Abstract
In the present report, we investigated changes in corticomotor excitability associated with unilateral knee dysfunction secondary to anterior cruciate ligament (ACL) injury. Ten participants, each with a previous history of unilateral ACL injury (median time post-injury 22 months) and eight healthy controls underwent transcranial magnetic stimulation (TMS) to assess excitability of the lower limb motor representation. Resting motor thresholds (RMTs) and stimulus response curves were measured at rest, while amplitude of motor evoked potentials and silent period duration were measured during active contraction. Correlations between these indices of excitability and three clinical measures of knee function were identified. Paired comparisons of indices by hemisphere revealed an asymmetry only in RMTs, which were significantly reduced on the side of injury in the ACL group. Correlations with clinical measures showed that the extent of quadriceps motor representation, as reflected by the steepness of SR curves, was strongly associated with quadriceps strength (r 2=0.71) on the injured side. The RMT asymmetry reported here in the context of ACL injury is consistent with other recent reports describing enhanced excitability of corticomotor projections targeting muscles adjacent to an immobilized or a painful joint. In such conditions, alterations in the quantity and quality of sensory feedback from the affected limb may underlie the rise in cortical excitability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Injury of the anterior cruciate ligament (ACL) is the most common knee pathology in sports and occupational activities [2, 44]. In the long term, ACL injuries often result in chronic knee instability and residual weakness in the quadriceps muscle [2]. This residual weakness can be substantial (15–40% decrease) persisting for months and even years following the initial injury and contributing ultimately to functional limitations [39, 41]. This persistent state of diminished ability to contract the musculature surrounding a joint after injuries has been referred to as “arthrogenic muscle inhibition (AMI)”[24]. At the neurophysiological level, AMI is thought to reflect an ongoing reflex inhibition resulting from damage or distension of joint structures, which affects the excitability of spinal interneurones and ultimately the ability to produce motor output [24, 33]. In the case of ACL injuries, the inability of affected individuals to fully activate the quadriceps in the context of maximal voluntary contraction (MVC) has been ascribed to AMI induced by sub-clinical joint effusion and reductions in joint afferent input [12, 16, 47, 51]. However, as shown recently by Mrachacz-Kersting et al. [30], the net contribution of reflex mechanisms to extensor torque at the knee in healthy persons is relatively small (~12%) at maximal or near maximal levels of effort, suggesting that additional mechanisms beyond reflex-mediated inhibition must be involved in persistent deficits of quadriceps strength in individuals who have recovered from an ACL injury. In this respect, several authors have stressed the possibility that alterations at the cortical level could also be present in this patient population and contribute to chronic deficits in muscle strength [15, 19, 42, 45, 46]. For instance, Urbach et al. [42] examined the extent of quadriceps activation during MVC in patients with confirmed ACL rupture using the interpolated twitch technique. Compared to healthy controls, patients with ACL rupture exhibited mild, but significant, deficits in voluntary activation in both the injured and uninjured legs, consistent with the existence of alterations at the cortical level. The high incidence of deficits in quadriceps activation was recently confirmed by Chmielewski et al. [10] in a sample of 100 consecutive highly active individuals examined 6–8 weeks after acute ACL rupture, with 43% of them exhibiting quadriceps inhibition in either the injured or the uninjured leg and 21% in both legs. Although little is known about the extent and nature of the cortical alterations that may affect the knee motor representation after ACL injury, evidence from TMS studies indicate that pain and restrictions imposed by bone and joint pathology can have profound effects on excitability at the motor cortical level [28, 49].
In the present study, using TMS to assess the excitability of the lower limb motor representation, we looked for possible asymmetries at the corticomotor level associated with unilateral knee dysfunction secondary to ACL injury. We also looked at possible relationships between indices of excitability on the injured side and clinical measures of knee function derived from a recent study involving the same patient population [21].
Materials and methods
Characteristics of study participants
The study participants consisted of a convenient sample of 18 young adults who were initially recruited for a study on weight discrimination. Of these, 10 participants presented with a history of unilateral knee dysfunction secondary to ACL injury (ACL group, mean age: 27±8 years, 6 females) in the months or years before testing (grade II–III knee sprain, 7 left knees, median time post-injury: 22 months; range 4–108 months). The remaining sample consisted of 8 healthy subjects without antecedent of knee injuries (control group, mean age: 23±3 years; 4 females). The University of Ottawa Human Research Ethics Board approved the study’s procedures and all participants provided informed consent prior to participation.
