Abstract
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is a distressing side effect that affects many patients undergoing emetogenic chemotherapy, despite the use of antiemetic medications. The purpose of this trial was to evaluate the efficacy and safety of gabapentin for the prevention of CINV during the first cycle of treatment in patients receiving moderately or highly emetogenic chemotherapy.
Methods
Eighty chemotherapy-naive patients, scheduled to receive moderately and highly emetogenic chemotherapy, were enrolled in this randomised, double-blind, placebo-controlled clinical trial. All patients received intravenous ondansetron 8 mg, dexamethasone 10 mg and ranitidine 50 mg before chemotherapy on day 1 and oral dexamethasone 4 mg twice a day on days 2 and 3. Patients were randomly assigned to take gabapentin 300 mg or placebo on the following schedule: 5 and 4 days before chemotherapy once daily, 3 and 2 days before chemotherapy twice daily, 1 day before to 5 days after chemotherapy thrice daily. The primary endpoint was complete overall protection from both vomiting and nausea over the course of the entire study (day 1 through day 5), and complete protection during the delayed period (24–120 h after chemotherapy).
Results
The proportion of patients achieving complete response improved from 40% to 62.5%, (p = 0.04) when comparing the control group and the gabapentin group, respectively. In the subset of patients who achieved complete control in the acute phase, the percentage of patients who achieved delayed complete control was higher in the gabapentin group (89.3 × 60.7%, p = 0.01). Adverse events did not significantly differ between study arms.
Conclusions
Gabapentin is a low-cost strategy to improve complete control of CINV, specially delayed CINV control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nausea and vomiting continue to be significant adverse effects of cancer treatment. They negatively affect patients' nutritional habits, ability to work and motivation to follow recommended antineoplastic treatment regimens [1].
Early attempts to control chemotherapy-induced nausea and vomiting (CINV) included corticosteroids and selective 5-hydroxytrytamine 3 (5-HT3) receptor antagonists [2–4]. The efficacy of 5-HT3 receptor antagonists is significantly improved when they are combined with corticosteroids [5, 6]. First-generation 5-HT3 receptor antagonists, such as ondansetron and granisetron, appear to be equally effective at preventing CINV at recommended doses [7, 8]. There is some evidence to suggest that palonosetron, a second-generation 5-HT3 receptor antagonist, is superior to first-generation 5-HT3 antagonists for the treatment of delayed emesis due to cisplatin-based chemotherapy [8–10].
Although significant progress has been made, the control of delayed emesis, defined by its occurrence more than 24 h after chemotherapy, remains an important challenge. In fact, some studies demonstrated that the addition of neurokinin-1 (NK-1) antagonists, including aprepitant and fosaprepitant, to corticosteroids and 5-HT3 receptor antagonists provides superior prevention of delayed CINV in patients receiving highly and moderately emetogenic chemotherapy [11–16]. However, the high cost of NK-1 antagonists and palonosetron precludes the wider use of these drugs in developing countries. Therefore, it is important to discover lower-cost strategies to improve CINV control.
Gabapentin is a y-aminobutyric acid analogue with an established history in treating epilepsy, chronic neuropathic pain, postoperative pain and postherpetic neuralgia [17–19]. There is only limited evidence of this drug's activity as an antiemetic agent. Two trials showed that gabapentin effectively suppressed postoperative nausea and vomiting [20, 21], but the mechanism by which this drug prevents CINV is unclear. A small open-label trial, without a placebo-control group, suggested that gabapentin was effective in reducing CINV. In this trial, nine patients with breast cancer, who had moderate to severe nausea in the first cycle of chemotherapy, received gabapentin in the subsequent cycles. Eight of the nine patients reported a reduction in nausea score after taking this drug [22].
The goal of this pilot study was to evaluate the potential role of gabapentin in the prevention of both acute and delayed CINV in cancer patients receiving highly and moderately emetogenic chemotherapy.
Patients and methods
This was a prospective, double-blind, placebo-controlled study conducted at our institution (Faculdade de Medicina da Fundação ABC and affiliated Hospitals) from April 2009 to April 2010. Patients and personnel involved in the study were blinded to the assigned treatment. The study was approved by the ethics committee of our institution. All the patients provided written informed consent. Clinical trial information can be found for the following: NCT01052844 [ClinicalTrials.gov]
Inclusion criteria
We included patients who were at least 18 years old, had an Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and were scheduled to receive their first cycle of moderately to highly emetogenic chemotherapy, which was defined as doses of cisplatin or doxurubicin equal to or greater than 60 and 50 mg/m2, respectively [23].
