Abstract
Industrial activities play a relevant role in environmental pollution since their wastes contain high concentrations of toxic elements that can add significant contamination to natural water and other water sources if no decontamination is previously applied. As toxic metals and metalloids are not biodegradable and tend to accumulate in living organisms, it is necessary to treat the contaminated industrial wastewaters prior to their discharge into the water bodies. There are different remediation techniques that have been developed to solve elemental pollution, but biosorption has arisen as a promising cleanup and low-cost biotechnology. Biosorption is governed by a variety of mechanisms including chemical binding, ion exchange, physisorption, precipitation, and oxide reduction. This review presents applications of biosorbents for metals and metalloids removal. Biomaterials including bacteria, fungi, algae, plant derivatives, agricultural wastes, and chitin–chitosan-based materials are considered. Also, bio-nano-hybrid materials, which have superlative sorption properties due to their high surface area coming from the nanomaterials structures and multifunctional capacity incorporated from the several types of chemical groups of biomaterials, are discussed. High metal removal percentages as high as 70–100% can be found in most works reported in the literature, which demonstrates the excellent performance obtained with biosorbents. These, as well as other important aspects linked to biosorption, are fully covered in the present review.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to their physical properties, metals have been defined as a group of opaque elements that form alloys, conduct heat and electricity and are usually malleable (Goyer 2004). Metalloids are those elements that show properties of both metals and nonmetals and are commonly represented by boron (B), silicon (Si), arsenic (As), antimony (Sb), and tellurium (Te) (Goldsmith 1982). Both metals and metalloids manifest different effects in processes involved in living organisms. Elements such as sodium (Na), potassium (K), calcium (Ca), zinc (Zn), iron (Fe), copper (Cu), magnesium (Mg), chromium (Cr), and selenium (Se) are considered essential for normal biochemical and cellular processes. However, some of them (Cu, Cr, Se) can be toxic at high concentrations (Smith et al. 2015). Additionally, metals and metalloids including As, mercury (Hg), cadmium (Cd), lead (Pb), thallium (Tl), vanadium (V), and nickel (Ni) are extremely toxic for living organism, even at trace concentrations, which cause severe damages in the normal functions of the organism (Maestri et al. 2010).
Metals can be introduced into the environment through natural and anthropogenic routes; however, the contribution of human activities is relevant to increase metal concentrations (Vijayaraghavan and Yun 2008b). There are many published works regarding the contamination of the environment by the presence of metals due to human activities (Bermudez et al. 2010; Cui et al. 2017; Manzano et al. 2015; Pandey et al. 2016). Thus, mining, refining of ores, combustion of fossil fuels, processed food industry, and other industrial processes play an important role in elemental contribution, as their wastes contain high concentrations of toxic metals that could be disposed to natural water and other water sources if no decontaminant treatment is previously applied (Ahmed and Ahmaruzzaman 2016). Since metals and metalloids are not degraded in the environment, they can be accumulated for a long time in ecosystems, and hence, they can be directly translated to food and drinking water available for population.
For the above-mentioned reasons, the concern of researchers regarding the environmental pollution is growing and intense work is being done to solve this problem. In this sense, several remediation techniques have been developed and applied to remove environmental contaminants, including biological treatment, reverse osmosis, coagulation/flocculation, electrochemical oxidation, and adsorption/biosorption (Dasgupta et al. 2015; Samer 2015; Yeung and Gu 2011). Regarding adsorption, activated carbon is the most common sorption material used for pollutant removal from wastewaters due to its outstanding properties, such as good pore structures and high specific surface area. However, the application of this adsorbent is not always feasible because of its high cost, being necessary the exploration of less expensive alternatives that can be also efficient for decontamination treatments. In this way, the concept of “biosorption” has been conceived as a promising cleanup, free of secondary pollution, and low-cost biotechnology, and researchers have therefore focused in the preparation of new biomaterials to remove environmental pollutants from contaminated matrices (Fomina and Gadd 2014).
Biosorption is one of the pillars of bioremediation, together with bioaccumulation and phytoremediation (Gavrilescu 2004). The concept of biosorption has been defined as the property of certain biomolecules or biomasses to bind and concentrate selected ions or other molecules from aqueous solutions (Volesky 2007). Contrary to bioaccumulation processes, which involve an active metabolic transport, biosorption is based on the use of non-living biomasses or biomolecules. Therefore, a passive remediation treatment is verified mainly due to the affinity between the biosorbent and the adsorbate, which represent the solid surface from the bio-substrate and the chemical contaminant that is accumulated at the interface, respectively (Volesky 2007).
In order to represent the worldwide development of biosorption research occurred during the last decades, the number of publications related with biosorption from 1980 to 2016 is exhibited in Fig. 1. Although a slight increase in the number of publications happened from 1980 to 2000, the highest growth has been observed from 2000 to 2016. These data reflect the interest of researchers and governments around the world to develop studies based on biosorption for the removal of pollutants from the environment.
Number of publications related to biosorption from 1980 to 2016. Data obtained from a Scopus search (http://www.scopus.com) performed with the term “biosorption” as a single search filter. A slight increase in the number of publications between 1980 and 2000 can be observed. However, the greatest growth in scientific contributions has been observed between 2000 and 2016. These data reflect a clear interest by researchers and governments around the world to develop studies based on biosorption for the elimination of pollutants from the environment
Although the biosorption is highly used due to its low cost, it offers other advantages over classical remediation technologies, such as a high efficiency, the reduction in chemical or biological sludges, the possibility of biosorbent reuse, and the recovery of metal after the biosorption process. In fact, biosorption has been catalogued as a bioremediation method comparable to ion exchange resin-based treatments to remediate metal ions (Volesky 2007). For this reason, this review will be focused on the state-of-the-art and know-how in biosorption science. The importance of biosorption for the encouragement of Green Chemistry will be detailed. Aspects including types of biosorbents and mechanisms involved in biosorption will be also commented and discussed. Finally, challenges, trends, and perspective-related biosorption of metals and metalloids will be exposed.
This article is an abridged version of the chapter published by Escudero et al. (2018) in the series Environmental Chemistry for a Sustainable World (https://www.springer.com/series/11480).
Biosorption for green chemistry development
It is a great challenge for scientists to develop safe and clean processes that can be framed in the concept of green chemistry and biosorbents representing an excellent tool to achieve that goal. The origin of the biomass used for removal of contaminants is an important factor to be considered in the frame of operations based on green chemistry. For instance, if the biomass consists on an agriculture/industry waste or an ubiquous and easily cultivated microorganism, the environmental and economic impact for obtaining the biosorbent is markedly minimized, which is in agreement with green chemistry goals. Several works have used wastes for removal of metals and metalloids from aqueous solutions (Habineza et al. 2017). Blázquez et al. studied the biosorption of Pb using olive stone and olive tree pruning, two industrial wastes of olive oil production (Blázquez et al. 2015). Vishan et al. also studied the biosorption of Pb, but using a bacterial strain isolated from compost of green waste, which proved to be an efficient, robust, and low-cost biosorbent (Vishan et al. 2017). Although one of the principles of green chemistry aims to state that it is better to prevent than cure, when bio-wastes are used for purposes of environmental decontamination, it is a favorable practice to treat the waste created previously.
The reutilization of the biomass in biosorption operations is a good experimental practice as it decreases the amount of sorption material and also the generation of wastes. Generally, it is possible to use a biosorbent for some cycles of biosorption–desorption (2–10 cycles are usually possible), minimizing not only the economic cost of the process, but also the environmental damage. For example, it has been reported the use of modified Auricularia Auricular matrix wastes for three biosorption/desorption cycles of Cd(II) (Song et al. 2017). After the sorption experiment, the biosorbent was put in contact with 0.1 mol/L of HCl and the mixture was stirred at 150 rpm for an hour at 293 K. Afterward, the biosorbent was washed with distilled water and dried until constant weight. The results of these experiments showed a reduction in the adsorption efficiency as the cycles were performed, observing the highest decrease between the first and the second cycle. Despite this variation, it was demonstrated that the biosorbent had a good potential reuse since it was useful to remove Cd(II) ions from aqueous solutions in all the assayed cycles. Some biosorbents can show disadvantages for their reuse, including poor mechanical strength, small particle size, mass loss after a regeneration step, and difficulty in separation from the aqueous phase. However, it is important to evaluate the possible reutilization of the biosorbent in order to promote the sustainable chemistry.
The immobilization of biomass on a solid supporting material represents another advantage for biosorption since it could improve mechanical strength, rigidity, porosity, and hence the performance of the biosorption (Dodson et al. 2015). Immobilized biomass can be implemented in automated systems, which helps to minimize the times elapsed for operations (washing, conditioning, cycles of biosorption and desorption). A large variety of polymeric compounds have been used for immobilization of biomass, including polysulfone, polyurethane, polyacrylamide, polyethyleneimine, and alginates. The selection of the support material can also be in accordance with the concept of green chemistry, although several characteristic such as solubility, biodegradability, and stability should be firstly considered in order to be suitable for the removal of contaminants from wastewaters.
When biosorption involves the removal of metals and metalloids, the analytical chemistry starts to acquire an important role on the contribution for an environmental friendly chemistry. It has been reported six basic strategies for greening analytical methods, which include the analysis of samples without previous treatment, the use of less polluting sample treatment, the miniaturization/automation of methods, the online decontamination of wastes, the use of alternative reagents, and the reduction in energy consumption (de la Guardia and Garrigues 2011). Fourier transform infrared spectroscopy (FTIR) is a vibrational spectroscopic technique usually used for the identification of functional groups present in biosorbents. Considering that it provides a high-quality spectral information of spectra, a direct analysis of sample without destruction, and generally avoids the use of solvents or reagents, it seems to be an optimal green analytical technique to characterize the biosorbent.
Mechanisms involved in elemental biosorption
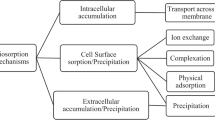
The mechanisms responsible for the metal binding differ according to the biomass type and the contaminant present in the environmental sample (Michalak et al. 2013). Due to the complexity of biomaterials, it is highly possible that several mechanisms can be present simultaneously (Fomina and Gadd 2014; Vijayaraghavan and Balasubramanian 2015). The removal of toxic metals and metalloids may involve both chemical and physical mechanisms (Vijayaraghavan and Yun 2008a). Physisorption is associated with the presence of Van der Waals’ attraction forces, whereas the chemisorption takes place as a result of relatively strong chemical bonding between adsorbates and biomass surface functional groups (Abdolali et al. 2014). Figure 2 shows different mechanisms involved in the removal of metals using biological substrates.
