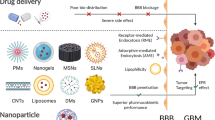
Abstract
Glioblastoma multiforme is a frequent tumor of the central nervous system in adults. Actual treatments allow a survival time of about 15 months, calling for advanced treatments. Here nanotechnologies have allowed drugs to cross the blood brain barrier and stay in glioblastoma multiforme tissues for a prolonged period of time. Actual research focus on active targeting of drugs in the glioblastoma multiforme tissue, and tracking the biodistribution of drugs by imaging. This chapter reviews nanotechnologies for targeted delivery of drugs in glioblastoma multiforme tissues. The potential of polymeric nanoparticles in the management of malignant gliomas is also discussed, with focus on coating and functionalization to cross the blood brain barrier.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Cancer is one of the diseases with the maximum mortality tolls in the world, amid major medical developments in the field of oncology (Padhi et al. 2015). There are many types of cancers (Fig. 12.1), out of which glioblastoma multiforme is one of the most severe and most frequent brain tumors of all human cancers in adults, responsible for about 50% of all primary gliomas (Fernandez et al. 2012). According to the World Health Organization, the prevalence is roughly 5–10 instances in a population of one lakh (Omuro and DeAngelis 2013). Bailey and Cushing initially identified glioblastoma multiforme in the year 1926, and they identified it as irregular glioblasts (glial cell growth) in the brain (MacKenzie 1927). A more frequently identified central nervous system tumor in adults is glioblastoma multiforme, which is specified as grade IV astrocytoma as described in the guidelines of the World Health Organization. Usually, the tumor shows dispersed boundaries and a large penetration into the surrounding healthy brain tissue of individual tumor cells that exacerbates surgical removal (Maher et al. 2001). In addition, conventional chemotherapy results in by low drug partitioning through the blood brain barrier leading to poor selectivity and lower therapeutic efficacy. Treatment methods presently offered have not proven very successful in improving the conditions of patients. It offers a median approximate life expectancy of just 12–16 months, presumably the presence of residual tumor cause the glioma to relapse.
Common types of brain tumors. Adapted from the Journal of Cancer Metastasis and Treatment, OEA Publishing Inc. from EL Amrawy et al. (2016), an open access article distributed under the creative commons attribution license that permits unrestricted use, distribution, and reproduction in any medium
Restricted and far less successful choices for the management of such extremely aggressive glioblastoma multiforme pushed the research scientists to develop new strategies furthering to improvement of existing technologies. Multiple options for overcoming the disadvantages of conventional delivery of anticancer agents to the brain through the use of nanocarriers have been implicated in the recent years. In curing the stated disease, nanoscience has played a vital role and specifically polymeric nanoparticles have shown the ability to penetrate the blood brain barrier and persist in glioblastoma multiforme tissues for a prolonged period of time. Nanoparticles are especially suitable carriers for deoxyribonucleic acid (DNA), ribonucleic acid (RNA), chemotherapeutic agents and proteins (Pitorre et al. 2020). Current nanotechnology based approaches aim to increase the active targeting of drug to the targeted tissues for the purpose of delivery of chemotherapeutic drugs and offer good imaging studies (Padhi et al. 2018; Behera and Padhi 2020).
The aim of the present chapter is to elaborate the progress in the field of nanotechnology that has helped enormously in the targeted delivery of chemotherapeutic drugs in glioblastoma multiforme tissues. The potential of polymeric nanoparticles in the management of malignant gliomas also will be addressed, along with the significance of their coating and functionalization for their ability to cross the blood brain barrier.
12.2 Glioblastoma
Glioblastoma multiforme remains as one of mankind’s biggest life threatening ailments and each year impacts lakhs of patients worldwide. Early in its pathogenesis, this malignancy infiltrates the brain and renders total neurosurgical resection which is nearly inevitable (Abrudan et al. 2014). Gliomas (54%) and primary brain tumors (16%) are categorized under glioblastoma multiforme. Malignant gliomas accounts for approximately 11,000 deaths globally (Behin et al. 2003). The standard treatment protocol pertains to surgical removal accompanied by temozolomide administered orally simultaneously with chemotherapeutic. The life expectancy of patients with glioblastoma multiforme following its preliminary identification is only 15 months (Roger Stupp et al. 2005). Hence, there arises a certain need to develop novel strategies for treating patients with glioblastoma multiforme. The lack of existing therapeutic strategies for malignant gliomas has so far been due to the presence of a subpopulation of malignant glioma cancer stem cells that have the strength to tolerate chemotherapy and ionizing radiation built on some of their distinctive attributes such as high anti-apoptotic protein expression, high ATP-binding cassette pump expression, and outstanding DNA properties (Stupp et al. 2005, 2010).
A bunch of nanoparticulate drug delivery systems, including polymeric nanoparticles, nanoemulsion, liposomes, iron oxide nanoparticles, and polymeric micelles have been widely studied as carriers for an array of drugs in the treatment of various disease conditions in the recent past (Patnaik et al. 2021; Behera et al. 2020a, b; Hassan et al. 2021) Passive and active targeting are the central approaches which are employed for targeting nanocarriers to specific sites (Padhi and Behera 2020). Passive targeting allows the accumulation of nanoparticles in tumor tissues owing to the typical attributes of the tumor microenvironment which is termed as enhanced permeability and retention effect (Verma et al. 2017). Enhanced permeability and retention effect allows the retention of nanomaterials in tumor tissues via passive targeting. At present, convection-enhanced delivery is applied to increase the uptake of nanomaterials into brain tumor tissues. Nanomaterials along with small-interfering RNA are used to suppress the gene function that makes glioblastoma multiforme highly aggressive. More importantly, these nanomaterials can be used to deliver chemotherapeutic agents specifically to the tumor tissues without causing systemic toxicity (Michael et al. 2018). A combination of conventional and nanotechnology-based therapies has provided promising outcomes in this regard (Abrudan et al. 2014).
12.3 Advances in the Development of Novel Therapeutics for Glioblastoma
Nanotechnology has revolutionized the preceding years in the drug delivery domain (Padhi et al. 2020). The past few years have witnessed major developments in the studies related to targeted therapies for amelioration of tumors. Owing to the specific chemical and physical characteristics that lead to precise distribution and accumulation of encapsulated drugs in precise organs and tissues, polymeric nanoparticles have proven as outstanding transport carriers for biologically active molecules or drugs (Abrudan et al. 2014). Polymeric nanoparticles employing biodegradable polymers like poly (ethylene glycol), and poly (butyl-cyanoacrylate) encapsulating an array of chemotherapeutic agents have garnered varied application and have resulted in improved survival rates. Polymeric nanoparticles have also demonstrated enhanced therapeutic efficacy with a reduction in adverse effects to the surrounding healthy tissues (Abrudan et al. 2014; Maier-Hauff et al. 2011; Khuroo et al. 2014).
Malignant gliomas are one among the deadly types of brain cancer. Particularly the administration of hydrophilic drugs in neat form leads to diminished targeted delivery at the tumor site due to inadequate blood brain barrier penetration. Furthermore, drugs of low molecular weight do not undergo sufficient accumulation in cancerous tissues and are characterized with a lower t1/2 in the systemic circulation. The nanoparticles may be engineered with suitable ligands for crossing the blood brain barrier leading to targeted delivery in the brain, thereby enhancing their therapeutic efficacy as compared to drugs in its native form. Surface decoration of polymeric nanoparticles with suitable ligand is known to improve the therapeutic effectiveness with reduced off-target side effects (Mahmoud et al. 2020).
