Abstract
The generation of renewable energy resources as an alternative to fossil fuels is essential to sustain the growing human population. Lignocellulosic biomass is considered an important renewable resource for various value-added compounds and biofuels, as the world is currently poised toward a carbohydrate-based economy. Analogous to petroleum refineries, biorefineries deal with the carbohydrate polymers (cellulose, hemicellulose) and aromatic compounds (lignin), which can be processed into different bioproducts. However, the complex architecture of crystalline cellulose, hemicellulose, and lignin creates high recalcitrance, which requires significant pretreatment steps. Thus, developing cost-effective pretreatment is crucial for the effective separation of the biomass components. In this chapter, first, the basic components of the lignocellulosic biomass have been briefly described followed by the various conventional physical and chemical pretreatment methods. In addition, the efficiency of different biomass-specific pretreatment operations and their combinations has been discussed in detail. Moreover, challenges of the pretreatment processes, like chemical recovery, inhibitory byproducts formation, prolonged and costly methods, and feedstock utilization are also highlighted. Overcoming the challenges has demonstrated the potentiality of the available pretreatment methods in the advanced biological refinery process for the production of biofuels and various value-added compounds.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Environmental pollution and climate changes are two major challenges of the present century, necessitating the use of non-fossil-based carbon-neutral fuel resources. Lignocellulose biomass (LCB) based feedstock presents one of the most suitable alternative resources for biofuels and value-added bioproducts in a sustainable manner. LCB has gained huge attention for promising biorefinery feedstock because of its ample abundance and lower cost than other biomass resources. However, conversion of LCB into useful biofuel or other value-added chemicals takes the integration of a series of chemical as well as biological procedures (Fig. 2.1). Considerable obstacles are involved in the effective utilization of LCB. The major hindrance to the conversion process is the complex organization of the structural components in the lignocellulosic biomass. The primary components of LCB are cellulose (C6H10O5)n, hemicellulose (C5H8O4)m, and lignin [C9H10O3(OCH3)0.9–1.7]x (Akhtar et al. 2016; Jørgensen et al. 2007; Kumar and Verma 2020a). This integral complexity of the plant cell wall leads to major recalcitrance against any kind of deconstruction of the LCB.
Schematic representation of the general pathway for biofuel and value-added chemical production in lignocellulose biorefinery. The lignocellulosic biomass undergoes steps like pretreatment and enzymatic hydrolysis for removal of lignin and depolymerization of cellulose and hemicellulose, releasing simple sugars for catalytic processing or fermentation for the production of biofuel or value-added chemicals
Pretreatment is the essential method performed upstream of the LCB to biofuel conversion, in order to overcome the recalcitrance and disrupt the complex organization of cellulose, hemicellulose, and lignin. Pretreatment is a vital component in converting LCB into liquid fuels and chemicals. Pretreatment aims to separate aromatic lignin and polysaccharides (cellulose and hemicellulose) and enhance accessibility to the hydrolytic enzymes for saccharification. The more the LCB is susceptible to hydrolysis, the more yield will be achieved for the fermentation process. Different types of pretreatment processes are employed to overcome the recalcitrance and breakdown of crystalline cellulose, opening the hemicellulose-cellulose surface with improved accessibility for further chemical conversion (Bhutto et al. 2017; Chen et al. 2017; Agrawal and Verma 2020; Bhardwaj et al. 2021). Notably, each pretreatment method has its specific consequence on the structure of the cellulose, hemicellulose, and lignin of the LCB. The physical and chemical changes of the LCB fractions are directly linked to the overall operation and effectiveness of the downstream process in terms of hydrolysis, substrate solubilization, fermentation rate, mixing, ethanol yield, etc. Thus, the choice of proper pretreatment method based on the LCB feedstock type and its effects on downstream steps is essential for the overall production of biofuels and chemicals.
In this chapter, first, the components of the LCB have been summarized followed by a discussion of the various conventional physical and chemical pretreatment methods. The challenges of different pretreatment methods are also highlighted along with the production of biofuels and various value-added compounds. Understanding the overall process helps in selecting the proper pretreatment method with subsequent operations based upon the type of feedstock, hydrolysis, and fermentation steps.
2.2 Components of Lignocellulosic Biomass
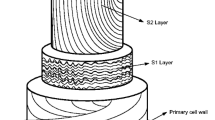
Plant cell wall mainly comprises polysaccharides such as cellulose, hemicellulose, aromatic polymer lignin, and a trace amount of pectin (Fig. 2.2) (Ververis et al. 2004). These complex composite materials provide the basic structural support to the plants. Cellulose remains in the core, surrounded by hemicellulose, whereas lignin appears at the outermost part of the plant material. Cellulose is a linear homopolymer of β-1, 4 linked glucose, whereas hemicellulose is a branched heterogeneous polysaccharide consisting of monomers like xylan, arabinoxylan, and others. Pectin is made up of complex polysaccharides such as α-linked galacturonic acid and rhamnose monomers. Lignin is also heterogeneous in nature and mainly contains alkyl-aromatic units forming a branched polymer. The composition of some popular lignocellulosic plant biomass is given in Table 2.1.
Composition of the lignocellulosic biomass: (a) Cellulose (40–50%), hemicellulose (20–40%), and lignin (18–35%) along with a trace amount of pectin, protein, extractives, and ash make up the lignocellulosic biomass. (b) Chemical structures of the major constituents of lignocellulosic biomass. Cellulose is a homopolymer of β-1,4-glycosidic linked glucan chains, whereas xylan is hemicellulose composed of β-1,4-linked xylose residues. Lignin is an aromatic compound consisting of mainly p-coumaryl, coniferyl, and sinapyl units
2.2.1 Cellulose
Cellulose is the most abundant organic matter available on earth, which represents the primary constituent of the plant cell wall. Out of all the components of the lignocellulosic biomass, cellulose represents a vast amount of fermentable sugar feedstock and a major focus of biofuel and value-added chemical generation on an industrial scale. However, it is more recalcitrant to the catalytic deconstruction process than other carbohydrate polymers. Cellulose is a linear chain of homopolysaccharides with anhydrous glucose units connected by β-1,4-glycosidic bonds.
Dimerization of glucose monomers through β-1,4-glycosidic bond forms cellobiose which is the basic structural unit of cellulose. The spatial organization of the β-1,4-glycosidic bonds inside the glucan chains gives rise to specific inter and intramolecular hydrogen bonding interactions imparting high crystallinity, making it insoluble in water or other common solvents (Bishop 2015; Kumar and Verma 2020b). Also, each cellulose chain comprises a reducing end at one end and a non-reducing end at the other (Fig. 2.3). During chain elongation, new glucose monomers are added to the non-reducing end. Proper knowledge of cellulose crystallinity is crucial for understanding the depolymerization process. The β-1,4 linked glucan chains form a highly ordered crystalline structure. It is reported from the X-ray diffraction data that the crystalline microfibrils contain 24–36 linear chains (Endler and Persson 2011; Fernandes et al. 2011). In some cases, loss of crystallinity results in disordered regions, which are known as amorphous cellulose. The highly ordered crystalline cellulose is separated by such amorphous regions. Interchain as well as intrachain hydrogen bondings due to the spatial organization of the hydroxyl groups of the glucose along with the different pyranose ring arrangements in the cellulose crystal give rise to four distinct crystalline allomorphs of cellulose. These are designated as cellulose I, II, III, and IV, respectively (Fig. 2.4). The difference is based on the crystal unit cell parameters such as unit cell length and unit cell angles, which defines the packing of cellulose chains in the crystal (Table 2.2).
Among all the cellulose allomorphs, cellulose I is predominantly found in nature. It is basically a mixture of two cellulosic forms, i.e., Iα and Iβ (Fig. 2.4). The Iβ form of cellulose has a monoclinic unit cell and is mostly found in higher plants like wood, cotton, and animals, whereas the Iα form has a triclinic unit cell and is found in a wide range of microorganisms like bacteria (Acetobacter xylinum) and algae (Glaucocystis nostochinearum). The cellulose Iβ form is gaining more interest, as this form is abundant in higher plants and serves as a potential feedstock for fermentable sugar generation. Cellulose II can be obtained from cellulose I by either alkali treatment or solubilization and regeneration (Mittal et al. 2011). The unit cell in cellulose II is also monoclinic as the Iβ. However, the chains in cellulose Iβ are parallel, whereas the chains in cellulose II are antiparallel. Cellulose I or II can be converted to cellulose III form by ammonia pretreatment which is more amorphous in nature. Allomorph III is subcategorized into cellulose IIII or IIIII based on the source of the conversion process. Due to reduced crystallinity, both the II and III allomorphs have greater enzyme accessible surface area that enhances the saccharification process (Chundawat et al. 2011; Payne et al. 2015). Cellulose IV can be produced by high-temperature treatment of cellulose III. This form of cellulose is less characterized. However, reports suggest that it is structurally similar to cellulose Iβ (Nishiyama 2009).