Mechanisms associated with biosorption of metals and metalloids. Chemical binding (complexation, chelation), ion exchange, physisorption, micro-precipitation and/or oxide reduction can be simultaneously present in the biosorption of metals and metalloids. COOH, NH2, OH, and SH are some functional groups often present in the surface of a biosorbent. Some examples of metal complexes that could be formed between a functional group of the biological substrate and a metal ion are showed. For example, the ion tetrachloromanganese (II) can be formed between chloride groups present on the biosorbent surface and manganese present in an aqueous solution. (

Chemical binding
Complexation
The metal removal from solutions may take place by a complex formation on the cell surface after the interaction between the metal ion and the active groups of the cell wall. A complex compound is a polyatomic molecule that takes place by association of one or several central atoms, which are metal cations, with a surrounding array of bound ligands. They are organic molecules or inorganic species (cations or anions) that contain free pair of electrons. Complex compounds can be neutral, positively or negatively charged, while bonding could be electrostatic, covalent, or a combination of both (Srivastava and Goyal 2010; Volesky 2003a). Figure 2 shows some examples of metal complexes that could be formed between a functional group of the biological substrate and a metal ion.
Chelation
Chelation is a process in which chelate compounds are formed. If one ligand is attached to the central atom through two or more coordinating atoms, the complex is called chelate (Javanbakht et al. 2014). Different biomasses have been reported to contain ligands useful for metal chelation. For example, Jaafar et al. reported that the removal of Cd(II) and Pb(II) ions occurred through the formation of a chelate with carboxyl groups of bacterial biomass of Deinococcus Radiodurans (Jaafar et al. 2016). It was elucidated by comparison of the FTIR spectra between the biomass and biomass with metal, where some changes were observed in the region of the peak at 1387 cm−1 attributed to C–O bond, which was shifted to 1392 and 1390 cm−1 when biomass was exposed to Cd(II) and Pb(II) ions, respectively.
Ion exchange
Ion exchange is known as a reversible interchange process that involves electrostatic interactions between cations present in the solution and the negatively charged groups of the cell wall or vice versa (Abdolali et al. 2014). The cell wall of biomass contains mainly polysaccharides as basic building blocks, and it is a well-established fact that bivalent metal ions are exchanged with counter ions of polysaccharides (He and Chen 2014; Veglio and Beolchini 1997). Indeed, biomaterials have numerous functional groups able to offer ion exchange sites, including –OH, NH2, –COOH, phosphate, sulfate, thiol or other groups (Escudero et al. 2016). It has been demonstrated that the biosorption of Cu(II) by Cystoseira crinitophylla biomass took place between the metal ions and the hydroxylic oxygens present in the polysaccharide chains of the brown algae (Christoforidis et al. 2015). The pH plays an important role in the ion exchange mechanism. In this work, it was verified that at low pH values, the biosorption capacity was dramatically reduced as result of the competition between metal ions and protons for the binding sites, while at basic pH values, there is risk of precipitation of Cu(II) as hydroxide.
Physisorption
As it was previously commented, physisorption is non-specific and involves weaker forces as compared to chemical mechanisms. The activation energy involved in physisorption processes is less than 1 kcal/gmol (Volesky 2003b). Even though the interaction energy is very weak (~ 10–100 meV) (Oura et al. 2003), physisorption plays an important role in the field of decontamination. Cid et al. reported that the biosorption of Cu(II) on the brown macroalgae Durvillaea Antarctica was due to a physisorption mechanism by forming an heterogeneous multilayer, followed by ion exchange mechanism (Cid et al. 2015). It is a good representation of the simultaneous mechanisms that can take place for the removal of toxic metals from aqueous solutions using biomass.
Micro-precipitation
The micro-precipitation mechanism occurs when the solubility of the sorbate reaches its limit. It is defined by the chemical interaction between the metal and the cell surface and is a process not depending on metabolism (Naja and Volesky 2011). Liu and co-workers have demonstrated that watermelon rind was an effective biosorbent for the removal of Cu(II), Zn(II), and Pb(II), and both ion exchange and micro-precipitation mechanisms were responsible by metal biosorption (Liu et al. 2012). Moreover, it has been also studied the removal of Pb(II) using bacterial biomass through several mechanisms, including physisorption, micro-precipitation as well as ion exchange (Jin et al. 2016).
Oxide reduction
An oxide reduction reaction can take place if there is one electron donor element and another that accepts them. However, another mechanism is often needed to finally eliminate metals using biomass. For instance, tea waste and date pits were investigated for their potential to remove toxic Cr(VI) ions from aqueous solution (Albadarin et al. 2013). The authors proved that the biosorption processes occurred by the initial biosorption of Cr onto positively charged functional groups of the biomass and then, by a reduction of Cr(VI) to Cr(III) in an acidic medium. FTIR confirmed –COOH, –NH2 and O–CH3 groups were involved in the biosorption and reduction in metals.
Biosorbents used for metal removal
Bacteria
According to the composition of their cell wall, bacteria are classified into Gram-positive and Gram-negative. Gram-positive bacteria are prokaryotic microorganisms characterized by the absence of outer membrane. Instead of this, they are surrounded by several layers of peptidoglycan, which is often densely functionalized with long anionic polymers, called teichoic acids (Young 2010). In contrast, Gram-negative bacteria show a thin layer of peptidoglycan cross-linked by short chains of amino acids, and an outer membrane rich in lipopolysaccharides (Young 2010). The composition of the cell wall plays a relevant role in metals and metalloids biosorption. In fact, it has been reported that functional groups including peptidoglycan, teichoic acids, phospholipids, lipopolysaccharides, and proteins are involved in elemental biosorption (Vijayaraghavan and Yun 2008b). Figure 3 shows a scheme of the main structural and chemical composition differences between both Gram-positive and Gram-negative bacteria.
Scheme of the structure and chemical composition of gram-negative (a) and gram-positive (b) bacteria. The group of Gram-positive bacteria is characterized by the absence of outer membrane and the presence of numerous layers of peptidoglycan, which is usually functionalized with long anionic polymers, named teichoic acids. Contrarily, Gram-negative bacteria show a thin layer of peptidoglycan cross-linked by short chains of amino acids, and an outer membrane rich in lipopolysaccharides. CM cytoplasmic membrane, FA fatty acid, LP lipoprotein, LPS lipopolysaccharide, P porin, PG peptidoglycan, PL phospholipid, PS periplasmic space, Pr protein, OM outer membrane
Within Gram-positive bacteria, Bacillus genus has been widely employed as biosorbent for removal of toxic metals, due to it is easy to be obtained, safety to humans, and environmental friendly. Table 1 shows a comparison on biosorption capacities of different micro- and macro-organisms used as biosorbents for toxic metal removal. Ahmad et al. studied the biosorption potential of Bacillus subtilis immobilized on calcium alginate gel beads for the removal of Cd from aqueous solutions (Ahmad et al. 2014). Batch biosorption experiments were developed in order to optimize experimental variables, including pH, initial concentration of Cd, contact time, and biosorbent dose. In agreement with previous works, the optimal pH value to reach the highest biosorption capacity was around 5.92. At this pH, interactions between the negatively charged surface of the biosorbent and the positive ions of Cd can occurr. The biosorbent showed a biosorption equilibrium capacity of 251.91 mg/g at 45 °C, which is fourfolds higher than that obtained for the removal of Cd using a blank composed by calcium alginate beads, thus exhibiting the advantages of immobilizing the bacterial biomass.
Bacillus thuringiensis strains have been applied for removal of europium (Eu) from aquatic ecosystems (Pan et al. 2017). The biosorbent showed to be efficient in terms of biosorption capacity (160 mg/g), besides exhibiting good regeneration and reusability. Bacillus genus has been also studied to remove Pb(II) from aqueous solutions (Ren et al. 2015). The results obtained from FTIR and EDS analyses suggest that Pb(II) could be covalently bind with C-, O-, N-, and P-containing functional groups present in the cell wall (carboxylate, hydroxyl, amino, and phosphate).
It is usual to chemically modify the surface of the biomass in order to improve the performance of the biosorbent. Kirova et al. studied the biosorption of Pb(II) from aqueous solutions by waste biomass of Streptomyces fradiae treated with NaOH (Kirova et al. 2015). FTIR spectra of treated S. fradiae showed several bands corresponding to functional groups that are able to bind Pb(II). Despite the chemical modification of the biomass, the biosorption capacity of this material was moderate (around 38 mg/g). Although the removal of Pb was affected by the presence of concomitant ions, the biomass resulted to be effective for biosorption purposes, due to the simplicity to be obtained and low cost.
Within Gram-negative bacteria, Pseudomonas genus has been one of the most used in biosorption studies. A recent work investigated the Pb(II) biosorption by a psychrotrophic strain of Pseudomonas sp. (Li et al. 2017a). With the aim of evaluating the effect of using Pb-resistant bacteria on the biosorption of this element, living and non-living Pb-resistant Pseudomonas strains were compared. It was observed that living strains showed higher biosorption capacity for Pb(II) than non-living bacteria (51 and 43 mg/g, respectively). However, both biosorbents demonstrated to be good alternatives to remediate Pb-contaminated aqueous matrices.
Due to the differences in the cell wall that divide bacteria into Gram-positive and Gram-negative groups, it would be interesting to answer whether one group of bacteria is better than the other for biosorption of metals and metalloids. Some contributions can be useful to clarify this point. It has been reported that functional groups present in Gram-negative bacteria are most exposed and available to remove toxic elements, contrarily to Gram-positive bacteria, which show low levels of surface available because of the densely cross-linked peptidoglycan layer (Joo et al. 2010). This statement arises from a study that compares Bacillus cereus and Pseudomonas aeruginosa strains for the biosorption and removal of Zn(II) from aqueous solutions (Joo et al. 2010). Although both bacterial groups were effective and low-cost biosorbents, the Gram-negative bacterium showed a higher biosorption capacity, which could be due to the different structures of the cell wall. Tsuruta et al. have previously studied the performance of Gram-negative bacteria, such as Bacillus megaterium, P. aeruginosa, and P. maltophilia, concluding that all of them are better materials to biosorb gold (Au) from aqueous solutions compared to Gram-positive bacteria (Tsuruta 2004). More recently, Oyetibo et al. developed equilibrium studies of Cd biosorption by bacterial strains isolated from polluted sites (Oyetibo et al. 2014). Four strains were evaluated, two Gram-negative (Pseudomonas aeruginosa, Burkholderia cepacia) and two Gram-positive bacteria (Corynebacterium kutscheri, Rhodococcus sp.). In this case, the experimental biosorption capacity was higher for Pseudomonas aeruginosa in comparison with the values obtained by Gram-positive bacteria. Nevertheless, Burkholderia cepacia showed less biosorption capacity than Corynebacterium and Rhodococcus genres. Therefore, although the wall cell structure of Gram-negative strains could facilitate the biosorption of some contaminants, this behavior is not always observed and depends on each system. In fact, it has been demonstrated that Gram-positive bacteria can be also useful to remove elemental pollutants from contaminated environments (Aryal and Liakopoulou-Kyriakides 2015).