In the context of the study on weight discrimination, all participants underwent clinical testing to assess subjective knee function, quadriceps strength and to obtain threshold values for differences in weight detected (see [21] for further details). This assessment was performed separately from the TMS session, usually on the same day but with a minimum 2-h interval between the two sessions. The clinical data derived from this assessment are summarized in Table 1 for the two groups of participants. In the ACL group, all participants reported some limitation on the injured side, as reflected by scores derived from the Knee Outcome Survey Activities of Daily Living (KOS-ADL) scale [26]. The majority (8/10) also exhibited reductions in quadriceps strength when torque produced during static MVC (n=3) were compared in the two legs (knee held at 45°, peak torque measured on a KIN-COM® 500H dynamometer, Chattanooga Group, TN 37343, USA). Finally, as we reported recently [21], the ability to detect small differences in weight during active leg lifting actions was also unilaterally decreased in participants of the ACL group.
Transcranial magnetic stimulation (TMS) and motor evoked potentials (MEP)
For TMS, participants were seated in a recording chair with 70° of hip flexion and 60° of knee flexion. Magnetic stimulation was produced using a MagStim 200 (The Magstim Company, Whitland, Dyfed, UK). The first few participants (n=4, ACL group) were tested using a 90-mm circular coil. However, a more efficient double-cone coil became available shortly after and the decision was made to continue with this new coil for the remaining participants (n=14). The fact that two different coils were used for testing was taken into account in the statistical analysis.
For MEP recordings, the quadriceps was selected as the target muscle. A pair of auto-adhesive disposable electrodes (1 cm2, AgCl) was placed along the mid-line over the distal one-third of the thigh, with the proximal electrode being placed at the border between rectus femoris and vastus lateralis, and the distal electrode being 2.5 cm caudal to that point. This placement, targeting the distal end of the rectus femoris, has been shown to be optimal to record evoked potentials in the quadriceps [18]. The electromyographic signal was amplified by 1,000 with a time constant of 3 ms and a low-pass filtering of 1 kHz using a polygraph amplifier (RMP-6004, Nihon-Kohden Corp.). Data acquisition was controlled using custom software in a PC running on Windows 98, equipped with an acquisition card (NI-DAQ PCI-6023, National Instrument Corp.). All signals were sampled at 2 kHz and saved for later analysis.
To determine the optimal site on the scalp to evoke activity in the contralateral quadriceps, the experimenter moved or tilted the coil (at first, the circular and then the double cone) in 1-cm step from the vertex, while stimulating at high intensity until reproducible MEPs could be obtained. For most subjects, the optimal site for the quadriceps was slightly anterior and lateral to the vertex. Once the optimal position was determined on the scalp a small adhesive marker was placed on the spot. The coil (either the circular or the double-cone) was maintained firmly in place using a custom-made coil-holder mounted at the back of the recording chair. Head movements were restrained during testing by means of a U-shaped cushion, which was fitted at the neck of the participant.
Assessment of corticomotor excitability
Resting motor threshold (RMT)
For RMT determination, we followed a procedure similar to that described by Mills and Nithi [29] that consists of eliciting a series of consecutive responses to determine an upper and a lower threshold, and then using the mean between the two as the threshold intensity. Because we investigated the lower limb in the present study, we used 7 instead of 10 consecutive responses to avoid too many stimuli. To speed up the process, the initial stimulator intensity used to locate the optimal spot on the scalp was first decreased in increments of 5% until no further MEPs could be evoked. The intensity was then gradually increased until a MEP reappeared. From this point, intensity was decreased in increments of 1% to find the maximal intensity that produced 0/7 responses in consecutive trials (lower threshold). Intensity was then gradually increased in 1% increments to find the minimal intensity that produced reliable MEPs of at least 25 μV in 7/7 consecutive trials (upper threshold). The RMT for each side was taken as the mean between the lower and upper threshold intensities. EMG activity was continuously monitored on an oscilloscope, at high gain, to ensure no voluntary muscular activity occurred during the procedure.
Stimulus–response (SR) curve
Stimulus–response curves display the relationship between the rise in MEP amplitude and the increase in TMS intensity [37]. For SR curve measurement, the stimulator intensity was initially set to the previously determined lower threshold value rounded down to the closest multiple of five (e.g. lower threshold = 58%, stimulator intensity set to 55%). Five MEPs were then recorded at this intensity. From this point, the stimulator output was increased gradually in 5% increments with 5 MEPs being recorded for each subsequent increase. The procedure ended when no further augmentation in MEP amplitude could be obtained after two successive increases in intensity (i.e. point of saturation). SR curves were obtained by plotting variations in MEP amplitude (mean, peak-to-peak) against corresponding increases in TMS intensity. As suggested by Ray et al. [36], we used simple linear regression analyses to compute the slope parameter of each SR curve. The slope summarizing the strength of the SR curve provided a single parameter for statistical comparisons.
