Abstract
The barriers in the delivery of the therapeutic agent to brain diseases are blood–brain barrier (BBB), blood-cerebrospinal fluid barrier, and cellular barriers. The above mentioned barriers limit the distribution of the therapeutic agent or drug delivery system, thereby affects the therapeutic efficacy. The route of administration is also an important factor in the drug delivery to the brain diseases. Therefore, there is unmet need for the development of drug delivery systems which will overcome the barriers and delivers the therapeutic agent to the brain diseases. This chapter is focused on various strategies used to overcome the barriers in drug delivery to the brain diseases. The application of energy and chemical substances such as osmotic agent and permeation enhancers has been studied. Other strategies, such as developing the prodrug and inclusion complex of therapeutic agents, have been explained. The application and limitations of the different routes of administration such as intravenous, intra-arterial, intranasal, intracerebral, and intracerebroventricular have been described. The drug delivery system in the nanoscale such as liposomes, nanoemulsion, polymeric nanoparticles, and dendrimer have been explored to overcome the limitations associated with drug delivery to the brain diseases. Specific examples are described in this chapter. Lastly, various ongoing clinical trials for drug targeting to the brain are listed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
The brain is one of the most vital organs, acting as the control center of the body. It is the key component of the central nervous system (CNS). Any small irregularities to this vital system might pose devastating consequences in one’s lifestyle, leading to diseases or disorders. Some of those conditions include epilepsy, Alzheimer’s, cerebrovascular diseases, neurodegenerative disorders such as Parkinson’s, HIV encephalopathy, and brain tumors. Most of these disease conditions require the drug substance to reach the intracranial target site for potential therapeutic effect. Despite billion-dollar investments and aggressive research for the cure, patients suffering from such brain relevant diseases/disorders outnumber those dying of heart diseases or other types of cancers [1, 2]. An arsenal of potent therapeutic agents developed over the last 50 years demonstrated promising effects in the laboratory, but their translation to the clinic has been very less successful. This failure is often not due to lack of drug potency but due to associated limitations in the therapeutic agent pharmacokinetics, off-target effects, and in the drug delivery methods. Some formidable impediments that regularly hamper effective drug delivery to the brain were identified in the early twentieth century, with blood-brain barrier (BBB) being the first [3]. Several novel research strategies have been proposed and investigated, since then, to overcome the deficiencies and challenges associated with conventional delivery mechanisms. This chapter briefly discusses those barriers impeding brain delivery followed by strategies to overcome associated challenges and concludes with summary of approved drug products as well as ongoing clinical trials.
11.2 Barriers and Approaches to Tailor BBB
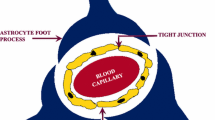
The BBB is the first physiological impediment to the delivery of drug molecules from the systemic circulation to the brain. It acts as a selectively permeable membrane, separating from the peripheral systemic circulation, to control and protect the brain microenvironment, i.e., neurons that are highly susceptible to changes in signaling neurotransmitters as well as extracellular ions. The same selectively hamper drug molecule’s penetration through the endothelium lining. This led to aggressive research to study distinguishable physiological characteristics between the capillary endothelial cell linings of the cerebral and systemic microvasculature. The first characteristic is the absence of pores and fenestrations with gaps in the cerebral endothelial cells when compared with systemic capillaries. Exchange of ions or therapeutic agents between blood and surrounding interstitium is commonly abetted by the membrane transporters. Tight penta-laminar arrangement of adjacent cerebral capillary endothelial cells are responsible for limited permeability of ions and small molecules and practically impermeable to peptides and macromolecules [4]. This was further confirmed by the measurements of ionic current flow across most systemic and cerebral capillary endothelium microvasculature [5]. In BBB disruption approach, the therapeutic agents are directly delivered to CNS by amending the integrity of tight junctions of BBB causing transient disruption of BBB. This BBB disruption can be achieved by two methods: (i) application of energy and (ii) use of chemical substances.
11.2.1 By Application of Energy
Electromagnetic radiations or ultrasound is used for disrupting BBB. These approaches offer the advantage of targeting a specific area of the brain. The radiation was used to tailor the properties of BBB which can be explored for drug or gene delivery [6]. The use of ultrasound for tailoring the properties of BBB for drug or gene therapy was summarized in the reported article [7]. Focused ultrasound when combined with microbubbles resulted in increase in permeability of BBB and the blood-tumor barrier (BTB), which helps in enhancing delivery of doxorubicin across BBB and BTB and increased the retention of the drug in the tissue up to 24 h [8].
The path of entry of drug molecules by ultrasound occurs by the following mechanisms:
-
(i)
Thermal lesions are formed which leads to alteration of permeability and opening of BBB.
-
(ii)
Small air-filled cavities are formed by the injected fluid in the luminal membrane of BBB which allows the drug to pass through them easily.
-
(iii)
Microbubbles are formed by ultrasound contrast agents, which increase in size and finally burst causing the opening of tight junctions.
