Abstract
Due to its richness and carbon neutrality, biomass is emerging as a useful resource in the hunt for renewable energy sources. The process of pyrolysis is widely used to transform biomass into useful hydrocarbons or alternative energy sources. Lignocellulosic biomass is a renewable energy source that is high in carbon. This review article describes the pyrolysis process, which includes biomass pre-treatment, pyrolysis mechanism, and process product upgrading. The pre-treatments of bio material using chemical and physical methods are investigated. One of the ineffective methods for transforming lignocellulosic biomass—which contains components of hemicellulose, cellulose, and lignin—into solids, liquids, and gases is pyrolysis. The pyrolysis conversion mechanism includes the production of char, depolymerization, fragmentation, and several secondary reactions. Research gaps are also identified, along with suggestions for improving pre-treatment methods. It is also described how the performance of the pyrolysis process is affected by the properties of the feedstock, operating parameters, and biomass kinds. Using a few research concepts, rapid progress in pyrolysis instrument identification is discussed. This study starts with a review of the chemistry of contemporary lignocellulose pyrolysis and moves on to the production of various products like oil, gas, and char.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Concerns over energy supply security and the negative environmental repercussions of using fossil fuels have made the search for alternate and sustainable energy sources extremely tempting in recent decades[1,2,3,4,5]. Most developing countries rely heavily on fossil fuels such as natural gas, coal, and petroleum products for power generation, transportation, and industry [6]. Socioeconomic stability is a predictor of long-term fuel supply. The inadequacy of fossil fuels for long-term viability and their negative environmental consequences necessitates the development of alternative renewable and sustainable energy sources[7, 8]. Because of its capacity to be converted into energy-efficient biofuels employing agrochemical, biochemical, physical, and thermochemical processes, biomass has long been regarded as one of the most promising renewable energy sources[9]. Virgin woods, wastes, energy crops, or agriculture leftovers are all lignocellulosic biomass sources [10]. Agricultural wastes are among the most important un-tapped bioenergy source [11]. Rice straw and husks, corncobs, bagasse, and wooden wastes are examples of these remnants. It would be amazing if these waste materials could be converted into high-quality fuels and energy [12]. The carbon sources in biomass are photosynthetic processes, like parts of a plant’s growth phase, and consume CO2 from the atmosphere.
Biomass is regarded as its most accessible source of carbon that is both sustainable and renewable for the manufacture of biofuels. Biomass is renewable, plentiful, and affordable [13], accounting for around 16% of global energy use annually. Organic materials generated by plants, animals, and other living entities, such as bacteria, are referred to as biomass [14]. Pyrolysis is the non-oxidative thermally decomposition of a lignocellulosic biomass matrix at a higher temperature. This process is classified as slow-pyro, rapid-pyro, or flash-pyro based on process characteristics such as heat rate, temperature, and solids residence time[15]. Due to its capacity to recover both the chemical and calorific value of the biomass feedstock, biomass pyrolysis has proven to be a feasible thermochemical conversion process [16]. The compositional and structural components of lignocellulose biomass are depicted in Fig. 1.
This conversion technology can provide bio-oils, gas, and char yields of up to 70%–95% of the original biomass weight [17]. Temperatures for biomass pyrolysis typically vary from 300℃ to 1000°C [18]. Pyrolysis systems are often energy-intensive, putting their economic viability in industrial applications at risk. Over the last few decades, research has focused on the development of energy-efficient pyrolysis devices that can be used for biomass pyrolysis[19]. Slow or quick pyrolysis is used to break down lignocellulosic materials, depending on the intended pyrolysate (liquid oil or biochar). Slow pyrolysis predominantly creates biochar, with residual tar and syngas seldom used; pyrolysis carbonisation is aided by a conventional heating element or microwave irradiation [20]. The ablative pyrolysis system is depicted schematically in Fig. 2. In contrast to slow pyrolysis, fast pyrolysis transforms lignocellulose biomass into mostly compounds [21]. Pyrolysis of lignocelluloses’ biomass, such as straw and wood, is now seen as a potential alternative to replacing petroleum and stimulating agricultural economies by producing sustainable fuels and chemical commodities. The following sections will go through the differences between slow and fast pyrolysis and how feedstock quality affects the outcome of both processes[22].
2 Thermochemical Conversions
It’s vital to remember that pyrolysis is one form of thermochemical conversion technology. It refers to converting anything utilizing chemical processes triggered by heat. The five basic modes of thermochemical conversion in wood combustion are evaporation (210°C), torrefaction (between 235°C and 320°C), pyrolysis (between 310°C and 660 °C), gasification (between 720°C and 830°C), or combustion (400°C–1900°C).
The moisture in the biomass evaporates at temperatures lower than 200 °C. This stage is crucial because the biomass will stay at 100 °C until the majority of the water has evaporated due to the high latent heat of vaporisation of water (2230 J/g). Mass and absorbency of the biomass must be thoroughly investigated because mass transportation limits may make it difficult for dense materials like hardwood to dry. Chopping and grinding can help to overcome heat and mass transport restrictions. The vapors generated in this stage are mostly water and have a white colour[23].
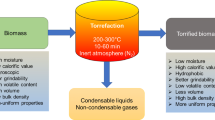
Torrefaction, commonly known as roasting, is the next thermochemical event. Coffee beans are produced using the same method. (When coffee beans are harvested from the plants, they are green!) Torrefaction is a kind of pyrolysis that takes place at low temperatures. Extractives and non-structural light chemicals break down and evaporate between 225°C and 300°C, whereas hemicellulose degenerates and vanishes. Lignocellulosic by-products begin to de-polymerize or generate fluid intermediate on surfaces of biomass cells wall at this temperature. Torrefaction is defined by the breakdown of large macromolecules like cellulose. It can be employed as the initial stage in a stand-alone technology or as a phase in pyrolysis, gasification, or combustion reactors[24]. Torrefaction is important for biomass transportation and combustion because it increases the combustion heating value (in mass terms) of the biomass by about 25% and reduces grinding energy by up to ten times, thanks to the lysis of amorphous cellulose zones that contribute to the formation of brittle fibers.
To put it another way, torrefied biomass produces 20% more heat than non-torrefied biomass, while milling it after torrefaction uses less energy. Torrefaction also produces organic gases, which can be burnt. Torrefaction fumes are acidic because acetic acid attached to the hemicellulose is liberated [25].
Pyrolysis occurs after torrefaction at temperatures ranging from 300°C to 650°C. According to a brief description, pyrolysis breaks down lignocellulose into smaller components. Before vaporizing from porous biomass units, smaller molecules (monomers or oligomers) may create a fluid intermediate. Products of pyrolysis that don’t make it out break down into small molecules or polymerize into char. It’s worth noting that as pyrolysis advances to generate char, the porosity of the biomass rises, lowering the solid’s density.
Pyrolysis is followed by gasification at temperatures ranging from 700°C to 850°C. During gasification, processes transform the carbonaceous solid (char) and pyrolysis vapours into carbon monoxide, water, methane, and hydrogen, resulting in a gas mixture known as “syngas.“ It is often created in oxygen-depleted conditions with a stoichiometric ratio of 15 to 28% for full combustion. Gasification is utilized as a stand-alone method in the chemical and energy sectors to produce hydrogen gas that may be used for various applications. Key processes in gasification include the carbon-oxygen reaction, the Boudouard process, the carbon water reaction, the hydrogenation reactions, the water-gas shifting reactions, or methanation reactions. When oxygen is present, combustion provides the heat needed to complete these reactions, a process known as autothermal gasification. Biomass may be heated externally (with water) to produce a large amount of hydrogen, a process known as external heat source gasification. Gasification is a significant factor for pyrolysis, especially if the heating is not uniform, with some areas of the biomass reaching gasification temperatures while others continue to warm. Steam gasification increases the surface area of biochar generated during physical activation techniques to produce activated carbons[26].
There’s also the issue of combustion. As we all know, combustion necessitates the presence of carbon, oxygen, and a source of high calorific value. Depending on the reactor’s moisture, temperature, air volume, and gas flow patterns, biomass combustion can reach temperatures above 2000 °C. Some pyrolysis vapours or syngas are burned in the vapour form in slow pyrolysis or direct gasification to send heat back to the solid biomass[27]. Char oxidation occurs when oxygen combines with the rich carbon residue left over after pyrolysis. Ash is a white powder that remains after the full combustion of biomass and is usually created by inorganic elements. Primary mass loss from pyrolysis lasts till 600°C, but secondary mass loss from char oxidation lasts until 1300°C [28]. Even though combustion does not occur within the pyrolysis reactor, it is typically an important part of the process. Slow pyrolysis reactors and quick pyrolysis systems generate heat from pyrolysis products (vapour or gases) to keep the process running[29]. Thermochemical reactions in the combustion of wood can be seen in Fig. 3.
3 The Influence of Lignocellulose Structure on Pyrolysis
A fundamental understanding of the characteristic of lignocellulosic’s biomass is essential to effectively demonstrate the mechanism of pyrolysis. In this section, we are discussing the property of lignocellulosic’s biomass. Lignocellulosic biomass is a complex biopolymer composed mostly of celluloses, hemicelluloses, or lignins[30]. Lignocellulosic biomass can be seen in Fig. 4. Lignocellulosic biomass generally contains 45 to 55% celluloses, 10–25% hemicelluloses, and 10–25% lignins. Cell walls of lignocellulosic are composed of cellulose, a linear polysaccharides polymers of glucose held together by a -1, 4-glycoside bond[31]. Varieties of hydroxyl group are found in the cellulosic chain, leading to hydrogen bond formation [32]. There are both amorphous’ (low crystallinity) and crystalline (high crystallinity) cellulose regions (low crystallinity)[33]. Hemicelluloses and pectins interact with cellulosic micro-fibrils, while lignins protect them [34]. In contrast to celluloses, hemicelluloses are more amorphous, unpredictable, and diversified. Pentose (arabinoses and xyloses ), hexose (glucose, galactoses, mannoses, rhamnoses, and fucoses), and uronic acid make up hemicelluloses (glucuronic acids, methyl glucuronic acids, and galacturonic acids)[35]. Because of the shorter and branch chain of hemicellulose, which allows the creation of a network with cellulosic micro-fibrils and lignins, the lignocellulose matrix is particularly rigid. Lignins are naturally occurring water-insoluble or optically inert chemical molecules. Propyl-phenol is an aromatic and hydrophobic amorphous biopolymer with a complicated aromatic structure[36]. Three important phenol-containing components are p-coumaryl, sinapyl alcohols, and coniferyl (Fig. 5). The C-O (-O-4, -O-4, and 4-O-5 links) and C-C (-5, 5–5, -1, - connections) linkage connect these components[37]. Lignins are also necessary for cross-linking celluloses and hemicelluloses, resulting in a hard and three-dimensional cell wall structure; furthermore, because of their higher carbon contents, lignin stores around 40% of the energy in lignocellulosic’s biomass. Softwoods generally contain more lignins than hardwoods, and most agricultural byproducts have more lignins than hardwoods [38].
4 Biomass Pyrolysis
Following the primary decomposition of biomass, condensable products are converted to low-molecular-weight vapors or char, resulting in pyrolysis products (Fig. 6). Biomass frequently breaks down the massive hydrocarbon particles into simpler hydrocarbons. Bio oils are a biomass-derived pyrolysis liquid containing trace levels of alkanes, aromatic chemicals, phenol derivatives, ester, ketone, ether, alcohol, and amine[39]. Several factors impact the chemical composition of bio-oils, including the kind of biomass utilized, processing parameters (temperature, heating duration, and residence time), and the condensing process (condensing technique as well as cooling rate) [40]. Pyrolysis is classified into three types based on the pace at which heat is applied and the kind of product selected (vapour, solids, or liquids) such as rapid, intermediate, and slow pyrolysis. The pyrolysis mode and operation circumstances can affect relative quantities of gas, liquid, and solid products [41]. The fast pyrolysis process, in which biomass is rapidly heated in the absence of O2 by introducing an inert-gases into reactions, may be carried out at a higher altitude of 500 -700 °C; feedstock reaches maximum temperature before breakdown processes begin[42]. For a quick char removal design, this method necessitates that feedstock be produced into tiny particle sizes. The average diameters of solid material like biomass, measured in microns, are referred to as particle size. Slow pyrolysis generates some solid and gas charcoal while employing moderate heating temperature, a lengthy vapor-residences rate, or a low temperature than fast-pyro; char is an end product of slower pyrolysis. Intermediate pyro processes may occur in temperatures ranging from 500 to 650 °C in fixed beds-pyrolysis reactors. Fast pyrolysis of biomass is the preferred approach due to rapid rates of reactions or higher yield of liquids product. Several studies have been conducted in the last 10 years on the rapid pyrolysis process, many researchers have looked at the distribution and combination of these primary biomass pyrolysis product’s (solid, liquid, and gas) produced by various types of bio mass using various operating’s settings or reactor designs. Temperature is one of the most important factors to consider during the pyrolysis process since it has a direct influence on production of bio-oils. At high temperature, char production diminishes considerably. This happens as a result of early deterioration of bio mass at higher temperature, followed by thermal breakdown that results in the formation of char. To reduce the oxygen and water content, viscosity, and acidity of bio-oils using several up-grading procedures. Furthermore, more progress was made by optimizing reaction conditions and improving realistic models for bio-oil production related to the kinetics of biomass thermal decomposition [43].
5 Reactor Configurations
The particle interactions in reactors, dependent on the pyrolysis reactors’ form, add several layers of complexity to pyrolysis operations [44]. Here are some examples of traditional pyrolysis reactors configuration:
5.1 Ablative Reactor
The pyrolysis action of an ablative reactor is analogous to butter melting on the surfaces of a frying pan. In these reactors, biomass is thermally “eroded” by pressing against heated reactor walls. Compression, the relative velocity of biomass on heating systems, or reactors temperatures all impact reaction rates. This reactor can handle large feedstocks and delivers good char abrasion[45]. The CNRS in Nancy, France, undertook breakthrough research on the relationship between pressure, temperature, and motion in an ablative pyrolysis reactor in the 1980s.
5.2 Auger Reactors
Auger reactor is a continuous tubular reactor that uses a spinning screw to elate solid biomass and transmit warmth along tubular reactor walls for pyrolysis. The screws serve two functions in this approach: first, this mixe the feeds, and second, it controls the biomass residences time in reactors. These reactor architectures are highly compact and, in some cases, even portable, allowing them to be used on a biomass production site or where biomass is easily available. Increasingly, on-site biomass conversions reduce operational expenses by removing the price of shipping feedstock to biorefineries [46]. Ingram et al. demonstrated the speedy pyrolysis of woody biomass carried out in an auger reactor. The bio-oils produced in their study were found to be equal to bio-oils produced using fluidized beds or vacuum pyrolysis procedures. However, if compared to the heat-transfer rate achieved in fluidized beds reactor, the approach demonstrated a low heat-transfer rate, showing that there is still space for improvement[47].
