Abstract
There are growing concerns that a significant reliance on fossil resources is not sustainable for the production of polymers. The all-out transition toward renewable resources for polymer production is inevitable. Polymers from renewable resources include cellulose, starch, chitosan, lignin, proteins, oils, common commodity polymers (e.g., polyethylene, polyethylene terephthalate), and microbial poly(ester)s. Fundamental research in the production, modification, property enhancement, and new applications of these materials is an important undertaking. In this chapter, existing advances in the exploitation of renewable resources to produce polymers are summarized.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
There are growing concerns over the sustainability of materials and chemicals from petrochemical-based resources for the threat to welfare, health, and the ecosystem (Kanagaratnam et al. 2013; Mac Kinnon et al. 2017; Koutinas et al. 2014; Sahoo et al. 2018). It is an enduring need to substitute polymers from fossil fuel-based resources with the more environmental congenial renewable resources (Gandini and Belgacem 2013). Contemporary progresses in the production and properties of polymers from renewable resources with better characteristics and possibly small expenses have stimulated the optimisms of replacing the traditional petrochemical-based polymers (Kumar et al. 2017; Saikia and Karak 2017; Nilsson et al. 2015). With the latest innovations in production technologies and the invention of new exploitable chemical building blocks, investigation in ecological polymers from renewable resources has increased dramatically (Lindblad et al. 2002).
The term “renewable” has become a major attention among the industries and citizens. The used feedstock may be termed as renewable when it is mobilized from resources which are biologically refilled on a human timescale; on the other hand, petrochemical resources take thousands of centuries to be built naturally (Gandini and Lacerda 2015), provided that new harvest balances cultivating bio-based feedstock can be called renewable. For instance, peat is not judged renewable due to sluggish restoration rate, and on the other hand, tropical hardwood can be termed as renewable when appropriately governed. Sustainability is becoming immensely important leading to create an attention in the use of renewable resource in polymer application (Tsanaktsis et al. 2015). Renewable resource-based polymer is a new era, which is entirely based on natural resources, significantly creating a paradigm shift in the properties of polymer-based materials (Wu 2015; Yan and Chen 2015; Das et al. 2015; Han et al. 2016; Zia et al. 2016; Lligadas et al. 2014). The environment is protected due to reduction in emission of harmful gasses by replacement of the fossil fuel and gas by green agricultural resources. Thus, the objective of this chapter is to present the contemporary research achievements by emphasizing the wide possibility of the renewable polymers for a huge number of functions.
Historically, the attainment of fossil fuels depended on cost-effective extensive manufacturing (Bruijnincx et al. 2015). The cheap refining competences offered an economical supply of building block compounds in the petroleum-based industry (Imre and Pukánszky 2015). Similarly, improvements in the development of renewable feedstocks (e.g., cellulosic) for biofuels should facilitate the production of low-cost elementary units for beneficial chemical and polymeric developments (Thakur et al. 2014).
Another aspect is that approximately 1.3 billion tons of waste are being generated all over the world each year and is expected to escalate to 4 billion tons by 2100 (Zhang 2016). Disposing of waste jeopardizes the people life and environment. Incineration of waste like plastic has tremendous impact on the environment, as burning of plastic tends to produce dioxin-like noxious substances (Baeumler et al. 2012). Hence, throwing away of waste resources and materials denotes ruining of huge amount of money (Vestapen et al. 2017). In case of traditional petroleum-based plastics, durability, which makes it a viable candidate, renders it vulnerable after disposal. Having immense resistance to microbial degradation, these materials are not biodegradable and substantially accumulate above the ground creating waste (Botello-Álvarez et al. 2018). The polymers from agro-based resources could conquer the restrictions of the petrochemical resources which are widely used.
2 Renewable Polymers Classifications
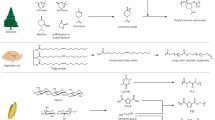
A wide spectrum of materials from renewable resources is already potentially available. Most importantly these resources are sustainable to provide a wealthy variety of building blocks and polymers that are presently offered from fossil fuel-based industry. Based on the source and synthesis technology, renewable polymers are classified into four major groups. Figure 1 represents a classification of renewable polymer which is based on synthesis source.
-
(i)
Polymers from vegetable resources, for example, agro-polymers (cellulose or starch)
-
(ii)
Polymers obtained from animal resources, for example, chitin and chitosan
-
(iii)
Polymers obtained from microbial action, for example, the polyhydroxyalkanoates (PHAs)
-
(iv)
Polymers from monomers which is chemically and conventionally synthesized (obtained from agro-resources), for example, polylactic acid (PLA), biopolyethylene (PE), biopolyethylene terephthalate (PET).
3 Some Polymers from Renewable Sources
An assessment of the contemporary polymer field dealing with renewable resources reveals an enormous global activity in both academic and industrial arena. Some renewable resource-based polymers are described on the basis of their preparation, processing, properties, and performance below.
3.1 Cellulose
Cellulose represents, by far, the most appealing candidate in terms of availability. Cellulose is the most abundant natural resource on earth since it represents the chief constituent of plant cell walls (French et al. 1993; Ketabchi et al. 2015, 2016; Hoque et al. 2016). For example, cellulose nanoparticles (CNPs) can abundantly be isolated from Kenaf fiber (Ketabchi et al. 2016). As the most plentiful biopolymer on earth, with some 1012 tons, cellulose is readily renewed. It also possesses unique properties and chemical reactivity. This polymer constituted of d-glucose residues as monomeric units (Fig. 2) (Chiellini et al. 2002a, b). Cellulose molecules are unbranched chains of up to 17,000 1,4 linked ß-d-glucose residues, but shorter chains occur under other circumstances. The two end groups of cellulose are not chemically matching, since one exhibits the “normal” C4–OH group (non-reducing end), while the other has a C1–OH component in equilibrium with the conforming aldehyde function (reducing end) (Gandini 2011a, b).
Chemical structure of cellulose (Chiellini et al. 2002a)
Cellulose is the dominant integral of woods which are used in an extensive variety of basic materials for buildings and furniture. Clean cellulose is usually branched out from plants exploiting various pulping procedures. Fibers from pulp are exploited in printing papers, industrial papers, household essentials such as toilet papers, paper towels, handkerchiefs, facial tissues, napkins, and so on.
This structure is responsible for the exclusive characteristics of cellulose. Because of the equatorial positions of the hydroxyls on the cellulose chain, they protrude laterally along the extended molecule and are readily available for hydrogen bonding. These hydrogen bonds cause the chains to group together in a highly ordered structure. The interchain hydrogen bonds in the crystalline regions are strong, giving the resultant structure good strength, corresponding high surface energy, high hydrophilic character, and insolubility in most solvents. They also prevent cellulose from melting (nonthermoplastic). Of course, the marked reactivity of cellulose is associated with the three OH groups present in each anhydroglucose units (AGU) (Petersen et al. 1999).
Most celluloses have a high degree of polymerization both as a function of the species and of the isolation and purification procedures. The latter operations often introduce other functionalities, like carbonyl and carboxyl groups, into the macromolecular backbone (Gandini 2011a, b). The glycosidic bonds in cellulose are strong, and this polymer is stable under a wide variety of reaction conditions. The purification step is also known to provide specific features to cellulose, including surface charge and additional chemical reactivity (Petersen et al. 1999).
Modified cellulose was the first polymer developed prior to the advancement of modern synthetic plastic industry. Celluloid from nitrocellulose was the first thermoplastic material invented in the nineteenth century (Shi et al. 2011). Technically, worthy chemical modifications of cellulose polymer generally engage reaction (i.e., characteristic of alcohols) with free hydroxyl groups in 2, 3, and 6 positions (Simon et al. 1998). Etherification and esterification of individual hydroxyl groups, of the polysaccharide backbone, are of specific significance for cellulose. The alteration of chemical nature of cellulose enables its synthesis with the same environments exercised for thermoplastic polymers. Several cellulosic derivatives are industrially handy such as cellulose acetate, ethyl cellulose, hydroxyethyl cellulose, and hydroxypropyl cellulose. These derivatives have been widely exploited for the fabrication of membranes and hollow fibers capable of immobilization of enzymes and in the practice of hemodialysis reverse osmosis and chromatographic supports (Lagoa et al. 1999; Hoenich and Stamp 2000). Furthermore, hydroxyalkyl cellulose and carboxymethyl celluloses are being used as a media for drug delivery and as wound dressing (Vert 2001).
The period of nanocellulose can be acknowledged with the inauguration of the third millennium (Klemm et al. 2011; Einchhorn 2011; Einchhorn et al. 2010; Dufresne 2017; Missoum et al. 2013; Habibi 2014). Some noticeable illustrated cellulosic nanoscale substances are enthusiastically investigated because of their exceptional characteristics and innovative functions in materials science: cellulose nanocrystals (CNC) (Habibi et al. 2010), nanofibrillated cellulose (CNF) (Siró and Plackett 2010), and bacterial cellulose (BC) (Pecoraro et al. 2008). For instance, the nanocellulosic fibrils are isolated from cellulose through high-pressure, high-temperature, and high-velocity impact homogenization, grinding, or microfluidization.
Cellulose xanthate in sulfuric acid or sodium sulfate solution is used to produce regenerated cellulose by extrusion or spinning (Shi et al. 2011). Cellophane film and rayon fiber are two important regenerated celluloses. Biodegradable cellophane is a transparent film with a low permeability to air, oil, and grease. Cellophane is still exploited these days in some specialized packaging films such as candy wraps, and industrial films as a base film for tapes. Rayon is an artificial redeveloped cellulose fiber formulated from a range of cellulosic natural resources such as wood, cotton, hemp, and bamboo (Kauffman 1993). Rayon fiber is employed mostly in apparels, bedding products, disposable personal sanitary objects including feminine pads and liners, furnishings, window treatment, medical products, tire cords, lighter fillings, and so on. Cellulose can also produce nanocomposites consisting of carboxymethyl cellulose, chitosan, and hydroxyapatite (HA) that can be used for load-bearing biomedical devices (Michael et al. 2016). Jiang et al. (2008) prepared a three-dimensional scaffold consisting of HA, chitosan, and carboxymethyl cellulose where the mechanical properties were seen to increase due to enhanced dispersion of HA in the polymer matrix.
