Abstract
Endogenous and exogenous cannabinoids modulate many physiological and pathological processes by binding classical cannabinoid receptors 1 (CB1) or 2 (CB2) or non-cannabinoid receptors. Cannabinoids are known to exert antiproliferative, apoptotic, anti-migratory and anti-invasive effect on cancer cells by inducing or inhibiting various signaling cascades. In this chapter, we specifically emphasize the latest research works about the alterations in endocannabinoid system (ECS) components in malignancies and cancer cell proliferation, migration, invasion, angiogenesis, autophagy, and death by cannabinoid administration, emphasizing their mechanism of action, and give a future perspective for clinical use.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cannabinoids are terpenophenolic compounds which are classified as plant-derived phytocannabinoids, endocannabinoids produced by humans and animals and synthetic forms produced in laboratory. These compounds have been extensively studied for their biological roles in physiological and pathological processes (Shah et al. 2021) including cell proliferation (Braile et al. 2021; Daris et al. 2019), migration (Daris et al. 2019; Kovalchuk and Kovalchuk 2020), invasion (Sledzinski et al. 2021; Tomko et al. 2020), angiogenesis (Lee et al. 2021; Wang and Multhoff 2021), autophagy (Hinz and Ramer 2019; Lee et al. 2021), and apoptosis (Leo and Abood 2021; Vecera et al. 2020). In recent years, many clinical studies concerning the cannabinoid management have been conducted on their relieving effect on chemotherapy-related nausea and vomiting, spasms, neuropathic pain, insomnia, and seizures (Mücke et al. 2018; Pauli et al. 2020; Sawtelle and Holle 2021). Therefore, this chapter focuses on the recent preclinical and clinical advances in the fields of cannabinoids and their effects on cellular mechanisms in healthy and cancerous cells.
2 Focus on Cannabinoids
2.1 Phytocannabinoids
Cannabis sativa L. (marijuana) plant comprises more than 100 psychoactive terpenophenolic compounds known as cannabinoids (Abrams and Guzman 2015; Bogdanovic et al. 2017; McAllister et al. 2015). Delta-9-tetrahydracannabinol (Δ9-THC), cannabidiol (CBD) and cannabigerol (CBG) are known as the major compounds among all phytocannabinoids (Pagano et al. 2021). Cannabinol (CBN), cannabichromene (CBC), cannabidiolic acid (CBDA), cannabinodiol (CBND), cannabielsoin (CBE), cannabicyclol (CBL), cannabitriol (CBT), and cannabidivarin (CBDV) are the other well-known minor phytocannabinoids (Walsh and Holmes 2022). Plasma concentration of Δ9-THC reaches its highest level at 1–6 h after the cannabis ingestion, and its half-life is approximately 20–30 h (Abrams and Guzman 2015). Maximum concentration of Δ9-THC reaches in 2–10 min after the cannabis inhalation and the levels decrease rapidly within 30 min (Abrams and Guzman 2015; Baglot et al. 2021).
2.2 The Endocannabinoid System (ECS)
The endocannabinoid system (ECS) comprises endogenous agonists called “endocannabinoids”, enzymes responsible for synthesizing and degrading endocannabinoids, and cannabinoid (CB) receptors (Lu and Mackie 2021; Pertwee 2012). Endocannabinoids are known as natural lipid mediators found in human body (Lu and Mackie 2021), and best characterized endocannabinoids anandamide (N-arachidonoylethanolamine, AEA) and 2-arachidonoyl glycerol (2-AG) generally act through classical CB1 and CB2 receptors (K. A. Johnson and Lovinger 2016; Martinez-Pena et al. 2021; Wu 2019). Besides CB1/2 receptors, both endogenous and exogenous cannabinoids may interact with other G-protein-coupled receptors, GPCR55, GPCR18, GPCR92 or GPCR12 (Biringer 2021; Irving et al. 2017; Pacher et al. 2020; Starowicz et al. 2007); transient receptor potential vanilloid (TRPV) channels TRPV1 or TRPV2 (Martinez-Pena et al. 2021; Petrosino et al. 2016), and nuclear peroxisome proliferator–activated receptor α (PPARα) (P. Morales and Jagerovic 2020; Muller et al. 2018) to regulate various physiological processes involving hemostasis and energy balance (Bellocchio et al. 2008; Martinez-Pena et al. 2021), appetite (Jager and Witkamp 2014; Wu 2019), memory and learning (Wu 2019), and control in nausea and vomiting (Parker et al. 2011; Sharkey et al. 2014). Anandamide is produced with the catalysis of N-acyl phosphatidylethanolamine (NAPE) by N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) (De Petrocellis and Di Marzo 2009; Lu and Mackie 2021; Pyszniak et al. 2016). 2-AG is synthesized by conversion to diacylglycerol by diacylglycerol lipase (DAGL) enzyme, depending on the activation of phospholipase C (PLC) (Battista et al. 2012; De Petrocellis and Di Marzo 2009; Martinez-Pena et al. 2021). Diacylglycerol is generally hydrolyzed with monoacylglycerol lipase (MAGL) or alpha/beta-hydrolase domain containing 6/12 (ABDH6/12) (Grabner et al. 2017; Lu and Mackie 2021; Moreno et al. 2019), and AEA is hydrolyzed by fatty acid amide hydrolase (FAAH) (De Petrocellis and Di Marzo 2009; Massi et al. 2013; Pyszniak et al. 2016). AEA and 2-AG are also hydrolyzed by cyclooxygenases (COX, e.g. COX-2) (Egmond et al. 2021; Lu and Mackie 2021; Maccarrone 2017; Urquhart et al. 2015), lipoxygenases (LOX, e.g. ALOX isoforms) (Egmond et al. 2021; Maccarrone 2017), cytochrome P450 (CYP-450) or monooxygenases as well (Lu and Mackie 2021; Pyszniak et al. 2016; Zelasko et al. 2015) (Fig. 1). AEA, oleoylethanolamide (OEA), and palmitoylethanolamide (PEA) are also hydrolyzed by N-acylethanolamide-hydrolyzing acid amidase (NAAA) (Lu and Mackie 2021; Pagano et al. 2021; Ramer et al. 2019).
Schematic representation of endocannabinoid synthesis and breakdown. ABHD6/12 α/β-hydrolase domain containing protein 6 or 12, COX-2 Cyclooxygenase 2, DAG 1,2 Diacylglycerol, DAGL Diacylglycerol lipase, FAAH Fatty acid amide hydrolase, MAGL Monoacylglycerol lipase, NAAA N-Acylethanolamide-hydrolysing acid amidase, NAPE N-Arachidonoyl phosphatidylethanolamine, NAPE-PLD NAPE phospholipase D, NAT N-Acyltransferase, PLC Phospholipase C
CB1 receptor is predominantly located in synaptic terminals in hippocampus, basal ganglia, cerebellum, and cerebral cortex in central nervous system (Egmond et al. 2021; Lu and Mackie 2021; Pacher et al. 2020; Smiarowska et al. 2022; Wu 2019), bronchial and bronchiolar epithelia in respiratory system (Boyacıoğlu et al. 2021; Smiarowska et al. 2022), uterus, ovary, follicular fluid, embryo and placenta in female reproductive system (Bilgic et al. 2017; Fonseca et al. 2018; Martinez-Pena et al. 2021; Scotchie et al. 2015), testis, vas deferens and prostate in male reproductive system (du Plessis et al. 2015; Walker et al. 2019), and duodenal subepithelial region in digestive system (Health Canada 2018; Lee et al. 2016; Smiarowska et al. 2022), whereas lymphocytes, monocytes, macrophages, mast cells, and natural killer cells carry CB2 receptor in immune system (Chakravarti et al. 2014; Compagnucci et al. 2013; Lu and Mackie 2021; Martinez-Pena et al. 2021). Our group previously revealed that CB1 and 2 receptors are present in bone marrow mononuclear cells and hematopoietic stem cells (Kose et al. 2018).
CB ligands interact with Gαi/o coupled receptors (Nogueras-Ortiz and Yudowski 2016) that in turn inhibit adenylyl cyclase enzyme, decrease cyclic adenosine monophosphate (cAMP) production, and activate the downstream mitogen-activating protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway, respectively (Abrams and Guzman 2015; Turgeman and Bar-Sela 2019). Those signaling cascades are directly related with cell proliferation, migration, and death balance (Egmond et al. 2021; Howlett 2005). CB1/2 receptor and MAGL gene deletions have been reported to cause a deceleration in the progression of various cancer types, and an increase in their expression might also trigger carcinogenesis (Hinz and Ramer 2019; Moreno et al. 2019) and other pathological conditions such as traumatic brain injury, stroke or drug addiction (D.-j. Chen et al. 2017; Gallego-Landin et al. 2021). CB2 receptor level increases in various neurological diseases such as Alzheimer’s disease (Aso and Ferrer 2016), depression (Onaivi et al. 2008), and Parkinson’s disease (Concannon et al. 2016) when compared to CB1.
2.3 Synthetic Cannabinoids
Synthetic cannabinoids are manufactured as functional analogues of phytocannabinoids and endocannabinoids not only binding to CB1 or CB2 receptors (Egmond et al. 2021; Lim et al. 2021; Mangal et al. 2021; Smiarowska et al. 2022) but also interacting with intracellular survival or apoptotic molecules (Pyszniak et al. 2016). Synthetic cannabinoids are also known as bioactive compounds when compared to natural cannabinoids (Mangal et al. 2021; Morales and Reggio 2019). Synthetic cannabinoid agonists cannot cross the blood–brain barrier despite Δ9-THC (Smiarowska et al. 2022). Non-specific CB1/2 agonists such as WIN55–212-2, HU-210, CP55–940, JWH-018 or KM-233; CB1 agonists like arachidonoylcyclopropylamide (ACPA), arachidonyl-2′chloroethylamide (ACEA) and methanandamide; CB1 antagonists such as SR141716 (also known as Rimonabant), and CB2 agonists such as CB65, JWH-133, and JWH-015 (K. A. Johnson and Lovinger 2016; Khan et al. 2016; Ladin et al. 2016; Pyszniak et al. 2016; Sledzinski et al. 2021; Velasco et al. 2016) have been developed to stimulate CB1/2 receptors pharmacologically. Synthetic cannabinoids have been substantially researched in preclinical studies for their antitumor properties involving suppression of proliferation, angiogenesis, invasion, migration and metastasis and stimulation of autophagy and apoptosis, through binding CB1 or CB2 receptors with a higher affinity (Pyszniak et al. 2016; Sledzinski et al. 2021; Velasco et al. 2016).
2.4 Cannabinoids in Healthy Vs Cancer Cell Behavior
Both endogenous and exogenous cannabinoids have crucial biological roles in many physiological and pathological processes (Shah et al. 2021). Cannabinoid agonists provide intracellular Ca2+ release for vascular (Howlett and Abood 2017), gastric (Mahavadi et al. 2014), and myometrial (Brighton et al. 2009) smooth muscle contraction via Gi/o-dependent PI3K, Src kinase, and extracellular signal-regulated kinase 1/2 (ERK1/2) activation under the regulation of CB1/2 receptors. Those agonists also regulate the reorganization of actin cytoskeleton through focal adhesion kinase (FAK) phosphorylation and Ras-Raf-MEK-ERK1/2 cascade (Dalton et al. 2013). There have been studies revealing their involvement in learning and memory (Smiarowska et al. 2022), circadian rhythm (Vaseghi et al. 2021), regulation of food intake (Silvestri and Di Marzo 2013; Silvestri et al. 2011), and homeostasis (Klumpers and Thacker 2018) and on-going large-scale studies including the use of cannabinoids for their antinociceptive (Good et al. 2019; Häuser et al. 2018; Lichtman et al. 2018), anti-inflammatory (Turcotte et al. 2015), neuroprotective (Minerbi et al. 2019), immunomodulatory (Das et al. 2019), and antiepileptic (Das et al. 2019; Moreno et al. 2019) properties.
The ECS ligands, AEA and 2-AG, or their metabolites may reach detectable picomolar plasma levels providing an equilibrium between the tissues and the circulation (Röhrig et al. 2019). However, they are known to be unstable in circulating system as being catalyzed by ECS enzymes in plasma under physiological conditions (Lanz et al. 2018). Our group also previously showed the presence and concentration of AEA and 2-AG metabolites in healthy rat plasma samples (Ozdurak et al. 2010). On the contrary, altered ECS components including enzymes and receptors are positively correlated with tumorigenesis (Daris et al. 2019; Drozd et al. 2022; Laezza et al. 2020; Pagano et al. 2021). Elevated CB1/2 receptor levels were demonstrated in breast (Caffarel et al. 2010; Pérez-Gómez et al. 2015), endometrial (Thangesweran Ayakannu et al. 2015; Guida et al. 2010), ovarian (Messalli et al. 2014), prostate (Chung et al. 2009; Cipriano et al. 2013; Singh et al. 2020) and non–small cell lung (NSCLC) cancers (Boyacıoğlu et al. 2021; Preet et al. 2011; Xu et al. 2019), melanoma (Carpi et al. 2017; Zhao et al. 2012), and hepatocellular carcinoma (Mukhopadhyay et al. 2015). Reduced protein expressions of NAPE-PLD, FAAH, and/or MAGL (Ramer et al. 2021) were positively correlated with AEA or 2-AG synthesis in colorectal (Chen et al. 2015; Sun et al. 2013), endometrial (Ayakannu et al. 2019), hepatocellular (Zhu et al. 2016) carcinoma, and glioma (Wu et al. 2012). The effect of cannabinoids on cell proliferation, migration, invasion, angiogenesis, autophagy, and death is schematized in Fig. 2 and will be discussed in detail below.