5.3 Fluidized-beds Reactor
Fluidized-beds reactor(FBR) is a mostly researched and successful pyrolysis reactor. In this reactor, bio mass particle is quickly heated by fraternization with streams of swirling sand unit at high temperature. A volatile mixture of sand and bio mass particles results in a higher mass or heat-transfer coefficient, creating an ideal setting for rapid pyrolysis. Heat is given to beds either directly (adding hot solid to beds) or indirectly (hot gas/steams delivered via a tube in beds) by external burning of bio-oils/char. Fluidized-beds reactors (FBR) also provide a straightforward method, are easy to scale up, and provide total control over your home time[48]. FBR is classified as (a) entrained, (b) bubbling, and (c) circulating. The suspension of bio mass in these reactors enables quick thermochemical conversion, which is the major issue. In a bubbling fluidized-beds reactor, the gas velocity is typically 1.7 to 1.9 times the minimum fluidization velocity. In entrained Fluidized-beds reactors, biomass particles might fall freely under gravity or get entrained due to actions of gas drag forces (EFBR)[49].
5.4 Rotating Cone (Bio mass Technology Groups)
The majority of the research in the development of rotating-cone reactor have been conducted by Bio mass Technology Groups and Twente University of Technology (BTG) [50]. In a rotating cone reactor, biomass is delivered to the bottom of revolving cones and spirals up the rotating cone’s walls due to centrifugal forces. Biomass may be heated fast because of excellent heat conduction from the wall or heated sands. The most notable advantage of this configuration is that no carrier gases are required in conveying vapours, which reduces operational expenses.
6 Biomass Pre-treatments
Pretreatment of biomass is a technique for facilitating conversions of bio mass into marketable commodities[51]. It improves process selectivity for a certain product by making bio mass polymer more approachable by opening up polymers fibre in improving cellulose, hemicellulose, and lignin conversion[52]. The pre-treatment procedure depicted in Fig. 7 is a simplified schematic.
6.1 Physical Pre-treatment
The particle size of the feedstocks has an impact on yields and qualities of the bio-oils generated throughout the manufacturing processes. Due to continuous temperature exchange inside molecules, tiny particles are favoured in quick pyrolysis, whereas insufficient temperature exchange in larger units centralizes in relative particle temperatures and results in a decreased liquid yield[53]. Many scholars have looked at the effects of woody biomass particles diameter on pyrolysis processes in literature[54]. The heat-resistance distances between particle surfaces and its core rise as particle size grow, preventing the pyro reactions from being accomplished by reducing heat transmission to bio mass [55]. Shen et al.[22] investigated the influence of particle diameter on bio oils yields in fluidized-beds reactors or discovered a 12–14% increase in bio-oils output when particle sizes were reduced from 1.5 mm to 0.3 mm. Among the most significant challenges with the pyrolysis process is the low density of fuel, which may have an impact on pyrolysis yield and composition. To address the low population density of bio mass, an increased density strategy can be used to make it denser for pyrolysis. High bio-oil yields have been achieved as a result of wood densification [51]. It might also be regarded as a good strategy for reducing the moisture content in bio mass and so improving the composition of bio oils [56]. Pelletizing, briquetting, and using a screw extruder are the most prevalent methods for biomass densification[57]. Dry-torrefaction (DT) is a thermal bio mass pre-treatments technique that may be conducted in three different modes depending on the applied temperature: moderate, mild, and severe. Light torrefaction occurs at 200℃, mild torrefaction occurs at 250℃, and severe torrefaction occurs at 290℃ and is primarily responsible for the breakdown of cellulose, hemicellulose, and lignin respectively [52]. Torrefaction improves bio mass structure, leading to greater bio-oils [58].
6.2 Chemical Pre-treatments
Chemical pre-treatments have been utilized to improve pyrolysis quality attributes by removing undesirable inorganic feedstock components. Chemical treatments such as acids and alkaline pre-treatments, hydrothermal pre-treatments, ammonia fiber expansions, and steam explosion have all been developed. Carbides, phosphates, sulfides, and chloride ions are the most common pollutants detected in biomass [59], all of which help in the production of the reaction products. Metals in bio mass, such as potash, sodium, magnesium, and calcium, may disturb the production of oil during pyrolysis. [60]. The inclusion of metal, according to most research, increases ash generation, as well as bio-oils instability and pyrolysis reactor corrosion.
Organic acids, Mineral acids, and diluted acids, such as chloric or sulphuric acid, have all been used as acidic pre-treatments [61]. Acid treatment has been found to remove hemicellulosic carbohydrates by generating hydroxyl acids. The creation of gypsum (CaSO4.2H2O), which is made of calcium sulfate dihydrate, is a disadvantage of acid pre-treatments. Acid pre-treatments, on the other hand, reduce ash content also, improve bio-oils characteristics. Alkali solution with high concentrations and low temperatures, such as Ca(OH)2, NaOH, and NH4OH, had been used in enhancing the biomass structure and partly removing hemicellulose and lignin from biomass. The most often utilized alkaline solutions for the alkaline pre-treatment procedure are NaOH and KOH. By disrupting the glycosidic and ester connections in lignin structures, alkaline pre-treatments at low temperatures have been shown in enhancing bio mass structure[62].
Wet Torrefaction (WT) is a pre-treatment method that uses highly pressurized water at a moderate ambient temperature of 200 °C − 250℃ and a pressure of 45 bar. High-pressure water penetrates biomass during this process, hydrating cellulose, solubilizing, and removing hemicellulose and a little amount of lignin. As a result, WT pre-treatment increases the quality of bio-oil by lowering the quantity of water generated by hemicellulose during pyro processes. Furfurals, phenolic compounds, and others sugar are abundant in the liquid produced by these reactions [63]. For wood preparation, a steam explosion has been created [64]. The wood chips are fed into the vessel at 275 °C, 35 bar for 2. minutes, after which pressure is increased up to 70 bar in 5 s. This approach simplifies the process of obtaining fuel for subsequent transformation. Figure 8 describes the correlation between non-treated and heat burst bio mass.
Ammonia fiber expansions (AFE) have indeed been proven to be an excellent pre-treatment approach for enhancing bio mass structure when bio mass is exposed to ammonium at extreme temps (90°C-140 °C) and pressures (220–3800 psi) [65]. AFE is a mechanical and biochemical reaction that occurs inside a reactor with high pressure and temperature control and fluid ammonium running through it. In a closed reactor, a 1:1 or 1:2 ratio of ammonia to biomass is combined for 10–60 min before being heated to the desired temperatures and pressures[66]. Biological pre-treatments are among the most energy-efficient pre-treatment procedures since it’s done at room temperatures and pressures with No chemicals and energy. The biomass pretreatments in this approach employ microbes and bacteria to break down the major connections between lignin, cellulose or lignin, and hemicellulose [67]. Table 1 lists all pros as well as disadvantages of several lignocellulosic biomass pre-treatments techniques. Mechanical pretreatment has the benefit of being able to manage a big volume of biomass. However, one of the disadvantages of this technology is that it consumes more energy. Furthermore, acid treatment might result in a high sugar yield; nevertheless, one of the disadvantages of this process is the cost of the acid. Ammonia fiber expansions (AFE) is a cost-effective method for processing agro-bio mass for high sugar yields, but it requires ammonium recovery [68].
7 Lignocellulosic Biomass-Based Pyrolysis
As the technology progresses from the lab to the market, studying the chemical processes of bio mass pyrolysis might aid with processing and reactor design. The mechanistic analysis of the pyrolysis processes is difficult because biomass has difficult chemical structures. As result, three primary components of bio mass, hemicellulose, cellulose, and lignin, are most commonly investigated in terms of pyrolysis behaviour [69]. Pyrolysis research has recently benefited from newly established analytical tools such as Py-GC–MS [70]. This approach, on the other hand, was unable to provide extensive information on pyrolysis processes. Dehydration, depolymerization, decarboxylation, and isomerization are all reactions that occur during the pyrolysis process.
The conduct of lignocellulosic bio mass component during pyrolysis is influenced through characteristics such as divergent rates of reaction or permanence of specific items, the link between Items, and the sensitivity of pyro by-products. During lignocellulose pyrolysis, several activities take place, however, they are usually condensed into three main steps: (a) evaporations of available humidities, (b) fundamental bio mass degradations, and (c) reaction (which include oil-cracking and re polymerization). During the initial stage, maximum biomass breakdown occurs around 200℃-400℃, resulting in solid char formation, while subsequent reactions occurring on the internal surface of biomass increase the temperature [71]. Hemicellulose decomposition, which is usually characterized by xylan, takes place between 250℃ to 350℃, on the other hand, cellulose-based pyro takes place between 325℃-400℃, with levoglucosan as a by-product, also lignins, most stable products, degrade at temperatures above 300℃ to 550℃. Table 2 shows the component examination of certain plant biomass samples in general.
7.1 Pyrolysis of Cellulose
Celluloses are supramolecular linear polysaccharides with long glucose chains linked by -1, 4-glycosidic linkages. It interacts with other molecules through inter-molecular and intra-molecular hydrogen bonds, that help in maintaining their crystal lattice and biochemical inertness. The glycosidic linkage in cellulose is fragile and prone to break in an acidic pH or at high temperatures[74]. At 300 °C, the fundamental reaction phase in cellulose pyrolysis begins, which includes breakdown and polymerization events and results in the synthesis of low molecular molecules such as glyceraldehyde, hydroxyl acetaldehyde, furan, formic acid, CO2, and H2O[75]. Decomposition of cellulose can be divided into two ways: first, involving dehydration of cellulose to yield “anhydrous-cellulose”; second cellulose is depolymerized to produce mostly laevo-glucose, with minuscule quantities of other anhydro-monosaccharides[76]. Low temperatures have been demonstrated to impact the initial breakdown step, resulting in less polymerization and the formation of “anhydrous-cellulose” or [77]. Nevertheless, in the high heating system, cellulosic disintegration was manifested as two opposing degrading events, which first produces predominantly charcoal and gases and the other which produces primarily tars. As a result, cellulose breakdown at higher temperatures and higher heating rates has become the most common method for cellulose decomposition.
7.2 Pyrolysis of Hemicellulose
Hemi-cellulose surrounds and joins cellulose fibers with a branch-rich, randomized amorphous form [78]. Some of the saccharides contained in hemicellulose include xylose, glucose, arabinose, mannose, and galactose. Hemicellulose chains are unstructured and ruptured at a temperature lesser than cellulose chains because cellulose’s crystalline structure must be destroyed thermally to free carboxyl at specific heat[79]. At 300 °C, the primary reaction phase in hemicellulose pyrolysis takes place, which involves breakdown and polymerization events and results in the synthesis of low molecular molecules such as glycolaldehyde, hydroxyl acetaldehyde, furan, formic acid, CO2, and H2O[80]. Furthermore, it stimulates glycosidic bond breakage, resulting in the creation of oligosaccharides, and then break downs into xylose, releasing CO2 and water. Cellulose biomass converts to bio-oils along with charcoal as the temperature rises beyond 800 °C. Through breaking and rearrangement of the depolymerized molecule, higher pyrolysis temperatures of xylose produced lightweight oxygenated molecules such as acetic acid, furfural, formic acid, and furan. In addition, the char generated contains active functional groups such as ketone, aldehyde, alcohol, and a carboxylic acid, as well as an Oxygen-containing ring structure created due to polymerization reaction of pyrolysis, intermediated developed throughout reactions sequence with the release of CO2, CO, and H2O[81].
7.3 Pyrolysis of Lignin
Lignin is an aromatic chemical that is found in lignocellulosic bio mass. It is regarded as a carbon-neutral aromatics substrate that is both renewable and sustainable[82]. The primary products of lignin pyrolysis include anisole, phenol, cresol, guaiacol, syringol, and others. When softwood lignin is pyrolyzed, guaiacol is the primary product. Similarly, pyrolysis of hardwoods lignin produces syringol and guaiacol derivatives. According to Zhao et al.[83], the quick pyrolysis of lignin leads to the generation of 39% guaiacol. Guaiacol is often regarded as a typical prototype of lignin’s derived bio-oil because it comprises two types of oxygen and carbon linkages, which are found in the bulk of lignin and its Product’s formed from lignin derivatives. The role of a catalyst has been determined by studying reactions in guaiacol. Whenever OH groups of phenol are transformed, they either follow the aromatic hydroxyl bond’s hydrogenolysis pathway or processes of hydrogenations of the same bond. Reaction’s routes are mostly determined by the catalyst’s composition. The hydrodeoxygenation reaction, which is catalyzed by demethylation/demethoxylation and dehydroxylation, is still the most important (kinetically) during guaiacol catalysis [84]. Hydrogenation of the benzene molecule happens after dihydroxylation[85].
8 Main Factor which Influences Pyrolysis Products Yields
8.1 Temperatures
Pyrolysis’s sophisticated thermal treatment procedure and temperatures have a considerable impact on the outcome. Thermally conversions of lignocellulosic’s waste occur at a temperature above 300 °C, and pyrolysis’ was classified as either rapid (700 to 1200 °C with the heating rate ranging from 100 to 200 °C/s) or slow (300 to 400 °C with a heating rate ranging from 0.1 to 1 °C/s) depending on heating-rate and Temperature [86]. Furthermore, temperatures have an impact on the qualities or dominance of final products (bio-oils, bio-char, and gas). Faster pyrolysis yields more bio-oils than char or gas, with yields distribution ranging from 50 to 70% bio-oils, 10 to 15% charcoals, and 10 to 20% gas outputs, respectively[87]. Slower pyrolysis at 300 to 400 °C, on other hand, yielded charcoals and traces quantities of liquid fuels and gases. [88]. Bio oils production peaks between 400 and 550 °C. The dispersion of bio-oils reduces after heating, and at temperatures above 700 °C, further breakdowns process converts char and bio-oils to gaseous products. The temperature has an impact on the compositions of bio-oils. Whenever temperatures are elevated from 300 to 500 and 600 °C to 800 °C, decarboxylations and dehydration occur, resulting in greater polar, carbon contents, aliphatic and aromatic compounds, and oxygenated hydro-carbons in bio oils [89]. The tar produced by heating green wastes at 600 to 800 °C is dominated by benzene or naphthalene. As temperature climbed, the ratio of large to tiny aromatic rings increased, but the oxygen-containing functional ring disappeared. The formation of hydrogen increases with increasing temperature.
8.2 Residence Times
One of the most important operational factors influencing product generation and dispersion during pyrolysis processes is residence times. A short residence time favored bio-oils generation by reducing secondary reactions. As the residence time rises, organic vapours produced by lignocellulosic bio mass are transformed to total gas and char. At temperature below 900 °C or 850 °C, increased residence times results in more tar or gas. Sweet-gums hardwood and raw-sorghums pyrolysis’ at 700- or 525 degrees Fahrenheit for 0.7–1.7 s and 0.2–0.9 s, respectively, led to a 24% and 31.8% drop in bio-oils yield. Ningbo et al.[90] discovered that higher temperatures and lengthy residence durations cause the production of phenol in bio-oils. 6-minute residences stay at 900 °C could result in a 6.5% energy profit.
8.3 Particles Size
The selection of biomass particle size is critical for optimal pyrolysis and product output[91]. The low thermal conductivity of biomass had a greater impact on its reaction rates and heat-transfer effects. Many studies recommend using smaller-size bio mass to achieve speedy and equal heating of bio mass [92]. If the diameter of the bio mass particle is very tiny, it would be thermally degraded very fastly, turning bio mass into persistent gas. This might affect bio-oils production during bio mass pyrolysis[93]. Fast pyrolysis breaks down tiny biomass units into inert gases, which are subsequently evacuated from reactors.
