Abstract
Oncology practitioners currently have very effective antiemetic agents in the form of 5-hydroxytryptamine-3 receptor antagonists, neurokinin-1 receptor antagonists, dexamethasone, and olanzapine for use in the prevention of chemotherapy-induced nausea and vomiting in patients receiving moderately or highly emetogenic chemotherapy. The choice of individual agents and the combination of agents should be dictated by the emetogenicity of the chemotherapy and patient risk factors. The available agents for the prevention of CINV appear to be safe and effective with few reported adverse events when used in the recommended doses.
The use of these agents in various clinical settings is described by established antiemetic guidelines from the Multinational Association of Supportive Care in Cancer and the European Society of Medical Oncology, the American Society of Clinical Oncology, and the National Comprehensive Cancer Network. These guidelines should be followed by practitioners in order to provide the highest possible quality of care for patients receiving chemotherapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Chemotherapy-induced nausea and vomiting (CINV) is associated with a significant deterioration in quality of life and is perceived by patients as a major adverse effect of the treatment [1,2,3]. Increased risk of CINV is associated with the type of chemotherapy administered (Table 46.1) and specific patient characteristics (Table 46.2) [3]. CINV can result in serious complications, such as weakness, weight loss, electrolyte imbalance, dehydration or anorexia, and is associated with a variety of complications, including fractures, oesophageal tears, decline in behavioural and mental status, and wound dehiscence [1,2,3]. Patients who are dehydrated, debilitated or malnourished, as well as those who have an electrolyte imbalance or those who have recently undergone surgery or radiation therapy are at greater risk of experiencing serious complications from CINV [1,2,3].
The type of chemotherapy to be given defines the degree of emetogenicity (Table 46.3) and the risk of CINV for patients. Table 46.4, 46.5 and 46.6 lists the emetogenicity of the various intravenous chemotherapy agents. Table 46.7 lists the emetogenicity of some of the oral chemotherapy agents. The type and number of antiemetics to be used for the control of CINV is dictated by whether the chemotherapy is of high, moderate, or low emetogenic potential.
Studies have suggested that physicians and nursing staff underestimated the CINV experienced by patients [4], and there is a significant financial impact of health care expenditures when CINV is not well controlled [5].
The use of first generation 5-hydroxytryptamine-3 (5-HT3) receptor antagonists plus dexamethasone has improved the control of CINV [3, 6]. Studies have also demonstrated improvement in the control of CINV with the use of a second-generation 5-HT3 receptor antagonist palonosetron [7], neurokinin-1 (NK-1) receptor antagonists (aprepitant, netupitant, and rolapitant) [8,9,10] and olanzapine, an antipsychotic that blocks multiple neurotransmitters in the central nervous system [11,12,13,14,15].
The primary endpoint used for studies evaluating various agents for the control of CINV has been complete response (no emesis, no use of rescue medication) over the acute (24 h post-chemotherapy), delayed (24–120 h) and overall (0–120 h) periods [3]. Studies have shown that the combination of a 5-HT3 receptor antagonist, dexamethasone and an NK−1 receptor antagonist have improved the control of emesis in patients receiving either highly emetogenic chemotherapy (HEC) or moderately emetogenic chemotherapy (MEC) over a 120-h period following chemotherapy administration [3, 7,8,9,10,11,12,13]. Many of these same studies have measured nausea as a secondary endpoint and have demonstrated that nausea has not been well controlled [16, 17].
Emesis is a well-defined event that is easily measured, but nausea may be more subjective and more difficult to measure. However, two well defined measures of nausea that appear to be effective and reproducible measurement tools are the visual analogue scale (VAS) and the Likert Scale [18]. The VAS is a scale from 0 to 10 or 0 to 100, with zero representing no nausea and 10 or 100 representing maximal nausea. The Likert Scale asks patients to rate nausea as ‘None, Mild, Moderate or Severe’.
Many studies have reported the secondary endpoint of ‘no significant nausea’ or ‘only mild nausea [3, 8, 17]. Studies that have reported ‘no nausea’ may be more useful in identifying the most effective available anti-nausea agents [14, 16].
Despite the introduction of more effective antiemetic agents, emesis and nausea remain a significant complication of chemotherapy. The purpose of this review is to evaluate the clinical agents available for the prevention and treatment of CINV. The use of these agents in various clinical settings is described using the recently established guidelines from the Multinational Association of Supportive Care in Cancer (MASCC) and the European Society of Medical Oncology [19], the American Society of Clinical Oncology (ASCO) [20], and the National Comprehensive Cancer Network (NCCN) guidelines [21]. The literature cited in the report consists of the primary clinical trials used for the United States Food & Drug Administration (FDA) approval of the various agents as well as recent comprehensive reviews.
1.1 Pathophysiology of Nausea and Vomiting
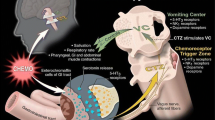
The sensation of nausea and the act of vomiting are protective reflexes that rid the intestine and stomach of toxic substances. The experience of nausea is subjective, and nausea may be considered a prodromal phase to the act of vomiting [18] although significant nausea may occur without vomiting. Vomiting consists of a pre-ejection phase, retching and ejection and is accompanied by shivering and salivation. Vomiting is triggered when afferent impulses from the cerebral cortex, chemoreceptor trigger zone (CTZ), pharynx and vagal afferent fibres of the gastrointestinal (GI) tract travel to the vomiting centre (VC), located in the medulla (Fig. 46.1). Efferent impulses then travel from the VC to the abdominal muscles, salivation centre, cranial nerves and respiratory centre, causing vomiting. It is thought that chemotherapeutic agents cause vomiting by activating neurotransmitter receptors located in the CTZ, GI tract and VC. The mechanisms of emesis are not well defined, but investigations suggest that emesis may be primarily mediated through neurotransmitters (serotonin, dopamine, substance P) in the GI tract and the central nervous system [18]. Figure 46.1 shows that chemotherapy agents may directly affect areas in the cerebral cortex, the medulla oblongata, or may stimulate the small intestine of the GI tract via the vagus nerve. A VC, termed the ‘central pattern generator’ by some authors [22], appears to be located in the lateral reticular formation of the medulla, which coordinates the mechanism of nausea and vomiting. An additional important area, also located in the medulla, is the CTZ in the area postrema near the fourth ventricle [22]. It is strongly suspected that the nucleus tractus solitarius (NTS) neurons lying ventrally to the area postrema initiate emesis [23]. This medullary area is a convergence point for projections arising from the area postrema and the vestibular and vagal afferents [23]. The NTS is a good candidate for the site of action of centrally acting antiemetics.
The main approach to the control of emesis has been to identify the active neurotransmitters and their receptors in the central nervous system and the GI tract that mediate the afferent inputs to the VC (Fig. 46.2). Agents that may block these neurotransmitter receptors in the CTZ, the VC or the GI tract may be useful in preventing or controlling emesis (Table 46.8).
Nausea is a difficult-to-describe, sick or queasy sensation, usually perceived as being in the stomach that is sometimes followed by emesis [18]. The experience of nausea is difficult to describe in another person. Nausea and emesis are not necessarily on a continuum. One can experience nausea without emesis and one can have sudden emesis without nausea. Nausea has been assumed to be the conscious awareness of unusual sensations in the ‘vomiting centre’ of the brainstem (Fig. 46.1), but the existence of such a centre and its relationship to nausea remain controversial [18].
The study of the receptors that are illustrated in Fig. 46.2 has guided the development of the antagonists to the serotonin and the substance P receptors with relative success in controlling emesis. It is not clear whether the serotonin and/or the substance P receptors are important in the control of nausea. Other receptors, such as dopaminergic, histaminic and muscarinic, may be the dominant receptors in the control of nausea [3, 16, 17].
1.2 Types of Chemotherapy-Induced Nausea and Vomiting (CINV)
Five categories are used to classify CINV: acute, delayed, anticipatory, breakthrough, and refractory. Nausea and vomiting may occur any time after the administration of chemotherapy, but the mechanisms appear different for CINV occurring in the first 24 h after chemotherapy in contrast to that which occurs in the period of 1–5 days after chemotherapy.
1.2.1 Acute CINV
The term acute-onset CINV refers to nausea and/or vomiting occurring within 24 h of chemotherapy administration [3] and usually peaks within the first 5, 6 h after the initiation of chemotherapy. The incidence of acute emesis and/or nausea reflects several treatment-related factors, including the environment in which chemotherapy is administered, the emetogenicity of the chemotherapy, the dosage of the emetogenic agents and patient-related factors [21].
1.2.2 Delayed CINV
Nausea and/or vomiting that develop more than 24 h after chemotherapy administration is known as delayed emesis and/or nausea. Typically occurring with administration of cisplatin, carboplatin, doxorubicin, or cyclophosphamide, delayed emesis/nausea is more common in those who experience acute emesis/nausea. Other predictive factors include the dose and the emetogenicity of the chemotherapeutic agent, patient sex and age, and protection against nausea and vomiting in previous cycles of chemotherapy [3, 21]. For cisplatin, which has been most extensively studied, delayed emesis reaches peak intensity 2–3 days subsequent to chemotherapy administration and can last up to a week [3, 19,20,21].
1.2.3 Breakthrough CINV
Vomiting and/or nausea that occurs within 5 days after chemotherapy despite prophylactic use of antiemetic agents and/or requires additional antiemetics (‘rescue’) is called breakthrough emesis
1.2.4 Refractory CINV
Vomiting and/or nausea occurring after chemotherapy in subsequent chemotherapy cycles when antiemetic prophylaxis and/or rescue have failed in earlier cycles is known as refractory emesis [3, 21].
1.2.5 Anticipatory CINV
If patients experience CINV, they may develop a conditioned response known as anticipatory nausea and/or vomiting, which occurs prior to the administration of chemotherapy in future chemotherapy cycles and is attributed to the adverse memory of prior CINV. Incidence rates for this type of nausea and vomiting range from 10% to 45%, with nausea occurring more frequently [3, 21].
2 Antiemetic Agents
2.1 Dopamine Receptor Antagonists
Dopamine receptors are known to exist in the CTZ, and this is the main area of activity of the dopamine antagonists, such as the phenothiazines and the butyrophenones (droperidol, haloperidol). However, a high level of blockade of the dopamine receptors results in extrapyramidal reactions, as well as disorientation and sedation, limiting the clinical use of these agents. Their current use is primarily to treat established nausea and emesis and not for CINV prophylaxis [21].
