Abstract
Among molecular structures, colloidal molecules have attracted the attention of the scientific community because of their distinct geometry (topology, size on a nanometer scale). In this field, dendrimers are an example of colloidal polymeric molecules which have a specific size and are identified and regulated by repeating units as generations. The dendrimers are very similar to the colloidal particles in geometric form and size, which is more evident in the higher generations of the colloidal dendrimers. On the other hand, the unique structural features of these macromolecules resemble them as macromolecular colloids. These properties including the high degree of freedom, the controllable molecular size and weight, the need for no initiator, the presence of end groups, the high drug transfer capacity, etc., have made dendrimers applicable to a variety of fields such as medicine, biomedical, pharmaceutical, catalyst, and so on.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dendrimers as globular nano-macromolecules are radially symmetric molecules with well-specified, uniform and integrated structure including tree-like arms or branches [1,2,3,4]. The structure of these materials has a profound influence on their physical and chemical properties. Due to their unique behaviors, they are used in a broad range of biomedical and industrial applications. Unbeatable features of dendrimer such as the same size, the highest degree of branch formation, water solubility and presence of internal cavities make them attractive for various applications [5, 6]. In addition, some dendrimers have natural medicinal activities such as antibacterial, antiviral and antitumor activities [7].
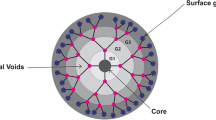
Although research on dendrimers began in 1970s, no successful method was reported by the late 1970s. In 1978, Buhleier et al. [5] introduced a group of synthetic cascading molecules. In 1984–1985, Tomalia et al. [7] reported the first real dendrimers, and in 1990, Hawker et al. [8] published a report on preparation of spherical molecules. Unlike linear polymers, these macromolecules do not entangle [9, 10] and have unusual viscosity behaviors such as low solution viscosity and functional groups can be either protected or exposed [11, 12]. These unique macromolecules are made up of three basic parts, namely a central core with at least two identical chemical functions, branches exuding from the core which are divided into several layers called generations and terminal groups which have important role in properties of dendrimers [13, 14].
Synthesis approaches
Divergent method
In this approach, dendrimer begins to grow from a multifunctional core through a step-by-step iterative addition of monomers [15, 16]. The advantages of this approach are the high rate of polymerization, modification and change in surface groups, and synthesis of high molecular weight dendrimers [17, 18]. However, side reactions occurring during synthesis are the most important disadvantage of this approach, especially in poly(amidoamine) dendrimers. Also, incomplete reactions occur in the end groups and lead to structural defects [19]. Other disadvantages of this approach include formation of some low molecular weight molecules, lack of diversity in group’s outer layers and high reaction temperature sensitivity which can cause reversible Michael addition reaction [20].
Convergent approach
Convergent approach was introduced to overcome the disadvantages of divergent approach [20]. In this approach, highly pure dendrons are produced firstly and then connected by a multifunctional core. This approach has several advantages including easy product purification and significant reduction in structural defect. Convergent approach suffers from high number of steps, difficulty in the synthesis of dendrimers with higher generations and reduced returns due to reduced reactivity of the central dendrons [15]. In convergent approach, the formation of higher generations is so difficult due to the occurrence of spatial inhibition in the reaction between dendrons and molecular nucleus [4]. Scheme 1 schematically shows the synthetic path for divergent and convergent approaches.

Reproduced with the permission of RSC, 2020 [19]
Synthetic path for divergent and convergent approaches
Click chemistry
Click chemistry approach is another method for the rapid and reliable synthesis of dendrimers. One of the salient features of this method is the high chemical performance of the reaction. Also, simple reaction conditions, readily available reagents, and benign used solvents are other features of this method [21].
Lego chemistry
Various methods have been discovered by scientists in order to simplify the synthetic method for the synthesis of dendrimers in terms of cost and synthesis time. Lego chemistry is one of the results of these excavations. This method has been reviewed several times, and the results showed that this method can increase the number of terminal groups from 48 to 250 in one step [21].
Types of dendrimers
Dendrimers based on their shape, peripheral groups and inner cavity can be divided into several types including PAMAM dendrimer [22], PPI dendrimer [23], liquid crystalline (LC) dendrimers [24], chiral dendrimers [25], peptide dendrimers [26], core–shell (tecto)dendrimers [27], glycodendrimers [28], etc. Herein, three types of dendrimers as the most important ones have been widely reviewed.
PAMAM dendrimers
The first successful effort to create and design dendritic structures by organic synthesis was made by Vogtle and colleagues [5]. Then, Tomalia and colleagues succeeded in synthesis of PAMAM dendrimer in the early 1980s [7]. PAMAM dendrimers owing to the combination of superficial amines and interior amide bonds are used in many biological applications [29]. Ease of use, low-cost synthesis of doped PAMAM dendrimers than other biological molecules such as antibodies and proteins of the same size and biocompatibility have made them interesting for biochemistry, nanotechnology and medicine [29,30,31]. These dendrimers are generally synthesized via divergent approach [5]. Core molecules which give rise to PAMAM dendrimers can vary but the most basic initiators are ammonia [32] and ethylenediamine [33]. As shown in Scheme 2, PAMAM dendrimers use the following iterative reactions to grow:

Reproduced with the permission of RSC, 2020 [4]
Synthetic route of PAMAM dendrimer
-
(1)
Michael addition of the amino-terminated compound onto methyl(meth)acrylate;
-
(2)
Amidation reaction between amine-containing compounds and product of stage (i) to achieve a new amino-terminated compound.
It should be noted that focal points of convergent-synthesized segment have been used to create unsymmetrical dendrimers [34] and dendrimers with various core functionalizations [35]. Their functionality is readily tailored, and their uniformity, size and highly reactive surfaces are the functional keys to their application [36].
Poly(propylene imine) (PPI) dendrimers
The first poly(propylene imine) dendrimer was synthesized by Vögtle et al., according to a repetitive reactions consisting of Michael addition of an amine to acrylonitrile and then reducing the nitrile groups to primary amines [37]. The fifth-generation PPI dendrimer is the highest generation that has been synthesized, and different cores such as EDA [38] and 1,4-diaminobutane (DAB) [39] are used to synthesize these dendrimers with different generations. Scheme 3 shows the synthesis steps of PPI dendrimer with EDA core. Different methods have been used for reduction of nitrile groups to amines as follows:

Reproduced with the permission of Springer, 2020 [43]
Synthetic route of PPI dendrimers
-
Using a heterogeneous hydrogenation catalyst (such as Raney nickel and cobalt), a pressure of 40 bar and a temperature of 70 °C [40];
-
Using hydrazinium monoformate and Raney nickel catalyst [41];
-
Using lithium aluminum hydride catalyst [42].
Phosphorous-based dendrimers
Rich structures of silicon- and phosphorus-based dendrimers are now available [44,45,46,47,48]. In recent years, because of the unprecedented attributes of phosphorous-based dendrimers such as catalyzing, materials science and medicine, they have been considered significantly [49, 50]. Several groups have described phosphate-based dendrimers using a divergent method [51, 52]. The first method was described by Regan et al. in 1990 [53]. They have synthesized a new family of dendrimers consisting of a central core and many branch points with quaternary phosphonium ion sites. In 1994, DuBois et al. [54] synthesized the first small dendrimers containing a phosphine at each branching point for electrochemical CO2 reduction. In 1999, Kakkar and colleagues obtained larger dendrimers containing phosphine group at any point in the branching process [55]. In 1994, Majoral and colleagues presented the first neutral phosphorus dendrimers [45]. They used the following two basic steps: the reaction of hydroxybenzaldehyde and a core with P–Cl or aldehyde functions and condensation of aldehyde groups with a phosphorhydrazide [56, 57]. The attendance of aldehyde end groups or P(S)Cl2 at each step can develop reactions [58,59,60,61,62,63,64,65]. However, the synthesis of these dendrimers is time-consuming. Majoral and colleagues succeeded in transforming the synthesis process into a single step using the classical method [65, 66]. They used (S)P(OC6H4CHO)3 as a core (G0). Thus, in the first step, G1 was easily synthesized with six terminal groups of diphenylphosphino end groups using reaction between core (G0) and three equiv. of AB2 (monomer 1). In the second step, G2 was synthesized (with 12 aldehyde terminal groups), using Staudinger reaction between G1 and six equiv. of the azide (monomer 2). Dendrimers were formed up to the fourth generation using these two monomers in four steps. Scheme 4 shows the structure of phosphorus dendrimers.
Structure of phosphorus-containing dendrimers [67]
Applications
In contrast to linear polymers, which are often randomly formed, dendrimers have a specific structure that comprises a central core with branches that are located radially. In the higher generations (above 4), their structure changes to three-dimensional and quasi-spherical. The major intramolecular forces in the dendrimer are covalent bonds, but other types of interactions (such as hydrogen bonds) are known as well. They also have the ability to trap guests within the molecular space because of the ability to move their branch structure. Although many other nanostructures provide a high surface area and can be used for drug delivery [68, 69], dendrimers have good control and flexibility for this purpose. These macromolecules are very useful for carrying materials and can be organized into different dimensions. Indoor spaces such as surface end groups can be used as centers for the integration of chemical functional groups. This characteristic of dendrimers makes them useful for various applications such as catalysts [70], medicine [71], drug delivery [72] and synthesis of nanoparticles [73].
