Abstract
Muscle glycogen is an important fuel source for contracting skeletal muscle, and it is well documented that exercise performance is impaired when the muscle’s stores of glycogen are exhausted. The role of carbohydrate (CHO) availability on exercise performance has been known for more than a century, while the specific role of muscle glycogen for muscle function has been known for half a century. Nonetheless, the precise cellular and molecular mechanisms by which glycogen availability regulates cell function and contractile-induced fatigue are unresolved. Alterations of pre-exercise muscle glycogen reserves by dietary and exercise manipulations or modifying the degree of dependency on endogenous glycogen during exercise have collectively established a close relationship between muscle glycogen and the resistance to fatigue. It is also apparent that glycogen availability regulates rates of muscle glycogenolysis and resynthesis, muscle glucose uptake, key steps in excitation-contraction coupling, and exercise-induced cell signaling regulating training adaptation. The present review provides both a historical and contemporary overview of the effects of exercise on muscle glycogen metabolism, addressing factors affecting glycogen use during exercise as well as the evolving concepts of how glycogen and glycolysis are integrated with cell function, skeletal muscle fatigue, and training adaptation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
It is remarkable how skeletal muscle fibers can adapt acutely to provide the necessary production of energy during exercise, where a several-fold elevated energy turnover can be sustained for hours or a more than a hundred-fold increase can be executed for minutes. This ability to balance the energy turnover during various types of exercise is achieved by an integration of different energy pathways and by an efficient regulatory system, ensuring that ATP resynthesis is closely matched to the ATP demand of exercise. By the end of the 1930s, it had already been established that both fat and carbohydrate (CHO) could be used as fuel sources for aerobic metabolism during exercise and that fuel used during exercise can be modified by dietary manipulation (Zuntz 1896; Frentzel and Reach 1901; Krogh and Lindhard 1920; Edwards et al. 1934; Christensen and Hansen 1939). It was also understood that CHO was the predominant metabolic substrate when exercising at high intensities and that a relation existed between increasing CHO utilization with increased intensity. Although these early studies had documented that CHO is a major substrate during exercise and that the diet plays an important role in endurance capacity, it was the introduction of the needle biopsy technique in the 1960s that initially demonstrated that work time to exhaustion is highly correlated with muscle glycogen concentration (Bergström et al. 1967). Furthermore, it was also established that muscle glycogen content can be easily altered by isocaloric diets with varied CHO content and that at exhaustion there was a near depletion of muscle glycogen. These seminal studies on the important role of muscle glycogen on prolonged submaximal exercise performance have since been confirmed and extended numerous times. It is also well established that endurance training increases the basal stores of muscle glycogen, as demonstrated in rodent muscle already in the 1930s (Palladin 1945) and later in humans (Taylor et al. 1992; Gollnick et al. 1974). Additionally, trained humans have higher muscle glycogen content and a lower carbohydrate utilization for a given absolute submaximal exercise intensity when compared to untrained subjects (Karlsson et al. 1974; Saltin et al. 1976).
Based on the fundamental findings that carbohydrate combustion is more efficient than fat combustion (~6.2 ATP per O2 and ~ 5.6 ATP per O2, respectively) (Krogh and Lindhard 1920; McGilvery 1975) and that the degradation of glycogen to lactate or CO2 and H2O in muscles provides a more rapid energy production than that provided by the utilization of fatty acids (~1, 0.5 and 0.24 μmoles ATP g−1 s−1, respectively) (Margaria et al. 1964), a large body of studies have been undertaken from around the mid-1960s to today to investigate which factors influence glycogen utilization during exercise and how this affects the function of skeletal muscle fibers and athletic performance. As discussed above, a fundamental observation is that exercise performance is impaired when the muscle’s stores of glycogen are exhausted. During exercise, glycogen is utilized and can be depleted to very low levels often reaching one-fifth to one-sixth of the pre-exercise level (Gollnick et al. 1974). This is observed in humans, who are unable to withstand exercise at or above moderate intensity for a prolonged time when the stores are depleted to very low levels (<150 mmol kg−1 dw) compared to pre-exercise levels of 500–900 mmol kg−1 dw (Bergström et al. 1967; Hermansen et al. 1967) even with carbohydrate supplementation during the exercise (Coyle et al. 1986; Rauch et al. 1995). Therefore, the understanding of factors affecting the graded utilization of glycogen during exercise is key to avoid unforeseen glycogen-dependent muscle fatigue. Indeed, the precise mechanisms underpinning the role of glycogen in muscle function and performance are far from understood.
With this in mind, the present chapter provides both a historical and contemporary overview of the regulation of muscle glycogen metabolism during exercise in humans. After an initial discussion of glycogen storage and regulatory processes of glycogenolysis, a critical review of the factors that modulate glycogen utilization during exercise (i.e., intensity, duration, training status, modality, fiber type, sex, subcellular location, and environmental factors) is then presented. Subsequently, the evolving concepts of how glycogen and glycolysis are integrated with cell function and skeletal muscle fatigue are discussed from both a biochemical and physiological context. Finally, we close by outlining how glycogen utilization may serve as a signal to regulate cell signaling processes associated with modulating the endurance phenotype (i.e., training adaptations).
5.2 Glycogen Storage and Regulation
5.2.1 Biochemistry of the Glycogen Particle and Its Turnover
Glycogen is a unique molecule among several glucose polymers found in nature with structural and energy storage functions. Polymers of glucose with structural function include chitin (polymer of n-acetylglucosamine, a derivative of glucose), predominantly in arthropods and fungi, and cellulose in plants, algae, and oomycetes, which represents the most abundant polymer on earth (Klemm et al. 2005). For energy storage, the main polymers of glucose are starch in plants and glycogen which is by far the most widespread form of storage, found in archaea, bacteria, and eukaryotes (from protozoa and fungi to mammals) (Ball et al. 2011). Synthesis of glycogen through digestion of other glucose polymers is possible in humans for which starch represents a digestible form, while other mammals (e.g., ruminants and ungulates) can also digest cellulose by endosymbiont bacteria in the gut. In this context, two facts make glycogen standout: (1) it is a highly evolutionarily conserved molecule in prokaryotes and from unicellular eukaryotes to mammals, and (2) it represents the largest storage form of energy for high metabolic power output processes in mammalian cells, for which different mechanisms have evolved to maximize its synthesis and storage. These facts attest its key role in energy storage and place it in the centerstage of cellular energy production in skeletal muscle for exercise.
The glycogen polysaccharide is termed as a glycogen molecule, granule, or particle (Fig. 5.1). Due to its physical association with several proteins, it has also been termed glycosome to indicate its more organelle-like structure (Rybicka 1996). When glucose enters the cell, it is phosphorylated to glucose 6-phosphate by hexokinase I and can either be metabolized in the glycolytic pathway or be added to existing glycogen particles by the action of phosphoglucomutase, UDP-glucose pyrophosphorylase, glycogen synthase, and branching enzyme (Roach et al. 2012). While the first two steps activate the glucose, the glycogen synthase catalyzes the reaction, where a ɑ-1,4-glycosidic linkage connects the new glycosyl units with the nonreducing end of a chain of glycosyl units. The chain grows to a size of 11–13 glycosyl units. The branching enzyme transfers a set of glycosyl units from a chain to another position of a chain by creating ɑ-1,6-glycosidic linkages. Since each chain branches out two times with new chains organized in concentric tiers, the number of glycosyl units in the glycogen particle increases exponentially from around 200 in a small particle of 4 tiers (diameter of 11 nm) to around 55,000 in the largest particle of 12 tiers (diameter of 42 nm). In resting skeletal muscles, the typical (around 80% of all particles observed) diameter of glycogen particles is 20–32 nm (Marchand et al. 2002; Nielsen et al. 2010; Hokken et al. 2020), corresponding to 1000 to 9000 glycosyl units per particle (Melendez-Hevia et al. 1993). This structure of the glycogen particle is probably optimized by evolution to increase solubility and decrease the osmotic effect (Melendez-Hevia et al. 1993). Despite this, the relatively high numerical density of glycogen particles equals an osmotic effect of around 3 grams of water per 1 gram of glycogen (Olsson and Saltin 1970), which can accumulate up to 2 kilograms of glycogen-dependent stored water in humans during glycogen-loaded conditions (Olsson and Saltin 1970; Shiose et al. 2016).
Schematic diagram of a glycogen particle (with four concentric tiers of chains of glycosyl units) and the turnover of glycosyl units to lactate generating a high metabolic power or to CO2 and H2O ensuring a high metabolic capacity. G glycogenin, GP glycogen phosphorylase, G1P glucose-1-phosphate, G6P glucose-6-phosphate, TCA tricarboxylic acid cycle, ETC electron transport chain
Although the true numerical density of glycogen particles cannot be investigated with the currently available techniques, measures of the size of the observable glycogen particles indicate that the utilization of glycogen during exercise is mainly attributable to a decrease in the average particle size (Marchand et al. 2007; Gejl et al. 2017a, b; Hokken et al. 2020); on the other hand, with training and diet interventions, the increase in resting glycogen content is ascribed to an increase in particle number (Nielsen et al. 2010; Jensen et al. 2021). Thus, in resting muscles, there may be a preferable size of particles, which could be the result of a trade-off between storage efficiency (larger particles store more glycosyl units per volume) and metabolic power (smaller particles possess a higher glycogenolytic rate) as also suggested by Shearer and Graham (2004). An increase in glycogen particle number has been recognized to require a de novo synthesis of the self-glycosylating protein backbone glycogenin (Roach et al. 2012), but recent studies have suggested an increase in glycogen particle number based on a mechanism, which is independent on glycogenin (Testoni et al. 2017; Visuttijai et al. 2020).
The degradation of glycogen is conducted by the action of glycogen phosphorylase, which cuts the ɑ-1,4-glycosidic linkages and liberates the glycosyl units to the glycolytic pathway. Interestingly, given the branched structure of the glycogen particle, around one third of the glycosyl units are in the outermost tier (Melendez-Hevia et al. 1993) and readily available for glycogen phosphorylase securing a fast mobilization of energy. A more pronounced degradation of the particle requires a coordinated action of glycogen debranching enzyme cutting the ɑ-1,6-glycosidic linkages. As discussed, the breakdown of muscle glycogen to produce glucose 1-phosphate is thus under the control of glycogen phosphorylase, and this reaction requires both glycogen and Pi as substrates. Phosphorylase, in turn, exists as a more active a form (which is under the control of phosphorylation by phosphorylase kinase) and also as a more inactive b form (which exists in a dephosphorylated form due to the action of protein phosphatase 1). Phosphorylase can be transformed via covalent modification (i.e., phosphorylation by phosphorylase kinase) as mediated through epinephrine (Roach et al. 2012). Additionally, Ca2+ is a potent positive allosteric regulator of phosphorylase kinase through binding to the calmodulin subunit (Jensen and Richter 2012), though glycolytic flux is not controlled by Ca2+ directly, but by factors related to energy state (Ørtenblad et al. 2009). During contractile activity, the increased accumulation of Pi as a result of increased ATP hydrolysis can increase the rate of glycogenolysis as it provides increased substrate required for the reaction. Furthermore, greater accumulations of free ADP and AMP can also subsequently fine-tune the activity of phosphorylase a through allosteric regulation (Howlett et al. 1998). When taken collectively, the regulation of glycogen phosphorylase is dependent on hormonal control, substrate availability, and local allosteric regulation, the precise contribution of which is dependent on the specific exercise challenge.
5.2.2 Measurement of Muscle Glycogen
The golden standard for determination of muscle glycogen is the biochemical technique where the glycosyl units are liberated by acid hydrolysis and the amount determined spectrophotometrically (Passonneau and Lowry 1993). This is performed in skeletal muscle biopsies in small specimens of ~10 mg (wet weight), and with small adjustments can also be applied to single fibers (Hintz et al. 1982). The latter approach makes it possible to directly combine glycogen content with other biochemical or physiological measures and to distinguish between different fiber types. Noninvasively, nuclear magnetic resonance (NMR) has been optimized to detect muscle glycogen signals (Taylor et al. 1992) and can serve as an alternative to the biochemical assessment when biopsies are unsuitable for the target group.
To investigate fiber-type-specific glycogen, a semi-quantitative histochemical periodic acid-Schiff (PAS) staining has been employed in a large body of studies. Here, the glycogen content is assessed by staining intensity from empty to full using a scale of 4–5 steps and compared with the myosin ATPase characteristics (Pearse 1961). The PAS staining is based on a reaction of periodic acid with all sugars in the muscle, and it is therefore not specific for glycogen. It has been suggested to be replaced by an antibody-based technique (Nakamura-Tsuruta et al. 2012; Skurat et al. 2017), which can also be used by dot blotting (Albers et al. 2015).
With the binding properties of glycogen particles to a reduced form of osmium, a protocol for staining glycogen in transmission electron microscopy has also been developed (de Bruijn 1973; Marchand et al. 2002). With this approach, the subcellular distribution of glycogen can be envisaged along with information on fiber types (based on Z-discs and M-band appearances) and other ultrastructural parameters (Sjöström et al. 1982b).
5.2.3 Inter-fiber Variability and Subcellular Differences
While most studies on glycogen content are conducted using homogenates of small pieces of muscle, a string of studies have shown large inter-fiber variability ranging from around 100 to 1000 mmol kg−1 dw (Essén and Henriksson 1974). With discrimination between fiber types based on myosin ATPase activity, it has repeatedly been shown that type 2 fibers contain about 10–30% more glycogen than type 1 fibers in human skeletal muscles (e.g., Essén and Henriksson 1974; Ball-Burnett et al. 1991; Greenhaff et al. 1993). At the subcellular level, glycogen particles are dispersed heterogeneously throughout the myoplasm with one large pool located in the intermyofibrillar space close to sarcoplasmic reticulum and mitochondria and two small pools located in the intramyofibrillar and subsarcolemmal spaces, respectively (Sjöström et al. 1982a; Fridén et al. 1985, 1989; Marchand et al. 2002, see Fig. 5.4, Sect. 5.3). Although all the pools are utilized during exercise taxing the endogenous glycogen stores (see later), their content seems not to be related in resting muscles (Nielsen et al. 2010), suggesting that local independent factors are involved in their regulation.
5.3 Utilization of Glycogen During Exercise
As alluded to earlier, a series of studies from Scandinavian researchers during the late 1960s and early 1970s collectively demonstrated the role of skeletal muscle glycogen as a key fuel source for prolonged exercise capacity, particularly when completed at higher exercise intensities (Bergström and Hultman 1966a, b; Bergström et al. 1967; Saltin and Karlsson 1971). For example, pioneering work from Bergström et al. (1967) highlighted the role of dietary carbohydrate in elevating muscle glycogen reserves and subsequently demonstrated that high pre-exercise muscle glycogen concentration (~890 mmol.kg−1 dw) extends exercise capacity at 75% of VO2max by a remarkable 320% (from 59 to 189 min) when compared to low (~170 mmol.kg−1 dw) muscle glycogen stores. It was later demonstrated that increasing exercise intensity results in an exponential increase in the rate of muscle glycogen utilization (Saltin and Karlsson 1971).
Further association between starting skeletal muscle glycogen and prolonged aerobic exercise performance (Karlsson and Saltin 1971) solidified the central role of skeletal muscle glycogen as key substrate for intense exercise of prolonged duration. These seminal studies paved the way for the next 50 years of research examining the dietary and exercise-related factors that can subsequently affect the pattern of glycogen utilization during exercise. Such factors have been the subject of a recent meta-analysis (Areta and Hopkins 2018) and are portrayed conceptually in Fig. 5.2. The text to follow provides a critical overview of factors that can affect glycogen utilization during exercise.