There has been a significant improvement in nanomedicine and cancer care over the past few decades. However, due to its complicated pathophysiology, cancer continues to be a daunting health issue. As shown by the american cancer society, the number of cancer occurrences is projected to increase to 27.5 million by the year 2040. The major brain tumors, which can seldom be healed, are among the most problematic malignant cancers, with a 5 years average lifespan. Gliomas are by far the most prevalent type of primary malignant brain tumor in adults (Lapointe et al. 2018). The primary factors that decide whether glioma cells belong to low grade (WHO I and II) or high grade (WHO III and IV) category are their capacity to penetrate and metastasize into surrounding tissues of brain. Gliomas have the ability to penetrate the underlying tissue, and hence it becomes difficult to define their margins. This results in the failure of conventional treatment approach to provide a curative effect. The prevalent chemical and physical barriers challenging the biological milieu possess a major challenge for the effective delivery of the drugs at the target site (Cornago et al. 2014; De Boer and Gaillard 2007). The barrier that prohibits suitable delivery of drugs across the brain is the blood brain tumor barrier and the blood brain barrier (Fig. 12.2). The multipotent stem cells that culminate into glioma cells are capable of self-renewal and often relapse (Binello and Germano 2011). With the advancement of targeted strategies for drug delivery to the brain, attempts have been explored to address physical barriers, however, all of these methods are found to be intrusive and hazardous with severe side effects. Utilizing polymeric nanoparticles for drug delivery and targeting is one among the innovative treatment options. Positive results for drug-loaded nanoparticles targeting gliomas are reported in many research studies, which are discussed later in this chapter. Further attempts should indeed be made to enhance these nanomedicines to improve their ability to target gliomas (Mahmoud et al. 2020).
Differences between blood brain barrier and blood brain tumor barrier. Blood brain barrier, a diffusion barrier, protects the brain and maintain brain’s homeostasis by controlling influx of blood components into the brain. Brain capillary endothelial cells and other cell types such as pericytes, astrocytes, and neuronal cells that play an important role in its function form the blood brain barrier. Tight junctions of brain capillary endothelial cells prevent paracellular transport of small and large water-soluble compounds (a). Under some pathological conditions like tumors, the structure and functions of blood brain barrier are altered. In such case, the barrier is called as blood-brain tumor barrier. In high-grade gliomas, the blood brain tumor barrier becomes disrupted and leaky in nature (b). Adapted from the Journal of Cancer Metastasis and Treatment, OEA Publishing Inc. from EL Amrawy et al. (2016), an open access article distributed under the creative commons attribution license that permits unrestricted use, distribution, and reproduction in any medium
12.4 Drug Delivery to the Brain
Paracellular permeability is not provided by the normal physiology of the blood brain barrier. In central nervous system diseases such as glioblastoma multiforme, however, it may take place if the blood brain barrier is damaged, which may allow drug distribution to the brain. Immune cells like leukocytes are transferred through chemotaxis and diapedesis processes to the brain parenchyma in conditions like neuroinflammation, or glioblastoma multiforme. This process could be exploited in the production of nanoparticles or drugs that could be phagocytosed by leukocytes and then transmitted into the brain. This has been shown to improve the effectiveness of free drugs and nanoparticles supplied by such a natural process, which is also regarded as the trojan horse mechanism. This process makes it possible to penetrate the brain with larger particles, but their larger size will also contribute to enhanced toxicity. To circumvent the blood brain barrier, there seems to be a range of choices some of which are mentioned below.
Intracerebroventricular delivery is done directly into the brain via an aggressive skull invasion technique and drug injection. To administer the medication via an outlet catheter, a pump or an implantable reservoir is employed. At high levels, they facilitate the flow for a steady drug supply. The procedure of intracerebroventricular is highly aggressive and may contribute to augmented intracranial pressure and infections.
Intraparenchymal/Intracerebral management includes injecting medications straight into the brain tissue either through stereotactic injection or through implant formulation, which can be inserted throughout resection surgical procedure (Gliadel®), or through stereotactic surgical treatment. The problem of this delivery technique is that the medication dissemination happens gradually from the injection/implantation site (penetrates only 2 mm inside) that helps the drug to escape.
Convection enhanced delivery is a surgical technique that is marginally less intrusive whereby catheters are positioned within the brain parenchyma interstitial space. Using a pump the solution of drug is administered inside the brain underneath a positive pressure gradient, resulting in a greater amount being visually presented for intracerebral/intraparenchymal treatment. Although this technique is also invasive and could increase the threat for patients like illness, tissue damage, and air bubbles. Besides, the solution of drug might escape into vulnerable parts of the brain, like subarachnoid space, because of the high pressure being utilized (Bennewitz and Saltzman 2009).
Intrathecal administration is regarded to be among the lowest medical interventions in which medicines are inserted through a lumbar puncture into another subarachnoid space of the spinal cord, touching the central nervous system parenchyma into the cerebral spinal fluid. Though, according to this process, potential adverse effects known as adverse immune responses and infections may follow. Furthermore, while the intracerebroventricular and intrathecal techniques may resolve the obstacles of cerebrospinal fluid and blood brain barrier, the glial cells and ependymal cell layer reside between the cerebrospinal fluid and the brain parenchyma, restricting the effectiveness of diffusion of the drug by these strategies to enter the brain parenchyma (Mahmoud et al. 2020).
Intratympanic administration uses the middle ear pathway to administer medications that are transmitted by pinocytosis, ultimately accessing the brain whereby they circumvent the blood-labyrinthine barrier. For therapeutics upto 1 μm scale, this route may be acceptable. Poly (D, L-lactide-co-glycolide) nanoparticles have been utilized with encouraging effectiveness to deliver drugs through this pathway.
Intranasal distribution is a method of drug delivery that is non-invasive and used for circumventing blood brain barrier into nasal cavity via spraying medications, whereby they disperse drugs extracellularly or by convection. Another direction is through the intra-neuronal transport of olfactory sensory neurons or trigeminal nerves, called intra-neuronal transport (Lochhead and Thorne 2012). In addition to becoming effective for customers, the intranasal route is advantageous in facilitating the rapid onset of action and preventing first-pass metabolism (Zhang et al. 2016).
Certain other blood brain barrier crossing strategies have been researched; many of these are intrusive, including the blood brain barrier’s osmotic opening. The trojan-horse technology, for example, which depends on the combination of drugs with genetically engineered proteins that could circumvent the blood brain barrier via receptor-mediated transport processes, has also been examined by other non-invasive methods. Such strategies are often rife with adverse effects, therefore more reliable, and less toxic methods are required to deliver medications to the brain to enhance the management of brain tumors (Busquets et al. 2015).
12.5 Polymeric Nanoparticles for Targeting Glioblastoma
Nanomedicine has equipped us with such a powerful candidate that can be used around the blood brain barrier to increase the absorption of drugs. It is because nanoparticles are capable of being loaded with drugs and functionalized with various ligands that allow the blood brain barrier targeting. By focusing the medication within or on the blood brain barrier surface, nanoparticles are suggested to carry out their operation in transmitting drugs through the blood brain barrier, resulting in a greater concentration gradient among brain and blood, facilitating drug’s passive diffusion throughout the brain. An important advancement in nanotechnology is the polymeric nanoparticles delivery by active targeting through ligands mediated surface modification that attaches to a specific entity on the cancer cell surface or to some other cells inside the body (Holmes 2013; Re et al. 2012). Examples of such nanoparticles for treatment of malignant gliomas by targeting are shown in Table 12.1. Either for the intracellular or extracellular drugs delivery, actively targeted nanoparticles needs to be explored. If directed to intracellular sites, nanoparticles are most successful (Mahmoud et al. 2020). Nanoparticles provide the potential to diffuse through the improved permeability and retention effect into the leaky vasculature of tumor tissues (Wicki et al. 2015). Three key tasks can be achieved for successful treatment of glioblastoma multiforme: (i) to enhance blood brain barrier crossing ability of chemotherapeutic agents, to infiltrate into brain tissue and deliver the therapeutic concentrations in the tumor tissue, (ii) to prevent or eliminate adverse effects, and (iii) to preserve therapeutic drug concentrations at the tumor site, to extend its half-life and to prevent rapid clearance (Wicki et al. 2015).