Some crucial properties of cellulose that influence its pretreatment and enzymatic scarification are described below:
-
(a)
Crystallinity index (CrI): It provides a measure of the compactness and reactivity of the cellulosic material which can be quantified by an X-ray diffraction pattern. For example, the CrI for cellulose type I can be obtained using the following method of Jayme and Knolle (Lenz 1994):
$$ CrI=1-\frac{h_{\mathrm{am}}}{h_{\mathrm{cr}}}=1-\frac{h_{\mathrm{am}}}{\left({h}_{\mathrm{tot}}-{h}_{\mathrm{am}}\right)} $$(2.1)where hcr is the height of the cellulose crystal in the 002 reflections with 2θ = 22.5°, ham is the height of the amorphous cellulose, and htot is defined as:
$$ {h}_{\mathrm{tot}}={h}_{\mathrm{cr}}+{h}_{\mathrm{am}} $$(2.2) -
(b)
Degree of Polymerization (DP): The degree of polymerization is another important parameter affecting enzymatic hydrolysis. It determines the availability of terminal and internal glycosidic bonds for both the exo- and endo-catalytic enzymes. Three specific DPs are generally considered such as the number average DP (DPN), the weighted average DP (DPW) and DP obtained from viscosity (DPV). The governing formulae are given below:
$$ {DP}_{\mathrm{N}}=\frac{M_{\mathrm{n}}}{MW_{\mathrm{glu}}}=\frac{\sum {N}_{\mathrm{i}}{M}_{\mathrm{i}}}{\sum {N}_{\mathrm{i}}}/{MW}_{\mathrm{glu}} $$(2.3)$$ {DP}_{\mathrm{W}}=\frac{M_{\mathrm{w}}}{MW_{\mathrm{glu}}}=\frac{\sum {N}_{\mathrm{i}}{M_{\mathrm{i}}}^2}{\sum {N}_{\mathrm{i}}}/{MW}_{\mathrm{glu}} $$(2.4)$$ {DP}_{\mathrm{V}}=\frac{M_{\mathrm{v}}}{MW_{\mathrm{glu}}}=\frac{\sum {N}_{\mathrm{i}}\eta }{\sum {N}_{\mathrm{i}}}/{MW}_{\mathrm{glu}} $$(2.5)where Mi is the molar mass of a given fraction I with Ni number of moles with viscosity η and MN, MW, MV, MWglu being the average molecular weight, weight-average molecular weight, viscosity-average molecular weight, and molecular weight of anhydroglucose, respectively. Cellodextrins with DP ranging from 2 to 6 are generally water soluble (Klemm et al. 1998). Solubility decreases with increasing DP of the polysaccharide. For example, glucan chains with DP 7–13 are weakly soluble (Zhang and Lynd 2003). However, DP greater than 30 is insoluble in common solvents.
-
(c)
Accessibility: Cellulases need to bind effectively to the solid surface with proper orientation of the cellulose for the initiation of hydrolysis. The microcrystalline structure of cellulose along with particle size and shape determines the probability of interaction between the enzyme and the glycosidic bonds. Thus, cellulose accessibility is a key factor in the saccharification step.
2.2.2 Hemicellulose
It is a complex branched biopolymer of pentose (C5) and hexose (C6) sugars, mostly D-xylose, D-galactose, L-arabinose, D-mannose, and D-glucose, having a short length of around 50 to 200 units. However, the composition of hemicellulose varies from different sources due to its heterogeneous nature. Hemicellulose isolated from the cell wall of rice endosperm reveals that it is composed of mainly xylose, arabinose, and glucose forming the polysaccharide moieties xyloglucan, β-glucan, and arabinoxylan, respectively (Shibuva and Misaki 1978; Bhardwaj et al. 2020; Bhardwaj and Verma 2021). The β-glucan contains both the 1,3 and 1,4 glycosidic linkages. The xylose is attached to the C6 position of the 1,4 linked glucan backbone to form the xyloglucan. The arabinoxylan shows a complex branched structure where the arabinose is connected to the 1,4 linked xylose chain. Acetate groups are attached to the hydroxyl functional groups of the sugar rings via an ester linkage. Hemicellulose mostly provides the bridge between core cellulosic parts with outer lignin. Researchers are focusing on hemicellulose for the production of value-added chemicals in biorefineries (Rambo et al. 2015).
2.2.3 Lignin
Lignin is a non-carbohydrate phenolic polymer, made up of a complex network of aromatic alcohols. The main components of this crosslinked heteropolymer are p-coumaryl, coniferyl, and sinapyl alcohols (Rubin 2008). The units are known as p-hydroxyphenyl, guaiacyl, and syringyl when present in a polymer chain. Lignin is bound to hemicellulose with covalent linkages to form an amorphous matrix that surrounds core cellulose microfibrils. Strong ether (C–O–C) and carbon–carbon (C–C) bonds in the lignin provide high mechanical strength to the plants. So far, lignin is unusable in the fermentation process for biofuel production and can be used as an alternative resource for value-added chemical production in the biorefinery (Radhakrishnan et al. 2021; Kumar et al. 2020).
2.3 Different Technologies for Lignocellulosic Biomass Pretreatment
For the production of lignocellulosic biofuels and other economically important chemicals, the first step is to overcome the heterogeneity of the raw biomass in a cost-effective manner. Several pretreatment techniques have been adopted to separate the cellulose from other biopolymeric mixtures followed by decrystallization of the cellulose microfibrils that will be subjected to the enzymatic hydrolysis in the subsequent steps. The primary goals of the pretreatment are the efficient separation of lignin, hemicellulose, and cellulose by disrupting the structural linkages and increasing the accessibility to the hydrolytic enzymes. Moreover, some other key targets in the pretreatment process are a reduction in the crystallinity index, the degree of polymerization of the cellulose, and particle size of biomass. Some of the key criteria for a “good” pretreatment method are (a) less degradation of the biomass, (b) lowering the inhibitory product formation, (c) minimizing energy input, (d) low-cost pretreatment agents and catalyst recycling, (e) overall cost-effectiveness. The pretreatment procedures can be broadly classified into three major categories: physical, chemical, and biological pretreatments. The chemical pretreatments are subcategorized into biochemical and thermochemical processes. The traditional physical and chemical pretreatment methods are discussed in the following sections.
2.3.1 Physical Pretreatment Process
The physical pretreatment processes are aimed to increase the overall surface area of the biomass and reduction in the particle size which in turn decreases the degree of polymerization of the material. The commonly available physical pretreatment methods are described below.
2.3.1.1 Milling
Milling is a mechanical process for lignocellulose pretreatment. The different types of milling used for the deconstruction of lignocellulosic biomass are ball milling, disk milling, two-roll milling, colloid milling, and hammer milling (Mood et al. 2013). The average particle size obtained from the milling process is around 10–30 mm. However, the high energy requirement of the process raises the production cost which is partially overcome by wet disk milling with comparatively lower energy consumption. In a comparative study on the effectiveness of wet disk and ball milling processes, it was found that the total glucose and xylose yield in the enzymatic hydrolysis step from the wet disk pretreated sugarcane bagasse were 49.3% and 36.7%, respectively, whereas the ball milling pretreated biomass gave rise to maximum yield of 78.7% and 72.1%, respectively (Hideno et al. 2009). Value-added chemicals such as methyl levulinate were made with a maximum yield of 64.92 mol%, using ball milling pretreatment by facilitating cellulose depolymerization (Chen et al. 2019).