3.2 Starch
Starch is the chief form of carbohydrate packing in green plants. It is the primary constituent of seeds, tubers, and roots. Starch is manufactured commercially from corn, wheat, rice, tapioca, potato, sago, and other sources. Starch contains six-membered ring glucose units (glucopyranose). There are two major types of starch molecular structure. In one type of structure, starch molecules are highly branched, known as amylopectin. It constitutes the major part of starch (i.e., up to 100% in waxy starches, 72% in normal maize starch, and 80% in potato starch). Amylopectin molecules are composed of chains of glucose units linked by a-1-4 linkages with branches of double helical a-1-6 linkages which gives the crystallinity of starch (Chauhan et al. 2000). The average branch chain length is 20–30 glucosidic units (Zdrahala 1997). The other type of starch structure is arranged by repeating units of 1-4-a-glucose with very few branches and is known as amylose. Both amylopectin and amylose structures are shown in Fig. 3.
Schematic representation of a amylopectin and b amylose structure (Chiellini et al. 2002a)
Apart from use in food applications, starch is utilized considerably as surface modifying agents in paper and textile industry, as adhesive in corrugated board industry. Other applications of starch include laundry, baby powders, oil production, and production of bioplastics or biodegradable polymers (Bemiller 1997; Hoque et al. 2013a). Relatively cheap polyhydroxy compounds were developed from starch for industrial applications. A suitable mixture of chemical reagents permits for the introduction of ionic charge into the starch molecules (Doane et al. 1992). Starch can be readily transformed to glucose from which a variety of cyclic and acyclic polyols, aldehydes, ketones, acids, esters, and ether can be attained with the introduction of anionic or cationic charge by adding suitable chemical reagents (Tharanathan 2005). Lately, cornstarch was utilized to produce adhesive by cross-linking with polyvinyl alcohol and hexamethoxymethylmelamine together with citric acid as catalyst (Imam et al. 1999).
Aqueous esterification of starch can be generated at low alkalinity, under controlled pH. These reactions can be controlled by regulating the temperature for a range of degree of substitution (DS = ≤ 0.2 to ≥ 2.4) (Tessler and Billmers 1996; Sagar and Merrill 1995; Mormann and Spitzer 2001). Starch ester can be produced in nonaqueous medium with the use of solvents such as pyridine. Here, pyridine is used to serve the dual purpose of catalyst and solvent (Maim et al. 1951). The reaction of starches with several oxidizing reagents (such as hypochlorite, permanganate, hydrogen peroxide, persulfate, periodate, and dichromate) reduces molecular weight and also increases the solubility of starch for paper and food applications (Thoma and Stewart 1965; Kruger 1989; Vikso-Nielsen et al. 2009). These oxidized starches have found a large application in adhesive manufacturing.
A high carboxyl and carbony1 containing starch-modified water-soluble products can be developed by a reactive extrusion process with hydrogen peroxide in the presence of ferrous-cupric catalyst (Wing and Willett 1997, 1999). In graft polymerization technique, a free radical is initiated on the starch backbone and then allowed to react with polymerizable vinyl or acrylic monomers with chemical or radioactive induction (Jyothi 2010; Meshram et al. 2009). Such starch graft polymers were suggested for the application as thickeners for aqueous system, flocculants, clarification aids for wastewaters, retention aids in paper making, and many other uses (Labet et al. 2007).
3.3 Lignin
Lignin is the second most plentiful natural polymer surpassed only by cellulose in nature. Naturally, lignin acts as a cement and has a supportive structural function by establishing hydrophobic structure to defend highly hydrophilic cellulose and hemicellulose segments. Free lignin is insoluble even in strong mineral acids and hydrocarbons (Goheen and Royt 1981; Pollak 1952). Lignin in wood can be made soluble in water by changing its polymerization state with physical or chemical treatment (Calvo-Flores and Dobado 2010). Lignin exhibits a complicated three-dimensional aromatic molecule composed of phenyl groups, depending on its origin (Adler 1977). The irregular molecular mass of this biopolymer is an outcome of the random cross‐linked polymerization of phenolic moieties by at least ten types of C–C and C–O–C bonds, initiating from radical‐coupling reactions between phenolic radicals. It is commonly acknowledged that there are three basic phenol products that are known as monolignols: 10 p‐coumaryl alcohol (M1H), coniferyl alcohol (M1G), and sinapyl alcohol (M1S) (Vanholme et al. 2008). Each monolignol produces p‐hydroxyphenyl, guaiacyl, and syringyl residues in the polymer (Fig. 4).
The lignin polymer is synthesized by peroxidase‐mediated dehydrogenation of monolignol units, giving a heterogeneous structure formed by basic units linked by C–C bonds and aryl–ether linkages with aryl–glycerol and β‐aryl ether (dos Santos Abreu et al. 2007). In general, lignin is obtained from biomass with an organic solvent/water/reagents mixture at a high temperature and pressure. The most abundant monolignol in hardwoods is sinapyl alcohol, and this leads to syringyl S-units in the lignin polymer (Lora and Aziz 1985). Lignin is separated by acid precipitation. However, only Alcell and Organocell lignins are commercially available (Piccolo et al. 1997). A partial hydrolysis of lignin can be carried out by treating wood with steam at high temperature and pressure (i.e., about 200 °C), followed by a sudden decompression in the presence of some chemicals (Focher et al. 1991). Under these circumstances, a water‐insoluble lignin material with a low level of carbohydrate and wood‐extractive impurities develops. Liquefaction of lignocellulosics was earlier performed by several hours of treatment at 300–400 °C in aqueous or organic solvents. Subsequently, liquefaction in organic solvents at temperatures of 240–270 °C without catalyst or at temperatures around 80–150 °C with acidic catalysts was developed with very high yield (90–95%) (Shirashi 1992). Lignin is used as dispersant, binders, humectants, emulsion stabilizers, sequestering agents, fuel source, concrete admixtures, and so on (Northey 1992).
Lignin surface and solubility characteristics can be modified with a wide range of reactions: alkylation, dealkylation, oxyalkylation, amination, carboxylation, acylation, halogenation, nitration, hydrogenolysis, methylolation, oxidation and reduction, sulfomethylation, sulfonation, silylation, phosphorylation, and nitroxide formation (Wang et al. 1992). The glass transition temperature, Tg, of lignin varies widely depending on the method of isolation and molecular weight. Chemical modification of lignin has mainly been used to dissolve lignin into organic solvents and to determine the functional groups of lignin in the synthesis of copolymers with modified lignins (Thielemans and Wool 2005).
The sulfite‐pulping procedure is used to employed to produce ligninsulfonates from waste liquid from softwood with salts of sulfurous acid (sulfites or bisulfites) (Lindsey and Tollens 1892). Ligninsulfonates can be used as binders, dispersant agents for pesticides, emulsifiers, and heavy‐metal sequestrants (Mathiasson and Kubat 1994; Browning 1955; Wetzel et al. 2006). Low‐cost binders from ligninsulfonates are commonly used in coal briquettes or ceramics, briquetting of mineral dust (fines, shavings, turnings), and wood‐related material such as plywood or particle boards (Lora and Glasser 2002). In craft pulping of wood, the lignin is made water soluble through the introduction of alkaline solution. Kraft lignin is a good precursor for the preparation of char and active carbon (Carrott and Carrott 2007). Furthermore, lignin is an economical basic material for carbon fiber production. Several synthetic methods exist for preparing carbon fiber from lignin (Kadla et al. 2002).
3.4 Chitin and Chitosan
Chitin, an important structural polysaccharide, is well known to consist of 2-acetamido-2-deoxy-β-d-glucose through a β (1 → 4) linkage (Rinaudo 2006) (Fig. 5). In fact, chitin exists in three different polymorphic forms (α, β, and γ) (Blackwell 1982; Rudall and Kenchington 1973; Tong and Yao 1997). Recent studies have confirmed that the γ form is a variant of α family (Atkins 1985). This naturally abundant mucopolysaccharide can be found in the cell walls of lower plants and fungi, insect cuticles and in the exoskeleton of arthropods and mollusks. An estimated billion tons of chitins are synthesized every year in nature (Singh and Ray 2000). It is highly hydrophobic and is insoluble in water and most organic solvents. It has low chemical reactivity. For its inertness, it gained less attention with respect to cellulose, remaining an almost unutilized resource. It is soluble in hexafluoro isopropanol, hexafluoroacetone, chloroalcohols in conjugation with aqueous solutions of mineral acids (French et al. 1993) and dimethylacetamide containing 5% lithium chloride.
Chitosan is the fully or partially N-deacetylated derivative of chitin (Fig. 6) (Sahoo and Nayak 2011). Chitosan is soluble in acetic acid and other organic solvents. It is more favorable in many applications than chitin. Chitin and chitosan are of commercial interest due to their high percentage of nitrogen (6.89%) compared to synthetically substituted cellulose (1.25%). Because of the presence of the amino functionality, chitin and chitosan could be suitably modified to impart desired properties and distinctive biological functions including solubility (Tharanathan and Kittur 2003; Kurita 2001; Gorochovceva and Makuška 2004).
Water-resistant adhesive proteins used by marine animals (mussels) to adhere to wet or submerged surface have stimulated the studies to confer water-resistant adhesive properties to semidilute solutions of chitosan. High viscosities and water-resistant adhesive strength of chitosan semidilute solutions have been obtained by tyrosinase catalyzed reaction with 3,4-dihydroxyphenethylamine (dopamine) or by reaction with glutaraldehyde (Nakajima et al. 1984). The deacetylation process is rarely complete, and most commercial and laboratory products tend to be a copolymer of N-acetylglucosamine and N-glucosamine repeat units. The ratio of the two repeating units depends on the source and preparation conditions of chitosan, but in many cases, the glucosamine units predominate.
