Abstract
Muscle glycogen is the main substrate during high-intensity exercise and large reductions can occur after relatively short durations. Moreover, muscle glycogen is stored heterogeneously and similarly displays a heterogeneous and fiber-type specific depletion pattern with utilization in both fast- and slow-twitch fibers during high-intensity exercise, with a higher degradation rate in the former. Thus, depletion of individual fast- and slow-twitch fibers has been demonstrated despite muscle glycogen at the whole-muscle level only being moderately lowered. In addition, muscle glycogen is stored in specific subcellular compartments, which have been demonstrated to be important for muscle function and should be considered as well as global muscle glycogen availability. In the present review, we discuss the importance of glycogen metabolism for single and intermittent bouts of high-intensity exercise and outline possible underlying mechanisms for a relationship between muscle glycogen and fatigue during these types of exercise. Traditionally this relationship has been attributed to a decreased ATP resynthesis rate due to inadequate substrate availability at the whole-muscle level, but emerging evidence points to a direct coupling between muscle glycogen and steps in the excitation–contraction coupling including altered muscle excitability and calcium kinetics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Muscle glycogen metabolism is notably elevated during high-intensity exercise leading to substantial declines after short duration. |
In addition to declines at the whole-muscle level, heterogeneity in cellular and subcellular depletion should be considered and may be a key aspect in a link between muscle glycogen and performance. |
Altered muscle glycogen content results in impaired single and repeated high-intensity exercise tolerance, only if glycogen is reduced below a certain critical level and no consistent effects of muscle glycogen supercompensation are apparent. |
The mechanisms for a coupling between muscle glycogen and performance may be mediated through key steps in the excitation–contraction coupling such as impaired muscle excitability and calcium regulation. |
1 Introduction
Since early studies by Christensen and Hansen [1] demonstrated new insights into the role of fat and carbohydrate metabolism during exercise, a strong relationship between muscle glycogen and performance during prolonged exercise has been well established [2,3,4,5,6,7]. In contrast, the effect of glycogen content on high-intensity (defined here as ≥ 100% VO2max) exercise performance is more ambiguous. During high-intensity exercise, glycogenolysis is the main source for adenosine triphosphate (ATP) resynthesis coupled with creatine phosphate (PCr) breakdown, especially during brief bursts of high-intensity exercise and with an expanded contribution from oxidative phosphorylation as exercise duration increases or when bouts are repeated [8,9,10,11]. This results in substantial PCr depletion and marked perturbations in the intracellular environment including H+ accumulation, increased formation of byproducts of ATP hydrolysis, altered ion homeostasis, increased levels of reactive oxidative species etc. which may all contribute significantly to fatigue development [12,13,14,15]. The relative importance and interactions between these fatiguing mechanisms are still under debate and have been covered in other papers [14,15,16,17,18]. The focus of the present review is to address the role of muscle glycogen metabolism in performance during high-intensity exercise, for example when high-intensity bouts are performed towards the end of a team sport match rather than in a non-fatigued state. In this regard, it is known that ~ 50% of the ATP turnover is fueled by glycogenolysis during a 6-s sprint [11], resulting in a notably high muscle glycogen metabolism. Accordingly, after high-intensity team sport exercise, such as ice hockey, muscle glycogen is reduced by more than 50% concomitant with symptoms of fatigue [19]. Furthermore, at this point, a large proportion of individual fast- and slow-twitch fibers are depleted of glycogen [19], which indicates that fiber-specific utilization of muscle glycogen and possibly the spatial distribution in different subcellular compartments may be key components in a potential link between fatigue development and muscle glycogen depletion [20, 21]. Accordingly, muscle glycogen localized in specific areas within muscle fibers have been suggested to retain distinct regulatory roles and depletion patterns [21,22,23,24,25,26]. This may be particularly important during high-intensity exercise where fatigue development typically manifests in advance of severe muscle glycogen depletion at the whole-muscle level and may be a central factor in previous observations of impaired muscle function instigated by only partially lowered muscle glycogen levels [27,28,29]. Nonetheless, results examining the impact of glycogen content on high-intensity exercise performance are divergent. For example, Hargreaves et al. [30] failed to establish any relationship between glycogen levels and ~ 75 s all-out cycling, whereas Balsom et al. [31] demonstrated that lowered initial glycogen content attenuated exercise tolerance when 6-s sprints interspersed by 30-s recovery intervals were repeated fifteen times.
Moreover, mechanisms linking muscle glycogen content to exercise tolerance are not fully elucidated. Historically, the detrimental effect of lowered muscle glycogen has been attributed to energy deficiency in terms of inadequate glycogen available for breakdown and ATP regeneration at the required rate for maintenance of muscle function at the whole-muscle level [14, 32]. However, this concept has been challenged, and evolving evidence points to a direct link between muscle glycogen content and steps in the excitation–contraction (E–C) coupling including factors related to muscle calcium (Ca2+) regulation and membrane excitability [22, 33,34,35,36]. Thus, effects of lowered muscle glycogen have been observed even after recovery periods where muscle ATP concentration would be normal and under experimental circumstances in vitro where global ATP and PCr levels can be maintained at near resting levels suggesting either a non-metabolic role of muscle glycogen or a localization–specific metabolic decline [27, 28, 34, 37,38,39].
Therefore, the objective of the present review is to examine the evidence linking muscle glycogen content and utilization to performance during high-intensity exercise and delineate possible underlying mechanisms.
2 Muscle Glycogen Storage and Utilization Patterns During High-Intensity Exercise
Glycogen is a readily mobilized storage form of carbohydrates in most cells with the majority stored in skeletal muscle (~ 400 g) and a smaller amount located in hepatocytes (~ 100 g) [40, 41]. Resting levels are 400–600 mmol·kg−1 dw depending on training status and with super compensated levels as high as 450–850 mmol·kg−1 dw [42]. On the other hand values of 200–300 mmol·kg−1 dw have been measured under low carbohydrate dietary conditions [42]. During exercise, these levels can be markedly reduced, but some glycogen (~ 10%) always remains in the muscle even following severe exercise [41]. Metabolism of muscle glycogen accelerates with exercise intensity and above ~ 75% VO2max, carbohydrate is the main substrate, due to a higher power of aerobic ATP generation by carbohydrate combustion [12, 43, 44]. At intensities near and above ~ 100% VO2max the anaerobic turnover of muscle glycogen increases substantially, resulting in a high ATP turnover, but at a high cost in terms of energy efficiency (~ 10 times less ATP per glucose molecule) and resulting in changes in the intracellular milieu [9, 12, 45, 46]. Hence, during a 6-s sprint with a peak intensity of ~ 300% VO2max, approximately 50% of the ATP production is provided by a rapid increase in anaerobic glycolysis accompanied by PCr hydrolysis and only a minor part covered by oxidative phosphorylation (< 10%) (see Fig. 1) [9, 11]. Likewise, during a 30-s all-out exercise scenario ~ 65 to 75% of the energy provision is fueled by anaerobic glycolysis [46, 47] resulting in a notably high muscle glycogen turnover. Thus, ~ 15% reductions have been demonstrated following single 6-s sprints and ~ 20 to 30% reductions after 30-s all-out exercise [9, 11, 46, 48]. Accordingly, high initial glycogenolytic rates of ~ 4.5 mmol glucosyl units·kg−1 dw·s−1 have been estimated and even higher glycogen breakdown rates of up to ~ 7 mmol glucosyl units·kg−1 dw·s−1 transiently during very intense exercise (resulting in glucose 6-phosphate accumulation) (see Fig. 2) [9, 11, 49]. However, already during 30-s maximal exercise, the rate of glycogenolysis/glycolysis is considerably lowered after the initial ~ 15-s concomitant with impaired power output and increased contribution from aerobic energy turnover [9, 11, 46]. Thus, with repeated bouts of high-intensity exercise, the rate of carbohydrate utilization is reduced even at the onset of exercise [46]. This has been proposed to relate to muscle acidosis, the increased reliance on aerobic energy metabolism, and/or a result of the decrease in work capacity and therefore energy requirements caused by fatigue development [9, 11, 49, 50]. Nonetheless, the turnover of muscle glycogen is elevated during high-intensity exercise leading to pronounced declines after short-duration exercise.
ATP turnover rates and muscle metabolism during a first (a) and third (b) bout of high-intensity isokinetic cycling separated by 4-min of rest. Adapted with permission from Parolin et al. [11]
3 Muscle Glycogen Utilization in Individual Fibers
Muscle glycogen is stored heterogeneously and likewise displays a heterogeneous and fiber-type specific depletion pattern depending on exercise mode, duration, and intensity [20, 25, 29, 32, 51,52,53,54,55]. Thus, during high-intensity exercise essentially all fibers are activated [56] resulting in simultaneous depletion of both slow and fast-twitch fibers with a higher breakdown rate in fast-twitch fibers [12, 19, 20, 48, 55]. For example, declines of 126 and 77 mmol glucosyl units·kg−1 dw in fast- and slow-twitch muscle, respectively, have been reported after 30-s sprinting [48]. However, while a significant amount of glycogen may remain in some individual fibers at cessation of exhaustive high-intensity exercise, a substantial number of fibers can be depleted, even if glycogen at the whole-muscle level remains only partially lowered. For instance, after intermittent team sport exercises such as soccer [57] and ice hockey [19], which are characterized by frequent bouts of high-intensity exercise, more than 50% of slow and fast-twitch fibers in the knee-extensor muscles were depleted or nearly depleted of glycogen (see Fig. 3). Moreover, Gollnick et al. [20] demonstrated that ~ 40% of fast-twitch fibers were low in glycogen after only six 1-min high-intensity exercise bouts. This depletion pattern of individual fibers is comparable to findings obtained following prolonged exercise where large numbers of individual fibers of both fiber-types are depleted of glycogen [7, 29, 52, 53, 55, 58, 59]. On that note, fiber-type differences in glycogen utilization have been linked to distinct molecular signaling responses and could be important for exercise type-specific adaptations such as in insulin sensitivity and mitochondrial morphology in fast and slow-twitch fibers [60]. Moreover, depletion of individual muscle fibers may locally attenuate the ability of these specific fibers to maintain adequate energy production and/or impair E-C coupling processes and subsequent force production to support high-intensity performance as will be discussed in detail in coming sections. Accordingly, higher post-exercise inosine monophosphate (IMP) concentrations have been observed in glycogen-depleted fibers, suggestive of metabolic alterations due to local substrate depletion [61]. In support, large heterogeneity in single fiber PCr and ATP concentrations has been found after exercise pointing to the importance of local energy metabolism in addition to considerations of energy availability at the whole-muscle level [56, 62, 63]. This heterogeneity may indeed be a major component of a potential link between muscle glycogen content and performance during high-intensity exercise in advance of whole-muscle glycogen depletion.
