Abstract
Introduction
Primary hyperparathyroidism (PHPT) results in increased bone turnover, resulting in bone mineral density (BMD) reduction and a predisposition towards fractures. Parathyroidectomy (PTX) is the only definitive cure.
Objective
The primary goals of this study were to investigate the impact of PTX on BMD in patients with PHPT and to identify factors associated with post-operative BMD improvement using a multivariate model.
Methods
Between 1999 and 2010, a total of 757 patients underwent PTX for treatment of PHPT; 123 patients had both a pre- and a post-operative dual-energy X-ray absorptiometry (DEXA) scan. A prospective database was queried to obtain information about patient demographics, medications, comorbidities, and pre- and post-operative laboratory values. A Cox regression model was used to stratify patients and to identify factors that independently predict BMD response following PTX in this patient population.
Results
Overall, mean percent change in BMD was +12.31 % at the spine, +8.9 % at the femoral neck (FN), and +8.5 % at the hip, with a mean follow-up of 2.3 ± 1.5 years. A total of 101 (82.1 %) patients had BMD improvement at their worst pre-operative site. In patients who improved, 69.9 % (n = 86) had >5 % increase. Factors associated with BMD improvement at the worst pre-operative site were as follows: male gender (hazard ratio [HR] 2.29; 95 % confidence interval [CI] 1.54–4.21); pre-operative BMD with T-score less than −2.0 (HR 1.89; 95 % CI 1.11–2.39); age <55 years (HR 1.74; 95 % CI 1.14–2.25); BMD DEXA scan at >2.5 years post-operatively (HR 1.71; 95 % CI 1.09–2.17); history of previous fracture (HR 1.24; 95 % CI 1.05–1.92); and private insurance (HR 1.18; 95 % CI 1.06–2.1). The use of bisphosphonates, estrogens, vitamin D supplementation, or tobacco; obesity; history of previous PTX, serum calcium or parathyroid hormone levels were not independently associated with post-operative BMD improvement.
Conclusion
Osteoporosis is one of the established National Institutes of Health criteria for PTX in asymptomatic patients with PHPT, but BMD improvement is not consistently seen during the post-operative period. Gender, age, more severe pre-operative bone disease, and insurance status were all predictors for greater BMD improvement following PTX. Further studies with a rigorous post-operative BMD regimen are needed in order to validate these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary hyperparathyroidism (PHPT) results in increased bone turnover, resulting in a reduction in bone mineral density (BMD) and a predisposition towards fractures. PHPT affects up to 1 % of the adult population, overwhelmingly the most frequent being women [1]. After menopause, there is a decrease in estrogen levels, with resultant bone loss [2]. In PHPT, elevated parathyroid hormone (PTH) increases osteoclastic activity and leads to a further decrease in BMD, resulting in osteopenia and osteoporosis. Osteoporosis, defined by the World Health Organization as a reduction in BMD of −2.5 standard deviations below peak bone mass (T-score −2.5) and osteopenia (T-score between −1 and −2.5) are associated with increased fracture risk. There is a high correlation between BMD, bone health, and fracture risk. [3] BMD is routinely measured with dual-energy X-ray absorptiometry (DEXA); and, in patients with PHPT and mild hypercalcemia, DEXA is often used to select patients for parathyroidectomy (PTX). [4] Patients with PHPT who remain untreated experience an ongoing decrease in BMD. [5] PTX has been found to improve BMD, but this improvement is not universal and is inconsistent across different populations. [6] [7] The goal of this study was to assess improvement in BMD after PTX in PHPT and stratify the change in BMD based upon patient factors such as gender, race, age, and other comorbidities.
Methods
Between 1999 and 2009, a total of 757 patients underwent PTX for treatment of PHPT at Emory University Hospital, Atlanta, GA, USA; 374 patients had a preoperative DEXA within 2 years of surgery and 154 patients had both a pre-operative and a post-operative DEXA scan (range 9 months to 8.2 years). After Institutional Review Board (IRB) approval, a prospective database was queried to obtain information about patient demographics, medications, comorbidities, and pre-operative and post-operative laboratory values (Table 1). BMD was measured at lumbar spine, femur, and total hip and recorded in absolute g/cm2 and as T-scores. DEXA scans were performed on a Hologic QDR4500 (Hologic, Bedford, MA, USA) for 94 % of patients and Lunar Prodigy Advance (General Electric, Fairfield, CT, USA) for 6 % of patients. BMD comparisons were only performed if the patient had a postoperative DEXA on the same machine as the initial preoperative DEXA. Due to missing data, only 123 patients were included in the final analysis, and the frequency of analyzed variables is displayed in Table 2. An initial comparison was performed between the patients with only a pre-operative DEXA (n = 374) and patients who underwent both a pre-operative and post-operative DEXA (n = 123) (Table 3). Body mass index (BMI) was calculated in kg/m2, and glomerular filtration rate (GFR) was calculated using MDRD based upon age, race, gender, and serum creatinine. BMD improvement was classified as a >5 % improvement in absolute BMD, and it was examined at lumbar spine, total hip, and femur.
