Abstract
Monoclonal antibodies (MAbs) have become a substantial part of many pharmaceutical company portfolios. However, the development process of MAbs for clinical use is quite different than for small-molecule drugs. MAb development programs require careful interdisciplinary evaluations to ensure the pharmacology of both the MAb and the target antigen are well-understood. Selection of appropriate preclinical species must be carefully considered and the potential development of anti-drug antibodies (ADA) during these early studies can limit the value and complicate the performance and possible duration of preclinical studies. In human studies, many of the typical pharmacology studies such as renal or hepatic impairment evaluations may not be needed but the pharmacokinetics and pharmacodynamics of these agents is complex, often necessitating more comprehensive evaluation of clinical data and more complex bioanalytical assays than might be used for small molecules. This paper outlines concerns and strategies for development of MAbs from the early in vitro assessments needed through preclinical and clinical development. This review focuses on how to develop, submit, and comply with regulatory requirements for MAb therapeutics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This manuscript provides an overview of the non-clinical and clinical development process for monoclonal antibodies (MAbs). |
Considerations for clinical pharmacology assessments are discussed, including translation of non-clinical pharmacology findings to clinical pharmacology. |
The underlying complexities of MAb pharmacokinetics and dynamics are reviewed. |
1 Introduction
Development of therapeutic monoclonal antibodies (MAbs) has been growing and is rapidly expanding into new therapeutic areas. Given technological advances that allow more carefully engineered MAbs with greater affinity and efficiency, the expansion in MAb and MAb-related compounds such as antibody–drug conjugates (ADCs), fusion proteins, and other derivatives are not likely to slow in the near future. MAbs can have multiple mechanisms of action [1, 2]; Fig. 1 presents an overall schematic of their potential modalities of action. For example, growth factors such as vascular endothelial growth factor may be over-expressed in tumor cells, promoting tumor growth, drug resistance, and metastasis. Antibodies directed against growth factors can disrupt the signaling pathways promoted by the growth factors either by binding to the antigen and removing it from circulation or by affecting the ability of the antigen to adapt a conformation necessary for signaling pathway functionality. Interference of such pathways can subsequently impact apoptosis and inhibit tumor proliferation. MAbs can also bind or inhibit antigens that facilitate metastasis or vascularization of tumors (e.g., bevacizumab). MAbs based on the IgG1 or IgG3 framework can make use of effector functions such as antibody-dependent cell cytotoxicity (ADCC) or complement-dependent cytotoxicity to remove aberrant cells [3, 4]. Due to their binding specificity, MAbs can also be used as targeting agents for cytotoxic small molecules [5] (e.g., ADCs) and, perhaps most importantly, can affect the immune system by either enhancing or suppressing it. Indeed, most MAbs in clinical development or on the market are indicated for treatment of patients with cancer and inflammatory/autoimmune disease [6] and therefore interact directly with the immune system.
Successful translational strategies for the development of MAbs should therefore promote understanding of the relationship between exposure and response with respect to both beneficial and deleterious effects from early stages of development. It is important to develop an understanding of the safety, pharmacokinetics, and pharmacodynamics of the MAb as well as influential factors that affect these attributes. Figure 2 depicts a generic development scheme for MAbs.
2 Non-Clinical Development
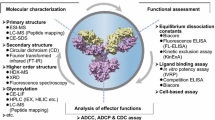
There have been substantial changes in development strategies for MAbs since the first antibody was approved in 1986 [7, 8]. Investigation of inherent risk for adverse immune-mediated drug reactions to MAbs in humans (e.g., infusion reactions, anaphylaxis, cytokine storms, immune suppression, and autoimmunity) requires more detailed exploration. Some of these changes are due to the adverse events observed with natalizumb, a MAb used for treatment of relapsing–remitting multiple sclerosis. Progressive multifocal leukoencephalopathy (PML) is a rare, debilitating, and frequently fatal viral infection affecting the central nervous system. PML primarily affects individuals with chronically and severely suppressed immune systems. The disease attracted attention following the 2005 discovery of significant PML risk associated with natalizumb [9]. Further concerns were raised with an anti-CD28 superagonist, where the novel MAb (TGN-1412) caused the first human dose group to experience a cytokine release storm with serious, nearly fatal outcomes during a clinical trial in the UK [10]. This study demonstrated that while MAbs usually have limited off-target toxicity, they still may have substantial target-related toxicity. In addition, this experience highlighted possible pitfalls in interpretation and extrapolation of non-clinical findings to clinical expectations. The profound adverse events observed in the TGN-1412 study underscored the importance in considering all available biological data, including knowledge of the comparative pharmacological effects in animals and humans, when evaluating the safety of MAbs and in the selection of the starting dose in humans. Given the increasing prominence of MAbs in drug development pipelines, careful attention should be paid to the current International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) S6 regulatory guideline on Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals [11] and its addendum [12], which provides guidance to ensure expedient and safe clinical evaluation of MAbs. Figure 3 lays out a general scheme of in vitro and non-clinical in vivo evaluations used for MAb development.
Timelines for initiation of non-clinical pharmacology and toxicology assessments. The development strategy and timelines for assessments are presented. Note that depending on the indication being evaluated, some studies such as juvenile/developmental and reproductive toxicology studies may either need to be moved to an earlier time (if the indication is pediatric) or may be omitted if such studies are not relevant. ADCC antibody-dependent cell cytotoxicity, CDC complement-dependent cytotoxicity, NK natural killer, PD pharmacodynamic, PK pharmacokinetic
Selection of a target antigen is the first step of MAb development. The selection of this target requires extensive knowledge of biological processes involved in specific disease pathology. These processes can then be used to frame an appropriate development strategy in order to ascertain the mechanism of action, binding specificity, affinity, kinetics, and potency of the target antigen as well as potential isoforms of the antigen. Understanding not only the role of the antigen in disease pathology but also determining if the target antigen has functional redundancy in other systems is necessary to determine likely drug effects of a MAb in clinical use. If the target antigen is part of a family of conserved proteins, then blockage or down-regulation of the antigen may not result in the desired pharmacological response. Thus, immunohistological evaluation of appropriate tissues to determine antigen expression, especially differential expression in target tissues versus normal tissues, is important to determine likely results of administration of a MAb targeting that antigen.
In addition, once a target is identified, appropriate engineering of the MAb can markedly improve its pharmacologic effect. For example, early forms of the antagonistic anti-proprotein convertase subtilisin kexin type 9 (PCSK9) MAbs showed potentially beneficial effects but also exhibited target-mediated clearance, resulting in dose-dependent pharmacokinetics that would potentially result in suboptimal exposure when the expression of antigen target is high. In order to improve the pharmacokinetics, an engineered pH-sensitive form of the anti-PCSK9 MAb was developed [13]. This engineered MAb exhibited a prolonged half-life and increased duration of pharmacologic activity. Anti-PCSK9 MAb binds with high affinity to PCSK9 in the plasma at pH 7.4, but the antibody–antigen complex dissociates at the endosomal pH of 5.5–6.0 in order to enhance lysosomal destruction of the target by avoiding neonatal Fc-receptor (FcRn)-mediated recycling of the MAb–target complex and reduce target-mediated degradation of the MAb.
2.1 In Vitro Evaluations of Monoclonal Antibody Binding
It is now possible to engineer MAbs with exquisite specificities and to fine-tune their biophysical properties for nearly any specified application. Depending on the desired functionality, antibody/antigen interactions can be long- or short-lived. Screening studies are used to eliminate MAbs with undesirable properties and to identify those with specific reaction-rate and affinity properties [14–16]. Biophysical screening is effective in evaluation of MAb cross-reactivity with orthologous antigens in non-human species. Orthologs are genes in different species that have evolved from a common ancestral gene through speciation. Orthologs generally retain the same function in the course of evolution but can impact the ability to evaluate MAb functionality in non-human studies. Implementation of effective biophysical screening strategies during early development allows drug candidate selection with respect to orthologous antigen activity for addressing potential safety concerns [17]. Effective screening is also important for the identification of antigens that are redundant as well as determination of orthologous antigen selectivity for selection of the relevant toxicology species [18]. There are three biophysical techniques for evaluation of MAb-binding kinetics, Biacore, kinetic exclusion assay (KinExA) and fluorescence-activated cell-sorting (FACS) that are frequently used to screen MAb candidates and these are briefly described below.
The Biacore assay is a primary technique used for determining antigen/antibody kinetics. In this assay, one reactant is immobilized to a biosensor surface, while the second binding agent flows across the surface. Binding kinetics can then be followed in real time by surface plasmon resonance (SPR). SPR is directly related to refractive index changes at the biosensor surface that occur during complex formation and dissociation [19, 20]. Changes in the refractive index at the surface where the binding interaction occurs are detected and recorded as RU (resonance units). Curves are generated from the RU trace (‘sensogram’) and are evaluated by fitting the RU data to well-defined binding models to determine the association rate constant (k on), the dissociation rate constant (k off), and the binding affinity (K D = k off/k on) [20, 21]. To fully evaluate the binding kinetics, five to eight different concentrations of analyte are evaluated. The challenges encountered during Biacore analysis, such as working with bispecific multivalent MAbs and designing and performing appropriate Biacore experiments, have been covered in several publications [22–24].
KinExA is another approach used for quantification of protein–ligand interactions [25]. KinExA and Biacore are complimentary approaches and are often used together to characterize MAb-binding kinetics [24]. KinExA incorporates the use of a flow spectrofluorimeter and is a solution-based assessment. With this approach, equilibrated solutions of antigen and antibody flow through a bead bed with immobilized antigen. The free antibody-binding site from the equilibrated solution then binds to the bead pack. The antibody bound to the bead pack is detected by a secondary fluorescently labeled specific polyclonal antibody. The percentage of the free antibody versus total antigen is used to generate a titration curve that can be fitted to a 1:1 equilibrium model to calculate the K D of the interaction. Binding rate constants (k on and k off) can also be determined using KinExA [24, 26].
