Abstract
Objective
Parathyroidectomy (PTx) has an established benefit in patients with symptomatic primary hyperparathyroidism (PHPT). However, its efficacy in mild asymptomatic PHPT has not been proven. This study aimed to systematically review and meta-analyze the best available evidence from randomized-controlled trials comparing the efficacy of PTx over conservative management (non-PTx) on skeletal outcomes [fractures and bone mineral density (BMD)], nephrolithiasis risk and quality of life (QoL) in patients with mild asymptomatic PHPT.
Methods
A comprehensive literature search was conducted in PubMed, Scopus and Cochrane databases, from conception to February 23, 2020. Data were extracted from the studies that fulfilled the eligibility criteria and were synthesized quantitatively (fixed or random effects model) as relative risks and percentage mean differences (MD) with 95% confidence intervals (CI). I2 index was employed for heterogeneity.
Results
Four studies were included in the meta-analysis. There was no difference in fracture risk between PTx and active surveillance. The PTx group demonstrated higher BMD [MD 3.55% (95% CI 1.81, 5.29) in lumbar spine and 3.44% (95% CI 1.39, 5.49) in total hip, without difference in femoral neck and forearm] and lower calcium concentrations (MD − 13.26%, 95% CI − 7.10, − 19.43) compared with the non-PTx group. No difference was observed between groups regarding nephrolithiasis or QoL indices, except for general health (higher in PTx group).
Conclusions
In patients with mild asymptomatic PHPT, PTx increases BMD and reduces serum calcium concentrations. However, its superiority over active surveillance in terms of fracture risk, nephrolithiasis and QoL cannot be supported by current data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary hyperparathyroidism (PHPT) is a common endocrine disorder characterized by hypercalcemia and increased (or inappropriately normal) serum parathyroid hormone (PTH) concentrations [1]. It is most commonly identified in postmenopausal women > 50 years old, with a prevalence of 1:1000 in the general population and a female-to-male ratio of 3:1 [2]. The most severe phenotype is symptomatic PHPT, which usually manifests with hypercalcemia-associated symptomatology (muscle weakness, fatigue, arrhythmias, constipation, depression) and is associated with increased risk of renal and skeletal complications, such as nephrolithiasis, nephrocalcinosis, renal failure, osteoporosis and increased risk of fractures. Nevertheless, asymptomatic disease constitutes the most common form of PHPT [1]. Moreover, a new entity called “normocalcemic PHPT” (NPHPT) has been recognized, characterized by high serum PTH concentrations with persistently normal albumin-corrected or ionized serum calcium concentrations, with 25-hydroxy-vitamin D [25(OH)D] concentrations > 20 ng/mL, to exclude secondary hyperparathyroidism [1].
Parathyroidectomy (PTx) is the most effective treatment for PHPT, especially in patients with symptomatic disease, since it effectively increases bone mineral density (BMD) and reduces the risk of fracture and nephrolithiasis [3, 4]. However, in subjects with asymptomatic PHPT, the decision for performing a PTx may be based on specific criteria. These include at least one of the following: (i) serum calcium concentrations > 1 mg/dL above the upper limit of normal; (ii) T-score ≤ − 2.5 assessed by dual-energy X-ray absorptiometry (DXA) or history of vertebral fracture; (iii) nephrocalcinosis or nephrolithiasis or creatinine clearance of < 60 mL/min/1.73 m2; (iv) urinary calcium excretion > 400 mg/24 h with a biochemical profile suggestive for increased nephrolithiasis risk; or (v) age < 50 years old [3]. The non-surgical approach is mainly adopted for cases with PHPT, who present contraindications to surgery or refuse PTx or who do not meet the above criteria [3]. However, it has not been clarified whether PTx is indeed superior to active surveillance or pharmaceutical intervention (anti-osteoporotic medications or calcimimetics) in cases with mild asymptomatic disease. The term “mild asymptomatic” encompasses PHPT cases without symptoms suggestive of hypercalcemia, fractures or nephrolithiasis, not fulfilling the above criteria for PTx [1, 3].
