[ {\frac{1}{{1 - {F^2}(t)}}} \right] \) versus t (Fig. 8) is a line whose slope, \( \frac{{{\pi^2}}}{{{R^2}_a}}{D_e} \), thus the diffusion coefficient, D e , can be calculated. The value of diffusion coefficient as calculated is shown in Table 4. The values of D e falls well within the values reported in literature, especially for chemisorptions system 10−9–10−17 m2/s (Srivastava et al. 2009).
3.9 Isotherm studies
The interaction between sorbate and sorbent are frequently interpreted using the Langmuir and the Freundlich isotherm models (Langmuir 1918; Freundlich 1906). The Langmuir model is based on the assumption of a structurally homogeneous sorbent where all sorption sites are identical and energetically equivalent and assuming monolayer sorption onto the sorbent surface. The Langmuir isotherm model is represented by the first equation in Table 5.
Webi and Chakravort (1974) expressed the essential characteristics and feasibility of the Langmuir isotherm in terms of a dimensionless constant, separation factor, or equilibrium parameter, R L (second equation in Table 5). If this value ranges in between 0 and 1, then the sorption process is favorable. For all the experimental data, the values of R L that were lying between 0 and 1 indicated favorable sorption (McKay et al. 1982).
The Freundlich isotherm model is applied to describe heterogeneous systems characterized by a heterogeneity factor of 1/n.This model describes reversible sorption and is not restricted to the formation of the monolayer. The Freundlich isotherm model is expressed by the third equation in Table 5. Table 5 indicates the Langmuir and Freundlich constants along with the statistical parameters. The values of n in the Freundlich constant lies between 1 and 10 for all biosorbents represent favorable sorption (Bhattacharya et al. 2008). From the Table 5 it can be concluded that the sorption of Pb(II) onto all the biosorbents follows the Freundlich adsorption isotherm model. Hyacinth roots had maximum Pb(II) ions sorption capacity (24.9376 mg/g) than other biosorbents studied.
3.10 Sorption energy calculation
The Langmuir or Freundlich isotherm does not give any idea about the sorption processes whether it is chemical or physical, but the Dubinin–Radushkevich (Dubinin et al. 1947) isotherm was used to predict the nature of sorption processes by calculating sorption energy. The linear from of the model is described as
From Fig. 9 a plot of ln\( {C_{\text{abs}}} \) versus \( {\varepsilon^2} \) gave a straight line from which the values of \( \lambda \) and \( {X_m} \)for all the sorbents were calculated. Mean sorption energy (E) was calculated using
where E is the energy required to adsorb 1 mol of Pb(II) ions from aqueous solution. The mean sorption energy, E is below 8 kJ/mol, then the sorption process can be explained by physical sorption, between 8 and 16 kJ/mol it can be explained by chemical sorption (Dubinin et al. 1947; Sawalha et al. 2006). The estimated values of E were 10.174, 11.293, 10.672, 10.391, 10.358, and 11.251 kJ/mol for rice straw, rice bran, rice husk, coconut shell, neem leaves, and hyacinth roots, respectively, which suggested the sorption process was chemical in nature.
3.11 Thermodynamic study
To study the thermodynamic behavior of Pb(II) ions, the thermodynamic parameters such as change in Gibbs free energy (\( \Delta {G^0} \)), enthalpy (\( \Delta {H^0} \)), and entropy (\( \Delta {S^0} \)) are calculated. The thermodynamic equilibrium constant (\( K_c^0 \)) are obtained by calculating apparent equilibrium constant (\( {K'_c} \)) at different temperature and initial Pb(II) ions concentration for each system and extrapolating to zero (Naiya et al. 2009a).
The change of Gibbs free energy (\( \Delta {G^0} \)), enthalpy (\( \Delta {H^0} \)), and entropy (\( \Delta {S^0} \)) for the biosorption processes are calculated by using the following equations
From the slope and intercept of the plot \( \ln K_c^0 \) versus 1/T, the values of \( \Delta {H^0} \)and \( \Delta {S^0} \) had been computed, while \( \Delta {G^0} \) was calculated using Eq. 17 and reported in Table 6. The results indicated that the biosorption processes were endothermic in nature. The heat of sorption values that were lying between 20.658 and 41.299 kJ mol−1 suggested that the processes were chemical in nature (Unlu and Ersoz 2007). The negative value of \( \Delta {G^0} \) at all temperatures indicated the feasibility of biosorption process. The positive value of \( \Delta {S^0} \) predicted the increased randomness at the solid/solution interface during the Pb(II) sorption process.
