Abstract
Objective
Parathyroidectomy (PTX) is the conclusive therapy for primary hyperparathyroidism (PHPT), but its effect on the risk of urolithiasis is inconclusive. We comprehensively reviewed the currently available research to investigate the impact of PTX on the likelihood of urolithiasis among individuals suffering PHPT.
Methods
Internet-based articles in English language released on Cochrane, PubMed, Scopus, Web of knowledge, and Embase up to September, 2023 were comprehensively reviewed. Each publication in contrast to the incidence, occurrence, or recurrence of urolithiasis after PTX versus medical treatment in PHPT patients was included. The outcome with pooled relative risks (RRs) and corresponding 95% confidence intervals (CIs) was examined employing DerSimonian and Laird’s model of random effects. To determine the range of the real effect size of a future study in 95% of all populations, a prediction interval (PI) was also established.
Results
Finally, ten studies involving 74,190 patients were included. Results from randomized-controlled trials (RCTs) and observational studies (OSs) both revealed that PTX did not substantially lessen the vulnerability of urolithiasis among individuals with PHPT (RCTs: pooled relative risk [RR] 0.42, 95%CI 0.13–1.41, p = 0.163; OSs: pooled RR 1.37, 95%CI 0.96 to 1.97, p = 0.084). The PI (RCT: 0.03 to 5.96; OSs: 0.44–4.20) containing 1.0 suggested the possibility of consistent results in future studies. Subgroup and sensitivity analyses supported the above findings, and no evidence showed publication bias.
Conclusion
Our analysis from the available RCTs or OSs did not give adequate or exact proof that the average effect of PTX lowers the incidence of urolithiasis among PHPT persons based on the random-effects model. Future research shall take into account the common effect of PTX as well as the prerequisites of preventive stone procedures, which will further help us assess the effectiveness of PTX in reducing kidney calculus comorbidity and develop techniques to avoid stone sequelae in these individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary hyperparathyroidism (PHPT), a prevalent endocrine illness, is characterized by excessive parathyroid hormone (PTH) production by multiple parathyroid glands. PHPT seems to be the third leading metabolic problem following diabetic mellitus and hypothyroidism [1, 2]. PHPT affects women nearly three times more frequently than males worldwide. Postmenopausal (post-M) women account for a sizable proportion of PHPT patients in Western countries, owing to both the earlier screening for osteoporosis [3] and estrogen withdrawal for parathyroid tumorigenesis and intestinal calcium absorption reductions [4, 5]. A large US investigation showed that PHPT attacked 0.86% of the total population [6]. The approximate incidence was 1 per 400 females and 1 per 1000 males from 1995 to 2010, and was 233 per 100,000 females and 85 per 100,000 males in 2010 [7]. Like in the United States [8, 9], individuals in Western Europe experiencing silent minor hypercalcemia are frequently identified with PHPT as per multichannel biochemical tests. PHPT was detected in 3.4% of postmenopausal women in Sweden according to a community health survey [10]. The incidence rates of PHPT in Scotland increased between 1986 and 2013, and from 1998 to 2006 [11, 12], because of increased disease knowledge, faster detection, and an increase in life expectancy. A community historical follow-up research project from 2007 to 2018 across Tayside, Scotland [13], discovered 4–6 cases of PHPT per 10,000 person-years on average each year. This population-based survey reveals a 0.84% occurrence of PHPT and confirms that PHPT seems to be a widespread metabolic problem in the general population, especially among postmenopausal females. The higher life expectancy of the Scottish population, particularly elderly people who are still most prone to PHPT, has led to a rise in predominance, despite stable incidence rates.