The primary endpoint of this study was to systematically investigate and meta-analyze the best available evidence from randomized-controlled trials (RCTs) regarding the efficacy of PTx on skeletal outcomes (fracture risk and BMD), compared with conservative management (non-PTx) in patients with mild asymptomatic PHPT. Secondary endpoints were the comparative efficacy of these approaches on serum calcium concentrations, nephrolithiasis risk and quality of life (QoL).
Materials and methods
Guidelines followed
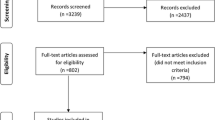
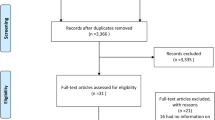
This systematic review followed the PRISMA guidelines [5]. A flow chart diagram is shown in Fig. 1. The present study has already been registered in the Prospective Register of Systematic Reviews (PROSPERO) System (PROSPERO ID: CRD42020153369).
Search strategy
The following PICO (Population, Intervention or Exposure, Comparison, Outcome) elements were applied as inclusion criteria for the systematic review: (i) population: asymptomatic patients with PHPT; (ii) intervention: non-PTx (conservative management, including either active surveillance or medical treatment); (iii) comparison group: PTx; (iv) outcome: fracture risk (primary outcome), BMD, serum and urinary calcium concentrations, nephrolithiasis and QoL (secondary endpoints). A systematic literature search for English publications in PubMed (Medline), Scopus, and Cochrane databases was performed, from conception up to February 23rd, 2020. The following search-string was used for PubMed, with appropriate modifications for the other two databases: (“Hyperparathyroidism, Primary”[MeSH] OR “primary hyperparathyroidism”[tiab]) AND (parathyroidectomy[tiab] OR (parathyroid*[tiab] AND (removal[tiab] OR surgery[tiab] OR operation[tiab])) OR “surgical management”[tiab] OR “surgical treatment”[tiab]) AND (“non-surgical management”[tiab] OR “non-surgical treatment”[tiab] OR “non surgical management”[tiab] OR “non surgical treatment”[tiab] OR “medical management”[tiab] OR “pharmaceutical management”[tiab] OR “medical treatment”[tiab] OR “pharmaceutical treatment”[tiab]OR surveillance[tiab] OR observation[tiab] OR bisphosphonate*[tiab] OR alendronate[tiab] OR risedronate[tiab] OR zoledronate[tiab] OR “zoledronic acid”[tiab] OR clodronate[tiab] OR pamidronate[tiab] OR etidronate[tiab] OR neridronate[tiab] OR SERM*[tiab] OR “selective estrogen receptor modulator*”[tiab] OR “selective-estrogen-receptor-modulator*”[tiab] OR raloxifene[tiab] OR bazedoxifene[tiab] OR estrogen[tiab] OR estradiol[tiab] OR calcitonin[tiab] OR denosumab[tiab] OR cincalcet[tiab] OR calcimimetic*[tiab] OR placebo[tiab]) NOT (Animal[MeSH] NOT Human[MeSH]) NOT (letter[pt] OR comment[pt] OR editorial[pt] OR Review[pt] OR “practice guideline”[ptyp] OR “case reports”[ptyp]).
Only RCTs meeting the inclusion criteria were retrieved. Observational studies, review articles and case reports were excluded. Furthermore, the references in all selected studies were manually searched to identify additional eligible trials. The main search was completed independently by three investigators (KV, SV and VP), who checked all the available articles. Any discrepancy was resolved by consultation of a fourth researcher, not involved in the initial procedure (PA). EndNote V9 was used as the reference manager software. Grey literature was searched through relevant sources, such as https://www.opengrey.eu, https://greylit.org and https://clinicaltrials.gov.
Trial selection
Inclusion criteria were as follows: (1) RCTs comparing PTx with conservative treatment in patients with mild asymptomatic PHPT, not fulfilling the criteria for surgery [3] (that is, cases without hypercalcemia-associated symptomatology and with albumin-corrected serum calcium < 1 mg/dL above the upper limit of normal, 24-h urinary calcium < 400 mg, age- and sex-matched BMD > − 2 SD, normal renal function and age > 50 years); (2) studies providing extractable data on fracture risk, BMD, serum and urinary calcium concentrations, nephrolithiasis and/or QoL in patients with asymptomatic PHPT.