3.12 FTIR studies
FTIR is used to investigate the changes in vibration frequency in the functional groups of the sorbents due to Pb(II) ions sorption. The sorbents were scanned in the spectral range of 4,000–400 cm−1. The functional group is one of the key factors to understand the mechanism of metal binding process on natural sorbents.
Figure 10 shows the FTIR plot for fresh and Pb(II) ions loaded coconut shell. Table 7 represented the shift from the wave number of dominant peak associated with the fresh and Pb(II) ions loaded sorbents in the FTIR plots. There were clear shift from wave number of 3,348.78 cm−1 (raw rice straw) to 3,296.13 cm−1 (metal loaded rice straw), 3,342.03 cm−1 (raw rice bran) to 3,334.31 cm−1 (metal loaded rice bran), 3,385.42 cm−1 (raw rice husk) to 3,465.04 cm−1 (metal loaded rice husk), and 3,328.53 cm−1 (raw hyacinth roots) to 3,273.90 cm−1 (metal loaded hyacinth roots) which indicated surface –OH group was one of the functional group responsible for sorption. Aliphatic C=O group was responsible for sorption of Pb(II) onto coconut shell only which was inferred from the shift of the peak more than 10 cm−1. Unsaturated group like alkene was also active on rice straw, rice bran, and rice husk.
Aromatic C–NO2 stretching was found to have major shift of wave number from 1,515.77 cm−1 (raw rice husk) to 1,532.46 cm−1 (metal loaded rice husk), 1,514.81 cm−1 (raw hyacinth root) to 1,541.10 cm−1 (metal loaded hyacinth root), and 1,515.46 cm−1 (raw neem leaves) to 1,557.15 cm−1 (metal loaded neem leaves) for the sorption of Pb(II). Alkane group was only responsible for Pb(II) ions sorption onto coconut shell as indicated in Table 7. Rice straw FTIR spectrum also showed intense bands around 1,321.00 cm−1 which shifted to 1,338.60 cm−1 for Pb(II) ions loaded rice straw. This was attributed that the carboxylate anion was responsible for the sorption on rice straw.
Si–O stretching was also responsible for Pb(II) sorption onto rice waste as indicated in Table 7. Sulfonic acid S=O stretching was found be to responsible for Pb(II) sorption onto hyacinth roots only.
Table 7 also indicated that the minor shift for the other band (aliphatic C–H, aldehyde C–H stretching, phosphate ester group, –SO3 group, and sulfonyl chloride group) which showed that these groups were not involved in the sorption process. Atomic radius of Pb(II) ions is 1.75 Å, and it fits well into the binding site of the biosorbents.
3.13 Desorption studies
The Pb(II) ions loaded biosorbents creates a serious disposal problem as it is hazardous for environment. This problem may be minimizing by regeneration process. In this process adsorbed Pb(II) ions elutriate to the solution and regenerated the biosorbents. The concentrated metal solution may be suitable for the recovery of Pb(II) ions. The regenerated biosorbent may be recycled for reuse to reduce the wastewater treatment cost. So regeneration process, i.e., desorption study was important and was carried out at different concentration of HNO3. Desorption experiments were performed maintaining the process condition similar to the batch experiments. It was evident from Table 8 that the maximum desorption efficiency was 95.76% for coconut shell. In the desorption process, H+ ions replace the Pb(II) ions on the metal-loaded biosorbents, thus acting as a cation exchanger.
3.14 Comparison studies
The sorption capacity of Pb(II) ions onto different biosorbents were compared with other biosorbents reported in literature and is shown in Table 9. The sorbents capacities vary due to the properties of each sorbent such as structure, functional groups, and surface area, etc.
3.15 Treatment of battery industries effluents
A wastewater containing Pb(II) ions are collected from a medium-sized battery industry at Kolkata, India. This wastewater has been used to remove the Pb(II) ions to determine the suitability of natural biosorbents in batch condition. The characteristics of the wastewater are shown in Table 10. The batch sorption studies were carried out under optimum conditions of pH, contact time, and sorbent dosage level for all the sorbents used in the study. The final results are reported in Table 10. It is clear from the table that the coconut shell is the most suitable biosorbent for the treatment of battery industries effluents containing Pb(II) ions. The coconut shell and hyacinth root as sorbents give Pb(II) ions concentration in the treated effluent below 0.1 mg/L which follows the norms of Indian Standard of the discharge of the wastewater containing Pb(II) ions (IS 10500 1992). The other ions present in the battery industry wastewater also removed to a small extent.