Exclusion criteria
We excluded patients with any severe concurrent illness other than cancer, gastrointestinal obstruction and active peptic ulcer disease; patients with a history of brain metastasis or of receiving radiation therapy; patients who regularly used corticosteroids, benzodiazepines, tricyclic antidepressant or cannabinoids; and patients who reported vomiting or the use of antiemetics in the 24 h prior to the administration of chemotherapy. We also excluded patients with any of the following laboratory measurements: serum aspartate aminotransferase, serum alanine aminotransferase or serum bilirubin levels more than twice the upper limit of normal.
Treatments
All patients received intravenous ondansetron 8 mg, dexamethasone 10 mg and ranitidine 50 mg before chemotherapy on day 1 and oral dexamethasone 4 mg twice a day on days 2 and 3. Patients were randomly assigned, with a block-balanced randomization list, to take gabapentin 300 mg or placebo on the following regimens, as described by Guttuso et al. [22]:
-
Five and 4 days before chemotherapy (days 5 and 4): once daily;
-
Three and 2 days before chemotherapy (days 3 and 2): twice daily;
-
One day before to 5 days after chemotherapy (days 1 to 5): thrice daily.
Definitions of CINV
Episodes of vomiting or retching were recorded by the patients on diary cards from the initiation of chemotherapy infusion (0 h) until the morning of day 6 (120 h). An emetic episode was defined as a single instance of vomiting or retching; distinct episodes were separated by at least 1 min. The use of rescue therapy, defined as any medication taken to treat established nausea or emesis, was also recorded. Permitted rescue medications included 5-HT3-antagonists, phenothiazines, butyrophenones and domperidone.
Nausea was assessed on a 100-mm horizontal visual-analogue scale in the patient diary with the heading: “How much nausea have you had over the past 24 h?” The left-hand end (0 mm) of the scale was labelled “no nausea” and the right-hand end was labelled “nausea as bad as it could be”. Every 24 h, the patients indicated the degree of nausea during the previous 24 h by placing a vertical mark on the line scale [24]. On days 2 to 6, daily telephone contact was made by study site personnel to confirm that patients were taking study medications appropriately and maintaining accurate record.
Complete protection from nausea and vomiting (CP) was defined as the absence of any episode of nausea or vomiting and no use of rescue medication. CP was further defined as either acute (ACP), when occurring during the first 24 h after chemotherapy; delayed (DCP), when occurring during the period from days 2 through 5 after chemotherapy; or overall, when occurring over the entire period of the study (first 120 h).
Adverse events were recorded up to the post-study visit, which occurred on day 6 after chemotherapy, using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 [25].
Patients answered the Functional Living Index-Emesis (FLIE) questionnaire before the initiation of chemotherapy infusion on day 1 and on day 6 after chemotherapy [26]. The FLIE questionnaire is a validated patient-reported measure of the impact of CINV on daily life [27].
Statistical methods
The primary endpoints of this study were complete protection from both vomiting and nausea during the entire period of study (days 1 to 5) and complete protection during the delayed period. The secondary endpoints were to evaluate the adverse events other than episodes of vomiting or nausea, and to evaluate the impact of nausea and vomiting on quality of life (QoL) using the FLIE questionnaire.
In order to calculate the time to first failure of emesis control, the time to first failure episode was also assessed. The first failure was defined as the occurrence of either vomiting, use of rescue medication or the occurrence of nausea, whichever came first.
Statistical analyses were carried out using SPSS 17.0. For all binary outcome efficacy measures, comparison between the gabapentin regimen and the control regimen was made using logistic regression. We evaluated associations between categorical variables using the chi-square test. Because we wanted to test whether the addition of gabapentin was superior to placebo, we used unicaudal significance tests with a significance threshold of p ≤ 0.05. For the time to first failure of emetic control, we employed Kaplan–Meier curves and log-rank test.
We estimated that with a total of 80 patients, we could, with a type 1 error of 0.05 and a power of 0.8, consider a 30% difference in the proportion of patients experiencing complete control of nausea and vomiting between arms.