8.4 Reactions Environment
Because pyrolysis processes are carried out in an inert-gases environment to avoid influences of oxygen, the reaction environment is critical. Another gas, like steams, carbon dioxide, hydrogens, carbon monoxide, methanes, or heliums, are being explored as reaction environments. According to Kan et al.[72], gases in reaction environments, could impact pyrolysis processes and product distribution. The organic oxygenated compound is made whenever steams are used in an inert atmosphere. Steams also prevent further cracking reactions [94]. In a methane gas reaction setting, the highest bio-oils yield is 58.7%. Similarly, CO generates a little number of bio-oils (49.6%), which is higher in the methoxy-containing compound which is larger than phenol. Whenever carbon dioxide is used in sweeping gases, it creates methoxy-containing mono-functionals phenol as well as char with various chemicals composition and increased surface areas [95]. These also increased the generation of permanent gas by allowing for more thermal cracking of volatiles and the consequent development of char. When hydrogen is used as an inert gas, greater heating value bio-oils are produced[96]. Furthermore, hydrogen radical reacts with oxygenated and olefin in a methane environment to form furan.
8.5 Bio Mass Moistures Content
The presence of a substantial amount of water in biomass is most commonly related to its moisture content. During pyrolysis, the water component of bio mass evaporates, leaving the bio mass fluid-free. Much research has demonstrated that bio mass with decreased moisture content yields a more cost-effective product[97]. Because too much water in the biomass might lead to the formation of tar. As a result, both the quality and quantity of pyrolytic products are lowered. Higher water content in biomass necessitates higher heat energy for drying, raising biomass temperatures. In contrast, the moisture content of biomass is a significant disadvantage in pyrolysis’. As a result, choosing bio mass with a moisture level of less than 30% is preferable[98].
9 Pyrolysis Product
The volatile product, which is turned into a fluid portion of the pyrolysis technique known as oil after condensation, is by far the most important pyro outcome. Hundreds of organic compounds can be identified in these bio-oils, including alcohols, ketones, phenols, aldehydes, and oligomers. Furthermore, pyrolysis processes produce ash, a solid by-product. Toxic metals could be present inside the pyrolysis solid substance, which was introduced to the biomass during the raw - materials collection and processing. Bio oils must be characterized to determine reactor design parameters, build kinetic parameters, and commercialize them.
9.1 Bio Oils
The pyrolysis procedure produces bio-oils, yellow to brown liquids with a stronger odour. Because of their poor properties, bio-oils are regarded as a clever blend of chemicals with severely restricted use. Because of its reduced calorific value, increased water content, or acidity, raw bio-oil can only be utilized as boiler fuel[99]. The chemical makeup of the produced bio-oils is heavily determined by the kind of biomass and the pyrolysis conditions. In most situations, water accounts for 15–35% of the weights of bio-oils. Water is unavoidable due to moisture in feedstocks and certain processes like dehydration which occurs during thermal decomposition of bio mass [100]. The inclusion of water in bio-oils is viewed negatively since it lowers heating values and promotes phase separation. It aids in lowering the pH of bio-oil. Bio oils have lower heating values (LHV) due to their increased water content and absence of oxygen[101]. Whenever pyrolysis’ temperature is raised to a certain maximum, the production of bio-oils increases, but then falls when temperatures are raised higher[64]. Despite this, it has been discovered that increasing the cellulose component of feedstocks enhances bio-oils output[102]. As a result, factors like bio mass types and operating conditions may have an impact on bio-oils yield[103]. The most common feedstocks identified to make bio-oils via pyro and hydro-thermal processes were rice husk, oil palm, cotton stalk, and palm kernel shell[104]. Table 3 compares several types of bio mass with their respective bio-oil outputs.
9.2 Bio-char
The solid product of lignocellulosic bio mass pyrolysis, biochar, is a highly carbonaceous substance with carbon contents ranging from 65 to 90%. The physical, as well as chemical properties of solid bio char, is substantially determined by the kind of feedstocks and process conditions, which influences its widespread use. By contracting the bio-oils production, the biochar yields were determined maximum in slower-pyrolysis (550 °C) [107]. Similarly, their microporous nature, high specific surface area, and cation exchange capacity improve their ability to filter and adsorb harmful contaminants[108]. These serve as a forerunner for mass manufacturing of activated Carbon because of their adsorptive capacity and physic-chemical activation [109]. They’ve also been used in catalysis, detoxication, composting of fermentation, and electrochemical energy packing. Because of its higher nutrient retention capacity in soils and ability to produce symbiotic habitats for micro-organisms, biochar may also be utilized as an organic fertilizer. As a consequence of biochar effects on production and carbon-sequestrations, it saves significantly more GHG than direct biomass burning [110]. According to Akom et al.[111], roughly 0.36 to 0.46 kg of biochar may be produced from 1 kilogramme of lignocellulosic bio mass, with a conversion rate of 36–45 per cent. Table 4 compare different form of bio mass with their relative’s bio char yield.
9.3 Bio-gases
CO, H2, CO2, hydrocarbon gas (C1-C4), and H2S were the most common gases produced during biomass pyrolysis[115]. Pyrolytic gases are classified into three types: in-combustible gas (CO2 or H2O), combustible gases (CH4 or CO), and nitrogen-containing gas (HCN or NH3) Lower pyrolysis’ temperature provides low gas yield, whereas high pyrolysis temperatures produce high gas yield. As temperatures rise, bio mass undergoes secondary reactions, resulting in the generation of pyrolytic gas. Furthermore, adding zeolite catalysts for pyrolysis’ at 500 °C increases the generation of pyrolysis gases. [116]. According to the literature, CO2 is usually generated as a result of the breakdown process. Carbonyl and carboxyl groups are mostly generated during the biomass pyrolysis process, whereas CO is primarily created. C-O-C and C = O bond is severed[117]. H2 is primarily created through the breakdowns of aromatic or C-H groups. However, at a lower temperature, CO2 and CO were the main gas produced. At high temperatures, CH4 is a significant result of lignin depolarization reactions. [118].
10 Applications of Lignocellulosic Derived Products
10.1 Biochar
10.1.1 Removal of Organics Pollutant
Biochar has been shown to lower the presence of pollutants in soil and their uptake by organisms living and developing in the soil [119]. Compared to wood-derived biochar generated at a low pyrolysis temperature, biochar produced at a substantially higher pyrolysis temperature had higher surface area and microporosity, which helped to reduce pesticide availability in soil. Even though the pollutant removal efficiency was greater with biochar unamended soil, the pesticides’ fixation capacity was still higher with biochar amended soil, making it less harmful to plants due to lower absorption [120]. However, when the amount of biochar in the amended soil increased, pesticide depletion increased [121]. Green waste-derived biochar was also found to greatly reduce the leaching effect of simazine and atrazine on groundwater [122]. It has also been discovered that electron-deficient cationic dyes may be electrostatically adsorbed onto electron-rich anionic biochar. At a higher pH, the polar antibiotic sulfamethazine is adsorbed better because a hydrogen bond is formed in-between it[123][124]. The COOH and OH groups of woody bio-char, while at a low or neutral pH, - electrons donor-acceptors interaction and cation-exchanges interactions are dominant interaction between bio char and SMZ [125]. As a result, it demonstrates how ph. levels influence adsorption.
10.1.2 Removal of Inorganic Pollutants
Inorganic compounds are very hazardous, posing a significant threat to public health and the environment [126]. Heavy metals are the most poisonous and carcinogenic of them [127], and most inorganic compounds found in industrial wastes and elsewhere are different from them[128]. Biochar generated at low pyrolysis temperatures often has a high carbon content, microporosity, and a variety of functional groups. However, the ion exchange process is still the most important feature for heavy metal removal[129]. The ability of biochar to reduce metals to a stable state is strongly reliant on its physicochemical properties [130]. The interaction of heavy metals is therefore very dependent on the pyrolysis temperature, the biomass from which it was produced, the pH, and the pace at which it was applied. Several investigations have been conducted on the effectiveness of heavy metal extraction in an aqueous system using biochar made from sewage sludge, agricultural wastes, and animal manure [131]. Cu+ 2, like other heavy metals, was attracted to functional groups like COOH or OH on surfaces of woody biochar, and its attraction was pH-dependent. The mechanism of adsorption is more inclined towards cationic exchange at lower pHs, and more inclined towards functional group interaction at higher pH. [132]. Many aspects of biochar, including C and O contents, polarity index, and so on, were influenced by Ph, which was important for high adsorption. Bio char that has been changed before to pyrolysis has been demonstrated to have a higher capacity for removing Cr+ 6 than bio char made from dung[133]. To conclude, the removal of inorganic contaminants is highly reliant on the procedure, feedstock type, and a variety of biochar physiochemical properties.
10.1.3 Removal of Dyes
Biochar is used as a sorbent to remove organic pollutants from wastewater[134]. Dyes and phenols are two of the most challenging organic pollutants to eliminate [112]. Dye wastewater is generated in a range of industrials process, including dyestuffs productions, dyeing’s, printings, or garment productions, and it is also used as a metallic detector and biological staining indicator[135]. The dye molecules and the biochar surface area interact electrostatically during dye removal processes[136]. In addition to electrostatics contact, pore filling is a common mechanism of interactions. Carbonaceous materials’ wide surface areas and pores size aided in the removal of organics contaminants due to the action of pore-filling. Dye effluent from garment dyeing operations accounts for 10–15% of all dyes used in the industry, amounting to 1.5–108 m3 each year[137]. Textile colors, for example, had been successfully removed using pyrolysis’ biochar generated from the macro alga, which is difficult to degrade because of their light or oxidizing agent stability, also their susceptibilities to aerobics digestion[138]. The dyes adsorptions isotherm or kinetic on MDBC800 is characterized by Freundlich isotherms and pseudo-second-orders models, respectively. MDBC800 has maximum dye adsorption potentials, with 5306.2 mg/g, particularly for Malachite Green[139]. According to these findings, rice husk hydrochar outperformed pyro-chars in terms of methylene blue, iodine, or copper adsorptions. The higher methylene-blue adsorption capability is thought to be because of improved oxygen functionality on surfaces of biochar, which make biochar very negatively charged overall. According to an independent study, the most important component which enhances methylene-blue adsorptions is cation exchange[140].
10.1.4 Farming Systems
Biochar application to agriculture has been promoted as a solution for mitigating climate change and improving soil quality. Biochar is produced by pyrolyzing uncontaminated or sustainable biomass sources, like forests, agricultural leftovers, livestock manure, or other organics waste, at low temperatures (300 -600 °C) in anoxic conditions. The resulting substance, that could be applied to soil, had significant levels of non-biodegradable carbon that may remain for hundreds to thousands of years. Carbon stability in biochar varies with pyro-input temperatures. Singh et al. [30] reported that the stable carbon content of biochar formed by slower pyrolysis ranged from 45% (poultry litter pyrolyzed at 400 degrees Celsius) to 92%. (Woods feed-stocks pyrolyded at 550 °C). Whenever it comes to carbon sequestration tactics, this approach’s USP is the far greater longer-term stability of carbon in biochar compared to normal methods for bio mass disposals[141]. Singh et al. reported that stable carbons level of slower-pyrolyzed biochar ranged from 50% (poultry litter pyrolyzed at 450 °C) to 94%. (Wood feed-stocks pyrolyzed at 600 degrees Celsius). Whenever it comes to carbon sequestration tactics, significantly greater long-term carbon stability in biochar compared to traditional bio mass disposal methods is this approach USP[142,143,144].
10.1.5 Climatic Change Impacts
Biochar production methods and subsequently biochar applications in agricultural soils, as indicated previously on several occasion in the text, could contribute to GHG emission reductions or helps battle climatic changes in a variety of ways. These are primarily accomplished by the stabilization of biomass carbon and its long-term storage in soils, as well as the recovery of energy from pyrolysis co-products. Reduced fertilizer requirements, reduced CH4 (mostly in rice-field) and N2O emission from soils increased crop production or avoided emission from bio mass disposal using ancient techniques all contributing to climatic change mitigations in the biochar system. Including a review of E-LCA research on biochar in paddy fields, carbon footprints of crops produced in bio-char-treated soils vary from 1.43 to 2.79 kg CO2-eq per kilogramme rice grain, demonstrating a considerable reduction compared to milled rice in soil lacking bio char inclusion. A larger range of the result is attributable to different assumptions and processes in the E-LCA study, like feedstock types, bio char stabilities in soils, bio char addition rates, soil GHG emission, energies, and fertilizer displacements implications, or methodological considerations. Carbon storage in biochar is dominating process in climatic effects of biochar systems, according to a contribution analysis undertaken in E-LCA research. This means, that biochar is made up of stable Carbon molecules that contribute considerably to stable soil organic carbon pools[113, 114, 145, 146].
10.2 Bio Oils
10.2.1 Fertilizers
Studies have demonstrated that fast pyrolysis bio-oils are effective for soil conditionings. The fact that Bio oils swiftly react with urea, ammonia, and other related compounds to produce organic nitrogen is a game changer here. These compounds polymerize and solidify when heated, resulting in stable products [147]. These substances are known as slow-release fertilizers. This higher-quality organics fertilizer is produced from wastes bio mass, which increases the economic feasibility of fast pyrolysis’. The new fertilizers are non-polluting to groundwater and don’t include -NH2 groups. As a result, expanding its use and manufacture in the agricultural sector may have significant positive impacts[148].
10.2.2 Resins
Bio oils contain a variety of oxygen and hydrogen organisms that produce instability and a high inclination to polymerize. However, because a large number of compounds containing oxygen groups are produced from fossil-based sources, Bio oils may be used to manufacture value-added chemicals at a low cost. Several tests have shown that Bio oils include several useful components such as phenols, aldehyde, and furan, which might be utilized in chemical products like Resorcinol Formaldehyde (RF) resin. RF resin is a wood structural material glue that may be set at room temperature. According to research, Bio-RF oil’s adhesive resin had the best flexural and tensile strength. According to studies, Bio oils generated from woodchips and waste paper have the ability to connect two metal plates with high tensile strength. Furthermore, Bio oils have adhesive characteristics and maximum tensile strength of bonding between two aluminum plates ranging from roughly 2520 N (Bio oils from spruce wood chips) to 2300 N, according to research (Bio oils from waste paper)[149].
10.2.3 High-value Chemicals
The majority of industrial and everyday chemicals are now derived from fossil fuel feedstocks. Nonetheless, in recent years, research on the synthesis and separation of compounds from bio-oils has been boosted[150]. A variety of methods have been used to separate chemicals from bio-oils. Among these, distillation and solvent extraction processes are two of the most well-known and widely used.
Solvent Extraction
As solvents for extracting chemicals from bio-oils, alkalines solution, ketone, ether, water, super-critical CO2, ethyl acetates, toluenes, and n-hexanes have all been explored[150]. In a technique, phenol was recovered from a bio-oil made from sawdust pyro using a solvent. Caustic soda was prepared after extracting the phenols with ethyl ether and 10% wt sodium phenoxide. Additionally, hydrochloric acid was employed to eliminate phenols[73]. Other studies extracted several organic compounds from forest debris bio-oils using n-hexane, water, and dichloromethane[105, 151].
