Abstract
CNS disorders are on the rise despite advancements in our understanding of their pathophysiological mechanisms. A major hurdle to the treatment of these disorders is the blood–brain barrier (BBB), which serves as an arduous janitor to protect the brain. Many drugs are being discovered for CNS disorders, which, however fail to enter the market because of their inability to cross the BBB. This is a pronounced challenge for the pharmaceutical fraternity. Hence, in addition to the discovery of novel entities and drug candidates, scientists are also developing new formulations of existing drugs for brain targeting. Several approaches have been investigated to allow therapeutics to cross the BBB. As the molecular structure of the BBB is better elucidated, several key approaches for brain targeting include physiological transport mechanisms such as adsorptive-mediated transcytosis, inhibition of active efflux pumps, receptor-mediated transport, cell-mediated endocytosis, and the use of peptide vectors. Drug-delivery approaches comprise delivery from microspheres, biodegradable wafers, and colloidal drug-carrier systems (e.g., liposomes, nanoparticles, nanogels, dendrimers, micelles, nanoemulsions, polymersomes, exosomes, and quantum dots). The current review discusses the latest advancements in these approaches, with a major focus on articles published in 2015 and 2016. In addition, we also cover the alternative delivery routes, such as intranasal and convection-enhanced diffusion methods, and disruption of the BBB for brain targeting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The blood–brain barrier is a physiological gatekeeper protecting the brain against toxins and harmful chemicals. |
Understanding the pathophysiological mechanisms involved in alterations to the blood–brain barrier during CNS disorders has led to the delivery of drugs by exploiting physiological transport systems that offer advantages in improved brain targeting. |
Innovations in nanotechnological tools, combined with physiological transport mechanism-based approaches, have generated promising formulations for CNS diseases. |
1 Introduction
The blood–brain barrier (BBB) is responsible for physiologically protecting the brain from exposure to toxins and ill effects. However, this physiological protective function of the BBB presents a key challenge to the pharmaceutical fraternity, with a need to circumvent it so as to deliver drugs to the brain in various CNS disorders.
The BBB is unique in nature because it has selective permeability (which also changes in some diseased conditions). The BBB is a complex system made up of a structurally distinct, continuous endothelial cell layer separating the blood from the extracellular fluid of the brain (Fig. 1). The luminal plasma membrane of the endothelial cells is directed towards the blood, while the abluminal plasma membrane faces the brain [1–4]. The presence of adhesion molecules and tight junctions between endothelial cells [5] and the low density of pinocytes [6] are some of the key structural features that make the BBB selectively permeable. Further, as age advances, the chances of the development of CNS disorders proportionately increases and the selective permeability of drugs aggravates the issues related to drug delivery and brain targeting. With advancing age, the BBB starts altering and contributes to neurodegenerative diseases [7]. Moreover, there is a decline in several processes related to functioning of the substantia nigra, resulting in an increased chance of developing Parkinson’s disease [8].
Advancements in technological tools and biomedical sciences have led to better understanding of not only the detailed structure of the BBB but also the pathophysiological mechanisms of CNS disorders. Moreover, there has been tremendous research in the field of nanomedicine-based drugs. Despite these, the distress caused by several CNS-related diseases is increasing [9, 10]. As per the Alzheimer’s Association, “Alzheimer’s disease is the 6th leading cause of death in United States” [11]. As per the Parkinson’s Disease Foundation, “more than 10 million people worldwide are living with Parkinson’s disease” [12]. Moreover, “the number of new cases of brain and other nervous system cancers was 6.4 per 100,000 men and women per year and the number of deaths was 4.3 per 100,000 men and women per year” as per the National Cancer Institute [13]. Additionally, other CNS disorders such as stroke, encephalopathy, meningitis, Huntington’s chorea, epilepsy, and psychological disorders also present a huge social and economic burden.
Drug discoveries continue to be made in plentiful numbers but even though these novel therapeutic molecules give good results in the early phase of drug development, they often fail to successfully clear subsequent clinical trials, owing in part to their inability to cross the BBB. The 2014 statistics of top global prescription drugs indicate that, out of the top ten molecules, only one drug is a CNS therapeutic; additionally, among the top ten global therapy areas in terms of sales, only two CNS therapy areas, i.e., pain- and mental health-related products, appear [14]. Considering these data, it appears that the CNS market is underperforming and the major reason for this is the inability of drugs to cross the BBB. However, this also provides the opportunity for researchers to develop novel formulations for CNS disorders. An overview of novel CNS drugs under clinical trials is depicted in Table 1 and a summary about newly approved drugs/formulations for CNS disorders is given in Table 2. We have previously reviewed several approaches for brain targeting [1]. In the current review, we outline the latest advancements in these approaches for brain targeting.
2 Physiological Transport Mechanisms
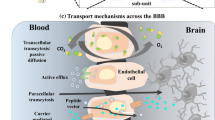
There are several transport mechanisms by which solutes/substances are physiologically conveyed across the BBB (see Fig. 2). Water-soluble agents are transported by simple diffusion through paracellular pathways. Small lipid-soluble agents such as corticosteroids and alcohol cross the BBB through transcellular routes. In addition to these, adsorptive-mediated transcytosis (AMT), receptor-mediated transport (RMT), inhibition of efflux pumps, cell-mediated endocytosis, and use of peptide vectors are some other mechanisms for transport across the BBB [1, 9, 47]. The details of these mechanisms being employed for targeting the brain are described in the upcoming section.
2.1 Adsorptive-Mediated Transcytosis
AMT involves electrostatic binding of polycationic peptides or proteins, acting as ligands, with micro-anionic moieties present at the luminal surface of the endothelial cells. Although AMT has lower binding affinities than RMT, it allows for higher binding capacities. Cationized albumin is an example of such a ligand, which was reported to be conjugated with deferasirox, an iron-chelating agent, for Alzheimer’s disease. In Alzheimer’s disease, iron plays an important role in oxidative stress and protein degradation, resulting into neuronal damage. Using in-vitro and in-vivo studies, the authors confirmed that such a system produced neuronal protection and a reduction in amyloid β-induced learning deficits, indicating efficient delivery of drugs to the brain via AMT [48]. Similarly, cationic bovine serum albumin was developed as an important ligand for preparing polyethylene glycol (PEG)-polylactic acid nanoparticles, which showed enhanced brain transcytosis across the BBB co-culture [49]. In another study, cationic bovine serum albumin was conjugated to engineered solid lipid nanoparticles (SLNs) containing the anti-cancer agent doxorubicin, depicting maximum transcytosis of the developed system to HNGC-1 cell lines and improved pharmacokinetics using the in-vivo system [50].
GM1 is a glycosphingolipid that is ubiquitously present on the endothelial surface and capable of acting as the transcytotic receptor for cholera toxin B. Ganglioside GM1-targeted polymersomes undergo transcytosis in brain endothelial cells, which represents an impending strategy for the treatment of brain-related disorders [51]. The delivery of ciprofloxacin as a model antibiotic was enhanced by conjugating cholesteryl chloroformate to Tat (amino acid sequence of the peptide: 49-57, YGRKKRRQRRR) peptide, which undergo AMT [52, 53]. A dual approach of receptor-mediated endocytosis along with AMT was adopted by Chen et al. [54, 55] by preparing lactoferrin-modified procationic liposomes of doxorubicin for glioma. Another such dual approach was adopted for the preparation of cationic albumin and mannose-modified-albumin conjugated doxorubicin nanoparticles for brain cancer. This system resulted in a reduction in tumor size in an orthotopic glioma model in rats [56].
2.2 Active Efflux Transport
The brain endothelial cells express several ATP-driven drug efflux pumps, such as multi-drug-resistant proteins and P-glycoprotein (P-gp), which are responsible for transporting drugs out of the brain to the blood. Thus, they serve as one of the barriers for brain targeting. Many multi-drug-resistant proteins can efflux out cationic, amphiphilic, as well as neutral compounds while P-gp is responsible for the efflux of lipophilic compounds [9, 57]. Thus, inhibition of these efflux pumps can serve as an important approach for brain targeting.
GNE-317 and GDC-0980 are two phosphatidylinositol 3-kinase PI3K/mechanistic target of rapamycin inhibitors that also inhibit efflux pumps, e.g., P-gp and breast cancer resistance protein (Bcrp), in different capacities. In an animal model of C57B6/J mice bearing intracranial GL261 tumors, there was a threefold greater penetration of GNE-317 compared with GDC-0980 in the brain, which makes them important in the treatment of invasive glioma [58].
Fingolimod (FTY720), a prodrug approved for multiple sclerosis, converts to an active phosphorylated compound that serves as an agonist at the sphingosine-1-phosphate receptor. Both sphingosine-1-phosphate and fingolimod are reported to inhibit P-gp [59]. Verapamil, paclitaxel, and loperamide are known substrates for P-gp and hence do not penetrate into the brain. When sphingosine-1-phosphate or fingolimod was co-administered, an increase in the brain uptake of radiolabeled verapamil, paclitaxel, and loperamide occurred [59]. This implies that co-administration of fingolimod or sphingosine-1-phosphate along with the drugs can lead to enhanced permeability of drugs to the brain.
Inhibition of P-gp as a strategy is also explored for the treatment of gliomas. Cediranib is a vascular endothelial growth factor receptor inhibitor being investigated for the treatment of a wide range of cancers including glioblastoma multiforme. Cediranib is a substrate for P-gp and hence its penetration across the BBB is limited. Concomitant administration of P-gp inhibitors LY335979 and the dual P-gp/Bcrp inhibitor GF120918 along with cediranib resulted in significantly increased brain concentrations in mice [60]. Similarly, concurrent administration of the dual P-gp/Bcrp inhibitor elacridar with sorafenib (a multi-targeted tyrosine kinase inhibitor being investigated for glioma) increased the penetration into the brain [61].
Pluronic® P85, a Pluronic® block copolymer, is one of the most widely studied P-gp inhibitors for its ability to enhance delivery of numerous anti-cancer agents such as etoposide, doxorubicin, and other drugs such as loperamide, digoxin, and valproic acid [62, 63]. Additionally, Pluronic® P85 inhibits multi-drug-resistant proteins MRP1 and MRP2, although the inhibition is less than at P-gp [63]. The penetration of a model drug rhodamine 123 was increased when Pluronic® P85 was used at a concentration less than the critical micelle concentration, but not when Pluronic® P85 was used at higher concentrations [64]. In addition to its P-pg-inhibiting property, Pluronic® P85 is also useful as a vehicle for drug delivery [65]. Moreover, Pluronic® block copolymers are reported to chemosensitize multi-drug-resistant tumors and increase the activity of doxorubicin by increasing pro-apoptotic signaling in cancer cells [66].
2.3 Carrier-Mediated Transport
Certain endogenous substances of our body such as glucose, vitamins, amino acids, and neuropeptides are transported into the brain via specific carriers present at the BBB. Certain drugs for which brain targeting is required are chemically modified so that their structure resembles the endogenous substance of the body and thereby they are being transported by the specific carrier via carrier-mediated transport.
One of the simplest and classic examples is converting dopamine to levodopa for the treatment of Parkinson’s disease. Dopamine cannot cross the BBB; however, levodopa is transported by a large neutral amino acid transporter [67].
Many solute carriers (SLC) such as SLC2, SLC7, and SLC16 are present on the BBB. Glucose transporters (GLUT) form a part of the SLC subgroups. Out of total glucose transporters located on BBB, GLUT1 is localized to both luminal and abluminal membranes of the BBB endothelial cells with the ratio of 1:4 respectively [68]. Antiepileptic prodrugs nipecotic acid and 7-chloronokynurenic acid were developed and they were transported by GLUT1 [69, 70]. Despite these, this approach has the limitation of only being able to transport the agents that are structurally similar to the endogenous ligands because they will only be transported by endogenous carriers. Moreover, even the size of the drug molecules should be similar to the endogenous ligands as the carriers are present on the cell membrane [71]. To overcome these limitations, designing peptide carriers is being trialed. K16apolipoprotein E (ApoE), a synthetic peptide carrier, was designed and synthesized. This carrier has a specific property of altering the permeability of the BBB temporarily. K16ApoE was pre-mixed with cetuximab and following this, seven different small molecule chemotherapeutic agents were injected. Use of K16ApoE as a carrier increased penetration across the BBB ranging from 34- to 92-fold for different drugs [72].
2.4 Receptor-Mediated Transport
In several diseased conditions, there is upregulation of specific receptors. RMT exploits this property for the active targeting of drugs. A prepared formulation is tagged with a ligand that is specific to the upregulated receptor, causing it to specifically bind to the receptor. Once ligand receptor binding occurs, receptor-mediated endocytosis takes place. This is followed by movement of the drug-receptor complex within the endothelial cytoplasm and finally exocytosis of the drug onto the abluminal surface. Several enzymes, lysosomes, and endosomes come into contact with the drug-receptor complex during the transit within the endothelial cytoplasm, which may or may not degrade the candidate molecule/drugs. Hence, a formulation that is stable in the presence of such enzymes and is not affected by lysosomes needs to be developed [9].
Recently, advancements in biomedical sciences and technological tools have led to a better and deeper understanding of the pathophysiology of the diseases. This has increased our knowledge regarding the upregulation of several receptors that can be targeted for the delivery of drugs across the BBB. The upcoming sections give an overview of some recently developed formulations with respect to the RMT approach.
2.4.1 Transferrin Receptors
Transferrin receptors are the most widely studied receptors for brain delivery [73]. As mentioned in the previous section, endosomal degradation of the drug-receptor complex can occur in the endothelial cytoplasm. Initially, it was understood that transferrin-transferrin receptor complexes are stable and do not undergo endosomal degradation and thus are easily transported towards the abluminal surface [74–76]. Some studies reported that anti-transferrin receptor antibodies clearly crossed the BBB and reached the brain tissue in higher concentrations than non-specific immunoglobulins [77, 78].