This method is mostly used as a diagnostic tool for imaging brain and brain tumors [9].
11.2.2 By the Usage of Chemical Substances
11.2.2.1 Osmosis-Mediated BBB Disruptions
In this method, chemical substances that are hyperosmolar/hypertonic to BBB cells are used. When the cells are in hypertonic solutions, the cellular fluids come out of the cells to maintain equilibrium, and as a result, cells shrivel leading to the opening of tight junctions in capillary endothelial cells. The most common osmotic agent used for this is mannitol, which has the potential to help drugs to cross BBB. It was also shown that mannitol increased the delivery of stem cells and growth factors across BBB [10].
However, this technique is limited due to the prolonged recovery period of BBB after disruption, which leads to an increase in intracranial pressure due to influx of macrophages and other molecules [11]. Increased intracranial pressure is a contraindication for brain tumor treatments. Other disadvantages are hemodynamic variability between patients and variable BBB disruption after repeated exposure to osmosis- or radiation-mediated disruption [12].
11.2.2.2 By Permeation Enhancers
The chemical substances such as bradykinin analogs and alkylglycerol act by increasing the permeability of BBB. The few examples are given below.
-
(a)
Bradykinin analogs: These substances stimulate B2 receptors, which lead to increase in intracellular calcium levels. This calcium activates actin/myosin fibers leading to leaching out junctional proteins and thereby loosens the tight junctions [13]. Cereport (RMP-7) is a bradykinin analog, which increased the delivery of loperamide to the brain resulting in the induction of analgesic effect [14].
-
(b)
Alkylglycerols: These are surfactant-like molecules. These agents disrupt BBB by destabilizing the membrane. Despite being a successful strategy, disrupting the BBB increases the risk of infection [15]. In normal brain and brain tumors, alkylglycerols enhanced the delivery of small and large compounds [16].
11.3 Approaches to Modify Therapeutic Agents
Despite being a successful strategy, disrupting the BBB increases the risk of infections. Various strategies have been exploited to achieve the goal of delivering the drug into the brain, which can be broadly classified into four parts: (i) bypassing the BBB, (ii) BBB disruption, (iii) modification of drug, and (iv) nanocarrier systems. For bypassing BBB, altering route of administration for both direct delivery and nasal pathway was studied. Physical and chemical approaches were utilized for disrupting BBB. Drug modifications including prodrug and inclusion complex followed by novel drug carrier systems were summarized below.
11.3.1 Drug Modification
11.3.1.1 Prodrug
Prodrugs are inactive drug or precursor of pharmacologically active substances which after enzymatic degradation releases or get converted into the active form of the drug within the body. A prodrug is developed by covering, masking, or altering the functional group of the parent molecule with another functional group and results in a new entity with unique physicochemical properties [17, 18]. These entities are acted upon by the enzymes present on the BBB to give active agents. It is a unique drug delivery strategy that improves the drugs’ solubility, stability, and absorption through the biological membrane and reduces premature metabolism. The prodrug was initially explored for hydrophilic drugs to enhance the nasal permeability of drug thus the nasal drug absorption and also to protect the drug from enzymatic degradation in the nasal cavity [19]. The lipophilic and biocompatible nature of promoiety makes it more suitable for nasal administration. Gambaryan and coworkers evaluated the delivery of antiparkinsonian drug dopamine in its prodrug form L-DOPA through nasal route by incorporation into the PLGA nanoparticles. The prodrug gets converted into the parent drug by enzymatic degradation with L-amino decarboxylase enzyme inside the brain. The in vivo investigation on animal model exhibited a significant improvement in the therapeutic potency of the drug and sustained drug action [20].
11.3.1.2 Inclusion Complex
The complexation between two molecules in which one serves as a cavity for the inclusion of another compound is known as an inclusion complex. It is based on the host-guest chemistry: the one who has the cavity or hole is called the host molecule, while the other which gets encapsulated into the cavity is known as guest molecule [21, 22]. Cyclodextrins are most commonly used host molecule in drug development. Owing to the unique characteristic feature, the hydrophilic outer surface and hydrophobic internal cavity, it can form a complex with a wide variety of drugs, both hydrophilic and lipophilic [23]. Furthermore, this approach overcomes the limitations of intranasal route solubility and enzymatic degradation and thus increases the bioavailability. Zhang formulated poloxamer/chitosan thermosensitive gel containing hydroxypropyl-β-cyclodextrin (HP-β-CD)-curcumin inclusion complex, to enhance the brain availability and antidepressant effect of curcumin through i.n. administration [24]. In pharmacokinetic studies, from thermoresponsive gel, AUC0–8 of curcumin was 1.62 and 1.28 times higher in plasma and hippocampus, respectively, when compared to i.v. administration, which shows that the system has high potential for clinical application.
11.4 Drug Products and Formulations Explored in Various Routes of Administration
The route of administration such as intravenous (i.v.), intra-arterial, intranasal (i.n), and intracerebroventricular has been used to deliver the therapeutic agent to the brain. The summary of commercially available drug products used for brain diseases via various route administrations is provided in Table 11.1.