Relative muscle glycogen utilization of individual fibers before and after high-intensity exercise (ice hockey). Adapted with permission from Vigh-Larsen et al. [19]
4 Muscle Glycogen Storage in Distinct Spatial Compartments
In addition to fiber-specific storage and depletion patterns, glycogen is deposited in specific compartments within muscle fibers, possibly exerting specialized regulatory and metabolic functions [21, 23, 24, 26, 64,65,66]. By two-dimensional electron microscopy images (see Fig. 4), three subcellular locations have been defined; (a) intermyofibrillar glycogen located between myofibrils in close contact with the longitudinal part of the sarcoplasmic reticulum (SR) and mitochondria, (b) intramyofibrillar glycogen located between the contractile filaments close to triadic junctions within the transverse tubular system (T-system) and (c) subsarcolemmal glycogen stored below the cell surface membrane (for reviews see Nielsen et al. [21] and Ørtenblad et al. [64]). Specifically, intramyofibrillar glycogen has repeatedly been demonstrated both in rodents and humans to be closely associated with exercise tolerance and muscle function [28, 39, 67, 68]. Most recently, Jensen et al. [68] demonstrated an association between this particular pool and endurance capacity in moderately trained subjects, which has also been demonstrated in highly trained skiers [28]. Also, more pronounced relative utilization of intramyofibrillar glycogen has been demonstrated after high-intensity [69] as well as prolonged exercise [70, 71]. Moreover, near-depleted levels of specifically intramyofibrillar glycogen have been observed after resistance exercise in fast-twitch fibers [72]. This may be facilitated by restricted diffusion capacity in the narrow triadic junctions, formed by the terminal cisternae of the SR, which lies along the T-system, clearly separated from mitochondria and intermyofibrillar glycogen, but with a high local energy turnover during repetitive contractions. Hence, the compartmentalized muscle cell with a high and fluctuating energy metabolism may necessitate rapidly available local energy provision, whereas utilization of distinct subcellular glycogen pools presumably reflects the activation of different ATPases dependent on contractile/exercise task [21]. However, little is known about the precise role of the glycogen pools in providing energy for specific ATPases and/or regulation of E-C coupling, though this can be speculated upon. Thus, intramyofibrillar glycogen located in the adjacent proximity to triadic junctions may be essential for energy supply for maintenance of muscle function in these specific areas. Moreover, both myofibrillar fractions (intra and intermyofibrillar glycogen) likely drive myosin-action crossbridge cycling [24], while intermyofibrillar glycogen additionally has been associated with SR Ca2+ uptake [39]. On the other hand, the subcarcolemmal fractions are supposedly involved in energy provision for Na+-K+-ATPases along the sarcolemma [25]. This localized compartmentalization of energy turnover is in line with findings reporting impaired muscle function in conditions of enzymatical removal of muscle glycogen or inhibition of glycogen phosphorylase, despite maintained globally cellular ATP and PCr concentrations [34, 35, 73, 74]. Together, the existence of different subcellular glycogen pools with distinct utilization, suggests that a link between muscle glycogen content and muscle function may be related to direct effects of localization-specific glycogen on muscle E–C coupling and contractile function.
Representative image of the spatial distribution of muscle glycogen from electron microscopy in musculus triceps brachii before (left) and after (right) ~ 4 min of high-intensity skiing. Black dots = glycogen particles; SS = subsarcolemmal; Intra = intramyofibrillar, IMF = intermyofibrillar. Adapted with permission from Gejl et al. [69]
5 Muscle Glycogen Content and High-Intensity Exercise Performance
The relationship between muscle glycogen content and high-intensity exercise performance has been investigated in numerous studies originally in the 1980s [75,76,77,78,79,80,81,82,83,84] and 1990s [29,30,31, 85,86,87,88,89,90,91,92], while only a few additional studies since then have provided additional insight [93,94,95,96,97,98,99]. The performance measures in these studies can be subdivided into (I) continuous high-intensity exercise (> 60 s duration), (II) single or repeated sprints (< 60 s duration) and (III) neuromuscular contractile performance (voluntary or electrically induced maximal or near-maximal contractions). Results of these studies are summarized in Tables 1, 2 and 3. It should be noted, however, that a major limitation in several of these studies is the lack of muscle glycogen measurements. Instead, classical exercise and dietary strategies have been commonly utilized and successful glycogen manipulations assumed on the basis of previous work. Therefore, the ability to draw conclusions on the relationship between muscle glycogen levels and performance is confounded by indirect assumptions of glycogen depletion based only on exercise and dietary manipulations.
5.1 Continuous High-Intensity Exercise
For continuous high-intensity exercise, we identified three studies measuring muscle glycogen concentrations [29, 30, 86]. In one study, applying the one-legged knee-extensor model, no initial relationship was determined between high-intensity exercise lasting ~ 3 min and muscle glycogen content which was 372 and 756 mmol·kg−1 dw in each leg following prior exercise and diet manipulations [29]. However, when a second bout was performed after a recovery period, with pre-exercise muscle glycogen concentrations lowered to 310 and 698 mmol·kg−1 dw, performance was impaired only in the low glycogen condition where post-exercise glycogen levels reached 125 mmol·kg−1 dw. This led the authors to conclude that above-normal muscle glycogen did not facilitate high-intensity exercise performance, whereas depletion below a certain threshold provoked impaired exercise tolerance. However, the glycogen manipulation was achieved through exhaustive exercise performed three- compared to one day prior to testing in the high and low glycogen legs, respectively, inducing a possible influence of persisting fatigue. Vandenberghe et al. [86] assessed the effect of elevated muscle glycogen stores (364 vs. 568 mmol·kg−1 dw pre-exercise) on muscle metabolism in an initial condition and repeated the same diet and exercise intervention in a second condition, expecting alterations of a similar magnitude, but observed no performance-enhancing effect in time-to-exhaustion at 125% VO2max. Similarly, Hargreaves et al. [30] observed no difference in 75-s exercise performance when muscle glycogen was manipulated (462 vs. 668 mmol·kg−1 dw pre-exercise), reaching 359 mmol·kg−1 dw post-exercise in the low glycogen condition. Thus, it seems that at least when muscle glycogen is only moderately altered, no effect is evident for continuous high-intensity performance. However, five other studies assessed the influence of glycogen-depleting exercise followed by diet manipulations without muscle biopsy sampling [76,77,78,79, 95]. In all these, time-to-exhaustion at 100–115% VO2max was substantially decreased (15–31%) in the low-carbohydrate trials. Moreover, the effect of enhanced carbohydrate intake with the purpose of elevating muscle glycogen content above resting levels was indirectly evaluated by the incorporation of a supercompensation condition [76,77,78,79, 89] and in only two investigations was a performance-enhancing effect observed [79, 89]. However, neither study was placebo-controlled and the order of exercise was not randomized by Maughan et al. [79]. Collectively, muscle glycogen seems to be important for continuous high-intensity exercise tolerance only if a certain degree of depletion is achieved, whereas loading the stores above normal levels imposes no consistent additional benefit. However, more research with measurements of muscle glycogen content is warranted.
5.2 Single and Repeated Sprint Ability
In three out of four studies with a measured lowering of muscle glycogen, performance declined in single or repeated sprint activities [31, 85, 93, 97]. For example, Balsom et al. [31] utilized 15 × 6-s sprints interspersed by 30-s of recovery and observed a 5% performance impairment in the last four exercise bouts in the low glycogen trial (180 vs. 397 mmol·kg−1 dw pre-exercise), reaching post-exercise glycogen values of 127 mmol·kg−1 dw, which is similar to findings by Gejl et al. [97]. Further, Rockwell et al. [93] performed repeated 60-s all-out efforts with 3-min recovery until a 30% decrease in average power and observed a nearly 40% shorter time-to-exhaustion when muscle glycogen was lowered (222 mmol·kg−1 dw pre-exercise and 118 mmol·kg−1 dw post-exercise). In contrast, Hargreaves et al. [85] observed no influence of altered muscle glycogen content on 30-s maximal cycling, but muscle glycogen content was only reduced to 350 mmol·kg−1 dw pre-exercise in the low condition. In line with this, Gejl et al. [69], observed no change in performance during 4 × 4-min supramaximal ergometer skiing time-trials with 45 min of recovery, with muscle glycogen levels of 575 and 383 mmol·kg−1 dw before the first- and the last bout, respectively.
In three additional studies, without muscle glycogen measurements, sprint performance deteriorated in low carbohydrate conditions [87, 88, 91], whereas one study found no change in performance, possibly explained by a low sample size [81]. Moreover, high-carbohydrate conditions were included in three investigations to elevate muscle glycogen stores, but no performance-improvement was observed in line with Hargreaves et al. [85]. Collectively, it appears that substantial reductions in muscle glycogen content is linked with impaired performance during both single and repeated sprint activities, whereas carbohydrate-loading seems to add no additional benefit, but the existing literature with actual muscle glycogen measurements is limited.
5.3 Assessments of Neuromuscular Function
No effects of lowered or elevated muscle glycogen content were observed in all but one investigation examining the relationship between glycogen content and neuromuscular function [75, 80, 83, 96, 99]. For example, Jacobs et al. [80] detected no differences in a muscle fatigue test consisting of repeated maximal isokinetic knee-extensor contractions when pre-exercise muscle glycogen was altered (205 vs. 412 vs. 812 mmol·kg−1 dw). Similarly, Symons et al. [75] performed quadriceps surface electrical stimulation at 50 Hz for 2 s, maximal voluntary isometric contractions, and a muscle fatigue test and no distinction in performance was observed despite marked differences in pre-exercise muscle glycogen storage (153 vs. 426 mmol·kg−1 dw). An equivalent pattern was reported by Skein et al. [96] also utilizing neuromuscular and muscle fatigue testing with repeated maximal contractions. In addition, Cheng and coworkers [99] observed no improved recovery of maximal voluntary- or electrically stimulated low- or high-frequency contractions by carbohydrate ingestion following exercise. Only one study reported altered neuromuscular performance in a purportedly lowered and super compensated muscle glycogen state, but without muscle biopsy sampling [82]. Hence, repeatedly stimulating the triceps surae muscle using trains at 20 Hz lasting 330 ms every s for 2 min resulted in a 7% reduction in performance in the low- and a 12% improvement in the high carbohydrate condition. In contrast, no effect was observed for trains at 10, 20, and 50 Hz or maximal voluntary contractions. Also during sustained isometric contractions, impaired performance was observed in two studies when muscle glycogen was lowered, however, effects of prior exercise were not accounted for [83, 84]. Finally, divergent findings appear for the capacity to perform resistance or power exercise in purpotedly lowered glycogen conditions as one study observed an impairment in total work capacity [98], whereas two studies did not show any effect [90, 94]. In a study by Leveritt et al. [92] a reduced performance was only observed for maximal isoinertial strength including multiple repetitions, but not in a limited series of brief isokinetic knee-extensions at different speeds, which likely poses less of a challenge to the glycogenolytic system.
