Abstract
The synergy of plants and microbes is one of the most interesting parts of holobiont research that yet have to be unwrapped before we can understand its implications in agriculture. Environmental stresses on plant ecology have further added to our curiosity in this context. Microorganisms are key players in benefitting plant health. This chapter mainly covers heavy metal and metalloid (HM)-induced phytotoxicity in different crops. We will be describing the role of soil-dwelling plant growth-promoting rhizobacteria (PGPR) in the mitigation of HM-induced damages in plants. We will also consider more generally the influential role of these microbes in biotic stress tolerance and the agricultural adoption of PGPR-involved strategies to combat HMs, which will help us provide adequate food for the world’s human population and the animals on which we depend for food, labor and companionship. Our starting point will be PGPR collected directly from the crop rhizosphere and associated with the lessening of HM content in crops, but excluding those intracellular endophytic microbes and those involved in PGPR-assisted phytoremediation. The principal rationale for these research efforts is to reduce the consumer’s health risks that are directly associated with the mobilisation or immobilisation of HMs inside plant cells. These microbes are possibly the best candidates for bioremediation because of their resilience and ability to withstand high HM levels, their mediation of the limiting effects that recalcitrant metals exert upon plant’s health, our successes of collaboration with the plants and microbes for biocontrol activities and microbial phytostimulation. This elaborative study covers the effect of 10 HMs (viz. Arsenic, Cadmium, Chromium, Cobalt, Copper, Lead, Manganese, Mercury, Nickel and Zinc) on crops and the HM-resistant PGPR discovered since 20 years. In addition, a general account of fundamental principles behind bacterial heavy metal resistance has been elaborated. Hence, this chapter will be of great interest especially to environmental microbiologists.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
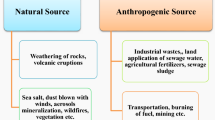
The global food crisis is one of the discernible situations that necessitate substantial attention. Due to high population growth (especially in China and India, the top two populated countries in the world) with a proportionate decrease in cultivable land, this catastrophe is becoming more acute daily. Apart from natural sources, several unplanned anthropogenic activities are known to generate an additional burden that jeopardises the environment and its ecosystem, contaminating its different components including soil and groundwater (Sharma and Archana 2016; Liu and Ma 2020). Heavy metal(loid)s (HMs) are one of the recalcitrant contaminants in agricultural fields that degrade the soil quality affecting the growth and crop yield, causing severe to chronic phytotoxicity. This might be due in part to the selection pressure that HMs impose on the soil-dwelling microbiome involved in phytostimulation and maintaining soil-biogeochemical cycling. However, certain microorganisms with their unequivocal properties combat HMs, developing an array of active or passive resistance mechanisms to survive in such a harsh environment (Chen et al. 2016; Tiwari and Lata 2018; Kotoky et al. 2019). There are successful candidates among them that have been found to colonise the soil area around the rhizosphere and rhizoplane (root surface) in response to enriched soil nutrients including the attractants released as root exudates from host plants. Host root exudates provide nutrients and act as signaling molecules to the colonisers to establish effective plant-microbe interactions. These exudates take the foremost part in controlling the diversity and composition of plant-associated soil microbial communities (Steinauer et al. 2016).
Plant growth-promoting rhizobacteria (PGPR) are group of free-living rhizobacterial communities that competitively colonise around the root surfaces stimulating plant growth by secreting a variety of phytostimulating substances and preventing some causes of host’s diseases in a sustainable manner (Kloepper 1978). Rhizobacterial plant growth-promoting (PGP) traits include 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity, phosphate solubilisation, indole-3- acetic acid (IAA) production, nitrogen fixation, siderophore production and many more. PGPR also protect plants from invading phytopathogens by secreting antibiotics, antifungal compounds, hydrocyanic acid (HCN), chitinase, etc. The PGPR strains with remarkable HM-withstanding property assist their immobile host to develop HM-tolerance for their combined survival in their contaminated habitat. These microbes are known as HM-resistant PGPR (HMR-PGPR). For several years, these PGPR strains have been isolated from the metal-contaminated rhizosphere of different crops including vegetables (Mitra et al. 2018a, b; Pramanik et al. 2017, 2018a, b; Khanna et al. 2019).
So, to ensure food security, the development of environmental cleanup methods is urgently needed to accomplish the reclamation of contaminated agricultural lands. Unlike the issue of organic pollutants, which sometimes seemed easier to resolve, mitigation of heavy metal contamination has been proving to be one of the more difficult tasks ever undertaken. Organic contaminants can be degraded. The metal pollutants are instead non-degradable in nature, and these contaminants can only be transformed into less toxic forms or removed by means that include accumulation and adsorption. Most of the conventional methods for remediation of heavy-metal-contaminated soil are physicochemical in nature which is expensive, ineffective, creates secondary pollutants and unsuitable for large areas (Quartacci et al. 2006). In this context, HM-resistant PGPR-induced bioremediation is one such approach which is inexpensive, effective, sustainable and ecofriendly. Unlike some non-PGPR microbial strains (Hu et al. 2007; Rehman et al. 2008; Muneer et al. 2009; Shakya et al. 2012; Liu et al. 2013; Davolos and Pietrangeli 2013) isolated from contaminated soil and groundwater, HM-resistant PGPR play a dual role in heavy metal bioremediation as well as plant growth promotion. Some of the non-PGPR strains have also been proven promising as potent bioremediators.
This chapter encompasses heavy metal and metalloid resistant plant growth-promoting rhizobacteria (HMR-PGPR), which are a functionally defined group of microorganisms, discovered during the last two decades that have been found to improve the growth of different crops across the world under different levels of HMs contamination. It covers latest information on diverse HMR-PGPR that exhibited various degrees of HM-resistance, different levels of release of plant growth-promoting substances and different capacities to accelerate plant growth by reducing HM stress-induced morpho-biochemical changes in the affected plants. A brief account of how biotic stress tolerance is facilitated by plant growth-promoting bacteria (PGPB), general HM resistant mechanisms, signaling cascades and genetically modified PGPR are also presented and discussed. Furthermore, we will provide some conclusions about the major obstacles to the application in HMR-PGPR in the field and future prospects of these strains. We will also discuss the times and places where non-HM resistant PGPR, metal-resistant plant growth-promoting bacteria (PGPB) and rhizobia have been advocated. Overall, this chapter is a substantial collection of information on heterogeneous microbial communities (especially HMR-PGPR) interacting with diverse hosts working in different soil types for crop improvement in a sustainable manner.
2 Heavy Metal(loid)-Induced Phytotoxicity in Crop Plants
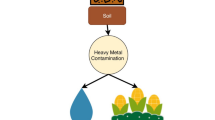
The incessant spread and increasing levels of HMs in agricultural soils have caused severe impairment of crops which not only results in reduced yield but also a serious toxic threat to the crop consumers. Plants, being immobile, are unable to escape from this stressful environment and uptake bioavailable non-essential HM cations into their plant cells along with essential soil nutrients. These HMs, upon surpassing certain threshold levels, impose severe cellular damages with various unusual morphological manifestations. The threshold level of HMs to induce phytotoxicity highly depends on plant species or even a particular cultivar. The uptake, translocation and cellular compartmentalisation of heavy metals may be governed by perhaps only one or just a few genes (Ernst 1996). Moreover, this also depends on the cationic forms of HMs. The observable external changes include reduction of seed germination, changes in root-shoot length and changes in root-shoot fresh and dry weight that ultimately decrease plant biomass (Table 22.1). As the root is directly exposed to the soil HMs, the root is the first organ encountered by toxic HMs, and the toxic effects follow into the shoots and other aerial parts of the plants. Affected root growth results in the poor acquisition of essential nutrients, and thereby an insufficient supply of nutrients to the photosynthetic cells in the aerial parts. To date, the members of Poaceae are the most studied crops on which the phytotoxic effects of different HMs have been investigated (Fig. 22.1). The phytotoxic consequences of all the ten HMs (viz. arsenic, cadmium, chromium, cobalt, copper, lead, manganese, mercury, nickel and zinc) discussed here have been studied on Poaceae (Fig. 22.1). After Poaceae, the HM-phytotoxicity studies have focused mainly on members of Fabaceae, Solanaceae and Brassicaceae, as predominant crops (Fig. 22.1). The less-studied families in the context with HM phytotoxicity are Amaryllidaceae, Euphorbiaceae, Amaranthaceae, Rosaceae, Linaceae, Malvaceae, Asteraceae and Cucurbitaceae (Fig. 22.1).
Among HMs, arsenic (As) is considered as an analog of phosphate (P) that competes with P-transporters in the root plasma membrane (Meharg and Macnair 1992). Although As-tolerance has been identified in a number of plant species (Meharg and Macnair 1992), elevated As-level has been found to negatively affect rice, maize, black gram, soybean, mung bean, cucumber, sorghum, barley, mustard, broccoli, pea and Chinese cabbage (Table 22.1). Biochemical changes identified in these crops include a reduction in photosynthetic pigments (chlorophyll, carotenoids), increased accumulation of reactive oxygen species (ROS), membrane lipid peroxidation, inhibition of ATP formation, enhanced proline and protein content and increased abscisic acid (ABA) synthesis (Table 22.1). Furthermore, altered activities of various cellular enzymes including RuBisCO, amylase, protease, catalase, peroxidase and other antioxidant enzymes are evident (Stoeva et al. 2005; Srivastava et al. 2017; Ghosh et al. 2018; Dong et al. 2020; Chauhan et al. 2020). Besides, As-mediated induction of cell death in root tips, proteomic alteration and disruption of normal cellular function have also been identified (Requejo and Tena 2006; Armendariz et al. 2016).
