Abstract
In nature, chitin is the second most plentiful and renewable polysaccharide and is present among versatile group of organisms from fungi and nematodes to arthropods and crustaceans. Enzymatic degradation is the preferable environmentally safe mode of bioprocessing of this inert biopolymer. Chitin-scavenging enzyme-producing sources are covering the living groups from prokaryotes to plants, viruses, vertebrates, and even human. Current-day biotechnologies have raised the development of bioprocesses by using microbes especially bacteria. Bacteria that produce chitinases are with varieties of habitats ranging from Antarctic soil to hot spring, crustacean waste site, animal gut, and endophytic ecosystems. Chitin metabolism is a necessary life-supporting goings-on in agronomic plant pests like fungi, insects, and parasitic nematodes which are negatively proportionate to the agricultural production systems. Placement of such potent chitinolytic bacteria for plant fortification against attacking pests is a well-practiced, biotechnologically equipped biocontrol strategy. By-products of chitin by enzymatic hydrolysis, like oligomers or monomers, have several applications in persuading the plant defense systems. Carrying the host-defensive activity to biocontrol potentiality against plant pests, bacteria with chitinolytic property also behaved as a plant growth-promoting biofertilizing employee in modern-day sustainable agricultural practices. In this context, the distribution of chitinase-producing bacteria according to their diversity of habitats is studied, and the less explored habitats can be an arsenal for biocontrolling agents against plant pests.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Chitin is the second most abundant biodegradable carbon substrate after cellulose, which exists naturally in the biosphere as a structural polysaccharide of β-(1,4)-linked N-acetyl-d-glucosamine (GlcNAc). In nature, chitin is available in two crystalline formats, α and β. In the case of α-chitin, it is the most copious crystalline form, and the linear chains of GlcNAc unit are assembled in an antiparallel fashion, commonly exemplified by the shrimps and crabs, fungi, and cysts of Entamoeba. On the other hand, β-chitin is made up of parallel chains of GlcNAc units and found in squid pens (Yan and Fong 2015; Jang et al. 2004). Overall, chitin is extensively distributed in nature, mainly as an organizational polysaccharide in fungal cell walls (predominantly in Ascomycota, Basidiomycota, and Chytridiomycota), exoskeletons of arthropods, external shells of crustaceans, egg shell, and gut lining of parasitic nematodes (Brzezinska et al. 2014; Lenardon et al. 2010). The applicable fields of chitin are biotechnologically noteworthy, from chemical, biochemical, food, and pharmaceutical (antimicrobial, anticholesterol, antitumor, drug delivery, dietary fiber, and wound healing) industries (Patil et al. 2000; Gooday 1999; Muzzarelli et al. 1999; Dixon 1995) to wastewater treatment and management (Flach et al. 1992).
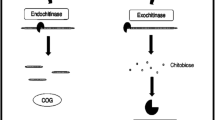
The insolubility of chitin and its inertness to chemical agents have amplified the exploration for substitute disposal methods such as biological processing. One such preferable practice is enzymatic treatment because of its uniformity toward the reaction and the products. Oligomers or monomers, by-products of chitin, have several applications in eclectic arenas (Patil et al. 2000). For such bio-based handling, chitinase comes first, and it acts to hydrolyze the β-1,4-glycosidic bonds between the N-acetyl-d-glucosamine residues that encompass a chitin chain (Henrissat 1999). Chitinases are classified into two types, exochitinases and endochitinases, based on their site and the nature of their hydrolyzed bonds (Henrissat 1999; Henrissat and Bairoch 1996). Endochitinases cleave chitin chains in random locations, generating low molecular weight oligomers, such as chitotriose, chitotetraose, and diacetylchitobiose. The exochitinases have been alienated into two subcategories: chitobiosidases which gradually release diacetylchitobiose from the non-reducing end of the chitin and β-N-acetylglucosaminidases, cleaving the oligomers of chitin (products of endochitinase), thereby producing monomers of glucosamine (Hamid et al. 2013).
Chitinases so far sequenced are also classified into glycoside hydrolase families (families 18, 19, and 20), constructed on the basis of amino acid sequence resemblance of their catalytic domains. The chitinases with different family backgrounds have dissimilar amino acid sequence and completely unlike three-dimensional (3D) structures (Perrakis et al. 1994; Henrissat 1991) and molecular mechanisms. Therefore, they are likely to have evolved from diverse lineages. The family 18 chitinases hydrolyze glycosidic bonds with the retention of anomeric configuration at C1 atom (Kramer and Koga 1986). The catalytic domains of these chitinases have a fold of barrel with a catalytic groove as demonstrated by 3D structural analysis of hevamine (Kramer and Muthukrishnan 1997). These chitinases catalyze the hydrolysis of Glc-N-Ac-Glc-N-Ac and Glc-N-Ac-Glc-N- linkages. These chitinases are inhibited by allosamidine, an isomer of N-acetyl glucosamine. On the other hand, the family 19 chitinases hydrolyze glycosidic bond with an inversion of anomeric configuration at C1 atom (Stinizi et al. 1993; Broglie et al. 1991). The catalytic domain of these chitinases has a fold of high helical content and structural similarity, including conserved core of the enzyme (Grison et al. 1996). They catalyze the hydrolysis of Glu-N-Ac and Gluc-N-Ac linkages only. The activity of these chitinases is insensitive to allosamidine. They catalyze the hydrolysis of chitin similar to acid-base mechanism (Grison et al. 1996; Desouza and Murray 1995). The conserved region of the catalytic domain of this family of chitinases resembles crystal structure of lysozyme (Terwisscha et al. 1996). Family 18 (subfamilies A, B, and C) includes chitinases derived mostly from fungi but also from bacteria, viruses, animals, insects, and plants. Family 19 comprises chitinases derived from plants (classes I, II, and IV), and several are derived from bacteria, e.g., Streptomyces griseus. Family 20 includes N-acetylglucosaminidase from Vibrio harveyi and N-acetylhexosaminidase from Dictyostelium discoideum and human (Brzezinska et al. 2014; Dahiya et al. 2006; Duo-Chuan 2006; Patil et al. 2000; Henrissat 1999). Largely, chitinases produced by a versatile group of living systems range from microbes like bacteria, fungi, and virus to insects, plants, and animals and are also present in human blood serum (Gohel et al. 2006).
Modern biotechnology has raised the development of bioprocesses to use microbes to produce value-added bio-chemicals like enzymes (Yan and Fong 2015). Chitinolytic microorganisms play an indispensable biogeochemical role in chitin bioprocessing (Ilangumaran et al. 2017). Chitinase-producing microorganisms exhibit their wide range of distribution in the environment. Not only they are present in extreme habitat like Antarctic soil, hot spring, and soda lake, but also their attendance was observed from crustaceans’ waste to gut system, rhizospheric soil, and endophytic domains. These workhorses of the chitinase production company are both the eukaryotic and prokaryotic types of microorganisms. Chitinolytic fungi comprise 25–60% of the entire mold fungi, but their figure is inferior to the digit of bacteria (Brzezinska et al. 2014). The majority of the fungi belong to Ascomycota, whereas in bacteria, Proteobacteria are dominant over Firmicutes, Actinobacteria, and Bacteroidetes (Fig. 18.1).
Apart from the chitinase-producing capability of fungi, it is also responsible for causing various plant diseases. Plant diseases cause massive loss to the plant population together with economically important crop plants, causing misery to human beings (late blight of potato by Phytophthora infestans and brown spot of rice by Helminthosporium oryzae lead to Irish and Bengal famines, respectively) (Agrios 2005). Fungal phytopathogens are the serious intimidations to the commercial crops like cereals, potatoes, vines, fruits, and vegetables and are orthodoxly demolished by chemical fungicides. But the extensive uses of chemical fungicides are presumed to be lethal for the beneficial insects and microorganisms in the habitat soil and invade the food chain through biomagnification, leading to metabolic disorders, massive mutation, and carcinogenic effect on human beings. But modern approaches like biological control through biomolecules like chitinases for aiding sustainable agriculture give a substitute environment-friendly policy for monitoring phytopathogens like insects, fungi, and nematodes (Gaurav et al. 2017; Brzezinska et al. 2014).
So, microorganisms from diversified natural resources with chitinolytic activity can open a new arena in biotechnological approaches as a “green fungicide” or “green insecticide” or as a whole “green pesticide” and also can be a treasure box for human welfare as it may replace the use of chemical fungicide and insecticide.
