Abstract
Salinity of the agriculture soil is the serious issue all over the world, and it is also an important environmental factor for reduction of growth and yield of agricultural crops. The density of more salt available in soil may alter the physiological and metabolic activities in the agricultural crops and reduce the growth and production of crops both qualitative and quantitative ways. For combating against soil salinity, many transgenic salt-tolerant crops have been developed but far too little is success. For solution, in the soils the use of plant growth-promoting rhizobacteria (PGPR) can reduce soil salinity, load of chemical fertilizers, and pesticide in the agricultural field, and improve soil health, seed germination, crop growth, and productivity under saline condition PGPR accepted as potential microbes that can tolerate various atmospheric circumstances like more temperature, pH, and saline soils. In the saline environment, many halophilic/halotolerant bacteria and plants/halophytes are observed/adapted and perform a significant role in saline soil ecosystem. Innumerable microfloral communities and halophytes contain salt-tolerant gene, and they perform as an essential protagonist in subsistence for extreme environmental condition especially salt. It can be concluded that PGPR can be used as a supportable, manageable, sustainable, and economical tool for salinity tolerance and productivity of crops/plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
The population of human will be expected to reach 9.8 billion until 2050 (Magallon and Dinneny 2019). In addition, the demand for food also be increased with the enhancing population, but this demand cannot be fulfilled without soil fertility, beneficial microorganisms, and essential nutrients of the soil (Poeplau et al. 2019; Chandra and Enespa 2017). Currently, many chemical fertilizers and pesticides are used in the soil for production of food; however, these ingredients can be increased for crop growth and productivity (Chandra and Enespa 2017), but simultaneously it increases soil salinity and also reduces soil fertility and beneficial microorganisms present in the soils (Rashid et al. 2016; Yang et al. 2019). The salinity in soil ecosystem is a major agrochemical/abiotic stress problem mainly in the semi-barren and waterless areas (Gu et al. 2016). Approximately, 65% of crop’s productivity is adversely affected by saline soil (Machado and Serralheiro 2017).
A significant role is played by microorganisms in the improvement of productive soil and crop production and yield. In addition, some ions (e.g., sodium (Na+) and potassium (K+)) also affect the growth of plant and microorganisms and ultimately increase the soil salinity (Yan et al. 2015). Besides these, the climate changes such as drought, shortage of water, low rainfall, and abrupt changes in temperature also increase the soil salinity (Chandra and Enespa 2016). Reactive oxygen species (ROS), hydrogen peroxide (H2O2), superoxide (O2), hydroxyl radicals (OH−), lipid peroxidation, and the integrity of the membrane are other parameters of soil salinity which are produced by the cellular response (Choudhury et al. 2017; Chakraborty et al. 2018; Singh et al. 2018). In the presence of soil salinity, organic matter, essential nutrients, and beneficial microorganisms are reduced and ultimately it negatively affects the crop’s productivity (Egamberdieva et al. 2017). Soil salinity reduces the root and shoots growth and finally decreases the crop’s productivity (Glick 2014). For the management of soil salinity, plants used various types of mechanisms (Schmidt et al. 2018). Among all mechanisms, osmolyte is a common mechanism used by the plant. Osmolytes provide protection to the plant cell organelles and also build up compatible solutes (Chakraborty et al. 2018; El-Esawi et al. 2019). Besides, the formation of free radicals stabilizes DNA, stress protein, and prolines during salt stress condition are other factors for survival and growth of the plant (Teh et al. 2016; Chandra and Enespa 2016). Moreover, antioxidant enzymes such as peroxidases (POX), superoxide dismutase (SOD), and catalase (CAT) also protect against salinity and toxicity (Joseph and Jini 2010; Caverzan et al. 2016).
However, these mechanisms are not good for a long time in the reduction of soil salinity; currently, it needs a viable method for reduction of soil salinity and improves the soil fertility and increases microbial population, plant growth, and yield at high saline condition (Ladeiro 2012; Shrivastava and Kumar 2015). Microorganisms play a significant role in the improvement of soil fertility, crop’s growth, and yield (Yan et al. 2015; Biswas and Paul 2017). Among all microbial group, plant growth-promoting rhizobacteria (PGPR) is an eco-friendly method for plant growth and sustainable agriculture by various ways such as the production of phytohormones, solubilization of minerals such as potassium, zinc, phosphate, and chelation of iron under saline condition (Verma et al. 2015; Yadav et al. 2015a, b, c; Habib et al. 2016; Ilangumaran and Smith 2017; Numan et al. 2018). This chapter describes the role of PGPR in the improvement of soil fertility and reduction of soil salinity and crop’s yield under saline condition. In addition, how halotolerant microbes and plants survive under saline condition has been also explained.
11.2 Halophiles, Classification, and Hypersaline Environments
Those microorganisms can propagate and maintain their spore cycle at more saline concentrations (≥150 g L−1/15%) known as halophile (Ollivier et al. 1994; Oren 2008). The halophile is categorized into three dissimilar groups on the beginning of different salt concentrations: 1) less (1–6% NaCl), temperate (7–15%), and more salt concentrated halophile (15–30%) (de Lourdes Moreno et al. 2013; Chandra and Singh 2014; Yadav et al. 2019a, 2015d). Different concentrations of salt occur in the soil, and these are found at various depths in the soil habitats. According to Or et al. (2007), salt concentration and their variability are found much more than water. In the saline environment, different plants are growing known as halotolerant (halophytes) at different concentrations of salts and recorded well adaptability and perform a key character in the biogeochemical cycles (Nabti et al. 2015; Etesami and Beattie 2018). Microbes play a major character in enhancement of herb adaptation at various saline habitats (Bringel and Couée 2015; Bang et al. 2018; Yadav et al. 2019a). However, a limited microbial diversity is found in the extreme soil habitats/hypersaline environments due to various environmental factors and high salt concentrations (Ulukanli and Digrak 2002; Chandra and Singh 2016; Yadav and Saxena 2018). Besides soil salinity, the saline environment is mainly found in the aquatic water such as lakes, river, pond, and sea (Sánchez-Porro et al. 2003). From saline environments, the food or food-based products, plants, and animals contain salts (Maturrano et al. 2006; Ventosa et al. 2015).
11.3 Halophilic/Halotolerant Microbial Diversity in Soil
Soil salinity affects the structure, composition of microbial species, and also bacteriological populations present in the rhizospheric regions of crops. These communities have different groups, which show modified structural and physiological properties under hypersaline condition (Bever et al. 2012; Mendes et al. 2013). However, bacterial communities are dominant as compared to other microbial communities (e.g., virus, fungi, protozoa, and algae), and it is found in the rhizospheric region of the plant under saline condition (Mukhtar et al. 2017; Yamamoto et al. 2018; Chandra and Enespa 2019b). Besides rhizosphere, bacterial communities are also recorded endophytic region, in salt lakes, river water, and root nodules (Albaggar 2014; Leite et al. 2017). But in the saline soil, bacterial communities do not define a similar group of phylogeny but signify a assemblage which has progressed in altered types of microorganisms that belong to the genera Actinopolyspora, Bacillus, Halomonas Micrococcus, Marinococcus, Pseudomonas, Salinicoccus, and Vibrio which are mainly found in the hypersaline region (Ventosa et al. 1998; Soto-Padilla et al. 2014; Chandra et al. 2014; Verma et al. 2017b; Yadav et al. 2018a, b, d). These genera belong to both Gram-positive and Gram-negative bacteria showing rod-, comma-, and cocci-shaped cell. However, Gram-negative bacteria appear to be dominant in saline environments (Ventosa et al. 1998; Canfora et al. 2014). In Gram-negative bacteria, root-nodulating bacteria showing root-colonizing property are considered to be a halotolerant group (Zahran 1997). These bacteria have capable of nitrogen fixation and improve soil fertility at high concentration. A halotolerant bacterium Swaminathania salitolerans gen. nov., sp. nov. was isolated from the rhizosphere, roots, and stems of mangrove-associated wild rice (Loganathan and Nair 2004). Another bacteria belonging to the genus of Azospirillum, Bacillus, Enterobacter, and Azotobacter were isolated from the different agricultural under saline soils (Alamri and Mostafa 2009; Fendrihan et al. 2017). The popular nitrogen-fixing bacterium Rhizobium is linked with marsh grass Spartina alterniflora as a halotolerant plant has also been isolated and identified from hypersaline condition (Bedre et al. 2016).
Besides, another nitrogen-fixing bacterium Bacillus was screened from salty soils of Egypt, and it showed acetylene reduction activity at 5% NaCl concentration (Zahran et al. 1995). The genus Azotobacter is the free-living nitrogen-fixing bacterium showing a significant role in different environmental conditions such as soil, water, and sediments at the high salt concentration (Akhter et al. 2012; Sahoo et al. 2014). Azotobacter strain isolated from agricultural crops showed high nitrogen-fixing ability at 30% NaCl. The nitrogen fixation efficiency of a bacterium A. vinellandii was decreased from nonsaline to saline condition as reported by Sahoo et al. (2014). Azospirillum halopraeferens was isolated and enhanced the growth of mangrove plant by root colonization irrigated with seawater (Bashan et al. 2000). A little information is available on the halotolerant microbial diversity isolated from saline soils as compared with hypersaline aquatic locales (Oren 2008; Yang et al. 2016).
11.4 Effect of Soil Salinity in the Soil Environment
The salinity soil is considered mainly as a major problem in the ecosystem because these problems increase continuously, disturbing biotic and abiotic soil constituents (Vandegehuchte et al. 2010; Bünemann et al. 2018). It also affects natural circumstances in the barren and semi-barren regions of an ecosystem. Excess of saline soils affects seriously on the micro- and macro-floral structure and on space where it lives (Getu 2009). Excess salt in the soils known as sodic soils contains sodium and chloride ions in the earthen constituent part (Bianco and Defez 2010). Due to insufficient discharge and drainage of irrigation water, salts accumulated in the soil (Cuevas et al. 2019). However, the chlorides, bicarbonates of calcium, sulfates, carbonates, magnesium, sodium, and potassium salts are present in the irrigation water (Warrence et al. 2002). The soil structure growth and the production of crops adversely are affected by salt concentration (Ondrasek et al. 2011; Shrivastava and Kumar 2015). On the bases of soil and groundwater practices generally, the salinity is of three types: transient, groundwater associated, and irrigation salinities (Greene et al. 2016; Chandra et al. 2020). Salinity affects both soil system and living organisms that are known as most severe abiotic environmental stress (Gupta and Huang 2014). The immediate consequences of soil are found for biological activity or conservation occurs within the pore space or on the surfaces of the particles that forms the pores (Indoria et al. 2017; Totsche et al. 2018). High salinity leads to negative effects on soil structure which is well known.
Soil dispersion and clay platelets to swell and aggregate are caused by elevated sodium concentrations (Warrence et al. 2002). Thus, in the binding of clay particles, the forces involved are dislocated under the stimulus of sodium ions. Clay particles to plug soil pores are caused due to the dispersion of soil (Arora and Dagar 2019). Therefore, the permeability of soil for water and air is reduced and forms apparent crusting (Kooistra and Tovey 1994; Greene and Hairsine 2004).
It is documented that the presence of water in the soil leads to the swelling of the soil particles with high smectite clay content, and the hydration of some minerals as a result of the reduction of the cross-sectional area of soil pores is documented (Mahrous et al. 2018). Under high sodium or low salt concentrations, this process is completed and it causes the mobilization of fine particles and diffusion within the pores (Mahrous et al. 2018; Chandra et al. 2020). The water and air will be obstructed within the soil structure and particles by the particles stored in the small pores (Schjønning et al. 2002).
11.5 Mechanisms for Adaptation of Microorganisms in the Hypersaline Environment
Phylogenetically, the microbial life is very diverse at high concentrations, and the salinity environments are occupied by halophilic and halotolerant microflora of all domains of life, such as archaea, bacteria, and eukarya (Oren 2008; Ma et al. 2010). Using this mechanisms these halophile microorganisms to tolerate the high salt concentrations, and in various cases to acclimatize their structure to alterations in high salinity in their environments, are miscellaneous as well (Oren 2008).
