Abstract
Excessive concentrations of F−, NO3− and other chemicals in drinking water can have adverse human health effects. In this study, groundwater quality and potential source distribution of F− and NO3− in a community in north-eastern Ghana, Bolgatanga were evaluated. In addition, human health risk associated with the drinking of groundwater by the residents was assessed. Ion chromatography technique was used to determine F−, Cl−, NO3−, and SO42−, potentiometric titrimetric technique was applied to analyze HCO3−, and flame atomic absorption spectrophotometry was used to quantify K+ and Na+. EDTA titrimetric analysis was used for the analysis of Mg2+ and Ca2+. The quality of groundwater for drinking was evaluated by applying the entropy of water quality index (EWQI) and comparing the analyzed data values of different chemicals detected in the water samples to the recommended ranges of the world health organization (WHO). Evaluation of health risks associated with various age groups (infants, children, and adults) was achieved based on estimation of hazard quotient and hazard index. The results of EWQI showed that 72.00% of groundwater samples were of medium quality (i.e. grade 3) and convenient for drinking purposes upon simple treatment. The groundwater quality of nine (18.00%) and five (10.00%) sampled sites were poor and extremely poor, respectively, and they were regarded as unsafe for drinking (grades 4 and 5). Also, the results obtained revealed that F− and NO3− concentrations in groundwater samples were in the ranged of 0.18–1.83 and 2.06–81.30 mg/L, respectively. Six out of fifty samples have high F− levels exceeding the permissible limits (1.5 mg/L), while 26 samples have high NO3− levels surpassing the recommended limits for drinking purposes according to WHO (10 mg/L). Human activities such as fertilizer application on farmlands, sewage discharge, etc. are the factors influencing enrichment of NO3− contamination. The fundamental source of F− in the groundwater is the dissolution of fluorite. Correlation coefficients obtained for F− and NO3− with other chemicals have further substantiated the sources of pollutants in the groundwater of the studied area. Generally, the three age categories are vulnerable to F− and NO3− contaminants in drinking groundwater of the sampled sites. The order of vulnerability is as follows: adults > children > infants. The results of the hazard index obtained suggested that all the samples are likely to pose negative health effects on children and adults. However, the proportions are relatively smaller for infants (36%). The results of this study indicate that immediate action needs to be taken to reduce groundwater pollution of F− and NO3− in the studied area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
One of the most necessary elements for human survival is water. Nevertheless, human development and inhabitants' growth impose constantly heightening pressures on the quality of water [1]. Howard et al. opined that the safety and nearness of drinking water have become an issue of concern all over the world [2]. This problem is more severe in rural settlements. Globally, it is estimated that 76% of rural inhabitants had means of getting clean and drinkable water; however, the estimated percentage for rural communities was far lesser as compared to urban zones (94% of the residents) [3]. In regards to Ghana, about 55% of its inhabitants have the means to continual safe drinking water [4]. Approximately 53% of the rural inhabitants in Ghana had no means of pipe-borne water [4]. In the light of these, there is a high-priority requirement to take measures to improve means to safe, feasible, and long-term drinking water in remote regions.
Around 20% of the world's fresh water supply is obtained from groundwater, and it has been the only source of potable water in most nations [5, 6]. Unfavorable health impacts could emanate from utilization (i.e. drinking, cooking, etc.) of water polluted with the chemicals. Even if the chemicals are from anthropogenic sources such as sewage, inorganic fertilizer, organic manure, etc., or natural sources including geochemical reactions, lithology, recharge water quality, velocity, the solubility of salts, etc. [1, 7]. Out of numerous pollutants in groundwater that could have negative health impacts on humans, fluoride and nitrate are more extensive. The levels of fluoride and nitrate in drinking water are crucial characteristics of hydro-geochemistry, due to their effects on human health. Fluoride is a vital microelement for human growth, development, and health. Arithmetically, a lesser amount (˂ 1.0 mg/L) in drinking water is usually recommended to have an advantageous impact on dental caries occurrence, specifically at young ages [8, 9]. However, long-term excessive exposure (> 1.5 mg/L) to fluoride may generate a multitude of unfavorable impacts such as increased rate of bone fractures, dental fluorosis, impaired thyroid function, skeletal fluorosis, etc. [8, 10]. The occurrence of fluoride in natural waters is chiefly as a free ion [1]. The fundamental source of fluoride in groundwater is the dissolution of fluoride-bearing minerals (fluorite [CaF2]) [11]. Globally, it is regarded that fluorosis is a health issue, which is plaguing millions of human beings in many corners of the world [13,14,15,16]. There has been a concern of nitrate pollution in shallow groundwater underneath farmland regions with characteristics of oxic geochemical conditions and well-drained soils [12]. Higher values of nitrate in groundwater have been extensively described in many studies [1, 13,14,15,16]. Blue-baby syndrome commonly known to people as methemoglobinemia is the most instantaneous life menacing impact of the exposure to nitrate via ingesting contaminated water (disease mainly for children) [17]. Other unfavorable impacts of nitrate accumulation have also been reported which are associated with two types of birth defects and fifteen kinds of cancer [1, 17].