However, other studies have since indicated that the transport of antibodies was constricted to brain endothelial cells only, suggesting that for antibody-like OX26, RMT was not capable of providing therapeutic concentrations of the drug across the BBB [79, 80]. Additionally, it was also reported that gold nanoparticles bound with 8D3 anti-transferrin receptor antibodies when administered intravenously in mice underwent RMT. However, most of the vesicles underwent endosomal degradation and only a few could reach the abluminal membrane [81].
To overcome this drawback, newer approaches that modified the antibody-binding strength have been used so as to mimic endogenous transferrin, so that brain uptake of the antibodies can occur [82–84]. Such a strategy was employed by Clark and Davis in which they prepared gold nanoparticles with a transferrin ligand. However, they used an acid-cleavable linkage between the transferring and nanoparticle core, which resulted in escape from endosomal degradation and increased transport to the parenchymal region [85].
Kang et al. [86] encapsulated dopamine into PEGylated liposomes that was then conjugated to the anti-transferrin receptor monoclonal antibody, OX26. Using a rat model of Parkinson’s disease, they found an eightfold increase in dopamine concentration in the brain tissue.
Transferrin receptor-mediated targeting for efficient delivery of small interfering RNA (siRNA) in glioma has also been reported. Layer-by-layer assembling of protamine/chondroitin sulfate/siRNA/cationic liposomes modified at the T7 peptide led to effective penetration of the nanoparticles into the core of the tumor when evaluated using U87 glioma cells and in-vivo imaging studies [87]. In another study for delivery of siRNA, a dual approach of RMT and cell-penetrating peptide was used. The authors prepared conjugated transferring peptide with transportan and myristic acid, which underwent transferrin RMT in glioma cell lines [88]. The detailed review of the transferrin-mediated brain targeting is reported elsewhere [73].
2.4.2 Folate Receptors
Folate receptor-mediated endocytosis and brain targeting is another approach with an advantage of not undergoing endosomal degradation, if prepared skillfully. Micelle-like nanoparticles for delivery of cytochrome c protein using redox-sensitive bonds between nanoparticles and folic acid resulted in efficient brain targeting as depicted in in-vitro and in-vivo brain tumor studies [89]. In another approach, elongated cellulose nanocrystals decorated with folic acid for targeted delivery of an anti-cancer agent were developed. The system underwent RMT as shown in cellular binding/uptake studies in human and rat brain tumor cells [90].
2.4.3 Lipoprotein Receptor-Related Protein
Alzheimer’s disease is associated with formation of the amyloid precursor protein and alterations in alpha-2-macroglobulin and apolipoproteins. Lipoprotein receptor-related proteins (LRPs) are signaling receptors serving as receptors for these three peptides. Thus, they play a role in the pathogenesis of Alzheimer’s disease. Moreover, these receptors are upregulated in malignant astrocytomas, including glioblastoma cells [91]. Paclitaxel conjugated with angiopep-2, a ligand for the LRP receptor, depicted to undergo receptor-mediated endocytosis, exerted beneficial effects in clinical trials of cancer patients. The formulation was also safe and well tolerated by the patients [92].
2.4.4 Scavenging Receptors
Scavenging receptors (SRs) are transmembrane glycoproteins serving as targets for modified forms of low-density lipoprotein, i.e., oxidized and acetylated low-density lipoprotein [93]. Out of several types of SRs, SRA1 and SRB1 are expressed on brain capillary endothelial cells [94, 95]. Peptide vector, PepFect 32, was decorated with angiopep-2 ligand for the delivery of plasmid DNA. Initially, authors speculated that because angiopep-2 is a ligand for LRP receptors, it undergoes RMT via LRP receptors [96]. However, when they carried out in-vitro assays, they confirmed that it undergoes RMT via both LRP receptors as well as class A and B SR [97].
2.4.5 Interleukin-13 Receptor α2
Interleukin-13 receptor α2 (IL-13Rα2) is one of the key receptors of the family of IL-13 cytokine receptors with no signaling functions [98]. IL-13Rα2 is the specific subtype that is up-regulated in glioblastoma multiforme and undergoes internalization upon being activated by a ligand [99]. Pep-1 (CGEMGWVRC) peptide is reported to deliver drugs across the BBB via IL-13Rα2-mediated endocytosis [100]. This work was further extended by developing copolymer nanoparticles conjugated with pep-1 for delivery of paclitaxel [101]. In-vitro studies with C6 glioma cells along with qualitative and quantitative in-vivo studies in the intracranial glioma-bearing mice model showed enhanced penetration of paclitaxel for glioma treatment [101]. In another dual targeting approach, polymeric nanoparticles were attached with arginylglycylaspartic acid (RGD) and IL-13 peptide. RGD serves as a ligand to the αvβ3 integrin receptor on the neovasculature and the IL-13 peptide serves as a ligand to IL-13Rα2. Such dual targeting not only results in internalization of the nanoparticles to a large extent but also results in clathrin-mediated endocytosis of the IL-13 peptide instead of macropinocytosis [102]. This is an example of using two ligands for two receptors.
Another tactic in this direction that has been recently developed is the use of single peptides serving as ligands at two receptors. dGR, which is the opposite sequence to RGD, was conjugated with octa-arginine. Octa-arginine is a cell-penetrating peptide and this conjugation forms a tandem peptide that serves as a ligand for both integrin αvβ3 and neuropilin-1 receptors. This tandem peptide was prepared into liposomes and encumbered with paclitaxel which showed an excellent anti-proliferative effect on tumor and cancer stem cells. This was additionally confirmed in the C6-bearing mice model in which there was an extensive anti-glioma effect [103].
2.4.6 Insulin Receptors
Insulin receptors belong to the family of tyrosine kinase-linked receptors and are responsible for the transport of insulin across the BBB [104]. During Alzheimer’s disease, amyloid β peptides compete with insulin for binding with insulin receptors leading to an impairment of glucose utilization in the brain [105]. Anti-human insulin receptor antibody 83-14 was conjugated with amphiphilic diblock copolymer poly(dimethylsiloxane)-block-poly(2-methyl-2-oxazoline) self-assembling polymersomes. The in-vitro studies using the hCMEC/D3 cell line revealed a specific uptake of the polymersomes and thereby a good targeting [106]. Despite this, as insulin receptors are involved in maintaining glucose homeostasis, targeting the insulin receptor is considered an unsafe approach.
2.4.7 Glutamate Receptors
Type 1 metabotropic glutamate receptors, a G-protein-coupled receptor, are implicated in several diseases such as epilepsy, anxiety, drug addiction, and pain. A bi-specific antibody comprising the IgG antibody against the type 1 glutamate receptor and a second antibody derived from the camelid single-domain antibody, which targets BBB transmigration, were developed. These antibodies exhibited good penetration and inhibition of thermal algesia [107].
2.5 Peptide Vector Strategies
As an auxiliary to the RMT approach, another approach of using peptides with cell-piercing properties is being investigated. In this approach, small peptides are conjugated with the drug and these peptides serve as vectors for transporting drugs with less elucidated mechanisms [108]. Böckenhoff et al. [109] designed and carried out a comparative study of five peptide vectors for the treatment of lysosomal storage disorders. They used lysosomal enzyme arylsulfatase A as an enzyme replacement therapy that was conjugated to five different vectors, e.g., Apo B, human immunodeficiency virus tat protein, Apo E-I, Apo E-II, and angiopep peptide. Out of these five vectors, Apo E-I and Apo E-II were found to be outstanding and showed the best results [109]. As mentioned in the previous section, Angiopep-2 serves as a ligand for RMT. A new molecule was established by conjugating angiopep-2 with an anti-HER2 monoclonal antibody for mammary gland cancer. Using the HER2-positive BT-474 cell line and BT-474 xenograft mice models, the authors reported an increased uptake of the molecule, which could be attributed to the increased penetrating capacity of the peptide vector [110].
The above-mentioned peptide vectors have directly been conjugated to the drug candidate/enzyme/peptide. However, the peptide vector strategy is not only limited to this. It has also been investigated for developing nanoparticles for the treatment of Alzheimer’s disease. The B6 peptide was conjugated with sialic acid-altered selenium nanoparticles. The authors carried out a battery of assays using an inductively coupled plasma-atomic emission spectroscope, confocal microscope, the transwell experiment, and a flow cytometer to determine transport efficiency in in-vitro models. The B6 peptide conjugation proved to be an encouraging strategy for enhanced brain targeting [111].
2.6 Cell-Mediated Transport
Transport of drugs using the ‘cells’ of the body itself is the policy of cell-mediated transport. These ‘cells’ of the body are specific cells such as macrophages, monocytes, and neutrophils, which are recruited during the inflammatory phase of any brain disorders and have good ability to commute through the circulation and reach the site of inflammation. These cells serve as ‘Trojan horses’ and freight the drug molecules across the BBB. Earlier studies report the use of hematopoietic stem cells for recruiting circulating monocytes to the CNS in lethally irradiated animals. However, this is not commercially viable as the use of irradiation leads to several lethal implications [112–114].
Recently, monocytes and monocyte-derived macrophages have been used. Out of these two, monocytes were better in homing ability. The authors also validated the study by investigating the ability of monocytes to ferry the superparamagnetic iron oxide nanoparticle SHP30 in the inflamed brain region, which showed good penetration [115]. Several other such reports are reviewed by Batrakova et al. [116]. Cell-mediated transport is associated with major drawbacks, e.g., poor drug-loading capacities, premature release of the loaded carriage, and inability of the cargo to selectively reach the site of action [116].
3 Drug-Delivery Approaches
3.1 Drug Delivery from Microspheres
Microspheres are small spherical solid particles, with diameters in the micrometer range, typically 1–1000 µm. The drug is either entrapped, dissolved, encapsulated, or attached to the polymeric matrix. They can be prepared by using various natural, semi-synthetic, and synthetic materials.
Activation of glucagon-like peptide-1 receptors in the brain provide a neuroprotective action. Chien et al. [117] developed long-lasting exendin-4 (glucagon-like peptide-1 analog, with a short half-life)-loaded poly(d,l-lactide-co-glycolide) (PLGA) microspheres for exploring its neuroprotective potential against cerebral ischemia in diabetic rats. The results of the study revealed that compared with a plain drug, drug-loaded PLGA microspheres showed a sustained higher level of exendin-4 in plasma and cerebrospinal fluid (CSF) for at least 2 weeks. This could be attributed to the gradual degradation of PLGA microspheres, which leads to improved diabetes mellitus-induced glycemia after a single subcutaneous administration.
In another study, Vera et al. [118] developed both micro- and nano-particulate drug-delivery systems for celecoxib for implantation in the brain (after partial/complete removal of the tumor) and crossing the BBB, respectively. In-vitro cell culture studies performed using SKN-AS and U373-MG cell lines revealed an anti-proliferative action of celecoxib microspheres, with half-maximal effective concentration values of 82.4 and 99.81 µM in SKN-AS and U373-MG cells, respectively. Further, administration of nanoparticles (coated with a surface active agent, polysorbate 80) to rats showed a significant increase in fluorescence imaging of the cerebral cortex region, depicting the potential of the developed system for brain targeting.
Recently, a new tyrosine kinase (anti-angiogenic) inhibitor, AZD2171, related to vascular endothelial growth factor signaling has shown tremendous results in the treatment of glioblastoma. The major drawbacks associated with AZD2171 were its low permeability through the BBB, high toxicity, and short half-life. To overcome these drawbacks, Shivinsky et al. [119] developed a modified PLGA microsphere for the treatment of glioblastoma. The results of the study revealed that the release of AZD2171 from the developed microspheres was as effective as a free drug in terms of inhibiting proliferation (in vitro) and endothelial growth. It also had a significant cytotoxic effect against glioblastoma cell lines (U87 and A172). Further, a complete tumor inhibition was achieved following a single treatment with AZD2171-loaded PLGA microspheres (6 mg/kg) administered locally adjacent to human U87 glioma tumors inoculated subcutaneously in nude mice. Although, the authors proposed that the developed system can be used for brain targeting, they have not used an orthotropic model of disease. Thus, further studies are required to provide the proof of concept for achieving brain targeting.
“Drug encapsulated aerosolized microspheres as a biodegradable, intelligent glioma therapy (DREAM BIG)” was developed by Floyd et al. [120] DREAM BIG delivers three chemotherapeutic agents directly to the brain surgical site by employing aerosolized bioadhesive and biodegradable microspheres after tumor excision. It comprises rhodamine B-loaded PLGA and immunoglobulin G-loaded polylactic acid microspheres. The ex-vivo application of developed systems on brain tissues showed encouraging results, demonstrating the potential of DREAM BIG therapy for the treatment of malignant gliomas.
3.2 Drug Delivery from Biodegradable Wafers
Gliadel® wafer (BCNU-PCPP:SA polymer) (BCNU, 1,3-bis(2-chloroethyl)-1-nitrosourea, Carmustine; Arbor Pharmaceuticals, LLC, Atlanta, GA, USA), is the only approved polymeric system by the US Food and Drug Administration for the treatment of brain tumors [1, 121, 122]. It offers three distinct features that are unlike other drug-delivery systems: (1) it delivers a high drug load locally to the tumor site; (2) it releases the drug in a sustained manner over prolonged periods of time; and (3) it is biodegradable in nature [123]. The Gliadel® wafer is not recommended for patients allergic to carmustine or any of the components of the Gliadel® wafer. If the patient is undergoing surgery for malignant glioma and implantation of the Gliadel® wafer, they should be monitored closely for known complications, including convulsions, infections, abnormal wound healing, and swelling of the brain. If not implanted properly, the Gliadel® wafer could block the flow of CSF and might cause abnormal accumulation of fluid in the brain (obstructive hydrocephalus). The Gliadel® wafer is not recommended in pregnant women as carmustine (active component) can cause harm to the fetus [124].