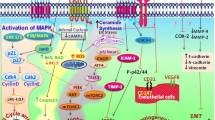
An overview of downstream signaling pathways in a cancer cell by various exogenous and endogenous cannabinoids via CB1, CB2, and TRPV1 receptors. Activation of those cannabinoid and non-cannabinoid receptors stimulates de novo ceramide synthesis which induces endoplasmic reticulum (ER) stress, p8, TRIB3, CHOP, and ATF-4. Activation of TRIB3 and mTORC-2 and inhibition of p-PI3K lead to prevention of Akt phosphorylation and, therefore, cell proliferation. Inhibited p-Akt also decreases mTORC-1 level and induces autophagy in cancer cell. Cannabinoids induce ERK1/2 which triggers p27 and p21 leading to cyclin D and E, cdc2, and cdk2 reduction and cell cycle arrest. Release of Ca2+ stimulates ROS production, activates ER stress, induces NOXA and Bax and mitochondrial cytochrome c release, which activates caspase 9 and 3 leading to apoptosis. Stimulated CB1/2 receptors inhibit invasion and migration by enhancing TIMPs, metastasis by reducing MMP2 and 9, and angiogenesis by inhibiting VEGF and Ang-2. Δ9-THC Delta-9-tetrahydracannabinol, 2-AG 2-Arachidonoylglycerol, ACPA Arachidonoyl cyclopropilamide, AEA Anandamide, Akt Protein kinase B, Ang-2 Angiotensin II, ATF-4 Activating transcription factor-4, Bax Bcl-2-associated X protein, Bcl-2 B-cell lymphoma 2, CaMKKβ Calcium ions/calmodulin-stimulated protein kinase kinase β, cAMP Cyclic adenosine monophosphate, CB1 Cannabinoid receptor 1, CB2 Cannabinoid receptor 2, CHOP C/EBP homologous protein, ERK1/2 Extracellular signal-regulated kinase 1/2, JNK c-Jun N-terminal kinase, MAPK Mitogen-activated protein kinase, MMP-2/9 Matrix metalloproteinase 2/9, mTORC-1/2 Mammalian target of rapamycin C-1/2, NOXA Phorbol-12-myristate-13-acetate-induced protein 1, p21 Cyclin-dependent kinase inhibitor 1, p27 Cyclin-dependent kinase inhibitor 1B, PI3K Phosphoinositide 3-kinase, PKA Protein kinase A, ROS Reactive oxygen species, SMAC Second mitochondria-derived activator of caspase, TIMP Tissue inhibitor of metalloproteinase, TRIB3 Tribbles pseudokinase 3, TRPV1 Transient receptor potential cation channel subfamily V member 1, VEGF Vascular endothelial growth factor, XIAP X-linked inhibitor of apoptosis
2.4.1 Cannabinoids in Cell Proliferation
Cannabinoids reduce proliferation of various cancer cells through cannabinoid or non-cannabinoid receptor mechanisms. Δ9-THC exerts anti-proliferative effect (Fowler 2015) on A549, H460, H1792, and SW-1573 NSCLC (Baram et al. 2019; Milian et al. 2020; Preet et al. 2008; Sarafian et al. 2008), LNCaP, 22RV1, DU-145, and PC-3 prostate cancer (De Petrocellis et al. 2013), Panc1, Capan2, BxPc3, and MiaPaCa2 pancreatic cancer (Carracedo et al. 2006), HeLa cervical cancer (Ramer and Hinz 2008), U266 and RPMI multiple myeloma (Nabissi et al. 2016), MDA-MB231 breast cancer (Hirao-Suzuki et al. 2019), HL60 acute myeloid leukemia (Katherine A. Scott et al. 2017), T98G, U87MG, and GL261 glioma (López-Valero et al. 2018; Scott et al. 2014), D283, D425, and PER547 medulloblastoma (Andradas et al. 2021), IC-1425EPN and DKFZ-EP1NS ependymoma (Andradas et al. 2021), and SF126, U251, and U87 glioblastoma (Marcu et al. 2010; Torres et al. 2011) cell lines through ERK1/2 activation, PI3K/Akt inhibition and Raf-1 translocation. Non-psychoactive natural CBD inhibits the proliferation of A549, H460, and primary NSCLC (Ramer et al. 2013), SKOV-3 ovarian cancer (Fraguas-Sánchez et al. 2020), MDA-MB231 breast cancer (McAllister et al. 2007; Nallathambi et al. 2018), U878MG, U373MG, SF126, U251, and U87 glioblastoma (Marcu et al. 2010; Singer et al. 2015; Torres et al. 2011), T acute lymphoblastic leukemia and Jurkat (Kalenderoglou et al. 2017), SUM159 triple negative breast cancer (Mohamad Elbaz et al. 2015), SK-N-SH neuroblastoma (Fisher et al. 2016), LNCaP and DU-145 prostate cancer (De Petrocellis et al. 2013), D283, D425, and PER547 medulloblastoma (Andradas et al. 2021), IC-1425EPN and DKFZ-EP1NS ependymoma (Andradas et al. 2021), and CaCo-2 and HCT116 colon adenocarcinoma (Aviello et al. 2012) cells by elevating p53, EGFR, ERK1/2, Akt, and C/EBP homologous protein (CHOP) and/or inhibiting transient receptor potential cation channel subfamily M (melastatin) member 8 (TRPM8). 2-AG and methanandamide reduce viability of PC-3 and primary prostate cancer cells by activating caspase-3 and ERK1/2 levels and by reducing Bcl-2 and Akt levels (Orellana-Serradell et al. 2015). CB1 inverse agonist Rimonabant (SR141716) inhibits proliferation of HCT116 and SW48 colon cancer cells by inducing cytochrome C release and TRAILR-1, −2, and −3 expressions and downregulating Bcl-2 and XIAP (Proto et al. 2017). Rimonabant also shows Wnt/β-catenin-mediated anti-proliferative effect on primary colon cancer stem cells in vitro (Fiore et al. 2018). Non-selective pan CB agonist WIN55,212–2 or JWH-133 has anti-proliferative effect on T98G, LN18, LN229, U251MG, and U87MG glioma cell lines by inducing intrinsic apoptotic pathway and DNA fragmentation (Ellert-Miklaszewska et al. 2021), LNCaP and PC-3 prostate cancer cells by downregulating PI3K/Akt/mTOR cascade (Morell et al. 2016), A549, SW-1573, A459, CALU1, H460 and H1299 NSCLC cells through PI3K/Akt and JNK pathways (Boyacıoğlu et al. 2021; Preet et al. 2011; Ravi et al. 2014; Vidinsky et al. 2012), and 786-O, SMKTR2, SMKT-R3, Caki-2, RCC-6, 769-P, Caki-1, and ACHN human renal carcinoma lines by stimulating cell cycle arrest at G0/G1 phase (Khan et al. 2018). WIN55,212–2 inhibits BEL7402 hepatocellular carcinoma cell line by inducing p27, downregulating cyclin D1 and, therefore, promoting cell cycle arrest at G0/G1 phase and reducing ERK1/2 protein expression (D. Xu et al. 2015). WIN55,212–2 diminishes the viability of A549 NSCLC cells by increasing DNA fragments in nucleus (Müller et al. 2017). Cannflavin A, a compound of Cannabis sativa, reduces the proliferation of T24 and TCCSUP bladder transitional cell carcinoma lines (Andrea M. Tomko et al. 2022). Our group also demonstrated that AEA and 2-AG decrease HEp-2 human laryngeal squamous cancer cell proliferation in vitro (Önay et al. 2022).
2.4.2 Cannabinoids in Cell Migration, Invasion, and Angiogenesis
Tumor growth and expansion are highly dependent on neovascularization, cancer cell migration, and metastasis (Laezza et al. 2020; Wang and Multhoff 2021). Anti-angiogenic, anti-invasive, and anti-metastatic activities of cannabinoids have been extensively tested to block the induction and expansion of tumor growth (Pagano et al. 2021; Ramer et al. 2021; Vecera et al. 2020). Those effects have been associated with various metalloproteinases, inhibitors, and adhesive molecules (Braile et al. 2021; Sledzinski et al. 2018; Wang and Multhoff 2021). JWH-133 prevents angiogenesis and migration of human umbilical vein endothelial cells (HUVECs) by activating tissue inhibitor of matrix metalloproteinases-1 (TIMP-1) besides inducing DNA fragmentation in A549, H460, and/or H358 NSCLC cells (Ramer et al. 2014; Vidinsky et al. 2012). CBD inhibits angiogenesis of HUVECs through vascular endothelial growth factor 1 or 2 (VEGF1/2), angiopoietin-2, urokinase-type plasminogen activator (uPA), and matrix metalloproteinase 2 or − 9 (MMP-2/9) blockage (Solinas et al. 2012). Selective CB1 agonist ACEA and JWH-133 inhibit invasion of U138 glioma cells (Tim Hohmann et al. 2017). Coincubation of selective CB1 receptor antagonist AM281 with ACEA significantly diminishes the invasion of LN229 glioblastoma cell line in vitro (T. Hohmann et al. 2019). CBD inhibits A549, H358, and/or H460 NSCLC cell invasion by decreasing plasminogen activator inhibitor-1 (PAI-1) expression (Ramer et al. 2010b) or by upregulating TIMP-1 (Ramer et al. 2012; Ramer et al. 2010a) or intercellular adhesion molecule-1 (ICAM-1) (Haustein et al. 2014; Ramer et al. 2012) levels. CBD also prevents epithelial growth factor (EGF)-induced migratory ability of 4 T1.2 and SUM159 triple-negative breast cancer by inhibiting MMP-2 and -9 expressions in addition to phosphorylated Akt (p-Akt) and ERK (Elbaz et al. 2015) or Ishikawa, PCEM004a and PCEM004b endometrial cancer lines (Marinelli et al. 2020) in vitro. Anti-invasive and anti-migratory effects of AEA have been revealed in U251 glioma cells in vitro (Ma et al. 2016). FAAH inhibitors arachidonoyl serotonin (AA-5HT) and URB597 diminish A549 cell metastasis and invasion via upregulation of TIMP-1 (Winkler et al. 2016). CB2 receptor agonist JWH-015 reduces migratory and invasive properties of M2-polarized macrophages when co-cultured with A549 NSCLC cells through inhibition of FAK, vascular cell adhesion molecule 1 (VCAM1) and MMP-2 expressions (Ravi et al. 2016). Anti-metastatic property of CBD, Δ9-THC, SR141716A, and/or SR144528 (CB2 receptor antagonist) has been established in MDA-MB231 breast cancer cells via Id1 downregulation (McAllister et al. 2011; Murase et al. 2014) and p-ERK and p38/MAPK upregulation (McAllister et al. 2011). WIN-55, 212–2 inhibits migration and metastasis of SGC7901 and AGS gastric cancer cell lines via COX-2, vimentin, and p-Akt downregulation and E-cadherin upregulation (Xian et al. 2016). Δ9-THC decreases motility of HEC-1B and AN3 CA endometrial cancer cells by inhibiting MMP-9 expression (Zhang et al. 2018) and U266 and RPMI multiple myeloma cells by reducing CXCR4 and CD147 (Nabissi et al. 2016).