The third analytical approach is the FACS method. FACS is a titration experiment that allows measurements of the K D of a MAb to a cell surface receptor and FACS is also used for ex vivo determinations of receptor occupancy and saturation by the MAb [27]. For assessment of MAb-binding kinetics, a constant number of receptor-bearing cells in solution are titrated with increasing concentrations of a MAb and allowed to reach equilibrium. A fluorescently labeled polyclonal antibody probe is used to detect cell-bound MAb by measuring the fluorescence of cells moving individually through an excitation laser. An equation, based on the multiple independent binding-site equations, relates mean fluorescence intensity (MFI) measured by FACS to the amount of cell-bound antibody [28]. The K D of the antibody to the cell receptor is estimated by fitting MFI as a function of the antibody concentration. Integrating receptor occupancy data using mathematical models can provide a means of testing hypotheses, as well as providing a basis for extrapolation of preclinical findings to humans [29].
As an example, Bates et al. [30] used this methodology to explore the role of binding kinetics on the neutralizing potency of the MAb palivizumab against respiratory syncytial virus (RSV). Investigating naturally occurring mutations of the RSV F protein, the target of palivizumab, indicated that while reduction of k on resulted in increased escape from MAb-mediated neutralization of RSV, changes in k off did not significantly affect neutralizing activity. These results were further supported by the observation that an increase in k on in motavizumab, a variant of palivizumab, resulted in enhanced potency against RSV.
2.2 In Vivo Non-Clinical Pharmacology and Toxicology Studies
In vivo pharmacologic and toxicologic evaluations comprise a major component of MAb development. The designs and needs for each type of assessment are somewhat different. For example, pharmacologic studies are typically conducted over a short duration of time and thus the formation of anti-drug antibodies (ADAs) in the animal species selected for assessment is less problematic than in long-term toxicologic assessments. Further, non-clinical pharmacology studies often require an animal model of a specific disease or condition, whereas toxicology studies generally do not. Thus, while some concerns for each type of study overlap, there is often somewhat different emphasis on these issues.
2.3 Non-Clinical Pharmacology Evaluations
Conducting pharmacologic studies in appropriate animal models can greatly enhance the translation of information across species by allowing models developed in preclinical species to be scaled to humans in order to guide dose selection for the first-in-human (FIH) study [17]. However, there are numerous considerations when making these translations. Extrapolation of pharmacokinetic data from animal models requires consideration of species differences in antibody salvage (e.g., FcRn interactions [31]), which supports the long circulating half-lives of MAbs in humans. This salvage is usually comparable even with fully human MAbs in non-human primates (NHPs) but is generally less effective in many lower species, resulting in faster clearance and a shorter half-life than would be expected in humans. Other differences can be found in the binding affinity of MAbs with antigens in lower species, which can also result in pharmacokinetic and pharmacodynamic behavior that is different in animals than would be expected in humans. Thus, extrapolation from NHP data where the pharmacokinetics are dose proportional is generally straightforward, while MAbs that do not exhibit dose-proportional or linear pharmacokinetics must be scaled allowing for differences in binding affinity. Target-mediated drug disposition (TMDD), where target binding affects MAb disposition behavior within the body, leads to non-linear, saturable distribution and elimination kinetics, with substantial between- and within-subject pharmacokinetic variability [32]. However, as long as relative binding affinity is accounted for, TMDD models built on preclinical data can be used to explore the potential impact of disease-related target load on pharmacokinetic variability. Lastly, human and humanized proteins are generally more immunogenic in animal species than in humans. While non-clinical studies are not generally predictive of immunogenicity in humans [33], it must be considered when planning non-clinical studies and when approaching FIH studies. For predictive assessments of immunogenicity in humans, computational methods for identification of T cell epitopes have been reported [34], but the predictive values of these tools and other strategies for predicting immunogenic potential in humans [35] are still being explored.
Determination of appropriate dose selection for clinical assessment includes the amount of drug delivered, frequency of administration, and treatment duration. These variables should be selected based on knowledge of the relationship between the dose, the concentration–time course and effects arising from that dose, and the likelihood of both beneficial and adverse consequences resulting from these exposure profiles. Pharmacokinetic/pharmacodynamic (PK/PD) models relate doses to concentrations and then describe concentration–effect relationships, facilitating the prediction of the time course of drug effects. PK/PD modeling provides robust support to a drug development program and can be implemented in the earliest stages of drug development and continued throughout all development phases [36]. In the discovery phase modeling provides insights into mechanism of action and enables selection of clinical candidate molecules based on pharmacokinetic properties and the predicted human therapeutic index. Translational PK/PD modeling can provide estimates of efficacious exposures, associated dose regimens, as well as implications of disease burden [37]. Thus, PK/PD modeling should be a part of non-clinical pharmacology assessments. This translational approach not only facilitates dose selection in human studies but can also be used to support dose selection for regulatory submissions.
2.4 Animal Models of Disease
Rodent models can play a central role for MAb discovery and development, as well as for translational strategies. For models of inflammatory disease, the rat model is frequently used for studies of rheumatoid arthritis (RA) pathogenesis and response to treatment [38]. While many adjuvants are used, pristane-induced arthritis in rats is an optimal model for RA as this mimics the chronic relapsing disease course associated with RA. Other models, such as the collagen-induced arthritis model, have also been used to evaluate anti-inflammatory MAbs [39], and is still used today [40, 41]. Data collected from preclinical pharmacology studies can be used to relate systemic MAb exposure to inflammation [42] as well as disease progression, which can subsequently be linked to human exposure and response [43, 44].
For cancer models, the human tumor xenograft model is the primary tool to evaluate in vivo effects on local growth, metastasis, or survival [45, 46]. Transgenic knock-in mice are genetically engineered to express the human ortholog of the antigen target, making this animal model an informative means to evaluate MAbs with poor or no cross-reactivity to the murine ortholog. Furthermore, the use of transgenic knock-in mice as an experimental model precludes the use of surrogate antibodies. However, xenograft models are often not fully representative of all stages of cancer progression [47, 48]. For example, transplanted tumors adapted to grow in animals often exhibit a higher proliferative capacity than the original patient tumor [49]. The vascularity of the transplanted tumor may also differ from the original tumor; transplanted tumors can exhibit improved blood supply and reduced necrosis [48, 50]. In addition, consideration must be made of immune competence, tumor heterogeneity, tumor microenvironment, and stromal components [51] as well as differences in immune system (e.g., ADCC, complement activation, targeting of natural killer [NK] cells) [52]. Despite these limitations, carefully conducted studies of tumor growth in the mouse model can be used to understand human response.
The use of PK/PD models of tumor growth applied to preclinical and clinical studies has been recently reviewed [53]. One of the most common markers of response in the oncology setting is tumor growth. Measurements of tumor size and other tumor-related biomarkers have been used to describe and quantify the progression of tumor-related disease and the response to treatment. Daydé et al. [54] reported on preclinical PK/PD evaluations to better understand the clinically observed inter-patient variability of rituximab, a MAb targeting CD20. The impact of tumor burden on pharmacokinetics and efficacy was tested in a murine syngenic lymphoma model expressing human CD20 sharing characteristics of the human disease, showing that high tumor burden increases rituximab clearance and subsequently reduces efficacy, a finding consistent with human results. Such PK/PD models, when appropriately scaled, can be useful to predict human exposure and response.
2.5 Toxicology Evaluations
A thorough understanding of the pharmacology and comparative immunology of a MAb in humans and animals is necessary to select pharmacologically relevant species for toxicology assessments, given limitations of the chosen animal species. Such studies must provide sufficient information to predict the immunological response and the risk of adverse events in humans and to select a safe human starting dose for the FIH clinical study based on the minimum anticipated biological effect level (MABEL).
MAbs are proteins, and their metabolism is well-defined (catabolism into constituent amino acids), so there is no need to evaluate the potential to form reactive or toxic metabolites [6]. Since they are limited by size to the extracellular space and do not interact directly with DNA, MAbs are not directly genotoxic. However, as previously noted, orthologous cross-reactivity, immunologic differences, and other issues must be considered in toxicology studies. Thus, the toxicology program for MAbs differs from those for small molecules. Figure 3 provides a generic overview of the timing of toxicology studies for MAb assessments during the drug development process.
During the design of any non-clinical safety program, the guidelines recognized or published by the ICH and regulatory bodies such as the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) should be consulted. Some of these guidelines, although not specifically addressing MAb safety evaluations, still provide important guidance on relevant test strategies/models and regulatory expectations. Table 1 lists many relevant documents released by the regulatory agencies to guide MAb development.