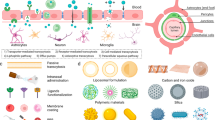
3.3 Drug Delivery from Colloidal Drug-Carrier Systems
Colloidal drug carriers include nanosized drug-carrier systems, e.g., liposomes, nanoparticles (polymeric, lipid and inorganic nanoparticles), nanogels, dendrimers, micelles, nanoemulsions, polymersomes, exosomes, and quantum dots. These carriers usually are in the nanometer range (1–1000 nm). As the minimum diameter of blood vessels is in the range of 6–9 µm, the nanocarriers could be well targeted to the organs via the blood stream. Further, surface functionalization ability using a targeting ligand provides an additional edge to these carriers to transport the drug across the BBB [125].
3.3.1 Liposomes
Liposomes are vesicular drug-delivery systems consisting of an aqueous inner core enclosed by uni-/multi-lamellar phospholipid bilayers. The phospholipid bilayers consist of naturally produced phosphatidylcholine, sphingomyelin, or glycerophospholipids. Liposomes can accommodate both hydrophobic and hydrophilic drugs in their phospholipid bilayers and aqueous core, respectively, thus making it a drug-delivery system of choice for systemic delivery of various therapeutics [125].
Liposomes are reported to improve the delivery of drugs through the BBB in a significant manner [126–130]. Chen et al. [131] developed a dual-targeted liposomal delivery system by using both a cell-penetrating peptide (TAT) and a specific targeting ligand (transferrin) for the treatment of brain glioma. They also evaluated the combination of synergistic chemotherapeutic agents, e.g., doxorubicin and paclitaxel. In-vitro, three-dimensional, tumor spheroid penetration assays and cellular uptake studies proved that liposomes conjugated with transferrin and the cell-penetrating peptide TAT (TF/TAT-LP) could effectively target brain endothelial and carcinoma cells with deep penetration through the endothelial monolayers. Further, an in-vivo imaging study proved that TF/TAT-LP possesses the highest tumor distribution property, which was further confirmed by fluorescent images of the brain section [131]. The results of the study revealed that the doxorubicin- and paclitaxel-loaded TF/TAT-LP (TF/TAT-PTX/DOX-LP) shows the best anti-glioma effect with improvement of glioma bearing survival time, compared with doxorubicin and paclitaxel when used separately.
In another study, sertraline (antidepressant)-loaded pegylated and glycosylated liposomes for brain targeting were developed by Harbi et al. [132]. The results of liposomal transport through mouse brain endothelial polyoma cells demonstrated greater capacity of the glycosylated liposomes to target the cerebellar than pegylated liposomes, owing to their higher density of GLUT1 and higher glucose utilization. This transport capacity of liposomes was confirmed further by the inhibitory action of both phenobarbital and cytochalasin B [132]. In-vitro studies of time-lapse live-cell imaging and flow cytometry using a C6 glioma cell model, and in-vivo near-infrared fluorescence imaging demonstrated that the developed glycosylated liposomes can be transported through the BBB by classical endocytosis, as well as by a specific transcytosis process [132].
Drug delivery to the brain offers various barrier systems such as enzymes present in the blood and brain capillary endothelial cells, BBB, and the blood–brain–tumor barrier (BBTB). To overcome these barriers Wei et al. [133] developed a surface-modified liposomal drug delivery system. Proteolytically stable peptides, (D)CDX (a D-peptide ligand of nicotine acetylcholine receptors on the BBB) and c(RGDyK) [a ligand of integrin, highly expressed on the BBTB and glioma cells], were evaluated for surface modifications of liposomes. The in-vitro cell uptake studies revealed that the developed dually labeled liposomes could target both tumor cells as well as brain capillary endothelial cells, and effectively traverse the BBTB and BBB monolayers, thus overcoming the enzymatic barrier and targeting three-dimensional tumor spheroids [133]. Its ability to target intracranial glioma was further verified in vivo by performing ex-vivo imaging and histological studies [133]. The results of the studies revealed that both (D)CDX- and c(RGDyK)-modified liposomes presented a better anti-glioma effect with prolonged median survival of nude mice bearing glioma compared with unmodified liposomes or liposomes modified with an individual peptide ligand.
3.3.2 Nanoparticles
3.3.2.1 Polymeric Nanoparticles
Polymers from natural as well as synthetic origins are used for the preparation of a polymeric nanoparticulate system. They are classified into two categories, nanospheres and nanocapsules, according to the drug-loading methods. In nanospheres, the drug molecules are dispersed throughout or just present on the surface of the polymeric matrix, while in the case of the nanocapsules, the drug molecules are surrounded by the polymeric shell (reservoir). A wide range of polymers has been investigated for their sustained and targeted drug-release profile [135].
Chitosan nanoparticles functionalized with PEG, modified and unmodified with monoclonal antibody OX26 (Cs-PEG-OX26) were prepared and characterized chemico-physically. The in-vivo evaluation of the developed system revealed that the brain uptake of OX26-conjugated nanoparticles was much higher than that of unmodified nanoparticles. This could be because of the prolonged circulation property attributed by PEG, the interaction between negatively charged brain endothelial cells and positively charged chitosan, and the affinity of OX26 for transferrin receptors [134].
Dual-peptide-functionalized albumin-based nanoparticles with tumor-targeting, cell-penetrating, and endolysosomal pH-responsive properties were proposed by Chen et al. [135]. The nanoparticles were prepared by electrostatic interaction between KALA (a cell-penetrating peptide) and cRGD-BSA (cRGD as a tumor-specific ligand). The prepared nanoparticulate system showed an enhanced inhibitory effect on the growth of tumor cells with αvβ3-integrin overexpression, revealing the potential of the system in the treatment of brain tumors.
A novel loperamide-loaded PLGA nanoparticle system was prepared using a nano-emulsification technique. In-vivo analgesic studies using the hot-plate method revealed that the drug-loaded PLGA nanoparticulate system was able to deliver the drug efficiently across the BBB, and that efficiency increases when a surface-modified nanoparticulate system is employed for achieving active targeting (a monoclonal antibody against the transferrin receptor) [136].
3.3.2.2 Lipid Nanoparticles
Lipid nanoparticles are a drug containing a solid lipophilic matrix with the particle size ranging from 100 to 1000 nm. The most commonly used nanoparticles, however, fall in the range of 150–300 nm. The lipid nanoparticles may be considered as derivatives of oil-in-water emulsions, wherein the liquid lipids of oil droplets are replaced by lipids that exist in a solid form at body temperature. The term ‘lipids’ when used in the context of lipid-based nanoparticulate delivery, may be any of the following: waxes, sterols, cholesterol, fat-soluble vitamins, monoglycerides, diglycerides, triglycerides, or phospholipids [137]. Most of the lipids used are generally regarded as safe by the US Food and Drug Administration except cetyl alcohol [138]. SLNs have been reported to deliver lipophilic drugs efficiently across the BBB.
Garanti et al. [139] evaluated the anti-cancer efficacy of asiatic acid-loaded SLNs against glioblastoma and reported higher cytotoxicity towards U87 MG cells than SVG P12 (human fetal glial) normal cells. The results of the MTT assay revealed a significant cytotoxic effect of drug-loaded SLNs on U87 MG cells compared with SVG P12 normal cells. The study also revealed that the cellular uptake of SLNs was facilitated by an energy-dependent endocytosis process (as evidenced by fluorescence imaging and flow cell cytometry).
Melanotransferrin antibody (MA) and tamoxifen (TX) were conjugated on etoposide (ETP)-entrapped SLNs (ETP-SLNs) to target the BBB and glioblastoma multiforme. The anti-proliferation efficacy against U87MG cells was found to be in the order of MA-TX-ETP-SLNs > TX-ETP-SLNs > ETP-SLNs > SLNs, revealing the potential of MA-TX-ETP-SLNs to deliver ETP across the BBB for the treatment of glioblastoma multiforme [140].
Galantamine hydrobromide, a promising acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease, is reported to be associated with cholinergic side effects. Its poor brain penetration results in lower bioavailability to the target site. With an aim to overcome these limitations, SLNs of galantamine hydrobromide were developed by employing biodegradable and biocompatible components. In-vivo evaluations of the developed system demonstrated significant memory restoration capability in cognitive-deficit rats in comparison with the naive drug. The developed carriers offered approximately twice the bioavailability to that of a plain drug, revealing the potential of the system in the treatment of Alzheimer’s disease [141].
Docetaxel-loaded lactoferin-appended SLNs were developed for the targeted delivery of the drug to the brain. Cytotoxicity studies of the developed system revealed an augmented apoptotic activity of lactoferin-conjugated SLNs compared with unconjugated SLNs and the pure drug. Further, the brain concentration of the drug was significantly elevated with the ligand-appended SLNs than with the marketed formulation, revealing the potential of the system for brain targeting [142].
Vincristine- and temozolomide-loaded SLNs and nanostructured lipid carriers were developed for the treatment of glioma. To provide a better treatment option for gliomatosis cerebri, the antitumor efficacy of SLNs vs. nanostructured lipid carriers was compared. The anti-tumor efficacies were evaluated using U87 malignant glioma cells and mice bearing the malignant glioma model. The results of the study revealed a significantly better glioma inhibition by nanostructured lipid carrier formulations compared with SLNs. Further, a dual drug application displayed the highest antitumor efficacy in vivo and in vitro than all the other formulations used [143]. Although the authors proposed that the developed system can be used for brain targeting, they have not used an orthotropic model of disease. Thus, further studies are required to provide the proof of concept for achieving brain targeting.
3.3.2.3 Inorganic Nanoparticles
Nanoformulations from inorganic materials, such as metal/metal oxides and silica, are used mainly for imaging/diagnostic purposes [144]. The surface of these nanoparticles can be functionalized to enhance penetration through the BBB [125]. Transferrin-appended, doxorubicin- and paclitaxel-loaded, magnetic silica PLGA nanoparticles were developed for the treatment of brain glioma. In-vivo therapeutic efficacy of drug-loaded nanoparticles using an intracranial U-87 MG-luc2 xenograft of BALB/c nude mice revealed that transferrin-conjugated doxorubicin- and paclitaxel-loaded nanoparticles exhibited the strongest anti-glioma activity as compared with doxorubicin and paclitaxel treatment alone with transferrin and in combination without transferrin [145].
PEG-modified carbon nanotubes showed good potential in crossing the BBB. Doxorubicin-loaded, oxidized multi-walled carbon nanotubes conjugated with PEG and angiopep-2 were developed. Intracellular tracking (in vitro) and fluorescence imaging (in vivo) revealed that the surface modifications of nanotubes with PEG and angiopep-2 established an ideal dual-targeted delivery system for both glioma cells and the BBB [125, 146].
Super-paramagnetic iron oxide nanoparticles (SPIONs) are another promising nanocarrier system proving their potential, owing to their inherent properties such as intrinsic magnetism, high safety potential, and ease of preparation and surface modifications. Among various nanocarriers reported, SPIONs are reported to achieve the highest drug-targeting potential. This could be attributed to the fact that an externally applied magnetic field applied to the targeted organ enhances the accumulation of magnetic nanoparticles in the drug site of action. Further, theranostic SPIONs have also been developed, making therapeutic delivery and diagnostics possible. PEGylated SPIONs, Tween SPIONs (surface of SPIONs modified with polysorbate 80), and recombinant human epidermal growth factor SPIONs have been successfully developed and evaluated for the targeted delivery of nanoparticles across the BBB [147–149].
Despite some favorable merits offered by SPIONs, there are some toxicological apprehensions that need to be taken into consideration such as the alteration in gene expression profiles, potential disturbance in iron homeostasis, oxidative stress and unpredictable cellular responses, and the induction of signaling pathways [150].
3.3.3 Nanogels
Nanogels, a new class of carrier systems for the delivery of drugs and biomacromolecules, have been proposed for crossing the BBB. A nanogel system for insulin delivery to the brain has been developed, taking into consideration the protective role of insulin in Alzheimer’s disease. A carboxyl-functionalized poly(N-vinyl pyrrolidone) nanogel system was produced by ionizing radiation as a substrate for the covalent attachment of insulin. The results of the study revealed that the nanogel system was a suitable vehicle for the delivery of insulin across the BBB and a very promising tool for the treatment of neurodegenerative diseases [151].
Baklaushev et al. [152] developed a cisplatin-loaded nanogel system for the treatment of intracranial gliomas 101/8. The developed nanogels were further conjugated with monoclonal antibodies specific to the membrane protein connexin 43 and brain-specific anion transporter 1. A significant reduction in tumor volume (magnetic resonance imaging volumetric analysis) was observed in rats when treated with the drug-loaded targeted nanogel system as compared with other formulations. In another study, a nanocarrier system appended with monoclonal antibodies specific to connexin 43 was proposed. The antitumor effect of the system was evaluated using the C6 glioma model. The results of the study revealed a significant reduction in the systemic toxicity of the drug when administered using the ligand-appended nanogels, in addition to effective inhibition of tumor growth, and significant prolongation in the lifespan of tumor-bearing animals [153].
3.3.4 Dendrimers
Dendrimers are enormously branched, three-dimensional, well-organized nanoscopic macromolecules (typically 5000–500,000 g/mol) [154]. The word ‘dendrimer’ arises from the Greek ‘dendron’, meaning ‘tree’ or ‘branch’, and meros meaning ‘part’. ‘Arboroles’ or ‘cascade polymers’ are the other names for dendrimers. The three-dimensional structure of dendrimers imparts them with a variety of distinctive properties, such as the presence of well-defined functional groups at the periphery, a globular nanoscaled shape, hydrophilic or hydrophobic cavities in the interior, and a low polydispersity index, and thus a wide range of potential applications [155].