2.4.3 Cannabinoids in Cell Autophagy and Death
Cannabinoids activate autophagy and apoptosis through CB1/2 or other non-cannabinoid receptors. Δ9-THC, WIN-55,212–2, and/or JWH-015 stimulate A549 and SW-1573 NSCLC cell apoptosis by inhibiting EGF-induced p-ERK, p-JNK, and p-Akt (Preet et al. 2008; Preet et al. 2011). Δ9-THC, JWH-015, CBD, AEA, and/or Met-F-AEA (combined with URB597) induce apoptosis of U87MG, U118MG, and T98G glioblastoma (Ivanov et al. 2020; Ivanov et al. 2017), A549 NSCLC (Ramer et al. 2013; Ravi et al. 2014), Ishikawa, Hec50co, MFE-280, and/or HEC-1a endometrial cancer (Fonseca et al. 2018; Marinelli et al. 2020), and HepG2 and HuH-7 hepatocellular liver carcinoma (Vara et al. 2011) cell lines through JNK, p38-MAPK phosphorylation, p-Akt inhibition, NF-κB phospho-p65 reduction, caspase-3/−7 activation or COX-2, and PPAR-γ upregulation. Cannflavin A promoted apoptosis of T24 bladder transitional cell carcinoma lines through caspase-3 cleavage in vitro (Tomko et al. 2022). We also previously demonstrated that specific CB1 receptor agonist ACPA induces A549, H1299, H358, and H838 NSCLC cell line apoptosis by inhibiting Akt/PI3K pathway, glycolysis, TCA cycle, amino acid synthesis, and urea cycle and by activating JNK cascade (Boyacıoğlu et al. 2021). LV50, a compound having high affinity to CB2 receptor, promotes apoptosis of Jurkat leukemia cells by inducing cleavage of caspase-3/−8 and PARP (Capozzi et al. 2018). CBD induces apoptosis of HCT116 and DLD-1 colorectal cancer cell lines through ROS-dependent Noxa activation (Jeong et al. 2019).
Autophagy is a self-degradative process involving packaging of cytoplasmic organelles called autophagosome (Chang 2020; Pagano et al. 2021). Cannabinoids are known to stimulate autophagy through various cellular mechanisms including ceramide accumulation by hydrolysis of sphingomyelin or de novo ceramide synthesis (Gómez del Pulgar et al. 2002; Lee et al. 2021; Pagano et al. 2021). Newly synthesized ceramide induces expressions of p38, CHOP, ATF-4, and TRIB3 (see Fig. 2), thus inhibiting PI3K/Akt cascade or activating Ca2+/calmodulin-dependent kinase kinase (CaCMKK) through ER stress (Das et al. 2019; Kabir et al. 2019; Ramer et al. 2021). Δ9-THC stimulates sphingolipid synthesis, dihydroceramide accumulation, and autophagosome and autolysosome production in U87MG glioma cell line (Hernández-Tiedra et al. 2016). CBD activates autophagy in Jurkat, MOLT-3, CCFR-CEM, K562, Reh, and RS4;11 leukemia cell lines via increasing LC3-II expression, damaging permeability of mitochondria and releasing cytochrome c (Olivas-Aguirre et al. 2019). Combined Δ9-THC, CBD, CBG, and CBN treatment induces autophagy of MCF-10A non-cancerous breast cell line by activating lipid synthesis, lysosomal vacuoles, and ER-stress-related chaperone protein glucose-regulated protein 78 (GRP78) expression (Schoeman et al. 2020). ACPA and CB2 receptor agonist GW405833 stimulates autophagy of Panc1 pancreatic cancer cells through AMPK activation and Akt/c-Myc inhibition (Dando et al. 2013). Δ9-THC treatment activates autophagy of CHL-1, A375, and SK-MEL-28 melanoma cells by elevating LC3-positive autophagosome and cytochrome-c levels (Armstrong et al. 2015).
2.5 Preclinical In Vivo Studies and Clinical Status of Cannabinoids
Preclinical in vivo studies show that cannabinoid administration leads to decrease in proliferation, migration, invasion, angiogenesis, autophagy, and death in pancreatic (Aizikovich 2020; Carracedo et al. 2006; Donadelli et al. 2011; Sharafi et al. 2019; Yang et al. 2020), lung (Ramer et al. 2012; Ramer et al. 2013; Ravi et al. 2016; Ravi et al. 2014; Winkler et al. 2016; Yasmin-Karim et al. 2018), breast (Elbaz et al. 2017; McAllister et al. 2011; Murase et al. 2014; Nasser et al. 2011), prostate (De Petrocellis et al. 2013; Morales et al. 2013; Morell et al. 2016; Qiu et al. 2019; Roberto et al. 2019), colorectal (Aviello et al. 2012; Borrelli et al. 2014; Deng et al. 2022; Kargl et al. 2013; Martínez-Martínez et al. 2016; Proto et al. 2017; Romano et al. 2014), brain (Gurley et al. 2012; López-Valero et al. 2018; Scott et al. 2014; Singer et al. 2015) and liver (Vara et al. 2013; Vara et al. 2011) cancers, melanoma (Armstrong et al. 2015; Glodde et al. 2015; Kenessey et al. 2012; Simmerman et al. 2019), multiple myeloma (Barbado et al. 2017), and neuroblastoma (Fisher et al. 2016) through PI3K/AKT/mTOR, ERK/MAPK and/or PAI-1 signaling pathways. Recent reports relating to the cannabinoids in various in vivo cancer models are presented in detail (Table 1).
2-AG: 2-Arachidonoyl glycerol; AA-5HT: Arachidonoyl serotonin; ACEA: Arachidonyl-2′chloroethylamide; ACF: Aberrant crypt foci; Akt: Protein kinase B; AM281: CB1-specific antagonist; AMPK: 5’ AMP-activated protein kinase; Bax: Bcl-2-associated X protein; BDS: Botanical cannabinoid extraction; CBD: Cannabidiol; CBG: Cannabigerol; Cyc D1: Cyclin D1; COX-2: Cyclooxygenase-2; CXCR4: C-X-C chemokine receptor type 4; DC: Dendritic cell; EGFR: Epidermal growth factor receptor; ERK: Extracellular signal-regulated kinase; GEM: Gemcitabin; GW9662: PPARγ antagonist; IGF-IR: Type 1 insulin-like growth factor receptor; IL6/10: Interleukin 6/10; iNOS: Inducible nitric oxide synthase; i.p.: intraperitoneal; i.t.: intratumoral; JWH-015: CB2-specific agonist; JWH-133: CB2-specific agonist; KM-233: Synthetic analogue of THC; MDSC: myeloid-derived suppressor cell; Met-F-AEA: Stable analogue of anandamide; MMP2/9: Matrix metalloproteinase 2/9; NFκB: Nuclear factor kappa B; O-1602: Cannabidiol analogue; O-1663: Resorcinol derivative; PAI-1: Plasminogen activator inhibitor-1; PAK1: P21-activated kinase-1; PM49: Synthetic cannabinoid quinone; p.o.: per oral; PPARγ: Peroxisome proliferator-activated receptor γ; p.t.: peritumoral; s.c.: subcutaneous; SR1 (or SR141716): CB1-specific antagonist (Rimonabant), SR144528: CB2-specific antagonist; STAT3: Signal transducer and activator of transcription 3; TIMP-1: Tissue inhibitor of matrix metalloproteinases-1; TNF-α: Tumor necrosis factor-alpha; URB597: FAAH inhibitor; βIII Tub: βIII Tubulin; Δ9-THC: Delta-9-tetrahydracannabinol.
Cannabinoid agonists are currently used in the treatment of obesity (Bi et al. 2020; McClements 2020) and as neuroprotective agents for various diseases (Gado et al. 2019) involving Parkinson’s (Celorrio et al. 2016; Cristino et al. 2020) and Alzheimer’s diseases and multiple sclerosis (Black et al. 2019; Novotna et al. 2011) in the clinic. An attention to the use of cannabinoids for medical applications has grown due to their antinociceptive (Brunetti et al. 2020; Bruni et al. 2018; Good et al. 2019; VanDolah et al. 2019) and antiepileptic (Billakota et al. 2019; Brunetti et al. 2020; VanDolah et al. 2019) effects and the modulatory roles in appetite, nausea, and vomiting (Strouse 2016; VanDolah et al. 2019; White 2019). Dronabinol (Abrams and Guzman 2015; Shah et al. 2020) and nabilone oral capsules (Abuhasira et al. 2018; Shah et al. 2020) have equal potency to cure chemotherapy-related nausea and vomiting when compared to the US Food and Drug Administration (FDA)-approved other antiemetic drugs. Clinical studies reveal the relieving effect of nabiximols, oromucosal spray with THC and CBD as active ingredients, on spasms and neuropathic pain in multiple sclerosis (Abuhasira et al. 2018; Lowe et al. 2021) and improvement in sleep disorders in patients with insomnia (Klumpers and Thacker 2018). FDA-approved Epidiolex, as CBD active ingredient, is currently used for seizures related to Lennox-Gastaut and Dravet syndromes (Abu-Sawwa and Stehling 2020; Levinsohn and Hill 2020; Steele et al. 2019). A phase I/II trial exploring the immune-modulatory and anti-inflammatory potency of CBD showed that it avoids graft versus host disease (GVHD) incidence when administered in addition to standard GVHD prophylaxis (Yeshurun et al. 2014). It is worth noting that SR141716 as an anorectic agent used for the obesity treatment was banned by the FDA due to its severe side effects (Khan et al. 2016; Shah et al. 2019). Studies consisting of the analgesic and anti-epileptic properties of cannabinoids on various diseases are shown in Table 2.
3 Future Perspectives for Cannabinoids as Prospective Agents for Cancer
Phytocannabinoids and endogenous and synthetic cannabinoids have been examined in preclinical research works and clinical trials to assess the therapeutic potential for various diseases including cancers. One of the key pitfalls occurs in the short half-lives and psychotropicity of cannabinoids. Therefore, it is crucial to use anti-cancer cannabinoids effective in triggering intrinsic apoptotic mechanisms at low doses without reaching central nervous system. Natural cannabis derivatives are clinically used for pain relief but the horizon should be expanded on their application as anti-tumor agents. Still, a major gap remains which needs to be filled by new research works to clarify the effects of cannabinoids on the tumor microenvironment. Moreover, outputs of in vitro molecular tests should be translated to in vivo models, since in vitro data does not precise the possible problems within the diseased animal as a whole. Preclinical randomized studies convey the therapeutic performance of cannabinoids on cellular mechanisms. Clinical trials including phase trials provide the assessment of personalized performance of different cannabinoid system agents before translation to clinic. A literature search of clinicaltrials.gov by September 2022 found 77 completed clinical studies about cannabis/cannabinoid use in mental disorders, psychotic disorders, pain, immune system diseases, gastrointestinal diseases, central nervous system diseases and various syndromes including Dravet, Tourette, and Lennox-Gastaut syndromes. In 31 out of 77 (40.26%) studies, cannabinoids were tested for their pain-relieving capability; 24 out of 31 searches have been confirmed in phase II/III clinical trials. Epidiolex, CBD oral solution, was approved by FDA on June 25, 2018, to alleviate the seizures observed in Lennox-Gastaut and Dravet syndromes. On the other hand, depression and suicide in patients caused withdrawal of CB1 antagonist rimonabant from the market. No clinical trial or approval has been reported for cannabinoids as anti-cancer therapeutics. As to future prospects, cannabinoids might be evaluated as potential chemotherapeutic drugs or effective adjunctive therapeutics to be used with chemotherapeutics or other targeted agents. However, further investigations are necessary to clarify the safety and potency of cannabinoids.