The frequency of PHPT among Chinese seniors and middle-aged people (n = 2451) was disclosed to be 0.2% [14]. Nevertheless, as a result of the rising measurement of ionized calcium level, the annual incidence and prevalence in nations outside North America and Europe are soaring, which seems to be linked to a shift in patient presentation of disease from having symptoms to being asymptomatic. Consequently, it is reasonable to expect that the incidence and prevalence of PHPT will rise globally [15]. Unlike in Western countries, PHPT was more common in premenopausal (pre-M) women in Asian countries, such as China [16] and India [17], which could be attributed to the disease's aggressive character in this region of the world, which is exacerbated by the related severe vitamin D deficiency [17].
PHPT clinically results in a number of co-morbidities, including osteoporosis, fractures, urolithiasis, muscle weakness, cardiac abnormality, psychiatric abnormality, cancers, and increased mortality [18]. Among them, urolithiasis with a reported prevalence of 3–5% [19] is a major concern for PHPT patients, and contributes to chronic kidney disease and reduced life quality [20, 21]. Urolithiasis can cause severe pain, infection, obstruction, and kidney damage, and its recurrence rate is high due to the lack of proper treatment [22]. Both parathyroidectomy (PTX) and medical treatment are proposed to decrease serum intact PTH (iPTH) and resolve PHPT. PTX can be administered to individuals experiencing hypercalciuria (24 h urine calcium > 400 mg), demonstrative kidney calculi disorder, or imaging-confirmed silent stones [23]. The mechanism underlying the decrease in stone formation following PTX is most likely due to the correction of hypercalcemia and hyperparathyroidism, both of which are known risk factors of stone development [24]. However, in America, the majority of PHPT patients are handled non-operatively, as only 50% of individuals suffering from renal stones and 38.9% of overall patients receive PTX within a year after PHPT diagnosis [25]. In addition, the effect of PTX on urolithiasis development in PHPT patients has not been well established. Some studies show that PTX reduces the likelihood of developing urinary stones by lowering serum calcium and PTH levels [26, 27], while other studies find no significant change in stone incidence after PTX [28, 29]. This inconsistency can be explained by the variations in research demographics and designs.
Given the concerns above, we carried out a meta-analysis to examine the possibility of urolithiasis among PHPT individuals treated by PTX versus medical method.
Methods
Search strategy and selection criteria
We looked for medical studies released prior to September 2023 in EMBASE, Web of Knowledge, PubMed, Scopus, and Cochrane pursuing Preferred Reporting Items for Systematic Reviews and Meta-Analyses [30]. The following search terms were used: “primary hyperparathyroidism”, or “PHPT”; “parathyroidectomy”, or “parathyroid gland surgery”, or “PTX”; “renal calculi”, or “kidney stones”, or “urolithiasis”, or “nephrolithiasis”. GS and XMW sought for possible studies individually, and any discord between them was resolved by GLT. We communicated with the researchers, whenever necessary, for clarification of the information being provided.
Inclusion and exclusion criteria
Reports that satisfied further prerequisites were included: (1) randomized-controlled trials (RCTs) or observational studies (OSs) involving adults (age ≥ 18) and (2) assessments of the likelihood of urolithiasis (incidence, prevalence, or recurrence) or provision of sufficient information for calculating relative risks (RRs) and 95% confidence intervals (CIs) comparing PTX-treated versus medically treated PHPT patients.
Studies were excluded if they were reviews, commentaries, editorials, letters, unpublished studies, quasi-experiments, animal studies, comparisons among many forms of PTX or various medicinal therapies, or comparisons between PTX treatments, or studies with a sample size less than 50 patients.
Data extraction and quality assessment
The titles and abstracts of all discovered papers were examined for consideration by two distinct reviewers. Full-text articles were obtained for further assessment. Discrepancies were resolved through discussion and consensus.
The selection of studies and retrieval of information followed a normal Cochrane process. The following information was collected: study methodology, representative sample, patient characteristics, period of follow-up, PTX technique, severity of PHPT, type of urolithiasis, and outcomes.
