Abstract
Renewable agricultural biomass derived chemicals, their modifications and uses have seen multiplicity in numerous applications and important processes with major impacts on the pursuit for eco-sustainability. Such applications range include the energy sector, chemistry, pharmacy, the textile industry, paints and coatings, plastic industry, to name but a few. This field of lignocellulosic derived chemicals interconnects several scientific disciplines ranging from agriculture, biochemistry, engineering, environmental sciences, forestry, pharmacy, medicine, etc. hence making it difficult to have a single expert view on these complicated interactions. Therefore, the idea to create a focused review, specifically, on FA (an important furanic compound) is the main objective of this article. FA and its resultant derivatives exhibits an array of capabilities and fascinating properties in various fields of applications. As a compound or with co-reactants, it finds interesting applications as base and/or intermediate chemical compound, hypergolic rocket fuels, in flame resistant composites and coatings used in aerospace, auto, and the built environment; it also finds application as mortars, cementitious grouts, impregnating materials, and sealants due to its exceptional resistance to common corrosive chemicals such as acids, alkalis and other solvents when it is cross-linked. Coupled with its environmental and economic benefits FA has proved to be a remarkable eco-sustainable bio-derived compound.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Chemicals obtained from inedible lignocellulosic agricultural biomass, has been noted to be one of the most promising environmentally benign, sustainable and industrially applicable alternatives to petroleum feedstock [1,2,3,4,5]. Hence, lignocellulosic biomass offers an enormous assortment of derivable chemical compounds capable of producing materials analogous to and even exceeding those derived from fossil chemicals [6,7,8,9,10,11]. Available data indicate that with commensurate policies and investments to promote the use of agricultural waste residues, there are associated benefits such as considerably reduction in the dependence on fossil derived chemicals [3, 4, 10, 11], increased job opportunities in the agricultural and allied sector [12], and consequent impact on energy security [2, 13]. Moreover, with the increasing concerns over the climatic impact of greenhouse effect coupled with the volatility in oil prices and attendant undesirable environmental issues of fossil hydrocarbons, many scientists agree that it is exigent and timely to consider the vast opportunities offered by non-edible agricultural lignocellulosic biomass [14,15,16].
Advances in agriculture and biotechnology has made it feasible to produce lignocellulosic biomass at far lower costs (per barrel of oil energy equivalence) than crude oil [2, 4, 17]. It is estimated that of the over 200-billion tons of lignocellulosic biomass produced on Earth yearly only about 3% is being harnessed by humans [1, 18]. Lignocellulosic is a complex hetero-matrix composition of about 75–85% cellulose, hemicellulose and lignin polymers; the remaining percentage comprises of proteins, pectin, lipids and other extractable non-structural materials [19, 20]. Although all its major constituents are of significant interest to many fields of science and technology, however, hemicellulose in particular has shown strides for further development towards more interesting applications. This is because it is a complex polymeric carbohydrate that is a rich source of pentosan (xylan or polypentose) with the chemical structure given in Fig. 1 and is the main feedstock for furfural the precursor chemical for furfuryl alcohol, FA, [21,22,23]. There are a variety of pre-treatment techniques for lignocellulosic agricultural biomass which can be grouped into four main categories; physical, chemical, biological and physiochemical techniques all specifically developed to modify the physical and chemical structure of lignocellulosic biomass in order to efficiently extract hemicellulose from cellulose without promotion of sugar degradation. Subsequently, these pre-treatment process remains the most expensive steps within the overall conversion process of lignocellulosic biomass to bio-based products [24].
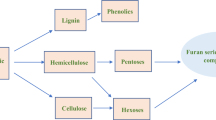
FA is noted as the most important derivative of furfural. The first reported laboratory synthesis of FA was in 1864 and was via the reduction of furfural with sodium or sodium amalgam [25,26,27]. However, it was Erdmann who described its properties and effects on laboratory animals in the introductory comment of his report in 1902, on the toxicity of FA as a constituent of coffee [28]. Its major industrial production began in 1934 when the Quaker Oats Company, Cedar Rapids USA, achieved a 99% conversion of furfural to FA using a copper-catalyst supported Na2O·xSiO2 system employed in the gas-phase hydrogenation of furfural [29, 30]. Currently, over 60% of annual production of furfural is converted to FA which finds a wide range of applications in many industries such as pharmaceuticals and manufacturing. Figure 2 shows a simplified an eco-sustainability chart showing how FA is derived from hemicellulose [23, 30, 31].
1.1 Physical Structure and Properties of FA
FA, is a mobile colourless or pale yellow liquid, however, upon exposure to prolonged daylight and air it becomes brown to dark-red. It has a characteristics mild odour reminiscent of almonds and will readily form an azeotrope with water at atmospheric pressure (80 wt% water, bp 98.5 °C). It exhibits a good solubility in many organic solvents such as tetrahydrofuran, chloroform, ether, acetone, and dimethylformamide, and freezes at minus 14.63 °C at a pressure of 1 atm, and boils at 170 °C. The chemical structure of FA is given in Fig. 3 [23, 25,26,27,28,29, 32,33,34].
2 FA Production
Industrially speaking, the two main commercial processes for the production of FA are the vapour-phase and liquid-phase processes via the selective-catalytic reduction or hydrogenation of furfural [6, 21, 23, 34,35,36,37,38]. Scheme 1 gives an overview of the catalytic hydrogenation of furfural to FA using copper chromite as catalyst [23].
2.1 Vapour Phase Process
Figure 4 shows the schematic process employed widely in industry for FA production via the vapour phase process [23]. The furfural feedstock is fed into a packed column 1 through an evaporator system by a dosed quantity of H2 introduced at the bottom of the reaction column 1 in a counter-current system of H2 flowing upwards and liquid furfural flowing downwards, the hydrogen gets saturated with the vapour pressure of furfural at 120 °C, a controlled circulation pump 2, and a heater 3 energised by steam maintains the furfural temperature at 120 °C. The resulting mixture of hydrogen and furfural vapour passes a demister pad 4 and a superheater 5 before it enters a tubular catalytic reactor 6 maintained at a temperature in the order 135 °C by means of hot oil. The tubes are filled with copper chromite pellets catalysing the desired reaction of furfural with hydrogen to form FA.
The reaction being slightly exothermic, liberates about 60.7 kJ/mol, hence the flowing oil acts as a cooling system. The gaseous mixture of reaction products enters a condensation system comprising a packed column 7, a pump 8, and a cooler 9. The pump circulates unrefined FA through the cooler 9 and unto the packing of column 7 where it meets a countercurrent of the gaseous products. From the latter stream, most of the condensables are liquefied. The remaining portion, consisting of unreacted hydrogen and the saturation quantities of the condensables at the column temperature, is recompressed by a ROOTS pump 10 and added to the hydrogen feed to check losses. A small bleed stream prevents a build-up of impurities. The condensed portion is fed into a reboiler system consisting of tank 11, a circulation pump 12, and a heater 13 energised by steam. The vapour produced by this system enters a packed vacuum distillation column 14. The head vapour of this column is liquefied by a condenser 15 maintained at reduced pressure by a vacuum pump 16. Most of the condensate is returned to the column as reflux, while the rest represents a small head fraction consisting of 2-methyl furan, unreacted furfural, and reaction water from the 2-methyl furan formation and polymerisation effects. The sump fraction is the purified FA [23, 30].
2.2 Liquid Phase Process
First reported in 1928 by the Quaker Oats Company the schematics in Fig. 5 depicts an old-fashioned, less sophisticated process for making FA via the Liquid-phase hydrogenation of furfural [23, 30]. In this process, the catalyst is employed as a slurry, and the hydrogenation carried out at pressure of 200-ATM and at a temperature of 120 °C. Furfural and a copper chromite catalyst are mixed in tank 1 by means of a circulation pump 2. Pump 3 feeds the slurry continuously through preheater 4 into a tubular bubble reactor 5. Hydrogen, from a water electrolysis plant, is injected by compressor 6. The mixture leaving the reactor flows through a cooler 7 into cyclone 8 where excess hydrogen is separated from the slurry and reinjected into the reactor feed stream by means of compressor 9. The slurry is depressurised in tank 10, a relatively small quantity of hydrogen thereby released vented into the ambient air. Pump 11 takes the depressurised slurry into an overflow sedimentation centrifuge 12 where most of the catalyst particles are separated from the liquid phase. Removal of the solids from the bowl is effected manually at appropriate intervals. The liquid phase flows into a still 13 topped by a rectification column 14.