All continuous variables between the groups were analyzed by a t test, and categorical variables were analyzed by a Fisher exact test. A Cox regression model was used to stratify patients and to identify factors that independently predict BMD improvement of 5 % following PTX in this patient population. Significance was defined as p value < 0.05. Statistical analysis was carried out with SPSS Statistics 17.0 (IBM, Armonk, NY, USA) and SAS Version 9.2 for Windows (SAS Institute, Cary, NC, USA).
Results
PTX was successful in 99.2 % (n = 122) of patients, with a mean age of 57.9 ± 13.6 years. At follow-up (range 9 months–8.2 years), mean serum calcium was 9.2 ± 0.62 md/dL and PTH 52 ± 17 pg/mL (normal reference range 10–65 pg/mL). The patients receiving a postoperative DEXA were similar to patients receiving preoperative DEXA only in gender, race, BMI, GFR, BMD, serum calcium, serum PTH, and serum phosphorus. However, patients receiving a postoperative DEXA had a mean age of 57.9 ± 13.6 compared with patients who had a preoperative DEXA only (53.1 ± 16.2) (p value = 0.0214) (Table 3).
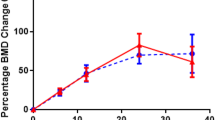
A total of 82.1 % (n = 101) of patients had an improvement in BMD; 12.2 % (n = 15) had 0–5 % improvement, 26.8 % (n = 33) had 5–10 % improvement, 23.6 % (n = 29) had 10–15 % improvement, and 19.5 % (n = 24) had >15 % improvement. The distributions in percent change in BMD from preoperative DEXA to postoperative DEXA at lumbar spine, femur, and total hip were −59 to 111 %, −53 to 158 %, and −20 to 74 %, respectively (Fig. 1). A total of 86.2 % (n = 106) of patients had a repeat BMD within 3 years of PTX; improvement in T-scores and absolute BMD was noted at years 1, 2, 3, 7, and 8 (Figs. 2 and 3). In the other years, there was either lack of improvement or the standard error of the mean was not substantive.
A linear regression model was used to compare the worst site of BMD in a patient with its respective change after PTX. Patients who presented with a lower BMD trended towards more improvement in BMD after PTX than patients who presented with a higher T-score (Fig. 4). This was observed in both T-score improvement and absolute BMD improvement. On univariate analysis, BMD improvement after PTX was associated with male gender; worst preoperative T-score; age <55 years; a DEXA performed >2.5 years after PTX; history of previous fracture; private insurance; use of bisphosphonates, estrogen agonists, and vitamin D supplementation. Tobacco use, BMI > 30, reoperative PTX, preoperative serum calcium, or PTH were not associated with BMD improvement. Based upon clinical value, due to missing data or a prevalence of <10 % in the dataset, the following variables were excluded from univariate analysis: chronic protein pump inhibitor (PPI) use, vitamin D deficiency, non-steroidal anti-inflammatory drug (NSAID) use, estrogen antagonist use, alcohol intake, steroid use, heart disease, and PTX failure. On multivariate analysis, BMD improvement was associated with male gender, worst preoperative T-score, age <55, a DEXA performed >2.5 years after PTX, history of previous fracture, and private insurance (Table 4).
Discussion
Bone density improves after PTX in >80 % of patients. In this study, we observed that bone density improvement is also associated with age, gender, severity of bone disease, and insurance status. This improvement was observed in spine, hip, and femoral head for up to 4 years post-PTX. Unique aspects of this study are the duration of follow-up (range 1–8 years) and the continued improvement in bone density for each year of follow-up.