Groundwater is highly essential in arid and semiarid territories because surface waters are normally in short supply. Bolgatanga, informally known to people as Bolga, is the capital town of Bolgantanga municipal in Ghana and shares a border with Burkina Faso. This town is a good description of a town where inhabitants depend on groundwater (borehole water) for everyday activities (e.g. drinking, cooking, bathing, etc.). Borehole well water is a type of shallow groundwater normally from depths between 20 and 200 m. Generally, boreholes diameter varies from 100 to 1000 mm and water is pumped to the surface via the main pipe 25–300 mm in diameter. However, in real life, the superficial type of groundwater is vulnerable to pollution from several origins in the alluvial plain. The predominant occupation in Bolga is agriculture and most farmers used fertilizers as inputs to boost their farm yields. These fertilizers (e.g. nitrate and phosphate fertilizers) on the farmland fields can fortuitously seep into shallow aquifers due to runoff and excessive erosion. Additionally, the basic information regarding the safety protocols of rural water for drinking does not exist in most of the towns and villages in Ghana for example Bolgatanga municipal. Furthermore, due to domestic sewage discharge and the excessive application of inorganic fertilizer in the farming community of Bolgatanga, the status of fluoride and nitrate in borehole well water can be high. Also, poisoning as a result of fluorine has become a cultural disease in the area due to the elevated content of fluoride ions in groundwater many years ago [4]. Notwithstanding the significant purpose of groundwater, studies into groundwater pollution as well as the health risk associated with residents living in this farming community with less population are lacking.
Because of these, this study considers the question “is groundwater (i.e. borehole well water) in the farming community of Bolgatanga Municipal, Ghana fit for drinking?” The study further proposes that the groundwater must be comprehensively assessed regularly to adequately answer the research question. To conduct the study, water samples were collected from borehole wells in the Bolgatanga municipality near farming areas to evaluate the groundwater quality by using two methods: (1) entropy water quality index (EWQI) and (2) comparative analyses of the values obtained and the recommended limits set by the World Health Organization (WHO). Also, correlation analyses were carried out to determine the potential sources of fluoride and nitrate pollution in the groundwater of the study area, and evaluate the risk of fluoride and nitrate pollution associated with human health through ingestion pathways for the various age groups.
2 Methodologies
2.1 Study Area
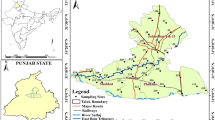
The study was conducted in the Bolgatanga district in the Upper East Region of Ghana. Its land area is approximately 729 km2 and has coordinates of 10° 47′ N0° 51′ W. It is bonded to the south by Talensi district, north by Bongo district, east and west by Nabdam and Kassena-Nankana districts respectively. Bolgatanga Municipal has total inhabitants of 236,753 with 57.25% female population and 42.75% male population according to Ghana Statistical Department. The natural vegetation of the municipal is composed of tree savannah, with acacia and baobab trees. During the dry season, the low vegetation becomes dried and can be burnt through the perennial bushfires. Annual mean rainfall is 965 mm whereas minimum temperature is 14 °C in December and maximum temperature is around 46 °C in March to April. As it is mentioned earlier, the major source of income is agriculture and the main agronomic crops grown are cereals (e.g. maize, rice, wheat, and sorghum), vegetables (e.g. tomatoes, pepper, etc.), leguminous crops (e.g. cowpea, Bambara groundnuts, groundnuts, soybean, etc.), and stem and root tubers (e.g. yam, sweet potato, etc.). Figure 1 illustrates the map of Bolgatanga Municipal showing the sampled sites.
2.2 Geology of the Study Area
Bolgatanga Municipal is underlain by two prime geologic formations: the pre-Cambrian basement complex and the paleozoic consolidated sedimentary formations. The pre-Cambrian basement complex rocks consist of crystalline igneous and metamorphic rocks which cover nearly 92% of the municipal.
2.3 Sampling
The technique for sampling collection was adapted by Chen et al. with little modification [1]. The location for sampling was categorized into 10 (i.e. GW1–GW10). In each category, 5 groundwater samples were collected from 5 representative borehole wells. Thus, 50 groundwater samples were collected from fifty borehole hand-pumping wells in November 2020. A portable handheld Global Positioning System device (GPS) was used to locate the site of the borehole wells in the field. Generally, the depth of the borehole wells sampled ranges from 8 to 32 m from the ground surface. The water from the borehole hand-pumping wells sampled is used for drinking, cooking, irrigation, etc. without any treatment. Samples were stored in clean polyethylene bottles. During sampling, polyethylene bottles were rigorously rinsed 3–5 times with borehole well water to be sampled. After sampling, the samples were stored in an ice chest at a temperature of 20 °C before physicochemical analysis. In situ analyses such as temperature, pH, and total dissolved solids (TDS) were carried out using transportable instruments according to the protocols of US EPA [18].
2.4 Chemical Analysis
The analysis of F−, Cl−, HCO3−, NO3−, SO42−, K+, Na+, Mg2+, and Ca2+ were performed at the laboratory of Ghana Food and Drugs Authority within 72 h of sampling. The potentiometric titrimetric technique was used to analyze HCO3−. The methodology given by the protocol [19] was followed without any modifications, except that the TRIS-H2SO4 buffer was at pH 8.2 instead of 8.6.
EDTA titrimetric analysis was used for the analysis of Mg2+, and Ca2+ according to the method proposed by US EPA [18]. 10 mL sample of the EDTA solution was pipetted into a conical flask. 10 mL of NH3 buffer solution and 1 mL of Eriochrome Black T indicator solution were added. The EDTA was titrated with magnesium chloride solution until the endpoint is reached (a permanent color change from blue to pink is observed). After standardization of EDTA solution, 100 mL of the groundwater sample for each sample point was placed into a 250 mL conical flask. 0.005 mol/L EDTA solutions were prepared by diluting the 0.05 mol/L EDTA solution by a factor of 1/10. The groundwater samples were titrated with 20 mL of the diluted EDTA. 10 mL of NH3 buffer and 1 mL of Eriochrome Black T indicator solution were added. 0.0025 mol/L MgCl2 solution was prepared by diluting 0.025 mol/L of MgCl2 by a factor of 1/10. Groundwater samples were titrated with 0.0025 mol/L MgCl2 solutions until a permanent pink color appears. The same procedure was used for Ca2+, except that CaCl2 solution was used instead of MgCl2 solution.
