Abstract
Recent advances in genomic technology have led to a better understanding of key molecular alterations that underlie glioblastoma (GBM). The current WHO-based classification of GBM is mainly based on histologic features of the tumor, which frequently do not reflect the molecular differences that describe the diversity in the biology of these lesions. The current WHO definition of GBM relies on the presence of high-grade astrocytic neoplasm with the presence of either microvascular proliferation and/or tumor necrosis. High-throughput analyses have identified molecular subtypes and have led to progress in more accurate classification of GBM. These findings, in turn, would result in development of more effective patient stratification, targeted therapeutics, and prediction of patient outcome. While consensus has not been reached on the precise nature and means to sub-classify GBM, it is clear that IDH-mutant GBMs are clearly distinct from GBMs without IDH1/2 mutation with respect to molecular and clinical features, including prognosis. In addition, recent findings in pediatric GBMs regarding mutations in the histone H3F3A gene suggest that these tumors may represent a 3rd major category of GBM, separate from adult primary (IDH1/2 wt), and secondary (IDH1/2 mut) GBMs. In this review, we describe major clinically relevant genetic and epigenetic abnormalities in GBM—such as mutations in IDH1/2, EGFR, PDGFRA, and NF1 genes—altered methylation of MGMT gene promoter, and mutations in hTERT promoter. These markers may be incorporated into a more refined classification system and applied in more accurate clinical decision-making process. In addition, we focus on current understanding of the biologic heterogeneity and classification of GBM and highlight some of the molecular signatures and alterations that characterize GBMs as histologically defined. We raise the question whether IDH-wild type high grade astrocytomas without microvascular proliferation or necrosis might best be classified as GBM, even if they lack the histologic hallmarks as required in the current WHO classification. Alternatively, an astrocytic tumor that fits the current histologic definition of GBM, but which shows an IDH mutation may in fact be better classified as a distinct entity, given that IDH-mutant GBM are quite distinct from a biological and clinical perspective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glial tumors can be divided into two major categories based on the degree of invasiveness into the surrounding brain tissue; gliomas with diffuse infiltration of the brain parenchyma are referred to as “diffuse gliomas”, to be contrasted with gliomas with more “circumscribed” growth behavior. Diffuse gliomas share the ability to infiltrate surrounding normal brain parenchyma, and unfortunately, inevitably recur even after gross total resection [136]. Given their extensive infiltrative nature, major goals for neurosurgery include cytoreduction, to the extent that is possible, as well as obtaining tissue for accurate diagnosis. Another feature of diffuse gliomas is the notion that low-grade tumors of WHO grade II over time not only recur but also tend to progress to high-grade (anaplastic) gliomas of WHO grade III and eventually secondary GBM of WHO grade IV, leading to rapid clinical deterioration. GBM is considered incurable, with a median survival of 15 months following aggressive combination of therapies including maximal-safe surgical resection, adjuvant radiation therapy (RT) with concurrent and adjuvant temozolomide (TMZ) treatment [154]. Many tumors respond poorly to conventional chemotherapy and radiation, and those for which tumor control is accomplished often lack a durable therapy response [107]. Therefore, development of new diagnostic approaches and especially more effective treatment strategies is urgently needed. Targeting driver molecular aberrations is the most promising therapeutic advancement, as seen with successes of “personalized” and targeted therapies in other cancer types. In this context, we provide in this review an update on the state of our knowledge in this field, focusing on how understanding of the molecular heterogeneity of GBM has been and could be utilized for classification of these tumors into molecular subtypes that could potentially improve outcomes for specific tumor subsets.
To date, gliomas are classified largely based on their histopathological characteristics and while clinical and radiological features of the tumors are at times taken into account, the present WHO classification is mainly based on histological features. Histologic criteria for high-grade infiltrating astrocytic tumor (at least grade III) include hypercellularity, nuclear atypia, and mitotic activity. Furthermore, a GBM diagnosis requires, in addition, either microvascular proliferation and/or tumor necrosis. However, many aspects of these histologic features remain poorly correlated with key molecular drivers and pathways. For example, the presence or absence of IDH mutations cannot be distinguished on pure morphologic grounds in GBM. In addition, among IDH wild-type high-grade gliomas (which account for over 90 % of GBM), the key molecular chromosomal changes are shared between histologic grade III (anaplastic astrocytoma) and GBM (histologic grade IV) tumors. In addition, clinical and biological variability is thought to exist within each grade and each tumor entity, suggesting that identification of molecular factors which contribute to this variation would be invaluable for the development of targeted therapies. In other words, histopathologically defined GBM in fact may represent multiple subtypes based on molecular features or signatures.
The emergence of molecular signatures in cancer can iteratively present a shift in the way diagnosis and treatment of malignancies are approached. In turn, molecular signatures that are found to either describe fundamental biologic behavior or correlate clinically with patient outcome—following administration of either cytotoxic or molecularly targeted agents—become candidates to enter classification criteria as circumstances warrant. Due to this shift, tumors are classified not only based on a static concept of how they “look” under the microscope, but rather by incorporating molecular markers relevant to current therapeutic modalities. Clear proof of principle for such approaches has been demonstrated in therapies targeting EGFR mutant non-small-cell lung cancer [103], HER2-amplified breast cancer [149], lung cancer harboring the EML4–ALK translocation [89], chronic myelogenous leukemia (CML) harboring the BCR–ABL translocation [36], and BRAF mutant melanoma [26]. Annotations of molecular alterations are more routinely being incorporated into histopathologic diagnosis where appropriate [34, 104] and have facilitated therapeutic decision making, progressively decreasing the time frame from target discovery to therapy [25]. Molecular initiatives, including the Cancer Genome Atlas (TCGA), have described fundamental aspects of the biologic underpinnings of GBM [18] and lower grade gliomas (TCGA network, unpublished data). The TCGA project was initiated by the NIH and is a comprehensive, coordinated, multicenter effort that applies multiple innovative genomic analysis tools to understand the genetics and epigenetics of cancer. More than 20 cancer types, including more than 10,000 samples, will undergo detailed genomic characterization and further incorporated with bioinformatic and data analysis components that will enable researchers to apply this information for prevention, diagnosis, and treatment of cancer. Unfortunately, although such molecular alterations have led to extensive clinical progress for many cancer types, to date these alterations have not been incorporated into clinical decision making where ultimately the subtype classification can be matched with efficacious therapeutic options. In addition, more detailed characterization of the genomic alterations that are clinically relevant still need to be established in GBM to fully implement and maximize information from these high-throughput genomic studies.
Clinical diagnosis of GBM
Based on guidelines of the World Health Organization (WHO) for classification of central nervous system tumors [101], diffuse gliomas are divided into three grades: WHO grades II, III, and IV, with WHO grade IV diffuse glioma being synonymous with GBM. Diffuse gliomas occur more commonly in adults than in children and are the most common intrinsic primary brain tumors that display a wide range of clinical behaviors, ranging from slow clinical progression in patients with WHO grade II tumors, to very short median survival times of 12–18 months in patients with WHO grade IV tumors (GBM); however, long-term survival extending the span of three years has been described in a fraction of GBM patients [88]. Diffuse gliomas of WHO grade II or III are further divided into several histologic entities, including astrocytoma/anaplastic astrocytoma, oligodendroglioma/anaplastic oligodendroglioma, and oligoastrocytoma/anaplastic oligoastrocytoma. The most common diffuse glioma, however, is GBM (WHO grade IV), accounting for 45–50 % of all primary intrinsic brain tumors [38, 101, 161], with the vast majority of GBMs arising de novo as “primary GBMs”. GBMs that develop by progression from a pre-existing glioma of WHO grade II or III are less common and are referred to as “secondary GBMs” [118]. Most primary GBMs manifest in elderly patients, while secondary GBMs most commonly affect younger patients prior to the age of 45 years.
Histopathologically, several patterns exist, including giant cell GBM, small cell GBM, and gliosarcoma. Gliosarcoma can be observed at initial diagnosis or at recurrence, and appears to have similar genetic aberrations as GBM, although MGMT methylation may be less frequently present [92], and EGFR mutations may be less common as well [56]. Another pattern that may be seen is termed “GBM with oligodendroglioma component” (GBM-O), where the tumor, at least regionally, appears similar to anaplastic oligodendroglioma. These tumors are easily distinguished from GBM by the presence or absence of 1p/19q co-deletion, which while controversial, in our view effectively defines this differential diagnosis as glioblastoma versus oligodendroglioma. Specifically, GBM-O is distinguished from anaplastic oligodendroglioma (AO) by the absence of 1p/19q deletion, and the presence of IDH mutation and 1p/19q deletion effectively defines AO and is therefore incompatible with the diagnosis of GBM-O. To put it a different way, high-grade gliomas with IDH mutation and whole-arm 1p/19q co-deletion should in our view be classified as AO grade III. For further discussion on this point, the reader is referred to the companion article on oligodendroglial tumors in the cluster in this issue of Acta Neuropathologica.
Integrated genomic analysis of GBM
Traditionally, GBM is separated into 2 major classes as “primary” and “secondary” GBM. Primary GBM was suggested as generally presenting without a known clinical precursor, while secondary GBM was a result of molecular progression and increased malignancy grade of a lower grade glioma over time. Ongoing and recent advances have demonstrated molecular correlates of these clinical definitions. For example, TERT promoter mutation, PTEN tumor suppressor gene mutation, and high-level gene amplification of certain proto-oncogenes—most commonly the epidermal growth factor receptor (EGFR) gene—are hallmark alterations in primary GBMs, while mutations of IDH1/2, TP53, and ATRX are frequent in secondary GBMs [97, 118]. Going further, several recent studies have utilized high-throughput genomic, epigenomic, and transcriptomic approaches for detailed molecular characterization of gliomas [18, 66, 166, 176]. The identification of distinctive and highly recurrent molecular alterations has begun to clarify some of this diversity and introduce new concepts in tumor classification. Further, these studies provide insights for improvement of current therapeutic strategies and development of a new paradigm for the management of this deadly malignancy.
Large-scale molecular profiling of diffuse gliomas has taken place in individual laboratories [13, 123, 129], at the national level in the US by TCGA network [23], and at the international level within the International Cancer Genome Consortium (ICGC) [74]. GBM was one of the early tumor types that was investigated by TCGA and characterization of the genome and transcriptome of these tumors has provided a detailed insight of their genomic landscape and revealed the major molecular alterations that may contribute to disease pathobiology and progression [18, 23, 166]. While many of the findings from TCGA were confirmatory and relied on the foundation set by prior studies, insights gained from TCGA data are partially based on the ability to integrate data from diverse molecular platforms (mRNA, miRNA, DNA copy number, mutational data, protein expression, DNA methylation) on a focused set of tumor samples. TCGA and other large-scale analyses have demonstrated that GBM, as histologically defined, is a heterogeneous tumor type at the molecular level and is potentially sub-classifiable into distinct biologic entities based on molecular pathogenesis and “driver” lesions (i.e., molecular changes that are required for tumorigenesis and progression). While such comprehensive genome-wide studies have provided useful insights for the characterization and classification of tumors, their experimental limitations need to be taken into consideration when drawing conclusions. Such limitations include the retrospective nature of the experimental design and the fact that patients involved in these studies were not uniformly treated. In addition, the impact of patient selection with respect to tumors with sufficient material for multidimensional profiling is unknown, as is the potential for bias from the fact that samples were derived primarily from academic oncology centers. Furthermore, although some prognostic markers are emerging from these studies, there is a great demand for the identification of bona fide predictive markers that would improve the treatment process for personalized care and these markers await identification. At this point, translation of key novel biomarkers discovered by initiatives such as TCGA into clinically useful tests is yet to be fully accomplished. That said, these efforts have led to improved understanding of the molecular signature of GBM and other diffuse gliomas and have revealed a number of consistent alterations in genes and pathways, including mutations in specific genes, modified pathway component expression signatures, and altered DNA methylation patterns [23, 117, 129, 166], but point to the still unmet need to incorporate these findings into the clinic to identify predictive markers to improve outcome for patients with GBM.
