Abstract
There are numerous physical, social, and psychological benefits of exercise, sport and play for youth athletes. However, dynamic activities come with a risk of injury that has yet to be abated, warranting novel therapeutics to promote injury-resistance and to keep an active lifestyle throughout the lifespan. The purpose of the present manuscript was to summarize the extant literature and potential connecting framework regarding youth brain development and neuroplasticity associated with musculoskeletal injury. This review provides the foundation for our proposed framework that utilizes the OPTIMAL (Optimizing Performance Through Intrinsic Motivation and Attention for Learning) theory of motor learning to elicit desirable biomechanical adaptations to support injury prevention (injury risk reduction), rehabilitation strategies, and exercise performance for youth physical activity and play across all facets of sport (Prevention Rehabilitation Exercise Play; PREP). We conclude that both young male and females are ripe for OPTIMAL PREP strategies that promote desirable movement mechanics by leveraging a unique time window for which their heightened state of central nervous system plasticity is capable of enhanced adaptation through novel therapeutic interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Problem: Anterior Cruciate Ligament Injuries in Youth
Sustaining an injury to the anterior cruciate ligament (ACL) can be physically, emotionally, and financially traumatic for a youth athlete, including potential premature closure to an athletic career [48] and greater long-term risk of osteoarthritis and reduced quality of life [126, 135]. Following the initial injury (i.e., primary), reports of subsequent ACL re-rupture or contralateral ACL injury (i.e., secondary) within five years are as high as 23%, with youth athletes under the age of 25 being predominantly susceptible [3, 158]. Even when loss of function is restored and secondary injury is avoided, recent evidence indicates that successful return to pre-injury level of activity following ACL reconstruction (ACLR) is lower than formerly thought; approximately 35% of individuals suffering an ACL injury fail to return to previous activity levels [107, 143, 156]. The high re-injury rate and inability to restore functional capability indicates that current standards of care including ACLR and rehabilitation may not adequately address the deficits that may have preceded and/or propagated the initial injury [28, 56, 71, 129]. Novel therapeutic methods are therefore warranted to reduce re-injury rates, increase return-to-play rates, and improve post-injury quality of activity, particularly in youth athletes who are most susceptible to primary and secondary injury [118, 132, 158].
Current rehabilitation strategies for ACL injury focus primarily on restoration of neuromuscular function through muscle strengthening and neuromuscular control exercises [68, 146]. Though multiple factors can contribute to primary and secondary ACL injury, neuromuscular function is most readily modifiable, whereas other potential deficiencies, such as bony anatomy or circadian hormones, cannot be easily altered. Likewise, the goals of neuromuscular function focused rehabilitation have traditionally included restoring bilateral symmetrical motion, introducing safer movement patterns, and avoiding positions thought to excessively strain the ACL, such as dynamic knee valgus, knee hyperextension, tibial internal rotation, or stiff legged landings exhibited by minimal hip and knee flexion angles [14, 29, 71, 142, 152]. These aims are commonly accomplished by specific exercises addressing range of motion, muscle strength, proprioception, joint stability, endurance, and functional movement [40]. However, evidence indicates that youth athletes are often unsuccessful in their attempts to safely make the transition from the clinic to the playing field [55, 61]. Specifically, rehabilitation programs can improve lower extremity movement patterns associated with injury when assessed in the lab [11, 111–114, 116], but these improved movement patterns do not readily transfer to sport [32].
A number of factors may contribute to lack of motor skill transfer, including noncompliance with rehabilitation limiting skill acquisition altogether and/or the relative contextual simplicity of clinical rehabilitation in comparison to the intense demands of a competitive sport environment (i.e., the neurocognitive challenges associated with defenders, moving balls, targets, etc.) [65, 109]. Further, some commonly used metrics, such as bilateral symmetry, may not be as important as once thought, as hop testing symmetry does not mirror quad strength symmetry and restoring bilateral hop symmetry is not always effective for reducing ACL-reinjury [67, 75, 82, 100, 157]. Conversely, quadriceps strength deficits are well documented post ACLR and may provide a related indicator of residual deficits following surgery [120]. Compounding the injured limbs relative strength deficits, the uninjured limb typically also suffers decrements in strength and power post-surgery, potentially leading to altered biomechanics and masking of limb to limb deficits [49, 117, 119]. Emerging neuromechanical evidence shows that post-injury altered biomechanics could, in part, result from unresolved alterations throughout the central nervous system (CNS) that affect both involved and uninvolved limbs following ACL injury and subsequent surgical reconstruction [81, 101, 145]. For instance, patients following ACLR exhibit differential knee-related brain activation in regions important for attention, vision, and sensorimotor integration compared to their non-injured peers [7, 8, 63], indicating aspects of normal CNS function may not be fully restored through rehabilitation. Thus, employing rehabilitation strategies that focus on addressing musculoskeletal system may restore symmetrical bilateral kinematics, but may overlook critical CNS impairments that allow for, and actually may underlie, subtle prolonged movement compensations.
The Solution: OPTIMAL PREP Strategies for Injury Resistant Movement
Recent evidence indicates that incorporating motor learning principles into rehabilitation protocols can improve landing mechanics for those recovering from ACL injury [53] and has been theorized to improve CNS function more broadly (non-specific to ACL injury) [160]. Considering that classic motor learning principles can be easily implemented through slight modifications in verbal instruction and feedback, such principles may be able to overcome the shortcomings of conventional ACL rehabilitation protocols by leveraging the brain’s capabilities to positively adapt by targeting specific neural mechanisms via its potential for neuroplasticity [30]. While the concept of applying motor learning principles to ACL injury rehabilitation and prevention have been presented in the literature [12, 51, 52, 54, 55], clinicians, coaches, instructors, and athletes do not commonly implement motor learning theory principles within their clinic, on the practice field, or during athletic competition [39, 41, 64, 77, 134, 138, 154, 162]. A recent review provided the potential neural correlates and applications for various motor learning principles to ACL rehabilitation [54], and another clearly described related aspects of motor learning for clinicians aiming to enhance adaptive neuroplasticity post-ACLR [43], but neither report discussed recommendations within the context of the OPTIMAL (Optimizing Performance Through Intrinsic Motivation and Attention for Learning) theory of motor learning [160]. Specifically, OPTIMAL theory identifies three distinct and partially independent motivational (enhanced expectancies, autonomy support) and attentional (external focus) motor learning factors/principles (i.e., “pillars”) theorized to leverage the capacity for CNS plasticity to achieve enhanced motor behavior through adaptive neuroplasticity [160]. Further, the pillars of OPTIMAL motor learning can be combined for more robust, additive effects [1, 23, 102, 103, 106, 128, 161], but a conceptual framework for application of OPTIMAL theory in the context of ACL injury management strategies in youth—inclusive of prevention, rehabilitation, and exercise performance more generally—has yet to be proposed. A better mechanistic understanding regarding the role of motor learning on the CNS may help practitioners ‘buy-in’ and implement such methods in practice. For instance, applying more targeted and motivational approaches may lead to a more confident recovery, thereby increasing patient motivation and adherence allowing for the dosage needed to restore functional ability after injury [46, 151].
The purpose of this manuscript was to summarize the extant literature regarding youth brain development, as well as neuroplasticity associated with ACL injury. Specifically, we aimed to provide a conceptual framework for how the application of the OPTIMAL theory pillars could be applied to capitalize on youth neuroplasticity and elicit desirable biomechanical adaptations to support injury prevention (specifically reduction of injury risk), optimize rehabilitation strategies, and enhance exercise performance to support youth play across all facets of sport (Prevention Rehabilitation Exercise Play; PREP). “Youth” was defined herein as ~ 6 to 25 years of age and inclusive of pre-adolescence/pubertal, adolescence/post pubertal, and early adulthood. We selected a lower bound age range of ~ 6 years old indicative of the age period in which youth generally exhibit the requisite motor competence to benefit from training/resistance/agility programs, possess sufficient level of physical and emotional maturity, and/or is ready for structured sports participation [10, 91–96, 98, 99, 105, 115]. An older bound age range of ~ 25 years old was included to be cognizant of the college-aged participants that constitute the majority of studies providing empirical data for the neuroplasticity of ACL injury [122] and OPTIMAL motor learning [160], yet still within the age window that is burdened by such musculoskeletal trauma [76, 164]. However, we emphasize the described age window should not be considered fixed or restricted based on chronological number in light of various growth/maturational factors [9, 97, 104]. In fact, neurological, developmental-related factors could potentially amplify the relative effectiveness of OPTIMAL PREP strategies in the younger cohort of our age window (pre to early-adolescence vs. late adolescence/early adulthood), but will require implementation of these programs and further investigation to clarify with certainty.
