Abstract
Upper tract urothelial carcinoma is a rare disease with complex treatment pathways. This disease typically affects elderly patients, except in those with hereditary disorders, and is associated with tobacco use and other environmental exposures. Patients can present with hematuria or renal colic due to obstruction, and the diagnosis is often made via imaging and endoscopic biopsy. Treatment modalities vary widely based on histology, location of disease, clinical stage, and patient comorbidities and may include endoscopic resection, intracavitary chemotherapy, radical resection, and systemic therapies. As this is a rare disease, there is no current consensus on surgical approach, bladder cuff management, or use of neoadjuvant chemotherapy. Systemic therapy with either chemotherapy or immunotherapy is the treatment of choice for metastatic disease, and metastasectomy may be indicated in some cases. Due to the high rates of bladder and systemic recurrence, these patients require close surveillance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Background
Epidemiology
Upper tract urothelial carcinoma (UTUC) accounts for approximately 10% of all urothelial cancers [1, 2]. Renal pelvis tumors are twice as common as ureteral tumors, and concomitant carcinoma in situ (CIS) occurs in up to 31% of cases [1]. This is usually a disease of the elderly with a mean age around 70 years [1]. Around 60% of patients present with invasive disease, including 10% with metastatic disease [1]. Histologically, pure urothelial carcinoma is most common, but variant histological subtypes are observed in up to 25% of cases, with squamous differentiation being the most common [3]. Variant histological subtypes are often associated with worse prognoses, especially the sarcomatoid, micropapillary, and small cell variants [3, 4].
Urothelial carcinoma (UC) can affect the entire urothelium and tends to be a multifocal disease. Consequently, it is not uncommon for patients to be diagnosed with synchronous or metachronous UC of the upper and lower tracts [5]. About 10% of patients with history of bladder cancer develop UTUC, whereas up to 50% of patients with UTUC subsequently develop bladder cancer [5]. Thus, depending on the patient’s risk status, assessment of the entire urothelium may be needed at various intervals during the management and surveillance of patients with UC.
Risk Factors
Tobacco use, aristolochic acid (AA) intake, alcohol consumption, occupational exposures (aniline dyes), anti-inflammatory medication (phenacetin), chemotherapeutics (cyclophosphamide), and genetic disorders are all risk factors for developing UTUC.
Tobacco use increases the risk of developing UTUC by as much as six-fold, and the risk increases with cumulative smoking exposure [6]. In addition, continued smoking is associated with worse prognosis in patients with a UTUC diagnosis, and smoking cessation can mitigate these adverse outcomes [7].
Aristolochic acid is a substance found in plants that has been attributed to advanced renal disease and UTUC. It irreversibly injures the renal cortex, leading to extensive interstitial fibrosis and end-stage renal disease [8]. Its exposure can either be through environmental contamination of agricultural products by aristolochic contaminated plants or ingestion of aristolochic based herbal remedies [8, 9]. The former is the likely etiology in cases of Balkan endemic nephropathy, where exposure to the substance occurs through contamination of locally grown wheat, while the latter was identified in individuals consuming high doses of AA in a herbal mix used in weight loss clinics [8, 10]. The association with UTUC was suspected in early reports of cellular atypia in patients with aristolochic induced nephropathy and case reports of patients with aristolochic induced nephropathy who developed urothelial cell carcinoma [11, 12]. One later study reported that up to half of patients with aristolochic induced nephropathy are subsequently found to have urothelial carcinoma [13].
Alcohol consumption has also been linked to UTUC. In a case-control study, alcohol consumption was significantly higher in patients with urothelial carcinoma (OR 1.23). Strengthening the causal link, a dose-response was observed with higher consumption associated with increasing risk [14].
Exposures to other substances such as analgesics, cyclophosphamide, and arsenic have been weakly associated with increased risk of developing UTUC, but results have been inconsistent [6, 9, 15,16,17].
Genetic Factors
The molecular changes observed in patients with UTUC are similar to those seen in bladder cancer [18]. The most common mutations seen in sporadic UTUC include alterations in FGFR3, KMT2D, KDM6A, STAG2, cdkN2A, TP53, PIK3CA, and TSC1 [19]. Additionally, mutations in the mismatch repair process can be associated with UTUC in patients with Lynch syndrome and can be present in around 5% of patients diagnosed with UTUC [20].
Lynch Syndrome
Upper tract urothelial carcinoma is the third most common cancer in Lynch syndrome [21]. Lynch syndrome is caused by germline mutations in the mismatch repair genes MLH1, MSH2, MSH6, or PMS2. Mutations in these genes lead to microsatellite errors during replication [22]. These patients are up to 22 times more likely to develop UTUC compared to the general population [21]. The Amsterdam Criteria I and II can be used to help identify families who are likely to have Lynch syndrome [23]. According to the EAU guidelines, the diagnosis of Lynch syndrome should be suspected in patients with one or more of the following: (1) metachronous Lynch syndrome-related cancer (colorectal, endometrial, ovarian, stomach, small intestine), (2) age <65 years with UTUC, (3) first-degree relative with a Lynch-related cancer younger than 50 years of age, or (4) two first-degree relatives with Lynch syndrome-related cancer [24]. If any of the above is suspected, patients can be referred for germline testing and individual/family genetic counseling for follow-up and evaluation for other Lynch-related cancers.
Diagnosis
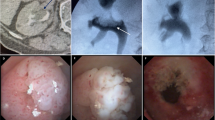
Evaluation for UTUC is most often prompted by presenting symptoms. Symptoms of UTUC can include microscopic hematuria, gross hematuria, flank pain, or constitutional symptoms in advanced cases [24]. The gold standard cross-sectional imaging test is a CT scan with intravenous contrast and delayed images (CT urogram), where the classic finding is a filling defect on delayed imaging (Fig. 20.1). CT urogram has high accuracy for diagnosing UTUC, with around 92% sensitivity and 95% specificity [25]. In patients who cannot undergo CT imaging, MRI with delayed imaging is also an option, though the sensitivity is lower at around 63% [26].
Another critical tool used in the diagnosis, management, and surveillance of patients with UTUC is cystoscopy. Assessing the bladder mucosa is important, as concomitant bladder cancer is present in up to 30% of patients presenting with UTUC [27]. Once the diagnosis of UTUC is suspected, pathological evaluation is important in determining management options. Pathologic specimens are obtained through either ureteroscopic biopsy or percutaneous biopsy. Ureteroscopy has the benefit of allowing direct visualization of the tumors (Fig. 20.2) and multiple techniques for obtaining tissue. These include ureteral brush biopsy, forceps biopsy, or basket biopsy [28]. Percutaneous biopsy approaches are typically performed by interventional radiology teams using ultrasound or CT-guided approaches. Though case reports exist describing possible tumor seeding after biopsy, studies have reported extremely low rates of seeding with percutaneous biopsy making this approach a good alternative when endoscopic biopsy attempts have failed [29, 30].
Urine tests can also be used in the evaluation of these patients. Urine cytology is less sensitive for UTUC than for bladder cancer; however cytology obtained from barbotaged urine can have sensitivities over 90% [31]. Fluorescence in situ hybridization (FISH) analysis is another urine-based biomarker, but the performance is suboptimal, with a sensitivity of 50% [32].
Enhanced Imaging Modalities
In cases where the suspected area is not clearly visualized, visualization can be supplemented with enhanced imaging modalities. These enhanced imaging modalities have demonstrated efficacy in the diagnosis of patients with urothelial cancer of the bladder, though there is less data available regarding use in the upper tracts [33, 34].
While the use of enhanced ureteroscopy in the upper tracts is not typical, some data suggest it may be a useful adjunct. Kata et al. assessed the diagnostic ability of blue light ureteroscopy and found that it detected more UTUC tumors than both CT urogram and white light ureteroscopy with over 95% of tumors detected on blue light [35, 36]. Narrow-band imaging (NBI) has also been evaluated for use in the upper tracts and has been shown to improve tumor detection by 23% [37]. While these technologies have demonstrated promising results in the detection and localization of UTUC, and especially CIS, their value as it relates to oncologic outcomes will need to be verified in the setting of one or more clinical trials.
Staging
Accurate staging may be difficult, due to limitations in obtaining sufficient samples during biopsy; however there are several nomograms that can be used that can help one predict the probability of invasive or non-organ confined disease with relatively good accuracy [38,39,40]. Staging is done using the TNM classification for staging tumors [41]:
- TX:
-
Primary tumor cannot be assessed.
- T0:
-
No evidence of primary tumor.
Ta: Non-invasive papillary carcinoma
Tis: Carcinoma in situ
- T1:
-
Tumor invades subepithelial connective tissue.
- T2:
-
Tumor invades muscularis.
- T3:
-
Tumor invades beyond muscularis into the periureteric fat (ureter).
Tumor invades beyond muscularis into the peripelvic fat or renal parenchyma (renal pelvis).
- T4:
-
Tumor invades adjacent organs or through the kidney into perinephric fat.
- NX:
-
Regional lymph nodes cannot be assessed.
- N0:
-
No regional lymph node metastasis.
- N1:
-
Metastasis in a single lymph node 2 cm or less in greatest dimension.
- N2:
-
Metastasis in a single lymph node 2 cm or greater in greatest dimension or multiple lymph nodes.
- M0:
-
No distant metastasis.
- M1:
-
Distant metastasis.
Prognosis
There are several factors that have been found to be prognostic in the management of patients with UTUC. In general, prognostic factors can be grouped into three categories: pre-operative/clinical factors, surgical/pathologic factors, and molecular markers [42].
Pre-operative Factors
Certain demographic factors and overall patient health have been found to be prognostic factors for UTUC. Age has been identified as an independent predictor of worse cancer-specific survival (CSS) and overall survival (OS) in patients with UTUC in retrospective studies [38]. In addition, elderly patients have been found to present with more aggressive disease [38, 42]. With regard to ethnicity, some data has suggested poorer survival outcomes in persons of color [1]. Other patient-level factors include poor performance status [39], obesity [43], and tobacco use [7, 40, 44].
There are several disease-specific factors that affect survival as well. Ureteral tumor location and multifocal tumors have been described as factors associated with worse prognosis in retrospective studies [45, 46]. However, studies have been inconsistent with regard to the effect of tumor location on survival outcomes [43]. High tumor stage is a known factor associated with worse outcomes in patients with UTUC, but there are limitations in predicting tumor stage prior to surgery. As discussed previously, several nomograms aim to decrease this limitation by using histological and radiological factors to predict invasive disease [47, 48]. With surgical timing, delay to definitive radical nephroureterectomy (RNU) >3 months in patients with high-risk disease has been associated with more aggressive pathological features and worse recurrence-free survival (RFS) and CSS in some studies, though these results have not been consistent [49,50,51]. Other factors that have been associated with worse oncological outcomes include pre-operative hydronephrosis [52], higher American Society of Anesthesiologist score [53], and previous/synchronous bladder cancer [54].
Surgical/Post-operative Factors
Pathological tumor stage is an important factor for the prognosis of patients with UTUC. Based on several studies, tumor stage has been found to be a significant factor associated with oncological outcomes, with a steep reduction in survival with advanced tumor stage. For instance, examples of 5-year CSS rates for pTa/pT1, pT2, pT3, and pT4 disease are >90%, 70–80%, 50–55%, and 0–35% [45, 55,56,57,58]. Tumor grade is also an established factor associated with survival, where high-grade disease is significantly associated with worse outcomes compared to low-grade disease [58]. Lymph node involvement is an important factor associated with worse RFS and CSS [55, 56] with lymph node-positive patients demonstrating significantly worse 5-year RFS and CSS, compared to patients with no evidence of lymph node involvement (29.0% vs 73.4% and 35.3% vs. 77.3%, respectively) [58]. Lymphovascular invasion is found in approximately 20% of patients after RNU and is associated with a greater risk of mortality and recurrence [59]. Another operative outcome that has important prognostic implications is the status of the soft tissue surgical margin at the time of RNU. In a multicenter review, a positive margin was associated with worse metastasis-free survival and CSS [60]. Other factors that have been associated with worse oncological outcomes include sessile tumor growth [61], larger tumor size [62], tumor multifocality [56], and tumor necrosis [63].
Molecular Markers
The prognostic role of multiple biomarkers has been investigated in UTUC. However, the rarity of the disease limits definitive conclusions related to these biomarkers. Examples of the types of markers include cell adhesion molecules (E-Cadherin, Beta-Catenin, Parvin-Beta, CD24), markers associated with microsatellite instability, cell differentiation (Uroplakin III), angiogenesis (HIF-1 alpha), cell cycle regulation (p53), cell proliferation (Ki-67, EGFR), apoptosis (Bcl-2), vascular invasion, PD-1 pathway, and inflammatory cells (C-reactive protein, leukocytes) [42].