Anions such as F−, Cl−, NO3−, and SO42− were quantified using a single ion chromatography technique [20]. An analytical column, a self-regenerating suppressor, and NaCO3 as the eluent were employed to separate the anions. Technique detection limits for the anions of interest are shown in Table 1. The precision of the technique was tested at five different concentration levels for each anion reference sample to ascertain the suitability of the technique for groundwater analysis. Reference samples were added to the groundwater and drinking water for recovery studies between two successive months. Precision was also evaluated as the relative standard deviation of both reproducibility (between-day and different concentrations) and repeatability (within-day) for groundwater samples. Standard deviation and relative standard deviation values obtained show that the technique is suitable for determining major anions in groundwater samples. Thus, 50 groundwater samples collected from the study area were evaluated.
Flame atomic absorption spectrophotometry (FAAS) was used to measure K+ and Na+ [21]. Standard solutions of K+ and Na+ were prepared to standardize and calibrate the instrument. The detection limits of the instrument for K+ and Na+ are depicted in Table 1. The analytical precision of 2.5% standard deviation for K+ and 3.2% standard deviation for Na+ was achieved indicating that the technique is effective for assessing K+ and Na+ in groundwater samples. Groundwater samples were filtered to remove suspended visible debris and transferred to clean test tubes for direct analysis. Each sample is replicated to get good repeatability. Table 1 depicts analytical techniques used, respective detection limits and the WHO guidelines values for the various chemical analyses.
2.5 Charge Balance Error (% CBE)
One of the primary laws of nature is that aqueous solutions should be electrically neutral. This implies that in real solutions, the total sum of all cations (positive charges) must equal the total sum of anions (negative charges). Nevertheless, analytical errors and/or unanalyzed components result in electrical imbalances. The three main causes of electrical imbalances include: using unfiltered samples that contain particulate matter which dissolve upon addition of chemical substance, lab errors during analysis, and when some dissolved major ions are not measured. Charge-balance error (% CBE) for each sample was estimated using the formula below to check the validity and quality of water samples [23]:
The expressions of all cations and anions are in mg/L% CBE as absolute values were 5% or less is considered as perfect for water analysis, although up to 10% is also considered appropriate. The validated check balance indicates that most of the groundwater samples have % CBE less than or equal to ± 5%. However, nine samples (GW1C, GW1D, GW2A, GW5C, GW6A, GW6D, GW9E, GW10A, and GW10B) their percentage charge balance were greater than ± 5% but less than ± 10% which were also considered acceptable in this study.
2.6 Entropy Water Quality Index (EWQI)
The water quality index introduced by Horton [24] has been widely accepted as a standard method for evaluating water quality. The notion of entropy was first suggested by Shannon [25]. Li et al. [26] used the entropy weight to the conventional water quality index and put forward the EWQI. The entropy water quality index is a more objective index of groundwater quality which helps to eliminate the human subjectivity influence when estimating the weight of evaluation indexes [27]. Due to its simplicity, accuracy, and consistency, the entropy water quality index has been extensively applied by a lot of researchers all over the world [28,29,30,31,32,33,34]. In this research, the entropy is used to define the groundwater and was quantified via six steps as follows:
Step 1: The initial groundwater quality matrix establishment. The initial matrix can be instituted depended on the chemistry analysis data of groundwater samples. Assume there are k groundwater samples, and each sample has l evaluation indexes, the initial groundwater quality matrix is X, then xij is the initial value of the jth evaluation indicator of the ith groundwater sample. In the present study, the values of k and l were 50 and 11, respectively,
Step 2: Groundwater quality matrix normalization. A significant variation usually occurs in the units and amount of grades of various groundwater quality indicators which results in a monumental variation in the weight estimated. Thus, Eq. (3) was used to normalize the initial groundwater quality index, where yij is the value of normalized data and (rij) jmin and (rij) jmax are the least and highest range of indicators j, respectively. Equation (4) was also used to characterize the standard groundwater quality matrix Y,
Step 3: Information entropy determination. Equation (5) was used to calculate the ratio pij of the index value j of sample i, and the correction factor 10–4 is used to ensure the significance of the equation. To estimate the information entropy ej of indicator j, Eq. (6) was used,
Step 4: Entropy weight quantification. The smaller the value of the information entropy ej is, the larger the control of the j index. The entropy weight of each indicator wj was derived based on Eq. (7),
Step 5: The quality rating scale determination. The quality rating scale qj of index j was evaluated according to Eq. (8), where cj denotes the content of index j (mg/L) and sj represents the standard limit of index j (mg/L) of drinking water quality based on Chinese criteria limit recommendation,
Step 6: Entropy water quality index (EWQI) computation. Equation (9) was used to compute EWQI,
In regards to EWQI, the quality of groundwater for human consumption can be grouped into five ranks, broadening from extremely poor to excellent water [21, 27]; extensive details are presented in Table 2.
2.7 Statistical Analyses
Statistical package for the social sciences (SPSS) version 20.0 was employed to carry out the correlation analysis of the various chemicals in the samples. That is, to measure the statistical dependence between two variables, Spearman's rank correlation was used [35].
2.8 Health Risk Evaluation
To comprehend the likelihood of unfavorable impacts on humans who may be exposed to chemicals in polluted water, health risk assessment is important. It is mostly the primary measure in safeguarding safety and health. To calculate for non-carcinogenic effects of contaminants, the empirical models put forward by US EPA [36] were employed.