Common pathways disrupted in GBM
In the past two decades, a large number of recurring molecular alterations have been identified in gliomas and particularly in GBMs, which enable characterization of diffuse gliomas and better understanding of glioma landscape and pathways that are disrupted in this malignancy. In the initial TCGA report, Sanger sequencing was combined with array-based platforms to analyze alterations in 601 genes from 91 samples. This study investigated the gene expression, DNA methylation, DNA copy number, in addition to coding and non-coding RNA expression profiles. Results from TCGA studies and contributions made by individual labs have revealed a number of genetic abnormalities and as a result, specific patterns have emerged that suggest the involvement of specific molecular and signaling pathways in the development and progression of glial tumors. These include loss of CDKN2A, RB1, and TP53 tumor suppressor genes, in addition to alterations in genes involved in these pathways or regulated by these tumor suppressor proteins [30, 63, 100, 131, 171]. Mutations in the IDH1, ATRX, and p53 genes are considered molecular hallmarks of diffuse and anaplastic astrocytomas (WHO grades II and III) as well as secondary GBMs [27, 77, 100, 106], and interestingly, TP53 mutations also occur in nearly all instances of the rare giant cell GBM variant [110]. Integrated genomic studies have revealed that in the majority of GBMs, the functions of p53 (87 % of GBM patients) and retinoblastoma (Rb) (78 % of GBM patients) pathways are disrupted either by mutations or gene copy number alterations [23]. In addition, mutations in genes encoding upstream regulators of Rb, but not necessarily the RB1 gene itself, have been known for some time to be characteristic of gliomas [23, 63, 123]. For example, in a fraction of anaplastic gliomas and particularly in GBMs, the CDKN2A gene is homozygously deleted; CDKN2A locus encodes both Ink4A and Arf proteins, which are crucial activators of Rb and p53, respectively [22, 123, 126, 163]. In addition, upstream repressors of p53 and Rb signaling pathways, such as Cdk4 (phosphorylates and inactivates Rb) and Mdm2 (p53 inhibitor), are often up-regulated by gene amplification, suggesting the involvement of alternative mechanisms for disruption of p53 and Rb signaling pathways, as observed in the majority of GBMs [23].
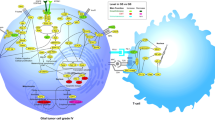
In addition to alterations in tumor suppressive pathways, activation of oncogenic pathways such as those involving receptor tyrosine kinases (RTKs) are well known to be one of the most common genetic alterations in malignant gliomas (Fig. 1). The role of these drivers of glioma has been demonstrated and their importance was revealed in a number of studies using mouse models. In adult GBMs, high-level genomic amplification (~40 %) occurs in the EGFR gene, often along with constitutively activating mutations in this protein’s ectodomain mainly through the variant III (vIII) deletion event [46, 93, 114, 123, 178]. Although the changes leading to the EGFRvIII mutation are complex and heterogeneous, they are considered late events following amplification of EGFR. Overall, EGFRvIII is found in approximately 30–50 % of glioma cases in which EGFR amplification is present. Histopathologically, the pattern recognized as “small cell” GBM is enriched for EGFRvIII-positive tumors [128]. In addition, in a smaller proportion of adult GBMs (~13 %), high-level amplification of the platelet-derived growth factor receptor alpha gene (PDGFRA) has also been detected [114]. Similarly, constitutively activating deletion mutants in PDGFRA have been demonstrated in receptor-amplified GBMs [121]. PDGFRA gene amplification appears to be a common genomic alteration in the RTK pathway, exerting a significant impact on pediatric GBMs and diffuse intrinsic pontine gliomas (DIPG) [125, 126, 182]. Although much less frequent in GBMs, high-level amplification of the MET proto-oncogene has also been shown [114, 123, 126]. More importantly, activating genetic alterations can occur simultaneously in multiple RTKs within individual GBMs, with distinct cellular subpopulations containing amplified receptor genes [151, 158]. This finding suggests that the targeting of single RTKs in an effort to neutralize oncogenic signaling may in some cases prove futile, and drugs targeting multiple RTKs activated in GBM may confer greater treatment efficacy in some settings as opposed to drugs targeting single RTKs. Finally, although high-level amplification of RTK genes is not frequent in WHO grade II and III gliomas, their pathogenesis is often associated with elevated PDGF signaling and PDGFRA phosphorylation [33, 52]. These findings suggest that the lack of success in anti-EGFR treatment trials of GBM may be in part due to the high degree of heterogeneity and complexity of RTK biology in gliomas.
Alterations in the RTK/RAS/PI3 K signaling pathway in GBM. Several genes that encode proteins involved in the RTK/RAS/PI3 K signaling pathway are considerably altered in GBM. Genes that are most frequently amplified in this pathway are epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor α (PDGFRA), two transmembrane receptors with tyrosine kinase activity. The most commonly deleted gene in the RTK pathway is phosphatase and tensin homolog (PTEN), a tumor suppressor that inhibits phosphatidylinositol-3 kinase (PI3 K) signaling such as retinoblastoma (RB1), a cell cycle inhibitor of PARK2, a regulator of dopaminergic cell death, and neurofibromin 1 (NF1), a negative regulator of the RAS signal transduction pathway. The most commonly mutated genes in this pathway are PTEN, NF1, EGFR, and PIK3R1, and PIK3CA. This figure was adapted from The Cancer Genome Atlas Research Network [23]
The majority of GBMs exhibit activation of the extended PI3 K–AKT–mTOR and RAS–MAPK signaling pathways [114] and these are therefore, considered to be common oncogenic alterations in these tumors. Deregulating mutations in these pathways include mutations in genes encoding either the catalytic (PIK3CA) or regulatory (PIK3R1) domains of PI3 K, which in turn induce the activity of these enzymes (~15 % of adult GBMs), as well as deletions and/or silencing mutations in PTEN, the primary negative regulator of the PI3 K-AKT signaling pathway (~30 % of cases). Beyond genetic alterations of PTEN, additional epigenetic and miRNA-based regulation of PTEN repression have also been described in diffuse gliomas, although they are more common in WHO grade II and III gliomas (50–60 %) [52, 71, 82, 108, 175]. Mutations in the Ras antagonist protein neurofibromin 1 (NF1) are thought to be the cause of neurofibromatosis type 1, a cancer predisposition syndrome mainly characterized by frequent neurofibromas and astrocytomas [55]. Recent studies, however, have demonstrated NF1 somatic gene mutation or deletion in 15–18 % of “primary” GBMs [23, 123], and a major conclusion from the TCGA effort was demonstration of a link between NF1 gene alteration and the mesenchymal GBM subclass (see below).
The identification of point mutations in codon 132 of isocitrate dehydrogenase I (IDH1) (and less commonly codon 172 of IDH2) in gliomas has provided a fundamental new insight into our understanding of the biology, as well as the molecular classification of these tumors [123]. Such mutations are frequent in WHO grade II and III diffuse gliomas (70–90 %) and secondary GBMs (85 %), but are rarely found in patients with traditionally referred to as “primary” GBMs (5 %) [58, 181]. While the distinction between primary and secondary pathways to GBM was originally based on the different clinical history, it has become evident that both are molecularly distinct GBM entities with absence or presence of IDH1/2 mutations being the most important molecular discriminator. Furthermore, IDH1/2 mutations are generally found to positively correlate with other genetic abnormalities common to diffuse gliomas such as TP53 and ATRX mutations in astrocytoma and 1p/19q co-deletion in oligodendroglial tumors, while they display an inverse correlation with EGFR gene amplification and monosomy of chromosome 10, alterations that more commonly occur in primary GBMs [181]. Therefore, the molecular pathways that lead to the development of low-grade gliomas and secondary GBMs that they evolve into are clearly distinct from those giving rise to primary GBMs. On this point, the prior designation of “primary GBM” is likely misleading, since IDH wild-type lower grade gliomas—especially anaplastic astrocytomas—are often genomically identical and likely represent precursors to IDH wild-type GBM. Therefore, “primary” GBM likely undergo molecular evolution from lower grade lesions (Brat et al. Comprehensive, integrative genomic analysis of diffuse lower grade gliomas, in press). As a first pass, while GBMs with IDH1/2 mutations are relatively uncommon, IDH1/2 mutant secondary GBMs represent a completely different biologic entity compared to the majority of GBMs which do not harbor mutations in IDH1/2, i.e., most primary GBMs. In addition, anaplastic astrocytomas that are IDH wild type are for practical purposes best considered as GBM, since these tumors show genomic hallmarks of GBM (loss of chromosome 10, gain of chromosome 7, and EGFR amplification) and clinically behave as GBMs.
Transcriptional subtypes of GBM
The availability of high-throughput genomic platforms for mRNA expression profiling since the late 1990s has resulted in some experience and published data attempting to identify patterns of gene expression and to codify these into subtypes, with subsequent correlative studies layering additional genetic and genomic aberrations. Initially, work on gliomas focused mainly on high-grade tumors such as GBM and comprehensive transcriptional analysis was used to identify molecular correlates for known clinical and/or pathological distinctions, such as the corresponding WHO grade, primary versus secondary GBM, and astrocytic versus oligodendroglial morphology [49, 80, 83, 96, 133, 137, 146, 160, 162]. Subsequent profiling studies have successfully identified distinct molecular signatures for diffuse gliomas and revealed specific subclasses within GBMs.
In 2006, Phillips et al. [129] examined differential expression of markers associated with clinical outcome and used K-means clustering to delineate gene signatures in WHO grade III and IV diffuse gliomas. Three major subclasses of GBM emerged based on this analysis: proneural, mesenchymal, and proliferative. This classification bares similarity to earlier sub-classification of prognostically relevant high-grade gliomas [47]. In addition, markers associated with one of the major subtypes (the mesenchymal signature), including YLK40 and VEGF, had previously been applied to distinguish GBMs from lower grade gliomas [48, 51, 137]. In its initial description, the proneural signature was shown to be associated with a better outcome (although later this was discovered to be confounded by the fact that IDH1/2 mutant gliomas invariably appear to be proneural) and expresses marker genes associated with neurogenesis. In a study by Aiguo et al. unsupervised analysis of transcriptome profiles from 159 glioma samples predicted two major groups of gliomas (oligodendroglioma-rich and GBM-rich) that were further separable into six hierarchically nested subtypes [95]. The initial TCGA expression profiling report described four GBM subtypes termed proneural, neural, classical, and mesenchymal [166]. This study also documented genomic associations, with classical, proneural, and mesenchymal tumors strongly enriched for aberrations in EGFR, PDGFRA and IDH1 or IDH2, and NF1 genes, respectively (Fig. 2).
Transcriptional subtypes of glioblastomas based on Phillips and Verhaak classification. Gene expression-based molecular classification of GBM into proneural, neural, classical, and mesenchymal subtypes by Verhaak et al. and into proneural, proliferative, and mesenchymal subtypes by Phillips et al. Integrated genomic analysis demonstrate patterns of somatic mutations and DNA copy number alterations. Aberrations in EGFR, NF1, and PDGFRA/IDH1 genes each define the classical, mesenchymal, and proneural subtypes, respectively
The proneural subtype is mainly described by mutations in PDGFRA or in IDH1/2, whereas the classical subtype is characterized by amplification/mutation of the EGFR gene, and mutations in neurofibromin 1 (NF1) are mainly found in the mesenchymal subtype. The proneural GBM is further subdivided into glioma CpG (G–CIMP)-positive and -negative subgroups based on the characteristic DNA methylation patterns that are directly linked to the IDH1/2 mutational status [117]. The mesenchymal signature is mainly regulated by the expression of the transcription factor signal transducer and activator of transcription 3 (STAT3), CCAAT/enhancer-binding protein-β (C/EBPβ), and transcriptional co-activator with PDZ-binding motif (TAZ), which have also been associated with poor clinical outcome [15, 45]. Furthermore, recent studies have shown that CTNND2 (encoding catenin-δ2) and RHPN2 function as negative and positive genetic regulators of mesenchymal transformation, respectively [32, 45]. Whether the proneural and mesenchymal signatures, as well as the other transcriptional subtypes of GBMs, could serve as predictors of patient outcome is being investigated by a number of groups. While overall the proneural subclass is associated with better outcome, a finer examination suggests that proneural GBMs can be subdivided into IDH1/2 mutant G-CIMP-positive and -negative subsets and once IDH1/2 G-CIMP status is controlled for, the proneural class has no prognostic advantage compared to other IDH1/2 wild-type GBMs [18].
While the two major subclasses, proneural and mesenchymal, appear to be reproducibly defined and characterized and may describe important biology, the implementation of these gene expression signatures into clinical diagnosis has not been accomplished. Indeed, subclass assignment has been shown to be unstable and change following surgical resection and radiochemotherapy [14, 129]. Moreover, a recent study analyzing expression signatures of single cells within GBM samples showed substantial intratumoral heterogeneity of expression subclasses within each tumor [124]. Based on these considerations, the mRNA expression profile of glial tumors may represent an average of a heterogeneous mix of transcriptional signatures and therefore, alternative aberrations, including the genomic and epigenomic profiles of tumors may represent more stable metrics for tumor classification.