Management
The management of patients with localized UTUC is typically dictated by the patient’s tumor risk status as well as the patient’s comorbidities. Risk stratification into low- or high-risk groups can typically be determined by the tumor’s histological and radiographic appearance [24]. Some studies and guidelines have suggested risk stratification as follows [24, 64]:
-
High-risk
-
Hydronephrosis
-
Tumor >2 cm
-
High-grade cytology
-
Multifocal
-
Prior radical cystectomy for high-grade disease
-
Variant histology
-
-
Low-risk
-
Unifocal
-
Tumor <2 cm
-
Low-grade cytology
-
Low-grade biopsy
-
Non-invasive on imaging
-
Management of localized UTUC can be split into two categories: kidney-sparing approaches and radical surgery. Low-risk patients are typically better suited for kidney-sparing techniques which include endoscopic procedures, percutaneous tumor resection, segmental ureteral resection, and intracavitary chemotherapy instillation.
Endoscopic Management
Endoscopic procedures including ureteroscopy with tumor ablation, fulguration, or resection are commonly used for both diagnostic and therapeutic purposes in patients with UTUC. Multiple studies have demonstrated the efficacy of endoscopic management, but most are limited by a retrospective and single-institutional design. In one study with a median follow-up of 54 months, 68% of patients had disease recurrence, and 19% eventually proceeded with RNU [65]. Fortunately, the vast majority of these recurrences (approximately 75%) can be retreated endoscopically using close surveillance strategies [66]. Therefore, appropriately selected patients with UTUC can be managed with endoscopic surgery, but recurrences often occur and about one-third of patients may be at risk for progression and eventually need radical nephroureterectomy.
A Special Note on Ureteroscopy
When ureteroscopy is planned as the management option in a patient with UTUC, there are a few key points to consider. The location of the tumor will dictate what kind of ureteroscope is used (i.e., flexible or semirigid). We prefer to start assessments by first placing a safety wire into the collecting system. If a small tumor is anticipated, a flexible ureteroscope can be advanced under fluoroscopic guidance over the wire to the area of disease taking care to ensure that the entire collecting system and ureter are inspected at some point during the case. The morphology and size of the tumor dictate the appropriate treatment option once the tumor is visualized. For tumors that appear papillary and on a stalk, a ureteroscopic biopsy forceps or stone basket can be used to remove the tumor. The remaining tumor burden should be ablated with holmium:YAG, neodymium:YAG, thulium laser ablation, or Bugbee fulguration. If a semirigid ureteroscope is used for a ureteral tumor, a ureteroscopic resectoscope can be used [67, 68]. This is helpful in cases with flat tumors or when the above options are not adequate for sampling. After completion of the treatment, a ureteral stent should be considered in cases of extensive manipulation, resection, or injury to the collecting system.
Percutaneous Surgery
When tumors are too large to be managed by ureteroscopy and renal preservation is desired, a percutaneous approach can be considered. Percutaneous access is obtained in standard fashion prior to the surgery or at the time of surgery by ultrasound or fluoroscopic approach. The percutaneous tract is then dilated to 30 Fr using balloon dilation system. Next, a rigid nephroscope is placed through sheath and used to identify and remove the tumor using a cold cup forceps [28]. If needed, a resectoscope can be used to resect and fulgurate the base of the tumor. Additional small tumors may occur throughout the collecting system and can be managed using a flexible nephroscope and ablative techniques. A second-look nephroscopy is often done a few days after the index surgery to ensure all tumor has been treated [28]. An 8–14 Fr nephrostomy tube or a ureteral stent can be left in place at the conclusion of the surgery at the surgeon’s discretion.
Segmental Ureteral Resection
When there is a large ureteral tumor burden that cannot be addressed endoscopically and/or renal preservation is imperative, segmental ureteral resection can be considered. In terms of efficacy of this approach, a population-based study found that 5-year cancer-specific mortality rates were similar between the RNU and segmental ureteral resection and this was not conditionally changed on multivariable analysis [69]. However, not unexpectedly, there are some data to suggest that this approach is associated with higher risk of recurrence. This is clearly due, at least in part, to the risk of metachronous recurrences in urothelium which is otherwise removed with RNU. In a systematic review and meta-analysis of 4797 total patients, segmental ureterectomy was associated with a shorter 5-year RFS despite presenting with lower stages and grades [70]. Thus, segmental resection is likely associated with a greater risk of recurrence compared to radical surgery, especially in patients with high-risk features.
Intracavitary Instillation
Adjuvant Therapy
UTUC has the propensity to recur, and studies have investigated methods to reduce the risk of recurrence, with similar goals as the methods used in bladder cancer. Intravesical adjuvant instillation with either chemotherapy or BCG is effective in reducing the risk of recurrence in patients with non-muscle-invasive bladder cancer [71]. Similar concepts have been investigated in UTUC with inconsistent results. However, the data are mostly from nonrandomized retrospective comparisons which are heavily biased toward the null due to confounding by indication [58, 72, 73]. Therefore, it is unclear what the true benefit of topical therapy is in the adjuvant setting.
Primary Ablative Therapy
MitoGel
For select cases when surgical resection is not an option, topical therapy can be considered as a primary ablative option. A recently published open-label, single-arm, phase 3 trial evaluated the safety and efficacy of a mitomycin-containing reverse thermal gel as a primary chemoablative agent for low-grade tumors. Seventy-one patients received at least one dose via retrograde stent (4 mg/mL, dosed according to volume in patient’s collecting system). Fifty-nine percent of these patients achieved a complete response on ureteroscopy at 3 months, and 70% showed durability at 12 months. This treatment was associated with some adverse effects including ureteral stenosis in 44% of patients [74]. Chemoablative therapy can be considered in patients with low-grade, large volume UTUC above the ureteropelvic junction where endoscopic management may be difficult and/or in those with significant comorbidities who cannot tolerate a major operation.
Radical Surgery
Radical nephroureterectomy remains the treatment of choice for many patients with UTUC. It is most often used in cases of patients with high-grade disease, independent of location, but is also used for those with low-grade tumors with high-risk features.
Low-Grade Disease
Radical nephroureterectomy is indicated as first-line treatment in patients with low-grade tumors with high-risk characteristics. These include hydronephrosis, size greater than 2 cm, multifocality, prior radical cystectomy for bladder cancer, or variant histology [75, 76]. These factors increase the risk of higher stage at time of surgery and need for more aggressive initial treatment for oncological safety. Recurrence-free survival and cancer-specific survival in patients with low-grade disease undergoing RNU are 88.3% and 89.1% at 5 years, respectively [58]. In addition, those with early recurrence despite adequate kidney sparing therapy may benefit from subsequent radical RNU. Those patients who fail initial endoscopic management and proceed to have RNU have similar 5-year OS compared to those who had immediate RNU (64% vs. 59%, respectively), and 43% had progression to, or occult, high-grade disease [72]. Additionally, RNU may be most appropriate for patients who are unable to have close follow-up [75, 76].
High-Grade Disease
The primary role of RNU is in those with localized high-grade disease. Margulis et al. showed a RFS and CSS for those with high-grade disease to be 57.2% and 63.1% at 5 years, respectively [58]. Patients found to have pT3 disease at time of RNU had a RFS of 48.0% and CSS of 54.0% at 5 years [58]. Those with pT4 had a RFS and CSS probability after RNU of 4.7% and 12.2% at 5 years, respectively [58].
Pre-operative Considerations
Preparing a patient to undergo RNU requires careful planning and patient education. Prompt surgery after diagnosis is important to avoid disease progression due to delays in treatment. We hesitate to argue for a definitive timepoint during which surgery should be performed since the predicted risk of metastatic disease increases with each day, but the general rule from the literature is that surgery should be performed within 12 weeks [50, 51, 72, 73, 77]. However, it is not likely that surgery at 11 weeks is substantially different in terms of risk than surgery at 13 weeks, and, in general, we advocate for surgery as soon as possible since delayed surgery is associated with decreased OS [73, 77].
Patients should have pre-operative evaluation with their primary care doctor for clearance and medical optimization. Patients who are actively smoking at time of diagnosis should be counseled on risks, and tobacco cessation should be encouraged. Tobacco use not only increases the risk for recurrence and mortality after RNU but is also associated with poor healing and risk of blood clot [7, 78]. Nutritional status should be optimized, diabetic sugar control evaluated, and obese patients encouraged to lose weight prior to surgery. Other important comorbidities that may contribute to anesthetic risks or post-operative recovery should be appropriately evaluated and treated prior to undergoing surgery.
Antimicrobial prophylaxis is recommended in patients undergoing renal surgery, and we typically use a single dose of a second-generation cephalosporin prior to incision. Urinalysis and culture prior to surgery and evaluation of prior positive cultures can aid in antibiotic selection. Any positive culture must be treated prior to surgery.
All patients undergoing RNU should be counseled on risk of dialysis. There is low risk of progression to dialysis in those patients with normally functioning kidneys and overall good health. However, loss of nephrons and chemotherapy may lead to chronic kidney disease (CKD) in even the healthiest of patients. Patients with GFR <60 mL/min, solitary functioning kidney, or proteinuria are referred to nephrology prior to surgery in our practice [79].
Pre-operative planning also requires evaluation of bloodwork and review of imaging. Complete blood count and basic metabolic panel should be obtained prior to surgery. A type and screen should be performed on all patients, and blood products should be prepared prior to start of surgery for certain cases. Pre-operative review of abdominal imaging is essential to identify number of ureters, arteries, and veins and prepare for any aberrant anatomy. Staging studies of the chest prior to surgery are critical as well.
Surgical Technique
Outcome Comparison for RNU Technique
Choosing a method for RNU depends on the surgeon’s comfort with the approach and patient characteristics. Current techniques include open, laparoscopic, and robot-assisted techniques. Whether open or minimally invasive, adherence to basic oncologic principles is paramount to decrease recurrence rates. Strict avoidance of entry into the urinary tract during dissection and complete ureteral resection is essential. Contact between tumor and instruments should be avoided, and the specimen must be removed en bloc using a protective system such as endobag to prevent tumor spillage or seeding [80]. Removal of the entire tumor with a clear margin is essential, as a positive surgical margin is a poor prognostic factor after RNU. The 5-year CSS and metastasis-free survival in patients with positive surgical margin compared to those with negative margin were shown to be 59.1% vs. 83.3% and 51.6% vs. 79.3%, respectively [81].
With the advent of minimally invasive surgery and increasing use of robotics in surgery, several groups have compared oncologic outcome and surgical outcomes based on surgical approach. While the quality of evidence varies, the results of these studies showed that RFS, CSS, OS, and bladder specific recurrence do not significantly differ based on open, laparoscopic, or robotic approach [82,83,84,85,86,87]. Walton et al. showed comparable outcomes between open and laparoscopic RNU; however, the laparoscopic RNU group had fewer node-positive patients compared to the open RNU group (2.9% vs. 6.8%, p = 0.041) [83]. One limitation of these studies is possible selection bias with physicians choosing laparoscopic approach for those with expected N0 disease or smaller tumors. When looking specifically at locally advanced disease (pT3/pT4), data is conflicting [88]. Ariane et al. showed 5-year RFS and CSS in pT3/pT4 patients who had laparoscopic RNU were comparable to open RNU [85]. Conversely, one of the few randomized prospective studies showed a significant difference in metastasis-free survival and CSS between open and laparoscopic RNU [89]. A total of 80 patients were randomized to either open or laparoscopic RNU, and, in patients with high-grade or pT3 disease, significantly better oncologic outcomes were seen in the open RNU group [89]. There is less available literature on comparison of robotic RNU to other approaches, but data suggest equivalent oncologic outcomes [86, 87, 90]. However, robotic RNU is associated with higher frequency of concurrent lymph node dissection when compared to laparoscopic RNU and fewer positive surgical margins when compared to open RNU [87]. Robotic-assisted RNU may become the surgical technique of choice with increasing access to robotic systems and training in residency as it does not seem to compromise oncologic control for most patients and enables lymphadenectomy compared to laparoscopic approaches. A final concern unique to minimally invasive approaches is trocar site or peritoneal seeding from pneumoperitoneum used for visualization. A few studies have reported rare retroperitoneal and trocar site tumor deposition after laparoscopic surgery, but departures from sound oncologic surgical principles were the likely cause in these cases [91, 92].
Minimally invasive and open RNU differs slightly in terms of peri-operative factors but has similar rates of complications. Blood loss appears to be higher in open RNU and surgical time longer in minimally invasive RNU [84, 85]. Time to discharge is significantly lower in laparoscopic or robotic RNU compared to open RNU, but robotic RNU is associated with the highest in-hospital costs [86, 89]. Post-operative complication rate after RNU is about 15%, and incidence does not differ based on operative approach [85]. Complications observed include wound infections, post-operative bleeding, ileus, incisional hernia, and pneumothorax [84]. The majority of complications (8.9%) observed were Clavien I and II, and a total of 4.3% of patients had Clavien III or higher complications [85].