The head vapours of the column are liquefied in condenser 15, the resulting distillate being partly returned to the column to effect rectification and partly collected in tank 16. This distillate is pure FA. Vacuum pump 17 maintains a reduced pressure to permit distillation at moderate temperatures [23, 30].
However, with the exception of producers in China, other large-scale commercial producers of FA such as Illovo (South Africa), and QO, Indo-Rama (Thailand), employ the vapour-phase process for the following reasons [23, 34].
-
1.
FA conversion proceeds at much lower temperatures and pressures compared to the liquid phase
-
2.
The lower temperatures give the added advantage of reducing the quantity of other by-products formed; hence yielding a higher crude grade of FA.
-
3.
Since the lower temperature impacts reduction of other by-products, it also has the advantage of consuming less furfural feedstock per approximately 0.5-kg of FA produced.
-
4.
Increased lifetime of catalyst employed.
Notwithstanding, the choice of FA synthesis method from furfural is largely dependent on the economics and environmental concerns. However, both gas-phase and liquid-phase catalytic processes have been successfully shown to have their respective advantages, inclusive of better yields and ease of obtaining refined FA in a single-continuous process [5, 30, 39,40,41,42,43]. Furthermore, a life cycle assessment (LCA) and life cycle costing (LCC) on the environmental and economic impact of furfuryl alcohol production using corncobs as raw material showed that increasing electricity consumption efficiency and furfural product yield, decreasing transportation distance from corncob buyers and suppliers, choosing the suitable corncob compression technique, and optimising the wastewater reuse system were the key contributing factors that resulted in reducing the overall environmental and economic impacts of this process [44].
3 Nature and Chemistry of FA
FA is a predominant member of the heterocyclic furan family [45, 46]. It is classified as a primary (1°) alcohol due to the typical characteristics of having one carbon-atom bonded to a carbon atom carrying the hydroxyl group. Although FA exhibits the chemical behaviour of primary alcohols however it exhibits an atypical chemical characteristics by readily reacting with strong acids to form a complex resinous material [46,47,48,49]. This peculiar ability of a supposedly primary alcohol has intrigued chemists, technologists, and scientists for decades and subsequently various attempts have been made to explain this phenomena [50,51,52,53].
FA is a very reactive chemical compound; this reactivity has been attributed to the regiospecific-discrimination against the C2 and/or C5 carbon by the highly dienophillic nature of the furanic system compared to its well-known homologous series thiophene and pyrrole as shown in Fig. 6 [54].
This prevailing dienic nature of the furanic ring is responsible for the peculiar atypical chemical reactivity nature of FA, a furan-bearing compound that is hugely influenced by its molecular orbital resonance [55]. This makes it possible for regioselective substitution reactions to occur at the C2 and/or C5 carbon positions when these are not substituted; thereby suggesting structure I in Fig. 7 as the dominant resonance structure [54, 56, 57].
3.1 Polycondensation Reactions of FA
Dunlop et al. and others demonstrated that the properties of FA changes significantly when in contact with acidic mediums. They further showed that under these conditions FA resinified and finally cured into a black insoluble bio-plastic [35, 49]. Pummerer et al. proposed that step-growth polymerisation is the predominant reaction pathway for acid catalysed FA via cationic active species, given in scheme 2, with resultant repeating units linked by methylene bridges as shown in Fig. 8 [58].
3.1.1 Complex Chemistry of FA Polycondensates
The complexity in the mechanism and products of FA polycondensates is well-known [46, 59]. The isolation of varied polyfurfuryl alcohol resins confirmed to consist of combination species such as 2-oxymethyl-5-furfuryl furan, 2-oxymethyl-5-(5ˊ-furfuryl)-furfuryl furan, di-furfuryl ether, di-2-furylmethane, formaldehyde, and levulinic acid, under acidic systems, are well documented [60,61,62]. Furthermore, it has been shown that polymers of FA catalysed by acids, non-acids (such as γ-alumina) or heat alone differ uniquely in chemical properties and compositions [63, 64]. Over the years, works by Krishnan et al. [65], Dunlop et al. [49], Gandini et al. [66], Choura et al. [53], and others have employed both mechanistic, theoretical, computer simulations, chemo-rheological, and kinetic studies in attempting to explain this puzzle, by either clear-cut evidences and/or tentatively. Equations 1–6 summarises the schemes and structures hitherto proposed by various studies for the polycondensates of FA.
3.1.1.1 Equation 1: Intermolecular Water Loss
Studies has shown that furan and its methylated homologues such as FA undergo cationic polymerisation which may be initiated either by a Brønsted or Lewis acid producing a complex, and sometimes irreproducible structures; and that the first-step in the polycondensation of FA involves the predomination of intermolecular dehydration from two monomeric units whereby the hydroxyl group of one monomer is attacked by the active α-hydrogen atom of another monomer eliminating water in the process subsequently linked by the methylene groups hence forming a dimer as shown in Scheme 3 [49, 53, 67,68,69].
3.1.1.2 Equation 2: Furan Chain Linkage
Preceding intermolecular dehydration is succeeded by further intermolecular dehydration leading to higher weight condensation products as shown in Fig. 9 (Scheme 4) [49, 67, 70, 71].
3.1.1.3 Equation 3: Ether Formation
The formation of furfuryl ether has been posited to result from possible combination of the methylol group, CH2OH, of two monomeric units instead of intermolecular dehydration proposed in Equation 1 thereby resulting in an ether linkage instead of a methylene bridge however still with the elimination of water (Scheme 5) [35, 67, 68, 72].
3.1.1.4 Equation 4: Hydrolytic Cleavage of Furan Ring
Hydrolytic cleavage of the furanic ring in the FA monomer has been shown to occur under certain conditions during the polycondensation process (Fig. 10) [72, 73].
3.1.1.5 Equation 5: Possible Crosslinking
It has been suggested that the crosslinking of FA polycondensates resins consists of a variant repeating-structural units and not a homogenous system as supposed. Furthermore, it was postulated that, possibly, formaldehyde is formed at certain stage which condenses with the intermediate products to form a complex polymer network [67, 71, 72].
3.1.1.6 Equation 6: Possible Crosslinking
Gandini et al. in their related studies have shown that the cationic polymerisation of furfuryl alcohol proceeded via the same pathway as the cationic polymerisation of 2-vinylfuran which stems from the ease of hydride abstraction associated with the C–H bond directly connected to the furan heterocycle. By using model compounds, it has been shown that the stabilisation of the ensuing carbenium ion, and its possibility of inducing a proton abstraction impacts a neutrally unsaturated moiety. It is the repetition of this sequences (repetitive cycles) that generates conjugated moieties, resulting in the growth of these unsaturated moieties and of the sites =CH– linking the furan to an unsaturated 2,5-dihydrofuran counterpart which immediately results in the chain coupling, after multiple unsaturations are formed. These are due to derived interchain cycloaddition between furan rings and conjugated structures accompanying the step-growth (polyaddition) mechanism, as shown in scheme 6 [53, 66, 74].
4 Selected Applications of FA
4.1 Rocket Fuels
Furfuryl Alcohol releases about 26 MJ/kg heat combustion when it burns, hence its use as an alternative hypergolic propellant for rocket engines [23, 29, 52, 75]. Kulkarni et al. demonstrated that rocket fuel blends consisting of 3-carene, norbornadiene, FA, ethylidene norbornene, and kerosene in different weight proportions exhibited good synergistic hypergolic ignition with red fuming nitric acid as oxidiser with almost no ignition delays. They concluded that these fuel blends exhibited high combustion efficiency of over 95% with very good performance comparable to, and even exceeding existing rocket fuels; coupled with the advantages of nontoxicity, eco-friendliness, safe handling and transporting [76]. Furthermore, Bhosale et al. showed that FA used in hypergolic ionic biofuel blend presented a low-cost, technologically promising, affordable, benign and high performance hypergolic fuel for applications in missile propulsions and satellite launch vehicles [77].