2.2 Serotonin (5-HT3) Receptor Antagonists
Serotonin receptors, specifically the 5-HT3 receptors, exist in the central nervous system and in the GI tract. The 5-HT3 receptor antagonists appear to act through both the central nervous system and the GI tract via the vagus and splanchnic nerves.
The introduction of 5-HT3 receptor antagonists for the prevention of CINV, as well as post-operative and radiotherapy-induced nausea and vomiting, has resulted in an improvement in supportive care [3]. Treatment guidelines for the prevention of CINV recommended by a number of international groups [19,20,21] suggest the use of a 5-HT3 receptor antagonist and dexamethasone alone or in combination with other antiemetics pre-chemotherapy for the prevention of acute CINV, and the use of dexamethasone alone or in combination with other antiemetics following chemotherapy for the prevention of delayed nausea and vomiting in patients receiving either moderately or highly emetogenic chemotherapy.
2.3 First-Generation 5-HT3 Receptor Antagonists
Table 46.9 shows the 5-HT3 receptor antagonists currently in use. The first-generation 5-HT3 receptor antagonists (dolasetron, granisetron, ondansetron, tropisetron [24], azasetron [25] and ramosetron [26] are equivalent in efficacy and toxicities when used in the recommended doses and compete only on an economic basis [27]. They have not been associated with major toxicities, with the most commonly reported adverse events being mild headache, constipation and, occasionally, mild diarrhea [3]. Azasetron and ramosetron are not available in North America and Europe and have not been compared extensively with the other 5-HT3 receptor antagonists. They are marketed primarily in Southeast Asia.
Differences in metabolism of the 5-HT3 receptor antagonists may occur due to genetic variability in individuals which may lead to a difference in response to these agents, but there have been no documented clinical reports of this phenomenon [3, 19,20,21, 24,25,26,27].
In 2006, Canada issued a drug alert for dolasetron, due to the potential of serious cardiovascular adverse events (cardiac arrhythmias) [28], stating that dolasetron was not indicated for use in children, but only for prevention of CINV in adults [28]. Subsequently, in 2010, the US FDA announced that the intravenous form of dolasetron should no longer be used to prevent CINV in any patient. New data suggested that dolasetron injection can increase the risk of developing a prolongation of the QT interval, which may potentially precipitate life-threatening ventricular arrhythmias [29].
In 2012, the FDA placed a restriction on the doses of intravenous ondansetron due to the risk of prolongation of the QT interval [30]. Patients who may be at particular risk for QT prolongation with ondansetron is those with congenital long QT syndrome, congestive heart failure, brady-arrhythmias, or patients taking concomitant medications that prolong the QT interval. The use of a single 32-mg intravenous dose of ondansetron should be avoided. New information indicates that QT prolongation occurs in a dose-dependent manner, and specifically at a single intravenous dose of 32 mg. The lower-dose intravenous regimen of 0.15 mg/kg every 4 h for three doses may be used in adults with CINV. However, no single intravenous dose of ondansetron should exceed 16 mg due to the risk of QT prolongation. The new information does not change any of the recommended oral dosing regimens for ondansetron, including the single oral dose of 24 mg for CINV [30].
Mason et al. [31] has reported that intravenous granisetron had no clinically significant effect on the QTc interval at supratherapeutic concentrations.
The first-generation 5-HT3 receptor antagonists have not been as effective against delayed emesis as they are against acute CINV [32,33,34]. The first-generation 5-HT3 receptor antagonists alone do not add significant efficacy to that obtained by dexamethasone in the control of delayed emesis [33]. Hickok et al. [34] reported that the first-generation 5-HT3 receptor antagonists used in the delayed period were no more effective than prochlorperazine in controlling nausea. The antiemetic effects of prochlorperazine can be attributed to post-synaptic dopamine receptor blockade in the CTZ. A meta-analysis [33] showed that there was neither clinical evidence nor considerations of cost effectiveness to justify using the first-generation 5-HT3 receptor antagonists beyond 24 h after chemotherapy for the prevention of delayed emesis. A number of studies have also demonstrated that there has been poor control of delayed nausea by the first-generation 5-HT3 receptor antagonists in patients receiving HEC or MEC [12, 35, 36].
2.4 Extended Release Granisetron
A randomized, double-blind, phase III clinical trial evaluated the antiemetic efficacy of transdermal granisetron compared to oral granisetron in patients receiving MEC and HEC [37]. There was no significant difference in the control of acute or delayed emesis between transdermal and oral granisetron. The data demonstrated that transdermal granisetron was effective and safe in the control of acute emesis induced by MEC and HEC [37].
APF530 is a new, subcutaneously (SC) administered polymeric formulation of granisetron that was developed to provide slow, controlled, and sustained release of granisetron to prevent both acute and delayed CINV associated with MEC and HEC [38]. APF530 consists of 2% granisetron and a polymer vehicle of tri(ethylene glycol) poly(orthoester) (TEG-POE) that undergoes controlled hydrolysis, resulting in slow, controlled, and sustained drug release. The novel biodegradable polymeric excipient is hydrolyzed in vivo, generating nontoxic biodegradable metabolites. This Biochronomer™ drug delivery system (Heron Therapeutics, Inc., Redwood City, CA) allows therapeutic levels of granisetron to be maintained for >5 days with a single subcutaneous injection. In a clinical study [38] in patients undergoing chemotherapy, single-dose APF530 (5–15 mg granisetron) administered SC in the abdomen provided circulating levels of granisetron within 30 min, a maximum plasma concentration at ∼24 h, and sustained therapeutic levels for >120 h. In a phase 3 noninferiority trial, the clinical efficacy of APF530 250 and 500 mg SC (containing granisetron 5 and 10 mg, respectively) was compared with that of the approved dose of palonosetron (0.25 mg intravenously) in combination with dexamethasone for prevention of acute and delayed CINV following single-day administration of MEC or HEC in patients with cancer. APF530 was noninferior to palonosetron with injection site reactions and constipation the most commonly reported adverse events [38]. In a QTc study, the APF530 formulation had no clinically significant effect on the QTc interval at supratherapeutic concentrations [31].
2.5 Second-Generation 5-HT3 Receptor Antagonists: Palonosetron
Palonosetron is a second-generation 5-HT3 receptor antagonist that has antiemetic activity at both central and GI sites [3, 6, 7]. In comparison with the first-generation 5-HT3 receptor antagonists, it has a higher potency, a significantly longer half-life and a different molecular interaction with 5-HT3 receptors [3, 6, 7, 39] (Table 46.10). Palonosetron studies suggest that it may have efficacy in controlling delayed CINV compared with the first-generation 5-HT3 receptor antagonists [3, 6, 7, 39].
Palonosetron demonstrated a 5-HT3 receptor binding affinity at least 30-fold higher than other 5-HT3 receptor antagonists [34]. Rojas et al. [40] reported that palonosetron exhibited allosteric binding and positive cooperativity when binding to the 5-HT3 receptor compared with simple bimolecular binding for both granisetron and ondansetron. Additional studies by Rojas et al. [40] suggested that palonosetron triggers 5-HT3 receptor internalization and causes prolonged inhibition of receptor function. Differences in binding and effects on receptor function may explain some differences between palonosetron and the first-generation 5-HT3 receptor antagonists [7, 40]. These differences may explain palonosetron’s efficacy in delayed CINV compared with the first-generation receptor antagonists [3, 7, 39].
In a systematic review and meta-analysis of all randomized controlled trials comparing a single dose of palonosetron with other 5-HT3 receptor antagonists, Botrel et al. [41] concluded that palonosetron was more effective than the first generation receptor antagonists in preventing acute and delayed CINV in patients receiving MEC or HEC, regardless of the use of concomitant corticosteroids. Schwartzberg et al. [42] concluded that palonosetron is more effective than the first generation 5-HT3 receptor antagonists in controlling CINV in the delayed and overall post-chemotherapy periods based on a pooled analysis of phase III clinical studies of palonosetron versus ondansetron, dolasetron, and granisetron. In an additional review, Popovic et al. [43] concluded that palonosetron is safer and more efficacious than the other 5-HT3 receptor antagonists. The international antiemetic guidelines [19,20,21] recommend palonosetron as the preferred 5-HT3 receptor antagonist.
The safety and tolerability of palonosetron has been well documented in multiple, large phase III trials. There were no clinically relevant differences seen among palonosetron, ondansetron, or dolasetron in laboratory, electrocardiographic, or vital sign changes over multiple cycles of chemotherapy [7, 39, 43,44,45]. The adverse reactions reported were the most common reactions reported for the 5-HT3 receptor antagonist drug class. There have been no reports of any adverse cardiac events with palonosetron, specifically no prolongation of the QT interval in healthy volunteers or patients receiving repeated cycles of emetogenic chemotherapy [7, 39, 43,44,45] Table 46.11 summarizes the reported adverse events of the antiemetic guideline directed serotonin antagonists.
There are no other second-generation 5-HT3 receptor antagonists on the market and there is no information available on other second-generation agents in development.
2.6 Dopamine-Serotonin Receptor Antagonists
Metoclopramide has antiemetic properties both in low doses as a dopamine antagonist and in high doses as a serotonin antagonist. The use of metoclopramide may be somewhat efficacious in relatively high doses (20 mg orally, three times daily) in the delayed period [46] but may result in sedation and extrapyramidal side effects [3, 21, 47].
Metoclopramide has been used both as a preventative agent for CINV [46] as well as a treatment for breakthrough CINV [21, 47].
In 2013, the European Medicines Agency issued use restrictions for metoclopramide due to the risk of
-
extrapyramidal disorders
-
involuntary movement disorders that may include muscle spasms
-
tardive dyskinesia
It was noted that the risk of side effects is increased at high doses or with long-term treatment. The review recommended that treatment duration be restricted to short-term use (up to 5 days) and that the maximum dose be limited in adults to 10 mg three times daily. It was also recommended that metoclopramide not be used in children under 1 year old [48].
The reduced dose of 10 mg three times daily may be less efficacious as a preventative agent for CINV and as a treatment for breakthrough CINV [3, 21, 46, 47].
2.7 Neurokinin (NK-1) Receptor Antagonists
Substance P is a mammalian tachykinin that is found in vagal afferent neurons innervating the brainstem NTS, which sends impulses to the VC [49]. Substance P induces vomiting and binds to NK1 receptors in the abdominal vagus, the NTS, and the area postrema [49]. Compounds that block NK1 receptors lessen emesis after cisplatin, ipecac, apomorphine and radiation therapy [49]. These observations have recently led to the development of NK1 receptor antagonists and the study of the role they may play in controlling CINV.