Conceptual representation of skeletal muscle glycogen utilization dynamics at different intensities and durations, based on mathematical modelling of data extracted from a large sample of published literature (Areta and Hopkins 2018). Note that the starting muscle glycogen has been set constant at 600 mmol. kg−1 dw for exercise of intensities ranging from equivalent to 40 to 120% of VO2max, ranging from 5 to 120 min duration
5.3.1 Duration and Intensity
Although the duration of exercise that can be maintained is inevitably dependent on exercise intensity, the effect of intensity and duration on muscle glycogen utilization can be analyzed independently. Glycogen utilization increases in accordance with increased exercise duration though it is noteworthy that the rates of utilization vary at different stages during exercise. For example, during the first ~20 min of exercise, the rates of glycogen utilization seem to be the highest, a factor that is likely related to higher activity of glycogenolytic enzymes and lower availability of other metabolic substrates (Green et al. 1995; Chesley et al. 1996; Dyck et al. 1996; Graham et al. 2001), over and above the fact that higher glycogen concentration per se is associated with increased glycogen utilization (see below substrate availability and glycogen utilization). Thereafter, rates of utilization during prolonged exercise seem to be rather constant until they reach low levels (~200 mmol. kg−1 dw) at which point the rates are then reduced significantly (Coyle et al. 1986; Bosch et al. 1993, 1996). Fatigue during prolonged submaximal exercise (i.e., 60–70% of VO2max) typically occurs after a duration of 2 h with glycogen at exhaustion within the range of ~100 to 200 mmol.kg−1 dw (Areta and Hopkins 2018). Indeed, early studies on muscle glycogen and athletic performance suggested that the inability to maintain high rates of work would coincide with theoretical muscle glycogen content of 70–120 mmol.kg−1 dw during an endurance competition of ~135 min (Karlsson and Saltin 1971). With this in mind, it is most likely that only competitive events lasting >90 min would benefit from CHO loading strategies that aim to super compensate pre-exercise muscle glycogen stores, where performance has been suggested to improve by ~2–3% (Hawley et al. 1997a, b).
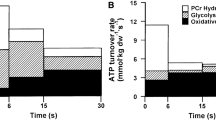
During intense exercise, ATP provision is achieved principally by the oxidation of carbohydrate, and muscle glycogen utilization increases exponentially with exercise intensity. At intensities ranging from 75% VO2max to near maximal workloads, glycogen is the main energy substrate (Saltin 1973; Gaitanos et al. 1993; Hultman and Greenhaff 1999). Thus, even a single 6 s or 30 s all-out sprint can reduce muscle glycogen by15 or 20–30%, respectively (Gaitanos et al. 1993; Bogdanis et al. 1996;Parolin et al. 1999). Such high glycogen utilization is achieved by estimated glycogenolytic rates of around 4.5 mmol glucosyl units.kg-1.dw.s−1 (Gaitanos et al. 1993; Parolin et al. 1999). During longer durations of high-intensity exercise, the glycogenolytic rate is known to decreases though it is noteworthy that muscle glycogen stores in both arms and legs are reduced by 20–25% during 4 min of high-intensity all-out cross-country sprint skiing (Gejl et al. 2014).
The first study to systematically investigate the effect of intensity demonstrated a clear exponential increase in muscle glycogen use (Fig. 5.3), with a disproportionate increase in rates at intensities close to VO2max and above (Saltin and Karlsson 1971). More recent studies addressing the contribution of different energy substrates using metabolic tracers show a clear disproportionate increase in reliance on skeletal muscle glycogen with increasing intensities when compared to all other fuel sources including intracellular lipids and plasma lipids and glucose (Romijn et al. 1993; van Loon et al. 2001). Corroborating these findings, a meta-analytic evaluation of the effect of exercise intensity on muscle glycogen use demonstrates moderate and large effects with increasing exercise intensity, albeit with substantial variation between studies (Areta and Hopkins 2018). The variation in glycogen use observed in different studies and individuals at the same intensity may be related to the fact that % VO2max is typically used as the “default” intensity parameter, and this parameter may not necessarily match the metabolic and substrate demands of the effort at the muscular level. Indeed, muscular oxidative capacity can vary greatly between individuals with the same VO2max (Holloszy 1973; Holloszy and Coyle 1984), and lactate threshold represents a more suitable method for which to match intensity within and between studies (Coyle et al. 1988; Poole et al. 2020).
Relationship between exercise intensity and rates of skeletal muscle glycogen utilization. Linear increases in exercise intensity (as % of VO2max equivalent) result in exponential increases in skeletal muscle glycogen utilization, as shown by early research (Figure redrawn from Saltin and Karlsson 1971)
Given that phosphorylase can be transformed via covalent modification (i.e., phosphorylation by phosphorylase kinase) mediated through epinephrine, it would be reasonable to expect that greater phosphorylase transformation from b to a may be one mechanism to explain increased glycogenolysis that is evident with increasing exercise intensity. This would also be logical given that sarcoplasmic Ca2+ levels would be increased with high-intensity exercise (given the need for more rapid cross-bridge cycling) and that Ca2+ is a potent positive allosteric regulator of phosphorylase kinase through binding to the calmodulin subunit. However, the percentage of phosphorylase in the more active a form does not appear to be increased with exercise intensity and in actual fact is decreased after only 10 min of high-intensity exercise, which may be related to the reduced pH associated with intense exercise (Howlett et al. 1998). Whereas this mechanism of transformation (mediated by Ca2+ signaling) may be in operation within seconds of the onset of contraction (Parolin et al. 1999), it appears that post-transformational mechanisms are in operation during more prolonged periods of high-intensity exercise given that glycogenolysis still occurs despite reduced transformation. In this regard, vital signals related to the energy status of the cell play a more prominent role. Indeed, as exercise intensity progresses from moderate- to high-intensity exercise, the rate of ATP hydrolysis increases so much so that there is a greater accumulation of ADP, AMP, and Pi, thus providing allosteric and substrate level control (Howlett et al. 1998).
One final consideration is that the rapid decrease in muscle glycogen at intensities close to and above VO2max most likely represents mere glycogenolysis, rather than oxidation of its glucose units. For example, all-out exercise of ~2 min at an intensity well above the VO2max decreases muscle glycogen by about 25% (Medbø 1993; Medbø et al. 2006), but the net oxidation of the glycogen utilized is estimated to be ~4–13% (Medbø 1993). In this scenario, most of it ends as lactate, of which half is released to the bloodstream and the other half is utilized for rapid muscle glycogen resynthesis within the muscle (Medbø et al. 2006). Bangsbo et al. (1991) estimated that between 13 and 27% of lactate released from high-intensity exercise was converted back to glycogen (Bangsbo et al. 1991). From a practical perspective, it is worth noting that despite the disproportionate increase of skeletal muscle glycogen with increased intensity, prolonged steady-state exercise results in increased absolute muscle glycogen utilization when compared with shorter high-intensity intervals (Impey et al. 2020).
5.3.2 Substrate Availability
Although exercise intensity and duration are key drivers of glycogen use, high or low glycogen availability can result in higher or lower glycogenolysis, respectively, despite divergent exercise intensities (Arkinstall et al. 2004). Due to the significant effects of nutrient availability on glycogen utilization dynamics and exercise performance, manipulation of substrate availability and skeletal muscle glycogen has therefore been a major area of focus in research. Indeed, given the relationship between high muscle glycogen and increased work capacity, a number of dietary practices manipulating substrate availability have been tested to maximize muscle glycogen stores or to minimize its use during exercise in order to further increase work capacity. The most noteworthy practices manipulating substrate availability are the increase of muscle glycogen itself, the increase in availability and oxidative capacity of lipids (through diet and intravenous provision of lipids or lipolytic agents), and the ingestion of glucose during exercise.
5.3.2.1 Increased Muscle Glycogen Stores and Glycogen Use
Skeletal muscle glycogen content is directly related to dietary carbohydrate as shown by seminal studies (Bergström et al. 1967; Gollnick et al. 1972), a finding that has systematically been corroborated in the literature (Areta and Hopkins 2018). Depleting muscle glycogen through exhaustive exercise followed by high carbohydrate intake has been shown to subsequently overshoot skeletal muscle glycogen reserves above normal resting values in the previously contracted muscle (Bergström and Hultman 1966a, b), a phenomenon that was termed as “glycogen supercompensation.” The precise molecular mechanisms underpinning the supercompensation effect remain an active area of research (Hingst et al. 2018). Since the original work documenting this effect, it is now accepted that prior depletion of muscle glycogen is not necessary for individuals who regularly practice aerobic-type exercise and that a taper of training load in conjunction with elevated dietary carbohydrate intake of ~10–12 g/kg of body mass/day can maximize skeletal muscle glycogen concentration within 24–48 h (Burke et al. 2017). Regardless, higher resting muscle glycogen leads to higher rates of muscle glycogen use during exercise, likely a reflection of substrate regulation of glycogen phosphorylase (Hargreaves et al. 1995). Indeed, when intensity and duration of exercise are kept constant, commencing exercise with high muscle glycogen (e.g., > 600 mmol.kg−1 dw) leads to higher muscle glycogen use compared to normal (e.g., ~450 mmol.kg−1 dw) or low (e.g., <300 mmol.kg−1 dw) starting muscle glycogen (Galbo et al. 1979; Hargreaves et al. 1995; Areta and Hopkins 2018). Accordingly, when examining performance testing data of prolonged endurance exercise of a set distance and workload, exercise is often finished with similar muscle glycogen levels despite significant differences in starting muscle glycogen (Karlsson and Saltin 1971; Sherman et al. 1981; Rauch et al. 1995; Tomcik et al. 2018).
5.3.2.2 Increased Lipid Availability and Glycogen Sparing
Given the comparatively unlimited storage of energy in the form of fat, increasing the use of lipids as a fuel source during exercise to “spare” muscle glycogen with the goal of improving physical performance has been a topic of intense research. Intravenous lipid infusion, lipolytic agents, high-fat meals, and high-fat diets have been all been used to test this hypothesis. Interventions using intralipid infusion and heparin have been consistently shown to spare muscle glycogen between ~15 and 45% at moderate and high intensities lasting 15–60 min (Vukovich et al. 1993; Dyck et al. 1993, 1996; Odland et al. 1998; Hawley 2002). The effects of a high-fat meal consumed prior to exercise, however, have been less consistent with studies ranging from 40% glycogen sparing to no glycogen sparing at all (Costill et al. 1977; Vukovich et al. 1993; Hawley 2002;). The possibility of sparing skeletal muscle glycogen via dietary fat intake has been expanded through the use of high-fat diets during days and weeks.
The use of high-fat diets consumed over durations greater than 3 days has been termed “fat adaptation” and is effective in increasing circulating free fatty acids (FFA), FFA uptake by muscle, and increased fat oxidation during exercise (Stellingwerff et al. 2006). The increased rates of fat oxidation, however, occur in parallel with decreased skeletal muscle glycogen due to reduced dietary carbohydrate intake (Hammond et al. 2019; Areta et al. 2020) and hence, as detailed in the previous section, lower muscle glycogen results in lower glycogen utilization. Nonetheless, 24 h of restoration of carbohydrate intake after days of fat adaptation permits skeletal muscle glycogen storage while still retaining the increased capacity to utilize higher amounts of fat during exercise and spare glycogen to some extent (Burke et al. 2000; Stellingwerff et al. 2006). However, fat adaptation protocols also decrease pyruvate dehydrogenase (PDH) activity (Stellingwerff et al. 2006), the enzyme that represents a rate-limiting step in the flux of glucose into mitochondrial ATP, and thereby this condition impairs the capacity of generating metabolic power through the oxidation of glycogen. Therefore, in the majority of the studies to date, fat adaptation has not been able to enhance physical performance in endurance exercise, likely due to impairment of the capacity for high-intensity exercise (Burke 2006). However, over prolonged exercise (>5 h), fat adaptation with carbohydrate restoration is potentially beneficial for performance (Rowlands and Hopkins 2002). In conclusion, increasing fat availability acutely and chronically has the capacity to reduce muscle glycogen use during exercise, likely due to the independent and combined effects of increasing fat utilization and decreasing the capacity to oxidize glucose. Nonetheless, these strategies do not seem conducive to promoting exercise performance.
5.3.2.3 CHO Supplementation During Exercise and Glycogen Sparing
The fact that carbohydrate consumption during exercise increases carbohydrate oxidation led to the idea that muscle glycogen would be spared as a fuel source at its expense (Coyle et al. 1986). This intuitive idea, however, has not been supported by research findings. When considering whole skeletal muscle glycogen, a meta-analytic evaluation of CHO supplementation during exercise (n = 24 studies) determined there was no glycogen-sparing effect (Areta and Hopkins 2018). It is important to highlight that this meta-analysis only evaluated the presence or absence of CHO consumption. Indeed, although the effects of consuming 0, 15, 30, and 60 g/h of CHO showed no differences in glycogen use during exercise (Smith et al. 2010), a more nuanced analysis should perhaps determine whether low, medium, and high amounts of carbohydrate (e.g., <30, ~60, and ≥ 90 g/h respectively) across different exercise durations can affect glycogen utilization. No unequivocal evidence exists to support sparing with specific fibers, with reports of no sparing in either fiber type (Coyle et al. 1986; Mitchell et al. 1989), sparing in type 2a fibers (De Bock et al. 2006), and sparing in type 1 fibers (Yaspelkis et al. 1993; Tsintzas et al. 1995, 1996; Tsintzas et al. 2001). Such differences between studies may be related to differences in CHO availability prior to exercise, the CHO feeding strategy, and contrasting exercise protocols related to duration, modality, and intensity. In a recent study, Fell et al. (2021) demonstrated that consuming 45 or 90 g/h of CHO (in the form of solids, gels, and fluids) does not spare glycogen use in type 1 or 2 fibers when compared with no CHO ingestion, as assessed during 3 h of steady-state cycling conducted at lactate threshold. Importantly, exercise was commenced after a 36 h CHO loading protocol of 12 g/kg as well as consumption of a pre-exercise meal of 2 g/kg, thus replicating nutritional practices of elite road cyclists. Despite the apparent consensus that CHO feeding does not exert glycogen sparing, future studies should also examine the effects of CHO feeding on utilization within the subcellular glycogen storage pools of both type 1 and 2 fibers.
5.3.3 Training Status
For a given absolute exercise intensity, longitudinal studies demonstrate that glycogen utilization is reduced with exercise training (Karlsson et al. 1974), an effect that is confined locally to the actual muscles that were trained (Saltin et al. 1976). The reduced glycogenolysis observed after training is not due to any change in phosphorylation transformation but rather allosteric mechanisms (Chesley et al. 1996). Indeed, exercise in the trained state is associated with reduced content of ADP, AMP, and Pi thereby providing a mechanism leading to reduced phosphorylase activity. Importantly, the reduced rates of glycogenolysis that are evident with training are also apparent despite the fact that training induces elevations in resting glycogen stores and that higher basal glycogen is normally associated with increased glycogen utilization. As such, local allosteric control exerts a more pronounced regulatory role than substrate-level regulation. On this basis, it is now accepted that glycogen utilization during exercise is inversely related to training status and that exercise in the trained state, at same absolute intensity, requires comparatively less muscle glycogen. However, despite this well-documented finding, a meta-analytic evaluation of the effects of training status on glycogen utilization has shown only a trivial or small effect in relation to increases of VO2max of 10 ml/kg/BM (Areta and Hopkins 2018).
This latter finding may be attributable to the fact that VO2max is not an ideal parameter of training status when assessing changes in local muscle metabolism, despite it being considered as the main parameter for maximal aerobic capacity. Rather, a parameter of training status (and indeed exercise intensity) that is more reflective of skeletal muscle oxidative capacity is likely a more suitable approach to assessing the changes in glycogen utilization associated with training. For example, as little as 3 days of prolonged (2 h) aerobic-type training induces glycogen sparing by ~60% when assessed during exercise undertaken at 65% VO2peak in healthy individuals (Green et al. 1995). As such, parameters of training status that are more indicative of the oxidative capacity of skeletal muscle (i.e., lactate threshold) are likely a better parameter for classification of status when determining the effects of training on skeletal muscle glycogen utilization. Indeed, individuals with comparable VO2max but who possess a “high” or “low” lactate threshold present with distinct differences in glycogen utilization when cycling for 30 min at 80% VO2max, i.e., subjects with a low threshold utilize more than twice as much muscle glycogen during 30 min exercise (Coyle et al. 1988).
In summary, substantial evidence demonstrate that a more trained skeletal muscle (i.e., with higher oxidative capacity) is less reliant on skeletal muscle glycogen use during exercise. However, there is a large variation in rates of muscle glycogen use in individuals when exercising at a percentage of their VO2max. The use of a parameter of intensity and training status representative of skeletal muscle oxidative capacity will be important to further characterize the estimation of muscle glycogen use during exercise.