Current NIH guidelines recommend PTX in “asymptomatic” PHPT patients when a T-score less than −2.5 [REF]. In this study, and based upon the multivariate analysis, patients with a T-score less than −2.5 had a larger improvement in BMD than those with T-score greater than −2.5. However, in patients with T-score greater than −2.5, there was also an observed improvement in T-score after PTX. Nearly 12 % of patients had no improvement in BMD when compared with their BMD on a DEXA prior to PTX. However, this does not take into account the bone loss that occurred between the time of BMD at initial DEXA and PTX; and BMD improvement could potentially be greater if the pre-operative DEXA was closer to the time of PTX. This also does not account for the bone loss that would have occurred if the patient was untreated for the duration between PTX and repeat DEXA. [8] Silverberg et al. [5] reported a substantial decrease in BMD in untreated “asymptomatic” patients with PHPT over a 10-year period. Therefore, patients may benefit from PTX even without BMD improvement with a cessation in bone deterioration.
Most studies that have reported an improvement in BMD after PTX have focused on post-menopausal women, as their risk of fracture is higher than that of other groups. [6] A unique feature of our study is that 25 % of our patients were men; and male gender was independently associated with BMD improvement (odds ratio [OR] 2.29; 95 % confidence interval [CI] 1.54–4.2.). Post-menopausal women were less likely to have an improvement in BMD. Ballem et al. [9] reported a greater improvement in BMD in males. However, there are conflicting reports in the literature. [10] Because estrogen plays a substantial role in BMD in women and its status can be a confounding variable, it is possible that men may be more likely to have a demonstrable improvement. However, in our study, 75 % of patients were female, and over 60 % of women also had an improvement in BMD. Physical activity and role of testosterone are two other factors that may account for differences, and that information was not available for analysis. Higher serum calcium, PTH, and urinary calcium levels were associated with improvement in BMD in a study by Dy et al. [10] In their cohort of 420 patients, 75 % had an improvement in BMD after PTX, with improvement more likely to be observed in patients with more severe disease. This observation was confirmed both by our multivariate analysis, T-score less than −2.0 was independently associated with improvement (OR 1.89; 95 % CI 1.11–2.39), and our linear regression analysis (Fig. 4) with correlation coefficient of 0.244. Thus, patients with severe bone disease are more likely to benefit in BMD improvement from PTX.
Although fracture risk after PTX was not an endpoint of analysis in this study, a previous history of fracture was associated with BMD improvement. This link could possibly be a marker of severity of disease and the benefit after PTX. In general, a decrease in BMD is associated with increased fracture risk. VanderWalde et al. [11] reported a 13 % decreased risk of fracture after PTX. However, only a few studies are dedicated to fracture risk and PTX. [12] With limited data, it is beyond the scope of this study to comment on post-operative fracture risk.
We have previously reported that private insurance plays a role in outcomes after PTX in secondary hyperparathyroidism in the dialysis population. [13] This is well supported in outcomes in dialysis, transplant, and vascular patients. The role of private insurance in disparity in outcomes after PTX is beyond the scope of the current analysis. However, it can be hypothesized that patients with private insurance are socioeconomically advantaged and healthier; and this may affect outcomes after PTX.
Nordenström et al. [14] reported upon an association between BMD improvement, pre-operative serum vitamin D levels, and GFR. They postulated that patients with a higher pre-operative serum vitamin D level may have increased bone turnover and thus have more ability to gain in bone mass. [14] In our study, neither pre-operative vitamin D level nor vitamin D supplementation at follow-up was associated with BMD improvement. A limitation of our study is that serum vitamin D levels were not available at follow-up for analysis. Other factors such as physical activity and routine exercise are known to affect BMD but were not available for analysis and are potential confounders. Another limitation of this study is that only 123 patients were analyzed when 757 patients underwent PTX. This adds the potential of selection bias. In order to address this issue, we compared patients with both pre-operative and post-operative DEXA with those patients with only a pre-operative DEXA (Table 2), and identified that patients who were older were more likely to have a post-operative DEXA. We can extrapolate that a younger cohort of patients was less likely to have a post-operative DEXA. From our study, a younger cohort is more likely to have a BMD improvement after PTX, thus we are potentially underestimating BMD improvement in a heterogeneous population. Low numbers of follow-up BMD information were available in years 5–8 after PTX; therefore, it is difficult to make conclusions for trajectory of BMD improvement after 4 years.
The cost of DEXA varies with institution and type of machine. The cost to a self-pay patient for a DEXA in 2006 was $US200 and therefore it does not accrue a prohibitive cost in the care of patients with PHPT. [15] Both pre-operative and post-operative DEXA are important in patients with PHPT. Although NIH criteria identify DEXA measurement of a T-score as a threshold in considering PTX in “asymptomatic” patients, we believe its greatest utility is in identifying patients for a more stringent follow-up with DEXA scan and adjunctive therapy to PTX. Based upon this analysis, a potential regimen for more intensive and earlier repeat DEXA scan can be instituted in patients with severe bone disease and those less likely to improve after PTX.