4.2 FA-Phenolic Binders
FA constitutes the sizeable portion in the widely used FURANFootnote 1 foundry binders, abounding mostly in patent literatures, consisting between 30–85% of total contents and generally used in three main variant combinations viz FA/UF (Urea formaldehyde), FA/PF (Phenol Formaldehyde) and FA/PF/UF system. With the added advantage of flexibility as FURAN foundry binders find applications in HOT-BOX, gas hardened processes and the traditional FURAN-NO-BAKE (FNB) system [29, 78].
4.2.1 FURAN NO-BAKE (FNB) Process
This was introduced in 1958 is a self-setting metal-casting system employing no heat application (cold setting binder system) but rather an acid catalyst (such as sulphuric, sulfonic and phosphoric acids) to initiate the hardening of the mould shown in Fig. 11 [79] at room temperature, where the setting time is controlled by the nature and amount of catalyst used. It is an eco-friendly, energy saving and efficient moulding and casting system, coupled with its superior shakeout characteristics and the ease for sand reclamation via thermal and/or mechanical reclamation procedure. Other advantages of the FBN include its relative low-costs, dimensional precision, rapid hardening rate, production competence, and abundance of the raw material needed and the ease of sustainability [29, 80]
4.2.2 FURAN HOT BOX Process
Developed by the Quaker Oats company unlike the FBN system involves the application of heat (usually between 180 and 270 °C) and latent acid catalysts (such as the solutions of urea or ammonium salts of strong acids). It is usually employed in both light (such as Aluminium) and heavy (such as bronze) metal casting and is appropriate for mass production. Generally speaking, the resins employed in this process are UF resins modified with about 20–50% FA copolymers and PF resins modified with urea with the addition of small amounts of corn flour and paraffin wax to facilitate a thorough mixing of the resin with the sand (usually within the range of 1–2.5% based on sand quantity employed). The resins are properly mixed with the sand and proportionate catalyst, and then blown into a heated mould (core boxes) to initiate the curing reaction [78, 81].
4.2.3 FURAN Gas Hardened Process
Also referred to the Cold-Box process is well suited for mass moulding of small moulds and cores employing sulphur dioxide (SO2) as catalyst in a closed-air system, at room temperature, which rapidly sets the FA-phenolic resin sand mix [82].
4.3 Wood Preservation
“Furfurylation” of wood is a chemical process by which commercial wood properties are improved using FA as a low-viscosity modifying agent to change the wood structure and chemistry so that it becomes less susceptible to biodegradation and resistant to chemical attack. The insitu complex polymerisation process within the wood system has been known as an eco-efficient “green” alternative for the previously employed toxic and hazardous compounds such as salts of copper, chromium and arsenic [83,84,85,86]. Furfurylated woods are known to be non-toxic materials suitable for internal and external applications where a high demand for performance and aesthetic characteristics are required [87]. Lande et al. demonstrated that furfurylated wood was completely resistant to attack in areas of high termite activity [88]. Similarly, Esteves et al. concluded that furfurylation of wood imparted hardness and improved the durability of the wood. They observed that the moisture behaviour of furfurylated wood decreased in relation to the wood equilibrium moisture content but had an increment in its dimensional stability; thereby enhancing reduction in anisotropy with no significant effect on the bending properties [89].
Dong et al. [90] in their work demonstrated a novel bio-based wood polymer nanocomposites successfully prepared from fast-growing poplar wood employing FA and nano-SiO2. They posited that SEM and FT-IR studies showed that the nano-SiO2 were incorporated in the wood and fixated on the wood cell via the effect of the polymerised FA this significantly improved the modulus of elasticity (MOR) of the wood. Furthermore, they showed that the thermal stabilisation and flame retardancy of the wood improved remarkably at 2.0% nano-SiO2 incorporation. In another study Hazarika et al. [91] investigated the properties of wood impregnated with melamine-formaldehyde-FA (MFFA) copolymer and montmorillionite (MMT) concluding that the wood exhibited improved higher dimensional stability, lower water uptake (%), enhanced resistance, and better mechanical properties such as flexural, tensile and hardness.
4.4 Pharmaceuticals
FA is a very vital pharmaceutical compound which finds use as a chemical intermediate compound such as for Vitamin C and Lysine production [92, 93]. The latter being an essential amino acids not synthesised biologically in the human body [42]. Once referred to as the “Herpes killer”, lysine is more biologically active in its L-configuration and is necessary for proper growth and development in children; it also helps adults to retain proper balance of nitrogen in the body. It is a well-known pharmaceutical drug useful in combating cold sores and virus infections; and has the chemical structure as shown in Fig. 12 [94, 95].
4.5 As an Industrial Solvent
Furfuryl Alcohol is a fine solvent which when used alone or in combination with other solvents finds application as a general cleaning solvent and paint softener. It also finds use as dispersant for dyes in the textile industry and finds application as solvents for many resinous materials [96–98]. Its solvent properties can easily be enhanced by slightly heating since its flashpoint is 75 °C [99].
4.6 Levulinic Acid
Levulinic acid (LA) is a versatile and valuable-building-block industrial chemical that is derived from FA. It was first produced in 1870 and has a well-known chemical structure that is depicted in Fig. 13 [100]. The United States Department of Energy ranked it amongst the top twelve value added chemicals derived from lignocellulosic biomass [101–103]. Pummerer and Gump suggested that the reaction leading to levulinic acid from FA proceeded by hydration and ring-opening reactions in the presence of strong acids such as HCl [104].
Although several attempts were reported for the production of LA from petroleum-based compounds, these approaches failed to be commercialised due to the high-cost and complex production processes involved [105]. Hence, the industrially cost-effective method employed commercially remains the renewable lignocellulosic feedstock such as FA [106]. It has been shown that when the conversion reaction of FA to LA is carried out in water, 80% yield was achieved; when performed in ketones (such as acetone and 2-butanone) a yield of 93% and above was achieved [101, 107].
4.7 Flavouring and Fragrances
Conversion of FA to its sulphur or nitrogen containing compound makes is considered a new route for inexpensive starting material for fragrances [108] and in the production of flavourings [34]. There is a huge market potential for food flavouring agents, perfumes and fragrances and it is this driving force that has advanced research and development into furan-based renewable chemical precursors and/or intermediates such as FA in the fragrance, perfumes, and flavour industries [109]. For example, furfuryl mercaptan (2-furanmethanethiol) an essential flavourant of coffee and a constituent of many foods and beverages is made from FA by reaction with thiourea in the presence of hydrogen chloride as shown in scheme 7 [68, 110].
Ethyl Maltol (hydroxyl ethyl pyrone) is a complex alcoholic heterocyclic compound which finds use as butterscotch, strawberry jammy, and brown sugar characteristic flavouring. It is another example of electrochemical reactions of FA that has been successfully converted into a commercial process for the production of flavours [111, 112].
4.8 Resins
FA resins (polymers and/or oligomers), alone or with co-reactants, find interesting applications in fields such as aerospace, scientific laboratories, and the auto industries. Due to their exceptional resistance to corrosive chemicals (e.g. acids, alkalis and other solvents), they are also used in built environment as cementitious grouts, mortars, coating, impregnating materials, and sealants [61, 72, 114,115,115]. These resins are also used in hospital operating floor coverings which demand low electrostatic resistivity to prevent electrostatic discharge from igniting flammable liquid substances often used in hospital environments [116]. When reinforced with fibre-glass, a material that is resistant to corrosion and heat distortion (at elevated temperature) with low flame and smoke emission level is obtained. This material finds applications in reinforced tanks, pipes, reaction vessels, vats, and ducts [72, 117,118,119,119]. For example, Lecite® mortar, an FA resin, developed by Electro Chemical Engineering & Manufacturing, Emmaus, Pa, United States, was used in the construction of the scrubbing tower [120].
FA resins also find wide-industrial applications as binder matrix in various fibre-reinforced composites in the auto, aerospace and construction industries, which exhibit almost the same corresponding physical properties as those manufactured from the dominant phenol resins [116]. Nu-Kast® pump, a product of Nukem Manufacturing United Sates, is an example of the outstanding versatility of FA resins. Cast entirely from FA monomer, this pump is light weight and compact, corrosion-proof inside and outside, great mechanical strength and resistant to severe shock with the ability to resist practically all commercial acid solutions, salts, alkalis, and organic solvents [121]. FA resins form gap-filling glues when modified with urea yielding a material with exceptional strength. These adhesives exhibit good flexibility, resist cracking and deterioration upon aging. They also show good resistance to shrinkage under high pressure and temperature [115].