Distillation
Distillation at high temperatures and air pressure can separate the high-value compounds in bio-oils [19]. The polymerization of reactive organics oxygen compounds like aromatics, aldehydes, and ketones occurs at high distillation temperatures due to a large concentration of reactive organics oxygen compounds like aromatics, aldehydes, and ketones. So as to reduce distillation temperatures and avoid unwanted Reaction’s, vacuum distillation is advised. Furthermore, it has been demonstrated that vacuum distillation improves separation efficiency and that this approach may separate smaller molecules such as acetic and formic acids, as well as hydroxy acetone[152].
10.2.4 Bio Oils as Boilers Fuel and in Heavy-duty Engines
Bio oils generated by the pyrolysis processes may be used as boiler and furnace fuels either directly or in combination with fossil fuels. Bio oils have major advantages as a boilers-fuel as compared to heavier fossil fuels. The burning of bio-oils produced little CO2, NOx, and SOx emissions, which could reduce air pollution and may remove the use of additional techniques in boilers to reduce NOx and SOx emissions.[153][154]. Co-feeding 2.5 wt percent bio-oils with heavy fuel oils, for example, reduced NO and SO2 concentrations by around 2.6 and 7.9%, respectively. The low nitrogen concentration (save in the leaves) and sulfur contents in bio mass, particularly sulfurs specie, led to this advantageous feature[106]. Many kinds of biomass did not include any sulfur species.
Direct Burning
According to research from European countries, direct combustion of bio-oils for heat generation may be economically competitive with the supply of fossil fuel resources. The overall cost of the equal quantity of heat generated by bio-oil-based boilers in Austria, Belgium, and Finland, for example, was 76, 50, and 65% of the cost of heat produced by fossil-fuels-based boilers, respectively. The difference was due to labour expenses, biomass pricing, and so on. In the United States, Red Arrow Products were the first to directly burn bio-oils to create heat. The plants had a capacity of 5 MWth and were powered by bio-oils, charcoal, and pyrolytic gases. The average emissions from their facility were 17.0% CO, 1.2% NOx, and 0.2% formaldehyde. Arsta District Heating Plant in Sweden is the first large-scale industry in Europe to use bio-oils for heating. They selected a fossil-fuels boiler (9 MW heat output capacity) and converted its fuels to bio-oils of diverse origin. Their studies showed that bio-oils may successfully replace fossil fuels in larger-scale boilers while retaining acceptable combustion performance.
Furthermore, they identified the need for newer bio-oil burner systems, which are more expensive than standard fossil fuel burners. In Finland, Fortum may burn more than 40 tonnes of bio-oils in another heating facility (1.5 MW heat capacity). Valtion Teknillinen Tutkimuskeskus (VTT) in Finland tested several bio-oils in a boiler with a heat output capacity of 4 MW. They discovered that optimal bio-oils pumping, air flow rate to bio-oils flow rate ratio and nozzle design may all have a significant impact on the boiler’s efficiency. Furthermore, the water and solid matter content of bio-oils, as well as their viscosity, have a major influence on combustion efficiency. The lack of any additives was the most striking aspect of these studies. Furthermore, the combustibility and emissions of different bio-oils differed. The flame created by bio-oils combustion was larger and lasted longer than the flame produced by mineral oil combustion. The more emissions there are, the more inhomogeneity and high water contents of bio-oils there are [155, 156].
Co-firings
To delay obstructions in the nozzle and sprays of the burner, the bio-oils was co-fed with fossil fuel like coals, diesel, and natural gas. Furthermore, co-firings with fossil fuel may enhance engine efficiencies while minimizing the cost of burner system and engine body modification whenever compared to direct burnings of bio-oils. Ormrod Diesels feed bio-oils derived from pyrolysis’ of woods and fuel into a diesel engine. The engine can run for up to 400 h without deteriorating performance. However, some deposits did build inside feeding pumps and injectors. The Italian researcher investigated the co-feeding of bio-oils with diesel up to 50%. They determined that the bio-miscibility of oils with diesel is low and that injectors are partially destroyed. The engine’s combustion efficiency, however, remained unaltered. The best range for blending bio-oils with diesel, according to Bari and Ge et al., might be 5–30 v percent. Furthermore, they were able to show that co-feeding 20% bio-oils with diesel resulted in no engine problems[19, 157, 158].
Burner Technology for bio-oils
Conventional industrial mineral oil burners are divided into two varieties depending on their structure: “mono-block” (suitable for boilers up to 15 MW) or “dual-block” (suited for boilers more than 10 MW). The atomization system is the primary difference between them. In mono-blocks, higher pressures are used to atomize fuels or inject them into combustion chambers. However, in dual-blocks, a second air blower is used. Mono-blocks technology is low-cost, simple, and compact.
However, erosions or injection blockages are both disadvantages. A mono-block burner may not be a viable option for bio-oil combustions because of the presence of a considerable quantity of solid chars in bio-oils. In contrast, atomizers may use both pressure and auxiliary media like compressed air and steams. In this case, atomization pressure may be reduced, putting less strain on the boiler’s pump, nozzle, and body. Furthermore, the dual-block system can handle higher solids concentrations in the oil than a mono-block system. Furthermore, the oil-to-medium ratio may be simply adjusted. However, the complexity and high cost of the dual-block technique may be considered drawbacks. Because bio-oils contain a substantial amount of solid char, the dual-block approach was recommended for boilers that utilize only bio-oils or co-fed bio-oils with fossil fuel. The atomization procedure is used in systems to lessen stresses on nozzles and pumps. Higher-pressure air was used to achieve atomization. Before combustion, bio-oils and atomization air is mixed together and fed at higher pressure into combustions reactors. This helps to keep the spray stream from being blocked too rapidly. To verify the combustion quality, a flames-scanner is installed on reactors. The flow rate of the air may be changed to increase the quality of the flame.
10.2.5 Bio Oils to Biofuels by Hydro-deoxygenation
Bio oils contain a higher concentration of water, acid, or heavier oligomer components, making them unstable or low in energy. As a result, it is difficult to store, transport, and immediately use bio-oils in vehicle engines. In this concept, hydro-deoxygenation is employed as a process to increase the property of bio-oils as a motor fuel. HDO’S is a catalytic process that decreases the oxygen content of bio-oils under high pressure and temperature conditions. In 1984, the first study of HDO’S of bio-oils was published in the open literature. In trials, hydro-desulfurization catalysts from the fossil-fuel refinery are used for HDO’S of bio-oils. However, the process was not commercialized due to the catalyst’s rapid deactivation (after only a few hours of operation). Alternative reactor designs and process improvements, like two-stage upgrades using lower-temperature HDO’S followed by moderate temperatures HDO’S, are available in addition to transition metals-based catalysts. HDO’S is developed to overcome issues of coking in bio-oil upgrading. Nonetheless, because of high coke production, the HDO’S processes suffer from fast catalysts deactivation. The principal causes of coke generation were stated to be aromatics and sugars. However, a more basic study is required to establish the influence of each of the key components of bio-oils on coking.
10.2.6 Carbonaceous Material from Bio-oils
Carbonaceous material had several applications in a number of sectors. Carbonaceous material could be generated from coals and biofuels. When considering environmental impacts or sustainability of the feedstock, the production of carbonaceous products from biomass is preferred. Furthermore, biomass often contains low levels of sulfur, and the nitrogen content of wood is far lower than that of coal. As a result, carbonaceous compounds generated from biomass have much lower sulfur and nitrogen levels. For many years, researchers have been exploring the production of activated carbon from biomass, which is also a mature commercialization technique. Activated carbon works effectively as an absorbent due to its abundance of micropores and mesopores. Nonetheless, with the exception of some kinds of biomass such as coconut shell and bamboo, the highly developed porous structure of activated carbon results in low mechanical strength.
Hu et al. developed a method for generating carbonaceous material that uses bio-oils or biochar as feedstocks to increase the mechanical strength of biomass-derived carbonaceous materials. This method makes use of the polymerization proclivity of bio-strong oil. Bio oils polymerization solid products are expected to fill pores of biochar, enhancing the strengths of final composites. However, Hu et al. showed that polymerization of bio-oils didn’t fill pores of biochar, but rather generated chemical connections with organic on the surfaces of biochar, boosting the strengths of carbon compounds. However, efficiencies of bio-oils polymerization are inadequate to create a significant yield of carbon compounds by cross polymerization with biochar. Furfurals are therefore used as agents to accelerate polymerization reaction rates. Their findings showed that furfurals could cross polymerize with both bio-oils or biochar, functioning as glue to bind organic in bio-oils and organic in biochar, resulting in higher strength carbon compounds. Their current findings merely imply that furfurals can be used to initiate or accelerate polymerization processes. However, the specific role of organic in bio-oils during polymerization, as well as the exact polymerization mechanism, are yet unknown[159].
10.3 Biogas
10.3.1 Vehicular Use
Biogas may be compressed and used as an alternative transportation fuel in light and heavy-duty vehicles utilizing the same current technique as compressed natural gas automobiles[160]. In numerous countries, biogas is viewed as an environmentally preferable alternative to diesel and gasoline for powering buses and other municipal vehicles. The sound level generated by methane-powered engines is normally lower than that produced by diesel engines, and the exhaust fume pollutants created by methane-powered engines are considered lower than those produced by diesel engines, with nitrogen oxide emissions being exceptionally low. Biogas combustion in mobile engines requires compression to high-pressure gas (> 3000 psi) and is best employed in fleet vehicles. A refueling station may be required to shorten the filling time and guarantee enough fuel storage.
10.3.2 Power Generation
Biogas production is often utilized to create power and heat. This can be accomplished by co-firing the product gas in standalone combined heat and power (CHP) facilities or large-scale power plants. Pyrolysis biogas is a flammable gas that may be used to create power in several equipments, including steam turbines, gas engines, and turbines. While the use of gas in boilers for steam cycles does not generally demand extensive gas treatment prior to power generation, the use of gas in gas engines necessitates a higher level of purification and preparation. One of the most critical parts of the process is the consistency and stability of the fuel provided to the internal combustion engine. It is ensured by the feedstock stability and precisely temperature-controlled treatment conditions of the green pyrolysis unit.
10.3.3 Diesel Production
Biogas can be used in the Fischer-Tropsch process to produce diesel, or it can be catalytically converted to methane, methanol, and dimethyl ether. If the syngas is cryogenically processed, it should be noted that due to carbon monoxide and nitrogen having very similar boiling points of -191.5 °C and − 195.79 °C, respectively, this technology has great difficulty recovering pure carbon monoxide if relatively large volumes of nitrogen are present. Carbon monoxide is eliminated selectively by complexing/decomplexing it with cuprous aluminum chloride (CuAlCl4) mixed in an organic solvent such as toluene. Purified carbon monoxide may be 99.9% pure, making it a great feedstock for the chemical industry. The system’s reject gas contains carbon dioxide, nitrogen, methane, ethane, and hydrogen. The reject gas is then processed with a pressure swing adsorption system to recover hydrogen, and the hydrogen and carbon monoxide are recombined in the appropriate ratio for catalytic methanol synthesis, Fischer-Tropsch diesel, and other uses. Cryogenic purification is not well adapted to generate fuel alone due to the significantly lower net energy gain.
10.3.4 Ethanol and Hydrogen Production
Ethanol as a fuel has considerable potential for lowering greenhouse gas emissions and reducing dependency on imported petroleum in the United States. While 100% replacement of petroleum-based fuels is unlikely, ethanol is predicted to replace up to one-third of domestic petroleum usage in the near future. Mesophilic bacteria are one method for converting syngas to ethanol. Henstra et al. show how bacteria produce ethanol via the acetyl-CoA pathway. During the process, hydrogen and carbon monoxide are oxidized, while carbon dioxide is reduced numerous times until methyl-tetrahydrofolate is formed. The attached methyl group is then converted to acetyl-CoA via acetyl-CoA synthase and carbon monoxide dehydrogenase, along with carbon monoxide produced by the cell’s reduction of carbon dioxide and CoA. After that, acetyl-CoA may be transformed into ethanol and a variety of other valuable molecules[161].
In an experiment, Younesi et al. demonstrated the synthesis of ethanol from syngas fermentation utilizing the bacterium Clostridium ljungdahlii. Younesi discusses the advantages of using microbes over pure chemical processes, the most significant of which are lower energy costs, higher yields, and the avoidance of equilibrium reactions. In preparation for the experiment, Clostridium ljungdahlii was grown and placed in serum bottles containing nutrients. Clostridium ljungdahlii culture media was then placed in sealed bioreactors to allow for anaerobic fermentation[162].
While ethanol has a lot of potential as an alternative fuel, hydrogen may be even more efficient. Hydrogen releases no new carbon into the atmosphere and produces no toxic byproducts. Because hydrogen is extremely volatile, the biggest impediments to its utilization as a fuel are transportation and storage issues. While hydrogen looks to be the perfect future fuel, further progress is needed to make hydrogen more portable and easier to store. These issues will very definitely be resolved in subsequent research. Rhodospirillum rubrum bacteria might be utilized to generate hydrogen from syngas. R. rubrum is a photosynthetic proteobacterium that uses the Water Gas Shift process to convert syngas to hydrogen. This reaction turns carbon monoxide and water into carbon dioxide and hydrogen. Najafpour et al. conducted several investigations to investigate R. the ability of rubrum to convert carbon monoxide to hydrogen while investigating the effects of substrate concentrations in growth media and the implications of using different bioreactors[163, 164].
Najafpour et al. cultivated R. rubrum in a serum bottle with various acetate concentrations in the medium in one experiment. After being exposed to syngas, R. rubrum used the Water Gas Shift reaction to convert carbon monoxide to hydrogen. Following a 120-hour incubation period, samples were obtained to evaluate the reactor’s cell dry weight, acetate concentrations, and carbon monoxide concentrations. At an acetate concentration of 1.5 g/L and a starting carbon monoxide pressure of 0.53 atm, 2.35 millimoles of hydrogen were produced, yielding a 98% hydrogen yield.
10.3.5 Biogas and Sanitation
NANOCLEAN projects are presently focusing on innovative ways to manufacture biogas more efficiently by incorporating iron oxide nanoparticles into organic waste treatment techniques. This technology has the potential to triple the yield of biogas. Faecal sludge is sludge produced by worksite sanitation systems. After collection and transportation, faecal sludge can be treated with sewage in a conventional treatment plant, or it can be treated independently in a faecal sludge treatment plant. Faecal sludge can be composted or treated with organic solid waste in an anaerobic digestion system. Biogas may be produced by the anaerobic digestion of faecal sludge. Excreta management that is appropriate and valorized through the production of biogas from faecal sludge aids in mitigating the effects of poorly managed excreta, such as waterborne infections and water and environmental pollution.