Bladder Cuff Resection
Approach to bladder cuff management is as varied as the approach to RNU. Oncologic surgical principles again are key to prevent tumor spillage and ensure good outcomes. Due to the vast number of approaches to bladder cuff management, the goals in this section will be to review those most frequently used. Depending on the technique planned, the ureter is typically clipped as distally as possible during the nephrectomy portion to prevent tumor spillage. In some cases, applying two clips and using electrocautery to transect the ureter between clips can be used. Cautery is thought to destroy any tumor cells at the site of transection and prevent tumor spillage. For large distal tumors that involve the ureteral orifice or protrude into the bladder, larger bladder cuff, en bloc partial, or radical cystectomy may be required to achieve an appropriate oncologic resection.
Endoscopic
There are multiple endoscopic approaches to bladder cuff management including the “pluck” technique and the intussusception technique as well as variations upon these techniques [93]. The “pluck” technique or transurethral resection of ureteral orifice should only be performed in the absence of bladder tumors and can be used for patients with proximal UTUC [94]. For all endoscopic techniques, the patient should be placed in dorsal lithotomy position. A resectoscope is inserted, and the bladder inspected to ensure intravesical absence of areas suspicious for tumor prior to initiating resection. The ureteral orifice on the ipsilateral side of disease is identified and resected aggressively with Collins knife through the intramural tunnel until fat is visualized. The patient is then repositioned for the nephrectomy portion, and once the ureter is dissected more proximally, it is able to be “plucked” as the distal dissection has already been performed. While this is not our preferred technique, operative time is reported to be the principal advantage of this technique compared to other bladder cuff techniques [95].
Another commonly described endoscopic technique is the intussusception technique. This is only indicated for tumors of the renal pelvis as it requires division of the ureter. A ureteral catheter is placed within the ureter, and, after dissection of the ureter and completion of the nephrectomy, two ties are placed around the distal ureter and the ureter is divided between. A resectoscope is then inserted and using a Collins knife, the bladder cuff incision is made. After the incision is made, the ureteral catheter is pulled into the bladder to allow for the ureter to intussuscept into the bladder and catheter, and specimens are then removed through the urethra. This approach is associated with an 18.7% failure rate for complete ureteral removal, and 15.6% of patients required a second incision for ureteral excision [96].
Open Technique
The open technique for bladder cuff resection can be used with either open or minimally invasive approaches for the nephrectomy portion of the surgery. The principal rationale for this technique in minimally invasive cohorts is that an open incision is going to be required for extraction anyhow. If a midline laparotomy or thoracoabdominal incision is not used, a second incision such as a Pfannenstiel, Gibson, or low midline incision can be used to access the bladder and ureter. After making the incision and bluntly dissecting the space of Retzius, the distal ureter is identified at the location where it crosses over the common iliac artery. Previously placed ureteral clips should be located after dissecting peritoneum off the ureter and vessels and mobilizing the ureter. The ureter is then dissected toward the intramural tunnel, ligating the superficial pedicle of the bladder to allow for better visualization and access to the intramural tunnel. If performing a completely extravesical approach, the ureter is dissected away from the detrusor muscle through the intramural tunnel until there appears to be a circumferential area of bladder mucosa surrounding the ureter. The bladder must be completely drained prior to resecting the bladder cuff. It is helpful to apply a stay stitch at one end of the planned cystotomy to prevent tissue from retracting. We have also found that placing Shallcross or other clamp across the planned bladder cuff and dividing on the bladder side of the clamp prevent tumor spillage and aid in manipulation of the tissues. Prior to division of the bladder cuff, it is important to ensure the contralateral ureteral orifice will not be resected. For a transvesical technique, the bladder is drained after dissection of distal ureter and anterior cystotomy made. The ipsilateral ureteral orifice is identified and dissected with a 1 cm bladder mucosal margin around the orifice. All cystotomies are then closed in a two-layer fashion using absorbable suture.
Laparoscopic Technique
Pure laparoscopic management of bladder cuff can be approached transvesically or via endoscopic stapler, though neither of these are our preferred technique. The transvesical technique is a minimally invasive approach that mimics the en bloc resection principles of open bladder cuff management [97]. The patient is first placed in lithotomy and bladder distended after inserting cystoscope. One to two 5 mm ports are placed into the bladder under direct visualization. The ureteral orifice is controlled by passing an endoloop around the opening. A ureteral catheter is then passed into the ureter to identify the intramural portion and ensure adequate bladder cuff excision. A Collins knife is typically used to incise the bladder cuff until adipose tissue is seen ensuring entry into the extravesical space. The orifice is then grasped with laparoscopic instrument and retracted into the bladder so the endoloop can be passed more proximally along the ureter. The ureteral catheter is then removed and endoloop tightened to prevent tumor spillage. This is a technically difficult option, and history of prior pelvic radiation or concurrent UCC of the bladder is a contraindication to this approach [98].
The laparoscopic stapling technique is used by many physicians due to the decreased operative time and avoidance of urinary tract entry. Prior to the laparoscopic portion of the case, cystoscopy is used to cauterize the ipsilateral ureteral orifice. Once the patient is repositioned and laparoscopic ports placed, the nephrectomy is performed prior to dissection of the distal ureter. The ureter is then dissected to intramural tunnel, ligating the lateral pedicle as needed. The detrusor is dissected off the ureter by applying traction to the ureter. Once dissection is completed, the ureter should be retracted as much as possible without avulsing the ureter with hopes of retracting a rim of bladder mucosa through the dissected intramural tunnel. The endovascular stapler is then placed as distally as possible. Some downsides of using this technique include incomplete pathologic evaluation of the bladder cuff and distal margin due to staples and high risk of incomplete bladder cuff and distal ureteral resection [99]. Using stapling device also poses the theoretical risk of stone encrustation of the staple line. A recent study by Tsivian et al. reported data on a variation of the laparoscopic technique in which a LigaSure device is used rather than stapler to avoid this issue [100].
Robotic Technique
Robotic RNU and bladder cuff excision have been increasing in popularity due to access to robotic equipment and increased robotic surgical training. While some studies report the transvesical technique with second cystotomy used to intravesically dissect the intramural ureter, most studies report use of an extravesical technique which is our preferred approach [101]. Patients are placed in a supine position with the arms tucked and the table flexed and rotated. This permits a side-docking approach and easy rotation of the boom when using the Da Vinci XI system. Ports can be placed in the traditional RNU template when planning on single docking (RNU and bladder cuff performed without repositioning the robot – though we do not hesitate to re-dock when exposure is suboptimal). For this technique, 8 mm ports are placed in a row after catheter insertion and bladder decompression (Fig. 20.3). The most superior port is placed about 2 fingerbreadths below the costal margin at the mid-clavicular line. As the more inferior ports are placed, each should be placed slightly more medially than the previous port to allow for bladder cuff dissection. If robotic stapler is planned to be used on the hilar structures, the larger 12 mm robotic port is often placed just left of the camera port to accommodate size of stapler. Inner cannula can be used to accommodate smaller instruments when stapler is not on the field. Assistant ports are placed along midline with 10–12 mm port just above the umbilicus and 5 mm port about 1 handbreadth superior to this. Adjustments may be needed depending upon patient size to avoid arm collisions. Other techniques have described success with assistant port placement in line with robotic trocars [102]. The single docking is beneficial in that it does not require reposition or more port placement, but this port positioning can make bladder cuff dissection more difficult, especially with the older Davinci Si models. A double docking technique, conversely, does require robotic repositioning and increased number of ports but improves bladder access and visualization. With the advent of the Da Vinci XI, the rotation of the boom is a very efficient process and can enable better triangulation in the pelvis when performing the distal ureteral dissection. For a right-sided RNU, the camera port is placed just above the level of the umbilicus with two robotic ports placed to the left of the camera about one handbreadth apart and one robotic port to the right of the camera (Fig. 20.3). These should all be placed close to the level of the umbilicus. Assistant ports are placed on the patient’s right. This set up is mirrored for left-sided nephroureterectomies.
Robotic port placement for nephroureterectomy. Dotted line indicates mid-clavicular line. Circular ports are robotic port sites, square ports are 5 mm ports, and triangular ports are 12 or 15 mm ports. The top image depicts port placement for single docking approach. Image depicts port placement for bladder cuff
No matter the port placement, the dissection and bladder cuff management are the same. After ports are placed, the ureter is identified as it crosses the common iliac and is dissected distally, ligating the lateral pedicle to the bladder for visualization. It is helpful to use the fourth arm to retract the bladder to the contralateral side for better visualization (Fig. 20.4). Once the intramural tunnel is identified, a clip is placed as distally as possible on the ureter to prevent tumor spillage during the subsequent dissection. The ureter is then placed on traction, and the detrusor is dissected off until a rim of bladder mucosa is seen clearly around the ureter. At this point, an absorbable stitch is placed at the superior aspect of the planned bladder cuff and tied. The bladder is drained via catheter. The fourth arm can be helpful in either retracting the bladder or retracting the suture upward. Bladder cuff incision is then initiated using robotic scissors and electrocautery as needed. After opening the incision partially, it can be helpful to close the cystotomy using the previously placed suture while visualization is optimal. In addition, the contralateral ureteral orifice can be identified through the cystotomy to prevent injury. Bladder cuff excision is completed alternating between incision and closure until the ureter is completely dissected free. The cystotomy is then closed in a second layer using absorbable suture. After this, a second set of robotic ports can be placed if using the double docking technique, and the surgeon will proceed with the nephrectomy portion of the procedure.
Robotic extravesical bladder cuff technique – (a) initial dissection of distal ureter and landmark anatomy. (b) Ureter is placed on traction to facilitate dissection of detrusor off the intramural tunnel. (c) Prior to incising the bladder, stitch is placed at the superior extent of the cystotomy to prevent retraction of the mucosa after incision is made. The fourth arm is placed on the bladder for better visualization. (d) View of cystotomy after a single layer of closure
Outcome Comparison
At present, there is no clearly superior bladder cuff technique from an oncologic perspective [93]. Data on bladder cuff recurrence vary based on the study and are likely dependent on operator skill and comfort level with the technique, disease characteristics, and length of follow-up. Current research suggests that recurrence within the bladder, which typically presents within the first 3–4 years after surgery, occurs in 21–40% of patients after RNU but is seen more frequently (30–64%) in patients with incomplete ureteral or bladder cuff excisions [103,104,105,106]. Therefore, complete resection is likely more important than the specific technique. There is some consensus, however, with multiple studies showing increased risk of bladder cuff recurrence in patients undergoing laparoscopic or endoscopic approaches [107, 108]. Endoscopic approaches, in particular, have fallen out of favor due to concerns regarding inadequate distal ureteral resection, prolonged exposure of the bladder to ureteral mucosa in the case of the intussusception technique, and seeding of the extravesical space [94, 96, 98, 105, 109, 110].
There is minimal data on the robotic bladder cuff approach with regard to bladder recurrences and oncologic outcomes. However, early studies report comparable OS and CSS as well as intravesical recurrence [86]. Looking at retroperitoneal recurrence and distant metastases, there appears to be no difference between the endoscopic and open techniques [111]. In addition, RFS and CSS do not appear to differ between open and endoscopic bladder cuff techniques [95]. Another factor to evaluate when comparing oncologic outcomes and prognosis based on distal bladder cuff management is positive margin rate [112]. A comparison of current bladder cuff literature by Phe et al. showed that the highest reported positive margin rates were for the laparoscopic stapling technique with rates as high as 25% [112]. As previously discussed, positive margins are a predictor for poor prognosis, suggesting that the laparoscopic technique is possibly inferior. Therefore, while there is no clear data to suggest a superior technique, laparoscopic stapling and endoscopic techniques should be used with caution due to higher rates of bladder recurrences and positive surgical margins. The open technique has not consistently been shown to be superior, but due to the need for extraction site incision for the kidney, this technique may provide the highest level of oncologic control with the lowest learning curve and without subjecting the patient to excess incisions, prolonged operative time, or repositioning.
Post-operative Care
Post-operative care for patients status post RNU follows typical renal surgery pathways. Patients who underwent flank incision or other open surgery approach may require a longer duration of pain control measures. Pain management should be multi-modal including acetaminophen, opioids, muscle relaxants, or other adjunct measures such as topical thermal therapy or abdominal binder. A multi-modal approach allows for decreased use of opioids and earlier mobility. In some cases, epidural or patient-controlled analgesia may be necessary.
Diet and activity should be initiated early. Clear liquids on the day of surgery with progression to solids as tolerated are the typical diet progression for patients regardless of operative approach. Patients should be encouraged to ambulate the day after surgery. Early ambulation and normal diet can lead to earlier discharge and improved recovery.
Thromboembolic and respiratory prophylaxis is essential for a successful recovery. Sequential compression devices or thromboembolic deterrent stockings (TED) are recommended post-operatively to avoid venous thromboembolic (VTE) events. Patients at higher risk for VTE should be placed on pharmacologic prophylaxis such a subcutaneous heparin in combination with mechanical prophylaxis measures. Early activity is also protective against VTE. Patients who undergo laparoscopic or open surgeries will often take shallow inspirations due to pain and may have decreased respirations secondary to opioid use. Deep breathing and use of incentive spirometer are recommended to prevent atelectasis and respiratory infection.