ACL Injury and Neuroplasticity in Youth: An Overlapping Time Window
The inclusive youth age window of ~ 6 to 25 is intended to overlap between periods when ACL injury prevalence and incidence are high and when youth are undergoing a state of heightened CNS plasticity. Data from the United Kingdom and Sweden show that ACL surgical repairs are most often performed in patients aged 20–29 [2, 3]. However, surgical rates in patients under 20 years old are rapidly increasing, with reports from 1997 to 2017 indicating more than a 20-fold increase [2]. The age window of surgical interventions for ACL injury is further modulated by sex. Prior to maturation, boys appear to be at greater risk of ACL injury; however, following puberty, ACL injury risk and incidence disproportionately increase in females [44, 73, 137]. Given these data, combined with secondary injury being most prevalent in youth under the age of 20 years old [158], youth may particularly stand to benefit from OPTIMAL PREP to enhance injury prevention programs for reduced risk for primary and secondary injury.
Throughout these time periods, and across the lifespan more generally, brain function and structure is malleable. However, during childhood and adolescence, many progressive and regressive functional and structural changes take place until more stable states are reached during young adulthood [148]. Specifically, longitudinal evidence from a large study of 387 subjects aged 3–27 years old, demonstrated that from early childhood to early adulthood white matter volume increases linearly (i.e., progressive change) and grey matter volume develops along a U-shaped curve (i.e., progressive and then regressive change) that peaks around age 8.5 years for females and 10.5 years for males [86]. These developmental changes result in the strengthening of connectivity between functionally-related, spatially distinct brain regions [155], as well as substantial reduction in synaptic density—otherwise known as synaptic pruning [130]—which accounts for grey matter loss during adolescence [74, 86]. Though males and females tend to follow similar trajectories of neurodevelopment, the age of peak regional grey matter when there is a switch from mostly growth to mostly synaptic pruning, is consistently 1–2 years earlier for females than males, but the rate of change is higher in males across childhood and adolescence [86]. Due to female neurodevelopmental trajectories peaking earlier than males, and physical maturational onsets earlier in females than males, researchers have tenuously suggested that the onset of puberty and sex hormones likely contribute to the earlier (younger age of onset) window for the onset of synaptic pruning [31, 45, 70].
At the individual level, the timeline for brain maturation varies; however, higher-order cognitive brain regions, such as the prefrontal cortex, generally mature after regions more important for sensorimotor control, such as the postcentral gyrus/primary somatosensory cortex [50]. Specifically, prospective longitudinal neuroimaging data (ranging from ~ 4 to ~ 21 years old) indicate that gray matter volume follows a “back-to-front” developmental trajectory as youth transition from childhood to early adulthood, such that occipital/parietal lobes develop prior to the frontal lobe [50]. These findings can be attributed to functional, evolutionary milestones necessitating the need for basic sensorimotor control before higher level (i.e., top-down) cognitive functioning. However, in the context of sports, successful top-down processing in addition to fundamental sensorimotor functioning is vital to maintain injury-resistance during play [37, 38, 150]. Though the plastic nature of the brain is maintained across the lifespan, the robust changes in brain and behavior during the younger years [131] likely support an ideal time window to apply interventions that capitalize on the increased potential to influence injury-resistant neuroplastic adaptations.
The requisite for efficient top-down processing for youth injury resistance can be seen on an athletic field where athletes must execute appropriate motor responses while navigating a physically and cognitively demanding environment. Complex sporting scenarios exemplify the importance of successful communication throughout the nervous system, as errors in higher-level processing can instantiate a traumatic injury. For instance, ACL injury events occur more readily when an athlete is cognitively distracted (e.g., an incoming ball, surrounded by defenders) [72, 83], plausibly due to the CNS failing to anticipate, prepare, and/or correct a high-risk knee position. Thus, perception–action loops, or the higher-order ability to integrate sensory information with past experiences and prepare and execute the appropriate motor response, are vital for injury resistance, warranting approaches that can provide developmental synchrony between top-down processing and sensorimotor control. One means to accomplish this may be through physical activity due to its capability to improve both neurocognitive function [5, 24, 144, 149] and sensorimotor control in adolescents [35, 110, 115]. Further, youth physical fitness is associated with grey matter and white matter microstructure profiles more similar to young adults [69]. Thus, therapeutics for youth that include physical activity may foster enhanced injury resistance by supporting development of top-down processing at earlier ages, or at least shift the maturational timeline to occur more in tandem with sensorimotor cortical maturation.
Indeed, unique training frameworks have been developed and supported for implementation in youth athletes aimed to reduce the likelihood of such injury scenarios by leveraging the beneficial effects of physical activity on neurophysiologic development [42, 44, 45, 95, 96, 110, 115, 127]. While motor coordination does develop naturally, creating optimal learning environments for physical activity and individualized motor learning strategies can further enhance automaticity of motor control and retention of motor behavior [79]. To accomplish optimal learning environments, limitations with respect to motor learning literature should also be considered. Specifically, many classic studies tuse terms such as “practice” or “acquisition” interchangeable with “training”, as they have relied on short-term interventions of one or two days and “retention/learning” assessments following rest periods as low as 20 min [139, 140] and typically not more than 24 h [78, 159, 160]. While still important contributions to the literature, the nuanced differences between motor performance and motor learning [79, 147] are not always clearly delineated, specifically as to whether a learned behavior may actually be retained for an extended time period. There is a critical need to create novel, individualized teaching and learning opportunities that promote long-term motor neurodevelopment and retention of training adaptations [6, 94, 127].
One example of such an environment is integrative neuromuscular training that incorporates general and specific strength and conditioning exercises, while also challenging neurocognitive and sensorimotor processes [110], that can reduce the risk of ACL injury [118]. Though the mechanistic influence of such programs on neurodevelopment has been primarily theoretical, the extant literature indicates that youth neuroplasticity is malleable and ripe for intervention-increased protective adaptations. The multimodal approaches combined in OPTIMAL theory may provide a pathway to enhance motor development in young athletes that increases injury-resistance across the lifespan. The recent proliferation of research into the neuroplasticity associated with ACL injury has revealed distinct alterations within the CNS (using methods including functional magnetic resonance imaging [fMRI], transcranial magnetic stimulation, etc.) (for a review see Neto et al. [122]), providing an opportunity to apply innovative techniques capable of treating both movement and CNS dysfunction simultaneously in youth, such as OPTIMAL PREP strategies. To support the subsequent neurophysiologic sections related to ACL injury, Fig. 1 is provided as general reference regarding the anatomy of the CNS related to neuromuscular control.
General overview of key central nervous system components involved in neuromuscular/sensorimotor control. Though nearly all brain regions play some role in human movement depending on various constraints, we color-coded six brain regions particularly important for lower extremity, closed kinetic chain sensorimotor control (Grooms et al. [59]). In light of unique, individual anatomical structure, we determined the location of each brain region by using standard probabilistic atlases of the human brain with various thresholds to support presentation. The blue line represents the corticospinal tract and efferent information where it innervates with the musculature, whereas the teal line represents afferent information traveling from the musculature through the dorsal column and into the brain
Neuroplasticity and ACL Injury
One factor driving the novel investigation of neuroplasticity and ACL injury stems from data indicates that these incidents typically occur in dynamic environments that simultaneously challenge multiple neural processing demands (e.g., integration of vision and proprioception inputs with cognitive decision-making when an athlete attempts to navigate through two defenders) [83, 84]. Indirect evidence of CNS dysfunction such as neurocognitive measures of reaction time and memory [150] indicates a potential predisposition for ACL injury that is unique from the classic neuromuscular [57] or biomechanical measures associated with ACL injury risk [71]. Further, emergent data of direct CNS dysfunction related to ACL injury (specifically within the brain and spinal cord) has proliferated and generally fall into one of three categories in order of most empirical evidence: (1) CNS alterations following ACLR (2) CNS alterations that are associated with high ACL injury-risk biomechanics (e.g., aberrant frontal plane biomechanics) and (3) CNS dysfunction identified prior to future ACL injury. While a scoping review of these three topics are outside the aims of this report (see Neto et al. [122] for such a review), we briefly describe each below to provide the foundation that supports the application of OPTIMAL PREP training strategies [34, 36].
Central Nervous System Alterations Following ACLR
Though a ligamentous injury such as an ACL rupture does not directly insult the CNS, disrupted afferent signaling from the damaged/reconstructed ligament can propagate alterations in spinal and supraspinal function.[121]. One technique capable of quantifying supraspinal dysfunction is transcranial magnetic stimulation (TMS), which has been used in patients following ACLR [66, 87–89, 101, 123, 133, 163]. In brief, TMS applies magnetic fields to stimulate brain nerve cells (typically over the primary motor cortex to produce a musculature response at rest, i.e., resting motor threshold) or during an activity, i.e., active motor threshold) quantified with electromyography. In turn, these techniques can estimate the excitability of intracortical and corticospinal neurons that innervate specific musculature [141]. Regarding ACLR, the primary muscle group investigated is the quadriceps via numerous TMS techniques including single- and paired-pulse TMS and demonstrating altered intracortical and corticospinal excitability after injury compared to uninjured controls [87, 123, 163]. Further, elevated intracortical inhibition and depressed corticospinal excitability in patients following ACLR has been associated with reduced quadriceps voluntary activation [88, 101, 133], yet are limited by their failure to examine more widespread brain dysfunction beyond the primary motor cortex.