Fibers made of chitin and chitosan are useful as absorbable sutures and wound dressing materials (Nakajima et al. 1984). Novel methods have been recently devised for the preparation of chitin threads for the fabrication of absorbable suture materials, dressings, and biodegradable substrates for the growth of human skin cells (keratinocytes and fibroblasts) (Tamura 2004). It has also been claimed that wound dressings made of chitin and chitosan fibers have applications in wastewater treatment. Here, the removal of heavy metal ions by chitosan through chelation has received much attention (Muzzarelli 1997). Yannas et al. proposed a design for artificial skin, applicable to long-term chronic use, focusing on a nonantigenic membrane, which performs as a biodegradable template for synthesis of neodermal tissue (Yannas et al. 1982).
Artificial kidney membranes prepared from modified and alburnin-blended chitosan membranes were found to be potential candidates for dialysis applications (Reinhart and Peppas 1984). Chitosan can provide ionic conductivity when dissolved in acetic acid. The transport of protons which gives conductivity in chitosan is thought to occur through many microvoids in the polymer. The choice of a more suitable electrode material may produce a better battery system (Arof et al. 1995).
3.5 Proteins
Proteins are built up of chain of amino acid residues joined by amide linkages with the presence of reactive groups such as amino, carboxyl, amido, hydroxyl, thiol, and imidazole groups. Proteins have a wide variety of applications in fermentation processes, coatings, encapsulant in the pharmaceutical and food industries, adhesives, surfactants, and plastics (De Graaf and Kolster 1998). Proteins can be used for making of slowly degrading packaging or mulching films (Chiellini et al. 2002a, b). Examples of industrial proteins are plant proteins, such as wheat and corn gluten, soy, pea, and potato proteins; and animal proteins, such as casein, whey, keratin, collagen, and gelatin. Recently, gelatin gets much technological appreciation for its solubility in hot water, polyampholyte character, availability in a wide range of viscosity, and thermally reversible gel formation. Gelatin is currently used in a variety of applications comprising manufacturing of pharmaceutical products, X-ray and photographic films development and food processing (De Graaf and Kolster 1998; Feughelman 2002).
Collagen is used as the basic material for the manufacturing of leather products (Yannas 1972). Proteins are also used for the fabrication of materials such as edible films, films and coating, adhesives, thermoplastics, and surfactants (Cuq et al. 1998; Kester 1986). Chemical modifications of proteins improve its economic consideration as thermoplastic or thermosetting materials (De Graaf et al. 1998). Esterification of protein carboxyl and amide groups by fatty alcohol that lead to a protein derivative with improved functional properties can result into a lower water sensitivity. In this case esterified protein becomes less soluble at basic pH compared to native proteins, indicating a hydrophobation effect (Ayhllon-Meixueiro et al. 2001).
3.6 Polylactic Acid (PLA)
Polylactic acid (PLA) is a biodegradable aliphatic polyester. Most importantly, PLA can be synthesized from bio-derived monomers. Lactide monomer is produced from lactic acid, which is produced by fermentation of a renewable agricultural source corn (Auras et al. 2004). Lactic acid is a chiral molecule available in the l and d stereoisomer forms (Fig. 7). Lactide is a cyclic dimer of lactic acid that has three possible stereoisomers: (i) l-lactide (LLA), which is composed of two l-lactic acids, (ii) d-lactide (DLA), which is composed of two d-lactic acids, and (iii) meso-lactide (MLA), which is composed of an l-lactic acid and a d-lactic acid (Kulkarni et al. 1971). Lactic acid in the l-form is actually produced by fermentation from nearly any renewable resources.

Adapted from Auras et al. (2004)
Synthesis of PLA from l- and d-lactic acids.
To produce lactic acid (2-hydroxy propionic acid) which is the single monomer of PLA, fermentation or chemical synthesis is used. Its two optically active configurations, the l(+) and d(−) stereoisomers, are produced by bacterial (heterofermentative and homofermentative) fermentation of carbohydrates. PLA can be made through a polymerization process from the lactic acid by polycondensation or ring-opening polymerization or can be made by direct methods such as azeotropic dehydration or enzymatic polymerization (Fig. 7) (Lasprilla et al. 2012). The direct polymerization and ring-opening polymerization methods are commonly exploited. Polycondensation reaction produces low molecular weight poly(lactic acid). Lactide ring-opening polymerization provides a direct and easy access to the corresponding high molecular weight polylactide. Industrially important high molecular weight PLA with a controlled optical and crystal property is formed with vacuum distillation of l-lactide, by ring-opening polymerization. Stannous octoate (bis 2-ethyl hexanoate, SnOct2) for the l-lactide ring-opening polymerization is used as active initiator, which in turn causes a low degree of racemization at high temperature (Pang et al. 2010).
Some unique properties (such as high mechanical strength, good appearance, good barrier, and low toxicity) of PLA have broadened its application. PLA exhibits rapid loss of molecular weight at processing temperature while undergoing thermal treatment. PLA is thermally unstable, and during thermal processing or under hydrolytic conditions, the ester linkages of PLA tend to degrade (Cheng et al. 2009). The degradation rate of PLA rapidly increases above the melting point, although it undergoes thermal degradation at temperatures lower than the melting point of the polymer. PLA films have good tensile strength, lower than polyethylene terephthalate (PET) but higher than polystyrene (PS). The permeability coefficients of O2 and CO2 are lower than PS and comparable to those of PET, in the context of barrier properties of PLA. PLA has the highest value in comparison to high-density polyethylene (HDPE), polypropylene (PP) and PS for tensile modulus and flexural modulus (Akbari et al. 2015).
Crystallinity influences many polymer properties including modulus, stiffness, tensile strength, hardness, melting point, and crease point. The crystallization rate of PLA at temperatures between 100 and 118 °C is very high (Drumright et al. 2000). It was also concluded that the high crystallization rate of PLA below 120 °C has to be ascribed to the high rate of radial growth of the spherulites (spherical semicrystalline regions inside nonbranched linear polymers) after measuring crystallization rates of PLA over a wide temperature range (Müller et al. 2015).
PLA applications include a wide variety of biomedical products (Hutmacher et al. 2008; Yousefi et al. 2015; Hoque et al. 2018), food packaging for meat and soft drinks, films for agro-industry, and nonwoven materials in hygiene products. PLA is approved by the US Food and Drug Administration (FDA) for the biomedical applications in the orthopedic area that include fixation screws, suture anchors, meniscal darts, suture reinforcements, and skin replacement materials (Jagur-Grodzinski 1999; Nair and Laurencin 2007). Tormala et al. proposed fully resorbable composites by reinforcing matrices with resorbable PLLA fibers and calcium phosphate-based glass fibers (Törmälä et al. 1991). One of the first commercially available fiber-formed bioresorbable medical products is based on copolymers of glycolic acid (GA) in combination with l-lactide (Vicryl) (Albertsson and Varma 2003). Porous PLA scaffolds have been found to be potential reconstruction matrices for damaged tissues and organs (Södergård and Stolt 2002; Avinc and Khoddami 2010; Nainar et al. 2014; Hoque et al. 2018). The capability of PLA to produce hybrid paper plastic packaging that is compostable and to recycle back to lactic acid by alcoholysis or hydrolysis has made it suitable for various applications. As a “green” food packaging polymer, PLA is now considered as the growing alternative. It has a wide range of application in the field of fresh products as thermoformed PLA containers are being used in retail markets as containers (Lunt 1998). Besides, PLA/natural fiber composites have been known for its potential applications in the areas of automotive parts, construction materials, food packaging, and medical devices (Asha et al. 2017).
3.7 Vegetable Oil-Derived Polymer
Vegetable oils, such as soybean oil, palm oil, and rapeseed oil, are extracted primarily from the seeds of oilseed plants and have a wide variety of applications: as foods, fuels (biofuels), lubricants, paints, cosmetics, pharmaceuticals, plasticizers, and construction materials (Hoque and Gee 2013). They are also attractive monomers for polymer chemistry due to their natural abundance and reactive functionality (Wang and Schuman 2013). From seeds of certain plants, vegetable oils are extracted, the mainstream of which are oil palm (Elaeis), soybean (Glycine max), sunflower (Helianthus), and oilseed rape (Brassica napus). These seeds have a wide range of applications and are produced on a very large scale (i.e., 156 megatons in 2012) (Zhu et al. 2016). Majority of them are used as food, biofuels, and chemical feedstocks. The key chemical building blocks of vegetable oils are triglycerides which is the triesters of glycerin and fatty acids. Of the five commonly occurring fatty acids, two are saturated (palmitic and stearic) and three are unsaturated (oleic, linoleic, and linolenic).
Triglyceride structure consisting of glycerol esterified by three long-chain aliphatic acids with variable number of carbon atoms (Fig. 8). During esterification, the number of C=C unsaturation in the chain makes the main difference for different types of fatty acid structures including the hydroxyl moieties (Pagliaro et al. 2007). Fatty acids account for 95% of the total weight of triglycerides and their content varies depending on the plant, the crop, the season, and the growing conditions (Fig. 8i). The structures of some frequently studied fatty acids are depicted in Fig. 8(ii). By transesterification reactions, they are commonly processed to produce glycerol and fatty esters. In terms of polymer production, glycerol is used as a precursor for the production of monomers such as epichlorohydrin and lactic acid. It is a widely used building block in polymer science finding application in the synthesis of polyurethanes, polyesters, or telomeres (Behr et al. 2008). On the other hand, fatty acids have been used for a long period for the development of polymeric structures, both directly and as building blocks for the synthesis of more sophisticated monomers (Behr et al. 2008).
The triglyceride oils need to be functionalized and separated to make well-defined polymers and monomers. By reactions at the olefin functional groups for unsaturated fatty acids, functionalization is achieved. Commonly used olefin functionalization reactions include epoxidation, isomerization, hydroformylation, reduction, and metathesis reactions to produce polyol (Williams and Hillmyer 2008). The polyurethanes are prepared by the reaction between a vegetable oil-derived polyol and various isocyanates. These polyurethane materials are characterized with particular attention being paid to their degradability and biocompatibility. The epoxy resin formation with epoxidized plant oils and fatty acids was the most vividly studied topic (Maisonneuve et al. 2013). The epoxidation of unsaturated fatty acids or triglycerides can be achieved in a straightforward reaction with, e.g., molecular oxygen, hydrogen peroxide as well as by chemo-enzymatic reactions. Additionally, acrylated epoxidized soybean oil as well as maleinized soy oil monoglyceride and maleinized hydroxylated oil were used to prepare composite materials with glass fibers as well as natural flax and hemp fibers (Maisonneuve et al. 2013).
