Abstract
Purpose of Review
We review the epidemiology, risk factors, diagnosis, and treatment of upper tract urothelial carcinoma (UTUC), with a distinction between the different risk groups.
Recent Findings
Endoscopic treatment with laser ablation of tumors has an evolving role in treating low-grade UTUC including select large and multifocal tumors, along with complementary topical chemotherapeutic treatment that can reach difficult intrarenal locations. Template lymphadenectomy is recommended in patients undergoing nephroureterectomy. A recent randomized control trial showed benefit of adjuvant chemotherapy after radical nephroureterectomy for locally advanced disease. Advances in immunologic therapy have shown promise in treating metastatic UTUC, and immunologic-based therapies have been incorporated into treatment regimens.
Summary
Notable progress has been made in both the surgical and medical treatment arms for UTUC, thus extending the reach of nephron-sparing therapy for those with localized disease and increasing overall survival for those with locally advanced disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epidemiology
Upper tract urothelial carcinoma (UTUC) designates lesions originating from the luminal space of the ureter and/or intrarenal collecting system including the renal pelvis, infundibula, and calyces. It is a relatively rare disease, which constitutes 5–10% of all urothelial carcinomas [1]. Disease occurrence is 2:100,000 in the western world and has demonstrated an upward trend due to advancement in imaging and endoscopic technology, as well as longer survival of patients with bladder cancer, from which UTUC often subsequently develops [2]. The mean age at diagnosis is between 68 and 73 years [3]. In the west, disease occurrence is higher in men compared to women at a ratio of 2:1, respectively, with similar treatment outcomes for both genders [4, 5]. In two-thirds of cases, the disease originates from the intrarenal collecting system (in the rest, it originates from the ureter), and in 1 in 4 cases, the disease is multifocal [6]. In 70% of cases, the pathological grade of the disease is high-grade, and there is evidence of concurrent carcinoma in situ in 10–36% of cases. In 60% of cases, the disease is locally advanced at diagnosis, in 7% of cases, the disease is metastatic, and in 20% of cases, there exists concurrent bladder cancer [7].
Genetics
Histologically, UTUC and bladder cancer are similar, but they differ genetically in levels of gene expression and silencing (such as RB1, TP53, HRAS), which may explain the higher prevalence of more advanced and more aggressive disease in UTUC compared to bladder cancer [8]. Although UTUC and bladder cancer share many clinical and pathological features, UTUC represents a unique disease entity with its own management recommendations.
Lynch syndrome (also known as HNPCC—hereditary non-polyposis colorectal cancer) is an autosomal dominant hereditary syndrome characterized by genetic mutations that impair DNA mismatch repair (MMR). The 5 main genes involved in Lynch syndrome are MSH-2, MLH-1, MSH-6, PMS-2, and EPCAM. Patients that have this syndrome are at an increased risk to develop multiple malignancies such as colorectal cancer, endometrial cancer, and UTUC, among others [9]. The risk to develop UTUC is the highest among patients with MSH-2 mutations [10]. Compared to sporadic UTUC cases, patients with UTUC and Lynch syndrome present on average, 8 years younger, more often have tumors in the ureter [11] and may be more susceptible to bilateral upper tract UTUC development, which is often metachronous [12]. A high index of suspicion for Lynch syndrome should exist in UTUC patients that are relatively young or have a personal or familial history of these syndrome-related malignancies, thus prompting consultation with a genetics counselor [9].
Risk Factors
The most significant risk factor for the development of UTUC in developed countries is smoking history, which increases the risk by 2–7.5-fold. People who smoke more than 2 packs a day have a 4.8-fold risk for the disease, while people who smoke less than half a pack per day have a 2.4-fold risk, which points towards an association between the disease and the amount of packs per-day [13]. A history of smoking is found in 53% of patients with UTUC [5]. The second most significant risk factor in developed countries is occupational exposure to aromatic amines, which is used in the paint, rubber, and textile industries. These substances are excreted in the urine and can cause chronic tubulointerstitial inflammation and expose the urothelium to carcinogens. Exposure for 7 years or more can cause an increased risk by 8.3-fold for UTUC, usually 20 years or more after exposure. The occupational exposure to these substances in developed countries has been dramatically reduced due to decreased use and implementation of strict safety guidelines [14].