MEP facilitation and silent period (SP) duration during active contraction
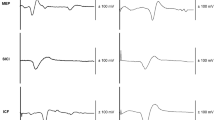
The last two indices were measured concomitantly during active contraction of the quadriceps. Participants were asked to extend the knee and to hold this position for the duration of a tone, which lasted 1,000 ms. In the course of the contraction, TMS was delivered at a fixed delay of 500 ms at an intensity corresponding to the upper motor threshold determined previously (see earlier). Eight trials were performed for each leg with a 15 s rest period between each. MEP amplitude (peak-to-peak) and durations of SP were recorded on each trial and averaged for each leg. The SP duration was defined as the interval between MEP onset and the return of EMG activity (i.e. 30% of peak-to-peak EMG activity prior to stimulation, see Fig. 3a).
Quadriceps H-reflex recording
For half of the participants in the ACL group, those in which an H-response could be evoked in the quadriceps, the amplitude of the reflex was measured to assess spinal excitability. For this measurement, the participants were seated with 50° of hip flexion and 60° of knee flexion [20]. After appropriate skin preparation, a pair of electrodes (2-cm inter-electrode distance) was positioned over the inferior third of the vastus lateralis, an area suitable for recording maximal H-reflex in the quadriceps [18]. The femoral nerve was stimulated with the active electrode (half-ball 2-cm diameter) positioned at the femoral triangle, 1–2 cm lateral to the femoral artery. The stimuli consisted of 1 ms square wave pulse generated by a S88 Stimulator (Grass Instrument CO, MA 02169, USA). The indifferent electrode was positioned over the buttock on the ipsilateral side. In each case, the intensity was adjusted so as to obtain a maximal H while eliciting a minimal M-response. Ten trials (75 ms epoch) were recorded and averaged for each leg to get amplitude (peak-to-peak) and latency measurements.
Statistical analysis
Given that two different coils were used to assess participants and the large inter-subject variability of TMS-derived indices of excitability [43], only paired comparisons between sides (i.e. injured vs. uninjured; right vs. left) were performed for the statistical analysis. Individual indices derived from each hemisphere (i.e. RMT, SR curve, MEP amplitude and SP duration) were compared using paired t tests to detect significant differences. Pearson’s product-moment correlation coefficients were also computed to look for possible associations between indices of excitability and clinical measures of knee function on the injured side (i.e. ratings of knee function, quadriceps strength and weight discrimination). All statistical tests were performed using GraphPad Prism version 4.00 for Windows, (GraphPad Software, San Diego, CA, USA, http://www.graphpad.com) with the level of significance being set at 0.05 for all comparisons.
Results
RMTs
The distribution of individual RMTs from both groups is shown in Fig. 1. In the ACL group, most participants exhibited lower threshold values on the injured, as compared to, the uninjured side. The cluster of higher values simply reflects the fact that some participants were tested with the less efficient circular coil (see Materials and methods). The asymmetry found in the distribution of RMTs was statistically significant (t=2.87, P=0.02). In contrast, RMTs determined in healthy controls were highly comparable in the two hemispheres (t=0.60, P=0.57).
Resting motor threshold (RMT). Distribution of RMTs, as determined from the quadriceps muscle on each side, in the two groups of participants. Note that the cluster of higher values in the ACL group simply reflects the fact that the first few participants in this group were tested with the less-efficient circular coil (see Materials and methods). *P<0.05, paired t test
SR curves
Examination of SR curves in the ACL group revealed no systematic patterns of variation with regards to the injured versus uninjured leg. Individual SR curves for the 10 ACL participants can be regrouped into three categories. Firstly, three participants presented SR curves similar to that shown in Fig. 2a, exhibiting steeper slopes on the uninjured, as opposed to, the injured side. Secondly, SR curves for four other participants showed the reverse pattern, exhibiting steeper slopes on the injured side, as exemplified in Fig. 2b. Finally, the remaining three participants presented a pattern closely resembling that seen in control participants (Fig. 2c), exhibiting similar SR curves on both sides. The absence of any systematic patterns in the ACL group can be further appreciated by examining Fig. 2d, where individual slope parameters computed from SR curves are given for each leg in the two groups.