Catalysis
In recent decades, researchers have performed many different works in the field of catalysts [74,75,76,77,78,79,80,81,82,83]. Dendrimers are unique macromolecules that can be accessed using chemical compounds from a variety of building blocks. Metal complexes as catalytic groups can be located in the dendrimer core to exploit microenvironment and selectivity factors of the dendritic shell [84]. Reymond and co-workers reported the first catalytic peptide dendrimers for an ester hydrolysis reaction [85]. Dendrimers are used to prepare a particular microenvironment to simplify catalyst separation and recovery [86, 87]. In recent studies, researchers have examined peptide dendrimers such as protein mimics, antiviral and anticancer agents, vaccines and drug and gene delivery systems [88, 89]. Douat-Casassus et al. [90] synthesized different peptide dendrimers based on the Fmoc-protected 3,5-diami-nobenzoic acid as a building block for the branching unit and bearing the catalytic triad amino acids serine, histidine, and aspartate at variable positions on the dendrimer branches. As catalysis is relatively easy to tune the structure, size and location of catalytically active sites, it can be one of the most promising applications of dendrimers [91]. Host dendrimers for metal nanoparticles are catalytically active for the following reasons [92]:
-
Dendrimers have a relatively uniform structure and composition;
-
Nanoparticles are stabilized within the dendrimer internal cavity, which prevents their aggregation during the catalytic reaction;
-
Nanoparticles inside the dendrimers are maintained by steric effects so that there is a significant portion of their inactive levels to participate in the catalytic reactions;
-
To control the access of small molecules to the encapsulated (catalytic) nanoparticles, the branches of dendrimer can be used as selective gates;
-
The hybrid nanocomposite solubility can be controlled with the dendrimer periphery.
Catalytic performance is measurable by sustainability, activity, selectivity, and recyclability. As shown in Scheme 5, this depends on the dendritic architecture including distinguishing periphery-, core- and focal point-functionalized dendrimers [93].

Reproduced with the permission of Elsevier, 2020 [95]
Various dendritic architectures: catalyst located at the periphery (a), internal core (b), focal point of a wedge (c) and periphery of a wedge (d)
In 2000, Chechik and Crooks [94] reported Pd-encapsulated dendrimer nanoparticles as highly selective and active fluorous biphasic catalysis. They showed that these catalysts could be easily recycled and used for multiple reactions. In 2001, Crooks et al. [95] synthesized metal nanoparticles-encapsulated dendrimers and evaluated their catalytic applications. In 2006, Hoover et al. [96] used dendrimer nanoparticles (DENs) as precursors of Pt–Cu catalysts where the effect of particle composition on heterogeneous catalysts was investigated. Karakhanov et al. [97] reported thermo-responsive ruthenium catalysts based on PPI dendrimers cross-linked with poly(ethylene glycol) diglycidyl ether. The results showed good chemical and physical attributes of the synthesized catalysts such as metal loading, mean particles size, surface structure, etc. Also, dendrimers-based catalysts have many other applications including membrane reactor [98,99,100,101], biphasic systems [102], “tea bag” [103] and ionic liquids [104, 105].
Medications and in pharmaceutical
Dendrimers are used for many medications and in pharmaceutical due to the well-defined 3D structure, surface functional groups and low size besides predetermined molecular weight [106, 107]. They are combined with drugs and bioactive molecules, and their internal cavities can also be changed for combination of hydrophobic and hydrophilic drugs [108, 109]. Modified surface end groups have also been used to attach antibodies and bioactive substances and increase reactivity and solubility [110, 111]. Mechanisms of interactions between drugs and dendrimers like other polymeric structures are categorized into three main classes, namely encapsulation, electrostatic interactions and covalent conjugations [112,113,114,115,116].
Drug delivery systems
The central core and its internal units of dendrimers create cavities as environment for drug placement. The solubility and chemical behavior of these macromolecules can be controlled by binding the target functional groups to their surface [110]. In 1982, Maciejewski proposed the use of dendrimers as molecular containers [117]. In 2005, Patri and colleagues synthesized fifth-generation PAMAM dendrimer conjugates with folic acid and then examined the solubility of dendrimer conjugates and compared the efficacy of covalently bounded methotrexate (MTX) onto fifth-generation PAMAM dendrimer [118]. In 2012, Wang and colleagues used PPI dendrimer with varying degrees of acetylation and encapsulated drugs including sodium methotrexate and doxorubicin [119]. Acetylation of more than 80% of functional groups significantly reduced cell cytotoxicity in MCF-7 and A549 cell lines. They found that the loading capacity of drug was proportional to the degree of acetylation and increasing degree of acetylation resulted in higher loading capacity of the drug. In 2014, Kesharwani et al. [120] loaded third-, fourth- and fifth-generation PPI dendrimers with melphalan drug under identical conditions. They found that increasing generation of dendrimer led to higher drug loading due to increased internal cavities of dendrimer [120]. In 2014, Pourjavadi et al. investigated pH-responsive magnetic nanoparticles to control the release of DOX. They have grown a third-generation PAMAM dendrimer on the surface of magnetic iron oxide nanoparticles. Then, surface amines were modified with poly(ethylene glycol) dimethyl ester and then loaded the DOX onto the surface [121]. In 2017, Golshan et al. [122] functionalized fifth-generation PPI dendrimer with folic acid to target DOX delivery at different pH values. In other works, surfaces of gold nanoparticles and cellulose nanocrystals were modified with fifth-generation PPI dendrimer and release behavior of DOX was investigated at different pH values [123, 124]. In 2019, Najafi et al. synthesized gold/dendrimer hybrid nanoparticles using fifth-generation PPI dendrimer and investigated DOX release behavior and found that drug cumulative release was increased with increasing grafting density of dendrimer [23].
Brain tumor
Throughout the world, cancer has appeared as a basic cause of mortality in the world. Among the various types of cancer, brain tumor has the highest risk for life. Different types of dendrimers such as PAMAM [125, 126], PPI [127, 128] and PLL [129, 130] are used to treat and diagnose brain tumors and other cancers [131]. The greatest obstacle is often not drug potency but the physical barriers present at distinguished interfaces containing the blood vessels of the brain (blood–brain barrier, BBB), the choroid plexus (blood–cerebrospinal fluid barrier, BCSFB) and the arachnoid layer of the meninges (blood–arachnoid layer), interpreting the typical circulatory routes of delivery as ineffective [132, 133]. Many chemotherapy drugs do not reach the brain because they are substrates of the efflux transporters at the BBB. To resolve this matter, delivering chemotherapy by nanocarriers presents an attractive method [134]. Dendrimers can be easily delivering the drugs across the BBB due to their size, higher drug loading and controlled drug release [135]. Dendrimers with targeting abilities cargoe drugs to the tumor sites and penetrate to brain after systemic administration [136]. Mishra et al. [137] demonstrated that hydroxyl-terminated fourth-generation PAMAM dendrimers provided site-specific delivery of small molecular drugs across the BBB and blood–CSF barriers. Xie et al. [138] synthesized PAMAM dendrimer composites and investigated the physiochemical properties and biological effects to achieve nasal brain transport. Teow et al. [139] showed 12-fold increased permeability of PTX compared to free drug and evaluated cytotoxicity using third-generation PAMAM dendrimers loaded with PTX. Sk et al. [140] showed that PAMAM dendrimers as drug carries increased the bioavailability of natural podophyllotoxin and estramustine conjugated with PAMAM dendrimer and increased the inhibitory activity of antimitotic agents on tubulin polymerization of glioma cell survival. Patel et al. [141] synthesized PTX-conjugated PPI dendrimer for brain delivery and also showed that the prepared conjugate had a long-term efficacy and low cytotoxicity. Somani et al. [142] checked brain delivery of plasmid DNA as medicine using third-generation PPI dendrimer anchored with transferrin (Tf). Results showed increasing uptake of plasmid DNA via Tf-conjugated PPI dendrimers.
Photodynamic therapy
Peng et al. [143] improved the photodynamic efficacy of hydrophobic porphyrin using PAMAM dendrimer–porphyrin conjugates with minimized side effects. Kojima et al. [144] investigated interactions of photosensitizers between PEG-attached PPI and PAMAM dendrimers for photodynamic therapy. Taratula et al. [145] showed that phthalocyanine-dendritic complex modified with PEG and targeting LHRH moiety had significant potential for NIR fluorescence image-guided drug delivery and photodynamic therapy. Narsireddy et al. [146] conjugated fourth-generation PAMAM dendrimer with a peptide for targeted in vivo photodynamic therapy. Lee and Kim [147] reported a hydrophilic nanoconjugate to enhance PDT efficacy by improving water solubility and intracellular uptake of Ce6.
MRI
Dendrimers are a class of compounds with great potential for use as MRI diagnostic or theranostic agents [148]. In the early 1990s, the first in vivo diagnostic imaging applications using dendrimer-based MRI contrast agents were demonstrated by Lauterbur et al. [149]. Wiener et al. introduced the first new class of dendrimer-based metal chelating as MRI contrast agent [149]. In 2001, Konda et al. [150] used folic acid-conjugated fourth-generation dendrimers as MRI contrast agent. Results showed longitudinal relaxation rate at T1 by over 100% in cells expressing the folate receptor, compared to untreated cells. Wang et al. [151] showed that a second-generation PPI dendrimer had higher relaxivity than the corresponding ammonia core PAMAM agent. Haribabu et al. used multifunctional G3 PAMAM dendrimers as T1 and T2 contrast agents for MRI [152].
X-ray contrast agent
X-ray is a useful imaging device for organs and tissues that is used in many clinical trials [153]. Guo et al. [154] modified fifth-generation PAMAM dendrimer with gold nanoparticles in different concentrations, and results demonstrated that these nanoparticles were more effective than iodine-based contrast agents for X-rays imaging. Liu et al. [155] offered the synthesis of fifth-generation PAMAM-stabilized silver nanoparticles for X-ray computed tomography (CT) imaging applications. Kojima et al. developed AuNPs-loaded PEGylated-PAMAM dendrimers for CT imaging [156,157,158]. Zhu et al. [159] used multifunctional AuNPs-trapped PAMAM dendrimer as a template for efficient targeting of cancer cells and X-ray attenuation.