In summary, no effects of muscle glycogen availability are observed when evaluating the performance of brief electrically or voluntarily induced single contractions, which may be explained by lower glycogenolytic stress in such brief contractions. Surprisingly, during the muscle fatigue tests, which included several sequential brief contractions, there were still no consistent effects of glycogen availability. Finally, divergent evidence is available for the impact of muscle glycogen content on resistance exercise capacity, and more research is needed to allow for firm conclusions.
6 Summary of the Relationship Between Muscle Glycogen Content and High-Intensity Performance
Collectively, it appears possible to produce maximal force for brief periods as in a single or limited series of maximal contractions in a lowered muscle glycogen state, whereas inconclusive evidence is available for resistance exercise capacity. In contrast, continuous or repeated high power outputs requiring maximal rates of glycogenolysis and producing large alterations in cellular metabolite and ion homeostasis, consistently results in impaired performance when glycogen is lowered. Moreover, it appears that a threshold for impaired performance of ~ 250 to 300 mmol·kg−1 dw is evident (see Fig. 5). This is consistent with results obtained from intermittent sporting activities where muscle glycogen has been linked to high-intensity exercise tolerance [43, 100,101,102]. Thus, the effects of lowered muscle glycogen may be important for end-game fatigue during team sports where muscle glycogen decreases substantially (below ~ 200 mmol·kg−1 dw) [19, 57, 102, 103]. Of note, these data represent whole-muscle glycogen content and do not delineate both fiber-type and subcellular heterogeneity in utilization as outlined above. Glycogen may also be critically lowered earlier during exercise in congested fixtures where a progressive reduction has been demonstrated in ice hockey and in situations of short recovery time, for example in soccer where a prolonged glycogen repletion pattern has been shown [70, 104, 105]. Moreover, athletes engaged in multiple daily high-intensity events may be prone to detrimental effects of lowered muscle glycogen content during subsequent exercise as well as athletes performing high-intensity training coupled with periods of energy restriction. On that note, it has been demonstrated that the rate of muscle glycogen resynthesis is elevated after high-intensity exercise during the initial stages of recovery (~ 0 to 2 h), likely attributable to elevated glycolytic intermediates and lactate production providing immediate substrate for glycogen resynthesis, at least transiently before returning to resting levels (for review see Pascoe et al. [106] and Jentjens et al. [107]).
Muscle glycogen levels in the low glycogen conditions in studies providing these values, while evaluating performance during continuous high-intensity exercise as well as single or repeated sprint ability compared to a normal glycogen control condition. * = reduced performance in the low glycogen condition (P ≤ 0.05). Full bars represent pre-exercise glycogen levels, whereas the striped area marks the amount of glycogen utilized in the studies providing these values. Area between dashed lines = approximate threshold for impaired exercise tolerance. Bangsbo et al. A = first exercise bout and Bangsbo et al. B = second exercise bout from Bangsbo et al. [29]
Importantly, several methodological limitations should be considered such as a lack of muscle glycogen measurements, placebo-control and non-randomized order of exercise. Moreover, the mode of glycogen-depleting exercise may be important since Jacobs et al. [108] reported that glycogen depletion of both slow- and fast-twitch quadriceps muscle was associated with attenuated maximal strength, whereas selective depletion of slow-twitch fibers did not impair performance. In the studies discussed above, prolonged exercise or a combination of prolonged and high-intensity exercise was applied. In addition, Karlsson et al. [109] found the deleterious effects of muscle glycogen depletion to be most pronounced in individuals with a high distribution of glycolytic fast-twitch fibers. Finally, the post-prandial state in which participants were tested differed which may influence blood glucose levels and affect exercise tolerance irrespective of muscle glycogen levels [110].
7 Mechanisms Linking Lowered Muscle Glycogen Content to Muscle Function
In the following sections suggested mechanisms delineating interactions between lowered muscle glycogen and muscle function will be discussed. These include altered substrate metabolism, Ca2+ regulation, muscle excitability, myofibrillar contractile function, and a brief discussion of other potential mechanisms.
7.1 Muscle Glycogen Content and Glycogenolytic/Glycolytic Rate
The temporal relationship between muscle glycogen content and exercise tolerance has traditionally been attributed to perturbations in energy status to preserve the ATP resynthesis rate required for optimal excitatory and contractile function provoked by depleted muscle glycogen stores during prolonged exercise [14, 32, 111]. In contrast, during high-intensity exercise, fatigue development manifests in advance of severe glycogen depletion with marked PCr degradation, H+ and phosphate accumulation and it has therefore been assumed that glycogen levels do not significantly affect energy metabolism under these conditions [12]. During prolonged exercise, the energy deficiency theory is supported by increased deamination of AMP to IMP and NH3 at the point of fatigue, indicative of increased reliance on the myokinase reaction including decreased pyruvate and tricarboxylic acid intermediates suggestive of inadequate energy supply [111,112,113,114]. On the contrary, no or only minor reductions in global levels of muscle ATP and non-depleted levels of PCr have been reported at fatigue during prolonged exercise, even in the presence of low glycogen levels [33, 111,112,113], which is in contrast to observations during high-intensity exercise where significant perturbations occur, especially in fast-twitch fibers [9, 11, 46, 56, 63]. Moreover, increases in IMP have been speculated to relate to the gradual activation of fast-twitch fibers during progressive exercise as even low-frequent stimulation induces major increases in IMP formation in rodent fast-twitch muscle despite sufficient glycogen availability [53, 115, 116]. However, increases in IMP occur in both slow- and fast-twitch fibers in humans, being most pronounced in glycogen-depleted fibers [61]. Further, Sahlin et al. [32] suggested that even small decreases in ATP, while not consistently observed at the whole-muscle level, may occur in restricted cell compartments or in specific glycogen-depleted fibers and result in substantial relative increases in ADP, AMP and Pi, which may affect muscle function rather than a decrease in ATP per se. This is in line with the previous results by Norman et al. [61] showing higher IMP accumulation in glycogen-depleted fibers and large PCr degradation in specific single fibers in human skeletal muscle indicating substantial local energy deficiency [62]. Thus, global measures of energy homeostasis may be inadequate for describing a link between muscle glycogen and fatigue since perturbations in individual fibers or within microenvironments in the highly structured muscle cells may occur. Notably, this may be relevant for high-intensity exercise since local energy restrictions caused by substrate depletion in specific areas could be important despite only moderately lowered global levels. Further, local energy disruptions may be augmented by the increasing muscle acidosis accumulating during high-intensity exercise as the combination of muscle acidosis and increased Pi has been suggested to act synergistically to exacerbate fatigue development [117]. However, the rate of H+ accumulation would be expected to be lower in a glycogen-depleted condition due to a potentially reduced muscle energy turnover and earlier onset of fatigue. For example, Bangsbo et al. [118], demonstrated that time-to-exhaustion, but also muscle H+ accumulation and absolute H+ values, were lower at exhaustion when repeating high-intensity exercise with lowered muscle glycogen content after a one hour recovery period.
During prolonged exercise muscle glycogen utilization rates have been demonstrated to associate with initial muscle glycogen concentrations in nearly all studies [6, 119,120,121,122], with few exceptions [123]. At high initial glycogen levels, glycogen phosphorylase activity is likely increased, whereas it is unclear whether low initial glycogen levels per se or other factors such as increased free fatty acid availability or hormonal alterations following low-carbohydrate diets (typically with a high-fat content) are the dominant factors that affects metabolism [10, 124, 125]. Moreover, Stellingwerff et al. [126] reported increased fat metabolism and a decrease in glycogenolysis after a 5-day high-fat diet despite restoration of carbohydrate stores during a final days diet. In vitro studies attempting to circumvent this, support a link between muscle glycogen content and glycogenolytic rate in some [127,128,129], but not all studies [130, 131]. In contrast, the glycogenolytic rate appears to be regulated irrespective of initial glycogen level during high-intensity exercise in vivo [75, 83, 86, 132,133,134,135], at least when muscle glycogen stores are not fully depleted. For example, Ren et al. [134] demonstrated that muscle glycogen breakdown was unaffected by initial glycogen content in the range of 155–350 mmol·kg−1 dw when imposing intense electrical stimulation to the quadriceps muscle which is similar to findings by Spencer et al. [135] during exercise at 95% VO2max despite elevated muscle IMP concentrations in a low glycogen condition. However, time-to-exhaustion was markedly deteriorated and it is difficult to ascertain whether a reduced glycogenolytic rate in the final stages of exercise affected performance [135]. Jacobs et al. [80] observed lower muscle lactate accumulation during intense knee-extensor exercise only when glycogen was severely lowered (~ 170 mmol·kg−1 dw), but this was not replicated in a subsequent study by Symons et al. [75] applying the same exercise modality. Collectively, a discrepancy exists for altered blood or muscle lactate accumulation during high-intensity exercise as some [76, 80, 88, 91, 95, 136], but not all studies support a relationship with muscle glycogen [75, 81, 85, 87, 137]. Nonetheless, changes in lactate concentrations may be a product of increased uptake by other tissues rather than a decreased rate of production and/or reflective of a lower total work performed and may therefore be a mediocre predictor of glycolytic rate [29].
The apparent discrepancy between prolonged and high-intensity exercise may be related to the rate of glycogen utilization during exercise which is ~ 0.5 and ~ 5 mmol glycosyl units·kg−1 dw·min−1 at 30 and 90% VO2max, respectively, but increases markedly to ~ 250 to 300 mmol glycosyl units·kg−1 dw·min−1 during maximal exercise at ~ 300 VO2max which is only sustainable for a few seconds [9, 11, 44, 59]. Thus, during sub-maximal exercise in a low glycogen condition, it may be possible to adapt metabolism to fuel availability and rely more on FFA oxidation and plasma glucose extraction, whereas high-intensity exercise demands maximal activation of the glycogenolytic system [10, 125, 138, 139]. This is triggered by marked metabolic disturbances, including elevated levels of ADP and Pi, AMP, IMP and NH3, in association with increased Ca2+ transients and possible regulation from muscle H+ concentration [50, 130, 138, 140,141,142]. Accordingly, Km values of phosphorylase b and a for glycogen are extremely low, i.e. less than 3 mmol glucosyl units·l−1 and it is, therefore, conceivable that glycogen concentration per se plays little role in limiting the rate of glycogenolysis [143]. However, due to the heterogeneous distribution of glycogen in muscle fibers, it cannot be excluded that subsaturating levels of glycogen can occur in specific cell areas which is difficult to capture with global measures of glycogenolytic rate.
In summary, energy deficiency caused by a decelerated glycogenolytic/glycolytic rate in a condition of low muscle glycogen may be implicated in fatigue development during high-intensity exercise. However, no conclusive evidence is presently available, and it is conceivable that energy deficiency may only cause a problem if muscle glycogen is severely lowered at the whole-muscle level or when distinct areas or individual fibers become depleted. Accordingly, the link between muscle glycogen and muscle fatigue, as well as underlying mechanisms explaining such coupling is not well understood. It has been proposed that muscle glycogen can affect several steps in E-C coupling including the propagation of action potentials along the sarcolemma and into the T-system, triggering Ca2+ release and initiation of crossbridge cycling [14] (see Fig. 6). Evidence for alterations in these steps will be discussed below.