Likewise, phytotoxicity of other HMs reported almost parallel kinds of morpho-biochemical dysfunctions (Table 22.1). Studies of cadmium (Cd)-induced phytotoxicity have focused mainly on rice, wheat, tomato, potato, cucumber, pea, lettuce and mung bean (Table 22.1). An upsurge of ethylene content in rice seedlings has been noticed in response to Cd stress (Mitra et al. 2018a; Pramanik et al. 2018a) that is linked to increased accumulation of H2O2, leading to cell apoptosis (Chmielewska-Bak et al. 2014). Cobalt (Co), one of the naturally occurring HMs in the earth’s crust, spreads through human activities as well, and that element is taken up by plants from the contaminated soil. However, information on Co-phytotoxicity is less available in the literature compared to As and Cd. Wheat, barley, oilseed rape, tomato and cauliflower have been studied so far to elucidate Co-induced phytotoxicity (Chatterjee and Chatterjee 2000, 2003; Li et al. 2009; Ozfidan-Konakci et al. 2020). Co was found to decrease plant growth, photosynthetic rate, water content, osmotic potential, stomatal conductance, transpiration rate and cause chlorosis that ultimately manifested as decreased plant biomass (Table 22.1). An exogenous application of CoCl2 was shown to decrease plant ethylene levels compared to controls (Pramanik et al. 2017, 2018a). The number of phytotoxicity studies on chromium (Cr), copper (Cu), mercury (Hg), manganese (Mn), nickel (Ni), lead (Pb) and zinc (Zn) on the more common crop plants is also impressive, with reporting of various morpho-biochemical malfunctions in plants.
3 Role of Heavy Metal(loid) Resistant Plant Growth-Promoting Rhizobacteria in Crop Improvement
Soil, being the sink of nutrients for plants, is also the chief source of contaminants. The information summarised in Table 22.1 provides an idea of observed intensification of heavy metal contamination and consequences of the major HM contaminants on some common crops. Plants have developed their own natural mechanisms to regulate the uptake, translocation and accumulation of HMs, which is known as natural phytoremediation. In reality, plants are not the only warriors that are exposed to and affected by soil HMs, and indeed there similarly exist some close neighbors like the rhizospheric microbial community that also have direct or indirect influences on plant growth. Phytoremediation is one of the safest, eco-friendly technologies and is often triggered by plant growth-promoting bacteria (PGPB) as a response to accelerated HM uptake and accumulation in the plant cells (Ullah et al. 2015). This concept of designing and promoting bacteria-assisted phytoremediation technology is not intended to be applied only in the case of agricultural crops that are consumed by humans, cattle or other animals to reduce the high chances of HM toxicity in the food chain (Fig. 22.2). Rather, the preferred usage of PGPR-mediated bioremediation would be in such cases where some specific group of PGPR reduce both the HM-induced phytotoxic effects and HM-uptake as well (Fig. 22.2). PGPR fall under a special group of fast-growing microorganisms which are a good instance of phytostimulating biological agents of natural occurrence. Since many years, soil microbiologists and environmentalists have been devoting their tireless efforts to isolate PGPR strains with greater efficiency of bioremediation and plant growth promotion, and to apply their discoveries about HM-contaminated soil for the benefit of sustainable agriculture (Table 22.2). Here, in this review, we will largely examine HM-resistant PGPR (involved in PGPR-mediated bioremediation) publications from the last two decades and present their results in brief (Table 22.2). We have considered only those HM-resistant PGPR strains which were tested for their plant growth-promoting activities on selected crops, with those microbes having been applied as bioinoculants either in laboratory conditions or in the field. It is evident from Table 22.2 that the phytotoxic effects mentioned in Table 22.1 have been significantly reduced by the use of HM-resistant PGPR.
One of the most vital and key representations of this chapter is the documentation of culture media for the isolation of HM-resistant PGPR. Proteobacteria seem to have been the most commonly isolated group from all the stated culture media. Yeast extract mannitol (YEM) medium has been the most preferable isolation medium, followed by Davis Mingioli (DM) medium with Cd (Fig. 22.3). From a critical analysis of the information presented in Table 22.2, we find that the diversity of the HM-resistant PGPR community covers only three bacterial groups, i.e. proteobacteria, firmicutes and actinobacteria, and it is prominantly dominated by proteobacteria (Fig. 22.4). Furthermore, proteobacteria is the most abundant PGPR member responsible for resistance to all the studied heavy metal(oid)s. Actinobacteria exhibit their remediational property only against Cd. The firmicutes are a set of PGPR sensitive to As, Hg and Zn (Fig. 22.5). Additionally, among the PGPR members, all the documented phenomenal PGP traits are mainly portrayed by the proteobacterial representatives, and actinobacterial agents are accountable only for their IAA and ACC deaminase producing capabilities (Fig. 22.6). Moreover, in case of firmicutes, they are the silent member in case of N2 fixation, potassium solubilisation, ammonia and HCN production. However, the firmicutes have exhibited ACC activity, P-solubilisation, siderophore activity and IAA production (Fig. 22.6).
Medium used for isolation of heavy metal(loid)-resistant PGPR. (CDM Chemically defined medium, KBM King’s B medium, TM+HM T-medium with HM, YEM+CD Yeast extract mannitol with Cd, NA Nutrient agar, TCS Tryptone casein soya, TYE Tryptone yeast extract, LB+MM Luria–Bertani minmal media, DM+CD Davis Mingioli with Cd, AM Ashby’s mannitol, YEM Yeast extract mannitol, LB+CD Luria–Bertani with Cd, DFN+CR Dworkin and Foster nutrient with Cr, USA Urease screening agar)
4 Genetically Modified Plant Growth-Promoting Rhizobacteria for Crop Enhancement
Natural components like the PGPR play an indispensable role in the advancement of sustainable agriculture and also serve as an imperishable treasure box for the environment. Considering the limitations of these natural bio-agents, the idea of using genetic modification approaches has attracted the attention of scientists with the goal of attaining greater desired efficiency. With the improvements achieved by genetically engineering PGPR, the heavy metal accumulating gene and the biocontrolling genes can be assembled to conduct enhanced bioremediation and potentially achieve biocontrol in the rhizospheric soil. In this context, for superior cadmium (Cd2+) bioaccumulation purpose, the phytochelatin synthase gene (PCSAT) from Arabidopsis thaliana was introduced into Mesorhizobium huakuii strain B3 and then set up as a symbiosis with M. huakuii strain B3 and Astragalus sinicus, whereupon a desired activity was noted accordingly (Sriprang et al. 2003). It was possible to carry out that project because the peptides like phytochelatins (PC) and metallothioneins (MT) exhibit high affinity towards a variety of heavy metals (Chaudhary and Shukla 2019). Furthermore, genetically transformed rhizobacterial strains demonstrated significant biocontrol potentiality over fungal phytopathogens (Sattiraju et al. 2019). In such cases, incorporation of a mini-Tn5 vector containing the complete operon for the biosynthesis of an antifungal metabolite phenazine- 1- carboxylic acid (PCA), within Pseudomonas fluorescens has been documented to accelerate the suppression of fungal diseases by the genetically engineered bacterial strain in comparison to the natural bacterial strain (Timms-Wilson et al. 2000). Similar kinds of approaches were reported from several studies where genetically engineered PGPR strains showed enhanced PGP traits as well as biocontrol efficiency (Bloemberg and Lugtenberg 2001) and can be exemplified by the integration of Cry-toxin-encoding cry1Ac7 gene from Bacillus thuringiensis, chitinase-encoding chiA gene from Serratia marcescens and ACC deaminase-producing gene from Enterobacter cloacae into rhizobacterial strains like Pseudomonas sp. (Sattiraju et al. 2019). The relocation of sss gene from biocontrol strain P. fluorescens WCS365 to other P. fluorescens rhizobacterial strains was found to improve the competitive root colonising efficiency (Dekkers et al. 2000). Apart from the genetically modified PGPR, transgenic plants also display greater PGP traits, especially higher ACC deaminase activity and heavy metal accumulation (Zhuang et al. 2007; Stearns et al. 2005; Nie et al. 2002). However, genetically modified PGPB are considered less effective in terms of their survival and proliferation as compared to non-transformed versions of the same organisms; and this decreased fitness may be due to overburden of metabolic load by the expression of foreign genes (Glick 2020).