Catheter management is partially dependent on bladder cuff approach and concerns for urine leak post-operatively. Jackson-Pratt drain is useful when placed intraoperatively to monitor for urine leak and fluid output. It is our practice to test drain creatinine the morning of post-operative day 1. If body fluid creatinine of the drain is normal, the drain is removed, and the catheter is removed the following week. A cystogram is often performed prior to catheter removal to ensure watertight bladder cuff closure and adequate healing prior to catheter removal. Patients with normal body fluid creatinine at time of discharge may not require a cystogram prior to catheter removal. Cystogram showing extravasation from bladder cuff indicates need of a longer course of drainage with the catheter and serial cystograms prior to removal. In patients with elevated drain creatinine at the time of discharge, longer drain course and monitoring of drain output are essential. Once drain output decreases and body fluid creatinine is consistent with serum, the drain can be safely removed, and cystogram performed. Providers should have high suspicion for urine leak in those patients with failure to progress or those who present with ileus even in presence of previously normal body fluid creatinine.
Lymph Node Dissection
Curative and Diagnostic Role
Lymph node dissection (LND) should be performed at the time of RNU in high-risk tumors. Regional lymph nodes are the most common metastatic site for UTUC, and up to 30% of patients with muscle invasive UTUC will present with positive lymph nodes at the time of surgery [113, 114]. Lymph node dissection for invasive urothelial carcinoma of the bladder is routinely performed as it may provide survival and prognostic benefit [115, 116]. For UTUC, however, it is unclear as to whether lymph node dissection is curative. Conclusions on the curative role of LND in UTUC are limited due to small study population, lack of well-defined patient selection criteria, lack of standardized LND template, and retrospective nature of studies. In one prospective study using standardized LND templates based on tumor location, patients who underwent LND for renal pelvis cancers pT2 or higher had significantly greater OS and CSS compared to those who did not undergo lymphadenectomy (OS 86.1% vs. 48.0%, CSS 89.8% vs. 51.7%) [117]. No survival improvement was seen in tumors localized to the ureter [117]. A large retrospective review of >2800 patients showed that there was no survival difference in patients who had pN0 compared to pNX disease [118]. This study is limited due to lack of standardized templates, retrospective nature, and possible selection bias of physicians choosing not to perform LND on low-risk patients. Multiple studies have shown that patients with muscle-invasive disease who were pN0 had significantly improved survival compared to those who were pNX [119, 120]. In the ≥pT2 population, 5-year disease-free survival and CSS in those who underwent LND compared to those who did not were 64% vs. 37% and 67% vs. 40%, respectively [121]. Therefore, the curative benefit of LND appears to be greatest in patients with muscle-invasive disease and those with enlarged nodes on imaging.
Lymph node dissection for UTUC is an excellent prognostic tool and helps to identify patients who would benefit from adjuvant systemic therapy since positive lymph nodes and extranodal extension are predictive of decreased survival [122,123,124]. Patients with pathologically node-positive disease, regardless of grade, had RFS and CSS rates of 29.0% and 35.3% at 5 years, respectively [58]. Those who have not undergone neoadjuvant chemotherapy with extranodal extension have been shown to have significantly higher disease recurrence (HR 2.0, 95% CI 1.44–2.78) and significantly higher cancer-specific mortality (HR 1.97, 95% CI 1.38–2.81) compared to those with positive lymph nodes without extranodal extension [122]. Therefore, extranodal extension appears to be a more powerful predictor of recurrence and poor survival compared to the number of positive nodes alone. Lymph node density has also been thought to be a prognostic factor with those patients having a lymph node density >30% having poorer outcomes [125]. Recent studies have failed to reproduce this finding [122].
Template
One of the main issues with current lymph node dissection research is lack of a standardized template. Fajkovic et al. showed that the median number of lymph nodes removed in regional lymphadenectomy is four and that there is no prognostic value to the number of nodes removed or number of positive nodes [122]. However, other studies have shown that the number of nodes removed does matter. For instance, to achieve a 75% probability of finding one or more positive lymph node, at least eight lymph nodes must be removed [126]. However, if the appropriate template is used, fewer number of nodes may be needed in the dissection for diagnostic and curative resection.
Regional template largely depends on location of tumor. In a study by Kondo et al., sites of tumor deposition via lymphatic spread are well defined (Table 20.1) [127]. Regional template based on this lymphatic spread should include ipsilateral hilar and adjacent paraaortic or paracaval nodes, for pelvic or proximal ureteral tumors, and should include pelvic nodes, for distal ureteral tumors [120, 127,128,129,130,131,132,133,134]. Extended templates are shown in Fig. 20.5 [117]. This differs from the regional template in the following ways: (1) renal pelvis tumor LND inferior boundary at the IMA, (2) addition of retrocaval nodes for right sided renal pelvis tumors, (3) addition of retrocaval nodes for upper 2/3 ureteral mass, and (4) inferior boundary of aortic bifurcation for upper 2/3 ureteral masses.
Complications
Complications of LND are often related to uncontrolled lymphatic drainage. Comparison between patients who had lymph node dissection at time of RNU compared to RNU alone showed higher incidence of lymphorrhea, chyle fistula, and thigh numbness [117]. The incidence of all grade complications is not increased by addition of LND at time of RNU [117].
Intravesical Therapy After Radical Nephroureterectomy
Patients with UTUC are high risk for recurrence within the bladder. Current research suggests recurrence within the bladder occurs in 21–40% of patients after RNU [103,104,105,106]. Therapies to avoid recurrence are highly sought after to avoid further surgeries, decrease risk for upper tract seeding in the contralateral solitary kidney, and prevent decreased renal function due to ureteral or bladder obstruction of the solitary kidney. Post-operative instillation of intravesical chemotherapy has been shown to decrease intravesical recurrence. Use of intravesical Mitomycin-c (40 mg in 40 mL) at the time of catheter removal after RNU leads to an 11% absolute risk reduction and 40% relative risk reduction of intravesical recurrence [106]. Instillation of piparubicin within 48 hours after RNU also leads to decreased recurrence rates [135]. A meta-analysis of five trials using intravesical chemotherapy after RNU showed similar risk reduction without serious adverse events related to intravesical therapy [136]. Only 20.5% of patients who received post-operative intravesical therapy had bladder recurrence compared to 36.7% having bladder recurrence in those who did not receive intravesical therapy [136]. Factors associated with increased risk of intravesical recurrence include advanced age, male gender, tumor within the ureter, higher tumor stage, concomitant CIS, lymph node involvement, and prior bladder cancer [107, 137]. Intravesical recurrence does not appear to affect CSS or OS in those with muscle-invasive upper tract disease. However, in those with non-muscle-invasive UTUC, bladder recurrence is associated with significantly decreased CSS and OS [138]. Current recommendation on timing of instillation is to perform the instillation within 10 days of surgery [80]. A cystogram may be performed prior to instillation, but is not necessary [106]. Therefore, while it is unclear if survival is improved with the use of intravesical therapy, use of MMC or other intravesical agents does significantly reduce bladder recurrence.
Systemic Chemotherapy
Neoadjuvant
Neoadjuvant chemotherapy (NAC) use in UTUC has been growing in popularity due to increasing studies showing beneficial effects for UC. Multiple retrospective reviews have shown favorable clinical and pathologic response to NAC. After receiving NAC, up to 80% of patients demonstrate clinical response on imaging studies [139]. Neoadjuvant chemotherapy is associated with significantly decreased incidence of advanced disease stage at time of RNU compared to those receiving RNU alone [140,141,142,143]. In a study by Matin et al., pathologic specimens were compared between subjects who were diagnosed with high-grade UTUC and underwent either RNU or NAC followed by RNU. The results showed significant difference in the incidence of pT2 and pT3 or higher-stage tumors favoring the NAC group, when compared to the RNU group (pT2, 48.8% vs. 65.4%; ≥pT3, 27.9% vs. 47.7%) [140]. A second retrospective study reported pathologic downstaging in 27% of patients with high-grade UTUC who received NAC [144]. In addition, pathologic complete response on RNU specimen has been observed in 6–15% of patients after neoadjuvant chemotherapy [140,141,142, 144].
Evaluation of patients with high-grade cT2-4 disease who underwent neoadjuvant chemotherapy showed that those with pathologic downstaging or pathologic complete response had improved OS [144]. In addition, a literature review by Loew et al. pooled results from six studies showing a 56% OS benefit for those receiving NAC [145]. Kubota et al. compared RFS, CSS, and OS in those patients receiving either NAC and RNU or RNU alone with locally advanced UTUC [146]. They found that NAC use is associated with significantly prolonged RFS and CSS but found no significant improvement in OS [146]. In summary, NAC appears to be effective for UTUC in patients with clinically advanced disease, high-grade disease on biopsy specimen, or other high-risk features. Further prospective trials are needed to define selection criteria demonstrating clear effect.
First-line NAC for UTUC is typically cisplatin-based, though carboplatin is frequently used in those patients who cannot tolerate cisplatin. The majority of studies showing improved oncologic outcomes combine patients receiving cisplatin (GC, MVAC, MVEC, MEP), carboplatin, and occasionally docetaxel-based chemotherapy into the NAC group [143,144,145,146]. Oncologic outcomes in these retrospective case control studies between gemcitabine/cisplatin versus gemcitabine/carboplatin have shown no difference in progression-free survival (PFS), CSS, or OS at about 40 months [143, 146]. When looking at quality of life during NAC, gemcitabine/cisplatin is associated with higher rates of gastrointestinal symptoms, decreased physical and functional quality of life, and fatigue compared to gemcitabine/carboplatin [147]. Research on this topic is limited due to infrequent use of neoadjuvant chemotherapy in an already rare disease and retrospective and observational nature of studies. We routinely recommend neoadjuvant chemotherapy in patients with high suspicion of muscle-invasive or locally advanced disease.
Adjuvant
Adjuvant chemotherapy (AC) for UTUC is more widely accepted than NAC due to larger volume of evidence and recent randomized trials showing oncologic benefit. Common chemotherapy used in the adjuvant setting is typically platinum-based though studies have included paclitaxel or other non-platinum-based regimens [145]. Multiple retrospective studies have failed to show that AC provides oncologic benefit in high-risk UTUC patients or have only shown improvement in OS and CSS in small subgroups such as those with LVI [148, 149]. One of the most recent trials, the POUT trial, is a phase 3, open-label, multi-center, randomized control trial of 255 muscle-invasive and/or lymph node-positive UTUC patients without metastases [150]. The patients were randomized to surveillance or four cycles platinum-based AC within 90 days from surgery. At 3 years, the RFS rate in the AC group was 71% compared to only 53% in the RNU alone group [150]. A total of 44% of patients within the AC group developed acute grade 3 or worse chemotherapy-related adverse events including decreased neutrophils or platelets, nausea or vomiting, and neutropenic fever [150]. A second randomized control trial published in 2019 showed improved OS, CSS, and PFS in high-risk UTUC patients who received GC after RNU compared to those who had RNU alone [151]. When looking at the combined results from AC trials in the meta-analysis by Loew et al., the pooled results for OS, CSS, and RFS for the AC group were significantly favorable at 0.77, 0.79, and 0.52, respectively [145]. Therefore, while initial studies did not show significant benefit in use of AC for UTUC, multiple recent randomized trials and retrospective trials have shown improved oncologic outcomes when using AC within the immediate post-operative period in patients with muscle-invasive disease, positive lymph nodes, or LVI [145, 150,151,152]. We routinely recommend patients to receive adjuvant platinum-based chemotherapy if they had not received it in neoadjuvant setting.
Surveillance
Patients with UTUC are at high risk for recurrence. This risk is present regardless of the treatment modality. It is recommended that these patients undergo cystoscopic surveillance as often as every 3 months for the first year and then at longer intervals afterward [24, 153]. In patients who have <pT2 disease who underwent renal preservation surgery, surveillance should include a combination of ureteroscopy and upper tract cross-sectional imaging with delayed phases at 3–12-month intervals. Concurrent cross-sectional chest imaging is recommended for higher-risk patient undergoing renal preservation surgery.
Metastatic UTUC
Primary Nephroureterectomy
There have been some studies that assess the role of RNU in the setting of metastatic disease, but its role is currently limited. An analysis by Moschini et al. reviewed data from an international, multicenter, multidisciplinary database and evaluated the impact of surgery on the primary tumor site on cancer-specific mortality and overall mortality in patients with metastatic urothelial cell carcinoma. There were 326 patients in the analysis of which 47 (14%) were treated with surgery of the primary site. Nineteen of these patients had a primary UTUC, while the remainder suffered from a primary bladder cancer. On multivariable analysis, surgery was associated with superior cancer-specific mortality (HR 0.59) compared to patients who only received chemotherapy [154]. This benefit was only seen in patients with a single metastatic site. Another study by Nazzani et al. reviewed data from the SEER database that included 1174 patients with metastatic UTUC, 38% of whom underwent RNU. The study found that surgery was a predictor for lower cancer-specific mortality which was confirmed on multivariable analysis and after inverse probability of treatment weighting adjustment [155]. However, retrospective studies evaluating the effect local therapy in patients with metastatic disease are highly influenced by immortal time bias, and caution should be exercised when interpreting these effect estimates. In our practice, a RNU is considered in select patients with metastatic UTUC who have demonstrated a favorable response after systematic therapy.