Fungi
Fungal biomasses have received great attention as biosorbent materials to remove toxic elements mainly because they are easy to grow and are available as industrial waste products (Fomina and Gadd 2014). The elemental biosorption by fungi takes place in the cell wall. Fungal cell walls are complex macromolecular structures consisting mainly on polysaccharides (80–90%) and proteins, lipids, and pigments as minority compounds (Fomina and Gadd 2014). Chitin is also a common constituent of fungal cell walls. This variety of structural components ensures many different functional groups that are able to bind metal ions of toxicological interest. Although fungi are a large group of eukaryotic microorganisms, three types have major importance in the field of biosorption: molds, mushrooms, and yeasts (Wang and Chen 2009).
Molds are composed of long and branched threads called hyphae, which form a tangled mass named mycelium (Pokethitiyook and Poolpak 2016). Vale and co-workers have evaluated the capacity of Aspergillus niger to adsorb and remove Cr(VI) and Zn(II) from wastewaters (Vale et al. 2016). Comparing infrared spectra before and after biosorption of metals, the authors demonstrated that hydroxyl groups were mainly responsible for the removal of Zn(II), while amine groups were involved in the biosorption of Cr(VI). A very low biosorption capacity was reported for both metals (3.8 and 4.9 mg/g for Zn(II) and Cr(VI), respectively), which could be countered by the fact of being an economical and easily obtainable biosorbent.
Some works have proposed that the implementation of metal oxides nanoparticles as solid supports can improve the biosorption capacity, the physical and chemical stability, and the lifetime of biosorbents (Bakircioglu et al. 2010; Mahmoud et al. 2011). Mahmoud et al. have reported the immobilization of three fungal biomass, Aspergillus ustus, Fusarium verticillioides, and Pencillium funiculosum, on SiO2 nanoparticles for the selective biosorption of Cr(III) and Cr(VI) from aqueous solutions (Mahmoud et al. 2015a). In this study, the researchers reported that only by controlling the pH value of the solution, the biosorbents were selective for speciation and extraction of Cr species. Thus, maximum biosorption efficiency of Cr(III) was detected at pH 7, while for Cr(VI) it was found at pH 2.0. The time to reach the equilibrium biosorption was very short (15 min) and the experimental data were in accordance by both Langmuir and Freundlich models. By the comparison of the three biosorbents, F. verticiloides immobilized on nanoparticles show the higher intensity of biosorption. Seawater and industrial wastewater samples were analyzed, reporting a removal percentage between 83.9 and 98.2% for Cr(III), and 80.6–99.8% for Cr(VI) species.
Mushrooms are macro-fungi big enough to be observed by the naked eye and to be picked up handly (Maurya et al. 2006). This class of fungi has been extensively applied in biosorption studies. Recently, Kariuki and co-authors have proposed the use of Lepiota hystrix for biosorption of Cu(II) and Pb(II) ions from aqueous solutions (Kariuki et al. 2017). The efficiency of biosorption was evaluated in batch experiments and the optimized procedure was applied in river water samples. The analysis of the FTIR spectrum indicated that an interaction between metal ions and fungi biomass occurs through hydroxyl, carboxyl, amine, and amide groups. In the desorption study, the percentages of recovery obtained with 0.1 mol/L HCl were around 70% and 50% for Cu(II) and Pb(II), respectively. The researchers suggested that it was because some extra mechanisms, besides ion exchange, could be involved in the biosorption of metal ions. In real samples, the percentage of adsorption was lower for both metals. This behavior could be attributed to high levels of competing cations and ligands present in natural waters. However, although the matrix effect played a negative role in terms of reducing the removal of the contaminants, the biosorption by mushrooms is still efficient to be applied in real contaminated matrices. In other recent work, the removal of Cr(VI) from industrial wastewater samples was evaluated using a fixed-bed column modified by immobilization of Auricularia auricula substrate (Zang et al. 2017). The biosorbent was chemically modified using cetyltrimethyl ammonium bromide and immobilized onto sodium alginate. The procedure to obtain the modified and immobilized biosorbent is detailed in Fig. 4. The biosorption capacity of this mushroom was significantly higher (p < 0.05) than that obtained using the unmodified substrate of fungi, at different Cr(VI) concentrations. This could be due to a reduction in the surface tension by cetyltrimethyl ammonium bromide molecules or to an increase in the number of positive charges on the fungal surface, thus leading to an enhanced biosorption of the anionic species of Cr(VI). Furthermore, a study on the regeneration and reuse of the biosorbent was developed, obtaining a removal of around 50% after three biosorption–desorption cycles.
Representation of the chemical modification of Auricularia auricula and its immobilization onto a solid support. The dried biomass was chemically modified using cetyltrimethyl ammonium bromide (CTAB), stirring at 150 rpm and 293 K during 24 h. Subsequently, the modified biomass was washed with distilled water and dried at 333 K overnight. Then, the modified biomass was immobilized onto sodium alginate beads using sodium alginate and calcium chloride at concentrations showed in the figure to finally obtain 3–5 mm beads of immobilized biosorbent
Regarding yeasts, Saccharomyces cerevisiae is a worldwide known unicellular fungi that has received increasing attention in the field of biosorption due to its capacity for metal biosorption. Zhao and co-workers used S. cerevisiae to remove Ag(I) ions from low-concentration aqueous solutions (Zhao et al. 2015). In this study, the authors highlighted the use of yeast without any modification and proved that, in comparison with other biosorbents, Ag(I) could be fastly adsorbed onto the biomass (60 min). Furthermore, the equilibrium was reached within 60 min and a removal percentage of 93% was achieved when the initial concentration of Ag(I) was lower than 100 mg/L. Different analytical techniques suggested that Ag(0) were deposited on the surface of yeast and the FTIR spectrum confirmed that functional groups of the biosorbent were responsible of the reduction of Ag(I) to Ag(0). Additionally, Mahmoud et al. obtained excellent extraction of Hg(II) in different real water samples (92–100%) (Mahmoud et al. 2015b). A magnetic solid-phase extraction (MSPE) procedure was developed using a composite made of activated carbon-immobilized nano-Fe3O4-impregnated Saccharomyces cerevisiae. The researchers showed that at pH values between 1 and 3, the highest removal of Hg(II) was achieved when activated carbon-immobilized-nano-Fe3O4 was used. However, at higher pH values, the biosorption of Hg(II) was 20% more efficient when S. cerevisiae was immobilized on the magnetic surface, which could be attributed to the marked increase in the surface area resulting from the presence of magnetic nanoparticles within the structure of the yeast.
Algae
Algae represent another class of biomaterials applied for biosorption and removal of toxic elements from contaminated matrices. Their cell wall is composed of polysaccharides, glycoproteins, and sometimes chitin as an external thin layer (Wurdack 1923). The functional groups present in the algae cell wall (e.g., –COOH, –NH2 groups) can be responsible of metal binding. These biological substrates have often an opportunistic life and can be easily cultivated under different laboratory conditions, thus generating biomasses with different functional groups that might be useful for the removal of various contaminants.
The biosorption of Tl(I) using green micro-algae from eutrophic water sources has been investigated by Birungi and co-workers (Birungi and Chirwa 2015). Three species were identified and evaluated for biosorption of Tl, including Scenedesmus acuminutus, Chlorella vulgaris, and Chlamydomonas reinhardtii. At the optimal conditions, all biosorbents show outstanding biosorption capacities (please, see Table 1), which demonstrates the potential of these biomaterials to treat toxic elements from the aqueous media.
Some pre-treatments are commonly used to enhance the biosorption capacity of algal biomasses. Although raw algae can efficiently remove metals from aqueous media, secondary pollution caused by the release of organic compounds from the algae has to be mentioned. It has been recently compared the use of raw and pre-treated 2-Hypnea Valentiae algae for the removal of Co(II) from aqueous solutions (Vafajoo et al. 2018). The pre-treatment of the raw biomass consisted on mixing 10 g of sieved algae with 1 L of 10% formaldehyde during 1 h at room temperature. Afterward, the biomass was separated from the aqueous media by filtration, washed with deionized water, and dried overnight at 60 °C. The authors suggested that this surface modification could not only prevent leaching of components from the algae, but also improve the stability of the biosorbent during the biosorption process. This was in agreement with the results of the study, which showed an improvement in the biosorption capacity using the pre-treated biomass in comparison with the raw algae (16.6 vs. 10.9 mg/g).
The char derived from the pyrolysis of algal biomass has been also explored in the biosorption field. Cho et al. proved that the char derived from Undaria pinnatifida macroalgae was efficient for the removal of Cu(II) from aqueous solutions (Cho et al. 2013). It was observed that physical activation of the biochar is enhanced by 25% the biosorption of Cu(II) in comparison with the char without activation. This is probably because the activation process could have increased the surface area of the biosorbent, meaning that a high number of new pores were generated and this caused an increase in exchangeable cations on the surface. An optimum pH value of 5.5 was chosen to remove Cu(II) ions from aqueous solutions considering both the effects that pH causes on the surface of the biochar and the speciation of Cu(II). More recently, Scenedesmus dimorphushas micro-algae biochar has been studied for biosorption of Co(II) from aqueous solutions (Bordoloi et al. 2017). This process was faster in the initial stages and kept constant after reaching the equilibrium, which suggest that the more available sites of the surface of the biosorbent were saturated and the vacant binding sites still available on the biochar were of difficult access for Co(II) ions.
Simultaneous biosorption of Se, As, and Mo using a modified algal-based biochar was studied by Johansson et al. (Johansson et al. 2016). Initially, biomass of Oedogonium sp. was exposed to FeCl3 solutions for 24 h at 20 °C on a shaker plate. Then, the Fe-treated biomass was converted to biochar by a slow pyrolysis stage. The biosorption process was successfully applied in Tarong Ash Water for the removal of Se, As, and Mo, despite the competitive oxoanions (e.g., SO −24 ) present in the real samples.