Dendrimer as molecular probe
In 2005, Cotle et al. reported the ensemble and single-molecule (SM) dynamics of Forster resonance energy transfer in a multichromophoric rigid polyphenylenic dendrimer [160]. Kim et al. [161] synthesized biocompatible fluorescent dendritic nanoprobes containing multiple covalently linked organic dyes for fluorescence imaging.
Gene therapy
Human diseases which are transmitted to specific cells by genetic material are diagnosed and treated by gene therapy [162]. Dendrimers are used in gene delivery because of monodispersity, functional groups and multivalence structures [163, 164]. Haensler et al. [165] used dendrimers for gene therapy. Li et al. [166] modified gold nanoparticles with fifth-generation PAMAM dendrimer for a safe delivery system as controlled gene delivery for breast cancer therapy. Wang et al. synthesized aptamer-conjugated PAMAM dendrimer nanoparticles for targeted gene delivery. Results showed that dendrimer improved cellular uptake in A549 cell line and enhanced gene transfection efficiency [167]. Luong et al. used PEGylated-PAMAM dendrimers for enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery [168]. Amreddy et al. [169] used folic acid-conjugated PAMAM dendrimer for targeted combined delivery of drug and gene to improve bioavailability and enhance therapeutic effects.
Waste water treatment
Different methods are used to treat wastewater which include dialysis [170], reverse osmosis [171], ion exchange [172], electrostatic interactions [173, 174], etc. Many adsorbents for wastewater treatment have limitations such as low absorption capacity, lack of economics of operation and fast adsorption rates [175,176,177]. Due to the large number of cavities between their branches, dendrimers have high absorbance properties for wastewater treatment [178], and also because of amine end groups, they are the best option to accumulate metal ions [177]. The first report for the removal of heavy metals from water and soil using dendrimers was presented by Diallo et al. [179]. Peng et al. [180] used amphoteric PAMAM dendrimers as a flocculent in treating wastewater. In 2013, Barakat et al. [181] synthesized PAMAM-modified TiO2 and examined the critical parameters which affect the ion removal including batch retention time, pH and metal ion concentration. Yuan et al. [182] evaluated the heavy-ion adsorption capacity of PAMAM-modified graphene oxide. Their results showed that dendrimers had a great ability to adsorb heavy ions, including Cu2+, Zn2+, Fe3+, Pb2+, Cr3+. In 2014, Hayati et al. [183] studied thermodynamic properties of dye removal from colored textile wastewater using PPI dendrimer. Results indicated that dendrimer was an environmentally friendly material and suitable for removing paint from colored textile sewage at various temperatures. In 2017, Peer et al. [184] examined the absorption of Cd(II), Pb(II) and Cu(II) from aqueous solution using PAMAM-modified graphene oxide. They also studied the effects of pH, the dose of adsorbent, the contact time, Cd(II), Pb(II) and Cu(II) ions concentration, temperature of aqueous solution and thermodynamic properties (enthalpy, entropy and Gibbs free energy).
Conclusions
Unlike linear polymers, dendrimers are nano-macromolecules which branch out of a core and all the branches eventually reach a central core. In synthesis of dendrimers, their molecular size and weight can be controlled. The presence of a large number of terminal branches increases the solubility and reactivity of the dendrimers. The solubility of dendrimers is strongly influenced by the nature of the surface groups. Initiators are not used to construct dendrimers, which causes their low toxicity. Dendrimers also have a high drug delivery capacity. The unique properties of dendrimers such as controlled size, monodispersity and reactive surface groups make these molecules ideal for medical applications including biomedical, drug delivery, catalysis, etc.
Abbreviations
- PPI:
-
Poly(propylene imine) dendrimer
- PAMAM:
-
Poly(amidoamine) dendrimer
- LC:
-
Liquid crystalline dendrimer
- Tecto:
-
Core–shell dendrimer
- MRI:
-
Magnetic resonance imaging
- EDA:
-
Ethylenediamine
- DAB:
-
1,4-Diaminobutane
- DOX:
-
Doxorubicin
- PLL:
-
Poly(l-lysine) dendrimers
- Tf:
-
Transferrin
- G:
-
Generation
- PEG:
-
Polyethylene glycol
- AuNPs:
-
Gold nanoparticles
- Cr:
-
Chromium
- DENs:
-
Dendrimer nanoparticles
- Pt:
-
Platinum
- Cu:
-
Copper
- Pd:
-
Palladium
- 3D:
-
3-Dimentional
- MCF-7:
-
Breast cancer
- A549:
-
Adenocarcinomic human alveolar basal epithelial cells
- BCSFB:
-
Blood–cerebrospinal fluid barrier
- BBB:
-
Blood–brain barrier
- Zn:
-
Zinc
- Fe:
-
Iron
- CT:
-
Computed tomography
- SM:
-
Single molecule
References
M. Banaei, M. Salami-Kalajahi, Synthesis of poly(2-hydroxyethyl methacrylate)-grafted poly(aminoamide) dendrimers as polymeric nanostructures. Colloid Polym. Sci. 293, 1553–1559 (2015). https://doi.org/10.1007/s00396-015-3559-y
Y. Cheng, L. Zhao, Y. Li, T. Xu, Design of biocompatible dendrimers for cancer diagnosis and therapy: current status and future perspectives. Chem. Soc. Rev. 40, 2673–2703 (2011). https://doi.org/10.1039/C0CS00097C
C. Dufès, I.F. Uchegbu, A.G. Schätzlein, Dendrimers in gene delivery. Adv. Drug Del. Rev. 57, 2177–2202 (2005). https://doi.org/10.1016/j.addr.2005.09.017
F. Samadaei, M. Salami-Kalajahi, H. Roghani-Mamaqani, M. Banaei, A structural study on ethylenediamine- and poly(amidoamine)-functionalized graphene oxide: simultaneous reduction, functionalization, and formation of 3D structure. RSC Adv. 5, 71835–71843 (2015). https://doi.org/10.1039/C5RA12086A
E. Buhleier, W. Wehner, F. Vögtle, “Cascade”- and “nonskid-chain-like” syntheses of molecular cavity topologies. Synthesis 78, 155–158 (1978). https://doi.org/10.1055/s-1978-24702
F. Najafi, M. Salami-Kalajahi, H. Roghani-Mamaqani, Synthesis of amphiphilic Janus dendrimer and its application in improvement of hydrophobic drugs solubility in aqueous media. Eur. Polym. J. 134, 109804 (2020). https://doi.org/10.1016/j.eurpolymj.2020.109804
D.A. Tomalia, H. Baker, J. Dewald, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder, P. Smith, A new class of polymers: starburst-dendritic macromolecules. Polym. J. 17, 117–132 (1985). https://doi.org/10.1295/polymj.17.117
C. Hawker, J.M.J. Fréchet, A new convergent approach to monodisperse dendritic macromolecules. J. Chem. Soc., Chem. Commun. (1990). https://doi.org/10.1039/C39900001010
D.A. Tomalia, J.M.J. Fréchet, Discovery of dendrimers and dendritic polymers: a brief historical perspective. J Polym. Sci. Polym. Chem. 40, 2719–2728 (2002). https://doi.org/10.1002/pola.10301
G.R. Newkome, Z. Yao, G.R. Baker, V.K. Gupta, Cascade molecules: a new approach to micelles. J. Org. Chem. 50, 2003–2004 (1985). https://doi.org/10.1021/jo00211a052
C.J. Hawker, J.M.J. Fréchet, Preparation of polymers with controlled molecular architecture: a new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 112, 7638–7647 (1990). https://doi.org/10.1021/ja00177a027
E. Abbasi, S.F. Aval, A. Akbarzadeh, M. Milani, H.T. Nasrabadi, S.W. Joo, Y. Hanifehpour, K. Nejati-Koshki, R. Pashaei-Asl, Dendrimers: synthesis, applications and properties. Nanoscale Res. Lett. 9, 247 (2014). https://doi.org/10.1186/1556-276X-9-247
D.A. Tomalia, The dendritic state. Mater. Today 8, 34–46 (2005). https://doi.org/10.1016/S1369-7021(05)00746-7
F. Samadaei, M. Salami-Kalajahi, H. Roghani-Mamaqani, Grafting of poly(acrylic acid) onto poly(amidoamine)-functionalized graphene oxide via surface-mediated reversible addition-fragmentation chain transfer polymerization. Int. J. Polym. Mater. Polym. Biomater. 65, 302–309 (2016). https://doi.org/10.1080/00914037.2015.1119686
R. Touzani, Dendrons, dendrimers new materials for environmental and science applications. J. Mater. Environ. Sci. 2, 201–214 (2011)