Data extraction
The following data from each eligible study were extracted and recorded: (i) first author; (ii) year of publication; (iii) country(-ies) in which the study was conducted; (iv) duration of the study; (v) total number and mean age of participants; (vi) number of new (total, vertebral, non-vertebral, hip) fractures, assessed either clinically or radiographically; (vii) BMD at baseline and at the end of study (expressed either as absolute values in g/cm2 or as T-scores); (viii) serum concentrations and 24 h-urinary calcium excretion at baseline and at the end of study; (ix) new events of nephrolithiasis; (x) QoL at baseline and at the end of study; (xi) number of patients from the conservative treatment group, who eventually needed PTx.
The following comparisons were made between PTx and non-PTx groups: (i) number of new (total, vertebral, non-vertebral and hip) fractures; (ii) percentage (%) change in lumbar spine (LS), femoral neck (FN), total hip (TH) and forearm BMD; (iii) percentage (%) change in serum concentrations and 24 h-urinary calcium excretion; (iv) number of patients with new kidney stone formation; (v) absolute change in QoL scores.
In case of missing data or ambiguities in study design or trial conduction, the study authors were contacted by e-mail to request additional information.
Statistical analysis
Random or fixed effects model was used for data synthesis (Mantel/Haenszel model) according to heterogeneity. Associations were reported as relative risks (RR, for fractures and nephrolithiasis), percentage mean differences (MD, for BMD and calcium) or standardized MD (SMD, for QoL) with 95% confidence intervals (CI). A p value < 0.05 was considered as statistically significant. Heterogeneity was tested with the Cochrane Chi-square test and the degree of heterogeneity was quantified by the I2 statistics. Heterogeneity was considered as “low” if I2 was < 30%, whereas values of 30–60% or > 60% were used to define “moderate” or “high” degree of heterogeneity, respectively. Publication bias was tested by the Begg–Mazumdar and Egger’s tests (with p values > 0.1 indicating the absence of publication bias). The risk of bias and the study quality assessment was performed by the Cochrane assessment tool for RCTs [RevMan software, version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014)].
Results
Descriptive data
The initial literature search yielded 717 results, after removing duplicates. Of those, after screening by title and abstract, 16 were selected as full-texts for eligibility (Fig. 1). Ten studies were excluded due to: (i) non-RCT design (n = 5); (ii) referring to another article, already included in the quantitative analysis (n = 4); (iii) including patients without mild PHPT phenotype (n = 1). Six studies were included in the qualitative and four in the quantitative analysis [6,7,8,9,10,11]. Three studies reported different datasets from the same population [8, 10, 11] and, therefore, only one was included in the quantitative analysis. In particular, the study by Bollerslev et al. [8] was used for QoL data extraction and calculation of total number of participants, whereas the study by Lundstam et al. [10] for fractures, serum calcium and PTH concentrations and nephrolithiasis data. Data regarding BMD were extracted from the study by Lundstam et al. [11]. The effect of PTx compared with active surveillance was tested in four studies [6, 7, 9, 11]. The descriptive characteristics of the included studies and their participants are presented in Table 1.
Of note, medical treatment (etidronate) compared with PTx was tested in only one RCT [12]. However, PHPT in these patients was not mild, since their T-scores ranged from − 4 to − 3 and, therefore, this study was excluded from the analysis. In this study, PTx increased LS BMD to a greater extent, compared with etidronate. However, no difference in total BMD was observed between groups. PTx was also beneficial in reducing serum calcium concentrations compared with etidronate. No RCT on other bisphosphonates or calcimimetics was identified. Definition of mild asymptomatic PHPT and the specific inclusion criteria used in each study are presented in Table 2.
