Abstract
Objective
To investigate the existence of predictive factors for concomitant, primary UUT-UCC and BC. Upper urinary tract urothelial cell carcinoma (UUT-UCC) is a pan-urothelial disease of the transitional epithelial cells. Although several studies have shown the association of bladder recurrence following UUT-UCC, little is known on the incidence of concomitant UUT-UCC and bladder cancer (BC) without previous BC.
Materials and methods
A retrospective review of 673 patients diagnosed and treated for UUT-UCC was performed. Patients with history of BC were excluded. We investigated age, sex, location of the upper tract tumor (calyx, renal pelvis, upper ureter, mid-ureter, lower ureter), multifocality, clinical symptoms, tumor grade and pathological stage. Contingency tables and chi-square test were used for categorical variables and analysis of variance (ANOVA) for quantitative variables.
Results
450 patients eligible for inclusion were identified. Of these, 76 (17 %) presented concomitant primary UUT-UCC and BC. Location of primary UUT-UCC was in calyx and/or renal pelvis in 25 patients (34 %), upper ureter 8 (11 %) and lower ureter 37 (49 %). In 6 patients (8 %), data were missing. Concomitant BC was found in 10, 18, and 33 % of patients with primary caliceal/renal pelvis, upper ureter and lower ureter UUT-UCC, respectively. On multivariate analysis, location of UUT-UCC was the only predictive factor for concomitant bladder tumor (OR: 1.7; 95 % CI, 1.007–2.906 p = 0.047).
Conclusions
Our findings suggest that the possibility of concomitant BC in primary diagnosed patient with UUT-UCC is as high as 33 % and mainly depends on upper tract tumor location.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that UUT-UCC is a pan-urothelial disease of the urothelial cells, which covers the luminal surface of the entire urinary tract extending from renal calyces to the proximal urethra. Furthermore, many UCCs are multifocal and synchronous tumors that can be detected both in the bladder and in the upper tracts at primary diagnosis [1–7].
The possibility of developing synchronous, multifocal UCC in the urinary tract may be explained by two theories: The first is the “Field Cancerization theory” [3] in which the multifocal development of cancer is secondary to the continuous exposure of the urothelium to carcinogens in the urine and the second is the “seeding or cancer cell implantation of cancers cells theory” [4] in which multiple carcinomas are the result of intraluminal spread from a single lesion.
The urinary bladder is the most frequent site of recurrence following primary treatment of UTT, with rates that vary from 15 to 50 % [6, 8–12]. Up to 80–90 % of bladder recurrences occur within the first 2–3 years from primary treatment of UUT-UCC [6, 9, 10, 13–15]. Although UUT-UCC is an uncommon pathology after treatment of primary bladder tumors, it is known that its incidence is higher in patients with high-grade disease and those with urinary reflux [16, 17].
Very little is known on the simultaneous diagnosis of primary upper tract tumors and bladder carcinoma, although its incidence appears to be low. Only in 8–13 % of cases, concurrent bladder cancer is present [1, 5]. Specifically, to our knowledge, there are no studies that evaluate clinical factors that predict the simultaneous presence of UUT-UCC and bladder UUC.
Accordingly, the objective of this study was to determine whether exist any clinical factors that predict the presence of concomitant bladder cancer in patients with no previous history of bladder cancer who were diagnosed with primary UUT-UCC.
Materials and methods
Study design and data collection description
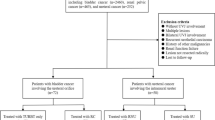
A retrospective analysis of 673 patients diagnosed and treated for UUT-UCC at our center from 1950 to 2008 was performed. Data collection and analysis were conducted in accordance with an Institutional Review Board (IRB) approved protocol. Patients were assessed and treated according to the Fundació Puigvert guidelines for the protection of human subjects recruited under institutional review board–approved protocols.