2.8.1 Chronic Daily Intake (CDI)
The average daily dose of each variable can be estimated by the Equation:
where CDI denotes exposure dose via intake of groundwater (mg/kg/day); ED signifies the exposure duration (/year); EF represents the exposure frequency (day/year); IR denotes ingestion rate of groundwater (L/day); Cw represents the concentration of a contaminant of interest in groundwater (mg/L); AT signifies the meantime for non-carcinogenic effects (days); and BW denotes the average body weight (kg) of inhabitants in Bolgatanga. One hundred inhabitants' body weights were taken and the average weight was recorded.
About 91% of residents in Bolgatanga municipal rely on groundwater for drinking, and therefore EF is allocated 365 days/year for inhabitants. The value of ED is 30 years for adults, 12 years for children, and 1 year for infants [1, 23]. The AT value is 365 days [20]. The human water ingestion rate (IR) in L/day is 0.3 L/day for infants [37], 1.00 L/day for children [38] and 3.53 L/day for adults [39]. In regards to body weight (BW), BW = 5 kg for infants [40], BW = 15 kg for children [38], and BW = 56.70 kg for adults.
2.8.2 Hazard Quotient (HQ)
Non-carcinogenic effects of each variable were calculated as hazard quotient (HQ) by equation [38]:
where RfD denotes the reference dose of a particular pollutant. From the database of Integrated Risk Information System, US EPA [41], the RfD of F− is 0.40 mg/kg/day and NO3− is 1.60 mg/kg/day. If HQ ≤ 1, then no adverse health effects are expected as a result of exposure, and if HQ > 1, then adverse health effects are possible.
2.8.3 Hazard Index (HI)
The integrated risk is often referred to as the hazard index. The HI of fluoride and nitrate in drinking water was estimated by summing the HQ value of fluoride and that of nitrate. It was calculated by Eq. 12 [36, 38]:
3 Results and Discussion
3.1 The Groundwater Quality Assessment
To give the general features of the borehole well water quality, all the parameters were statistically analyzed. The minimum, the average, the maximum, and the standard deviation for each parameter are shown in Table 3. The suitability of borehole water (groundwater) for drinking was evaluated by,
-
(a)
Applying the entropy water quality index. The entropy water quality index (EWQI) technique was applied to evaluate the groundwater quality (borehole well water) in a farming community of Bolgatanga Municipal for drinking, and pH, TDS, F−, NO3−, Cl−, HCO3−, SO42−, K+, Mg2+, Ca2+, and Na were considered. Ghana's drinking water quality standards are inappropriate due to the failure of the water resources commission (WRC) management to establish a permanent standard drinking water quality criteria limit for assessment of water quality in Ghana. Therefore, Chinese drinking water quality criteria were adapted as the criteria limit for groundwater quality determination in the farming community of Bolgatanga Municipal. Based on the distribution range of the EWQI value, the groundwater quality level was evaluated. The results obtained from EWQI estimation ranged from 43.22 to 189.04, with an average value of 96.00 (Table 3). Therefore, there is no excellent groundwater sampled (grade 1) in a community in north-eastern Ghana, Blogatanga. Out of 50 samples collected, 72.00% of groundwater samples were in grade 3 (i.e. of medium quality and convenient for drinking purposes) according to the categorization of EWQI (Table 2). The groundwater quality of nine (18.00%) and five (10.00%) samples were found to be poor and extremely poor, respectively and they were regarded as unsafe for drinking (grades 4 and 5). In the farming community of Bolgatanga Municipal, the borehole well water (shallow groundwater) in most areas is of medium water quality, and after simple treatment; the water can be fit for drinking. However, the samples with high values of EWQI were detected in an area closed to refuse dump and cabbage and sorghum farming. Therefore, groundwater pollution may be usually attributed to sewage discharge and nitrogenous fertilizer.
-
(b)
The values obtained for different chemicals detected in the water samples were compared to the recommended ranges of WHO [10, 22] (Table 1).
One of the essential parameters of potable water is total dissolved solids (TDS) which are usually considered as an indicator of water palatability. According to WHO, freshwater with TDS less than 1000.00 mg/L is generally admissible to be consumed, even though, admissibility may depend on other important standards [22]. Based on the results shown in Table 3, huge differences in TDS values existed between the various sampling stations. TDS vary from 602.43 to 2072.33 mg/L with an average value of 1176.73 mg/L, and 64.00% of the samples (i.e. 32 samples) were above the allowable limits (1000.00 mg/L) for TDS [22]. The influence of longer residence time of groundwater in the aquifer, solubilization, and ion exchange may be the reason for higher TDS levels in some groundwater sampled stations [42].
The acidity or alkalinity of a solution is the general term used to explain pH [22]. The values of pH in all sampled stations ranged between 6.97 and 8.11 with an average value of 7.61, indicating a slightly basic condition of groundwater. The values of pH for all the sampled stations are within the acceptable range (6.50–8.50).
The level of the anions of the groundwater follows the trend: F− ˂ NO3− ˂ Cl− ˂ HCO3− ˂ SO42− with average percentages of 0.10, 3.11, 18.89, 38.30, and 39.60%, respectively. The common anion in groundwater is SO42−; however, high levels of SO42− may result in a laxative impact on the human system when associated with excess Mg2+ in drinking water [43]. According to the guidelines of WHO, the limit of SO42− in drinking water recommended is 250 mg/L [10]. Nevertheless, the level of SO42− in the studied area ranged from 106.71 to 708.33 mg/L. 38.00% of the analyzed samples are beyond the maximum permissible limit of SO42− for drinking. One of the extremely stable ions in water is chloride which may be obtained from the leaching of sedimentary rock and soil, weathering, and domestic effluents [34, 44]. According to WHO, the acceptable limit of Cl− in drinking water is 250 mg/L. The level of Cl− in the water samples ranged from 60.32 to 333.91 mg/L with an average value of 118.21 mg/L. Three out of the fifty samples have high levels of chloride ion exceeding the acceptable limit therefore most of the borehole wells are suitable for drinking. The unexpected increase in Cl− level in the groundwater samples may be ascribed to the influence of anthropogenic pollution. HCO3− content in borehole well water samples varied from 106.72 to 401.22 mg/L with an average value of 239.62 mg/L. 11 samples which constitute 22% of samples collected exhibit levels of HCO3− exceeding the acceptable limit of WHO (i.e. 300.00 mg/L).