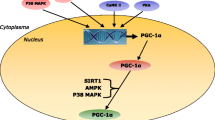
Simplified overview of possible interactions between muscle glycogen storage size and muscle function through direct associations between muscle glycogen and steps in E–C coupling and cross-bridge cycling (e.g. localization-specific metabolic perturbations and/or regulation from glycogen storage size) and/or indirectly through reduced glycogenolytic rate and impaired whole-muscle energy homeostasis including accelerated metabolite accumulation. NKA = Na+-K+-ATPase
7.2 Muscle Glycogen and Muscle Excitability
Muscle excitability is paramount for muscle contractility [13] and Na+-K+-ATPases are important regulators of excitability when the homeostasis of the cell interior and resting membrane potential is challenged by large Na+ and K+ fluxes during high-intensity exercise [13, 144, 145]. The extracellular K+ accumulation increases with exercise intensity reaching interstitial levels of 10–14 mM [144, 146, 147]. At such K+ concentrations slow-inactivation of voltage-gated Na+ channels may occur, impairing action-potential propagation and muscle function [148,149,150], although several mechanisms interact during exercise to counteract the depressive effects of elevated extracellular levels of K+ [151,152,153]. In this regard, multiple studies have demonstrated a downturn in maximal Na+-K+-ATPase activity following both prolonged and intense exercise [154,155,156,157,158,159,160] (for review see McKenna et al. [18]) although contradictory findings and methodological questions have been raised for the in vitro measurements of Na+-K+-ATPase activity [161,162,163,164,165]. Nonetheless, a possible decrease in Na+-K+-ATPase activity following exhaustive exercise may be partly associated with lowered muscle glycogen levels (and the inability to supply ATP locally) and consequently an impaired ability of muscle fibers to regulate ion fluxes. This interplay is noteworthy since it has been demonstrated that Na+-K+-ATPases in the T-system of skeletal muscle, where ionic perturbations may be most severe during exercise [149], preferentially utilize ATP derived from glycolysis [36, 166, 167]. Thus, stimulation of glycolytic ATP production in mechanically skinned fibers decreased the repriming period in partly depolarized fibers, indicative of increased Na+-K+-ATPase activity, whereas the addition of pyruvate as substrate for the TCA cycle did not elicit any up-regulations in excitability [36]. Further, inhibition of glycolysis has been shown to increase intracellular Na+ concentrations, likewise indicative of attenuated Na+-K+-ATPase activity, whereas blockage of Na+-K+-ATPases by ouabain substantially lowers the glycogenolytic/glycolytic rate [167, 168]. Furthermore, enzymatical removal of glycogen or inhibition of glycogen phosphorylase has been shown to increase the repriming period in rat fast-twitch muscle, even despite globally normal ATP concentrations [34, 35]. This supports the possibility of glycogen functioning as a direct regulator of muscle excitability, or alternately, that muscle glycogen may be essential for supplying ATP to the T-system and triad junctions with limited diffusional capacity and a large density of Na+-K+-ATPases [34, 35, 64]. However, from a mechanistic perspective, it is unclear whether altered Na+-K+ fluxes and muscle excitability are directly associated with changes in Na+-K+-ATPase activity since other ion fluxes partake in the control of muscle excitability. Thus, muscle-specific chloride channels (ClC-1) exert a counter-regulatory effect on muscle excitability during exercise (for reviews see Baekgaard et al. [169] and Imbrici et al. [170]). The ClC-1 channels are finely regulated by the metabolic state of the muscle and it is therefore possible that muscle glycogen can be implicated in the activation/deactivation of these channels. However, direct experimental evidence to support a potential relationship to muscle glycogen stores is required [169].
In summary, changes in muscle excitability, likely influenced by altered Na+-K+-ATPase activity in concert with possible perturbations in other ion channel systems may precipitate muscle fatigue during high-intensity exercise in a glycogen-depleted state, although more direct evidence in exercising humans and on exact regulatory mechanisms is warranted.
7.3 Muscle Glycogen and Ca2+ Regulation
In skeletal muscle, Ca2+ released from the SR is an integral part of muscle function through initiation of cross-bridge cycling and activation of metabolism. Although Ca2+ release as such is not an energy-consuming process, glycogenolytic complexes including glycogen-regulating and glycolytic enzymes are associated with the SR, advocating for a connection between muscle glycogen metabolism and Ca2+ regulation [65, 66, 171,172,173,174]. Accordingly, the calcium release channels (RyR1) are modulated by ATP levels activating the channels by directly binding to one or more sites at the RyR1 proteins [175,176,177,178]. Accumulation of the byproducts of ATP hydrolysis during muscle contractions (especially ADP and AMP) on the other hand acts as competitive weak agonists interfering with optimal ATP and RYR1 associations, changing the open probability of these channels. Indeed, experiments in humans [28, 97, 105, 179] and animal models [27, 37,38,39, 73, 74] with one exception [180] have demonstrated that amended Ca2+ kinetics may be an important determinant linking lowered muscle glycogen content to deteriorated muscle function. For example, early experiments by Chin and Allen [27] applied electrical stimulation to intact mouse skeletal muscle to deplete the muscle glycogen stores. Subsequent recovery, in absence or presence of glucose resulted in only partial recovery of force and a sustained lowering of glycogen in the glucose-free trial. In contrast, full recovery of force and substantial glycogen resynthesis was observed in the glucose condition accompanied by differences in Ca2+ release [27]. In later human experiments involving elite cross-country skiers Ørtenblad et al. [28] and subsequently Gejl et al. [97] confirmed that muscle glycogen was linked with Ca2+ kinetics as recovery with or without glucose resulted in substantial differences in glycogen levels and SR Ca2+ release rate. Specifically, intramyofibrillar glycogen was linked with Ca2+ kinetics, and not subsarcolemmal nor intermyofibrillar stores [28]. The association of intramyofibrillar glycogen and SR Ca2+ release rate has subsequently been confirmed in vitro by single fiber measurements [39, 67]. In a study by Nielsen et al. [39] a positive relationship between intramyofibrillar glycogen content and exercise capacity and an inverse link between intermyofibrillar glycogen content and half-relaxation time was demonstrated. This suggests that Ca2+ uptake, which in contrast to Ca2+ release is an energy-consuming process, can also be adversely impaired by lowered muscle glycogen. Moreover, this intriguingly points to distinct effects of specific muscle glycogen storage sites. In support, an accelerated reduction in Vmax of the Ca2+ ATPase and both Ca2+ release and uptake has been demonstrated in exercising humans when initiating exercise with lowered muscle glycogen [179]. In more recent observations by Gejl et al. [97], peak power output during repeated high-intensity exercise and SR Ca2+ release was impaired in a low glycogen condition, further supporting a link between muscle glycogen, Ca2+ kinetics and high-intensity exercise performance. In this regard, a critical threshold of ~ 250 to 300 mmol·kg−1 dw has been suggested to compromise calcium kinetics which fits well with the observed threshold for impaired exercise performance observed in the present review and previous observations of increased cell signaling below this level of glycogen storage [28, 97, 179, 181]. Importantly, muscle glycogen may impair muscle function independent of muscle cell global ATP level, as demonstrated in experiments with mechanically skinned fibers where global ATP can be kept high and constant, while glycogen levels are manipulated [34, 35, 39, 73]. For example, the ability of mechanically skinned toad fibers to respond to T-system depolarization was impaired in a low glycogen condition despite the bathing solution being rich in ATP and PCr [73]. This suggests either a non-metabolic role of muscle glycogen or that muscle glycogen may exert a direct localization–specific metabolic effect through storage of muscle glycogen in specific subcellular areas, providing immediately available energy for ATP resynthesis, which may be critical for muscle function [64]. This may serve as a potential feedback mechanism between muscle glycogen storage size and muscle excitability and SR Ca2+ release to prevent excessive ATP depletion of the cell and protect intracellular homeostasis [28]. Alternately, a possible structural role of muscle glycogen has been proposed, as glycogen depletion may result in dissociation of the glycogenolytic complexes from the SR, changing its structural integrity and/or through phosphorylation/de-phosphorylation changes of the Ca2+ release channels and glycogenolytic enzymes [174, 182,183,184]. Accordingly, a regulatory phosphatase protein subunit appears to couple these complexes to the SR [185]. Moreover, evidence of intracellular redistribution of the active form of glycogen phosphorylase has been suggested, acting as a safety mechanism to inhibit glycogenolysis when muscle glycogen levels are critically low [186]. Finally, AMP-activated protein kinase (AMPK) has a glycogen binding domain, and it has been hypothesized that glycogen depletion results in inhibition of the glycogen-pool of AMPK, suggesting that energy availability and energy-sensing are interrelated, although these hypotheses have not yet been thoroughly studied [187,188,189,190,191,192].
In summary, substantial evidence indicates an important modulating effect of muscle glycogen content on SR Ca2+ regulation and muscle function, either through a direct metabolic role, possibly in relation to compartmentalized energy turnover and/or indirectly through feedback regulation from muscle glycogen metabolism. It is therefore credible, that altered SR Ca2+ kinetics can affect the muscle fatigue response during high-intensity exercise although exact mechanisms remain inconclusive.
7.4 Muscle Glycogen and Myofibrillar Function
The majority of the literature describing the effects of low glycogen has been focused on the relationship between muscle glycogen and key steps in the E-C coupling preceding the actual myofibrillar contraction. However, a decline in muscle glycogen at the whole-muscle level or in local myofibrillar fractions (inter and intramyofibrillar glycogen) could reduce the ATP provision for myosin ATPase and/or accelerate the formation of Pi which has been demonstrated to exert substantial inhibitory effects on the contractile machinery [14, 17]. Thus, muscle glycogen depletion may be hypothesized to affect the contractile function if lowered muscle glycogen is metabolically coupled to muscle fatigue. In a study by Chin et al. [27] an association was observed between changes in muscle glycogen and alterations in Ca2+ sensitivity and maximal Ca2+-activated force-generating capacity of myofibrils from mouse skeletal muscle. The magnitude of this alteration of myofibrillar function was similar in the glycogen-depleted state as in the baseline condition but occurred markedly faster during the contraction protocol suggesting that reduced ATP provision and PCr depletion were accelerated. Similarly using mouse skeletal muscle, Helander et al. [37] reported a delayed decrease in myofibrillar Ca2+ sensitivity after recovery in the presence of glucose, which is likely to be mediated via increased muscle glycogen. Contrasting these findings others have failed to observe any decline in maximally Ca2+ activated force in a lowered muscle glycogen condition using mechanically skinned fibers from cane toad [38, 73] or rat EDL muscle [74]. However, in two of these latter studies, the global levels of ATP and PCr were maintained high and constant [73, 74], whereas in the third study glycogen was only moderately lowered and measurements of intracellular Mg2+, which are thought to reflect intracellular ATP levels, were unchanged [38]. Thus, differences in diffusional restrictions compared to the T-system and restricted triadic junctions may have enabled cytosolic ATP and PCr availability to support myofibrillar function during these experiments. Therefore, more investigations are needed measuring myofibrillar function in conditions of low glycogen especially in human single fibers to provide unequivocal conclusions.