Polymeric nanoparticles are known as colloidal nanoparticles of submicronic size that are employed as drug carriers in which drugs are either are attached to the surface or encapsulated inside the core. Several types of polymers, like poly (butyl-cyanoacrylate), poly (lactic acid), poly (glycolic acid), poly (ε-caprolactone), poly (amino acids), and poly (lactic-co-glycolic acid); that are used in the formulation of nanoparticles. Owing to low toxicity profile and biocompatible properties, poly (lactic-co-glycolic acid), poly (lactic acid) and poly (glycolic acid) are by far the most commonly used polymers in the brain targeted drug delivery. They convert into lactic acid and glycolic acid, which join the pathway of kreb’s cycle, whereby their metabolites are extracted from the body as carbon dioxide and water. Polymeric nanoparticles have benefits over traditional nanoparticles, like enhanced kinetics of drug release, improved drugs compatibility, no phospholipid-like oxidation problems, and enhanced shelf life. An acknowledgment of the crystallinity, stability, and molecular weight of the polymers, and also the drug’s physicochemical properties, is needed to successfully synthesize polymeric nanoparticles for brain drug delivery (Mahmoud et al. 2020).
Kreuter et al. formulated polymeric nanoparticles to deliver drugs to the brain. Blood brain barrier penetration of dalargin was substantially improved by formulating it into nanoparticles employing poly (butyl cyanoacrylate). In 2001, the same p80 coated dalargin-loaded poly (butyl cyanoacrylate) nanoparticles were used by Kreuter et al. to increase brain tissue penetration. This nanoparticle composition has been used to inject certain drugs into the brain, including loperamide as well as doxorubicin (Kreuter 2001). Calvo et al. formulated nanoparticles of poly (ethylene glycol) – (poly (hexadecyl cyanoacrylate)) that showed significantly better brain buildup according to the p80 formulation, which might be attributable to passive diffusion or macrophage intake (Calvo et al. 2001). On the surface of nanoparticles, the poly (ethylene glycol) coating density influenced the degree to which they circumvent the blood brain barrier (Mahmoud et al. 2020).
For brain distribution of chemotherapeutic drugs, a multitude of distinct nanoparticle compositions were examined. In subsequent years, a major focus has been paid to nanoformulations for the glioblastoma multiforme management. The usage of nanoparticles for brain delivery can improve the probability of drugs crossing the blood brain barrier whilst minimizing unspecific aggregation in certain tissues. For instance, if correlated with free gadolinium, gadolinium-loaded nanoparticles improved the level of gadolinium by 100 folds. With the optimization of entrapment efficiency, drug loading, and drug release profile, the development of nanoparticles has increased in the last couple of years.
Advances in the stealth capabilities of nanoparticles have also strengthened their protection against protein agglutination in the blood, enabling them to avoid blood cleaning from the reticuloendothelial system. Ligand-modified surface nanoparticles have been documented to allow the imaging of nanoparticles for brain tumors as well. In an attempt to safeguard nanoparticles from plasma protein binding and reticulo endothelial system uptake, PEGylation approach for nanoparticles has been commonly utilized. Nanoparticles provide the brain with a non-invasive drug delivery means. To be much more efficient with decreased toxicity, nanoparticles for brain targeting needs to fulfill some significant tasks. The criteria involves nanocarriers to be biodegradable, non-toxic, no blood aggregation, greater encapsulation efficiency, extended circulation time, and the ability to cross the blood brain barrier (Tian et al. 2014).
Paclitaxel is a chemotherapeutic agent that was in a nanoparticle formulation composed of poly (lactic-co-glycolic acid). The entrapment allowed for the repossession of paclitaxel (toxic chemotherapeutic agent) only within the nanoparticles before the enhanced permeation and retention effect, which reduced systemic toxicity and entered the tumor tissue. Though enhanced permeation and retention effect is extremely advantageous for nanoparticulate drug delivery, this passive mechanism helps the nanocarriers to penetrate the glioblastoma multiforme tissue. Polymeric nanoparticles which do not penetrate cancerous tissues tends to retain in the liver, kidney, and spleen reticuloendothelial tissue (Mehrotra and Tripathi 2015; Pérez-Martínez et al. 2011). Convection-enhanced delivery is a tool to preserve a pressure gradient throughout interstitial infusions. It has been used to improve the paclitaxel loaded polymeric nanoparticles delivery to the brain parenchymal cells. It has been demonstrated that convection-enhanced delivery greatly enhances the distribution of small and large molecules inside the brain (Michael et al. 2018; Zhou et al. 2013).
Enhanced cytotoxicity of paclitaxel – loaded nanoparticles was reported when a ligand specific to the transmembrane human epidermal growth factor receptor 2; extracellular domain was employed as compared to non-targeted nanoparticles. This increase in cytotoxicity was due to enhanced cellular absorption of the targeted nanoparticles. Due to the drawbacks associated with the use of multi-ligands in a single nanosystem for tumor cells targeting, many researchers have chosen to employ a single ligand. Multi-ligands affect the release of drugs as well as the mobility of nanoparticles. Also, the targeting efficacy of the nanoparticles is often seen to be decreased by interaction amongst ligands and/or competitive binding. It is understood that transferrin receptors as well as low-density protein-related lipoprotein receptor are over-expressed in glioma cells. Using binding the angiopep and anti-transferrin ligands to its exterior surface, these two receptors were targeted by polymeric nanoparticles to reach glioma cells. The most frequently described receptor-mediated transport mechanism is the transferrin receptor that ensures successful cellular uptake.
Using an in vitro blood brain barrier model, Chang et al. showed that the transferring coated poly (lactic-co-glycolic acid) nanoparticles showed 20-folds improvement in poly (lactic-co-glycolic acid) uptake relative to uncoated polymeric nanoparticles. The absorption of transferrin- poly (lactic-co-glycolic acid) nanoparticles by the blood brain barrier occurred by endocytosis pathway. The main drawback with transferrin as a ligand is its competition with endogenous transferrin for receptor binding. It may contribute to a decrease in cellular uptake by the tumor cells. An antibody directed against transferrin was used as an alternative ligand to the endogenous transferrin because it binds to the epitope of transferrin receptor, which is located at a higher position as compared to the transferrin binding position. Consequently, even if they do not interact with the transferrin intake process, nanoparticles get less binding rivalry. This improves their cellular uptake and hence their therapeutic efficacy. To improve brain uptake, various antibodies like 8D3 (both anti-mouse TfR mAbs), OX26 (anti-rat TfR mAbs), and R17–217 are being established. Rmalho et al. have produced temozolomide – mediated receptor loaded poly (lactic-co-glycolic acid) nanoparticles functionalized with OX26 mAbs for glioblastoma multiforme treatment. Especially the cellular internalization of OX26 mAbs nanoparticles was greatly improved as compared to the poly (lactic-co-glycolic acid) nanoparticles with no mAbs (Mahmoud et al. 2020).