2.7.1 Aprepitant
Aprepitant is an NK-1 receptor antagonist that blocks the emetic effects of substance P [3, 8, 50]. When combined with a standard regimen of the corticosteroid dexamethasone and a 5-HT3 receptor antagonist, aprepitant is effective in the prevention of CINV in patients receiving cisplatin based HEC [3, 50]. This regimen is recommended in the guidelines of multiple international groups for the control of CINV in patients receiving HEC [19,20,21].
Combined data from two large phase III trials of aprepitant plus a first-generation 5-HT3 receptor antagonist and dexamethasone for the prevention of CINV in patients receiving HEC demonstrated an improvement in complete response when aprepitant was added to ondansetron and dexamethasone, but there was no improvement in nausea when the pooled data was analysed for sex (no nausea, overall period: 46% for women, aprepitant group, 38% for women, control group; 50% for men, aprepitant group, 44% for men, control group) [51]. Using the same pooled data, a separate analysis [52] showed a statistical but small improvement in no nausea with the use of aprepitant (no nausea, overall period: 48%, aprepitant group; 42%, control group).
In a similar study involving breast cancer patients receiving cyclophosphamide and doxorubicin or epirubicin, aprepitant was added to ondansetron and dexamethasone for the prevention of CINV. The addition of aprepitant to the 5-HT3 receptor antagonist plus dexamethasone improved the complete response, but there was no improvement in nausea (no nausea, overall period: 33% aprepitant group; 33% control group) [36].
Palonosetron and aprepitant have been combined with dexamethasone for the prevention of CINV in a phase II study of 58 patients who received doxorubicin and cyclophosphamide [53]. This three-drug antiemetic regimen was found to be safe and highly effective in preventing emesis and rescue in the acute, delayed and overall periods, but there was poor control of nausea (no nausea, overall period: 30%).
2.7.2 Fosaprepitant
Fosaprepitant (also known as MK-0517 and L-758,298) is a water-soluble phosphoryl pro-drug for aprepitant that, when administered intravenously, is converted to aprepitant within 30 min via the action of ubiquitous phosphatases. The pharmacological effect of fosaprepitant is attributed to aprepitant. Due to the rapid conversion of fosaprepitant to the active form (aprepitant) by phosphatase enzymes, it is expected to provide the same aprepitant exposure in terms of area under the curve (AUC) and a correspondingly similar antiemetic effect [54, 55]. Studies have demonstrated that a single dose of intravenous fosaprepitant, 150 mg on day 1 of cisplatin chemotherapy, was noninferior to a 3-day oral regimen of aprepitant in the prevention of CINV in the 120 h post-chemotherapy [55].
Both standard 3-day dosing of aprepitant and single-dose fosaprepitant have been demonstrated to be well tolerated after ondansetron and dexamethasone in patients receiving cisplatin [55]. The tolerability profiles of the two regimens were similar, except for a higher incidence of infusion-site adverse events and significantly more thrombophlebitis with intravenous fosaprepitant. Higher incidence of infusion-site adverse events was observed in a retrospective review of 98 patients treated with fosaprepitant [56].
Aprepitant is metabolized extensively by liver enzymes, primarily CYP3A4. CYP3A4 inhibitors can increase aprepitant exposure, and CYP3A4 inducers can reduce aprepitant exposure [57]. Aprepitant is also both an inducer and a moderate inhibitor of CYP3A4 [58]. Consequently, the potential for drug-drug interactions exists when aprepitant is coadministered with other drugs that are metabolized by CYP enzymes, including chemotherapeutic agents [59]. Results from several clinical efficacy trials and pharmacokinetic studies showed that most drug-drug interactions with aprepitant had little or no clinical consequence and that no differences in severe adverse events were noted between treatment arms with or without aprepitant [52, 59]. Aprepitant had minimal effect on the area under the curve (AUC) of several chemotherapeutic agents tested, including cyclophosphamide, docetaxel, and vinorelbine [59]. Coadministration of aprepitant causes a significant increase in the AUC of some corticosteroids, including a 2.2-fold increase in dexamethasone and a 2.5-fold increase in oral methylprednisolone, necessitating up to 50% dose reduction of these drugs [59]. Aprepitant causes reduced AUC of oral contraceptives, and this has prompted the recommendation of a secondary barrier contraceptive for patients receiving aprepitant [59]. Ifosfamide and aprepitant are both substrates of CYP3A4, and theoretical questions have been raised as to whether aprepitant could be potentially involved in rare cases of ifosfamide encephalopathy, but no clinical data exist demonstrating an association [8, 57, 59].
Recently, the success of the use of NK-1 receptor antagonists with 5-HT3 receptor antagonists and dexamethasone in preventing emesis in patients receiving single day highly emetogenic chemotherapy [3, 6] prompted the use of the NK-1 receptor antagonist aprepitant combined with a 5-HT3 receptor antagonist and dexamethasone in patients receiving multi-day, high dose chemotherapy prior to SCT. A number of Phase III studies have been reported with the use of the NK-1 aprepitant added to a 5-HT3 receptor antagonists and dexamethasone [60,61,62,63] in patients receiving multi-day, high dose chemotherapy prior to autologous or allogeneic stem cell transplant (SCT). In a randomized, placebo-controlled, phase III clinical trial, Stiff et al. [62] randomized 179 patients receiving multi-day, high dose chemotherapy prior to SCT for autologous and allogeneic transplants to aprepitant or placebo in combination with ondansetron and dexamethasone prior to chemotherapy. There was a significant improvement in emesis with the use of aprepitant, but no difference in the use of rescue medications or nausea. No adverse events were noted with the use of aprepitant.
Schmitt et al. [60] randomized 362 patients receiving 2 days of high dose melphalan chemotherapy prior to SCT for autologous transplants to aprepitant or placebo in combination with granisetron and dexamethasone prior to chemotherapy and post chemotherapy. There was a significant improvement in complete response with the use of aprepitant, but no difference in the use of rescue medications or nausea. No adverse events were noted with the use of aprepitant. Svanberg and Birgegard [63] randomized 96 patients receiving multi-day, high dose chemotherapy prior to SCT for autologous transplants to aprepitant or placebo in combination with tropisetron and a corticosteroid prior to chemotherapy and post chemotherapy. There was a significant improvement in emesis with the use of aprepitant, but no difference in the use of rescue medications or nausea. No adverse events were noted with the use of aprepitant.
Pielichowski et al. [61] used aprepitant, palonosetron, and dexamethasone to prevent nausea and vomiting following BEAM chemotherapy before autologous hematopoietic stem cell transplantation for patients with non-Hodgkin’s or Hodgkin’s lymphoma. Emesis was improved in the acute and delayed phases post chemotherapy compared to historical controls who received ondansetron or palonosetron plus dexamethasone alone.
One retrospective study and two prospective (phase II, III) studies, each with a small number of patients (25–40 patients) also demonstrated improvement in emesis with the addition of aprepitant to a 5-HT3 receptor antagonist with or without a corticosteroid in patients receiving autologous or allogeneic stem cell transplant [64,65,66].
As a result of the studies cited above, the 2017 ASCO and the 2017 MASCC/ESMO antiemetic guidelines have recommended the use of a NK-1 receptor antagonist, a 5-HT3 receptor antagonist, and dexamethasone as the preferred prophylaxis for patients receiving high-dose, multi-day chemotherapy prior to autologous or allogeneic stem cell transplantation [20, 67]. The studies discussed above have demonstrated that the addition of aprepitant to a 5-HT3 receptor antagonist and dexamethasone result in improved control of emesis post chemotherapy, but not nausea. The control of nausea remains a significant patient problem, not only in multi-day, high-dose chemotherapy, but also in single day highly emetogenic chemotherapy. Neither 5-HT3 receptor antagonists, nor aprepitant appear to be effective anti-nausea agents in the post chemotherapy period [3, 6, 16, 17].
2.7.3 Cinvanti
Cinvanti is a substance P/neurokinin-1 (NK-1) receptor antagonist, approved by the FDA on November 9, 2017, indicated in adults, in combination with other antiemetic agents, for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of HEC including high-dose cisplatin and nausea and vomiting associated with initial and repeat courses of MEC.
Cinvanti is a polysorbate 80-free, intravenous formulation of the NK1 receptor antagonist fosaprepitant indicated for the prevention of acute and delayed CINV. Cinvanti does not contain polysorbate 80 or any other synthetic surfactant. Pharmaceutical formulations containing polysorbate 80 have been linked to hypersensitivity reactions, including anaphylaxis and irritation of blood vessels resulting in infusion-site pain [68]. Cinvanti was approved based on data demonstrating the bioequivalence of Cinvanti to fosaprepitant supporting its efficacy for the prevention of acute and delayed CINV following HEC and MEC. Results from two pivotal randomized, crossover bioequivalence studies of Cinvanti and fosaprepitant IV showed subjects receiving Cinvanti reported fewer adverse events than those receiving fosaprepitant, including substantially fewer infusion-site reactions [68].
2.7.4 Netupitant/NEPA
Netupitant is a NK-1 receptor antagonist approved by the FDA in 2014 for the prevention of chemotherapy-induced nausea and vomiting. In vitro and in vivo pharmacologic characterization demonstrated that Netupitant inhibits substance P in NK-1 receptors but was inactive for NK-2 and NK-3 receptors. This was demonstrated with intrathecal injections in mice, and intraperitoneally in both mice and gerbils. In all assays, aprepitant exhibited similar effects [69].
Netupitant behaves as a brain penetrant, is orally active, and is a potent and selective NK-1 antagonist [69, 70]. Rossi et al. [69] and Spinelli et al. [70] reported that positive emission tomography results demonstrate that netupitant is a potent agent targeting NK-1 receptors. It appears to have a high degree of occupancy (90%) for a long duration (96 h) when given as a single oral dose and appears to be well tolerated [69,70,71]. Netupitant has a high binding affinity, and a long half-life of 90 h compared to a 9–13 h half-life of aprepitant [8, 9, 69,70,71]. It is metabolized by CYP3A4 and is a moderate inhibitor of CYP3A4 [9, 69,70,71]. Due to netupitant’s interaction with CYP3A4, it potentially could increase the concentration of docetaxel when administered simultaneously. However, netupitant would be expected to have similar interactions as aprepitant which has been shown not to cause any clinically significant alterations in the pharmacokinetics of docetaxel or of its toxicity (adverse events and neutropenia) compared with administration of docetaxel alone in cancer patients [59].