2.6 Species Selection
The structural specificity of most immune receptors targeted by MAbs makes the selection of an appropriate animal model challenging. The primary consideration for selection of a relevant species is the requirement for data from the animal to accurately predict likely adverse effects in humans. Risk prediction of adverse reactions and dose selection for FIH clinical trials is based on preclinical safety assessment in at least one pharmacologically relevant animal species [11, 12]. As a rule, the main toxicological liabilities of a MAb are related to its pharmacological effects. For example, MAbs that cause immunosuppression (such as those used to treat inflammatory conditions) can increase risk of opportunistic bacterial, fungal or parasitic infection, chronic viral infection, and virally induced cancers. MAbs that activate immune cells, such as T cells, NK cells, or B cells, could also activate autopathogenic cells, resulting in autoimmunity such as is seen with the anti-CD52 MAb alemtuzumab [55] and the anti-CTLA-4 (cytotoxic T lymphocyte-associated protein 4) MAb ipilizumab [56]. However, toxicity can also result from binding to target antigen in tissues other than those necessary for therapeutic effect. The skin toxicity (acneiform rash) observed with cetuximab (anti-EGFR [epidermal growth factor receptor] MAb) [57] is attributed to the expression of the targeted antigens in skin. Thus, the selected animal species should express the target antigen sufficiently on appropriate tissues. The species antigen must be recognized and bound by the MAb with sufficient affinity to allow evaluation of pharmacological activity. Furthermore, the antigen target should play a similar role in both the selected species and humans. The binding of a MAb to its target antigen at normal levels of tissue expression does not always mean the species is relevant, or that there is pharmacological activity. Approximately 10 % of MAbs that cross-reacted with NHP antigens showed less than 10 % potency in that species [58]. Therefore, it is important to show that the MAb is pharmacologically active through functional potency assays. Ideally, the target should be modulated in a manner similar to humans and, to fully assess species relevance, activation of downstream signaling pathways or effector function may require investigation [17]. When MAbs possess agonistic properties, further characterization of potency and downstream signaling pathways may also be necessary to establish whether the toxicological consequences of target modulation can be fully investigated in preclinical models [17].
Thus, selection of a relevant species must be considered and justified for each MAb in development individually. Studies may be conducted in only one species of animal, particularly if only one relevant animal model can be identified, but regulatory authorities may request assessments in more than one species [59]. There are situations when an appropriate species cannot be identified, for example if the target is not expressed in animals. In these scenarios, additional approaches such as use of surrogate antibody(s) that have similar characteristics to the MAb or generation of transgenic animals that express antigen need to be considered. Transgenic animal models must be characterized for antigen expression and functional integrity. Preclinical studies should be supplemented by ex vivo investigations in human and animal cells and tissues to determine the relative potency of the MAb in humans versus the selected species, and to characterize the pharmacological activity.
Owing to their genetic and pharmacological similarity to humans, NHPs are the most commonly selected animal model for safety assessment of MAbs [18], although NHP should not be used simply as a ‘default’ but rather should be justified. For reasons of practicality, the cynomolgus monkey is the preferred NHP species. Occasionally, marmosets and rhesus macaques are used. In some rare cases, the only naturally occurring species in which certain very selective MAbs exhibit pharmacological activity is the chimpanzee. However, toxicity studies of chimpanzees involving terminal investigations are justified only in exceptional circumstances due to ethical considerations. NHPs are normally the most relevant model for evaluating the safety of MAbs, because the pharmacological activity in NHPs often resembles that in humans more strongly than that in lower species such as dogs, rabbits, and rodents. In addition, the NHP immune system is generally more similar to humans than the lower species. Furthermore, human and humanized MAbs are less likely to be immunogenic following long-term dosing in NHPs than in lower species. It is important to assess the immunogenicity and the production of ADAs, including neutralizing antibodies, by the selected species. ADA formation can make it difficult to achieve sufficient MAb concentrations to assess pharmacological activity and toxicity. A recent review [58] offers strategies for minimizing the use of NHP in preclinical assessments.
2.7 Dose and Regimen Selection
Pharmacodynamic endpoints, if available, should be routinely assessed in toxicity studies to demonstrate pharmacological activity of the MAb in vivo. Generally, repeat-dose toxicity studies using dose levels and exposures representing multiples of the starting dose and highest dose in humans, with a dosing duration of 4 or sometimes 13 weeks (depending on the duration of exposure in the FIH study) followed by an exposure-free recovery period of 4–8 weeks (the duration depends on the predicted duration of exposure and pharmacological activity) are used for generating data to support human entry. The dosing duration in FIH studies for non-life-threatening indications is usually limited to the dosing duration covered in the animal studies [11, 12]. However, for life-threatening indications, such as in oncology, treatment duration in FIH studies may exceed preclinical coverage in animal studies considerably. Most of these considerations are applicable to MAbs in general.
3 Clinical Development
As for all novel therapeutics, the clinical development of MAb-based therapeutics is focused on establishing the safety and efficacy of the applied therapeutic and on determining the optimal dose for the most preferable highest risk/benefit ratio. Due to their inherent and often complex pharmacokinetic and pharmacodynamic properties (Fig. 4), however, antibody-based therapeutics often require specific considerations within their clinical development, some of which are highlighted in the following sections.

(modified from Chirmule et al. [62]; reproduced with permission of Springer)
Multiple clearance pathways affecting the pharmacokinetics of a monoclonal antibody (MAb). Depicted is a typical two-compartment pharmacokinetic model for a MAb with administration of a dose (D) that may undergo pre-systemic degradation (degradation rate constant [k deg]), concentrations of the MAb in the central (Ab1) and peripheral (Ab2) compartment, and interdepartmental clearance (Q). The pharmacokinetic model includes two linear clearance pathways representative of unspecific proteolytic degradation, one from the central compartment (CL1) and one from the peripheral compartment (CL2), as well as recycling through the neonatal Fc-receptor (FcRn)-mediated salvage pathway (recycling rate constant [K rmr]). Added to these clearance pathways is, on the right-hand side, a target-mediated disposition pathway that constitutes interaction of the MAb with its pharmacologic target receptor, which is in a homeostatic equilibrium of synthesis and degradation (rate constants k syn and k deg). The dynamic equilibrium for the formation of the resulting MAb–receptor complex (Ab–R) is determined through the association rate constant k on and the dissociation rate constant k off. The formation of Ab–R not only elicits the pharmacologic effect but also triggers degradation of the complex. Thus, target binding and subsequent Ab–R degradation constitute an additional clearance pathway for the MAb (CL3). The left-hand side of the graphic depicts the effect of an immune response to the MAb resulting in anti-drug antibody (ADA) formation. Again, the circulating concentration of the ADA is determined by a homeostatic equilibrium between its formation rate (k formation) and a catabolic turnover process (rate constant [k cat]). The ADA response results in the formation of immune complexes with the drug (ADA–Ab), dependent on the dissociation constant K d. Dependent on the size and structure of the immune complexes, endogenous elimination pathways through the reticuloendothelial system may be triggered, most likely via Fcγ-mediated endocytosis. Thus, immune complex formation and subsequent degradation may constitute an additional clearance pathway (CL4) for MAbs
3.1 Early Stage Clinical Development
3.1.1 Prediction of Human Pharmacokinetics/Pharmacodynamics from Preclinical Species
The extrapolation of pharmacokinetic and pharmacodynamic results from preclinical species to humans and thus the selection of safe starting doses for MAbs remains challenging. On one hand, this is often related to the lack of cross-reactivity of the MAb to its molecular target in different species. While a human MAb against a human target often cross-reacts with its ortholog in NHPs, it rarely cross-reacts with the orthologs of rodents or other preclinical species. This becomes especially relevant if the MAb undergoes TMDD. On the other hand, non-target-related disposition processes may also show large species-specific differences. For example, FcRn exhibits substantial affinity differences between mice and humans. Mouse FcRn has a 2.4-fold higher binding affinity to human IgG molecules than to human FcRn [60]. As a result, the FcRn-mediated recycling pathway for human IgG is more efficient in mice than in humans, thereby resulting in a lower clearance of human IgG in mice than would be expected solely based on allometric size differences between mice and humans. As a consequence, inclusion of mouse pharmacokinetic data in allometric scaling approaches for MAb clearance may lead to substantial overestimation of human MAb clearance [61]. In appreciation of these challenges, investigations into the prediction of MAb clearance through non-target-related elimination pathways in humans have recommended the use of only one species, the cynomolgus monkey as a NHP, to make allometry-based clearance predictions using an allometric exponent of 0.85 [61].
For target-mediated clearance pathways, differences among species in target binding affinity as well as target expression and density need to be considered beyond size differences accounted for using allometric scaling [43]. While binding affinities are often available from in vitro assays, target expression levels in different tissues of different species are in most cases not known. As a consequence, exposure predictions between different species for MAbs that undergo TMDD usually fall short at those dose levels and associated concentrations where the target-mediated, non-linear elimination pathway is not saturated.
In addition, human MAbs administered to preclinical species have a high potential of immunogenicity as they are recognized as foreign proteins to which the animal’s B cells do not have tolerance. As a consequence, ADAs may by formed in the animals under prolonged therapy and may affect the observed pharmacokinetic and PK/PD properties of the MAb [62].
3.1.2 First-in-Human Dose Selection
There are several alternative approaches coexisting that are frequently used in FIH dose predictions. The classic approach for determining the maximum recommended starting dose (MRSD) is based on the No Observed Adverse Effect Level (NOAEL) dose determination and subsequent human equivalent dose estimation according to the FDA guidance document [63]. In response to the TGN-1412 disaster, additional approaches for the estimation of safe human starting doses were developed [64]. Of particular mention is the MABEL approach published by the EMA [65], as well as receptor occupancy-based approaches. While the MABEL approach had been specifically designed for ‘high-risk medicinal products’ with, for example, novel mechanisms of action or high species specificity, today, most clinical development programs use several or all of these approaches simultaneously under consideration of the ‘totality of evidence’ to develop a FIH dose selection rationale for a particular MAb-based therapeutic. A recent reintroduction of TGN-1412 into clinical testing at appropriate doses determined using these approaches indicated that the compound, when appropriately dosed, is safe and well-tolerated, thereby providing evidence regarding the appropriateness of the MABEL approach [66]. However, when selecting a starting dose, one should consider not only the exposure–response relationship but also the liability associated with that response.