Stimulus-response (SR) curves in the quadriceps at rest. Examples of individual SR curves, with associated slope parameters, obtained from ACL group participants (a, b) and one healthy control (c). d Distribution of individual slope parameters computed from each leg in the two groups. Note that the absence of any systematic pattern of variation with regard to the injured versus uninjured leg in the ACL group
MEP facilitation and SP duration during active contraction
An example of MEP recorded during active contraction of the quadriceps and the subsequent period of EMG silence following the TMS pulse is shown in Fig. 3a. In this subject, as for the majority in the ACL group, no major difference was seen in the traces between the injured and uninjured leg. In fact, as evident in the distributions illustrated in Fig. 3b and c, both the MEP amplitude and the SP duration were very similar in the two sides of both groups. Accordingly, no side-to-side difference was detected in either group.
MEP facilitation and silent period (SP) duration during active contraction of the quadriceps. a Example of a facilitated MEP with the subsequent SP recorded from the injured and uninjured leg of an ACL group participant. b, c Distribution of individual variations in MEP amplitude and SP duration measured from each leg in the two groups
Quadriceps H-reflex
Among the participants in the ACL group who had a measurable quadriceps H-reflex, there was a tendency for the reflex to be smaller on the injured side than on the uninjured side. An example of such reduction in H-reflex amplitude is shown in Fig. 4a. The tendency for H-reflex to be smaller on the side of injury is also apparent in Fig. 4b, although the difference did not reach statistical significance owing to the small number of observations (t=2.475, P=0.07).
Quadriceps H-reflex. a Example of quadriceps H-reflex recorded, respectively, from the injured and uninjured leg of an ACL group participant (overly of 10 trials each). b Distribution of H-reflex amplitudes measured in selected ACL group participants. Note that the tendency for the H-reflex to be diminished in amplitude on the injured side
Correlations with clinical measures
As shown in Table 2, corticomotor indices of excitability were only poorly correlated with subjective ratings of knee function. Slightly higher r values were obtained for the relationships with quadriceps strength and acuity thresholds for weight discrimination, although only the correlation with SR curves and strength came out as significant. Indeed, the slope parameters derived from SR curves showed a strong positive correlation with individual variations in quadriceps strength. To further appreciate this relationship, individual slope values computed for each leg have been plotted against corresponding strength values for the two groups of participants in Fig. 5. It can be seen in that participants exhibiting steeper slopes (Fig. 5a) on the injured side were also those that produced greater torque in the quadriceps during MVC. In contrast, no such association could be seen on the uninjured side of the same participants, nor in healthy control (Fig. 5b).
Correlations with knee function. Relationship between individual slope parameters derived from SR curves and variations in quadriceps strength in the ACL (a) and control (b) groups. Note the strong association between the two variables for data collected on the injured side in the ACL group, which is absent for data collected on the uninjured side and in the legs of control participants. The data point with asterisks “*” correspond to ACL participants with no deficit in quadriceps strength on the injured side
Discussion
In the present study, bilateral comparisons of corticomotor indices of excitability in individuals with antecedents of unilateral ACL injury revealed no major inter-hemispheric asymmetries in the lower limb motor representations, except for RMTs, which were found to be significantly reduced on the hemisphere contralateral to the injured leg. In addition, a strong association was found between the steepness of SR curves on the injured side and individual variations in quadriceps strength.
RMTs and SR curves at rest
Resting motor thresholds and SR curves provide slightly different, but complementary, information about the excitability of a given motor representation. The RMT reflects the neuronal excitability of the most excitable elements under the coil, whereas the SR curve provides information about the spatial distribution of excitable elements under the coil [1, 40]. In terms of mapping, RMTs thus provide information about the central core region of a motor representation, while the steepness of SR curves reflect the extent of the motor representation [1, 6]. In the ACL group, the reduction of RMTs observed on the injured side was not accompanied by a parallel systematic increase in the steepness of the SR curve, which suggests a change in the excitability of the central core region of the quadriceps rather than an expansion of its representation. The steepness of the SR curve is also an index of the strength of corticospinal projections to a given motoneurones pool [4, 13, 37] and, as such, the variations observed in the ACL group might have reflected the inherent variability in the incidence and severity of “quadriceps activation failure” reported recently by Chmielewski et al. [10] in their survey of 100 consecutive cases. These authors concluded that, while the overall incidence of quadriceps inhibition was high after ACL rupture, a substantial proportion of subjects (~60%) remain unaffected and those affected showed as much frequent inhibition on the injured side as to the uninjured side [10]. Such variability may thus account for the present observation that some participants of the ACL group showed steeper SR curves on the injured side as opposed to the uninjured side, while others showed the exact reverse pattern. It remains that the reductions seen in RMT were quite systematic in the ACL group and likely reflective of a change in cortical excitability. The possibility that the observed reductions might have reflected changes in the excitability of spinal motoneurones is unlikely, given that participants tended to exhibit signs of inhibition rather than facilitation, as judged from H-reflex measurements. Besides, persistent reflex-inhibition of the quadriceps motoneurone pool appears to be a common pathophysiological feature in knee ligament injuries [15, 33, 46]. Such considerations reinforce our contention that reductions of RMTs in the ACL group were largely attributable to changes in excitability at the cortical level.