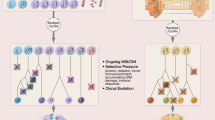
Progression of IDH-wild type and IDH-mutant gliomas. IDH-mutant gliomas (right) go through an ordered sequence of genetic modifications. Upon acquisition of IDH1/2 mutations and hypermethylation of CpG islands (CIMP) in the glial progenitor cells, a subset of these cells acquires secondary mutations in TP53 and ATRX, which result in the development of astrocytomas and eventual progression to ‘secondary’ glioblastoma. Co-deletion of 1p and 19q occurs in the other subset of glial cells, along with TERT promoter mutation, leading to formation of oligodendrogliomas. IDH-wild type gliomas (left) progress via acquisition of different molecular alterations and most commonly present as glioblastoma. However, the designation as ‘primary’ glioblastoma may not be entirely accurate, as IDH-wild type lower grade astrocytoma, although not common, is well-described
Clinically relevant genetic and epigenetic abnormalities in GBM
Isocitrate dehydrogenase 1 and 2 (IDH1/2) genes
One of the most important discoveries resulting from high-throughput genomic studies, which has led to remodeling of our understanding of gliomas including GBMs, was the identification of mutations in the metabolic enzymes isocitrate dehydrogenase 1 and 2 (IDH1/2) [123]. In this landmark paper, the majority of tumor samples bearing this mutation (5/6) were classified as secondary GBMs, suggesting that IDH1/2 mutation could serve as a genetic marker for this GBM type. Mutant IDH1/2 alleles identified in gliomas result in enzymes with a neomorphic function [31], whereby the mutant enzymes have acquired the ability to catalyze the NADPH-dependent reduction of α-KG to the (R)-enantiomer of 2-hydroxyglutarate (2-HG), that is the same stereoisomer of 2-HG seen in D-2-HG. In fact, Dang et al. [31] showed that IDH1/2 mutant cells had high levels of 2-HG, as is also found in primary IDH1 mutant gliomas and in the serum of IDH1/2 mutant acute myeloid leukemia (AML) patients [54, 169].
It is thought that expression of mutant IDH1/2 proteins results in inhibition of α-KG-dependent dioxygenases by 2-HG. Enzymes that are α-KG dependent regulate a number of physiological processes such as hypoxia sensing, histone demethylation, and changes in DNA methylation, among others [98]. A distinctive and nearly invariable feature of IDH1/2 mutant gliomas is the glioma CpG island methylator phenotype (G-CIMP) [117]. Baysan et al. [12] applied unsupervised clustering of TCGA methylation data from 368 GBM samples, showing that G-CIMP-positive expression signatures were linked with mutant IDH1 expression and correlated with better prognosis. For the detection of IDH1/2 mutant gliomas—where approximately 15 % of cases are not detected using the IDH-R132H antibody—DNA sequencing of antibody-negative cases has provided more accurate diagnosis and prediction of patient outcome and prognosis. This is especially useful in younger GBM patients, as IDH1/2 mutation is more common in this patient group.
In addition to providing insights about the origin of gliomas, the mutational status of IDH1/2 serves as a prognostic marker in patients with WHO grade II and III gliomas [58, 123, 141, 181] and GBMs [172]. While IDH1/2 wild-type GBMs (as well as most anaplastic gliomas that do not have an IDH mutation) exhibit a pattern of genetic changes that are associated with primary GBMs—such as gain of chromosome 7, loss of chromosome 10, and EGFR amplification—this pattern is not characteristic of IDH1/2 mutant GBMs. Unresolved issues remain, related to understanding of the specific driver molecular changes in IDH1/2 wild-type GBM, made complex by the fact that some of the prototypical changes include gains and losses of whole chromosomes or chromosomal arms (e.g., losses of chromosome 10 and the short arm of chromosome 9, and gain of chromosome 7). Intertwined within this issue is the fact that the expression subtypes within IDH1/2 wild-type GBM (e.g., proneural versus mesenchymal) often have largely similar genomic changes, with the exception of the classical subtype which harbors large-scale amplification of EGFR at the genomic level. One recent study attempted to address this issue in an interesting manner by using mathematical modeling of genomic changes in IDH1/2 wild-type/G-CIMP-negative GBM using data available from TCGA coupled with experimental mouse modeling. Their results suggested that gain of PDGFA (chromosome 7) and loss of PTEN (chromosome 10) are likely initial driver events, and that a hierarchy of expression subtypes exists. It is also likely that PDGFA drives a proneural phenotype, which can be followed by loss of NF1 function to promote a subsequent mesenchymal phenotype [122]. Taken together, the collective data clearly show, although histologically similar, IDH1/2-mutant and wild-type GBMs are clearly distinct diseases on a genomic basis and understanding the biological contribution of these mutations may help in the diagnosis and design of treatment strategies (Fig. 3). On this point, the development of therapies specific for mutant IDH1/2 appears to be a practical lead for molecularly driven therapies and show promising pre-clinical results, either as a small molecular inhibitor or as a vaccine approach targeting the R132H protein as a tumor-specific neoantigen [135, 144]. Additional clinical development of these approaches, including plans to address blood–brain barrier penetration of these targeting agents, is likely to yield important information and hopefully therapeutic advances in the coming years.
Epidermal growth factor receptor (EGFR) and EGFRvIII
Approximately 40 % of primary GBMs carry amplification of the EGFR gene [70, 76, 143]. In addition, about 50 % of GBMs with EGFR amplification also harbor a mutation in this gene that codes for EGFRvIII—a constitutively active variant of EGFR that is supposed to promote tumor growth and is potentially associated with a worse clinical outcome [86, 87]. Interestingly, established prognostic factors in GBM, e.g., the Radiation Therapy Oncology Group’s recursive partitioning analysis (RTOG-RPA) class, were not predictive of outcome in EGFRvIII-positive GBMs [127]. The EGFRvIII mutation involves an intragenic gene rearrangement that is generated by an in-frame deletion of exons 2–7, which encode part of the extracellular domain of this protein [40, 62, 147]. A number of studies have shown that ectopic overexpression of EGFRvIII in glioma cell lines results in constitutive autophosphorylation and activation of the Shc–Grb2–Ras and class I PI3 K pathways [69, 113], induces tumorigenicity [69], cell proliferation [113], and resistance to apoptosis through modulation of Bcl-X L gene expression [111]. Interestingly, the tumorigenic effects of EGFRvIII overexpression are not recapitulated by overexpression of the wild-type EGFR. Furthermore, both EGFRvIII and wild-type EGFR proteins have been detected in the nucleus and are thought to drive transcriptional and signaling pathways that contribute to cell proliferation and DNA damage repair [168]. Notably, although EGFRvIII is well known to induce cell proliferation, it is only expressed in a fraction of GBM cells [116]. A number of recent studies have suggested a model for functional heterogeneity, where a small number of EGFRvIII-positive cells not only drive their own proliferation but also enhance the proliferation of their neighboring cells that express wild-type EGFR. In a study conducted by Inda et al. it was found that wild-type EGFR-expressing cells exhibit accelerated proliferation due to a paracrine mechanism driven by EGFRvIII-expressing cells. They demonstrated that human glioma tissues, glioma cell lines, glioma stem cells, and immortalized mouse Ink4a/Arf (−/−) astrocytes expressing EGFRvIII also expressed cytokines such as IL-6 and/or leukemia inhibitory factor (LIF), which in turn activate the cytokine co-receptor gp130, and thereby, induce the expression of wild-type EGFR in the neighboring cells [73]. Therefore, intratumoral heterogeneity and cooperativity may be the key for EGFRvIII function in GBM. Alterations in the EGFR gene have been found in other cancer types such as non-small-cell lung cancer (NSCLC), but the type of genetic alterations found in EGFR in GBM are distinct from those associated with other cancers. For example, focal EGFR amplification occurs at an extremely high rate in gliomas (>20 copies) and the majority of other mutations—such as the EGFRvIII mutation and missense mutations—are located in the extracellular domain [23, 93], while in most non-glioma cancers they are found in the intracellular domain [75]. It should be noted that EGFR amplification and EGFRvIII expression may not persist in cultured cells as in primary tumors, but recent studies have successfully passaged EGFRvIII-expressing GBM xenografts both in vivo as well as in vitro by growing them in stem cell culture conditions [153]. Therefore, long-term EGFRvIII expression may in fact be possible and is associated with differentiation and/or the developmental stage of the tumor.
The EGFRvIII mutation has become clinical relevance as this deletion mutation generates a novel peptide sequence that may serve as an immunogenic tumor-specific target, which can be exploited in a peptide-based vaccination strategy. Initial results from single-arm trials employing EGFRvIII-specific vaccination provided promising results in comparison to historical controls [138]. The efficacy of EGFRvIII-targeted vaccination in newly diagnosed GBM patients is currently being investigated in the prospective randomized ACT IV trial (EUDRA-CT#: 2011-006068-32).
Platelet-derived growth factor alpha (PDGFRA)
In approximately 30 % of human gliomas, expression of genes associated with platelet-derived growth factor receptor (PDGFR) signaling and genes involved in oligodendrocyte development (OLIG2, NKX2-2, and PDGF), are observed and are thought to be hallmarks of the proneural signature in GBM [17]. Amplification of the alpha-type PDGFR (PDGFRA) gene is found in 15 % of all tumors, mainly in the proneural subtype of GBM [129, 166] and approximately 40 % of tumors harboring gene amplification contain an intragenic deletion in this gene, termed PDGFRA Δ8,9 [28], where in-frame deletion of 243 base pairs of exons 8 and 9 results in a truncated extracellular domain [121]. In addition to this deletion, in-frame gene fusion of the extracellular domain of KDR/VEGFR-2 and the intracellular domain of PDGFRA has also been found, and both of these mutant proteins were shown to be constitutively active, display transforming ability and could be inhibited using inhibitors of PDGFRA. Point mutations in PDGFRA have also been detected but are generally rare [23]. In addition, PDGFR signaling can be activated upon up-regulation of PDGF ligands (A–D) in approximately 30 % of glioma tumor samples and cell lines. The expression of PDGFRB, however, seems to be limited to proliferating endothelial cells in GBM [33, 43, 64, 99, 150].
Similar to EGFR and EGFRvIII, amplification of PDGF and PDGFR seems to promote aggressive glioma growth. Assanah et al. [3, 4] demonstrated that transduction of cells of the subventricular zone (SVZ) of the lateral ventricle of neonatal rats with a retrovirus expressing PDGF yielded large, diffuse tumors that resembled GBM. They found that in these tumors, both infected and uninfected PDGFRα+-expressing progenitors massively proliferated, suggesting that PDGF expression leads to tumor formation through both autocrine and paracrine signaling mechanisms, driving the evolution of heterogeneous malignant gliomas. These results raise the possibility that cells distinct from the initially transformed cells of origin within the tumor environment can eventually become tumor cells and suggest a model of glioma evolution that is different from the generally accepted view of linear gliomagenesis [44].
Neurofibromatosis type 1 gene (NF1)
Large-scale sequencing analysis by the TCGA has shown that in approximately 15 % of glioma samples the NF1 gene is inactivated by genetic loss or mutation [123], and NF1 mutations are most common in the mesenchymal subtype of GBM [166]. Inactivation of NF1 protein can also arise from excessive proteasomal degradation mediated by hyperactivation of PKC [23, 109]. Neurofibromin 1 is the product of NF1 gene and is a tumor suppressor that negatively regulates Ras and mTOR signaling pathways in astrocytomas. In fact, experiments using NF1-deficient primary murine astrocytes have revealed that loss of NF1 causes increased cell proliferation and migration that is dependent on hyperactivation of mTOR mediated by Ras signaling. In this setting, mTOR induces rapamycin-sensitive activation of Rac1 GTPase, independent of elongation factor 4E-binding protein 1(4EBP-1)/S6 kinase (S6 K) [140]. Stat3 is another downstream target of NF1 that is regulated in an mTORC1- and Rac1-dependent manner and increases cyclinD1 expression [9].