Despite the efforts made in the biosorption field over the last years, advances have been mainly focused in laboratory-scale studies (Park et al. 2010). Most biosorption systems have shown limited industrial applications because industrial effluents are complex matrices and the presence of concomitants in these samples could deteriorate the performance of the biosorption. However, some contributions are taking the biosorption a step forward. For instance, the biosorption of Zn(II) from industrial effluents using the brown seaweed Fucus vesiculosus and sugar beet pulp was evaluated from laboratory tests to a pilot approach (Castro et al. 2017). Pilot experiments were made using a high feed rate to implement a reactor on an industrial-scale, and large glass columns were built to evaluate the applicability of the biosorption for the treatment of wastewaters at this scale. Taking into account that sugar beet pulp is a biosorbent less expensive than brown algae, a combination of both biomasses at different ratios of pulp/algae was evaluated to reduce the cost of the process (1:2, 1:1, and 2:1). It was observed that the columns percolated suitably in all cases and results showed that the most cost-effective option to treat Zn(II) ions would be the column packed with 1:1 pulp/algae biosorbent. The demonstrated ability of the pilot-scale biosorption for the efficient removal of Zn(II) ions from real wastewaters turns the biosorption into a very adequate process for large-scale applications.
Plant derivatives and agricultural wastes
Both plant derivatives and agricultural wastes have been widely used as potential biosorbents for the removal of metals and metalloids (Jain et al. 2016). Their cell wall consists on cellulose as the main polysaccharide, and other components such as proteins, hemicellulose, and lipids (Nguyen et al. 2013). These biosorbents are one of the more economic options to remove contaminants and it can be considered as environmental friendly due to their fast biodegradation. Furthermore, they are renewable, available in abundance, non-toxic and some of them can be reused throughout several biosorption–desorption cycles (Dhir 2014).
Jain et al. have used Jatropha curcas seed coat and fruit coat for Cd(II) biosorption (Jain et al. 2015). The results of FTIR indicated that Cd(II) binding was mainly due to the functional groups –OH, –NH, –COOH, and –CO present on the biosorbent surface. It was observed an increase in metal biosorption when increasing the biosorbent dose, which could be related with a major number of binding sites and surface area. However, at higher biosorbent doses, less biosorption capacities were obtained, which might be due to electrostatic interactions between cells and interference between the binding sites at high biosorbent dose. Biosorption capacities around 14 ad 17 mg/g were obtained for fruit coat and seed, respectively.
A chemical pre-treatment has been proposed in tomato wastes using 3% (v/v) HCl for Cu(II) removal from aqueous solutions (Yargıç et al. 2015). The results showed that the highest removal of metal ions was obtained at pH 8 (around 92%). The evaluated kinetic models reflected that pseudo-second-order kinetic model was the most suitable, indicating that the rate controlling mechanism for the biosorption was chemisorption. The pseudo-second-order kinetics has also described adequately the biosorption of Pb(II) and Cd(II) onto the surfaces of untreated Barbula lambarenensis and modified with sodium tripolyphosphate and ethylene glycol (Okoli et al. 2016). The authors reported that the use of sodium tripolyphosphate increased the specific surface area of the raw biosorbent in approximately 10 times, which was reflected in the biosorption capacities reported in Table 1. The tripolylphosphate group is a common chelating agent, and its use for the pre-treatment of the raw biosorbent generated extra tripolylphosphate groups on the surface of the biosorbent, causing an increase in the biosorption capacities. From the FTIR results, it was observed that the ethylene glycol biosorbent showed additional hydroxyl groups from ethylene glycol in comparison with the raw material, which resulted useful to adsorb metal ions.
Jiang et al. proposed the synthesis of a magnetic biosorbent using Litchi chinensis peels for the removal of Pb(II) from aqueous solutions (Jiang et al. 2015). The biosorbent was synthesized by adding Fe3O4 magnetic nanoparticles powder to the biomass, followed by the addition of sodium triphosphate. Under optimal experimental conditions, the biosorbent showed a biosorption capacity of 3.39 mg/g, using an initial metal concentration of 50 mg/L and adsorbent dosage of 5 g/L. One of the major advantages of this work is that the biosorbent containing the metal can be rapidly and easily separated from the solutions using an external magnetic field, avoiding time consuming steps such as centrifugations or filtrations.
Recently, Safinejad and co-workers have also been used a magnetic biosorbent prepared by adhering Fe3O4 on the surface of an agricultural waste for biosorption Pb(II) from aqueous solutions (Safinejad et al. 2017). In this case, shells of walnut fruit were chosen as biosorbent. The magnetic biosorbent showed a remarkable capacity of regeneration. The authors stated that after 10 biosorption–desorption cycles, the biosorbent preserved its characteristics without losing its magnetic properties. Besides, real waste samples were analyzed and about 98% of Pb(II) was removed during the first 10 min of the biosorption. The metal biosorption was quite fast and could be completed in the first minutes due to the absence of an internal diffusion resistance.
The removal of pollutants using activated carbons prepared from agricultural waste material has been also reported by several researchers (Gupta et al. 2015; Sayʇili et al. 2015). Under controlled conditions, the wastes can be converted into activated carbon through pyrolysis with or without chemical activating agents. Biosorption using these types of biosorbents have been found to be renewable and at the same time shows high efficiency, ease of operation and low costs (Okman et al. 2014). Van Thuan et al. have proposed the use of KOH-activated carbon from banana peel for the biosorption of Cu(II), Ni(II), and Pb(II) ions (Van Thuan et al. 2017). The authors used the response surface methodology (RSM) to optimize the experimental variables, including the initial concentration of metal ions, dose of biosorbent, and pH of solution. The biosorption model resulting from the quadratic equations proved to be statistically significant and the predictive potential of the model was also successful. In other work, Ghasemi and co-workers have studied the use of fig sawdust as a precursor for the production of activated carbon by chemical activation with H3PO4 for the removal of Pb(II) from drain water samples (Ghasemi et al. 2014). The authors proposed the formation of Cπ-Pb(II) complex and ion exchange reactions as the main mechanisms responsible for the biosorption of the metal by activated carbon.
An appealing study based on the removal of Cr species from water samples has been recently proposed by Khaskheli et al. (2016). Agricultural wastes of Okra leaves were evaluated for the biosorption of Cr(III) and Cr(VI). The leaves were ground, sieved to obtain small particle sizes, and finally treated with HCl. It was found that the highest removal capacity of Cr(III) was 221.17 mg/g at pH 4.0 and 81.94 mg/g at pH 2.0 for Cr(VI). Under optimal conditions, the biosorbent was able to remove around 90% of Cr from spiked real water samples.
Chitin–chitosan-based materials
Chitin is a linear biopolymer composed by 2-acetamido-2-deoxy-d-glucopyranose (GlcNAc) and 2-amino-2-deoxy-d-glucopyranose (GlcN) units. Chitin is normally obtained from shrimp and crab shells, which are wastes from the seafood industries, by sequential steps of demineralization, deproteinization, deodorization, and drying (Moura et al. 2015). Chitosan, in turn, is the product of alkaline deacetylation of chitin. During the deacetylation reaction, the chitin acetamido groups are converted into amino groups. If the acetylated units are higher than 60% of the biopolymer chain, we have chitin. If the de-acetylated units are higher than 60% of the biopolymer chain, we have chitosan (Fiamingo et al. 2017). Chitin and chitosan present a series of interesting physicochemical characteristics, which are responsible for their unlimited potential of application (Dotto and Pinto 2017). The elemental biosorption on chitin or chitosan is normally studied in batch systems and is dependent of some factors (Cadaval et al. 2017). The most important factors are the solution pH, deacetylation degree, and metal speciation (Guibal 2004). Generally, the interactions of metals with chitin/chitosan-based materials can occur by (I) complexation on nitrogen (free electronic doublet), (II) formation of ternary complexes and (III) ion exchange/electrostatic attraction (Guibal 2004). At pH lower than 6.7, the amino groups are susceptible to be protonated, turning chitosan into a polycationic material able to interact with negatively charged metals, like Cr (Cadaval et al. 2013), V (Cadaval et al. 2016), or As (Boddu et al. 2008), by electrostatic attraction or ion exchange. It is evident that higher deacetylation degrees contribute for these interactions. On the other hand, when pH is higher than 6.7, the amino groups are non-ionized and they possess a free electronic doublet on N atom able to interact with metal cations, like Ag (Zhang et al. 2015), Cd (Hu et al. 2017), Co (Dotto et al. 2015), Cu (Hu et al. 2017), Hg (Kyzas and Deliyanni 2013), Ni (Monier et al. 2010) and Pb (Hu et al. 2017). Regarding OH groups, it is accepted that they essentially contribute to stabilizing metal binding on amine groups (Guibal 2004).
Table 2 shows the biosorption capacities of different chitin–chitosan-based materials for different metals and metalloids. Some conclusions can be obtained from this Table 2: (1) It is corroborated that chitin/chitosan-based materials can be used as alternative biosorbents to remove metals from aqueous media, (2) It is demonstrated that several types of chemical and physical modifications can be performed in these biopolymers in order to improve its biosorption potential, (3) The biosorption of metals and metalloids on chitin/chitosan-based materials is normally investigated from 293 to 333 K, (4) For all investigated elements, the biosorption was favored under neutral or acid conditions and (5) The biosorption capacities vary in a wide range and are extremely dependent of the experimental conditions.
Bio-nano-hybrids materials
Bio-nano-hybrids materials are formed by the assembly of molecular species of biological origin and inorganic nano-substrates (Ruiz-Hitzky et al. 2008). These emerging materials are used in several fields, such as tissues engineering and new materials with enhanced functional and structural properties (Darder et al. 2007; Dujardin and Mann 2002; Ruiz-Hitzky and Darder 2006).
Nanotechnology is being introduced in the environmental field, particularly as it relates to the combination of biological substrates with nanomaterials, for the development of new hybrid bio-nano-materials with high retention capacity of metals (Khanra et al. 2012; Mahmoud et al. 2013). Within the bio-nano-hybrids materials that are being synthesized and evaluated to remove pollutants from the environment, it can be found those formed resulting from the combination of polysaccharides with nanomaterials. For instance, bio-nano-hybrid materials of nano-hydroxyapatite chitin (n-HApC) and nano-hydroxyapatite chitosan (n-HApCs) have been synthesized and applied for the removal of Fe(III) from aqueous solutions (Kousalya et al. 2010). The materials were prepared following the precipitation method, mixing a solution of ammonium dihydrogen phosphate with a mixture of Ca(NO3)2 solution and chitin or chitosan in the ratio 3:2. The obtained precipitate was rinsed with water up to reach a neutral pH, and dried at 150 °C. It has to be mentioned that the synthesized bio-nano-hybrid materials showed higher biosorption capacities than nano-hydroxyapatite only.