5.3.4 Exercise Mode
5.3.4.1 Running vs. Cycling
When comparing glycogen utilization within the vastus lateralis muscle, it has been consistently demonstrated that cycling induces greater absolute utilization than when running at a matched relative exercise intensity and duration. For example, Arkinstall et al. (2004) compared glycogen utilization in the vastus lateralis of moderately trained males during 60 minutes of cycling and running at lactate threshold and observed an absolute utilization of approximately 220 and 120 mmol.kg−1 dw, respectively. Accordingly, a recent meta-analysis demonstrated that absolute glycogen utilization is “small but very likely” reduced during matched protocols of running versus cycling (i.e., relative exercise intensity and duration), where the expected reduction in running equates to 70 mmol.kg−1 dw (Areta and Hopkins 2018). Such differences between modalities are, of course, reflective of greater recruitment of the vastus lateralis muscle during cycling when compared with running. The role of muscle recruitment in modulating glycogen utilization is also evident during running where both the absolute and rate of glycogen utilization are higher in the gastrocnemius muscle when compared with the vastus lateralis (Costill et al. 1974). This pattern of greater glycogen utilization within the gastrocnemius versus vastus lateralis when running is evident in both males and females during moderate- and high-intensity exercise protocols (Impey et al. 2020).
5.3.4.2 Resistance Exercise
Resistance exercise sessions typically consist of several sets (interspaced by pauses of 1–4 min) of repeated near maximal force productions. Here, the short duration and the high intensity of each force production necessitate a predominant use of the rapid energy systems taxing glycogen and creatine phosphate as substrates. During recovery periods between sets, however, other substrates such as intramuscular triglycerides can also contribute to the overall energy provision (Koopman et al. 2006). The most important factor for the utilization of glycogen during resistance exercise seems to be the total work performed rather than the exercise intensity (Robergs et al. 1991). Typical high-volume resistance exercise sessions decrease skeletal muscle glycogen by about 20–40% (100–250 mmol.kg−1 dw) (Tesch et al. 1986; Essén-Gustavsson and Tesch 1990; Robergs et al. 1991; Pascoe et al. 1993; MacDougal et al. 1999; Haff et al. 2003; Harber et al. 2010; Samuelson et al. 2016), but, in contrast to endurance exercise, the decrease is larger in type 2 fibers than in type 1 fibers (Tesch et al. 1998; Koopman et al. 2006; Morton et al. 2019).
5.3.5 Lower vs Upper Body
Vastus lateralis of the quadriceps has been the muscle predominantly sampled in research on skeletal muscle glycogen and used as a model that is normally extrapolated to all other muscles. However, several lines of evidence point to different intrinsic regulations of metabolism in the upper and lower body. These differences include a higher relative fat oxidation in the leg compared with arm exercise (Helge et al. 2008; Larsen et al. 2009), and a higher mitochondrial oxygen flux is present in the vastus lateralis compared to the arm (deltoid) muscle measured by high-resolution respirometry (Larsen et al. 2009). Further, it is well recognized that lactate release is higher during arm compared with leg exercise of comparable intensity (Jensen-Urstad and Ahlborg 1992; Jensen-Urstad et al. 1993). Thus, Ahlborg and Jensen-Urstad (1991) had two groups performing arm cranking and leg cycling, respectively, and demonstrated a higher relative carbohydrate utilization during arm cranking due to both a higher muscle glycogenolysis and a higher glucose uptake, while arms also had a higher lactate release (Ahlborg and Jensen-Urstad 1991). In a later study, the glucose uptake was not different between limbs when expressed per muscle volume, whereas the lactate release was noticeably higher in the arm than in the leg (Jensen-Urstad et al. 1993). Overall, this suggests that exercising arm muscle displays a different metabolic response compared with leg muscle, with a higher glycogen utilization and lactate release compared to leg muscle during same relative exercise intensity, implying a possible qualitative difference between muscles from the arm vs. the leg. However, another explanation for the differences described above may simply be that the upper body musculature, including the arm muscle, resides in less trained musculature than that of the lower body, as in the leg muscles (see Sect. 5.3.3). Still, when arm-trained athletes are exercising with either arm or leg exercise at the same relative intensity, there was a markedly higher lactate release during arm exercise (Jensen-Urstad and Ahlborg 1992). Also, in highly trained cross-country skiers, a net lactate release from arms and uptake by legs has been demonstrated during exercise involving both the upper and lower body (van Hall et al. 2003). In line with this, direct comparisons of the highly trained arm and leg muscles of elite cross-country skiers reveal that despite same mitochondrial volume percentage and citrate synthase activity in the legs and arms, the muscles exhibited clear difference in their enzyme-linked ability to oxidize fatty acids (HAD capacity) and a fourfold higher intramyocellular lipid volume contents in leg muscles (Ørtenblad et al. 2018; Koh et al. 2018). These data point to a clear limb difference in metabolism between the leg and the arm, which cannot be explained by training status or different fiber-type distributions. Taken together, the current data suggests that when exercising at the same relative intensity, arm muscle has a higher muscle glycogen use and lactate release as compared to the leg. This is also apparent in subjects with trained both upper and lower body.
5.3.6 Temperature
Exercise in the heat results in exaggerated fatigue concomitant with major alterations in several physiological and metabolic factors. The majority of the research conducted on the effects of heat stress on energy metabolism during exercise has demonstrated a shift toward increased carbohydrate use. During exercise in the heat, the rate of muscle glycogen degradation is significantly increased (Fink et al. 1975; Febbraio et al. 1994) with an increase in both carbohydrate oxidation and lactate accumulation at a given exercise intensity, while the muscle glucose uptake and utilization appear to be unaltered during exercise in the heat, despite hyperglycemia and an augmented liver glucose output. Thus, the increase in carbohydrate utilization is largely explained by an increased muscle glycogenolysis observed via both aerobic and anaerobic energy turnover. The mechanisms thought to be responsible for the enhanced muscle glycogenolysis likely are due to increased sympatho-adrenal response and increased muscle temperature (Hargreaves et al. 1996). Although exercise in the heat increases the intramuscular glycogen utilization (Fink et al. 1975; Febbraio et al. 1994; Hargreaves et al. 1996), depletion seems not to be the cause of fatigue during exercise in the heat. Thus, a general observation is that muscle glycogen stores are not critically low at fatigue and exhaustion, i.e., <250–300 mmol.kg−1 dw, suggesting that exercise in the heat is terminated before available glycogen stores have been limiting. Further, the total amount of carbohydrate oxidized during exercise in the heat is relatively low as exercise time is shorter than exercising in moderate or low temperatures (Fink et al. 1975; Febbraio et al. 1994; Galloway and Maughan 1997). Still, diet-induced increase in muscle glycogen before exercise is associated with enhanced exercise capacity in the heat, and carbohydrate ingestion during exercise increases exercise capacity in the heat (Pitsiladis and Maughan 1999; Carter et al. 2003). These outcomes cannot be explained by direct effects on either hyperthermia or substrate depletion but may exert an ergogenic effect related to factors peripheral to the muscle. Taken together, exercising in the heat results in an increase in intramuscular glycogen utilization; however, fatigue in these circumstances appears to be related to factors other than muscle glycogen per se.
5.3.7 Altitude
As the contribution of carbohydrate is determined by the exercise intensity, the hypoxic exposure experienced at altitude causes, in most studies, an increase in relative carbohydrate utilization, when exercising at the same absolute intensity as at sea level (Young et al. 1991; Brooks et al. 1991; Brooks 1992; Roberts et al. 1996a, b; Lundby et al. 2004; Katayama et al. 2009), but not all (Braun et al. 2000; O'Hara et al. 2017; Matu et al. 2018). A more direct comparison of possible effects on substrate utilization during exercise at hypoxic conditions can be gained by comparing exercise at sea level and at altitude, matched for the same relative intensities. A meta-analysis of the effects of exposure to hypoxia during exercise matched for relative intensities, compared with normoxia, demonstrated no consistent change in the relative contribution of carbohydrate to the total energy yield (Griffiths et al. 2019). This has been evidently demonstrated in a study where subjects exercised at both the same absolute and relative intensity as at sea level and under acute hypoxia, before and after 4 weeks exposure to 4100 m altitude (Lundby and Van Hall 2002). Submaximal substrate utilization was unchanged with acute and chronic hypoxia when exercising at the same relative intensity, while the carbohydrate utilization was increased when exercising at the same absolute intensity. Further, 4 weeks of acclimatization to altitude did not affect substrate utilization, also confirming data from rodents (McClelland et al. 1998). Overall, these studies demonstrate that relative work intensity is the main factor determining fuel selection during exercise and prolonged hypoxia does not cause a significant shift in fuel selection. However, little is known about the relative contributions of muscle glycogen during hypoxia.
5.3.8 Sex Differences
Sex-based differences in substrate metabolism during endurance exercise are well documented in that it is now recognized that females have reduced reliance on whole-body CHO metabolism (typically reflective of reduced liver glycogen metabolism) to support energy production (Devries 2016). Although not always consistent, there is also some evidence that females use less glycogen than males. For example, when assessed during the luteal phase, females use less glycogen in the vastus lateralis muscle (25%) compared with males, as assessed during 90 minutes of cycling at 65% VO2peak (Devries et al. 2006). When tested in the mid-follicular phase, it has also been reported that females use less glycogen (25%) in the vastus lateralis muscle than males when running a set distance of 15.5 km on a treadmill at 65% VO2peak (Tarnopolsky et al. 1990). When completing a 16 km road run undertaken at lactate threshold in the mid-follicular phase, we also recently reported that females use less glycogen in both the vastus lateralis (30%) and gastrocnemius muscles (20%) when compared with males (Impey et al. 2020). It is, of course, difficult to offer definitive mechanisms underpinning such differences in local muscle metabolism owing to the challenge of matching resting glycogen concentration, training status, total work done, or distance covered between sexes (i.e., thus reflective of exercise intensity and duration). Additionally, variations in phase of the menstrual cycle studied as well as the use of contraceptives also make it difficult to compare between studies. On the basis that studies examining the impacts of sex hormones (e.g., estrogen and progesterone) have generally indicated they exert minimal regulatory effects on muscle glycogen utilization (Devries 2016), it is possible that the aforementioned factors may indeed play a more subtle but influential role. A recent meta-analysis demonstrated that sex-based differences in glycogen utilization are indicative of a “likely small” reduction on absolute glycogen utilization of approximately 30 mmol.kg−1 dw (Areta and Hopkins 2018). From a practical perspective, we therefore suggest that such magnitudes of differences in glycogen utilization are unlikely to require sex-specific nutritional strategies and that both males and females should simply ensure they commence their training or competitive scenario with sufficient glycogen stores to meet the subsequent metabolic demand.
5.3.9 Subcellular Compartmentalization
In the above sections, the utilization of glycogen has been described based on mixed muscle homogenates or histochemical-defined specific fiber types. This view assumes a uniform utilization of glycogen within the muscle fibers, i.e., with no spatial compartmentalization of metabolic reactions and no existence of local gradients of ions and metabolites. A wealth of studies have shown that this assumption is not valid and, in contrast, demonstrated that the muscle fibers’ interior is extremely crowded (high concentrations of, e.g., proteins, metabolites, and ions) with limited free diffusion, but with a highly developed compartmentalized network of enzymatic reactions (e.g., Srere 1967; Sear 2019). Within this crowded interior, glycogen particles are dispersed in distinct compartments. The definition of these compartments is man-made and may be limited by the typical 2D portray of a muscle fiber. However, the studies from independent research groups (Sjöström et al. 1982a; Marchand et al. 2002; Nielsen et al. 2011) have all defined three clearly separated compartments (Fig. 5.4): (1) the intermyofibrillar space (between the myofibrils); (2) the intramyofibrillar space (within the myofibrils); and (3) the subsarcolemmal space (just beneath the surface membrane). In addition, some studies have addressed glycogen particles in physical interaction with the sarcoplasmic reticulum (Wanson and Drochmans 1968; Goldstein et al. 1985; Tammineni et al. 2020) or have discriminated between intramyofibrillar glycogen located either in the I- or A-band of the sarcomere (Fridén et al. 1985, 1989). In the subsarcolemmal space, glycogen particles have been described as perinuclear (Caulfield and Klionsky 1959), nuclear (Sun et al. 2019), or lysosomal (Viragh et al. 1982). Since quantitative data is available only for the three clearly separated compartments, this will form the basis for the discussion below.
Representative transmission electron micrographs showing the typical pattern of glycogen storage (glycogen particles are the black dots) at pre- (a) and post-prolonged exhaustive exercise (b). Mi, mitochondria. The glycogen particles located within the sarcomere often close to the Z-disc and as strings between the filaments are termed intramyofibrillar glycogen (thick arrow). The particles located close to mitochondria (Mi) between the myofibrils are termed intermyofibrillar glycogen (thin arrows). The images are collected as a part of the project described in Nielsen et al. 2011
Of the three compartments, intramyofibrillar glycogen are utilized relatively most during various types of exercise (Marchand et al. 2007; Nielsen et al. 2011; Gejl et al. 2017a, b, c; Jensen et al. 2020b; Hokken et al. 2020, Fig. 5.4). In a recent study where participants cycled at 75% of ̇VO2max, exhaustion was associated with mixed muscle homogenate glycogen concentration well above zero (120 mmol kg dw), but with intramyofibrillar glycogen levels close to zero in about 60% of the type 1 fibers (Jensen et al. 2020b). In comparison, subsarcolemmal and intermyofibrillar glycogen levels were close to zero in only 40 and 10% of the type 1 fibers, respectively, clearly suggesting a link between the depletion of intramyofibrillar glycogen and exhaustion. However, in a few exceptions, intramyofibrillar glycogen was not found to be preferentially utilized, which forms some basis for the understanding of compartmentalized glycogen utilization. Firstly, with repeated high-intensity exercise (4 × 4 min sprint skiing), only intermyofibrillar glycogen was utilized during the fourth exercise bout (Gejl et al. 2017a, b, c), indicating that the energy production by degradation of intramyofibrillar glycogen can be replaced by another energy source if the exercise is repeated. This could be from intermyofibrillar glycogen degradation, which in absolute terms was utilized more during the fourth exercise bout, or from phosphocreatine, which is also localized within the myofibrils (Wallimann and Eppenberger 1985) and known to super-compensate in response to repeated exercise (Sahlin et al. 1997). Secondly, if subsarcolemmal glycogen is super-compensated, its utilization rate is increased concomitant with a reduced utilization of intramyofibrillar glycogen (Jensen et al. 2020b). This implies the existence of a mechanism linking subsarcolemmal glycogen with intramyofibrillar glycogen utilization. Thirdly, after resistance exercise, intermyofibrillar glycogen was preferentially utilized in type 1 fibers, which contrasts to type 2 fibers, where glycogen was utilized from all compartments (Hokken et al. 2020). The resistance exercise was characterized by work periods interspaced by 2–4 min pauses, which may facilitate some resynthesis of glycogen. With a low rate of glycogen utilization in type 1 fibers, a small preferential resynthesis of intramyofibrillar glycogen during rest periods may almost equal the degradation during work, which underscores that net results (pre-post) from intermittent exercises should be carefully interpreted.
5.4 Glycogen Depletion and Fatigue
Most of the studies investigating the role of glycogen in muscle fatigue are based on associations or correlational findings, but the causative effect and mechanisms explaining glycogen depletion-induced fatigue are not clear. To definitively address this experimentally, these human studies with correlative findings should be combined with mechanistic in vitro studies, but while glycogen can be removed enzymatically by amylase, it cannot be instantly added exogenously. Due to these inherent limitations in research designs, our understanding of the role of glycogen in muscle fatigue must include careful interpretations of the available data.
5.4.1 Correlations with Performance
Pioneering research by A.V. Hill suggested that glycogen was the sole fuel for muscle work and that lactate was necessary to activate the muscle (e.g., Hill 1913). Today, it is widely known that ATP generated through several metabolic pathways is the fuel for muscle work and that Ca2+ ions ultimately mediate switching off the brake on actin filaments and facilitate cross-bridge formation, which is the main feat of ATP consumption of contracting skeletal muscle. Although Hill unintendedly exaggerated the role of glycogen in skeletal muscle, much evidence still points toward glycogen as a key fuel source mediating these processes. In the following sections, we review the literature examining the role of glycogen for performance during prolonged, short-term, and resistance exercise.
5.4.1.1 Prolonged Exercise (60–180 Min)
When performance is evaluated as maximal work conducted at a fixed protocol of working intensity (time to exhaustion), most (Bergström et al. 1967; Galbo et al. 1979; Lamb et al. 1994; Walker et al. 2000; McInerney et al. 2005; Duhamel et al. 2006c; Alghannam et al. 2016; Jensen et al. 2020b), but not all (Madsen et al. 1990), studies demonstrate improved performance with high pre-exercise glycogen stores. Closed-end tests (e.g., time trials) show small effects (Karlsson and Saltin 1971; Widrick et al. 1993; Rauch et al. 1995) or no effect (Sherman et al. 1981; Hawley et al. 1997a, b; Burke et al. 2000; Tomcik et al. 2018) of high pre-exercise glycogen stores on performance. Independent of test protocol, the characteristics of the studies showing a positive effect of elevated glycogen levels are that the participants either worked for 90–180 min and/or had large differences (>50%) in pre-exercise glycogen levels between the experimental conditions.