Abbreviations
- 2-AG:
-
2-Arachidonoyl glycerol
- AA-5HT:
-
Arachidonoyl serotonin
- ABDH6/12:
-
Alpha/beta-hydrolase domain containing 6/12
- ACEA:
-
Arachidonyl-2′chloroethylamide
- ACF:
-
Aberrant crypt foci
- ACPA:
-
Arachidonoyl cyclopropilamide
- AEA:
-
Anandamide
- AKT:
-
Protein kinase B
- AMPK:
-
5’ AMP-activated protein kinase
- ANG-2:
-
Angiotensin II
- ASD :
-
Autism spectrum disorder
- ATF-4 :
-
Activating transcription factor-4
- BAX :
-
Bcl-2-associated X protein
- BCL-2 :
-
B-cell lymphoma 2
- BDS:
-
Botanical cannabinoid extraction
- CAMKKβ :
-
Calcium ions/calmodulin-stimulated protein kinase kinase β
- CAMP :
-
Cyclic adenosine monophosphate
- CB :
-
Cannabinoid
- CB1 :
-
Cannabinoid receptor 1
- CB2 :
-
Cannabinoid receptor 2
- CBC:
-
Cannabichromene
- CBD:
-
Cannabidiol
- CBDA:
-
Cannabidiolic acid
- CBDV:
-
Cannabidivarin
- CBE:
-
Cannabielsoin
- CBG :
-
Cannabigerol
- CBL:
-
Cannabicyclol
- CBN :
-
Cannabinol
- CBND:
-
Cannabinodiol
- CBT:
-
Cannabitriol
- CHOP :
-
C/EBP homologous protein
- COX:
-
Cyclooxygenase
- CXCR4:
-
C-X-C chemokine receptor type 4
- CYC D1:
-
Cyclin D1
- CYP-450:
-
Cytochrome P450
- DAGL:
-
Diacylglycerol lipase
- DC:
-
Dendritic cell
- DS :
-
Dravet syndrome
- ECS:
-
Endocannabinoid system
- EGF :
-
Epithelial growth factor
- EGFR:
-
Epidermal growth factor receptor
- ER:
-
Endoplasmic reticulum
- ERK1/2:
-
Extracellular signal-regulated kinase 1/2
- FAAH:
-
Fatty acid amid hydrolase
- FAK:
-
Focal adhesion kinase
- FDA:
-
Food and Drug Administration
- GEM:
-
Gemcitabin
- GPR:
-
G-protein coupled receptor
- GRP78 :
-
Chaperone protein glucose-regulated protein 78
- GVHD :
-
Graft versus host disease
- HUVEC:
-
Human umbilical vein endothelial cell
- ICAM-1 :
-
Intercellular adhesion molecule-1
- IGF-IR:
-
Type 1 insulin-like growth factor receptor
- IL6/10:
-
Interleukin 6/10
- iNOS:
-
Inducible nitric oxide synthase
- JNK :
-
c-Jun N-terminal kinase
- MAGL:
-
Monoacylglycerol lipase
- MAPK:
-
Mitogen-activating protein kinase
- MDSC:
-
Myeloid-derived suppressor cell
- MMP-2/9 :
-
Matrix metalloproteinase 2/9
- MS :
-
Multiple sclerosis
- MTORC-1/2 :
-
Mammalian target of rapamycin C-1/2
- NAAA:
-
N-Acylethanolamide-hydrolysing acid amidase
- NAPE:
-
N-acyl phosphatidylethanolamine
- NAPE-PLD:
-
N-acyl phosphatidylethanolamine phospholipase D
- NAT:
-
N-Acyltransferase
- NFκB:
-
Nuclear factor kappa B
- NOXA :
-
Phorbol-12-myristate-13-acetate-induced protein 1
- NSCLC:
-
Non–small cell lung cancer
- OEA:
-
Oleoylethanolamide
- P21 :
-
Cyclin-dependent kinase inhibitor 1
- P27 :
-
Cyclin-dependent kinase inhibitor 1B
- PAI-1:
-
Plasminogen activator inhibitor 1
- PAK1:
-
P21-activated kinase 1
- PEA:
-
Palmitoylethanolamide
- PI3K:
-
Phosphoinositide 3 kinase
- PKA :
-
Protein kinase A
- PLC:
-
Phospholipase C
- PPARα :
-
Peroxisome proliferator–activated receptor α
- PPARγ:
-
Peroxisome proliferator–activated receptor γ
- ROS :
-
Reactive oxygen species
- SMAC :
-
Second mitochondria-derived activator of caspase
- STAT3:
-
Signal transducer and activator of transcription 3
- TIMP :
-
Tissue inhibitor of metalloproteinase
- TNF-α:
-
Tumor necrosis factor-alpha
- TRIB3 :
-
Tribbles pseudokinase 3
- TRPV :
-
Transient receptor potential cation channel subfamily V member
- UPA :
-
Urokinase-type plasminogen activator
- VCAM1 :
-
Vascular cell adhesion molecule 1
- VEGF :
-
Vascular endothelial growth factor
- XIAP :
-
X-linked inhibitor of apoptosis
- βIII Tub:
-
βIII Tubulin
- Δ9-THC:
-
Delta-9-tetrahydracannabinol
References
Abrams DI, Guzman M (2015) Cannabis in cancer care. Clin Pharmacol Ther 97(6):575–586. https://doi.org/10.1002/cpt.108
Abuhasira R, Shbiro L, Landschaft Y (2018) Medical use of cannabis and cannabinoids containing products - regulations in Europe and North America. Eur J Intern Med 49:2–6. https://doi.org/10.1016/j.ejim.2018.01.001
Abu-Sawwa R, Stehling C (2020) Epidiolex (Cannabidiol) primer: frequently asked questions for patients and caregivers. J Pediatr Pharmacol Ther 25(1):75–77. https://doi.org/10.5863/1551-6776-25.1.75
Aizikovich A (2020) Anticancer effect of new cannabinoids derived from Tetrahydrocannabinolic acid on PANC-1 and AsPC-1 human pancreas tumor cells. J Pancreat Cancer 6(1):40–44. https://doi.org/10.1089/pancan.2020.0003
Andradas C, Byrne J, Kuchibhotla M, Ancliffe M, Jones AC, Carline B et al (2021) Assessment of Cannabidiol and Δ9-Tetrahydrocannabiol in mouse models of Medulloblastoma and Ependymoma. Cancers 13(2). https://doi.org/10.3390/cancers13020330
Aran A, Harel M, Cassuto H, Polyansky L, Schnapp A, Wattad N et al (2021) Cannabinoid treatment for autism: a proof-of-concept randomized trial. Mol Autism 12(1):6. https://doi.org/10.1186/s13229-021-00420-2
Armstrong JL, Hill DS, McKee CS, Hernandez-Tiedra S, Lorente M, Lopez-Valero I et al (2015) Exploiting cannabinoid-induced cytotoxic autophagy to drive melanoma cell death. J Invest Dermatol 135(6):1629–1637. https://doi.org/10.1038/jid.2015.45
Aso E, Ferrer I (2016) CB2 cannabinoid receptor as potential target against Alzheimer’s disease. Front Neurosci 10. https://doi.org/10.3389/fnins.2016.00243
Aviello G, Romano B, Borrelli F, Capasso R, Gallo L, Piscitelli F et al (2012) Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J Mol Med (Berl) 90(8):925–934. https://doi.org/10.1007/s00109-011-0856-x
Ayakannu T, Taylor AH, Willets JM, Konje JC (2015) The evolving role of the endocannabinoid system in gynaecological cancer. Hum Reprod Update 21(4):517–535. https://doi.org/10.1093/humupd/dmv022
Ayakannu T, Taylor AH, Bari M, Mastrangelo N, Maccarrone M, Konje JC (2019) Expression and function of the endocannabinoid modulating enzymes fatty acid amide hydrolase and N-Acylphosphatidylethanolamine-specific phospholipase D in endometrial carcinoma. Front Oncol 9:1363. https://doi.org/10.3389/fonc.2019.01363
Baglot SL, Hume C, Petrie GN, Aukema RJ, Lightfoot SHM, Grace LM et al (2021) Pharmacokinetics and central accumulation of delta-9-tetrahydrocannabinol (THC) and its bioactive metabolites are influenced by route of administration and sex in rats. Sci Rep 11(1):23990. https://doi.org/10.1038/s41598-021-03242-7
Baram L, Peled E, Berman P, Yellin B, Besser E, Benami M et al (2019) The heterogeneity and complexity of cannabis extracts as antitumor agents. Oncotarget 10(41):4091–4106. https://doi.org/10.18632/oncotarget.26983
Barbado MV, Medrano M, Caballero-Velázquez T, Álvarez-Laderas I, Sánchez-Abarca LI, García-Guerrero E et al (2017) Cannabinoid derivatives exert a potent anti-myeloma activity both in vitro and in vivo. Int J Cancer 140(3):674–685. https://doi.org/10.1002/ijc.30483
Battista N, Di Tommaso M, Bari M, Maccarrone M (2012) The endocannabinoid system: an overview. Front Behav Neurosci 6. https://doi.org/10.3389/fnbeh.2012.00009
Bellocchio L, Cervino C, Pasquali R, Pagotto U (2008) The endocannabinoid system and energy metabolism. J Neuroendocrinol 20(6):850–857. https://doi.org/10.1111/j.1365-2826.2008.01728.x
Bi GH, Galaj E, He Y, Xi ZX (2020) Cannabidiol inhibits sucrose self-administration by CB1 and CB2 receptor mechanisms in rodents. Addict Biol 25(4):e12783. https://doi.org/10.1111/adb.12783
Bilgic E, Guzel E, Kose S, Aydin MC, Karaismailoglu E, Akar I et al (2017) Endocannabinoids modulate apoptosis in endometriosis and adenomyosis. Acta Histochem 119(5):523–532. https://doi.org/10.1016/j.acthis.2017.05.005
Billakota S, Devinsky O, Marsh E (2019) Cannabinoid therapy in epilepsy. Curr Opin Neurol 32(2):220–226. https://doi.org/10.1097/wco.0000000000000660
Biringer RG (2021) Endocannabinoid signaling pathways: beyond CB1R and CB2R. J Cell Commun Signaling 15(3):335–360. https://doi.org/10.1007/s12079-021-00622-6
Black N, Stockings E, Campbell G, Tran LT, Zagic D, Hall WD et al (2019) Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry 6(12):995–1010. https://doi.org/10.1016/s2215-0366(19)30401-8
Bogdanovic V, Mrdjanovic J, Borisev I (2017) A review of the therapeutic antitumor potential of cannabinoids. J Altern Complement Med 23(11):831–836. https://doi.org/10.1089/acm.2017.0016
Borrelli F, Pagano E, Romano B, Panzera S, Maiello F, Coppola D et al (2014) Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a cannabis-derived non-psychotropic cannabinoid. Carcinogenesis 35(12):2787–2797. https://doi.org/10.1093/carcin/bgu205
Boyacıoğlu Ö, Bilgiç E, Varan C, Bilensoy E, Nemutlu E, Sevim D et al (2021) ACPA decreases non-small cell lung cancer line growth through Akt/PI3K and JNK pathways in vitro. Cell Death Dis 12(1):56. https://doi.org/10.1038/s41419-020-03274-3
Braile M, Marcella S, Marone G, Galdiero MR, Varricchi G, Loffredo S (2021) The interplay between the immune and the endocannabinoid Systems in Cancer. Cell 10(6). https://doi.org/10.3390/cells10061282
Brighton PJ, McDonald J, Taylor AH, Challiss RAJ, Lambert DG, Konje JC, Willets JM (2009) Characterization of anandamide-stimulated cannabinoid receptor signaling in human ULTR myometrial smooth muscle cells. Mol Endocrinol 23(9):1415–1427. https://doi.org/10.1210/me.2009-0097
Brunetti P, Lo Faro AF, Pirani F, Berretta P, Pacifici R, Pichini S, Busardò FP (2020) Pharmacology and legal status of cannabidiol. Ann Ist Super Sanita 56(3):285–291. https://doi.org/10.4415/ann_20_03_06
Bruni N, Della Pepa C, Oliaro-Bosso S, Pessione E, Gastaldi D, Dosio F (2018) Cannabinoid delivery Systems for Pain and Inflammation Treatment. Molecules (Basel, Switzerland) 23(10):2478. https://doi.org/10.3390/molecules23102478
Caffarel MM, Andradas C, Mira E, Pérez-Gómez E, Cerutti C, Moreno-Bueno G et al (2010) Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol Cancer 9:196. https://doi.org/10.1186/1476-4598-9-196
Capozzi A, Mattei V, Martellucci S, Manganelli V, Saccomanni G, Garofalo T et al (2018) Anti-proliferative properties and Proapoptotic function of new CB2 selective cannabinoid receptor agonist in Jurkat leukemia cells. Int J Mol Sci 19(7). https://doi.org/10.3390/ijms19071958
Carpi S, Fogli S, Polini B, Montagnani V, Podestà A, Breschi MC et al (2017) Tumor-promoting effects of cannabinoid receptor type 1 in human melanoma cells. Toxicol In Vitro 40:272–279. https://doi.org/10.1016/j.tiv.2017.01.018
Carracedo A, Gironella M, Lorente M, Garcia S, Guzman M, Velasco G, Iovanna JL (2006) Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res 66(13):6748–6755. https://doi.org/10.1158/0008-5472.Can-06-0169
Celorrio M, Fernández-Suárez D, Rojo-Bustamante E, Echeverry-Alzate V, Ramírez MJ, Hillard CJ et al (2016) Fatty acid amide hydrolase inhibition for the symptomatic relief of Parkinson’s disease. Brain Behav Immun 57:94–105. https://doi.org/10.1016/j.bbi.2016.06.010
Chakravarti B, Ravi J, Ganju RK (2014) Cannabinoids as therapeutic agents in cancer: current status and future implications. Oncotarget 5(15):5852–5872. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4171598/
Chang NC (2020) Autophagy and stem cells: self-eating for self-renewal. Front Cell Dev Biol 8. https://doi.org/10.3389/fcell.2020.00138
Chen L, Chen H, Li Y, Li L, Qiu Y, Ren J (2015) Endocannabinoid and ceramide levels are altered in patients with colorectal cancer. Oncol Rep 34(1):447–454. https://doi.org/10.3892/or.2015.3973