The basic mechanisms for adaptation of microorganisms in the hypersaline environment are given below:
-
Biological membranes of the microorganism are absorptive to water containing salt. Consequently, the movement of water inside and outside of microbial cells is possessed by changes in ionic activity between cytoplasm and external medium (Murínová and Dercová 2014; Watson 2015).
-
The bacterial cell maintains high osmotic pressure under saline condition; therefore, it is another strategy for adaptation mechanism (Weinisch et al. 2018).
-
The high concentrations of inorganic salts inside the microbial cell are accumulated and achieved the osmotic balance. The sodium ions are left out from cells in all three domains of life, and inside the cell the salt strategy is based on KCl rather than NaCl as a main salt of intracellular organism (Oren 2002).
-
Di-myoinositol-1, 1-phosphate, cyclic 2,3-diphosphoglycerate, α-diglycerol phosphate, mannosylglycerate, and mannosylglyceramide are compatible solutes which are very strong water structure formers and are excepted from the hydration shell of proteins, thus alleviating the hydration shell and decreasing the water activity coefficients (Gunde-Cimerman et al. 2018).
-
In many extremophiles, such low-molecular weight compounds are accumulated to increase the concentrations of salts but also as a reply to other ecological alterations such as temperature stress.
-
Di-myoinositol-1, 1-phosphate, cyclic 2, 3-diphosphoglycerate, α-diglycerol phosphate, mannosylglycerate, and mannosylglyceramide are the examples of organic compatible solutes in thermophiles and in psychrophiles (da Costa and Santos 2009).
-
Mostly, at low salt concentration, the microorganisms are endured and also accumulate salts inside the cell in the form of solutes from outside medium (Shrivastava and Kumar 2015).
11.5.1 Mechanism of Salt Tolerance
The microbial population in the rhizosphere decreases severely due to increase in pH and salinity (Ibekwe et al. 2010). In hypersaline atmosphere the microbes inhabits using “compatible solute strategy” having capability to strong osmotic pressure to resist the salt stress (Pikuta et al. 2007; Chandra and Singh 2017). Choline, betaine, proline, glutamic acid, and other amino acids are the compatible solutes stored by various halophilic bacteria at high concentrations without interfering with cellular processes (Poolman and Glaasker 1998).
11.5.2 Characteristics and Function of Compatible Solutes
The HPLC and NMR methods are followed for the determination and production of compatible solutes in various archaea and bacteria (Roberts 2005a, b). The compounds in limited numbers comprise the bacteria such as sugars (trehalose), polyols (glycerol and glucosyl glycerol), free amino acids (proline and glutamate), offshoots thereof (proline, betaine, and ectoine), quaternary amines and their sulfonium analogs (glycine betaine, carnitine, and dimethylsulfoniopropionate), sulfate esters (choline-O-sulfate), and N-acetylated diamino acids and small peptides (N-acetylornithine and N-acetylglutaminylglutamine amide) (Kempf and Bremer 1998). Generally, the compatible solutes do not carry a net charge at physiological pH due to their high molecular solubility (Galinski 1993). The vital cellular functions such as DNA replication, DNA–protein interactions, and the cellular metabolic machinery without disturbing the solutes can reach high intracellular concentrations in disparity to mineral salts (Wang and Levin 2009; Long et al. 2018). Compatible solutes such as glycine, betaine, and proline increase the cytoplasmic volume and water content freely of the cells at high osmolality, and their accumulation uninterruptedly permitted proliferation of cells under unfavorable conditions (Kohler et al. 2015).
Various halotolerant nitrogen-fixing bacteria accumulate electrolytes such as K+ glutamate, as enzymes, ribosomes, and transport proteins of these bacteria require high level of potassium for stability and activity using salt in strategy mechanism (Da Costa et al. 1998a, b). But within the cell physiology, organic solute accumulations are more compatible (Ventosa et al. 1998; Wood et al. 2001). The organic solutes have two mechanisms under saline conditions for their mode of actions: firstly to increase the intracellular osmotic strength and secondly to stabilization; the cellular macromolecules are proposed (Yancey et al. 1982; Csonka 1989; Chandra and Enespa 2019a). After adding these solutes in bacterial culture, the drastic stimulation in growth rate is observed in cells in high osmolality media (Gouffi et al. 1998). Higher internal concentrations of solutes accumulated in the alleviation of osmolality (Patchett et al. 1992). The glucose is oxidized in Entner–Doudoroff pathway modifications by the mostly halotolerant organisms (Fig. 11.1), the synthesis of compatible solutes after formation of pyruvate, and its further oxidation by pyruvate oxidoreductase in tricarboxylic acid cycle (TCA) (Kindzierski et al. 2017).

(Figure adopted by Saum and Müller 2008)
Synthesis of compatible solutes: Proline, ectoine, and glutamine under stress conditions
In salt-tolerant bacteria, the accumulation of organic solutes has been found to require genetic initiation (Roberts 2005a, b). In response to osmotic stress in Bacillus sp., intracellular proline to increase rapidly has been observed and the corresponding genes were detected, respectively, proB, proA, and proC encoding γ-glutamyl kinase (γ-GK), γ-glutamyl-phosphate reductase (γ-GPR), and pyrroline-5-carboxylate (P5C) reductase (Pérez-Arellano et al. 2010). L-aspartokinase (Ask), L-2,4-diaminobutyric acid transaminase (EctB), L-2,4-diaminobutyric acid acetyltransferase (EctA), and L-ectoine synthase (EctC) encoding the structural gene and detected for biosynthesis of major harmonious solute like ectoine in Halobacillus dabanensis (Reshetnikov et al. 2006; Czech et al. 2019). Choline or choline-O-sulfate oxidized enzymatically into glycine betaine due to involvement of four genes betI, betC, betB, and betA well characterized at molecular level and organized into one operon (Osteras et al. 1998; Stöveken et al. 2011). Various halotolerant nitrogen-fixing bacteria are also observed in the cell for the maintaining of the balance of Na+ and K+ ions (Hanin et al. 2016; Thomas and Apte 1984). A cytoplasmic KCL concentration is maintained by bacteria similar to that of the surrounding medium in order to attain an osmotic equilibrium (Kraegeloh et al. 2005). The Na+/H+ antiporter performance is a major character in homeostasis of pH and Na+ in cells that interchange Na+ for H+ (Suárez et al. 2008). The genes that are proved to be involved in halotolerance in nitrogen-fixing bacteria either through knockout studies or through overexpression studies are framed in Table 11.1.
11.5.3 Exchange of Solutes/Ions
Many solutes/ions are present in the soils and perform an important character in the existence of microorganisms in the presence of soil salinity (Shrivastava and Kumar 2015). However, more solutes or ions containing soils can decrease microbial population in the rhizospheric region of plants (Aung et al. 2018). Several microbes reside in hypersaline environment condition proficient passionate osmotic pressure, and thus use compatible solute strategy or salt-in strategy to resist salt stress (Oren 2011). Choline, betaine, proline, glutamic acid, and other amino acids compatible solutes accumulated in most of the bacteria at high salinity without interfering with cellular procedures (Wood et al. 2001).
11.5.4 Mechanism of Salt-Dependent Lipid Changes
The lipid content present in the microbial plasma membrane shows special character for the survival of stress environmental condition. The phospholipids of Pseudomonas halosaccharolytica contain glucosyl phosphatidylglycerol, phosphatidylglycerol, diphosphatidylglycerol, and phosphatidylethanolamine which are responsible for growth under high saline condition (Li et al. 2016), and this result indicates increase of phosphatidylglycerol and reduction in phosphatidylethanolamine (Hiramatsu et al. 1980). Later, Hara and Masui (1985) observed that pulse-chase labeling of lipids with several radioactive originators showed that the rate of synthesis of phosphatidylethanolamine was inhibited by an increase in salt concentration, but the rate of phosphatidylglycerol synthesis was unaffected. The deficiency of motivation of phosphatidylglycerol creation by salt does not settle with compositional data. The radiolabeling experimentations were performed with nongrowing, starved cells, whereas the compositions of lipids were resolute directly on cells collected from culture media (Hara and Masui 1985). The inhibition of phosphatidylethanolamine creation leads to an upsurge in phosphatidylglycerol comfortable in the microbial cell because of the bifurcated phospholipid biosynthetic pathway going inside the cell (Sohlenkamp and Geiger 2016). A similar type of study was performed by Ohno et al. (1979); the little amount of NaCl did not affect the growing bacteria due to the presence of glucosyl phosphatidylglycerol. However, survival mechanisms of halophilic bacteria due to membrane lipid composition cannot judge very easily; this is a very difficult process (Oren 2008). The lots of chemicals, labor, and time may be taken to well understand the interaction between bacterial lipid membrane and salt medium (Pichler and Emmerstorfer-Augustin 2018).
11.5.5 Salt-Tolerant Genes of Bacteria
Many microorganisms contain salt-tolerant gene and perform an important character in survival for extreme environmental condition especially salt (Holmberg and Bülow 1998). The bacterial spores of Bacillus thuringiensis israelensis, B. sphaericus, and B. subtilis contain osmotolerant protein, i.e., small acid-soluble spore protein (SASP) coded by an ssp gene and this gene can survive at the high salt concentration (Cucchi and Rivas 1995). Cucchi and Rivas (1995) reported a sspE gene from B. subtilis and is introduced into another host bacterium B. thuringiensis israelensis strain 4Q2 and observed 65–650 times higher level of salt-tolerant property as compared to natural B. thuringiensis israelensis. In addition, this bacterium does not cause any side effects in living organisms as well as environments. Some other genes such as ectA (diaminobutyric acid acetyltransferase), ectB (diaminobutyric acid aminotransferase), and ectC (ectoine synthase) genes are reported in B. halodurans and showed in the survival of stress tolerance (Reshetnikov et al. 2011).
There are two genes, namely, GspM and EchM have recognized from a metagenomic collection organized from water sample of pond (Kapardar et al. 2010). GspM gene displays comparison with stress proteins, and another gene EchM showed similarity with enoyl-CoA hydratases and both genes were identified to be responsible for halotolerant at high concentration and have latent solicitation in generating halotolerant recombinant bacteria or transgenic crops (Kapardar et al. 2010). The two genes were further isolated from Rhizobium sp. BL3 and showed hyper-salt-tolerant ability (Payakapong et al. 2006). Hence, many microbes from rhizosphere can be exploited to isolate novel gene for salt tolerance and their potential application in the plant genetic engineering or plant growth under saline environment condition.
11.5.6 Salt-Tolerant Genes of Yeast
The two genes HAL1 and HAL3 were isolated and showed overexpressed gene from yeast (Saccharomyces cerevisiae) and also increased the halotolerant capability by a decreasing intracellular Na+ and enhanced internal K+ concentration during salt stress (Ferrando et al. 1995; Locascio et al. 2019). Further, the gene HAL1 has been introduced into tomato crop by Agrobacterium tumefaciens-mediated transformation which improves salt tolerance of the transgenic tomato and enhances the growth and productivity (Gisbert et al. 2000). An enzyme mitogen-activated protein kinase (MAPK) coded by a gene HOG1 shows an important role in the osmoregulatory pathway in S. cerevisiae (O’Rourke and Herskowitz 1998). This gene is also responsible for salt tolerance in Torulopsis versatilis (Wang et al. 2014). A delightful mutant strain Torulopsis versatilis T5 showing salt-tolerant ability was fashioned from wild-type T. versatilis (T) consuming genome trundling and further isolated two genes T5HOG1 and THOG1, demonstrating upturn of salt tolerance in T. versatilis (Cao et al. 2011). Moreover, overexpression of T5HOG1 and THOG1 enhanced the acceptance of salt in S. cerevisiae (Cao et al. 2011).
11.5.7 Salt-Tolerant Genes of Plants
A wide range of cruel ecological circumstances such as salinity, heat, cold, drought, and insect attack are normally exposed in plants. Plants have established altered methods being in sessile nature to survive grow and develop under speedily altering environmental conditions (Hayat et al. 2012). For these mechanisms, plants regulate genes for transcription which are known as transcriptomics under stress conditions (Shu et al. 2018). The genes for regulation of transcription play different roles under stressful environmental conditions. However, during the reproductive and seedling stages, plants have more sessile to stress and the stress response studies express novel genes or proteins with imperative roles in plant anxiety reworking during these growth stages (Verma et al. 2016a, b). However, the word salinity acceptance comes from one or more genes that reduce the uptake of the salt content from the soil and the conveyance of salt through the plant (Munns 2005, 1993).