11.4.1 Intravenous Delivery
The brain has extensive blood supply with a network of capillaries constituting around 20 m2 area. The neurons are also well connected with the blood vessels. Hence this approach is considered to have great potential to deliver drugs to the brain.
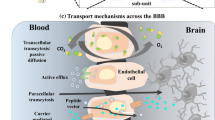
This route also bypasses first-pass metabolism. However, due to rapid metabolism and clearance of drugs from extracellular fluid, there is little accumulation of drug in the brain. Thus, drug accessibility to the brain by this route is significantly affected by its half-life, metabolism rate, permeability across BBB (Fig. 11.1, [25]), and level of nonspecific binding to plasma proteins. In addition to the conventional methods, several strategies such as nanoparticles, liposomes, polymeric micelles, and nanoemulsions were proposed to overcome the difficulties posed by BBB (Fig. 11.1, [25]). The delivery of drugs to the brain using nanoparticle systems is influenced by the physicochemical properties (composition, nature of entrapped drug, shape), modification of surface, and pharmacokinetic parameters. The characteristics of nanoparticles were represented in Fig. 11.2. Few strategies were elaborated below in which liposomes were applied via i.v. delivery. The other types of nanocarriers such as polymeric nanoparticles [26], polymeric micelles [27], and nanoemulsions [28] administered via i.v. route were reported.
Schematic of different mechanisms for BBB crossing. (Figure reproduced with permission from [25])
Nanoparticle characteristics influencing systemic delivery and blood-brain barrier (BBB) passage. (Figure reproduced with permission from [29])
Chen Z-L et al. showed liposomes modified with transferrin (Tf) promote α-mangostin (α-M) to overcome the BBB [30]. α-M is used for the treatment of Alzheimer’s disease (AD) [31]. But its activity is compromised due to poor penetration of drug through the BBB. Liposomes were prepared by thin-film hydration method. The average particle size of the Tf(α-M) liposome was 196.3 ± 7.09 nm, PDI of 0.211 ± 0.034, and zeta potential of −22.23 ± 2.87 mV. In brain imaging studies, in place of α-M, dye DiR was used and injected into the rats via the tail vein. It was observed that after 2 h of treatment, Tf-DiR liposomes showed higher fluorescence in the brain than only DiR liposomes and DiR solution groups.
When compared to unmodified liposomes and α-M solution, Tf-modified liposomes delivered more α-M into the brain, which suggests the role of Tf in the transportation of α-M into the brain. In pharmacokinetic studies, it was observed that Tf (α-M) liposome group (Dose 5mg/kg) that the t1/2, MRT, AUC O-T, and AUC O-α values were higher when compared with α-M solution, which shows that it is a suitable dosage form for α-M delivery.
Glutathione targeted pegylated (GSH-PEG) liposomes were formulated to deliver amyloid-targeting antibody fragments (VHH-pa2H) to the brain by bypassing BBB. 1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and egg-yolk phosphatidylcholine (EYPC) were used to formulate two different GSH-PEG liposomes by post-insertion method [32]. The average size of the GSH-PEG DMPC liposomal VHH-pa2H-DTPA was ~110 nm and PDI of ~0.105, and the size of GSH-PEG EYPC liposomal VHH-pa2H-DTPA was ~108 nm and PDI of ~0.061. Unencapsulated VHH-pa2H-DTPA-111In showed significantly lower AUC (P < 0.05) when compared to encapsulated VHH-pa2H-DTPA-111In GSH-PEG DMPC and GSH-PEG-EYPC. In later liposomes, VHH-pa2H-DTPA-111In GSH-PEG-EYPC showed higher AUC (P < 0.05), against VHH-pa2H-DTPA-111In GSH-PEG-DMPC. Biodistribution studies are carried out in APP/PS1 and wild-type animals. Both liposomal formulations showed significant higher retention of the tracer in excised perfused brain when compared with free VHH-pa2H (P < 0.05). In these two tested phospholipids, GSH-PEG EYPC showed better activity than GSH-PEG DMPC. Only after single injection of the tracer VHH-pa2H encapsulated GSH-PEG-EYPC liposomes showed high cellular uptake in the brain.
Jiang and coworkers conjugated glioma-homing peptide (Pep-1) to pegylated polyamidoamine (PAMAM) dendrimer nanoparticles which were evaluated in glioblastoma multiforme (GBM) as targeted delivery system [26]. In U87MG tumor-bearing mice, targeted nanoparticles fluorescence intensity at glioma site was 2.02 times higher than nontargeted system, and also they concluded that targeted delivery system showed high accumulation and penetration into the tumor.
11.4.2 Intra-arterial Delivery
The brain has a predominantly high oxygen demand. It denotes almost one-fifth of the body’s total oxygen consumption at rest. Therefore, the brain is abundantly furnished by arteries. There are mainly two paired arteries that are responsible for blood supply to the brain: the vertebral arteries and internal carotid arteries. They arise in the neck and ascend to the cranium.