4.9 Polymer Concretes
FA is used to produce non-petroleum based high-quality polymer concrete with very good properties such as resistance to acid, and alkali, heat stability, faster curing time, improved strength and bonding factors when compared to Portland cement concretes [122]. FA polymer concretes are usually employed in aggressive environments such as corrosion resistant baths, chemical resistant floorings, channel pipes and structural materials in nonferrous metallurgical plants [123]. Muthukumar et al. demonstrated that low viscosity of FA resin used in polymer concretes resulted to low binder content with cost effective formulations hence a competitive advantage over other conventional binders such as epoxy and polyesters resins employed in production of polymer concretes [124].
A demonstrated water-compatible polymer concrete materials developed from FA used in rapid repaid repair systems for airport runways, in all-weather conditions, has been reported. The resulting surfaced runway was reported to exhibit commensurate durability and compressive strength of the original surface. The formulation was shown to polymerise and cure within 20-min exhibiting a compressive strength of 20 MPa. It proved to be stable even under adverse chemical conditions and withstood temperatures of up to 200 °C. Further tests on the rehabilitated road pothole slabs demonstrated that the concrete can resist high stresses under repeated loads successfully. These FA-polymer concrete could be installed in less than 30-min, under any weather condition, thereby reducing the cost of man-hours [125, 126].
4.10 Wood Adhesives
FA also finds application as a resin for wood adhesive. It has been shown that composite boards were prepared using wood powder as matrix and FA or prepolymers of FA (oligomeric systems) as binder with hydrogen peroxide/ferrous ion or nitric acid as an activator. The study demonstrated that the tensile strength and water resistance of the oligomeric systems were superior to that obtained with monomeric FA. Furthermore it was shown that the degree of polymerisation of the oligomeric FA influenced the properties of the wood composite and that the addition of the activator to the binder instead of the matrix system yielded better results, further suggesting that the activation proceeds primarily through the binder oxidation. The study further demonstrated that using of acetone-soluble fraction of pre-oxidised oligomeric FA as binder gave impressive results, and the boards exhibited a tensile strength over 50% above reference phenol/resorcinol/formaldehyde (PRF) boards [127].
Abdullah et al. [128] in their work developed an eco-friendly and formaldehyde-free wood adhesive from tannin-FA renewable materials. A more recent work on FA-aldehyde plywood adhesive resins showed that comparatively FA-glyoxal (FAG) resin showed satisfactory results for plywood composite boards. It was demonstrated that the dry strength, 24-h wet strength and 2-h boiled-water wet strength were 1.02 MPa, 1.36 MPa and 1.46 MPa respectively, which is significantly higher than the standard requirements (≥ 0.7 MPa). Furthermore since the glyoxal is non-toxic and non-volatile it demonstrates that FAG resin can be considered a more eco-friendly and sustainable alternative to the FA-formaldehyde adhesives [129].
4.11 Carbon-Carbon Materials
When FA resins are pyrolysed above 450 °C they yield glassy-porous carbons which has been used in mesoporous absorbent systems. At higher pyrolysis temperatures of up to 1000 °C, high-grade carbon materials are produced which are industrially employed in carbon-carbon composites materials such as brakes and clutches, rocket motors, heatshield, aero-engine components, high-grade military gears and hardware, as well as biomedical devices [70, 130–132]. FA also finds application in the production of nano-porous membranes for desalination of brackish and seawater [133, 134].
4.12 Foams
Basso et al. in their studies have shown the possibility of producing a cheap and eco-friendly formaldehyde-free rigid foams with outstanding thermal performance from FA and tannin [135]. In a related work, Basso et al. successfully developed mixed phenolic-polyurethane-type rigid foams using tannin-furfuryl alcohol natural materials co-reacted with polymeric isocyanate which the method can be adapted for industrial continuous lines production, thus, opening up new possibilities for large-scale manufacture of these sustainable foams. The underlying technology for such tannin-based foams is on a self-blowing process with mild exothermic reaction due to the self-condensation reaction of the FA under acidic conditions thereby initiating rapid evaporation, at ambient temperature, of the organic volatile during hardening [136]. Similarly, relative low cost furanic foams (consisting only of FA systems) exhibiting excellent thermal stability under high temperatures has been investigated. These FA foams finds interesting applications such as in foundries to bind the sand of moulds and/or cores for casting engine heads and other kind of steel tools [137]. Tondi et al. has demonstrated the upscaling of eco-sustainable tannin foams. These bio-derived tannin systems have similar reactivity than phenol and when co-reacted with FA produces polymers suitable for a wide range of applications such as in waste water remedial [138]. Similarly, carbon foams with improved thermal conductivity and mechanical properties were prepared from tannin-based resin and exfoliated graphite used as filler. These organic-carbon foams were first prepared by suspending exfoliated graphite in an aqueous solution of tannin, FA, formaldehyde, diethyl ether and para-toulene-4-sulphonic acid at room temperature. These carbon foams find varied applications ranging from templates for preparation of the metallic and ceramic foams currently used in industry to electrodes and insulating liners for high temperature applications up to 2500 °C [139].
Furthermore, Jinwoo et al. has shown that low-cost mesocellular carbon foams from FA can be used in catalysts supports, high performance adsorbent systems for bulky pollutants, and in highly efficient electrode materials [140]. FA has also been employed in the production of environmentally benign polyols which have found applications as replacement for petroleum-based polyols in polyurethane foams [141].
4.13 Composites
TRB Lightweight Structures Ltd has developed a biocomposite resin based carbon reinforced polymer (CFRP) sandwich panel door leaf shown in Fig. 14 from the “prepreg” of FA oligomeric systems, which contains 100% recycled foam core. This is the first of its kind bi-composite railway carriage door to fully meet the most demanding fire, smoke and toxic fumes (FST) specifications in subterranean rail applications (overground and underground rail use). This biocomposite easily passed the BS 6853 and BS 476, as well as being EN 45545 HL3 compliant [142].
FA was employed in the materials used by the United States space agency in their space shuttle thermal protection systems (TPS). Reinforced carbon-carbon was produced from cured graphite fabric that was impregnated with phenolic resin laid up in complex shaped moulds. After the parts were rough trimmed it was impregnated with FA and pyrolysed converting the resin polymer to carbon. The impregnation and pyrolysis is done multiple times to increase density which also resulted in improved, mechanical and flame retardant properties of these parts [143].
Wang et al. [144] reported a robust, environmental-friendly method to synthesise polymer/clay aerogel nanocomposites materials from low density FA oligomeric systems and clay. Polymer/clay aerogels find applications ranging from catalyst supports, packaging, thermal insulation, absorption and structural applications.
Graphene/titanium carbide composites were synthesised employing sol-gel infiltration and spark plasma sintering (SPS). FA was used as the polymerisable carbon source. The graphene used was casted into a sponge-like shape consisting of three-dimensional network of graphene sheet whilst the sol-gel infiltration synthesis method allowed for the formation of nano-structured ceramics inside the porous structure of the graphene networks, hence forming the composites. Titanium-carbide (TiC) composites are ultra-high temperature ceramics (UHTC) with low thermal expansivity and density (4.93 g/cm3), high melting points (3067 °C), high Vickers hardness (28–35 GPa), high Young’s modulus (410–450 GPa) and high thermal and electrical conductivity. Their investigated applications includes usage as cutting tools, refractory components, super-computers, electronic elements, in aerospace engineering and so on [145, 146].
Ebrahimi et al. has reported the preparation of FA functionalised carbon nanotube (CNT) and epoxide novolac resin composites with high char yield. The epoxidised novolac resin (ENR) composites exhibited high thermal stability and char residue. The study demonstrated that modification of oxidised CNTs with FA resulted in improved dispersion in the resin matrix [147].
4.14 Sundry Applications
Nobuo et al. described a process for producing diamond powder by a shock compressing method using FA as a carbon precursor [148]. FA has also been used in the production of bio-based nanocomposites, batteries and nuclear-grade graphitic rods for use in nuclear plants [71, 149, 150].
