Abstract
Flax (Linum usitatissimum L.), one of the oldest cultivated crops, continues to be widely grown for oil, fiber and food. Furthermore, the plants show a metal tolerance dependent on species so is ideal for research. Present study was conducted to find out the influence of copper (Cu) toxicity on plant biomass, growth, chlorophyll content, malondialdehyde (MDA) contents, proline production, antioxidative enzymes and metal up taken by L. usitatissimum from the soil grown under mixing of Cu-contaminated soil with natural soil by 0:1 (control), 1:0, 1:1, 1:2 and 1:4. Results revealed that, high concentration of Cu in the soil affected plant growth and development by reducing plant height, plant diameter and plant fresh and dry biomass and chlorophyll contents in the leaves compared with the control. Furthermore, Cu in excess causes generation of reactive oxygen species (ROS) such as superoxide radical (O–) and hydroxyl radicals (OH), which is manifested by high malondialdehyde (MDA) and proline contents also. The increasing activities of superoxidase dismutase (SOD) and peroxidase (POD) in the roots and leaves of L. usitatissimum are involved in the scavenging of ROS. Results also showed that L. usitatissimum also has capability to revoke large amount of Cu from the contaminated soil. As Cu concentration in the soil increases, the final uptake of Cu concentration by L. usitatissimum increases. Furthermore, the soil chemical parameters (pH, electrical conductivity and cation exchange capacity) were increasing to highest levels as the ratio of Cu concentration to the natural soil increases. Thus, Cu-contaminated soil is amended with the addition of natural soil significantly reduced plant growth and biomass, while L. usitatissimum is able to revoke large amount of Cu from the soil and could be grown as flaxseed and a potential candidate for phytoremediation of Cu.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal contamination have become a severe issue in all over the world especially in China due to industrialization and globalization and many other documented cases such as smelters, foundries and agriculture (Yang et al. 2018; Rehman et al. 2019a). High concentration of heavy metals in the agricultural land may cause adverse effect on plants, causes reduction in crop yield and plant productivity and also affect soil organisms (Khan et al. 2016; Singh et al. 2018). Furthermore, high concentration of heavy metals affect not only plants but their metabolic activities and their biological redistribution through pollution of water, air and soil (Wuana and Okieimen 2011; Volland et al. 2014; ul Hassan et al. 2017). Cadmium (Cd), copper (Cu), lead (Pb) and mercury (Hg) are considered as major heavy metal pollutants, which mostly occur in the soil with high anthropogenic pressure (Nagajyoti et al. 2010; Song et al. 2017). Among these major heavy metal pollutants, Cu is an important element, which is also an essential element required in many physiological processes such as photosynthesis, respiration and N2 fixation (Sun et al. 2014; Chen et al. 2015; Husak 2015). Moreover, Cu is considered as a trace element, essential for the plants but in minute quantity, while lack of Cu concentration causes many disorders in the plants (Verma and Bhatia 2014; Zlobin et al. 2015). Uptake and translocation of Cu in plant species mainly depend on levels of Cu supply and growth conditions Saleem et al. (2019a). However, Cu in cells need to be kept at low levels because excessive Cu can induce alterations in the DNA, cell membrane integrity, respiration, photosynthesis, and enzyme activity which may lead to growth inhibition and endangered survival of plants (Aggarwal et al. 2012; Nair and Chung 2015; Saleem et al. 2019b). The reduction in plant biomass is a common response in plants when exposed to excess Cu. The major causes of high concentration of Cu in the soil are the use of Cu-containing herbicides, pesticides, fungicides and industrial effluents, sewage sludge and mining (Mahmud et al. 2013; Yang et al. 2014; Rizwan et al. 2016). Cu in excess causes the generation of reactive oxygen species, which are toxic for the plant tissues, and these species scavenge by the activities of antioxidants such as superoxide dismutase (SOD) and peroxidase (POD), which also play a significant role in reducing Cu toxicity in Boehmeria nivea L. and Zea mays L. (Murakami and Ae 2009; Rehman et al. 2019a). Oxidative stress in terms of lipid peroxidation causes disturbance to many metabolic pathways and damage to membrane-bounded organelles in the cell (Halliwell and Gutteridge 2015; Liu et al. 2015). Proline is an important amino acid and believed to be a single molecule, which helps in activating many physiological and molecular responses in the plants even in stress conditions. It was observed that, when plants undergo Cu stress condition, they accumulate high contents of proline in their tissue to overcome metal stress (Szabados and Savouré 2010; Ku et al. 2012).
Recently, there are many physical-chemical technologies for the remediation of toxic pollutants and petroleum hydrocarbons from the contaminated soil. These technologies include soil washing, thermal desorption and incineration (Zhu et al. 2016; Yang et al. 2018). Most of these techniques are expensive to implement at full scale and require continuous monitoring and control for optimum performance. Some of the techniques simply move the contamination elsewhere and may create significant risks in the excavation, handling and transport of hazardous materials. But phytoremediation (use of green plants for remediation of contaminated soil) is an option to perform such operations using natural biological activity (Ashraf et al. 2019). Furthermore, this technique is relatively cheap, eco-friendly and scientifically approved, have a high public acceptance and can be carried out in vast fields (Zaheer et al. 2015). This technique is successful not only to accumulate heavy metals from the soil but extra nutrients and organic matter can also remediate with the technique. There are many types of phytoremediation (phytotransformation, rhizosphere bioremediation, phytostabilization, phytoextraction and rhizofiltration), but phytoextraction is most common as it is the use of green plants sown under contaminated soil and able to accumulate plants in their above ground parts of the plant (Vangronsveld et al. 2009; Habiba et al. 2015). This technique is relatively cheap than soil excavation and treatment or disposal (Tahmasbian and Sinegani 2016; Yahaghi et al. 2018). Recently, many plant species including flax (Linum usitatissimum L.) has been used for phytoextraction of different heavy metals such as Zn, Cu, Hg and As (Smolinska 2015; Pajević et al. 2016; Sidhu et al. 2018; Lajayer et al. 2019). Many previous studies also suggested that L. usitatissimum is an ideal candidate for the phytoremediation of different heavy metals due to many unique characteristics such as huge biomass and many biochemical activities (Griga et al. 2003a, b; Hancock et al. 2012). However, L. usitatissimum is used as flaxseed or fibrous crop and has many industrial applications like solid yarns, twine, cordage, hoses, wood shives and tires (Najmanova et al. 2012; Wróbel-Kwiatkowska et al. 2012). Flax seed is used as food, pharmaceutical, as a component in compound feed and its oil for the production of varnishes and paints. Linseed provides more options for use than most other crops. The main product of linseed is seed. Linseed is used whole or slightly crushed for the production of bread and rolls, adding to the dough, making bakery products. (Vrbová et al. 2013; Yurkevich et al. 2017).