Salinity tolerance is a very complex process that is recycled by plants to regulate (up-regulation or down-regulation) the manufacture of specific gene products in the form of RNA or proteins (Gupta and Huang 2014). This process has been accepted at different stages of central dogma technologies like from initiation of RNA processing, post-transcriptional modification, and initiation translation to post-translational modification of proteins in living organisms especially plants (Zhao et al. 2017). Understanding the transcription or translation of plants delivers thorough knowledge about the gene expression at the mRNA level. The summary of transcriptional or translational level is widely used for isolation and identification of candidate genes involved in stress responses (Xiao et al. 2017).
Transcriptome profiling is the screening processes which down-regulated or up-regulated the transcription processes that are enormous evidence about salt-tolerant genes till now. Further, a genomic method gives an important role in cloning, encoding, screening, and identifying these genes (Lodish et al. 2000). Under salt stress condition, the expression of gene is altered by transcript issues and those up- or down-regulated the expression of the gene in plants or microorganisms by these are most important switches (Lodish et al. 2000).
A gene bZIP was identified and showed up-regulation gene expression in wheat crop under insistent salt stress disorder and gene expression of down-regulation in salt-tolerant variety of wheat crop (Hayano-Kanashiro et al. 2009). The osmotic regulating and ROS-scavenging genes mostly are salt tolerance genes and also up-regulated in salinity toleant species (Amirbakhtiar et al. 2019). According to study, more than 10 genes showed up-regulated genes in halophytes plant species Spartina alterniflora under saline condition. Under saline condition, more than 10 genes showed up-regulated genes in Spartina alterniflora halophytes plant species, and most of the genes were found to osmotic regulation process among them (Bedre et al. 2016).
11.6 Mechanisms of Plant Growth Promotion of Halophilic Bacteria
11.6.1 Nitrogen Fixation Under Salt Stress Condition
At global level in arid and semi-arid regions, salinity is a serious issue for agriculture. Growth promotion and photosynthesis rate at various stages of plants affected by salinity stress (Magallon and Dinneny 2019). The production of salt-sensitive crops such as legumes is affected by salt stress particularly since these plants depend on nitrogen requirement for symbiotic N2 fixation (Hussain et al. 2010; Kour et al. 2019b, c, d). The crop productivity mainly depends on the deprived mutual association of nodulation in bacteria and ultimately decreases in nitrogen fixation capacity (Mengel et al. 2001). Vicia faba, Phaseolus vulgaris, and Glycine max legume plants are more salt-tolerant species than another leguminous plant Pisum sativum (Mengel et al. 2001). V. faba crop fixed more nitrogen under saline condition due to the presence of rhizobia inside the root nodules and it has been seen (Mengel et al. 2001). Prosopis, Acacia, and Medicago sativa are the other salt-tolerant leguminous plants but these are less halotolerant than the leguminous plants (Joseph et al. 2015). Rhizobium sp. performs a very significant character in symbiosis with plants and nodulation process but, in the presence of salt, inhibits the initial process of rhizobium–legume symbiosis (Maróti and Kondorosi 2014). However, in several reports, the effect of salt stress on nodulation and nitrogen fixation of legumes have been observed (Maróti and Kondorosi 2014). In the presence of salt, the capability of N2-fixation reduces and is documented to a decrease in the respiration of the nodules and minimize in cytosolic production protein, especially leghaemoglobin by nodulation (Zahran 1999). Saline stress negatively affected on N2 fixation by legumes is related to the salt-induced decline directly in dry weight and nitrogen content in plant shoot (Delgado et al. 1994).
Glycine betaine is the osmoprotective substances which perform an imperative character in the maintenance of nitrogenase activity in bacteroides under salinity stress (Normand et al. 2015). The halotolerant Rhizobium sp. enhanced the growth, nodulation, and fixed N2 content in Acacia ampliceps plant containing 200 mM NaCl concentration in the sand culture medium (Egamberdieva et al. 2013) and one more halotolerant Rhizobium sp. designed N2 fixing symbiosis more effective with soybean than other salt-sensitive strain of bacteria (Egamberdieva et al. 2013). Further, the isolated rhizobial strains from Acacia nilotica showed tolerance to 850 mM NaCl concentration formed effective N2-fixing nodules on Acacia trees grown at 150 mM NaCl (Zahran 1999). The salt-tolerant Rhizobium strains produce nodulation in legumes and form effective N2 fixing symbiosis capability in the soil under moderate halophile environment observed in the result (Zahran 1999). Therefore, the booster of salt-tolerant rhizobia strains in the rhizosphere of leguminous crop can enhance the N2 fixation ability under saline condition. However, host tolerance legume to NaCl is a very key element in influencing the achievement of harmonious Rhizobium strains to form symbiosis successfully under the halophilic environment (Egamberdieva et al. 2013).
11.6.2 Phytohormone Production Under Saline Condition
Phytohormones are natural organic compounds which enhance the growth and productivity of cultivars at very less concentrations. These phytohormones support the distinction and improvement of plant growth by the regulation of various progressions. Generally, the phytohormones at plants root locality are the microbial origin recommended for a functional reply in the host crop (Verma et al. 2016a, b; Enespa and Chandra 2019). Indole-3 acetic acid (IAA), gibberellic acid, abscisic acid (ABA), cytokinins, and other plant growth regulators produced by NaCl-tolerant rhizobacteria outwardly maintain the rooting with augmented number of roots, increase root length, shoot length, and number of root tips, and finally lead to increase in the uptake of nutrients and thus progress plant fitness under saline environmental circumstances (Verma et al. 2016a, b). Bacillus and Pseudomonas strains belong to IAA production that improved the growth of soybean crop at 100 mM NaCl concentration by the increasing antioxidant activity and decreasing the lipid peroxidation (Kumari et al. 2015). Furthermore, an isolated bacterium produced osmotolerant IAA displayed to increase the sprouting of rice seeds in salinity stress are reported (Jha and Subramanian 2013).
11.6.3 ACC Deaminase Production Under Saline Condition
A volatile phytohormone known as ethylene has capacity for growth promotion of plant at very less quantity like nodulations and improvement of various asexual plant parts, rooting, cuttings, and also twisted in the transduction of a signal for the appreciation of saline stress ecosystem (Saravanakumar and Samiyappan 2007). However, a large amount of ethylene is produced under abiotic environmental ecosystem and in the presence of this substrate can inhibit the root growth, shoot growth, and productivity of plants (Morgan and Drew 1997). Some chemical substrates such as aminoethoxyvinylglycine and cobalt ions act as an inhibitor of ethylene synthesis (Arora et al. 2017).
However, these chemical substrates are too much expensive and also can harm plants and environment. Halotolerant rhizobacteria showing plant growth-promoting characters contain aminocyclopropane-1-carboxylate (ACC) deaminase which splits ACC into ammonia and α-ketobutyrate, thereby reducing the near of ethylene in stressed plants (Habib et al. 2016). In the presence of ACC deaminase-producing bacteria, plant 1-aminocyclopropane-1-carboxylate is sequestrated and ruined by the cells of bacteria to fund energy and nitrogen, enhancing the plant growth under saline ecosystem (Tiwari et al. 2018).
The rhizospheric bacteria which belong to Gram-positive and Gram-negative genera such as Arthrobacter, Bacillus, Brevibacterium, Corynebacterium, Exiguobacterium, Halomonas, Micrococcus, Oceanimonas, Planococcus, and Zhihengliuella have been widely reported for ACC deaminase activity under saline conditions and have recognized as a potential role in enhancement of growth under saline ecosystem through ACC deaminase activity (Siddikee et al. 2015; Yadav et al. 2019c, d, e). Pseudomonas simiae strain AU5 is the mutant bacterium overproduced ACC deaminase documented to alleviate salt stress in mung bean plants as compared to wild strain P. simiae AU5 and observed decrease the concentration of ethylene and salt-induced membrane (bacteria and plants) damage (Kumari et al. 2016).
11.6.4 Under Salt Condition Phosphate Solubilization
Phosphorus (P) is an indispensable mineral after nitrogen for the growth of plant promotion as it and essential of dissimilar biomolecules such as nucleic acids, nucleotides, phospholipids, and phosphoproteins (Sharma et al. 2013). In the presence of salinity, uptake of P in plants is reduced and deficiency of P is appeared in the form of symptoms such as dark bluish-green in color with leaves and stem becoming purplish, etc. (Sharma et al. 2013). Mostly, insoluble forms of phosphorus in soils, i.e., organic and inorganic phosphate, have less mobilization in the soils (Sharma et al. 2013). Insoluble organic and inorganic phosphate conversion can be possible due to species of rhizobacteria and also helps in the translocation of P from soil to roots. For the solubilization of insoluble phosphates, many rhizobacteria show one of the several mechanisms such as reactions of ion-exchange, chelation, acidification, and the production organic acids of low molecular weight such as gluconic acids (Kalayu 2019; Rana et al. 2019a, b; Verma et al. 2017a). The halotolerant rhizobacteria to be vital for the mobilization of plant nutrients in several types and reduced the acceptability of inorganic fertilizers (Jiang et al. 2019).
However, phosphate solubilization is a common process in the rhizosphere by rhizobacteria that upsurge the mineral accessibility to crop (Jiang et al. 2019). An important role played by the rhizospheric bacteria to the regulation of P from less available forms and are essential for sustaining P is voluntarily available pools. Upadhyay et al. (2011) reported rhizobacterial strains to have well-organized solubilizing ability of phosphate even up to high saline (6% NaCl concentration) condition and enhanced plant growth under similar condition. For example, Pseudomonas inoculated in the rhizosphere of Zea mays crop showed salt tolerance under 6% NaCl stress condition and increased the crop growth at same salt condition (Bano and Fatima 2009). Additionally, Herbaspirillum seropedicae and Burkholderia sp. are the phosphate dissolving bacteria; treated plants recorded 1.5–21% dry weight as a compared to control plant under saline condition. Afterward, the better germination of root and shoot growth as compared with control plant after being exposed to NaCl inoculated Azospirillum in lettuce seeds (Carrozzi et al. 2012). P. simiae solubilizes phosphate by producing acid phosphatase activity along with volatile compounds that enhanced plant storage protein and uptake of P in soybean plants under 100 mM NaCl saline ecosystem (Vaishnav et al. 2015).
11.6.5 Antioxidative Response Under Salt Condition
The compounds inhibit oxidation reaction known as an antioxidant, and this is a chemical/biochemical process that can produce free radicals (Lü et al. 2010). The oxidative stress is caused by the abiotic environmental factor like drought and saline soil and resulted in the formation of reactive oxygen species (ROS) such as singlet oxygen (O2), hydrogen peroxide (H2O2), and hydroxyl radical (−OH) that damage cellular membranes, proteins, and DNA (Nita and Grzybowski 2016). When the level of ROS increases, this causes oxidative damage to biomolecules such as lipoproteins and at last leads to the death of plants (Sharma et al. 2012). However, some major antioxidative enzymes such as superoxide dismutase (SOD), peroxidase (POX), and catalase (CAT) are produced by rhizospheric bacteria such as Streptococcus. Proteamaculans, and Rhizobium leguminosarum, and non-antioxidant enzymes/compounds like ascorbic acid, tocopherols, and glutathione contribute in ROS-scavenging mechanism (Sharma et al. 2012). Mycorrhizal-inoculated lettuce plants showed higher superoxide dismutase (SOD) activity and protect the plant in the presence of antioxidant under drought stress condition (Ruiz-Lozano 2003).
Salt resistance plants have been associated to more effective antioxidant schemes, and a salt-tolerant bacterium P. simiae strain AU enriched antioxidants (peroxidase and catalase) and gene expression in soybean plants when treated with 100 mM NaCl stress disorder (Vaishnav et al. 2016; Chandra and Enespa 2019c). Drought stress effects in maize plants are alleviated by Pseudomonas spp. drought-tolerant rhizobacteria due to decrease in the antioxidant enzyme activity (Afridi et al. 2019). The catalase and peroxidase activity boosted the non-inoculated crops during saline soil, whereas Azospirillum brasilense inoculated plants showed lower enzyme activity and expressively ameliorated the deleterious effects of salinity (Omar et al. 2009).