3.1.3 Selection of Dose-Adjustment Metrics
As many of the first-generation MAb-based therapeutics had anti-cancer indications, their dosing was often adjusted based on a body size measure such as body weight or body surface area as this had historically also been done for small-molecule anti-cancer agents [67]. To address the question of whether size-adjusted dosing or fixed (flat) dosing is the more appropriate approach in early clinical development, Wang and colleagues [68] performed a retrospective analysis on 12 marketed MAbs. While systemic exposures were similar for both approaches at the population level, the correct dosing approach based on the presence or absence of body size as a determinant of the clearance of the MAb became highly relevant for patients with extreme body sizes relative to the general population. The latter has, for example, also been suggested for the MAb cetuximab in a separate analysis [69]. Body size was found to be a metric for many MAb dose regimens despite the fact that information in the documents submitted to regulatory agencies did not identify body size as a predictive factor [70]. Hence, Wang and colleagues [68] recommend starting in early clinical development with fixed dosing as long as no data are available regarding whether body size is a predictor for MAb clearance. Once sufficient clinical data has been accumulated, a population pharmacokinetic analysis should assess whether body size is a clinically relevant covariate for MAb exposure and the dosing algorithm should be adjusted accordingly. In addition, consideration of alternative metrics for dose adjustment, such as the use of albumin, which is commonly identified as being predictive of MAb clearance [71–73] and often has a substantial impact as well as being reflective of disease severity, might also be considered for dose adjustments. Furthermore, for those MAbs that exhibit TMDD and non-linear pharmacokinetics, loading or ‘induction’ dose strategies may be appropriate to saturate or clear available antigen targets. Alternatively, dose adjustments based on antigen expression may be considered, such as is used with omalizumab [74].
3.1.4 Choice of the Route of Administration
Since all approaches to make MAb-based therapeutics orally bioavailable have so far been unsuccessful to achieve clinically relevant systemic exposures, MAb-based therapeutics are still given by parenteral administration, preferably by intravenous or subcutaneous injection or infusion. The subcutaneous route has several practical advantages over intravenous injection, including easier administration in ambulatory or home care settings without the need for venous access, reduced risk of injection, and the lack of need for skilled healthcare providers for the administration. Major limitations of subcutaneous administration are the limited volume that can be administered (although the addition of recombinant human hyaluronidase, which temporarily degrades hyaluronan, may allow subcutaneous delivery of larger volumes that are otherwise not feasible and improve bioavailability of subcutaneously administered MAbs [75]) as well as the potentially substantial presystemic degradation. As therapeutic proteins are to an increasing degree taken up into the lymphatic system with increasing molecular weight via convective transport, degradation during the passage through the lymphatic system can substantially limit the systemic bioavailability of a subcutaneously administered MAb [76]. Physiologically based pharmacokinetic modeling suggests that the elimination rate during lymphatic transport and the transit time for the drug movement from the administration site through the lymphatic system into the systemic circulation are the major determinants for bioavailability, leading in many cases to the typical bioavailability range of 50–70 % for subcutaneously administered MAbs [77, 78].
3.1.5 Healthy Volunteers Versus Patients
While FIH studies are routinely performed in healthy volunteers to assess the safety and pharmacokinetics of the investigational drug, many of the initial clinical studies of first-generation MAb-based therapeutics were performed in patients. This was predominantly driven by the fact that many of the first-generation MAbs were developed for cancer indications and most FIH studies in the field of oncology, particularly for cytotoxic drugs, have traditionally been performed in patients. With the advent of MAb development for non-cancer indications and the growing experience and usually high tolerability of MAb-based therapeutics with regard to acute toxicity, FIH studies for MAbs are increasingly performed in populations of healthy volunteers [79]. For many MAbs, individual doses of even up to several grams were well-tolerated and dose-limiting toxicity could often not be reached in phase I dose-escalation studies [80]. This is largely driven by the fact that humanized or human MAbs as IgG molecules usually have no or only very limited acute off-target toxicity, similar to the experiences observed for immunoglobulin therapy [81]. If dose-limiting acute toxicity is observed for MAb-based therapeutics, then it is usually related to on-target effects. Thus, the rationale for selecting patients versus healthy individuals for FIH studies should be guided by whether the putative mechanism of action for the MAb on-target effects may lead to severe, unacceptable toxicity even below the potential maximum tolerated dose.
3.2 Drug Interactions
In our current age of polypharmacy and combination pharmacotherapy, MAbs are frequently not administered alone but concurrently with other medications, including small-molecule drugs and other therapeutic proteins. This raises the potential for drug–drug interactions affecting the pharmacokinetics and thus systemic exposure of one of the concomitantly used agents. While for many years there was a perception that therapeutic proteins do not exhibit drug interactions, this notion has been revised with the more recent development of immunomodulatory agents, particularly the experience with the anti-interleukin (IL)-6 MAb tocilizumab [82]. While the potential for direct interactions of drug-metabolizing enzymes or transport proteins of small-molecule drugs with therapeutic proteins is generally low, indirect interactions have been observed particularly for therapeutic proteins that are cytokines or cytokine modulators, such as many immunomodulatory MAbs. This interaction has also been described as a drug–disease interaction [83]. In general, the immunomodulatory therapeutic protein increases or decreases the inflammatory status in the body and with that induces down- or upregulation of acute-phase proteins such as drug-metabolizing enzymes and transporters. Small-molecule drugs that are substrates to these enzymes and transporters would thus be affected by the change in expression and subsequent activity of these drug disposition mechanisms and might need dose adjustment. For tocilizumab, initiation of therapy resulted in a decrease of the chronic inflammatory condition in the body and thus allowed previously suppressed drug-metabolizing enzymes and transporters to reach homeostatic expression levels. As a consequence, the systemic exposure of simvastatin decreased by 57 % within 2 weeks of initiation of tocilizumab therapy [84].
The most frequent drug interactions where the protein is the victim and the small molecule is the perpetrator are mechanistically related to changes in target-mediated clearance or changes in immunogenicity-related clearance [85]. For the anti-CD11a antibody efalizumab, oral administration of triple immunosuppressive therapy consisting of cyclosporine, sirolimus, and prednisone in transplant recipients reduced efalizumab clearance by approximately 50 %, presumably through reduction of CD11a-positive circulating T cells responsible for the target-mediated elimination pathway [86]. Similarly, co-administration of methotrexate has been reported to reduce adalimumab clearance by 29–44 % [87] in patients with RA. The latter has been suggested to be driven by a reduction in the immunogenicity-related clearance of adalimumab secondary to exposure to the immunosuppressant methotrexate [88]. Alternatively, methotrexate has been reported to reduce Fcγ receptor expression in vitro, which is involved in another elimination pathway for many MAbs [89].
Less mechanistically expected drug–drug interactions have also been described. The recombinant human keratinocyte growth factor palifermin, for example, has been reported to be displaced from its binding to the keratinocyte growth factor (KGF) receptor. Heparin co-administration increased the palifermin area under the plasma concentration–time curve (AUC) 4- to 5-fold and decreased its half-life by 40–45 %, suggesting an approximately 70–80 % decrease in palifermin clearance and volume of distribution. However, these changes in the pharmacokinetics of palifermin during co-administration of heparin did not affect the pharmacodynamic effect of palifermin, or the anticoagulant activity of heparin, and did not lead to increased safety findings [90].
The FDA guidance document on drug–drug interactions provides a decision tree on the situations in which in vivo drug–drug interaction studies for therapeutic proteins would be warranted [91]. In general, drug–drug interactions between therapeutic proteins and small-molecule drugs are less extensive than, for example, interactions between small-molecule drugs. Most of the time, changes in systemic exposure due to the effect of therapeutic proteins do not result in exposure changes of more than 2-fold, rarely necessitating dose adjustments.
3.3 Special Populations
3.3.1 Patients with Renal Impairment
Although the kidneys may contribute to the proteolytic degradation of therapeutic proteins including MAbs similar to other organs, MAbs are not expected to be subject to renal excretion due to their large molecular weight of 150 kDa [92]. Only antibody fragments that have a molecular weight below the cutoff value for glomerular filtration of approximately 60 kDa are expected to undergo renal excretion [93]. This is, for example, the case for the Fab fragment ranibizumab used to treat age-related macular degeneration, which has a molecular weight of 48 kDa. Ranibizumab undergoes substantial renal elimination and thus renal impairment decreases the clearance for this kind of compound [94]. In contrast, no effect of renal impairment would be expected for intact MAbs. While formal pharmacokinetic studies in patients with renal impairment are scarce, case reports for a variety of MAbs, including bevacizumab, cetuximab, rituximab and trastuzumab, support this expected lack of effect of renal impairment on MAb disposition [93]. Therefore, renal impairment studies are usually not included in the clinical pharmacology package of MAb development projects. A recently published study performed based on regulatory requests further confirms this notion: the MAb elotuzumab targeting signaling lymphocyte activation molecule F7 in combination with lenalidomide and dexamethasone in patients with multiple myeloma did not show any differences in peak plasma concentrations or systemic exposure in patients with severe renal impairment (creatinine clearance <30 mL/min, not requiring dialysis) or patients with end-stage renal disease requiring dialysis compared with patients with normal renal function. The study concluded that elotuzumab may be administered without dose adjustment for renal function [95]. However, in some circumstances, renal elimination of intact MAbs must be considered, particularly in pathologic conditions in which the glomerular filtration barrier is compromised. For example, in focal segmental glomerulosclerosis, which results in loss of glomerular function, renal clearance of adalimumab, a MAb targeting tumor necrosis factor (TNF), was identified [96]. In this study, the authors noted that both renal and non-renal clearance contributed to an enhanced total clearance of adalimumab. Similarly, for patients with systemic lupus erythematosus, who often have proteinuria, IgG clearance can be altered [97]. In this work, the authors found an association between increasing baseline proteinuria and increasing clearance, which they indicated may be clinically relevant with very high proteinuria levels. Thus, for patients with proteinuria, renal clearance can comprise a portion of total clearance of MAbs.