A relevant fraction within nanomaterials is represented by those made of carbon structures. In recent years, its application in the removal of toxic metals has been extensively investigated, with the use of diamonds, fullerenes, carbon nanotubes, graphene, carbon nanofibers, nanocouples, and nanotrompets (Ihsanullah et al. 2016; Liu et al. 2017; Pirveysian and Ghiaci 2018; Zhao et al. 2017). Several studies on the combination of microorganisms with carbon nanotubes have been developed for technological applications such as contaminant removal, bio-battery production, and electronic devices (Fu et al. 2017; Li 2016; Xue et al. 2017). Graphene has been also used to synthesized a bio-nano-hybrid material made of sulfur-graphene oxide nanosheets for the removal of Pb(II), Cd(II), Ni(II), and Zn(II) ions from aqueous solutions (Pirveysian and Ghiaci 2018). The presence of sulfur in the hybrid material was demonstrated by EDS and XPS techniques. In order to improve the efficiency of the sorbent, the hybrid material was coated with a mesoporous shell of TiO2 or SiO2. Although the hybrid material proved to be useful for the removal of metal ions from aqueous solutions, coating of the bio-nano-hybrid material with TiO2 seems to improve the biosorption capacity, which could be associated with the higher surface area of the coated biosorbent.
In addition, the use of nanoparticles, and particularly magnetic nanoparticles, is of great interest for the development of remediation processes that are based on the use of microorganisms as biosorbents. Among the outstanding properties of these nanoparticles, they have ability to give magnetization to living cells of microorganisms (Tian et al. 2010). The material resulting from the interaction of microorganisms-magnetic nanoparticles not only shows the advantage of acquire super-paramagnetic character of magnetic nanoparticles, but also exhibit good biosorption capacity, toward both metals and toward organic compounds (Ji et al. 2010; Tian et al. 2010). Thus, they also become excellent alternatives for the removal of contaminants from aqueous matrices. An additional advantage of magnetized cells is that, upon acquiring magnetic properties, they can be easily removed from the aqueous phase by application of a magnetic field. Different procedures have been proposed for the magnetization of microbial cells, either by treatment with magnetic fluids, covalent immobilization on magnetic transporters, specific interaction with immunomagnetic particles, cell cross-linking in the presence of magnetic particles, among others (Pospiskova et al. 2013). For instance, Rao et al. synthesized a hybrid material using Fe nanoparticles to confer magnetic properties to yeast cells of Yarrowia lipolytica. The cells modified with phyto-inspired Fe0/Fe3O4 nanoparticles were applied to the removal of Cr(VI) ions from aqueous solutions (Rao et al. 2013). Alternatively, Safarik et al. synthesized Kluyveromyces fragilis cells magnetized with a magnetic ferrofluid. A volume of 1 mL of ferrofluid was added to 3 mL of the yeast cells suspension in diluted acetic acid and the resulting suspension was mixed during 1 h at room temperature. The excess of ferrofluid was removed by sequential washes with acetic acid and water, until the supernatant was clear. The magnetized yeasts were then captured using a magnet and stored in water at 4 °C until its use.
Conclusions, trends, and perspectives
At present, a wide variety of biosorbents have been successfully applied for the removal of metal and metalloids pollutants from the environment, offering generally good removal percentages of contaminants and biosorption capacities. Typical materials that are used in decontamination processes, such as microorganisms, agricultural wastes, and plant derivatives will continue to be used as biosorbents because of the great advantages they have shown, i.e., low cost of material, biodegradability, reutilization for several biosorption–desorption cycles and the possibility of developing environmental friendly processes. However, the fast introduction of nanotechnology in several science areas is quickly offering new nanomaterials and nanoparticles that have special physical chemistry properties. This opens the possibility of preparing novel biosorption materials resulting from the combination of nanomaterials with biomass (microorganisms, bacteria, fungi, etc.) or by functionalization of their surface with selected biomolecules, which could provide higher retention capacity, physical and chemical stability, and add remarkable advantages (e.g., magnetic separation) for more efficient and straightforward application of these novel bio-nanohybrids materials in future large-scale processes. These notorious advantages could lead, in a near future, to a partial replacement of conventional biosorption materials by bio-nanohybrids materials. Furthermore, one of the main disadvantages of classical biosorbents is related to the lack of selectivity toward a specific metal, and even worse, toward a particular chemical species of an element. For this reason, there is a trend toward the development of new biomaterials that are capable to improve these negative aspects. In addition to nanotechnology, genetic engineering will continue to acquire a protagonist role in the decontamination field based on biosorption processes, as manipulated cells could speed up the removal of pollutants, show increased biosorption capacity and enhance the selectivity of the biosorbents toward specific metal and metalloids. Therefore, as time will go on, it is expected that new and better biosorption materials will progressively be appeared in the literature.
References
Abdolali A, Guo WS, Ngo HH, Chen SS, Nguyen NC, Tung KL (2014) Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: a critical review. Biores Technol 160:57–66. https://doi.org/10.1016/j.biortech.2013.12.037
Abdul Mujeeb VM, Alikutty P, Muraleedharan K (2014) Synthesis, characterization and vanadium (V) sorption studies on some chitosan derivatives. J Water Proc Eng 4:143–148. https://doi.org/10.1016/j.jwpe.2014.09.010
Adamczuk A, Kołodyńska D (2015) Equilibrium, thermodynamic and kinetic studies on removal of chromium, copper, zinc and arsenic from aqueous solutions onto fly ash coated by chitosan. Chem Eng J 274:200–212. https://doi.org/10.1016/j.cej.2015.03.088
Ahmad MF, Haydar S, Bhatti AA, Bari AJ (2014) Application of artificial neural network for the prediction of biosorption capacity of immobilized Bacillus subtilis for the removal of cadmium ions from aqueous solution. Biochem Eng J 84:83–90. https://doi.org/10.1016/j.bej.2014.01.004
Ahmed MJK, Ahmaruzzaman M (2016) A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J Water Proc Eng 10:39–47. https://doi.org/10.1016/j.jwpe.2016.01.014
Albadarin AB, Mangwandi C, Walker GM, Allen SJ, Ahmad MNM, Khraisheh M (2013) Influence of solution chemistry on Cr(VI) reduction and complexation onto date-pits/tea-waste biomaterials. J Environ Manage 114:190–201. https://doi.org/10.1016/j.jenvman.2012.09.017
Anirudhan TS, Rijith S (2012) Synthesis and characterization of carboxyl terminated poly(methacrylic acid) grafted chitosan/bentonite composite and its application for the recovery of uranium(VI) from aqueous media. J Environ Radioact 106:8–19. https://doi.org/10.1016/j.jenvrad.2011.10.013
Aryal M, Liakopoulou-Kyriakides M (2015) Bioremoval of heavy metals by bacterial biomass. Environ Monit Assess 187:4173–4180. https://doi.org/10.1007/s10661-014-4173-z
Bakircioglu Y, Bakircioglu D, Akman S (2010) Biosorption of lead by filamentous fungal biomass-loaded TiO2 nanoparticles. J Hazard Mat 178:1015–1020. https://doi.org/10.1016/j.jhazmat.2010.02.040
Barriada JL, Herrero R, Prada-Rodríguez D, Sastre de Vicente ME (2008) Interaction of mercury with chitin: a physicochemical study of metal binding by a natural biopolymer. React Funct Polym 68:1609–1618. https://doi.org/10.1016/j.reactfunctpolym.2008.09.002
Bermudez GM, Moreno M, Invernizzi R, Pla R, Pignata ML (2010) Heavy metal pollution in topsoils near a cement plant: the role of organic matter and distance to the source to predict total and HCL-extracted heavy metal concentrations. Chemosphere 78:375–381. https://doi.org/10.1016/j.chemosphere.2009.11.012
Birungi ZS, Chirwa EM (2015) The adsorption potential and recovery of thallium using green micro-algae from eutrophic water sources. J Hazard Mater 299:67–77. https://doi.org/10.1016/j.jhazmat.2015.06.011
Blázquez G, Ronda A, Martín-Lara MA, Pérez A, Calero M (2015) Comparative study of isotherm parameters of lead biosorption by two wastes of olive-oil production. Water Sci Technol 72:711–720. https://doi.org/10.2166/wst.2015.153
Boddu VM, Abburi K, Talbott JL, Smith ED, Haasch R (2008) Removal of arsenic (III) and arsenic (V) from aqueous medium using chitosan-coated biosorbent. Water Res 42:633–642. https://doi.org/10.1016/j.watres.2007.08.014
Bordoloi N, Goswami R, Kumar M, Kataki R (2017) Biosorption of Co (II) from aqueous solution using algal biochar: kinetics and isotherm studies. Biores Technol 244:1465–1469. https://doi.org/10.1016/j.biortech.2017.05.139