Results
Between April 2009 and April 2010, 80 patients were enrolled in this study, of whom 40 were randomised to the experimental group (gabapentin) and 40 were randomised to the control group (placebo). The baseline characteristics of eligible patients were similar between groups (Table 1).
The majority of patients were female (93.75%) and were scheduled to receive a moderately emetogenic chemotherapeutic regimen of doxorubicin and cyclophosphamide for the treatment of breast cancer (91.25%).
The primary endpoint of overall complete response (Table 2) was reported by significantly more patients in the gabapentin group than in the control group (65% vs. 42.5%; p = 0.04).
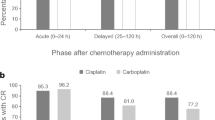
The rates of acute control of nausea and vomiting (first 24 h) were similar across both arms (70%). Complete protection during the delayed phase occurred in 21 patients (52.5%) in the control group and in 28 patients (70%) in the gabapentin group (p = 0.108) (Fig. 1).
Complete control during the acute phase and study group (placebo or gabapentin) were the only independent factors for achieving overall complete response by logistic regression (p = 0.01 and 0.04, respectively).
Among the patients who achieved complete control during the acute phase, significantly more patients in the gabapentin group achieved complete control during delayed phase (Fig. 2). We did not observe, however, a significantly higher complete control of delayed vomiting in the gabapentin group as compared to placebo (p = 0.07)
Figure 3 shows the Kaplan–Meier curve for the time to first failure after the initiation of chemotherapy. During the first 24 h, the curves of gabapentin and placebo were similar; however, after the first 24 h, more patients in the placebo group experienced a failure than in the gabapentin group, although this difference was not statistically significant (p = 0.09).
On the FLIE questionnaire, there was no significant difference between the treatment groups. No serious adverse events were observed, although one patient discontinued medication in the gabapentin group due to sleepiness (Table 3).
Discussion
The current pilot trial demonstrated that the addition of gabapentin to ondansetron and dexamethasone during the first cycle of moderately and highly emetogenic chemotherapy significantly improved complete control of CINV compared to placebo. Although the differences were not significant for the complete control of vomiting, complete control of nausea, and no rescue therapy, the trends observed for each of these favoured the gabapentin treatment group.
Most of the patients in this study were female and received doxorubicin and cyclophosphamide, a moderately emetogenic regimen. In this study, we achieved complete control of CINV in 65% of patients using gabapentin. Interestingly, War et al. treated patients subjected to moderately emetogenic chemotherapy with a combination of aprepitant, ondansetron and dexamethasone and achieved complete control of CINV in 51% of patients [28]. In that study, the complete response rate of the control group was similar to the complete response rate obtained in our study (42% and 42.5%, respectively).
In our study, response rates in the acute phase were similar between both groups. The overall complete CINV control superiority of gabapentin is probably related to its positive effects in the delayed phase of CINV control, even though we observed no statistically significant differences in delayed CINV control with gabapentin. Interestingly, in the subset of patients who achieved complete control in the acute phase, the percentage of patients who achieved delayed complete control was significantly higher in the gabapentin group (Fig. 2). Hence, gabapentin appears play a role in the prevention of delayed CINV.
The role of gabapentin in preventing CINV and vomiting was less clear prior to this pilot study. Guttuso et al. reported the results of a small open-label study of nine patients with nausea and vomiting after the first cycle of moderately emetogenic chemotherapy. The authors postulated that the mitigation of the tachykinin neurotransmitter might play a role in the prevention of CINV; however, the real mechanism of action of gabapentin as an antiemetic agent is not known [22].
In our study, gabapentin was extremely well tolerated, displaying a side-effect profile similar to that of the placebo. Only one patient stopped gabapentin treatment, 1 day before the end of the trial due to sleepiness.
Our study, however, has limitations. We included mainly women with breast cancer who received moderately emetogenic chemotherapy. Therefore, future studies should include more men, patients with different tumours and also subjects scheduled to receive highly emetogenic chemotherapy.
Emesis is an autonomic reflex controlled by multiple neurotransmitter systems. Blocking both the 5-HT3 receptor and substance P/NK1 receptors has been demonstrated to reduce CINV in patients receiving chemotherapy. A three-drug regimen of 5-HT3 receptor antagonists (ondansetron, granisetron, palonosetron), NK-1 antagonists (aprepitant, casopitant) and corticosteroids (dexamethasone) is currently the standard treatment for the prevention of CINV in patients receiving moderately emetogenic chemotherapy [29, 30].