5 Plant Growth-Promoting Rhizobacteria in Biotic Stress Tolerance
The rhizosphere is a phenomenal environment where the plant-beneficial microbes especially the bacteria renowned as rhizobacteria, colonise and steadily perform several plant growth-promoting activities by means of facilitating nutrient availability and assimilation, and help conquer over disease-instigating microbes (Pérez-Montaño et al. 2014). The plant growth-promoting activities of these beneficial rhizobacteria include nitrogen fixation, solubilisation of minerals like phosphorus, production of ACC-deaminase and other plant growth regulators like auxins, gibberellins and cytokinins. Biocontrol properties are one of the key characteristic features of these PGPR (Kloepper 1978). Their antagonistic potentiality against phytopathogens is mainly categorised according to activities like the production of siderophores, lytic enzymes, antibiotics, bacteriocins, volatile organic compounds (VOC), hydrogen cyanide (HCN) and their ability to obstruct bacterial quorum sensing (Aloo et al. 2019; Pérez-Montaño et al. 2014; Kumar and Dubey 2012). Apart from these capabilities, PGPR also induce systemic resistance (ISR) proficiency which can help suppress pathogenicity that other microbes exhibit against host plants, and PGPR do as well improve the sustainability of agricultural systems (Beneduzi et al. 2012). Among the reported PGPR genera, Pseudomonas sp., Bacillus sp. and Streptomyces sp. are the warhorses in the avenue of biocontrol of phytopathogens (Table 22.3; Arrebola et al. 2019). Moreover, the rhizobacterial phyla involved in this job are dominated by proteobacteria, firmicutes and actinobacteria (Fig. 22.7). The bio-protecting efficiency of PGPR are not only restricted to countering the pathogenic microbial members of the rhizosphere community like fungi and bacteria, but are also promising as agents against metazoan phytopathogens like insects and nematodes (Table 22.3; Fig. 22.8).
The biological control of phytopathogens by the PGPR group of organisms does in many ways strengthen both plant and soil health. Rhizobacterial secretion of siderophores is among the mechanisms exhibited by the PGPR members that are antagonistic against other microoganisms. The actions of siderophores are based upon their chelation of iron which inhibits iron-dependent nutritional or energetic processes in those other microbes (Chaiharn et al. 2009). In iron-limiting soil environments, the binding of iron by siderophore-producing rhizobacteria can also boost up the availability of iron to those plants that are able to accumulate siderophore-bound iron (Tank et al. 2012). Apart from iron chelation, siderophores can bind with other heavy metals like Cd, Cu, Pb, Al and Zn which in turn diminishes the stress to plants that may be imposed by those other heavy metals (Ahemad and Kibret 2014). PGPR additionally produce various defensive lytic enzymes such as chitinase, glucanase, cellulase, protease, chitosanase, peroxidase, catalase, phenolic lyase, superoxide dismutase, etc. (Aloo et al. 2019) which can act to protect plants from the pathogens. Pathogens responsible for several plant diseases are directly liable for plant growth inhibition and these are mainly fungi and insects (Banerjee and Mandal 2019). The lytic enzymes like chitinase, chitosanase, glucanase and cellulases produced by PGPR act in a straight line biocontrol mechanism against the chitin and glucan cell wall components of those fungi and insects. Disease control management by the PGPR is additionally accomplished not only by means of antibiotics produced like zwittermicin, mycosubtilin, gramicidin S, polymyxin B, bacilysin, rhizocticins, etc. but also by bacteriocins (Saraf et al. 2014; Haggag 2008; Leclere et al. 2005; Chin-A-Woeng et al. 2003). Enhancement of plant defense mechanisms by a combination of ISR plus biocontrol ability was also validated by studies of several PGPR that produce VOCs (Shafi et al. 2017; Cao et al. 2011). The occurrence of such dual potentiality can be exemplified by VOCs like 2, 3-butanediol, isoprene and acetoin that are produced by different PGPR (Lee et al. 2015; Ryu et al. 2004). Plant pathogens can also be controlled by many PGPR via HCN production, a recognised VOC which disrupts the electron transport system that leads to blocking the energy supply of the pathogens (Patel and Minocheherhomji 2018).
In recent years, biocontrol has become an emerging and promising technological approach in developing sustainability in agriculture with optimism both for its comprehensive potentiality against various types of plant pathogens as well as its being an efficient alternative resource over chemical fungicides and pesticides. In addition, several PGPR have been documented for their ability to remediate heavy metals in agricultural fields. There are indeed many published reports on heavy metal remediation by the PGPR (Table 22.2); although reporting on the combinational effect of HM bioremediation cum biocontrol activity by PGPR is very scarce. Two such examples of combined activity by PGPR are Alcaligenes sp. and Pseudomonas aeruginosa, where nickel and manganese bioremediations were testified along with aptitude for biocontrol of phytopathogens like Aspergillus niger, A. flavus, Fusarium oxysporum, Alternaria alternata, Cercospora arachichola and Metarhizium anisopliae (Sayyed and Patel 2011). There is some justifiable optimism that the application of this kind of heavy metal remediating cum biocontrolling PGPR in agricultural fields will replace the usage of chemical pesticides and fertilisers, which in turn will decrease the bioaccumulation of hazardous chemicals into agronomic plants and passage of these contaminants further up the biological chain, leading to a more environmentally safe and affordable agriculture in terms of human welfare. However, the effective biocontrol property of PGPR against invading phytopathogens is subject to the considerations of soil type, host plant species and influential holobiont microbial community in the rhizosphere (Subrahmanyam et al. 2020).
6 Mechanism of Heavy Metal(loid) Resistance by Plant Growth-Promoting Rhizobacteria
Plant-associated HM-resistant PGPR are more profoundly present in heavy-metal-contaminated soil, as evidenced by many earlier publications (Pandey et al. 2010; Chen et al. 2016; Treesubsuntorn et al. 2018; Pramanik et al. 2017, 2018a, b; Mitra et al. 2018a, b). Such PGPR strains are known to develop resistance mechanisms in adaptation to the different HM ions present in their habitats (Table 22.4). The various known survival strategies which metal tolerant species have used to combat HMs are summarised in Table 22.4. These include active transport of metal ions (efflux/influx) by the presence of a group of specific membrane bound, cytoplasmic or periplasmic metal transporters (Nies 2003; Yang et al. 2019), production of biodegradable metal chelators like siderophores (Sinha and Mukherjee 2008; Dimkpa et al. 2008), intracellular bioaccumulation and biosorption (Chen et al. 2016; Treesubsuntorn et al. 2018; Pramanik et al. 2017, 2018a, b; Mitra et al. 2018a, b; Pal and Sengupta 2019), enzymatic oxidation and reduction metal transformations (Chatterjee et al. 2009; Pramanik et al. 2016; Ghosh et al. 2018; Kamaruzzaman et al. 2019), extracellular complexation by the secretion of extracellular polysaccharides (EPSs) (Gupta and Diwan 2017), etc. (Table 22.4). The genetic determinants of metal resistance can be localised either in chromosomal or extrachromosomal genetic elements.
Heavy metals most commonly exist in the form of cations which can form many unspecific complexes. Among all these, a few HM cations are important biological trace elements (such as Mn2+, Zn2+, Cu2+, Ni2+, Mo2+, Co2+) used in regulating several important biochemical reactions. The intracellular passage of different HMs is, in fact, governed by two opposite types of uptake systems. The first of these systems is constitutively expressed, fast, unspecific and uses a variety of substrates, while the second system is inducible, slow and highly specific for substrates (Nies 1999). The main driving force for the first system is an electrochemical gradient across the plasma membrane, and for the second system it is the energy generated by ATP hydrolysis (Nies and Silver 1995). The constitutive and unspecific nature of the first kind of system causes most of the HM-toxicity in bacteria as it continuously accumulates a heavy metal even if the cell already contains a high concentration of that same HM (Nies and Silver 1995). After a metal has been accumulated beyond threshold levels, HMs impart several toxic effects such as inhibition of enzyme actions due to the binding of Hg2+, Cd2+ and Ag2+ to -SH groups, generation of oxidative stress and inhibition of the activity of sulphate and phosphate compounds by structurally related chromate and arsenate, respectively. Briefly, there are six widely known heavy metal resistance mechanisms in bacteria, they are: (1) exclusion of HMs by permeability barriers, (2) extracellular sequestration, (3) intracellular sequestration, (4) enzymatic detoxification of HMs, (5) active transport or efflux system of HMs and (6) reduction in HM sensitivity of cellular targets.
However, the details of many heavy metal resistance mechanisms used by PGPR are still to be fully explored, and we will have to unravel the genetic mysteries behind metal-PGPR interactions to effectively apply them for HM-bioremediation.
7 Constraints in the Application of Plant Growth-Promoting Rhizobacteria
Although the PGPR strains far discovered have proven promising in controlled laboratory conditions, their efficacy in reality is contingent on how they act in field conditions. During the last few decades, a number of PGPR strains have been discovered around the world but few reached the ultimate goal of having utility for farmers. In contrast to the laboratory, the reality of field work is one of non-optimal conditions that may or may not be favouarbale for the survival and proliferation of the PGPR strains (Glick 2020). The existence and growth of field-applied PGPR strains indeed depends on a vast range of adverse environmental factors that need to be overcome so that the microbes take part in assisting plant growth-promotion activities in contaminated soil (Fig. 22.9). It is not an easy task to achieve successful application of such PGPR strains even if they hold a bunch of potentially beneficial traits for the crop plants. Apart from following government-enforced guidelines, one of the major constraints in field application is soil type and it directly influences the survival and growth of the microbial communities (Fig. 22.9). To introduce a genetically engineered orgainsm, we need to give special attention to the fact that government legislation varies from country-to-country. Soil parameters such as compaction, oxygen content, pH and temperature are also crucial in this respect because they can affect the functioning of the microbes. In contrast to wild type indigenous strains, the genetically modified organisms are often less adaptive perhaps as a consequence of burdensome metabolic demands due to the expression and perhaps overexpression of foreign DNA (Glick 2020). In addition, PGPR strains often do not have equal abilities to compete with soil-borne phytopathogens and other antagonistic soil microbial communities, the PGPR strains sometimes do not have the capacities to tolerate a wide range of soil contaminants, and habituation to growing in nutrient-rich media under laboratory conditions may have resulted in functional loss of active genes that previously made the microbes suitable in contaminated rhizopshere environments (Glick 2020; Fig. 22.9).