As cortical activity reflects a balance between inhibitory and excitatory circuits [22], electroencephalography (EEG) provides a means to supplement TMS-driven responses by measuring synaptic electrical activity throughout the cortex. Like TMS, there are numerous methods (e.g., at rest, during isometric muscular contractions, peripheral nerve stimulation) and techniques including somatosensory-evoked potentials and spectral analyses that have successfully demonstrated altered CNS function following ACL injury [7, 8, 25, 85, 108, 124, 125, 153]. For instance, by using EEG and asking patients following ACLR to complete joint position and force reproduction tasks, researchers have identified alterations in frontal theta and parietal alpha-2 frequency bands compared to the unaffected contralateral limb and/or controls [7, 8], potentially indicating a less efficient allocation of CNS resources towards somatosensory and attentional processing. Though these findings supplement those from TMS by showing CNS dysfunction that extends beyond the primary motor cortex, EEG is limited by recordings of superficial brain activity that precludes insight into potential subcortical functioning that is critical for motor control, such as the basal ganglia shown in Fig. 1.
fMRI and MRI allow for the measure of cortical and subcortical brain structure and function with higher spatial resolution relative to other described methods. Like the aforementioned instrumentation, numerous methods and techniques are possible by means of fMRI and MRI. These include and are not limited to studying brain function at rest and during movement via the blood oxygen level dependent signal, and brain structure at rest as determined by relative cortical thickness and white matter connectivity, all of which can be analyzed over the whole brain or isolated to regions of interest. With respect to ACLR, task-based fMRI paradigms of unilateral knee flexion and extension movements have identified regional differences in blood oxygen level dependent signal activation/connectivity between those with ACLR and/or ACL deficient and controls [26, 62, 63, 80, 89]. Results from these studies have led researchers to hypothesize a framework whereby, following an ACL injury, patients switch from a sensory-motor to a visual-motor brain activation strategy for knee motor control [58, 63]. Supported by findings of increased activity in regions important for vision relative to sensorimotor control, these findings indicate that patients following ACLR rely more heavily on visual-proprioceptive processing following injury [21, 63], potentially due to internally-focused, visually guided and largely feedforward rehabilitation strategies with focus of attention on the joint or surrounding musculature. Recently, MRI-derived methods such as diffusion weighted imaging have even been combined with TMS to reveal neurostructural alterations for patients following ACLR, with less primary motor cortex excitability and alterations to the anisotropic/diffusion properties of the corticospinal tract observed [90].
CNS Alterations that are Associated with High ACL Injury-Risk Biomechanics
Emergent evidence has shown that athletes with poor neuromuscular control that have not experienced an injury or may have yet to experience an injury as signified by high external peak knee abduction moments during a drop vertical jump, eliciting resting-state electrocortical activity that may signify the CNS cannot effectively transition from rest to move states [19]. Technological improvements have advanced this line of research from resting to active states by developing MRI-compatible motion capture systems that can now be used to capture lower extremity biomechanics concurrent with CNS function derived from fMRI [4, 15]. Preliminary findings from our laboratory have revealaed increased out-of-plane (frontal) knee angle associated with altered brain activity in regions important for attention, sensorimotor control, and sensorimotor integration while no similar in-plane (sagittal) neural correlates were identified [33]. Further confirming the CNS linkages to aberrant movement, preliminary findings demonstrated that overlapping, aberrant movement-associated increases in brain activity within the lingual gyrus were observed between two separate cohorts of youth soccer athletes completing a simulated bilateral leg press during fMRI [27]. Aberrant biomechanics were identified for one cohort by increased bilateral frontal plane knee loads during a drop vertical jump, and increased frontal plane motion during the actual fMRI task was identified using MRI-compatible motion analyses for the second cohort [27, 60]. Cumulatively, the emergent literature with simultaneous measurement of CNS function with lower extremity biomechanics indicate distinct neural linkages associated with high ACL injury risk biomechanics.
Central Nervous System Dysfunction Prior to ACL Injury
Variations of the aforementioned methods and techniques have also emerged as relevant approaches to investigate CNS alterations prospective to the ACL injury event. To our knowledge, only two preliminary studies have prospectively used such direct measures—specifically resting-state fMRI—to evaluate CNS dysfunction prior to an ACL injury [37, 38]. Both studies, one with high school boys’ football and one with high school girls’ soccer, revealed reduced functional connectivity between regions important for sensorimotor control—some of which that are shown in Fig. 1—in athletes who went on to injury, compared to their uninjured peers. It was surmised that reduced functional connectivity, defined as the temporal correlation of the residual BOLD signal between spatially distinct brain regions [13], represented a potential predisposition for ACL injury, possibly reflecting poor sensorimotor CNS function that impeded neuromuscular control. The prospective findings relating CNS function with aberrant biomechanics indicate that CNS function should be considered along with biomechanical and muscular function for injury prevention strategies [35, 61, 136].
Collectively, data from TMS, EEG, and fMRI/MRI related to ACL injury have revealed distinct alterations within the CNS that could be targeted through neural mechanistic approaches, particularly in youth who are ripe for intervention-increased protective brain adaptations. Specifically, the robust behavioral literature supporting the OPTIMAL theory “pillars” of motor learning, as well as the theorized neural mechanisms of each principle, could potentially be used to uniquely target the neuroplasticity surrounding ACL injury [36]. Further literature provides more tangible examples of how OPTIMAL PREP strategies, such as “augmented” neuromuscular training [16–18] could be used to promote injury resistance [34].
Broad Application of OPTIMAL PREP Strategies in Youth
To enhance the clinical applicability, we have focused the current commentary on the neuroscience of ACL injury. Despite the noted sex- and age-related factors of neurodevelopment and ACL injury more broadly, OPTIMAL PREP strategies are designed to be agnostic to sex. However, OPTIMAL PREP strategies may demonstrate amplified effectiveness if implemented at the earliest ages, but future research and supporting data is warranted to delineate and optimize the best timing to apply these interventional strategies. Further, we emphasize that the provided framework is also applicable for enhancing motor control for injury prevention, performance enhancement, and management of other youth musculoskeletal conditions such as patellofemoral pain or juvenile fibromyalgia. The opportunity to apply OPTIMAL PREP training strategies across the spectrum of youth populations is grounded in their heightened CNS plasticity more broadly [50, 131, 148, 155], making them uniquely suited for motor learning adaptations that can reduce injury risk and enhance injury recovery. For instance, children with developmental coordination disorder—a condition characterized by maladaptive motor development [47]—who completed a trail-tracing test elicited an altered brain activation profile [165] that shared similarities to patients following ACLR completing knee flexion and extension movements [63]. Specifically, both patient populations demonstrated increased activity in various occipital-parietal regions compared to controls when completing their respective sensorimotor-based tasks [63, 165]. OPTIMAL PREP strategies provide limitless potential for practitioners to apply techniques that target the aforementioned neural alterations [34]. For example, a clinician could aim to reweight their patients’ brain activity in favor of sensorimotor activation using an external focus of attention [36].
Further, these strategies are relevant to motor control more generally and are designed to be agnostic to ACL injury. Specifically, the targeted population for OPTIMAL PREP strategies does not need to share an “overlapping” neural dysfunction to that reviewed for ACL injury, or even have measurable CNS dysfunction to begin with. For instance, a “visual-motor” brain activation strategy for knee motor control is present following ACLR [58, 63], but is not required for OPTIMAL PREP strategies to potentially be effective. Normal age-related structural development from childhood to adolescence of white matter fiber direction and gray matter volume important for sensorimotor control are intricately linked, potentially due to synergism between white matter expansion and gray matter contraction by means of myelination and synaptic/dendritic maturational processes [20]. The motivational pillars of OPTIMAL theory that include autonomy support and enhanced expectancies may be uniquely suited to exploit these neurodevelopmental processes as part of PREP strategies by releasing dopamine to modulate pre- to-post-synaptic transmission between neurons for enhanced sensorimotor control [36, 160]. Please see Fig. 2 that summarizes our theoretical framework to apply OPTIMAL PREP strategies for injury resistance by capitalizing on their heightened CNS plasticity.
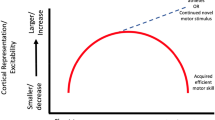
Potential for OPTIMAL PREP strategies to accelerate injury resistance in youth: capitalizing on a unique time window of heightened central nervous system plasticity (~ 6 to 25 years of age). The line chart illustrates potential responsiveness to ACL injury risk reduction and neuromuscular training interventions across age/maturational status. *Responsiveness is operationally defined as subsequent risk for an ACL injury following completion of such a program; y-axis is approximate, but conceptually derived from relevant data in females that did not incorporate OPTIMAL PREP strategies [132]. Purple lines represent potential responsiveness if intervention is implemented in adulthood (light purple), and the potential for relatively enhanced responsiveness if OPTIMAL PREP strategies are additively incorporated (dark purple). Green lines represent responsiveness if intervention is implemented in adolescence (light green), and the potential for amplified responsiveness if OPTIMAL PREP strategies are additively incorporated (dark green). Brain images with yellow lines depict heightened synaptic pruning [130], and progressively increased blue brain shading represents the trajectory of gray matter maturation from parietal-occipital to frontal lobes [50]. †Central nervous system plasticity is generally considered most robust and responsive during “youth” versus middle and late adulthood. However, the immense variability of neuroplastic changes (e.g., cellular, structural, functional; often context- or individual-specific) require future investigation within the context of OPTIMAL PREP application before more defined age windows can be clarified
Summary and Future Directions
We have summarized the extant literature regarding brain development, neuroplasticity, and ACL injury within the context of a youth athlete. CNS linkages between aberrant movement and ACL injury, combined with youth neurodevelopment more broadly, indicate a unique window to apply OPTIMAL PREP strategies to achieve injury-resistance during play. Specifically, the positive brain adaptations we anticipate in response to OPTIMAL PREP strategies are designed to be applicable to a variety of populations throughout the formative developmental years for those with and without movement disorders.