5.4.1.2 Short-Term Exercise (<15 Min)
If the pre-exercise glycogen level is well above the utilization level, the current consensus is that there are no effects of above-normal levels of glycogen on short-term exercise performance. This is based on tests of time to exhaustion at 125% (Vandenberghe et al. 1995) and 85% (Lambert et al. 1994) of VO2max as well as a 75 sec all-out time trial (Hargreaves et al. 1997). However, if the pre-exercise glycogen level is very low, both repeated 6 sec all-out sprint (Balsom et al. 1999) and repeated one-legged intense exercise (Bangsbo et al. 1992) performance are impaired. Collectively, muscle glycogen seems to be important for both continuous high-intensity exercise tolerance (>60 s duration) and single or repeated sprint performance (<60 s duration), only if a substantial degree of depletion is achieved, whereas loading the stores above normal levels imposes no consistent additional benefit (Vigh-Larsen et al. 2021).
5.4.1.3 Resistance Exercise
Several studies have found that a carbohydrate-restricted diet is not associated with reduced strength and power output (i.e., Mitchell et al. 1997; Sawyer et al. 2013), suggesting that resistance exercise performance is not related to the muscle glycogen levels. This is in accordance with one study, where a very low pre-exercise glycogen level did not affect maximal voluntary isometric contraction or peak force during 50 repetitions (Symons and Jacobs 1989). While this is corroborated by the finding that only around 40% of the glycogen stores in the active muscles are utilized during typical resistance exercise protocols (Tesch et al. 1986; Essén-Gustavsson and Tesch 1990; Robergs et al. 1991; MacDougal et al. 1999), a recent study demonstrated, however, that a local pool (intramyofibrillar) of glycogen in type 2 fibers is very low after resistance exercise (Hokken et al. 2020), suggesting that fatigue development of some type 2 fibers may limit resistance exercise performance.
5.4.2 A Causal Link to Fatigue?
The causal link between glycogen depletion and impaired performance is most likely multifactorial, but studies on isolated muscles from rodents (Chin and Allen 1997) and amphibians (Stephenson et al. 1999) clearly suggest a local factor within the muscles. To our knowledge, till date, only one paper has questioned this finding. Here, mice were lacking glycogen due to a whole-body gys1 (glycogen synthase) deletion and showed surprisingly normal exercise capacity (Pederson et al. 2005). However, only around 10% of the mice survived birth suggesting a survival phenotype, which may not be comparable to a wild-type phenotype, and a later study using inducible skeletal muscle-specific gys1 deletion showed considerable reduced exercise capacity (Xirouchaki et al. 2016). Thus, the lack of glycogen inevitable leads to diminished muscle function. Nonetheless, the mechanisms explaining why and how glycogen and glycolytic rate are integrated with cell function are far from understood.
According to one’s intuition and the recognized “energy crisis” theory, the association between low muscle glycogen levels and impaired contractile function is that low glycogen causes a slowed glycogenolytic and glycolytic flux, compromising the required rate of ATP regeneration for the sustained muscle function during a given intensity (Green 1991; Sahlin et al. 1998). Consequently, the muscle is unable to maintain an adequate ATP supply to one or more of the processes involved in E-C coupling, leading to impaired muscle function, i.e., fatigue. This is supported by observations of whole muscle cell PCr decreases along with an increase in free ADP and IMP following prolonged glycogen depleting exercise (Norman et al. 1988; Sahlin et al. 1997). However, this theory is challenged by both in vitro and in vivo studies demonstrating a strong association between low glycogen and decreased muscle function even after recovery periods, where ATP levels would be normal (Bangsbo et al. 1992; Chin and Allen 1997). Moreover, muscular fatigue is also observed even when glycogen is far from depleted in different conditions where glycogen is decreased prior to the start of exercise (Duhamel et al. 2006a, b; Ørtenblad et al. 2011). Also, low glycogen affects muscle function under experimental circumstances in vitro where global ATP and PCr levels can be maintained at near resting levels (Kabbara et al. 2000; Helander et al. 2002; Nielsen et al. 2009). Together, these series of experiments do not provide experimental support for the energy crisis hypothesis.
It is noteworthy, however, that the ATP concentration inside cells may not be uniform at a subcellular level (Jones 1986). The highly organized muscle cell forms many compartments and hence microenvironments with high ATPase activity, and restricted diffusional access of metabolites and observations on whole muscle experiments or fiber preparations does not rule out a role of glycogen in maintaining a subcellular compartment energy status. Such a functional compartmentalization of glycolytic metabolism is known in a variety of tissues, including skeletal muscle. In this way, the model that has evolved is that glycolytic-derived ATP regulates key steps in the muscle excitation-contraction (E-C) coupling by delivering ATP in the microenvironment of the triad junction (Han et al. 1992; Korge and Campbell 1995). This is particularly significant in the muscle triad junction between the transverse tubular system and the sarcoplasmic reticulum (SR), with a diffusional restricted space around 12 nm wide and with a high metabolic activity (Dulhunty 1984). Consistent with the notion of compartmentalized glycolysis, most of the glycolytic enzymes are associated with membranes of intracellular compartments such as the SR (Wanson and Drochmans 1972; Entman et al. 1980; Xu and Becker 1998). Furthermore, glycogen is stored in particles located in specific regions of the muscle fiber, and variable utilization of these depots occurs during exercise, with the depot localized near the triad region being repeatedly associated with muscle function and whole-body exhaustion (see Sect. 5.3.9). Physiologically, this organization places muscular energy stores in close proximity to their site of utilization and provides support for the emerging concept for functionally compartmentalized energetic networks, ensuring an efficient energy transfer and signal transduction between energy production and utilization in different cellular compartments (Korge and Campbell 1995; Saks et al. 2008; Nielsen and Ørtenblad 2013). The following sections will focus on the experimental evidence of how glycogenolytically/glycolytically derived products preferentially regulate key steps in the muscle E-C coupling, i.e., SR Ca2+ regulation and muscle excitability, thus providing an explanation of the observed association between muscle glycogen contents and fatigue.
5.4.2.1 SR Ca2+ Regulation
Contraction of skeletal muscle is governed by the series of events in the E-C coupling, in which the Ca2+ release and uptake from the sarcoplasmic reticulum (SR) are an integral part through initiation and termination of the cross-bridge cycling. The SR Ca2+ release is triggered through an action potential (AP) activation of the voltage-sensor molecules in the t-system membrane, which open the SR Ca2+ release channels (RyR), leading to a rise in intracellular free concentration ([Ca2+]i) and generation of force by the contractile apparatus (Melzer et al. 1995; Stephenson 1996).
Both direct and indirect evidence point to a modulating role of glycogen availability on SR Ca2+ handling, as demonstrated in animal (Chin and Allen 1997; Stephenson et al. 1999; Kabbara et al. 2000; Barnes et al. 2001; Helander et al. 2002; Nielsen et al. 2009) and human models (Gejl et al. 2014; Duhamel et al. 2006b; Ørtenblad et al. 2011). Using both single fibers and muscle bundles, Chin and Allen (1997) elegantly demonstrated that muscle force and [Ca2+]i are associated with muscle glycogen content. Thus, through the manipulation of glucose availability in the recovery phase after fatiguing contractions, it was shown that a reduced resting level of glycogen was associated with a faster decrease in tetanic [Ca2+]i and force during subsequent contractions. These results have subsequently been confirmed (Kabbara et al. 2000; Helander et al. 2002; Nielsen et al. 2014), and together, experiments on rodent muscle suggest that the change in tetanic [Ca2+]i associated with fatigue and recovery has a component that is glycogen dependent.
The mechanisms linking low muscle glycogen with decreased [Ca2+]i has further been elucidated by direct measures of SR vesicle Ca2+ release rate (Duhamel et al. 2006a, b, c; Ørtenblad et al. 2011). These studies on SR vesicles from the human muscle, where glycogen levels have been modulated by the diet either before or during the recovery phase after exercise, demonstrate a clear association between the SR vesicle Ca2+ release rate and muscle glycogen levels (Duhamel et al. 2006a, b; Ørtenblad et al. 2011; Gejl et al. 2014; Watanabe and Wada 2019). Importantly, there seems to be a critical level of muscle glycogen at around 250–300 mmol.kg−1 dw below which the SR Ca2+ release rate is impaired (Duhamel et al. 2006a; Ørtenblad et al. 2011; Gejl et al. 2014). Such data explain why minor decreases in muscle glycogen do not cause significant impairments in SR Ca2+ release rate and why exhaustive exercise starting with a low or high muscle glycogen store decreases or improves the endurance performance, respectively, possibly affected by SR Ca2+ regulation (Ørtenblad and Nielsen 2015; Ørtenblad et al. 2013).
The use of the mechanically skinned fiber preparation has provided a unique means to investigate the possible interactions between glycogen and basic muscle function (Lamb and Stephenson 2018). Indeed, the selective removal of the sarcolemma allows for the study of muscle function and the effects of glycogen content per se while maintaining the cellular architecture and control of the intracellular milieu, i.e., keeping PCr and ATP high and constant. Studies using the mechanically skinned fiber have provided experimental evidence that low glycogen content in the muscle fiber is associated with force depression during repeated voltage sensor activated contractions in most studies (Stephenson et al. 1999; Barnes et al. 2001; Watanabe and Wada 2019), but not all (Goodman et al. 2005), as well as AP-induced contractions (Nielsen et al. 2009: Jensen et al. 2020a).
Taken together, emerging evidence suggests that low muscle glycogen affects the SR Ca2+ release and in turn [Ca2+]i and muscle function despite global ATP being held constant. This conclusion supports the concept of a functionally compartmentalized energetic network regulating key steps in the muscle E-C coupling. In support hereof are the observations (from both mechanically skinned fiber preparation, intact mouse fibers, and human SR vesicles) that the specific pool of intramyofibrillar glycogen within the myofibrils is associated with SR Ca2+ release and [Ca2+]i (Nielsen et al. 2009; Ørtenblad et al. 2011; Nielsen et al. 2014). At present, little is known about the precise mechanism(s) which links glycogen levels in the muscle with SR Ca2+ release rate.
In skeletal muscle, Ca2+ is released from the SR Ca2+ stores via the specific Ca2+ channels (RyR1), located at the junctional SR of the triad, which ensures efficient Ca2+ release to the contractile proteins. In relation to the role of glycogen affecting the SR Ca2+ release, low glycogen may in the compartmentalized cell lead to changes in metabolic status, especially in triad region with the RyR localization. This may lead to increase in free [Mg2+] and decrease in free [ATP], which are strong regulators of the RyR1, also in the physiological range of changes (Lamb and Stephenson 1994; Blazev and Lamb 1999). Furthermore, glycolytic intermediates as fructose 1,6-bisphosphate have been demonstrated to increase the open probability of the RyR channels (Han et al. 1992). However, low glycogen has also been demonstrated to modulate the Ca2+ release rate in isolated vesicles without restricted metabolic space and during resting metabolic conditions. This may indicate a crucial role of the metabolic machinery associated with the SR. Also, the RyR protein contains numerous phosphorylation sites, which may be affected by PKA and Ca2+-calmodulin-dependent kinase II in fast twitch fibers (Fill and Copello 2002). However, the impact of phosphorylation and/or dephosphorylation on single RyR channel behavior and the role of glycogen and energy status are at present not fully unraveled.
5.4.2.2 Muscle Excitability and Na, K-Pump
With repeated intense activation, a change in the electrochemical gradients for K+ can cause a substantial membrane depolarization leading to failure of excitation and SR Ca2+ release and an ensuing decrease in force responses (Sejersted and Sjøgaard 2000; Clausen and Nielsen 2007), although several mechanisms interact during exercise to counteract the depressive effects of elevated extracellular levels of K+ (Pedersen et al. 2003; de Paoli et al. 2007, 2010). There is a reasonably well-established relationship between glycolytic-derived ATP and Na, K-pump activity, and evidence exists supporting that glycolysis and the Na, K-pump are functionally coupled. This seems to be an evolutionary conserved metabolic coupling and has been observed in several tissue types, including the mammalian erythrocytes (Schrier 1966; Mercer and Dunham 1981; Kennedy et al. 1986), axons (Caldwell et al. 1960), cardiac myocytes (Philipson and Nishimoto 1983; Hasin and Barry 1984; MacLeod 1989), and skeletal muscle (Clausen 1965; James et al. 1999; Okamoto et al. 2001; Jensen et al. 2020a). In line with this, many tissues generate pyruvate and lactate under aerobic conditions (aerobic glycolysis) in a process linking glycolytic ATP supply to the activity of the Na, K-pump (Brooks 1986; Dhar-Chowdhury et al. 2007). Indeed, aerobic glycolysis and glycogenolysis occur in resting, well-oxygenated skeletal muscles and is closely linked to stimulation of active membrane Na, K-pump transport due to epinephrine release (James et al. 1996, 1999a, b; Bundgaard et al. 2003; Levy et al. 2005). The role of glycolysis and oxidative phosphorylation in providing fuel to the Na, K-pump in the skeletal muscle was investigated using the Na, K-pump inhibitor ouabain in resting rat extensor digitorum longus muscles, demonstrating that Na, K-pump activity is only impaired when the glycolysis is inhibited (Okamoto et al. 2001). A tight coupling between the glycogenolytic rate and Na, K-pump activity is further demonstrated by the observation that intracellular Na+ decreases if glycogen breakdown is stimulated with epinephrine at rest, while ouabain significantly attenuates glycogen utilization (James et al. 1999b). The data suggest that in the skeletal muscle, glycolysis is the predominant source of the fuel for the Na, K-pump. Further, a direct link between muscle glycogenolysis and Na, K-pump activity was demonstrated by the observation of decreased glycogen utilization in resting muscle when the muscle Na, K-pump activity was blocked with ouabain (Clausen 1965). Moreover, lactic acid production was increased in proportion to activation of the Na, K-pump. Taken together, these data indicate a clear association of glycogenolytic/glycolytically derived ATP on active cation transport across the muscle membranes. Indeed, a direct link between energy state and excitability of the muscle was demonstrated by blocking cross-bridge cycling and SR Ca2+ release with the cross-bridge cycling blocker BTS (N-benzyl-p-toluene sulphonamide) and dantrolene, respectively, thereby conserving energy during repeated electrical stimulations, which in turn improved the ability of muscles to maintain excitability during high-frequency stimulation (Macdonald et al. 2007).
The effects of glycogenolytically derived ATP on muscle excitability are further substantiated in mechanically skinned fibers with a high and constant global ATP, demonstrating that glycogenolytically derived energy is associated with fiber contractile endurance irrespective of global ATP levels. Thus, enzymatically lowering glycogen by 70% led to a reduction in both voltage sensors-activated and AP-induced forces in the skinned fibers, with a larger decrease in the AP force by the glycogen lowering. These data suggest that low glycogen and glycogenolytic rate affects t-system polarization and excitability, as the voltage sensor inactivation is displaced to markedly more positive Vm values compared with the AP inactivation (Ørtenblad and Stephenson 2003; Nielsen et al. 2004) and thereby force production will be less affected by voltage sensor compared to AP activation. For a more direct estimate of the Na, K-pump function, one can quantify the membrane ability to respond to two closely spaced AP. The repriming time until the second pulse generates an AP is in turn dependent on the Na, K-pump function. A depolarization of the t-system increases the repriming time as expected; however, the addition of phosphoenolpyruvate, which increases glycolytic ATP resynthesis, decreases t-system repriming period (Dutka and Lamb 2007a, b). Also, when glycogen is enzymatically lowered by glucoamylase treatment, the repriming period increases (Watanabe and Wada 2019; Jensen et al. 2020a). This was confirmed by the use of glycogen phosphorylase inhibitors and glycogen lowering treatment in mechanically skinned fibers, which invariably prolonged repriming time, strongly indicating an attenuated Na, K-pump activity (Jensen et al. 2020a). Collectively, substantial evidence indicates a tight coupling between glycogenolytic-glycolytic-derived ATP production on Na-K-pump function, with low glycogen or inhibited GP in turn leading to an attenuated t-system excitability and ultimately force production.