Intra-arterial drug delivery across the BBB may offer the advantages, i.e., reduction in the dose of the drug to be delivered, targeted drug delivery, higher drug availability to the site of action, as well as the decreased drug exposure to the unintended sites as compared to the i.v. route [33]. Despite these advantages, BBB targeting has been limited due to the cerebral blood movement as well as the varying degree of intactness of the BBB in the diseases when presented with the drug solution. Nanoparticulate drug delivery through the carotid artery may help in overcoming the shortcomings of conventional drug delivery. The important factors affecting the efficiency of such delivery systems involve an interplay between the pharmacokinetic, pharmacodynamic, and hydrodynamic factors.
Liposome-encapsulated antibody against ICAM-1 (intercellular adhesion molecule-1) when administered through the carotid artery resulting in a significant increase in uptake in TNFα induced inflamed areas of the mice brain (much higher than the healthy brain) as compared to the vehicle delivery through the jugular vein. Additionally, these ~150 nm nanoparticles showed more than 100-fold higher uptake as compared to the anti-IgG tagged liposome besides being significantly biodistributed into the brain as compared to lungs. The specific uptake of the immuno-targeted nanocarrier may be attributed to the overexpression of the ICAM-1 on the luminal surface of the inflamed tissue of the brain. This approach of combining the targeted nanocarrier along with the intra-arterial catheter-based local delivery may be utilized for the delivery of the therapeutics against the inflamed brain etiologies [34].
PAMAM (polyamidoamine)-based generation 4 dendrimers loaded with deferoxamine tuned to 5 nm in size, and the neutral surface charge was tested for their uptake in the mice glioma brain via intra-arterial as well as i.v. The intra-arterial route-based delivery showed a significant improvement in the brain uptake of these carriers (after 1 h and after 24 h) than the i.v. delivery in case of intact BBB as well as mannitol-induced disrupted BBB. Such dendrimer-based approaches may be utilized for the delivery of the chemotherapeutic agents as well as other therapeutic agents across the BBB [35]. However, the design of such delivery vehicles needs to establish a careful balance between the physicochemical components as well as the associated physiological risks (such as cerebral lesions) of this delivery route [36].
11.4.3 Intranasal
The intranasal (i.n.) route of administration of drugs for drug delivery to the brain is being used extensively due to direct transport of drug from the submucosa of the nose to cerebrospinal fluid (CSF) compartment of the brain. This route offers advantages of evading BBB and first-pass metabolism. The nasal epithelium is highly permeable and allows rapid drug absorption to the brain due to high blood flow owing to its large surface area and porous membrane. This route delivers many advantages like reduced dose, self-administration, improved patient compliance (noninvasive), and compatibility for the delivery of wide variety of therapeutic agents. However, it has some limitations like damaging nasal mucosa, irritation, rapid clearance by mucociliary clearance system, elimination by systemic absorption, and interference due to patient conditions like nasal congestion.
The nasal cavity is divided into three regions: vestibular, respiratory, and olfactory region. The vestibular region is the first region that is highly enriched with ciliated cells and mucus, which are engaged in mucociliary clearance. Most of the drug administered in this region is lost due to mucociliary clearance. The respiratory region, which covers the major portion of the nasal cavity, is highly vascularized, and it is the major site of drug absorption into systemic circulation. Compounds enter the bloodstream by transcellular/paracellular passive absorption−/carrier-mediated transport/transcytosis pathways. The olfactory region, next to respiratory region, is the foremost site, from which drug can be absorbed directly into the brain by different mechanisms like transcellular, paracellular, olfactory, and trigeminal neural pathways.
Zheng and coworkers encapsulated novel β-sheet breaker peptide, H102 into liposomes and administered i.n. in mice for the treatment of Alzheimer’s disease [37]. H102 liposomes were prepared by thin-film hydration method. Liposomes have a mean particle size of 112.2 ± 6.4 nm, surface charge of −2.96 ± 0.38 mV, and PDI of 0.185 ± 0.012.
In plasma, after administering i.n. H102 liposome, H102 was found after 90 min, whereas for i.n. H102 solution, it was found only up to 45 min, and it was not detectable after 5 min by i.v. route. The i.n. H102 liposomes significantly increased the absolute and relative bioavailabilities, thereby suggesting increased the nasal absorption of H102. In hippocampus, the AUC of H102 liposomes was 2.92 times higher than that of the i.n. H102 solution group which shows that the formulation can cross BBB. In Morris water maze test, compared to i.v., i.n. H102 solution, i.n. H102 liposomes effectively amended spatial memory. In Alzheimer’s disease, typical pathological indication is formation of Aβ plaque. After i.n. H102 liposome injection, the size and quantity of Aβ plaque were decreased and were close to that of sham group.