The levels of cations follow the trend: K+ ˂ Mg2+ ˂ Ca2+ ˂ Na+ with an average percentage of 1.57, 15.38, 41.29, and 41.76%. The level of K+ and Na+ detected in the study area ranged from 2.52 to 14.11 mg/L with a mean value of 4.46 mg/L and 79.31–273.02 mg/L with a mean value of 118.90 mg/L, respectively. The levels of K+ in the borehole well were generally low compared with other cations (i.e. Mg2+, Ca2+, and Na+). Based on WHO guidelines, five of the samples have high levels of K+ (> 10 mg/L) surpassing the permissible limit (i.e. WHO limit for K+ in drinking water is 10.00 mg/L). Six samples have high levels of Na+ (> 200 mg/L) exceeding the acceptable limit established by WHO. Chen et al. opined that cation exchange and dissolution of evaporates for example silicates and halite may be attributed to the high level of Na+ in the borehole wells [1]. The content of Mg2+ in samples varied from 21.33 to 71.07 mg/L with an average value of 43.78 mg/L. 45 samples, which constitute 90% of the total sampling sites have high levels of Mg2+ (> 30.00 mg/L) as compared to the permissible limit set by WHO. The concentrations of Ca2+ in investigated groundwater samples varied between 66.52 and 162.97 mg/L with an average value of 117.61 mg/L. 2 out of 50 samples were observed to be within the acceptable limit (75.00 mg/L) for Ca2+ in drinking water set by WHO. Enrichment of Mg2+ and Ca2+ in groundwater samples could result from the water being categorized as hard water.
3.2 Fluoride (F−) Contamination
One of the primary trace elements in groundwater is fluoride. The levels of fluoride in the study area varied from 0.18 to 1.83 mg/L with an average value of 0.69 mg/L. Six of the samples have concentrations higher (> 1.50 mg/L) than the acceptable limit for fluoride in drinking water according to WHO. The pH values (6.97–8.11) for the borehole well water in the study area serve as a favorable environment for F− enrichment [6]. The increase of the dissolution of F− bearing minerals such as CaF2 in groundwater is attributable to high pH values [37]. In a situation whereby groundwater is in alkaline or weakly alkaline medium, the increase in the concentration of OH− will lead chemical reaction 1 (CR1) to move to the left, resulting in the corresponding dissolution of CaF2 as shown in Equation CR2 [9],
Li et al. opined that the quantity of F− discharged into borehole well waters is influenced by the degree of saturation of fluorite and calcite and the levels of Ca2+, Na+, and HCO3− in groundwater [45]. Positive inter-relationships between Ca2+ and F− could promote the weathering of fluorite (CaF2); nevertheless, as shown in Table 4 and Fig. 2. The results indicate a significant weak negative correlation of calcium ions with fluoride (rs = − 0.026, p ˂ 0.05) in the groundwater samples. This means that high levels of Ca2+ in the groundwater restrict the dissolution of CaF2. The contents of F− have a corresponding increase with the elevated Na+ in the borehole well because high Na+ levels accelerate the discharge of F− or exchanging of F− by hydroxyl ion [46]. However, different observations were made in the current study. A negative correlation between Na+ and F− (rs = − 0.054) was observed in the water samples. Furthermore, high levels of HCO3− may subdue the level of Ca2+ in borehole well, encouraging the enrichment of F− (Chemical reaction 3) [9],
However, a negative inter-relationship between HCO3− and F− (rs = − 0.580) is observed in the borehole well water samples. The variability could be attributed to the recharge and mixing of different recharge waters [47].
3.3 Nitrate (NO3 −) Contamination
The levels of NO3− in the borehole well water samples ranged from 2.06 to 81.30 mg/L with a mean value of 19.44 mg/L. The number of samples analyzed with high NO3− concentrations surpassing the permissible limits (10.00 mg/L) of WHO is twenty-six. Generally, the content of NO3− greater than 3.00 mg/L is attributed to human activities [1, 48]. Except for samples GW5C, GW5E, and GW6A, all the groundwater samples analyzed had NO3− concentrations beyond the threshold. Fabro et al. reported that the concentrations of NO3− in groundwater are usually associated with agricultural practices [15]. In the Bolgatanga setting, the type of inorganic fertilizers farmers used to apply on their farm fields includes ammonium bicarbonate, sulfate, and urea. Through the process of nitrification, NH4 obtained from inorganic fertilizer can be quickly transformed to NO3−. Even though the application of nitrogenous fertilizer is to improve crops yield, not all fertilizer applied is used by plants. Zhao et al. reported that the maximum efficiency of N uptake from added chemical fertilizers is approximately 50% [49]. This suggests that around half of the applied nitrogenous fertilizer can either be lost via volatilization, denitrification, and leaching or remained within the soil compartment. Shallow groundwater serves the favorable atmosphere for leaching of NO3− into groundwater. Therefore, an intensive input of N fertilizer to the soil will lead to an extensive spread of NO3− contamination of groundwater by leaching. Generally, a positive correlation between NO3− and SO42− is considered as an influence of N-fertilizer input [50]. Although, a positive correlation was observed between NO3− and SO42− in this study, nevertheless, it was significant (Table 4). Other sources other than N-fertilizer may also influence nitrate levels in the groundwater. Wastes generated from human activities are likely to contribute to the high level of NO3− in groundwater. The sewage system structure is not properly planned in the study area. Field observation indicated that inhabitants usually dumped the untreated used water into drainage ditches or on the ground and these discharges end up into nearby groundwater. Furthermore, Pacheco et al. and Chen et al. opined that the value of correlation coefficient between NO3− and Cl− > + 0.35 could likely be due to a common source for wastewater contamination [1, 51, 52]. It was observed from Table 4 that there is a positive correlation (rs = 0.227) between NO3− and Cl− although the correlation value is below + 0.35 and not significant. However, it confirms that there has been some contribution of domestic waste in regards to NO3− contamination of the groundwater in the study area.