Two reviewers (GS and XMW) individually appraised the merits of articles. With the Risk of Bias Tool developed by the Cochrane Collaboration, the risk of bias (low, unclear or high) in six categories was judged for each RCT with three tiers per category: sequence generation; allocation hiding; blinding participants, personnel, and outcome assessors; partial outcome data; selective result reporting; and other biases. Provided a minimum of one category was "high risk of bias," the general likelihood of bias was high, while it was low when all categories remained “low risk” [31]. The quality of OSs was surveyed using the Newcastle–Ottawa Scale (NOS). The NOS consists of three quality criteria for cohort studies: choice, consistency, and result. The maximum rating for each of these three categories is 4, 2, and 3 stars, respectively. Therefore, the highest quality comprises 9 stars, and a study featuring 6 stars or more is considered to be of good quality [31]. The 11-item Agency for Healthcare Research and Quality (AHRQ) checklist can be used to evaluate the quality of cross-sectional research articles [32]. The possible answers for each item are "Yes," "No," and "Unclear". If the response to a question is "yes," one point is awarded; otherwise, no point is given. A study with a total score of 0–5, 6–7, and 8–11 is deemed to be of low, moderate, and high quality, respectively. Disagreements were resolved through dialogue.
Statistical analysis
We combined dichotomous outcomes (e.g., probability of urolithiasis) utilizing relative risk (RR) with 95%CI. With I2 statistic, heterogeneity across studies was quantified as minor (I2 < 25%), modest (25–50%), or significant (≥ 50%). Considering the intrinsic variation with research methodology, we predetermined individual analysis for the RCTs and OSs. For RCTs (or OSs), the primary or secondary analysis comprised all the included trials. Each participant who started to receive therapy, whether he/she completed it or not, was counted as a patient in the RCTs that employed the intent-to-treat criterion. A prediction interval (PI) was calculated to indicate the range of a true effect size of a future study in 95% of all populations [33]. Given the anticipated heterogeneity of OSs, we subjected the patients with or without prior record of urolithiasis at the time of PHPT diagnosis to distinct subanalysis. We also ran sensitivity tests to clarify if the heterogeneity may be attributed to observable sources. Funnel plot inequality was studied with visual representation, and Begg's, and Egger's assessment when at least five studies were available for investigation. All analyses except for the PI were finished on Review Manager 5.3.5 from the Cochrane Collaboration with STATA 14.0 (STATA College Station, USA). The PI were performed with R software Version 4.1.3. The significance threshold was p < 0.05, except for publication bias test (p < 0.10).
Results
Study selection and characteristics
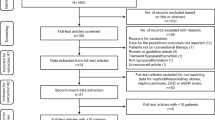
We first found 3366 possibly relevant publications, of which 31 were retrieved for further inspection. Two RCTs [34, 35] represented an identical group or organization, but their results were different. One study [36] was removed from the meta-analysis, because its reported occurrence rates of urolithiasis were zero in both the PTX and medical therapy groups. Finally, four RCTs [34, 35, 37, 38] with 485 patients as well as six OSs (one cross-sectional study[39] and five cohort studies [40,41,42,43,44]) with 73,705 individuals were included (Fig. 1). The four RCTs had sample sizes between 50 and 191 patients, follow-up periods from 1.0 to 10.0 years, 79.24% to 92.00% females, and mean age of 63.10 to 64.89 years. Individuals with mild PHPT were enrolled in all RCTs.
In the six OSs, the sample sizes ranged from 265 to 44,978 patients, the follow-up period ranged from 1.15 to 8.01 years, the female percentage was 12.2% to 78.20%, and the mean age was 52.02 to 67.50 years. Apart from two investigations on multinational origins, the remaining nine trials were from either the United States or European countries. Table 1 outlines the key elements of the ten studies.
Quality assessment
Figure S1 and Table S1 show the methodological quality ratings of RCTs and OSs, respectively. As a result of the absence of respondent blindness or hiding of assignment, research staff, and result judgement, we classified the four RCTs as a medium level of bias risk. Three of the five CSs have NOS scores ≥ 7 (mean of 8.2). According to AHRQ, the cross-sectional study was of intermediate quality (Table S1).