In order to evaluate preoperative predictive factors of concomitant bladder cancer and primary UUT-UCC, the study population was divided into two groups: (a) patients with primary UUT-UCC and simultaneous bladder cancer and (b) patients with primary UUT-UCC without bladder cancer.
Inclusion/exclusion criteria
Inclusion criteria were primary UUT-UCC treated either with radical or with conservative approach, and the presence of concomitant bladder cancer. Exclusion criteria were previous history of bladder cancer.
The following variables were investigated: age, sex, location of the tumor in the upper tract (calyx, renal pelvis, upper ureter, mid-ureter, lower ureter), multifocality (solitary/multiple), clinical symptoms (microscopic hematuria, gross hematuria, flank pain and urosepsis), radiological findings both on i.v. urography and on CT scan (normal, hydronephrosis, filling defect, non-functioning kidney, renal or pelvic mass), tumor grade and pathological stage. Location of primary upper tract tumor was confirmed by pathology specimen.
Tumors were staged according to the TNM classification 2002 and graded according the 1973 World Health Organization classification and then revisited according to last WHO and TNM classification.
Statistical analysis
Variables were described as mean and standard deviation for quantitative analysis, and as percentage and case load number for categorical analysis. Contingency tables and chi-square test were used for categorical variables analysis, and analysis of variance (ANOVA) was used for quantitative variables studies. Finally, a multivariate approach was performed using binary logistic regression and the forward stepwise method with the likelihood ratio (LR) for the choice of variables.
The final model was evaluated by the Hosmer–Lemenshow test.
The software used was SPSS (V18.0).
Results
Between June 1950 and September 2008, 673 patients, 551 men and 122 women (81.9 and 18.1 %, respectively), underwent radical nephroureterectomy (RNU) with removal of bladder-cuff for upper urinary tract tumor (UUTT) or a conservative approach with endoscopic resection of tumor. UUTT were located in calyx and renal pelvis in 348 patients, upper ureter 156 patients and lower ureter 40 patients. A total of 30 patients presented with concomitant tumors of calyx/pelvis and upper ureter: 12 with cancer in the upper and lower ureter, 16 with tumors of the calyx/pelvis and lower ureter and 10 with tumors of the calyx/pelvis as well as the upper and lower ureter (61 missing data). The mean age of this cohort was 65 years (SD: 10.82 and range: 27–91 years).
A total of 223 (33 %) patients were excluded from the study due to a previous history of bladder cancer; therefore, the review identified 450 patients suitable for the study. Of these patients, 76 (17 %) were found to have concomitant primary UUT-UCC and bladder cancer, and 374 (83 %) were found to have solely UUT-UCC. Demographic and radiological findings of the two groups are shown in Tables 1 and 2. Specifically, in the group of patients with UUT-UCC and concomitant bladder cancer (76 patients), there were 64 men (84 %) and 12 women (16 %). The mean age was 66 ± 11 years. The location of the primary UUT-UCC was found to be in the calyx and/or renal pelvis in 25 patients (34 %), in the upper ureter in 8 patients (11 %) and in the lower ureter in 37 patients (49 %). In 6 patients with concomitant bladder cancer (8 %), the location of the tumor was not recorded.
When looking at the overall population (450 patients), concomitant bladder cancer was found in 10, 18 and 33 % of patients with primary caliceal/renal pelvic, upper ureteral or lower ureteral UUT-UCC, respectively (Table 2). If we compare patients with or without concomitant bladder cancer, there were 52.8 % of tumors located in the lower ureter in patients with concomitant bladder cancer versus 22.6 % in those without.
Both on univariate and on multivariate analysis, age, sex, clinical symptoms, multifocality, grade and pathological stage did not predict concomitant bladder tumor presence. In particular, regarding pathological stage, non-muscle-invasive tumor was present in 220 patients, while 195 patients presented with muscle-invasive UUT-UCC (35 patients missing data); tumor grade was G1 in 19 patients (4.2 %), G2 in 190 (42.2 %) patients and G3 in 184 (40.9 %) patients (57 patients no grade applicable). Data on radiological findings (both on IVU and on CT scan) like hydronephrosis, filling defect, non-functioning kidney and renal or pelvic mass were not significant for predicting the presence of concomitant BC.