M. Golshan, E. Rostami-Tapeh-Esmail, M. Salami-Kalajahi, H. Roghani-Mamaqani, A review on synthesis, photophysical properties, and applications of dendrimers with perylene core. Eur. Polym. J. 137, 109933 (2020). https://doi.org/10.1016/j.eurpolymj.2020.109933
B. Razavi, R. Abbaszadeh, M. Salami-Kalajahi, H. Roghani-Mamaqani, Multi-responsive poly(amidoamine)-initiated dendritic-star supramolecular structures containing UV cross-linkable coumarin groups for smart drug delivery. J. Mol. Liq. (2020). https://doi.org/10.1016/j.molliq.2020.114138
S. Tripathy, L. Baro, M.K. Das, Dendrimer chemistry and host–guest interactions for drug targeting. Int. J. Pharm. Sci. Res. 5, 16–25 (2014). https://doi.org/10.13040/IJPSR.0975-8232.5(1).16-25
M. Sowinska, Z. Urbanczyk-Lipkowska, Advances in the chemistry of dendrimers. New J. Chem. 38, 2168–2203 (2014). https://doi.org/10.1039/C3NJ01239E
T.M. Miller, T.X. Neenan, Convergent synthesis of monodisperse dendrimers based upon 1,3,5-trisubstituted benzenes. Chem. Mater. 2, 346–349 (1990). https://doi.org/10.1021/cm00010a006
S. Svenson, D.A. Tomalia, Dendrimers in biomedical applications—reflections on the field. Adv. Drug Deliv. Rev. 64, 102–115 (2012). https://doi.org/10.1016/j.addr.2012.09.030
F. Najafi, M. Salami-Kalajahi, H. Roghani-Mamaqani, A. Kahaie-Khosrowshahi, A comparative study on solubility improvement of tetracycline and dexamethasone by poly (propylene imine) and polyamidoamine dendrimers: an insight into cytotoxicity and cell proliferation. J. Biomed. Mater. Res. A (2019). https://doi.org/10.1002/jbm.a.36830
F. Najafi, M. Salami-Kalajahi, H. Roghani-Mamaqani, A. Kahaie-Khosrowshahi, Effect of grafting ratio of poly(propylene imine) dendrimer onto gold nanoparticles on the properties of colloidal hybrids, their DOX loading and release behavior and cytotoxicity. Colloids Surf. B-Biointerfaces 178, 500–507 (2019). https://doi.org/10.1016/j.colsurfb.2019.03.050
J.S. Choi, D.K. Joo, C.H. Kim, K. Kim, J.S. Park, Synthesis of a barbell-like triblock copolymer, poly(l-lysine) dendrimer–block-poly(ethylene glycol)–block-poly(l-lysine) dendrimer, and its self-assembly with plasmid DNA. J. Am. Chem. Soc. 122, 474–480 (2000). https://doi.org/10.1021/ja9931473
Z. Weng, F. Zaera, Synthesis of chiral dendrimer-encapsulated nanoparticle (DEN) catalysts. Top. Catal. 61, 902–914 (2018). https://doi.org/10.1007/s11244-018-0955-9
Y. Cao, G.K. Nguyen, S. Chuah, J.P. Tam, C.F. Liu, Butelase-mediated ligation as an efficient bioconjugation method for the synthesis of peptide dendrimers. Bioconjugate Chem. 27, 2592–2596 (2016). https://doi.org/10.1021/acs.bioconjchem.6b00538
F. Chen, L. Kong, L. Wang, Y. Fan, M. Shen, X. Shi, Construction of core–shell tecto dendrimers based on supramolecular host–guest assembly for enhanced gene delivery. J. Mater. Chem. B 5, 8459–8466 (2017). https://doi.org/10.1039/C7TB02585H
S. Yang, H. Zhang, Q. Liu, S. Sun, P. Lei, Z. Zhao, Y. Wang, The synthesis and biological evaluation of chondroitin sulfate E glycodendrimers. Future Med. Chem. 11, 1403–1415 (2019). https://doi.org/10.4155/fmc-2019-0011
R. Esfand, D.A. Tomalia, Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov. Today 6, 427–436 (2001). https://doi.org/10.1016/S1359-6446(01)01757-3
C.C. Lee, J.A. MacKay, J.M. Fréchet, F.C. Szoka, Designing dendrimers for biological applications. Nat. Biotechnol. 23, 1517–1526 (2005). https://doi.org/10.1038/nbt1171
D.A. Tomalia, L.A. Reyna, S. Svenson, Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem. Soc. Trans. 35, 61–67 (2007). https://doi.org/10.1042/BST0350061
X. Liu, C. Liu, C.V. Catapano, L. Peng, J. Zhou, P. Rocchi, Structurally flexible triethanolamine-core poly(amidoamine) dendrimers as effective nanovectors to deliver RNAi-based therapeutics. Biotechnol. Adv. 32, 844–852 (2014). https://doi.org/10.1016/j.biotechadv.2013.08.001
D.A. Tomalia, H. Baker, J. Dewald, M. Hall, G. Kallos, S. Martin, P. Smith, Dendritic macromolecules: synthesis of starburst dendrimers. Macromolecules 19, 2466–2468 (1986). https://doi.org/10.1021/ma00163a029
J.W. Lee, J.H. Kim, B.K. Kim, J.H. Kim, W.S. Shin, S.H. Jin, Convergent synthesis of PAMAM dendrimers using click chemistry of azide-functionalized PAMAM dendrons. Tetrahedron 62, 9193–9200 (2006). https://doi.org/10.1016/j.tet.2006.07.030
E.N. Augustus, E.T. Allen, A. Nimibofa, W. Donbebe, A review of synthesis, characterization and applications of functionalized dendrimers. Am. J. Polym. Sci. 7, 8–14 (2017). https://doi.org/10.5923/j.ajps.20170701.02
X.C. Shen, J. Zhou, X. Liu, J. Wu, F. Qu, Z.L. Zhang, L. Peng, Importance of size-to-charge ratio in construction of stable and uniform nanoscale RNA/dendrimer complexes. Org. Biomol. Chem. 5, 3674–3681 (2007). https://doi.org/10.1039/B711242D
E. Buhleier, W. Wehner, F. Vögtle, “Cascade”- and “nonskid-chain-like” syntheses of molecular cavity topologies. Synthesis 9, 155–158 (1978). https://doi.org/10.1055/s-1978-24702
S. García-Gallego, L. Díaz, J.L. Jiménez, R. Gómez, F.J. de la Mata, M.A. Muñoz-Fernández, HIV-1 antiviral behavior of anionic PPI metallo-dendrimers with EDA core. Eur. J. Med. Chem. 98, 139–148 (2015). https://doi.org/10.1016/j.ejmech.2015.05.026
I.J. Majoros, C.R. Williams, D.A. Tomalia, J.R. Baker Jr., New dendrimers: synthesis and characterization of POPAM − PAMAM hybrid dendrimers. Macromolecules 41, 8372–8379 (2008). https://doi.org/10.1021/ma801843a
U. Gupta, S.K.D. Dwivedi, H.K. Bid, R. Konwar, N.K. Jain, Ligand anchored dendrimers based nanoconstructs for effective targeting to cancer cells. Int. J. Pharm. 393, 186–197 (2010). https://doi.org/10.1016/j.ijpharm.2010.04.002
S. Gowda, D.C. Gowda, Application of hydrazinium monoformate as new hydrogen donor with Raney nickel: a facile reduction of nitro and nitrile moieties. Tetrahedron 58, 2211–2213 (2002). https://doi.org/10.1016/S0040-4020(02)00093-5
L.H. Amundsen, L.S. Nelson, Reduction of nitriles to primary amines with lithium aluminum hydride1. J. Am. Chem. Soc. 73, 242–244 (1951). https://doi.org/10.1021/ja01145a082
S.J. Alavi, L. Gholami, S. Askarian, M. Darroudi, A. Massoudi, M. Rezaee, R.K. Oskuee, Hyperbranched–dendrimer architectural copolymer gene delivery using hyperbranched PEI conjugated to poly(propyleneimine) dendrimers: synthesis, characterization, and evaluation of transfection efficiency. J. Nanopart. Res. 19, 49 (2017). https://doi.org/10.1007/s11051-017-3739-4
A. Morikawa, M. Kakimoto, Y. Imai, Synthesis and characterization of new polysiloxane starburst polymers. Macromolecules 24, 3469–3474 (1991). https://doi.org/10.1021/ma00012a001
N. Launay, A.M. Caminade, R. Lahana, J.P. Majoral, A general synthetic strategy for neutral phosphorus-containing dendrimers. Angew. Chem. Int. Ed. 33, 1589–1592 (1994). https://doi.org/10.1002/anie.199415891
C. Galliot, C. Larre, A.M. Caminade, J.P. Majoral, Regioselective stepwise growth of dendrimer units in the internal voids of a main dendrimer. Science 277, 1981–1984 (1997). https://doi.org/10.1126/science.277.5334.1981
J.W. Knapen, A.W. van der Made, J.C. de Wilde, P.W. van Leeuwen, P. Wijkens, D.M. Grove, G. van Koten, Homogeneous catalysts based on silane dendrimers functionalized with arylnickel(II) complexes. Nature 372, 659–663 (1994). https://doi.org/10.1038/372659a0
L.L. Zhou, J. Roovers, Synthesis of novel carbosilane dendritic macromolecules. Macromolecules 26, 963–968 (1993). https://doi.org/10.1021/ma00057a013
J.P. Majoral, A.M. Caminade, What to do with Phosphorus in Dendrimer Chemistry, in New Aspects in Phosphorus Chemistry II, ed. by J.G. Verkade, J.P. Majoral (Springer, Berlin, 2003), pp. 111–159. https://doi.org/10.1007/3-540-46100-0_5
J.P. Majoral, A.M. Caminade, V. Maraval, The specific contribution of phosphorus in dendrimer chemistry. Chem. Commun. (2002). https://doi.org/10.1039/b207194k
D.C. Tully, J.M. Fréchet, Dendrimers at surfaces and interfaces: chemistry and applications. Chem. Commun. (2001). https://doi.org/10.1039/B104290B
S.M. Grayson, J.M. Frechet, Convergent dendrons and dendrimers: from synthesis to applications. Chem. Rev. 101, 3819–3868 (2001). https://doi.org/10.1021/cr990116h