A lot of investigations have been reported about the potential of L. usitatissimum for phytoremediation of toxic heavy metals (Belkhadi et al. 2010; Kaplan et al. 2015; Praczyk et al. 2015), but very limited studies have explored on Cu toxicity on L. usitatissimum. Furthermore, this unique study also provide the information regarding growth and biochemical response of L. usitatissimum under mixing of Cu-contaminated soil with natural soil collected from two different sites of China. To the best of our knowledge, this study is among the few studies that focus on the metal tolerance and accumulation among fibrous crops in order to investigate their suitability for metal-contaminated sites. Therefore, the important goals of our study are (1) to determine the effect of Cu toxicity on seedlings growth of L. usitatissimum and (2) to evaluate the phytoremediation potential of L. usitatissimum under Cu-contaminated soil.
Materials and methods
Plant material and experimental treatments
Cu-contaminated soil was collected from a Cu mining area of Baisha Village, DaYe County, Hubei, China (115.20′E, 29.85′N), at depth of 0–20 cm, while natural soil was collected from experimental stations of Huazhong Agricultural University, Wuhan, China (114.20′E, 30.28′N). The following are the main agrochemical properties of the tested loam soil: pH 6.87, electrical conductivity (EC) of 269 μs cm−1, cation exchange capacity (CEC) of 14.99 cmol kg−1, organic matter of 3.96 g kg−1, total nitrogen of 0.16 g kg−1, total phosphorus of 1.97 g kg−1, total potassium of 12.25 g kg−1 and total Cu of 2153 mg kg−1, while the physicochemical properties of natural soil were as follows: pH 6, EC 2 dS cm−1, 22 g kg−1 organic matter, 23.16 mg kg−1 exchangeable K, 0.23 g kg−1 total P, 20 g kg−1 total N and total Cu 38 mg kg−1.These soils were mixed in the ratio of Cu-contaminated soil to natural soil, i.e., 0:1 (control), 1:0, 1:1, 1:2 and 1:4, dried under the shade and passed through the sieves of 5 mm before starting a pot experiment. After the mixing of two different soils, the pots were equilibrated for 2 weeks by one cycle of saturation with distilled water and air-drying. The seeds of flax Longya 10 were sown in 16 kg of pots (30-cm-tall × 40-cm-wide) on 15 November 2018 (Fig. 1). Each treatment was arranged in a completely randomized design (CRD) with six replications and three plants in each pot. Weeding, irrigation with Cu-free water and other necessary intercultural operations were done when needed. Pots were placed in a glasshouse, where plants received natural light, with day/night temperature of 10/2 °C and day/night humidity of 80/90%. At the time of harvest, shoot and root samples of each variety were collected separately and cleaned thoroughly with tap water and rinsed with 0.1 M HCl solution followed by several rinses with deionized water. Shoot and root samples were processed after oven dried at 65 °C for 72 h, and some samples were stored in refrigerator at − 80 °C. Post-harvest soil samples were collected separately from each pot. All chemicals used were of analytical grade, procured from Sinopharm Chemical Reagent Co., Ltd.
Sampling and data collection
All plants were wrapped for different biological traits on mid of the January 2019 (60 days after seed sowing). On that day, leaves and roots samples were picked during 09:00–10:30 a.m. The samples were washed with distilled water, immediately placed in liquid nitrogen and stored in a freezer at low temperature (− 80 °C) for further analysis. Five plants per each treatment were selected for morphological traits, which were uprooted with the roots. Plant height was measured by measuring root length and shoot length of the plant. Plant diameter (mm) was measured at 10 cm above from the surface of soil using digital vernier caliper (ST22302 SG Tools, Hangzhou, China). Plant fresh weight was measured by measuring shoot weight and root weight together with the help of digital weighting balance. Later, plant samples (roots together with shoots) were dried in an oven at 105 °C for 1 h, then at 65 °C for 72 h to determine their dry weight. Roots were immersed in 20 mM Na2EDTA for 15–20 min to remove Cu adhered to the surface of roots. Then, roots were washed thrice with distilled water and finally once with de-ionized water and dried for further analysis.
Determination of chlorophyll contents
Chlorophyll content from fresh plant leaves was determined according to Lichtenthaler (1987) and expressed in mgg−1 FW.
Determination of MDA, proline content and antioxidant enzymes
The liquid nitrogen–chilled 500-mg leaf samples were used to obtain enzyme extract. The leaf samples were normalized with 4 mL of chilled 50 mM potassium-phosphate (K-P buffer), having pH 7, in a pre-cooled mortar and pester. The enzyme extract was transferred into Eppendorf tubes after centrifuging at 11,500×g (4 °C) for 20 min. The method given by Chen and Pan (1996) was performed to measure activity of superoxide dismutase (SOD) and expressed as Ug−1 FW. The activity of peroxidase enzyme (POD) was measured as described by Sakharov and Ardila (1999) and expressed as Ug−1 FW. The method described by Heath and Packer (1968) was adopted to measure the concentration of lipid peroxidation (MDA). Proline contents were measured by the procedure of Bates et al. (1973) and expressed as μgg−1 FW. The MDA and proline contents were expressed as μmolesg−1 FW.