Diagnosis
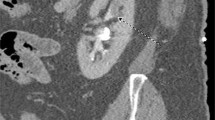
The diagnosis of UTUC is often made following abdominal-pelvic cross-sectional imaging for symptoms such as gross hematuria or flank pain but is also made in otherwise asymptomatic patients with microhematuria, those with history of bladder cancer, or less often as an incidental finding (Fig. 1A). In one study, among 168 patients with UTUC, the most common presenting symptom was hematuria in 73% of cases, followed by flank pain in 32% of cases [15]. For workup of hematuria, we perform a urine cytology test to check for the presence of malignant cells and cystoscopy to check for a urethral or bladder source of bleeding and visualize the ureteral orifice for bloody efflux. We also perform an upper urinary tract imaging test with a CTU (computerized tomography urography) that has a sensitivity and specificity for UTUC of 92% and 95%, respectively [16], or an MRU (magnetic resonance urography). MRU has a lower sensitivity (75% in tumors smaller than 2 cm) but is used in cases with a contraindication for IV contrast or a desire to avoid ionizing radiation [16]. These cross-sectional modalities help rule out overtly metastatic or locally advanced disease and are essential for at least preliminary clinical staging.
A Cross-sectional CT urogram demonstrates 2.5 cm intrarenal tumor originating from the anterior surface of the renal pelvis (black arrow). B Retrograde pyelogram demonstrating same tumor as filling defect (white arrow). C Retrograde pyelogram showing complete clearance of tumor in one setting. D Digital ureteroscopic view of upper tract tumor prior to endoscopic treatment. E Same tumor with application of neodymium:YAG laser shows coagulation effect. F Ureteroscopic view of collecting system showing tumor clearance after laser resection
When there is a suspicion for a lesion in the upper urinary tract, ureterorenoscopy is performed to visualize the entire collecting system, collect ipsilateral urine cytology, identify any lesions, and perform biopsies (Fig. 1B–D). The accuracy of biopsies taken during ureterorenoscopy is notoriously low in determining disease stage (level of penetrance to the urinary tract wall) and was reported to be as low as 58.6% [17]. Nonetheless, in determining the grade of the disease, the endoscopic biopsy was reported to be as high as 94%. Of note, the technique in which the biopsy is taken can determine its accuracy and reliability, and the use of a flat-wire basket is the most accurate, compared to cup biopsy devices [18]. High-grade disease was found to correlate to invasion to the muscular wall with a positive predictive value of 60%. In cases of a sub-epithelial invasion of the tumor, the positive predictive value for muscular invasion is 86% [19]. It is essential for a pathological report on an UTUC biopsy to contain the grade of the tumor.
In addition to gathering the above information during ureteroscopy, the surgeon can appreciate the feasibility of endoscopic treatment on a case-by-case basis, which is an essential first step in determining if a patient is a candidate for nephron-sparing treatment. There is evidence that in cases of a clear lesion on a CTU/MRU test with a urine cytology test positive for malignancy, a radical surgery may be performed, and the ureterorenoscopy can be omitted [17]. Concerns have been raised about the possible disadvantages of ureteroscopic evaluation of UTUC including the promotion of metastatic disease by pyelovenous backflow of tumor cells, delay to NU, and promotion of eventual bladder tumor development [20]. Multiple groups have shown that metastatic disease is not promoted in those UTUC patients undergoing diagnostic URS prior to NU versus patients who were treated with immediate NU, even when controlled for tumor grade, after being followed with cross-sectional imaging for a mean of 34–50 months [21, 22]. No differences were seen in rate of eventual metastatic disease or overall survival. Intravesical tumor development has been inconsistently reported as higher in those undergoing URS prior to NU versus those that do not [23,24,25]. What is consistent in all studies, however, is that there is no difference in cancer-specific survival for up to 41–64 months follow-up. Therefore, the benefit of ureteroscopy in terms of providing more accurate staging information and offering a nephron-sparing approach needs to be weighed against the possible increased rate of eventual bladder tumor development, which will not impact cancer-specific survival. In a recent review of 2,380 patients treated for UTUC, diagnostic ureterorenoscopy was a part of the diagnostic process in 49.7% of patients, demonstrating the lack of uniformity in approach [5].