4 Conclusions
Removal of Pb(II) ions from aqueous solutions and battery industry wastewater has been carried out using six low-cost biosorbents in the batch process. The optimum pH was found to be 5. The statistical parameter indicated that the adsorption process follows the pseudo-second-order model for all the sorbents, but it was intraparticle diffusion for rice husk. Film diffusion and intraparticle diffusion were jointly responsible for Pb(II) ions sorption. The effective diffusivity calculated by using Vermeulen’s approximation indicated that the interaction between Pb(II) ions on natural sorbents was chemical in nature. All the sorption processes follow the Freundlich sorption isotherm model. Hyacinth roots had maximum Pb(II) ions (24.9376 mg/g) sorption capacities than other biosorbents studied. Sorption energy calculated from D-R showed the chemisorptions process for all the sorbents. The thermodynamic study indicated the sorption process was spontaneous and endothermic in nature. FTIR studies indicated that the different functional groups were responsible for Pb(II) binding process. Desorption experiments were performed using different concentration of HNO3 to reuse the biosorbents. The maximum desorption efficiency was found for coconut shell, and it was comparable for other biosorbents. Natural sorbents can be used as an effective removal Pb(II) ions from industrial wastewater containing Pb(II) ions.
Abbreviations
- a 1 :
-
Elovich constant which gives an idea of the reaction rate constant
- b :
-
Langmuir constant (in liters per milligram)
- B :
-
Time-dependent factor
- b 1 :
-
Elovich constants and represents the rate of chemisorption at zero coverage
- C :
-
Intraparticle diffusion constant
- C a :
-
Pb(II) ions concentration on the sorbent at equilibrium (in milligrams per liter)
- Cabs :
-
The amount of Pb(II) adsorbed onto sorbent surface (in moles per gram)
- C e :
-
Pb(II) ions concentration in solution at equilibrium (in milligrams per liter)
- C 0 :
-
Initial Pb(II) ions concentration (in milligrams per liter)
- C t :
-
Pb(II) ions concentration at time t (in milligrams per liter)
- D e :
-
Effective diffusion coefficient of adsorbates in the sorbent phase (in square meters per second)
- E :
-
Mean sorption energy (in kilojoules per mole)
- F(t):
-
Ratio of amount of Pb(II) ions adsorbed per gram of sorbent at any time to that of at equilibrium time
- ∆G 0 :
-
Gibbs free energy (in kilojoules per mole)
- ∆H 0 :
-
Enthalpy (in kilojoules per mole)
- K 1 :
-
Lagergren rate constant (per minute)
- K 2 :
-
Pseudo-second-order rate constant (in milligrams per gram per minute)
- K i :
-
Intraparticle rate constant (in milligrams per gram per square root of minute)
- K bq :
-
The constant obtained by multiplying q max and b
- \( K_c^0 \) :
-
Thermodynamic equilibrium constant
- \( {K\prime_c} \) :
-
Apparent equilibrium constant
- M :
-
Mass of the sorbent per unit volume (in grams per liter)
- m s :
-
Amount of sorbent added in gram
- n :
-
Freundlich constants intensity of sorption (in milligrams per gram)/(in milligrams per liter)1/n
- n 1 :
-
An integer
- q e :
-
Amount adsorb per gram of the sorbent at equilibrium
- q max :
-
Maximum sorption capacity (in milligrams per gram)
- q t :
-
Amount (in milligrams) adsorb per gram of sorbent
- q tm :
-
Amount (in milligrams) adsorb per gram of sorbent from model
- \( q_{ \propto } \) :
-
Amount (in milligrams) adsorb per gram of sorbent at infinite time
- r 2 :
-
Correlation coefficient
- R :
-
Ideal gas constant in kilojoules per mole per kelvin
- R L :
-
Separation factor
- R a :
-
Radius of the sorbent particle (in meter)
- S S :
-
External surface area of the sorbent per unit volume (per meter)
- ∆S 0 :
-
Entropy (in kilojoules per mole kelvin)
- t :
-
Time (in minutes)
- T:
-