5.4.2.3 Insights from McArdle Patients
Insights into the role and importance of muscle glycogen can be gained from McArdle patients (glycogen storage disease type V) who lack glycogen phosphorylase and thereby not able to catalyze the breakdown of glycogen into glucose-1-phosphate in muscle fibers (McArdle 1951; Santalla et al. 2014). McArdle patients’ have low peak work capacities and exercise intolerance and a suppressed plasma lactate concentration during exercise (De Stefano et al. 1996; Ørngreen et al. 2015). The exercise intolerance is especially evident during the onset of exercise, during high-intensity exercise and high force output contractions with smaller muscle groups (Lucia et al. 2008; Santalla et al. 2014). These patients may provide a unique paradigm to gain insight to the role of muscle glycogen per se in muscle function and exercise tolerance. Interestingly, a higher surface electromyography signal is measured during submaximal contractions in McArdle patients, which is indicative of a need to activate larger muscle mass for a given force output, suggesting reduced muscle excitability (Santalla et al. 2014). Interestingly, McArdle patients have less Na+-K+-ATPases as compared to controls (Haller et al. 1998). Taken together, insights from McArdle patients are in line with the idea of glycolytic/glycogenolytic-derived ATP as required for muscle E-C coupling, possibly by a tight coupling between glycogenolytic/glycolytic rate and Na+-K+-ATPase activity.
5.5 Muscle Glycogen as a Regulator of Skeletal Muscle Training Adaptations
In addition to the well-documented role as a metabolic substrate for ATP provision, it is becoming increasingly accepted that the glycogen granule can also act as a regulator of training adaptations (Impey et al. 2018; Impey et al. 2016). Accordingly, the concept of deliberately training with reduced pre-exercise muscle glycogen availability in an attempt to enhance the activation of the molecular signaling pathways that regulate mitochondrial biogenesis (the so-called train low paradigm) has received significant research in the previous decade (Burke et al. 2018). In this regard, findings from acute exercise studies demonstrate that commencing exercise with “reduced” pre-exercise muscle glycogen concentration upregulates cell signaling pathways with putative roles in the regulation of both the nuclear and mitochondrial genomes (Pilegaard et al. 2002; Wojtaszewski et al. 2003; Yeo et al. 2010; Bartlett et al. 2013; Psilander et al. 2013). Furthermore, repeated bouts of train-low exercise can subsequently augment many hallmark muscle adaptations inherent to the endurance phenotype (as reviewed in Impey et al. 2018). For example, the strategic periodization of dietary CHO in order to commence exercise with low muscle glycogen (during 3–10 weeks of training) enhances mitochondrial enzyme activity and protein content (Hansen et al. 2005; Morton et al. 2009; Yeo et al. 2008) and whole body and intra-muscular lipid metabolism (Hulston et al. 2010) and in some instances improves exercise capacity (Hansen et al. 2005) and performance (Marquet et al. 2016a, b), though performance enhancing effects are not always evident (Yeo et al. 2008; Hulston et al. 2010; Burke et al. 2017; Gejl et al. 2017a, b; 2018). As such, the train-low paradigm and wider CHO periodization strategies have subsequently gained increased recognition among athletic populations (Stellingwerff 2012; Burke et al. 2018; Impey et al. 2018). It should also be noted that some of the enhanced adaptations associated with “train-low” (at least in the twice per day training model) may be due to performing two consecutive training sessions in close proximity to one another, as opposed to the effects of low pre-exercise muscle glycogen per se (Andrade-Souza et al. 2020).
Skeletal muscle glycogen may exert its regulatory effects upon training adaptation through modulation of the AMP-activated protein kinase (AMPK)-peroxisome proliferator-activated receptor γ coactivator 1α (PGC- 1α) signaling axis. For example, exercise-induced AMPKα2 activity (Wojtaszewski et al. 2003), phosphorylation (Yeo et al. 2010), and nuclear abundance (Steinberg et al. 2006) are all augmented under conditions of reduced pre-exercise muscle glycogen. These effects may be partly mediated through the glycogen-binding domain present on the β-subunit of AMPK (McBride and Hardie 2009; McBride et al. 2009). Commencing acute exercise with reduced muscle glycogen also potentiates the phosphorylation of the tumor suppressor protein, p53 (Bartlett et al. 2013), that in turn may coordinate regulation of the mitochondrial genome, through modulation of mitochondrial transcription factor A (Tfam). It is noteworthy that training with low muscle glycogen also increases epinephrine, stimulates lipolysis, increases circulating free fatty acids (FFAs), and therefore augments both whole-body and intramuscular lipid oxidation (Hearris et al. 2018, 2020; Maunder et al. 2021). In this way, it is possible that FFAs may also regulate the enhanced adaptations associated with training low through acting as stimulatory signaling molecules for PPARδ signaling.
Given that the enhanced training response associated with train-low is potentially mediated by muscle glycogen availability, it is pertinent to consider the absolute glycogen concentrations required to facilitate the response. In relation to research design, high glycogen trials are commonly commenced with muscle glycogen concentrations between 400 and 600 mmol.kg−1 dw and remain above 300 mmol.kg−1 dw after exercise (Wojtaszewski et al. 2003; Roepstorff et al. 2005; Bartlett et al. 2013). In such instances, these researchers observed attenuated (Wojtaszewski et al. 2003) or abolished (Roepstorff et al. 2005; Bartlett et al. 2013) activation of cell signaling pathways. For example, we have previously observed that both AMPK and p53-related signaling is reduced when exercise is commenced and finished with high (reducing from ~500 to ~300 mmol.kg−1 dw, respectively) versus low (reducing from ~150 to ~50 mmol.kg−1 dw, respectively) muscle glycogen (Bartlett et al. 2013). A detailed examination of available train low studies (see Impey et al. 2018) indeed demonstrates that adaptations associated with CHO restriction are particularly evident when the absolute pre-exercise muscle glycogen concentration permits exercise-induced depletion to post-exercise concentrations that are ≤200–300 mmol.kg−1 dw. Accordingly, we also observed that comparable cell signaling responses occur if exhaustive (Hearris et al. 2019) and non-exhaustive (Hearris et al. 2020) exercise is finished with similar absolute post-exercise muscle glycogen concentrations (i.e., 100–300 mmol.kg−1 dw), despite commencing exercise with graded levels of muscle glycogen concentration (i.e., 300–600 mmol.kg−1 dw). On this basis, it was suggested that the absolute post-exercise muscle glycogen concentration may be a more influential factor than pre-exercise muscle glycogen concentration in relation to modulation of exercise-induced cell signaling. In keeping with this hypothesis, CHO feeding during exercise attenuates AMPK-mediated signaling but only when glycogen sparing occurs (Akerstrom et al. 2006). Furthermore, restoring post-exercise glycogen levels to >500 mmol.kg−1 dw attenuates exercise-induced changes in gene expression (Pilegaard et al. 2005). From a practical perspective, such data collectively suggest that distinct differences in post-exercise muscle glycogen concentration would likely be required between low (e.g., <200 mmol.kg−1 dw) and high (> 300 mmol.kg−1 dw) glycogen conditions in order to achieve any potential physiological advantage of reduced muscle glycogen availability in relation to augmenting training adaptation. While the optimal approach to CHO periodization remains to be determined, further research is also required to examine the role of the specific subcellular storage pools of glycogen as a regulator of skeletal muscle cell signaling pathways. Moreover, despite the theoretical rationale for carefully scheduled periods of “training-low,” it is noteworthy that definitive evidence supporting that this approach to training induces superior improvements in performance is currently limiting. In this regard, there is also a requirement to utilize experimental testing protocols (e.g., exercise durations of 3–6 h) in which the physiological adaptations associated with training low (i.e., increased oxidative capacity and lipid oxidation) may actually manifest as improved exercise performance.
5.6 Concluding Remarks and Future Directions
Glycogen is an important fuel source for contracting skeletal muscle to sustain high metabolic power output during exercise, and modern research has also shown it to be far more than just an inert energy source. Early research determined a clear direct association between the capacity to withstand intense exercise for prolonged periods and starting muscle glycogen, showing that the onset of fatigue coincides with the depletion of glycogen to very low levels, even with exogenous carbohydrate supplementation. In addition to exercise intensity and duration, baseline muscle glycogen and training status have also been identified as important factors in determining its rate of use, with other factors such as sex substrate availability and environment also having some impact. Glycogen use has shown to be divergent in different fiber types, and more recent research has identified specific subcellular loci of glycogen (namely, intramyofibrillar, intermyofibrillar, and subsarcolemmal), with possible diverse utilization and metabolic function depending on their localization. The mechanisms explaining how glycogen and glycolytic rate are integrated with muscle function are far from understood; however, emerging evidence link glycogen availability with key steps in the excitation-contraction coupling. In addition, it is also apparent that glycogen availability can exert important effects on metabolic regulation and exercise-induced cell signaling, regulating training adaptation. Thus, glycogen has a far more diverse function than just being an energy storage. Despite over 100 years of research in carbohydrate metabolism, fundamental aspects of muscle glycogen metabolism and regulation continue to challenge the scientific community and remain an evergreen area of exciting research. At several levels, we appear to have a complete understanding of the physiology of muscle glycogen, and yet at others, we know very little. Indeed, many aspects of muscle glycogen metabolism in exercise and cell function warrant further knowledge. Table 5.1 lists possible future studies further generating our understanding of muscle glycogen metabolism and regulation, as well as the causal link underlying the association between glycogen availability in the etiology of muscle fatigue and key steps in muscle function and regulator of training adaptations.
References
Ahlborg G, Jensen-Urstad M (1991) Metabolism in exercising arm vs. leg muscle. Clin Physiol 11:459–468
Akerstrom TC, Birk JB, Klein DK, Erikstrup C, Plomgaard P, Pedersen BK, Wojtaszewski JF (2006) Oral glucose ingestion attenuates exercise-induced activation of 5′-AMP-activated protein kinase in human skeletal muscle. Biochem Biophys Res Commun 342:949–955
Albers PH, Pedersen AJT, Birk JB, Kristensen DE, Vind BF, Baba O, Nøhr J, Højlund K, Wojtaszewski J (2015) Human muscle fiber type-specific insulin signaling: impact of obesity and type 2 diabetes. Diabetes 64:485–497
Alghannam AF, Jedrzejewski D, Tweddle MG, Gribble H, Bilzon J, Thompson D, Tsintzas K, Betts JA (2016) Impact of muscle glycogen availability on the capacity for repeated exercise in man. Med Sci Sports Exerc 48:123–131
Andrade-Souza VM, Ghiarone T, Sansonio A, Santos Silva KA, Tomazini F, Enrico Perri F, Saner N, Kuang K, Bertuzzi R, Leandro CG, Bishop DJ, Lima-Silva AE (2020) Exercise twice-a-day potentiates markers of mitochondrial biogenesis in men. FASEB J 34:1602–1609
Areta JL, Hopkins WG (2018) Skeletal muscle glycogen content at rest and during endurance exercise in humans a meta-analysis. Sports Med 48:2091–2102
Areta JL, Iraki J, Owens DJ, Joanisse S, Philp A, Morton JP, Hallén J (2020) Achieving energy balance with a high-fat meal does not enhance skeletal muscle adaptation and impairs glycemic response in a sleep-low training model. Exp Physiol:EP088795
Arkinstall MJ, Bruce CR, Clark SA, Rickards CA, Burke LM, Hawley JA (2004) Regulation of fuel metabolism by preexercise muscle glycogen content and exercise intensity. J Appl Physiol 97(6):2275–2283
Ball S, Colleoni C, Cenci U, Raj JN, Tirtiaux C (2011) The evolution of glycogen and starch metabolism in eukaryotes gives molecular clues to understand the establishment of plastid endosymbiosis. J Exp Bot 62(6):1775–1801
Ball-Burnett M, Green HJ, Houston ME (1991) Energy metabolism in human slow and fast twitch fibres during prolonged cycle exercise. J Physiol 437:257–267
Balsom PD, Gaitanos GC, Söderlund K, Ekblom B (1999) High-intensity exercise and muscle glycogen availability in humans. Acta Physiol Scand 165:337–345
Bangsbo J, Gollnick PD, Graham TE, Saltin B (1991) Substrates for muscle glycogen synthesis in recovery from intense exercise in man. J Physiol 434:423–440
Bangsbo J, Graham TE, Kiens B, Saltin B (1992) Elevated muscle glycogen and anaerobic energy production during exhaustive exercise in man. J Physiol 451:205–227
Barnes M, Gibson LM, Stephenson DG (2001) Increased muscle glycogen content is associated with increased capacity to respond to T-system depolarisation in mechanically skinned skeletal muscle fibres from the rat. Pflugers Arch 442(1):101–106
Bartlett, J. D., Louhelainen, J., Iqbal, Z., Cochran, A. J., Gibala, M. J., Gregson, W., . . . Morton, J. P. (2013). Reduced carbohydrate availability enhances exercise-induced p53 signaling in human skeletal muscle: implications for mitochondrial biogenesis. American journal of physiology. Regulatory, Integrative and Comparative Physiology, 304, R450–R458
Bergström J, Hultman E (1966a) The effect of exercise on muscle glycogen and electrolytes in normals. Scand J Clin Lab Invest 18(1):16–20
Bergström J, Hultman E (1966b) Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man. Nature 210:309–310
Bergström J, Hermansen L, Hultman E, Saltin B (1967) Diet, muscle glycogen and physical performance. Acta Physiol Scand 71:140–150
Blazev R, Lamb GD (1999) Low [ATP] and elevated [Mg2+] reduce depolarization-induced Ca2+ release in rat skinned skeletal muscle fibres. J Physiol 520(Pt 1):203–215
Bogdanis GC, Nevill ME, Boobis LH, Lakomy HK (1996) Contribution of phosphocreatine and aerobic metabolism to energy supply during repeated sprint exercise. J Appl Physiol 80(3):876–884
Bosch AN, Dennis SC, Noakes TD (1993) Influence of carbohydrate loading on fuel substrate turnover and oxidation during prolonged exercise. J Appl Physiol 74(4):1921–1927
Bosch AN, Weltan SM, Dennis SC, Noakes TD (1996) Fuel substrate kinetics of carbohydrate loading differs from that of carbohydrate ingestion during prolonged exercise. Metabolism 45(4):415–423