2 Diversity of Culturable Chitinase-Producing Bacteria
Microorganisms utilize composite chitin molecule as carbon and energy source by hydrolyzing it into simple sugars known as the chitinase producers (Gaurav et al. 2017). Several natural resources are used for isolation of chitinase-producing bacteria and fungi. Such natural resources are like soil, water, shrimp shell waste, crab cell waste, fishing fields, seafood-processing industries, plant endophytes, and gut systems. The soil resources reflect great variations like agricultural, rhizospheric, mangrove, and Antarctic soils. The water resources are like hot spring, soda lake, Lonar lake, freshwater lake, marine water, and shrimp-cultivating ponds. Among the gut systems, both the vertebrate (fish and bat) and invertebrate (insect, earthworm) are explored. Chitinolytic bacterial flora consists of both the Gram-positive and Gram-negative types with respect to all the isolated fields. Among the reported culturable bacterial diversity, Proteobacteria is the predominant one (56.46%) followed by Firmicutes (27.75%), Actinobacteria (8.61%), Bacteroidetes (6.69%), and Deinococcus-Thermus (0.47%) (Fig. 18.1). Culturable microorganisms possess chitinase production with habitat specificity and are listed in Table 18.1.
2.1 Chitinase-Producing Bacteria Isolated from Soil
Reports regarding chitinase-producing soil bacteria are studied in detail so far. A list of soil bacteria with chitinolytic activity are given in Table 18.1. Among the reported bacterial diversity, Proteobacteria is the dominant group (52.27%) over the Firmicutes (22.72%), Bacteroidetes (12.5%), Actinobacteria (11.36%), and Deinococcus-Thermus (1.13%) (Fig. 18.2).
2.2 Chitinase-Producing Bacteria Isolated from Different Water Bodies
Chitinase-producing bacteria are also reported from various water bodies such as shrimp ponds, marine water, Lonar lake, hot spring, and moat water. Among them, shrimp-cultivating ponds are the potent container of the chitinolytic bacteria. The list of chitinase-producing bacteria isolated from different water bodies are presented in Table 18.2. In the middle of all reported bacterial variations from the different water bodies, Proteobacteria is the mostly rich group of bacteria (57.14%) followed by Firmicutes (28.57%) and Actinobacteria (14.28%) (Fig. 18.3).
2.3 Chitinase-Producing Bacteria Isolated from Crab Shell Waste
Crab cells are made up of chitin. Therefore, promising chitinase-producing bacteria can be isolated from these wastes. Reports regarding the chitinolytic bacteria from crab cell wastes are recorded in Table 18.3. Bacterial diversity in this area is commanded by Proteobacteria (60%), and the rest of the representatives are from Firmicutes (20%) and Bacteroidetes (20%) (Fig. 18.4).
2.4 Chitinase-Producing Bacteria Isolated from Shrimp Shell Waste
Shrimp shell wastes are the major sources of chitin as they are made up of chitinous exoskeleton. Reports regarding the bacteria isolated from the shrimp shell waste are enlisted in Table 18.4. Data regarding the bacterial diversity from the shrimp shell waste are dominated by Proteobacteria (66.66%) over the Actinobacteria (16.66%) and Firmicutes (16.66%), as shown in Fig. 18.5.
2.5 Chitinase-Producing Endophytic Bacteria
Endophytic bacteria with chitinase production ability are reported from economically important crop plants like potato, maize, and brassica. A list is given in the Table 18.5. In this area, most of the chitinolytic bacteria are from the Proteobacteria (40%), Firmicutes (40%), and Actinobacteria (20%) (Fig. 18.6).
2.6 Chitinase-Producing Gut Bacteria
Chitinase production by the gut bacteria is reported among the invertebrates and vertebrates. Among the invertebrates, insect and earthworm are the only reports where chitinolytic symbiotic gut microbes are observed (Tables 18.7 and 18.8). Fish and bat are the two vertebrates where chitinase-producing gut bacteria (Tables 18.6 and 18.9) are studied so far. Here, the reported gut bacteria are listed in Table 18.6.
2.6.1 Chitinase-Producing Fish Gut Bacteria (Table 18.6)
2.6.2 Chitinase-Producing Insect Gut Bacteria (Table 18.7)
2.6.3 Chitinase-Producing Earthworm Gut Bacteria (Table 18.8)
2.6.4 Chitinase-Producing Gut Bacteria of Bat (Table 18.9)
Chitinase-producing bacteria from different natural resources are stated in this chapter. There are many reports available in regard to soil and water. Reports in relation to shrimp shell waste and crab cell waste are plenty, but gut bacterial reports for chitinase production are limited only in two groups, i.e., insect and earthworm (invertebrates) and fish and bat members (vertebrates). There are vast resources of unexplored fields in relation to chitinase-producing gut microbes. Therefore, gut microorganisms possessing chitinolytic activity can be a hidden tool toward the biotechnological approaches (Figs. 18.7, 18.8, 18.9 and 18.10).
3 Their Role in the Habitat
Microorganisms which utilize merged chitin molecule as carbon and energy source by hydrolyzing it into simple sugars are known as the chitinase producers (Gaurav et al. 2017). Their wide-ranging abundance has already been stated earlier. Chitinolytic microbes can be isolated from the habitats on the basis of availability of their food material like chitin. Such habitats cover from shrimp shell waste area and crab shell dumping zone to soil, water, gut environments, and so on. Microbial residency on these types of locale plays an indispensable role to simplify the rigid chitin which subsequently produces oligomers and monomers, and these products cause several beneficial benefits toward the residing environment, chiefly as the biofertilizing, biocontrolling, and biowaste managing agents.
Among the natural resources, crustacean biowastes exclusively shrimp and crab shells have the maximum chitin content up to 60% (Chakrabarti 2002; Wang et al. 2006; Kandra et al. 2012). Annually, around 1011 tons of chitinous ingredients are produced in the aquatic environment, but there is no considerable addition of chitin in the ocean sediments as the chitinolytic microorganisms in the aquatic ecosystem basically degrade them (Ghorbel-Bellaaj et al. 2012; Halder et al. 2012). So, the microbial population belonging to these habitats like marine water, shrimp shell waste, and crab shell waste exhibits a significant chitin-reducing activity as they utilize these biowastes as nutritional resources. Evidences are also available in support of the bacterial type isolated from these habitats (Tables 18.2, 18.3, and 18.4). Microbial residents in such type of habitats are also serving as an environmentally autoregulated biowaste management agent. Marine microorganisms have established inimitable metabolic and physiological abilities to harvest novel metabolites which are not often existing in microbes of terrestrial origin. Away from their bio-recycling capability, some marine bacteria have a good potential for the control of fungal phytopathogens and mycotoxins (Kong 2018).
Reports concerning the chitinase-producing microorganisms isolated from the variable soil environments are numerous and listed in Table 18.1. The presence of such kind of microbes plays several advantageous characters in that type of soil atmosphere. From antifungal assets are through chitinase production to plant growth-promoting properties like phosphate and zinc solubilization ability, indole 3-acetic acid and siderophore production, seed germination enhancing ability, etc. (Sarbadhikary and Mandal 2017; Kejela et al. 2017; Patel and Arcahna 2017; Adhikari et al. 2017). In the current scenario, the participation of microbial inoculants as biofertilizers and biocontrol agents in the agriculture industry has been growing noticeably. Microbial inoculants are favored to reduce environmental toxicity instigated by chemicals and pesticides.
In the case of gut ambience, the presence of such type of microorganisms strictly depends upon the food habit of the host because they take part in the host’s digestion and nutritive processes. Microbes that degrade the dietary compounds can retain, proliferate, and establish symbiosis, and the others that are unable to degrade are washed out (Banerjee et al. 2017). In the later part of this endeavor, it can be observed that several reports are available related to gut microbes of insect, earthworm, fish, and bat that can hydrolyze chitin. These hosts are the consumers of chitinous materials, and it can be assumed that these gut microbes play a role in their digestion, vitamin synthesis, and antifungal activity with their chitinolytic efficiency (Dillon and Dillon 2004; Genta et al. 2006).
So, in this framework, the role of the chitinase producers in their habitats stands with a great biotechnological importance for modern-day sustainable agriculture, which leads to a pronounced human welfare phenomenon by replacing ecotoxic chemical fertilizers, fungicides, and pesticides.