Temperature in kelvin
- TDS:
-
Total dissolves solid
- t 0 :
-
Elovich constant equals to 1/(a 1·b 1)
- V :
-
Volume of the solution (in liters)
- X m :
-
Maximum sorption capacity of sorbent (in millimoles per gram)
- β :
-
Mass transfer coefficient (in centimeters per second)
- λ :
-
Constant related to sorption energy (in square moles per square kilojoule)
- ε :
-
Polanyi potential (in square kilojoules per square mole)
- \( \chi_t^2 \) :
-
Chi-square value (\( \chi_t^2 = \sum {\frac{{{{({q_t} - {q_{{tm}}})}^2}}}{{{q_{{tm}}}}}} \))
Reference
Ali I (2010) The quest for active carbon adsorbent substitutes: inexpensive adsorbents for toxic metal ions removal from wastewater. Separ Purif Rev 39:95–171
Ali I, Gupta VK (2007) Advances in water treatment by adsorption technology. Nat Protoc 1:2661–2667
APHA, AWWA, WEF (1998) Standard methods for examination of water and wastewater, 20th edn. APHA, New York
Bhattacharya AK, Naiya TK, Mandal SN, Das SK (2008) Adsorption, kinetics and equilibrium studies on the removal of Cr(VI) from aqueous solutions using different low-cost adsorbents. Chem Eng J 137(3):529–541
Blazquez G, Calero M, Hernainz F, Tenorio G, Martin-Lara MA (2010) Equilibrium biosorption of lead(II) from aqueous solutions by solid waste from. Chem Eng J 160:615–622
Boyd GE, Adamson AW, Myers LS (1947) The exchange adsorption of ions from aqueous solutions by organic zeolites. II. Kinetic. J Am Chem Soc 69:2836–2848
Cimino G, Passerini A, Toscano G (2000) Removal of toxic cations and Cr(VI) from aqueous solutions by hazelnut shell. Water Res 34:2955–2962
Conrad K, Hansen HCB (2007) Sorption of zinc and lead on coir. Bioresour Technol 98:89–97
Cruz-Olivares J, Pérez-Alonso C, Barrera-Diaz C, Lَpez G, Balderas-Hernandez P (2010) Inside the removal of lead(II) from aqueous solutions by De-Oiled Allspice Husk in batch and continuous processes. J Hazard Mater 181:1095–1101
Dahiya S, Tripathi RM, Hegde AG (2008) Biosorption of lead and copper from aqueous solutions by pre-treated crab and arca shell biomass. Bioresour Technol 99:179–187
Dubinin MM, Zaverina ED, Radushkevich LV (1947) Sorption and structure of active carbons I. Adsorption of organic vapors. Zhurnal Fizicheskoi Khimii 21:1351–1362
Freundlich H (1906) Adsorption in solution. PhysChemie 57:384–410
Gupta VK, Ali I (2004) Removal of lead and chromium from wastewater using bagasse fly ash—a sugar industry waste. J Colloid Interface Sci 27:321–328
Gupta VK, Andimranali (2008) Removal of endosulfan and methoxychlor from water on carbonslurry. Environ Sci Technol 42:766–770
Gupta VK, Rastogi A (2008a) Biosorption of lead from aqueous solutions by green algae Spirogyra species: kinetics and equilibrium studies. J Hazard Mater 152:407–414
Gupta VK, Rastogi A (2008b) Biosorption of lead(II) from aqueous solutions by non-living algal biomass Oedogonium sp. and Nostoc sp.—a comparative study. Colloids Surf B Biointerfaces 64:170–178
Gupta VK, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402
Gupta VK, Sharma S (2003) Removal of zinc from aqueous solutions using bagasse fly ash: a low cost adsorbent. Ind Eng Chem Res 42:6619–6624
Gupta VK, Rastogi A, Dwivedi MK, Mohan D (1997) Process development for the removal of zinc and cadmium from wastewater using slag—a blast furnace waste material. Separ Sci Tech 32(17):2883–2912
Gupta VK, Mohan D, Sharma S (1998) Removal of lead from wastewater using bagasse fly ash—a sugar industry waste material. Separ Sci Tech 33:1331–1343
Gupta VK, Mohan D, Sharma S, Park KT (1999) Removal of chromium(VI) from electroplating industry wastewater using bagasse fly ash—a sugar industry waste material. Environmentalist 19:129–136
Gupta VK, Gupta M, Sharma S (2001) Process development for the removal of lead and chromium from aqueous solutions using red mud—an aluminium industry waste. Water Res 35:1125–1134