3.3.2 Patients with Hepatic Impairment
The effect of hepatic impairment on MAb disposition has been investigated much less than renal impairment. The guidance document of the EMA on the clinical investigation of the pharmacokinetics of therapeutic proteins states in general terms that reduced hepatic function may decrease the elimination of a protein for which hepatic degradation is an important elimination pathway [98]; however, no specific examples are provided. For MAbs, unspecific proteolytic degradation usually occurs throughout the body, particularly in endothelial cells in organs with large capillary beds, including the intestines and the skin. Thus, while the liver may contribute to the elimination of MAbs, it is usually not the major elimination organ [99]. Therefore, hepatic impairment would not be expected to have a major effect on the pharmacokinetics of MAbs. For panitumumab, a MAb against EGFR, it could indeed be shown that mild-to-moderate hepatic dysfunction had no effect on the panitumumab pharmacokinetics [100]; this has been confirmed in clinical case reports [101]. Combination therapy with cetuximab and bevacizumab has even been considered as a treatment option for patients with hepatic metastases of colorectal cancer who cannot be treated with standard chemotherapy regimens due to impaired liver metabolism of cytotoxic substances [102].
3.3.3 Pediatric Patients
Many clinical pharmacology programs for MAbs are faced with the challenge to extend the development and application of these therapeutic proteins to pediatric patients of various age ranges. This is largely driven by requirements by the FDA for the early integration of a pediatric study plan (PSP), and by the EMA to develop a pediatric investigation plan (PIP) [103]. There is a growing body of experience with the therapeutic use of MAbs in pediatric patients, including infants and neonates. Palivizumab, for example, is exclusively used in the prevention of RSV infections from postnatal age 0 to 2 years in premature neonates. Other MAbs approved for pediatric and adult indications include adalimumab, infliximab, basiliximab, eculizumab, tocilizumab, omalizumab, daclizumab, and canakinumab [104]. Extrapolating clinical results from adult to pediatric populations is complicated by differences in the underlying diseases and indications as well as a lack of in-depth understanding of the drug disposition mechanisms and their developmental trajectories in pediatric patients. Other complications include differences in body composition and rapidly changing body size. This topic has been reviewed by Edlund et al. [106]. While many differences in pharmacokinetic behaviors can be accounted for by size differences alone, a lack of understanding in the differences between adult and children with regard to drug disposition processes unique to MAbs creates uncertainty and potential inaccuracies in extrapolations from adult to pediatric patients. Some of these processes include the FcRn salvage pathway for MAbs, MAb transport in the lymphatic system after subcutaneous administration, and the target expression and density for MAbs that undergo TMDD [107]. This becomes especially relevant if physiologically based pharmacokinetic modeling approaches are employed to facilitate these extrapolations [108, 109].
Dose selection for pediatric use is often performed by body weight- or body surface area-based dosing, including allometrically based dose adjustments. While these mechanisms can often address adequate dose adjustments over a wide size range, they fall short if there are non-size-related differences in systemic clearance or if the effect of body size is not linearly related to MAb clearance [70], resulting in lower exposure in pediatric patients than in adults (see Fig. 5) and subsequent therapeutic failure [110]. In this situation dose adjustments may be necessary, and they are often difficult to perform as they may require extensive calculations to determine the dose, which may be prone to errors. A practical alternative that has achieved increasing acceptance is the approach of tiered fixed dosing, where defined body size strata are used to assign dosages to specific subgroups of the pediatric population. This methodology is simple to use for the practitioner and has been used for MAbs such as basiliximab, adalimumab, and tocilizumab [104].
Simulated area under the plasma concentration–time curve at steady state (AUCss) following intravenous infusion of infliximab 5 mg/kg every 8 weeks. This figure, generated using the pharmacokinetic model reported by Xu et al. [140], shows a median AUCss that is 50 % lower for subjects aged 2–5 years and 25 % lower for subjects aged 6–17 years than for adults
3.3.4 Elderly Patients
The effects of advanced age can impact safety and efficacy of biologics. The effect of age on the pharmacokinetic properties of a protein appear to be due to changes in endothelial and macrophage function and, to a lesser extent, to changes in organ blood flow. Alterations of the immune response in elderly patients have been associated with increased amounts of memory and alloreactive T cells as well as altered cytokine responses [105], which can impact both on the pharmacokinetics and pharmacodynamics of a protein therapeutic agent. In addition, there is a higher incidence of infections and malignancies in the elderly population in general so evaluations of safety and efficacy in elderly patients should be conducted.
3.4 The Effect of Disease and Disease Stage
It is not uncommon for MAb clearance to vary. Many MAbs undergo TMDD, which results in non-linear, saturable pharmacokinetic behavior. If antigen expression is reflective of disease burden, then there is often a time-dependent component of clearance such that the clearance slows over time in patients who respond to drug. A MAb exemplifying this type of behavior is alemtuzumab, a humanized IgG1 MAb targeting the CD52 antigen, which is a glycoprotein found on the cell surface of lymphocytes. Alemtuzumab was initially approved to treat B cell chronic lymphocytic leukemia (B-CLL). In this patient population, alemtuzumab clearance is highly dependent on tumor burden, with very non-linear pharmacokinetic behavior that becomes somewhat more linear as the white cell count decreases on repeated administration [111]. However, in multiple sclerosis, which has a lower antigen burden, the pharmacokinetics are linear [112]. Similar dependencies were seen with trastuzumab, a MAb targeting the her2-neu antigen expressed on the surface of tumor cells, when used to treat metastatic breast cancer [113]; higher clearance was seen when trastuzumab was used to treat advanced gastric cancer [114]. Even with MAbs that do not exhibit TMDD, clearance has been reported to change across different indications (Table 2).
Co-morbidity can also contribute to altered MAb clearance. For example, diabetic co-morbidity is relatively common in the treatment of plaque psoriasis and psoriatic arthritis with ustekinumab (approximately 10.6 % of patients in the pivotal trials exhibited this co-morbidity) [115]. In patients with diabetic co-morbidity, clearance was often increased. The mechanistic basis of the alteration of clearance may be due to altered non-enzymatic glycosylation of IgG molecules in diabetic patients [116]. Other disease-mediated alterations in MAb clearance may occur as well. For example, IgG is lost to the gut in patients with inflammatory bowel disease, with clearance being related to lesion severity [117]. This finding of protein-losing enteropathy contributing to MAb clearance was later confirmed by Brandse et al. [118] and is thought to contribute to loss of therapeutic response.
3.5 Late-Stage Clinical Development
During phase II and III of clinical development, treatment duration increases and the dose regimens examined narrow. In most cases, trials are conducted against the standard of care to assess efficacy. There is a need to characterize the safety and efficacy of new MAbs in the target patient population and it is important to get both pharmacokinetic and pharmacodynamic data from patients with a wide range of disease severity.
3.6 Immunogenicity
It is common that at least some patients in any clinical trial will have been treated previously with MAbs. However, it should be noted that patients who have developed ADAs to other MAbs may be more likely to develop ADAs to the new MAb. This propensity has been seen, for example, when patients on infliximab who develop ADAs switch to adalimumab [119]. The cause for this is not clear, although the ADAs developed to the first MAb are not cross-reactive to the new MAb. A generally heightened immunoreactivity in some patients compared with others, as well as genetic factors such as human leukocyte antigen (HLA) genotype have been discussed as potential underlying reasons [120]. Indeed, the development of ADA is a key component of clinical development and it is important to ensure that the ADA assays are sensitive, selective, and quantitative early in development. In addition, dose regimens should be selected to minimize the likelihood of ADA development. Two therapeutic strategies have been associated with a reduction in ADA formation in inflammatory disease: (1) use of MAbs in a regularly scheduled regimen rather than episodic administration; and (2) concomitant administration of immunosuppressive agents (e.g., azathioprine, mercaptopurine, or methotrexate) with the MAb [121]. However, these options may be less feasible for MAbs used to treat cancer due, for example, to a higher frequency of adverse events in this therapeutic area. Furthermore, the treatment duration for antineoplastic MAbs is expected to be of much shorter duration than for chronic inflammatory conditions. However, protracted periods of time where the circulating MAb concentration is below the limit of assay quantification have been associated with ADAs, even when dosing is regularly scheduled [122]. Thus, dose selection should aim to minimize this for most patients. In order to minimize the likelihood of loss of response, many rheumatologists and gastroenterologists are beginning to implement therapeutic drug monitoring as part of their practice, allowing dose adjustments to be made to reduce the likelihood of ADAs and to maintain response [123, 124].
3.7 Population Pharmacokinetic/Pharmacodynamic Modeling
Modeling and simulation have become important tools for integrating data, knowledge, and mechanisms to make rational decisions about drug use and development. The development of these models provides a framework for predicting the time course of exposure and response following different dose regimens. Such models can be used for simulation to evaluate competing study designs, allowing more information to be captured more efficiently [2, 125, 126]. Such models can also be used to support dose regimen selection during drug development and regulatory review [44]. There has been a widespread adoption of population modeling methods that can quantitate and explain variability in drug exposure and response in the pharmaceutical industry. All drugs exhibit between-subject variability in both exposure and response. Population-based modeling is aimed at identifying and quantifying that variability. Understanding the influence of factors such body weight, age, genotype, renal or hepatic function, and concomitant medications on drug exposure and response is important in refining dosage recommendations and improving the safety and efficacy of an agent by appropriately controlling variability in drug exposure. A basic review of this approach was published by Mould and Upton [127]. Patient factors commonly identified as being predictive of pharmacokinetic and pharmacodynamic variability for MAbs are generally body size, albumin, ADAs, disease and disease severity, and concomitant medications [2, 128].