Chen D-J, Gao M, Gao F-F, Su Q-X, Wu J (2017) Brain cannabinoid receptor 2: expression, function and modulation. Acta Pharmacol Sin 38(3):312–316. https://doi.org/10.1038/aps.2016.149
Chung SC, Hammarsten P, Josefsson A, Stattin P, Granfors T, Egevad L et al (2009) A high cannabinoid CB1 receptor immunoreactivity is associated with disease severity and outcome in prostate cancer. Eur J Cancer 45(1):174–182. https://doi.org/10.1016/j.ejca.2008.10.010
Cipriano M, Haggstrom J, Hammarsten P, Fowler CJ (2013) Association between cannabinoid CB1 receptor expression and Akt Signalling in prostate cancer. PLoS One 8(6). https://doi.org/10.1371/journal.pone.0065798
Compagnucci C, Di Siena S, Bustamante MB, Di Giacomo D, Di Tommaso M, Maccarrone M et al (2013) Type-1 (CB1) cannabinoid receptor promotes neuronal differentiation and maturation of neural stem cells. PLoS One 8(1):e54271. https://doi.org/10.1371/journal.pone.0054271
Concannon RM, Okine BN, Finn DP, Dowd E (2016) Upregulation of the cannabinoid CB2 receptor in environmental and viral inflammation-driven rat models of Parkinson’s disease. Exp Neurol 283(Pt A):204–212. https://doi.org/10.1016/j.expneurol.2016.06.014
Cristino L, Bisogno T, Di Marzo V (2020) Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol 16(1):9–29. https://doi.org/10.1038/s41582-019-0284-z
Dalton GD, Peterson LJ, Howlett AC (2013) CB1 cannabinoid receptors promote maximal FAK catalytic activity by stimulating cooperative signaling between receptor tyrosine kinases and integrins in neuronal cells. Cell Signal 25(8):1665–1677. https://doi.org/10.1016/j.cellsig.2013.03.020
Dando I, Donadelli M, Costanzo C, Dalla Pozza E, D’Alessandro A, Zolla L, Palmieri M (2013) Cannabinoids inhibit energetic metabolism and induce AMPK-dependent autophagy in pancreatic cancer cells. Cell Death Dis 4:e664. https://doi.org/10.1038/cddis.2013.151
Daris B, Verboten MT, Knez Z, Ferk P (2019) Cannabinoids in cancer treatment: therapeutic potential and legislation. Bosn J Basic Med Sci 19(1):14–23. https://doi.org/10.17305/bjbms.2018.3532
Das S, Kaul K, Mishra S, Charan M, Ganju RK (2019) Cannabinoid signaling in cancer. In AN Bukiya (Ed) Recent advances in cannabinoid physiology and pathology (Vol. 1162). pp 51–61)
De Petrocellis L, Di Marzo V (2009) An introduction to the endocannabinoid system: from the early to the latest concepts. Best Pract Res Clin Endocrinol Metab 23(1):1–15. https://doi.org/10.1016/j.beem.2008.10.013
De Petrocellis L, Ligresti A, Schiano Moriello A, Iappelli M, Verde R, Stott CG et al (2013) Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: pro-apoptotic effects and underlying mechanisms. Br J Pharmacol 168(1):79–102. https://doi.org/10.1111/j.1476-5381.2012.02027.x
Deng Y-M, Zhao C, Wu L, Qu Z, Wang X-Y (2022) Cannabinoid Receptor-1 suppresses M2 macrophage polarization in colorectal cancer by downregulating EGFR. Cell Death Discovery 8(1):273. https://doi.org/10.1038/s41420-022-01064-8
Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J et al (2016) Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol 15(3):270–278. https://doi.org/10.1016/s1474-4422(15)00379-8
Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R et al (2017) Trial of Cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med 376(21):2011–2020. https://doi.org/10.1056/NEJMoa1611618
Devinsky O, Patel AD, Thiele EA, Wong MH, Appleton R, Harden CL et al (2018) Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology 90(14):e1204–e1211. https://doi.org/10.1212/wnl.0000000000005254
Donadelli M, Dando I, Zaniboni T, Costanzo C, Dalla Pozza E, Scupoli MT et al (2011) Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Dis 2:e152. https://doi.org/10.1038/cddis.2011.36
Drozd M, Marzęda P, Czarnota J, Dobrzyński M, Skubel T, Dudek I, Rybak N (2022) The potential of cannabinoids in the treatment of lung cancer. J Edu Health Sport 12(8):1100–1110. https://doi.org/10.12775/JEHS.2022.12.08.094
du Plessis SS, Agarwal A, Syriac A (2015) Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J Assist Reprod Genet 32(11):1575–1588. https://doi.org/10.1007/s10815-015-0553-8
Egmond N, Straub VM, Stelt M (2021) Targeting endocannabinoid signaling: FAAH and MAG lipase inhibitors. Annu Rev Pharmacol Toxicol 61(1):441–463. https://doi.org/10.1146/annurev-pharmtox-030220-112741
Elbaz M, Nasser MW, Ravi J, Wani NA, Ahirwar DK, Zhao H et al (2015) Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol Oncol 9(4):906–919. https://doi.org/10.1016/j.molonc.2014.12.010
Elbaz M, Ahirwar D, Ravi J, Nasser MW, Ganju RK (2017) Novel role of cannabinoid receptor 2 in inhibiting EGF/EGFR and IGF-I/IGF-IR pathways in breast cancer. Oncotarget 8(18):29668–29678. https://doi.org/10.18632/oncotarget.9408
Ellert-Miklaszewska A, Ciechomska IA, Kaminska B (2021) Synthetic cannabinoids induce autophagy and mitochondrial apoptotic pathways in human glioblastoma cells independently of deficiency in TP53 or PTEN tumor suppressors. Cancers 13(3):419. Retrieved from https://www.mdpi.com/2072-6694/13/3/419
Fiore D, Ramesh P, Proto MC, Piscopo C, Franceschelli S, Anzelmo S et al (2018) Rimonabant kills colon cancer stem cells without inducing toxicity in Normal colon organoids. Front Pharmacol 8. https://doi.org/10.3389/fphar.2017.00949
Fisher T, Golan H, Schiby G, PriChen S, Smoum R, Moshe I et al (2016) In vitro and in vivo efficacy of non-psychoactive Cannabidiol in neuroblastoma. Curr Oncol 23(11):15–22. Retrieved from https://www.mdpi.com/1718-7729/23/11/2893
Fonseca BM, Correia-da-Silva G, Teixeira NA (2018) Cannabinoid-induced cell death in endometrial cancer cells: involvement of TRPV1 receptors in apoptosis. J Physiol Biochem 74(2):261–272. https://doi.org/10.1007/s13105-018-0611-7
Fowler C (2015) Delta9-tetrahydrocannabinol and cannabidiol as potential curative agents for cancer: a critical examination of the preclinical literature. Clin Pharmacol Therapeutics 97(6):587–596. https://doi.org/10.1002/cpt.84
Fraguas-Sánchez AI, Fernández-Carballido A, Simancas-Herbada R, Martin-Sabroso C, Torres-Suárez AI (2020) CBD loaded microparticles as a potential formulation to improve paclitaxel and doxorubicin-based chemotherapy in breast cancer. Int J Pharm 574:118916. https://doi.org/10.1016/j.ijpharm.2019.118916
Gado F, Meini S, Bertini S, Digiacomo M, Macchia M, Manera C (2019) Allosteric modulators targeting cannabinoid cb1 and cb2 receptors: implications for drug discovery. Future Med Chem 11(15):2019–2037. https://doi.org/10.4155/fmc-2019-0005
Gallego-Landin I, García-Baos A, Castro-Zavala A, Valverde O (2021) Reviewing the role of the endocannabinoid system in the pathophysiology of depression. Front Pharmacol 12. https://doi.org/10.3389/fphar.2021.762738
Glodde N, Jakobs M, Bald T, Tüting T, Gaffal E (2015) Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci 138:35–40. https://doi.org/10.1016/j.lfs.2015.04.003
Gómez del Pulgar T, Velasco G, Sánchez C, Haro A, Guzmán M (2002) De novo-synthesized ceramide is involved in cannabinoid-induced apoptosis. Biochem J 363(Pt 1):183–188. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1222465/
Good P, Haywood A, Gogna G, Martin J, Yates P, Greer R, Hardy J (2019) Oral medicinal cannabinoids to relieve symptom burden in the palliative care of patients with advanced cancer: a double-blind, placebo controlled, randomised clinical trial of efficacy and safety of cannabidiol (CBD). BMC Palliat Care 18(1):110. https://doi.org/10.1186/s12904-019-0494-6
Grabner GF, Zimmermann R, Schicho R, Taschler U (2017) Monoglyceride lipase as a drug target: at the crossroads of arachidonic acid metabolism and endocannabinoid signaling. Pharmacol Ther 175:35–46. https://doi.org/10.1016/j.pharmthera.2017.02.033
Guida M, Ligresti A, De Filippis D, D’Amico A, Petrosino S, Cipriano M et al (2010) The levels of the endocannabinoid receptor CB2 and its ligand 2-Arachidonoylglycerol are elevated in endometrial carcinoma. Endocrinology 151(3):921–928. https://doi.org/10.1210/en.2009-0883
Gurley SN, Abidi AH, Allison P, Guan P, Duntsch C, Robertson JH et al (2012) Mechanism of anti-glioma activity and in vivo efficacy of the cannabinoid ligand KM-233. J Neuro-Oncol 110(2):163–177. https://doi.org/10.1007/s11060-012-0958-5
Häuser W, Finn DP, Kalso E, Krcevski-Skvarc N, Kress H-G, Morlion B et al (2018) European pain federation (EFIC) position paper on appropriate use of cannabis-based medicines and medical cannabis for chronic pain management. Eur J Pain 22(9):1547–1564. https://doi.org/10.1002/ejp.1297
Haustein M, Ramer R, Linnebacher M, Manda K, Hinz B (2014) Cannabinoids increase lung cancer cell lysis by lymphokine-activated killer cells via upregulation of ICAM-1. Biochem Pharmacol 92(2):312–325. https://doi.org/10.1016/j.bcp.2014.07.014
Health Canada (2018) Information for health care professionals: Cannabis (marihuana, marijuana) and the cannabinoids. In
Hernández-Tiedra S, Fabriàs G, Dávila D, Salanueva Í, Casas J, Montes LR et al (2016) Dihydroceramide accumulation mediates cytotoxic autophagy of cancer cells via autolysosome destabilization. Autophagy 12(11):2213–2229. https://doi.org/10.1080/15548627.2016.1213927
Hinz B, Ramer R (2019) Anti-tumour actions of cannabinoids. Br J Pharmacol 176(10):1384–1394. https://doi.org/10.1111/bph.14426
Hirao-Suzuki M, Takeda S, Watanabe K, Takiguchi M, Aramaki H (2019) Δ9-tetrahydrocannabinol upregulates fatty acid 2-hydroxylase (FA2H) via PPARα induction: a possible evidence for the cancellation of PPARβ/δ-mediated inhibition of PPARα in MDA-MB-231 cells. Arch Biochem Biophys 662:219–225. https://doi.org/10.1016/j.abb.2018.12.011
Hohmann T, Grabiec U, Ghadban C, Feese K, Dehghani F (2017) The influence of biomechanical properties and cannabinoids on tumor invasion. Cell Adhes Migr 11(1):54–67. https://doi.org/10.1080/19336918.2016.1183867
Hohmann T, Feese K, Greither T, Ghadban C, Jäger V, Dehghani F, Grabiec U (2019) Synthetic cannabinoids influence the invasion of glioblastoma cell lines in a cell- and receptor-dependent manner. Cancers 11(2). https://doi.org/10.3390/cancers11020161
Howlett AC (2005) Cannabinoid receptor signaling. Handb Exp Pharmacol 168:53–79. https://doi.org/10.1007/3-540-26573-2_2
Howlett AC, Abood ME (2017) CB1 and CB2 receptor pharmacology. Adv Pharmacol (San Diego, Calif) 80:169–206. https://doi.org/10.1016/bs.apha.2017.03.007
Irving A, Abdulrazzaq G, Chan SLF, Penman J, Harvey J, Alexander SPH (2017) Chapter seven - cannabinoid receptor-related orphan G protein-coupled receptors. In: Kendall D, Alexander SPH (eds) Advances in pharmacology, vol 80. Academic Press, pp 223–247
Ivanov VN, Wu J, Hei TK (2017) Regulation of human glioblastoma cell death by combined treatment of cannabidiol, γ-radiation and small molecule inhibitors of cell signaling pathways. Oncotarget 8(43):74068–74095. https://doi.org/10.18632/oncotarget.18240
Ivanov VN, Grabham PW, Wu C-C, Hei TK (2020) Inhibition of autophagic flux differently modulates cannabidiol-induced death in 2D and 3D glioblastoma cell cultures. Sci Rep 10(1):2687. https://doi.org/10.1038/s41598-020-59468-4