The importance of the asymmetry in RMTs reported here needs to be emphasized since motor thresholds tend to be quite symmetrical between homologous pairs of muscles in healthy persons [11, 29]. In fact, this was the case for our group of healthy controls. Thus, this asymmetry likely reflects a genuine inter-hemispheric difference in the excitability of the central core representation of the quadriceps muscle. To a certain extent, the present finding of reduced RMTs in an individual with ACL injuries is reminiscent of the changes in cortical excitability reported after limb deafferentation secondary to clinical amputations or induced by ischemic nerve blockade. In such deafferentation, TMS indices of excitability are typically increased in the motor representation of muscles proximal to the deprived area with reduced RMTs, increased MEP size and enlarged motor output maps (reviewed in [9]). This increased excitability is thought to reflect changes primarily at the motor cortical level via reductions in GABAergic inhibition [7].
Evidently, knee ligament injuries cannot be compared to amputations, but nevertheless such injuries are almost invariably associated with some degrees of sensory deficit due to a loss in mechanoreceptive innervation of the joint capsule [23]. In investigating this issue in our group of participants, we were able to recently show that their ability to detect differences in weight during active leg lifting was affected on the injured side, suggesting a deficit in the appreciation of force signals arising from the quadriceps [21]. This concurs with other reports describing mild to moderate deficits in the ability to sense static joint position and detect movement in patients with ACL injuries [3, 17, 38]. It is therefore conceivable that ACL lesions, by partially depriving the corticomotor representation of the knee from sensory inputs arising from the joint capsule, may trigger plasticity changes similar to those induced by total deafferentation. In keeping with this suggestion, two recent reports have provided evidence that corticomotor excitability can be enhanced in clinical conditions characterized by joint pathology leading to sensorimotor restriction, and therefore producing some forms of sensory deprivation. Zanette et al. [49], for instance, reported that the excitability of the corticomotor projections to hand muscles was unilaterally increased immediately after a period of limb immobilization for wrist fractures. Similar to the present study RMTs were reduced on the immobilized side, but unlike the present study SR curves changed in parallel with RMTs (i.e. increased slopes on the immobilized side). Interestingly, the inter-hemispheric asymmetry in RMTs was still detectable in four patients when tested 1 month later at follow-up, while SR curves tended to normalize. A pattern similar in many respects to findings of our ACL injury group participants in the chronic stage. The other report by On et al. [32] showed that TMS evoked larger MEPs in the quadriceps on the affected side in patients with chronic patellofemoral pain syndrome without concomitant changes in M-wave or in F-wave, suggesting an increased excitability at the cortical level. It is worth noting that in both reports, as in the present study, the studied participants were free of acute pain symptoms and joint effusion at the time of testing. Thus, it seems that increased excitability of corticomotor projections targeting muscles adjacent to a pathological joint is a common feature in many clinical conditions wherein the quantity and quality of sensory feedback from a limb is altered as a result of pain, disuse or restrictions.
MEP facilitation and SP duration during active contraction
MEP facilitation during active contraction reflects enhanced excitability both at the cortical and spinal level, through the increase in the size and number of descending excitatory volleys and lowering of the firing threshold of spinal motoneurones via incoming peripheral afferents [22]. Since voluntary activity is known to normalize motor cortical excitability, any changes measured at rest that persist during active contraction are likely due to long-term modifications in synaptic efficacy [37]. In the ACL group, like in healthy controls, no inter-limb asymmetry was detected in the amplitude of facilitated MEPs, which indicates that changes in synaptic efficacy of corticospinal transmission were unlikely in our group of participants with ACL injury. The preserved ability to produce MEP facilitation during low-level contractions of the quadriceps also indicates that the quality and the quantity of descending volleys was apparently unaffected in this force range, in spite of the presence of deficits in muscle strength at maximal effort. The present observation is consistent with that of Zanette et al. [49] who reported that inter-hemispheric asymmetries in SR curves were abolished during active contraction in their group of patients after wrist immobilization.