Braun B, Mawson JT, Muza SR et al (2000) Women at altitude: carbohydrate utilization during exercise at 4,300 m. J Appl Physiol 88:246–256
Brooks GA (1986) Lactate production under fully aerobic conditions: the lactate shuttle during rest and exercise. Fed Proc 45(13):2924–2929
Brooks GA (1992) Increased glucose dependency in circulatory compensated hypoxia. In: Houston C, Coates J (eds) Hypoxia and mountain medicine. Queen City, Burlington, VT, pp 213–226
Brooks GA, Butterfield GE, Wolfel RR et al (1991) Increased dependence on blood glucose after acclimatization to 4,300 m. J Appl Physiol 70:919–927
Bundgaard H, Kjeldsen K, Suarez Krabbe K, van Hall G, Simonsen L, Qvist J, Hansen CM, Moller K, Fonsmark L, Lav Madsen P, Klarlund Pedersen B (2003) Endotoxemia stimulates skeletal muscle Na+-K+-ATPase and raises blood lactate under aerobic conditions in humans. Am J Physiol Heart Circ Physiol 284(3):H1028–H1034
Burke LM (2006) “Fat adaptation” for athletic performance: the nail in the coffin? J Appl Physiol 100(1):7–8
Burke LM, Angus DJ, Cox GR, Cummings NK, Febbraio MA, Gawthorn K, Hawley JA, Minehan M, Martin DT, Hargreaves M (2000) Effect of fat adaptation and carbohydrate restoration on metabolism and performance during prolonged cycling. J Appl Physiol 89(6):2413–2421
Burke LM, Loon LJC, Hawley JA (2017) Postexercise muscle glycogen resynthesis in humans. J Appl Physiol 122(5):1055–1067
Burke LM, Hawley JA, Jeukendrup A, Morton JP, Stellingwerff T, Maughan RJ (2018) Toward a common understanding of diet–exercise strategies to manipulate fuel availability for training and competition preparation in endurance sport. Int J Sport Nutr Exerc Metab 28:451–463
Caldwell PC, Hodgkin AL, Keynes RD, Shaw TL (1960) The effects of injecting energy-rich phosphate compounds on the active transport of ions in the giant axons of Loligo. J Physiol 152:561–590
Carter J, Jeukendrup AE, Mundel T et al (2003) Carbohydrate supplementation improves moderate and high-intensity exercise in the heat. Pflugers Arch 446:211–219
Caulfield J, Klionsky B (1959) Myocardial ischemia and early infarction: an electron microscopy study. Amer J Path 35:489–523
Chesley A, Heigenhauser GJ, Spriet LL (1996) Regulation of muscle glycogen phosphorylase activity following short-term endurance training. Am J Phys 270(2 Pt 1):E328–E335
Chin ER, Allen DG (1997) Effects of reduced muscle glycogen concentration on force, Ca2+ release and contractile protein function in intact mouse skeletal muscle. J Physiol 498:17–29
Clausen T (1965) The relationship between the transport of glucose and cations across cell membranes in isolated tissues. I. Stimulation of glycogen deposition and inhibition of lactic acid production in diaphragm, induced by ouabain. Biochim Biophys Acta 109:164–171
Clausen T, Nielsen OB (2007) Potassium, Na+,K+-pumps and fatigue in rat muscle. J Physiol 584(Pt 1):295–304
Costill DL, Jansson E, Gollnick PD, Saltin B (1974) Glycogen utilization in leg muscles of men during level and uphill running. Acta Physiol Scand 91:475–481
Costill DL, Coyle E, Dalsky G, Evans W, Fink W, Hoopes D (1977) Effects of elevated plasma FFA and insulin on muscle glycogen usage during exercise. J Appl Physiol Respir Environ Exerc Physiol 43(4):695–699
Coyle EF, Coggan AR, Hemmert MK, Ivy JL (1986) Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J Appl Physiol 61(1):165–172
Coyle EF, Coggan AR, Hopper MK, Walters TJ (1988) Determinants of endurance in well-trained cyclists. J Appl Physiol. (Bethesda, Md.: 1985), 64(6):2622–2630
De Bock K, Derave W, Ramaekers M, Richter EA, Hespel P (2006) Fiber type-specific muscle glycogen sparing due to carbohydrate intake before and during exercise. J Appl Physiol 102(1):183–188
de Bruijn WC (1973) Glycogen, its chemistry and morphologic appearance in the electron microscope. I. a modified OsO 4 fixative which selectively contrasts glycogen. J Ultrastruct Res 42:29–50
de Paoli FV, Overgaard K, Pedersen TH, Nielsen OB (2007) Additive protective effects of the addition of lactic acid and adrenaline on excitability and force in isolated rat skeletal muscle depressed by elevated extracellular K+. J Physiol 581:829–839
de Paoli FV, Ørtenblad N, Pedersen TH, Jørgensen R, Nielsen OB (2010) Lactate per se improves the excitability of depolarized rat skeletal muscle by reducing the cl- conductance. J Physiol 588(Pt 23):4785–4794
De Stefano N, Argov Z, Matthews PM et al (1996) Impairment of muscle mitochondrial oxidative metabolism in McArdles’s disease. Muscle Nerve 19(6):764–769
Devries MC (2016) Sex-based differences in endurance exercise muscle metabolism: impact on exercise and nutritional strategies to optimize health and performance in woman. Exp Physiol 101:243–249
Devries MC, Hamadeh MJ, Phillips SM, Tarnopolsky MA (2006) Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise. Am J Physiol Regul Intergr Comp Physiol 291:1120–1128
Dhar-Chowdhury P, Malester B, Rajacic P, Coetzee WA (2007) The regulation of ion channels and transporters by glycolytically derived ATP. Cell Mol Life Sci 64:3069–3083
Duhamel TA, Green HJ, Perco JG, Ouyang J (2006a) Comparative effects of a low-carbohydrate diet and exercise plus a low-carbohydrate diet on muscle sarcoplasmic reticulum responses in males. Am J Physiol Cell Physiol 291:C607–C617
Duhamel TA, Green HJ, Perco JG, Ouyang J (2006b) Effects of prior exercise and a low-carbohydrate diet on muscle sarcoplasmic reticulum function during cycling in women. J Appl Physiol 101:695–706
Duhamel TA, Perco JG, Green HJ (2006c) Manipulation of dietary carbohydrates after prolonged effort modifies muscle sarcoplasmic reticulum responses in exercising males. Am J Physiol Regul Integr Comp Physiol 291:R1100–R1110
Dulhunty AF (1984) Heterogeneity of T-tubule geometry in vertebrate skeletal muscle fibres. J Muscle Res Cell Motil 5:333–347
Dutka TL, Lamb GD (2007a) Na+-K+ pumps in the transverse tubular system of skeletal muscle fibers preferentially use ATP from glycolysis. Am J Physiol-Cell Physiol 293:C967–C977
Dutka TL, Lamb GD (2007b) Transverse tubular system depolarization reduces tetanic force in rat skeletal muscle fibers by impairing action potential repriming. Am J Physiol Cell Physiol 292:C2112–C2121
Dyck DJ, Putman CT, Heigenhauser GJ, Hultman E, Spriet LL (1993) Regulation of fat-carbohydrate interaction in skeletal muscle during intense aerobic cycling. Am J Phys 265(6 Pt 1):E852–E859
Dyck DJ, Peters SJ, Wendling PS, Chesley A, Hultman E, Spriet LL (1996) Regulation of muscle glycogen phosphorylase activity during intense aerobic cycling with elevated FFA. Am J Phys 270(1 Pt 1):E116–E125
Edwards HT, Margaria R, Dill DB (1934) Metabolic rate, blood sugar, and the utilization of carbohydrate. Am J Phys 58:203–209
Entman ML, Keslensky SS, Chu A, Van Winkle B (1980) The sarcoplasmic reticulum-glycogenolytic complex in mammalian fast twitch skeletal muscle. J Biol Chem 255:6245–6252
Essén B, Henriksson J (1974) Glycogen content of individual muscle fibres in man. Acta Physiol Scand 90:645–647
Essén-Gustavsson B, Tesch PA (1990) Glycogen and triglyceride utilization in relation to muscle metabolic characteristics in men performing heavy-resistance exercise. Eur J Appl Physiol 61:5–10
Febbraio MA, Snow RJ, Hargreaves M et al (1994) Muscle metabolism during exercise and heat stress in trained men: effect of acclimation. J Appl Physiol 76:589–597
Fell JM, Hearris MA, Ellis DG, Moran J, Jevons EFP, Owens DJ, Strauss JA, Cocks MC, Louis JB, Shepherd SO, Morton JP (2021) Carbohydrate intake improves exercise capacity but does not affect subcellular lipid droplet morphology, AMPK and p53 signalling in human skeletal muscle. J Physiol 599:2823–2849
Fill M, Copello JA (2002) Ryanodine receptor calcium release channels. Physiol Rev 82(4):893–922
Fink WJ, Costill DL, Van Handel PJ (1975) Leg muscle metabolism during exercise in the heat and cold. Eur J Appl Physiol 34:183–190
Frentzel J, Reach F (1901) Untersuchungen zur Frage nach der Quelle der Muskelkraft. Pflugers Arch 83:477–508
Fridén J, Seger J, Ekblom B (1989) Topographical localization of muscle glycogen: an ultrahistochemical study in the human vastus lateralis. Acta Physiol Scand 135:381–391
Fridén J, Seger J, Ekblom B (1985) Implementation of periodic acid-thiosemicarbazide-silver proteinate staining for ultrastructural assessment of muscle glycogen utilization during exercise. Cell Tissue Res 242:229–232
Gaitanos GC, Williams C, Boobis LH, Brooks S (1993) Human muscle metabolism during intermittent maximal exercise. J Appl Physiol 75(2):712–719
Galbo H, Holst JJ, Christensen NJ (1979) The effect of different diets and of insulin on the hormonal response to prolonged exercise. Acta Physiol Scand 107:19–32
Galloway SDR, Maughan RJ (1997) Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med Sci Sports Exerc 29:1240–1249
Gejl K, Hvid LG, Ulrik Frandsen U, Jensen K, Sahlin K, Ørtenblad N (2014) Muscle glycogen content modifies SR Ca2+ release rate in elite endurance athletes. Med Sci Sports Ex 46(3):496–505
Gejl KD, Ørtenbad N, Andersson E, Plomgaard P, Holmberg HC, Nielsen J (2017a) Local depletion of glycogen with supra-maximal exercise in human skeletal muscle fibres. J Physiol 595:2809–2821
Gejl KD, Thams L, Hansen M, Rokkedal-Lausch T, Plomgaard P, Nybo L, Larsen FJ, Cardinale DA, Jensen K, Holmberg HC, Vissing K, Ørtenblad N (2017b) No superior adaptations to carbohydrate periodization in elite endurance athletes. Med Sci Sports Exerc 49(12):2486–2497
Gejl KD, Ørtenblad N, Andersson E, Plomgaard P, Holmberg HC, Nielsen J (2017c) Local depletion of glycogen with supra-maximal exercise in human skeletal muscle fibres. J Physiol 595(9):2809–2821
Gejl KD, Vissing K, Hansen M, Thams L, Rokkedal-Lausch T, Plomgaard P, Meinild Lundby AK, Nybo L, Jensen K, Holmberg HC, Ørtenblad N (2018) Changes in metabolism but not myocellular signaling by training with CHO-restriction in endurance athletes. Physiol Rep 6(17):e13847
Goldstein MA, Murphy DL, Van Winkle WB, Entman ML (1985) Cytochemical studies of a glycogen-sarcoplasmic reticulum complex. J Muscle Res Cell Motility 6:177–187
Gollnick PD, Piehl K, Saubert CW, Armstrong RB, Saltin B (1972) Diet, exercise, and glycogen changes in human muscle fibers. J Appl Physiol 33(4):421–425
Gollnick PD, Piehl K, Saltin B (1974) Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J Physiol 241:45–57
Goodman C, Blazev R, Stephenson G (2005 Sep) Glycogen content and contractile responsiveness to T-system depolarization in skinned muscle fibres of the rat. Clin Exp Pharmacol Physiol 32(9):749–756
Graham TE, Adamo KB, Shearer J, Marchand I, Saltin B (2001) Pro- and macroglycogenolysis: relationship with exercise intensity and duration. J Appl Physiol 90(3):873–879
Green HJ (1991) How important is endogenous muscle glycogen to fatigue in prolonged exercise? Can J Physiol Pharmacol 69(2):290–297
Green HJ, Ball-Burnett M, Jamieson G, Cadefau J, Cussó R (1995) Metabolic adaptations to short-term training are expressed early in submaximal exercise. Can J Physiol Pharmacol 73(4):474–482
Greenhaff PL, Söderlund K, Ren JM, Hultman E (1993) Energy metabolism insingle human muscle fibres during intermittent contraction with occluded circulation. J Physiol 460:443–453
Griffiths A, Shannon OM, Matu J, King R, Deighton K, O'Hara JP (2019) The effects of environmental hypoxia on substrate utilisation during exercise: a meta-analysis. J Int Soc Sports Nutr 16(1):10
Haff GG, Lehmkuhl MJ, McCoy LB, Stone MH (2003) Carbohydrate supplementation and resistance training. Strength Cond Res 17(1):187–196
Haller RG, Clausen T, Vissing J (1998) Reduced levels of skeletal muscle Na+K+ -ATPase in McArdle disease. Neurology 50:37–40
Hammond KM, Sale C, Fraser W, Tang J, Shepherd SO, Strauss JA, Close GL, Cocks M, Louis J, Pugh J, Stewart C, Sharples AP, Morton JP (2019) Post-exercise carbohydrate and energy availability induce independent effects on skeletal muscle cell signalling and bone turnover: implications for training adaptation. J Physiol 597(18):4779–4796
Han JW, Thieleczek R, Varsanyi M, Heilmeyer LM Jr (1992) Compartmentalized ATP synthesis in skeletal muscle triads. Biochemistry 31:377–384
Hansen AK, Fischer CP, Plomgaard P, Andersen JL, Saltin B, Pedersen BK (2005) Skeletal muscle adaptation: training twice every second day vs. training once daily. J Appl Physiol 98(1):93–99
Harber MP, Crane JD, Douglass MD, Weindel KD, Trappe TA, Trappe SW, Fink WF (2010) Resistance exercise reduces muscular substrates in women. Int J Sports Med 29(9):719–725
Hargreaves M, McConell G, Proietto J (1995) Influence of muscle glycogen on glycogenolysis and glucose uptake during exercise in humans. J Appl Physiol 78(1):288–292
Hargreaves M, Angus D, Howlett K et al (1996) Effect of heat stress on glucose kinetics during exercise. J Appl Physiol 81:1594–1597
Hargreaves M, Finn JP, Withers RT, Halbert JA, Scroop GC, Mackay M, Snow RJ, Carey MF (1997) Effect of muscle glycogen availability on maximal exercise performance. Eur J Appl Physiol 75:188–192
Hasin Y, Barry WH (1984) Myocardial metabolic inhibition and membrane potential, contraction, and potassium uptake. Am J Phys 247:H322–H329
Hawley JA (2002) Effect of increased fat availability on metabolism and exercise capacity. Med Sci Sports Exerc 34(9):1485–1491
Hawley JA, Palmer GS, Noakes TD (1997a) Effects of 3 days of carbohydrate supplementation on muscle glycogen content and utilisation during a 1-h cycling performance. Eur J Appl Physiol Occup Physiol 75:407–412
Hawley JA, Schabort EJ, Noakes TD, Dennis SC (1997b) Carbohydrate-loading and exercise performance. Sports Med 24(2):73–81
Hearris MA, Hammond KM, Fell JM, Morton JP (2018) Regulation of muscle glycogen metabolism during exercise: implications for endurance performance and training adaptations. Nutrients 10(3):298
Hearris MA, Hammond KM, Seaborne RA, Stocks B, Shepherd SO, Philp A, Sharples AP, Morton JP, Louis JB (2019) Graded reductions in preexercise muscle glycogen impair exercise capacity but do not augment skeletal muscle cell signaling: implications for CHO periodization. J Appl Physiol 126(6):1587–1597
Hearris MA, Owens DJ, Strauss JA, Shepherd SO, Sharples AP, Morton JP, Louis JB (2020) Graded reductions in pre-exercise glycogen concentration do not augment exercise-induced nuclear AMPK and PGC-1α protein content in human muscle. Exp Physiol 105(11):1882–1894
Helander I, Westerblad H, Katz A (2002 Jun) Effects of glucose on contractile function, [Ca2+]i, and glycogen in isolated mouse skeletal muscle. Am J Physiol Cell Physiol 282(6):C1306–C1312
Helge JW, Damsgaard R, Overgaard K, Andersen JL, Donsmark M, Dyrskog SE, Hermansen K, Saltin B, Daugaard JR (2008) Low-intensity training dissociates metabolic from aerobic fitness. Scand J Med Sci Sports 18:86–94
Hermansen L, Hultman E, Saltin B (1967) Muscle glycogen during prolonged severe exercise. Acta Physiol 71(2–3):129–139
Hill AV (1913) The energy degraded in the recovery processes of stimulated muscles. J Physiol 46(1):28–80