Metastasectomy
Data on surgical resection of metastatic disease is very limited, but some retrospective studies have shed light on this management approach. For instance, Siefker-Radtke et al. reported outcomes on 31 patients with metastatic urothelial cell carcinoma who underwent metastasectomy. Sites of metastasis included the lung, distant lymph nodes, brain, and subcutaneous tissue. All visible gross disease was resected in 97% of patients. The 5-year survival after metastasectomy was 33% suggesting that surgery may provide some survival benefit in these patients [156]. Another study by Lehmann et al. reported the German exposure of metastasectomy across 15 centers. This included 44 patients with metastatic bladder or UTUC who underwent complete resection of detectable metastasis. The 5-year OS after surgery was 28% which again suggests that there may be a survival benefit with surgery in patients with metastatic urothelial cell carcinoma [157]. There have been additional studies that have reported an oncological benefit of surgical resection in patients with metastatic UTUC after systematic therapy, where optimal patients are those who have had a favorable response and/or have limited areas of metastasis [158, 159]. We consider surgical resection of metastatic sites in highly selected motivated patients who have a favorable response to systemic therapy and have oligometastatic disease.
Systemic Therapy
First-Line Therapy
Platinum-Based Chemotherapy
Urothelial carcinoma is generally regarded as chemo-sensitive disease. Cisplatin-based combination chemotherapy is typically the first-line option in managing patients with metastatic UTUC. Data on platinum-based chemotherapy for UTUC is mostly extrapolated from studies assessing advanced urothelial cell cancer studies that involve bladder cancer. Most first-line regimens include gemcitabine/cisplatin (GC) or dose-dense methotrexate/vinblastine/doxorubicin/cisplatin (ddMVAC). MVAC was historically the first-line regimen, as this demonstrated complete remission in around 36% of patients with metastatic urothelial cell carcinoma but was associated with toxicity, with significant rates of grade 3+ myelosuppression, mucositis, sepsis, and some reports of drug-related deaths [160]. Both GC and ddMVAC have been associated with, at least similar or in the case of ddMVAC, superior, oncological outcomes compared to MVAC. In addition, these regimens are much better tolerated than standard MVAC [161, 162]. With regard to efficacy of cisplatin-based chemotherapy in metastatic UTUC, a study by Moschini et al. evaluated three EORTC studies that assessed efficacy of MVAC and/or GC in patients with advanced urothelial cell carcinoma and investigated whether tumor location affected survival in these patients. In 1039 patients, progression-free and overall survival did not differ between bladder and UTUC, suggesting that outcomes between the two disease processes are similar after treatment with cisplatin-based chemotherapy [163]. However most of the patients with UTUC may have previously undergone a RNU and may not have suitable kidney function. As a result, these patients may receive carboplatin instead of cisplatin. The two drugs are not considered equivalent in efficacy; however carboplatin is very active in this disease [164]. The overall goal of chemotherapy is to achieve disease remission or stabilization which can help improve survival of the patient. Most patients will progress and succumb to their disease after subsequent lines of therapy.
Checkpoint Inhibitors
Checkpoint inhibitors are antibodies designed to target the programmed death (PD-1) pathway and prevent tumor cells from binding PD-1/PD-L1 receptors/ligands on T cells. As a result, these immune cells can be activated and exhibit their normal functions that include stimulating cytokine release and cytotoxic activity of tumor cells [165].
There are currently five immune checkpoint inhibitors approved for advanced urothelial carcinoma including pembrolizumab, atezolizumab, nivolumab, durvalumab, and avelumab. Pembrolizumab and atezolizumab are also approved for first-line cisplatin-ineligible patients who have high expression of PD-L1 in tumor-infiltrating immune cells. Pembrolizumab was approved based on KEYNOTE-052 which was a multicenter single-arm phase 2 study that assessed first-line pembrolizumab in cisplatin-ineligible patients with metastatic urothelial cancer [166]. Three-hundred and seventy-four patients were enrolled, 59 of whom had UTUC. In the patients with UTUC, 13 (22%) achieved an objective radiological response. In the entire cohort, including advanced metastatic bladder cancer, disease control was achieved in 173 (47%) of patients, and 17 (5%) achieved a complete response. A PD-L1 expression cutoff of 10% was associated with a higher frequency of response to therapy. Sixty-two percent of patients experienced an adverse effect, with 16% experiencing a grade 3 or worse complication. Another study assessed the effects of atezolizumab, a PD-L1 inhibitor, in patients with metastatic urothelial cell carcinoma. This study similarly focused on patients with metastatic urothelial carcinoma who were previously untreated and cisplatin ineligible. In 119 patients who received therapy, the objective response rate was 23%, and complete response was 9%. In the 33 patients with UTUC, there was an objective response in 13 (39%) patients. This drug was well-tolerated overall with 35% of patients experiencing some form of adverse effect [167].
In the subsequent large phase III trials exploring checkpoint inhibitors irrespective of patients’ PD-L1 expression status and after prior exposure to chemotherapy, response rates were in the range of 15–20%. The response rates were quite similar among other agents (Table 20.2).
Immunotherapy can also be used in the maintenance setting, after achieving response to chemotherapy or at the time of progression irrespective of patients’ PD-L1 expression status. Avelumab is the only checkpoint inhibitor approved for maintenance therapy in patients with advanced urothelial carcinoma. The approval was based on a large phase III clinical trial randomizing 700 patients, who had received platinum-based chemotherapy and had no progression, to avelumab and best supportive care vs best supportive care alone. The trial met its primary end point with median overall survival in the avelumab arm 21 months vs 14 months (HR 0.69, p = 0.001) in the supportive care. The adverse effects were consistent with known toxicities of avelumab [173].
Immune checkpoint inhibitors provide potential to achieve a complete remission in a small group of patients and thus are integral to the treatment paradigm of patients with advanced UC despite very low response rates. First-line checkpoint inhibitors are generally used in clinical practice in patients with low volume disease. These agents may not cause immediate debulking of the cancer as observed with more traditional cytotoxic therapy [169]. For this reason, patients who are symptomatic from their disease and are suitable for chemotherapy are best served with combination chemotherapy in the beginning of the treatment and then can transition to immunotherapy after they have achieved some degree of response as a maintenance strategy. Immune checkpoint therapy brings its own set of challenges with autoimmune side effects which require an astute physician to diagnose and treat them in a timely fashion with immunosuppressive medication as they can potentially become life-threatening [174].
Second-Line Therapy
Enfortumab is a medication in a new class of therapies called antibody drug conjugates. This is a highly sophisticated pharmacologic system designed to deliver cytotoxic payloads using an antibody directed toward the specific tumor antigens. Enfortumab delivers monomethyl auristatin E (MMAE), a microtubule inhibitor that inhibits cell division, to tumors expressing nectin-4, which is overexpressed in UC cells. It is approved for patients with advanced urothelial carcinoma who had previously received platinum-based chemotherapy and an immune checkpoint inhibitor. The drug was investigated in a large phase II clinical trial in patients who were previously heavily treated. Forty-four percent patients had a response with 12% achieving complete response. The response onset was very quick and was observed across all subgroups. Estimated median PFS was 5.8 months. This is a significant improvement over traditional second-line cytotoxic therapy. Despite targeting the chemotherapy to the tumor cell, the drug has significant toxicities including fatigue, alopecia, neuropathy, rash, decreased appetite, and dysgeusia. Few patients also reported severe Steven-Johnson-like syndrome and neutropenia. However, no toxicity-related death was reported [175].
Erdafitinib is another new in class FGFR3 inhibitor which is approved for advanced urothelial carcinoma patients, harboring alterations in FGFR3, who had progressed after platinum-based chemotherapy. This is a potent oral small molecule tyrosine kinase inhibitor of FGFR1-4. The drug was evaluated in an uncontrolled single arm phase II study in patients harboring these alterations. Forty percent of the patients had confirmed response with 3% patients achieving complete response. Median PFS was 5.5 months. Treatment-related grade 3 or high side effects were observed in 46% of the patients including hyponatremia (11%), stomatitis (10%), and asthenia (7%). Other less common but significant toxicity includes hyperphosphatemia, retinal detachment, and skin toxicity [176].
Salvage or Third-Line Therapy
In patients who require additional systemic therapy following recurrence or progression after initial therapy for metastatic disease, there are several options that have been investigated. Historically, systemic chemotherapy has been administered in this setting. One option that had been commonly used in Europe was vinflunine, a microtubule inhibitor. A study by Bellmunt et al. assessed its efficacy in this setting. This was a phase 3 trial that compared vinflunine plus supportive care vs supportive care after disease progression following platinum-based therapy [177]. The study included 370 patients who were randomly assigned to the two treatment modalities. On multivariable analysis, vinflunine was associated with significantly greater survival benefit, reducing the death risk by 23%, and the median survival for this regimen was longer than in those who only received supportive care (6.9 vs 4.3 months). Other agents that have been studied include gemcitabine, pemetrexed, and taxanes, as single agents, and have demonstrated similar effects [178,179,180]. Combination chemotherapeutic options have also been reported in this setting and have generally achieved better response rates compared to single agents, though with the cost of increased adverse effects. Examples of combination therapy include paclitaxel/gemcitabine and paclitaxel/carboplatin [181, 182].
If patients previously responded to cisplatin-based chemotherapy, repeating a cisplatin-based regimen may be an effective option. For instance, Han et al. assessed the efficacy of standard MVAC in the setting of patients who failed first-line gemcitabine/cisplatin. The overall response rate was 30% and a complete response was achieved in 6.7% of patients. The median OS was 10.9 months. This was not without toxicities, as 63.3% of patients experienced a grade 3 neutropenia and 30% experienced a grades 3–4 thrombocytopenia [183]. Another study by Edeline et al. assessed accelerated dose MVAC in the setting of patients who failed gemcitabine-platinum therapy. There were 45 patients who were reviewed, 61% of whom experienced a response, with 10% achieving a complete response. The median OS was 14.2 months. Regarding toxicities, 69% of patients experienced a grades 3–4 adverse reaction [184]. In general, there are very few patients who would be suitable for any chemotherapy after initial lines of therapy. We always encourage patients with good performance status to explore clinical trial or offer palliative care.
Abbreviations
- AC:
-
Adjuvant chemotherapy
- CSS:
-
Cancer-specific survival
- CIS:
-
Carcinoma in situ
- CI:
-
Confidence interval
- ddMVAC:
-
Dose-dense methotrexate, vinblastine, doxorubicin, cisplatin
- GC:
-
Gemcitabine/cisplatin
- HR:
-
Hazard ratio
- LND:
-
Lymph node dissection
- MVAC:
-
Methotrexate, vinblastine, doxorubicin, cisplatin
- NAC:
-
Neoadjuvant chemotherapy
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- RNU:
-
Radical nephroureterectomy
- RFS:
-
Recurrence-free survival
- UTUC:
-
Upper tract urothelial carcinoma
- UC:
-
Urothelial carcinoma
References
Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int. 2011;107(7):1059–64.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
Rink M, Robinson BD, Green DA, Cha EK, Hansen J, Comploj E, et al. Impact of histological variants on clinical outcomes of patients with upper urinary tract urothelial carcinoma. J Urol. 2012;188(2):398–404.
Desai FS, Nongthombam J, Singh LS. Retrospective evaluation of risk factors and immunohistochemical findings for pre-neoplastic and neoplastic lesions of upper urinary tract in patients with chronic nephrolithiasis. Asian Pac J Cancer Prev. 2015;16(18):8293–8.
Azemar MD, Comperat E, Richard F, Cussenot O, Roupret M. Bladder recurrence after surgery for upper urinary tract urothelial cell carcinoma: frequency, risk factors, and surveillance. Urol Oncol. 2011;29(2):130–6.
Pommer W, Bronder E, Klimpel A, Helmert U, Greiser E, Molzahn M. Urothelial cancer at different tumour sites: role of smoking and habitual intake of analgesics and laxatives. Results of the Berlin Urothelial Cancer Study. Nephrol Dial Transplant. 1999;14(12):2892–7.
Rink M, Xylinas E, Margulis V, Cha EK, Ehdaie B, Raman JD, et al. Impact of smoking on oncologic outcomes of upper tract urothelial carcinoma after radical nephroureterectomy. Eur Urol. 2013;63(6):1082–90.
Cosyns JP. Aristolochic acid and ‘Chinese herbs nephropathy’ - a review of the evidence to date. Drug Saf. 2003;26(1):33–48.
Colin P, Koenig P, Ouzzane A, Berthon N, Villers A, Biserte J, et al. Environmental factors involved in carcinogenesis of urothelial cell carcinomas of the upper urinary tract. BJU Int. 2009;104(10):1436–40.