The included studies were published between 2004 and 2017. Two studies were conducted in the USA [6, 9], one in Italy [7] and one was multicenter (Denmark, Sweden, Norway) [11]. The number of participants ranged from 18 to 191, providing a total number of 312, 154 of which were managed surgically (PTx group) and 158 conservatively (active surveillance; non-PTx group), although the number of patients providing data for each outcome was much lower. Mean participants’ age was 64.2 ± 7.9 years and mean follow-up time 28.5 (range 6–60) months. With respect to vitamin D and calcium supplementation, two studies reported no use of calcium [7, 9] or vitamin D supplements [9], whereas, in one study [11], both calcium and vitamin D supplementation were allowed. There were no reports on calcium or vitamin D supplementation in one study [6].There were no reports of concomitant use of anti-osteoporotic agents by the participants, except for the study by Bollerslev et al. [8], in which 16.7% of patients in the PTx and 12.6% in the non-PTx group continued to receive estrogen or bisphosphonates. Mean baseline 25(OH)D concentrations differed across studies, being 20.2 ± 9.5 vs. 20.6 ± 10.8 ng/mL (Rao et al. [6]), 13.4 ± 6.7 vs. 17.5 ± 10.9 ng/mL (Ambrogini et al. [7]), 47.4 ± 19.9 vs. 41.9 ± 7 ng/mL (Perrier et al. [9]), and 16.4 ± 6 vs. 18.4 ± 6 ng/mL (Lundstam et al. [11]) in PTx-group vs. non-PTx group, respectively.
Data on fracture incidence
Fracture incidence was reported in three studies [6, 7, 10], all of which provided data on vertebral fracture rate, whereas non-vertebral fracture data were available only in two studies [6, 10] (follow-up time 1–5 years). With respect to hip fractures, no new fracture occurred in the study by Rao et al. [6], which was the only one with available data.
There was no difference in fracture incidence between PTx and non-PTx groups, although a tendency for reduction was observed in the former group (RR 0.34, 95% CI 0.11, 1.10, p = 0.07, I2 = 0% for total fractures; RR 0.15, 95% CI 0.02, 1.23, p = 0.08, I2 = 0% for vertebral fractures). The respective forest plots are presented in Fig. 2. When assessment of fracture risk was based on per-protocol analysis, a lower risk for total fractures was demonstrated for PTx compared with non-PTx group (RR 0.26, 95% CI 0.08, 0.83, p = 0.02, I2 = 0%).
Percentage changes in BMD
With regard to BMD changes, three studies provided data on LS and forearm (distal third or ultra-distal radius) [6, 7, 11] and two on either FN [6, 11] or TH [6, 7]. In general, higher LS and TH BMD values were observed in patients undergoing PTx compared with those under active surveillance (MD 3.55%, 95% CI 1.81, 5.29, p < 0.01, I2 = 98% for LS BMD; MD 3.44%, 95% CI 1.39, 5.49, p = 0.001, I2 = 99%, for TH BMD). The difference between groups with regard to FN was marginally insignificant (MD 2.89%, 95% CI 0.06, 5.71, p = 0.05, I2 = 100%) and non-significant regarding forearm BMD (MD 1.06%, 95% CI 1.30, 3.43, p = 0.38, I2 = 100%). These data are illustrated in Fig. 3.
Changes in serum calcium concentrations and 24-h urinary calcium excretion
Mild hypercalcemia was recorded in all studies, but with serum calcium concentrations not exceeding the threshold of 1 mg/dL above the upper limit of normal (Table 1). Three studies [7, 9, 10] reported data on serum calcium concentrations at baseline and at the end of study in both groups. One of them [10] provided data on serum calcium adjusted for albumin, another one [7] provided data on ionized serum calcium, whereas another study [9] did not clarify this correction. Data analysis demonstrated a mean reduction of 13.26% (95% CI 7.10, 19.43, p < 0.001, I2 = 100%) in the PTx compared with non-PTx group (Fig. 4a). In the study by Ambrogini et al. [7], 23/24 patients who underwent PTx showed normalization of serum calcium, whereas it remained stable in 27/28 patients followed without PTx (one patient in each group developed hypercalcemia), after 1 year. In the study by Perrier et al. [9], all patients remained eucalcemic after PTx, whereas there was no change in serum calcium concentrations in the observation group (6 months of follow-up). In the study by Lundstam et al. [10], serum calcium normalized in all patients following PTx, whereas it decreased mildly in the active surveillance group (5 years of follow-up).