Hingst JR, Bruhn L, Hansen MB, Rosschou MF, Birk JB, Fentz J, Foretz M, Viollet B, Sakamoto K, Færgeman NJ, Havelund JF, Parker BL, James DE, Kiens B, Richter EA, Jensen J, Wojtaszewski JFP (2018) Exercise-induced molecular mechanisms promoting glycogen supercompensation in human skeletal muscle. Molecular Metabolism
Hintz CS, Chi MM-Y, Fell RD, Ivy JL, Kaiser KK, Lowry CV, Lowry OH (1982) Metabolite changes in individual rat muscle fibers during stimulation. Am J Physiol Cell Physiol 242:C218–C228
Hokken R, Laugesen S, Aagaard P, Suetta C, Frandsen U, Ørtenblad N, Nielsen J (2020) Subcellular localization- and fibre type-dependent utilization of muscle glycogen during heavy resistance exercise in elite power and Olympic weightlifters. Acta Physiol (Oxf) e13561
Holloszy JO (1973) Biochemical adaptations to exercise: aerobic metabolism. Exerc Sport Sci Rev 1:45–71
Holloszy JO, Coyle EF (1984) Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol 56(4):831–838
Howlett RA, Parolin ML, Dyck DJ, Jones NL, Heigenhauser GJ, Spriet LL (1998) Regulation of skeletal muscle glycogen phosphorylase and PDH at varying exercise power outputs. Am J Phys 275:R418–R425
Hulston CJ, Venables MC, Mann CH, Martin C, Philp A, Baar K, Jeukendrup AE (2010) Training with low muscle glycogen enhances fat metabolism in well-trained cyclists. Med Sci Sports Exerc 42:2046–2055
Hultman, Greenhaff (1999) Role of submaximal exercise in promoting creatine and glycogen accumulation in human skeletal muscle. J Appl Physiol 87(2):598–604
Impey SG, Hammond KM, Shepherd SO, Sharples AP, Stewart C, Limb M et al (2016) Fuel for the work required: a practical approach to amalgamating train-low paradigms for endurance athletes. Physiol Rep 4:e12803
Impey SG, Hearris MA, Hammond KM, Bartlett JD, Louis J, Close GL, Morton JP (2018) Fuel for the work required: a theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Sports Med 48:1031–1048
Impey SG, Jevons E, Mees G, Cocks M, Strauss J, Chester N, Laurie I, Target D, Hodgson A, Shepherd SO, Morton JP (2020) Glycogen utilization during running: intensity, sex, and muscle-specific responses. Med Sci Sports Exerc 52(9):1966–1975
James JH, Fang CH, Schrantz SJ, Hasselgren PO, Paul RJ, Fischer JE (1996) Linkage of aerobic glycolysis to sodium-potassium transport in rat skeletal muscle. Implications for increased muscle lactate production in sepsis. J Clin Invest 98(10):2388–2397
James JH, Luchette FA, McCarter FD, Fischer JE (1999a) Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet 354(9177):505–508
James JH, Wagner KR, King J-K, Leffler RE, Upputuri RK, Balasubramanian A, Friend LA, Shelly DA, Paul RJ, Fischer JE (1999b) Stimulation of both aerobic glycolysis and Na+-K+-ATPase activity in skeletal muscle by epinephrine or amylin. Am J Physiol Endocrinol Metab 277:E176–E186
Jensen TE, Richter EA (2012) Regulation of glucose and glycogen metabolism during and after exercise. J Physiol 590(5):1069–1076
Jensen R, Nielsen J, Ørtenblad N (2020a) Inhibition of glycogenolysis prolongs action potential repriming period and impairs muscle function in rat skeletal muscle. J Physiol 598(4):789–803
Jensen R, Ørtenblad N, Stausholm MH, Skjærbæk MC, Larsen DN, Hansen M, Holmberg HC, Plomgaard P, Nielsen J (2020b) Heterogeneity in subcellular muscle glycogen utilisation during exercise impacts endurance capacity in men. J Physiol 598:4271–4292
Jensen R, Ørtenblad N, Stausholm MH, Skjærbæk MC, Larsen DN, Hansen M, Holmberg HC, Plomgaard P, Nielsen J (2021) Glycogen supercompensation is due to increased number, not size, of glycogen particles in human skeletal muscle. Exp Physiol 106:1272–1284
Jensen-Urstad M, Ahlborg G (1992) Is the high lactate release during arm exercise due to a low training status? Clin Physiol 12:487–496
Jensen-Urstad M, Ahlborg G, Sahlin K (1993) High lactate and NH3 release during arm vs. leg exercise is not due to beta-adrenoceptor stimulation. J Appl Physiol 74:2860–2867
Jones DP (1986) Intracellular diffusion gradients of O2 and ATP. Am J Phys 250:C663–C675
Kabbara AA, Nguyen LT, Stephenson GM, Allen DG (2000) (2000). Intracellular calcium during fatigue of cane toad skeletal muscle in the absence of glucose. J Muscle Res Cell Motil 21(5):481–489
Karlsson J, Saltin B (1971) Diet, muscle glycogen, and endurance performance. J Appl Physiol 31(2):203–206
Karlsson J, Nordesjō LO, Saltin B (1974) Muscle glycogen utilization during exercise after physical training. Acta Physiol 90:210–217
Katayama K, Goto K, Ishida K, Ogita F (2009) Substrate utilization during exercise and recovery at moderate altitude. Metabolism 59(7):959–966
Kennedy BG, Lunn G, Hoffman JF (1986 Jan) Effects of altering the ATP/ADP ratio on pump-mediated Na/K and Na/Na exchanges in resealed human red blood cell ghosts. J Gen Physiol 87(1):47–72
Klemm D, Heublein B, Fink H-P, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed Engl 44(22):3358–3393
Koh HE, Ørtenblad N, Winding KM, Hellsten Y, Mortensen SP, Nielsen J (2018) High-intensity interval, but not endurance training induces muscle fiber type-specific subsarcolemmal lipid droplet size reduction in type 2 diabetic patients. Am J Physiol Endocrinol Metab 315(5):E872–E884
Koopman R, Manders RJ, Jonkers RA, Hul GB, Kuipers H, van Loon LJ (2006) Intramyocellular lipid and glycogen content are reduced following resistance exercise in untrained healthy males. Eur J Appl Physiol 96:525–534
Korge P, Campbell KB (1995) The importance of ATPase microenvironment in muscle fatigue: a hypothesis. Int J Sports Med 16:172–179
Krogh A, Lindhard J (1920) The relative value of fat and carbohydrate as sources of muscular energy: with appendices on the correlation between standard metabolism and the respiratory quotient during rest and work. Biochem J 14:290–363
Lamb GD, Stephenson DG (1994) Effects of intracellular pH and [Mg2+] on excitation-contraction coupling in skeletal muscle fibres of the rat. J Physiol 478(Pt 2):331–339
Lamb GD, Stephenson DG (2018) Measurement of force and calcium release using mechanically skinned fibers from mammalian skeletal muscle. J Appl Physiol 125(4):1105–1127
Lambert EV, Speechly DP, Dennis SC, Noakes TD (1994) Enhanced endurance in trained cyclists during moderate intensity exercise following 2 weeks adaptation to a high fat diet. Eur J Appl Physiol Occup Physiol 69:287–293
Larsen S, Ara I, Rabol R, Andersen JL, Boushel R, Dela F, Helge JW (2009) Are substrate use during exercise and mitochondrial respiratory capacity decreased in arm and leg muscle in type 2 diabetes? Diabetologia 52:1400–1408
Levy B, Gibot S, Franck P, Cravoisy A, Bollaert PE (2005) Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet 365(9462):871–875
Lucia A, Nogales-Gadea G, Perez M et al (2008) McArdle disease: what do neurologists need to know? Nat Clin Pract Neurol 4(10):568–577
Lundby C, Van Hall G (2002) Substrate utilization in sea level residents during exercise in acute hypoxia and after 4 weeks of acclimatization to 4100 m. Acta Physiol Scand 176(3):195–201
Lundby C, Calbet JAL, van Hall G, Saltin B, Sander M (2004) Pulmonary gas exchange at maximal exercise in Danish lowlanders during 8 wk of acclimatization to 4100 m and in high-altitude Aymara natives. Am J Phys Regul Integr Comp Phys 287(5):R1202–R1208
Macdonald WA, Ørtenblad N, Nielsen OB (2007) Energy conservation attenuates the loss of skeletal muscle excitability during intense contractions American. Journal of Physiology (Endocrin and Metab) 292:E771–E778
MacDougal JD, Ray S, Slae DG, McCartney N, Lee P, Garner S (1999) Muscle substrate utilization and lactate production during weightlifting. Can J Appl Physiol 24(3):209–215
MacLeod KT (1989) Effects of hypoxia and metabolic inhibition on the intracellular sodium activity of mammalian ventricular muscle. J Physiol 416:455–468
Madsen K, Pedersen PK, Rose P, Richter EA (1990) Carbohydrate supercompensation and muscle glycogen utilization during exhaustive running in highly trained athletes. Eur J Appl Physiol Occup Physiol 61:467–472
Marchand I, Chorneyko K, Tarnopolsky M, Hamilton S, Shearer J, Potvin J, Graham TE (2002) Quantification of subcellular glycogen in resting human muscle: granule size, number, and location. J Appl Physiol 93:1598–1607
Marchand I, Tarnopolsky M, Adamo KB, Bourgeois JM, Chorneyko K, Graham TE (2007) Quantitative assessment of human muscle glycogen granules size and number in subcellular locations during recovery from prolonged exercise. J Physiol 580(2):617–628
Margaria R, Cerretelli P, Mangili F (1964) Balance and kinetics of anaerobic energy release during strenuous exercise in man. J Appl Physiol 19:623–628
Marquet LA, Brisswalter J, Louis J, Tiollier E, Burke LM, Hawley JA, Hausswirth C (2016a) Enhanced endurance performance by periodization of carbohydrate intake: “sleep low” strategy. Med Sci Sports Exerc 48:663–672
Marquet LA, Hausswirth C, Molle O, Hawley JA, Burke LM, Tiollier E, Brisswalter J (2016b) Periodization of carbohydrate intake: short- term effect on performance. Nutrients 8:755
Matu J, Gonzalez JT, Ispoglou T, Duckworth L, Deighton K (2018) The effects of hypoxia on hunger perceptions, appetite-related hormone concentrations and energy intake: a systematic review and meta-analysis. Appetite 125:98–108
Maunder E, Bradley HE, Deane CS, Hodgson AB, Jones M, Joanisse S, Turner AM, Breen L, Philp A, Wallis GA (2021) Effects of short term graded dietary carbohydrate intake on intramuscular and whole body metabolism during moderate intensity exercise. J Appl Physiol 131(1):376–387
McArdle B (1951) Myopathy due to a defect in muscle glycogen breakdown. Clin Sci 10:20
McBride A, Hardie DG (2009) AMP-activated protein kinase–a sensor of glycogen as well as AMP and ATP? Acta Physiol 196:99–113
McBride A, Ghilagaber S, Nikolaev A, Hardie DG (2009) The glycogen-binding domain on the AMPK β subunit allows the kinase to act as a glycogen sensor. Cell Metab 9:23–34
McClelland GB, Hochachka PW, Weber JM (1998) Carbohydrate utilization during exercise after high-altitude acclimation: a new perspective. Proc Natl Acad Sci 95:10288–10293
McGilvery RW (1975) The use of fuels for muscular work. In: Howald H et al (eds) Metabolic adaptation to prolonged physical exercise. Springer, Basel
McInerney P, Lessard SJ, Burke LM, Coffey VG, Lo Giudice SL, Southgate RJ, Hawley JA (2005) Failure to repeatedly supercompensate muscle glycogen stores in highly trained men. Med Sci Sports Exerc 37(3):404–411
Medbø JI (1993) Glycogen breakdown and lactate accumulation during high-intensity cycling. Acta Physiol Scand 149(1):85–89
Medbø JI, Jebens E, Noddeland H, Hanem S, Toska K (2006) Lactate elimination and glycogen resynthesis after intense bicycling. Scand J Clin Lab Invest 66(3):211–226
Melendez-Hevia E, Wadell TG, Shelton ED (1993) Optimization of molecular design in the evolution of metabolism: the glycogen molecule. Biochem J 295:477–483
Melzer W, Herrmann-Frank A, Lüttgau HC (1995) The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta 1241(1):59–116
Mercer RW, Dunham PB (1981 Nov) Membrane-bound ATP fuels the Na/K pump. Studies on membrane-bound glycolytic enzymes on inside-out vesicles from human red cell membranes. J Gen Physiol 78(5):547–568
Mitchell JB, Costill DL, Houmard JA, Fink WJ, Pascoe DD, Pearson DR (1989) Influence of carbohydrate dosage on exercise performance and glycogen metabolism. J Appl Physiol 67(5):1843–1849
Mitchell JB, DiLauro PC, Pizza FX, Cavender DL (1997) The effect of preexercise carbohydrate status on resistance exercise performance. Int J Sport Nutr 7(3):185–196
Morton JP, Croft L, Bartlett JD, MacLaren DPM, Reilly T, Evans L et al (2009) Reduced carbohydrate availability does not modulate training-induced heat shock protein adaptations but does upregulate oxidative enzyme activity in human skeletal muscle. J Appl Physiol 106:1513–1521
Morton RW, Sonne MW, Zuniga AF, Mohammad IYZ, Jones A, McGlory C, Keir PJ, Potvin JR, Philips SM (2019) Muscle fibre activation is unaffected by load and repetition duration when resistance exercise is performed to task failure. J Physiol 597:4601–4613
Nakamura-Tsuruta S, Yasuda M, Nakamura T, Shinoda E, Furuyashiki T, Kakutani R, Takata H, Kato Y, Ashida H (2012) Comparative analysis of carbohydrate-binding specificities of two anti-glycogen monoclonal antibodies using ELISA and surface plasmon resonance. Carbohydr Res 350:49–54
Nielsen J, Ørtenblad N (2013) Physiological aspects of the subcellular localization of glycogen in skeletal muscle (review). Appl Physiol Nutr Metab 38(2):91–99
Nielsen OB, Ørtenblad N, Lamb GD, Stephenson DG (2004) Excitability of the T-tubular system in rat skeletal muscle: roles of K+ and Na+ gradients and Na+-K+ pump activity. J Physiol 557:133–146
Nielsen J, Schrøder HD, Rix CG, Ørtenblad N (2009) Distinct effects of subcellular glycogen localization on tetanic relaxation time and endurance in mechanically skinned rat skeletal muscle fibres. J Physiol 587:3679–3690
Nielsen J, Mogensen M, Vind BF, Sahlin K, Højlund K, Schrøder HD, Ørtenblad N (2010) Increased subsarcolemmal lipids in type 2 diabetes: effect of training on localization of lipids, mitochondria, and glycogen in sedentary human skeletal muscle. Am J Physiol Endocrinol Metab 298:E706–E713
Nielsen J, Holmberg HC, Schrøder HD, Saltin B, Ørtenbald N (2011) Human skeletal muscle glycogen utilization in exhaustive exercise: role of subcellular localization and fibre type. J Physiol 589(11):2871–2885
Nielsen J, Cheng AJ, Ørtenblad N, Westerblad H (2014) Subcellular distribution of glycogen and decreased tetanic Ca2+ in fatigued single intact mouse muscle fibres. J Physiol 592(9):2003–2012
Norman B, Sollevi A, Jansson E (1988) Increased IMP content in glycogen-depleted muscle fibres during submaximal exercise in man. Acta Physiol Scand 133(1):97–100
Odland LM, Heigenhauser GJF, Wong D, Hollidge-Horvat MG, Spriet LL (1998) Effects of increased fat availability on fat-carbohydrate interaction during prolonged exercise in men. Am J Phys Regul Integr Comp Phys 274(4):R894–R902
O'Hara JP, Woods DR, Mellor A, Boos C, Gallagher L, Tsakirides C, Arjomandkhah NC, Holdsworth DA, Cooke CB, Morrison DJ, Preston T, King RF (2017) A comparison of substrate oxidation during prolonged exercise in men at terrestrial altitude and normobaric normoxia following the coingestion of 13C glucose and 13C fructose. Physiol Rep 5(1):e13101
Okamoto K, Wang W, Rounds J, Chambers EA, Jacobs DO (2001) ATP from glycolysis is required for normal sodium homeostasis in resting fast-twitch rodent skeletal muscle. Am J Physiol Endocrinol Metab 281:E479–E488
Olsson K-E, Saltin B (1970) Variation in total body water with muscle glycogen changes in man. Acta Physiol Scand 80:11–18
Ørngreen MC, Jeppesen TD, Taivassalo T, Hauerslev S, Preisler N, Heinicke K, Haller RG, Vissing J, van Hall G (2015) Lactate and energy metabolism during exercise in patients with blocked Glycogenolysis (McArdle disease). J Clin Endocrinol Metab 100(8):E1096–E1104
Ørtenblad N, Stephenson GD (2003) A novel signalling pathway originating in mitochondria modulates muscle membrane excitability. J Physiol 548:139–145
Ørtenblad N, Nielsen J (2015) Muscle glycogen and cell function–location, location, location. Scand J Med Sci Sports Suppl 4:34–40