References
Abdollahipour R, Valtr L, Wulf G. Optimizing bowling performance. J Mot Learn Dev. 2020;8(2):233–44. https://doi.org/10.1123/jmld.2019-0017.
Abram SG, Price AJ, Judge A, Beard DJ. Anterior cruciate ligament (ACL) reconstruction and meniscal repair rates have both increased in the past 20 years in England: hospital statistics from 1997 to 2017. Br J Sports Med. 2020;54(5):286–91. https://doi.org/10.1136/bjsports-2018-100195.
Ahldén M, Samuelsson K, Sernert N, Forssblad M, Karlsson J, Kartus J. The Swedish National Anterior Cruciate Ligament Register: a report on baseline variables and outcomes of surgery for almost 18,000 patients. Am J Sports Med. 2012;40(10):2230–5. https://doi.org/10.1177/0363546512457348.
Anand M, Diekfuss JA, Bonnette S, Hurn M, Short I, Grooms DR, Myer GD. Validity assessment of a single camera MRI-compatible motion capture system for use with lower extremity neuroimaging paradigms. Int J Sports Phys Ther. 2020. (in press).
Ardoy DN, Fernandez-Rodriguez JM, Jimenez-Pavon D, Castillo R, Ruiz JR, Ortega FB. A physical education trial improves adolescents' cognitive performance and academic achievement: the EDUFIT study. Scand J Med Sci Sports. 2014;24(1):e52–61. https://doi.org/10.1111/sms.12093.
Balyi I, Hamilton A. Long-Term Athlete Development: Trainability in children and adolescents. Windows of opportunity. Optimal trainability. Victoria, BC: National Coaching Institute British Columbia & Advanced Training and Performance Ltd; 2004.
Baumeister J, Reinecke K, Schubert M, Weib M. Altered electrocortical brain activity after ACL reconstruction during force control. J Orthop Res. 2011;29(9):1383–9. https://doi.org/10.1002/jor.21380.
Baumeister J, Reinecke K, Weiss M. Changed cortical activity after anterior cruciate ligament reconstruction in a joint position paradigm: an EEG study. Scand J Med Sci Sports. 2008;18(4):473–84. https://doi.org/10.1111/j.1600-0838.2007.00702.x.
Baxter-Jones AD, Eisenmann JC, Sherar LB. Controlling for maturation in pediatric exercise science. Pediatr Exerc Sci. 2005;17(1):18–30.
Behm DG, Faigenbaum AD, Falk B, Klentrou P. Canadian Society for Exercise Physiology position paper: resistance training in children and adolescents. Appl Physiol Nutr Metab. 2008;33(3):547–61. https://doi.org/10.1139/h08-020.
Bell DR, Oates DC, Clark MA, Padua DA. Two-and 3-dimensional knee valgus are reduced after an exercise intervention in young adults with demonstrable valgus during squatting. J Athl Train. 2013;48(4):442–9. https://doi.org/10.4085/1062-6050-48.3.16.
Benjaminse A, Gokeler A, Dowling AV, Faigenbaum A, Ford KR, Hewett TE, Onate JA, Otten B, Myer GD. Optimization of the anterior cruciate ligament injury prevention paradigm: novel feedback techniques to enhance motor learning and reduce injury risk. J Orthop Sports Phys Ther. 2015;45(3):170–82. https://doi.org/10.2519/jospt.2015.4986.
Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn Reson Med. 1995;34(4):537–41. https://doi.org/10.1002/mrm.1910340409.
Boden BP, Dean GS, Feagin JA, Garrett WE. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–8.
Bonnette S, Anand M, Barber KD, DiCesare CA, Diekfuss JA, Grooms DR, Kiefer AW, Kitchen K, Reddington D, Riehm C, Riley MA, Schille A, Shafer J, Thomas S, Myer GD. The future of ACL prevention and rehabilitation: Integrating technology to optimize personalized medicine. Aspetar Sports Med J. 2020;9:72–7.
Bonnette S, DiCesare CA, Diekfuss JA, Grooms DR, MacPherson RP, Riley MA, Myer GD. Advancing anterior cruciate ligament injury prevention using real-time biofeedback for amplified sensorimotor integration. J Athl Train. 2019;54(9):985–6. https://doi.org/10.4085/1062-6050-54.083.
Bonnette S, DiCesare CA, Kiefer AW, Riley MA, Barber-Foss KD, Thomas S, Diekfuss JA, Myer GD. A technical report on the development of a real-time visual biofeedback system to optimize motor learning and movement deficit correction. J Sports Sci Med. 2020;19(1):84–94.
Bonnette S, DiCesare CA, Kiefer AW, Riley MA, Barber Foss KD, Thomas S, Kitchen K, Diekfuss JA, Myer GD. Injury risk factors integrated into self-guided real-time biofeedback improves high-risk biomechanics. J Sport Rehab. 2019;28(8):831–9. https://doi.org/10.1123/jsr.2017-0391.
Bonnette S, Diekfuss JA, Grooms DR, Kiefer AW, Riley MA, Riehm C, Moore C, Barber Foss KD, DiCesare CA, Baumeister J, Myer GD. Electrocortical dynamics differentiate athletes exhibiting low- and high-ACL injury risk biomechanics. Psychophysiology. 2020. https://doi.org/10.1111/psyp.13530.
Bray S, Krongold M, Cooper C, Lebel C. Synergistic effects of age on patterns of white and gray matter volume across childhood and adolescence. eNeuro. 2015;2(4):e0003–15. https://doi.org/10.1523/ENEURO.0003-15.2015.
Calvert GA, Hansen PC, Iversen SD, Brammer MJ. Detection of audio-visual integration sites in humans by application of electrophysiological criteria to the BOLD effect. Neuroimage. 2001;14(2):427–38. https://doi.org/10.1006/nimg.2001.0812.
Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154(1):1–10. https://doi.org/10.1007/s00221-003-1684-1.
Chua LK, Wulf G, Lewthwaite R. Onward and upward: optimizing motor performance. Hum Mov Sci. 2018;60:107–14. https://doi.org/10.1016/j.humov.2018.05.006.
Costigan SA, Eather N, Plotnikoff RC, Hillman CH, Lubans DR. High-intensity interval training for cognitive and mental health in adolescents. Med Sci Sports Exerc. 2016;48(10):1985–93. https://doi.org/10.1249/mss.0000000000000993.
Courtney C, Rine RM, Kroll P. Central somatosensory changes and altered muscle synergies in subjects with anterior cruciate ligament deficiency. Gait Posture. 2005;22(1):69–74. https://doi.org/10.1016/j.gaitpost.2004.07.002.
Criss CR, Onate JA, Grooms DR. Neural activity for hip-knee control in those with anterior cruciate ligament reconstruction: a task-based functional connectivity analysis. Neurosci Lett. 2020;730:134985. https://doi.org/10.1016/j.neulet.2020.134985.
Criss CR, Grooms DR, Diekfuss JA, Ellis JD, Thomas S, DiCesare CA, Bonnette S, Yuan W, Dudley JA, Schneider DK, Berz K, Myer GD. Simulated landing neural correlates of anterior cruciate ligament injury risk biomechanics. J Athl Train. 2019;54(9):989–1003. https://doi.org/10.4085/1062-6050-54.081.
Culvenor AG, Barton CJ. ACL injuries: the secret probably lies in optimising rehabilitation. Br J Sports Med. 2018;52(22):1416–8. https://doi.org/10.1136/bjsports-2017-098872.
Dai B, Mao M, Garrett WE, Yu B. Biomechanical characteristics of an anterior cruciate ligament injury in javelin throwing. J Sport Health Sci. 2015;4(4):333–40. https://doi.org/10.1016/j.jshs.2015.07.004.
Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72(3):443–54. https://doi.org/10.1016/j.neuron.2011.10.008.
de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev. 2006;82(4):257–66. https://doi.org/10.1016/j.earlhumdev.2005.10.013.
DiCesare CA, Kiefer AW, Bonnette SH, Myer GD. High-risk lower-extremity biomechanics evaluated in simulated soccer-specific virtual environments. J Sport Rehabil. 2019;29(3):294-300. https://doi.org/10.1123/jsr.2018-0237.