The efficiency of a dendrimer-based formulation for a poorly water-soluble antipsychotic agent, haloperidol, in targeting the brain via intranasal and intraperitoneal administration routes was evaluated by Katare et al. [156]. The results revealed a 100-fold increase in the aqueous solubility of the drug in the developed formulation. Further, the formulation was assessed via different routes of administration for behavioral (cataleptic and locomotor) responses, and for haloperidol distribution in plasma and brain tissues. The developed dendrimer system displayed a considerably higher distribution of drug in both plasma and brain as compared with the control formulation delivered by intraperitoneal injection. In addition, the drug-loaded dendrimer formulation, when administered by the intranasal route, produced a behavioral response similar to that of the intraperitoneal injection, but at a 6.7 times lower dose.
When treated with nanomedicines, the major challenge for glioblastoma multiforme therapies is poor penetration and subsequent retention of nanoparticles in the glioblastoma parenchyma. The size of the nanoparticle can considerably influence the delivery efficiency in the tumor tissue. Decreasing the nanoparticle size can improve the nanoparticle penetration in tumor tissue but decrease the nanoparticle retention effect. Therefore, small nanoparticles with a high retention effect in tumors are urgently needed for effective glioblastoma multiforme drug delivery. In lieu of this, Zhao et al. [157] developed the CREKA (fibrin-binding peptide), peptide-appended, PEGylated, PAMAM dendrimer nanoparticulate system for the treatment of glioblastoma multiforme. In-vivo studies in glioblastoma multiforme bearing nude mice revealed that the peptide-modified dendrimer nanoparticulate system showed deeper penetration and higher accumulation in glioblastoma tissues compared with unmodified nanoparticles.
In another study, Zarebkohan et al. [158] developed serine-arginine-leucine (SRL)-appended PAMAM dendrimers for gene (DNA) delivery to the brain. In-vitro studies showed that SRL-modified nanoparticles have good transfection efficacy and low toxicity. After intravenous administration, the ligand-appended nanoparticles were observed to cross the BBB and enter the brain parenchyma, revealing the potential of the system for gene delivery to the brain.
3.3.5 Micelles
Micelles are a spherical colloidal system consisting of a hydrophobic core and a hydrophilic surface. They are composed of amphiphilic block copolymers, which tend to aggregate in aqueous solutions to form micelles [125]. Docetaxel-loaded, transferrin-appended, vitamin E TPGS (d-α-tocopheryl polyethylene glycol 1000 succinate) micelles were developed by Sonali et al. [159] for the treatment of brain cancer. Results showed that the particle sizes of the non-targeted and targeted micelles were <20 nm, with about 85% encapsulation efficiency. The drug release from transferrin-conjugated micelles was sustained for >24 h with 50% of drug release. The in-vivo results indicated that transferrin-targeted TPGS micelles could be a promising tool for achieving brain targeting of drugs, owing to its nano size, solubility enhancement, and improvement in permeability compared with the unmodified micelles and marketed formulation.
Quercetin-loaded lyophilized polymeric micelles were developed by Wang et al. [160] for the treatment of brain glioma. Results showed that the particle size of quercetin-loaded freeze-dried nanomicelles ranged from 20 to 80 nm, with an efficient sustained-release profile. Increased intracellular uptake into Caco-2 cells with low cytotoxicity, efficient penetration of BBB, and powerful cytotoxicity on C6 glioma cells were observed. The in-vivo studies in tumor-bearing mice revealed that the developed drug-loaded polymeric micelles are accumulated into the tumor tissues, and demonstrate considerable anti-tumor efficacy (which improved the survival rate of the animals).
Myricetin-loaded Pluronic P123/F68 micelles functionalized with chitosan were developed for the treatment of glioblastoma cancer. The results of the study revealed that drug-loaded micelles enhances the cellular uptake and antitumor activity compared with free myricetin in vitro, with a considerably improved anticancer effect in vivo following efficient transport across the BBB [161]. Although the authors proposed that the system can be used for brain targeting, they did not used an orthotropic model of disease. Thus, further studies are required to provide the proof of concept for achieving brain targeting.
In another study, Wang et al. [162] developed a myricetin-loaded mixed micelle system using sodium dodecyl sulfate, Pluronic F68, and Labrasol for the delivery of the drug across the BBB. The average size of the myricetin-loaded mixed micelle was found to be 96.3 nm, with a negatively charged potential and spherical shape. The results of the study revealed that drug-loaded mixed micelles depicted a higher preference for the brain than the free drug alone, suggesting that the developed system could promote absorption and increase relative bioavailability of drugs.
3.3.6 Nanoemulsions
Nanoemulsions are thermodynamically stable, heterogenously nanosized dispersions of water-in-oil or oil-in-water preparations stabilized by means of an emulsifying agent, i.e., a surfactant and co-surfactant [125].
A risperidone-loaded nanoemulsion was developed as a promising carrier for brain-targeted drug delivery. The optimized risperidone-loaded nanoemulsions contain sodium oleate in the aqueous phase and polysorbate 80, poloxamer 188, or Solutol® HS15 as a co-emulsifier with a mean size of 160 nm, size distribution of <0.15, and zeta potential around −50 mV. The nanoemulsion was produced by hot homogenization and their ability to improve risperidone delivery to the brain was assessed in rats. The results of the study revealed that nanoemulsion co-stabilized using Tween 80 and displayed 1.4- to 7.4-fold higher brain availability of risperidone compared with other nanoemulsions and the drug solution, suggesting the potential of the system for brain targeting [163].
In another study, a saquinavir-loaded oil-in-water nanoemulsion system was proposed for achieving brain targeting. The saquinavir bioavailability, as well as distribution in different organ systems, was examined. Compared with the aqueous suspension, saquinavir concentrations in systemic circulation, when administered in flax-seed oil nanoemulsions (100–200 nm), were found to be threefold higher. The oral bioavailability and distribution to the brain (a potential sanctuary site for human immunodeficiency virus) were significantly enhanced with saquinavir delivered in the nanoemulsion formulation. Saquinavir in a flax-seed oil nanoemulsion showed maximum plasma concentration and area under the plasma concentration-time curve values five and threefold higher in the brain, respectively, as compared with an aqueous suspension, revealing an enhanced rate and extent of aqueous suspension absorption following oral administration of nanoemulsions [164].
3.3.7 Polymersomes
Polymersomes are a bi-layered nanosized vesicular system consisting of amphiphilic block copolymers [165]. They are made from synthetic amphiphilic block copolymers by employing an aqueous solvent, aqueous/organic system, or organic solvent. Amphiphilic block copolymers with a hydrophilic fraction of 35 ± 10% tend to aggregate to form polymersomes [125].
Doxorubicin-loaded, transferrin-conjugated biodegradable polymersomes were developed by Pang et al. [166]. Drug-loaded biodegradable polymersomes were prepared by the nanoprecipitation technique, followed by conjugation with transferrin with an average diameter of 107 nm. Compared with free drug and drug-loaded polymersomes, transferrin-conjugated drug-loaded polymersomes exhibited the highest cytotoxic activity against C6 glioma cells. Further, results of a pharmacodynamics study revealed a noteworthy reduction in tumor volume and a considerable increment in the median survival rate in the group of animals treated with transferrin-conjugated biodegradable polymersomes.
A novel PEG-PLGA polymersome-based nanosystem was proposed by Yu et al. [167]. The surface of the system was further functionalized using lactoferrin (Lf-POS). Three formulations of Lf-POS, with different densities of lactoferrin on the surface of polymersomes, were prepared and characterized. The results of brain delivery in mice demonstrated that the optimized number of lactoferrin conjugated per polymersome was found to be 101. This obtains the greatest BBB permeability surface area product and percentage of injected dose per gram of brain, revealing the potential of the system for brain delivery.
Des-octanoyl ghrelin and folate conjugated with polymersomal doxorubicin (GFP-D) was developed for the effective treatment of brain tumors. In-vitro testing using C6 glioma cells and a BBB model demonstrated that the developed ligand-appended polymersome showed a robust penetrating-targeting function for drug delivery. In-vivo antitumor experiments showed that the ligand-appended polymersome gave a much better anti-glioma effect and presented a significant improvement in the overall survival of the tumor-bearing mice compared with the treatments with free doxorubicin, liposomal doxorubicin, polymersomal doxorubicin, or single ligand-conjugated polymersomal doxorubicin [168].
3.3.8 Exosomes
Exosomes are lipid vesicles secreted intracellularly by inward folding of the multi-vesicular body of the membrane and, thereafter, they ooze out of the plasma membrane of the cell [169–171]. These exosomes are secreted by all the different types of cells and hence they are found in ample numbers in body fluids such as saliva, blood, and urine [172]. As they are components from the cell membrane, a circular bubble shape, and nanosized, they have the characteristics for ease of migration from one cell to another and release their content like microRNAs and proteins [173].
Several CNS drug candidates are being developed using exosome-based delivery. In one such study, exosomes were prepared from an EL-4 mouse lymphoma cell line and curcumin, which is a hydrophobic polyphenol, and were loaded by simple incubation [174]. Currently, there are two clinical trials being carried out for curcumin-loaded exosomes for the treatment of cancer [171]. The advantages offered by exosomes as a vehicle for drug delivery include (1) small molecules and biologicals can be encapsulated into the exosomes, (2) exosomes can cross physiological barriers, and target tissue through their natural surface proteins, (3) they provide broad distribution in biological fluids, thus improving circulation time and efficacy, and (4) they offer an improved safety profile and selectivity over polymeric nanoparticles and liposomes [170, 173, 175–179].
3.3.9 Quantum Dots
The majority of brain-targeted drug-delivery systems take aim at the receptors positioned on the endothelial cells of the brain capillaries. Paris-Robidas et al. [180] developed Ri7 (monoclonal antibodies) conjugated quantum dots for targeted delivery to the murine transferrin receptors. Specific transferrin receptor-mediated endocytosis of the system was confirmed using N2A and nEnd5 cells. In-vivo studies in mice depicted a fourfold increase in the volume of distribution in brain tissues as compared with control animals [180]. Immunofluorescence analysis showed that Ri7 quantum dots were sequestered throughout the cerebral vasculature 30 min, 1 h, and 4 h post-injection, with a decline of signal intensity after 24 h. Transmission electron microscopic studies confirmed that Ri7 quantum dots were extensively internalized by the brain capillary endothelial cells, averaging 37 ± 4 Ri7 quantum dots/cell 1 h after injection [180]. Most quantum dots within the brain capillary endothelial cells were observed in small vesicles (58%), with a smaller proportion detected in tubular structures or in multi-vesicular bodies. Parenchymal penetration of Ri7 quantum dots was extremely low and comparable with control IgG. The results of the study showed that systemically administered, Ri7 quantum dot complexes undergo extensive endocytosis by brain capillary endothelial cells, thus revealing the potential of the novel therapeutic system for brain endothelial cell drug delivery [180].
3.4 Drug Delivery from Microchips
Microchips can be used for either single- or multiple-drug delivery to the brain [181, 182]. Microchips comprise pumps, valves, and channels, and are regulated by electrochemical dissolution [182] or time-dependent biodegradation [183]. Scott et al. [184] demonstrated the potential use of these microchips for delivery of temozolomide in a rodent gliosarcoma model. The results of the study revealed that the drug was delivered from the system in a predictable manner, leading to prolonged survival of the animals compared with the oral therapy. The main drawbacks associated with this systems are (1) refilling of the device, (2) possible changes in the magnetic fields, and (3) malfunctioning of the electronic system [181].
4 Intranasal Delivery to the Brain
When administered intranasally, drugs reach the CSF and olfactory bulb in a significant quantity via the olfactory sensory neurons. The route through which the drug reaches the CSF and brain tissues after intranasal administration is shown in Fig. 3 [1]. Further, it has been reported that the uptake of drug in CSF and brain tissues depends on the lipophilicity and molecular weight of the drug [185–190]. Advantages and disadvantages offered by the intranasal drug-delivery system are depicted in Fig. 4a, b [191].
Recently, liposomes loaded with a novel β-sheet breaker peptide (H102) were developed for the treatment of Alzheimer’s disease. The results of the study revealed a consistent penetration of the liposomes into the Calu-3 cell monolayers. When administered intranasally, the system delivered the drug load effectively to the brain tissues. The area under the plasma concentration-time curve of H102-loaded liposomes in the hippocampus was reported to be 2.92-fold higher than that of the solution group. Further, as compared with the intranasal solution, the developed liposomal system provided a remarkable improvement in spatial memory impairment in the Alzheimer’s disease rat model. It also inhibited further plaque deposition and increased the activities of choline acetyltransferase and insulin-degrading enzyme, revealing the potential of the systems for the treatment of Alzheimer’s disease [192].
A deferoxamine mesylate-loaded microparticulate system for intranasal delivery to the brain was proposed by Rassu et al. [193]. Drug-loaded microparticles were prepared using methyl-β-cyclodextrin and chitosan chloride by employing a spray-drying technique. After intranasal administration to rats, the drug uptake into the CSF was found to be 3.83 ± 0.68 μg/mL and 14.37 ± 1.69 μg/mL for chitosan chloride and methyl-β-cyclodextrin, respectively, after 30 min of insufflation of microparticles, revealing the potential of the systems for the nose-to-brain uptake of the drug and thereby limiting its systemic exposure.