Collectively, low muscle glycogen may affect myofibrillar function through metabolite accumulation secondary to low glycogen levels, whereas the effects of localized energy deficiency is inadequately described and not fully understood.
7.5 Other Potential Factors
In addition to the above interactions between muscle glycogen and muscle function that may affect performance during high-intensity exercise other related mechanisms have been suggested. Thus, central fatigue in response to lowered muscle glycogen levels has been proposed based on altered self-paced performance during prolonged- as well as intermittent high-intensity exercise in lowered glycogen conditions, even in situations where subjects were unaware of the fact that muscle glycogen was manipulated [96, 193]. However, these links have not yet been well-examined and may in part be explained by hypoglycemia-related central fatigue appearing concomitant with decreased muscle glycogen levels [96, 110, 194, 195]. Interestingly, reductions in brain glycogen have been demonstrated in animals model which may be another mechanism by which glycogen can affect the central nervous system irrespective of muscle glycogen levels although no evidence for such a link is available in exercising humans [196, 197].
8 Conclusion
In conclusion, lowering skeletal muscle glycogen content can alter muscle function and provoke impairments in performance during high-intensity exercise, whereas elevated muscle glycogen stores provide no additional performance-enhancing benefit. However, more high-quality studies in exercising humans are needed to confirm these relationships. The apparent exacerbated muscle fatigue in a lowered glycogen condition is likely multifactorial and may associate with multiple key steps in the E-C coupling, such as modified cell excitability and Ca2+ kinetics, and may be mediated by a direct metabolic role (provision of ATP) likely through localization–specific energy metabolism and/or indirectly through feedback regulation from muscle glycogen stores, including a possible link to the central nervous system. Accordingly, depletion of individual muscle fibers and specific subcellular compartments may be essential in explaining the accelerated fatigue development in a lowered glycogen condition, even in advance of whole-muscle glycogen depletion.
9 Future Research
Since many questions remain open, future research should seek to expand these knowledge gaps through experiments further detailing the regulation of muscle function and metabolism in relation to muscle glycogen storage size. Thus, experiments concerning systemic whole-body exercise to fiber-type specific, whole-muscle and single fiber measurements, including expanded analyses of subcellular substrate metabolism and protein regulation/translocation and even measures of brain glycogen metabolism and central fatigue responses would be interesting research areas. Suggested key areas could be human single fiber metabolism and fatigue responses of slow and fast-twitch fibers as well as measures of in vivo metabolism using nuclear magnetic resonance spectroscopy of skeletal muscle and brain tissue. Specifically, the role and utilization of the compartmentalized glycogen pools, for distinct ATPases and steps in E-C coupling, warrant further investigation. Also, placebo-controlled trials with actual measurements of muscle glycogen metabolism are evidently needed to unequivocally determine the effect of muscle glycogen on high-intensity exercise performance.
References
Christensen EH, Hansen O. Arbeitsfähigkeit und ernärung (Physical performance and nutrition). Skandinavishes Archiv für Physiolgie. 1939;81:160–71.
Hargreaves M, Hawley JA, Jeukendrup A. Pre-exercise carbohydrate and fat ingestion: effects on metabolism and performance. J Sports Sci. 2004;22(1):31–8.
Bergstrom J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967;71(2):140–50.
Hermansen L, Hultman E, Saltin B. Muscle glycogen during prolonged severe exercise. Acta Physiol Scand. 1967;71(2):129–39.
Karlsson J, Saltin B. Diet, muscle glycogen, and endurance performance. J Appl Physiol. 1971;31(2):203–6.
Gollnick PD, Piehl K, Saubert CW, Armstrong RB, Saltin B. Diet, exercise, and glycogen changes in human muscle fibers. J Appl Physiol. 1972;33(4):421–5.
Costill DL, Gollnick PD, Jansson ED, Saltin B, Stein EM. Glycogen depletion pattern in human muscle fibres during distance running. Acta Physiol Scand. 1973;89(3):374–83.
Hargreaves M. Skeletal muscle metabolism during exercise in humans. Clin Exp Pharmacol Physiol. 2000;27(3):225–8.
Gaitanos GC, Williams C, Boobis LH, Brooks S. Human muscle metabolism during intermittent maximal exercise. J Appl Physiol (1985). 1993;75(2):712–9.
Jensen TE, Richter EA. Regulation of glucose and glycogen metabolism during and after exercise. J Physiol. 2012;590(5):1069–76.
Parolin ML, Chesley A, Matsos MP, Spriet LL, Jones NL, Heigenhauser GJ. Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. Am J Physiol. 1999;277(5):E890-900.
Hultman E, Greenhaff PL. Skeletal muscle energy metabolism and fatigue during intense exercise in man. Sci Prog. 1991;75(298 Pt 3–4):361–70.
Clausen T, Nielsen OB. The Na+, K(+)-pump and muscle contractility. Acta Physiol Scand. 1994;152(4):365–73.
Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88(1):287–332.
Fitts RH. The role of acidosis in fatigue: pro perspective. Med Sci Sports Exerc. 2016;48(11):2335–8.
Cheng AJ, Place N, Westerblad H. Molecular basis for exercise-induced fatigue: the importance of strictly controlled cellular Ca(2+) handling. Cold Spring Harb Perspect Med. 2018;8(2):a029710.
Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74(1):49–94.
McKenna MJ, Bangsbo J, Renaud JM. Muscle K+, Na+, and Cl disturbances and Na+-K+ pump inactivation: implications for fatigue. J Appl Physiol (1985). 2008;104(1):288–95.
Vigh-Larsen JF, Ermidis G, Rago V, Randers MB, Fransson D, Nielsen JL, et al. Muscle metabolism and fatigue during simulated ice hockey match-play in elite players. Med Sci Sports Exerc. 2020;52(10):2162–71.
Gollnick PD, Armstrong RB, Sembrowich WL, Shepherd RE, Saltin B. Glycogen depletion pattern in human skeletal muscle fibers after heavy exercise. J Appl Physiol. 1973;34(5):615–8.
Nielsen J, Ørtenblad N. Physiological aspects of the subcellular localization of glycogen in skeletal muscle. Appl Physiol Nutr Metab. 2013;38(2):91–9.
Ørtenblad N, Westerblad H, Nielsen J. Muscle glycogen stores and fatigue. J Physiol. 2013;591(18):4405–13.
Marchand I, Chorneyko K, Tarnopolsky M, Hamilton S, Shearer J, Potvin J, et al. Quantification of subcellular glycogen in resting human muscle: granule size, number, and location. J Appl Physiol (1985). 2002;93(5):1598–607.
Friden J, Seger J, Ekblom B. Topographical localization of muscle glycogen: an ultrahistochemical study in the human vastus lateralis. Acta Physiol Scand. 1989;135(3):381–91.
Friden J, Seger J, Ekblom B. Implementation of periodic acid-thiosemicarbazide-silver proteinate staining for ultrastructural assessment of muscle glycogen utilization during exercise. Cell Tissue Res. 1985;242(1):229–32.
Sjostrom M, Friden J, Ekblom B. Fine structural details of human muscle fibres after fibre type specific glycogen depletion. Histochemistry. 1982;76(4):425–38.
Chin ER, Allen DG. Effects of reduced muscle glycogen concentration on force, Ca2+ release and contractile protein function in intact mouse skeletal muscle. J Physiol. 1997;498(Pt 1):17–29.
Ørtenblad N, Nielsen J, Saltin B, Holmberg HC. Role of glycogen availability in sarcoplasmic reticulum Ca2+ kinetics in human skeletal muscle. J Physiol. 2011;589(Pt 3):711–25.
Bangsbo J, Graham TE, Kiens B, Saltin B. Elevated muscle glycogen and anaerobic energy production during exhaustive exercise in man. J Physiol. 1992;451:205–27.
Hargreaves M, Finn JP, Withers RT, Halbert JA, Scroop GC, Mackay M, et al. Effect of muscle glycogen availability on maximal exercise performance. Eur J Appl Physiol Occup Physiol. 1997;75(2):188–92.
Balsom PD, Gaitanos GC, Soderlund K, Ekblom B. High-intensity exercise and muscle glycogen availability in humans. Acta Physiol Scand. 1999;165(4):337–45.
Sahlin K, Tonkonogi M, Soderlund K. Energy supply and muscle fatigue in humans. Acta Physiol Scand. 1998;162(3):261–6.
Green HJ. How important is endogenous muscle glycogen to fatigue in prolonged exercise? Can J Physiol Pharmacol. 1991;69(2):290–7.
Jensen R, Nielsen J, Ørtenblad N. Inhibition of glycogenolysis prolongs action potential repriming period and impairs muscle function in rat skeletal muscle. J Physiol. 2020;598(4):789–803.
Watanabe D, Wada M. Effects of reduced muscle glycogen on excitation-contraction coupling in rat fast-twitch muscle: a glycogen removal study. J Muscle Res Cell Motil. 2019;40(3–4):353–64.
Dutka TL, Lamb GD. Na+-K+ pumps in the transverse tubular system of skeletal muscle fibers preferentially use ATP from glycolysis. Am J Physiol Cell Physiol. 2007;293(3):C967–77.
Helander I, Westerblad H, Katz A. Effects of glucose on contractile function, [Ca2+]i, and glycogen in isolated mouse skeletal muscle. Am J Physiol Cell Physiol. 2002;282(6):C1306–12.
Kabbara AA, Nguyen LT, Stephenson GM, Allen DG. Intracellular calcium during fatigue of cane toad skeletal muscle in the absence of glucose. J Muscle Res Cell Motil. 2000;21(5):481–9.
Nielsen J, Schroder HD, Rix CG, Ørtenblad N. Distinct effects of subcellular glycogen localization on tetanic relaxation time and endurance in mechanically skinned rat skeletal muscle fibres. J Physiol. 2009;587(Pt 14):3679–90.
Hearris MA, Hammond KM, Fell JM, Morton JP. Regulation of muscle glycogen metabolism during exercise: implications for endurance performance and training adaptations. Nutrients. 2018;10(3):298.
Murray B, Rosenbloom C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr Rev. 2018;76(4):243–59.
Areta JL, Hopkins WG. Skeletal muscle glycogen content at rest and during endurance exercise in humans: a meta-analysis. Sports Med. 2018;48(9):2091–102.
Saltin B. Metabolic fundamentals in exercise. Med Sci Sports. 1973;5(3):137–46.
Saltin B, Karlsson J. Muscle glycogen utilization during work of different intensities. In: Pernow B, Saltin B, editors. Muscle metabolism during exercise. New York: Plenum pub; 1971. p. 289–99.