Risk of urolithiasis
The combined relative risk (RR) of urolithiasis following PTX versus medical treatment in the four RCTs was 0.42 (95%CI 0.13–1.41; p = 0.163) in the random-effects model, showing no heterogeneity. The PI was 0.03 to 5.96. The six relevant OSs had similar minuscule-integrated estimates regarding the impact (pooled RR, 1.37; 95%CI 0.96–1.97; p = 0.084; PI: 0.44 to 4.20; Fig. 2), with notable heterogeneity (I2 = 85.7%; p < 0.001). History of urolithiasis at PHPT diagnosis yielded similar results by separate subanalyses (Fig. 3). Sensitivity analysis showed that heterogeneity did not vanish after removing individual studies. Neither Egger’s nor Begg’s tests showed clear systematic bias in the urolithiasis risk analyses (both p > 0.1; Fig. S2).
Discussion
The current meta-analysis provides a thorough examination of the relationship between PTX and urinary stone development in PHPT patients. Results show that neither RCTs nor OSs prove PTX significantly reduce the vulnerability of urolithiasis among PHPT patients. The PI (RCT: 0.03–5.96; OSs: 0.44–4.20) containing 1.0 suggested the possibility of consistent results in future studies. Subgroup analyses were carried out to determine if the risk of urolithiasis varied with the history of urolithiasis when PHPT was first diagnosed. Separate subanalyses revealed underwhelming results for the history of kidney stones during PHPT diagnosis. The finding is similar with the majority of previous studies, which reveal no substantial reduction in the risk of urolithiasis after PTX in PHPT patients. Moreover, the risk of urolithiasis was elevated in individuals receiving PTX versus medical therapy, but not significantly (pooled RR, 1.37; 95% CI 0.96–1.97; p = 0.084). Multivariable analysis by Vestergard et al. [40] also found that PTX-treated patients had a higher incident of any kidney stone. They thought that the reason was due to the residual treatment selection bias with more advanced disease (a large preexisting stone burden and worse PHPT biochemical profiles) in surgically treated patients. Posen et al. [39] discovered individuals with history of renal stones had a 50% likelihood of recurring episodes, whether they received PTX or not. Even if they remained hypercalcaemic and hypercalciuric, patients without history of calculi were unlikely to produce stones.
At the same hand, because stones that appear soon after PTX may be formed before the therapy, the advantages regarding operative treatment may emerge only after long time. According to Seib et al., patients experiencing kidney calculi had a twofold adjusted likelihood of a clinically noteworthy recurrence following PTX, as opposed to those receiving non-operative treatment [42]. However, the occurrence of kidney stones following PTX decreased over time. They also discovered no discernible difference in the probability for fresh stone occurrence based on therapy for PHPT individuals without experiencing kidney calculi. It is implied that PTX has no meaningful advantage on the principal avoidance of kidney calculi and that PTX reduces or delays the prospective likelihood of reoccurring kidney calculi, but does not prevent kidney stones.
Another probable explanation is that the formation of urolithiasis is a systemic disorder. Hypercalciuria is a factor, but it is not the only way PHPT patients develop urolithiasis. Reducing PHPT-related risk factors, such as hypercalcaemia, phosphate excretion, and urine pH after PTX, may have a favorable influence on the recurrence of urolithiasis. Other factors influencing stone formation besides hypercalcaemia include low urine volume, elevated pH, oxalate and citrate excretion, younger age and male gender, and predisposing genetic factors [45, 46]. Apart from hypercalciuria, the lithogenic factors associated with PHPT patients may differ depending on the demographic and geographic region analyzed [47]. Despite a successful PTX, the overall risk of urolithiasis is still higher because of other co-occurring factors [29]. Future research shall take into account diet modification, pharmaceutical intervention, as well as the elimination of risk factors for urolithiasis formation, regardless of whether they undergo PTX.