As for the SP, its duration provides information regarding the excitability of intra-cortical inhibitory circuits in the motor cortex [6]. The initial phase of the SP (0–50 ms) corresponds to the spinal refractory period, while the later phase (<50 ms) corresponds to the suppression of cortical excitability and is believed to reflect long lasting GABAergic inhibition [8]. The SP is prolonged following strokes and in response to certain drugs like l-Dopa [27, 35], while it is shortened in Alzheimer’s and Parkinson’s disease [31, 34]. In this experiment, no difference was noted in terms of SP duration between the two limbs in the two groups of participants thus indicating that intra-cortical inhibitory mechanisms mediating the SP do not appear to be affected following ACL injuries. More studies with larger samples of participants and using paired-pulse TMS paradigms will be necessary to further address issues regarding impairments in corticocortical circuitry in the case of ACL injury.
Relationship with clinical measures
Of the three clinical measures of knee function, only measurements of quadriceps strength showed evidence of relationship with the indices of excitability derived from the injured leg. The absence of correlations with ratings of knee function and thresholds for weight discrimination is not surprising, given the recognized difficulties in linking changes in excitability measured at the corticomotor level with those measured at the behavioural level [40]. In this respect, the positive correlation found between individual variations in the slope of SR curves and quadriceps strength seems intriguing. At first sight, such an association seems physiologically plausible, given that the steepness of the slope in SR curves is also a reflection of the strength of corticospinal projections [4, 13, 37]. At the representational level, the correlation implies that individuals having a larger distribution of excitable elements within the motor representation also produce greater torques in the quadriceps during MVC. Chmielewski et al. [10], in their series of observations on quadriceps activation after unilateral ACL rupture, also found a relationship between electrophysiological indices of voluntary activation in the quadriceps and muscle strength. Interestingly, and much like in the present study, this relationship was evident only on the injured side in individuals showing impaired voluntary activation in the quadriceps. One can only speculate about the functional significance of the association found here between SR curves and quadriceps strength in the ACL group but it is tempting to link the correlation with the reduction seen in RMTs on the injured side. From a functional point of view, the two findings may be seen as an adaptation of the corticospinal system in response to long-standing unilateral knee dysfunction. In terms of strategy, shifting from a more semi-automatic mode of control to a more voluntary-driven mode of control over the knee musculature might be an important coping mechanism after ACL injuries. Indeed, while normal locomotion does not depend heavily on descending projections from the corticospinal tract [5], specialized locomotor activities, for instance those requiring precise placement of the foot, do rely heavily on corticospinal projections to reset the central pattern generators [14]. In subjective reports, individuals with ACL injuries often allude to the fact that they are now more “aware” of their knee during activities of daily living and avoid large forces and positions that are too extreme. In studies on the kinematics and kinetics of gait patterns individuals with ACL injuries display many subtle changes. For example, greater activation of hamstrings and gastrocnemius muscles is thought to reflect voluntary interventions to increase tibial external rotation and knee valgus to prevent further injury [25, 50]. Altogether, these observations support the notion that voluntary driven control over the thigh musculature is an important coping mechanism in individuals who have sustained unilateral knee ligament injuries. Thus, individuals who preserve and maintain the strength of corticospinal projections by exerting more overt control over the quadriceps might be more capable of coping with external demands in the context of sports and leisure activities. Such a possibility certainly merits further exploration from larger sample sizes, comparing “copers” versus “non-copers” in terms of the relationships between changes in corticomotor excitability and modifications of muscle strength.