Bogdanis GC, Nevill ME, Lakomy HK, Boobis LH. Power output and muscle metabolism during and following recovery from 10 and 20 s of maximal sprint exercise in humans. Acta Physiol Scand. 1998;163(3):261–72.
Bogdanis GC, Nevill ME, Boobis LH, Lakomy HK. Contribution of phosphocreatine and aerobic metabolism to energy supply during repeated sprint exercise. J Appl Physiol (1985). 1996;80(3):876–84.
Jones NL, McCartney N, Graham T, Spriet LL, Kowalchuk JM, Heigenhauser GJ, et al. Muscle performance and metabolism in maximal isokinetic cycling at slow and fast speeds. J Appl Physiol (1985). 1985;59(1):132–6.
Greenhaff PL, Nevill ME, Soderlund K, Bodin K, Boobis LH, Williams C, et al. The metabolic responses of human type I and II muscle fibres during maximal treadmill sprinting. J Physiol. 1994;478(Pt 1):149–55.
Spriet LL. Anaerobic metabolism in human skeletal muscle during short-term, intense activity. Can J Physiol Pharmacol. 1992;70(1):157–65.
Spriet LL, Lindinger MI, McKelvie RS, Heigenhauser GJ, Jones NL. Muscle glycogenolysis and H+ concentration during maximal intermittent cycling. J Appl Physiol (1985). 1989;66(1):8–13.
Vollestad NK, Tabata I, Medbo JI. Glycogen breakdown in different human muscle fibre types during exhaustive exercise of short duration. Acta Physiol Scand. 1992;144(2):135–41.
Vollestad NK, Blom PC. Effect of varying exercise intensity on glycogen depletion in human muscle fibres. Acta Physiol Scand. 1985;125(3):395–405.
Vollestad NK, Vaage O, Hermansen L. Muscle glycogen depletion patterns in type I and subgroups of type II fibres during prolonged severe exercise in man. Acta Physiol Scand. 1984;122(4):433–41.
Gollnick PD, Armstrong RB, Saubert CW, Sembrowich WL, Shepherd RE, Saltin B. Glycogen depletion patterns in human skeletal muscle fibers during prolonged work. Pflugers Arch. 1973;344(1):1–12.
Essen B. Intramuscular substrate utilization during prolonged exercise. Ann N Y Acad Sci. 1977;301:30–44.
Krustrup P, Soderlund K, Mohr M, Gonzalez-Alonso J, Bangsbo J. Recruitment of fibre types and quadriceps muscle portions during repeated, intense knee-extensor exercise in humans. Pflugers Arch. 2004;449(1):56–65.
Krustrup P, Mohr M, Steensberg A, Bencke J, Kjaer M, Bangsbo J. Muscle and blood metabolites during a soccer game: implications for sprint performance. Med Sci Sports Exerc. 2006;38(6):1165–74.
Ball-Burnett M, Green HJ, Houston ME. Energy metabolism in human slow and fast twitch fibres during prolonged cycle exercise. J Physiol. 1991;437:257–67.
Gollnick PD, Piehl K, Saltin B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J Physiol. 1974;241(1):45–57.
Kristensen DE, Albers PH, Prats C, Baba O, Birk JB, Wojtaszewski JF. Human muscle fibre type-specific regulation of AMPK and downstream targets by exercise. J Physiol. 2015;593(8):2053–69.
Norman B, Sollevi A, Jansson E. Increased IMP content in glycogen-depleted muscle fibres during submaximal exercise in man. Acta Physiol Scand. 1988;133(1):97–100.
Sahlin K, Soderlund K, Tonkonogi M, Hirakoba K. Phosphocreatine content in single fibers of human muscle after sustained submaximal exercise. Am J Physiol. 1997;273(1 Pt 1):C172–8.
Karatzaferi C, de Haan A, Ferguson RA, van Mechelen W, Sargeant AJ. Phosphocreatine and ATP content in human single muscle fibres before and after maximum dynamic exercise. Pflugers Arch. 2001;442(3):467–74.
Ørtenblad N, Nielsen J. Muscle glycogen and cell function—location, location, location. Scand J Med Sci Sports. 2015;25(Suppl 4):34–40.
Wanson JC, Drochmans P. Role of the sarcoplasmic reticulum in glycogen metabolism. Binding of phosphorylase, phosphorylase kinase, and primer complexes to the sarcovesicles of rabbit skeletal muscle. J Cell Biol. 1972;54(2):206–24.
Wanson JC, Drochmans P. Rabbit skeletal muscle glycogen. A morphological and biochemical study of glycogen beta-particles isolated by the precipitation-centrifugation method. J Cell Biol. 1968;38(1):130–50.
Nielsen J, Cheng AJ, Ørtenblad N, Westerblad H. Subcellular distribution of glycogen and decreased tetanic Ca2+ in fatigued single intact mouse muscle fibres. J Physiol. 2014;592(9):2003–12.
Jensen R, Ørtenblad N, Stausholm MH, Skjaerbaek MC, Larsen DN, Hansen M, et al. Heterogeneity in subcellular muscle glycogen utilisation during exercise impacts endurance capacity in men. J Physiol. 2020;598(19):4271–92.
Gejl KD, Ørtenblad N, Andersson E, Plomgaard P, Holmberg HC, Nielsen J. Local depletion of glycogen with supramaximal exercise in human skeletal muscle fibres. J Physiol. 2017;595(9):2809–21.
Nielsen J, Holmberg HC, Schroder HD, Saltin B, Ørtenblad N. Human skeletal muscle glycogen utilization in exhaustive exercise: role of subcellular localization and fibre type. J Physiol. 2011;589(Pt 11):2871–85.
Marchand I, Tarnopolsky M, Adamo KB, Bourgeois JM, Chorneyko K, Graham TE. Quantitative assessment of human muscle glycogen granules size and number in subcellular locations during recovery from prolonged exercise. J Physiol. 2007;580(Pt. 2):617–28.
Hokken R, Laugesen S, Aagaard P, Suetta C, Frandsen U, Ørtenblad N, et al. Subcellular localization- and fibre type-dependent utilization of muscle glycogen during heavy resistance exercise in elite power and Olympic weightlifters. Acta Physiol (Oxf). 2021;231(2):e13561.
Stephenson DG, Nguyen LT, Stephenson GM. Glycogen content and excitation-contraction coupling in mechanically skinned muscle fibres of the cane toad. J Physiol. 1999;519(Pt 1):177–87.
Barnes M, Gibson LM, Stephenson DG. Increased muscle glycogen content is associated with increased capacity to respond to T-system depolarisation in mechanically skinned skeletal muscle fibres from the rat. Pflugers Arch. 2001;442(1):101–6.
Symons JD, Jacobs I. High-intensity exercise performance is not impaired by low intramuscular glycogen. Med Sci Sports Exerc. 1989;21(5):550–7.
Greenhaff PL, Gleeson M, Maughan RJ. Diet-induced metabolic acidosis and the performance of high intensity exercise in man. Eur J Appl Physiol Occup Physiol. 1988;57(5):583–90.
Greenhaff PL, Gleeson M, Maughan RJ. The effects of dietary manipulation on blood acid-base status and the performance of high intensity exercise. Eur J Appl Physiol Occup Physiol. 1987;56(3):331–7.
Greenhaff PL, Gleeson M, Whiting PH, Maughan RJ. Dietary composition and acid-base status: limiting factors in the performance of maximal exercise in man? Eur J Appl Physiol Occup Physiol. 1987;56(4):444–50.
Maughan RJ, Poole DC. The effects of a glycogen-loading regimen on the capacity to perform anaerobic exercise. Eur J Appl Physiol Occup Physiol. 1981;46(3):211–9.
Jacobs I. Lactate concentrations after short, maximal exercise at various glycogen levels. Acta Physiol Scand. 1981;111(4):465–9.
Wootton SAWC. Influence of carbohydrate-status on performance during maximal exercise. Int J Sports Med. 1984;5:126–7.
Young K, Davies CT. Effect of diet on human muscle weakness following prolonged exercise. Eur J Appl Physiol Occup Physiol. 1984;53(1):81–5.
Sahlin K, Broberg S, Katz A. Glucose formation in human skeletal muscle. Influence of glycogen content. Biochem J. 1989;258(3):911–3.
Maughan RJ. Effects of prior exercise on the performance of intense isometric exercise. Br J Sports Med. 1988;22(1):12–5.
Hargreaves M, McKenna MJ, Jenkins DG, Warmington SA, Li JL, Snow RJ, et al. Muscle metabolites and performance during high-intensity, intermittent exercise. J Appl Physiol (1985). 1998;84(5):1687–91.
Vandenberghe K, Hespel P, Vanden Eynde B, Lysens R, Richter EA. No effect of glycogen level on glycogen metabolism during high intensity exercise. Med Sci Sports Exerc. 1995;27(9):1278–83.
Jenkins DG, Palmer J, Spillman D. The influence of dietary carbohydrate on performance of supramaximal intermittent exercise. Eur J Appl Physiol Occup Physiol. 1993;67(4):309–14.
Casey A, Short AH, Curtis S, Greenhaff PL. The effect of glycogen availability on power output and the metabolic response to repeated bouts of maximal, isokinetic exercise in man. Eur J Appl Physiol Occup Physiol. 1996;72(3):249–55.
Pizza FX, Flynn MG, Duscha BD, Holden J, Kubitz ER. A carbohydrate loading regimen improves high intensity, short duration exercise performance. Int J Sport Nutr. 1995;5(2):110–6.
Mitchell JB, DiLauro PC, Pizza FX, Cavender DL. The effect of preexercise carbohydrate status on resistance exercise performance. Int J Sport Nutr. 1997;7(3):185–96.
Langfort J, Zarzeczny R, Pilis W, Nazar K, Kaciuba-Uscitko H. The effect of a low-carbohydrate diet on performance, hormonal and metabolic responses to a 30-s bout of supramaximal exercise. Eur J Appl Physiol Occup Physiol. 1997;76(2):128–33.
Leveritt M, Abernethy PJ. Effects of carbohydrate restriction on strength performance. J Strength Cond Res. 1999;13:52–7.
Rockwell MS, Rankin JW, Dixon H. Effects of muscle glycogen on performance of repeated sprints and mechanisms of fatigue. Int J Sport Nutr Exerc Metab. 2003;13(1):1–14.
Hatfield DL, Kraemer WJ, Volek JS, Rubin MR, Grebien B, Gomez AL, et al. The effects of carbohydrate loading on repetitive jump squat power performance. J Strength Cond Res. 2006;20(1):167–71.
Lima-Silva AE, Pires FO, Bertuzzi R, Silva-Cavalcante MD, Oliveira RS, Kiss MA, et al. Effects of a low- or a high-carbohydrate diet on performance, energy system contribution, and metabolic responses during supramaximal exercise. Appl Physiol Nutr Metab. 2013;38(9):928–34.
