Abstract
Groundwater samples were collected from 105 wells in the Jiroft aquifer in southeastern Iran in 2017 to assess the suitability of the groundwater for potable use using water quality indices including the Canadian Council of Ministers of the Environment water quality index (CCMEWQI) and the modified drinking water quality index (MDWQI). Groundwater quality was also assessed by a number of statistical methods and the use of piper diagrams. The assessment of the groundwater quality using the CCMEWQI indicated that 79% of the groundwater samples fell in the “excellent” class for drinking usage. The results of the assessment with the MDWQI indicated that all samples could be categorized as having an “excellent” quality for potable use. An assessment of the groundwater data with principal component analysis produced two principal components that accounted for 86.88% of the variance of the data. Factor 1 had strong positive loadings for Na+, Ca2+, Cl−, SO42−, TH and TDS. This factor is likely to be linked to the combined effects of aquifer mineral–groundwater reactions and the result of evaporative concentration. Factor 2 had a strong positive loading for HCO3−. HCO3− is likely to have originated from carbonate dissolution and the microbial respiration of organic carbon in the soil profile. About of 80% of the water wells that were categorized in cluster 1 were found to be suitable for drinking water usage. A piper diagram indicated that Ca2+, Na+, HCO3−+CO32− and SO42− were the dominant ions in samples that were categorized as belonging to cluster 1. In cluster 2, Na+, SO42− and Cl− were the dominant ions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The combined effects of climate change, population growth, and contamination from anthropogenic and geogenic sources can reduce the availability of water resources for a variety of uses in many regions of the world (Ebrahimi et al. 2013). In particular, the reduction of precipitation in many arid and semi-arid regions has greatly reduced the availability of surface water resources and increased groundwater abstraction to meet the increasing demand for drinking water in many countries such as Iran (Dehghanzadeh et al. 2015). Consequently, it is important that the chemical characteristics of groundwater resources are carefully assessed to find the suitability of the groundwater for potable usage (Mosaferi et al. 2014). Investigations are usually carried out using a number of geochemical and statistical tools which can determine baseline groundwater quality (Edmunds et al. 2003).
There are a number of approaches that can be used to assess groundwater quality (Panagopoulos et al. 2012) including the use of multivariate statistical techniques (Razmkhah et al. 2010) such as principal component analysis (PCA) and hierarchical cluster analysis (HCA). HCA and PCA are useful methods of categorizing groundwater samples on the basis of the similarity of their chemical compositions and can help assess the degree to which groundwater quality parameters are inter-related. PCA can help identify important underlying factors that describe the pattern of correlations between chemical parameters. Cluster analysis can be used to group samples on the basis of their similarity and the results of such an analysis are usually presented in a dendrogram (Dehghanzadeh et al. 2015).
This study aimed to evaluate the chemical characteristics of groundwater resources of Jiroft County located in an arid region in southeastern Iran where groundwater is the main source of drinking water (Faryabi et al. 2010). Faryabi et al. (2010) previously evaluated the factors influencing groundwater chemical quality using statistical and hydrochemical methods in the Jiroft plain in 2010. Variations of groundwater quality over time in the area were also assessed by Sardoo and Azareh (2017) which indicated there was a significant degradation of the quality of groundwater in the Jiroft plain between 2002 and 2012.
However, to the best of our knowledge, there have been no significant investigations of groundwater quality in the Jiroft plain since 2012. Consequently, the current study was undertaken to fill this information gap. The suitability of groundwater for drinking purposes in this study was assessed by using two water quality indices: the index developed by the Canadian Council of Ministers of the Environment (CCME); and the modified drinking water quality index (MDWQI).
Additionally, PCA and HCA approaches were used in this study to distinguish between the influence of natural processes as well as man-made pollution on the quality of groundwater resources. Finally, the hydrochemical characteristics of the assessed groundwater resources were determined using piper diagrams.