References
Abbruzzese G, Trompetto C (2002) Clinical and research methods for evaluating cortical excitability. J Clin Neurophysiol 19:307–321
Arnold JA, Coker TP, Heaton LM, Park JP, Harris WD (1979) Natural history of anterior cruciate tears. Am J Sports Med 7:305–313
Beard DJ, Kyberd PJ, Fergusson CM, Dodd CA (1993) Proprioception after rupture of the anterior cruciate ligament. An objective indication of the need for surgery? J Bone Joint Surg Br 75:311–315
Boroojerdi B, Battaglia F, Muellbacher W, Cohen LG (2001) Mechanisms influencing stimulus-response properties of the human corticospinal system. Clin Neurophysiol 112:931–937
Capaday C, Lavoie BA, Barbeau H, Schneider C, Bonnard M (1999) Studies of the corticospinal control of human walking: I. responses to focal transcranial mangetic stimulation of the motor cortex. J Neurophysiol 81:129–139
Chen R (2000) Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve Suppl 9:S26–S32
Chen R, Corwell B, Yaseen Z, Hallett M, Cohen LG (1998) Mechanisms of cortical reorganization in lower-limb amputees. J Neurosci 18:3443–3450
Chen R, Lozano AM, Ashby P (1999) Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res 128:539–542
Chen R, Cohen LG, Hallett M (2002) Nervous system reorganization following injury. Neuroscience 111:761–773
Chmielewski TL, Stackhouse S, Axe MJ, Snyder-Mackler L (2004) A prospective analysis of incidence and severity of quadriceps inhibition in a consecutive sample of 100 patients with complete acute anterior cruciate ligament rupture. J Orthop Res 22:925–930
Civardi C, Boccagni C, Vicentini R, Bolamperti L, Tarletti R, Varrasi C, Monaco F, Cantello R (2001) Cortical excitability and sleep deprivation: a transcranial magnetic stimulation study. J Neurol Neurosurg Psychiatr 71:809–812
Cole KJ, Daley BJ, Brand RA (1996) The sensitivity of joint afferents to knee translation. Sportverletz Sportschaden 10:27–31
Devanne H, Lavoie BA, Capaday C (1997) Input–output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114:329–338
Drew T (1988) Motor cortical cell discharge during voluntary gait modification. Brain Res 457:181–187
Elmqvist LG, Lorentzon R, Johansson C, Fugl-Meyer AR (1988) Does a torn anterior cruciate ligament lead to change in the central nervous drive of the knee extensors? Eur J Appl Physiol Occup Physiol 58:203–207
Ferrell WR (1980) The adequacy of stretch receptors in the cat knee joint for signalling joint angle throughout a full range of movement. J Physiol 299:85–99
Friden T, Roberts D, Zatterstrom R, Lindstrand A, Moritz U (1999) Proprioceptive defects after an anterior cruciate ligament rupture—the relation to associated anatomical lesions and subjective knee function. Knee Surg Sports Traumatol Arthrosc 7:226–231
Garland SJ, Gerilovsky L, Enoka RM (1994) Association between muscle architecture and quadriceps femoris H-reflex. Muscle Nerve 17:581–592
Gauffin H, Pettersson G, Tegner Y, Tropp H (1990) Function testing in patients with old rupture of the anterior cruciate ligament. Int J Sports Med 11:73–77
Guiheneuc P, Ginet J (1974) Study of the Hoffman Reflex obtained at the level of the quadriceps muscle of normal human subjects. Electroencephalogr Clin Neurophysiol 36:225–231
Heroux ME, Tremblay F (2005) Weight discrimination after anterior cruciate ligament injury: a pilot study. Arch Phys Med Rehabil 86:1362–1368
Hess CW, Mills KR, Murray NMF (1986) Magnetic stimulation of the human brain: facilitation of motor responses by voluntary contraction of ipsilateral and contralateral muscles with additional observations in an amputee. Neurosci Lett 71:235–240
Hogervorst T, Brand RA (1998) Mechanoreceptors in joint function. J Bone Joint Surg Am 80:1365–1378
Hopkins JT, Ingersoll CD (2000) Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J Sport Rehabil 9:135–159
Houk JC, Yack HJ (2003) Associations of knee angles, moments and function among subjects that are healthy and anterior cruciate ligament deficient (Acld) during straight ahead and crossover cutting activities. Gait Posture 18:126–138
Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD (1998) Development of a patient-reported measure of function of the knee. J Bone Joint Surg 80:1132–1145
Kukowski B, Haug B (1992) Quantitative evaluation of the silent period, evoked by trancranial magnetic stimulation during sustained muscle contraction, in normal man and in patients with stroke. Electroencephalogr Clin Neurophysiol 32:373–378
