Abstract
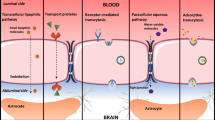
Brain cancers, especially glioblastomas (GBM), are among the most deadly human tumors due to high proliferation rates, invasion into functioning brain parenchyma, genomic instability, cellular and molecular heterogeneity, and more. The brain exhibits a uniquely complex physiology and utilizes specialized mechanisms, including blood-brain barrier formation, to prevent the influx of many molecules from the external environment, including most systemically administered chemotherapeutics. Nanoparticles are particularly promising for drug delivery to the brain due to their small size, ability to encapsulate drugs, controlled drug release profiles, and their potential to be actively targeted to cells and structures within the brain. In this chapter, we outline why targeted nanotechnology is poised to overcome the brain’s specialized barriers to drug delivery and introduce some of the cell surface molecules, including the fibroblast growth factor-inducible 14 (Fn14) receptor, employed for targeting nanodrug carriers to GBM. Importantly, we review an advanced nanotherapeutic formulation developed by our team designed for optimal (1) brain tumor accumulation and penetration by reducing nonspecific interactions with blood plasma proteins and extracellular matrix proteins (off-target effects) and (2) brain tumor targeting by increasing specific interactions with targeting molecules that are overexpressed on cancer cells (on-target effects). These decreased adhesivity, receptor-targeted (DART) nanoparticles were developed for GBM treatment by balancing and maintaining low nonspecific adhesivity and high Fn14 receptor binding.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359(5):492–507
Gladson CL, Prayson RA, Liu WM (2010) The pathobiology of glioma tumors. Annu Rev Pathol 5:33–50
Cloughesy TF, Cavenee WK, Mischel PS (2014) Glioblastoma: from molecular pathology to targeted treatment. Annu Rev Pathol 9:1–25
Aldape K et al (2015) Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol 129(6):829–848
Louis DN et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820
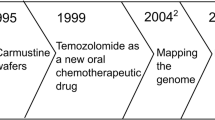
Stupp R et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Woodworth GF et al (2014) Emerging insights into barriers to effective brain tumor therapeutics. Front Oncol 4:126
Galicich JH, French LA, Melby JC (1961) Use of dexamethasone in treatment of cerebral edema associated with brain tumors. J Lancet 81:46–53
Shibamoto Y et al (1990) Supratentorial malignant glioma: an analysis of radiation therapy in 178 cases. Radiother Oncol 18(1):9–17
Stewart LA (2002) Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 359(9311):1011–1018
Brem H et al (1995) Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet 345(8956):1008–1012
Brem H et al (1995) The safety of interstitial chemotherapy with BCNU-loaded polymer followed by radiation therapy in the treatment of newly diagnosed malignant gliomas: phase I trial. J Neuro-Oncol 26(2):111–123
Valtonen S et al (1997) Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery 41(1):44–48; discussion 48-9
Westphal M et al (2003) A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-Oncology 5(2):79–88
Westphal M et al (2006) Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir 148(3):269–275. discussion 275
Giese A et al (2003) Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol 21(8):1624–1636
Vehlow A, Cordes N (2013) Invasion as target for therapy of glioblastoma multiforme. Biochim Biophys Acta 1836(2):236–244
van Tellingen O et al (2015) Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat 19:1–12
Armulik A et al (2010) Pericytes regulate the blood-brain barrier. Nature 468(7323):557–561
Pardridge WM (2005) The blood-brain barrier: bottleneck in brain drug development. NeuroRx 2(1):3–14
Obermeier B, Daneman R, Ransohoff RM (2013) Development, maintenance and disruption of the blood-brain barrier. Nat Med 19(12):1584–1596
Groothuis DR (2000) The blood-brain and blood-tumor barriers: a review of strategies for increasing drug delivery. Neuro-Oncology 2(1):45–59
Tate MC, Aghi MK (2009) Biology of angiogenesis and invasion in glioma. Neurotherapeutics 6(3):447–457
Plog BA, Nedergaard M (2018) The Glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol 13:379–394