10.3.6 Pyrolysis’ Gases Chromatography/Mass Spectrometry
Py-GC/MS is a chemicals analysis technology that first thermally degrades a substance to generate small and more analytically relevant fragments which could then be separated on GC’s columns or identified in an MS’s instruments. Pyrolysate aggregates and other side products are infrequent since Py-GC/MS decomposes instantly. As a result, chemically unmodified pyrolysate could be studied. Py-GC/MS may be used to investigate resin deterioration, evaluate volatile additives, and analyze the composition of polymers compounds that were difficult to dissolve in solvents. It may be used to investigate polymer, polymer blend, or copolymer which are difficult to investigate using the standard method. However, because a large number of specimens cannot fit within the sample tube, analyzing trace components is difficult. The sample is placed in the microfurnace’s inactive sample container, dropped into the reactors-core, which is wrapped in a higher frequency coil and filled with helium as carrier gases through a switch, and then pyrolyzed. The pyrolysis results were generally consistent due to the small temperature change produced by the sample holder’s smaller capacity.
Single-Shots Py-GC/MS
The simplest fundamental Py-GC/MS method, known as one shot, requires only one heating to accomplish the thermal breakdown. Thermal breakdown occurs at temperatures ranging from 50 to 1000 °C. After the thermal breakdown, samples are vaporized and put in a gas chromatograph. GC was used to separate the gas, and MS is used to quantify it. The advantage of this method is that it can analyze all of the original sample’s components in a single measurement. This means that the component ratio of original solids may be computed. Lacquer has a breakdown temperature of 500 °C, and dimethylpolysiloxane or phenyl methylpolysiloxane GC columns were utilized. Because of its catechol composition, lacquer film has an extraordinarily high polarity. As a result, detection will be diminished if an exceedingly polar column is used. Furthermore, because urushiol is un-detectable at a temperature below 280 °C, the oven temperature must be increased and a heat-resistant column utilized.
Evolved Gases Analysis
EGA (evolved gases analysis) is a method for analyzing gases that are created when a heated substance decomposes or desorbs. A short column and evolved gas sensing distinguish the mechanical setup (EGD). Because many organic macromolecules disintegrate at temperatures lower than 1000 °C, a restricted temperature range of 50-1000 °C is used. In lacquers film investigation, temperature ranges are always set to 50–650 °C, and gases chromatographs oven were adjusted to generate a continuous temperature increase of 10 °C per minute from 50 to 650 °C. EGA examination decomposes a lacquers coating in three phases, according to our findings: low (100 -200 °C), medium (200 -300 °C), and high (300 -500 °C). The first m/z = 60 peaks at lower temperatures (100 -200 °C) were shorter carboxylic acids with various carbon chains. Sugar decomposition was assumed to be responsible for the second peak at medium (200 -300 °C). The other two peaks, m/z = 108 and m/z = 123 were assumed to be the original thermal breakdown peaks of urushiol at high temperatures (300 -500 °C). The peak location of acetone powders, that is acetone insoluble materials removed with acetone, was often in line with the second EGA peak, suggesting that the acetone powder contains a lot of sugars.
Double-Shot Pyrolysis
EGA data can be used to execute double-shot pyrolysis. Simple chromatograms are generated because volatile and non-volatile substances may be examined individually. Double-shot pyrolysis gradually heats samples and examines gases produced only in a certain range. This approach is quite beneficial for determining the properties of plastic and rubbers. There are two types of Py-GC/MS measurements: direct or derivatives. Because high polar chemicals, like urushiol, tend to adhere to GC’s columns and impair analytical sensitivity, using thermal desorptions additive, like tetramethyl ammonium hydroxides (TMAH), that may be examined individually or pyrolyzed with polymers, could be advantageous. This approach is very effective for components with clearly identifiable thermal breakdown points and is good for eliminating a plasticizer that has already been added to samples or solvents which has remained in very smaller amounts. This method, however, can’t be used when samples correspond to energies of heating. Lacquer sap may contain a variety of other admixtures like tung oils, rosins, and tars. The lacquers film and TMAH are combined in a sample cup and pyrolyzed at 500 °C.
11 The Effect of Lignocelluloses Pyrolysis’ on Environments and Economy
11.1 Potential Commercialization Advantage
Pyrolysis’ of lignocellulosic’s biomass is viewed as a longer-term sustainable alternative source of energy in the future of population expansion, increasing energy use, depleting fossil resources, and global warming. As a result, further research is needed to accelerate the commercialization of pyrolysis products. Priority number one is to increase the qualities of pyrolysis’ bio-oils. The qualities or consistency of commercialized pyrolysis bio-oils should be preserved. It is possible if oil had a high degree of non-homogeneity and monomer with lower sub-atomic masses. The monomer of lignins found in biomass produces higher-atomic-weights defined pairs in pyrolysis oils. As a result, biomass with lower lignin content is more desirable because it lowers heavier atomic weight or intensifies pyrolysis oils, resulting in more homogenous fluid[165]. Although water contents (hydrophilic) decrease NOx while enhancing the quality of the bio-oils stream, it too implies that bio-oils are not soluble in water, implying its hydrophobic qualities, which diminishes biofuel’s heating estimates. Whenever bio-oils are stored for an extended period, their thicknesses might rise because of an increase in viscosities. This might have a negative influence on oils if it has to be utilized as a viable fuel. As a result, in order to address this issue, bio-oils shall be modified to improve their qualities. The commercial feasibility of pyrolysis technologies determines their advancement. Due to increased production costs, pyrolysis items are currently unable to compete competitively with a nonrenewable energy source. The pyrolysis’ breakthroughs shall overcome numerous specialized or non-specialized hurdles before they can be commercialized and used in industry. Manufacturing pyrolysis products is substantially less expensive than producing petroleum products. The reactors are the most significant components in a pyrolysis facility, however, it just accounts for 10–15% of overall expenses. Sorting and pounding biomass, stacking or chopping biomass, items assortment or capacity, or other expenditures are incurred. The expenditures generated by a pyrolysis plant would most likely be separated into two categories: capital working or speculative or other expenses. All capital or permanent expenditures include pyrolysis units, fundamental gear, feedstock supplies and capabilities, and office developments (lands, transports, buildings, etc.). The fixed costs are mostly dictated by the kind of biomass feedstocks, facility sizes, and amount of innovation. Unpredictable expenditures include biomass harvesting or feedstock maintenance, utilities, and product transfers. The financial potential of pyrolysis biorefineries is defined by their annual productivity or volumes of biomass processed. Although lignocellulosic biomass has a lower energy density (18 MJ/kg) than petroleum-derived fuel (42.9 MJ/kg), this may be mitigated by large expenses associated with feedstock supply and pyrolysis logistics. Compared to charcoals, which is the major output of slower pyrolysis, fast pyrolysis of lignocellulosic’s biomass is notably favourable economically since it creates higher-rates products such as bio-oils.
11.2 Industrial Application or Environmental Repercussion
Lignocellulosic or other biomass is mostly abundant renewable energy resource on the globe and is recognized as a dependable source of renewable liquids, solid, or gas fuel. Since the beginning of time, mankind has exploited lignocellulosic biomass acres to manufacture raw-material, chemicals, or fuels, that are currently obtained from natural gases and petroleums. However, because coals, natural gases, and petroleums are finite resources that will be depleted in near future, lignocellulosic’s bio-mass energy is being recognized, concentrated, and supported. As a result, much research and experiments to improve energy production from lignocellulosic’s biomass have been done. Many large-scale lignocellulosic’s biomass-based pyrolysis’ factories are in operation. Pyrolysis’ has a number of detrimental environmental implications. There have been several research on quick pyrolysis processes and providing series, but there has not been a comprehensive evaluation of various useful series configurations in terms of their environmental performance and economics when considering a variety of end-products or integrating possibilities. Every year, 160 billion tonnes of lignins are produced worldwide, of which lesser than 5% is used to make lignin-derived goods, while the other 95% is either cooked for energy or dumped in landfills[166]. Lignin combustion directly poses a considerable environmental concern. When processes of converting lignocellulosic’s biomass into bioethanol are coupled with bio-refinery technology, particularly lignin bio-refinery, it could effectively cut GHG emission while remaining cost-effective. Due to the biodegradability of lignins, turning lignin wastes into several valuable products may be a feasible alternative to a typical petrochemical-based refinery. However, lignin valorization is restricted due to its complicated structure, and as it degrades, lignin fractions become very reactive, making them susceptible to the condensation process[167]. In Joensuu, Finland, circulatory Fluidized-beds reactors are integrated into combined heating and power network. It proved to be advantageous in terms of heat integration, logistics, and other productivity-related advantages. Heat integration might help to reduce emissions. Several studies propose hydroprocessing after fast pyrolysis’ due to economic and environmental benefits. Hydrogen sources are important in terms of greenhouse gas emissions (GHG) in this process, with natural-gases hydrogen accounting for around half of total GHG emissions. Each palm oil company formed in Malaysia generated around 5 kg of dry biomass. According to Ghanaian statistics from 2008, lignocellulosic’s biomass produced over 72.20 TJ of energy [168]. Table 5 shows some pyrolysis units worldwide.
12 Advantage of Lignocellulosic’s Bio-mass
Lignocellulosic biomass is composed of several components with varying physical or chemical characteristics. It is a fibrous component of plant materials that lack starches. The term lignocelluloses refer to the three major constituents found in most plants: celluloses, hemicelluloses, and lignins. Lignocelluloses are a complex matrix composed of polysaccharides, phenolic-polymer, and protein. Celluloses, major components of terrestrial plants’ cell-wall, are glucans polysaccharides with enormous energy reserves and significant potential for conversion into biofuels. The ability of first-generation bio-mass (produced mostly from food crops like grain, sugar-beet, or oil-seed) to achieve oil-product substitution, climate change mitigation, and economic growth goals is limited. Their sustainable production is being scrutinized, as is the potential of creating undue competition for the land and water required for food and fiber production. The combined consequences of these concerns have increased interest in developing non-food biofuels. Lignocellulosic feedstocks include cereal straw, bagasse, forest wastes, and purpose-grown energy crops such as vegetative grasses and short rotation forests. These second-generation biomasses may avoid many of the problems that afflicted first-generation biomass and, in the long term, may bring greater cost-cutting opportunities.
The most abundant possible feedstock is lignocellulosic biomass, which consists of agricultural waste (corn stover, crop straws, and bagasse), herbaceous crops (alfalfa, switchgrass), short rotation woody crops, forestry residues, waste paper, and other wastes (municipal and industrial). Bioethanol production from these feedstocks might be an interesting way to dispose of these residues. Importantly, there is no detrimental impact on food security from lignocellulosic biomass resources. Furthermore, in terms of energy security, environmental concerns, employment opportunities, agricultural expansion, and foreign exchange, bioethanol is vital for both rural and urban areas. The polysaccharides cellulose and hemicellulose, as well as lignin, make up the majority of lignocellulosic biomass. Because polysaccharide is more stable and pentose sugar is not readily fermentable by Saccharomyces cerevisiae, the use of lignocellulosic’s bio-mass is more difficult than the use of first-generation feed-stocks. Polysaccharides shall first be hydrolyzed, or broken-down, down into simpler sugar using acids or enzymes before lignocellulosic biomass may be converted to biofuels. To address such issues, several biotechnology-based approaches are being used, like the development of Saccharomyces cerevisiae strain capable of fermenting pentoses sugar, the use of alternative yeast specie capable of naturally fermenting pentoses sugar, and the engineering of an enzyme capable of breaking down celluloses and hemicelluloses into a simpler sugar.
Throughout the EU, including Denmark, Spain, and Sweden, a pilot facility for lignocellulosic’s biomass processing had been constructed. In Ottawa, Canada, Iogen Corporation built the world’s largest demonstration facility for making lignocellulose ethanol (from wheat, barley straws, and maize stovers), with a capacity of 2.5 Ml. Many additional processing plants are either operational or under construction across the world.
Because it can be produced faster and at a cheaper cost than food crops, lignocellulosic biomass has an economic advantage over other agriculturally significant biofuel feedstocks such as corn starch, soybeans, and sugar cane. Lignocellulosic biomass was an important component of a major food crop; it is a plant non-edible half that is now underutilized but has the potential to be used for a variety of uses. In short, biofuels made from lignocellulosic biomass hold the key to addressing society’s basic needs without jeopardizing the country’s food supply.
13 Challenges and Limitations
Pyrolysis is seen as a promising alternative for turning lignocellulosic biomass into valuable Products such as renewables liquids gasoline. Different kinds of research had been conducted to precisely examine its kinetics, process simulations, and detailed characterization of biofuels in order to understand its precise mechanisms. In contrast, despite a comprehensive grasp of the mechanics of the pyrolysis processes and recent improvements in this technology, various factors, beginning with the teething period, must be worked out to overcome those issues. One of the main challenges with the first biomass pre-treatment process is the lack of a coherent and consistent strategy for biomass pre-treatments. External pre-treatments methods like compression, densification, and Torre faction, as well as synthetic pre-treatments methods like acids, alkalines, hydro-thermal, steams explosion, and wet Torre faction, had all been investigated and documented, but still, no investigation over the use of ionic liquids pre-treated lignocellulosic biomass in pyrolysis has been done. Ionic liquids could have a favourable influence on the pyrolysis process and allow for greater efficiency. Another major issue for the processes is the quality of the value-added product. Because of higher oxygen and moisture concentrations, the resultant bio-oils are not appropriate for direct use as a combusting fuel in an automobile engine. As a result, a suitable technique for extracting water and oxygen must be created in order to generate higher-grade bio-oils comparable to current petroleum-derived fuel. So far, numerous enhanced bio-oil technologies, such as solvent extraction, emulsification, hydro deoxygenation, catalytic procedures, and steamy reforming, have been recorded. However, bio-oils output in these techniques is highly erratic, providing further limits to bio-oils generation. Bio oils products must be improved while still producing a higher yield. Another issue is a lack of understanding of the connection between preliminary precursor (raw materials) and total plant functioning.
Reactors are critical components of the pyrolysis process, helping in the main transformation of lignocellulosic biomass. As a result, reactor architecture, heat transfers, and temperature control are crucial for pyrolysis performances. Pyrolysis reactor designs include fluidized beds, flowing fluids beds, ablative reactors, rotating cones reactors, twin screw reactors, and vacuum reactors. Each reactor configuration has its own set of limitations. The necessity for size decreased biomass (shredded or pulverized) as a pre-requisite before thermal degradations are one of the primary difficulties in permanent fluid beds. Particles with sizes as tiny as 2–3 mm are frequently suggested. Even though a fixed fluid bed reactor is a simple reactor with great temperature control, the need for speedy char separation to minimize vapour cracking is a significant challenge. The circulating fluid bed’s primary challenge is that the hydrodynamics are highly complex and must be researched. Similarly, the char combustion in the reactor’s second compartment requires close attention. Another limitation of the approach is the scarcity of substantial studies on heat transport in the larger-scale system. The pilot-scale rotating cone reactor, which runs on wood waste and has a capacity of 250 kg/h, is now operational. The key hurdles with rotating cones reactor are particle sizes less than 6 mm and biomass with lesser than 10% moisture contents. The reactors in ablative reactors are highly sophisticated, and scaling up may be costly because of the vast surface required. Another disadvantage of this reactor is that the heat transmission to the reactor limits the reaction speeds.
To maintain the consistency of the pyrolysis process, overall energies and materials balance must be improved for long-term or lucrative applications. To generate higher-quality bio-oil with a high yield, the process must be economically optimized. Alternative catalysts for zeolites, like calcium oxides and magnesium oxides, must be researched or investigated for bio-oils de-oxygenation. More research is needed to manufacture phenolics-richer bio-oil or improve processes by addressing the inclusion of inorganic salt and carbon-based catalyst in cellulosic biomass.