11.6.6 Siderophore Production Under Salt Condition
In the chelation of micronutrients, siderophore plays an imperative character such as iron even under limiting conditions and with the redox activity it serves as a cofactor of many enzymes (Ahmed and Holmström 2014; Chandra and Enespa 2016). Several studies are reported on Bacillus to be a good siderophore producer (Kesaulya et al. 2018). Production of siderophores in the rhizosphere by bacteria also helps in dissolving of other ingredients, for example, P, zinc, potassium, and the availability of various ionic ingredients to the plant through chelation of iron from precipitated form (Sharma et al. 2013; Ahmed and Holmström 2014). In the soils, a huge amount of iron is existent, but in an extremely unsolvable ferric hydroxide form, hence the performances of iron as a limiting factor for promotion of plants growth even in ironic soil. However, ferrous (Fe++) iron is oxidized into ferric (Fe+++) form by oxidation process (Kesaulya et al. 2018). Under the biological ecosystem, the ferric ions are inexplicable which forms its achievement by microorganisms, a considerable challenge in the soils (Colombo et al. 2014). Siderophores play important roles in the development of plant growth by rhizospheric microorganisms (Ahmed and Holmström 2014). Plants and bacteria mediate competition using existence of siderophore that results in exclusions of fungal pathogens and other microbial competitors in the rhizosphere by a reduction in the availability of iron for their survival (Ahmed and Holmström 2014).
11.6.7 Halophilic Microbes as Biocontrol Agents
The production of crop yield potentially increased, and its diseases controlled biologically from rhizospheric microflora. Inhibition of phytopathogens using rhizobacteria compromises a more sustainable method to control infection as compared to harmful chemical-based methods (Compant et al. 2010; Etesami and Alikhani 2018). Under the saline condition, a halophilic microbe plays an important role in maintaining morphology, physiology, and reduction in soil salinity and also increases plant susceptibility against phytopathogens (Table 11.2) (Etesami and Beattie 2018).
Halophilic microbes use to hostage the injurious properties of plant pathogens through different mechanisms. Halophilic microbes produce one or more antimicrobial metabolites that act as antifungal, antibacterial, antiviral, antioxidant, cytotoxic, phytotoxic, and/or antitumor mediators (Olanrewaju et al. 2017). Bacillus and Pseudomonas bacterial genera secreted this type of metabolites. Halophilic microbes are also able to produce enzymes such as lipase, cellulase, β-1, 3-glucanase, chitinase, and protease which can degrade cell wall and fungal growth (Husson et al. 2017; Vaddepalli et al. 2017). Halophilic microbes compete for nutritive ingredients or for sites binding on roots of plants, and this type of antagonism reduces the growth of phytopathogen or mandatory destroyed proliferation of plant–pathogen (Olanrewaju et al. 2017). Halophilic microbes such as Alcaligenes, Aeromonas, Bacillus, Rhizobium, and Pseudomonas can produce hydrogen cyanide production, and the presence of this chemical substance may control phytopathogens (El-Rahman et al. 2019; Suman et al. 2016; Verma et al. 2018; Yadav et al. 2018c).
Halophilic microbes activate induced systemic resistance and enhance immunity against phytopathogens (Olanrewaju et al. 2017). Halophilic microbes disrupt signaling pathways of phytopathogens by quorum quenching approach. For interference of signal pathways to minimize pathogen virulence, some specific degrading enzymes, such as lactonase, are responsible (Olanrewaju et al. 2017). Halophilic microbes synthesized siderophore and inhibited the proliferation phytopathogens due to decrease in the iron availability to phytopathogens (Ahmed and Holmström 2014). The halophilic microbes provide biocontrol of phytopathogens by the production of antibiotics and antifungal metabolic substances. Fusarium sambucinum, F. roseum var. sambucinum, F. oxysporum, F. moniliforme, F. graminearum, Penicillium citrinum, Aspergillus flavus, and Botrytis cinerea are phytopathogenic fungi that are controlled by halophilic rhizospheric bacteria B. subtilis, B. cereus, B. pumilus, B. licheniformis, C. alkalitolerans, Halomonas elongate, and Halobacillus halophilus, Halobacillus faecis, Salinicoccus roseus (Ahmed and Holmström 2014; Olanrewaju et al. 2017; El-Rahman et al. 2019).
11.7 Role of Halophilic Microbes in Sustainable Agriculture
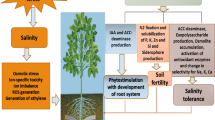
Chemical fertilizers and pesticides are commonly used by the farmers for improvement of soil fertility, growth, and productivity of crops under salt-based and non-salt-based ecosystem (Ju et al. 2018). But their regular use causes an adverse effect on living organism and soils (Bernardes et al. 2015). Apart from these, chemical fertilizers remediate in the crop which feed by the organisms and ultimately reach to top consumers and cause numerous diseases (Gonçalves et al. 2014). However, many transgenic salt-tolerant crops have been developed but far too little is successful (Bharti et al. 2016). An alternative method is available which could replace chemical fertilizers and pesticides and also improve soil health, seed germination, crop growth, and productivity by rhizospheric bacteria (Vejan et al. 2016). These rhizospheric bacteria enhance the growth and improvement of plants either straight or circuitously by colonizing the plant root (Vejan et al. 2016; Kour et al. 2019b; Yadav et al. 2019b).
The uninterrupted character of PGPRs involves the fixation of nitrogen (N2) secretion of metabolites, for instance, the indole-acetic acid (IAA) production, ammonia, solubilization of phosphate, siderophore, and zinc (Ahemad and Kibret 2014; Chandra and Enespa 2016). Indirect growth promotion can be observed in the prevention and reduction of phytopathogens in plants through biocontrol mechanism. In this mechanism, PGPRs produce some lytic enzymes for fungal pathogens (cellulase, β-1, 3 glucanase, chitinase, and 1-aminocyclopropane-1-carboxylate (ACC) deaminase), reduction of iron (Fe) from the soil/rhizosphere and hydrogen cyanide (HCN), salicylic acid, antibiotics, or antifungal compounds (Odoh 2017; Chandra and Enespa 2019a, b, c). Besides, PGPRs also accepted as capable rhizobacteria that can tolerant environmental stresses such as high salt, high temperature, and pH (Ahemad and Kibret 2014).
The plant growth-promoting rhizobacteria enhance nutrient availability that includes nitrogen fixation and phosphate-solubilizing microorganisms. In indirect means, it reduces the deleterious effect of plant pathogens on crop yield (Ahemad and Kibret 2014). It shows antagonism against phytopathogenic microorganisms by producing siderophore (Vejan et al. 2016). PGPR have been developed and used as biofertilizers. Biofertilizers containing these PGPRs are economical, environment-friendly, and potentially renewable source of necessary enriched plant nutrients that makes it an excellent substitute of harmful fertilizers and chemical (Vejan et al. 2016).
The mechanism-based action can be differentiated into three dissimilar groups, i.e, (1) Biofertilizer, containing PGPR having N2 fixation and P solubilization capability, (2) biopesticide, containing PGPR that inhibits the growth of phytopathogenic microorganisms, and (3) phytostimulator, containing PGPR that have ability to produce phytohormones (Vejan et al. 2016). Various agronomically imperative PGPR include the species, such as Alcaligenes sp., Caulobacter, Serratia, Erwinia, Bacillus, Enterobacter, Phyllobacterium sp., and Bacillus thuringiensis, Hyphomicrobium, Azotobacter, Azospirillum, and Acetobacter (Sharma et al. 2013; Ahemad and Kibret 2014; Vejan et al. 2016; Kour et al. 2019a; Verma et al. 2016a, b). The PGPR used as bio-pesticides and biofertilizers for supportable farming have augmented enormously all over the world. The useful properties of PGPR on the improvement and the production of crops have been studied and reported by worldwide on a wide variety of crops such as pulses, vegetables, cereals, and oilseed crops (Gouda et al. 2018). Numerous PGPRs belonging to genera Pseudomonas, Bacillus, Azospirillum, and Enterobacter have been screened from the rhizospheric habitat of various economically important crops and were reported for their synergistic effect on plant growth promotion (Egamberdiyeva et al. 2001).
11.8 Conclusions and Future Prospects
Halophilic microbes are isolated from saline soils or rhizosphere of halophytic plants and shows plant growth-promoting characters directly like the production of IAA, solubilization of phosphate, production of siderophore, fixation of N2, deaminase ACC activity, or indirect ways by controlling phytopathogens under saline condition. However, the habitats of halophilic microbes may be rhizosphere, endophytic, or phyllosphere, and these microbes can augment the biomass and productivity of crops using the halophytic and halotolerant crops. The inoculation of halotolerant microbes in the rhizosphere of crops is a viable strategy for eco-friendly approach and supportable improvement of crop in salt-related farming, which consist of cultivation of crops in dry and semidry regions. Several possibilities of study would move us earlier to accepting these approaches for salt-related cultivation. Knowledge of plant–microbe interactions facilitates policies for the protection of crops and saline soil remediation, and this type of interactions is also observed in the area for ecological appreciative of microbes, which promotes halophyte to adaptability in salinity-rich environment.