Diekfuss JA, Anand M, Grooms DR, Slutsky-Ganesh AB, Bonnette, S, Barber Foss KD, DiCesare CA, Hunnicutt JL, MyerGD. Novel brain mechanisms regulating anterior cruciate ligament injury risk biomechanics utilizing a motion analysis system integrated with functional magnetic resonance imaging during lower extremity movement. National Athletic Trainers’ Association Clinical Symposia & Athletic Training Exposition. 2020, July 13–16 (Virtual due to COVID-19).
Diekfuss JA, Bonnette S, Hogg JA, Riehm C, Grooms DR, Singh H, Anand M, Slutsky AB, Wilkerson G, Myer GD. Practical training strategies to apply neuro-mechanistic motor learning principles to facilitate adaptations towards injury-resistant movement in youth. J Sci Sport Exerc. 2020. https://doi.org/10.1007/s42978-020-00083-0.
Diekfuss JA, Grooms DR, Bonnette S, DiCesare CA, Thomas S, MacPherson RP, Ellis JD, Kiefer AW, Riley MA, Schneider DK, Gadd B, Kitchen K, Barber Foss KD, Dudley JA, Yuan W, Myer GD. Real-time biofeedback integrated into neuromuscular training reduces high-risk knee biomechanics and increases functional brain connectivity: a preliminary longitudinal investigation. Psychophysiology. 2020. https://doi.org/10.1111/psyp.13545.
Diekfuss JA, Grooms DR, Hogg JA, Singh H, Slutsky AB, Bonnette S, Riehm C, Anand M, Nissen KS, Wilkerson G, Myer GD. Targeted application of motor learning theory to leverage youth neuroplasticity for enhanced injury-resistance and exercise performance: OPTIMAL PREP. J Sci Sport Exerc. 2020. https://doi.org/10.1007/s42978-020-00085-y.
Diekfuss JA, Grooms DR, Nissen KS, Schneider DK, Foss KDB, Thomas S, Bonnette S, Dudley JA, Yuan W, Reddington DL, Ellis JD, Leach J, Gordon M, Lindsey C, Rushford K, Shafer C, Myer GD. Alterations in knee sensorimotor brain functional connectivity contributes to ACL injury in male high-school football players: a prospective neuroimaging analysis. Braz J Phys Ther. 2020,24(5):415–23. https://doi.org/10.1016/j.bjpt.2019.07.004.
Diekfuss JA, Grooms DR, Yuan W, Dudley J, Barber Foss KD, Thomas S, Ellis JD, Schneider DK, Leach J, Bonnette S, Myer GD. Does brain functional connectivity contribute to musculoskeletal injury? A preliminary prospective analysis of a neural biomarker of ACL injury risk. J Sci Med Sport. 2019;22(2):169–74. https://doi.org/10.1016/j.jsams.2018.07.004.
Diekfuss JA, Raisbeck LD. Focus of attention and instructional feedback from NCAA division 1 collegiate coaches. J Mot Learn Dev. 2016;4(2):262–73. https://doi.org/10.1123/jmld.2015-0026.
Dragicevic-Cvjetkovic D, Jandric S, Bijeljac S, Palija S, Manojlovic S, Talic G. The effects of rehabilitation protocol on functional recovery after anterior cruciate ligament reconstruction. Med Arch. 2014;68(5):350. https://doi.org/10.5455/medarh.2014.68.350-352.
Durham K, Van Vliet PM, Badger F, Sackley C. Use of information feedback and attentional focus of feedback in treating the person with a hemiplegic arm. Physiother Res Int. 2009;14(2):77–90. https://doi.org/10.1002/pri.431.
Faigenbaum AD, Lloyd RS, Myer GD. Youth resistance training: past practices, new perspectives, and future directions. Pediatr Exerc Sci. 2013;25(4):591–604. https://doi.org/10.1123/pes.25.4.591.
Faltus J, Criss CR, Grooms DR. Shifting focus: a clinician's guide to understanding neuroplasticity for anterior cruciate ligament rehabilitation. Curr Sports Med Rep. 2020;19(2):76–83. https://doi.org/10.1249/jsr.0000000000000688.
Fayad LM, Parellada JA, Parker L, Schweitzer ME. MR imaging of anterior cruciate ligament tears: is there a gender gap? Skelet Radiol. 2003;32(11):639–46. https://doi.org/10.1123/pes.25.4.591.
Ford P, De Ste Croix M, Lloyd R, Meyers R, Moosavi M, Oliver J, Till K, Williams C. The long-term athlete development model: physiological evidence and application. J Sports Sci. 2011;29(4):389–402. https://doi.org/10.1080/02640414.2010.536849.
Frosch K, Habermann F, Fuchs M, Michel A, Junge R, Schmidtmann U, Stürmer K. Is prolonged ambulatory physical therapy after anterior cruciate ligament-plasty indicated? Comparison of costs and benefits. Der Unfallchirurg. 2001;104(6):513–8. https://doi.org/10.1007/s001130170114.
Geuze RH. Postural control in children with developmental coordination disorder. Neural Plast. 2005;12(2–3):183–96. https://doi.org/10.1155/NP.2005.183.
Giugliano DN, Solomon JL. ACL tears in female athletes. Phys Med Rehabil Clin N Am. 2007;18(3):417–38. https://doi.org/10.1016/j.pmr.2007.05.002.
Goerger BM, Marshall SW, Beutler AI, Blackburn JT, Wilckens JH, Padua DA. Anterior cruciate ligament injury alters preinjury lower extremity biomechanics in the injured and uninjured leg: the JUMP-ACL study. Br J Sports Med. 2015;49(3):188–95. https://doi.org/10.1136/bjsports-2013-092982.
Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101(21):8174. https://doi.org/10.1073/pnas.0402680101.
Gokeler A, Benjaminse A, Hewett TE, Paterno MV, Ford KR, Otten E, Myer GDD. Feedback techniques to target functional deficits following anterior cruciate ligament reconstruction: implications for motor control and reduction of second injury risk. Sports Med. 2013;43(11):1065–74. https://doi.org/10.1007/s40279-013-0095-0.
Gokeler A, Benjaminse A, Seil R, Kerkhoffs G, Verhagen E. Using principles of motor learning to enhance ACL injury prevention programs. Sports Orthop Traumatol. 2018;34(1):23–30. https://doi.org/10.1007/s40279-019-01058-0.
Gokeler A, Benjaminse A, Welling W, Alferink M, Eppinga P, Otten B. The effects of attentional focus on jump performance and knee joint kinematics in patients after ACL reconstruction. Phys Ther Sport. 2015;16(2):114–20. https://doi.org/10.1016/j.ptsp.2014.06.002.
Gokeler A, Neuhaus D, Benjaminse A, Grooms DR, Baumeister J. Principles of motor learning to support neuroplasticity after ACL injury: implications for optimizing performance and reducing risk of second ACL injury. Sports Med. 2019;49:853–65. https://doi.org/10.1007/s40279-019-01058-0.
Gokeler A, Seil R, Kerkhoffs G, Verhagen E. A novel approach to enhance ACL injury prevention programs. J Exp Orthop. 2018;5(1):22. https://doi.org/10.1186/s40634-018-0137-5.
Grindem H, Arundale AJ, Ardern CL. Alarming underutilisation of rehabilitation in athletes with anterior cruciate ligament reconstruction: four ways to change the game. Br J Sports Med. 2018;52(18):1162–3. https://doi.org/10.1136/bjsports-2017-098746.
Grindstaff TL, Jackson KR, Garrison JC, Diduch DR, Ingersoll CD. Decreased quadriceps activation measured hours prior to a noncontact anterior cruciate ligament tear. J Orthop Sports Phys Ther. 2008;38(8):502–7. https://doi.org/10.2519/jospt.2008.2761.
Grooms D, Appelbaum G, Onate J. Neuroplasticity following anterior cruciate ligament injury: a framework for visual-motor training approaches in rehabilitation. J Orthop Sports Phys Ther. 2015;45(5):381–93. https://doi.org/10.2519/jospt.2015.5549.
Grooms DR, Diekfuss JA, Ellis JD, Yuan W, Dudley JA, Barber Foss KD, Thomas S, Altaye M, Haas L, Williams B, Lanier JM, Bridgewater K, Myer GD. A novel approach to evaluate brain activation for lower extremity motor control. J Neuroimaging. 2019;29(5):580–8. https://doi.org/10.1111/jon.12645.
Grooms DR, Diekfuss JA, Ellis JD, Thomas S, DiCesare CA, Bonnette S, Yuan W, Dudley JA, Schneider DK, Berz K, Riley MA, Criss CR, Myer GD. Sensorimotor neural correlates of anterior cruciate ligament injury risk biomechanics. 2019; Abstracts: ACL Research Retreat VIII, Greensboro, NC, March 14-16, 2019, national conference, podium presentation. https://doi.org/10.4085/1062-6050-54.081.