In another study, a piperine-encapsulated chitosan nanoparticulate system was developed to achieve nose-to-brain targeting. Alzheimer’s disease was induced in male Wistar rats, on which full behavioral and biochemical testing was conducted. Piperine nanoparticles showed a significant improvement in cognitive functions as compared with donepezil (standard drug), with an additional advantage of its dual mechanism, i.e., antioxidant effect and acetylcholinesterase inhibition [194].
PLGA and SLN systems were developed for nose-to-brain delivery of tarenflurbil (TFB) for the treatment of Alzheimer’s disease. Pharmacokinetic studies suggested improved circulation behavior of nanoparticles and the absolute bioavailability was found to be in the order: TFB-PLGA nanoparticles (intranasal) > TFB-SLNs (intranasal) > TFB solution (intranasal) > TFB suspension (oral). Brain-targeting efficiency was determined in terms of percentage drug targeting efficiency (%DTE) and drug transport percentage (DTP). The highest %DTE (287.24) and DTP (65.18) were observed for TFB-PLGA nanoparticles followed by TFB-SLNs (%DTE: 183.15 and DTP: 45.41) among all other tested groups. The above results proved that therapeutic concentrations of TFB could be transported directly to the brain via the olfactory pathway after intranasal administration of polymeric and lipidic nanoparticles [195].
5 Convection-Enhanced Diffusion Delivery
In convection-enhanced diffusion, drug distribution takes place via bulk flow where a catheter is implanted in the brain that is connected to a pump and that drives fluid flow to the brain at a prescribed infusion rate [196]. This type of delivery system is being studied in clinical trials in high-grade gliomas [1].
Many potential therapeutic agents have been investigated for convection-enhanced diffusion delivery for the treatment of brain glioma [197]. These include nanoparticle conjugates [198–201], liposomes [202–204], viral particles [205, 206], oligonucleotides [207, 208], and gene therapies [202, 205, 206]. Liposomal formulations can significantly reduce unwanted side effects to normal tissues, permit greater volume of distribution of the drug, and decrease the clearance rate of tissues, thus providing a prolonged exposure of drug to the targeted region [203].
Similar merits are offered by the polymeric nanoparticles, in that they can be conjugated to numerous chemotherapeutic agents in addition to a contrast agent and can be fabricated for optimal convection characteristics (<100 nm) [198]. Although, many preclinical trials have been conducted, none of the specific vehicles have been proved to be reliable and very few have succeeded in reaching clinical trials.
6 Disruption of the BBB
6.1 Osmotic (Chemical or Hypertonic Shock) Disruption
Mannitol, the most commonly explored osmotic agent, shrinks the endothelial cells hyperosmotically, thus opening the endothelial tight junction, which further leads to passive diffusion of large molecules across the BBB [1, 209, 210]. Recently, it has been used in combination with nanoparticles [211], cellular delivery [212], peptides [213], and gene delivery [214, 215]. However, apart from its beneficial effects, risk factors are also associated with mannitol therapy such as structural brain damage, expression of heat shock proteins and microembolisms, altered glucose uptake, abnormal neuronal function, and passage of plasma proteins [1, 216–218].
Apart from mannitol, there have been other chemical agents explored for the disruption of the BBB; namely, borneol [219], polydixylitol [220], and lyophosphatic acid [221]. Although, studies involving chemical agents for the disruption of the BBB are ongoing, inconsistent efficacy and safety aspects have led to the development of less invasive physical modes of disruption.
6.2 Biochemical BBB Disruption (Administration of Vasoactive Substances)
In-vivo studies in animal models revealed the selective potential of the vasoactive amines [bradykinin (and its analog RMP-7, receptor-mediated permeabilizer) and histamine] in opening of the BBTB. Bradykinin was reported to increase the permeability of the endothelial tight junction by activating the B2 receptor of the endothelial cells [1, 222]. Recently, Cote et al. [223] explored the potential of co-administration of kinin B1R and B2R in tumor-selective disruption of BBB. The results of the study revealed that co-administration of kinin B1R and a B2R agonist delivered the anticancer agent carboplatin more efficiently compared with the agonist alone. Further, the survival rate of glioma-bearing rats was also found to be higher, owing to the selective targeting of the anticancer agent; thus, revealing the potential of the system in improving the brain bioavailability and therapeutic efficacy of the anticancer agent. Recently, the bradykinin analog, RMP-7, along with transferrin was used to prepare liposomes containing nerve growth factor for the treatment of Alzheimer’s disease. The developed formulation was evaluated using astrocyte-regulated, human brain microvascular endothelial cells, which showed increased permeability across the BBB [224].
In addition to bradykinin and histamine, adenosine is another vasoactive substance that has been explored for enhancing BBB permeability. Adenosine acts on adenosine 2A receptors, which belong to the family of G-protein-coupled receptors located on the brain capillary endothelial cells. Recently, it has been reported that activation of adenosine 2A receptors leads to the opening of tight junctions, which results in enhanced permeability of BBB [225]. However, this opening is temporary and transient. Gao et al. [226] designed a series of agonists of adenosine 2A receptors (nanoagonists), which resulted in the opening of tight junctions. Imaging studies indicated that model drug uptake was increased in vivo and the opening of the tight junction lasted between 0.5 and 2 h. The next challenge for this group was to regulate the opening of the tight junctions only in the ischemic region and not the other unaffected areas of the brain. Subsequently, they developed other nanoagonists, which resulted in the enhanced delivery of neuroprotectants specifically into ischemic regions efficiently [227].
6.3 BBB Disruption by Alkylglycerols
Another approach for disruption of the BBB employs various types of alkylglycerols. It has been reported that the extent of disruption of the BBB depends on the number and length of glycerols and alkyl groups, respectively, present in the chemical structure. Although the exact mechanism(s) of disruption are unknown, preliminary results reveal a momentary breakdown of endothelial tight junctions, resulting in an increase in permeability [1, 228–230].
Hulper et al. [231] explored the potential of two alkylglycerols, 1-O-pentylglycerol and 2-O-hexyldiglycerol, on barrier properties of cultured brain microvascular endothelial cells. An in-vitro BBB model consisting of primary cultures of rat brain endothelial cells, co-cultured with rat cerebral glial cells was used in the study. The results of the study revealed that the permeability was only of a short duration and was reversible. Further, it did not have any impact on the tight junction strand complexity. The study authors concluded that the rearrangement of proteins and alterations in the shape of cells depicts the involvement of the cytoskeleton in the action of alkylglycerols.
6.4 Ultrasound-Facilitated Disruption
Recently, focused ultrasound (FU), a non-invasive approach for the disruption of the BBB, has been gaining tremendous interest among the scientific community. FU uses ultrasound (‘sonications’) in combination with pre-formed microbubbles (available commercially as a contrast imaging agent for ultrasound) [209, 232]. Microbubbles (1–10 μm) consist of a semi-solid gas (perfluorocarbon)-encapsulated albumin or lipid core that are constrained to the vasculature. They concentrate the effect of ultrasound to the microvasculataure, thus reducing the level of exposure of FU required to produce a biological effect [233]. The efficiency of FU was investigated for the delivery of small molecules [234, 235], macromolecules [236], DNA [237, 238], siRNA [239], and gene delivery using viral vectors [240, 241].
It was evidenced from the tumor efficacy models and preliminary efficiency models that FU could be an effective tool for drug delivery to the CNS; although efforts to optimize these protocols are still ongoing. Definity® microbubbles (1.1- to 3.3-μm lipid particles with octafluoropropane-encapsulated gas) have been used in numerous studies to investigate the effects of various experimental parameters on FU. Parameters such as duration of sonication, repetition frequency of pulse, concentration of microbubble, pulse length, and influence of standing waves and pressure are all considered to be significant for both efficacy and safety [209, 236, 242–245]. Although in recent studies minor toxicity of FU to the brain has been reported with no neurotoxicity issues, the clinical application of this method still needs to be viewed cautiously [246–248].
7 Conclusions and Future Perspectives
Accessing the brain tissue by circumventing the BBB has been one of the greatest challenges for formulation scientists. Progress in the field of biomedical sciences and technology has led to remarkable advancements in brain targeting. Exploiting the inherent mechanisms for the transport of drugs such as adsorptive-mediated transcytosis, inhibition of active efflux pumps, receptor mediated transport, and cell-mediated endocytosis have provided promising results. Technological improvements have led to encouraging outcomes using liposomes, nanoparticles, and dendrimers. Alternative routes past the BBB have also shown significant potential for being commercialized.
Furthermore, to achieve a constructive brain-targeted drug-delivery system, some basic concerns need to be addressed in the nearby future, such as (1) factors affecting the in-vivo performance of the system should be well explicated and evaluated (e.g., in the case of in-vivo studies that describe the treatment of glioblastoma, the BBB in tumors is less tight than in healthy brain tissue; this may influence the outcome of the studies), (2) more emphasis should be focused on the use of biodegradable materials, which get eliminated easily from the brain, (3) a considerable improvement needs to be made in targeting the efficiency of such systems before their clinical application, (4) hydrophobic drugs may leak from the delivery system and subsequently enter the brain, thus proof of the penetration of the drug into the brain does not (necessarily) mean penetration of the delivery system itself, and (5) a uniform method of preparation should be developed for the development of a nanoparticulate system to achieve a more homogenous and predictable product. The essence of evolving a successful delivery method lies in contemplating not only effective brain targeting formulations but also less toxic and safe therapies so as to enable a better and healthy future for the patients.
References
Patel MM, Goyal BR, Bhadada SV, et al. Getting into the brain: approaches to enhance brain drug delivery. CNS Drugs. 2009;23:35–58.
Brightman MW. Morphology of blood–brain interfaces. Exp Eye Res. 1977;25(Suppl. 1):1–25.
Schlosshauer B. The blood–brain barrier: morphology, molecules, and neurothelin. Bioassays. 1993;15:341–6.
Ricci M, Blasi P, Giovagnoli S, Rossi C. Delivering drugs to the central nervous system: a medicinal chemistry or a pharmaceutical technology issue? Curr Med Chem. 2006;13:1757–75.
Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol. 2005;25:5–23.
Stewart PA. Endothelial vesicles in the blood–brain barrier: are they related to permeability? Cell Mol Neurobiol. 2000;20:149–63.
Marques F, Sousa JC, Sousa N, Palha JA. Blood–brain-barriers in aging and in Alzheimer’s disease. Mol Neurodegener. 2013;8:38.
Reev A, Simcox E, Turnbull D. Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res Rev. 2014;14:19–30.
Chen Y, Liu L. Modern methods for delivery of drugs across the blood–brain barrier. Adv Drug Deliv Rev. 2012;64:640–65.
Neuwelt E, Abbott NJ, Abrey L, et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7:84–96.
Alzheimer’s Association. Alzheimer’s disease facts and figures. http://www.alz.org/facts/. Accessed 29 Aug 2016.
Parkinson’s Disease Foundation. http://www.pdf.org/en/parkinson_statistics. Accessed 29 Aug 2016.
National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Program. http://seer.cancer.gov/statfacts/html/brain.html. Accessed 29 Aug 2016.
Lindsley CW. 2014 global prescription medication statistics: strong growth and CNS well represented. ACS Chem Neurosci. 2015;6(4):505–6.
Hottinger AF, Aissa AB, Espeli V, et al. Phase I study of sorafenib combined with radiation therapy and temozolomide as first-line treatment of high-grade glioma. Br J Cancer. 2014;110(11):2655–61.
Karajannis MA, Legault G, Fisher MJ, et al. Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro Oncol. 2014;16(10):1408–16.
Taylor JW, Dietrich J, Gerstner ER, et al. Phase 2 study of bosutinib, a Src inhibitor, in adults with recurrent glioblastoma. J Neurooncol. 2015;121(3):557–63.
Balaña C, Gil MJ, Perez P, et al. Sunitinib administered prior to radiotherapy in patients with non-resectable glioblastoma: results of a phase II study. Target Oncol. 2014;9(4):321–9.
Raizer JJ, Grimm SA, Rademaker A, et al. A phase II trial of PTK787/ZK 222584 in recurrent or progressive radiation and surgery refractory meningiomas. J Neurooncol. 2014;117(1):93–101.
Cacciavillano W, Sampor C, Venier C, et al. A phase I study of the anti-idiotype vaccine racotumomab in neuroblastoma and other pediatric refractory malignancies. Pediatr Blood Cancer. 2015;62(12):2120–4.
Curtin F, Perron H, Kromminga A, et al. Preclinical and early clinical development of GNbAC1, a humanized IgG4 monoclonal antibody targeting endogenous retroviral MSRV-Env protein. MAbs. 2015;7(1):265–75.
Solomon MT, Miranda N, Jorrín E, et al. Nimotuzumab in combination with radiotherapy in high grade glioma patients: a single institution experience. Cancer Biol Ther. 2014;15(5):504–9.
Chheda MG, Wen PY, Hochberg FH, et al. Vandetanib plus sirolimus in adults with recurrent glioblastoma: results of a phase I and dose expansion cohort study. J Neurooncol. 2015;121(3):627–34.
Nygaard HB, Wagner AF, Bowen GS, et al. A phase Ib multiple ascending dose study of the safety, tolerability, and central nervous system availability of AZD0530 (saracatinib) in Alzheimer’s disease. Alzheimers Res Ther. 2015;7(1):35.
Coric V, Salloway S, van Dyck CH, et al. Targeting prodromal Alzheimer disease with avagacestat: a randomized clinical trial. JAMA Neurol. 2015;72(11):1324–33.
Ryan ML, Falk DE, Fertig JB, et al. A phase 2, double-blind, placebo-controlled randomized trial assessing the efficacy of ABT-436, a novel V1b receptor antagonist, for alcohol dependence. Neuropsychopharmacology. 2016 Oct 19. doi:10.1038/npp.2016.214. (Epub ahead of print).