Gupta VK, Singh P, Rahman N (2004) Adsorption behavior of Hg(II), Pb(II) and Cd(II) from aqueous solution on Duolite C-433: a synthetic resin. J Colloid Interface Sci 275:398–402
Gupta VK, Ali I, Saini VK (2007) Adsorption studies on the removal of Vertigo Blue 49 and Orange DNA13 from aqueous solutions using carbon slurry developed from a waste material. J Colloid Interface Sci 315:87–93
Gupta VK, Carrott PJM, Carrott MMLR, Suhas (2009) Low-cost adsorbents: growing approach to wastewater treatment—a review. Crit Rev Environ Sci Tech 39:783–842
Gupta VK, Rastogi A, Nayak A (2010a) Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material. J Colloid Interface Sci 342:135–141
Gupta VK, Rastogi A, Nayak A (2010b) Biosorption of nickel onto treated alga (Oedogonium hatei): application of isotherm and kinetic models. J Colloid Interface Sci 342:533–539
Gupta VK, Agarwal S, Saleh TA (2011) Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J Hazard Mater 185:17–23
Han R, Zhang J, Zou W, Shi J, Lui H (2005) Equilibrium biosorption isotherm for lead ion on chaff. J Hazard Mater 125:266–271
Hasana SH, Srivastavaa P, Talatb M (2010) Biosorption of lead using immobilized Aeromonas hydrophila biomass in up flow column system: factorial design for process optimization. J Hazard Mater 177:312–322
Ho YS, McKay G (2002) Application of kinetic models to the sorption of copper(II) onto peat. Adsorp Sci Technol 20(8):797–815
Ho YS, Mckay G, Wase DAJ, Foster CF (2000) Study of the sorption of divalent metal on to peat. Adsorp Sci Technol 18:639–650
Imamoglu M, Tekir O (2008) Removal of copper (II) and lead (II) ions from aqueous solutions by adsorption on activated carbon from a new precursor hazelnut husks. Desalination 228:108–113
IS 10500 (1992) Drinking water specification (reaffirmed 1993). http://www.hppcb.nic.in/EIAsorang/Spec.pdf. Accessed 09 Aug 2007
Kalavathy MH, Karthikeyan T, Rajgopal S, Miranda LR (2005) Kinetic and isotherm studies of Cu(II) adsorption onto H3PO4-activated rubber wood sawdust. J Colloid Interface Sci 292:354–362
Khalid LB, Girgis BS, Tawfik T (2001) Decomposition of H2O2 on activated carbon obtained from olive stones. J Chem Technol Biotechnol 76:132–1140
Khan TA, Singh V, Ali I (2009) Sorption of Cd(II), Pb(II), and Cr(VI) metal ions from wastewater using bottom fly ash as a low cost sorbent. J Environ Protect Sci 3:124–132
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar 24:1–39
Lalhruaitluanga H, Jayaram K, Prasad MNV, Kumar KK (2010) Lead(II) adsorption from aqueous solutions by raw and activated charcoals of Melocanna baccifera Roxburgh (bamboo)—a comparative study. J Hazard Mater 175:311–318
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lee SH, Jung CH, Chung H, Lee MY, Yang JW (1998) Removal of heavy metals from aqueous solution by apple residues. Process Biochem 33:205–211
Li Q, Zhai J, Zhang W, Wang M, Zhou J (2007) Kinetic studies of adsorption of Pb(II), Cr(III) and Cu(II) from aqueous solutions by saw dust and modified peanut husk. J Hazard Mater 141:163–167
Liao D, Zheng W, Li X, Yang Q, Yue X, Guo L, Zeng G (2010) Removal of lead(II) from aqueous solutions using carbonate hydroxyapatite extracted from eggshell waste. J Hazard Mater 177:126–130
Low MJD (1960) Kinetics of chemisorption of gases on solids. Chem Rev 60:267–312
Low KS, Lee CK, Liew SC (2000) Sorption of cadmium and lead from aqueous solutions by spent grain. Process Biochem 36:59–64
Mahmoud M, Osman MM, Hafez OF, Hegazi AH, Elmelegy E (2010) Removal and pre concentration of lead (II) and other heavy metals from water by alumina adsorbents developed by surface-adsorbed-dithizone. Desalination 251:123–130
Mckay G, Otterburn MS, Sweeney AG (1981) Surface mass transfer processes during colour removal from effluent using silica. Water Res 15:327–331