In the study by Rao et al. [6], there were no reports of progression to hypercalcemia in patients randomized to non-PTx, during two years of follow-up. However, three of 28 (10.7%) patients under active surveillance eventually required PTx due to complications, such as nephrolithiasis, pancreatitis and neuromuscular symptomatology (fatigue, irritability, and depression). In the study by Ambrogini et al. [7], no patient developed hypercalcemia and there was no need for PTx during follow-up (1 year). However, in the study by Lundstam et al. [10], 12 out of 73 (16.4%) non-PTx subjects, underwent surgery during the 5-year follow-up, mainly due to hypercalcemia (exact number not reported).
With regard to 24-h urinary calcium, data were available from two studies [6, 7], in both of which these were < 400 mg/24-h. There was a significant decrease in 24-h urinary calcium with PTx, whereas no change was observed in the non-PTx group [6, 7]. In the study by Ambrogini et al. [7], four out of 26 non-PTx patients (15.4%) developed marked hypercalciuria (> 400 mg/24 h) during follow-up, most of which had baseline concentrations of > 300 mg/24 h.
Nephrolithiasis incidence
Regarding nephrolithiasis, no difference was observed between groups (RR 0.53, 95% CI 0.10, 2.87, p = 0.46, I2 = 0%). These data were available from three studies [6, 7, 10] and are presented in Fig. 4b.
QoL assessment
QoL was assessed in four studies [6,7,8,9] through five different scales. The Short Form (36) Health Survey (SF-36) score was used in two studies [6, 7], whereas a generic SF-36 form in another study [8]. Symptom checklist revised questionnaire provided data on psychosocial well-being in two studies [6, 7], whereas comprehensive psychopathological rating scale in one study [8]. The study by Perrier et al. [9] tested neuropsychological function through a variety of scales demonstrating no significant results between the compared groups.
Meta-analysis was based on SF-36 score data, from either original or generic form, using SMD in both groups. No difference in all (physical and social function, physical and emotional role function, mental health, vitality and bodily pain) but one (general health, which was higher in the PTx group) indices, were observed between groups (data presented in Supplementary Fig. 1).
Discussion
The present meta-analysis demonstrated a beneficial effect of PTx on BMD and serum calcium concentrations over active surveillance in patients with mild asymptomatic PHPT. However, there was no difference between groups in terms of fracture risk, although a tendency for a reduction with PTx should be mentioned. Moreover, no difference was observed between groups regarding the risk for nephrolithiasis or QoL indices (except for general health, which seems to be improved after PTx).
In general, the bone-protective efficacy of PTx in asymptomatic PHPT cases has long been established [3]. In patients unwilling to undergo a PTx or having contraindications to surgery, medical treatment seems to offer bone-protection compared with placebo, since it increases BMD (estrogen, bisphosphonates), although data on fracture risk are lacking [13, 14]. Cinacalcet is also effective in normalizing calcium concentrations, alone or with bisphosphonates [14]. Comparative data from non-RCTs show predominance of PTx over bisphosphonates with regard to bone mass [15] and fracture risk [16]. Denosumab seems also to be effective in PHPT by increasing BMD, conferring greater benefit compared with patients with primary osteoporosis [17].
Mild asymptomatic form constitutes the “lion’s share” of all PHPT cases, characterized by low risk of complications (i.e., osteoporosis, nephrolithiasis, neuromuscular/neuropsychiatric complications, deterioration in QoL) compared with classical PHPT [1,2,3]. It mostly manifests with mild hypercalcemia (< 12 mg/dL) or even with normal calcium concentrations (NPHPT). The latter has a prevalence of 0.1–8.9% [18, 19] and is characterized by an intermediate biochemical phenotype between classical PHPT and controls (normocalcemia with normal PTH concentrations) [19, 20]. Intermittent mild hypercalcemia may be also observed during follow-up in > 50% NPHPT patients [19]. In cases of mild asymptomatic PHPT, including NPHPT, the benefits of PTx are obscure, with studies yielding inconsistent results [1, 3]. Therefore, caregivers may also consider the non-surgical approach in such cases.