References
Abd_Allah EF, Alqarawi AA, Hashem A, Radhakrishnan R, Al-Huqail AA, Al-Otibi FO, Malik JA, Alharbi RI, Egamberdieva D (2018) Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J Plant Inter 13(1):37–44
Afridi MS, Mahmood T, Salam A, Mukhtar T, Mehmood S, Ali J, Chaudhary HJ (2019) Induction of tolerance to salinity in wheat genotypes by plant growth promoting endophytes: Involvement of ACC deaminase and antioxidant enzymes. Plant Physiol Biochem 139:569–577
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Uni Sci 26(1):1–20
Ahmed E, Holmström SJ (2014) Siderophores in environmental research: roles and applications. Microb. Biotech 7(3):196–208
Akhtar SS, Andersen MN, Naveed M, Zahir ZA, Liu F (2015) Interactive effect of biochar and plant growth-promoting bacterial endophytes on ameliorating salinity stress in maize. Funct Plant Biol 42(8):770–781
Akhter MS, Hossain SJ, Amir-Hossain SK, Datta RK (2012) Isolation and characterization of salinity tolerant Azotobacter sp. Greener J Biol Sci 2(3):43–51
Alamri SA, Mostafa YS (2009) Effect of nitrogen supply and Azospirillum brasilense sp-248 on the response of wheat to seawater irrigation. Saudi J Bio Sci 16(2):101–107
Albaggar A (2014) Investigation of bacterial community composition and abundance in a lowland arable catchment (Doctoral dissertation, University of East Anglia)
Amirbakhtiar N, Ismaili A, Ghaffari MR, Firouzabadi FN, Shobbar ZS (2019) Transcriptome response of roots to salt stress in a salinity-tolerant bread wheat cultivar. PLoS One 14(3):0213305
Arora S, Singh AK, Singh YP (eds) (2017) Bioremediation of salt affected soils: an Indian perspective. Springer
Arora S, Dagar JC (2019) Salinity tolerance indicators. In: Dagar J, Yadav R, Sharma P (eds) Research developments in saline agriculture. Springer, Singapore
Aung K, Jiang Y, He SY (2018) The role of water in plant–microbe interactions. Plant J 93(4):771–780
Bang C, Dagan T, Deines P, Dubilier N, Duschl WJ, Fraune S, Hentschel U, Hirt H, Hülter N, Lachnit T, Picazo D (2018) Metaorganisms in extreme environments: do microbes play a role in organismal adaptation. Zoology 127:1–9
Bano A, Fatima M (2009) Salt tolerance in Zea mays (L). following inoculation with Rhizobium and Pseudomonas. Biol Fert Soils 45(4):405–413
Bashan Y, Moreno M, Troyo E (2000) Growth promotion of the seawater-irrigated oilseed halophyte Salicornia bigelovii inoculated with mangrove rhizosphere bacteria and halotolerant Azospirillum spp. Biol Fert Soils 32(4):265–272
Bedre R, Mangu VR, Srivastava S, Sanchez LE, Baisakh N (2016) Transcriptome analysis of smooth cordgrass (Spartina alterniflora Loisel), a monocot halophyte, reveals candidate genes involved in its adaptation to salinity. BMC Genom 17(1):657
Bernardes MF, Pazin M, Pereira LC, Dorta DJ (2015) Impact of pesticides on environmental and human health. Toxicology Studies-Cells, Drugs and Environment (Andreazza C y Scola G Eds.). InTech, Croacia 8:195–233
Bever JD, Platt TG, Morton ER (2012) Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Ann Rev Microbiol 66:265–283
Bharti B, Kumar S, Lee HN, Kumar R (2016) Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci Rep 30(6):32355
Bharti N, Barnawal D, Maji D, Kalra A (2015) Halotolerant PGPRs prevent major shifts in indigenous microbial community structure under salinity stress. Microb Ecol 70(1):196–208
Bharti N, Yadav D, Barnawal D, Maji D, Kalra A (2013) Exiguobacterium oxidotolerans, a halotolerant plant growth promoting rhizobacteria, improves yield and content of secondary metabolites in Bacopa monnieri (L.) Pennell under primary and secondary salt stress. Biotech 29(2):379–387
Bianco C, Defez R (2010) Improvement of phosphate solubilization and Medicago plant yield by an indole-3-acetic acid-overproducing strain of Sinorhizobium meliloti. Appl Env Microb 76(14):4626–4632
Biswas J, Paul AK (2017) Diversity and production of extracellular polysaccharide by halophilic microorganisms. Biodiversity Int J 1:00006
Bringel F, Couée I (2015) Pivotal roles of phyllosphere microorganisms at the interface between plant functioning and atmospheric trace gas dynamics. Front Microb 22:6–486
Bünemann EK, Bongiorno G, Bai Z, Creamer RE, De Deyn G, de Goede R, Fleskens L, Geissen V, Kuyper TW, Mäder P, Pulleman M (2018) Soil quality–a critical review. Soil Biol Biochem 120:105–125
Canfora L, Bacci G, Pinzari F, Papa GL, Dazzi C, Benedetti A (2014) Salinity and bacterial diversity: to what extent does the concentration of salt affect the bacterial community in a saline soil. PLoS One 9(9):106662
Cao XX, Meng M, Wang YY, Wang CL, Hou LH (2011) Identification of salt-tolerant gene HOG1 in Torulopsis versatilis. Biotech Let 33(7):1449
Carrozzi LE, Creus CM, Barassi CA, Monterubbianesi G, Di Benedetto A (2012) Reparation of aged lettuce (Lactuca sativa) seeds by osmotic priming and Azospirillum brasilense inoculation. Botany 90(12):1093–1102
Caverzan A, Casassola A, Brammer SP (2016) Antioxidant responses of wheat plants under stress. Genet Mol Biol 39(1):1–6
Chakraborty K, Basak N, Bhaduri D, Ray S, Vijayan J, Chattopadhyay K, Sarkar RK (2018) Ionic basis of salt tolerance in plants: nutrient homeostasis and oxidative stress tolerance. In: Plant nutrients and abiotic stress tolerance. Springer, Singapore, pp 325–362
Chandra P, Barsainya M, Singh DP (2014) A fourier transform infrared (FTIR) spectroscopic study on cellular changes in the Marinococcus Luteus sslb 1 under different salinity regime. Int J Pharm Bio Sci 5:848–854
Chandra P, Enespa (2016) Applications and mechanisms of plant growth-stimulating rhizobacteria. In: Plant-microbe interaction: an approach to sustainable agriculture. Springer, Singapore, pp 37–62
Chandra P, Enespa (2017) Microbial volatiles as chemical weapons against pathogenic fungi. In: Volatiles and food security. Springer, Singapore, pp 227–254
Chandra P, Enespa (2019a) Mycoremediation of environmental pollutants from contaminated soil. In: Varma A, Choudhary D (eds) Mycorrhizosphere and pedogenesis. Springer, Singapore
Chandra P, Enespa (2019b) Fungal enzymes for bioremediation of contaminated soil. In: Yadav A, Singh S, Mishra S, Gupta A (eds) Recent advancement in white biotechnology through fungi. Fungal Biology. Springer, Cham
Chandra P, Enespa (2019c) Soil–Microbes–Plants: interactions and ecological diversity. In: Varma A, Tripathi S, Prasad R (eds) Plant microbe interface. Springer, Cham
Chandra P, Enespa, Kumar M (2020) Contribution of microbes in the renovation of wetlands. In: Upadhyay A, Singh R, Singh D (eds) Restoration of wetland ecosystem: a trajectory towards a sustainable environment. Springer, Singapore
Chandra P, Singh DP (2014) Removal of Cr (VI) by a halotolerant bacterium Halomonas sp. CSB 5 isolated from Sambhar salt Lake Rajasthan (India). Cell Mol Biol 60:64–72
Chandra P, Singh DP (2016) Isolation of alkaliphilic bacterium Citricoccus alkalitolerans CSB1: an efficient biosorbent for bioremediation of tannery waste water. Cell Mol Biol 62(3):135
Chandra P, Singh E (2017) Applications and mechanisms of plant growth-stimulating rhizobacteria. In: Choudhary D, Varma A, Tuteja N (eds) Plant-microbe interaction: an approach to sustainable agriculture. Springer, Singapore
Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90(5):856–867
Chu TN, Tran BT, Van Bui L, Hoang MT (2019) Plant growth-promoting rhizobacterium Pseudomonas PS01 induces salt tolerance in Arabidopsis thaliana. BMC Res Notes 12(1):11
Colombo C, Palumbo G, He JZ, Pinton R, Cesco S (2014) Review on iron availability in soil: interaction of Fe minerals, plants, and microbes. J Soils Sediments 14(3):538–548
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo-and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42(5):669–678
Csonka LN (1989) Physiological and genetic responses of bacteria to osmotic stress. Microbiol Mol Biol Rev 53(1):121–147
Cucchi A, de Rivas CS (1995) ssp genes and spore osmotolerance in Bacillus thuringiensis israelensis and Bacillus sphaericus. Curr Microbiol 31(4):228–233
Cuevas J, Daliakopoulos IN, del Moral F, Hueso JJ, Tsanis IK (2019) A review of soil-improving cropping systems for soil salinization. Agronomy 9(6):295
Czech L, Höppner A, Kobus S, Seubert A, Riclea R, Dickschat JS, Bremer E (2019) Illuminating the catalytic core of ectoine synthase through structural and biochemical analysis. Sci Rep 9(1):364
da Costa MS, Santos H (2009) Compatible solutes in microorganisms that grow at high temperature. Extremophile 5(3):265
da Costa MS, Santos H, Galinski EA (1998a) An overview of the role and diversity of compatible solutes in Bacteria and Archaea. In: Antranikian G (eds) Biotechnology of extremophiles. Advances in biochemical engineering/biotechnology, vol 61. Springer, Berlin
da Costa MS, Santos H, Galinski EA (1998b) An overview of the role and diversity of compatible solutes in Bacteria and Archaea. In: Biotechnology of extremophiles. Springer, Berlin, pp 117–153
de Lourdes Moreno M, Pérez D, García M, Mellado E (2013) Halophilic bacteria as a source of novel hydrolytic enzymes. Life 3(1):38–51
Delgado MJ, Ligero F, Lluch C (1994) Effects of salt stress on growth and nitrogen fixation by pea, faba-bean, common bean and soybean plants. Soil Biol Biochem 26(3):371–376
Egamberdieva D, Berg G, Lindström K, Räsänen LA (2013) Alleviation of salt stress of symbiotic Galega officinalis L. (goat’s rue) by co-inoculation of Rhizobium with root-colonizing Pseudomonas. Plant Soil 369:453–465
Egamberdieva D, Davranov K, Wirth S, Hashem A, Abd_Allah EF (2017) Impact of soil salinity on the plant-growth–promoting and biological control abilities of root associated bacteria. Saudi J Biolog Sci 24(7):1601–1608
Egamberdiyeva D, Davranov KD, Höflich G (2001) Influence of growth-promoting bacteria from Uzbekistan and Germany on the growth and nutrient uptake of cotton and wheat on different soils. Plant nutrition. Springer, Dordrecht, pp 674–675
El-Esawi MA, Al-Ghamdi AA, Ali HM, Alayafi AA (2019) Azospirillum lipoferum FK1 confers improved salt tolerance in chickpea (Cicer arietinum L.) by modulating osmolytes, antioxidant machinery and stress-related genes expression. Environ Exp Bot 159:55–65
El-Rahman AA, Shaheen HA, El-Aziz RM, Ibrahim DS (2019) Influence of hydrogen cyanide-producing rhizobacteria in controlling the crown gall and root-knot nematode, Meloidogyne incognita. Egyptian J Biolog Pest Control 29(1):41
Enespa, Chandra P (2019) Fungal community for novel secondary metabolites. In: Yadav A, Singh S, Mishra S, Gupta A (eds) Recent advancement in white biotechnology through fungi. Fungal biology. Springer, Cham
Etesami H, Alikhani HA (2018) Bacillus species as the most promising bacterial biocontrol agents in rhizosphere and endorhiza of plants grown in rotation with each other. Eur J Plant Pathol 150(2):497–506
Etesami H, Beattie GA (2018) Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front Microbiol 8(9):148
Fendrihan S, Constantinescu F, Sicuia O, Dinu S (2017) Azospirillum strains as biofertilizers and biocontrol agents-a practical review. J Adv Agric 7(3):1096–1108
Ferrando A, Kron SJ, Rios G, Fink GR, Serrano R (1995) Regulation of cation transport in Saccharomyces cerevisiae by the salt tolerance gene HAL3. Mol Cellular Biol 15(10):5470–5481
Galinski EA (1993) Compatible solutes of halophilic eubacteria: molecular principles, water-solute interaction, stress protection. Experientia 49(6–7):487–496
Getu M (2009) Ethiopian floriculture and its impact on the environment. Mizan Law Rev 3(2):240–270
Gisbert C, Rus AM, Boları́n MC, López-Coronado JM, Arrillaga I, Montesinos C, Caro M, Serrano R, Moreno V (2000) The yeast HAL1 gene improves salt tolerance of transgenic tomato. Plant Physiol 123(1):393–402