14 Future Perspective
Lignocellulose pyrolysis products can be considered a sustainable and ecologically friendly carbon-based materials. Many gaps and ambiguities exist in the field of biochar synthesis and valorization, necessitating further investigation as a novel and exciting research topic. To identify the link between biochar structural characteristics and their properties, extensive study is necessary. In some cases, the introduction of some free radicals and functional groups, as well as the formation of some defective structures, will be crucial in boosting the value of biochar. The structure of biochar must be exposed at both the macroscopic and microscopic levels; reliable qualitative and quantitative methodologies are required to characterize the results. Furthermore, the application of molecular mechanics and other theoretical techniques can assist in the building of biochar’s spatial structure, allowing us to acquire a deeper understanding of the biochar structure at the molecular level. To optimize biochar structure and characteristics, a fundamental knowledge of the conversion process is essential. Despite much research on the subject, the specific reaction pathways remain unknown. Priority should be given to the formation of functional groups during biomass conversion and the generation of biochar porous structures. Kinetic studies at the microscopic and macroscopic levels are both necessary and advantageous in understanding biomass conversion to biochar. Product qualities are heavily influenced by preparation conditions such as temperatures, atmospheres, feedstock compositions, and so on. Before evaluating their linkages, each of these situations’ influence mechanisms must be disclosed individually. Various methods, including in situ activation and biomass pretreatment, can also be used to improve the properties of biochar. Coordination of the biochar synthesis process with these techniques can further improve the biochar properties. The inclusion of functional groups, heteroatoms, metal atoms, and other components into biochar may enhance its unique properties, and unique biochar may be formed by surface modification and pore management. Furthermore, the development of novel and environmentally friendly biochar matrix composites with large surface areas and specific surface functions is a promising option. It should be noted that the potential ecotoxicity of the introduced materials should be evaluated before their usage.
Pyrolysis, which uses the energy from biogas generation from agricultural wastes to transform crop leftovers into biochar, has many environmental and economic benefits. It not only provides an appealing alternative for minimizing air pollution caused by the open burning of crop residues, but it also provides a beneficial agriculture sustainable model for reusing agricultural wastes. Because these fresh biomasses have a high moisture content, extra energy is required for baking and pyrolysis if they are used directly as feedstock for biochar synthesis. It is more efficient to use them as raw materials in a marsh gas tank for biogas generation. In comparison to agricultural residues, animal manures and household trash have a comparatively high N content, which might offer a favourable C/N ratio for microorganism development and hence increase biogas generation. Furthermore, biogas residue contains a high concentration of nutritious components that may be reused as fertilizer. Crop residue, unlike wood, has specific properties such as low density, higher mass, thin form, and flexible structure. As a result, employing a standard spiral conveyor to feed crop debris into a pyrolysis kiln is challenging. A larger volume of agricultural debris necessitates an abnormally large volume of the kiln. Although the old method of cutting agricultural residue might result in feeding via a spiral conveyor, it needs more effort and energy. Furthermore, chopped crop debris may be too bulky to be pyrolyzed in a volume-limited kiln. There is no present method that can manufacture biochar on a big scale from agricultural leftovers.
15 Conclusion
The growing demand for sustainable biofuels and biomolecules made from biomass waste has sparked extensive study into biomass-based pyrolysis. Studies that analyze the various kinds of biomass used in pyrolysis and their impact on the pyrolytic products produced have been published. The focus of this investigation, however, is on lignocellulosic biomass and pyrolysis due to its biochemical nature (lignins, hemicellulose, and cellulose). Investigations on the complexity of lignocellulosic biomass and its effects on the process of conversion into fuels and chemicals were conducted. Along with this issue, the economic viability and long-term effects of commercializing the pyrolysis technology were examined. During the pyrolysis process, three different forms of fuels are produced: fluid (bio-oils), pyrolysis-gas (hydro or synthesis gas), or solids (biochar). The bio-oils created during the pyrolysis processes may eventually take the place of conventional fossil fuels. Furthermore, during biomass pyrolysis, hundreds of biochemicals may be produced. The production and quality of pyrolytic products can be increased. It is advised that the processing system be modified to incorporate fuels and chemical synthesis in order to make pyrolysis procedures commercially practical.
References
Dhyani V, Bhaskar T (2018) A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew Energy 129:695–716. https://doi.org/10.1016/j.renene.2017.04.035
Zadeh ZE, Abdulkhani A, Aboelazayem O, Saha B (2020) Recent Insights into Lignocellulosic Biomass Pyrolysis: A Critical Review on Pretreatment, Characterization, and Products Upgrading. Processes 8:799. https://doi.org/10.3390/pr8070799
K N Y TPD, Varjani PSSKRYK (2021) Lignocellulosic biomass-based pyrolysis: A comprehensive review. Chemosphere 286:131824. https://doi.org/10.1016/j.chemosphere.2021.131824
Liu C, Wang H, Karim AM, Sun J, Wang Y (2014) Catalytic fast pyrolysis of lignocellulosic biomass. Chem Soc Rev 43:7594–7623. https://doi.org/10.1039/C3CS60414D
Mushtaq F, Mat R, Ani FN (2014) A review on microwave assisted pyrolysis of coal and biomass for fuel production. Renew Sustain Energy Rev 39:555–574. https://doi.org/10.1016/j.rser.2014.07.073
Mushtaq F, Abdullah TAT, Mat R, Ani FN (2015) Optimization and characterization of bio-oil produced by microwave assisted pyrolysis of oil palm shell waste biomass with microwave absorber. Bioresour Technol 190:442–450. https://doi.org/10.1016/j.biortech.2015.02.055
Gupta M, Savla N, Pandit C, Pandit S, Gupta PK, Pant M et al Use of biomass-derived biochar in wastewater treatment and power production: A promising solution for a sustainable environment.Sci Total Environ2022:153892. https://doi.org/10.1016/j.scitotenv.2022.153892
Gupta R, Pandit C, Pandit S, Gupta PK, Lahiri D, Agarwal D et al (2022) Potential and future prospects of biochar-based materials and their applications in removal of organic contaminants from industrial wastewater. J Mater Cycles Waste Manag 24:852–876. https://doi.org/10.1007/s10163-022-01391-z
Rawat K, BIOMASS TO FUEL (2016) :CONVERSION TECHNIQUES, p.155–94
Rout TK (2013) Pyrolysis of coconut shell. MTech.
Durak H (2016) Pyrolysis of Xanthium strumarium in a fixed bed reactor: Effects of boron catalysts and pyrolysis parameters on product yields and character. Energy Sources Part Recovery Util Environ Eff 38:1400–1409. https://doi.org/10.1080/15567036.2014.947446
Worasuwannarak N, Sonobe T, Tanthapanichakoon W (2007) Pyrolysis behaviors of rice straw, rice husk, and corncob by TG-MS technique. J Anal Appl Pyrolysis - J ANAL APPL PYROL 78:265–271. https://doi.org/10.1016/j.jaap.2006.08.002
Dai L, Wang Y, Liu Y, Ruan R, He C, Yu Z et al (2019) Integrated process of lignocellulosic biomass torrefaction and pyrolysis for upgrading bio-oil production: A state-of-the-art review. Renew Sustain Energy Rev 107:20–36. https://doi.org/10.1016/j.rser.2019.02.015
Fakayode OA, Aboagarib EAA, Zhou C, Ma H (2020) Co-pyrolysis of lignocellulosic and macroalgae biomasses for the production of biochar - A review. Bioresour Technol 297:122408. https://doi.org/10.1016/j.biortech.2019.122408
Papari S, Hawboldt K (2015) A review on the pyrolysis of woody biomass to bio-oil: Focus on kinetic models. Renew Sustain Energy Rev 52:1580–1595. https://doi.org/10.1016/j.rser.2015.07.191
Zhao X, Zhou H, Sikarwar VS, Zhao M, Park A-HA, Fennell PS et al (2017) Biomass-based chemical looping technologies: the good, the bad and the future. Energy Environ Sci 10:1885–1910. https://doi.org/10.1039/C6EE03718F
Mohan D, Pittman CU, Steele PH (2006) Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy Fuels 20:848–889. https://doi.org/10.1021/ef0502397
Zhang H, Gao Z, Ao W, Li J, Liu G, Fu J et al (2017) Microwave-assisted pyrolysis of textile dyeing sludge using different additives. J Anal Appl Pyrolysis 127:140–149. https://doi.org/10.1016/j.jaap.2017.08.014
Czernik S, Bridgwater AV (2004) Overview of Applications of Biomass Fast Pyrolysis Oil. Energy Fuels 18:590–598. https://doi.org/10.1021/ef034067u
Nasuha N, Hameed BH (2011) Adsorption of methylene blue from aqueous solution onto NaOH-modified rejected tea. Chem Eng J 166:783–786. https://doi.org/10.1016/j.cej.2010.11.012
Venderbosch R, Prins W (2010) Fast pyrolysis technology development. Biofuels Bioprod Biorefining 4:178–208. https://doi.org/10.1002/bbb.205
Shen J, Wang XS, Garcia-Perez M, Mourant D, Rhodes MJ, Li C-Z (2009) Effects of particle size on the fast pyrolysis of oil mallee woody biomass. Fuel 88:1810–1817. https://doi.org/10.1016/j.fuel.2009.05.001
Griffin MB, Iisa K, Wang H, Dutta A, Orton KA, French RJ et al (2018) Driving towards cost-competitive biofuels through catalytic fast pyrolysis by rethinking catalyst selection and reactor configuration. Energy Environ Sci 11:2904–2918. https://doi.org/10.1039/C8EE01872C
Qin F, Zhang C, Zeng G, Huang D, Tan X, Duan A (2022) Lignocellulosic biomass carbonization for biochar production and characterization of biochar reactivity. Renew Sustain Energy Rev 157:112056. https://doi.org/10.1016/j.rser.2021.112056
Phanphanich M, Mani S (2011) Impact of torrefaction on the grindability and fuel characteristics of forest biomass. Bioresour Technol 102:1246–1253. https://doi.org/10.1016/j.biortech.2010.08.028
Kumar A, Jones DD, Hanna MA (2009) Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2:556–581. https://doi.org/10.3390/en20300556
Vo TA, Tran QK, Ly HV, Kwon B, Hwang HT, Kim J et al (2022) Co-pyrolysis of lignocellulosic biomass and plastics: A comprehensive study on pyrolysis kinetics and characteristics. J Anal Appl Pyrolysis 163:105464. https://doi.org/10.1016/j.jaap.2022.105464
Nussbaumer T (2003) Combustion and co-combustion of biomass: fundamentals, technologies, and primary measures for emission reduction. Energy Fuels 17. https://doi.org/10.1021/ef030031q
Kh K, Xl B, Rc MR (2014) The effect of low-concentration oxygen in sweep gas during pyrolysis of red oak using a fluidized bed reactor. Fuel 124:49–56
Stöcker M (2008) Biofuels and biomass-to-liquid fuels in the biorefinery: catalytic conversion of lignocellulosic biomass using porous materials. Angew Chem Int Ed Engl 47:9200–9211. https://doi.org/10.1002/anie.200801476
Sienkiewicz M, Kucinska-Lipka J, Janik H, Balas A (2012) Progress in used tyres management in the European Union: a review. Waste Manag 32:1742–1751. https://doi.org/10.1016/j.wasman.2012.05.010
Zheng Y, Zhao J, Xu F, Li Y (2014) Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog Energy Combust Sci 42:35–53. https://doi.org/10.1016/j.pecs.2014.01.001
M’barek I, Slimi H, AlSukaibi AKD, Alimi F, Lajimi RH, Mechi L et al (2022) Cellulose from Tamarix aphylla’s stem via acetocell for cadmium adsorption. Arab J Chem 15:103679. https://doi.org/10.1016/j.arabjc.2021.103679
Klemm D, Heublein B, Fink H-P, Bohn A, Cellulose (2005) Fascinating Biopolymer and Sustainable Raw Material. Angew Chem Int Ed 44:3358–3393. https://doi.org/10.1002/anie.200460587
Alonso DM, Wettstein SG, Dumesic JA (2012) Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem Soc Rev 41:8075–8098. https://doi.org/10.1039/C2CS35188A
Bu Q, Lei H, Zacher AH, Wang L, Ren S, Liang J et al (2012) A review of catalytic hydrodeoxygenation of lignin-derived phenols from biomass pyrolysis. Bioresour Technol 124:470–477. https://doi.org/10.1016/j.biortech.2012.08.089
Zakzeski J, Bruijnincx PCA, Jongerius AL, Weckhuysen BM (2010) The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev 110:3552–3599. https://doi.org/10.1021/cr900354u
Calvo-Flores FG, Dobado JA (2010) Lignin as Renewable Raw Material. Chemsuschem 3:1227–1235. https://doi.org/10.1002/cssc.201000157
Sangaré D, Bostyn S, Moscosa Santillán M, García-Alamilla P, Belandria V, Gökalp I (2022) Comparative pyrolysis studies of lignocellulosic biomasses: Online gas quantification, kinetics triplets, and thermodynamic parameters of the process. Bioresour Technol 346:126598. https://doi.org/10.1016/j.biortech.2021.126598
Di Blasi C (2008) Modeling chemical and physical processes of wood and biomass pyrolysis. Prog Energy Combust Sci 34:47–90. https://doi.org/10.1016/j.pecs.2006.12.001
Lu Q, Li W-Z, Zhu X-F (2009) Overview of fuel properties of biomass fast pyrolysis oils. Energy Convers Manag 50:1376–1383. https://doi.org/10.1016/j.enconman.2009.01.001
Doumer ME, Arízaga GGC, da Silva DA, Yamamoto CI, Novotny EH, Santos JM et al (2015) Slow pyrolysis of different Brazilian waste biomasses as sources of soil conditioners and energy, and for environmental protection. J Anal Appl Pyrolysis 113:434–443. https://doi.org/10.1016/j.jaap.2015.03.006
Jeong YW, Choi SK, Choi YS, Kim SJ (2015) Production of biocrude-oil from swine manure by fast pyrolysis and analysis of its characteristics. Renew Energy 79:14–19
Yu J, Paterson N, Blamey J, Millan M (2017) Cellulose, xylan and lignin interactions during pyrolysis of lignocellulosic biomass. Fuel 191:140–149. https://doi.org/10.1016/j.fuel.2016.11.057
Lede J, Panagopoulos J, Li H, Villermaux J (1985) Fast Pyrolysis of Wood: Direct Measurement and Study of Ablation Rate. Fuel 64:1514–1520. https://doi.org/10.1016/0016-2361(85)90365-5
Badger P, Fransham P Use of mobile fast pyrolysis plants to densify biomass and reduce biomass handling costs—A preliminary assessment 2006. https://doi.org/10.1016/J.BIOMBIOE.2005.07.011
Ingram L, Mohan D, Bricka M, Steele P, Strobel D, Crocker D et al (2008) Pyrolysis of Wood and Bark in an Auger Reactor: Physical Properties and Chemical Analysis of the Produced Bio-oils. Energy Fuels 22:614–625. https://doi.org/10.1021/ef700335k
Jp B, Ys C, Sk C, Yw J (2014) Fast pyrolysis of Douglas fir by using tilted-slide reactor and characteristics of biocrude-oil fractions. Renew Energy 65:7–13
Hornung A (2014) Transformation of Biomass: Theory to Practice, https://doi.org/10.1002/9781118693643
Wagenaar BM, Venderbosch R, Carrasco J, Strenziok R, Aa BJ (2008) Rotating Cone Bio-Oil Production and Applications, p. 1268–80. https://doi.org/10.1002/9780470694954.ch105
Rollag SA, Lindstrom JK, Brown RC (2020) Pretreatments for the continuous production of pyrolytic sugar from lignocellulosic biomass. Chem Eng J 385:123889. https://doi.org/10.1016/j.cej.2019.123889
Gageanu I, Voicu G, Bunduchi G, Bracacescu C (2016)EXPERIMENTAL RESEARCH ON THE PROCESS OF PELLETING SALIX VIMINALIS DEPENDING ON HUMIDITY AND GRANULATION,
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M et al (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686. https://doi.org/10.1016/j.biortech.2004.06.025
A M-M AM (2019) Laboratory studies on the influence of biomass particle size on pyrolysis and combustion using TG GC/MS. Fuel 252:635–645
Qh C, Hj RG, Gs L, Fc YXL (2018) Understanding of formation mechanisms of fine particles formed during rapid pyrolysis of biomass. Fuel 216:538–547
Muazu RI, Borrion A, Stegemann J (2017) Life cycle assessment of biomass densification systems. Biomass Bioenergy 107:384–397
Di Blasi C, Branca C, Lombardi V, Ciappa P, Di Giacomo C (2013) Effects of Particle Size and Density on the Packed-Bed Pyrolysis of Wood. Energy Fuels 27:6781–6791. https://doi.org/10.1021/ef401481j
Mamvura TA, Danha G (2020) Biomass torrefaction as an emerging technology to aid in energy production. Heliyon 6:e03531. https://doi.org/10.1016/j.heliyon.2020.e03531
Neupane S, Adhikari S, Wang Z, Ragauskas AJ, Pu Y (2015) Effect of torrefaction on biomass structure and hydrocarbon production from fast pyrolysis. Green Chem 17:2406–2417. https://doi.org/10.1039/C4GC02383H
Cm PGVG (2018) Effect of alkali metal ions presence on the products of xylan steam assisted slow pyrolysis. Fuel 216:36–43
Isahak WNRW, Hisham MWM, Yarmo MA, Yun Hin T (2012) A review on bio-oil production from biomass by using pyrolysis method. Renew Sustain Energy Rev 16:5910–5923
Cao B, Wang S, Hu Y, Abomohra AE-F, Qian L, He Z et al (2019) Effect of washing with diluted acids on Enteromorpha clathrata pyrolysis products: Towards enhanced bio-oil from seaweeds. Renew Energy 138:29–38
Kumar R, Strezov V, Weldekidan H, He J, Singh S, Kan T et al (2020) Lignocellulose biomass pyrolysis for bio-oil production: A review of biomass pre-treatment methods for production of drop-in fuels. Renew Sustain Energy Rev 123:109763. https://doi.org/10.1016/j.rser.2020.109763
Lynam J, Reza MT, Lynam J, Uddin MH, Coronella C, Reza MT, Lynam JG, Uddin MH, Coronella CJ (2013) Hydrothermal carbonization: Fate of inorganics. Biomass Bioenerg. 49, 86–94. Biomass Bioenergy 2013;49:86–94
Tabil L, Adapa P, Kashaninejad M (2011) Biomass Feedstock Pre-Processing – Part 1: Pre-Treatment. IntechOpen. https://doi.org/10.5772/17086
Sundaram V, Muthukumarappan K, Kamireddy SR (2015) Effect of ammonia fiber expansion (AFEX™) pretreatment on compression behavior of corn stover, prairie cord grass and switchgrass. Ind Crops Prod 74:45–54. https://doi.org/10.1016/j.indcrop.2015.04.027
Mathew AK, Parameshwaran B, Sukumaran RK, Pandey A (2016) An evaluation of dilute acid and ammonia fiber explosion pretreatment for cellulosic ethanol production. Bioresour Technol 199:13–20. https://doi.org/10.1016/j.biortech.2015.08.121
Vasco-Correa J, Ge X, Li Y (2016) Biological Pretreatment of Lignocellulosic Biomass. Biomass Fractionation Technol. Lignocellul. Feedstock Based Biorefinery. Elsevier Inc., pp 561–585. https://doi.org/10.1016/B978-0-12-802323-5.00024-4.