Skein M, Duffield R, Kelly BT, Marino FE. The effects of carbohydrate intake and muscle glycogen content on self-paced intermittent-sprint exercise despite no knowledge of carbohydrate manipulation. Eur J Appl Physiol. 2012;112(8):2859–70.
Gejl KD, Hvid LG, Frandsen U, Jensen K, Sahlin K, Ørtenblad N. Muscle glycogen content modifies SR Ca2+ release rate in elite endurance athletes. Med Sci Sports Exerc. 2014;46(3):496–505.
Oliver JM, Almada AL, Van Eck LE, Shah M, Mitchell JB, Jones MT, et al. Ingestion of high molecular weight carbohydrate enhances subsequent repeated maximal power: a randomized controlled trial. PLoS ONE. 2016;11(9):e0163009.
Cheng AJ, Chaillou T, Kamandulis S, Subocius A, Westerblad H, Brazaitis M, et al. Carbohydrates do not accelerate force recovery after glycogen-depleting followed by high-intensity exercise in humans. Scand J Med Sci Sports. 2020;30(6):998–1007.
Akermark C, Jacobs I, Rasmusson M, Karlsson J. Diet and muscle glycogen concentration in relation to physical performance in Swedish elite ice hockey players. Int J Sport Nutr. 1996;6(3):272–84.
Bangsbo J, Norregaard L, Thorsoe F. The effect of carbohydrate diet on intermittent exercise performance. Int J Sports Med. 1992;13(2):152–7.
Bendiksen M, Bischoff R, Randers MB, Mohr M, Rollo I, Suetta C, et al. The Copenhagen Soccer Test: physiological response and fatigue development. Med Sci Sports Exerc. 2012;44(8):1595–603.
Green HJ, Daub BD, Painter DC, Thomson JA. Glycogen depletion patterns during ice hockey performance. Med Sci Sports. 1978;10(4):289–93.
Montgomery DL. Physiology of ice hockey. Sports Med. 1988;5(2):99–126.
Krustrup P, Ørtenblad N, Nielsen J, Nybo L, Gunnarsson TP, Iaia FM, et al. Maximal voluntary contraction force, SR function and glycogen resynthesis during the first 72 h after a high-level competitive soccer game. Eur J Appl Physiol. 2011;111(12):2987–95.
Pascoe DD, Gladden LB. Muscle glycogen resynthesis after short term, high intensity exercise and resistance exercise. Sports Med. 1996;21(2):98–118.
Jentjens R, Jeukendrup A. Determinants of post-exercise glycogen synthesis during short-term recovery. Sports Med. 2003;33(2):117–44.
Jacobs I, Kaiser P, Tesch P. Muscle strength and fatigue after selective glycogen depletion in human skeletal muscle fibers. Eur J Appl Physiol Occup Physiol. 1981;46(1):47–53.
Karlsson J, Sjodin B, Jacobs I, Kaiser P. Relevance of muscle fibre type to fatigue in short intense and prolonged exercise in man. Ciba Found Symp. 1981;82:59–74.
Nybo L. CNS fatigue and prolonged exercise: effect of glucose supplementation. Med Sci Sports Exerc. 2003;35(4):589–94.
Sahlin K, Katz A, Broberg S. Tricarboxylic acid cycle intermediates in human muscle during prolonged exercise. Am J Physiol. 1990;259(5 Pt 1):C834–41.
Norman B, Sollevi A, Kaijser L, Jansson E. ATP breakdown products in human skeletal muscle during prolonged exercise to exhaustion. Clin Physiol. 1987;7(6):503–10.
Broberg S, Sahlin K. Adenine nucleotide degradation in human skeletal muscle during prolonged exercise. J Appl Physiol (1985). 1989;67(1):116–22.
Sahlin K, Broberg S, Ren JM. Formation of inosine monophosphate (IMP) in human skeletal muscle during incremental dynamic exercise. Acta Physiol Scand. 1989;136(2):193–8.
Gollnick PD, Karlsson J, Piehl K, Saltin B. Selective glycogen depletion in skeletal muscle fibres of man following sustained contractions. J Physiol. 1974;241(1):59–67.
Meyer RA, Terjung RL. AMP deamination and IMP reamination in working skeletal muscle. Am J Physiol. 1980;239(1):C32–8.
Nelson CR, Debold EP, Fitts RH. Phosphate and acidosis act synergistically to depress peak power in rat muscle fibers. Am J Physiol Cell Physiol. 2014;307(10):C939–50.
Bangsbo J, Graham T, Johansen L, Strange S, Christensen C, Saltin B. Elevated muscle acidity and energy production during exhaustive exercise in humans. Am J Physiol. 1992;263(4 Pt 2):R891–9.
Sherman WM, Costill DL, Fink WJ, Miller JM. Effect of exercise-diet manipulation on muscle glycogen and its subsequent utilization during performance. Int J Sports Med. 1981;2(2):114–8.
Bosch AN, Dennis SC, Noakes TD. Influence of carbohydrate loading on fuel substrate turnover and oxidation during prolonged exercise. J Appl Physiol (1985). 1993;74(4):1921–7.
Putman CT, Spriet LL, Hultman E, Lindinger MI, Lands LC, McKelvie RS, et al. Pyruvate dehydrogenase activity and acetyl group accumulation during exercise after different diets. Am J Physiol. 1993;265(5 Pt 1):E752–60.
Hargreaves M, McConell G, Proietto J. Influence of muscle glycogen on glycogenolysis and glucose uptake during exercise in humans. J Appl Physiol (1985). 1995;78(1):288–92.
Madsen K, Pedersen PK, Rose P, Richter EA. Carbohydrate supercompensation and muscle glycogen utilization during exhaustive running in highly trained athletes. Eur J Appl Physiol Occup Physiol. 1990;61(5–6):467–72.
Costill DL, Coyle E, Dalsky G, Evans W, Fink W, Hoopes D. Effects of elevated plasma FFA and insulin on muscle glycogen usage during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(4):695–9.
Hargreaves M. Muscle glycogen and metabolic regulation. Proc Nutr Soc. 2004;63(2):217–20.
Stellingwerff T, Spriet LL, Watt MJ, Kimber NE, Hargreaves M, Hawley JA, et al. Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration. Am J Physiol Endocrinol Metab. 2006;290(2):E380–8.
Hespel P, Richter EA. Glucose uptake and transport in contracting, perfused rat muscle with different pre-contraction glycogen concentrations. J Physiol. 1990;427:347–59.
Hespel P, Richter EA. Mechanism linking glycogen concentration and glycogenolytic rate in perfused contracting rat skeletal muscle. Biochem J. 1992;284(Pt 3):777–80.
Richter EA, Galbo H. High glycogen levels enhance glycogen breakdown in isolated contracting skeletal muscle. J Appl Physiol (1985). 1986;61(3):827–31.
Spriet LL, Berardinucci L, Marsh DR, Campbell CB, Graham TE. Glycogen content has no effect on skeletal muscle glycogenolysis during short-term tetanic stimulation. J Appl Physiol (1985). 1990;68(5):1883–8.
Vandenberghe K, Richter EA, Hespel P. Regulation of glycogen breakdown by glycogen level in contracting rat muscle. Acta Physiol Scand. 1999;165(3):307–14.
Klausen K, Sjogaard G. Glycogen stores and lactate accumulation in skeletal muscle of man during intense bicycle exercise. Scand J Sports Sci. 1980;2(1):7–12.
Boobis LH, Williams C, Wootton SA. Influence of sprint training on muscle metabolism during brief maximal exercise in man. J Physiol. 1983;342:36–7.
Ren JM, Broberg S, Sahlin K, Hultman E. Influence of reduced glycogen level on glycogenolysis during short-term stimulation in man. Acta Physiol Scand. 1990;139(3):467–74.
Spencer MK, Katz A. Role of glycogen in control of glycolysis and IMP formation in human muscle during exercise. Am J Physiol. 1991;260(6 Pt 1):E859–64.
Greenhaff PL, Gleeson M, Maughan RJ. The effects of a glycogen loading regimen on acid-base status and blood lactate concentration before and after a fixed period of high intensity exercise in man. Eur J Appl Physiol Occup Physiol. 1988;57(2):254–9.
Greenhaff PL, Gleeson M, Maughan RJ. The effects of diet on muscle pH and metabolism during high intensity exercise. Eur J Appl Physiol Occup Physiol. 1988;57(5):531–9.
Spriet LL. New insights into the interaction of carbohydrate and fat metabolism during exercise. Sports Med. 2014;44(Suppl 1):S87-96.
Sahlin K, Harris RC. Control of lipid oxidation during exercise: role of energy state and mitochondrial factors. Acta Physiol (Oxf). 2008;194(4):283–91.
Sahlin K, Sallstedt EK, Bishop D, Tonkonogi M. Turning down lipid oxidation during heavy exercise–what is the mechanism? J Physiol Pharmacol. 2008;59(Suppl 7):19–30.
Hargreaves M. Exercise, muscle, and CHO metabolism. Scand J Med Sci Sports. 2015;25(Suppl 4):29–33.
Ørtenblad N, Macdonald WA, Sahlin K. Glycolysis in contracting rat skeletal muscle is controlled by factors related to energy state. Biochem J. 2009;420(2):161–8.
Newsholme EA, Start C. Regulation in metabolism. Toronto: Wiley; 1973.
Juel C, Pilegaard H, Nielsen JJ, Bangsbo J. Interstitial K(+) in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Integr Comp Physiol. 2000;278(2):R400–6.
Clausen T. Quantification of Na+, K+ pumps and their transport rate in skeletal muscle: functional significance. J Gen Physiol. 2013;142(4):327–45.
Nordsborg N, Mohr M, Pedersen LD, Nielsen JJ, Langberg H, Bangsbo J. Muscle interstitial potassium kinetics during intense exhaustive exercise: effect of previous arm exercise. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R143–8.
Mohr M, Nordsborg N, Nielsen JJ, Pedersen LD, Fischer C, Krustrup P, et al. Potassium kinetics in human muscle interstitium during repeated intense exercise in relation to fatigue. Pflugers Arch. 2004;448(4):452–6.
Cairns SP, Flatman JA, Clausen T. Relation between extracellular [K+], membrane potential and contraction in rat soleus muscle: modulation by the Na+-K+ pump. Pflugers Arch. 1995;430(6):909–15.
Sejersted OM, Sjogaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev. 2000;80(4):1411–81.
Ruff RL. Sodium channel slow inactivation and the distribution of sodium channels on skeletal muscle fibres enable the performance properties of different skeletal muscle fibre types. Acta Physiol Scand. 1996;156(3):159–68.
Pedersen TH, Clausen T, Nielsen OB. Loss of force induced by high extracellular [K+] in rat muscle: effect of temperature, lactic acid and beta2-agonist. J Physiol. 2003;551(Pt 1):277–86.
de Paoli FV, Overgaard K, Pedersen TH, Nielsen OB. Additive protective effects of the addition of lactic acid and adrenaline on excitability and force in isolated rat skeletal muscle depressed by elevated extracellular K+. J Physiol. 2007;581(Pt 2):829–39.