K. Rengan, R. Engel, Phosphonium cascade molecules. J. Chem. Soc., Chem. Commun. (1990). https://doi.org/10.1039/C39900001084
A. Miedaner, C.J. Curtis, R.M. Barkley, D.L. DuBois, Electrochemical reduction of CO2 catalyzed by small organophosphine dendrimers containing palladium. Inorg. Chem. 33, 5482–5490 (1994). https://doi.org/10.1021/ic00102a022
M. Petrucci-Samija, V. Guillemette, M. Dasgupta, A.K. Kakkar, A new divergent route to the synthesis of organophosphine and metallodendrimers via simple acid–base hydrolytic chemistry. J. Am. Chem. Soc. 121, 1968–1969 (1999). https://doi.org/10.1021/ja983132p
N. Launay, A.M. Caminade, J.P. Majoral, Synthesis and reactivity of unusual phosphorus dendrimers. A useful divergent growth approach up to the seventh generation. J. Am. Chem. Soc. 117, 3282–3283 (1995). https://doi.org/10.1021/ja00116a037
M. Slany, M. Bardaji, M.J. Casanove, A.M. Caminade, J.P. Majoral, B. Chaudret, Dendrimer surface chemistry. Facile route to polyphosphines and their gold complexes. J. Am. Chem. Soc. 117, 9764–9765 (1995). https://doi.org/10.1021/ja00143a023
M.L. Lartigue, M. Slany, A.M. Caminade, J.P. Majoral, Phosphorus-containing dendrimers: synthesis of macromolecules with multiple tri-and tetrafunctionalization. Chem. Eur. J. 2, 1417–1426 (1996). https://doi.org/10.1002/chem.19960021114
N. Launay, M. Slany, A.M. Caminade, J.P. Majoral, Phosphorus-containing dendrimers Easy access to new multi-difunctionalized macromolecules. J. Org. Chem. 61, 3799–3805 (1996). https://doi.org/10.1021/jo960045b
M. Bardají, M. Kustos, A.M. Caminade, J.P. Majoral, B. Chaudret, Phosphorus-containing dendrimers as multidentate ligands: palladium, platinum, and rhodium complexes. Organometallics 16, 403–410 (1997). https://doi.org/10.1021/om9606101
M. Bardaji, A.M. Caminade, J.P. Majoral, B. Chaudret, Ruthenium hydride and dihydrogen complexes with dendrimeric multidentate ligands. Organometallics 16, 3489–3497 (1997). https://doi.org/10.1021/om970092
D. Prévôté, A.M. Caminade, J.P. Majoral, Phosphate-, phosphite-, ylide-, and phosphonate-terminated dendrimers. J. Organ. Chem. 62, 4834–4841 (1997). https://doi.org/10.1021/jo9701750
J.P. Majoral, A.M. Caminade, Divergent approaches to phosphorus-containing dendrimers and their functionalization, in Dendrimers (Springer, Berlin, 1998), pp. 79–124. https://doi.org/10.1007/3-540-69779-9_3
A.M. Caminade, R. Laurent, B. Chaudret, J.P. Majoral, Phosphine-terminated dendrimers: synthesis and complexation properties. Coordin. Chem. Rev. 178, 793–821 (1998). https://doi.org/10.1016/S0010-8545(98)00057-5
L. Brauge, G. Magro, A.M. Caminade, J.P. Majoral, First divergent strategy using two AB2 unprotected monomers for the rapid synthesis of dendrimers. J. Am. Chem. Soc. 123, 6698–6699 (2001). https://doi.org/10.1021/ja0029228
S. Fruchon, M. Poupot, L. Martinet, C.O. Turrin, J.P. Majoral, J.J. Fournié, R. Poupot, Anti-inflammatory and immunosuppressive activation of human monocytes by a bioactive dendrimer. J. Leukoc. Biol. 85, 553–562 (2009). https://doi.org/10.1189/jlb.0608371
S. Fruchon, R. Poupot, The ABP dendrimer, a drug-candidate against inflammatory diseases that triggers the activation of interleukin-10 producing immune cells. Molecules 23, 1272 (2018). https://doi.org/10.3390/molecules23061272
E. Abdollahi, A. Khalafi-Nezhad, A. Mohammadi, M. Abdouss, M. Salami-Kalajahi, Synthesis of new molecularly imprinted polymer via reversible addition fragmentation transfer polymerization as a drug delivery system. Polymer 143, 245–257 (2018). https://doi.org/10.1016/j.polymer.2018.03.058
S.M. Modarresi-Saryazdi, V. Haddadi-Asl, M. Salami-Kalajahi, N,N′-methylenebis(acrylamide)-crosslinked poly(acrylic acid) particles as doxorubicin carriers: a comparison between release behavior of physically loaded drug and conjugated drug via acid-labile hydrazone linkage. J. Biomed. Mater. Res. A 106, 342–348 (2018). https://doi.org/10.1002/jbm.a.36240
A.M. Caminade, P. Servin, R. Laurent, J.P. Majoral, Dendrimeric phosphines in asymmetric catalysis. Chem. Soc. Rev. 37, 56–67 (2008). https://doi.org/10.1039/B606569B
J.B. Wolinsky, M.W. Grinstaff, Therapeutic and diagnostic applications of dendrimers for cancer treatment. Adv. Drug Del. Rev. 60, 1037–1055 (2008). https://doi.org/10.1016/j.addr.2008.02.012
S. Mignani, J. Rodrigues, H. Tomas, M. Zablocka, X. Shi, A.M. Caminade, J.P. Majoral, Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem. Soc. Rev. 47, 514–532 (2018). https://doi.org/10.1039/C7CS00550D
F. Najafi, N. Ghasemian, M. Safari, M. Salami-Kalajahi, Poly(propylene imine) dendrimer as reducing agent for chloroauric acid to fabricate and stabilize gold nanoparticles. J. Phys. Chem. Solids 148, 109682 (2021). https://doi.org/10.1016/j.jpcs.2020.109682
A. Ghorbani-Choghamarani, Z. Taherinia, Synthesis of peptide nanofibers decorated with palladium nanoparticles and its application as an efficient catalyst for the synthesis of sulfides via reaction of aryl halides with thiourea or 2-mercaptobenzothiazole. RSC Adv. 6, 59410–59421 (2016). https://doi.org/10.1039/C6RA02264B
A. Ghorbani-Choghamarani, Z. Taherinia, The first report on the preparation of peptide nanofibers decorated with zirconium oxide nanoparticles applied as versatile catalyst for the amination of aryl halides and synthesis of biaryl and symmetrical sulfides. New J. Chem. 41, 9414–9423 (2017). https://doi.org/10.1039/C7NJ00628D
A. Ghorbani-Choghamarani, Z. Taherinia, Synthesis of biaryls using palladium nanoparticles immobilized on peptide nanofibers as catalyst and hydroxybenzotriazole as novel phenylating reagent. Chin. J. Catal. 38, 469–474 (2017). https://doi.org/10.1016/S1872-2067(17)62586-X
Z. Taherinia, A. Ghorbani-Choghamarani, Cu(I)–PNF, an organic-based nanocatalyst, catalyzed C–O and C–S cross-coupling reactions. Can. J. Chem. 97, 46–52 (2019). https://doi.org/10.1139/cjc-2017-0733
Z. Taherinia, A. Ghorbani-Choghamarani, M. Hajjami, Decorated peptide nanofibers with Cu nanoparticles: an efficient catalyst for the multicomponent synthesis of chromeno [2, 3-d] pyrimidin-8-amines, quinazolines and 2H-indazoles. ChemistrySelect 4, 2753–2760 (2019). https://doi.org/10.1002/slct.201803412
Z. Taherinia, A. Ghorbani-Choghamarani, M. Hajjami, Peptide nanofiber templated zinc oxide nanostructures as non-precious metal catalyzed N-arylation of amines, one-pot synthesis of imidazoheterocycles and fused quinazolines. Catal. Lett. 149, 151–168 (2019). https://doi.org/10.1007/s10562-018-2580-4
A. Ghorbani-Choghamarani, Z. Taherinia, Eco-friendly synthesis of 3-aminoimidazo [1, 2-a] pyridines via a one-pot three-component reaction in PEG catalyzed by peptide nanofibers: as hydrogen-bonding organocatalyst. J. Iran. Chem. Soc. 17, 59–65 (2020). https://doi.org/10.1007/s13738-019-01744-w
A. Ghorbani-Choghamarani, Z. Taherinia, Chiral cobalt-peptide metal-organic framework (Co-P-MOF): as an efficient and reusable heterogeneous catalyst for the asymmetric sulfoxidative cross-coupling reaction using polysulfinylpiperazine. Synth. Met. 263, 116362 (2020). https://doi.org/10.1016/j.synthmet.2020.116362
R.H. Hudson, A. Ghorbani-Choghamarani, Oligodeoxynucleotides incorporating structurally simple 5-alkynyl-2′-deoxyuridines fluorometrically respond to hybridization. Org. Biomol. Chem. 5, 1845–1848 (2007). https://doi.org/10.1039/B705805E
N.J. Wells, A. Basso, M. Bradley, Solid-phase dendrimer synthesis. Pept. Sci. 47, 381–396 (1998). https://doi.org/10.1002/(SICI)1097-0282(1998)47:5%3c381:AID-BIP5%3e3.0.CO;2-F
A.M. Caminade, R. Laurent, Homogeneous catalysis with phosphorus dendrimer complexes. Coordin. Chem. Rev. 389, 59–72 (2019). https://doi.org/10.1016/j.ccr.2019.03.007
A. Esposito, E. Delort, D. Lagnoux, F. Djojo, J.L. Reymond, Catalytic peptide dendrimers. Angew. Chem. 115, 1419–1421 (2003). https://doi.org/10.1002/ange.200390326