4 Potential Applications
Microbial enzymes are relatively more stable than corresponding enzymes derived from plants or animals (Wiseman 1995). Enzymes of microbial origin have been used in various industries for many centuries. Enzymes from microbial sources are widely used in industrial processes mainly because of their low cost, large productivity, vast availability, chemical stability, and flexibility (Banerjee et al. 2016), and bacterial chitinases are such kind of biomolecules. Chitinase enzyme has received increased attention due to its wide range of biotechnological applications, especially in agriculture for biocontrol of phytopathogenic fungi and harmful insects (Kuddus and Ahmad 2013). Chitinases are with immense importance in the biotechnology and bioprocessing ranges; because of their versatile potentiality as pesticide (against fungi, insects, and nematodes), they induce plant disease resistance, alternative petroleum feedstock, waste water management, marine by-products treatment (shrimp shell waste and sea food degradation), pharmaceutical industry activities (chitosaccharides), protoplast isolation from fungi and yeast, and preparation of single-cell protein (Kumar et al. 2018; Mao et al. 2017; Ilangumaran et al. 2017; Honda et al. 2017; Wang and Liang 2017; Aggarwal et al. 2015; Brzezinska et al. 2014; Halder et al. 2013; Mubarik et al. 2010).
4.1 Induce Plant Defense System
Biocontrol activities and plant growth-promoting potentialities are not only synchronized by the bacterial chitinolytic property but also obtained by the derivatives of chitin molecules. Their operational machineries are the outcome in direct antimicrobial responsibilities, stimulation of plant defense responses, and plant metabolic activity (El Hadrami et al. 2010; Ramírez et al. 2010). Chitosan has the capability to prevent the growth of a variety of bacteria and fungi (Rabea et al. 2003; El Hadrami et al. 2010; Xia et al. 2011; Sharp 2013). The antimicrobial potentiality of chitosan is known for its cationic features, which disrupt potassium signaling cascade in pathogens. Furthermore, chitosan interrupts membrane integrity of vacuoles and endomembrane organelles in fungal pathogens (Rabea et al. 2003; Sharp 2013). One such example was investigated by O’Herlihy et al. (2003) where chitosan exhibits the inhibitory activity against Phytophthora capsici and P. infestans. Another improvised nanotechnology-based work has been revealed by Chandra et al. (2015) where the chitosan nanoparticles (CNP) are capable of inducing and augmenting immune response in plants. CNP-treated leaves of Camellia sinensis produced substantial progress in the plant’s innate immune response by the induction of defense enzyme activity, upregulation of defense-related genes including that of several antioxidant enzymes, and elevation of the levels of total phenolics (Fig. 18.11).
Chitin oligosaccharides perform as pathogen-associated molecular patterns (PAMPs) due to their structural resemblance to the ingredients of pathogen cell wall in various plant pathosystems. PAMPs are accepted by host transmembrane pattern recognition receptors (PRRs), which signal defense corridors of induced systemic resistance (ISR) and systemic acquired resistance (SAR) (Eckardt 2008; Zipfel 2009). As a result, when real pathogen occurrence happens, the plant disease resistance mechanisms deliberate boosted protection against it. Thus, chitin derivatives attained from microbial degradation of crustaceans shells can be applied as elicitors of innate and systemic immune responses in plants (Benhamou 1996; Jones and Dangl 2006). The chitinous extracts assembled from microbial degradation were applied to induce disease resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato DC3000 and Botrytis cinerea (Ilangumaran et al. 2017).
4.2 Antifungal Activity of Bacterial Chitinase
Awareness in biological control has amplified over the past decades. The necessity for the replacements of chemical fungicides arises because of their penetration into the food chain which leads to the human health hazard and establishes resistant phytopathogens and also accelerates environmental contamination in parallel. Recently, biological control has been dedicated on bacteria-producing mycolytic enzymes, exclusively chitinases, recognized to hydrolyze chitin, a key element of fungal cell walls. In this context, antagonistic bacterial chitinases provide an environmentally sound substitute to synthetic chemicals because of their perceived safety and inferior environmental impact. Biological control policies have turned into an imperative attitude for aiding sustainable agriculture (Brzezinska et al. 2014; Berg and Hallmann 2006). Commercial biocontrol representatives mainly belong to the spore-forming bacteria because of their durability in a diversity of formulations and field environments for an extended period even under adverse situations (Subbanna et al. 2018).
The mainstream of pathogenic fungi comprises chitin and β-(1,3) glucan in their cell walls (Bartnicki-Garcia 1968), and disbanding or disruption of these organizational polymers has negative consequences over the growth and differentiation of fungi (Poulose 1992). One of the key antagonist apparatuses used by the biocontrol agents for these types of phytopathogens is the enzymatic disintegration of cell walls heading to leakage of fungal protoplasm (Lim et al. 1991; Kim and Chung 2004). Cell wall-degrading enzymes, especially chitinolytic enzyme-producing biocontrolling bacteria, are able to effectively control plant pathogenic fungi in this way (Broglie et al. 1991; Ordentlich et al. 1988) (Fig. 18.12). Abilities of bacteria to produce antifungal chitinase are widely known (Table 18.10), and the majority of biocontrolling bacteria belong to Proteobacteria (55.88%), Firmicutes (23.52%), Actinobacteria (19.11%), and Bacteroidetes (1.47%).
Degradation of fungal (Rhizopus stolonifer) cell wall by chitinase-producing bacterial strains (Ghosh et al. 2015)
4.3 Insecticidal Activity of Bacterial Chitinase
Insect infestation is a major issue of many agronomic crops. Insects attack more than 500 plant species belonging to 63 plant families. Insects are the vector of plant virus member especially of the geminivirus group. Some diseases associated with the whitefly are lettuce necrotic yellows, irregular ripening of tomato, silver leaf of squash, cotton leaf curl, tobacco leaf curl, and cassava mosaic. Meanwhile, chitin-scavenging enzymes are applied to renovate chitin-holding raw material into biotechnologically serviceable apparatuses; they are a significant concern of chemical and pharmaceutical activities (Aggarwal et al. 2015).
In insects, the dynamic configurations such as exoskeleton, appendages, peritrophic membrane, etc. are made up of chitin as a chief structural element. Therefore, the growth and development are intensely administrated by building and transformation of these chitinous assemblies (Merzendorfer and Zimoch 2003). Thus, addition of chitinolytic enzymes can interrupt in the basic functional progressions similar to ecdysis and redevelopment of peritrophic membrane. Reports suggest chitinase enhanced destruction to the peritrophic membrane of the insect gut (Subbanna et al. 2018). In that way, the creation of a less operative barricade results in appreciable decline in feeding and reduction in the proficiency of digestive procedure, nutritional consumption, and growth. Apart from the straight destruction of peritrophic membrane, chitinases can also perform physical malformations in midgut epithelial cells, like bloating, elongations, and creations of several vacuoles (Terra and Ferreira 2005; Otsu et al. 2003; Gongora et al. 2001; Wiwat et al. 2000).
As the exo-skeletal and other portions of the insects are made up of chitin, prospective chitinolytic bacterial isolates are taking place as a promising biopesticide in the field of improvised biotechnology (Singh et al. 2016). Biocontrol of such insects through potent chitinolytic bacteria is reported so far and can be applied as insecticides to control these plant pests (Merzendorfer and Zimoch 2003). According to Aggarwal et al. (2015), a potent chitinase producer, Serratia marcescens, demonstrates the highest mortality range of Spodoptera litura larvae up to the level 70.8%. Another evidence shows the efficiency of Bacillus cereus as a biocontrol agent upon agronomic pest like Bemisia tabaci (Mubarik et al. 2010). The potentiality of exoskeleton degradation of the whitefly treated with chitinase isolated from B. cereus is given in Fig. 18.13. Keeping the evidences alive, Otsu et al. (2003) exhibit that chitinase-secreting Alcaligenes paradoxus KPM-012A was exploited as a biocontrol agent of phytophagous ladybird beetles Epilachna vigintioctopunctata. The use of biocontrol agent Bacillus thuringiensis H1 has a promising effect on different stages of Musca domestica lifecycles (Salama et al. 2016).
Degradation of whitefly (Bemisia tabaci) exoskeleton with Bacillus cereus chitinase [(a) Control whitefly; (b) day 1, treatment with bacterial chitinase; (c) day 3, degradation of insect exoskeleton] (Mubarik et al. 2010)
Reports regarding the significant plant pest control by the chitinolytic bacteria are reported in such forms like larval developmental control of pests and can be exemplified by Trichoplusia ni (Broadway et al. 1998), Helicoverpa armigera (Chandrasekaran et al. 2012; Singh et al. 2016), and Malacosoma neustria (Danismazoglu et al. 2015) and sucking pests like Myzus persicae (Broadway et al. 1998; Rahbe and Febvay 1993), Bemisia argentifolii, Hypothenemus hampei (Broadway et al. 1998), and Hypothenemus hampei (Martínez et al. 2012).