8 Conclusion
Heavy metal(loid)-affected agricultural crops have benefitted for many years from the application of indigenous HM-resistant PGPR. Although there are a lot of constraints associated with the application of these microorganisms, their great diversity and natural abundance in contaminated soil offers a ray of hope as we explore their potential role in agriculture. Recent advancements in bioremediation strategies have given us cause for optimism. But, before field application, these PGPR should be verified for their degree of metal resistance, their level of plant growth-promoting traits, and obviously their ability to reduce HM-content in plant parts under controlled conditions. Henceforth, these PGPR are naturally dwelling microflora that should be isolated, enriched and applied for sustainable agriculture in HM-contaminated fields.

Contributing authors of this book chapter
References
Aafi NE, Brhada F, Dary M et al (2012) Rhizostabilization of metals in soils using Lupinus luteus inoculated with the metal resistant rhizobacterium Serratia sp. MSMC541. Int J Phytoremediat 14(3):261–274
Achouak W, Sutra L, Heulin T et al (2000) Pseudomonas brassicacearum sp. nov. and Pseudomonas thivervalensis sp. nov., two root-associated bacteria isolated from Brassica napus and Arabidopsis thaliana. Int J Syst Evol Micr 50(1):9–18
Adaikkalam V, Swarup S (2002) Molecular characterization of an operon, cueAR, encoding a putative P1-type ATPase and a MerR-type regulatory protein involved in copper homeostasis in Pseudomonas putida. Microbiology 148:2857–2867
Adediran GA, Ngwenya BT, Mosselmans JFW et al (2015) Mechanisms behind bacteria induced plant growth promotion and Zn accumulation in Brassica juncea. J Hazard 283:490–499
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ Sci 26:1–20
Akanbi-Gada MA, Ogunkunle CO, Vishwakarma V et al (2019) Phytotoxicity of nano-zinc oxide to tomato plant (Solanum lycopersicum L.): Zn uptake, stress enzymes response and influence on non-enzymatic antioxidants in fruits. Environ Technol Innov 14:100325
Akinci IE, Akinci S, Yilmaz K (2010) Response of tomato (Solanum lycopersicum L.) to lead toxicity: Growth, element uptake, chlorophyll and water content. Afr J Agric Res 5(6):416–423
Almaghrabi OA, Massoud SI, Abdelmoneim TS (2013) Influence of inoculation with plant growth promoting rhizobacteria (PGPR) on tomato plant growth and nematode reproduction under greenhouse conditions. Saudi J Biol Sci 20:57–61
Aloo BN, Makumba BA, Mbega ER (2019) The potential of bacilli rhizobacteria for sustainable crop production and environmental sustainability. Microbiol Res 219:26–39
Andersen JB, Koch B, Nielsen TH et al (2003) Surface motility in Pseudomonas sp. DSS73 is required for efficient biological containment of the root-pathogenic microfungi Rhizoctonia solani and Pythium ultimum. Microbiology 149:37–46
Armendariz AL, Talano MA, Oller ALW et al (2015) Effect of arsenic on tolerance mechanisms of two plant growth-promoting bacteria used as biological inoculants. J Environ Sci 33:203–210
Armendariz AL, Talano MA, Travaglia C et al (2016) Arsenic toxicity in soybean seedlings and their attenuation mechanisms. Plant Physiol Biochem 98:119–127
Arrebola E, Tienda S, Vida C et al (2019) Fitness features involved in the biocontrol interaction of pseudomonas chlororaphis with host plants: the case study of PcPCL1606. Front Microbiol 10:719
Ashwini N, Srividya S (2014) Potentiality of Bacillus subtilis as biocontrol agent for management of anthracnose disease of chilli caused by Colletotrichum gloeosporioides OGC1. 3. Biotech 4:127–136
Banerjee S, Mandal NC (2019) Diversity of chitinase-producing bacteria and their possible role in plant pest control. In: Satyanarayana T, Das SK, Johri BN (eds) Microbial diversity in ecosystem sustainability and biotechnological applications. Springer, Singapore, pp 457–491
Belimov AA, Kunakova AM, Safronova VI et al (2004) Employment of rhizobacteria for the inoculation of barley plants cultivated in soil contaminated with lead and cadmium. Microbiology 73:99–106
Beneduzi A, Ambrosini A, Passaglia LM (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol 35:1044–1051
Bensidhoum L, Nabti E, Tabli N et al (2016) Heavy metal tolerant Pseudomonas protegens isolates from agricultural well water in northeastern Algeria with plant growth promoting, insecticidal and antifungal activities. Eur J Soil Biol 75:38–46
Bhattacharyya C, Bakshi U, Mallick I et al (2017) Genome-guided insights into the plant growth promotion capabilities of the physiologically versatile Bacillus aryabhattai strain AB211. Front Microbiol 8:411
Bjelić D, Marinković J, Tintor B et al (2018) Antifungal and plant growth promoting activities of indigenous rhizobacteria isolated from maize (Zea mays L.) rhizosphere. Commun Soil Sci Plan 49:88–98
Bloemberg GV, Lugtenberg BJ (2001) Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol 4:343–350
Bull CT, Shetty KG, Subbarao KV (2002) Interactions between myxobacteria, plant pathogenic fungi, and biocontrol agents. Plant Dis 86:889–896
Calderón CE, Pérez-García A, de Vicente A et al (2013) The dar genes of Pseudomonas chlororaphis PCL1606 are crucial for biocontrol activity via production of the antifungal compound 2-hexyl, 5-propyl resorcinol. Mol Plant Microbe In 26:554–565
Calzada Urquiza C, Arvizu Hernández I, Cruz Medina JA et al (2016) Identification by MALDI-TOF mass spectrometry of mercury-resistant bacteria associated with the rhizosphere of an apple orchard. Geomicrobiol J 34:176–182
Cao Q, Hu Q-H, Khan S et al (2007) Wheat phytotoxicity from arsenic and cadmium separately and together in solution culture and in a calcareous soil. J Hazard 148(1-2):377–382
Cao Y, Zhang Z, Ling N et al (2011) Bacillus subtilis SQR 9 can control Fusarium wilt in cucumber by colonizing plant roots. Biol Fert Soils 47:495–506
Carlot M, Giacomini A, Casella S (2002) Aspects of plant-microbe interactions in heavy metal polluted soil. Acta Biotechnol 22:13–20
Carrasco JA, Armario P, Pajuelo E et al (2005) Isolation and characterisation of symbiotically effective Rhizobium resistant to arsenic and heavy metals after the toxic spill at the Aznalcollar pyrite mine. Soil Biol Biochem 37(6):1131–1140
Castillo UF, Strobel GA, Ford EJ et al (2002) Munumbicins, wide-spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedia nigriscans. Microbiology 48:2675–2685
Cavalca L, Zanchi R, Corsini A et al (2010) Arsenic-resistant bacteria associated with roots of the wild Cirsium arvense (L.) plant from an arsenic polluted soil, and screening of potential plant growth-promoting characteristics. Syst Appl Microbiol 33:154–164
Chaiharn M, Chunhaleuchanon S, Lumyong S (2009) Screening siderophore producing bacteria as potential biological control agent for fungal rice pathogens in Thailand. World J Microbiol Biot 25:1919–1928
Chatterjee J, Chatterjee C (2000) Phytotoxicity of cobalt, chromium and copper in cauliflower. Environ Pollut 109(1):69–74
Chatterjee J, Chatterjee C (2003) Management of phytotoxicity of cobalt in tomato by chemical measures. Plant Sci 164(5):793–801
Chatterjee C, Dube B, Sinha P et al (2004) Detrimental effects of lead phytotoxicity on growth, yield, and metabolism of rice. Commun Soil Sci Plan 35(1-2):255–265
Chatterjee S, Sau GB, Mukherjee SK (2009) Plant growth promotion by a hexavalent chromium reducing bacterial strain, Cellulosimicrobium cellulans KUCr3. World J Microbiol Biotechnol 25:1829–1836
Chaudhary T, Shukla P (2019) Bioinoculants for bioremediation applications and disease resistance: innovative perspectives. Indian J Microbiol 59:129–136
Chauhan R, Awasthi S, Indoliya Y et al (2020) Transcriptome and proteome analyses reveal selenium mediated amelioration of arsenic toxicity in rice (Oryza sativa L.). J Hazard 390:122122
Chen XH, Koumoutsi A, Scholz R et al (2007) Comparative analysis of the complete genome sequence of the plant growth–promoting bacterium Bacillus amyloliquefaciens FZB42. Nat Biotechnol 25:1007–1014
Chen Y, Chao Y, Li Y et al (2016) Survival strategies of the plant-associated bacterium Enterobacter sp. strain EG16 under cadmium stress. Appl Environ Microbiol 82:1734–1744
Chidambaram A, Sundaramoorthy P, Murugan A et al (2009) Chromium induced cytotoxicity in blackgram (Vigna mungo L.). J Environ Health Sci Eng 6(1):17–22