Table 5 depicts the monomial hazard quotient of F− and NO3− calculated in each sampled station, while Table 6 shows the aggregate risk (hazard index) computed from each sampled site. The values of hazard quotient for fluoride contents at sampled stations range from 0.027 to 0.555 for infants, 0.34 to 3.66 for children, and 1.23 to 8.55 for adults, respectively. The ranges of hazard quotient for nitrate levels in all sampled sites were in the range of 0.02–3.05 for infants, 1.03–35.52 for children, and 2.41–94.93 for adults, respectively. In regards to integrated risk (hazard index), the values varied from 0.112 to 3.140 for infants, 1.45–41.88 for children, and 3.39–97.76 for adults, respectively. The hazard quotient induced by fluoride via ingestion approach is lower as compared to that caused by nitrate for different growth stage categories. Nitrate contributes around 86.83% of the total non-carcinogenic risk. Based on the results (Tables 5 and 6), adults are most susceptible to F− and NO3− contaminants in drinking borehole well water followed by children, the high values of HQ and HI for adults and children may be due to the average age considered in the computation of HQ and HI as well as the ingestion rate. In general, all three age categories are vulnerable to fluoride and nitrate pollutants in drinking borehole well water. For infants, 18 sampled sites (36%) have high hazard index values surpassing the safety limit (> 1), indicating that non-carcinogenic risk may occur via drinking water. In the case of children and adults, all the sampled sites (50 samples) have high hazard index values exceeding the recommended limit (> 1), indicating that all the samples may cause non-carcinogenic risk to children and adults via ingestion approach (drinking water). Due to the high hazard index scores (> 1) obtained, the borehole well water sampled may pose unfavorable health impacts on infants, children, and adults populations, respectively.
3.4 Groundwater Quality Management Sustainability
Due to the significance of groundwater for maintaining the life of humans and plants, the sustainable development and management of groundwater resources is crucial, specifically in poor water quality areas. The outcomes of groundwater quality and human risk assessments have suggested that groundwater in the studied area is not safe for human consumption. Thus, some management plans are proposed to improve the quality of groundwater, lessen the risk of human health, and intensify the sustainable groundwater quality management in the studied region.
-
1.
Excessive application of nitrogenous fertilizer should be minimized to reduce the concentrations of nitrate in groundwater. Farmers can also opt for organic fertilizers and biodegradable waste to help reduce nitrate levels in groundwater.
-
2.
In addition, enhancing scientific research, upgrading the monitoring network, strengthening cooperation and sharing of data is essential and logical for guaranteeing sustainable groundwater development.
-
3.
Unfortunately, surface water samples were not available in the study area, therefore there is no comparative analysis of the correlation and differences between groundwater quality and surface water quality, and it is wished that additional research can be performed in the future.
-
4.
The residents should minimize the use of borehole well water as the sole source for drinking purposes and rely on alternate sources such as rainwater. Rainwater harvesting is one of the game-changer to reduce the consumption of fluoride and nitrate accumulation in the human system.
4 Conclusion
In this present study, various physicochemical parameters of 50 shallow groundwater samples were examined to evaluate the groundwater quality for drinking and fluoride and nitrate contamination, and the health risks it poses to inhabitants of Bolgatanga Municipal, Ghana. The results of the study can be outlined as follows:
-
1.
Based on the values obtained for EWQI, most of the water samples were of medium water quality, and thus, the groundwater at various sites fit for drinking after simple treatment.
-
2.
In regards to WHO comparison, The F− levels range from 0.18 to 1.83 mg/L with an average value of 0.61 mg/L. Six of the samples have high concentrations of fluoride (> 1.5 mg/L) exceeding the safety limit for fluoride in drinking water set by WHO. The concentrations of NO3− in groundwater samples varied between 2.06 and 81.30 mg/L with a mean value of 19.44 mg/L. Twenty-six of the water samples have high NO3− concentrations exceeding the acceptable limits (10 mg/L).
-
3.
Concerning health risk assessment, the order of people's vulnerability to F− and NO3- age-wise is as follows: adults > children > infants. 18 of the sampled sites have high hazard index values as compared to the safety limit (> 1), suggesting that non-carcinogenic risk may occur through ingestion pathways for infants. It is noted that all the sampled sites have high hazard index values than the safety limit, indicating that all the sampled sites could give rise to non-carcinogenic risk through ingestion approach for children and adults.
-
4.
It can be deduced that inorganic fertilization on farmlands, sewage discharges, and the chemical processes in soil within the study area is the prime influence of groundwater contamination.
-
5.
It is believed that the inhabitants may experience dental and bone fluorosis in their lifetime if they continue to use groundwater for drinking purposes without treatment.