Putro JN, Soetaredjo FE, Lin S-Y, Ju Y-H, Ismadji S (2016) Pretreatment and conversion of lignocellulose biomass into valuable chemicals. RSC Adv 6:46834–46852. https://doi.org/10.1039/C6RA09851G
Liu Q, Wang S, Zheng Y, Luo Z, Cen K (2008) Mechanism study of wood lignin pyrolysis by using TG–FTIR analysis. J Anal Appl Pyrolysis 82:170–177. https://doi.org/10.1016/j.jaap.2008.03.007
Yaashikaa PR, Senthil Kumar P, Varjani SJ, Saravanan A (2019) Advances in production and application of biochar from lignocellulosic feedstocks for remediation of environmental pollutants. Bioresour Technol 292:122030. https://doi.org/10.1016/j.biortech.2019.122030
Kan T, Strezov V, Evans TJ (2016) Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew Sustain Energy Rev 57:1126–1140. https://doi.org/10.1016/j.rser.2015.12.185
Ke L, Wu Q, Zhou N, Xiong J, Yang Q, Zhang L et al (2022) Lignocellulosic biomass pyrolysis for aromatic hydrocarbons production: Pre and in-process enhancement methods. Renew Sustain Energy Rev 165:112607. https://doi.org/10.1016/j.rser.2022.112607
Dai L, Wang Y, Liu Y, He C, Ruan R, Yu Z et al (2020) A review on selective production of value-added chemicals via catalytic pyrolysis of lignocellulosic biomass. Sci Total Environ 749:142386. https://doi.org/10.1016/j.scitotenv.2020.142386
Zheng M, Wang Z, Li X, Qiao X, Song W, Guo L (2016) Initial reaction mechanisms of cellulose pyrolysis revealed by ReaxFF molecular dynamics. Fuel 177:130–141. https://doi.org/10.1016/j.fuel.2016.03.008
Abdulkhani A, Marvast EH, Ashori A, Karimi AN (2013) Effects of dissolution of some lignocellulosic materials with ionic liquids as green solvents on mechanical and physical properties of composite films. Carbohydr Polym 95:57–63. https://doi.org/10.1016/j.carbpol.2013.02.040
Greenhalf CE, Nowakowski DJ, Harms AB, Titiloye JO, Bridgwater AV (2012) Sequential pyrolysis of willow SRC at low and high heating rates Implications for selective pyrolysis. Fuel 93:692–702
Ma Z, Chen D, Gu J, Bao B, Zhang Q (2015) Determination of pyrolysis characteristics and kinetics of palm kernel shell using TGA–FTIR and model-free integral methods. Energy Convers Manag 89:251–259
Huang X, Cheng D, Chen F, Zhan X (2016) Reaction pathways of hemicellulose and mechanism of biomass pyrolysis in hydrogen plasma: A density functional theory study. Renew Energy 96:490–497
Yang H, Li S, Liu B, Chen Y, Xiao J, Dong Z et al (2020) Hemicellulose pyrolysis mechanism based on functional group evolutions by two-dimensional perturbation correlation infrared spectroscopy. Fuel 267:117302. https://doi.org/10.1016/j.fuel.2020.117302
Zhou X, Li W, Mabon R, Broadbelt LJ (2017) A Critical Review on Hemicellulose Pyrolysis. Energy Technol 5:52–79. https://doi.org/10.1002/ente.201600327
Cui Y, Wang W, Chang J (2019) Study on the Product Characteristics of Pyrolysis Lignin with Calcium Salt Additives. Materials 12:1609. https://doi.org/10.3390/ma12101609
Zhao C, Kou Y, Lemonidou AA, Li X, Lercher JA (2009) Highly Selective Catalytic Conversion of Phenolic Bio-Oil to Alkanes. Angew Chem Int Ed 48:3987–3990. https://doi.org/10.1002/anie.200900404
Yang H, Yan R, Chen H, Zheng C, Lee DH, Liang DT (2006) In-Depth Investigation of Biomass Pyrolysis Based on Three Major Components: Hemicellulose, Cellulose and Lignin. Energy Fuels 20:388–393. https://doi.org/10.1021/ef0580117
Saidi M, Samimi F, Karimipourfard D, Nimmanwudipong T, Gates BC, Rahimpour MR (2013) Upgrading of lignin-derived bio-oils by catalytic hydrodeoxygenation. Energy Environ Sci 7:103–129. https://doi.org/10.1039/C3EE43081B
Tripathi M, Sahu JN, Ganesan P (2016) Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew Sustain Energy Rev 55:467–481. https://doi.org/10.1016/j.rser.2015.10.122
Bridgwater AV (2003) Renewable fuels and chemicals by thermal processing of biomass. Chem Eng J 91:87–102. https://doi.org/10.1016/S1385-8947(02)00142-0
Demirbas A, Arin G (2002) An Overview of Biomass Pyrolysis. Energy Sources 24:471–482. https://doi.org/10.1080/00908310252889979
Ateş F, Işıkdağ MA (2008) Evaluation of the Role of the Pyrolysis Temperature in Straw Biomass Samples and Characterization of the Oils by GC/MS. Energy Fuels 22:1936–1943. https://doi.org/10.1021/ef7006276
Ningbo G, Baoling L, Aimin L, Juanjuan L (2015) Continuous pyrolysis of pine sawdust at different pyrolysis temperatures and solid residence times. J Anal Appl Pyrolysis 114:155–162. https://doi.org/10.1016/j.jaap.2015.05.011
Soria-Verdugo A (2020) Chapter 8 - Pyrolysis of sludge and biomass residues. In: Olivares JA, Puyol D, Melero JA, Dufour J (eds) Wastewater Treat. Residues Resour. Biorefinery Prod. Biofuels, Elsevier, pp 155–181. https://doi.org/10.1016/B978-0-12-816204-0.00008-4.
Guedes RE, Luna AS, Torres AR (2018) Operating parameters for bio-oil production in biomass pyrolysis: A review. J Anal Appl Pyrolysis 129:134–149. https://doi.org/10.1016/j.jaap.2017.11.019
Islam MR, Parveen M, Haniu H (2010) Properties of sugarcane waste-derived bio-oils obtained by fixed-bed fire-tube heating pyrolysis. Bioresour Technol 101:4162–4168. https://doi.org/10.1016/j.biortech.2009.12.137
Pütün E, Ateş F, Pütün AE (2008) Catalytic pyrolysis of biomass in inert and steam atmospheres. Fuel 87:815–824. https://doi.org/10.1016/j.fuel.2007.05.042
Guizani C, Escudero Sanz FJ, Salvador S (2014) Effects of CO2 on biomass fast pyrolysis: Reaction rate, gas yields and char reactive properties. Fuel 116:310–320. https://doi.org/10.1016/j.fuel.2013.07.101
Zhang H, Xiao R, Huang H, Xiao G (2009) Comparison of non-catalytic and catalytic fast pyrolysis of corncob in a fluidized bed reactor. Bioresour Technol 100:1428–1434. https://doi.org/10.1016/j.biortech.2008.08.031
Johari K, Saman N, Song ST, Cheu SC, Kong H, Mat H (2016) Development of coconut pith chars towards high elemental mercury adsorption performance - Effect of pyrolysis temperatures. Chemosphere 156:56–68. https://doi.org/10.1016/j.chemosphere.2016.04.114
Lee XJ, Lee LY, Gan S, Thangalazhy-Gopakumar S, Ng HK (2017) Biochar potential evaluation of palm oil wastes through slow pyrolysis: Thermochemical characterization and pyrolytic kinetic studies. Bioresour Technol 236:155–163. https://doi.org/10.1016/j.biortech.2017.03.105
Li P, Shi X, Jiang L, Wang X, Song J, Fang S et al (2022) Synergistic enhancement of bio-oil quality through hydrochloric or acetic acid-washing pretreatment and catalytic fast pyrolysis of biomass. Ind Crops Prod 187:115474. https://doi.org/10.1016/j.indcrop.2022.115474
Bridgwater AV (2012) Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 38:68–94. https://doi.org/10.1016/j.biombioe.2011.01.048
Khuenkaeo N, Tippayawong N (2020) Production and characterization of bio-oil and biochar from ablative pyrolysis of lignocellulosic biomass residues. Chem Eng Commun 207:153–160. https://doi.org/10.1080/00986445.2019.1574769
Rahman AA, Sulaiman F, Abdullah N (2015) Effect of temperature on pyrolysis product of empty fruit bunches. AIP Conf Proc 1657:v
Wang Y, Akbarzadeh A, Chong L, Du J, Tahir N, Awasthi MK (2022) Catalytic pyrolysis of lignocellulosic biomass for bio-oil production: A review. Chemosphere 297:134181. https://doi.org/10.1016/j.chemosphere.2022.134181
Lahijani P, Mohammadi M, Mohamed AR, Ismail F, Lee KT, Amini G (2022) Upgrading biomass-derived pyrolysis bio-oil to bio-jet fuel through catalytic cracking and hydrodeoxygenation: A review of recent progress. Energy Convers Manag 268:115956. https://doi.org/10.1016/j.enconman.2022.115956
Shah Z, Cataluña R, Ceschi M, da Silva R Separation of Phenol from Bio-oil Produced from Pyrolysis of Agricultural Wastes.Mod Chem Appl 2017;Vollume5. https://doi.org/10.4172/2329-6798.1000199
Lehto J, Oasmaa A, Solantausta Y, Kytö M, Chiaramonti D (2014) Review of fuel oil quality and combustion of fast pyrolysis bio-oils from lignocellulosic biomass. Appl Energy 116:178–190. https://doi.org/10.1016/j.apenergy.2013.11.040
Li Y, Xing B, Ding Y, Han X, Wang S (2020) A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass. Bioresour Technol 312:123614. https://doi.org/10.1016/j.biortech.2020.123614
Alabdrabalnabi A, Gautam R, Mani Sarathy S (2022) Machine learning to predict biochar and bio-oil yields from co-pyrolysis of biomass and plastics. Fuel 328:125303. https://doi.org/10.1016/j.fuel.2022.125303
Czajczyńska D, Anguilano L, Ghazal H, Krzyżyńska R, Reynolds AJ, Spencer N et al (2017) Potential of pyrolysis processes in the waste management sector. Therm Sci Eng Prog 3:171–197. https://doi.org/10.1016/j.tsep.2017.06.003
Lee Y, Park J, Ryu C, Gang KS, Yang W, Park Y-K et al (2013) Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500°C. Bioresour Technol 148:196–201. https://doi.org/10.1016/j.biortech.2013.08.135
Akom A, Fanyin - Martin A, Boateng CO-, Otoo E, Dawoe E (2020) Yield and Cost of Biochar Produced by a Locally Fabricated Reactor. J Agric Environ Sci 9. https://doi.org/10.15640/jaes.v9n2a1
Enaime G, Baçaoui A, Yaacoubi A, Lübken M (2020) Biochar for Wastewater Treatment—Conversion Technologies and Applications. Appl Sci 10:3492. https://doi.org/10.3390/app10103492
Cowie A, Woolf D, Gaunt J, Brandão M, de la Cowie RA (2015) R and A. Biochar, carbon accounting and climate change. Biochar Environ. Manag. 2nd ed., Routledge;
Mattila T, Grönroos J, Judl J, Korhonen M-R (2012) Is biochar or straw-bale construction a better carbon storage from a life cycle perspective? Process Saf Environ Prot 90:452–458. https://doi.org/10.1016/j.psep.2012.10.006
González A, Goikolea E, Barrena JA, Mysyk R (2016) Review on supercapacitors: Technologies and materials. Renew Sustain Energy Rev 58:1189–1206
Lewandowski W, Januszewicz K, Kosakowski W (2019) Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—A review. J Anal Appl PYROLYSIS 25–53. https://doi.org/10.1016/j.jaap.2019.03.018
Ee JL, Yk K (2020) Recent advances in the catalytic pyrolysis of microalgae. Catal Today 355:263–271
Zhan H, Zhuang X, Song Y, Liu J, Li S, Chang G et al (2019) A review on evolution of nitrogen-containing species during selective pyrolysis of waste wood-based panels. Fuel 253:1214–1228. https://doi.org/10.1016/j.fuel.2019.05.122
Herath I, Kumarathilaka P, Al-Wabel MI, Abduljabbar A, Ahmad M, Usman ARA et al (2016) Mechanistic modeling of glyphosate interaction with rice husk derived engineered biochar. Microporous Mesoporous Mater 225:280–288. https://doi.org/10.1016/j.micromeso.2016.01.017
Zhang K, Cheng X, Dang H, Ye C, Zhang Y, Zhang Q (2013) Linking litter production, quality and decomposition to vegetation succession following agricultural abandonment. Soil Biol Biochem 57:803–813. https://doi.org/10.1016/j.soilbio.2012.08.005
Lu H, Zhang W, Yang Y, Huang X, Wang S, Qiu R (2012) Relative distribution of Pb2 + sorption mechanisms by sludge-derived biochar. Water Res 46:854–862. https://doi.org/10.1016/j.watres.2011.11.058