Materials and methods
Study area and water sampling
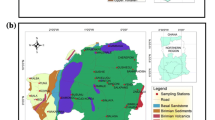
Groundwater sampling for this study was carried out in Jiroft County which is located in Kerman Province in southeastern Iran (28°40′3.32″N, 57°44′15.02″E) (Fig. 1). This county is located in an arid area near the Lout Desert. Jiroft plain is located 550–800 m above sea level (Sardoo and Azareh 2017). The average annual precipitation in the plain is estimated about 190 mm (Abbasnejad and Abbasnejad 2013). In 2017, the year of study, Jiroft County had a population of 130,429 according to the Statistical Center of Iran (2019). Groundwater is the only source of drinking water in this area.
Groundwater was sampled from 105 deep wells located in the Jiroft aquifer in 2017. Wells were randomly selected. The Jiroft aquifer was artesian before 2003, but excessive groundwater abstraction since then has caused the drying of artesian wells. The aquifer is confined under the central clay flat, but it is unconfined in alluvial fans (Abbasnejad and Abbasnejad 2013). All samples were collected in the polyethylene 1-L bottles that were washed with distilled water and dried before sampling. The wells were pumped for a period of 5–10 min before the samples were collected. Water samples were maintained at room temperature in a dark place before being subjected to chemical analysis. Chemical analysis was done as soon as possible.
Analysis of the chemical parameters
Ten parameters including pH, electrical conductivity (EC), sodium, magnesium, calcium, bicarbonate, sulfate, chloride, total hardness (TH) and total dissolved solids (TDS) were measured using the standard APHA methods (EatonAD 2017). Anions and cations were measured by ion chromatography (Metrohm 8580 Professional IC, Switzerland). The values of pH and EC were tested using a pH meter (WTW model) and an EC meter (ESI model). Values of TH and TDS were analyzed using the EDTA titrimetric and gravimetric method, respectively (Yousefi et al. 2017).
Data quality assurance and quality control (QA/QC)
In this study, blanks and real samples were used for each type of chemical analysis. Analyses were done in triplicate. All solutions and reagents were obtained from Merck Company. The method detection limits (MDLs) were obtained by using three standard deviations to the average of the analyzed blank. The MDLs were 0.05 (μg/m3) and the recovery efficiencies for water-soluble ions gained 97–133%.
Spatial analysis
The spatial distribution of groundwater quality parameters measured in the study area was displayed through GIS maps in ArcGIS (version 10.1). The kriging interpolation procedure was used to create independent raster layers for cations, anions and TDS values. The regions with minimum and maximum cations, anions and TDS were highlighted using the 6-D stretch method and these were classified with a color signifying system, (higher concentrations with red and lower values in a green color).
Assessment of water quality for drinking purpose
A water quality index is a useful approach to summarize complex water quality data and simplify its communication to the general public. In the current study, two water quality indices (the index developed by the Canadian Council of Ministers of the Environment, CCME, and the modified drinking water quality index, MDWQI) were used to investigate the suitability of groundwater for drinking usage in the studied area.
The CCME index is based on the equations that were defined by the British Columbia Ministry of Environment, Lands and Parks and then modified by Alberta Environment. The mentioned index is based on three factors: (F1) scope; the percentage of parameters that do not meet the standards; (F2) frequency, the percentage of tests that do not meet standards or failed tests; and (F3) amplitude, the amount by which the failed test values do not meet the standards. The values of the CCME index were calculated according to Eqs. (1–5) (Tyagi et al. 2013):
In this study, the modified drinking water quality index (MDWQI) was also used to classify groundwater resources. MDWQI is a modified form of the CCME water quality index that applies weighting factors to the input parameters. The MDWQI can be developed through the following four steps (Mohebbi et al. 2013):
-
1.
The water quality parameters of interest are selected. The determination of the MDWQI requires that regulated measurements of water quality parameters are undertaken and that the national water quality criteria exist.
-
2.
The weight factors for selected water quality parameters are selected, as different parameters have a dissimilar effect on the suitability of drinking water for use. The MDWQI is then calculated by applying the weight factors to the selected parameters. In this study, these factors were determined based on values developed in a previous study (Mohebbi et al. 2013) using the Delphi technique.