To the best of our knowledge, the present meta-analysis is the fourth one published heretofore. The first on this concept was published in 2010 by Sankaran et al. [21], focusing only on skeletal outcomes. The authors included both cohorts and RCTs. However, few of them had compared PTx with non-PTx (i.e., most reported comparative data of medical treatment over placebo). Briefly, the authors concluded that anti-resorptive therapy produces comparable effect with PTx on BMD (the effect of the latter seems to be overestimated in observational studies) and the untreated mild PHPT does not lead to rapid bone loss [21].
The second meta-analysis was published in 2016 by Singh Ospina et al., which compared the efficacy of PTx with that of active surveillance in patients with mild asymptomatic PHPT regarding fracture and nephrolithiasis incidence, as well as QoL indices [22]. In alignment with the present study, no difference was found between PTx and non-PTx groups. However, this meta-analysis included both RCTs and cohort studies. In contrast to the present study, data synthesis from RCTs did not show any difference in BMD between groups (n = 2). However, in this context, the present meta-analysis included the study by Ambrogini et al. [7] (n = 3) and focused on percentage (%) mean differences in BMD, instead of absolute BMD values.
The third systematic review and meta-analysis was published in 2018 by Zhang et al., which focused on the long-term skeletal outcomes after PTx compared with non-PTx in patients with PHPT [23]. This meta-analysis showed anti-fracture efficacy of PTx over non-PTx, as well as improvement in BMD in LS and FN. However, it must be highlighted that the authors erroneously meta-analyzed data from the same population as belonging to different studies. This was the case both for RCTs and cohort studies [8, 10, 11, 24, 25]. Moreover, the cohort study group was characterized by high heterogeneity, due to inclusion of one study, which compared PTx with medical treatment (bisphosphonates) [26] and another with a mixed population of symptomatic and asymptomatic PHPT [27]. Therefore, the results of this meta-analysis should be interpreted with caution.
With regard to gender, there were no distinct data for males and females across studies (the proportion of males was 10–22%). Interestingly, in the study by Amborgini et al. [7], the results regarding BMD changes and QoL did not change when males were excluded from the analysis. In general, limited data exist on this concept. In a retrospective study (n = 123; 25.2% men), male gender was independently associated with BMD improvement in patients with PHPT undergoing PTx (hazard ratio 2.29, 95% CI 1.54–4.2) [28]. However, in another retrospective comparative study in patients with PHPT (n = 417; 22.3% males), men were significantly younger and more frequently symptomatic (including nephrolithiasis and osteoporosis) compared with women. However, there was no gender difference in surgical referral among symptomatic and asymptomatic patients (84.6% in men vs. 84.9% in women) [29].
The main strength of the present meta-analysis is that it included only RCTs and it covered the wide clinical PHPT phenotype (skeletal complications, changes in biochemical profile, including both serum and urinary calcium, nephrolithiasis and QoL).
However, certain limitations should be recognized. First, the lack of significance with respect to fracture risk may be attributed to the low number of fractures recorded in both groups, although the per-protocol analysis demonstrated a reduction in total fracture risk after PTx. Therefore, a potential benefit of PTx cannot be excluded. This may be further supported by the benefit of PTx on BMD, as a surrogate outcome for fractures. Second, the fracture incidence was the primary endpoint only in one study [10]. Third, the follow-up time in the included studies was relatively short to draw safe conclusions. Longer observation may lead some patients under active surveillance to surgery. Fourth, the heterogeneity regarding fracture ascertainment among studies should be acknowledged. The study by Rao et al. [6] and Lundstam et al. [10] had performed plain radiographs to identify asymptomatic vertebral fractures, whereas the study by Ambrogini et al. [7] reported clinical vertebral fractures. These limitations would also apply to nephrolithiasis, since a renal ultrasound was not performed routinely during the follow-up period in most studies. Fifth, the heterogeneity in baseline 25(OH)D concentrations among studies should also be taken under consideration. Sixth, the studies included in the meta-analysis do not adequately address the way of exclusion of a familial form of PHPT. Seventh, the heterogeneity across studies, regarding the method of 25(OH)D and PTH assessment, should also be recognized. Eighth, the heterogeneity across studies regarding the device used for DXA scan, the different physical principles for X-ray emission sources and the lack of cross-calibration between the instruments used, should also be taken into account.