Jager G, Witkamp RF (2014) The endocannabinoid system and appetite: relevance for food reward. Nutr Res Rev 27(1):172–185. https://doi.org/10.1017/S0954422414000080
Jeong S, Yun HK, Jeong YA, Jo MJ, Kang SH, Kim JL et al (2019) Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer Lett 447:12–23. https://doi.org/10.1016/j.canlet.2019.01.011
Johnson KA, Lovinger DM (2016) Presynaptic G protein-coupled receptors: gatekeepers of addiction? Front Cell Neurosci 10. https://doi.org/10.3389/fncel.2016.00264
Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT (2010) Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manag 39(2):167–179. https://doi.org/10.1016/j.jpainsymman.2009.06.008
Johnson JR, Lossignol D, Burnell-Nugent M, Fallon MT (2013) An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manag 46(2):207–218. https://doi.org/10.1016/j.jpainsymman.2012.07.014
Kabir MF, Kim H-R, Chae H-J (2019) Endoplasmic reticulum stress and autophagy. Endoplasmic Reticulum
Kalenderoglou N, Macpherson T, Wright KL (2017) Cannabidiol reduces leukemic cell size – but is it important? Front Pharmacol 8. https://doi.org/10.3389/fphar.2017.00144
Kargl J, Haybaeck J, Stančić A, Andersen L, Marsche G, Heinemann A, Schicho R (2013) O-1602, an atypical cannabinoid, inhibits tumor growth in colitis-associated colon cancer through multiple mechanisms. J Mol Med (Berl) 91(4):449–458. https://doi.org/10.1007/s00109-012-0957-1
Kavia RB, De Ridder D, Constantinescu CS, Stott CG, Fowler CJ (2010) Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Mult Scler 16(11):1349–1359. https://doi.org/10.1177/1352458510378020
Kenessey I, Bánki B, Márk A, Varga N, Tóvári J, Ladányi A et al (2012) Revisiting CB1 receptor as drug target in human melanoma. Pathol Oncol Res 18(4):857–866. https://doi.org/10.1007/s12253-012-9515-y
Khan MI, Sobocinska AA, Czarnecka AM, Krol M, Botta B, Szczylik C (2016) The therapeutic aspects of the endocannabinoid system (ECS) for cancer and their development: from nature to laboratory. Curr Pharm Des 22(12):1756–1766
Khan MI, Sobocińska AA, Brodaczewska KK, Zielniok K, Gajewska M, Kieda C et al (2018) Involvement of the CB2 cannabinoid receptor in cell growth inhibition and G0/G1 cell cycle arrest via the cannabinoid agonist WIN 55,212–2 in renal cell carcinoma. BMC Cancer 18(1):583. https://doi.org/10.1186/s12885-018-4496-1
Klumpers L, Thacker D (2018) A brief background on cannabis: from plant to medical indications. J AOAC Int 102:412. https://doi.org/10.5740/jaoacint.18-0208
Kose S, Aerts-Kaya F, Kopru CZ, Nemutlu E, Kuskonmaz B, Karaosmanoglu B et al (2018) Human bone marrow mesenchymal stem cells secrete endocannabinoids that stimulate in vitro hematopoietic stem cell migration effectively comparable to beta-adrenergic stimulation. Exp Hematol 57:30–41.e31. https://doi.org/10.1016/j.exphem.2017.09.009
Kovalchuk O, Kovalchuk I (2020) Cannabinoids as anticancer therapeutic agents. Cell Cycle (Georgetown, Tex) 19(9):961–989. https://doi.org/10.1080/15384101.2020.1742952
Ladin DA, Soliman E, Griffin L, Van Dross R (2016) Preclinical and clinical assessment of cannabinoids as anti-cancer agents. Front Pharmacol 7(361):361. https://doi.org/10.3389/fphar.2016.00361
Laezza C, Pagano C, Navarra G, Pastorino O, Proto MC, Fiore D et al (2020) The endocannabinoid system: a target for cancer treatment. Int J Mol Sci 21(3). https://doi.org/10.3390/ijms21030747
Lanz C, Mattsson J, Stickel F, Dufour JF, Brenneisen R (2018) Determination of the endocannabinoids anandamide and 2-Arachidonoyl glycerol with gas chromatography-mass spectrometry: analytical and Preanalytical challenges and pitfalls. Med Cannabis Cannabinoids 1(1):9–18. https://doi.org/10.1159/000489032
Lee Y, Jo J, Chung HY, Pothoulakis C, Im E (2016) Endocannabinoids in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 311(4):G655–g666. https://doi.org/10.1152/ajpgi.00294.2015
Lee XC, Werner E, Falasca M (2021) Molecular mechanism of autophagy and its regulation by cannabinoids in cancer. Cancers 13(6). https://doi.org/10.3390/cancers13061211
Leo LM, Abood ME (2021) CB1 cannabinoid receptor signaling and biased signaling. Molecules (Basel, Switzerland) 26(17). https://doi.org/10.3390/molecules26175413
Levinsohn EA, Hill KP (2020) Clinical uses of cannabis and cannabinoids in the United States. J Neurol Sci 411:116717. https://doi.org/10.1016/j.jns.2020.116717
Lichtman AH, Lux EA, McQuade R, Rossetti S, Sanchez R, Sun W et al (2018) Results of a double-blind, randomized, placebo-controlled study of Nabiximols Oromucosal spray as an adjunctive therapy in advanced cancer patients with chronic uncontrolled pain. J Pain Symptom Manag 55(2):179–188.e171. https://doi.org/10.1016/j.jpainsymman.2017.09.001
Lim KJH, Lim YP, Hartono YD, Go MK, Fan H, Yew WS (2021) Biosynthesis of nature-inspired unnatural cannabinoids. Molecules (Basel, Switzerland) 26(10). https://doi.org/10.3390/molecules26102914
López-Valero I, Saiz-Ladera C, Torres S, Hernández-Tiedra S, García-Taboada E, Rodríguez-Fornés F et al (2018) Targeting glioma initiating cells with a combined therapy of cannabinoids and temozolomide. Biochem Pharmacol 157:266–274. https://doi.org/10.1016/j.bcp.2018.09.007
Lowe H, Toyang N, Steele B, Bryant J, Ngwa W (2021) The endocannabinoid system: a potential target for the treatment of various diseases. Int J Mol Sci 22(17). https://doi.org/10.3390/ijms22179472
Lu HC, Mackie K (2021) Review of the endocannabinoid system. Biol Psychiatry-Cognitive Neurosci Neuroimaging 6(6):607–615. https://doi.org/10.1016/j.bpsc.2020.07.016
Lynch ME, Cesar-Rittenberg P, Hohmann AG (2014) A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manag 47(1):166–173. https://doi.org/10.1016/j.jpainsymman.2013.02.018
Ma C, Wu TT, Jiang PC, Li ZQ, Chen XJ, Fu K et al (2016) Anti-carcinogenic activity of anandamide on human glioma in vitro and in vivo. Mol Med Rep 13(2):1558–1562. https://doi.org/10.3892/mmr.2015.4721
Maccarrone M (2017) Metabolism of the endocannabinoid anandamide: open questions after 25 years. Front Mol Neurosci 10. https://doi.org/10.3389/fnmol.2017.00166
Mahavadi S, Sriwai W, Huang J, Grider JR, Murthy KS (2014) Inhibitory signaling by CB1 receptors in smooth muscle mediated by GRK5/β-arrestin activation of ERK1/2 and Src kinase. Am J Physiol-Gastrointestinal Liver Physiol 306(6):G535–G545. https://doi.org/10.1152/ajpgi.00397.2013
Mangal N, Erridge S, Habib N, Sadanandam A, Reebye V, Sodergren MH (2021) Cannabinoids in the landscape of cancer. J Cancer Res Clin Oncol 147(9):2507–2534. https://doi.org/10.1007/s00432-021-03710-7
Marcu JP, Christian RT, Lau D, Zielinski AJ, Horowitz MP, Lee J et al (2010) Cannabidiol enhances the inhibitory effects of delta9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol Cancer Ther 9(1):180–189. https://doi.org/10.1158/1535-7163.Mct-09-0407
Marinelli O, Morelli MB, Annibali D, Aguzzi C, Zeppa L, Tuyaerts S et al (2020) The effects of Cannabidiol and prognostic role of TRPV2 in human endometrial cancer. Int J Mol Sci 21(15). https://doi.org/10.3390/ijms21155409
Martínez-Martínez E, Martín-Ruiz A, Martín P, Calvo V, Provencio M, García JM (2016) CB(2) cannabinoid receptor activation promotes colon cancer progression via AKT/GSK3β signaling pathway. Oncotarget 7(42):68781–68791. https://doi.org/10.18632/oncotarget.11968
Martinez-Pena AA, Perono GA, Gritis SA, Sharma R, Selvakumar S, Walker OS et al (2021) The impact of early life exposure to cannabis: the role of the endocannabinoid system. Int J Mol Sci 22(16). https://doi.org/10.3390/ijms22168576
Massi P, Solinas M, Cinquina V, Parolaro D (2013) Cannabidiol as potential anticancer drug. Br J Clin Pharmacol 75(2):303–312. https://doi.org/10.1111/j.1365-2125.2012.04298.x
McAllister SD, Christian RT, Horowitz MP, Garcia A, Desprez PY (2007) Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol Cancer Ther 6(11):2921–2927. https://doi.org/10.1158/1535-7163.Mct-07-0371
McAllister SD, Murase R, Christian RT, Lau D, Zielinski AJ, Allison J et al (2011) Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res Treat 129(1):37–47. https://doi.org/10.1007/s10549-010-1177-4
McAllister SD, Soroceanu L, Desprez P-Y (2015) The antitumor activity of plant-derived non-psychoactive cannabinoids. J Neuroimmune Pharmacol 10(2):255–267. https://doi.org/10.1007/s11481-015-9608-y
McClements DJ (2020) Enhancing efficacy, performance, and reliability of cannabis edibles: insights from lipid bioavailability studies. Annu Rev Food Sci Technol 11(1):45–70. https://doi.org/10.1146/annurev-food-032519-051834
McCoy B, Wang L, Zak M, Al-Mehmadi S, Kabir N, Alhadid K et al (2018) A prospective open-label trial of a CBD/THC cannabis oil in dravet syndrome. Ann Clin Transl Neurol 5(9):1077–1088. https://doi.org/10.1002/acn3.621
Mersiades AJ, Tognela A, Haber PS, Stockler M, Lintzeris N, Simes J et al (2018) Oral cannabinoid-rich THC/CBD cannabis extract for secondary prevention of chemotherapy-induced nausea and vomiting: a study protocol for a pilot and definitive randomised double-blind placebo-controlled trial (CannabisCINV). BMJ Open 8(9):e020745. https://doi.org/10.1136/bmjopen-2017-020745
Messalli EM, Grauso F, Luise R, Angelini A, Rossiello R (2014) Cannabinoid receptor type 1 immunoreactivity and disease severity in human epithelial ovarian tumors. Am J Obstet Gynecol 211(3):234.e231–234.e236. https://doi.org/10.1016/j.ajog.2014.04.004
Milian L, Mata M, Alcacer J, Oliver M, Sancho-Tello M, Martín de Llano JJ et al (2020) Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS One 15(2):e0228909. https://doi.org/10.1371/journal.pone.0228909
Minerbi A, Häuser W, Fitzcharles MA (2019) Medical cannabis for older patients. Drugs Aging 36(1):39–51. https://doi.org/10.1007/s40266-018-0616-5
Morales P, Jagerovic N (2020) Novel approaches and current challenges with targeting the endocannabinoid system. Expert Opin Drug Discovery 15(8):917–930. https://doi.org/10.1080/17460441.2020.1752178
Morales P, Reggio PH (2019) CBD: a new Hope? ACS Med Chem Lett 10(5):694–695. https://doi.org/10.1021/acsmedchemlett.9b00127
Morales P, Vara D, Goméz-Cañas M, Zúñiga MC, Olea-Azar C, Goya P et al (2013) Synthetic cannabinoid quinones: preparation, in vitro antiproliferative effects and in vivo prostate antitumor activity. Eur J Med Chem 70:111–119. https://doi.org/10.1016/j.ejmech.2013.09.043
Morell C, Bort A, Vara D, Ramos-Torres A, Rodríguez-Henche N, Díaz-Laviada I (2016) The cannabinoid WIN 55,212-2 prevents neuroendocrine differentiation of LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis 19(3):248–257. https://doi.org/10.1038/pcan.2016.19
Moreno E, Cavic M, Krivokuca A, Casado V, Canela E (2019) The endocannabinoid system as a target in cancer diseases: are we there yet? Front Pharmacol 10(339):339. https://doi.org/10.3389/fphar.2019.00339
Mücke M, Weier M, Carter C, Copeland J, Degenhardt L, Cuhls H et al (2018) Systematic review and meta-analysis of cannabinoids in palliative medicine. J Cachexia Sarcopenia Muscle 9(2):220–234. https://doi.org/10.1002/jcsm.12273