4.4 Antagonistic Effect Against Nematodes
Apart from the antifungal and insecticidal fitness, the chitinolytic bacteria also exhibit their nematicidal property against the plant parasites. Nematodes are key agricultural pests of potatoes and in some other crops. Economic crop miscarriage can happen when the nematode population in soil is extraordinarily high. Chemical nematicides are operative but are very toxic to humans and are environmentally hurtful. In search of such alternative, certain bacteria can diminish nematode mobility (Stirling 1984), while other bacteria are on the right path and can produce combinations lethal to plant-parasitic nematodes (Sikora 1991; Spiegel et al. 1991; Oostendorp and Sikora 1990). One such investigation is chitinase-producing soil isolates like Chromobacterium sp. UP1 and Stenotrophomonas maltophilia MI-12, which inhibited egg hatch of the potato cyst nematode, Globodera rostochiensis, up to 70% as the main constituent of the eggshell of G. rostochiensis is chitin (Cronin et al. 1997; Clarke and Hennessy 1976).
Nematode eggs are mainly composed of chitin as the chief structural ingredient. This chitinous facility offers resistance counter to chemical and biological nematicides (Wharton 1980). Chitinases are known to affect egg hatching of many parasitic nematodes like Meloidogyne hapla (Mercer et al. 1992), M. incognita (Lee and Kim 2015; Nguyen et al. 2007; Jung et al. 2002), M. javanica (Spiegel et al. 1991), and M. arenaria (Kalaiarasan et al. 2006) by disfiguring and vandalizing the egg shells, leading to either suppression of hatching (Cronin et al. 1997; Lee and Kim 2015) or premature exposure of juveniles which are ineffectual to persist in soil environment (Jung et al. 2002). However, some studies reported discrepancy in susceptibility of eggs and juvenile to chitinases.
In connection with antifungal, insecticidal, and nematicidal properties, there is an upsurge of attention to evolve environment-friendly plant pest-controlling substitutions like chitinase-producing bacteria. This investigation was conducted to travel the unexplored areas of chitinolytic microbes’ hub and their possible application as a green pesticide.
5 Conclusions
Chitin in the environment is both abundant and prevalent at the same time. Actually, it is the second most abundant biodegradable biopolymer on earth, next to cellulose. Chitin is found in many lifeforms, such as shells (shrimps and crabs), exoskeletons and gut linings of arthropods (crustaceans and insects), and cell walls of several fungi, including some yeasts and structural framework unit of some protista as well as of nematode eggs. The biomolecules that can solubilize that inflexible chitin are known as chitinases. Chitinase can be produced from bacteria, fungi, viruses, plants, and human also. Plant chitinase is produced as a PR protein in response to its defense mechanisms. Bacterial chitinases are recorded from different natural resources like diverse soil and water habitats and shrimp and crab shell waste and also from altered gut systems. Numerous varieties of soil environments are the residence of so many types of chitinolytic bacterial groups. The variants of soil backgrounds are ranging from Antarctic to mangrove, vineyard, agricultural field, and rhizospheric soils of several categories like tea, mango, wheat, maize, rice, and pepper plants. Chitinase-producing bacteria are the resident among the wide range of water bodies from marine to freshwater, hot spring, irrigation well, Lonar lake, shrimp pond, and moat water. These chitinolytic bacteria are the dwellers not only of soil and water but also of shrimp and crab waste dumping area. Interestingly, they are also reported as plant endophytes of agronomic plant parts like root, stem, and leaves. Apart from the rhizospheric soil appearance to endophytic residence, chitinase producers are also present in both the vertebrate and invertebrate gut environments such as fish, bat, insect, and earthworm.
In connection with the abundance of the chitinolytic bacteria in both the endophytic and the endozoic manner, it can be stated that these chitinase-producing bacteria can deliver metabolic competences, necessary nutrients, and protection against pathogens through enzymatic performances which seem to share evolutionary trends. Many microbial genomes possess different genes encoding chitinolytic enzymes, which have been extensively investigated, but studies regarding the use of microorganisms that utilize insoluble chitin as a carbon source in the area of gut system are sparse. Study of chitinolytic gut microflora is in its infancy; only a few have been studied in adequate detail. As there is versatility within the animal population in terms of population size, habitat, feeding habit, etc., it may be expected that gut microflora can be a gem container consisting of several chitinase producers.
Reported investigations regarding the uses of chitinases and potent chitinolytic microorganisms especially bacteria in the biotechnologically advanced sustainable agriculture are receiving immense attention. From the biocontrol potentiality to biofertilizing ability, these microorganisms approach a new bio-based concept that can reduce the use of chemical fungicides, pesticides, and fertilizers with the assistance of such natural chitinase producers. These chitinolytic bacteria can, therefore, be used as a raw material in biotechnology for environmentally safe and affordable agriculture that leads to human welfare.
6 Future Perspectives
-
Fungicidal and insecticidal activity of bacterial chitinase may supplement the use of chemical fungicides and insecticides.
-
Bioaccumulation of fungicide and insecticide in agronomic crop fields leads to human health risk by biomagnification.
-
Inductive plant defense mechanism through the by-products of microbial chitinases like chitooligomers and monomers will secure more pest control potentiality.
-
Formulation of microbes as biofertilizers with capabilities like plant growth-promoting traits can create a novel biotechnologically advanced agronomic tool.
References
Abdallah RAB, Stedel C, Garagounis C, Nefzi A, Jabnoun-Khiareddine H, Jabnoun-Khiareddine KK, Daami-Remadi M (2017) Involvement of lipopeptide antibiotics and chitinase genes and induction of host defense in suppression of Fusarium wilt by endophytic Bacillus spp. in tomato. Crop Prot 99:45–58
Adhikari M, Yadav DR, Kim SW, Um YH, Kim HS, Lee SC, Song JY, Kim HG, Lee YS (2017) Biological control of bacterial fruit blotch of watermelon pathogen (Acidovorax citrulli) with rhizosphere associated bacteria. Plant Pathol J 33:170–183
Aggarwal C, Paul S, Tripathi V, Paul B, Khan MA (2015) Chitinase producing Serratia marcescens for biocontrol of Spodoptera litura (Fab) and studies on its chitinolytic activities. Ann Agric Res (New Series) 36:132–137
Agrios GN (2005) Plant diseases caused by fungi. In: Plant pathology, 5th edn. Elsevier Academic Press, London, pp 386–593
Ahmadi KJA, Yazdi T, Najafi MF, Shahverdi AR, Faramarzi MA, Zarrini G, Behravn J (2008) Optimization of medium and cultivation condition for chitinase production by the newly isolated: Aeromonas sp. Biotechnology 7:266–272
Ajayi AA, Onibokun EA, George FOA, Atolagbe OM (2016) Isolation and characterization of chitinolytic bacteria for chitinase production from the African Catfish, Clarias gariepinus (Burchell, 1822). Res J Microbiol 11:119–125
Alhasawi A, Appanna VD (2017) Enhanced extracellular chitinase production in Pseudomonas fluorescens: biotechnological implications. AIMS Bioeng 4:366–375