Soil analysis
Soil samples were collected from each pot at post-harvest stage, air dried and passed through 0.1-mm nylon for further analysis. Soil pH, EC and CEC were measured according to Lu (2000). The bio-available Cu was measured according to Houben et al. (2013). The digestion of soil samples were performed in a microwave oven operating system (Milestone, ETHOS D) with an energy output 0–400 W (0–100% potency, respectively). Approximately 0.100 g of dry soil samples were placed into the Teflon microwave digestion vessels, and then 3 ml of H3PO4 and 2 ml of HCLO4 were added to each sample. Plant samples were digested using the optimized microwave programs. After cooling to room temperature, the digested samples were diluted to a final volume of 25 ml with deionized water. Blank samples were prepared simultaneously. These solutions were stored in a refrigerator at 4 °C until the analysis was carried out. The total contents of Cu in the digests were determined by graphite furnace atomic absorption spectrophotometer (AAS) model Agilent 240 FS-AA equipped with deuterium background correctors, a graphite furnace GF95 and an auto-sampler (Rehman et al. 2019c). The replicates were taken for each sample. The determination of Cu removal (%) was measured by using the formula of Tanhan et al. (2007):
where C0 refers to the initial concentration of a heavy metal and C1 refers to its final concentration.
Statistical analysis
The data recorded were statistically analyzed using Statistix 8.1 Analytical Software, Tallahassee, FL, USA. Testing showed that all plant- or soil-related data were approximately normally distributed. Thus, the differences between treatments were determined using analysis of variance, and the least significant difference test (P ≤ 0.05) was used for multiple comparisons between treatment means. Pearson’s correlation analysis was performed to quantify relationships between various analyzed variables. Graphical presentation was carried out using SigmaPlot 12.5. The R Studio was used to calculate Pearson’s correlation.
Results
Growth, biomass and chlorophyll contents of L. usitatissimum
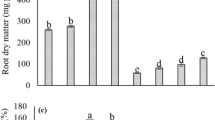
Results regarding plant height, plant diameter, fresh and dry biomass of L. usitatissimum were presented in the Table 1. These results showed that addition of Cu concentration to natural soil causes significant (P ≤ 0.05) decrease in plant growth and development when compared with control. It was observed that maximum plant height was observed in the plants, which grown without any addition of Cu in the soil (control), while addition of 1:0, 1:1, 1:2 and 1:4 Cu concentration to natural soil causes reduction by 60, 52, 40 and 26%, respectively. Similarly, plant diameter was decreased by 56, 48, 36 and 20% by adding of Cu-contaminated soil to natural soil in the ratio of 1:0, 1:1, 1:2 and 1:4, respectively, compared with control, i.e., 0:1. Fresh and dry biomasses of the plant significantly (P ≤ 0.05) decreased by adding of Cu-contaminated soil into the natural soil. Compared with the control, plant fresh weight was decreased by 67, 56, 42 and 21%, while plant dry weight was decreased by 80, 52, 40 and 20% by mixing of Cu-contaminated soil to natural soil at 1:0, 1:1, 1:2 and 1:4, respectively. Total chlorophyll contents in the leaves were also affected when Cu-contaminated soil were added to the natural soil (Table 1). The results were showed that addition of Cu-contaminated soil to natural soil by 1:0, 1:1, 1:2 and 1:4 decreased chlorophyll contents in the leaves by 61, 40, 21 and 7%, respectively, compared with the plants grown in natural soil without concentration of Cu.
Lipid peroxidation, proline and antioxidant enzymes
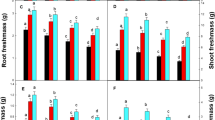
Lipid peroxidation in term of malondialdehyde (MDA) contents was also determined from the roots and leaves of L. usitatissimum (Fig. 2a). It was noticed that addition of Cu-contaminated soil to the natural soil significantly (P ≤ 0.05) increased MDA contents in the roots and leaves of L. usitatissimum, which is the indication of oxidative damage occur in the plants due to phytotoxicity of Cu concentration. Compared with the control, the contents of MDA were increased by 508, 421, 284 and 163% in the roots and 386, 235, 136 and 60% in the leaves by mixing of Cu-contaminated soil to natural soil at 1:0, 1:1, 1:2 and 1:4, respectively. Similarly, proline contents were also increased in the plants as concentration of Cu increased in the soil (Fig. 2b). The contents of proline increased by 23, 14, 10 and 5% in the roots while increased by 16, 10, 6 and 3% in the leaves when Cu-contaminated soil was mixed with natural soil at:0, 1:1, 1:2 and 1:4, respectively, compared with control, i.e., 0:1.
Effect of different levels of Cu-contaminated soil mixed with natural soil on MDA (a), proline (b), SOD (c) and POD (d) in the roots and leaves of L. usitatissimum. Values in the table are just one harvest. Mean ± SD (n = 3). Different letters within a column indicate significant difference between the treatments (P < 0.05). Relative radiance of plastic filter used: Ck control (soil without Cu concentration), T1 (Cu-contaminated soil is mixed with natural soil by 1:0), T2 (Cu-contaminated soil is mixed with natural soil by 1:1), T3 (Cu-contaminated soil is mixed with natural soil by 1:2) and T4 (Cu-contaminated soil is mixed with natural soil by 1:4)
In the present study, the activity of superoxidase dismutase (SOD) and peroxidase (POD) were also investigated under the mixing of two different soils (Fig. 2c, d). These results suggested that addition of Cu-contaminated soil to natural soil increased the activities of antioxidants in the roots and leaves of L. usitatissimum. The activity of SOD increased by 443, 353, 303 and 231% in the roots while increased by 604, 536, 434 and 286% in the leaves when Cu-contaminated soil was mixed with natural soil at 1:0, 1:1, 1:2 and 1:4, respectively, compared with control, i.e., 0:1. Compared with the control, the activity of POD increased by 350, 248, 183 and 159% in the roots while increased by 499, 344, 314 and 283% in the leaves by mixing of Cu-contaminated soil to natural soil at 1:0, 1:1, 1:2 and 1:4, respectively.