Conclusions
In patients with mild asymptomatic PHPT, PTx is superior to active surveillance in increasing BMD and reducing serum calcium concentrations. However, there is no evidence to suggest that PTx is beneficial in terms of fracture, nephrolithiasis prevention and QoL in these patients. In any case, more data from comparative studies with longer follow-up intervals are needed to further designate the best approach to this sub-population of PHPT. Moreover, the calculation of serum calcium/phosphate ratio, which may further contribute in the correct diagnosis and management of PHPT [30], (especially the normocalcemic forms) should be evaluated in future studies.
References
Walker MD, Silverberg SJ (2018) Primary hyperparathyroidism. Nat Rev Endocrinol 14:115–125
Clarke BL (2013) Epidemiology of primary hyperparathyroidism. J Clin Densitom 16:8–13
Bilezikian JP, Brandi ML, Eastell R, Silverberg SJ, Udelsman R, Marcocci C, Potts JT Jr (2014) Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab 99:3561–3569
Wilhelm SM, Wang TS, Ruan DT et al (2016) The American Association of Endocrine Surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg 151:959–968
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8:336–341
Rao DS, Phillips ER, Divine GW, Talpos GB (2004) Randomized controlled clinical trial of surgery versus no surgery in patients with mild asymptomatic primary hyperparathyroidism. J Clin Endocrinol Metab 89:5415–5422
Ambrogini E, Cetani F, Cianferotti L et al (2007) Surgery or surveillance for mild asymptomatic primary hyperparathyroidism: a prospective, randomized clinical trial. J Clin Endocrinol Metab 92:3114–3121
Bollerslev J, Jansson S, Mollerup CL et al (2007) Medical observation, compared with parathyroidectomy, for asymptomatic primary hyperparathyroidism: a prospective, randomized trial. J Clin Endocrinol Metab 92:1687–1692
Perrier ND, Balachandran D, Wefel JS et al (2009) Prospective, randomized, controlled trial of parathyroidectomy versus observation in patients with “asymptomatic” primary hyperparathyroidism. Surgery 146:1116–1122
Lundstam K, Heck A, Mollerup C et al (2015) Effects of parathyroidectomy versus observation on the development of vertebral fractures in mild primary hyperparathyroidism. J Clin Endocrinol Metab 100:1359–1367
Lundstam K, Heck A, Godang K et al (2017) Effect of surgery versus observation: skeletal 5-year outcomes in a randomized trial of patients with primary HPT (the SIPH Study). J Bone Miner Res 32:1907–1914
Horiuchi T, Onouchi T, Inoue J, Shionoiri A, Hosoi T, Orimo H (2002) A strategy for the management of elderly women with primary hyperparathyroidism: a comparison of etidronate therapy with parathyroidectomy. Gerontology 48:103–108
Grey AB, Stapleton JP, Evans MC, Tatnell MA, Reid IR (1996) Effect of hormone replacement therapy on bone mineral density in postmenopausal women with mild primary hyperparathyroidism. A randomized, controlled trial. Ann Intern Med 125:360–368
Leere JS, Karmisholt J, Robaczyk M, Vestergaard P (2017) Contemporary medical management of primary hyperparathyroidism: a systematic review. Front Endocrinol (Lausanne) 8:79
Tournis S, Fakidari E, Dontas I et al (2014) Effect of parathyroidectomy versus risedronate on volumetric bone mineral density and bone geometry at the tibia in postmenopausal women with primary hyperparathyroidism. J Bone Miner Metab 32:151–158
Yeh MW, Zhou H, Adams AL, Ituarte PH, Li N, Liu IL, Haigh PI (2016) The relationship of parathyroidectomy and bisphosphonates with fracture risk in primary hyperparathyroidism: an observational study. Ann Intern Med 164:715–723