Louveau A et al (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523(7560):337–341
Bellail AC et al (2004) Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol 36(6):1046–1069
Nicholson C, Tao L (1993) Hindered diffusion of high molecular weight compounds in brain extracellular microenvironment measured with integrative optical imaging. Biophys J 65(6):2277–2290
Diaz RJ et al (2014) Focused ultrasound delivery of Raman nanoparticles across the blood-brain barrier: potential for targeting experimental brain tumors. Nanomedicine 10(5):1075–1087
Alonso MJ (2004) Nanomedicines for overcoming biological barriers. Biomed Pharmacother 58(3):168–172
Jain RK, Stylianopoulos T (2010) Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol 7(11):653–664
Hersh DS et al (2016) Evolving drug delivery strategies to overcome the blood brain barrier. Curr Pharm Des 22(9):1177–1193
Calvo P et al (2001) Long-circulating PEGylated polycyanoacrylate nanoparticles as new drug carrier for brain delivery. Pharm Res 18(8):1157–1166
Blanco E, Shen H, Ferrari M (2015) Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol 33(9):941–951
Wadajkar AS et al (2017) Tumor-targeted nanotherapeutics: overcoming treatment barriers for glioblastoma. Wiley Interdiscip Rev Nanomed Nanobiotechnol 9(4)
Pourgholi F et al (2016) Nanoparticles: novel vehicles in treatment of glioblastoma. Biomed Pharmacother 77:98–107
Householder KT et al (2015) Intravenous delivery of camptothecin-loaded PLGA nanoparticles for the treatment of intracranial glioma. Int J Pharm 479(2):374–380
Ambruosi A et al (2006) Biodistribution of polysorbate 80-coated doxorubicin-loaded [14C]-poly(butyl cyanoacrylate) nanoparticles after intravenous administration to glioblastoma-bearing rats. J Drug Target 14(2):97–105
Nance E et al (2014) Non-invasive delivery of stealth, brain-penetrating nanoparticles across the blood-brain barrier using MRI-guided focused ultrasound. J Control Release 189:123–132
Byrne JD, Betancourt T, Brannon-Peppas L (2008) Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev 60(15):1615–1626
Peer D et al (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2(12):751–760
Yang Z et al (2010) A review of nanoparticle functionality and toxicity on the central nervous system. J R Soc Interface 7(Suppl 4):S411–S422
Cheng Y et al (2014) Blood-brain barrier permeable gold nanoparticles: An efficient delivery platform for enhanced malignant glioma therapy and imaging. Small 10(24):5137–5150
Barbu E et al (2009) The potential for nanoparticle-based drug delivery to the brain: overcoming the blood-brain barrier. Expert Opin Drug Deliv 6(6):553–565
Kreuter J et al (2002) Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J Drug Target 10(4):317–325
Zensi A et al (2009) Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J Control Release 137(1):78–86
Ren T et al (2009) Preparation and therapeutic efficacy of polysorbate-80-coated amphotericin B/PLA-b-PEG nanoparticles. J Biomater Sci Polym Ed 20(10):1369–1380
Samad A, Sultana Y, Aqil M (2007) Liposomal drug delivery systems: an update review. Curr Drug Deliv 4(4):297–305
Chastagner P et al (2015) Phase I study of non-pegylated liposomal doxorubicin in children with recurrent/refractory high-grade glioma. Cancer Chemother Pharmacol 76(2):425–432
Fabel K et al (2001) Long-term stabilization in patients with malignant glioma after treatment with liposomal doxorubicin. Cancer 92(7):1936–1942
Linot B et al (2014) Use of liposomal doxorubicin-cyclophosphamide combination in breast cancer patients with brain metastases: a monocentric retrospective study. J Neuro-Oncol 117(2):253–259
Qin J et al (2014) cRGD mediated liposomes enhanced antidepressant-like effects of edaravone in rats. Eur J Pharm Sci 58:63–71
Ying X et al (2010) Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Control Release 141(2):183–192
Padfield E, Ellis HP, Kurian KM (2015) Current therapeutic advances targeting EGFR and EGFRvIII in glioblastoma. Front Oncol 5:5
Eller JL et al (2002) Activity of anti-epidermal growth factor receptor monoclonal antibody C225 against glioblastoma multiforme. Neurosurgery 51(4):1005–1013; discussion 1013-4