Chin-A-Woeng TF, Bloemberg GV, Lugtenberg BJ (2003) Phenazines and their role in biocontrol by Pseudomonas bacteria. New Phytol 157:503–523
Chmielewska-Bak J, Lefèvre I, Lutts S, Kulik A, Deckert J (2014) Effect of cobalt chloride on soybean seedlings subjected to cadmium stress. Acta Soc Bot Pol 83(3)
Cho U-H, Park J-O (2000) Mercury-induced oxidative stress in tomato seedlings. Plant Sci 156(1):1–9
Chou T-S, Chao Y-Y, Huang W-D et al (2011) Effect of magnesium deficiency on antioxidant status and cadmium toxicity in rice seedlings. J Plant Physiol 168(10):1021–1030
Danish S, Kiran S, Fahad S et al (2019) Alleviation of chromium toxicity in maize by Fe fortification and chromium tolerant ACC deaminase producing plant growth promoting rhizobacteria. Ecotoxicol Environ Saf 185:109706
Dary M, Chamber-Pérez M, Palomares A et al (2010) “In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J Hazard 177(1-3):323–330
Das S, Jean JS, Chou ML et al (2016) Arsenite-oxidizing bacteria exhibiting plant growth promoting traits isolated from the rhizosphere of Oryza sativa L.: implications for mitigation of arsenic contamination in paddies. J Hazard Mater 302:10–18
Dave R, Tripathi RD, Dwivedi S et al (2013) Arsenate and arsenite exposure modulate antioxidants and amino acids in contrasting arsenic accumulating rice (Oryza sativa L.) genotypes. J Hazard Mater 262:1123–1131
Davolos D, Pietrangeli B (2013) A molecular study on bacterial resistance to arsenic-toxicity in surface and underground waters of Latium (Italy). Ecotoxicol Environ Saf. 96:1–9
Dekkers LC, Mulders IH, Phoelich CC et al (2000) The sss colonization gene of the tomato-Fusarium oxysporum f. sp. radicis-lycopersici biocontrol strain Pseudomonas fluorescens WCS365 can improve root colonization of other wild-type Pseudomonas spp. bacteria. Mol Plant Microbe Interact 13:1177–1183
Deshwal VK, Pandey P, Kang SC et al (2003) Rhizobia as a biological control agent against soil borne plant pathogenic fungi. Indian J Exp Biol 41:1160–1164
Dias MC, Monteiro C, Moutinho-Pereira J et al (2013) Cadmium toxicity affects photosynthesis and plant growth at different levels. Acta Physiol Plant 35(4):1281–1289
Dimkpa C, Svatoš A, Merten D, Büchel G, Kothe E (2008) Hydroxamate siderophores produced by streptomyces acidiscabies E13 bind nickel and promote growth in cowpea (Vigna unguiculata L.) under nickel stress. Can J Microbiol 54(3):163–172
Dong Y, Gao M, Song Z et al (2020) Microplastic particles increase arsenic toxicity to rice seedlings. Environ Pollut 259:113892
Eleftheriou EP, Michalopoulou VA, Adamakis I-DS (2015) Aberration of mitosis by hexavalent chromium in some Fabaceae members is mediated by species-specific microtubule disruption. Environ Sci Pollut Res 22(10):7590–7599
Ernst WHO (1996) Phytotoxicity of heavy metals. In: Rodriguez-Barrueco C (ed) Fertilizers and environment. Developments in plant and soil sciences, vol 66. Springer, Dordrecht, pp 423–430
Erturk FA, Agar G, Arslan E et al (2014) Determination of genomic instability and DNA methylation effects of Cr on maize (Zea mays L.) using RAPD and CRED-RA analysis. Acta Physiol Plant 36(6):1529–1537
Etesami H, Alikhani HA (2017) Evaluation of gram-positive rhizosphere and endophytic bacteria for biological control of fungal rice (Oryza sativa L.) pathogens. Eur J Plant Pathol 147:7–14
Faria JM, Teixeira DM, Pinto AP et al (2020) Toxic levels of manganese in an acidic Cambisol alters antioxidant enzymes activity, element uptake and subcellular distribution in Triticum aestivum. Ecotoxicol Environ Saf 193:110355
Fatnassi IC, Chiboub M, Saadani O et al (2015) Impact of dual inoculation with Rhizobium and PGPR on growth and antioxidant status of Vicia faba L. under copper stress. CR Biol 338(4):241–254
Führs H, Hartwig M, Molina LEB et al (2008) Early manganese-toxicity response in Vigna unguiculata L.–a proteomic and transcriptomic study. Proteomics 8(1):149–159
Ganesan V (2008) Rhizoremediation of cadmium soil using a cadmium-resistant plant growth-promoting rhizopseudomonad. Curr Microbiol 56(4):403–407
Ghosh R, Barman S, Khatun J et al (2016a) Biological control of Alternaria alternata causing leaf spot disease of Aloe vera using two strains of rhizobacteria. Biol Control 97:102–108
Ghosh R, Barman S, Mukherjee R et al (2016b) Role of phosphate solubilizing Burkholderia spp. for successful colonization and growth promotion of Lycopodium cernuum L.(Lycopodiaceae) in lateritic belt of Birbhum district of West Bengal, India. Microbiol Res 183:80–91
Ghosh PK, Maiti TK, Pramanik K et al (2018) The role of arsenic resistant Bacillus aryabhattai MCC3374 in promotion of rice seedlings growth and alleviation of arsenic phytotoxicity. Chemosphere 211:407–419
Gill RA, Zang L, Ali B et al (2015) Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 120:154–164
Glick BR (2020) Issues regarding the use of PGPB. In: Beneficial plant-bacterial interactions. Springer, Cham
Gontia-Mishra I, Sapre S, Sharma A et al (2016) Alleviation of mercury toxicity in wheat by the interaction of mercury-tolerant plant growth-promoting rhizobacteria. J Plant Growth Regul 35:1000–1012
Gopalakrishnan S, Humayun P, Kiran BK et al (2011) Evaluation of bacteria isolated from rice rhizosphere for biological control of charcoal rot of sorghum caused by Macrophomina phaseolina (Tassi) Goid. World J Microbiol Biotechnol 27:1313–1321
Goupil P, Souguir D, Ferjani E et al (2009) Expression of stress-related genes in tomato plants exposed to arsenic and chromium in nutrient solution. J Plant Physiol 166(13):1446–1452
Guo J, Chi J (2014) Effect of Cd-tolerant plant growth-promoting rhizobium on plant growth and Cd uptake by Lolium multiflorum Lam. and Glycine max (L.) Merr. in Cd-contaminated soil. Plant Soil 375(1-2):205–214
Gupta P, Diwan B (2017) Bacterial exopolysaccharide mediated heavy metal removal: a review on biosynthesis, mechanism and remediation strategies. Biotechnol Rep 13:58–71
Gupta A, Kumar M, Goel R (2004) Bioaccumulation properties of nickel-, cadmium-, and chromium-resistant mutants of Pseudomonas aeruginosa NBRI 4014 at alkaline pH. Biol Trace Elem Res 99:269–277
Haggag WM (2008) Isolation of bioactive antibiotic peptides from Bacillus brevis and Bacillus polymyxa against Botrytis grey mould in strawberry. Arch Phytopathol Plant Prot 41:477–491
Han H, Wang Q, He L-y et al (2018) Increased biomass and reduced rapeseed Cd accumulation of oilseed rape in the presence of Cd-immobilizing and polyamine-producing bacteria. J Hazard 353:280–289
Hao X, Xie P, Zhu YG et al (2015) Copper tolerance mechanisms of Mesorhizobium amorphae and its role in aiding phytostabilization by Robinia pseudoacacia in copper contaminated soil. Environ Sci Technol 49:2328–2340
Hassanein WA, Awny NM, El-Mougith AA et al (2009) Characterization and antagonistic activities of metabolite produced by Pseudomonas aeruginosa Sha8. J Appl Sci Res 5:392–403
Hu Q, Dou M, Qi H et al (2007) Detection, isolation, and identification of cadmium-resistant bacteria based on PCR-DGGE. J Environ. Sci. 19:1114–1119
Huang HC, Erickson RS (2007) Effect of seed treatment with Rhizobium leguminosarum on Pythium damping-off, seedling height, root nodulation, root biomass, shoot biomass, and seed yield of pea and lentil. J Phytopathol 155:31–37
Imtiyaz S, Agnihotri RK, Ganie SA et al (2014) Biochemical response of Glycine max (L.) Merr. to cobalt and lead stress. J Stress Physiol Biochem 10(3):259–272
Ishtiaq S, Mahmood S (2012) Phytotoxicity of nickel and its accumulation in tissues of three Vigna species at their early growth stages. J Appl Bot Food Qual 84(2):223
Islam F, Yasmeen T, Arif M et al (2016) Combined ability of chromium (Cr) tolerant plant growth promoting bacteria (PGPB) and stress alleviator (salicylic acid) in attenuation of chromium stress in maize plants. Plant Physiol Biochem 108:456–467
Jahan MS, Guo S, Baloch AR et al (2020) Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol Environ Saf 197:110593
Kamaruzzaman MA, Abdullah SRS, Hasan HA et al (2019) Potential of hexavalent chromium-resistant rhizosphere bacteria in promoting plant growth and hexavalent chromium reduction. J Environ Biol 40:427–433
Kamnev AA, Antonyuk LP, Kulikov LA et al (2004) Monitoring of cobalt (II) uptake and transformation in cells of the plant-associated soil bacterium Azospirillum brasilense using emission Mössbauer spectroscopy. BioMetals 17:457–466