Using genetically engineered mouse models, it was found that targeted homozygous loss of NF1 in astrocytes is not sufficient to induce tumor formation, although it is sufficient to increase cell growth both in vitro and in vivo [8]. Furthermore, NF1 −/− astrocytes were shown to develop optic gliomas in NF1 +/− brains of mice [7, 184] and low levels of cAMP expression in the stroma cause induction of optic glioma formation in genetically engineered mouse models of NF1 [170]. Other studies using genetically engineered mouse models have shown that loss of NF1 in glial cells, in combination with a germ line TP53 mutation, results in astrocytomas [183] and further progress to GBM upon deletion of PTEN [90]. More recent work has revealed that the same combination of genetic alterations in these tumor suppressor genes in neural stem/progenitor cells is necessary and sufficient to induce astrocytoma formation [1]. Loss of NF1 gene function has been implicated in the development of the mesenchymal phenotype for GBM [166]. These findings emphasize the heterogeneity and the contribution of cell type-specific effects of various genetic alterations to the development of GBM.
MGMT promoter methylation
Promoters of several genes at specific loci are hypermethylated in GBM and frequently result in altered expression of tumor suppressor genes, such as cyclin-dependent kinase inhibitor 2A (CDKN2A), RB1, PTEN, and TP53, among others [2, 6, 29, 112]. One of the clinically most important DNA methylation markers in GBMs is the promoter of MGMT (encoding O 6-methylguanine-DNA methyltransferase), which is found in approximately 40 % of primary GBM patients and is associated with transcriptional silencing of the MGMT gene. Hypermethylation of the MGMT promoter was demonstrated to serve as a predictive marker for alkylating chemotherapy in GBMs [61, 174]. MGMT is a DNA repair enzyme and modulation of sensitivity to alkylating agents can be explained by the ability of this enzyme to restore guanine from O-6-methylguanine, which is the type of genomic lesion induced by alkylating agents used for chemotherapy drugs such as temozolomide (TMZ) (Fig. 4). A number of clinical trials and cohort studies have shown that promoter methylation of the MGMT gene is associated with prolonged progression-free and overall survival in patients who were treated with alkylating agents [41, 60, 61, 65, 172].
MGMT promoter methylation as a predictive marker for TMZ treatment. TMZ is an oral alkylating agent used as a chemotherapeutic treatment for GBMs. TMZ causes DNA lesions such as O6-methylguanine (O6-meG), and N3-methyladenine and N7-methylguanine (N3-meA, N7-meG). O6-meG DNA methyltransferase (MGMT) restores the guanine to normal by removing the O6-alkylguanine, and thereby, promoting tumor cell survival. MGMT function may be impaired by gene deletion or suppression of its expression by hypermethylation of its promoter. Specifically in glioblastomas, IDH1/2 mutations cause the CpG island methylator phenotype (CIMP) which may involve MGMT methylation as part of this phenomenon. Loss of MGMT-mediated DNA repair may lead to DNA strand breaks, apoptosis, autophagy, and tumor cell death
A seminal trial conducted by the EORTC examined concurrent/adjuvant TMZ treatment during and after radiotherapy compared to radiotherapy alone for newly diagnosed GBM patients [155]. While the trial was overall positive, analysis of a subset of samples in this trial showed that patients with glioma tumors harboring MGMT promoter methylation benefited from chemotherapy almost exclusively [61]. Similar results have been found in elderly patients, showing improved outcome with chemotherapy treatment in MGMT promoter-methylated tumors, while worse survival was associated with unmethylated tumors [105, 174], suggesting that MGMT promoter methylation is not a prognostic, but instead a predictive marker. Additional work has reconfirmed the predictive value of MGMT promoter methylation for response to chemotherapy in IDH1/2 wild-type GBMs, while this marker is prognostic, albeit more commonly, in IDH1/2 mutant anaplastic gliomas [165, 173]. It is important to note that there is extensive overlap between MGMT methylation status and G-CIMP, and while MGMT methylation is present in a subset of G-CIMP-negative GBMs, it is found in almost all cases of G-CIMP-positive tumors [5]. Importantly, MGMT promoter methylation is a clinically important predictive marker for guiding adjuvant therapy in elderly GBM patients [61, 105, 119, 174]. In this patient group, MGMT methylation status has emerged as a predictive marker to determine best therapy and inclusion of TMZ. In this setting, the MGMT promoter methylation status helps to stratify patients into those who should be treated with radiotherapy only, i.e., patients with MGMT promoter unmethylated tumors, and those who should be treated with TMZ chemotherapy or combined TMZ/radiotherapy, i.e., patients with MGMT promoter-methylated tumors [61, 105, 119, 174]. However, the importance of MGMT methylation testing in non-elderly GBM patients remains a matter of debate, as these patients are often treated with TMZ regardless of methylation status. However, MGMT methylation status may be useful in these patients to distinguish pseudoprogression (PsPD) from true progression [16]. PsPD is a pathological phenomenon in malignant glioma patients that are treated with combination radiotherapy and TMZ. PsPD generally occurs within a few months from radiochemotherapy and appears as an increase in tumor size in radiological imaging; however, it is not accompanied with worsening of the neurological signs and symptoms. PsPD was recorded in 21 (91 %) of 23 patients with methylated MGMT promoter and 11 (41 %) of 27 patients with unmethylated MGMT promoter (P = 0.0002). In pediatric gliomas, both the frequency (16–50 %) [20, 35, 94, 152] and the prognostic or predictive significance of MGMT silencing remain to be determined [35, 94].
Epigenome-wide analysis of DNA methylation patterns in glioma tumors has improved our understanding of glioma biology and has contributed to the advancement of tumor classification [117, 156]. Recently, algorithms have been developed that enable assessment of the three biomarkers, 1p/19q co-deletion, G-CIMP status, and MGMT promoter methylation, using Illumina Infinium HumanMethylation450 (450 K) data [5, 117, 156]. Hybridization of tumor DNA to these arrays allows one to profile methylation of up to 450,000 CpG sites distributed across the human genome as well as analyzing genome-wide copy number changes [5, 68, 156]. In addition, this method is suitable for analysis of formalin-fixed and paraffin-embedded (FFPE) tissue samples [68]. Wiestler et al. [177] assessed the reliability and value of this technology and demonstrated its diagnostic and prognostic accuracy in determining G-CIMP, 1p/19q co-deletion, and MGMT promoter methylation status in the biomarker cohort of the prospective NOA-04 trial. Further optimization and elucidation of MGMT methylation testing may yield additional clinical relevance of this important biomarker.
hTERT promoter mutation
Human telomerase is a ribonucleoprotein that regulates the length of telomeric DNA at the ends of chromosomes and therefore, plays an important role in cellular immortalization and oncogenesis. One of the hallmarks of cancer is deregulation of telomere maintenance and this process is regulated by the enzyme telomerase, which is active in 90 % of all advanced cancers. Telomerase reverse transcriptase (TERT) is the catalytic subunit of the telomerase complex and its expression is associated with poor outcome in most tumors such as breast cancer, sarcomas, and brain tumors [37, 50, 102, 139, 159]. Recent findings have established frequent mutations in the promoter of TERT in a number of cancer types, including melanomas, liposarcomas, bladder cancer, and gliomas [53, 67, 81, 86, 167]. Interestingly, genomic analysis of gliomas has shown that TERT promoter mutations occur in 70–80 % of primary GBMs and in more than 70 % of oligodendrogliomas, but are less frequent in IDH1/2 mutant diffuse and anaplastic astrocytomas as well as IDH1/2 mutant (secondary) GBMs that instead carry frequent ATRX mutations [11, 72, 115]. TERT promoter mutations are also rare in pediatric GBMs characterized by histone H3.3 (H3F3A) mutations, which often are associated with TP53 and ATRX/DAXX mutations [160].
Recently, Killela et al. [81] assessed the association between IDH1/2 mutation and TERT promoter mutations across several glioma subtypes. The joint influence of IDH1/2 mutation and TERT promoter mutation on overall survival (OS) was examined and three common glioma subtypes were delineated; astrocytomas of WHO grade II and III, oligodendrogliomas of WHO grade II and III, and GBMs. In general, TERT promoter mutations predicted poorer OS in GBMs without IDH1/2 mutations. Additional studies by Simon et al. and Labussiere et al. demonstrated that TERT promoter mutation signature could serve as a novel independent prognostic factor for poor outcome in primary GBMs. Their findings, however, suggest that the prognostic effect of TERT promoter mutation is independent of the mutation status of IDH1/2 in GBMs [91, 148]. On the other hand, TERT promoter mutations maybe associated with longer survival in patients with IDH1/2 mutant gliomas, as they are closely linked with the prognostically favorable 1p/19q co-deletion in oligodendroglial tumors [3, 152]. Analysis of TERT promoter mutation also serves as a novel prognostic marker for primary GBM patients and more recently, combined analysis of TERT promoter mutation, EGFR amplification, and IDH1/2 mutation has enabled identification of distinct classes of adult GBM [81]. TERT promoter mutation testing may have a dual role in molecular classification of gliomas based on IDH1/2 mutation status: within the IDH1/2 mutant tumors, TERT mutation could possibly serve as a surrogate/confirmatory marker for 1p/19q co-deletion, as the two are highly correlated. For the purposes of IDH1/2 wild-type GBMs, TERT mutation appears to be found in the majority of cases, but those which do not have promoter mutations (referred to in a recent report as “triple negative” (negative for all 3 markers: IDH mutation, 1p/19q co-deletion, and TERT mutation) may be clinically distinct from those GBMs which are “single-positive” (TERT-mutant only) [39].
BRAF mutation
Activating missense mutations at the BRAF hotspot codon 600, most commonly the V600E, are common in several neuroepithelial tumors, including pleomorphic xanthoastrocytoma and one-third of gangliogliomas, and occasional pilocytic astrocytoma [142]. In GBMs, BRAF V600E mutations have been detected in approximately 5 % of the cases [85, 142]. A higher frequency of BRAF mutation has been reported in GBMs with histological features of epitheloid differentiation, i.e., “epitheloid GBMs”, which preferentially manifest in children and young adults and carry BRAF V600E mutations in more than 50 % of the cases (7 of 13) [84]. While treatment of pediatric low-grade astrocytoma patients with sorafenib, a multikinase inhibitor targeting BRAF, VEGFR, PDGFR, and c-KIT, resulted in unexpected acceleration of tumor growth, even in patients with BRAF mutant tumors [79], a recent case report showed complete regression of a BRAF V600E mutant pediatric GBM following treatment with the BRAF inhibitor vemurafenib [134]. Thus, molecular testing for BRAF mutation, either by DNA sequencing or by immunohistochemistry using a BRAF V600E-specific antibody [24], may uncover a potentially active novel targeted therapy option in a small fraction of GBM patients.
Comparison of molecular features of GBMS in pediatric versus adult patients
Childhood GBM is much less common in absolute numbers than the adult form; however, it is relatively a much more frequent primary CNS tumor as a proportion of all brain tumors (children 0–19 years: GBMs, 2.9 %, malignant gliomas NOS, 11.7 %; all age groups: GBMs 15.4 %) [120]. The 2-year survival rate for GBM in children is approximately 12 %, making this disease a leading cause of cancer-related deaths in children [19]. A number of studies have indicated that distinct genetic mechanisms play a role in the pathogenesis of pediatric and adult GBMs [42, 57, 126, 157, 179] and although in-depth analysis have demonstrated alterations in three key signaling pathways—including TP53, PI3 K/Akt, and Rb—and identified discrete transcriptional subtypes in adults, little is known about alterations in these pathways in pediatric GBMs. Two recent studies have attempted to identify somatic mutations specific to GBM patients who are younger than 19 years of age at the time of diagnosis. These studies were the first ones to discover somatic mutations in the histone H3.3-alpha-thalassemia X-linked mental retardation protein (ATRX)–death domain-associated protein (DAXX) chromatin remodeling pathway that lead to changes in the chromatin architecture and play a major role in pediatric GBM pathogenesis in approximately 44 % of tumors [145, 180]. Recurrent somatic mutations in H3F3A, the gene which encodes the replication-independent histone 3 (H3) variant H3.3, result in amino acid changes mainly in two residues within the histone tail; K27 M or G24R/G34 V. This mutation was found predominantly in GBM and was more prevalent in children than adults; however, recent findings point to approximately 5 % of adult GBM patients also carrying this lesion [132] and likewise, H3F3A mutation in adult GBM patients is associated with ATRX mutations. In addition, somatic mutations in TP53 were found in 54 % of all cases and in 86 % of cases harboring mutations in H3F3A and/or ATRX. The mutations in H3.3/ARTX/DAXX/TP53 were also found to associate with changes in the telomere lengthening and specific gene expression profiles, suggesting that changes in the chromatin architecture contribute to the pathogenesis of childhood GBM. Other studies that analyzed molecular profiles of pediatric high-grade gliomas (HGG) have also suggested the existence of molecularly diverse subsets of pediatric GBMs [42, 57, 125, 126]. Another alteration found in pediatric GBMs is a higher amplification frequency of the PDGFRA gene that is associated with activation of a PDGFRA-driven gene expression signature [125, 126, 130].