The comprehensive search method based on typical Cochrane protocols, and the relatively large sample size, are two strengths of this review. However, there are some restrictions. First, we are unable to uncover unreleased documents that may have skewed our findings. Second, because the meta-analysis is based partially on OSs, the possibility of confounding cannot be totally eliminated. Third, the included studies differ in patient characteristics, research design, and follow-up duration, which can have contributed to the observed heterogeneity. Furthermore, no data regarding the initial degree of hypercalciuria or related stone risk factors were gathered, which can have influenced the results.
Conclusions
In conclusion, based on the random-effects model, our investigation from the available RCTs or OSs does not provide adequate or exact proof that the average effect of PTX lowers the likelihood of urolithiasis among PHPT individuals. However, this finding must be regarded with caution, because the true variation in effects between studies could be attributable to uncharacterised or unexplained factors. Future research shall take into account the common effect of PTX as well as the prerequisites of preventive stone procedures, which will further help us assess the effectiveness of PTX in reducing kidney calculus comorbidity and develop techniques to avoid stone sequelae in these individuals.
Data availability
The datasets used and analyzed in this investigation are accessible from the corresponding author in response to a legitimate request.
References
Gasparri G (2017) Updates in primary hyperparathyroidism. Updates Surg 69(2):217–223
Zhu CY, Sturgeon C, Yeh MW (2020) Diagnosis and management of primary hyperparathyroidism. JAMA 323(12):1186–1187
Silverberg SJ et al (2014) Current issues in the presentation of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab 99(10):3580–3594
Rubin MR et al (2003) Raloxifene lowers serum calcium and markers of bone turnover in postmenopausal women with primary hyperparathyroidism. J Clin Endocrinol Metab 88(3):1174–1178
Nordin BE et al (2004) Effect of age on calcium absorption in postmenopausal women. Am J Clin Nutr 80(4):998–1002
Press DM, Siperstein AE, Berber E, Shin JJ, Metzger R, Monteiro R, Mino J, Swagel W, Mitchell JC (2013) The prevalence of undiagnosed and unrecognized primary hyperparathyroidism: a population-based analysis from the electronic medical record. Surgery 154:1232–1237. https://doi.org/10.1016/j.surg.2013.06.051. (discussion 1237-1238)
Yeh MW, Ituarte PH, Zhou HC et al (2013) Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab 98(3):1122–1129
Griebeler ML, Kearns AE, Ryu E, Hathcock MA, Melton LJ 3rd, Wermers RA (2015) Secular trends in the incidence of primary hyperparathyroidism over five decades (1965–2010). Bone 73:1–7
Minisola S, Arnold A, Belaya Z et al (2022) Epidemiology, pathophysiology, and genetics of primary hyperparathyroidism. J Bone Miner Res 37(11):2315–2329
Lundgren E, Hagström EG, Lundin J, Winnerbäck K, Roos J, Ljunghall S, Rastad J (2002) Primary hyperparathyroidism revisited in menopausal women with serum calcium in the upper normal range at population-based screening 8 years ago. World J Surg 26:931–936. https://doi.org/10.1007/s00268-002-6621-0
Yu N, Donnan PT, Murphy MJ, Leese GP (2009) Epidemiology of primary hyperparathyroidism in Tayside, Scotland. UK Clin Endocrinol (Oxf) 71(4):485–493
Collier A, Portelli M, Ghosh S, Nowell S, Clark D (2017) Primary hyperparathyroidism: Increasing prevalence, social deprivation, and surgery. Endocr Res 42(1):31–35