D. Astruc, F. Chardac, Dendritic catalysts and dendrimers in catalysis. Chem. Rev. 101, 2991–3024 (2001). https://doi.org/10.1021/cr010323t
F. Diederich, B. Felber, Supramolecular chemistry of dendrimers with functional cores. Proc. Natl. Acad. Sci. 99, 4778–4781 (2002). https://doi.org/10.1073/pnas.052568099
A.M. Muhanna, E. Ortiz-Salmerón, L. Garcı́a-Fuentes, J.J. Giménez-Martı́nez, A. Vargas-Berenguel, Synthesis of peptide dendrimers based on a β-cyclodextrin core with guest binding ability. Tetrahedron Lett. 44, 6125–6128 (2003). https://doi.org/10.1016/s0040-4039(03)01432-1
C.H. Tung, S. Mueller, R. Weissleder, Novel branching membrane translocational peptide as gene delivery vector. Bioorg. Med. Chem. 10, 3609–3614 (2002). https://doi.org/10.1016/S0968-0896(02)00248-1
E. Delort, T. Darbre, J.L. Reymond, A strong positive dendritic effect in a peptide dendrimer-catalyzed ester hydrolysis reaction. J. Am. Chem. Soc. 126, 15642–15643 (2004). https://doi.org/10.1021/ja044652p
U. Boas, J.B. Christensen, P.M. Heegaard, Dendrimers in medicine and biotechnology: new molecular tools. R. Soc. Chem. (2006). https://doi.org/10.1039/9781847552679
Y. Niu, R.M. Crooks, Dendrimer-encapsulated metal nanoparticles and their applications to catalysis. Comptes Rendus Chim. 6, 1049–1059 (2003). https://doi.org/10.1016/j.crci.2003.08.001
J.N. Reek, S. Arevalo, R. van Heerbeek, P.C. Kamer, P.W. Van Leeuwen, Dendrimers in catalysis. Adv. Catal. 49, 71–151 (2006). https://doi.org/10.1016/S0360-0564(05)49002-1
V. Chechik, R.M. Crooks, Dendrimer-encapsulated Pd nanoparticles as fluorous phase-soluble catalysts. J. Am. Chem. Soc. 122, 1243–1244 (2000). https://doi.org/10.1021/ja9936870
R.M. Crooks, M. Zhao, L. Sun, V. Chechik, L.K. Yeung, Dendrimer-encapsulated metal nanoparticles: synthesis, characterization, and applications to catalysis. Acc. Chem. Res. 34, 181–190 (2001). https://doi.org/10.1021/ar000110a
N.N. Hoover, B.J. Auten, B.D. Chandler, Tuning supported catalyst reactivity with dendrimer-templated Pt − Cu nanoparticles. J. Phys. Chem. B 110, 8606–8612 (2006). https://doi.org/10.1021/jp060833x
E. Karakhanov, A. Maximov, A. Zolotukhina, Y. Kardasheva, M. Talanova, Thermo-responsive ruthenium dendrimer-based catalysts for hydrogenation of the aromatic compounds and phenols. J. Inorg. Organometal. Polym. Mater. 26, 1264–1279 (2016). https://doi.org/10.1007/s10904-016-0399-2
N. Brinkmann, D. Giebel, G. Lohmer, M.T. Reetz, U. Kragl, Allylic substitution with dendritic palladium catalysts in a continuously operating membrane reactor. J. Catal. 183, 163–168 (1999). https://doi.org/10.1006/jcat.1999.2462
N.J. Hovestad, E.B. Eggeling, H.J. Heidbüchel, J.T. Jastrzebski, U. Kragl, W. Keim, G. van Koten, Selective hydrovinylation of styrene in a membrane reactor: use of carbosilane dendrimers with hemilabile P, O ligands. Angew. Chem. Int. Ed. 38, 1655–1658 (1999). https://doi.org/10.1002/(SICI)1521-3773(19990601)38:11%3c1655:AID-ANIE1655%3e3.0.CO;2-2
D. De Groot, B.F. de Waal, J.N. Reek, A.P. Schenning, P.C. Kamer, E.W. Meijer, P.W. van Leeuwen, Noncovalently functionalized dendrimers as recyclable catalysts. J. Am. Chem. Soc. 123, 8453–8458 (2001). https://doi.org/10.1021/ja005774u
N.J. Pijnenburg, H.P. Dijkstra, G. van Koten, R.J.K. Gebbink, SCS-pincer palladium-catalyzed auto-tandem catalysis using dendritic catalysts in semi-permeable compartments. Dalton Trans. 40, 8896–8905 (2011). https://doi.org/10.1039/C1DT10502G
J.K. Kassube, L.H. Gade, Immobilisation of the pyrphos ligand on soluble hyperbranched supports and use in rhodium-catalysed hydrogenation in ionic liquids. Adv. Synth. Catal. 351, 739–749 (2009). https://doi.org/10.1002/adsc.200900016
M. Gaab, S. Bellemin-Laponnaz, L.H. Gade, “Catalysis in a tea bag”: synthesis, catalytic performance and recycling of dendrimer-immobilised bis-and trisoxazoline copper catalysts. Chem. Eur. J. 15, 5450–5462 (2009). https://doi.org/10.1002/chem.200900504
G. Ou, L. Xu, B. He, Y. Yuan, Enhanced stability of charged dendrimer-encapsulated Pd nanoparticles in ionic liquids. Chem. Commun. (2008). https://doi.org/10.1039/B806163G
A. Arunchander, S.G. Peera, V. Parthiban, S. Akula, T. Kottakkat, S.D. Bhat, A.K. Sahu, Dendrimer confined Pt nanoparticles: electro-catalytic activity towards the oxygen reduction reaction and its application in polymer electrolyte membrane fuel cells. RSC Adv. 5, 75218–75228 (2015). https://doi.org/10.1039/c5ra15233j
M. Banaei, M. Salami-Kalajahi, A “grafting to” approach to synthesize low cytotoxic poly(aminoamide)-dendrimer-grafted Fe3O4 magnetic nanoparticles. Adv. Polym. Technol. 37, 943–948 (2018). https://doi.org/10.1002/adv.21741
S. Tripathy, M.K. Das, Dendrimers and their applications as novel drug delivery carriers. J. Pharm. Sci. 3, 142–149 (2013). https://doi.org/10.7324/JAPS.2013.3924
M. Najlah, S. Freeman, M. Khoder, D. Attwood, A. D’Emanuele, In vitro evaluation of third generation PAMAM dendrimer conjugates. Molecules 22, 1661 (2017). https://doi.org/10.3390/molecules22101661
F. Soltani, M. Ramezani, S. Amel Farzad, A. Mokhtarzadeh, M. Hashemi, Comparison study of the effect of alkyl-modified and unmodified PAMAM and PPI dendrimers on solubility and antitumor activity of crocetin. Artif. Cells Nanomed. Biotechnol. 45, 1356–1362 (2017). https://doi.org/10.1080/21691401.2016.1236805
M. Soler, P. Mesa-Antunez, M.C. Estevez, A.J. Ruiz-Sanchez, M.A. Otte, B. Sepulveda, L.M. Lechuga, Highly sensitive dendrimer-based nanoplasmonic biosensor for drug allergy diagnosis. Biosens. Bioelectron. 66, 115–123 (2015). https://doi.org/10.1016/j.bios.2014.10.081
D.H. Nguyen, L.G. Bach, D.H. Nguyen Tran, V.D. Cao, T.N.Q. Nguyen, T.T.H. Le, T.T.H. Thi, Partial surface modification of low generation polyamidoamine dendrimers: gaining insight into their potential for improved carboplatin delivery. Biomolecules 9, 214 (2019). https://doi.org/10.3390/biom9060214
M. Fallahi-Sambaran, M. Salami-Kalajahi, E. Dehghani, F. Abbasi, Investigation of different core–shell toward Janus morphologies by variation of surfactant and feeding composition: a study on the kinetics of DOX release. Colloids Surf. B-Biointerfaces 170, 578–587 (2018). https://doi.org/10.1016/j.colsurfb.2018.06.064
N.K. Jain, U. Gupta, Application of dendrimer–drug complexation in the enhancement of drug solubility and bioavailability. Expert Opin. Drug Metab. Toxicol. 4, 1035–1052 (2008). https://doi.org/10.1517/17425255.4.8.1035
G. Nikravan, V. Haddadi-Asl, M. Salami-Kalajahi, Synthesis of dual temperature- and pH-responsive yolk–shell nanoparticles by conventional etching and new deswelling approaches: DOX release behavior. Colloids Surf. B-Biointerfaces 165, 1–8 (2018). https://doi.org/10.1016/j.colsurfb.2018.02.010
A. Brownlie, I.F. Uchegbu, A.G. Schätzlein, PEI-based vesicle-polymer hybrid gene delivery system with improved biocompatibility. Int. J. Pharm. 274, 41–52 (2004). https://doi.org/10.1016/j.ijpharm.2003.12.029
M. Fallahi-Samberan, M. Salami-Kalajahi, E. Dehghani, F. Abbasi, Investigating Janus morphology development of poly(acrylic acid)/poly(2-(dimethylamino)ethyl methacrylate) composite particles: an experimental study and mathematical modeling of DOX release. Microchem. J. 145, 492–500 (2019). https://doi.org/10.1016/j.microc.2018.11.017
M. Maciejewski, Concepts of trapping topologically by shell molecules. J. Macromol. Sci. A 17, 689–703 (1982). https://doi.org/10.1080/00222338208062416
A.K. Patri, J.F. Kukowska-Latallo, J.R. Baker Jr., Targeted drug delivery with dendrimers: comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv. Drug Deliv. Rev. 57, 2203–2214 (2005). https://doi.org/10.1016/j.addr.2005.09.014
F. Wang, X. Cai, Y. Su, J. Hu, Q. Wu, H. Zhang, Y. Cheng, Reducing cytotoxicity while improving anti-cancer drug loading capacity of polypropylenimine dendrimers by surface acetylation. Acta Biomater. 8, 4304–4313 (2012). https://doi.org/10.1016/j.actbio.2012.07.031