Another major difference between adult and pediatric GBM is the concomitant gain of chromosome 7 and loss of chromosome 10 in most adult tumors (on average 84 %) [18, 23]. Additional genomic abnormalities that occur at higher frequency in adult than in childhood GBMs include gains of chromosomes 19 and 20, and losses that affect chromosomes 9p, 22q, 13q, 14q, and 6q [18, 23]. Such chromosomal imbalances are generally found at lower frequency in childhood GBMs, and a proportion of these tumors (~15 %) does not contain any detectable copy number alterations [10, 78, 126, 156]. Pediatric tumors also display more frequent gain of chromosome arm 1q compared to the adult counterparts, while they rarely harbor gain of chromosome 7 and loss of chromosome 10 (Fig. 5) [10, 126]. In addition, TERT promoter mutations occur at a much lower rate (3–11 %) in pediatric GBMs [81, 86], which instead frequently display mutations in the H3.3/ATRX/DAXX and consequent alternative lengthening of telomeres (ALT) [59, 145]. With respect to DNA methylation signatures in pediatric GBMs, Sturm et al. [156] performed genomic DNA methylation profiling of 59 pediatric and 77 adult tumors and identified distinct epigenetic GBM subgroups that were closely linked to specific genetic alterations. One of the identified subtypes was the IDH1/2 mutant group, which is directly associated with global hypermethylation (G-CIMP positive), while the H3F3A–G34 group is linked to a hypomethylated signature of the genome (G-CIMP negative). In light of the identification of distinct genetic and epigenetic differences between pediatric and adult GBMs, and the recently identified correlation between these changes, it would be essential to fully understand the molecular differences between the adult and pediatric tumors to establish treatments specifically targeting GBMs in the two age groups. The substantial molecular differences between pediatric and adult tumors suggest that pediatric GBMs are in fact distinct entities on a biologic level. Even though the histology overlaps between pediatric and adult GBM, the genetic signatures indicate that these should not be “lumped” together into a single entity. In addition, recent data show that tumors morphologically classified as GBM in children actually represent very distinct subsets, based on molecular criteria (Illumina 450 k methylation profiling, DNA copy number analysis, and mutational analysis). Specifically, pediatric GBM that showed evidence of amplification a known oncogene and/or K27 M mutation in histone H3.3 showed a particularly poor prognosis, while tumors without evidence of these genetic lesions were prognostically more favorable, with a 3-year overall survival rate of approximately 70 % [87].
Major classes of glioma based on differences in pediatric or adult genomic alterations. Somatic mutations in the histone H3.3/ATRX/DAXX chromatin remodeling pathway are mainly found in pediatric glioma patients. Higher amplification frequency of PDGFRA gene and frequent gain of chromosome 1q are also common in pediatric gliomas. In adults, gain of chromosome 7 and loss of chromosome 10 are highly prevalent, in addition to TERT promoter mutation, EGFR amplification, and IDH1/2 mutation
Conclusion
Recently, aberrations in genes and molecular pathways in GBMs have provided a biological basis to establish appropriate clinically relevant biomarkers and point to the need for development of new therapeutic opportunities. We are at a point where progress in molecular classification of GBMs has provided useful insights for the development of more effective targeted therapeutics. Several clinically relevant molecular markers are well established and serve in the clinic as standard of care for patients diagnosed with glioma tumors. For example, the status of MGMT promoter methylation in GBMs (especially those detected in elderly patients), 1p and 19q co-deletions in anaplastic oligodendrogliomas, and IDH1/2 mutations, now play major roles in tumor diagnostics and/or clinical decision making [21, 105, 164, 174]. Meanwhile, multiplatform analyses of the genetic, epigenetic, and transcriptional profiles have proven useful in refining the classification of brain tumors and predicting patient outcome. Recent studies on pediatric GBM have demonstrated that these tumors, which are frequently driven by epigenetic changes in histone H3.3, may represent a 3rd major category of GBM, in addition to IDH1/2 mutant (secondary) and IDH1/2 wild-type (primary) GBMs in adults. With these molecular insights, it is hoped that further improvements in molecular assays would bring them to the clinic and be sought after as clinically indicated. These techniques might soon become more widely available, easier to standardize, and become more cost effective. Furthermore, the current histology-based diagnosis of brain tumors will increasingly be supplemented with molecular diagnostic tests to enable a biology-based classification and improve patient stratification that will hopefully be incorporated in carefully designed clinical trials. It is hoped that this approach of precision diagnostics–therapeutics can lead to step-by-step improvements of outcome where effective therapeutics are appropriately “matched” with molecularly defined patient subsets. Even with the current excitement in molecular classification, we remain a significant distance from substantive improvements, and ultimately a cure, for patients with GBM. Proper classification and biologic understanding, while a key feature of personalized therapy, is only one component and in itself is of limited value unless matched by parallel successes in the development of companion drugs and modalities for the overall goal of improved patient outcomes.
References
Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, Alvarez-Buylla A, Parada LF (2009) Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell 15:45–56. doi:10.1016/j.ccr.2008.12.006
Amatya VJ, Naumann U, Weller M, Ohgaki H (2005) TP53 promoter methylation in human gliomas. Acta Neuropathol 110:178–184. doi:10.1007/s00401-005-1041-5
Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J, Canoll P (2006) Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci Off J Soc Neurosci 26:6781–6790. doi:10.1523/JNEUROSCI.0514-06.2006
Assanah MC, Bruce JN, Suzuki SO, Chen A, Goldman JE, Canoll P (2009) PDGF stimulates the massive expansion of glial progenitors in the neonatal forebrain. Glia 57:1835–1847. doi:10.1002/glia.20895
Bady P, Sciuscio D, Diserens AC, Bloch J, van den Bent MJ, Marosi C, Dietrich PY, Weller M, Mariani L, Heppner FL et al (2012) MGMT methylation analysis of glioblastoma on the Infinium methylation BeadChip identifies two distinct CpG regions associated with gene silencing and outcome, yielding a prediction model for comparisons across datasets, tumor grades, and CIMP-status. Acta Neuropathol 124:547–560. doi:10.1007/s00401-012-1016-2
Baeza N, Weller M, Yonekawa Y, Kleihues P, Ohgaki H (2003) PTEN methylation and expression in glioblastomas. Acta Neuropathol 106:479–485. doi:10.1007/s00401-003-0748-4
Bajenaru ML, Hernandez MR, Perry A, Zhu Y, Parada LF, Garbow JR, Gutmann DH (2003) Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res 63:8573–8577
Bajenaru ML, Zhu Y, Hedrick NM, Donahoe J, Parada LF, Gutmann DH (2002) Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol Cell Biol 22:5100–5113
Banerjee S, Byrd JN, Gianino SM, Harpstrite SE, Rodriguez FJ, Tuskan RG, Reilly KM, Piwnica-Worms DR, Gutmann DH (2010) The neurofibromatosis type 1 tumor suppressor controls cell growth by regulating signal transducer and activator of transcription-3 activity in vitro and in vivo. Cancer Res 70:1356–1366. doi:10.1158/0008-5472.CAN-09-2178
Bax DA, Mackay A, Little SE, Carvalho D, Viana-Pereira M, Tamber N, Grigoriadis AE, Ashworth A, Reis RM, Ellison DW et al (2010) A distinct spectrum of copy number aberrations in pediatric high-grade gliomas. Clin Cancer Res Off J Am Assoc Cancer Res 16:3368–3377. doi:10.1158/1078-0432.CCR-10-0438
Baylin SB, Esteller M, Rountree MR, Bachman KE, Schuebel K, Herman JG (2001) Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum Mol Genet 10:687–692
Baysan M, Bozdag S, Cam MC, Kotliarova S, Ahn S, Walling J, Killian JK, Stevenson H, Meltzer P, Fine HA (2012) G-cimp status prediction of glioblastoma samples using mRNA expression data. PLoS One 7:e47839. doi:10.1371/journal.pone.0047839
Beroukhim R, Getz G, Nghiemphu L, Barretina J, Hsueh T, Linhart D, Vivanco I, Lee JC, Huang JH, Alexander S et al (2007) Assessing the significance of chromosomal aberrations in cancer: methodology and application to glioma. Proc Natl Acad Sci USA 104:20007–20012. doi:10.1073/pnas.0710052104
Bhat KP, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, Wani K, Heathcock L, James JD, Goodman LD et al (2013) Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell 24:331–346. doi:10.1016/j.ccr.2013.08.001
Bhat KP, Salazar KL, Balasubramaniyan V, Wani K, Heathcock L, Hollingsworth F, James JD, Gumin J, Diefes KL, Kim SH et al (2011) The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes Dev 25:2594–2609. doi:10.1101/gad.176800.111
Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F, Andreoli A et al (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol Off J Am Soc Clin Oncol 26:2192–2197. doi:10.1200/JCO.2007.14.8163
Brennan C, Momota H, Hambardzumyan D, Ozawa T, Tandon A, Pedraza A, Holland E (2009) Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS One 4:e7752. doi:10.1371/journal.pone.0007752
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH et al (2013) The somatic genomic landscape of glioblastoma. Cell 155:462–477. doi:10.1016/j.cell.2013.09.034
Broniscer A, Gajjar A (2004) Supratentorial high-grade astrocytoma and diffuse brainstem glioma: two challenges for the pediatric oncologist. Oncologist 9:197–206
Buttarelli FR, Massimino M, Antonelli M, Lauriola L, Nozza P, Donofrio V, Arcella A, Oliva MA, Di Rocco C, Giangaspero F (2010) Evaluation status and prognostic significance of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation in pediatric high grade gliomas. Child’s Nervous Sys ChNS Off J Int Soc Pediatr Neurosurg 26:1051–1056. doi:10.1007/s00381-010-1191-1
Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, Fink K, Souhami L, Laperriere N, Curran W et al (2013) Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol Off J Am Soc Clin Oncol 31:337–343. doi:10.1200/JCO.2012.43.2674
Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR, Silver JS, Stark PC, Macdonald DR, Ino Y et al (1998) Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 90:1473–1479
Cancer Genome Atlas Research N (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068. doi:10.1038/nature07385
Capper D, Preusser M, Habel A, Sahm F, Ackermann U, Schindler G, Pusch S, Mechtersheimer G, Zentgraf H, von Deimling A (2011) Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol 122:11–19. doi:10.1007/s00401-011-0841-z
Chabner BA (2011) Early accelerated approval for highly targeted cancer drugs. New Engl J Med 364:1087–1089. doi:10.1056/NEJMp1100548
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M et al (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. New Eng J Med 364:2507–2516. doi:10.1056/NEJMoa1103782
Chung R, Whaley J, Kley N, Anderson K, Louis D, Menon A, Hettlich C, Freiman R, Hedley-Whyte ET, Martuza R et al (1991) TP53 gene mutations and 17p deletions in human astrocytomas. Genes Chromosom Cancer 3:323–331