Gabapentin may be a cheaper alternative to NK-1 antagonists. The cost of one course of gabapentin/cycle of chemotherapy in our country is US$25.00, whereas the cost of aprepitant is US$345.00. For each cycle of chemotherapy, we can save US$ 315.00, which translates into about US$ 1.512.000.00 yearly savings in our service. This is a special issue in developing countries, where the cost of medication may impact a patient's ability to use effective therapy [31].
We feel that the results of our study encourage the design of a larger randomised study comparing gabapentin and NK-1 antagonists.
References
Osoba D, Zee B, Pater J, Warr D, Latreille J, Kaizer L (1997) Determinants of postchemotherapy nausea and vomiting in patients with cancer. Quality of Life and Symptom Control Committees of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 15(1):116–123
Cubeddu LX, Hoffmann IS, Fuenmayor NT, Finn AL (1990) Efficacy of ondansetron (GR 38032F) and the role of serotonin in cisplatin-induced nausea and vomiting. N Engl J Med 322(12):810–816
Kaasa S, Kvaløy S, Dicato MA, Ries F, Huys JV, Royer E, Carruthers L (1990) A comparison of ondansetron with metoclopramide in the prophylaxis of chemotherapy-induced nausea and vomiting: a randomized, double-blind study. International Emesis Study Group. Eur J Cancer 26(3):311–314
Markman M, Sheidler V, Ettinger DS, Quaskey SA (1984) Antiemetic efficacy of dexamethasone. Randomized, double-blind, crossover study with prochlorperazine in patients receiving cancer chemotherapy. N Engl J Med 311(9):549–552
Roila F, Tonato M, Cognetti F, Cortesi E, Favalli G, Marangolo M, Amadori D, Bella MA, Gramazio V, Donati D et al (1991) Prevention of cisplatin-induced emesis: a double-blind multicenter randomized crossover study comparing ondansetron and ondansetron plus dexamethasone. J Clin Oncol 9(4):675–678
Hesketh PJ, Harvey WH, Harker WG, Beck TM, Ryan T, Bricker LJ, Kish JA, Murphy WK, Hainsworth JD, Haley B (1994) A randomized, double-blind comparison of intravenous ondansetron alone and in combination with intravenous dexamethasone in the prevention of high-dose cisplatin-induced emesis. J Clin Oncol 12(3):596–600
del Giglio A, Soares HP, Caparroz C, Castro PC (2000) Granisetron is equivalent to ondansetron for prophylaxis of chemotherapy-induced nausea and vomiting: results of a meta-analysis of randomized controlled trials. Cancer 89(11):2301–2308
Billio A, Morello E, Clarke MJ (2010) Serotonin receptor antagonists for highly emetogenic chemotherapy in adults. Cochrane Database Syst Rev 1:CD006272
Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, Macciocchi A, Grunberg S; 99–04 Palonosetron Study Group (2003) Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer 98(11):2473–2482
Gralla R, Lichinitser M, Van Der Vegt S, Sleeboom H, Mezger J, Peschel C, Tonini G, Labianca R, Macciocchi A, Aapro M (2003) Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 14(10):1570–1577
Rapoport BL, Jordan K, Boice JA, Taylor A, Brown C, Hardwick JS et al (2010) Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: a randomized, double-blind study. Support Care Canc 18(4):423–431
Roila F, Rolski J, Ramlau R, Dediu M, Russo MW, Bandekar RR et al (2009) Randomized, double-blind, dose-ranging trial of the oral neurokinin-1 receptor antagonist casopitant mesylate for the prevention of cisplatin-induced nausea and vomiting. Ann Oncol 20(11):1867–1873
Hesketh PJ, Warr DG, Street JC, Carides AD (2010) Differential time course of action of 5-HT(3) and NK (1) receptor antagonists when used with highly and moderately emetogenic chemotherapy (HEC and MEC). Support Care Canc. doi:10.1007/s00520-010-0944-4
Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R et al (2003) The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study Group. J Clin Oncol 21(22):4112–4119
Yamauchi T, Kaneko S, Yagi K, Sase S (2006) Treatment of partial seizures with gabapentin: double-blind, placebo-controlled, parallel-group study. Psychiatry Clin Neurosci 60(4):507–515