2.3.1.2 Extrusion
Extrusion is another thermo-physical pretreatment method, where the biomass is passed through an extruder with moderate to high temperatures. The rapid mixing and excessive shearing in the chamber lead to the breakdown of the crystalline structure and produce short fibers which increase the enzymatic accessibility of the biomass in the hydrolysis step. For example, extrusion with temperature up to 80 °C, screw speed of 350 rpm, and moisture content of 40% provided a yield of 94.8% glucose conversion by the enzymatic hydrolysis from the pretreated soybean hulls (Yoo et al. 2011). Recently, extrusion has been combined with cellulases for obtaining sugar conversion yield of at least 94% at 50 °C (Gatt et al. 2019). This process doesn’t produce any effluent which is an advantage in terms of disposal cost reduction. Continuous operating process without washing and the possibility of easy scale-up have made extrusion an industrially important pretreatment process.
2.3.1.3 Pyrolysis
Pyrolysis is the process of deconstruction of lignocellulosic biomass at high temperatures. This pretreatment approach has been adopted by many industries. When lignocellulosic materials are subjected to temperatures as high as 300–800 °C or more, the cellulose crystallinity is gradually decreased with the production of residual char and gaseous compounds (Fisher et al. 2002). For example, 50–55 (wt%) liquid fraction, 25–27 (wt%) char, and other gaseous compounds were produced from hardwood pyrolysis at a temperature of 200–275 °C for 30–45 min followed by 450 °C for 1 h (Ortega et al. 2011). However, the decomposition is significantly less at lower temperatures. It is reported that more than 80–85% cellulose conversion can be achieved by coupling mild acid hydrolysis with pyrolysis. Cellulose decomposition can also be performed by introducing sodium carbonate or zinc chloride as a catalyst in the process. Catalytic pyrolysis shows a significant rise in the organic liquid yield of 42 wt% in pretreated biomass than raw biomass at a process temperature of 600 °C (Persson and Yang 2019).
2.3.1.4 Microwave
The complex organization of cellulose and other components of the lignocellulosic biomass can also be subjected to microwave irradiation rather than conventional heating for the deconstruction process. Microwaves produce high-frequency electromagnetic field that generates heat when interacting with an object containing mobile charges. The difference with conventional heating is that heat transfer occurs through the surface of the objects, whereas microwave introduces high molecular collision in the cellulose because of dielectric polarization which leads to the breakdown of the cellulose ultra-structure, separation of lignin/hemicellulose and finally enhancing the accessibility of the material for the hydrolytic enzymes. For example, 30% and 12% lignin removal were obtained in corn straw and rice husk, respectively, with microwave pretreatment (Diaz et al. 2015). Currently, microwave-assisted pretreatment (with irradiation at 800 W and 152 °C for 45 s) was carried out using choline chloride and lactic acid deep eutectic solvent (ChCl: LA DES) where 85%–87% pure lignin was recovered while the cellulose existed in the solid fraction (Chen and Wan 2018). In another study, researchers have reported microwave pretreatment using choline chloride and oxalic acid dihydrate deep eutectic solvent (microwave irradiation at 800 W and 80 °C for 3 min) with a pure lignin recovery of 96% (Liu et al. 2017a).
2.3.1.5 Freeze Pretreatment
Freezing treatment is a newly introduced approach where the material is stored in the refrigerator for a specific time which significantly increases the cellulase performance before the hydrolysis step. It is found that the enzymatic hydrolysis was enhanced from 48% to 84% on a freeze pretreated rice straw (Chang et al. 2011). Low environmental impact and absence of chemical hazards make the process promising.
The main advantage of the physical pretreatment method is that the particle size of the biomass is reduced which enables efficient heat and mass transfer during further processing. On the other hand, cellulose crystallinity and degree of polymerization are decreased, thus providing higher surface area and pore size during the hydrolysis step for improved sugar yield. However, the major limitations of these processes are the high energy requirement and equipment maintenance cost which make them economically inefficient.
2.3.2 Physico-Chemical Pretreatment Process
2.3.2.1 Steam Explosion
Steam explosion is a thermo-mechano-chemical pretreatment method that involves the thermal steam-heating to degrade the lignocellulosic components, the mechanical shearing force due to sudden pressure drop, and chemical autohydrolysis for glycosidic bond cleavage. In this method, the biomass is subjected to saturated steam at a temperature of around 160 °C–260 °C at a high pressure of ~0.69 to 4.83 MPa for a few seconds to minutes followed by exposing it to the atmospheric pressure. This explosive decompression results in the disintegration of the lignocellulosic matrix. Hemicellulose hydrolysis along with lignin transformation at high-temperature treatment exposes the cellulose surface to the cellulolytic enzyme machinery in the subsequent steps. The efficiency of this process depends on the particle size, temperature, the residence time of the material, and total moisture content inside the chamber. The efficiency of the process can be greatly enhanced by the introduction of catalysts such as sulfuric acid (H2SO4), sulfur dioxide (SO2), or carbon dioxide (CO2) which accelerate hydrolysis and removal of hemicellulose. Steam explosion pretreatment at 177 °C for 5 min of banana lignocellulosic biomass gave rise to 91% glucose yield in enzymatic hydrolysis (Guerrero et al. 2017). Moreover, pretreatment including wheat straw, miscanthus, and poplar has shown the role of steam explosion in reducing hemicellulose content and breaking down the lignin cross-linkages for improved enzymatic hydrolysis (Auxenfans et al. 2017). The main advantage of this technology is that due to the partial lignin and hemicellulose solubilization, the sugar yield is improved during the enzymatic hydrolysis. On the other hand, no requirement of recycling and less environmental impact make this process industrially feasible. However, there is a possibility of forming fermentation inhibitors at high temperatures. In case of incomplete deconstruction of the biomass, soluble lignin may get precipitated and hinder effective hydrolysis. Considerable energy requirement due to the maintenance of high temperature and pressure increases the overall cost of the process.
2.3.2.2 Ammonia Fiber Explosion (AFEX)
It is a thermochemical pretreatment method that is based on the same principle as steam explosion. Here, the lignocellulosic biomass is treated with liquid ammonia at high pressure (1.72–2.06 MPa) and moderate temperature (60 °C–120 °C) for a specified time (~30 min). Then the pressure is swiftly dropped which results in the quick expansion of the compressed ammonia gas that breaks the matrix of the lignin–carbohydrate complex followed by degradation of the fibers. Ammonia pretreatment produces solid materials, whereas steam explosion gives rise to the slurry. It is notable that AFEX doesn’t release any sugar due to less solubility of hemicellulose but it disintegrates the components to increase the surface area of the biopolymeric mixture for higher enzymatic accessibility. AFEX pretreatment is proven to be more effective for the pretreatment of low lignin-containing biomass such as alfalfa stems, wheat straw, rice straw, switchgrass, corn stover, etc. than hardwood and softwood materials with more lignin. AFEX process optimization can be done by varying the following parameters, i.e., the amount of ammonia to biomass loading ratio, water loading, temperature, the residence time of the biomass, and the moisture content. For example, ammonia to biomass loading ratio of 2:1 along with 120% moisture content and 5 min of residence time at 140 °C temperature were found to be optimal for bioethanol production from sorghum bagasse (Li et al. 2010). In a recent study, the efficacy of both the steam explosion and AFEX was explored for the production of animal feeds and biofuel feedstocks in a sugarcane-based biorefinery approach. It was shown that up to 3368 L and 4360 L of ethanol per hectare of sugarcane cultivated land could be generated from steam explosion and AFEX pretreated sugarcane residues, respectively (Mokomele et al. 2018). AFEX increases the effective surface for enzymatic hydrolysis and enhances the sugar yield. Besides, no inhibitory by-products are formed during the process, and no biomass washing or detoxification steps are required. However, this pretreatment method is not effective for high lignin-containing biomass and softwoods. The corrosive and hazardous properties of AFEX pretreatment require a controlled environment and costly equipment. The cost of ammonia is another concern that makes the process highly expensive at large scale (Agbor et al. 2011).