NEPA is an oral fixed-dose combination of netupitant and palonosetron which has been employed in phase II and phase III clinical trials for the prevention of CINV in patients receiving the chemotherapy combination of an anthracycline and cyclophosphamide and HEC [9, 72,73,74]. The clinical trials demonstrated that NEPA (300 mg of netupitant plus 0.50 mg of palonosetron) plus dexamethasone significantly improved the prevention of CINV compared to the use of palonosetron and dexamethasone alone in patients receiving either HEC [72] or a combination of an anthracycline and cyclophosphamide [73]. The significant improvement in the delayed period (24–120 h) and the overall period (0–120 h) post chemotherapy was maintained over multiple cycles of chemotherapy [74]. Adverse events (hiccups, headache, constipation) were few in number (≤ 3.5%) and were mild to moderate in severity [9, 72,73,74]. No cardiac adverse events were noted.
On October 10, 2014, oral NEPA (Akynzeo) was approved by the US FDA to treat nausea and vomiting in patients undergoing cancer chemotherapy [75].
2.7.5 Rolapitant
Rolapitant is a high affinity, highly-selective NK-1 receptor antagonist [76] It penetrates the central nervous system following oral administration, and it has a high affinity for the human NK-1 receptor and is highly selective over the human NK-2 and NK-3 receptor subtypes. It is a functionally competitive antagonist and reversed NK-1 agonist-induced foot tapping in a gerbil animal model following both intravenous and oral intravenous and oral administration [76]. Rolapitant reverses both apomorphine and cisplatin-induced emesis in ferrets [76].
The pharmacokinetics of rolapitant demonstrates that it has a long half-life (approximately 180 h) with high affinity (Ki = 0·66 nM) for the NK-1 receptor [76, 77], and it does not induce or inhibit CYP3A4. Poma et al. [77] reported that rolapitant and its major metabolite SCH720 881 do not affect the pharmacokinetics of midazolam, a sensitive cytochrome P450 3A4 substrate. Rolapitant does not induce CYP3A4, and single oral doses of rolapitant, co-administered with midazolam were safe and well tolerated. Administration of rolapitant, unlike other NK-1 receptor antagonists aprepitant and netupitant, does not require dose adjustment of concomitantly administered drugs metabolized by CYP34A.
Rolapitant is a moderate CYP2D6 inhibitor suggesting that there could be potential interactions with metoprolol or venlafaxine.
A phase I clinical trial in 14 healthy volunteers demonstrated that a 180 mg rolapitant dose provided ≥90% NK-1 receptor occupancy in the brain for up to 5 days following a single dose [10, 78]. A phase II randomized, double-blind, active-controlled dose-finding study showed that a 180 mg dose of rolapitant plus granisetron and dexamethasone was safe and effective in the prevention of CINV in patients receiving HEC [10, 79]. Complete response was significantly improved with rolapitant compared to placebo with all patients receiving ondansetron and dexamethasone.
The 180 mg dose of rolapitant was used in three large phase III clinical trials which demonstrated that rolapitant, granisetron, and dexamethasone significantly improved complete response compared to granisetron and dexamethasone alone in patients receiving MEC and HEC [10, 80, 81]. Approximately 80% of the patients in the MEC study [80] received a combination of an anthracycline and cyclophosphamide chemotherapy or carboplatin chemotherapy. There were no serious adverse events in the clinical trials, and there were no differences in the number of adverse events in the rolapitant or control arms.
On September 2, 2015, the US FDA approved oral Rolapitant (Varubi) for the prevention of nausea and vomiting associated with cancer chemotherapy. On October 25, 2017, the FDA approved an intravenous form of rolapitant equivalent to the oral form. Intravenous rolapitant is an emulsion, which is polysorbate 80 free.
2.8 Safety and Tolerability of Neurokinin-1 Receptor Antagonists
A ten-year review of the safety and efficacy of aprepitant and fosaprepitant [8] demonstrated that these agents are well tolerated, and there appear to be no major systemic adverse events associated with their use. In comparison studies, aprepitant treated patients have had patterns and incidences of adverse events similar to those associated with standard control antiemetic therapy [8] (Table 46.12). Both standard 3-day dosing of aprepitant and single-dose fosaprepitant have been demonstrated to be well tolerated after ondansetron and dexamethasone in patients receiving cisplatin [55]. The tolerability profiles of the two regimens appear similar, except for a higher incidence of infusion-site adverse events and significantly more phlebitis with intravenous fosaprepitant [56]. Higher incidence of infusion-site adverse events was observed in a retrospective review of 98 patients treated with fosaprepitant [56] and in randomized, cross-over bioequivalence studies of cinvanti and fosaprepitant in normal volunteers [68].
The recent studies on rolapitant and netupitant have also demonstrated a low level of adverse events, not different from comparison control antiemetic therapy, in patients receiving either MEC or HEC [72, 73, 80, 81] (Table 46.12). Headache, constipation, hiccups, and fatigue appear to be the most commonly reported events.
dos Santos et al. [82] reported that in a retrospective review of sixteen studies of the NK-1 receptor antagonists, the incidence of severe infection increased from 2% to 6% in the NK-1 receptor antagonist group in three RCTs with a total of 1480 patients. The increased infection rate was not seen in the other thirteen studies and was not reported in a ten-year review of aprepitant [8] or the recent phase III clinical trials of netupitant [72, 73] or rolapitant [80, 81]. A recent meta-analysis by Zhang et al. [83] reported that NK-1 receptor antagonist-based triple regimens were effective in the prevention of chemotherapy-induced nausea and vomiting with few significant toxicities.
2.8.1 Dexamethasone
Dexamethasone has been an effective antiemetic in controlling both acute and delayed CINV when combined with 5-HT3 receptor antagonists and NK-1 receptor antagonists and it is essentially the main corticosteroid used as an antiemetic [19,20,21]. Dexamethasone added to a 5-HT3 receptor antagonist improves the control of acute CINV, and it has been used as a single agent or in combination with NK-1 receptor antagonists in an attempt to control delayed CINV [19,20,21].
Concern has been expressed with the potential toxicity of the use of multiple-day dexamethasone to control CINV [84]. Patients receiving dexamethasone as prophylaxis for CINV reported moderate to severe problems with insomnia, hyperglycemia, indigestion, epigastric discomfort, agitation, increased appetite, weight gain and acne [84]. Some studies have demonstrated that dexamethasone use might be decreased from multiple days to 1 day in an antiemetic regime when used with other agents which are effective in controlling CINV in both the acute and the delayed periods [13, 21, 85, 86].
Celio et al. [85] used palonosetron in combination with a 1 day versus 3 days of dexamethasone to prevent CINV in patients receiving MEC. There was no improvement in complete response or no nausea over the 5-day overall period with the use of 3 days of dexamethasone versus 1 day of dexamethasone. A similar study [86] using palonosetron plus dexamethasone for 1 day versus 3 days for breast cancer patients receiving an anthracycline and cyclophosphamide chemotherapy showed similar results: no improvement in complete response or in no nausea over the 5-day overall period with the use of 3 days versus 1 day of dexamethasone.
Navari et al. [13, 21] reported that 4 days of olanzapine with 1 day of a 5-HT3 receptor antagonist and 1 day of dexamethasone was effective in the prevention of CINV in patients receiving HEC.
2.8.2 Olanzapine
Olanzapine is an atypical antipsychotic agent of the thiobenzodiazepine class and was approved by the FDA for the treatment of the manifestations of psychotic disorders in 1996 [87, 88] with a generic formulation becoming available in 2011. This drug blocks multiple neurotransmitter receptors including dopaminergic (D1, D2, D3, D4 brain receptors), serotonergic (5-HT2a, 5-HT2c, 5-HT3, 5-HT6 receptors), catecholaminergic (alpha1 adrenergic receptors), acetylcholinergic (muscarinic receptors), and histaminergic (H1 receptors) [89]. Olanzapine has five times the affinity for 5-HT2 receptors than for D2 receptors [90]. The effect of olanzapine on the serotonin-mediated 5-HT2C receptor as well as other dopamine and serotonin receptors may explain, in part, its efficacy in alleviating nausea and vomiting.
A benefit of olanzapine is that it is not a cytochrome P450 inhibitor and thus appears to have fewer drug interactions than many other drugs [89, 90]. Common side effects are sedation and weight gain [91]. The sedation is short term and may be dose dependent [92] The weight gain can occur after higher doses given over a period of months and can lead to diabetes mellitus when given for a period of greater than 6 months [93].
Phase III clinical trials have demonstrated the effectiveness of olanzapine in the prevention of CINV [12,13,14,15, 94]. Olanzapine improved the control of nausea and emesis when added to azasetron and dexamethasone compared to azasetron and dexamethasone alone in patients receiving MEC and HEC [12]. Olanzapine, palonosetron, and dexamethasone improved the control of nausea compared to aprepitant, palonosetron, and dexamethasone in patients receiving HEC [13]. This antiemetic regimen has been recommended by the NCCN guidelines as an option for the prevention of CINV in patients receiving HEC [21].
The National Cancer Institute recently approved a multi-institutional phase III clinical trial (Alliance A221301) for the prevention of CINV in patients receiving highly emetogenic chemotherapy using olanzapine plus standard antiemetics compared to placebo plus standard antiemetics [14]. The trial was based on substantial evidence that this drug is helpful for preventing chemotherapy-induced nausea and vomiting [11,12,13] and for treating nausea/vomiting that had occurred as a result of chemotherapy [47].This randomized, double blind, phase III trial was performed in chemotherapy naïve patients receiving cisplatin, ≥70 mg/m2, or cyclophosphamide-anthracycline-based chemotherapy, comparing olanzapine (OLN) to placebo (PLA) in combination with aprepitant (APR), a 5-HT3 receptor antagonist (5-HT3), and dexamethasone (DEX). The OLN regimen was 10 mg of oral OLN, 125 mg oral APR, a 5-HT3, and oral DEX 12 mg pre-chemotherapy, day 1, and 10 mg/day of oral OLN on days 2–4 post-chemotherapy, 80 mg oral APR, days 2, 3 post chemotherapy, and 8 mg oral DEX, days 2–4 post chemotherapy. The PLA regimen was oral placebo, day 1, and oral placebo on days 2–4 post chemotherapy, with the APR, 5-HT3, and DEX pre and post-chemotherapy being the same as in the OLN regimen. Fosaprepitant (150 mg IV), day 1 could be substituted for the oral aprepitant. Palonosetron, ondansetron, or granisetron were the permitted 5-HT3 options. Nausea was measured on a 0–10 visual analogue scale, with 0 being no nausea at all and 10 being nausea as bad as it can be.