Vegetable oils symbolize an excellent foundation for obtaining aliphatic diacids or diesters and diamines for polyamides production. Partially fatty acid-based polyamides from azelaic acid (PA-6,9 and PA-6,6,9), sebacic acid (PA-6,10), dimer fatty acids, and brassylic acid have been synthesized from different researchers (Meier et al. 2007). Polyamide synthesis from plant oils already found industrial application in the preparation of Nylon-11, since the necessary monomer, 11-amino-undecanoic acid, can be obtained from castor oil by further chemical modification of 10-undecenoic acid (Maisonneuve et al. 2013).
The radical polymerization of acrylated or maleinized plant oil derivatives was investigated to produce plant oil-based thermosets. Another way is the direct cross-linking of soybean oil with polybutadiene or p-dinitrosobenzene and of linseed oil with phenolic resins. In this regard recently, the synthesis of polyurethane foams and the development of biocompatible adhesives were also carried out from plant oil (Meier et al. 2007; Yilmaz and Kusefoğlu 2005; Mutlu and Kusefoglu 2009). Moreover, the great potential of fatty acids as building blocks for olefin metathesis polymerization (ADMET) is now materialized commercially (de Espinosa and Meier 2011). On the other hand, the production of monomers and polymers by thiol–ene coupling reactions with fatty acid derivatives is a newly growing area (Black and Rawlins 2009). These multifunctional thiols and enes were synthesized by ring opening of epoxidized soybean oil with polythiols or allyl alcohol, respectively. These products with high functionality provided good coating film properties by UV curing in the presence of petrochemical-based enes or thiols.
3.8 Terpenes
Terpenes and terpenoids are derived from plants. Both terpenes and terpenoids have a common isoprene unit (2-methyl-1,4-butadiene) as a common carbon skeleton building block in their chemical structures. The best-known polyterpene is natural rubber. Other types of terpenes are being investigated as monomers for polymer production, although on a much smaller scale. One of them is turpentine, which is extracted from pine trees (Pinus spp.) and is composed mainly of α-pinene (45–97%) and β-pinene (0.5–28%), and limonene, which is extracted from the peel of citrus fruits (De Carvalho and da Fonseca 2006). The general formula of monoterpene is C10H16 (Erman 1985). Among the huge variety, α-pinene, β-pinene, limonene, and myrcene are the most common types of terpenes which can be readily isolated in viable amounts from turpentine.
Among terpenes, β-pinene is by far the most sustainable monomer because of the unhindered availability of its alkenyl unsaturation. Its cationic polymerization has been extensively studied, first with classical Lewis-acid initiators and then using quasi-living systems (Silvestre and Gandini 2008). Myrcene was polymerized through a double mechanism consisting of a cyclization step through ring-closing metathesis followed by its cationic activation to give a high molecular weight polymer (Kobayashi et al. 2009). The synthesis of polyamides and polyurethanes was also done from limonene-based diamines and diamides prepared by a similar click strategy involving thiolamine-hydrochlorides (Firdaus and Meier 2013). Monomer limonene oxide was copolymerized with CO2 using β-diiminate zinc complexes to yield polycarbonate structures (Byrne et al. 2004). A polyester network, with ultra-small pores exploited for gas separation application, was synthesized from triterpene as monomer by the isolation of betulin (which bear two OH groups) from birch bark and its polycondensation with 1,3,5-benzenetricarbonyl trichloride (Jeromenok et al. 2011).
3.9 Furans
Furan derivatives are present in nature in a wide category of structures. Furan is one of the major representatives of the five-member unsaturated heterocycle family. Furan displays the lowest aromatic and the highest dienic character. In furan chemistry alkylation, halogenation, sulfonation, and nitration occur regioselectively at C2 and/or C5. A typical consequence in macromolecular synthesis is the ease with which furan, and some of its derivatives undergo the Diels–Alder (DA) reaction as dienes (Gandini and Belgacem 1997; Moreau et al. 2004; Gandini 2010, 2011a, b; Manfredi and Rivero 2012; Hou et al. 1998; Sutton et al. 2013).
Some of the novel furan monomers displayed characteristics entirely compatible with their conventional petrochemical alternatives. 2-Furyl oxirane monomer can take part in anionic polymerization with the initiation by a very weak nucleophiles like amines and alcohols, for the synthesis of block, star, and grafted copolymers (Gandini and Belgacem 1997; Moreau et al. 2004; Gandini 2010, 2011a, b; Manfredi and Rivero 2012; Hou et al. 1998; Sutton et al. 2013).
Polyamides were made by “direct condensation” through the polycondensation of both 2,5- and 3,4-dicarboxylic acids with a number of aromatic diamines (Gandini and Belgacem 1997; Moreau et al. 2004; Gandini 2010, 2011a, b; Manfredi and Rivero 2012; Hou et al. 1998; Sutton et al. 2013). These polyamides showed regular structures, high molecular weights and interesting crystallization and thermal properties. Furans resulting from furfural (F) can be condensed with aldehydes and ketones to produce difuran monomers bearing different terminal functionalities. Polyesters, polyamides, and polyurethanes were prepared with these monomers by step-growth polymerizations. When hydroxymethylfurfural (HMF) was used to prepare novel furan monomers, a new family of polycondensation structures arose and their macromolecules displayed remarkable properties (Gandini and Belgacem 1997; Moreau et al. 2004; Gandini 2010, 2011a, b; Manfredi and Rivero 2012; Hou et al. 1998; Sutton et al. 2013). Other materials based on the polymerization of furan monomers include photosensitive macromolecules with applications in the printing industry, conjugated semiconducting polymers with luminescent features, polymer electrolytes for solid-state batteries based on the combination of furan and chitosan chemistries, and insulating foams (Gandini and Belgacem 1997; Moreau et al. 2004; Gandini 2010, 2011a, b; Manfredi and Rivero 2012; Hou et al. 1998; Sutton et al. 2013).
3.10 Polyhydroxyalkanoates
Biodegradable polyhydroxyalkanoates (PHAs) plastics are produced by bacteria in several grades with the difference in composition and molecular weight (Braunegg et al. 1998). The bacteria produces the PHAs from a carbon source that is when overfed, the PHA polymer is formed and on the contrary the polymer is consumed when the bacteria are short of a feeding source. PHAs have properties similar to those of some polyolefins. They are fully and rapidly biodegraded under appropriate conditions. The simplest PHA is polyhydroxybutyrate (PHB) polymer and the only type of PHAs relevant for practical applications (Braunegg 2002). The first step of bacterial synthesis of PHB consists in the conversion of a selected carbon source to acetate. Then, an enzyme cofactor is attached via the formation of a thioester bond. The enzyme, called coenzyme A (CoA), is a universal carrier of acyl groups in biosynthesis, and acetyl-CoA is a basic metabolic molecule found in all PHA-producing organisms by the sequential action of three enzymes, 3-ketothiolase, acetoacetyl-CoA reductase, and PHA synthase (Choi and Lee 1999). A dimer acetoacetyl-CoA is formed via reversible condensation and subsequently reduced to a monomer unit (R)-3-hydroxybutyryl-CoA. PHB is formed via the polymerization of the latter, maintaining the asymmetric center (Macrae and Wilkinson 1958).
Appropriate exchanged propiolactones can be used to produce PHAs chemically using aluminum or zinc alkyl catalysts with water as the cocatalyst. In terms of stereoregularity, synthetic PHAs can be made almost identical to the corresponding biopolymers (Chodak 2008). This resemblance results even in the excellent biodegradability of the material (Araki and Hayase 1979). However, high lactone monomer costs are the major limitation for industrially application of the synthetic PHAs (Philip et al. 2007).
The structure of PHAs is based on polyester macromolecules bearing optically active carbon atoms. The crystalline structure of PHB is orthorhombic with dimensions of the basic crystalline cell a = 5.76 Å, b = 13.20 Å, c = 5.96 Å. A partially planar zigzag structure can be formed as a result of the mechanical uniaxial load from the amorphous phase between lamellae (Yamane et al. 2001; Orts et al. 1990). PHB is a completely biodegradable, highly hydrophobic thermoplastics, containing almost 80% crystallinity, with high melting temperature, resistance to organic solvents and possessing excellent mechanical strength and modulus resembling that of polypropylene (Pollet and Avérous 2011). Noticeable brittleness, very low deformability, high propensity to a rapid thermal degradation, difficult processing by conventional thermoplastic technologies (mainly due to fast thermal degradation), and rather high price compared to other high-volume plastics may be named as the major factors hindering a wider application of PHB (Follain et al. 2014).
In order to improve properties of PHB, random copolymers have been prepared by replacing the methyl group on the PHB main chain by ethyl or longer substituents with the help of changing the substrate on which the bacteria grow (Doi et al. 1987). Bacterial synthesis using sodium octanoate as a sole carbon source was reported to result in a formation of copolymer with the majority of polyhydroxyoctanoate segments (Gagnon et al. 1992). An application of epoxidized soybean oil has been reported recently for plasticizing blends of PHBV and cellulose acetate butyrate for packaging applications. The material with longer side chains is claimed to be suitable for using as degradability enhancers for poly(olefins) (Marschessault et al. 1990). In another study, it is claimed that the ductility of PHB can be improved significantly by hot rolling treatment (Barham and Keller 1986). In case of chemical modification, poly(hydroxy ether) prepared from Bisphenol A was reported to react with PHB by transesterification during annealing at 180 °C (Yuan and Ruckenstein 1998). In another case, grafting of acrylic acid onto PHB and PHBV was initiated by gamma irradiation (Mitomo et al. 1996).