Soil characteristics and extractable Cu concentration in soil
In the present study, different agrochemical properties of soil such as pH, electrical conductivity (EC) and cation exchange capacity (CEC) at post-harvesting stage of the plants were also determined (Table 2). These results suggesting that addition of different ratios of Cu-contaminated soil to the natural soil significantly (P ≤ 0.05) increased soil properties compared with control. Our results revealed that soil pH increased by 9, 6, 3 and 2% when Cu-contaminated soil mixed with natural soil at 1:0, 1:1, 1:2 and 1:4, respectively, compared with control, i.e., 0:1. Compared with control, soil EC increased by 33, 22, 13 and 6% while CEC increased by 40, 34, 14 and 8% by mixing of Cu-contaminated soil to natural soil at 1:0, 1:1, 1:2 and 1:4, respectively.
In the present study, Cu concentration at post-harvest stage of L. usitatissimum from the soil were also measured (Table 3). Increasing Cu concentration in the soil causes more uptake of Cu by the plants. After harvesting L. usitatissimum, the final concentration of Cu was less than the initial concentration of Cu. The results suggested that maximum Cu concentration was up taken by the plant grown under 1:0 (Cu concentration mixed with natural soil), i.e., 36 mg kg−1 Cu while minimum Cu concentration was up taken by the plants grown under 1:4 (Cu concentration mixed with natural soil), i.e., 11 mg kg−1 Cu compared with the plants grown without any concentration of Cu. These results also suggested that L. usitatissimum has potential to revoke Cu in ranging from 22 to 27% from Cu-contaminated soil.
Correlation between growth parameters, chlorophyll contents and Cu up taken by L. usitatissimum from the soil
The Pearson correlation analysis was carried out to quantify the relationship between various studied parameters (Fig. 3). Cu up taken from the soil is negatively correlated with plant height, plant diameter, plant fresh weight, plant dry weight and total chlorophyll contents in the leaves of L. usitatissimum. However, all other growth attributes are positively correlated with each other but negatively correlated with Cu up taken from the soil. This correlation reflected the close connection between Cu uptake and growth in L. usitatissimum.
Discussion
For the past few decades, industrialization and urbanization are the main causes of increasing heavy metal concentration in agricultural land, which also have effects on ecosystem (Manousaki et al. 2008; Murakami and Ae 2009; Nagajyoti et al. 2010; Rehman et al. 2019b). Heavy metal contamination is toxic for the plants, but heavy metal such as Cu is also required by the plants for normal growth and development (Xu et al. 2006; Li et al. 2018; Saleem et al. 2019). Although Cu is a micronutrient for the plants, excess Cu may cause toxicity, which ultimately affects crop yield and plant productivity (Bouazizi et al. 2010). Normal concentration of Cu in the plants is 20–30 mg kg−1 of dry mass. The Cu level accumulated by the plants must be transported and distributed to various parts of the plants for healthy growth and development. Contrastingly, high concentration of Cu in the plant tissues/cell causes chlorosis, inhibited root growth, bronzing and necrosis (Stojek 2013; Waters and Armbrust 2013). In the present study, addition of Cu-contaminated soil to the natural soil causes significant reduction in plant growth and biomass (Table 1). However, the reduction in the growth of L. usitatissimum under high levels of Cu suggested the Cu-induced toxicity at its elevated concentrations. Phytotoxicity of Cu-affected plant growth and composition has been showed by many studies (Habiba et al. 2015; Zaheer et al. 2015; Race et al. 2016). Reduction in plant biomass is a common response in plants exposed to an excess of Cu (Pietrzak and Uren 2011). These findings are in agreement with Li et al. (2018) who reported significant reductions in the biomass of Cu-treated plants of Brassica napus L. The observed reduction in plant dry mass is also in conformity with similar investigations on Festuca arudinacea L. and Lolium perenne L. seedlings exposed to Cu stress (Zhao et al. 2010). Increasing Cu concentration in the soil has also been reported to reduce both shoot and root growth in Arabidopsis thaliana L. (Kolbert et al. 2012). Liu et al. (2015) adds additional supporting evidence to our present findings.
Determination of chlorophyll contents from the leaves are important biological parameter for the evaluation of plant stress. It was observed that increasing concentration of Cu in the soil adversely affects leaf chlorophyll (Rehman et al. 2019b). In addition to plant growth and biomass, leaf chlorophyll contents were decreased by Cu toxicity (Table 1). Decrease in chlorophyll contents might be result of displacement of Mg required for chlorophyll biosynthesis or ultra-structural alteration of chloroplast under metal toxicity. This reduction in chlorophyll contents might be due to the inhibited activities of various enzymes associated with chlorophyll biosynthesis (Martins and Mourato 2006; Zvezdanović et al. 2007). Similar results were showed by Sánchez-Pardo et al. (2014) when they studied Lupinus albus L. and Glycine max L. under Cu stress.