Cadaval T, Câmara A, Dotto G, Pinto L (2013) Adsorption of Cr(VI) by chitosan with different deacetylation degrees. Des Water Treat 51:7690–7699. https://doi.org/10.1080/19443994.2013.778797
Cadaval T, Dotto G, Seus E, Mirlean N, Pinto L (2016) Vanadium removal from aqueous solutions onto chitosan films. Des Water Treat 57:16583–16591. https://doi.org/10.1080/19443994.2015.1079741
Cadaval T, Vieira M, Câmara A, Dotto G, Pinto L (2017) Application of chitosan based materials for dyes/metals removal, chitosan based materials and its applications. Bentham Science Publishers, Sharjah, pp 154–180. https://doi.org/10.2174/9781681084855117030010
Castro L, Blazquez ML, Gonzalez F, Munoz JA, Ballester A (2017) Biosorption of Zn(II) from industrial effluents using sugar beet pulp and F. vesiculosus: from laboratory tests to a pilot approach. Sci Total Environ 598:856–866. https://doi.org/10.1016/j.scitotenv.2017.04.138
Cho HJ, Baek K, Jeon J-K, Park SH, Suh DJ, Park Y-K (2013) Removal characteristics of copper by marine macro-algae-derived chars. Chem Eng J 217:205–211. https://doi.org/10.1016/j.cej.2012.11.123
Christoforidis AK, Orfanidis S, Papageorgiou SK, Lazaridou AN, Favvas EP, Mitropoulos A (2015) Study of Cu(II) removal by Cystoseira crinitophylla biomass in batch and continuous flow biosorption. Chem Eng J 277:334–340. https://doi.org/10.1016/j.cej.2015.04.138
Cid H, Ortiz C, Pizarro J, Barros D, Castillo X, Giraldo L, Moreno-Piraján JC (2015) Characterization of copper (II) biosorption by brown algae Durvillaea antarctica dead biomass. Adsorption 21:645–658. https://doi.org/10.1007/s10450-015-9715-3
Côrtes LN, Tanabe EH, Bertuol DA, Dotto GL (2015) Biosorption of gold from computer microprocessor leachate solutions using chitin. Waste Manag 45:272–279. https://doi.org/10.1016/j.wasman.2015.07.016
Cui J-L, Luo C-L, Tang CW-Y, Chan T-S, Li X-D (2017) Speciation and leaching of trace metal contaminants from e-waste contaminated soils. J Hazard Mat 329:150–158. https://doi.org/10.1016/j.jhazmat.2016.12.060
Darder M, Aranda P, Ruiz-Hitzky E (2007) Bionanocomposites: a new concept of ecological, bioinspired, and functional hybrid materials. Adv Mat 19:1309–1319. https://doi.org/10.1002/adma.200602328
Dasgupta J, Sikder J, Chakraborty S, Curcio S, Drioli E (2015) Remediation of textile effluents by membrane based treatment techniques: a state of the art review. J Environ Manage 147:55–72. https://doi.org/10.1016/j.jenvman.2014.08.008
de la Guardia M, Garrigues S (2011) Challenges in green analytical chemistry. RSC Publishing, Valencia. https://doi.org/10.1039/9781849732963
Dhir B (2014) Potential of biological materials for removing heavy metals from wastewater. Environ Sci Pollut Res 21:1614–1627. https://doi.org/10.1007/s11356-013-2230-8
Dodson JR, Parker HL, García AM, Hicken A, Asemave K, Farmer TJ, He H, Clark JH, Hunt AJ (2015) Bio-derived materials as a green route for precious and critical metal recovery and re-use. Green Chem 17:1951–1965. https://doi.org/10.1039/c4gc02483d
Donia AM, Atia AA, Elwakeel KZ (2007) Recovery of gold(III) and silver(I) on a chemically modified chitosan with magnetic properties. Hydrometallurgy 87:197–206. https://doi.org/10.1016/j.hydromet.2007.03.007
Dotto GL, Pinto L (2017) General considerations about chitosan, chitosan based materials and its applications. Bentham Science Publishers, Sharjah, pp 3–33
Dotto GL, Cunha JM, Calgaro CO, Tanabe EH, Bertuol DA (2015) Surface modification of chitin using ultrasound-assisted and supercritical CO2 technologies for cobalt adsorption. J Hazard Mat 295:29–36. https://doi.org/10.1016/j.jhazmat.2015.04.009
Dujardin E, Mann S (2002) Bio-inspired materials chemistry. Adv Mat 14:775–788. https://doi.org/10.1002/1527-2648(20020717)4:7%3c461:AID-ADEM461%3e3.0.CO;2-K
Escudero LB, Maniero M, Agostini E, Smichowski PN (2016) Biological substrates: green alternatives in trace elemental preconcentration and speciation analysis. TrAC Trends Anal Chem 80:531–546. https://doi.org/10.1016/j.trac.2016.04.002
Escudero LB, Quintas PY, Wuilloud RG, Dotto GL (2018) Biosorption of metals and metalloids. In: Crini G, Lichtfouse E (eds) Green adsorbents for pollutant removal: innovative materials. Springer, Cham, pp 35–86. https://doi.org/10.1007/978-3-319-92162-4_2
Fan C, Li K, Li J, Ying D, Wang Y, Jia J (2017) Comparative and competitive adsorption of Pb(II) and Cu(II) using tetraethylenepentamine modified chitosan/CoFe2O4 particles. J Hazard Mat 326:211–220. https://doi.org/10.1016/j.jhazmat.2016.12.036
Fiamingo A, Delezuk J, Corrêa R, Campana-Filho S (2017) Chitosan based materials and its applications, obtention processes and main characteristics. Bentham Science Publishers, Sharjah, pp 34–48. https://doi.org/10.2174/97816810848551170301
Fomina M, Gadd GM (2014) Biosorption: current perspectives on concept, definition and application. Bioresour Technol 160:3–14. https://doi.org/10.1016/j.biortech.2013.12.102
Fu K, Yao Y, Dai J, Hu L (2017) Progress in 3D printing of carbon materials for energy-related applications. Adv Mat. https://doi.org/10.1002/adma.201603486
Gavrilescu M (2004) Removal of heavy metals from the environment by biosorption. Eng Life Sci 4:219–232. https://doi.org/10.1002/elsc.200420026
Ghasemi M, Naushad M, Ghasemi N, Khosravi-fard Y (2014) A novel agricultural waste based adsorbent for the removal of Pb(II) from aqueous solution: kinetics, equilibrium and thermodynamic studies. J Ind Eng Chem 20:454–461. https://doi.org/10.1016/j.jiec.2013.05.002
Goldsmith RH (1982) Metalloids. J Chem Educ 59:526–527. https://doi.org/10.1021/ed059p526
Goyer R (2004) Issue paper on the human health effects of metals. Environmental Protection Agency, Seatle
Guibal E (2004) Interactions of metal ions with chitosan-based sorbents: a review. Sep Purif Technol 38:43–74. https://doi.org/10.1016/j.seppur.2003.10.004
Gupta VK, Nayak A, Bhushan B, Agarwal S (2015) A critical analysis on the efficiency of activated carbons from low-cost precursors for heavy metals remediation. Crit Rev Environ Sci Technol 45:613–668. https://doi.org/10.1080/10643389.2013.876526
Habineza A, Zhai J, Ntakirutimana T, Qiu FP, Li X, Wang Q (2017) Heavy metal removal from wastewaters by agricultural waste low-cost adsorbents: hindrances of adsorption technology to the large scale industrial application—a review. Desal Water Treat 78:192–214. https://doi.org/10.5004/dwt.2017.20581
He J, Chen JP (2014) A comprehensive review on biosorption of heavy metals by algal biomass: materials, performances, chemistry, and modeling simulation tools. Biores Technol 160:67–78. https://doi.org/10.1016/j.biortech.2014.01.068
Hu C, Zhu P, Cai M, Hu H, Fu Q (2017) Comparative adsorption of Pb(II), Cu(II) and Cd(II) on chitosan saturated montmorillonite: kinetic, thermodynamic and equilibrium studies. Appl Clay Sci 143:320–326. https://doi.org/10.1016/j.clay.2017.04.005
Ihsanullah Abbas A, Al-Amer AM, Laoui T, Al-Marri MJ, Nasser MS, Khraisheh M, Atieh MA (2016) Heavy metal removal from aqueous solution by advanced carbon nanotubes: critical review of adsorption applications. Sep Purif Technol 157:141–161. https://doi.org/10.1016/j.seppur.2015.11.039
Jaafar R, Al-Sulami A, Al-Taee A, Aldoghachi F, Suhaimi N, Mohammed S (2016) Biosorption of some heavy metals by Deinococcus radiodurans isolated from soil in Basra governorate-Iraq. J Bioremediat Biodegrad 7:1–4. https://doi.org/10.4172/2155-6199.1000332
Jain N, Johnson TA, Kumar A, Mishra S, Gupta N (2015) Biosorption of Cd(II) on jatropha fruit coat and seed coat. Environ Monit Assess 187:411–423. https://doi.org/10.1007/s10661-015-4658-4
Jain CK, Malik DS, Yadav AK (2016) Applicability of plant based biosorbents in the removal of heavy metals: a review. Environ Process 3:495–523. https://doi.org/10.1007/s40710-016-0143-5
Javanbakht V, Alavi SA, Zilouei H (2014) Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Sci Technol 69:1775–1787. https://doi.org/10.2166/wst.2013.718
Javanbakht V, Ghoreishi SM, Habibi N, Javanbakht M (2016) A novel magnetic chitosan/clinoptilolite/magnetite nanocomposite for highly efficient removal of Pb(II) ions from aqueous solution. Powder Technol 302:372–383. https://doi.org/10.1016/j.powtec.2016.08.069
Ji YQ, Hu YT, Tian Q, Shao XZ, Li J, Safarikova M, Safarik I (2010) Biosorption of strontium ions by magnetically modified yeast cells. Sep Sci Technol 45:1499–1504. https://doi.org/10.1080/01496391003705664
Jiang R, Tian J, Zheng H, Qi J, Sun S, Li X (2015) A novel magnetic adsorbent based on waste litchi peels for removing Pb(II) from aqueous solution. J Environ Manage 155:24–30. https://doi.org/10.1016/j.jenvman.2015.03.009
Jin Y, Wang X, Zang T, Hu Y, Hu X, Ren G, Xu X, Qu J (2016) Biosorption of lead(II) by Arthrobacter sp. 25: process optimization and mechanism. J Microbiol Biotechnol 26:1428–1438. https://doi.org/10.4014/jmb.1603.03074
Johansson CL, Paul NA, de Nys R, Roberts DA (2016) Simultaneous biosorption of selenium, arsenic and molybdenum with modified algal-based biochars. J Environ Manage 165:117–123. https://doi.org/10.1016/j.jenvman.2015.09.021
Joo JH, Hassan SHA, Oh SE (2010) Comparative study of biosorption of Zn2+ by Pseudomonas aeruginosa and Bacillus cereus. Int Biodeter Biodegr 64:734–741. https://doi.org/10.1016/j.ibiod.2010.08.007