Chamorro A, Amaro S. Castellanos M; URICO-ICTUS Investigators. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): a randomised, double-blind phase 2b/3 trial. Lancet Neurol. 2014;13(5):453–60.
AmBiosome® (amphotericin B) liposome for injection [product information]. https://www.ambisome.com/. Accessed 18 Nov 2016.
Titan Pharmaceuticals. http://www.titanpharm.com/pipeline/probuphine. Accessed 18 Nov 2016.
Zinbryta™ (dacaluzumab), 150 mg subcutaneous injection [product information]. https://www.zinbryta.com/. Accessed 18 Nov 2016.
Fycompa™ (perampal), tablets, oral suspension [product information]. https://fycompa.com/. Accessed 18 Nov 2016.
Evomela® (melphalan) [product information]. http://www.evomela.com/. Accessed 18 Nov 2016.
Briviact® (brivacetam) [product information]. https://www.briviact.com/. Accessed 18 Nov 2016.
Onzetra® Xsail (sumatriptan nasal powder) 11 mg per nose piece [product information]. https://www.onzetra.com/. Accessed 18 Nov 2016.
Zembrace™ Symtouch™ (sumatriptan injection) 3 mg [product information]. http://www.zembrace.com/. Accessed 18 Nov 2016.
Adzenys XR-OD™ (amphetamine) extended-release orally disintegrating tablets [product information]. http://adzenysxrodt.com/. Accessed 18 Nov 2016.
Rytary® (carbidopa and levodopa) extended-release capsules [product information]. https://rytary.com/. Accessed 18 Nov 2016.
Duopa® carbidopa/levodopa enteral suspension 4.63 mg/20 mg per ml [product information]. https://www.duopa.com/. Accessed 18 Nov 2016.
Aptension XR® methylphenidate HCl extended-release capsules [product information]. http://www.aptensioxr.com/. Accessed 18 Nov 2016.
Unitixin (dinuuximab) injection [product information]. https://www.unituxin.com/. Accessed 18 Nov 2016.
Invega Trinza® paliperidone palmitate extended-release injectable suspension [product information]. https://www.invegatrinzahcp.com/. Accessed 18 Nov 2016.
Rexulti® brexpiprazole 2 mg tablets [product information]. https://www.rexulti.com/us/mdd. Accessed 18 Nov 2016.
Spritam® (levetiracetam) tablets for oral suspension [product information]. https://www.spritam.com/. Accessed 18 Nov 2016.
Vraylar® (cariprazine) capsules [product information]. http://www.vraylar.com/. Accessed 18 Nov 2016.
Aristada® aripiprazole lauroxil extended-release injectable suspension [product information]. https://www.aristada.com/. Accessed 18 Nov 2016.
Belbuca™ (buprenorphine) buccal film [product information]. https://www.belbuca.com/patient/. Accessed 18 Nov 2016.
Terasaki T, Tsuji A. Drug delivery to the brain utilizing blood–brain barrier transport systems. J Control Release. 1994;29:163–9.
Kamalinia G, Khodagholi F, Shaerzadeh F, et al. Cationic albumin-conjugated chelating agent as a novel brain drug delivery system in neurodegeneration. Chem Biol Drug Des. 2015;86:1203–14.
Lu W, Wan J, She Z, Jiang X. Brain delivery property and accelerated blood clearance of cationic albumin conjugated pegylated nanoparticle. J Control Release. 2007;118:38–53.
Agarwal A, Agrawal H, Tiwari S, et al. Cationic ligand appended nanoconstructs: a prospective strategy for brain targeting. Int J Pharm. 2011;12(421):189–201.
Stojanov K, Georgieva JV, Brinkhuis RP, et al. In vivo biodistribution of prion- and GM1-targeted polymersomes following intravenous administration in mice. Mol Pharm. 2012;9:1620–7.
Liu L, Guo K, Lu J, et al. Biologically active core/shell nanoparticles self-assembled from cholesterol-terminated PEG-TAT for drug delivery across the blood–brain barrier. Biomaterials. 2008;29:1509–17.
Liu L, Venkatraman SS, Yang YY, et al. Polymeric micelles anchored with TAT for delivery of antibiotics across the blood–brain barrier. Biopolymers. 2008;90:617–23.
Chen H, Tang L, Qin Y, et al. Lactoferrin-modified procationic liposomes as a novel drug carrier for brain delivery. Eur J Pharm Sci. 2010;40:94–102.
Chen H, Qin Y, Zhang Q, et al. Lactoferrin modified doxorubicin-loaded procationic liposomes for the treatment of gliomas. Eur J Pharm Sci. 2011;44:164–73.
Byeon HJ, le Thao Q, Lee S, et al. Doxorubicin-loaded nanoparticles consisted of cationic- and mannose-modified-albumins for dual-targeting in brain tumors. J Control Release. 2016;225:301–13.
Fromm MF. P-glycoprotein: a defense mechanism limiting oral bioavailability and CNS accumulation of drugs. Int J Clin Pharmacol Ther. 2000;38:69–74.
Becker CM, Oberoi RK, McFarren SJ, et al. Decreased affinity for efflux transporters increases brain penetrance and molecular targeting of a PI3K/mTOR inhibitor in a mouse model of glioblastoma. Neuro Oncol. 2015;17:1210–9.
Cannon RE, Peart JC, Hawkins BT, et al. Targeting blood–brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci USA. 2012;109:15930–5.
Wang T, Agarwal S, Elmquist WF. Brain distribution of cediranib is limited by active efflux at the blood–brain barrier. J Pharmacol Exp Ther. 2012;341:386–95.
Agarwal S, Sane R, Ohlfest JR, Elmquist WF. The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J Pharmacol Exp Ther. 2011;336:223–33.
Batrakova EV, Li S, Miller DW, Kabanov AV. Pluronic P85 increases permeability of a broad spectrum of drugs in polarized BBMEC and Caco-2 cell monolayers. Pharm Res. 1999;16:1366–72.
Batrakova EV, Li S, Li Y, et al. Effect of pluronic P85 on ATPase activity of drug efflux transporters. Pharm Res. 2004;21:2226–33.
Batrakova EV, Han HY, Miller DW, Kabanov AV. Effects of pluronic P85 unimers and micelles on drug permeability in polarized BBMEC and Caco-2 cells. Pharm Res. 1998;15:1525–32.
Kabanov AV, Batrakova EV, Miller DW. Pluronic block copolymers as modulators of drug efflux transporter activity in the blood–brain barrier. Adv Drug Deliv Rev. 2003;55:151–64.
Sharma AK, Zhang L, Li S, et al. Prevention of MDR development in leukemia cells by micelle-forming polymeric surfactant. J Control Release. 2008;131:220–7.
Mena I, Cotzias GC. Protein intake and treatment of Parkinson’s disease with levodopa. N Engl J Med. 1975;292:181–4.
Cornford EM, Hyman S, Swartz BE. The human brain glut1 glucose transporter: Ultrastructural localization to the blood–brain barrier endothelia. J Cereb Blood Flow Metab. 1994;14:106–12.
Bonina FP, Arenare L, Palagiano F, et al. Synthesis, stability, and pharmacological evaluation of nipecotic acid prodrugs. J Pharm Sci. 1999;88:561–7.
Bonina FP, Arenare L, Ippolito R, et al. Synthesis, pharmacokinetics and anticonvulsant activity of 7-chlorokynurenic acid prodrugs. Int J Pharm. 2000;202:79–88.
Tsuji A, Tamai I. Carrier-mediated or specialized transport of drugs across the blood–brain barrier. Adv Drug Deliv Rev. 1999;36:277–90.
Sarkar G, Curran GL, Sarkaria JN, et al. Peptide carrier-mediated non-covalent delivery of unmodified cisplatin, methotrexate and other agents via intravenous route to the brain. PLoS One. 2014;9:e97655.
Johnsen KB, Moos T. Revisiting nanoparticle technology for blood–brain barrier transport: unfolding at the endothelial gate improves the fate of transferrin receptor-targeted liposomes. J Control Release. 2016;222:32–46.
Fishman JB, Rubin JB, Handrahan JV, et al. Receptor-mediated transcytosis of transferrin across the blood–brain barrier. J Neurosci Res. 1987;18:299–304.
Raub TJ, Newton CR. Recycling kinetics and transcytosis of transferrin in primary cultures of bovine brain microvessel endothelial cells. J Cell Physiol. 1991;149:141–51.
Descamps L, Dehouck MP, Torpier G, Cecchelli R. Receptor-mediated transcytosis of transferrin through blood–brain barrier endothelial cells. Am J Physiol. 1996;270(4 Pt 2):H1149–58.
Friden PM, Walus LR, Musso GF, et al. Anti-transferrin receptor antibody and antibody-drug conjugates cross the blood–brain barrier. Proc Natl Acad Sci USA. 1991;88:4771–5.
Pardridge WM, Buciak JL, Friden PM. Selective transport of an anti-transferrin receptor antibody through the blood–brain barrier in vivo. J Pharmacol Exp Ther. 1991;259:66–70.
Moos T, Morgan EH. Restricted transport of anti-transferrin receptor antibody (OX26) through the blood–brain barrier in the rat. J Neurochem. 2001;79:119–29.
Gosk S, Vermehren C, Storm G, Moos T. Targeting anti-transferrin receptor antibody (OX26) and OX26-conjugated liposomes to brain capillary endothelial cells using in situ perfusion. J Cereb Blood Flow Metab. 2004;24:1193–204.
Cabezón I, Manich G, Martín-Venegas R, et al. Trafficking of gold nanoparticles coated with the 8D3 anti-transferrin receptor antibody at the mouse blood–brain barrier. Mol Pharm. 2015;12:4137–45.
Yu YJ, Zhang Y, Kenrick M, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med. 2011;3:84ra44.
Pardridge WM. Blood–brain barrier drug delivery of IgG fusion proteins with a transferrin receptor monoclonal antibody. Expert Opin Drug Deliv. 2015;12:207–22.
Niewoehner J, Bohrmann B, Collin L, et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. 2014;81:49–60.
Clark AJ, Davis ME. Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proc Natl Acad Sci USA. 2015;112:12486–91.
Kang YS, Jung HJ, Oh JS, Song DY. Use of PEGylated immunoliposomes to deliver dopamine across the blood–brain barrier in a rat model of Parkinson’s disease. CNS Neurosci Ther. 2016;22(10):817–23.
Wei L, Guo XY, Yang T, et al. Brain tumor-targeted therapy by systemic delivery of siRNA with transferrin receptor-mediated core-shell nanoparticles. Int J Pharm. 2016;510:394–405.
Youn P, Chen Y, Furgeson DY. A myristoylated cell-penetrating peptide bearing a transferrin receptor-targeting sequence for neuro-targeted siRNA delivery. Mol Pharm. 2014;11:486–95.
Morales-Cruz M, Cruz-Montañez A, Figueroa CM, et al. Combining stimulus-triggered release and active targeting strategies improves cytotoxicity of cytochrome c nanoparticles in tumor cells. Mol Pharm. 2016;13:2844–54.
Dong S, Cho HJ, Lee YW, Roman M. Synthesis and cellular uptake of folic acid-conjugated cellulose nanocrystals for cancer targeting. Biomacromolecules. 2014;15:1560–7.
Yamamoto M, Ikeda K, Ohshima K, et al. Increased expression of low density lipoprotein receptor-related protein/alpha2-macroglobulin receptor in human malignant astrocytomas. Cancer Res. 1997;57:2799–805.
Xiao G, Gan LS. Receptor-mediated endocytosis and brain delivery of therapeutic biologics. Int J Cell Biol. 2013;2013:703545.
Peiser L, Gordon S. The function of scavenger receptors expressed by macrophages and their role in the regulation of inflammation. Microbes Infect. 2001;3:149–59.
Srivastava RA. Scavenger receptor class B type I expression in murine brain and regulation by estrogen and dietary cholesterol. J Neurol Sci. 2003;210:11–8.
de Boer AG, van der Sandt IC, Gaillard PJ. The role of drug transporters at the blood–brain barrier. Annu Rev Pharmacol Toxicol. 2003;43:629–56.
Srimanee A, Regberg J, Hällbrink M, et al. Peptide-based delivery of oligonucleotides across blood–brain barrier model. Int J Pept Res Ther. 2014;20:169–78.
Srimanee A, Regberg J, Hällbrink M, et al. Role of scavenger receptors in peptide-based delivery of plasmid DNA across a blood–brain barrier model. Int J Pharm. 2016;500:128–35.
Ribas A, Butterfield LH, Glaspy JA, Economou JS. Current developments in cancer vaccines and cellular immunotherapy. J Clin Oncol. 2003;21:2415–32.
Madhankumar AB, Slagle-Webb B, Wang X, et al. Efficacy of interleukin-13 receptor-targeted liposomal doxorubicin in the intracranial brain tumor model. Mol Cancer Ther. 2009;8:648–54.
Wang B, Lv L, Wang Z, et al. Nanoparticles functionalized with Pep-1 as potential glioma targeting delivery system via interleukin 13 receptor α 2-mediated endocytosis. Biomaterials. 2014;35:5897–907.
Wang B, Lv L, Wang Z, et al. Improved anti-glioblastoma efficacy by IL-13Rα2 mediated copolymer nanoparticles loaded with paclitaxel. Sci Rep. 2015;5:16589.
Gao X, Qian J, Zheng S, et al. Overcoming the blood–brain barrier for delivering drugs into the brain by using adenosine receptor nanoagonist. ACS Nano. 2014;8:3678–89.
Liu Y, Mei L, Xu C, et al. Dual receptor recognizing cell penetrating peptide for selective targeting, efficient intratumoral diffusion and synthesized anti-glioma therapy. Theranostics. 2016;6:177–91.