Cationic liposomes made from L-α-phosphatidylcholine and dihexadecylmethylhydroxyethylammonium bromide were analyzed for BBB crossing by utilizing the i.n. route [38]. Cationic liposomes were used to treat organophosphorus poisoning, which results from exposure to organophosphorus agents (OP). The acute toxicity of OP agents was observed due to the inhibition of acetylcholinesterase. They used 2-PAM which is cholinesterase reactivator as a model drug in formulation of cationic liposome for the treatment of organophosphorus poisoning. The hydrodynamic diameter of 2-PAM loaded liposomes was 1142 ± 2 nm with zeta potential of +6 ± 0.2 mV and PDI of 0.2 ± 0.03. Rhodamine B encapsulated cationic liposomes applied i.n. showed higher rhodamine absorption in the brain.
For the reactivation of brain AChE, rat model was used. Organophosphate paraoxon (POX) (0.8 × LD50) was used as AChE inhibitor. 2-PAM (7 mg/kg) loaded cationic liposomes when introduced i.n. showed 12 ± 1% reactivation of brain AChE, whereas the free 2PAM was failed in reactivation of AChE.
In other studies, paroxetine was delivered i.n. in rats using nanoemulsion formulation, which showed 2.57 times in an increase in permeation when delivered using nanosuspension orally [39]. Behavior activities also improved drastically when nanoformulation applied i.n. by increasing glutathione levels and decreasing the increased TBARS in Wistar rats. Also, efavirenz, which is an antiretroviral drug, was formulated in solid lipid nanoparticles and administered i.n. in adult Wistar albino rats, which showed 150-fold increase in brain targeting and 70 times higher absorption potential when compared with orally administered marketed formulation [40].
11.4.4 Intracerebral
Drugs can be directly administered in the brain by intracerebral administration by injection and implants. Drugs can be injected as bolus or infusion. However, the bolus injection is hampered by the limited diffusion coefficient of drugs through brain parenchyma which results in slower movement of compounds. Intracerebral infusion requires the insertion of a catheter into the brain. This strategy can be improved by convection-enhanced delivery, which involves positive hydrostatic pressure. A positive pressure gradient is created through an infusion pump, which enables the administration of drug through catheter and helps the administered drug to penetrate further into target tissue.
Although the intracerebral route of administration results in high local concentrations of the drug, the drug release kinetics from the carrier, physiochemical microenvironment at the injection site, as well as diffusion-based uptake through the brain parenchyma govern the efficacy of the product. Drug delivery through this approach not only reduces the systemic toxicity of drug by avoiding the BBB path but also may be associated with neurotoxicity at the site of delivery due to the lack of efficient diffusion-based drug uptake and presence of high localized concentrations of the drug [41].
Surface decoration of PLGA nanoparticles with high degree of pegylation and loaded with paclitaxel was evaluated against 9 L gliosarcoma-bearing Harlan F344 rats. These nanoparticles having a size of 70 nm and near zeta potential (−2 mV) exhibited 100-fold increase in the tumor uptake with a significant reduction in the tumor growth as well as improved bio-distribution in the tumor parenchymal cells as compared to the non-pegylated components. This improved intra-tumor distribution may serve in development of better treatment of brain tumors as well as other brain disorders [42].
The immune cell components of the central nervous system, microglia, have been implicated for their role in the destruction and degeneration of the neurons in brain diseases such as multiple sclerosis, Parkinsonian disease, and Alzheimer’s disease [43]. The progress of the disease may be arrested by the use of clodronate, which propagates the apoptosis of the microglia and microglia-induced activation of chemokines, cytokines, and proteases. The intracerebral injection of the liposomal clodronate resulted in a significant improvement in the uptake by the parenchymal cells with the subsequent reduction in the microglial population as compared with i.v. injection. However, the liposomal drug delivery through this route showed the toxicity to brain cells [44].
Additionally, the controlled release of the drug delivered through intracerebral delivery may be achieved by the use of polymeric biodegradable wafers, nanofibers, and depots [45]. The USFDA and EMEA approved product Gliadel™ (carmustine wafer) prepared using polifeprosan 20 copolymer is one such example used as an adjunct to other chemotherapeutics in newly diagnosed gliomas as well as glioblastoma [46]. The blood carmustine levels are detected 24 h postimplantation with the drug reaching Cmax within 3 h after carmustine release [47].
11.4.5 Intracerebroventricular Injection
The drug is directly introduced into the ventricles of the brain after intracerebroventricular (ICV) injection. The ventricles of the brain allow the diffusion of drug into parenchymal cells of the brain through the interaction of CSF contents with interstitial fluid. However, as the rate of CSF turnover is much faster than the drug diffusion, drug is more prone to enter general circulation than targeted cites in the brain. This CSF turnover depends on size of the individual and volume of CSF. For instance, adults have more CSF than children and thus longer turnover time. Therefore, the drug concentrations vary largely with individuals. Furthermore, this route leads to higher drug exposure at the ependymal surface and is effective if the target receptor is located near the ependymal surface.