Another technique for improving the blood brain barrier’s absorption of nanoparticles is polymer coating that enhances cellular uptake process. Kreuter showed that i.v. injected doxorubicin-loaded p80-coated nanoparticles had a 40% more cure rate in rats with intracranially transplanted glioblastoma multiformes. Albeit not thoroughly elucidated, he hypothesized that endocytosis by the endothelial cells lining the brain blood capillaries may be the underlying mechanism for transporting the nanoparticles around the blood brain barrier. The coating of p80 nanoparticles resulted in the surface adsorption of apolipoprotein E from blood plasma on them. The nanosized particles then imitated low-density lipoprotein particles and were thus able to communicate with the low-density lipoprotein receptor, contributing to improved endothelial cell uptake (Kreuter 2001). The first polymeric nanoparticles for blood brain barrier absorption were studied in 1995 by Schröder et al. for hexapeptide dalargin-loaded nanoparticles by poly (butyl cyanoacrylate) nanoparticles coated with p80 were noted. Wohlfart et al. showed that poly (lactic-co-glycolic acid) nanoparticles-coated with poloxamer 188 permitted the doxorubicin delivery at therapeutically efficient concentrations across blood brain barrier. Because of the poloxamer 188 coating, the reason for the transport through the blood brain barrier was hypothesized to be the adsorption of blood apolipoproteins (ApoE or ApoA-I) on the surface of nanoparticles. Manlioovskaya et al. showed that through clathrin-mediated endocytosis, the nanoparticles were taken up by human primary glioblastoma cells (U87). They also showed that doxorubicin was released from the nanoparticles through diffusion instead of intracellular degradation (Demeule et al. 2008). The research thus showed that poly (lactic-co-glycolic acid) nanoparticles coated with poloxamer 188 could increase the targeting of such chemotherapeutic drugs for brain tumors.
Angiopep is one more successful lipophorin-receptor ligand that is used for delivery of drugs to central nervous system (Demeule et al. 2008). In contrast to transferrin, the transcytosis potential and parenchymal aggregation of angiopep-2 is much higher. A series of research studies have verified the potential of angiopep to promote blood brain barrier absorption of polymeric nanoparticles (Mahmoud et al. 2020) for enhancing the paclitaxel delivery to glioma cells. Xin et al. formulated nanoparticles with dual-targeting approach. Angiopep-PEG-PCL nanoparticles, relative to non-targeted poly (ethylene glycol)-poly (ε-caprolactone) nanoparticles, were strongly endocytosed by human primary glioblastoma (U87) cells. These nanoparticles in 3D glioma spheroids displayed a greater amount of penetration, distribution, and aggregation as well as enhanced therapeutic effectiveness when in U87 tumor-carrying mice (Xin et al. 2012).
12.6 Peptide-Receptor as a Dual-Targeting Drug Delivery Approach
The use of receptors present on tumor cells for the targeting of nanomedicines is one technique to improve glioblastoma multiforme management. One such example is the low-density lipoprotein receptor (cell-surface receptor) that is expressed by blood brain barrier cells and over-expressed by glioblastoma cells. Angiopep-2, a 19 amino acid peptide that specifically binds to the low-density lipoprotein receptor, has been shown to improve the blood brain barrier delivery of wide chemotherapy agents when evaluated in both in vitro and in vivo models (Pitorre et al. 2020).
Xin et al. investigated the concept of developing dual-targeted angiopep-2 modified nanoparticles. The restructured nanoparticles need to first traverse the blood brain barrier and then target the tumor cells (Xin et al. 2011). Angiopep-2-conjugated poly (ethylene glycol)-poly (ε-caprolactone) nanoparticles were fabricated by coupling of angiopep-2 and maleimide- poly (ethylene glycol)-poly (ε-caprolactone)copolymer. Paclitaxel (PTX) was used as a model drug in the said system. The encapsulation ratio and angiopep-2-paclitaxel loading coefficient decreased without a targeting ligand relative to paclitaxel loaded nanoparticles. The formulations were evaluated in nude mice implanted with intracranial tumor U87 MG upon intravenous injection. The findings indicated that angiopep-2-paclitaxel nanoparticles aggregation was much greater than paclitaxel nanoparticles in the brain of tumor bearing mice. The finding was supported by an ex vivo assessment of the expurgated tissues (liver, heart, kidney, spleen, and lung) that showed selective brain tumor deposition by the targeted nanoparticles (Pitorre et al. 2020). In the presence of low-density lipoprotein receptors in both tumor cells and blood brain barrier, differences in the absorption of angiopep-2-conjugated nanoparticles may be associated with peptide-induced infiltration as compared to non-conjugated nanoparticles. Similar authors examined the bioavailability of angiopep-2-poly (ethylene glycol)- paclitaxel nanoparticles utilizing a three dimensional glioma cell culture model. Angiopep-2-oly (ethylene glycol)-paclitaxel nanoparticles transcytosis through blood brain barrier cells shadowed by tumor cell endocytosis was shown by low-density lipoprotein receptor recognition, verifying the dual-targeting approach (Xin et al. 2012).
The evaluation of anti-tumor effectiveness was done in vivo in U87 MG tumor-bearing mouse model. In contrast to the control group treated with saline, tumor inhibition levels were 20.5%, 36.1%, and 65.6%, while mice were given poly (ethylene glycol)-paclitaxel nanoparticles; angiopep-2-poly (ethylene glycol)-paclitaxel nanoparticles or taxol respectively. Furthermore, the median survival time was 37 days for mice treated with angiopep-2-poly (ethylene glycol)-paclitaxel nanoparticles, which was substantially higher as compared to the poly (ethylene glycol)-paclitaxel nanoparticles or taxol treated mice. Altogether, the findings indicated the potential of the dual-targeting method using angiopep-2 conjugated nanoparticles. Besides, after i.v. infusion of conjugated non-loaded nanoparticles (100 mg/kg/day) over a week, acute toxicity was not observed in the liver, hematological system, brain and kidney parenchyma (Xin et al. 2011).
12.7 Dual-Targeting of Both Glioma and Neovascular Cells
Of all solid tumors, glioblastoma multiforme is one of the most studied one, and neovascularization has a major role in glioma development (Pitorre et al. 2020). Zhang et al. have established an interesting dual-targeting strategy by developing nanoparticles to target neovascular cells while delivering paclitaxel to control tumor cells. It has been shown that Enhanced green fluorescent protein (EGFP-EGF1), a fusion protein, binds tissue factor uniquely to neovascular and tumor cells. Poly (ethylene glycol)-poly (lactic acid) nanoparticles in the size range of 105 nm was formulated by emulsion-solvent evaporation process, which was evaluated in cells that express tissue factor. An improved in vivo absorption of functionalized nanoparticles in extravascular and neovascular tumor cells was observed 4 h after intravenous administration relative to non-functionalized nanoparticles. Also, the median survival time for control animals with functionalized nanoparticles was longer (41 days) compared to the non-functionalized nanoparticles treated animals (21–27 days), taxol (13 days), and saline (14 days) (Mei et al. 2010; Zhang et al. 2014).
12.8 Aptamer-Peptide Conjugates as a Dual-Targeting Delivery System
Gao et al. designed a targeted delivery method capable of crossing the blood brain barrier. Poly (ethylene glycol)-polycaprolactone nanoparticles loaded with docetaxel were formulated using the emulsion solvent evaporation method and TGNYKALHPHNG (TGN), a 12 amino acid peptide and an aptamer (AS1411) was grafted to the surface of the nanoparticles to boost uptake across the blood brain barrier and target tumor cells, respectively (Gao et al. 2012, 2014). Utilizing mouse brain endothelial cells, the nanoparticles in vitro tumor-targeting efficacy was investigated. In contrast with the AS1411- nanoparticles and non-grafted nanoparticles, nanoparticles grafted with both TGN and AS1411 showed a greater brain uptake, which indicated a TGN mediated uptake of nanoparticles via blood brain barrier. Nanoparticles modified with TGN were identified in tumor cells as well as in healthy brain tissue, whereas TGN and AS1411 modified NPs both were primarily found within the glioblastoma multiforme cells. The outcomes of the nanoparticles uptake into the glioblastoma multiforme verified the formulation’s dual-targeting efficiency. The enhanced in vivo effect of the dual-targeting approach i.e. mice bearing the tumor treated with AS1411-TGN nanoparticles was shown to have an enhanced survival time by 36 days comparable to TGN- nanoparticles (31 days) or AS1411- nanoparticles (30 days) treated mice (Pitorre et al. 2020).