When a plant undergoes stress condition, the dynamic equilibrium of reactive oxygen species (ROS) is disturbed and causes generation of these species, which causes ROS accumulation in the cell/tissues and causes lipid peroxidation (Sgherri et al. 2007; Rizwan et al. 2016). This accumulation of ROS is toxic for the plants and may cause cellular damage. Although, the accumulation of ROS is a common feature of the plants under stress environment, which induced oxidative damage in the plants (Andrade et al. 2010; Thounaojam et al. 2012). Heavy metal accumulation in the cell/tissues is involved in the direct or indirect generation of ROS in the following ways: (1) direct transfer of electrons, (2) disturbance of metabolic pathways and (3) reduced activities of antioxidants (Sun et al. 2010; Halliwell and Gutteridge 2015). Antioxidants such as superoxidase dismutase (SOD) and peroxidase (POD) are involved in the scavenging of ROS (Meng et al. 2007; Liu et al. 2018). The SOD catalyzes the dismutation of superoxide to H2O2, and molecular oxygen and POD decomposes H2O2 by oxidation of cosubstrates (Chandrasekhar and Ray 2017). In the present study, high Cu concentration in the soil causes oxidative stress by increasing MDA contents, and, due to generation of ROS, antioxidants (SOD and POD) come into play for the scavenging of ROS (Fig. 2). Upregulation of activity of these enzymes shows the capacity of plants to scavenge excessive ROS in the cells. Increasing activities of antioxidants under high concentration of Cu in the soil indicated that L. usitatissimum could tolerate Cu stress by enhancing antioxidative defense system. When plants undergo heavy metal stress, they believed to accumulate high contents of proline in their tissue to reduce metal toxicity as showed by Ku et al. (2012) in Nicotiana benthamiana L. In the present study, significant increase in antioxidant enzyme activities can be considered as an indicator of increased ROS production and mitigation (Upadhyay and Panda 2010; Shin et al. 2012). Goswami and Das (2016) studied Calandula officinalis L. under Cu stress and noticed that phytotoxicity of Cu induced oxidative damage, which was overcome by increasing activities of antioxidants. Production of antioxidant enzymes (SOD and POD) in L. usitatissimum, consequently, serves as an approach to strengthen cell antioxidant system and overcome the risk of ROS production due to metal stress (Rout and Sahoo 2013).
In the present study, a significant (P ≤ 0.05) change in the chemical properties of soil at post-harvest stage was observed. Results from the present study revealed that addition of Cu-contaminated soil to the natural soil increased soil pH, EC and CEC (Table 2). The increased in soil pH, EC and CEC at post-harvest stage might be due to the high physiochemical properties of soil in Cu-contaminated soil. The similar soil (Cu-contaminated soil) was used by Rehman et al. (2019a), but they added rice straw biochar (RSB) in it to reduce phytotoxicity of Cu and noticed that addition of RSW significantly increased plant growth and biomass while reduced the metal accumulation by the plants. But our objective is accumulation of Cu by the plant to remove heavy metals from the soil. Although very few literature are available of mixing of two different soils taken from two different sites, we have observed that addition of Cu-contaminated to natural soil affected plant growth and composition (Table 1). Furthermore, L. usitatissimum is a potential candidate to remove heavy metals from the metal-contaminated soil studied by many researchers (Szynkowska et al. 2009; Hradilová et al. 2010; Smykalova et al. 2010; Vrbová et al. 2013). Our results also suggested that L. usitatissimum seedlings have ability to remove Cu from the Cu-contaminated soil ranging from 22 to 27%. Hosman et al. (2017) studied L. usitatissimum plants under different concentrations of lead (Pb), cadmium (Cd) and zinc (Zn) and showed that L. usitatissimum has ability to revoke high concentration of heavy metals from the soil. Similar findings were obtained by Amna et al. (2015) and Uddin Nizam et al. (2016) when they studied L. usitatissimum under the concentration of different heavy metals and noticed that L. usitatissimum has potential to revoke huge amount of heavy metals from the soil and can be used as phytoremediation for different heavy metals under metal-contaminated soil.
Conclusions
Study concludes that L. usitatissimum could be considered as a phytoremediator plant and classified as an accumulator for the tested Cu with different mechanisms and considered a good accumulator. Furthermore, L. usitatissimum has a considerable potential to cope with high concentration of Cu in the soil due to active antioxidative defense mechanism. However, addition of Cu-contaminated soil to natural soil significantly reduced plant height, plant diameter and fresh and dry biomass of the plant and chlorophyll contents in the leaves. Moreover, excess Cu in the soil also induces oxidative damage in L. usitatissimum, which overcomes by the activities of antioxidants (SOD and POD). Chemical properties (pH, EC and CEC) of soil are also affected by addition of Cu as post-harvesting stage of the plant. Contrastingly, L. usitatissimum has potential to cope huge amount of Cu from the soil and can be used as a heavy metal accumulator for metal-contaminated soil. However, future research is needed on the effects of Cu stress on quality of both fiber and flaxseed from L. usitatissimum. Moreover, potential for L. usitatissimum in remediation of soils polluted with heavy metals should be tested under field conditions.
References