Mukhopadhyay B, Schuebel K, Mukhopadhyay P, Cinar R, Godlewski G, Xiong K et al (2015) Cannabinoid receptor 1 promotes hepatocellular carcinoma initiation and progression through multiple mechanisms. Hepatology 61(5):1615–1626. https://doi.org/10.1002/hep.27686
Müller L, Radtke A, Decker J, Koch M, Belge G (2017) The synthetic cannabinoid WIN 55,212-2 elicits death in human cancer cell lines. Anticancer Res 37(11):6341. Retrieved from http://ar.iiarjournals.org/content/37/11/6341.abstract
Muller C, Morales P, Reggio PH (2018) Cannabinoid ligands targeting TRP channels. Front Mol Neurosci 11:487. https://doi.org/10.3389/fnmol.2018.00487
Murase R, Kawamura R, Singer E, Pakdel A, Sarma P, Judkins J et al (2014) Targeting multiple cannabinoid anti-tumour pathways with a resorcinol derivative leads to inhibition of advanced stages of breast cancer. Br J Pharmacol 171(19):4464–4477. https://doi.org/10.1111/bph.12803
Nabissi M, Morelli MB, Offidani M, Amantini C, Gentili S, Soriani A et al (2016) Cannabinoids synergize with carfilzomib, reducing multiple myeloma cells viability and migration. Oncotarget 7(47):77543–77557. https://doi.org/10.18632/oncotarget.12721
Nallathambi R, Mazuz M, Namdar D, Shik M, Namintzer D, Vinayaka AC et al (2018) Identification of synergistic interaction between cannabis-derived compounds for cytotoxic activity in colorectal cancer cell lines and colon polyps that induces apoptosis-related cell death and distinct gene expression. Cannabis Cannabinoid Res 3(1):120–135. https://doi.org/10.1089/can.2018.0010
Nasser MW, Qamri Z, Deol YS, Smith D, Shilo K, Zou X, Ganju RK (2011) Crosstalk between chemokine receptor CXCR4 and cannabinoid receptor CB2 in modulating breast cancer growth and invasion. PLoS One 6(9):e23901. https://doi.org/10.1371/journal.pone.0023901
Nogueras-Ortiz C, Yudowski GA (2016) The multiple waves of cannabinoid 1 receptor signaling. Mol Pharmacol 90(5):620–626. https://doi.org/10.1124/mol.116.104539
Novotna A, Mares J, Ratcliffe S, Novakova I, Vachova M, Zapletalova O et al (2011) A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex(®) ), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol 18(9):1122–1131. https://doi.org/10.1111/j.1468-1331.2010.03328.x
Olivas-Aguirre M, Torres-López L, Valle-Reyes JS, Hernández-Cruz A, Pottosin I, Dobrovinskaya O (2019) Cannabidiol directly targets mitochondria and disturbs calcium homeostasis in acute lymphoblastic leukemia. Cell Death Dis 10(10):779. https://doi.org/10.1038/s41419-019-2024-0
Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L et al (2008) Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann N Y Acad Sci 1139(1):434–449
Önay Ö, Köse S, Süslü N, Korkusuz P, Nemutlu E, Aydın C, Hoşal Ş (2022) Human laryngeal squamous cell carcinoma cell line release of endogenous anandamide and 2-arachidonoylglycerol, and their antiproliferative effect via exogenous supplementation: an in vitro study. Cell Tissue Bank 23(1):93–100. https://doi.org/10.1007/s10561-021-09917-9
Orellana-Serradell O, Poblete CE, Sanchez C, Castellon EA, Gallegos I, Huidobro C et al (2015) Proapoptotic effect of endocannabinoids in prostate cancer cells. Oncol Rep 33(4):1599–1608. https://doi.org/10.3892/or.2015.3746
Ozdurak RH, Seker T, Korkusuz P, Korkusuz F (2010) Quantification of anandamide and 2-Arachidonylglycerol in plasma samples: a short, non-toxic HPLC method and sample storage. Turkish J Biochem-Turk Biyokimya Dergisi 35(3):279–284. Retrieved from <Go to ISI>://WOS:000282700000019
Pacher P, Kogan NM, Mechoulam R (2020). Beyond THC and endocannabinoids. In P. A. Insel (Ed.), Annual review of pharmacology and toxicology (Vol. 60, pp. 637–659)
Pagano C, Navarra G, Coppola L, Bifulco M, Laezza C (2021) Molecular mechanism of cannabinoids in cancer progression. Int J Mol Sci 22(7). https://doi.org/10.3390/ijms22073680
Parker LA, Rock EM, Limebeer CL (2011) Regulation of nausea and vomiting by cannabinoids. Br J Pharmacol 163(7):1411–1422. https://doi.org/10.1111/j.1476-5381.2010.01176.x
Pauli CS, Conroy M, Vanden Heuvel BD, Park SH (2020) Cannabidiol drugs clinical trial outcomes and adverse effects. Front Pharmacol 11:63. https://doi.org/10.3389/fphar.2020.00063
Pérez-Gómez E, Andradas C, Blasco-Benito S, Caffarel MM, García-Taboada E, Villa-Morales M et al (2015) Role of cannabinoid receptor CB2 in HER2 pro-oncogenic signaling in breast cancer. JNCI: J Nat Cancer Inst 107(6). https://doi.org/10.1093/jnci/djv077
Pertwee RG (2012) Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philos Trans R Soc Lond Ser B Biol Sci 367(1607):3353–3363. https://doi.org/10.1098/rstb.2011.0381
Petrosino S, Schiano Moriello A, Cerrato S, Fusco M, Puigdemont A, De Petrocellis L, Di Marzo V (2016) The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Br J Pharmacol 173(7):1154–1162. https://doi.org/10.1111/bph.13084
Preet A, Ganju RK, Groopman JE (2008) Delta9-tetrahydrocannabinol inhibits epithelial growth factor-induced lung cancer cell migration in vitro as well as its growth and metastasis in vivo. Oncogene 27(3):339–346. https://doi.org/10.1038/sj.onc.1210641
Preet A, Qamri Z, Nasser MW, Prasad A, Shilo K, Zou XH et al (2011) Cannabinoid receptors, CB1 and CB2, as novel targets for inhibition of non-small cell lung cancer growth and metastasis. Cancer Prev Res (Phila) 4(1):65–75. https://doi.org/10.1158/1940-6207.Capr-10-0181
Proto MC, Fiore D, Piscopo C, Franceschelli S, Bizzarro V, Laezza C et al (2017) Inhibition of Wnt/β-catenin pathway and histone acetyltransferase activity by Rimonabant: a therapeutic target for colon cancer. Sci Rep 7(1):11678. https://doi.org/10.1038/s41598-017-11688-x
Pyszniak M, Tabarkiewicz J, Luszczki JJ (2016) Endocannabinoid system as a regulator of tumor cell malignancy - biological pathways and clinical significance. Onco Targets Ther 9:4323–4336. https://doi.org/10.2147/OTT.S106944
Qiu C, Yang L, Wang B, Cui L, Li C, Zhuo Y et al (2019) The role of 2-arachidonoylglycerol in the regulation of the tumor-immune microenvironment in murine models of pancreatic cancer. Biomed Pharmacother 115:108952. https://doi.org/10.1016/j.biopha.2019.108952
Ramer R, Hinz B (2008) Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J Natl Cancer Inst 100(1):59–69. https://doi.org/10.1093/jnci/djm268
Ramer R, Merkord J, Rohde H, Hinz B (2010a) Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem Pharmacol 79(7):955–966. https://doi.org/10.1016/j.bcp.2009.11.007
Ramer R, Rohde A, Merkord J, Rohde H, Hinz B (2010b) Decrease of plasminogen activator Inhibitor-1 may contribute to the anti-invasive action of Cannabidiol on human lung cancer cells. Pharm Res 27(10):2162–2174. https://doi.org/10.1007/s11095-010-0219-2
Ramer R, Bublitz K, Freimuth N, Merkord J, Rohde H, Haustein M et al (2012) Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. FASEB J 26(4):1535–1548. https://doi.org/10.1096/fj.11-198184
Ramer R, Heinemann K, Merkord J, Rohde H, Salamon A, Linnebacher M, Hinz B (2013) COX-2 and PPAR-gamma confer cannabidiol-induced apoptosis of human lung cancer cells. Mol Cancer Ther 12(1):69–82. https://doi.org/10.1158/1535-7163.Mct-12-0335
Ramer R, Fischer S, Haustein M, Manda K, Hinz B (2014) Cannabinoids inhibit angiogenic capacities of endothelial cells via release of tissue inhibitor of matrix metalloproteinases-1 from lung cancer cells. Biochem Pharmacol 91(2):202–216. https://doi.org/10.1016/j.bcp.2014.06.017
Ramer R, Schwarz R, Hinz B (2019) Modulation of the endocannabinoid system as a potential anticancer strategy. Front Pharmacol 10(430). https://doi.org/10.3389/fphar.2019.00430
Ramer R, Wittig F, Hinz B (2021) The endocannabinoid system as a pharmacological target for new cancer therapies. Cancers 13(22). https://doi.org/10.3390/cancers13225701
Ravi J, Sneh A, Shilo K, Nasser MW, Ganju RK (2014) FAAH inhibition enhances anandamide mediated anti-tumorigenic effects in non-small cell lung cancer by downregulating the EGF/EGFR pathway. Oncotarget 5(9):2475–2486. https://doi.org/10.18632/oncotarget.1723
Ravi J, Elbaz M, Wani NA, Nasser MW, Ganju RK (2016) Cannabinoid receptor-2 agonist inhibits macrophage induced EMT in non-small cell lung cancer by downregulation of EGFR pathway. Mol Carcinog 55(12):2063–2076. https://doi.org/10.1002/mc.22451
Roberto D, Klotz LH, Venkateswaran V (2019) Cannabinoid WIN 55,212-2 induces cell cycle arrest and apoptosis, and inhibits proliferation, migration, invasion, and tumor growth in prostate cancer in a cannabinoid-receptor 2 dependent manner. Prostate 79(2):151–159. https://doi.org/10.1002/pros.23720
Röhrig W, Achenbach S, Deutsch B, Pischetsrieder M (2019) Quantification of 24 circulating endocannabinoids, endocannabinoid-related compounds, and their phospholipid precursors in human plasma by UHPLC-MS/MS. J Lipid Res 60(8):1475–1488. https://doi.org/10.1194/jlr.D094680
Romano B, Borrelli F, Pagano E, Cascio MG, Pertwee RG, Izzo AA (2014) Inhibition of colon carcinogenesis by a standardized Cannabis sativa extract with high content of cannabidiol. Phytomedicine 21(5):631–639. https://doi.org/10.1016/j.phymed.2013.11.006
Sarafian T, Montes C, Harui A, Beedanagari SR, Kiertscher S, Stripecke R et al (2008) Clarifying CB2 receptor-dependent and independent effects of THC on human lung epithelial cells. Toxicol Appl Pharmacol 231(3):282–290. https://doi.org/10.1016/j.taap.2008.05.001
Sawtelle L, Holle LM (2021) Use of cannabis and cannabinoids in patients with cancer. Ann Pharmacother 55(7):870–890. https://doi.org/10.1177/1060028020965224
Scheffer IE, Halford JJ, Miller I, Nabbout R, Sanchez-Carpintero R, Shiloh-Malawsky Y et al (2021) Add-on cannabidiol in patients with Dravet syndrome: results of a long-term open-label extension trial. Epilepsia 62(10):2505–2517. https://doi.org/10.1111/epi.17036
Schoeman R, Beukes N, Frost C (2020) Cannabinoid combination induces cytoplasmic Vacuolation in MCF-7 breast cancer cells. Molecules (Basel, Switzerland) 25(20):4682. Retrieved from https://www.mdpi.com/1420-3049/25/20/4682
Scotchie JG, Savaris RF, Martin CE, Young SL (2015) Endocannabinoid regulation in human endometrium across the menstrual cycle. Reprod Sci 22(1):113–123. https://doi.org/10.1177/1933719114533730
Scott KA, Dalgleish AG, Liu WM (2014) The combination of cannabidiol and Δ9-tetrahydrocannabinol enhances the anticancer effects of radiation in an orthotopic murine glioma model. Mol Cancer Ther 13(12):2955–2967. https://doi.org/10.1158/1535-7163.Mct-14-0402
Scott KA, Dalgleish AG, Liu WM (2017) Anticancer effects of phytocannabinoids used with chemotherapy in leukaemia cells can be improved by altering the sequence of their administration. Int J Oncol 51(1):369–377. https://doi.org/10.3892/ijo.2017.4022
Serpell M, Ratcliffe S, Hovorka J, Schofield M, Taylor L, Lauder H, Ehler E (2014) A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain 18(7):999–1012. https://doi.org/10.1002/j.1532-2149.2013.00445.x
Shah S, Gupta A, Kumar P (2019) Emerging role of cannabinoids and synthetic CB1/CB2 receptor agonists in cancer treatment and chemotherapy-associated cancer management. J Cancer Res Ther. https://doi.org/10.4103/jcrtJCRT_488_18