Kaluzova M et al (2015) Targeted therapy of glioblastoma stem-like cells and tumor non-stem cells using cetuximab-conjugated iron-oxide nanoparticles. Oncotarget 6(11):8788–8806
An Z et al (2018) Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene 37(12):1561–1575
Frederick L et al (2000) Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res 60(5):1383–1387
Hadjipanayis CG et al (2010) EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res 70(15):6303–6312
Kim C, Ye F, Ginsberg MH (2011) Regulation of integrin activation. Annu Rev Cell Dev Biol 27:321–345
Mattern RH et al (2005) Glioma cell integrin expression and their interactions with integrin antagonists: Research Article. Cancer Ther 3a:325–340
Reardon DA et al (2008) Cilengitide: an integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin Investig Drugs 17(8):1225–1235
Jiang X et al (2013) Integrin-facilitated transcytosis for enhanced penetration of advanced gliomas by poly(trimethylene carbonate)-based nanoparticles encapsulating paclitaxel. Biomaterials 34(12):2969–2979
Dufes C, Al Robaian M, Somani S (2013) Transferrin and the transferrin receptor for the targeted delivery of therapeutic agents to the brain and cancer cells. Ther Deliv 4(5):629–640
Rosager AM et al (2017) Transferrin receptor-1 and ferritin heavy and light chains in astrocytic brain tumors: expression and prognostic value. PLoS One 12(8):e0182954
Pang Z et al (2011) Enhanced intracellular delivery and chemotherapy for glioma rats by transferrin-conjugated biodegradable polymersomes loaded with doxorubicin. Bioconjug Chem 22(6):1171–1180
Lam FC et al (2018) Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat Commun 9(1):1991
Lillis AP, Mikhailenko I, Strickland DK (2005) Beyond endocytosis: LRP function in cell migration, proliferation and vascular permeability. J Thromb Haemost 3(8):1884–1893
Pang Z et al (2010) Lactoferrin-conjugated biodegradable polymersome holding doxorubicin and tetrandrine for chemotherapy of glioma rats. Mol Pharm 7(6):1995–2005
Ruan S et al (2015) Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials 37:425–435
Benarroch EE (2014) Brain glucose transporters: implications for neurologic disease. Neurology 82(15):1374–1379
Patching SG (2017) Glucose transporters at the blood-brain barrier: function, regulation and gateways for drug delivery. Mol Neurobiol 54(2):1046–1077
Devraj K et al (2011) GLUT-1 glucose transporters in the blood-brain barrier: differential phosphorylation. J Neurosci Res 89(12):1913–1925
Jiang X et al (2014) Nanoparticles of 2-deoxy-D-glucose functionalized poly(ethylene glycol)-co-poly(trimethylene carbonate) for dual-targeted drug delivery in glioma treatment. Biomaterials 35(1):518–529
Oliveira R et al (2005) Contribution of gap junctional communication between tumor cells and astroglia to the invasion of the brain parenchyma by human glioblastomas. BMC Cell Biol 6(1):7
Schulz R et al (2015) Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacol Ther 153:90–106
Bates DC et al (2007) Connexin43 enhances glioma invasion by a mechanism involving the carboxy terminus. Glia 55(15):1554–1564
Chekhonin VP et al (2012) Targeted delivery of liposomal nanocontainers to the peritumoral zone of glioma by means of monoclonal antibodies against GFAP and the extracellular loop of Cx43. Nanomedicine 8(1):63–70
Cheng E et al (2013) TWEAK/Fn14 Axis-targeted therapeutics: moving basic science discoveries to the clinic. Front Immunol 4:473
Winkles JA (2008) The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discov 7(5):411–425
Burkly LC (2014) TWEAK/Fn14 axis: the current paradigm of tissue injury-inducible function in the midst of complexities. Semin Immunol 26(3):229–236
Burkly LC, Michaelson JS, Zheng TS (2011) TWEAK/Fn14 pathway: an immunological switch for shaping tissue responses. Immunol Rev 244(1):99–114
Perez JG et al (2016) The TWEAK receptor Fn14 is a potential cell surface portal for targeted delivery of glioblastoma therapeutics. Oncogene 35(17):2145–2155
Tran NL et al (2003) The human Fn14 receptor gene is up-regulated in migrating glioma cells in vitro and overexpressed in advanced glial tumors. Am J Pathol 162(4):1313–1321
Tran NL et al (2006) Increased fibroblast growth factor-inducible 14 expression levels promote glioma cell invasion via Rac1 and nuclear factor-kappaB and correlate with poor patient outcome. Cancer Res 66(19):9535–9542