12.9 Routes of Administration of Nanoparticles in the Treatment of Malignant Gliomas
For nanoparticles engineered to manage brain tumors, there have been three major routes of administration: (i) direct brain delivery (ii) direct systemic brain delivery and (iii) indirect systemic brain delivery. Direct brain delivery ensures nanoparticles injection directly into the brain, which bypasses the blood brain barrier. Convection-enhanced delivery was used specifically in brain tissue to infuse a nanoparticle suspension. Convection-enhanced delivery was used by Lollo et al. to deliver paclitaxel loaded lipid nanocapsules into mice brain. The findings indicated that for lipid nanocapsule-treated mice, the total survival period was substantially higher than that of free paclitaxel-treated mice (Allard et al. 2010; Lollo et al. 2015). Fourniols et al. reported the direct injection of polymerizable hydrogel containing micelles loaded with temozolomide into the brain using a syringe through an incision drilled into the skull. The temozolomide - loaded injection micelles were well tolerated and the hydrogel improved the drug release profile. The key drawbacks of direct brain delivery included the contamination risk and the necessity to regulate essential factors like osmolarity and pH, which can lead to serious brain injury if not optimized (Huynh et al. 2012).
Specific systemic brain delivery involves the administration of nanoparticles directly via the carotid artery into the bloodstream, which are transferred to the brain, eliminating the rest of the systemic circulation. Compared to convection-enhanced delivery, this approach has demonstrated enhanced existence with a decreased risk of brain injury. Huynh et al., using both direct systemic brain delivery and convection-enhanced delivery in glioblastoma multiforme-inflicted rats, administered the nanoparticles loaded with ferrociphenol. Compared to convection-enhanced delivery community’s survival of 24 days, direct systemic delivery provided a survival time period of 28 days. The findings showed that direct systemic delivery relative to direct brain delivery may provide a small improvement in survival time spans (Huynh et al. 2011, 2012).
Indirect systemic delivery is required for the further introduction of nanoparticles into systemic circulation through administration routes requiring absorption, such as nasal, oral, peritoneal and topical administration. Non-invasiveness and patient compliance are the main benefits of oral administration. Two different curcumin preparations (nanoparticles and plain suspension) were orally administered to rats and were evaluated in rat intestinal model ex vivo. The observations revealed that the nanoparticles bioavailability was 12 times higher compared to the single suspension of the neat drug. Intraperitoneal administration is commonly used as an alternative technique for the administration of the medication into peritoneal tissue. It can be used for delivering massive doses and in situations when a vein for direct systemic delivery is difficult to locate (Verreault et al. 2015).
12.10 Challenges Related to Nanotherapy of Malignant Gliomas
12.10.1 Reticulo Endothelial System
The mononuclear phagocyte system, often referred to as reticulo endothelial system, has cellular and non-cellular components. The administered nanosystems are often recognized by the reticulo endothelial system leading to an induction of cytokine cascade that causes inflammation and the circulating phagocytes may induce the removal of nanoparticles. Besides, macromolecules like proteins and lipids binds to the nanoparticles surface creating a biological corona that is identified and discharged from the bloodstream by the immune system. Surface modified nanoparticles are not recognized by the reticulo endothelial system, which helps in overcoming the said challenge and enables their presence in the bloodstream for extended durations. Surface modification is achieved by using zwitter ionic ligands such as glutathione, PEGylation, or cysteine. In research conducted by Choi et al., the results inferred the fact that the use of neutral dihydrolipoic acid – connected polyethylene glycol; or zwitter ionic (cysteine) coating material for quantum dot coating prohibited serum protein adsorption and inhibited renal clearance. An in vivo study indicated that the use of PEGylated human serum albumin nanoparticles loaded with paclitaxel achieved extended systemic circulation by more than 96 h and improved tumor aggregation leading to improved anti-cancer efficacy and extended animal life expectancy (Mahmoud et al. 2020).
12.10.2 Renal System
The biggest challenge faced by the nanoparticles the systemic circulation is the renal clearance. Nanoparticles greater than 8 nm in size may find difficulties crossing the glomerular filtration barrier. Besides, cationic nanoparticles of 2–6 nm would show greater renal clearance than anionic or neutral nanoparticles of similar size because the glomerular basement membrane is negatively charged (von Roemeling et al. 2017). The shape of a nanoparticle could also influence renal clearance. Improved clearance of rod-shaped nanoparticles with a diameter of 0.8–1.2 nm were reported by Ruggiero et al. (Ruggiero et al. 2010). Optimization strategies may be employed by formulation scientists to design biodegradable nanoparticles that may be resistant to renal clearance. Nevertheless, before entering their target site, this may lead to premature release of the therapeutic drugs (Mahmoud et al. 2020).
12.10.3 Blood Brain Barrier
The blood brain barrier comprises of tight junctions that restricts access of nanoparticles into the brain. Nanoparticles with conjugated ligands, were readily internalized by blood brain barrier through the receptor-mediated endocytosis (Mahmoud et al. 2020).
12.10.4 Pathophysiological Barriers in Cancer
Nature of cancer, its location, stage, and patient’s traits are the important characteristics that affect the composition and structure of tumor extracellular matrix and its vasculature (von Roemeling et al. 2017). These properties stand as major hurdle in achieving suitable penetration of the nanoparticles in the solid tumors. Delivery of drugs to the tumor cells involves the transport of nanoparticles through blood vessels, crossing the interstitial space to reach the tumor site. This delivery is affected by the morphological differentiations between the tumor and normal cells and/or tissues. The abnormal tumor tissue environment leads to leaky vessels, abnormal blood flow, abnormal lymphatic vessels and vascular hyperpermeability. All of these factors contribute to interstitial hypertension, thereby hindering the diffusion process. Two major strategies have been extensively utilized to enhance the drug delivery, namely normalization of tumor vasculature by using antiangiogenic agents that repairs the imbalance between overexpressed proangiogenic and antiangiogenic factors in tumor tissues, and second is normalization of tumor matrix that is based on degradation of collagen and glycosaminoglycan to improve the nanoparticles penetration (Alexandrakis et al. 2004; Batchelor et al. 2007; Blanco et al. 2015; Boucher et al. 1990; Jain 2005; Mahmoud et al. 2020). Smart nanoparticles are being fabricated which can react to environmental conditions and enable better bioavailability for therapy (Mahmoud et al. 2020).