Amin A, Ali SW, Arshad R, Nadeem S, Ali S (2011) Characterization of chitinolytic bacterial strains isolated from local habitat. Mycopathologia 9:51–55
Anuradha V, Revathi K (2013) Purification and characterization of bacterial chitinase isolated from crustacean shells. Int J Pure Appl Biosci 4:78–82
Aounallah MA, Slimene-Debez IB, Djebali K, Gharbi D, Hammami M, Azaiez S, Limam F, Tabbene O (2017) Enhancement of exochitinase production by Bacillus licheniformis AT6 strain and improvement of N-acetylglucosamine production. Appl Biochem Biotechnol 181:650–666
Askarian F, Zhou Z, Olsen RE, Sperstad S, Ringo E (2012) Culturable autochthonous gut bacteria in Atlantic salmon (Salmo salar L.) fed diets with or without chitin. Characterization by 16S rRNA gene sequencing, ability to produce enzymes and in vitro growth inhibition of four fish pathogens. Aquaculture 326–329:1–8
Banerjee S, Mukherjee A, Dutta D, Ghosh K (2015) Evaluation of chitinolytic gut microbiota in some carps and optimization of culture conditions for chitinase production by the selected bacteria. J Microbiol Biotechnol Food Sci 5:12–19
Banerjee S, Maiti TK, Roy RN (2016) Identification and product optimization of amylolytic Rhodococcus opacus GAA 31.1 isolated from gut of Gryllotalpa africana. J Genet Eng Biotechnol 14:133–141
Banerjee S, Maiti TK, Roy RN (2017) Protease production by thermo-alkaliphilic novel gut isolate Kitasatospora cheerisanensis GAP 12.4 from Gryllotalpa africana. Biocatal Biotransform 35:168–176
Bansode VB, Bajekal SS (2006) Characterization of chitinase from microorganisms isolated from lonar lake. Indian J Biotechnol 5:357–363
Bao-Qin H, Chang-Ying YU, Wan-Shun LIU, Ji-xun DAI (2004) Purification and inhibition fungal growth of chitinases from Vibrio pacini. Wuhan Univ J Natl Sci 9:973–978
Bartnicki-Garcia S (1968) Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol 22:87–108
Benhamou N (1996) Elicitor-induced plant defense pathways. Trends Plant Sci 1:233–240
Berg G, Hallmann J (2006) Control of plant pathogenic fungi with bacterial endophytes. In: Schulz B, Boyle C, Sieber TN (eds) Microbial root endophytes. Springer, Berlin/Heidelberg, pp 53–69
Bhushan B (1998) Isolation, purification, characterization and scaleup production of a thermostable chitinase from an alkalophilic microorganism. Ph.D. thesis, Department of Microbiology, Punjab University, Chandigarh
Broadway RM, Gongora C, Kain WC, Sanderson JP, Monroy JA, Bennett KC, Warner JB, Hoffmann MP (1998) Novel chitinolytic enzymes with biological activity against herbivorous insects. J Chem Ecol 24:985–998
Broglie K, Chet I, Holliday M, Cressrnan R, Biddle P, Knowlton S, Mauvais C, Broglie R (1991) Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science 254:1194–1197
Brzezinska MS, Jankiewicz U, Walczak M (2013) Biodegradation of chitinous substances and chitinase production by the soil actinomycete Streptomyces rimosus. Int Biodeterior Biodegradation 84:104–107
Brzezinska MS, Jankiewicz U, Burkowska A, Walczak M (2014) Chitinolytic microorganisms and their possible application in environmental protection. Curr Microbiol 68:71–81
Chakrabarti R (2002) Carotenoprotein from tropical brown shrimp shell waste by enzymatic process. Food Biotechnol 16:81–90
Chandra S, Chakraborty N, Dasgupta A, Panda K, Acharya K (2015) Chitosan nanoparticles: a positive modulator of innate immune responses in plants. Sci Rep 5:15195
Chandrasekaran R, Revathi K, Nisha S, Kirubakaran SA, Sathish-Narayanan S, Senthil-Nathan S (2012) Physiological effect of chitinase purified from Bacillus subtilis against the tobacco cutworm Spodoptera litura Fab. Pestic Biochem Physiol 104:65–71
Chang WT, Chen YC, Jao CL (2007) Antifungal activity and enhancement of plant growth by Bacillus cereus grown on shellfish chitin wastes. Bioresour Technol 98:1224–1230
Chrisnasari R, Yasaputera S, Christianto P, Santoso VI, Pantjajani T (2016) Production and characterization of chitinases from thermophilic bacteria isolated from prataan hot spring, East Java. J Math Fund Sci 48:149–163
Clarke AJ, Hennessy J (1976) The distribution of carbohydrates in cysts of Heterodera rostochiensis. Nematologica 22:190–195
Cronin D, Moenne-Loccoz Y, Dunne C, O’Gara F (1997) Inhibition of egg hatch of the potato cyst nematode Globodera rostochiensis by chitinase-producing bacteria. Eur J Plant Pathol 103:433–440
Dahiya N, Tewari R, Tiwari RP, Hoondal GS (2005) Chitinase from Enterobacter sp. NRG4: its purification, characterization and reaction pattern. Electron J Biotechnol 8:134–145
Dahiya N, Tewari R, Hoondal GS (2006) Biotechnological aspects of chitinolytic enzymes: a review. Appl Microbiol Biotechnol 25:1–10
Danismazoglu M, Demir I, Sezen K, Muratoglu H, Nalçacioglu R (2015) Cloning and expression of chitinase A, B, and C (chiA, chiB, chiC) genes from Serratia marcescens originating from Helicoverpa armigera and determining their activities. Turk J Biol 39:78–87
Das P, Kumar P, Kumar M, Solanki R, Kapur MK (2017) Purification and molecular characterization of chitinases from soil actinomycetes. Afr J Microbiol Res 11:1086–1102
Desouza MM, Murray MK (1995) An estrogen-dependent sheep oviductal glycoprotein has glycan linkages typical of sialomucins and does not contain chitinase activity. Biol Reprod 53:1517–1526
Dey A, Ghosh K, Hazra N (2016) Evaluation of extracellular enzyme-producing autochthonous gut bacteria in walking catfish, Clarias batrachus (L.). J Fish 4:345–352
Dillon RJ, Dillon VM (2004) The gut bacteria of insects: nonpathogenic interactions. Annu Rev Entomol 49:71–92
Dixon B (1995) Using fungal dressings to heal wounds. Biotechnology 13:120–121
Duo-Chuan L (2006) Review of fungal chitinases. Mycopathologia 163:345–360
Eckardt NA (2008) Chitin signaling in plants: insights into the perception of fungal pathogens and rhizobacterial symbionts. Plant Cell 20:241–243
El Hadrami A, Adam LR, El Hadrami I, Daayf F (2010) Chitosan in plant protection. Mar Drugs 8:968–987
Flach J, Pilet PE, Jolles P (1992) What’s new in chitinases research? Experientia (Basel) 48:701–716
Frankowski J, Lorito M, F Scala F (2001) Purification and properties of two chitinolytic enzymes of Serratia plymuthica HRO-C48. Arch Microbiol 176:421–426
Gaurav R, Tang J, Jadhav J (2017) Novel chitinase producer Bacillus pumilus RST25 isolated from the shellfish processing industry revealed antifungal potential against phyto-pathogens. Int Biodeterior Biodegradation 125:228–234
Genta FA, Dillon RJ, Terra WR, Ferreira C (2006) Potential role for gut microbiota in cell wall digestion and glucoside detoxification in Tenebrio molitor larvae. J Insect Physiol 52:593–601
Ghasemi S, Ahmadian G, Jelodar NB, Rahimian H, Ghandili S, Dehestani A, Shariati P (2010) Antifungal chitinases from Bacillus pumilus SG2: preliminary report. World J Microbiol Biotechnol 26:1437–1443
Ghorbel-Bellaaj O, Manni L, Jellouli K, Hmidet N, Nasri M (2012) Optimization of protease and chitinase production by Bacillus cereus SV1 on shrimp shell waste using statistical experimental design. Biochemical and molecular characterization of the chitinase. Ann Microbiol 62:1255–1268
Ghosh R, Barman S, Mukhopadhyay A, Mandal NC (2015) Biological control of fruit-rot of jackfruit by rhizobacteria and food grade lactic acid bacteria. Biol Control 83:29–36
Gohel V, Chaudhary T, Vyas P, Chhatpar HS (2004) Isolation and identification of marine chitinolytic bacteria and their potential in antifungal biocontrol. Indian J Exp Biol 42:715–720
Gohel V, Singh A, Vimal M, Ashwini P, Chhatpar HS (2006) Bioprospecting and antifungal potential of chitinolytic microorganisms. Afr J Biotechnol 5:54–72