Navari RM (2007) (2007) Fosaprepitant (MK-0517): a neurokinin-1 receptor antagonist for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin Investig Drugs 16(12):1977–1985
Herrstedt J, Apornwirat W, Shaharyar A, Aziz Z, Roila F, Van Belle S et al (2009) Phase III trial of casopitant, a novel neurokinin-1 receptor antagonist, for the prevention of nausea and vomiting in patients receiving moderately emetogenic chemotherapy. J Clin Oncol 27(32):5363–5369
Srivastava U, Kumar A, Saxena S, Mishra AR, Saraswat N, Mishra S (2010) Effect of preoperative gabapentin on postoperative pain and tramadol consumption after minilap open cholecystectomy: a randomized double-blind, placebo-controlled trial. Eur J Anaesthesiol 27(4):331–335
Irving G, Jensen M, Cramer M, Wu J, Chiang YK, Tark M et al (2009) Efficacy and tolerability of gastric-retentive gabapentin for the treatment of postherpetic neuralgia: results of a double-blind, randomized, placebo-controlled clinical trial. Clin J Pain 25(3):185–192
Khademi S, Ghaffarpasand F, Heiran HR, Asefi A (2010) Effects of preoperative gabapentin on postoperative nausea and vomiting after open cholecystectomy: a prospective randomized double-blind placebo-controlled study. Med Princ Pract 19(1):57–60
Mohammadi SS, Seyedi M (2008) Effects of gabapentin on early postoperative pain, nausea and vomiting in laparoscopic surgery for assisted reproductive technologies. Pak J Biol Sci 11(14):1878–1880
Guttuso T Jr, Roscoe J, Griggs J (2003) Effect of gabapentin on nausea induced by chemotherapy in patients with breast cancer. Lancet 361(9370):1703–1705
Naeim A, Dy SM, Lorenz KA, Sanati H, Walling A, Asch SM (2008) Evidence-based recommendations for cancer nausea and vomiting. J Clin Oncol 26(23):3903–3910
Navari RM, Reinhardt RR, Gralla RJ, Kris MG, Hesketh PJ, Khojasteh A et al (1999) Reduction of cisplatin-induced emesis by a selective neurokinin-1-receptor antagonist. L-754,030 Antiemetic Trials Group. N Engl J Med 340(3):190–195
The National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 available online at http://evs.nci.nih.gov/ftp1/CTCAE/About.html, accessed July 27, 2010
Decker GM, DeMeyer ES, Kisko DL (2006) Measuring the maintenance of daily life activities using the functional living index-emesis (FLIE) in patients receiving moderately emetogenic chemotherapy. J Support Oncol 4(1):35–41, 52
Martin AR, Pearson JD, Cai B et al (2000) Validation of a 5-day recall version of the Functional Living Index-Emesis (FLIE) quality of life questionnaire for chemotherapy-induced emesis. Qual Life Res 9:18
Warr DG, Hesketh PJ, Gralla RJ, Muss HB, Herrstedt J, Eisenberg PD, Raftopoulos H, Grunberg SM, Gabriel M, Rodgers A, Bohidar N, Klinger G, Hustad CM, Horgan KJ, Skobieranda F (2005) Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 23(24):5851
Wickham R (2010) Best practice management of CINV in oncology patients: II. Antiemetic guidelines and rationale for use. J Support Oncol 8(2 Suppl 1):10–15
Herrstedt J, Roila F (2009) Chemotherapy-induced nausea and vomiting: ESMO clinical recommendations for prophylaxis. Ann Oncol 20(Suppl 4):156–158
Briesacher BA, Gurwitz JH, Soumerai SB (2007) Patients at-risk for cost-related medication nonadherence: a review of the literature. J Gen Intern Med 22(6):864–871
Conflicts of interest
The authors indicated no potential conflicts of interest. All authors had full control of all primary data and agree to allow the journal to review the data if requested.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Esm 1
(PDF 112 kb)
Rights and permissions
About this article
Cite this article
Cruz, F.M., de Iracema Gomes Cubero, D., Taranto, P. et al. Gabapentin for the prevention of chemotherapy- induced nausea and vomiting: a pilot study. Support Care Cancer 20, 601–606 (2012). https://doi.org/10.1007/s00520-011-1138-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-011-1138-4