Shah SA, Gupta AS, Kumar P (2020) Emerging role of cannabinoids and synthetic cannabinoid receptor 1/cannabinoid receptor 2 receptor agonists in cancer treatment and chemotherapy-associated cancer management. J Can Res Ther. Retrieved from Preprint at http://www.cancerjournal.net/preprintarticle.asp?id=263538
Shah S, Gupta A, Kumar P (2021) Emerging role of cannabinoids and synthetic cannabinoid receptor 1/cannabinoid receptor 2 receptor agonists in cancer treatment and chemotherapy-associated cancer management. J Cancer Res Ther 17(1):1–9. https://doi.org/10.4103/jcrt.JCRT_488_18
Sharafi G, He H, Nikfarjam M (2019) Potential use of cannabinoids for the treatment of pancreatic cancer. J Pancreat Cancer 5(1):1–7. https://doi.org/10.1089/pancan.2018.0019
Sharkey KA, Darmani NA, Parker LA (2014) Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur J Pharmacol 722:134–146. https://doi.org/10.1016/j.ejphar.2013.09.068
Silvestri C, Di Marzo V (2013) The endocannabinoid system in energy homeostasis and the Etiopathology of metabolic disorders. Cell Metab 17(4):475–490. https://doi.org/10.1016/j.cmet.2013.03.001
Silvestri C, Ligresti A, Di Marzo V (2011) Peripheral effects of the endocannabinoid system in energy homeostasis: adipose tissue, liver and skeletal muscle. Rev Endocrine Metabolic Disorders 12(3):153–162. https://doi.org/10.1007/s11154-011-9167-3
Simmerman E, Qin X, Yu JC, Baban B (2019) Cannabinoids as a potential new and novel treatment for melanoma: a pilot study in a murine model. J Surg Res 235:210–215. https://doi.org/10.1016/j.jss.2018.08.055
Singer E, Judkins J, Salomonis N, Matlaf L, Soteropoulos P, McAllister S, Soroceanu L (2015) Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis 6(1):e1601. https://doi.org/10.1038/cddis.2014.566
Singh K, Jamshidi N, Zomer R, Piva TJ, Mantri N (2020) Cannabinoids and prostate cancer: a systematic review of animal studies. Int J Mol Sci 21(17):6265. Retrieved from https://www.mdpi.com/1422-0067/21/17/6265
Sledzinski P, Zeyland J, Slomski R, Nowak A (2018) The current state and future perspectives of cannabinoids in cancer biology. Cancer Med 7(3):765–775. https://doi.org/10.1002/cam4.1312
Sledzinski P, Nowak-Terpilowska A, Zeyland J (2021) Cannabinoids in medicine: cancer, immunity, and microbial diseases. Int J Mol Sci 22(1). https://doi.org/10.3390/ijms22010263
Smiarowska M, Bialecka M, Machoy-Mokrzynska A (2022) Cannabis and cannabinoids: pharmacology and therapeutic potential. Neurol Neurochir Pol 56(1):4–13. https://doi.org/10.5603/PJNNS.a2022.0015
Solinas M, Massi P, Cantelmo A, Cattaneo M, Cammarota R, Bartolini D et al (2012) Cannabidiol inhibits angiogenesis by multiple mechanisms. Br J Pharmacol 167(6):1218–1231. https://doi.org/10.1111/j.1476-5381.2012.02050.x
Starowicz K, Nigam S, Di Marzo V (2007) Biochemistry and pharmacology of endovanilloids. Pharmacol Ther 114(1):13–33. https://doi.org/10.1016/j.pharmthera.2007.01.005
Steele G, Arneson T, Zylla D (2019) A comprehensive review of cannabis in patients with cancer: availability in the USA, general efficacy, and safety. Curr Oncol Rep 21(1):10. https://doi.org/10.1007/s11912-019-0757-7
Strouse TB (2016) Cannabinoids in medical practice. Cannabis Cannabinoid Res 1(1):38–43. https://doi.org/10.1089/can.2015.0010
Sun H, Jiang L, Luo X, Jin W, He Q, An J et al (2013) Potential tumor-suppressive role of monoglyceride lipase in human colorectal cancer. Oncogene 32(2):234–241. https://doi.org/10.1038/onc.2012.34
Taylor L, Crockett J, Tayo B, Morrison G (2019) A phase 1, open-label, parallel-group, single-dose trial of the pharmacokinetics and safety of Cannabidiol (CBD) in subjects with mild to severe hepatic impairment. J Clin Pharmacol 59(8):1110–1119. https://doi.org/10.1002/jcph.1412
Tomko AM, Whynot EG, Ellis LD, Dupre DJ (2020) Anti-cancer potential of cannabinoids, terpenes, and flavonoids present in cannabis. Cancers 12(7). https://doi.org/10.3390/cancers12071985
Tomko AM, Whynot EG, Dupré DJ (2022) Anti-cancer properties of cannflavin A and potential synergistic effects with gemcitabine, cisplatin, and cannabinoids in bladder cancer. J Cannabis Res 4(1):41. https://doi.org/10.1186/s42238-022-00151-y
Torres S, Lorente M, Rodriguez-Fornes F, Hernandez-Tiedra S, Salazar M, Garcia-Taboada E et al (2011) A combined preclinical therapy of cannabinoids and Temozolomide against glioma. Mol Cancer Ther 10(1):90–103. https://doi.org/10.1158/1535-7163.Mct-10-0688
Turcotte C, Chouinard F, Lefebvre JS, Flamand N (2015) Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J Leukoc Biol 97(6):1049–1070. https://doi.org/10.1189/jlb.3RU0115-021R
Turgeman I, Bar-Sela G (2019) Cannabis for cancer - illusion or the tip of an iceberg: a review of the evidence for the use of cannabis and synthetic cannabinoids in oncology. Expert Opin Investig Drugs 28(3):285–296. https://doi.org/10.1080/13543784.2019.1561859
Urquhart P, Nicolaou A, Woodward DF (2015) Endocannabinoids and their oxygenation by cyclo-oxygenases, lipoxygenases and other oxygenases. Biochimica et Biophysica Acta (BBA) – Mol Cell Biol Lipids 1851(4):366–376. https://doi.org/10.1016/j.bbalip.2014.12.015
VanDolah HJ, Bauer BA, Mauck KF (2019) Clinicians’ guide to Cannabidiol and hemp oils. Mayo Clin Proc 94(9):1840–1851. https://doi.org/10.1016/j.mayocp.2019.01.003
Vara D, Salazar M, Olea-Herrero N, Guzman M, Velasco G, Diaz-Laviada I (2011) Anti-tumoral action of cannabinoids on hepatocellular carcinoma: role of AMPK-dependent activation of autophagy. Cell Death Differ 18(7):1099–1111. https://doi.org/10.1038/cdd.2011.32
Vara D, Morell C, Rodríguez-Henche N, Diaz-Laviada I (2013) Involvement of PPARγ in the antitumoral action of cannabinoids on hepatocellular carcinoma. Cell Death Dis 4(5):e618. https://doi.org/10.1038/cddis.2013.141
Vaseghi S, Arjmandi-Rad S, Nasehi M, Zarrindast MR (2021) Cannabinoids and sleep-wake cycle: the potential role of serotonin. Behav Brain Res 412:113440. https://doi.org/10.1016/j.bbr.2021.113440
Vecera L, Gabrhelik T, Prasil P, Stourac P (2020) The role of cannabinoids in the treatment of cancer. Bratislava Med J-Bratislavske Lekarske Listy 121(1):79–95. https://doi.org/10.4149/bll_2020_012
Velasco G, Sanchez C, Guzman M (2016) Anticancer mechanisms of cannabinoids. Curr Oncol (Toronto, Ont) 23(2):S23–S32. https://doi.org/10.3747/co.23.3080
Vidinsky B, Gal P, Pilatova M, Vidova Z, Solar P, Varinska L et al (2012) Anti-proliferative and anti-angiogenic effects of CB2R agonist (JWH-133) in non-small lung cancer cells (A549) and human umbilical vein endothelial cells: an in vitro investigation. Folia Biol 58(2):75–80. Retrieved from <Go to ISI>://WOS:000303140600005
Walker OLS, Holloway AC, Raha S (2019) The role of the endocannabinoid system in female reproductive tissues. J Ovarian Res 12(1):3. https://doi.org/10.1186/s13048-018-0478-9
Walsh KB, Holmes AE (2022) Pharmacology of minor cannabinoids at the cannabinoid CB1 receptor: isomer- and ligand-dependent antagonism by Tetrahydrocannabivarin. Receptors 1(1):3–12. Retrieved from https://www.mdpi.com/2813-2564/1/1/2
Wang F, Multhoff G (2021) Repurposing Cannabidiol as a potential drug candidate for anti-tumor therapies. Biomol Ther 11(4). https://doi.org/10.3390/biom11040582
White CM (2019) A review of human studies assessing Cannabidiol’s (CBD) therapeutic actions and potential. J Clin Pharmacol 59(7):923–934. https://doi.org/10.1002/jcph.1387
Winkler K, Ramer R, Dithmer S, Ivanov I, Merkord J, Hinz B (2016) Fatty acid amide hydrolase inhibitors confer anti-invasive and antimetastatic effects on lung cancer cells. Oncotarget 7(12):15047–15064. https://doi.org/10.18632/oncotarget.7592
Wu J (2019) Cannabis, cannabinoid receptors, and endocannabinoid system: yesterday, today, and tomorrow. Acta Pharmacol Sin 40(3):297–299. https://doi.org/10.1038/s41401-019-0210-3
Wu X, Han L, Zhang X, Li L, Jiang C, Qiu Y et al (2012) Alteration of endocannabinoid system in human gliomas. J Neurochem 120(5):842–849. https://doi.org/10.1111/j.1471-4159.2011.07625.x
Xian X, Huang L, Zhang B, Wu C, Cui J, Wang Z (2016) WIN 55,212-2 inhibits the epithelial mesenchymal transition of gastric cancer cells via COX-2 signals. Cell Physiol Biochem 39(6):2149–2157. Retrieved from https://www.karger.com/DOI/10.1159/000447910
Xu D, Wang J, Zhou Z, He Z, Zhao Q (2015) Cannabinoid WIN55, 212-2 induces cell cycle arrest and inhibits the proliferation and migration of human BEL7402 hepatocellular carcinoma cells corrigendum in /mmr/13/1/1054. Mol Med Rep 12(6):7963–7970. https://doi.org/10.3892/mmr.2015.4477
Xu SH, Ma HC, Bo YH, Shao MJ (2019) The oncogenic role of CB2 in the progression of non-small-cell lung cancer. Biomed Pharmacother 117:109080. https://doi.org/10.1016/j.biopha.2019.109080
Yang Y, Huynh N, Dumesny C, Wang K, He H, Nikfarjam M (2020) Cannabinoids inhibited pancreatic cancer via P-21 activated kinase 1 mediated pathway. Int J Mol Sci 21(21):8035. Retrieved from https://www.mdpi.com/1422-0067/21/21/8035
Yasmin-Karim S, Moreau M, Mueller R, Sinha N, Dabney R, Herman A, Ngwa W (2018) Enhancing the therapeutic efficacy of cancer treatment with cannabinoids. Front Oncol 8(114):114. https://doi.org/10.3389/fonc.2018.00114
Yeshurun M, Shpilberg O, Levy-Assaraf M, Herscovici K, Dreyer J, Peck A et al (2014) Cannabidiol an innovative strategy for graft versus host disease prevention: an update of a phase I/II study. Biol Blood Marrow Transplant 20(2):S283–S284. https://doi.org/10.1016/j.bbmt.2013.12.476
Zelasko S, Arnold WR, Das A (2015) Endocannabinoid metabolism by cytochrome P450 monooxygenases. Prostaglandins Other Lipid Mediat 116-117:112–123. https://doi.org/10.1016/j.prostaglandins.2014.11.002
Zhang Y, Zheng W, Shen K, Shen W (2018) Δ9-tetrahydrocannabinol inhibits epithelial-mesenchymal transition and metastasis by targeting matrix metalloproteinase-9 in endometrial cancer. Oncol Lett 15(6):8527–8535. https://doi.org/10.3892/ol.2018.8407
Zhao Z, Yang J, Zhao H, Fang X, Li H (2012) Cannabinoid receptor 2 is upregulated in melanoma. J Cancer Res Ther 8(4):549–554. https://doi.org/10.4103/0973-1482.106534
Zhu W, Zhao Y, Zhou J, Wang X, Pan Q, Zhang N et al (2016) Monoacylglycerol lipase promotes progression of hepatocellular carcinoma via NF-κB-mediated epithelial-mesenchymal transition. J Hematol Oncol 9(1):127. https://doi.org/10.1186/s13045-016-0361-3
Acknowledgements
This work received no external funding.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a conflict of interest.
Ethical Approval
The authors declare that this article does not contain any study with human participants or animals.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Boyacıoğlu, Ö., Korkusuz, P. (2022). Cannabinoids as Prospective Anti-Cancer Drugs: Mechanism of Action in Healthy and Cancer Cells. In: Turksen, K. (eds) Cell Biology and Translational Medicine, Volume 19. Advances in Experimental Medicine and Biology(), vol 1410. Springer, Cham. https://doi.org/10.1007/5584_2022_748
Download citation
DOI: https://doi.org/10.1007/5584_2022_748
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-28419-9
Online ISBN: 978-3-031-28420-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)