References
Chen J, Wu H, Qian H, Gao Y (2016) Assessing nitrate and fluoride contaminants in drinking water and their health risk of rural residents living in a semiarid region of Northwest China. Expo Health. https://doi.org/10.1007/s12403-016-0231-9
Howard GJB, Pedley S, Schmoll O, Chorus I, Berger P (2006) Groundwater and public health. IWA Publishing, London
World Health Organization (WHO)/United Nations Children’s Fund (UNICEF) (2010) Progress on sanitation and drinking-water 2010 update Joint monitoring programme for water supply and sanitation. Geneva
Asare EA (2021) Impact of the illegal gold mining activities on Pra River of Ghana on the distribution of potentially toxic metals and naturally occurring radioactive elements in agricultural land soils. Chem Afr 1–18. https://doi.org/10.1007/s42250-021-00285-1
Villholth KG, Rajasooriyar LD (2010) Groundwater resources and management challenges in Sri Lanka-An overview. Water Resour Manage 24:1489–1513. https://doi.org/10.1007/s11269-009-9510-6
United Nations Educational Scientific and Cultural Organization (UNESCO) (2004) Groundwater resources of the world and their use. In: Zektser IS, Everett LG (eds), Published in 2004 by the United Nations Educational, Scientific and Cultural Organization 7, place de Fontenoy, 75352 Paris 07 SP. ISBN 92-9220-007-0
Li P, Wu J, Qian H, Zhang Y, Yang N, Jing L, Yu P (2016) Hydrogeo chemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger Desert, Northwest China. Expo Health. https://doi.org/10.1007/s12403-016-0193-y
World Health Organization (WHO) (1984) Guidelines for drinking water quality, vol 2. Health criteria and other supporting information, World Health Organization, Geneva
Adimalla N, Venkatayogi S, Das SVG (2019) Assessment of fluoride contamination and distribution: a case study from a rural part of Andhra Pradesh, India. Appl Water Sci 9:94
World Health Organization (WHO) (2004) Guidelines for drinking water quality. World Health Organization, Geneva
Li P, Wu J, Qian H, Wu J (2014) Hydrogeochemistry and quality assessment of shallow groundwater in the southern part of the Yellow River alluvial plain (Zhongwei section), Northwest China. Earth Sci Res J 18(1):27–38
Chen J, Wu H, Qian H (2016) Groundwater nitrate contamination and associated health risk for the rural communities in an agricultural area of Ningxia, northwest China. Expo Health. https://doi.org/10.1007/s12403-016-0208-8
Thorburn PJ, Biggs JS, Weier KL, Keating BA (2003) Nitrate in groundwater of intensive agricultural areas in coastal Northeastern Australia. Agric Ecosyst Environ 94:49–58
Wu J, Sun Z (2015) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Expo Health. https://doi.org/10.1007/s12403-015-0170-x
Fabro AYR, Avila JGP, Alberich MVE, Sansores SAC, Camargo-Valero MA (2015) Spatial distribution of nitrate health risk associated with groundwater use as drinking water in Merida, Mexico. Appl Geogr 65:49–57. https://doi.org/10.1016/j.apgeog.2015.10.004
Chen TB, Zen XB, Hu QX (2002) Utilization efficiently of chemical fertilizers among different counties of China. Acta Geogr Sin 57:531–538
Forman D, Al-Dabbagh S, Doll R (1985) Nitrates, nitrites and gastric cancer in Great Britain. Nature 313:620–625
U.S. Environmental Protection Agency (1995) Groundwater sampling—a workshop Summary, Dallas, Texas, November 30–December 2, 1993; EPA/600/R-94/205
Borkovec M (2012) TP Titration rev, Travaux pratiques de chimie analytique II pour chimistes
Wu J, Lu J, Wen XH, Zhang ZH, Lin YC (2019) Severe nitrate pollution and health risks of coastal aquifer simultaneously influenced by saltwater intrusion and intensive anthropogenic activities. Arch Environ Contam Toxicol 77:79–87
Bazeli J, Ghalehaskar S, Morovati M, Soleimani H, Masoumi S, Rahmani Sani A, Saghi MH, Rastegar A (2020) Health risk assessment techniques to evaluate non-carcinogenic human health risk due to fluoride, nitrite and nitrate using Monte Carlo simulation and sensitivity analysis in Groundwater of Khaf County, Iran. Int J Environ Anal Chem 1:1–21
World Health Organization (WHO) (2011) Guidelines for drinking water quality, vol 4. World Health Organization, Geneva
Singh G, Rishi MS, Herojeet R, Kaur L, Sharma K (2020) Evaluation of groundwater quality and human health risks from fluoride and nitrate in semi-arid region of northern India. Environ Geochem Health 42:1833–1862
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37:300–306
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27:623–656
Li PY, Qian H, Wu JH (2010) Groundwater quality assessment based on improved water quality index in Pengyang County, Ningxia, Northwest China. J Chem 7:S209–S216
Chen F, Yao L, Mei G, Shang Y, Xiong F, Ding Z (2021) Groundwater quality and potential human health risk assessment for drinking and irrigation purposes: a case study in the semiarid region of North China. Water 13:783
Wang D, Wu J, Wang Y, Ji Y (2020) Finding high-quality groundwater resources to reduce the hydatidosis incidence in the Shiqu County of Sichuan Province, China: analysis, assessment, and management. Expo Health 12:307–322
Subba RN, Sunitha B, Adimalla N, Chaudhary M (2020) Quality criteria for groundwater use from a rural part of Wanaparthy District, Telangana State, India, through ionic spatial distribution (ISD), entropy water quality index (EWQI) and principal component analysis (PCA). Environ Geochem Health 42:579