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microb Res 169(1):30–39
Gonçalves AC Jr, Nacke H, Schwantes D, Coelho GF (2014) Heavy metal contamination in brazilian agricultural soils due to application of fertilizers. Environ Risk Assess Soil Contam 26(1):105–135
Gouda S, Kerry RG, Das G, Paramithiotis S, Shin HS, Patra JK (2018) Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microb Res 206:131–140
Gouffi K, Pichereau V, Rolland JP, Thomas D, Bernard T, Blanco C (1998) Sucrose Is a Nonaccumulated Osmoprotectant inSinorhizobium meliloti. J Bacteriol 180(19):5044–5051
Greene R, Timms W, Rengasamy P, Arshad M, Cresswell R (2016) Soil and aquifer salinization: toward an integrated approach for salinity management of groundwater. Integrated groundwater management. Springer, Cham, pp 377–412
Greene RS, Hairsine PB (2004) Elementary processes of soil–water interaction and thresholds in soil surface dynamics: a review. Earth Surf Process Landf J Br Geomorphol Res Group 29(9):1077–1091
Gu H, Ma C, Gu J, Guo J, Yan X, Huang J, Guo Z (2016) An overview of multifunctional epoxy nanocomposites. J Mat Chem 4(25):5890–5906
Gunde-Cimerman N, Plemenitaš A, Oren A (2018) Strategies of adaptation of microorganisms of the three domains of life to high salt concentrations. FEMS Microbiol Rev 42(3):353–375
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomic 2014:1–18
Habib SH, Kausar H, Saud HM (2016) Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. Biomed Res Int 2016:1–10
Hanin M, Ebel C, Ngom M, Laplaze L, Masmoudi K (2016) New insights on plant salt tolerance mechanisms and their potential use for breeding. Front Plant Sci 7:1787
Hara H, Masui M (1985) Effect of NaCl concentration on the synthesis of membrane phospholipid in a halophilic bacterium. FEMS Microb Ecol 1(5):279–282
Hayano-Kanashiro C, Calderón-Vázquez C, Ibarra-Laclette E, Herrera-Estrella L, Simpson J (2009) Analysis of gene expression and physiological responses in three Mexican maize landraces under drought stress and recovery irrigation. PLoS One 4(10):e7531
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7(11):1456–1466
Hiramatsu T, Yano I, Masui M (1980) Effect of NaCl concentration on the protein species and phospholipid composition of the outer membrane in a moderately halophilic bacterium. FEMS Microbiol Lett 7(4):289–292
Holmberg N, Bülow L (1998) Improving stress tolerance in plants by gene transfer. Trend Plant Sci 3(2):61–66
Hussain N, Sarwar G, Schmeisky H, Al-Rawahy S, Ahmad M (2010) Salinity and drought management in legume crops. Climate change and management of cool season grain legume crops. Springer, Dordrecht, pp 171–191
Husson F, Lê S, Pagès J (2017) Exploratory multivariate analysis by example using R. Chapman and Hall/CRC, p 8
Ibekwe AM, Poss JA, Grattan SR, Grieve CM, Suarez D (2010) Bacterial diversity in cucumber (Cucumis sativus) rhizosphere in response to salinity, soil pH, and boron. Soil Biol Biochem 42(4):567–575
Ilangumaran G, Smith DL (2017) Plant growth promoting rhizobacteria in amelioration of salinity stress: a systems biology perspective. Front Plant Sci 23(8):1768
Inaba M, Sakamoto A, Murata N (2001) Functional expression in Escherichia coli of low-affinity and high-affinity Na+ (Li+)/H+ antiporters of Synechocystis. J Bacteriol 183(4):1376–1384
Indoria AK, Rao CS, Sharma KL, Reddy KS (2017) Conservation agriculture–a panacea to improve soil physical health. Curr Sci 112:1–10
Jha Y, Subramanian RB (2013) Paddy plants inoculated with PGPR show better growth physiology and nutrient content under saline condition. Chil J Agric Res 73(3):213–219
Jha Y, Subramanian RB, Patel S (2011) Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol Plant 33(3):797–802
Jiang H, Qi P, Wang T, Chi X, Wang M, Chen M, Chen N, Pan L (2019) Role of halotolerant phosphate-solubilizing bacteria on growth promotion of peanut (Arachis hypogaea) under saline soil. Ann App Biol 174(1):20–30
Joe MM, Devaraj S, Benson A, Sa T (2016) Isolation of phosphate solubilizing endophytic bacteria from Phyllanthus amarus Schum and Thonn: evaluation of plant growth promotion and antioxidant activity under salt stress. J App Res Med Arom Plant 3(2):71–77
Joseph B, Jini D (2010) Insight into the role of antioxidant enzymes for salt tolerance in plants. Int J Bot 6(4):456–464
Joseph S, Murphy DJ, Bhave M (2015) Identification of salt tolerant Acacia species for saline land utilisation. Biologia 70(2):174–182
Ju I, Wj B, Md S, Ia O, Oj E (2018) A review: biofertilizer-a key player in enhancing soil fertility and crop productivity. J Microbiol Biotechnol Rep 7:2
Kalayu G (2019) Phosphate solubilizing microorganisms: promising approach as biofertilizers. Int J Agron 2019:1–7
Kapardar RK, Ranjan R, Grover A, Puri M, Sharma R (2010) Identification and characterization of genes conferring salt tolerance to Escherichia coli from pond water metagenome. Bioresour Technol 101(11):3917–3924
Kempf B, Bremer E (1998) Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch Microbiol 170(5):319–330
Kesaulya H, Hasinu JV, Tuhumury GN (2018) Potential of Bacillus spp produces siderophores insuppressing thewilt disease of banana plants. In: IOP Conference Series: Earth and Environmental Science, vol 102, p 012016
Kindzierski V, Raschke S, Knabe N, Siedler F, Scheffer B, Pflüger-Grau K, Kunte HJ (2017) Osmoregulation in the halophilic bacterium Halomonas elongata: a case study for integrative systems biology. PLoS One 12(1):e0168818
Klähn S, Marquardt DM, Rollwitz I, Hagemann M (2009) Expression of the ggpPS gene for glucosylglycerol biosynthesis from Azotobacter vinelandii improves the salt tolerance of Arabidopsis thaliana. J Exp Bot 60(6):1679–1689
Kohler C, Lourenço RF, Bernhardt J, Albrecht D, Schüler J, Hecker M, Gomes SL (2015) A comprehensive genomic, transcriptomic and proteomic analysis of a hyperosmotic stress sensitive α-proteobacterium. BMC Microbiol 15(1):71
Kooistra MJ, Tovey NK (1994) Effects of compaction on soil microstructure. Dev Agric Eng 11:91–111
Kour D, Rana KL, Kumar A, Rastegari AA, Yadav N, Yadav AN, Gupta VK (2019a) Extremophiles for hydrolytic enzymes productions: biodiversity and potential biotechnological applications. In: Molina G, Gupta VK, Singh BN, Gathergood N (eds) Bioprocessing for biomolecules production. Wiley, USA, pp 321–372
Kour D, Rana KL, Yadav AN, Yadav N, Kumar V, Kumar A, Sayyed RZ, Hesham AE-L, Dhaliwal HS, Saxena AK (2019b) Drought-tolerant phosphorus-solubilizing microbes: biodiversity and biotechnological applications for alleviation of drought stress in plants. In: Sayyed RZ, Arora NK, Reddy MS (eds) Plant growth promoting rhizobacteria for sustainable stress management: volume 1: rhizobacteria in abiotic stress management. Springer Singapore, Singapore, pp 255–308. https://doi.org/10.1007/978-981-13-6536-2_13
Kour D, Rana KL, Yadav N, Yadav AN, Kumar A, Meena VS, Singh B, Chauhan VS, Dhaliwal HS, Saxena AK (2019c) Rhizospheric microbiomes: biodiversity, mechanisms of plant growth promotion, and biotechnological applications for sustainable agriculture. In: Kumar A, Meena VS (eds) Plant growth promoting rhizobacteria for agricultural sustainability: from theory to practices. Springer Singapore, Singapore, pp 19–65. https://doi.org/10.1007/978-981-13-7553-8_2
Kour D, Rana KL, Yadav N, Yadav AN, Singh J, Rastegari AA, Saxena AK (2019d) Agriculturally and industrially important fungi: current developments and potential biotechnological applications. In: Yadav AN, Singh S, Mishra S, Gupta A (eds) Recent advancement in white biotechnology through fungi, volume 2: perspective for value-added products and environments. Springer International Publishing, Cham, pp 1–64. https://doi.org/10.1007/978-3-030-14846-1_1
Kraegeloh A, Amendt B, Kunte HJ (2005) Potassium transport in a halophilic member of the bacteria domain: identification and characterization of the K+ uptake systems TrkH and TrkI from Halomonas elongata DSM 2581T. J Bacteriol 187(3):1036–1043
Kumari S, Vaishnav A, Jain S, Varma A, Choudhary DK (2015) Bacterial-mediated induction of systemic tolerance to salinity with expression of stress alleviating enzymes in soybean (Glycine max L. Merrill). J Plant Growth Regul 34(3):558–573
Kumari S, Vaishnav A, Jain S, Varma A, Choudhary DK (2016) Title. World J Microbiol. World J Microbiol Biotech 32(1):1–10
Ladeiro B (2012) Saline agriculture in the 21st century: using salt contaminated resources to cope food requirements. J Bot 310705–310707
Laloknam S, Tanaka K, Buaboocha T, Waditee R, Incharoensakdi A, Hibino T, Takabe T (2006) Halotolerant cyanobacterium Aphanothece halophytica contains a betaine transporter active at alkaline pH and high salinity. Appl. Environ. Microbiol. 72(9):6018–6026
Leite J, Fischer D, Rouws LF, Fernandes-Júnior PI, Hofmann A, Kublik S, Schloter M, Xavier GR, Radl V (2017) Cowpea nodules harbor non-rhizobial bacterial communities that are shaped by soil type rather than plant genotype. Front Plant Sci 20(7):2064
Lentes CJ, Mir SH, Boehm M, Ganea C, Fendler K, Hunte C (2014) Molecular characterization of the Na+/H+-antiporter NhaA from Salmonella typhimurium. PLoS One 9(7):e101575
Li C, Tan BK, Zhao J, Guan Z (2016) In vivo and in vitro synthesis of phosphatidylglycerol by an Escherichia coli cardiolipin synthase. J Biol Chem 291(48):25144–25153
Li HQ, Jiang XW (2017) Inoculation with plant growth-promoting bacteria (PGPB) improves salt tolerance of maize seedling. Russ J Plant Physiol 64(2):235–241
Liu J, Tang L, Gao H, Zhang M, Guo C (2019) Enhancement of alfalfa yield and quality by plant growth-promoting rhizobacteria under saline-alkali conditions. J Sci Food Agric 99(1):281–289
Locascio A, Andrés-Colás N, Mulet JM, Yenush L (2019) Saccharomyces cerevisiae as a tool to investigate plant potassium and sodium transporters. Int J Mol Sci 20(9):2133
Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J (2000) Molecular cell biology 4th edition. National Center for Biotechnology Information, Bookshelf
Loganathan P, Nair S (2004) Swaminathania salitolerans gen. nov., sp. nov., a salt-tolerant, nitrogen-fixing and phosphate-solubilizing bacterium from wild rice (Porteresia coarctata Tateoka). Int J Syst Evol Microbiol 54(4):1185–1190
Long X, Tian J, Liao X, Tian Y (2018) Adaptations of Bacillus shacheensis HNA-14 required for long-term survival under osmotic challenge: a multi-omics perspective. RSC Adv 8(48):27525–27536
Lü JM, Lin PH, Yao Q, Chen C (2010) Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med 14(4):840–860
Ma Y, Galinski EA, Grant WD, Oren A, Ventosa A (2010) Halophiles 2010: life in saline environments. Appl Environ Microbiol 76(21):6971–6981
Machado R, Serralheiro R (2017) Soil salinity: effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 3(2):30
Magallon KJ, Dinneny JR (2019) Environmental stress: salinity Ruins a Plant’s Day in the Sun. Curr Biol 29(10):360–362
Mahrous M, Šegvić B, Zanoni G, Khadka S, Senadheera S, Jayawickrama P (2018) The role of clay swelling and mineral neoformation in the stabilization of high plasticity soils treated with the fly ash-and metakaolin-based geopolymers. Mineral 8(4):146
Mandon K, Østerås M, Boncompagni E, Trinchant JC, Spennato G, Poggi MC, Le Rudulier D (2003) The Sinorhizobium meliloti glycine betaine biosynthetic genes (betICBA) are induced by choline and highly expressed in bacteroids. Mol Plant-Microbe Interact 16(8):709–719
Maróti G, Kondorosi à (2014) Nitrogen-fixing Rhizobium-legume symbiosis: are polyploidy and host peptide-governed symbiont differentiation general principles of endosymbiosis? Front Microbiol 5. https://doi.org/10.3389/fmicb.2014.00326