Bogusz A, Oleszczuk P, Dobrowolski R (2015) Application of laboratory prepared and commercially available biochars to adsorption of cadmium, copper and zinc ions from water. Bioresour Technol 196:540–549. https://doi.org/10.1016/j.biortech.2015.08.006
Lima DR, Lima EC, Umpierres CS, Thue PS, El-Chaghaby GA, da Silva RS et al (2019) Removal of amoxicillin from simulated hospital effluents by adsorption using activated carbons prepared from capsules of cashew of Para. Environ Sci Pollut Res 26:16396–16408. https://doi.org/10.1007/s11356-019-04994-6
Elhleli H, Mannai F, ben Mosbah M, Khiari R, Moussaoui Y (2020) Biocarbon Derived from Opuntia ficus indica for p-Nitrophenol Retention. Processes 8:1242. https://doi.org/10.3390/pr8101242
Nelissen V, Ruysschaert G, Müller-Stöver D, Bodé S, Cook J, Ronsse F et al (2014) Short-Term Effect of Feedstock and Pyrolysis Temperature on Biochar Characteristics, Soil and Crop Response in Temperate Soils. Agronomy 4:52–73. https://doi.org/10.3390/agronomy4010052
Cao X, Ma L, Gao B, Harris W (2009) Dairy-Manure Derived Biochar Effectively Sorbs Lead and Atrazine. Environ Sci Technol 43:3285–3291. https://doi.org/10.1021/es803092k
Khan S, Chao C, Waqas M, Arp HPH, Zhu Y-G (2013) Sewage Sludge Biochar Influence upon Rice (Oryza sativa L) Yield, Metal Bioaccumulation and Greenhouse Gas Emissions from Acidic Paddy Soil. Environ Sci Technol 47:8624–8632. https://doi.org/10.1021/es400554x
Bolan NS, Choppala G, Kunhikrishnan A, Park J, Naidu R (2013) Microbial transformation of trace elements in soils in relation to bioavailability and remediation. Rev Environ Contam Toxicol 225:1–56. https://doi.org/10.1007/978-1-4614-6470-9_1
Dhahri R, Yılmaz M, Mechi L, Alsukaibi AKD, Alimi F, ben Salem R et al (2022) Optimization of the Preparation of Activated Carbon from Prickly Pear Seed Cake for the Removal of Lead and Cadmium Ions from Aqueous Solution. Sustainability 14:3245. https://doi.org/10.3390/su14063245
Kanjanarong J, Giri BS, Jaisi DP, Oliveira FR, Boonsawang P, Chaiprapat S et al (2017) Removal of hydrogen sulfide generated during anaerobic treatment of sulfate-laden wastewater using biochar: Evaluation of efficiency and mechanisms. Bioresour Technol 234:115–121. https://doi.org/10.1016/j.biortech.2017.03.009
Xu G, Sun J, Shao H, Chang SX (2014) Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity.Ecol Eng
Smith P (2016) Soil carbon sequestration and biochar as negative emission technologies. Glob Change Biol 22:1315–1324. https://doi.org/10.1111/gcb.13178
Wu Q, Zhang Y, Cui M-H, Liu H, Liu H, Zheng Z et al (2022) Pyrolyzing pharmaceutical sludge to biochar as an efficient adsorbent for deep removal of fluoroquinolone antibiotics from pharmaceutical wastewater: Performance and mechanism. J Hazard Mater 426:127798. https://doi.org/10.1016/j.jhazmat.2021.127798
ben Mosbah M, Mechi L, Khiari R, Moussaoui Y (2020) Current State of Porous Carbon for Wastewater Treatment. Processes 8:1651. https://doi.org/10.3390/pr8121651
Guimarães T, Luciano VA, Silva MSV, de Carvalho Teixeira AP, da Costa MM, Lopes RP (2022) Biochar-iron composites: An efficient material for dyes removal. Environ Nanotechnol Monit Manag 17:100645. https://doi.org/10.1016/j.enmm.2022.100645
Nuanhchamnong C, Kositkanawuth K, Wantaneeyakul N (2022) Granular waterworks sludge-biochar composites: Characterization and dye removal application. Results Eng 14:100451. https://doi.org/10.1016/j.rineng.2022.100451
Park C, Rosenblat JD, Brietzke E, Pan Z, Lee Y, Cao B et al (2019) Stress, epigenetics and depression: A systematic review. Neurosci Biobehav Rev 102:139–152. https://doi.org/10.1016/j.neubiorev.2019.04.010
Kar S, Santra B, Kumar S, Ghosh S, Majumdar S (2022) Sustainable conversion of textile industry cotton waste into P-dopped biochar for removal of dyes from textile effluent and valorisation of spent biochar into soil conditioner towards circular economy. Environ Pollut 312:120056. https://doi.org/10.1016/j.envpol.2022.120056
Chen C-L, Hermans L, Viswanathan MC, Fortun D, Aymanns F, Unser M et al (2018) Imaging neural activity in the ventral nerve cord of behaving adult Drosophila. Nat Commun 9:4390. https://doi.org/10.1038/s41467-018-06857-z
Huff M, da Silveira WA, Carnevali O, Renaud L, Hardiman G (2018) Systems Analysis of the Liver Transcriptome in Adult Male Zebrafish Exposed to the Plasticizer (2-Ethylhexyl) Phthalate (DEHP). Sci Rep 8:2118. https://doi.org/10.1038/s41598-018-20266-8
Patra D, Patra BR, Pattnaik F, Hans N, Kushwaha A (2022) Chapter 5 - Recent evolution in green technologies for effective valorization of food and agricultural wastes. In: Hussain CM, Singh S, Goswami L, editors. Emerg. Trends Approaching Zero Waste, Elsevier; p. 103–32. https://doi.org/10.1016/B978-0-323-85403-0.00001-3
Lehmann J, Abiven S, Kleber M, Pan G, Singh BP, Sohi SP et al (2015) Persistence of biochar in soil. Biochar Environ. Manag. 2nd ed., Routledge;
Cheng C-H, Lehmann J, Thies JE, Burton SD (2008) Stability of black carbon in soils across a climatic gradient. J Geophys Res Biogeosciences 113. https://doi.org/10.1029/2007JG000642
Singh BP, Cowie AL, Smernik RJ (2012) Biochar Carbon Stability in a Clayey Soil As a Function of Feedstock and Pyrolysis Temperature. Environ Sci Technol 46:11770–11778. https://doi.org/10.1021/es302545b
Zhang A, Cheng G, Hussain Q, Zhang M, Feng H, Dyck M et al (2017) Contrasting effects of straw and straw–derived biochar application on net global warming potential in the Loess Plateau of China. Field Crops Res 205:45–54. https://doi.org/10.1016/j.fcr.2017.02.006
Paustian K, Lehmann J, Ogle S, Reay D, Robertson GP, Smith P (2016) Climate-smart soils. Nature 532:49–57. https://doi.org/10.1038/nature17174
Fernandez-Akarregi AR, Makibar J, Cueva F, Brañas J, del Campo P, Piskorz J et al (2010) High Quality Fertilizers Based on Biomass Pyrolysis Bio-Oil and Char. Eur Biomass Conf Exhib Proc. 18th EU BC&E-Lyon 2010:1522–6 https://doi.org/10.5071/18thEUBCE2010-VP2.7.23
Fahmi R, Bridgwater AV, Thain SC, Donnison IS, Morris PM, Yates N (2007) Prediction of Klason lignin and lignin thermal degradation products by Py–GC/MS in a collection of Lolium and Festuca grasses. J Anal Appl Pyrolysis 80:16–23. https://doi.org/10.1016/j.jaap.2006.12.018
Ren X, Cai H, Du H, Chang J (2017) The Preparation and Characterization of Pyrolysis Bio-Oil-Resorcinol-Aldehyde Resin Cold-Set Adhesives for Wood Construction. Polymers 9:E232. https://doi.org/10.3390/polym9060232
Hu X, Zhang Z, Gholizadeh M, Zhang S, Lam CH, Xiong Z et al (2020) Coke Formation during Thermal Treatment of Bio-oil. Energy Fuels 34:7863–7914. https://doi.org/10.1021/acs.energyfuels.0c01323
Oasmaa A, Kuoppala E, Solantausta Y (2003) Fast Pyrolysis of Forestry Residue. 2. Physicochemical Composition of Product Liquid. Energy Fuels 17:433–443. https://doi.org/10.1021/ef020206g
Zhang X-S, Yang G-X, Jiang H, Liu W-J, Ding H-S (2013) Mass production of chemicals from biomass-derived oil by directly atmospheric distillation coupled with co-pyrolysis. Sci Rep 3:1120. https://doi.org/10.1038/srep01120
Bridgwater T (2018) Challenges and Opportunities in Fast Pyrolysis of Biomass: Part I. Johns Matthey Technol Rev 62:118–130. https://doi.org/10.1595/205651318X696693
Hou S, Li Z, Wang H (2016) A Fast Algorithm to Generate Feasible Solution of Production Facilities Layout Based on Plane Segmentation. Math Probl Eng 2016:e1712376. https://doi.org/10.1155/2016/1712376
Brammer JG, Lauer M, Bridgwater AV (2006) Opportunities for biomass-derived “bio-oil” in European heat and power markets. Energy Policy 34:2871–2880. https://doi.org/10.1016/j.enpol.2005.05.005
Chiaramonti D, Prussi M, Buffi M, Tacconi D (2014) Sustainable bio kerosene: Process routes and industrial demonstration activities in aviation biofuels. Appl Energy 136:767–774. https://doi.org/10.1016/j.apenergy.2014.08.065
Chiaramonti D, Bonini M, Fratini E, Tondi G, Gartner K, Bridgwater AV et al (2003) Development of emulsions from biomass pyrolysis liquid and diesel and their use in engines—Part 2: tests in diesel engines. Biomass Bioenergy 25:101–111. https://doi.org/10.1016/S0961-9534(02)00184-8
Bari S (2014) Performance, combustion and emission tests of a metro-bus running on biodiesel-ULSD blended (B20) fuel. Appl Energy 124:35–43. https://doi.org/10.1016/j.apenergy.2014.03.007
Hu X, Nango K, Bao L, Li T, Hasan MDM, Li C-Z (2019) High yields of solid carbonaceous materials from biomass. Green Chem 21:1128–1140. https://doi.org/10.1039/C8GC03153C
Mao S, Shu R, Guo F, Bai J, Xu L, Dong K et al (2022) Fuel gas production from microwave-induced biomass pyrolysis using char-supported metal composites as both catalysts and microwave absorbers. Int J Hydrog Energy 47:25309–25321. https://doi.org/10.1016/j.ijhydene.2022.05.260
Henstra AM, Sipma J, Rinzema A, Stams AJM (2007) Microbiology of synthesis gas fermentation for biofuel production. Curr Opin Biotechnol 18:200–206. https://doi.org/10.1016/j.copbio.2007.03.008
Younesi H, Najafpour G, Mohamed AR (2005) Ethanol and acetate production from synthesis gas via fermentation processes using anaerobic bacterium, Clostridium ljungdahlii. Biochem Eng J 27:110–119. https://doi.org/10.1016/j.bej.2005.08.015
Najafpour G, Younesi H, Mohamed AR (2004) Effect of organic substrate on hydrogen production from synthesis gas using Rhodospirillum rubrum, in batch culture. Biochem Eng J 2:123–130. https://doi.org/10.1016/j.bej.2004.06.001
Younesi H, Najafpour G, Ku Ismail KS, Mohamed AR, Kamaruddin AH (2008) Biohydrogen production in a continuous stirred tank bioreactor from synthesis gas by anaerobic photosynthetic bacterium: Rhodopirillum rubrum. Bioresour Technol 99:2612–2619. https://doi.org/10.1016/j.biortech.2007.04.059
Jahirul MI, Rasul MG, Chowdhury AA, Ashwath N (2012) Biofuels Production through Biomass Pyrolysis —A. Technological Rev Energies 5:4952–5001. https://doi.org/10.3390/en5124952
Rajesh Banu J, Kavitha S, Yukesh Kannah R, Poornima Devi T, Gunasekaran M, Kim S-H et al (2019) A review on biopolymer production via lignin valorization. Bioresour Technol 290:121790. https://doi.org/10.1016/j.biortech.2019.121790
Han J, Elgowainy A, Dunn JB, Wang MQ (2013) Life cycle analysis of fuel production from fast pyrolysis of biomass. Bioresour Technol 133:421–428. https://doi.org/10.1016/j.biortech.2013.01.141
Duku MH, Gu S, Hagan EB (2011) A comprehensive review of biomass resources and biofuels potential in Ghana. Renew Sustain Energy Rev 15:404–415. https://doi.org/10.1016/j.rser.2010.09.033
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pandit, C., Pandit, S., Pant, M. et al. A Concise Review on the Synthesis, and Characterization of the Pyrolytic Lignocellulosic Biomass for Oil, Char and Gas Production: Recent Advances and its Environmental Application. Chemistry Africa 6, 2237–2263 (2023). https://doi.org/10.1007/s42250-022-00512-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00512-3