Four hundred one patients were enrolled with 380 patients evaluable (192 patients receiving the OLN regimen and 188 patients receiving the PLA regimen). The proportion of patients with no nausea was significantly improved for the OLN regimen compared to the PLA regimen for the acute period (24 h post-chemotherapy) (74% vs. 45%, p = 0.002), for the delayed period (25–120 h post-chemotherapy) (42% vs. 25%, p = 0.002), and for the overall period (0–120 h) (37% vs. 22%, p = 0.002). Complete response (CR) (no emesis, no rescue medications) was significantly improved in OLN compared to PLA patients for the acute (86% vs. 65%, p < 0.001), the delayed (67% vs. 52%, p = 0.007), and the overall periods (64% vs. 41%, p < 0.001). There were no Grade 3 or 4 toxicities. No nausea, the primary endpoint, and complete response, a secondary endpoint, were significantly improved with OLN compared to PLA [14]. Based on the results of this study [14], the NCCN, ASCO, and MASCC/ESMO amtiemetic guidelines have recommended the use of olanzapine, a 5-HT3 receptor antagonist, a NK-1 receptor antagonist, and dexamethasone as the preferred prophylaxis for the prevention of CINV in patients receiving HEC [19,20,21].
A recent study has compared olanzapine to metoclopramide for the treatment of breakthrough emesis and nausea in patients receiving HEC and guideline-directed antiemetic prophylaxis. Olanzapine was significantly better than metoclopramide for the treatment of breakthrough emesis and nausea. This was the first phase III study on the treatment of breakthrough emesis and nausea [47]. Based on this study [47], olanzapine has been recommended for use for the treatment of breakthrough chemotherapy-induced nausea and vomiting by both the NCCN antiemetic guidelines [21] and ASCO antiemetic guidelines [20].
2.8.3 Gabapentin
Gabapentin is a gamma aminobutyric acid (GABA) analogue that has been used for the treatment of seizures, chronic neuropathic pain, CINV, and post-herpetic neuralgia [95, 96]. The mechanism of action exerted by gabapentin is unknown. Gabapentin is structurally related to the neurotransmitter GABA, but it does not interact with GABA receptors, is not converted metabolically into GABA or a GABA agonist, and is not an inhibitor of GABA uptake or degradation [96].
Guttuso et al. [97]. reported an improvement in CINV in six of nine breast cancer patients when gabapentin was used to prevent nausea. Cruz et al. [98] added gabapentin to ondansetron, dexamethasone and ranitidine to prevent CINV in patients receiving HEC. The complete response was significantly improved in the patients receiving gabapentin but nausea was not significantly improved (no nausea, overall: 62% vs. 45%).
A phase III double-blind, placebo-controlled study of gabapentin for the prevention of chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy has been reported. All patients received a 5-HT3 receptor antagonist and dexamethasone prior to chemotherapy and dexamethasone post chemotherapy. Patients were randomized to 5 days of gabapentin or placebo starting with the day of chemotherapy. In this study, gabapentin did not significantly improve delayed CINV [99].
2.8.4 Cannabinoids
Studies in animal models have suggested that delta-9-tetrahydrocannabinoid (dronabinol) selectively acts on CB1 receptors in specific regions of the dorsal vagal complex to inhibit emesis [100, 101]. A few reported studies have explored this mechanism in patients [102, 103]. Meiri et al. [102] looked at the efficacy of dronabinol versus ondansetron in patients receiving chemotherapy for a wide variety of neoplasms. Dronabinol and ondansetron were similarly effective antiemetic treatments in 61 patients receiving MEC and HEC.
Nabilone is a synthetic cannabinoid, a racemic mixture of isomers, that mimics the main ingredient of cannabis (dronabinol). A recent review of the published English literature on the use of oral nabilone in the treatment of CINV concluded that cannabinoids do not add to benefits of the 5-HT3 receptor antagonists [103].
At this time, there is insufficient data to support the routine use of dronabinol or nabilone [103,104,105] as preventative antiemetics in all chemotherapeutic regimens. Limited data suggest that dronabinol may be effective for some patients in the breakthrough CINV setting [20, 104, 105]. Further study of the scope of cannabinoid’s potential efficacy is warranted.
2.8.5 Ginger
Ginger is an herbal supplement that has been used for reducing the severity of motion sickness, pregnancy-induced nausea and post-operative nausea and vomiting [106]. The mechanism of action by which ginger might exert antiemetic effects is unclear. Animal studies have described enhanced GI transport, anti-5-hydroxytryptamine activity and possible central nervous system antiemetic effects. Human experiments to determine the mechanism of action show varying results regarding gastric motility and corpus motor response [106].
Pillai et al. [106] added ginger to ondansetron and dexamethasone in children and young adults receiving HEC and reported a reduction in the severity of acute and delayed CINV, but all patients had some nausea in days 1–4 post-chemotherapy. Zick et al. [107] reported that ginger provided no additional benefit for reduction of the prevalence or severity of acute or delayed CINV when given with 5-HT3 receptor antagonists and/or aprepitant in 162 cancer patients receiving chemotherapy. Ryan et al. [108] gave ginger before and after chemotherapy administration to 644 patients receiving a wide variety of chemotherapy regimens and found a reduction in nausea during the first day of chemotherapy.
At present, the available studies do not support ginger as an agent to recommend for the prevention of CINV. There are ongoing studies to determine if there is a role for ginger in the prevention of chemotherapy-induced nausea and vomiting [109].
3 Clinical Management of CINV
3.1 Principles in the Management of CINV
International antiemetic guidelines [19,20,21] form the basis for the recommendations for the management of CINV. As new information and new studies emerge, these guidelines will evolve to provide the highest quality evidence-based clinical practice.
3.1.1 Single-Day Chemotherapy (Table 46.13)
For patients receiving HEC, current evidence suggests the following [19,20,21].
-
Pre-chemotherapy—olanzapine with any of the 5-HT3 receptor antagonists, plus an NK-1 receptor antagonist plus dexamethasone The guidelines suggest that the combination of cyclophosphamide and doxorubicin should be considered as HEC and the appropriate preventative agents should be used.
-
Post-chemotherapy—olanzapine with or without dexamethasone or dexamethasone alone.
For patients receiving MEC, current evidence suggests the following [19,20,21].
-
Pre-chemotherapy—the 5-HT3 receptor antagonist palonosetron plus dexamethasone. If palonosetron is not available, ondansetron or granisetron may be employed.
-
Post-chemotherapy—dexamethasone on days 2–4.
Antiemetic guidelines of the past have included the available oral first-generation 5-HT3 receptor antagonists as optional therapy for the prevention of delayed emesis, but the level of evidence supporting this practice is low [34, 110]. The first-generation 5-HT3 receptor antagonists are no longer recommended for use post-chemotherapy [19,20,21].
For patients receiving low emetogenic chemotherapy, a single agent in the form of a 5-HT3 receptor antagonist, dexamethasone, or a phenothiazine, depending on the clinical situation, should be used pre-chemotherapy, and an antiemetic following chemotherapy should be given only as needed.
3.1.2 Treatment of Breakthrough CINV
Phenothiazine, metoclopramide, dexamethasone or olanzapine may be effective in the treatment of breakthrough nausea and vomiting [21]. A 5-HT3 receptor antagonist may also be effective unless a patient presents with nausea and vomiting that developed following the use of a 5-HT3 receptor antagonist as prophylaxis for chemotherapy or radiotherapy-induced emesis. It is very unlikely that breakthrough nausea and vomiting will respond to an agent in the same drug class after unsuccessful prophylaxis with an agent with the same mechanism of action.
Patients who develop nausea or vomiting post-chemotherapy (days 1–5) despite adequate prophylaxis should be considered for treatment with a regimen of 3 days of oral or sublingual olanzapine or oral metoclopramide. A recently completed phase III study demonstrated that oral olanzapine (10 mg/day for 3 days) was significantly better than oral metoclopramide (10 mg three times daily for 3 days) in controlling both emesis and nausea in patients receiving HEC who developed breakthrough CINV despite guideline-directed prophylactic antiemetics [20, 47].
It is important to note that aprepitant has been approved as an additive agent to a 5-HT3 receptor antagonist and dexamethasone for the prevention of CINV. It has not been studied and should not be used to treat breakthrough nausea and vomiting.
3.1.3 Refractory CINV
Patients who develop CINV during subsequent cycles of chemotherapy when antiemetic prophylaxis has not been successful in controlling CINV in earlier cycles should be considered for a change in the prophylactic antiemetic regimen. If anxiety is considered to be a major patient factor in the CINV, a benzodiazepine such as lorazepam or aprazolam can be added to the prophylactic regimen. If the patient is receiving HEC, olanzapine (days 1–4) may be added to a prophylactic regimen of a 5-HT3 receptor antagonist, a NK-1 receptor antagonist, and dexamethasone [14, 19,20,21] or substituted for a NK-1 receptor antagonist in combination with a 5-HT3 receptor antagonists and dexamethasone [13, 21]. If the patient is receiving MEC, an NK-1 receptor antagonist may be added to a palonosetron and dexamethasone antiemetic regimen [21].
3.1.4 Anticipatory CINV
In order to prevent the occurrence of anticipatory CINV, patients should be counseled prior to the initial course of treatment concerning their ‘expectations’ of CINV. Patients should be informed that very effective prophylactic antiemetic regimens will be used and that 70–75% of patients will have a complete response (no emesis, no use of rescue medications). Patients risk factors for CINV should be carefully evaluated, and the most effective prophylactic antiemetic regimen for the patient’s specific type of chemotherapy should be used prior to the first course of chemotherapy in order to obtain the optimum control of CINV during the first course of chemotherapy. If CINV is effectively controlled during the first chemotherapy cycle, it is likely that the patient will have effective control during subsequent cycles of the same chemotherapy. If the patient has a poor experience with CINV in the first cycle, it may be more difficult to control CINV in subsequent chemotherapy cycles, and refractory and/or anticipatory CINV may occur. The use of anti-anxiety medications such as lorazepam or another benzodiazepine may be considered for excess anxiety prior to the first course of chemotherapy in order to obtain an optimum outcome and prevent anticipatory CINV. If anticipatory CINV occurs despite the use of prophylactic antiemetics, behavioural therapy might be considered [111, 112].