12.10.5 Multidrug Resistance
Multidrug resistance entails drug release outside the cells, either inherited or acquired from long-term drug exposure, causing a reduction in efficacy and concentration of drugs within the cell lumen. Cancer cells can be resistant to some chemotherapeutic agent viz. taxanes, anthracyclines, and vinca alkaloids, which when ejected by cancer cells causes increased toxicity to healthy cells (Szakács et al. 2006). Multidrug resistance probably occurs from overexpressed P-glycoprotein which is an ATP-binding cassette transporter (present in brain, liver and placenta) that functions as efflux pump with an ability of binding several hydrophobic drugs and also plays a role of protecting vital organs from toxins (Aller et al. 2009; Gottesman et al. 2002). Other multidrug resistance associated proteins includes multidrug resistance – associated protein-1 and the breast cancer resistance protein (Fletcher et al. 2010). Efflux pump inhibitors such as verapamil (covera) and cyclosporine have been investigated and are emerging as first-generation antagonists (Dean et al. 2005). Addressing multidrug resistance in cancer has involved the exploitation of nanoparticles drug delivery systems in encapsulating chemotherapy drugs. Liposomes and nanoparticles encapsulating doxorubicin and verapamil have been formulated for the targeted inhibition of P-glycoprotein (Wu et al. 2007).
12.11 Conclusion
Malignant gliomas are some of the most violent tumor types, which do not react to most traditional chemotherapy and radiation therapies. This is actually due to the blood brain barrier’s selective nature, which prohibits most particles, particularly therapeutics, from accessing the brain. In addition, traditional glioma management techniques only enable patients to live for a certain time period while dealing with harmful adverse effects that arise primarily from the invasiveness of treatment methods. Nanodrug delivery system is a non-invasive and versatile therapy area that enables the development of nanometer-size materials to serve as drug delivery systems. Such engineered nanoparticles only targets the over-expressed receptors on tumor tissues while sparing normal tissues, leading to reduced adverse effects. The positive pre-clinical data has formed the base for the suitable application of nanosystems in the clinical usage. FDA approval has been obtained for the application of nanoparticles for the intravenous route, which offers advantages for the management of metastasized tumors. Thanks to their biocompatible and biodegradable actions within the human body and the limitless shapes and features that can be manipulated into, polymeric nanoparticles are attracting further interest in the malignant glioma treatment. As previously discussed in this analysis, polymeric nanoparticles can be especially beneficial once PEGylated. Even more, efforts are required to optimize the scale up techniques, drug loading ability and drug release pattern, considering the physiological obstacles and various physicochemical properties of drugs that may impede their performance.
References
Abrudan C, Florian IS, Baritchii A, Soritau O, Dreve S, Tomuleasa C, Petrushev B (2014) Assessment of temozolomide action encapsulated in chitosan and polymer nanostructures on glioblastoma cell lines. Rom Neurosurg 21(1):19–30. https://doi.org/10.2478/romneu-2014-0002
Alexandrakis G, Brown EB, Tong RT, McKee TD, Campbell RB, Boucher Y, Jain RK (2004) Two-photon fluorescence correlation microscopy reveals the two-phase nature of transport in tumors. Nat Med 10:203–207. https://doi.org/10.1038/nm981
Allard E, Jarnet D, Vessières A, Vinchon-Petit S, Jaouen G, Benoit JP, Passirani C (2010) Local delivery of ferrociphenol lipid nanocapsules followed by external radiotherapy as a synergistic treatment against intracranial 9L glioma xenograft. Pharm Res 27(1):56–64. https://doi.org/10.1007/s11095-009-0006-0
Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL (2009) Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 323:1718–1722. https://doi.org/10.1126/science.1168750
Batchelor TT, Sorensen AG, di Tomaso E, Zhang W-T, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen P-J, Zhu M (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11:83–95. https://doi.org/10.1016/j.ccr.2006.11.021
Behera A, Padhi S (2020) Passive and active targeting strategies for the delivery of the camptothecin anticancer drug: a review. Environ Chem Lett 18:1557–1567. https://doi.org/10.1007/s10311-020-01022-9
Behera A, Mittu B, Padhi S, Singh A (2020a) Antimicrobial efficacy of essential oil nanoemulsions. In: Dhull S, Chawla P, Kaushik R (eds) Nanotechnological approaches in food microbiology. Taylor & Francis, pp 294–309
Behera A, Mittu B, Padhi S, Patra N, Singh J (2020b) Bimetallic nanoparticles: green synthesis, applications, and future perspectives. In: Abd-Elsalam K (ed) Multifunctional hybrid nanomaterials for sustainable agri-food and ecosystems. Elsevier, pp 639–682
Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY (2003) Primary brain tumours in adults. Lancet 361(9354):323–331. Lancet. https://doi.org/10.1016/S0140-6736(03)12328-8
Bennewitz MF, Saltzman WM (2009) Nanotechnology for delivery of drugs to the brain for epilepsy. Neurotherapeutics 6(2):323–336. https://doi.org/10.1016/j.nurt.2009.01.018
Binello E, Germano IM (2011) Targeting glioma stem cells: a novel framework for brain tumors. Cancer Sci 102(11):1958–1966). NIH Public Access. https://doi.org/10.1111/j.1349-7006.2011.02064.x
Blanco E, Shen H, Ferrari M (2015) Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol 33:941–951. https://doi.org/10.1038/nbt.3330
Boucher Y, Baxter LT, Jain RK (1990) Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res 50:4478–4484
Busquets MA, Espargaro A, Sabaté R, Estelrich J (2015) Magnetic nanoparticles cross the blood-brain barrier: when physics rises to a challenge. Nanomaterials 5(4):2231–2248. MDPI AG. https://doi.org/10.3390/nano5042231
Calvo P, Gouritin B, Chacun H, Desmaile D, D’Angelo J, Noel JP, Georgin D, Fattal E, Andreux JP, Couvreur P (2001) Long-circulating pegylated polycyanoacrylate nanoparticles as new drug carrier for brain delivery. Pharm Res 18(8):1157–1166. https://doi.org/10.1023/A:1010931127745
Chang J, Jallouli Y, Kroubi M, Yuan XB, Feng W, Kang CS, Pu PY, Betbeder D (2009) Characterization of endocytosis of transferrin-coated PLGA nanoparticles by the blood-brain barrier. Int J Pharm 379(2):285–292. https://doi.org/10.1016/j.ijpharm.2009.04.035
Cornago M, Garcia-Alberich C, Blasco-Angulo N, Vall-llaura N, Nager M, Herreros J, Comella JX, Sanchis D, Llovera M (2014) Histone deacetylase inhibitors promote glioma cell death by G2 checkpoint abrogation leading to mitotic catastrophe. Cell Death Dis 5(10):e1435. https://doi.org/10.1038/cddis.2014.412
Cui Y, Zhang M, Zeng F, Jin H, Xu Q, Huang Y (2016) Dual-targeting magnetic PLGA nanoparticles for codelivery of paclitaxel and curcumin for brain tumor therapy. ACS Appl Mater Interfaces 8(47):32159–32169. https://doi.org/10.1021/acsami.6b10175