Aggarwal A, Sharma I, Tripathi B, Munjal A, Baunthiyal M, Sharma V (2012) Metal toxicity and photosynthesis. Photosynthesis: overviews on recent progress and future perspectives, 229–236
Amna MS, Syed JH, Munis MFH, Chaudhary HJ (2015) Phyto-extraction of nickel by Linum usitatissimum in association with Glomus intraradices. Internat J Phytor 17:981–987
Andrade SA, Gratão PL, Azevedo RA, Silveira AP, Schiavinato MA, Mazzafera P (2010) Biochemical and physiological changes in jack bean under mycorrhizal symbiosis growing in soil with increasing Cu concentrations. Environ Exp Bot 68:198–207
Ashraf S, Ali Q, Zahir ZA, Ashraf S, Asghar HN (2019) Phytoremediation: environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotox Environ Safe 174:714–727
Bates LS, Waldren RP, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Belkhadi A, Hediji H, Abbes Z, Nouairi I, Barhoumi Z, Zarrouk M, Chaïbi W, Djebali W (2010) Effects of exogenous salicylic acid pre-treatment on cadmium toxicity and leaf lipid content in Linum usitatissimum L. Ecotox Environ Safe 73:1004–1011
Bouazizi H, Jouili H, Geitmann A, El Ferjani E (2010) Copper toxicity in expanding leaves of Phaseolus vulgaris L.: antioxidant enzyme response and nutrient element uptake. Ecotox Environ Safe 73:1304–1308
Chandrasekhar C, Ray JG (2017) Copper accumulation, localization and antioxidant response in Eclipta alba L. in relation to quantitative variation of the metal in soil. Acta Physiol Plant 39:205
Chen C-N, Pan S-M (1996) Assay of superoxide dismutase activity by combining electrophoresis and densitometry. Botan Bullet Acad Sinica 37
Chen J, Shafi M, Li S, Wang Y, Wu J, Ye Z, Peng D, Yan W, Liu D (2015) Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of Moso bamboo (Phyllostachys pubescens). Sci Report 5:13554
Goswami S, Das S (2016) Copper phytoremediation potential of Calandula officinalis L. and the role of antioxidant enzymes in metal tolerance. Ecotox Environ Safe 126:211–218
Griga M, Bjelkova M, Tejklova E (2003a) Phytoextraction of heavy metals by fibre crops: Linum usitatissimum L. case study. Proceedings of the 2nd European Bioremediation Conference, Chania, Crete, TU Crete pp 353-356
Griga M, Bjelkova M, Tejklová E (2003b) Potential of flax (Linum usitatissimum L.) for heavy metal phytoextraction and industrial processing of contaminated biomass-a review. Risk assessment and sustainable land management using plants in trace element-contaminated soils. Centre INRA Bordeaux-Aquitaine, Villenave d’Ornon, France 174-180
Habiba U, Ali S, Farid M, Shakoor MB, Rizwan M, Ibrahim M, Abbasi GH, Hayat T, Ali B (2015) EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ Sci Pollut R 22:1534–1544
Halliwell B, Gutteridge JM (2015) Free radicals in biology and medicine. Oxford University Press, NewYork, pp 888
Hancock LM, Ernst CL, Charneskie R, Ruane LG (2012) Effects of cadmium and mycorrhizal fungi on growth, fitness, and cadmium accumulation in flax (Linum usitatissimum; Linaceae). Amer J Bot 99:1445–1452
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hosman ME, El-Feky SS, Mohamed M, Shaker EM (2017) Mechanism of phytoremediation potential of flax (Linum usitatissimum L.) to Pb, Cd and Zn. Asian J Plant Sci Re 7:30–40
Houben D, Evrard L, Sonnet P (2013) Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 92:1450–1457
Hradilová J, Řehulka P, Řehulková H, Vrbová M, Griga M, Brzobohatý B (2010) Comparative analysis of proteomic changes in contrasting flax cultivars upon cadmium exposure. Electrophoresis 31:421–431
Husak V (2015) Copper and copper-containing pesticides: metabolism, toxicity and oxidative stress. J Vasyl Stef Precarp Nat Uni 2:38–50
Kaplan ME, Simmons ER, Hawkins JC, Ruane LG, Carney JM (2015) Influence of cadmium and mycorrhizal fungi on the fatty acid profile of flax (Linum usitatissimum) seeds. J Sci Food Agric 95:2528–2532
Khan SU, A-u K, Shah A-u-HA, Shah SM, Hussain S, Ayaz M, Ayaz S (2016) Heavy metals content, phytochemical composition, antimicrobial and insecticidal evaluation of Elaeagnus angustifolia. Toxicol Ind Health 32:154–161
Kolbert Z, Pető A, Lehotai N, Feigl G, Erdei L (2012) Long-term copper (Cu 2+) exposure impacts on auxin, nitric oxide (NO) metabolism and morphology of Arabidopsis thaliana L. Plant Growth Regul 68:151–159
Ku H-M, Tan C-W, Su Y-S, Chiu C-Y, Chen C-T, Jan F-J (2012) The effect of water deficit and excess copper on proline metabolism in Nicotiana benthamiana. Biol Plant 56:337–343
Lajayer BA, Moghadam NK, Maghsoodi MR, Ghorbanpour M, Kariman K (2019) Phytoextraction of heavy metals from contaminated soil, water and atmosphere using ornamental plants: mechanisms and efficiency improvement strategies. Environ Sci Pollut R 26:8468–8484
Li L, Zhang K, Gill RA, Islam F, Farooq MA, Wang J, Zhou W (2018, 2018) Ecotoxicological and interactive effects of copper and chromium on physiochemical, ultrastructural, and molecular profiling in Brassica napus L. BioMed Res Internat
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes, methods in enzymology. Elsevier:350–382
Liu Q, Zheng L, He F, Zhao F-J, Shen Z, Zheng L (2015) Transcriptional and physiological analyses identify a regulatory role for hydrogen peroxide in the lignin biosynthesis of copper-stressed rice roots. Plant Soil 387:323–336
Liu J, Wang J, Lee S, Wen R (2018) Copper-caused oxidative stress triggers the activation of antioxidant enzymes via ZmMPK3 in maize leaves. PLoS One 13:e0203612
Lu R (2000): Analytical methods of soil agrochemistry (in Chinese), China Agriculture Technology Press, pp 305–336
Mahmud S, Hassan MM, Moniruzzaman M, Biswas N, Rahman MM, Haque ME (2013) Study on the accumulation of copper from soil by shoots and roots of some selective plant species. Internat J Biosc 3:68–75