2.3.2.3 CO2 Explosion
The CO2 explosion is another explosion-based method similar to steam explosion and AFEX for the pretreatment of lignocellulosic biomass. In this process, supercritical CO2 is used with a temperature and pressure greater than its critical temperature and pressure values (31 °C and 7.4 MPa, respectively). At this condition, liquid and gases can coexist, and CO2 shows liquid-like density as well as gas-like transport properties with the ability to penetrate the lignocellulosic pores facilitating the deconstruction process. When it is used along with water, carbonic acid is formed which accelerates the polymer hydrolysis. After mild treatment, explosive release of CO2 due to the pressure drop destroys the architecture of hemicellulose and lignin and exposes enzyme accessible surface area for cellulose hydrolysis. It is shown that significantly higher amounts of reducing sugar (84.7%) are obtained from supercritical CO2 (165 °C and 21.37 MPa for a residence time of 30 mins) pretreated aspen wood than the untreated biomass (14.5%) in the hydrolysis process (Kim and Hong 2001). In a recent study, supercritical CO2 (50 °C–80 °C and 17.5–25.0 MPa pressure for 12 h–60 h) has been used for pretreating corn stover and corn cob, which resulted in around three to four-fold higher sugar yield than the untreated feedstocks during the enzymatic hydrolysis (Zhao et al. 2019). Low operating temperature with no inhibitory/toxic product formation and complete removal of CO2 by depressurization have made this process promising for scale-up purpose. The CO2 can be readily obtained as a by-product from many industrial processes. However, the low process efficiency for different lignocellulose resources and high-pressure equipment rises the capital cost significantly (Agbor et al. 2011).
2.3.2.4 Liquid Hot Water Pretreatment
Liquid hot water (LHW) pretreatment is an efficient pretreatment method for improving enzymatic hydrolysis in a chemical-free manner for biomass conversion. In this process, liquid water is used as a reaction medium at a high temperature (160 °C–220 °C), and pressure is controlled to keep the water in a liquid state. This method has been shown to be effective for the pretreatment of sugarcane bagasse, corncobs, corn stover, wheat straw, etc. For example, wheat pretreatment using LHW gave rise to glucose and xylose yields of 91% and 80%, respectively (Pérez et al. 2008). In another study, alkali-catalyzed (0.5% aqueous NaOH at 170 °C) LHW pretreated bamboo yielded 30.9 g/100 g reducing sugars during hydrolysis and 9.6 g ethanol/100 g bamboo after fermentation (Yang et al. 2019b). LHW pretreatment with corncobs for 10 min at 160 °C significantly enhanced solubilization of hemicellulose (pentose yield of 58.8%) and lignin removal (60%), resulting in 73.1% glucose recovery in the hydrolysis step (Imman et al. 2018). This is an environment-friendly approach with less or no inhibitory by-product formation due to low process temperature and results in significant sugar recovery from both hemicellulose and cellulose. But, there is high energy and water requirement, which limits the application of the process and it needs further optimization for commercial applications.
2.3.2.5 Irradiation
Irradiation is another approach for lignocellulosic biomass pretreatment. This is generally coupled with other conventional pretreatment processes. Application of electron beam or gamma (γ) rays along with mechanical crushing, thermal or chemical pretreatments has shown enhanced enzymatic hydrolysis in comparison with pretreatment in absence of such irradiation. Pretreatment with an electron beam decreases the cellulose crystallinity and increases the enzymatic hydrolysis. In the case of ionizing irradiation, long- and short-lived radicals are formed that accelerate the deconstruction of biomass by chain scission and cross-linking (Khan et al. 2006). Hydrothermal pretreatment of sugarcane bagasse along with a 50 kGy electron beam has shown to increase the saccharification yield of glucose by 20% (Duarte et al. 2012). Application of electron beam (7.5 kGy) in mild alkali soaked Artemisia ordosica gave an increased yield of reducing sugars as 520.67 mg/g under optimal conditions (Xiang et al. 2017). In a recent study, significantly reduced particle size and low shear rate were achieved using γ-irradiation at 800 kGy along with thermal or dilute acid pretreatment where the sugar yield was found to be around 251 g/L (Liu et al. 2017b). Though electron beam or gamma (γ) rays enhance the enzymatic saccharification, the industrial application of irradiation is limited due to the high cost of installation and environmental concerns. However, these technologies can be used in combination with other methods for designing an optimized pretreatment process.
2.3.2.6 Ultrasonication
Ultrasonic radiation can also be used to deconstruct the complex architecture of lignocellulosic biomass. The basic mechanism of high-frequency ultrasonication in any liquid is the formation of bubbles followed by its sudden collapse at the solid surface, inducing a microjet streaming known as asymmetric cavitation. These cavities enhance the enzymatic accessibility at the substrate surface. At a fixed level of enzyme and substrate concentration, sonication at 40%, 60%, and 80% amplitudes (Idiss: 16.2, 32.2, and 43.4 W/cm2, respectively) has shown increased enzymatic hydrolysis by 15%, 24%, and 54%, respectively, in comparison to the mechanical agitation alone (Szabo and Csiszar 2017). Ultrasonication can also be used along with other chemical pretreatment agents such as acid-alkali, ionic liquids, etc. For example, ultrasonication in combination with HCl pretreatment resulted in significantly higher delignification in deenanath grass (80.4%) and hybrid napier grass (82.1%) than that of acid pretreatment alone (Mohapatra et al. 2017). Another study shows that ultrasonication pretreatment along with NaOH in rice straw can degrade hemicellulose and lignin without much-affecting cellulose and generated 2.73 g/L of reducing sugars during hydrolysis (Wu et al. 2017). Ultrasonication is also a green approach and proved to be efficient at a laboratory scale. However, the main disadvantage is that it exhibits different efficacy toward a variety of lignocellulose biomass. Thus an optimized cost-effective technology development is still challenging. Moreover, it can be applied in combination with other methods for hybrid pretreatment of lignocellulose biomass.
2.3.3 Chemical Pretreatment Process
2.3.3.1 Ozonolysis
Ozone can be used as a potential oxidant to pretreat lignocellulosic biomass. It has the ability of delignification along with degradation of hemicellulose which leads to exposing the inner cellulosic core to the hydrolytic enzymes, accelerating the hydrolysis by 4–5 folds. Its powerful oxidant properties allow the breakdown and effective removal of lignin without producing any toxic by-products that may create inhibitory effects in downstream processes. As the process is operated in ambient conditions, no extra cost is required like other pretreatment methods which are carried out at high temperature and pressure. The enzymatic hydrolysis yield was increased from 29% to 88.6% after ozonolysis in wheat straw (García-Cubero et al. 2009). It was recently demonstrated that ozonolysis could effectively remove 20% lignin with 31% sugar yield through enzymatic hydrolysis from water-submerged municipal waste (Rosen et al. 2019). In another work, eucalyptus branches were subjected to ozonolysis with significant delignification (26.63%) and saccharification yield (68%) (Andersen et al. 2019). However, the high cost of ozone along with large requirements has limited its use on an industrial scale.
2.3.3.2 Acid Hydrolysis
Acid pretreatment of lignocellulosic biomass has been extensively used in industries. Strong acids like sulfuric, nitric, and hydrochloric acid are employed to remove lignin and hydrolyze the hemicellulose and cellulose for providing greater accessibility to the enzymes. Acid pretreatment can be performed in either concentrated acid at low temperature or a diluted acid at high temperature. The main advantages of this technique are the solubilization of hemicellulose and the precipitation of lignin at the same time. For example, acid pretreatment of coastal bermudagrass with 1.2% H2SO4 at 140 °C for 30 min retention time gave rise to a total sugar yield of 94% in the hydrolysis step (Redding et al. 2011). However, during the process, the degradation of monosaccharides leads to the formation of many unwanted inhibitory by-products such as 5-hydroxymethylfurfural (HMF) and 2-furfuraldehyde (Saha 2004). Moreover, high-temperature treatments sometimes produce furfural which gets degraded into formic or levulinic acids. These inhibitory compounds create a severe impact affecting the efficiency of the hydrolytic enzymes. Toxicity, acid recovery, and corrosiveness of the equipment are some of the considerable drawbacks of this approach. Current industrial processes are focused on reducing the inhibitory by-products by using diluted acid treatments.
2.3.3.3 Alkali Hydrolysis
Alkaline pretreatment is one of the common industrial methods for delignification. Alkaline agents like sodium hydroxide (NaOH), ammonium hydroxide (NH4OH), calcium hydroxide (Ca(OH)2), potassium hydroxide (KOH), etc. are used for pretreatment of lignocellulosic residues. Alkaline pretreatment induces solvation and saponification. These cause the deformation of complex networks of lignin, decrystallization of cellulose, cellulose swelling, and fractionation of lignin by breaking the ester linkages between lignin and other hemicelluloses in the complex network, thus promoting the solubilization of these components and enhancing the activity of the catalytic enzymes. For example, NaOH pretreatment (4–40 g/100 g dry straw) was found to decrease xylan content up to 46.37% and resulted in 64.55% glucose yield (Wan et al. 2011). Moreover, sodium carbonate (Na2CO3) pretreatment was found to yield significant total sugar (71.7%), glucan (76.1%), and xylan (73.2%) from rice straw (Yang et al. 2012). Alkaline pretreatment is very much effective for lignin removal and cellulose decrystallization and thereby increasing the enzyme accessible surface area. The energy requirement is also low due to the low process temperature. However, long residence time makes the neutralization step very difficult before the downstream and increases the overall cost of the process.