Grooms DR, Kiefer AW, Riley MA, Ellis JD, Thomas S, Kitchen K, DiCesare CA, Bonnette S, Gadd B, Barber Foss KD, Yuan W, Silva P, Galloway R, Diekfuss JA, Leach J, Berz K, Myer GD. Brain-behavior mechanisms for the transfer of neuromuscular training adaptions to simulated sport: initial findings from the train the brain project. J Sport Rehabil. 2018;27(5):1–5. https://doi.org/10.1123/jsr.2017-0241.
Grooms DR, Page S, Onate JA. Brain activation for knee movement measured days before second anterior cruciate ligament injury: neuroimaging in musculoskeletal medicine. J Athl Train. 2015;50(10):1005–10. https://doi.org/10.4085/1062-6050-50-10-02.
Grooms DR, Page SJ, Nichols-Larsen DS, Chaudhari AM, White SE, Onate JA. Neuroplasticity associated with anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2017;47(3):180–9. https://doi.org/10.2519/jospt.2017.7003.
Halperin I, Chapman DW, Martin DT, Abbiss C, Wulf G. Coaching cues in amateur boxing: an analysis of ringside feedback provided between rounds of competition. Psychol Sport Exerc. 2016;25:44–50. https://doi.org/10.1016/j.psychsport.2016.04.003.
Herman DC, Barth JT. Drop-jump landing varies with baseline neurocognition: implications for anterior cruciate ligament injury risk and prevention. Am J Sports Med. 2016;44(9):2347–53. https://doi.org/10.1177/0363546516657338.
Héroux ME, Tremblay F. Corticomotor excitability associated with unilateral knee dysfunction secondary to anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):823–33. https://doi.org/10.1007/s00167-006-0063-4.
Herrington L, Ghulam H, Comfort P. Quadriceps strength and functional performance after anterior cruciate ligament reconstruction in professional soccer players at time of return to sport. J Strength Cond Res. 2018. https://doi.org/10.1519/JSC.0000000000002749.
Herrington L, Myer G, Horsley I. Task based rehabilitation protocol for elite athletes following anterior cruciate ligament reconstruction: a clinical commentary. Phys Ther Sport. 2013;14(4):188–98. https://doi.org/10.1016/j.ptsp.2013.08.001.
Herting MM, Chu X. Exercise, cognition, and the adolescent brain. Birth Defects Res. 2017;109(20):1672–9. https://doi.org/10.1002/bdr2.1178.
Herting MM, Gautam P, Spielberg JM, Dahl RE, Sowell ER. A longitudinal study: changes in cortical thickness and surface area during pubertal maturation. PLoS One. 2015;10(3):e0119774–e01197740119774. https://doi.org/10.1371/journal.pone.0119774.
Hewett TE, Myer GDD, Ford KR, Heidt RS Jr, Colosimo AJ, McLean SG, van den Bogert AJ, Paterno MV, Succop P. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501. https://doi.org/10.1177/0363546504269591.
Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43(6):417–22. https://doi.org/10.1136/bjsm.2009.059162.
Huston LJ, Greenfield MLV, Wojtys EM. Anterior cruciate ligament injuries in the female athlete: potential risk factors. Clin Orthop Relat Res. 2000;372(372):50–63. https://doi.org/10.1097/00003086-200003000-00007.
Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. https://doi.org/10.1016/0006-8993(79)90349-4.
Ithurburn MP, Longfellow MA, Thomas S, Paterno MV, Schmitt LC. Knee function, strength, and resumption of preinjury sports participation in young athletes following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2019;49(3):145–53. https://doi.org/10.2519/jospt.2019.8624.
Janssen K, Orchard J, Driscoll T, van Mechelen W. High incidence and costs for anterior cruciate ligament reconstructions performed in Australia from 2003–2004 to 2007–2008: time for an anterior cruciate ligament register by Scandinavian model? Scand J Med Sci Sports. 2012;22(4):495–501. https://doi.org/10.1111/j.1600-0838.2010.01253.x.
Johnson L, Burridge JH, Demain SH. Internal and external focus of attention during gait re-education: an observational study of physical therapist practice in stroke rehabilitation. Phys Ther. 2013;93(7):957–66. https://doi.org/10.2522/ptj.20120300.
Kal E, Prosée R, Winters M, van der Kamp J. Does implicit motor learning lead to greater automatization of motor skills compared to explicit motor learning? A systematic review. PLoS One. 2018;13(9):e0203591. https://doi.org/10.1371/journal.pone.0203591.
Kantak SS, Winstein CJ. Learning–performance distinction and memory processes for motor skills: a focused review and perspective. Behav Brain Res. 2012;228(1):219–31. https://doi.org/10.1016/j.bbr.2011.11.028.
Kapreli E, Athanasopoulos S, Gliatis J, Papathanasiou M, Peeters R, Strimpakos N, Van Hecke P, Gouliamos A, Sunaert S. Anterior cruciate ligament deficiency causes brain plasticity: a functional MRI study. Am J Sports Med. 2009;37(12):2419–26. https://doi.org/10.1177/0363546509343201.
Konishi Y, Aihara Y, Sakai M, Ogawa G, Fukubayashi T. Gamma loop dysfunction in the quadriceps femoris of patients who underwent anterior cruciate ligament reconstruction remains bilaterally. Scand J Med Sci Sports. 2007;17(4):393–9. https://doi.org/10.1111/j.1600-0838.2006.00573.x.
Kotsifaki A, Korakakis V, Whiteley R, Van Rossom S, Jonkers I. Measuring only hop distance during single leg hop testing is insufficient to detect deficits in knee function after ACL reconstruction: a systematic review and meta-analysis. Br J Sports Med. 2020;54(3):139–53. https://doi.org/10.1136/bjsports-2018-099918.
Krosshaug T, Nakamae A, Boden BP, Engebretsen L, Smith G, Slauterbeck JR, Hewett TE, Bahr R. Mechanisms of anterior cruciate ligament injury in basketball. Am J Sports Med. 2007;35(3):359–67. https://doi.org/10.1177/0363546506293899.
Krosshaug T, Slauterbeck JR, Engebretsen L, Bahr R. Biomechanical analysis of anterior cruciate ligament injury mechanisms: three-dimensional motion reconstruction from video sequences. Scand J Med Sci Sports. 2007;17(5):508–19. https://doi.org/10.1111/j.1600-0838.2006.00558.x.
Lavender A, Laurence A, Bangash I, Smith R. Cortical evoked potentials in the ruptured anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 1999;7(2):98–101. https://doi.org/10.1007/s001670050129.
Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–73. https://doi.org/10.1016/j.neuroimage.2007.03.053.
Lepley A, Gribble P, Thomas A, Tevald M, Sohn D, Pietrosimone B. Quadriceps neural alterations in anterior cruciate ligament reconstructed patients: a 6-month longitudinal investigation. Scand J Med Sci Sports. 2015;25(6):828–39. https://doi.org/10.1111/sms.12435.
Lepley AS, Ericksen HM, Sohn DH, Pietrosimone BG. Contributions of neural excitability and voluntary activation to quadriceps muscle strength following anterior cruciate ligament reconstruction. Knee. 2014;21(3):736–42. https://doi.org/10.1016/j.knee.2014.02.008.
Lepley AS, Grooms DR, Burland JP, Davi SM, Kinsella-Shaw JM, Lepley LK. Quadriceps muscle function following anterior cruciate ligament reconstruction: systemic differences in neural and morphological characteristics. Exp Brain Res. 2019;1–12: https://doi.org/10.1007/s00221-019-05499-x.
Lepley AS, Ly MT, Grooms DR, Kinsella-Shaw JM, Lepley LK. Corticospinal tract structure and excitability in patients with anterior cruciate ligament reconstruction: a DTI and TMS study. Neuroimage Clin. 2020;25:102157. https://doi.org/10.1016/j.nicl.2019.102157.
Lloyd RS, Cronin JB, Faigenbaum AD, Haff GG, Howard R, Kraemer WJ, Micheli LJ, Myer GD, Oliver JL. National Strength and Conditioning Association position statement on long-term athletic development. J Strength Cond Res. 2016;30(6):1491–509. https://doi.org/10.1519/JSC.0000000000001387.
Lloyd RS, Faigenbaum AD, Myer G, Stone M, Oliver J, Jeffreys I, Pierce K. UKSCA position statement: Youth resistance training. Prof Strength Cond. 2012;26:26–39.
Lloyd RS, Meyers RW, Oliver JL. The natural development and trainability of plyometric ability during childhood. Strength Cond J. 2011;33(2):23–32. https://doi.org/10.1519/SSC.0b013e3182093a27.
Lloyd RS, Oliver JL. The youth physical development model: a new approach to long-term athletic development. Strength Cond J. 2012;34(3):61–72. https://doi.org/10.1519/SSC.0b013e31825760ea.
Lloyd RS, Oliver JL, Faigenbaum AD, Howard R, Croix MBDS, Williams CA, Best TM, Alvar BA, Micheli LJ, Thomas DP. Long-term athletic development-part 1: a pathway for all youth. J Strength Cond Res. 2015;29(5):1439–50. https://doi.org/10.1519/JSC.0000000000000756.