Injection blow-molded bottles for packaging of biodegradable containers for hair shampoo or motor oil and disposable razor handles were produced with PHB (Ramsay and Ramsay 1990; Dalev et al. 1998). Excellent barrier properties against gas permeation of isotropic PHB foils may be considered for application in foodstuffs packaging (Basta 1984). PHB-coated paper has been shown to be completely biodegradable and also easier to recycle than conventionally coated paper (Chodak 2002). A direct electrostatic coating technique is possible to use for depositing PHB on a low dielectric substrate such as paper (Marchessault et al. 1993). PHB fibers were considered to be mainly used for the production of scaffolds (Van de Velde and Kiekens 2002). PHB composites with apatite can be used as biodegradable bone fracture fixations or even as bone repair materials (Ni and Wang 2002).
3.11 Polycarbonates
As carbon dioxide (CO2) is abundant and inexpensive, it is an interesting synthetic feedstock for the production of polymers. CO2 fixation through reaction with the highly reactive three-membered epoxide ring to afford cyclic or polymeric carbonates is an extensive field of research (Kember et al. 2011; Miao et al. 2008). The copolymerization involves the alternating insertion of carbon dioxide and epoxide in the growing polymer chain. The use of a catalyst is required in order to achieve the selective synthesis of polycarbonates with high yield and under relatively mild conditions of temperature and CO2 pressure. In the copolymerization cycle, a Lewis acidic metal species binds and ring opens an epoxide, generating a metal alkoxide species. CO2 introduces into the metal alkoxide bond giving a metal carbonate, and this group can combine and ring open a further molecule of epoxide; duplication of the cycle ultimately directs to the production of high molecular weight polycarbonate (Coates and Moore 2004). A variety of homogeneous and heterogeneous Lewis acidic metal complexes initiate the polymerization, including Zn(II), Co(II), and Cr(III) alkoxides, carboxylates, and carbonates (Cohen et al. 2005). It was also found that the addition of phenylphosphonic acid [PhP(O)(OH)2] or phosphoric acid [H3PO4] as chain transfer agent in CO2–PO copolymerization catalyzed by a cobalt salen complex produced flame-retarding poly(propylene carbonate)s (Cohen et al. 2005).
The mechanical properties of polycarbonates can differ significantly as a function of the nature of the epoxide used in the copolymerization with CO2. Poly(cyclohexene carbonate) is a brittle material at room temperature, whereas poly(ethylene carbonate) displays rubber-like properties (Taherimehr and Pescarmona 2014). The low thermal degradation temperature of polycarbonates is problematic for processing by common thermoplastic techniques such as molding, mixing, or extrusion. The use of co-monomers and end groups has improved the thermal stability (Hwang et al. 2003).
Polycarbonate polyols of low molecular weight may be suitable to prepare foams, coatings, and adhesives, whereas high molecular weight polycarbonates may be used as rigid plastics or elastomers (Zhu et al. 2016). Recently, it was shown in a discovery that poly(propylene carbonate) (PPC) could be used in the application as organic filler in packaging material and containers as with some modification it exhibited good adhesion, smooth processability, and better thermal degradability (Allen 2014). PPC has potential application in medical implants for its production of nontoxic consequences by enzymatic degradation in the body (Kim et al. 2008). PPC can also be used in combination with other polymers (such as starch) to make biodegradable plastics (Luinstra 2008).
3.12 Biopolyethylene (PE) and Biopolyethylene Terephthalate (PET)
Polyethylene (PE) is an important engineering polymer traditionally produced from petroleum-based resources. PE is now being manufactured from dehydration of ethanol produced by microbial fermentation of plant-based renewable resources. Bioethanol is developed from sugar cane, sugar beet, maize, wood, wheat, corn, and other plant wastes through microbial strain and biological fermentation process (Babu et al. 2013). At the end of the fermentation process, ethanol is distilled in order to remove water and to yield azeotropic mixture of hydrous ethanol. Ethanol is then dehydrated at high temperatures over a solid catalyst to produce ethylene and, subsequently, polyethylene (Chen et al. 2007). Polyethylene from natural resources has exactly the same chemical, physical, and mechanical properties as petrochemical polyethylene. These bio-based PE grades are mainly targeted toward food packing, cosmetics, personal care, automotive parts, and toys.
For partially bio-based polyethylene terephthalate (PET) (30 or 70%) production, one of the two fossil precursors {e.g., ethylene glycol (EG) and purified terephthalic acid (PTA)} is substituted with a bio-based material. Distinctively for a fully bio-based PET, both precursors are produced from biomass (Tuck et al. 2012). PTA accounts for approximately 70% of the weight of PET resin, while EG contributes to the rest. In the case of bio-based PET, para-xylene is produced from bioisobutanol by fermentation of cellulosic materials. PTA is currently produced from the oxidation of aromatic para-xylene. Then, PTA is condensed with ethylene glycol to make PET. Bio-based versions of PTA and EG can be produced from a variety of biomass materials (Mülhaupt 2013). Currently, PET is being used for clothing and beverage bottles on a huge scale.
4 Conclusions and Future Perspectives
Undoubtedly, renewability of polymer material is an increasing new point to maintain the sustainability of ecosystem. As the foreseeable limit of fossil feedstocks and the increasing the environmental concerns, it is necessary to develop novel bio-renewable resource-based polymer materials on a large scale. Therefore, readjusting in carbon cycle is taken into serious consideration by the researchers. In this process, renewability of polymers is an escalating new fact to uphold the sustainability of the ecosystem. Polymers from renewable resources will perform a continual growing task in the common plastic goods as well as in medical products. Apart from economic and environmental advantages, the new property outcomes are evitable in the renewable base polymers. Essential research attempts in this field are set to persist. It is obligatory at this stage in the development of this renewable research field to assemble an abundant database in preparation for the gradual declining of fossil resources. This will eventually enable us to pick wise decisions and choices for industrial implementation.
References
Adler E (1977) Lignin chemistry—past, present and future. Wood Sci Technol 11(3):169–218
Akbari A, Majumder M, Tehrani A (2015) Polylactic acid (PLA) carbon nanotube nanocomposites. In: Handbook of polymer nanocomposites. Processing, performance and application. Springer, Berlin, pp 283–297
Albertsson AC, Varma IK (2003) Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 4(6):1466–1486
Allen SD (2014) U.S. Patent No. 8,748,555. U.S. Patent and Trademark Office, Washington, DC
Araki T, Hayase S (1979) Biodegradability of synthetic β-substituted poly-β-esters. J Polym Sci Polym Chem Ed 17(6):1877–1881
Arof L, Subban RHY, Radhakrishna S (1995) In: Prasad PN (ed) Polymer and other advanced materials: emerging technologies and business. p 539
Asha AB, Sharif A, Hoque ME (2017) Interface interaction of jute fiber reinforced PLA biocomposites for potential applications. In: Jawaid M, Salit MS, Alothman OY (eds) Green biocomposites: design and applications. Springer, Switzerland
Atkins E (1985) Conformations in polysaccharides and complex carbohydrates. J Biosci 8(1–2):375–387
Auras R, Harte B, Selke S (2004) An overview of polylactides as packaging materials. Macromol Biosci 4(9):835–864
Avinc O, Khoddami A (2010) Overview of poly(lactic acid)(PLA) fibre. Fibre Chem 42(1):68–78
Ayhllon-Meixueiro F, Tropini V, Silvestre F (2001) Fatty esterification of plant proteins. In: Biorelated polymers. Springer, Boston, pp 231–236
Babu RP, O’connor K, Seeram R (2013) Current progress on bio-based polymers and their future trends. Prog Biomater 2(1):8
Baeumler A, Ijjasz-Vasquez E, Mehndiratta S (2012) Sustainable low-carbon cities in China: why it matters and what can be done. In: Sustainable low-carbon city development in China, pp 39–67
Barham PJ, Keller A (1986) The relationship between microstructure and mode of fracture in polyhydroxybutyrate. J Polym Sci Part B Polym Phys 24(1):69–77
Basta N (1984) Biopolymers challenge petrochemicals. High Technol 4(2):66–70
Behr A, Eilting J, Irawadi K, Leschinski J, Lindner F (2008) Improved utilisation of renewable resources: new important derivatives of glycerol. Green Chem 10(1):13–30
Bemiller JN (1997) Starch modification: challenges and prospects. Starch-Stärke 49(4):127–131
Black M, Rawlins JW (2009) Thiolene UV-curable coatings using vegetable oil macromonomers. Eur Polym J 45(5):1433–1441
Blackwell J (1982) The macromolecular organization of cellulose and chitin. In: Cellulose and other natural polymer systems. Springer, Boston, pp 403–428