Risk Stratification
The approach to the management of non-metastatic UTUC patients begins with determining the risk group of the patient—either a low-risk or a high-risk group [26, 27], a stratification that was adopted in 2013 by the EAU. The stratification is made according to disease grade on cytology and biopsy, disease stage according to CT/MRI (less than T2 versus T2 and above), unifocal or multifocal distribution, tumor size (less than 2 cm versus 2 cm and larger), or signs of advanced disease such as hydronephrosis or history of radical cystectomy (Fig. 2) [28••]. According to contemporary guidelines, to risk-stratify disease as low risk, it is mandatory to perform a diagnostic ureteroscopy with urine cytology and a tissue biopsy. However, to risk-stratify the disease as high risk, it is enough to have a high-grade voided urine cytology or have a history of a radical cystectomy for high-grade disease. The spectrum of management includes (1) radical surgery with resection of the ipsilateral kidney and the ureter (radical nephroureterectomy); (2) resection of an involved segment of the ureter; and (3) local endoscopic treatments mainly with laser ablation of the tumor. In the last several years, following the advancement in endoscopic surgery and laser technologies, and the gained experience with endoscopic treatments, the definition of the risk groups has changed, mostly in tumor size [28••]. Several recent publications have demonstrated good outcomes with endoscopic treatment even for large and multi-focal tumors as long as the disease is low-grade [29,30,31]. In the past, renal preserving treatments were offered only to patients with a single-functioning kidney, chronic renal failure, or bilateral disease, to avoid the risk for dialysis after nephrectomy. According to the European Association of Urology (EAU) guidelines published in 2020, renal preserving treatments should be offered to patients in the low-risk group, regardless of the status of the contralateral kidney [28••]. In a review paper published by the EAU in 2016, including 6,190 patients with low-grade non-invasive disease, outcomes after radical nephroureterectomy were similar to endoscopic treatment and segmental ureterectomy [32]. In addition, a large portion of patients with UTUC has other medical conditions such as hypertension (61%), hyperlipidemia (36%), type 2 diabetes (21%), ischemic heart disease (33%), and pulmonary disease (21%). These patients are at a higher risk for complications after radical major surgery. In a multi-center study of 731 patients who had undergone radical nephroureterectomy, there was a 38% complication rate after surgery, with 8% complication grade 3 and above according to the Clavien-Dindo classification [33]. This high complication rate has increased the motivation for renal preserving treatments in this disease. Nonetheless, following renal preservation treatments, there is a high local recurrence rate, which dictates a strict endoscopic follow-up of the upper urinary tract. For these local recurrences, endoscopic treatment is usually feasible and does not result in an increased oncologic risk [29,30,31].
Risk stratification of non-metastatic UTUC. [28••]
Treatment of Low-Risk Group
The spectrum of treatments for the low-risk group includes the nephron-sparing treatments: segmental ureterectomy, retrograde or antegrade ureterorenoscopy with laser treatment, and topical ablative treatment.
Segmental Ureterectomy
This involves resection of the involved segment of the ureter with a wide margin and local lymphadenectomy. In cases where the involved segment is the distal ureter, along with resection, a ureteroneocystostomy is performed. The oncologic outcome is better for resection of the distal ureter than for the mid and proximal ureter [32]. After surgery, there is a need to follow-up on the ipsilateral ureter and kidney in the form of cross-sectional imaging with ureteroscopic evaluation for any suspicious findings.
Endoscopic Treatment
This includes retrograde and/or antegrade ureterorenoscopy with biopsy and treatment of any visible tumors with laser energy or electrocautery [29, 30] (Fig. 1E–F). The risk for local recurrence can approach 70–80% and not surprisingly increases with longer follow-up. The risk for pathological progression in terms of grade is relatively low between 2 and 15% [29, 31, 34] but has been reported as high as 31% in a series with mean tumor size of 3.0 cm [30]. Therefore, patients with relatively larger sized lesions, even if low grade, need to be counseled on the higher rate of grade progression. Younger, healthier patients with normal contralateral kidneys will be better served with NU, while those with solitary kidneys, renal insufficiency or prohibitive comorbidities for NU, can still benefit from a nephron-sparing option.
Due to the relatively high risk of local recurrence, it is common practice to perform strict ureteroscopic surveillance on the affected upper urinary tract. The surveillance protocol usually includes a ureterorenoscopy every 3 months in the first year after the diagnosis, bi-annually in the 2nd year, and annually thereafter. Every local recurrence usually resets the protocol. In every surveillance procedure, one must survey the urinary bladder, ureter, renal pelvis, and calyces, along with urine samples for cytology, a biopsy of any suspicious lesions, and laser treatment of all visible lesions. This endoscopic surveillance needs to continue indefinitely unless there is evidence of a local invasion, pathologic progression (to high-grade disease), local recurrence in high volume (which represents a more aggressive disease), or inability to continue endoscopic treatments due to anatomic considerations such as prohibitive ureteral strictures or difficult to reach locations such as an inaccessible calyx with tumor due to infundibular stenosis. In these cases, the patient is referred for a radical nephroureterectomy. In the past several years, the spectrum of disease that can be treated endoscopically has widened. Nonetheless, endoscopic management of UTUC is an ensemble of an accurate diagnosis, surgical experience with proper endourological equipment, and a clear knowledge of the limitations of this treatment approach. In addition to periodical ureteroscopy, a CTU/MRU is performed annually to evaluate the contralateral side for any suspicious lesions and to rule out local or metastatic progression, which may alter the treatment and consider radical surgery or systemic treatment [28••].