2.3.3.4 Organosolv Pretreatment
In this approach, lignocellulosic biomass is heated in the presence of organic liquids and a water mixture (ethanol-water, ethylene glycol, benzene-water, etc.). A significant amount of lignin and some amount of hemicellulose get dissolved in the process while the solid cellulose part is left in the medium. A wide range of organic compounds such as ethanol, methanol, glycerol, tetrahydrofurfuryl alcohol, dimethyl sulfoxide, ethers, ketone, etc. has been used to study the solvent extraction process (Thring et al. 1990). Pretreatment of wheat straw using aqueous glycerol has shown cellulose recovery of 95% along with 70% lignin (Sun and Chen 2007). For a purpose of utilizing whole lignocellulose biomass in biorefinery, ethanol organosolv pretreatment (50% (v/v) ethanol and 1% (w/v) sulfuric acid catalyst) was carried out with simultaneous generation of organosolv lignin (12 g), furfural (7.9 g), glucose (37.1 g), and hemicellulose-derived sugars (11.4 g) (Choi et al. 2019). In a recent study, hybrid Pennisetum (HP) biomass was pretreated with different organosolv (tetrahydrofurfuryl alcohol (THFA), γ-valerolactone (GVL), ethanol, and acetone) under 100 °C for 2 h which resulted in significant-high glucan yield (80.8%), and THFA was reported to be most effective among the organosolv for improved hydrolysis and lignin recovery (Tan et al. 2019). In another work, the use of ethanol-water resulted in 86% glucan and 62% lignin removal in Eucalyptus nitens bark (Romaní et al. 2019). This method is effective for the pretreatment of softwood materials with high lignin content and also for efficient fractionation of pure cellulose, hemicellulose, and lignin. Organic solvents with a low boiling point can be easily recovered and recycled. However, solvents with high boiling points need high-pressure conditions, and the huge cost of the solvents makes the process expensive. The solvents must be well separated before the downstream process or else the growth of the fermentative microbes can be inhibited.
2.3.3.5 Oxidative Lime Pretreatment (OLP)
Lime is an effective oxidative agent for lignocellulose pretreatment for reducing the lignin content and enhancing better cellulose recovery. The efficiency of the delignification of thermal lime pretreatment can be greatly enhanced by introducing oxygen as an oxidant in the process. OLP effectively removes lignin and hemicellulose acetyl content from the biomass which makes the substrates more accessible to hydrolysis. Recent studies have shown that 68% and 98% of total xylan and glucan have been recovered, respectively, when switchgrass is pretreated using OLP at 110 °C and 6.89 bar for 240 min in combination with milling (Falls and Holtzapple 2011). In another study, lime pretreatment of corn stover resulted in 87.5% lignin removal along with a yield of 93.2% glucose and 79.5% xylose during the hydrolysis (Kim and Holtzapple 2005). One major disadvantage of this process is that a huge amount of water is required for washing out the calcium salts. Moreover, the cost of downstream processing also makes it expensive.
2.3.3.6 Ionic Liquid Treatment
The chemical pretreatments discussed above mainly consist of derivatizing solvents which cause chemical modification to the cellulose, reducing cellulose yield in the pure form. In recent years, Ionic Liquids (ILs) are considered a non-derivatizing solvent and have shown huge potential for the pretreatment of lignocellulosic biomass. ILs are eco-friendly and often referred to as “Green Solvents” (Wasserscheid and Keim 2000). The specialty of IL is that cellulose and lignin can be solubilized simultaneously. Ionic liquids are organic salts generally composed of a relatively large cation and a smaller anion, with a melting point of less than 100 °C (Rogers and Seddon 2003). IL has been proved to be a potential pretreatment agent due to its high thermal stability, low vapor pressure, and non-derivatizing solvent properties. Several ILs such as 1-ethyl-3-methylimidazolium-acetate ([EMIM][Ac]), 1-ethyl-3- methylimidazolium diethyl phosphate ([EMIM][DEP]), 1-allyl-3-methylimidazolium-chloride ([AMIM][Cl]), and 1-butyl-3-methylimidazolium chloride ([BMIM][Cl]) are some of the effective ILs to dissolve lignocellulosic biomass (Fig. 2.5) (Zhao et al. 2008). However, [EMIM][Ac] shows one of the best promising cellulose recovery among all the ionic liquids (Sun et al. 2009).
Pretreatment of wheat straw at 120 °C for 6 h using [EMIM][Ac] resulted in near 100% (w/w) sugar recovery (da Costa Lopes et al. 2013). [EMIM][Ac] along with buffer solution is used to successfully remove ~50% lignin from the wood chip biomass (Moniruzzaman and Ono 2012). To study the enzymatic saccharification, IL pretreated eucalyptus was subjected to enzymatic hydrolysis, and a nine-fold rise in cellulose conversion was observed (Uju et al. 2012). A high glucose yield of around 95.2% was found when the sugarcane bagasse was pretreated with IL and regenerated in water followed by enzymatic hydrolysis. Studies have been performed to optimize the process parameters of IL pretreatment which reported that dissolution can be enhanced by 50% using 67% aqueous-[EMIM][Ac] solvent mixture at 150 °C for 90 min, generating ~81% fermentable sugar (Fu and Mazza 2011). Several computational, as well as experimental studies, have been performed to understand the underlying mechanism of the process. The key factors governing the interaction between IL and lignocellulosic biomass are the cationic and anionic properties which vary with different ILs, the temperature, and the time of the pretreatment process. 13C high-resolution NMR spectroscopy of cellulose oligomers in [BMIM][Cl] has revealed that the cellulose chains adopt specific conformational changes in IL environment (Moulthrop et al. 2005). Another study using 13C and 35/37Cl NMR relaxation in [BMIM][Cl] revealed that the hydroxyl protons of the cellulose chains interact with the Cl− ions during the dissolution (Remsing et al. 2006). High temperature (~450 K similar to the industrial pretreatment temperature) vacuum molecular dynamics simulation study of crystalline Iβ cellulose has shown a temperature-dependent behavior of cellulose microcrystal where significant deviation in the crystal unit cell parameters has been found due to the change in the rotamers of the hydroxymethyl group conformation (Bergenstråhle et al. 2007). This change affects the stable hydrogen bonding network in the cellulose which facilitates the formation of hydrogen bonding between the anionic species of the IL and the hydroxyl group of cellulose chains. At the same time, the hydrophobic interaction occurs between the imidazolium ring of the cation and cellulose. These interactions break the stable inter- and intra-hydrogen bonds present within the lignocellulosic biomass, leading to the dissolution of the complex (Wahlström and Suurnäkki 2015). IL pretreatment increases the porosity of the biomass which makes it highly susceptible to enzymatic hydrolysis. Despite having so many attractive features of ILs, the main challenges of IL pretreatment are its high cost, recyclability, and recovery of ILs. To optimize the process, researchers are trying to use different binary mixtures along with IL during the pretreatment. In a recent study, it has been reported that the molecular behavior of [Emim][Ac]/water mixtures and its interaction with cellulose microcrystal reveals that 50–80% [EMIM][Ac] can be effective for both cellulose and lignin dissolution process (Manna et al. 2021; Manna and Ghosh 2019; Shi et al. 2014). Researchers have also used several organic solvents (N,N-dimethylacetamide, N,N-dimethylformamide, dimethyl sulfoxide, and pyridine) as diluents to improve the viscosity of IL for process optimization (Yang et al. 2019a). Progress has been made for a simultaneous pretreatment and hydrolysis process for cost-effective IL pretreatment technology (Husson et al. 2018).
2.4 Current Challenges in the Pretreatment Methods
At a process level, pretreatments are a multi-scale and non-homogeneous system where LCB interacts with the other components of physical and chemical agents. The complex heterogeneous nature of the LCB affects the overall reaction process leading to changes in yield in different pretreatment processes. This complexity has limited many of the traditional physical and chemical pretreatment processes in an experimental stage. Some of the key problems in pretreatment processes are given below:
-
1.