Botello-Álvarez JE, Rivas-García P, Fausto-Castro L, Estrada-Baltazar A, Gomez-Gonzalez R (2018) Informal collection, recycling and export of valuable waste as transcendent factor in the municipal solid waste management: a Latin-American reality. J Clean Prod 182:485–495
Braunegg G (2002) Sustainable poly(hydroxyalkanoate)(PHA) production. In: Degradable polymers. Springer, Dordrecht, pp 235–293
Braunegg G, Lefebvre G, Genser KF (1998) Polyhydroxyalkanoates, biopolyesters from renewable resources: physiological and engineering aspects. J Biotechnol 65(2–3):127–161
Browning WC (1955) Lignosulfonate stabilized emulsions in oil well drilling fluids. J Petrol Technol 7(06):9–15
Bruijnincx PC, Rinaldi R, Weckhuysen BM (2015) Unlocking the potential of a sleeping giant: lignins as sustainable raw materials for renewable fuels, chemicals and materials. Green Chem 17(11):4860–4861
Byrne CM, Allen SD, Lobkovsky EB, Coates GW (2004) Alternating copolymerization of limonene oxide and carbon dioxide. J Am Chem Soc 126(37):11404–11405
Calvo-Flores FG, Dobado JA (2010) Lignin as renewable raw material. Chemsuschem 3(11):1227–1235
Carrott PJM, Carrott MR (2007) Lignin–from natural adsorbent to activated carbon: a review. Biores Technol 98(12):2301–2312
Chauhan GS, Mahajan S, Guleria LK (2000) Polymers from renewable resources: sorption of Cu2+ ions by cellulose graft copolymers. Desalination 130(1):85–88
Chen G, Li S, Jiao F, Yuan Q (2007) Catalytic dehydration of bioethanol to ethylene over TiO2/γ-Al2O3 catalysts in microchannel reactors. Catal Today 125(1–2):111–119
Cheng Y, Deng S, Chen P, Ruan R (2009) Polylactic acid (PLA) synthesis and modifications: a review. Front Chem China 4(3):259–264
Chiellini E, Chiellini F, Cinelli P (2002a) Polymers from renewable resources. In: Degradable polymers. Springer, Dordrecht, pp 163–233
Chiellini E, Cinelli P, Antone SD, Ilieva VI (2002b) Environmentally degradable polymeric materials (EDPM) in agricultural applications—an overview. Polimery 47(7–8):538–544
Chodak I (2002) Polyhydroxyalkanoates: properties and modification for high volume applications. In: Degradable polymers. Springer, Dordrecht, pp 295–319
Chodak I (2008) Polyhydroxyalkanoates: origin, properties and applications. In: Monomers, polymers and composites from renewable resources. pp 451–477
Choi J, Lee SY (1999) Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl Microbiol Biotechnol 51(1):13–21
Coates GW, Moore DR (2004) Discrete metal-based catalysts for the copolymerization of CO2 and epoxides: discovery, reactivity, optimization, and mechanism. Angew Chem Int Ed 43(48):6618–6639
Cohen CT, Chu T, Coates GW (2005) Cobalt catalysts for the alternating copolymerization of propylene oxide and carbon dioxide: combining high activity and selectivity. J Am Chem Soc 127(31):10869–10878
Cuq B, Gontard N, Guilbert S (1998) Proteins as agricultural polymers for packaging production. Cereal Chem 75(1):1–9
Dalev P, Vassileva E, Mark JE, Fakirov S (1998) Enzymatic degradation of formaldehyde-crosslinked gelatin. Biotechnol Tech 12(12):889–892
Das O, Sarmah AK, Bhattacharyya D (2015) A sustainable and resilient approach through biochar addition in wood polymer composites. Sci Total Environ 512:326–336
De Carvalho CC, da Fonseca MMR (2006) Biotransformation of terpenes. Biotechnol Adv 24(2):134–142
de Espinosa LM, Meier MA (2011) Plant oils: the perfect renewable resource for polymer science?! Eur Polym J 47(5):837–852
De Graaf LA, Kolster P (1998) Industrial proteins as a green alternative for ‘petro’ polymers: potentials and limitations. In: Macromolecular symposia, vol 127, no 1. Hüthig & Wepf Verlag, Basel, pp 51–58
De Graaf LA, Kolster P, Vereijken JM (1998) Modification of wheat gluten for non-food applications. Plant proteins from European crops. Springer, Berlin, pp 335–339
Doane WM, Swanson CL, Fanta GF (1992) Emerging polymeric materials based on starch. In: Rowell RM, Scultz TP, Narayan R (eds) Emerging technologies for materials and chemicals from biomass, ACS symposium series (USA) 476, pp 197–230
Doi Y, Kunioka M, Nakamura Y, Soga K (1987) Biosynthesis of copolyesters in Alcaligenes eutrophus H16 from carbon-13 labeled acetate and propionate. Macromolecules 20(12):2988–2991
dos Santos Abreu H, do Nascimento AM, Maria MA (2007) Lignin structure and wood properties. Wood Fiber Sci 31(4):426–433
Drumright RE, Gruber PR, Henton DE (2000) Polylactic acid technology. Adv Mater 12(23):1841–1846
Dufresne A (2017) Nanocellulose: from nature to high performance tailored materials. Walter de Gruyter GmbH & Co KG, Berlin
Einchhorn SJ (2011) Cellulose nanowhiskers: promising materials for advanced applications. Soft Matter 7(2):303–315
Einchhorn SJ, Dufresne A, Aranguren MM, Capadona JR, Rowan SJ, Weder C et al (2010) Review: current international research into cellulose nanofibres and composites. J Mater Sci 45:1–33
Erman WF (1985) In: Gassman PG (ed) Chemistry of the monoterpenes, Part A. Studies in organic chemistry. Marcel Dekker, New York, pp 929–1033
Feughelman M (2002) Natural protein fibers. J Appl Polym Sci 83(3):489–507
Firdaus M, Meier MA (2013) Renewable polyamides and polyurethanes derived from limonene. Green Chem 15(2):370–380
Focher B, Marzetti A, Crescenzi V (eds) (1991) Steam explosion techniques: fundamentals and industrial applications. In: Proceedings of the international workshop on steam explosion techniques: fundamentals and industrial applications, Milan, Italy, 20–21 Oct 1988. CRC Press, Boca Raton
Follain N, Chappey C, Dargent E, Chivrac F, Crétois R, Marais S (2014) Structure and barrier properties of biodegradable polyhydroxyalkanoate films. J Phys Chem C 118(12):6165–6177
French AD, Bertoniere NR, Battista OA, Cuculo JA, Gray DG (1993) Cellulose, Chapter in: Kirk-Othmer encyclopedia of chemical technology, 4th edn., vol. 5. Wiley, New York, pp 476–496
Gagnon KD, Lenz RW, Farris RJ, Fuller RC (1992) The mechanical properties of a thermoplastic elastomer produced by the bacterium Pseudomonas oleovorans. Rubber Chem Technol 65(4):761–777
Gandini A (2010) Furans as offspring of sugars and polysaccharides and progenitors of a family of remarkable polymers: a review of recent progress. Polym Chem 1(3):245–251
Gandini A (2011a) Furan monomers and their polymers: synthesis, properties and applications. In: Biopolymers—new materials for sustainable films and coatings, pp 179–209
Gandini A (2011b) The irruption of polymers from renewable resources on the scene of macromolecular science and technology. Green Chem 13(5):1061–1083
Gandini A, Belgacem MN (1997) Furans in polymer chemistry. Prog Polym Sci 22(6):1203–1379
Gandini A, Belgacem MN (2013) The state of the art of polymers from renewable resources. In: Handbook of biopolymers and biodegradable plastics, pp 71–85
Gandini A, Lacerda TM (2015) From monomers to polymers from renewable resources: recent advances. Prog Polym Sci 48:1–39
Goheen DH, Royt CR (1981) Lignin. Kirk-othmer encyclopedia of polymer science and technology, vol 14, 3rd edn. Wiley, New York, pp 294–312
Gorochovceva N, Makuška R (2004) Synthesis and study of water-soluble chitosan-O-poly(ethylene glycol) graft copolymers. Eur Polym J 40(4):685–691
Habibi Y (2014) Key advances in the chemical modification of nanocelluloses. Chem Soc Rev 43(5):1519–1542
Habibi Y, Lucia LA, Rojas OJ (2010) Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev 110(6):3479–3500
Han Y, Yuan L, Li G, Huang L, Qin T, Chu F, Tang C (2016) Renewable polymers from lignin via copper-free thermal click chemistry. Polymer 83:92–100
Hoenich NA, Stamp S (2000) Clinical investigation of the role of membrane structure on blood contact and solute transport characteristics of a cellulose membrane. Biomaterials 21(3):317–324
Hoque ME, Gee LP (2013) Biodiesel from plant resources—sustainable solution to ever increasing fuel oil demands. J Sustain Bioenergy Syst 3:163–170
Hoque ME, Ye TJ, Yong LC, Dahlan KM (2013) Sago starch-mixed low-density polyethylene (LDPE) biodegradable polymer: synthesis and characterization. J Mater Article ID 365380, 7 pages. https://doi.org/10.1155/2013/365380
Hoque ME, Khan AM, Islam MS, Asim M, Jawaid M, Othman OA (2016) The effect of natural degradation on the mechanical and morphological properties of tropical woods. Cellul Chem Technol 50(7–8):723–730
Hoque ME, Daei JMG, Khalid M (2018) Next generation biomimetic bone tissue engineering matrix from poly(l-lactic acid) PLA/calcium carbonate composites doped with silver nanoparticles. Curr Anal Chem 14(3):268–277
Hou XL, Cheung HY, Hon TY, Kwan PL, Lo TH, Tong SY, Wong HN (1998) Regioselective syntheses of substituted furans. Tetrahedron 54(10):1955–2020
Hutmacher DW, Hoque ME, Wong YS (2008) Design, fabrication and physical characterization of scaffolds made from biodegradable synthetic polymers in combination with RP systems based on melt extrusion. In: Bidanda B, Bartolo P (eds) Virtual prototyping & bio manufacturing in medical applications. Springer, USA
Hwang Y, Jung J, Ree M, Kim H (2003) Terpolymerization of CO2 with propylene oxide and ε-caprolactone using zinc glutarate catalyst. Macromolecules 36(22):8210–8212
Imam SH, Mao L, Chen L, Greene RV (1999) Wood adhesive from crosslinked poly(vinyl alcohol) and partially gelatinized starch: preparation and properties. Starch-Stärke 51(6):225–229