3.1.5 Multi-Day Chemotherapy and High-Dose Chemotherapy with Stem Cell or Bone Marrow Transplantation
The success of the use of NK-1 receptor antagonists with 5-HT3 receptor antagonists and dexamethasone in preventing emesis in patients receiving single day highly emetogenic chemotherapy [3, 6] prompted the use of the NK-1 receptor antagonist aprepitant combined with a 5-HT3 receptor antagonist and dexamethasone in patients receiving multi-day, high dose chemotherapy prior to SCT. A number of Phase II and III studies have been reported with the use of the NK-1 aprepitant added to a 5-HT3 receptor antagonists and dexamethasone [60,61,62,63,64,65,66] in patients receiving multi-day, high dose chemotherapy prior to autologous or allogeneic stem cell transplant (SCT). As a result of these studies, the 2017 ASCO and the 2017 MASCC/ESMO antiemetic guidelines have recommended the use of a NK-1 receptor antagonist, a 5-HT3 receptor antagonist, and dexamethasone as the preferred prophylaxis for patients receiving high-dose, multi-day chemotherapy prior to autologous or allogeneic stem cell transplantation [20, 67]. The recent studies [60,61,62,63,64,65,66] have demonstrated that the addition of aprepitant to a 5-HT3 receptor antagonist and dexamethasone resulted in improved control of emesis post chemotherapy, but not nausea. The control of nausea remains a significant patient problem, not only in multi-day, high-dose chemotherapy, but also in single day highly emetogenic chemotherapy. Neither 5-HT3 receptor antagonists, nor the NK-1 receptor antagonists appear to be effective anti-nausea agents in the post chemotherapy period [3, 6, 14, 16].
4 Prevention and Treatment of Nausea
The current data in the literature from multiple large studies suggest that the first or second-generation 5-HT3 receptor antagonists and the NK-1 receptor antagonists have not been effective in the control of nausea in patients receiving either MEC or HEC, despite the marked improvement in the control of emesis with these agents [16,17,18]. It appears that neither the serotonin nor the substance P receptors may be important in mediating nausea. Phase III studies with olanzapine have demonstrated very good control of both emesis and nausea in patients receiving either MEC or HEC [12,13,14]. Preliminary small studies with gabapentin, cannabinoids and ginger are inconclusive in defining their role, if any, in the prevention of nausea. At this time, olanzapine appears to have high potential for the prevention of both emesis and nausea in patients receiving MEC or HEC [12,13,14]. If patients are having difficulty with significant nausea, consideration should be given to including olanzapine in their prophylactic antiemetic regimen [12,13,14]. Olanzapine may also be efficacious in the treatment of breakthrough nausea [47].
5 Conclusions and Future Directions
The introduction of the 5-HT3 receptor antagonists combined with the use of dexamethasone significantly improved the prevention of acute emesis in patients receiving MEC or HEC. The 5-HT3 receptor antagonists have been safe and well tolerated with a minority of patients experiencing a mild headache, mild diarrhea, or mild constipation. There have been concerns with the potential of the prolongation of the QT interval with the use of the 5-HT3 receptor antagonists. These concerns have resulted in the FDA recommendations of discontinuing the use of dolasetron for the prevention of CINV and a restriction of the higher intravenous doses of ondansetron. Granisetron and palonosetron appear to have much less potential for prolongation of the QT interval with no restrictions by the FDA on their use. The prevention of chemotherapy-induced nausea and emesis in the delayed period have not been effective with the use of the first generation 5-HT3 receptor antagonists, and they are no longer recommended for use as prophylaxis in the delayed period The second generation 5-HT3 receptor antagonist palonosetron may be more effective in the prevention of nausea and emesis in the delayed period.
Dexamethasone has improved the control of CINV in the acute and delayed periods when used in combination with other antiemetics. Patients have experienced insomnia and varying degrees of gastric irritability with the use of dexamethasone. Some studies have demonstrated effective prevention of CINV in patients receiving MEC or the combination of an anthracycline and cyclophosphamide chemotherapy with the use of palonosetron plus 1 day versus 3 days of dexamethasone. In addition, studies have demonstrated that olanzapine, palonosetron, and 1 day of dexamethasone may be effective in the prevention of CINV in patients receiving HEC.
The use of the NK-1 receptor antagonists in combination the 5-HT3 receptor antagonists and dexamethasone has significantly improved the control of emesis in the acute and delayed phase in patients receiving HEC. Aprepitant, fosaprepitant, netupitant, and rolapitant have been shown to be safe and effective in phase III clinical trials with few adverse events. Aprepitant, fosaprepitant, and netupitant are metabolized by the liver enzyme CYP3A4 and are moderate inhibitors of CYP3A4, potentially resulting in drug interactions. There have been few, if any, clinical adverse events attributable to CYP3A interactions with these NK-1 receptor antagonists. Rolapitant does not induce CYP3A4.
Olanzapine, a US FDA approved antipsychotic, has been shown to be safe and effective in preventing both nausea and emesis in patients receiving MEC or HEC. With the exception of mild sedation, which appears to be well tolerated, there have been no reported adverse events associated with the use of olanzapine on the day of chemotherapy or days 2–4 post chemotherapy. Olanzapine also appears to be an effective agent in the treatment of breakthrough emesis and nausea.
Recent phase III studies have demonstrated that the addition of aprepitant to a 5-HT3 receptor antagonist and dexamethasone has improved the control on emesis in patients received high dose, multi-day chemotherapy prior to stem cell transplant. The recent updated antiemetic guidelines have recommended the use of this three drug regimen for high dose, multi-day chemotherapy.
Oncology practitioners currently have very effective antiemetic agents for the prevention of CINV in patients receiving MEC or HEC. The choice of individual agents and the combination of agents should be dictated by the emetogenicity of the chemotherapy that is to be administered and patient risk factors. Antiemetic choices should be guided by the international antiemetic guidelines. The available agents for the prevention of CINV appear to be safe and effective with few reported adverse events.
The first generation 5-HT3 receptor antagonists ondansetron and granisetron have similar efficacy and compete only on an economic basis. Both are available as generics. The second generation 5-HT3 receptor antagonist palonosetron is the recommended 5-HT3 receptor antagonist by some of the international guidelines; it is not yet available in generic form. When used in the recommended doses, these agents should be safe with few adverse events.
Dexamethasone should be used in conjunction with the 5-HT3 receptor antagonists, and consideration should be given to using it on the day of chemotherapy only in conjunction with other effective antiemetics to minimize any adverse events.
At present, there is only one definitive published clinical trial reporting a direct comparison of the efficacy and safety of the various NK-1 receptor antagonists (aprepitant, fosaprepitant, cinvanti, netupitant, rolapitant). Zhang et al. reported a phase III randomized, double-blind clinical trial in patients receiving cisplatin-based chemotherapy [113] in which 828 patients were randomized to receive NEPA plus dexamethasone or aprepitant, granisetron and dexamethasone. The primary endpoint of complete response (no emesis, no rescue) demonstrated that there was no difference in the two regimens, both of which were well tolerated.
There are some pharmacokinetic differences between rolapitant and the other commercially available, oral NK-1 receptor antagonists. Rolapitant has a longer half-life (180 h) than aprepitant (9–13 h) and netupitant (90 h) which may be important in multiple-day chemotherapy clinical settings. Future studies may determine if this may be an important clinical issue.
Rolapitant does not induce or inhibit CYP3A4, unlike the other NK-1 receptor antagonists, aprepitant and netupitant. Among the class of NK-1 receptor antagonists, this unique feature corresponds to a reduced propensity of drug interactions that may decrease the need for dose modifications of other drugs metabolized by CYP3A4 when administered concomitantly with rolapitant. A rolapitant antiemetic regimen may simplify medical management of some oncology patients, who may be receiving multiple medications.
Based on the available clinical trial data, the NK-1 receptor antagonists have significantly improved the prevention of acute and delayed emesis in patients receiving HEC and have few adverse events. There is little evidence, however, that these agents are effective in controlling nausea. Although there appear to be other NK-1 receptor antagonists in development, there does not appear to be any which are pending regulatory approval in the near future.
Olanzapine appears to an effective agent in the control of emesis and nausea when combined with other antiemetic agents. At present, olanzapine appears to be the only current effective agent for the control of nausea. Nausea appears to be an important and prevalent clinical issue, despite the control of emesis with the 5-HT3 receptor antagonists, dexamethasone, and the NK-1 receptor antagonists. When used for a period of 4 days (pre- and post- chemotherapy), olanzapine is associated with only mild sedation.
The current antiemetics that are recommended by the various international antiemetic guidelines are safe and effective in the prevention of chemotherapy-induced nausea and vomiting when used in the recommended doses. These guidelines should be followed by practitioners in order to provide the highest possible quality of care for patients receiving chemotherapy.
Clinical Cases
Case Study 1:
-
Patient is a 65-year-old man, former 2 pack/d smoker who quit 1 year ago. He sees his primary care physician for a cough that has lasted 4 weeks
-
Chest X-ray reveals a poorly differentiated mass confined to the upper right lobe, and a CT/PET scan shows a tumor measuring 4.5 × 2.0 cm and possible intrapulmonary lymph node involvement, with no evidence of distant metastasis
-
Surgical resection with mediastinal lymph node dissection is performed. Pathology reveals stage II adenocarcinoma
-
After discussion of adjuvant chemotherapy options with the treatment team, a regimen of paclitaxel and carboplatin is selected. Although a cisplatin regimen would be first choice for most patients at this stage, it is contraindicated in this patient because he has moderate bilateral hearing impairment
-
Adjuvant therapy regimen:
-
Paclitaxel 200 mg/m2 IV over 3 h
-
Carboplatin AUC 6 mg/mL/min IV over 45–60 min
-
Repeat every 21 days for 4 cycles
Which CINV prophylactic regimen would you recommend?
-
Palonosetron/dexamethasone
-
Netupitant/palonosetron/dexamethasone
-
Prochlorperazine
-
Metoclopramide
-
NK-1 RA + 5-HT3 + Dex + Olanzapine
Is this chemotherapy regimen moderately or highly emetogenic chemotherapy?
-
Case Study 2:
-
48-year-old mother of 3 diagnosed with invasive ductal carcinoma, HER2−/ER-/PR- tumor
-
Underwent a lumpectomy and axillary dissection
-
Histopathology revealed 3-cm primary tumor and involvement in 3 of 18 lymph nodes
-
Agrees to a “dose-dense” doxorubicin/cyclophosphamide followed by paclitaxel and radiation therapy
-
Risk factors for emesis include hyperemesis of pregnancy, low alcohol intake
-
Receives ondansetron/aprepitant/dexamethasone for prophylaxis and dexamethasone for Days 2 and 3
-
Develops nausea and vomiting (Breakthrough CINV) on Day 4 after chemotherapy
-
How would you treat the Breakthrough CINV?