On multivariate analysis, the location of UUT-UCC in the distal ureter was the only predictive factor for the presence of a concomitant bladder tumor (OR: 1.7; 95 % CI, 1.007–2.906 p = 0.047) (Table 2).
Discussion
UUT-UCC is a rare disease with an estimated annual incidence in Western countries about one or two new cases per 100,000 inhabitants [18]. Probably because of that reason and similarities with urothelial carcinoma of the bladder, we have been managing that pathology for many years as bladder cancer. In the recent years, we have realized the natural history of UUT-UUCs differs from that of bladder cancer: 60 % of UUT-UCCs are invasive at diagnosis though only 15–25 % of bladder tumors are invasive at presentation; furthermore, as compared to bladder cancer, the peak incidence is at a later age at 70–80 years [1, 5, 19]. Although there is a lack of data in the current literature to provide strong recommendations, recent multicenter studies have motivated the European Association of Urology (EAU) Guideline Group on urothelial cell carcinoma of the upper urinary tract to publish new guides to aid clinicians in their daily practice [20]; this document represents the first real Guidelines for UUT-UUC.
In accord with several previously published reports [21, 22], this study revealed tumors within the renal pelvis are more common than ureteral lesions (pelvis/calyx 285 patients; 58 upper ureter; 114 lower ureter; populations of patients without previous BC) and also a similar percentage of multifocality, more than one lesion in UUT, around 10–20 % [23]. The prognostic significance of UUT tumor location is controversial. However, several studies have suggested that ureteral disease often confers a worse prognosis compared with renal pelvic tumors, with an associated higher risk of local recurrence and mortality [7, 24]. Recent multicenter studies have shown that there is no difference in outcomes between patients with renal pelvic tumors and those with ureteral tumors following nephroureterectomy. This finding confirms that only pT stage, grade and lymph node status were associated with disease recurrence and cancer-specific survival [25].
The most common site of recurrence is the bladder representing about 30–51 % of all recurrences [25, 26], whereas recurrence in the contralateral upper tract is observed in only 2–6 % of cases [27, 28]. Both upper urinary tract recurrence after treatment of bladder cancer and bladder recurrence after treatment of upper urinary tract TCC have been well documented but only a few studies have reported concurrence of UUT-UCC and BC; this is estimated to occur in 8–13 % of cases [25, 26]. Additionally, the analysis of risk factors and the incidence of primary UUT tumors and simultaneous bladder tumors in the absence of a previous history of bladder cancer have been poorly evaluated with no subsequent literature looking at this analysis depending on UUT tumor location.
In our series, location of the upper tract tumor was identified as the only predictive factor on univariate and multivariate analyses for simultaneous bladder cancer in patients with primary UUT-UCC. In these patients, the possibility of having a simultaneous urothelial bladder cancer is progressively higher as the ureteral tumor gets closer to the bladder. Tumors located in the renal pelvis/calices had a 10 % possibility of diagnosis concomitant bladder cancer; however, tumors located in the lumbar and sacral ureter had 18 and 33 % possibility, respectively, of diagnosis concomitant bladder cancer (p < 0.001) (Table 2).
The evidence that the closer the location of the UUT-UCC to the bladder, the higher the incidence of bladder cancer favors the seeding or cancer cell implantation theory [4]. This theory had already been shown from the clinical point of view with a much higher incidence of tumors in the bladder after UUTT than UUTT after bladder cancer and with several basic research studies shoving monoclonality in this multifocal disease [29].