-
3.
After the weight values are selected, the values of the MDWQI are calculated in a similar way to the CCMEWQI considering factors F1–F3. The values of F1 and F2 were calculated according to Eqs. (6–7):
F3 was determined from the excursion amount of violator parameters and then the normalized sum of excursions (NSE) by Eqs. (8–10), respectively, as follows:
where wqi is the weight factor of an intended parameter, wqj is t weight factor of a violator parameter, Nqi is the number of tested parameters, Nqj is the number of violator parameter tests, m is the number of intended parameters and n is the number of violator parameters, Eqj is the value of excursion of a violator parameter, and Cqj and SVqj are the amount of the violator parameter and its standard value, respectively. Finally, the values for both water quality indices were calculated from Eq. 11 (Mohebbi et al. 2013; Tyagi et al. 2013):
In this study, ten water quality parameters were selected to develop the values of CCMEWQI and MDWQI for groundwater samples. The water quality standards and the assigned weight factors of input parameters that were used for calculating the CCMEWQI and MDWQI are given in Table 1. The classification of the groundwater samples according to the calculated values of the CCMEWQI and MDWQI is described in Table 2 (Mohebbi et al. 2013; Tyagi et al. 2013).
Hydrochemical analysis
The chemical composition of groundwater samples was classified using piper diagrams that were compiled using the AqQA software package.
Multivariate statistical analysis
The water quality parameters that were measured in groundwater samples and their inter-relationships were investigated by the descriptive statistics (including means, maximum and minimum values, and values of standard deviation) and correlation analysis. The data were also assessed using PCA and HCA to identify the underlying factors that influence the groundwater quality in the region.
The variables that were selected for assessment using PCA were Na+, Mg2+, Ca2+, HCO3−, Cl−, SO42−, TDS and total hardness. The factors were extracted with a minimum acceptable eigenvalue of 1. All multivariate statistical analysis was done using R software version 3.5.2.
Results and discussion
Chemical characteristics of the groundwater resources
Descriptive statistics for the investigated chemical parameters are reported in Table 3. As it can be seen in this table, the EC values in the studied area ranged from 240 to 3180 μs/cm. These values were more than those reported by Mosaferi1 et al. (2014) in rural communities in the northwestern part of Iran (110 to 1750 μs/cm). Also, Baghvand et al. (2010) reported the EC values in the range of 1987–12,751 μs/cm in an aquifer in Iran central desert. The range and average values of TH in the groundwater samples were 65–740 and 242.14 ± 124.76 mg/L as CaCO3, respectively. Consequently, the analyzed samples could be classified as being “hard” water. The pH values of groundwater samples varied between 6.8 and 8.60, with a mean value of 7.7 ± 0.4.
Based on the spatial distribution of the parameters in Fig. 2, anions, cations and TDS had more levels in the southwest area of Jiroft aquifer.
Chemical parameters were compared to the national standard. The extent to which the measured concentrations deviated from the respective water quality standards for each parameter is shown in Fig. 3. According to this figure, the values of pH and Mg2+ had the lowest and highest deviations, respectively, from the Iran national water quality standard (Institute of Standards and Industrial Research of Iran 2009). The values of Ca2+ in all sampling points were in accordance with the national standard for this parameter.
Water quality indices for drinking usage
The values of CCMEWQI and MDWQI were used to assess the quality of water for drinking usage. The classification of the suitability of groundwater samples for drinking usage based on the values of the CCMEWQI and the MDWQI is shown in Fig. 4. Based on the results from the CCMEWQI assessment, 79% of the studied wells are classified as having “excellent” quality for drinking usage. The percentage of samples having “good”, “fair” and “marginal” suitability for use, as the drinking water source was found to be 9%, 10% and 2%, respectively. No groundwater samples were classified in the category of “poor quality”.
Based on an assessment of the MDWQI, all groundwater samples could be categorized as being in the “excellent” class with regard to their suitability for use for drinking water (Fig. 4). So, it can be concluded that CCMEWQI is a more stringent index to classify groundwater resources. According to the CCMEWQI, 80% of groundwater wells in the study area would be classified as having an “excellent” quality with regard to their suitability for potable use.