Imre B, Pukánszky B (2015) From natural resources to functional polymeric biomaterials. Eur Polym J 68:481–487
Jagur-Grodzinski J (1999) Biomedical application of functional polymers. React Funct Polym 39(2):99–138
Jeromenok J, Böhlmann W, Antonietti M, Weber J (2011) Intrinsically microporous polyesters from betulin-toward renewable materials for gas separation made from birch bark. Macromol Rapid Commun 32(22):1846–1851
Jiang L, Li Y, Wang X, Zhang L, Wen J, Gong M (2008) Preparation and properties of nano-hydroxyapatite/chitosan/carboxymethyl cellulose composite scaffold. Carbohydr Polym 74(3):680–684
Jyothi AN (2010) Starch graft copolymers: novel applications in industry. Compos Interfaces 17(2–3):165–174
Kadla JF, Kubo S, Gilbert RD, Venditti RA (2002) Lignin-based carbon fibers. In: Chemical modification, properties, and usage of lignin. Springer, Boston, MA, pp 121–137
Kanagaratnam S, Hoque ME, Sahri MM, Spowage A (2013) Investigating the effect of deforming temperature on the oil-binding capacity of palm oil based shortening. J Food Eng 118(1):90–99
Kauffman GB (1993) Rayon: the first semi-synthetic fiber product. J Chem Educ 70(11):887
Kember MR, Buchard A, Williams CK (2011) Catalysts for CO2/epoxide copolymerisation. Chem Commun 47(1):141–163
Kester J (1986) Edible films and coatings: a review. Food Technol 40(12):47–59
Ketabchi MR, Khalid M, Hoque ME, Ratnam CT, Walvekar R (2015) Eco-friendly and cost-effective isolation of cellulose microfibres and nano-crystals from kenaf fibres. In: Proceedings, 13th international conference on environment, ecosystems and development (EED 2015), 23–25 Apr 2015, Kuala Lumpur, Malaysia
Ketabchi MR, Khalid M, Ratnam CT, Manicham S, Walvekar R, Hoque ME (2016) Sonosynthesis of cellulose nanoparticles (CNP) from kenaf fiber: effects of processing parameters. Fibers Polym 17(9):1352–1358
Kim G, Ree M, Kim H, Kim IJ, Kim JR, Im Lee J (2008) Biological affinity and biodegradability of poly(propylene carbonate) prepared from copolymerization of carbon dioxide with propylene oxide. Macromol Res 16(5):473–480
Klemm D, Kramer F, Moritz S, Lindström T, Ankerfors M, Gray D, Dorris A (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed 50(24):5438–5466
Kobayashi S, Lu C, Hoye TR, Hillmyer MA (2009) Controlled polymerization of a cyclic diene prepared from the ring-closing metathesis of a naturally occurring monoterpene. J Am Chem Soc 131(23):7960–7961
Koutinas AA, Chatzifragkou A, Kopsahelis N, Papanikolaou S, Kookos IK (2014) Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 116:566–577
Kruger LH (1989) U.S. Patent No. 4,838,944. U.S. Patent and Trademark Office, Washington, DC
Kulkarni RK, Moore EG, Hegyeli AF, Leonard F (1971) Biodegradable poly(lactic acid) polymers. J Biomed Mater Res 5(3):169–181
Kumar S, Krishnan S, Samal SK, Mohanty S, Nayak SK (2017) Itaconic acid used as a versatile building block for the synthesis of renewable resource based resins and polyesters for future prospective: a review. Polym Int 66(10):1349–1363
Kurita K (2001) Controlled functionalization of the polysaccharide chitin. Prog Polym Sci 26(9):1921–1971
Labet M, Thielemans W, Dufresne A (2007) Polymer grafting onto starch nanocrystals. Biomacromolecules 8(9):2916–2927
Lagoa R, Murtinho D, Gil MH (1999) Membranes of cellulose derivatives as supports for immobilization of enzymes. In: Imam SH, Greene RV, Zaidi BR (eds) Biopolymers—utilizing nature’s, advanced, materials. Acs symposium series 723, ACS, Washington DC, pp 228–234
Lasprilla AJ, Martinez GA, Lunelli BH, Jardini AL, Maciel Filho R (2012) Poly-lactic acid synthesis for application in biomedical devices—a review. Biotechnol Adv 30(1):321–328
Lindblad MS, Liu Y, Albertsson AC, Ranucci E, Karlsson S (2002) Polymers from renewable resources. In: Degradable aliphatic polyesters. Springer, Berlin, pp 139–161
Lindsey JB, Tollens B (1892) Ueber Holz-Sulfitflüssigkeit und Lignin. Justus Liebigs Annalen der Chemie 267(2–3):341–366
Lligadas G, Tüzün A, Ronda JC, Galià M, Cádiz V (2014) Polybenzoxazines: new players in the bio-based polymer arena. Polym Chem 5(23):6636–6644
Lora JH, Aziz S (1985) Organosolv pulping: a versatile approach to wood refining. Tappi (United States) 68(8)
Lora JH, Glasser WG (2002) Recent industrial applications of lignin: a sustainable alternative to nonrenewable materials. J Polym Environ 10(1–2):39–48
Luinstra GA (2008) Poly(propylene carbonate), old copolymers of propylene oxide and carbon dioxide with new interests: catalysis and material properties. Polym Rev 48(1):192–219
Lunt J (1998) Large-scale production, properties and commercial applications of polylactic acid polymers. Polym Degrad Stab 59(1–3):145–152
Mac Kinnon M, Brouwer J, Samuelsen S (2018) The role of natural gas and its infrastructure in mitigating greenhouse gas emissions, improving regional air quality, and renewable resource integration. Prog Energy Combust Sci 64:62–92. https://doi.org/10.1016/j.pecs.2017.10.002
Macrae RM, Wilkinson JF (1958) The influence of culture conditions on polyhydroxybutyrate synthesis by Bacillus megaterium. In: Proceedings of the royal society of Edinburgh, vol 27. pp 73–78
Maim CJ, Mench JW, Kendall DL, Hiatt GD (1951) Aliphatic acid esters of cellulose. Preparation by acid-chloride-pyridine procedure. Ind Eng Chem 43(3):684–688
Maisonneuve L, Lebarbé T, Grau E, Cramail H (2013) Structure–properties relationship of fatty acid-based thermoplastics as synthetic polymer mimics. Polym Chem 4(22):5472–5517
Manfredi LB, Rivero G (2012) Furan resins: synthesis, characterization and applications. Chem Phys Res J 5(1/2):45
Marchessault RH, Rioux P, Saracovan I (1993) Direct electrostatic coating of paper. Nordic Pulp Paper Res J (Sweden) 8(1):211–216
Marschessault RH, Monasterios CJ, Morin FG, Sundararajan PR (1990) Chiral poly(β-hydroxyalkanoates): an adaptable helix influenced by the alkane side-chain. Int J Biol Macromol 12(2):158–165
Mathiasson A, Kubat DG (1994) Lignin as binder in particle boards using high frequency heating. Holz als Roh-und Werkstoff 52(1):9
Meier MA, Metzger JO, Schubert US (2007) Plant oil renewable resources as green alternatives in polymer science. Chem Soc Rev 36(11):1788–1802
Meshram MW, Patil VV, Mhaske ST, Thorat BN (2009) Graft copolymers of starch and its application in textiles. Carbohydr Polym 75(1):71–78
Miao CX, Wang JQ, He LN (2008) Catalytic processes for chemical conversion of carbon dioxide into cyclic carbonates and polycarbonates. Open Org Chem J 2(1)
Michael FM, Khalid M, Walvekar R, Ratnam CT, Ramarad S, Siddiqui H, Hoque ME (2016) Effect of nanofillers on the physico-mechanical properties of load bearing bone implants. Mater Sci Eng C 67:792–806
Missoum K, Belgacem MN, Bras J (2013) Nanofibrillated cellulose surface modification: a review. Materials 6(5):1745–1766
Mitomo H, Sasaoka T, Yoshii F, Makuuchi K, Saito T (1996) Radiation-induced graft polymerization of acrylic acid onto poly(3-hydroxybutyrate) and its copolymer. Sen’i Gakkaishi 52(11):623–626
Moreau C, Belgacem MN, Gandini A (2004) Recent catalytic advances in the chemistry of substituted furans from carbohydrates and in the ensuing polymers. Top Catal 27(1–4):11–30
Mormann W, Spitzer D (2001) Silylation of OH‐polymers by reactive extrusion. In: Macromolecular symposia, vol 176, no 1. WILEY‐VCH Verlag GmbH, Weinheim, pp 279–288
Mülhaupt R (2013) Green polymer chemistry and bio-based plastics: dreams and reality. Macromol Chem Phys 214(2):159–174
Müller AJ, Ávila M, Saenz G, Salazar J (2015) Crystallization of PLA-based materials. Poly(lactic acid) science and technology: processing, properties, additives and applications. Royal Society of Chemistry, London, pp 68–98
Mutlu H, Kusefoglu SH (2009) Synthesis and characterization of polymers from soybean oil and p-dinitrosobenzene. J Appl Polym Sci 113(3):1925–1934
Muzzarelli RAA (1997) Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell Mol Life Sci CMLS 53(2):131–140
Nainar SMM, Begum S, Ansari MNM, Hoque ME, Aini SS, Ng MH, Ruszymah BHI (2014) Effect of compatibilizers on in vitro biocompatibility of PLA-HA bioscaffold. Bioinspired Biomimetic Nanobiomater 3(4):208–216
Nair LS, Laurencin CT (2007) Biodegradable polymers as biomaterials. Prog Polym Sci 32(8–9):762–798
Nakajima M, Atsumi K, Kifune K (1984) Development of absorbable sutures from chitin. In: Chitin, chitosan, and related enzymes. Academic Press, New York, pp 407–410
Ni J, Wang M (2002) In vitro evaluation of hydroxyapatite reinforced polyhydroxybutyrate composite. Mater Sci Eng C 20(1–2):101–109
Nilsson TY, Wagner M, Inganäs O (2015) Lignin modification for biopolymer/conjugated polymer hybrids as renewable energy storage materials. Chemsuschem 8(23):4081–4085
Northey RA (1992) In: Rowell RM, Scultz TP, Narayan R (eds) Low cost uses of lignin, in emerging technologies for materials and chemicals from biomass, ACS symposium series 476. ACS, Washington, D.C., pp l46–175
Orts WJ, Marchessault RH, Bluhm TL, Hamer GK (1990) Observation of strain-induced β form in poly(β-hydroxyalkanoates). Macromolecules 23(26):5368–5370
Pagliaro M, Ciriminna R, Kimura H, Rossi M, Della Pina C (2007) From glycerol to value-added products. Angew Chem Int Ed 46(24):4434–4440
Pang X, Zhuang X, Tang Z, Chen X (2010) Polylactic acid (PLA): research, development and industrialization. Biotechnol J 5(11):1125–1136