Pardridge WM, Eisenberg J, Yang J. Human blood–brain barrier insulin receptor. J Neurochem. 1985;44:1771–8.
Xie L, Helmerhorst E, Taddei K, et al. Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. J Neurosci. 2002;22:RC221.
Dieu LH, Wu D, Palivan CG, et al. Polymersomes conjugated to 83-14 monoclonal antibodies: in vitro targeting of brain capillary endothelial cells. Eur J Pharm Biopharm. 2014;88:316–24.
Webster CI, Caram-Salas N, Haqqani AS, et al. Brain penetration, target engagement, and disposition of the blood–brain barrier-crossing bispecific antibody antagonist of metabotropic glutamate receptor type 1. FASEB J. 2016;30:1927–40.
Vivès E, Schmidt J, Pèlegrin A. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim Biophys Acta. 2008;1786:126–38.
Böckenhoff A, Cramer S, Wölte P, et al. Comparison of five peptide vectors for improved brain delivery of the lysosomal enzyme arylsulfatase A. J Neurosci. 2014;34:3122–9.
Regina A, Demeule M, Tripathy S, et al. ANG4043, a novel brain-penetrant peptide-mAb conjugate, is efficacious against HER2-positive intracranial tumors in mice. Mol Cancer Ther. 2015;14:129–40.
Yin T, Yang L, Liu Y, et al. Sialic acid (SA)-modified selenium nanoparticles coated with a high blood–brain barrier permeability peptide-B6 peptide for potential use in Alzheimer’s disease. Acta Biomater. 2015;25:172–83.
Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. Faseb J. 2004;18:998–1000.
Bechmann IL, Goldmann J, Kovac AD, et al. Circulating monocytic cells infiltrate layers of anterograde axonal degeneration where they transform into microglia. Faseb J. 2005;19:647–9.
Priller J, Flügel A, Wehner T, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–61.
Tong HI, Kang W, Davy PM, et al. Monocyte trafficking, engraftment, and delivery of nanoparticles and an exogenous gene into the acutely inflamed brain tissue: evaluations on monocyte-based delivery system for the central nervous system. PLoS One. 2016;11:e0154022.
Batrakova EV, Gendelman HE, Kabanov AV. Cell-mediated drug delivery. Expert Opin Drug Deliv. 2011;8:415–33.
Chien CT, Jou MJ, Cheng TY, et al. Exendin-4-loaded PLGA microspheres relieve cerebral ischemia/reperfusion injury and neurologic deficits through long-lasting bioactivity-mediated phosphorylated Akt/eNOS signaling in rats. J Cereb Blood Flow Metab. 2015;35:1790–803.
Vera M, Barcia E, Negro S, et al. New celecoxib multiparticulate systems to improve glioblastoma treatment. Int J Pharm. 2014;473:518–27.
Shivinsky A, Bronshtein T, Haber T, Machluf M. The effect of AZD2171- or sTRAIL/Apo2L-loaded polylactic-co-glycolic acid microspheres on a subcutaneous glioblastoma model. Biomed Microdevices. 2015;17:69.
Floyd JA, Galperin A, Ratner BD. Drug encapsulated aerosolized microspheres as a biodegradable, intelligent glioma therapy. J Biomed Mater Res A. 2016;104:544–52.
Brem H, Gabikian P. Biodegradable polymer implants to treat brain tumors. J Control Release. 2001;74:63–7.
Newcomb R, Abbruscato TJ, Singh T, et al. Bioavailability of ziconotide in brain: influx from blood, stability and diffusion. Peptides. 2000;21:491–501.
Panigrahi M, Das PK, Parikh PM. Brain tumor and Gliadel wafer treatment. Indian J Cancer. 2011;48:11–7.
Gliadel. http://gliadel.com/patient/how-gliadel-is-used.php. Accessed 18 Nov 2016.
Li X, Tsibouklis J, Weng T, et al. Nano carriers for drug transport across the blood brain barrier. J Drug Target. 2016;19:1–12.
Elenna B, Alexander VK. Polymers for CNS drug delivery. Pharm Tech Europe 2007;19(5):23–31.
Umezawa F, Eto Y. Liposome targeting to mouse brain: mannose as a recognition marker. Biochem Biophys Res Commun. 1988;153:1038–44.
Aoki H, Kakinuma K, Morita K, et al. Therapeutic efficacy of targeting chemotherapy using local hyperthermia and thermosensitive liposome: evaluation of drug distribution in a rat glioma model. Int J Hyperther. 2004;20:595–605.
Chekhonin VP, Zhirkov YA, Gurina OI, et al. PEGylated immunoliposomes directed against brain astrocytes. Drug Deliv. 2005;12:1–6.
Pardridge WM. Tyrosine hydroxylase replacement in experimental Parkinson’s disease with transvascular gene therapy. NeuroRx. 2005;2:129–38.
Chen X, Yuan M, Zhang Q, et al. Synergistic combination of doxorubicin and paclitaxel delivered by blood brain barrier and glioma cells dual targeting liposomes for chemotherapy of brain glioma. Curr Pharm Biotechnol. 2016;17:636–50.
Harbi I, Aljaeid B, El-Say KM, Zidan AS. Glycosylated sertraline-loaded liposomes for brain targeting: QbD study of formulation variabilities and brain transport. AAPS PharmSciTech. 2016;17(6):1404–20.
Wei X, Gao J, Zhan C, et al. Liposome-based glioma targeted drug delivery enabled by stable peptide ligands. J Control Release. 2015;218:13–21.
Monsalve Y, Tosi G, Ruozi B, et al. PEG-g-chitosan nanoparticles functionalized with the monoclonal antibody OX26 for brain drug targeting. Nanomedicine (Lond). 2015;10:1735–50.
Chen B, He XY, Yi XQ, et al. Dual-peptide-functionalized albumin-based nanoparticles with ph-dependent self-assembly behavior for drug delivery. ACS Appl Mater Interfaces. 2015;7:15148–53.
Fornaguera C, Dols-Perez A, Calderó G, et al. PLGA nanoparticles prepared by nano-emulsion templating using low-energy methods as efficient nanocarriers for drug delivery across the blood–brain barrier. J Control Release. 2015;211:134–43.
Attama AA, Momoh MA, Philip F. Lipid nanoparticulate drug delivery systems: a revolution in dosage form design and development. In: Sezer AD, editor. Recent Advances Novel Drug Carrier Systems. Croatia (European Union): In Tech; 2012. doi:10.5772/50486.
Shah R, Eldridge D, Palombo E, Harding I. Lipid nanoparticle: production, characterization and stability. 1st ed. Geneva: Springer International Publishing; 2015.
Garanti T, Stasik A, Burrow AJ, et al. Anti-glioma activity and the mechanism of cellular uptake of asiatic acid-loaded solid lipid nanoparticles. Int J Pharm. 2016;500(1–2):305–15.
Kuo YC, Wang IH. Enhanced delivery of etoposide across the blood–brain barrier to restrain brain tumor growth using melanotransferrin antibody- and tamoxifen-conjugated solid lipid nanoparticles. J Drug Target. 2016;24:645–54.
Misra S, Chopra K, Sinha VR, Medhi B. Galantamine-loaded solid-lipid nanoparticles for enhanced brain delivery: preparation, characterization, in vitro and in vivo evaluations. Drug Deliv. 2016;23:1434–43.
Singh I, Swami R, Pooja D, et al. Lactoferrin bioconjugated solid lipid nanoparticles: a new drug delivery system for potential brain targeting. J Drug Target. 2016;24:212–23.
Wu M, Fan Y, Lv S, et al. Vincristine and temozolomide combined chemotherapy for the treatment of glioma: a comparison of solid lipid nanoparticles and nanostructured lipid carriers for dual drugs delivery. Drug Deliv. 2015;27:1–6.
Yang Hu. Nanoparticle-mediated brain-specific drug delivery, imaging and diagnosis. Pharm Res. 2010;27:1759–71.
Cui Y, Xu Q, Chow PK, et al. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials. 2013;34:8511–20.
Ren J, Shen S, Wang D, et al. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 2012;33:3324–33.
Liu DF, Qian C, An YL, et al. Magnetic resonance imaging of post-ischemic blood–brain barrier damage with PEGylated iron oxide nanoparticles. Nanoscale. 2014;6:15161–7.
Huang Y, Zhang B, Xie S, et al. Superparamagnetic iron oxide nanoparticles modified with Tween 80 pass through the intact blood–brain barrier in rats under magnetic field. ACS Appl Mater Inter. 2016;8:11336–41.
Shevtsov MA, Nikolaev BP, Yakovleva LY, et al. Superparamagnetic iron oxide nanoparticles conjugated with epidermal growth factor (SPION-EGF) for targeting brain tumors. Int J Nanomed. 2014;9:273–87.
Laurent S, Saei AA, Behzadi S, et al. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: opportunities and challenges. Expert Opin Drug Deliv. 2014;11:1449–70.
Picone P, Ditta LA, Sabatino MA, et al. Ionizing radiation-engineered nanogels as insulin nanocarriers for the development of a new strategy for the treatment of Alzheimer’s disease. Biomaterials. 2016;80:179–94.
Baklaushev VP, Nukolova NN, Khalansky AS, et al. Treatment of glioma by cisplatin-loaded nanogels conjugated with monoclonal antibodies against Cx43 and BSAT1. Drug Deliv. 2015;22:276–85.
Nukolova NV, Baklaushev VP, Abakumova TO, et al. Targeted delivery of cisplatin by connexin 43 vector nanogels to the focus of experimental glioma C6. Bull Exp Biol Med. 2014;157:524–9.
Madaan K, Kumar S, Poonia N, et al. Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci. 2014;6(3):139–50.
Noriega-Luna B, Godínez LA, Rodríguez FJ, et al. Applications of dendrimers in drug delivery agents, diagnosis, therapy, and detection. J Nanomaterials. 2014;2014:Article ID 507273. (19 pages).
Katare YK, Daya RP, Sookram Gray C, et al. Brain targeting of a water insoluble antipsychotic drug haloperidol via the intranasal route using PAMAM dendrimer. Mol Pharm. 2015;12:3380–8.
Zhao J, Zhang B, Shen S, et al. CREKA peptide-conjugated dendrimer nanoparticles for glioblastoma multiforme delivery. J Colloid Interface Sci. 2015;450:396–403.
Zarebkohan A, Najafi F, Moghimi HR, et al. Synthesis and characterization of a PAMAM dendrimer nanocarrier functionalized by SRL peptide for targeted gene delivery to the brain. Eur J Pharm Sci. 2015;78:19–30.
Sonali, Agrawal P, Singh RP, et al. Transferrin receptor-targeted vitamin E TPGS micelles for brain cancer therapy: preparation, characterization and brain distribution in rats. Drug Deliv. 2016;23:1788–98.
Wang G, Wang JJ, Chen XL, et al. Quercetin-loaded freeze-dried nanomicelles: improving absorption and anti-glioma efficiency in vitro and in vivo. J Control Release. 2016;235:276–90.
Wang G, Wang JJ, Tang XJ, et al. In vitro and in vivo evaluation of functionalized chitosan-Pluronic micelles loaded with myricetin on glioblastoma cancer. Nanomedicine. 2016;12:1263–78.
Wang G, Wang JJ, Li F, To SS. Development and evaluation of a novel drug delivery: Pluronics/SDS mixed micelle loaded with myricetin in vitro and in vivo. J Pharm Sci. 2016;105:1535–43.
Đorđević SM, Cekić ND, Savić MM, et al. Parenteral nanoemulsions as promising carriers for brain delivery of risperidone: design, characterization and in vivo pharmacokinetic evaluation. Int J Pharm. 2015;493:40–54.
Vyas TK, Shahiwala A, Amiji MM. Improved oral bioavailability and brain transport of saquinavir upon administration in novel nanoemulsion formulations. Int J Pharm. 2008;347:93–101.
Discher BM, Won YY, Ege DS, et al. Polymersomes: tough vesicles made from diblock copolymers. Science. 1999;284:1143–6.
Pang Z, Gao H, Yu Y, et al. Enhanced intracellular delivery and chemotherapy for glioma rats by transferrin-conjugated biodegradable polymersomes loaded with doxorubicin. Bioconjug Chem. 2011;22:1171–80.
Yu Y, Jiang X, Gong S, et al. The proton permeability of self-assembled polymersomes and their neuroprotection by enhancing a neuroprotective peptide across the blood–brain barrier after modification with lactoferrin. Nanoscale. 2014;6:3250–8.
Chen YC, Chiang CF, Chen LF, et al. Polymersomes conjugated with des-octanoyl ghrelin and folate as a BBB-penetrating cancer cell-targeting delivery system. Biomaterials. 2014;35:4066–81.
Yang T, Martin P, Fogarty B, et al. Exosome delivered anticancer drugs across the blood–brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32:2003–14.
Kooijmans SA, Vader P, van Dommelen SM, et al. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomed. 2012;7:1525–41.
Lai RC, Yeo RW, Tan KH, Lim SK. Exosomes for drug delivery: a novel application for the mesenchymal stem cell. Biotechnol Adv. 2013;31:543–51.
van den Boorn JG, Dassler J, Coch C, et al. Exosomes as nucleic acid nanocarriers. Adv Drug Deliv Rev. 2013;65:331–5.
Lakhaland S, Wood MJ. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. BioEssays News Rev Mol Cell Dev Biol. 2011;33:737–41.
Zhuang X, Xiang X, Grizzle W, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther J Am Soc Gene Ther. 2011;19:1769–79.
Gonzales PA, Pisitkun T, Hoffert JD, et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol. 2009;20:363–79.
Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–5.
Jang SC, Kim OY, Yoon CM, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698–710.