De Boer AG, Gaillard PJ (2007) Strategies to improve drug delivery across the blood-brain barrier. Clin Pharmacokinet 46(7):553–576. Clin Pharmacokinet. https://doi.org/10.2165/00003088-200746070-00002
Dean M, Fojo T, Bates S (2005) Tumour stem cells and drug resistance. Nat Rev Cancer 5:275–284. https://doi.org/10.1038/nrc1590
Demeule M, Regina A, Ché C, Poirier J, Nguyen T, Gabathuler R, Castaigne JP, Béliveau R (2008) Identification and design of peptides as a new drug delivery system for the brain. J Pharmacol Exp Ther 324(3):1064–1072. https://doi.org/10.1124/jpet.107.131318
Ding H, Inoue S, Ljubimov AV, Patil R, Portilla-Arias J, Hu J, Konda B, Wawrowsky KA, Fujita M, Karabalin N, Sasaki T, Black KL, Holler E, Ljubimova JY (2010) Inhibition of brain tumor growth by intravenous poly (β-L-malic acid) nanobioconjugate with pH-dependent drug release. Proc Natl Acad Sci U S A 107(42):18143–18148. https://doi.org/10.1073/pnas.1003919107
ELAmrawy F, Othman AA, Adkins C, Helmy A, Nounou MI (2016) Tailored nanocarriers and bioconjugates for combating glioblastoma andother brain tumors. J Cancer Metastasis Treat 2:112–122
Fernandez AP, Serrano J, Amorim MA, Pozo-Rodrigalvarez A, Martinez-Murillo R (2012) Adrenomedullin and nitric oxide: implications for the etiology and treatment of primary brain tumors. CNS Neurol Disord Drug Targets 10(7):820–833. https://doi.org/10.2174/187152711798072374
Fletcher JI, Haber M, Henderson MJ, Norris MD (2010) ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer 10:147–156. https://doi.org/10.1038/nrc2789
Fornaguera C, Dols-Perez A, Calderó G, García-Celma MJ, Camarasa J, Solans C (2015) PLGA nanoparticles prepared by nano-emulsion templating using low-energy methods as efficient nanocarriers for drug delivery across the blood-brain barrier. J Control Release 211:134–143. https://doi.org/10.1016/j.jconrel.2015.06.002
Ganipineni LP, Ucakar B, Joudiou N, Riva R, Jérôme C, Gallez B, Danhier F, Préat V (2019) Paclitaxel-loaded multifunctional nanoparticles for the targeted treatment of glioblastoma. J Drug Target 27(5–6):614–623. https://doi.org/10.1080/1061186X.2019.1567738
Gao H, Qian J, Cao S, Yang Z, Pang Z, Pan S, Fan L, Xi Z, Jiang X, Zhang Q (2012) Precise glioma targeting of and penetration by aptamer and peptide dual-functioned nanoparticles. Biomaterials 33(20):5115–5123. https://doi.org/10.1016/j.biomaterials.2012.03.058
Gao H, Zhang S, Yang Z, Cao S, Jiang X, Pang Z (2014) In vitro and in vivo intracellular distribution and anti-glioblastoma effects of docetaxel-loaded nanoparticles functioned with IL-13 peptide. Int J Pharm 466(1–2):8–17. https://doi.org/10.1016/j.ijpharm.2014.03.012
Geldenhuys W, Wehrung D, Groshev A, Hirani A, Sutariya V (2015) Brain-targeted delivery of doxorubicin using glutathione-coated nanoparticles for brain cancers. Pharm Dev Technol 20(4):497–506. https://doi.org/10.3109/10837450.2014.892130
Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP–dependent transporters. Nat Rev Cancer 2:48–58. https://doi.org/10.1038/nrc706
Hassan N, Firdaus S, Padhi S, Ali A, Iqbal Z (2021) Investigating natural antibiofilm components: a new therapeutic perspective against candidal vulvovaginitis. Med Hypotheses 148:110515. https://doi.org/10.1016/j.mehy.2021.110515
Holmes D (2013) The next big things are tiny. Lancet Neurol 12(1):31. Lancet Publishing Group. https://doi.org/10.1016/S1474-4422(12)70313-7
Huynh NT, Morille M, Bejaud J, Legras P, Vessieres A, Jaouen G, Benoit JP, Passirani C (2011) Treatment of 9L gliosarcoma in rats by ferrociphenol-loaded lipid nanocapsules based on a passive targeting strategy via the EPR effect. Pharm Res 28(12):3189–3198. https://doi.org/10.1007/s11095-011-0501-y
Huynh NT, Passirani C, Allard-Vannier E, Lemaire L, Roux J, Garcion E, Vessieres A, Benoit JP (2012) Administration-dependent efficacy of ferrociphenol lipid nanocapsules for the treatment of intracranial 9L rat gliosarcoma. Int J Pharm 423(1):55–62. https://doi.org/10.1016/j.ijpharm.2011.04.037
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307:58–62. https://doi.org/10.1126/science.1104819
Jain A, Jain A, Garg NK, Tyagi RK, Singh B, Katare OP, Webster TJ, Soni V (2015) Surface engineered polymeric nanocarriers mediate the delivery of transferrin-methotrexate conjugates for an improved understanding of brain cancer. Acta Biomater 24:140–151. https://doi.org/10.1016/j.actbio.2015.06.027
Khuroo T, Verma D, Talegaonkar S, Padhi S, Panda A, Iqbal Z (2014) Topotecan–tamoxifen duple PLGA polymeric nanoparticles: investigation of in vitro, in vivo and cellular uptake potential. Int J Pharm 473:384–394. https://doi.org/10.1016/j.ijpharm.2014.07.022
Kreuter J (2001) Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev 47(1):65–81. https://doi.org/10.1016/S0169-409X(00)00122-8
Lapointe S, Perry A, Butowski NA (2018) Primary brain tumours in adults. Lancet 392(10145):432–446. Lancet Publishing Group. https://doi.org/10.1016/S0140-6736(18)30990-5
Li J, Feng L, Fan L, Zha Y, Guo L, Zhang Q, Chen J, Pang Z, Wang Y, Jiang X, Yang VC, Wen L (2011) Targeting the brain with PEG-PLGA nanoparticles modified with phage-displayed peptides. Biomaterials 32(21):4943–4950. https://doi.org/10.1016/j.biomaterials.2011.03.031
Lollo G, Vincent M, Ullio-Gamboa G, Lemaire L, Franconi F, Couez D, Benoit JP (2015) Development of multifunctional lipid nanocapsules for the co-delivery of paclitaxel and CpG-ODN in the treatment of glioblastoma. Int J Pharm 495(2):972–980. https://doi.org/10.1016/j.ijpharm.2015.09.062
MacKenzie D (1927) A classification of the tumours of the glioma group on a histogenetic basis, with a correlated study of prognosis. By Percival Bailey and Harvey Cushing. Medium 8vo. Pp. 175, with 108 illustrations. 1926. Philadelphia, London, and Montreal: J. B. Lippinco. Br J Surg 14(55):554–555. https://doi.org/10.1002/bjs.1800145540
Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA (2001) Malignant glioma: genetics and biology of a grave matter. Genes Dev 15(11):1311–1333. Genes Dev. https://doi.org/10.1101/gad.891601
Mahmoud BS, Alamri AH, McConville C (2020) Polymeric nanoparticles for the treatment of malignant gliomas. Cancers 12(1):1–28. https://doi.org/10.3390/cancers12010175
Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Orawa H, Budach V, Jordan A (2011) Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neuro-Oncol 103(2):317–324. https://doi.org/10.1007/s11060-010-0389-0
Malinovskaya Y, Melnikov P, Baklaushev V, Gabashvili A, Osipova N, Mantrov S, Ermolenko Y, Maksimenko O, Gorshkova M, Balabanyan V, Kreuter J, Gelperina S (2017) Delivery of doxorubicin-loaded PLGA nanoparticles into U87 human glioblastoma cells. Int J Pharm 524(1–2):77–90. https://doi.org/10.1016/j.ijpharm.2017.03.049
Mehrotra N, Tripathi RM (2015) Short interfering RNA therapeutics: Nanocarriers, prospects and limitations. IET Nanobiotechnol 9(6):386–395. Institution of Engineering and Technology. https://doi.org/10.1049/iet-nbt.2015.0018