Results of multivariate statistical analysis
Figure 5 displays the results of the Pearson correlation for ten chemical parameters measured in groundwater samples. The EC–TDS pair had the highest correlation coefficient (0.999), while HCO3−-TDS and HCO3−-EC showed negligible correlations with each other (0.052). The pH values demonstrated a negative correlation with all parameters. Also, HCO3− and Na+, HCO3− and Cl−, HCO3− and SO42− exhibited negative correlations with each other.
The results of the PCA of chemical parameters are shown graphically in Fig. 6, and the full assessment is shown in Table 4. Two principal components were extracted from the groundwater quality dataset which described about 86.88% of the total sample variance (Table 4). The variables Na+, Ca2+, Cl−, SO42−, TH and TDS had strong positive loadings on factor 1. This factor could be likely linked to the combined effects of aquifer mineral–groundwater reactions and evaporative concentration. A strong positive loading could be observed through HCO3− on factor 2. HCO3− is likely to have originated from carbonate dissolution and the microbial respiration of organic carbon in the soil profile. Mg2+ showed a strong positive loading on factor 1 as well as on factor 2.
Also, Fig. 7 depicts the results of the HCA for the 105 groundwater wells in a dendrogram. It shows that the groundwater samples can be categorized into two distinct clusters based on TDS values. In terms of TDS concentration, about 80% of the wells were categorized in cluster 1 (Fig. 7a). Wells classified in this cluster had TDS levels lower than 850 mg/L (where the recommended limit in Iran for TDS = 1500 mg/L). Consequently, these wells would potentially be suitable for drinking water usage provided that other potentially toxic chemical constituents meet the national water quality guidelines. Cluster 2 included 21 groundwater wells, in which the TDS values were higher than 850 mg/L, and mostly higher than 1000 mg/L (and up to 2060 mg/L). Groundwater samples in this cluster could be classified as brackish water and would generally not meet the Iranian drinking water standard for this parameter. A dendrogram for the wells in terms of average linkage between groups is illustrated in Fig. 7b.
Hydrochemical characteristics
The hydrochemical characteristics of groundwater samples are shown in a piper trilinear diagram in Fig. 8. This diagram indicates that the cations Ca2+ and Na+ and anions HCO3−+CO32− and SO42− were the dominant ions in groundwater samples that belong to cluster 1. In cluster 2, Na+, SO42− and Cl− were the main ions. The dominant ions in cluster 1 are (Ca2+, Na+, HCO3−+CO32− and SO42−) and in cluster 2 are (Na+, SO42− and Cl−). These associations reveal the geochemical evolution of groundwater in the studied aquifer as the changing the cation composition from Ca2+/HCO3 with a low salinity near the recharge area to Ca2+, Na+, HCO3−+CO32−, Cl− and SO42− dominant along a groundwater flow paths. It could be explained by ion exchange processes (Faryabi et al. 2010; Walter et al. 2017), gypsum and halite dissolution/precipitation kinetics in the bedrock and Na-rich plagioclase weathering (Faryabi et al. 2010).
Conclusions
In the current study, the chemical characteristics of groundwater were evaluated in Jiroft County in southeastern Iran and the suitability of the water for potable use was assessed using two water quality indices (the CCME and MD water quality indices).
Chemical analysis indicated that groundwater from the study area had a high level of hardness and had pH values that ranged between 6.8 and 8.60, with a mean value of 7.7 ± 0.4. Of the parameters measured in groundwater, the values of pH and Mg2+ were found to have the smallest and largest deviations from the Iran national water quality standards. The assessment indicated that 80% and 100% of the groundwater samples in the study area were suitable for drinking usage based on the results of assessment with the CCME and MD water quality indices, respectively.