P. Kesharwani, R.K. Tekade, N.K. Jain, Generation dependent cancer targeting potential of poly(propyleneimine) dendrimer. Biomaterials 35, 5539–5548 (2014). https://doi.org/10.1016/j.biomaterials.2014.03.064
A. Pourjavadi, S.H. Hosseini, M. Alizadeh, C. Bennett, Magnetic pH-responsive nanocarrier with long spacer length and high colloidal stability for controlled delivery of doxorubicin. Colloids Surf. B-Biointerfaces 116, 49–54 (2014). https://doi.org/10.1016/j.colsurfb.2013.12.048
M. Golshan, M. Salami-Kalajahi, H. Roghani-Mamaqani, M. Mohammadi, Synthesis of poly(propylene imine) dendrimers via homogeneous reduction process using lithium aluminium hydride: bioconjugation with folic acid and doxorubicin release kinetics. Appl. Organomet. Chem. 31, e3789 (2017). https://doi.org/10.1002/aoc.3789
M. Golshan, M. Salami-Kalajahi, M. Mirshekarpour, H. Roghani-Mamaqani, M. Mohammadi, Synthesis and characterization of poly (propylene imine)-dendrimer-grafted gold nanoparticles as nanocarriers of doxorubicin. Colloids Surf. B-Biointerfaces 155, 257–265 (2017). https://doi.org/10.1016/j.colsurfb.2017.04.029
M. Golshan, M. Salami-Kalajahi, H. Roghani-Mamaqani, M. Mohammadi, Poly(propylene imine) dendrimer-grafted nanocrystalline cellulose: doxorubicin loading and release behavior. Polymer 117, 287–294 (2017). https://doi.org/10.1016/j.polymer.2017.04.047
Y. Bae, E.S. Green, G.Y. Kim, S.J. Song, J.Y. Mun, S. Lee, J.S. Choi, Dipeptide-functionalized polyamidoamine dendrimer-mediated apoptin gene delivery facilitates apoptosis of human primary glioma cells. Int. J. Pharm. 515, 186–200 (2016). https://doi.org/10.1016/j.ijpharm.2016.09.083
Y.K. Katare, R.P. Daya, C. SookramGray, R.E. Luckham, J. Bhandari, A.S. Chauhan, R.K. Mishra, Brain targeting of a water insoluble antipsychotic drug haloperidol via the intranasal route using PAMAM dendrimer. Mol. Pharm. 12, 3380–3388 (2015). https://doi.org/10.1021/acs.molpharmaceut.5b00402
V. Gajbhiye, N.K. Jain, The treatment of glioblastoma xenografts by surfactant conjugated dendritic nanoconjugates. Biomaterials 32, 6213–6225 (2011). https://doi.org/10.1016/j.biomaterials.2011.04.057
H.K. Patel, V. Gajbhiye, P. Kesharwani, N.K. Jain, Ligand anchored poly(propyleneimine) dendrimers for brain targeting: comparative in vitro and in vivo assessment. J. Colloid Interface Sci. 482, 142–150 (2016). https://doi.org/10.1016/j.jcis.2016.07.047
L.M. Kaminskas, B.D. Kelly, V.M. McLeod, G. Sberna, D.J. Owen, B.J. Boyd, C.J.H. Porter, Characterisation and tumour targeting of PEGylated polylysine dendrimers bearing doxorubicin via a pH labile linker. J. Control. Release 152, 241–248 (2011). https://doi.org/10.1016/j.jconrel.2011.02.005
K.T. Al-Jamal, W.T. Al-Jamal, S. Akerman, J.E. Podesta, A. Yilmazer, J.A. Turton, K. Kostarelos, Systemic antiangiogenic activity of cationic poly-l-lysine dendrimer delays tumor growth. Proc. Natl. Acad. Sci. U.S.A. 107, 3966–3971 (2010). https://doi.org/10.1073/pnas.0908401107
M. Bredel, J. Zentner, Brain-tumour drug resistance: the bare essentials. Lancet Oncol. 3, 397–406 (2002). https://doi.org/10.1016/S1470-2045(02)00786-6
W.M. Pardridge, The blood–brain barrier: bottleneck in brain drug development. NeuroRx 2, 3–14 (2005). https://doi.org/10.1602/neurorx.2.1.3
W.M. Pardridge, Drug transport across the blood–brain barrier. J. Cereb. Blood Flow Metab. 32, 1959–1972 (2012). https://doi.org/10.1038/jcbfm.2012.126
V. Mishra, P. Kesharwani, Dendrimer technologies for brain tumor. Drug Discov. Today 21, 766–778 (2016). https://doi.org/10.1016/j.drudis.2016.02.006
R.K. Jain, T. Stylianopoulos, Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 7, 653–664 (2010). https://doi.org/10.1038/nrclinonc.2010.139
N. Dwivedi, J. Shah, V. Mishra, M.C.I. Mohd Amin, A.K. Iyer, R.K. Tekade, P. Kesharwani, Dendrimer-mediated approaches for the treatment of brain tumor. J. Biomater. Sci. Polym. Ed. 27, 557–580 (2016). https://doi.org/10.1080/09205063.2015.1133155
M.K. Mishra, C.A. Beaty, W.G. Lesniak, S.P. Kambhampati, F. Zhang, M.A. Wilson, W.A. Baumgartner, Dendrimer brain uptake and targeted therapy for brain injury in a large animal model of hypothermic circulatory arrest. ACS Nano 8, 2134–2147 (2014). https://doi.org/10.1021/nn404872e
H. Xie, L. Li, Y. Sun, Y. Wang, S. Gao, Y. Tian, L. Zhang, An available strategy for nasal brain transport of nanocomposite based on PAMAM dendrimers via in situ gel. Nanomaterials 9, 147 (2019). https://doi.org/10.3390/nano9020147
H.M. Teow, Z. Zhou, M. Najlah, S.R. Yusof, N.J. Abbott, A. D’emanuele, Delivery of paclitaxel across cellular barriers using a dendrimer-based nanocarrier. Int. J. Pharm. 441, 701–711 (2013). https://doi.org/10.1016/j.ijpharm.2012.10.024
U.H. Sk, D. Dixit, E. Sen, Comparative study of microtubule inhibitors–estramustine and natural podophyllotoxin conjugated PAMAM dendrimer on glioma cell proliferation. Eur. J. Med. Chem. 68, 47–57 (2013). https://doi.org/10.1016/j.ejmech.2013.07.007
S.K. Patel, V. Gajbhiye, N.K. Jain, Synthesis, characterization and brain targeting potential of paclitaxel loaded thiamine-PPI nanoconjugates. J. Drug Target. 20, 841–849 (2012). https://doi.org/10.3109/1061186X.2012.719231
S. Somani, D.R. Blatchford, O. Millington, M.L. Stevenson, C. Dufès, Transferrin-bearing polypropylenimine dendrimer for targeted gene delivery to the brain. J. Control. Rel. 188, 78–86 (2014). https://doi.org/10.1016/j.jconrel.2014.06.006
C.L. Peng, P.S. Lai, C.H. Chiu, C.C. Chang, T.Y. Lai, M.J. Shieh, Synthesis and photodynamic efficacy of PAMAM-photosensitiser conjugates in vitro. NSTI-Nanotech. 2, 384–387 (2006)
C. Kojima, Y. Toi, A. Harada, K. Kono, Preparation of poly(ethylene glycol)-attached dendrimers encapsulating photosensitizers for application to photodynamic therapy. Bioconjugate Chem. 18, 663–670 (2007). https://doi.org/10.1021/bc060244u
O. Taratula, C. Schumann, M.A. Naleway, A.J. Pang, K.J. Chon, O. Taratula, A multifunctional theranostic platform based on phthalocyanine-loaded dendrimer for image-guided drug delivery and photodynamic therapy. Mol. Pharm. 10, 3946–3958 (2013). https://doi.org/10.1021/mp400397t
A. Narsireddy, K. Vijayashree, M.G. Adimoolam, S.V. Manorama, N.M. Rao, Photosensitizer and peptide-conjugated PAMAM dendrimer for targeted in vivo photodynamic therapy. Int. J. Nanomed. 10, 6865–6878 (2015). https://doi.org/10.2147/IJN.S89474
S.R. Lee, Y.J. Kim, Hydrophilic chlorin e6-poly(amidoamine) dendrimer nanoconjugates for enhanced photodynamic therapy. Nanomaterials 8, 445 (2018). https://doi.org/10.3390/nano8060445
M.T. McMahon, J.W. Bulte, Two decades of dendrimers as versatile MRI agents: a tale with and without metals. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 10, e1496 (2018). https://doi.org/10.1002/wnan.1496
E. Wiener, M.W. Brechbiel, H. Brothers, R.L. Magin, O.A. Gansow, D.A. Tomalia, P.C. Lauterbur, Dendrimer-based metal chelates: a new class of magnetic resonance imaging contrast agents. Magn. Reson. Med. 31, 1–8 (1994). https://doi.org/10.1002/mrm.1910310102
S.D. Konda, M. Aref, S. Wang, M. Brechbiel, E.C. Wiener, Specific targeting of folate–dendrimer MRI contrast agents to the high affinity folate receptor expressed in ovarian tumor xenografts. Magn. Reson. Mater. Phys., Biol. Med. 12, 104–113 (2001). https://doi.org/10.1007/BF02668091
S.J. Wang, M. Brechbiel, E.C. Wiener, Characteristics of a new MRI contrast agent prepared from polypropyleneimine dendrimers, generation 2. Investig. Radiol. 38, 662–668 (2003). https://doi.org/10.1097/01.rli.0000084887.47427.75
V. Haribabu, A.S. Farook, N. Goswami, R. Murugesan, A. Girigoswami, Optimized Mn-doped iron oxide nanoparticles entrapped in dendrimer for dual contrasting role in MRI. J. Biomed. Mater. Res. B 104, 817–824 (2016). https://doi.org/10.1002/jbm.b.33550
W.A. Kalender, X-ray computed tomography. Phys. Med. Biol. 51, R29 (2006). https://doi.org/10.1088/0031-9155/51/13/R03
C. Peng, H. Wang, R. Guo, M. Shen, X. Cao, M. Zhu, X. Shi, Acetylation of dendrimer-entrapped gold nanoparticles: synthesis, stability, and X-ray attenuation properties. J. Appl. Polym. Sci. 119, 1673–1682 (2011). https://doi.org/10.1002/app.32845