Maturrano L, Santos F, Rosselló-Mora R, Antón J (2006) Microbial diversity in Maras salterns, a hypersaline environment in the Peruvian Andes. Appl Env Microbiol 72(6):3887–3895
Mendes R, Garbeva P, Raaijmakers JM (2013) The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev 37(5):634–663
Mengel K, Kirkby EA, Kosegarten H, Appel T (2001) Nitrogen. Principles of plant nutrition. Springer, Dordrecht, pp 397–434
Mitschke J, Georg J, Scholz I, Sharma CM, Dienst D, Bantscheff J, Hess WR (2011) An experimentally anchored map of transcriptional start sites in the model cyanobacterium Synechocystis sp. PCC6803. Proc Natl Acad Sci 108(5):2124–2129
Morgan PW, Drew MC (1997) Ethylene and plant responses to stress. Physiol Plant 100(3):620–630
Mukhtar S, Ishaq A, Hassan S, Mehnaz S, Mirza MS, Malik KA (2017) Comparison of microbial communities associated with halophyte (Salsola stocksii) and non-halophyte (Triticum aestivum) using culture-independent approaches. Pol J Microbiol 66(3):353–364
Munns R (1993) Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses. Plant Cell Environ 16(1):15–24
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167(3):645–663
Murínová S, Dercová K (2014) Response mechanisms of bacterial degraders to environmental contaminants on the level of cell walls and cytoplasmic membrane. Int J Microbiol 2014:1–16
Nabti E, Schmid M, Hartmann A (2015) Application of halotolerant bacteria to restore plant growth under salt stress. Halophiles. Springer, Cham, pp 235–259
Nita M, Grzybowski A (2016) The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxidative Med Cell Longev 2016:1–23
Nogales J, Campos R, BenAbdelkhalek H, Olivares J, Lluch C, Sanjuan J (2002) Rhizobium tropici genes involved in free-living salt tolerance are required for the establishment of efficient nitrogen-fixing symbiosis with Phaseolus vulgaris. Mol Plant-Microbe Interact 15(3):225–232
Normand P, Caumette P, Goulas P, Pujic P, Wisniewski-Dyé F (2015) Adaptations of prokaryotes to their biotopes and to physicochemical conditions in natural or anthropized environments. Environmental microbiology: fundamentals and applications. Springer, Dordrecht, pp 293–351
Numan M, Bashir S, Khan Y, Mumtaz R, Shinwari ZK, Khan AL, Khan A, Ahmed AH (2018) Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: a review. Microbiol Res 209:21–32
O’Rourke SM, Herskowitz I (1998) The Hog1 MAPK prevents cross talk between the HOG and pheromone response MAPK pathways in Saccharomyces cerevisiae. Genes Dev 12(18):2874–2886
Odoh CK (2017) Plant growth promoting rhizobacteria (PGPR): a bio protectant bio inoculant for sustainable agrobiology. A review. Int J Adv Res Biol Sci 4:123–142
Ohno Y, Yano I, Masui M (1979) Effect of NaCl concentration and temperature on the phospholipid and fatty acid compositions of a moderately halophilic bacterium, Pseudomonas halosaccharolytica. J Biochem 85(2):413–421
Olanrewaju OS, Glick BR, Babalola OO (2017) Mechanisms of action of plant growth promoting bacteria. World J Microbiol Biotech 33(11):197
Ollivier B, Caumette P, Garcia JL, Mah RA (1994) Anaerobic bacteria from hypersaline environments. Microbiol Mol Biol Rev 58(1):27–38
Omar MN, Osman ME, Kasim WA, El-Daim IA (2009) Improvement of salt tolerance mechanisms of barley cultivated under salt stress using Azospirillum brasilense. Salinity and water stress. Springer, Dordrecht, pp 133–147
Ondrasek G, Rengel Z, Veres S (2011) Soil salinisation and salt stress in crop production. Abiotic stress in plants-Mech Adapt. https://doi.org/10.5772/22248
Or D, Smets BF, Wraith JM, Dechesne A, Friedman SP (2007) Physical constraints affecting bacterial habitats and activity in unsaturated porous media-a review. Adv Water Resour 30(6–7):1505–1527
Oren A (2002) Diversity of halophilic microorganisms: environments, phylogeny, physiology, and applications. J Ind Microbiol Biotechl 28(1):56–63
Oren A (2008) Microbial life at high salt concentrations: phylogenetic and metabolic diversity. Sal Syst 4(1):2
Oren A (2011) Thermodynamic limits to microbial life at high salt concentrations. Environ Microbiol 13(8):1908–1923
Osteras M, Boncompagni E, Vincent N, Poggi MC, Le Rudulier D (1998) Presence of a gene encoding choline sulfatase in Sinorhizobium meliloti bet operon: choline-O-sulfate is metabolized into glycine betaine. Proc Natl Acad Sci 95(19):11394–11399
Patchett RA, Kelly AF, Kroll RG (1992) Effect of sodium chloride on the intracellular solute pools of Listeria monocytogenes. Appl Environ Microbiol 58(12):3959–3963
Payakapong W, Tittabutr P, Teaumroong N, Boonkerd N, Singleton PW, Borthakur D (2006) Identification of two clusters of genes involved in salt tolerance in Sinorhizobium sp. strain BL3. Symbiosis 41(1):47–53
Peng J, Wu D, Liang Y, Li L, Guo Y (2019) Disruption of acdS gene reduces plant growth promotion activity and maize saline stress resistance by Rahnella aquatilis HX2. J B Microbiol 59(4):402–411
Pérez-Arellano I, Carmona-Álvarez F, Martínez AI, Rodríguez-Díaz J, Cervera J (2010) Pyrroline-5-carboxylate synthase and proline biosynthesis: from osmotolerance to rare metabolic disease. Protein Sci 19(3):372–382
Pichler H, Emmerstorfer-Augustin A (2018) Modification of membrane lipid compositions in single-celled organisms-from basics to applications. Method 147:50–65
Pikuta EV, Hoover RB, Tang J (2007) Microbial extremophiles at the limits of life. Crit Rev Microbiol 33(3):183–209
Poeplau C, Helfrich M, Dechow R, Szoboszlay M, Tebbe CC, Don A, Geerts R (2019) Increased microbial anabolism contributes to soil carbon sequestration by mineral fertilization in temperate grasslands. Soil Biol Biochem 130:167–176
Poolman B, Glaasker E (1998) Regulation of compatible solute accumulation in bacteria. Mol Microbiol 29(2):397–407
Rana KL, Kour D, Sheikh I, Dhiman A, Yadav N, Yadav AN, Rastegari AA, Singh K, Saxena AK (2019a) Endophytic fungi: biodiversity, ecological significance and potential industrial applications. In: Yadav AN, Mishra S, Singh S, Gupta A (eds) Recent advancement in white biotechnology through fungi, vol 1. Diversity and enzymes perspectives. Springer, Switzerland, pp 1–62
Rana KL, Kour D, Sheikh I, Yadav N, Yadav AN, Kumar V, Singh BP, Dhaliwal HS, Saxena AK (2019b) Biodiversity of endophytic fungi from diverse niches and their biotechnological applications. In: Singh BP (ed) Advances in endophytic fungal research: present status and future challenges. Springer International Publishing, Cham, pp 105–144. https://doi.org/10.1007/978-3-030-03589-1_6
Rashid MI, Mujawar LH, Shahzad T, Almeelbi T, Ismail IM, Oves M (2016) Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol Res 183:26–41
Reshetnikov AS, Khmelenina VN, Mustakhimov II, Kalyuzhnaya M, Lidstrom M, Trotsenko YA (2011) Diversity and phylogeny of the ectoine biosynthesis genes in aerobic, moderately halophilic methylotrophic bacteria. Extremophile 15(6):653
Reshetnikov AS, Khmelenina VN, Trotsenko YA (2006) Characterization of the ectoine biosynthesis genes of haloalkalotolerant obligate methanotroph “Methylomicrobium alcaliphilum 20Z”. Arch Microbiol 184(5):286–297
Roberts MF (2005a) Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Syst 1(1):5–30
Roberts MF (2005b) Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Syst 1(1):5
Ruiz-Lozano JM (2003) Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 13(6):309–317
Sahoo RK, Ansari MW, Pradhan M, Dangar TK, Mohanty S, Tuteja N (2014) A novel Azotobacter vinellandii (SRI Az 3) functions in salinity stress tolerance in rice. Plant Signal Behav 9(7):511–523
Sánchez-Porro C, Martin S, Mellado E, Ventosa A (2003) Diversity of moderately halophilic bacteria producing extracellular hydrolytic enzymes. J Appl Microbiol 94(2):295–300
Saravanakumar D, Samiyappan R (2007) ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J Appl Microbiol 102(5):1283–1292
Saum SH, Müller V (2008) Regulation of osmoadaptation in the moderate halophile Halobacillus halophilus: chloride, glutamate and switching osmolyte strategies. Sal Syst 4(1):4
Schjønning P, Munkholm LJ, Moldrup P, Jacobsen OH (2002) Modelling soil pore characteristics from measurements of air exchange: the long-term effects of fertilization and crop rotation. Euro J Soil Sci 53(2):331–339
Schmidt RR, Weits DA, Feulner CF, van Dongen JT (2018) Oxygen sensing and integrative stress signaling in plants. Plant Physiol 176(2):1131–1142
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1–26
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2(1):587
Shi-Ying Z, Cong F, Yong-xia W, Yun-sheng X, Wei X, Xiao-Long C (2018) Salt-tolerant and plant growth-promoting bacteria isolated from high-yield paddy soil. Canad J Microbiol 64(12):968–978
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22(2):123–131
Shu Y, Li W, Zhao J, Liu Y, Guo C (2018) Transcriptome sequencing and expression profiling of genes involved in the response to abiotic stress in Medicago ruthenica. Genet Mol Biol 41(3):638–648
Siddikee MA, Sundaram S, Chandrasekaran M, Kim K, Selvakumar G, Sa T (2015) Halotolerant bacteria with ACC deaminase activity alleviates salt stress effect in canola seed germination. J Korean Soc Appl Biol Chem 58(2):237–241
Silini A, Cherif-Silini H, Yahiaoui B (2016) Growing varieties durum wheat (Triticum durum) in response to the effect of osmolytes and inoculation by Azotobacter chroococcum under salt stress. Afr J Microbiol Res 10(12):387–399
Singh R, Upadhyay AK, Chandra P, Singh DP (2018) Sodium chloride incites reactive oxygen species in green algae Chlorococcum humicola and Chlorella vulgaris: Implication on lipid synthesis, mineral nutrients and antioxidant system. Bioresour Technol 270:489–497
Singh RP, Jha P, Jha PN (2015) The plant-growth-promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat (Triticum aestivum) under salt stress. J Plant Physiol 184:57–67
Sohlenkamp C, Geiger O (2016) Bacterial membrane lipids: diversity in structures and pathways. FEMS Microbiol Rev 40(1):133–159
Soto-Padilla MY, Valenzuela-Encinas C, Dendooven L, Marsch R, Gortáres-Moroyoqui P, Estrada-Alvarado MI (2014) Isolation and phylogenic identification of soil haloalkaliphilic strains in the former Texcoco Lake. Int J Env Health Res 24(1):82–90
Stöveken N, Pittelkow M, Sinner T, Jensen RA, Heider J, Bremer E (2011) A specialized aspartokinase enhances the biosynthesis of the osmoprotectants ectoine and hydroxyectoine in Pseudomonas stutzeri A1501. J Bacteriol 193(17):4456–4468
Stritzler M, Elba P, Berini C, Gomez C, Ayub N, Soto G (2018) High-quality forage production under salinity by using a salt-tolerant AtNXH1-expressing transgenic alfalfa combined with a natural stress-resistant nitrogen-fixing bacterium. J Biotech 276:42–45
Suman A, Yadav AN, Verma P (2016) Endophytic microbes in crops: diversity and beneficial impact for sustainable agriculture. In: Singh D, Abhilash P, Prabha R (eds) Microbial inoculants in sustainable agricultural productivity, research perspectives. Springer, India, pp 117–143. https://doi.org/10.1007/978-81-322-2647-5_7
Suárez R, Wong A, Ramírez M, Barraza A, Orozco MDC, Cevallos MA, Iturriaga G (2008) Improvement of drought tolerance and grain yield in common bean by overexpressing trehalose-6-phosphate synthase in rhizobia. Mol Plant-Microbe Interact 21(7):958–966
Teh CY, Shaharuddin NA, Ho CL, Mahmood M (2016) Exogenous proline significantly affects the plant growth and nitrogen assimilation enzymes activities in rice (Oryza sativa) under salt stress. Acta Physiol Plant 38(6):151