Finan and coworkers tested intracerebroventricular route for the treatment of edema in mice [48]. They have used the chondroitinase ABC enzyme, which degrades chondroitin sulfate proteoglycan, which is responsible for edema. After the treatment, the ipsilateral water fraction was decreased to 0.54%, which indicates more than half of the edema induced by trauma was overcome. The treatment was selective as water fraction is not effected in uninjured animals when compared to injured animals. ICV method was far superior due to its improved patient compliance and efficient when compared to intraparenchymal injection and i.v. delivery. There are certain limitations for this method, such as murine brain is smaller than human brain, and here treatment was given within 5 min, which is impractical in treating humans.
Mutations in SMN1 gene result in spinal muscular atrophy, which is an autosomal recessive neuromuscular disease. In neonatal SMNΔ7mice, by administering scAAV9 vector, which is expressing a codon-optimized version of the human SMN1 cDNA under the control of PGK promotor in ICV space, therapeutic efficiency was measured [49]. All tested mice showed enhanced life span and growth in a dose-dependent manner with longest median survival of 346 days at dose of 3 × 1013 vg/kg. When ICV dose is co-administered with i.v. injection at different ratios, it didn’t improve survival. In biodistribution studies, after 90 days of postinjection, it was observed that CNS transduction is achieved through vector release by ICV route, while i.v. administration transudes principally in peripheral organs (lung and heart). One more critical advantage of gene therapy by ICV route is need of steroid treatment or immune suppression is hugely reduced. Different routes of brain targeting methods outlined in Table 11.2.
11.5 Clinical Advancements and Ongoing Clinical Trials
The nanocarrier systems have been studied in clinical trials. List of some ongoing clinical trials for brain targeting of the drug using various delivery approaches is mentioned in Table 11.3.
The ongoing clinical trials for the delivery of drugs to the brain and preclinical studies suggest that these delivery systems or approaches will be promising for the treatment of brain disorders.
11.6 Summary
The brain is almost inaccessible for most of the drugs, macromolecules, and other foreign materials due to the presence of BBB. This reduces the drug concentrations at the site of action and thereby the effectiveness of therapies and makes the treatment of CNS/brain disorders challenging. The use of chemical agents and prodrug showed promising results. Currently, the targeted drug delivery system-based therapies administered via different routes demonstrated promising findings while overcoming the BBB. The extensive work has been done in the last two decades on the use of different drug carrier systems, route of administration, and various surface-acting ligands specific to the brain receptors to successfully deliver the drugs to the brain. In this chapter, we have discussed the strategies to overcome the challenges associated with the delivery of drug/s and use of route of administration. After all such claims of numerous drug delivery strategies, till now not even one is commercially available for clinical application. However, newer strategies are being tested in ongoing clinical trials. The reason behind is most of the drug delivery approaches are successful in preliminary trials and do not show promising results in the clinical trials. Along with this, the safety profile of the carrier system, drug release behavior, encapsulation efficiency, permeability across BBB, and bioavailability are the major challenges to resolve.
References
Abbott NJ, Romero IA (1996) Transporting therapeutics across the blood-brain barrier. Mol Med Today 2:106–113
Kibiuk LV, Stuart D, Miller M (2008) Brain facts: a primer on the brain and nervous system. The Society for Neuroscience
Banks WA (2016) From blood–brain barrier to blood–brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov 15:275
Pardridge WM (1988) Recent advances in blood-brain barrier transport. Annu Rev Pharmacol Toxicol 28:25–39
Burke M, Langer R, Brim H (1999) Central nervous system: drug delivery to treat. The Encyclopedia of controlled drug delivery. Wiley, New York, pp 184–212
Nordal RA, Wong CS (2005) Molecular targets in radiation-induced blood-brain barrier disruption. Int J Radiat Oncol Biol Phys 62:279–287
O’Reilly MA, Hynynen K (2012) Ultrasound enhanced drug delivery to the brain and central nervous system. Int J Hyperth 28:386–396
Park J, Aryal M, Vykhodtseva N, Zhang YZ, McDannold N (2017) Evaluation of permeability, doxorubicin delivery, and drug retention in a rat brain tumor model after ultrasound-induced blood-tumor barrier disruption. J Control Release 250:77–85