In their work Nanaji et al. [151] demonstrated that utilizing FA as an alternative source of carbon precursor (for the first time) a smart, efficient and cost-effective methodology employing a modified evaporation induced self-assembly (EISA), strategy was used to synthesise mesoporous carbon (MC), which exhibited excellent textural parameters, employed in super-capacitors. They showed that the resulting carbon synthesised with the modified EISA method exhibited a higher specific surface area with large pore volume and more ordered graphitic carbon. The wettability studies demonstrated that the functionalised mesoporous carbon surface had superior hydrophilic properties as compared against the non-functionalised mesoporous carbon film surface. In a related study Gao et al. [152] also has developed a boron-doped mesoporous carbons (BOMCs) for making of super-capacitors. Different boron contents were prepared by nano-casting using silica KIT-6, FA and boric acid as the template, carbon, and boron sources respectively.
Furfuryl Alcohol modified melamine sponge (MS) for high-efficient oil spill clean-up and recovery has been reported by Feng et al. The FA modified MS exhibited excellent hydrophobicity, improved thermal and mechanical properties, and showed excellent oil sorption capacities (75–160 g/g for various oils or organic solvents) and better recyclability capabilities; thus proposing such FA modified MS as potential candidates for high efficient absorbents for oil-water separation. The further demonstrated that FA modified commercial MS can be synthesised using a simple non-toxic and expensive modifying agents or solution [153].
FA functionalised water-soluble graphene dispersions, fabricated by the exfoliation of graphite by Diels–Alder cycloaddition reaction has been reported by Zhang et al. The study demonstrated that the high-quality graphene-FA so produced exhibited no significant structural defects less than a few layers. Furthermore, positing that facile procedure so reported could be used for the synthesis of versatile functional graphene with other organic group on the surface of graphene and the hydroxyl groups of FA for a variety of applications [154]. Furthermore, FA has been reportedly used in the manufacture of esters, synthetic fibres and rubbers [97, 107, 155].
5 Conclusion
Owing to the ever increasing call for eco-sustainable chemicals and materials, FA derived from non-edible lignocellulosic biomass has continued to show increasing potential as a choice alternative to fossil-derived chemicals and materials in many industrial and materials applications as shown in this review article.
Non-edible lignocellulosic agricultural biomass offers us the bottomless opportunity for a cheaper, greener and eco-sustainable abundant resources. Coupled with advances in science and technology this eco-sustainable resources can be harnessed cheaply and effectively thereby reducing the overdependence on depleting and comparatively expensive fossil derived chemicals, mitigate greenhouse gas emissions, improve local economies and ensure energy security.
FA has been used to present the vast opportunities that chemicals from non-edible lignocellulosic agricultural residues can offer mankind; coupled with the intriguing chemistry and nature of FA reactions with co-reactants or alone, it avails scientists and technologists the capability to modify, tailor and transform it into materials and chemicals to meet specific end-use and applications with interesting properties not obtainable in fossil derived chemicals and materials.
Notes
FURAN is a common terminology used to refer to binders containing furfuryl alcohol and either urea or phenol formaldehyde or mixtures of both.
References
Lucia LA (2008) Lignocellulosic biomass: a potential feedstock to replace petroleum. BioResources 3:981–982
Huber GW (2008) Breaking the chemical and engineering barriers to lignocellulosic biofuels. University of Massachusetts Amherst, Washington D.C
Binder JB, Raines RT (2009) Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals. J Am Chem Soc 131:1979–1985. https://doi.org/10.1021/ja808537j
Isikgor FH, Becer CR (2015) Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym Chem 6:4497–4559. https://doi.org/10.1039/C5PY00263J
Nanao H, Murakami Y, Sato O, Yamaguchi A, Hiyoshi N, Shirai M (2017) Furfuryl alcohol and furfural hydrogenation over activated carbon-supported palladium catalyst in presence of water and carbon dioxide. Chem Sel 2:2471–2475. https://doi.org/10.1002/slct.201700382
Carraher CE, Sperling LH (1986) Renewable-Resource Materials, 1st edn. Springer International Publishing, United States. https://doi.org/10.1007/978-1-4613-2205-4
Amarasekara AS, Mittal V (2012) Renewable polymers. Synthesis, processing, and technology. Scrivener Publishing LLC, Beverly
Pizzi A, Ibeh C (2014) Aminos, handbook of thermoset plastics. In: Doduik H, Goodman SH (eds) Third. Elsevier Inc., Amsterdam, p 795
Yao K, Tang C (2013) Controlled polymerization of next-generation renewable monomers and beyond. Macromolecules 46:1689–1712. https://doi.org/10.1021/ma3019574
Gandini A (2008) Polymers from renewable resources: a challenge for the future of macromolecular materials. Macromolecules 41:9491–9504. https://doi.org/10.1021/ma801735u
Spiridon I, Popa VI (2008) Hemicelluloses: major sources, properties and applications. Elsevier Inc, Amsterdam
Hall KO, Chuck A, Sang M (2013) Economic transformation & job creation: the Caribbean experience. Trafford Publishing, Bloomington
Phalan B (2009) The social and environmental impacts of biofuels in Asia: an overview. Appl Energy 86:S21–S29. https://doi.org/10.1016/j.apenergy.2009.04.046
Frankel EG (2007) A world beyond petroleum. Springer, New York
Mahesh S, Vedamurthy AB (2003) Conventional fuels and enviromental impact. In: Biotechnology - 4: Including recombinant DNA technology enviromental biotechnology, and cell culture. New age international. New Delhi, India, pp 106–109
Mcnally R, Levi M (2013) The era of volatile oil prices. JSTOR 1:1–8
George A, Brandt A, Tran K, Zahari SMSNS, Klein-Marcuschamer D, Sun N, Sathitsuksanoh N, Shi J, Stavila V, Parthasarathi R, Singh S, Holmes BM, Welton T, Simmons BA, Hallett JP (2015) Design of low-cost ionic liquids for lignocellulosic biomass pretreatment. Green Chem 17:1728–1734. https://doi.org/10.1039/C4GC01208A
Tojo S, Hirasawa T (2013) Research approaches to sustainable biomass systems. Elsevier Inc, Amsterdam
Chen H (2014) Biotechnology of lignocellulose: theory and practice. Springer, New York
Bajpai P (2016) Pretreatment of lignocellulosic biomass for biofuel production. Springer, New York
Machado G, Leon S, Santos F, Lourega R, Dullius J, Mollmann ME, Eichler P (2016) Literature review on furfural production from lignocellulosic biomass. Nat Resour 07:115–129. https://doi.org/10.4236/nr.2016.73012
Anwar Z, Gulfraz M, Irshad M (2014) Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J Radiat Res Appl Sci 7:163–173. https://doi.org/10.1016/j.jrras.2014.02.003
Zeitsch KL (2000) The chemistry and technology of furfural and its many by-products. Elsevier B.V, Amsterdam
Mussatto SI, Dragone GM (2016) Biomass pretreatment, biorefineries, and potential products for a bioeconomy development. In: Mussatto SI (ed) Biomass fractionation technologies for a lignocellulosic feedstock based biorefinery. Elsevier Inc., Amsterdam, pp 1–22