Manousaki E, Kadukova J, Papadantonakis N, Kalogerakis N (2008) Phytoextraction and phytoexcretion of Cd by the leaves of Tamarix smyrnensis growing on contaminated non-saline and saline soils. Environ Res 106:326–332
Martins LL, Mourato MP (2006) Effect of excess copper on tomato plants: growth parameters, enzyme activities, chlorophyll, and mineral content. J Plant Nutr 29:2179–2198
Meng Q, Zou J, Zou J, Jiang W, Liu D (2007) Effect of Cu2+ concentration on growth, antioxidant enzyme activity and malondialdehyde content in garlic (Allium sativum L.). Acta Biol Cracov Ser Bot 49:95–101
Murakami M, Ae N (2009) Potential for phytoextraction of copper, lead, and zinc by rice (Oryza sativa L.), soybean (Glycine max [L.] Merr.), and maize (Zea mays L.). J Hazar Material 162:1185–1192
Nagajyoti PC, Lee KD, Sreekanth T (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Nair PMG, Chung IM (2015) Study on the correlation between copper oxide nanoparticles induced growth suppression and enhanced lignification in Indian mustard (Brassica juncea L.). Ecotox Environ Safe 113:302–313
Najmanova J, Neumannova E, Leonhardt T, Zitka O, Kizek R, Macek T, Mackova M, Kotrba P (2012) Cadmium-induced production of phytochelatins and speciation of intracellular cadmium in organs of Linum usitatissimum seedlings. Ind Crop Prod 36:536–542
Pajević S, Borišev M, Nikolić N, Arsenov DD, Orlović S, Župunski M (2016) Phytoextraction of heavy metals by fast-growing trees: a review, phytoremediation. Springer, pp 29–64
Pietrzak U, Uren N (2011) Remedial options for copper-contaminated vineyard soils. Soil Research 49:44–55
Praczyk M, Heller K, Silska G, Baraniecki P (2015) Analysis of accumulation of cadmium in seeds of selected breeding linseed (Linum usitatissimum L.) genotypes cultivated for medicinal purposes. Herba Polonica 61:19–30
Race M, Marotta R, Fabbricino M, Pirozzi F, Andreozzi R, Cortese L, Giudicianni P (2016) Copper and zinc removal from contaminated soils through soil washing process using ethylenediaminedisuccinic acid as a chelating agent: a modeling investigation. J Environ Chem Engin 4:2878–2891
Rehman M, Liu L, Bashir S, Saleem MH, Chen C, Peng D, Siddique KH (2019a) Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (Boehmeria nivea L.) grown as forage in aged copper-contaminated soil. Plant Physiol Biochem 138:121–129
Rehman M, Liu L, Wang Q, Saleem MH, Bashir S, Ullah S, Peng D (2019b) Copper environmental toxicology, recent advances, and future outlook: a review. Environ Sci Pollut R:1–14
Rehman M, Maqbool Z, Peng D, Liu L (2019c) Morpho-physiological traits, antioxidant capacity and phytoextraction of copper by ramie (Boehmeria nivea L.) grown as fodder in copper-contaminated soil. Environ Sci Pollut R 26:5851–5861
Rizwan M, Ali S, Abbas T, Zia-ur-Rehman M, Hannan F, Keller C, Al-Wabel MI, Ok YS (2016) Cadmium minimization in wheat: a critical review. Ecotox Environ Safe 130:43–53
Rout JR, Sahoo SL (2013) Antioxidant enzyme gene expression in response to copper stress in Withania somnifera L. Plant Growth Regul 71:95–99
Sakharov IY, Ardila GB (1999) Variations of peroxidase activity in cocoa (Theobroma cacao L.) beans during their ripening, fermentation and drying. Food Chem 65:51–54
Saleem MH, Fahad S, Khan SU, Ahmar S, Khan MHU, Rehman M, Maqbool Z, Liu L (2019a) Morpho-physiological traits, gaseous exchange attributes, and phytoremediation potential of jute (Corchorus capsularis L.) grown in different concentrations of copper-contaminated soil. Ecotox Environ Safe. https://doi.org/10.1016/j.ecoenv.2019.109915
Saleem MH, Ali S, Seleiman MF, Rizwan M, Rehman M, Akram NA, Liu L, Alotaibi M, Al-Ashkar I, Mubushar M (2019b) Assessing the correlations between different traits in copper-sensitive and copper-resistant varieties of jute (Corchorus capsularis L.). Plants 8:545
Sánchez-Pardo B, Fernández-Pascual M, Zornoza P (2014) Copper microlocalisation and changes in leaf morphology, chloroplast ultrastructure and antioxidative response in white lupin and soybean grown in copper excess. J Plant Res 127:119–129
Sgherri C, Quartacci MF, Navari-Izzo F (2007) Early production of activated oxygen species in root apoplast of wheat following copper excess. J Plant Physiol 164:1152–1160
Shin L-J, Lo J-C, Yeh K-C (2012) Copper chaperone antioxidant protein1 is essential for copper homeostasis. Plant Physiol 159:1099–1110
Sidhu GPS, Bali AS, Singh HP, Batish DR, Kohli RK (2018) Ethylenediamine disuccinic acid enhanced phytoextraction of nickel from contaminated soils using Coronopus didymus (L.) Sm. Chemosphere 205:234–243
Singh PK, Wang W, Shrivastava AK (2018) Cadmium-mediated morphological, biochemical and physiological tuning in three different Anabaena species. Aquat Toxicol 202:36–45
Smolinska B (2015) Green waste compost as an amendment during induced phytoextraction of mercury-contaminated soil. Environ Sci Pollut R 22:3528–3537
Smykalova I, Vrbova M, Tejklova E, Vetrovcova M, Griga M (2010) Large scale screening of heavy metal tolerance in flax/linseed (Linum usitatissimum L.) tested in vitro. Ind Crop Prod 32:527–533
Song B, Zeng G, Gong J, Liang J, Xu P, Liu Z, Zhang Y, Zhang C, Cheng M, Liu Y (2017) Evaluation methods for assessing effectiveness of in situ remediation of soil and sediment contaminated with organic pollutants and heavy metals. Environ Int 105:43–55
Stojek M (2013) The concentration of molybdenum and copper in rocks, soils and plants in the area of Jabłonki (eastern Beskids Mts.). Environ Prot Nat 24:13–17
Sun B-Y, Kan S-H, Zhang Y-Z, Deng S-H, Wu J, Yuan H, Qi H, Yang G, Li L, Zhang X-H (2010) Certain antioxidant enzymes and lipid peroxidation of radish (Raphanus sativus L.) as early warning biomarkers of soil copper exposure. J Hazard Mater 183:833–838