Eller-Vainicher C, Palmieri S, Cairoli E, Goggi G, Scillitani A, Arosio M, Falchetti A, Chiodini I (2018) Protective effect of denosumab on bone in older women with primary hyperparathyroidism. J Am Geriatr Soc 66:518–524
Pawlowska M, Cusano NE (2015) An overview of normocalcemic primary hyperparathyroidism. Curr Opin Endocrinol Diabetes Obes 22:413–421
Schini M, Jacques RM, Oakes E, Peel NFA, Walsh JS, Eastell R (2020) Normocalcemic hyperparathyroidism: study of its prevalence and natural history. J Clin Endocrinol Metab 105(4):e1171–e1186
Palermo A, Naciu AM, Tabacco G et al (2020) Clinical, biochemical and radiological profile of normocalcemic primary hyperparathyroidism. J Clin Endocrinol Metab 105:dgaa174
Sankaran S, Gamble G, Bolland M, Reid IR, Grey A (2010) Skeletal effects of interventions in mild primary hyperparathyroidism: a meta-analysis. J Clin Endocrinol Metab 95:1653–1662
Singh Ospina N, Maraka S, Rodriguez-Gutierrez R et al (2016) Comparative efficacy of parathyroidectomy and active surveillance in patients with mild primary hyperparathyroidism: a systematic review and meta-analysis. Osteoporos Int 27:3395–3407
Zhang L, Liu X, Li H (2018) Long-term skeletal outcomes of primary hyperparathyroidism patients after treatment with parathyroidectomy: a systematic review and meta-analysis. Horm Metab Res 50:242–249
VanderWalde LH, Liu IL, O’Connell TX, Haigh PI (2006) The effect of parathyroidectomy on bone fracture risk in patients with primary hyperparathyroidism. Arch Surg 141:885–889 ((discussion 889-891))
VanderWalde LH, Liu IL, Haigh PI (2009) Effect of bone mineral density and parathyroidectomy on fracture risk in primary hyperparathyroidism. World J Surg 33:406–411
Fang WL, Tseng LM, Chen JY, Chiou SY, Chou YH, Wu CW, Lee CH (2008) The management of high-risk patients with primary hyperparathyroidism—minimally invasive parathyroidectomy vs. medical treatment. Clin Endocrinol (Oxf) 68:520–528
Vestergaard P, Mosekilde L (2003) Cohort study on effects of parathyroid surgery on multiple outcomes in primary hyperparathyroidism. BMJ 327:530–534
Sharma J, Itum DS, Moss L, Li C, Weber C (2014) Predictors of bone mineral density improvement in patients undergoing parathyroidectomy for primary hyperparathyroidism. World J Surg 38:1268–1273
Castellano E, Attanasio R, Boriano A et al (2017) Sex difference in the clinical presentation of primary hyperparathyroidism: influence of menopausal status. J Clin Endocrinol Metab 102:4148–4152
Madeo B, Kara E, Cioni K et al (2017) Serum calcium to phosphorous (Ca/P) ratio is a simple, inexpensive, and accurate tool in the diagnosis of primary hyperparathyroidism. JBMR Plus 2:109–117
Acknowledgements
The authors would like to thank Prof. Marcocci for providing additional data from their study, which were included in the analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Panagiotis Anagnostis, Konstantina Vaitsi, Stavroula Veneti, Victoria Potoupni, Eustathios Kenanidis, Eleftherios Tsiridis, Theodosios S. Papavramidis and Dimitrios G. Goulis declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

40618_2020_1447_MOESM1_ESM.jpg
Supplementary Figure 1. Forest plot for the outcome of quality of life [A) physical function, B) social function, C) physical role function, D) emotional role function, E) mental health, F) vitality, G) bodily pain, H) general health]. (JPG 185 kb)

Rights and permissions
About this article
Cite this article
Anagnostis, P., Vaitsi, K., Veneti, S. et al. Efficacy of parathyroidectomy compared with active surveillance in patients with mild asymptomatic primary hyperparathyroidism: a systematic review and meta-analysis of randomized-controlled studies. J Endocrinol Invest 44, 1127–1137 (2021). https://doi.org/10.1007/s40618-020-01447-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01447-7