Ono N (2001) Synthesis of heterocyclic compounds, vol 1. Springer, New York
NIIR (2005) Furfuryl alcohol: resins. Synthetic resins techonology handbook. Asia Pacific Business Press Inc., New Delhi, pp 423–438
National Institute for Occupational Safety and Health (1977) Criteria for a recommended standard: occupational exposure to refined petroleum solvents. Centres for Disease Control and Prevention, United States, pp 77–179. https://www.cdc.gov/niosh/nioshtic-2/00073145.html
Erdmann E (1902) Zur Charakteristik des Furfuralkohols. Berichte der Dtsch Chem Gesellschaft 35:1855–1862. https://doi.org/10.1002/cber.190203502121
IFC (2018) Industrial development of furfuryl alcohol. http://www.furan.com/furfuryl_alcohol_historical_overview.html. Accessed 16 Jun 2018
Mariscal R, Maireles-Torres P, Ojeda M, Sádaba I, López Granados M (2016) Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ Sci 9:1144–1189. https://doi.org/10.1039/C5EE02666K
Perez RF, Fraga MA (2014) Hemicellulose-derived chemicals: one-step production of furfuryl alcohol from xylose. Green Chem 16:3942. https://doi.org/10.1039/C4GC00398E
Winter R (2009) A consumer’s dictionary of food additives, seventh. Three Rivers Press, New York
Duffey HR, Barrett H (1951) Furfural and other furan compounds. Kirk Othmer Encycl Chem Technol 995–996(1002–03):1007–1008
United States International Trade Commission (2001) Furfuryl alcohol from China and Thailand. US International Trade Commission, Washington D.C., United States
von Wissell L, Tollens B (1893) XXXIII. Ueber den Furfur- oder Furalkohol und einige Derivate desselben. Justus Liebigs Ann Chem 272:291–306. https://doi.org/10.1002/jlac.18932720305
Carraher CE, Acid F (1978) Synthesis of furfuryl alcohol. J Chem Educ 55:269–270
Gong W, Chen C, Zhang Y, Zhou H, Wang H, Zhang H, Zhang Y, Wang G, Zhao H (2017) Efficient synthesis of furfuryl alcohol from H2-hydrogenation/transfer hydrogenation of furfural using sulfonate group modified Cu catalyst. ACS Sustain Chem Eng 5:2172–2180. https://doi.org/10.1021/acssuschemeng.6b02343
Brownlee HJ, Miner CS (1948) Industrial development of furfural. Ind Eng Chem 40:201–204. https://doi.org/10.1021/ie50458a005
Seo G, Chon H (1981) Hydrogenation of furfural over copper-containing catalysts. J Catal 67:424–429. https://doi.org/10.1016/0021-9517(81)90302-X
Egeblad K, Rass-Hansen J, Marsden CC, Taarning E, Hviid Christensen C (2009) Heterogeneous catalysis for production of value-added chemicals from biomass. In: Spivey JJ, Dooley KM (eds) Catalysis: Volume 21. The Royal Society of Chemistry, pp 13–50. https://doi.org/10.1039/b712664f
Gallezot P (2013) Metal catalysts for the conversion of biomass to chemicals. In: New and future developments in catalysis: catalytic biomass conversion. Elsevier Science Publishers B.V., pp 1–27. https://doi.org/10.1016/C2010-0-68566-X
Sa J, Srebowata A (2016) Hydrogenation with low-cost transition metals. CRC Press, Boca Raton
Heynderickx MP, Thybaut JW, Poelman H, Poelman D, Marin GB (2010) Applied catalysis B: environmental kinetic modeling of the total oxidation of propane over CuO–CeO2/g-Al2O3. Appl Catal B Environ 95:26–38. https://doi.org/10.1016/j.apcatb.2009.11.018
Hong J, Zhou J, Hong J (2015) Environmental and economic impact of furfuralcohol production using corncob as a raw material. Int J Life Cycle Assess 20:623–631. https://doi.org/10.1007/s11367-015-0854-2
Gandini A (1990) Polymers and oligomers containing furan rings. In: ACS Symposium Series, vol 433, pp 195–208. https://doi.org/10.1021/bk-1990-0433.ch017
Choura M, Belgacem NM, Gandini A (1996) Acid-catalyzed polycondensation of furfuryl alcohol: mechanisms of chromophore formation and cross-linking. Macromolecules 29:3839–3850. https://doi.org/10.1021/ma951522f
Hein M, Arena S (2010) Foundations of college chemistry, alternate, thirteenth. Wiley, New York
Kelter PB, Mosher MD, Scott A (2008) Chemistry: the practical science, vol 10. Houghton Mifflin Company, Boston
Dunlop AP, Peters FN Jr (1942) The nature of furfuryl alcohol. Ind Eng Chem 34:814. https://doi.org/10.1021/ie50391a010
Rathi A, Chanda M (1974) Kinetics of solution polymerisation of furfuryl alcohol. Appl Polym 18:1541–1548
Gonzalez R, Figueroa JM, Gonzalez H (2001) Furfuryl alcohol polymerization by iodine in methylene chloride. Eur Polym J 38:287–297
Kim T, Assary RS, Marshall CL, Gosztola DJ, Curtiss LA, Stair PC (2011) Acid-catalyzed furfuryl alcohol polymerization: characterizations of molecular structure and thermodynamic properties. ChemCatChem 3:1451–1458. https://doi.org/10.1002/cctc.201100098
Choura M, Belgacem NM, Gandini A (1997) The acid-catalyzed polycondensation of furfuryl alcohol: old puzzles unravelled. Macromol Symp 122:263–268. https://doi.org/10.1002/masy.19971220141
Spiridon I, Popa VI (2008) Hemicelluloses: major sources, properties and applications. Elsevier Inc, Amsterdam
McKillip WJ (1989) Chemistry of furan polymers. In: Adhesives from renewable resources. ACS Symposium Series, pp 408–423. https://doi.org/10.1021/bk-1989-0385.ch029
Belen’kii LI (1975) Direction and some characteristics of electrophilic substitution reactions in thiophene and furan series. Bull Acad Sci USSR Div Chem Sci 24:279–291. https://doi.org/10.1007/BF00925770
Belen’kii LI (1980) Activity and selectivity in the electrophilic substitution of five-membered heterorings (review). Chem Heterocycl Compd 16:1195–1210. https://doi.org/10.1007/BF00501819
Pummerer R, Gump W (1923) Über die Aufspaltung des Furfurylalkohols und den Mechnanismus der Lävulinsäure-Bildung aus Hexosen. Berichte der Dtsch Chem Gesellschaft Abteilung B 66:999–1008. https://doi.org/10.1002/cber.19230560502
Guigo N, Mija A, Vincent L, Sbirrazzuoli N (2007) Chemorheological analysis and model-free kinetics of acid catalysed furfuryl alcohol polymerization. Phys Chem Chem Phys 9:5359. https://doi.org/10.1039/b707950h
Conley RT, Metil I (1963) An investigation of the structure of furfuryl alcohol polycondensates with infrared spectroscopy. J Appl Polym Sci 7:37–52. https://doi.org/10.1002/app.1963.070070104
Wewerka EM, Loughran ED, Walters KL (1971) A study of the low molecular weight components of furfuryl alcohol polymers. J Appl Polym Sci 15:1437–1451
Barr JB, Wallon SB (1971) The chemistry of furfuryl alcohol resins. J Appl Polym Sci 15:1079–1090. https://doi.org/10.1002/app.1971.070150504
Alamos L, Wewerka EM (1971) Study of the γ-alumina polymerization of furfuryl alcohol. J Polym Sci Part A 1 Polym Chem 9:2703–2715. https://doi.org/10.1002/pol.1971.150090923
Iroegbu AO, Hlangothi SP (2018) Effects of the type of catalyst on the polymerisation mechanism of furfuryl alcohol and its resultant properties. Chem Africa 5:6. https://doi.org/10.1007/s42250-018-0017-5
Krishnan TA, Chanda M (1975) Kinetics of polymerisation of furfuryl alcohol in aqueous solution. Die Angew Makromol Chem 43:145–156. https://doi.org/10.1002/apmc.1975.050430110
Gandini A, Belgacem MN (1997) Furans in polymer chemistry. Prog Polym Sci 22:1203–1379. https://doi.org/10.1016/S0079-6700(97)00004-X
Fink JK (2013) Reactive polymers fundamentals and applications: a concise guide to industrial polymers, 2nd edn. https://doi.org/10.1016/C2012-0-02516-1
Elvers B, Ellis BW (2016) Ullmann’s encyclopedia of industrial chemistry, 7th edn. Wiley, New York
Dunlop AP, Peters Jr. FN (1953) The Furans - American Chemical Society Monographs No. 119, 1st edn. Reinhold Publishing Corp., New York, United States.