Kariuki Z, Kiptoo J, Onyancha D (2017) Biosorption studies of lead and copper using rogers mushroom biomass ‘Lepiota hystrix’. S Afr J Chem Eng 23:62–70. https://doi.org/10.1016/j.sajce.2017.02.001
Khanra P, Kuila T, Kim NH, Bae SH, Yu DS, Lee JH (2012) Simultaneous bio-functionalization and reduction of graphene oxide by baker’s yeast. Chem Eng J 183:526–533. https://doi.org/10.1016/j.cej.2011.12.075
Khaskheli M, Memon S, Chandio Z, Jatoi W, Mahar M, Khokhar F (2016) Okra leaves—agricultural waste for the removal of Cr(III) and Cr(VI) from contaminated water. Am J Anal Chem 7:395–409. https://doi.org/10.4236/ajac.2016.74037
Kirova G, Velkova Z, Stoytcheva M, Hristova Y, Iliev I, Gochev V (2015) Biosorption of Pb(II) ions from aqueous solutions by waste biomass of streptomyces fradiae pretreated with NaOH. Biotechnol Biotechnol Equip 29:689–695. https://doi.org/10.1080/13102818.2015.1036775
Kousalya GN, Rajiv Gandhi M, Sairam Sundaram C, Meenakshi S (2010) Synthesis of nano-hydroxyapatite chitin/chitosan hybrid biocomposites for the removal of Fe(III). Carbohydr Polym 82:594–599. https://doi.org/10.1016/j.carbpol.2010.05.013
Kyzas GZ, Deliyanni EA (2013) Mercury(II) removal with modified magnetic chitosan adsorbents. Molecules (Basel, Switzerland) 18:6193–6214. https://doi.org/10.3390/molecules18066193
Li H (2016) Application of porous carbon macrostructures for water purification. Prog Chem 28:1462–1473. https://doi.org/10.7536/PC160305
Li M, Zhang Z, Li R, Wang JJ, Ali A (2016) Removal of Pb(II) and Cd(II) ions from aqueous solution by thiosemicarbazide modified chitosan. Int J Biol Macromol 86:876–884. https://doi.org/10.1016/j.ijbiomac.2016.02.027
Li D, Xu X, Yu H, Han X (2017a) Characterization of Pb2+ biosorption by psychrotrophic strain Pseudomonas sp. I3 isolated from permafrost soil of Mohe wetland in Northeast China. J Environ Manage 196:8–15. https://doi.org/10.1016/j.jenvman.2017.02.076
Li R, Liang W, Li M, Jiang S, Huang H, Zhang Z, Wang JJ, Awasthi MK (2017b) Removal of Cd(II) and Cr(VI) ions by highly cross-linked thiocarbohydrazide-chitosan gel. Int J Biol Macromol 104:1072–1081. https://doi.org/10.1016/j.ijbiomac.2017.07.005
Liu X, Zhang L (2015) Insight into the adsorption mechanisms of vanadium(V) on a high-efficiency biosorbent (Ti-doped chitosan bead). Int J Biol Macromol 79:110–117. https://doi.org/10.1016/j.ijbiomac.2015.04.065
Liu C, Ngo HH, Guo W (2012) Watermelon rind: agro-waste or superior biosorbent? Appl Biochem Biotechnol 167:1699–1715. https://doi.org/10.1007/s12010-011-9521-7
Liu YP, Zhang Q, Ren J, Guo J, Cai ZJ (2017) Preparation of polyhydroxybutyrate/carbon nanotubes composite nanofiber membrane and their adsorption performance for heavy metal ions. Acta Polym Sin 0:820–829. https://doi.org/10.11777/j.issn1000-3304.2017.16217
Maestri E, Marmiroli M, Visioli G, Marmiroli N (2010) Metal tolerance and hyperaccumulation: costs and trade-offs between traits and environment. Environ Exp Bot 68:1–13. https://doi.org/10.1016/j.envexpbot.2009.10.011
Mahmoud ME, Yakout AA, Abdel-Aal H, Osman MM (2011) Enhanced biosorptive removal of cadmium from aqueous solutions by silicon dioxide nano-powder, heat inactivated and immobilized Aspergillus ustus. Desalination 279:291–297. https://doi.org/10.1016/j.desal.2011.06.023
Mahmoud ME, Yakout AA, Abdel-Aal H, Osman MM (2013) Immobilization of Fusarium verticillioides fungus on nano-silica (NSi-Fus): a novel and efficient biosorbent for water treatment and solid phase extraction of Mg(II) and Ca(II). Biores Technol 134:324–330. https://doi.org/10.1016/j.biortech.2013.01.171
Mahmoud ME, Yakout AA, Abdel-Aal H, Osman MM (2015a) Speciation and selective biosorption of Cr(III) and Cr(VI) using nanosilica immobilized-fungi biosorbents. J Environ Eng 141:1–9. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000899
Mahmoud ME, Ahmed SB, Osman MM, Abdel-Fattah TM (2015b) A novel composite of nanomagnetite-immobilized-baker’s yeast on the surface of activated carbon for magnetic solid phase extraction of Hg(II). Fuel 139:614–621. https://doi.org/10.1016/j.fuel.2014.09.002
Manzano BC, Roberto MM, Hoshina MM, Menegario AA, Marin-Morales MA (2015) Evaluation of the genotoxicity of waters impacted by domestic and industrial effluents of a highly industrialized region of Sao Paulo State, Brazil, by the comet assay in HTC cells. Environ Sci Pollut Res Int 22:1399–1407. https://doi.org/10.1007/s11356-014-3476-5
Maurya NS, Mittal AK, Cornel P, Rother E (2006) Biosorption of dyes using dead macro fungi: effect of dye structure, ionic strength and pH. Biores Technol 97:512–521. https://doi.org/10.1016/j.biortech.2005.02.045
Michalak I, Chojnacka K, Witek-Krowiak A (2013) State of the art for the biosorption process—a review. Appl Biochem Biotechnol 170:1389–1416. https://doi.org/10.1007/s12010-013-0269-0
Monier M, Ayad DM, Wei Y, Sarhan AA (2010) Adsorption of Cu(II), Co(II), and Ni(II) ions by modified magnetic chitosan chelating resin. J Hazard Mat 177:962–970. https://doi.org/10.1016/j.jhazmat.2010.01.012
Moura JM, Farias BS, Rodrigues DAS, Moura CM, Dotto GL, Pinto LAA (2015) Preparation of chitosan with different characteristics and its application for biofilms production. J Polym Environ 23:470–477. https://doi.org/10.1007/s10924-015-0730-y
Naja G, Volesky B (2011) The mechanism of metal cation and anion biosorption. In: Kotrba P, Mackova M, Macek T (eds) Microbial biosorption of metals. Springer, Dordrecht, pp 19–58. https://doi.org/10.1007/978-94-007-0443-5
Nguyen ML, Juang R-S (2015) Modification of crosslinked chitosan beads with histidine and Saccharomyces cerevisiae for enhanced Ni(II) biosorption. J Taiwan Inst Chem Eng 56:96–102. https://doi.org/10.1016/j.jtice.2015.03.033
Nguyen TAH, Ngo HH, Guo WS, Zhang J, Liang S, Yue QY, Li Q, Nguyen TV (2013) Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Biores Technol 148:574–585. https://doi.org/10.1016/j.biortech.2013.08.124
Okman I, Karagöz S, Tay T, Erdem M (2014) Activated carbons from grape seeds by chemical activation with potassium carbonate and potassium hydroxide. Appl Surf Sci 293:138–142. https://doi.org/10.1016/j.apsusc.2013.12.117
Okoli CP, Diagboya PN, Anigbogu IO, Olu-Owolabi BI, Adebowale KO (2016) Competitive biosorption of Pb(II) and Cd(II) ions from aqueous solutions using chemically modified moss biomass (Barbula lambarenensis). Environ Earth Sci 76:33–37. https://doi.org/10.1007/s12665-016-6368-9
Oura K, Lifshits V, Saranin A, Zotov A, Katayama M (2003) Atomic structure of surfaces with adsorbates, surface science. Springer, Berlin. https://doi.org/10.1007/978-3-662-05179-5_9
Oyetibo GO, Ilori MO, Obayori OS, Amund OO (2014) Equilibrium studies of cadmium biosorption by presumed non-viable bacterial strains isolated from polluted sites. Int Biodeter Biodegr 91:37–44. https://doi.org/10.1016/j.ibiod.2014.03.004
Padilla-Rodríguez A, Hernández-Viezcas JA, Peralta-Videa JR, Gardea-Torresdey JL, Perales-Pérez O, Román-Velázquez FR (2015) Synthesis of protonated chitosan flakes for the removal of vanadium (III, IV and V) oxyanions from aqueous solutions. Microchem J 118:1–11. https://doi.org/10.1016/j.microc.2014.07.011
Pan X, Wu W, Lü J, Chen Z, Li L, Rao W, Guan X (2017) Biosorption and extraction of europium by Bacillus thuringiensis strain. Inorg Chem Commun 75:21–24. https://doi.org/10.1016/j.inoche.2016.11.012
Pandey B, Suthar S, Singh V (2016) Accumulation and health risk of heavy metals in sugarcane irrigated with industrial effluent in some rural areas of Uttarakhand, India. Process Saf Environ Prot 102:655–666. https://doi.org/10.1016/j.psep.2016.05.024
Park D, Yun Y-S, Park JM (2010) The past, present, and future trends of biosorption. Biotechnol Bioprocess Eng 15:86–102. https://doi.org/10.1007/s12257-009-0199-4
Pirveysian M, Ghiaci M (2018) Synthesis and characterization of sulfur functionalized graphene oxide nanosheets as efficient sorbent for removal of Pb2+, Cd2+, Ni2+ and Zn2+ ions from aqueous solution: a combined thermodynamic and kinetic studies. Appl Surf Sci 428:98–109. https://doi.org/10.1016/j.apsusc.2017.09.105
Pokethitiyook P, Poolpak T (2016) Biosorption of heavy metal from aqueous solutions. In: Ansari AA, Gill SS, Gill R, Lanza GR, Newman L (eds) Phytoremediation: management of environmental contaminants, vol 3. Springer, Cham, pp 113–141. https://doi.org/10.1007/978-3-319-40148-5
Pospiskova K, Prochazkova G, Safarik I (2013) One-step magnetic modification of yeast cells by microwave-synthesized iron oxide microparticles. Lett Appl Microbiol 56:456–461. https://doi.org/10.1111/lam.12069
Ramesh A, Hasegawa H, Sugimoto W, Maki T, Ueda K (2008) Adsorption of gold(III), platinum(IV) and palladium(II) onto glycine modified crosslinked chitosan resin. Biores Technol 99:3801–3809. https://doi.org/10.1016/j.biortech.2007.07.008
Rao A, Bankar A, Kumar AR, Gosavi S, Zinjarde S (2013) Removal of hexavalent chromium ions by Yarrowia lipolytica cells modified with phyto-inspired Fe0/Fe3O4 nanoparticles. J Contam Hydrol 146:63–73. https://doi.org/10.1016/j.jconhyd.2012.12.008