Yangand J, Aschner M. Developmental aspects of blood–brain barrier (BBB) and rat brain endothelial (RBE4) cells as in vitro model for studies on chlorpyrifos transport. Neurotoxicology. 2003;24:741–5.
Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther J Am Soc Gene Ther. 2010;18:1606–14.
Paris-Robidas S, Brouard D, Emond V, et al. Internalization of targeted quantum dots by brain capillary endothelial cells in vivo. J Cereb Blood Flow Metab. 2016;36:731–42.
Chaichana KL, Pinheiro L, Brem H. Delivery of local therapeutics to the brain: working toward advancing treatment for malignant gliomas. Ther Deliv. 2015;6:353–69.
Santini JT Jr, Cima MJ, Langer R. A controlled-release microchip. Nature. 1999;397:335–8.
Richards Grayson AC, Choi IS, Tyler BM, et al. Multi-pulse drug delivery from a resorbable polymeric microchip device. Nat Mater. 2003;2:767–72.
Scott AW, Tyler BM, Masi BC, et al. Intracranial microcapsule drug delivery device for the treatment of an experimental gliosarcoma model. Biomaterials. 2011;32:2532–9.
Illum L. Nasal drug delivery: possibilities, problems and solutions. J Control Release. 2003;87:187–98.
Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11:1–18.
Quay SC. Successful delivery of apomorphine to the brain following intranasal administration demonstrated in clinical study. New York: PR Newswire; 2001.
Fehm HL, Perras B, Smolnik R, et al. Manipulating neuropeptidergic pathways in humans: a novel approach to neuropharmacology. Eur J Pharmacol. 2000;405:43–54.
Perras B, Pannenborg H, Marshall L, et al. Beneficial treatment of agerelated sleep disturbances with prolonged intranasal vasopressin. J Clin N Psychopharmacol. 1999;19:28–36.
Perras B, Marshall L, Köhler G, et al. Sleep and endocrine changes after intranasal administration of growth hormone-releasing hormone in young and aged humans. Psychoneuroendocrinology. 1999;24:743–57.
Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64:614–28.
Zheng X, Shao X, Zhang C, et al. Intranasal H102 peptide-loaded liposomes for brain delivery to treat Alzheimer’s disease. Pharm Res. 2015;32:3837–49.
Rassu G, Soddu E, Cossu M, et al. Solid microparticles based on chitosan or methyl-β-cyclodextrin: a first formulative approach to increase the nose-to-brain transport of deferoxamine mesylate. J Control Release. 2015;201:68–77.
Elnaggar YS, Etman SM, Abdelmonsif DA, Abdallah OY. Intranasal piperine-loaded chitosan nanoparticles as brain-targeted therapy in Alzheimer’s disease: optimization, biological efficacy, and potential toxicity. J Pharm Sci. 2015;104:3544–56.
Muntimadugu E, Dhommati R, Jain A, et al. Intranasal delivery of nanoparticle encapsulated tarenflurbil: a potential brain targeting strategy for Alzheimer’s disease. Eur J Pharm Sci. 2016;92:224–34.
Krauze MT, Saito R, Noble C, et al. Effects of the perivascular space on convection-enhanced delivery of liposomes in primate putamen. Exp Neurol. 2005;196:104–11.
Vogelbaum MA, Aghi MK. Convection-enhanced delivery for the treatment of glioblastoma. Neuro Oncol. 2015;17(Suppl. 2):ii3–8.
Bernal GM, LaRiviere MJ, Mansour N, et al. Convection-enhanced delivery and in vivo imaging of polymeric nanoparticles for the treatment of malignant glioma. Nanomedicine. 2014;10:149–57.
Corem-Salkmon E, Ram Z, Daniels D, et al. Convection-enhanced delivery of methotrexate-loaded maghemite nanoparticles. Int J Nanomed. 2011;6:1595–602.
Hadjipanayis CG, Machaidze R, Kaluzova M, et al. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010;70:6303–12.
Sawyer AJ, Saucier-Sawyer JK, Booth CJ, et al. Convection-enhanced delivery of camptothecin-loaded polymer nanoparticles for treatment of intracranial tumors. Drug Deliv Transl Res. 2011;1:34–42.
Voges J, Reszka R, Gossmann A, et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol. 2003;54:479–87.
Dickinson PJ, LeCouteur RA, Higgins RJ, et al. Canine model of convection-enhanced delivery of liposomes containing CPT-11 monitored with real-time magnetic resonance imaging: laboratory investigation. J Neurosurg. 2008;108:989–98.
MacKay JA, Deen DF, Szoka FC Jr. Distribution in brain of liposomes after convection enhanced delivery; modulation by particle charge, particle diameter, and presence of steric coating. Brain Res. 2005;1035:139–53.
White E, Bienemann A, Pugh J, et al. An evaluation of the safety and feasibility of convection-enhanced delivery of carboplatin into the white matter as a potential treatment for high-grade glioma. J Neurooncol. 2012;108:77–88.
Barua NU, Woolley M, Bienemann AS, et al. Convection-enhanced delivery of AAV2 in white matter: a novel method for gene delivery to cerebral cortex. J Neurosci Methods. 2013;220:1–8.
Bogdahn U, Hau P, Stockhammer G, et al. Trabedersen Glioma Study Group. Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol. 2011;13(1):132–42.
Bogdahn U, Hau P, Stockhammer G, Trabedersen Glioma Study Group, et al. Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study. Neuro Oncol. 2010;12:401–8.
Crawford L, Rosch J, Putnam D. Concepts, technologies, and practices for drug delivery past the blood–brain barrier to the central nervous system. J Control Release. 2016;240:251–6.
Borlongan CV, Glover LE, Sanberg PR, Hess DC. Permeating the blood brain barrier and abrogating the inflammation in stroke: implications for stroke therapy. Curr Pharm Des. 2012;18:3670–6.
Sun Z, Worden M, Wroczynskyj Y, et al. Magnetic field enhanced convective diffusion of iron oxide nanoparticles in an osmotically disrupted cell culture model of the blood–brain barrier. Int J Nanomed. 2014;9:3013–26.
Okuma Y, Wang F, Toyoshima A, et al. Mannitol enhances therapeutic effects of intra-arterial transplantation of mesenchymal stem cells into the brain after traumatic brain injury. Neurosci Lett. 2013;554:156–61.
Yao ST, May CN. Intra-carotid angiotensin II activates tyrosine hydroxylase expressing rostral ventrolateral medulla neurons following blood–brain barrier disruption in rats. Neuroscience. 2013;24:148–56.
Foley CP, Rubin DG, Santillan A, et al. Intraarterial delivery of AAV vectors to the mouse brain after mannitol mediated blood brain barrier disruption. J Control Release. 2014;196:71–8.
Hwang DW, Son S, Jang J, et al. A brain-targeted rabies virus glycoprotein-disulfide linked PEI nanocarrier for delivery of neurogenic microRNA. Biomaterials. 2011;32:4968–75.
Salahuddin TS, Johansson BB, Kalimo H, Olsson Y. Structural changes in the rat brain after carotid infusions of hyperosmolar solutions: an electron microscopic study. Acta Neuropathol. 1988;77:5–13.
Salahuddin TS, Johansson BB, Kalimo H, Olsson Y. Structural changes in the rat brain after carotid infusions of hyperosmolar solutions: a light microscopic and immunohistochemical study. Neuropathol Appl Neurobiol Neuropept. 1988;14:467–82.
Miller G. Drug targeting: breaking down barriers. Science. 2002;297:1116–8.
Yan-Yu X, Qi-Neng P, Zhi-Peng C. The enhancing effect of synthetical borneol on the absorption of tetramethylpyrazine phosphate in mouse. Int J Pharm. 2007;337:74–9.
Garg P, Pandey S, Seonwoo H, et al. Hyperosmotic polydixylitol for crossing the blood brain barrier and efficient nucleic acid delivery. Chem Commun. 2015;51:3645–8.
On NH, Savant S, Toews M, Miller DW. Rapid and reversible enhancement of blood–brain barrier permeability using lysophosphatidic acid. J Cereb Blood Flow Metab. 2013;33:1944–54.
Matsukado K, Inamura T, Nakano S, et al. Enhanced tumor uptake of carboplatin and survival in glioma-bearing rats by intracarotid infusion of the bradykinin analog, RMP-7. Neurosurgery. 1996;39:125–33.
Côté J, Savard M, Neugebauer W, et al. Dual kinin B1 and B2 receptor activation provides enhanced blood–brain barrier permeability and anticancer drug delivery into brain tumors. Cancer Biol Ther. 2013;14:806–11.
Kuo YC, Chou PR. Neuroprotection against degeneration of SK-N-MC cells using neuron growth factor-encapsulated liposomes with surface cereport and transferrin. J Pharm Sci. 2014;103:2484–97.
Kim DG, Bynoe MS. A2A adenosine receptor regulates the human blood–brain barrier permeability. Mol Neurobiol. 2015;52:664–78.
Gao H, Xiong Y, Zhang S, et al. RGD and interleukin-13 peptide functionalized nanoparticles for enhanced glioblastoma cells and neovasculature dual targeting delivery and elevated tumor penetration. Mol Pharm. 2014;11:1042–52.
Zheng S, Bai YY, Liu Y, et al. Salvaging brain ischemia by increasing neuroprotectant uptake via nanoagonist mediated blood brain barrier permeability enhancement. Biomaterials. 2015;66:9–20.
Zhang Y, Miller DW. Pathways for drug delivery to the central nervous system. In: Wang B, Siahaan T, Soltero RA, editors. Drug delivery: principles and applications. Hoboken: Wiley Interscience; 2005. p. 29–56.
Erdlenbruch B, Alipour M, Fricker G, et al. Alkylglycerol opening of the blood–brain barrier to small and large fluorescence markers in normal and C6 glioma-bearing rats and isolated rat brain capillaries. Br J Pharmacol. 2003;140:1201–10.
Erdlenbruch B, Jendrossek V, Eibl H, Lakomek M. Transient and controllable opening of the blood–brain barrier to cytostatic and antibiotic agents by alkylglycerols in rats. Exp Brain Res. 2000;135:417–22.
Hülper P, Veszelka S, Walter FR, et al. Acute effects of short-chain alkylglycerols on blood–brain barrier properties of cultured brain endothelial cells. Br J Pharmacol. 2013;169:1561–73.
Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imagingguided focal opening of the blood–brain barrier in rabbits. Radiology. 2001;220(3):640–6.
Aryal M, Arvanitis CD, Alexander PM, McDannold N. Ultrasound-mediated blood–brain barrier disruption for targeted drug delivery in the central nervous system. Adv Drug Deliv Rev. 2014;72:94–109.
Yang FY, Lin YL, Chou FI, et al. Pharmacokinetics of BPA in gliomas with ultrasound induced blood–brain barrier disruption as measured by microdialysis. PLoS One. 2014;9:e100104.
Park J, Zhang Y, Vykhodtseva N, et al. The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound. J Control Release. 2012;162:134–42.
Choi JJ, Selert K, Gao Z, et al. Noninvasive and localized blood–brain barrier disruption using focused ultrasound can be achieved at short pulse lengths and low pulse repetition frequencies. J Cereb Blood Flow Metab. 2011;31:725–37.
Huang Q, Deng J, Wang F, et al. Targeted gene delivery to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Exp Neurol. 2012;233:350–6.
Huang Q, Deng J, Xie Z, et al. Effective gene transfer into central nervous system following ultrasound-microbubbles-induced opening of the blood–brain barrier. Ultrasound Med Biol. 2012;38:1234–43.
Burgess A, Huang Y, Querbes W, et al. Focused ultrasound for targeted delivery of siRNA and efficient knockdown of Htt expression. J Control Release. 2012;163:125–9.
Alonso A, Reinz E, Leuchs B, et al. Focal delivery of AAV2/1-transgenes into the rat brain by localized ultrasound-induced BBB Opening. Mol Ther Nucleic Acids. 2013;2:e73.
Hsu PH, Wei KC, Huang CY, et al. Noninvasive and targeted gene delivery into the brain using microbubble-facilitated focused ultrasound. PLoS One. 2013;8:e57682.
O’Reilly MA, Waspe AC, Ganguly M, Hynynen K. Focused-ultrasound disruption of the blood–brain barrier using closely-timed short pulses: influence of sonication parameters and injection rate. Ultrasound Med Biol. 2011;37:587–94.
O’Reilly MA, Huang Y, Hynynen K. The impact of standing wave effects on transcranial focused ultrasound disruption of the blood–brain barrier in a rat model. Phys Med Biol. 2010;55:5251–67.
Samiotaki G, Konofagou EE. Dependence of the reversibility of focused ultrasound induced blood–brain barrier opening on pressure and pulse length in vivo. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60:2257–65.
Yang FY, Lin GL, Horng SC, et al. Pulsed high intensity focused ultrasound enhances the relative permeability of the blood tumor barrier in a glioma-bearing rat model. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58:964–70.
Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6:268–86.
Aryal M, Vykhodtseva N, Zhang YZ, McDannold N. Multiple sessions of liposomal doxorubicin delivery via focused ultrasound mediated blood–brain barrier disruption: a safety study. J Control Release. 2015;204:60–9.
Vykhodtseva N, McDannold N, Hynynen K. Progress and problems in the application of focused ultrasound for blood–brain barrier disruption. Ultrasonics. 2008;48(4):279–96.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this review.
Conflict of interest
Mayur M. Patel and Bhoomika M. Patel declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Patel, M.M., Patel, B.M. Crossing the Blood–Brain Barrier: Recent Advances in Drug Delivery to the Brain. CNS Drugs 31, 109–133 (2017). https://doi.org/10.1007/s40263-016-0405-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-016-0405-9