Inagaki M, Kang F, Toyoda M, Konno H (2014) Carbon materials for adsorption of molecules and ions. Elsevier, Amsterdam
Wewerka EM (1968) Investigation of the polymerization of furfuryl alcohol with gel permeation chromatography. Cell Chem Technol 12:1671–1681
Schmitt CR (1974) Polyfurfuryl alcohol resins. Polym Plast Technol Eng 3:121–158. https://doi.org/10.1080/03602557408545025
Wewerka EM (1968) Investigation of the polymerization of furfuryl alcohol with gel permeation chromatography. Cell Chem Technol 12:1671–1681. https://doi.org/10.1002/app.1968.070120716
Belgacem NM, Gandini A (2018) Monomers, polymers and composites from renewable resources, 1st edn. Elsevier, Amsterdam
Engineers NBC (2010) Industrial alcohol technology handbook. Asia Pacific Business Press Inc, New Delhi
Kulkarni SG, Bagalkote VS, Patil SS, Kumar UP, Kumar VA (2009) Theoretical evaluation and experimental validation of performance parameters of new hypergolic liquid fuel blends with red fuming nitric acid as oxidizer. Propellants Explos Pyrotech 34:520–525. https://doi.org/10.1002/prep.200800061
Bhosale MVK, Kulkarni SG, Kulkarni PS (2016) Ionic liquid and biofuel blend: a low–cost and high performance hypergolic fuel for propulsion application. Chem Sel 1:1921–1925. https://doi.org/10.1002/slct.201600358
Peter B (2001) Foundry technology, second. Butterworth-Heinemann, Oxford
ASK Chemicals (2018) Foundry management and technology. https://www.foundrymag.com/moldscores/ask-chemicals-buys-hexion-s-eu-foundry-chemicals. Accessed 24 Nov 2018
Acharya SG, Vadher JA, Sheladiya M (2016) A furan no-bake binder system analysis for improved casting quality. Int J Met 10:491–499. https://doi.org/10.1007/s40962-016-0059-x
Pizzi A (2018) Urea and melamine aminoresin adhesives. In: Pizzi A, Lakshmi KM (eds) Handbook of adhesive technology, third. CRC Press, Boca Raton, p 295
Gandini A, Belgacem NM (2014) Furans. In: Dodiuk H, Goodman SH (eds) Handbook of thermoset plastics, thrid. William Andrew, Oxford, pp 93–110
Lande S, Eikenes M, Westin M, Schneider MH (2008) Furfurylation of wood: chemistry, properties, and commercialization. In: ACS Symposium Series, vol 982, pp 337–355. https://doi.org/10.1021/bk-2008-0982.ch020
Goldstein I, Dreher W (1960) Stable furfuryl alcohol impregnating solutions. Ind Eng Chem 52:57–58
Hägglund E (1951) Chemistry of wood. Academic Press, New York
John MJJ, Thomas S (2012) Natural polymer volume 2: Nanocomposites. Green Chemistry Series 17. The Royal Society of Chemistry. https://doi.org/10.1039/9781849735315
Eseyin AE, Steele PH (2015) An overview of the applications of furfural and its derivatives. Int J Adv Chem 3:42. https://doi.org/10.14419/ijac.v3i2.5048
Lande S, Westin M, Schneider M (2004) Properties of furfurylated wood. Scand J For Res 19:22–30. https://doi.org/10.1080/0282758041001915
Esteves B, Nunes L, Pereira H (2011) Properties of furfurylated wood (Pinus pinaster). Eur J Wood Wood Prod 69:521–525. https://doi.org/10.1007/s00107-010-0480-4
Dong Y, Yan Y, Zhang S, Li J, Wang J (2015) Flammability and physical–mechanical properties assessment of wood treated with furfuryl alcohol and nano-SiO2. Eur J Wood Wood Prod 73:457–464. https://doi.org/10.1007/s00107-015-0896-y
Hazarika A, Maji TK (2013) Effect of different crosslinkers on properties of melamine formaldehyde-furfuryl alcohol copolymer/montmorillonite impregnated softwood (Ficus hispida). Polym Eng Sci 53:1394–1404. https://doi.org/10.1002/pen.23391
Sidi Mohamed B, Périgaud C, Mathé C (2017) Revaluation of biomass-derived furfuryl alcohol derivatives for the synthesis of carbocyclic nucleoside phosphonate analogues. Beilstein J Org Chem 13:251–256. https://doi.org/10.3762/bjoc.13.28
Chen X, Zhang L, Zhang B, Guo X, Mu X (2016) Highly selective hydrogenation of furfural to furfuryl alcohol over Pt nanoparticles supported on g-C3N4 nanosheets catalysts in water. Sci Rep 6:28558. https://doi.org/10.1038/srep28558
Braverman ER (2003) The healing nutrients within: facts, findings, and new research on amino acids, 3rd edn. Basic Health Publications Inc., Laguna Beach, United States
Intratec Solutions (2016) l-Lysine-HCl production from glucose—cost analysis—lysine E12A. https://www.intratec.us/analysis/lysine-e12a. Accessed 15 Oct 2018
U.S. Environmental Protection Agency (2001) Guide to industrial assessments for pollution prevention and energy efficiency. https://www3.epa.gov/npdes/pubs/pretreatment_industrial_assessments.pdf. Accessed 13 Aug 2018
Cheremisinoff NP (2003) Industrial solvents handbook, revised and expanded, second. Marcel Dekker Inc, New York
NPCS Board of Consultants & Engineers (2009) Handbook on textile auxiliaries, dyes and dye intermediates technology. Asia Pacific Business Press Inc, New Delhi, p 736
Popendorf W (2006) Industrial hygiene control of airborne chemical hazards. CRC Press, Boca Raton
Ghorpade V, Hanna M (1997) Industrial applications for levulinic acid. In: Cambell GM, Webb C, Mckee SL (eds) Cereals. Springer, Boston, MA, pp 49–50. https://doi.org/10.1007/978-1-4757-2675-6_7
Werpy TA, Holladay JE, White JF (2004) Top value added chemicals from biomass: I. results of screening for potential candidates from sugars and synthesis gas. http://www.osti.gov/servlets/purl/926125-eeUkhS/
van de Graaf WD, Lange J (2007) Process for the conversion of furfuryl alcohol into levulinic acid or alkyl levulinate. p 19. https://patents.google.com/patent/US20070049771A1/en. Accessed 18 Aug 2018
Mellmer MA, Gallo JMR, Martin Alonso D, Dumesic JA (2015) Selective production of levulinic acid from furfuryl alcohol in THF solvent systems over H-ZSM-5. ACS Catal 5:3354–3359. https://doi.org/10.1021/acscatal.5b00274
Pummerer R, Gump W (1923) Ber die Atliapaltung des Furfurallcoho und den Meohanifmu der Lbwhe & um-Bildung aum Hexdeen. Berichte der Dtsch Chem Geselkchaft 56:999–1008
Li X, Jia P, Wang T (2016) Furfural: a promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal 6:7621–7640. https://doi.org/10.1021/acscatal.6b01838
Bozell JJ, Moens L, Elliott DC, Wang Y, Neuenscwander GG, Fitzpatrick SW, Bilski RJ, Jarnefeld JL (2000) Production of levulinic acid and use as a platform chemical for derived products. Resour Conserv Recycl 28:227–239. https://doi.org/10.1016/S0921-3449(99)00047-6
Van Der Waal JC, De Jong E (2016) Avantium chemicals: the high potential for the levulinic product tree. In: Domínguez de María P (ed) Industrial biorenewables: a practical viewpoint. Wiley, New York, pp 97–120
Squadrito G, Andaloro L, Ferraro M, Antonucci V (2014) Hydrogen fuel cell technology. Elsevier, Amsterdam
Centi G, Perathoner S (2013) Chapter 16 - Catalytic Transformation of CO2 to fuels and chemicals, with reference to biorefineries. In: Triantafyllidis K, Lappas A, Stocker M (eds) The role of catalysis for the sustainable production of bio-fuels and bio-chemicals. Elsevier, Amsterdam, pp 529–555
Blank I, Pascual EC, Devaud S, Fay LB, Stadler RH, Yeretzian C, Goodman BA (2002) Degradation of the coffee flavor compound furfuryl mercaptan in model Fenton-type reaction systems. J Agric Food Chem 50:2356–2364. https://doi.org/10.1021/jf011329m
Lawrence B, Mookheyee B, Wills B (1988) Developments in food sciences, flavors and fragrances: a world perspective. In: Proceedings of the 10th international congress of essential oils, fragrances, and flavors. Elsevier Inc., Washington D.C., p 848