PTX is associated with BMD improvement in both severe and milder forms of PHPT, with >80 % of patients demonstrating improvement in a heterogeneous population. Age, gender, and severity of bone disease are potential factors that may predict BMD improvement. However, these parameters should not be used as a determinant to perform a PTX. PTX results in both biochemical and overall health improvement. [16] We interpret these data to support the rationale for PTX in patients with both osteoporosis and osteopenia. This information can also be used for improved counseling for the patients and to stratify consideration of additives to surgical interventions such as pharmacotherapy.
References
Bilezkian JP, Potts JT, Fuleihan GH, Kleerekoper M et al (2002) Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Clin Endocrinol Metab 87:5353–5361
Francucci CM, Ceccoli L, Caudarell R, Rilli S, Boscaro M (2010) Skeletal effect of natural early menopause. J Endocrinol Invest 33(7 Suppl):39–44
Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312(7041):1254–1259
Bilezikian JP, Potts JT Jr, Gel-H Fuleihan, Kleerekoper M, Neer R, Peacock M et al (2002) Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Clin Endocrinol Metab 87:5353–5361
Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP (1999) A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med 341:1249–1255
Christiansen P, Steiniche T, Brixen K, Hessov I, Melsen F, Heickendorff L, Mosekilde L (1999) Primary hyperparathyroidism: effect of parathyroidectomy on regional bone mineral density in Danish patients: a three-year follow-up study. Bone 25(5):589–595
Hagström E, Lundgren E, Mallmin H, Rastad J, Hellman P (2006) Positive effect of parathyroidectomy on bone mineral density in mild asymptomatic primary hyperparathyroidism. J Int Med 259(2):191–198
Silverberg SJ, Gartenberg F, Jacobs TP, Shane E, Siris E, Staron RB et al (1995) Longitudinal measurements of bone density and biochemical indices in untreated primary hyperparathyroidism. J Clin Endocrinol Metab 80:723–728
Ballem N, Greene AB, Parikh RT, Berber E, Siperstein A, Milas M (2008) Appreciation of osteoporosis among men with hyperparathyroidism. Endocr Pract 14(7):820–831
Dy B, Grant CS, Wermers RA, Kearns AE, Huebner M, Harmsen WS, Thompson GB, Farley DR, Richard ML (2012) Changes in bone mineral density after surgical intervention for primary hyperparathyroidism. Surgery 152:1051–1058
VanderWalde LH, Liu I, Haigh PI (2009) Effect of bone mineral density and parathyroidectomy on fracture risk in primary hyperparathyroidism. World J Surg 33(3):406–411. doi:10.1007/s00268-008-9720-8
Khosla S, Melton RA, Crowson C, O’Fallon WM, Riggs LB (1999) Primary hyperparathyroidism and the risk of fracture: a population-based study. J Bone Miner Res 14(10):1700–1707
Sharma J, Raggi P, Kutner N, Bailey J, Zhang R, Huang Y, Herzog CA, Weber C (2012) Improved long-term survival of dialysis patients after near-total parathyroidectomy. J Am Coll Surg 214(4):400–407
Nordenström E, Westerdahl J, Bergenfelz A (2004) Recovery of bone mineral density in 126 patients after surgery for primary hyperparathyroidism. World J Surg 28(4):502–507. doi:10.1007/s00268-004-7274-y
The Lewin Group, Inc (2007) Assessing the costs of DXA services in the office-based setting. Final report. Available from: http://www.aace.com/advocacy/leg/pdfs/DXExecutiveSummary.pdf Accessed 5 Sep 2013]
Heyliger A, Tangpricha V, Weber C, Sharma J (2009) Parathyroidectomy decreases systolic and diastolic blood pressure in hypertensive patients with primary hyperparathyroidism. Surgery 146(6):1042–1047
Acknowledgments
The authors would like to thank Roberto Pacifici, MD and David B. Arkin, MD from the Department of Endocrinology at Emory University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, J., Itum, D.S., Moss, L. et al. Predictors of Bone Mineral Density Improvement in Patients Undergoing Parathyroidectomy for Primary Hyperparathyroidism. World J Surg 38, 1268–1273 (2014). https://doi.org/10.1007/s00268-014-2555-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-014-2555-6