Gongora CE, Wang S, Barbehenn RV, Broadway RM (2001) Chitinolytic enzymes from Streptomyces albidoflavus expressed in tomato plants: effects on Trichoplusia ni. Entomol Expe Appl 99:193–204
Gooday GW (1999) Aggressive and defensive roles for chitinases. EXS 87:157–169
Grison R, Besset BG, Schneider M, Lucante N, Olsen L, Leguay JJ, Toppan A (1996) Field tolerance to fungal pathogens of Brassica napus constitutively expressing a chimeric chitinase gene. Nat Biotechnol 14:643–646
Halder SK, Maity C, Jana A, Pati BR, Mondal KC (2012) Chitinolytic enzymes from the newly isolated Aeromonas hydrophila SBK1: study of the mosquitocidal activity. BioControl 57:441–449
Halder SK, Maity C, Jana A, Das A, Paul T, Das Mohapatra PK, Pati B, Mondal KC (2013) Proficient biodegradation of shrimp shell waste by Aeromonas hydrophila SBK1 for the concomitant production of antifungal chitinase and antioxidant chitosaccharides. Int Biodeterior Biodegradation 79:88–97
Hamid R, Khan MA, Ahmad M, Ahmad MM, Abdin MZ, Musarrat J (2013) Chitinases: an update. J Pharm Bioallied Sci 5:21–29
Hammami I, Siala R, Jridi M, Ktari N, Nasri M, Triki MA (2013) Partial purification and characterization of chiIO8, a novel antifungal chitinase produced by Bacillus cereus IO8. J Appl Microbiol 115:358–366
Han Y, Yang B, Zhang F, Miao X, Li Z (2009) Characterization of antifungal chitinase from marine Streptomyces sp. DA11 associated with South China sea sponge Craniella Australiensis. Mar Biotechnol 11:132–140
Han JH, Park GC, Kim KS (2018) Antagonistic evaluation of Chromobacterium sp. JH7 for biological control of Ginseng root rot caused by Cylindrocarpon destructans. Mycobiology 45:370–378
Henrissat B (1991) A classification of glycosyl hydrolases based on amino acid sequence similarities. J Biochem 280:309–316
Henrissat B (1999) Classification of chitinases modules. EXS 87:137–156
Henrissat B, Bairoch A (1996) Updating the sequence-based classification of glycosyl hydrolases. Biochem J 316:695–696
Honda K, Kimura K, Ninh PH, Taniguchi H, Okano K, Ohtake H (2017) In vitro bioconversion of chitin to pyruvate with thermophilic enzymes. J Biosci Bioeng 124:294–301
Huang L, Garbulewska E, Sato K, Kato Y, Nogawa M, Taguchi G, Shimosaka M (2012) Isolation of genes coding for chitin-degrading enzymes in the novel chitinolytic bacterium, Chitiniphilus shinanonensis, and characterization of a gene coding for a family 19 chitinase. J Biosci Bioeng 113:293–299
Ilangumaran G, Stratton G, Ravichandran S, Potin P, Asiedu S, Prithiviraj B (2017) Microbial degradation of lobster shells to extract chitin derivatives for plant disease management. Front Microbiol 8:1–14, 781
Indiragandhi P, Anandham R, Madhaiyan M, Poonguzhali S, Kim GH, Saravanan VS, Tongmin S (2007) Cultivable bacteria associated with larval gut of prothiofos-resistant, prothiofos-susceptible and field-caught populations of diamondback moth, Plutella xylostella and their potential for, antagonism towards entomopathogenic fungi and host insect nutrition. J Appl Microbiol 103:2664–2675
Itoi S, Okamura T, Koyama Y, Sugita H (2006) Chitinolytic bacteria in the intestinal tract of Japanese coastal fishes. Can J Microbiol 52:1158–1163
Jafari S, Aghaei SS, Afifi-Sabet H, Shams-Ghahfarokhi M, Jahanshiri Z, Gholami-Shabani M, Shafiei-Darabi S, Razzaghi-Abhyaneh M (2018) Exploration, antifungal and antiaflatoxigenic activity of halophilic bacteria communities from saline soils of Howze-Soltan playa in Iran. Extremophiles 22:87–98
Jain S, Tuteja N, Vaishnav A, Kumari S, Choudhary SD, Varma A (2017) Chitinolytic Bacillus-mediated induction of jasmonic acid and defense-related proteins in soybean (Glycine max l. merrill) plant against Rhizoctonia solani and Fusarium oxysporum. J Plant Growth Regul 36:200–214
Jang MK, Kong BG, Jeong YI, Lee CH, Nah JW (2004) Physicochemical characterization of α-chitin, β-chitin and γ-chitin separated from natural resources. J Polym Sci Part A Polym Chem 42:3423–3432
Jankiewicz U, Brzezinska MS, Saks E (2012) Identification and characterization of a chitinase of Stenotrophomonas maltophilia, a bacterium that is antagonistic towards fungal phytopathogens. J Biosci Bioeng 113:30–35
Jiang X, Chen D, Hong SH, Wang W, Chen SH, Zou SH (2012) Identification, characterization and functional analysis of a GH18 chitinase from Streptomyces roseolus. Carbohydr Polym 87:2409–2415
Jones JD, Dangl JL (2006) The plant immune system. Nature 444:323–329
Joo GJ (2005) Purification and characterization of an extracellular chitinase from the antifungal biocontrol agent Streptomyces halstedii. Biotechnol Lett 27:1483–1486
Joo WC, Yun UJ, Park HD (1996) Isolation of chitin-utilizing bacterium and production of its extracellular chitinase. J Microbiol Biotechnol 6:439–444
Jung WJ, Jung SJ, An KN, Jin YL, Park RD, Kim FY, Shon BK, Kim TH (2002) Effect of chitinase-producing Paenibacillus illinoisis KJA-424 on egg hatching of root-knot nematode (Meloidogyne incognita). J Microbiol Biotechnol 12:865–871
Kalaiarasan P, Lakshmanan PL, Rajendran G, Samiyappan R (2006) Chitin and chitinolytic biocontrol agents for the management of root knot nematode, Meloidogyne arenaria in groundnut (Arachis hypogaea L.) cv. Co3. Indian J Nematol 36:181–186
Kamil Z, Rizk M, Saleh M, Moustafa S (2007) Isolation and identification of rhizosphere soil chitinolytic bacteria and their potential in antifungal biocontrol. Glob J Mol Sci 2:57–66
Kamil FH, Saeed EE, El-Terabily KA, AbuQamar SF (2018) Biological control of mango dieback disease caused by Lasiodiplodia theobromae using streptomycete and non streptomycete actinobacteria in the United Arab Emirates. Front Microbiol 9:829–848
Kandra P, Challa MM, Padma Jyothi HK (2012) Efficient use of shrimp waste: present and future trends. Appl Microbiol Biotechnol 93:17–29
Kavitha A, Vijayalakshmi M (2011) Partial purification and antifungal profile of chitinase produced by Streptomyces tendae TK-VL_333. Ann Microbiol 61:597–603
Kejela T, Thakkar VR, Patel RR (2017) A novel strain of Pseudomonas inhibits Colletotrichum gloeosporioides and Fusarium oxysporum infections and promotes germination of Coffee. Rhizosphere 4:9–15
Keshavarz-Tohid V, Taheri P, Muller D, Prigent-Combaret C, Vacheron J, Taghavi SM, Tarighi S, Moenne-Loccoz Y (2017) Phylogenetic diversity and antagonistic traits of root and rhizosphere pseudomonads of bean from Iran for controlling Rhizoctonia solani. Res Microbiol 168:760–772
Kim PI, Chung KC (2004) Production of an antifungal protein for control of Colletotrichum lagenarium by Bacillus amyloliquefaciens MET 0908. FEMS Microbiol Lett 234:177–183
Kong Q (2018) Marine microorganisms as biocontrol agents against fungal phytopathogens and mycotoxins. Biocontrol Sci Tech 28:77–93
Kramer KJ, Koga D (1986) Insect chitin, Physical state synthesis, degradation and metabolic regulation. Insect Biochem 16:851–877
Kramer KJ, Muthukrishnan S (1997) Insect chitinases: molecular biology and potential use as biopesticides. Insect Biochem Mol Biol 27:887–900
Kuddus SM, Ahmed RIZ (2013) Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. J Genet Eng Biotechnol 11:39–46
Kumar A, Kumar D, George N, Sharma P, Gupta N (2018) A process for complete biodegradation of shrimp waste by a novel marine isolate Paenibacillus sp. AD with simultaneous production of chitinase and chitin oligosaccharides. Int J Biol Macromol 1:263–272
Lazado CC, Caipang CMA, Kiron V (2012) Enzymes from the gut bacteria of Atlantic cod, Gadus morhua and their influence on intestinal enzyme activity. Aquac Nutr 10:23–31