H. Liu, H. Wang, R. Guo, X. Cao, J. Zhao, Y. Luo, X. Shi, Size-controlled synthesis of dendrimer-stabilized silver nanoparticles for X-ray computed tomography imaging applications. Polym. Chem. 1, 1677–1683 (2010). https://doi.org/10.1039/C0PY00218F
Y. Umeda, C. Kojima, A. Harada, H. Horinaka, K. Kono, PEG-attached PAMAM dendrimers encapsulating gold nanoparticles: growing gold nanoparticles in the dendrimers for improvement of their photothermal properties. Bioconjugate Chem. 21, 1559–1564 (2010). https://doi.org/10.1021/bc1001399
C. Kojima, Y. Umeda, M. Ogawa, A. Harada, Y. Magata, K. Kono, X-ray computed tomography contrast agents prepared by seeded growth of gold nanoparticles in PEGylated dendrimer. Nanotechnology 21, 245104 (2010). https://doi.org/10.1088/0957-4484/21/24/245104
C. Kojima, S.H. Cho, E. Higuchi, Gold nanoparticle-loaded PEGylated dendrimers for theragnosis. Res. Chem. Intermed. 38, 1279–1289 (2012). https://doi.org/10.1007/s11164-011-0466-9
J. Zhu, L. Zheng, S. Wen, Y. Tang, M. Shen, G. Zhang, X. Shi, Targeted cancer theranostics using alpha-tocopheryl succinate-conjugated multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials 35, 7635–7646 (2014). https://doi.org/10.1016/j.biomaterials.2014.05.046
M. Cotlet, T. Vosch, S. Habuchi, T. Weil, K. Müllen, J. Hofkens, F. De Schryver, Probing Intramolecular Förster resonance energy transfer in a naphthaleneimide − peryleneimide − terrylenediimide-based dendrimer by ensemble and single-molecule fluorescence spectroscopy. J. Am. Chem. Soc. 127, 9760–9768 (2005). https://doi.org/10.1021/ja042656o
Y. Kim, S.H. Kim, M. Tanyeri, J.A. Katzenellenbogen, C.M. Schroeder, Dendrimer probes for enhanced photostability and localization in fluorescence imaging. Biophys. J. 104, 1566–1575 (2013). https://doi.org/10.1016/j.bpj.2013.01.052
R.C. Mulligan, The basic science of gene therapy. Science 260, 926–932 (1993). https://doi.org/10.1126/science.8493530
D.A. Tomalia, Birth of a new macromolecular architecture: dendrimers as quantized building blocks for nanoscale synthetic organic chemistry. Aldrichim. Acta 37, 39–57 (2004). https://doi.org/10.1016/j.progpolymsci.2005.01.007
H.S. Parekh, The advance of dendrimers—a versatile targeting platform for gene/drug delivery. Curr. Pharm. Des. 13, 2837–2850 (2007). https://doi.org/10.2174/138161207781757024
J. Haensler, F.C. Szoka Jr., Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjugate Chem. 4, 372–379 (1993). https://doi.org/10.1021/bc00023a012
C. Li, F. Xia, K. Wang, C. Wang, P. Xu, H. Zhang, D. Cui, Dendrimer-modified gold nanorods as high efficient controlled gene delivery release system under near-infrared light irradiation. Nano Biomed. Eng. 9, 82–95 (2017). https://doi.org/10.5101/nbe.v9i1.p82-95
H. Wang, X. Zhao, C. Guo, D. Ren, Y. Zhao, W. Xiao, W. Jiao, Aptamer-dendrimer bioconjugates for targeted delivery of miR-34a expressing plasmid and antitumor effects in non-small cell lung cancer cells. PLoS ONE 10, e0139136 (2015). https://doi.org/10.1371/journal.pone.0139136
D. Luong, P. Kesharwani, R. Deshmukh, M.C.I.M. Amin, U. Gupta, K. Greish, A.K. Iyer, PEGylated PAMAM dendrimers: enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 43, 14–29 (2016). https://doi.org/10.1016/j.actbio.2016.07.015
N. Amreddy, A. Babu, J. Panneerselvam, A. Srivastava, R. Muralidharan, A. Chen, R. Ramesh, Chemo-biologic combinatorial drug delivery using folate receptor-targeted dendrimer nanoparticles for lung cancer treatment. Nanomed. Nanotechnol. Biol. Med. 14, 373–384 (2018). https://doi.org/10.1016/j.nano.2017.11.010
B. Imran, S.J. Khan, I.A. Qazi, M. Arshad, Removal and recovery of sodium hydroxide (NaOH) from industrial wastewater by two-stage diffusion dialysis (DD) and electrodialysis (ED) processes. Desalin. Water Treat. 57, 7926–7932 (2016). https://doi.org/10.1080/19443994.2015.1048742
C.Y. Tang, Q.S. Fu, A.P. Robertson, C.S. Criddle, J.O. Leckie, Use of reverse osmosis membranes to remove perfluorooctane sulfonate (PFOS) from semiconductor wastewater. Environ. Sci. Technol. 40, 7343–7349 (2006). https://doi.org/10.1021/es060831q
D. Kavak, M. Demir, B. Başsayel, A.S. Anagün, Factorial experimental design for optimizing the removal of lead ions from aqueous solutions by cation exchange resin. Desalin. Water Treat. 51, 1712–1719 (2013). https://doi.org/10.1080/19443994.2012.714640
S.-A. Safavi-Mirmahalleh, M. Salami-Kalajahi, H. Roghani-Mamaqani, Adsorption kinetics of methyl orange from water by pH-sensitive poly(2-(dimethylamino)ethyl methacrylate)/nanocrystalline cellulose hydrogels. Environ. Sci. Pollut. Res. 27, 28091–28103 (2020). https://doi.org/10.1007/s11356-020-09127-y
S.-A. Safavi-Mirmahalleh, M. Salami-Kalajahi, H. Roghani-Mamaqani, Effect of surface chemistry and content of nanocrystalline cellulose on removal of methylene blue from wastewater by poly(acrylic acid)/nanocrystalline cellulose nanocomposite hydrogels. Cellulose 26, 5603–5619 (2019). https://doi.org/10.1007/s10570-019-02490-1
Z. Abousalman-Rezvani, P. Eskandari, H. Roghani-Mamaqani, H. Mardani, M. Salami-Kalajahi, Grafting light-, temperature, and CO2-responsive copolymers from cellulose nanocrystals by atom transfer radical polymerization for adsorption of nitrate ions. Polymer 182, 121830 (2019). https://doi.org/10.1016/j.polymer.2019.121830
Z. Abousalman-Rezvani, P. Eskandari, H. Roghani-Mamaqani, M. Salami-Kalajahi, Synthesis of coumarin-containing multi-responsive CNC-grafted and free copolymers with application in nitrate ion removal from aqueous solutions. Carbohydr. Polym. 225, 115247 (2019). https://doi.org/10.1016/j.carbpol.2019.115247
O. Valdés, C. Vergara, F.M. Nachtigall, Z. Lopez-Cabaña, J. Tapia, L.S. Santos, PAMAM built-on-silicon wafer thin-layer extraction devices for selective metal contamination detection. Tetrahedron Lett. 57, 2468–2473 (2016). https://doi.org/10.1016/j.tetlet.2016.04.063
D. Gajjar, R. Patel, H. Patel, P.M. Patel, Removal of heavy metal ions from water by hydroxyl terminated triazine-based dendrimer. Desalin. Water Treat. 55, 1209–1219 (2015). https://doi.org/10.1080/19443994.2014.922446
M.S. Diallo, L. Balogh, A. Shafagati, J.H. Johnson, W.A. Goddard, D.A. Tomalia, Poly(amidoamine) dendrimers: a new class of high capacity chelating agents for Cu(II) ions. Environ. Sci. Technol. 33, 820–824 (1999). https://doi.org/10.1021/es980521a
X.C. Peng, X.H. Peng, S.M. Liu, J.Q. Zhao, Synthesis and properties of new amphoteric poly (amidoamine) dendrimers. Exp. Polym. Lett. 3, 510–517 (2009). https://doi.org/10.3144/expresspolymlett.2009.63
M.A. Barakat, M.H. Ramadan, M.A. Alghamdi, S.S. Algarny, H.L. Woodcock, J.N. Kuhn, Remediation of Cu(II), Ni(II), and Cr(III) ions from simulated wastewater by dendrimer/titania composites. J. Environ. Manag. 117, 50–57 (2013). https://doi.org/10.1016/j.jenvman.2012.12.025
F. Zhang, B. Wang, S. He, R. Man, Preparation of graphene-oxide/polyamidoamine dendrimers and their adsorption properties toward some heavy metal ions. J. Chem. Eng. Data 59, 1719–1726 (2014). https://doi.org/10.1021/je500219e
B. Hayati, M. Arami, A. Maleki, E. Pajootan, Thermodynamic properties of dye removal from colored textile wastewater by poly (propylene imine) dendrimer. Desalin. Water Treat. 56, 97–106 (2015). https://doi.org/10.1080/19443994.2014.931529
F.E. Peer, N. Bahramifar, H. Younesi, Removal of Cd(II), Pb(II) and Cu(II) ions from aqueous solution by polyamidoamine dendrimer grafted magnetic graphene oxide nanosheets. J. Taiwan Inst. Chem. Eng. 87, 225–240 (2018). https://doi.org/10.1016/j.jtice.2018.03.039
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Najafi, F., Salami-Kalajahi, M. & Roghani-Mamaqani, H. A review on synthesis and applications of dendrimers. J IRAN CHEM SOC 18, 503–517 (2021). https://doi.org/10.1007/s13738-020-02053-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-02053-3