The EAU guidelines recommend cystoscopy (Grade A) in all the patients diagnosed of UUT-UCC in order to rule-out concomitant BC [20]. Our article confirms a 10 % incidence of BC in patients with primary UUT-UCC localized in the upper urinary tract, and that one in 3 of patients diagnosed with UCC in the distal ureter will have concomitant BC. Following surgical treatment, it is also mandatory a closed bladder surveillance with cystoscopy and urinary cytology for at least 5 years [20] because of the possibility to develop a BC in the follow-up.
In our experience, in some cases in which we found concurrent bladder tumor with UTUC, a TURBT was performed in conjunction with UUT surgery (endoscopic resection, RNU, Ureterectomy), mostly in tumors that seemed to be non-muscle invasive during the TURB. The finding of concurrent BC has not changed the indication of UUT surgery but sometimes has changed the surgical approach in order to minimize the risk of tumor dissemination by providing, in case of concomitant bladder and upper tract tumor, bladder radical surgery if tumor is muscle invasive.
The main result of our study (location of primary UUT tumor is a predictive factor of concomitant bladder cancer) will not change the management of UUT tumors but may change future follow-up strategies for patients with primary UUT located in the lower urinary tract.
A limitation of the study is that, even if we give a new predictive factor of concomitant BC, this will not going to change daily practise because cystoscopy has always to be done when we diagnose a primary UTT. We still do not know whether the locations of the UTT will influence bladder recurrence and/or change the follow-up schedule of the bladder.
Another limitation of the study involves the retrospective nature of this review with some data missing from the earlier proportion of this series (back to 1950).
Conclusions
We found that 17 % of patients with UUT-UCC and without a previous history of bladder cancer had a synchronous bladder tumor. In our data, tumor location in the upper urinary tract appears to be the only predictive factor for the presence of concomitant bladder cancer, becoming progressively higher as the upper tract tumor gets closer to the bladder.
We consider cystoscopy mandatory in the staging of UUT-UCC because the risk of a concurrent lesion in the bladder is not negligible. Early identification of a synchronous tumor may allow for more informed management options and better surgical planning.
References
Hall MC, Womack S, Sagalowsky Al et al (1998) Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30 years of experience in 252 patients. Urology 52:594–601
Kirkali Z, Chan T, Manoharam M et al (2005) Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 66:4–34
Harris AL, Neal DE (1992) Bladder cancer-field versus clonal origin. N Engl J Medical 326:759–761
Carcia SB, Park HS, Novelli M et al (1999) Field cancerization, clonally and epithelial stem cells: the spread of mutated clones in epithelial sheets. J Pathol 187:61–81
Olgac S, Mazumdar M, Dalbagni G et al (2004) Urothelial carcinoma of the renal pelvis: a clinicopathologic study of 130 cases. Am J Surg Pathol 28:1545–1552
van der Poel HG, Antonini N, van Tinteren H et al (2005) Upper urinary tract cancer. Location is correlated with prognosis. Eur Urol 48:438–444
Park S, Hong B, Kim CS et al (2004) The impact of tumor location on prognosis of transitional cell carcinoma of the upper urinary tract. J Urol 171:621–625
Hisataki T, Miyao N, Masumori N et al (2000) Risk factors for the development of bladder cancer after upper tract urothelial cancer. Urology 55:663–667
Kang CH, Yu TJ, Hsieh HH et al (2003) The development of bladder tumors and controlateral upper urinary tract tumors after primary transitional cell carcinoma of the upper urinary tract. Cancer 98:1620–1626
Mullerad M, Russo P, Golijanin D et al (2004) Bladder cancer as a prognostic factor for upper tract transitional cell carcinoma. J Urol 172(6 Pt1):2177–2181