4 Global Development Challenges
In 2008, 80 % of approved marketing applications for drugs and biologics contain data from foreign clinical trials [129]. Currently, more than half of clinical trial subjects and sites are located outside of the USA, with Western Europe accounting for the most foreign clinical trial subjects and sites. Based on the increase in foreign clinical investigators conducting clinical trials under investigational new drug applications (INDs) over the last decade, reliance on foreign clinical trials for MAbs is likely to grow. However, there are times when the results of a clinical trial differ between countries in which the study was conducted [130]. There are several possible reasons for these differences, including genotype differences in the study population [131], different underlying health issues/risk factors such as obesity, diabetes, smoking and alcohol consumption, diet and other factors, different standards of care including the stage of disease at diagnosis, and unexplained differences. Care should always be taken when evaluating data from multiple countries to assess whether baseline risk factors can explain some outcome discrepancies. Broad differences in health risk factors such as smoking or obesity can change the underlying etiology of the disease and the subsequent response to new therapeutic agents.
5 Conclusions
This review covers the basic concepts of the non-clinical and clinical drug development process for MAbs. Owing to their complex pharmacology, the development of each MAb must be tailored to the specific needs and concerns of the individual drug. During the development process, there is a need for diverse expertise and close multi-professional and multi-disciplinary collaboration within the development team. In addition, the drug manufacturing processes, which were not covered in this review, require special consideration.
References
Cragg MS, French RR, Glennie MJ. Signaling antibodies in cancer therapy. Curr Opin Immunol. 1999;11(5):541–7.
Mould DR. Using pharmacometrics in the development of biological therapeutic biological agents. In: Ette E, Williams P, editors. Pharmacometrics: the science of quantitative pharmacology. Hoboken: Wiley; 2007. p. 993–1033
Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23(9):1147–57.
Natsume A, In M, Takamura H, Nakagawa T, Shimizu Y, Kitajima K, et al. Engineered antibodies of IgG1/IgG3 mixed isotype with enhanced cytotoxic activities. Cancer Res. 2008;68:3863–72.
Ricart AD, Tolcher AW. Technology insight: cytotoxic drug immunoconjugates for cancer therapy. Nat Clin Pract Oncol. 2007;4(4):245–55.
Brennan FR, Morton LD, Spindeldreher S, Kiessling A, Allenspach R, Hey A, et al. Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies. MAbs. 2010;2(3):233–55.
Wordell CJ. Biotechnology update. Hosp Pharm. 1991;26:897–900.
Ortho Pharmaceutical Corporation. OKT3. Summary basis of approval of muromonab-CD3. Freedom of Information Services, FDA; 1986.
Pavlovic D, Patera AC, Nyberg F, Gerber M, Liu M; Progressive Multifocal Leukeoncephalopathy Consortium. Progressive multifocal leukoencephalopathy: current treatment options and future perspectives. Ther Adv Neurol Disord. 2015;8(6):255–73.
Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–28.
ICH. Guidance for industry. S6 preclinical safety evaluation of biotechnology-derived pharmaceuticals. 1997 Jul. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm074957.pdf. Accessed 7 Jan 2016.
ICH. Guidance for industry. S6 addendum to preclinical safety evaluation of biotechnology-derived pharmaceuticals. 2012 May. http://www.fda.gov/downloads/Drugs/…/Guidances/UCM194490.pdf. Accessed 7 Jan 2016.
Chaparro-Riggers J, Liang H, DeVay RM, Bai L, Sutton JE, Chen W, et al. Increasing serum half-life and extending cholesterol lowering in vivo by engineering antibody with pH-sensitive binding to PCSK9. J Biol Chem. 2012;287(14):11090–7.
Säfsten P, Klakamp SL, Drake AW, Karlsson R, Myszka DG. Screening antibody-antigen interactions in parallel using Biacore A100. Anal Biochem. 2006;353(2):181–90.
Leonard P, Säfsten P, Hearty S, McDonnell B, Finlay W, O’Kennedy R. High throughput ranking of recombinant avian scFv antibody fragments from crude lysates using the Biacore A100. J Immunol Methods. 2007;323(2):172–9.
Canziani GA, Klakamp S, Myszka DG. Kinetic screening of antibodies from crude hybridoma samples using Biacore. Anal Biochem. 2004;325(2):301–7.
Tabrizi MA, Roskos LK. Preclinical and clinical safety of monoclonal antibodies. Drug Discov. Today. 2007;12:540–7.
Chapman K, Pullen N, Graham M, Ragan I. Preclinical safety testing of monoclonal antibodies: the significance of species relevance. Nat Rev Drug Discov. 2007;6(2):120–6.
Rich RL, Myszka DG. Advances in surface plasmon resonance biosensor analysis. Curr Opin Biotechnol. 2000;11:54–61.
Karlsson R, Falt A. Experimental design for kinetic analysis of protein–protein interactions with surface plasmon resonance biosensors. J Immunol Methods. 1997;200:121–33.
Morton TA, Myszka DG. Kinetic analysis of macromolecular interactions using surface plasmon resonance biosensors. Methods Enzymol. 1998;295:268–94.
Roskos L. Molecular engineering II: antibody affinity (Chapter 7). In: Dubel S, editor. Handbook of therapeutic antibodies. Vol. 1. Weinheim: Wiley–VCH; 2007. pp. 45–169.
Drake AW, Tang ML, Papalia GA, Landes G, Haak-Frendscho M, Klakamp SL. Biacore surface matrix effects on the binding kinetics and affinity of an antigen/antibody complex. Anal Biochem. 2012;429(1):58–69.
Drake AW, Myszka DG, Klakamp SL. Characterizing high-affinity antigen/antibody complexes by kinetic- and equilibrium-based methods. Anal. Biochem. 2004;328:35–43.
Blake RC 2nd, Pavlov AR, Blake DA. Automated kinetic exclusion assays to quantify protein binding interactions in homogeneous solution. Anal Biochem. 1999;272(2):123–34.
Darling RJ, Brault P-A. Kinetic exclusion assay technology: characterization of molecular interactions. Assay Drug Dev Technol. 2004;2:647–57.
Mould DR, Davis CB, Minthorn EA, Kwok DC, Elliott MJ, Luggen ME, et al. A population pharmacokinetic-pharmacodynamic analysis of single doses of clenoliximab in patients with rheumatoid arthritis. Clin Pharmacol Ther. 1999;66(3):246–57.
Drake AW, Klakamp SL. A rigorous multiple independent binding site model for determining cell-based equilibrium dissociation constants. J Immunol Methods. 2007;318(1–2):147–52.
Spilker ME, Singh P, Vicini P. Mathematical modeling of receptor occupancy data: a valuable technology for biotherapeutic drug development. Cytometry B Clin Cytom. 2016;90(2):230–6. doi:10.1002/cyto.b.21318.
Bates JT, Keefer CJ, Slaughter JC, Kulp DW, Schief WR, Crowe JE Jr. Escape from neutralization by the respiratory syncytial virus-specific neutralizing monoclonal antibody palivizumab is driven by changes in on-rate of binding to the fusion protein. Virology. 2014;454–455:139–44.
Junghans RP. Finally! The Brambell receptor (FcRB). Mediator of transmission of immunity and protection from catabolism for IgG. Immunol Res. 1997;16(1):29–57.
Glassman PM, Balthasar JP. Mechanistic considerations for the use of monoclonal antibodies for cancer therapy. Cancer Biol Med. 2014;11(1):20–33.
European Medicinal agency (EMEA). Guideline on immunogenicity assessment of biotechnology-derived therapeutic proteins (Doc. Ref. EMEA/CHMP/BMWP/14327/2006). London: EMEA; 2006.
De Groot AS, Moise L. Prediction of immunogenicity for therapeutic proteins: state of the art. Curr Opin Drug Discov Devel. 2007;10(3):332–40.
Stas P, Lasters I. Strategies for preclinical immunogenicity assessment of protein therapeutics. IDrugs. 2009;12(3):169–73.
Meibohm B, Derendorf H. Pharmacokinetic/pharmacodynamic studies in drug product development. J Pharm Sci. 2002;91(1):18–31.
Gibbs JP. Prediction of exposure-response relationships to support first-in-human study design. AAPS J. 2010;12(4):750–8.
Holmdahl R, Lorentzen JC, Lu S, Olofsson P, Wester L, Holmberg J, et al. Arthritis induced in rats with nonimmunogenic adjuvants as models for rheumatoid arthritis. Immunol Rev. 2001;184:184–202.
Knoerzer DB, Karr RW, Schwartz BD, Mengle-Gaw LJ. Collagen-induced arthritis in the BB rat. Prevention of disease by treatment with CTLA-4-Ig. J Clin Invest. 1995;96(2):987–93.
Lon HK, Liu D, DuBois DC, Almon RR, Jusko WJ. Modeling pharmacokinetics/pharmacodynamics of abatacept and disease progression in collagen-induced arthritic rats: a population approach. J Pharmacokinet Pharmacodyn. 2013;40(6):701–12.
Lon HK, Liu D, Zhang Q, DuBois DC, Almon RR, Jusko WJ. Pharmacokinetic-pharmacodynamic disease progression model for effect of etanercept in Lewis rats with collagen-induced arthritis. Pharm Res. 2011;28(7):1622–30.
Earp JC, Dubois DC, Almon RR, Jusko WJ. Quantitative dynamic models of arthritis progression in the rat. Pharm Res. 2009;26(1):196–203.
Dong JQ, Salinger DH, Endres CJ, Gibbs JP, Hsu CP, Stouch BJ, et al. Quantitative prediction of human pharmacokinetics for monoclonal antibodies: retrospective analysis of monkey as a single species for first-in-human prediction. Clin Pharmacokinet. 2011;50(2):131–42.
Roy A, Mould DR, Wang XF, Tay L, Raymond R, Pfister M. Modeling and simulation of abatacept exposure and interleukin-6 response in support of recommended doses for rheumatoid arthritis. J Clin Pharmacol. 2007;47(11):1408–20.