Karthik C, Oves M, Sathya K et al (2016) Isolation and characterization of multi-potential Rhizobium strain ND2 and its plant growth-promoting activities under Cr (VI) stress. Arch Agron Soil Sci 63:1058–1069
Karthik C, Oves M, Sathya K et al (2017a) Isolation and characterization of multi-potential Rhizobium strain ND2 and its plant growth-promoting activities under Cr (VI) stress. Arch Agron Soil Sci 63:1058–1069
Karthik C, Elangovan N, Kumar TS et al (2017b) Characterization of multifarious plant growth promoting traits of rhizobacterial strain AR6 under Chromium (VI) stress. Microbiol. Res 204:65–71
Kavita B, Shukla S, Kumar GN et al (2008) Amelioration of phytotoxic effects of Cd on mung bean seedlings by gluconic acid secreting rhizobacterium Enterobacter asburiae PSI3 and implication of role of organic acid. World J Microbiol Biotechnol 24:2965–2972
Khan MR, Khan MM (2010) Effect of varying concentration of nickel and cobalt on the plant growth and yield of chickpea. Aust J Basic & Appl Sci 4(6):1036–1046
Khanna K, Jamwal VL, Kohli SK et al (2019) Role of plant growth promoting Bacteria (PGPRs) as biocontrol agents of Meloidogyne incognita through improved plant defense of Lycopersicon esculentum. Plant Soil 436:325–345
Khatun S, Ali MB, Hahn E-J et al (2008) Copper toxicity in Withania somnifera: growth and antioxidant enzymes responses of in vitro grown plants. Environ Exp Bot 64(3):279–285
Kloepper JW (1978) Plant growth-promoting rhizobacteria on radishes. In: Proceeding of the 4th Internet. Conf. on Plant Pathogenic Bacter, Station de Pathologie Vegetale et Phytobacteriologie, INRA, Angers, France
Kotoky R, Nath S, Maheshwari DK et al (2019) Cadmium resistant plant growth promoting rhizobacteria Serratia marcescens S2I7 associated with the growth promotion of rice plant. Environment Sustain 2:135–144
Kuffner M, De Maria S, Puschenreiter M et al (2010) Culturable bacteria from Zn-and Cd-accumulating Salix caprea with differential effects on plant growth and heavy metal availability. J Appl Microbiol 108(4):1471–1484
Kumar P (2012) Ph.D. thesis. Gurukul Kangri University, Haridwar, India
Kumar P, Dubey RC (2012) Plant growth promoting rhizobacteria for biocontrol of phytopathogens and yield enhancement of Phaseolus vulgaris. J Curr Pers Appl Microbiol 1:38
Labra M, Grassi F, Imazio S et al (2004) Genetic and DNA-methylation changes induced by potassium dichromate in Brassica napus L. Chemosphere 54(8):1049–1058
Lamhamdi M, Bakrim A, Aarab A et al (2011) Lead phytotoxicity on wheat (Triticum aestivum L.) seed germination and seedlings growth. CR Biol 334(2):118–126
Leclere V, Béchet M, Adam A et al (2005) Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism's antagonistic and biocontrol activities. Appl Environ Microbiol 71:4577–4584
Lee BD, Dutta S, Ryu H et al (2015) Induction of systemic resistance in Panax ginseng against Phytophthora cactorum by native Bacillus amyloliquefaciens HK34. J Ginseng Res 39:213–220
Li H-F, Gray C, Mico C et al (2009) Phytotoxicity and bioavailability of cobalt to plants in a range of soils. Chemosphere 75(7):979–986
Li X, Li D, Yan Z et al (2018) Biosorption and bioaccumulation characteristics of cadmium by plant growth-promoting rhizobacteria. RSC Adv 8:30902–30911
Ligon JM, Hill DS, Hammer PE et al (2000) Natural products with antifungal activity from Pseudomonas biocontrol bacteria. Pest Manag Sci 56:688–695
Liu Y, Ma R (2020) Human health risk assessment of heavy metals in groundwater in the luan river catchment within the north china plain. Geofluids 2020., Article ID 8391793:1–7
Liu D, Zou J, Meng Q et al (2009) Uptake and accumulation and oxidative stress in garlic (Allium sativum L.) under lead phytotoxicity. Ecotoxicology 18(1):134–143
Liu Q, Guo H, Li Y et al (2013) Acclimation of arsenic-resistant Fe(II)-oxidizing bacteria in aqueous environment. Int Biodeterior Biodegradation. 76:86–91
Lombardi L, Sebastiani L (2005) Copper toxicity in Prunus cerasifera: growth and antioxidant enzymes responses of in vitro grown plants. Plant Sci 168(3):797–802
Marrugo-Negrete J, Durango-Hernández J, Pinedo-Hernández J et al (2016) Mercury uptake and effects on growth in Jatropha curcas. Int J Environ Sci 48:120–125
Mathew DC, Ho Y-N, Gicana RG et al (2015) A rhizosphere-associated symbiont, Photobacterium spp. strain MELD1, and its targeted synergistic activity for phytoprotection against mercury. PLoS One 10(3):e0121178
Mazen MM, El-Batanony NH, Abd El-Monium MM et al (2008) Cultural filtrate of Rhizobium spp. and arbuscular mycorrhiza are potential biological control agents against root rot fungal diseases of faba bean. Glob J Biotechnol Biochem 3:32–41
Meharg AA, Macnair MR (1992) Suppression of the high affinity phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L. J Exp Bot 43(4):519–524
Mitra S, Pramanik K, Sarkar A et al (2018a) Bioaccumulation of cadmium by Enterobacter sp. and enhancement of rice seedling growth under cadmium stress. Ecotoxicol Environ Saf 156:183–196
Mitra S, Pramanik K, Ghosh PK et al (2018b) Characterization of Cd-resistant Klebsiella michiganensis MCC3089 and its potential for rice seedling growth promotion under Cd stress. Microbiol Res 210:12–25
Mohammed AF, Oloyede AR, Odeseye AO (2020) Biological control of bacterial wilt of tomato caused by Ralstonia solanacearum using Pseudomonas species isolated from the rhizosphere of tomato plants. Arch Phytopathol Plant Prot 53:1–16
Mossa A-W, Young SD, Crout NM (2020) Zinc uptake and phyto-toxicity: Comparing intensity-and capacity-based drivers. Sci Total Environ 699:134314
Muneer B, Rehman A, Shakoori FR et al (2009) Evaluation of Consortia of Microorganisms for Efficient Removal of Hexavalent Chromium from Industrial Wastewater. Bull Environ Contam Toxicol. 82:597–600
Naik MM, Pandey A, Dubey SK (2011) Lead-enhanced siderophore production and alteration in cell morphology in a Pb-resistant Pseudomonas aeruginosa strain 4EA. Curr Microbiol 62:409–414
Namdjoyan S, Kermanian H, Soorki AA et al (2017) Interactive effects of salicylic acid and nitric oxide in alleviating zinc toxicity of Safflower (Carthamus tinctorius L.). Ecotoxicology 26(6):752–761
Nazir H, Asghar HN, Zahir ZA et al (2016) Judicious use of kinetin to improve growth and yield of rice in nickel contaminated soil. Int J Phytoremediation 18(7):651–655
Nie L, Shah S, Rashid A et al (2002) Phytoremediation of arsenate contaminated soil by transgenic canola and the plant growth-promoting bacterium Enterobacter cloacae CAL2. Plant Physiol Biochem 40:355–361
Nie J, Pan Y, Shi J et al (2015) A comparative study on the uptake and toxicity of nickel added in the form of different salts to maize seedlings. Int J Env Res Pub He 12(12):15075–15087
Nies DH (1999) Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51:730–750
Nies DH (2003) Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev 27(2–3):313–339
Nies DH, Silver S (1995) Ion efflux systems involved in bacterial metal resistances. J. Ind. Microbiol 14:186–199
Oller ALW, Talano MA, Agostini E (2013) Screening of plant growth-promoting traits in arsenic-resistant bacteria isolated from the rhizosphere of soybean plants from Argentinean agricultural soil. Plant Soil 369:93–102
Oves M, Khan MS, Zaidi A (2013) Chromium reducing and plant growth promoting novel strain Pseudomonas aeruginosa OSG41 enhance chickpea growth in chromium amended soils. Eur J Soil Biol 56:72–83
Ozfidan-Konakci C, Yildiztugay E, Elbasan F et al (2020) Hydrogen sulfide (H2S) and nitric oxide (NO) alleviate cobalt toxicity in wheat (Triticum aestivum L.) by modulating photosynthesis, chloroplastic redox and antioxidant capacity. J Hazard 388:122061
Pal AK, Sengupta C (2019) Isolation of Cadmium and Lead Tolerant Plant Growth Promoting Rhizobacteria: Lysinibacillus varians and Pseudomonas putida from Indian Agricultural Soil. Soil Sediment Contam 28:601–629
Pandey V, Dixit V, Shyam R (2009) Chromium effect on ROS generation and detoxification in pea (Pisum sativum) leaf chloroplasts. Protoplasma 236(1–4):85–95
Pandey S, Saha P, Barai PK, Maiti TK (2010) Characterization of a Cd2+ −resistant strain of Ochrobactrum sp. isolated from slag disposal site of an iron and steel factory. Curr Microbiol 61(2):106–111
Patel TS, Minocheherhomji FP (2018) Plant growth promoting Rhizobacteria: blessing to agriculture. Int J Pure App Biosci 6:481–492