Jelakovic B, Karanovic S, Vukovic-Lela I, Miller F, Edwards KL, Nikolic J, et al. Aristolactam-DNA adducts are a biomarker of environmental exposure to aristolochic acid. Kidney Int. 2012;81(6):559–67.
Cosyns JP, Jadoul M, Squifflet JP, De Plaen JF, Ferluga D, van Ypersele de Strihou C. Chinese herbs nephropathy: a clue to Balkan endemic nephropathy? Kidney Int. 1994;45(6):1680–8.
Cosyns JP, Jadoul M, Squifflet JP, Van Cangh PJ, van Ypersele de Strihou C. Urothelial malignancy in nephropathy due to Chinese herbs. Lancet. 1994;344(8916):188.
Nortier JL, Martinez MM, Schmeiser HH, Arlt VM, Bieler CA, Petein M, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med. 2000;342(23):1686–92.
Zaitsu M, Kawachi I, Takeuchi T, Kobayashi Y. Alcohol consumption and risk of upper-tract urothelial cancer. Cancer Epidemiol. 2017;48:36–40.
Yang MH, Chen KK, Yen CC, Wang WS, Chang YH, Huang WJ, et al. Unusually high incidence of upper urinary tract urothelial carcinoma in Taiwan. Urology. 2002;59(5):681–7.
Saint-Jacques N, Parker L, Brown P, Dummer TJ. Arsenic in drinking water and urinary tract cancers: a systematic review of 30 years of epidemiological evidence. Environ Health. 2014;13:44.
Brenner DW, Schellhammer PF. Upper tract urothelial malignancy after cyclophosphamide therapy: a case report and literature review. J Urol. 1987;137(6):1226–7.
Lee JY, Kim K, Sung HH, Jeon HG, Jeong BC, Seo SI, et al. Molecular characterization of urothelial carcinoma of the bladder and upper urinary tract. Transl Oncol. 2018;11(1):37–42.
Sfakianos JP, Cha EK, Iyer G, Scott SN, Zabor EC, Shah RH, et al. Genomic characterization of upper tract urothelial carcinoma. Eur Urol. 2015;68(6):970–7.
Metcalfe MJ, Petros FG, Rao P, Mork ME, Xiao L, Broaddus RR, et al. Universal point of care testing for Lynch syndrome in patients with upper tract urothelial carcinoma. J Urol. 2018;199(1):60–5.
Roupret M, Yates DR, Comperat E, Cussenot O. Upper urinary tract urothelial cell carcinomas and other urological malignancies involved in the hereditary nonpolyposis colorectal cancer (lynch syndrome) tumor spectrum. Eur Urol. 2008;54(6):1226–36.
van der Post RS, Kiemeney LA, Ligtenberg MJ, Witjes JA, Hulsbergen-van de Kaa CA, Bodmer D, et al. Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. J Med Genet. 2010;47(7):464–70.
Vasen HF, Mecklin JP, Khan PM, Lynch HT. The international collaborative group on hereditary non-polyposis colorectal cancer (ICG-HNPCC). Dis Colon Rectum. 1991;34(5):424–5.
Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. 2018;73(1):111–22.
Janisch F, Shariat SF, Baltzer P, Fajkovic H, Kimura S, Iwata T, et al. Diagnostic performance of multidetector computed tomographic (MDCTU) in upper tract urothelial carcinoma (UTUC): a systematic review and meta-analysis. World J Urol. 2020;38(5):1165–75.
Takahashi N, Glockner JF, Hartman RP, King BF, Leibovich BC, Stanley DW, et al. Gadolinium enhanced magnetic resonance urography for upper urinary tract malignancy. J Urol. 2010;183(4):1330–65.
Cosentino M, Palou J, Gaya JM, Breda A, Rodriguez-Faba O, Villavicencio-Mavrich H. Upper urinary tract urothelial cell carcinoma: location as a predictive factor for concomitant bladder carcinoma. World J Urol. 2013;31(1):141–5.
Samson P, Smith AD, Hoenig D, Okeke Z. Endoscopic management of upper urinary tract urothelial carcinoma. J Endourol. 2018;32(S1):S10–S6.
Joseph JP, Potretzke TA, Packiam V, Sharma V, Toussi A, Miest TS, et al. Percutaneous image-guided core needle biopsy for upper tract urothelial carcinoma. Urology. 2020;135:95–100.
Huang SY, Ahrar K, Gupta S, Wallace MJ, Ensor JE, Krishnamurthy S, et al. Safety and diagnostic accuracy of percutaneous biopsy in upper tract urothelial carcinoma. BJU Int. 2015;115(4):625–32.
Malm C, Grahn A, Jaremko G, Tribukait B, Brehmer M. Diagnostic accuracy of upper tract urothelial carcinoma: how samples are collected matters. Scand J Urol. 2017;51(2):137–45.
Johannes JR, Nelson E, Bibbo M, Bagley DH. Voided urine fluorescence in situ hybridization testing for upper tract urothelial carcinoma surveillance. J Urol. 2010;184(3):879–82.
Fradet Y, Grossman HB, Gomella L, Lerner S, Cookson M, Albala D, et al. A comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of carcinoma in situ in patients with bladder cancer: a phase III, multicenter study. J Urol. 2007;178(1):68–73; discussion.
Mukherjee P, George AJP, Yadav BK, Jeyaseelan L, Kumar RM, Mukha RP, et al. The impact of narrow band imaging in the detection and resection of bladder tumor in transitional cell carcinoma of the bladder: a prospective, blinded, sequential intervention randomized controlled trial. Urology. 2019;128:55–61.
Kata SG, Aboumarzouk OM, Zreik A, Somani B, Ahmad S, Nabi G, et al. Photodynamic diagnostic ureterorenoscopy: a valuable tool in the detection of upper urinary tract tumour. Photodiagn Photodyn Ther. 2016;13:255–60.
Ahmad S, Aboumarzouk O, Somani B, Nabi G, Kata SG. Oral 5-aminolevulinic acid in simultaneous photodynamic diagnosis of upper and lower urinary tract transitional cell carcinoma - a prospective audit. BJU Int. 2012;110(11 Pt B):E596–600.
Traxer O, Geavlete B, de Medina SG, Sibony M, Al-Qahtani SM. Narrow-band imaging digital flexible ureteroscopy in detection of upper urinary tract transitional-cell carcinoma: initial experience. J Endourol. 2011;25(1):19–23.
Shariat SF, Godoy G, Lotan Y, Droller M, Karakiewicz PI, Raman JD, et al. Advanced patient age is associated with inferior cancer-specific survival after radical nephroureterectomy. BJU Int. 2010;105(12):1672–7.
Martinez-Salamanca JI, Shariat SF, Rodriguez JC, Chromecki TF, Ficarra V, Fritsche HM, et al. Prognostic role of ECOG performance status in patients with urothelial carcinoma of the upper urinary tract: an international study. BJU Int. 2012;109(8):1155–61.
Crivelli JJ, Xylinas E, Kluth LA, Rieken M, Rink M, Shariat SF. Effect of smoking on outcomes of urothelial carcinoma: a systematic review of the literature. Eur Urol. 2014;65(4):742–54.
Brierly JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. Wiley-Blackwell; 2017.
Lughezzani G, Burger M, Margulis V, Matin SF, Novara G, Roupret M, et al. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur Urol. 2012;62(1):100–14.
Isbarn H, Jeldres C, Shariat SF, Liberman D, Sun M, Lughezzani G, et al. Location of the primary tumor is not an independent predictor of cancer specific mortality in patients with upper urinary tract urothelial carcinoma. J Urol. 2009;182(5):2177–81.
Ehdaie B, Chromecki TF, Lee RK, Lotan Y, Margulis V, Karakiewicz PI, et al. Obesity adversely impacts disease specific outcomes in patients with upper tract urothelial carcinoma. J Urol. 2011;186(1):66–72.
Akdogan B, Dogan HS, Eskicorapci SY, Sahin A, Erkan I, Ozen H. Prognostic significance of bladder tumor history and tumor location in upper tract transitional cell carcinoma. J Urol. 2006;176(1):48–52.
Ouzzane A, Colin P, Xylinas E, Pignot G, Ariane MM, Saint F, et al. Ureteral and multifocal tumours have worse prognosis than renal pelvic tumours in urothelial carcinoma of the upper urinary tract treated by nephroureterectomy. Eur Urol. 2011;60(6):1258–65.
Chen XP, Xiong GY, Li XS, Matin SF, Garcia M, Fang D, et al. Predictive factors for worse pathological outcomes of upper tract urothelial carcinoma: experience from a nationwide high-volume centre in China. BJU Int. 2013;112(7):917–24.
Favaretto RL, Shariat SF, Savage C, Godoy G, Chade DC, Kaag M, et al. Combining imaging and ureteroscopy variables in a preoperative multivariable model for prediction of muscle-invasive and non-organ confined disease in patients with upper tract urothelial carcinoma. BJU Int. 2012;109(1):77–82.
Boorjian S, Ng C, Munver R, Palese MA, Vaughan ED Jr, Sosa RE, et al. Impact of delay to nephroureterectomy for patients undergoing ureteroscopic biopsy and laser tumor ablation of upper tract transitional cell carcinoma. Urology. 2005;66(2):283–7.
Sundi D, Svatek RS, Margulis V, Wood CG, Matin SF, Dinney CP, et al. Upper tract urothelial carcinoma: impact of time to surgery. Urol Oncol. 2012;30(3):266–72.
Waldert M, Karakiewicz PI, Raman JD, Remzi M, Isbarn H, Lotan Y, et al. A delay in radical nephroureterectomy can lead to upstaging. BJU Int. 2010;105(6):812–7.
Ito Y, Kikuchi E, Tanaka N, Miyajima A, Mikami S, Jinzaki M, et al. Preoperative hydronephrosis grade independently predicts worse pathological outcomes in patients undergoing nephroureterectomy for upper tract urothelial carcinoma. J Urol. 2011;185(5):1621–6.
Berod AA, Colin P, Yates DR, Ouzzane A, Audouin M, Adam E, et al. The role of American Society of Anesthesiologists scores in predicting urothelial carcinoma of the upper urinary tract outcome after radical nephroureterectomy: results from a national multi-institutional collaborative study. BJU Int. 2012;110(11 Pt C):E1035–40.
Mullerad M, Russo P, Golijanin D, Chen HN, Tsai HH, Donat SM, et al. Bladder cancer as a prognostic factor for upper tract transitional cell carcinoma. J Urol. 2004;172(6 Pt 1):2177–81.
Lughezzani G, Jeldres C, Isbarn H, Sun M, Shariat SF, Alasker A, et al. Nephroureterectomy and segmental ureterectomy in the treatment of invasive upper tract urothelial carcinoma: a population-based study of 2299 patients. Eur J Cancer. 2009;45(18):3291–7.
Novara G, De Marco V, Gottardo F, Dalpiaz O, Bouygues V, Galfano A, et al. Independent predictors of cancer-specific survival in transitional cell carcinoma of the upper urinary tract: multi-institutional dataset from 3 European centers. Cancer. 2007;110(8):1715–22.
Park J, Ha SH, Min GE, Song C, Hong B, Hong JH, et al. The protective role of renal parenchyma as a barrier to local tumor spread of upper tract transitional cell carcinoma and its impact on patient survival. J Urol. 2009;182(3):894–9.
Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115(6):1224–33.
Kikuchi E, Margulis V, Karakiewicz PI, Roscigno M, Mikami S, Lotan Y, et al. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J Clin Oncol. 2009;27(4):612–8.
Colin P, Ouzzane A, Yates DR, Audenet F, Pignot G, Arvin-Berod A, et al. Influence of positive surgical margin status after radical nephroureterectomy on upper urinary tract urothelial carcinoma survival. Ann Surg Oncol. 2012;19(11):3613–20.
Langner C, Hutterer G, Chromecki T, Rehak P, Zigeuner R. Patterns of invasion and histological growth as prognostic indicators in urothelial carcinoma of the upper urinary tract. Virchows Arch. 2006;448(5):604–11.
Simone G, Papalia R, Loreto A, Leonardo C, Sentinelli S, Gallucci M. Independent prognostic value of tumour diameter and tumour necrosis in upper urinary tract urothelial carcinoma. BJU Int. 2009;103(8):1052–7.
Zigeuner R, Shariat SF, Margulis V, Karakiewicz PI, Roscigno M, Weizer A, et al. Tumour necrosis is an indicator of aggressive biology in patients with urothelial carcinoma of the upper urinary tract. Eur Urol. 2010;57(4):575–81.
Roupret M, Colin P, Yates DR. A new proposal to risk stratify urothelial carcinomas of the upper urinary tract (UTUCs) in a predefinitive treatment setting: low-risk versus high-risk UTUCs. Eur Urol. 2014;66(2):181–3.