Lloyd RS, Oliver JL, Faigenbaum AD, Howard R, Croix MBDS, Williams CA, Best TM, Alvar BA, Micheli LJ, Thomas DP. Long-term athletic development, part 2: barriers to success and potential solutions. J Strength Cond Res. 2015;29(5):1451–64. https://doi.org/10.1519/01.JSC.0000465424.75389.56.
Lloyd RS, Oliver JL, Faigenbaum AD, Myer GD, Croix MBDS. Chronological age vs. biological maturation: implications for exercise programming in youth. J Strength Cond Res. 2014;28(5):1454–64. https://doi.org/10.1519/JSC.0000000000000391.
Lloyd RS, Oliver JL, Meyers RW, Moody JA, Stone MH. Long-term athletic development and its application to youth weightlifting. Strength Cond J. 2012;34(4):55–66. https://doi.org/10.1519/SSC.0b013e31825ab4bb.
Lloyd RS, Read P, Oliver JL, Meyers RW, Nimphius S, Jeffreys I. Considerations for the development of agility during childhood and adolescence. Strength Cond J. 2013;35(3):2–11. https://doi.org/10.1519/SSC.0b013e31827ab08c.
Losciale JM, Bullock G, Cromwell C, Ledbetter L, Pietrosimone L, Sell TC. Hop testing lacks strong association with key outcome variables after primary anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med. 2020;48(2):511–22. https://doi.org/10.1177/0363546519838794.
Luc-Harkey BA, Harkey MS, Pamukoff DN, Kim RH, Royal TK, Blackburn JT, Spang JT, Pietrosimone B. Greater intracortical inhibition associates with lower quadriceps voluntary activation in individuals with ACL reconstruction. Exp Brain Res. 2017;235(4):1129–37. https://doi.org/10.1007/s00221-017-4877-8.
Makaruk H, Porter JM, Bodasińska A, Palmer S. Optimizing the penalty kick under external focus of attention and autonomy support instructions. Eur J Sport Sci. 2020;1–9. https://doi.org/10.1080/17461391.2020.1720829.
Makaruk H, Porter JM, Sadowski J, Bodasińska A, Zieliński J, Niźnikowski T, Mastalerz A. The effects of combining focus of attention and autonomy support on shot accuracy in the penalty kick. PLoS One. 2019;14(9):e0213487. https://doi.org/10.1371/journal.pone.0213487.
Malina RM, Bouchard C, Bar-Or O. Growth, maturation, and physical activity. Champaign: Human Kinetics; 2004.
Malina RM, Cumming SP, Morano PJ, Barron M, Miller SJ. Maturity status of youth football players: a noninvasive estimate. Med Sci Sports Exerc. 2005;37(6):1044–52.
Marchant DC, Carnegie E, Wood G, Ellison P. Influence of visual illusion and attentional focusing instruction in motor performance. Int J Sport Exerc Psychol. 2019;17(6):659–69. https://doi.org/10.1080/1612197X.2018.1441165.
McCormack RG, Hutchinson MR. Time to be honest regarding outcomes of ACL reconstructions: should we be quoting 55–65% success rates for high-level athletes? Br J Sports Med. 2016;50(19):1167–8. https://doi.org/10.1136/bjsports-2016-096324.
Miao X, Huang H, Hu X, Li D, Yu Y, Ao Y. The characteristics of EEG power spectra changes after ACL rupture. PLoS One 2017;12(2). https://doi.org/10.1371/journal.pone.0170455.
Monfort SM, Pradarelli JJ, Grooms DR, Hutchison KA, Onate JA, Chaudhari AM. Visual-spatial memory deficits are related to increased knee valgus angle during a sport-specific sidestep cut. Am J Sports Med. 2019;47(6):1488–95. https://doi.org/10.1177/0363546519834544.
Myer GD, Faigenbaum AD, Chu DA, Falkel J, Ford KR, Best TM, Hewett TE. Integrative training for children and adolescents: techniques and practices for reducing sports-related injuries and enhancing athletic performance. Phys Sportsmed. 2011;39(1):74–84. https://doi.org/10.3810/psm.2011.02.1854.
Myer GD, Ford KR, Brent JL, Hewett TE. The effects of plyometric vs. dynamic stabilization and balance training on power, balance, and landing force in female athletes. J Strength Cond Res. 2006;20(2):345. https://doi.org/10.1519/R-17955.1.
Myer GD, Ford KR, Hewett TE. Rationale and clinical techniques for anterior cruciate ligament injury prevention among female athletes. J Athl Train. 2004;39(4):352.
Myer GD, Ford KR, McLean SG, Hewett TE. The effects of plyometric versus dynamic stabilization and balance training on lower extremity biomechanics. Am J Sports Med. 2006;34(3):445–55. https://doi.org/10.1177/0363546505281241.
Myer GD, Ford KR, Palumbo JP, Hewett TE. Neuromuscular training improves performance and lower-extremity biomechanics in female athletes. J Strength Cond Res. 2005;19(1):51–60. https://doi.org/10.1519/13643.1.
Myer GD, Lloyd RS, Brent JL, Faigenbaum AD. How young is “too young” to start training? ACSM's Health Fit J. 2013;17(5):14. https://doi.org/10.1249/FIT.0b013e3182a06c59.
Myer GD, Paterno MV, Ford KR, Hewett TE. Neuromuscular training techniques to target deficits before return to sport after anterior cruciate ligament reconstruction. J Strength Cond Res. 2008;22(3):987–1014. https://doi.org/10.1519/JSC.0b013e31816a86cd.
Myer GD, Paterno MV, Ford KR, Quatman CE, Hewett TE. Rehabilitation after anterior cruciate ligament reconstruction: criteria-based progression through the return-to-sport phase. J Orthop Sports Phys Ther. 2006;36(6):385–402. https://doi.org/10.2519/jospt.2006.2222.
Myer GD, Sugimoto D, Thomas S, Hewett TE. The influence of age on the effectiveness of neuromuscular training to reduce anterior cruciate ligament injury in female athletes: a meta-analysis. Am J Sports Med. 2013;41(1):203–15. https://doi.org/10.1177/0363546512460637.
Myer GDD, Schmitt LC, Brent JL, Ford KR, Barber Foss KD, Scherer BJ, Heidt RS Jr, Divine JG, Hewett TE. Utilization of modified NFL combine testing to identify functional deficits in athletes following ACL reconstruction. J Orthop Sports Phys Ther. 2011;41(6):377–87. https://doi.org/10.2519/jospt.2011.3547.
Nagai T, Schilaty ND, Laskowski ER, Hewett TE. Hop tests can result in higher limb symmetry index values than isokinetic strength and leg press tests in patients following ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2020;28(3):816–22. https://doi.org/10.1007/s00167-019-05513-3.
Needle AR, Lepley AS, Grooms DR. Central nervous system adaptation after ligamentous injury: a summary of theories, evidence, and clinical interpretation. Sports Med. 2017;47(7):1271–88. https://doi.org/10.1007/s40279-016-0666-y.
Neto T, Sayer T, Theisen D, Mierau A. Functional brain plasticity associated with ACL injury: a scoping review of current evidence. Neural Plast. 2019;2019. https://doi.org/10.1155/2019/3480512.
Norte GE, Hertel J, Saliba SA, Diduch DR, Hart JM. Quadriceps neuromuscular function in patients with anterior cruciate ligament reconstruction with or without knee osteoarthritis: a cross-sectional study. J Athl Train. 2018;53(5):475–85. https://doi.org/10.4085/1062-6050-102-17.
Ochi M, Iwasa J, Uchio Y, Adachi N, Kawasaki K. Induction of somatosensory evoked potentials by mechanical stimulation in reconstructed anterior cruciate ligaments. J Bone Jt Surg Br. 2002;84(5):761–6. https://doi.org/10.1302/0301-620x.84b5.12584.
Ochi M, Iwasa J, Uchio Y, Adachi N, Sumen Y. The regeneration of sensory neurones in the reconstruction of the anterior cruciate ligament. J Bone Jt Surg Br. 1999;81(5):902–6. https://doi.org/10.1302/0301-620x.81b5.9202.
Øiestad BE, Holm I, Engebretsen L, Risberg MA. The association between radiographic knee osteoarthritis and knee symptoms, function and quality of life 10–15 years after anterior cruciate ligament reconstruction. Br J Sports Med. 2011;45(7):583–8. https://doi.org/10.1136/bjsm.2010.073130.
Oliver JL, Lloyd RS. Long-term athlete development and trainability during childhood: a brief review. Prof Strength Cond J. 2012;26:19–24.
Pascua LA, Wulf G, Lewthwaite R. Additive benefits of external focus and enhanced performance expectancy for motor learning. J Sports Sci. 2015;33(1):58–66. https://doi.org/10.1080/02640414.2014.922693.
Paterno MV, Schmitt LC, Ford KR, Rauh MJ, Myer GD, Huang B, Hewett TE. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38(10):1968–78. https://doi.org/10.1177/0363546510376053.
Petanjek Z, Judaš M, Šimić G, Rašin MR, Uylings HB, Rakic P, Kostović I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci. 2011;108(32):13281–6. https://doi.org/10.1073/pnas.1105108108.
Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, DeRosse P, Zhang J-P, Giorgio A, Qiu D, Tapert SF. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr Bull. 2012;38(6):1308–17. https://doi.org/10.1093/schbul/sbs054.
Petushek EJ, Sugimoto D, Stoolmiller M, Smith G, Myer GD. Evidence-based best-practice guidelines for preventing anterior cruciate ligament injuries in young female athletes: a systematic review and meta-analysis. Am J Sports Med. 2019;47(7):1744–53. https://doi.org/10.1177/0363546518782460.
Pietrosimone BG, Lepley AS, Ericksen HM, Clements A, Sohn DH, Gribble PA. Neural excitability alterations after anterior cruciate ligament reconstruction. J Athl Train. 2015;50(6):665–74. https://doi.org/10.4085/1062-6050-50.1.11.
Porter J, Wu W, Partridge J. Focus of attention and verbal instructions: strategies of elite track and field coaches and athletes. Sport Sci Rev. 2010;19(3–4):77–89. https://doi.org/10.2478/v10237-011-0018-7.
Poulsen E, Goncalves GH, Bricca A, Roos EM, Thorlund JB, Juhl CB. Knee osteoarthritis risk is increased 4–6 fold after knee injury—a systematic review and meta-analysis. Br J Sports Med. 2019;53(23):1454–63. https://doi.org/10.1136/bjsports-2018-100022.
Powers CM, Fisher B. Mechanisms underlying ACL injury-prevention training: the brain-behavior relationship. J Athl Train. 2010;45(5):513–5. https://doi.org/10.4085/1062-6050-45.5.513.
Prince JS, Laor T, Bean JA. MRI of anterior cruciate ligament injuries and associated findings in the pediatric knee: changes with skeletal maturation. Am J Roentgenol. 2005;185(3):756–62. https://doi.org/10.2214/ajr.185.3.01850756.
Raisbeck L, Yamada M, Diekfuss JA. Focus of attention in trained distance runners. Int J Sports Sci Coach. 2018;13(6):1143–9. https://doi.org/10.1177/1747954118798396.
Raisbeck LD, Diekfuss JA, Wyatt W, Shea JB. Motor imagery, physical practice, and memory: the effects on performance and workload. Percept Mot Skills. 2015;121(3):691–705. https://doi.org/10.2466/23.25.PMS.121c23x6.
Raisbeck LD, Wyatt WR, Shea JB. A two process memory-based account for mental and physical practice differences. J Mot Behav. 2012;44(2):115–24. https://doi.org/10.1080/00222895.2012.654525.
Rossini PM, Barker A, Berardelli A, Caramia M, Caruso G, Cracco R, Dimitrijević M, Hallett M, Katayama Y, Lücking C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91(2):79–92. https://doi.org/10.1016/0013-4694(94)90029-9.
Schmitz RJ, Kulas AS, Perrin DH, Riemann BL, Shultz SJ. Sex differences in lower extremity biomechanics during single leg landings. Clin Biomech (Bristoal, Avon). 2007;22(6):681–8. https://doi.org/10.1016/j.clinbiomech.2007.03.001.
Shah VM, Andrews JR, Fleisig GS, McMichael CS, Lemak LJ. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233–9. https://doi.org/10.1177/0363546510372798.
Sibley BA, Etnier JL. The relationship between physical activity and cognition in children: a meta-analysis. Pediatr Exerc Sci. 2003;15(3):243–56. https://doi.org/10.1123/PES.15.3.243.
Silfies SP, Vendemia JM, Beattie PF, Stewart JC, Jordon M. Changes in brain structure and activation may augment abnormal movement patterns: an emerging challenge in musculoskeletal rehabilitation. Pain Med. 2017;18(11):2051–4. https://doi.org/10.1093/pm/pnx190.
Silva F, Ribeiro F, Oliveira J. Effect of an accelerated ACL rehabilitation protocol on knee proprioception and muscle strength after anterior cruciate ligament reconstruction. Arch Exerc Health Dis. 2012;3:139–44. https://doi.org/10.5628/aehd.v3i1-2.113.
Soderstrom NC, Bjork RA. Learning versus performance: an integrative review. Perspect Psychol Sci. 2015;10(2):176–99. https://doi.org/10.1177/1745691615569000.
Spear LP. Adolescent neurodevelopment. J Adolesc Health. 2013;52(2):S7–13. https://doi.org/10.1016/j.jadohealth.2012.05.006.
Subramanian SK, Sharma VK, Arunachalam V, Radhakrishnan K, Ramamurthy S. Effect of structured and unstructured physical activity training on cognitive functions in adolescents—a randomized control trial. J Clin Diagn Res. 2015;9(11):Cc04–Cc09. https://doi.org/10.7860/jcdr/2015/14881.6818.
Swanik CB, Covassin T, Stearne DJ, Schatz P. The relationship between neurocognitive function and noncontact anterior cruciate ligament injuries. Am J Sports Med. 2007;35(6):943–8. https://doi.org/10.1177/0363546507299532.
Treacy S, Barron O, Brunet M, Barrack R. Assessing the need for extensive supervised rehabilitation following arthroscopic ACL reconstruction. Am J Orthop (Belle Mead, NJ). 1997;26(1):25–9.
Tsai L-C, Powers CM. Increased hip and knee flexion during landing decreases tibiofemoral compressive forces in women who have undergone anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41(2):423–9. https://doi.org/10.1177/0363546512471184.
Valeriani M, Restuccia D, Lazzaro VD, Franceschi F, Fabbriciani C, Tonali P. Central nervous system modifications in patients with lesion of the anterior cruciate ligament of the knee. Brain. 1996;119(5):1751–62. https://doi.org/10.1093/brain/119.5.1751.
van der Graaff E, Hoozemans M, Pasteuning M, Veeger D, Beek PJ. Focus of attention instructions during baseball pitching training. Int J Sports Sci Coach. 2018;13(3):391–7. https://doi.org/10.1177/1747954117711095.
van Duijvenvoorde ACK, Achterberg M, Braams BR, Peters S, Crone EA. Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. Neuroimage. 2016;124(Pt A):409–20. https://doi.org/10.1016/j.neuroimage.2015.04.069.
Walden M, Hagglund M, Magnusson H, Ekstrand J. ACL injuries in men's professional football: a 15-year prospective study on time trends and return-to-play rates reveals only 65% of players still play at the top level 3 years after ACL rupture. Br J Sports Med. 2016;50(12):744–50. https://doi.org/10.1136/bjsports-2015-095952.
Welling W, Benjaminse A, Lemmink K, Gokeler A. Passing return to sports tests after ACL reconstruction is associated with greater likelihood for return to sport but fail to identify second injury risk. Knee. 2020;27(3):949–57. https://doi.org/10.1016/j.knee.2020.03.007.
Wiggins AJ, Grandhi RK, Schneider DK, Stanfield D, Webster KE, Myer GD. Risk of secondary injury in younger athletes after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Am J Sports Med. 2016;44(7):1861–76. https://doi.org/10.1177/0363546515621554.
Wulf G. Attentional focus and motor learning: a review of 15 years. Int Rev Sport Exerc Psychol. 2013;6(1):77–104. https://doi.org/10.1080/1750984X.2012.723728.
Wulf G, Lewthwaite R. Optimizing performance through intrinsic motivation and attention for learning: the OPTIMAL theory of motor learning. Psychon Bull Rev. 2016;23:1382–414. https://doi.org/10.3758/s13423-015-0999-9.
Wulf G, Lewthwaite R, Cardozo P, Chiviacowsky S. Triple play: additive contributions of enhanced expectancies, autonomy support, and external attentional focus to motor learning. Q J Exp Psychol. 2018;71(4):824–31. https://doi.org/10.1080/17470218.2016.1276204.
Yamada M, Diekfuss JA, Raisbeck L. Motor behavior literature fails to translate: a preliminary investigation into coaching and focus of attention in recreational distance runners. Int J Exerc Sci. 2020;13(5):789–801.
Zarzycki R, Morton SM, Charalambous CC, Marmon A, Snyder-Mackler L. Corticospinal and intracortical excitability differ between athletes early after ACLR and matched controls. J Orthop Res. 2018;36(11):2941–8. https://doi.org/10.1002/jor.24062.
Zbrojkiewicz D, Vertullo C, Grayson JE. Increasing rates of anterior cruciate ligament reconstruction in young Australians, 2000–2015. Med J Aust. 2018;208(8):354–8.
Zwicker JG, Missiuna C, Harris SR, Boyd LA. Brain activation of children with developmental coordination disorder is different than peers. Pediatrics. 2010;126(3):e678–686686. https://doi.org/10.1542/peds.2010-0059.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diekfuss, J.A., Hogg, J.A., Grooms, D.R. et al. Can We Capitalize on Central Nervous System Plasticity in Young Athletes to Inoculate Against Injury?. J. of SCI. IN SPORT AND EXERCISE 2, 305–318 (2020). https://doi.org/10.1007/s42978-020-00080-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42978-020-00080-3