Efforts have been made to lower the relatively high local recurrence rate after endoscopic treatment. The use of different topical agents to reduce local recurrences has been studied, mostly based on the experience established in bladder instillations such as Mitomycin C, BCG, and Gemcitabine. These treatments are delivered to the upper urinary tract using a percutaneous nephrostomy tube directly into the kidney, or through a retrograde ureteral catheter, and are given after complete laser eradication of all visible tumors. When using these instillations as an adjuvant treatment for endoscopic laser treatment, there was a somewhat lower local recurrence rate, but the rate remains high (around 50%) and does not seem to change the surveillance protocols. This, in addition to the technical challenge of repeatedly delivering the agents to the upper urinary tract, has caused the use of these instillations to be relatively limited [35, 36].
Topical Ablative Treatment
Recently, a thermo-reversible hydrogel containing Mitomycin C was developed and showed a 59% complete response rate in treating low-grade tumors up to 15 mm in size. This agent takes a liquid form at room temperature and is delivered through an open-ended ureteral catheter to the renal pelvis. Shortly after injection into the kidney, the agent receives heat from its surroundings, which causes its consistency to change into a gel. The gel slowly elutes in the upper urinary tract for about 6 h, during which the mucosa is adequately exposed to Mitomycin C [37••]. This hydrogel is an important tool in the armamentarium in treating UTUC, especially valuable in cases with a high recurrence rate or for tumors difficult to reach and treat ureteroscopically. Importantly, the ureteral stricture rate observed with this gel is not negligible [37••], cost is significant, and durability of treatment response is still being elucidated at the time of this report. Although it should not be considered a substitute for complete or near complete endoscopic resection, in accord with NCCN guidelines [38], this hydrogel may serve to further expand the reach of renal preserving treatment options for UTUC.
Treatment of the High-Risk Group
The treatment of choice for the high-risk group is radical nephroureterectomy of the ipsilateral side, including a bladder cuff excision. The reason for resecting the entire ipsilateral urinary tract is the risk of eventual development of luminal tumors in the remnant ureteral stump, which was observed in 42% of UTUC patients undergoing only a nephrectomy in a report from 1976 [39]. Nephroureterectomy can be performed in an open, laparoscopic, or robotic approach [40]. Studies on the surgical treatment of advanced disease (T3/T4, and N +) have shown better oncologic outcomes for the open approach [41]. For patients with high-risk disease who are not fit for radical surgery due to comorbidities or unwilling to undergo a radical surgery, endoscopic laser treatment may be offered on a palliative basis for local control and to treat hematuria or upper tract obstruction. In a retrospective study of 160 patients with UTUC treated endoscopically, 16 patients had high-grade disease and were treated palliatively. In 15 out of the 16, metastatic progressions occurred, with median overall survival of 29.2 months (range 6.5–52.6) [34].
Lymphadenectomy
The role of lymphadenectomy during radical nephroureterectomy has recently evolved from controversial to recommended according to contemporary guidelines from the European Association of Urology [28••] and the National Comprehensive Cancer Network [38]. Involvement of lymph nodes with UTUC was reported in 20–30% of cases with a primary tumor in the renal pelvis or the upper ureter and 10% involvement in cases with a primary tumor in the distal ureter [42]. The anatomical location of the involved lymph nodes depends on the location of the tumor along the urinary tract, which determines the lymphadenectomy template [42, 43]. Current NCCN guidelines recommend the following regional lymphadenectomy templates for high-grade tumors: for left-sided renal pelvic and upper ureteral tumors, paraaortic lymph nodes; for right-sided renal pelvic and upper ureteral tumors, paracaval lymph nodes; and for most mid-ureteral tumors and distal ureteral tumors, common iliac, external iliac, obturator, and hypogastric lymph nodes [38]. Template-based lymph node dissection during NU has been reported to improve cancer-specific survival and reduce local recurrence rates in patients with higher stage disease [44]. Given the inaccuracies of preoperative staging for UTUC [45], lymph node dissections are recommended for all patients undergoing NU, whenever technically feasible.
Post-operative Bladder Instillation
Intravesical occurrence of bladder cancer after nephroureterectomy was reported to be 15–50% for patients with no history of bladder cancer, which sets the need for periodic cystoscopic evaluation after surgery [46]. In an effort to reduce these occurrences, a single early post-operative intravesical instillation of chemotherapy has been studied. In a large multicenter prospective randomized trial among 284 patients, a single dose of Mitomycin C before urethral catheter removal after surgery was tested against the standard treatment and showed 11% absolute risk reduction for bladder recurrence in the 12 months following nephroureterectomy [47]. It is important to note that extravasation of chemotherapeutic agents can cause severe complications such as peritonitis [48, 49]. Therefore, bladder instillations should be given only in cases where extravasation is not suspected; otherwise, a cystogram should be performed to rule it out.