Ren G, Jin Y, Zhang C, Gu H, Qu J (2015) Characteristics of Bacillus sp. PZ-1 and its biosorption to Pb(II). Ecotoxicol Environ Saf 117:141–148. https://doi.org/10.1016/j.ecoenv.2015.03.033
Ruiz-Hitzky E, Darder M (2006) Special issue on trends in biohybrid nanostructured materials. Curr Nanosci 2:153–294. https://doi.org/10.2174/1573413710602030153
Ruiz-Hitzky E, Darder M, Aranda P (2008) An introduction to bio-nanohybrid materials, bio-inorganic hybrid nanomaterials. Wiley-VCH Verlag GmbH & Co. KGaA, Hoboken, pp 1–40. https://doi.org/10.1002/9783527621446.ch1
Safinejad A, Chamjangali MA, Goudarzi N, Bagherian G (2017) Synthesis and characterization of a new magnetic bio-adsorbent using walnut shell powder and its application in ultrasonic assisted removal of lead. J Environ Chem Eng 5:1429–1437. https://doi.org/10.1016/j.jece.2017.02.027
Saleh AS, Ibrahim AG, Abdelhai F, Elsharma EM, Metwally E, Siyam T (2017) Preparation of poly(chitosan-acrylamide) flocculant using gamma radiation for adsorption of Cu(II) and Ni(II) ions. Radiat Phys Chem 134:33–39. https://doi.org/10.1016/j.radphyschem.2017.01.019
Samer M (ed) (2015) Biological and chemical wastewater treatment processes, wastewater treatment engineering. InTech, London. https://doi.org/10.5772/61250
Sayʇili H, Güzel F, Önal Y (2015) Conversion of grape industrial processing waste to activated carbon sorbent and its performance in cationic and anionic dyes adsorption. J Clean Prod 93:84–93. https://doi.org/10.1016/j.jclepro.2015.01.009
Schleuter D, Gunther A, Paasch S, Ehrlich H, Kljajic Z, Hanke T, Bernhard G, Brunner E (2013) Chitin-based renewable materials from marine sponges for uranium adsorption. Carbohydr Polym 92:712–718. https://doi.org/10.1016/j.carbpol.2012.08.090
Shaker MA (2015) Adsorption of Co(II), Ni(II) and Cu(II) ions onto chitosan-modified poly(methacrylate) nanoparticles: dynamics, equilibrium and thermodynamics studies. J Taiwan Inst Chem Eng 57:111–122. https://doi.org/10.1016/j.jtice.2015.05.027
Smith KS, Balistrieri LS, Todd AS (2015) Using biotic ligand models to predict metal toxicity in mineralized systems. Appl Geochem 57:55–72. https://doi.org/10.1016/j.apgeochem.2014.07.005
Song T, Liang J, Bai X, Li Y, Wei Y, Huang S, Dong L, Qu J, Jin Y (2017) Biosorption of cadmium ions from aqueous solution by modified Auricularia Auricular matrix waste. J Mol Liq 241:1023–1031. https://doi.org/10.1016/j.molliq.2017.06.111
Songkroah C, Nakbanpote W, Thiravetyan P (2004) Recovery of silver-thiosulphate complexes with chitin. Process Biochem 39:1553–1559. https://doi.org/10.1016/S0032-9592(03)00284-X
Srivastava S, Goyal P (2010) Biosorption: mechanistic aspects. In: Novel biomaterials. Environmental science and engineering. Springer, Berlin, pp 47–50. https://doi.org/10.1007/978-3-642-11329-1_7
Tian Y, Ji C, Zhao M, Xu M, Zhang Y, Wang R (2010) Preparation and characterization of baker’s yeast modified by nano-Fe3O4: application of biosorption of methyl violet in aqueous solution. Chem Eng J 165:474–481. https://doi.org/10.1016/j.cej.2010.09.037
Tsuruta T (2004) Biosorption and recycling of gold using various microorganisms. J Gen Appl Microbiol 50:221–228. https://doi.org/10.2323/jgam.50.221
Vafajoo L, Cheraghi R, Dabbagh R, McKay G (2018) Removal of cobalt (II) ions from aqueous solutions utilizing the pre-treated 2-Hypnea Valentiae algae: equilibrium, thermodynamic, and dynamic studies. Chem Eng J 331:39–47. https://doi.org/10.1016/j.cej.2017.08.019
Vale MS, do Nascimento RF, Leitão RC, Santaella ST (2016) Cr and Zn biosorption by Aspergillus niger. Environ Earth Sci 75:462–468. https://doi.org/10.1007/s12665-016-5343-9
Van Thuan T, Quynh BTP, Nguyen TD, Ho VTT, Bach LG (2017) Response surface methodology approach for optimization of Cu2+, Ni2+ and Pb2+ adsorption using KOH-activated carbon from banana peel. Surf Interface 6:209–217. https://doi.org/10.1016/j.surfin.2016.10.007
Veglio F, Beolchini F (1997) Removal of metals by biosorption: a review. Hydrometallurgy 44:301–316. https://doi.org/10.1016/S0304-386X(96)00059-X
Vijayaraghavan K, Balasubramanian R (2015) Is biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions. J Environ Manage 160:283–296. https://doi.org/10.1016/j.jenvman.2015.06.030
Vijayaraghavan K, Yun Y-S (2008a) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291. https://doi.org/10.1016/j.biotechadv.2008.02.002
Vijayaraghavan K, Yun YS (2008b) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291. https://doi.org/10.1016/j.biotechadv.2008.02.002
Vishan I, Sivaprakasam S, Kalamdhad A (2017) Biosorption of lead using Bacillus badius AK strain isolated from compost of green waste (water hyacinth). Environ Technol 38:1812–1822. https://doi.org/10.1080/09593330.2017.1298674
Volesky B (2003a) Biosorption process simulation tools. Hydrometallurgy 71:179–190. https://doi.org/10.1016/S0304-386X(03)00155-5
Volesky B (2003b) Sorption and biosorption. BV Sorbex, St. Lambert
Volesky B (2007) Biosorption and me. Water Res 41:4017–4029. https://doi.org/10.1016/j.watres.2007.05.062
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226. https://doi.org/10.1016/j.biotechadv.2008.11.002
Wang L, Peng H, Liu S, Yu H, Li P, Xing R (2012) Adsorption properties of gold onto a chitosan derivative. Int J Biol Macromolec 51:701–704. https://doi.org/10.1016/j.ijbiomac.2012.06.010
Wen Y, Tang Z, Chen Y, Gu Y (2011) Adsorption of Cr(VI) from aqueous solutions using chitosan-coated fly ash composite as biosorbent. Chem Eng J 175:110–116. https://doi.org/10.1016/j.cej.2011.09.066
Wurdack ME (1923) Chemical composition of the walls of certain algae, vol 141. Ohio State University, Columbus
Xu C, Wang J, Yang T, Chen X, Liu X, Ding X (2015) Adsorption of uranium by amidoximated chitosan-grafted polyacrylonitrile, using response surface methodology. Carbohydr Polym 121:79–85. https://doi.org/10.1016/j.carbpol.2014.12.024
Xue XY, Cheng R, Shi L, Ma Z, Zheng X (2017) Nanomaterials for water pollution monitoring and remediation. Environ Chem Lett 15:23–27. https://doi.org/10.1007/s10311-016-0595-x
Yang R, Su Y, Aubrecht KB, Wang X, Ma H, Grubbs RB, Hsiao BS, Chu B (2015) Thiol-functionalized chitin nanofibers for As(III) adsorption. Polymer 60:9–17. https://doi.org/10.1016/j.polymer.2015.01.025
Yargıç AŞ, Yarbay Şahin RZ, Özbay N, Önal E (2015) Assessment of toxic copper(II) biosorption from aqueous solution by chemically-treated tomato waste. J Clean Prod 88:152–159. https://doi.org/10.1016/j.jclepro.2014.05.087
Yeung AT, Gu YY (2011) A review on techniques to enhance electrochemical remediation of contaminated soils. J Hazard Mat 195:11–29. https://doi.org/10.1016/j.jhazmat.2011.08.047
Young KD (2010) Bacterial cell wall. Wiley, Chichester. https://doi.org/10.1002/9780470015902.a0000297.pub2
Yu Z, Dang Q, Liu C, Cha D, Zhang H, Zhu W, Zhang Q, Fan B (2017) Preparation and characterization of poly(maleic acid)-grafted cross-linked chitosan microspheres for Cd(II) adsorption. Carbohydr Polym 172:28–39. https://doi.org/10.1016/j.carbpol.2017.05.039
Zang T, Cheng Z, Lu L, Jin Y, Xu X, Ding W, Qu J (2017) Removal of Cr(VI) by modified and immobilized Auricularia auricula spent substrate in a fixed-bed column. Ecol Eng 99:358–365. https://doi.org/10.1016/j.ecoleng.2016.11.070
Zhang L, Yang S, Han T, Zhong L, Ma C, Zhou Y, Han X (2012) Improvement of Ag(I) adsorption onto chitosan/triethanolamine composite sorbent by an ion-imprinted technology. Appl Surf Sci 263:696–703. https://doi.org/10.1016/j.apsusc.2012.09.143
Zhang L, Xia W, Teng B, Liu X, Zhang W (2013) Zirconium cross-linked chitosan composite: preparation, characterization and application in adsorption of Cr(VI). Chem Eng J 229:1–8. https://doi.org/10.1016/j.cej.2013.05.102
Zhang M, Helleur R, Zhang Y (2015) Ion-imprinted chitosan gel beads for selective adsorption of Ag+ from aqueous solutions. Carbohydr Polym 130:206–212. https://doi.org/10.1016/j.carbpol.2015.05.038
Zhao Y, Wang D, Xie H, Won SW, Cui L, Wu G (2015) Adsorption of Ag(I) from aqueous solution by waste yeast: kinetic, equilibrium and mechanism studies. Bioprocess Biosyst Eng 38:69–77. https://doi.org/10.1007/s00449-014-1244-z
Zhao D, Zhang Q, Xuan H, Chen Y, Zhang K, Feng S, Alsaedi A, Hayat T, Chen C (2017) EDTA functionalized Fe3O4/graphene oxide for efficient removal of U(VI) from aqueous solutions. J Colloid Interface Sci 506:300–307. https://doi.org/10.1016/j.jcis.2017.07.057
Zhou L, Shang C, Liu Z, Huang G, Adesina AA (2012) Selective adsorption of uranium(VI) from aqueous solutions using the ion-imprinted magnetic chitosan resins. J Colloid Interface Sci 366:165–172. https://doi.org/10.1016/j.jcis.2011.09.069
Acknowledgements
The authors would like to thank National Council for Scientific and Technological Development (CNPq), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Tecnológica (FONCYT) (Project PICT-2015-1338), and Universidad Nacional de Cuyo (Project 06/M031) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Escudero, L.B., Quintas, P.Y., Wuilloud, R.G. et al. Recent advances on elemental biosorption. Environ Chem Lett 17, 409–427 (2019). https://doi.org/10.1007/s10311-018-0816-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-018-0816-6