Cutress ML, Stewart GD, Wells-Cole S, Phipps S, Thomas BG, Tolley DA. Long-term endoscopic management of upper tract urothelial carcinoma: 20-year single-centre experience. BJU Int. 2012;110(11):1608–17.
Krambeck AE, Thompson RH, Lohse CM, Patterson DE, Segura JW, Zincke H, et al. Endoscopic management of upper tract urothelial carcinoma in patients with a history of bladder urothelial carcinoma. J Urol. 2007;177(5):1721–6.
Moore K, Khastgir J, Ghei M. Endoscopic management of upper tract urothelial carcinoma. Adv Urol. 2009;2009:620604.
Liatsikos EN, Dinlenc CZ, Kapoor R, Smith AD. Transitional-cell carcinoma of the renal pelvis: ureteroscopic and percutaneous approach. J Endourol. 2001;15(4):377–83; discussion 97.
Jeldres C, Lughezzani G, Sun M, Isbarn H, Shariat SF, Budaus L, et al. Segmental ureterectomy can safely be performed in patients with transitional cell carcinoma of the ureter. J Urol. 2010;183(4):1324–9.
Veccia A, Antonelli A, Checcucci E, Falagario U, Carrieri G, Guruli G, et al. Segmental ureterectomy for upper tract urothelial carcinoma: a systematic review and meta-analysis of comparative studies. Clin Genitourin Cancer. 2020;18(1):E10–20.
Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO joint guideline: American Urological Association. Available from: http://www.auanet.org/guidelines/non-muscle-invasive-bladder-cancer-(aua/suo-joint-guideline-2016.
Gadzinski AJ, Roberts WW, Faerber GJ, Wolf JS Jr. Long-term outcomes of immediate versus delayed nephroureterectomy for upper tract urothelial carcinoma. J Endourol. 2012;26(5):566–73.
Lee JN, Kwon S, Choi G, Kim H, Kim T-H, Kwon TG, et al. Impact of surgical wait time on oncologic outcomes in upper urinary tract urothelial carcinoma. J Surg Oncol. 2014;110
Kleinmann N, Matin SF, Pierorazio PM, Gore JL, Shabsigh A, Hu B, et al. Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): an open-label, single-arm, phase 3 trial. Lancet Oncol. 2020;21(6):776–85.
Roupret M, Colin P, Yates D. A new proposal to risk stratify urothelial carcinomas of the upper urinary tract (UTUCs) in a predefinitive treatment setting: low-risk versus high-risk UTUCs. Eur Urol. 2013;66
Seisen T, Colin P, Rouprêt M. Risk-adapted strategy for the kidney-sparing management of upper tract tumours. Nat Rev Urol. 2015;12(3):155–66.
Xia L, Taylor BL, Pulido JE, Guzzo TJ. Impact of surgical waiting time on survival in patients with upper tract urothelial carcinoma: a national cancer database study. Urol Oncol. 2018;36(1):10.e5–10.e22.
Simsir A, Sarsik B, Cureklibatir I, Sen S, Gunaydin G, Cal C. Prognostic factors for upper urinary tract urothelial carcinomas: stage, grade, and smoking status. Int Urol Nephrol. 2011;43(4):1039–45.
Smith JA, Howards SS, Preminger GM, Hinman F. Hinman’s atlas of urologic surgery. 3rd ed. Philadelphia: Elsevier/Saunders; 2012. xxix, 1151 p.
Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, et al. European Association of Urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur Urol. 2015;68(5):868–79.
Colin P, Ouzzane A, Yates DR, François A, Pignot G, Arvin-Berod A, et al. Influence of positive surgical margin status after radical nephroureterectomy on upper urinary tract urothelial carcinoma survival. Ann Surg Oncol. 2012;19(11):3613–20.
Favaretto RL, Shariat SF, Chade DC, Godoy G, Kaag M, Cronin AM, et al. Comparison between laparoscopic and open radical nephroureterectomy in a contemporary group of patients: are recurrence and disease-specific survival associated with surgical technique? Eur Urol. 2010;58(5):645–51.
Walton TJ, Novara G, Matsumoto K, Kassouf W, Fritsche HM, Artibani W, et al. Oncological outcomes after laparoscopic and open radical nephroureterectomy: results from an international cohort. BJU Int. 2011;108(3):406–12.
Ni S, Tao W, Chen Q, Liu L, Jiang H, Hu H, et al. Laparoscopic versus open nephroureterectomy for the treatment of upper urinary tract urothelial carcinoma: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2012;61(6):1142–53.
Ariane MM, Colin P, Ouzzane A, Pignot G, Audouin M, Cornu JN, et al. Assessment of oncologic control obtained after open versus laparoscopic nephroureterectomy for upper urinary tract urothelial carcinomas (UUT-UCs): results from a large French multicenter collaborative study. Ann Surg Oncol. 2012;19(1):301–8.
Clements MB, Krupski TL, Culp SH. Robotic-assisted surgery for upper tract urothelial carcinoma: a comparative survival analysis. Ann Surg Oncol. 2018;25(9):2550–62.
Rodriguez JF, Packiam VT, Boysen WR, Johnson SC, Smith ZL, Smith ND, et al. Utilization and outcomes of nephroureterectomy for upper tract urothelial carcinoma by surgical approach. J Endourol. 2017;31(7):661–5.
Peyronnet B, Seisen T, Dominguez-Escrig JL, Bruins HM, Yuan CY, Lam T, et al. Oncological outcomes of laparoscopic nephroureterectomy versus open radical Nephroureterectomy for upper tract urothelial carcinoma: an European Association of Urology Guidelines Systematic Review. Eur Urol Focus. 2019;5(2):205–23.
Simone G, Papalia R, Guaglianone S, Ferriero M, Leonardo C, Forastiere E, et al. Laparoscopic versus open nephroureterectomy: perioperative and oncologic outcomes from a randomised prospective study. Eur Urol. 2009;56(3):520–6.
Aboumohamed AA, Krane LS, Hemal AK. Oncologic outcomes following robot-assisted laparoscopic nephroureterectomy with bladder cuff excision for upper tract urothelial carcinoma. J Urol. 2015;194(6):1561–6.
Rouprêt M, Smyth G, Irani J, Guy L, Davin JL, Saint F, et al. Oncological risk of laparoscopic surgery in urothelial carcinomas. World J Urol. 2009;27(1):81–8.
Ong AM, Bhayani SB, Pavlovich CP. Trocar site recurrence after laparoscopic nephroureterectomy. J Urol. 2003;170(4 Pt 1):1301.
Macejko AM, Pazona JF, Loeb S, Kimm S, Nadler RB. Management of distal ureter in laparoscopic nephroureterectomy--a comprehensive review of techniques. Urology. 2008;72(5):974–81.
Abercrombie GF, Eardley I, Payne SR, Walmsley BH, Vinnicombe J. Modified nephro-ureterectomy. Long-term follow-up with particular reference to subsequent bladder tumours. Br J Urol. 1988;61(3):198–200.
Walton TJ, Sherwood BT, Parkinson RJ, Obakponovwe O, Thomas SA, Taylor MC, et al. Comparative outcomes following endoscopic ureteral detachment and formal bladder cuff excision in open nephroureterectomy for upper urinary tract transitional cell carcinoma. J Urol. 2009;181(2):532–9.
Giovansili B, Peyromaure M, Saighi D, Dayma T, Zerbib M, Debre B. Stripping technique for endoscopic management of distal ureter during nephroureterectomy: experience of 32 procedures. Urology. 2004;64(3):448–52; discussion 52.
Gill IS, Soble JJ, Miller SD, Sung GT. A novel technique for management of the en bloc bladder cuff and distal ureter during laparoscopic nephroureterectomy. J Urol. 1999;161(2):430–4.
Wein AJ, Kavoussi LR, Campbell MF. Campbell-Walsh urology / editor-in-chief, Alan J. Wein ; [editors, Louis R. Kavoussi ... et al.]. 10th ed. Philadelphia: Elsevier Saunders; 2012.
Steinberg JR, Matin SF. Laparoscopic radical nephroureterectomy: dilemma of the distal ureter. Curr Opin Urol. 2004;14(2):61–5.
Tsivian A, Benjamin S, Sidi AA. A sealed laparoscopic nephroureterectomy: a new technique. Eur Urol. 2007;52(4):1015–9.
Nanigian DK, Smith W, Ellison LM. Robot-assisted laparoscopic nephroureterectomy. J Endourol. 2006;20(7):463–5; discussion 5–6.
Zargar H, Krishnan J, Autorino R, Akca O, Brandao LF, Laydner H, et al. Robotic nephroureterectomy: a simplified approach requiring no patient repositioning or robot redocking. Eur Urol. 2014;66(4):769–77.
Raman JD, Ng CK, Boorjian SA, Vaughan ED Jr, Sosa RE, Scherr DS. Bladder cancer after managing upper urinary tract transitional cell carcinoma: predictive factors and pathology. BJU Int. 2005;96(7):1031–5.
Zigeuner RE, Hutterer G, Chromecki T, Rehak P, Langner C. Bladder tumour development after urothelial carcinoma of the upper urinary tract is related to primary tumour location. BJU Int. 2006;98(6):1181–6.
Xylinas E, Rink M, Cha EK, Clozel T, Lee RK, Fajkovic H, et al. Impact of distal ureter management on oncologic outcomes following radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol. 2014;65(1):210–7.
O’Brien T, Ray E, Singh R, Coker B, Beard R. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C trial). Eur Urol. 2011;60(4):703–10.
Xylinas E, Kluth L, Passoni N, Trinh QD, Rieken M, Lee RK, et al. Prediction of intravesical recurrence after radical nephroureterectomy: development of a clinical decision-making tool. Eur Urol. 2014;65(3):650–8.
Saika T, Nishiguchi J, Tsushima T, Nasu Y, Nagai A, Miyaji Y, et al. Comparative study of ureteral stripping versus open ureterectomy for nephroureterectomy in patients with transitional carcinoma of the renal pelvis. Urology. 2004;63(5):848–52.
Jones DR, Moisey CU. A cautionary tale of the modified “pluck” nephroureterectomy. Br J Urol. 1993;71(4):486.
Arango O, Bielsa O, Carles J, Gelabert-Mas A. Massive tumor implantation in the endoscopic resected area in modified nephroureterectomy. J Urol. 1997;157(5):1839.
Li WM, Shen JT, Li CC, Ke HL, Wei YC, Wu WJ, et al. Oncologic outcomes following three different approaches to the distal ureter and bladder cuff in nephroureterectomy for primary upper urinary tract urothelial carcinoma. Eur Urol. 2010;57(6):963–9.
Phe V, Cussenot O, Bitker MO, Roupret M. Does the surgical technique for management of the distal ureter influence the outcome after nephroureterectomy? BJU Int. 2011;108(1):130–8.
Zigeuner R, Pummer K. Urothelial carcinoma of the upper urinary tract: surgical approach and prognostic factors. Eur Urol. 2008;53(4):720–31.
Arancibia MF, Bolenz C, Michel MS, Keeley FX Jr, Alken P. The modern management of upper tract urothelial cancer: surgical treatment. BJU Int. 2007;99(5):978–81.
Leissner J, Hohenfellner R, Thuroff JW, Wolf HK. Lymphadenectomy in patients with transitional cell carcinoma of the urinary bladder; significance for staging and prognosis. BJU Int. 2000;85(7):817–23.
Herr HW, Bochner BH, Dalbagni G, Donat SM, Reuter VE, Bajorin DF. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol. 2002;167(3):1295–8.
Kondo T, Hara I, Takagi T, Kodama Y, Hashimoto Y, Kobayashi H, et al. Template-based lymphadenectomy in urothelial carcinoma of the renal pelvis: a prospective study. Int J Urol. 2014;21(5):453–9.
Lughezzani G, Jeldres C, Isbarn H, Shariat SF, Sun M, Pharand D, et al. A critical appraisal of the value of lymph node dissection at nephroureterectomy for upper tract urothelial carcinoma. Urology. 2010;75(1):118–24.
Roscigno M, Shariat SF, Margulis V, Karakiewicz P, Remzi M, Kikuchi E, et al. Impact of lymph node dissection on cancer specific survival in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy. J Urol. 2009;181(6):2482–9.
Brausi MA, Gavioli M, De Luca G, Verrini G, Peracchia G, Simonini G, et al. Retroperitoneal lymph node dissection (RPLD) in conjunction with nephroureterectomy in the treatment of infiltrative transitional cell carcinoma (TCC) of the upper urinary tract: impact on survival. Eur Urol. 2007;52(5):1414–8.
Roscigno M, Cozzarini C, Bertini R, Scattoni V, Freschi M, Da Pozzo LF, et al. Prognostic value of lymph node dissection in patients with muscle-invasive transitional cell carcinoma of the upper urinary tract. Eur Urol. 2008;53(4):794–802.
Fajkovic H, Cha EK, Jeldres C, Donner G, Chromecki TF, Margulis V, et al. Prognostic value of extranodal extension and other lymph node parameters in patients with upper tract urothelial carcinoma. J Urol. 2012;187(3):845–51.