Soto-Pedre E, Newey PJ, Leese GP (2023) Stable incidence and increasing prevalence of primary hyperparathyroidism in a population-based study in Scotland. J Clin Endocrinol Metab 108:e1117
Yan ST, Tian H, Li CL et al (2007) A preliminary survey of primary hyperparathyroidism in middle-aged and elderly Beijing Chinese. Zhonghua Nei Ke Za Zhi 46(8):651–653
Wermers RA (2023) The Incidence of Primary Hyperparathyroidism in the Current Era: Have We Finally Reached a Steady State. J Clin Endocrinol Metab 108:e1749
Castellano E et al (2017) Sex Difference in the Clinical Presentation of Primary Hyperparathyroidism: Influence of Menopausal Status. J Clin Endocrinol Metab 102(11):4148–4152
Arya AK et al (2021) Differences in Primary Hyperparathyroidism Between Pre- and Postmenopausal Women in India. Endocr Pract 27(7):710–715
Clifton-Bligh PB, Nery ML, Supramaniam R et al (2015) Mortality associated with primary hyperparathyroidism. Bone 74:121–124
Lui MS, Fisher JC, Underwood HJ, Patel KN, Ogilvie JB (2022) Stones left unturned: Missed opportunities to diagnose primary hyperparathyroidism in patients with nephrolithiasis. Surgery 171(1):23–28
Haley WE, Enders FT, Vaughan LE, Mehta RA, Thoman ME, Vrtiska TJ, Krambeck AE, Lieske JC, Rule AD (2016) Kidney Function After the First Kidney Stone Event. Mayo Clin Proc 91:1744–1752. https://doi.org/10.1016/j.mayocp.2016.08.014
Serna J, Talwar R, Ziemba JB (2020) Health-related quality of life in renal stone formers: are we improving. Curr Opin Urol 30:190–195. https://doi.org/10.1097/MOU.0000000000000716
Khan AA, Hanley DA, Rizzoli R et al (2017) Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporos Int 28(1):1–19
Bilezikian JP, Brandi ML, Eastell R, Silverberg SJ, Udelsman R, Marcocci C, Potts JT Jr (2014) Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab 99:3561–3569. https://doi.org/10.1210/jc.2014-1413
Pak CY (1979) Effect of parathyroidectomy on crystallization of calcium salts in urine of patients with primary hyperparathyroidism. Invest Urol 17:146–148
Seib CD, Meng T, Suh I, Cisco RM, Lin DT, Morris AM, Trickey AW, Kebebew E (2021) Undertreatment of primary hyperparathyroidism in a privately insured US population: Decreasing utilization of parathyroidectomy despite expanding surgical guidelines. Surgery 169:87–93. https://doi.org/10.1016/j.surg.2020.04.066
Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP (1999) A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med 341:1249–1255. https://doi.org/10.1056/NEJM199910213411701
Mollerup CL, Vestergaard P, Frøkjaer VG, Mosekilde L, Christiansen P, Blichert-Toft M (2002) Risk of renal stone events in primary hyperparathyroidism before and after parathyroid surgery: controlled retrospective follow up study. BMJ 325:807. https://doi.org/10.1136/bmj.325.7368.807
Frøkjaer VG, Mollerup CL (2002) Primary hyperparathyroidism: renal calcium excretion in patients with and without renal stone sisease before and after parathyroidectomy. World J Surg 26:532–535. https://doi.org/10.1007/s00268-001-0262-6
Rowlands C et al (2013) Recurrent urolithiasis following parathyroidectomy for primary hyperparathyroidism. Ann R Coll Surg Engl 95(7):523–528
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Lu RJ, Zhu SM, Tang FL, Zhu XS, Fan ZD, Wang GL, Jiang YF, Zhang Y (2017) Effects of vitamin D or its analogues on the mortality of patients with chronic kidney disease: an updated systematic review and meta-analysis. Eur J Clin Nutr 71:683–693. https://doi.org/10.1038/ejcn.2017.59
Guo Y, Lv Y, Liu X, Wang G (2023) Association between heavy metal mercury in body fluids and tissues and diabetes mellitus: a systematic review and meta-analysis. Ann Transl Med 11:114. https://doi.org/10.21037/atm-22-6404