Each pretreatment method has its own limitations. Some limitations of the traditional pretreatment methods are summarized in Table 2.3. However, a proper evaluation method needs to be developed in terms of economic feasibility and productivity to access the pretreatment processes.
-
2.
The understanding of a physical or chemical pretreatment technology on the basis of the reaction mechanism is limited. More research needs to be done on the fundamentals to reveal the influence of particular pretreatment on the structure and digestion of cellulose and hemicellulose. The knowledge can be utilized to develop a suitable reaction model or optimization of the existing technologies.
-
3.
Most of the pretreatment technologies primarily consider the cellulose hydrolysis, rate of hydrolysis, overall glucose yield, and lignin removal for the effectiveness of the process. However, it doesn’t provide insight into the physical chemistry perspective in the reaction process. Thus, further, development is necessary.
-
4.
Even if some methods are effective to the laboratory scale, the scale-up becomes more challenging due to the high cost, the requirement of optimal process conditions, and involved environmental concerns.
2.5 Conclusion
Pretreatment is the key step in the deconstruction of LCB for producing any biofuel or value-added chemicals. The effects of the traditional pretreatment methods on the different components of LCB have been discussed in detail. The crystallinity of the cellulose and removal of lignin are crucial for the effective enzymatic hydrolysis and overall sugar yield. Though few of the processes can help significant yield, the scale-up for industrial application is still a challenge to overcome. The challenge can be addressed in two ways. Either the existing techniques should be improved, or else research should be done on developing new eco-friendly, cost-effective, efficient pretreatment methods. Besides, an investigation needs to be done on LCB components to design efficient separation methods. Moreover, when a single pretreatment technique is used, it raises various technological drawbacks, environmental concerns, high-operation costs, and compromised productivity. Thus, the initiative should be taken to combine multiple pretreatment methods including physical and chemical processes. For example, mechanical crushing–electronic radiation–alkali treatment, mechanical crushing–microwave–chemical processing, microwave-IL treatment, mechanical crushing–chemical treatment–steam explosion, and others. By means of a combined pretreatment method, the deconstruction of lignocellulosic biomass can be significantly improved in terms of accessible surface area to the enzyme, efficient hydrolysis, and production of biofuel and value-added chemicals in a cost-effective manner.
Abbreviations
- AFEX:
-
Ammonia Fiber Explosion
- CrI:
-
Crystallinity index
- DA:
-
Dilute acid
- DP:
-
Degree of polymerization
- GR:
-
Gamma irradiation
- ILs:
-
Ionic liquids
- LCB:
-
Lignocellulosic biomass
- LHW:
-
Liquid hot water
- MWR:
-
Microwave radiation
- OS:
-
Organosolvent
- SE:
-
Steam explosion
- WO:
-
Wet oxidation
References
Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB (2011) Biomass pretreatment: fundamentals toward application. Biotechnol Adv 29(6):675–685
Agrawal K, Verma P (2020) Production optimization of yellow laccase from Stropharia sp. ITCC 8422 and enzyme-mediated depolymerization and hydrolysis of lignocellulosic biomass for biorefinery application. In: Biomass conversion and biorefinery, pp 1–20
Akhtar N, Gupta K, Goyal D, Goyal A (2016) Recent advances in pretreatment technologies for efficient hydrolysis of lignocellulosic biomass. Environ Progress Sustain Energy 35(2):489–511
Andersen SLF, Castoldi R, Garcia JAA, Bracht A, Peralta RA, de Lima EA, Helm CV, de Fátima Peralta Muniz Moreira R, Peralta RM (2019) Improving enzymatic Saccharification of Eucalyptus grandis branches by ozone pretreatment. Wood Sci Technol 53(1):49–69
Auxenfans T, Crônier D, Chabbert B, Paës G (2017) Understanding the structural and chemical changes of plant biomass following steam explosion pretreatment. Biotechnol Biofuels 10(1):36
Bergenstråhle M, Berglund LA, Mazeau K (2007) Thermal response in crystalline Iβ cellulose: a molecular dynamics study. J Phys Chem B 111(30):9138–9145
Bhardwaj N, Verma P (2021) Microbial xylanases: a helping module for the enzyme biorefinery platform. In: Srivastava N, Srivastava M (eds) Bioenergy research: evaluating strategies for commercialization and sustainability, pp 129–152
Bhardwaj N, Kumar B, Agrawal K, Verma P (2020) Bioconversion of rice straw by synergistic effect of in-house produced ligno-hemicellulolytic enzymes for enhanced bioethanol production. Bioresour Technol Rep 10:100352
Bhardwaj N, Kumar B, Agrawal K, Verma P (2021) Current perspective on production and applications of microbial cellulases: a review. Bioresour Bioprocess 8(1):1–34
Bhutto AW, Qureshi K, Harijan K, Abro R, Abbas T, Bazmi AA, Karim S, Yu G (2017) Insight into progress in pre-treatment of lignocellulosic biomass. Energy 122:724–745
Bishop CA (2015) Vacuum deposition onto webs, films and foils. 3rd edn
Bridgeman TG, Jones JM, Shield I, Williams PT (2008) Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 87(6):844–856
Chang KL, Thitikorn-amorn J, Hsieh JF, Bay Ming O, Chen SH, Ratanakhanokchai K, Huang PJ, Chen ST (2011) Enhanced enzymatic conversion with freeze pretreatment of rice straw. Biomass Bioenergy 35(1):90–95
Chen Z, Wan C (2018) Ultrafast fractionation of lignocellulosic biomass by microwave-assisted deep eutectic solvent pretreatment. Bioresour Technol 250:532–537
Chen H, Liu J, Chang X, Chen D, Xue Y, Liu P, Lin H, Han S (2017) A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process Technol 160:196–206
Chen X, Zhang Y, Mei J, Zhao G, Lyu Q, Xue L, Lyu H, Han L, Xiao W (2019) Ball milling for cellulose depolymerization and alcoholysis to produce methyl levulinate at mild temperature. Fuel Process Technol 188:129–136
Choi JH, Jang SK, Kim JH, Park SY, Kim JC, Jeong H, Kim HY, Choi IG (2019) Simultaneous production of glucose, furfural, and ethanol organosolv lignin for total utilization of high recalcitrant biomass by organosolv pretreatment. Renew Energy 130:952–960
Chundawat SPS, Beckham GT, Himmel ME, Dale BE (2011) Deconstruction of lignocellulosic biomass to fuels and chemicals. Annu Rev Chem Biomol Eng 2:121–145
da Costa Lopes AM, João KG, Rubik DF, Bogel-Łukasik E, Duarte LC, Andreaus J, Bogel-Łukasik R (2013) Pre-treatment of lignocellulosic biomass using ionic liquids: wheat straw fractionation. Bioresour Technol 142:198–208
Diaz AB, de Souza Moretti MM, Bezerra-Bussoli C, da Costa Carreira Nunes C, Blandino A, da Silva R, Gomes E (2015) Evaluation of microwave-assisted pretreatment of lignocellulosic biomass immersed in alkaline glycerol for fermentable sugars production. Bioresour Technol 185:316–323
Dijkerman R, Bhansing DCP, Op Den Camp HJM, Van Der Drift C, Vogels GD (1997) Degradation of structural polysaccharides by the plant cell-wall degrading enzyme system from anaerobic fungi: an application study. Enzym Microb Technol 21(2):130–136
Duarte CL, Ribeiro MA, Oikawa H, Mori MN, Napolitano CM, Galvão CA (2012) Electron beam combined with hydrothermal treatment for enhancing the enzymatic convertibility of sugarcane bagasse. Radiat Phys Chem 81(8):1008–1011
Endler A, Persson S (2011) Cellulose synthases and synthesis in Arabidopsis. Mol Plant 4(2):199–211