Clarke ID, Dirks PB (2003) A human brain tumor-derived PDGFR-alpha deletion mutant is transforming. Oncogene 22:722–733. doi:10.1038/sj.onc.1206160
Costello JF, Berger MS, Huang HS, Cavenee WK (1996) Silencing of p16/CDKN2 expression in human gliomas by methylation and chromatin condensation. Cancer Res 56:2405–2410
Costello JF, Plass C, Arap W, Chapman VM, Held WA, Berger MS, Su Huang HJ, Cavenee WK (1997) Cyclin-dependent kinase 6 (CDK6) amplification in human gliomas identified using two-dimensional separation of genomic DNA. Cancer Res 57:1250–1254
Dang L, Jin S, Su SM (2010) IDH mutations in glioma and acute myeloid leukemia. Trends Mol Med 16:387–397. doi:10.1016/j.molmed.2010.07.002
Danussi C, Akavia UD, Niola F, Jovic A, Lasorella A, Pe’er D, Iavarone A (2013) RHPN2 drives mesenchymal transformation in malignant glioma by triggering RhoA activation. Cancer Res 73:5140–5150. doi:10.1158/0008-5472.CAN-13-1168-T
Di Rocco F, Carroll RS, Zhang J, Black PM (1998) Platelet-derived growth factor and its receptor expression in human oligodendrogliomas. Neurosurgery 42:341–346
Dias-Santagata D, Akhavanfard S, David SS, Vernovsky K, Kuhlmann G, Boisvert SL, Stubbs H, McDermott U, Settleman J, Kwak EL et al (2010) Rapid targeted mutational analysis of human tumours: a clinical platform to guide personalized cancer medicine. EMBO Mol Med 2:146–158. doi:10.1002/emmm.201000070
Donson AM, Addo-Yobo SO, Handler MH, Gore L, Foreman NK (2007) MGMT promoter methylation correlates with survival benefit and sensitivity to temozolomide in pediatric glioblastoma. Pediatr Blood Cancer 48:403–407. doi:10.1002/pbc.20803
Druker B (2001) Signal transduction inhibition: results from phase I clinical trials in chronic myeloid leukemia. Semin Hematol 38:9–14
Ducrest AL, Szutorisz H, Lingner J, Nabholz M (2002) Regulation of the human telomerase reverse transcriptase gene. Oncogene 21:541–552. doi:10.1038/sj.onc.1205081
Dunn GP, Rinne ML, Wykosky J, Genovese G, Quayle SN, Dunn IF, Agarwalla PK, Chheda MG, Campos B, Wang A et al (2012) Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev 26:756–784. doi:10.1101/gad.187922.112
Eckel-Passow J, Lachance D, Walsh K, Decker P, Sicotte H, Pekmezci M, Molinaro A, Rice T, Kosel M, Smirnov I et al (2014) TERT promoter mutation, IDH mutation and 1p/19q codeletion define five glioma molecular groups with specific clinical characteristics and germline variant associations. Neuro-Oncology; Abstracts from the 19th Annual Scientific Meeting of the Society for Neuro-Oncology, City
Ekstrand AJ, Sugawa N, James CD, Collins VP (1992) Amplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the N- and/or C-terminal tails. Proc Natl Acad Sci USA 89:4309–4313
Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG (2000) Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. New Eng J Med 343:1350–1354. doi:10.1056/NEJM200011093431901
Faury D, Nantel A, Dunn SE, Guiot MC, Haque T, Hauser P, Garami M, Bognar L, Hanzely Z, Liberski PP et al (2007) Molecular profiling identifies prognostic subgroups of pediatric glioblastoma and shows increased YB-1 expression in tumors. J Clin Oncol Off J Am Soc Clin Oncol 25:1196–1208. doi:10.1200/JCO.2006.07.8626
Fleming TP, Saxena A, Clark WC, Robertson JT, Oldfield EH, Aaronson SA, Ali IU (1992) Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res 52:4550–4553
Fomchenko EI, Dougherty JD, Helmy KY, Katz AM, Pietras A, Brennan C, Huse JT, Milosevic A, Holland EC (2011) Recruited cells can become transformed and overtake PDGF-induced murine gliomas in vivo during tumor progression. PLoS One 6:e20605. doi:10.1371/journal.pone.0020605
Frattini V, Trifonov V, Chan JM, Castano A, Lia M, Abate F, Keir ST, Ji AX, Zoppoli P, Niola F et al (2013) The integrated landscape of driver genomic alterations in glioblastoma. Nat Genet 45:1141–1149. doi:10.1038/ng.2734
Frederick L, Wang XY, Eley G, James CD (2000) Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res 60:1383–1387
Freije WA, Castro-Vargas FE, Fang Z, Horvath S, Cloughesy T, Liau LM, Mischel PS, Nelson SF (2004) Gene expression profiling of gliomas strongly predicts survival. Cancer Res 64:6503–6510. doi:10.1158/0008-5472.CAN-04-0452
Fuller GN, Hess KR, Rhee CH, Yung WK, Sawaya RA, Bruner JM, Zhang W (2002) Molecular classification of human diffuse gliomas by multidimensional scaling analysis of gene expression profiles parallels morphology-based classification, correlates with survival, and reveals clinically-relevant novel glioma subsets. Brain Pathol 12:108–116
Fuller GN, Rhee CH, Hess KR, Caskey LS, Wang R, Bruner JM, Yung WK, Zhang W (1999) Reactivation of insulin-like growth factor binding protein 2 expression in glioblastoma multiforme: a revelation by parallel gene expression profiling. Cancer Res 59:4228–4232
Gertler R, Rosenberg R, Stricker D, Friederichs J, Hoos A, Werner M, Ulm K, Holzmann B, Nekarda H, Siewert JR (2004) Telomere length and human telomerase reverse transcriptase expression as markers for progression and prognosis of colorectal carcinoma. J Clin Oncol Off J Am Soc Clin Oncol 22:1807–1814. doi:10.1200/JCO.2004.09.160
Godard S, Getz G, Delorenzi M, Farmer P, Kobayashi H, Desbaillets I, Nozaki M, Diserens AC, Hamou MF, Dietrich PY et al (2003) Classification of human astrocytic gliomas on the basis of gene expression: a correlated group of genes with angiogenic activity emerges as a strong predictor of subtypes. Cancer Res 63:6613–6625
Gorovets D, Kannan K, Shen R, Kastenhuber ER, Islamdoust N, Campos C, Pentsova E, Heguy A, Jhanwar SC, Mellinghoff IK et al (2012) IDH mutation and neuroglial developmental features define clinically distinct subclasses of lower grade diffuse astrocytic glioma. Clin Cancer Res Off J Am Assoc Cancer Res 18:2490–2501. doi:10.1158/1078-0432.CCR-11-2977
Griewank KG, Murali R, Schilling B, Scholz S, Sucker A, Song M, Susskind D, Grabellus F, Zimmer L, Hillen U et al (2013) TERT promoter mutations in ocular melanoma distinguish between conjunctival and uveal tumours. Br J Cancer 109:497–501. doi:10.1038/bjc.2013.312
Gross S, Cairns RA, Minden MD, Driggers EM, Bittinger MA, Jang HG, Sasaki M, Jin S, Schenkein DP, Su SM et al (2010) Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med 207:339–344. doi:10.1084/jem.20092506
Gutmann DH, Rasmussen SA, Wolkenstein P, MacCollin MM, Guha A, Inskip PD, North KN, Poyhonen M, Birch PH, Friedman JM (2002) Gliomas presenting after age 10 in individuals with neurofibromatosis type 1 (NF1). Neurology 59:759–761
Han SJ, Yang I, Tihan T, Prados MD, Parsa AT (2010) Primary gliosarcoma: key clinical and pathologic distinctions from glioblastoma with implications as a unique oncologic entity. J Neurooncol 96:313–320. doi:10.1007/s11060-009-9973-6
Haque T, Faury D, Albrecht S, Lopez-Aguilar E, Hauser P, Garami M, Hanzely Z, Bognar L, Del Maestro RF, Atkinson J et al (2007) Gene expression profiling from formalin-fixed paraffin-embedded tumors of pediatric glioblastoma. Clin Cancer Res Off J Am Assoc Cancer Res 13:6284–6292. doi:10.1158/1078-0432.CCR-07-0525
Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, Felsberg J, Wolter M, Mawrin C, Wick W et al (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 118:469–474. doi:10.1007/s00401-009-0561-9
Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, Bettegowda C, Rodriguez FJ, Eberhart CG, Hebbar S et al (2011) Altered telomeres in tumors with ATRX and DAXX mutations. Science 333:425. doi:10.1126/science.1207313
Hegi ME, Diserens AC, Godard S, Dietrich PY, Regli L, Ostermann S, Otten P, Van Melle G, de Tribolet N, Stupp R (2004) Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res Off J Am Assoc Cancer Res 10:1871–1874
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. New Eng J Med 352:997–1003. doi:10.1056/NEJMoa043331
Heimberger AB, Hlatky R, Suki D, Yang D, Weinberg J, Gilbert M, Sawaya R, Aldape K (2005) Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res Off J Am Assoc Cancer Res 11:1462–1466. doi:10.1158/1078-0432.CCR-04-1737
Henson JW, Schnitker BL, Correa KM, von Deimling A, Fassbender F, Xu HJ, Benedict WF, Yandell DW, Louis DN (1994) The retinoblastoma gene is involved in malignant progression of astrocytomas. Annals Neurol 36:714–721. doi:10.1002/ana.410360505
Hermanson M, Funa K, Hartman M, Claesson-Welsh L, Heldin CH, Westermark B, Nister M (1992) Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res 52:3213–3219
Herrlinger U, Rieger J, Koch D, Loeser S, Blaschke B, Kortmann RD, Steinbach JP, Hundsberger T, Wick W, Meyermann R et al (2006) Phase II trial of lomustine plus temozolomide chemotherapy in addition to radiotherapy in newly diagnosed glioblastoma: UKT-03. J Clin Oncol Off J Am Soc Clin Oncol 24:4412–4417. doi:10.1200/JCO.2006.06.9104
Hoadley KA, Yau C, Wolf DM, Cherniack AD, Tamborero D, Ng S, Leiserson MD, Niu B, McLellan MD, Uzunangelov V et al (2014) Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell 158:929–944. doi:10.1016/j.cell.2014.06.049
Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K et al (2013) TERT promoter mutations in familial and sporadic melanoma. Science 339:959–961. doi:10.1126/science.1230062
Hovestadt V, Remke M, Kool M, Pietsch T, Northcott PA, Fischer R, Cavalli FM, Ramaswamy V, Zapatka M, Reifenberger G et al (2013) Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathol 125:913–916. doi:10.1007/s00401-013-1126-5
Huang HS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD, Huang CM, Gill GN, Wiley HS, Cavenee WK (1997) The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem 272:2927–2935
Hurtt MR, Moossy J, Donovan-Peluso M, Locker J (1992) Amplification of epidermal growth factor receptor gene in gliomas: histopathology and prognosis. J Neuropathol Exp Neurol 51:84–90
Huse JT, Brennan C, Hambardzumyan D, Wee B, Pena J, Rouhanifard SH, Sohn-Lee C, le Sage C, Agami R, Tuschl T et al (2009) The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev 23:1327–1337. doi:10.1101/gad.1777409
Ideraabdullah FY, Vigneau S, Bartolomei MS (2008) Genomic imprinting mechanisms in mammals. Mutat Res 647:77–85. doi:10.1016/j.mrfmmm.2008.08.008
Inda MM, Bonavia R, Mukasa A, Narita Y, Sah DW, Vandenberg S, Brennan C, Johns TG, Bachoo R, Hadwiger P et al (2010) Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev 24:1731–1745. doi:10.1101/gad.1890510
International Cancer Genome C, Hudson TJ, Anderson W, Artez A, Barker AD, Bell C, Bernabe RR, Bhan MK, Calvo F, Eerola I et al (2010) International network of cancer genome projects. Nature 464:993–998. doi:10.1038/nature08987
Janne PA, Engelman JA, Johnson BE (2005) Epidermal growth factor receptor mutations in non-small-cell lung cancer: implications for treatment and tumor biology. J Clin Oncol Off J Am Soc Clin Oncol 23:3227–3234. doi:10.1200/JCO.2005.09.985
Jaros E, Perry RH, Adam L, Kelly PJ, Crawford PJ, Kalbag RM, Mendelow AD, Sengupta RP, Pearson AD (1992) Prognostic implications of p53 protein, epidermal growth factor receptor, and Ki-67 labelling in brain tumours. Br J Cancer 66:373–385
Jiao Y, Killela PJ, Reitman ZJ, Rasheed AB, Heaphy CM, de Wilde RF, Rodriguez FJ, Rosemberg S, Oba-Shinjo SM, Nagahashi Marie SK et al (2012) Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget 3:709–722
Jones C, Perryman L, Hargrave D (2012) Paediatric and adult malignant glioma: close relatives or distant cousins? Nature reviews. Clin Oncol 9:400–413. doi:10.1038/nrclinonc.2012.87