-
IV fluids
-
Dexamethasone
-
Olanzapine
-
All of the above
-
How would you modify the patient’s antiemetic regimen for the next chemotherapy cycle?
-
Add dolasetron
-
Add olanzapine
-
Add fosaprepitant
-
None of the above
-
Case Study 3:
-
55-year-old woman with advanced colorectal cancer
-
Social history: Former smoker, nondrinker
-
Medical history: Currently receiving treatment for hypertension, dyslipidemia, and insomnia
-
Chemotherapy regimen: FOLFOX + bevacizumab IV every 14 days
-
Scheduled for second cycle of chemotherapy, but experienced nausea/vomiting several days after initiation of first cycle
-
Antiemetic prophylaxis with ondansetron and dexamethasone
-
What is the best option to improve the patient’s control of CINV in cycle 2 of FOLFOX?
-
Switch ondansetron to granisetron
-
Increase prochlorperazine dosing in the delayed phase
-
Add fosaprepitant to the prophylactic regimen
-
Administer olanzapine as a rescue medicine
-
Both c and d
-
Abbreviations
- ASCO:
-
American Society of Clinical Oncology
- CINV:
-
Chemotherapy-induced nausea and vomiting
- CTZ:
-
Chemoreceptor trigger zone
- FDA:
-
Food & Drug Administration
- GI:
-
Gastrointestinal
- 5-HT3:
-
5-hydroxytryptamine-3
- HEC:
-
Highly emetogenic chemotherapy
- MEC:
-
Moderately emetogenic chemotherapy
- MASCC:
-
Multinational Association of Supportive Care in Cancer
- NCCN:
-
National Comprehensive Cancer Network
- NTS:
-
Nucleus Tractus solitarius
- NK-1:
-
Neurokinin-1
- VAS:
-
Visual analogue scale
- VC:
-
Vomiting Centre
References
Bloechl-Daum B et al (2006) Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol 24:4472–4478
Cohen L et al (2007) Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer 15:497–503
Navari RM, Aapro M (2016) Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med 374:1356–1367
Grunberg SM (2004) Chemotherapy-induced nausea and vomiting: prevention, detection, and treatment—how are we doing? J Support Oncol 2:1–10
Broder MS et al (2014) The impact of 5HT3RA use on cost and utilization in patients with chemotherapy-induced nausea and vomiting: systematic review of the literature. Am Health Drug Benefits 7:171–182
Navari RM (2013) Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs 73:249–262
Navari RM (2014) Palonosetron for the treatment of chemotherapy-induced nausea and vomiting. Expert Opin Pharmacother 15:2599–2608
Aapro M, Carides A, Rapoport BL (2015) Aprepitant and fosaprepitant: a ten-year review of efficacy and safety. Oncologist 20:450–458
Navari RM (2015) Profile of netupitant/palonosetron fixed dose combination (NEPA) and its potential in the treatment of chemotherapy-induced nausea and vomiting (CINV). Drug Des Devel Ther 9:155–161
Navari RM (2015) Rolapitant for the treatment of chemotherapy induced nausea and vomiting. Expert Rev Anticancer Ther 15:1127–1133
Navari RM et al (2007) A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting. Support Care Cancer 15:1285–1291
Tan L et al (2009) Clinical research of olanzapine for the prevention of chemotherapy-induced nausea and vomiting. J Exp Clin Cancer Res 28:1–7
Navari RM, Gray SE, Kerr AC (2011) Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 9:188–195
Navari RM et al (2016) Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med 375:134–142
Mukhopadhyay S et al (2017) Role of olanzapine in chemotherapy-induced nausea and vomiting on platinum-based chemotherapy patients: a randomized controlled study. Support Care Cancer 25:145–154
Navari RM (2012) Treatment of chemotherapy-induced nausea. Commun Oncol 9:20–26
Ng TL, Hutton B, Clemons M (2015) Chemotherapy-induced nausea and vomiting: time for more emphasis on nausea? Oncologist 20:576–583
Stern RM, Koch KL, Andrews PLR (2011) Nausea: mechanisms and management. Oxford University Press, New York
Molassiotis A et al (2017) MASCC/ESMO antiemetic guidelines: introduction to the 2016 guideline update. Support Care Cancer 25:267
Hesketh PJ et al (2017) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 35:3240–3261
NCCN Clinical Practice Guidelines in Oncology version 2.2017. Antiemesis. National Comprehensive Cancer Network (NCCN) [online]. Available from URL: http://www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf. Accessed June, 2017
Koga T, Fukuda H (1992) Neurons in the nucleus of the solitary tract mediating inputs from Vagal afferents and the area postrema in the pattern generator in the emetic act in dogs. Neurosci Res 14:366–379
Yates BJ et al (1994) Organization of the vestibular inputs to nucleus tractus solitarius and adjacent structures in cat brain stem. Am J Phys 267:974–983
Simpson K, Spencer CM, McClellan KJ (2000) Topisetron: an update of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs 59:1297–1315
Kimura E et al (1996) Study on clinical effect of a continuous intravenous infusion of azasetron against nausea and vomiting induced by anticancer drugs including CDDP. Gan To Kagaku Ryoho 23:477–481
Taguchi T et al (1999) Usefulness of ramosetron hydrochloride on nausea and vomiting in CMF or CEF therapy for breast cancer. Gan To Kagaku Ryoho 26:1163–1170
Hesketh PJ (2000) Comparative review of 5-HT3 receptor antagonists in the treatment of acute chemotherapy-induced nausea and vomiting. Cancer Investig 18:163–173
World Health Organization (2006) Dolasetron mesylate and serious cardiovascular reactions, vol 20. WHO Drug Information, p 185
U.S. Food and Drug Information. FDA drug safety communication: abnormal heart rhythms associated with use of anzemet (dolasetron mesylate) [online]. Available from URL: http://www.fda.gov/Drugs.DrugSafety/usm237081.htm. Accessed 15 June 2017
Ondansetron [online], Available from URL: www.drugs.com/fda/ondansetron. Accessed 15 June July 2017
Mason JW et al (2014) A randomized, placebo-controlled, four-period cross-over definitive QT study of the effects of APF530 exposure, high-dose intravenous granisetron, and moxifloxacin on QTC prolongation. Cancer Manag Res 6:183–189
Roila F et al (2005) Delayed emesis: moderately emetogenic chemotherapy. Support Care Cancer 13:104–108
Geling O, Eichler H (2005) Should 5-Hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol 23:1289–1294
Hickok JT et al (2005) 5-HT3 receptor antagonists versus perchlorperazine for control of delayed nausea caused by doxorubicin: a URCC CCOP randomized controlled trial. Lancet Oncol 6:765–772
Saito M et al (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for the prevention of nausea and vomiting during chemotherapy: a double-blind, double dummy, randomized, comparative phase III trial. Lancet Oncol 10:115–124
Warr DG et al (2005) Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 23:2822–2830
Boccia RV et al (2011) Efficacy and tolerability of transdermal granisetron for the control of chemotherapy-induced nausea and vomiting associated with moderately and highly emetogenic multi-day chemotherapy: a randomized, double-blind, phase III study. Support Care Cancer 19:1609–1617
Raftopoulos H et al (2015) Comparison of an extended-release formulation of granisetron (APF530) versus palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: results of a prospective, randomized, double blind, noninferiority phase III trial. Support Care Cancer 23:723–732
Eisenberg P et al (2004) Efficacy, safety, and pharmacokinetics of palonosetron in patients receiving highly emetogenic, cisplatin-based chemotherapy: a dose-ranging, clinical study. Ann Oncol 15:330–337
Rojas C et al (2010) Palonosetron triggers 5-HT3 receptor internalization and causes prolonged inhibition of receptor function. J Pharmacol 626:193–199
Botrel T et al (2011) Efficacy of palonosetron compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic treatment: systematic review and meta-analysis. Support Care Cancer 19:823–832
Schwartzberg L et al (2014) Pooled analysis of phase III studies of palonosetron versus ondansetron, dolasetron, and granisetron in the prevention of chemotherapy-induced nausea and vomiting. Support Care Cancer 22:469–477
Popovic M et al (2014) Efficacy and safety of palonosetron for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer 22:1485–1497
Yavas C et al (2012) Acute effect of palonosetron electrocardiographic parameters in cancer patients; a prospective study. Support Care Cancer 20:2343–2347
Aogi K et al (2012) A phase III open-label study to assess safety and efficacy of palonosetron for preventing chemotherapy-induced nausea and vomiting (CINV) in repeated cycles of emetogenic chemotherapy. Support Care Cancer 20:1507–1514
Roila F et al (2014) Aprepitant versus metoclopramide, both combined with dexamethasone, for the preventing cisplatin-induced delayed emesis: a randomized, double-blind study. J Clin Oncol 32(5s (suppl)):abstr 9503
Navari RM, Nagy CK, Gray SE (2013) Olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer 21:1655–1663
European Medicine Agency Metoclopramide use recommendations, 26 July 2013. www.ema.europa.eu. Accessed 15 June 2017
Diemunsch P, Grelot L (2000) Potential of substance P antagonists as antiemetics. Drugs 60:533–546
Sankhala KK et al (2009) Prevention of chemotherapy induced nausea and vomiting: a focus on aprepitant. Expert Opin Drug Metab Toxicol 12:1607–1614
Hesketh PJ et al (2006) Combined data from two phase III trials of the NK-1 antagonist aprepitant plus a 5HT3 antagonist and a corticosteroid for prevention of chemotherapy-induced nausea and vomiting: effect of gender on treatment response. Support Care Cancer 14:354–360
Warr DG et al (2005) The oral NK1 antagonist aprepitant for the prevention of acute and delayed chemotherapy-induced nausea and vomiting: pooled data from two randomized, double-blind, placebo controlled trials. Eur J Cancer 41:1278–1285
Grote T et al (2006) Combination therapy for chemotherapy-induced nausea and vomiting in patients receiving moderately emetogenic chemotherapy: palonosetron, dexamethasone, and aprepitant. J Support Oncol 4:403–408
Navari RM (2007) Fosaprepitant (MK-0517): a neurokinin-1 receptor antagonist for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin Investig Drugs 16:1977–1985
Grunberg S et al (2011) Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: randomized, double-blind study protocol—EASE. J Clin Oncol 29:1495–1501
Leal AD et al (2014) Fosaprepitant-induced phlebitis: a focus on patients receiving doxorubicin/cyclophosphamide therapy. Support Care Cancer 22:1313–1317