Liepert J, Tegenthoff M, Malin JP (1995) Changes of cortical motor area size during immobilization. Electroencephalogr Clin Neurophysiol 97:382–386
Mills KR, Nithi KA (1997) Corticomotor threshold to magnetic stimulation: normal values and repeatability. Muscle Nerve 20:570–576
Mrachacz-Kersting N, Sinkjaer T (2003) Reflex and non-reflex torque responses to stretch of the human knee extensors. Exp Brain Res 151:72–81
Nakashima K, Wang Y, Shimoda M, Sakuma K, Takahashi K (1995) Shortened silent period produced by magnetic cortical stimulation in patients with Parkinson’s disease. J Neurol Sci 130(2):209–214
On AY, Uludag B, Taskiran E, Ertekin C (2004) Differential corticomotor control of a muscle adjacent to a painful joint. Neurorehabil Neural Repair 18:127–133
Palmieri RM, Tom JA, Edwards JE, Weltman A, Saliba EN, Mistry DJ, Ingersoll CD (2004) Arthrogenic muscle response induced by an experimental knee joint effusion is mediated by pre- and post-synaptic spinal mechanisms. J Electromyogr Kinesiol 14:631–640
Perreti A, Grossi D, Fragassi N, Lanzillo B, Nolano M, Pisacreta AI (1996) Evaluation of the motor cortex by magnetic stimulation in patients with Alzheimer disease. J Neurol Sci 135(1):31–37
Proiori A, Berardelli A, Inghilleri M, Polidori L, Manfredi M (1994) Electromyographic silent period after transcranial brain stimulation in Huntington’s disease. Mov Disord 9:178–182
Ray J, McNamara B, Boniface S (2002) Acquisition and expression of proximal and distal upperlimb stimulus-reponse curves to transcranial magnetic stimulation. Muscle Nerve 25:202–206
Ridding MC, Rothwell JC (1997) Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalogr Clin Neurophysiol 105:340–344
Risberg MA, Beynnon BD, Peura GD, Uh BS (1999) Proprioception after anterior cruciate ligament reconstruction with and without bracing. Knee Surg Sports Traumatol Arthrosc 7:303–309
Rudolph KS, Axe MJ, Buchanan TS, Scholz JP, Snyder-Mackler L (2001) Dynamic stability in the anterior cruciate ligament deficient knee. Knee Surg Sports Traumatol Arthrosc 9:62–71
Siebner HR, Rothwell J (2003) Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res 148:1–16
Snyder-Mackler L, Delitto A, Bailey SL, Stralka SW (1995) Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am 77A:1166–1173
Urbach D, Nebelung W, Weiler H, Awiszus F (1991) Bilateral deficit of voluntary quadriceps muscle activation after unilateral Acl tear. Med Sci Sports Exerc 31:1691–1696
Wassermann EM (2002) Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol 113:1165–1171
Wojtys EM, Huston LJ (2000) Longitudinal effects of anterior cruciate ligament injury and patellar tendon autograft reconstruction on neuromuscular performance. Am J Sports Med 28:336–344
Wojtys EM, Huston LJ (1994) Neuromuscular performance in normal and anterior cruciate ligament-deficient lower extremities. Am J Sports Med 22:89–104
Young A (1993) Current issues in arthrogenous inhibition. Ann Rheum Dis 52:829–834
Young A, Stokes M (1986) Reflex inhibition of muscle activity and the morphological consequences of inactivity. In: Saltin B (ed) Biochemistry of exercise VI, Human Kinetics Publishers, Champaign, pp 531–544
Zanette G, Tinazzi M, Bonato C, di Summa A, Manganotti P, Polo A, Fiaschi A (1997) Reversible changes of motor cortical outputs following immobilization of the upper limb. Electroencephalogr Clin Neurophysiol 105:269–279
Zanette G, Manganotti P, Fiaschi A, Tamburin S (2004) Modulation of motor cortex excitability after upper limb immobilization. Clin Neurophysiol 115:1264–1275
Zang L, Shiavi RG, Limbird TJ, Minorik JM (2003) Six degrees-of-freedom kinematics of Acl deficient knees during locomiotion—compensatory mechanism. Gait Posture 17:34–42
Zimny ML (1988) Mechanoreceptors in articular tissues. Am J Anat 182:16–32
Acknowledgements
Part of this work served as a partial fulfillment for a Master degree in Human Kinetics by M. E. Héroux. M.E. Héroux received a Graduate Scholarship from the Ministry of Colleges and Universities of Ontario during the completion of this study. François Tremblay is supported by Natural Science and Engineering Research Council of Canada. The authors wish to thank Lisa Francis for her help in revising the manuscript for English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Héroux, M.E., Tremblay, F. Corticomotor excitability associated with unilateral knee dysfunction secondary to anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthr 14, 823–833 (2006). https://doi.org/10.1007/s00167-006-0063-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-006-0063-4