Pecoraro É, Manzani D, Messaddeq Y, Ribeiro SJ (2008) Bacterial cellulose from Glucanacetobacter xylinus: preparation, properties and applications. In: Monomers, polymers and composites from renewable resources, pp 369–383
Petersen K, Nielsen PV, Bertelsen G, Lawther M, Olsen MB, Nilsson NH, Mortensen G (1999) Potential of biobased materials for food packaging. Trends Food Sci Technol 10(2):52–68
Philip S, Keshavarz T, Roy I (2007) Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J Chem Technol Biotechnol Int Res Process Environ Clean Technol 82(3):233–247
Piccolo RS, Santos F, Frollini E (1997) Sugar cane bagasse lignin in resol-type resin: alternative application for ligninphenol-formaldehyde resins. J Macromol Sci Part A 34(1):153–164
Pollak A (1952) Lignin. Kirk-Othmer encyclopedia of chemical technology, 1st edn., vol 8. Wiley, New York, pp 327–338
Pollet E, Avérous L (2011) Production, chemistry and properties of polyhydroxyalkanoates. In: Biopolymers—new materials for sustainable films and coatings. pp 65–86
Ramsay JA, Ramsay BA (1990) Poly-beta-hydroxyalkanoic acids(PHAs): unique, microbially produced thermoplastics. Appl Phycol Forum 7(3):1–5
Reinhart CT, Peppas NA (1984) Solute diffusion in swollen membranes. Part II. Influence of crosslinking on diffusive properties. J Membr Sci 18:227–239
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31(7):603–632
Rudall KM, Kenchington W (1973) The chitin system. Biol Rev 48(4):597–633
Sagar AD, Merrill EW (1995) Properties of fatty-acid esters of starch. J Appl Polym Sci 58(9):1647–1656
Sahoo D, Nayak PL (2011) Chitosan: the most valuable derivative of chitin. Biopolym Biomed Environ Appl 129–166
Sahoo S, Mohanty S, Nayak SK (2018) Biobased polyurethane adhesive over petroleum based adhesive: use of renewable resource. J Macromol Sci Part A 55(1):36–48
Saikia A, Karak N (2017) Renewable resource based thermostable tough hyperbranched epoxy thermosets as sustainable materials. Polym Degrad Stab 135:8–17
Shi B, Topolkaraev V, Wang J (2011) Biopolymers, processing, and biodegradation. In: Renewable and sustainable polymers. American Chemical Society, Washington, pp 117–132
Shirashi N (1992) Liquefaction of lignocellulosics in organic solvents and its applications. In: Rowell RM, Scultz TP, Narayan R (eds) Emerging technologies for materials and chemicals from biomass, ACS symposium series 476, pp 136–145
Silvestre AJ, Gandini A (2008) Terpenes: major sources, properties and applications. In: Monomers, polymers and composites from renewable resources, pp 17–38
Simon J, Müller HP, Koch R, Müller V (1998) Thermoplastic and biodegradable polymers of cellulose. Polym Degrad Stab 59(1–3):107–115
Singh DK, Ray AR (2000) Biomedical applications of chitin, chitosan, and their derivatives. J Macromol Sci Part C Polym Rev 40(1):69–83
Siró I, Plackett D (2010) Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose 17(3):459–494
Södergård A, Stolt M (2002) Properties of lactic acid based polymers and their correlation with composition. Prog Polym Sci 27(6):1123–1163
Sutton AD, Waldie FD, Wu R, Schlaf M, Louis A III, Gordon JC (2013) The hydrodeoxygenation of bioderived furans into alkanes. Nat Chem 5(5):428
Taherimehr M, Pescarmona PP (2014) Green polycarbonates prepared by the copolymerization of CO2 with epoxides. J Appl Polym Sci 131(21)
Tamura H (2004) Destruction of rigid crystalline structure to prepare chitin solution. Adv Chitin Sci 7:84–87
Tessler MM, Billmers RL (1996) Preparation of starch esters. J Environ Polym Degrad 4(2):85–89
Thakur VK, Thakur MK, Raghavan P, Kessler MR (2014) Progress in green polymer composites from lignin for multifunctional applications: a review. ACS Sustain Chem Eng 2(5):1072–1092
Tharanathan RN (2005) Starch—value addition by modification. Crit Rev Food Sci Nutr 45(5):371–384
Tharanathan RN, Kittur FS (2003) Chitin—the undisputed biomolecule of great potential. Crit Rev Food Sci Nutr 43(1):61–87
Thielemans W, Wool RP (2005) Lignin esters for use in unsaturated thermosets: lignin modification and solubility modeling. Biomacromolecules 6(4):1895–1905
Thoma JA, Stewart L (1965) In: Whistler RL, Pashall EF (eds) Starch: chemistry and technology, vol 1. Academic Press, New York, pp 209–249
Tong H, Yao SN (1997) Study of the microstructure and the microtopography for shell of crab and lobster. J Anal Sci 13(3):206–209
Törmälä P, Vasenius J, Vainionpää S, Laiho J, Pohjonen T, Rokkanen P (1991) Ultra-high-strength absorbable self-reinforced polyglycolide (SR-PGA) composite rods for internal fixation of bone fractures: in vitro and in vivo study. J Biomed Mater Res 25(1):1–22
Tsanaktsis V, Terzopoulou Z, Exarhopoulos S, Bikiaris DN, Achilias DS, Papageorgiou DG, Papageorgiou GZ (2015) Sustainable, eco-friendly polyesters synthesized from renewable resources: preparation and thermal characteristics of poly(dimethyl-propylene furanoate). Polym Chem 6(48):8284–8296
Tuck CO, Pérez E, Horváth IT, Sheldon RA, Poliakoff M (2012) Valorization of biomass: deriving more value from waste. Science 337(6095):695–699
Van de Velde K, Kiekens P (2002) Biopolymers: overview of several properties and consequences on their applications. Polym Testing 21(4):433–442
Vanholme R, Morreel K, Ralph J, Boerjan W (2008) Lignin engineering. Curr Opin Plant Biol 11(3):278–285
Vert M (2001) Biopolymers and artificial biopolymers in biomedical applications, an overview. In: Biorelated polymers. Springer, Boston, MA, pp 63–79
Vestapen B, Pawa F, Dortman B, Bagastyo A, Pratono AH, Rahmani P, Zurbrugg C (2017) Municipal solid waste management: market-driven upcycling of urban solid waste in Indonesia. In: Mahadwartha PA, Pratono AH, Verstappen BM, Zurbrügg C (eds) Generating value from organic waste (pp. 1–5), Master of Management Program, Faculty of Business and Economics, Universitas Surabaya, Surabaya, Indonesia
Vikso-Nielsen A, Andersen C, Pedersen S, Hjort C (2009) U.S. Patent No. 7,618,795. U.S. Patent and Trademark Office, Washington, DC
Wang R, Schuman TP (2013) Vegetable oil-derived epoxy monomers and polymer blends: a comparative study with review. Express Polym Lett 7(3):272–292
Wang J, Manley RSJ, Feldman D (1992) Synthetic polymer-lignin copolymers and blends. Prog Polym Sci 17(4):611–646
Wetzel S, Duchesne LC, Laporte MF (2006) Bioproducts from Canada’s forests: new partnerships in the bioeconomy. Springer Science & Business Media, Berlin
Williams CK, Hillmyer MA (2008) Polymers from renewable resources: a perspective for a special issue of polymer reviews. Polym Rev 48(1):1–10
Wing RE, Willett JL (1997) Water soluble oxidized starches by peroxide reactive extrusion. Ind Crops Prod 7(1):45–52
Wing RE, Willett JL (1999) In Imam SH, Greene RY, Zaidi BR (eds) Thermochemical processes for derivatization of starches with different amylose content in biopolymers—utilizing nature’s, advanced, materials. ACS symposium series 723, ACS, Washington DC, pp 55–64
Wu CS (2015) Renewable resource-based green composites of surface-treated spent coffee grounds and polylactide: characterisation and biodegradability. Polym Degrad Stab 121:51–59
Yamane H, Terao K, Hiki S, Kimura Y (2001) Mechanical properties and higher order structure of bacterial homo poly(3-hydroxybutyrate) melt spun fibers. Polymer 42(7):3241–3248
Yan N, Chen X (2015) Don’t waste seafood waste: turning cast-off shells into nitrogen-rich chemicals would benefit economies and the environment. Nature 524(7564):155–158
Yannas IV (1972) Collagen and gelatin in the solid state. J Macromol Sci Rev Macromol Chem 7(1):49–106
Yannas IV, Burke JF, Orgill DP, Skrabut EM (1982) Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science 215(4529):174–176
Yilmaz M, Kusefoğlu SH (2005) Gelation of soybean oil with polybutadiene. J Appl Polym Sci 96(6):2240–2246
Yousefi AM, Hoque ME, Prasad RGSV, Uth N (2015) Current strategies in multiphasic scaffold design for osteochondral tissue engineering: a review. J Biomed Mater Res Part A 103(7):2460–2481
Yuan Y, Ruckenstein E (1998) Miscibility and transesterification of phenoxy with biodegradable poly(3-hydroxybutyrate). Polymer 39(10):1893–1897
Zdrahala RJ (1997) Thermoplastic starch revisited. Structure/property relationship for “dialed‐in” biodegradability. In: Macromolecular symposia, vol 123, no 1. Hüthig & Wepf Verlag, Basel, pp 113–121
Zhang MSY (2016) Low-carbon indicator system–sino: evaluating low-carbon city development level in China, Doctoral dissertation, University of Duisburg-Essen
Zhu Y, Romain C, Williams CK (2016) Sustainable polymers from renewable resources. Nature 540(7633):354
Zia KM, Noreen A, Zuber M, Tabasum S, Mujahid M (2016) Recent developments and future prospects on bio-based polyesters derived from renewable resources: a review. Int J Biol Macromol 82:1028–1040
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sharif, A., Hoque, M.E. (2019). Renewable Resource-Based Polymers. In: Sanyang, M., Jawaid, M. (eds) Bio-based Polymers and Nanocomposites . Springer, Cham. https://doi.org/10.1007/978-3-030-05825-8_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-05825-8_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-05824-1
Online ISBN: 978-3-030-05825-8
eBook Packages: EngineeringEngineering (R0)