Karajannis MA, Legault G, Fisher MJ, Milla SS, Cohen KJ, Wisoff JH, Harter DH, Goldberg JD, Hochman T, Merkelson A et al (2014) Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro-oncology 16:1408–1416. doi:10.1093/neuonc/nou059
Karcher S, Steiner HH, Ahmadi R, Zoubaa S, Vasvari G, Bauer H, Unterberg A, Herold-Mende C (2006) Different angiogenic phenotypes in primary and secondary glioblastomas. Int J Cancer J Int Cancer 118:2182–2189. doi:10.1002/ijc.21648
Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA Jr, Friedman AH, Friedman H, Gallia GL, Giovanella BC et al (2013) TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA 110:6021–6026. doi:10.1073/pnas.1303607110
Kim H, Huang W, Jiang X, Pennicooke B, Park PJ, Johnson MD (2010) Integrative genome analysis reveals an oncomir/oncogene cluster regulating glioblastoma survivorship. Proc Natl Acad Sci USA 107:2183–2188. doi:10.1073/pnas.0909896107
Kim S, Dougherty ER, Shmulevich I, Hess KR, Hamilton SR, Trent JM, Fuller GN, Zhang W (2002) Identification of combination gene sets for glioma classification. Mol Cancer Ther 1:1229–1236
Kleinschmidt-DeMasters BK, Aisner DL, Birks DK, Foreman NK (2013) Epithelioid GBMs show a high percentage of BRAF V600E mutation. Am J Surg Pathol 37:685–698. doi:10.1097/PAS.0b013e31827f9c5e
Knobbe CB, Reifenberger J, Reifenberger G (2004) Mutation analysis of the Ras pathway genes NRAS, HRAS, KRAS and BRAF in glioblastomas. Acta Neuropathol 108:467–470. doi:10.1007/s00401-004-0929-9
Koelsche C, Sahm F, Capper D, Reuss D, Sturm D, Jones DT, Kool M, Northcott PA, Wiestler B, Bohmer K et al (2013) Distribution of TERT promoter mutations in pediatric and adult tumors of the nervous system. Acta Neuropathol 126:907–915. doi:10.1007/s00401-013-1195-5
Korshunov A, Ryzhova M, Hovestadt V, Bender S, Sturm D, Capper D, Meyer J, Schrimpf D, Kool M, Northcott PA et al (2015) Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol 129:669–678. doi:10.1007/s00401-015-1405-4
Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G et al (2007) Long-term survival with glioblastoma multiforme. Brain J Neurol 130:2596–2606. doi:10.1093/brain/awm204
Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Janne PA, Costa DB et al (2010) Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. New Eng J Med 363:1693–1703. doi:10.1056/NEJMoa1006448
Kwon CH, Zhao D, Chen J, Alcantara S, Li Y, Burns DK, Mason RP, Lee EY, Wu H, Parada LF (2008) Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res 68:3286–3294. doi:10.1158/0008-5472.CAN-07-6867
Labussiere M, Boisselier B, Mokhtari K, Di Stefano AL, Rahimian A, Rossetto M, Ciccarino P, Saulnier O, Paterra R, Marie Y et al (2014) Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology 83:1200–1206. doi:10.1212/WNL.0000000000000814
Lee D, Kang SY, Suh YL, Jeong JY, Lee JI, Nam DH (2012) Clinicopathologic and genomic features of gliosarcomas. J Neurooncol 107:643–650. doi:10.1007/s11060-011-0790-3
Lee JC, Vivanco I, Beroukhim R, Huang JH, Feng WL, DeBiasi RM, Yoshimoto K, King JC, Nghiemphu P, Yuza Y et al (2006) Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in the extracellular domain. PLoS Med 3:e485. doi:10.1371/journal.pmed.0030485
Lee JY, Park CK, Park SH, Wang KC, Cho BK, Kim SK (2011) MGMT promoter gene methylation in pediatric glioblastoma: analysis using MS-MLPA. Child’s nervous system: ChNS. Off J Int Soc Pediatr Neurosurg 27:1877–1883. doi:10.1007/s00381-011-1525-7
Li A, Walling J, Ahn S, Kotliarov Y, Su Q, Quezado M, Oberholtzer JC, Park J, Zenklusen JC, Fine HA (2009) Unsupervised analysis of transcriptomic profiles reveals six glioma subtypes. Cancer Res 69:2091–2099. doi:10.1158/0008-5472.CAN-08-2100
Liang Y, Diehn M, Watson N, Bollen AW, Aldape KD, Nicholas MK, Lamborn KR, Berger MS, Botstein D, Brown PO et al (2005) Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proc Natl Acad Sci USA 102:5814–5819. doi:10.1073/pnas.0402870102
Liu XY, Gerges N, Korshunov A, Sabha N, Khuong-Quang DA, Fontebasso AM, Fleming A, Hadjadj D, Schwartzentruber J, Majewski J et al (2012) Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol 124:615–625. doi:10.1007/s00401-012-1031-3
Loenarz C, Schofield CJ (2008) Expanding chemical biology of 2-oxoglutarate oxygenases. Nat Chem Biol 4:152–156. doi:10.1038/nchembio0308-152
Lokker NA, Sullivan CM, Hollenbach SJ, Israel MA, Giese NA (2002) Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res 62:3729–3735
Louis DN (1994) The p53 gene and protein in human brain tumors. J Neuropathol Exp Neurol 53:11–21
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. doi:10.1007/s00401-007-0243-4
Lu L, Zhang C, Zhu G, Irwin M, Risch H, Menato G, Mitidieri M, Katsaros D, Yu H (2011) Telomerase expression and telomere length in breast cancer and their associations with adjuvant treatment and disease outcome. Breast Cancer Res BCR 13:R56. doi:10.1186/bcr2893
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG et al (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. New Eng J Med 350:2129–2139. doi:10.1056/NEJMoa040938
MacConaill LE, Campbell CD, Kehoe SM, Bass AJ, Hatton C, Niu L, Davis M, Yao K, Hanna M, Mondal C et al (2009) Profiling critical cancer gene mutations in clinical tumor samples. PLoS One 4:e7887. doi:10.1371/journal.pone.0007887
Malmstrom A, Gronberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, Abacioglu U, Tavelin B, Lhermitte B, Hegi ME et al (2012) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 13:916–926. doi:10.1016/S1470-2045(12)70265-6
Mashiyama S, Murakami Y, Yoshimoto T, Sekiya T, Hayashi K (1991) Detection of p53 gene mutations in human brain tumors by single-strand conformation polymorphism analysis of polymerase chain reaction products. Oncogene 6:1313–1318
Masui K, Cloughesy TF, Mischel PS (2012) Review: molecular pathology in adult high-grade gliomas: from molecular diagnostics to target therapies. Neuropathol Appl Neurobiol 38:271–291. doi:10.1111/j.1365-2990.2011.01238.x
McBride SM, Perez DA, Polley MY, Vandenberg SR, Smith JS, Zheng S, Lamborn KR, Wiencke JK, Chang SM, Prados MD et al (2010) Activation of PI3 K/mTOR pathway occurs in most adult low-grade gliomas and predicts patient survival. J Neurooncol 97:33–40. doi:10.1007/s11060-009-0004-4
McGillicuddy LT, Fromm JA, Hollstein PE, Kubek S, Beroukhim R, De Raedt T, Johnson BW, Williams SM, Nghiemphu P, Liau LM et al (2009) Proteasomal and genetic inactivation of the NF1 tumor suppressor in gliomagenesis. Cancer Cell 16:44–54. doi:10.1016/j.ccr.2009.05.009
Meyer-Puttlitz B, Hayashi Y, Waha A, Rollbrocker B, Bostrom J, Wiestler OD, Louis DN, Reifenberger G, von Deimling A (1997) Molecular genetic analysis of giant cell glioblastomas. Am J Pathol 151:853–857
Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ (1998) Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci USA 95:5724–5729
Nakamura M, Yonekawa Y, Kleihues P, Ohgaki H (2001) Promoter hypermethylation of the RB1 gene in glioblastomas. Lab Investig J Tech Method Pathol 81:77–82
Narita Y, Nagane M, Mishima K, Huang HJ, Furnari FB, Cavenee WK (2002) Mutant epidermal growth factor receptor signaling down-regulates p27 through activation of the phosphatidylinositol 3-kinase/Akt pathway in glioblastomas. Cancer Res 62:6764–6769
Network TC (2013) Corrigendum: comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 494:506. doi:10.1038/nature11903
Ng HH, Bird A (1999) DNA methylation and chromatin modification. Curr Opin Genet Dev 9:158–163
Nishikawa R, Sugiyama T, Narita Y, Furnari F, Cavenee WK, Matsutani M (2004) Immunohistochemical analysis of the mutant epidermal growth factor, deltaEGFR, in glioblastoma. Brain Tumor Pathol 21:53–56
Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, Pan F, Pelloski CE, Sulman EP, Bhat KP et al (2010) Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17:510–522. doi:10.1016/j.ccr.2010.03.017
Ohgaki H, Kleihues P (2013) The definition of primary and secondary glioblastoma. Clin Cancer Res Off J Am Assoc Cancer Res 19:764–772. doi:10.1158/1078-0432.CCR-12-3002
Olson RA, Brastianos PK, Palma DA (2011) Prognostic and predictive value of epigenetic silencing of MGMT in patients with high grade gliomas: a systematic review and meta-analysis. J Neurooncol 105:325–335. doi:10.1007/s11060-011-0594-5
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J (2014) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-oncology 16(Suppl 4):1–63. doi:10.1093/neuonc/nou223
Ozawa T, Brennan CW, Wang L, Squatrito M, Sasayama T, Nakada M, Huse JT, Pedraza A, Utsuki S, Yasui Y et al (2010) PDGFRA gene rearrangements are frequent genetic events in PDGFRA-amplified glioblastomas. Genes Dev 24:2205–2218. doi:10.1101/gad.1972310
Ozawa T, Riester M, Cheng YK, Huse JT, Squatrito M, Helmy K, Charles N, Michor F, Holland EC (2014) Most human non-GCIMP glioblastoma subtypes evolve from a common proneural-like precursor glioma. Cancer Cell 26:288–300. doi:10.1016/j.ccr.2014.06.005
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812. doi:10.1126/science.1164382
Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL et al (2014) Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344:1396–1401. doi:10.1126/science.1254257
Paugh BS, Broniscer A, Qu C, Miller CP, Zhang J, Tatevossian RG, Olson JM, Geyer JR, Chi SN, da Silva NS et al (2011) Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J Clin Oncol Off J Am Soc Clin Oncol 29:3999–4006. doi:10.1200/JCO.2011.35.5677
Paugh BS, Qu C, Jones C, Liu Z, Adamowicz-Brice M, Zhang J, Bax DA, Coyle B, Barrow J, Hargrave D et al (2010) Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol Off J Am Soc Clin Oncol 28:3061–3068. doi:10.1200/JCO.2009.26.7252
Pelloski CE, Ballman KV, Furth AF, Zhang L, Lin E, Sulman EP, Bhat K, McDonald JM, Yung WK, Colman H et al (2007) Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J Clin Oncol Off J Am Soc Clin Oncol 25:2288–2294. doi:10.1200/JCO.2006.08.0705
Perry A, Aldape KD, George DH, Burger PC (2004) Small cell astrocytoma: an aggressive variant that is clinicopathologically and genetically distinct from anaplastic oligodendroglioma. Cancer 101:2318–2326. doi:10.1002/cncr.20625
Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L et al (2006) Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9:157–173. doi:10.1016/j.ccr.2006.02.019
Puget S, Philippe C, Bax DA, Job B, Varlet P, Junier MP, Andreiuolo F, Carvalho D, Reis R, Guerrini-Rousseau L et al (2012) Mesenchymal transition and PDGFRA amplification/mutation are key distinct oncogenic events in pediatric diffuse intrinsic pontine gliomas. PLoS One 7:e30313. doi:10.1371/journal.pone.0030313
Reifenberger J, Reifenberger G, Liu L, James CD, Wechsler W, Collins VP (1994) Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. Am J Pathol 145:1175–1190
Reuss DE, Sahm F, Schrimpf D, Wiestler B, Capper D, Koelsche C, Schweizer L, Korshunov A, Jones DT, Hovestadt V et al (2014) ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol. doi:10.1007/s00401-014-1370-3
Rickman DS, Bobek MP, Misek DE, Kuick R, Blaivas M, Kurnit DM, Taylor J, Hanash SM (2001) Distinctive molecular profiles of high-grade and low-grade gliomas based on oligonucleotide microarray analysis. Cancer Res 61:6885–6891
Robinson GW, Orr BA, Gajjar A (2014) Complete clinical regression of a BRAF V600E-mutant pediatric glioblastoma multiforme after BRAF inhibitor therapy. BMC Cancer 14:258. doi:10.1186/1471-2407-14-258
Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, Tsoi J, Clark O, Oldrini B, Komisopoulou E et al (2013) An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 340:626–630. doi:10.1126/science.1236062
Sahm F, Capper D, Jeibmann A, Habel A, Paulus W, Troost D, von Deimling A (2012) Addressing diffuse glioma as a systemic brain disease with single-cell analysis. Arch Neurol 69:523–526. doi:10.1001/archneurol.2011.2910
Sallinen SL, Sallinen PK, Haapasalo HK, Helin HJ, Helen PT, Schraml P, Kallioniemi OP, Kononen J (2000) Identification of differentially expressed genes in human gliomas by DNA microarray and tissue chip techniques. Cancer Res 60:6617–6622
Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, Gilbert MR, Herndon JE 2nd, McLendon RE, Mitchell DA et al (2010) Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol Off J Am Soc Clin Oncol 28:4722–4729. doi:10.1200/JCO.2010.28.6963
Sanders RP, Drissi R, Billups CA, Daw NC, Valentine MB, Dome JS (2004) Telomerase expression predicts unfavorable outcome in osteosarcoma. J Clin Oncol Off J Am Soc Clin Oncol 22:3790–3797. doi:10.1200/JCO.2004.03.043
Sandsmark DK, Zhang H, Hegedus B, Pelletier CL, Weber JD, Gutmann DH (2007) Nucleophosmin mediates mammalian target of rapamycin-dependent actin cytoskeleton dynamics and proliferation in neurofibromin-deficient astrocytes. Cancer Res 67:4790–4799. doi:10.1158/0008-5472.CAN-06-4470
Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, El Hallani S, Boisselier B, Mokhtari K, Hoang-Xuan K et al (2009) Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol Off J Am Soc Clin Oncol 27:4150–4154. doi:10.1200/JCO.2009.21.9832
Schindler G, Capper D, Meyer J, Janzarik W, Omran H, Herold-Mende C, Schmieder K, Wesseling P, Mawrin C, Hasselblatt M et al (2011) Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol 121:397–405. doi:10.1007/s00401-011-0802-6
Schlegel J, Merdes A, Stumm G, Albert FK, Forsting M, Hynes N, Kiessling M (1994) Amplification of the epidermal-growth-factor-receptor gene correlates with different growth behaviour in human glioblastoma. Int J Cancer J Int Cancer 56:72–77
Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, Menn O, Osswald M, Oezen I, Ott M et al (2014) A vaccine targeting mutant IDH1 induces antitumour immunity. Nature 512:324–327. doi:10.1038/nature13387
Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tonjes M et al (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482:226–231. doi:10.1038/nature10833
Shai R, Shi T, Kremen TJ, Horvath S, Liau LM, Cloughesy TF, Mischel PS, Nelson SF (2003) Gene expression profiling identifies molecular subtypes of gliomas. Oncogene 22:4918–4923. doi:10.1038/sj.onc.1206753
Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H, Makino K, Saya H, Hirano H, Kuratsu J et al (2003) Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res 63:6962–6970
Simon M, Hosen I, Gousias K, Rachakonda S, Heidenreich B, Gessi M, Schramm J, Hemminki K, Waha A, Kumar R (2014) TERT promoter mutations: a novel independent prognostic factor in primary glioblastomas. Neuro-oncology. doi:10.1093/neuonc/nou158
Slamon D, Pegram M (2001) Rationale for trastuzumab (Herceptin) in adjuvant breast cancer trials. Semin Oncol 28:13–19
Smith JS, Wang XY, Qian J, Hosek SM, Scheithauer BW, Jenkins RB, James CD (2000) Amplification of the platelet-derived growth factor receptor-A (PDGFRA) gene occurs in oligodendrogliomas with grade IV anaplastic features. J Neuropathol Exp Neurol 59:495–503
Snuderl M, Fazlollahi L, Le LP, Nitta M, Zhelyazkova BH, Davidson CJ, Akhavanfard S, Cahill DP, Aldape KD, Betensky RA et al (2011) Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell 20:810–817. doi:10.1016/j.ccr.2011.11.005
Srivastava A, Jain A, Jha P, Suri V, Sharma MC, Mallick S, Puri T, Gupta DK, Gupta A, Sarkar C (2010) MGMT gene promoter methylation in pediatric glioblastomas. Child’s nervous system: ChNS. Off J Int Soc Pediatr Neurosurg 26:1613–1618. doi:10.1007/s00381-010-1214-y
Stockhausen MT, Broholm H, Villingshoj M, Kirchhoff M, Gerdes T, Kristoffersen K, Kosteljanetz M, Spang-Thomsen M, Poulsen HS (2011) Maintenance of EGFR and EGFRvIII expressions in an in vivo and in vitro model of human glioblastoma multiforme. Exp Cell Res 317:1513–1526. doi:10.1016/j.yexcr.2011.04.001
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. doi:10.1016/S1470-2045(09)70025-7
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Eng J Med 352:987–996. doi:10.1056/NEJMoa043330
Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C, Pfaff E, Tonjes M, Sill M, Bender S et al (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22:425–437. doi:10.1016/j.ccr.2012.08.024
Suri V, Das P, Pathak P, Jain A, Sharma MC, Borkar SA, Suri A, Gupta D, Sarkar C (2009) Pediatric glioblastomas: a histopathological and molecular genetic study. Neuro-oncology 11:274–280. doi:10.1215/15228517-2008-092
Szerlip NJ, Pedraza A, Chakravarty D, Azim M, McGuire J, Fang Y, Ozawa T, Holland EC, Huse JT, Jhanwar S et al (2012) Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc Natl Acad Sci USA 109:3041–3046. doi:10.1073/pnas.1114033109
Tabori U, Ma J, Carter M, Zielenska M, Rutka J, Bouffet E, Bartels U, Malkin D, Hawkins C (2006) Human telomere reverse transcriptase expression predicts progression and survival in pediatric intracranial ependymoma. J Clin Oncol Off J Am Soc Clin Oncol 24:1522–1528. doi:10.1200/JCO.2005.04.2127
Tanwar MK, Gilbert MR, Holland EC (2002) Gene expression microarray analysis reveals YKL-40 to be a potential serum marker for malignant character in human glioma. Cancer Res 62:4364–4368
Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, Villano JL (2014) Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomark Prevent Pub Am Assoc Cancer Res Cosponsored Am Soc Prevent Oncol 23:1985–1996. doi:10.1158/1055-9965.EPI-14-0275
Tso CL, Freije WA, Day A, Chen Z, Merriman B, Perlina A, Lee Y, Dia EQ, Yoshimoto K, Mischel PS et al (2006) Distinct transcription profiles of primary and secondary glioblastoma subgroups. Cancer Res 66:159–167. doi:10.1158/0008-5472.CAN-05-0077
Ueki K, Ono Y, Henson JW, Efird JT, von Deimling A, Louis DN (1996) CDKN2/p16 or RB alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Res 56:150–153
van den Bent MJ, Brandes AA, Taphoorn MJ, Kros JM, Kouwenhoven MC, Delattre JY, Bernsen HJ, Frenay M, Tijssen CC, Grisold W et al (2013) Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol Off J Am Soc Clin Oncol 31:344–350. doi:10.1200/JCO.2012.43.2229
van den Bent MJ, Erdem-Eraslan L, Idbaih A, de Rooi J, Eilers PH, Spliet WG, den Dunnen WF, Tijssen C, Wesseling P, Sillevis Smitt PA et al (2013) MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic Oligodendrogliomas and Oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res Off J Am Assoc Cancer Res 19:5513–5522. doi:10.1158/1078-0432.CCR-13-1157
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP et al (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110. doi:10.1016/j.ccr.2009.12.020
Vinagre J, Almeida A, Populo H, Batista R, Lyra J, Pinto V, Coelho R, Celestino R, Prazeres H, Lima L et al (2013) Frequency of TERT promoter mutations in human cancers. Nature Commun 4:2185. doi:10.1038/ncomms3185
Wang SC, Hung MC (2009) Nuclear translocation of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clin Cancer Res Off J Am Assoc Cancer Res 15:6484–6489. doi:10.1158/1078-0432.CCR-08-2813
Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, Cross JR, Fantin VR, Hedvat CV, Perl AE et al (2010) The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17:225–234. doi:10.1016/j.ccr.2010.01.020
Warrington NM, Gianino SM, Jackson E, Goldhoff P, Garbow JR, Piwnica-Worms D, Gutmann DH, Rubin JB (2010) Cyclic AMP suppression is sufficient to induce gliomagenesis in a mouse model of neurofibromatosis-1. Cancer Res 70:5717–5727. doi:10.1158/0008-5472.CAN-09-3769
Watanabe T, Yokoo H, Yokoo M, Yonekawa Y, Kleihues P, Ohgaki H (2001) Concurrent inactivation of RB1 and TP53 pathways in anaplastic oligodendrogliomas. J Neuropathol Exp Neurol 60:1181–1189
Weller M, Felsberg J, Hartmann C, Berger H, Steinbach JP, Schramm J, Westphal M, Schackert G, Simon M, Tonn JC et al (2009) Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol Off J Am Soc Clin Oncol 27:5743–5750. doi:10.1200/JCO.2009.23.0805
Wick W, Meisner C, Hentschel B, Platten M, Schilling A, Wiestler B, Sabel MC, Koeppen S, Ketter R, Weiler M et al (2013) Prognostic or predictive value of MGMT promoter methylation in gliomas depends on IDH1 mutation. Neurology 81:1515–1522. doi:10.1212/WNL.0b013e3182a95680
Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, Nikkhah G, Papsdorf K, Steinbach JP, Sabel M et al (2012) Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol 13:707–715. doi:10.1016/S1470-2045(12)70164-X
Wiencke JK, Zheng S, Jelluma N, Tihan T, Vandenberg S, Tamguney T, Baumber R, Parsons R, Lamborn KR, Berger MS et al (2007) Methylation of the PTEN promoter defines low-grade gliomas and secondary glioblastoma. Neuro-oncology 9:271–279. doi:10.1215/15228517-2007-003
Wiestler B, Capper D, Hovestadt V, Sill M, Jones DT, Hartmann C, Felsberg J, Platten M, Feiden W, Keyvani K et al (2014) Assessing CpG island methylator phenotype, 1p/19q codeletion, and MGMT promoter methylation from epigenome-wide data in the biomarker cohort of the NOA-04 trial. Neuro-oncology. doi:10.1093/neuonc/nou138
Wiestler B, Capper D, Sill M, Jones DT, Hovestadt V, Sturm D, Koelsche C, Bertoni A, Schweizer L, Korshunov A et al (2014) Integrated DNA methylation and copy-number profiling identify three clinically and biologically relevant groups of anaplastic glioma. Acta Neuropathol 128:561–571. doi:10.1007/s00401-014-1315-x
Wong AJ, Ruppert JM, Bigner SH, Grzeschik CH, Humphrey PA, Bigner DS, Vogelstein B (1992) Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci USA 89:2965–2969
Wong KK, Tsang YT, Chang YM, Su J, Di Francesco AM, Meco D, Riccardi R, Perlaky L, Dauser RC, Adesina A et al (2006) Genome-wide allelic imbalance analysis of pediatric gliomas by single nucleotide polymorphic allele array. Cancer Res 66:11172–11178. doi:10.1158/0008-5472.CAN-06-2438
Wu G, Broniscer A, McEachron TA, Lu C, Paugh BS, Becksfort J, Qu C, Ding L, Huether R, Parker M et al (2012) Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 44:251–253. doi:10.1038/ng.1102
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ et al (2009) IDH1 and IDH2 mutations in gliomas. New Eng J Med 360:765–773. doi:10.1056/NEJMoa0808710
Zarghooni M, Bartels U, Lee E, Buczkowicz P, Morrison A, Huang A, Bouffet E, Hawkins C (2010) Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol Off J Am Soc Clin Oncol 28:1337–1344. doi:10.1200/JCO.2009.25.5463
Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, Messing A, Parada LF (2005) Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell 8:119–130. doi:10.1016/j.ccr.2005.07.004
Zhu Y, Harada T, Liu L, Lush ME, Guignard F, Harada C, Burns DK, Bajenaru ML, Gutmann DH, Parada LF (2005) Inactivation of NF1 in CNS causes increased glial progenitor proliferation and optic glioma formation. Development 132:5577–5588. doi:10.1242/dev.02162
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aldape, K., Zadeh, G., Mansouri, S. et al. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol 129, 829–848 (2015). https://doi.org/10.1007/s00401-015-1432-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-015-1432-1