Hung KC et al (2022) Association of preoperative vitamin D deficiency with the risk of postoperative delirium and cognitive dysfunction: A meta-analysis. J Clin Anesth 79:110681
Lundstam K, Heck A, Mollerup C et al (2015) Effects of parathyroidectomy versus observation on the development of vertebral fractures in mild primary hyperparathyroidism. J Clin Endocrinol Metab 100(4):1359–1367
Pretorius M, Lundstam K, Heck A et al (2022) Mortality and Morbidity in Mild Primary Hyperparathyroidism: Results From a 10-Year Prospective Randomized Controlled Trial of Parathyroidectomy Versus Observation. Ann Intern Med 175(6):812–819
Rao DS, Wallace EA, Antonelli RF et al (2003) Forearm bone density in primary hyperparathyroidism: long-term follow-up with and without parathyroidectomy. Clin Endocrinol (Oxf) 58(3):348–354
Ambrogini E et al (2007) Surgery or surveillance for mild asymptomatic primary hyperparathyroidism: a prospective, randomized clinical trial. J Clin Endocrinol Metab 92(8):3114–3121
Rao DS, Phillips ER, Divine GW, Talpos GB (2004) Randomized controlled clinical trial of surgery versus no surgery in patients with mild asymptomatic primary hyperparathyroidism. J Clin Endocrinol Metab 89(11):5415–5422
Posen S, Clifton-Bligh P, Reeve TS, Wagstaffe C, Wilkinson M (1985) Is parathyroidectomy of benefit in primary hyperparathyroidism. Q J Med 54(215):241–251
Vestergaard P, Mosekilde L (2003) Cohort study on effects of parathyroid surgery on multiple outcomes in primary hyperparathyroidism. BMJ 327(7414):530–534
Seib CD et al (2021) Association of Parathyroidectomy With 5-Year Clinically Significant Kidney Stone Events in Patients With Primary Hyperparathyroidism. Endocr Pract 27(9):948–955
Seib CD, Ganesan C, Arnow KD et al (2022) Kidney Stone Events Following Parathyroidectomy vs Nonoperative Management for Primary Hyperparathyroidism. J Clin Endocrinol Metab 107(7):e2801–e2811
Huang SY et al (2022) Parathyroidectomy for nephrolithiasis in primary hyperparathyroidism: Beneficial but not a panacea. Surgery 171(1):29-34
Axelsson KF et al (2022) Analysis of Comorbidities, Clinical Outcomes, and Parathyroidectomy in Adults With Primary Hyperparathyroidism. JAMA Netw Open 5(6):e2215396
Rejnmark L, Vestergaard P, Mosekilde L (2011) Nephrolithiasis and renal calcifications in primary hyperparathyroidism. J Clin Endocrinol Metab 96(8):2377–2385
Bilezikian JP et al (2009) Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab 94(2):335–339
Mollerup CL, Lindewald H (1999) Renal stones and primary hyperparathyroidism: natural history of renal stone disease after successful parathyroidectomy. World J Surg 23(2):173–175 (discussion 176)
Acknowledgements
The authors would like to thank all authors of the included studies in our meta-analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
As the study was a meta-analysis based on the existing population-based studies, we did not apply for the approval of institutional review board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11255_2023_3882_MOESM1_ESM.tif
Supplementary file1 Risk of bias assessment of randomized controlled trials. PHPT, primary hyperparathyroidism (TIF 1024 KB)
11255_2023_3882_MOESM2_ESM.tif
Supplementary file2 Funnel plot for effect of PTX treatment versus medical treatment on risk of urolithiasis (TIF 727 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Shi, G., Li, G. et al. Systematic review of the risk of urolithiasis following parathyroidectomy in patients with primary hyperparathyroidism. Int Urol Nephrol 56, 1217–1225 (2024). https://doi.org/10.1007/s11255-023-03882-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03882-w