Pérez-Montaño F, Alías-Villegas C, Bellogín RA et al (2014) Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol Res 169:325–336
Pramanik K, Ghosh PK, Ghosh A et al (2016) Characterization of PGP Traits of a Hexavalent Chromium Resistant Raoultella sp. Isolated from the Rice Field near Industrial Sewage of Burdwan District, WB. India. Soil Sed Contam 25(3):313–331
Pramanik K, Mitra S, Sarkar A et al (2017) Characterization of cadmium-resistant Klebsiella pneumoniae MCC 3091 promoted rice seedling growth by alleviating phytotoxicity of cadmium. Environ Sci Pollut Res 24:24419–24437
Pramanik K, Mitra S, Sarkar A et al (2018a) Alleviation of phytotoxic effects of cadmium on rice seedlings by cadmium resistant PGPR strain Enterobacter aerogenes MCC 3092. J Hazard 351:317–329
Pramanik K, Mitra S, Sarkar A et al (2018b) Characterization of a Cd2+-resistant plant growth promoting rhizobacterium (Enterobacter sp.) and its effects on rice seedling growth promotion under Cd2+-stress in vitro. Agric Nat Resour 52(3):215–221
Prasanna L, Eijsink VG, Meadow R et al (2013) A novel strain of Brevibacillus laterosporus produces chitinases that contribute to its biocontrol potential. Appl Microbiol Biotechnol 97:1601–1611
Priya MS (2015) Biomanagement of rice root knot nematode, Meloidogyne graminicola Golden and Brichfield in aerobic rice. Int J Manag Soc Sci 3:591–598
Przemieniecki SW, Kurowski TP, Damszel M et al (2018) Effectiveness of the Bacillus sp. SP-A9 strain as a biological control agent for spring wheat (Triticum aestivum L.). J Agric Sci Technol 20:609–619
Quartacci MF, Argilla A, Baker AJM et al (2006) Phytoextraction of metals from a multiply contaminated soil by Indian mustard. Chemosphere 63:918–925
Rafique A, Amin A, Latif Z (2015) Screening and characterization of mercury-resistant nitrogen fixing bacteria and their use as biofertilizers and for mercury bioremediation. Pak J Zool 47:1271–1277
Rahman H, Sabreen S, Alam S et al (2005) Effects of nickel on growth and composition of metal micronutrients in barley plants grown in nutrient solution. J Plant Nutr 28(3):393–404
Rangeshwaran R, Prasad RD (2000) Isolation and evaluation of rhizospheric bacteria for biological control of chickpea wilt pathogens. J Biol Control 14:9–15
Rehman A, Zahoor A, Muneer B et al (2008) Chromium tolerance and reduction potential of a Bacillus sp.ev3 Isolated from metal contaminated wastewater. Bull Environ Contam Toxicol 81:25–29
Reiss A, Jørgensen LN (2017) Biological control of yellow rust of wheat (Puccinia striiformis) with Serenade® ASO (Bacillus subtilis strain QST713). Crop Prot 93:1–8
Requejo R, Tena M (2006) Maize response to acute arsenic toxicity as revealed by proteome analysis of plant shoots. Proteomics 6(S1):S156–S162
Rizvi A, Khan MS (2018) Heavy metal induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen fixing Azotobacter chroococcum. Ecotoxicol Environ Saf 157:9–20
Rodriguez E, Azevedo R, Fernandes P et al (2011) Cr (VI) induces DNA damage, cell cycle arrest and polyploidization: a flow cytometric and comet assay study in Pisum sativum. Chem Res Toxicol 24(7):1040–1047
Rodríguez-Llorente ID, Gamane D, Lafuente A et al (2010) Cadmium biosorption properties of the metal-resistant Ochrobactrum cytisi Azn6. 2. Eng Life Sci 10(1):49–56
Ryu CM, Farag MA, Hu CH et al (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026
Sądej W, Żołnowski AC, Ciećko Z et al (2020) Evaluation of the impact of soil contamination with mercury and application of soil amendments on the yield and chemical composition of Avena sativa L. J Environ Sci Health A 55(1):82–96
Sagardoy R, Morales F, López-Millán AF et al (2009) Effects of zinc toxicity on sugar beet (Beta vulgaris L.) plants grown in hydroponics. Plant Biol 11(3):339–350
Sager SMA, Wijaya L, Alyemeni MN et al (2020) Impact of different cadmium concentrations on two Pisum sativum L. genotypes. Pak J Bot 52(3):821–829
Sahu GK, Upadhyay S, Sahoo BB (2012) Mercury induced phytotoxicity and oxidative stress in wheat (Triticum aestivum L.) plants. Physiol Mol Biol Pla 18(1):21–31
Saleem MH, Fahad S, Khan SU et al (2020) Copper-induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China. Environ Sci Pollut Res 27(5):5211–5221
Santos EF, Santini JMK, Paixão AP et al (2017) Physiological highlights of manganese toxicity symptoms in soybean plants: mn toxicity responses. Plant Physiol Biochem 113:6–19
Saraf M, Pandya U, Thakkar A (2014) Role of allelochemicals in plant growth promoting rhizobacteria for biocontrol of phytopathogens. Microbiol Res 169:18–29
Sattiraju KS, Kotiyal S, Arora A et al (2019) Plant growth-promoting microbes: contribution to stress management in plant hosts. In: Sobti R, Arora N, Kothari R (eds) Environmental biotechnology: for sustainable future. Springer, Singapore, pp 199–236
Sayyed RZ, Patel PR (2011) Biocontrol potential of siderophore producing heavy metal resistant Alcaligenes sp. and Pseudomonas aeruginosa RZS3 vis-a-vis organophosphorus fungicide. Indian J Microbiol 51:266–272
Schwartz AR, Ortiz I, Maymon M et al (2013) Bacillus simplex—a little known PGPB with anti-fungal activity—alters pea legume root architecture and nodule morphology when coinoculated with Rhizobium leguminosarum bv. viciae. Agronomy 3:595–620
Selva Kumar S, Ram Krishna Rao M, Deepak Kumar R et al (2013) Biocontrol by plant growth promoting rhizobacteria against black scurf and stem canker disease of potato caused by Rhizoctonia solani. Arch Phytopathol Plant Protect 46:487–502
Selvaraj S, Ganeshamoorthi P, Anand T et al (2014) Evaluation of a liquid formulation of Pseudomonas fluorescens against Fusarium oxysporum f. sp. cubense and Helicotylenchus multicinctus in banana plantation. BioControl 59:345–355
Seneviratne M, Gunaratne S, Bandara T et al (2016) Plant growth promotion by Bradyrhizobium japonicum under heavy metal stress. S Afr J Bot 105:19–24
Shafi J, Tian H, Ji M (2017) Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol Biotechnol Equip 31:446–459
Shahid M, Dumat C, Pourrut B et al (2014) Assessing the effect of metal speciation on lead toxicity to Vicia faba pigment contents. J Geochem Explor 144:290–297
Shakya S, Pradhan B, Smith L et al (2012) Isolation and characterization of aerobic culturable arsenic-resistant bacteria from surface water and groundwater of Rautahat District, Nepal. J Environ Manag 95:S250–S255
Sharaff M, Kamat S, Archana G (2017) Analysis of copper tolerant rhizobacteria from the industrial belt of Gujarat, western India for plant growth promotion in metal polluted agriculture soils. Ecotoxicol Environ Saf 138:113–121
Sharma RK, Archana G (2016) Cadmium minimization in food crops by cadmium resistant plant growth promoting rhizobacteria. Appl. Soil Ecol. 107:66–78
Shiyab S, Chen J, Han FX et al (2009) Phytotoxicity of mercury in Indian mustard (Brassica juncea L.). Ecotoxicol Environ Saf 72(2):619–625
Shri M, Kumar S, Chakrabarty D et al (2009) Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol Environ Saf 72(4):1102–1110
Sinha S, Mukherjee SK (2008) Cadmium–induced siderophore production by a high Cd-resistant bacterial strain relieved Cd toxicity in plants through root colonization. Curr Microbiol 56(1):55–60
Someya N, Sato Y, Yamaguchi I et al (2007) Alleviation of nickel toxicity in plants by a rhizobacterium strain is not dependent on its siderophore production. Commun Soil Sci Plant Anal 38:1155–1162
Sriprang R, Hayashi M, Ono H et al (2003) Enhanced accumulation of Cd2+ by a Mesorhizobium sp. transformed with a gene from Arabidopsis thaliana coding for phytochelatin synthase. Appl Environ Microbiol 69(3):1791–1796
Srivastava S, Sinha P, Sharma YK (2017) Status of photosynthetic pigments, lipid peroxidation and anti-oxidative enzymes in Vigna mungo in presence of arsenic. J Plant Nutr 40(3):298–306
Stearns JC, Shah S, Greenberg BM et al (2005) Tolerance of transgenic canola expressing 1-aminocyclopropane-1-carboxylic acid deaminase to growth inhibition by nickel. Plant Physiol Biochem 43:701–708
Steinauer K, Chatzinotas A, Eisenhauer N (2016) Root exudate cocktails: the link between plant diversity and soil microorganisms? Ecol Evol. 6(20):7387–7396
Stoeva N, Berova M, Zlatev Z (2005) Effect of arsenic on some physiological parameters in bean plants. Biol Plant 49(2):293–296