Hierarchical cluster analysis of the groundwater samples produced two distinct clusters. The assessment of these clusters with a piper diagram indicated that the first cluster had a chemical composition dominated by the cations Ca2+ and Na+ and the anions HCO3−+CO32− and SO42−. In cluster 2, Na+ and SO42− and Cl− were the dominant cation and anions, respectively.
References
Abbasnejad A, Abbasnejad B (2013) Subsidence hazard zoning of Jiroft plain, southeast Iran. Int J Eng Res Dev 7:61–68
Baghvand A, Nasrabadi T, Bidhendi GN, Vosoogh A, Karbassi A, Mehrdadi N (2010) Groundwater quality degradation of an aquifer in Iran central desert. Desalination 260:264–275
Dehghanzadeh R, Hir NS, Sis JS, Taghipour H (2015) Integrated assessment of spatial and temporal variations of groundwater quality in the eastern area of Urmia Salt Lake Basin using multivariate statistical analysis. Water Resour Manag 29:1351–1364
Eaton AD C (2017) Standard Methods for the examination of water and wastewater. American Public Health Association (APHA), Washington, D.C.
Ebrahimi A, Faraji M, Sadani M, Amin MM, Hajian M, Pourzamani HR (2013) Determination of BTEX concentration in the groundwater of east region of Isfahan using passive sampling method. Int J Environ Health Eng 2:29
Edmunds W, Shand P, Hart P, Ward R (2003) The natural (baseline) quality of groundwater: a UK pilot study. Sci Total Environ 310:25–35
Faryabi M, Kalantari N, Negarestani A (2010) Evaluation of factors influencing groundwater chemical quality using statistical and hydrochemical methods in Jiroft plain. GEOSCIENCES 20 115 To 120
Institute of Standards and Industrial Research of Iran (2009) Drinking water - Physical and chemical specifications
Statistical Center of Iran (2019) https://www.amar.org.ir. Accessed 27 Apr 2019
Mohebbi MR, Saeedi R, Montazeri A, Vaghefi KA, Labbafi S, Oktaie S, Abtahi M, Mohagheghian A (2013) Assessment of water quality in groundwater resources of Iran using a modified drinking water quality index (DWQI). Ecol Ind 30:28–34
Mosaferi M, Pourakbar M, Shakerkhatibi M, Fatehifar E, Belvasi M (2014) Quality modeling of drinking groundwater using GIS in rural communities, northwest of Iran. J Environ Health Sci 12:99
Panagopoulos GP, Bathrellos GD, Skilodimou HD, Martsouka F (2012) Mapping urban water demands using multi-criteria analysis and GIS. Water Resour Manag 26:1347–1363
Razmkhah H, Abrishamchi A, Torkian A (2010) Evaluation of spatial and temporal variation in water quality by pattern recognition techniques: a case study on Jajrood River (Tehran, Iran). J Environ Manag 91:852–860
Sardoo FS, Azareh A (2017) Evaluating the trend of changes in groundwater quality parameters (Case Study: Jiroft Plain). Eur J Sustain Dev 6:201–209
Tyagi S, Sharma B, Singh P, Dobhal R (2013) Water quality assessment in terms of water quality index. Am J Water Resour 1:34–38
Walter J, Chesnaux R, Cloutier V, Gaboury D (2017) The influence of water/rock–water/clay interactions and mixing in the salinization processes of groundwater. J Hydrol Reg Stud 13:168–188
Yousefi M, Saleh HN, Mohammadi AA, Mahvi AH, Ghadrpoori M, Suleimani H (2017) Data on water quality index for the groundwater in rural area Neyshabur County, Razavi province, Iran. Data Br 15:901–907
Acknowledgements
This work was part of an M.S. project of Fatemeh Eslami (First author), a student of the Tehran University of Medical Sciences (TUMS). The authors thank the TUMS and Iran water resources management for the technical support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eslami, F., Yaghmaeian, K., Mohammadi, A. et al. An integrated evaluation of groundwater quality using drinking water quality indices and hydrochemical characteristics: a case study in Jiroft, Iran. Environ Earth Sci 78, 314 (2019). https://doi.org/10.1007/s12665-019-8321-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8321-1