Lute KD, May KF Jr, Lu P, Zhang H, Kocak E, Mosinger B, et al. Human CTLA4 knock-in mice unravel the quantitative link between tumor immunity and autoimmunity induced by anti-CTLA-4 antibodies. Blood. 2005;106(9):3127–33.
Lee H, Zahra D, Vogelzang A, Newton R, Thatcher J, Quan A, et al. Human C5aR knock-in mice facilitate the production and assessment of anti-inflammatory monoclonal antibodies. Nat Biotechnol. 2006;24(10):1279–84.
Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs. 1999;17(4):343–59.
Hood JD, Cheresh DA. Building a better Trap. Proc Natl Acad Sci USA. 2003;100(15):8624–5.
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57.
Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417–27.
Wartha K, Herting F, Hasmann M. Fit-for purpose use of mouse models to improve predictivity of cancer therapeutics evaluation. Pharmacol Ther. 2014;142(3):351–61.
Loisel S, Ohresser M, Pallardy M, Daydé D, Berthou C, Cartron G, et al. Relevance, advantages and limitations of animal models used in the development of monoclonal antibodies for cancer treatment. Crit Rev Oncol Hematol. 2007;62(1):34–42.
Ribba B, Holford NH, Magni P, Trocóniz I, Gueorguieva I, Girard P, et al. A review of mixed-effects models of tumor growth and effects of anticancer drug treatment used in population analysis. CPT Pharmacometrics Syst Pharmacol. 2014;7(3):e113. doi:10.1038/psp.2014.12.
Daydé D, Ternant D, Ohresser M, Lerondel S, Pesnel S, Watier H, et al. Tumor burden influences exposure and response to rituximab: pharmacokinetic-pharmacodynamic modeling using a syngeneic bioluminescent murine model expressing human CD20. Blood. 2009;113(16):3765–72.
Coles AJ, Wing M, Smith S, Coraddu F, Greer S, Taylor C, et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet. 1999;354(9191):1691–5.
Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30(8):825–30.
Bria E, Cuppone F, Milella M, Verma S, Carlini P, Nistico C, et al. Trastuzumab cardiotoxicity: biological hypotheses and clinical open issues. Expert Opin Biol Ther. 2008;8:1963–71.
Chapman K, Pullen N, Coney L, Dempster M, Andrews L, Bajramovic J, et al. Preclinical development of monoclonal antibodies: considerations for the use of non-human primates. MAbs. 2009;1(5):505–16.
Schweiterman W. Regulating biopharmaceuticals under CDER versus CBER: an insider’s perspective. Drug Discov Today. 2006;11:945–51.
Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol. 2001;13(12):1551–9.
Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? MAbs. 2011;3(1):61–6.
Chirmule N, Jawa V, Meibohm B. Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy. AAPS J. 2012;14(2):296–302.
Department of Health and Human Services, Food and Drug Administration. Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Rockville: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER); 2005.
Lowe PJ, Tannenbaum S, Wu K, Lloyd P, Sims J. On setting the first dose in man: quantitating biotherapeutic drug-target binding through pharmacokinetic and pharmacodynamic models. Basic Clin Pharmacol Toxicol. 2010;106(3):195–209.
Muller PY, Milton M, Lloyd P, Sims J, Brennan FR. The minimum anticipated biological effect level (MABEL) for selection of first human dose in clinical trials with monoclonal antibodies. Curr Opin Biotechnol. 2009;20(6):722–9.
Tabares P, Berr S, Romer PS, Chuvpilo S, Matskevich AA, Tyrsin D, et al. Human regulatory T cells are selectively activated by low-dose application of the CD28 superagonist TGN1412/TAB08. Eur J Immunol. 2014;44(4):1225–36.
Nolting A, Fox F, Kovar A. Clinical drug development of cetuximab, a monoclonal antibody. In: Meibohm B, editor. Pharmacokinetics and pharmacodynamics of biotech drugs. Weinheim: Wiley-VCH; 2006. p. 353–71.
Wang DD, Zhang S, Zhao H, Men AY, Parivar K. Fixed dosing versus body size-based dosing of monoclonal antibodies in adult clinical trials. J Clin Pharmacol. 2009;49(9):1012–24.
Dirks NL, Nolting A, Kovar A, Meibohm B. Population pharmacokinetics of cetuximab in patients with squamous cell carcinoma of the head and neck. J Clin Pharmacol. 2008;48(3):267–78.
Mould DR, Green B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. BioDrugs. 2010;24(1):23–39.
Fasanmade AA, Adedokun OJ, Olson A, Strauss R, Davis HM. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010;48(5):297–308.
Rosario M, Dirks NL, Gastonguay MR, Fasanmade AA, Wyant T, Parikh A, et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther. 2015;42(2):188–202.
Lu JF, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol. 2008;62:779–86.
Genentech, Inc. Xolair® prescribing information. South San Francisco: Genentech, Inc.; 2014.
Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv. 2007;4(4):427–40.
Tang L, Persky AM, Hochhaus G, Meibohm B. Pharmacokinetic aspects of biotechnology products. J Pharm Sci. 2004;93(9):2184–204.
Zhao L, Ji P, Li Z, Roy P, Sahajwalla CG. The antibody drug absorption following subcutaneous or intramuscular administration and its mathematical description by coupling physiologically based absorption process with the conventional compartment pharmacokinetic model. J Clin Pharmacol. 2013;53(3):314–25.
Gill KL, Gardner I, Li L, Jamei M. A bottom-up whole-body physiologically based pharmacokinetic model to mechanistically predict tissue distribution and the rate of subcutaneous absorption of therapeutic proteins. AAPS J. 2016;18(1):156–70.
Tranter E, Peters G, Boyce M, Warrington S. Giving monoclonal antibodies to healthy volunteers in phase 1 trials: is it safe? Br J Clin Pharmacol. 2013;76(2):164–72.
Tosi D, Laghzali Y, Vinches M, Alexandre M, Homicsko K, Fasolo A, et al. Clinical development strategies and outcomes in first-in-human trials of monoclonal antibodies. J Clin Oncol. 2015;33(19):2158–65.
Cherin P, Marie I, Michallet M, Pelus E, Dantal J, Crave JC, et al. Management of adverse events in the treatment of patients with immunoglobulin therapy: a review of evidence. Autoimmun Rev. 2016;15(1):71–81.
Zhang X, Brennan BJ. Disease-drug-drug interaction assessment for tocilizumab—a monoclonal antibody against interleukin-6 receptor to treat patients with rheumatoid arthritis. In: Zhou H, Meibohm B, editors. Drug-drug interactions for therapeutic biologics. Hoboken: Wiley; 2013. p. 191–205.
Zhou H, Meibohm B. Drug-drug interactions for therapeutic biologics. Hoboken: Wiley; 2013.
Schmitt C, Kuhn B, Zhang X, Kivitz AJ, Grange S. Disease-drug-drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacol Ther. 2011;89(5):735–40.
Lu D, Girish S, Theil FP, Joshi A. Pharmacokinetic and pharmacodynamic based drug interactions for therapeutic proteins. In: Zhou H, Meibohm B, editors. Drug-drug interactions for therapeutic biologics. Hoboken: Wiley; 2013. p. 5–37.
Joshi A, Bauer R, Kuebler P, White M, Leddy C, Compton P, et al. An overview of the pharmacokinetics and pharmacodynamics of efalizumab: a monoclonal antibody approved for use in psoriasis. J Clin Pharmacol. 2006;46(1):10–20.
Seitz K, Zhou H. Pharmacokinetic drug-drug interaction potentials for therapeutic monoclonal antibodies: reality check. J Clin Pharmacol. 2007;47(9):1104–18.
Bartelds GM, Wijbrandts CA, Nurmohamed MT, Stapel S, Lems WF, Aarden L, et al. Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann Rheum Dis. 2007;66(7):921–6.
Wijngaarden S, van Roon JA, van de Winkel JG, Bijlsma JW, Lafeber FP. Down-regulation of activating Fcgamma receptors on monocytes of patients with rheumatoid arthritis upon methotrexate treatment. Rheumatology. 2005;44(6):729–34.
Yang BB, Gillespie B, Smith B, Smith W, Lissmats A, Rudebeck M, et al. Pharmacokinetic and pharmacodynamic interactions between palifermin and heparin. J Clin Pharmacol. 2015;55(10):1109–18.
DHHS, Food and Drug Administration. Guidance for industry: drug interaction studies - study design, data analysis, implications for dosing, and labeling recommendations. Silver Spring: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER); 2012.
Rathi C, Meibohm B. Clinical pharmacology of bispecific antibody constructs. J Clin Pharmacol. 2015;55 (Suppl 3):S21–8.
Meibohm B, Zhou H. Characterizing the impact of renal impairment on the clinical pharmacology of biologics. J Clin Pharmacol. 2012;52(1 Suppl):54S–62S.
Zhang Y, Yao Z, Kaila N, Kuebler P, Visich J, Maia M, et al. Pharmacokinetics of ranibizumab after intravitreal administration in patients with retinal vein occlusion or diabetic macular edema. Ophthalmology. 2014;121(11):2237–46.
Berdeja J, Jagannath S, Zonder J, Badros A, Kaufman JL, Manges R, et al. Pharmacokinetics and safety of elotuzumab combined with lenalidomide and dexamethasone in patients with multiple myeloma and various levels of renal impairment: results of a phase Ib study. Clin Lymphoma Myeloma Leuk. 2015;16(3):129–38.
Roberts BV, Susano I, Gipson DS, Trachtman H, Joy MS. Contribution of renal and non-renal clearance on increased total clearance of adalimumab in glomerular disease. J Clin Pharmacol. 2013;53(9):919–24.