Murrell DH, Zarghami N, Jensen MD, Chambers AF, Wong E, Foster PJ (2016) Evaluating changes to blood-brain barrier integrity in brain metastasis over time and after radiation treatment. Transl Oncol 9:219–227
Gonzales-Portillo GS, Sanberg PR, Franzblau M, Gonzales-Portillo C, Diamandis T, Staples M et al (2014) Mannitol-enhanced delivery of stem cells and their growth factors across the blood–brain barrier. Cell Transplant 23:531–539
Rapoport SI (2000) Osmotic opening of the blood–brain barrier: principles, mechanism, and therapeutic applications. Cell Mol Neurobiol 20:217–230
Bellavance M-A, Blanchette M, Fortin D (2008) Recent advances in blood–brain barrier disruption as a CNS delivery strategy. AAPS J 10:166–177
Azad TD, Pan J, Connolly ID, Remington A, Wilson CM, Grant GA (2015) Therapeutic strategies to improve drug delivery across the blood-brain barrier. Neurosurg Focus 38:E9
Emerich DF, Snodgrass P, Pink M, Bloom F, Bartus RT (1998) Central analgesic actions of loperamide following transient permeation of the blood brain barrier with Cereport™ (RMP-7)1Published on the World Wide Web on 30 June 1998.1. Brain Res 801:259–266
Hülper P, Veszelka S, Walter FR, Wolburg H, Fallier-Becker P, Piontek J et al (2013) Acute effects of short-chain alkylglycerols on blood-brain barrier properties of cultured brain endothelial cells. Br J Pharmacol 169:1561–1573
Erdlenbruch B, Alipour M, Fricker G, Miller DS, Kugler W, Eibl H et al (2003) Alkylglycerol opening of the blood–brain barrier to small and large fluorescence markers in normal and C6 glioma-bearing rats and isolated rat brain capillaries. Br J Pharmacol 140:1201–1210
Prokai-Tatrai K, Prokai L (2011) Prodrug design for brain delivery of small-and medium-sized neuropeptides. In: Merighi A (eds) Neuropeptides. Methods in molecular biology (Methods and Protocols), vol 789. Humana Press, New York City
Li Y, Zhou Y, Jiang J, Wang X, Fu Y, Gong T et al (2015) Mechanism of brain targeting by Dexibuprofen prodrugs modified with ethanolamine-related structures. J Cereb Blood Flow Metab 35:1985–1994
Tirucherai GS, Yang C, Mitra AK (2001) Prodrugs in nasal drug delivery. Expert Opin Biol Ther 1:49–66
Gambaryan PY, Kondrasheva IG, Severin ES, Guseva AA, Kamensky AA (2014) Increasing the efficiency of Parkinson’s disease treatment using a poly(lactic-co-glycolic acid) (PLGA) based L-DOPA delivery system. Exp Neurobiol 23:246–252
Buchwald P, Bodor N (2016) Brain-targeting chemical delivery systems and their Cyclodextrin-based formulations in light of the contributions of Marcus E. Brewster. J Pharm Sci 105:2589–2600
Muankaew C, Loftsson T (2018) Cyclodextrin-based formulations: a non-invasive platform for targeted drug delivery. Basic Clin Pharmacol Toxicol 122:46–55
Lisnyak YV, Martynov AV, Baumer VN, Shishkin OV, Gubskaya AV (2007) Crystal and molecular structure of β-cyclodextrin inclusion complex with succinic acid. J Incl Phenom Macrocycl Chem 58:367–375
Zhang Y (2018) Enhancing antidepressant effect of Poloxamer/chitosan thermosensitive gel containing Curcumin-Cyclodextrin inclusion complex. Int J Polym Sci 2018:1–8
Chen Y, Liu L (2012) Modern methods for delivery of drugs across the blood–brain barrier. Adv Drug Deliv Rev 64:640–665
Jiang Y, Lv L, Shi H, Hua Y, Lv W, Wang X et al (2016) PEGylated Polyamidoamine dendrimer conjugated with tumor homing peptide as a potential targeted delivery system for glioma. Colloids Surf B: Biointerfaces 147:242–249
Soni S, AK B, Rk S, Maitra A (2006) Delivery of hydrophobised 5-fluorouracil derivative to brain tissue through intravenous route using surface modified nanogels. J Drug Target 14:87–95
Yadav S, Gattacceca F, Panicucci R, Amiji MM (2015) Comparative biodistribution and pharmacokinetic analysis of cyclosporine-a in the brain upon intranasal or intravenous Administration in an oil-in-water nanoemulsion formulation. Mol Pharm 12:1523–1533
Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L, Bernardino L (2016) Nanoparticle-mediated brain drug delivery: overcoming blood–brain barrier to treat neurodegenerative diseases. J Control Release 235:34–47
Chen Z-L, Huang M, Wang X-R, Fu J, Han M, Shen Y-Q et al (2016) Transferrin-modified liposome promotes α-mangostin to penetrate the blood–brain barrier. Nanomedicine 12:421–430
Wang Y, Xia Z, Xu J-R, Wang Y-X, Hou L-N, Qiu Y et al (2012) α-Mangostin, a polyphenolic xanthone derivative from mangosteen, attenuates β-amyloid oligomers-induced neurotoxicity by inhibiting amyloid aggregation. Neuropharmacology 62:871–881
Rotman M, Welling MM, Bunschoten A, de Backer ME, Rip J, Nabuurs RJA et al (2015) Enhanced glutathione PEGylated liposomal brain delivery of an anti-amyloid single domain antibody fragment in a mouse model for Alzheimer’s disease. J Control Release 203:40–50