Neoadjuvant Therapy
Unlike the situation regarding locally advanced bladder cancer, neoadjuvant chemotherapy for high-risk UTUC has never yet been proven beneficial with a prospective randomized controlled trial. Nevertheless, several retrospective studies have been published showing that neoadjuvant chemotherapy leads to pathological downstaging, occasional pathological complete responses [50], and increased survival compared to well-matched historical controls which were treated with immediate NU [51]. The chemotherapeutic treatment can be given before nephroureterectomy, while the patient still has both the kidneys and usually better kidney function. In a review of 16 retrospective or single-arm studies, complete pathologic response was observed in 11% of cases and a partial response in 43% of cases. The hazard ratio for disease-specific survival was 0.38, which represented a 62% benefit for those who received neoadjuvant chemotherapy compared to nephroureterectomy alone [52]. Although chemotherapy regimens were not completely uniform, the vast majority were platinum-based regimens, either MVAC (methotrexate, vinblastine, doxorubicin, cisplatin) or GC (gemcitabine, cisplatin). Several prospective trials on neoadjuvant therapies are ongoing, including immunologic-based therapies.
Adjuvant Therapy
One of the most significant limitations for this type of treatment is the fact that after nephroureterectomy, only 20% of patients have kidney function sufficient enough for cisplatin-based chemotherapy. Cisplatin is the agent on which most of the chemotherapeutic regimens for urothelial carcinoma are based (when using a GFR cut-off of 60 ml/min) [38]. Nonetheless, there is mounting evidence showing the benefit of adjuvant chemotherapy and recently, immunotherapy as well. In a review of 18 studies, including 5,659 patients, there was a hazard ratio of 0.79 in favor of cancer-specific survival after adjuvant chemotherapy and radical nephroureterectomy, compared to radical nephroureterectomy alone [52]. A phase III open-label randomized controlled trial (POUT trial) evaluated the benefit of adjuvant chemotherapy after radical nephroureterectomy (initiated within 90 days of surgery) compared to radical nephroureterectomy alone [53••]. The study included 261 patients from 57 hospitals in the UK (132 patients in the chemotherapy arm and 129 patients in the surveillance arm), staged as pT2-T4/pN0-N3/M0 or any pathologic stage with /N1-3/M0. The chemotherapy regimen included four 21-day cycles of gemcitabine on days 1 and 8 and cisplatin on day 1 (or carboplatin when eGFR < 50 ml/min). The study found a significant improvement in disease-free survival and metastasis-free survival after a median follow-up of 30.3 months (hazard ratios of 0.45 and 0.48, respectively). Three-year event-free estimates were 71% and 46%, and grade 3 or worse acute treatment-related adverse events occurred in 44% and 4% of patients in the chemotherapy and surveillance arms, respectively.
In a recently published phase 3, multicenter, randomized controlled trial, the benefit of adjuvant nivolumab was compared to the one of placebo [54••]. The study included 353 patients who received nivolumab and 356 who received placebo. Median disease-free survival was 20.8 and 10.8 months in the nivolumab and placebo groups, respectively. Treatment-related adverse events of grade 3 or worse were observed in 17.9% and 7.2% of patients in the nivolumab and placebo groups, respectively. Two treatment-related deaths occurred (one case of pneumonitis and one of bowel perforation). Several other prospective trials for adjuvant therapy are currently ongoing, including immunologic therapies.
Treatment of Metastatic Disease
As mentioned earlier, 7% of cases are metastatic at diagnosis and are referred to medical oncology for systemic treatments. In addition, some patients develop metastatic progression from initially localized disease [7]. The evidence for the efficacy of systemic treatments for UTUC is based mostly on experience observed from treatments of bladder cancer patients [55]. The first-line therapy is cisplatin-based chemotherapy [56]. In the past several years, there has been major progress in understanding immunologic mechanisms involved in bladder cancer and UTUC, and several agents have been developed. In particular, drugs based on PD-1 (programmed death-1) and PD-L1 (programmed death-ligand 1) have shown efficacy in treating urothelial carcinomas. The role of these agents in the oncologic treatment regimens is increasing in all lines of therapy. Currently, in cisplatin-ineligible patients with metastatic UTUC, pembrolizumab or atezolizumab may be offered even as first-line therapy [57, 58].
Conclusions
Although UTUC is a relatively rare malignancy, its treatment has shown significant recent advancements. Experience in endoscopic treatments continues to grow, along with improvements in laser and endoscopic technology and a novel topical chemotherapeutic agent, all of which are continuously changing the treatment guidelines for low-grade non-metastatic disease. Nephroureterectomy is still considered the gold standard for high-risk disease and should be accompanied by template lymphadenectomy whenever possible. Recent guidelines for metastatic disease have immunologic therapy incorporated into all lines of therapy. The role of new immunologic-based therapies is increasing, and several trials are ongoing regarding its role in different points of therapy.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Siegel RL, Miller KD, Jemal A. Cancer statistics 2020. CA Cancer J Clin. 2020;70(1):7–30.