Fortin SP et al (2012) Cdc42 and the guanine nucleotide exchange factors Ect2 and trio mediate Fn14-induced migration and invasion of glioblastoma cells. Mol Cancer Res 10(7):958–968
Hersh DS et al (2018) Differential expression of the TWEAK receptor Fn14 in IDH1 wild-type and mutant gliomas. J Neuro-Oncol 138(2):241–250
Hersh DS et al (2018) The TNF receptor family member Fn14 is highly expressed in recurrent glioblastoma and in GBM patient-derived xenografts with acquired temozolomide resistance. Neuro-Oncology 20(10):1321–1330
Bouras A, Kaluzova M, Hadjipanayis CG (2015) Radiosensitivity enhancement of radioresistant glioblastoma by epidermal growth factor receptor antibody-conjugated iron-oxide nanoparticles. J Neuro-Oncol 124(1):13–22
Jiang X et al (2011) Self-aggregated pegylated poly (trimethylene carbonate) nanoparticles decorated with c(RGDyK) peptide for targeted paclitaxel delivery to integrin-rich tumors. Biomaterials 32(35):9457–9469
Jiang X et al (2013) Solid tumor penetration by integrin-mediated pegylated poly(trimethylene carbonate) nanoparticles loaded with paclitaxel. Biomaterials 34(6):1739–1746
Wadajkar AS et al (2017) Decreased non-specific adhesivity, receptor targeted (DART) nanoparticles exhibit improved dispersion, cellular uptake, and tumor retention in invasive gliomas. J Control Release 267:144–153
Li Y et al (2012) A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials 33(15):3899–3908
Porru M et al (2014) Medical treatment of orthotopic glioblastoma with transferrin-conjugated nanoparticles encapsulating zoledronic acid. Oncotarget 5(21):10446–10459
Kim SS et al (2014) The clinical potential of targeted nanomedicine: delivering to cancer stem-like cells. Mol Ther 22(2):278–291
Jain A et al (2015) Surface engineered polymeric nanocarriers mediate the delivery of transferrin-methotrexate conjugates for an improved understanding of brain cancer. Acta Biomater 24:140–151
Kim SS et al (2015) Encapsulation of temozolomide in a tumor-targeting nanocomplex enhances anti-cancer efficacy and reduces toxicity in a mouse model of glioblastoma. Cancer Lett 369(1):250–258
Xin H et al (2012) Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials 33(32):8167–8176
Thorne RG, Nicholson C (2006) In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci U S A 103(14):5567–5572
Nance EA et al (2012) A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med 4(149):149ra119
Dancy JG et al (2016) Non-specific binding and steric hindrance thresholds for penetration of particulate drug carriers within tumor tissue. J Control Release 238:139–148
Schneider CS et al (2015) Minimizing the non-specific binding of nanoparticles to the brain enables active targeting of Fn14-positive glioblastoma cells. Biomaterials 42:42–51
Cheng CJ et al (2015) A holistic approach to targeting disease with polymeric nanoparticles. Nat Rev Drug Discov 14(4):239–247
Lesniak MS, Brem H (2004) Targeted therapy for brain tumours. Nat Rev Drug Discov 3(6):499–508
Nance E et al (2014) Brain-penetrating nanoparticles improve paclitaxel efficacy in malignant glioma following local administration. ACS Nano 8(10):10655–10664
Zhang C et al (2017) Convection enhanced delivery of cisplatin-loaded brain penetrating nanoparticles cures malignant glioma in rats. J Control Release 263:112–119
Zhou J et al (2013) Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proc Natl Acad Sci U S A 110(29):11751–11756
Acknowledgments
This work was supported, in part, by the National Institutes of Health (R37 CA218617 (A.J.K.), K08 NS09043 (G.F.W.), RO1 NS107813 (G.F.W.), an Institutional Research Grant IRG-97-153-10 (A.S.W.) from the American Cancer Society. A.S.W. and N.P.C. were supported in part by NIH Training Grant T32 CA154274.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Wadajkar, A.S. et al. (2021). Surface-Modified Nanodrug Carriers for Brain Cancer Treatment. In: Agrahari, V., Kim, A., Agrahari, V. (eds) Nanotherapy for Brain Tumor Drug Delivery. Neuromethods, vol 163. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1052-7_5
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1052-7_5
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1051-0
Online ISBN: 978-1-0716-1052-7
eBook Packages: Springer Protocols