Falls M, Holtzapple MT (2011) Oxidative lime pretreatment of Alamo switchgrass. Appl Biochem Biotechnol 165(2):506–522
Fernandes AN, Thomas LH, Altaner CM, Philip C, Trevor Forsyth V, Apperley DC, Kennedy CJ, Jarvis MC (2011) Nanostructure of cellulose microfibrils in spruce wood. Proc Natl Acad Sci U S A 108(47):E1195–E1203
Fisher T, Hajaligol M, Waymack B, Kellogg D (2002) Pyrolysis behavior and kinetics of biomass derived materials. J Anal Appl Pyrolysis 62(2):331–349
Fu D, Mazza G (2011) Aqueous ionic liquid pretreatment of straw. Bioresour Technol 102(13):7008–7011
García-Cubero MT, González-Benito G, Indacoechea I, Coca M, Bolado S (2009) Effect of ozonolysis pretreatment on enzymatic digestibility of wheat and rye straw. Bioresour Technol 100(4):1608–1613
Gardiner ES, Sarko A (1985) Packing analysis of carbohydrates and polysaccharides. 16. The crystal structures of celluloses IV I and IV II. Can J Chem 63(1):173–180
Gatt E, Khatri V, Bley J, Barnabé S, Vandenbossche V, Beauregard M (2019) Enzymatic hydrolysis of corn crop residues with high solid loadings: new insights into the impact of bioextrusion on biomass deconstruction using carbohydrate-binding modules. Bioresour Technol 282:398–406
Guerrero AB, Ballesteros I, Ballesteros M (2017) Optimal conditions of acid-catalysed steam explosion pretreatment of banana lignocellulosic biomass for fermentable sugar production. J Chem Technol Biotechnol 92(9):2351–2359
Hideno A, Inoue H, Tsukahara K, Fujimoto S, Minowa T, Inoue S, Endo T, Sawayama S (2009) Wet disk milling pretreatment without sulfuric acid for enzymatic hydrolysis of Rice straw. Bioresour Technol 100(10):2706–2711
Howard RL, Abotsi E, Janse van Rensburg EL, Howard S (2003) Lignocellulose biotechnology: issues of bioconversion and enzyme production. Afr J Biotechnol 2(12):702–733
Husson E, Auxenfans T, Herbaut M, Baralle M, Lambertyn V, Rakotoarivonina H, Rémond C, Sarazin C (2018) Sequential and simultaneous strategies for biorefining of wheat straw using room temperature ionic liquids, xylanases and cellulases. Bioresour Technol 251:280–287
Imman S, Laosiripojana N, Champreda V (2018) Effects of liquid hot water pretreatment on enzymatic hydrolysis and physicochemical changes of corncobs. Appl Biochem Biotechnol 184(2):432–443
Jørgensen H, Kristensen JB, Felby C (2007) Enzymatic conversion of lignocellulose into fermentable sugars: challenges and opportunities. Biofuels Bioprod Biorefin 1(2):119–134
Kasthuraiah K, Sai Kishore N (2017) Lignocellulosic biofuels—challenges and potentials. Int J Pharma Biosci 8(1):376–381
Khan F, Ahmad SR, Kronfli E (2006) Γ-radiation induced changes in the physical and chemical properties of lignocellulose. Biomacromolecules 7(8):2303–2309
Kim S, Holtzapple MT (2005) Lime pretreatment and enzymatic hydrolysis of corn stover. Bioresour Technol 96(18 SPEC ISS):1994–2006
Kim KH, Hong J (2001) Supercritical CO2 pretreatment of lignocellulose enhances enzymatic cellulose hydrolysis. Bioresour Technol 77(2):139–144
Klemm D, Philipp B, Heinze T, Heinze U, Wagenknecht W (1998) Comprehensive cellulose chemistry, Fundamentals and analytical methods, vol I. Wiley-VCH, Hoboken
Kuhad RC, Singh A, Eriksson KE (1997) Microorganisms and enzymes involved in the degradation of plant fiber cell walls. Adv Biochem Eng Biotechnol 57:45–125
Kumar B, Verma P (2020a) Enzyme mediated multi-product process: a concept of bio-based refinery. Ind Crop Prod 154:112607
Kumar B, Verma P (2020b) Application of hydrolytic enzymes in biorefinery and its future prospects. In: Srivastava N, Srivastava M, Mishra PK, Gupta VK (eds) Microbial strategies for techno-economic biofuel production. Clean energy production technologies. Springer, Singapore, pp 59–83
Kumar B, Bhardwaj N, Verma P (2020) Microwave assisted transition metal salt and orthophosphoric acid pretreatment systems: generation of bioethanol and xylo-oligosaccharides. Renew Energy 158:574–584
Langan P, Nishiyama Y, Chanzy H (2001) X-ray structure of mercerized cellulose II at 1 Å resolution. Biomacromolecules 2(2):410–416
Lee J (1997) Biological conversion of lignocellulosic biomass to ethanol. J Biotechnol 56(1):1–24
Lenz RW (1994) Cellulose, structure, accessibility and reactivity, by H. A. Krässig, Gordon and Breach Publishers, 5301 Tacony Street, Philadelphia, PA, 1993; Xvi + 376 Pp. Price: $260.00. J Polym Sci A Polym Chem 32(12):2401–2401
Li H, Kim NJ, Jiang M, Kang JW, Chang HN (2009) Simultaneous saccharification and fermentation of lignocellulosic residues pretreated with phosphoric acid-acetone for bioethanol production. Bioresour Technol 100(13):3245–3251
Li BZ, Balan V, Yuan YJ, Dale BE (2010) Process optimization to convert forage and sweet sorghum bagasse to ethanol based on ammonia fiber expansion (AFEX) pretreatment. Bioresour Technol 101(4):1285–1292
Liu Y, Chen W, Xia Q, Guo B, Wang Q, Liu S, Liu Y, Li J, Haipeng Y (2017a) Efficient cleavage of lignin–carbohydrate complexes and ultrafast extraction of lignin oligomers from wood biomass by microwave-assisted treatment with deep eutectic solvent. ChemSusChem 10(8):1692–1700
Liu Y, Guo L, Wang L, Wang Z, Zhou H (2017b) Irradiation pretreatment facilitates the achievement of high total sugars concentration from lignocellulose biomass. Bioresour Technol 232:270–277
Manna B, Ghosh A (2019) Dissolution of cellulose in ionic liquid and water mixtures as revealed by molecular dynamics simulations. J Biomol Struct Dyn 37(15):3987–4005
Manna B, Datta S, Ghosh A (2021) Understanding the dissolution of softwood lignin in ionic liquid and water mixed solvents. Int J Biol Macromol 182:402–412
Mittal A, Katahira R, Himmel ME, Johnson DK (2011) Effects of alkaline or liquid-ammonia treatment on crystalline cellulose: changes in crystalline structure and effects on enzymatic digestibility. Biotechnol Biofuels 4:41
Mohapatra S, Dandapat SJ, Thatoi H (2017) Physicochemical characterization, modelling and optimization of ultrasono-assisted acid pretreatment of two Pennisetum sp. using Taguchi and artificial neural networking for enhanced delignification. J Environ Manag 187:537–549
Mokomele T, da Costa Sousa L, Bals B, Balan V, Goosen N, Dale BE, Görgens JF (2018) Using steam explosion or AFEX™ to produce animal feeds and biofuel feedstocks in a biorefinery based on sugarcane residues. Biofuels Bioprod Biorefin 12(6):978–996
Moniruzzaman M, Ono T (2012) Ionic liquid assisted enzymatic delignification of wood biomass: a new ‘green’ and efficient approach for isolating of cellulose fibers. Biochem Eng J 60:156–160
Mood SH, Golfeshan AH, Tabatabaei M, Jouzani GS, Najafi GH, Gholami M, Ardjmand M (2013) Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew Sust Energ Rev 27:77–93
Moulthrop JS, Swatloski RP, Moyna G, Rogers RD (2005) High-resolution 13C NMR studies of cellulose and cellulose oligomers in ionic liquid solutions. Chem Commun 40(12):1557–1559
Nishiyama Y (2009) Structure and properties of the cellulose microfibril. J Wood Sci 55(4):241–249
Nishiyama Y, Langan P, Chanzy H (2002) Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 124(31):9074–9082
Nishiyama Y, Sugiyama J, Chanzy H, Langan P (2003) Crystal structure and hydrogen bonding system in cellulose Iα from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 125(47):14300–14306
Okeke BC, Obi SKC (1994) Lignocellulose and sugar compositions of some agro-waste materials. Bioresour Technol 47(3):283–284