Lee YS, Kim KY (2015) Statistical optimization of medium components for chitinase production by Pseudomonas fluorescens strain HN1205: role of chitinase on egg hatching inhibition of root-knot nematode. Biotechnol Equip 29:470–478
Leiva J, Opazo R, Remond C, Uribe E, Velez A, Romero J (2017) Characterization of the intestinal microbiota of wild-caught and farmed fine flounder (Paralichthys adspersus). Lat Am J Aquat Res 45:370–378
Lenardon MD, Munro CA, Gow NAR (2010) Chitin synthesis and fungal pathogenesis. Curr Opin Microbiol 13:416–423
Li Z, Ye X, Peilin C, Kai J, Jie Z, Fei W, Weiliang D, Yan H, Zhengguang Z, Zhongli C (2017) Antifungal potential of Corallococcus sp. strain EGB against plant pathogenic fungi. Biol Control 110:10–17
Lim HS, Kim YS, Kim SD (1991) Pseudomonas stutzeri YPL-1 genetic transformation and antifungal mechanism against Fusarium solani, an agent of plant root rot. Appl Environ Microbiol 57:510–516
Liu D, Cai J, Xie C-C, Liu CH, Chen YH (2010) Purification and partial characterization of a 36-kDa chitinase from Bacillus thuringiensis spp. colmeri, and its biocontrol potential. Enzym Microb Technol 46:252–256
Mao X, Guo N, Sun J, Xue C (2017) Comprehensive utilization of shrimp waste based on biotechnological methods: a review. J Clean Prod 143:814–823
Martínez CP, Echeverri C, Florez JC, Gaitan AL, Gongora CE (2012) In vitro production of two chitinolytic proteins with an inhibiting effect on the insect coffee berry borer, Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae) and the fungus Hemileia vastatrix the most limiting pests of coffee crops. AMB Express 2:22–33
Melent’ev AI, Aktuganov GE, Galimzyanova NF (2001) The role of chitinase in the antifungal activity of Bacillus sp. 739. Microbiology 70:548–552
Mercer CF, Greenwood DR, Grant JL (1992) Effect of plant and microbial chitinases on the eggs and juveniles of Meloidogyne hapla Chitwood. Nematologica 38:227–236
Merzendorfer H, Zimoch L (2003) Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J Exp Biol 206:4393–4412
Mubarik NR, Mahagiani I, Anindyaputri A, Santoso S, Rusmana I (2010) Chitinolytic bacteria isolated from chili rhizosphere: chitinase characterization and its application as biocontrol for whitefly (Bemisia tabaci genn.). Am J Agric Biol Sci 5:430–435
Muzzarelli RA, Mattioli-Balmonte M, Pugnaloni A, Biagini G (1999) Biochemistry, histology and clinical uses of chitins and chitosans in wound healing. EXS 87:251–264
Nguyen VN, Kim YJ, Oh KT, Jung WJ, Park RD (2007) The role of chitinase from Lecanicillium antillanum B-3 in parasitism to root-knot nematode Meloidogyne incognita eggs. Biocontrol Sci Tech 17:1047–1058
O’Herlihy EA, Duffy EM, Cassells AC (2003) The effects of arbuscular mycorrhizal fungi and chitosan sprays on yield and late blight resistance in potato crops from microplants. Folia Geobot 38:201–207
Ong LGA, Lam HK, Lim MY, Tan TX (2017) Process optimization on chitinase production by locally isolated Enterobacter sp. and Zymomonas sp. Int J Chem Eng Appl 8:286–289
Oostendorp M, Sikora RA (1990) In vitro interrelationships between rhizosphere bacteria and Heterodera schachtii. Rev Nematol 13:269–274
Ordentlich A, Elad Y, Chet I (1988) Rhizosphere colonization by Serratia marcescens for the control of Sclerotium rolfsii. Soil Biol Biochem 19:747–751
Otsu Y, Matsuda Y, Shimizu H, Ueki H, Mori H, Fujiwara K, Nakajima T, Miwa A, Nonomura T, Sakuratani Y, Tosa Y, Mayama S, Toyoda H (2003) Biological control of phytophagous ladybird beetles Epilachna vigintioctopunctata (Col., Coccinellidae) by chitinolytic phylloplane bacteria Alcaligenes paradoxus entrapped in alginate beads. J Appl Entomol 127:441–446
Padder SA, Bhat ZA, Dar GH, Mohiddin FA (2017) Impact of plant growth promoting bacterial root endophytes on growth and nutrient status of brown sarson (Brassica rapa L.). Int J Pure App Biosci 5:638–651
Patel JK, Arcahna G (2017) Diverse culturable diazotrophic endophytic bacteria from Poaceae plants show cross-colonization and plant growth promotion in wheat. Plant Soil 417:99–116
Patil RS, Ghomade V, Deshpande MV (2000) Chitinolytic enzymes: an exploration. Enzym Microb Technol 26:473–483
Paulsen SS, Andersen B, Gram L, Machado H (2016) Biological potential of chitinolytic marine bacteria. Mar Drug 14:1–17
Perrakis A, Tews I, Dauter Z, Oppenheim A, Chet I, Wilson K, Vorgias C (1994) Crystal structure of a bacterial chitinase at 2.3 A° resolution. Structure 15:1169–1180
Poulose AJ (1992) Biotechnology and fungal control. In: Koller W (ed) Target sites of fungicide action. CRC Press, Boca Raton, pp 311–318
Prapagdee B, Kuekulvong C, Mongkolsuk S (2008) Antifungal potential of extracellular metabolites produced by Streptomyces hygroscopicus against phytopathogenic fungi. Int J Biol Sci 4:330–337
Prasanna L, Eijsink VG, Meadow R, Gåseidnes S (2013) A novel strain of Brevibacillus laterosporus produces chitinases that contribute to its biocontrol potential. Appl Microbiol Biotechnol 97:1601–1611
Prasanna ND, Vijayalakshmi K, Seshagirirao K, Shaheen SK (2014) Characterization of antifungal compounds produced by Pseudomonas stutzeri EGB3 isolated from gut of earthworm gut (Eisenia foetida). J Microbiol Antimicrobiol 6:57–65
Rabea EI, Badawy MET, Stevens CV, Smagghe G, Steurbaut W (2003) Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4:1457–1465
Rahbe Y, Febvay G (1993) Protein toxicity to aphids: an in vitro test on Acyrthosiphon pisum. Entomol Exp Appl 67:149–160
Ramírez MÁ, Rodríguez AT, Alfonso L, Peniche C (2010) Chitin and its derivatives as biopolymers with potential agricultural applications. Biotecnol Apl 27:270–276
Ravikumar M, Perinbam K (2016) Production, optimization and characterization of chitin deacetylase from marine bacteria Bacillus cereus TK19. J Acad Indus Res 5:72–76
Rishad KS, Jisha MS (2016) Screening of halophilic bacteria producing extracellular hydrolytic enzymes from valanthakad mangroves, Kochi, Kerala. J Microbiol Biotechnol Res 6:1–15
Salama EM, Ismail IA, Khattab AA (2016) Combined effect of a chitinase producing bacteria and Bacillus thuringiensis against Musca domestica (Diptera: Muscidae) larvae. Int J ChemTech Res 9:131–141
Sarbadhikary SB, Mandal NC (2017) Field application of two plant growth promoting rhizobacteria with potent antifungal properties. Rhizosphere 3:170–175
Seo DJ, Lee YS, Kim KY, Jung WJ (2016) Antifungal activity of chitinase obtained from Paenibacillus ehimensis MA2012 against conidial of Colletotrichum gloeosporioides in vitro. Microb Pathog 96:10–14
Setia NI, Suharjono (2015) Chitinolytic assay identification of bacteria isolated from shrimp waste based on 16S rDNA sequences. Adv Microbiol 5:541–548
Shaikh SS, Wani SJ, Sayyed RJ, Thakur R, Gukati A (2018) Production, purification and kinetics of chitinase of Stenotrophomonas maltophilia isolated from rhizospheric soil. Indian J Exp Biol 56:274–278
Sharp RG (2013) A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 3:757–793
Shivaji S, Reddy GSN, Chattopadhyay (2017) Bacterial biodiversity, cold adaptation and biotechnological importance of bacteria occurring in Antarctica. Proc Indian Natl Sci Acad 83:327–352
Sikora RA (1991) The concept of using plant health promoting rhizobacteria for the biological control of plant parasitic nematodes. In: Keel C, Koller B, Defago G (eds) Plant growth promoting rhizobacteria: progress and prospects. IOBC/WPRS Bulletin, Switzerland, pp 3–10
Singh PP, Shin YC, Park CS, Chung YR (1999) Biological control of fusarium Wilt of cucumber by chitinolytic bacteria. Phytopathology 89:92–99