Struemper H, Chen C, Cai W. Population pharmacokinetics of belimumab following intravenous administration in patients with systemic lupus erythematosus. J Clin Pharmacol. 2013;53(7):711–20.
European Medicines Agency. Guideline on the clinical investigation of the pharmacokinetics of therapeutic proteins. London: European Medicines Agency; 2007.
Meibohm B. Pharmacokinetics and pharmacodynamics of peptide and protein therapeutics. In: Crommelin DJA, Sindelar RD, Meibohm B, editors. Pharmaceutical biotechnology: fundamentals and applications. New York: Springer; 2013. p. 101–132
Amgen Inc. Vectibix (Panitumumab) prescribing information. Thousand Oaks: Amgen Inc.; 2015.
Krens LL, Baas JM, de Jong FA, Guchelaar HJ, Gelderblom H. Pharmacokinetics of panitumumab in a patient with liver dysfunction: a case report. Cancer Chemother Pharmacol. 2014;73(2):429–33.
Moosmann N, Laessig D, Michaely HJ, Schulz C, Heinemann V. Effective second-line treatment with cetuximab and bevacizumab in a patient with hepatic metastases of colorectal cancer and hyperbilirubinemia. Onkologie. 2007;30(10):509–12.
Xu Z, Davis HM, Zhou H. Rational development and utilization of antibody-based therapeutic proteins in pediatrics. Pharmacol Ther. 2013;137(2):225–47.
Zhang Y, Wei X, Bajaj G, Barrett JS, Meibohm B, Joshi A, et al. Challenges and considerations for development of therapeutic proteins in pediatric patients. J Clin Pharmacol. 2015;55(Suppl 3):S103–15.
Pascher A, Pratschke J, Neuhaus P, Tullius SG. Modifications of immune regulations with increasing donor and recipient age. Ann Transplant. 2004;9(1):72–3.
Edlund H, Melin J, Parra-Guillen ZP, Kloft C. Pharmacokinetics and pharmacokinetic-pharmacodynamic relationships of monoclonal antibodies in children. Clin Pharmacokinet. 2015;54(1):35–80.
Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28(6):507–32.
Barrett JS, Della Casa Alberighi O, Laer S, Meibohm B. Physiologically based pharmacokinetic (PBPK) modeling in children. Clin Pharmacol Ther. 2012;92(1):40–9.
Laer S, Barrett JS, Meibohm B. The in silico child: using simulation to guide pediatric drug development and manage pediatric pharmacotherapy. J Clin Pharmacol. 2009;49(8):889–904.
Falaiye TO, Mitchell KR, Lu Z, Saville BR, Horst SN, Moulton DE, et al. Outcomes following infliximab therapy for pediatric patients hospitalized with refractory colitis-predominant IBD. J Pediatr Gastroenterol Nutr. 2014;58(2):213–9.
Mould DR, Baumann A, Kuhlmann J, Keating MJ, Weitman S, Hillmen P, et al. Population pharmacokinetics-pharmacodynamics of alemtuzumab (Campath) in patients with chronic lymphocytic leukaemia and its link to treatment response. Br J Clin Pharmacol. 2007;64(3):278–91.
Garnock-Jones KP. Alemtuzumab: a review of its use in patients with relapsing multiple sclerosis. Drugs. 2014;74(4):489–504.
Bernadou G, Campone M, Merlin JL, Gouilleux-Gruart V, Bachelot T, Lokiec F, et al. Influence of tumour burden on trastuzumab pharmacokinetics in HER2 positive non-metastatic breast cancer. Br J Clin Pharmacol. 2016;81(5):941–8. doi:10.1111/bcp.12875.
Cosson VF, Ng VW, Lehle M, Lum BL. Population pharmacokinetics and exposure-response analyses of trastuzumab in patients with advanced gastric or gastroesophageal junction cancer. Cancer Chemother Pharmacol. 2014;73(4):737–47.
Zhou H, Theil F-P, editors. ADME and translational pharmacokinetics/pharmacodynamics of therapeutic proteins: applications in drug discovery and development. Hoboken: Wiley; 2016. p. 129.
Kaneshige H. Nonenzymatic glycosylation of serum IgG and its effect on antibody activity in patients with diabetes mellitus. Diabetes. 1987;36(7):822–8.
Kapel N, Meillet D, Favennec L, Magne D, Raichvarg D, Gobert JG. Evaluation of intestinal clearance and faecal excretion of alpha 1-antiproteinase and immunoglobulins during Crohn’s disease and ulcerative colitis. Eur J Clin Chem Clin Biochem. 1992;30(4):197–202.
Brandse JF, van den Brink GR, Wildenberg ME, van der Kleij D, Rispens T, Jansen JM, et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology. 2015;149(2):350–5.
Bartelds GM, Wijbrandts CA, Nurmohamed MT, Stapel S, Lems WF, Aarden L, et al. Anti-infliximab and anti-adalimumab antibodies in relation to response to adalimumab in infliximab switchers and anti-tumour necrosis factor naive patients: a cohort study. Ann Rheum Dis. 2010;69(5):817–21.
Barbosa MD, Vielmetter J, Chu S, Smith DD, Jacinto J. Clinical link between MHC class II haplotype and interferon-beta (IFN-beta) immunogenicity. Clin Immunol. 2006;118(1):42–50.
Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther. 2012;91(4):635–46.
Mould DR. The pharmacokinetics of biologics: a primer. Dig Dis. 2015;33(suppl 1):61–9.
Mould DR. Why therapeutic drug monitoring is needed for monoclonal antibodies and how do we implement this? Clin Pharmacol Ther. 2016;99(4):351–4. doi:10.1002/cpt.278.
Mould DR, Dubinsky MC. Dashboard systems: pharmacokinetic/pharmacodynamic mediated dose optimization for monoclonal antibodies. J Clin Pharmacol. 2015;55(Suppl 3):S51–9.
Whiting B, Kelman AW, Grevel J. Population pharmacokinetics: theory and clinical application. Clin Pharmacokinet. 1986;11:387–401.
Zhang L, Pfister M, Meibohm B. Concepts and challenges in quantitative pharmacology and model-based drug development. AAPS J. 2008;10(4):552–9.
Mould DR, Upton RN. Basic concepts in population modeling, simulation and model-based drug development. CPT Pharmacometrics Syst Pharmacol. 2012;1:e6.
Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(10):633–59.
Department of Health and Human Services, Office of Inspector General. Challenges to FDA’s ability to monitor and inspect foreign clinical trials. http://oig.hhs.gov/oei/reports/oei-01-08-00510.pdf. Accessed 2 Feb 2016.
Chang WC, Midodzi WK, Westerhout CM, Boersma E, Cooper J, Barnathan ES, et al. Are international differences in the outcomes of acute coronary syndromes apparent or real? A multilevel analysis. J Epidemiol Community Health. 2005;59(5):427–33.
McGraw J, Waller D. Cytochrome P450 variations in different ethnic populations. Expert Opin Drug Metab Toxicol. 2012;8(3):371–82.
Kavanaugh A, St Clair EW, McCune WJ, Braakman T, Lipsky P. Chimeric anti-tumor necrosis factor-alpha monoclonal antibody treatment of patients with rheumatoid arthritis receiving methotrexate therapy. J Rheumatol. 2000;27(4):841–50.
Xu Z, Seitz K, Fasanmade A, Ford J, Williamson P, Xu W, et al. Population pharmacokinetics of infliximab in patients with ankylosing spondylitis. J Clin Pharmacol. 2008;48(6):681–95.
Fasanmade AA, Adedokun OJ, Ford J, Hernandez D, Johanns J, Hu C, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol. 2009;65(12):1211–28.
Fasanmade AA, Adedokun OJ, Blank M, Zhou H, Davis HM. Pharmacokinetic properties of infliximab in children and adults with Crohn’s disease: a retrospective analysis of data from 2 phase III clinical trials. Clin Ther. 2011;33(7):946–64.
Anasetti C, Hansen JA, Waldmann TA, Appelbaum FR, Davis J, Deeg HJ, et al. Treatment of acute graft-versus-host disease with humanized anti-Tac: an antibody that binds to the interleukin-2 receptor. Blood. 1994;84(4):1320–7.
Mould DR, Sweeney KR. The pharmacokinetics and pharmacodynamics of monoclonal antibodies–mechanistic modeling applied to drug development. Curr Opin Drug Discov Devel. 2007;10(1):84–96.
Vincenti F, Lantz M, Birnbaum J, Garovoy M, Mould D, Hakimi J, et al. A phase I trial of humanized anti-interleukin 2 receptor antibody in renal transplantation. Transplantation. 1997;63(1):33–8.
Othman AA1, Tran JQ, Tang MT, Dutta S. Population pharmacokinetics of daclizumab high-yield process in healthy volunteers: integrated analysis of intravenous and subcutaneous, single- and multiple-dose administration. Clin Pharmacokinet. 2014;53(10):907–18.
Xu Z, Mould D, Hu C, Ford J, Keen M, Davis HM, et al. Population pharmacokinetic analysis of infliximab pediatrics using integrated data from six clinical trials. Clin Pharmacol Drug Dev. 2012;4:203.
Acknowledgments
The authors would like to thank the reviewers for their thoughtful consideration of this manuscript.
Author contributions
Drs. Mould and Meibohm contributed equally to the writing of this review article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr Mould is president of Projections Research Inc., a consulting company for the pharmaceutical industry. Dr Meibohm provides consultancy services to multiple entities in the pharmaceutical industry.
Funding
No sources of funding were used to assist in the preparation of this manuscript.
Rights and permissions
About this article
Cite this article
Mould, D.R., Meibohm, B. Drug Development of Therapeutic Monoclonal Antibodies. BioDrugs 30, 275–293 (2016). https://doi.org/10.1007/s40259-016-0181-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-016-0181-6