Soria F, Shariat SF, Lerner SP, Fritsche HM, Rink M, Kassouf W, et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J Urol. 2017;35(3):379–87.
Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973–2005. BJU Int. 2011;107:1059–64.
Shariat SF, Favaretto RL, Gupta A, Fritsche HM, Matsumoto K, Kassouf W, et al. Gender differences in radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol. 2011;29(4):481–6.
Baard J, Cormio L, Cavadas V, Alcaraz A, Shariat SF, de la Rosette J, et al. Contemporary patterns of presentation, diagnostics and management of upper tract urothelial cancer in 101 centers: the Clinical Research Office of the Endourological Society Global upper tract urothelial carcinoma registry. Curr Opin Urol. 2021;31(4):354–62.
Favaretto RL, Shariat SF, Chade DC, Godoy G, Adamy A, Kaag M, et al. The effect of tumor location on prognosis in patients treated with radical nephroureterectomy at Memorial Sloan-Kettering Cancer Center. Eur Urol. 2010;58:574–80.
Browne BM, Stensland KD, Moynihan MJ, Canes D. An analysis of staging and treatment trends for upper tract urothelial carcinoma in the National Cancer Database. Clin Genitourin Cancer. 2018;16(4):e743–50.
Sfakianos JP, Cha EK, Iyer G, Scott SN, Zabor EC, Shah RH, et al. Genomic characterization of upper tract urothelial carcinoma. Eur Urol. 2015;68(6):970–7.
Mork M, Hubosky SG, Rouprêt M, Margulis V, Raman J, Lotan Y, et al. Lynch syndrome: a primer for urologists and panel recommendations. J Urol. 2015;194(1):21–9.
van der Post RS, Kiemeney LA, Ligtenberg MJL, Witjes JA, Hulsbergen van de Kaa CA, Bodmer D, et al. Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. J Med Genet. 2010;47(7):464–70a.
Crockett DG, Wagner DG, Holmäng S, Johansson SL, Lynch HT. Upper urinary tract carcinoma in Lynch syndrome cases. J Urol. 2011;185(5):1627–30.
Hubosky SG, Boman BM, Charles S, Bibbo M, Bagley DH. Ureteroscopic management of upper tract urothelial carcinoma (UTUC) in patients with Lynch syndrome (hereditary nonpolyposis colorectal cancer syndrome). BJU Int. 2013;112(6):813–9.
Rink M, Xylinas E, Margulis V, Cha EK, Ehdaie B, Raman JD, et al. Impact of smoking on oncologic outcomes of upper tract urothelial carcinoma after radical nephroureterectomy. Eur Urol. 2013;63(6):1082–90.
Shinka T, Miyai M, Sawada Y, Inagaki T, Okawa T. Factors affecting the occurrence of urothelial tumors in dye workers exposed to aromatic amines. Int J Urol. 1995;2(4):243–8.
Inman Brant A, Tran Viet-Tan, Fradet Yves, Lacombe Louis. Carcinoma of the upper urinary tract: predictors of survival and competing causes of mortality. Cancer. 2009;115(13):2853–62a.
Janisch F, Shariat SF, Baltzer P, Fajkovic H, Kimura S, Iwata T, et al. Diagnostic performance of multidetector computed tomographic (MDCTU) in upper tract urothelial carcinoma (UTUC): a systematic review and meta-analysis. World J Urol. 2020;38(5):1165–75.
Vashistha V, Shabsigh A, Zynger DL. Utility and diagnostic accuracy of ureteroscopic biopsy in upper tract urothelial carcinoma. Arch Pathol Lab Med. 2013;137(3):400–7.
Kleinmann N, Healy KA, Hubosky SG, Margel D, Bibbo M, Bagley DH. Ureteroscopic biopsy of upper tract urothelial carcinoma: comparison of basket and forceps. J Endourol. 2013;27(12):1450–4.
Margolin EJ, Matulay JT, Li G, Meng X, Chao B, Vijay V, et al. Discordance between ureteroscopic biopsy and final pathology for upper tract urothelial carcinoma. J Urol. 2018;199(6):1440–5.
Sung HH, Jeon HG, Han DH, Jeong BC, Seo SI, Lee HM, et al. Diagnostic ureterorenoscopy is associated with increased intravesical recurrence following radical nephroureterectomy in upper tract urothelial carcinoma. PLoS One. 2015;10(11):e0139976.