Thomas J, Apte SK (1984) Sodium requirement and metabolism in nitrogen-fixing cyanobacteria. J Biosci 6(5):771–794
Tiwari G, Duraivadivel P, Sharma SPH (2018) 1-Aminocyclopropane-1-carboxylic acid deaminase producing beneficial rhizobacteria ameliorate the biomass characters of Panicum maximum Jacq. by mitigating drought and salt stress. Sci Rep 30(1):17513
Totsche KU, Amelung W, Gerzabek MH, Guggenberger G, Klumpp E, Knief C, Lehndorff E, Mikutta R, Peth S, Prechtel A, Ray N (2018) Microaggregates in soils. J Plant Nutr Soil Sci 181(1):104–136
Ulukanli Z, Digrak M (2002) Alkaliphilic micro-organisms and habitats. Turk J Biol 26(3):181–191
Upadhyay SK, Singh JS, Singh DP (2011) Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedo 21(2):214–222
Vaddepalli P, Fulton L, Wieland J, Wassmer K, Schaeffer M, Ranf S, Schneitz K (2017) The cell wall-localized atypical β-1, 3 glucanase ZERZAUST controls tissue morphogenesis in Arabidopsis thaliana. Development 144(12):2259–2269
Vaishnav A, Kumari S, Jain S, Varma A, Choudhary DK (2015) Putative bacterial volatile-mediated growth in soybean (Glycine max L. Merrill) and expression of induced proteins under salt stress. J App Microbiol 119(2):539–551
Vaishnav A, Kumari S, Jain S, Varma A, Tuteja N, Choudhary DK (2016) PGPR‐mediated expression of salt tolerance gene in soybean through volatiles under sodium nitroprusside. J Basic Microbiol 56(11):1274–1288
Vandegehuchte ML, de la Peña E, Bonte D (2010) Relative importance of biotic and abiotic soil components to plant growth and insect herbivore population dynamics. PLoS One 5(9):e12937
Verma P, Yadav AN, Khannam KS, Panjiar N, Kumar S, Saxena AK, Suman A (2015) Assessment of genetic diversity and plant growth promoting attributes of psychrotolerant bacteria allied with wheat (Triticum aestivum) from the northern hills zone of India. Ann Microbiol 65:1885–1899
Verma P, Yadav AN, Khannam KS, Kumar S, Saxena AK, Suman A (2016a) Molecular diversity and multifarious plant growth promoting attributes of Bacilli associated with wheat (Triticum aestivum L.) rhizosphere from six diverse agro-ecological zones of India. J Basic Microbiol 56:44–58
Verma V, Ravindran P, Kumar PP (2016b) Plant hormone-mediated regulation of stress responses. BMC Plant Biol 16(1):86
Verma P, Yadav AN, Khannam KS, Saxena AK, Suman A (2017a) Potassium-solubilizing microbes: diversity, distribution, and role in plant growth promotion. In: Panpatte DG, Jhala YK, Vyas RV, Shelat HN (eds) Microorganisms for green revolution: volume 1: microbes for sustainable crop production. Springer Singapore, Singapore, pp 125–149. https://doi.org/10.1007/978-981-10-6241-4_7
Verma P, Yadav AN, Kumar V, Singh DP, Saxena AK (2017b) Beneficial plant-microbes interactions: biodiversity of microbes from diverse extreme environments and its impact for crop improvement. In: Singh DP, Singh HB, Prabha R (eds) Plant-microbe interactions in agro-ecological perspectives: volume 2: microbial interactions and agro-ecological impacts. Springer Singapore, Singapore, pp 543–580. https://doi.org/10.1007/978-981-10-6593-4_22
Verma P, Yadav AN, Kumar V, Khan MA, Saxena AK (2018) Microbes in termite management: potential role and strategies. In: Khan MA, Ahmad W (eds) Termites and sustainable management: volume 2-economic losses and management. Springer International Publishing, Cham, pp 197–217. https://doi.org/10.1007/978-3-319-68726-1_9
Vejan P, Abdullah R, Khadiran T, Ismail S, Nasrulhaq Boyce A (2016) Role of plant growth promoting rhizobacteria in agricultural sustainability-a review. Mole 29(5):573
Ventosa A, de la Haba RR, Sánchez-Porro C, Papke RT (2015) Microbial diversity of hypersaline environments: a metagenomic approach. Curr Opin Microbiol 25:80–87
Ventosa A, Nieto JJ, Oren A (1998) Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev 62(2):504–544
Wai Liew C, Illias RM, Muhammad Mahadi N, Najimudin N (2007) Expression of the Na+/H+ antiporter gene (g1-nhaC) of alkaliphilic Bacillus sp. G1 in Escherichia coli. FEMS Microbiol Lett 276(1):114–122
Wang JD, Levin PA (2009) Metabolism, cell growth and the bacterial cell cycle. Nat Rev Microbiol 7(11):822–827
Wang X, Wang C, Wang C, Cao X, Hou L (2014) Torulopsis versatilis strains with increased salt tolerance carry mutations in the glycerol transporter gene FPS 1. Int J Food Sci Tech 49(3):673–678
Warrence NJ, Bauder JW, Pearson KE (2002) Basics of salinity and sodicity effects on soil physical properties. Departement of Land Resources and Environmental Sciences, Montana State University-Bozeman, MT, pp 1–29
Watson H (2015) Biological membranes. Essay Biochem 15(59):43–69
Wei W, Jiang J, Yang SS (2004) Mutagenesis and complementation of relA from Sinorhizobium meliloti 042BM as a salt tolerance involvement gene. Ann Microbiol 54:317–324
Weinisch L, Kühner S, Roth R, Grimm M, Roth T, Netz DJA, Filker S (2018) Identification of osmoadaptive strategies in the halophile, heterotrophic ciliate Schmidingerothrix salinarum. PLoS Biol 16(1):e2003892
Wood JM, Bremer E, Csonka LN, Kraemer R, Poolman B, van der Heide T, Smith LT (2001) Osmosensing and osmoregulatory compatible solute accumulation by bacteria. Comp Biochem Physiol Part A Mol Int Physiol 130(3):437–460
Xiao JP, Zhang LL, Zhang HQ, Miao LX (2017) Identification of genes involved in the responses of tangor (C. reticulata × C. sinensis) to drought stress. BioMed Res Int 2017:1–15
Yadav AN, Sachan SG, Verma P, Saxena AK (2015a) Prospecting cold deserts of north western Himalayas for microbial diversity and plant growth promoting attributes. J Biosci Bioeng 119:683–693
Yadav AN, Sachan SG, Verma P, Tyagi SP, Kaushik R, Saxena AK (2015b) Culturable diversity and functional annotation of psychrotrophic bacteria from cold desert of Leh Ladakh (India). World J Microbiol Biotechnol 31:95–108
Yadav AN, Sharma D, Gulati S, Singh S, Kaushik R, Dey R, Pal KK, Saxena AK (2015c) Haloarchaea endowed with phosphorus solubilization attribute implicated in phosphorus cycle. Sci Rep 5:12293
Yadav AN, Verma P, Kumar M, Pal KK, Dey R, Gupta A, Padaria JC, Gujar GT, Kumar S, Suman A, Prasanna R, Saxena AK (2015d) Diversity and phylogenetic profiling of niche-specific Bacilli from extreme environments of India. Ann Microbiol 65:611–629
Yadav AN, Saxena AK (2018) Biodiversity and biotechnological applications of halophilic microbes for sustainable agriculture. J Appl Biol Biotechnol 6:48–55
Yadav AN, Gulati S, Sharma D, Singh RN, Rajawat MVS, Kumar R, Dey R, Pal KK, Kaushik R, Saxena AK (2019a) Seasonal variations in culturable archaea and their plant growth promoting attributes to predict their role in establishment of vegetation in Rann of Kutch. Biologia 74:1031–1043. https://doi.org/10.2478/s11756-019-00259-2
Yadav AN, Kour D, Sharma S, Sachan SG, Singh B, Chauhan VS, Sayyed RZ, Kaushik R, Saxena AK (2019b) Psychrotrophic microbes: biodiversity, mechanisms of adaptation, and biotechnological implications in alleviation of cold stress in plants. In: Sayyed RZ, Arora NK, Reddy MS (eds) Plant growth promoting rhizobacteria for sustainable stress management: volume 1: rhizobacteria in abiotic stress management. Springer Singapore, Singapore, pp 219–253. https://doi.org/10.1007/978-981-13-6536-2_12
Yadav AN, Mishra S, Singh S, Gupta A (2019c) Recent advancement in white biotechnology through fungi volume 1: diversity and enzymes perspectives. Springer International Publishing, Cham
Yadav AN, Singh S, Mishra S, Gupta A (2019d) Recent advancement in white biotechnology through fungi. Volume 2: perspective for value-added products and environments. Springer International Publishing, Cham
Yadav AN, Singh S, Mishra S, Gupta A (2019e) Recent advancement in white biotechnology through fungi. Volume 3: perspective for sustainable environments. Springer International Publishing, Cham
Yadav AN, Kumar V, Prasad R, Saxena AK, Dhaliwal HS (2018a) Microbiome in crops: diversity, distribution and potential role in crops improvements. In: Prasad R, Gill SS, Tuteja N (eds) Crop improvement through microbial biotechnology. Elsevier, USA, pp 305–332
Yadav AN, Verma P, Kumar S, Kumar V, Kumar M, Singh BP, Saxena AK, Dhaliwal HS (2018b) Actinobacteria from rhizosphere: molecular diversity, distributions and potential biotechnological applications. In: Singh B, Gupta V, Passari A (eds) New and future developments in microbial biotechnology and bioengineering. USA, pp 13–41. https://doi.org/10.1016/b978-0-444-63994-3.00002-3
Yadav AN, Verma P, Kumar V, Sangwan P, Mishra S, Panjiar N, Gupta VK, Saxena AK (2018c) Biodiversity of the Genus Penicillium in different habitats. In: Gupta VK, Rodriguez-Couto S (eds) New and future developments in microbial biotechnology and bioengineering, Penicillium system properties and applications. Elsevier, Amsterdam, pp 3–18. https://doi.org/10.1016/b978-0-444-63501-3.00001-6
Yadav AN, Verma P, Sachan SG, Kaushik R, Saxena AK (2018d) Psychrotrophic microbiomes: molecular diversity and beneficial role in plant growth promotion and soil health. In: Panpatte DG, Jhala YK, Shelat HN, Vyas RV (eds) Microorganisms for green revolution-volume 2: microbes for sustainable agro-ecosystem. Springer, Singapore, pp 197–240. https://doi.org/10.1007/978-981-10-7146-1_11
Yamamoto K, Shiwa Y, Ishige T, Sakamoto H, Tanaka K, Uchino M, Tanaka N, Oguri S, Saitoh H (2018) Tsushima S (2018) Bacterial diversity associated with the rhizosphere and endosphere of two halophytes: Glaux maritima and Salicornia europaea. Front Microbiol 9:2878
Yan N, Marschner P, Cao W, Zuo C, Qin W (2015) Influence of salinity and water content on soil microorganisms. Int Soil Water Con Res 3(4):316–323
Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217(4566):1214–1222
Yang J, Ma LA, Jiang H, Wu G, Dong H (2016) Salinity shapes microbial diversity and community structure in surface sediments of the Qinghai-Tibetan Lakes. Sci Rep 26(6):25078
Yang XD, Ali A, Xu YL, Jiang LM, Lv GH (2019) Soil moisture and salinity as main drivers of soil respiration across natural xeromorphic vegetation and agricultural lands in an arid desert region. Catena 177:126–133
Zahran HH (1997) Diversity, adaptation and activity of the bacterial flora in saline environments. Biol Fert Soil 25(3):211–223
Zahran HH (1999) Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 63(4):968–989
Zahran HH, Ahmad MS, Afkar EA (1995) Isolation and characterization of nitrogen-fixing moderate halophilic bacteria from saline soils of Egypt. J Basic Microbiol 35(4):269–275
Zerrouk IZ, Benchabane M, Khelifi L, Yokawa K, Ludwig-Müller J, Baluska F (2016) A Pseudomonas strain isolated from date-palm rhizospheres improves root growth and promotes root formation in maize exposed to salt and aluminum stress. J Plant Physiol 191:111–119
Zhao BS, Roundtree IA, He C (2017) Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol 18(1):31
Zhao G, Kong W, Weatherspoon-Griffin N, Clark-Curtiss J, Shi Y (2011) Mg 2+ facilitates leader peptide translation to induce riboswitch-mediated transcription termination. EMBO J 30(8):1485–1496
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Enespa, Prakash, J., Chandra, P. (2020). Halophilic Microbes from Plant Growing Under the Hypersaline Habitats and Their Application for Plant Growth and Mitigation of Salt Stress. In: Yadav, A., Singh, J., Rastegari, A., Yadav, N. (eds) Plant Microbiomes for Sustainable Agriculture. Sustainable Development and Biodiversity, vol 25. Springer, Cham. https://doi.org/10.1007/978-3-030-38453-1_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-38453-1_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-38452-4
Online ISBN: 978-3-030-38453-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)