Overgaard K, Nielsen OB. Activity-induced recovery of excitability in K(+)-depressed rat soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2001;280(1):R48-55.
Leppik JA, Aughey RJ, Medved I, Fairweather I, Carey MF, McKenna MJ. Prolonged exercise to fatigue in humans impairs skeletal muscle Na+-K+-ATPase activity, sarcoplasmic reticulum Ca2+ release, and Ca2+ uptake. J Appl Physiol (1985). 2004;97(4):1414–23.
Fowles JR, Green HJ, Tupling R, O’Brien S, Roy BD. Human neuromuscular fatigue is associated with altered Na+-K+-ATPase activity following isometric exercise. J Appl Physiol (1985). 2002;92(4):1585–93.
Fraser SF, Li JL, Carey MF, Wang XN, Sangkabutra T, Sostaric S, et al. Fatigue depresses maximal in vitro skeletal muscle Na(+)-K(+)-ATPase activity in untrained and trained individuals. J Appl Physiol (1985). 2002;93(5):1650–9.
Aughey RJ, Clark SA, Gore CJ, Townsend NE, Hahn AG, Kinsman TA, et al. Interspersed normoxia during live high, train low interventions reverses an early reduction in muscle Na+, K +ATPase activity in well-trained athletes. Eur J Appl Physiol. 2006;98(3):299–309.
Petersen AC, Murphy KT, Snow RJ, Leppik JA, Aughey RJ, Garnham AP, et al. Depressed Na+-K+-ATPase activity in skeletal muscle at fatigue is correlated with increased Na+-K+-ATPase mRNA expression following intense exercise. Am J Physiol Regul Integr Comp Physiol. 2005;289(1):R266–74.
Sandiford SD, Green HJ, Duhamel TA, Perco JG, Schertzer JD, Ouyang J. Inactivation of human muscle Na+-K+-ATPase in vitro during prolonged exercise is increased with hypoxia. J Appl Physiol (1985). 2004;96(5):1767–75.
Aughey RJ, Murphy KT, Clark SA, Garnham AP, Snow RJ, Cameron-Smith D, et al. Muscle Na+-K+-ATPase activity and isoform adaptations to intense interval exercise and training in well-trained athletes. J Appl Physiol (1985). 2007;103(1):39–47.
Jannas-Vela S, Brownell S, Petrick HL, Heigenhauser GJF, Spriet LL, Holloway GP. Assessment of Na+/K+ ATPase activity in small rodent and human skeletal muscle samples. Med Sci Sports Exerc. 2019;51(11):2403–9.
Juel C, Hostrup M, Bangsbo J. The effect of exercise and beta2-adrenergic stimulation on glutathionylation and function of the Na, K-ATPase in human skeletal muscle. Physiol Rep. 2015;3(8):e12515.
Juel C, Nordsborg NB, Bangsbo J. Purinergic effects on Na, K-ATPase activity differ in rat and human skeletal muscle. PLoS ONE. 2014;9(3):e91175.
Juel C, Nordsborg NB, Bangsbo J. Exercise-induced increase in maximal in vitro Na-K-ATPase activity in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2013;304(12):R1161–5.
Juel C. Maximal Na(+)-K(+)-ATPase activity is upregulated in association with muscle activity. J Appl Physiol (1985). 2012;112(12):2121–3.
Dhar-Chowdhury P, Malester B, Rajacic P, Coetzee WA. The regulation of ion channels and transporters by glycolytically derived ATP. Cell Mol Life Sci. 2007;64(23):3069–83.
James JH, Wagner KR, King JK, Leffler RE, Upputuri RK, Balasubramaniam A, et al. Stimulation of both aerobic glycolysis and Na(+)-K(+)-ATPase activity in skeletal muscle by epinephrine or amylin. Am J Physiol. 1999;277(1):E176–86.
Okamoto K, Wang W, Rounds J, Chambers EA, Jacobs DO. ATP from glycolysis is required for normal sodium homeostasis in resting fast-twitch rodent skeletal muscle. Am J Physiol Endocrinol Metab. 2001;281(3):E479–88.
Baekgaard Nielsen O, de Paoli FV, Riisager A, Pedersen TH. Chloride channels take center stage in acute regulation of excitability in skeletal muscle: implications for fatigue. Physiology (Bethesda). 2017;32(6):425–34.
Imbrici P, Altamura C, Pessia M, Mantegazza R, Desaphy JF, Camerino DC. ClC-1 chloride channels: state-of-the-art research and future challenges. Front Cell Neurosci. 2015;9:156.
Entman ML, Keslensky SS, Chu A, Van Winkle WB. The sarcoplasmic reticulum-glycogenolytic complex in mammalian fast twitch skeletal muscle. Proposed in vitro counterpart of the contraction-activated glycogenolytic pool. J Biol Chem. 1980;255(13):6245–52.
Xu KY, Becker LC. Ultrastructural localization of glycolytic enzymes on sarcoplasmic reticulum vesticles. J Histochem Cytochem. 1998;46(4):419–27.
Lees SJ, Chen YT, Williams JH. Glycogen debranching enzyme is associated with rat skeletal muscle sarcoplasmic reticulum. Acta Physiol Scand. 2004;181(2):239–45.
Lees SJ, Franks PD, Spangenburg EE, Williams JH. Glycogen and glycogen phosphorylase associated with sarcoplasmic reticulum: effects of fatiguing activity. J Appl Physiol (1985). 2001;91(4):1638–44.
Laver DR. Regulation of ryanodine receptors from skeletal and cardiac muscle during rest and excitation. Clin Exp Pharmacol Physiol. 2006;33(11):1107–13.
Laver DR, Lenz GK, Lamb GD. Regulation of the calcium release channel from rabbit skeletal muscle by the nucleotides ATP, AMP, IMP and adenosine. J Physiol. 2001;537(Pt 3):763–78.
Popova OB, Baker MR, Tran TP, Le T, Serysheva II. Identification of ATP-binding regions in the RyR1 Ca(2)(+) release channel. PLoS ONE. 2012;7(11):e48725.
Ogawa H, Kurebayashi N, Yamazawa T, Murayama T. Regulatory mechanisms of ryanodine receptor/Ca(2+) release channel revealed by recent advancements in structural studies. J Muscle Res Cell Motil. 2020 Feb 10. Epub ahead of print.
Duhamel TA, Perco JG, Green HJ. Manipulation of dietary carbohydrates after prolonged effort modifies muscle sarcoplasmic reticulum responses in exercising males. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R1100–10.
Goodman C, Blazev R, Stephenson G. Glycogen content and contractile responsiveness to T-system depolarization in skinned muscle fibres of the rat. Clin Exp Pharmacol Physiol. 2005;32(9):749–56.
Impey SG, Hearris MA, Hammond KM, Bartlett JD, Louis J, Close GL, et al. Fuel for the work required: a theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Sports Med. 2018;48(5):1031–48.
Cuenda A, Nogues M, Henao F, Gutierrez-Merino C. Interaction between glycogen phosphorylase and sarcoplasmic reticulum membranes and its functional implications. J Biol Chem. 1995;270(20):11998–2004.
Favero TG. Sarcoplasmic reticulum Ca(2+) release and muscle fatigue. J Appl Physiol (1985). 1999;87(2):471–83.
Shearer J, Graham TE. New perspectives on the storage and organization of muscle glycogen. Can J Appl Physiol. 2002;27(2):179–203.
Sacchetto R, Bovo E, Donella-Deana A, Damiani E. Glycogen- and PP1c-targeting subunit GM is phosphorylated at Ser48 by sarcoplasmic reticulum-bound Ca2+-calmodulin protein kinase in rabbit fast twitch skeletal muscle. J Biol Chem. 2005;280(8):7147–55.
Prats C, Gomez-Cabello A, Hansen AV. Intracellular compartmentalization of skeletal muscle glycogen metabolism and insulin signalling. Exp Physiol. 2011;96(4):385–90.
Graham TE. Glycogen: an overview of possible regulatory roles of the proteins associated with the granule. Appl Physiol Nutr Metab. 2009;34(3):488–92.
Graham TE, Yuan Z, Hill AK, Wilson RJ. The regulation of muscle glycogen: the granule and its proteins. Acta Physiol (Oxf). 2010;199(4):489–98.
Hoffman NJ, Whitfield J, Janzen NR, Belhaj MR, Galic S, Murray-Segal L, et al. Genetic loss of AMPK-glycogen binding destabilises AMPK and disrupts metabolism. Mol Metab. 2020;41:101048.
Janzen NR, Whitfield J, Hoffman NJ. Interactive roles for AMPK and glycogen from cellular energy sensing to exercise metabolism. Int J Mol Sci. 2018;19(11):3344.
McBride A, Ghilagaber S, Nikolaev A, Hardie DG. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab. 2009;9(1):23–34.
McBride A, Hardie DG. AMP-activated protein kinase–a sensor of glycogen as well as AMP and ATP? Acta Physiol (Oxf). 2009;196(1):99–113.
Rauch HG, St Clair Gibson A, Lambert EV, Noakes TD. A signalling role for muscle glycogen in the regulation of pace during prolonged exercise. Br J Sports Med. 2005;39(1):34–8.
Karelis AD, Smith JW, Passe DH, Peronnet F. Carbohydrate administration and exercise performance: what are the potential mechanisms involved? Sports Med. 2010;40(9):747–63.
Williams JH, Batts TW, Lees S. Reduced muscle glycogen differentially affects exercise performance and muscle fatigue. Int Scholarly Res Notices. 2013;2013:371235
Matsui T, Soya M, Soya H. Endurance and brain glycogen: a clue toward understanding central fatigue. Adv Neurobiol. 2019;23:331–46.
Matsui T, Soya S, Okamoto M, Ichitani Y, Kawanaka K, Soya H. Brain glycogen decreases during prolonged exercise. J Physiol. 2011;589(Pt 13):3383–93.
Acknowledgements
We would like to acknowledge the contribution to the current project from the Danish Ministry of Culture, the Danish Elite Sports Organization Team Danmark and by The Novo Nordisk Foundation grant to Team Danmark (the PRoKIT research network).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The work was supported by the Danish Ministry of Culture (Grant no. FPK.2016-0054), the Danish Elite Sports Organization Team Danmark and by The Novo Nordisk Foundation grant to Team Danmark.
Conflict of interest
No conflicts of interest to declare.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
JFV-L and MM conceived the article. JFV-L conducted the literature search and wrote the first manuscript drafts. All authors made substantial contributions to the conception of the manuscript including critical revisions and contributions. All authors read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Vigh-Larsen, J.F., Ørtenblad, N., Spriet, L.L. et al. Muscle Glycogen Metabolism and High-Intensity Exercise Performance: A Narrative Review. Sports Med 51, 1855–1874 (2021). https://doi.org/10.1007/s40279-021-01475-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-021-01475-0