Singh AK, Singh A, Joshi P (2016) Combined application of chitinolytic bacterium Paenibacillus sp. D1 with low doses of chemical pesticides for better control of Helicoverpa armigera. Int J Pest Manag 62:222–227
Someya N, Nakajima M, Hirayae K, Hibi T, Akutsu K (2001) Synergistic antifungal activity of chitinolytic enzymes and prodigiosin produced by the biocontrol bacterium Serratia marcescens strain B2 against the gray mold pathogen, Botrytis cinerea. J Gen Plant Pathol 67:312–317
Someya N, Ikeda S, Morohoshi T, Noguchi Tsujimoto M, Yoshida T, Sawada H, Ikeda T, Tsuchiya K (2011) Diversity of culturable chitinolytic bacteria from rhizospheres of agronomic plants in Japan. Microbes Environ 26:7–14
Song YS, Seo DJ, Jung WJ (2017) Identification, purification, and expression patterns of chitinase from psychrotolerant Pedobacter sp. PR-M6 and antifungal activity in vitro. Microb Pathog 107:62–68
Spiegel Y, Cohn E, Galper S, Sharon E, Chet I (1991) Evaluation of a newly isolated bacterium, Pseudomonas chitinolytica sp. nov., for controlling the root-knot nematode Meloidogyne javanica. Biocontrol Sci Tech 1:115–125
Sridevi M, Mallaiah KV (2008) Factors effecting chitinase activity of Rhizobium sp. from Sesbania sesban. Biologia 63:307–312
Stinizi A, Heitz T, Prasad V, Wiedermann-Merdinoglu S, Geoffroy P, Legrand M, Fritig B (1993) Plant pathogenesis-related proteins and their role in defence against pathogens. Biochimie 75:687–706
Stirling G (1984) Biological control of Meloidogyne javanica with Bacillus penetrans. Phytopathology 74:55–60
Subbanna ARNS, Rajasekhara H, Mishra KK, Pattanayak A (2018) Pesticidal prospectives of chitinolytic bacteria in agricultural pest management. Soil Biol Biochem 116:52–66
Sugita H, Ito Y (2006) Identification of intestinal bacteria from Japanese flounder (Paralichthys olivaceus) and their ability to digest chitin. Lett Appl Microbiol 43:336–342
Suma K, Podile AR (2013) Chitinase A from Stenotrophomonas maltophilia shows transglycosylation and antifungal activities. Bioresour Technol 133:213–220
Sun X, Li J, Du J, Xiao H, Ni J (2018) Cellulomonas macrotermitis sp. nov., a chitinolytic and cellulolytic bacterium isolated from the hindgut of a fungus-growing termite. Antonie Van Leeuwenhoek 111:471–478
Tabli N, Rai A, Bensidhoum L, Palmieri G, Gogliettino M, Cocca E, Consiglio C, Cillo F, Bubici G, Nabti E (2018) Plant growth promoting and inducible antifungal activities of irrigation well water-bacteria. Biol Control 117:78–86
Tarabily KA, Soliman MH, Nassar AH, Hassani HA, Sivasithamparam K, McKenna F, Hardy GE (2000) Biological control of Sclerotinia minor using a chitinolytic bacterium and actinomycetes. Plant Pathol 49:573–583
Tariq AL, Chandran SR, Reyaz AL (2017) Molecular characterization and antifungal activity of extracellular chitinolytic enzyme producing Paenibacillus elgii TS33 isolated from shrimp shell waste. Int J Pharma Res Health Sci 5:2064–2069
Tasharrofi N, Adrangi S, Fazeli M, Rastegar H, Khoshayand MR, Faramarzi MA (2011) Optimization of chitinase production by Bacillus pumilus using Plackett-Burman design and response surface methodology. Iran J Pharm Res 10:759–768
Terra WR, Ferreira C (2005) Biochemistry of digestion. In: Gilbert LI, Iatrou K, Gill SS (eds) Comprehensive molecular insect science biochemistry and molecular biology. Elsevier, London, pp 171–224
Terwisscha Van Scheltinga AC, Henning M, Dijkstra BW (1996) The 1.8 A° resolution structure of hevamine, a plant chitinase/lysozyme and analysis of the conserved sequence and structure motifs of glycosyl hydrolase family 18. J Mol Biol 262:243–257
Thakkar A, Patel R, Thakkar J (2016) Partial purification and optimization of chitinase form Bacillus spp. J Microbiol Biotech Res 6:26–33
Thiagarajan V, Revathia R, Aparanjinib K, Sivamanic P, Girilala M, Priyad CS, Kalaichelvan PT (2011) Extra cellular chitinase production by Streptomyces sp. PTK19 in submerged fermentation and its lytic activity on Fusarium oxysporum PTK2 cell wall. Int J Curr Sci 1:30–44
Thomas JP, Prithiga P, Vijayalakshmi P (2018) Studies on chitinolytic bacteria in intestinal tract of marine fishes and shrimps. Int J Recent Sci Res 9:26277–26282
Tran DM, Sugimoto H, Nguyen DA, Watanabe T, Suzuki K (2018) Identification and characterization of chitinolytic bacteria isolated from a freshwater lake. Biosci Biotechnol Biochem 82:343–355
Vaidya RJ, Shah IM, Vyas PR, Chhatpar HS (2001) Production of chitinase and its optimization from a novel isolate Alcaligenes xylosoxydans: potential antifungal biocontrol. World J Microbiol Biotechnol 17:62–69
Vandana UK, Chopra A, Choudhury A, Adapa D, Mazumder PB (2018) Genetic diversity and antagonistic activity of plant growth promoting bacteria, isolated from tea-rhizosphere: a culture dependent study. Biomed Res 29:853–864
Vida C, Cazorla FM, de Vicente A (2017) Characterization of biocontrol bacterial strains isolated from a suppressiveness-induced soil after amendment with composted almond shells. Res Microbiol 168:583–593
Vincy V, Shoba VM, Viveka S, Vijaya TM, Rani GJB (2014) Isolation and characterization of chitinase from bacteria of shrimp pond. Eur J Exp Biol 4:78–82
Wang SL, Liang TW (2017) Microbial reclamation of squid pens and shrimp shells. Res Chem Intermed 43:3445–3462
Wang SL, Lin TY, Yen YH, Liao HF, Chen YJ (2006) Bioconversion of shellfish chitin wastes for the production of Bacillus subtilis W-118 chitinase. Carbohydr Res 341:2507–2515
Wang Q, Duan B, Yang R, Zhao Y, Zhang L (2015) Screening and identification of chitinolytic actinomycetes and study on the inhibitory activity against turfgrass root rot disease fungi. J Biosci Med 3:56–65
Wharton D (1980) Nematode eggshells. Parasitology 81:447–463
Whitaker JO, Dannelly HK, Prentice DA (2004) Chitinase in insectivorous bats. J Mammal 85:15–18
Wiseman A (1995) Introduction to principles. In: Wiseman A (ed) Handbook of enzyme biotechnology. Ellis Horwood Ltd./T.J. Press Ltd., Padstow/Cornwall, pp 3–8
Wiwat C, Thaithanun S, Pantuwatana S, Bhumiratana A (2000) Toxicity of chitinase-producing Bacillus thuringiensis sp. kurstaki HD-1 toward Plutella xylostella. J Invertebr Pathol 76:270–277
Xia W, Liu P, Zhang J, Chen J (2011) Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll 25:170–179
Yan Q, Fong SS (2015) Bacterial chitinase: nature and perspectives for sustainable bioproduction. Bioresour Bioprocess 2:31–39
Yandigeri MS, Malviya N, Solanki MK, Shrivastava P, Sivakumar G (2015) Chitinolytic Streptomyces vinaceusdrappus S5MW2 isolated from Chilika lake, India enhances plant growth and biocontrol efficacy through chitin supplementation against Rhizoctonia solani. World J Microbiol Biotechnol 31:1217–1225
Zarei M, Aminzadeh S, Zolgharnein H, Safahieh A, Daliri M, Noghabi KA, Ghoroghi A, Motallebi A (2011) Characterization of a chitinase with antifungal activity from a native Serratia marcescens B4A. Braz J Microbiol 42:1017–1029
Zipfel C (2009) Early molecular events in PAMP-triggered immunity. Curr Opin Plant Biol 12:414–420
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Banerjee, S., Mandal, N.C. (2019). Diversity of Chitinase-Producing Bacteria and Their Possible Role in Plant Pest Control. In: Satyanarayana, T., Das, S., Johri, B. (eds) Microbial Diversity in Ecosystem Sustainability and Biotechnological Applications. Springer, Singapore. https://doi.org/10.1007/978-981-13-8487-5_18
Download citation
DOI: https://doi.org/10.1007/978-981-13-8487-5_18
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8486-8
Online ISBN: 978-981-13-8487-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)