Kulp DA, Bagley DH. Does flexible ureteropyeloscopy promote local recurrence of transitional cell carcinoma? J Endourol. 1994;8:111–3.
Hendin BN, Streem SB, Levin HS, Klein EA, Novick AC. Impact of diagnostic ureteroscopy on long-term survival in patients with upper tract transitional cell carcinoma. J Urol. 1999;161(3):783–5.
Sankin A, Tin AL, Mano R, Chevinsky M, Jakubowski C, Sfaklanos JP, et al. Impact of ureteroscopy before nephroureterectomy for upper tract urothelial carcinoma on oncologic outcomes. Urology. 2016;94:148–53.
Luo HL, Kang CH, Chen YT, Chuang YC, Lee WC, Cheng YT, et al. Diagnostic ureteroscopy independently correlates with intravesical recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma. Ann Surg Oncol. 2013. https://doi.org/10.1245/s10434-013-3000-z.
Ishikawa S, Abe T, Shinohara N, Harabayashi T, Sazawa A, Maruyama S, et al. Impact of diagnostic ureteroscopy on intravesical recurrence and survival in patients with urothelial carcinoma of the upper urinary tract. J Urol. 2010;184:883.7.
Seisen T, Colin P, Rouprêt M. Risk-adapted strategy for the kidney-sparing management of upper tract tumours. Nat Rev Urol. 2015;12(3):155–66.
Rouprêt M, Colin P, Yates DR. A new proposal to risk stratify urothelial carcinomas of the upper urinary tract (UTUCs) in a predefinitive treatment setting: low-risk versus high-risk UTUCs. Eur Urol. 2014;66(2):181–3.
••Rouprêt M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat E, et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2020 Update. Eur Urol. 2021;79(1):62–79 (These guidelines dedicated for upper tract urothelial carcinoma is frequently updated, and provide current diagnosis and treatment recommendations graded as strong/weak level of recommendation for all disease stages.).
Villa L, Haddad M, Capitanio U, Somani BK, Cloutier J, Doizi S, et al. Which patients with upper tract urothelial carcinoma can be safely treated with flexible ureteroscopy with holmium:YAG laser photoablation? Long-term results from a high-volume institution. J Urol. 2018;199(1):66–73.
Scotland KB, Kleinmann N, Cason D, Hubbard L, Tanimoto R, Healy KA, et al. Ureteroscopic management of large ≥2 cm upper tract urothelial carcinoma: a comprehensive 23-year experience. Urology. 2018;121:66–73.
Shvero A, Abu-Ghanem Y, Laufer M, Dotan ZA, Zilberman DE, Mor Y, et al. Endoscopic treatment for large multifocal upper tract urothelial carcinoma. J Urol. 2021;205(4):1039–46.
Seisen T, Peyronnet B, Dominguez-Escrig JL, Bruins HM, Yuan CY, Babjuk M, et al. Oncologic outcomes of kidney-sparing surgery versus radical nephroureterectomy for upper tract urothelial carcinoma: a systematic review by the EAU non-muscle invasive bladder cancer guidelines panel. Eur Urol. 2016;70(6):1052–68.
Lin YK, Deliere A, Lehman K, Harpster LE, Kaag MG, Raman JD. Critical analysis of 30-day complications following radical nephroureterectomy for upper tract urothelial carcinoma. Can J Urol. 2014;21(4):7369–73.
Grasso M, Fishman AI, Cohen J, Alexander B. Ureteroscopic and extirpative treatment of upper urinary tract urothelial carcinoma: a 15-year comprehensive review of 160 consecutive patients. BJU Int. 2012;110(11):1618–26.
Metcalfe M, Wagenheim G, Xiao L, Papadopoulos J, Navai N, Davis JW, et al. Induction and maintenance adjuvant Mitomycin C topical therapy for upper tract urothelial carcinoma: tolerability and intermediate term outcomes. J Endourol. 2017;31(9):946–53.
Giannarini G, Kessler TM, Birkhäuser FD, Thalmann GN, Studer UE. Antegrade perfusion with bacillus Calmette-Guerin in patients with non-muscle-invasive urothelial carcinoma of the upper urinary tract: who may benefit? Eur Urol. 2011;60(5):955–60.
••Kleinmann N, Matin SF, Pierorazio PM, Gore JL, Shabsigh A, Hu B, et al. Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): an open-label, single-arm, phase 3 trial. Lancet Oncol. 2020;21(6):776-85. In this multi-institute clinical trial, the authors found that a novel thermal gel containing mitomycin-C is effective in ablating low-grade UTUC in 59%, which may increase the kidney preservation rate for this group of patients.
42a Flaig TW, Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, et al. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Bladder Cancer, Upper GU Tract Tumors, version 3.2021.