Ortega JV, Renehan AM, Liberatore MW, Herring AM (2011) Physical and chemical characteristics of aging pyrolysis oils produced from hardwood and softwood feedstocks. J Anal Appl Pyrolysis 91(1):190–198
Payne CM, Knott BC, Mayes HB, Hansson H, Himmel ME, Sandgren M, Ståhlberg J, Beckham GT (2015) Fungal cellulases. Chem Rev 115(3):1308–1448
Pérez JA, Ballesteros I, Ballesteros M, Sáez F, Negro MJ, Manzanares P (2008) Optimizing liquid hot water pretreatment conditions to enhance sugar recovery from wheat straw for fuel-ethanol production. Fuel 87(17–18):3640–3647
Persson H, Yang W (2019) Catalytic pyrolysis of demineralized lignocellulosic biomass. Fuel 252:200–209
Radhakrishnan R, Patra P, Das M, Ghosh A (2021) Recent advancements in the ionic liquid mediated lignin valorization for the production of renewable materials and value-added chemicals. Renew Sust Energ Rev 149:111368
Rambo MKD, Schmidt FL, Ferreira MMC (2015) Analysis of the lignocellulosic components of biomass residues for biorefinery opportunities. Talanta 144(11):696–703
Redding AP, Wang Z, Keshwani DR, Cheng JJ (2011) High temperature dilute acid pretreatment of coastal Bermuda grass for enzymatic hydrolysis. Bioresour Technol 102(2):1415–1424
Remsing RC, Swatloski RP, Rogers RD, Moyna G (2006) Mechanism of cellulose dissolution in the ionic liquid 1-n-butyl-3- methylimidazolium chloride: a 13C and 35/37Cl NMR relaxation study on model systems. Chem Commun 12:1271–1273
Rogers RD, Seddon KR (2003) Ionic liquids—solvents of the future? Science 302(5646):792–793
Romaní A, Larramendi A, Yáñez R, Cancela Á, Sánchez Á, Teixeira JA, Domingues L (2019) Valorization of Eucalyptus nitens bark by organosolv pretreatment for the production of advanced biofuels. Ind Crop Prod 132:327–335
Rosen Y, Mamane H, Gerchman Y (2019) Short ozonation of lignocellulosic waste as energetically favorable pretreatment. Bioenergy Res 12(2):292–301
Rubin EM (2008) Genomics of cellulosic biofuels. Nature 454(7206):841–845
Saha BC (2004) Lignocellulose biodegradation and applications in biotechnology. In: Lignocellulose biodegradation, vol. 889. ACS symposium series. American Chemical Society
Saini JK, Saini R, Tewari L (2015) Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3 Biotech 5(4):337–353
Sarko A, Southwick J, Hayashi J (1976) Packing analysis of carbohydrates and polysaccharides. 7. Crystal structure of cellulose IIII and its relationship to other cellulose polymorphs. Macromolecules 9(5):857–863
Shi J, Balamurugan K, Parthasarathi R, Sathitsuksanoh N, Zhang S, Stavila V, Subramanian V, Simmons BA, Singh S (2014) Understanding the role of water during ionic liquid pretreatment of lignocellulose: co-solvent or anti-solvent? Green Chem 16(8):3830–3840
Shibuva N, Misaki A (1978) Structure of hemicellulose isolated from rice endosperm cell wall: mode of linkages and sequences in xyloglucan, β-glucan and Arabinoxylan. Agric Biol Chem 42(12):2267–2274
Sun F, Chen H (2007) Evaluation of enzymatic hydrolysis of wheat straw pretreated by atmospheric glycerol autocatalysis. J Chem Technol Biotechnol 82(11):1039–1044
Sun N, Rahman M, Qin Y, Maxim ML, Rodríguez H, Rogers RD (2009) Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem 11(5):646–665
Szabo OE, Csiszar E (2017) Some factors affecting efficiency of the ultrasound-aided enzymatic hydrolysis of cotton cellulose. Carbohydr Polym 156:357–363
Tan X, Zhang Q, Wang W, Zhuang X, Deng Y, Yuan Z (2019) Comparison study of organosolv pretreatment on hybrid Pennisetum for enzymatic saccharification and lignin isolation. Fuel 249:334–340
Thring RW, Chornet E, Overend RP (1990) Recovery of a solvolytic lignin: effects of spent liquor/acid volume ratio, acid concentration and temperature. Biomass 23(4):289–305
Uju YS, Nakamoto A, Goto M, Tokuhara W, Noritake Y, Katahira S, Ishida N, Nakashima K, Ogino C, Kamiya N (2012) Short time ionic liquids pretreatment on lignocellulosic biomass to enhance enzymatic saccharification. Bioresour Technol 103(1):446–452
Ververis C, Georghiou K, Christodoulakis N, Santas P, Santas R (2004) Fiber dimensions, lignin and cellulose content of various plant materials and their suitability for paper production. Ind Crop Prod 19(3):245–254
Wahlström RM, Suurnäkki A (2015) Enzymatic hydrolysis of lignocellulosic polysaccharides in the presence of ionic liquids. Green Chem 17(2):694–714
Wan C, Zhou Y, Li Y (2011) Liquid hot water and alkaline pretreatment of soybean straw for improving cellulose digestibility. Bioresour Technol 102(10):6254–6259
Wasserscheid P, Keim W (2000) Ionic liquids—new ‘solutions’ for transition metal catalysis. Angew Chem Int Ed Engl 39(21):3772–3789
Wu H, Dai X, Zhou SL, Gan YY, Xiong ZY, Qin YH, Ma J, Yang L, Wu ZK, Wang TL, Wang WG, Wang CW (2017) Ultrasound-assisted alkaline pretreatment for enhancing the enzymatic hydrolysis of rice straw by using the heat energy dissipated from ultrasonication. Bioresour Technol 241:70–74
Xiang Y, Xiang Y, Wang L (2017) Electron beam irradiation to enhance enzymatic saccharification of alkali soaked Artemisia ordosica used for production of biofuels. J Environ Chem Eng 5(4):4093–4100
Yang L, Cao J, Jin Y, Chang HM, Jameel H, Phillips R, Li Z (2012) Effects of sodium carbonate pretreatment on the chemical compositions and enzymatic saccharification of rice straw. Bioresour Technol 124:283–291
Yang F, Wang X, Chen Q, Tan H (2019a) Improvement of the properties of 1-ethyl-3-methylimidazolium acetate using organic solvents for biofuel process. J Mol Liq 284:82–91
Yang H, Shi Z, Xu G, Qin Y, Deng J, Yang J (2019b) Bioethanol production from bamboo with alkali-catalyzed liquid hot water pretreatment. Bioresour Technol 274:261–266
Yoo J, Alavi S, Vadlani P, Amanor-Boadu V (2011) Thermo-mechanical extrusion pretreatment for conversion of soybean hulls to fermentable sugars. Bioresour Technol 102(16):7583–7590
Zhang YHP, Lynd LR (2003) Cellodextrin preparation by mixed-acid hydrolysis and chromatographic separation. Anal Biochem 322(2):225–232
Zhao H, Baker GA, Song Z, Olubajo O, Crittle T, Peters D (2008) Designing enzyme-compatible ionic liquids that can dissolve carbohydrates. Green Chem 10(6):696–670
Zhao MJ, Xu QQ, Li GM, Zhang QZ, Zhou D, Yin JZ, Zhan HS (2019) Pretreatment of agricultural residues by supercritical CO2 at 50–80 °C to enhance enzymatic hydrolysis. J Energy Chem 31:39–45
Acknowledgments
Authors are thankful to Department of Science and Technology (Grant No. CRG/2020/002080), Department of Biotechnology (Grant No. BT/RLF/Re-entry/06/2013) and Scheme for Promotion of Academic and Research Collaboration (SPARC), MHRD, Govt. of India (Grant No. SPARC/2018-2019/P265/SL). Pradipta Patra appreciates the support from the Department of Science and Technology(DST) (INSPIRE, India for the award of fellowships, DST). Manali Das thanks thesupport from the Council of Scientific and Industrial Research (CSIR).
Competing Interests
All the authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Manna, B., Das, M., Patra, P., Ghosh, A. (2022). Insight into Various Conventional Physical and Chemical Methods for the Pretreatment of Lignocellulosic Biomass. In: Verma, P. (eds) Thermochemical and Catalytic Conversion Technologies for Future Biorefineries. Clean Energy Production Technologies. Springer, Singapore. https://doi.org/10.1007/978-981-19-4316-4_2
Download citation
DOI: https://doi.org/10.1007/978-981-19-4316-4_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4315-7
Online ISBN: 978-981-19-4316-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)