Ørtenblad N, Macdonald WA, Sahlin K (2009) Glycolysis in contracting rat skeletal muscle is controlled by factors related to energy state. Biochem J 420:161–168
Ørtenblad N, Nielsen J, Saltin B, Holmberg HC (2011) Role of glycogen availability in sarcoplasmic reticulum Ca2+ kinetics in human skeletal muscle. J Physiol 589(3):711–725
Ørtenblad N, Westerblad H, Nielsen J (2013) Muscle glycogen stores and fatigue (review). J Physiol 591(18):4405–4413
Ørtenblad N, Nielsen J, Boushel R, Söderlund K, Saltin B, Holmberg H-C (2018) The muscle fiber profiles, mitochondrial content and enzyme activities of the exceptionally well-trained arm and leg muscles of elite cross-country skiers. Front Physiol 9:1031
Palladin AV (1945) The biochemistry of muscle training. Science 102:576–578
Parolin ML, Chesley A, Matsos MP, Spriet LL, Jones NL, Heigenhauser GJF (1999) Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. Am J Phys 277:E890–E900
Pascoe DD, Costill DL, Fink WJ, Robergs RA, Zachwieja JJ (1993) Glycogen resynthesis in skeletal muscle following resistive exercise. Med Sci Sports Exerc 25(3):349–354
Passonneau JV, Lowry OH (1993) Enzymatic analysis, vol 2. The Humana Press Inc., New Jersey, USA
Pearse AGE (1961) Carbohydrates. In: Histochemistry, theoretical and applied, Boston, Little, Brown and Company, pp 228–280
Pedersen TH, Clausen T, Nielsen OB (2003) Loss of force induced by high extracellular [K+] in rat muscle: effect of temperature, lactic acid and beta2-agonist. J Physiol 551:277–286
Pederson BA, Schroeder JM, Parker GE, Smith MW, DePaoli-Roach AA, Roach PJ (2005) Glucose metabolism in mice lacking muscle glycogen synthase. Diabetes 54:3466–3473
Philipson KD, Nishimoto AY (1983) ATP-dependent Na+ transport in cardiac sarcolemmal vesicles. Biochim Biophys Acta 733:133–141
Pilegaard H, Keller C, Steensberg A, Helge JW, Pedersen BK, Saltin B, Darrell Neufer P (2002) Influence of pre-exercise muscle glycogen content on exercise-induced transcriptional regulation of metabolic genes. J Physiol 541:261–271
Pilegaard H, Osada T, Andersen LT, Helge JW, Saltin B, Neufer PD (2005) Substrate availability and transcriptional regulation of metabolic genes in human skeletal muscle during recovery from exercise. Metabolism 54:1048–1055
Pitsiladis YP, Maughan RJ (1999) The effects of exercise and diet manipulation on the capacity to perform prolonged exercise in the heat and in the cold in trained humans. J Physiol 517:919–930
Poole DC, Rossiter HB, Brooks GA, Gladden LB (2020) The anaerobic threshold: 50+ years of controversy. J Physiol JP279963
Psilander N, Frank P, Flockhart M, Sahlin K (2013) Exercise with low glycogen increases PGC-1α gene expression in human skeletal muscle. Eur J Appl Physiol 113:951–963
Rauch LH, Rodger I, Wilson GR, Belonje JD, Dennis SC, Noakes TD, Hawley JA (1995) The effects of carbohydrate loading on muscle glycogen content and cycling performance. Int J Sport Nutr 5(1):25–36
Roach PJ, DePaoli-Roach AA, Hurley TD, Tagliabracci VS (2012) Glycogen and its metabolism: some new developments and old themes. Biochem J 441:763–787
Robergs RA, Pearson DR, Costill DD, Fink WJ, Pascoe DD, Benedict MA, Lambert CP, Zachweija JJ (1991) Muscle glycogenolysis during different intensities of weight-resistance exercise. J Appl Physiol 70(4):1700–1706
Roberts AC, Butterfield GE, Cymerman A, Reeves JT, Wolfel EE, Brooks GA (1996a) Acclimatization to 4,300-m altitude decreases reliance on fat as a substrate. J Appl Physiol 81:1762–1771
Roberts AC, Reeves JT, Butterfield GE et al (1996b) Altitude and b-blockade augments glucose utilization during submaximal exercise. J Appl Physiol 80:605–615
Roepstorff C, Halberg N, Hillig T, Saha AK, Ruderman NB, Woj- taszewski JF, Richter EA, Kiens B. (2005) Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am J Physiol Endocrinol Metab 288:E133–E142
Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR (1993) Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Phys 265(3 Pt 1):E380–E391
Rowlands DS, Hopkins WG (2002) Effects of high-fat and high-carbohydrate diets on metabolism and performance in cycling. Metab Clin Exp 51(6):678–690
Rybicka KK (1996) Glycosomes--the organelles of glycogen metabolism. Tissue Cell 28:253–265
Sahlin K, Söderlund K, Tonkonogi M, Hirakoba K (1997) Phosphocreatine content in single fibers of human muscle after sustained submaximal exercise. Am J Phys 273(1 Pt 1):C172–C178
Sahlin K, Tonkonogi M, Söderlund K (1998) Energy supply and muscle fatigue in humans. Acta Physiol Scand 162(3):261–266
Saks V, Beraud N, Wallimann T (2008) Metabolic compartmentation–a system level property of muscle cells. Int J Mol Sci 9:751–767
Saltin B (1973) Metabolic fundamentals in exercise. Med Sci Sports 5(3):137–146
Saltin B, Karlsson J (1971) Muscle glycogen utilization during work of different intensities. In: Pernow B, Saltin B (eds) Muscle metabolism during exercise, vol 11. Springer, US, pp 289–299
Saltin B, Nazar K, Costill DL, Stein E, Jansson E, Essen B, Gollnick PD (1976) The nature of the training response: peripheral and central adaptations of one-legged exercise. Acta Physiol Scand 96:289–305
Samuelson H, Moberg M, Apró W, Ekblom B, Blomstrand E (2016) Intake of branched-chain or essential amino acids attenuates the elevation in muscle levels of PGC-1α4 mRNA caused by resistance exercise. Am J Physiol Endocrinol Metab 311(1):246–251
Santalla A, Nogales-Gadea G, Ørtenblad N, Brull A, de Luna N, Pinós T, Lucia A (2014) McArdle disease: a unique study model in sports medicine. (review). Sports Med 44(11):1531–1544
Sawyer JC, Wood RJ, Davidson PW, Collins SM, Matthews TD, Gregory SM, Paolone VJ (2013) Effects of a short-term carbohydrate restricted diet on strength and power performance. J Strength Cond Res 27:2255–2262
Schrier SL (1966 Jan) Organization of enzymes in human erythrocyte membranes. Am J Phys 210(1):139–145
Sear RP (2019) Diffusionphoresis in cells: a general nonequilibrium, nonmotor mechanism for the metabolism-dependent transport of particles in cells. Phys Rev let 122:128101
Sejersted OM, Sjøgaard G (2000) Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev 80:1411–1481
Sherman WM, Costill DL, Fink WJ, Miller JM (1981) Effect of exercise-diet manipulation on muscle glycogen and its subsequent utilization during performance. Int J Sports Med 2:114–118
Shearer J, Graham T (2004) Novel aspects of skeletal muscle glycogen and its regulation during rest and exercise. Exerc Sport Sci Rev 32:120–126
Shiose K, Yamada Y, Motonaga K, Sagayama H, Higaki Y, Tanaka H, Takahashi H (2016) Segmental extracellular and intracellular water distribution and muscle glycogen after 72-h carbohydrate loading using spectroscopic techniques. J Appl Physiol 121(1):205–211
Sjöström M, Fridén J, Ekblom B (1982a) Fine structural details of human muscle fibres after fibre type specific glycogen depletion. Histochemistry 76:425–438
Sjöström M, Kidman S, Larsén KH, Ängquist KA (1982b) Z- and M-band appearance in different Histochemically defined types of human skeletal muscle fibers. J Histochem Cytochem 30(1):1–11
Skurat AV, Segvich DM, DePaoli-Roach AA, Roach PJ (2017) Novel method for detection of glycogen in cells. Glycobiology 27(5):416–424
Smith JW, Zachwieja JJ, Péronnet F, Passe DH, Massicotte D, Lavoie C, Pascoe DD (2010) Fuel selection and cycling endurance performance with ingestion of [13C]glucose: evidence for a carbohydrate dose response. J Appl Physiol 108(6):1520–1529
Srere PA (1967) Enzyme concentrations in tissues. Science 158(3803):936–937
Steinberg GR, Watt MJ, McGee SL, Chan S, Hargreaves M, Febbraio MA et al (2006) Reduced glycogen availability is associated with increased AMPKα2 activity, nuclear AMPKα2 protein abundance, and GLUT4 mRNA expression in contracting human skeletal muscle. Appl Physiol Nutr Metab 31:302–312
Stellingwerff T (2012) Case study: nutrition and training periodization in three elite marathon runners. Int J Sport Nutr Exer Metab 22(5):392–400
Stellingwerff T, Spriet LL, Watt MJ, Kimber NE, Hargreaves M, Hawley JA, Burke LM (2006) Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. American Journal of Physiology-Endocrinology and Metabolism 290(2):E380–E388
Stephenson DG (1996) Molecular cogs in machina carnis. Clin Exp Pharmacol Physiol 23(10–11):898–907
Stephenson DG, Nguyen LT, Stephenson GM (1999) Glycogen content and excitation-contraction coupling in mechanically skinned muscle fibres of the cane toad. J Physiol 519:177–187
Sun RC, Dukhande VV, Zhou Z, Young LEA, Emanuelle S, Brainson CF, Gentry MS (2019) Nuclear glycogenolysis modulates histone acetylation in human non-small cell lung cancers. Cell Metab 30(5):903–916.e7
Symons JD, Jacobs I (1989) High-intensity exercise performance is not impaired by low intramuscular glycogen. Med Sci Sports Exerc 21(5):550–557
Tammineni ER, Kraeva N, Figueros L, Manno C, Ibarra CA, Klip A, Riazi S, Rios E (2020) Intracellular calcium leak lowers glucose storage in human muscle, promoting hyperglycemia and diabetes. eLife 9:e53999
Tarnopolsky LJ, MacDougall JD, Atkinson SA, Tarnopolsky MA, Sutton JR (1990) Gender differences in substrate for endurance exercise. J Appl Physiol 68:302–308
Taylor R, Price TB, Rothman DL, Shulman RG, Shulman GI (1992) Validation of 13C NMR measurement of human skeletal muscle glycogen by direct biochemical assay of needle biopsy samples. Magn Reson Med 27(1):13–20
Tesch PA, Colliander EB, Kaiser P (1986) Muscle metabolism during intense, heavy resistance exercise. Eur J Appl Physiol 55:362–366
Tesch PA, Ploutz-Snyder LL, Yström L, Castro MJ, Dudley GA (1998) Skeletal muscle glycogen loss evoked by resistance exercise. J Strength Cond Res 12(2):67–73
Testoni G, Duran J, García-Rocha M, Vilaplana F, Serrano AL, Sebastián D, López-Soldado I, Sullivan MA, Slebe F, Vilaseca M, Munoz-Cánoves P, Guinovart JJ (2017) Lack of glycogenin causes glycogen accumulation and muscle function impairment. Cell Metab 26:256–266
Tomcik KA, Camera DM, Bone JL, Ross ML, Jeacocke NA, Tachtsis B, Senden J, LJC VANL, Hawley JA and Burke LM. (2018) Effects of Creatine and carbohydrate loading on cycling time trial performance. Med Sci Sports Exerc 50:141–150
Tsintzas OK, Williams C, Boobis L, Greenhaff P (1995) Carbohydrate ingestion and glycogen utilization in different muscle fibre types in man. J Physiol 489(Pt 1):243–250
Tsintzas OK, Williams C, Boobis L, Greenhaff P (1996) Carbohydrate ingestion and single muscle fiber glycogen metabolism during prolonged running in men. J Appl Physiol 81(2):801–809
Tsintzas K, Williams C, Constantin-Teodosiu D, Hultman E, Boobis L, Clarys P, Greenhaff P (2001) Phosphocreatine degradation in type I and type II muscle fibres during submaximal exercise in man: effect of carbohydrate ingestion. J Physiol 537(1):305–311
Van Hall G, Jensen-Urstad M, Rosdahl H, Holmberg HC, Saltin B, Calbet JA (2003) Leg and arm lactate and substrate kinetics during exercise. Am J Physiol Endocrinol Metab 284(1):E193–E205
van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ (2001) The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol 536(Pt 1):295
Vandenberghe K, Hespel P, Vanden Eynde B, Lyssens R, Richter EA (1995) No effect of glycogen level on glycogen metabolism during high intensity exercise. Med Sci Sports Exerc 27:1278–1283
Vigh-Larsen JF, Ørtenblad N, Spriet LL, Overgaard K, Mohr M (2021) Muscle glycogen metabolism and high-intensity exercise performance–a narrative review. Sports Med 51(9):1855–1874
Virágh S, Szabó E, Challice CE (1982) Glycogen-containing lysosomes and glycogen loss in the cardiomyocytes of embryonic and neonatal mice. Adv Myocardiol 3:553–561
Visuttijai K, Hedberg-Oldfors C, Thomsen C, Glamuzina E, Kornblum C, Tasca G, Hernandez-Lain A, Sandstedt J, Dellgren G, Roach P, Oldfors A (2020) Glycogenin is deispensable for glycogen synthesis in human muscle, and glycogen deficiency causes polyglucosan storage. J Clin Endocrinol Metab 105:557–566
Vukovich MD, Costill DL, Hickey MS, Trappe SW, Cole KJ, Fink WJ (1993) Effect of fat emulsion infusion and fat feeding on muscle glycogen utilization during cycle exercise. J Appl Physiol 75(4):1513–1518
Walker JL, Heigenhauser GJ, Hultman E, Spriet LL (2000) Dietary carbohydrate, muscle glycogen content, and endurance performance in well-trained women. J Appl Physiol (Bethesda, Md: 1985) 88:2151–2158
Wallimann T, Eppenberger HM (1985) Localization and function of M-line-bound creatine kinase. J Muscle Res Cell Motility 6:239–285
Wanson JC, Drochmans P (1968) Rabbit skeletal muscle glycogen. A morphological and biochemical study of glycogen betaparticles isolated by the precipitation-centrifugation method. J Cell Biol 38:130–150
Wanson JC, Drochmans P (1972) Role of the sarcoplasmic reticulum in glycogen metabolism. J Cell Biol 54:206–224
Watanabe D, Wada M (2019) Effects of reduced muscle glycogen on excitation–contraction coupling in rat fast-twitch muscle: a glycogen removal study. J Muscle Res Cell Motil
Widrick JJ, Costill DL, Fink WJ, Hickey MS, McConell GK, Tanaka H (1993) Carbohydrate feedings and exercise performance: effect of initial muscle glycogen concentration. J Appl Physiol (Bethesda, Md: 1985) 74:2998–3005
Wojtaszewski JFP, MacDonald C, Nielsen JN, Hellsten Y, Hardie DG, Kemp BE et al (2003) Regulation of 5′AMP- activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab 284:E813–E822
Xirouchaki CE, Mangiafico SP, Bate K, Ruan Z, Huang AM, Tedjosiswoyo BW, Lamont B, Pong W, Favaloro J, Blair AR, Zajac JD, Proietto J, Andrikopoulos S (2016) Impaired glucose metabolism and exercise capacity with muscle-specific glycogen synthase 1 (gys1) deletion in adult mice. Mol Metab 5:221–232
Xu KY, Becker LC (1998) Ultrastructural localization of glycolytic enzymes on sarcoplasmic reticulum vesticles. J Histochem Cytochem 46:419–427
Yaspelkis BB, Patterson JG, Anderla PA, Ding Z, Ivy JL (1993) Carbohydrate supplementation spares muscle glycogen during variable-intensity exercise. J Appl Physiol 75(4):1477–1485
Yeo WK, Paton CD, Garnham AP, Burke LM, Carey AL, Hawley JA (2008) Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens. J Appl Physiol 105:1462–1470
Yeo WK, McGee SL, Carey AL, Paton CD, Garnham AP, Hargreaves M, Hawley JA (2010) Acute signalling responses to intense endurance training commenced with low or normal muscle glycogen. Exp Physiol 95:351–358
Young AJ, Young PM, McCullough RE, Moore LG, Cymerman A, Reeves JT (1991) Effect of betaadrenergic blockade on plasma lactate concentration during exercise at high altitude. Eur J Appl Physiol Occup Physiol 63:315–322
Zuntz N (1896) Über die Rolle des Zuckers im tierischen Stoffwechsel. Arch Physiol:538–542
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ørtenblad, N., Nielsen, J., Morton, J.P., Areta, J.L. (2022). Exercise and Muscle Glycogen Metabolism. In: McConell, G. (eds) Exercise Metabolism. Physiology in Health and Disease. Springer, Cham. https://doi.org/10.1007/978-3-030-94305-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-94305-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94304-2
Online ISBN: 978-3-030-94305-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)