Dolf De Rovira S (2008) Dictionary of flavors. Wiley, New York
Payne C (1952) Cements. Ind Eng Chem 44:2292–2295
Norton AJ (1948) Furan Resins. Ind Eng Chem 40:236–238. https://doi.org/10.1021/ie50458a011
Oats Quaker (1957) Quaker oats furfuryl alcohol. Ind Eng Chem 49:64A–65A
Board N (2002) Modern technology of synthetic resins & their applications. Asia Pacific Business Press Inc, New Delhi
Fink JL (2017) Furan resins. React Polym 40:307–320
Norton AJ (1948) Furan Resins. React Polym 40:236–238. https://doi.org/10.1021/ie50458a011
Oats Quaker (1962) Corrosion problems solved with QO furfuryl alcohol. Chem Eng News 40:32
Oats Quaker (1953) Furfuryl alcohol resins are prominent in corrosion resistant construction. Ind Eng Chem 31:4475
Oats Quaker (1959) Furfuryl alcohol resins. Ind Eng Chem 30:277
Sugama T, Kukacka LE, Horn W (1981) Properties of water-compatible furfuryl alcohol polymer concrete. Cem Concr Res 11:497–506. https://doi.org/10.1016/0008-8846(81)90079-X
Solovjov GK, Trambovetsky VP, Kruger D (1994) Furan resin polymer concrete in the commonwealth of independent states (CIS). ACI Mater J 91:158–160. https://doi.org/10.14359/4567
Muthukumar M, Mohan D (2005) Studies on furan polymer concrete. J Polym Res 12:231–241. https://doi.org/10.1007/s10965-004-3206-7
Sugama T, Kukacka LE, Horn W (1981) Water-compatible polymer concrete materials for use in rapid repair systems for airport runways. US Department of Energy and Environment, New York. https://apps.dtic.mil/dtic/tr/fulltext/u2/a102873.pdf
Kruger D (1987) Development of furfuryl alcohol polymer concrete for South African applications. University of Johannesburg, Johannesburg
Dao LT, Zavarin E (1996) Chemically activated furfuryl alcohol-based wood adhesives I. The role of furfuryl alcohol. Holzforschung 50:470–476. https://doi.org/10.1515/hfsg.1996.50.5.470
Abdullah UHB, Pizzi A (2013) Tannin-furfuryl alcohol wood panel adhesives without formaldehyde. Eur J Wood Wood Prod 71:131–132. https://doi.org/10.1007/s00107-012-0629-4
Xi X, Wu Z, Pizzi A, Gerardin C, Lei H, Du G (2018) Furfuryl alcohol-aldehyde plywood adhesive resins. J Adhes. https://doi.org/10.1080/00218464.2018.1519435
Savage G (1993) Carbon-carbon composites, first. Chapman & Hall, London
Kimuraf S (1974) Graphitization of carbon fibre/glassy carbon composites. Carbon N Y 12:249–258. https://doi.org/10.1177/002199837300700109
Olabisi O, Adewale K (2016) Handbook of thermoplastics, 2nd edn. CRC Press, Boca Raton, United States
Lonsdale HK (1985) Membrane technology and applications. Wiley, New York
Zheng HY, Zhu YL, Teng BT, Bai ZQ, Zhang CH, Xiang HW, Li YW (2006) Towards understanding the reaction pathway in vapour phase hydrogenation of furfural to 2-methylfuran. J Mol Catal A Chem 246:18–23. https://doi.org/10.1016/j.molcata.2005.10.003
Basso MC, Li X, Fierro V, Pizzi A, Giovando S, Celzard A (2011) Green, formaldehyde-free, foams for thermal insulation. Adv Mater Lett 2:378–382. https://doi.org/10.5185/amlett.2011.4254
Basso MC, Pizzi A, Lacoste C, Delmotte L, Al-Marzouki FM, Abdalla S, Celzard A (2014) MALDI-TOF and13C NMR analysis of tannin-furanic-polyurethane foams adapted for industrial continuous lines application. Polymers (Basel) 6:2985–3004. https://doi.org/10.3390/polym6122985
Tondi G, Pizzi A, Pasch H, Celzard A, Rode K (2008) MALDI-ToF investigation of furanic polymer foams before and after carbonization: aromatic rearrangement and surviving furanic structures. Eur Polym J 44:2938–2943. https://doi.org/10.1016/j.eurpolymj.2008.06.029
Tondi G, Link M, Kolbitsch C, Lesacher R, Petutschnigg A (2016) Pilot plant up-scaling of tannin foams. Ind Crops Prod 79:211–218. https://doi.org/10.1016/j.indcrop.2015.11.013
Jana P, Fierro V, Pizzi A, Celzard A (2014) Biomass-derived, thermally conducting, carbon foams for seasonal thermal storage. Biomass Bioenerg 67:312–318. https://doi.org/10.1016/j.biombioe.2014.04.031
Lee J, Sohn K, Hyeon T (2002) Low-cost and facile synthesis of mesocellular carbon foams. Chem Commun. https://doi.org/10.1039/b208642e
van den Martien O, Bos H (2010) Composite Based on Natural Resources. In: Mussig J (ed) Industrial applications of natural fibres: structure, properties and technical applications. Wiley, New York, pp 432–442
Bio-Based News (2018) TRB develops new FST rated biocomposite rail carriage door leaf. http://news.bio-based.eu/trb-develops-new-fst-rated-biocomposite-rail-carriage-door-leaf/. Accessed 15 Nov 2018
Pirolini A (2014) Thermal protection systems. https://www.azom.com/article.aspx?ArticleID=11443. Accessed 12 Nov 2018
Wang T, Sun H, Long J, Wang YZ, Schiraldi D (2016) Biobased poly(furfuryl alcohol)/clay aerogel composite prepared by a freeze-drying process. ACS Sustain Chem Eng 4:2601–2605. https://doi.org/10.1021/acssuschemeng.6b00089
Wang X, Lu M, Qiu L, Huang H, Li D, Wang H, Cheng YB (2016) Graphene/titanium carbide composites prepared by sol–gel infiltration and spark plasma sintering. Ceram Int 42:122–131. https://doi.org/10.1016/j.ceramint.2015.08.017
Bae ST, Shin H, Jung HS, Hong KS (2009) Synthesis of titanium carbide nanoparticles with a high specific surface area from a TiO2 core-sucrose shell precursor. J Am Ceram Soc 92:2512–2516. https://doi.org/10.1111/j.1551-2916.2009.03243.x
Ebrahimi H, Roghani-Mamaqani H, Salami-Kalajahi M, Shahi S, Abdollahi A (2018) Preparation of furfuryl alcohol-functionalized carbon nanotube and epoxidized novolac resin composites with high char yield. Polym Compos 39:E1231–E1236. https://doi.org/10.1002/pc.24812
Setaka N (1981) Process for producing diamond powder by shock compression. https://patents.google.com/patent/US4377565. Accessed 12 Aug 2018
Nishi Y (2014) Past, present and future of lithium-ion batteries. can new technologies open up new horizons? In: Pstoia G (ed) Lithium-ion batteries: advances and applications. Elsevier, Amsterdam, pp 21–39
Thakur VK, Thakur MK, Asokan P (2017) Hybrid polymer composite materials. WoodHead Publishing, Cambridge
Nanaji K, Jyothirmayi A, Varadaraju U, Rao TN, Anandan S (2017) Facile synthesis of mesoporous carbon from furfuryl alcohol-butanol system by EISA process for supercapacitors with enhanced rate capability. J Alloys Compd 723:488–497. https://doi.org/10.1016/j.jallcom.2017.06.231
Gao J, Wang X, Zhang Y, Liu J, Lu Q, Liu M (2016) Boron-doped ordered mesoporous carbons for the application of supercapacitors. Electrochim Acta 207:266–274. https://doi.org/10.1016/j.jep.2017.05.029
Feng Y, Wang Y, Wang Y, Yao J (2017) Furfuryl alcohol modified melamine sponge for highly efficient oil spill clean-up and recovery. J Mater Chem A 5:21893–21897. https://doi.org/10.1039/c7ta06966a
Zhang J, Wang W, Peng H, Qian J, Ou E, Xu W (2017) Water-soluble graphene dispersion functionalized by diels-alder cycloaddition reaction. J Iran Chem Soc 14:89–93. https://doi.org/10.1007/s13738-016-0960-5
Kelland MA (2010) Production chemicals for the oil and gas industry. CRC Press, Boca Raton
Acknowledgement
We appreciate the Polymer and Physical Chemistry Unit at the Centre for Rubber Science and Technology, CRST, Nelson Mandela University, South Africa, for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iroegbu, A.O., Hlangothi, S.P. Furfuryl Alcohol a Versatile, Eco-Sustainable Compound in Perspective. Chemistry Africa 2, 223–239 (2019). https://doi.org/10.1007/s42250-018-00036-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-018-00036-9