Mckay G, Blair HS, Gardener JR (1982) Adsorption of dyes on chitin. I. Equilibrium studies. J Appl Polym Sci 27:3043–3057
Meunier N, Laroulandie J, Blais JF, Tyagi RD (2003) Cocoa shells for heavy metal removal from acidic solutions. Bioresour Technol 90:255–263
Naiya TK, Bhattacharya AK, Das SK (2009a) Adsorption of Cd(II) and Pb(II) from aqueous solutions on activated alumina. J Colloid Interface Sci 333:14–26
Naiya TK, Bhattacharya AK, Mandal SN, Das SK (2009b) The sorption of lead(II) ions on rice husk ash. J Hazard Mater 163:1254–1264
Pehlivan E, Altun T, Parlayici S (2009) Utilization of barley straws as biosorbents for Cu2+ and Pb2+ ions. J Hazard Mater 164:982–986
Qin LH, Zhang FB, Zang GL, Zhang SH (2007) Kinetics and mechanism of the sorption of NTS macroporous adsorption resin. Chem Industry Eng 24(3):245–248
Reddy DHK, Harinatha Y, Seshaiah K, Reddy AVR (2010) Biosorption of Pb(II) from aqueous solutions using chemically modified Moringa oleifera tree leaves. Chem Eng J 162:626–634
Reichenberg D (1953) Properties of ion-exchange resins in relation to their structure. III. Kinetics of exchange. J Am Chem Soc 75:589–597
Saeeda A, Iqbala M, Akhtarb MW (2005) Removal and recovery of lead(II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk). J Hazard Mater B117:65–73
Sawalha MF, Peralta-Videa JR, Romero-Gonzalez J, Gardea-Torresdey JL (2006) Biosorption of Cd(II), Cr(III), and Cr(VI) by saltbush (Atriplex canescens) biomass: thermodynamic and isotherm studies. J Colloid Interface Sci 300:100–104
Seeber G, Buchmeiser MR, Bonn GK, Bertsch T (1998) Determination of airborne, volatile amines from polyurethane foams by sorption onto a high-capacity cation-exchange resin based on poly (succinic acid). J Chromatogr A 809(1–2):121–129
Srivastava SK, Gupta VK, Mohan D (1997) Removal of lead and chromium by activated slag—a blast-furnace waste. J Environ Eng 123:461–468
Srivastava VC, Mall ID, Mishra IM (2006) Characterization of mesoporous rice husk ash (RHA) and adsorption kinetics of metal ions from aqueous solution onto RHA. J Hazard Mater B134:257–267
Srivastava VC, Mall ID, Mishra IM (2009) Competitive adsorption of cadmium(II) and nickel(II) ions from aqueous solution onto rice husk ash. Chem Eng Proc 48(1):370–379
Teng H, Hsieh CT (1999) Activation energy for oxygen chemisorption on carbon at low temperature. Ind Eng Chem Res 38:292–297
Unlu N, Ersoz M (2007) Removal of heavy metal ions by using dithiocarbamated-sporopollenin. Separ Purif Tech 52:461–469
Vermeulen T (1953) Theory for irreversible and constant pattern solid diffusion. Ind Eng Chem 45(8):1664–1670
Wang L, Zhang J, Zhao R, Li Y, Li C, Zhang C (2010) Adsorption of Pb(II) on activated carbon prepared from Polygonum orientale Linn.: kinetics, isotherms, pH, and ionic strength studies. Bioresour Technol 101:5808–5814
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civ Eng 89:31–60
Webi TW, Chakravort RK (1974) Pore and solid diffusion models for fixed bed absorbers. AICHE J 20:228–238
Zaki AB, El-Sheikh MY, Evans J, EI-Safty SA (2000) Kinetics and mechanism of the sorption of some aromatic amines onto amberlite IRA-904 anion-exchange resin. J Colloid Interface Sci 221:58–63
Acknowledgment
The authors acknowledge DST, Govt. of India for the financial support for the project work and fellowship to Mr. B. Singha (file no. DST/WTI/2K9/141, dated 19.05.2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor Vinod Kumar Gupta
Rights and permissions
About this article
Cite this article
Singha, B., Das, S.K. Removal of Pb(II) ions from aqueous solution and industrial effluent using natural biosorbents. Environ Sci Pollut Res 19, 2212–2226 (2012). https://doi.org/10.1007/s11356-011-0725-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-011-0725-8