Joshi S, Ellis JA, Emala CW (2014) Revisiting intra-arterial drug delivery for treating brain diseases or is it “déjà-vu, all over again”? J Neuroanaesthesiol Crit Care 1:108–115
Marcos-Contreras OA, Brenner JS, Kiseleva RY, Zuluaga-Ramirez V, Greineder CF, Villa CH et al (2019) Combining vascular targeting and the local first pass provides 100-fold higher uptake of ICAM-1-targeted vs untargeted nanocarriers in the inflamed brain. J Control Release 301:54–61
Lesniak W, Chu C, Jablonska A, Azad BB, Pomper M, Walczak P et al (2019) PET-CT shows advantage of Intra-arterial vs. Intravenous delivery of PAMAM Dendrimers for targeting the brain but their accumulation is transient. J Nucl Med 60:116
Argibay B, Trekker J, Himmelreich U, Beiras A, Topete A, Taboada P et al (2017) Intraarterial route increases the risk of cerebral lesions after mesenchymal cell administration in animal model of ischemia. Sci Rep 7:40758
Zheng X, Shao X, Zhang C, Tan Y, Liu Q, Wan X et al (2015) Intranasal H102 peptide-loaded liposomes for brain delivery to treat Alzheimer’s disease. Pharm Res 32:3837–3849
Pashirova TN, Zueva IV, Petrov KA, Lukashenko SS, Nizameev IR, Kulik NV et al (2018) Mixed cationic liposomes for brain delivery of drugs by the intranasal route: the acetylcholinesterase reactivator 2-PAM as encapsulated drug model. Colloids Surf B: Biointerfaces 171:358–367
Pandey YR, Kumar S, Gupta BK, Ali J, Baboota S (2015) Intranasal delivery of paroxetine nanoemulsion via the olfactory region for the management of depression: formulation, behavioural and biochemical estimation. Nanotechnology 27:025102
Gupta S, Kesarla R, Chotai N, Misra A, Omri A (2017) Systematic approach for the formulation and optimization of solid lipid nanoparticles of efavirenz by high pressure homogenization using design of experiments for brain targeting and enhanced bioavailability. Biomed Res Int 2017:5984014
Huang M, Gu X, Gao X (2019) Nanotherapeutic strategies for the treatment of neurodegenerative diseases. In: Brain targeted drug delivery system. Academic Press, San Diego, pp 321–356
Nance E, Zhang C, Shih T-Y, Xu Q, Schuster BS, Hanes J (2014) Brain-penetrating nanoparticles improve paclitaxel efficacy in malignant glioma following local administration. ACS Nano 8:10655–10664
Bachiller S, Jiménez-Ferrer I, Paulus A, Yang Y, Swanberg M, Deierborg T et al (2018) Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front Cell Neurosci 12:488
Han X, Li Q, Lan X, Leena E-M, Ren H, Wang J (2019) Microglial depletion with Clodronate liposomes increases Proinflammatory cytokine levels, induces astrocyte activation, and damages blood vessel integrity. Mol Neurobiol 56(9):6184–6196
Norouzi M (2018) Recent advances in brain tumor therapy: application of electrospun nanofibers. Drug Discov Today 23:912–919
Ashby LS, Smith KA, Stea B (2016) Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: a systematic literature review. World J Surg Oncol 14:225
Guerin C, Olivi A, Weingart JD, Lawson HC, Brem H (2004) Recent advances in brain tumor therapy: local intracerebral drug delivery by polymers. Investig New Drugs 22:27–37
Finan JD, Cho FS, Kernie SG, Morrison B (2016) Intracerebroventricular administration of chondroitinase ABC reduces acute edema after traumatic brain injury in mice. BMC Res Notes 9:160
Armbruster N, Lattanzi A, Jeavons M, Van Wittenberghe L, Gjata B, Marais T et al (2016) Efficacy and biodistribution analysis of intracerebroventricular administration of an optimized scAAV9-SMN1 vector in a mouse model of spinal muscular atrophy. Mol Ther Methods Clin Dev 3:16060
Wang Z, Zhao Y, Jiang Y, Lv W, Wu L, Wang B et al (2015) Enhanced anti-ischemic stroke of ZL006 by T7-conjugated PEGylated liposomes drug delivery system. Sci Rep 5:12651
Boche M, Pokharkar V (2017) Quetiapine nanoemulsion for intranasal drug delivery: evaluation of brain-targeting efficiency. AAPS PharmSciTech 18:686–696
Acknowledgments
The authors would like to acknowledge the Department of Pharmaceutics and Drug Delivery, School of Pharmacy, University of Mississippi, USA, for providing start-up support to Dr. Chougule’s lab.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kotha, A.K., Ghosh, S., Komanduri, N., Wang, R., Bhowmick, S., Chougule, M.B. (2019). Approaches in Barriers, Modifications, Route of Administrations, and Formulations of Therapeutic Agents for Brain Delivery. In: Misra, A., Shahiwala, A. (eds) Novel Drug Delivery Technologies. Springer, Singapore. https://doi.org/10.1007/978-981-13-3642-3_11
Download citation
DOI: https://doi.org/10.1007/978-981-13-3642-3_11
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3641-6
Online ISBN: 978-981-13-3642-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)