Strong DW, Pearse HD, Tank ES, Hodges CV. The ureteral stump after nephroureterectomy. J Urol. 1976;115(6):654–5.
Rodriguez JF, Packiam VT, Boysen WR, Johnson SC, Smith ZL, Smith ND, et al. Utilization and outcomes of nephroureterectomy for upper tract urothelial carcinoma by surgical approach. J Endourol. 2017;31(7):661–5.
Simone G, Papalia R, Guaglianone S, Ferriero M, Leonardo C, Forastiere E, et al. Laparoscopic versus open nephroureterectomy: perioperative and oncologic outcomes from a randomised prospective study. Eur Urol. 2009;56(3):520–6.
Kondo T, Nakazawa H, Ito F, Hashimoto Y, Toma H, Tanabe K. Primary site and incidence of lymph node metastases in urothelial carcinoma of upper urinary tract. Urology. 2007;69(2):265–9.
Matin SF, Sfakianos JP, Espiritu PN, Coleman JA, Spiess PE. Patterns of lymphatic metastases in upper tract urothelial carcinoma and proposed dissection templates. J Urol. 2015;194(6):1567–74.
Dominguez-Escrig JL, Peyronnet B, Seisen T, Bruins HM, Yuan CY, Babjuk M, et al. Potential benefit of lymph node dissection during radical nephroureterectomy for upper tract urothelial carcinoma: a systematic review by the European Association of Urology Guidelines Panel of non-muscle-invasive bladder cancer. Eur Urol Focus. 2019;5(2):224–41.
Yee A, Cha E, Sfakianos J, Kim P, Friedman F, Sternberg I, et al. MP77–14 can pre-operative CT identify positive lymph nodes in patients with upper tract urothelial carcinoma? J Urol. 2014;191:e917.
Matsui Y, Utsunomiya N, Ichioka K, Ueda N, Yoshimura K, Terai A, et al. Risk factors for subsequent development of bladder cancer after primary transitional cell carcinoma of the upper urinary tract. Urology. 2005;65(2):279–83.
O'Brien T, Ray E, Singh R, Coker B, Beard R. British Association of Urological Surgeons Section of Oncology. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C Trial). Eur Urol. 2011;60(4):703–10.
Koya MP, Simon MA, Soloway MS. Complications of intravesical therapy for urothelial cancer of the bladder. J Urol. 2006;175(6):2004–10.
Mertens LS, Meinhardt W, Rier WB, Nooter RI, Horenblas S. Extravasation of intravesical chemotherapy for non-muscle-invasive bladder cancer. Urol Int. 2012;89(3):332–6.
Matin SF, Margulis V, Kamat A, Wood CG, Grossman HB, Brown GA, et al. Incidence of downstaging and complete remission after neoadjuvant chemotherapy for high-risk upper tract transitional cell carcinoma. Cancer. 2010;116:3127–34.
Porten S, Siefker-Radtke MD, Xiao L, Margulis V, Kamat A, Wood CG, et al. Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma. Cancer. 2014;120:1794–9.
Leow JJ, Chong YL, Chang SL, Valderrama BP, Powles T, Bellmunt J. Neoadjuvant and adjuvant chemotherapy for upper tract urothelial carcinoma: a 2020 systematic review and meta-analysis, and future perspectives on systemic therapy. Eur Urol. 2021;79(5):635–54.
••Birtle A, Johnson M, Chester J, Jones R, Dolling D, Bryan RT, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet. 2020;395(10232):1268-77. In this prospective randomized trial, the authors show that adjuvant chemotherapy following radical nephroureterectomy for locally advanced UTUC improves disease-free survival.
•• Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384(22):2102-14. In this randomized prospective trial, the immunologic agent nivolumab was shown to be effective in the adjuvant setting in increasing disease-free survival by 10 months compared to placebo.
Moschini M, Shariat SF, Rouprêt M, De Santis M, Bellmunt J, Sternberg CN, et al. Impact of primary tumor location on survival from the European Organization for the Research and Treatment of Cancer Advanced Urothelial Cancer Studies. J Urol. 2018;199(5):1149–57.
von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068–77.
Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76.
Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–92.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Asaf Shvero declares that he has no conflict of interest. Scott G. Hubosky has received compensation for service as a consultant from Boston Scientific Corporation, and his institution (Sidney Kimmel Medical College at Thomas Jefferson University Hospital) participated in the OLYMPUS trial of UroGen’s UGN-101 (Jelmyto™), completely under local institutional review board approval.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Genitourinary Cancers
Rights and permissions
About this article
Cite this article
Shvero, A., Hubosky, S.G. Management of Upper Tract Urothelial Carcinoma. Curr Oncol Rep 24, 611–619 (2022). https://doi.org/10.1007/s11912-021-01179-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-021-01179-8