Suarez Moreno ZR, Vinchira-Villarraga DM, Vergara-Morales DI et al (2019) Plant-growth promotion and biocontrol properties of three Streptomyces spp. isolates to control bacterial rice pathogens. Front Microbiol 10:290
Subrahmanyam G, Kumar A, Sandilya SP, Chutia M Yadav AN (2020) Diversity, plant growth promoting attributes, and agricultural applications of rhizospheric microbes. In: Plant microbiomes for sustainable agriculture. Springer, Cham, pp 1–52
Sundaramoorthy P, Chidambaram A, Ganesh KS et al (2010) Chromium stress in paddy: (i) nutrient status of paddy under chromium stress;(ii) phytoremediation of chromium by aquatic and terrestrial weeds. CR Biol 333(8):597–607
Syu C-H, Huang C-C, Jiang P-Y et al (2015) Arsenic accumulation and speciation in rice grains influenced by arsenic phytotoxicity and rice genotypes grown in arsenic-elevated paddy soils. J Hazard 286:179–186
Tabatabaei FS, Saeedizadeh A (2017) Rhizobacteria cooperative effect against Meloidogyne javanica in rhizosphere of legume seedlings. Hell Plant Prot J 10:25–34
Tank N, Rajendran N, Patel B et al (2012) Evaluation and biochemical characterization of a distinctive pyoverdin from a Pseudomonas isolated from chickpea rhizosphere. Braz J Microbiol 43:639–648
Timms-Wilson TM, Ellis RJ, Renwick A et al (2000) Chromosomal insertion of phenazine-1-carboxylic acid biosynthetic pathway enhances efficacy of damping-off disease control by Pseudomonas fluorescens. Mol Plant Microbe Interact 13:1293–1300
Tiwari S, Lata C (2018) Heavy metal stress, signaling, and tolerance due to plant-associated microbes: an overview. Front Plant Sci 9(452):1–12
Tiwari S, Pandey S, Chauhan PS et al (2017) Biocontrol agents in co-inoculation manages root knot nematode [Meloidogyne incognita (Kofoid & White) Chitwood] and enhances essential oil content in Ocimum basilicum L. Ind Crops Prod 97:292–301
Treesubsuntorn C, Dhurakit P, Khaksar G, Thiravetyan P (2018) Effect of microorganisms on reducing cadmium uptake and toxicity in rice (Oryza sativa L.). Environ Sci Pollut Res 25(26):25690–25701
Tripathi M, Munot HP, Shouche Y et al (2005) Isolation and functional characterization of siderophore-producing lead-and cadmium-resistant Pseudomonas putida KNP9. Curr Microbiol 50(5):233–237
Ullah A, Heng S, Hussain MF et al (2015) Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ Exp Bot 117:28–40
Uzair B, Kausar R, Bano SA et al (2018) Isolation and molecular characterization of a model antagonistic Pseudomonas aeruginosa divulging in vitro plant growth promoting characteristics. Biomed Res Int 2018:1–7
Vinit-Dunand F, Epron D, Alaoui-Sossé B et al (2002) Effects of copper on growth and on photosynthesis of mature and expanding leaves in cucumber plants. Plant Science Plant Sci 1:53–58
Vivas A, Biro B, Ruiz-Lozano JM et al (2006) Two bacterial strains isolated from a Zn-polluted soil enhance plant growth and mycorrhizal efficiency under Zn-toxicity. Chemosphere 62:1523–1533
Wang Q, Xiong D, Zhao P et al (2011) Effect of applying an arsenic-resistant and plant growth–promoting rhizobacterium to enhance soil arsenic phytoremediation by Populus deltoides LH05-17. J Appl Microbiol 111(5):1065–1074
Wang T, Wang S, Tang X et al (2020) Isolation of urease-producing bacteria and their effects on reducing Cd and Pb accumulation in lettuce (Lactuca sativa L.). Environ Sci Pollut Res Int 27(8):8707–8718
Wani PA, Khan MS (2010) Bacillus species enhance growth parameters of chickpea (Cicer arietinum L.) in chromium stressed soils. Food Chem Toxicol 48(11):3262–3267
Wani PA, Khan MS (2013) Nickel detoxification and plant growth promotion by multi metal resistant plant growth promoting Rhizobium species RL9. Bull Environ Contam Toxicol 91:117–124
Wani PA, Khan MS, Zaidi A (2007) Effect of metal tolerant plant growth promoting Bradyrhizobium sp.(vigna) on growth, symbiosis, seed yield and metal uptake by greengram plants. Chemosphere 70:36–45
Wani PA, Khan MS, Zaidi A (2008a) Effects of heavy metal toxicity on growth, symbiosis, seed yield and metal uptake in pea grown in metal amended soil. Bull Environ Contam Toxicol 81:152–158
Wani PA, Khan MS, Zaidi A (2008b) Impact of zinc-tolerant plant growth-promoting rhizobacteria on lentil grown in zinc-amended soil. Agron Sustain Dev 28(3):449–455
Wani PA, Zaidi A, Khan MS (2009) Chromium reducing and plant growth promoting potential of Mesorhizobium species under chromium stress. Bioremediat J13:121–129
Wu SC, Peng XL, Cheung KC et al (2009) Adsorption kinetics of Pb and Cd by two plant growth promoting rhizobacteria. Bioresour Technol. 100:4559–4563
Wu J, Guo J, Hu Y et al (2015) Distinct physiological responses of tomato and cucumber plants in silicon-mediated alleviation of cadmium stress. Front Plant Sci 6:453
Wu B, He T, Wang Z et al (2020) Insight into the mechanisms of plant growth promoting strain SNB6 on enhancing the phytoextraction in cadmium contaminated soil. J Hazard 385:121587
Xiang N, Lawrence KS, Kloepper JW et al (2017) Biological control of Heterodera glycines by spore-forming plant growth-promoting rhizobacteria (PGPR) on soybean. Plos ONE 12:e0181201
Xiao L, Xie CC, Cai J et al (2009) Identification and characterization of a chitinase-produced Bacillus showing significant antifungal activity. Curr Microbiol 58:528–533
Xiong T, Zhang T, Dumat C et al (2018) Airborne foliar transfer of particular metals in Lactuca sativa L.: Translocation, phytotoxicity, and bioaccessibility. Environ Sci Pollut Res Int 26(20):20064–20078
Xu J, Yang L, Wang Z et al (2006) Toxicity of copper on rice growth and accumulation of copper in rice grain in copper contaminated soil. Chemosphere 62(4):602–607
Xu D, Chen Z, Sun K et al (2013) Effect of cadmium on the physiological parameters and the subcellular cadmium localization in the potato (Solanum tuberosum L.). Ecotoxicol Environ Saf 97:147–153
Yang E, Sun L, Ding X et al (2019) Complete genome sequence of Caulobacter flavus RHGG3 T, a type species of the genus Caulobacter with plant growth-promoting traits and heavy metal resistance. 3 Biotech 9(2):42
Yoon Y, Lee W-M, An Y-J (2015) Phytotoxicity of arsenic compounds on crop plant seedlings. Environ Sci Pollut Res 22(14):11047–11056
Zaidi S, Musarrat J (2004) Characterization and nickel sorption kinetics of a new metal hyper-accumulator Bacillus sp. J Environ Sci Health A Tox Hazard Subst Environ Eng 39(3):681–691
Zaidi S, Usmani S, Singh BR et al (2006) Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 64(6):991–997
Zhou L, Yuen G, Wang Y et al (2016) Evaluation of bacterial biological control agents for control of root-knot nematode disease on tomato. Crop Prot 84:8–13
Zhou J, Ren J, Wang X et al (2017) Ascorbic Acid Alleviates Toxicity Induced by Excess Copper in Brassica campestris Ssp. Chinensis Makino. Commun Soil Sci Plan 48(6):656–664
Zhuang X, Chen J, Shim H et al (2007) New advances in plant growth-promoting rhizobacteria for bioremediation. Environ Int 33:406–413
Zribi K, Djébali N, Mrabet M et al (2011) Physiological responses to cadmium, copper, lead, and zinc of Sinorhizobium sp. strains nodulating Medicago sativa grown in Tunisian mining soils. Ann Microbiol 62:1181–1188
Zurdo-Pineiro JL, Rivas R, Trujillo ME et al (2007) Ochrobactrum cytisi sp. nov., isolated from nodules of Cytisus scoparius in Spain. Int J Syst Evol Micr 57(4):784–788
Acknowledgements
KP and SB are thankful for financial assistance from the University Grants Commission, India for UGC—Dr. D. S. Kothari Post-Doctoral Fellowship [No.F.4-2/2006 (BSR)/BL/19-20/0072 dated October 21, 2019] and Department of Biotechnology, India for granting DBT Twinning Project [No. BT/PR25738/NER/95/1329/2017 dated December 24, 2018], respectively. DM acknowledges DST-PURSE program, Visva-Bharati for financial support.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pramanik, K., Banerjee, S., Mukherjee, D., Saha, K.K., Maiti, T.K., Mandal, N.C. (2021). Beneficial Role of Plant Growth-Promoting Rhizobacteria in Bioremediation of Heavy Metal(loid)-Contaminated Agricultural Fields. In: Hurst, C.J. (eds) Microbes: The Foundation Stone of the Biosphere. Advances in Environmental Microbiology, vol 8. Springer, Cham. https://doi.org/10.1007/978-3-030-63512-1_22
Download citation
DOI: https://doi.org/10.1007/978-3-030-63512-1_22
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-63511-4
Online ISBN: 978-3-030-63512-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)