Roupret M, Hupertan V, Seisen T, Colin P, Xylinas E, Yates DR, et al. Prediction of cancer specific survival after radical nephroureterectomy for upper tract urothelial carcinoma: development of an optimized postoperative nomogram using decision curve analysis. J Urol. 2013;189(5):1662–9.
Roscigno M, Brausi M, Heidenreich A, Lotan Y, Margulis V, Shariat SF, et al. Lymphadenectomy at the time of nephroureterectomy for upper tract urothelial cancer. Eur Urol. 2011;60(4):776–83.
Bolenz C, Shariat SF, Fernandez MI, Margulis V, Lotan Y, Karakiewicz P, et al. Risk stratification of patients with nodal involvement in upper tract urothelial carcinoma: value of lymph-node density. BJU Int. 2009;103(3):302–6.
Roscigno M, Shariat SF, Freschi M, Margulis V, Karakiewizc P, Suardi N, et al. Assessment of the minimum number of lymph nodes needed to detect lymph node invasion at radical nephroureterectomy in patients with upper tract urothelial cancer. Urology. 2009;74(5):1070–4.
Kondo T, Nakazawa H, Ito F, Hashimoto Y, Toma H, Tanabe K. Primary site and incidence of lymph node metastases in urothelial carcinoma of upper urinary tract. Urology. 2007;69(2):265–9.
Skinner D. The pelvic ureter. J R Soc Med. 1978;71(7):541.
McCarron JP, Mills C, Vaughn ED Jr. Tumors of the renal pelvis and ureter: current concepts and management. Semin Urol. 1983;1(1):75–81.
Heney NM, Nocks BN, Daly JJ, Blitzer PH, Parkhurst EC. Prognostic factors in carcinoma of the ureter. J Urol. 1981;125(5):632–6.
Grabstald H, Whitmore WF, Melamed MR. Renal pelvic tumors. JAMA. 1971;218(6):845–54.
Cummings KB. Nephroureterectomy: rationale in the management of transitional cell carcinoma of the upper urinary tract. Urol Clin North Am. 1980;7(3):569–78.
Batata MA, Whitmore WF, Hilaris BS, Tokita N, Grabstald H. Primary carcinoma of the ureter: a prognostic study. Cancer. 1975;35(6):1626–32.
Batata M, Grabstald H. Upper urinary tract urothelial tumors. Urol Clin North Am. 1976;3(1):79–86.
Ito A, Shintaku I, Satoh M, Ioritani N, Aizawa M, Tochigi T, et al. Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: the THP Monotherapy Study Group Trial. J Clin Oncol. 2013;31(11):1422–7.
Fang D, Li XS, Xiong GY, Yao L, He ZS, Zhou LQ. Prophylactic intravesical chemotherapy to prevent bladder tumors after nephroureterectomy for primary upper urinary tract urothelial carcinomas: a systematic review and meta-analysis. Urol Int. 2013;91(3):291–6.
Elalouf V, Xylinas E, Klap J, Pignot G, Delongchamps NB, Saighi D, et al. Bladder recurrence after radical nephroureterectomy: predictors and impact on oncological outcomes. Int J Urol. 2013;20(11):1078–83.
Yamashita S, Ito A, Mitsuzuka K, Tochigi T, Namima T, Soma F, et al. Clinical implications of intravesical recurrence after radical nephroureterectomy for upper urinary tract urothelial carcinoma. Int J Urol. 2016;23(5):378–84.
Meng X, Chao B, Vijay V, Silver H, Margolin EJ, Balar A, et al. High response rates to neoadjuvant chemotherapy in high-grade upper tract urothelial carcinoma. Urology. 2019;129:146–52.
Matin SF, Margulis V, Kamat A, Wood CG, Grossman HB, Brown GA, et al. Incidence of downstaging and complete remission after neoadjuvant chemotherapy for high-risk upper tract transitional cell carcinoma. Cancer. 2010;116(13):3127–34.
Liao RS, Gupta M, Schwen ZR, Patel HD, Kates M, Johnson MH, et al. Comparison of pathological stage in patients treated with and without neoadjuvant chemotherapy for high risk upper tract urothelial carcinoma. J Urol. 2018;200(1):68–73.
Almassi N, Gao T, Lee B, Stein RJ, Haber GP, Ornstein MC, et al. Impact of neoadjuvant chemotherapy on pathologic response in patients with upper tract urothelial carcinoma undergoing extirpative surgery. Clin Genitourin Cancer. 2018;16(6):e1237–e42.
Hosogoe S, Hatakeyama S, Kusaka A, Hamano I, Iwamura H, Fujita N, et al. Platinum-based neoadjuvant chemotherapy improves oncological outcomes in patients with locally advanced upper tract urothelial carcinoma. Eur Urol Focus. 2018;4(6):946–53.
Martini A, Daza J, Poltiyelova E, Gul Z, Heard JR, Ferket BS, et al. Pathological downstaging as a novel endpoint for the development of neoadjuvant chemotherapy for upper tract urothelial carcinoma. BJU Int. 2019;124:665–71.
Leow JJ, Chong YL, Chang SL, Valderrama BP, Powles T, Bellmunt J. Neoadjuvant and adjuvant chemotherapy for upper tract urothelial carcinoma: a 2020 systematic review and meta-analysis, and future perspectives on systemic therapy. Eur Urol. 2021;79(5):635–54.
Kubota Y, Hatakeyama S, Tanaka T, Fujita N, Iwamura H, Mikami J, et al. Oncological outcomes of neoadjuvant chemotherapy in patients with locally advanced upper tract urothelial carcinoma: a multicenter study. Oncotarget. 2017;8(60):101500–8.
Fukushi K, Narita T, Hatakeyama S, Yamamoto H, Soma O, Matsumoto T, et al. Quality-of-life evaluation during platinum-based neoadjuvant chemotherapies for urothelial carcinoma. Int J Clin Oncol. 2017;22(2):366–72.
Lee KS, Kim KH, Yoon YE, Choi KH, Yang SC, Han WK. Impact of adjuvant chemotherapy in patients with upper tract urothelial carcinoma and lymphovascular invasion after radical nephroureterectomy. Korean J Urol. 2015;56(1):41–7.
Hellenthal N, Shariat S, Margulis V, Karakiewicz P, Roscigno M, Bolenz C, et al. Adjuvant chemotherapy for high risk upper tract urothelial carcinoma: results from the upper tract urothelial carcinoma collaboration. J Urol. 2009;182:900–6.
Birtle A, Johnson M, Chester J, Jones R, Dolling D, Bryan RT, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet. 2020;395(10232):1268–77.
Luo Y, Feng BF, Wei DC, Han YL, Li MC, Zhao JH, et al. [Prospective controlled observation of effect of adjuvant chemotherapy on survival and prognosis of high-risk upper tract urothelial carcinoma patients underwent radical nephroureterectomy]. Zhonghua Yi Xue Za Zhi. 2019;99(40):3158–63.
Cho KS, Joung JY, Seo HK, Cho IC, Chung HS, Chung J, et al. Renal safety and efficacy of cisplatin-based chemotherapy in patients with a solitary kidney after nephroureterectomy for urothelial carcinoma of the upper urinary tract. Cancer Chemother Pharmacol. 2011;67(4):769–74.
Bladder cancer: national comprehensive cancer network. Available from: https://www.nccn.org/professionals/physician_gls/PDF/bladder.pdf.
Moschini M, Xylinas E, Zamboni S, Mattei A, Niegisch G, Yu EY, et al. Efficacy of surgery in the primary tumor site for metastatic urothelial cancer: analysis of an international, multicenter, multidisciplinary database. Eur Urol Oncol. 2020;3(1):94–101.
Nazzani S, Preisser F, Mazzone E, Marchioni M, Bandini M, Tian Z, et al. Survival effect of nephroureterectomy in metastatic upper urinary tract urothelial carcinoma. Clin Genitourin Cancer. 2019;17(3):e602–e11.
Siefker-Radtke AO, Walsh GL, Pisters LL, Shen Y, Swanson DA, Logothetis CJ, et al. Is there a role for surgery in the management of metastatic urothelial cancer? The M. D. Anderson experience. J Urol. 2004;171(1):145–8.
Lehmann J, Suttmann H, Albers P, Volkmer B, Gschwend JE, Fechner G, et al. Surgery for metastatic urothelial carcinoma with curative intent: the German experience (AUO AB 30/05). Eur Urol. 2009;55(6):1293–9.
Miller RS, Freiha FS, Reese JH, Ozen H, Torti FM. Cisplatin, methotrexate and vinblastine plus surgical restaging for patients with advanced transitional cell carcinoma of the urothelium. J Urol. 1993;150(1):65–9.
Dodd PM, McCaffrey JA, Herr H, Mazumdar M, Bacik J, Higgins G, et al. Outcome of postchemotherapy surgery after treatment with methotrexate, vinblastine, doxorubicin, and cisplatin in patients with unresectable or metastatic transitional cell carcinoma. J Clin Oncol. 1999;17(8):2546–52.
Sternberg CN, Yagoda A, Scher HI, Watson RC, Geller N, Herr HW, et al. Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium. Efficacy and patterns of response and relapse. Cancer. 1989;64(12):2448–58.
von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–8.
von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068–77.
Moschini M, Shariat SF, Roupret M, De Santis M, Bellmunt J, Sternberg CN, et al. Impact of primary tumor location on survival from the European Organization for the Research and Treatment of Cancer advanced urothelial cancer studies. J Urol. 2018;199(5):1149–57.
Linardou H, Aravantinos G, Efstathiou E, Kalofonos C, Anagnostopoulos A, Deliveliotis C, et al. Gemcitabine and carboplatin combination as first-line treatment in elderly patients and those unfit for cisplatin-based chemotherapy with advanced bladder carcinoma: phase II study of the Hellenic Co-operative Oncology Group. Urology. 2004;64(3):479–84.
Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517–9.
Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–92.
Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76.
Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–20.
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–26.
Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–22.
Powles T, O’Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(9):e172411.
Patel MR, Ellerton J, Infante JR, Agrawal M, Gordon M, Aljumaily R, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19(1):51–64.
Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–30.
Kottschade L, Brys A, Peikert T, Ryder M, Raffals L, Brewer J, et al. A multidisciplinary approach to toxicity management of modern immune checkpoint inhibitors in cancer therapy. Melanoma Res. 2016;26(5):469–80.
Rosenberg JE, O’Donnell PH, Balar AV, McGregor BA, Heath EI, Yu EY, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol. 2019;37(29):2592–600.
Loriot Y, Necchi A, Park SH, Garcia-Donas J, Huddart R, Burgess E, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381(4):338–48.
Bellmunt J, Theodore C, Demkov T, Komyakov B, Sengelov L, Daugaard G, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27(27):4454–61.
McCaffrey JA, Hilton S, Mazumdar M, Sadan S, Kelly WK, Scher HI, et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol. 1997;15(5):1853–7.
Joly F, Tchen N, Chevreau C, Henry-Amar M, Priou F, Chinet-Charrot P, et al. Clinical benefit of second line weekly paclitaxel in advanced urothelial carcinoma (AUC): a GETUG phase II study. J Clin Oncol. 2004;22(14):411s-s.
Sweeney CJ, Roth BJ, Kabbinavar FF, Vaughn DI, Arning M, Curiel RE, et al. Phase II study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J Clin Oncol. 2006;24(21):3451–7.
Kouno T, Ando M, Yonemori K, Matsumoto K, Shimizu C, Katsumata N, et al. Weekly paclitaxel and carboplatin against advanced transitional cell cancer after failure of a platinum-based regimen. Eur Urol. 2007;52(4):1115–22.
Otto T, Bex A, Krege S, Walz PH, Rubben H. Paclitaxel-based second-line therapy for patients with advanced chemotherapy-resistant bladder carcinoma (M1): a clinical phase II study. Cancer. 1997;80(3):465–70.
Han KS, Joung JY, Kim TS, Jeong IG, Seo HK, Chung J, et al. Methotrexate, vinblastine, doxorubicin and cisplatin combination regimen as salvage chemotherapy for patients with advanced or metastatic transitional cell carcinoma after failure of gemcitabine and cisplatin chemotherapy. Br J Cancer. 2008;98(1):86–90.
Edeline J, Loriot Y, Culine S, Massard C, Albiges L, Blesius A, et al. Accelerated MVAC chemotherapy in patients with advanced bladder cancer previously treated with a platinum-gemcitabine regimen. Eur J Cancer. 2012;48(8):1141–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Olson, K.M., Faraj, K.S., Singh, P., Tyson, M.D. (2022). Treatment of Upper Tract Urothelial Carcinoma. In: Stratton, K.L., Morgans, A.K. (eds) Urologic Oncology. Springer, Cham. https://doi.org/10.1007/978-3-030-89891-5_20
Download citation
DOI: https://doi.org/10.1007/978-3-030-89891-5_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-89890-8
Online ISBN: 978-3-030-89891-5
eBook Packages: MedicineMedicine (R0)