Sun X-H, Yu G, Li J-T, Jia P, Zhang J-C, Jia C-G, Zhang Y-H, Pan H-Y (2014) A heavy metal-associated protein (AcHMA1) from the halophyte, Atriplex canescens (Pursh) Nutt., confers tolerance to iron and other abiotic stresses when expressed in Saccharomyces cerevisiae. Int J Mol Sci 15:14891–14906
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Szynkowska M, Rybicki E, Leśniewska E, Pawlaczyk A, Paryjczak T, Matyjas-Zgondek E (2009) Influence of production progress on the heavy metal content in flax fibers. Chem Pap 63:537–542
Tahmasbian I, Sinegani AAS (2016) Improving the efficiency of phytoremediation using electrically charged plant and chelating agents. Environ Sci Pollut R 23:2479–2486
Tanhan P, Kruatrachue M, Pokethitiyook P, Chaiyarat R (2007) Uptake and accumulation of cadmium, lead and zinc by Siam weed [Chromolaena odorata (L.) King & Robinson]. Chemosphere 68:323–329
Thounaojam TC, Panda P, Mazumdar P, Kumar D, Sharma G, Sahoo L, Sanjib P (2012) Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem 53:33–39
Uddin Nizam M, Mokhlesur Rahman M, Kim J-E (2016) Phytoremediation potential of Kenaf (Hibiscus cannabinus L.), Mesta (Hibiscus sabdariffa L.), and jute (Corchorus capsularis L.) in arsenic-contaminated soil. Korean J Environ Agri 35:111–120
ul Hassan Z, Ali S, Rizwan M, Hussain A, Akbar Z, Rasool N, Abbas F (2017) Role of zinc in alleviating heavy metal stress, essential plant nutrients. Springer, pp 351–366
Upadhyay R, Panda SK (2010) Zinc reduces copper toxicity induced oxidative stress by promoting antioxidant defense in freshly grown aquatic duckweed Spirodela polyrhiza L. J Hazard Mater 175:1081–1084
Vangronsveld J, Herzig R, Weyens N, Boulet J, Adriaensen K, Ruttens A, Thewys T, Vassilev A, Meers E, Nehnevajova E (2009) Phytoremediation of contaminated soils and groundwater: lessons from the field. Environ Sci Pollut R 16:765–794
Verma A, Bhatia S (2014) Analysis of some physicochemical parameters and trace metal concentration present in the soil around the area of Pariccha thernal power station in Jhansi. India. Int J Inno Res Sci Eng Technol 3:10482–10488
Volland S, Bayer E, Baumgartner V, Andosch A, Lütz C, Sima E, Lütz-Meindl U (2014) Rescue of heavy metal effects on cell physiology of the algal model system Micrasterias by divalent ions. J Plant Physiol 171:154–163
Vrbová M, Kotrba P, Horáček J, Smýkal P, Švábová L, Větrovcová M, Smýkalová I, Griga M (2013) Enhanced accumulation of cadmium in Linum usitatissimum L. plants due to overproduction of metallothionein α-domain as a fusion to β-glucuronidase protein. Plant Cell Tissue Organ Cult 112:321–330
Waters BM, Armbrust LC (2013) Optimal copper supply is required for normal plant iron deficiency responses. Plant Signal Behav 8:e26611
Wróbel-Kwiatkowska M, Czemplik M, Kulma A, Żuk M, Kaczmar J, Dymińska L, Hanuza J, Ptak M, Szopa J (2012) New biocomposites based on bioplastic flax fibers and biodegradable polymers. Biotech Progr 28:1336–1346
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Isrn Ecology 2011
Xu J, Yang L, Wang Z, Dong G, Huang J, Wang Y (2006) Toxicity of copper on rice growth and accumulation of copper in rice grain in copper contaminated soil. Chemosphere 62:602–607
Yahaghi Z, Shirvani M, Nourbakhsh F, De La Pena TC, Pueyo JJ, Talebi M (2018) Isolation and characterization of Pb-solubilizing bacteria and their effects on Pb uptake by Brassica juncea: implications for microbe-assisted phytoremediation. J Microbiol Biotechnol 28:1156–1167
Yang W-d, Wang Y-y, Zhao F-l, Ding Z-l, Zhang X-c, Zhu Z-q, Yang X-e (2014) Variation in copper and zinc tolerance and accumulation in 12 willow clones: implications for phytoextraction. J Zhejiang Univ Sci B 15:788–800
Yang Z, Shi W, Yang W, Liang L, Yao W, Chai L, Gao S, Liao Q (2018) Combination of bioleaching by gross bacterial biosurfactants and flocculation: A potential remediation for the heavy metal contaminated soils. Chemosphere 206:83–91
Yurkevich OY, Kirov IV, Bolsheva NL, Rachinskaya OA, Grushetskaya ZE, Zoschuk SA, Samatadze TE, Bogdanova MV, Lemesh VA, Amosova AV (2017) Integration of physical, genetic, and cytogenetic mapping data for cellulose synthase (CesA) genes in flax (Linum usitatissimum L.). front. Plant Sci 8:1467
Zaheer IE, Ali S, Rizwan M, Farid M, Shakoor MB, Gill RA, Najeeb U, Iqbal N, Ahmad R (2015) Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotox Environ Safe 120:310–317
Zhao S, Liu Q, Qi Y, Duo L (2010) Responses of root growth and protective enzymes to copper stress in turfgrass. Acta Biol Cracov Ser Bot 52:7–11
Zhu H, Chen C, Xu C, Zhu Q, Huang D (2016) Effects of soil acidification and liming on the phytoavailability of cadmium in paddy soils of central subtropical China. Environ Pollut 219:99–106
Zlobin I, Kholodova V, Rakhmankulova Z, Kuznetsov VV (2015) Brassica napus responses to short-term excessive copper treatment with decrease of photosynthetic pigments, differential expression of heavy metal homeostasis genes including activation of gene NRAMP4 involved in photosystem II stabilization. Photosynth Res 125:141–150
Zvezdanović J, Marković D, Nikolić G (2007) Different possibilities for the formation of complexes of copper and zinc with chlorophyll inside photosynthetic organelles: chloroplasts and thylakoids. J Serbian Chem Soci 72
Funding
This research was supported by China Agriculture Research System project (CARS-16-E10) and the National Natural Science Foundation of China (31571717).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saleem, M.H., Fahad, S., Khan, S.U. et al. Copper-induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China. Environ Sci Pollut Res 27, 5211–5221 (2020). https://doi.org/10.1007/s11356-019-07264-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07264-7