Chen J, Huang Q, Lin Y, Fang Y, Qian H, Liu R, Ma H (2019) Hydrogeochemical characteristics and quality assessment of groundwater in an irrigated region, Northwest China. Water 11:96
Eslami F, Yaghmaeian K, Mohammadi A, Salari M, Faraji M (2019) An integrated evaluation of groundwater quality using drinking water quality indices and hydrochemical characteristics: a case study in Jiroft, Iran. Environ Earth Sci 78:314
Ji Y, Wu J, Wang Y, Elumalai V, Subramani T (2020) Seasonal variation of drinking water quality and human health risk assessment in Hancheng City of Guanzhong Plain, China. Expo Health 12:469–485
Karunanidhi D, Aravinthasamy P, Deepali M, Subramani T, Bellows BC, Li P (2020) Groundwater quality evolution based on geochemical modeling and aptness testing for ingestion using entropy water quality and total hazard indexes in an urbanindustrial area (Tiruppur) of Southern India. Environ Sci Pollut Res:1–16
Su Z, Wu J, He X, Elumalai V (2020) Temporal changes of groundwater quality within the groundwater depression cone and prediction of confined groundwater salinity using grey markov model in Yinchuan area of Northwest China. Expo Health 12:447–468
Siegel S (1956) Nonparametric statistics for the behavioural sciences. McGraw-Hill Book Company Inc, New York
USEPA (1989) Risk assessment guidance for superfund, vol I., Human health evaluation manual (Part A) Office of Emergency and Remedial Response. Washington, DC
Zhang YT, Wu JH, Xu B (2018) Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loses region, northwest China. Environ Earth Sci 77:12
Asare EA, Zaini BA, Wahi RB, Droepenu EK, Wilson F (2019) Validation of the atomic absorption spectroscopy (AAS) for heavy metal analysis and geochemical exploration of sediment samples from the Sebangan River. Adv Analy Chem 9(2):23–33
Milton AH, Rahman H, Smith W, Shrestha R, Dear K (2006) Water consumption patterns in rural Bangladesh: are we underestimating total arsenic load. J Water Health 4:431–436
Yang X, Li Y, Ma G, Hu X et al (2005) Study on weight and height of the Chinese people and the differences between 1192 and 2002. Chin J Epidemiol 26:489–493
US EPA (2012) Integrated risk information system. United States Environmental Protection Agency. http://cfpub.epa.gov/ncea/. Accessed 3May 2012
Wu JH, Sun ZC (2016) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, Mid-West China. Expo Health 8:311–329
Subba Rao N, Vidyasagar G, Surya Rao P, Bhanumurthy P (2015) Assessment of hydrogeochemical processes in a coastal region: application of multivariate statistical model. J Geol Soc India 84:494–500
Prasanth SVS, Magesh NS, Jitheshlal KV, Chandrasekar N, Gangadhar K (2012) Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal stretch of Alappuzha District, Kerala, India. Appl Water Sci 2:165–175
Li Q, Zhou J, Zhou Y, Bai C, Tao H, Jia R, Ji Y, Yang G (2014) Variation of groundwater hydrochemical characteristics in the plain area of the Tarim Basin, Xinjiang Region China. Environ Earth Sci 72(11):4249–4263
Wu J, Li P, Qian H (2015) Hydrochemical characterization of drinking groundwater with special reference to fluoride in an arid area of China and the control of aquifer leakage on its concentrations. Environ Earth Sci 73(12):8575–8588
Li P, Qian H, Wu J, Chen J, Zhang Y, Zhang H (2014) Occurrence and hydrogeochemistry of fluoride in alluvial aquifer of Weihe River, China. Environ Earth Sci 71:3133–3145. https://doi.org/10.1007/s12665-013-2691-6
Burkart MR, Kolpin DW (1993) Hydrogeologic and land-use factors associated with herbicide and nitrate occurrence in near-surface aquifers. J Environ Qual 22:646–656
Zhao B, Li X, Liu H, Wang B, Zhu P, Huang SM, Bao D, Li Y, So H (2011) Results from long-term fertilizer experiments in China: the risk of groundwater pollution by nitrate. NJAS-Wageningen J Life Sci 58(3–4):177–183
Esmaeili A, Moore F, Keshavarzi B (2014) Nitrate contamination in irrigation groundwater, Isfahan, Iran. Environ Earth Sci 72:2511–2522. https://doi.org/10.1007/s12665-014-3159-z
Pacheco J, Marin L, Cabrera A, Steinich B, Escolero O (2001) Nitrate temporal and spatial patterns in 12 water-supply wells, Yucatan, Mexico. Env Geol 40:708–715
Umezawa Y, Hosono T, Onodera S, Siringand F, Buapenge S, Delinomf R, Yoshimizug C, Tayasuh I, Nagatah T, Taniguchia M (2008) Sources of nitrate and ammonium contamination in groundwater under developing Asian megacities. Sci Total Environ 404:361–376
Acknowledgements
The authors would like to acknowledge the Ghana Environmental Protection Agency (Ghana EPA) and Ghana Food & Drugs Authority (GFDA) for non-financial supports.
Funding
The authors received no financial support for this research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declared no potential conflict of interest concerning the research, authorship, and/or publication of this article.
Data Availability
The dataset used during this study is available from the corresponding author on reasonable request.
Rights and permissions
About this article
Cite this article
Asare, E.A., Klutse, C.K. & Opare-Boafo, M.S. Assessment of Groundwater Quality, Source Distribution of Fluoride and Nitrate, and Associate Human Health Risk in a Community in North-Eastern Ghana, Bolgatanga. Chemistry Africa 5, 173–188 (2022). https://doi.org/10.1007/s42250-021-00290-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-021-00290-4