Drug interactions of aprepitant [slide presentation]. Available at http://www.fda.gov/ohrms/dockets/ac/03/slides/3928s1_03_fda-jarugula.ppt. Accessed 9 June 2017
Scientific discussion: this module reflects the initial scientific discussion for the approval of Emend. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-Scientific_Discussion/human/000527/WC500026534.pdf. Accessed 9 June 2017
Aapro MS, Walko CM (2010) Aprepitant: drug-drug interactions in perspective. Ann Oncol 21:2316–2323
Schmitt T et al (2014) Aprepitant, granisetron, and dexamethasone for prevention of chemotherapy-induced nausea and vomiting after high-dose melphalan in autologous transplantation for multiple myeloma: results of a randomized, placebo-controlled phase III trial. J Clin Oncol 32:3413–3420
Pielichowski W et al (2011) A triple-drug combination to prevent nausea and vomiting following BEAM chemotherapy before autologous hematopoietic stem cell transplantation. Transplant Proc 43:3107–3110
Stiff PJ et al (2013) Prevention of nausea and vomiting associated with stem cell transplant: results of a prospective, randomized trial of aprepitant used with highly emetogenic preparative regimens. Biol Blood Marrow Transplant 19:49–55
Svanberg A, Bergegard C (2015) Addition of aprepitant to standard antiemetic regimen continued for seven days after chemotherapy for stem cell transplantation provide significant reduction of vomiting. Oncologia 89:31–36
Uchida M et al (2013) Effectiveness and safety of antiemetic aprepitant in Japanese patients receiving high-dose chemotherapy prior to autologous hematopoietic stem cell transplantation. Biol Pharm Bull 36:819–824
Sakuri M et al (2014) Efficacy of aprepitant in preventing nausea and vomiting due to high-dose melphalan–based conditioning for allogeneic hematopoietic stem cell transplantation. Int J Hematol 99:457–462
Bechtel T et al (2014) Aprepitant for the control of delayed nausea and vomiting associated with the use of high-dose melphalan for autologous peripheral blood stem cell transplants in patients with multiple myeloma: a phase II study. Support Care Cancer 22:2911–2916
Einhorn LH et al (2017) 2016 updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following chemotherapy, and breakthrough nausea and vomiting. Support Care Cancer 25:303–308
Ottoboni T et al (2017) Bioequivalence of HTX-019 (aprepitant IV)(Cinvanti) and fosaprepitant in healthy subjects. Hematology/Oncology Pharmacy Association Annual Conference, March 29–April 1, 2017, Anaheim, CA
Rizzi A et al (2012) In vitro and in vivo pharmacological characterization of the novel NK-1 receptor selective antagonist Netupitant. Peptides 37:86–97
Spinelli T, Calcagneli S, Giuliano C (2014) Netupitant PET imaging and ADME studies in humans. J Clin Pharmacol 54:97–108
National Cancer Institute Drug Dictionary. Available from http://www.cancer.gov/drugdictionary. Accessed 15 June 2017
Hesketh PJ et al (2014) Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol 25:1340–1346
Aapro M et al (2014) A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol 25:1328–1333
Gralla RJ et al (2014) A phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol 25:1333–1339
FDA News Release. FDA approves Akynzeo for nausea and vomiting associated with cancer chemotherapy. Available from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements. Accessed 9 June 2017
Duffy RA et al (2012) Rolapitant (SCH 619734): a potent, selective and orally active neurokinin NK-1 receptor antagonist with centrally-mediated antiemetic effects in ferrets. Pharmacol Biochem Behav 102:95–100
Poma A et al (2013) Rolapitant and its major metabolite do not affect the pharmacokinetics of midazolam, a sensitive cytochrome P450 3A4 substrate. Support Care Cancer 21:S154. Abstract 441
Poma A et al (2014) Phase 1 positron emission tomography (PET) study of the receptor occupancy of rolapitant, a novel NK-1 receptor antagonist. J Clin Oncol 32:abstr e20690
Rapoport B et al (2015) Study of rolapitant, a novel, long acting, NK-1 receptor antagonist, for the prevention of chemotherapy-induced nausea and vomiting (CINV) due to highly emetogenic chemotherapy (HEC). Support Care Cancer 23:3281–3288
Schwartzberg L et al (2015) Safety and efficacy assessment of rolapitant for the prevention of chemotherapy-induced nausea and vomiting following administration of moderately emetogenic chemotherapy in cancer patients in a randomized phase 3 trial. Lancet Oncol 16:1071–1078
Rapoport B et al (2015) Safety and efficacy assessment of rolapitant for the prevention of chemotherapy-induced nausea and vomiting following administration of cisplatin-based highly emetogenic chemotherapy in cancer patients in two randomized phase 3 trials. Lancet Oncol 16:1079–1089
dos Santos LV et al (2012) Neurokinin-1 receptor antagonists for chemotherapy-induced nausea and vomiting: a systematic review. J Natl Cancer Inst 104:1280–1292
Zhang Y et al (2016) Neurokinin-1 receptor antagonist-based triple regimens in preventing chemotherapy-induced nausea and vomiting: a network meta-analysis. J Natl Cancer Inst 109:djw217. https://doi.org/10.1093/jnci/djw217
Vardy J et al Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer 1999, 94:1011–1015
Celio L et al (2011) Palonosetron in combination with 1-day versus 3-day dexamethasone for prevention of nausea and vomiting following moderately emetogenic chemotherapy: a randomized, multi-center, phase III trial. Support Care Cancer 19:1217–1225
Aapro M et al (2010) Double-blind, randomized, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol 21:1083–1088
Fulton B, Goa KL (1999) Olanzapine: a review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs 53:281–298
Kando JC et al (1997) Olanzapine: a new antipsychotic agent with efficacy in the management of schizophrenia. Ann Pharmacother 31:1325–1334
Bymaster FP et al (1996) Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 14:87–96
Stephenson CME, Pilowski LS (1999) Psychopharmacology of olanzapine: a review. Br J Psychiatry 174:52–58
Petersen AB et al (2014) Adverse effects associated with high dose therapy in patients admitted to inpatient psychiatric care. Clin Toxicol 52:39–43
Passik S et al (2004) A phase I trial of olanzapine (Zyprexa) for the prevention of delayed emesis in cancer patients receiving chemotherapy. Cancer Investig 22:383–388
Goldstein LE et al (1999) New-onset diabetes mellitus and diabetic ketoacidosis associated with olanzapine treatment. Psychosomatics 40:438–443
Mizukami N et al (2014) Olanzapine for the prevention of chemotherapy-induced nausea and vomiting in patients receiving highly or moderately emetogenic chemotherapy: a randomized, double-blind placebo-controlled study. J Pain Symptom Manag 47:542–550
Davis MP (2016) New therapies for antiemetic prophylaxis for chemotherapy. J Community Support Oncol 14:11–20
Irving G et al (2009) Efficacy and tolerability of gastric-retentive gabapentin for the treatment of post-herpetic neuralgia: results of a double-blind, randomized, placebo-controlled clinical trial. Clin J Pain 25:185–192
Guttuso T, Roscoe J, Griggs J (2003) Effect of gabapentin on nausea induced by chemotherapy in patients with breast cancer. Lancet 361:1703–1705
Cruz FM et al (2011) Gabapentin for the prevention of chemotherapy-induced nausea and vomiting: a pilot study. Support Care Cancer 20:601–606
Barton DL et al (2014) Phase III double-blind, placebo-controlled study of gabapentin for the prevention of delayed chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy, NCCTG N08C3 (Alliance). Cancer 120:3575–3583
Van Sickle MD et al (2005) Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310:329–332
Darmani NA (2001) Delta-9-tetrahydrocannabinol differentially supresses cisplatin-induced emesis and indices of motor function via cannabinoid CB1 receptors in the least shrew. Pharmacol Biochem Behav 69:239–249
Meiri E et al (2007) Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin 23:533–543
Davis MP (2008) Oral nabilone capsules in the treatment of chemotherapy-induced nausea and vomiting and pain. Expert Opin Investig Drugs 17:85–95
May MB, Grode AE (2016) Dronabinaol for chemotherapy-induced nausea and vomiting unresponsive to antiemetics. Cancer Manag Res 8:49–55
Badowski ML (2017) A review of oral cannabinoids and medical marijuana for the treatment of CINV: a focus on pharmacokinetic variability and pharmacodynamics. Cancer Chemother Pharmacol 80:441–449
Pillai AK et al (2011) Anti-emetic effect of ginger powder versus placebo as an add-on therapy in children and young adults receiving highly emetogenic chemotherapy. Pediatr Blood Cancer 56:234–238
Zick SM et al (2009) Phase II trial of encapsulated ginger as a treatment for chemotherapy-induced nausea and vomiting. Support Care Cancer 17:563–572
Ryan JL et al (2012) Ginger reduces acute chemotherapy-induced nausea: a URCC CCOP study of 576 patients. Support Care Cancer 20:1479–1489
Bossi P et al (2016) Searching for evidence to support the use of ginger in the prevention of chemotherapy-induced nausea and vomiting. J Altern Complement Med 22:486–488
Geling O, Eichler H (2005) Should 5-Hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol 23:1289–1294
Navari RM (2016) Should behavioral therapy be used as the primary treatment to control anticipatory vomiting? HemeOnc Today 17:13
Navari RM (2017) Cancer patients at high risk for chemotherapy-induced nausea and vomiting – prediction assessments/tools. Expert Rev Qual Cancer Care 2:102–108
Zhang L et al (2017) A randomized phase 3 study evaluating the efficacy of single-dose NEPA, a fixed antiemetic combination of netupitant and palonosetron, versus an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly Emetogenic chemotherapy (HEC). Ann Oncol 29:452–458. https://doi.org/10.1093/annonc/mdx698
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Navari, R.M. (2019). Chemotherapy Induced Nausea and Vomiting. In: De Mello, R., Mountzios, G., Tavares, Á. (eds) International Manual of Oncology Practice. Springer, Cham. https://doi.org/10.1007/978-3-030-16245-0_46
Download citation
DOI: https://doi.org/10.1007/978-3-030-16245-0_46
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16244-3
Online ISBN: 978-3-030-16245-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)