Terakawa T, Miyake H, Muramaki M et al (2008) Risk factors for intravesical recurrence after surgical management of transitional cell carcinoma of the upper urinary tract. Urology 71:123–127
Matsui Y, Utsunomiya N, Ichioka K et al (2005) Risk factors for subsequent development of bladder cancer after primary transitional cell carcinoma of the upper urinary tract. Urology 65(2):279–283
Raman JD, Ng CK, Boorjian SA et al (2005) Bladder cancer after managing upper urinary tract transitional cell carcinoma: predictive factors and pathology. BJU INT 96(7):1031–1035
Bariol SV, Stewart GD, McNeill SA et al (2004) Oncological control following laparoscopic nephroureterectomy: 7 year outcome. J Urol 172(5 Pt1):1805–1808
Salvador Bayarri J, Rodriguez-Villamil L, Imperatore V et al (2002) Bladder neoplasm after nephroureterectomy: does the surgery of the lower ureter transurethral resection or open surgery influence the evolution? Eur Urol 41(1):30–33
Amar AD, Das S (1985) Upper urinary tract transitional cell carcinoma in patients with bladder carcinoma and associated vesicoureteral reflux. J Urol 133(3):468–471
De Torres Mateos JA, Bañus Gassol JM, Palou Redorta J et al (1987) Vesicoureteral reflux and upper urinary tract transitional cell carcinoma after trans-urethral resection of recurrent superficial bladder carcinoma. J Urol 138(1):49–51
Jemal A, Siegel R, Ward E et al (2009) Cancer statistics. CA Cancer J Clin 59:225–249
Babjuk M, Oosterlinck W, Sylvester R et al (2008) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol 54(2):303–314
Rouprêt M, Zigeuner R, Palou J et al (2011) European Guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol 59:584–594
Huben RP, Mounzer AM, Murphy GP (1998) Tumor grade and stage as prognostic variables in upper tract urothelial tumors. Cancer 62:2016–2020
Murphy DM, Zincke H, Furlow WL (1980) Primary grade1 transitional cell carcinoma of the renal pelvis and ureter. J Urol 123:629–631
Faveretto RL, Shariat SF, Chade DC et al (2010) The effect of tumor location on prognosis in patients treated with radical nephroureterectomy at Memorial Sloan-Kettering Cancer Center. Eur Urol 58:574–580
Akdogan B, Dogan HS, Eskicorapci SY et al (2006) Prognostic significance of bladder tumor history and tumor location in upper urinary tract transitional cell carcinoma. J Urol 176:48–52
Ramman JD, Ng CK, Scherr DS et al (2010) Impact of tumor location on prognosis for patients with upper tract urothelial carcinoma managed by radical nephroureterectomy. Eur Urol 57:1072–1079
Azemar MD, Comperat E, Richard F et al (2011) Bladder recurrence after surgery for upper urinary tract urothelial cell carcinoma: frequency, risk factors, and surveillance. Urol Oncol 29(2):130–6 [Epub 2009 Sep 17]
Li WM, Shen JT, Li CC et al (2010) Oncologic outcomes following three different approaches to the distal ureter and bladder cuff in nephroureterectomy for primary upper urinary tract urothelial carcinoma. Eur Urol 57(6):963–969
Novara G, De Marco V, Dalpiaz O et al (2009) Independent predictors of contralateral metachronous upper urinary tract transitional cell carcinoma after nephroureterectomy: multi-institutional dataset from three European centers. Int J Urol 16(2):187–191
Catto JW, Hartmann A, Stoehr R et al (2006) Multifocal urothelial cancers with the mutator phenotype are of monoclonal origin and require panurothelial treatment for tumor clearance. J Urol 175(6):2323–2330
Acknowledgments
I.Gich for the statistical analysis, from the Department of Epidemiology, Hospital Sant a Creu I Sant Pau, Barcelona, Spain.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cosentino, M., Palou, J., Gaya, J.M. et al. Upper urinary tract urothelial cell carcinoma: location as a predictive factor for concomitant bladder carcinoma. World J Urol 31, 141–145 (2013). https://doi.org/10.1007/s00345-012-0877-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-012-0877-2