Mei H, Shi W, Pang Z, Wang H, Lu W, Jiang X, Deng J, Guo T, Hu Y (2010) EGFP-EGF1 protein-conjugated PEG-PLA nanoparticles for tissue factor targeted drug delivery. Biomaterials 31(21):5619–5626. https://doi.org/10.1016/j.biomaterials.2010.03.055
Michael JS, Lee B-S, Zhang M, Yu JS (2018) Nanotechnology for treatment of glioblastoma multiforme. J Transl Intern Med 6(3):128–133. https://doi.org/10.2478/jtim-2018-0025
Omuro A, DeAngelis LM (2013) Glioblastoma and other malignant gliomas: a clinical review. JAMA – J Am Med Assoc 310(17):1842–1850. JAMA. https://doi.org/10.1001/jama.2013.280319
Padhi S, Behera A (2020) Nanotechnology based targeting strategies for the delivery of camptothecin. In: Ankit S, Panda Amulya K, Eric L (eds) Pharmaceutical technology for natural products delivery, impact of nanotechnology. Springer Nature, Cham, pp 243–272
Padhi S, Mirza M, Verma D, Khuroo T, Panda A, Talegaonkar S et al (2015) Revisiting the nanoformulation design approach for effective delivery of topotecan in its stable form: an appraisal of its in vitro behavior and tumor amelioration potential. Drug Del 23:2827–2837. https://doi.org/10.3109/10717544.2015.1105323
Padhi S, Kapoor R, Verma D, Panda A, Iqbal Z (2018) Formulation and optimization of topotecan nanoparticles: in vitro characterization, cytotoxicity, cellular uptake and pharmacokinetic outcomes. J Photochem Photobiol B Biol 183:222–232. https://doi.org/10.1016/j.jphotobiol.2018.04.022
Padhi S, Nayak A, Behera A (2020) Type II diabetes mellitus: a review on recent drug based therapeutics. Biomed Pharmacother 131:110708. https://doi.org/10.1016/j.biopha.2020.110708
Patnaik S, Gorain B, Padhi S, Choudhury H, Gabr G, Md S et al (2021) Recent update of toxicity aspects of nanoparticulate systems for drug delivery. Eur J Pharm Biopharm 161:100–119. https://doi.org/10.1016/j.ejpb.2021.02.010
Pérez-Martínez FC, Guerra J, Posadas I, Ceña V (2011) Barriers to non-viral vector-mediated gene delivery in the nervous system. Pharm Res 28(8):1843–1858. Springer. https://doi.org/10.1007/s11095-010-0364-7
Pitorre M, Simonsson C, Bastiat G, Salomon C, Pitorre M, Simonsson C, Bastiat G, Salomon C (2020) Nanovectors for neurotherapeutic delivery Part II: polymeric nanoparticles to cite this version. In: Pillay V (ed) OMICS Advances in neurotherapeutic delivery technologies. https://www.researchgate.net/publication/308118004_Nanovectors_for_Neurotherapeutic_Delivery_Part_II_Polymeric_Nanoparticles
Ramalho MJ, Sevin E, Gosselet F, Lima J, Coelho MAN, Loureiro JA, Pereira MC (2018) Receptor-mediated PLGA nanoparticles for glioblastoma multiforme treatment. Int J Pharm 545(1–2):84–92. https://doi.org/10.1016/j.ijpharm.2018.04.062
Re F, Gregori M, Masserini M (2012) Nanotechnology for neurodegenerative disorders. Maturitas 73(1):45–51. Maturitas. https://doi.org/10.1016/j.maturitas.2011.12.015
Ruggiero A, Villa CH, Bander E, Rey DA, Bergkvist M, Batt CA, Manova-Todorova K, Deen WM, Scheinberg DA, McDevitt MR (2010) Paradoxical glomerular filtration of carbon nanotubes. Proc Natl Acad Sci U S A 107(27):12369–12374. https://doi.org/10.1073/pnas.0913667107
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996. https://doi.org/10.1056/NEJMoa043330
Stupp R, Tonn JC, Brada M, Pentheroudakis G (2010) High-grade malignant glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 21(SUPPL. 5):190–193. https://doi.org/10.1093/annonc/mdq187
Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM (2006) Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5:219–234. https://doi.org/10.1038/nrd1984
Tian G, Yin W, Jin J, Zhang X, Xing G, Li S, Gu Z, Zhao Y (2014) Engineered design of theranostic upconversion nanoparticles for tri-modal upconversion luminescence/magnetic resonance/X-ray computed tomography imaging and targeted delivery of combined anticancer drugs. J Mater Chem B 2(10):1379–1389. https://doi.org/10.1039/c3tb21394c
Verma D, Thakur P, Padhi S, Khuroo T, Talegaonkar S, Iqbal Z (2017) Design expert assisted nanoformulation design for co-delivery of topotecan and thymoquinone: optimization, in vitro characterization and stability assessment. J Mol Liq 242:382–394. https://doi.org/10.1016/j.molliq.2017.07.002
Verreault M, Wehbe M, Strutt D, Masin D, Anantha M, Walker D, Chu F, Backstrom I, Kalra J, Waterhouse D, Yapp DT, Bally MB (2015) Determination of an optimal dosing schedule for combining Irinophore C™ and temozolomide in an orthotopic model of glioblastoma. J Control Release 220(Pt A):348–357. https://doi.org/10.1016/j.jconrel.2015.10.053
von Roemeling C, Jiang W, Chan CK, Weissman IL, Kim BY (2017) Breaking down the barriers to precision cancer nanomedicine. Trends Biotechnol 35:159–171. https://doi.org/10.1016/j.tibtech.2016.07.006
Wicki A, Witzigmann D, Balasubramanian V, Huwyler J (2015) Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release 200:138–157. Elsevier. https://doi.org/10.1016/j.jconrel.2014.12.030
Wu J, Lee A, Lu Y, Lee RJ (2007) Vascular targeting of doxorubicin using cationic liposomes. Int J Pharm 337:329–335. https://doi.org/10.1016/j.ijpharm.2007.01.003
Xin H, Jiang X, Gu J, Sha X, Chen L, Law K, Chen Y, Wang X, Jiang Y, Fang X (2011) Angiopep-conjugated poly(ethylene glycol)-co-poly(ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials 32(18):4293–4305. https://doi.org/10.1016/j.biomaterials.2011.02.044
Xin H, Sha X, Jiang X, Zhang W, Chen L, Fang X (2012) Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials 33(32):8167–8176. https://doi.org/10.1016/j.biomaterials.2012.07.046
Zhang B, Wang H, Liao Z, Wang Y, Hu Y, Yang J, Shen S, Chen J, Mei H, Shi W, Hu Y, Pang Z, Jiang X (2014) EGFP-EGF1-conjugated nanoparticles for targeting both neovascular and glioma cells in therapy of brain glioma. Biomaterials 35(13):4133–4145. https://doi.org/10.1016/j.biomaterials.2014.01.071
Zhang TT, Li W, Meng G, Wang P, Liao W (2016) Strategies for transporting nanoparticles across the blood-brain barrier. Biomater Sci 4(2):219–229. Royal Society of Chemistry. https://doi.org/10.1039/c5bm00383k
Zhou J, Patel TR, Sirianni RW, Strohbehn G, Zheng MQ, Duong N, Schafbauer T, Huttner AJ, Huang Y, Carson RE, Zhang Y, Sullivan DJ, Piepmeier JM, Saltzman WM (2013) Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proc Natl Acad Sci U S A 110(29):11751–11756. https://doi.org/10.1073/pnas.1304504110
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Patil, P.H., Pardeshi, C.V., Surana, S.J. (2022). Polymeric Nanoparticles to Target Glioblastoma Tumors. In: Padhi, S., Behera, A., Lichtfouse, E. (eds) Polymeric nanoparticles for the treatment of solid tumors. Environmental Chemistry for a Sustainable World, vol 71. Springer, Cham. https://doi.org/10.1007/978-3-031-14848-4_12
Download citation
DOI: https://doi.org/10.1007/978-3-031-14848-4_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-14847-7
Online ISBN: 978-3-031-14848-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)